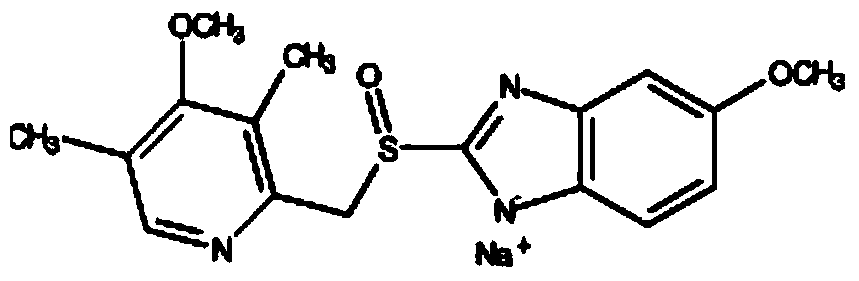
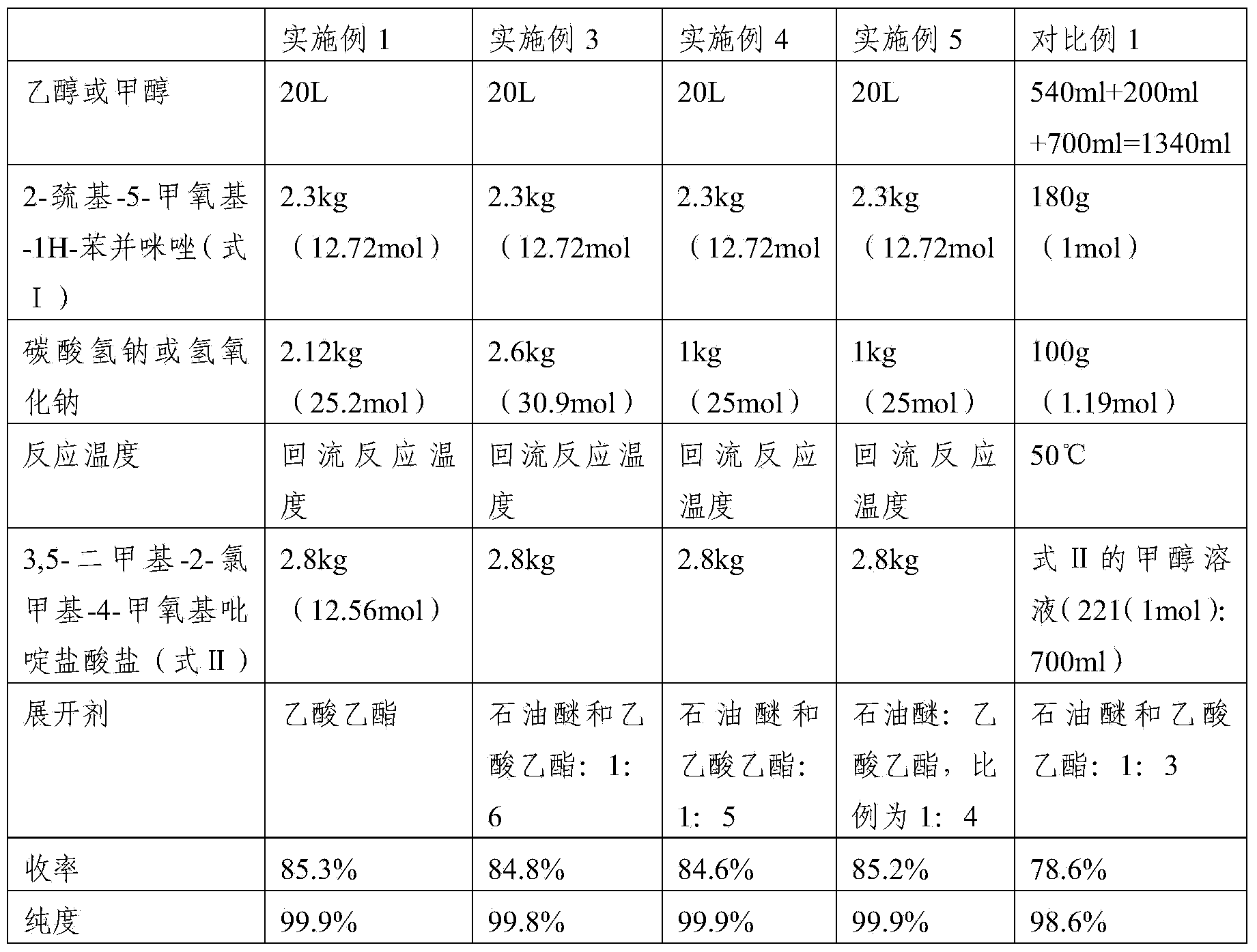
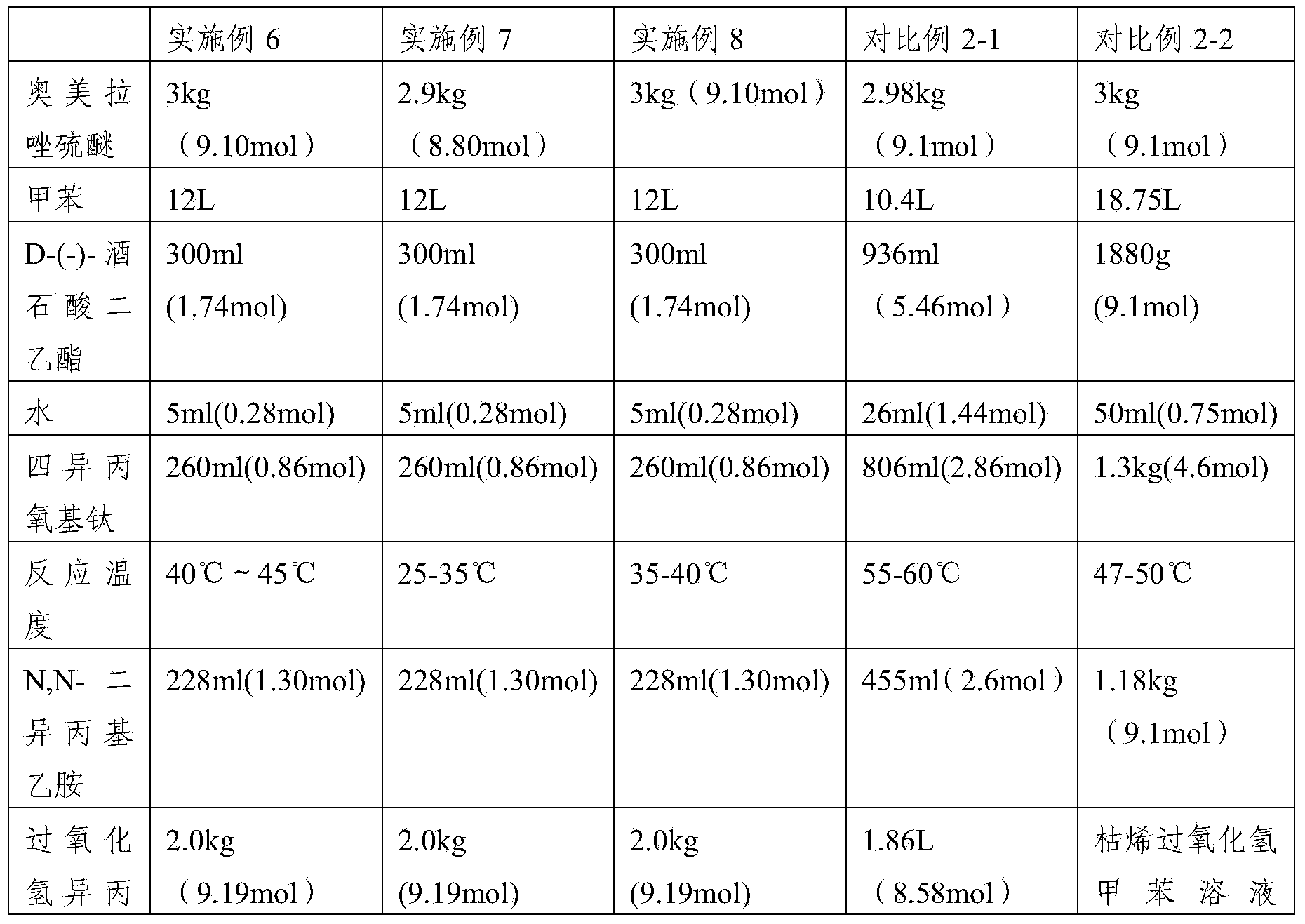
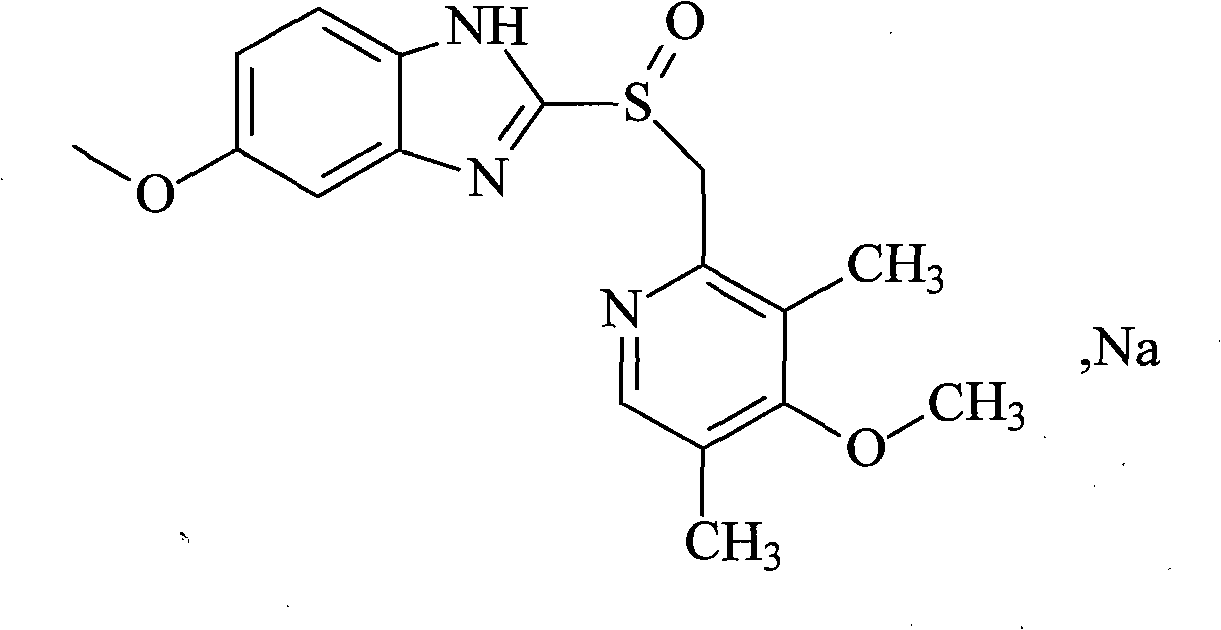
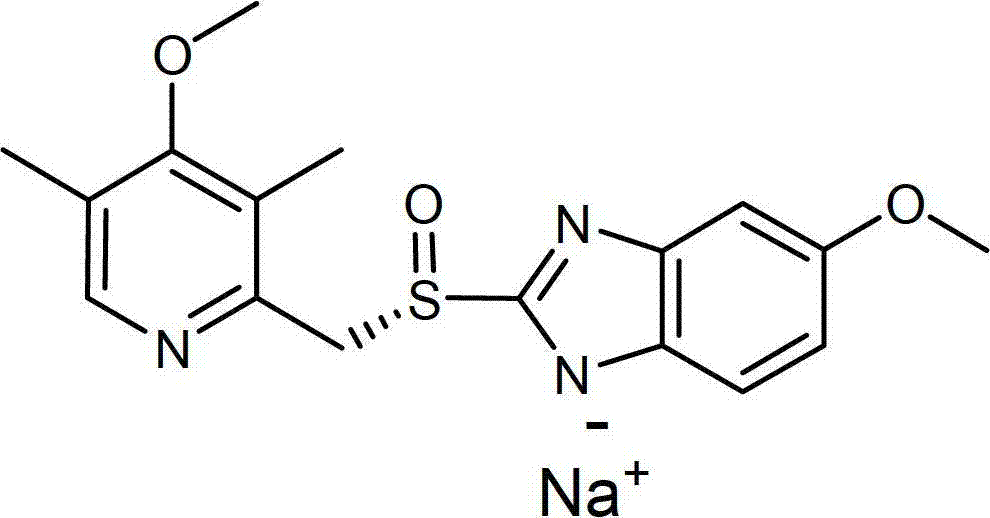
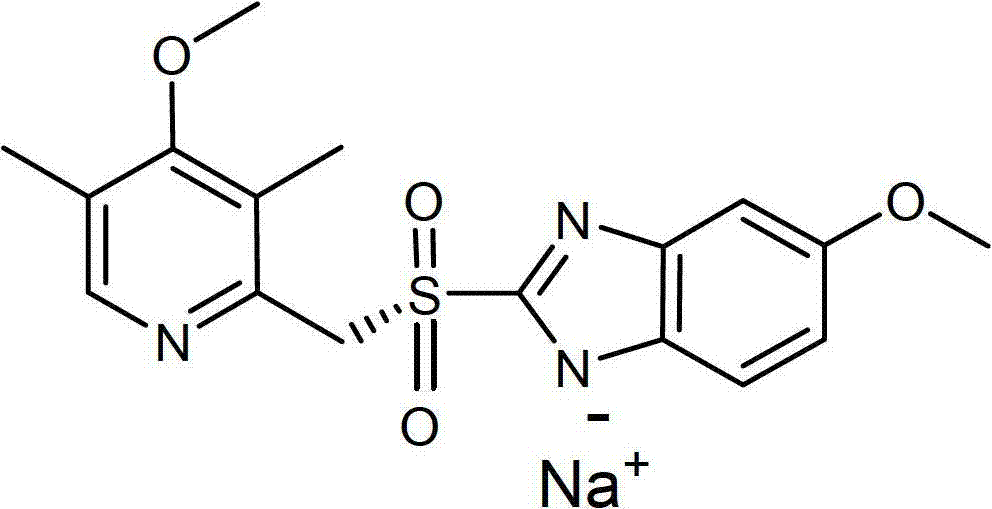
Patents
Literature
172 results about "Esomeprazole Sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
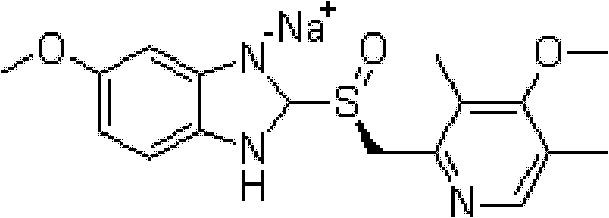
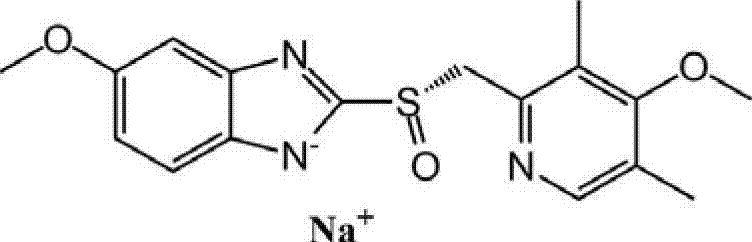
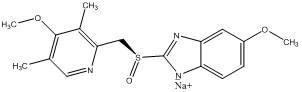
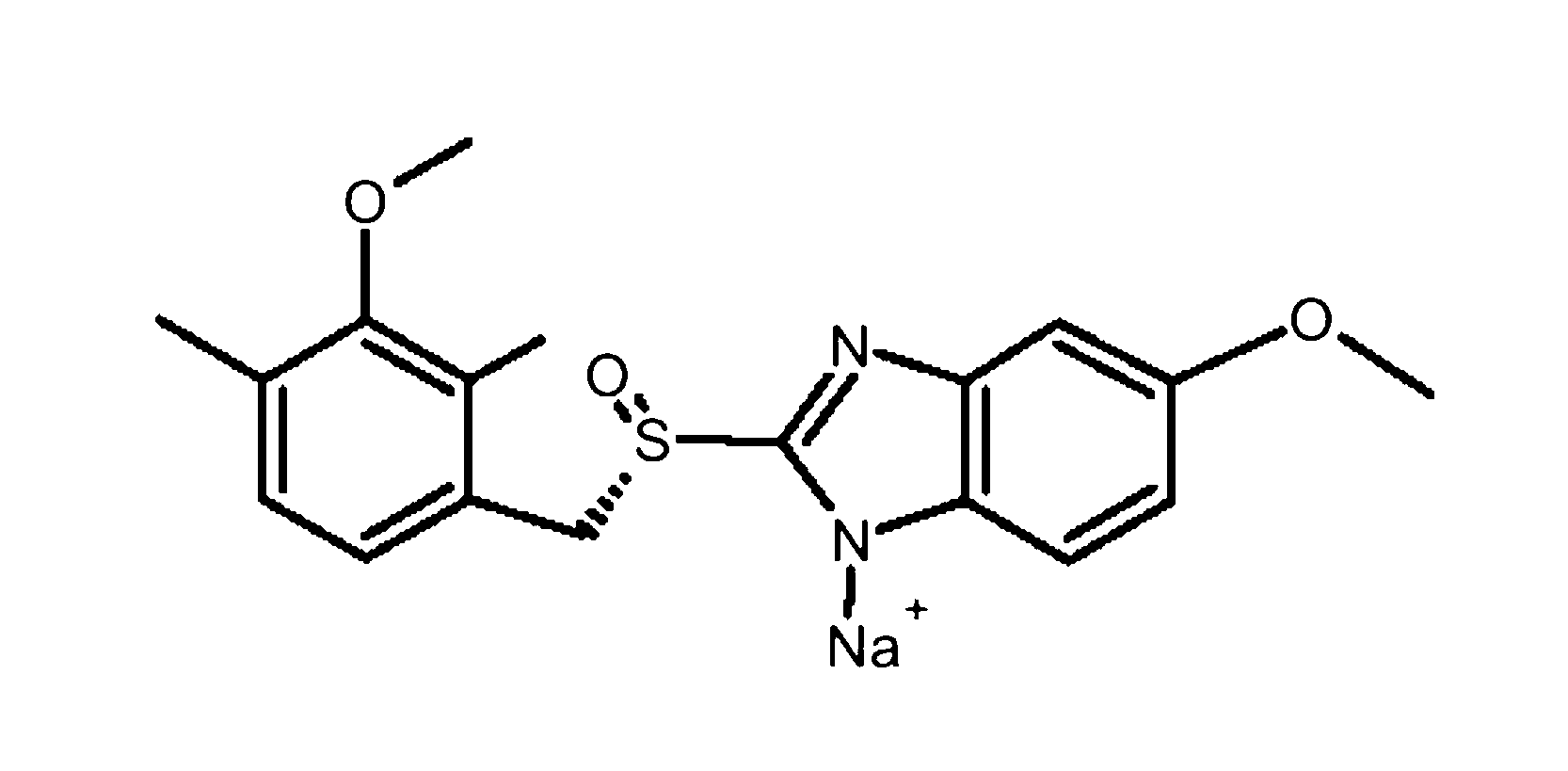
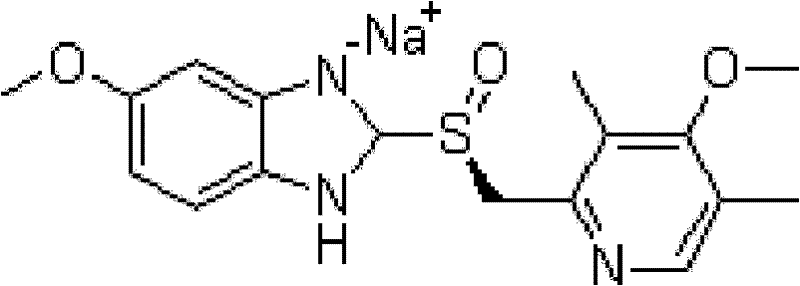
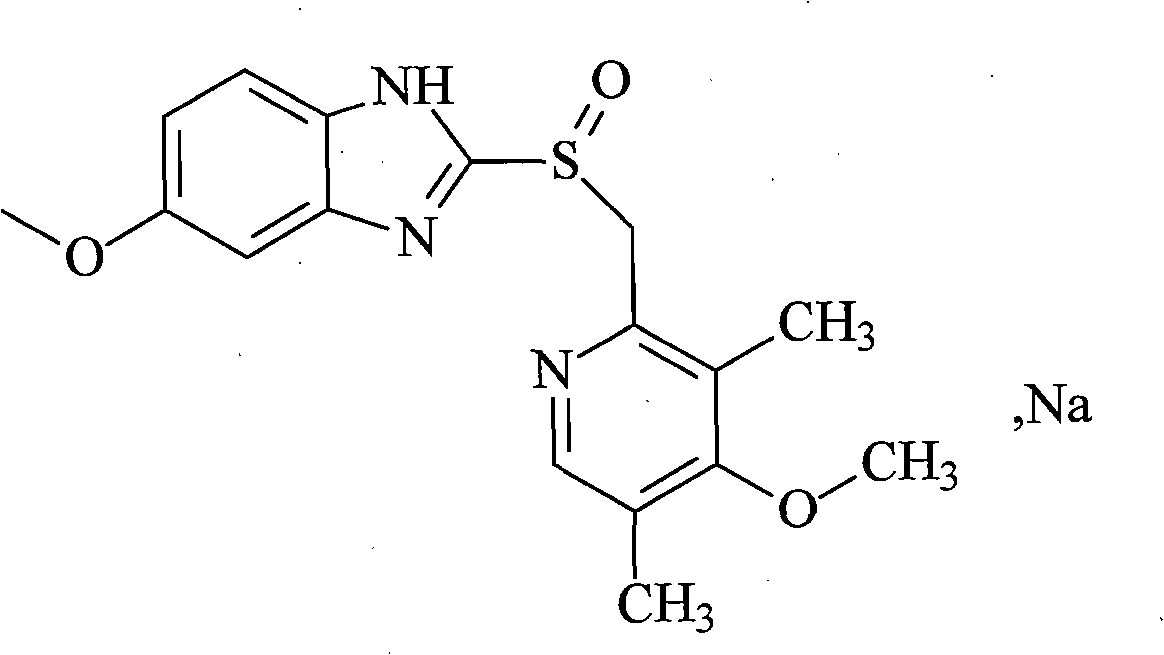
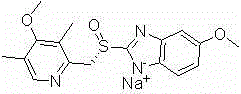
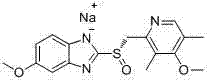
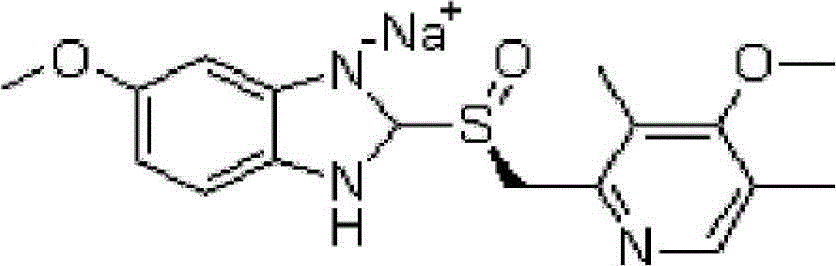
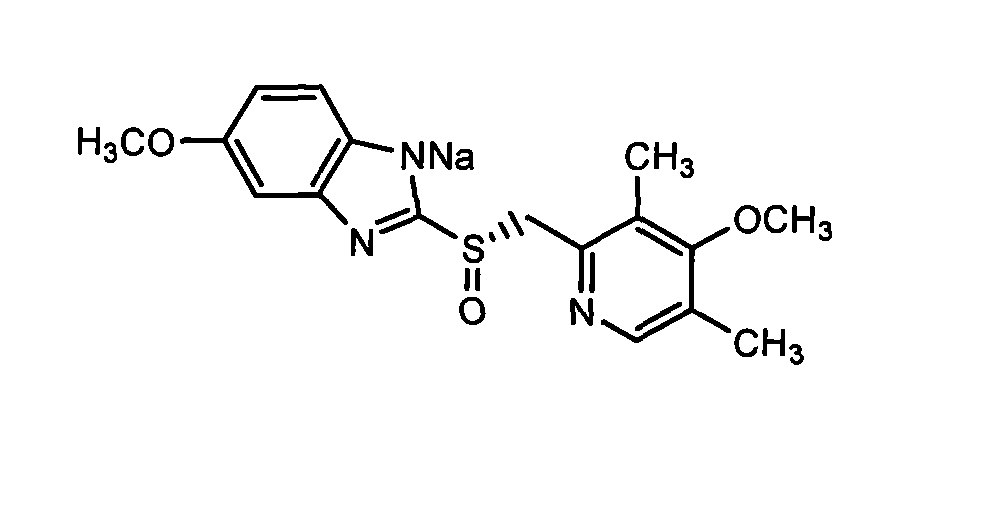
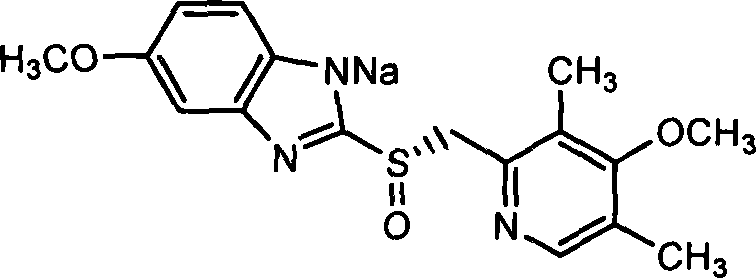
The sodium salt of the S-isomer of omeprazole, with gastric proton pump inhibitor activity. In the acidic compartment of parietal cells, esomeprazole is protonated and converted into the active achiral sulfenamide; the active sulfenamide forms one or more covalent disulfide bonds with the proton pump hydrogen-potassium adenosine triphosphatase (H+/K+ ATPase), thereby inhibiting its activity and the parietal cell secretion of H+ ions into the gastric lumen, the final step in gastric acid production. H+/K+ ATPase is an integral membrane protein of the gastric parietal cell.
Esomeprazole sodium bicarbonate composition
InactiveCN102078616AImprove complianceQuick effectOrganic active ingredientsDigestive systemSodium bicarbonateEsomeprazole Sodium
