Patents
Literature
1857 results about "Treated patient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
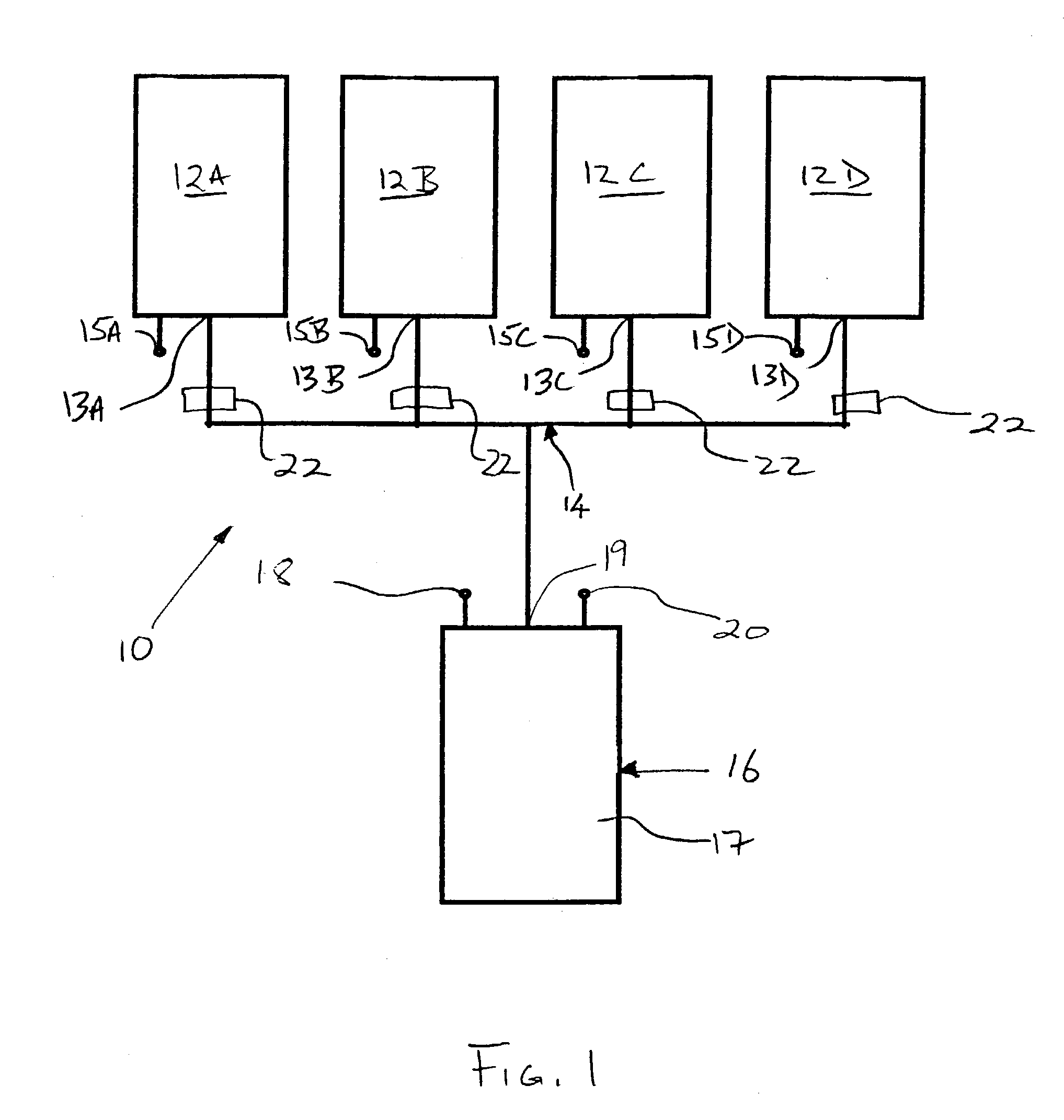
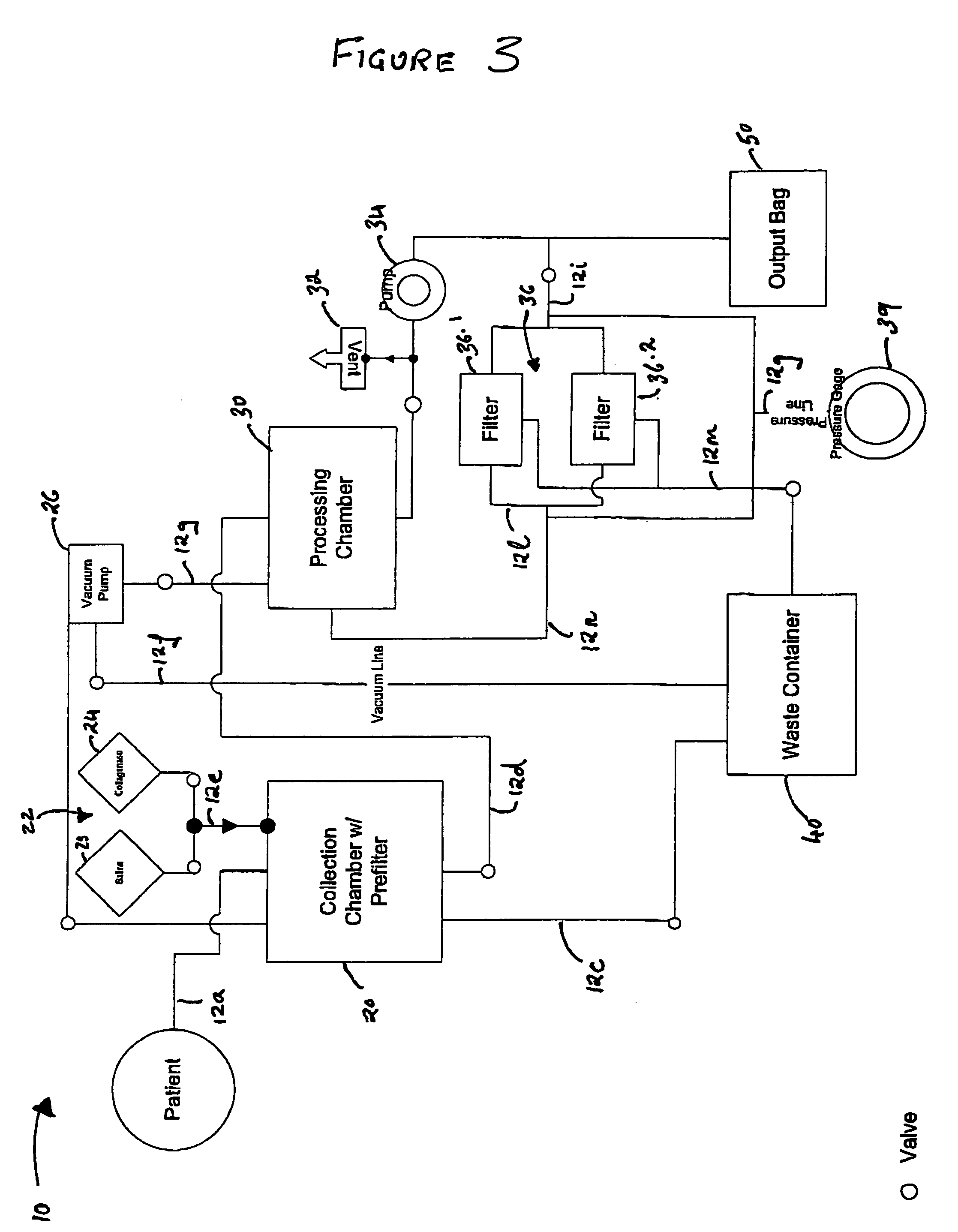
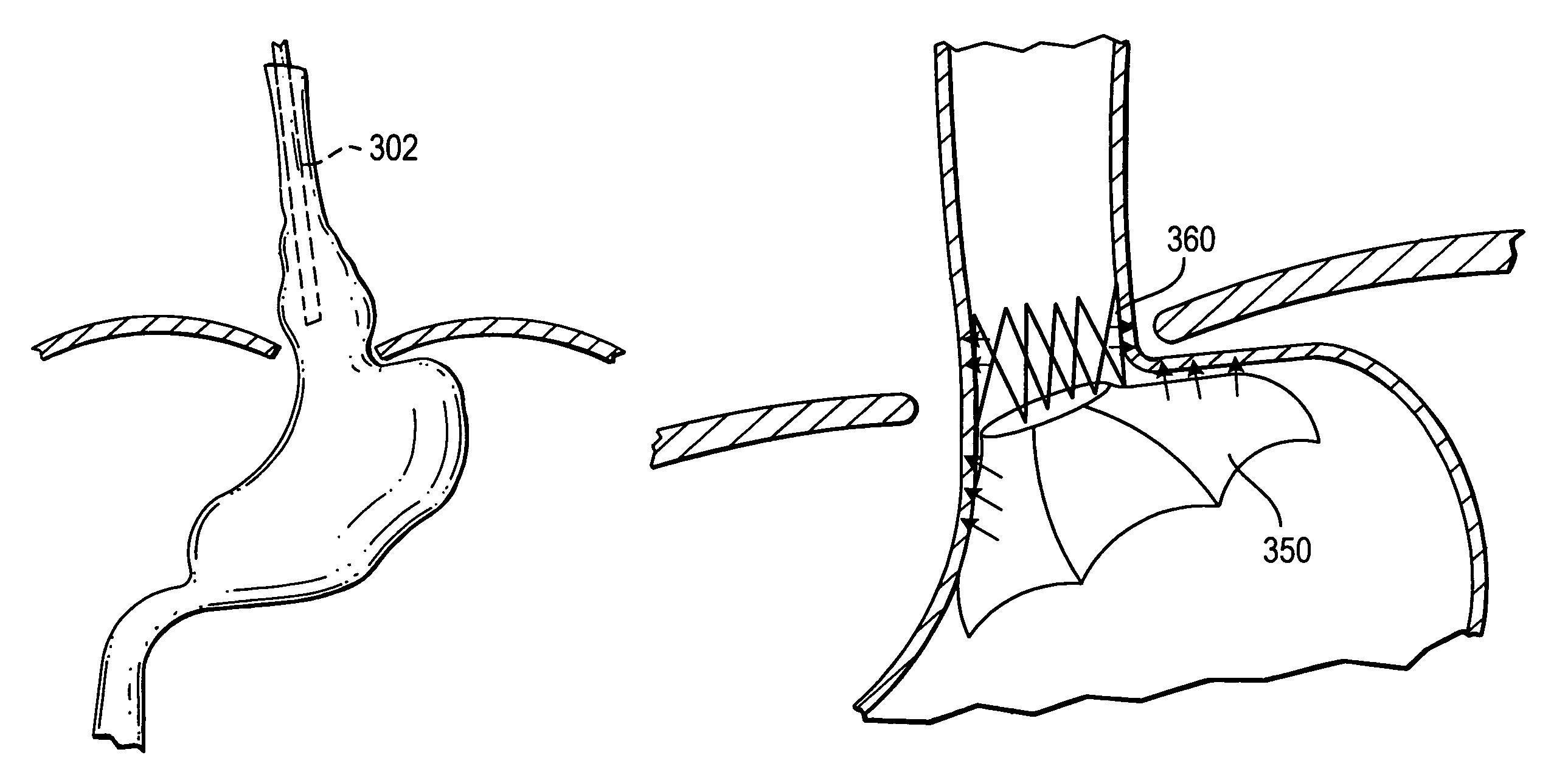
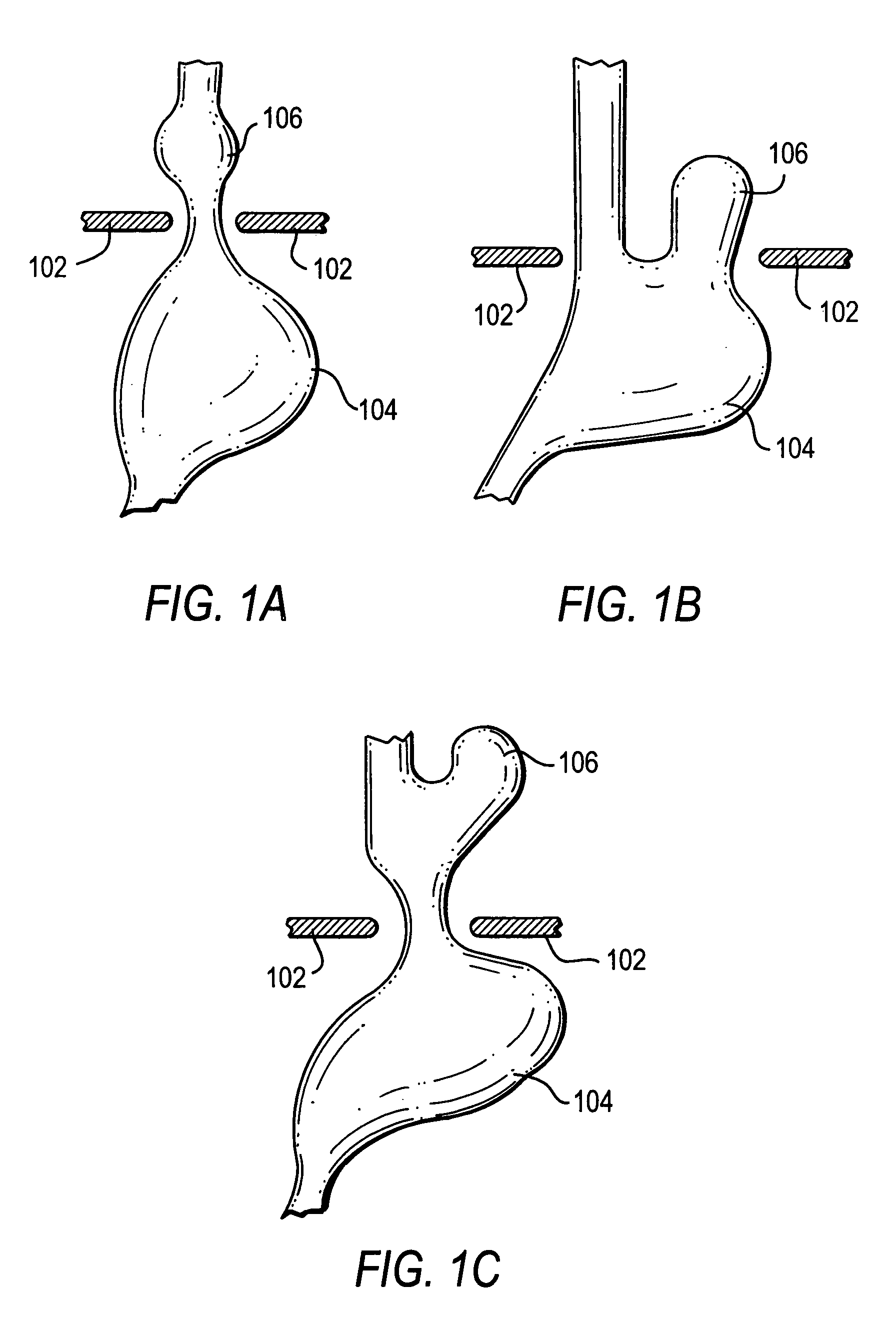
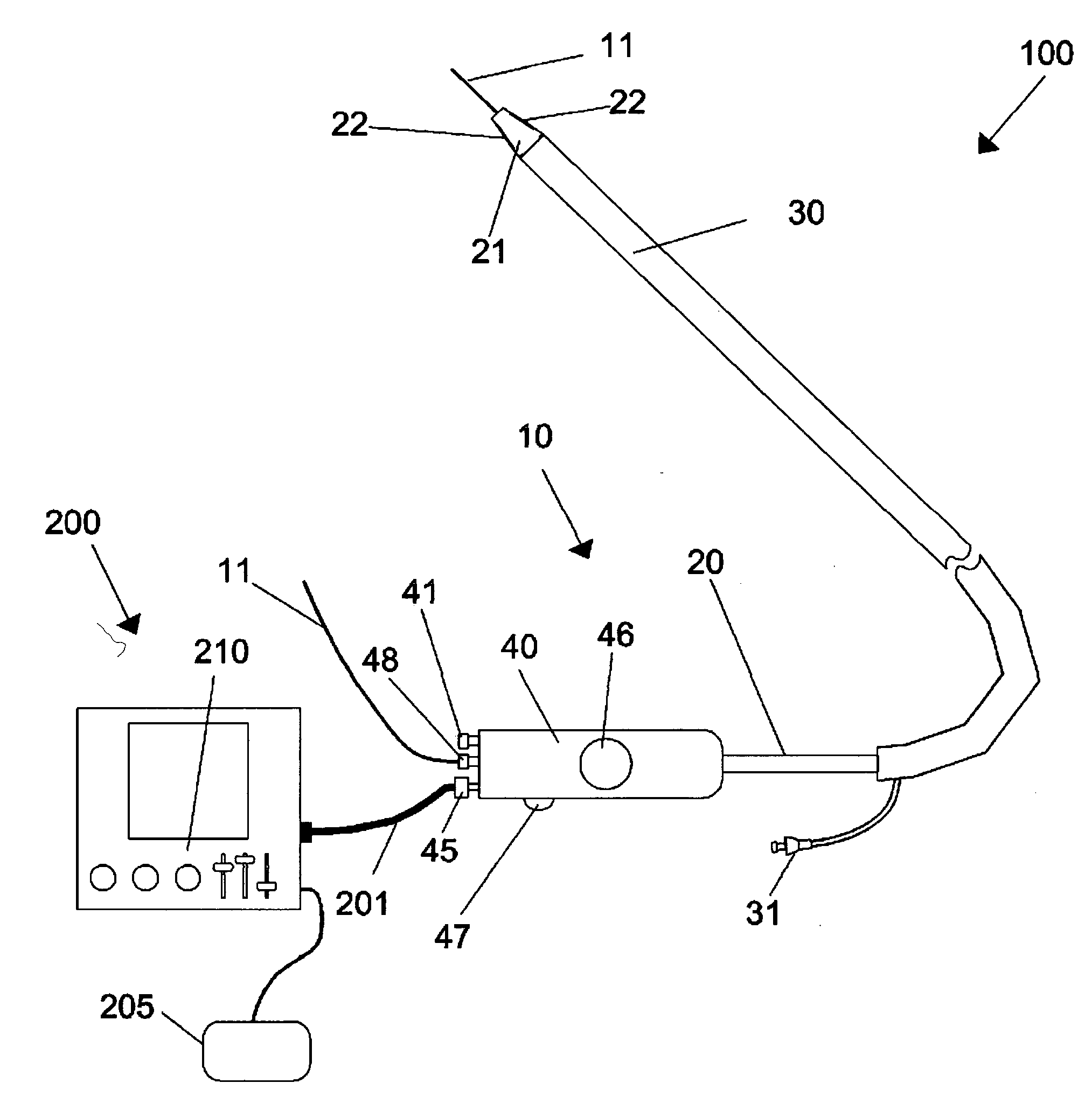
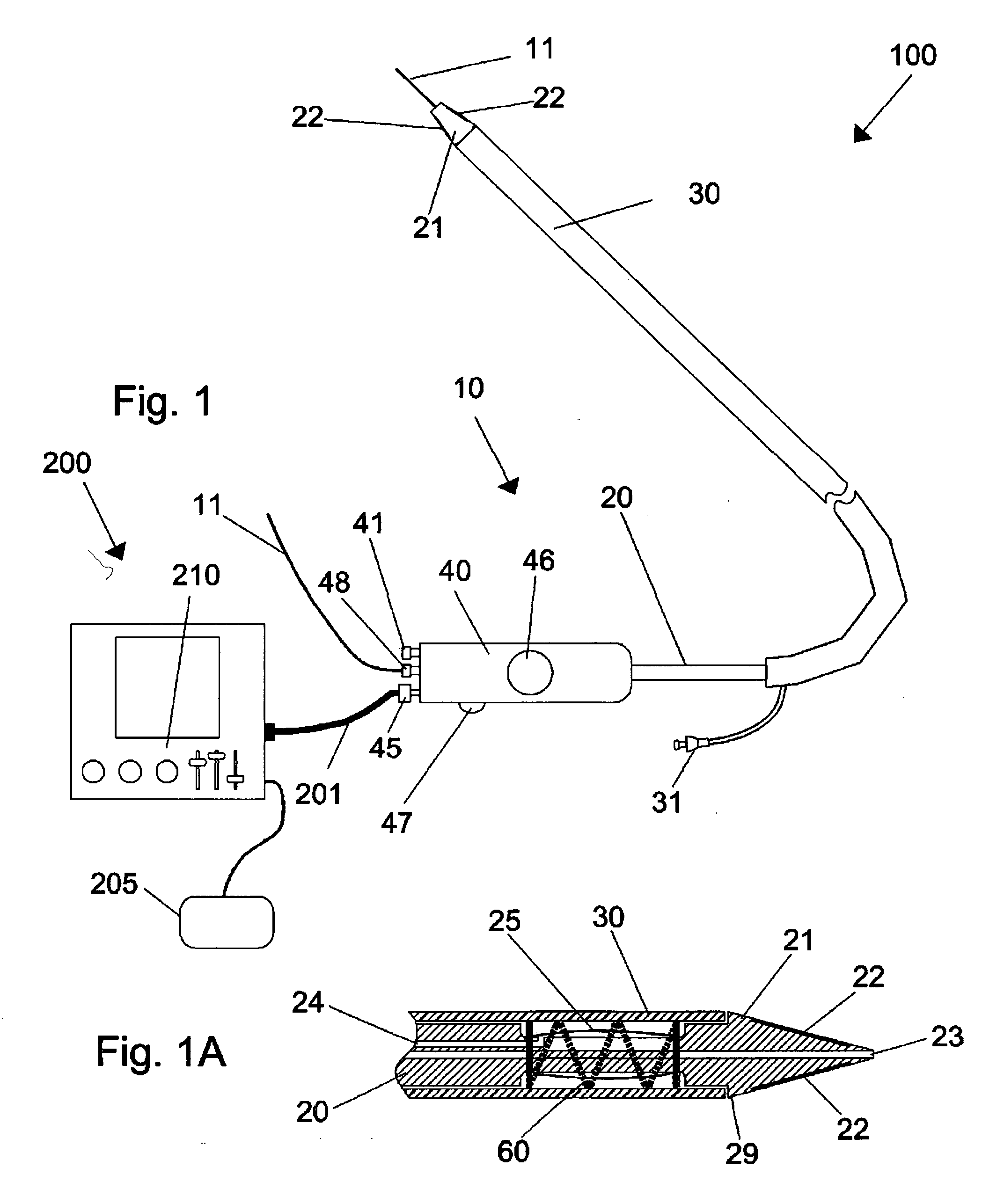
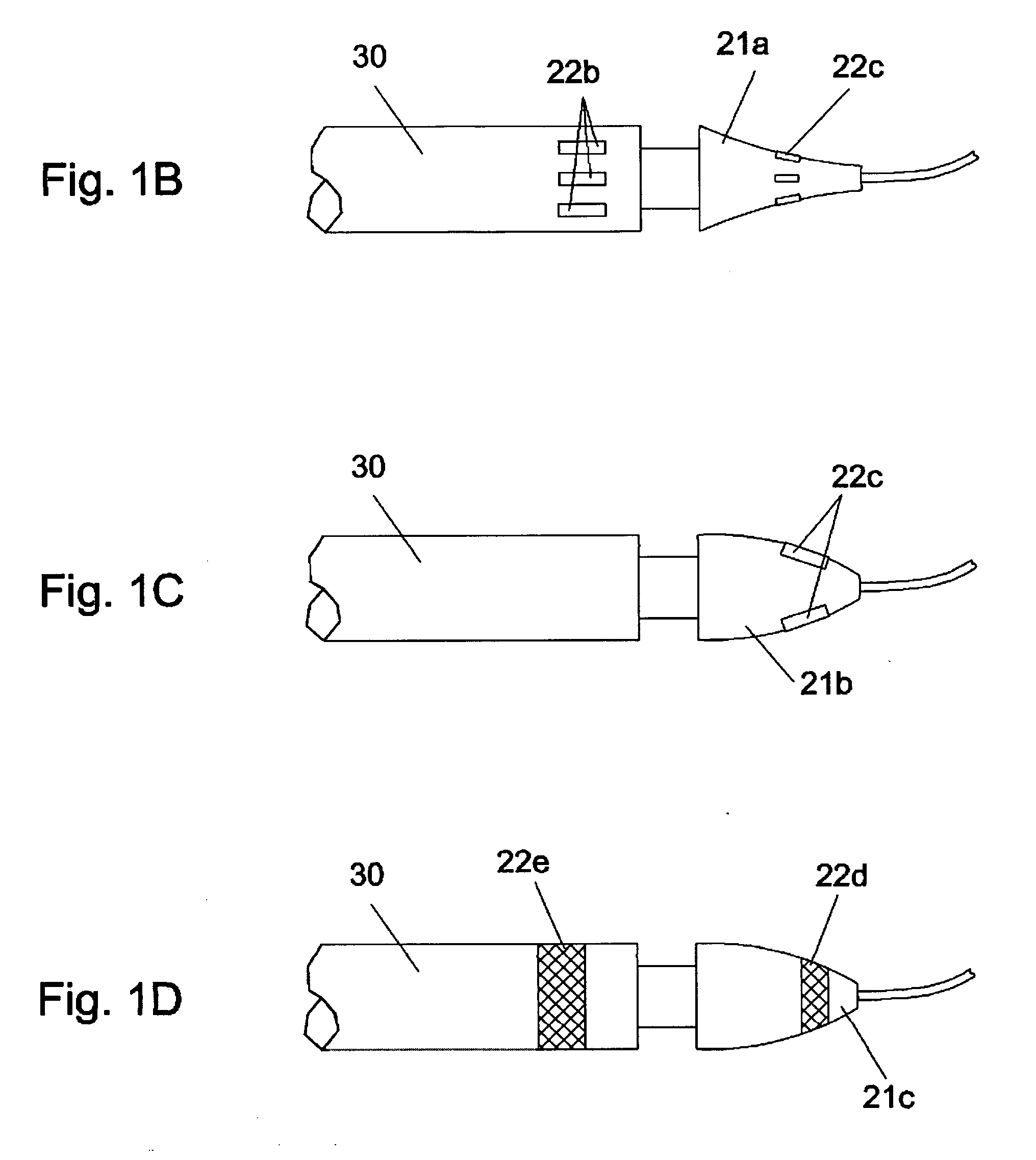
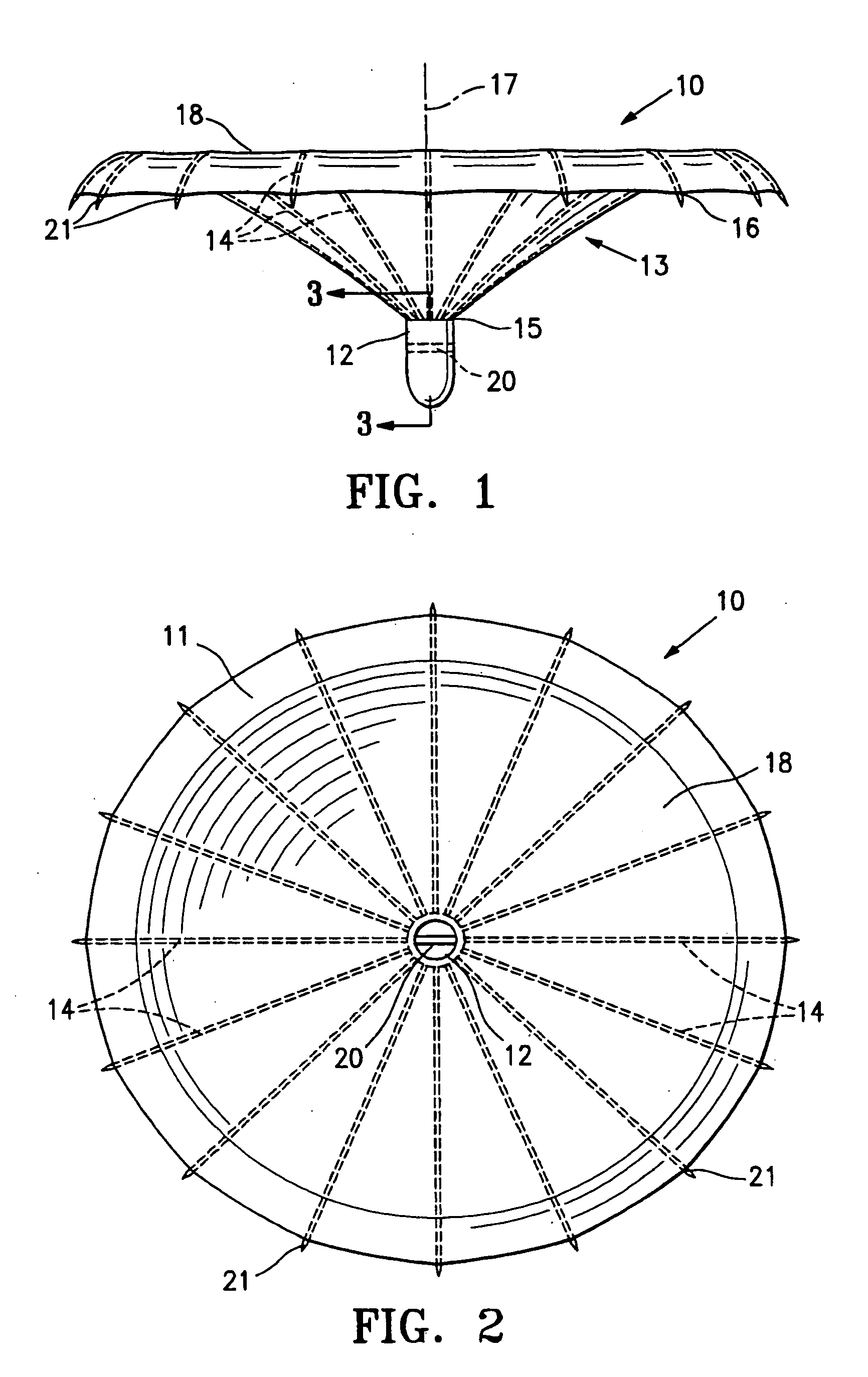
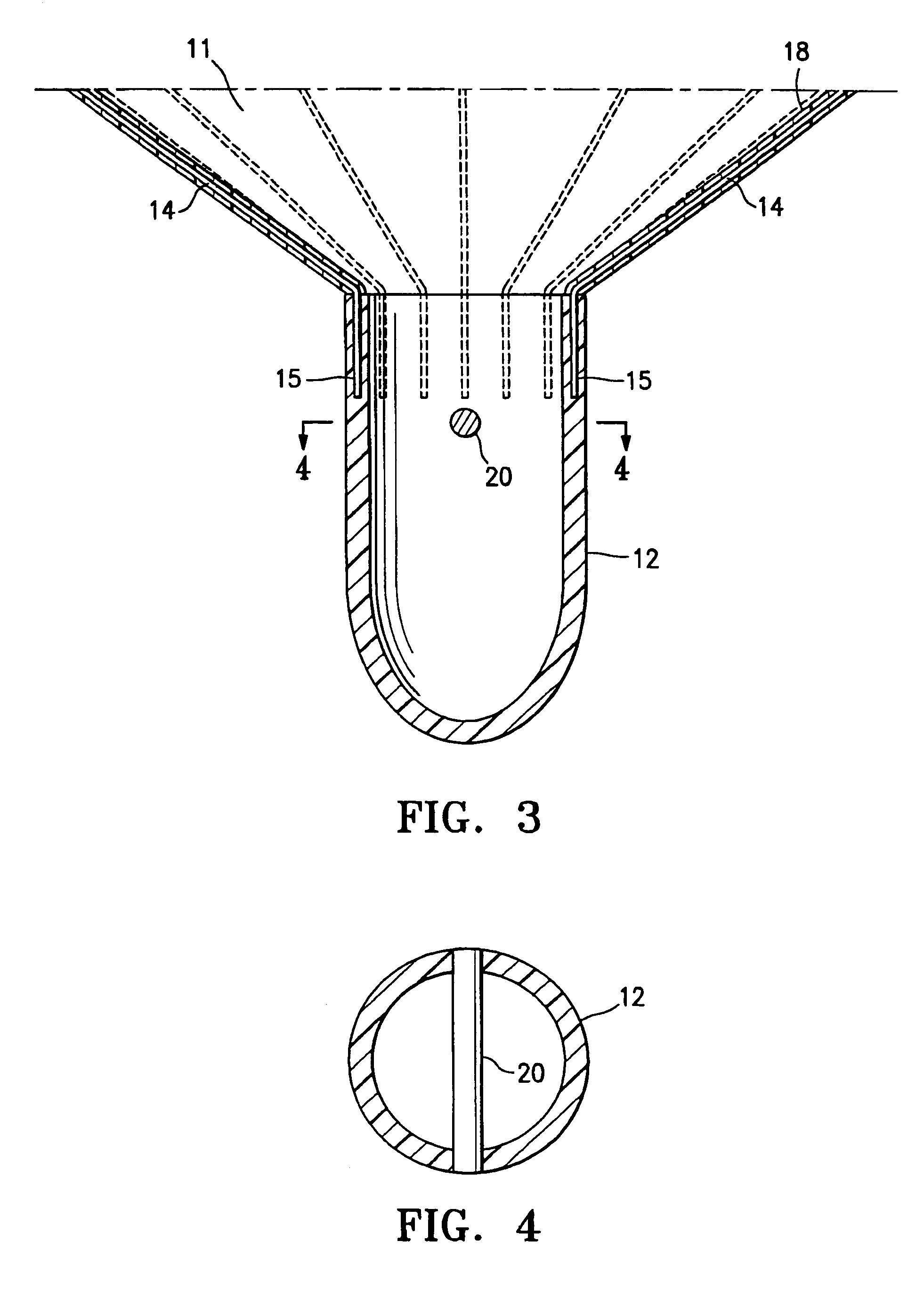
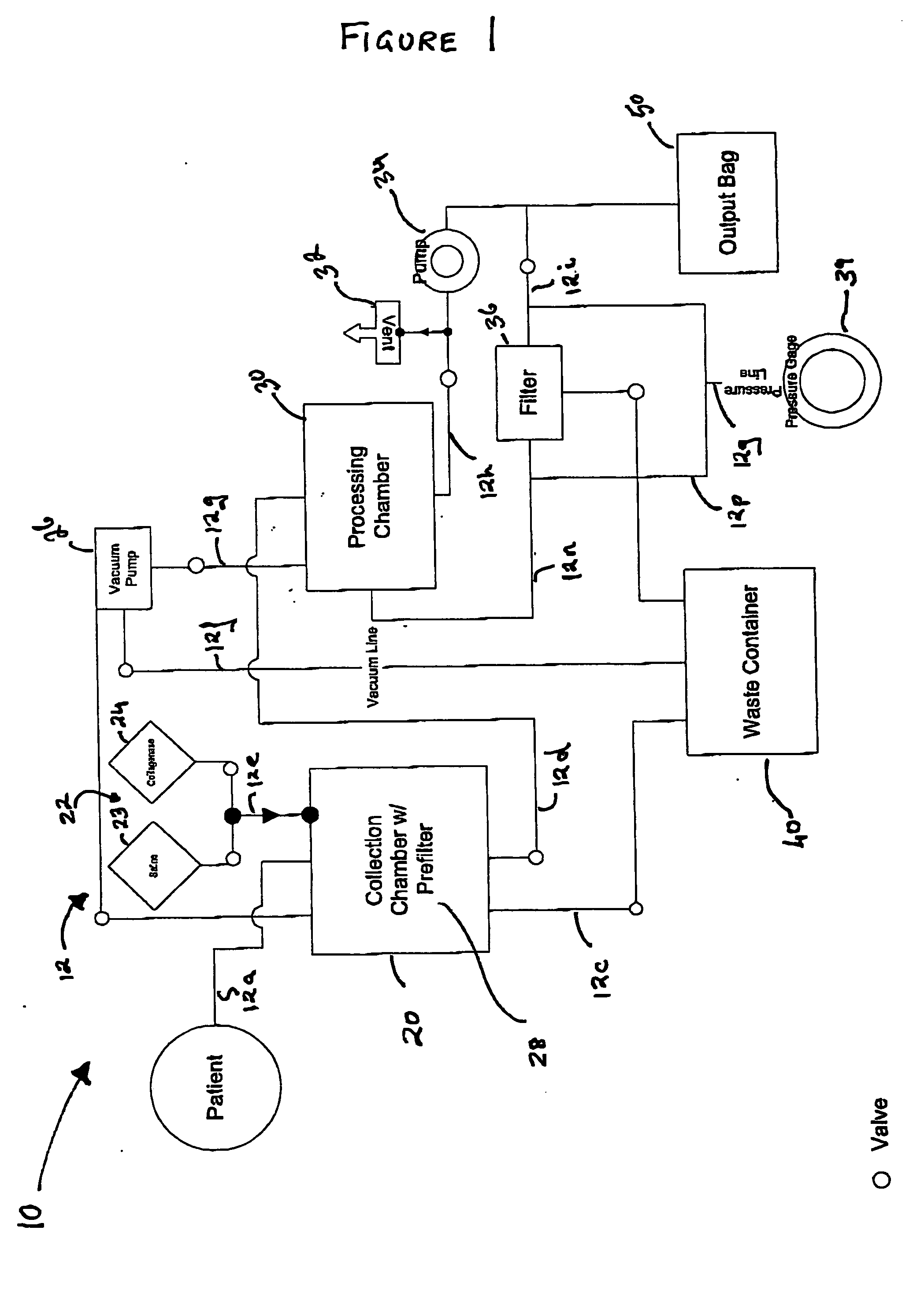
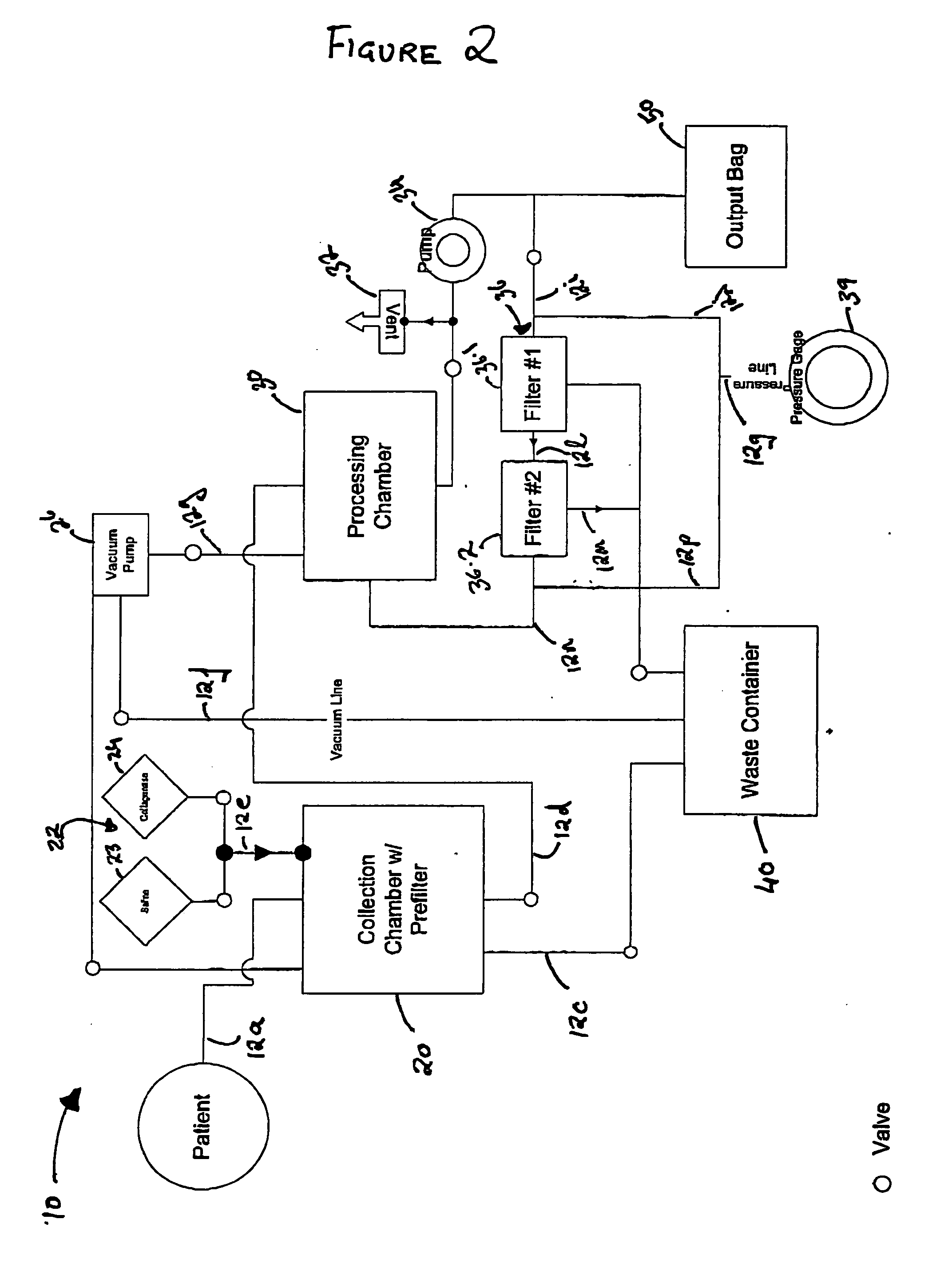
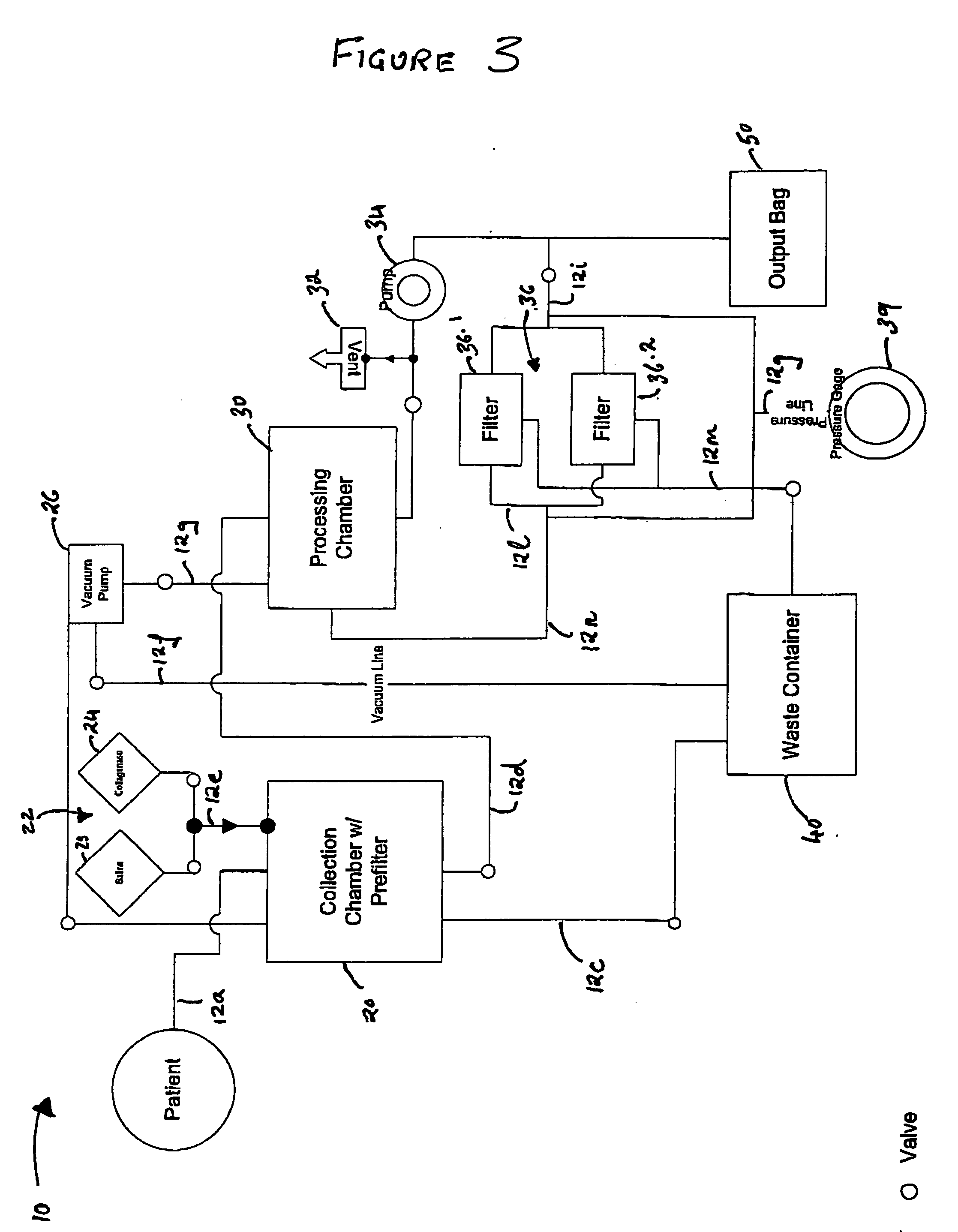
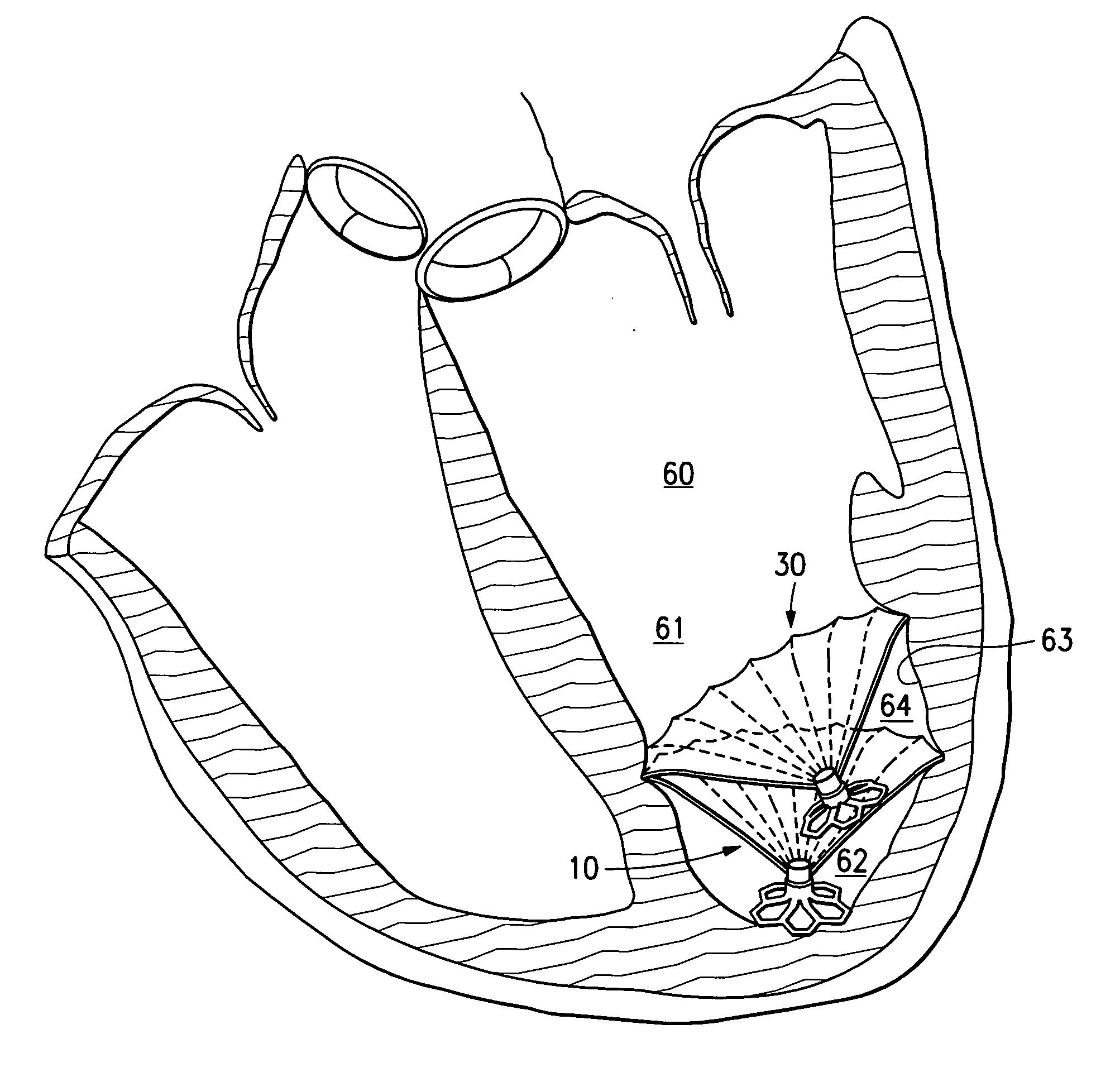
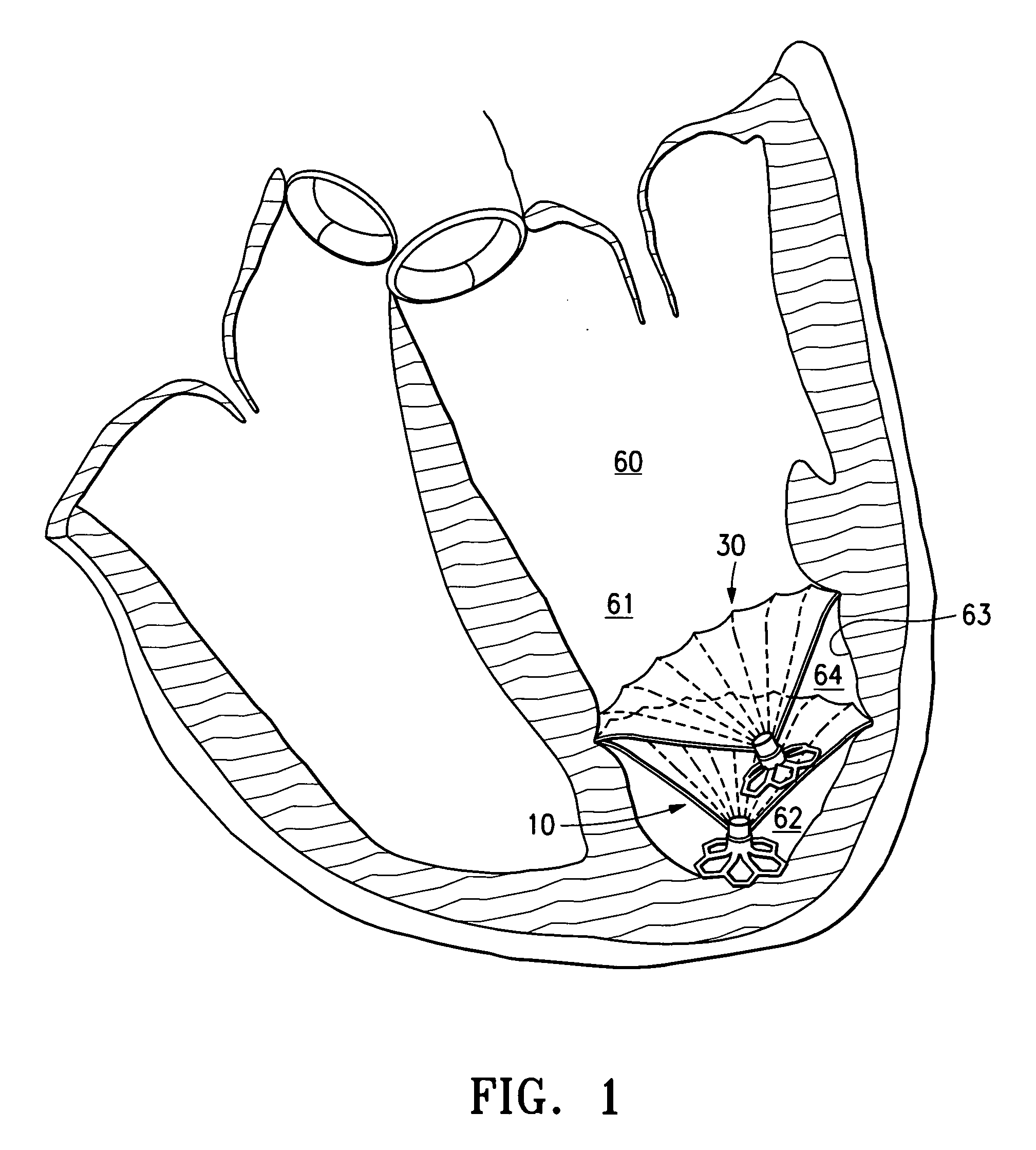
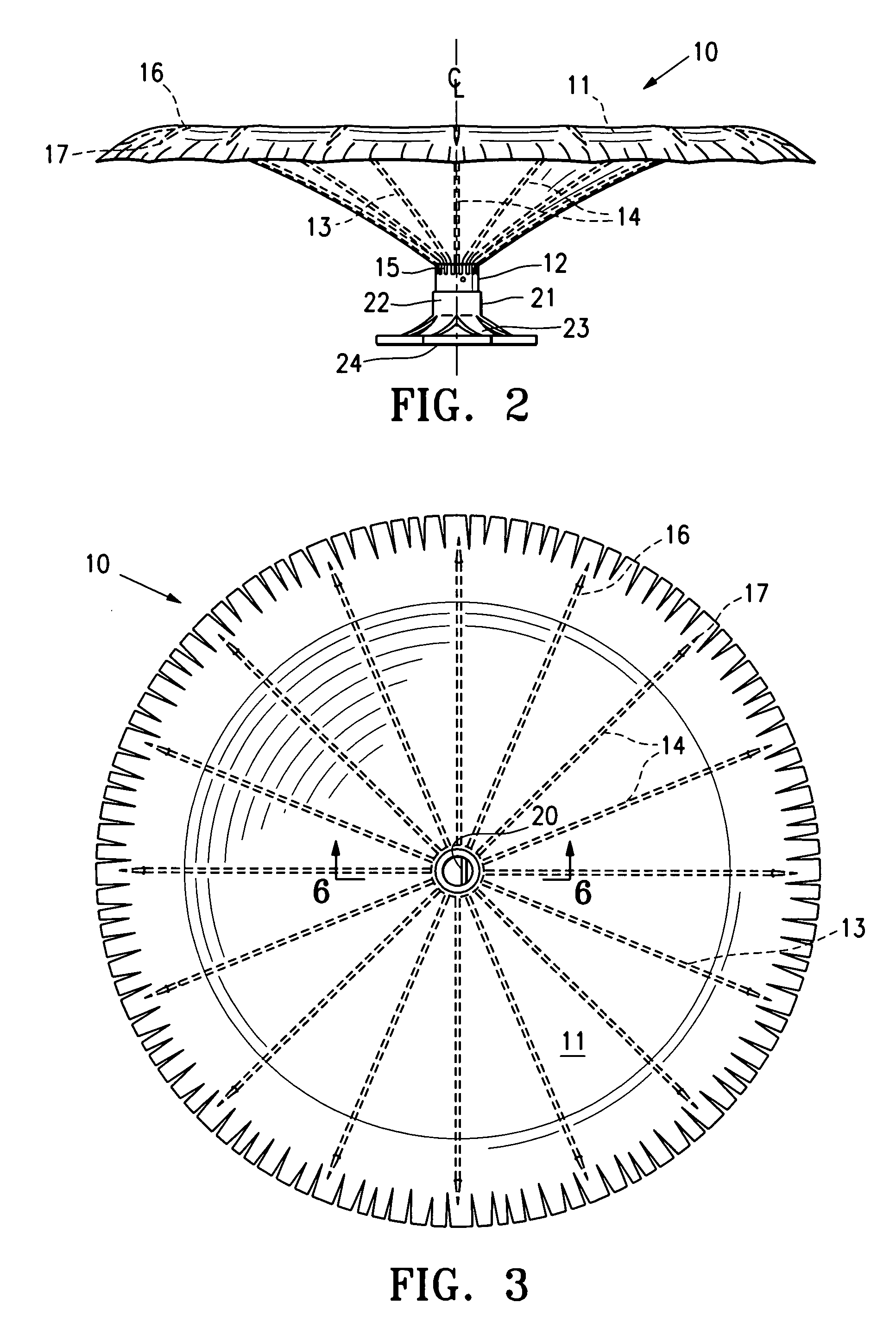
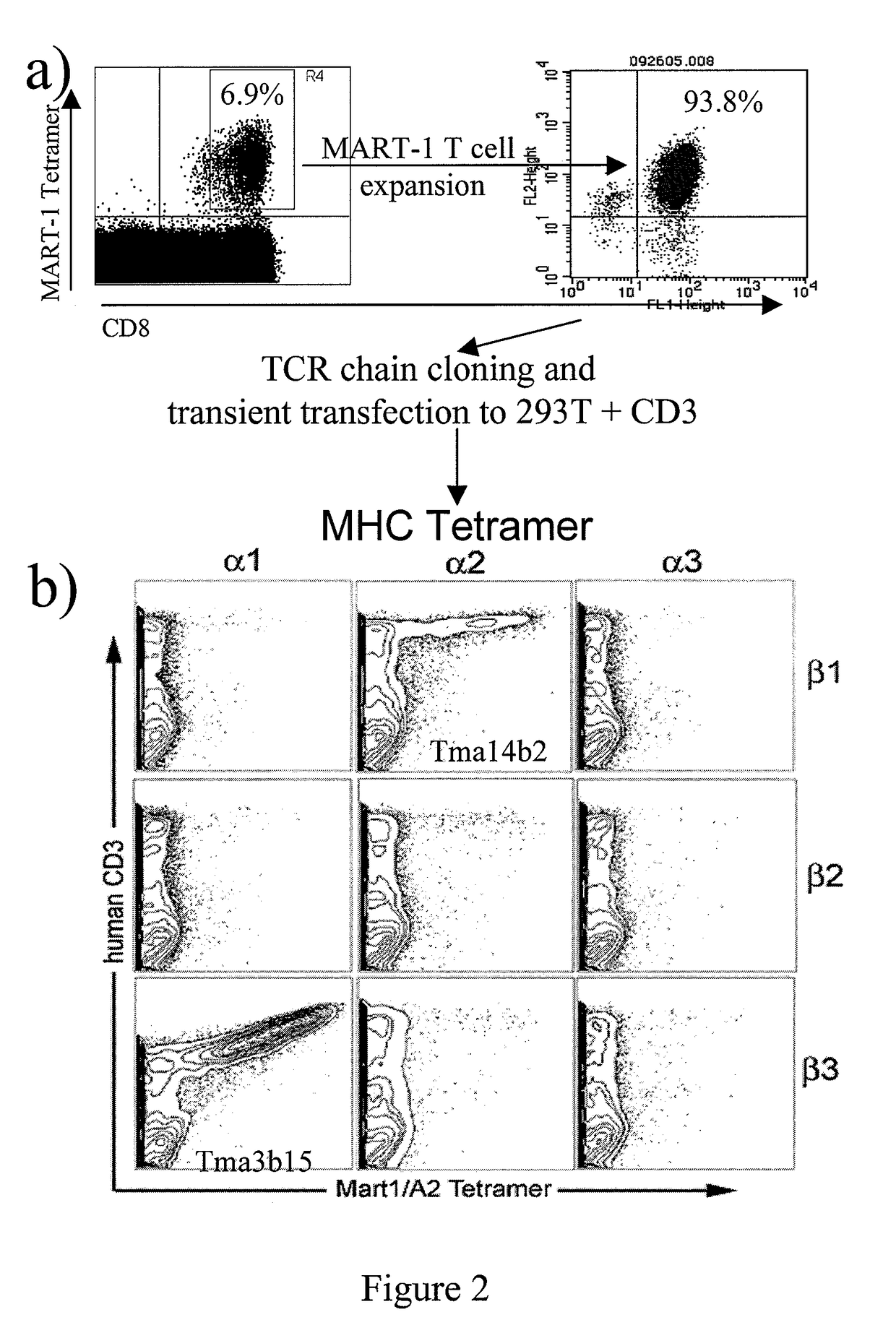
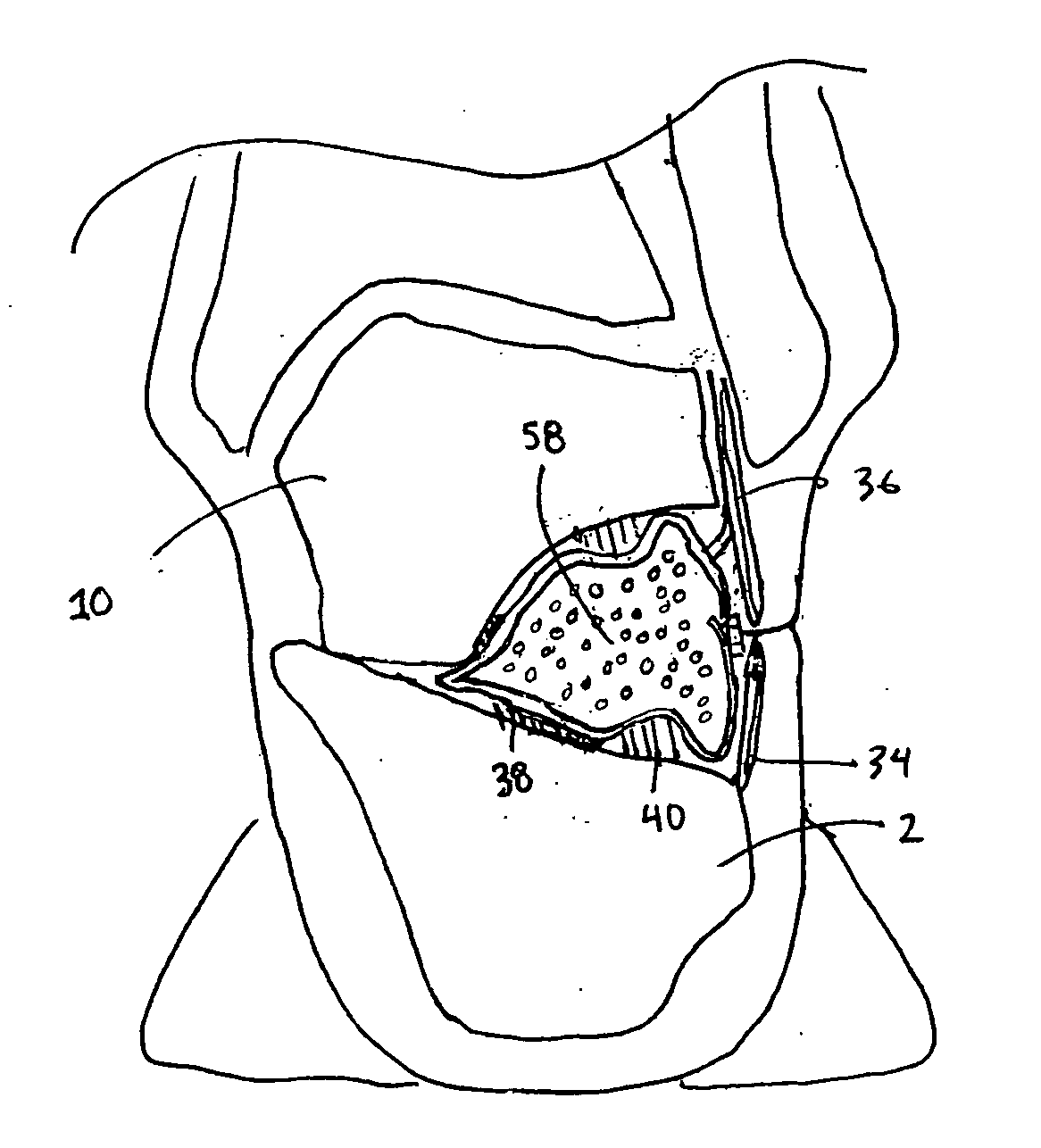
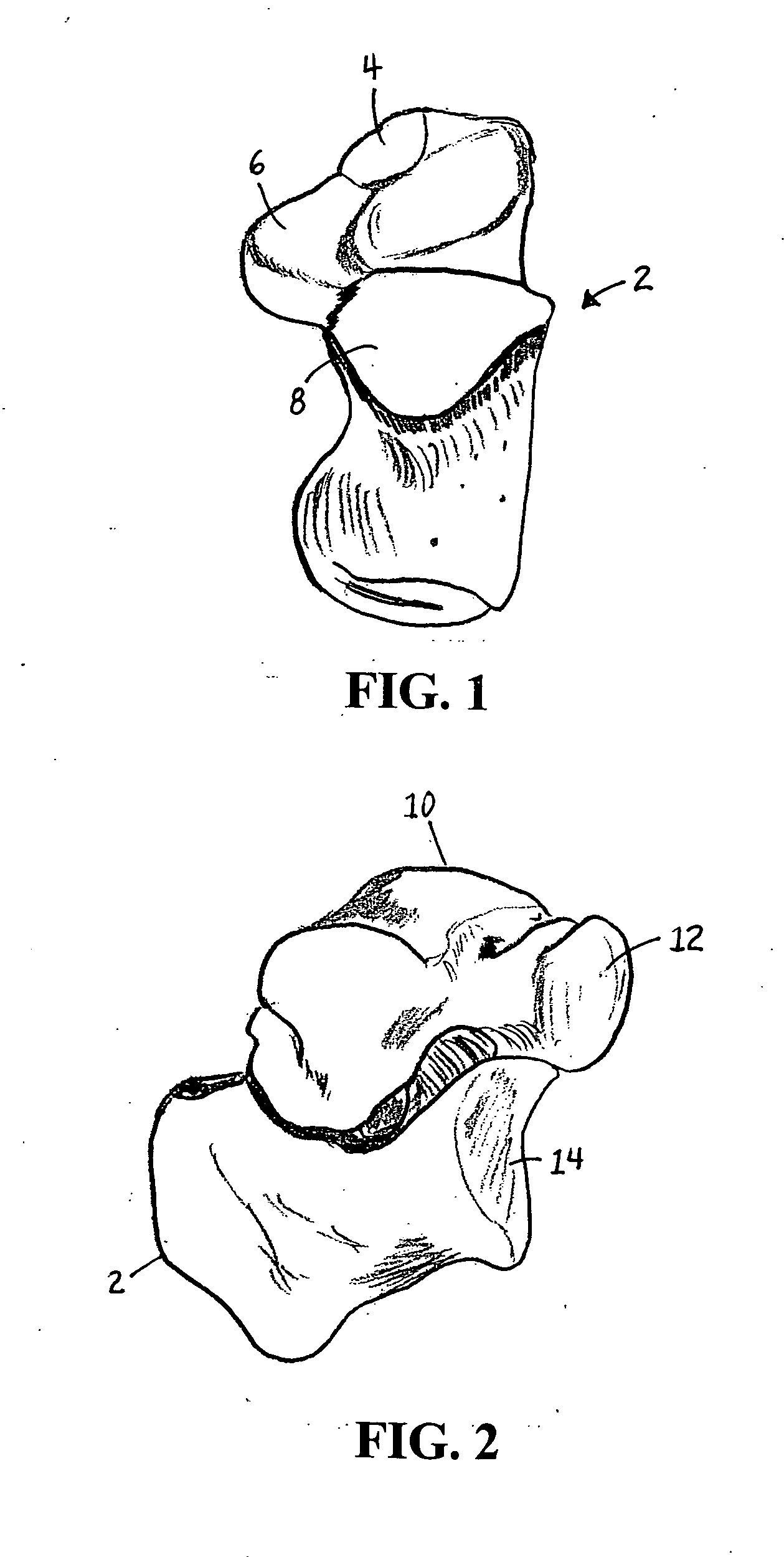
Methods and apparatus for improving the function of biological passages
ActiveUS6960233B1Reduces the hiatal herniaStrengthens the function of the lower esophageal sphincterTubular organ implantsInsertion stentEsophageal wall
Several methods and apparatus are available for treating patients that suffer from both gastro-esophageal reflux disorder and hiatal hernias. In order to treat these patients, an endoscopic probe may be used to push the herniated stomach below the diaphragm. In some embodiments, a two-part stent comprising a funnel stent and a reverse stent may be deployed to prevent a future re-herniation of the stomach and to reduce the annulus of the lower esophageal sphincter. In some embodiments, a reverse stent with perforating barbs may be deployed, in which the barbs penetrate the esophageal wall and attach to the diaphragm. In some embodiments, the crus muscles may be sutured together to reduce the hiatus size and a reverse stent may be deployed to reduce the annulus of the lower esophageal sphincter.
