Oxcarbazepine sustained release tablet and preparation method thereof
A gentle, tablet-core technology, applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, pill delivery, etc., can solve problems such as increased potassium gas conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
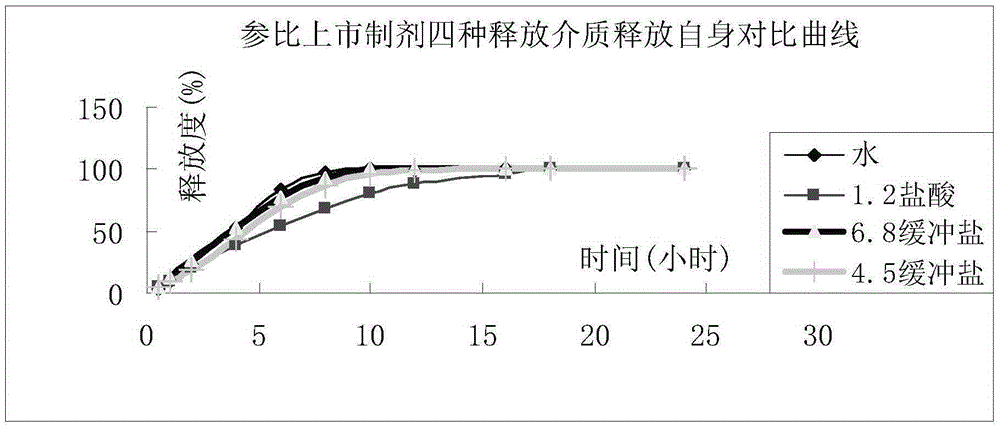
Image
Examples
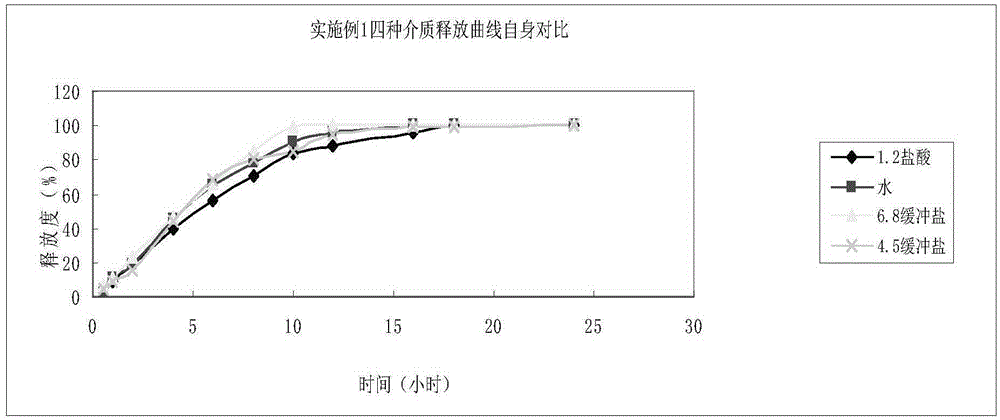
Embodiment 1
[0051] According to the amount of 100 tablets, weigh 60g of oxcarbazepine, add 1.3g of colloidal silicon dioxide, mix evenly, and then perform jet milling, then add 9g of microcrystalline cellulose, mix and then perform jet milling, and the particle size of the mixture is controlled at D90 of 25 μm ., D50 is 15 μm, D5 is 5 μm, then add microcrystalline cellulose 10g, hypromellose K4M12g, L100-55 4.5g, PVP3g, mix well, add an appropriate amount of purified water to 24 mesh wet granulation, and dry at 50-55°C When the water content reaches 3%, the granules are sized at 24 meshes, and then 1.2g of KG-802, 0.5g of magnesium stearate, and 1.5g of colloidal silicon dioxide are added, mixed evenly, and then compressed into tablets. The theoretical tablet weight is 1.03g, and the tablet hardness is It is controlled at 10-19kg, and then coated with a gastric-soluble coating powder containing an opacifying agent to form a mixed solution, and the theoretical weight gain is 2-4%.
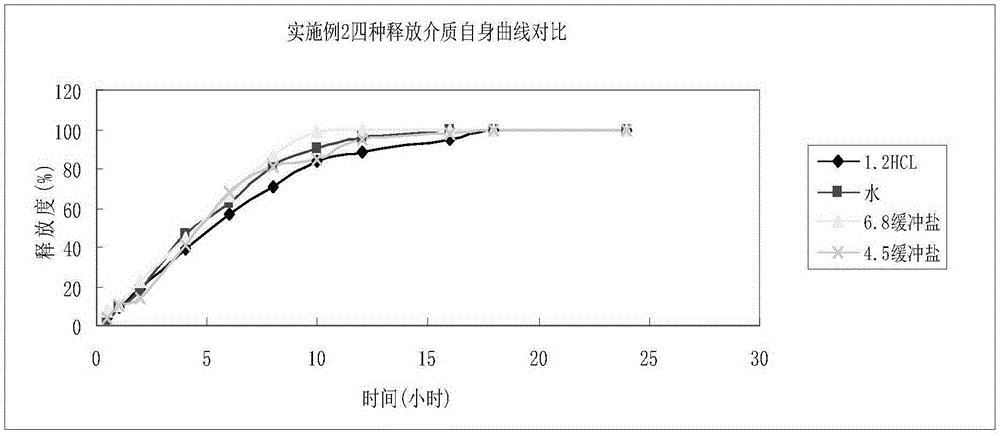
Embodiment 2
[0053] According to the amount of 100 tablets, weigh 60g of oxcarbazepine, add 1.0g of colloidal silicon dioxide, mix evenly, and then perform jet milling, then add 10g of microcrystalline cellulose, mix and then perform jet milling, and the particle size of the mixture is controlled at D90 of 25 μm ., D50 is 15 μm, D5 is 5 μm, then add microcrystalline cellulose 10g, hypromellose K15M7g, L100-55 7.5g, PVP3g, mix well, add an appropriate amount of purified water to 24 mesh wet granulation, and dry at 50-55°C When the water content is 3%, the granules are granulated at 24 mesh, and then 1.2g of KG-802, 3g of magnesium stearate, and 1.5g of colloidal silicon dioxide are added, mixed evenly, and then pressed into tablets. The theoretical tablet weight is 1.042g, and the tablet hardness is controlled. At 10-19 kg, the stomach-soluble coating powder containing an opacifying agent is formulated into a mixed solution for coating, and the theoretical weight gain is 2-4%.
Embodiment 3
[0055] According to the amount of 100 tablets, weigh 60g of oxcarbazepine, add 0.8g of colloidal silicon dioxide, mix evenly, and then perform jet milling, then add 6g of microcrystalline cellulose, mix and then perform jet milling, and the particle size of the mixture is controlled at D90 of 25 μm ., D50 is 15 μm, D5 is 5 μm, then add microcrystalline cellulose 8g, hypromellose K15M7g, L100-55 7.5g, PVP 2g, mix well, add an appropriate amount of purified water to 24 mesh wet granulation, and dry at 50-55°C When the water content is 3%, the granules are granulated at 24 mesh, and then 1.3g of KG-802, 0.5g of magnesium stearate, and 1.5g of colloidal silicon dioxide are added, mixed evenly, and then compressed into tablets. The theoretical tablet weight is 0.946g, and the tablet hardness is It is controlled at 10-19kg, and then coated with a gastric-soluble coating powder containing an opacifying agent to form a mixed solution, and the theoretical weight gain is 2-4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com