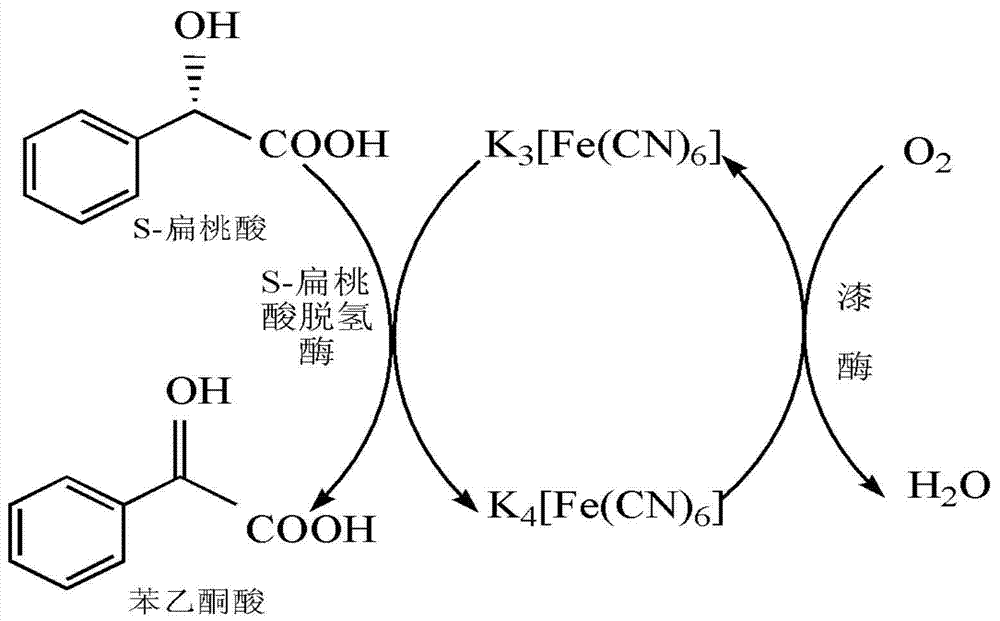
Method for preparing benzoyl formic acid and R-mandelic acid by coupling reaction of S- mandelic acid dehydrogenase and laccase
A mandelic acid dehydrogenase and coupling reaction technology, applied in the direction of fermentation, etc., can solve the problems of lack of ferricyanide experimental data, etc., and achieve the effects of convenient industrial production, low environmental pressure and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Reaction system: 50ml NaH with pH6.5 2 PO 4 - Citric acid (NaH 2 PO 4 Mixed with citric acid) buffer containing 10mmol / L racemic mandelic acid (commercially available), 0.3mmol / L sodium ferrocyanide, 50U S-mandelate dehydrogenase, 25U laccase. After shaking and reacting at 140rpm at 30°C for 10 hours, 49.9% of the racemic mandelic acid was consumed, and all the consumed S-mandelic acid was converted into acetophenone acid. The pH of the reaction solution was adjusted to below 2.0, and acetophenone acid and R- Mandelic acid, ether was distilled off, the mixture of acetophenone acid and R-mandelic acid was separated by vacuum distillation at 85°C and 13.33 pascals to obtain acetophenone acid and R-mandelic acid respectively.
Embodiment 2
[0021] Reaction system: 50ml NaH with pH6.5 2 PO 4 - Citric acid (NaH 2 PO 4 Mixed with citric acid) buffer containing 10mmol / L racemic mandelic acid (commercially available), 0.1mmol / L potassium ferrocyanide, 50U S-mandelate dehydrogenase, 25U laccase. After shaking and reacting at 140rpm at 30°C for 10 hours, 49.3% of the racemic mandelic acid was consumed, and all the consumed S-mandelic acid was converted into acetophenone acid. The pH of the reaction solution was adjusted to below 2.0, and acetophenone acid and R- Mandelic acid, ether was distilled off, the mixture of acetophenone acid and R-mandelic acid was separated by vacuum distillation at 85°C and 13.33 pascals to obtain acetophenone acid and R-mandelic acid respectively.
Embodiment 3
[0023] Reaction system: 50ml NaH with pH6.5 2 PO 4 - Citric acid (NaH 2 PO 4 Mixed with citric acid) buffer containing 5mmol / L racemic mandelic acid (commercially available), 1mmol / L sodium ferrocyanide, 50U S-mandelate dehydrogenase, 25U laccase. After reacting at 30°C for 12 hours, the consumption of racemic mandelic acid was 49.5%, and all the consumed S-mandelic acid was converted into acetophenone acid. Adjust the pH of the reaction solution to below 2.0, and diethyl ether extracted acetophenone acid and R-mandelic acid. , Distilled to remove ether, the mixture of acetophenone acid and R-mandelic acid was separated by vacuum distillation at 85°C 13.33 Pascals to obtain acetophenone acid and R-mandelic acid, adjust the pH of the reaction solution to below 2.0, and diethyl ether extracted phenethyl Keto acid and R-mandelic acid, ether was removed by distillation, the mixture of acetophenone acid and R-mandelic acid was separated by vacuum distillation at 85°C and 13.33 p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com