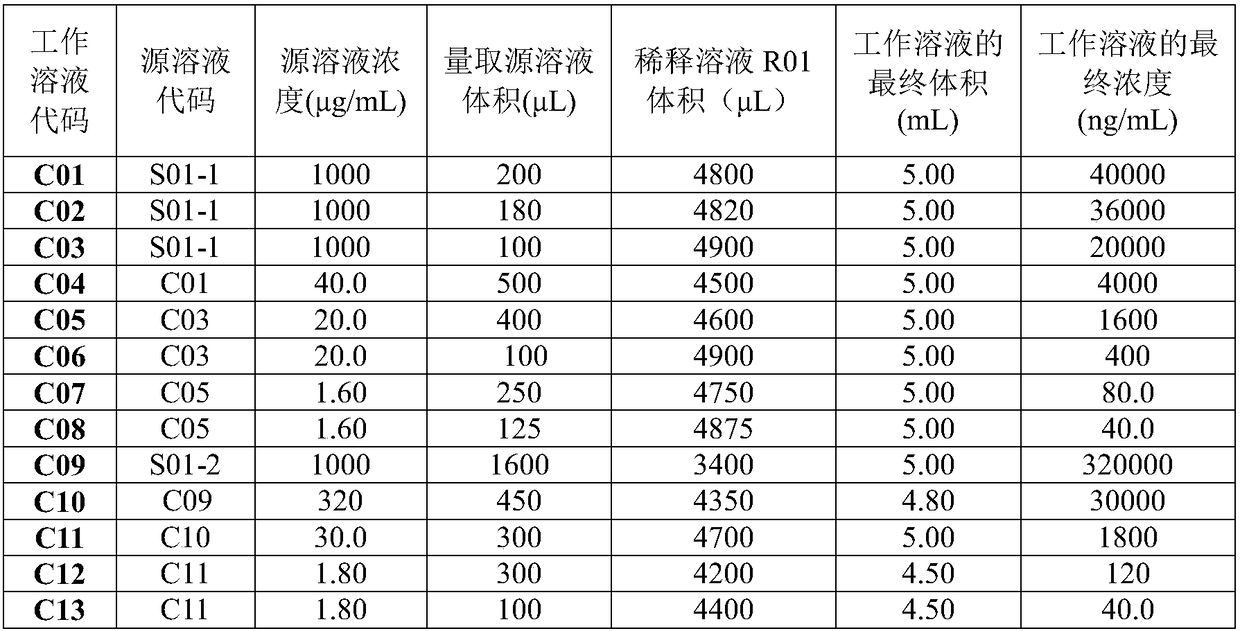
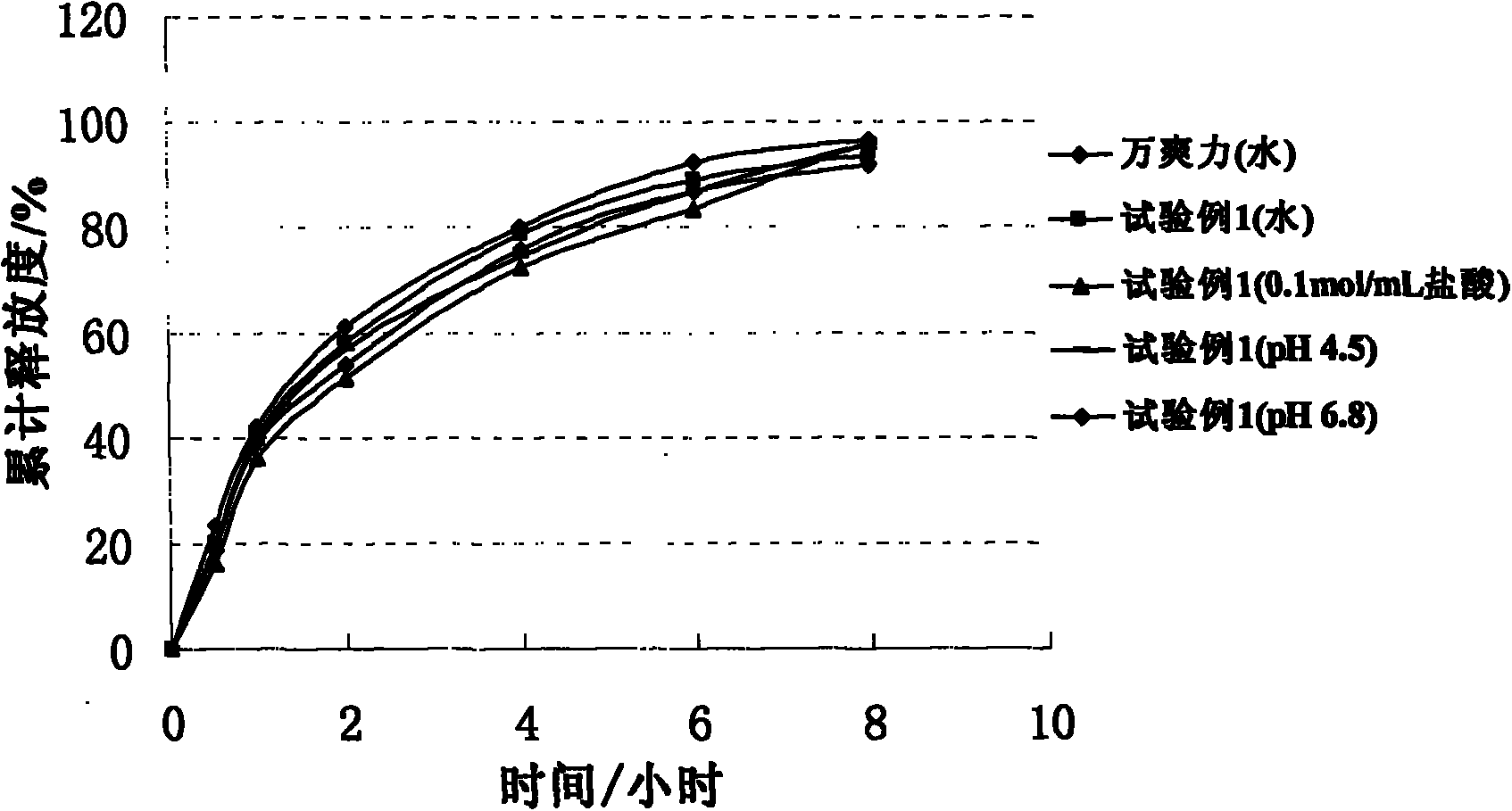
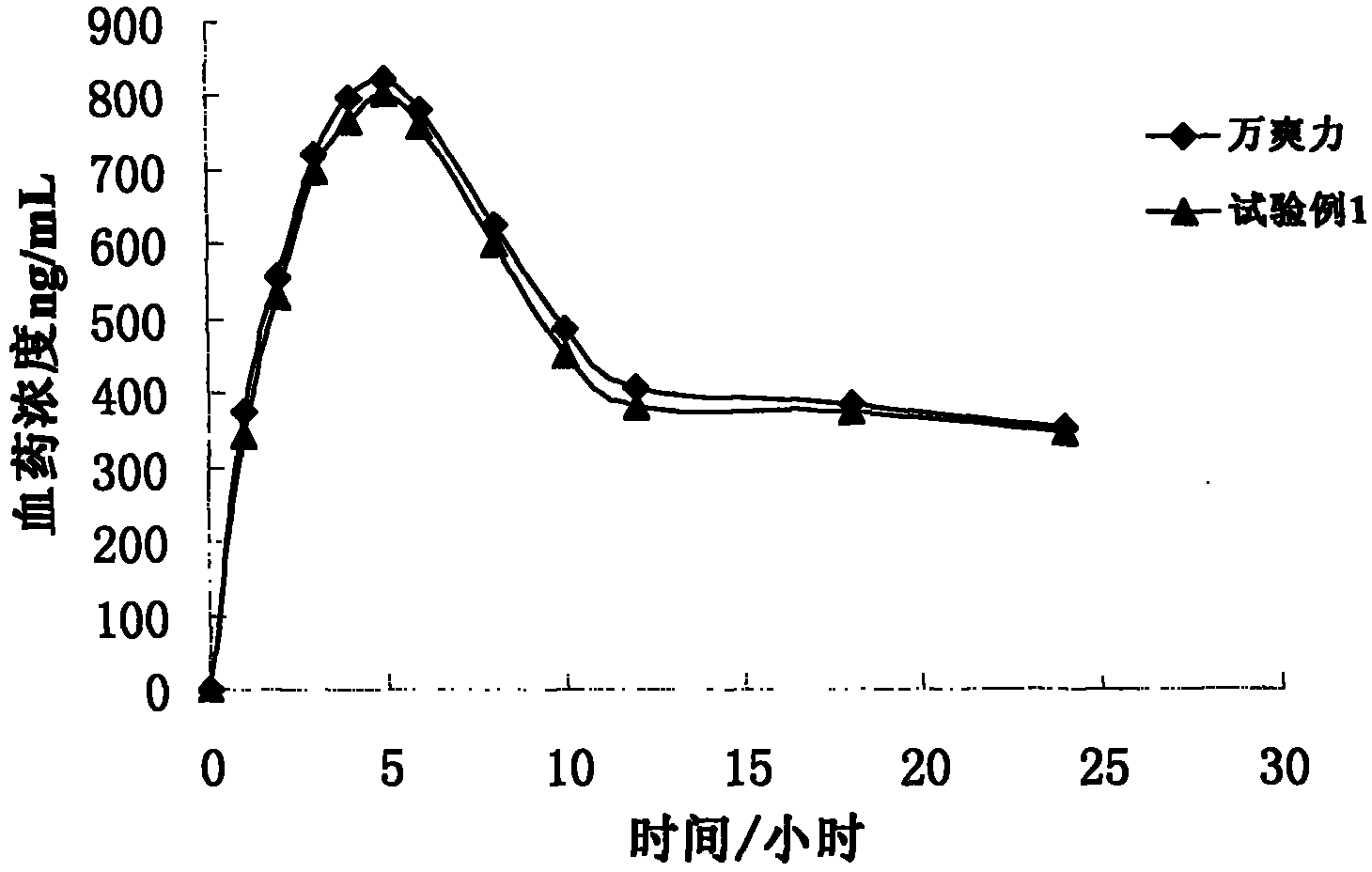
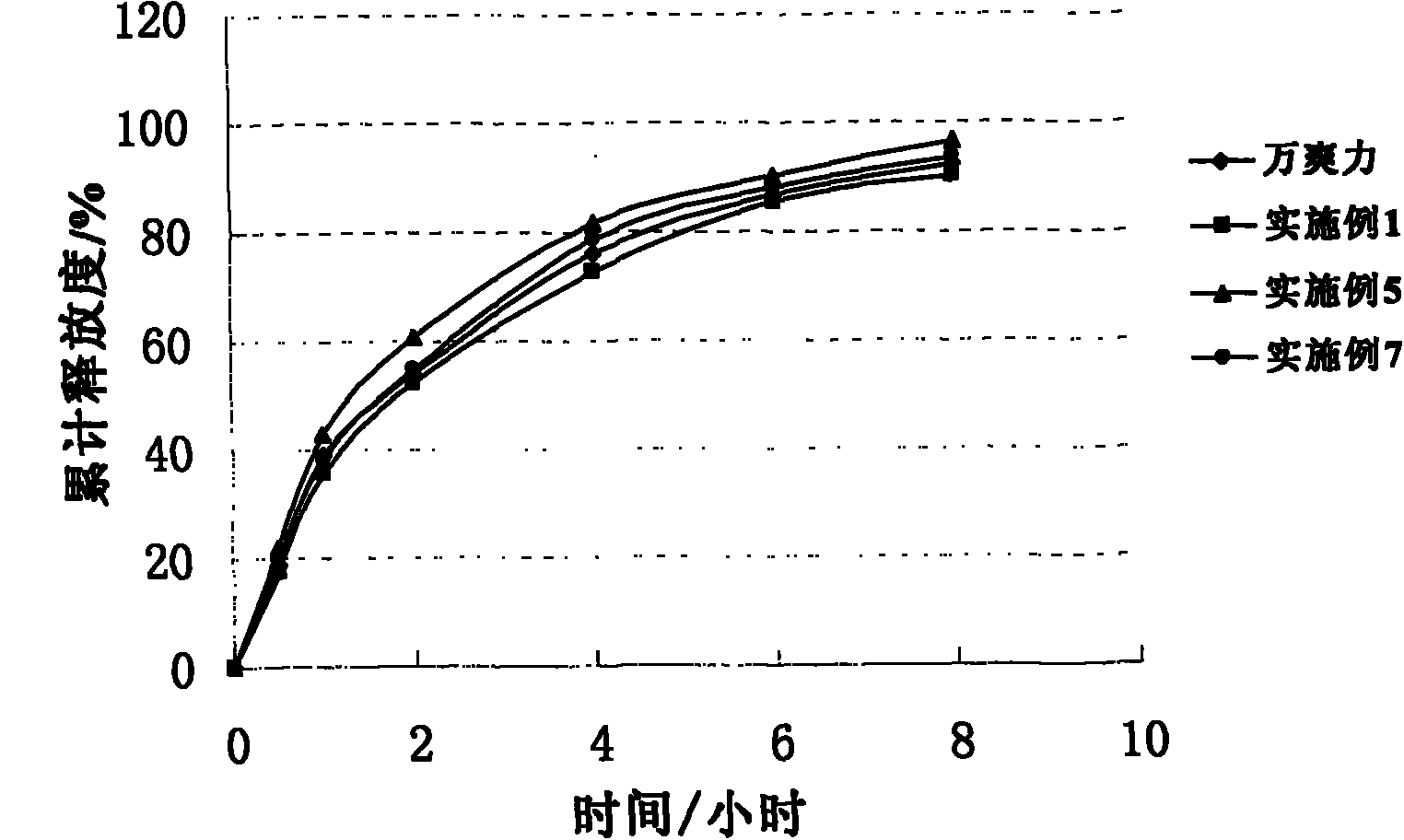
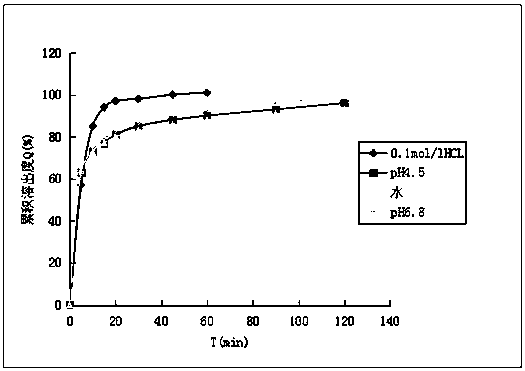
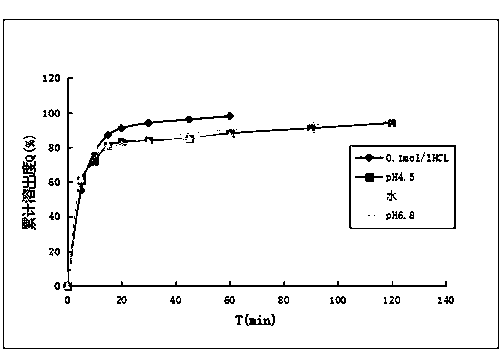
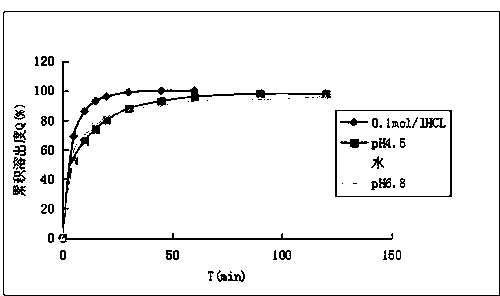
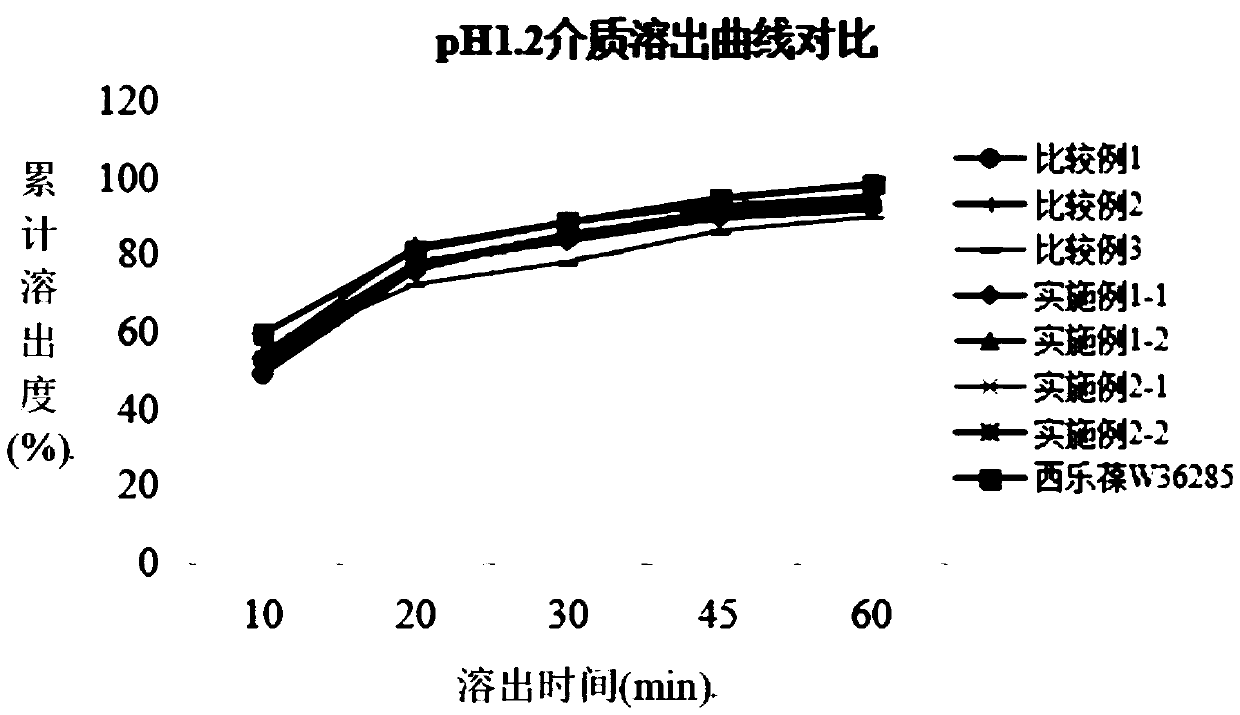
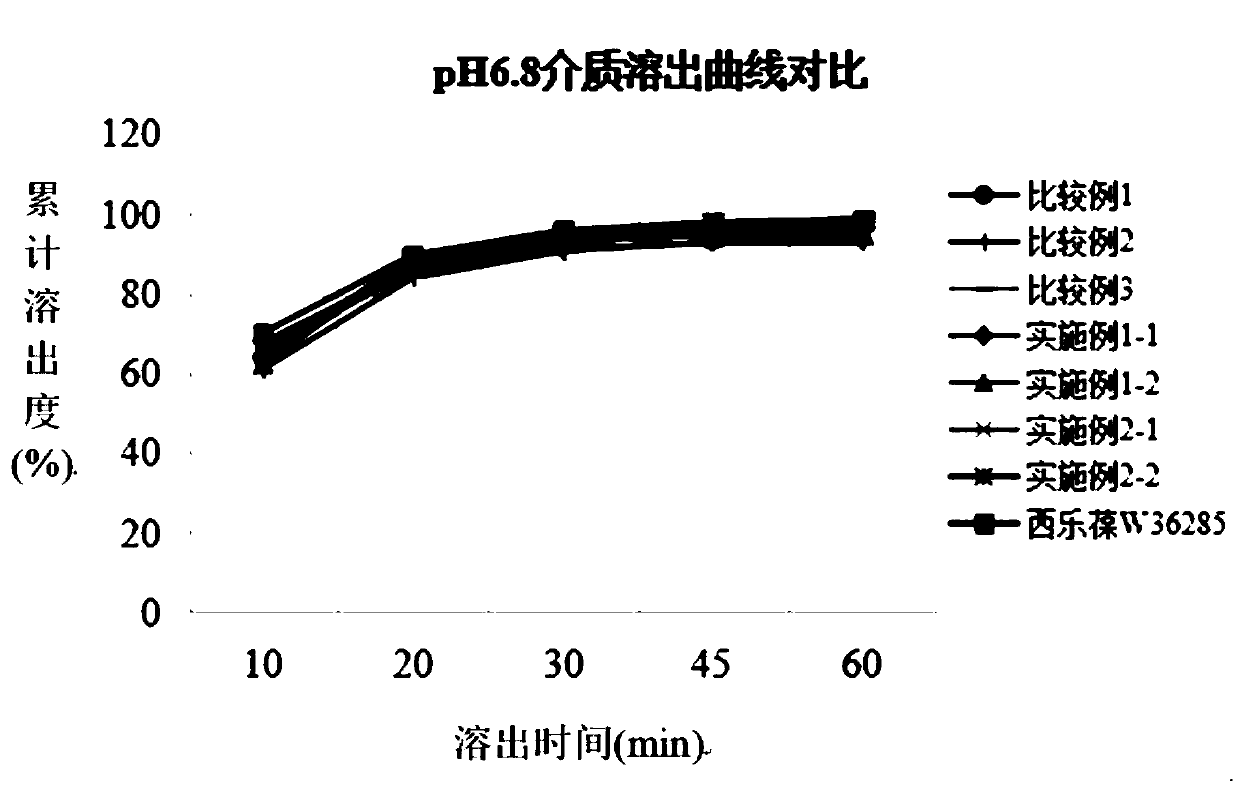
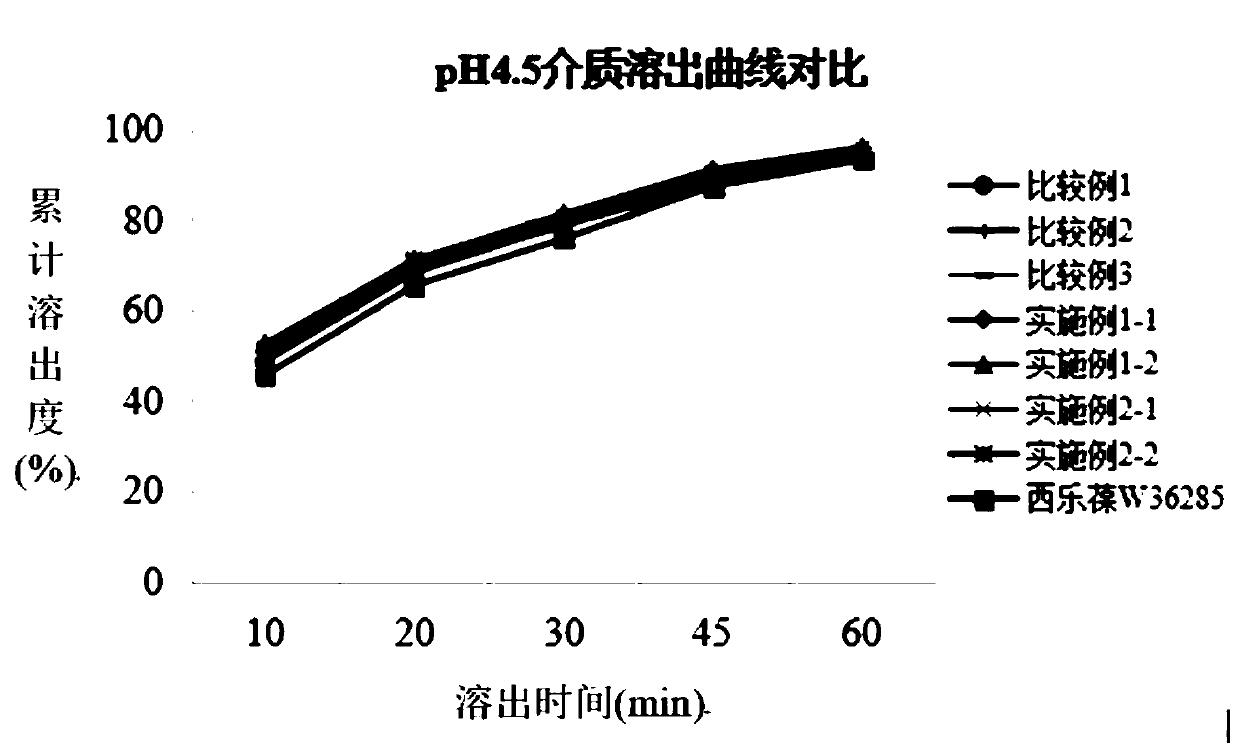
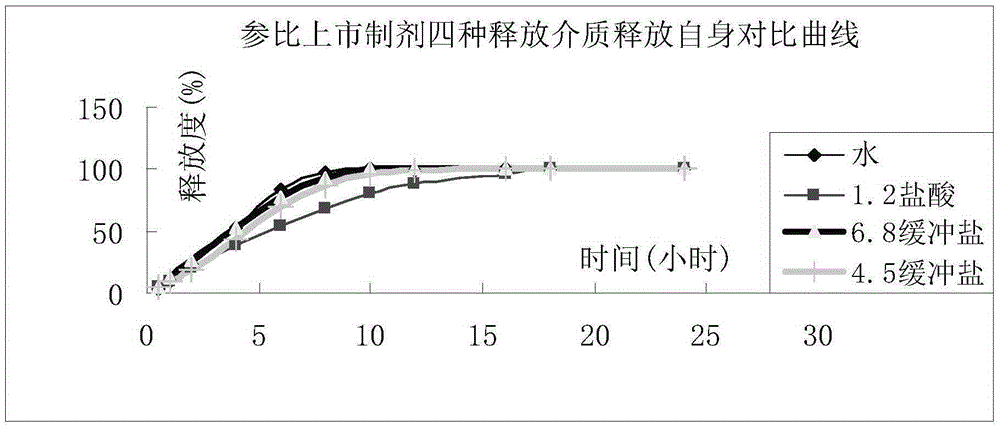
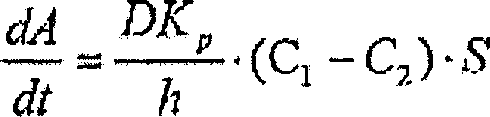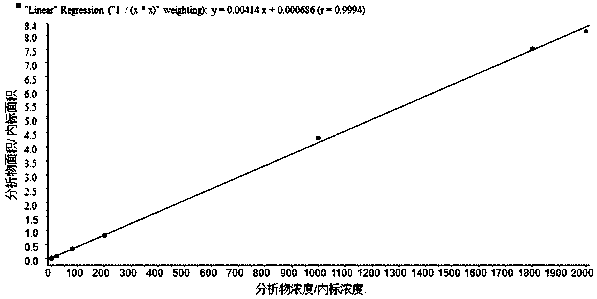Patents
Literature
127 results about "Bioequivalence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
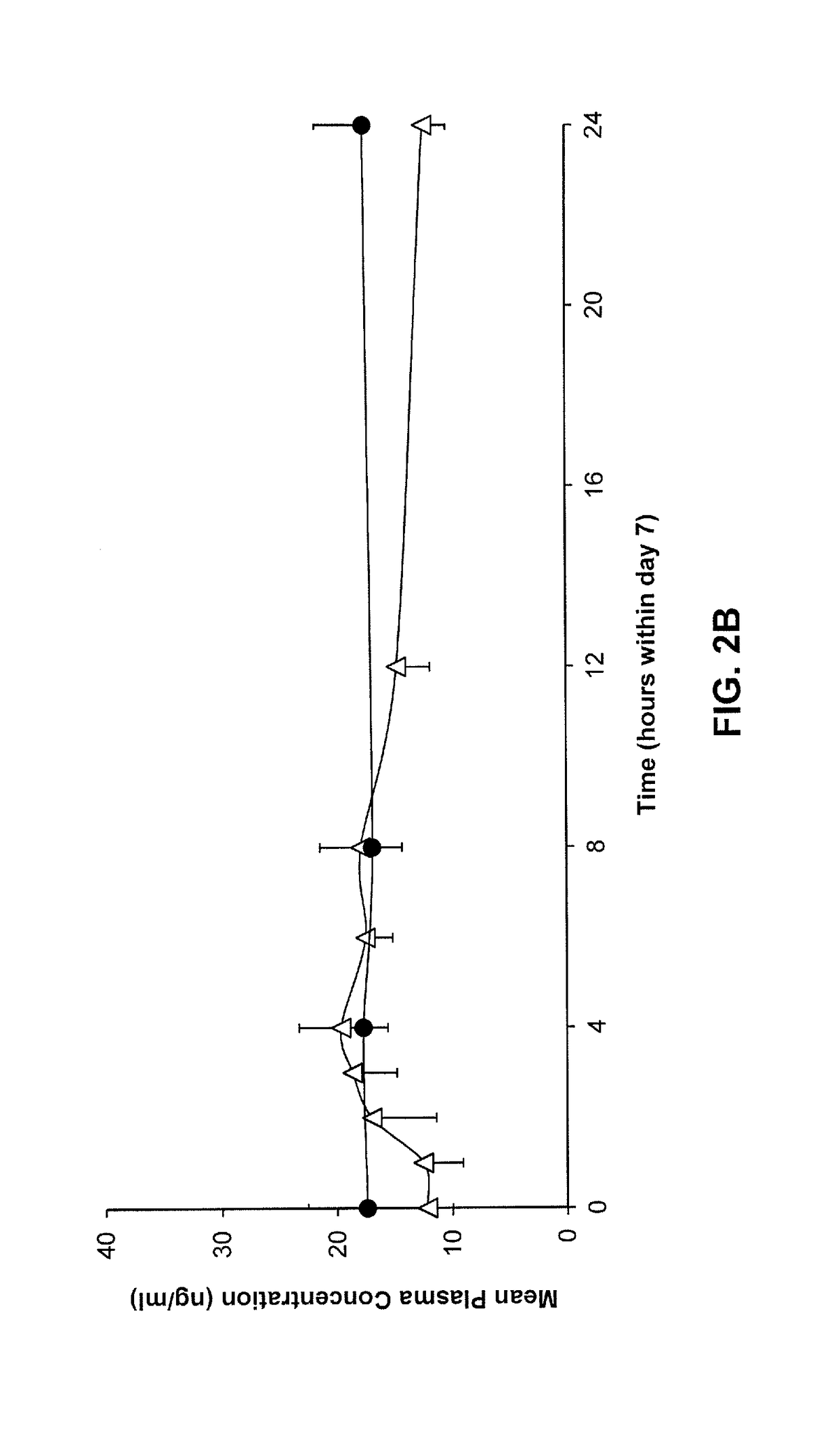
Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same.
