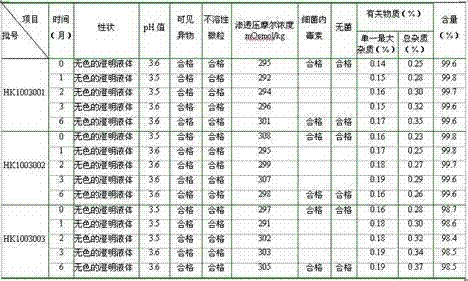
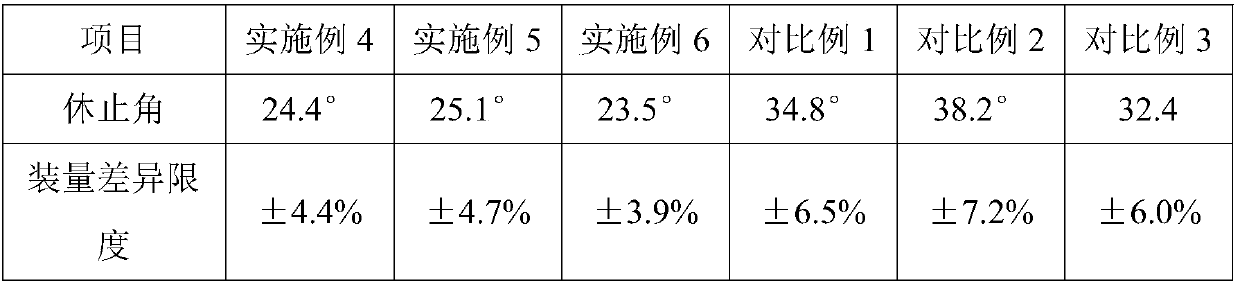
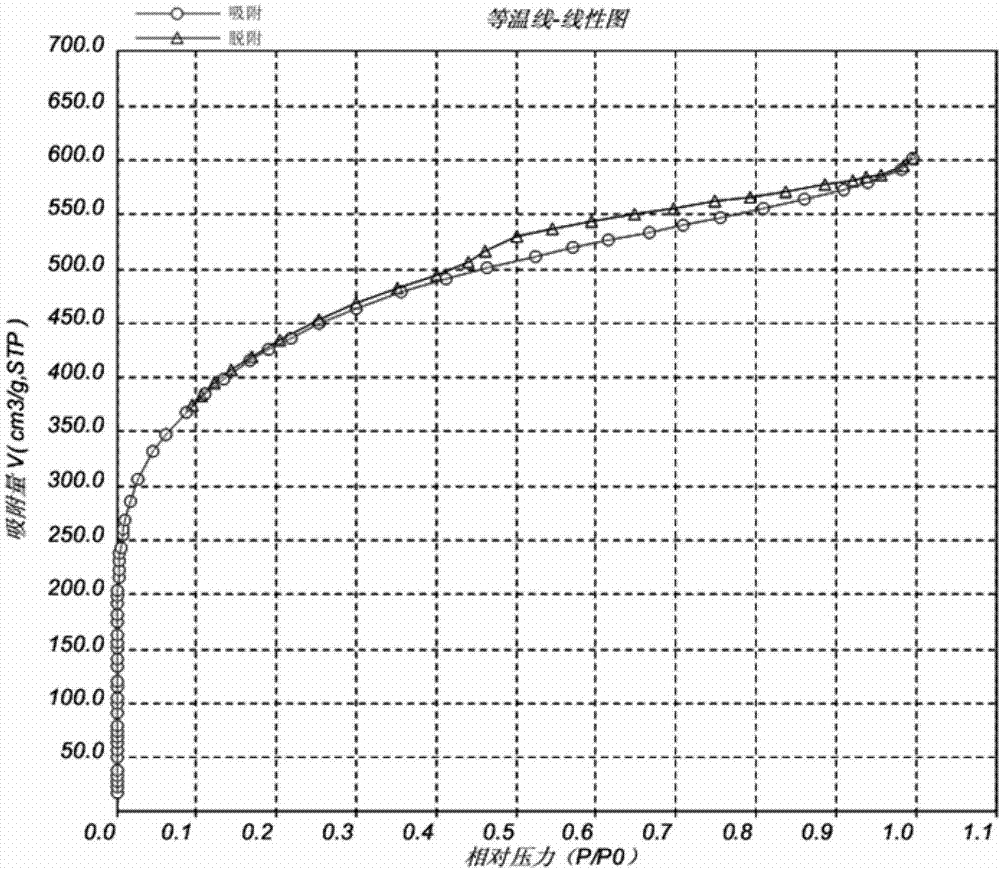
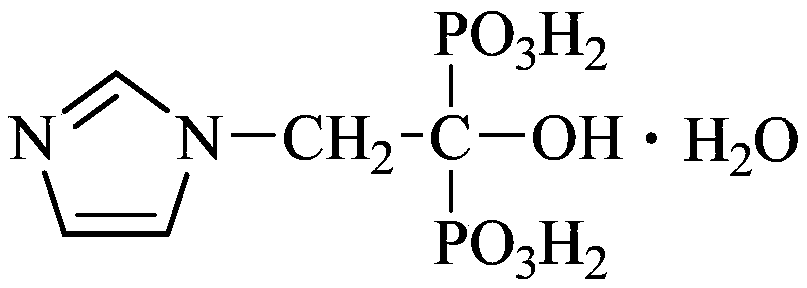Patents
Literature
44 results about "Medicinal charcoal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
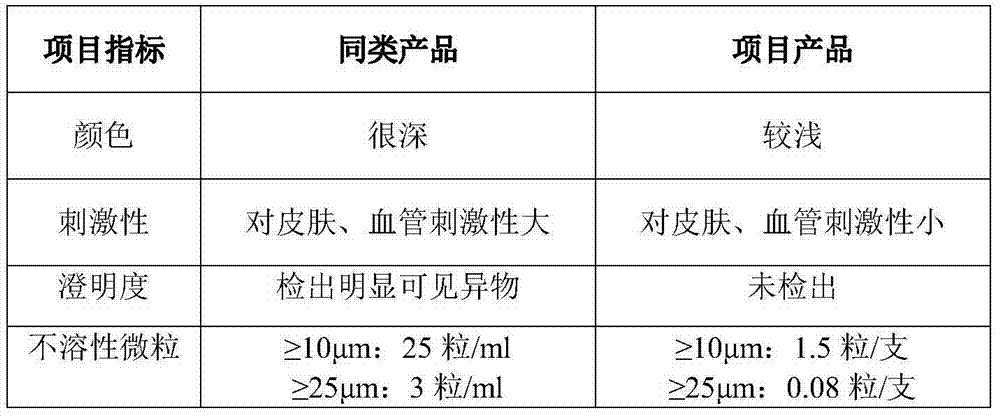
Charcoal also is used to relieve itching related to kidney dialysis treatment and to treat poisoning or drug overdose. Charcoal may also be used for other purposes not listed in this medication guide.