The invention relates to an oral medicinal composition formulation for treating peptic ulcer. The composition comprises esomeprazole, antiacid sodium bicarbonate and a medicinally acceptable carrier, wherein the esomeprazole contains an acid-sensitive proton pump inhibitor; and the antiacid sodium bicarbonate can improve the stability of the proton pump inhibitor. The invention also relates to a preparation method of the medicinal composition. Two active ingredients are integrated into one fixed unit formulation, so that response is quick, a medicinal scheme is simplified in the process of treating the peptic ulcer, and the compliance of a patient is improved.
Owner:BEIJING HONGWAN PHARMA TECH
Esomeprazole sodium freeze-dried powder injection and preparation method thereof
ActiveCN102357082AFeel comfortableGood molding effectOrganic active ingredientsPowder deliveryEsomeprazole SodiumMedicine
The invention belongs to the field of medicinal preparations, and mainly provides freeze-dried powder injection consisting of esomeprazole sodium, a propping agent, a metal ion chelant and a proper amount of pH regulator, and a preparation method for the injection. The freeze-dried powder injection has the advantages of compact form, high stability and small toxic and side effects, and is suitable for clinical application.
Owner:NANJING YOKO PHARMA
Industrial method for refining esomeprazole sodium salt
The invention relates to an industrial method for refining esomeprazole sodium salt. The method is mainly characterized by comprising the following steps of: suspending the esomeprazole sodium salt in a hot poor solvent in an amount which is 1 to 10 times weight of the esomeprazole sodium salt, slowly adding a good solvent such as methanol and ethanol in an amount which is 0.5 to 1.5 times weightof the mixture, filtering while heat to obtain a clarified solution, cooling to room