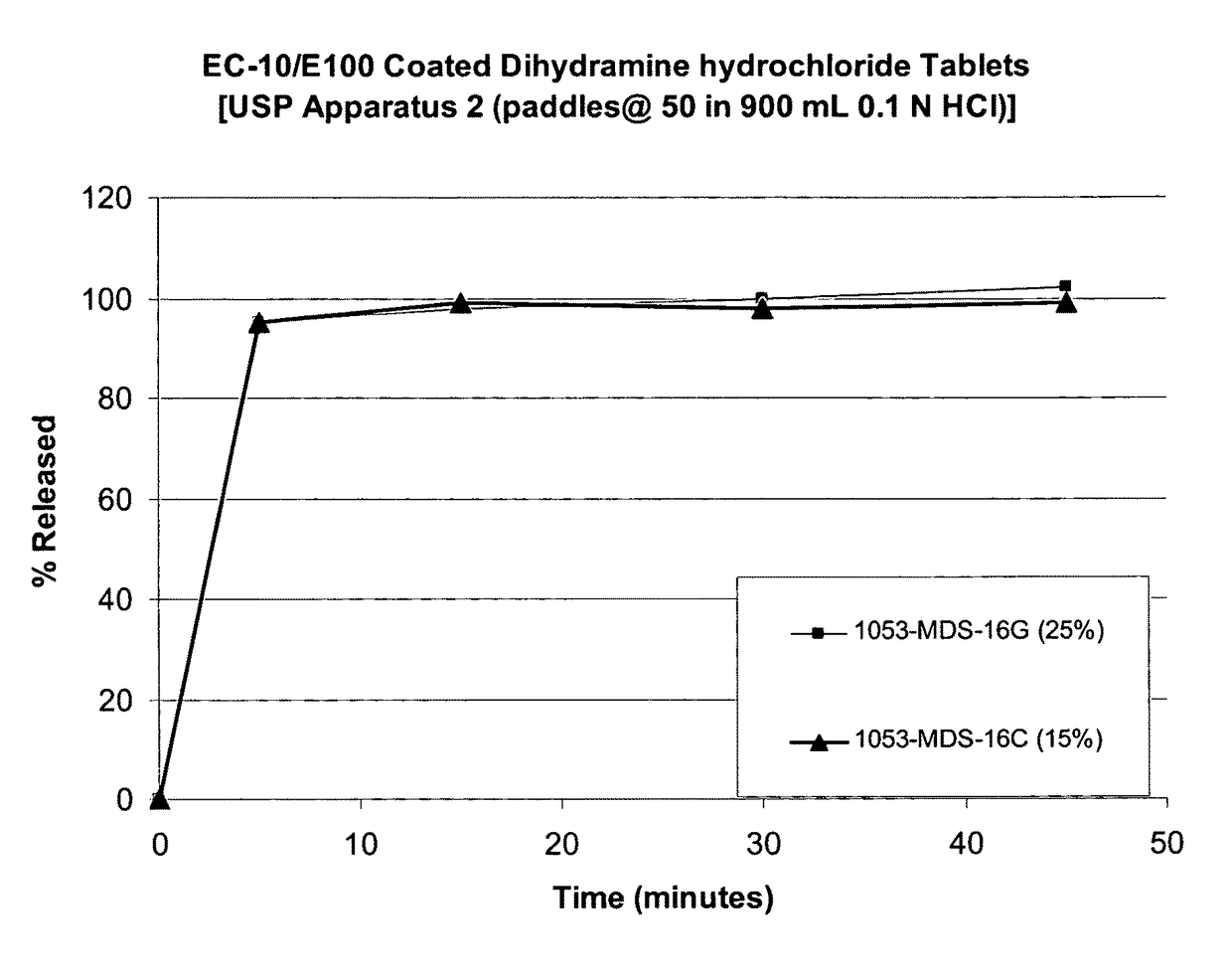
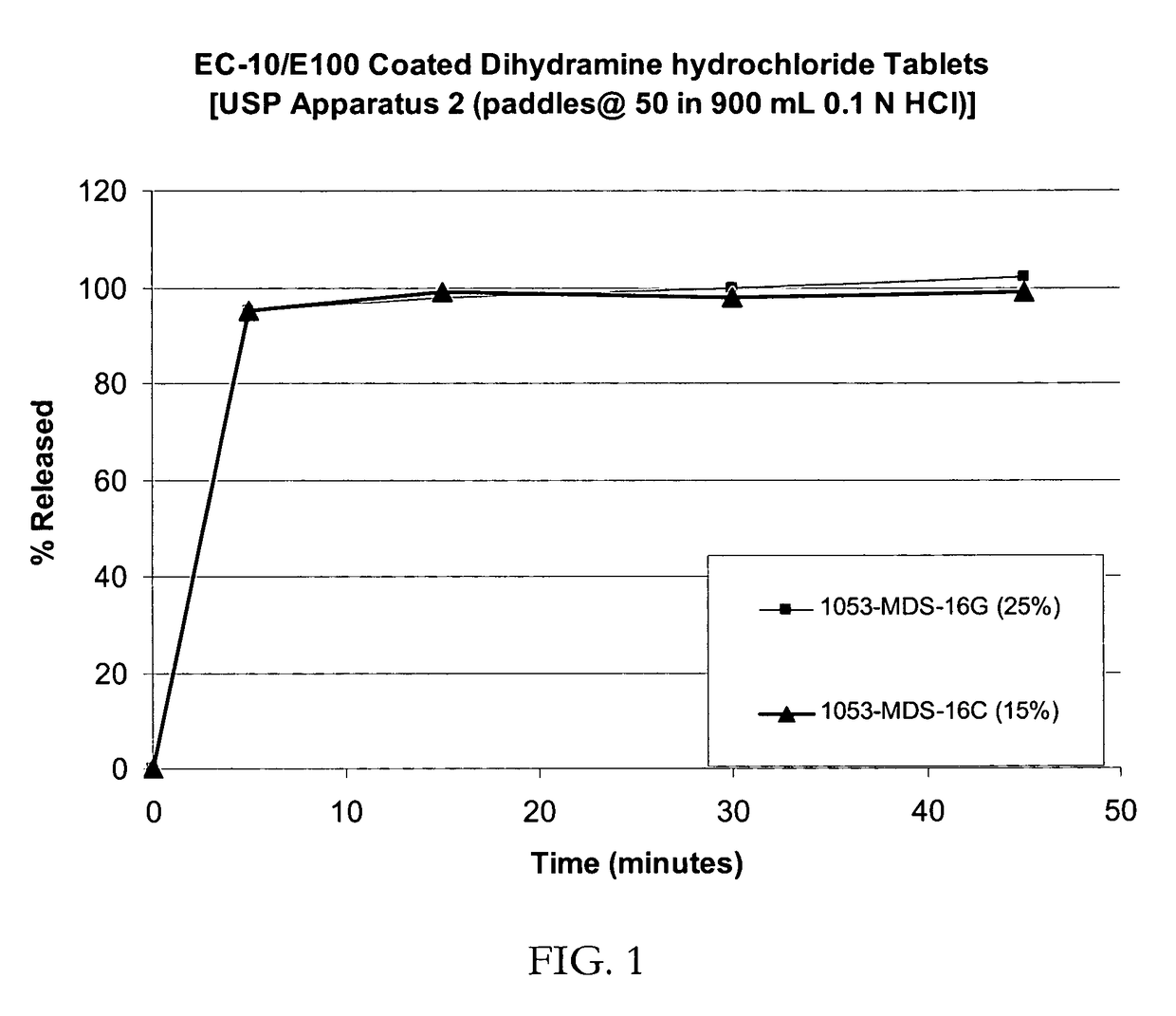
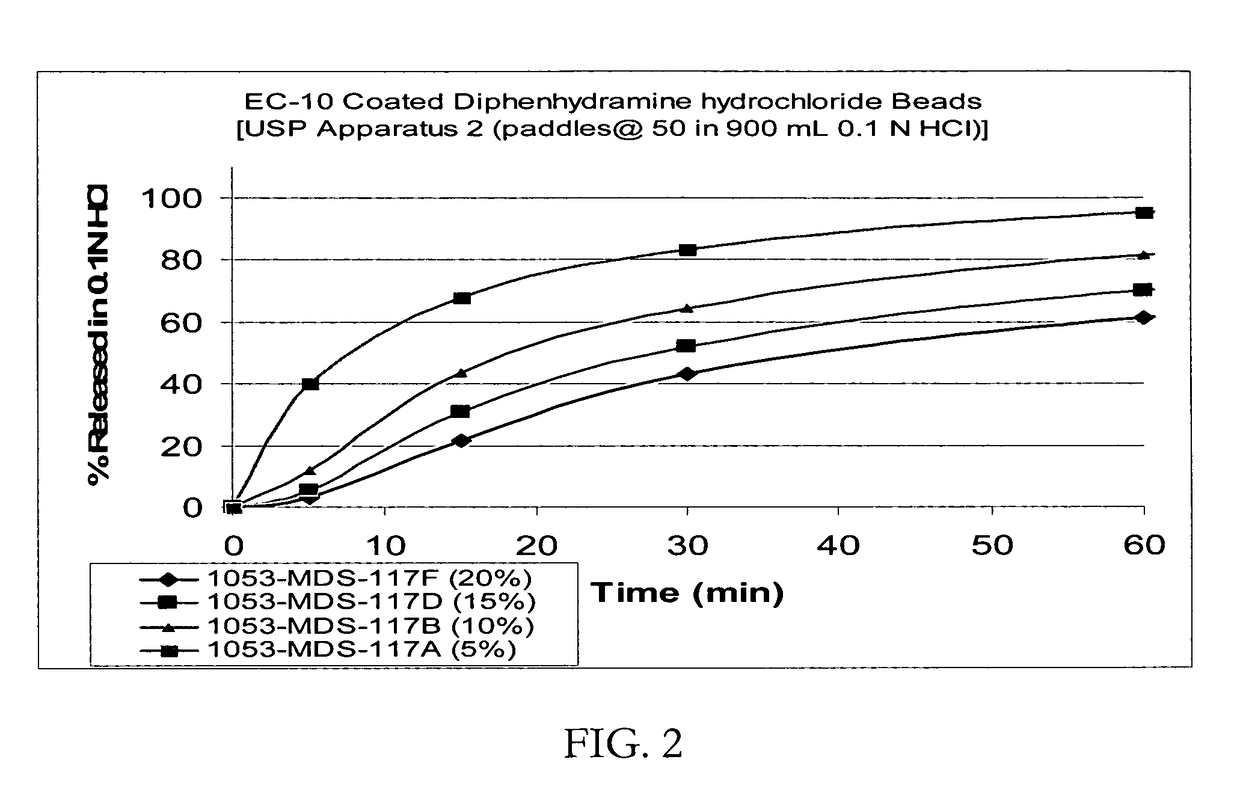
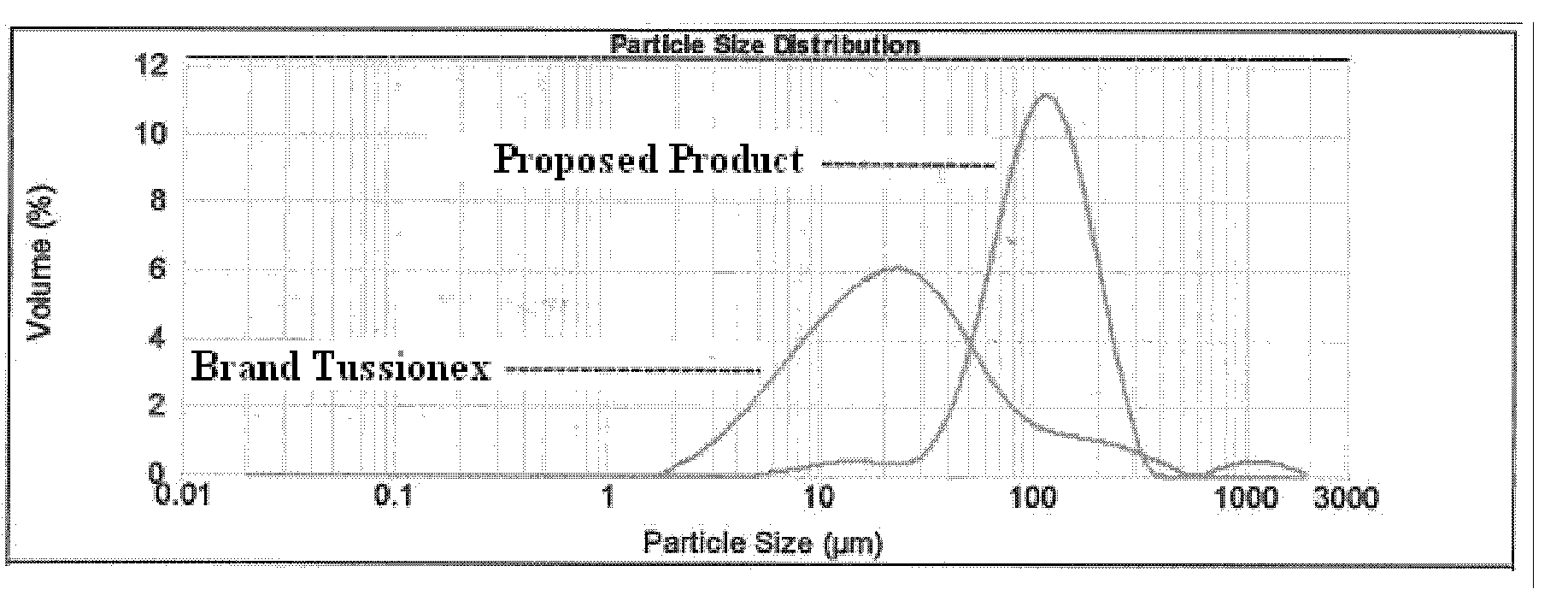
Taste-masked pharmaceutical compositions
ActiveUS20060078614A1Effective taste-maskingRapid/complete releasePill deliveryAdditive ingredientOrally disintegrating tablet
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredient(s), rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates on contact with saliva in the buccal cavity in about 60 seconds forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing the active) applied with a taste-masking membrane comprising a combination of water-insoluble and gastrosoluble polymers release not less than about 60% of the dose is in the stomach in about 30 minutes, thus maximizing the probability of achieving bioequivalence to the reference IR product having rapid onset of action (short Tmax). A process for preparing such compositions for oral administration using conventional fluid-bed equipment and rotary tablet press is also disclosed.
Owner:ADARE PHARM INC
Bioequivalence determination using expression profiling
The present invention provides methods to use expression profiles to determine the bioequivalence of two or more pharmaceutically equivalent drug formulations. In addition, this invention provides methods to select drug formulations that are able to substitute for standard drug formulations without change in clinical efficacy. In other embodiments, this invention provides computer systems, kits and databases for carrying out the methods of the invention.