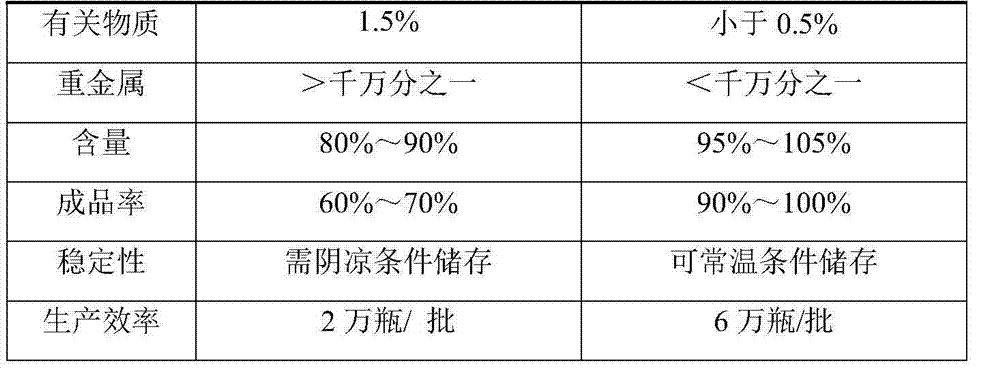
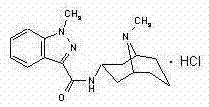
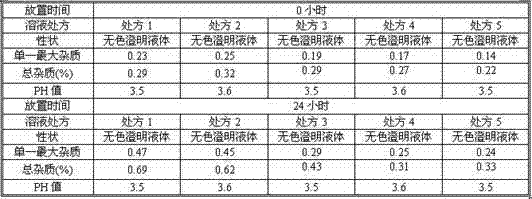
Fasudil hydrochloride pharmaceutical composition for injection
InactiveCN102266343AFix stability issuesHigh yieldOrganic active ingredientsPharmaceutical delivery mechanismPharmaceutical drugMedicinal chemistry
The invention discloses a pharmaceutical composition of fasudil hydrochloride for injection. The fasudil hydrochloride injection is composed of fasudil hydrochloride, cysteine hydrochloride and sodium chloride, each containing hydrochloric acid method Sudil 15-60mg, cysteine hydrochloride mg, sodium chloride mg. The preparation method is as follows: take 90% of the prescribed amount of water for injection, at a temperature of 55-65°C, add the prescribed amount of cysteine hydrochloride, stir to dissolve; add the prescribed amount of fasudil hydrochloride, stir until dissolved, and pour into Then add the sodium chloride of recipe quantity in the solution, stir until dissolving completely; Measure initial pH value, according to initial pH value, adjust pH value range with 4% sodium hydroxide solution and 10% cysteine hydrochloride solution in 5.5- 6.5; add medicinal charcoal to the mixture, stir; suction filter, add water for injection to the full amount, mix evenly; fine filter; fill; sterilize; The pharmaceutical composition of fasudil hydrochloride has good light stability, no crystallization and clarity, and good stability. The invention has the advantages of improving product yield, reducing cost, realizing industrialization, and better clinical application. more obvious advantages.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Medicine for preparing smokeless moxibustion therapy medicinal charcoal
InactiveCN1438020ASolve pollutionEliminate infringementDevices for heating/cooling reflex pointsUnknown materialsDrug herbalTraditional medicine
The preparation method of medicinal charcoal for making smokeless moxibustion includes: extruding the raw material medicine into the block medicine, the carbonizing said block medicine to obtain medicinal charcoal, had best extrude the raw material medicine into hollow roll form, at the same time of extruding the raw material medicine into the form of hollow roll the medicinal charcoal oil is collected. The above-mentioned raw material can be single Chinese medicinal material or Chinese medicine composition suitable for making moxibustion. Said invention can completely resolve the problem of environmental pollution resulted from moxibustion smoke and dust.
Owner:罗东海
Chinese and western medicinal composition for treating empyrosis
ActiveCN101850000ARapid epithelial repairPain relieverHydroxy compound active ingredientsTetracycline active ingredientsEpitheliumRepair tissue
Owner:张十方
Method for preparing levofloxacin hydrochloride sodium chloride injection
InactiveCN103479522ASubstance reductionHigh clarityAntibacterial agentsOrganic active ingredientsWater bathsSodium Chloride Injection
The invention provides a method for preparing levofloxacin hydrochloride sodium chloride injection. By researching a liquid medicine preparation method, medicinal charcoal pretreatment and a terminal sterilization process, product quality is ensured by the aid of technologies of a concentrated solution and diluted solution method, charcoal slurry preparation from medicinal charcoal, water bath sterilization and the like, production is facilitated, obtained finished products have higher stability, so that the storage life of the finished products can be prolonged, and clinical use effects are better.
Owner:HAINAN HOTMED TIANYA PHARMA
Tropisetron hydrochloride medicament composition for injection
InactiveCN102302495AFix stability issuesHigh yieldInorganic non-active ingredientsPharmaceutical delivery mechanismPharmaceutical drugMedicinal chemistry
The invention discloses a tropisetron hydrochloride medicament composition for injection. The medicament composition consists of tropisetron hydrochloride, sodium chloride and citric acid, and is characterized in that the weight ratio of tropisetron hydrochloride to citric acid is 1:(0.001-5). The preparation method of the medicament composition comprises the following steps: taking 95% of prescription amount of water for injection; introducing dioxide at the temperature of 30-40 DEG C until the pH value is at the rang of 3.0-4.0; adding prescription amount of sodium chloride and citric acid, stirring and dissolving; adding prescription amount of tropisetron hydrochloride, and stirring to dissolve completely; adding medicinal charcoal in the solution, and standing after stirring uniformly; filtering in vacuum, supplementing water for injection to full amount, and mixing uniformly; measuring the initial pH value, and regulating the pH value to the range of 3.0-4.0 with 4% sodium hydroxide solution and 10% citric acid solution according to the initial pH value; carrying out fine filtering; filling; sterilizing; carrying out lamp inspection; and warehousing, thus the finished product is obtained. The tropisetron hydrochloride medicament composition has good stability to light, has good stability, and has the obvious advantages for improving the product yield, lowering cost, implementing industrialization, and realizing clinical application better.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Preparation method of potassium aspartate crude drug
InactiveCN103664668ASimple processEase of industrial productionOrganic compound preparationAmino-carboxyl compound preparationBiochemical engineeringSpray dried
The invention provides a preparation method of a potassium aspartate crude drug. The preparation method specifically comprises the following steps: (a), adding proper amount of purified water into a liquor preparation tank, adding a potassium salt under stirring, adding aspartic acid in a fractional manner while stirring after all potassium salt is dissolved, and reacting under stirring; (b), detecting that a pH value is between 5.8-8.2, and regulating by using proper amount of the potassium salt or aspartic acid if the detected value deviates from 5.8-8.2; (c), adding medicinal charcoal and stirring for 20 minutes-30 minutes; (d), enabling medical liquor to pass through a decarburization filter, filtering by a 0.45-mu m filter, monitoring and recording that pressure of the 0.45-mu m filter is between a range of 0.15 MPa-0.2 MPa, adding the purified water into the liquor preparation tank after the medical liquor is filtered out, feeding filtrate into a buffer tank by once to twice, and clarifying the filtrate; (e), spray-drying, discharging and sub-packaging.