Owner:TORAX MEDICAL
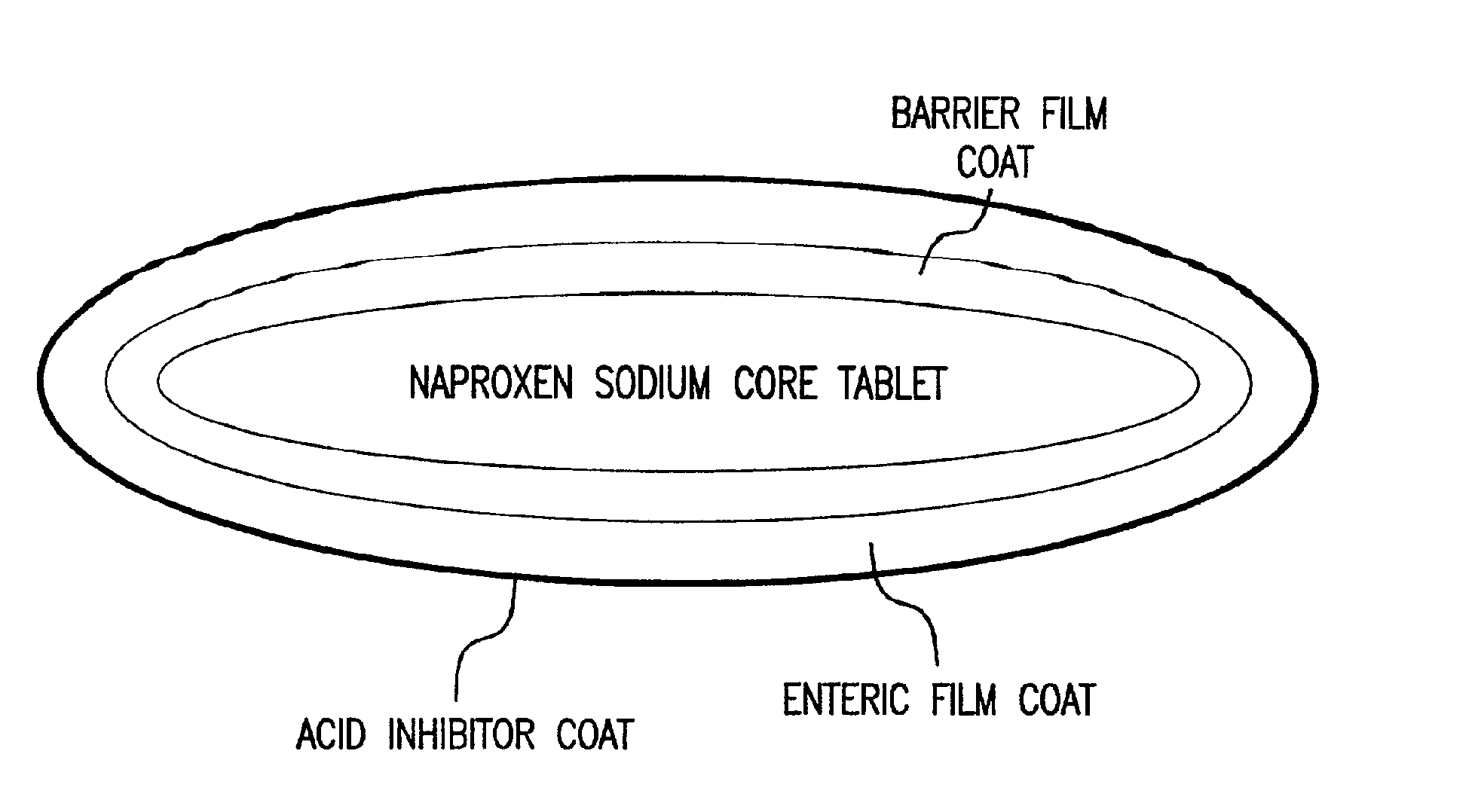
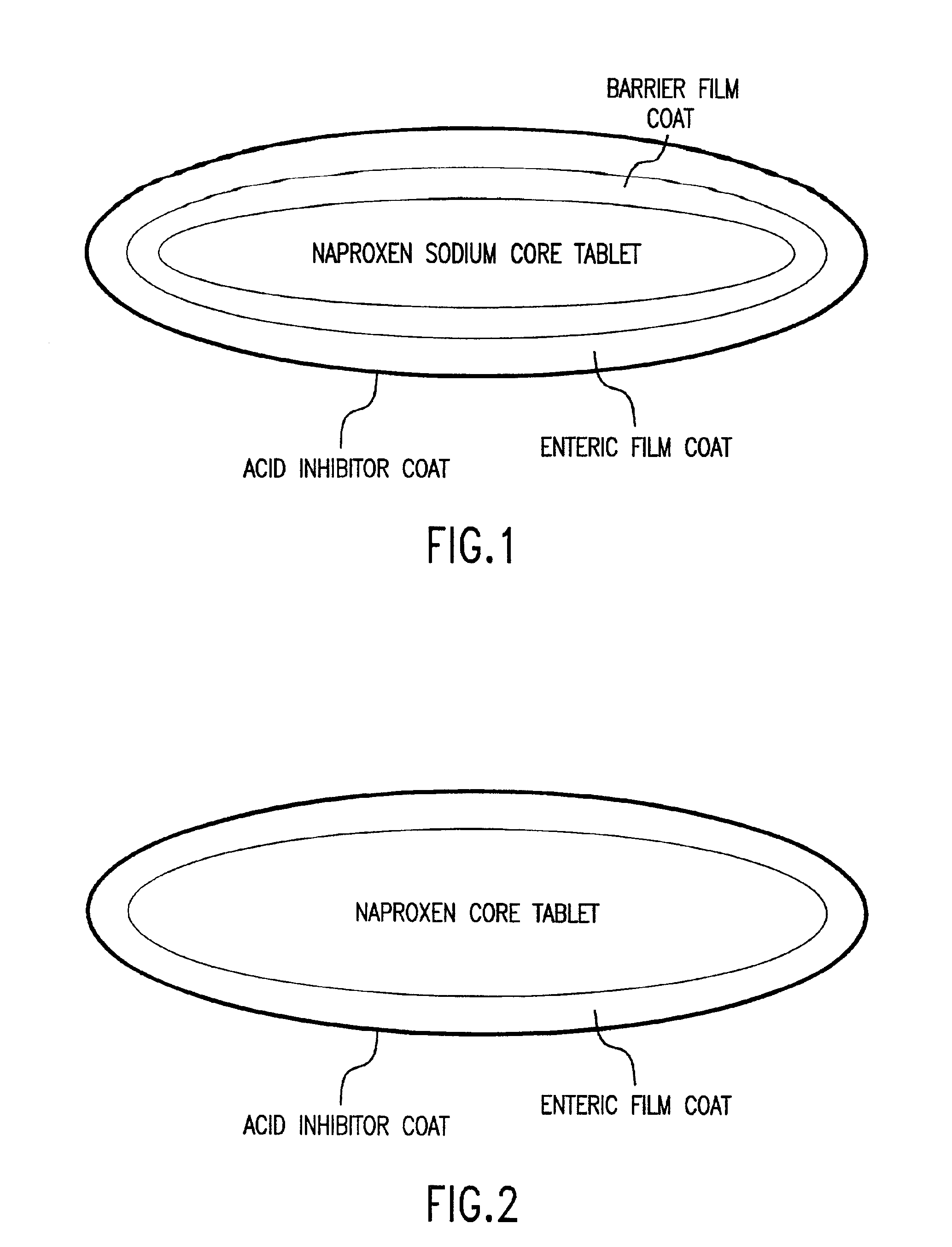
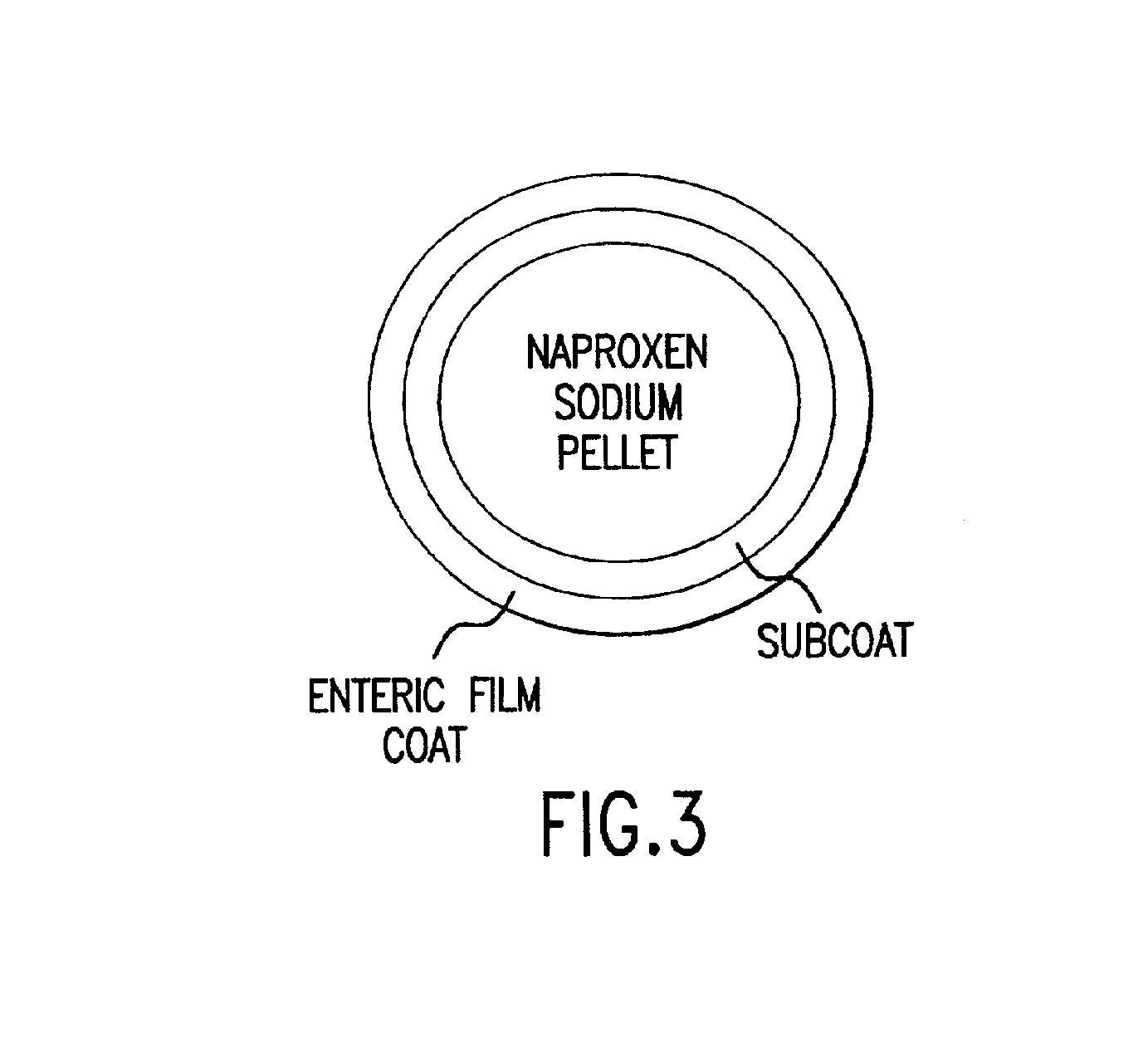
Pharmaceutical compositions for the coordinated delivery of NSAIDs
The present invention is directed to drug dosage forms that release an agent that raises the pH of a patient's gastrointestinal tract, followed by a non-steroidal anti-inflammatory drug. The dosage form is designed so that the NSAID is not released until the intragastric pH has been raised to a safe level. The invention also encompasses methods of treating patients by administering this coordinated release, gastroprotective, antiarthritic / analgesic combination unit dosage form to achieve pain and symptom relief with a reduced risk of developing gastrointestinal damage such as ulcers, erosions and hemorrhages.
Owner:NUVO PHARMA IRELAND DESIGNATED ACTIVITY CO
Devices, systems, and methods for energy assisted arterio-venous fistula creation
InactiveUS20060111704A1Avoid bleedingProviding tensionStentsSurgical instruments for heatingRESPIRATORY DISTRESS SYNDROME ADULTDisease
Devices, systems and methods are disclosed for the formation of an arteriovenous fistula. Embodiments include catheter apparatus including an ablation element for creating and / or modifying the fistula. The devices, systems and methods can be used to treat patients with one or more numerous ailments including chronic obstructive pulmonary disease, congestive heart failure, hypertension, hypotension, respiratory failure, pulmonary arterial hypertension, lung fibrosis and adult respiratory distress syndrome.
Owner:EDWARDS LIFESCIENCES CORP
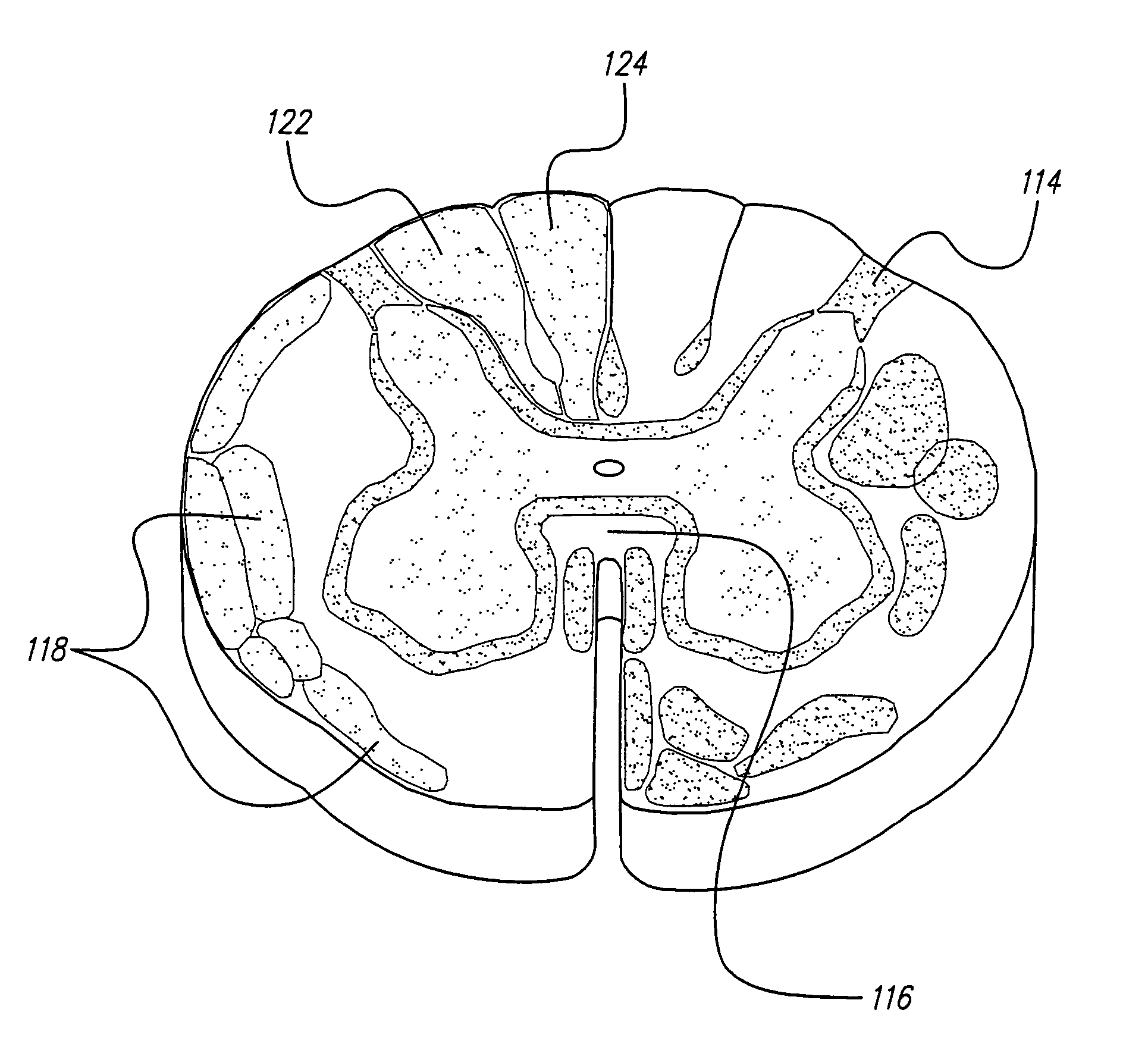
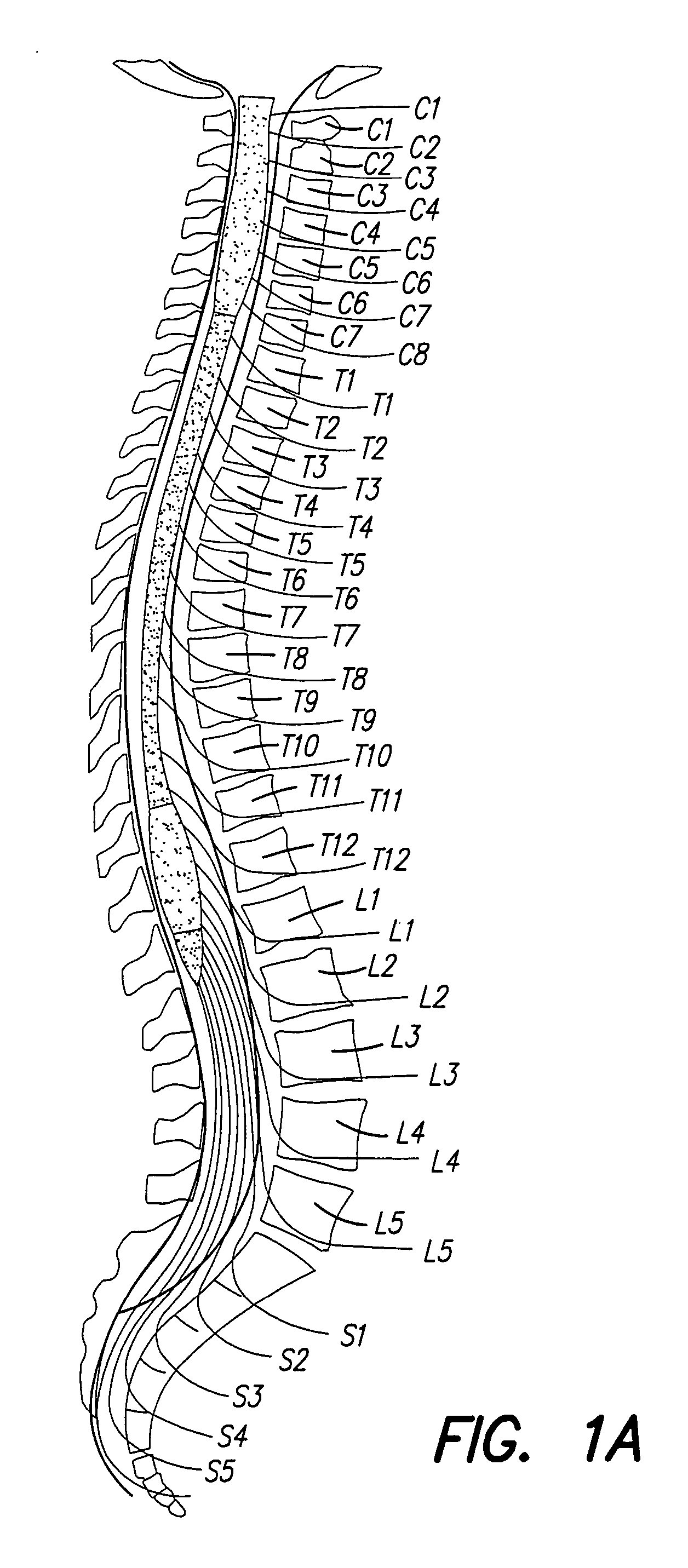
Methods for implanting a spinal cord stimulator
InactiveUS20050119713A1Decrease excitement of targeted nerve rootsIncrease the areaElectrotherapyElectric power transmissionSpinal column
Methods for implanting spinal cord stimulators are provided, including implanting at least one electrode in an anterolateral area of the spine. Stimulation provided by the stimulator(s) may be used to treat patients with chronic pain. The stimulator(s) use a power source / storage device, such as a rechargeable battery. Periodic recharging of such a power source / storage device is accomplished, for example, by inductive coupling with an external applience. The stimulators provide means of stimulating a nerve(s) or other tissue when desired, without the need for external appliances during the stimulation session. When necessary, external appliances are used for the transmission of data to and / or from the stimulator(s) and for the transmission of power, if necessary. In a preferred embodiment, the system is capable of open- and closed-loop operation. In closed-loop operation, at least one implant includes at least one sensor, and the sensed condition is used to adjust stimulation parameters.
Owner:BOSTON SCI NEUROMODULATION CORP
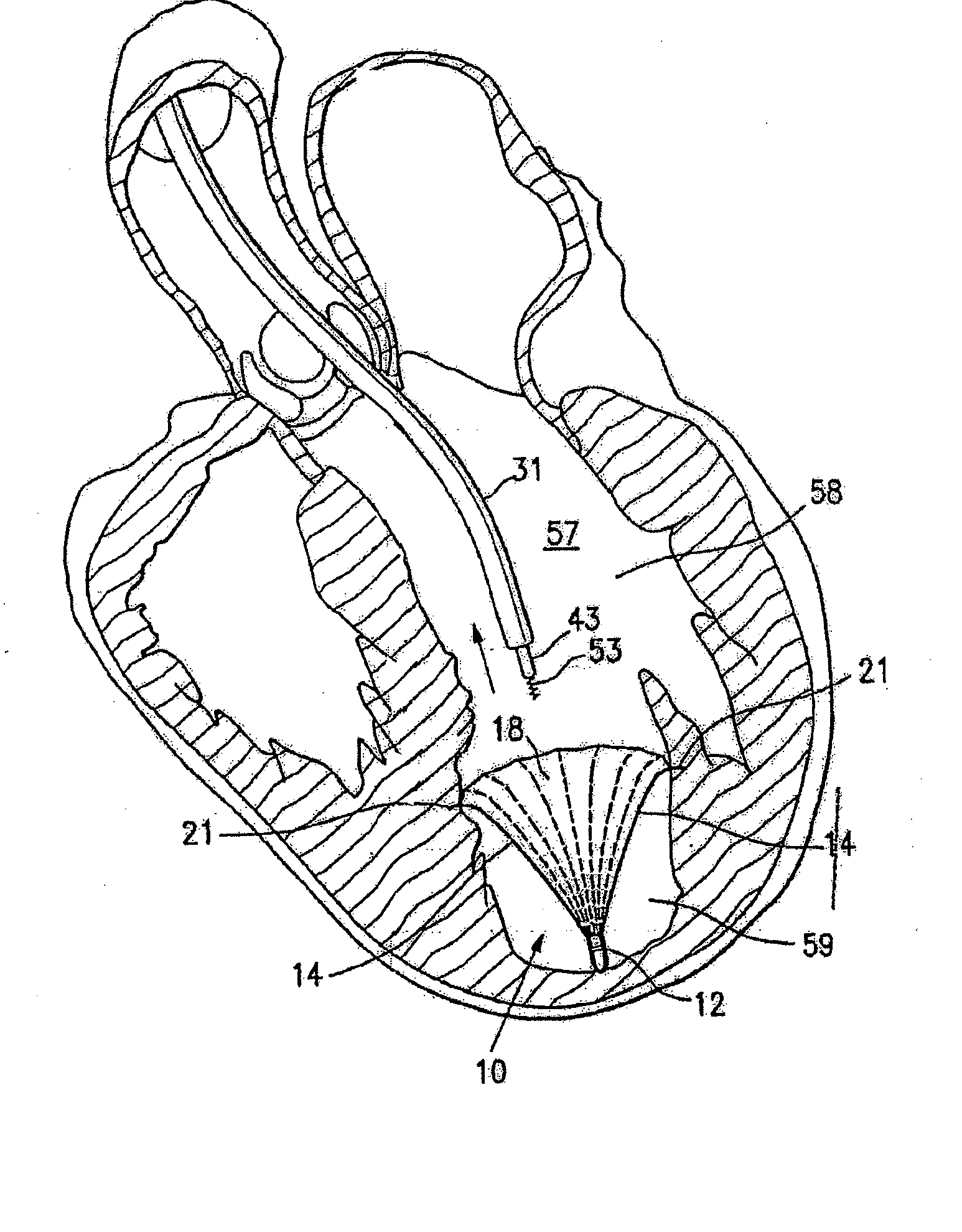
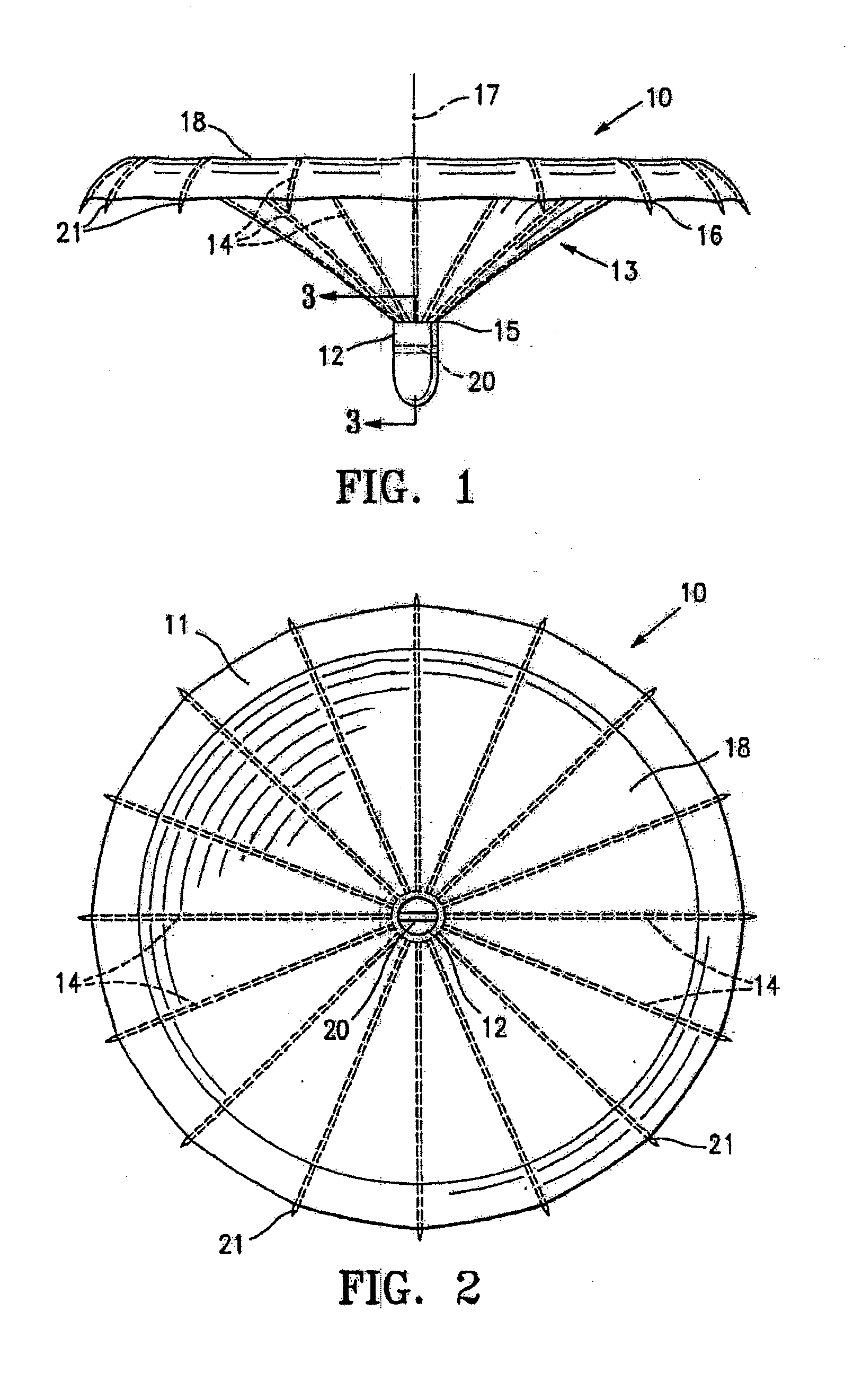
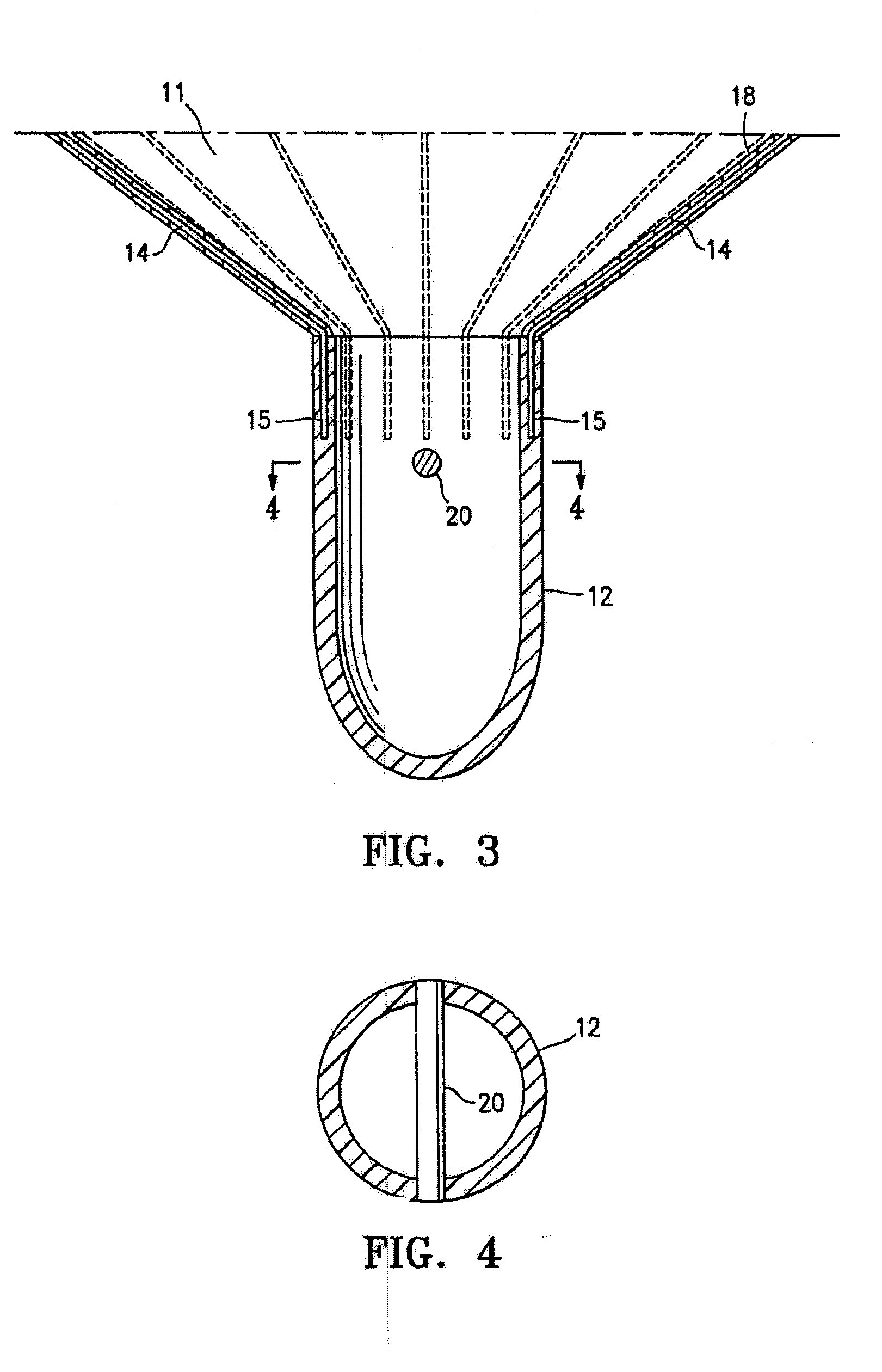
Ventricular partitioning device
ActiveUS20050154252A1Lower the volumeImprove ejection fractionHeart valvesHeart stimulatorsHeart chamberNon traumatic
This invention is directed to a partitioning device for separating a patient's heart chamber into a productive portion and a non-productive portion. The device is particularly suitable for treating patients with congestive heart failure. The partitioning device has a reinforced, expandable membrane which separates the productive and non-productive portions of the heart chamber and a support or spacing member extending between the reinforced membrane and the wall of the patient's heart chamber. The support or spacing member has a non-traumatic distal end to engage the ventricular wall.
