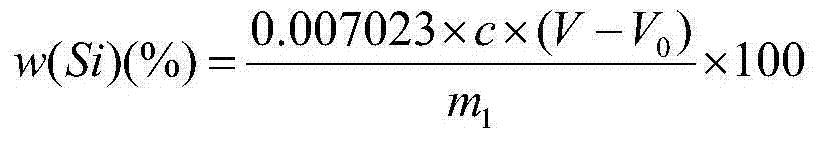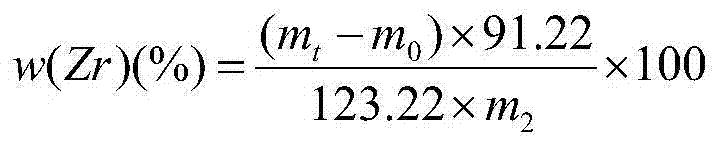Patents
Literature
476 results about "Mandelic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
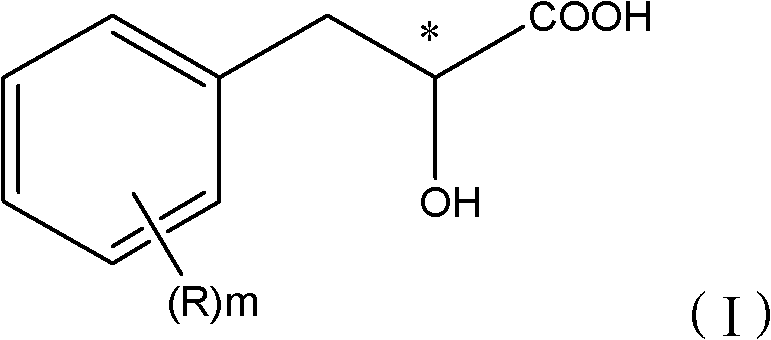
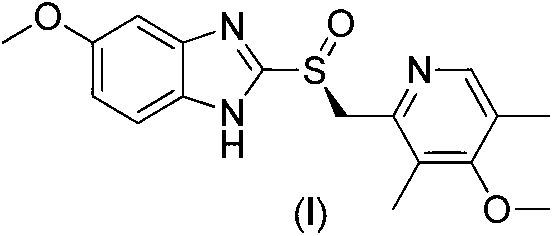
Mandelic acid is an aromatic alpha hydroxy acid with the molecular formula C₆H₅CH(OH)CO₂H. It is a white crystalline solid that is soluble in water and polar organic solvents. It is a useful precursor to various drugs. The molecule is chiral. The racemic mixture is known as paramandelic acid.
Method for screening stereoselective alpha-hydroxy acid dehydrogenase
InactiveCN102660631AOvercoming time-consuming and laboriousOvercoming demandsMicrobiological testing/measurementWater bathsAlpha hydroxy acid
The invention provides a method for screening stereoselective alpha-hydroxy acid dehydrogenase, comprising the following steps: (1) dissolving a sample to be detected containing alpha-hydroxy acid dehydrogenase in a buffer with pH of 6-9, adding an alpha-hydroxy acid chiral monomer as a substrate, and carrying out conversion reaction in water-bath at 20-50 DEG C; and (2) after the conversion reaction, adding the conversion solution in an FeCl3 developer solution to conduct color development reaction for 5-30 min, when the color development reaction finishes, judging the result according to the color appeared in the reaction solution. The method has no need to carry out derivatization on the substrate which is intend to screen, can rapidly identify the dehydrogenase activity and optical selectivity of the selected microbe by using simple colourimetry, has the advantages of simple screening flow, fast speed, low request for devices, strong versatility and the like, and offers great conveniences to the obtainment of optically pure products by using racemic mandelic acid and related derivatives as substrates to carry out resolution through biological enzymatic method.
Owner:ZHEJIANG UNIV OF TECH
Titanium-complex-containing liquid fertilizer, preparation method thereof, and solid fertilizer containing the same
The invention relates to a preparation method of a titanium-complex-containing liquid fertilizer, which comprises the following steps: 1) dissolving titanyl sulfate or titanium sulfate in water; 2) adding a ligand A; 3) adding a ligand B; and 4) aging, wherein the ligand A and the ligand B are independently selected from tartaric acid, citric acid, malic acid, mandelic acid and lactic acid, and the ligand A and the ligand B are different; the ligand A, the ligand B and Ti (IV) meet the following relationship: 2 <= (the total mole number of the ligand A and the ligand B): the mole number of Ti (IV) <= 12. The invention also relates to the liquid fertilizer prepared by the method, and a solid fertilizer containing the liquid fertilizer. The titanium-complex-containing liquid fertilizer uses few ligands, has higher stability, and is still clear and transparent when being put for more than one year, and the concentration of titanium ion in a solution is almost unchanged, so the liquid fertilizer is of an excellent functional fertilizer.
Owner:SINOFERT HOLDINGS +1
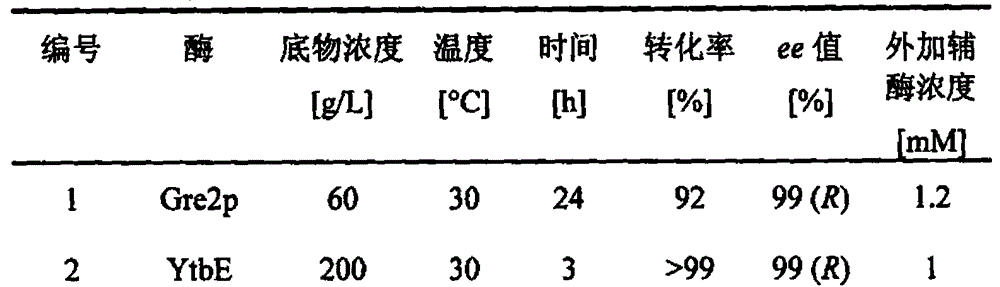
Carbonyl reductase mutant as well as gene and application thereof
InactiveCN104099305AImprove thermal stabilityIncreased reductase activityBacteriaOxidoreductasesMethyl o-chloromandelateMandelic acid
The invention relates to a carbonyl reductase CgKR1 mutant, a coding gene of the mutant, a recombinant expression vector containing the gene of the carbonyl reductase mutant, a recombinant expression transformant, a recombinase, a preparation method of the recombinase, and an application of the carbonyl reductase mutant to asymmetric reduction of ketonic ester for preparation of optically pure chiral hydroxyl ester, such as catalysis of o-cyano methyl phenylglyoxylate for asymmetric reduction to prepare (R)-o-chloro mandelic acid methyl ester. Compared with wild enzymes, the carbonyl reductase mutant has the advantages that the thermal stability is substantially improved, and the catalytic activity of part of the mutant to the o-chlorobenzoic acid formyl methyl ester is also obviously improved. The multiple mutants can be applied to catalysis of the ketonic ester for asymmetric reduction to prepare the optical purely-chiral hydroxyl ester, such as catalysis of the o-cyano methyl phenylglyoxylate for asymmetric reduction to prepare the optically pure (R)-o-chloro mandelic acid methyl ester. The carbonyl reductase mutants have the very good industrial application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
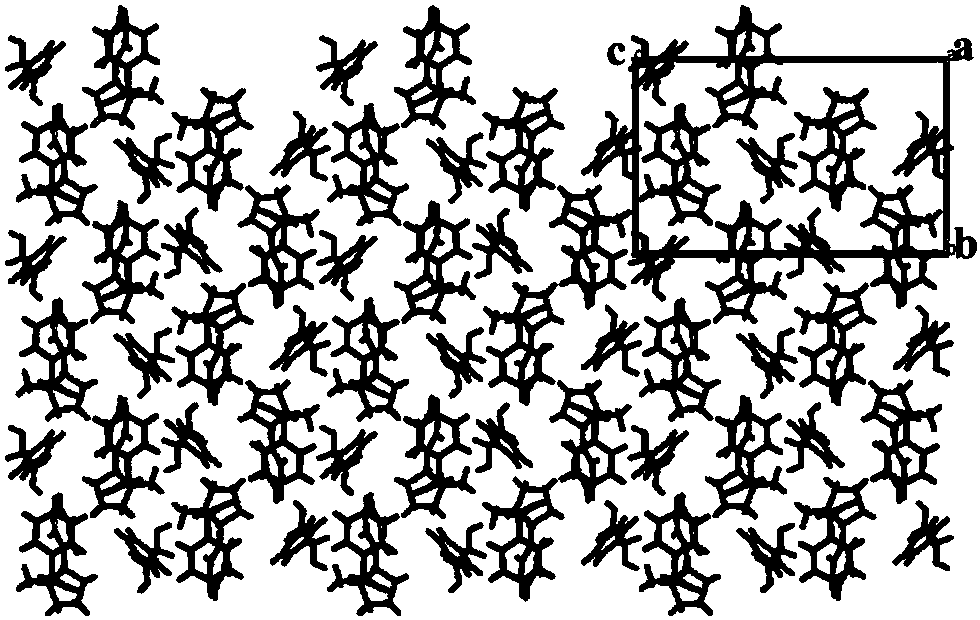
Nicotine-mandelate complex crystal, preparation method thereof and tobacco product containing same
InactiveCN108285441AGood sustained release effectLess irritatingTobacco treatmentOrganic compound preparationCrystal systemWater baths
The invention discloses a nicotine-mandelate complex crystal. A crystal molecular formula of the nicotine-mandelate complex crystal is C26H30N2O6; the nicotine-mandelate complex crystal belongs to anorthorhombic crystal system; a space group is P212121, and unit cell parameters are: a=9.5074(5) angstrom, b=12.7246(9) angstrom, c=20.4101(1) angstrom, and alpha=beta=gamma=90.00 degrees. The invention further discloses a preparation method of the nicotine-mandelate complex crystal, comprising the following steps: a, measuring and fetching nicotine and mandelic acid, dissolving the mandelic acidin a solvent, obtaining a saturated solution, placing the saturated solution of the mandelic acid in a water bath environment, stirring and adding the nicotine dropwise; b, transferring the reaction liquid obtained in step a to a light resistant container, performing ultrasonic treatment, shielding the reaction product from light, placing the reaction product at room temperature, enabling the reaction product to volatilize, and obtaining a nicotine mandelate crystal. The complex crystal has an obviously sustained-release effect in artificially simulated saliva. The invention further disclosestobacco products containing the nicotine-mandelate complex crystal. The tobacco products include gum-based chewing tobacco, a bagged mouth cigarette, an electronic cigarette liquid or a heated non-combustible cigarette.
Owner:CHINA TOBACCO YUNNAN IND
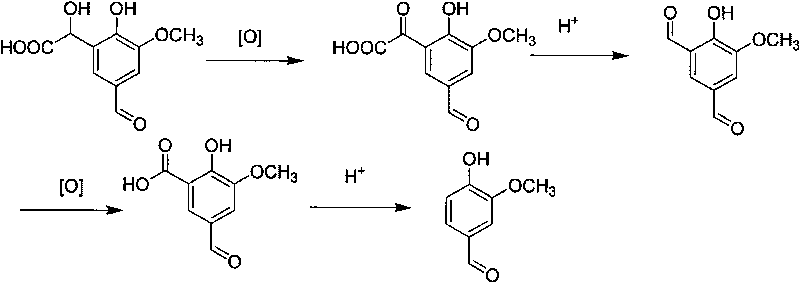
Method for converting 2-hydroxyl-3-methoxy-5-aldehyde mandelic acid into vanillin
InactiveCN101712605AAchieve emission standardsOrganic compound preparationWater/sewage treatment by ion-exchangeGlyoxylic acidEconomic benefits
The invention relates to a method for converting 2-hydroxyl-3-methoxy-5-aldehyde mandelic acid into vanillin, which sequentially comprises the steps of adsorbing by macroporous resin, oxidizing, acidizing, reoxidizing, reacidizing, and the like. The method integrates wastewater treatment and reactant recycling and realizes the wastewater treatment by the macroporous resin. Aromatic compounds in the wastewater are recycled by combining an oxidizing and acidizing process to produce a target product-vanillin. The invention realizes the standard discharge of the wastewater, realizes the recycling of the wastewater generated during producing the vanillin by a glyoxylic acid method, and improves economic benefits.
