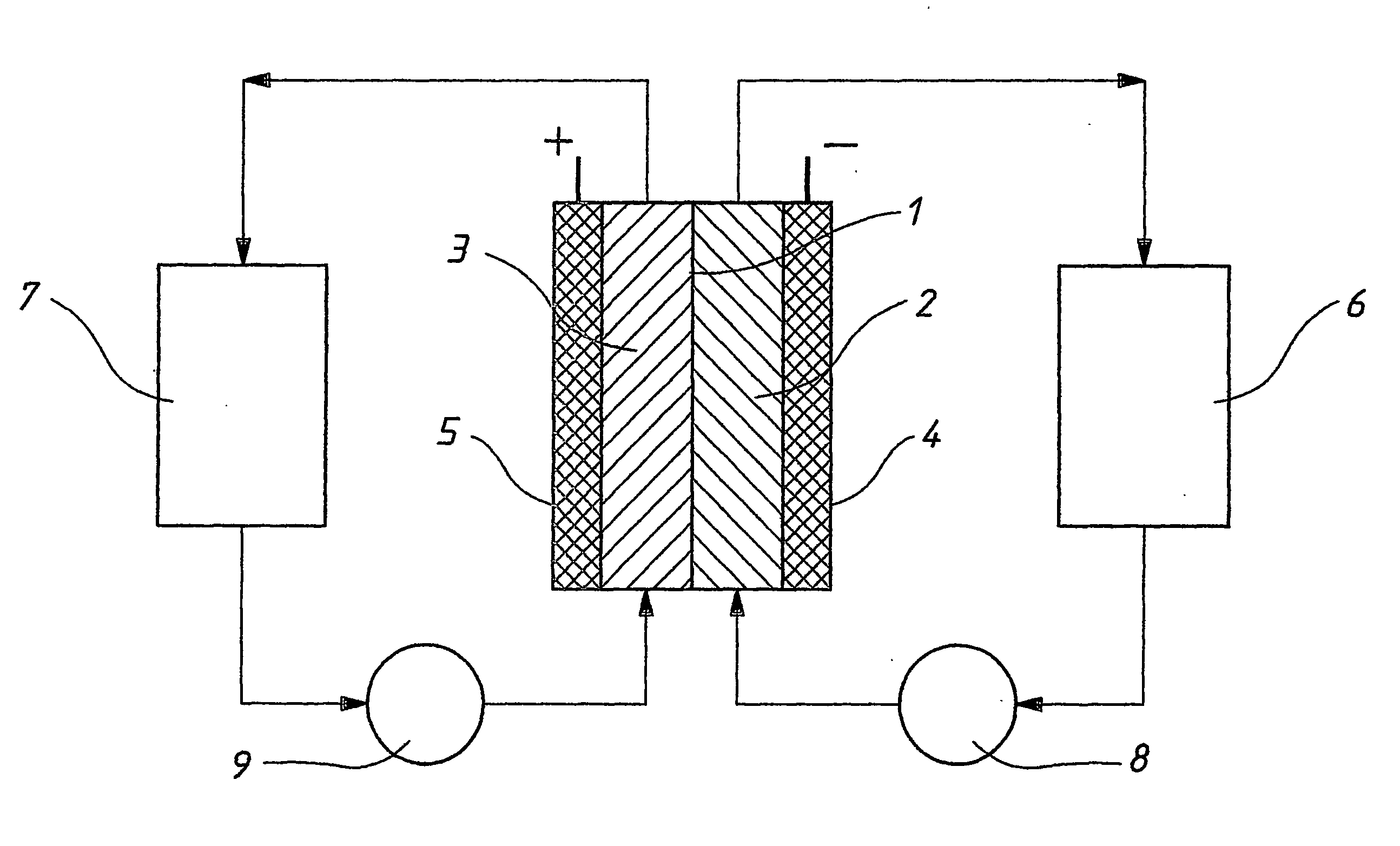
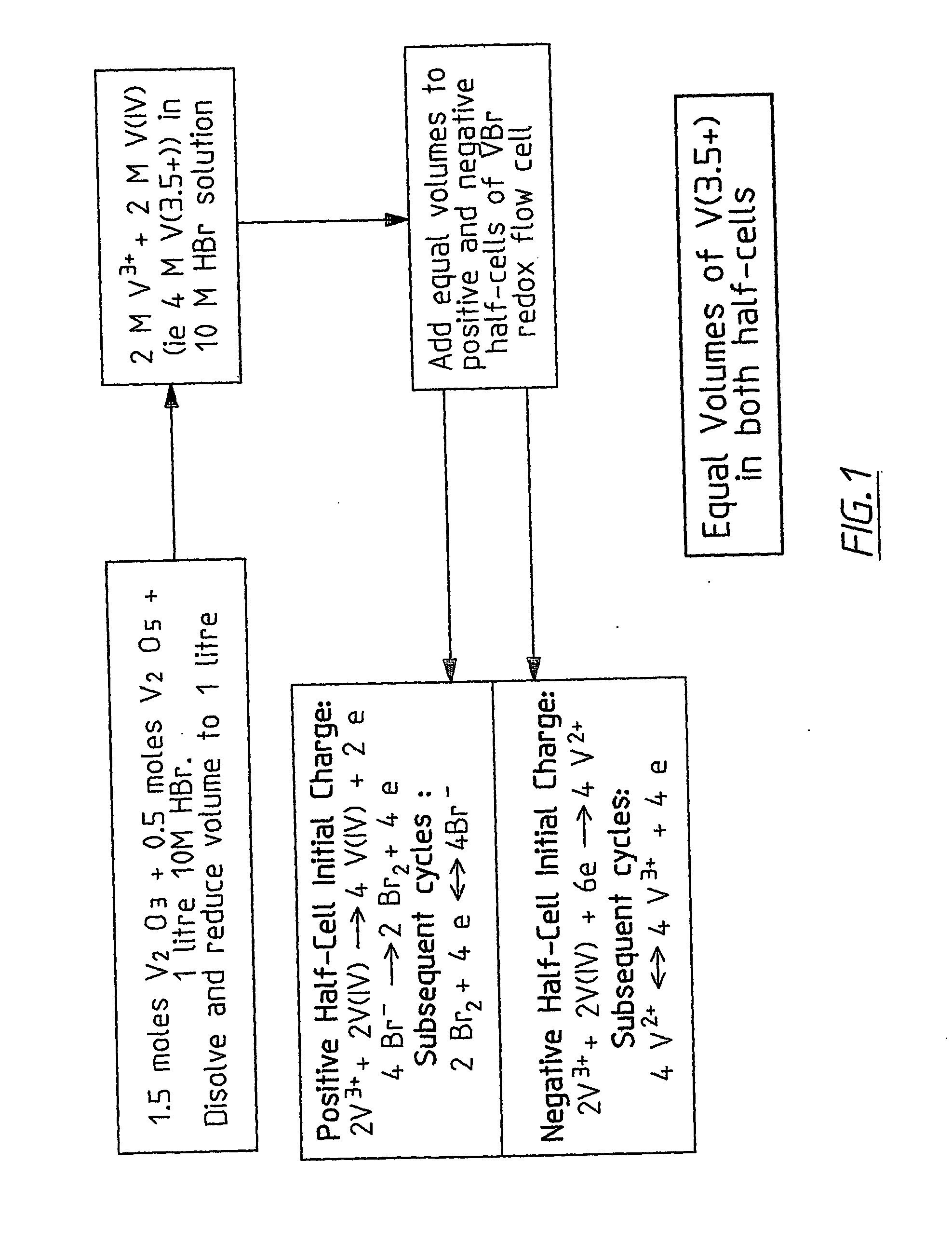
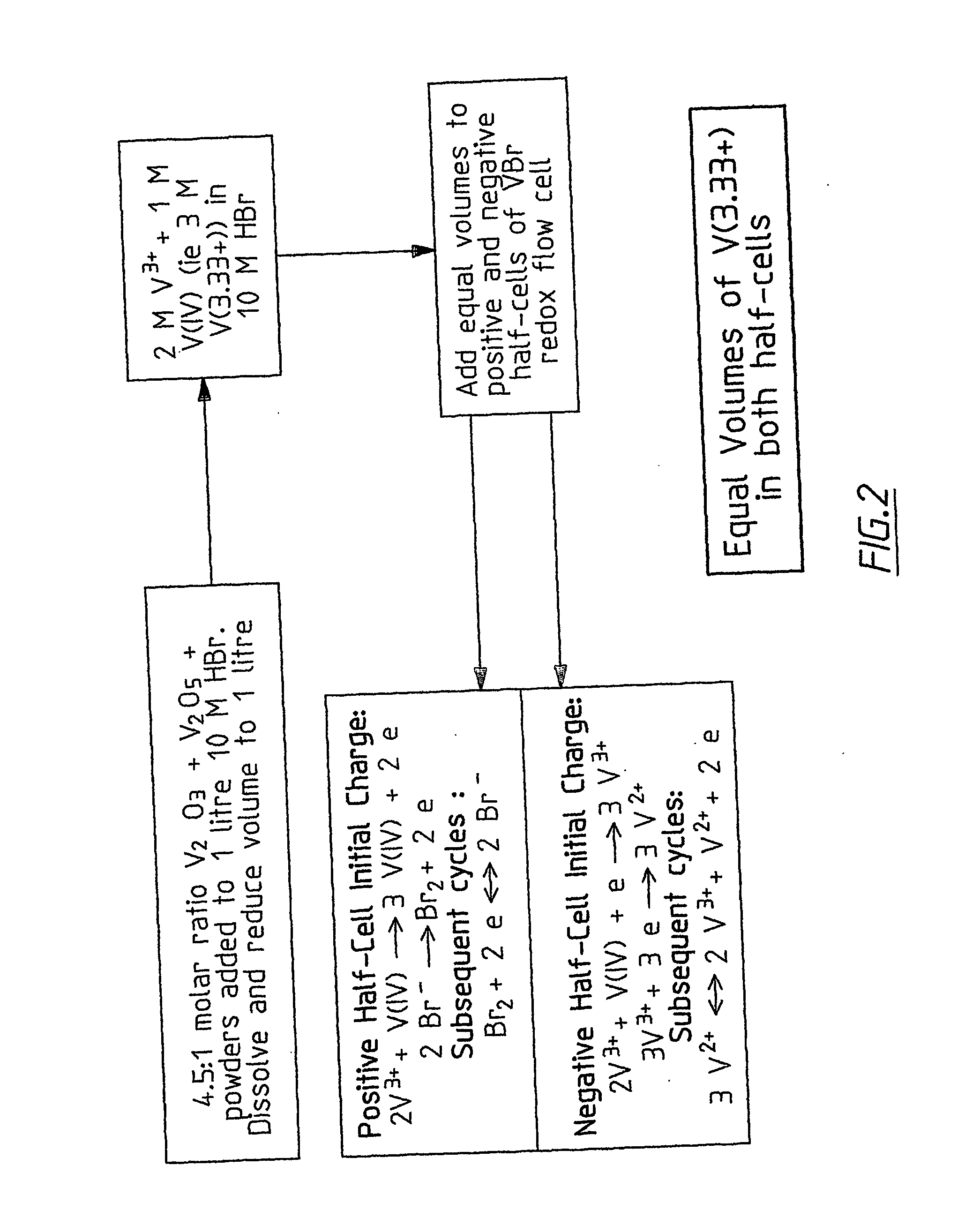
Novel vanadium halide redox flow battery
a redox flow battery and vanadium halide technology, applied in the direction of non-aqueous electrolyte cells, indirect fuel cells, cell components, etc., can solve the problems of reducing the life of cell components, causing bromine gas emission, etc., and achieve the effect of avoiding excessive bromine generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0166]FIG. 6 shows the initial charge-discharge curve for a vanadium halide redox flow cell containing 60 ml in each half-cell of a 2 M V(3.5+) solution in 6 M Br− plus 2 M Cl−. The cell employed a Gore Select P-03430 membrane. The cell was initially charged to a voltage of 1.25 V and discharged to a lower voltage limit of 0.25 V at a constant current of 1.0 Amp. The ratio of the initial charge time to the discharge time is seen to be approximately 1.5 showing that during the initial charge, 1.5 electrons per mole of vanadium are used in converting the