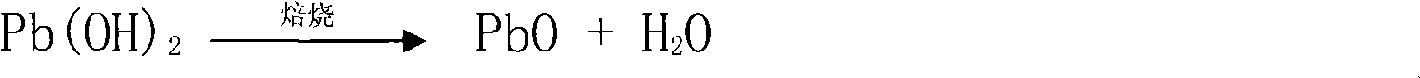Patents
Literature
1574 results about "Lead oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
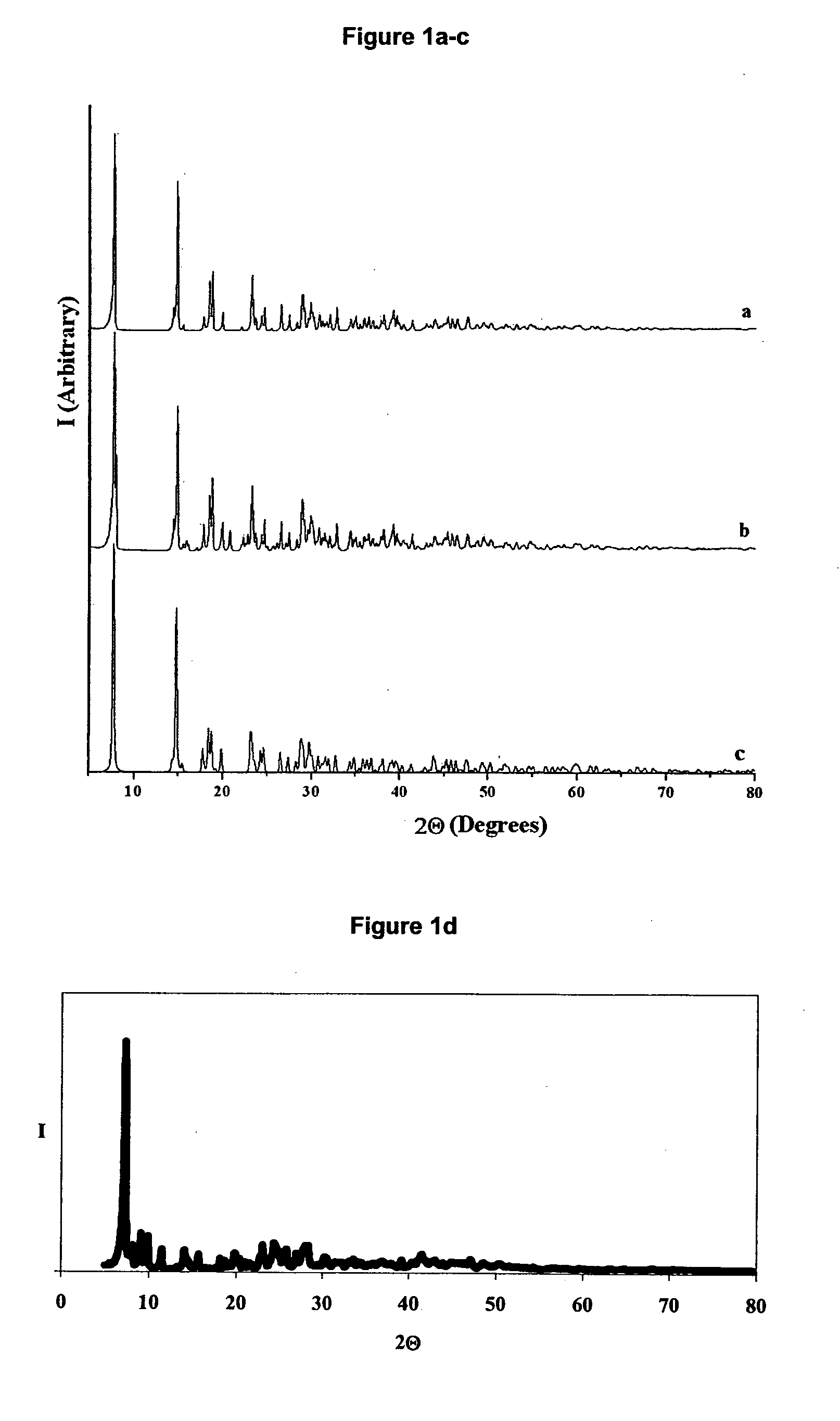
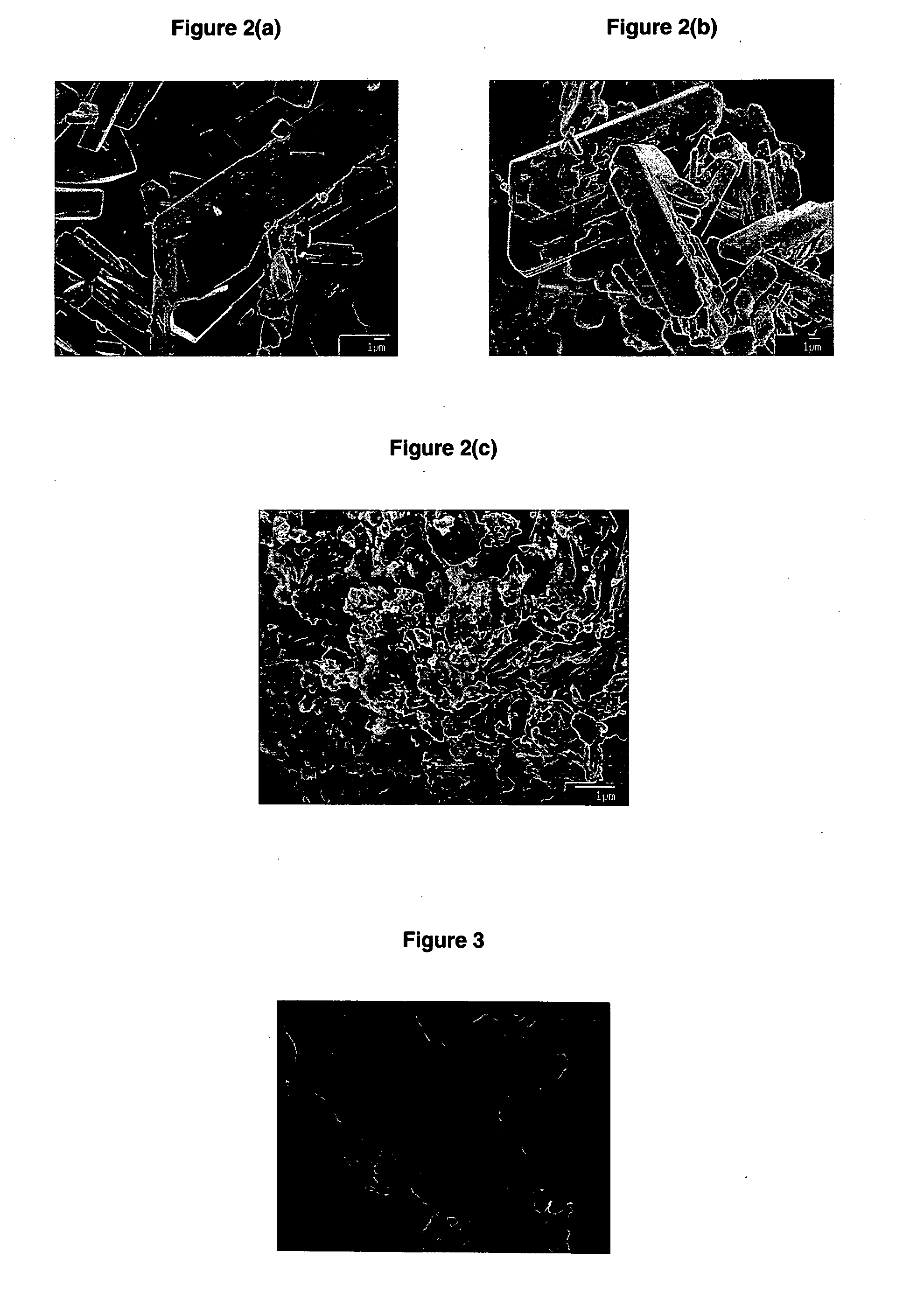
Lead oxides are a group of inorganic compounds with formulas including lead (Pb) and oxygen (O).
Optical fiber with quantum dots
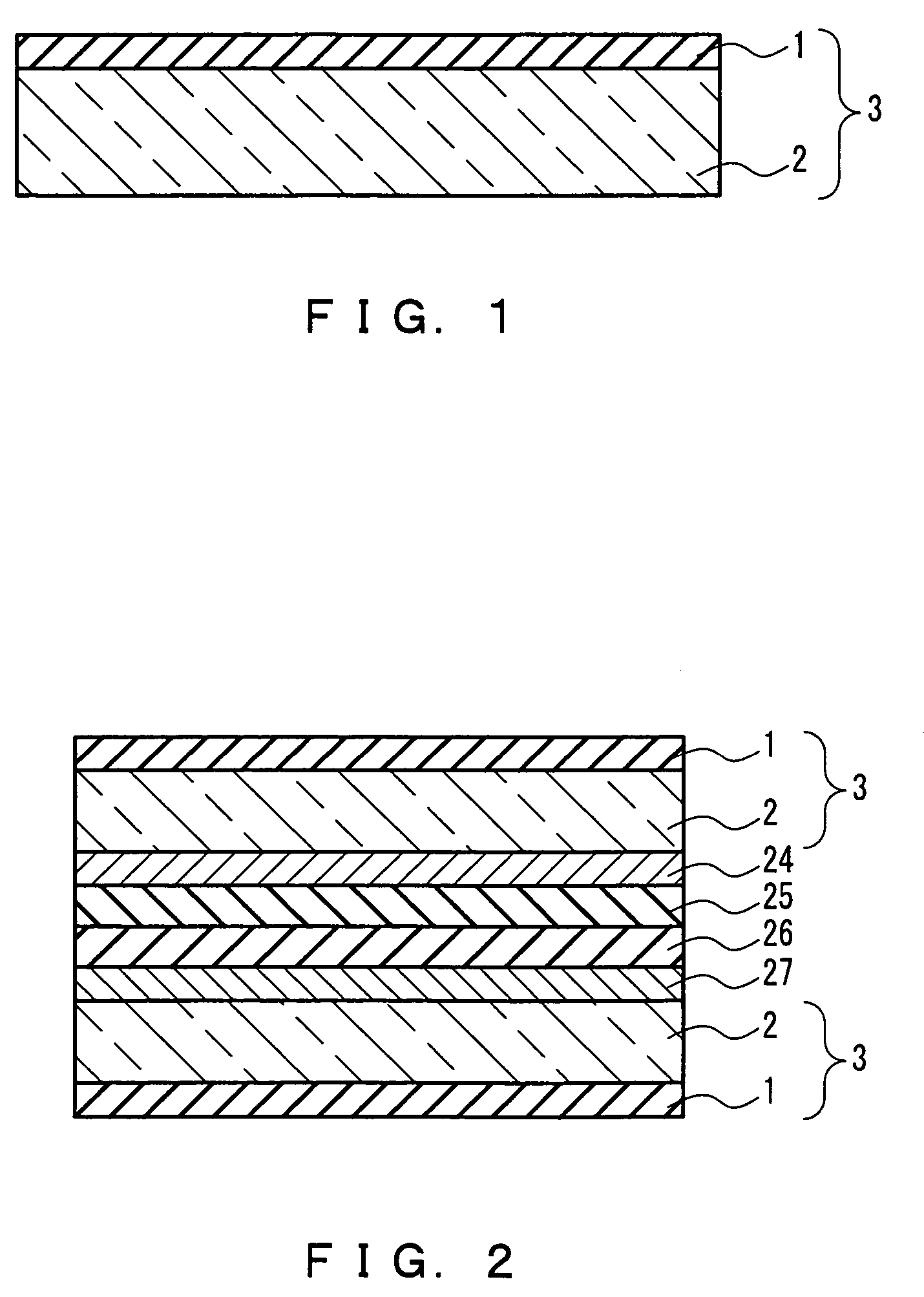
Holey optical fibers (e.g. photonic fibers, random-hole fibers) are fabricated with quantum dots disposed in the holes. The quantum dots can provide light amplification and sensing functions, for example. When used for sensing, the dots will experience altered optical properties (e.g. altered fluorescence or absorption wavelength) in response to certain chemicals, biological elements, radiation, high energy particles, electrical or magnetic fields, or thermal / mechanical deformations. Since the dots are disposed in the holes, the dots interact with the evanescent field of core-confined light. Quantum dots can be damaged by high heat, and so typically cannot be embedded within conventional silica optical fibers. In the present invention, dots can be carried into the holes by a solvent at room temperature. The present invention also includes solid glass fibers made of low melting point materials (e.g. phosphate glass, lead oxide glass) with embedded quantum dots.
Owner:LAMBDA LABORATORY INSTRUMENTS +1
Optical fiber with quantum dots
Holey optical fibers (e.g. photonic fibers, random-hole fibers) are fabricated with quantum dots disposed in the holes. The quantum dots can provide light amplification and sensing functions, for example. When used for sensing, the dots will experience altered optical properties (e.g. altered fluorescence or absorption wavelength) in response to certain chemicals, biological elements, radiation, high energy particles, electrical or magnetic fields, or thermal / mechanical deformations. Since the dots are disposed in the holes, the dots interact with the evanescent field of core-confined light. Quantum dots can be damaged by high heat, and so typically cannot be embedded within conventional silica optical fibers. In the present invention, dots can be carried into the holes by a solvent at room temperature. The present invention also includes solid glass fibers made of low melting point materials (e.g. phosphate glass, lead oxide glass) with embedded quantum dots.