Owner:DRESSMAN MARLENE MICHELLE +3
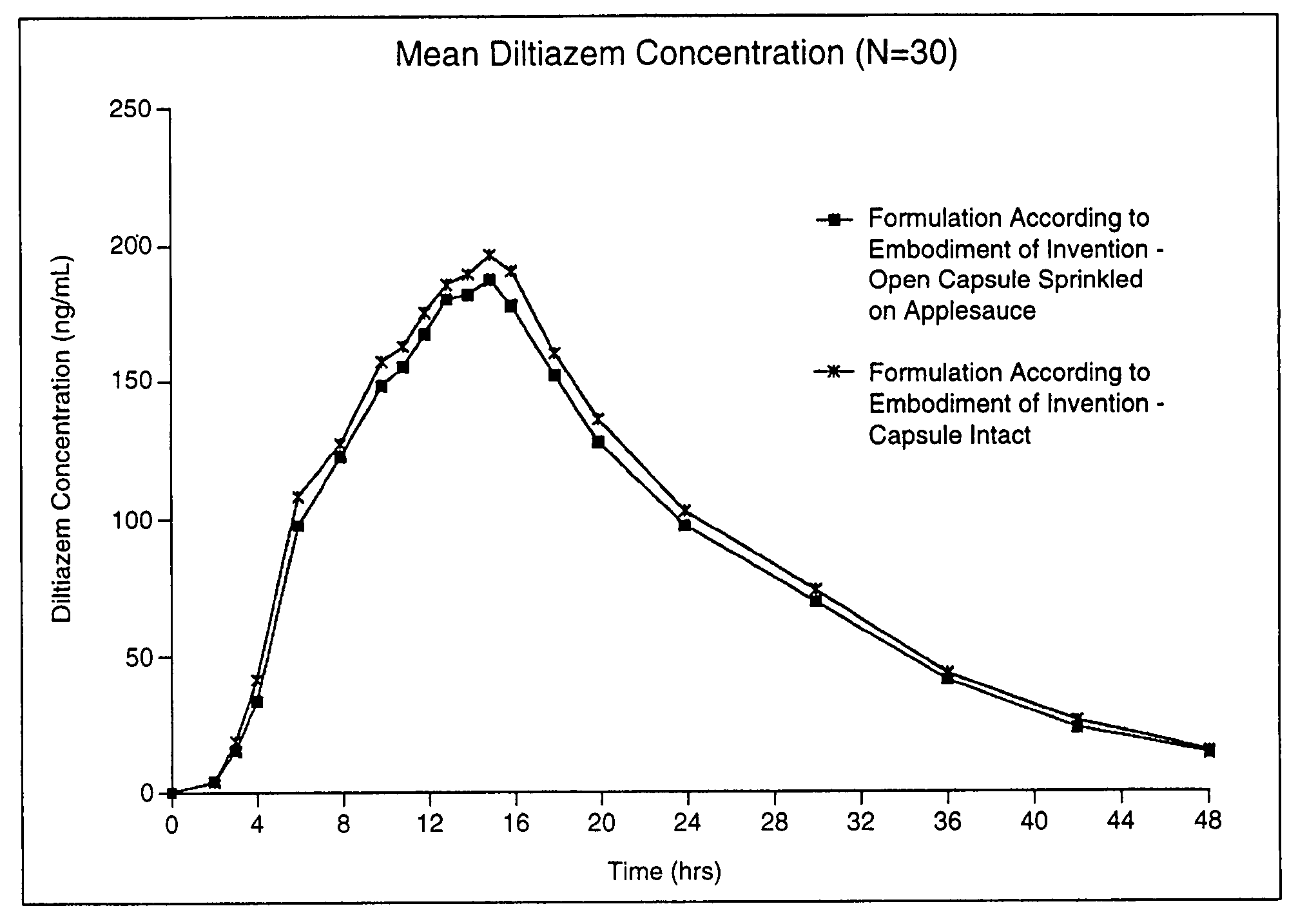
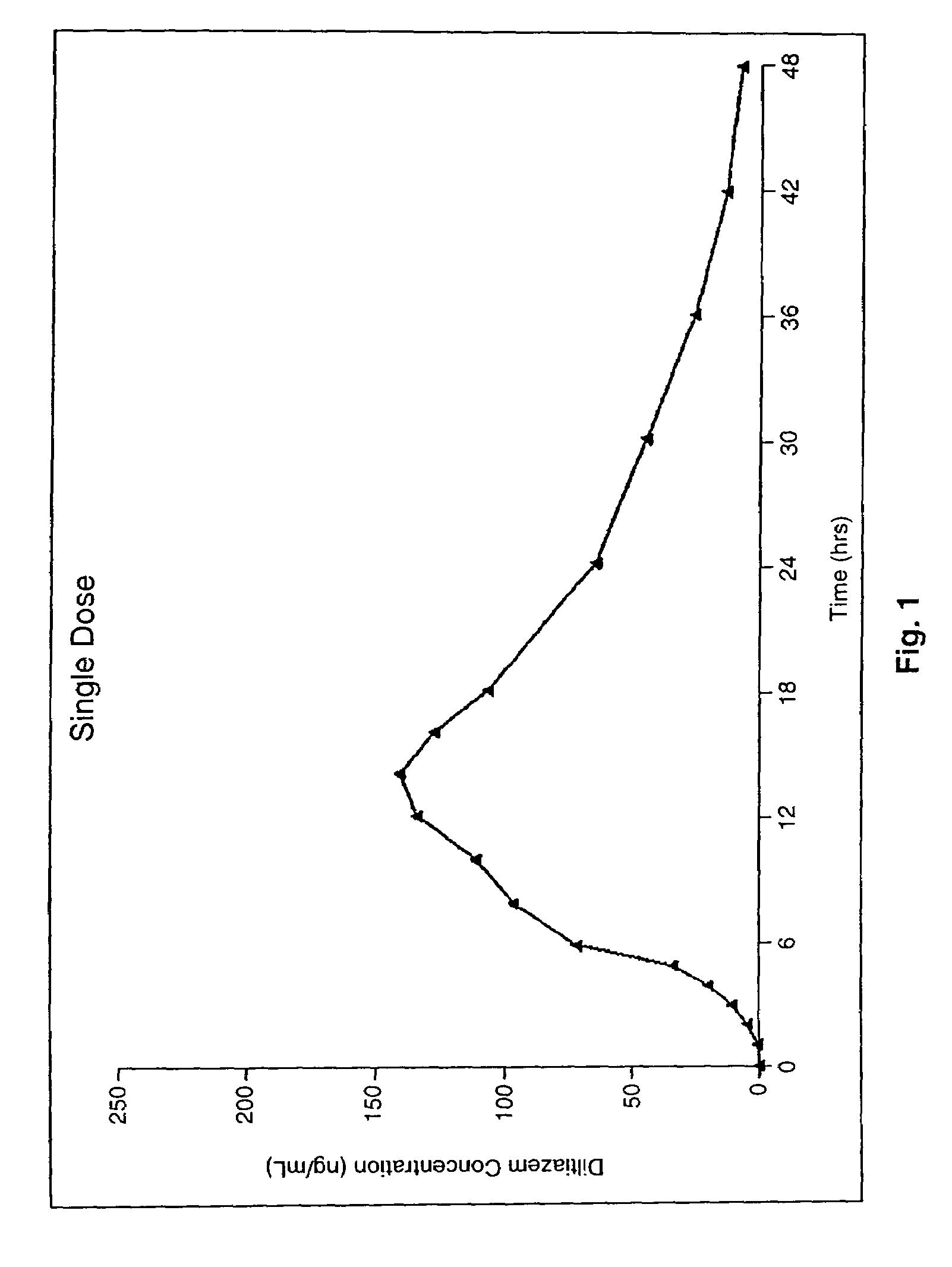
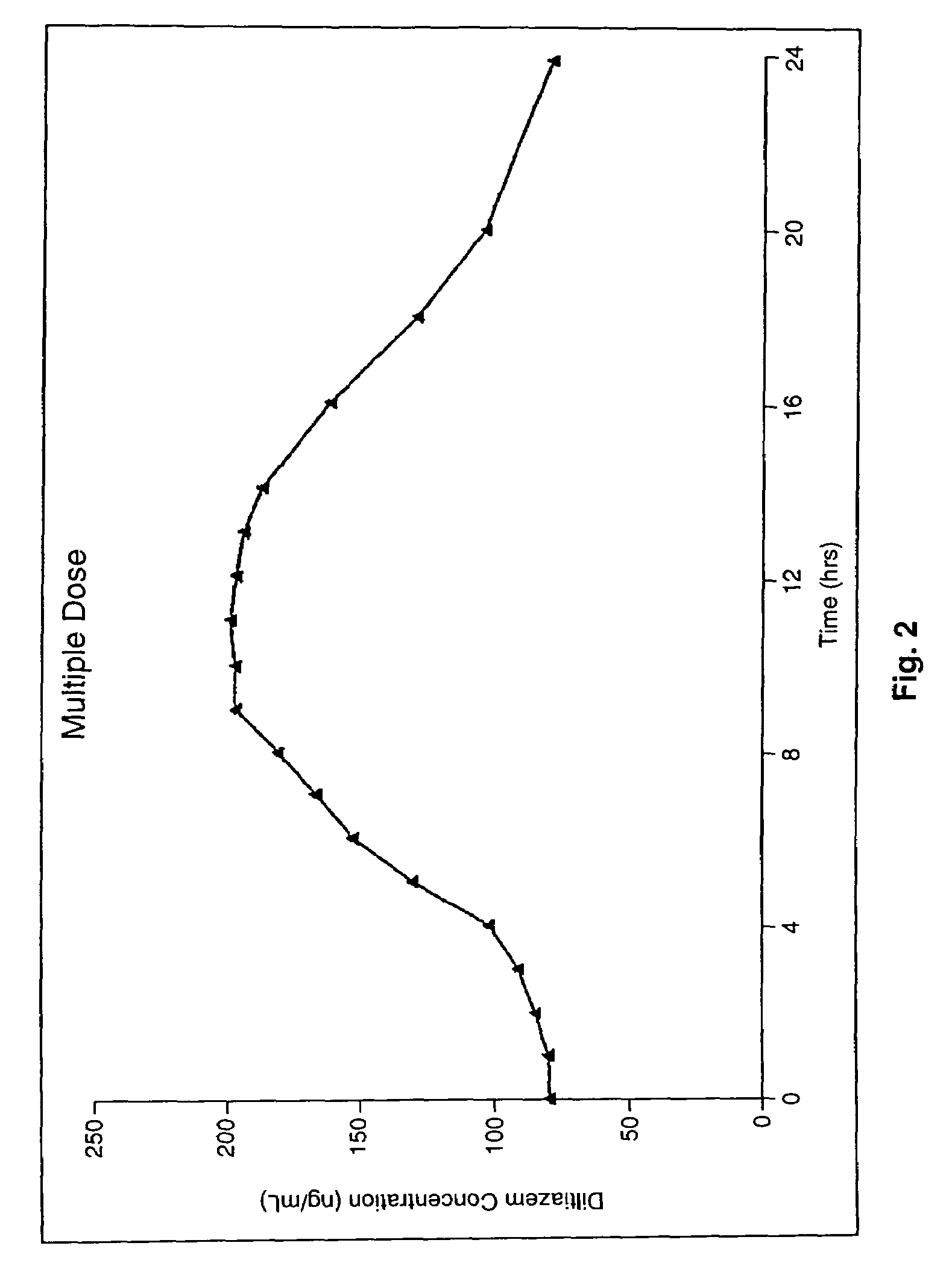
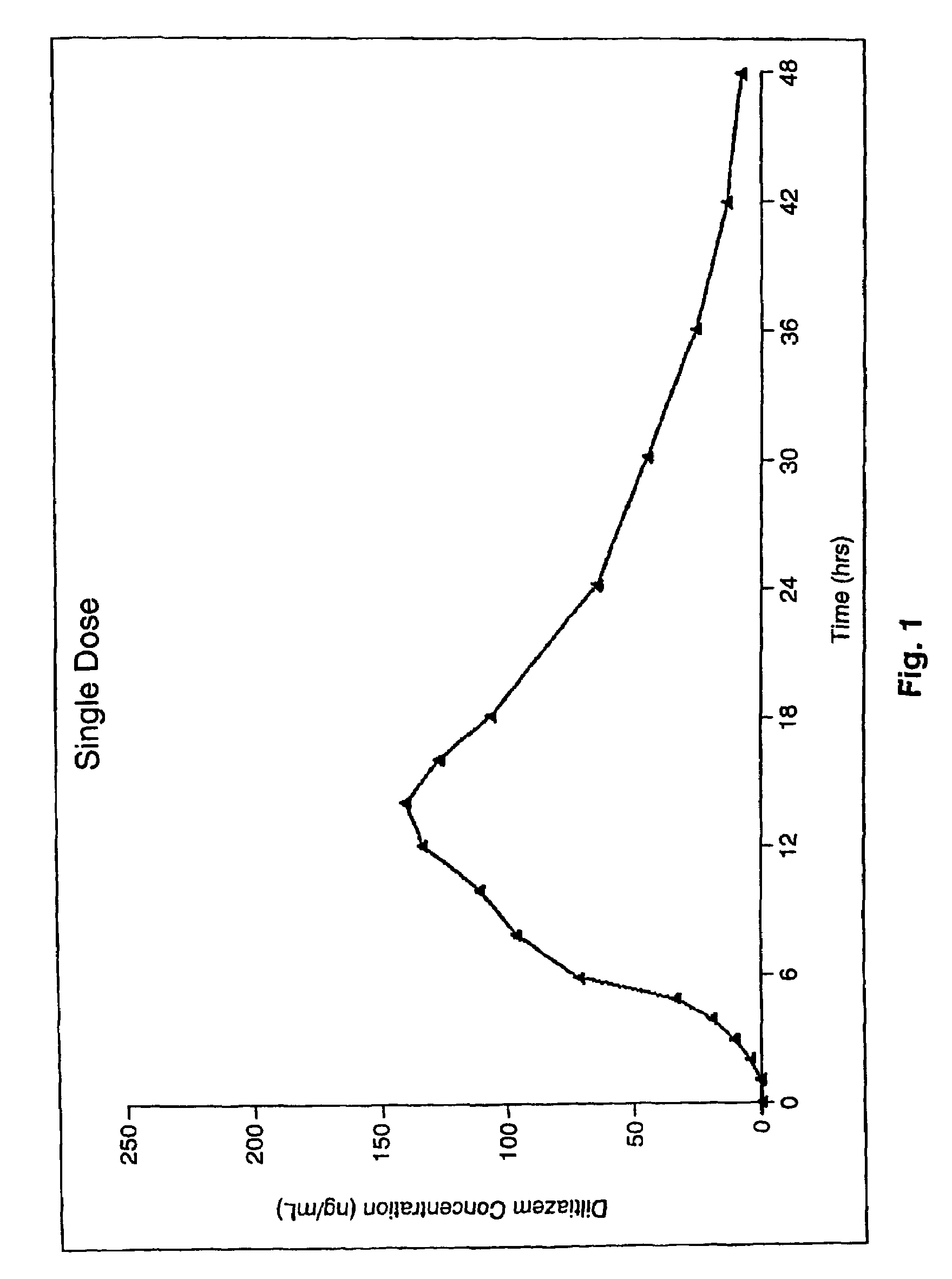
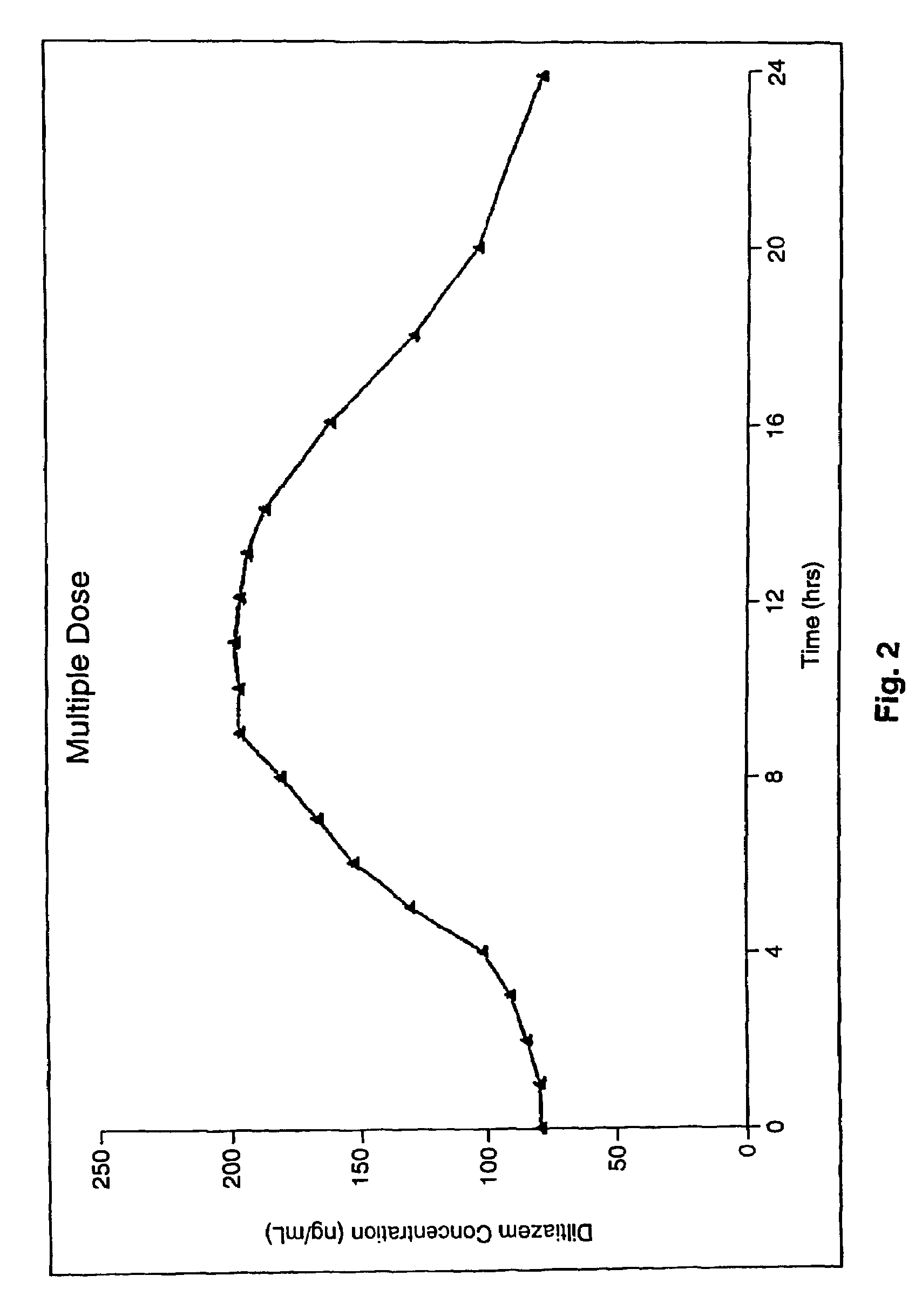
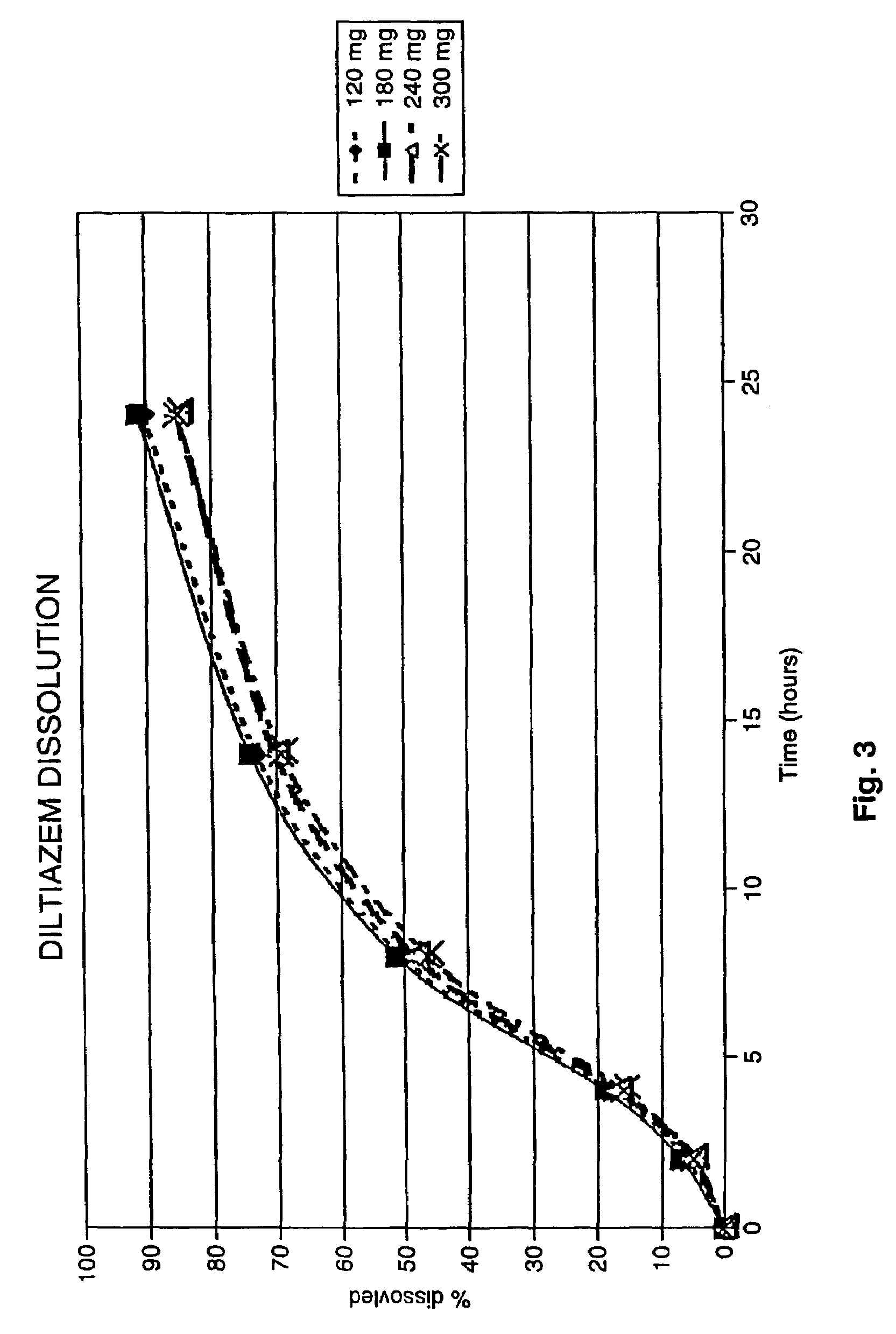
Chronotherapeutic diltiazem formulations and the administration thereof
InactiveUS7108866B1Maintain the solubility of the DiltiazemImprove bioavailabilityInorganic non-active ingredientsPill deliveryPharmacyControlled release
A controlled-release Galenical preparation of pharmaceutically acceptable Diltiazem including the pharmaceutically acceptable salts thereof, suitable for evening dosing every 24 hours containing from about 120 mg to about 540 mg or more (as desired) of the form of Diltiazem associated with excipients to provide controlled (sustained) release of the form of Diltiazem for providing a Cmax of Diltiazem in the blood at between about 10 hours and about 15 hours after administration, the preparation comprising the form of Diltiazem in oral sustained-release dosage form in which the Diltiazem is adapted to be released after administration over a prolonged period of time and exhibits when given to humans(i) a higher bioavailability when given at night compared to when given in the morning without food according to FDA guidelines or criteria and(ii) bioequivalence when given in the morning with and without food according to the same FDA guidelines or criteria.
Owner:VALEANT INT BERMUDA
Chronotherapeutic diltiazem formulations and the administration thereof
InactiveUS7348028B2Improve bioavailabilityMaintain the solubility of the DiltiazemPowder deliveryPill deliveryPharmacyControlled release
A method of treating or preventing myocardial ischemia in a patient in need thereof comprising administration of a controlled-release Galenical preparation of pharmaceutically acceptable Diltiazem including the pharmaceutically acceptable salts thereof, suitable for evening dosing every 24 hours containing from about 180 mg to about 420 mg of the form of Diltiazem associated with excipients to provide controlled (sustained) release of the form of Diltiazem for providing a Cmax of Diltiazem in the blood at between about 10 hours and about 17 hours after administration, the preparation comprising the form of Diltiazem in oral sustained-release dosage form in which the Diltiazem is adapted to be released after administration over a prolonged period of time and exhibits when given to humans(i) a higher bioavailability when given at night compared to when given in the morning without food according to FDA guidelines or criteria and(ii) bioequivalence when given in the morning with and without food according to the same FDA guidelines or criteria.
