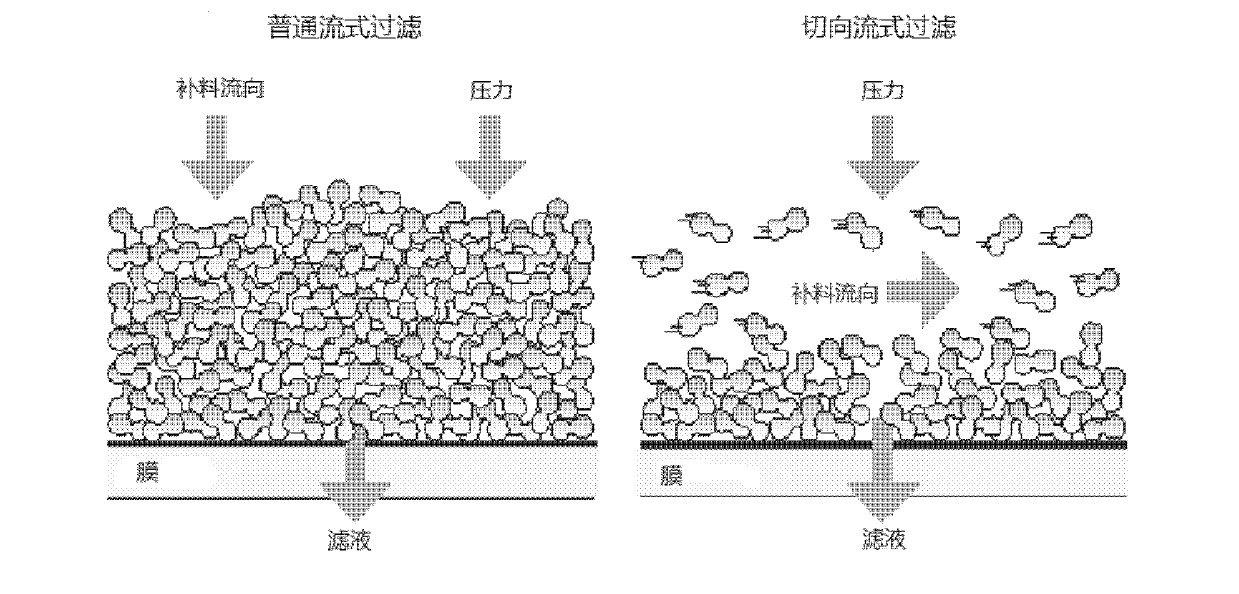
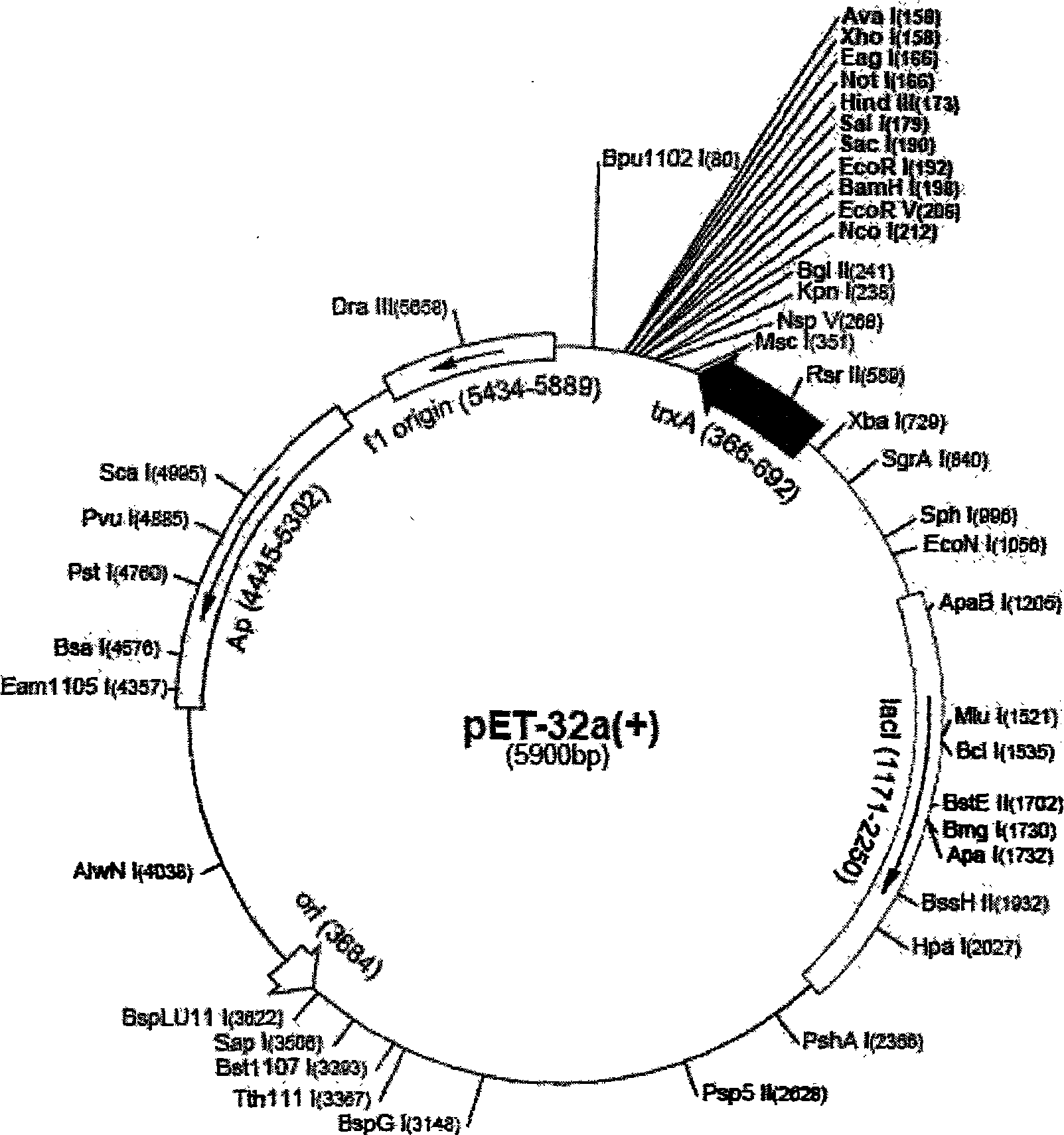
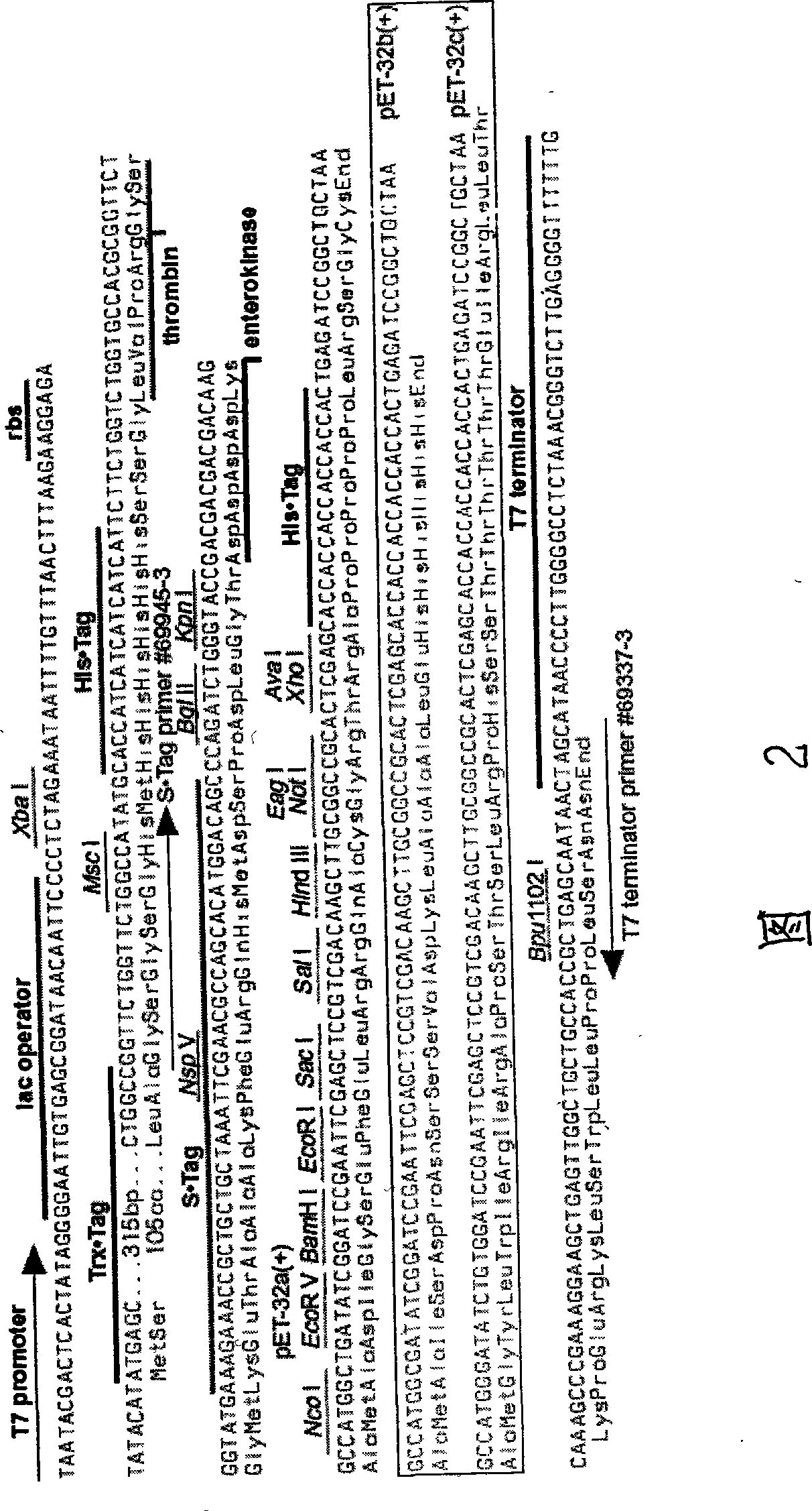
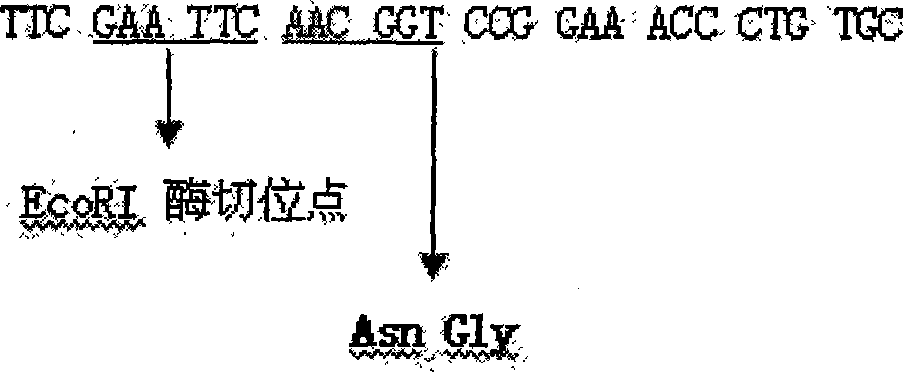Patents
Literature
268 results about "Fusion Protein Expression" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The expression of the fusion protein may be affected by a variety of factors such as the (a) E. coli strain, (b) cell growth conditions (e.g. temperature, aeration, cell density, IPTG concentration, etc.), (c) toxicity of the target protein, (d) codon usage and (e) structure and stability of mRNA.