temperature, separating out a solid, filtering, washing and performing vacuum drying. The method is easy and convenient to implement, and inorganic impurities and organic impurities contained in the crude product of esomeprazole sodium salt, including a peroxide sulphone impurity and an R-esomeprazole impurity can be effectively removed. The esomeprazole sodium salt refined by the method has the content of over 99.5 percent, and has the single impurity content of less than 0.1 percent.
Owner:NANJING YOKO PHARMA
Industrial production method of high-purity esomeprazole sodium
ActiveCN102321071AReduce the impactOxidation reaction time is shortOrganic chemistryOrganic basePotassium hydroxide
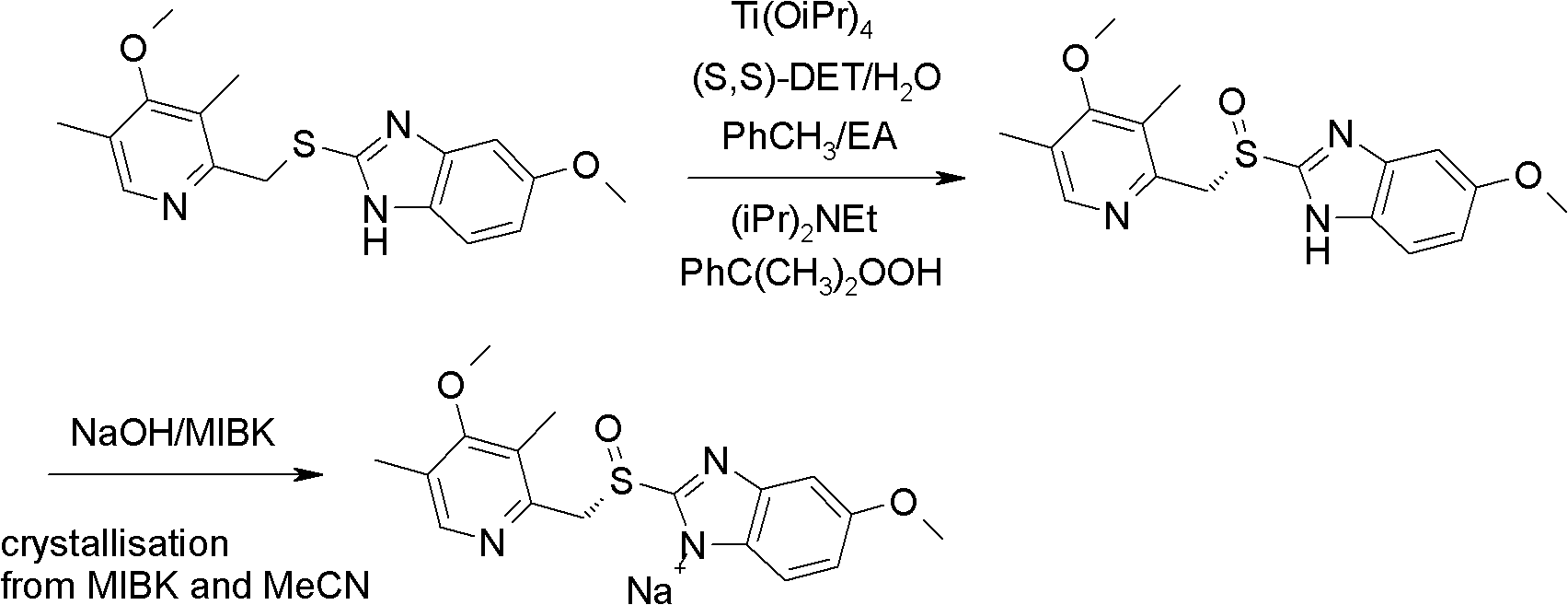
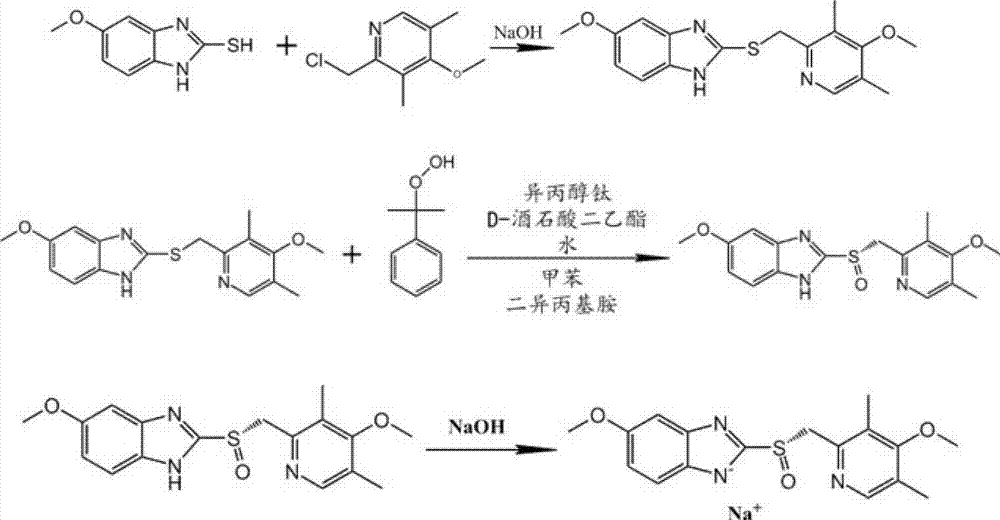
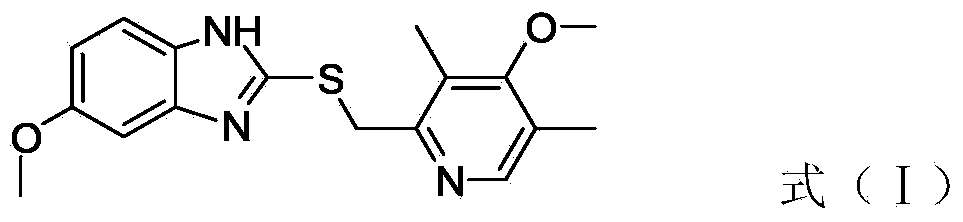
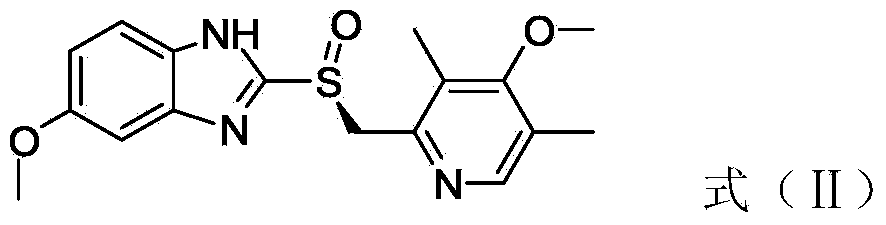
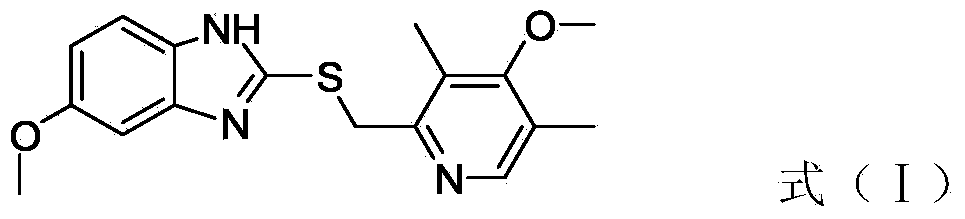
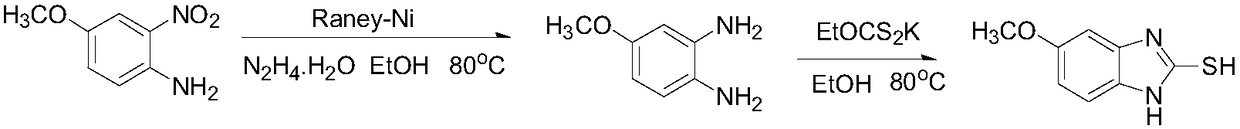
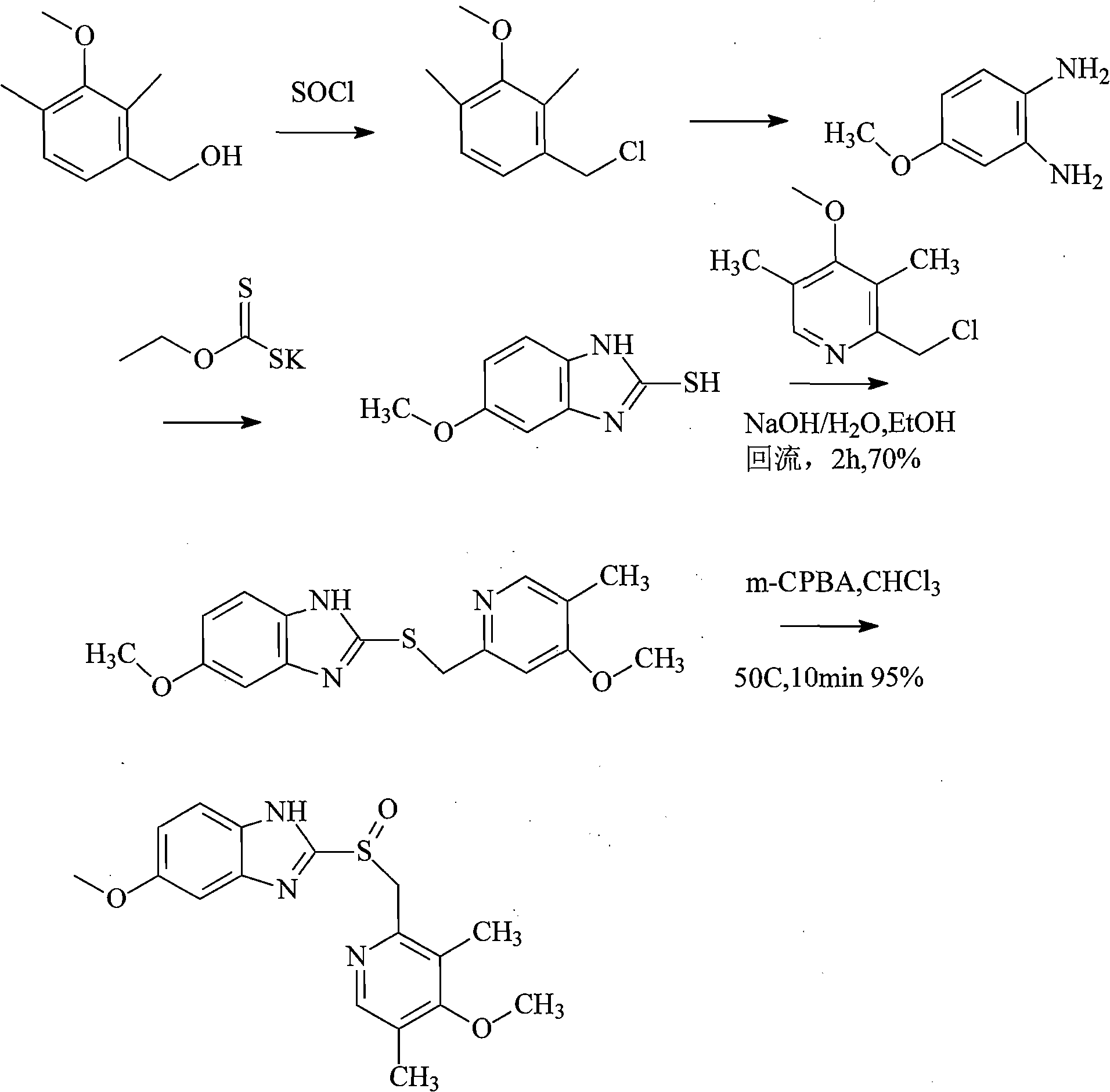
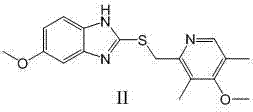
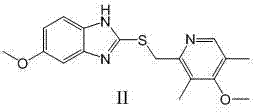
The invention relates to an industrial production method of high-purity esomeprazole sodium. The industrial production method is characterized by comprising the following steps: mixing a raw material 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H-benzimidazole with a solvent for dissolving 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H-benzimidazole; and successively adding water, D-diethyl tartrate and titanium iso-propoxide as well as an inorganic base, then adding cumene hydroperoxide, adding methanol or ethanol after reaction, filtering, carrying out posttreatment and salifying to prepare high-purity esomeprazole sodium, wherein the inorganic base