Owner:CHANTILLY BIOPHARMA
Method of formulating and designing liquid drug suspensions containing ion exchange resin particles
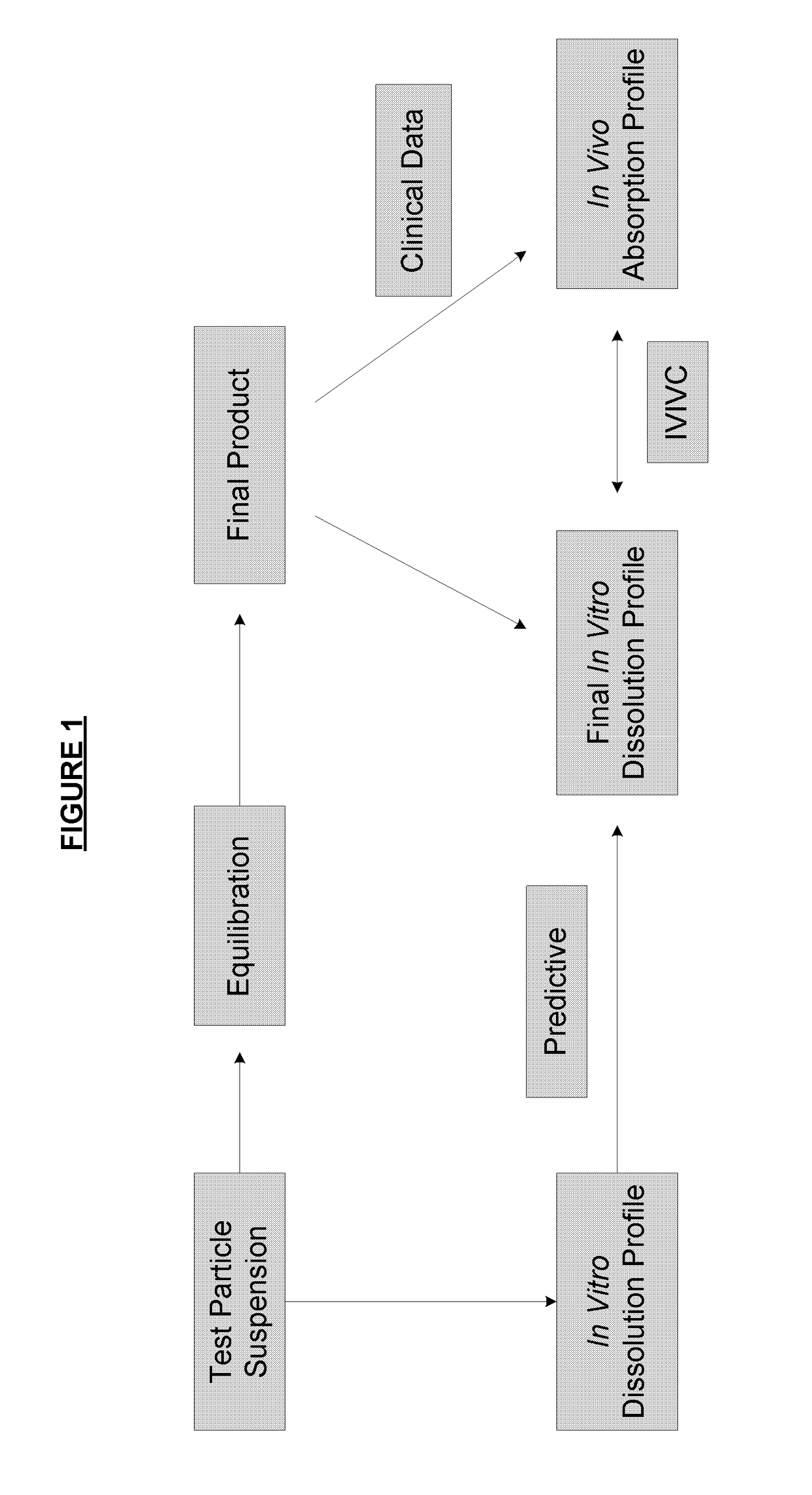
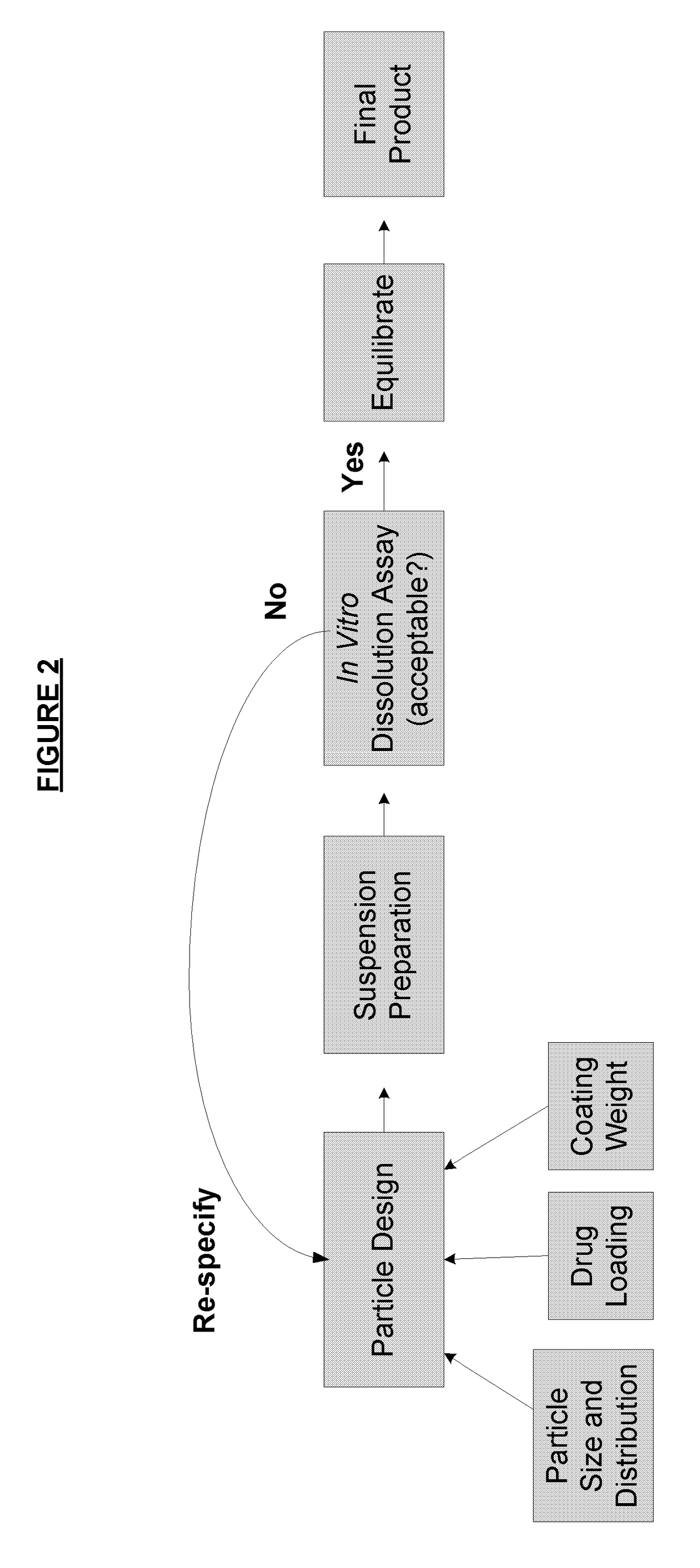
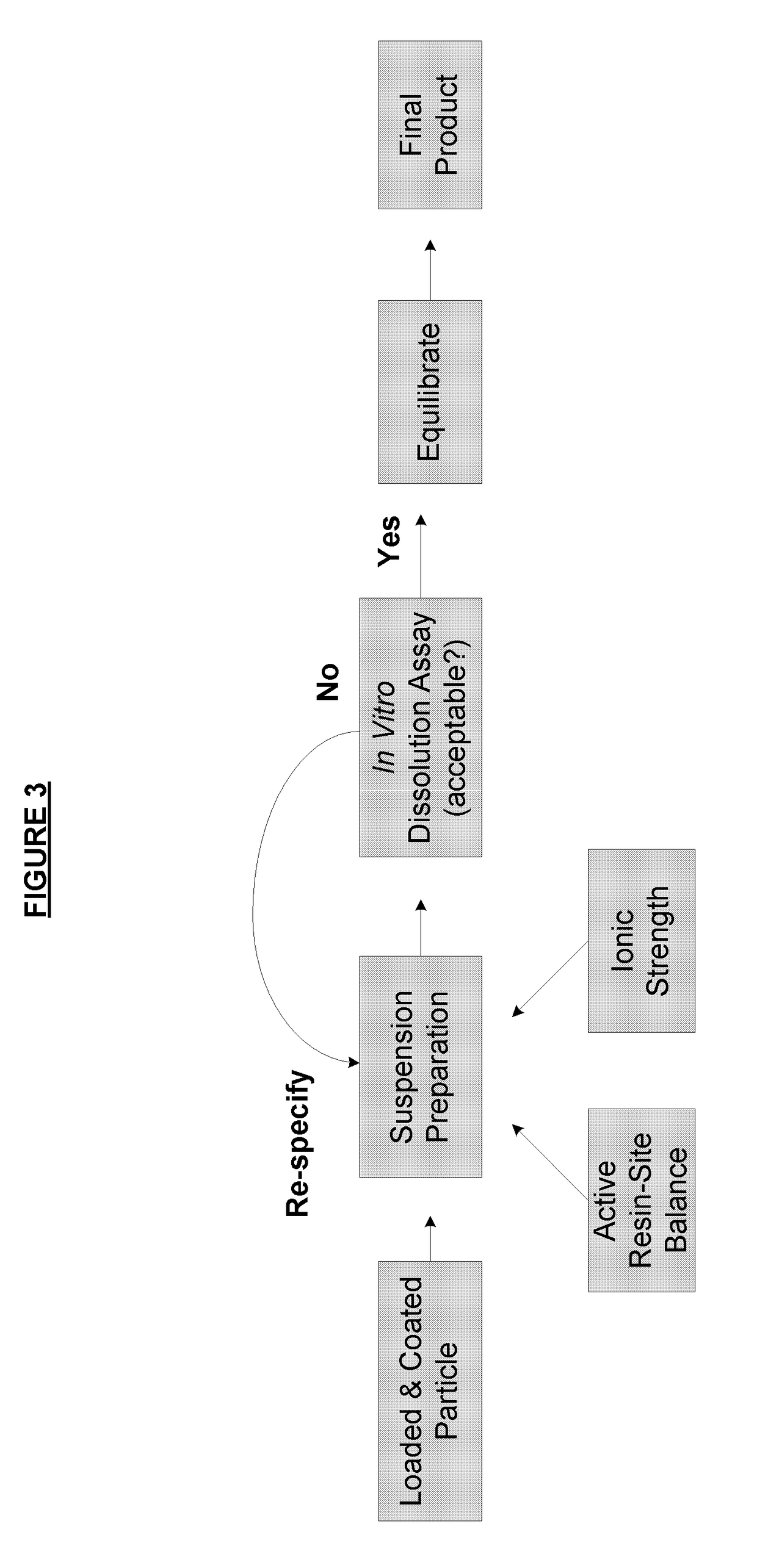
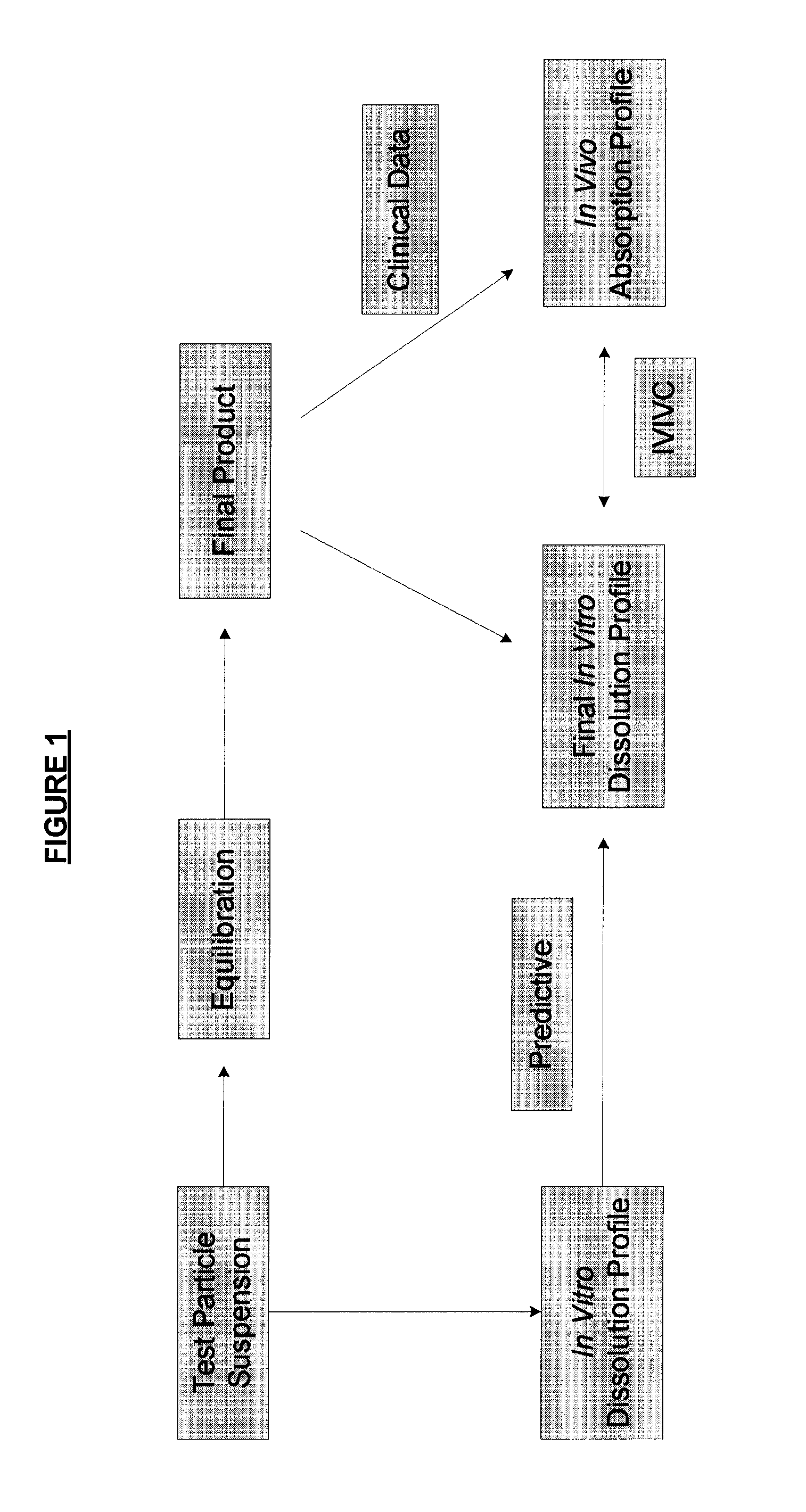
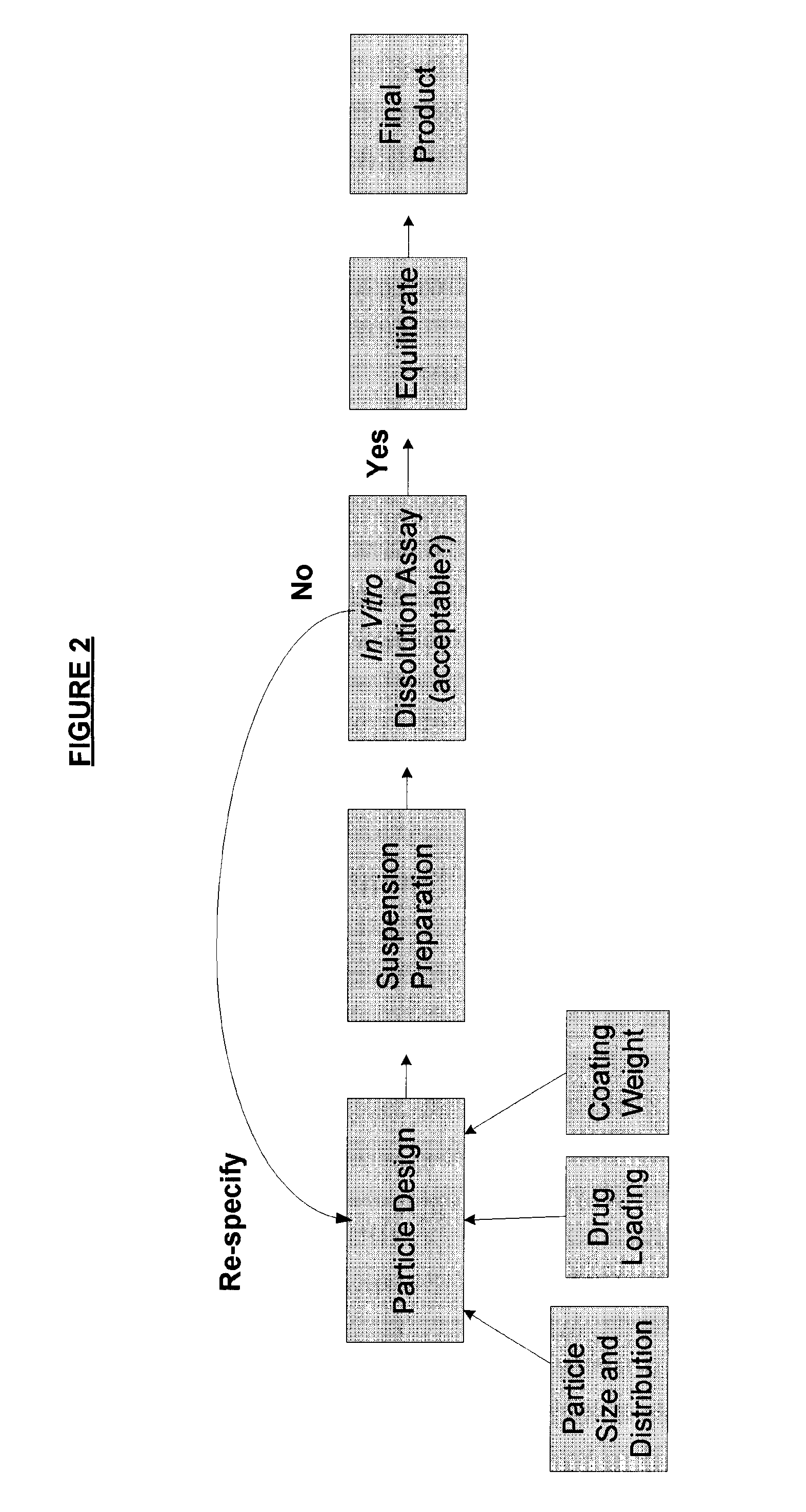
ActiveUS8470375B1Maintaining bioequivalenceMaintaining bioavailabilityDispersion deliverySolution deliveryQuality controlDissolution
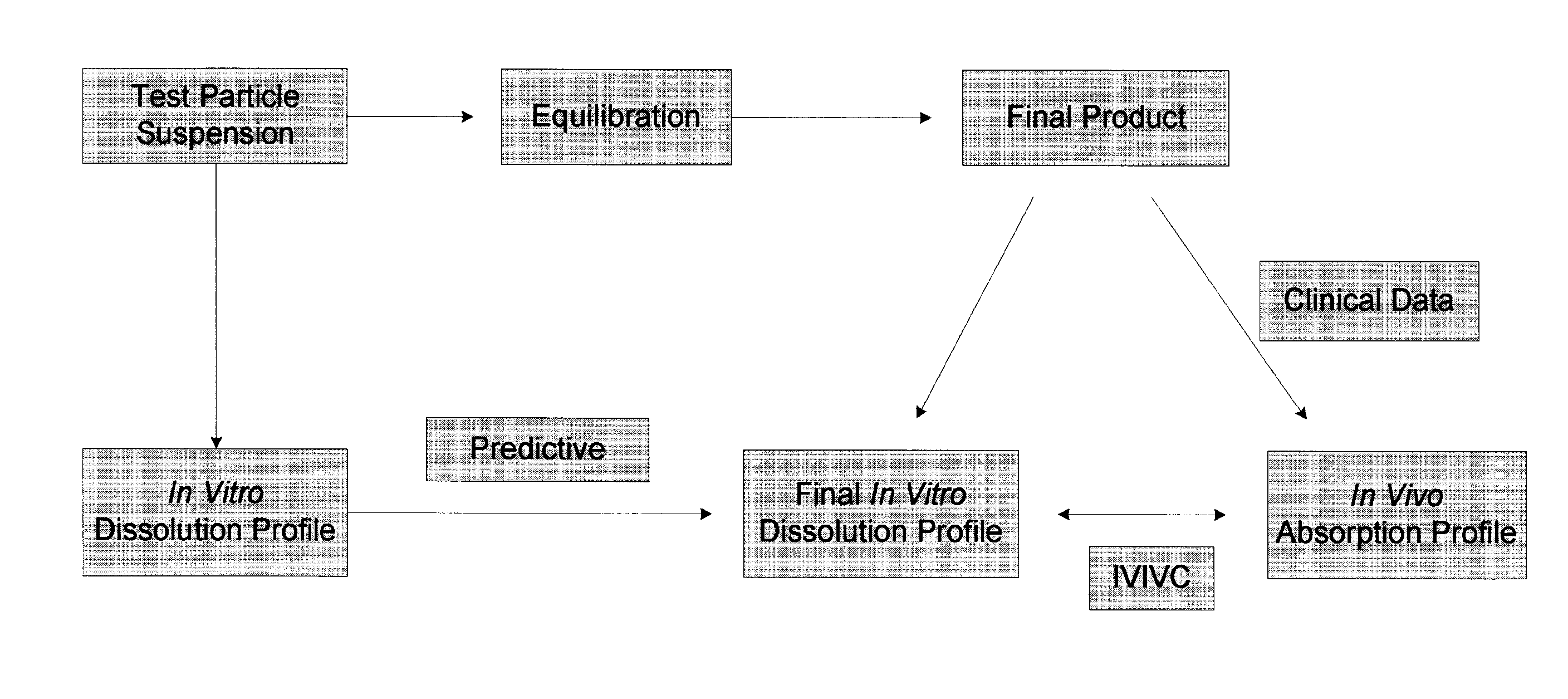
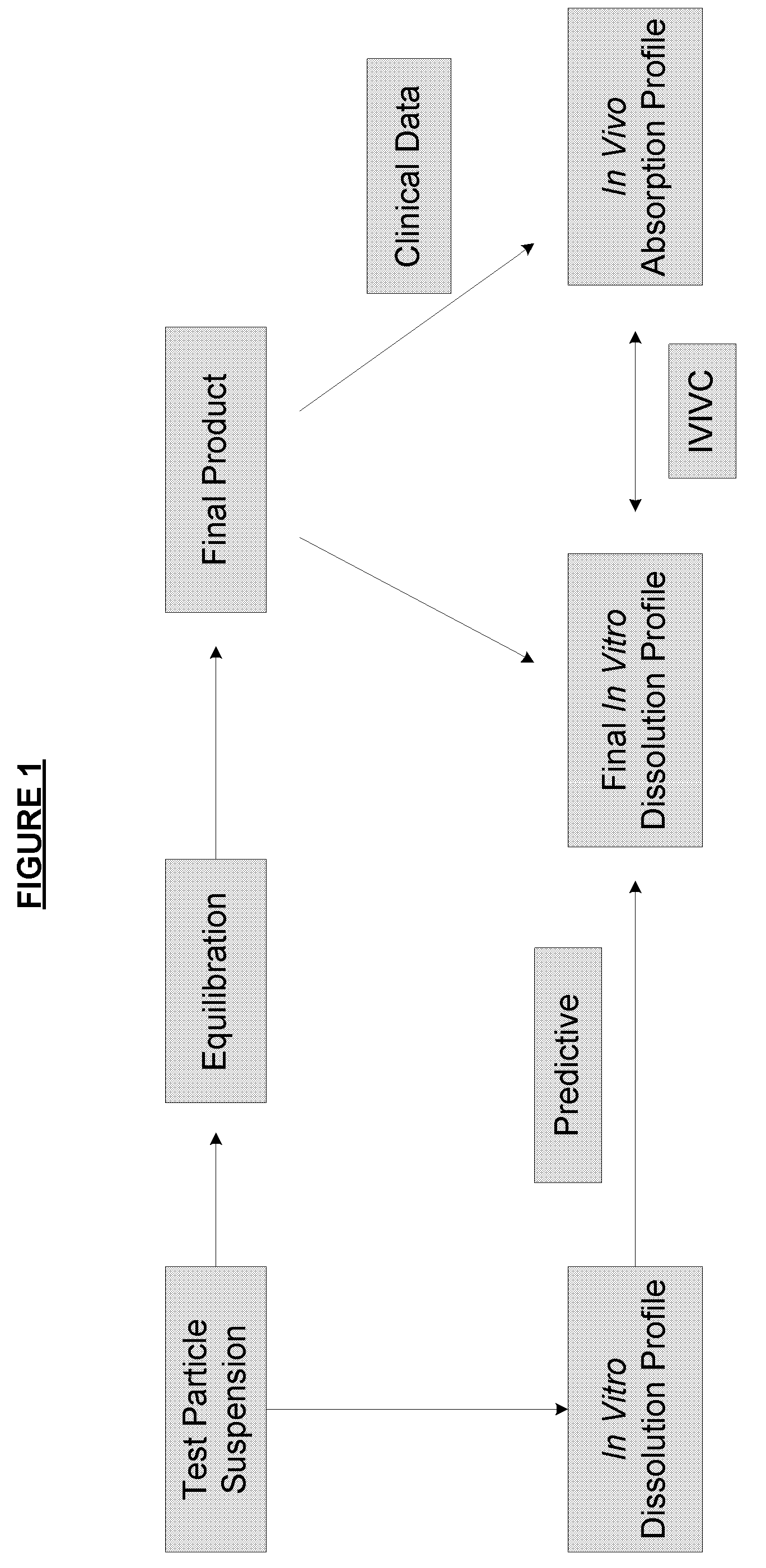
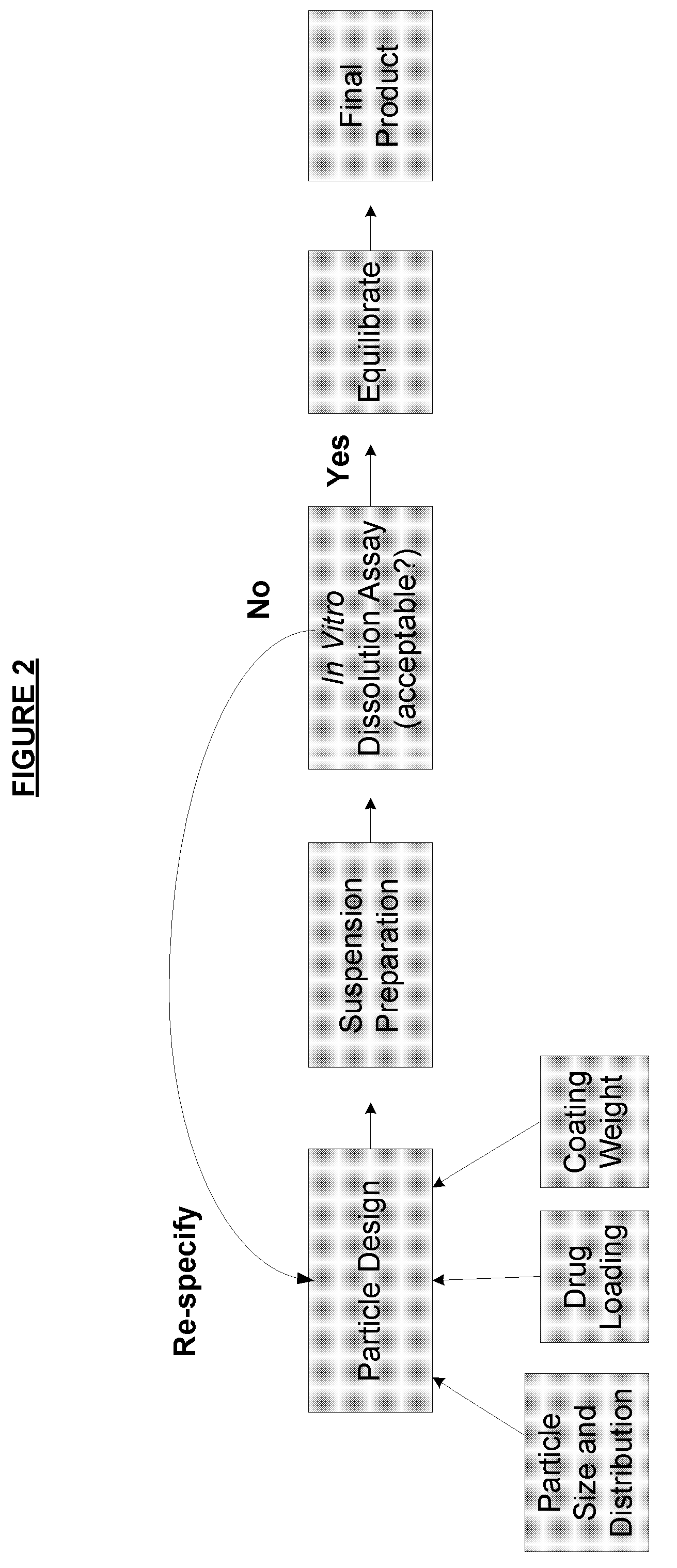
The invention relates to the formulation and quality control of liquid drug suspensions. In particular, the invention relates to methods of formulating liquid suspensions comprising drug-containing resin particles. The invention also relates to methods of confirming the acceptability of drug-containing resin particles for use in formulating liquid drug suspensions. The invention further relates to methods of formulating liquid suspensions in which drug-containing resin particles, the liquid suspension, or both are modified to achieve a desired in vitro dissolution profile. The invention also relates to a novel dissolution method and methods of predicting in vivo bioequivalence based on in vitro dissolution methods.