Owner:EDWARDS LIFESCIENCES CORP
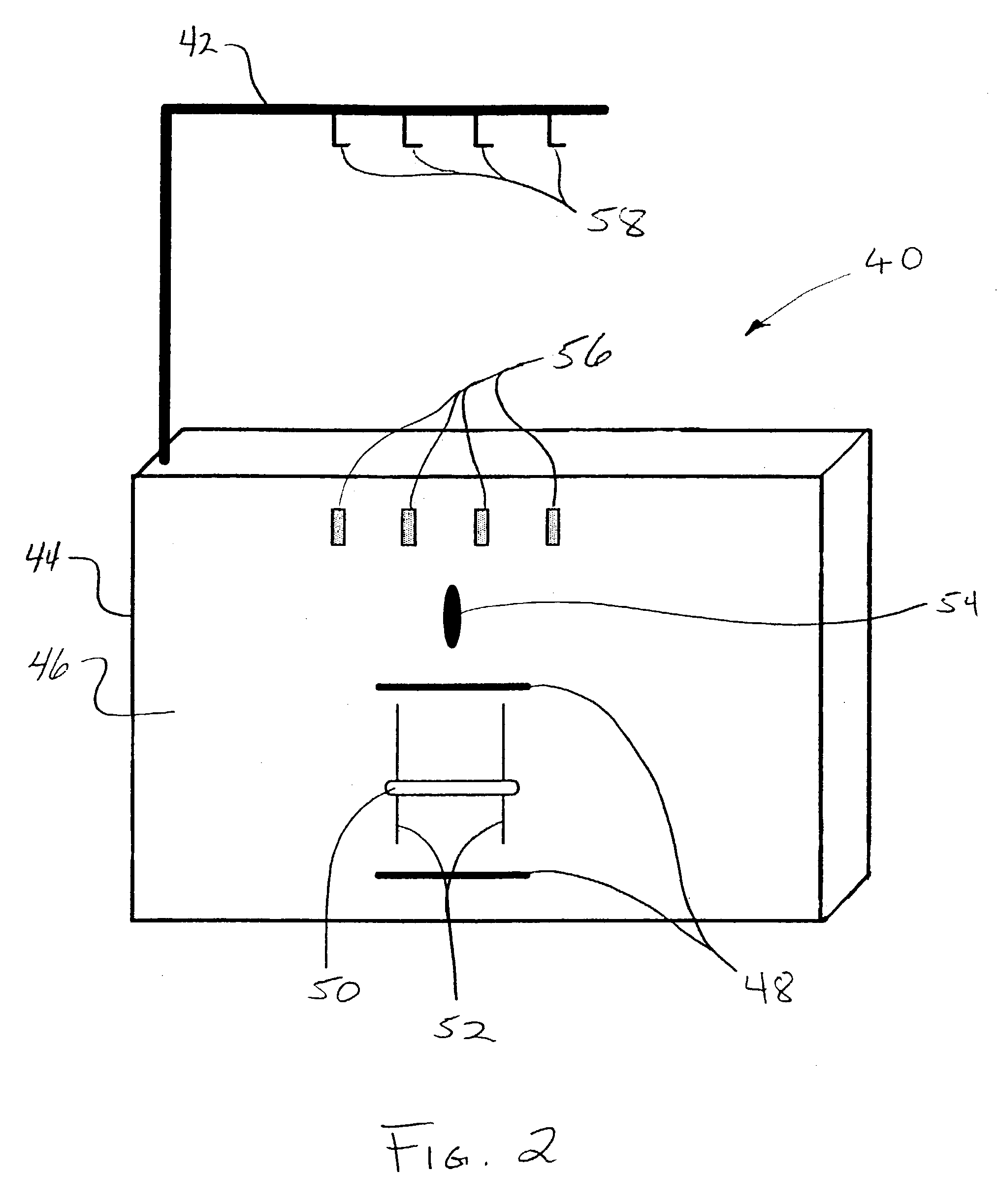
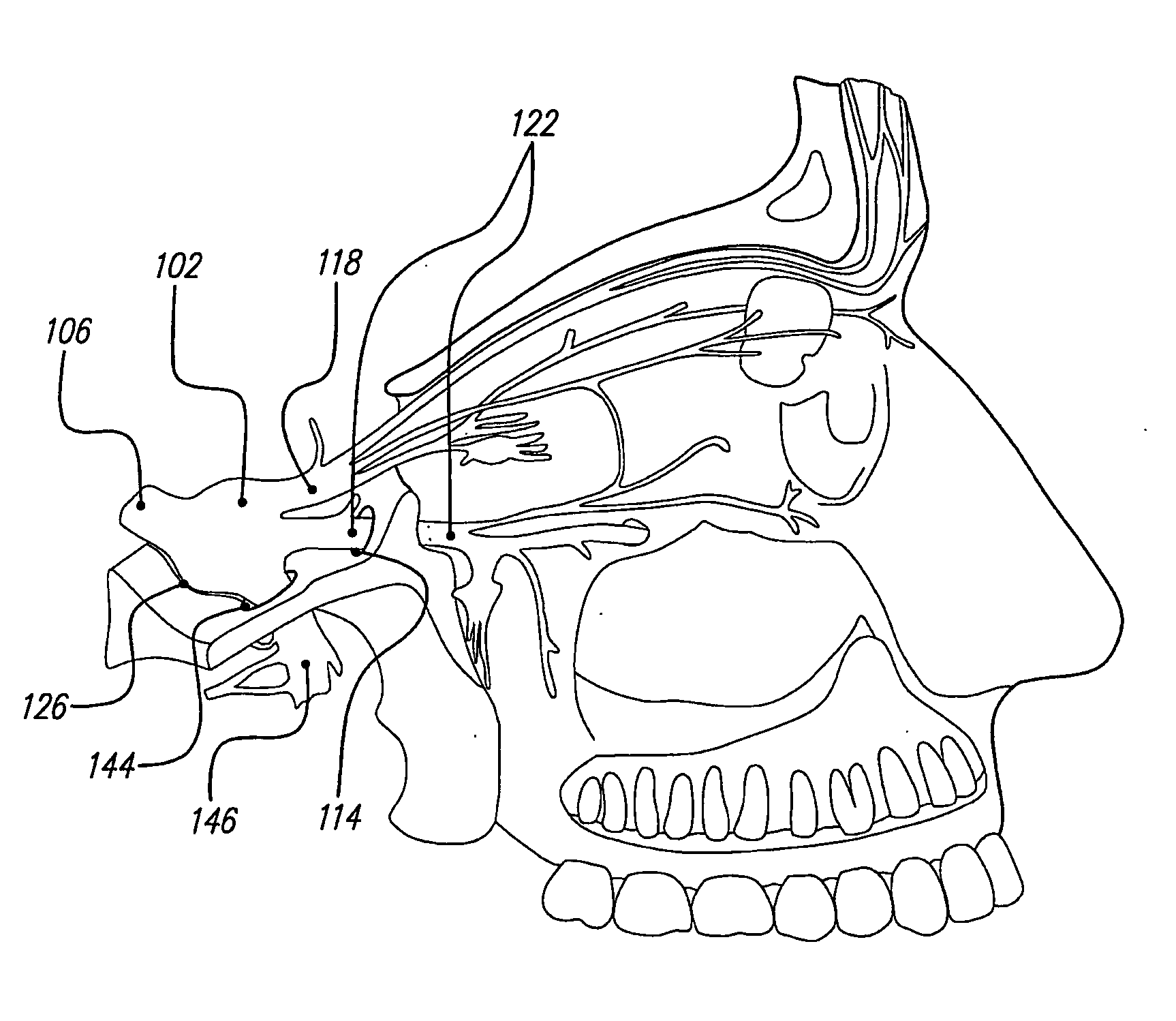
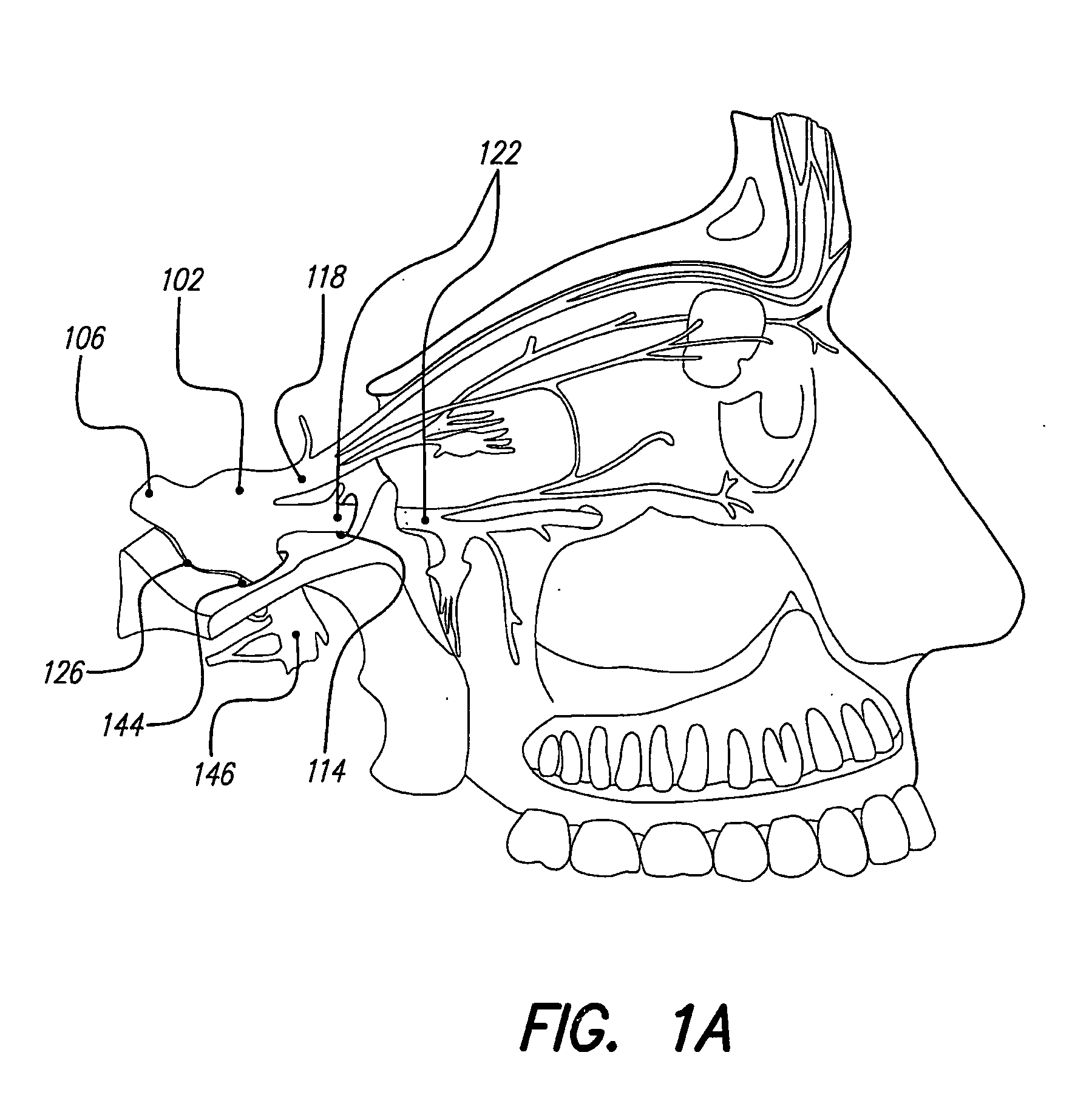
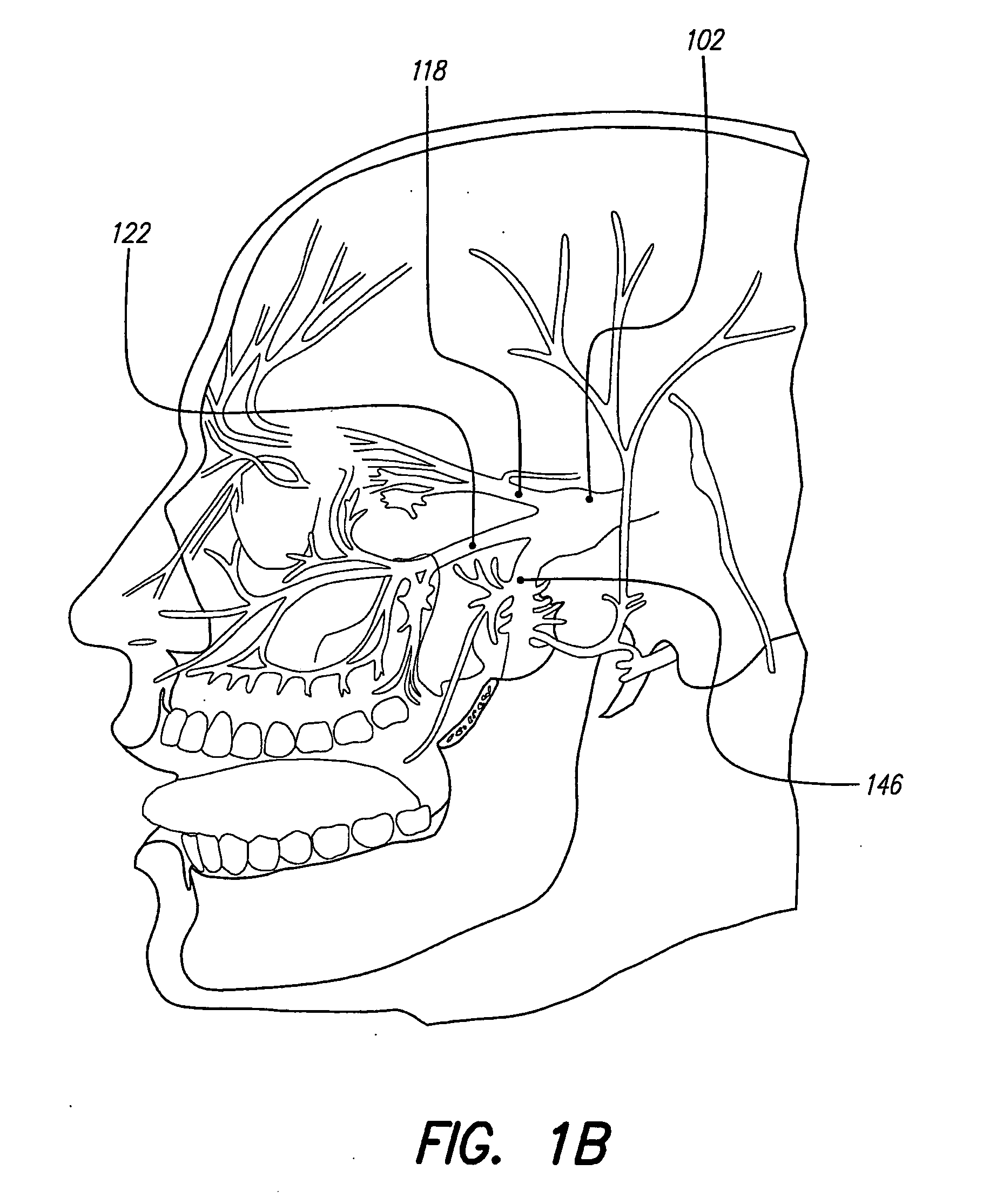
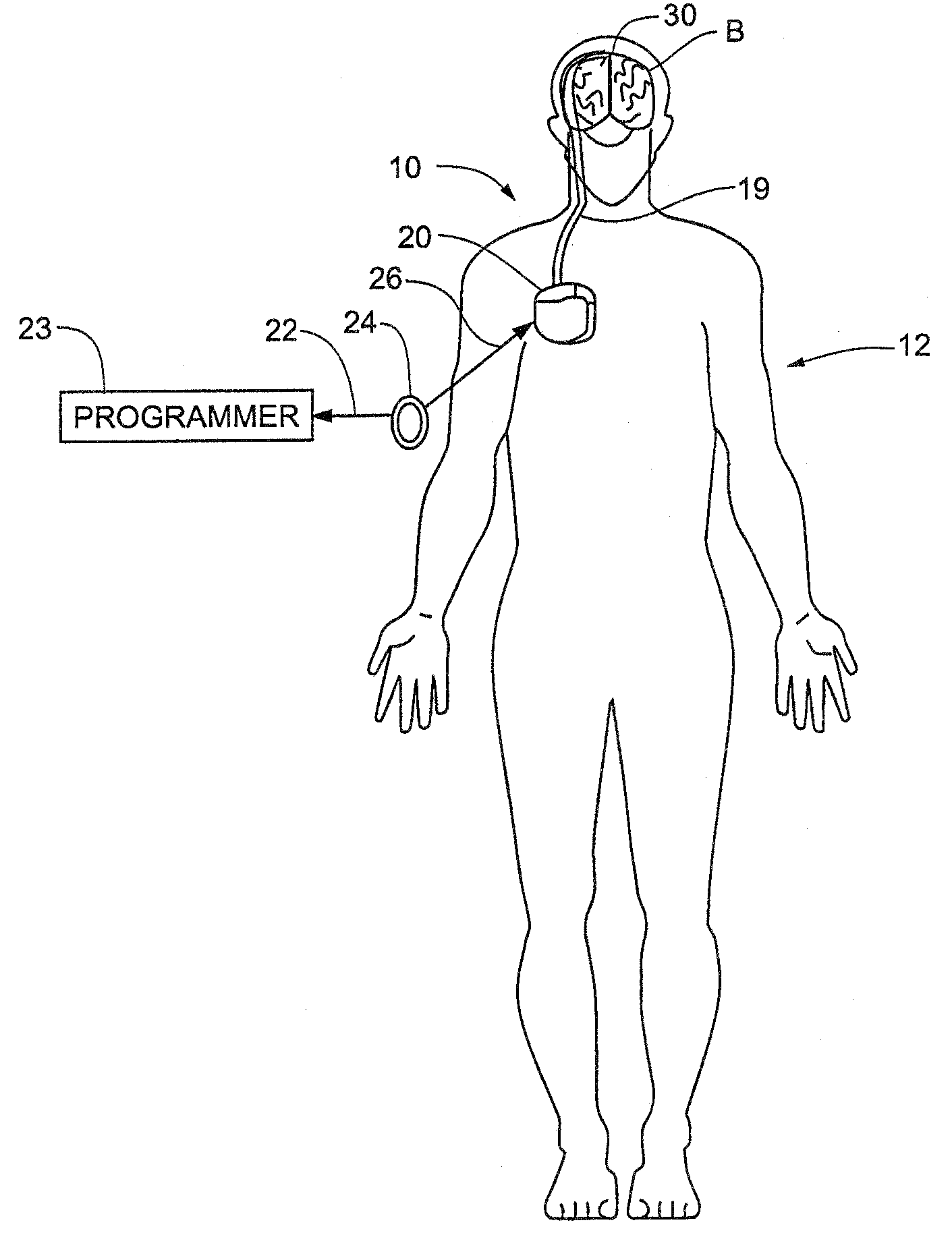
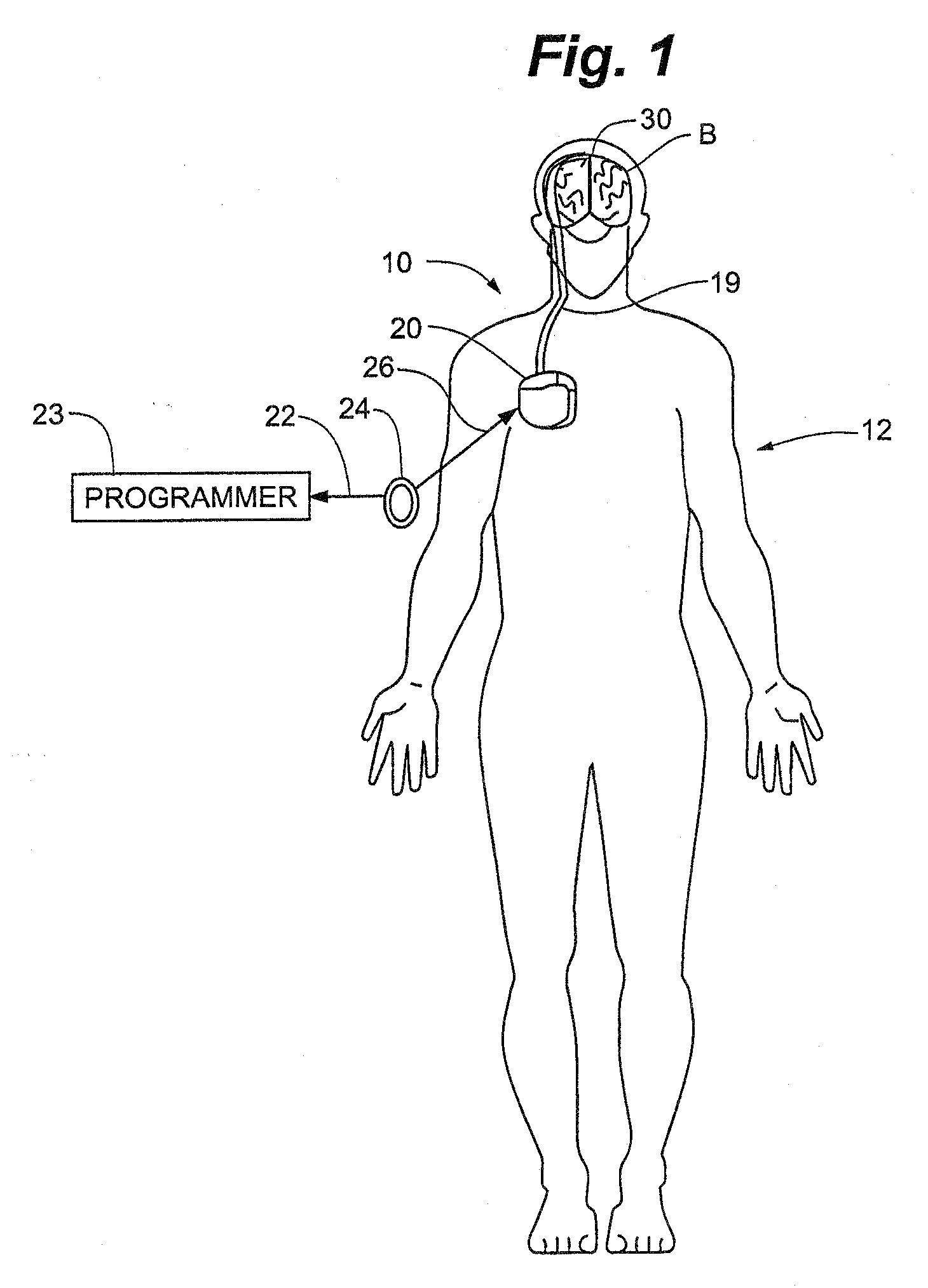
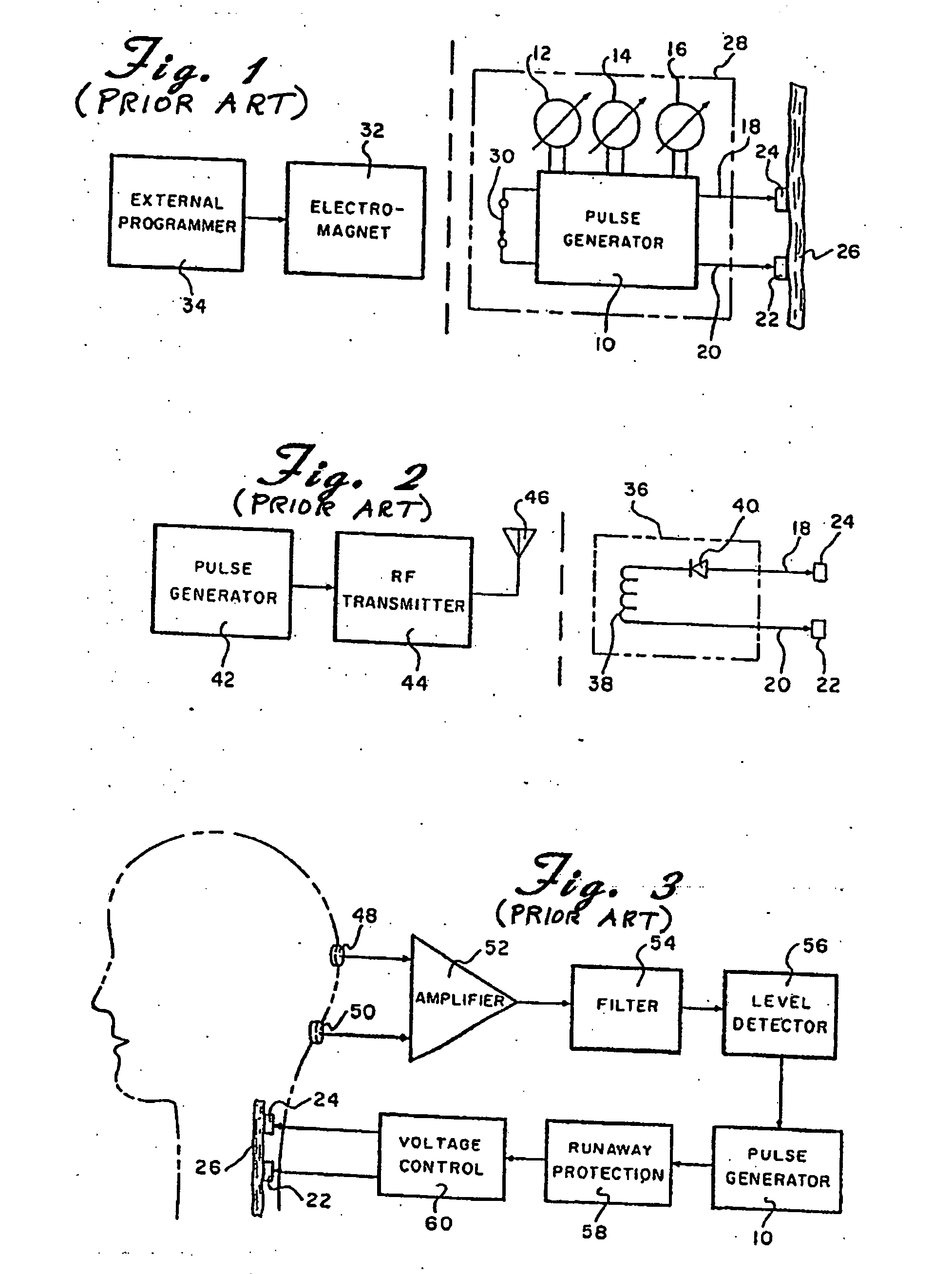
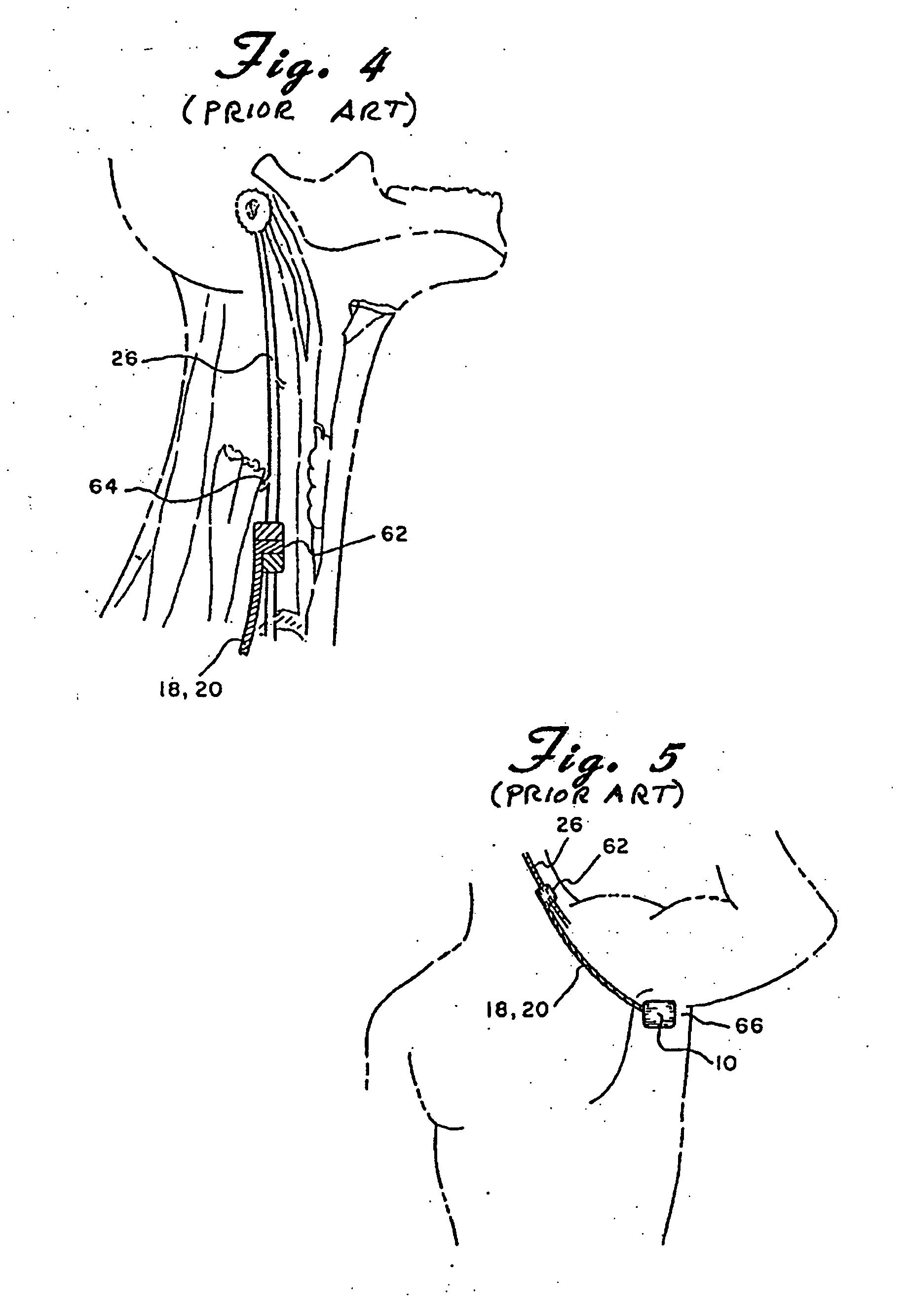
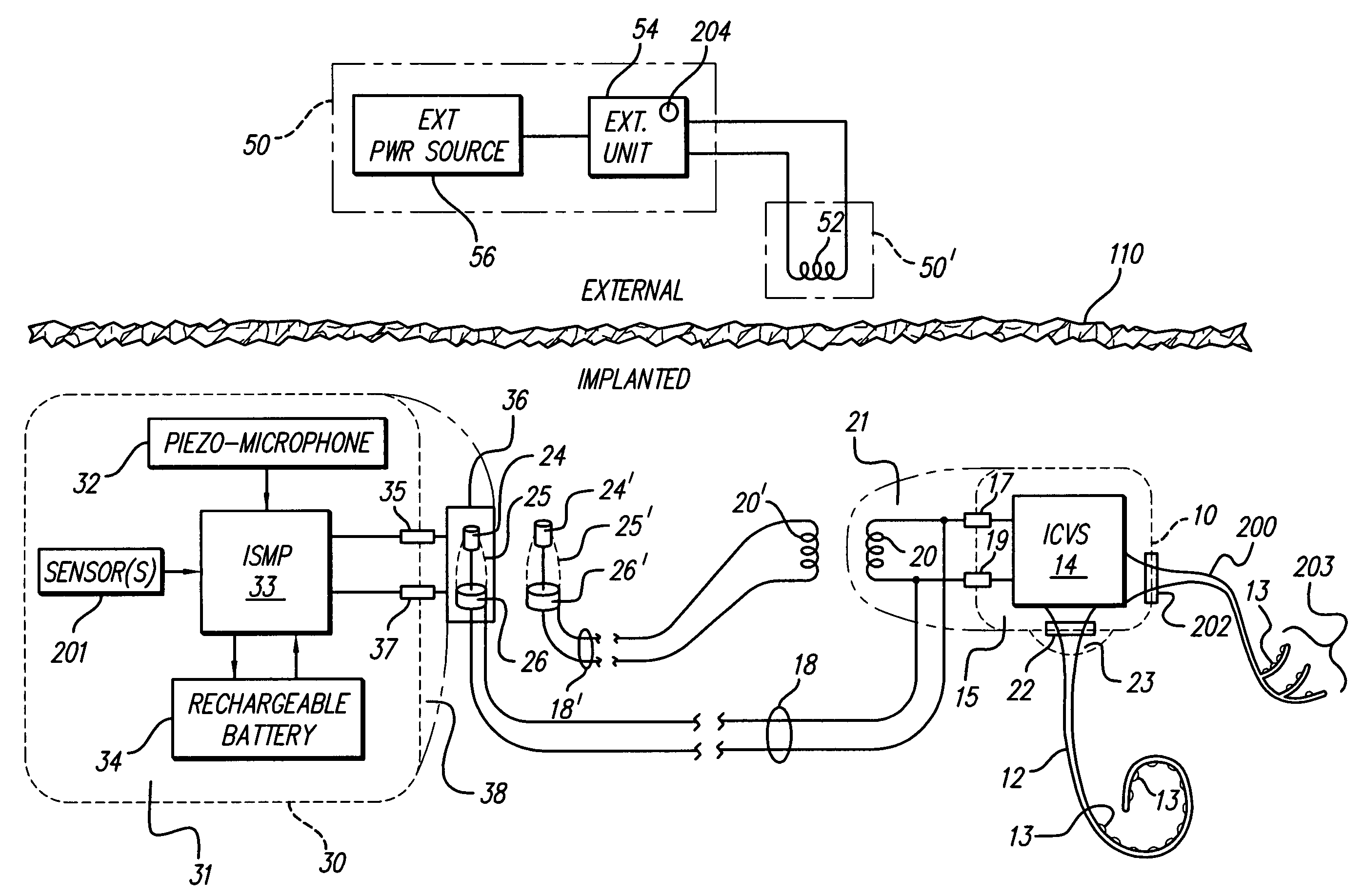
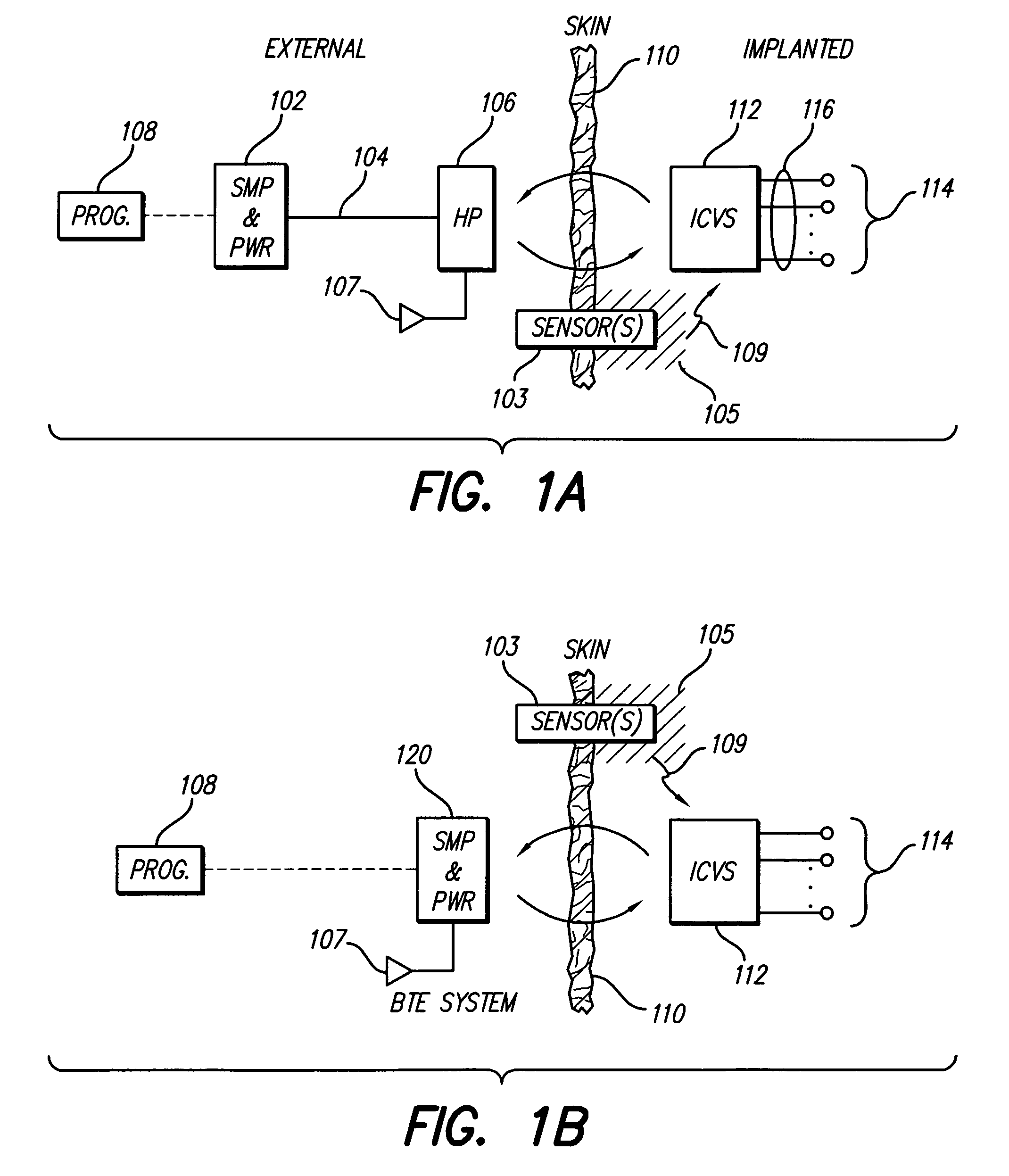
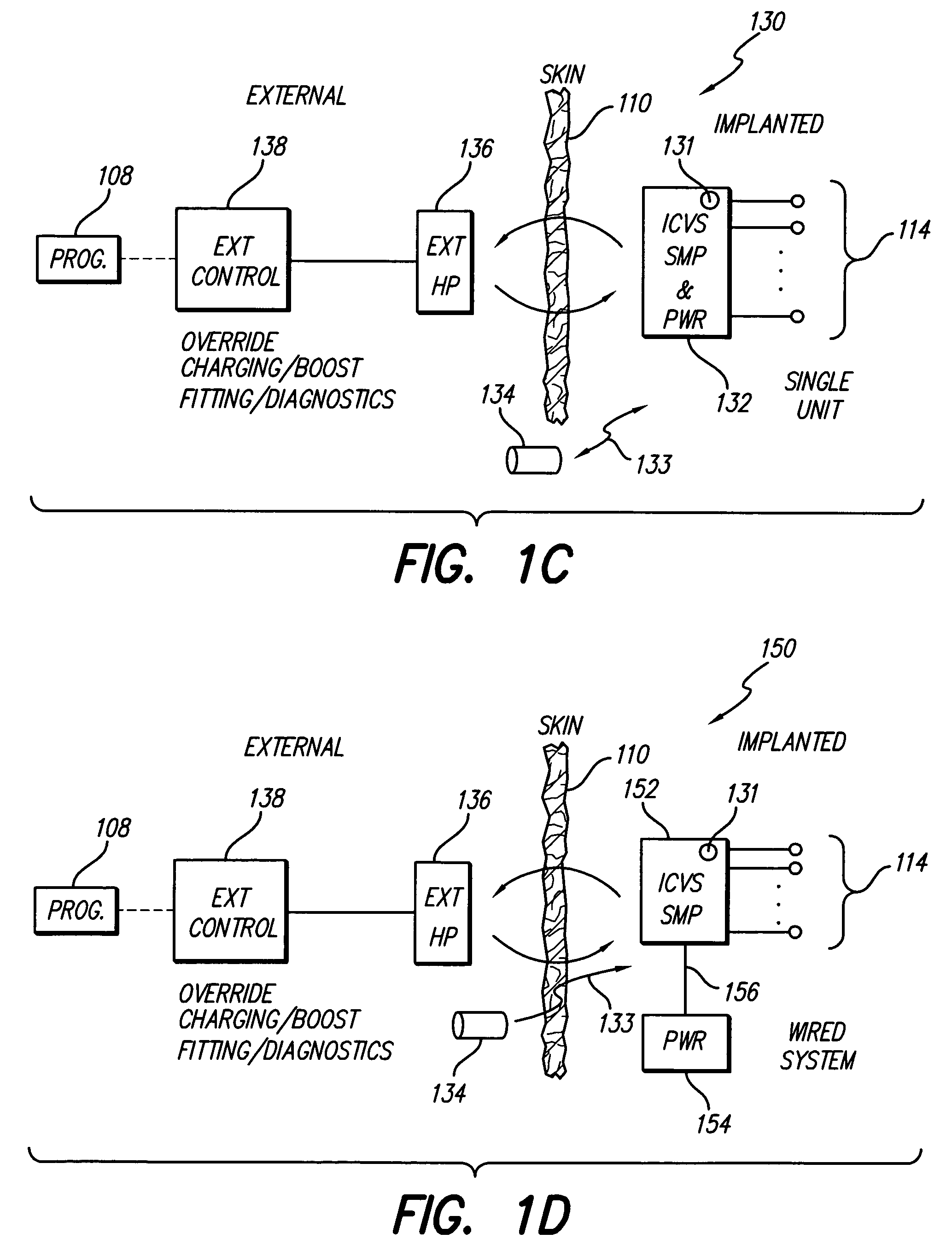
Skull-mounted electrical stimulation system and method for treating patients
ActiveUS20060293723A1Extension of timeMinimal discomfortHead electrodesMedical devicesDrugs infusionClosed loop
A system and method for applying electrical stimulation or drug infusion to nervous tissue of a patient to treat epilepsy, movement disorders, and other indications uses at least one implantable system control unit (SCU) (110), including an implantable signal / pulse generator (IPG) and one or more electrodes (152, 152′). The IPG is implanted in the mastoid area (143) of the skull (140) and communicates with at least one external appliance (230), such as a Behind-the-Ear (BTE) unit (100). In a preferred embodiment, the system is capable of open- and closed-loop operation. In closed-loop operation, at least one SCU includes a sensor, and the sensed condition is used to adjust stimulation parameters.
Owner:BOSTON SCI NEUROMODULATION CORP
Ventricular partitioning device
InactiveUS20060030881A1Lower the volumeImprove ejection fractionOcculdersSurgical veterinaryHeart chamberNon traumatic
This invention is directed to a partitioning device for separating a patient's heart chamber into a productive portion and a non-productive portion. The device is particularly suitable for treating patients with congestive heart failure. The partitioning device has a frame-reinforced, expandable membrane which separates the productive and non-productive portions of the heart chamber. The proximal ends of the ribs of the frame have tissue penetrating elements about the periphery thereof which are configured to penetrate tissue lining the heart wall at an angle approximately perpendicular to a longitudinal axis of the partitioning device. The partitioning device has a hub with a non-traumatic distal end to engage the ventricular wall.
Owner:EDWARDS LIFESCIENCES CORP
Method and Apparatus for the Treatment of Movement Disorders
A method, apparatus, and system for treating patients suffering from movement disorders having the ability to determine one or more biomarkers indicative of a disease state. In some embodiments, the biomarker may be used as a closed-loop feedback signal to control the delivery of therapy (such as electrical stimulation or drug therapy), and which may also be used as an indication of therapy effectiveness. One embodiment uses electrodes placed in the brain to measure EEG or local field potential (LFP) signals, from which the one or more biomarkers may be determined.