Owner:沈阳药联科技创新有限公司
Coenzyme A for injection and production process thereof
InactiveCN102397259AFull appearanceImproved freeze-drying processOrganic active ingredientsPowder deliveryFreeze-dryingCoenzyme A biosynthesis
The invention provides a coenzyme A for injection and a production process thereof. The coenzyme A for injection consists of a coenzyme A, mannitol, dextran-40, calcium gluconate, sodium bisulfite, medicinal charcoal and fresh water for injection. The production process comprises the following steps of: adding the dextran-40, mannitol and calcium gluconate into the water for injection for dissolving, adding the medicinal charcoal, and cooking to boiling state; filtering and decarburizing the solution with a titanium stick, and filtering; mixing a sodium bisulfite aqueous solution with the filtered and decarburized solution, regulating the pH value with hydrochloric acid, replenishing the water for injection for preparing, and filtering with a microporous filtering film to obtain a coenzyme A solution for injection; and pre-freezing the coenzyme A solution for injection, subliming and drying to obtain a coenzyme A freeze-dried powder injection. In a preparation method, a freeze drying process is improved, the freeze drying time is shortened, and the production efficiency is increased greatly. The obtained freeze-dried powder injection has plump appearance, a clarified solution and high product content stability, and is quickly dissolved in water.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Colored medicinal charcoal film coated tablet preparation method
ActiveCN101103997AEasy to acceptImprove clinical complianceDigestive systemCarbon active ingredientsColoredExcipient
The invention discloses a preparation method of painted medicinal carbon film-coating tablet produced by the raw material of medicinal carbon and medicinal excipients. The invention is characterized in that painted medicinal carbon film coating tablets are produced in the preparation method, thus the visual defect of black medicinal carbon is overcome and the painted tablets are easy to be accepted by patients. The smooth and round form of the painted medicinal carbon film coating tablet can help effectively guarantee the tablet quality and improve the clinical compliance of patients.
Owner:河北长天药业有限公司
Hippocampal multi-penis pills and preparation method thereof
ActiveCN109602836AImprove liquidityGood hygroscopicityInanimate material medical ingredientsBird material medical ingredientsPenisSucrose
The invention belongs to the technical field of pharmaceuticals, and in particular relates to hippocampal multi-penis pills and a preparation method thereof. The hippocampus multi-penis pills of the invention include pellets and coating materials. The pellets include the following raw materials in parts by weight: hippocampi, geckos, semen allii tuberosi, herba cynomorii, pilose antler (dehaired),fructus psoraleae (processed), fennel (processed), semen cuscutae (processed), flatstem milkvetch seeds (processed), fructus corni (processed), bighead atractylodes rhizome (stir-fried), cortex eucommiae (salted), red ginseng, clove fruits, radix achyranthis bidentatae, poria cocos, Chinese yams, radix astragali, radix angelicae sinensis, fossil fragments (calcined), licorice (processed), cinnamon, sparrow brains, Chinese magnoliavine fruits, Chinese wolfberry fruits, dog penis, donkey penis, ox penis, mink penis, prepared rehmannia roots, radix aconiti carmichaeli (processed), herba cistanche, radix morindae officinalis, herba epimedii and ethanol; and the coating materials include medicinal charcoal, sucrose, peach gum, and cera chinensis. The hippocampus multi-penis pills of the invention improve the fluidity of drug powder, improve the hygroscopicity of the drug powder, solve the problem of weight difference, and have short dissolution time.
Owner:LIAONING ORIENTAL PHARMA
Method for preparing potassium aspartate injection
InactiveCN103655461AGuaranteed stabilityAvoid pollutionOrganic active ingredientsMetabolism disorderEnvironmental engineeringInjection solution
The invention provides a method for preparing a potassium aspartate injection. The method comprises the following steps: (a) adding injection water accounting for 60-70 percent of the total volume of the preparation liquid into a concentrating preparation tank; (2) adding potassium aspartate into the concentrating preparation tank, stirring for 20-30 minutes until the potassium aspartate is completely dissolved, detecting and regulating the pH value to 4.5-9.5; (c) adding medicinal charcoal, stirring for 20-40 minutes at 70-90 DEG C, filtering the liquid medicine into a diluting preparation tank through a decarburization filter and a precise filter when the liquid medicine is hot; (d) adding injection water into the concentrating preparation tank, feeding the mixture to the diluting preparation tank through a filter to enable the liquid medicine in the diluting preparation tank to achieve the specific total volume of the preparation liquid; (e) stirring the liquid medicine in the diluting preparation tank for 5-10 minutes to lead the liquid medicine to be uniform, introducing the liquid medicine through a sterilization filter, and sampling to make intermediate inspection; (f) encapsulating and sterilizing.
Owner:沈阳药联科技创新有限公司
Procaine hydrochloride injection and its production technology
InactiveCN102366399AImprove stabilityAvoid hydrolysisOrganic active ingredientsInorganic non-active ingredientsMedicinal chemistryInjection solution
The invention belongs to the field of medicinal preparations, and provides a procaine hydrochloride injection and its preparation method. 1000 ml of the procaine hydrochloride injection provided by the invention contains 20g of procaine hydrochloride, 9g of common salt, 0.5g of medicinal charcoal and the balance being fresh injection water. The preparation method of the procaine hydrochloride injection is characterized by comprising the following steps of: a, adding common salt into injection water for dissolution, adding procaine hydrochloride, and stirring for dissolution; b, adjusting pH of a liquid medicine obtained from the step a to 4.0-4.5 by the use of 1 mol / L of hydrochloric acid; c, adding injection water into a liquid medicine obtained from the step b up to the full amount, adding medicinal charcoal, stirring for 10 minutes, standing for 20 minutes, and filtering through a titanium rod filter and 0.45 micron and 0.22 micron of a cartridge type filter; d, placing a sealed intermediate product into a sterilization cabinet, using circulating vapor of 100 DEG C, and sterilizing for 30 minutes. The procaine hydrochloride injection provided by the invention is more stable.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Scopolamine hydrobromide injection and production technology
ActiveCN102366402AIncrease profitFinished product content is stableNervous disorderDigestive systemThird generationFresh water
The invention discloses a preparation method of scopolamine hydrobromide injection, comprising the following steps that: (a) technology formula: each 1000 ml of scopolamine hydrobromide injection comprises 0.3 g of scopolamine hydrobromide, 9 g sodium chloride, 0.5 g of medicinal charcoal, and the balance consisting of fresh water for injection; (b) sodium chloride is added and dissolved in the water for injection, scopolamine hydrobromide is added, stirring is carried out until the scopolamine hydrobromide dissolve completely, and 1 mol / L of hydrobromic acid is used to adjust the pH value of the liquid medicine to 3.4-3.6; (c) the medicinal charcoal is added in the liquid medicine in the step (b) with stirring for 10 min and standing for 20 min, filtering and decarburization are carried out through a titanium stick; (d) filtering is carried out on the liquid medicine in the step (c) through a 0.45 mum cartridge filter and a 0.22 mum cartridge filter; and (e) the intermediate products are filled and sealed, then putting the filled and sealed products in a sterilization cabinet, and sterilization is carried out through circulating steam with the temperature of 100 DEG C for 30 min. According to the invention, the utilization rate of raw material is raised, and the prepared products have stable content of finished product.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Method for preparing active carbon for relieving or neutralizing effect of alcohol
InactiveCN103043661AEffective absorptionFood safetyCarbon compoundsCarbon active ingredientsActivated carbonAlcohol
The invention provides a method for preparing active carbon for relieving or neutralizing the effect of alcohol. The method comprises: using wood, shells, mao bamboo or coal as raw material, to customize active carbon with certain aperture distribution, of which the specific surface area is 1100-2000m2 / g, refining the customized active carbon, and adding 8-20% of aqueous alkali and the active carbon into a reaction vessel; introducing steam to heat the aqueous alkali until the temperature rises to 90+-5 DEG C, insulating, and dehydrating when the temperature falls up to less than 55 DEG C; washing with tap water and then dehydrating for several times; adding the dehydrated material into 6-15% of acid solution for pickling, introducing steam to heat the acid solution until the temperature rises to 65+-6 DEG C, insulating, adding cold water, and dehydrating when the temperature falls up to less than 55 DEG C; washing with tap water and then dehydrating for several times; washing with pure water and then dehydrating, to regulate the pH value in a range of 6.5-9.5. The active carbon prepared by the method has an obvious effect for relieving or neutralizing the effect of alcohol, accords with the standard of GB / T 13803.4-1999 of activated carbon for refinement of injection and the standard of medicinal charcoal in the Chinese Pharmacopoeia: 2010 edition, and accords with an edible hygienic standard.