Owner:NEOS THERAPEUTICS LP
Vilazodone hydrochloride composition and preparation method thereof
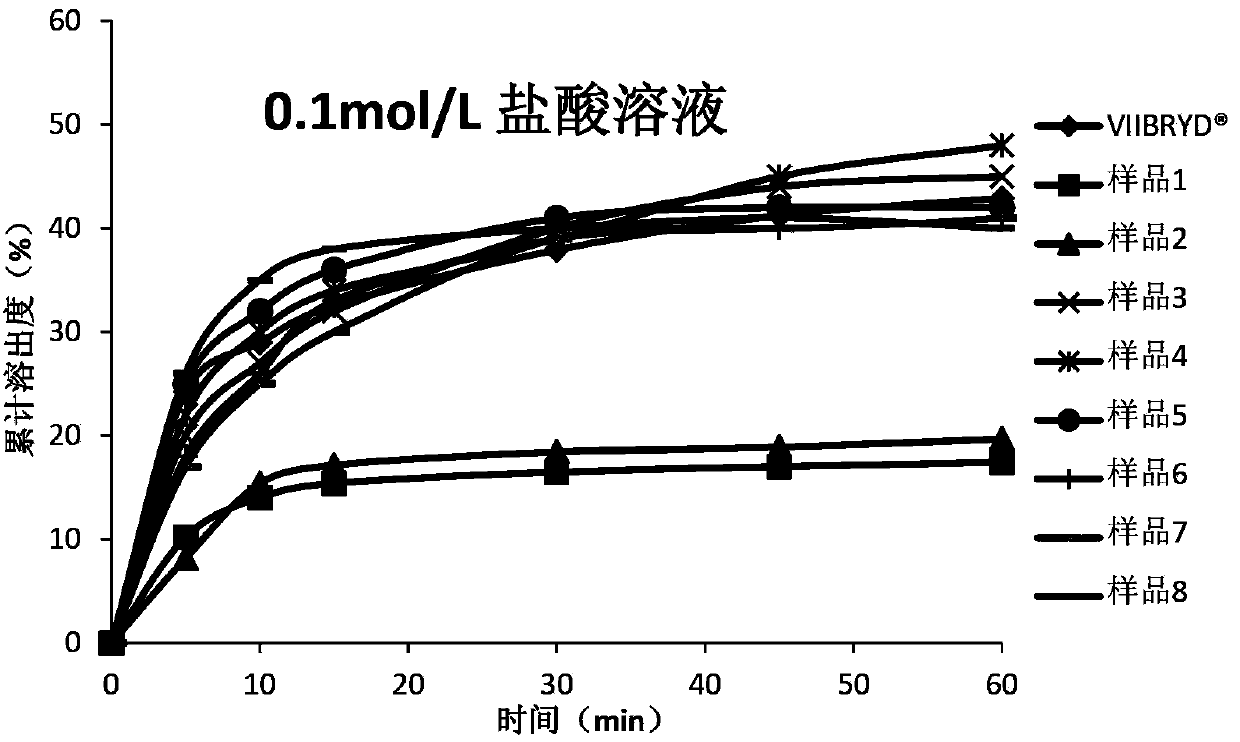
ActiveCN104116741ASolving In Vitro Dissolution ProblemsImprove bioavailabilityOrganic active ingredientsNervous disorderPharmaceutical medicineMicrocarrier
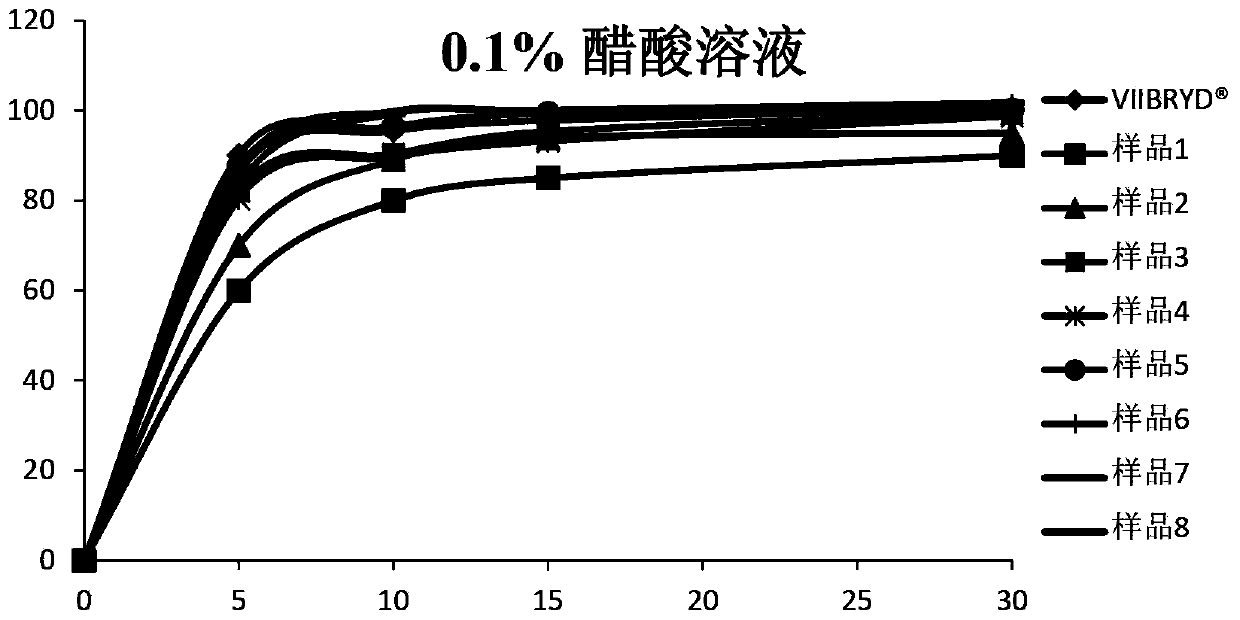
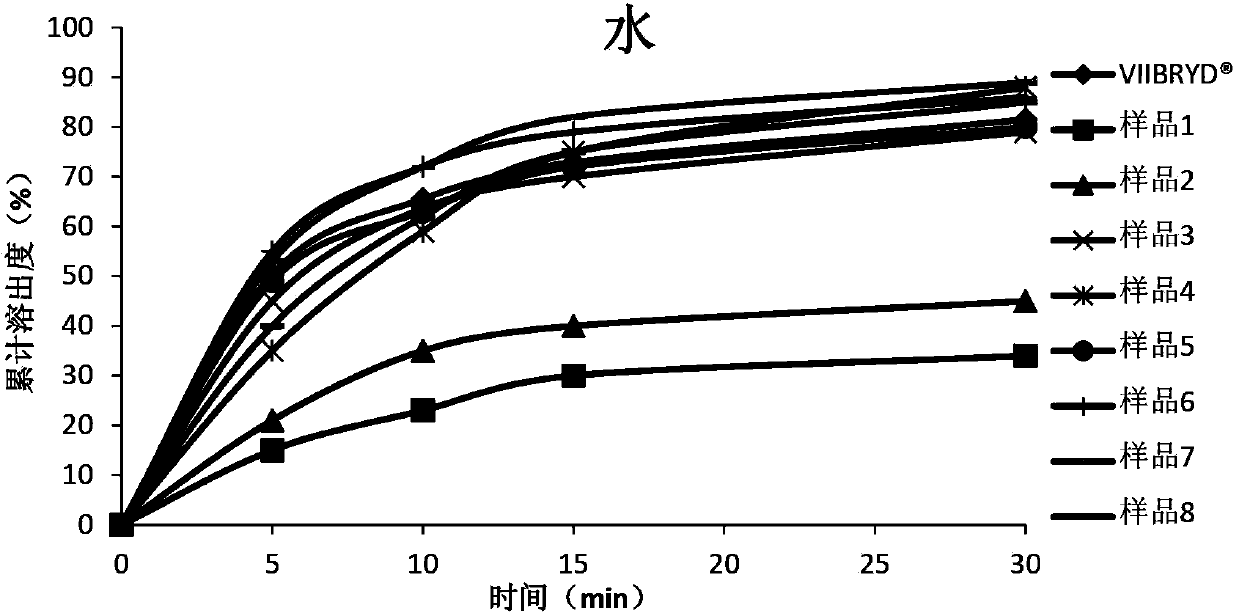
The invention relates to a vilazodone hydrochloride composition and a preparation method thereof. The composition includes vilazodone hydrochloride, a microcarrier and pharmaceutically acceptable excipients for an oral solid preparation. The preparation method is as below: mixing and crushing vilazodone hydrochloride and the microcarrier, and evenly mixing the mixture with pharmaceutically acceptable excipients for oral solid preparation. The obtained composition preparation has greatly improved dissolution in vitro, and in vivo bioavailability reaching bioequivalence to VIIBRYD.
Owner:JIANGSU SIMCERE PHARMA
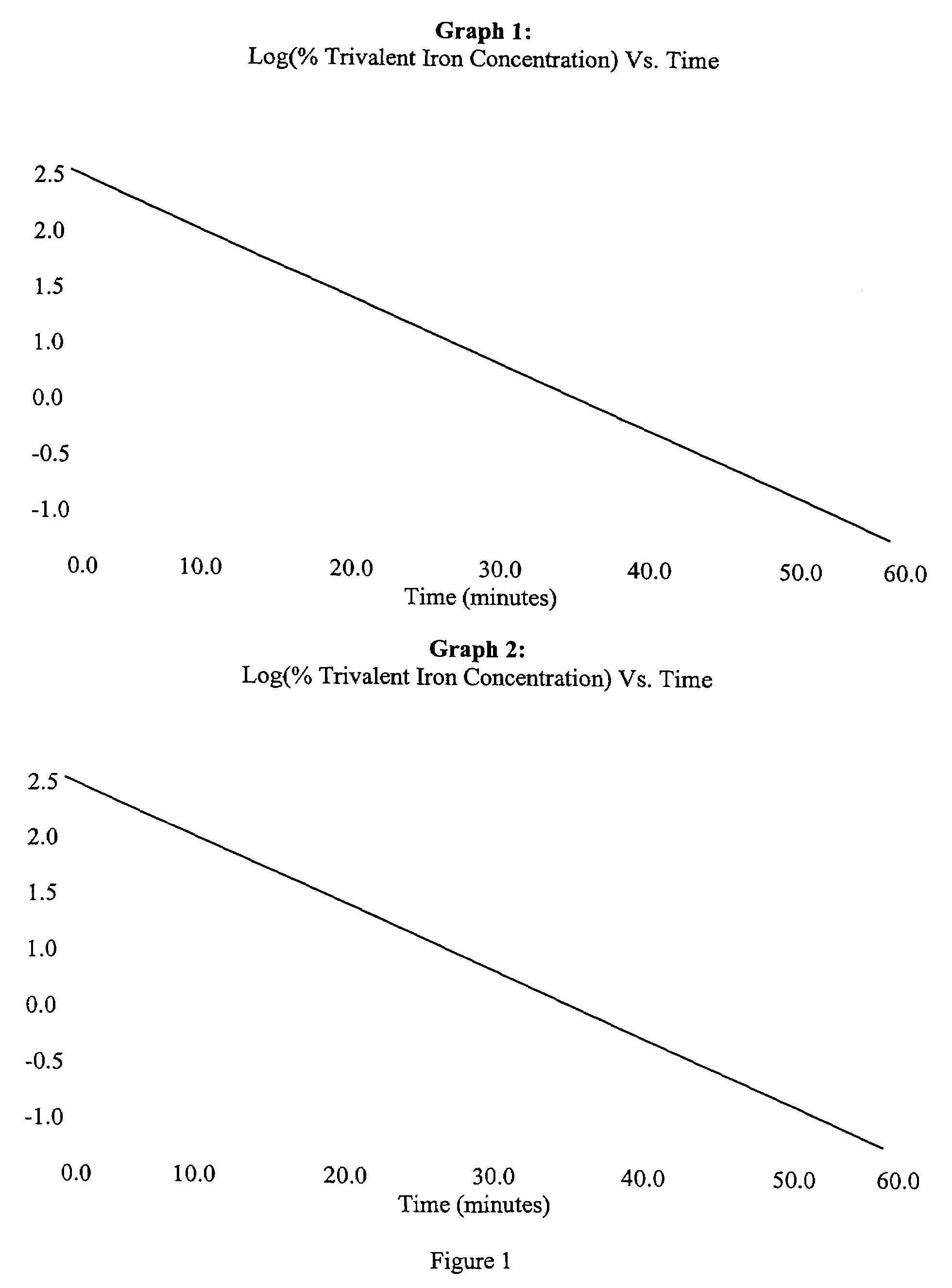
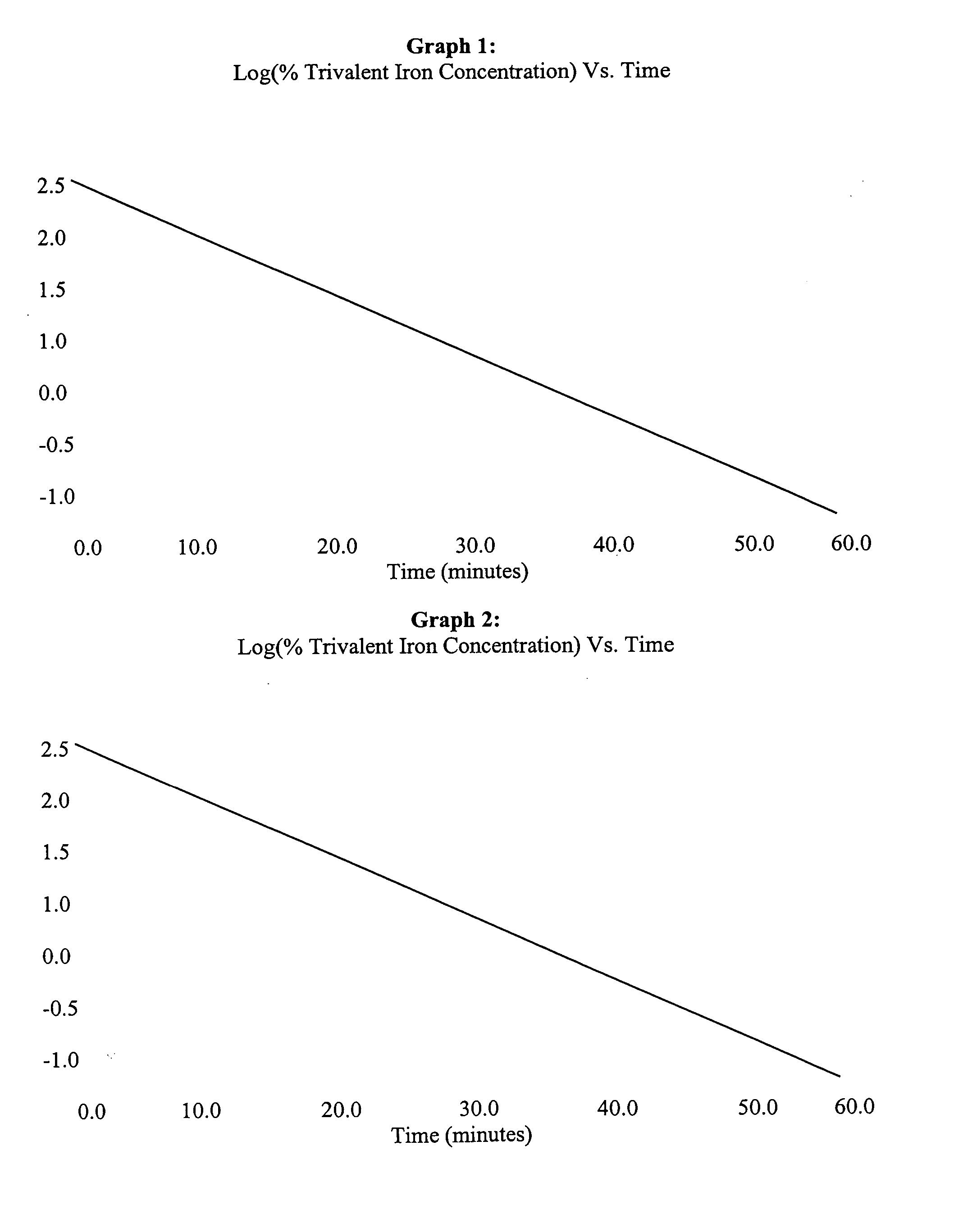
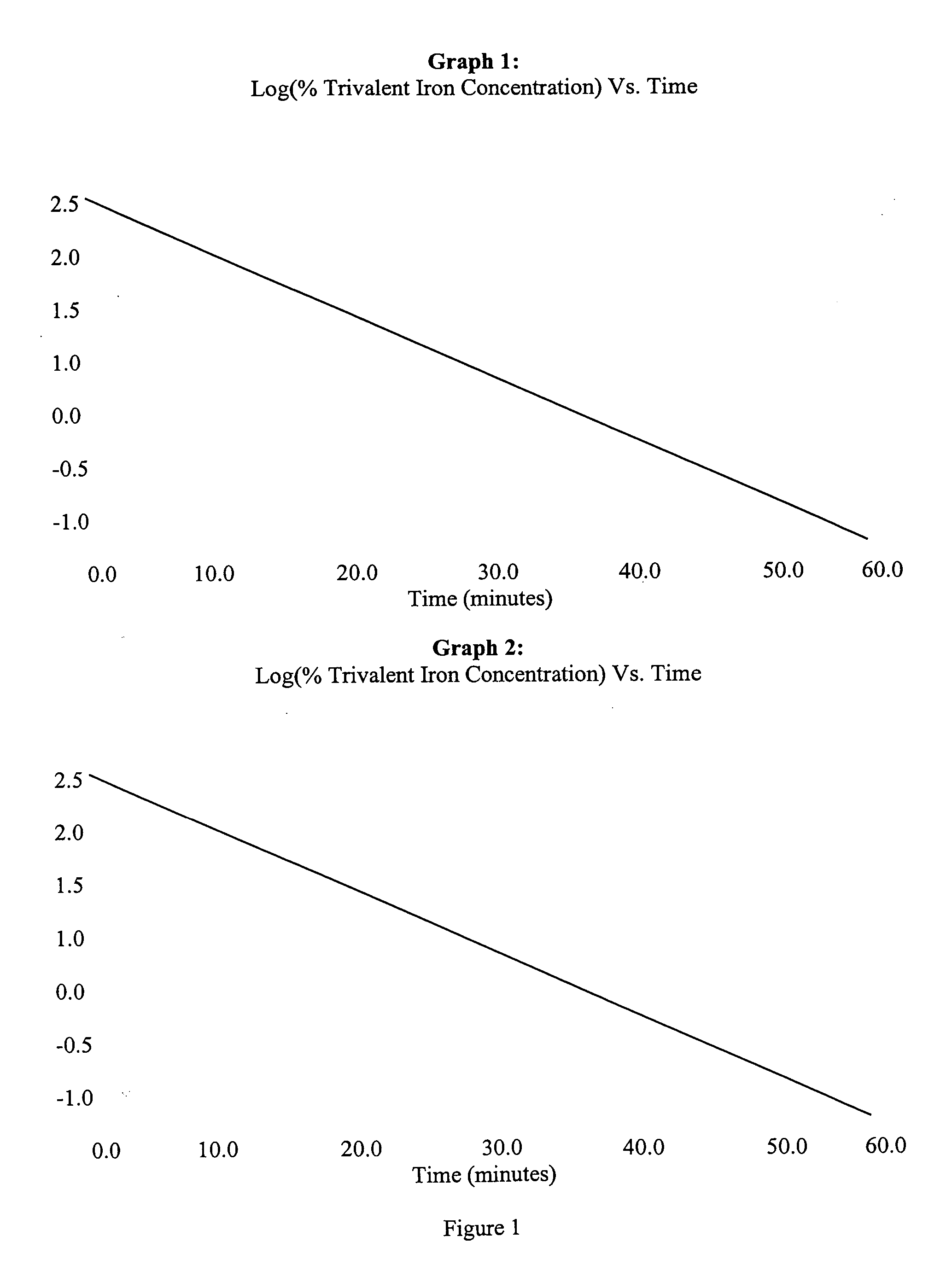
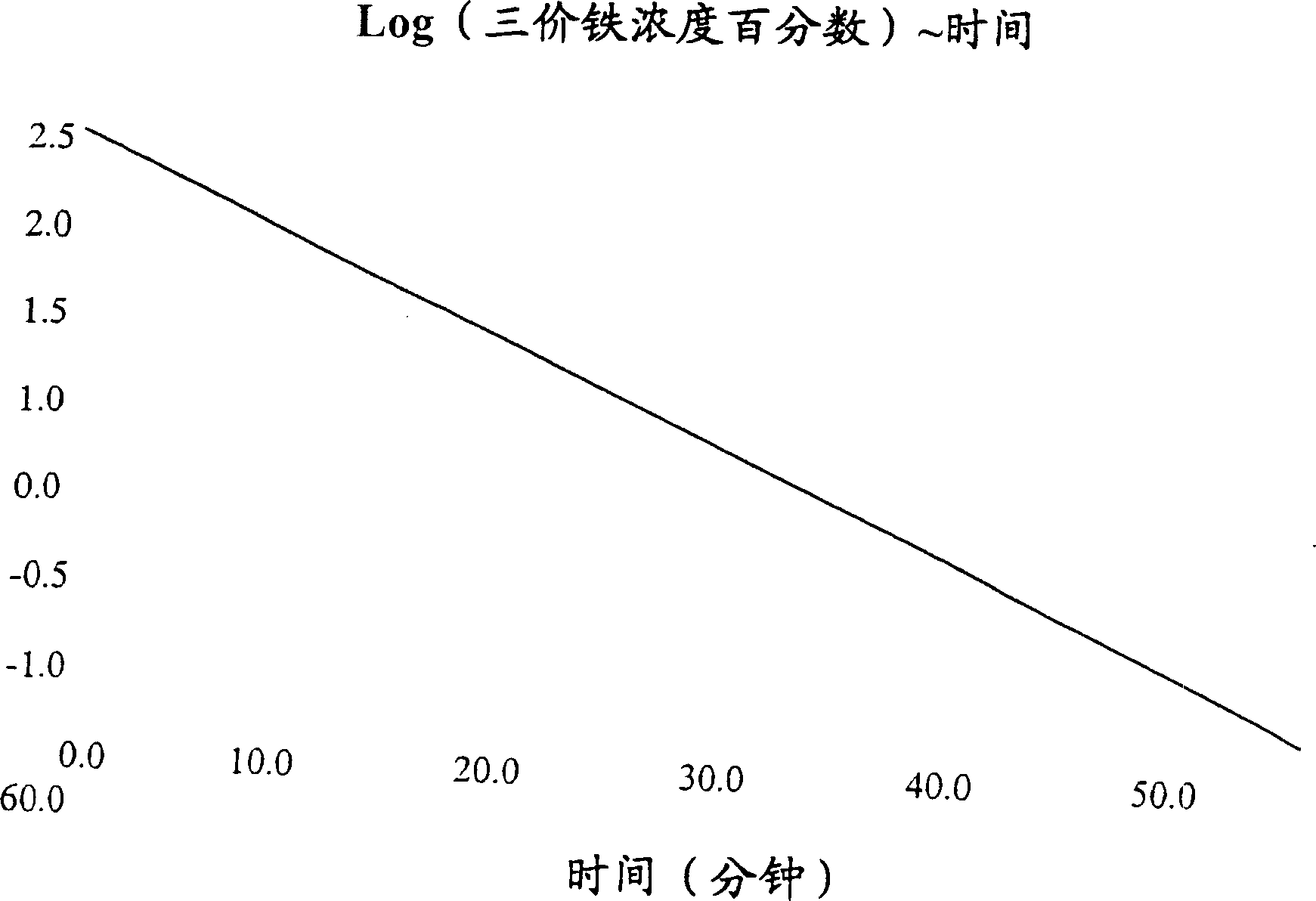
Bioequivalence test for iron-containing formulations
InactiveUS6911342B2Uniform particle sizeAnalysis using chemical indicatorsMaterial analysis by optical meansIron supplementQuality control
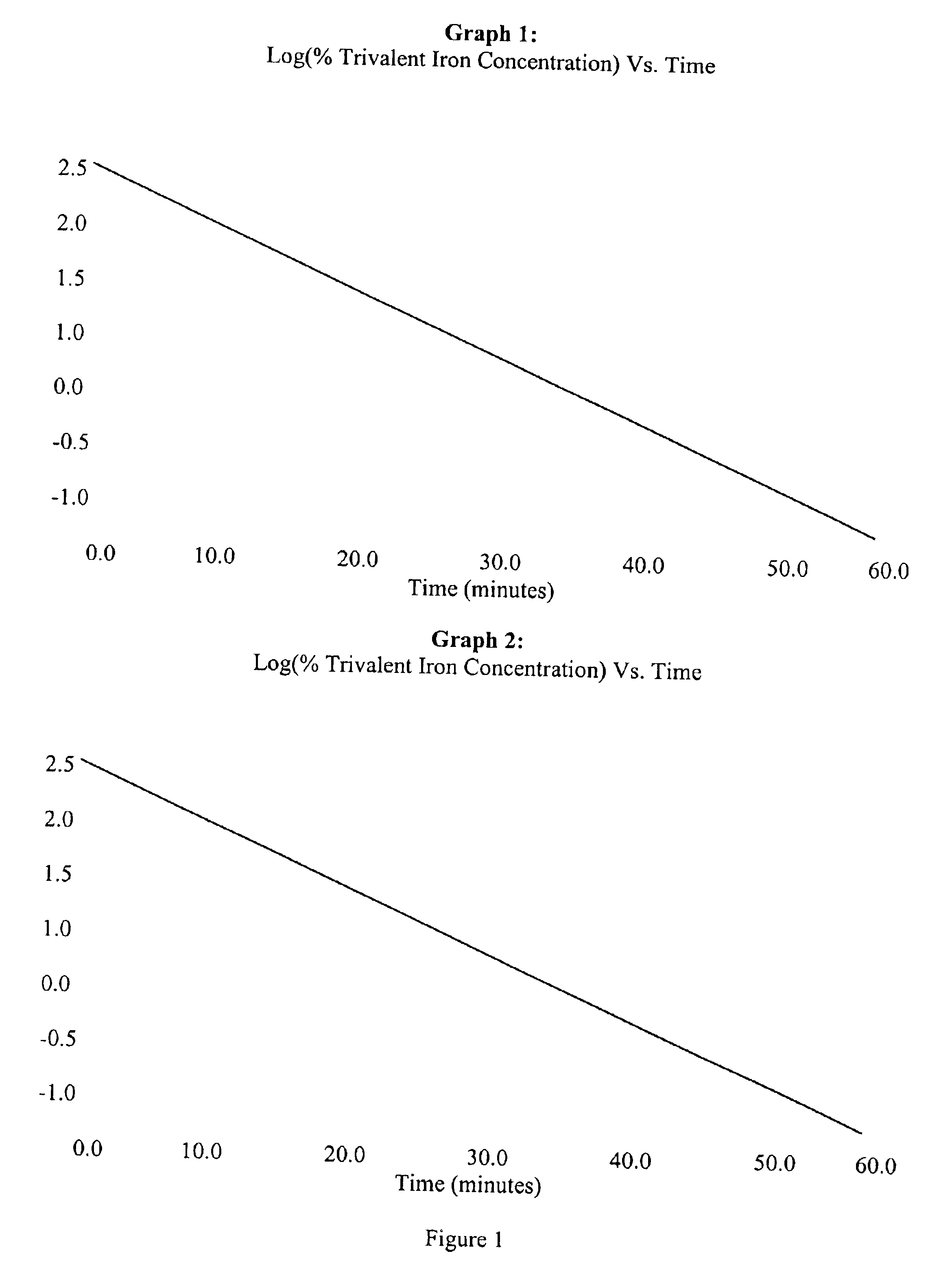
A rapid method for assessing the bioequivalence of iron in iron-supplement formulations, particularly iron-sucrose formulations is described, which is based upon the kinetics of reduction of iron (III) to iron (II) in a sample of the formulation. Quality control methods and associated kits also are described.