Owner:LAMBDA LABORATORY INSTRUMENTS +1
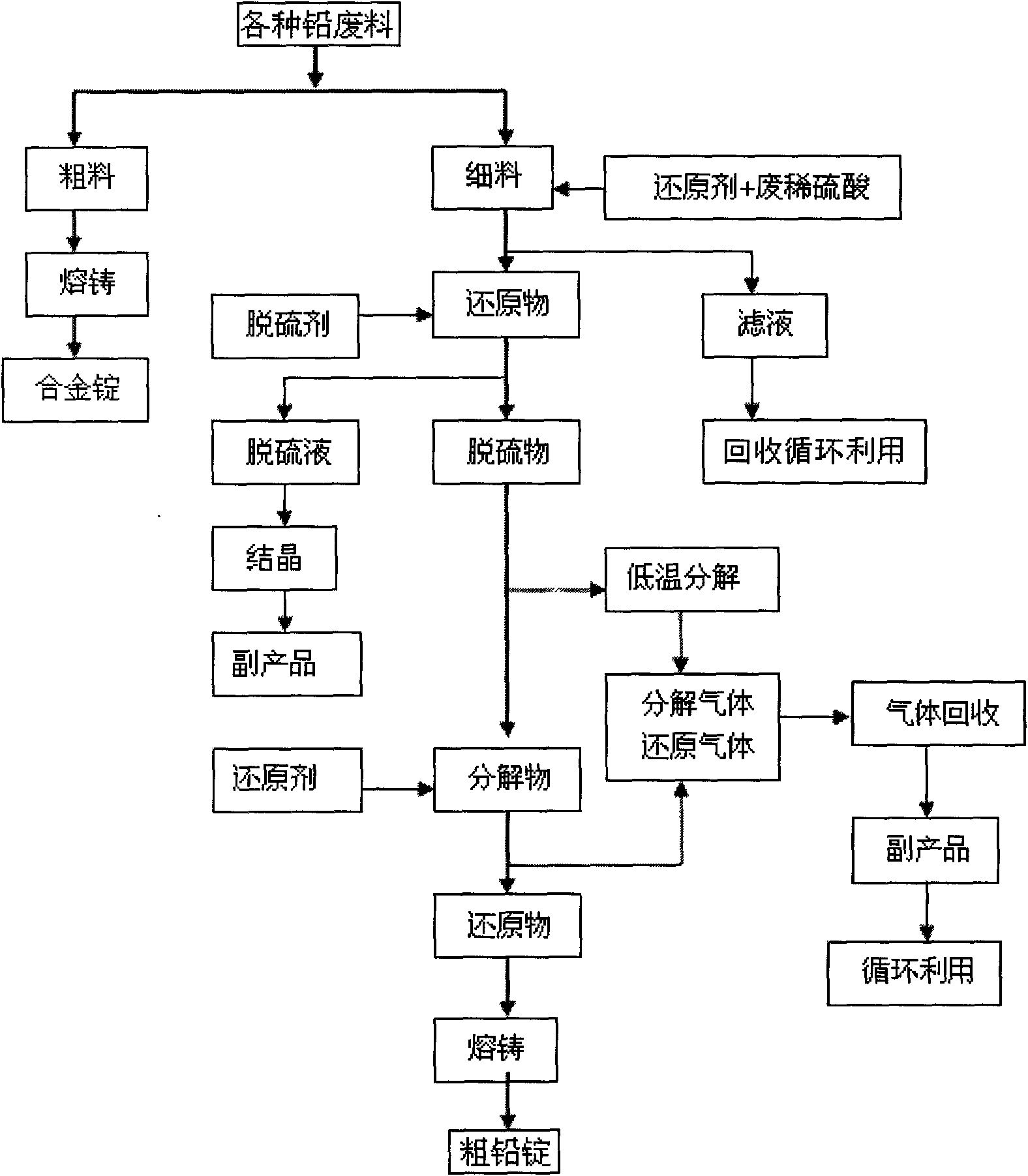
Waste lead recovering method for lead-acid storage batteries
InactiveCN101608264AAvoid harmLower decomposition temperaturePhotography auxillary processesProcess efficiency improvementLead dioxideEngineering
The invention discloses a waste lead recovering method for lead-acid storage batteries. The method comprises the following steps: fine stuff such as diachylon and the like are added in a reaction kettle with a stirring device; reducing agent (FeSO4) and dilute sulfuric acid are simultaneously added; stirring reaction is carried out at the temperature of 50-60 DEG C for 50-70 minutes so as to reduce lead dioxide into lead sulfate; the lead sulfate is added into the reaction kettle with the stirring device; water is simultaneously added into the reaction kettle for size mixing; then sodium carbonate is added; desulfuration is carried out at the temperature of 50-60 DEG C so as to obtain solid lead carbonate; the lead carbonate is put into a smelting furnace and then decomposed at the temperature of 320-350 DEG C so as to obtain lead oxide; and reducing agent (carbon) is added into the smelting furnace to reduce the lead oxide into metal lead at the temperature of 700-800 DEG C. The method recovers the lead by means of the combination of the wet and the dry processes, thereby avoiding the harm to the environment caused by lead dust, lead vapor, lead skim, sulfur dioxide gas, and the like by adopting fire smelting. The method has the advantages of high lead recovery rate, low energy consumption and no environment pollution.
Owner:张天任
Process for recovering lead oxides from exhausted batteries
ActiveUS7507496B1Speed up the processObtained inexpensivelySolvent extractionPrimary cell maintainance/servicingLead dioxideLead oxide
A process for recovering lead oxides from the spent paste of exhausted lead acid batteries. The process provides heating the spent paste with an alkali hydroxide solution at elevated temperatures prior to calcinations. Calcination is at various temperatures so that either lead mono-oxide, lead dioxide or red lead is obtained as the principal product. There is also provided the use of the lead oxide to prepare the paste for positive and negative electrodes or other lead compounds.
Owner:RETRIEV TECH +1
Method for producing lead oxide by recovering waste lead-acid batteries based on atom economy way
ActiveCN103146923AImprove recycling efficiencyMeet the needs of high-purity PbOProcess efficiency improvementChemical industryLead oxide
The invention provides a method for producing lead oxide by recovering waste lead-acid batteries based on an atom economy way and belongs to the field of chemical industry for clearing and recovering waste lead-acid batteries. The method comprises the steps of: heating lead paste and lead powder of the lead-acid batteries, and then carrying out solid phase mixing reaction, sodium hydroxide alkaline desulfurization and sodium hydroxide leaching to directly obtain a lead-bearing alkaline solution and filter residue; and carrying out purification and cooling crystallization on the solution so as to obtain high-purity lead oxide and a by-product sodium sulfate so as to eliminate the defect that a large quantity of chemical raw materials are required to be consumed in a conventional synthesis process of the lead oxide so that the method is a clean and energy-saving new technology and has a large-scale industrial application prospect.
Owner:BEIJING UNIV OF CHEM TECH
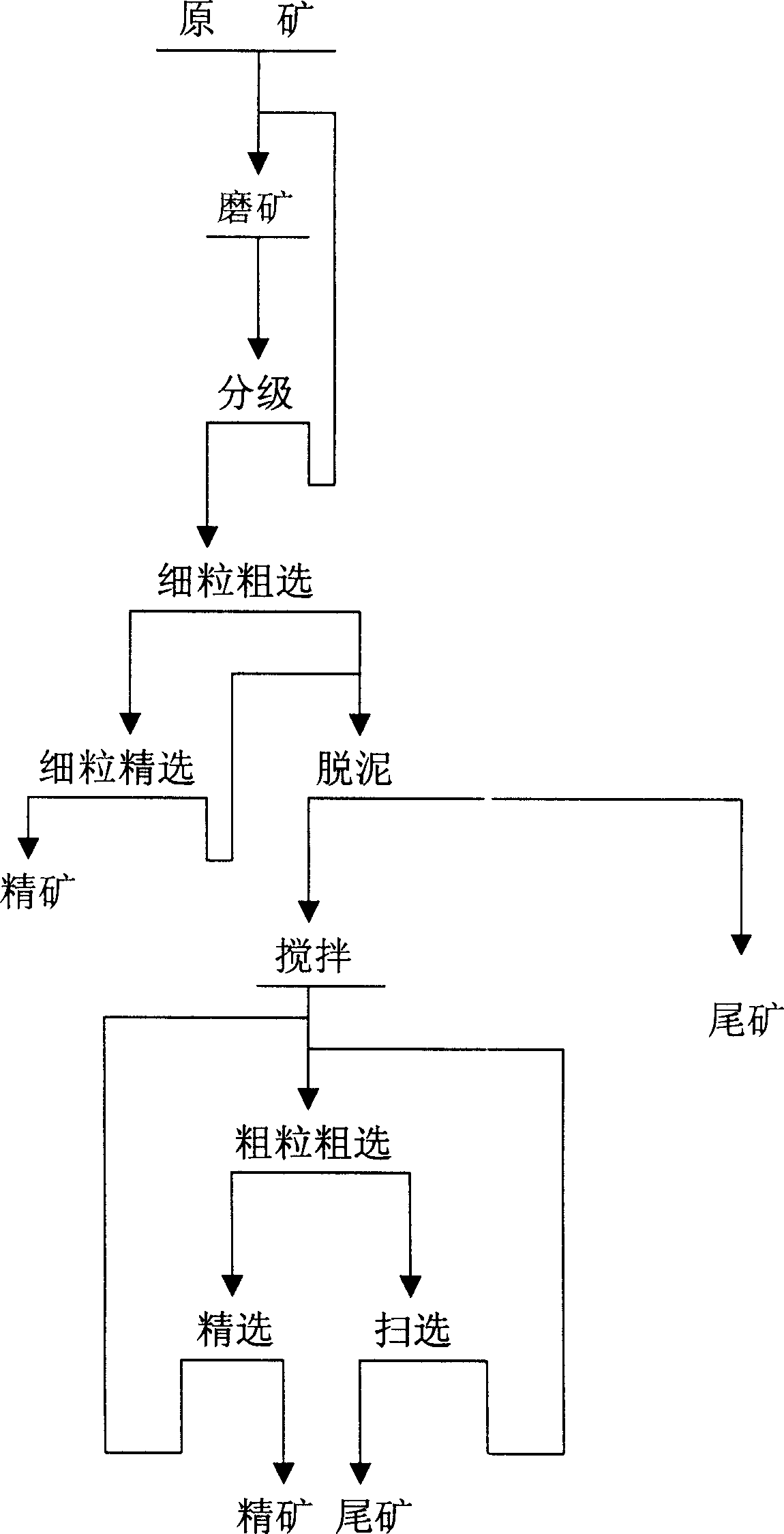
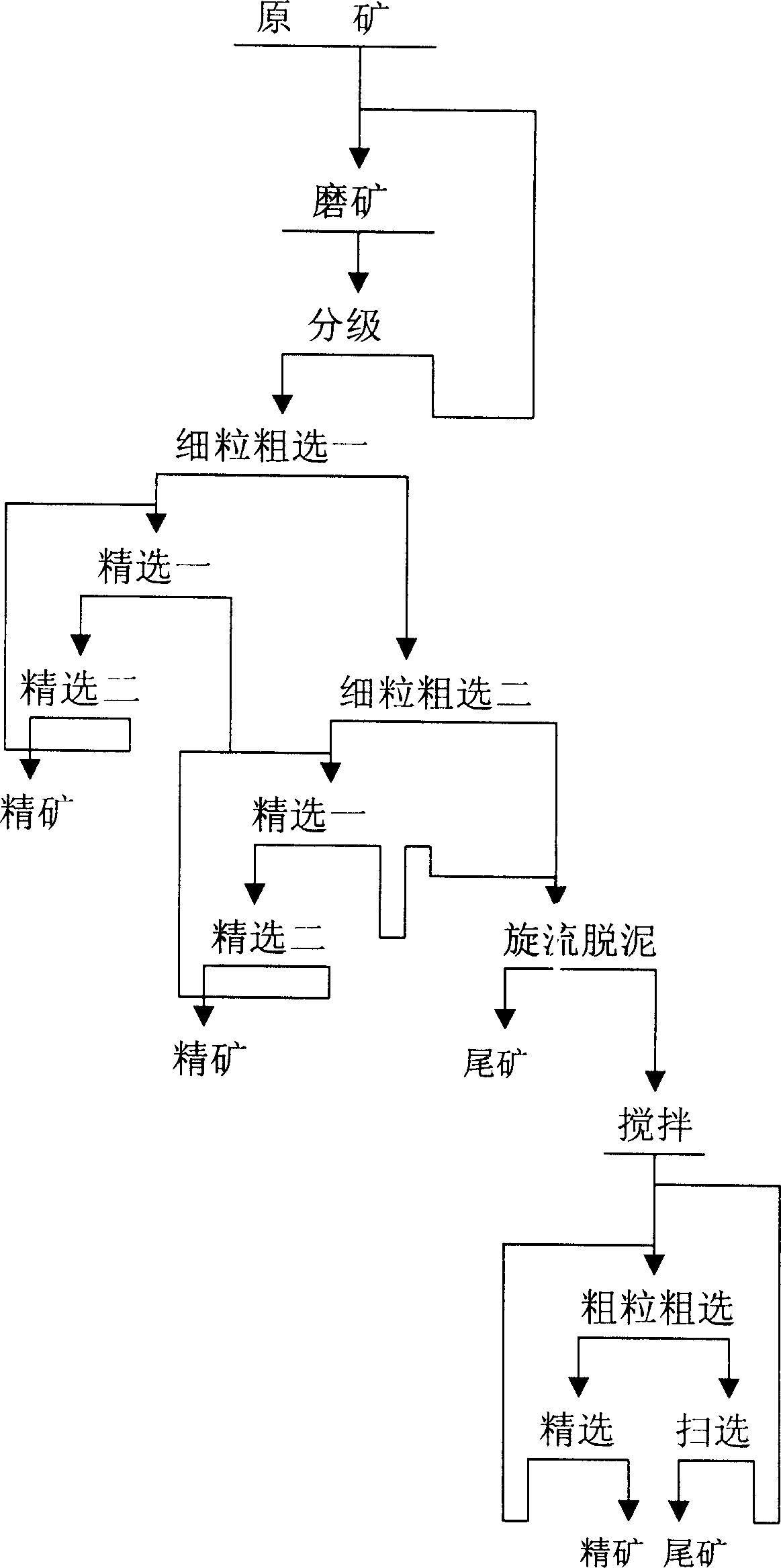
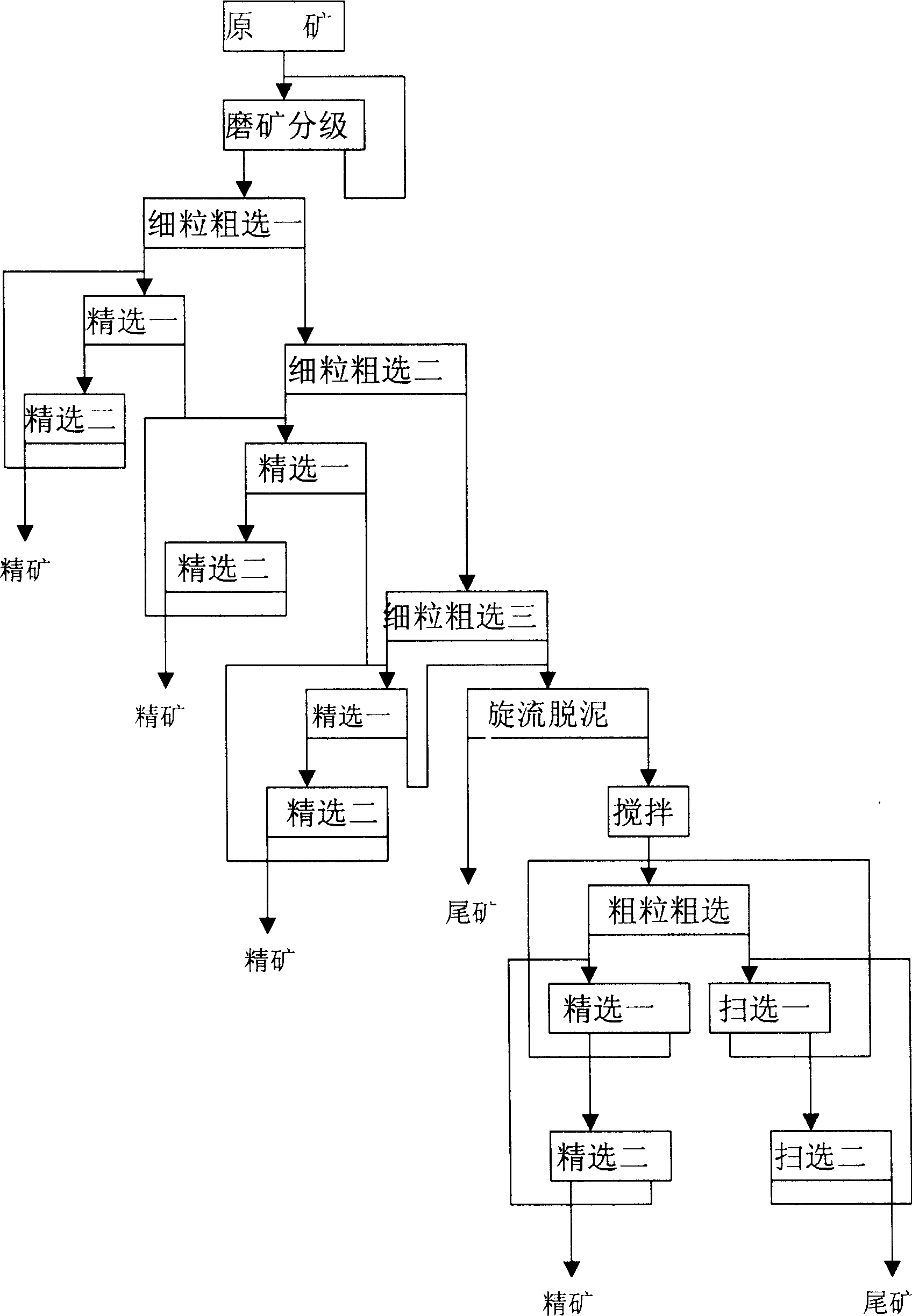
Beneficiation method for zinc oxide mine
InactiveCN1820853ASolve the problem of difficult flotation of argillaceous zinc oxide oreSolve the problem of flotationFlotationWet separationMineral SourcesLead oxide
The beneficiation method for zinc oxide ore includes milling, lead oxide floating, 1-3 grade of fine grain roughing of the tailings after lead oxide floating, 1-3 times of beneficiation for each roughed concentrate, roughing the middlings of the last time and the middlings of the first beneficiation, and final precise floating to obtain the concentrate. The present invention solves the technological problem of floating muddy zinc oxide ore by means of first floating of small grains and then the large grains, and has high zinc oxide ore recovering rate, reduced zinc oxide content in the tailings, lowered floating agent consumption and effective utilization of muddy zinc oxide ore.A
Owner:陈铁 +1
Ultrafine lead oxide prepared by using waste lead plaster and preparation method thereof
InactiveCN103374657AReduce energy consumptionSimple ingredientsReclaiming serviceable partsLead oxidesFiltrationTwo step
The invention discloses an ultrafine lead oxide prepared by using a waste lead plaster and a preparation method thereof. The preparation method comprises the following steps of: carrying out desulphurization process by mixing the waste lead plaster with an aqueous solution containing a composite desulfurizer for reaction; carrying out filtration to remove the desulphurization filtering solution to obtain the desulfurated lead plaster (filter residue); carrying out a leaching and crystal transformation process by adding a citric acid solution and a reducing agent into the desulfurated lead plaster obtained in the process, and carrying out filtration, washing, and drying to obtain the lead citrate after the desulfurated lead plaster reacts with the citric acid solution; carrying out a roasting process by roasting the lead citrate to obtain the ultrafine lead oxide. According to the preparation method disclosed by the invention, the ultrafine lead oxide is prepared from the waste lead storage lead plaster; a two-step leaching process is adopted; the filtering solution is simple in ingredient and can be recycled; a side product is recycled from the desulphurization solution. The preparation method disclosed by the invention is low in energy consumption, simple in equipment, high in lead recycling rate, and high in ultrafine lead product quality, and has the characteristics of good resource recycling effect, environmentally-friendly and pollution-free production process, and capability of clean production.