Owner:MEDTRONIC INC
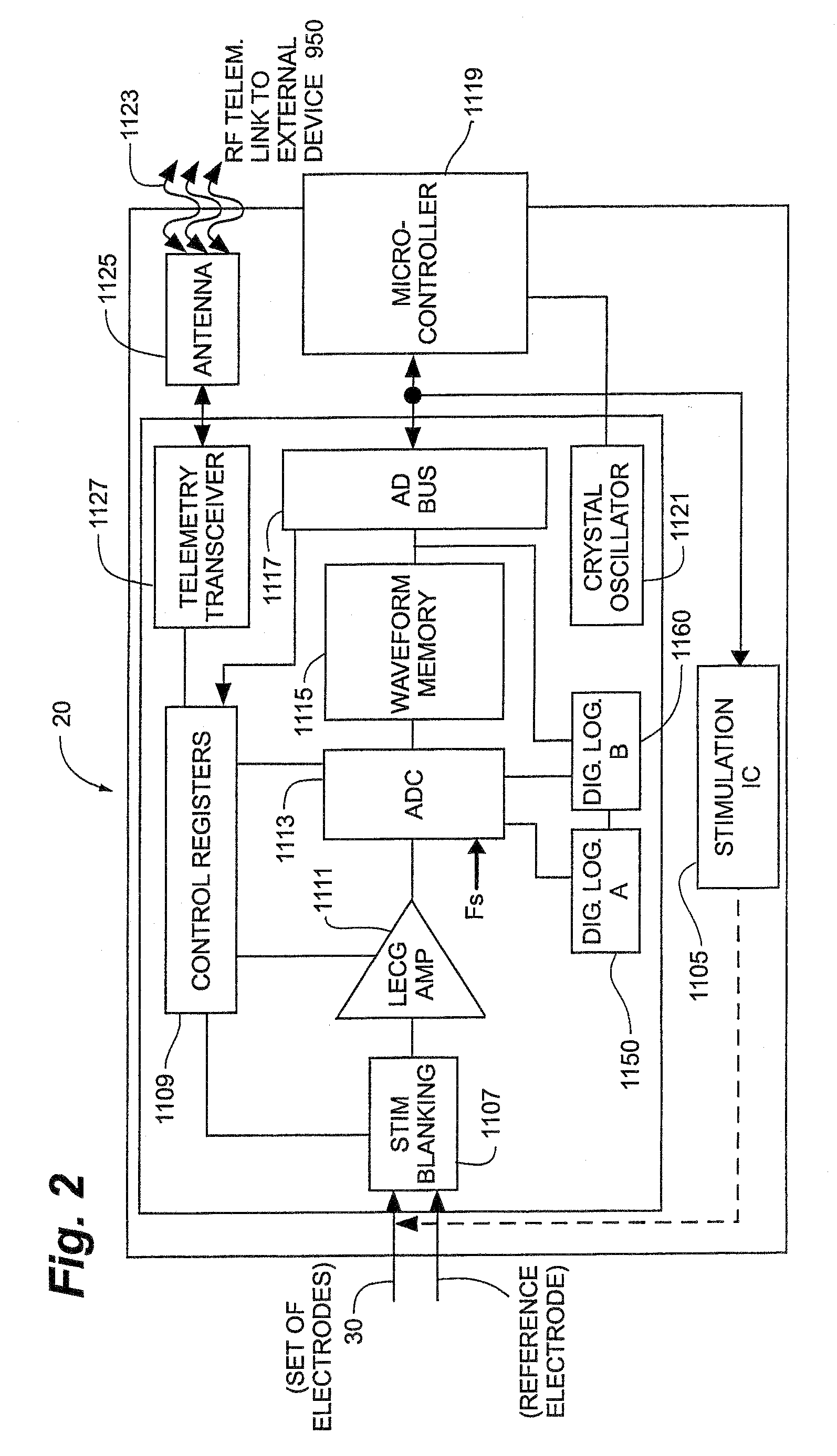
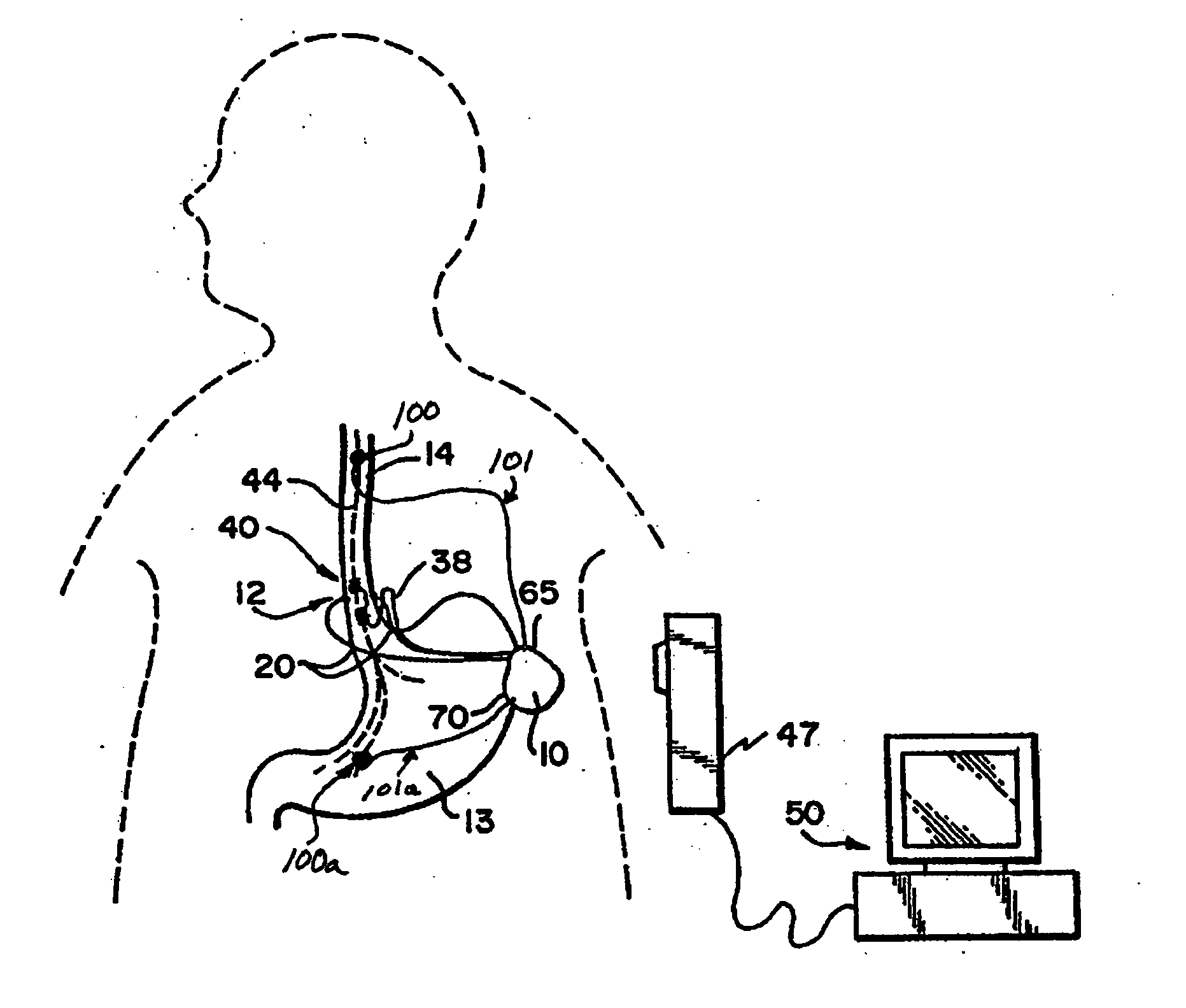
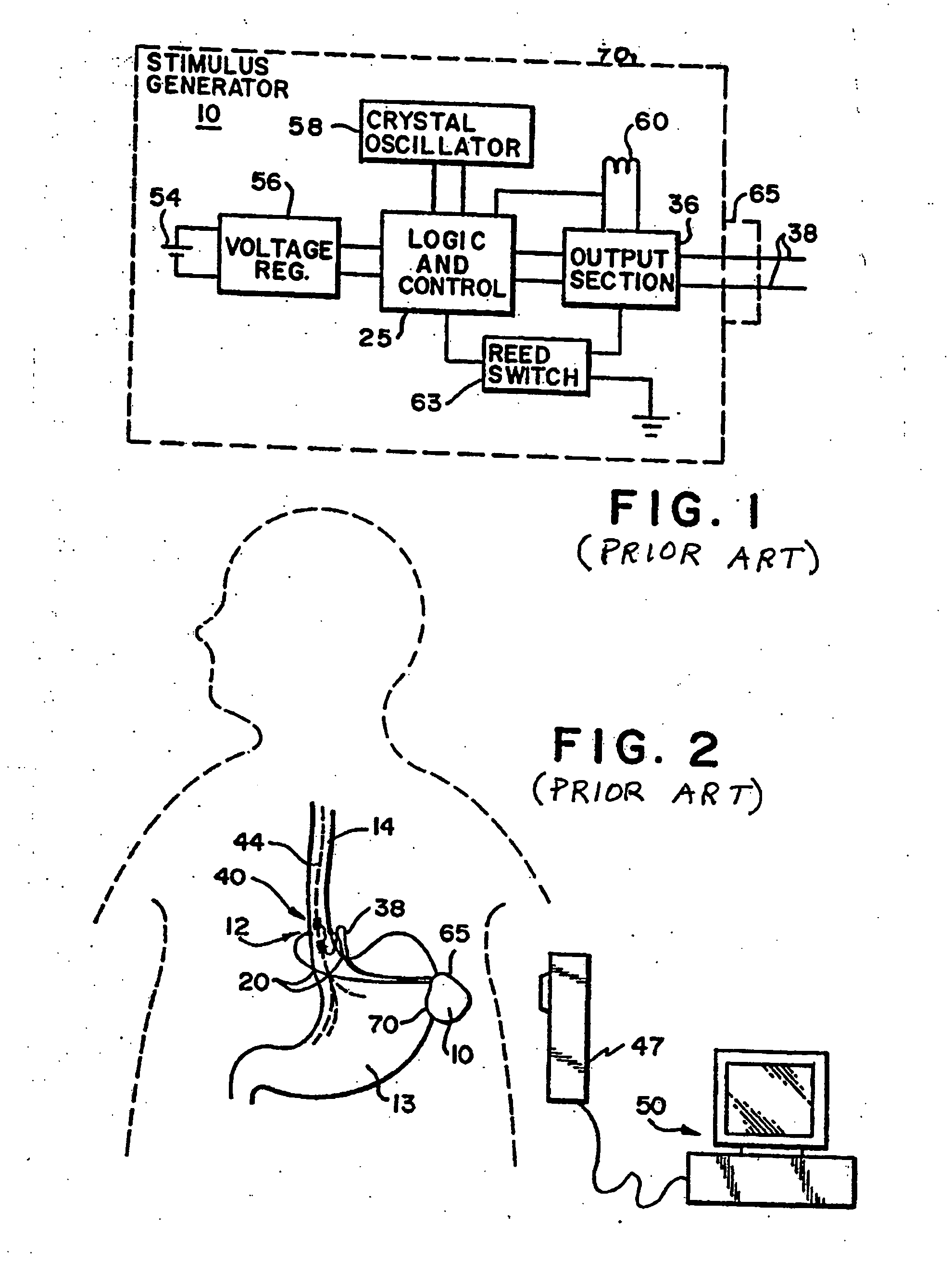
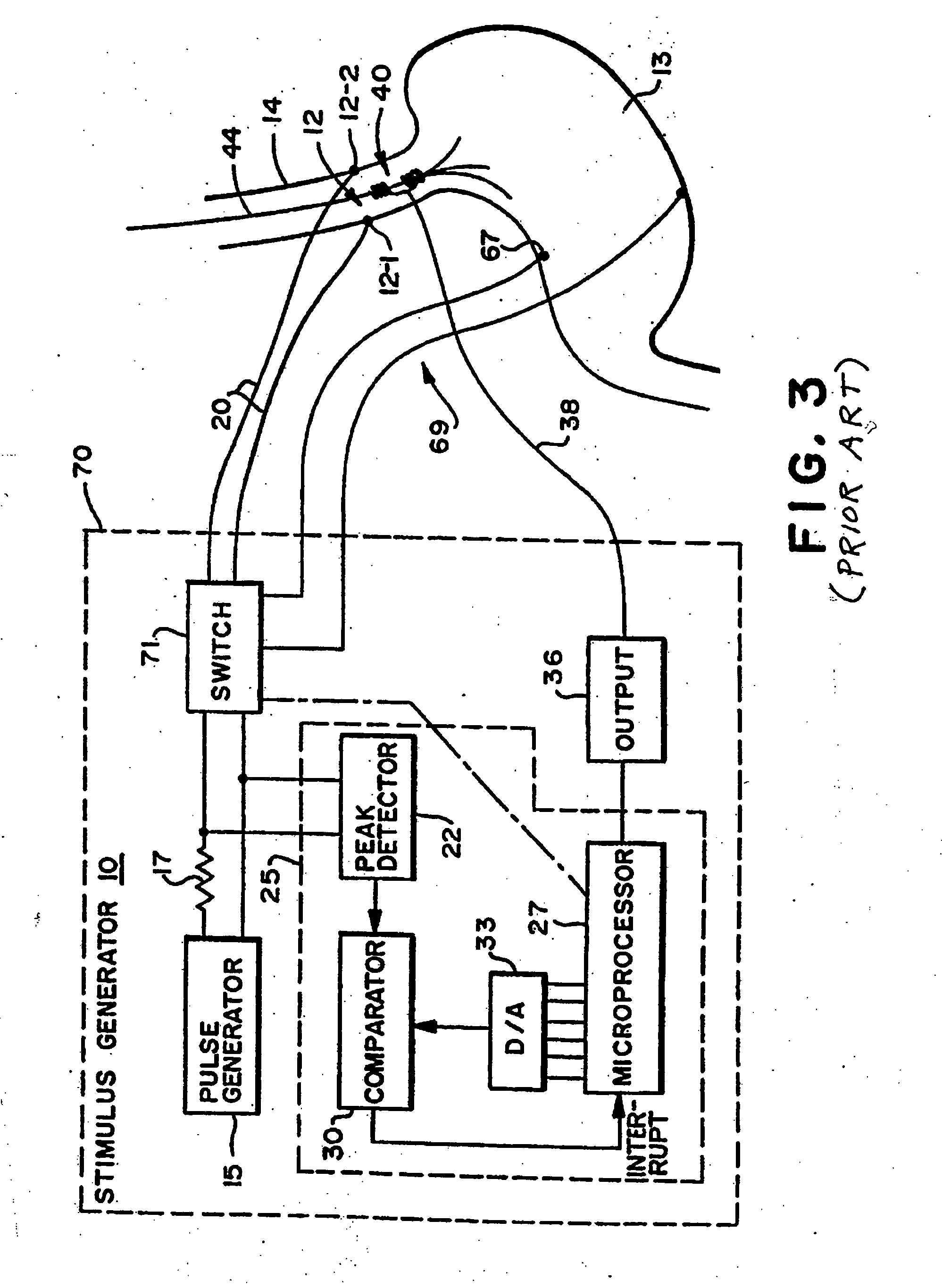
Obesity and eating disorder stimulation treatment with neural block
A method and apparatus for treating patients suffering from obesity or eating disorders by applying a predetermined stimulating signal to the patient's vagus nerve appropriate to alleviate the condition and by applying a neural conduction block to the vagus nerve at a blocking site with the neural conduction block selected to at least partially block nerve impulses on the vagus nerve at the blocking site.
Owner:RESHAPE LIFESCIENCES INC
Methods of using adipose tissue-derived cells in augmenting autologous fat transfer
ActiveUS20050025755A1Rapid and reliableHigh yieldBiocideElectrotherapyBreast augmentationAutologous Fat Transfer
Methods of treating patients for conditions such as breast augmentation, soft tissue defects, and urinary incontinence, are described. The methods include removing adipose tissue from a patient, processing a portion of the adipose tissue to obtain a substantially isolated population of regenerative cells, mixing the regenerative cells with another portion of adipose tissue to form a composition, and administering the composition to the patient from which the adipose tissue was removed.
Owner:LOREM VASCULAR PTE LTD
Movement disorder stimulation with neural block
InactiveUS20050070970A1Ameliorate disorderReduce adverse effectsInternal electrodesExternal electrodesBlocking nerveSignal on
A method and apparatus for treating patients suffering from involuntary movement disorders (including epilepsy) by stimulating a selected cranial nerve of the patient with an electrical signal applied to induce a signal at brain by applying an electrical signal at the nerve to ameliorate the disorder and by applying a neural conduction block at the nerve selected to at least partially block nerve impulses on said nerve at a blocking site and reduce adverse effects of said signal on an organ.
Owner:RESHAPE LIFESCIENCES INC
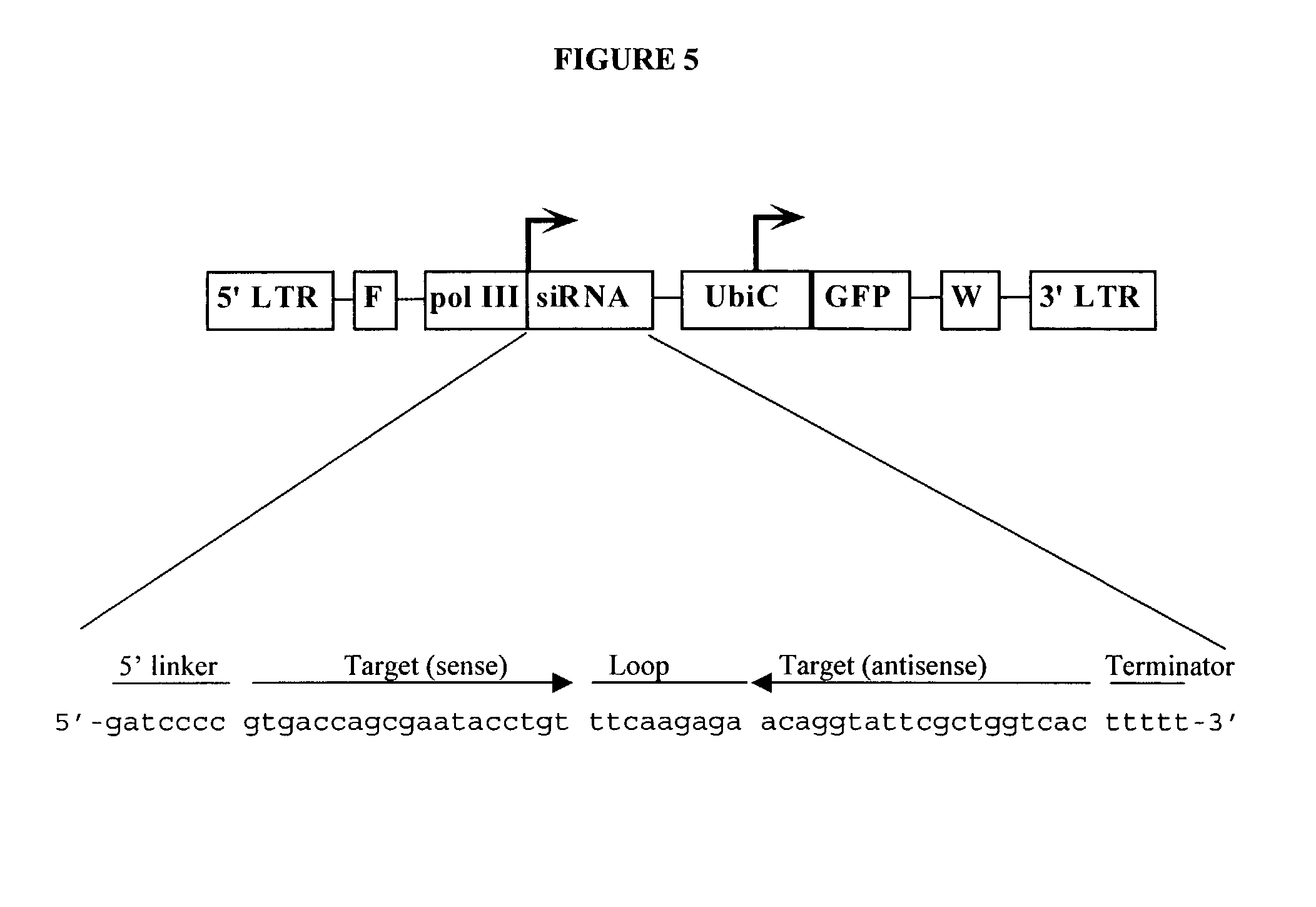
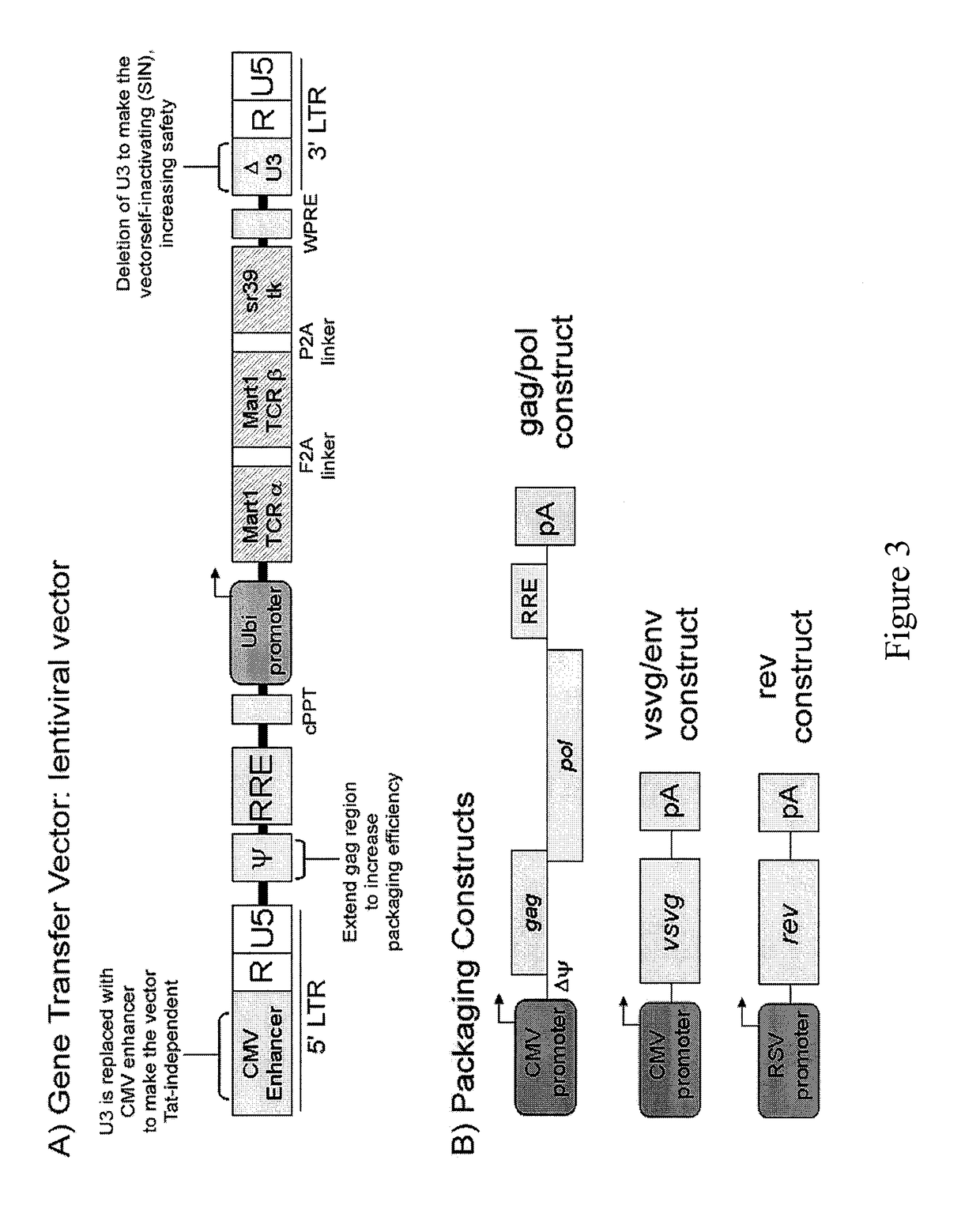
Method for expression of small antiviral RNA molecules within a cell
ActiveUS20030059944A1Prevents sequenceHigh expressionOrganic active ingredientsPeptide/protein ingredientsGeneticsViral life cycle
In one aspect, the invention provides methods and compositions for the expression of small RNA molecules within a cell using a retroviral vector. The methods can be used to express double stranded RNA complexes. Small interfering RNA (siRNA) can be expressed using the methods of the invention within a cell, that interfere with a viral life cycle by down regulating either the viral genome, a viral genome transcript, or a host cell that. In another aspect the invention provides methods for treating patients having suffering from infection, particularly infection with HIV.
