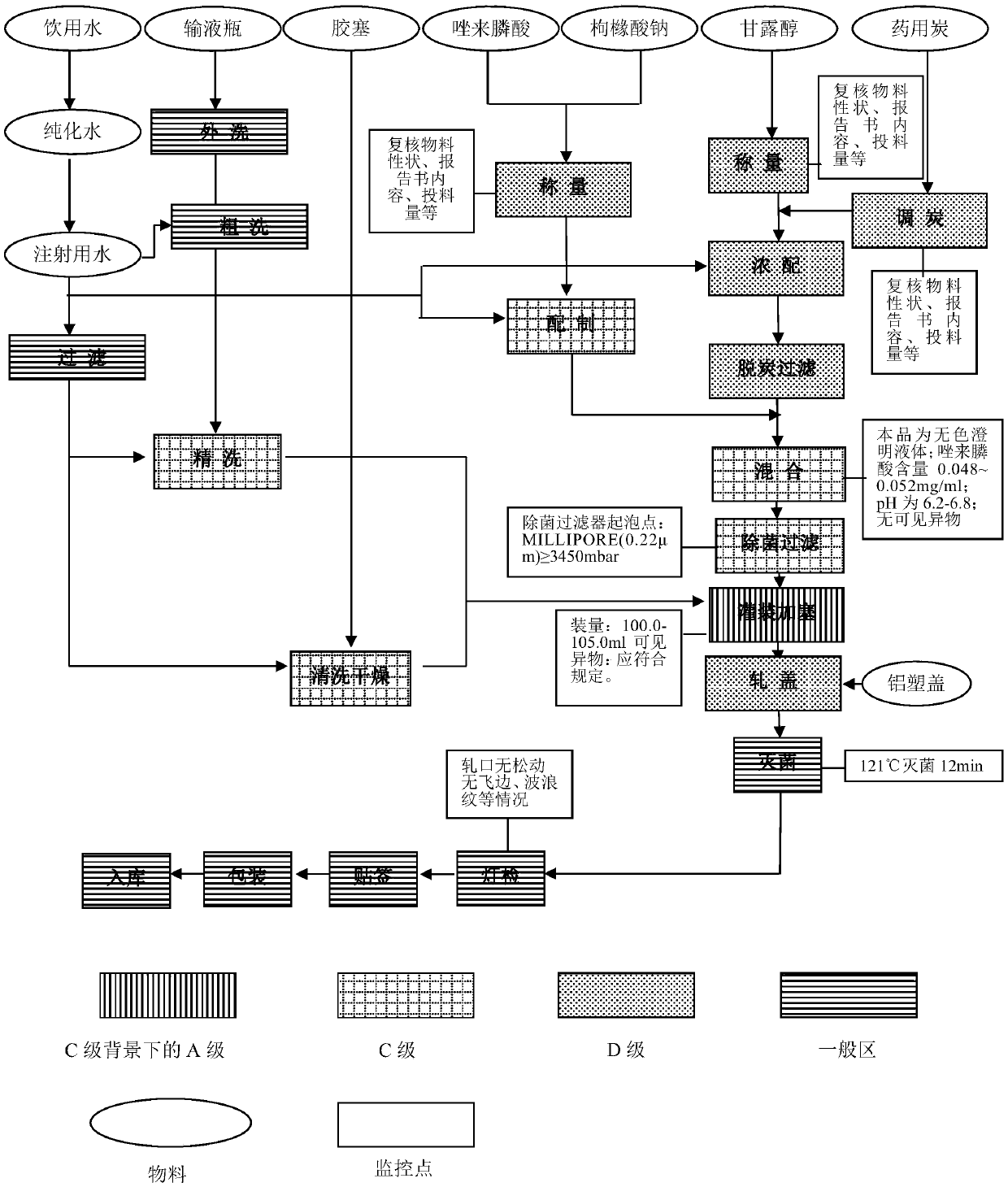
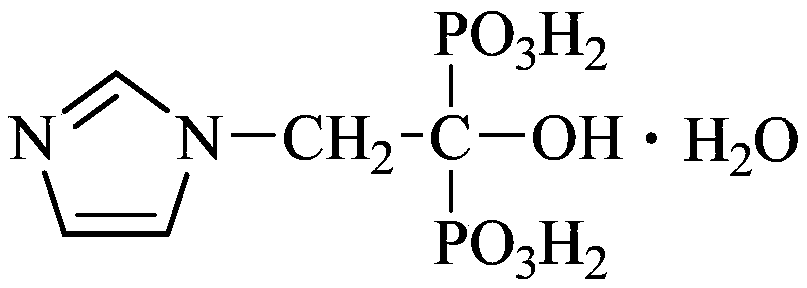Production process of zoledronic acid injection
A technology for the production of zoledronic acid, which is applied in the field of production technology for zoledronic acid injection, can solve the problems of unfavorable large-scale production and complicated procedures, and achieve the effects of high production efficiency, simple procedures and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The production process one of embodiment 1 zoledronic acid injection:
[0031] (1) Preparation process
[0032] Concentrated formulation: Inject 40% of the prescribed amount of fresh water for injection into the concentrated formulation tank, wet the weighed mannitol with fresh water for injection, transfer it to the concentrated formulation tank, stir until visually dissolved, and press 0.03 of the total volume % (W / V) Add medicinal charcoal, set the volume to the concentrated volume (60% of the prescription volume), stir at 70°C for 25 minutes, decarbonize in circulation for 15 minutes, and cool the liquid to 50°C. Pour all the liquid medicine into the dilute tank. Rinse the concentrated preparation tank with 150L water for injection and put it into the thin preparation tank.
[0033] Dilute preparation: Pour zoledronic acid and sodium citrate into a stainless steel barrel, stir and dissolve with fresh water for injection (less than 40% of the prescription volume). ...
Embodiment 2
[0042] The production process two of embodiment 2 zoledronic acid injection:
[0043] (1) Preparation process
[0044] Concentrated formulation: Inject 40% of the prescribed amount of fresh water for injection into the concentrated formulation tank, wet the weighed mannitol with fresh water for injection, transfer it to the concentrated formulation tank, stir until visually dissolved, and press 0.03 of the total volume % (W / V) Add medicinal charcoal, set the volume to the concentrated volume (60% of the prescription volume), stir at 75°C for 20min, decarbonize in circulation for 20min, and cool the liquid to 55°C. Pour all the liquid medicine into the dilute tank. Rinse the concentrated preparation tank with 150L water for injection and put it into the thin preparation tank.
[0045] Dilute preparation: Pour zoledronic acid and sodium citrate into a stainless steel barrel, stir and dissolve with fresh water for injection (less than 40% of the prescription volume). Transfer ...
Embodiment 3
[0054] Embodiment 3 Production process three of zoledronic acid injection:
[0055] (1) Preparation process
[0056] Concentrated formulation: Inject 40% of the prescribed amount of fresh water for injection into the concentrated formulation tank, wet the weighed mannitol with fresh water for injection, transfer it to the concentrated formulation tank, stir until visually dissolved, and press 0.03 of the total volume % (W / V) Add medicinal charcoal, set the volume to the concentrated volume (60% of the prescription volume), stir at 80°C for 15 minutes, decarbonize in circulation for 25 minutes, and cool the liquid to 60°C. Pour all the liquid medicine into the dilute tank. Rinse the concentrated preparation tank with 150L water for injection and put it into the thin preparation tank.
[0057] Dilute preparation: Pour zoledronic acid and sodium citrate into a stainless steel barrel, stir and dissolve with fresh water for injection (less than 40% of the prescription volume). T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com