POLYMERIC IMMUNOGLOBULIN FUSION PROTEINS THAT TARGET LOW AFFINITY FCyRECEPTORS
InactiveUS20090117133A1Small size range and conformationPromote generationSenses disorderPeptide/protein ingredientsLow affinityNACHT domain
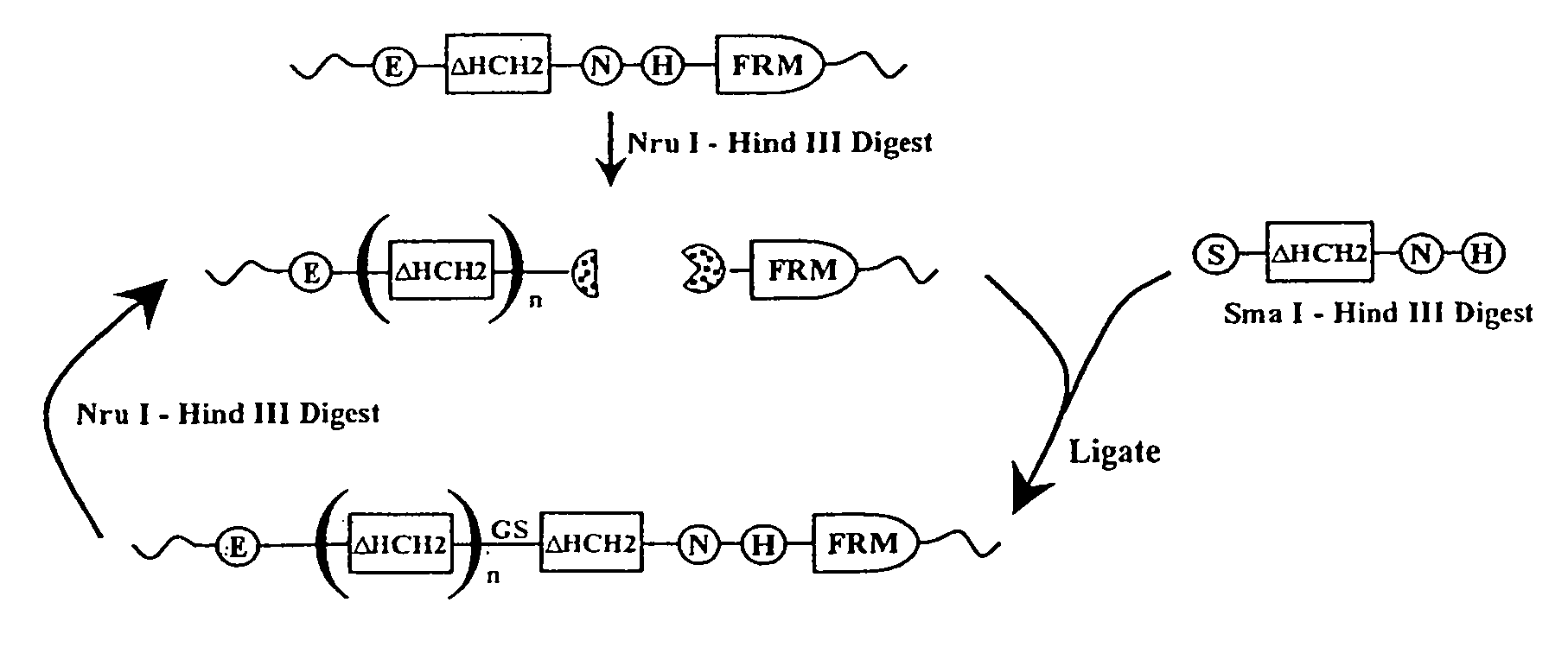
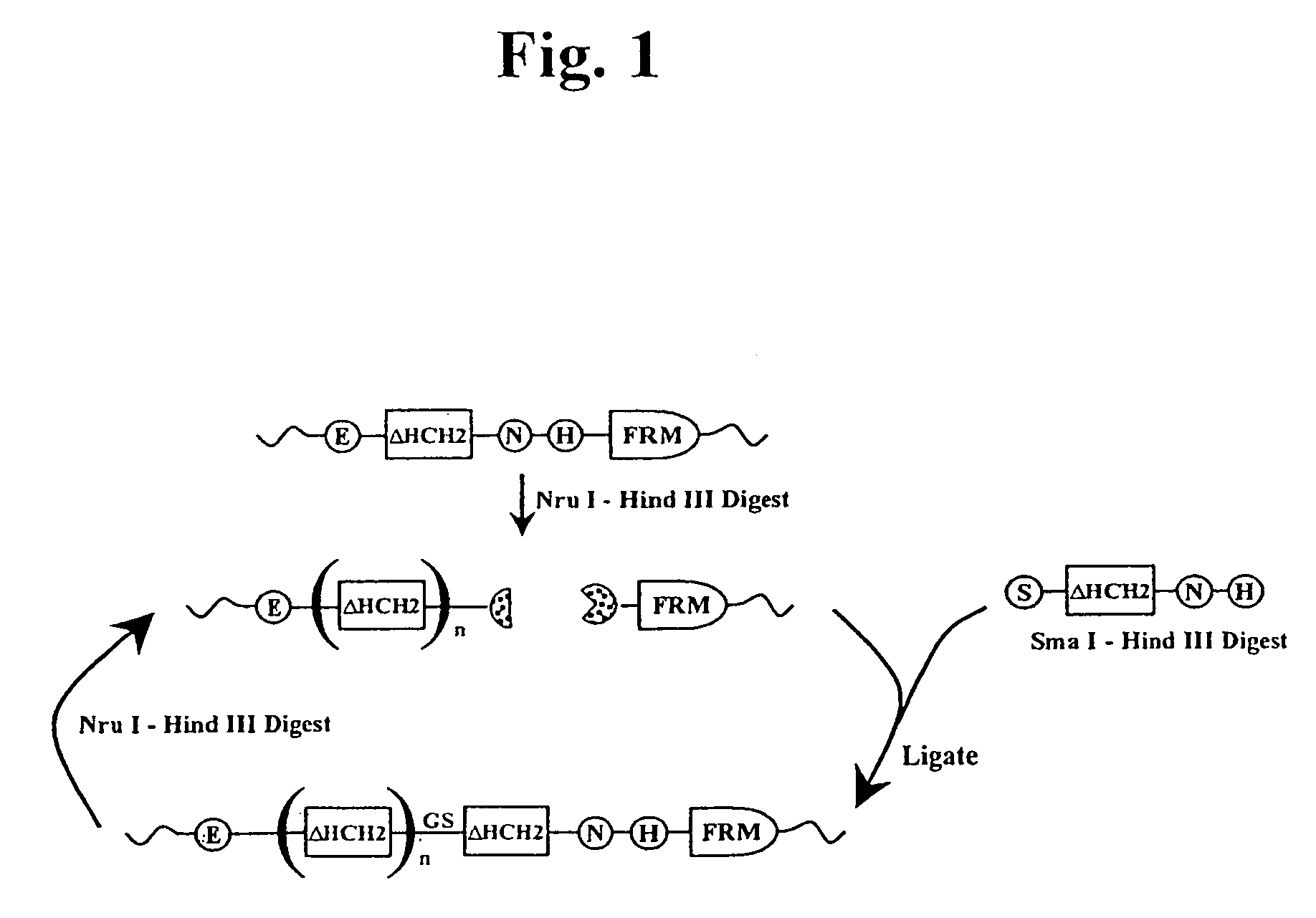
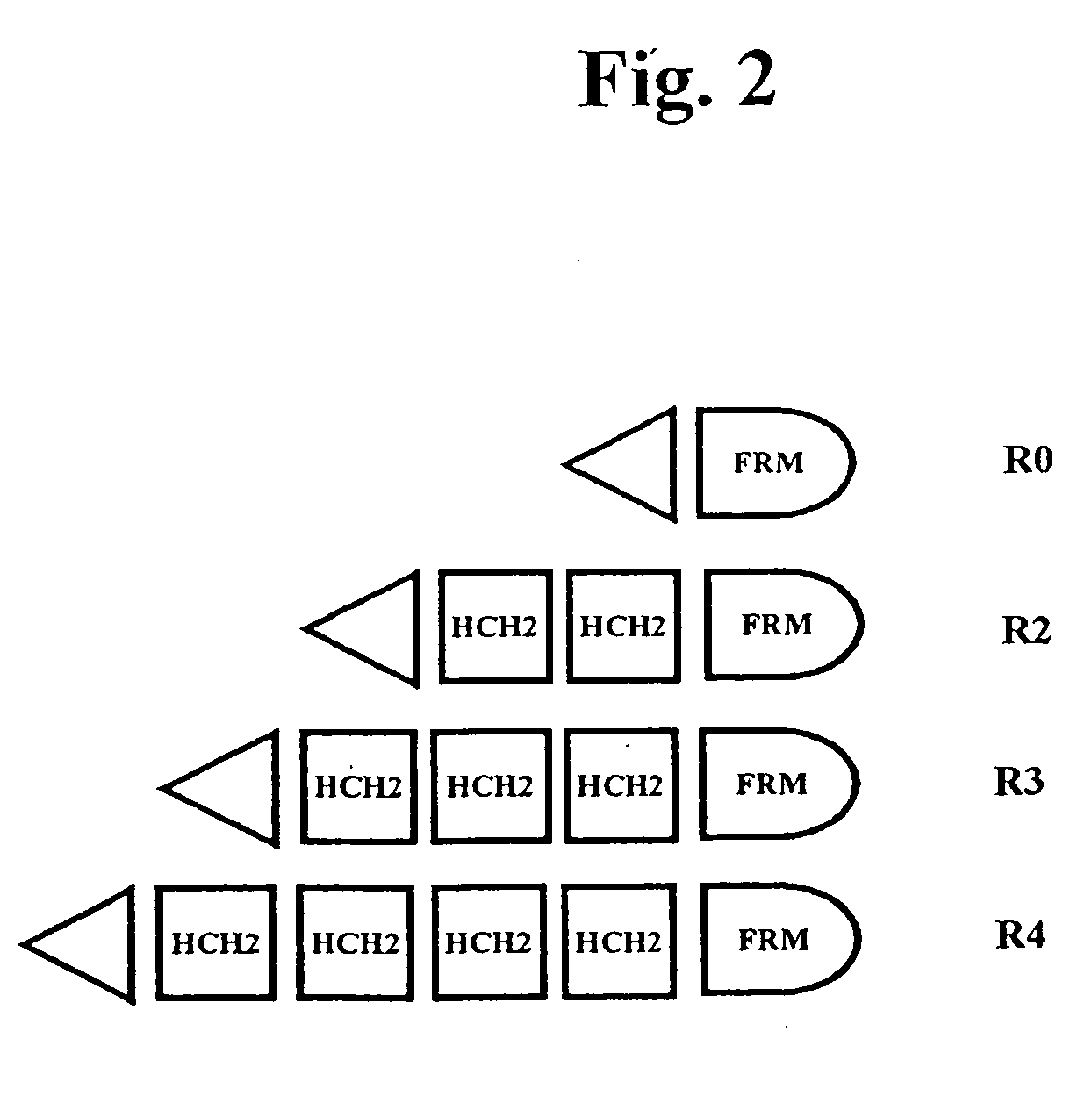
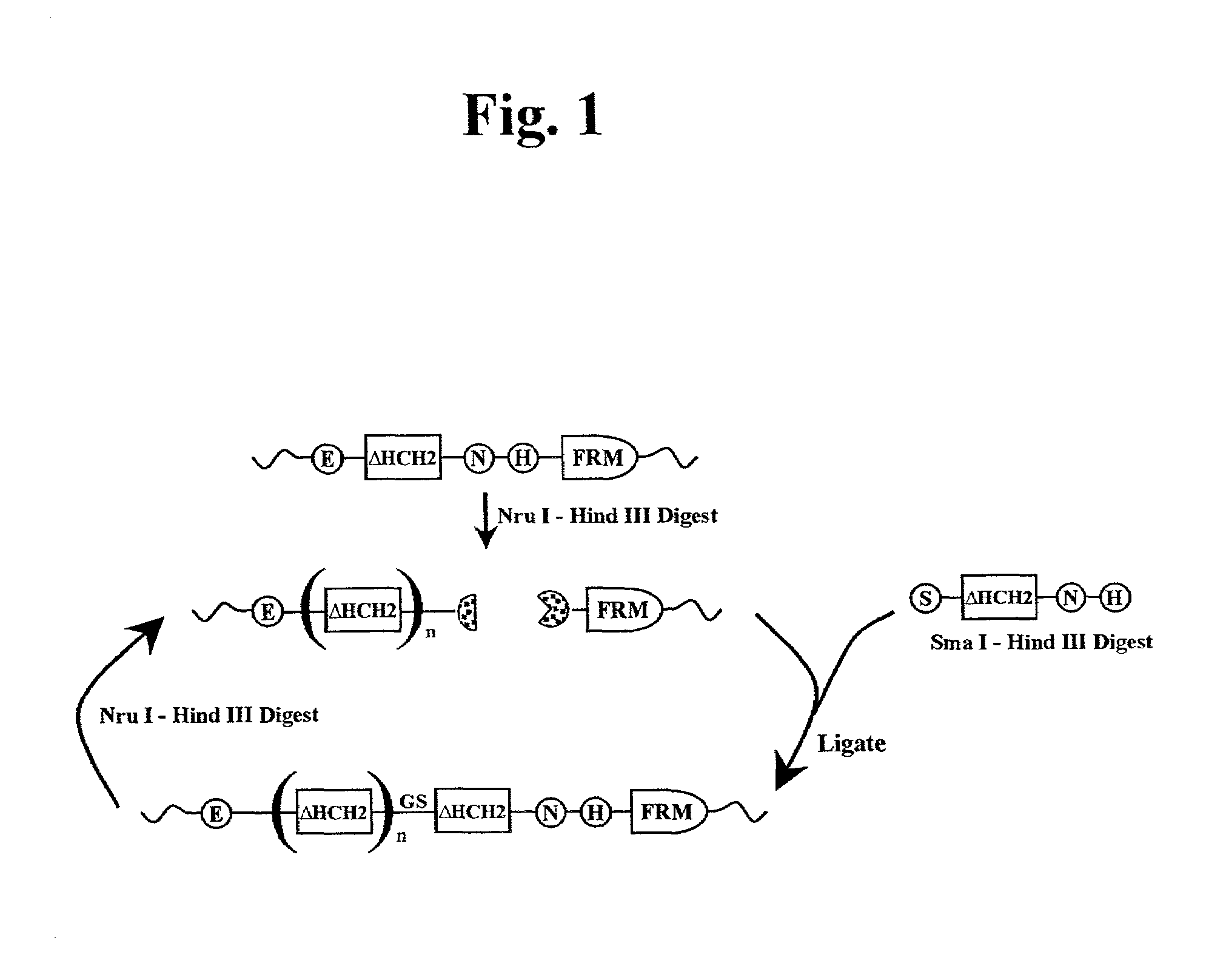
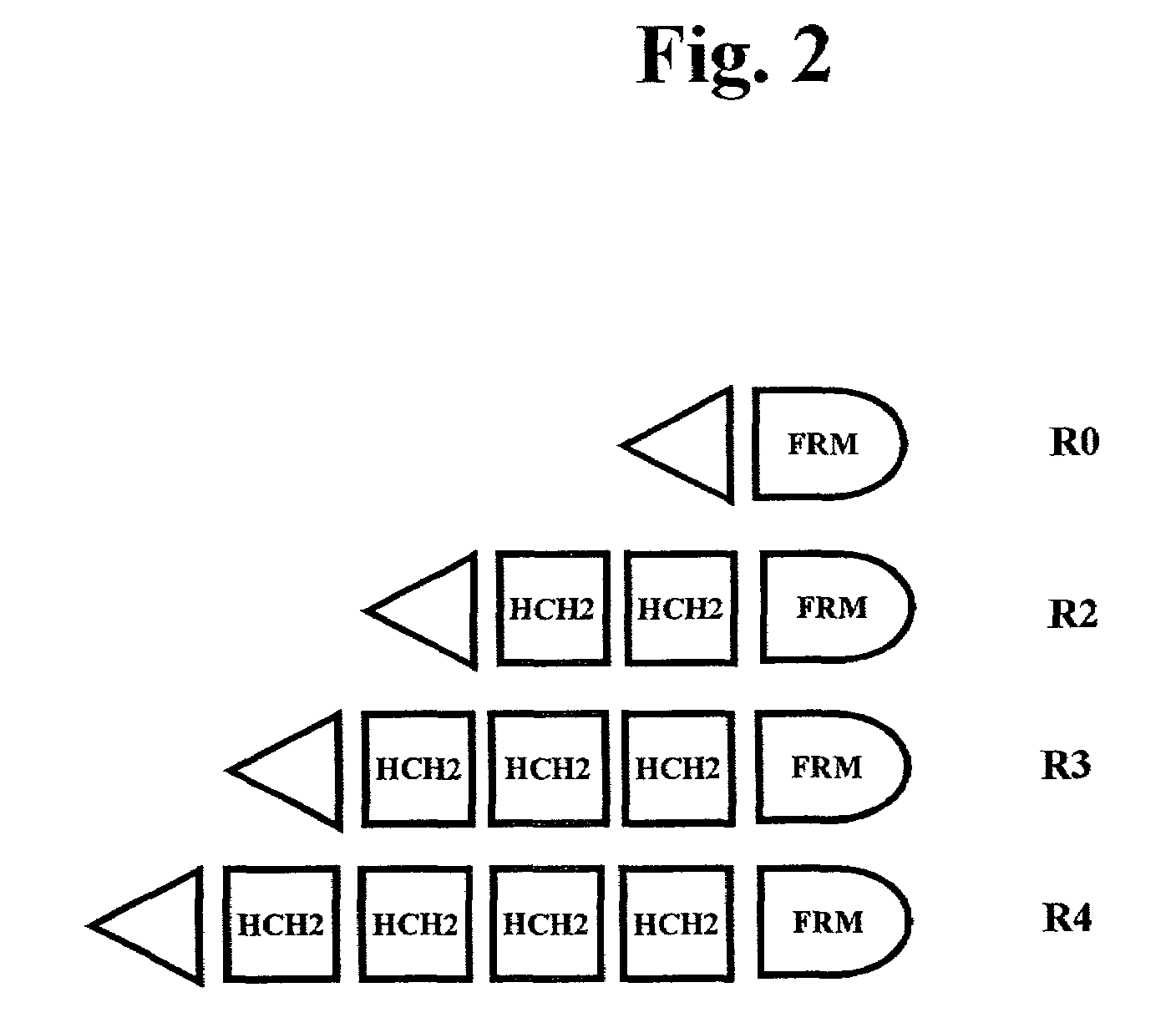
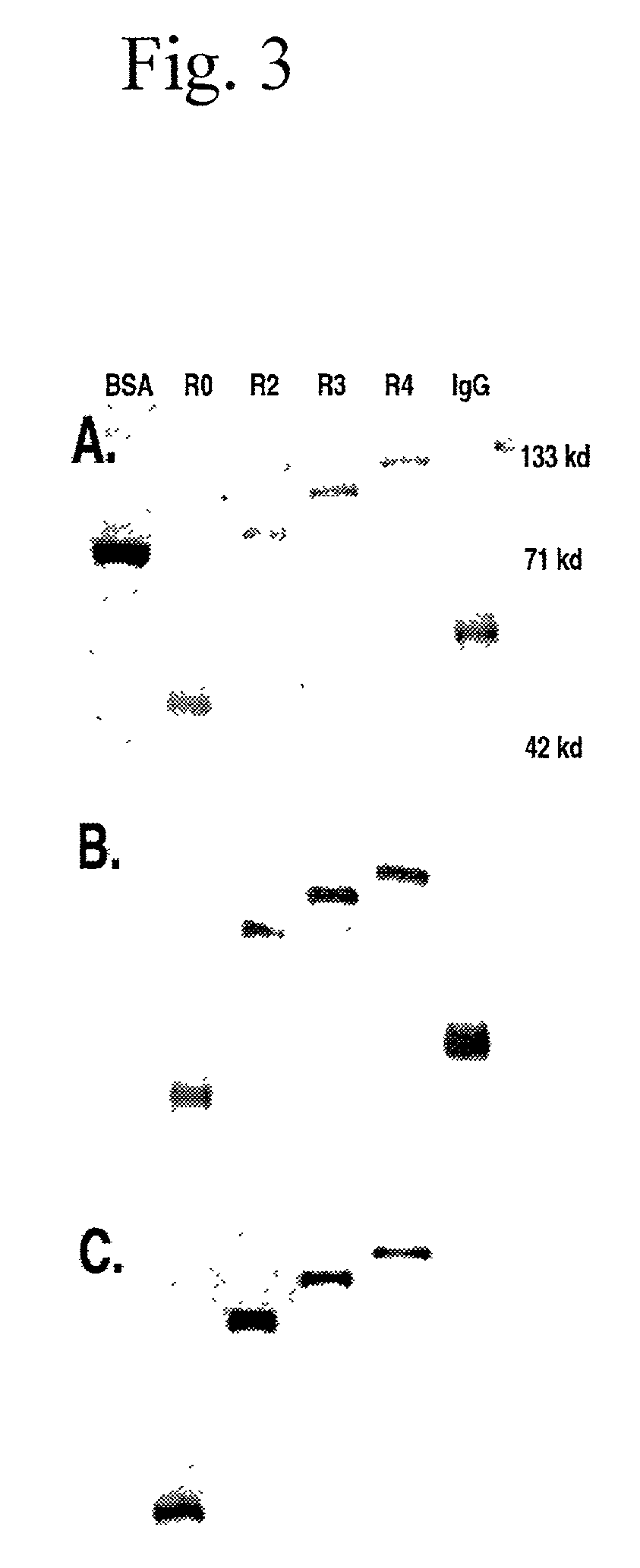
The present invention concerns a family of nucleic acids, polypeptides and cloning vectors which direct expression of fusion proteins that can mimic aggregated IgG (AIG) and immune complex function with respect to their interactions with FcγR and which allow for the inclusion and targeting of a second protein domain to cells expressing FcγR. This was accomplished by expressing multiple linear copies of the hinge and CH2 domains (HCH2) of human IgG1 fused to the framework region of human IgG1. Convenient restriction sites allow for the facile introduction of additional amino-terminal domains. Methods for treating patients using fission proteins are also disclosed. The HCH2 polymers described here represent a new strategy in the design of recombinant proteins for the therapeutic targeting of FcγR in autoimmune disorders.