Owner:承德鑫永晟炭业有限公司
Arginine hydrochloride injection and preparation method thereof
InactiveCN107115286AEfficient removalQuality improvementOrganic active ingredientsNervous disorderLiquid storage tankSand filter
The invention discloses an arginine hydrochloride injection and a preparation method thereof. It includes the following steps: ①adding 50% water for injection and arginine hydrochloride in the concentrated preparation tank, stirring and dissolving; then adding medicinal charcoal, stirring evenly, boiling for 30 minutes, and standing to cool; ②filtering with Suzhou sand After rod back filtration and decarbonization to clarity, filter into the dilute tank; ③add water for injection to the full amount, and stir evenly; ④filter through Suzhou sand filter rod, 0.45μm polyethersulfone and 0.22μm polyethersulfone fins until clarity; ⑤ Take a sample to test the intermediate content and pH value. If the intermediate content and pH value are qualified, filter the liquid medicine into the liquid storage tank; Filling liquid storage container for filling; ⑦ filling and sealing; ⑧ sterilization: temperature 115 ℃, time 30 minutes. The arginine hydrochloride injection prepared by the invention can effectively remove impurities such as raw materials, and has stable and reliable quality.
Owner:SHANGHAI XINYI JINZHU PHARMA
Ibuprofen medicine composition for injection
ActiveCN103027890ASolution destructionAchieve sterilization effectOrganic active ingredientsAntipyreticArginine glutamateIbuprofen arginine
The invention discloses an ibuprofen medicine composition for injection, which consists of ibuprofen and arginine glutamate, and is characterized in that the weight ratio of the ibuprofen to the arginine glutamate is 1:1.44. A preparation method of the medicine composition comprises the following steps of: taking injection water with weight being 70 percent of prescribed dosage, adding the arginine glutamate with prescribed dosage at 70-80DEG C, and agitating the mixture for dissolution; adding the ibuprofen with prescribed dosage and agitating the mixture till the ibuprofen is fully dissolved; adding medicinal charcoal into the solution, evenly agitating the mixture and standing the mixture; conducting suction filtration, replenishing the injection water to full dosage and evenly mixing the solution; determining the initial PH (potential of hydrogen) value, and regulating the pH value to 7.7 plus or minus 0.2 by using 4% sodium hydroxide solution and 10% hydrochloric acid solution according to the initial pH value; conducting refined filtration and filling; conducting sterilization; conducting lamp inspection; and warehousing the product to obtain the finished product. The ibuprofen medicine composition has high light stability, is reliable in quality and has more obvious advantages for product yield improvement, cost reduction, industrialization realization and better clinical application.
