Preparation process of freeze-dry powder injection containing esomeprazole sodium
A technology of esomeprazole sodium and freeze-dried powder injection, which is applied in the field of pharmaceutical preparations, can solve problems such as lack of preparation samples, large pH value changes, and poor solution clarity, and achieve good resolubility, low water content, and low cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
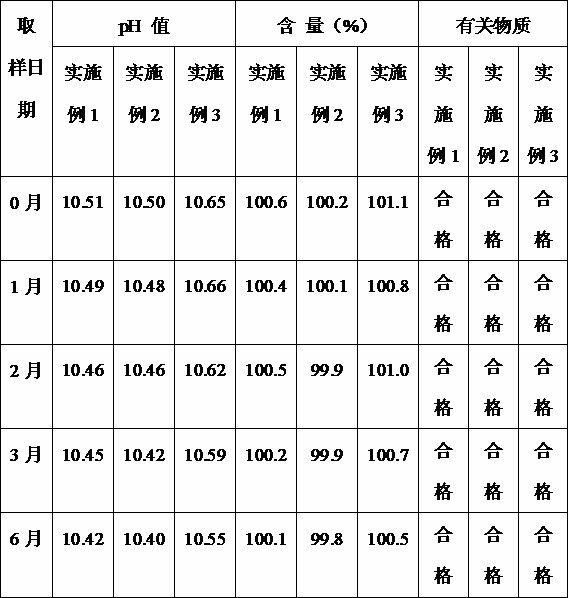
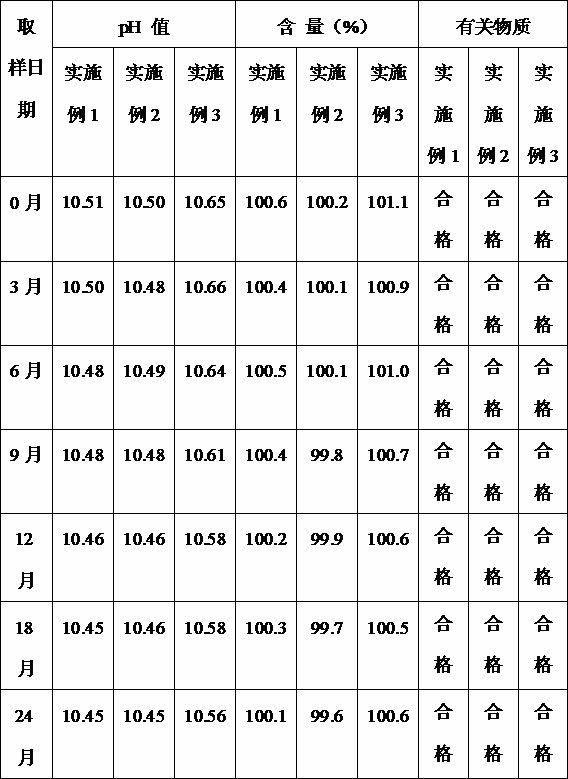
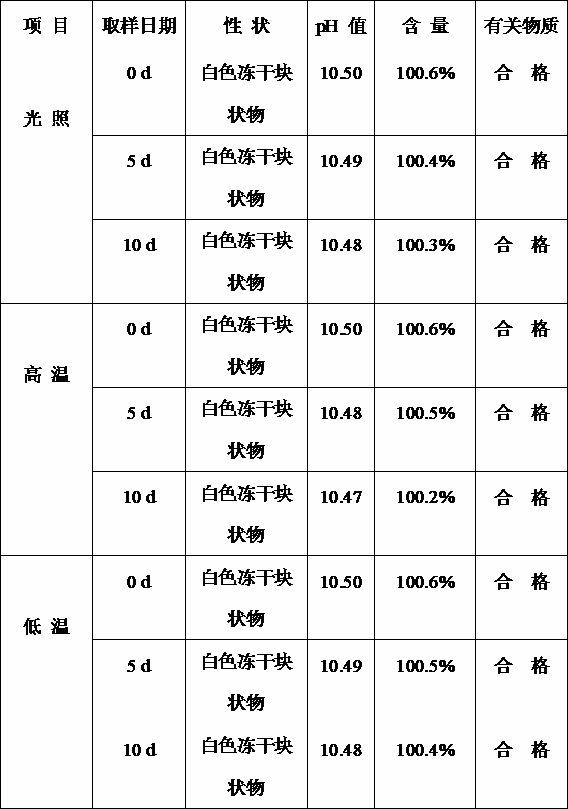
Examples
Embodiment 1
[0025] Prescription: (Specification: 40mg)
[0026] Esomeprazole sodium 100g (calculated as esomeprazole)
[0027] Mannitol 400g
[0028] Anhydrous sodium sulfite 10g
[0030] Add water for injection to 5000ml
[0031] Prepare 2500 sticks
[0032] Preparation:
[0033] Weigh the prescribed amount of mannitol, anhydrous sodium sulfite, calcium sodium edetate and dissolve them in fresh water for injection with a total volume of 80%, add the prescribed amount of esomeprazole sodium and stir until completely dissolved, add 10g of medicine Use charcoal, heat to 60°C, stir and adsorb at constant temperature for 20 minutes, and decarbonize and filter with a 0.8μm titanium rod. Use 1mol / L sodium hydroxide solution to adjust the pH of the filtrate to 10.6, and add water for injection to the full amount. After 0.45μm and 0.22μm two-stage precision filter for sterilization and filtration, the filtrate is checked for intermediates. After passing th...
Embodiment 2
[0035] Prescription: (Specification: 40mg)
[0036] Esomeprazole sodium 100g (calculated as esomeprazole)
[0037] Mannitol 400g
[0040] Add water for injection to 5000ml
[0041] Prepare 2500 sticks
[0042] Preparation:
[0043] Weigh the prescribed amount of mannitol, sodium bisulfite, calcium sodium edetate and dissolve them in fresh water for injection with a total volume of 80%, add the prescribed amount of esomeprazole sodium and stir until completely dissolved, add 12g Medicinal charcoal, stirred and adsorbed for 30 minutes, decarbonized and filtered with a 0.8μm titanium rod. Use 1mol / L sodium hydroxide solution to adjust the pH of the filtrate to 10.6, and add water for injection to the full amount. After 0.45μm and 0.22μm two-stage precision filter for sterilization and filtration, the filtrate is checked for intermediates. After passing the aseptic filling, the filling volume is controlled at 2...
Embodiment 3
[0045] Prescription: (Specification: 40mg)
[0046] Esomeprazole sodium 100g (calculated as esomeprazole)
[0047] Mannitol 300g
[0048] Sodium pyrosulfite 15g
[0049] Sodium Calcium EDTA 5g
[0050] Add water for injection to 5000ml
[0051] Prepare 2500 sticks
[0052] Preparation:
[0053]Weigh the prescribed amount of mannitol, sodium pyrosulfite, and calcium sodium edetate and dissolve them in fresh water for injection with a total volume of 80%, add the prescribed amount of esomeprazole sodium and stir until completely dissolved, add 8g of medicinal Charcoal, stirred and adsorbed for 40 minutes, decarbonized and filtered with a 0.8 μm titanium rod. Adjust the pH of the filtrate to 10.8 with 0.5mol / L sodium hydroxide solution, and add water for injection to the full amount. After 0.45μm and 0.22μm two-stage precision filter for sterilization and filtration, the filtrate is checked for intermediates. After passing the aseptic filling, the filling volume is contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com