Owner:JIAXING ZHONGHUA CHEM
Polishing composition and polishing method
A polishing composition includes fumed alumina, alumina other than fumed alumina, colloidal silica, a first organic acid, a second organic acid, an oxidizing agent, and water. When the second organic acid is citric acid, the first organic acid is preferably malic acid, while when the second organic acid is malic acid, the first organic acid is preferably citric acid. When the second organic acid is succinic acid, iminodiacetic acid, itaconic acid, maleic acid, malonic acid, crotonic acid, gluconic acid, glycolic acid, lactic acid, or mandelic acid, the first organic acid is preferably either citric acid or malic acid. The polishing composition can be suitably used for polishing the surface of a substrate for a magnetic disk.
Owner:FUJIMI INCORPORATED
New green synthesizing process for ketocoumaran compound
The invention discloses a new green synthesizing process for a ketocoumaran compound. The synthesizing process comprises the following steps: in the absence of a solvent, performing condensation reaction of a mandelic acid compound and hydroquinol under the action of a little amount of acid catalyst to obtain a full condensed intermediate, wherein the acid catalyst is selected from one of 10 to 37 percent concentrated hydrochloric acid, 60 to 98 percent concentrated sulfuric acid, fuming sulfuric acid, 80 to 98 percent phosphoric acid, polyphosphoric acid, p-toluenesulfonic acid, trifloromethanesulfonic acid, benzoic acid, ammonium acetate, ammonium chloride, KF, TiC14, NiCl2, CuSO4, Cu(OAc)2, ZnCl2, AlCl3, Fe(NO3)3, FeCl3 and SnCl2; and performing oxidation reaction of full condensed intermediate under the action of an oxidant to obtain a final product ketocoumaran compound. The synthesizing process of the invention is simple, and has low energy consumption and little environmental pollution.
Owner:ZHEJIANG UNIV OF TECH
Esomeprazole and preparation method of magnesium trihydrate of esomeprazole
The invention provides esomeprazole and a preparation method of magnesium trihydrate of the esomeprazole. The preparation method includes the following steps of subjecting racemization omeprazole and inorganic base to acid-base neutralization reaction in an alcoholic solution to obtain racemization omeprazole sodium salt; dissolving omeprazole sodium salt, organic metal coordination agents, chelating agents and organic base in an organic solvent for complex reaction to obtain esomeprazole complex; subjecting S-mandelic acid and the esomeprazole complex to condensation reaction to obtain an esomeprazole mandelate compound; dissolving the esomeprazole mandelate compound in an acetone solution, and performing filtering to obtain