Patents
Literature
688 results about "Analgesic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An analgesic or painkiller is any member of the group of drugs used to achieve analgesia, relief from pain. Analgesic drugs act in various ways on the peripheral and central nervous systems. They are distinct from anesthetics, which temporarily affect, and in some instances completely eliminate, sensation. Analgesics include paracetamol (known in North America as acetaminophen or simply APAP), the nonsteroidal anti-inflammatory drugs (NSAIDs) such as the salicylates, and opioid drugs such as morphine and oxycodone.
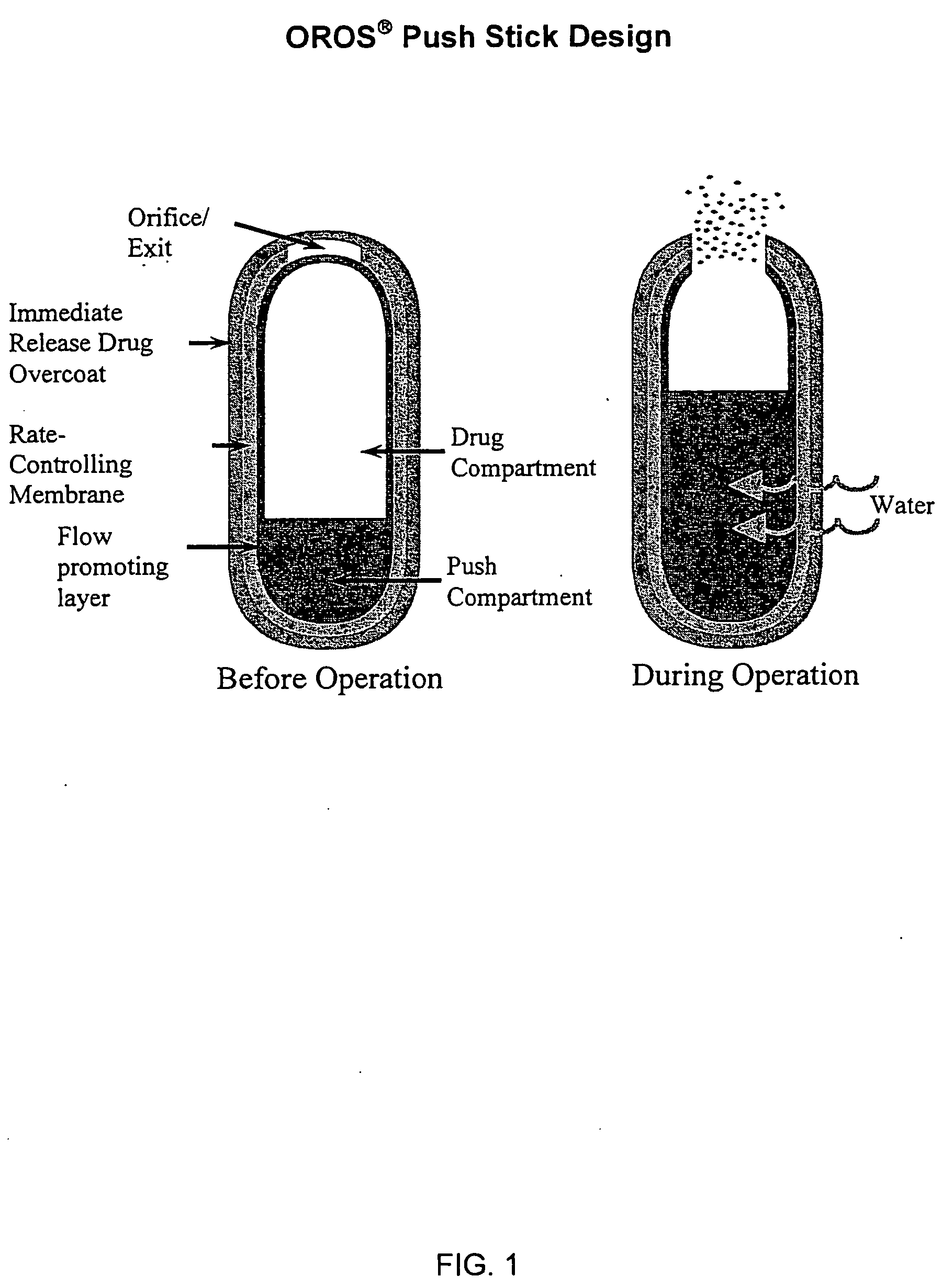
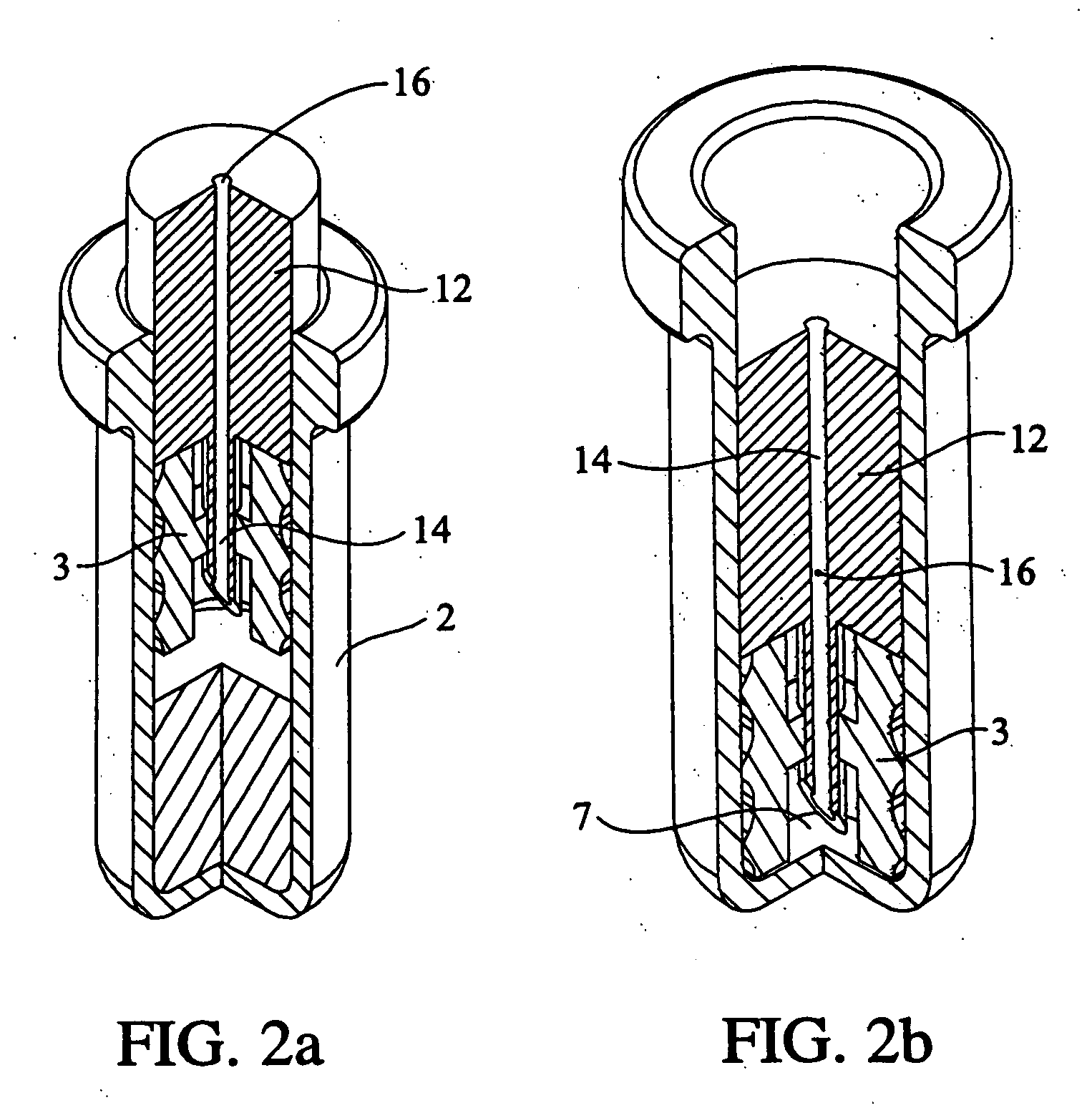
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS20030124185A1Analgesic and euphoric effect be reduce and eliminateCompromise integrityPowder deliveryPill deliveryOpioid antagonistDrug
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Particulate acellular tissue matrix
A method of processing an acellular tissue matrix to give a particulate acellular tissue matrix includes: cutting sheets of dry acellular tissue matrix into strips; cryofracturing the dry acellular tissue matrix strips at cryogenic temperatures; separating the resulting particles by size at cryogenic temperatures; and freeze drying the fraction of particles desired size to remove any moisture that may have been absorbed to give a dry particulate acellular tissue matrix. Rehydration of the dry particulate acellular tissue matrix may take place just prior to use. The particulate acellular tissue may be applied to a recipient site, by way of injection, spraying, layering, packing, in-casing or combinations thereof. The particulate acellular tissue may further include growth and stimulating agents selected from epidermal growth factor, fibroblast growth factor, nerve growth factor, keratinocyte growth factor, platelet derived growth factor, vasoactive intestinal peptide, stem cell factor, bone morphogetic proteins, chondrocyte growth factor and combinations thereof. Other pharmaceutically active compounds may be combined with the rehydrated particulate material including: analgesic drugs; hemostatic drugs; antibiotic drugs; local anesthetics and the like to enhance the acceptance of the implanted particulate material. The particulate material product may also be combined with stem cells selected from mesenchymal stem cells, epidermal stem cells, cartilage stem cells, hematopoietic stem cells and combinations thereof.