is one of potassium carbonate, sodium carbonate, sodium hydroxide and potassium hydroxide. By using the method in the invention, the defects of high cost and serious environment pollution which are caused by using the organic base in the prior art are solved, and the defects of difficult posttreatment, poor repeatability and difficult industrialization in the prior art are solved simultaneously. According to the invention, the inorganic base is used as the raw material, thus the industrial production method has the advantages of low cost, little environment pollution, short reaction time and high product purity, is easy to operate and industrially produce.
Owner:NANJING HAIRUN PHARM CO LTD
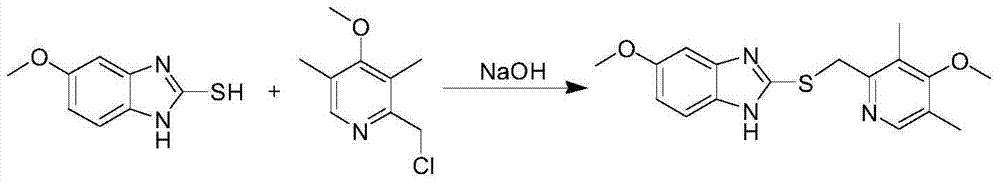
Method for synthesizing esomeprazole sodium
The invention discloses a method for synthesizing esomeprazole sodium. The method comprises the steps as follows: preparing 5-methoxy-2-(4-methoxy-3,5-dimethyl-2-pyridyl) methylthio-1H-benzimidazole, namely prochirality thioether; preparing crude esomeprazole sodium; refining the crude esomeprazole sodium; adding the prepared prochirality thioether and dried methylbenzene into D-(-) diethyl tartrate and water by stirring, adding titanium isopropylate, and stirring; and adding diisopropylamine at constant temperature, stirring, dropwise adding cumyl hydroperoxide with the mass concentration of 80%, ending the reaction, extracting, salifying, concentrating, washing and carrying out vacuum drying to obtain a crude product, and refining the crude product to obtain the esomeprazole sodium. The method is low in cost, toxicity and pollution, easy to operate, short in reaction time, high in product purity and easy for industrial production.