V(3.5+) solution to V2+ in the negative half-cell while in the positive half-cell, V(III) ions are first oxidised to V(IV) followed by the oxidation of Br− ions to Br3−. Subsequent charge-discharge cycles gave average charge and discharge time ratios of between 1.05 and 1.1, corresponding to coulombic efficiencies of 95 to 91%.
example 2
[0167] A cell employing a solution of 3 M vanadium (III) / (IV) bromide as the active material in both half-cells was set up and evaluated as follows:
[0168] The 50:50 vanadium(III) / vanadium (IV) bromide mixture (referred to as 3 M V(3.5+) was prepared by dissolving the required amounts of vanadium trioxide and pentoxide powders in 8 M hydrobromic acid. Hydrochloric acid was also added to bring the final solution chloride concentration to 1.5 M.
[0169]FIG. 7 shows the cell voltage versus time curves obtained during the charging and discharging cycling of the cell containing 60.0 mls of the solution in each half-cell and employing a Gore Select P-03430 membrane. The cell was charged to a voltage of 1.6 V and discharged to a lower voltage limit of 0.25 V at a constant current of 2.0 Amp. The theoretical charge and discharge time, assuming complete reaction of the vanadium ions in the negative half-cell was calculated as 2.4 hours. This compares with measured charge and discharge times o...
example 3
[0170] A 3 M V(3.5+) solution was prepared by combining a 3:1 mole ratio of V2O3:V2O5 powders as follows:
[0171] Mass V2O3 used: 168.64 g
[0172] Mass V2O5 used: 62.21 g
[0173] 1000 ml of 8M HBr was mixed with 150 ml 10 M HCl, stirred and heated around to 80° C. The V2O3 powder was slowly added to the HBr / HCl mixture, followed by slow addition of the V2O5 powder. The solution was then boiled to around 150° C. for about 1 hour. The final volume was approximately 1010 ml. During the vanadium oxide dissolution, negligible bromine was detected. When the same process was repeated by simultaneously adding the vanadium trioxide and vanadium pentoxide powders to the HBr / HCl mixture, however, bromine gas was observed to form as the vanadium pentoxide oxidised the bromide ions to bromine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com