Owner:AMERICAN REGENT INC
Methods of Formulating and Designing Liquid Drug Suspensions Containing Ion Exchange Resin Particles
The invention relates to the formulation and quality control of liquid drug suspensions. In particular, the invention relates to methods of formulating liquid suspensions comprising drug-containing resin particles. The invention also relates to methods of confirming the acceptability of drug-containing resin particles for use in formulating liquid drug suspensions. The invention further relates to methods of formulating liquid suspensions in which drug-containing resin particles, the liquid suspension, or both are modified to achieve a desired in vitro dissolution profile. The invention also relates to a novel dissolution method and methods of predicting in vivo bioequivalence based on in vitro dissolution methods.
Owner:NEOS THERAPEUTICS LP
Analytical Methods for Measuring Synthetic Progesterone
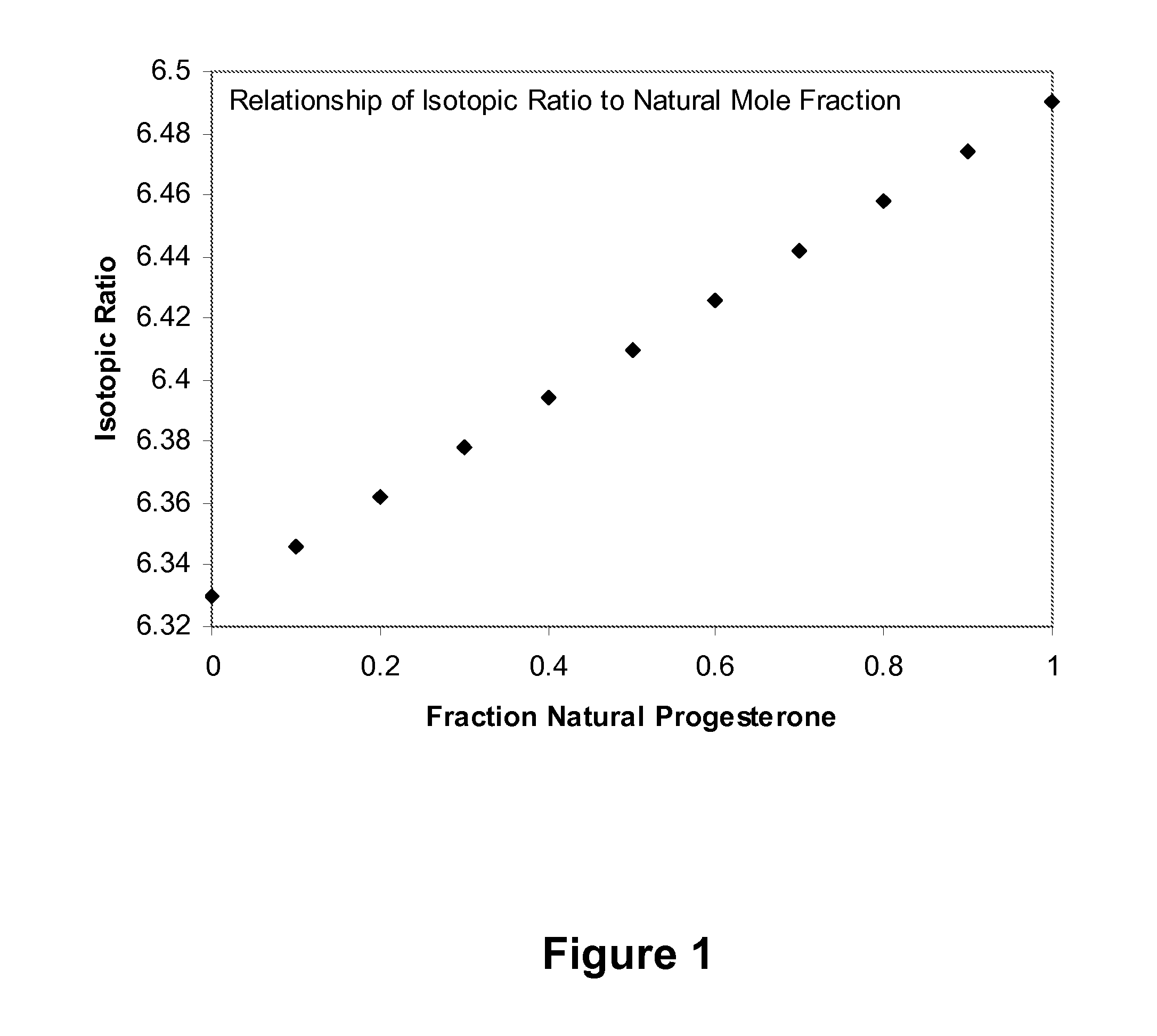
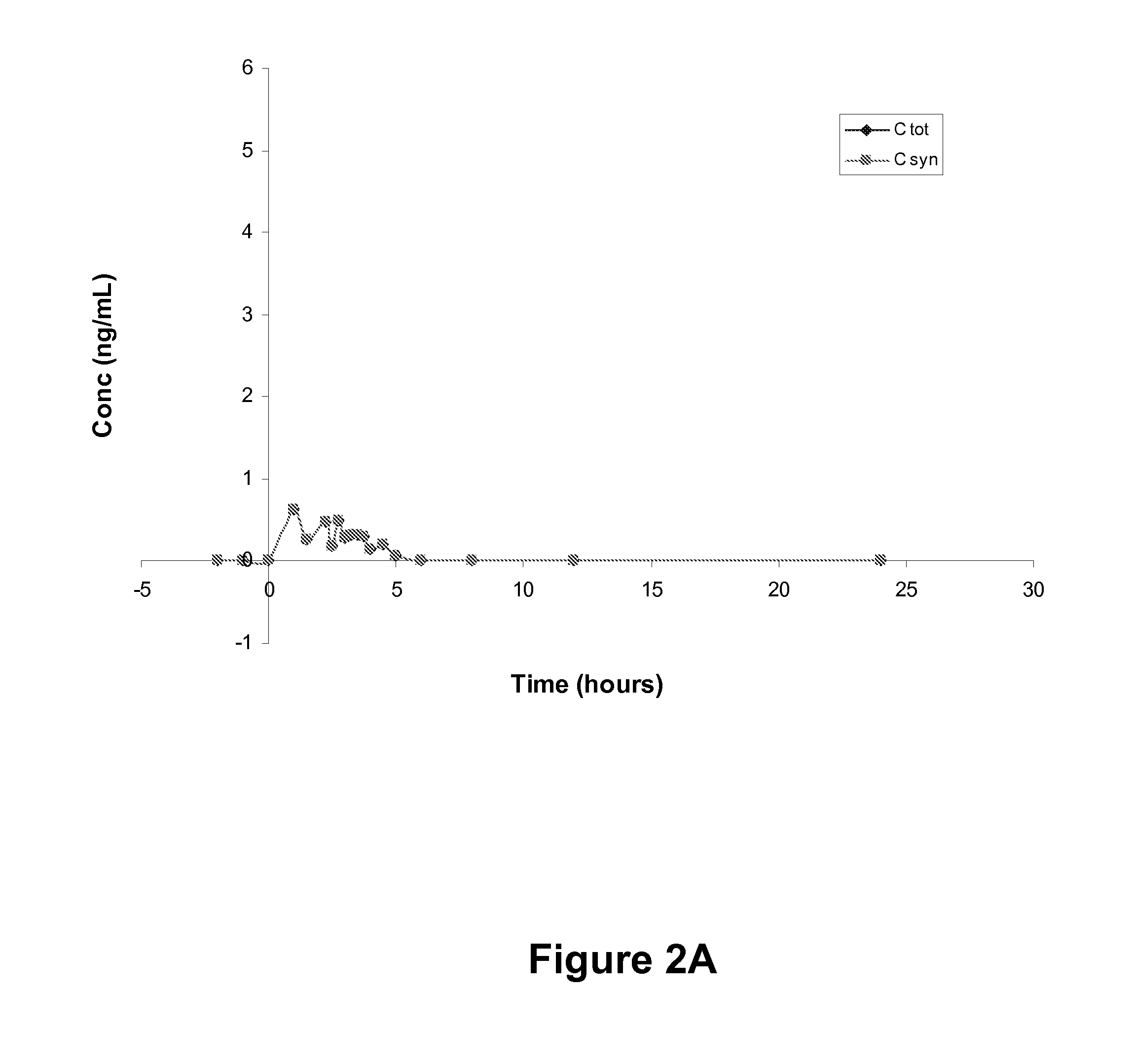
InactiveUS20100304426A1Limited advancementLimited approvalMicrobiological testing/measurementBiological testingAnalyteMass Spectrometry-Mass Spectrometry
Embodiments relating to methods, processes and systems for measuring progesterone are provided. In particular, methods permit measurement and quantification of synthetic and / or endogenous progesterone from a progesterone-containing blood fluid sample by measuring a progesterone carbon isotope ratio by mass spectrometry and calculating the fraction of synthetic progesterone in the sample from the isotope ratio. Also provided are methods of evaluating bioequivalence of a synthetic progesterone composition using any of the methods provided herein. In an embodiment, methods of precise measurements of plasma levels are described for detection of progesterone analytes such as total progesterone, endogenous animal progesterone, and synthetic progesterone. Correcting for fluctuations in endogenous progesterone levels following application of synthetic progesterone allows a significant reduction in the number of test subjects required to evaluate bioequivalence of a synthetic progesterone composition.
Owner:TOLMAR INC
Flupentixol and melitracen medicinal composition and preparation thereof
ActiveCN109674754AImprove stabilityLower levelOrganic active ingredientsNervous disorderMELITRACEN HYDROCHLORIDEAntioxidant
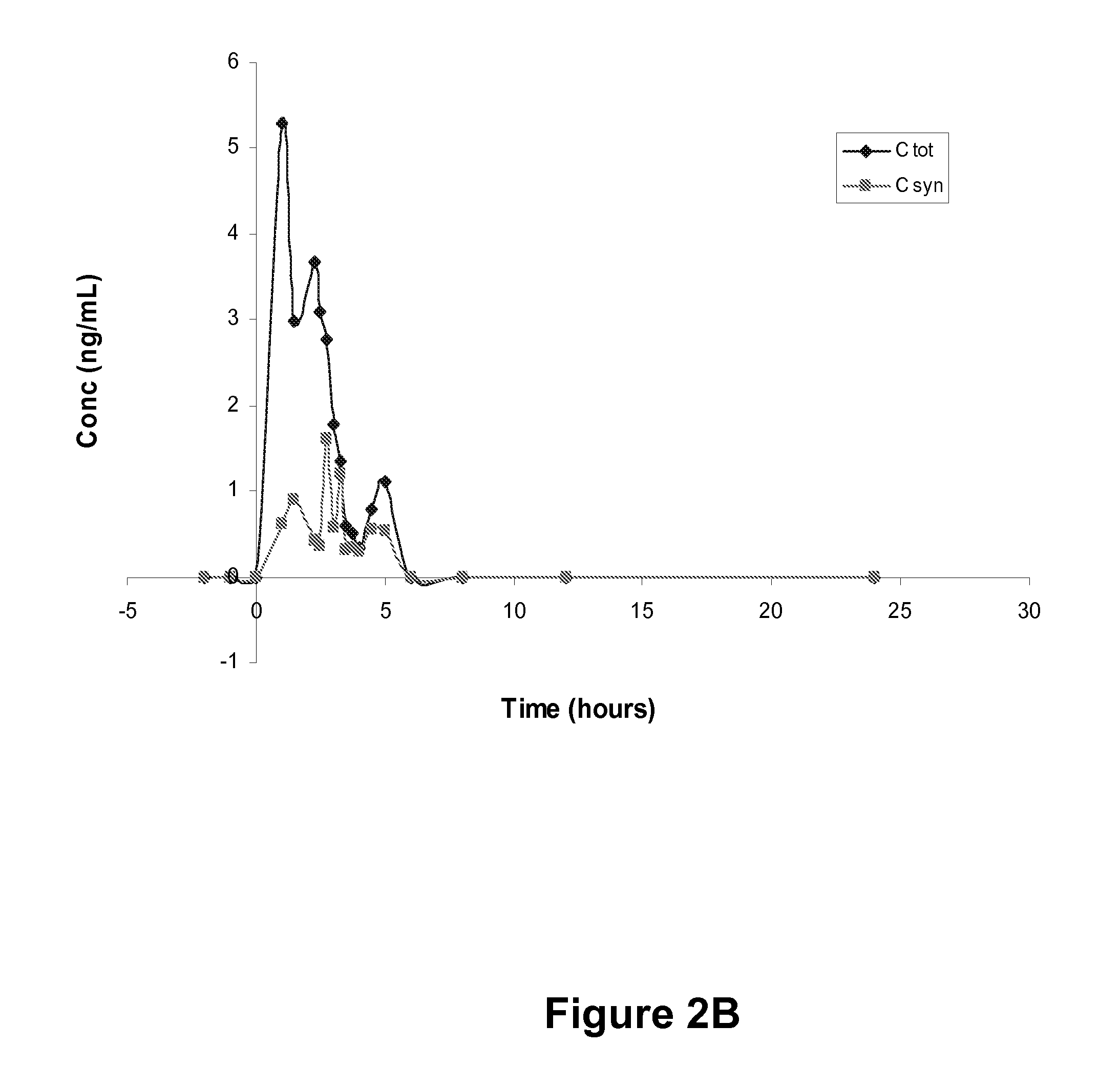
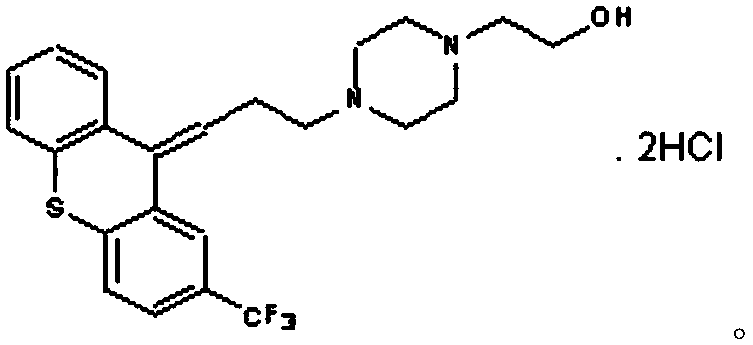
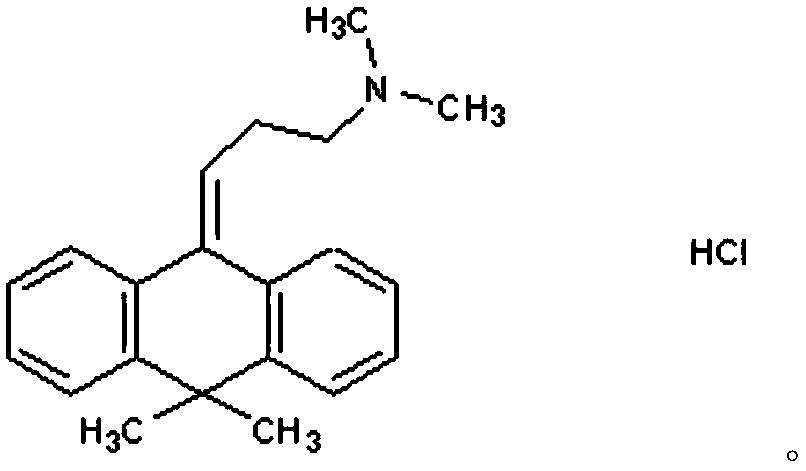
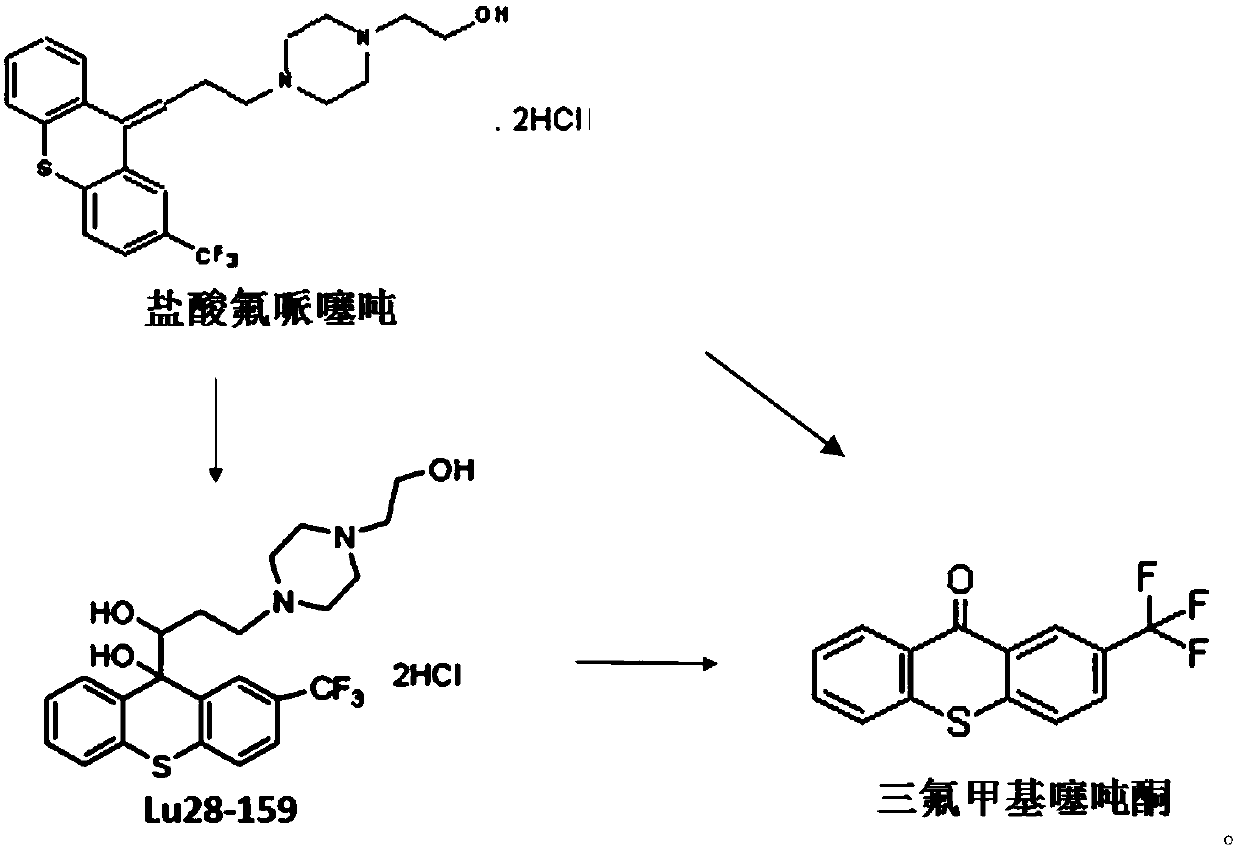
The invention relates to a flupentixol and melitracen medicinal composition and a preparation thereof, and belongs to the technical field of pharmaceutical preparations. The flupentixol and melitracenmedicinal composition is prepared from flupentixol dihydrochloride, melitracen hydrochloride and an antioxidant. The flupentixol and melitracen medicinal composition disclosed by the invention has the advantages that by adding the antioxidant, the stability of a flupentixol and melitracen compound preparation can be effectively enhanced, and impurities such as Lu28-159 and trifluoromethyl thioxanthone produced by degrading flupentixol can be effectively controlled, so that impurity level is reduced and the safety and the effectiveness of a product are ensured; flupentixol and melitracen medicinal tablets prepared by the preparation method disclosed by the invention have similar in-vitro dissolution behavior and bioequivalence with original research tablets Deanxit.
Owner:GUANGDONG SCI FINDER PHARMA TECH CO LTD +1
Taste-masked pharmaceutical compositions
ActiveUS9884014B2Increase probabilityEffective taste-maskingPill deliveryWater insolubleOrally disintegrating tablet
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredient(s), rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates on contact with saliva in the buccal cavity in about 60 seconds forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing the active) applied with a taste-masking membrane comprising a combination of water-insoluble and gastrosoluble polymers release not less than about 60% of the dose is in the stomach in about 30 minutes, thus maximizing the probability of achieving bioequivalence to the reference IR product having rapid onset of action (short Tmax). A process for preparing such compositions for oral administration using conventional fluid-bed equipment and rotary tablet press is also disclosed.
