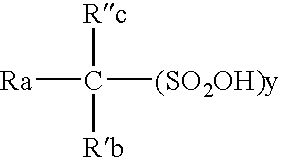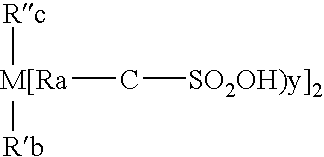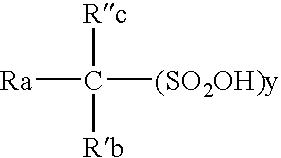High purity electrolytic sulfonic acid solutions
a technology of sulfonic acid and high purity, applied in the field of high purity sulfonic acid, can solve the problems of poor corrosion resistance of deposited metal, etc., and achieve the effect of effective us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0071] A. Commercially available 70% methanesulfonic acid (MSA) that contains a high concentration of methylmethanethiosulfonate, [MMTS, (CH3SO2SCH3)] was purified by the following procedure: [0072] (1) 250 ml of 70% methanesulfonic acid containing 12 parts-per-million (ppm) was placed into a 500 ml beaker. [0073] (2) 30% hydrogen peroxide solution, 1.125 grams, was added and the MSA was heated to 140 degrees Fahrenheit for three hours. [0074] (3) The methanesulfonic acid solution was cooled to room temperature and analyzed for MMTS.
[0075] The concentration of MMTS after the hydrogen peroxide treatment was non-detectable by gas chromatography methods. After purification the MSA was diluted to 15% by the addition of water.
[0076] B. A sample of 15% methanesulfonic acid (MSA) made according to Example 1A was electrolyted using a pure tin anode, an insoluble iridium oxide-coated titanium cathode. During electrolysis, tin dissolved into the 15% MSA. Trichloromethylmethylsulfone (TCMS) ...
example 2
[0077] A sample of 15% methanesulfonic acid (MSA) made as in Example 1(A) was electrolyted using a pure tin anode, an insoluble iridium oxide-coated titanium cathode.
[0078] During electrolysis, tin dissolved into the 15% MSA. Methylmethanethiosulfonate (MMTS) was incrementally added and the headspace gas above the electrochemical cell was smelled for detectable odor. After about 0.25 ppm of MMTS was added, an odor was noticeable. MMTS was seen to decompose using chromatographic techniques to primarily dimethyldisulfide (DMDS) in the electrolysis cell. Methylmercaptan (CH3SH) and dimethylsulfide (DMS) were also observed after electrolysis, both odorous impurities.
example 3
[0079] A sample of 15% methanesulfonic acid (MSA) made as in Example 1(A) was electrolytesdusing a pure tin anode, an insoluble iridium oxide-coated titanium cathode.
[0080] During electrolysis, tin dissolved into the 15% MSA. Methylmethanethiosulfonate (MMTS) was incrementally added up to 0.85 ppm. An odor was detectable. To this solution was added 1.0 ppm of Tricloromethylsulfone (TCMS). A very pungent odor was observed indicating a synergy between the two impurities in producing the undesirable odor.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com