Owner:LIFECELL
Tamper resistant dosage forms
ActiveUS20090081290A1Reduces and prevents stickingBiocidePowder deliveryOpioid analgesicsDosage form
The present invention relates to pharmaceutical dosage forms, for example to a tamper resistant dosage form including an opioid analgesic, and processes of manufacture, uses, and methods of treatment thereof.
Owner:PURDUE PHARMA LP
Sustained release opioid formulations and method of use
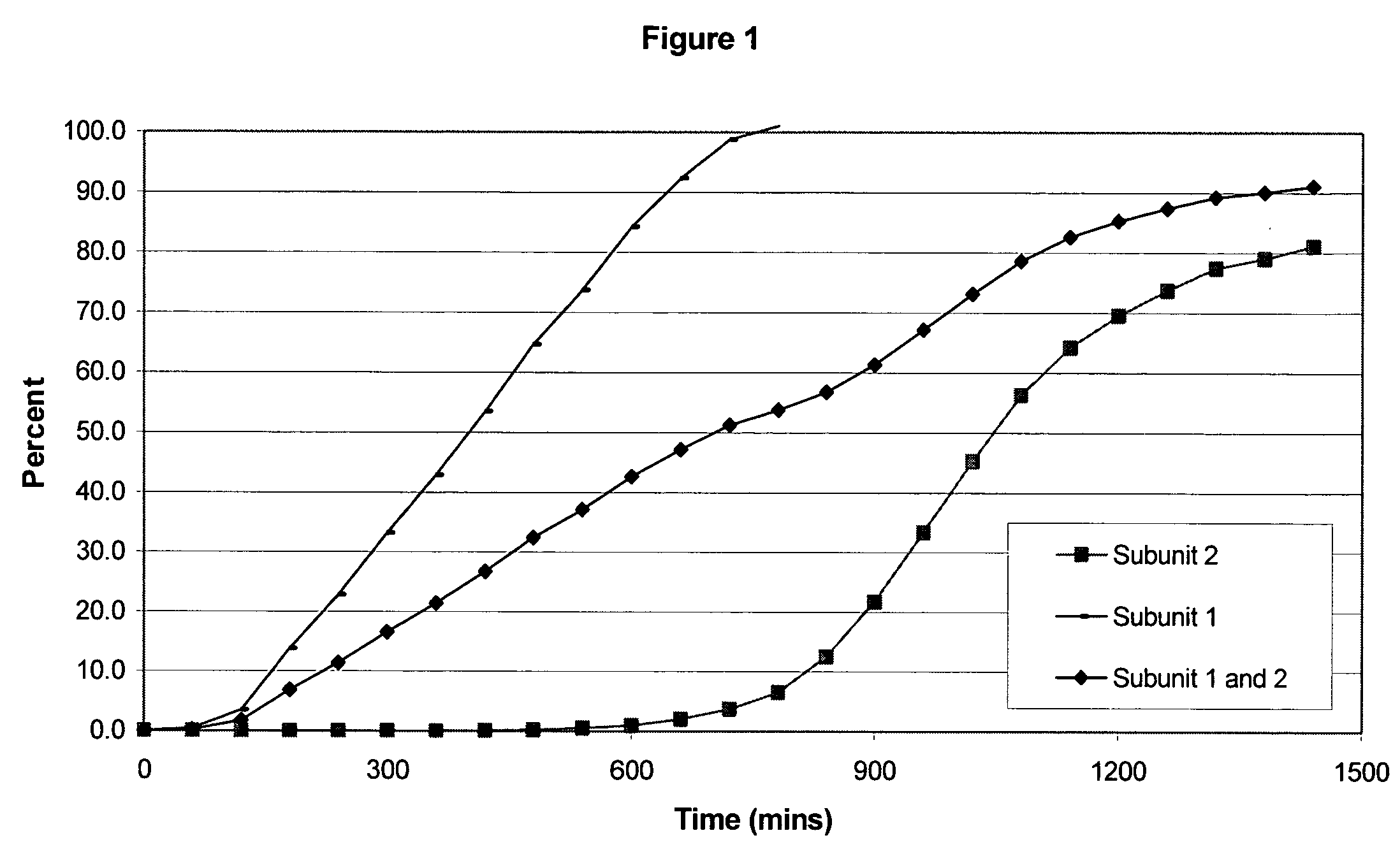
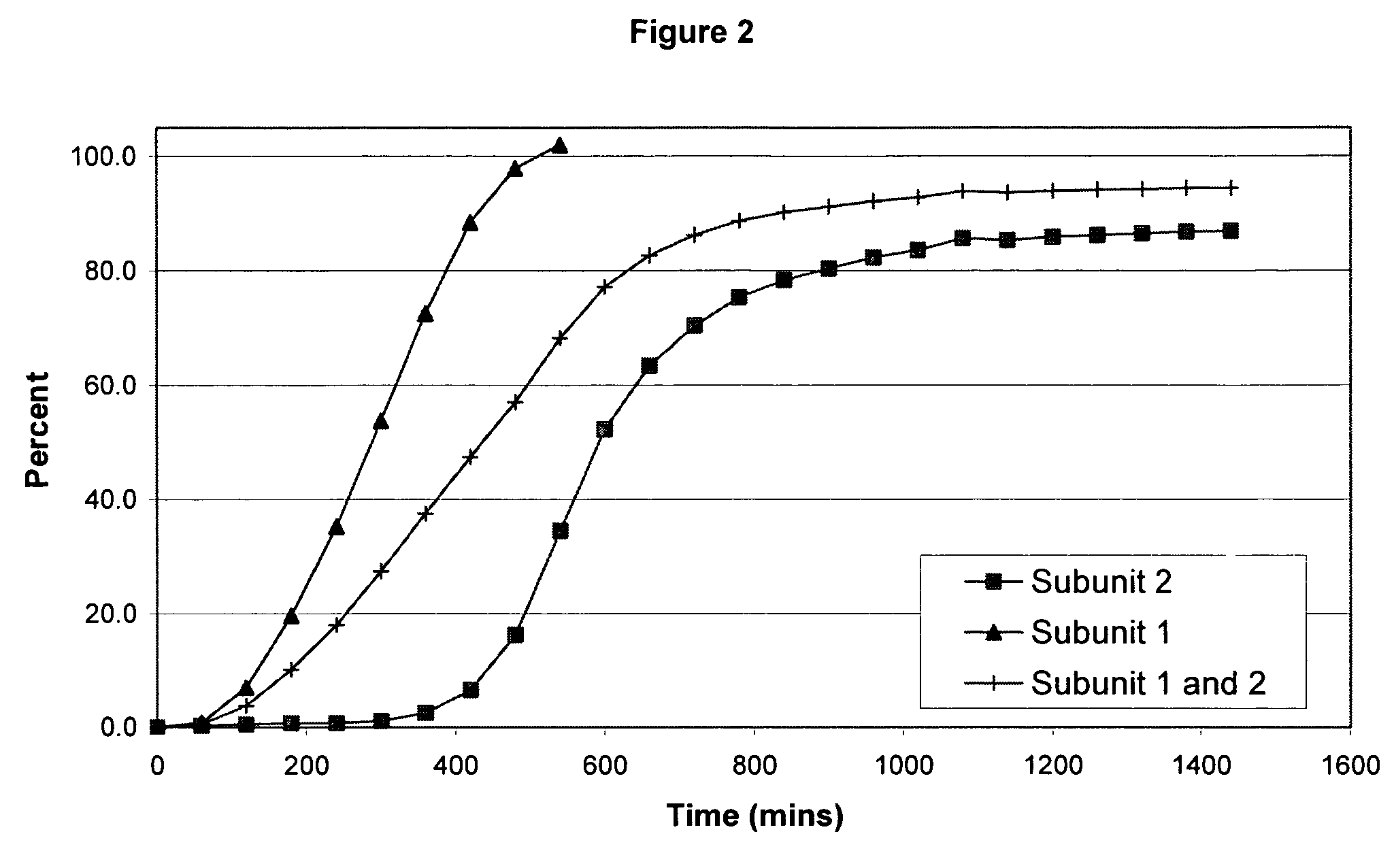
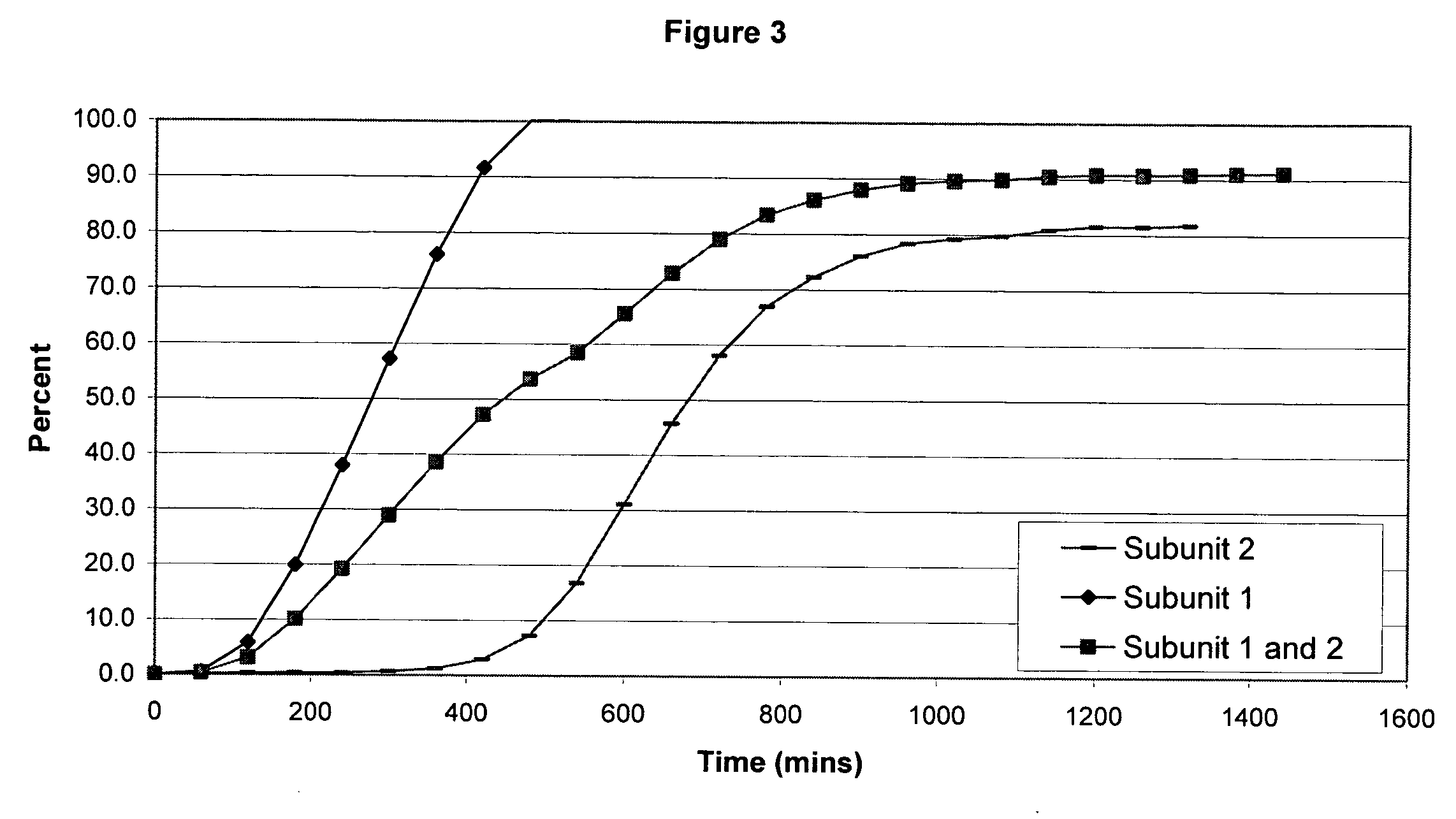
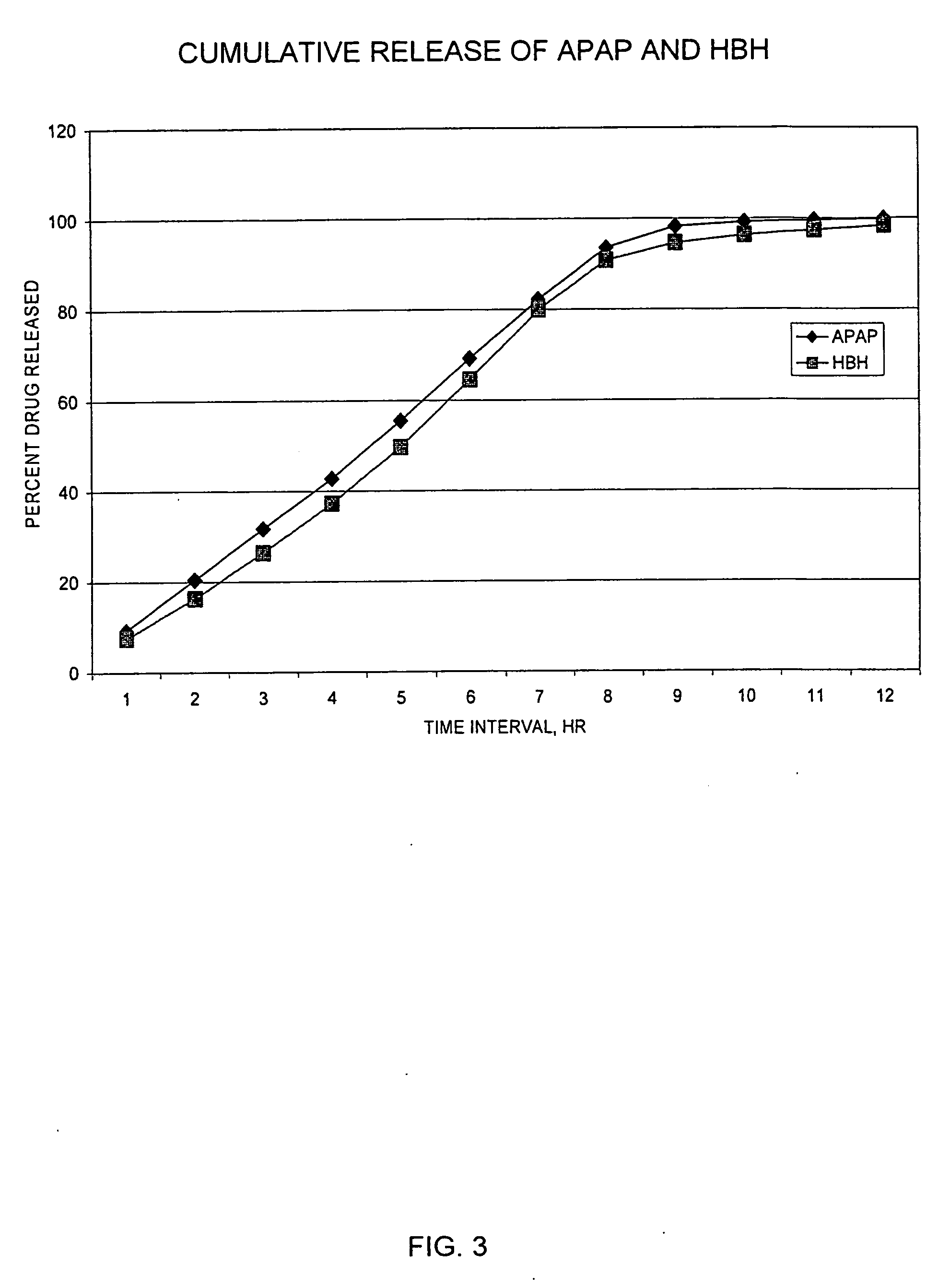