S-omeprazole-S-mandelate compound; and suspending the S-omeprazole-S-mandelate compound in a first solvent to obtain a suspension solution, and adjusting potential of hydrogen (pH) of the suspension solution to be 8-10 to obtain the esomeprazole, wherein the first solvent includes 30-32v / v% of an alkaline aqueous solution and 68-70v / v% of an organic solvent. By means of the preparation method, the technical problem that the yield and the purity of the esomeprazole in prior art are low is solved.
Owner:HUNAN FANGSHENG PHARMACEUTICAL CO LTD
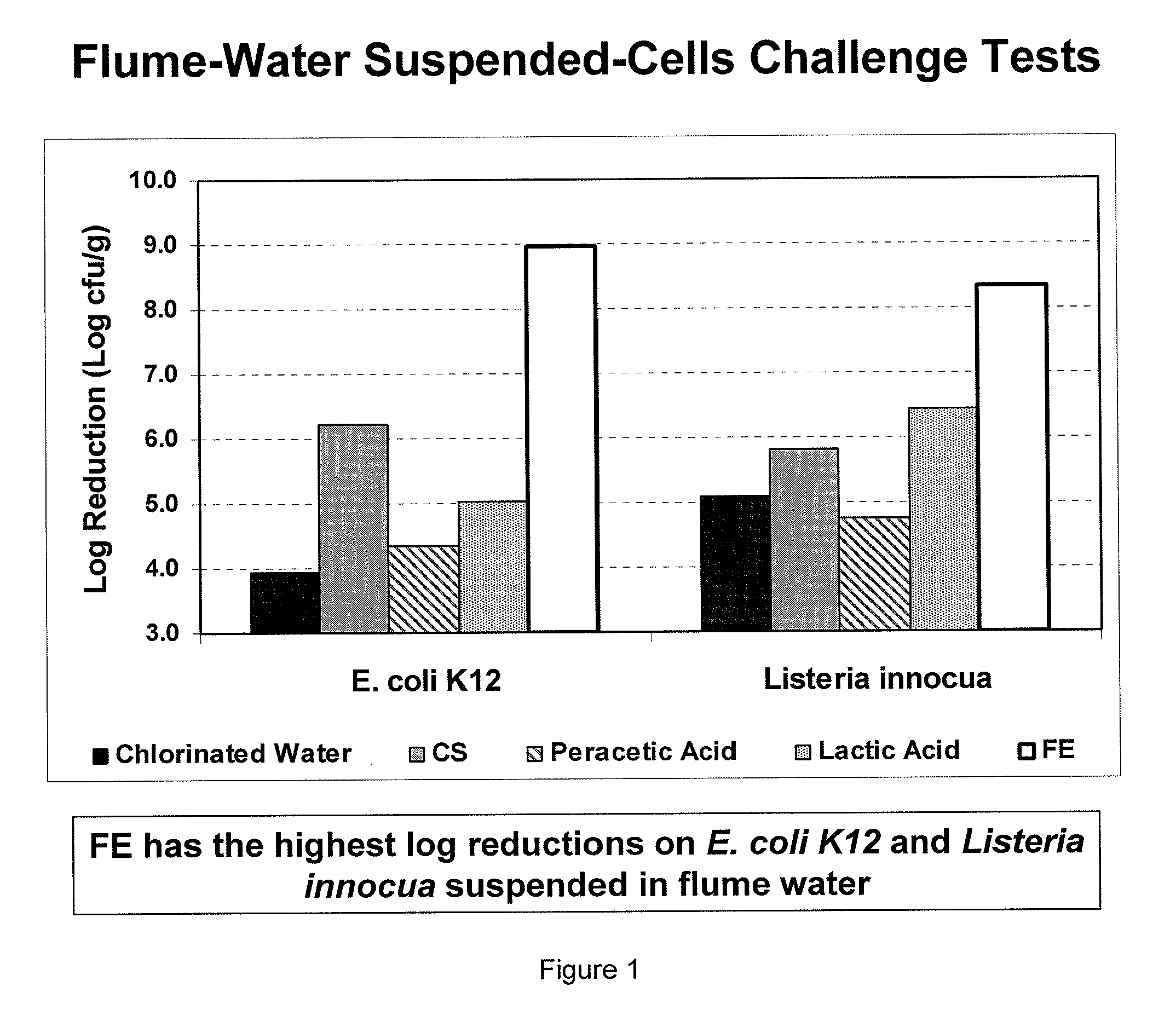
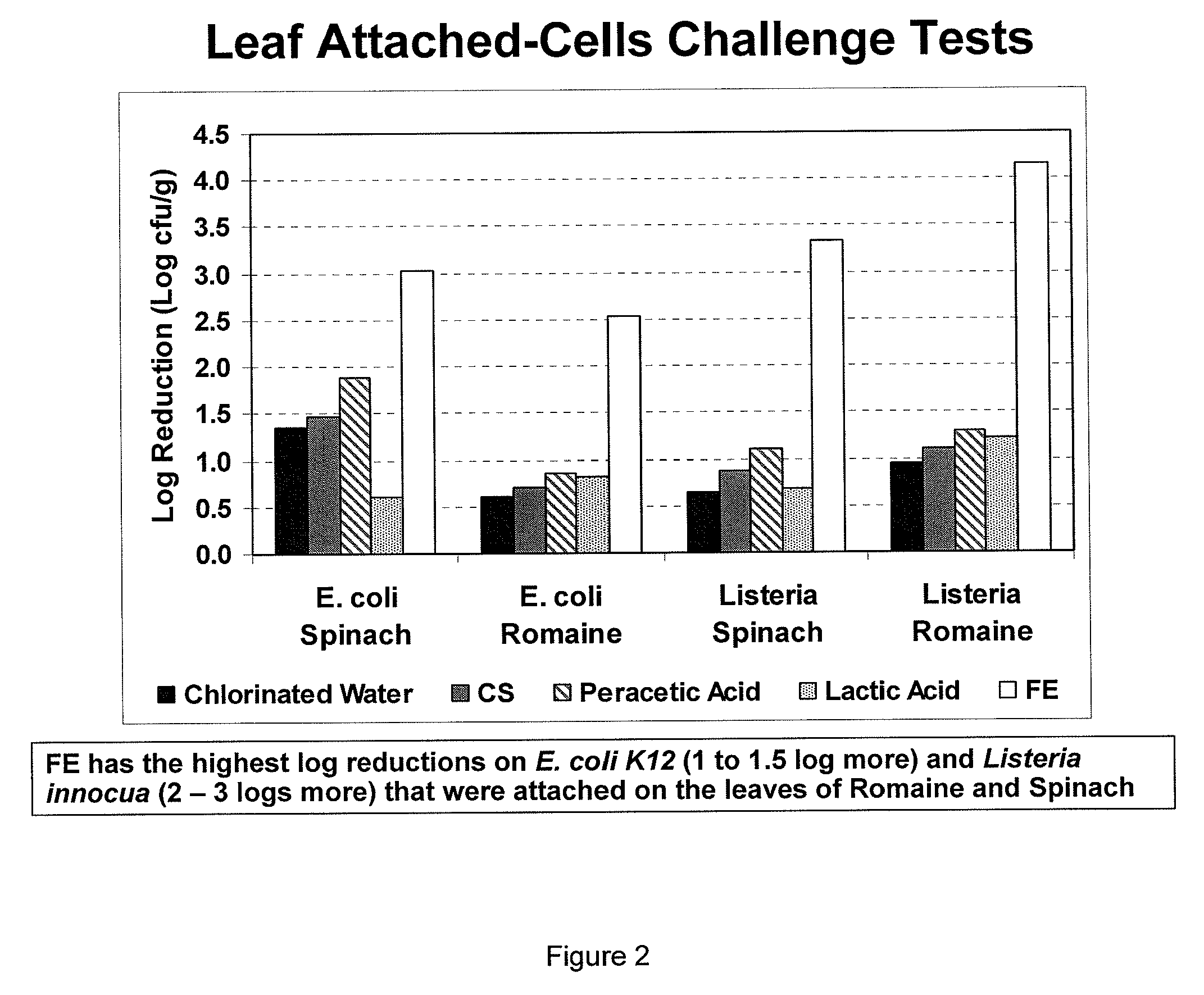
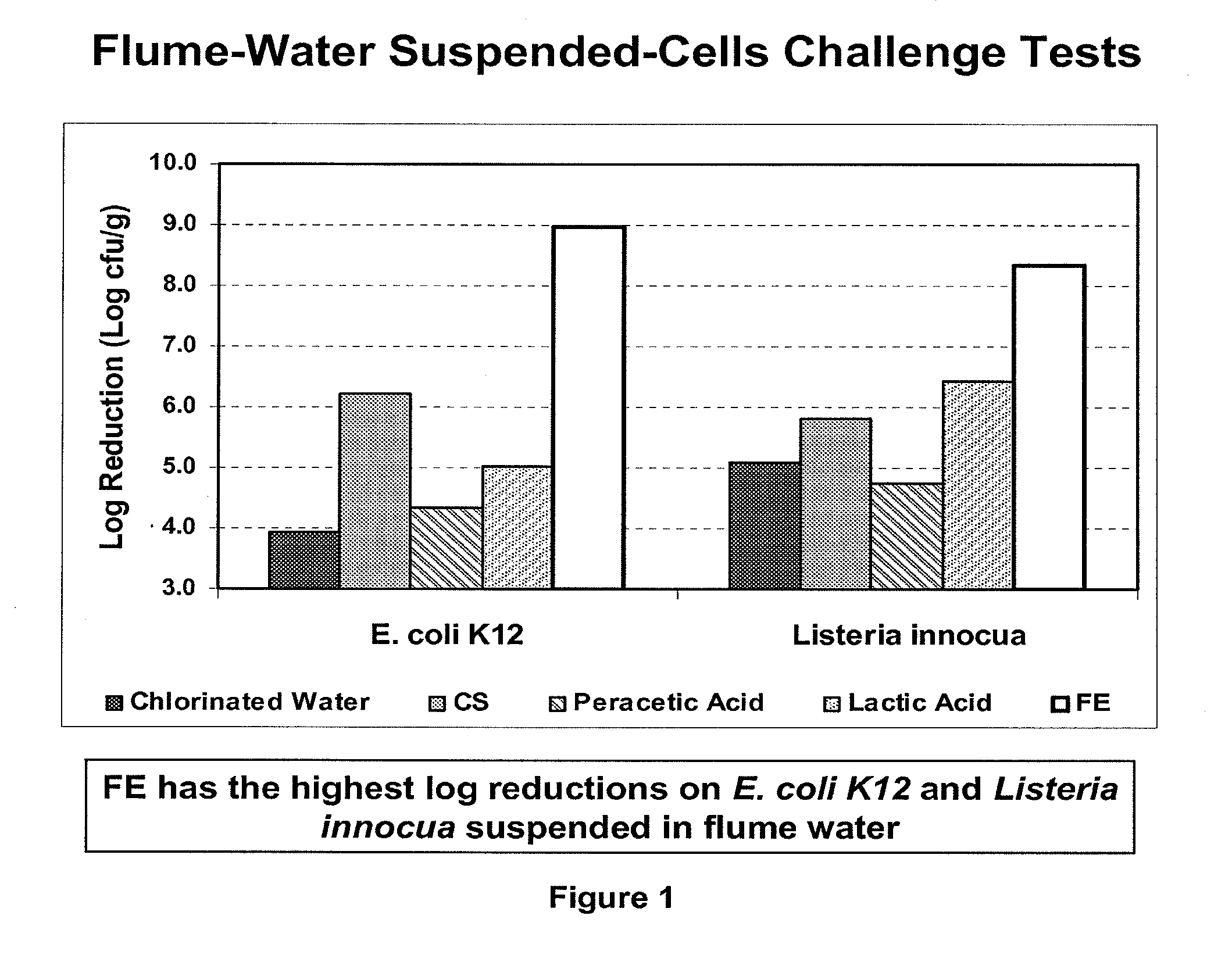
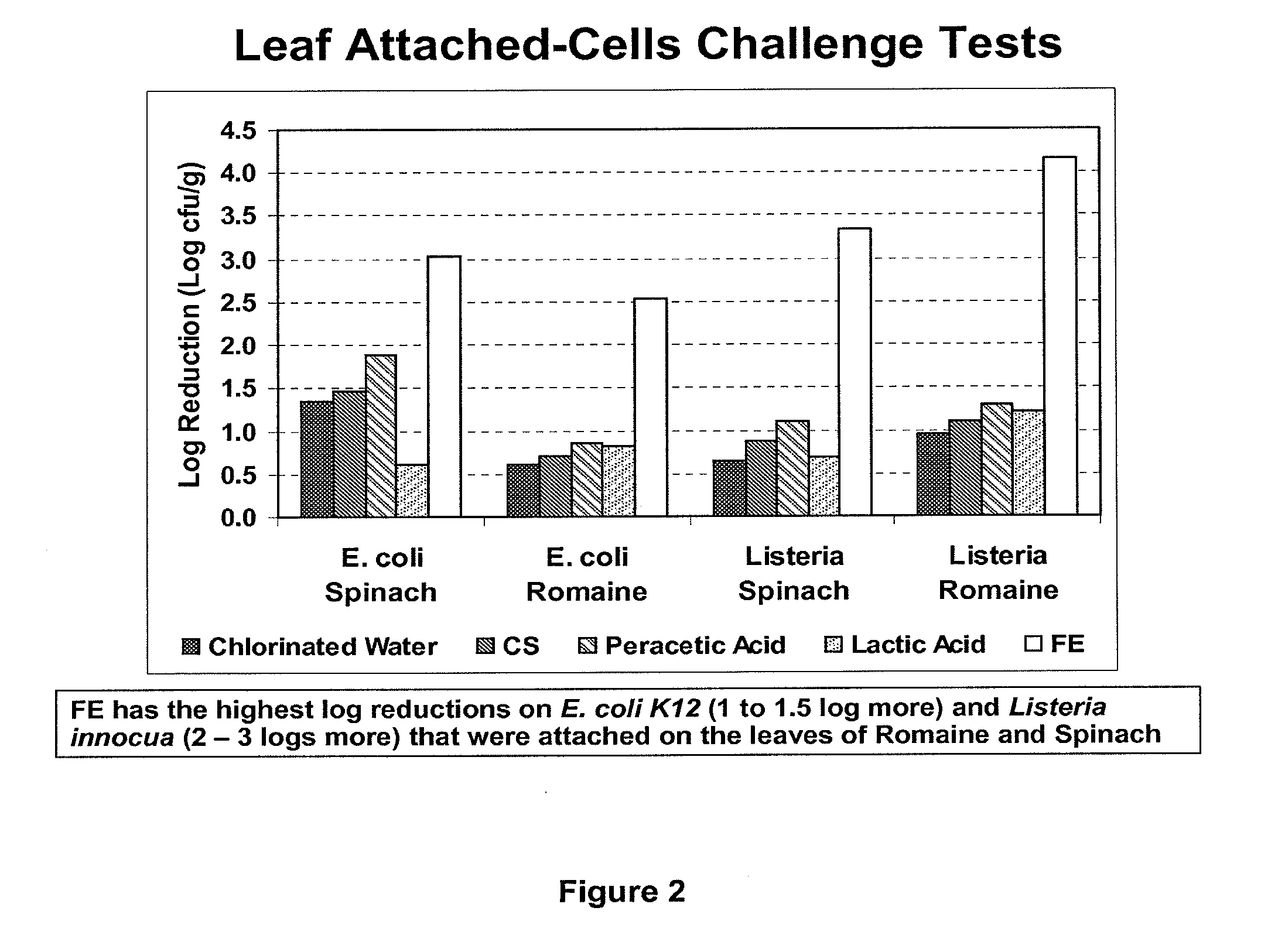
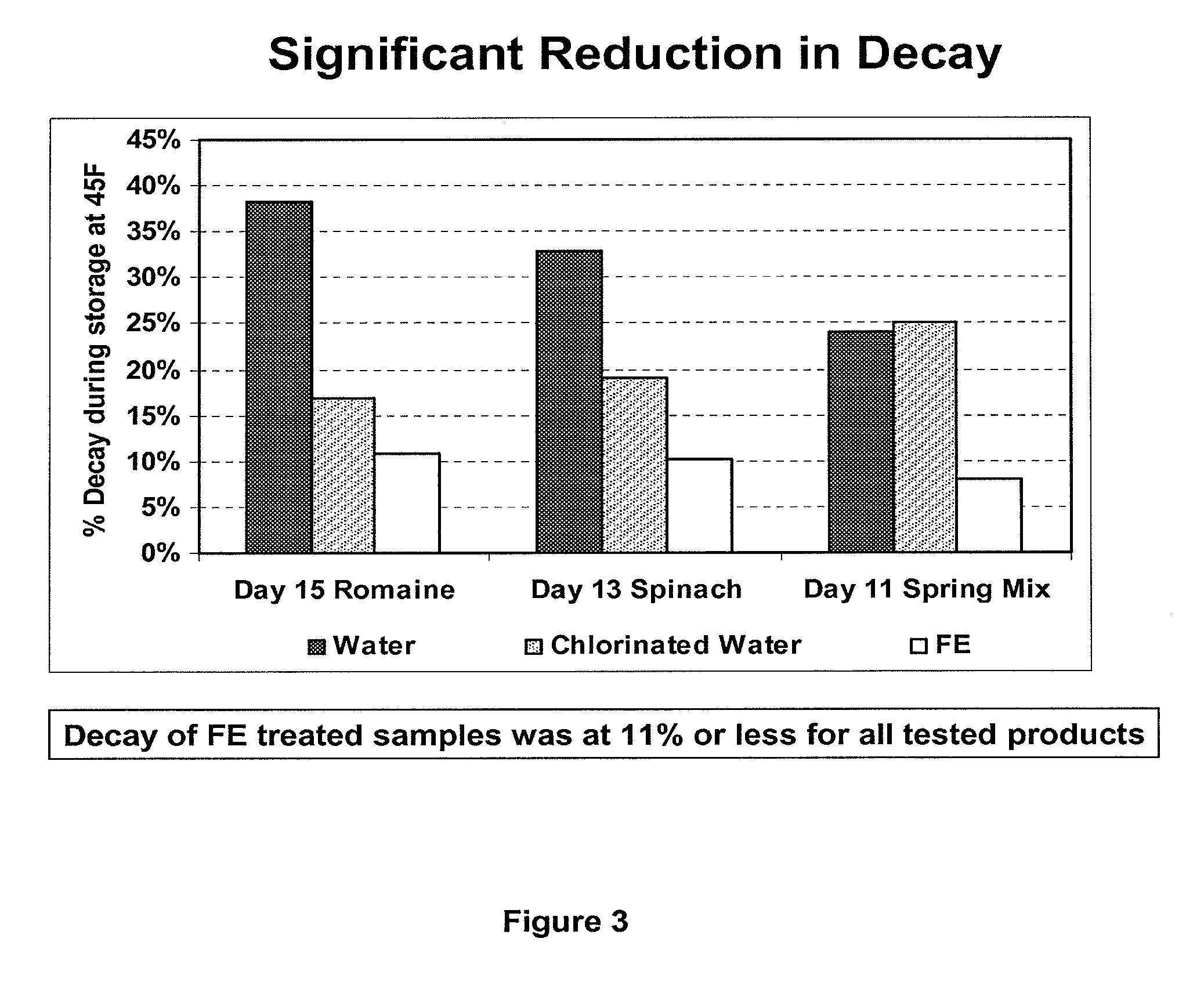
Peracid and 2-hydroxy organic acid compositions and methods for treating produce
InactiveUS20090324789A1Prevent spoilageMaintain qualityBiocideMilk preservationMicroorganismOrganic acid
Owner:FRESH EXPRESS
Compsns-and methods for trapping and inactivating pathogenic microbes and spermatozoa
Antimicrobial and contraceptive compositions and methods which prevent and / or reduce the risk of transmission of sexually transmitted diseases through sexual activity as well as prevent and / or reduce the risk of pregnancy are provided. The compositions contain (1) a matrix-forming agent, (2) a bio-adhesive agent, (3) a buffering agent, (4) optionally a humectant, (5) optionally a preservative, and (6) water; wherein the composition is suitable for application within the vagina; wherein the compositions form a semisolid matrix on contact with ejaculate (thereby trapping ejaculated microbes and spermatozoa); wherein the composition causes hardening of cervical mucus (thereby decreasing the probability of sperm entry); wherein the composition forms a bio-adhesive layer over vaginal surfaces (thereby preventing or reducing the risk of contact of STD-causing microbes with the vaginal surfaces); wherein the composition maintains an acidic vaginal pH of less than about 5 in the presence of semen ejaculated from the male; and wherein the composition does not significantly impair the natural microbiological balance within the vagina. The antimicrobial and contraceptive compositions may also contain additional antimicrobial and / or contraceptive agents (e.g., nonoxynol-9, octoxynol-9, benzalkonium chloride, phosphorylated hesperidins, sulfonated hesperidins, polystyrene sulfonates, substituted benzenesulfonic acid formaldehyde co-polymers, H2SO4-modified mandelic acids, povidone iodine, itraconazole, ketoconazole, metronidazole, clotrimazole, fluconazole, teraconazole, miconazole, tinidazole, iconazole, chloramphenicol, nystatin, cyclopiroxolamine, and the like).