Owner:湖北金洋冶金股份有限公司 +1
Electrical power storage devices
ActiveUS20110311876A1Large specific surface areaMaterial nanotechnologyNon-insulated conductorsElectrical batteryCarbon nanofiber
An electrical storage device includes high surface area fibers (e.g., shaped fibers and / or microfibers) coated with carbon (graphite, expanded graphite, activated carbon, carbon black, carbon nanofibers, CNT, or graphite coated CNT), electrolyte, and / or electrode active material (e.g., lead oxide) in electrodes. The electrodes are used to form electrical storage devices such as electrochemical batteries, electrochemical double layer capacitors, and asymmetrical capacitors.
Owner:JOHNSON CONTROLS AUTOBATTERIE GMBH & CO KGAA +1
Method for recovering lead oxide by waste lead-acid storage battery
InactiveCN101514395AReduce energy consumptionReduce pollutionProcess efficiency improvementLiquid wasteFiltration
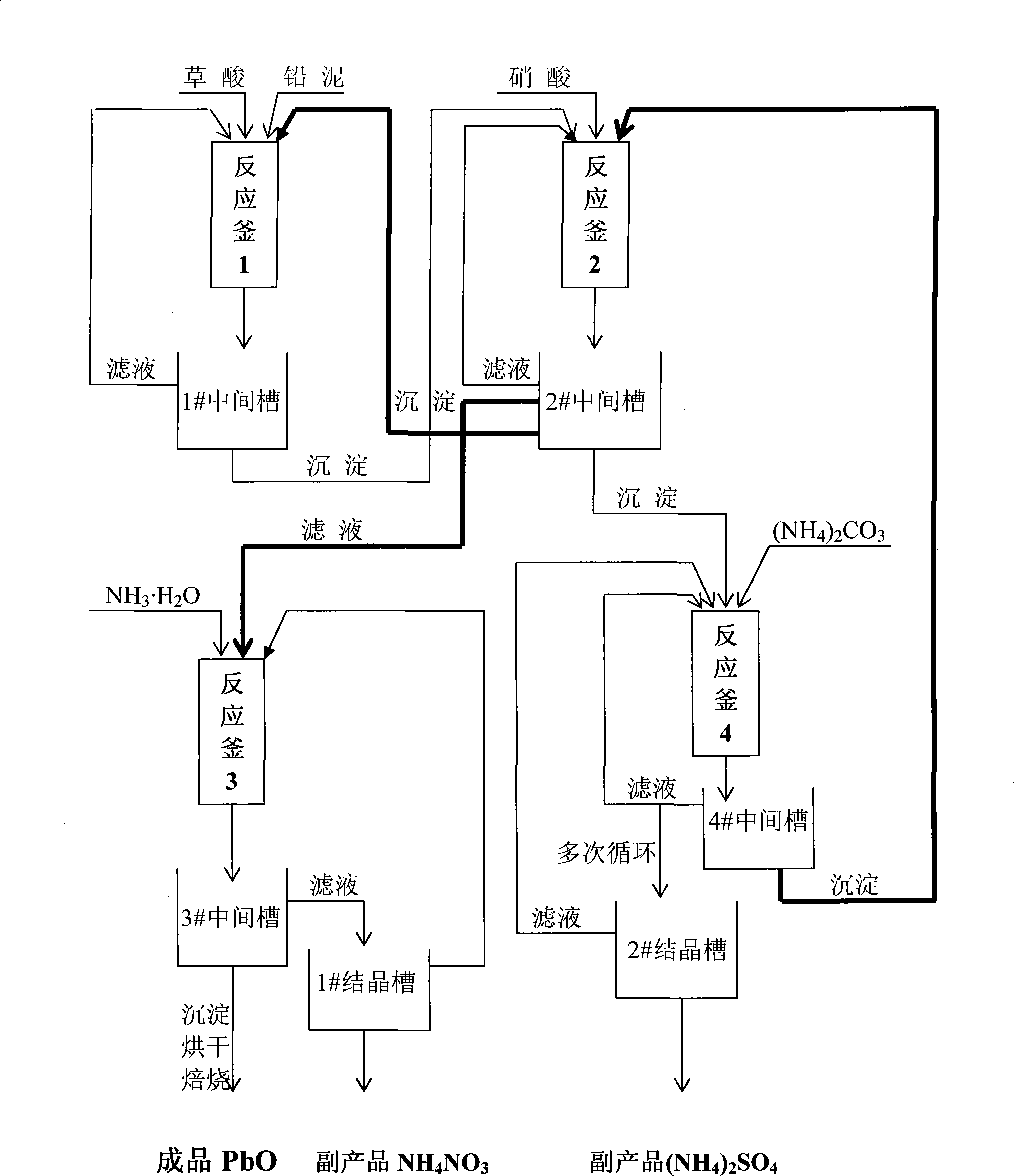
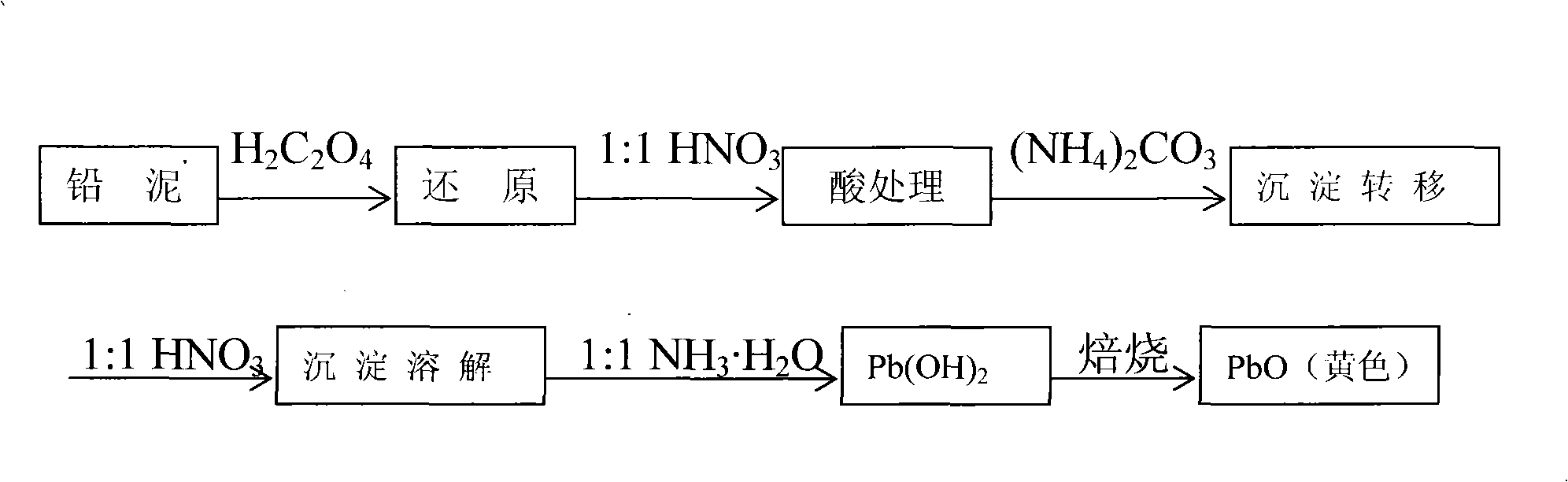
A method for recovering lead oxide by a waste lead-acid storage battery is disclosed. The waste lead-acid storage battery is crushed together with lead slime subsequent to acid cleaning, a grid plate and a filler comprising the lead slime are obtained by screening, the grid plate is fused-cast to an alloy ingot, the filler and the lead slime are ball-milled, and fine stuff is added with saturated oxalic acid solution for reaction at 25-65 DEG C and then for filtering and depositing; the deposition is then treated by excessive 30% nitric acid at the temperature of 40-45 DEG C for subsequent filtration and deposition, and the deposition is reacted with 4wt% sal volatile at the temperature of 25-65 DEG C for subsequent filtration and deposition; the deposition is added into recovered HNO3 to be dissolved at the temperature 40-45 DEG C until no bubble is generated, the filtered filtrate is added with 25% ammonia for reaction, filtration, washing and deposition to be neutral, and the lead oxide is obtained by drying and roasting. Recoverable nitramine and ammonium sulfate are recycled in all the filtrates in the technologies; thereby discharging no waste liquid. The utilization rate of raw material is 90.1-92.1%, the yield is 95.0-96.7%, and the content of PbO is 98.0-98.9%.
Owner:DALIAN WUHUATIANBAO TECH DEV
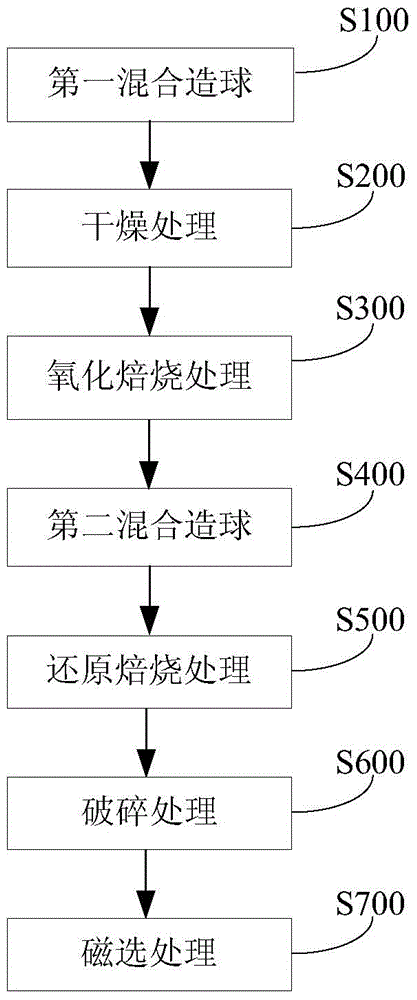
Method for separating valuable metals from copper slag
InactiveCN104404260AReduce consumptionAchieve recyclingProcess efficiency improvementFayaliteAdhesive
The invention discloses a method for separating valuable metals from copper slag. The method comprises the steps: (1) performing primary mixing palletizing on the copper slag and an adhesive to obtain copper slag pellets; (2) performing drying treatment on the copper slag pellets; (3) performing oxidation roasting treatment on the dried copper slag pellets so as to convert fayalite in the copper slag into ferric oxide; (4) performing secondary mixing palletizing on the copper slag pellets subjected to oxidization roasting and a mixture containing a reducing agent, an additive and an adhesive to obtain a pellet material; (5) performing reducing roasting on the pellet material so as to obtain reduced pellets and smoke containing zinc oxide and lead oxide; (6) smashing the reduced pellets to obtain a mixture containing metal iron powder and tailings; (7) performing magnetic separation on the mixture containing the metal iron powder and the tailings to respectively obtain the metal iron powder and the tailings. According to the method disclosed by the invention, iron, zinc and lead can be effectively separated from the copper slag, and the grade of obtained metal iron is higher.
Owner:JIANGSU PROVINCE METALLURGICAL DESIGN INST
Battery paste
A battery paste is disclosed. One such paste consists essentially of at least one lead oxide (i.e., an uncalcined oxide of lead) and at least one lead oxide sulfate, sufficient water to moisten the paste, and from 0.02 percent to 15 percent based on the weight of the lead oxide plus the weight of the lead oxide sulfate, calculated as the lead oxide, of glass fibers having an average diameter not greater than 15 micron. Another paste consists essentially of at least one lead oxide and at least one lead oxide sulfate, sufficient water to moisten the paste, and from 1 percent to 15 percent based on the weight of the lead oxide plus the weight of the lead oxide sulfate, calculated as the lead oxide, of glass fibers of a specific composition that enables specific beneficial ions to diffuse into the paste during the life of the battery.A method for producing such a battery paste and a delivery system for adding the additives that are added into the paste is also disclosed. The method comprises charging a part of the water and a part of the special composition glass fibers desired in the paste to a mechanical mixer, mixing the water and fibers, adding the lead oxide or oxides desired in the paste to the mixer, mixing the water, glass fibers and lead oxide or oxides until essentially all of the free water in the mixer has been mixed with the lead oxide or oxides, adding the rest of the water required to moisten the paste to the desired consistency and the sulfuric acid required to form the lead oxide sulfate or sulfates, and mixing the paste.The delivery system is the charging to a paste batch of a glass fiber mat that has been impregnated with the other required additives in such a proportion that a certain size / weight of the mat provides all the additional ingredients.
Owner:HOLLINGSWORTH VOSE
Cold-resistant rubber cable sheath material and preparation method
ActiveCN102399397AImprove low temperature performanceImprove flame retardant performancePlastic/resin/waxes insulatorsInsulated cablesParaffin waxActive agent
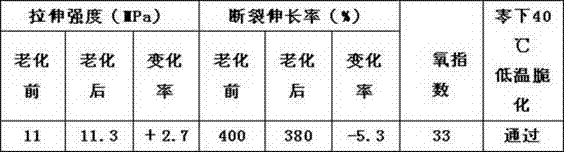
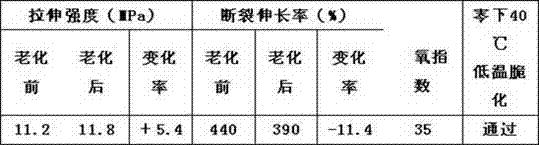
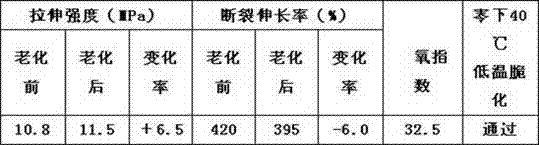
The invention discloses a cold-resistant rubber cable sheath material and a preparation method. The material comprises chlorinated polyethylene, ethylene-propylene-diene monomer (EPDM) rubber, lead oxide, calcium carbonate, N-isopropyl benzene-N'-phenyl p-phenylenediamine serving as an anti-aging agent, antimonous oxide, magnesium oxide, paraffin hydrocarbon oil, chlorinated paraffin, paraffin, carbon black, settled white carbon black, talc powder, gamma-aminopropyl triethoxysilane serving as a surfactant, dicumyl peroxide serving as a vulcanizing agent and triallyl isocyanurate serving as a co-vulcanizing agent. The low temperature performance of the material is improved by blending the chlorinated polyethylene and the EPDM rubber, and meanwhile, the flame-retardant performance of the rubber sheath material is improved by synergy of the antimonous oxide serving as a flame retardant and the chlorinated paraffin; experiments show that the rubber sheath material can pass low-temperature embrittlement test of 40 DEG C below zero, has excellent low-temperature resistance, has the oxygen index of more than 32 and has good flame-retardant performance; and the preparation method is simple and strong in operability.