Owner:TIANJIN HANKANG PHARMA BIOTECH
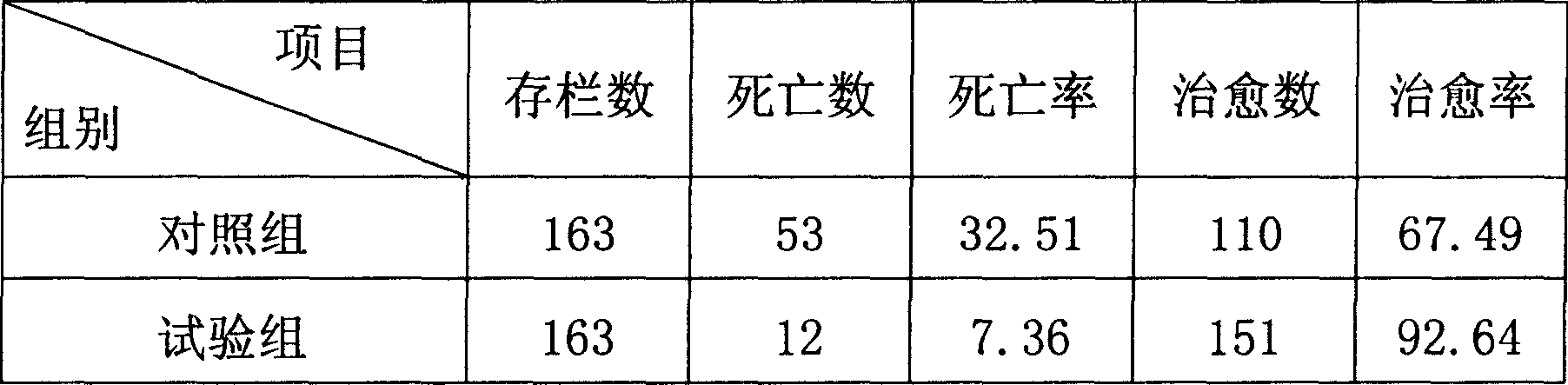
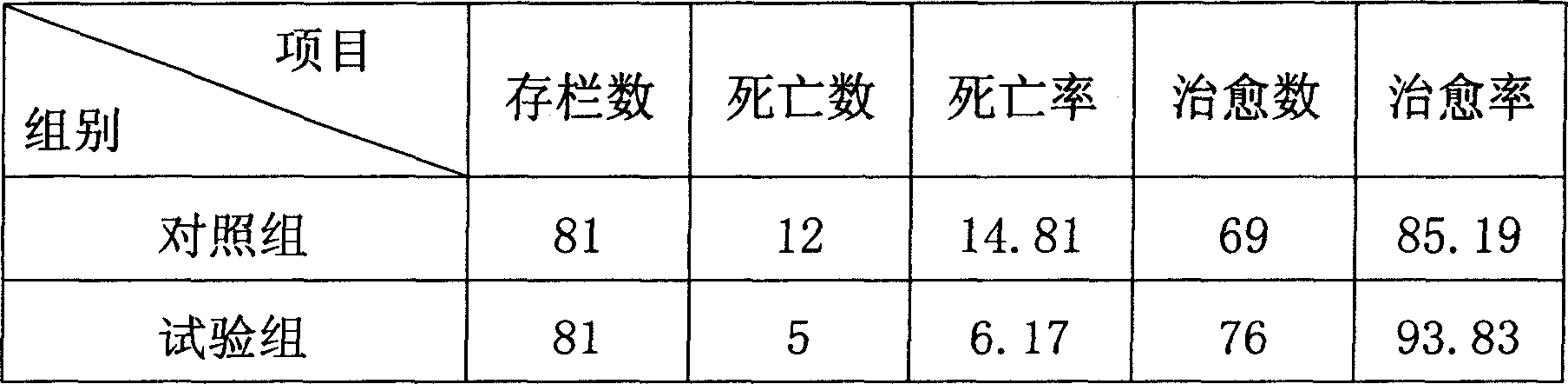
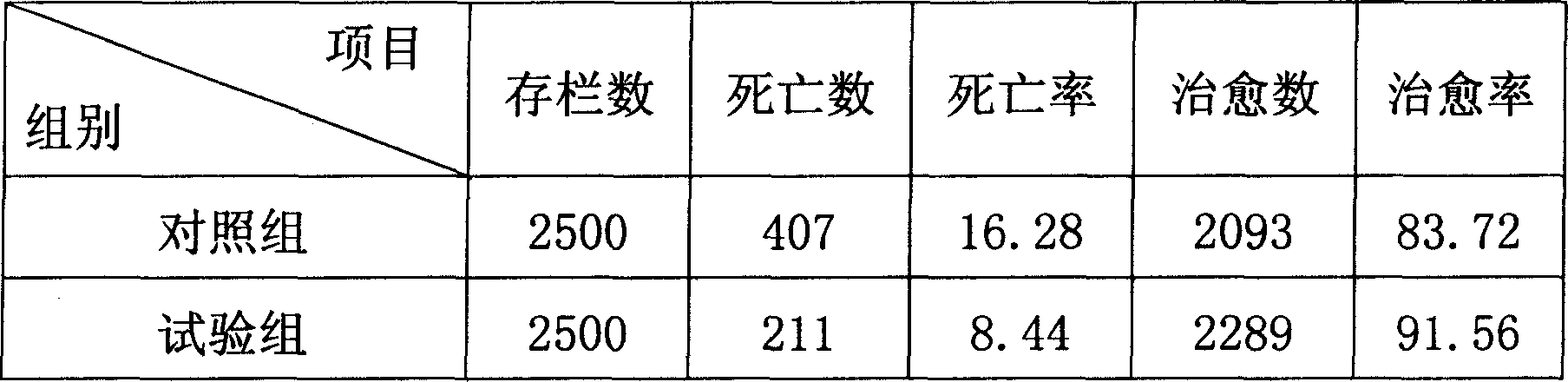
Medicine for preventing and controlling diarrhea of livestock and birds
InactiveCN1899532AReduce stimulationIncreased frequency of bowel movementsDigestive systemInanimate material medical ingredientsCurative effectSugar
The medicine for preventing and controlling diarrhea of livestock and poultry is prepared with medicinal charcoal, fried kaoliang, sodium humate and talc in certain weight proportion, and through crushing, grinding and mixing. Before application, the medicine in 1-2 wt% is mixed into feed for animal to take. At the same time, water with sugar and salt is added for replenishing body fluid and raising curative effect. The medicine has high curative rate, no adverse reaction, no medicine residue, no drug resistance and other advantages, and is suitable for various kinds of livestock and poultry.
Owner:HEBEI AGRICULTURAL UNIV.
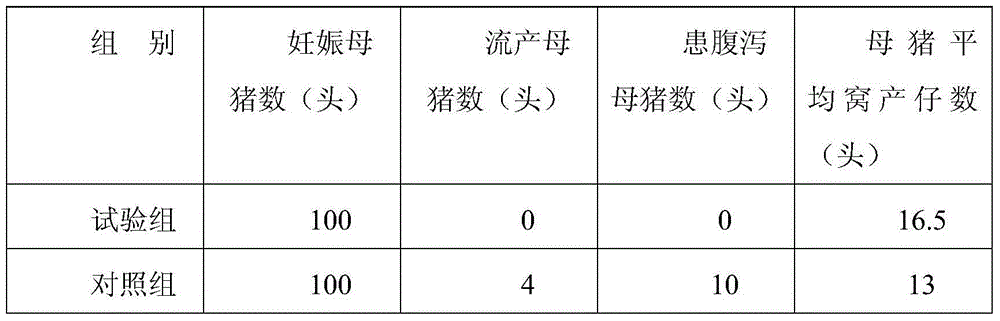
Pregnant sow feed for control of diarrhea and producing method thereof
InactiveCN104366040APrevention of stillbirthPrevent diarrheaFood processingAnimal feeding stuffAnimal scienceOyster
The invention discloses a pregnant sow feed for control of diarrhea and a producing method thereof, and the pregnant sow feed comprises the following raw materials by weight: 150-180 parts of barley protein powder, 140-160 parts of corn flour, 120-160 parts of wheat bran, 8-12 parts of herseconvolvuli linnaeus chrysalis powder, 10-15 parts of herse convolvuli chrysalis powder, 2-3 parts of radix puerariae, 1-2 parts of scutellaria baicalensis, 1-2 parts of coptis chinensis, 1-2 parts of radix paeoniae alba, 1-2 parts of prepared rhizome of rehmannia, 0.5-1 part of garlic, 0.5-1 part of raw ginger, 8-15 parts of chicken liver, 0.5-1 part of fish meat, 7-9 parts of green pepper, 1-2 parts of coriander, 2-3 parts of soy sauce, 0.5-1 part of vinegar, 10-14 parts of yellow rice, 8-13 parts of sago, 2-4 parts of mango dices, 1-2 parts of grape flesh, 1-3 parts of coptis chinensis leaf, 1-3 parts of emulsified fat, 4-8 parts of diatomite, 5-10 parts of talcum powder, 4-8 parts of medicinal charcoal, 1-2 parts of calcined oyster powder, 1-2 parts of calcined dragon bone powder, 10-15 parts of a food attractant, and proper amount of water. The pregnant sow feed effectively reduces economic loss of farmers.