Owner:RUSH UNIV MEDICAL CENT
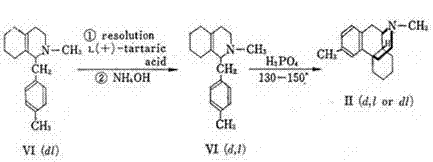
Preparation method and application of glycopyrronium bromide chiral antipode
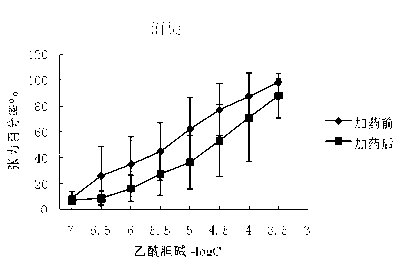
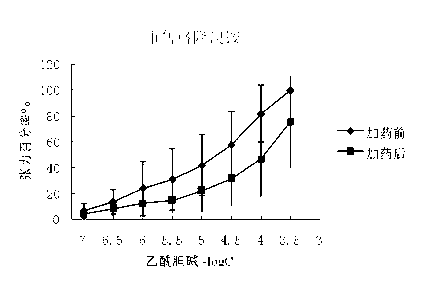
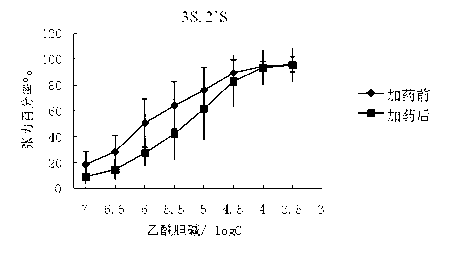
The invention belongs to the technical field of medicine, and discloses a preparation method of (3S,2'S), (3S,2'R), (3R,2'R) and (3R,2'S) four type chiral monomers of muscarine receptor antagonist racemic medicine glycopyrronium bromide. The method comprises the following steps: resolving racemic alpha-cyclopentylmandelic acid by a chemical resolution method by using L-Tyrosine methyl ester and (R)-alpha-phenylethylamine as resolution reagents to respectively prepare (S)-alpha-cyclopentylmandelic acid and (R)-alpha-cyclopentylmandelic acid; and carrying out esterification reaction to respectively obtain chiral intermediates (S) / (R)-alpha-cyclopentylmethyl mandelate. L / D-malic acid used as the raw material is subjected to four reaction steps, including condensation, carbonyl reduction, catalytic hydrogenation or transfer hydrogenation reduction debenzylation, and reduction alkylation or alkylogen alkylation, in a chiral synthesis mode to obtain another important chiral intermediate (S) / (R)-N-methyl-3-hydroxypyrrolidine. The chiral intermediate is subjected to ester exchange and quaterisation to respectively obtain the four (3S,2'S), (3S,2'R), (3R,2'R) and (3R,2'S) type glycopyrronium bromide chiral monomers. The result indicates that the (3R,2'S)-glycopyrronium bromide has the strongest cholinergic antagonistic action.
Owner:SHENYANG PHARMA UNIVERSITY +1
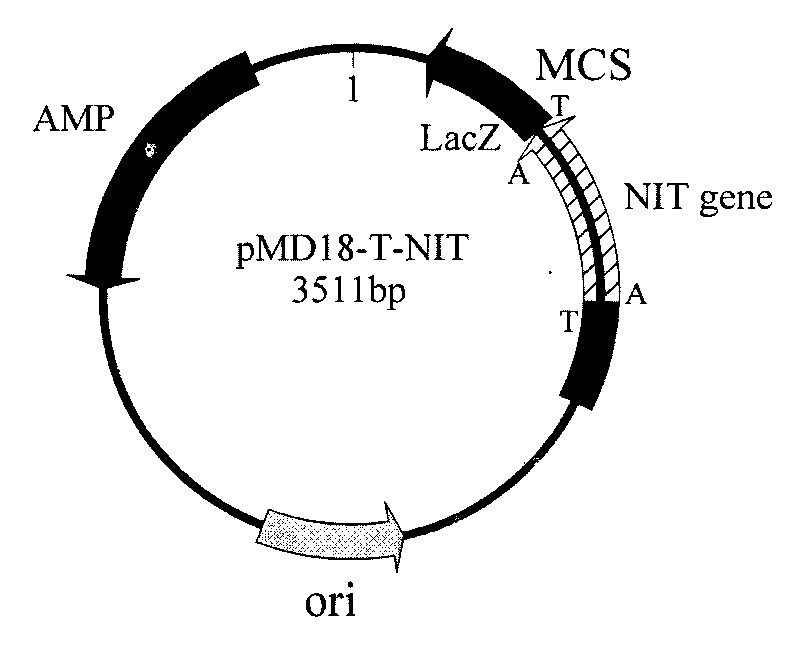
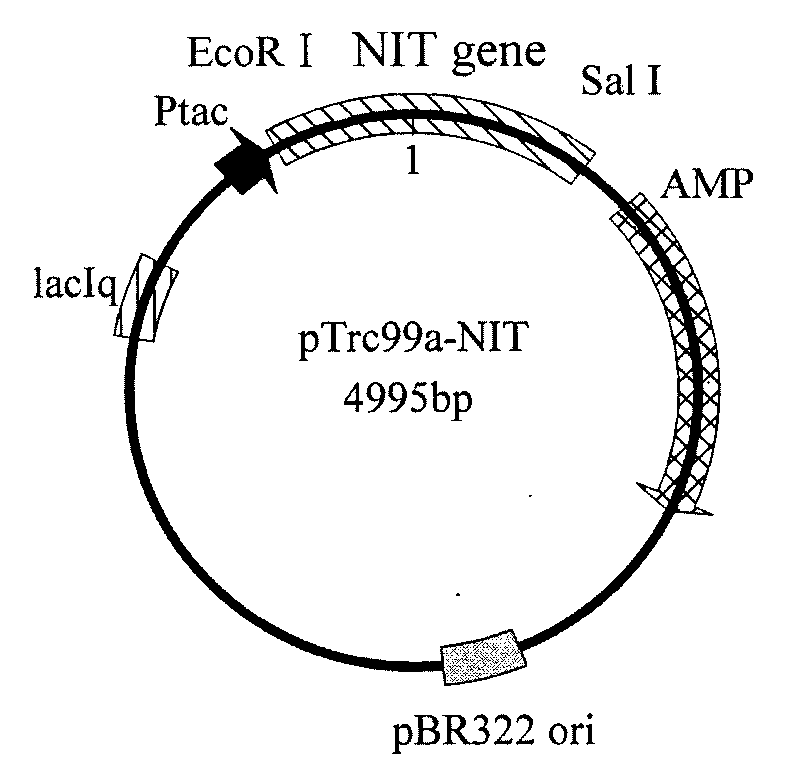
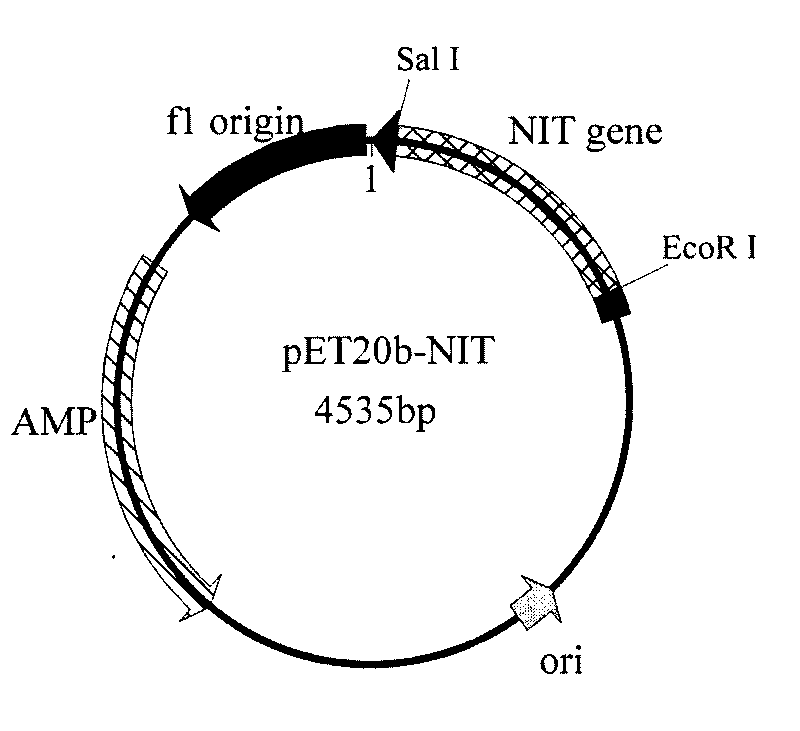
Nitrilase gene, vector, engineering bacteria and application thereof
The invention provides a nitrilase gene coding nitrilase, a recombinant vector containing the gene, a recombinant gene engineering bacteria obtained by converting the recombinant vector and application thereof in preparing recombinant nitrilase. The nitrilase gene can be connected with an expression vector for construction to obtain endoenzyme expression recombinant plasmid containing the gene or secretion expression recombinant plasmid, and then the endoenzyme expression recombinant plasmid containing the gene or the secretion expression recombinant plasmid is respectively and correspondingly converted to a colibacillus bacterial strain to obtain recombinant colibacillus; the recombinant colibacillus contains recombinant nitrilase and can recombine colibacillus into an enzyme resource for biological catalysis and conversion. The recombinant nitrilase serves as the enzyme for conversion, and racemisation mandelonitrile, acrylonitrile, iminodiacetonitrile or 2,2-dimethylcyclopropane carbonitrile and the like serve as a substrate for converting to react and prepare corresponding R-mandelic acid, crylic acid, iminodiacetic acid or chiral 2,2-dimethylcyclopropane formic acid and the like.
Owner:ZHEJIANG UNIV OF TECH
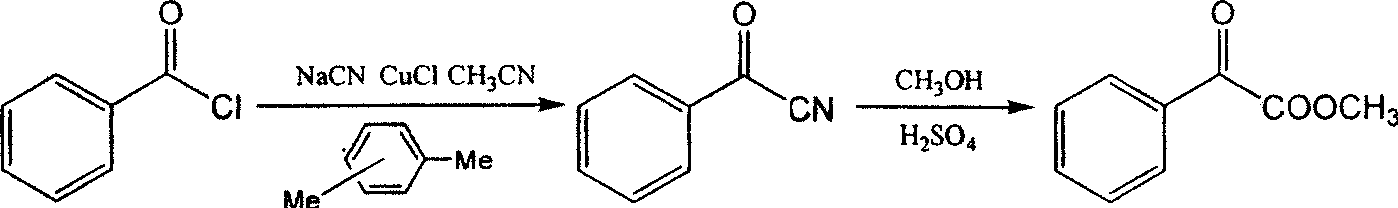
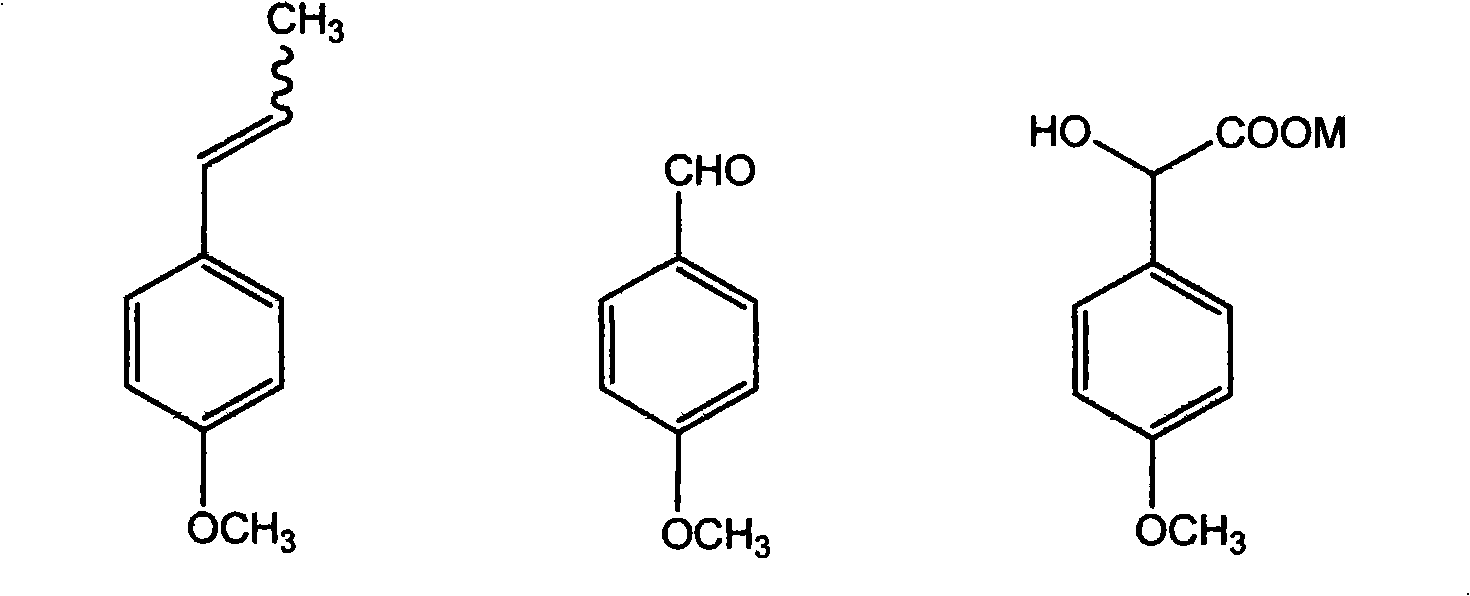
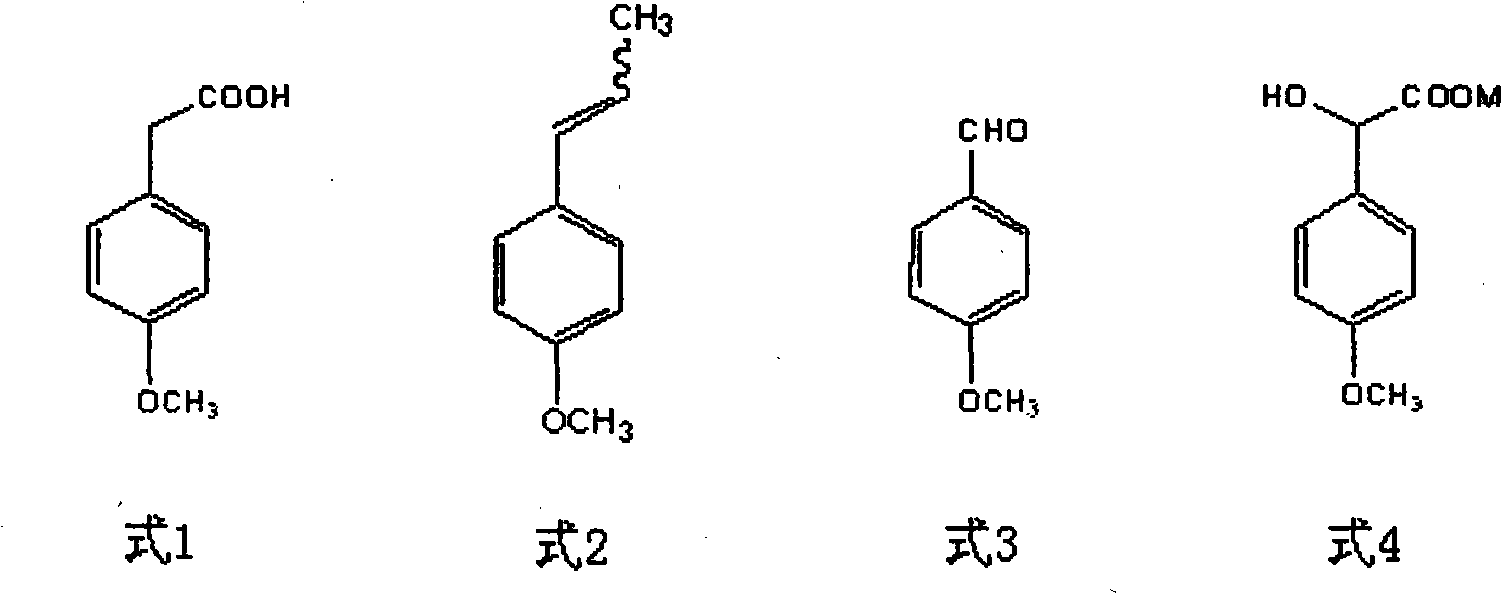
Method for preparing p-methoxypheny-lethyl acid from natural anethole
InactiveCN101298416ANo pollution in the processSimple reaction conditionsOrganic compound preparationCarboxylic preparation by oxidationP-methoxyphenylacetic acidPetrochemical
The invention discloses a method for preparing methoxyphenylacetic acid by natural anethole; the natural anethole is taken as a raw material and generated into the anisicaldehyde by oxidation reaction, then anisic mandelic acid (salt) is generated by the insertion reaction of carbine; finally the methoxyphenylacetic acid is obtained by reduction. The method has the advantages of reproducible raw material, simple operation and high yield, etc.; furthermore, the method can prepare the methoxyphenylacetic acid which can replace the source of petrochemical industry.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Method for producing R-(-)- benzoglycolic acid
InactiveCN101220382AHigh ee valueNon-volatileMicroorganism based processesFermentationWater bathsFiltration
The invention relates to a preparation method of R-(-)-mandelic acid and the steps are that: mandelic acid ethyl ester is taken and added in a reactor, then a ionic liquid, a tris(hydroxymethyl)aminoethane-hydrochloric acid buffer solution and a lipase catalyst are added; each milliliter of the ionic liquid is added with 0.1g to 0.3g of the mandelic acid ethyl ester, the amount of lipase is 2.5 percent to 7.5 percent of the weight of the mandelic acid ethyl ester, the ionic liquid: the buffer solution is equal to 1: 1 to 8 accounted by volume ratio, the pH scope of the buffer solution is 6 to 8.5; then reaction is carried out for 5.0 to 10 hours in a constant temperature water bath shaker, filtration is carried out for enzyme removal and water removal, the solids of the mandelic acid and the mandelic acid ethyl ester are obtained by ethyl acetate extraction and rotation evaporation; then n-hexane is added and the R-(-)-mandelic acid is obtained by filtration. The preparation method of the R-(-)-mandelic acid of the invention has simple technique, mild reaction conditions and small environmental pollution; the ionic liquid is taken as a reaction medium, thereby greatly shortening reaction time, the obtained product has high stereoselectivity, the ee value of the product is 60 percent at most, and the yield can achieve 92 percent.