Owner:ITERATIVE THERAPEUTICS
Polymeric immunoglobulin fusion proteins that target low-affinity Fcγreceptors
ActiveUS7511121B2Improve effectivenessModerating disease severitySenses disorderPeptide/protein ingredientsFusion Protein ExpressionLow affinity
The present invention concerns a family of nucleic acids, polypeptides and cloning vectors which direct expression of fusion proteins that can mimic aggregated IgG (AIG) and immune complex function with respect to their interactions with FcγR and which allow for the inclusion and targeting of a second protein domain to cells expressing FcγR. This was accomplished by expressing multiple linear copies of the hinge and CH2 domains (HCH2) of human IgG1 fused to the framework region of human IgG1. Convenient restriction sites allow for the facile introduction of additional amino-terminal domains. Methods for treating patients using fusion proteins are also disclosed. The HCH2 polymers described here represent a new strategy in the design of recombinant proteins for the therapeutic targeting of FcγR in autoimmune disorders.
Owner:ITERATIVE THERAPEUTICS
Human pancreas hyperglycemiacin relative peptide-2 analogue
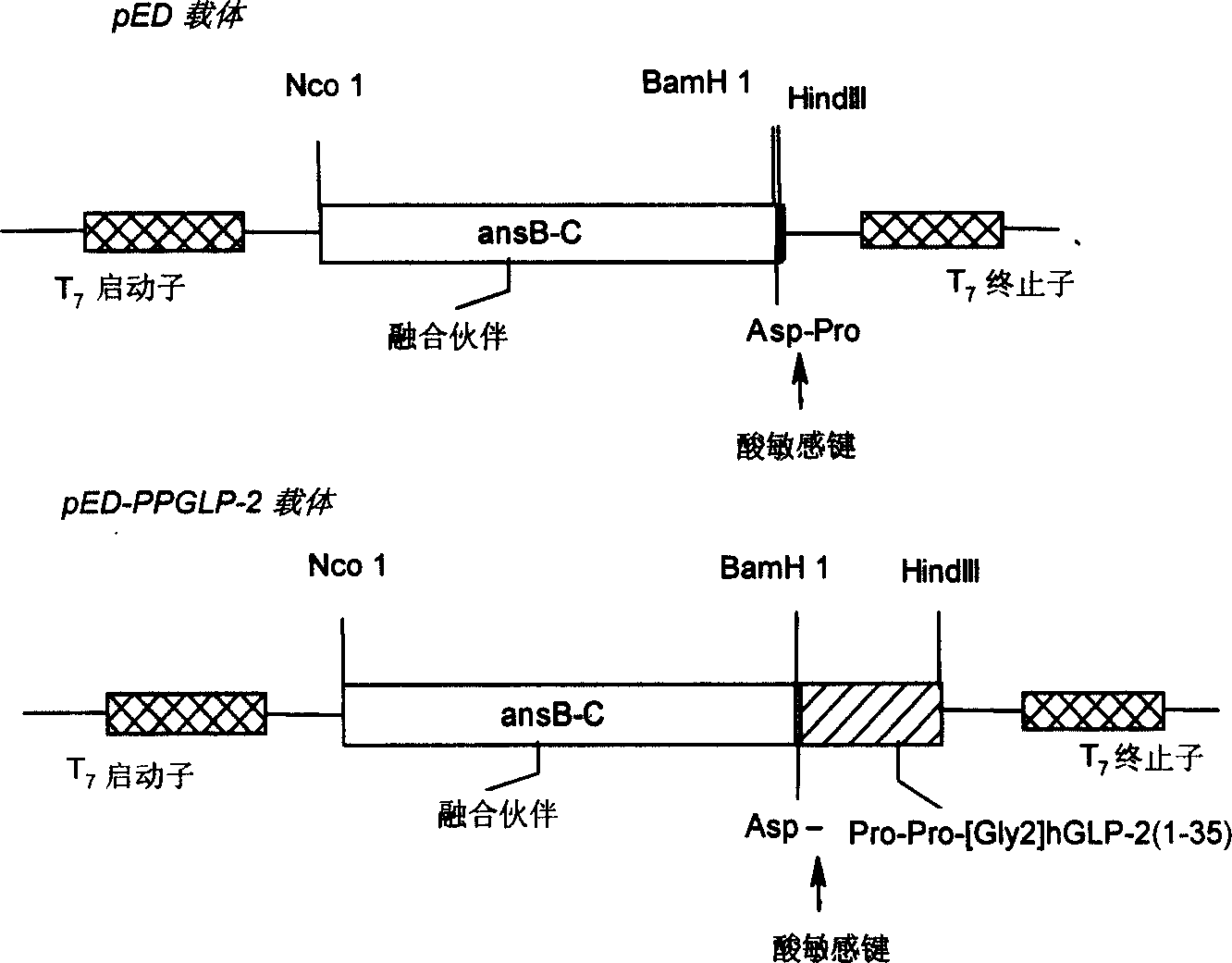
A human glucagon associated peptide-2 analog Pro-Pro-h[Gly2]GLP-2(1-35) for treating the short bowel syndrome and malabsorption of stomach and intestine is prepared through configuring genetic engineering bacterium for effective expression of Pro- Pro-h[Gly2]GLP-2(1-35) in colibacillus cell, splitting, washing, dissolving urea, depositing in alcohol, separating fusion protein, hydrolyzing it, chromatography by DEAE-52 column, separating by HPLC, and freeze drying.
Owner:CHINA PHARM UNIV
Establishing method of pig immunoglobulin Fc fragment-swine classical fever E2 fusion protein in CHO cell strain, as well as preparation method and application of fusion protein
ActiveCN106519041ASsRNA viruses positive-senseAntibody mimetics/scaffoldsFusion Protein ExpressionVaccine Production
The invention relates to a vaccine production technology in the technical field of biology, in particular to a CHO cell strain which is established by utilizing a gene engineering means and is used for expressing recombinant protein PigFC-pigSCFVE2, and a preparation method and application of the recombinant protein. The recombinant fusion protein PigFC-pigSCFVE2 provided by the invention is A1) or A2) shown as follows, wherein A1) is protein of which the amino acid sequence is as shown in SEQ ID No.2, and A2) is protein which is obtained by substituting, losing and / or adding one or several amino acid residues in the amino acid sequence of the protein of the A1) and has PigFC-pigSCFVE2 activity. A monoclonal cell strain which is obtained through the method and capable of carrying out secretory expression on PigFC-pigSCFVE2 is higher in fusion protein expression quantity, fusion protein obtained through affinity separation and purification of an antibody can be combined with a monoclonal antibody, animals can be immunized, the immunity of a generated neutralizing antibody is higher than that of a present market product, the fusion protein can be used for swine classical fever preventive vaccine, and the production cost and the immunity failure loss can be reduced.
Owner:TANGSHAN YIAN BIOLOGICAL ENG CO LTD
High-yield reactor for protein production, and production method and application thereof
InactiveCN103305417AReduce abnormal structural changesHigh yieldBioreactor/fermenter combinationsBiological substance pretreatmentsPerfusion CultureFiber
The invention discloses a high-yield reactor, and a production method and an application thereof. The high-yield reactor comprises a bioreactor and an ATF perfusion apparatus connected with the bioreactor. The protein production method using the high-yield reactor comprises the following steps: 1, connecting the ATF perfusion apparatus with the bioreactor, and completing an offline disinfection or online disinfection program; 2, inoculating seed cells to the bioreactor to a work volume according to the density, and sampling and detecting the number and biochemical indexes of the cells each 24h; 3, starting the ATF apparatus program for perfusion culture when the density of living cells increases to a certain density; and 4, collecting a culture solution in the bioreactor or filtered through hollow fibers in the ATF perfusion apparatus, and purifying to obtain required protein products. The high-yield reactor has the advantages of great shortening of the technological exploitation time of monoclonal antibody or fusion protein expression, production cost reduction, benefiting for the project registering and reporting and the project period shortening, and acceleration of the accomplishment application speeds of the biopharmacy industry.
Owner:WUXI BIOLOGICS CO LTD
Lactococcus promoters and uses thereof
ActiveUS8759088B2Increase the number ofHigh expressionBiocideBacteriaHeterologousFusion Protein Expression
The invention is in the field of molecular biology, and relates to recombinant engineering and protein expression. More in particular, the invention relates to nucleic acids for recombinant expression of proteins comprising sequences derived from Lactococcus and useful as promoters. The invention further relates to vectors comprising the nucleic acids and host cells transformed therewith. The invention also covers the use of host cells comprising the nucleic acids or vectors for expressing heterologous or homologous proteins; and also for delivery, especially therapeutic delivery, of the said proteins to subjects.
Owner:INTREXON ACTOBIOTICS NV
Process for improved protein expression by strain engineering
ActiveUS20140162279A1Increasing recombinant protein productionReduce energy consumptionMicrobiological testing/measurementFermentationBiotechnologyFusion Protein Expression
This invention is a process for improving the production levels of recombinant proteins or peptides or improving the level of active recombinant proteins or peptides expressed in host cells. The invention is a process of comparing two genetic profiles of a cell that expresses a recombinant protein and modifying the cell to change the expression of a gene product that is upregulated in response to the recombinant protein expression. The process can improve protein production or can improve protein quality, for example, by increasing solubility of a recombinant protein.