Owner:JIANGSU HENGTONG POWER CABLE
Lead regeneration method for recovering lead paste from waste lead acid storage battery by wet method
ActiveCN102618884AAvoid churnHigh recovery ratePhotography auxillary processesProcess efficiency improvementElectrolysisLead dioxide
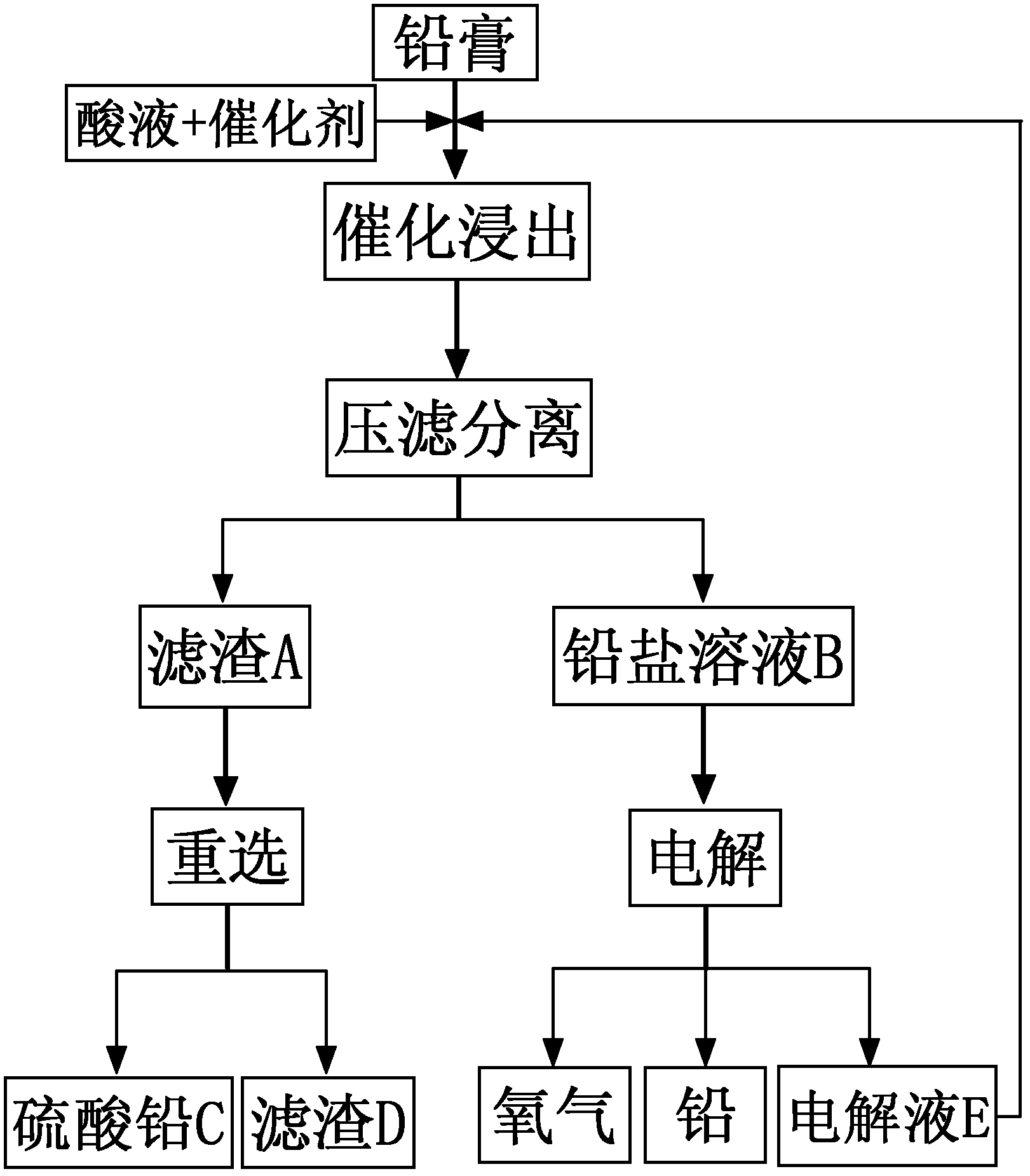
The invention discloses a lead regeneration method for recovering lead paste from a waste lead acid storage battery by a wet method, and belongs to the field of lead regeneration from the lead paste of the waste lead acid battery. The lead regeneration method comprises the following steps of: adding the lead paste into an acid liquor reaction kettle containing a catalyst to perform oxidation reduction reaction on lead and lead dioxide in the lead paste to obtain soluble lead salt solution; meanwhile, reacting lead oxide with acid to obtain lead salt; separating to obtain insoluble filter residue mainly containing soluble lead salt solution and lead sulfate; and electrolyzing the lead salt solution to obtain lead, oxygen and acidic electrolyte, wherein the electrolyte can be returned to a leaching process for recycling, and the filter residue A is treated by the conventional reselection process to obtain the lead sulfate and the filter residue D. According to the lead regeneration method, the production cost is reduced, and high comprehensive lead recovery rate is obtained.
Owner:北京绿色引领环保科技研究院有限公司
Enamel glaze and preparation process thereof
The invention discloses an enamel glaze which belongs to the technical field of ceramic materials, and the enamel glaze comprises a colored glaze basic glaze, an enamel glaze basic glaze and coloring pigments. The invention further discloses a preparation process of the enamel glaze and an application of the enamel glaze. The preparation process can improve the firing temperature of the enamel colored glaze, expand the firing range of the glaze, improve the viscosity of the enamel colored glaze and ensure that coloring metal oxides can not be diffused or the color can not be dimmed due to the flowing of the glaze by optimizing the formula of the enamel colored glaze, adopting an appropriate amount of zinc oxide for replacing heavy metal flux materials, such as lead oxide and the like, integrating the applicable color development performances of a variety of coloring materials and introducing a rare earth compound of yttrium oxide. The preparation process can further ensure that the coloring metal oxides in the enamel glaze can not be oxidized by oxygen or water vapor in the air through the way of underglaze color, thereby further ensuring that a product can not change the color or fade forever, closing heavy metals contained in the coloring oxides for avoiding solventing-out and solving the problem of heavy metal pollution.
Owner:SHENZHEN YONG FENG YUAN IND
Metal modified active carbon fiber electrode and method for removing nitrate thereby
The invention, belonging to water treatment technical field, discloses the electric pole used for removing nitrate in water. The invention uses one of noble metals (palladium, platinum, gold, rhodium, and ruthenium) and one of non noble metals (copper, tin, indium, zinc and silver) to modify the activated carbon fiber and make the electric pole. The method uses the said electric pole as negative pole, and uses graphite and lead oxide as anode, deoxidizing the nitrate at the condition of energisation. The electric pole is cheap and high activity, and the invention is simple operation, convenient management and suits for small-scale decentralized feedwater treatment.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
