Method for producing optical homochiral amygdalic acid
A technology of mandelic acid and methyl mandelic acid, which is applied in the field of chemical biosynthesis to produce optically pure chiral mandelic acid, and can solve the problems that the S-type synthesis has not been reported yet.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
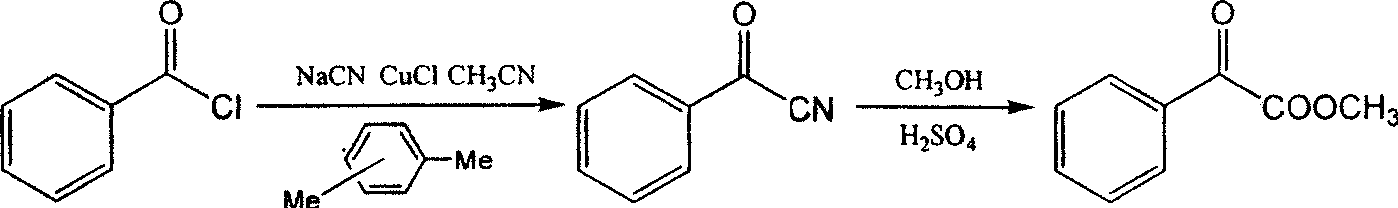
[0037] 1. Synthesis of benzoyl formate: Add 40mL (0.347moL) of benzoyl chloride, 20g (0.408moL) of NaCN, 10g (0.1moL) of CuCl, 8.5mL of xylene and 5mL of acetonitrile into a 250mL round-bottomed flask in sequence, and place at 130 °C and heated to reflux for 5 hours. Cool to room temperature after the reaction, filter with suction, and wash with xylene. The resulting filtrate was distilled under reduced pressure, and the fraction at 112-117°C (reduced pressure) was collected to obtain 18.6 g (0.142 moL) of benzoylnitrile, with a yield of 41%, and mp of 32°C.
[0038] Carefully add 10 mL of concentrated sulfuric acid to 30 mL of absolute ethanol dropwise, add 6.5 g of benzoyl nitrile, heat and reflux for 24 hours, after the reflux reaction is completed, distill the ethanol under reduced pressure, pour the concentrated solution into cold water at 0°C, and use Extracted with ethyl acetate, washed the organic layer with 5% sodium carbonate solution and water respectively, dried o...
Embodiment 2
[0050] 1. Synthesis of benzoyl formate: Add 80mL (0.694moL) of benzoyl chloride, 40g (0.816moL) of NaCN, 20g (0.2moL) of CuCl, 17mL of xylene and 10mL of acetonitrile into a round-bottomed flask in sequence, and heat at 130°C Reflux for 6 hours. Cool to room temperature after the reaction, filter with suction, and wash with xylene. The resulting filtrate was distilled under reduced pressure, and the fraction at 112-117°C (43mmHg) was collected to obtain 38.1g (0.29moL) of benzoylnitrile, with a yield of 42%, and mp32°C.
[0051] Carefully add 10mL of concentrated sulfuric acid to 30mL of absolute ethanol dropwise, add 7.1g of benzoyl nitrile, heat and reflux for 28 hours, after the reflux reaction is complete, evaporate the ethanol under reduced pressure, pour the concentrated solution into cold water at 5°C, and use Extract with ethyl acetate, wash the organic layer with 5% sodium carbonate solution and water respectively, dry over anhydrous sodium carbonate, distill off the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com