Owner:KAMP PHARMA
Granulating and coating process of esomeprazole magnesium contained in esomeprazole magnesium enteric-coated tablet
ActiveCN103040774AGuaranteed insolublePromote dissolutionOrganic active ingredientsPill deliveryEnteric-coated granulesEsomeprazole Sodium
The invention provides a granulating and coating process of esomeprazole magnesium contained in esomeprazole magnesium enteric-coated tablets. The granulating and coating process comprises the following steps of: firstly preparing esomeprazole magnesium granules; then preparing esomeprazole magnesium enteric-coated granules sequentially through isolating layer coating and enteric-coated layer coating; and finally blending adjuvants with the esomeprazole magnesium enteric-coated granules and tabletting to prepare the esomeprazole magnesium enteric-coated tablets. An appropriate granulating method comprises the following steps of: crushing and grinding the esomeprazole magnesium and the adjuvants into powder, and then uniformly mixing; mixing with an adhesive to obtain a water solution, stirring for 8-10 minutes in a wet type granulator to prepare appropriate granules; drying at 40-45 DEG C, and screening to obtain the granules with grain size being between 40 meshes and 80 meshes. According to the process including granulating and coating of raw materials, the enteric-coated granules are prepared firstly and then blended with the adjuvants and finally tabletting is carried out, pellets are not used, the content uniformity of the prepared esomeprazole magnesium enteric-coated tablet product is greatly enhanced, and the problem of unqualified uniformity of the product content caused by excessive material flowability in an original process is solved.
Owner:SHANGHAI SINE WANXIANG PHARMA
Esomeprazole magnesium injection liquid
InactiveCN101513387AConvenient for clinical operationImprove bioavailabilityOrganic active ingredientsDigestive systemPatient needReflux
The invention discloses an esomeprazole magnesium injection liquid capable of treating gastroesophageal reflux disease. At present, esomeprazole magnesium in the market is only tablets, and the patient needs to take the esomeprazole magnesium for 1 to 3 times per day; and because the dosage taken by the patient per day is large and the frequency is high, side effects generated by the medicine are large, too. In order to solve the problems, the invention aims to provides the esomeprazole magnesium injection liquid with convenient clinical use, high bioavailability and low price, which can make the medicine quickly reach effective treatment concentration in vivo through direct intravenous injection or intramuscular injection, reduce first-pass effect of the medicament in liver and improve the bioavailability of the medicament in vivo. The esomeprazole magnesium injection liquid provided by the invention mainly comprises the esomeprazole magnesium or pharmaceutically acceptable salts thereof and solvent for injection; and 1 ml of the injection liquid comprises 10 to 200 mg of the esomeprazole magnesium or the pharmaceutically acceptable salts thereof, and the pH of the injection liquid is between 3.0 and 8.0.
Owner:李铁军
Preparation method for high-purity esomeprazole sodium
ActiveCN103288801ASolve the prone to titanium complex suspensionSolve the difficulty of splittingOrganic chemistrySodium bicarbonateOmeprazole Sodium
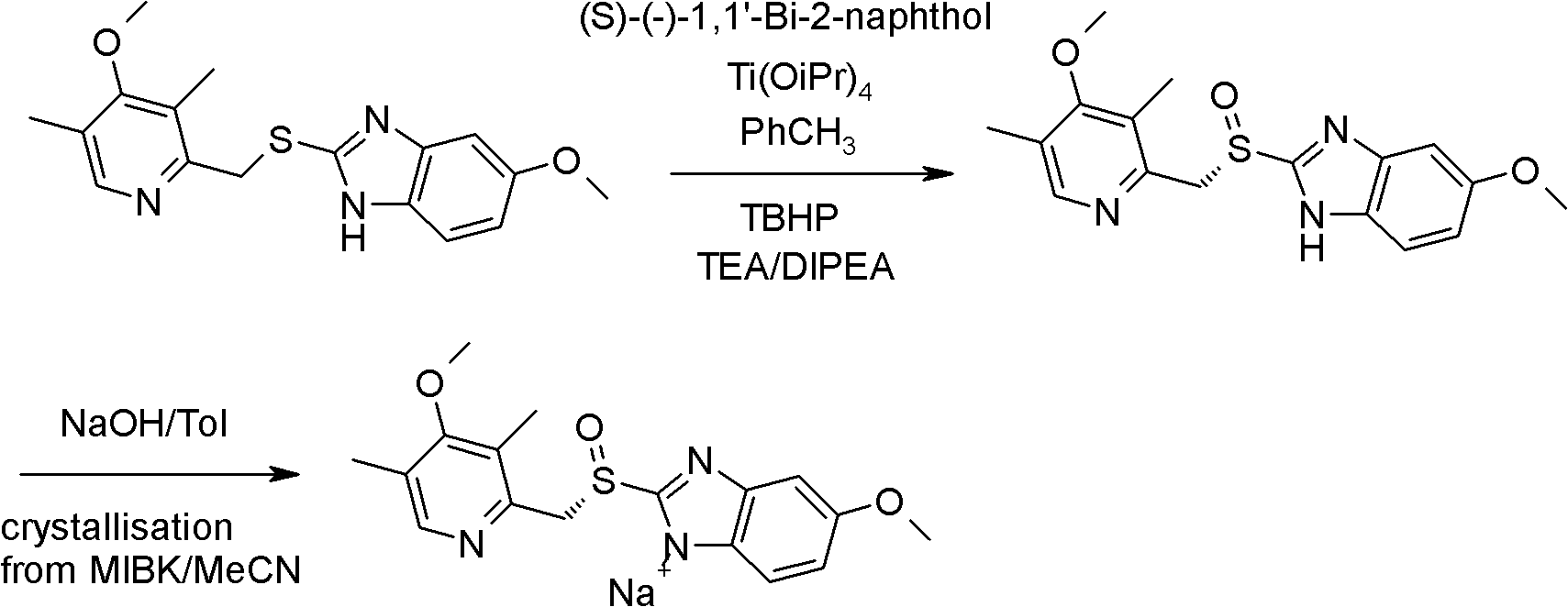
The invention discloses a preparation method for high-purity esomeprazole sodium. The preparation method comprises the steps of: including and splitting esomeprazole sodium and D-(-)-diethyl tartrate, titanium iso-propylate, triethylamine and L-(+)-mandelic acid in the presence of a proper amount of water, and separating to obtain an inclusion complex; dissolving the inclusion complex with ethyl acetate, washing inclusion complex with sodium carbonate water solution, carrying out ammonia hydroxide eluting on an ethyl acetate layer, slowly regulating the pH value to 6-7 with glacial acetic acid, then extracting with dichloromethane, and concentrating to obtain crude esomeprazole free alkali product; carrying out silica gel adsorption and elution on the crude product to obtain a pure esomeprazole free alkali product; and enabling the pure product and the methanol-ethanol-acetonitrile solution of sodium hydroxide to form salt, and then crystallizing with isopropyl ether to obtain the high-purity esomeprazole sodium. According to the preparation method, the difficulties that when inclusion and splitting are carried out, the titanium complex suspension body are difficult to split and the ammonia complex of titanium is difficult to remove can be solved, the industrialization production can be realized, the industrialized production cost is low, the product purity is high, the yield is high, and no harmful gas is generated.
Owner:SICHUAN BAILI PHARM CO LTD
Pharmaceutical composition containing esomeprazole sodium, and preparation method thereof
ActiveCN102813651AEasy to shapeReduce contentOrganic active ingredientsPowder deliveryEsomeprazole SodiumPharmaceutical drug
Owner:CHENGDU GUOHONG PHARMA
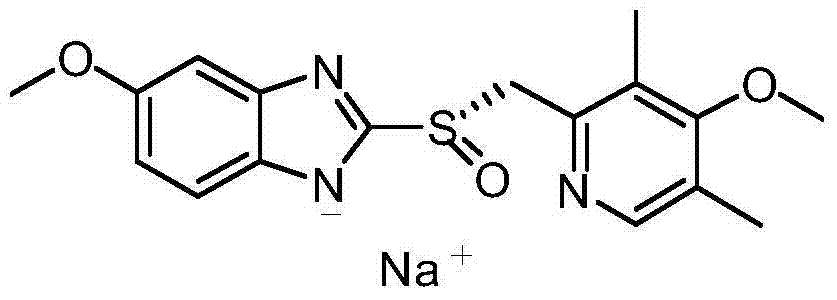
Esomeprazole sodium hemihydrate
The invention belongs to the technical field of medicine, and particularly relates to an esomeprazole sodium hemihydrate and a preparation method thereof; the esomeprazole sodium of the invention comprises a hemihydrate, and has the following advantages that: the chemical purity is 99.9%; the maximal impurity content is less than one thousandth; the optical purity is up to 99.96%ee; and the stability is good. The invention also relates to an application of a composition of the hydrate in the preparation of medicaments for treating stomach burning sensation caused by non-erosive gastroesophageal reflux, and erosive esophagitis.
Owner:TIANJIN HANRUI PHARMA
Preparation method of esomeprazole magnesium
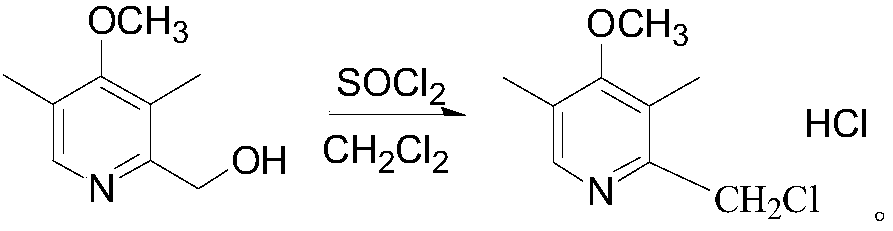
InactiveCN103936714AHigh yieldMild reaction conditionsOrganic chemistryEsomeprazole Sodium4-methoxypyridine
The invention discloses a preparation and refinement method of esomeprazole magnesium. The preparation and refinement method comprises the following steps: condensing 2-chloromethyl-3, 5-dimethyl-4-methoxyl pyridine hydrochloride and 2-sulfydryl-5-methoxy-benzimidazole serving as starting materials, carrying out improved sharpless asymmetric oxidation so as to prepare esomeprazole sodium, then carrying out salt displacement so as to prepare esomeprazole magnesium, and finally refining to obtain high-purity esomeprazole magnesium. The preparation method is mild in reaction conditions, simple to operate, good in repeatability and high in yield and facilitates industrial production. The chromatographic purity of esomeprazole magnesium prepared by the method is above 99.8%; the optical purity of esomeprazole magnesium reaches above 99.6%; esomeprazole magnesium is stable in morphology and can meet medicinal requirements.
Owner:北京华禧联合科技发展有限公司
Preparation of injection esomeprazole sodium
ActiveCN103006585AIncrease profitReduce manufacturing costPowder deliveryOrganic active ingredientsEsomeprazole SodiumActivated carbon
The invention discloses preparation of injection esomeprazole sodium. The preparation comprises the following steps of: (a) adding partial injection water into a basic liquid, adding edetate disodium, dissolving and cooling to a temperature of between 10 and 20 DEG C, introducing nitrogen and adding esomeprazole sodium, stirring until the mixture is completely dissolved, adding activated carbon, stirring, decarbonizing and filtering; (b) adding injection at a temperature of between 10 and 20 DEG C to account for 80 to 95 percent of the preparation amount, introducing nitrogen, regulating pH value to be between 11.0 and 11.8 with alkali, adding injection water at a temperature of between 10 and 20 DEG C to the total preparation amount, filtering by using a filter with filter element of 0.22mu m, subpackaging in penicillin bottles, partly covering rubber plugs, and lyophilizing. The finished product prepared by the preparation is more stable in quality, and the production cost is remarkably reduced, so that the preparation is advantageous to mass production, and is good in market prospect, and worthy of popularization.