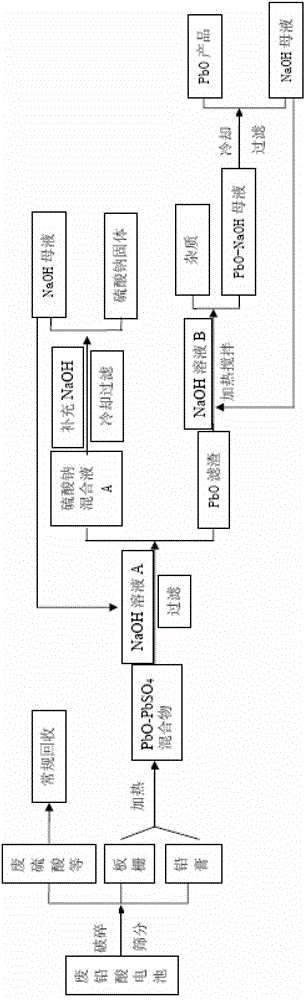
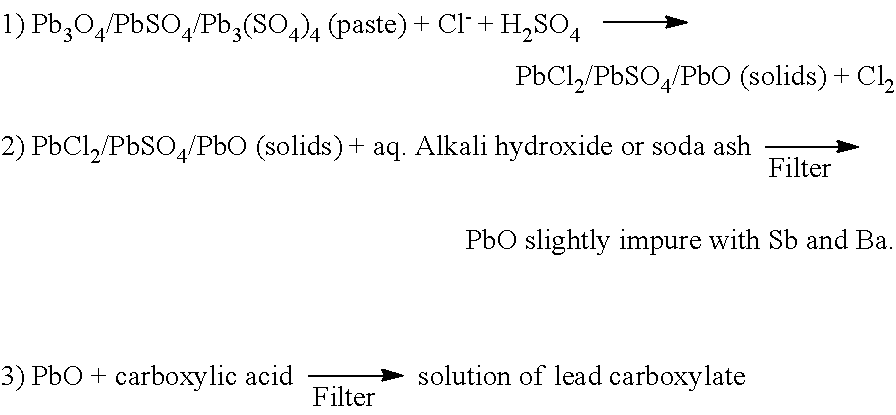
Reclaiming of lead in form of high purity lead compound from recovered electrode paste slime of dismissed lead batteries and/or of lead minerals
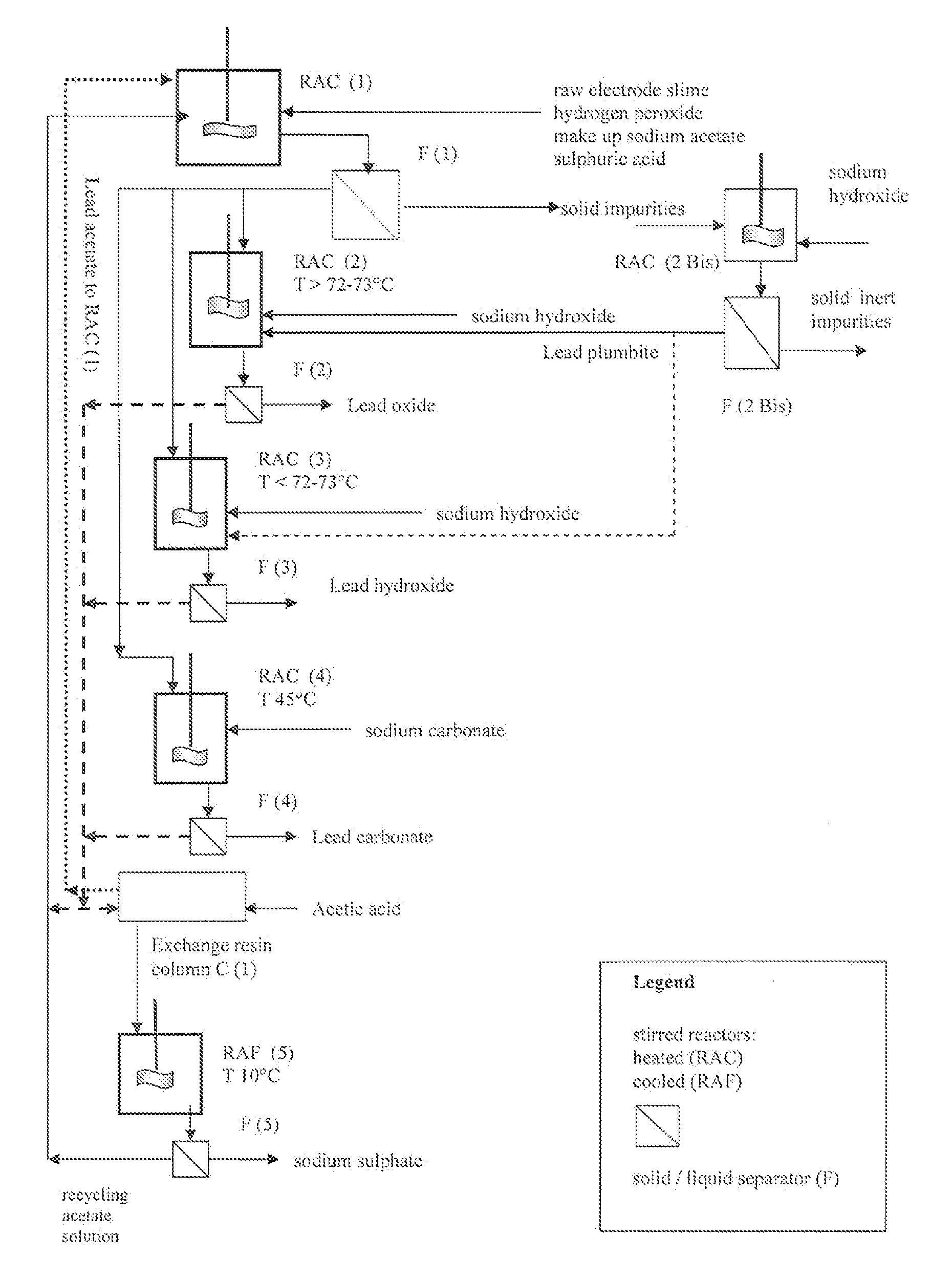
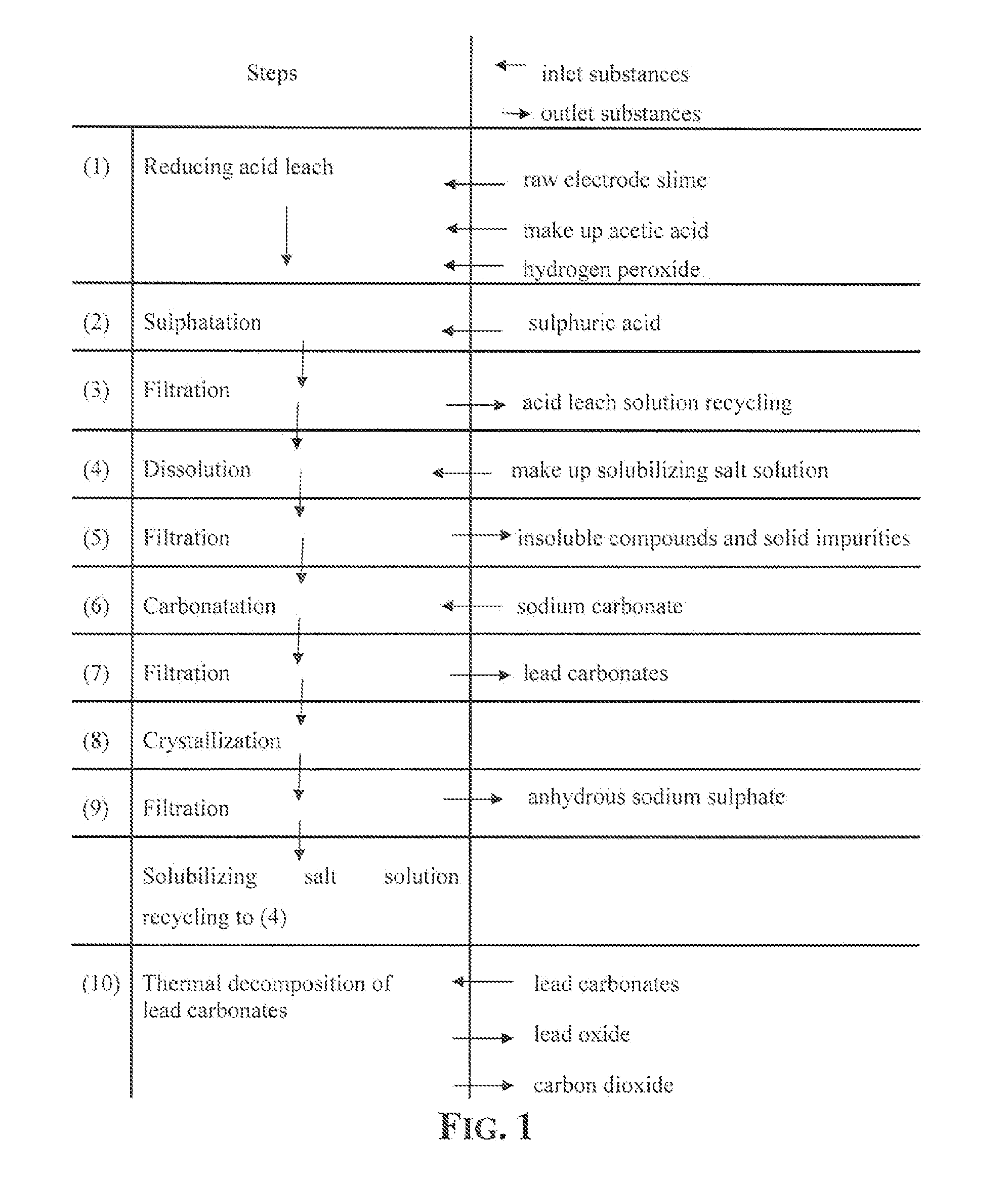
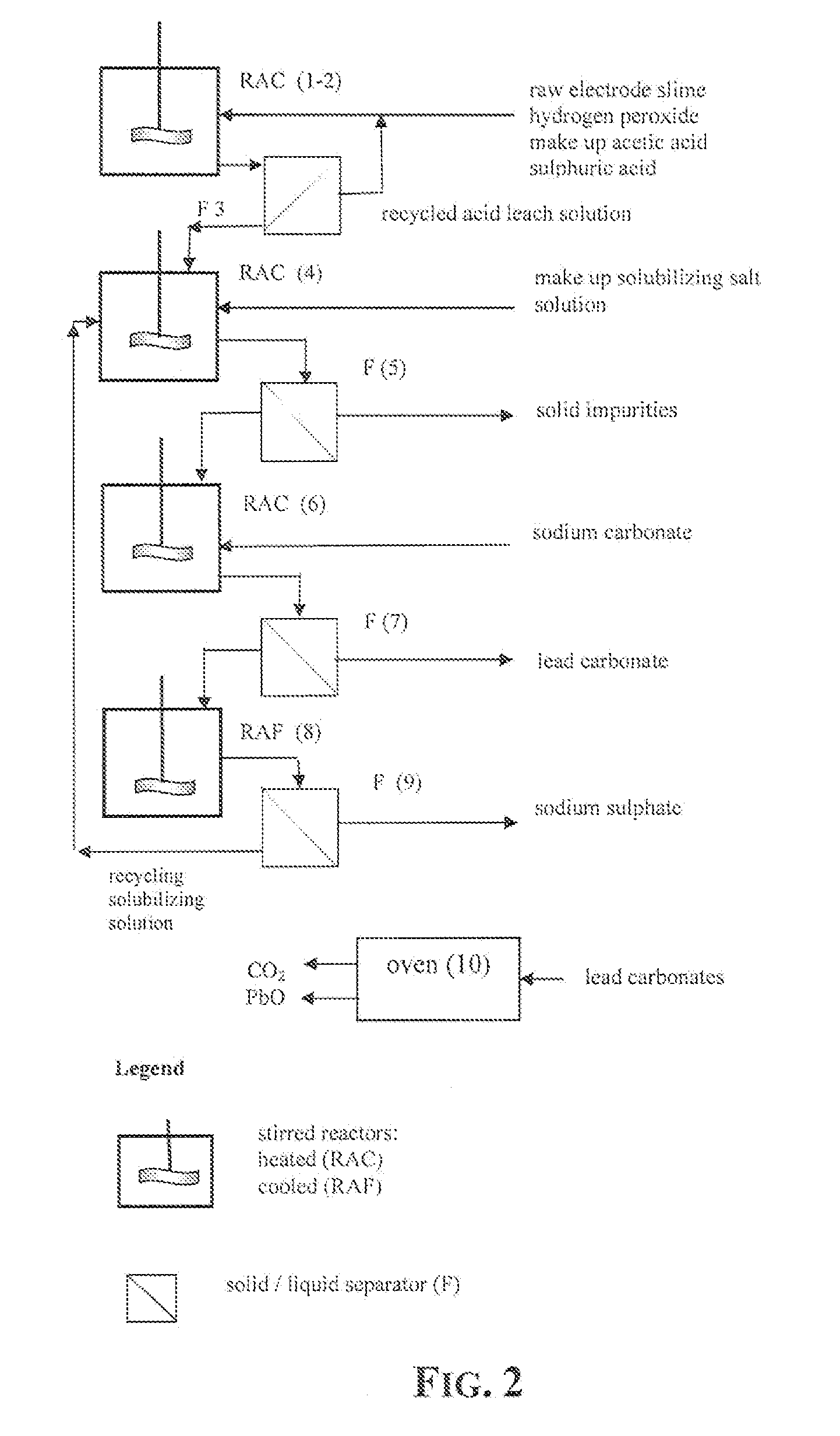
An outstandingly low environmental impact wet process recovers the lead content of an electrode slime and / or of lead minerals in the valuable form of high purity lead oxide or compound convertible to highly pure lead oxide by heat treatment in oven at relatively low temperature, perfectly suited for making active electrode pastes of new batteries or other uses. The process basically comprises the following treatments:a) suspending the impure lead containing material in an aqueous bath containing at least a lead oxide dissolving acid;b) reducing any insoluble lead dioxide to lead oxide by introducing in the suspension either hydrogen peroxide, a sulphite or sulphurous anhydride;c) converting all dissolved lead oxide to lead sulphate in the aqueous bath;d) obtaining a solution of lead sulphate obtained in an aqueous solution containing an acetate salt;e) precipitating and separating a purified lead compound in the form of either carbonate / oxycarbonate or of oxide / or hydroxide by adding to said acetate salt solution a carbonate salt or a hydroxide of the same cation of said acetate salt, respectively.Exemplary flow sheets according to several alternative embodiments and related processing plant diagrams are disclosed.
Owner:MILLBROOK LEAD RECYCLING TECH
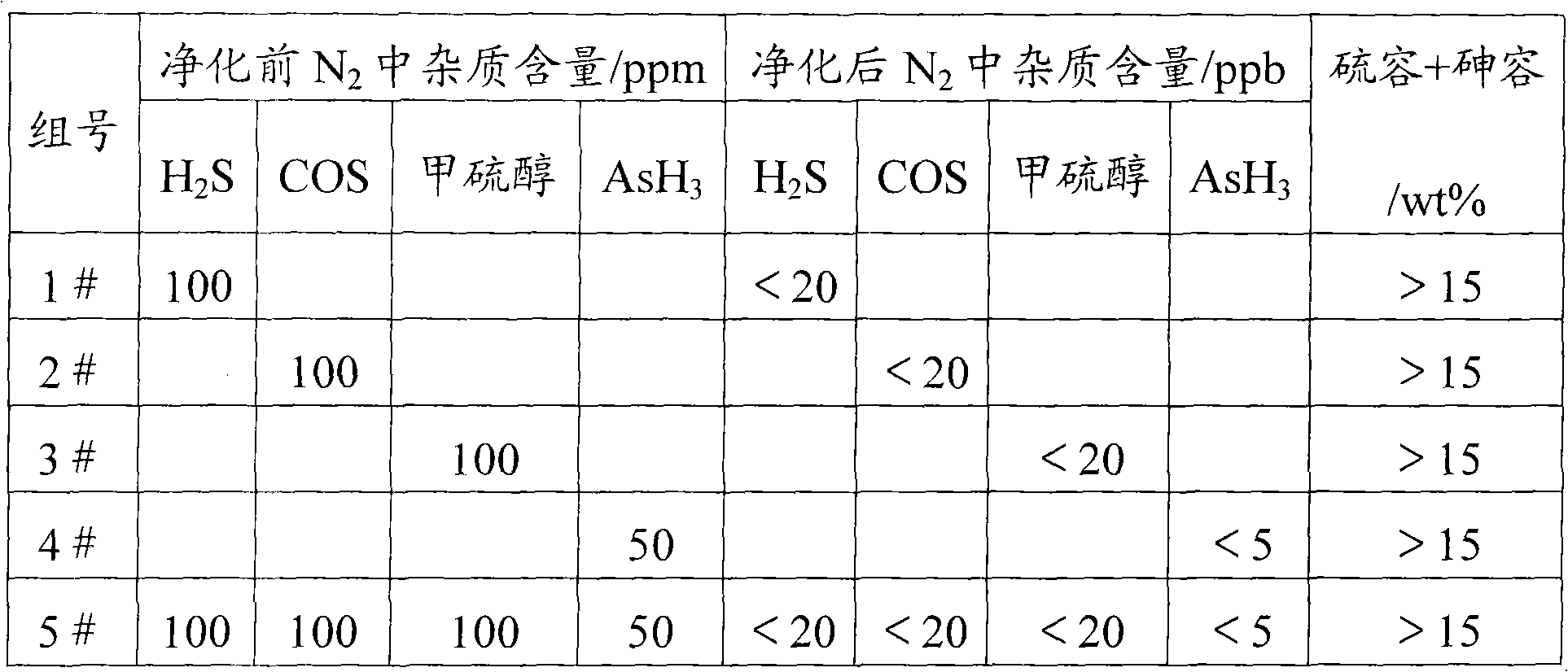
Normal temperature compound desulfuration and dearsenization agent and preparation method thereof
ActiveCN101591554AHigh desulfurization and arsenic removal efficiencyFull accessRefining with metal oxidesActive componentSulfur
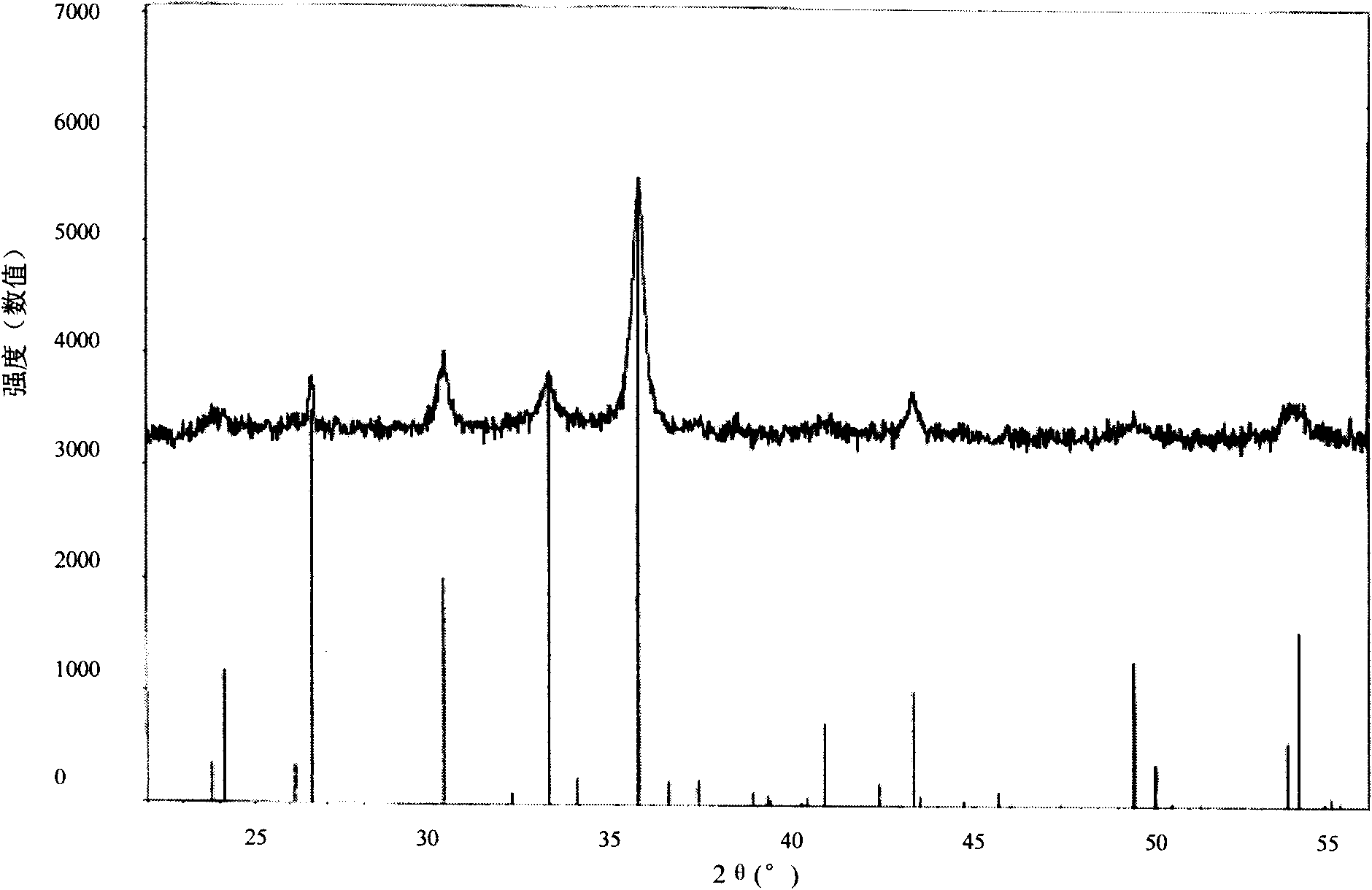
The invention relates to a desulfuration and dearsenization agent, which comprises a carrier and active components; wherein, the carrier can be formed by a single carrier, or by two or more carriers, and the carrier accounts for 10-80wt% of the total weight of the desulfuration and dearsenization agent; the active components comprises 0.1-40wt% of lead oxide, 0.1-60wt% of magnetic Fe21.333O32 and0.1-40wt% of copper oxide. The invention further discloses a preparation method of the desulfuration and dearsenization agent. The desulfuration and dearsenization agent prepared by the invention has high sulfur capacity and arsenic capacity with desulfuration and dearsenization rate being higher than 99%.
Owner:BEIJING SJ ENVIRONMENTAL PROTECTION & NEW MATERIAL CO LTD
Lead recycling
The present invention describes a method of recycling lead from lead containing waste, the method comprising the steps of mixing the battery paste with aqueous citric acid solution so as to generate lead citrate; isolating lead citrate from the aqueous solution; and converting the lead citrate to lead and / or lead oxide.