Owner:HEBEI UNIV OF TECH
Preparation method for high-purity esomeprazole sodium
ActiveCN103288801ASolve the prone to titanium complex suspensionSolve the difficulty of splittingOrganic chemistrySodium bicarbonateOmeprazole Sodium
The invention discloses a preparation method for high-purity esomeprazole sodium. The preparation method comprises the steps of: including and splitting esomeprazole sodium and D-(-)-diethyl tartrate, titanium iso-propylate, triethylamine and L-(+)-mandelic acid in the presence of a proper amount of water, and separating to obtain an inclusion complex; dissolving the inclusion complex with ethyl acetate, washing inclusion complex with sodium carbonate water solution, carrying out ammonia hydroxide eluting on an ethyl acetate layer, slowly regulating the pH value to 6-7 with glacial acetic acid, then extracting with dichloromethane, and concentrating to obtain crude esomeprazole free alkali product; carrying out silica gel adsorption and elution on the crude product to obtain a pure esomeprazole free alkali product; and enabling the pure product and the methanol-ethanol-acetonitrile solution of sodium hydroxide to form salt, and then crystallizing with isopropyl ether to obtain the high-purity esomeprazole sodium. According to the preparation method, the difficulties that when inclusion and splitting are carried out, the titanium complex suspension body are difficult to split and the ammonia complex of titanium is difficult to remove can be solved, the industrialization production can be realized, the industrialized production cost is low, the product purity is high, the yield is high, and no harmful gas is generated.
Owner:SICHUAN BAILI PHARM CO LTD
Peracid and 2-hydroxy organic acid compositions and methods for sanitation and disease prevention
Methods and compositions for treating living surfaces to control microorganisms are provided. The method treats produce by contacting a living surface with an aqueous solution comprising i) an organic peracid of the formula RC(O)OOH wherein R is methyl, ethyl, n-propyl, or s-propyl; ii) a 2-hydroxy organic acid selected from tartaric acid, citric acid, malic acid, mandelic acid, and lactic acid; and iii) water.
Owner:FRESH EXPRESS
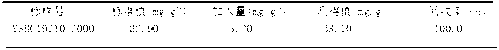
Method for determining alumina content in iron ore
InactiveCN103105396ARapid determinationThe result is accurateMaterial analysis by observing effect on chemical indicatorSodium acetatePotassium hydroxide
The invention relates to a detection method, in particular to a method for determining the alumina content in deposited iron briquette rock ore. The method includes: using potassium hydroxide as a fusing agent to fuse an iron ore sample in a high temperature furnace, conducting acidification by nitric acid, letting aluminum ions enter the solution, adding mandelic acid and excess ethylenediamine tetraacetic acid disodium salt (EDTA) standard solution to shelter titanium, iron, aluminum and other metal ions, and in an acetic acid-sodium acetate buffer solution with pH of 4, taking 1-(2-pyridylazo)-2-naphthol as an indicator, carrying out titration on the residual ethylenediamine tetraacetic acid disodium salt (EDTA) standard solution by means of a copper sulfate standard solution, adding a sodium fluoride solution to replace the ethylenediamine tetraacetic acid disodium salt (EDTA) standard solution cooperating with the aluminum ions, and then carrying out titration by a copper sulfate standard solution, thus obtaining the alumina content. The method provided in the invention has the characteristics of simplicity, rapidity, low cost and good repeatability, etc.
Owner:YUNNAN PHOSPHATE CHEM GROUP CORP
Biological preparation method for (1R, 2S)-2-(3,4-difluorophenyl) cyclopropylamine D-mandelate (I)
ActiveCN106701840AEfficient productionAmino preparation from aminesOrganic compound preparationAcetic acidEthyl ester
The invention provides a biological preparation method for (1R, 2S)-2-(3,4-difluorophenyl) cyclopropylamine D-mandelate (I). Specifically, the method disclosed by the invention comprises the following steps: (a) taking a compound of a formula VI as a substrate in a liquid reaction system, and carrying out an asymmetric addition reaction in the presence of coenzymes under catalysis of carbonyl reductase so as to form a compound of a formula V; (b) enabling the compound of the formula V to react with triethyl phosphonoacetate so as to obtain a compound of a formula IV; and (c) performing ammonolysis and Hofmann degradation on the compound of the formula IV and salifying with D-mandelic acid, thereby obtaining the compound of the formula VI. The invention further provides a reaction system which comprises (i) an aqueous solvent; (ii) a substrate which is the compound of the formula IV; (iii) coenzymes; (iv) carbonyl reductase; (v) a cosubstrate; and (vi) enzymes used for coenzymes regeneration. The structural formula is as shown in the specification.