Owner:PFENEX
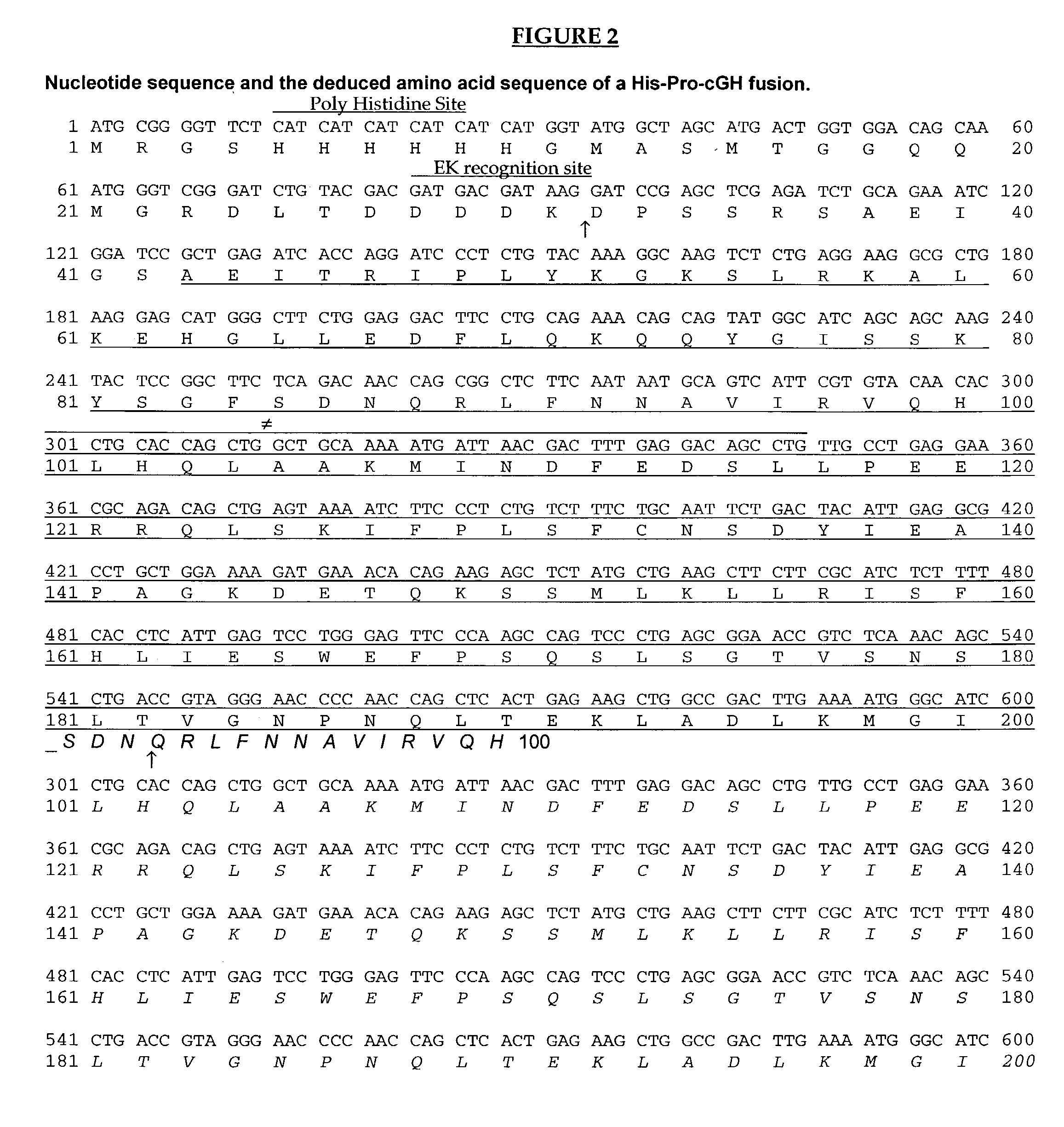
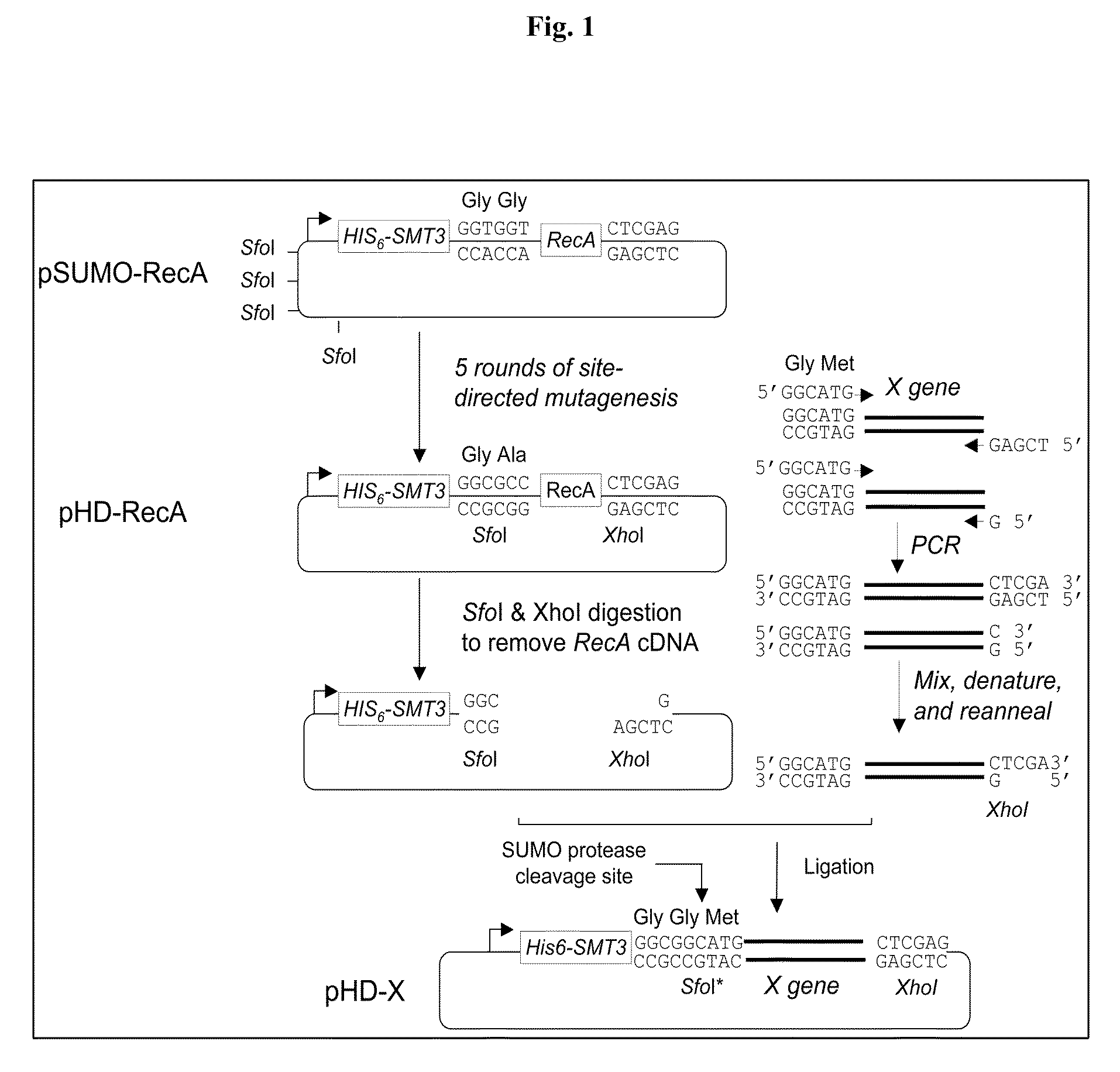
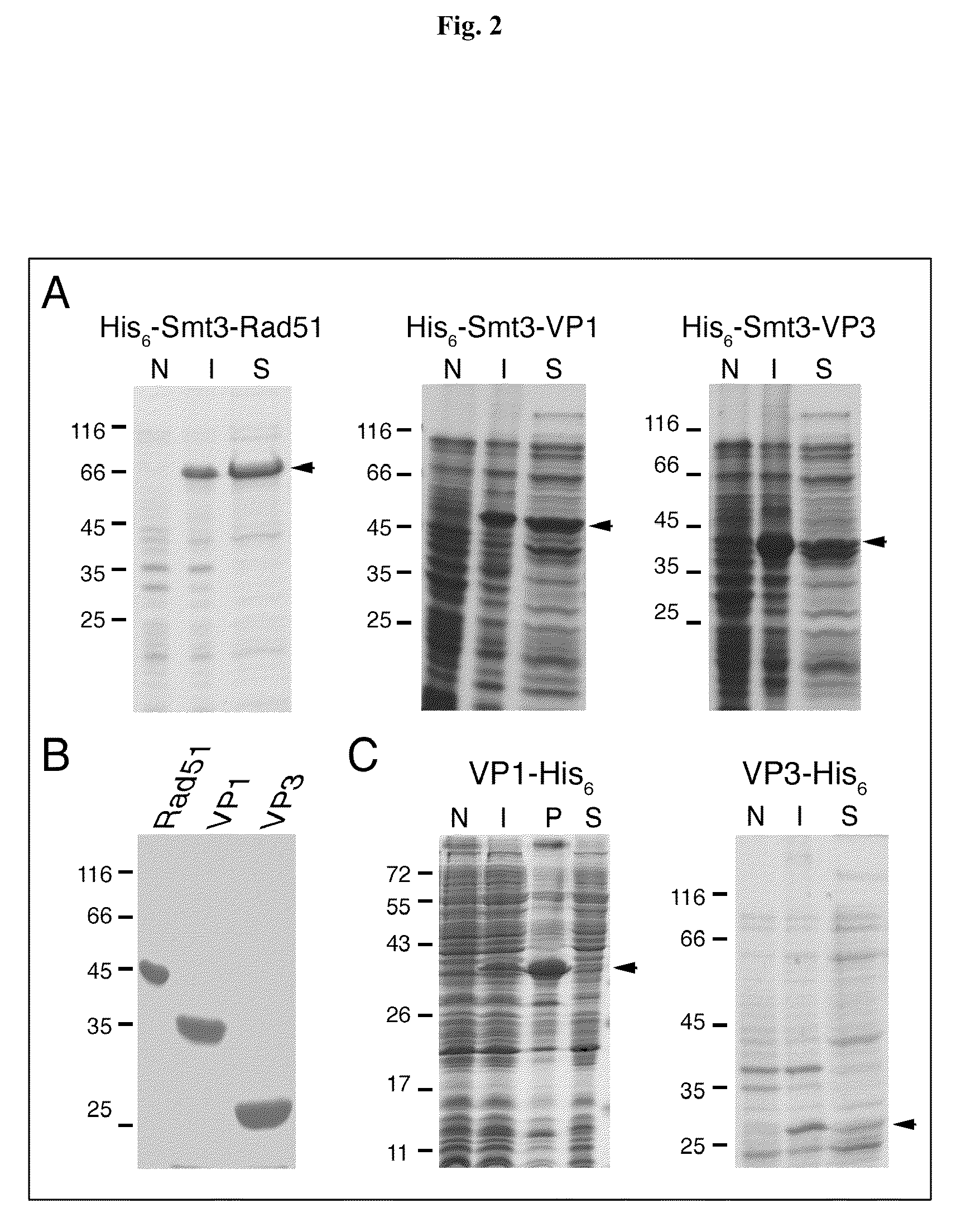
SUMO Fusion Protein Expression System for Producing Native Proteins
ActiveUS20090280535A1Peptide/protein ingredientsImmunoglobulinsFusion Protein ExpressionProtein formation
Owner:ACAD SINIC
Prepn of metallothionein
InactiveCN1348010AImprove compatibilityImprove production efficiencyFermentationEscherichia coliFusion Protein Expression
The present invention is one gene engineering process of preparing metallothionein in high yield. The process incldues constituting the expression plasmid or its mutant gene, transferring and fermentation culture in engineering colibacillus, induction and adding metal ion before further culture, collecting thallus; ultrasonic crushing thallus, centrifugation, static adsorption with affinity chromatographic column of glutathione-agarose gel 4B, incision with thrombase on the column, column chromatograhic desalting, gradient salt elution and collecting destination protein. The present inventioncan obtain destination protein with purity over 95 %. Using the said method to prepare 6 mutant proteins of monkey metallothionein I can obtain yield and purity similar to that in wild recombinant protein.
Owner:FUDAN UNIV
Recombinant immunogenic compositions and methods for protecting against lethal infections from Bacillus anthracis
ActiveUS7201912B2Reduce in quantityLow costBacterial antigen ingredientsSnake antigen ingredientsProtective antigenFusion Protein Expression
Recombinant immunogenic compositions and methods for protecting against lethal infections from Bacillus anthracis having a variant of recombinant Bacillus anthracis protective antigen (rPA) and a variant of recombinant Bacillus anthracis lethal factor (rLF). These proteins may be expressed separately or as a fusion protein. The recombinant proteins are produced in an avirulent strain of Bacillus anthracis that overproduces the desired antigens. The compositions and methods induce the animal host to produce antibodies against a virulent strain of Bacillus anthracis.
Owner:EMERGENT BIODEFENSE OPERATIONS LANSING
Method for high efficiency expressing destination protein
InactiveCN1869214AIncrease productionSimple methodRecombinant DNA-technologyFermentationFusion Protein ExpressionBiology
This invention discloses method of high efficiency expressing target albumen. Amalgamation albumen expression quantity can be greatly improved by using two steps culture strategy, and the culture temperature of the expression target albumen CHO cell is stabilized. The method in this invention is simple, and easy to realized, its efficiency is high, and cost is not improved. It has good application value not only to laboratory using or commercialization generating.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Method and uses for expressing polypeptide in endosperm using cereal non-storage protein as fusion vector
ActiveCN1884517AImprove solubilityBiologically activeInsulin-like growth factorsFermentationGenetically modified riceFusion Protein Expression
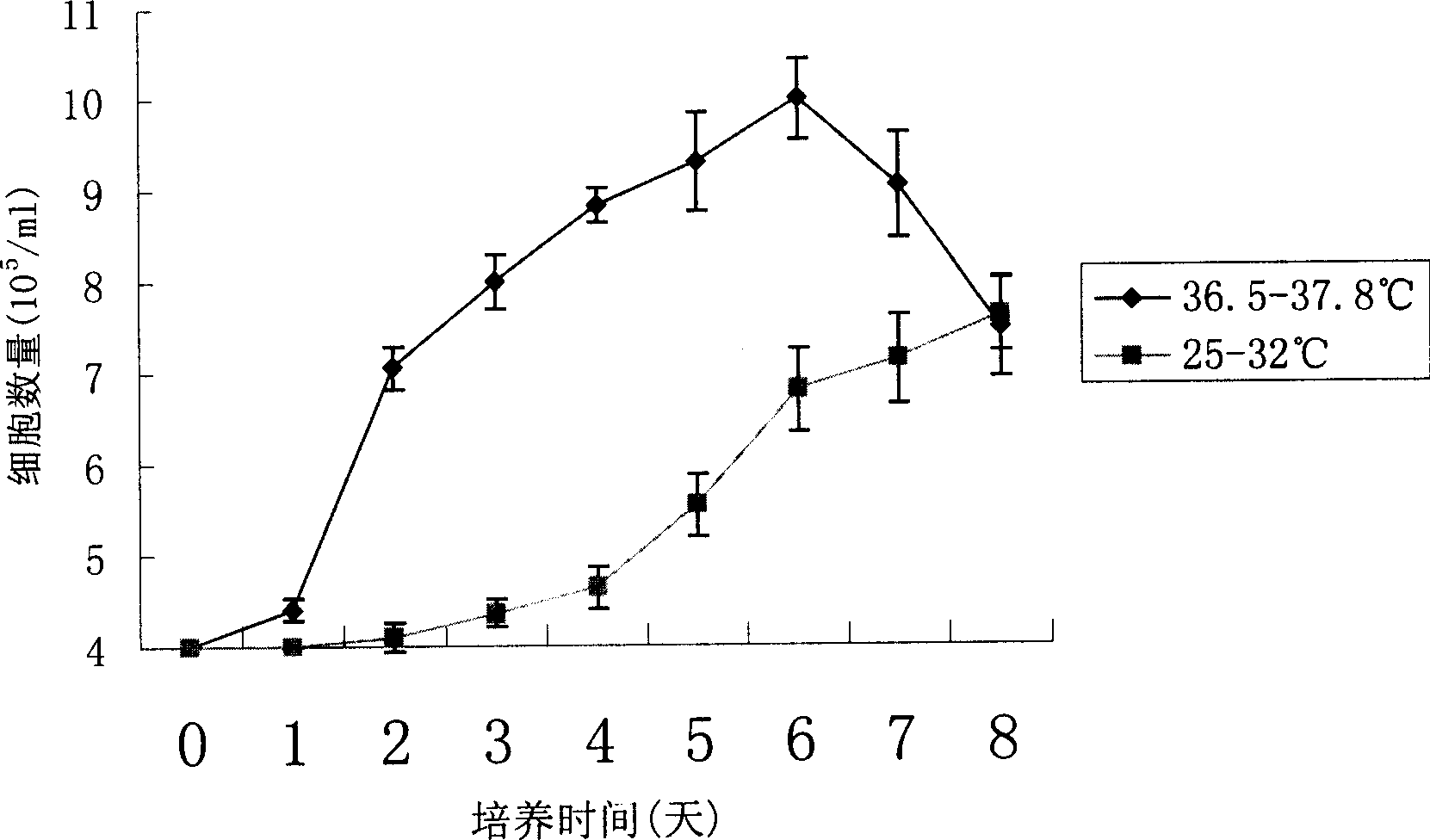
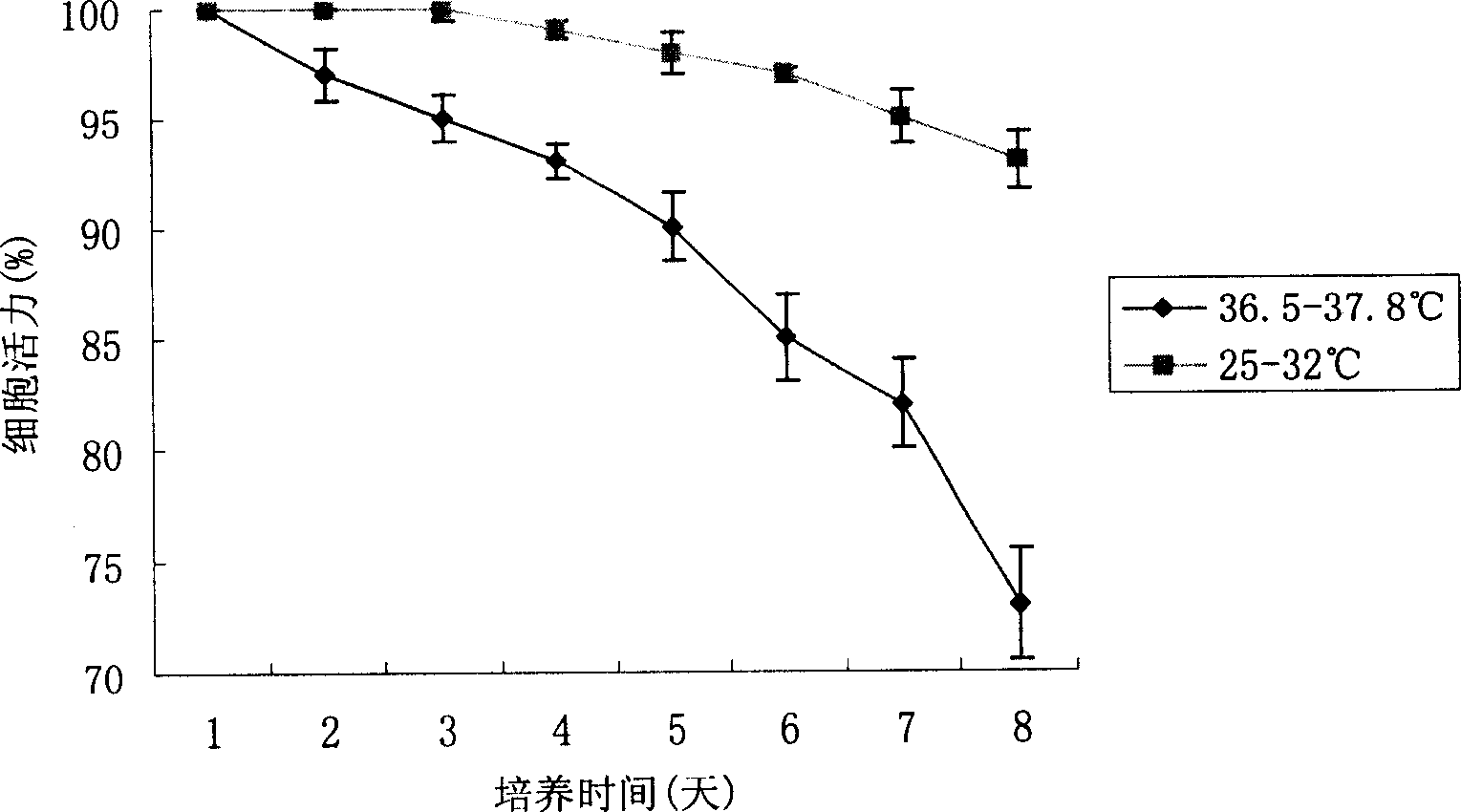