Owner:CAMBRIDGE ENTERPRISE LTD
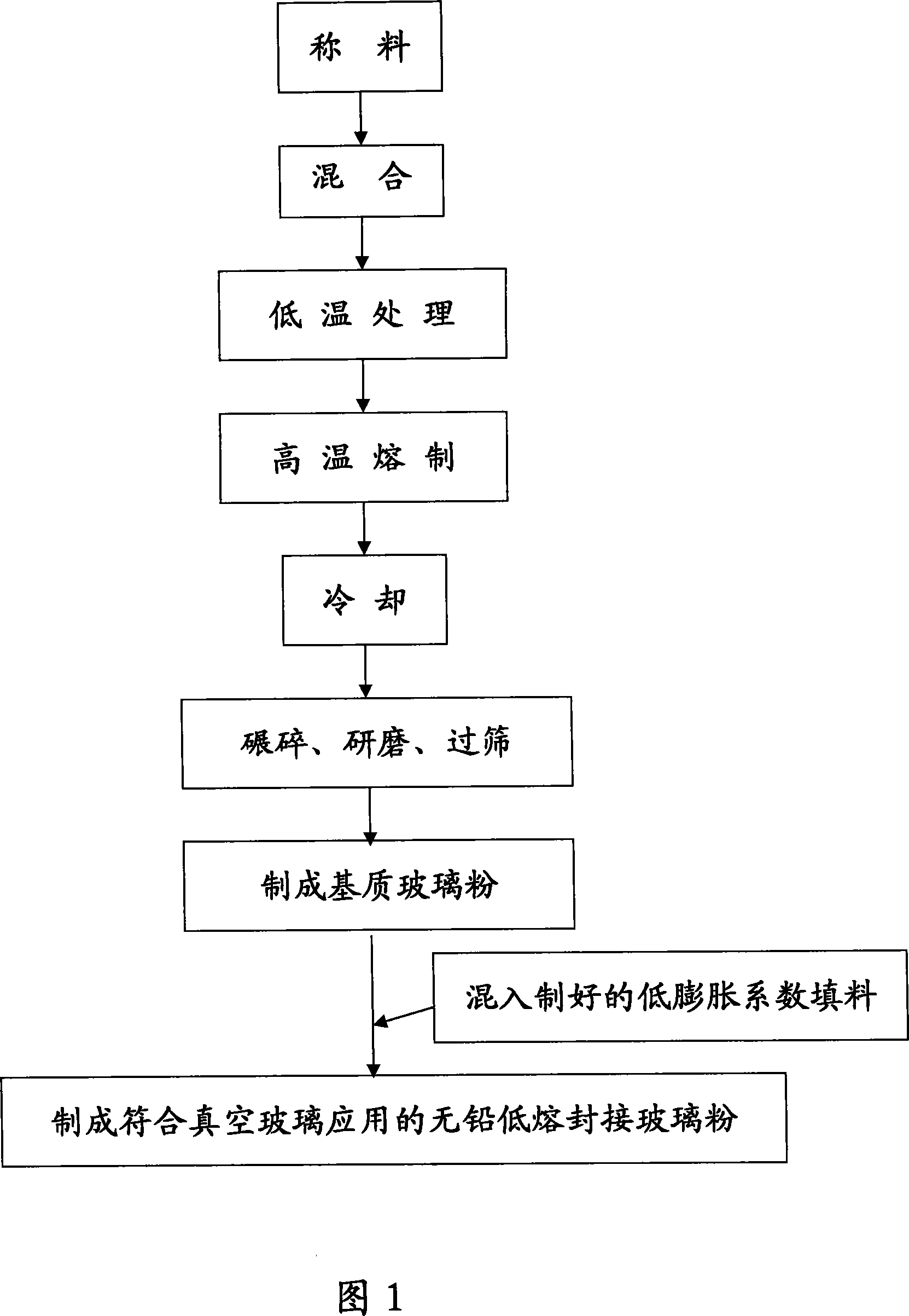
No-lead RE doped sealing glass powder with low smelting point and its production process
The present invention discloses one kind of no-lead RE doped sealing glass powder with low smelting point for vacuum glass sealing and its preparation process. The no-lead sealing glass powder has P2O5, SnO and ZnO as basic glass oxides and mixed RE oxide of La, Yt and Nd added, with the weight ratio between the basic glass oxides and the mixed RE oxide being (30.0-381.5) to (0.12-19.0). The no-lead sealing glass powder of the present invention with doping mixed RE oxide has lowered glass smelting and sealing temperature, raised chemical stability and lowered thermal expansion coefficient of glass as well as lowered environmental pollution. It is used in sealing electronic device and element and vacuum glass.
Owner:CHINA BUILDING MATERIALS ACAD
Recovery of high purity lead oxide from lead acid battery paste
There is provided a process for recovering high purity litharge PbO from spent lead acid battery paste at low temperatures and the further preparation of highly pure lead oxides and Pb(OH)2.
Owner:RETRIEV TECH
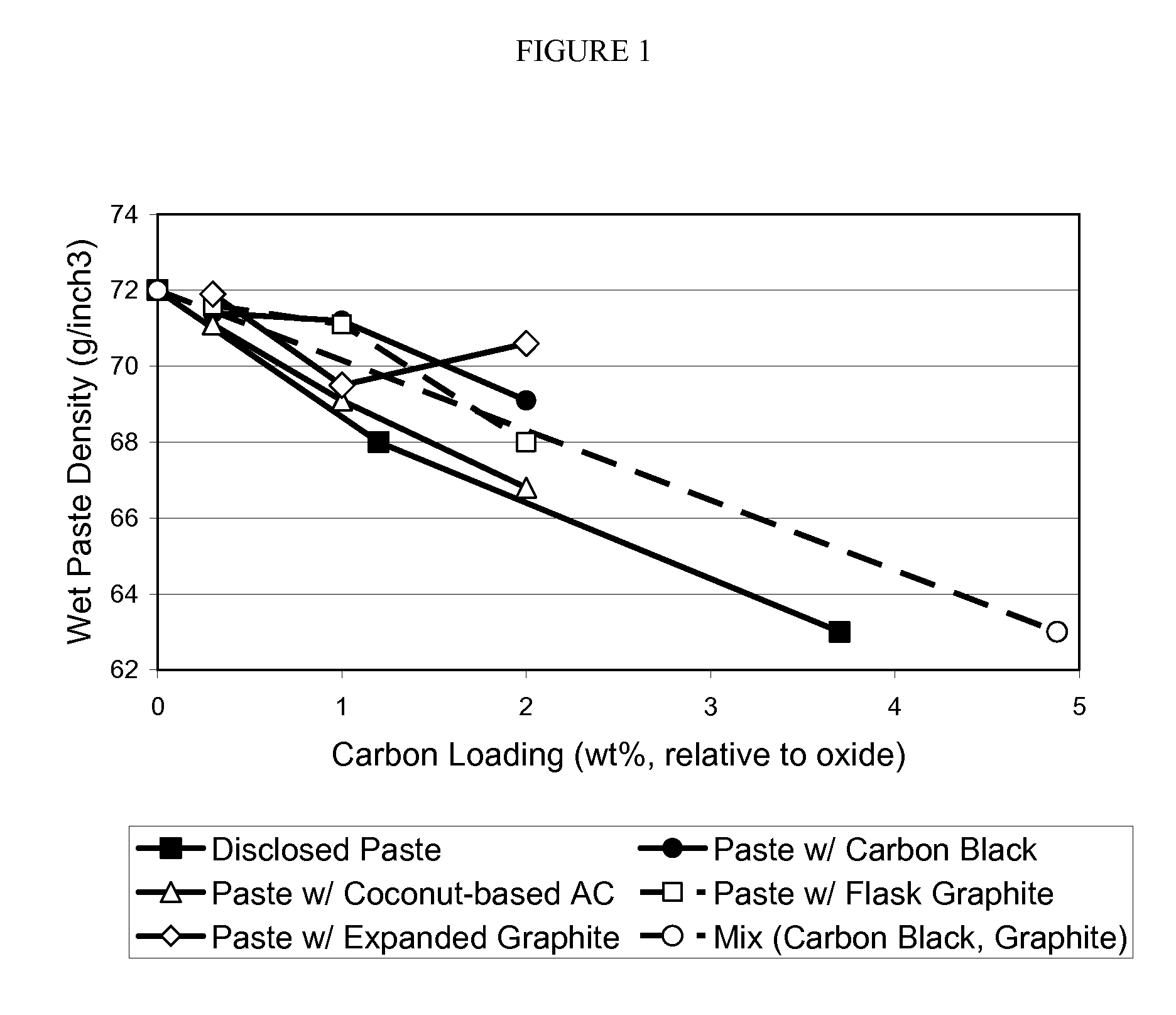
Enhanced negative plates for lead acid batteries
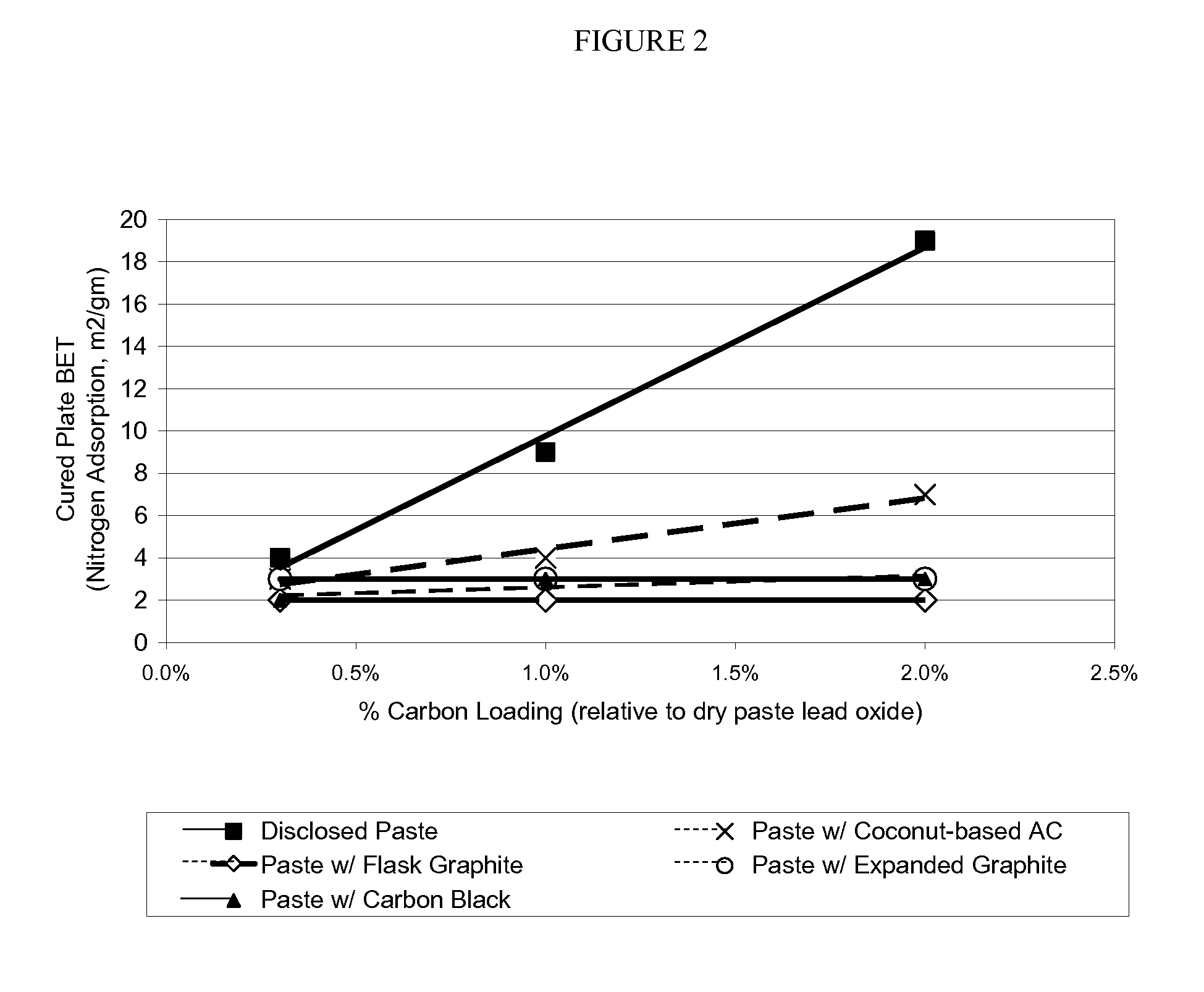
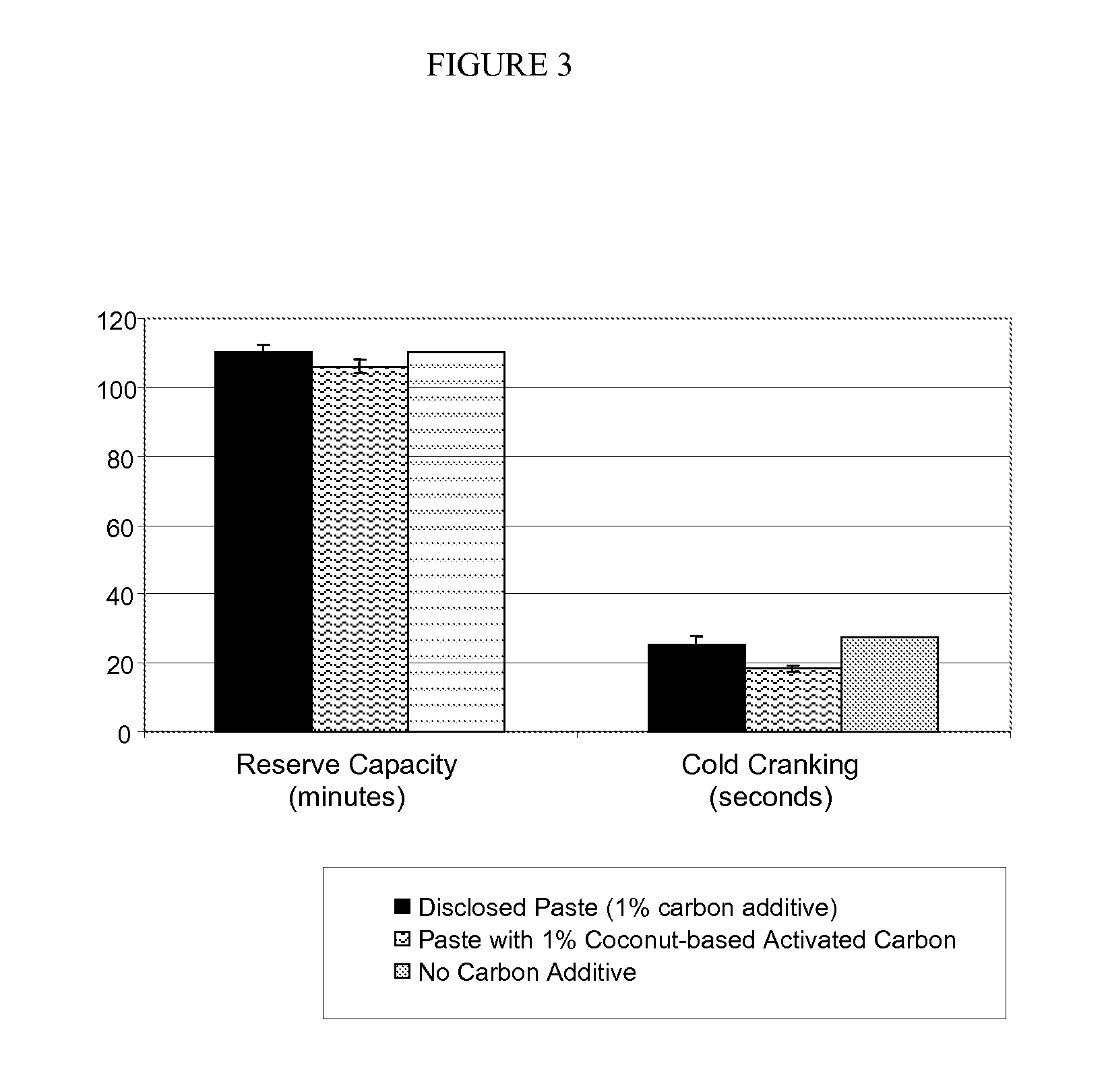
InactiveUS20100015531A1Reduced paste densityIncreased BET surface areaLead-acid accumulatorsLead-acid accumulator electrodesActivated carbonLead oxide
A paste for negative plate of lead acid battery is disclosed that has a reduced paste density, yet provides a negative plate with substantially increased BET surface area and consequently the battery with enhanced performance. The disclosed paste comprises an activated carbon additive having a mesopore volume of greater than about 0.1 cm3 / g and a mesopore size range of about 20 angstroms to about 320 angstroms as determined by DFT nitrogen adsorption isotherm. The cured negative plate made of the disclosed paste has a BET surface area of about 9 m2 / g and 19 m2 / g when the carbon loading level of the paste is about 1% and 2% weight, respectively relative to dry paste lead oxide. The battery including the negative plate made of the disclosed paste maintains the performance such as charge capacity and cycle life, despite containing less lead.