Owner:ADARE PHARM INC
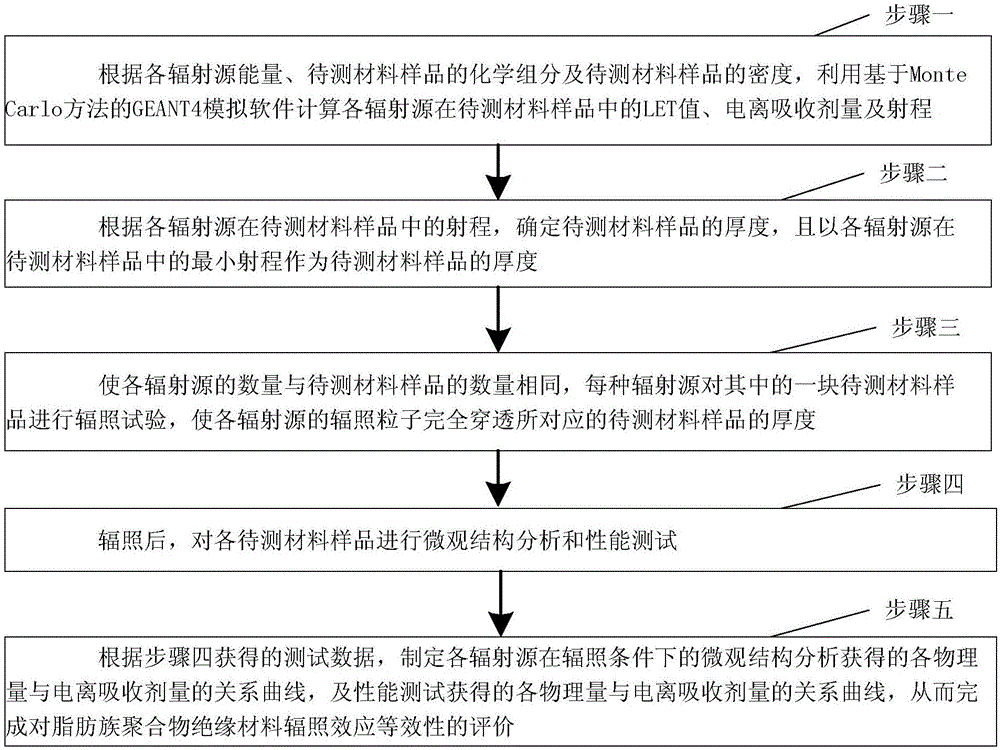
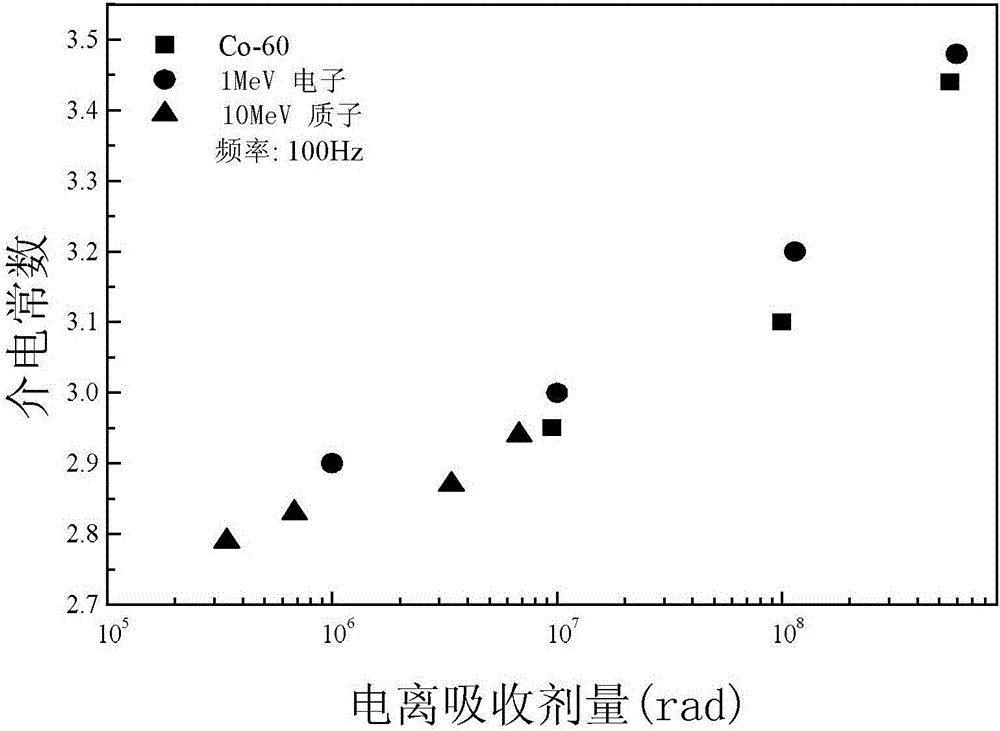
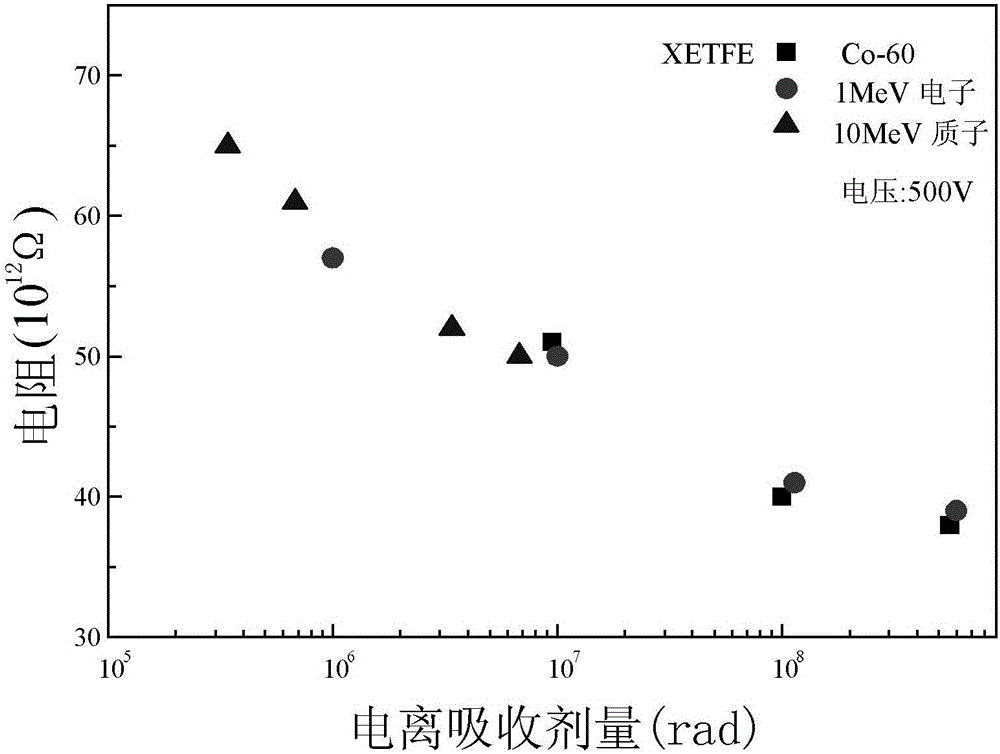
Space radiation effect bioequivalence evaluation method of aliphatic polymer insulation materials for space navigation
ActiveCN106404651AImprove accuracyEasy to operateWeather/light/corrosion resistanceStructure analysisPolymer insulation
The invention provides a space radiation effect bioequivalence evaluation method of aliphatic polymer insulation materials for space navigation, and relates to a bioequivalence evaluation method of the displacement radiation effect of different radiation sources of aliphatic polymer insulation materials for particle radiation environment. The problem of great evaluation error of the existing aliphatic polymer space radiation effect evaluation method for space navigation is solved. The LET value, the ionospheric absorption dosage and the ejection range of each radiation source in a sample of a material to be tested can be calculated; according to the ejection range of each radiation source in the sample of the material to be tested, the thickness of the sample of the material to be tested is determined, so that each radiation source performs radiation test correspondingly to one tested material, so that the radiation particles of each radiation source completely penetrate through the thickness of the corresponding sample of the material to be tested; after the radiation, a relationship curve of each physical quantity and the ionospheric absorption agent quantity obtained through microcosmic structure analysis of each radiation source under the radiation condition, and a relationship curve of each physical quantity and the ionospheric absorption agent quantity obtained through performance test are made. The method is used for evaluating the aliphatic polymer insulation materials.
Owner:HARBIN INST OF TECH
Bioequivalence test for iron-containing formulations
InactiveUS7169359B2Pharmaceutical non-active ingredientsSynthetic polymeric active ingredientsQuality controlIron supplement
A rapid method for assessing the bioequivalence of iron in iron-supplement formulations, particularly iron-sucrose formulations is described, which is based upon the kinetics of reduction of iron (III) to iron (II) in a sample of the formulation. Quality control methods and associated kits also are described.
Owner:AMERICAN REGENT INC
Methods of formulating and designing liquid drug suspensions containing ion exchange resin particles
The invention relates to the formulation and quality control of liquid drug suspensions. In particular, the invention relates to methods of formulating liquid suspensions comprising drug-containing resin particles. The invention also relates to methods of confirming the acceptability of drug-containing resin particles for use in formulating liquid drug suspensions. The invention further relates to methods of formulating liquid suspensions in which drug-containing resin particles, the liquid suspension, or both are modified to achieve a desired in vitro dissolution profile. The invention also relates to a novel dissolution method and methods of predicting in vivo bioequivalence based on in vitro dissolution methods.
Owner:NEOS THERAPEUTICS LP
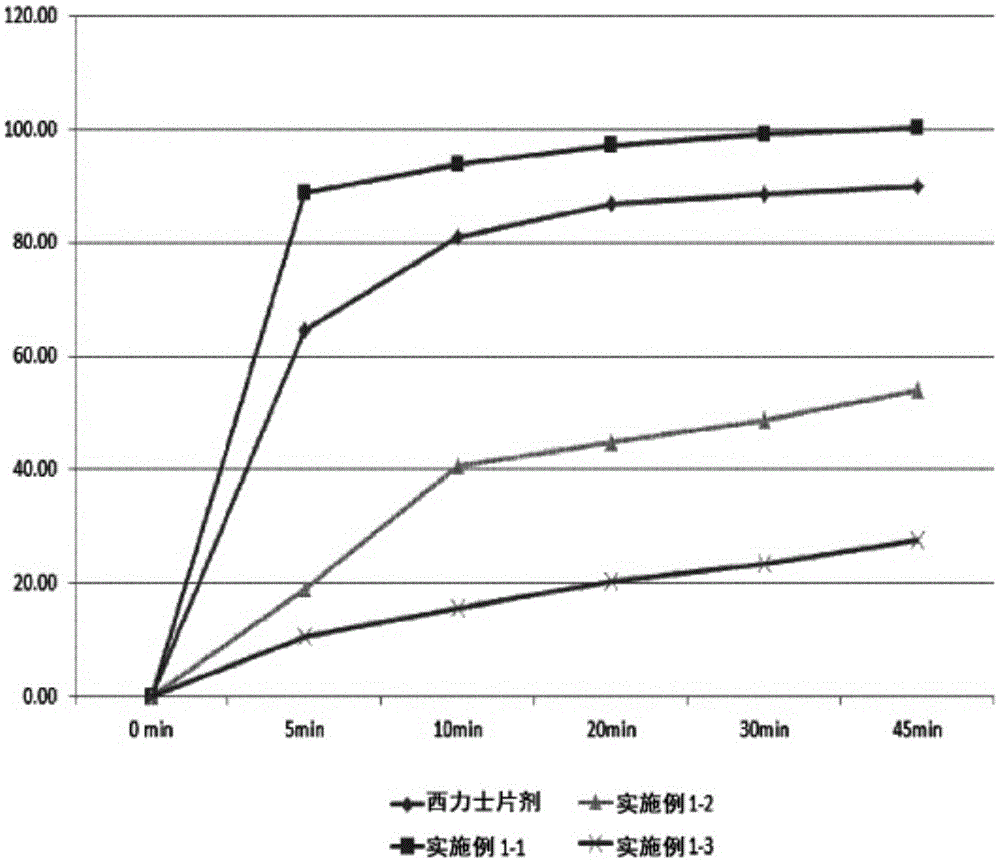
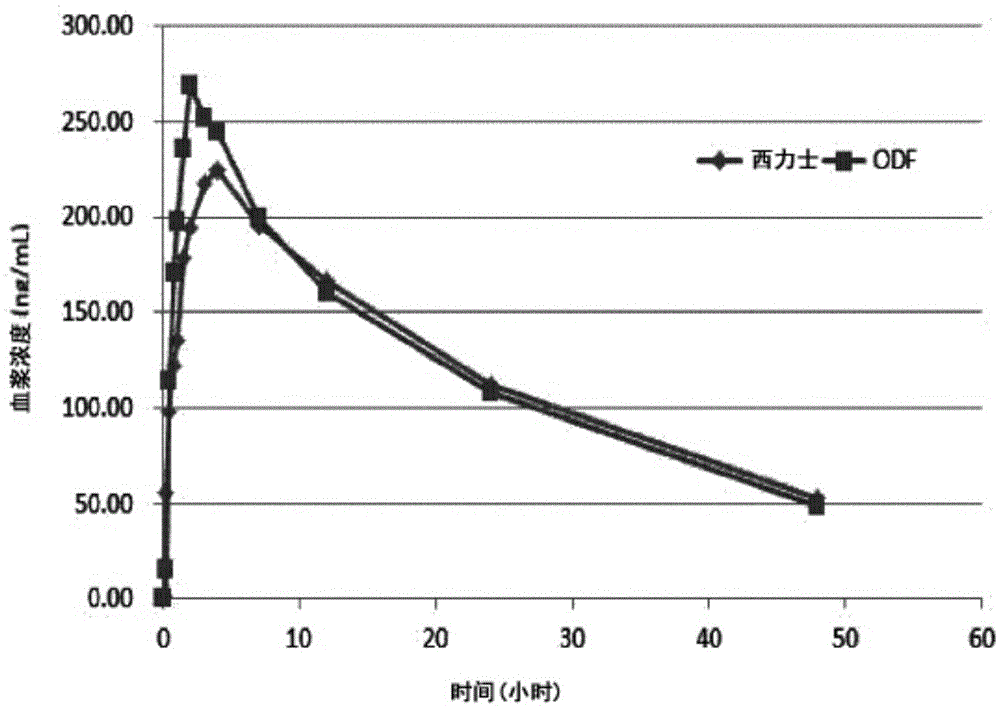
Oral disintegrating film formulation containing tadalafil and preparation method therefor
ActiveCN105611918AImprove stabilityNo oral absorptionOrganic active ingredientsPharmaceutical non-active ingredientsTadalafilFilm-forming agent
The present invention provides an oral disintegrating film formulation comprising: tadalafil as an active ingredient; and a combination of fluran and polyvinylpyrrolidone as a film forming agent. The oral disintegrating film formulation of the present invention does not exhibit buccal absorption, and can be formulated so as to exhibit bioequivalence to a tadalafil-containing tablet. In addition, the oral disintegrating film formulation of the present invention exhibits good stability.
Owner:WOOSHIN LABOTTACH CO LTD
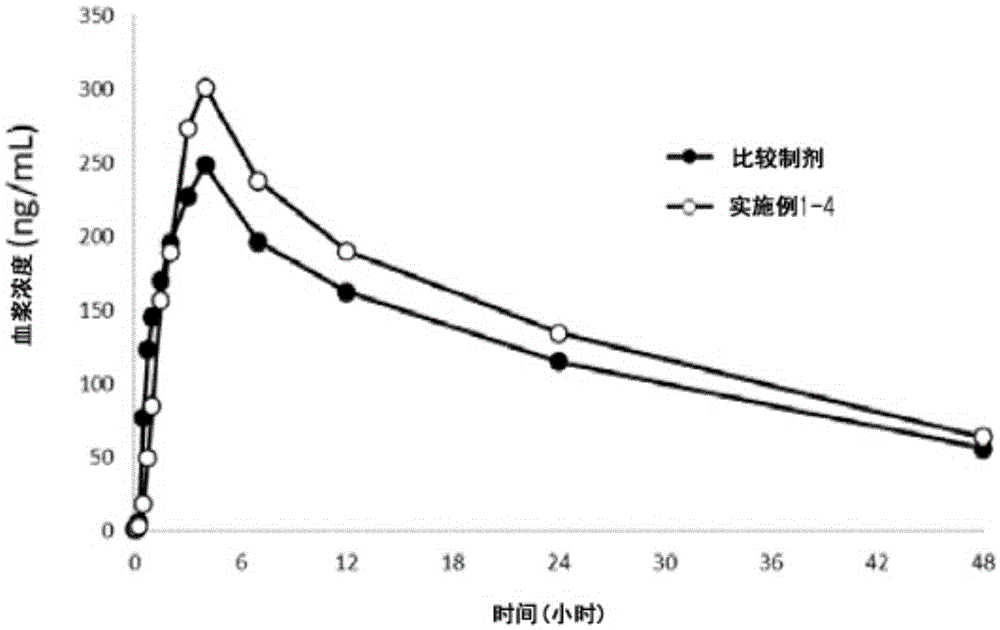
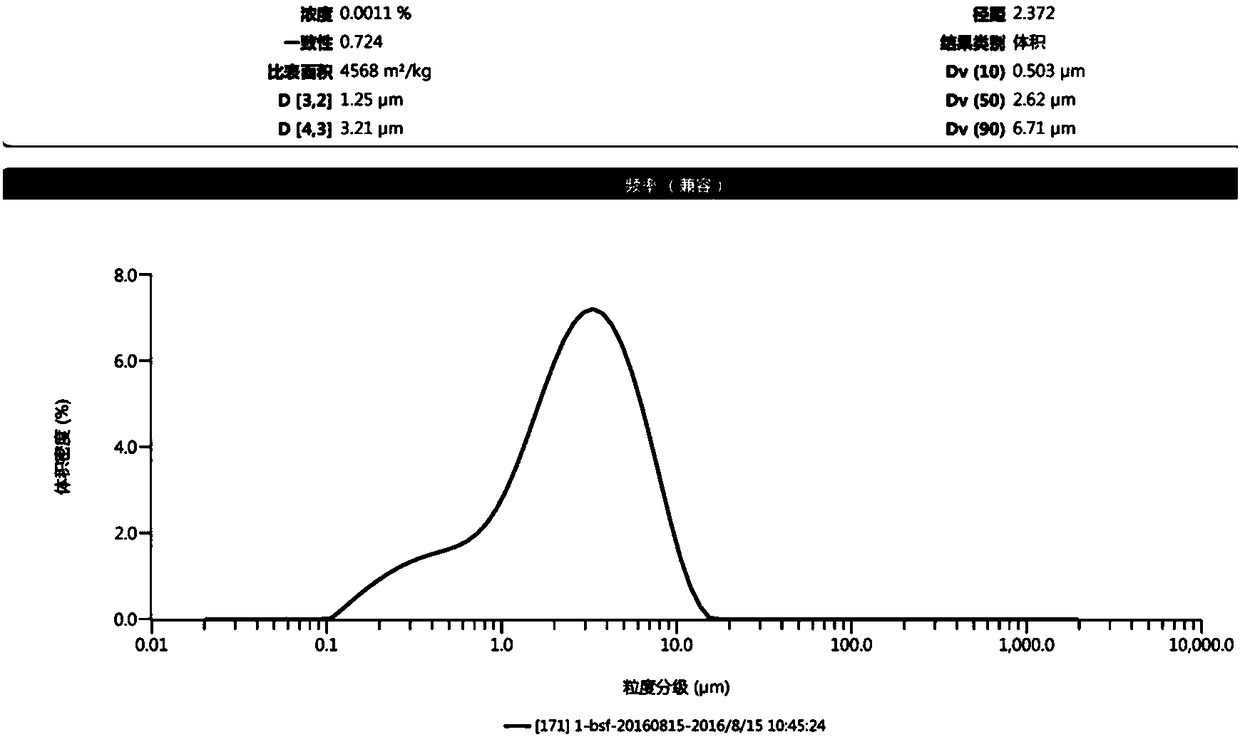
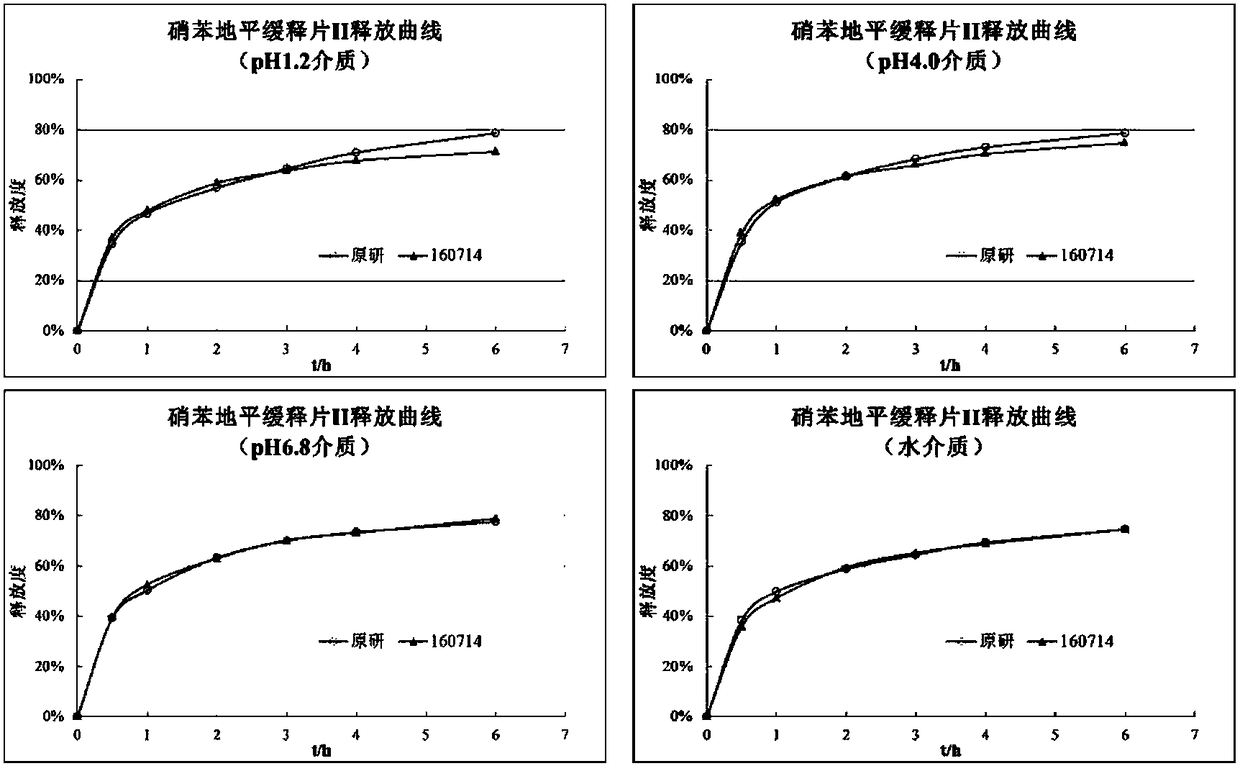
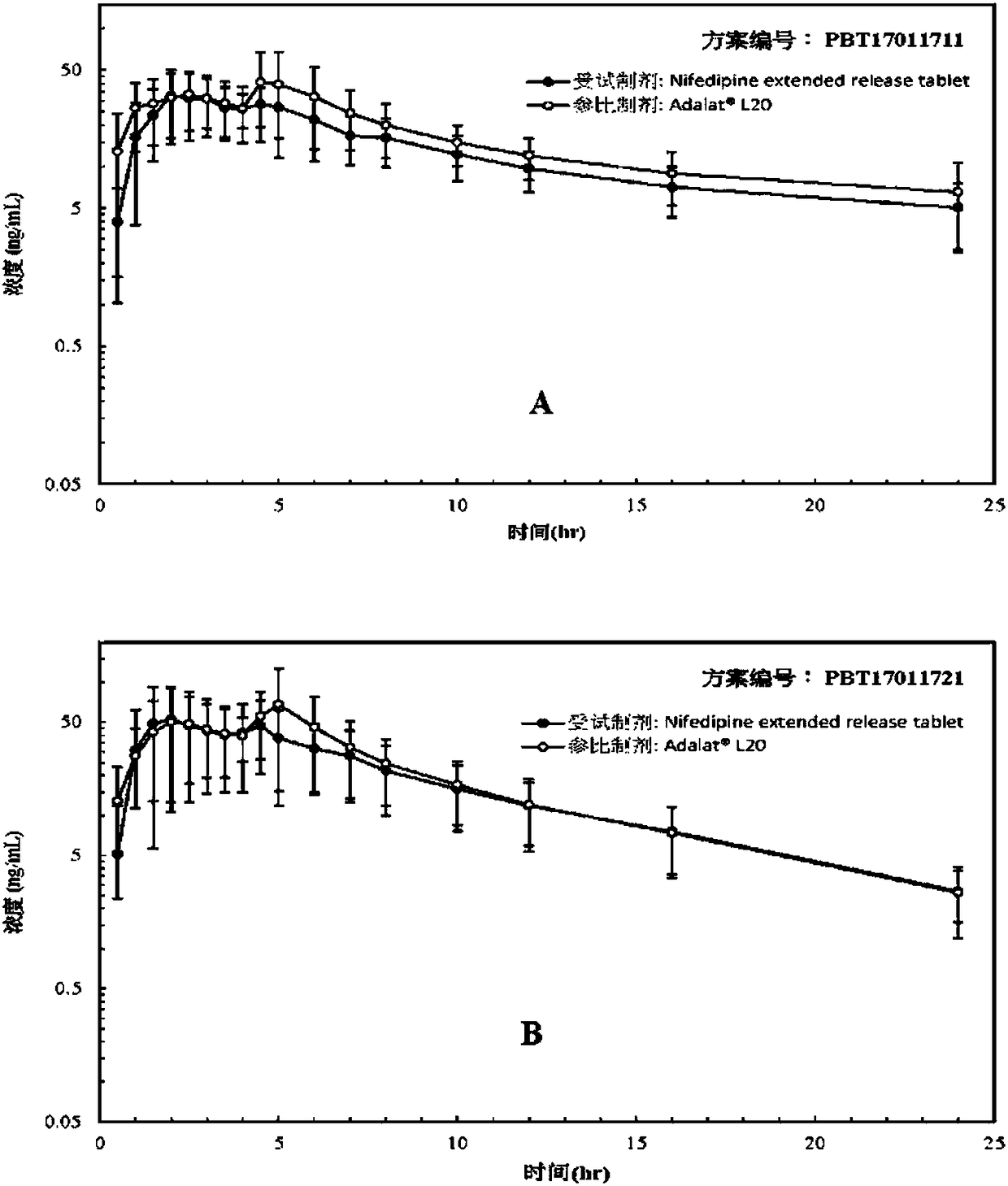
Nifedipine sustained release tablets and preparation method thereof
ActiveCN108186593AIn line with industrial production conditionsBreaking down the technical barriers of original researchOrganic active ingredientsPharmaceutical non-active ingredientsNifedipineSustained Release Tablet
The invention discloses a nifedipine sustained release tablet and a preparation method thereof. The grain diameter D90 of nifedipine is 5-10 mu m, and a tablet core is prepared from, in percentage byweight, 25% of nifedipine, 5%-10% of retardant composition, 0.5%-1.5% of tween 80 and the balance of other auxiliary materials. In order to guarantee the storage quality of the tablet core, the tabletis colored and coated, and the weight gain of a coat is 4%-5%. Initiative formula composition is adopted, a sustained release mode is innovated, and the nifedipine sustained release tablet capable ofcontinuously releasing a drug within 12 h is prepared, two times of human body pre-BE (bioequivalence) (12 cases, empty stomach / full stomach) research prove the BE of the product, and besides, a dissolution method related in vitro and in vivo is developed. The drug release principle and process monopoly of the nifedipine sustained release tablet are broken through, the adopted formula and preparation process are simpler and more controllable, facilitate industrial production in China and provide more clinic choices for patients and doctors, and the medication burden of the patients is reduced.