Owner:哈药集团股份有限公司 +1
Esomeprazole sodium composition used for injection and its preparation method
ActiveCN102440965AInhibition releaseAvoid hidden dangersOrganic active ingredientsPowder deliveryEthylenediamineForeign matter
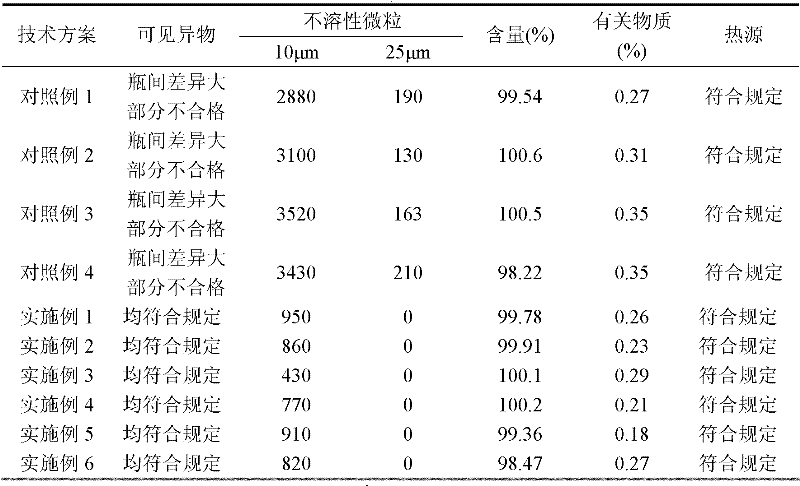
The invention provides an Esomeprazole sodium composition used for injection and its preparation method, the Esomeprazole sodium composition is prepared by the following steps: 1) liquid preparation: selecting esomeprazole sodium and ethylenediamine tetraacetic acid or ethylenediamine tetraacetic acid salt as raw materials, wherein the weight ratio of esomeprazole sodium to ethylenediamine tetraacetic acid or ethylenediamine tetraacetic acid salt is 1:0.01-0.1, weighting a proper amount of raw materials into a preparation tank, and adding water for injection to the weight of 23.47-234.7 timesof esomeprazole sodium, stirring and uniformly mixing the solution, adjusting the pH value of 10.0-12.5; 2) activated carbon finishing; 3) adsorbing; 4) sterile filtrating and split charging; 5) vacuum freeze-drying to obtain the esomeprazole sodium composition used for injection. The esomeprazole sodium composition used for injection and the preparation method are capable of ensuring the heat source, visible foreign matter and insoluble particulate to accord with the requirements of the injection for the injection preparations such as esomeprazole sodium preparation liquid with high pH value.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
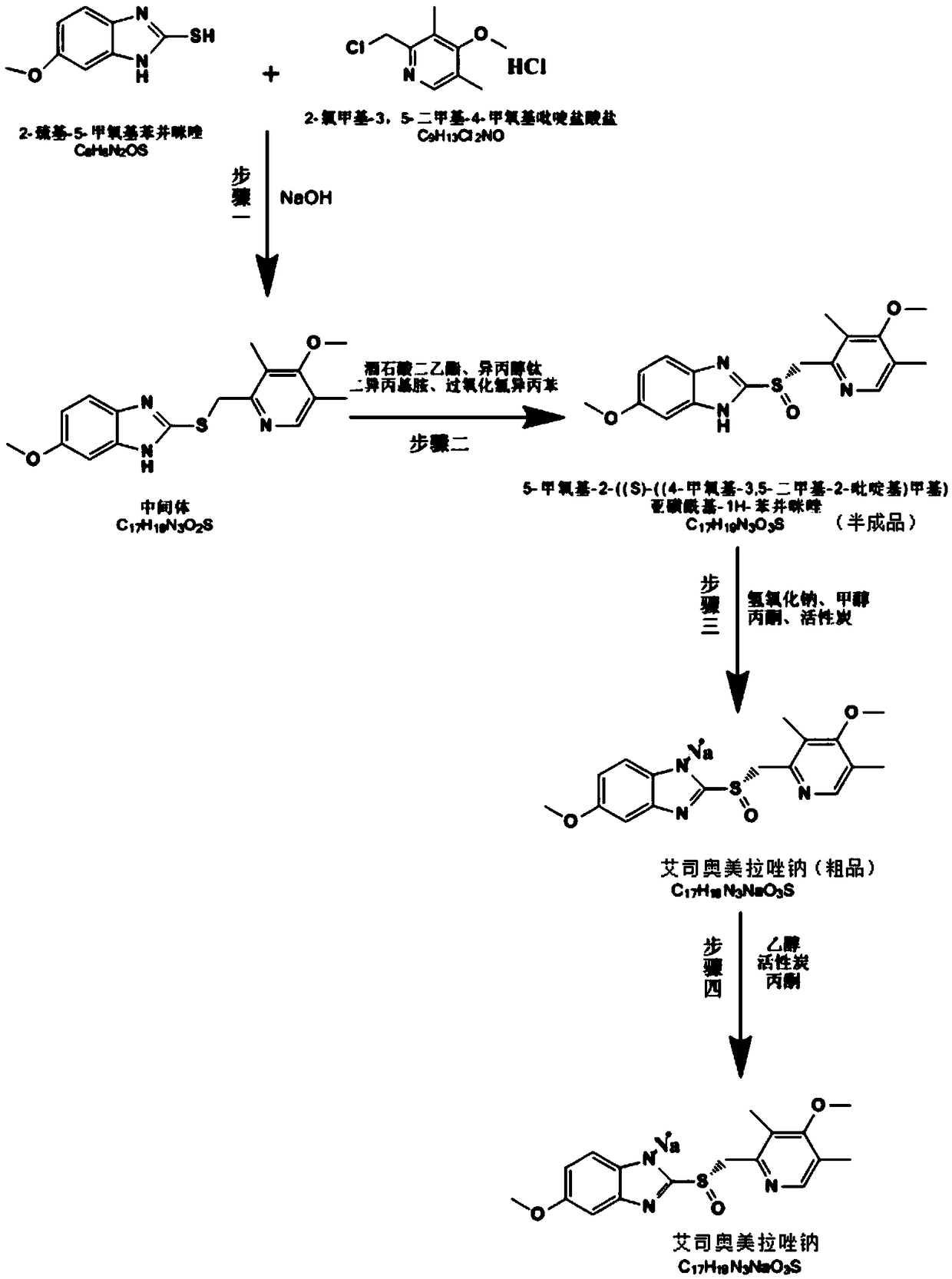
Method for synthesizing and refining esomeprazole sodium
ActiveCN103570686AConvenient sourceSimple and fast operationOrganic chemistryEsomeprazole SodiumTetraisopropyl titanate
The invention relates to a method for synthesizing and refining esomeprazole sodium. The method comprises the following several steps: (1) carrying out condensation by taking 2-sulfydryl-5-methoxybenzimidazole and 2-chloromethyl-3.5-dimethyl-4-methoxypyridine hydrochloride as raw materials to generate prochiral thioether; (2) synthesizing the prochiral thioether into an esomeprazole crude product under the action of D-(-) diethyl tartrate, water, tetraisopropyl titanate, diisopropylethylamine and cumene hydroperoxide, and refining to generate esomeprazole potassium salt; (3) dissolving the esomeprazole potassium salt, then transforming into esomeprazole sodium salt, and refining to obtain a final product. The process of the esomeprazole sodium has the advantages of easiness for operation, good reaction repeatability and higher yield; the obtained product is high in purity and suitable for industrialized production.
Owner:哈药集团股份有限公司 +1
Esomeprazole sodium and lyophilized preparation comprising same
InactiveCN109456306AImprove manufacturing precisionPreparation precision safetyOrganic active ingredientsPowder deliveryOmeprazole SodiumEsomeprazole Sodium
The invention provides esomeprazole sodium and a lyophilized preparation comprising the same. A preparation method of esomeprazole sodium includes following steps: 1), synthesizing an intermediate; 2), synthesizing esomeprazole sodium; 3), roughly preparing esomeprazole sodium; 4), finely preparing esomeprazole sodium. Esomeprazole sodium prepared by the method is higher in preparation accuracy and safer to use; the preparation process is easier to control, and step decomposition brings convenience to quality control of middle steps, so that ensuring of preparation accuracy of esomeprazole sodium is facilitated, and drug prepared from esomeprazole sodium is safer.
Owner:SHANXI PUDE PHARMA CO LTD
Injection esomeprazole composition and preparation method thereof
ActiveCN103222962AUniform particle sizeHigh encapsulation efficiencyPowder deliveryOrganic active ingredientsEsomeprazole SodiumMicrosphere
The invention relates to an injection esomeprazole composition. The injection esomeprazole composition comprises an active component and assisting agents, the active component is esomeprazole or esomeprazole sodium or an esomeprazole crystalline hydrate, the injection esomeprazole composition also comprises slow release microspheres containing the active component, the slow release microspheres comprise 10-30wt% of the active component, 60-80wt% of a biodegradable carrier material and 0.2-10wt% of a stabilizer, and the assisting agents comprise an excipient and a pH adjusting agent; and the injection esomeprazole composition is obtained through lyophilizing the active component, the assisting agents, the slow release microspheres and an aqueous solution having a pH value in a range of 11.0-12.0. The injection esomeprazole composition is a dual-controlled-release proton pump inhibitor releasing drugs two times, increases the treatment indexes of the drugs, and raises the qualities of preparation products.
Owner:苏州特瑞药业股份有限公司
Refining method and synthesis method of esomeprazole
ActiveCN103936715AEffective dissolutionImprove solubilityOrganic chemistryEsomeprazole SodiumOrganic solvent
The invention relates to a refining method and a synthesis method of esomeprazole. The refining method comprises the following steps: firstly, suspending a crude esomeprazole product in 2-5 times of good organic solvents, stirring at low temperature, filtering to remove the impurities, slowly adding 10-20 times of adverse organic solvents, stirring to crystallize over night, and centrifuging to dry, thereby obtaining a refined esomeprazole product. The refining method of the esomeprazole is stable and controllable in process, simple and convenient to operate and easy to industrialize; the prepared esomeprazole is hihg in purity, high in yield, high in optical purity and stable in component, and happening of adverse reaction accidents is greatly avoided.