Owner:HUAIBEI ZHENGXING BIOLOGICAL FEED
Production process of zoledronic acid injection
InactiveCN110812324AGood production process stabilitySimple processOrganic active ingredientsPharmaceutical delivery mechanismOfficinalProcess engineering
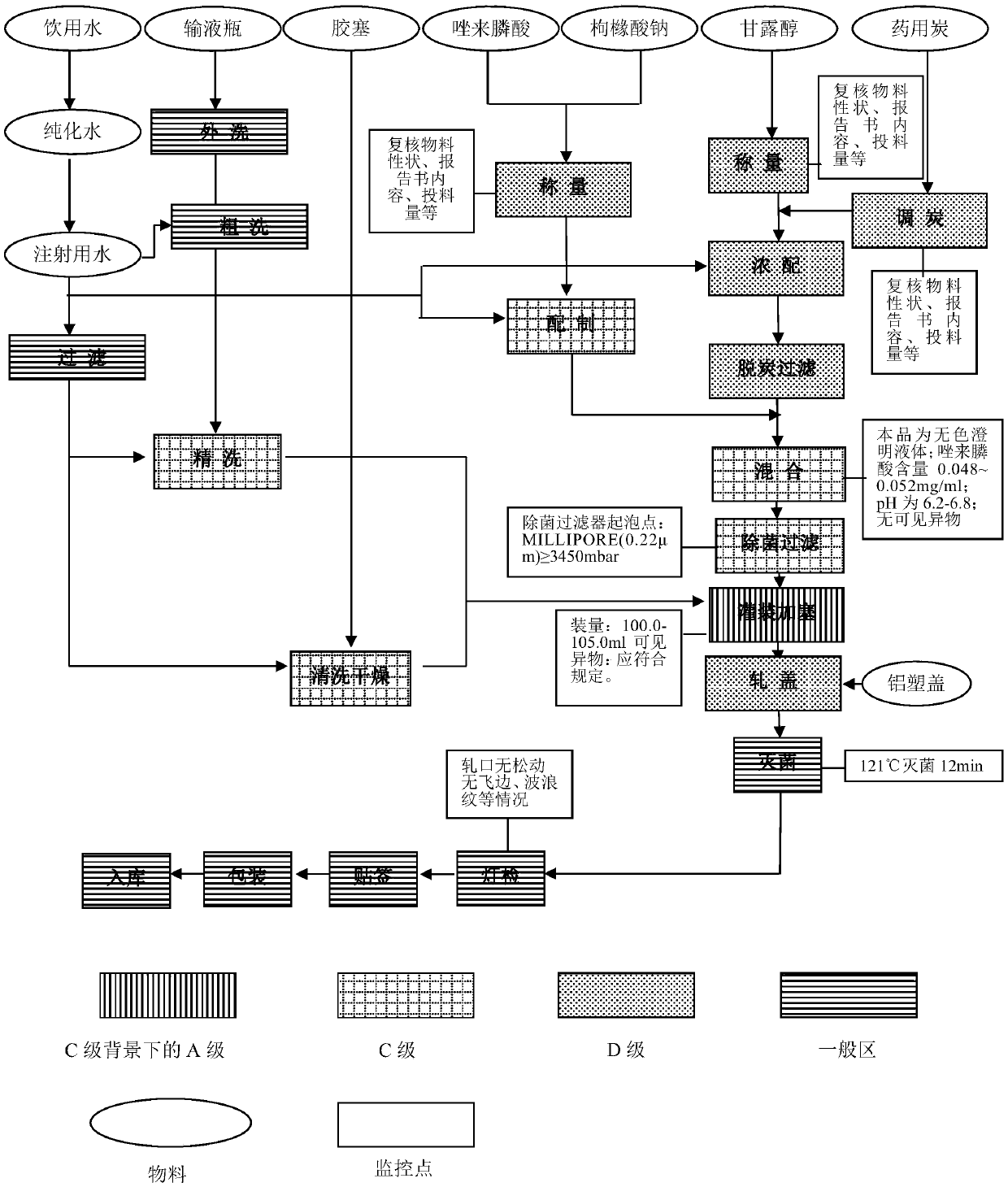
The invention provides a production process of a zoledronic acid injection. The production process comprises the following steps of (1) preparing an osmotic pressure regulating agent solution: dissolving a prescription amount of an osmotic pressure regulating agent by water for injection, adding medicinal charcoal, and performing decarbonization to obtain the osmotic pressure regulating agent solution; (2) preparing a zoledronic acid and pH regulating agent solution: dissolving a prescription amount of zoledronic acid and a pH regulating agent by the water for injection to obtain the zoledronic acid and pH regulating agent solution; (3) preparing a prescription solution: uniformly mixing the obtained osmotic pressure regulating agent solution and the zoledronic acid and pH regulating agentsolution, dissolving the solution to a constant volume of a prescription volume by using the water for injection, and performing filtration to obtain the zoledronic acid prescription solution with apH value being 6.2 to 6.8; and (4) performing sterilization and packaging: taking the zoledronic acid prescription solution obtained in the step (3), and performing sterilization and packaging to obtain the zoledronic acid injection. The production process of the zoledronic acid injection provided by the invention has the advantages that the stability is high; work procedures are simple; the production efficiency is high; the quality is controllable; and the industrialization can be easily realized.
Owner:YANGTZE RIVER PHARM GRP SICHUAN HAIRONG PHARM CO LTD +1
Feed additive capable of resisting diarrhoea for suckling pigs, as well as preparation method and application of feed additive
InactiveCN106551134ADiarrhea Prevention and ControlCompatibility is scientific and reasonableAnimal feeding stuffAccessory food factorsFood additiveAnimal science
The invention discloses a feed additive capable of resisting diarrhoea for suckling pigs. The feed additive is prepared from the following components in parts by weight: 1-5 parts of selenizing oligomerization aminopolysaccharide, 20-30 parts of cooked sorghum flour, 5-10 parts of medicinal charcoal, 1-5 parts of alumen, 1-5 parts of licorice root powder, 1-5 parts of citric acid, 1-3 parts of carvacrol and 50-60 parts of feed-grade montmorillonite powder. The feed additive capable of resisting diarrhoea for the suckling pigs disclosed by the invention is scientific and reasonable in compatibility, all the components are in synergistic reaction, and the feed additive is low in price, extensive in adaptability, free from toxic and side effects, capable of effectively preventing and controlling the diarrhoea of the suckling pigs, and suitable for popularization and application. The invention also provides a preparation method of the feed additive capable of resisting the diarrhoea for the suckling pigs. The preparation method is simple in technological steps, easy to implement, low in production cost, free from special requirements for equipment, and suitable for industrialized mass production.
Owner:ZHEJIANG OCEAN UNIV
Method for preparing Sparfloxacin
The invention discloses a method for preparing Sparfloxacin. The method comprises the steps of sequentially adding cis-2,6-dimethylpiperazine, 5-amino-1-cyclopropyl-6,7,8-trifluoro-1,4-dihydro-4-oxo-3-quinoline carboxylic acid and an aprotic polar solvent into a reaction tank according to a feeding ratio, performing a condensation reaction so as to obtain a wet crude product of Sparfloxacin, carrying out baking on the wet crude product of Sparfloxacin so as to control the water content of the wet crude product of Sparfloxacin, adding the dried Sparfloxacin crude product, an alkaline aqueous solution, water, hydrochloric acid, EDTA and medicinal charcoal into a reaction tank according to a feeding ratio, carrying out heating and stirring, carrying out cooling after dissolving is completed, carrying out discharging, centrifuging and spin-drying so as to obtain a wet Sparfloxacin product, subjecting the wet Sparfloxacin product to drying and performing crushing, thereby obtaining a finished product. As a whole, the method has the advantages that the product is good in stability and easy to treat in medicine processing, and the preparation process is simple.
Owner:河南精康制药有限公司
Chinese medicine for treating enteritis and colitis
ActiveCN101019938AEffective treatmentEliminate side effectsDigestive systemCarbon active ingredientsSide effectCurative effect
The Chinese medicine for treating enteritis and colitis is prepared with the materials including aucklandia root, white atractylodes rhizome, fructus evodiae, malaytea scurfpea fruit, Chinese goldthread, medicinal charcoal, vitamin B1 and metronidazole, and through depurating, washing and drying aucklandia root, white atractylodes rhizome, fructus evodiae, malaytea scurfpea fruit and Chinese goldthread, crushing to form 100-200 mesh powder; grinding medicinal charcoal, vitamin B1 and metronidazole into 100-200 mesh powder; mixing in certain weight ratio; encapsulating and packing. The Chinese medicine has obvious curative effect, no toxic side effect and other advantages.
Owner:武建军
Crucian feed for preventing and treating diseases and improving umami taste of flesh of crucian and preparation method thereof
InactiveCN104322927AStrong disease resistanceOvercoming easily sickFood processingAnimal feeding stuffDiseaseBiotechnology
The invention discloses a crucian feed for preventing and treating diseases and improving an umami taste of flesh of crucian and a preparation method thereof. The feed comprises following raw materials, by weight, 90-110 parts of corn flour, 70-80 parts of soya bean powder, 40-50 parts of fermented blood powder, 40-50 parts of oat bran, 20-30 parts of oat leaves, 15-20 parts of purple cabbage juice, 20-25 parts of tomato juice, 12-18 parts of cucumber juice, 6-9 parts of hance brandisia herb, 4-6 parts of vegetable protein milk, 2-3 parts of radix isatidis, 1-2 parts of pulsatilla chinensis, 1-2 parts of fructus viticis leaves, 1-2 parts of endothelium corneum gigeriae galli, 1-2 parts of loquat flowers, 0.5-1 part of sugar, 3-5 parts of rice wine, 5-8 parts of buckwheat flour, 2-3 parts of whole milk powder, 2-3 parts of medicinal charcoal, 3-4 parts of leaf mould, 2-4 parts of talcum powder, 8-15 parts of a phagostimulant and a proper amount of water. The feed is rich in nutrition, is strong in anti-disease capability, enables meat quality of the crucian to be greatly improved after being eaten by the crucian, and enables the meat of the crucian to be fine in mouthfeel and delicious in taste.