Owner:MEADWESTVACO CORP
Method for recovering lead oxide from waste lead plaster
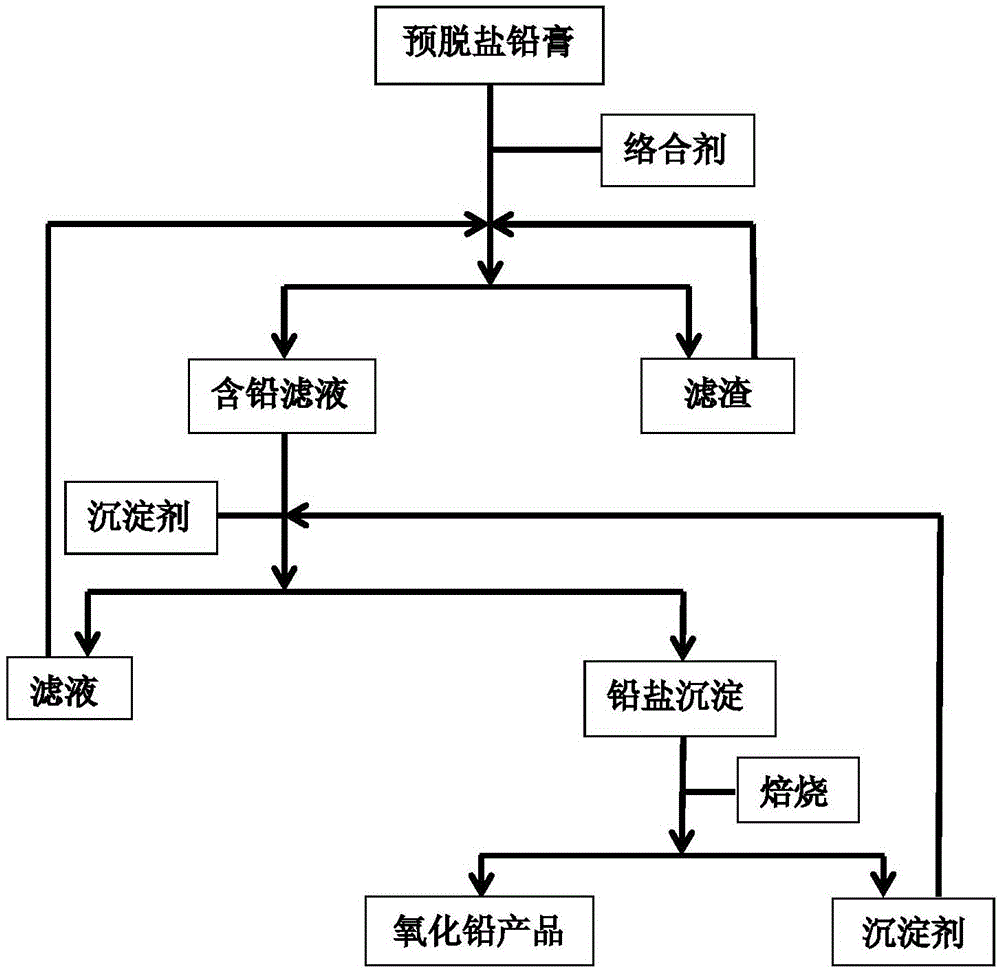
ActiveCN104141045AAtom economy is highIn line with the principles of atom economyLead-acid accumulatorsLead monoxideLead saltLead oxide
The invention provides a method for recovering lead oxide from waste lead plaster. The method comprises the following steps: a, dissolving pre-desalted lead plaster by using a complexing agent solution, and reacting all PbOs with a complexing agent to generate complexing ions so as to obtain a lead-containing solution and filter residues; b, adding a precipitating agent into the lead-containing solution, reacting the precipitating agent with the lead complexing ions to generate lead salt precipitation and the regenerated complexing agent; and c, roasting the lead salt precipitation to obtain lead oxide, and regenerating the precipitating agent. The method can be widely suitable for lead plaster formed by mixing various sources, the process conditions are relatively mild, a process is relatively environment-friendly, and the final recovery rate of lead oxide can reach more than 99%, so that the method has very high application values in the industry of recovery treatment of waste lead-acid batteries.
Owner:BEIJING UNIV OF CHEM TECH
Organic luminescence device and its production method
InactiveUS20050122039A1Excellent gas barrier propertiesDischarge tube luminescnet screensLayered productsWater vaporOxygen
An organic luminescence device uses a substrate with a gas-barrier film in which a gas-barrier film containing an amorphous oxide and at least two kinds of oxides selected from the group consisting of boron oxide, phosphorus oxide, sodium oxide, potassium oxide, lead oxide, titanium oxide, magnesium oxide, and barium oxide is formed on a substrate. The selected two kinds of oxides are a combination of an oxide of an element having a large atomic radius and an oxide of an element having a small atomic radius. The substrate is made of glass or plastic. As a result, the organic luminescence device using a substrate excellent in gas-barrier capability to prevent the infiltration of oxygen, water vapor, etc. from outside is provided.
Owner:PANASONIC CORP
High density nanowire arrays in glassy matrix
InactiveUS20070131269A1Cost-effectivelySmall footprintThermoelectric device detailsThermoelectric device junction materialsThermoelectric materialsFiber
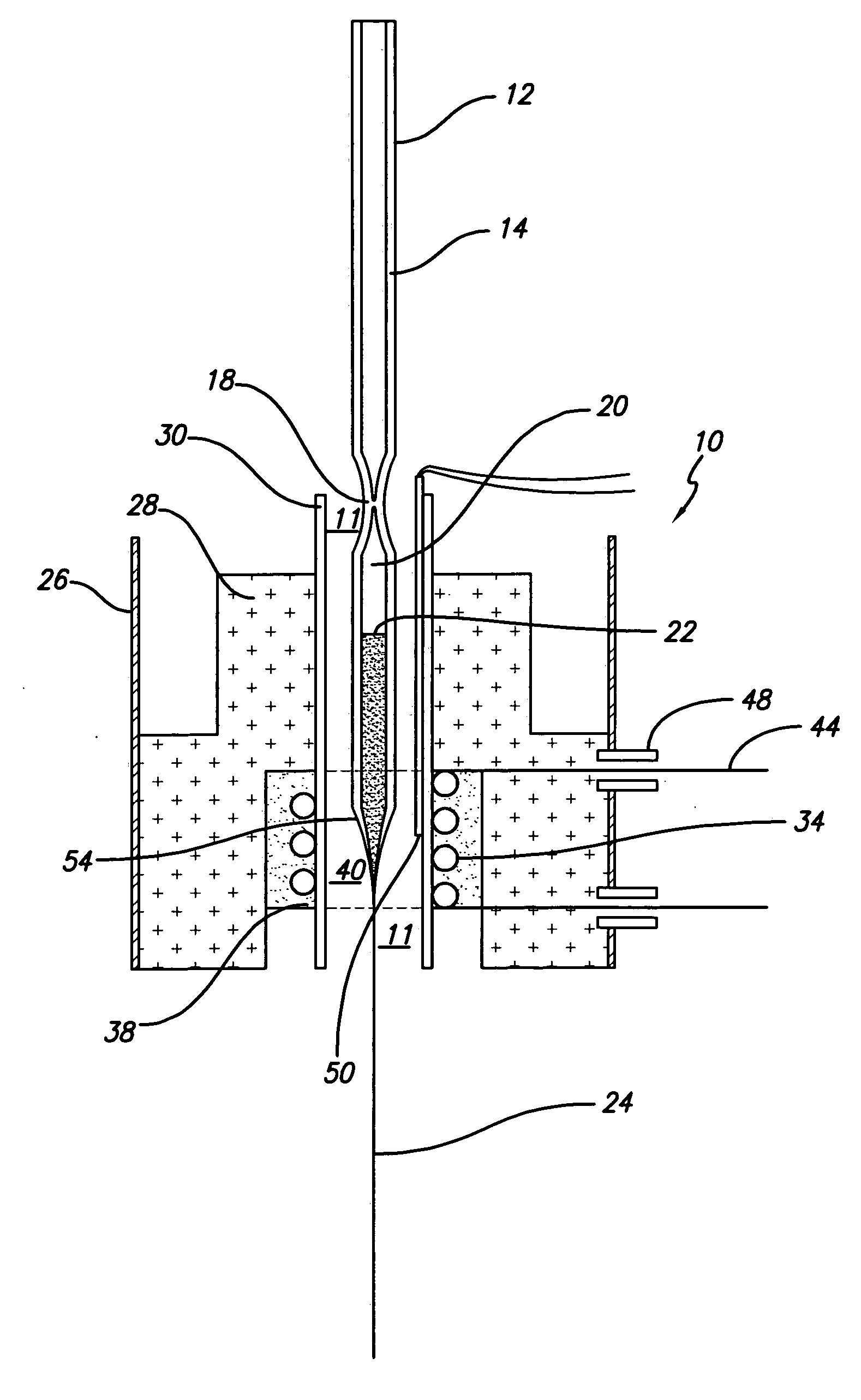
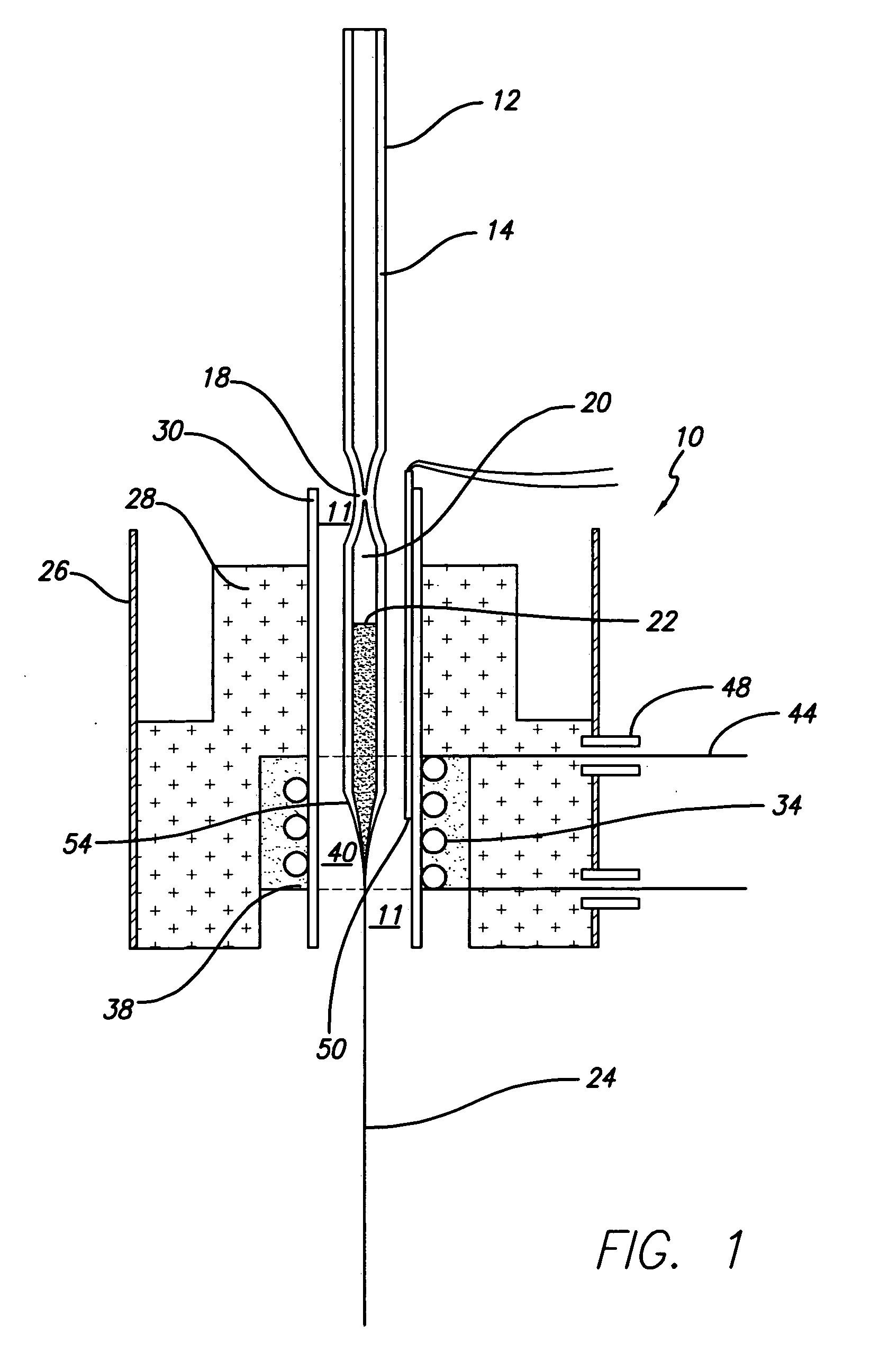
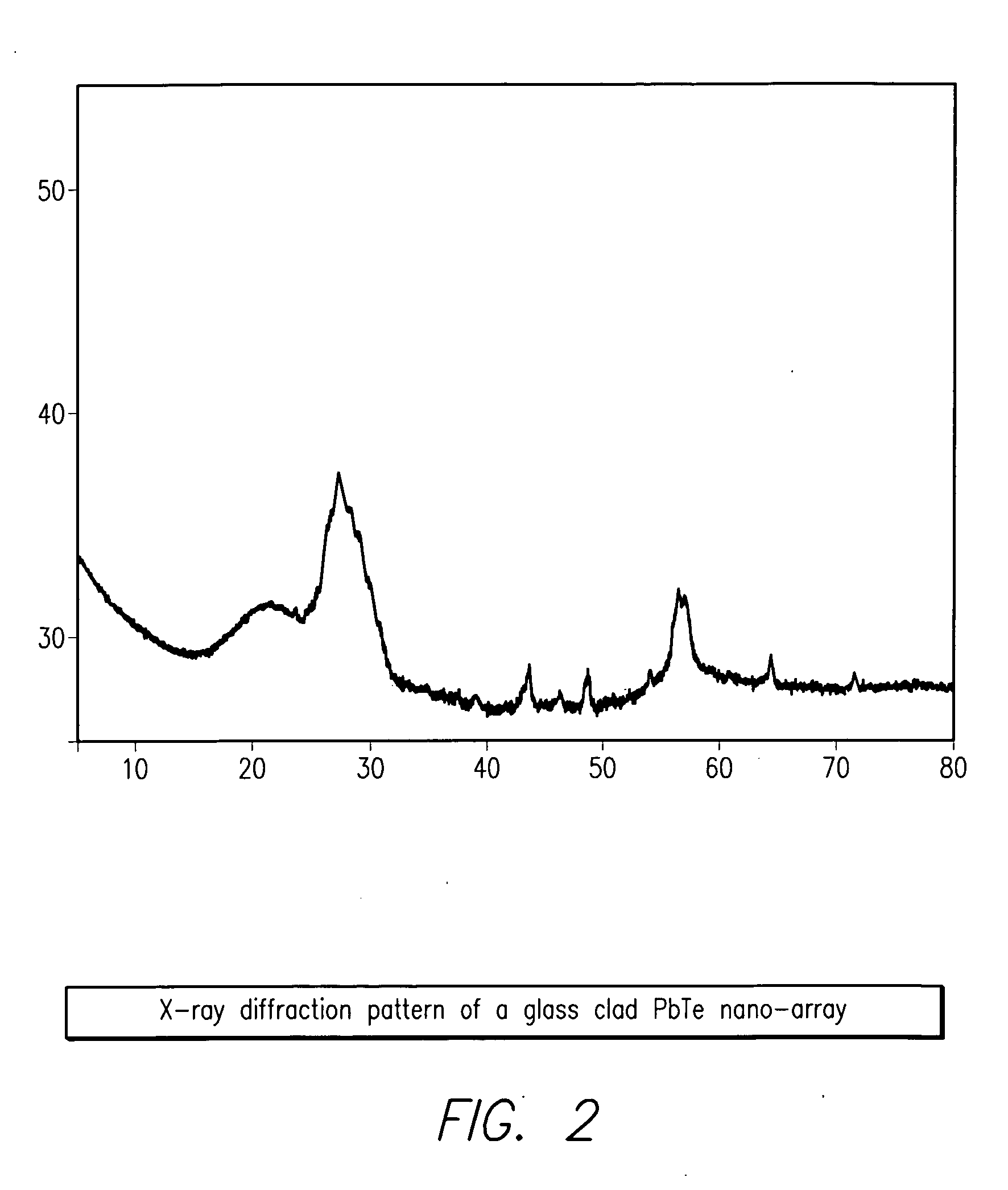
The present invention provides high density nanowire arrays in a glassy matrix comprising one or more thermoelectric fibers embedded in an electrically insulating material such that the thermoelectric material exhibits quantum confinement. According to the preferred embodiment of the invention, the thermoelectric material comprises PbTe and the glassy matrix comprises an electrically insulating material comprising a binary, ternary or higher component glass such as pyrex, borosilcate, aluminosilicate, quartz. The glass may also be formed from multiple constituents but not limited to lead oxide, tellurium dioxide and silicon dioxide, alumina, calcium oxide etc.