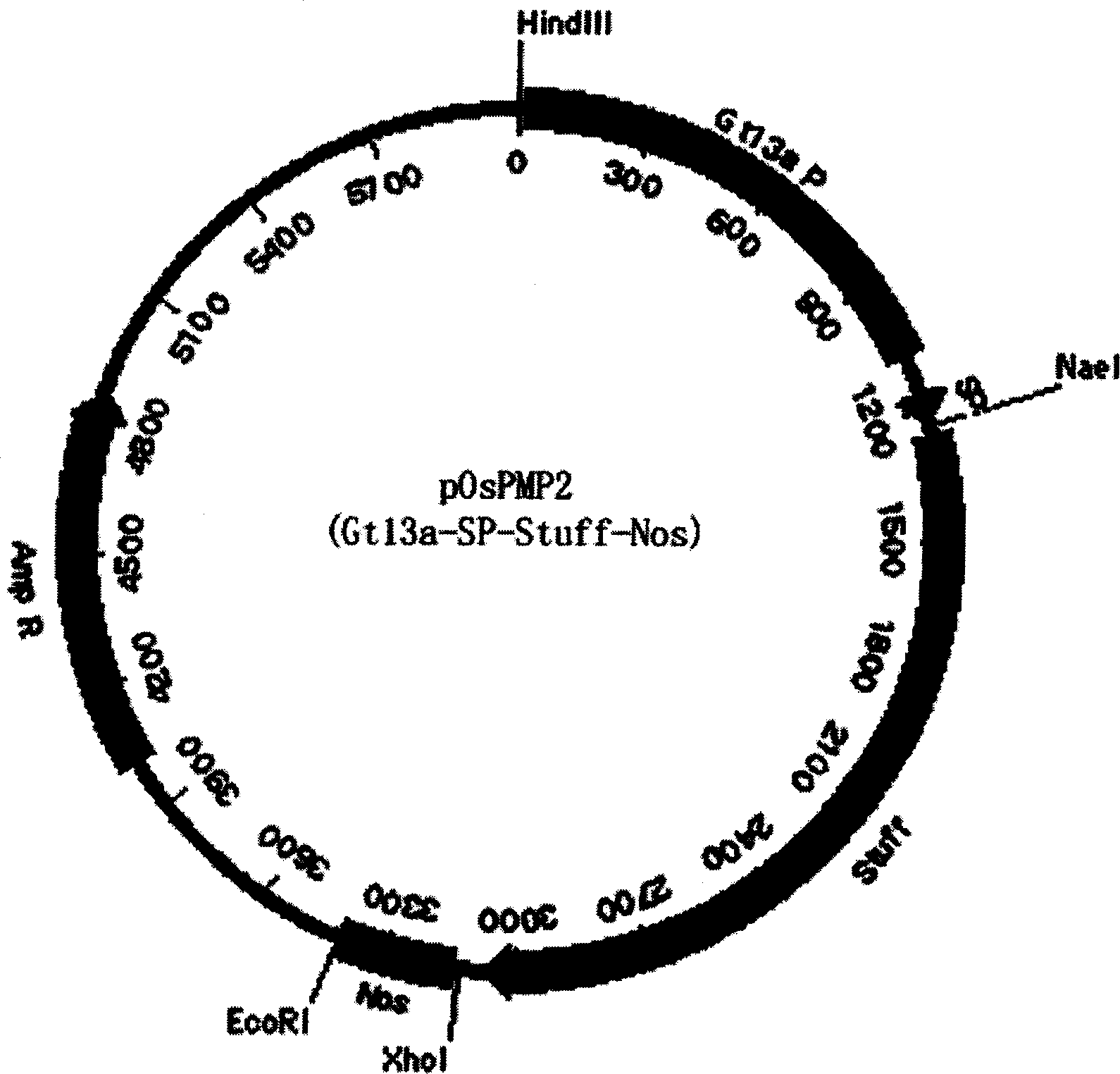
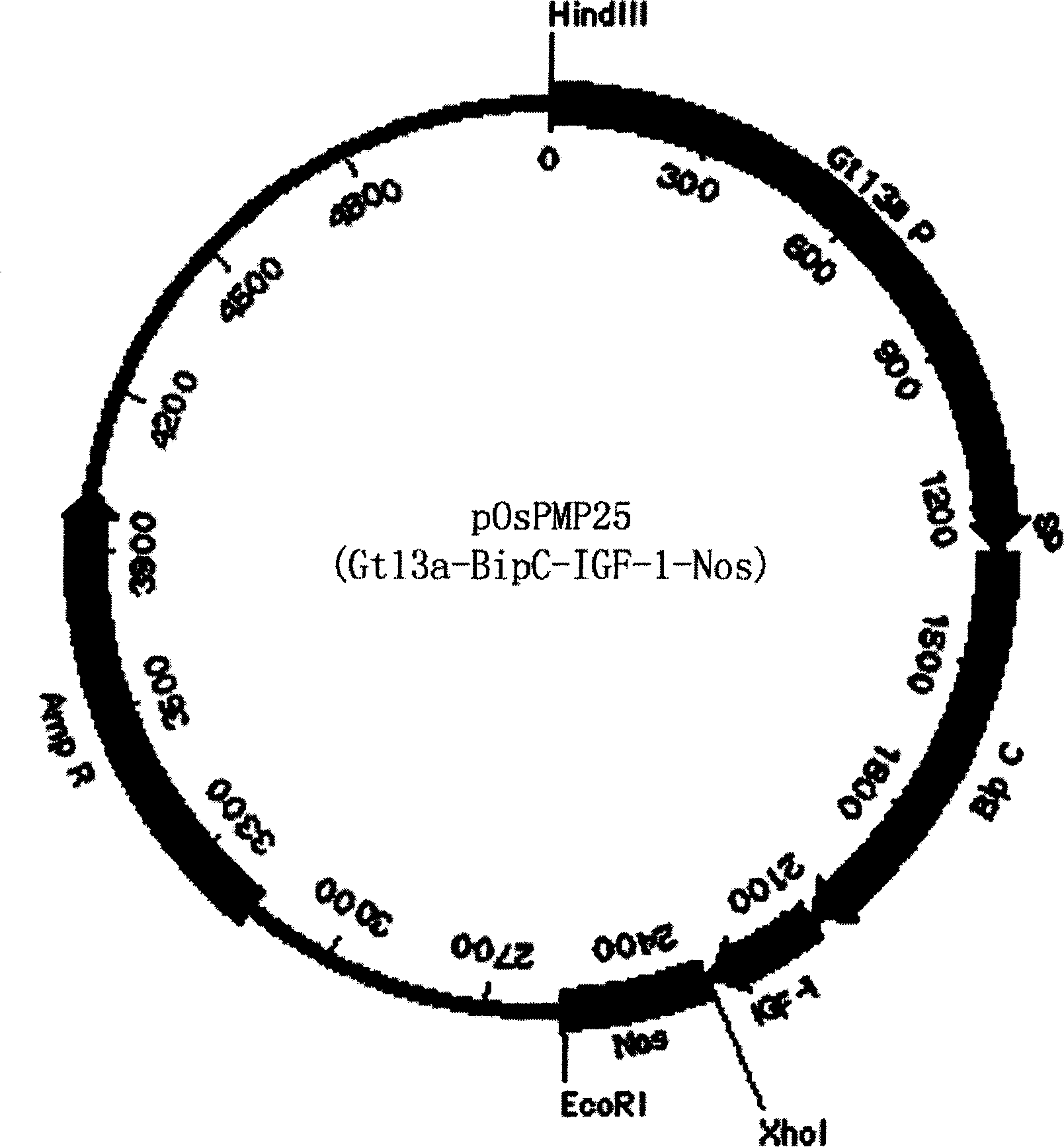
The invention discloses a method of using corn non-storage protein as molten carrier in endosperm expression polypeptide and its application, which includes: first obtaining rice astopic promoter and signal peptide; secondarily constructing rice albuminous cell specific expression carrier; thirdly using rice preference codon to prepare rice non-storage protein Bip gene C-end which is used as molten carrier, wheat PD1 gene C end and para-insulin growth factor-1 gene; fourthly constructing expression fusion protein carrier; fifthly obtaining genetically modified rice and barley plant with more than 0.3 % seed dry weight of their fusion protein expression. The invention is of simple process, convenient operation and low cost, and can be used in corn endosperm to express various polypeptides.
Owner:WUHAN HEALTHGEN BIOTECHNOLOGY CORP
Gene recombinant human active basic fibroblast growth factor fusion protein, preparation method thereof and application thereof
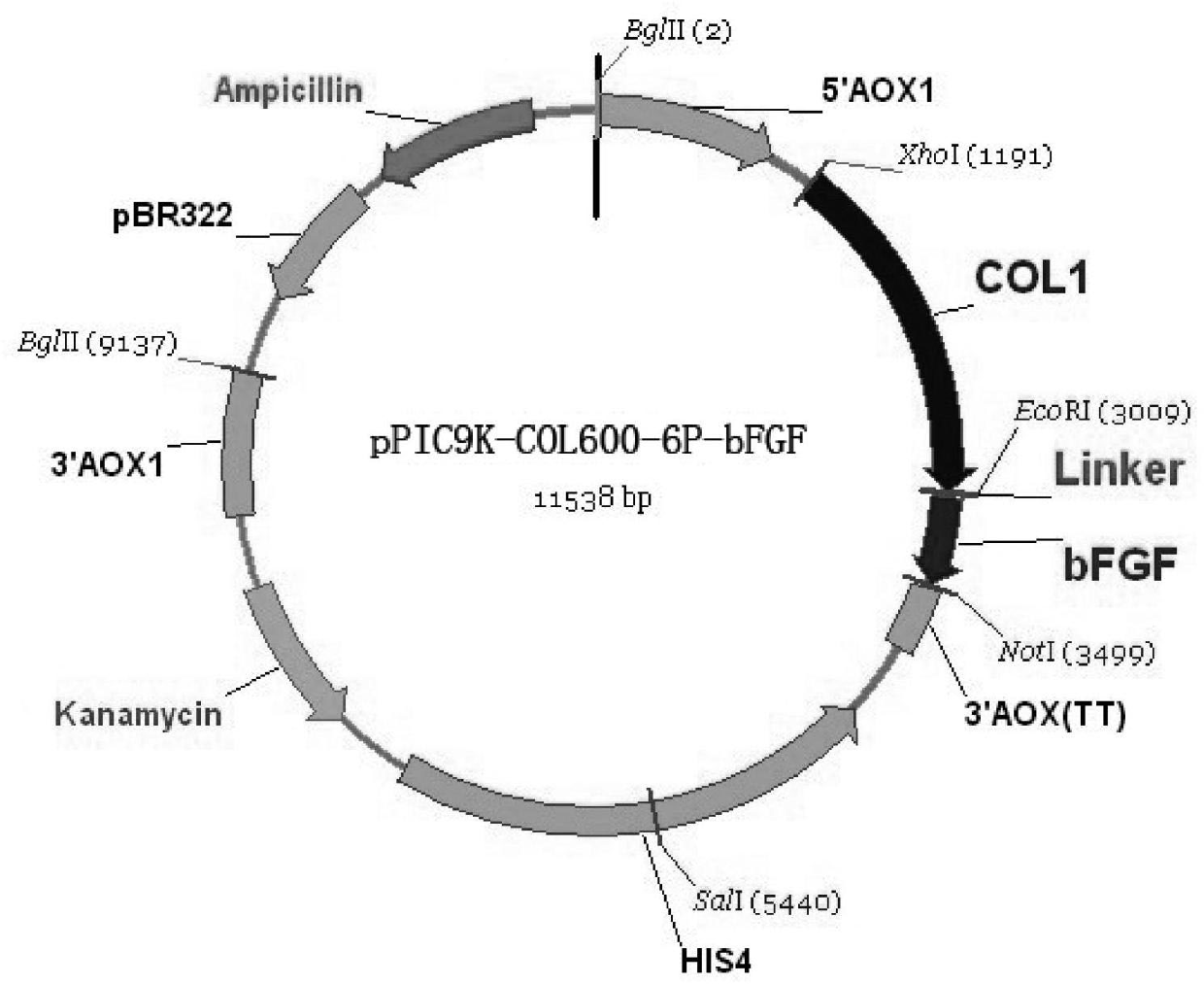
The invention provides a gene recombinant human active basic fibroblast growth factor fusion protein, wherein the whole length of the fusion protein is 762 amino acids, the nitrogen end is I type human-like collagen 500-1099 peptide and jointly has 600 amino acids, the carbon end is human basic fibroblast growth factor and jointly has 154 amino acids, and two peptides are connected with each other by glutamic acid, phenylalanine and 6 amino acids-containing flexible peptide. A preparation method of the gene recombinant human active basic fibroblast growth factor fusion protein comprises the following steps of: constructing a gene recombinant human active basic fibroblast growth factor fusion protein expression vector, electrically transforming pichia pastoris, screening multi-copy inserting recon, fermenting the gene recombinant human active basic fibroblast growth factor fusion protein, and purifying the gene recombinant human active basic fibroblast growth factor fusion protein. Thegene recombinant human active basic fibroblast growth factor fusion protein is used for preparing the cosmetics.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
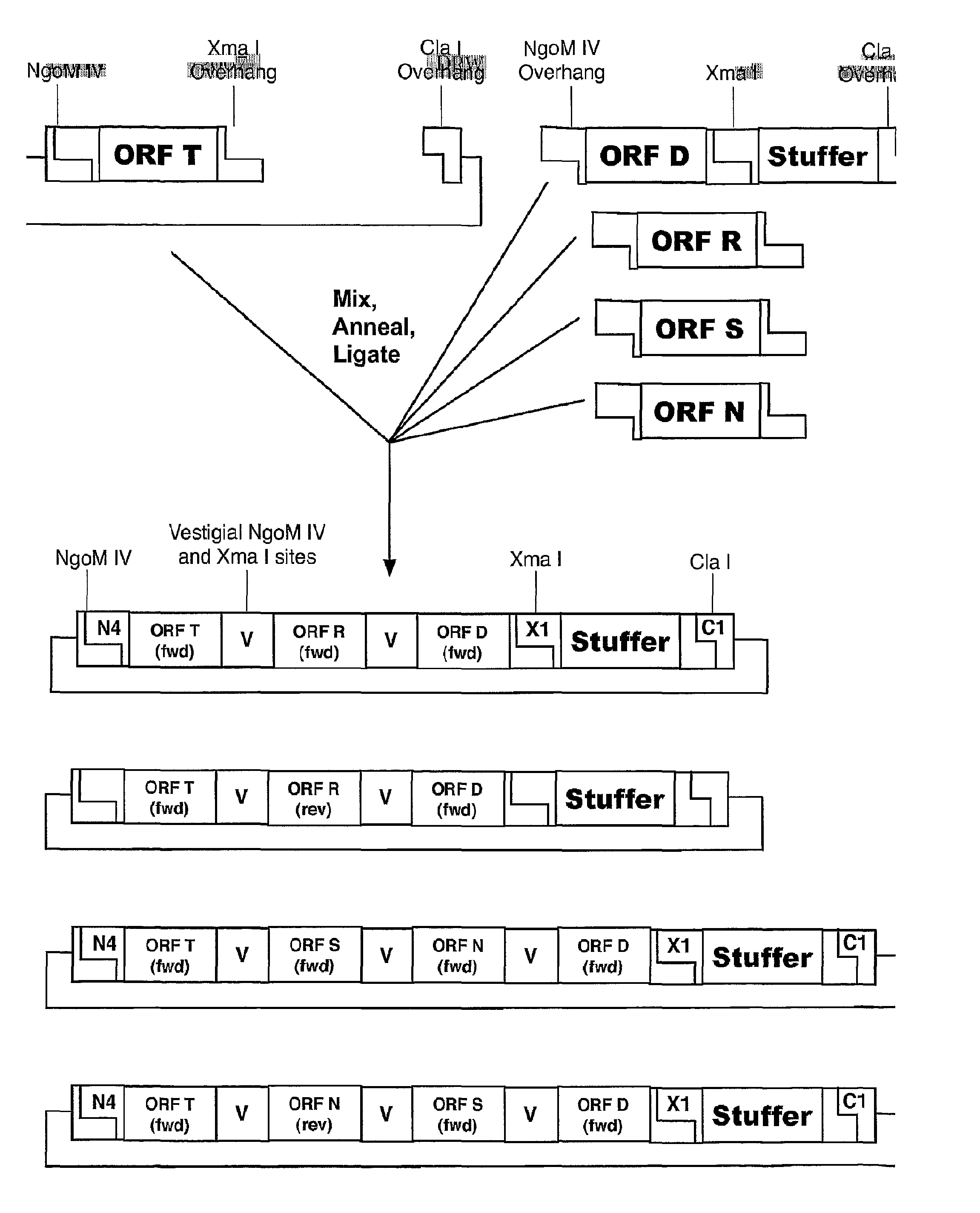
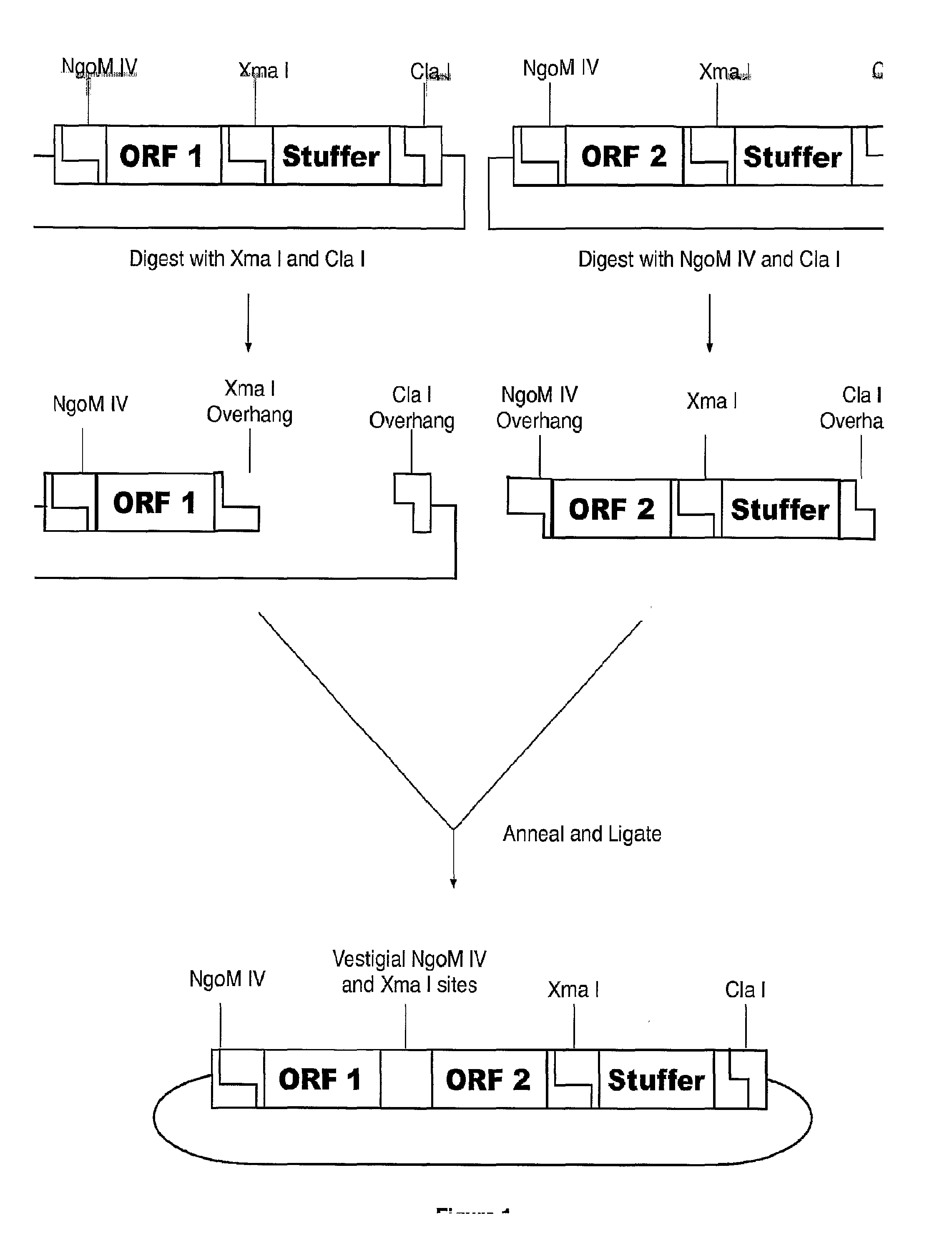
Methods of Making Modular Fusion Protein Expression Products
InactiveUS20090123973A1The process is simple and fastSugar derivativesBacteriaFusion Protein ExpressionOpen reading frame
The invention relates to methods of making modular chimeric protein expression products and compositions utilized in the methods. In particular, the invention relates to sequential, directional cloning of polynucleotides encoding polypeptide modules. Each clonable element or module contains an open reading frame of interest flanked by predetermined restriction sites. The methods include using modules and vectors containing these modules as starting materials for recombinant DNA techniques. One advantage of the invention is that it allows for many variations of fusion proteins to be made quickly and easily without needing to design and evaluate each subsequent cloning step.
Owner:PRECIGEN INC