Owner:CALIFORNIA INST OF TECH
Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds
Compounds of Formula I-a and all pharmaceutically-acceptable forms thereof, are described herein. The variables R1, R2, R3, Z1, Q, and A shown in Formula I-a are defined herein. Pharmaceutical compositions containing one or more compounds of Formula I-a, or a pharmaceutically acceptable form of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents are provided herein. Methods of treating patients suffering from certain diseases responsive to inhibition of tyrosine kinase activity are also given. In certain embodiments the diseases are responsive to inhibition of Btk activity and / or B-cell proliferation. Such methods comprise administering to such patients an amount of a compound of Formula I-a effective to reduce signs or symptoms of the disease. These diseases include cancer, an autoimmune and / or inflammatory disease, or an acute inflammatory reaction. Thus methods of treatment include administering a sufficient amount of a compound or salt as provided herein to decrease the symptoms or slow the progression of these diseases. Other embodiments include methods of treating other animals, including livestock and domesticated companion animals, suffering from a disease responsive to inhibition of kinase activity. Methods of treatment include administering a compound of Formula I-a as a single active agent or administering a compound of Formula I-a in combination with one or more other therapeutic agent. A method for determining the presence of Btk in a sample, comprising contacting the sample with a compound or form thereof of Formula I-a under conditions that permit detection of Btk activity, detecting a level of Btk activity in the sample, and therefrom determining the presence or absence of Btk in the sample.
Owner:GILEAD CONNENTICUT INC
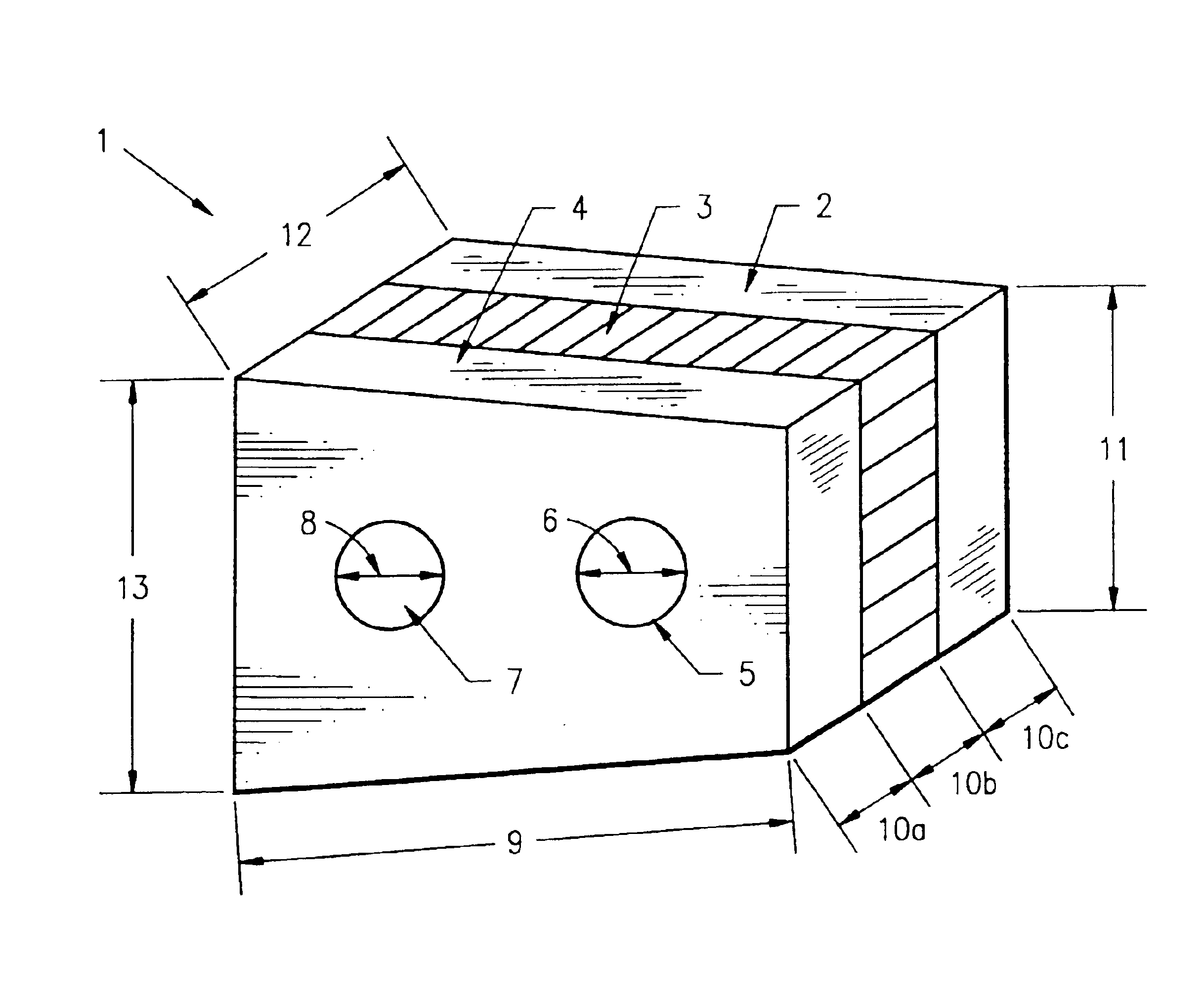
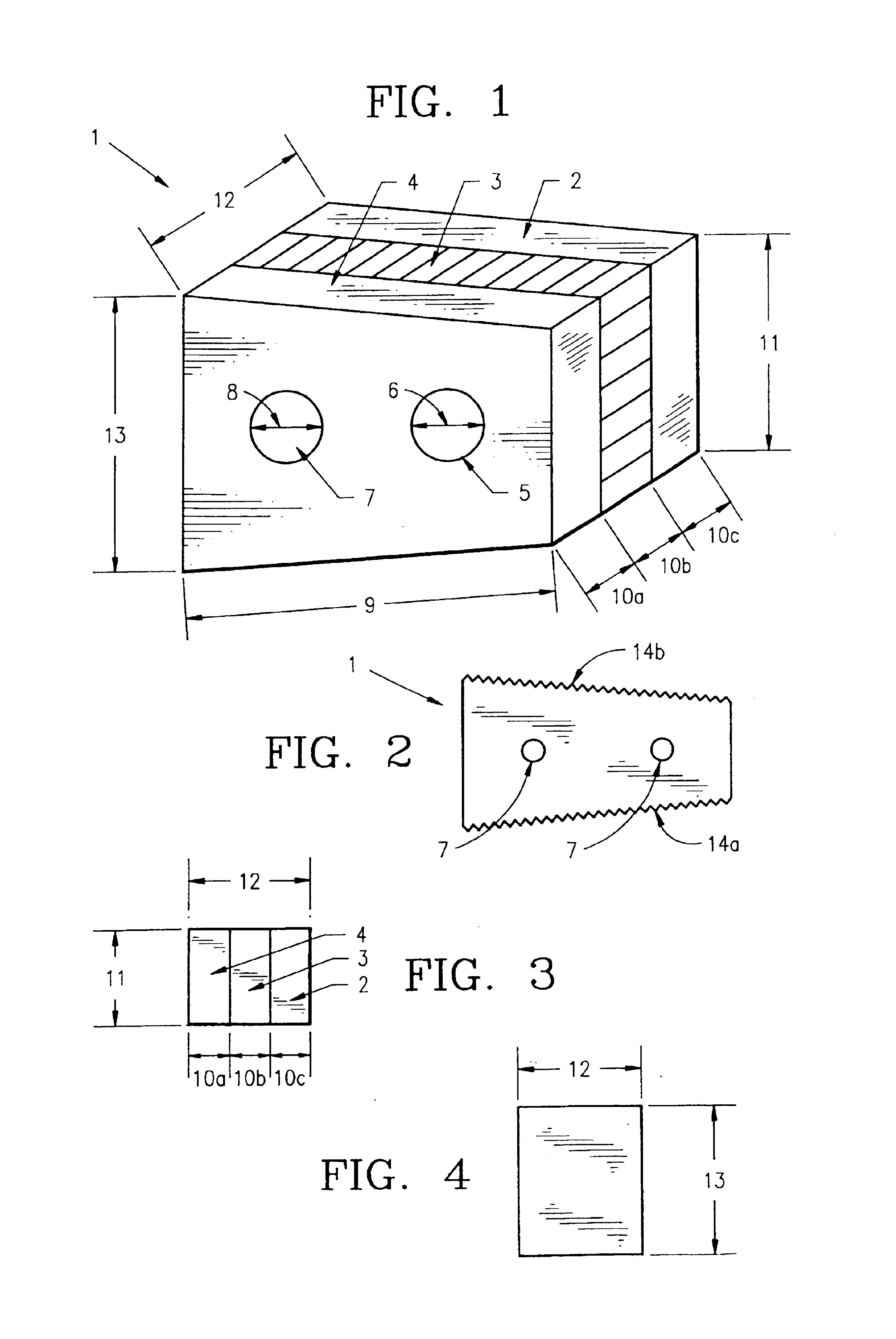
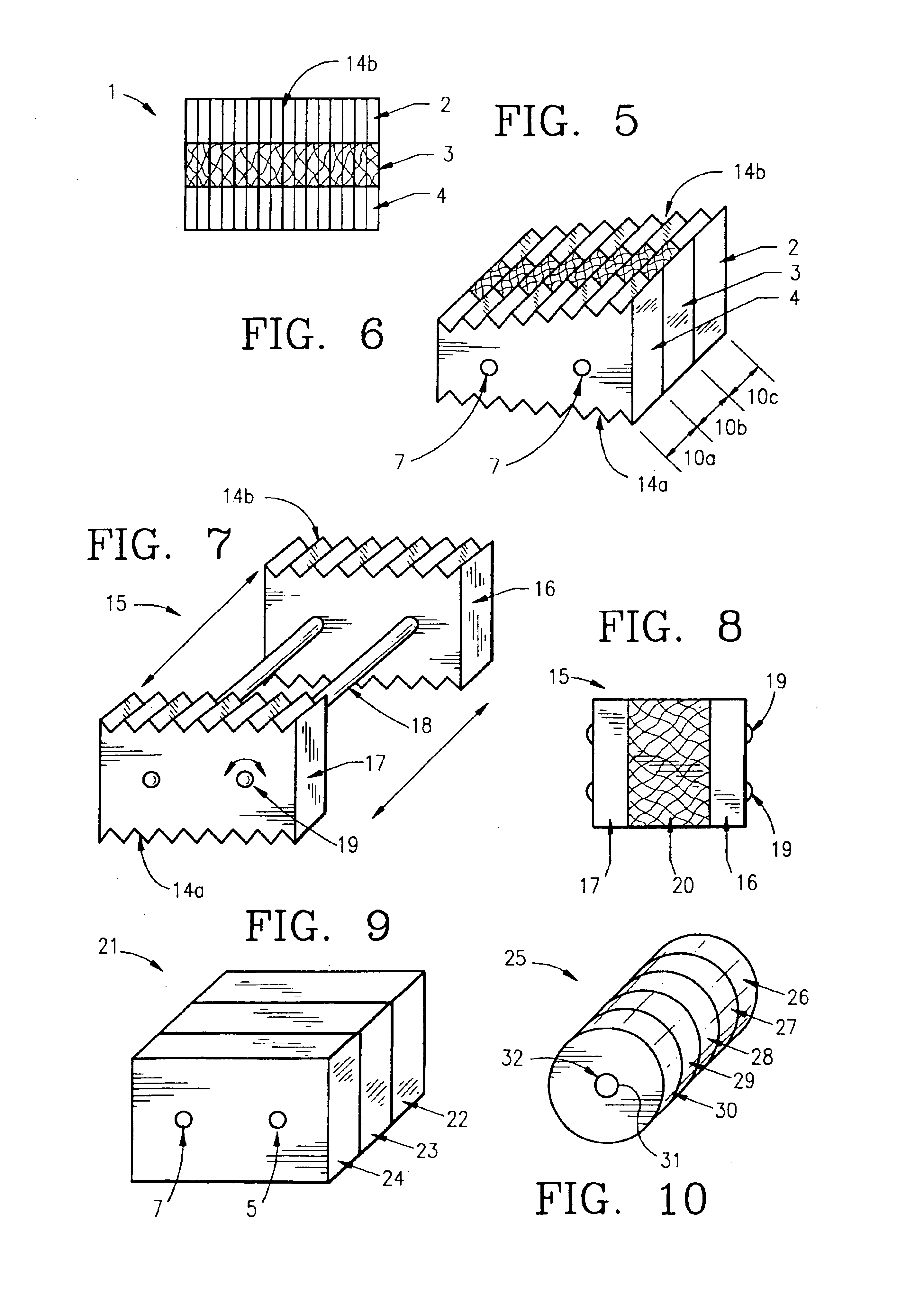
Composite bone graft, method of making and using same
InactiveUS6902578B1Avoid significant donor site morbidityHigh mechanical strengthBone implantJoint implantsDiseaseOssicular Prosthesis Implantation
The invention is directed to a composite bone graft for implantation in a patient, and methods of making and using the composite bone graft, along with methods for treating patients by implanting the composite bone graft at a site in a patient. The composite bone graft includes two or more connected, discrete, bone portions, and includes one or more biocompatible connectors which hold together the discrete bone portions to form the composite bone graft. The composite bone graft may include one or more textured bone surfaces. The textured surface preferably includes a plurality of closely spaced protrusions, preferably closely spaced continuous protrusions. The composite bone graft is useful for repairing bone defects caused by congenital anomaly, disease, or trauma, in a patient, for example, for restoring vertical support of the anterior and / or posterior column. Implantation of the composite bone graft results in improved graft stability and osteoinductivity, without a decrease in mechanical strength. The composite bone graft does not shift, extrude or rotate, after implantation. The present composite bone graft can be appropriately sized for any application and can be used to replace traditional non-bone prosthetic implants.
Owner:LIFENET HEALTH
Multiple partitioning devices for heart treatment
ActiveUS20060014998A1Lower the volumeReduce stressHeart valvesHeart stimulatorsHeart chamberCongestive heart failure chf
This invention is directed to a system and method for partitioning a patient's heart chamber into a productive portion and a non-productive portion which are particularly suitable for treating patients with congestive heart failure. The partitioning system has a plurality of partitioning devices with reinforced, expandable membranes which separate the productive and non-productive portions of the heart chamber. When deployed within the patient's heart chamber, the second partitioning device is off-set from the deployed first partitioning device to cover a region of the wall defining the patient's heart chamber which is not covered by the first partitioning device. The multiple partitioning devices may be independent from each other or may be interconnected, e.g. a tether or strand.
Owner:EDWARDS LIFESCIENCES CORP
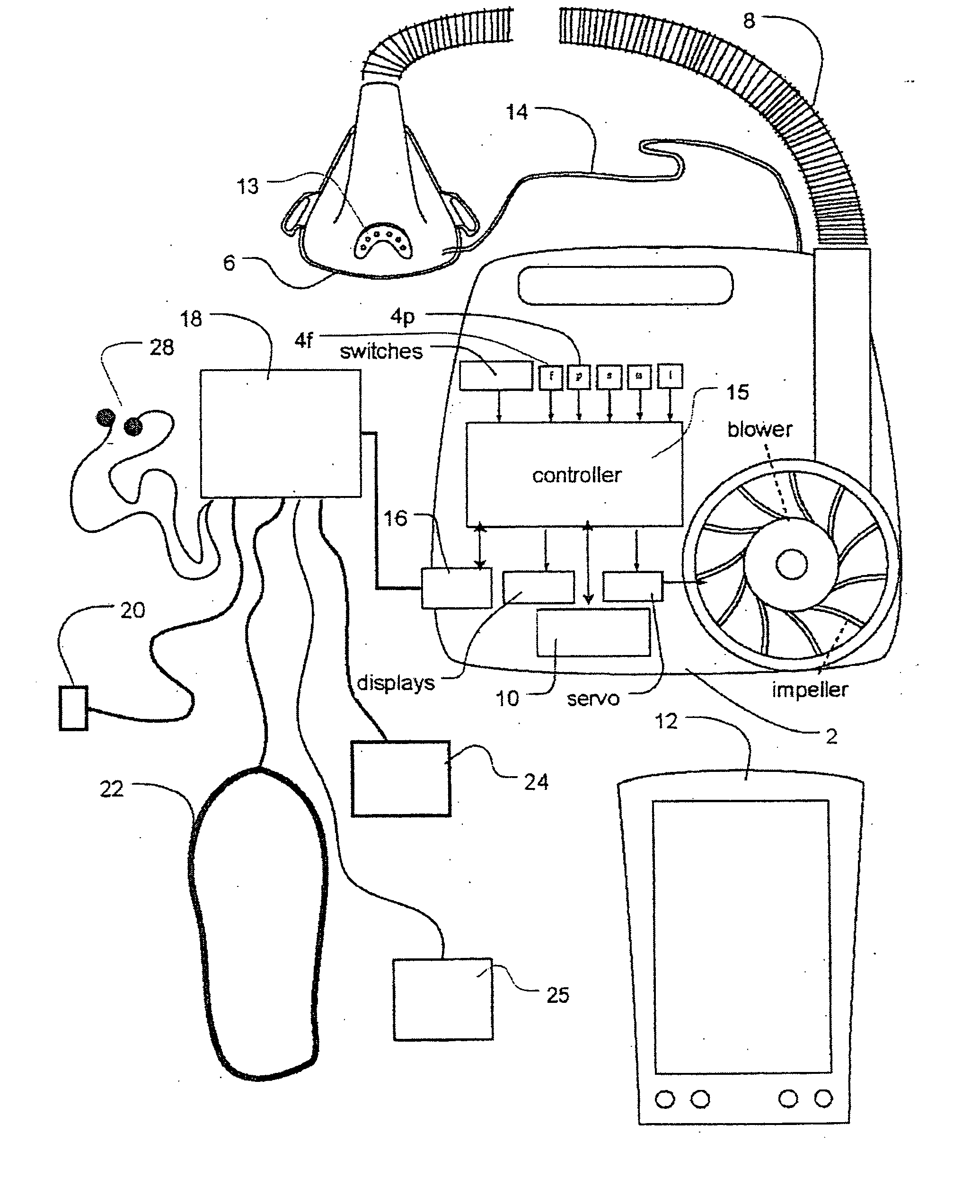
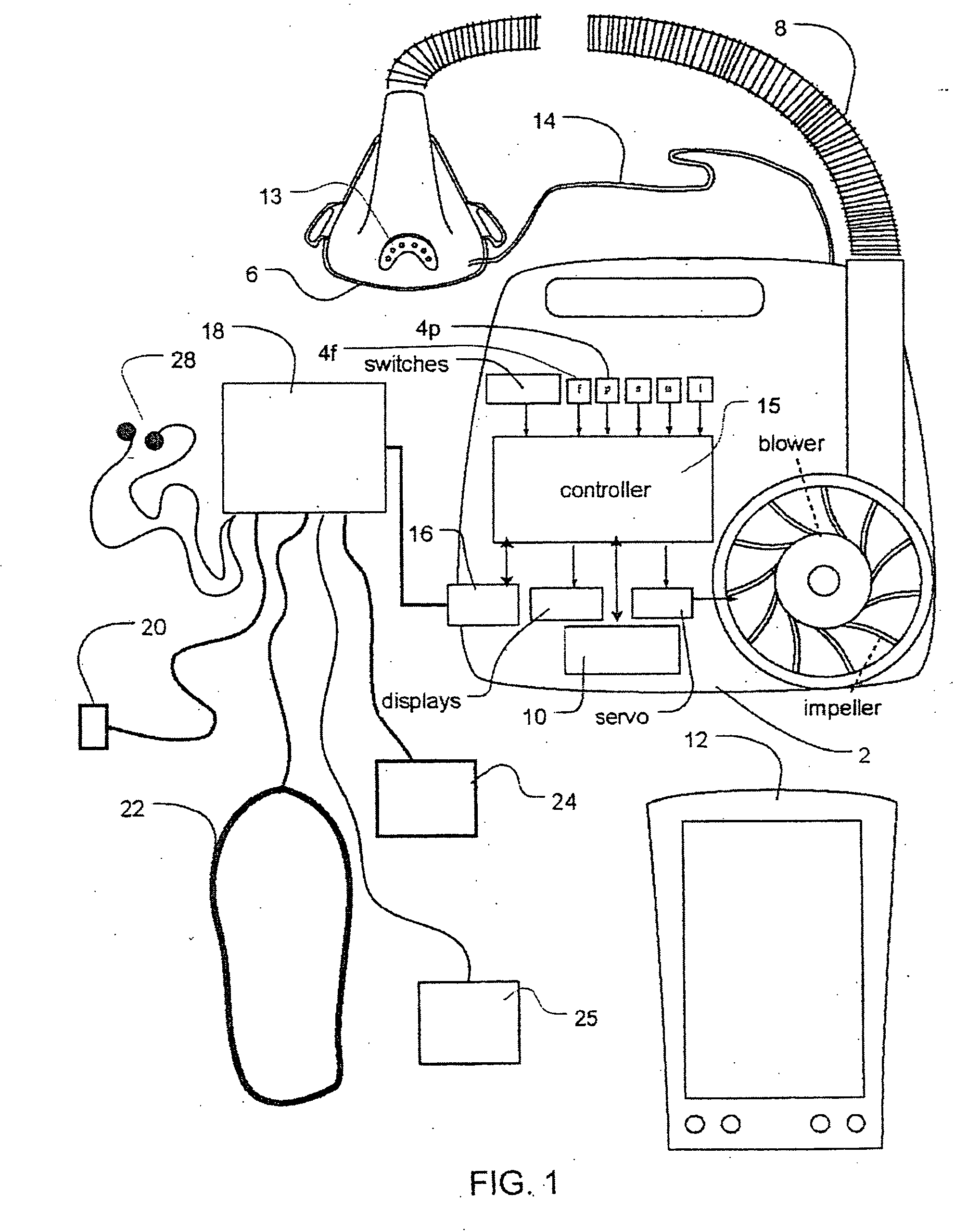
Methods and apparatus for heart failure treatment
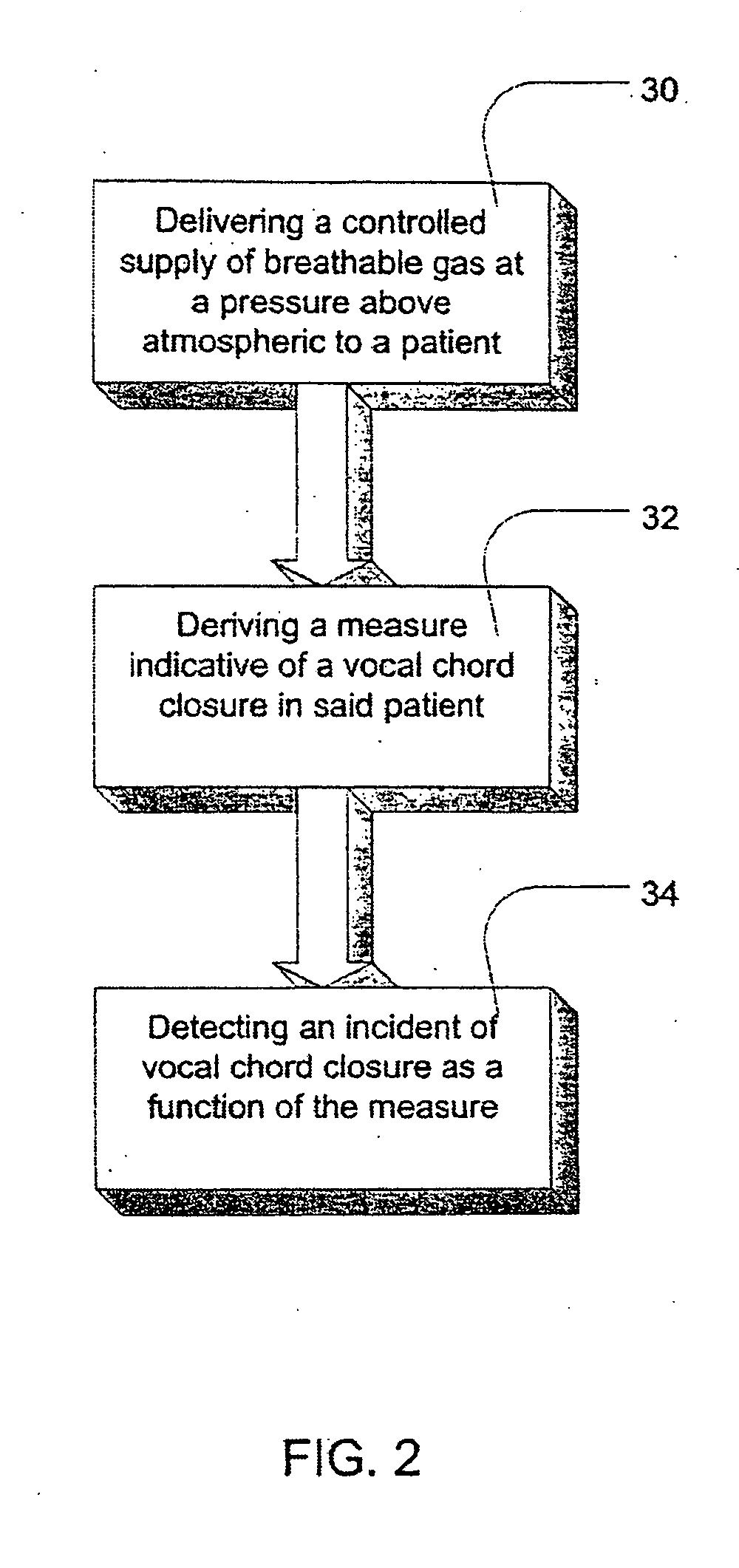
InactiveUS20060084877A1Treatment level is not increasedIncrease pressureElectrocardiographyRespiratory masksCardiac failure therapyDisease cause
Methods and apparatus for assessing the condition of and treating patients for heart failure by the delivery of continuous positive airway pressure are disclosed. Airflow of the patient is measured to determine occurrences of central apneas. A heart failure index is calculated from a count of the number of central apneas that have occurred. The present heart failure index can be compared with a previously calculated heart failure index to determine how the patient's heart failure disease has changed and how it should be treated.