Owner:ZT3 TECH INC
Method for recycling waste lead-acid cells to directly produce lead oxide
ActiveCN103014347AImprove recycling efficiencyMeet the needs of high-purity PbOLead oxidesProcess efficiency improvementLead oxideEngineering
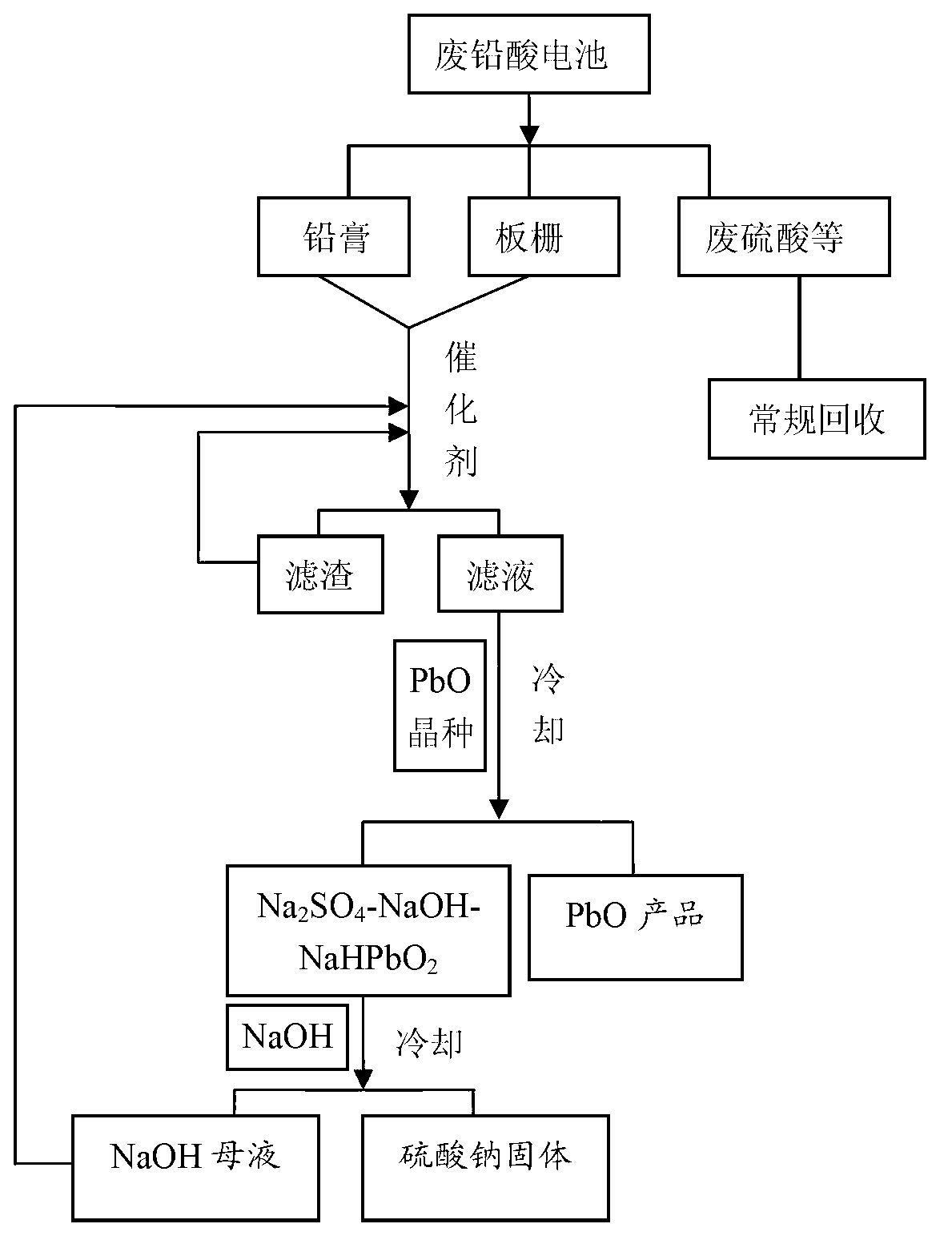
The invention discloses a method for recycling waste lead-acid cells to directly produce lead oxide, belonging to the wet-process metallurgical field of recycling lead from lead-containing materials and waste lead-acid cells. The method for recycling waste lead-acid cells to directly produce lead oxide comprises the following steps of: carrying out a reaction on diachylum, lead powder and sodium hydroxide solution in the presence of catalyst to obtain a mixed solution containing NaHPbO2, sodium sulfate and sodium hydroxide, and un-reacted lead powder and impurities, and separating the mixed solution; cooling and filtering the mixed solution to obtain PbO crystals, and an alkaline solution containing Na2SO4 and the residual NaHPbO2; adding the NaOH to the solution, re-cooling the mixture to separate out sodium sulfate solid and obtain the NaOH solution containing the residual NaHPbO2; and carrying out re-dissolving-filtering-re-crystallizing to the PbO in the NaOH solution with mass concentration of 15-50% to obtain the pure lead oxide solid. The lead recovery ratio is generally between 98.5% and 99.2%, and the purity of the lead oxide is as high as 99.99% or higher.
Owner:BEIJING UNIV OF CHEM TECH
Method of recovering sodium sulfate from lead-bearing desulfurized waste liquid
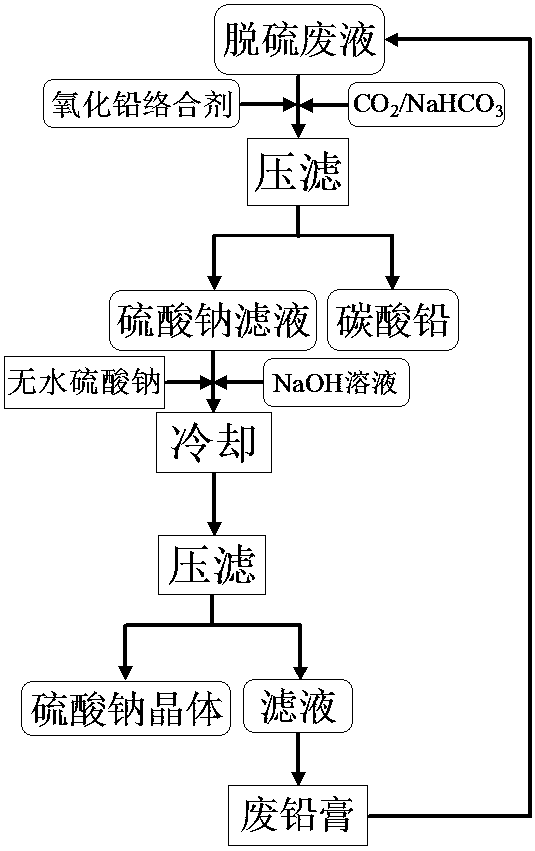
ActiveCN103771459AStrong complexing abilityEliminate secondary pollutionAlkali metal sulfite/sulfate purificationLead carbonateLead oxide
The invention discloses a method of recovering sodium sulfate from a lead-bearing desulfurized waste liquid. The method is characterized by comprising the following steps: after adding a complexing agent into the lead plaster-bearing desulfurized waste liquid, forming a lead carbonate precipitate of lead complex ions in the liquor by adopting carbon dioxide or sodium hydrogen carbonate, and then carrying out solid-liquid separation to obtain a sodium sulfate filtrate and the lead carbonate precipitate; adding anhydrous sodium sulfate and sodium hydroxide into the sodium sulfate filtrate obtained by separating lead carbonate, and cooling at low temperature to separate out sodium sulfate crystals; and returning the filtrate without the sodium sulfate crystals again for desulfurization of lead oxide-bearing wastes.
Owner:BEIJING UNIV OF CHEM TECH
A kind of copper red glaze and its production method and the method for making ceramic product with it
The invention discloses copper red glaze and a production method thereof, and a method for preparing a ceramic product therefrom. The copper red glaze is prepared from parent glaze, a coloring agent and a stabilizing agent, wherein the parent glaze is prepared from the following components in parts by weight: 26 to 32 parts of quartz, 24 to 30 parts of feldspar, 10 to 14 parts of limestone, 12 to16 parts of barium carbonate, 4 to 8 parts of clay, 0 to 4 parts of zinc oxide and 0 to 4 parts of sodium borate; the coloring agent is one or a mixture of a plurality of copper oxide, cuprous oxide and copper carbonate, and the adding amount of the coloring agent is 1 to 4 parts by weight; the stabilizing agent is one or a mixture of a plurality of stannic oxide, ferric oxide, lead oxide and silicon carbide, and the adding amount of the stabilizing agent is 3 to 6 parts by weight. According to the invention, the copper red glaze is bright crimson red, the ceramic product produced from the copper red glaze has high yield and low cost, and can be used for producing domestic ceramic and display art ceramic products.
Owner:广东文化长城集团股份有限公司
High-energy lead-acid storage battery cathode plate diachylon and preparation method thereof
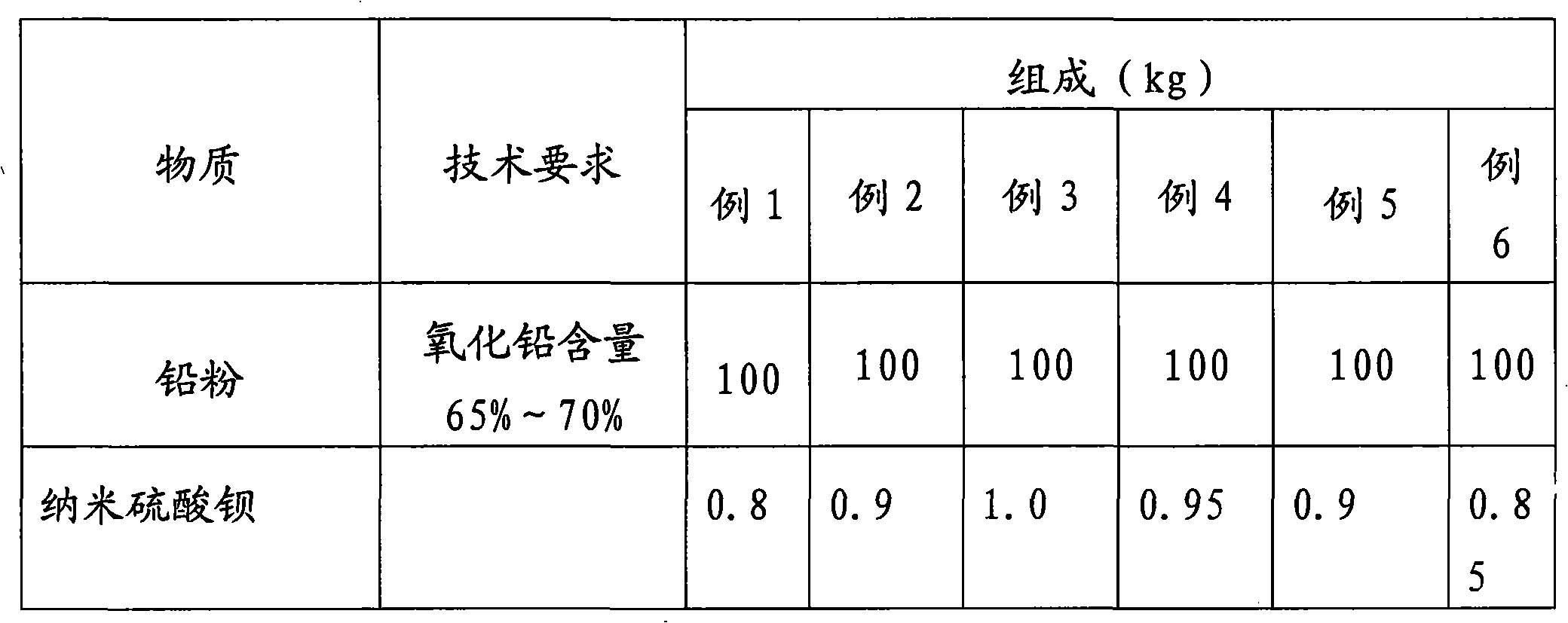
ActiveCN101937991ALow conductivityImprove the utilization rate of active substancesLead-acid accumulator electrodesPolyesterFiber
The invention provides a high-energy lead-acid storage battery cathode plate diachylon and a preparation method thereof. The diachylon comprises lead powder, pure water, analytical pure sulfuric acid and an auxiliary material, wherein the lead powder comprises 65-70 percent of lead oxide, the density of the analytical pure sulfuric acid is 1.400g / cm<3>, and the pure water has the conductivity of smaller than 0.3 mus / cm; and the auxiliary material comprises the following components based on the weight proportion of the lead powder: 0.8-1 percent of nano barium sulphate, 0.1-0.3 percent of modified sodium lignosulphonate, 0.1-0.3 percent of tannin, 0.08-0.1 percent of polyester short fiber, 0.2-0.3 percent of carbon black and 0.3-1 percent of poly-alpha-olefin base oil. When diachylon is mixed, 10-15kg of water, 8-12kg of analytical pure sulfuric acid and the auxiliary material based on the weight proportion of the lead powder are added into 100kg of lead powder. The invention provides the high-energy lead-acid storage battery cathode plate diachylon and the preparation method thereof.
Owner:张家口保胜新能源科技有限公司
Lead recycling
ActiveUS20100040938A1Simple methodOrganic chemistryPrimary cell maintainance/servicingCITRATE ESTERLead oxide
The present invention describes a method of recycling lead from lead containing waste, the method comprising the steps of mixing the battery paste with aqueous citric acid solution so as to generate lead citrate; isolating lead citrate from the aqueous solution; and converting the lead citrate to lead and / or lead oxide.
Owner:CAMBRIDGE ENTERPRISE LTD
Method for preparing superfine lead oxide by using electrode active materials of wasted lead acid batteries
ActiveCN102747227ALong-lasting useReduce the risk of contaminationLead monoxideWaste accumulators reclaimingEconomic benefitsLead oxide
The invention discloses a method for preparing superfine lead oxide by using electrode active materials of wasted lead acid batteries. According to the invention, the superfine powder disclosed herein can be directly used for producing new lead acid batteries by the prior art, lead acid batteries can be recycled, and the method is a production technology which accords with circular economy principles. The method can eliminate the pollution generated by the waste lead acid batteries at the source, has the characteristics of low production cost and high economic benefit, and is suitable for existing lead acid battery production enterprises to let practical and affordable lead acid batteries be used with persistence and reassurance.
Owner:JIANGSU HUAFU STORAGE NEW TECH DEV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com