Owner:SHANGHAI INST OF PHARMA IND +1
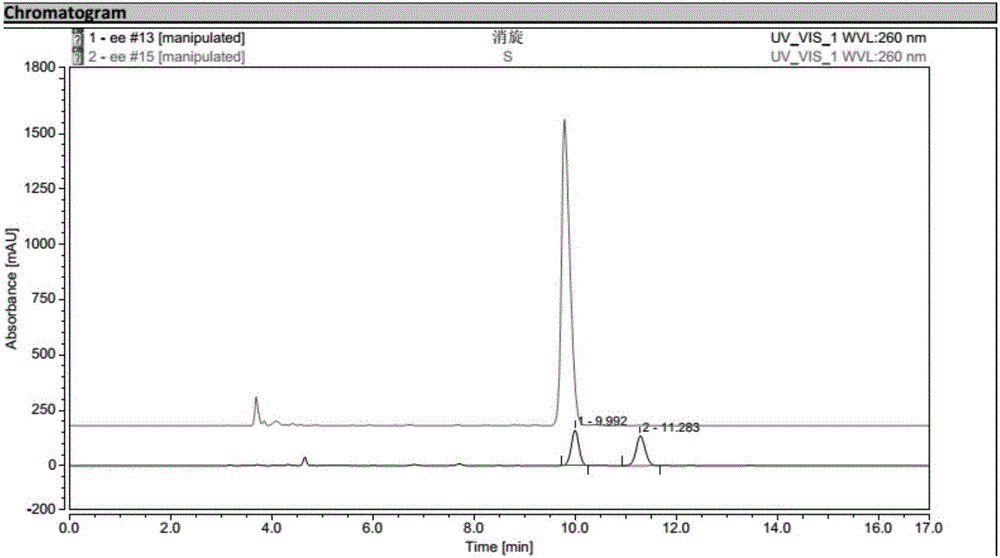
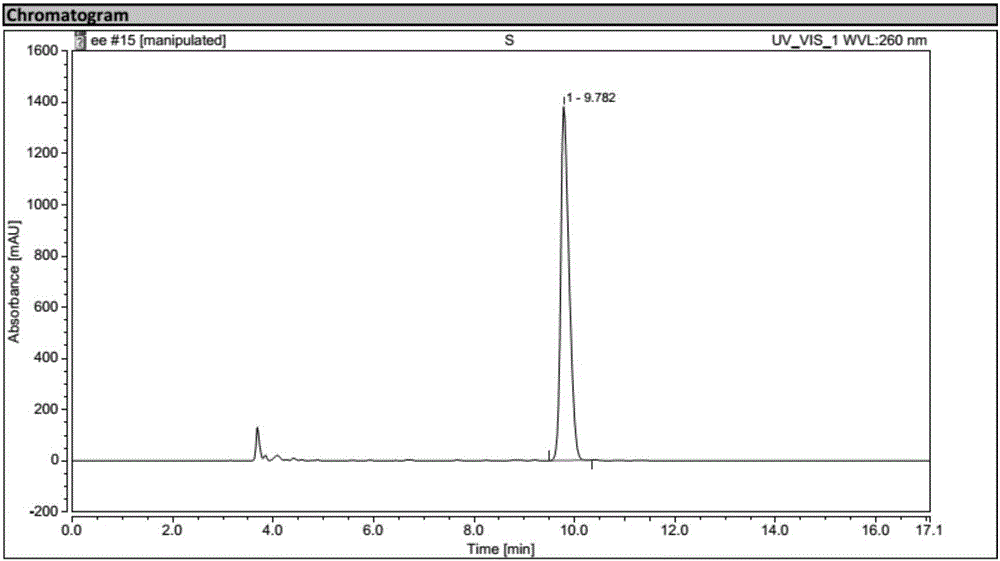
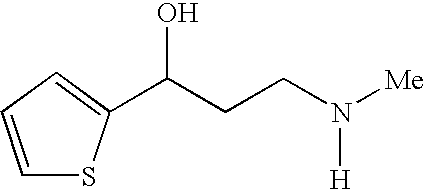
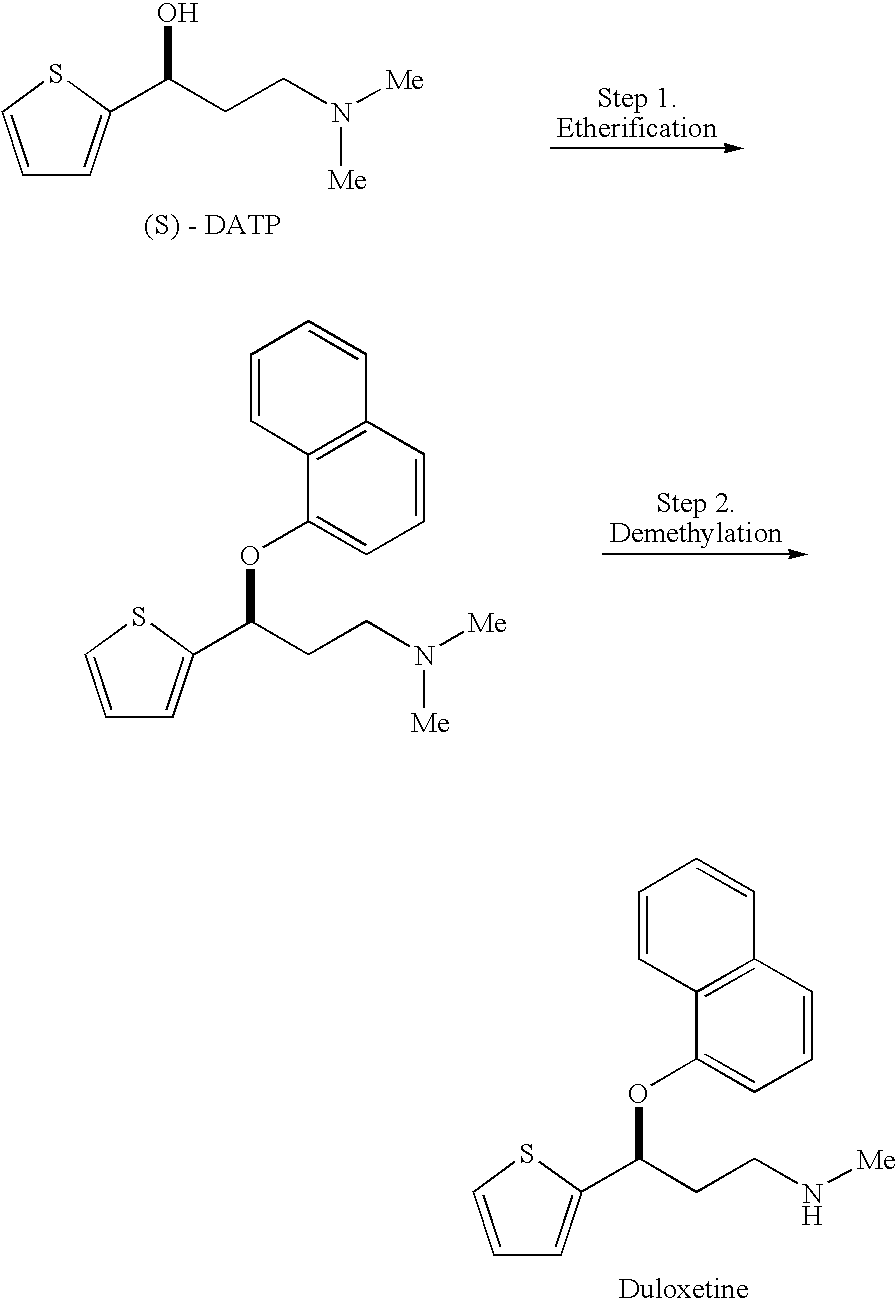
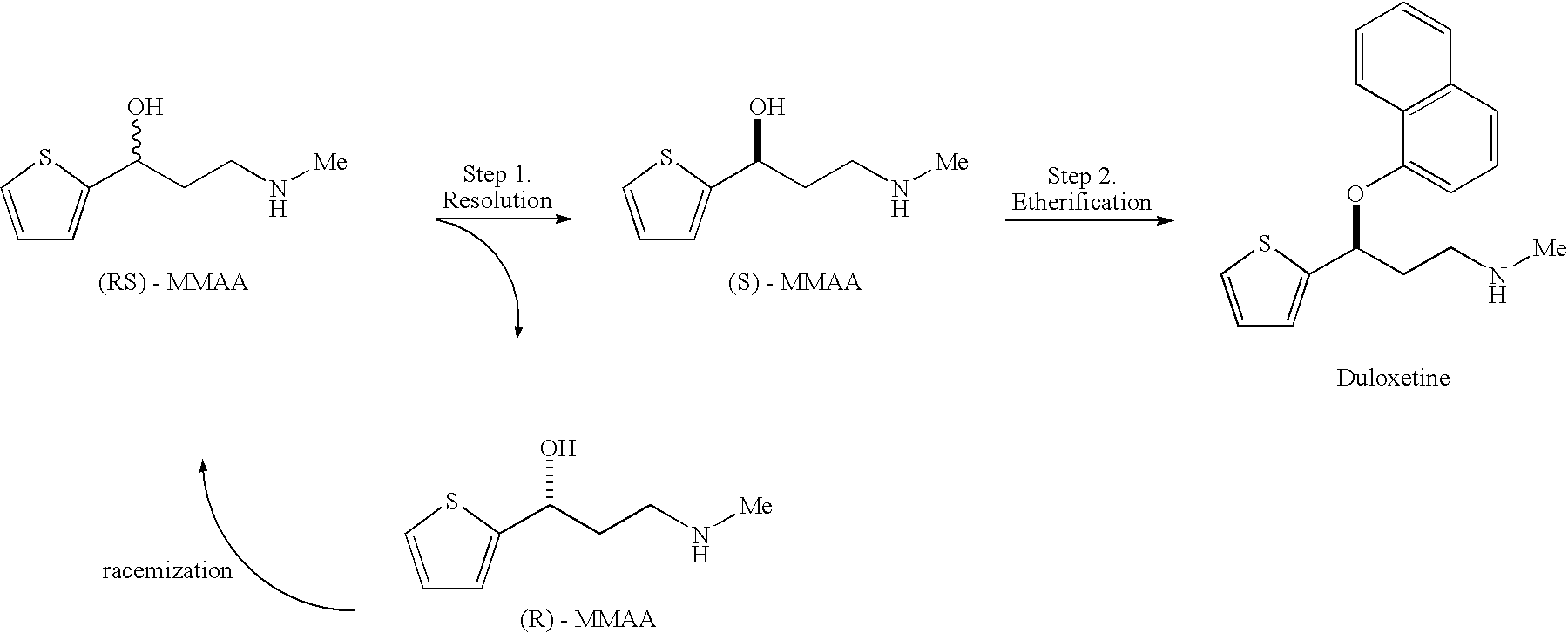
Process for preparing optically active 3-(methylamino)-1-(2-thienyl) propan-1-ol and intermediates for preparation
InactiveUS20060063943A1Ease of industrial applicationHigh optical purityOrganic chemistryDuloxetineMandelic acid
Disclosed is a process for commercial preparation of 3-(methylamino)-1-(2-thienyl)propan-1-ol (hereinafter abbreviated as “MMAA”) of the formula below: The process is carried out by the diastereomeric salt formation method using optically active mandelic acid or its derivatives, or an optically active tartaric acid derivative as the resolving agent. The product compound, diastereomeric satls, is useful as the intermediate for producing pharmaceuticals, such as duloxetine.
Owner:YAMAKAWA CHEM IND
Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds
A process and diastereomeric salts useful for the optical resolution of racemic α-[4-(1,1-dimethylethyl)phenyl]-4-(hydroxydiphenylmethyl)-1 -piperidinebutanol, 4-[4-[4-(hydroxydiphenylmethyl)-1 -piperidinyl]-1-hydroxybutyl]-α,α-dimethylbenzeneacetic acid and lower alkyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]-α,α-dimethylbenzeneacetates. The process comprises placing into solution a chiral resolving agent, either (+) / (−)-di-paratoluoyltartaric acid or (−) / (+)-mandelic acid, in an amount equimolar to a compound corresponding to the desired enantiomer of the above compound, precipitating the resulting diastereomeric salt between the chiral resolving agent and the target enantiomer and separating the enantiomer.
Owner:MERRELL DOW PHARMA INC
Preparation method of single chiral metal-organic framework material with chiral separation and photoinduction functions
InactiveCN103193831AEasy to operateImprove efficiencyOxygen-containing compound preparationOrganic compound preparationChemical industryCarboxyl radical
The invention provides a preparation method of a single chiral metal-organic framework material with chiral separation and photoinduction functions, belonging to the technical field of chemical industry. A single chiral ligand (S)-H4L: (S)-3,3'-ditertbutyl-5,5'-bis(3,5-dicarboxyphenyl)-6,6'-dimethyl-2,2'-dihydroxy-1,1'-biphenyl is synthesized and then mixed with metal salts, the mixture is dissolved by N,N-dimethylformamide and water, acetate is added to react, after reaction, the product is cooled to a room temperature, and then the cooled product is washed by ether for several times, and dried in the air to obtain the finish product. The method is relatively simple and easy to operate, and ideal in separation effects for mandelic ester compounds, and has functions of inducing asymmetric cyclization of tropolone ether.
Owner:SHANGHAI JIAO TONG UNIV
Preparation method for graphene water-based paint and adhesive with improved light resistance
InactiveCN104098980AStrong light resistanceImprove adhesionNon-macromolecular adhesive additivesMacromolecular adhesive additivesPolymer scienceAcrylic resin
The invention relates to a preparation method for graphene water-based paint and adhesive with improved light resistance. The preparation method for the graphene water-based paint and adhesive with the improved light resistance includes that adding water, emulsifying agent and acrylic amide to a reaction vessel, heating to 40 degrees centigrade, stirring for 30 minutes, adding monomer A, emulsifying for 40 to 70 minutes, starting to fill reflux water after heating to 75 degrees centigrade, adding initiating agent for 1 to 2 hours after heating to 80 degrees centigrade, reacting for 1 to 2 hours after finishing adding, adding 6 beta-hydroxy-4-alkene-3-bean sterone and cantharidin, and stirring to react for 3 hours to obtain nuclear emulsion; simultaneously adding monomer B and triggering agent to the nuclear emulsion for 1 to 3 hours, stirring to react for 1 hour at 90 degrees centigrade after finishing adding, adding coupling agent grafted graphene solution and 3-methoxy-4-hydroxy mandelic acid, adding cross-linking agent, reacting for 2 to 3 hours at 70 to 85 degrees centigrade, cooling to 40 degrees centigrade, adding emulsifying agent, reacting for 70 minutes, adding sodium citrate, sodium borohydride and 2, 4-diamido-6-hydroxy pyrimidine, reacting for 1 hour at 80 degrees centigrade, and adding ammonia water to regulate the pH value to 7 to 8 to obtain the light-resistant water-based paint and adhesive. The preparation method for the graphene water-based paint and adhesive with the improved light resistance is capable of greatly improving the light resistance of acrylic resin and avoiding the disadvantage that the acrylic resin emulsion film is yellow.
