Method of recovering sodium sulfate from lead-bearing desulfurized waste liquid
A desulfurization waste liquid, sodium sulfate technology, applied in the direction of alkali metal sulfite/sulfate purification, etc., can solve problems such as limitation and neglect of recovery of sodium sulfate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
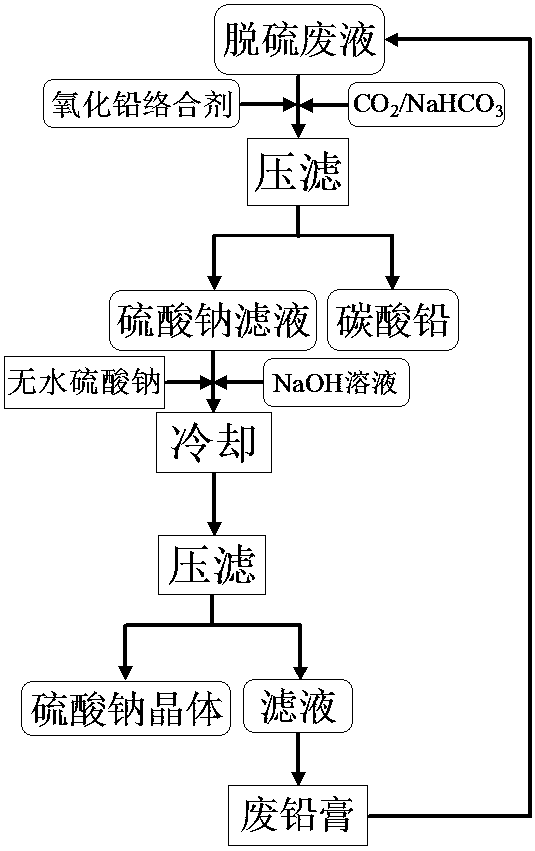
[0031] Take 10 liters of 2.20 mol / L NaOH solution to desulfurize the waste lead plaster of blue sky electric vehicle and recover the desulfurization waste liquid. Through conventional chemical titration analysis, the desulfurization waste liquid mainly contains 1.02 mol / L sodium sulfate and 0.15 mol / L sodium hydroxide, and contains 0.002 mol / L lead ions. This waste liquid reclaims sodium sulfate, lead carbonate, and the process of realizing NaOH solution regeneration is as follows:
[0032] (1) Add 0.065 mol / L sodium acetate as lead oxide complexing agent to the desulfurization waste liquid, then add 0.0195 mol sodium bicarbonate to the solution for carbonation, so that the lead ions in the solution form lead carbonate precipitation;
[0033] (2) Carry out solid-liquid separation subsequently, obtain sodium sulfate filtrate and lead carbonate precipitation;
[0034] (3) Add 280 g of anhydrous sodium sulfate and 2.4 L of NaOH solution with a concentration of 12 mol / L to the so...
Embodiment 2
[0038] Take 10 liters of 2.50 mol / L NaOH solution to desulfurize the waste lead plaster of Fengfan lead-acid battery and recover the desulfurization waste liquid. Through conventional chemical titration analysis, the desulfurization waste liquid mainly contains 1.13 mol / L sodium sulfate and 0.21 mol / L of NaOH, while containing 0.004 mol / L of lead ions. This waste liquid reclaims sodium sulfate, lead carbonate, and the process of realizing NaOH solution regeneration is as follows:
[0039] (1) After adding 0.025 mol / L nitrilotriacetic acid as a lead oxide complexing agent to the desulfurization waste liquid, 0.039 mol carbon dioxide is introduced into the solution for carbonation, so that the lead ions in the solution form lead carbonate precipitates;
[0040] (2) Carry out solid-liquid separation subsequently, obtain sodium sulfate filtrate and lead carbonate precipitation;
[0041] (3) add 426g anhydrous sodium sulfate and 2.3 L concentration in the sodium sulfate filtrate af...
Embodiment 3
[0045] Take 5 liters of 3.0 mol / L NaOH solution to desulfurize the lead-containing flue ash obtained from ordinary lead-acid battery smelting and recover the desulfurization waste liquid. According to conventional chemical titration analysis, the desulfurization waste liquid mainly contains 1.33 mol / L sodium sulfate and 0.12 mol / L NaOH, and also contains 0.002 mol / L lead ion and 0.001 mol / L lead sulfate suspended. This waste liquid reclaims sodium sulfate, lead carbonate, and the process of realizing NaOH solution regeneration is as follows:
[0046] (1) Add 0.06 mol / L ethanolamine to the desulfurization waste liquid as a lead complexing agent, and then add 0.0145 mol sodium bicarbonate to the solution for carbonation, so that the lead ions and lead sulfate in the solution are converted into more insoluble lead carbonate precipitation;
[0047] (2) Carry out solid-liquid separation subsequently, obtain sodium sulfate filtrate and lead carbonate precipitation;
[0048] (3) Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com