Owner:HEILONGJIANG ZBD PHARMA +1
Esomeprazole sodium refining method
InactiveCN103420979AEasy to operateEase of industrial productionOrganic chemistryEsomeprazole SodiumAlcohol
The invention relates to an esomeprazole sodium refining method. The esomeprazole sodium refining method provided by the invention is used in the refining of an esomeprazole sodium crude product synthesized from a prochiral compound sulfide through an asymmetric oxidation reaction. The refining method comprises the steps that: the esomeprazole sodium crude product synthesized through the asymmetric oxidation reaction is adopted as a raw material, and is dissolved by using low-grade organic ketone; acetonitrile is added; after crystallization and precipitation, filtering is carried out, such that a refined product after first crystallization is obtained; the refined product is subjected to heating and dissolving by using low-grade organic alcohol; the mixture is cooled; and after crystallization and precipitation, filtering is carried out, such that a refined product after second crystallization is obtained. Optical purity of the esomeprazole sodium refined product is improved from 92% of the crude product to higher than 99.8%, and content is higher than 99.5%.
Owner:SHANHE PHARMA GUANGZHOU CITY
Esomeprazole sodium compound and preparation method thereof
The invention relates to a purified esomeprazole sodium compound and a preparation method thereof. The preparation method comprises the following steps: 1) dissolving the crude products of esomeprazole sodium in water, stirring the materials fully to dissolve esomeprazole sodium, ensuring the solution to pass through a macroporous adsorption resin, using mobile phase for elution and purification and collecting the eluent; 2) pumping ammonia into or adding ammonia water to the eluent to process the eluent for several minutes to 8 hours, carrying out stirring in the processing course, filteringout the separated precipitate to obtain filtrate which is aqueous solution containing esomeprazole sodium and optionally heating the aqueous solution to remove the residual ammonia; 3) adding alcoholsolvents to the aqueous solution, controlling the temperature for recrystallization, centrifuging and washing the separated crystals and drying the crystals to obtain the fine products of esomeprazole sodium; and 4) optionally returning the crystallization mother solution to the step 3), namely adding the crystallization mother solution to the aqueous solution obtained in the step 2) together with the alcohol solvents. By adopting the method, the purity of the crude products of esomeprazole sodium can be substantially improved, the quality of the preparation product can be improved and the toxic and side effects can be reduced.
Owner:HAINAN LINGKANG PHARMA CO LTD
Esomeprazole sodium polymorph, preparation method and application thereof
ActiveCN102746272AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistryEsomeprazole SodiumCombinatorial chemistry
The invention belongs to the field of medicinal chemistry, and specifically to an esomeprazole sodium polymorph, a preparation method and an application thereof. The present invention discloses an esomeprazole sodium polymorph having good stability and excellent solubility, wherein a preparation process of the esomeprazole sodium polymorph is easily industrialized, quality reproducibility is good, and product purity is easy to control.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Preparation process of freeze-dry powder injection containing esomeprazole sodium
InactiveCN102657622AReduce moisture contentLow content of related substancesOrganic active ingredientsPowder deliveryAntioxidantFreeze-drying
The invention relates to a preparation process of a freeze-dry powder injection containing esomeprazole sodium, and also provides the esomeprazole sodium for injection, which has the advantages of safe quality and high stability. The preparation process comprises the following steps of: dissolving the esomeprazole sodium, an excipient, a metal ion complexing agent, an antioxidant and the like in injection water; adding a pH value adjusting agent to adjust the pH of mixed solution; adding medicinal carbon to decolorize; filtering; canning the filtrate; partially plugging; performing freeze-drying; pressing a plug under vacuum; rolling a cover; performing lamp inspection; and packaging to obtain a finished product. The preparation process is rational in prescription design; the defects of instability of the esomeprazole sodium upon heating and poor clarity of solution after redissolution are effectively overcome; and a prepared product has the advantages of high stability, high redissolution and the like and is suitable for industrial production.
Owner:KAMP PHARMA
Method for purifying esomeprazole sodium
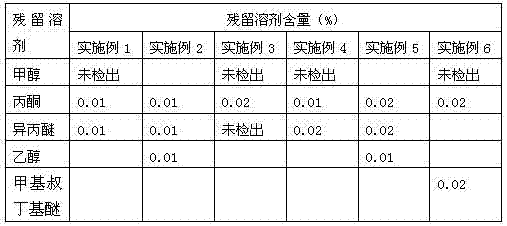
The invention relates to a method for purifying esomeprazole sodium, which comprises the following steps: after dissolving an esomeprazole sodium crude product in 0.5-10 times of an alcohol solvent, adding a poor solvent, filtering to obtain a solid, dissolving the solid in 0.5-10 times of acetone, crystallizing until the system becomes turbid, adding a poor solvent, filtering, and drying to finally obtain the sample. The method can remove abundant residual solvents which can not be easily removed in the prior art to obtain the high-purity low-solvent-residue high-yield product, and is suitable for industrial production.
Owner:四川尚锐生物医药有限公司
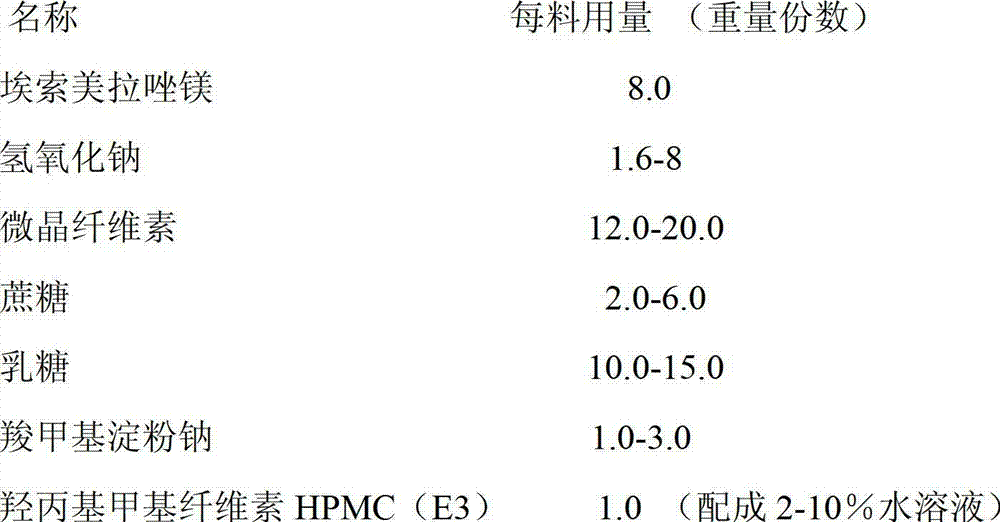
Pharmaceutical composition containing esomeprazole sodium and preparation method thereof
InactiveCN108785259AInhibit growthReduced stabilityPowder deliveryOrganic active ingredientsOmeprazole SodiumDesorption
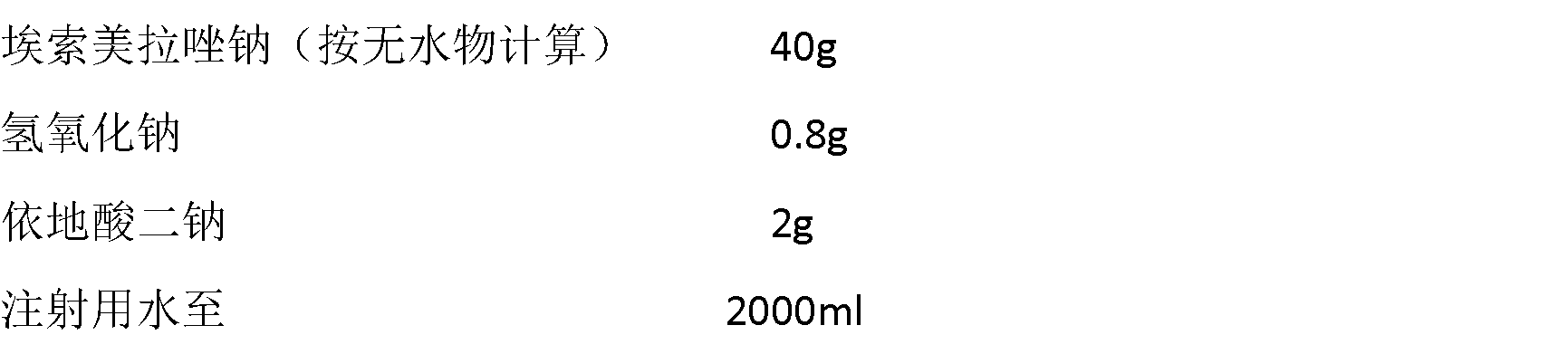
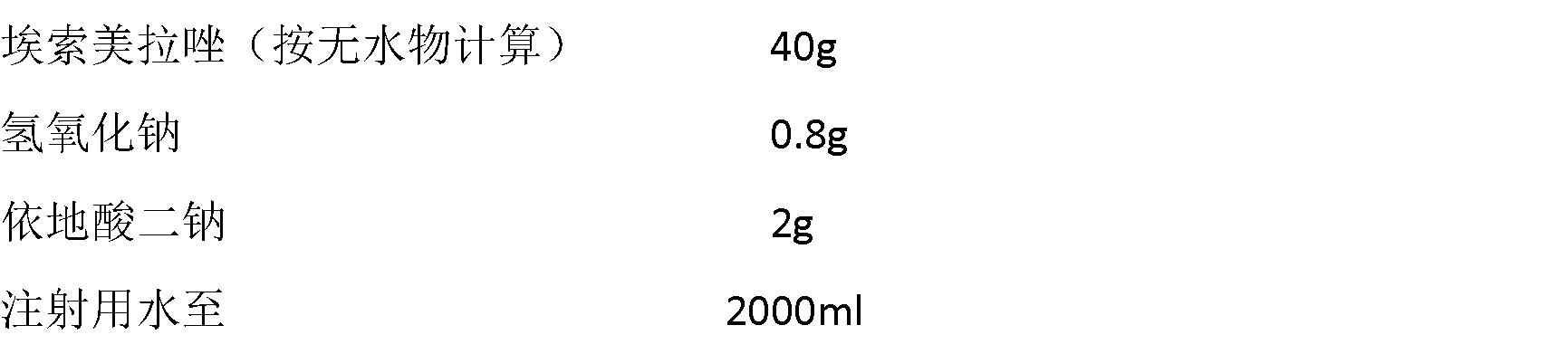
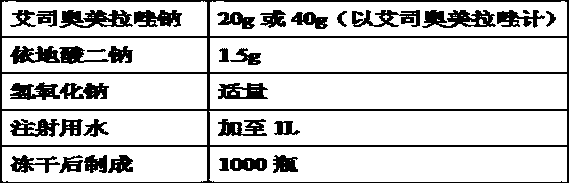
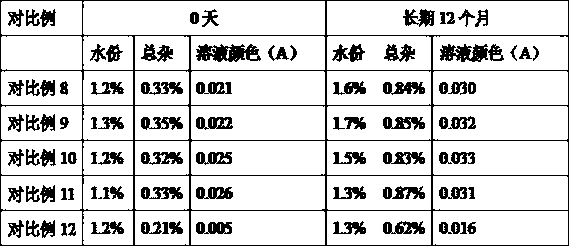
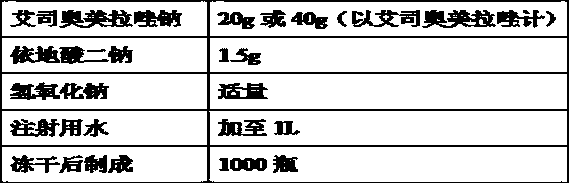
The invention relates to a pharmaceutical composition containing esomeprazole sodium and a preparation method thereof. The pharmaceutical composition is freeze-dried powder for injection, and for every 1000 bottles, the pharmaceutical composition is composed of the following pharmaceutical ingredients of 20-40 grams of the esomeprazole sodium, 1.5 grams of disodium edetate, and 1 liter of water for injection. The preparation method of the pharmaceutical composition containing the esomeprazole sodium includes the steps of liquid preparation and freeze drying, wherein in the process of liquid preparation, the esmeprazole sodium, the disodium edetate, and the water for injection are mixed to prepare a liquid of a pH of 11.0 to 11.5; the step of freeze drying includes freeze control 1, freezecontrol 2, sublimation drying 1, sublimation drying 2 and desorption drying. The pharmaceutical composition containing the esomeprazole sodium has various advantages, such as having low moisture content, few metal ions and colored impurities and good stability, and having no significant increase in impurities accompanied by changes in solution color after long-term placement.