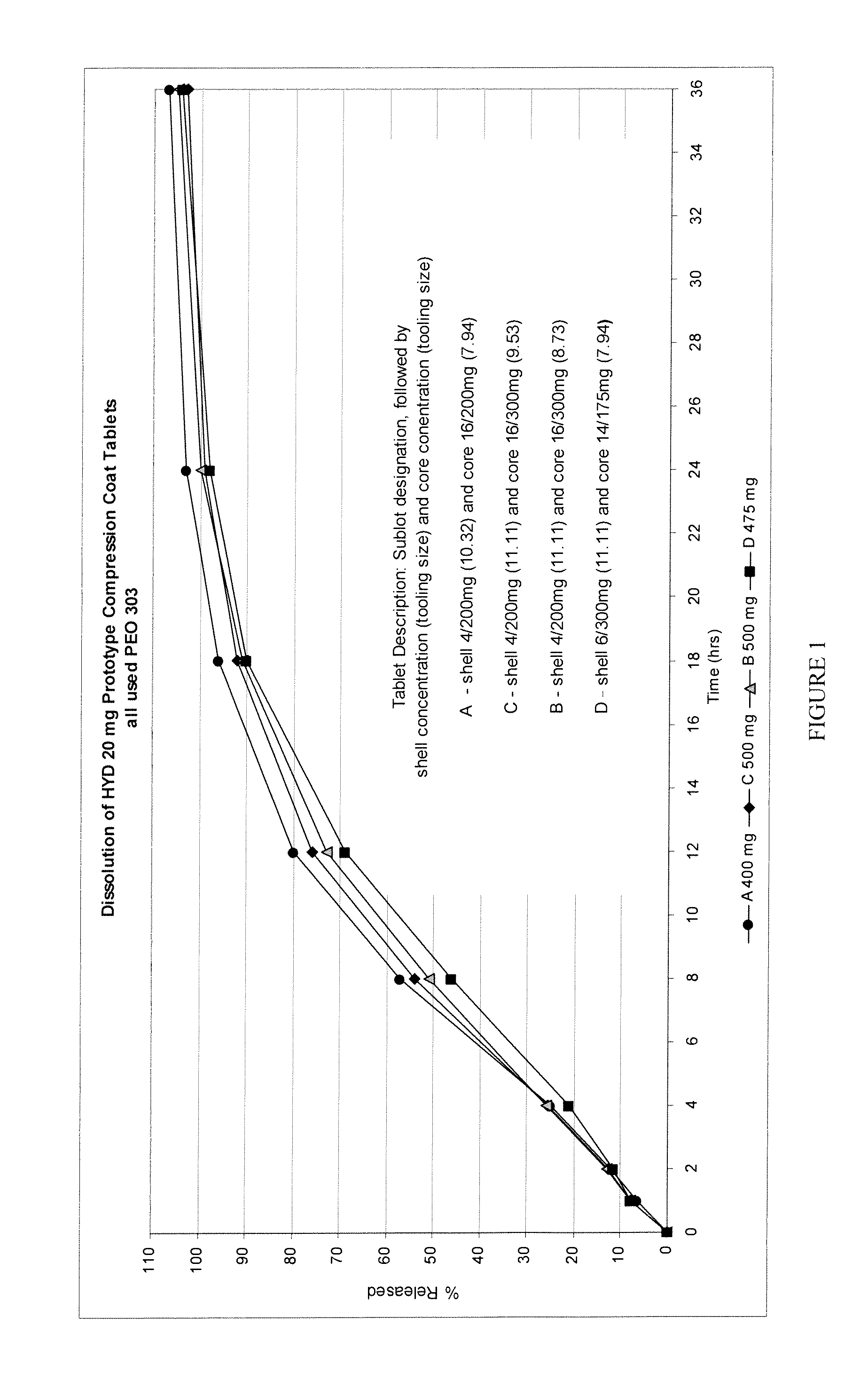
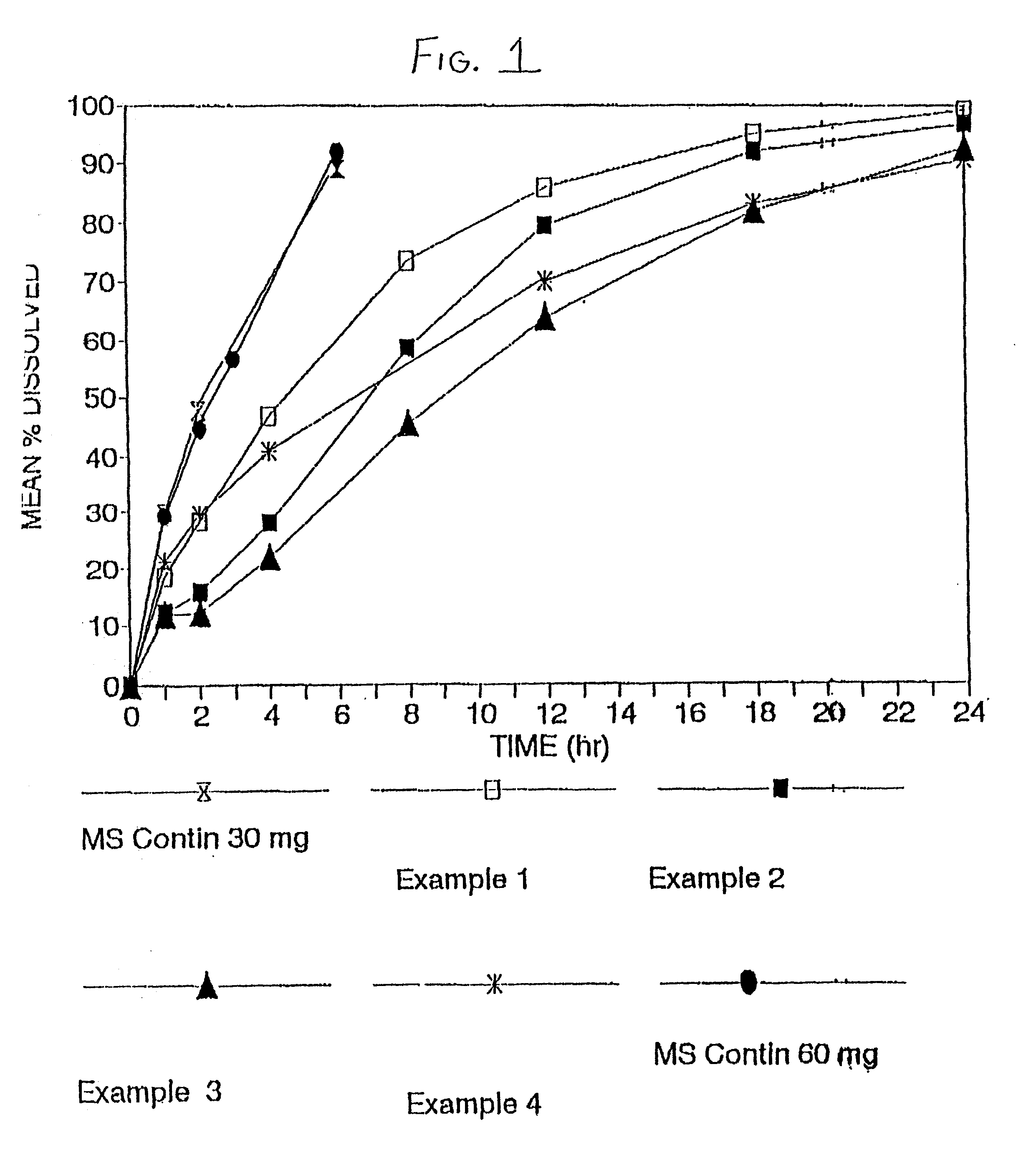
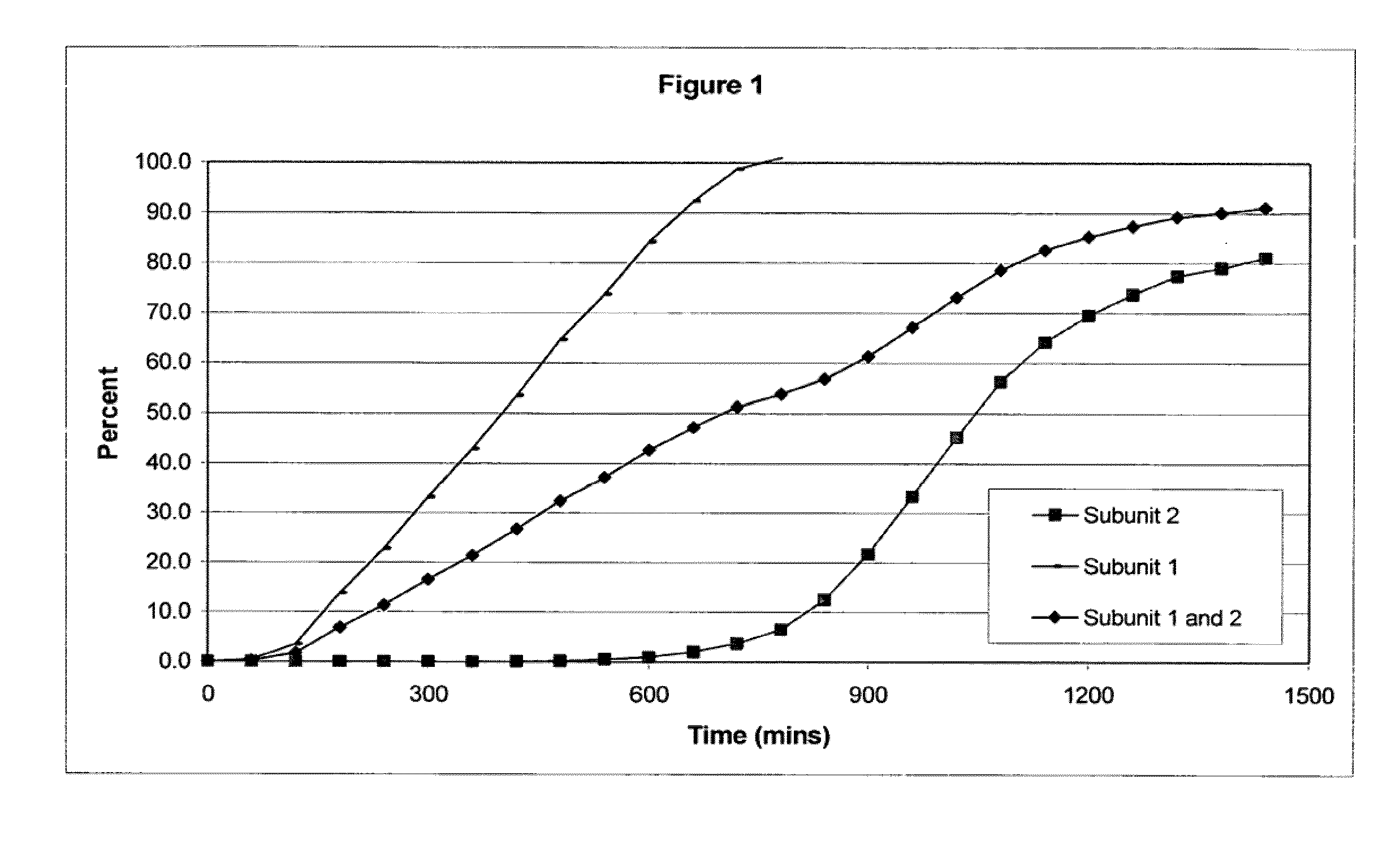
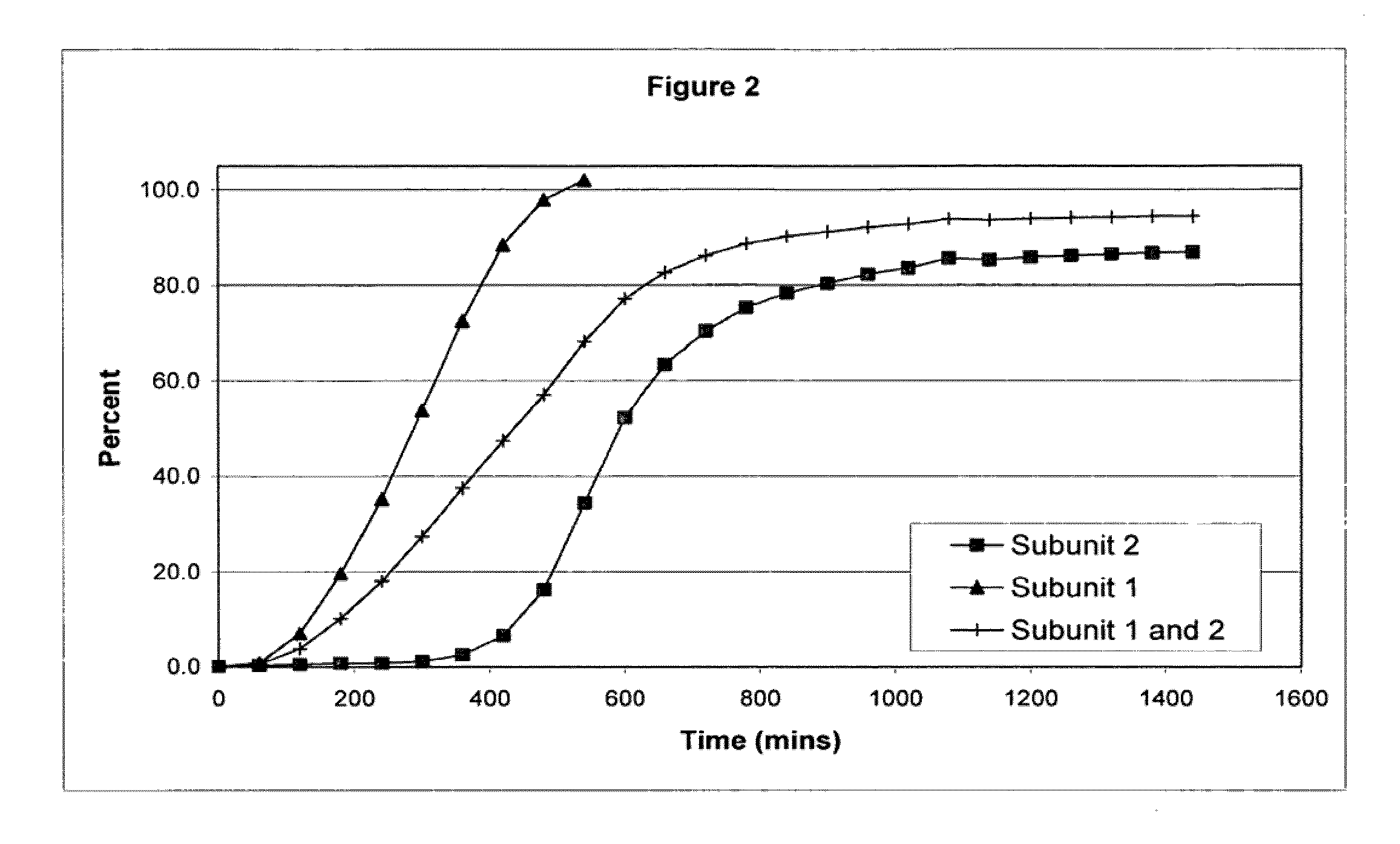
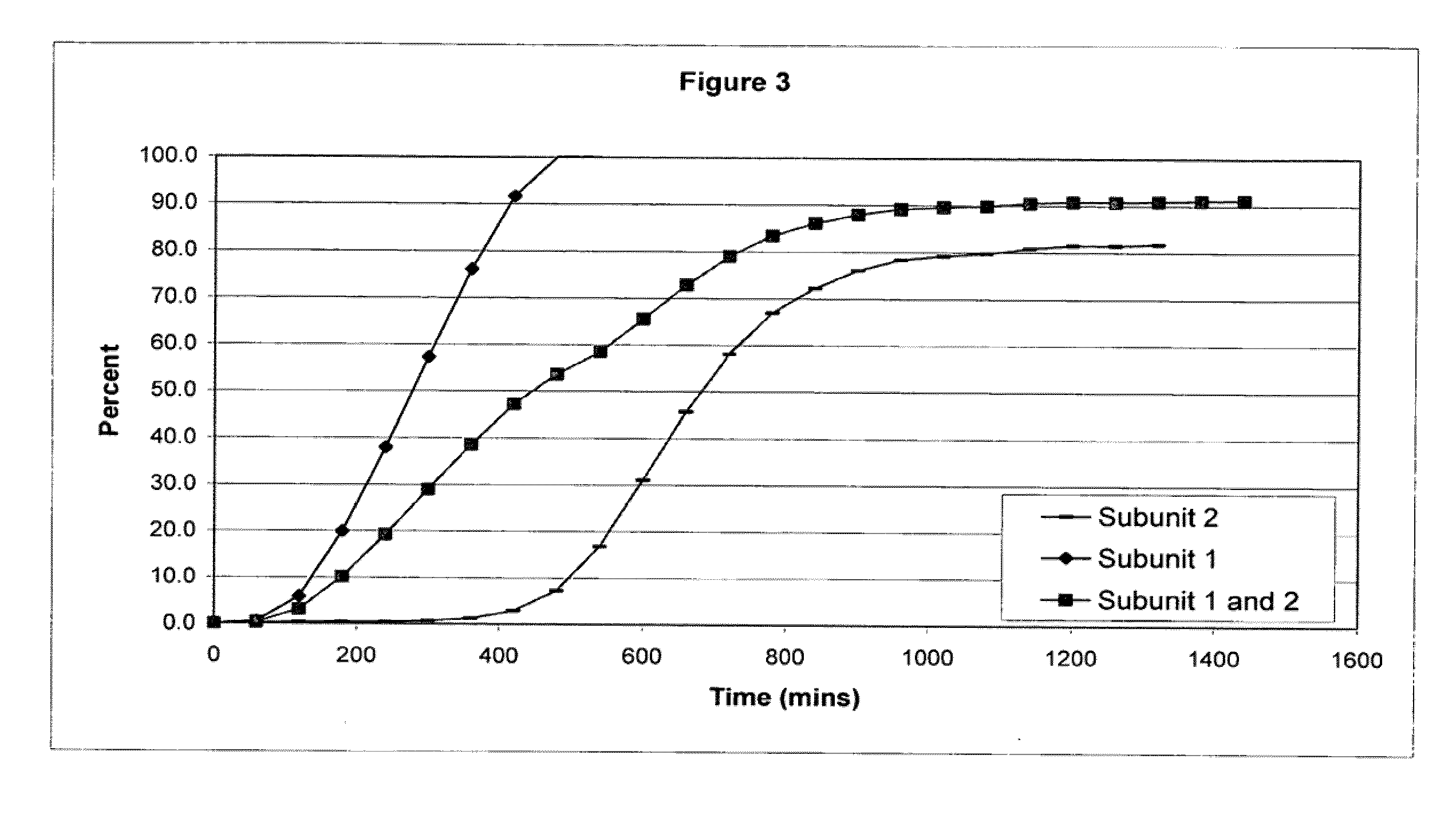
The invention combines two different subunits with different release profiles in