Owner:GUANGZHOU XIANGHAI BIOTECH CO LTD
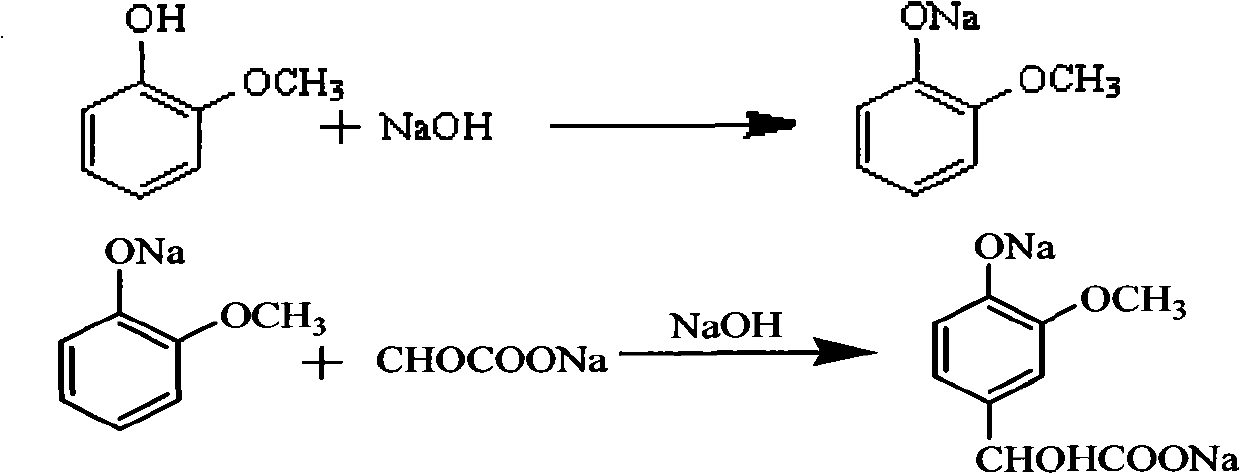
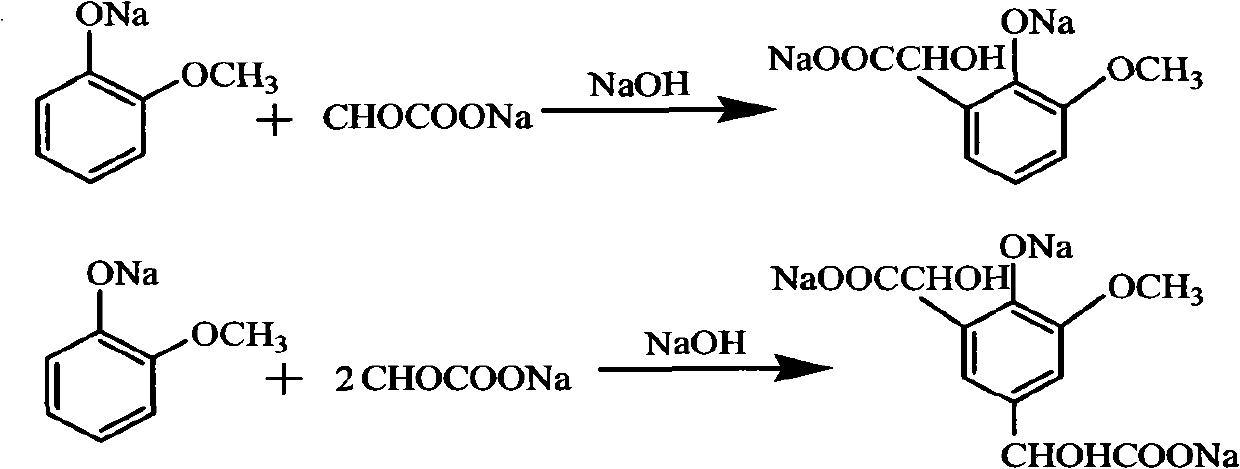
Method for preparing 3-methoxy-4-hydroxy mandelic acid
ActiveCN102086151APrevent oxidationIncrease profitOrganic compound preparationCarboxylic compound preparationReaction temperatureGuaiacol
A method for preparing 3-methoxy-4-hydroxy mandelic acid adopts guaiacol and glyoxylic acid as raw materials, and comprises the following steps: slowly adding a 30 wt% sodium hydroxide solution into the glyoxylic acid solution with stirring, allowing the mixed solution to react at a reaction temperature of 0-50 DEG C and a pH value of 1-7 to obtain a sodium glyoxylate solution; adding the guaiacol into water with stirring and under the protection of nitrogen gas, slowly adding the 30 wt% sodium hydroxide solution, allowing the mixed solution to react at a reaction temperature of 0-50 DEG C and a pH value of 11-12 to obtain guaiacol sodium; slowly and dropwisely adding the sodium glyoxylate solution into the guaiacol sodium solution with stirring and under the protection of nitrogen gas, with the mole ratio of the sodium glyoxylate and the guaiacol sodium being 1:1.2-2, allowing the mixed solution to react at a reaction temperature of 0-20 DEG C and a pH value of 11-12, 30-90 min afterthe dropwise addition, stirring the mixed solution for 1-3 hours, stopping the reaction at the reaction temperature for 24-36 hours, then acidifying the reaction solution by a 50 wt% sulfuric acid solution to control the pH value to be 4-5. The yield of the reaction is above 95 wt%.
Owner:PETROCHINA CO LTD
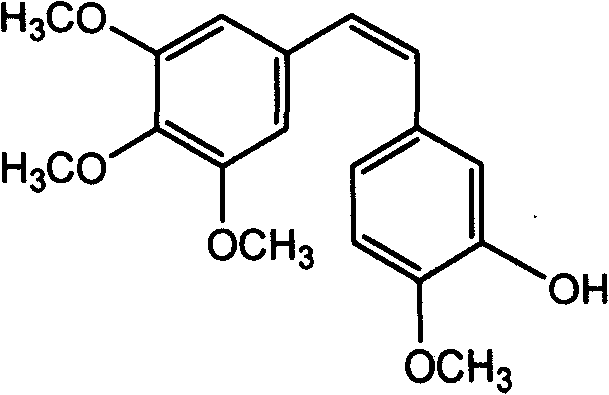
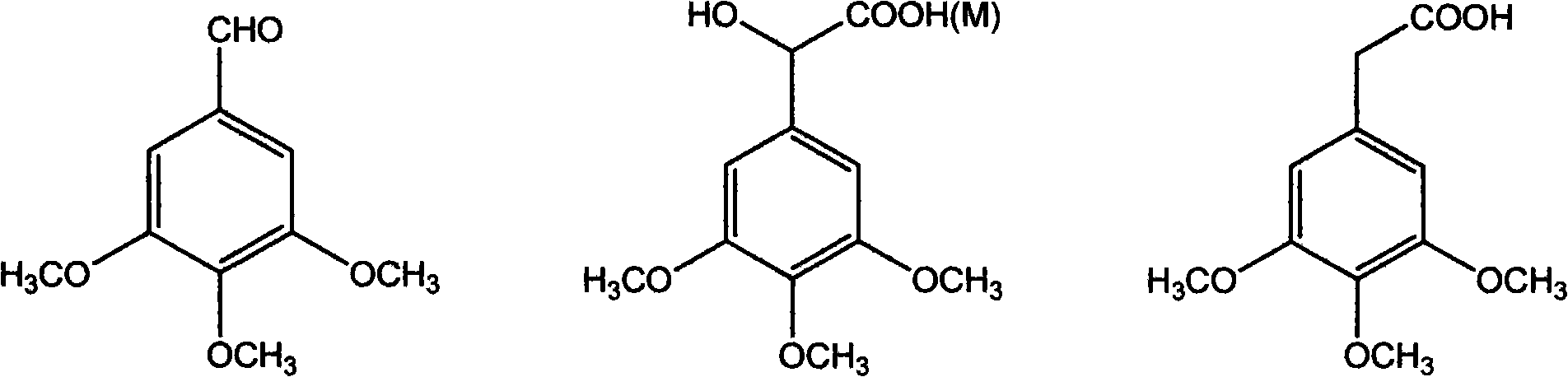
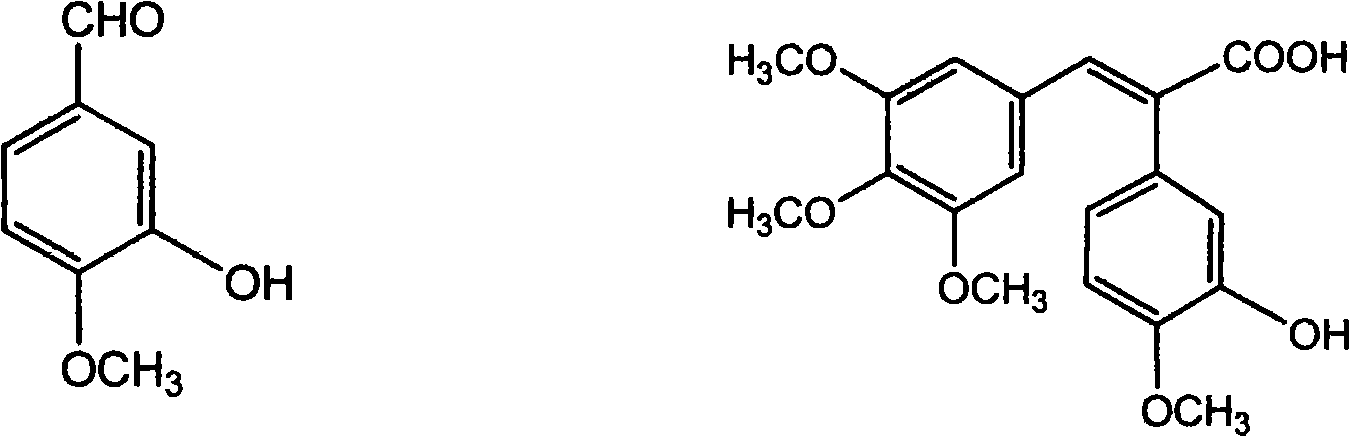
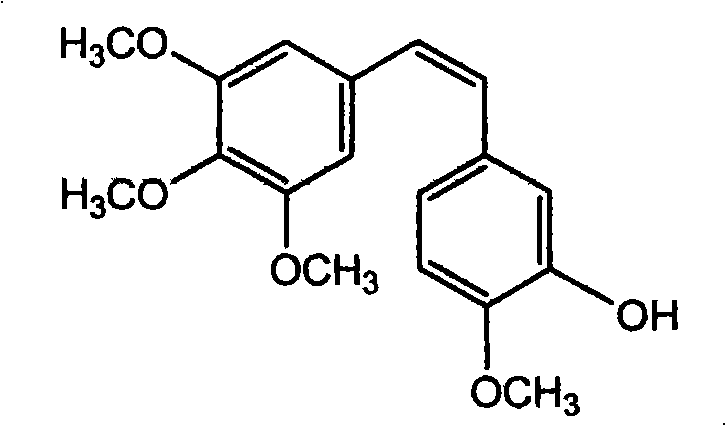
Method of preparing (Z)-3'-hydroxyl-3,4,4',5-tetramethoxy toluylene
InactiveCN101402555AGood sustainable development abilityCost-effectiveOrganic chemistryOrganic compound preparationPetrochemicalMandelic acid
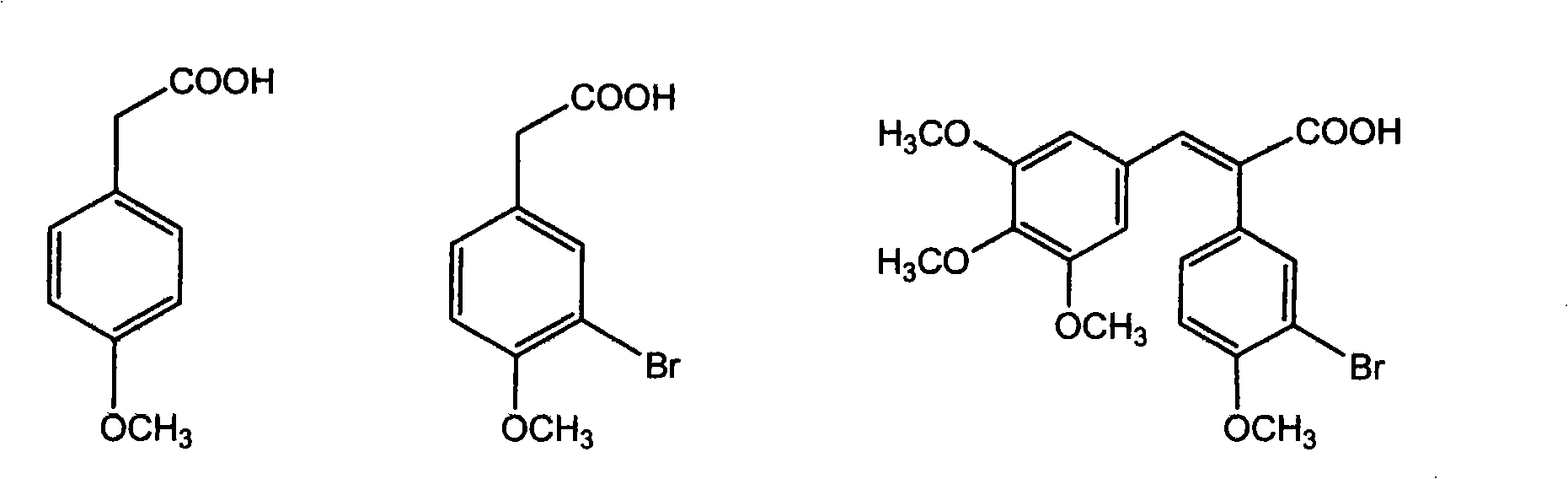
The invention discloses a method for preparing (Z)-3'-hydroxy radical -3,4,4',5-tetra- methoxyl diphenyl ethylene by the material of renewable natural plant resource. Naturally sourced 3,4,5-tri-methoxybenzaldehyde (a derivative extracted from Chinese gall) is taken as the raw material; and 3,4,5-tri-methoyl mandelic acid is obtained through a dichlorocarbene insertion reaction and is reduced to obtain 3,4,5-tri-methoyl phenylacetic acid. The compound can have a Perkin reaction with naturally sourced isovanillin to construct a cis-form diphenyl ethylene backbone, and the (Z)-3'-hydroxy radical -3,4,4',5-tetra- methoxyl diphenyl ethylene is obtained after a decarboxylic reaction. The method adopts the renewable resource-isovanillin and the 3,4,5-tri-methoxybenzaldehyde rich in China to replace increasingly exhausted petrochemical materials, thereby having a good sustainable development capability and remarkable economic, environmental and ecological benefits.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
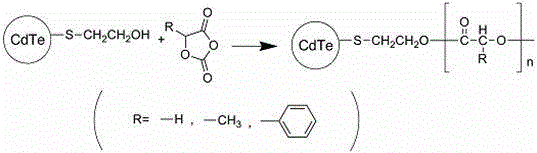
Poly-a-hydroxy acid modified CdTe quantum dot and preparation method thereof
InactiveCN104371730ARaw materials are easy to getLow costLuminescent compositionsPolymer modifiedMandelic acid
The invention discloses a preparation method of a poly-a-hydroxy acid modified CdTe quantum dot. The preparation method comprises the following steps: preparing a CdTe quantum dot with a mercaptoethanol modified surface by taking the mercaptoethanol as a ligand and adopting a water-phase method; generating a-hydroxy acid oxygen acid anhydride polymerization by adopting hydroxy contained in the surface of the CdTe quantum dot; and introducing poly-a-hydroxy acid, namely polylactic acid, polyglycolic acid, poly (mandelic acid) and the like, to the surface of the CdTe quantum dot so as to realize the polymer modification on the CdTe quantum dot. The poly-a-hydroxy acid modified CdTe quantum dot has the advantages of good hydrophilicity and stability, excellent fluorescence property, good biocompatibility and the like and can be widely applied to the field of biomedicine as a novel fluorescent nanoprobe.
Owner:YUNNAN MINZU UNIV
Method for producing optical homochiral amygdalic acid
This invention discloses a method for producing optical pure chiral mandelic acid. Use methyl benzoyl formate or the derivative of phenylglyoxylic acid as the bottom thing, the microorganism's cell of yeasts or mould whitlying etc. as catalyst, alternatively reduces the carbonyl in the bottom thing to the hydroxyl to get the chiral mandelic acid relying mainly on a kind of type (usually as R a type). This invention adopts chemistry-biology combination craft , with low cost, the chiral purity is high, easy to realize production for industrialization, it is easy to popularize and apply.
Owner:SHANDONG UNIV
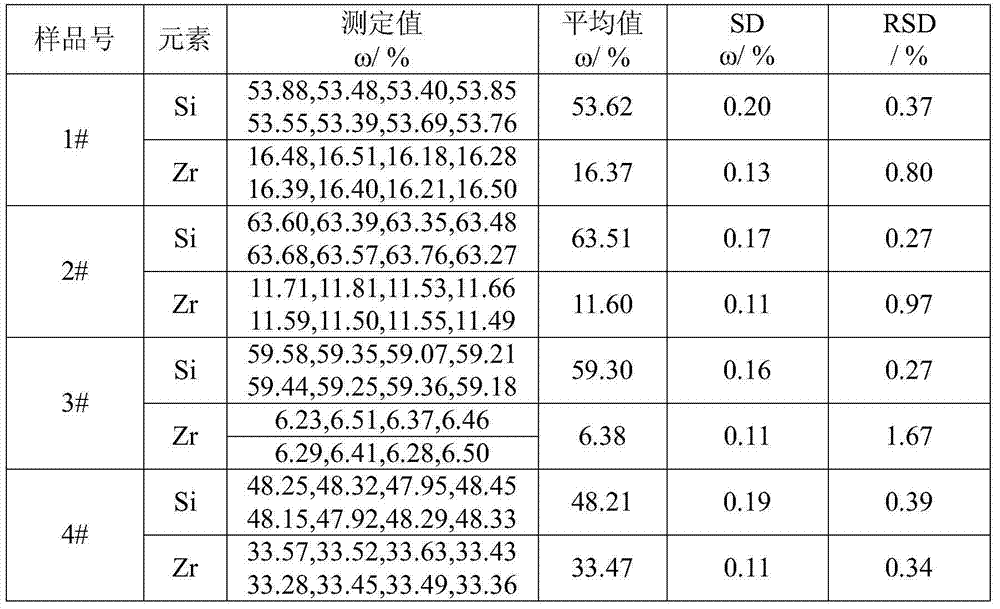
Method for determining content of silicon and zirconium in silicozirconium alloy