Owner:CHENGDU GUOHONG PHARMA
Esomeprazole sodium polymorph and application of esomeprazole sodium polymorph in drugs for injection
InactiveCN102746273AImprove solubilityImprove stabilityOrganic active ingredientsOrganic chemistryEsomeprazole SodiumDisease
The present invention relates to an esomeprazole sodium polymorph and an application of the esomeprazole sodium polymorph in drugs for injection. According to the present invention, esomeprazole sodium compositions for injection have characteristics of good stability and high purity, a preparation process of the esomeprazole sodium compositions is easily industrialized, and the esomeprazole sodium compositions can be used for treatments of diseases related to gastric acid parasecretion, wherein the esomeprazole sodium compositions are prepared from the esomeprazole sodium polymorph having good stability and excellent solubility in the present invention.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Esomeprazole sodium lyophilized powder injection and preparation method thereof
InactiveCN102973524AImprove stabilityAvoid degradationPowder deliveryOrganic active ingredientsEsomeprazole SodiumUltrafiltration
The invention provides an esomeprazole sodium lyophilized powder injection for injection and a preparation method thereof. The weight ratio of esomeprazole sodium to edetate disodium and / or sodium calcium edentate is 1:(0.01-0.1), and the pH value of the esomeprazole sodium lyophilized powder injection is between 9.5 and 11.5. The preparation method comprises the following steps of: 1) weighing and dissolving edetate disodium and / or sodium calcium edentate in the prescription amount in injection water, stirring to dissolve, and regulating the pH value of sodium hydroxide liquid to be between 10.5 and 12.5; and 2) weighing esomeprazole sodium in the prescription amount, adding into liquid prepared in the step 1), stirring at room temperature to completely dissolve the liquid, and filling the injection water till full dose is reached; 3) performing ultrafiltration on the liquid medicament prepared in the step 2) to remove pyrogen; 4) sterilizing and filtering the liquid medicament which is subjected to ultrafiltration; and 5) filling and lyophilizing. The product prepared by the preparation method of esomeprazole sodium lyophilized powder injection has low related substances, and the substance does not remarkably increase in the storing process, and the content is not remarkably reduced.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Preparation process of the sodium salt of esomeprazole
InactiveCN102770423AOrganic active ingredientsOrganic chemistryBenzimidazole derivativeEsomeprazole Sodium
Preparation process of the sodium salt of a 2-(2-pyridylmethylsulfinyl)- benzimidazole derivative compound It comprises a preparation process of esomeprazole sodium substantially free of sulfone impurity comprising the steps of: a) combining either esomeprazole with a (C3-C8)-ketone or a mixture thereof, a sodium alkoxide, and a (C1-C5)-alcohol, or esomeprazole sodium with a (C3-C8)-ketone or a mixture thereof and a (C1-C5)-alcohol; and b) recovering the esomeprazole sodium formed from the reaction media by filtration.
Owner:ESTEVE QUIMICA
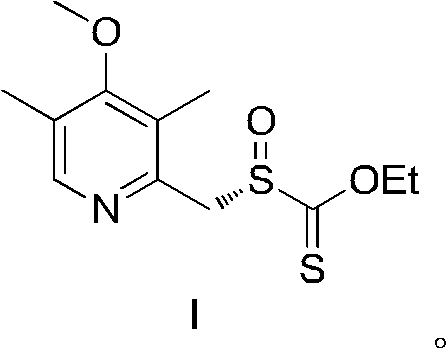
Novel chiral sulfoxide compound and method for preparing esomeprazole by using novel chiral sulfoxide compound
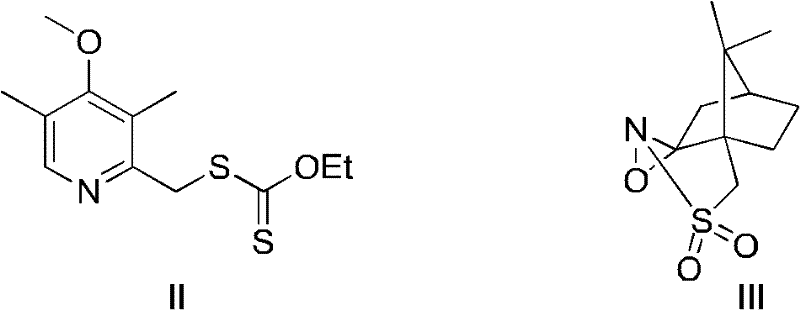
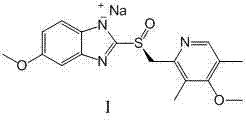
The invention discloses a (S)-(((4-methoxy-3,5-dimethyl pyridine-2-yl)methyl)sulfinyl) ethyl thioformate compound, a preparation method of the compound and a process for preparing esomeprazole by using the compound. The compound and the preparation method have the remarkable advantages that: raw materials for synthesis are cheap and can be easily obtained; the reaction conditions are moderate; the yield of the prepared esomeprazole is high; the optical purity is high; the operation is safe; the environment is slightly polluted; and the compound is more suitable for industrial large-scale production.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Method for enhancing production yield and rate of esomeprazole
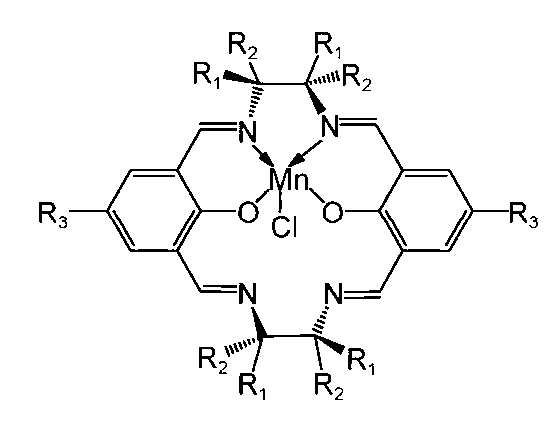
The invention aims to enhance the conversion and generation yield and rate of esomeprazole from omeprazole precursor-thioether through a chiral catalyst Mn(salen). The method comprises the following steps: weighing a right amount of esomeprazole precursor-thioether, evenly mixing with dichloromethane, adding the chiral catalyst Mn(salen) and D-(-)diethyl tartrate to carry out chiral conversion, adding cumene hydroperoxide for oxidation to generate esomeprazole, filtering to remove the chiral catalyst, distilling out dichloromethane to obtain an esomeprazole crude product, and finally, crystallizing with acetone to obtain the esomeprazole pure product. High-performance liquid chromatography is utilized to detect the content. The chiral catalyst Mn(salen) can enhance the generation yield of esomeprazole sodium by 50%, and save the reaction time by 2 hours or so. The method is simple to operate, and has the advantages of low reagent toxicity, low required initial raw material cost and high product yield.
Owner:CP PHARMA QINGDAO CO LTD
Industrial production method of esomeprazole
InactiveCN104496964AReduce manufacturing costEasy to solveOrganic chemistryEsomeprazole SodiumSulfur Ethers
The invention provides an industrial production method of esomeprazole. According to the industrial production method, under the action of a phase transfer catalyst and strong alkali, omeprazole sulfur ether is prepared through condensation; through low-temperature asymmetric oxidation, the generation of nitric oxide and sulfone is reduced, an organic solution of esomeprazole (as shown in the formula III) is prepared, the organic solution can be directly fed into a sodium alcoholate solution, three steps namely asymmetric oxidation aftertreatment, salting and crystallization and refining are completed once, a finished esomeprazole product is prepared in multiple batches, the purity of the finished esomeprazole product is greater than 99.8%, the isomer of the finished esomeprazole product is less than 0.05%, the individual impurity of the finished esomeprazole product is less than 0.1%, and the medicine grade raw material medicine requirements are met. By adopting the preparation method, the step of extracting and purifying esomeprazole and esomeprazole sodium is canceled, the production period is shortened, the production cost is greatly reduced, the possibility that the sulfone impurity is increased in the production process is avoided, the preparation method is safe and reliable, simple and easy to operate, stable in salting and good in repeatability, and the product quality is greatly improved.
Owner:合肥远志医药科技开发有限公司
Preparation method of high-purity esomeprazole sodium salt
ActiveCN102993177ASimple stepsSuitable for industrial purification productionOrganic chemistryEsomeprazole SodiumRoom temperature
The invention provides a refining method of industrial esomeprazole sodium salt. The preparation method is characterized by comprising the following steps: adding esomeprazole sodium salt to methanol, the amount of which is 1.6-4 times of that of the esomeprazole sodium salt; stirring and dissolving the esomeprazole sodium salt under the room temperature; filtering the mixture to obtain a settled solution; cooling the settled solution at 0 DEG C and stirring the settled solution for 2-4 hours; filtering and drying the precipitate; using poor solvents such as acetone, acetonitrile, isopropyl ether and the like for heating and refluxing the mixture for 2-4 hours; cooling the mixture to room temperature, and filtering and drying the mixture. Through the preparation method, high-purity esomeprazole sodium salt can be obtained, single purity content does not exceed 0.1%, the moisture content is smaller than 1%; and the moisture content does not exceed 5% after placing the mixture for 12 hours at 40 DEG C under the relative humidity of 80%.
Owner:科贝源(北京)生物医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com