Owner:南京百思福医药科技有限公司
Sugar-free oral transmucosal solid dosage forms and uses thereof
The present invention is directed to oral solid dosage forms. The solid dosage forms are sugar-free, and comprise a pharmaceutical agent and a suitable pharmaceutically acceptable excipient. Preferably, the solid dosage forms of the present invention are bioequivalent to a sugar-containing solid dosage form. Bioequivalence is preferably obtained by incorporating an ionizing agent, more preferably in the form of a buffer system, into the solid dosage forms, in an amount sufficient to maintain a portion of the pharmaceutical agent, upon dissolution of said composition in saliva, in an ionized state.
Owner:CEPHALON INC
Quantitative analysis method for tadalafil in human blood plasma
PendingCN108627576AHigh degree of automationGood repeatabilityComponent separationTadalafilOperability
The invention discloses a quantitative analysis method for tadalafil in human blood plasma. The quantitative analysis method comprises: (1) preparation of a standard working solution; (2) sample treatment; (3) production of a standard curve; and (4) quantitative analysis, wherein a test sample is treated according to the step (3), and calculating is performed according to the standard curve equation obtained in the step (3) to obtain the concentration of tadalafil in the test sample. According to the present invention, the concentration of tadalafil in the human blood plasma is determined by using the liquid chromatography-mass spectrometry (HPLC-MS / MS) technology, such that the concentration of tadalafil in the human blood plasma is accurately quantitated; the chromatographic column, thedissolving liquid, the mobile phase, the mass spectrometry conditions and the like are reasonably selected, and the flow rate, the elution mode, the mass spectrometry conditions and other process conditions are optimized, such that the advantages of simple operation, high repeatability, good accuracy and high operability can be achieved; and the method can be directly used for the sample detectionanalysis in bioequivalence.
Owner:武汉宏韧生物医药股份有限公司
Bioequivalence test for iron-containing formulations
InactiveUS20050123504A1Uniform particle sizePharmaceutical non-active ingredientsSynthetic polymeric active ingredientsBioequivalenceQuality control
A rapid method for assessing the bioequivalence of iron in iron-supplement formulations, particularly iron-sucrose formulations is described, which is based upon the kinetics of reduction of iron (III) to iron (II) in a sample of the formulation. Quality control methods and associated kits also are described.
Owner:AMERICAN REGENT INC
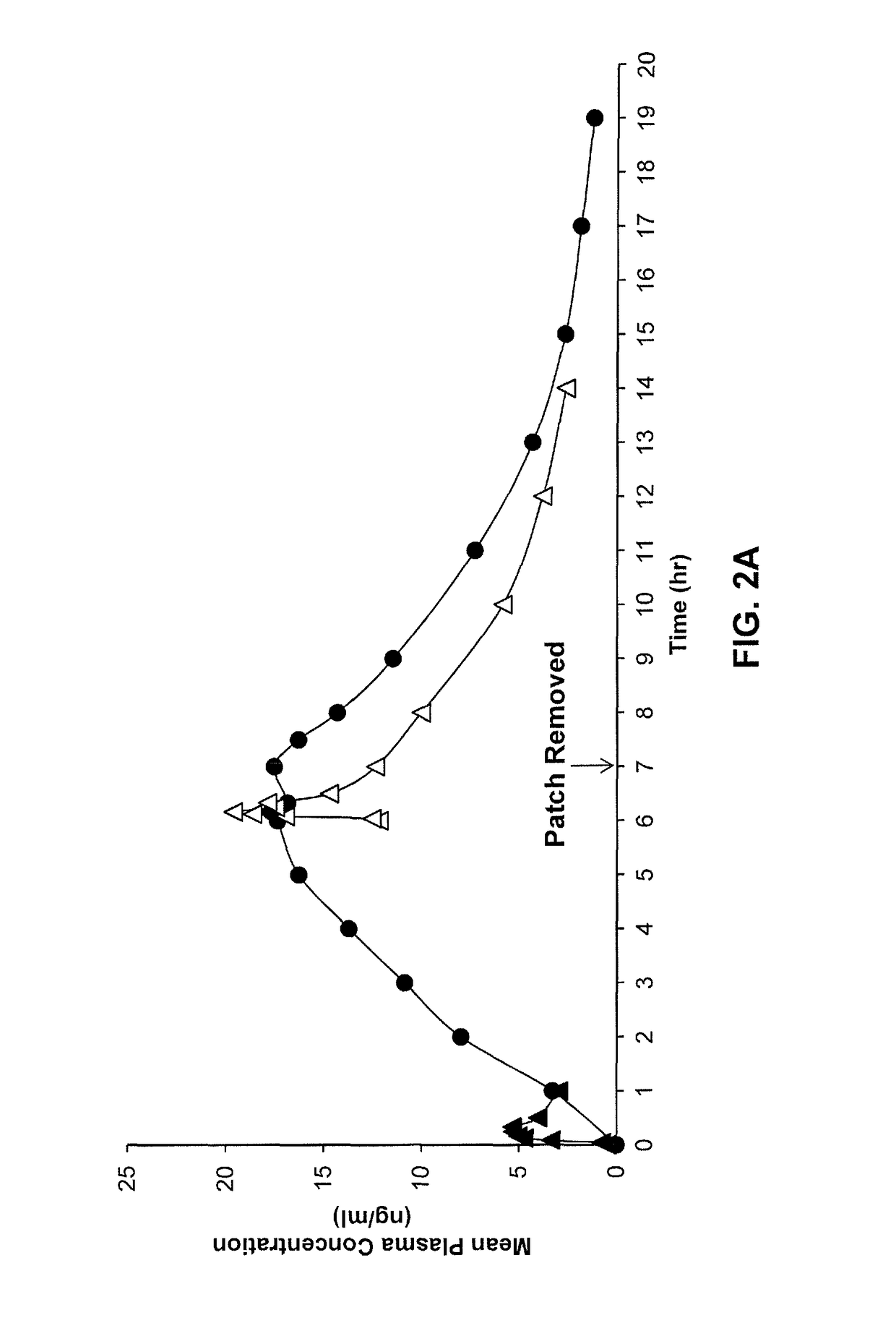
Transdermal delivery systems with pharmacokinetics bioequivalent to oral delivery
A method for delivering a therapeutic agent to a subject from a transdermal delivery system is described, where the therapeutic agent (i) has a half-life in the blood when delivered orally of greater than about 48 hours and (ii) is for the treatment of a chronic condition. The transdermal delivery system achieves transdermal delivery of the therapeutic agent at steady state that is bioequivalent to administration of the therapeutic agent orally, wherein bioequivalency is established by (a) a 90% confidence interval of the relative mean Cmax and AUC of the therapeutic agent administered from the transdermal delivery system and via oral delivery between 0.70 and 1.43 or between 0.80 and 1.25, or (b) a 90% confidence interval of the ratios for AUC and Cmax of the therapeutic agent administered from the transdermal delivery system and via oral delivery between 0.70 and 1.43 or between 0.80 and 1.25.
Owner:CORIUM LLC
A method of simultaneously detecting the content of artesunate and the content of dihydroartemisinin in animal blood plasma
InactiveCN103940918AHigh purityImprove concentrationComponent separationSolid phase extractionSolvent
The invention discloses a method of simultaneously detecting the content of artesunate and the content of dihydroartemisinin in animal blood plasma. The method comprises steps of: extracting a blood plasma sample with a solvent, processing the sample liquid with a solid phase extraction technology, adding the sample liquid into a small processed solid-phase extraction column, washing the column with acetic acid and a methanol-acetic acid solution, eluting with ethyl acetate and 1-chorobutane, collecting the eluate, blowing the eluate to dry with nitrogen, dissolving residue with methanol so as to obtain a sample solution to be tested, and accurately measuring the content of the artesunate and the content of the dihydroartemisinin in the sheep blood plasma by utilization of HPLC-MS / MS. The lowest detectable limit of the artesunate is 0.1 ng*mL<-1>. The lowest detectable limit of the dihydroartemisinin is 1 ng*mL<-1>. The method reduces influences of impurities, enriches the sample concentration and is high in specificity and good in separation effect. Linearity, stability, reproducibility, and recovery tests of the method satisfy good technical requirements. The method lays methodology foundations for research of pharmacokinetics and bioequivalence of the medicine inside animals.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Method for preparing isosorbide mononitrate sustained-release capsules
ActiveCN106138012ASolve the crystallization problemAvoid easy cakingGranular deliveryMicrocapsulesAdjuvantSustained Release Capsule
The invention belongs to the field of medicine industry and particularly relates to a method for preparing isosorbide mononitrate sustained-release capsules. The method for preparing the isosorbide mononitrate sustained-release capsules, provided by the invention, comprises the steps: (1) preparing isosorbide mononitrate quick-release micropellets; (2) preparing isosorbide mononitrate sustained-release micropellets; (3) carrying out mixing: mixing the isosorbide mononitrate quick-release micropellets with the isosorbide mononitrate sustained-release micropellets, and carrying out encapsulation, thereby obtaining the product. Shown by experimental determination, the product prepared by the technical scheme provided by the invention is good in stability, the problem, i.e., crystallization of isosorbide mononitrate is solved, and the problems of the existing processes that principal drugs and adjuvants are prone to caking and heated softening, the removal of residual solvent is not facilitated, and the micropellets are adhered are also avoided. Shown by in-vitro dissolution and in-vivo biological equivalent experimental investigations, the isosorbide mononitrate sustained-release capsules provided by the invention have bioequivalence compared with original triturates.
Owner:广东隆信制药有限公司
Trimetazidine hydrochloride sustained release tablet taking glyceryl behenate as framework material and preparation method of trimetazidine hydrochloride sustained release tablet
InactiveCN104274419ASimple processEase of industrial productionOrganic active ingredientsSenses disorderTrimetazidine DihydrochlorideSustained Release Tablet
The invention belongs to the field of sustained-release drug preparations, and particularly relates to a trimetazidine hydrochloride sustained release tablet taking glyceryl behenate as a framework material and a preparation method of the trimetazidine hydrochloride sustained release tablet. According to the main technical scheme disclosed by the invention, a sustained-release preparation is prepared from trimetazidine hydrochloride as an effective component, only glyceryl behenate as a sustained-release framework material and auxiliary materials such as a small amount of release speed regulator. According to the trimetazidine hydrochloride sustained release tablet provided by the invention, an in vitro release rate experiment shows that the drug release is not affected by the pH environment, compared with commercially available 'vasorel' (trimetazidine dihydrochloride tablet), the trimetazidine hydrochloride sustained release tablet has good similarity (f2 is greater than 65); and the Beagle pharmacokinetic experiment in dogs shows that the trimetazidine hydrochloride sustained release tablet has bioequivalence in comparison with 'vasorel'. One trimetazidine hydrochloride sustained release tablet provided by the invention is taken twice a day, and the trimetazidine hydrochloride sustained release tablet is convenient to take, has good medicine compliance in patients, and is capable of keeping steady state plasma concentration for a long period of time. The preparation method disclosed by the invention is simple and stable in process, and is easily put into volume production.
Owner:广东省中药研究所
Method for detecting quetiapine in human plasma by HPLCMS-MS combination
InactiveCN109900820ASame retention timeGood reproducibilityComponent separationQuetiapineRetention time
The invention belongs to the field of biological analysis and particularly relates to a method for detecting quetiapine in human plasma by HPLCMS-MS combination. The method comprises steps of (1), human plasma sample pretreatment; (2), liquid chromatography-mass spectrum combined detection, mobile phase A and mobile phase B are utilized as a mixed mobile phase for gradient elution, the mobile phase A is acetonitrile-water, the mobile phase B is ammonium acetate-water; and (3), determination of the concentration of the quetiapine in the human plasma. The method is advantaged in that deuteratedfumaric acid quetiapine is used as an internal marker, gradient elution is performed through utilizing an InertSustain C18 column, the deuterated internal marker and a determinand have the same retention time, chemical properties and matrix effects, and reproducibility and accuracy of concentration detection of the deuterated fumaric acid quetiapine in plasma are good. The method is used for evaluating bioequivalence of each quetiapine dosage form.
Owner:NANJING HEALTHNICE MEDICAL TECH +2
Fluconazole tablets and preparation method thereof
InactiveCN108578377APromote dissolutionGood bioequivalenceOrganic active ingredientsAntimycoticsAdhesiveFiller Excipient
The invention belongs to the technical field of medicine preparations and particularly relates to fluconazole tablets and a preparation method thereof. The fluconazole tablets comprise the following raw materials in parts by weight: 40 to 50 parts of fluconazole, 15 to 40 parts of a filling agent, 2 to 15 parts of a disintegrating agent, 5 to 10 parts of an adhesive and 0.2 to 2 parts of a lubricant, wherein the filling agent comprises sodium alginate, microcrystalline cellulose and anhydrous calcium hydrogen phosphate; the disintegrating agent comprises croscarmellose sodium; the adhesive ispovidone K30; and the lubricant is magnesium stearate. The fluconazole tablets have the advantages of high compressibility, controllable friability and dissolubility, rapidness in disintegration, excellent stability and dissolution performance, high bioequivalence and high bioavailability.
Owner:TIANJIN PHARMA GROUP XINZHENG
Concentration determining method of arecoline hydrobromide in animal blood plasma
InactiveCN103940935AMeet the analysis and testing requirementsHigh detection sensitivityComponent separationInternal standardBlood plasma
The invention relates to a concentration determining method of arecoline hydrobromide in animal blood plasma. The method applies high performance liquid chromatography-tandem mass spectrum technology for concentration determination of the arecoline hydrobromide in the animal blood plasma. Beta-pinene is adopted as an internal standard substance, and the concentration of the arecoline hydrobromide in the animal blood plasma can be accurately determined. The high performance liquid chromatography-tandem mass spectrum process which is rapid, accurate and sensitive is adopted for the concentration determination of the arecoline hydrobromide in the animal blood plasma. The method is high in specificity and good in separation effect. Linearity, stability, reproducibility, and recovery tests of the method satisfy good technical requirements. The method lays methodology foundations for research of pharmacokinetics and bioequivalence of the medicine inside animals.
Owner:LANZHOU INST OF ANIMAL SCI & VETERINARY PHARMA OF CAAS
Bioequivalence test for iron-containing formulations
InactiveCN1685227AOrganic active ingredientsAnalysis using chemical indicatorsSucroseIRON PREPARATIONS
Owner:VIFOR (INT) AG
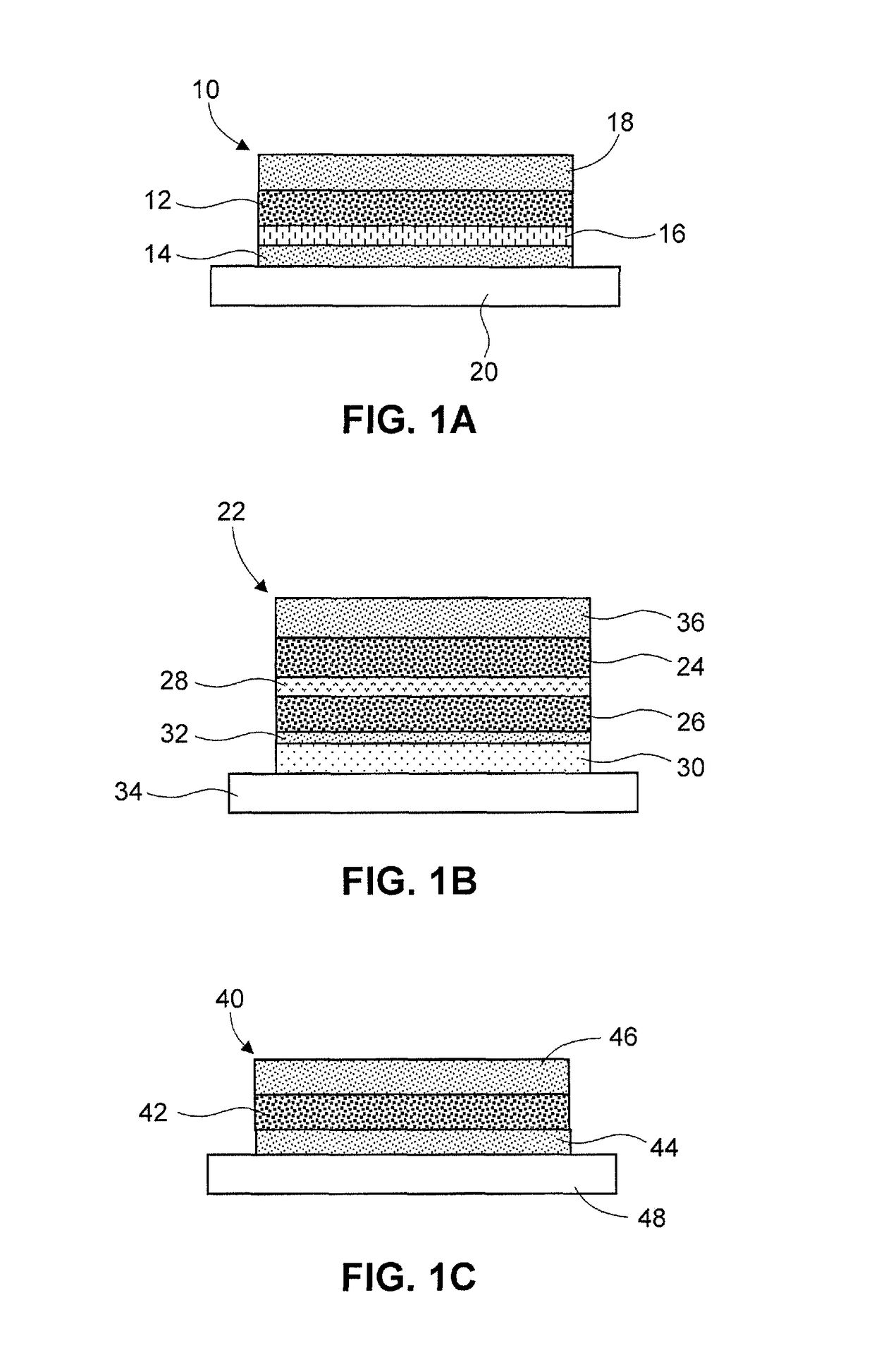
Seven day drug in adhesive transdermal delivery
ActiveUS9408802B1Conveniently and effectively deliverPatient compliance is goodAdhesive dressingsSheet deliveryCross-linked polyethyleneDrug in adhesive
A transdermal formulation and method of treatment includes providing an active pharmaceutical ingredient, a poly-isobutylene or silicone PSA, optionally, an oil, a crosslinked polyvinylpyrrolidone, and optionally, silicon dioxide, wherein said formulation is prepared on a backing in a three layer transdermal delivery system formulated to provide transdermal delivery and therapeutic levels for up to seven days and optionally with an overlay system required to show bioequivalence.
Owner:PROSOLUS PHARM LP
Solid dispersion method of celecoxib and preparation method of celecoxib capsules
ActiveCN110604722AEffective dispersionHigh densityOrganic active ingredientsPowder deliveryLACTOSE MONOHYDRATEMechanical milling
The invention provides a solid dispersion method of celecoxib and a preparation method of celecoxib capsules, and the solid dispersion method comprises the following steps: mixing the celecoxib with pharmaceutical adjuvants to obtain raw and auxiliary material mixed powder, and carrying out ultramicro jet milling and / or mechanical milling on the raw and auxiliary material mixed powder, wherein thepharmaceutical adjuvants at least comprise lactose monohydrate and lauryl sodium sulfate. The solid dispersion method disclosed by the invention not only overcomes the characteristic of poor preparation of a celecoxib preparation, but also solves the problem of slow dissolution of the celecoxib, and the preparation is good in stability, so that the preparation is consistent with an original developed preparation (Celebrex) in prescription and dissolution, and the quality consistency and bioequivalence of the celecoxib preparation and the original developed preparation (Celebrex) are ensured.
Owner:SHANDONG CHUANGXIN PHARMA RES & DEV
Oxcarbazepine sustained release tablet and preparation method thereof
InactiveCN106727391ASolve solubilityGuaranteed bioequivalenceNervous disorderPharmaceutical non-active ingredientsSustained Release TabletReference product
The invention relates to an oxcarbazepine sustained release tablet and a preparation method thereof. According to the method adopted by the invention, raw materials are specially treated together with an auxiliary material first to prepare oxcarbazepine composition, and the particle size of the oxcarbazepine composition is strictly controlled. KG-802 is added into an extra auxiliary material. According to the oxcarbazepine sustained release tablet prepared with the method provided by the invention, the use of a large amount of a cosolvent namely sodium lauryl sulfate in an original developed preparation is avoided, the toxicity of a preparation finished product to a human body after taking is reduced, and the pollution to the environment in a preparation process is reduced; and compared with a reference product on sale, release curves in release media with different pH values can keep similar, the bioequivalence of an in-vitro test is ensured, and the release curves of the release media with different pH values are more similar to one another in comparison with the preparation on sale. The oxcarbazepine sustained release tablet provided by the invention is prepared by adopting a conventional granulation and tabletting process, the process is simple, and the oxcarbazepine sustained release tablet is suitable for large-scale production.
Owner:浙江四维医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com