novel sustained-release oral dosage forms. In particular, the oral dosage forms include a subunit that comprises an opioid analgesic and a sustained-release material, wherein the dissolution rate in-vitro of the subunit, when measured by the standard USP Drug Release test of U.S. Pharmacopeia XXVI (2003) <724>, is less than about 10% within about 6 hours and at least about 60% within about 24 hours; less than about 10% within about 8 hours and at least about 60% within about 24 hours; less than about 10% within about 10 hours and at least about 60% within about 24 hours; or less than about 10% within about 12 hours and at least about 60% within about 24 hours; the dosage form providing a duration of therapeutic effect of about 24 hours.
Owner:ALPHARMA PHARMA
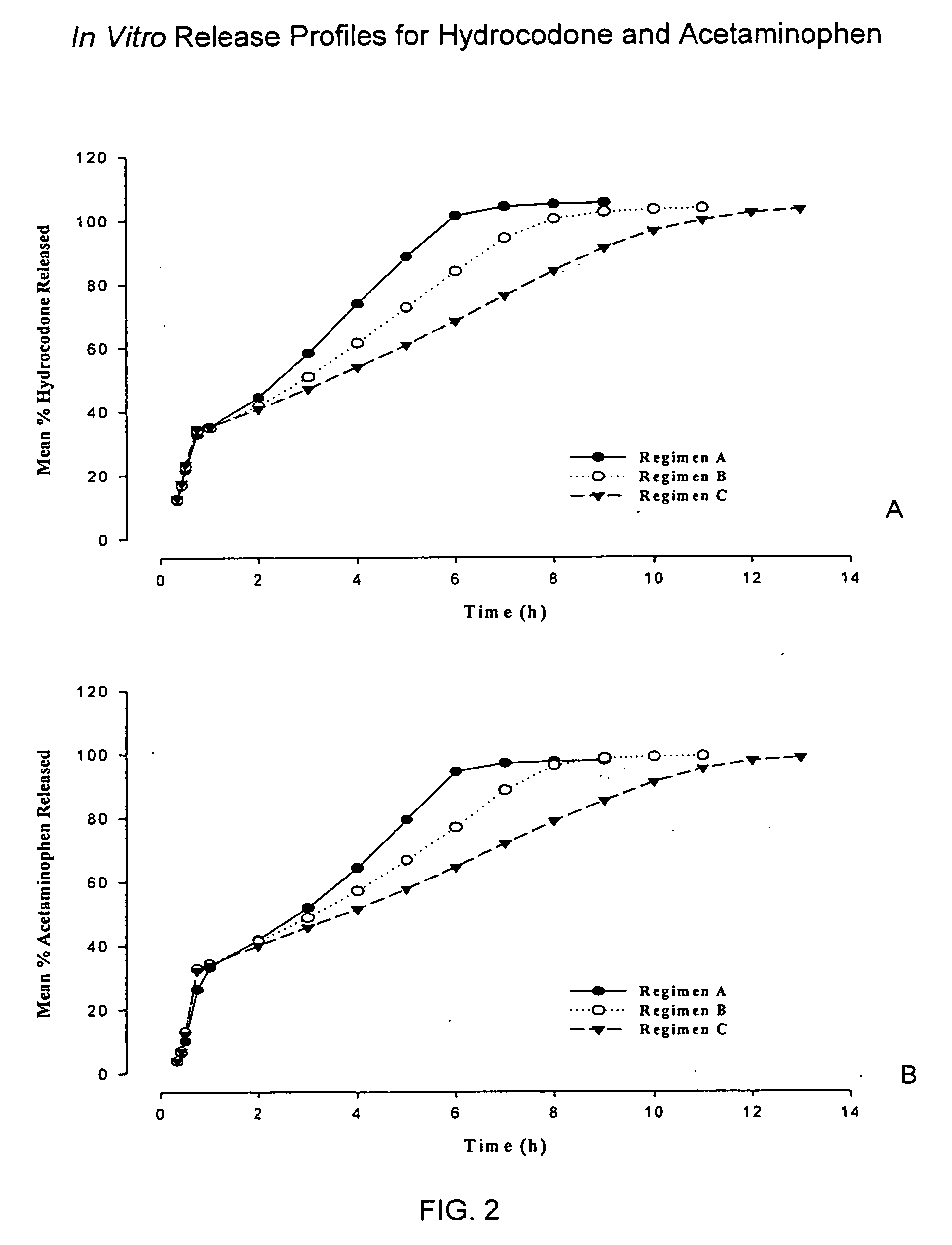
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