Owner:ANHUI CHENNUO FEED GREASE PROCESSING
Traditional Chinese medicine composition for relieving scald
InactiveCN107625832ASave materialNo side effectsCarbon active ingredientsUnknown materialsSide effectChinese drug
The invention relates to a traditional Chinese medicine composition for relieving scald and relates to the technical field of traditional Chinese medicines. The traditional Chinese medicine composition for relieving scald comprises, by weight, 5 to 12 parts of radix sanguisorbae, 3 to 9 parts of pain relieving tablets, 4 to 8 parts of cortex phellodendri, 6 to 16 parts of medicinal charcoal, 5 to15 parts of elephant skin, 5 to 11 parts of furazolidone, 3 to 9 parts of griseofulvin, 5 to 10 parts of Cape jasmine and 8 to 16 parts of chrysanthemum. The traditional Chinese medicine composition has good effects of relieving scald and has no side effect.
Owner:覃益祥
Prescription of Huanyuan Injection and its preparation process and usage
InactiveCN1323690COvercoming major side effectsEasy to acceptPharmaceutical delivery mechanismBlood disorderFormularyOfficinal
The invention relates to a traditional Chinese preparation for treating acute haemorrhagia apoplexy, the process for preparation and use thereof, wherein the preparation comprises astragalus root, dried rehmannia root, achyranthes root, F68, medicinal charcoal, 95% ethanol, and water for injection.
Owner:哈尔滨博发康安电站设备有限公司
Ibuprofen medicine composition for injection
ActiveCN103027890BSolve insolubleHigh yieldOrganic active ingredientsAntipyreticArginine glutamateIbuprofen arginine
The invention discloses an ibuprofen medicine composition for injection, which consists of ibuprofen and arginine glutamate, and is characterized in that the weight ratio of the ibuprofen to the arginine glutamate is 1:1.44. A preparation method of the medicine composition comprises the following steps of: taking injection water with weight being 70 percent of prescribed dosage, adding the arginine glutamate with prescribed dosage at 70-80DEG C, and agitating the mixture for dissolution; adding the ibuprofen with prescribed dosage and agitating the mixture till the ibuprofen is fully dissolved; adding medicinal charcoal into the solution, evenly agitating the mixture and standing the mixture; conducting suction filtration, replenishing the injection water to full dosage and evenly mixing the solution; determining the initial PH (potential of hydrogen) value, and regulating the pH value to 7.7 plus or minus 0.2 by using 4% sodium hydroxide solution and 10% hydrochloric acid solution according to the initial pH value; conducting refined filtration and filling; conducting sterilization; conducting lamp inspection; and warehousing the product to obtain the finished product. The ibuprofen medicine composition has high light stability, is reliable in quality and has more obvious advantages for product yield improvement, cost reduction, industrialization realization and better clinical application.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Medicine for preventing and controlling diarrhea of livestock and birds
InactiveCN100512861CReduce stimulationIncreased frequency of bowel movementsDigestive systemInanimate material medical ingredientsMedicineCurative effect
The medicine for preventing and controlling diarrhea of livestock and poultry is prepared with medicinal charcoal, fried kaoliang, sodium humate and talc in certain weight proportion, and through crushing, grinding and mixing. Before application, the medicine in 1-2 wt% is mixed into feed for animal to take. At the same time, water with sugar and salt is added for replenishing body fluid and raising curative effect. The medicine has high curative rate, no adverse reaction, no medicine residue, no drug resistance and other advantages, and is suitable for various kinds of livestock and poultry.
Owner:HEBEI AGRICULTURAL UNIV.
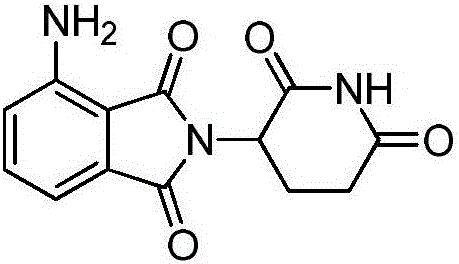
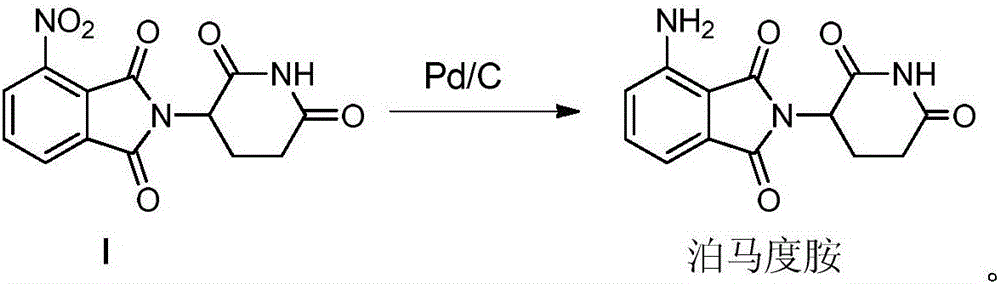
Preparation method for high-purity pomalidomide
A preparation method for high-purity pomalidomide comprises the following steps: (1) adding 3-nitro-N-(2,6-dioxo-3-piperidyl)phthalimide, palladium carbon and N-methyl pyrrolidone to a reaction kettle, vacuumizing, then introducing hydrogen gas, carrying out suction filtration after a reaction is complete, adding medicinal charcoal to the filtrate, stirring, heating, and carrying out suction filtration; and (2) adding an alkaline aqueous solution to the filtrate finally obtained in the step (1), stirring, then adding purified water, cooling and crystallizing, carrying out suction filtration, adding purified water to the obtained filter cake, stirring, carrying out suction filtration, washing, and drying to obtain the high-purity pomalidomide product. The preparation method provided by the invention has the advantages of simple operation and mature and stable technology, and can significantly improve the quality of pomalidomide; the purity of the obtained product is greater than 99.5%, the contents of single impurities are all less than 0.1%, and the medicinal requirements are met.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Preparation method of anesthetic
ActiveCN112156093AImprove cooling effectIncrease flow rateOrganic active ingredientsAnaestheticsSodium acetateArticaine Hydrochloride
The invention relates to the related technical field of anesthetics, and particularly discloses a preparation method of an anesthetic. The preparation method mainly comprises the following steps: (1)adding injection water with a temperature of 75-80 DEG C into a material mixing tank; (2) adjusting a target temperature of a thermostat to 25-30 DEG C, and pumping liquid nitrogen into an inlet section of a gas guide channel; (3) after the temperature is stabilized at the target temperature, weighing materials, adding sodium metabisulfite and disodium edetate into a feeding hopper, and then adding sodium acetate and acetic acid; and then adding articaine hydrochloride and sodium chloride, and dissolving the materials in the injection water; (4) adding medicinal charcoal into the feeding hopper, performing uniform stirring, stopping pumping liquid nitrogen, performing standing for 5-10 minutes, and performing circulating charcoal removal for 10-15 minutes; (5) preparing a 0.1 mol / L HCl solution, and dissolving weighed epinephrine into the prepared hydrochloric acid solution; and (6) adding the solution obtained in the step (5) into the material solution obtained in the step (4), continuously adding injection water, and performing filtering with a 0.22 [mu]m micro-porous filter element. The method can accelerate the cooling speed of the injection water.