Owner:RESMED LTD
Peripheral seal for a ventricular partitioning device
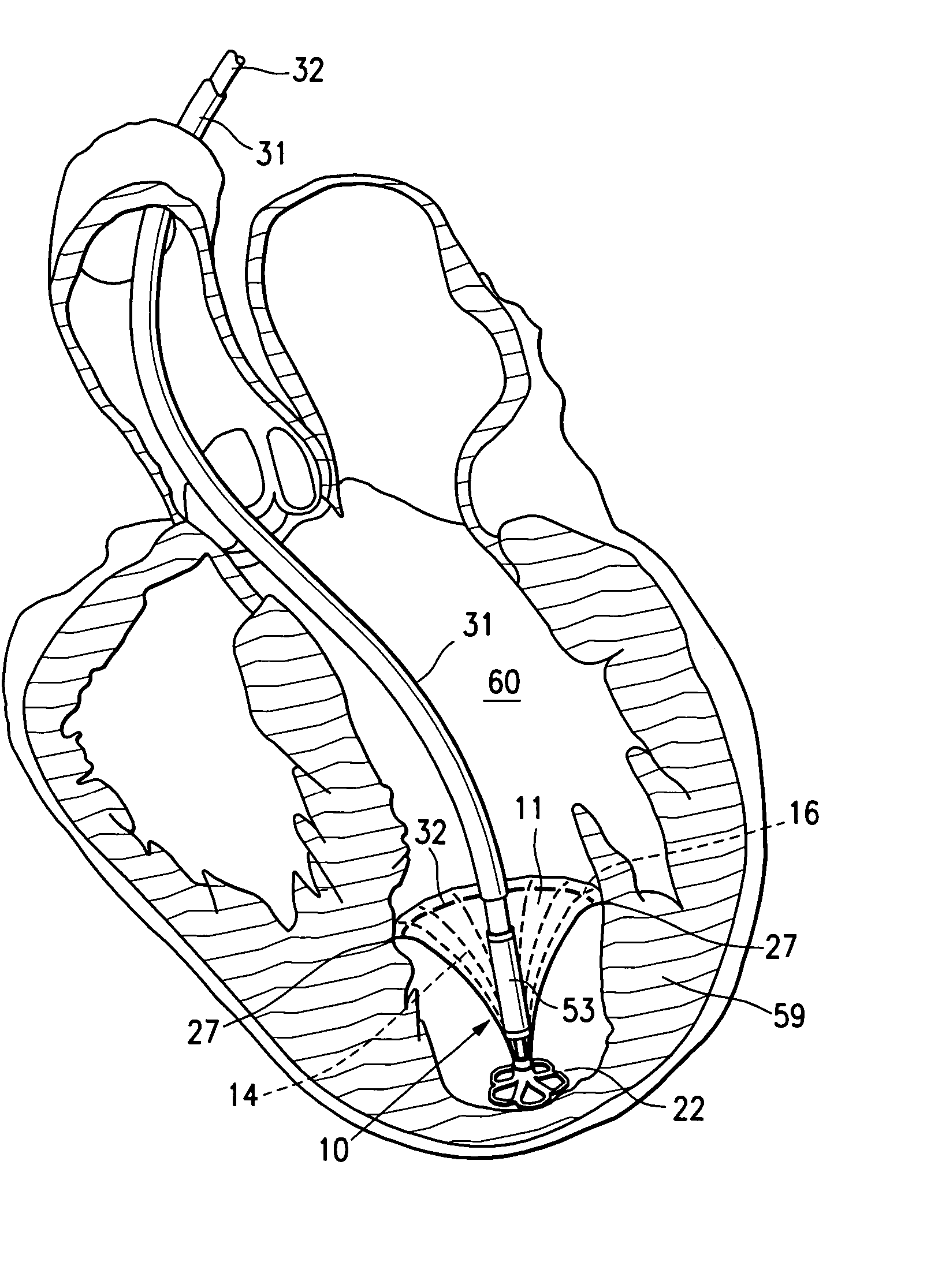
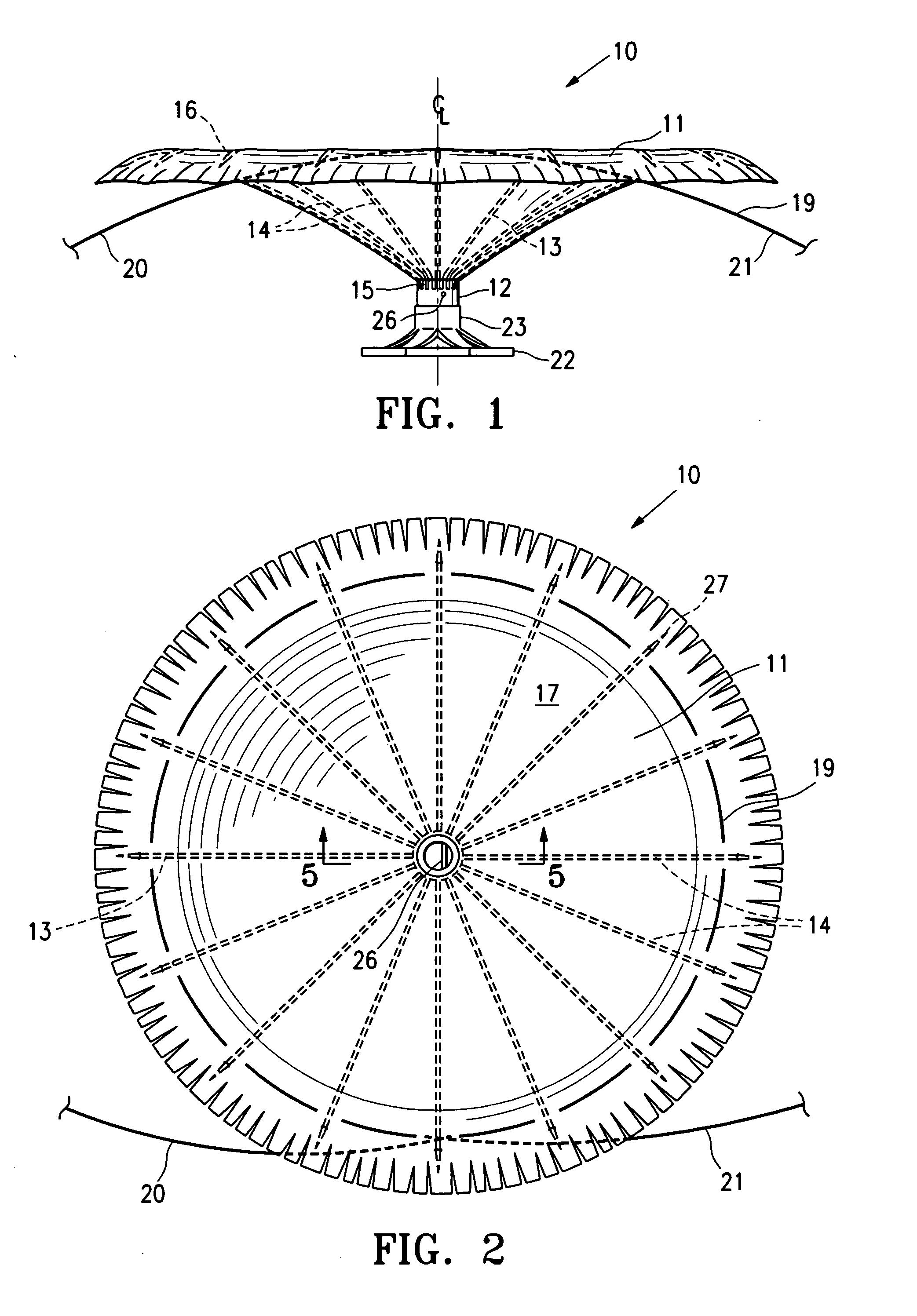
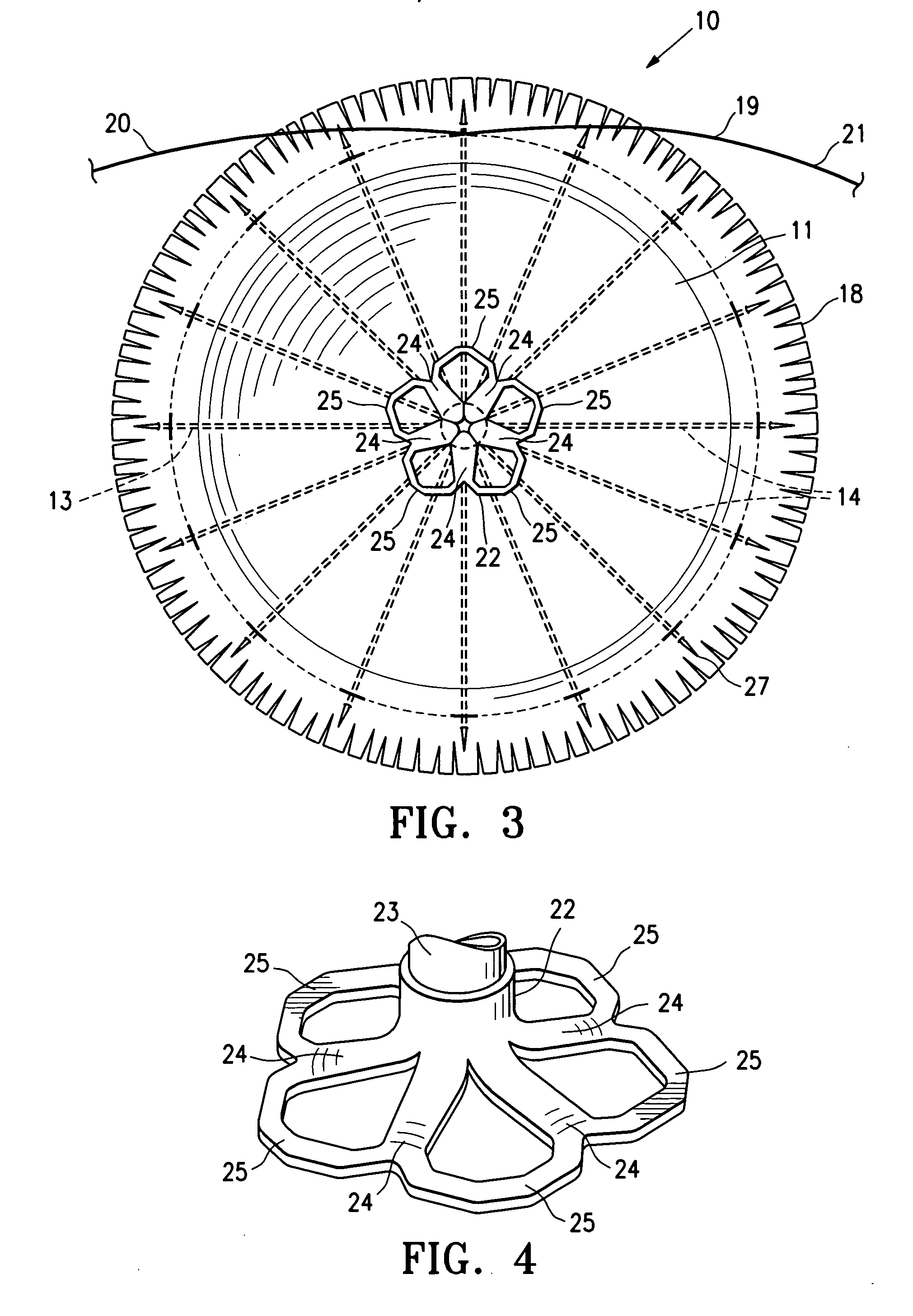
ActiveUS20060281965A1Lower the volumeImprove ejection fractionHeart stimulatorsOcculdersHeart chamberCongestive heart failure chf
This invention is directed to a partitioning device for separating a patient's heart chamber into a productive portion and a non-productive portion which is suitable for treating patients with heart disease, particularly congestive heart failure. The partitioning device has a reinforced membrane with outwardly biased members to help seal the periphery of the membrane against the wall of the patient's heart chamber. In one embodiment, the outwardly biased member is an expansive strand that extends between adjacent ribs of an expandable frame which reinforces the membrane. In another embodiment, the outwardly biased member is a hydrophilic body such as foam which swells upon contact with body fluid such as blood in the heart chamber. The reinforced membrane has a central hub with a distally extending support stem with a plurality of feet which extend radially from a centerline axis and preferably have ends that are aligned in a common plane. The ends of the pods which extend radially away from the centerline axis may be interconnected by flexible struts and / or webs.
Owner:EDWARDS LIFESCIENCES CORP
Laminar ventricular partitioning device
InactiveUS20080071298A1Lower the volumeReduce stressCeramic shaping apparatusOcculdersHeart chamberNon traumatic
Owner:EDWARDS LIFESCIENCES CORP
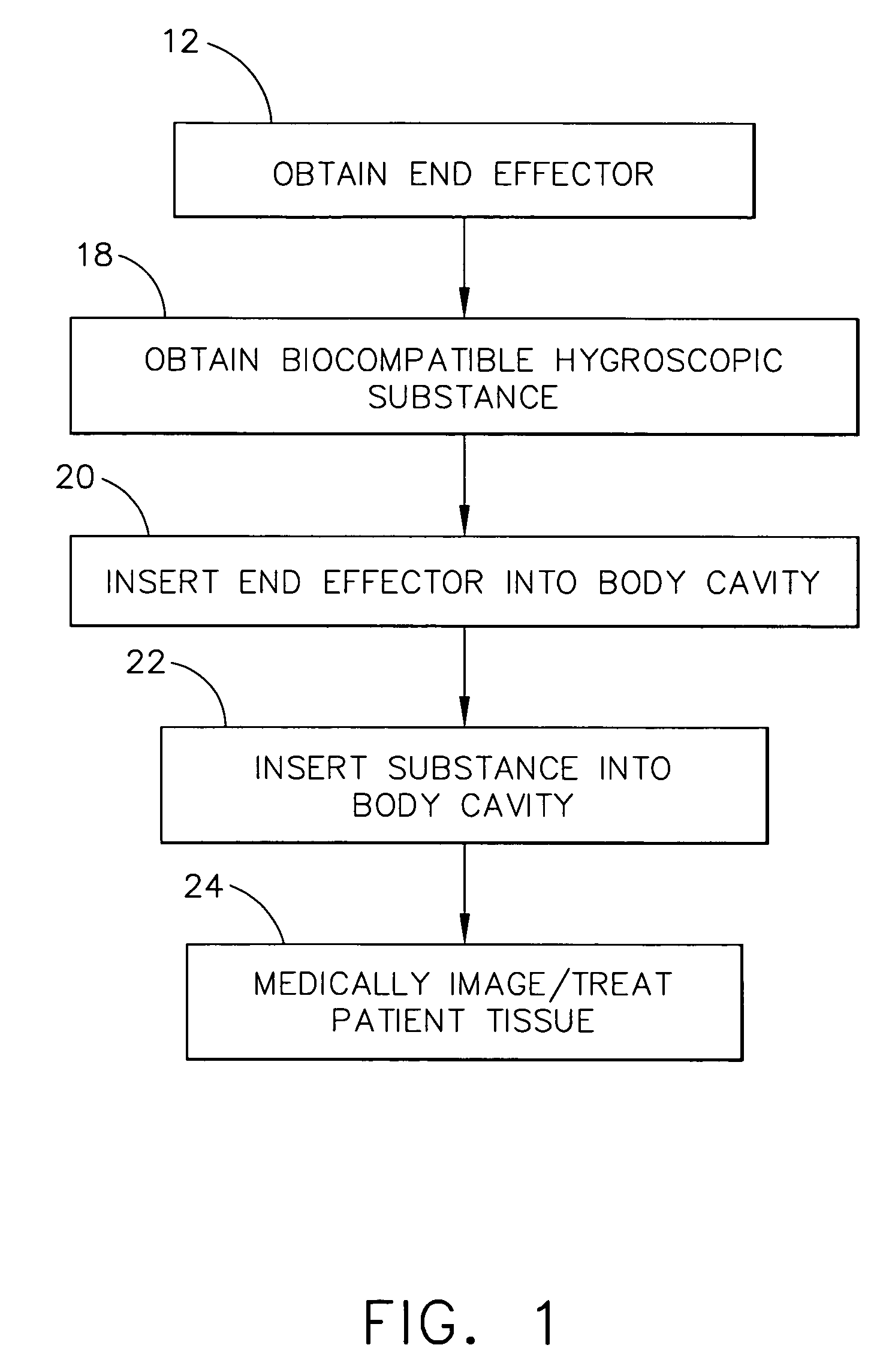
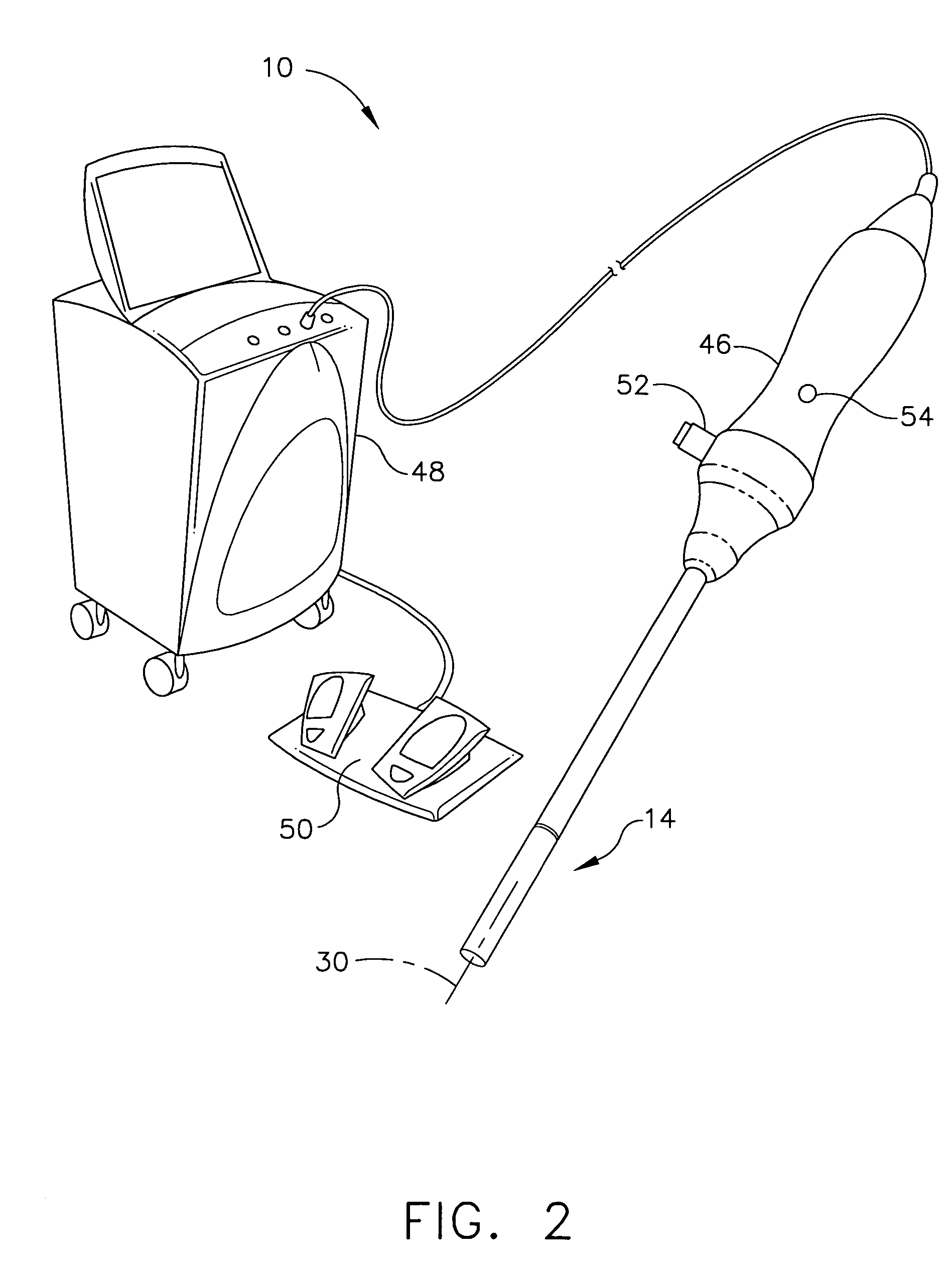
Intra-cavitary ultrasound medical system and method
A method for medically employing ultrasound within a body cavity of a patient. An end effector is obtained having a medical ultrasound transducer assembly. A biocompatible hygroscopic substance is obtained having a non-expanded anhydrous state and having an expanded and fluidly-loculated hydrated state. The end effector, including the transducer assembly, and the substance in substantially its anhydrous state are inserted into a body cavity (such as endoscopically inserted into a uterus) of a patient. The transducer assembly is used to medically image and / or medically treat patient tissue (such as stopping blood flow to, and / or ablating, a uterine fibroid). A system for medically employing ultrasound includes the end effector and the substance. In another system, the end effector includes the substance. The substance in its hydrated state expands inside the body cavity providing acoustic coupling between the wall of the body cavity and the transducer assembly.
Owner:CILAG GMBH INT
Methods of using adipose tissue-derived cells in the treatment of cardiovascular conditions
Adipose derived regenerative cells are used to treat patients, including patients with cardiovascular conditions, diseases or disorders. Methods of treating patients include processing adipose tissue to deliver a concentrated amount of regenerative cells, e.g., stem and / or progenitor cells, obtained from the adipose tissue to a patient. The methods may be practiced in a closed system so that the stem cells are not exposed to an external environment prior to being administered to a patient. Accordingly, in a preferred method, adipose derived regenerative cells are placed directly into a recipient along with such additives necessary to promote, engender or support a therapeutic cardiovascular benefit.
Owner:LOREM VASCULAR PTE LTD
Expandable catheter system for peri-ostial injection and muscle and nerve fiber ablation
ActiveUS20120271277A1Improve control and treatmentTime efficient and safeElectrocardiographySurgical needlesCapital equipmentLeft atrium
At the present time, physicians often treat patients with atrial fibrillation (AF) using radiofrequency (RF) catheter systems to ablate conducting tissue in the wall of the Left Atrium of the heart around the ostium of the pulmonary veins. These systems are expensive and take time consuming to use. The present invention circular ablation system CAS includes a multiplicity of expandable needles that can be expanded around a central axis and positioned to inject a fluid like ethanol to ablate conductive tissue in a ring around the ostium of a pulmonary vein quickly and without the need for expensive capital equipment. The expansion of the needles is accomplished by self-expanding or balloon expandable structures. The invention includes centering means so that the needles will be situated in a pattern surrounding the outside of the ostium of a vein. Also included are members that limit the distance of penetration of the needles into the wall of the left atrium, or the aortic wall. The present invention also has an important application to ablate tissue around the ostium of one or both renal arteries, for the ablation of the sympathetic nerve fibers and / or other afferent or efferent nerves going to or from each kidney in order to treat hypertension.
Owner:ABLATIVE SOLUTIONS INC
Treatment of obesity by bilateral vagus nerve stimulation
A method of treating patients for compulsive overeating includes stimulating left and right branches of the patient's vagus nerve simultaneously with electrical pulses in a predetermined sequence of a first period in which pulses are applied continuously, alternating with a second period in which no pulses are applied. The electrical pulses are preferably applied to the vagus nerve at a supradiaphragmatic location.
Owner:CYBERONICS INC
Dual cochlear/vestibular stimulator with control signals derived from motion and speech signals
A system for treating patients affected both by hearing loss and by balance disorders related to vestibular hypofunction and / or malfunction, which includes sensors of sound and head movement, processing circuitry, a power source, and an implantable electrical stimulator capable of stimulating areas of the cochlea and areas of the vestibular system.
Owner:THE JOHNS HOPKINS UNIVERSITY SCHOOL OF MEDICINE
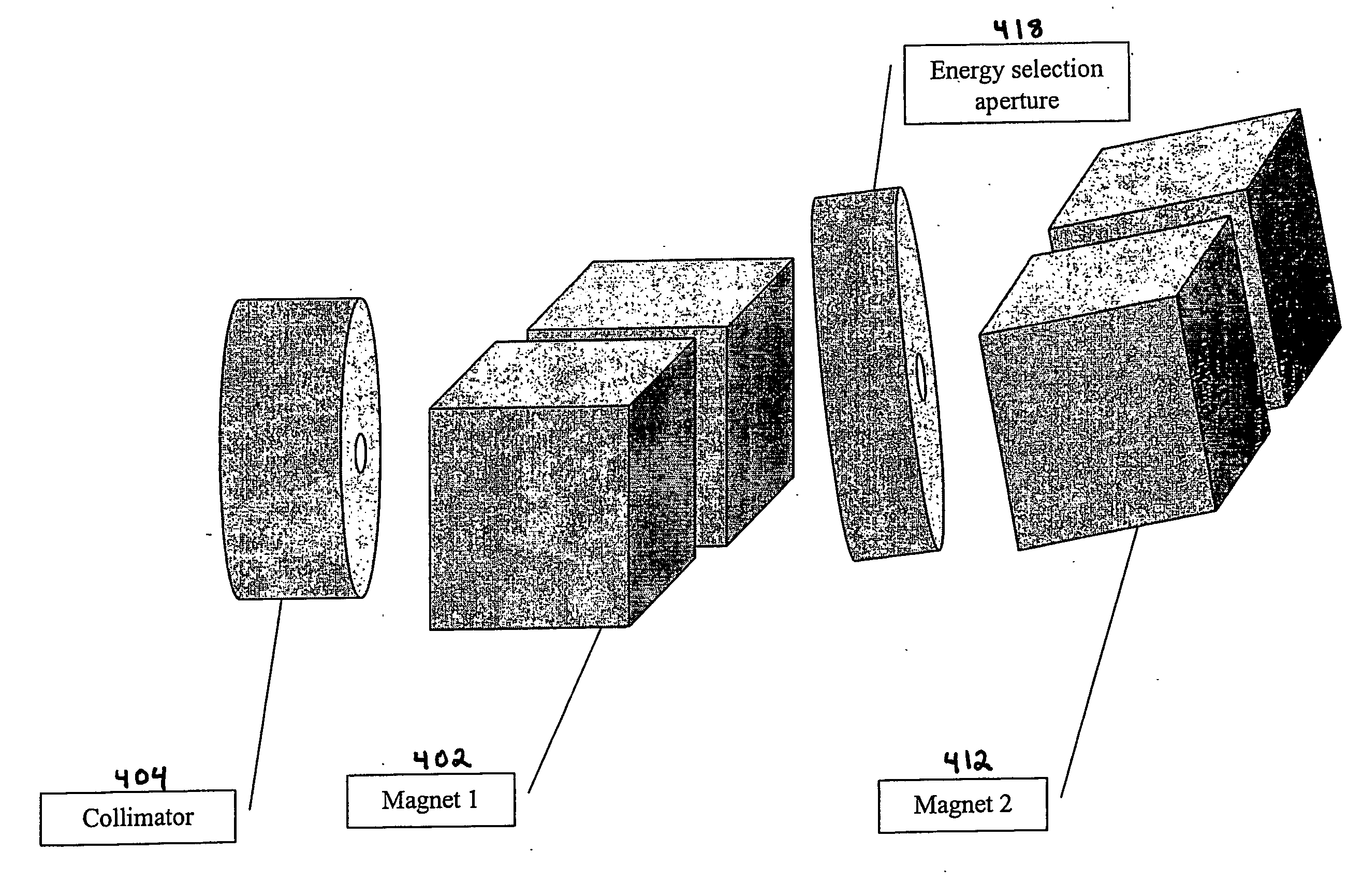
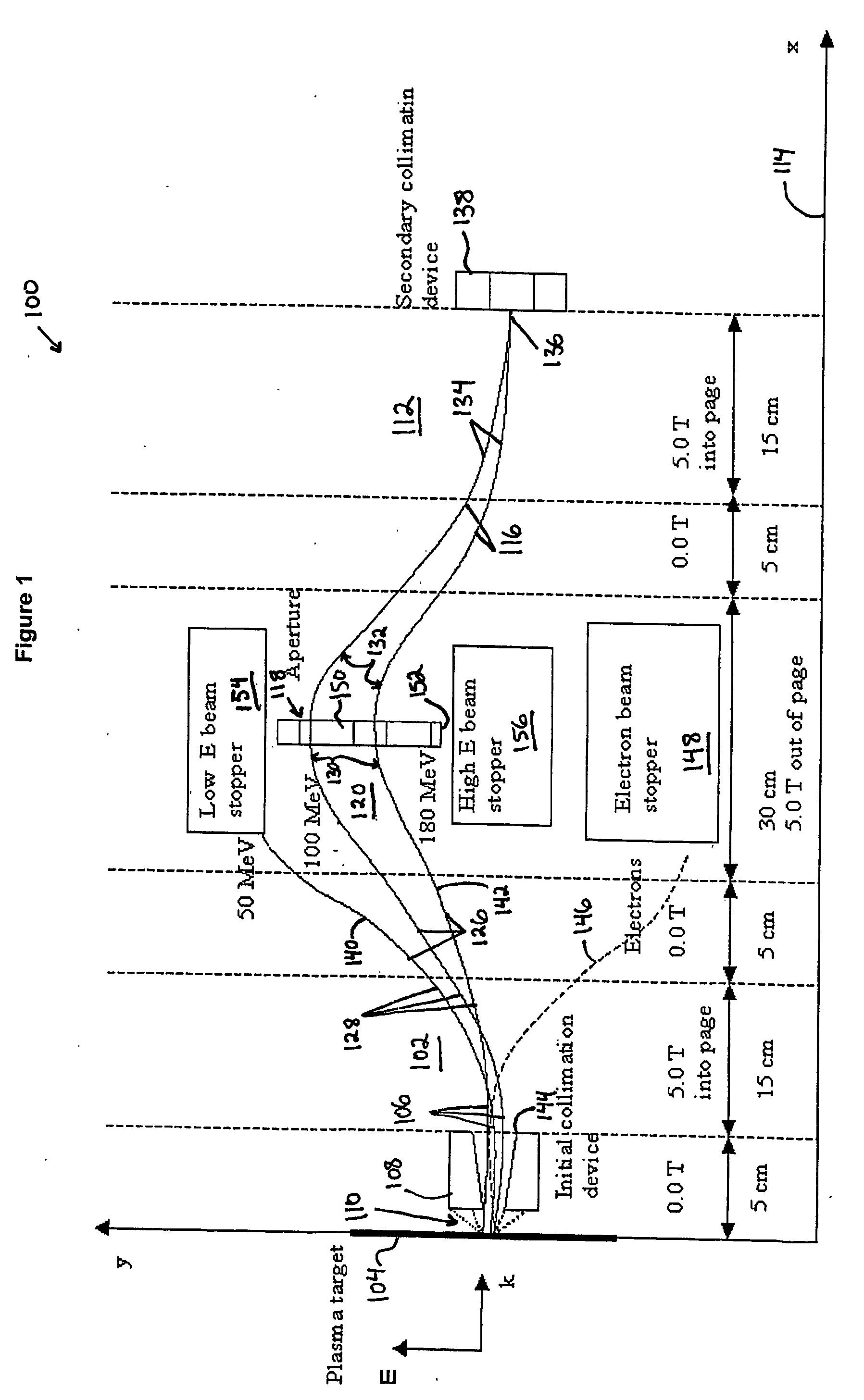
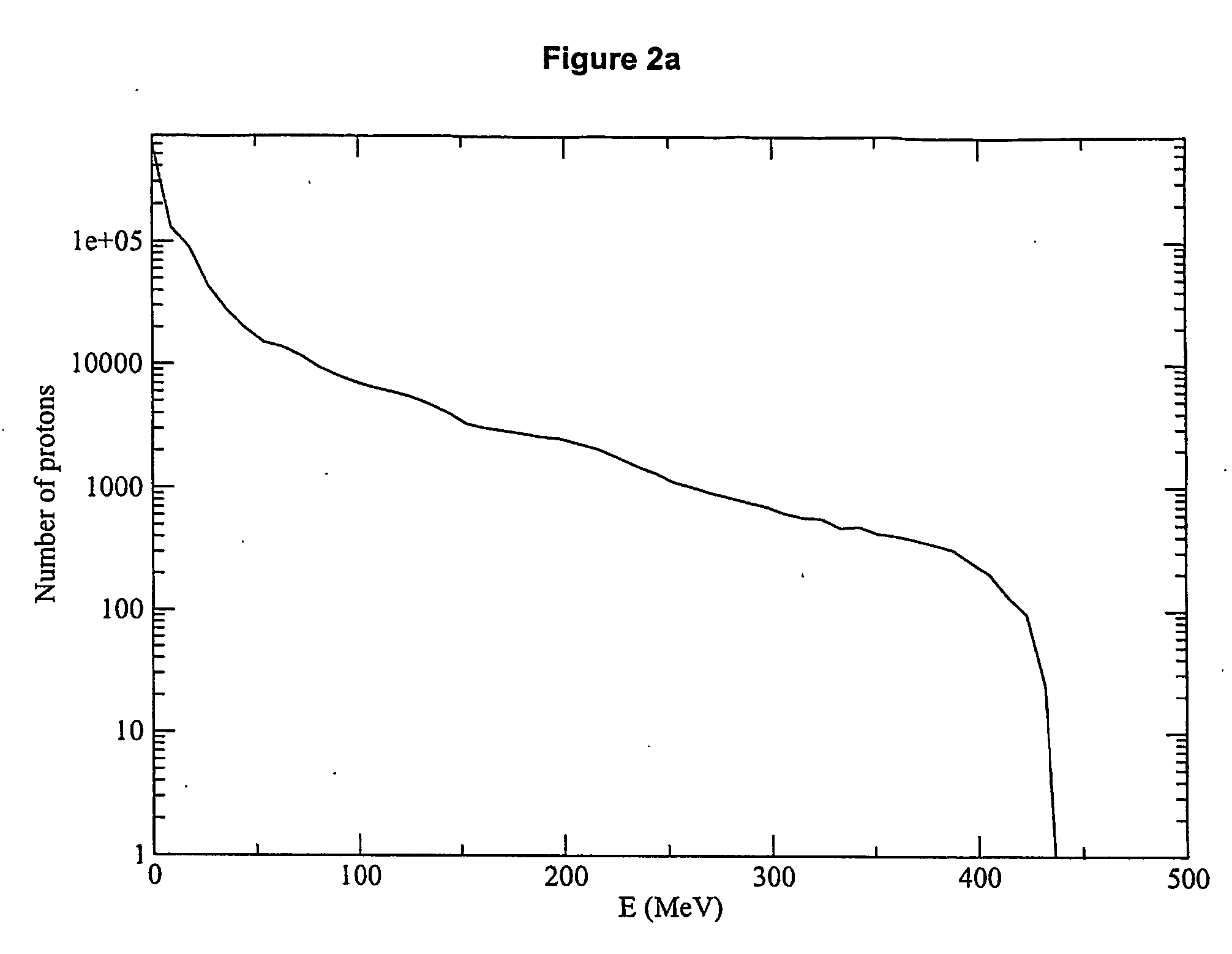
High energy polyenergetic ion selection systems, ion beam therapy systems, and ion beam treatment centers
InactiveUS20060145088A1High energySuitable as therapeuticThermometer detailsBeam/ray focussing/reflecting arrangementsHigh energyIon beam
Devices and methods are provided for generating laser-accelerated high energy polyenergetic positive ion beams that are spatially separated and modulated based on energy level. The spatially separated and modulated high energy polyenergetic positive ion beams are used for radiation therapy. In addition, methods are provided for treating patients in radiation treatment centers using therapeutically suitable high energy polyenergetic positive ion beams that are provided by spatially separating and modulating positive ion beams. The production of radioisotopes using spatially separated and modulated laser-accelerated high energy polyenergetic positive ion beams is also provided.