The invention relates to a method for determining content of silicon and zirconium in silicozirconium alloy, and belongs to the technical field of material chemical analysis. The method comprises the following steps: decomposing a sample with nitric acid and hydrofluoric acid to convert the silicon into fluosilicic acid; adding potassium fluoride to generate potassium fluosilicate precipitation; filtering, washing and adding hot water to decompose the potassium fluosilicate precipitation to release hydrofluoric acid; using bromothymol blue or phenolphthalein as indicator to carry out titration with a sodium hydroxide standard solution until the solution turns to blue or light red as an end point; calculating the content of silicon; dissolving the sample with nitric acid and hydrofluoric acid, carrying out perchloric acid smoking, reacting zirconium with mandelic acid in a hot hydrochloric acid medium to generate an insoluble mandelic acid white precipitation; calcining the precipitation at 850-900 DEG C; weighing in the form of oxide; and conducting conversion to obtain the content of zirconium. The method for determining silicon and zirconium content in silicozirconium alloy has not been reported so far, and the method provided by the invention solves the problem, has the advanategs of simpleness, fastness, high accuracy and good repeatability, and is favorable for popularization and application.
Owner:INST OF RES OF IRON & STEEL JIANGSU PROVINCE
Method for preparing (Z)-3'-hydroxy-3,4,4',5-tetramethoxy diphenyl ethylene from regenerative natural plant resource
InactiveCN101353296AGood sustainable development abilityCost-effectiveOrganic chemistryOrganic compound preparationPetrochemicalMandelic acid
The invention discloses a preparation method for (Z)-3'-hydroxyl-3,4,4',5-tetramethoxyl diphenyl ethylene. A diphenyl ethylene framework structure is built by the Perkin reaction method, and natural aniseed fat-soluble components and propenyl anisole (anethole) are taken as raw materials, and oxidized to obtain anisaldehyde; dichlorocarbene insertion reaction is carried out on the anisaldehyde to obtain p-methoxyl-mandelic acid which is reduced to obtain methoxyl-phenylacetic acid, the methoxyl-phenylacetic acid is brominized to obtain 3-bromo-4-methoxyl-phenylacetic acid. The compound and the natural 3,4,5-trimethoxybenzaldehyde (nutgall extract derivative) carry out Perkin reaction to build a cis-form diphenyl ethylene framework which is further converted and decarboxylated by functional groups to obtain the (Z)-3'-hydroxyl-3,4,4',5-tetramethoxyl diphenyl ethylene. The raw materials of the invention are reproducible natural resources-anethole which are rich in China and 3,4,5-trimethoxybenzaldehyde, and replace non-renewable petrochemical materials which are used by the prior art and become less and less so that the method has the advantages of good sustainable development capability as well as remarkable economic, environmental and ecological benefits.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Polishing composition and polishing method of substrate for memory and hard disk
InactiveCN1357586AIncrease capacityImprove storage densityOther chemical processesLapping machinesSuccinic acidMolybdic acid
A polishing composition for a substrate to be used for a memory hard disk, which comprises the following components (a) to (d):(a) water,(b) at least one compound selected from the group consisting of a polyoxyethylene polyoxypropylene alkyl ether and a polyoxyethylene polyoxypropylene copolymer,(c) at least one compound selected from the group consisting of nitric acid, nitrous acid, sulfuric acid, hydrochloric acid, molybdic acid, sulfamic acid, glycine, glyceric acid, mandelic acid, malonic acid, ascorbic acid, glutamic acid, glyoxylic acid, malic acid, glycolic acid, lactic acid, gluconic acid, succinic acid, tartaric acid, maleic acid and citric acid, and their salts, and(d) at least one abrasive selected from the group consisting of aluminum oxide, silicon dioxide, cerium oxide, zirconium oxide, titanium oxide, silicon nitride and silicon carbide.
Owner:FUJIMI INCORPORATED
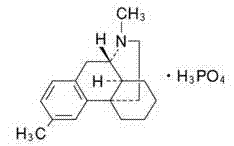
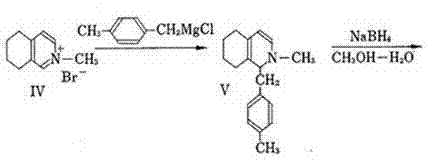
Preparation method of dimethylmorphinan phosphate used as cough medicine
InactiveCN102241630AReduction of chiral resolution processHigh yieldOrganic chemistryO-Phosphoric AcidIsoquinoline
The invention discloses a preparation method of dimethylmorphinan phosphate used as a cough medicine. The method comprises the following steps: removing L-mandelic acid from (S)-l-(4-methylbenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline-L-mandelate to obtain (S)-l-(4-methylbenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline, performing methylation by using the (S)-l-(4-methylbenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline chiral material as a raw material, and then performing cyclization reaction together with phosphoric acid under the conditions of heating and decompression to obtain (9S,13S,14S)-3,17-dimethylmorphinan monophosphate with three chiral centers, namely dimethylmorphinan phosphate; and desalting, rectifying, crystallizing, salifying, drying, and packaging to obtain the refined dimethylmorphinan phosphate product. The method has the characteristics of simple process, low cost, high yield, high product purity, short production cycle and the like, thereby being a synthetic route suitable for industrial mass production.
Owner:HANGZHOU BAOLING GROUP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
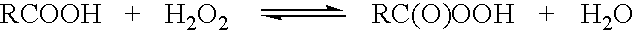


![Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds](https://images-eureka.patsnap.com/patent_img/0b0c3f56-53f8-44bf-b54d-80c6f7053051/US20060014793A1-20060119-C00001.png)
![Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds](https://images-eureka.patsnap.com/patent_img/0b0c3f56-53f8-44bf-b54d-80c6f7053051/US20060014793A1-20060119-C00002.png)
![Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds](https://images-eureka.patsnap.com/patent_img/0b0c3f56-53f8-44bf-b54d-80c6f7053051/US20060014793A1-20060119-C00003.png)