Bacterial spores
InactiveUS20050232947A1Easy to produceLow costAntibacterial agentsPolypeptide with localisation/targeting motifAntigenFusion Protein Expression
The invention provides a spore genetically modified with genetic code comprising at least one genetic construct encoding an antigen and a spore coat protein as a chimeric gene, said genetically modified spore having said antigen expressed as a fusion protein with said spore coat protein.
Owner:ROYAL HOLLOWAY & BEDFORD NEW COLLEGE
Method of producing virus-like particles of picornavirus using a small-ubiquitin-related fusion protein expression system
A method for producing picornaviral capsid protein complexes (e.g., picornavirus like particles) in E. coli using a small-ubiquitin-related fusion protein expression system and an E. coli strain used in practicing this method. Also disclosed is use of the picornaviral capsid protein complexes like thus prepared for eliciting immune responses.
Owner:ACAD SINIC
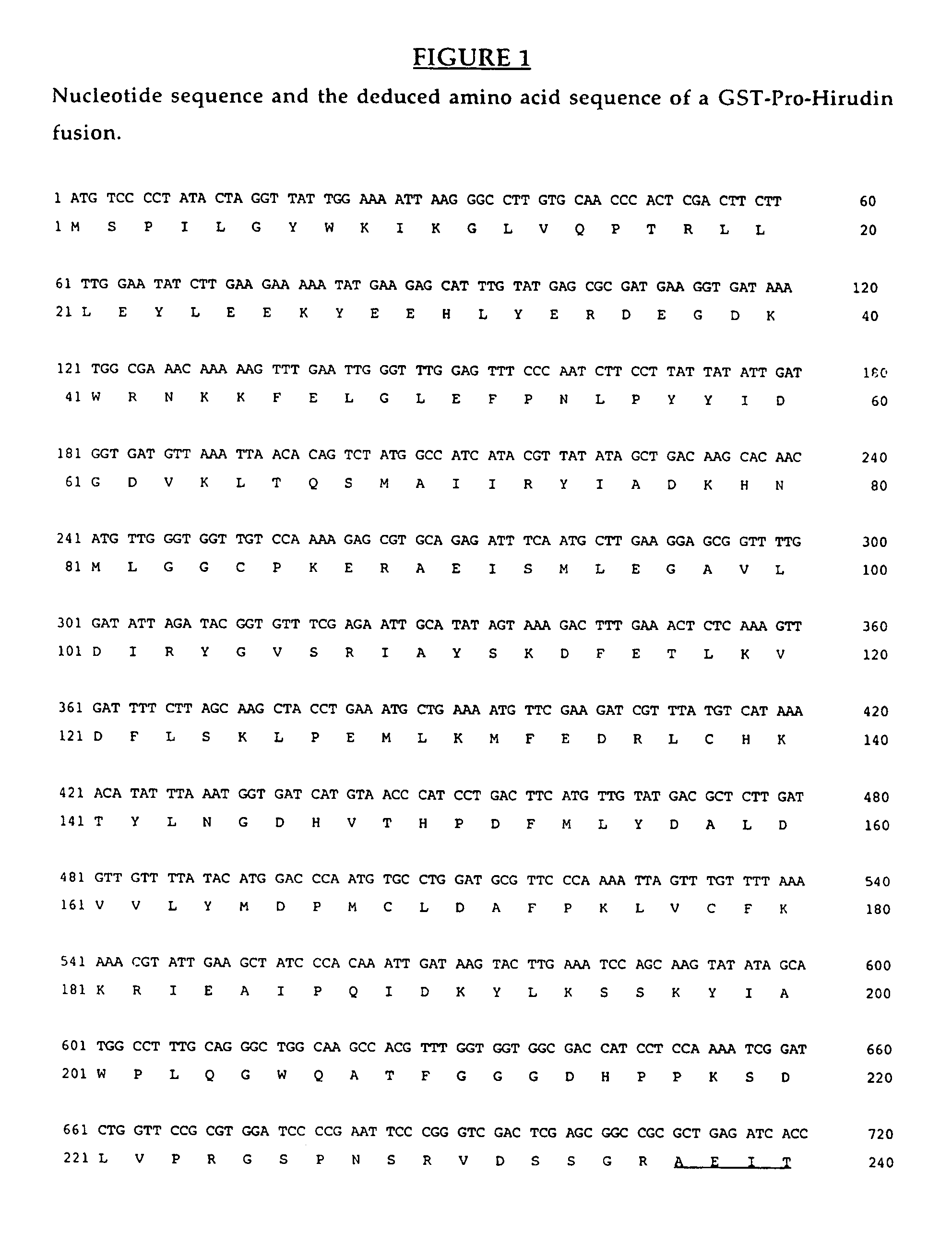
Method for cleavage of fusion proteins
InactiveUS7531325B2Readily apparentPolypeptide with localisation/targeting motifHydrolasesFusion Protein ExpressionImproved method
An improved method for recovering recombinantly produced polypeptides is described. The method involves expressing the recombinant polypeptide as a fusion protein with a pro-peptide. The pro-peptide-polypeptide fusion protein can be cleaved and the recombinant polypeptide released under the appropriate conditions.
Owner:SEMBIOSYS GENETICS INC
Tumor necrosis factor receptor related proteins Tango-63d and Tango-63e
InactiveUS20060069246A1Improve purification effectExtended half-lifeOrganic active ingredientsFungiFusion Protein ExpressionScreening method
The invention relates to the discovery and characterization of Tango-63d, Tango-63e genes and the polypeptides they encode. Tango-63d and Tango-63e are two novel polypeptides within the tumor necrosis factor (TNF) receptor superfamily. The invention encompasses nucleic acid molecules encoding nucleic acids and polypeptides of the invention, or mutant forms thereof that encode dysfunctional receptor polypeptides, vectors containing these nucleic acid molecules, cells harboring recombinant DNA molecules encoding nucleic acids or polypeptides of the invention, or mutant forms thereof, host fusion proteins that include functional or dysfunctional polypeptides of the invention, transgenic animals that express nucleic acids or polypeptides of the invention, screening methods and therapeutic methods employing the nucleic acid molecules and polypeptides described above, substantially purified nucleic acids and polypeptides of the invention, and therapeutic compositions containing these nucleic acid molecules and polypeptides.
Owner:MILLENNIUM PHARMA INC
Method for producing and cleaving a fusion proteins with an n-terminal chymosin pro-peptide
An improved method for recovering recombinantly produced polypeptides is described. The method involves expressing the recombinant polypeptide as a fusion protein with a pro-peptide. The pro-peptide-polypeptide fusion protein can be cleaved and the recombinant polypeptide released under the appropriate conditions.