Owner:INST FOR CANCER RES
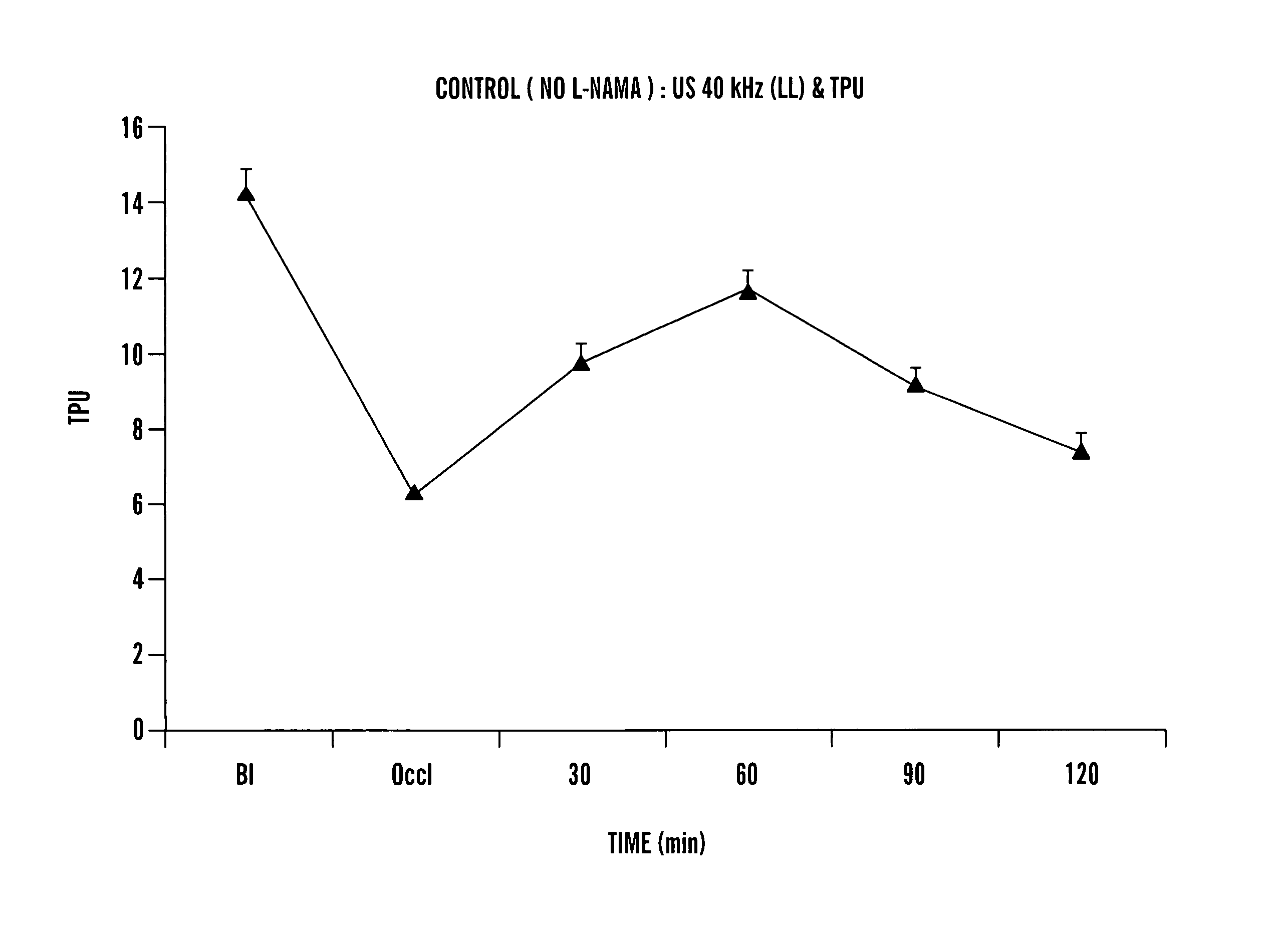
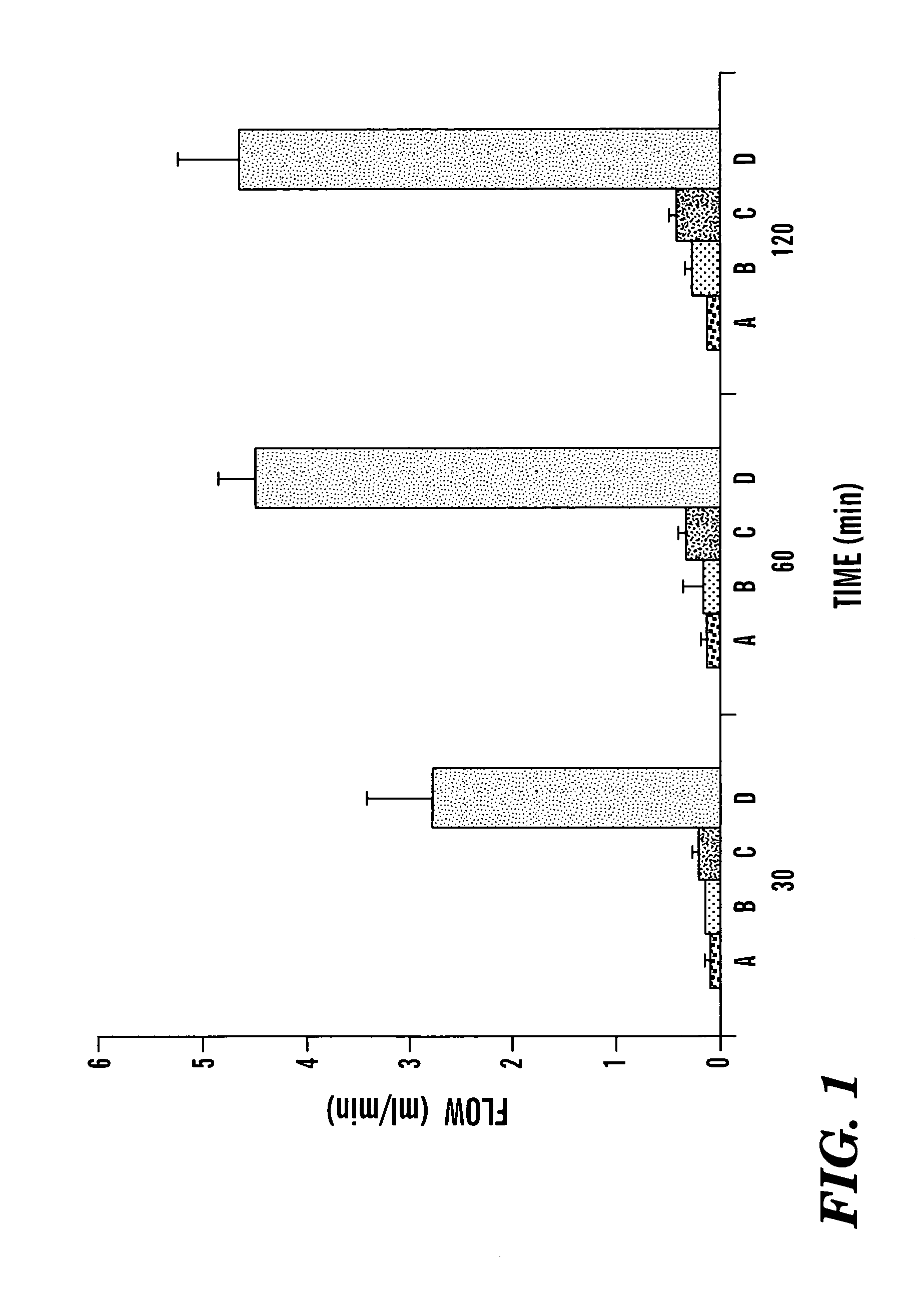
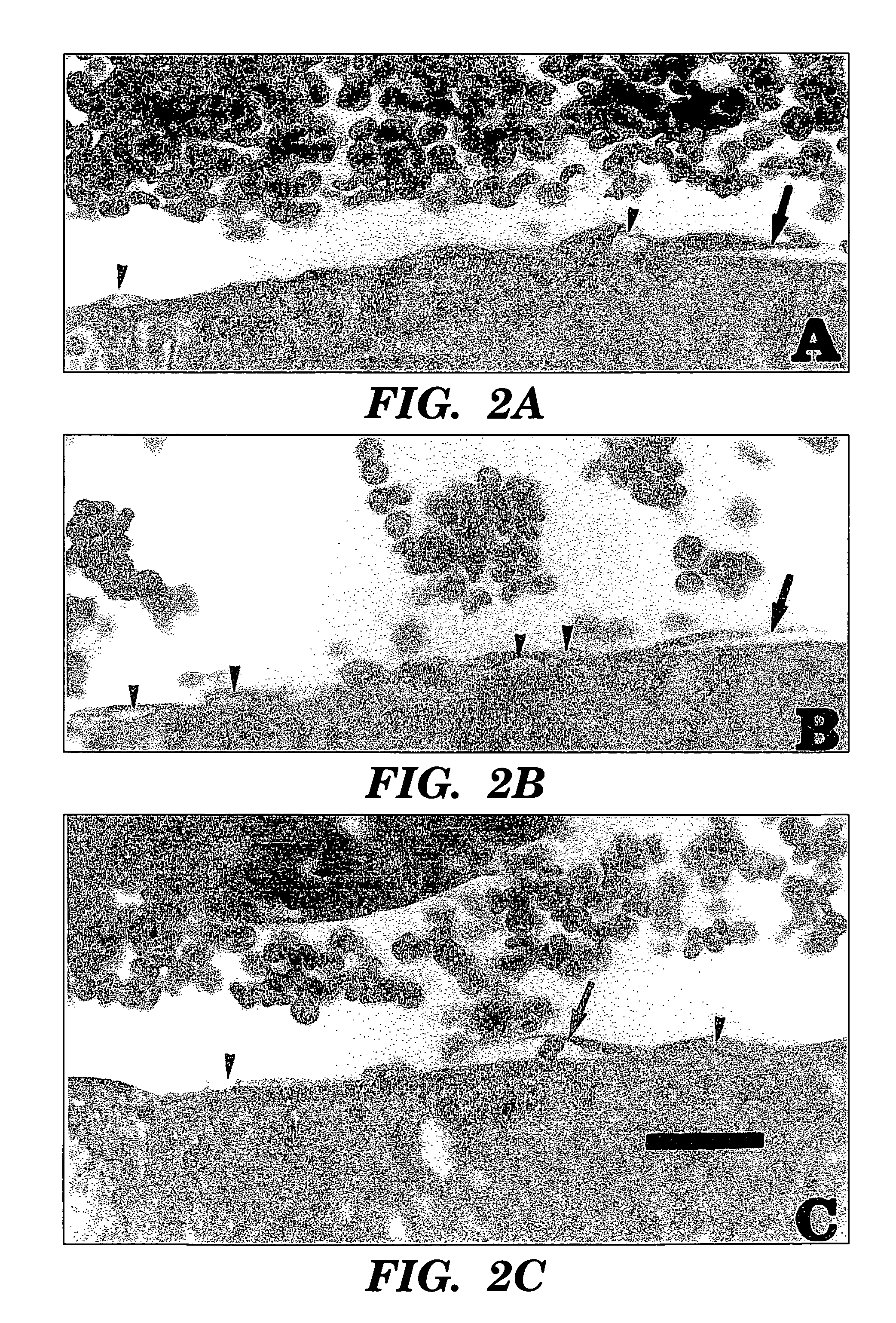
Method of treating a patient with a neurodegenerative disease using ultrasound
InactiveUS7211054B1Promote wound healingPromote healingUltrasound therapyChiropractic devicesDiseaseNitric oxide
The present invention provides methods for treating patients with ultrasound to promote healing. In particular, the present invention provides a method for improving blood flow to ischemic tissue by applying ultrasound to ischemic tissue. The invention also provides a method for increasing nitric oxide production in tissue by applying ultrasound to tissue.
Owner:UNIVERSITY OF ROCHESTER
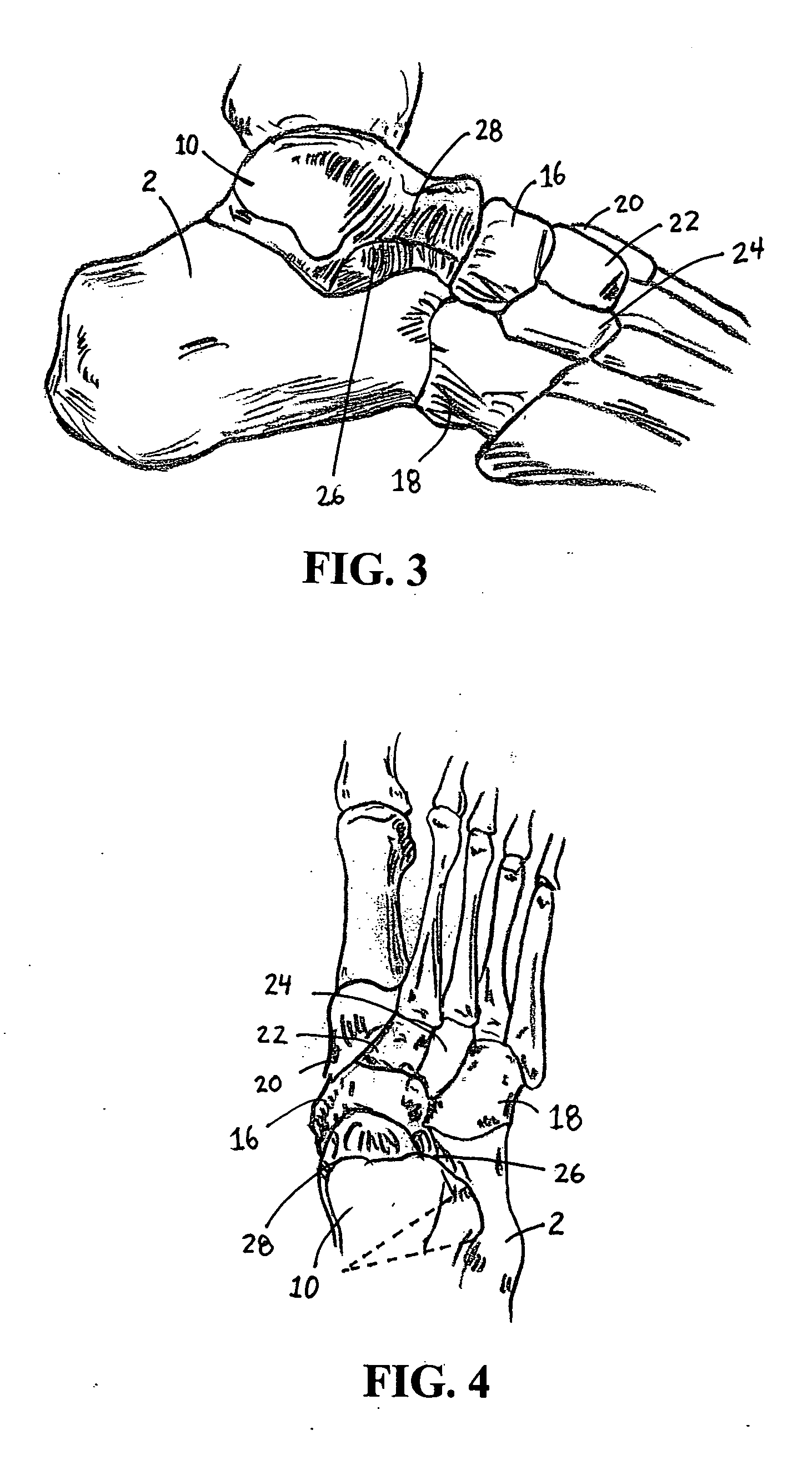
Catheter deliverable foot implant and method of delivering the same
InactiveUS20050197711A1Quality improvementAltering range of motionAnkle jointsToe jointsFirst metatarsalRange of motion
Methods and devices are disclosed for manipulating alignment of the foot to treat patients with flat feet, posterior tibial tendon dysfunction and metatarsophalangeal joint dysfunction. An enlargeable implant is positioned in or about the sinus tarsi and / or first metatarsal-phalangeal joint of the foot. The implant is insertable by minimally invasive means and enlarged through a catheter or needle. Enlargement of the implant alters the range of motion in the subtalar or first metatarsal-phalangeal joint and changes the alignment of the foot or toe.
Owner:CACHIA VICTOR V
Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds
ActiveUS7393848B2Prevent proliferationInhibit the activity of BtkBiocideOrganic chemistryActive agentBruton's tyrosine kinase
Compounds of Formula Iand all pharmaceutically acceptable forms thereof, are described herein.The variables R1, R2, R3, Z2, and Q, shown in Formula I are defined herein.Pharmaceutical compositions containing one or more compounds of Formula I, or a pharmaceutically acceptable form of such compounds, and one or more pharmaceutically acceptable carriers, excipients, or diluents are provided herein.Methods of treating patients suffering from certain diseases responsive to inhibition of tyrosine kinase activity are also given. In certain embodiments the diseases are responsive to inhibition of Btk activity and / or B-cell proliferation. Such methods comprise administering to such patients an amount of a compound of Formula I effective to reduce signs or symptoms of the disease. These diseases include cancer, an autoimmune and / or inflammatory disease, or an acute inflammatory reaction. Thus methods of treatment include administering a sufficient amount of a compound or salt as provided herein to decrease the symptoms or slow the progression of these diseases.Other embodiments include methods of treating other animals, including livestock and domesticated companion animals, suffering from a disease responsive to inhibition of kinase activity.Methods of treatment include administering a compound of Formula I as a single active agent or administering a compound of Formula I in combination with one or more other therapeutic agent.A method for determining the presence of Btk in a sample, comprising contacting the sample with a compound or form thereof of Formula I under conditions that permit detection of Btk activity, detecting a level of Btk activity in the sample, and therefrom determining the presence or absence of Btk in the sample.
Owner:GILEAD CONNENTICUT INC
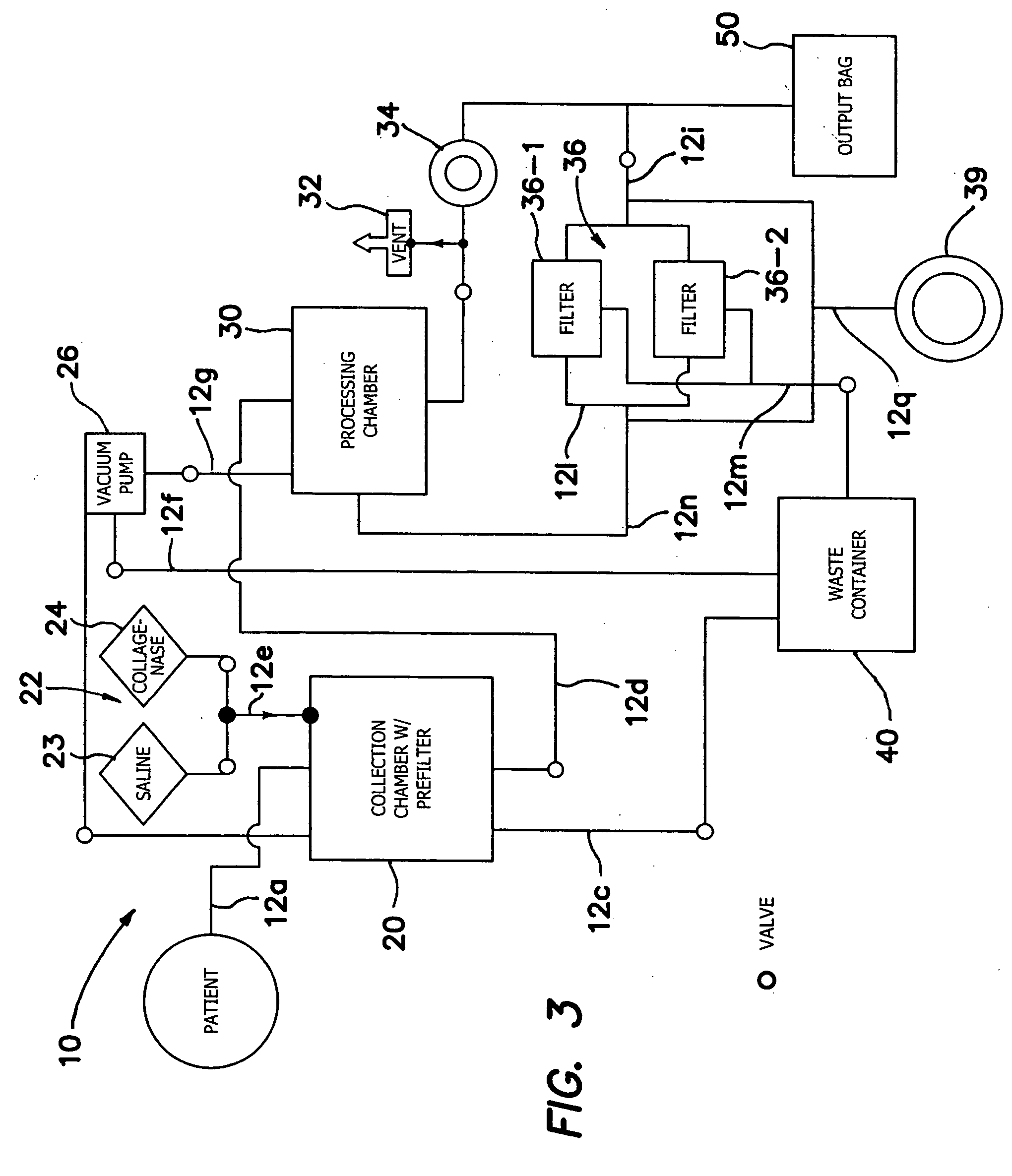
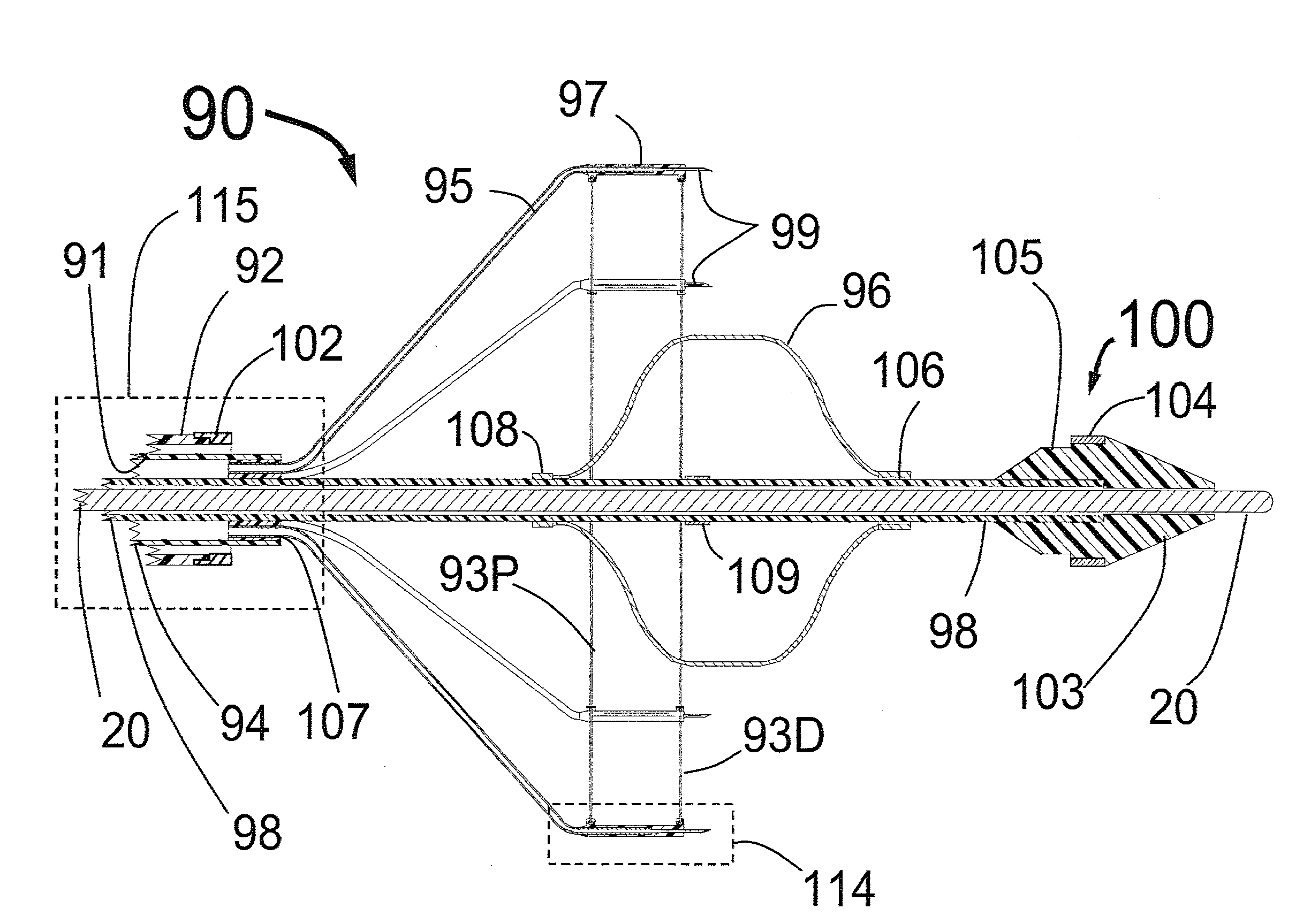
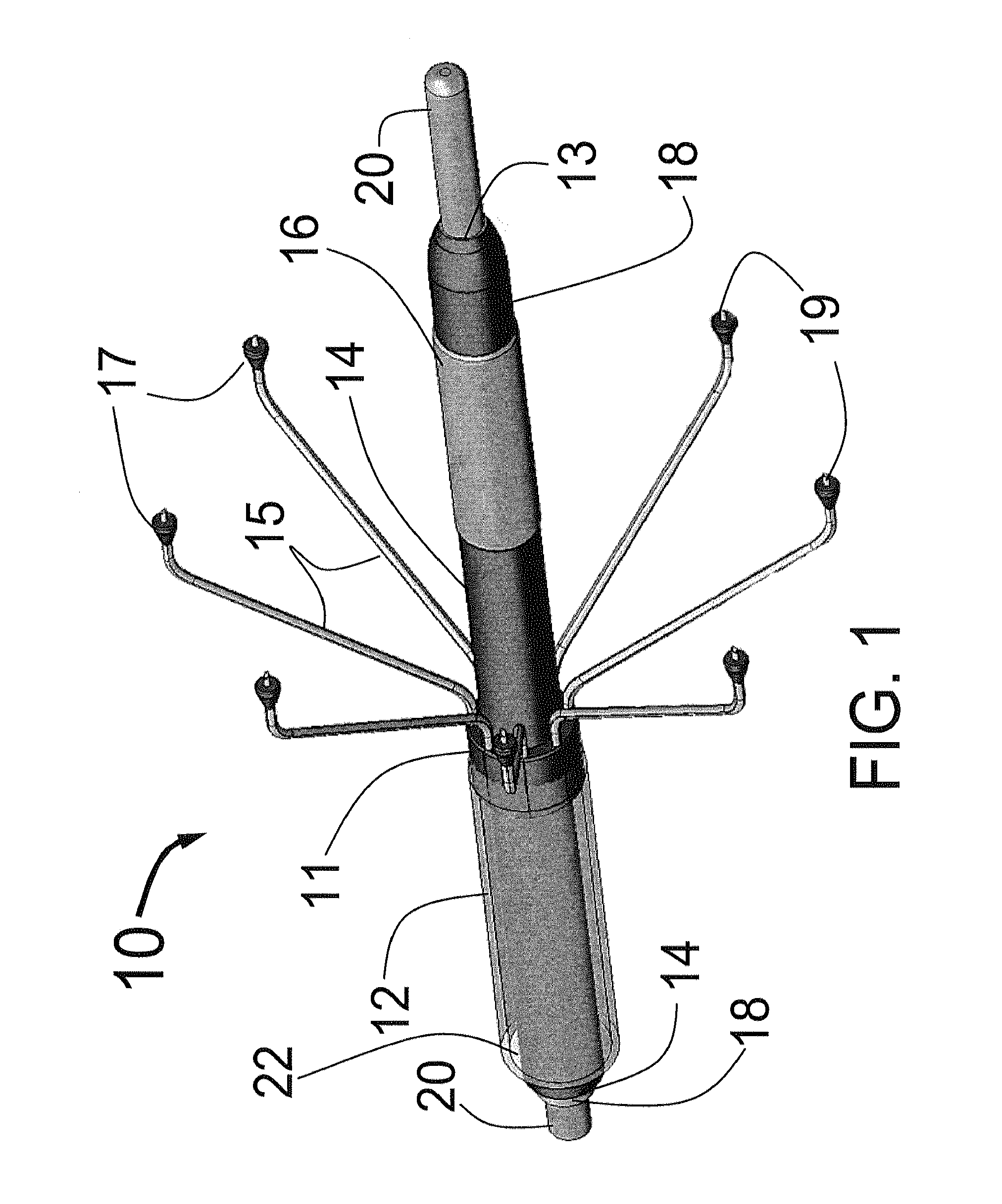
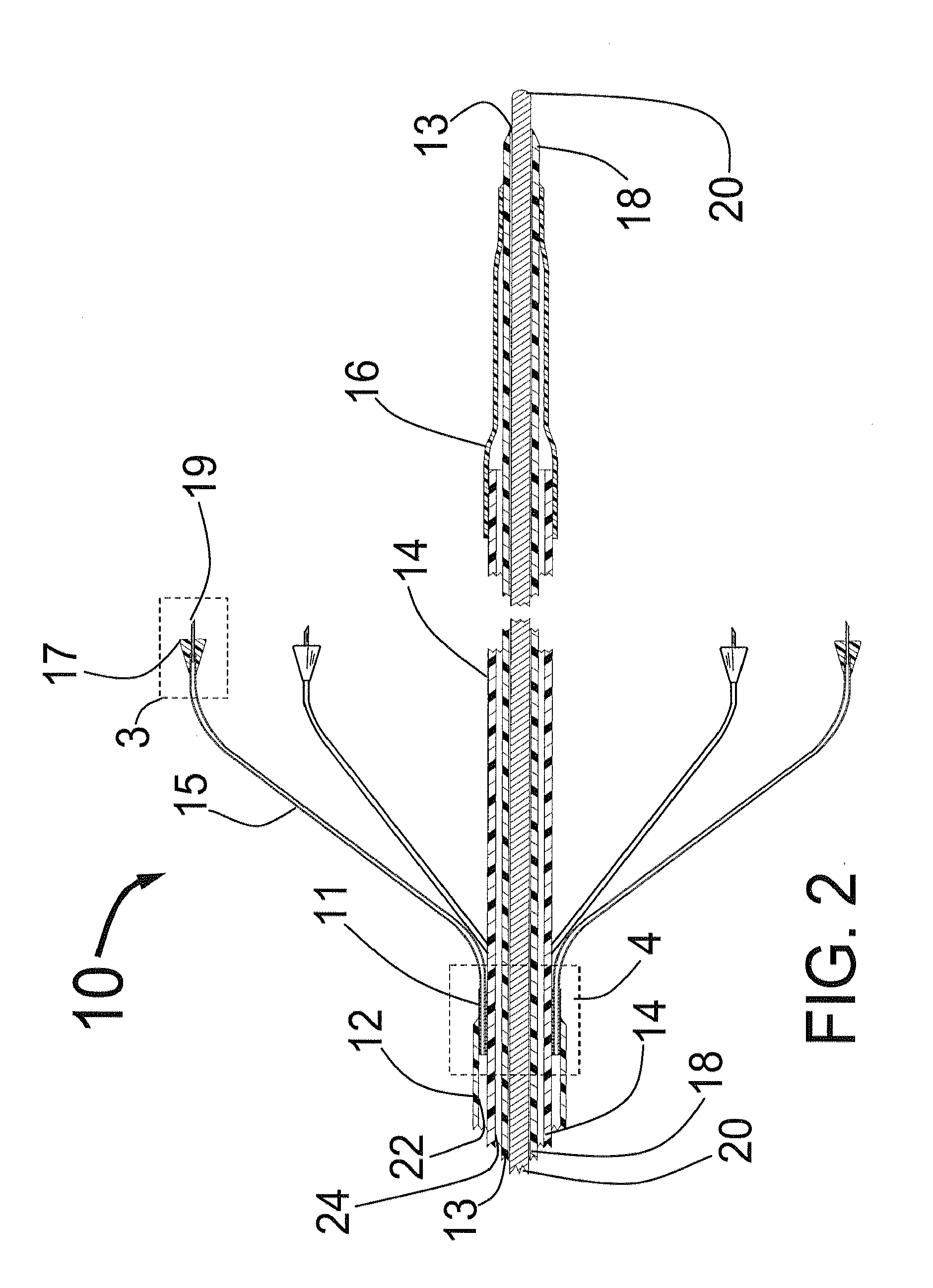
Systems and methods for treating patients with collagen-rich material extracted from adipose tissue
InactiveUS20030162707A1Cosmetic preparationsConnective tissue peptidesBrown adipose tissueWhite adipose tissue
Compositions, methods, and systems are disclosed for using collagen-rich material, derived from adipose tissue, that is placed directly into a recipient along with such additives necessary to promote, engender, or support a therapeutic, structural, or cosmetic benefit. The compositions may be obtained during the course of a single surgical procedure, and may be administered to the patient immediately after adipose tissue is removed from a patient, such as within hours or days from being withdrawn from the patient.
Owner:LOREM VASCULAR PTE LTD
Methods of using adipose tissue-derived cells in augmenting autologous fat transfer
ActiveUS7651684B2Diminished and non-existent needRapid and reliable deviceBiocideElectrotherapyAutologous Fat TransferBreast augmentation
Methods of treating patients for conditions such as breast augmentation, soft tissue defects, and urinary incontinence, are described. The methods include removing adipose tissue from a patient, processing a portion of the adipose tissue to obtain a substantially isolated population of regenerative cells, mixing the regenerative cells with another portion of adipose tissue to form a composition, and administering the composition to the patient from which the adipose tissue was removed.
Owner:LOREM VASCULAR PTE LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ead034c7-c473-4c4b-9966-87a09aefc546/US20050090499A1-20050428-C00001.png)
![Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ead034c7-c473-4c4b-9966-87a09aefc546/US20050090499A1-20050428-C00002.png)
![Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds Certain imidazo[1,2-a]pyrazin-8-ylamines and method of inhibition of bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ead034c7-c473-4c4b-9966-87a09aefc546/US20050090499A1-20050428-C00003.png)
![Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ebbaacdc-083b-4261-bc4a-f1b6b1a1c3a3/US07393848-20080701-C00001.png)
![Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ebbaacdc-083b-4261-bc4a-f1b6b1a1c3a3/US07393848-20080701-C00002.png)
![Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds Certain heterocyclic substituted imidazo[1,2-A]pyrazin-8-ylamines and methods of inhibition of Bruton's tyrosine kinase by such compounds](https://images-eureka.patsnap.com/patent_img/ebbaacdc-083b-4261-bc4a-f1b6b1a1c3a3/US07393848-20080701-C00003.png)