Owner:GUIZHOU BEIKE FACTORR BIOTECH CO LTD
Sodium cantharidate and preparation method thereof
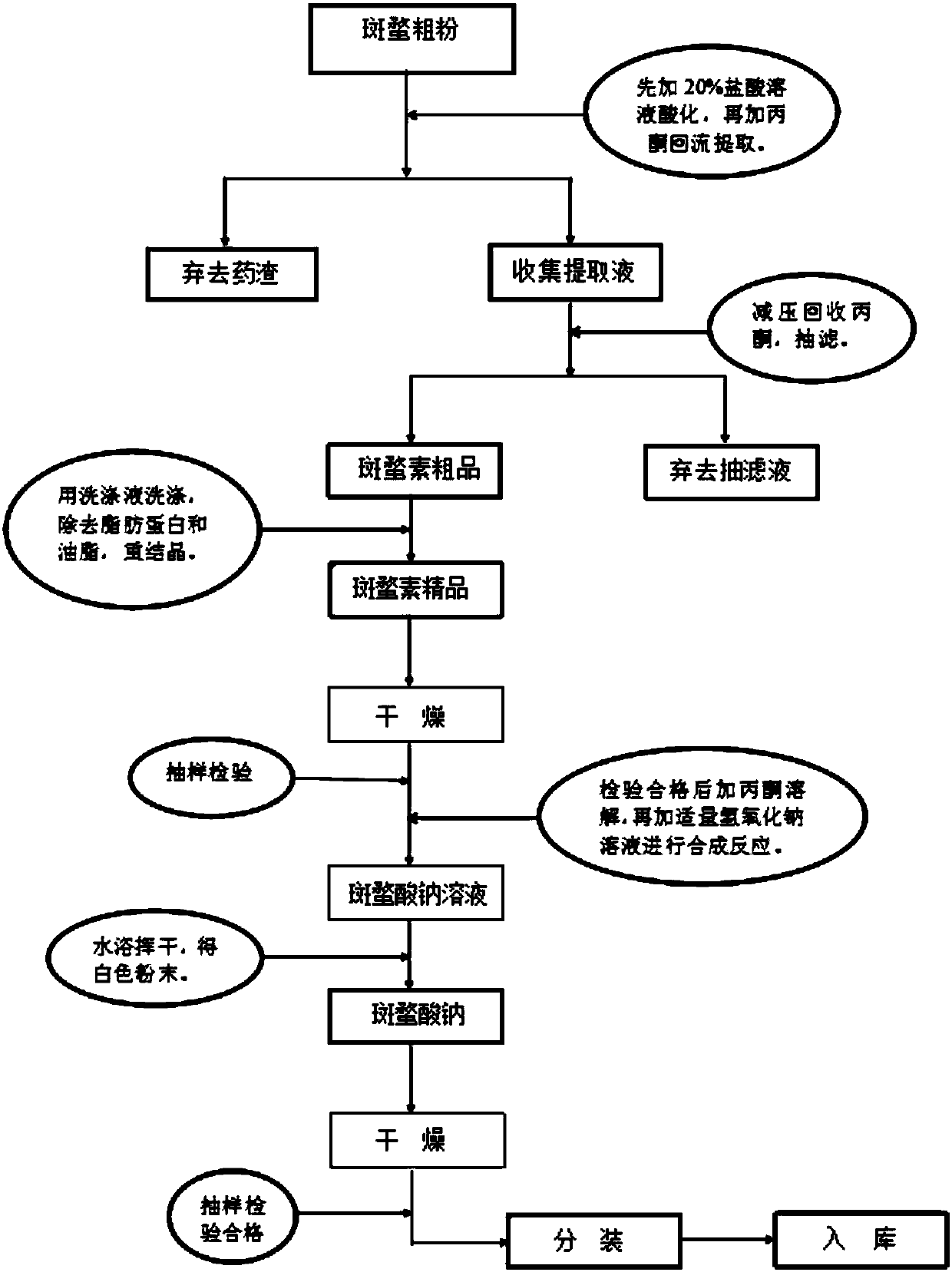
InactiveCN107936036AHigh purityDissolves excellently in waterOrganic chemistryFiltrationResource utilization
The invention discloses a sodium cantharidate and a preparation method thereof, which comprises the following components: mylabris, hydrochloric acid, acetone, absolute ethanol, petroleum ether, sodium hydroxide and medicinal charcoal; the sodium cantharidate and the preparation method thereof are weighed The coarse powder of cantharidin is extracted by reflux in an explosion-proof glass dipping tank, the extract is collected and the filtrate is discarded, washed and impurities are removed to obtain the crude cantharidin, which is dissolved in acetone and then recrystallized. The acetone is evaporated until the crystals are separated out, and the obtained product is obtained by suction filtration. Cantharidin high-quality goods, slowly drip into sodium hydroxide solution, after the reaction is complete, add medicinal charcoal aqueous solution, and suction filter while it is hot to obtain sodium cantharidate solution, then dry it, and actually make sodium cantharidate fine powder The purity is high, and the effect of dissolving in water is better, so that the utilization rate of resources in the process of use is higher, and the bioabsorption rate also increases accordingly. The preparation method uses exquisite materials, and the actual production capacity is outstanding. Chemical recovery rate.
Owner:GUIZHOU BAIQIANG PHARMA
Method for preparing active pharmaceutical ingredient of magnesium aspartate hydrochloride
InactiveCN106916075ASimple processSuitable for industrial productionOrganic compound preparationAmino-carboxyl compound preparationCentrifugationFiltration
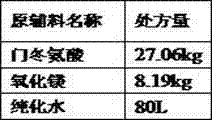
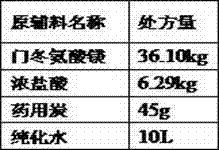
The invention discloses a method for preparing an active pharmaceutical ingredient of magnesium aspartate hydrochloride. The method comprises the following specific steps: (a) adding a formula specified volume of purified water into a solution preparation tank, carrying out heating, adding weighed magnesium oxide into the tank with stirring, adding aspartic acid into the tank in batches with stirring, and continuing to stir until the solution is clarified after adding is completed; (b) carrying out suction filtration on the reaction solution while the reaction solution is hot, carrying out depressurized concentration on filtrate so as to remove 1 / 3 to 1 / 2 of water, naturally cooling remaining liquid to room temperature so as to precipitate a large amount of white solids, and carrying out centrifugation; (c) carrying out drying, so as to obtain magnesium aspartate solids; (d) adding a specified amount magnesium aspartate and a specified volume of water into a reactor, carrying out heating with the temperature of 40 DEG C, carrying out stirring for 20 minutes, adding a hydrochloric solution into the reactor in batches, and continuing to stir until the reaction solution is clarified; (e) adding medicinal charcoal (0.1% W / V), and carrying out stirring with heating; (f) filtering medicated liquid through a decarbonization filter and a 0.45-microgram filter; and (g) carrying out spray drying, carrying out discharging, carrying out crushing, and carrying out subpackaging.
Owner:LIAONING YAOLIAN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com