Owner:SEMBIOSYS GENETICS INC
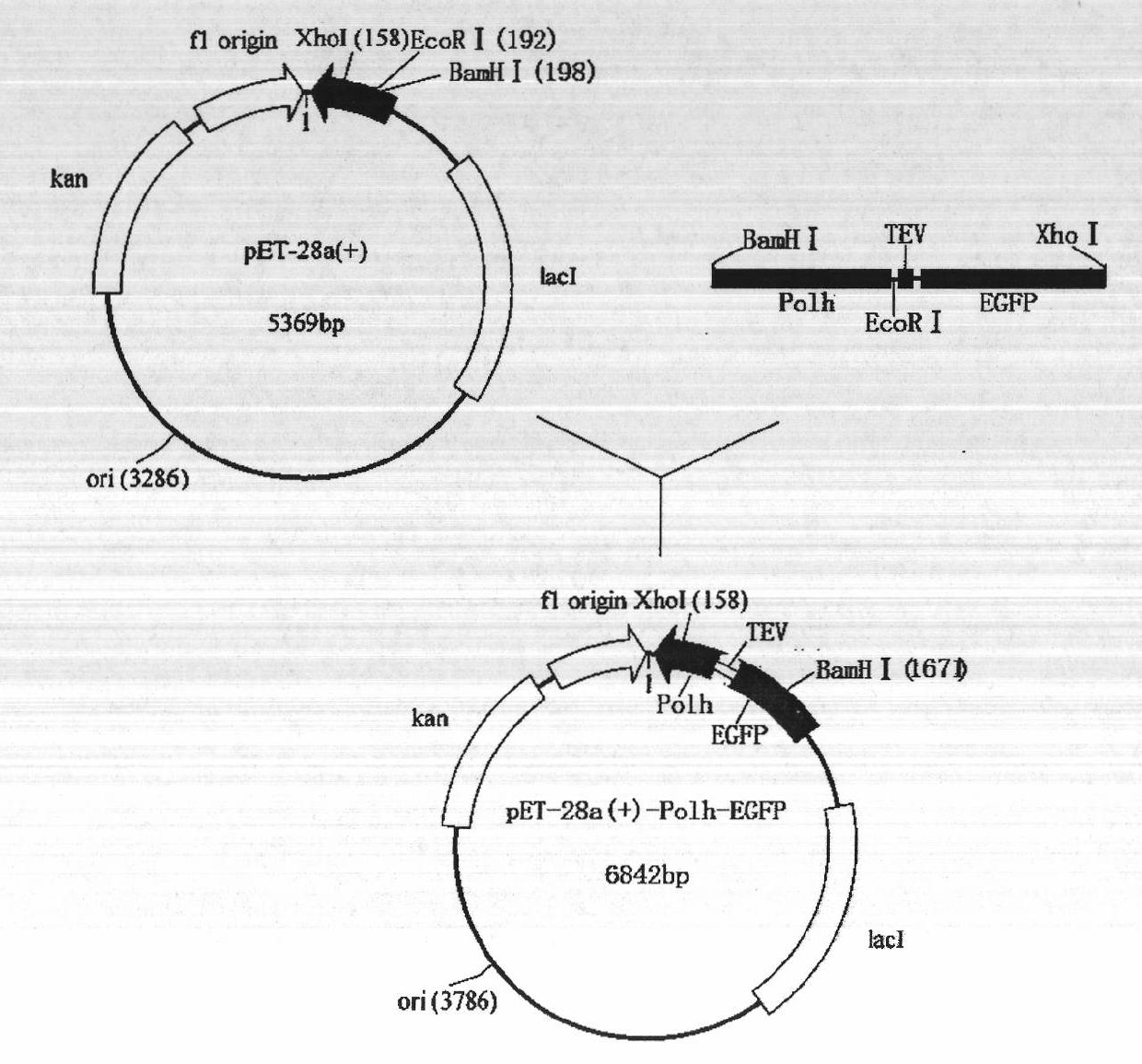
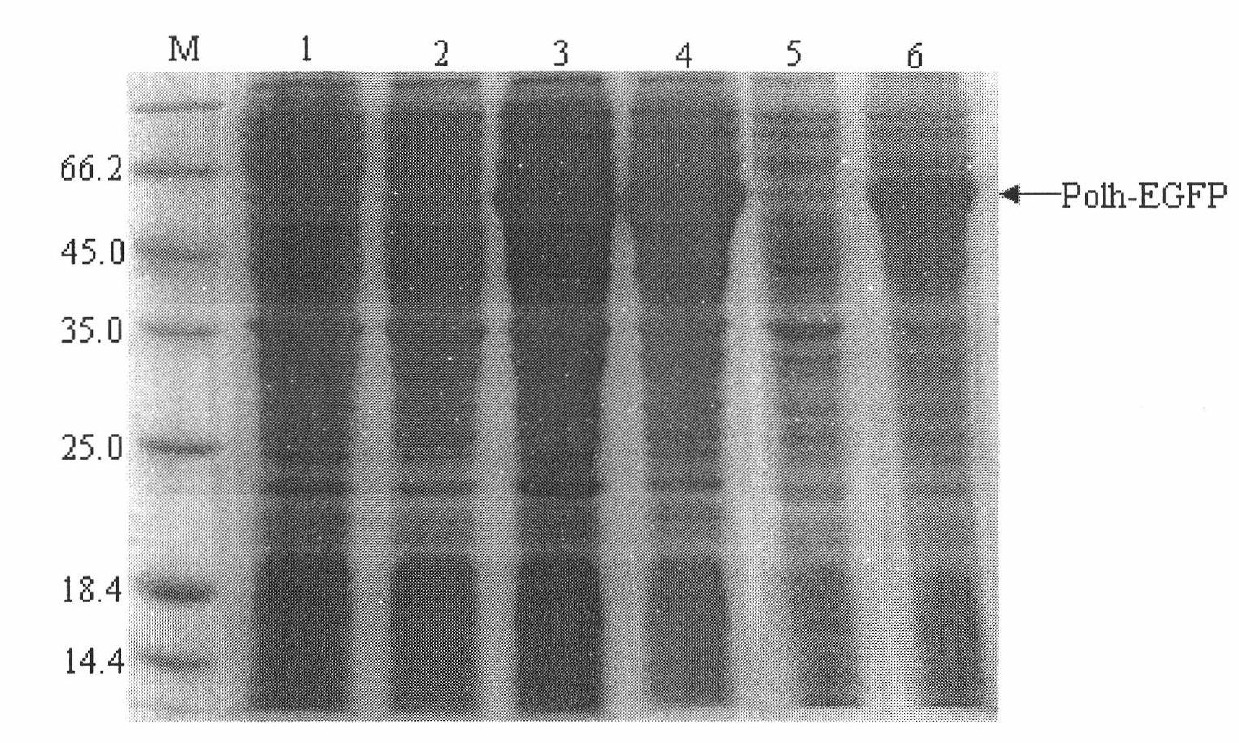
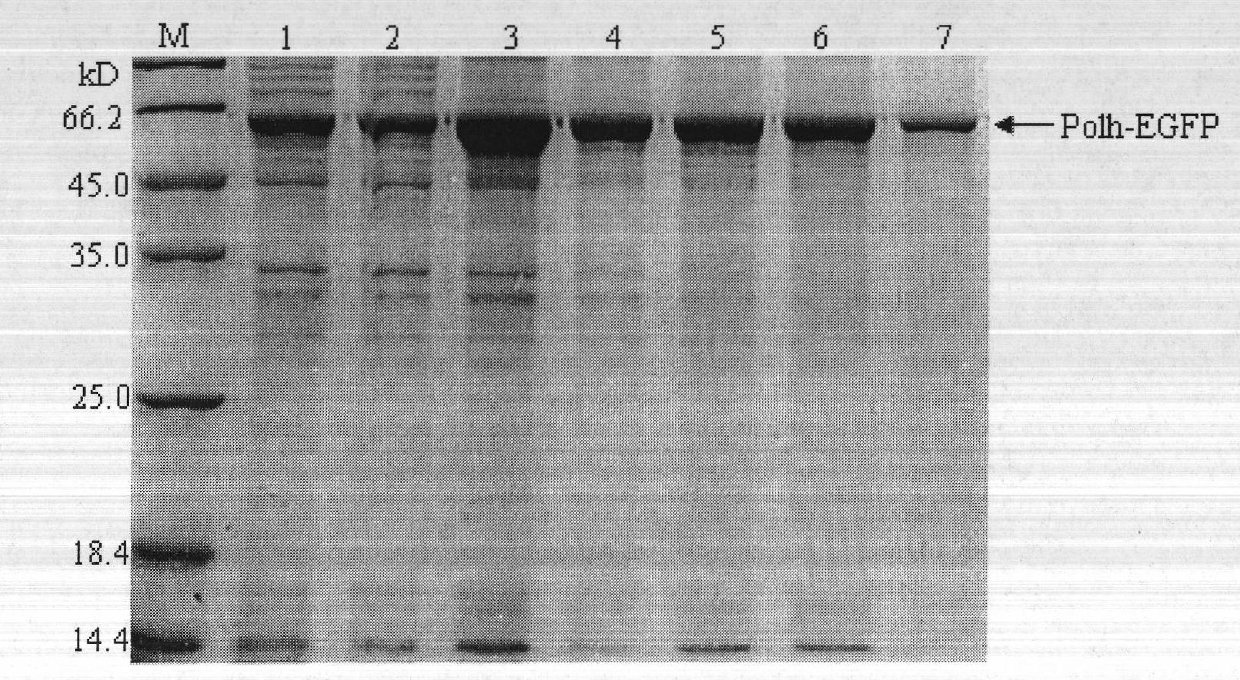
Method for purifying fusion protein based on bombyx mori baculovirus polyhedron dissolving characteristic
The invention relates to a method for expressing and purifying fusion protein based on bombyx mori baculovirus polyhedron dissolving characteristic and a high-efficiency fusion expression vector for implementing the fusion protein expression and purification method. The method comprises the following steps of: constructing a bombyx mori polyhedrin gene (Po1h) and a target protein gene into a prokaryotic expression vector and introducing a protease enzyme digestion site between the bombyx mori polyhedrin gene (Po1h) and the target protein gene so as to perform fusion expression in a prokaryotic expression bacterium; due to the solubility characteristics that the polyhedrin is dissolved under the alkaline condition and precipitated under the neutral condition, washing the precipitate, which is obtained after ultrasonic induction of the thallus, by using buffer solutions with different pH values; dissolving the precipitate collected finally by using a buffer solution with high pH value; centrifuging, adjusting the pH value of the collected supernate to be neutral; centrifugally collecting the precipitate which is the fusion protein obtained through separation and purification; performing enzyme digestion on specific protease and performing purification, performing nickel column purification on the polyhedrin tag of the fusion protein to obtain the target protein, so that the fusion protein purification method based on bombyx mori baculovirus polyhedron dissolving characteristic and prokaryotic expression is formed.
Owner:TIANJIN YAOYU BIOLOGICAL TECH
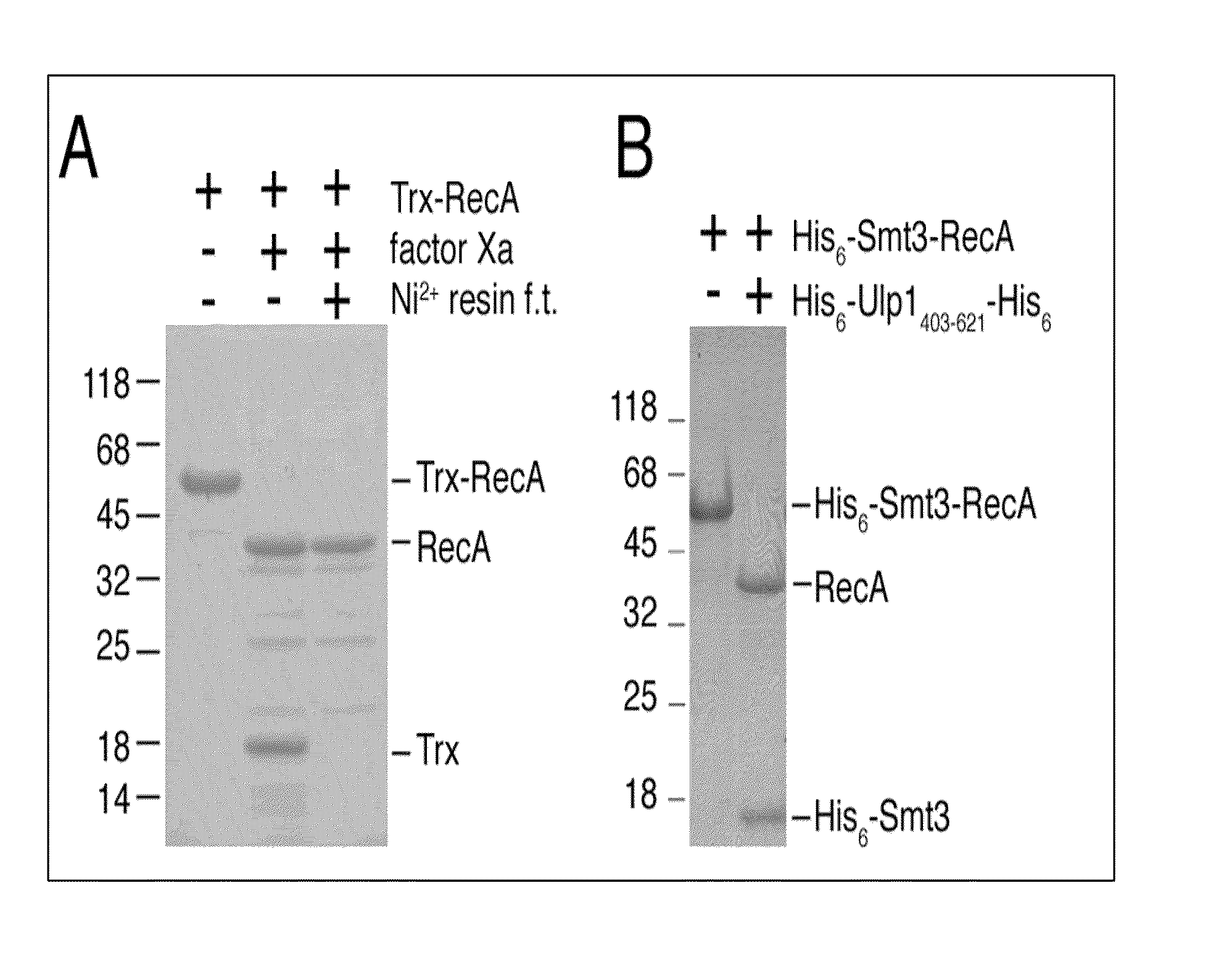
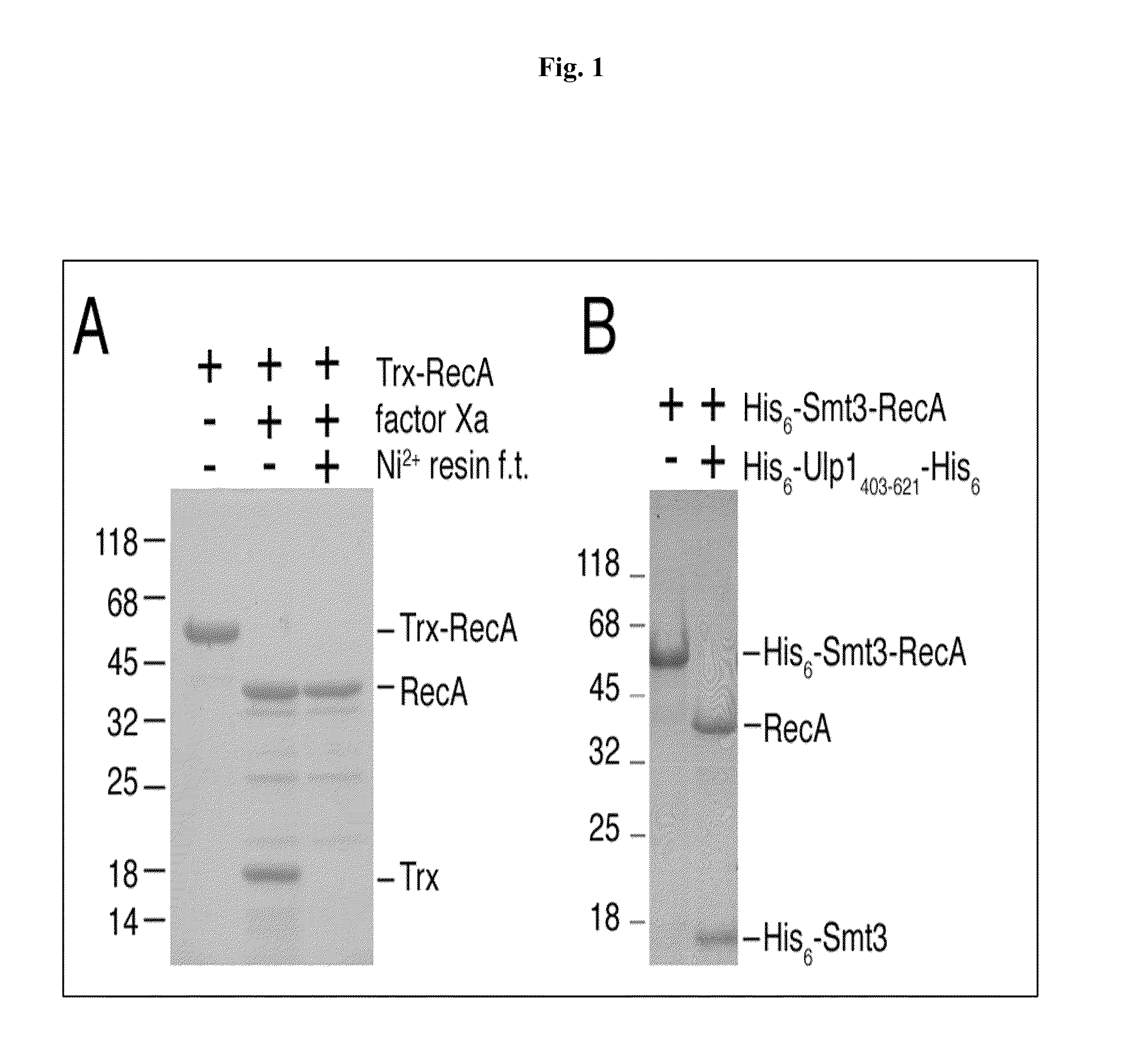
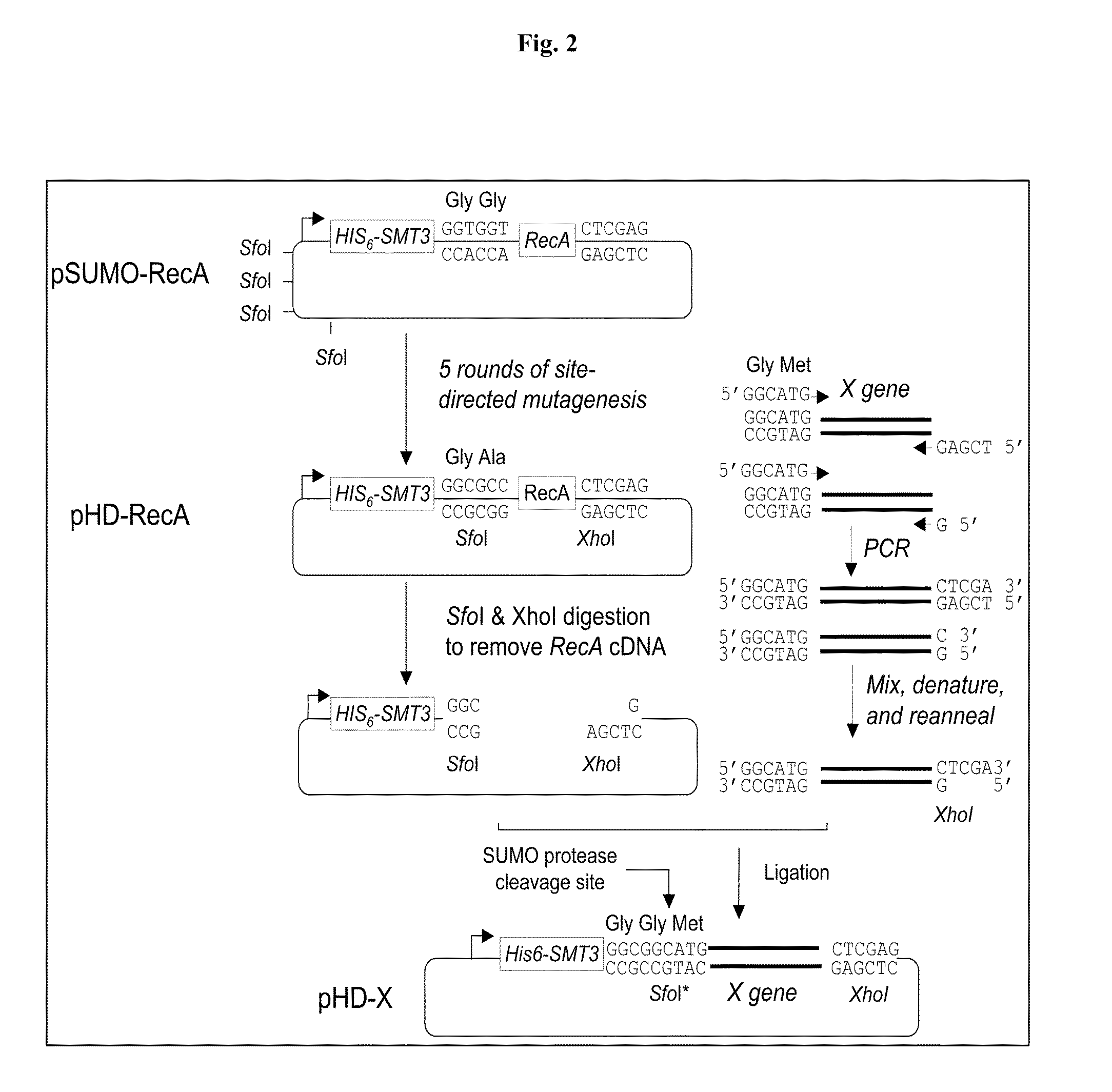
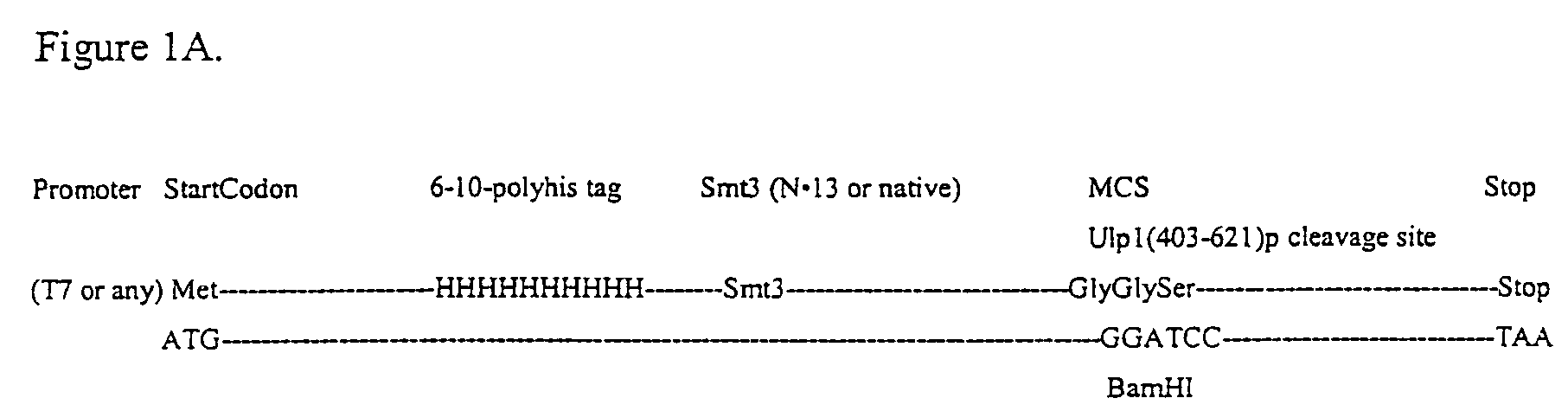
Rapidly cleavable SUMO fusion protein expression system for difficult to express proteins
A recombinant expression system for the expression of a poly amino acid, peptide or protein is provided. The poly amino acid of interest is expressed as a fusion protein that includes an amino acid sequence recognized and cleaved by a Ulp1 protease. The amino acid sequence joined to the poly amino acid of interest is preferably from a SUMO (small ubiquitin-like molecule) protein. This sequence imparts favorable solubility and refolding properties to the fusion protein. A purification tag may also be incorporated into the fusion protein for ease of isolation. The Ulp1 protease used to cleave the fusion protein may be the Ulp1 protease or the active Ulp1 protease fragment, Ulp1(403-621). The Ulp1 protease rapidly and specifically cleaves the fusion proteins of the invention at the Ulp1 cleavage site. The amino acid sequence recognized by a Ulp1 protease is cleaved asymetrically to leave only an N-terminal serine joined to the poly amino acid of interest. This recombinant expression system is particularly advantageous for expression and rapid and highly specific cleavage and purification of poly amino acids that have low solubilities or are difficult to express in other systems.
Owner:CORNELL RES FOUNDATION INC
Preparation of Soluble Capsid Proteins of Picornaviruses Using SUMO Fusion Technology
InactiveUS20090280531A1Simple and efficientSsRNA viruses positive-senseSugar derivativesFusion Protein ExpressionPicornavirus
A method of producing a soluble capsid protein of a picornavirus using a novel and efficient SUMO fusion protein expression system.
Owner:ACAD SINIC
Bovine lactoferricin and preparation method thereof
InactiveCN102993296AHeat resistantHas a broad-spectrum antibacterial effectBacteriaTransferrinsFusion Protein ExpressionPepsin
The invention relates to bovine lactoferricin and a preparation method thereof. Bovine lactoferricin (Lfcin B) is 25 amino acid residues hydrolyzed from a bovine lactoferrin (BLF) N-terminal (17-41) by pepsin, and has a molecular weight of approximately 3.1kd. The preparation method comprises the steps of: (2) recombinant expression vector construction; (2) fusion protein expression induction; and (3) protein separation and purification. Bovine lactoferricin has the advantages that: Lfcin B has the highest activity among all the Lfcins, and an antibacterial effect 400 times that of bovine lactoferrin, such that Lfcin B can play an important role in gastrointestinal tracts.
Owner:GUANGZHOU GLAM BIOTECH
HIV-1 yesisting polypeptide C22, its coding sequence and prepn process
The present invention relates to one kind of HIV-1 resisting polypeptide C22, and its expression precursor, coding DNA sequence, monomer polypeptide medicine composite and preparation process. The polypeptide C22 is prepared through expression with fusion protein to obtain the polypeptide precursor, and cleaving the precursor to obtain polypeptide C22. The preparation process is simple, high in final product yield and suitable for industrial production.
Owner:TONGJI UNIV
Method of preparing recombinant small molecular protein or polypeptide with hirudin as fusion partner
InactiveCN103819546APlant peptidesFusions for enhanced expression stability/foldingFusion Protein ExpressionProtein target
The invention provides a new method of fusion expressing a small molecular protein (polypeptide). The new method is characterized by comprising the following steps: using hirudin as a fusion partner (label), splicing the protein (polypeptide) with a small molecular mesh to the downstream of the hirudin as the fusion partner to carry out fusion expression, designing a connecting peptide (which contains protease or a chemical cutting site or intein as a self-cuttable protein intron) between the fusion partner and a target protein (polypeptide), and releasing the target protein (polypeptide) by restriction enzyme digestion or chemical cutting or induced self cutting after fusion protein expression. The new method has the advantages that (1) as the hirudin as the fusion partner (label) is smaller (with the molecular weight of 7Kd), the rate of the protein (polypeptide) with the small molecular mesh accounting for a fusion protein can be effectively increased, and the yield of the target small molecular protein (polypeptide) is finally increased; (2) the hirudin still has the anticoagulant activity after being fused as the fusion partner (label), and the expression and the purification of the fusion protein can be conveniently detected and traced in real time.
Owner:CHINA PHARM UNIV
Fusion proteins, recombinant bacteria, and exosporium fragments for animal health and aquaculture
Fusion proteins, recombinant Bacillus cereus family members that express fusion proteins, and exosporium fragments derived from spores of the recombinant Bacillus cereus family members are provided. Compositions comprising the spores or exosporium fragments are also provided. Methods involving the use of spores of recombinant Bacillus cereus family members and exosporium fragments derived from spores of a recombinant Bacillus cereus family member in the fields of animal health and aquaculture are provided. In particular, methods are for provided for using such spores or exosporium fragments for protecting an animal or an aquatic organism from a pathogen. Methods are also provided for using exosporium fragments for producing an immunogenic response in an aquatic animal. Products for use inprotecting animals from pathogens are also provided, including adhesive patches, wound dressings, insert trays for livestock footbaths, hoof bandages, feed, feed additives, and insect foggers.
Owner:SPOGEN BIOTECH INC
Process for producing recombinant insulin-like growth factor-1(IGF-1) amalgamation protein
InactiveCN101429519AEasy to placePromote formationBacteriaMicroorganism based processesBiotechnologyHydroxylamine
The invention discloses a method for preparing recombined human insulin growth factor-1(IGF-1) fused protein, which belongs to the field of biological medicine field in biological technique. The invention adopts the genetic engineering fungus fermentation method for production: a. designing and synthesizing recombining human IGF-1 fused protein gene; b. constructing an expression vector of the recombined human IGF-1 fused protein; c. utilizing the expression vector to convert the host to construct the genetic engineering fungus; and d. utilizing the genetic engineering fungus to ferment the recombined human IGF-1 fused protein. In the method, according to the characteristics that the first amino acid of the natural IGF-1 is glycine(GLy, G), a leading peptide is added before the natural IGF-1, and a hydroxylamine specific cracking part(Asn-Gly peptide bond) is designed between the leading peptide and the natural IGF-1, thereby making the expression products stable and lowering purification cost. In the method, the fused protein in serial expression is used, wherein the N end is thioredoxin, and the C end is IGF-1, so that the expression products are more stable, and the separation method is simple and cheap. The method has wide application prospect.
Owner:FUJIAN MEDICAL UNIV
Gene for coding aflatoxin degradation enzyme and method for obtaining high-efficiency aflatoxin degradation enzyme
InactiveCN103555745AHydrolasesMicroorganism based processesEscherichia coliFusion Protein Expression
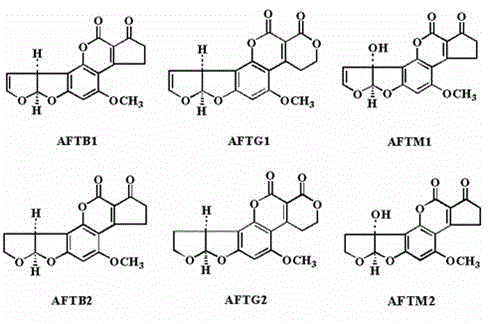
The invention discloses a gene for coding an aflatoxin degradation enzyme and a method for obtaining a high-efficiency aflatoxin degradation enzyme. The nucleotide sequence of the gene for coding the aflatoxin degradation enzyme is shown as SEQ ID NO. 1. The method for obtaining the high-efficiency aflatoxin degradation enzyme comprises the following steps: cloning the gene shown as the SEQ ID NO. 1 to a carrier pCold-II; converting to Escherichia coli BL21-PG-Tf2 to obtain expression thallus of the aflatoxin degradation enzyme; inducing expression of the expression thallus by using tetracycline and IPTG (isopropyl-beta-d-thiogalactoside); collecting the thallus; crushing; purifying by using a fusion protein expressed tag on the pCold-II to obtain the high-efficiency aflatoxin degradation enzyme. The purity of the obtained aflatoxin degradation enzyme reaches over 95 percent by optimizing the expression carrier and the expression condition, and the efficiency for degrading the aflatoxin B1 reaches 161.7 nmol / min / mg.pr.
Owner:HUAZHONG NORMAL UNIV
Adiponectin-Glucagon-like peptide-1-like peptide recombinant protein expression vector and construction
InactiveCN1737150AKeep spaceMaintain biological activityHybrid peptidesVector-based foreign material introductionFusion Protein ExpressionGlycine
Disclosed is an adiponectin-Glucagon-like peptide-1-like peptide recombinant protein expression vector and construction, wherein adiponectin and GLP-1-A encoded gene are obtained by utilizing PCR technology, the method comprises obtaining adiponectin through amplification of adiponectin spherical domain gene, then obtaining GLP-1-A encoded gene by mutating codons encoding No. 8 amino acid alanine in GLP-1-(7-37) encoded gene, then cloning GLP-1-A and adiponectin encoded genes directionally to expression vector by utilizing enzyme digestion technology, obtaining adiponectin-glucagon-like peptide-1 analogue peptide fusion protein expression vector, finally expressing adiponectin-GLP-1-A fusion protein through transforming protein expression bacteria.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com