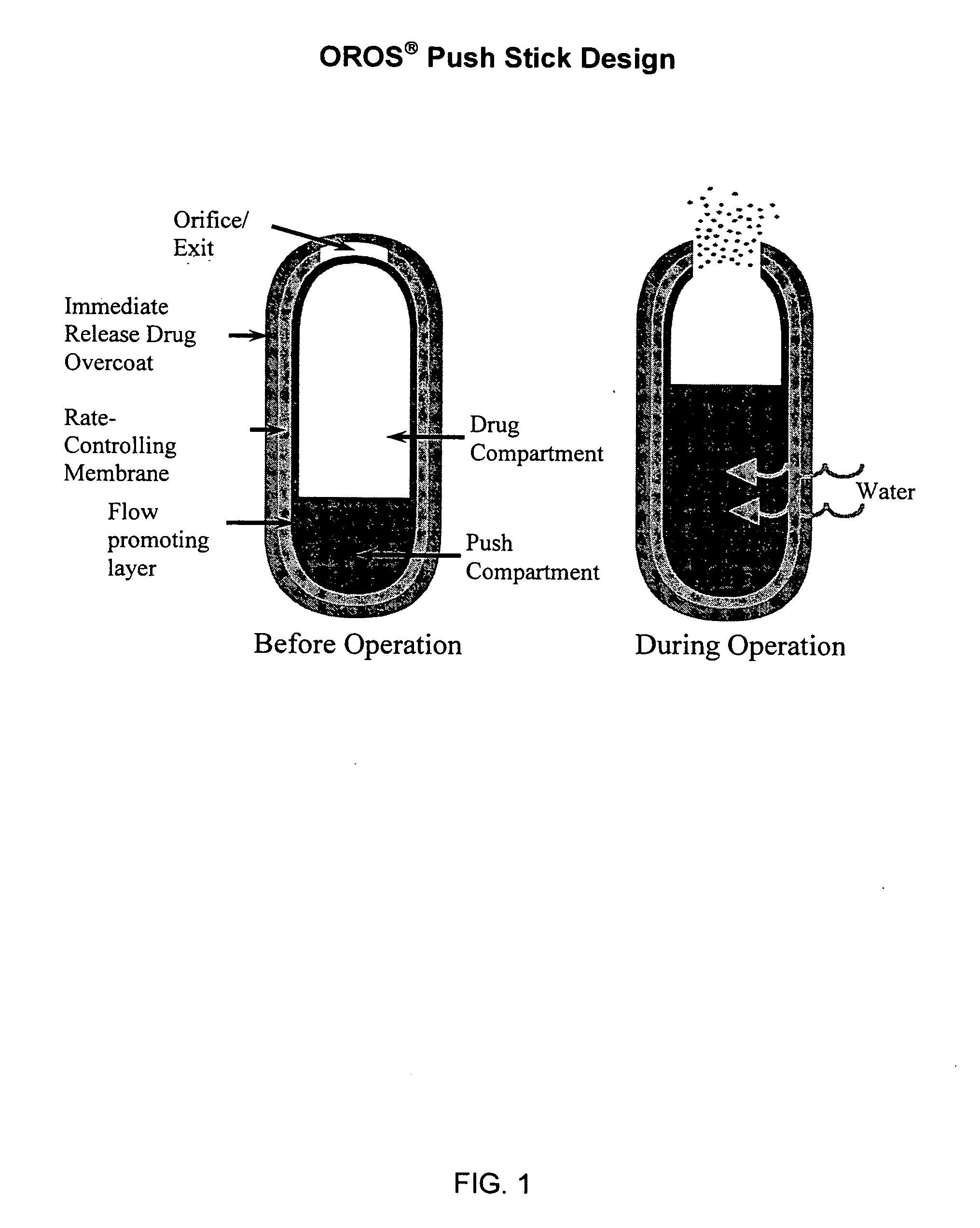
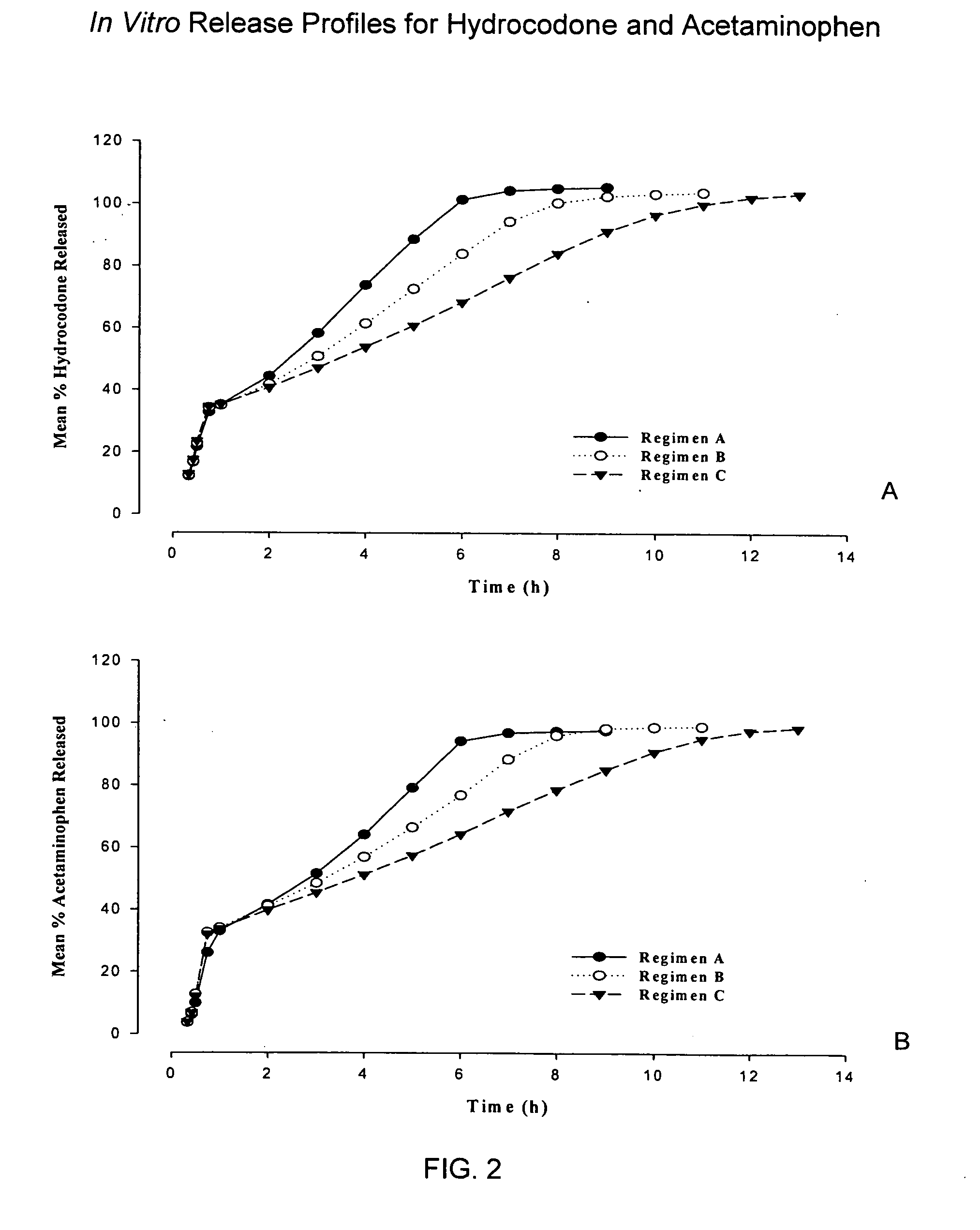
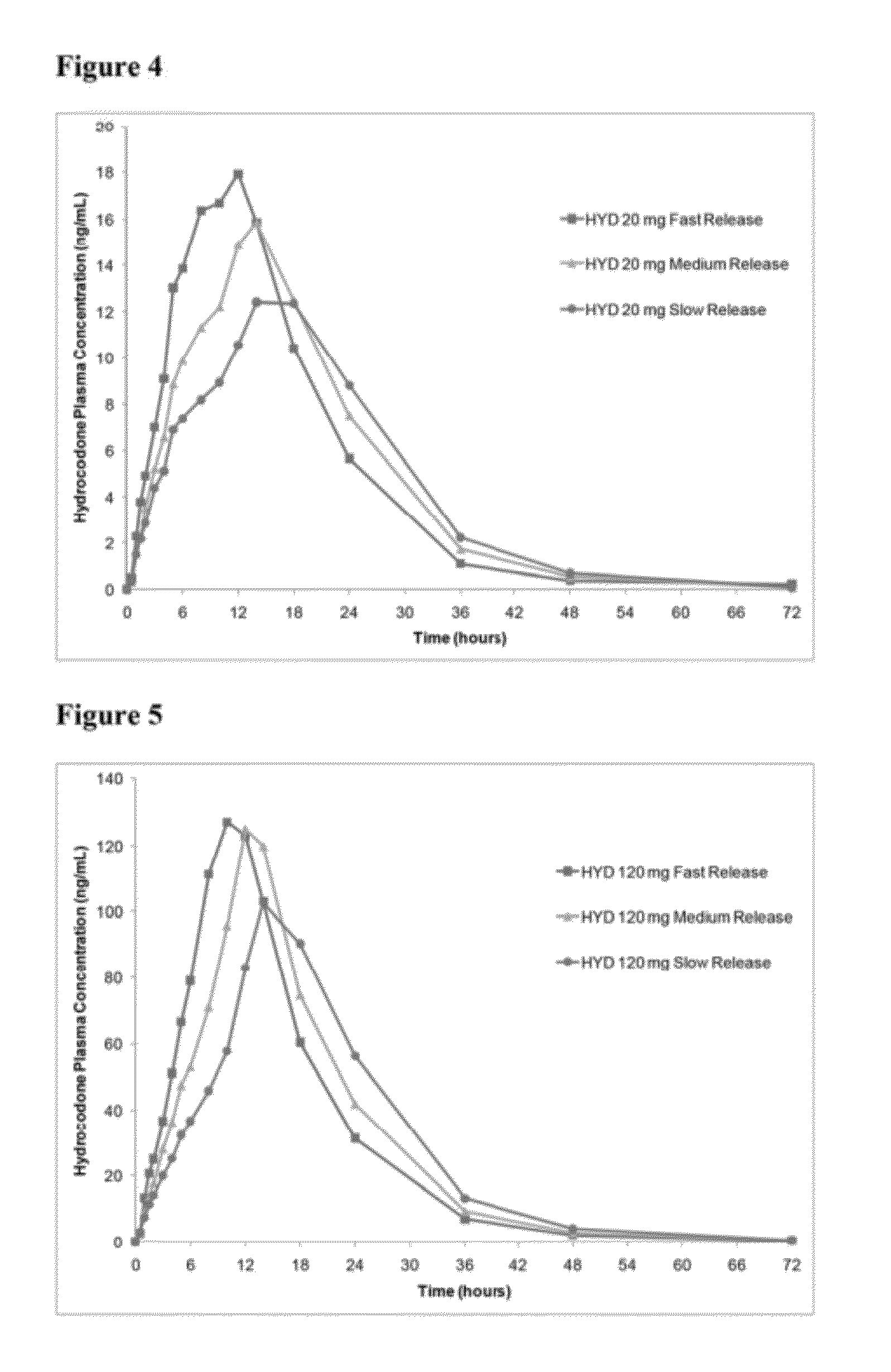
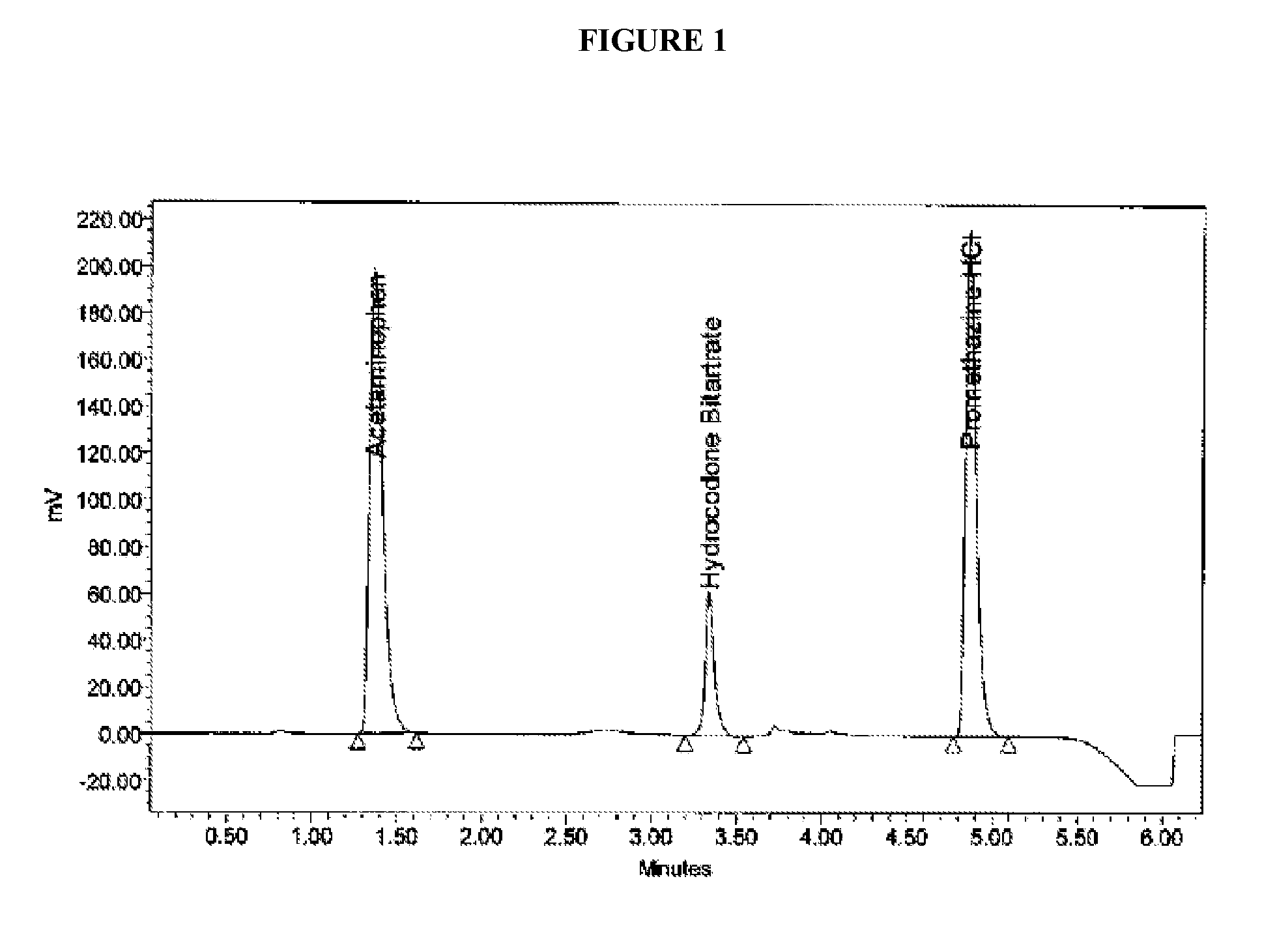
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Formulations of nonopioid and confined opioid analgesics
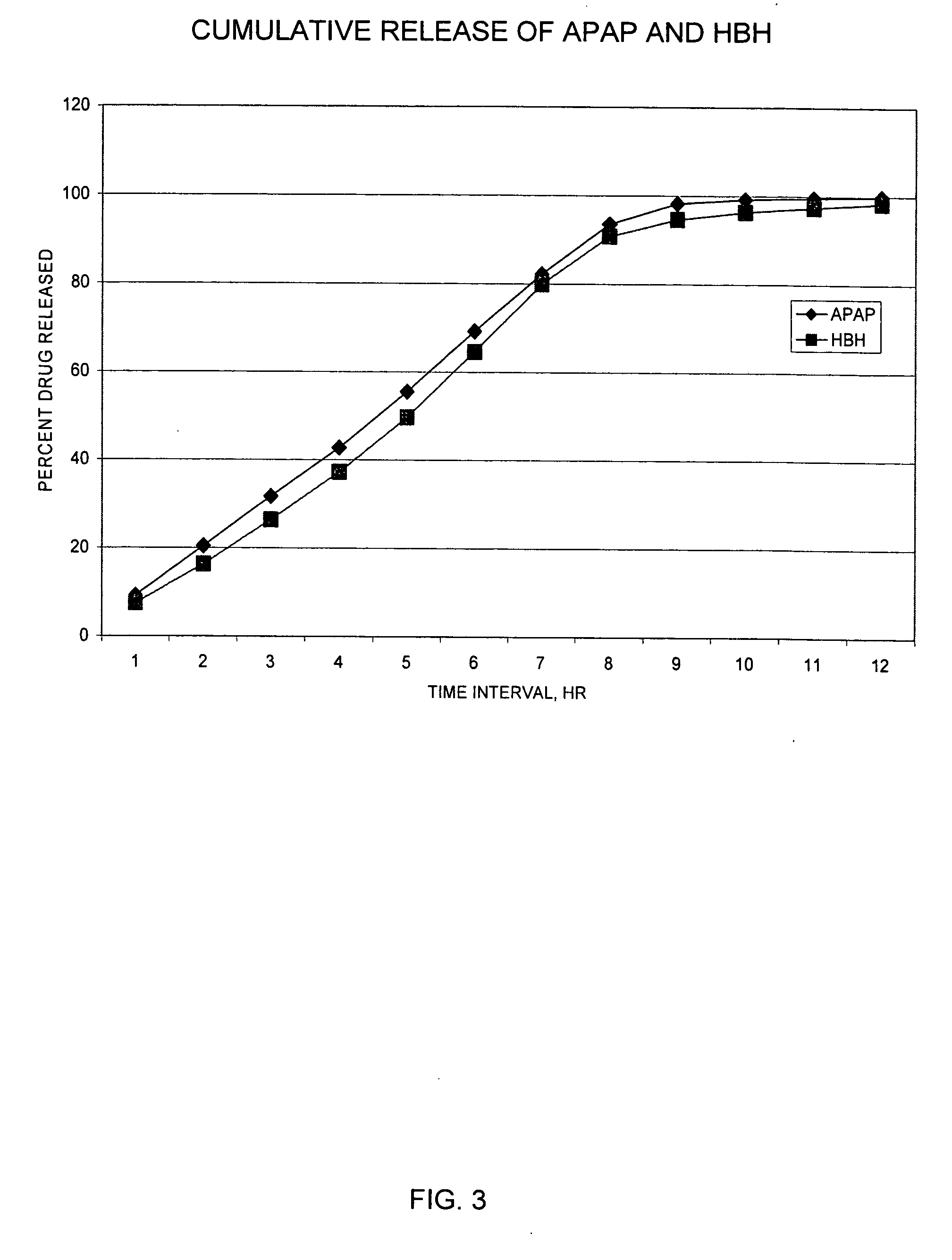
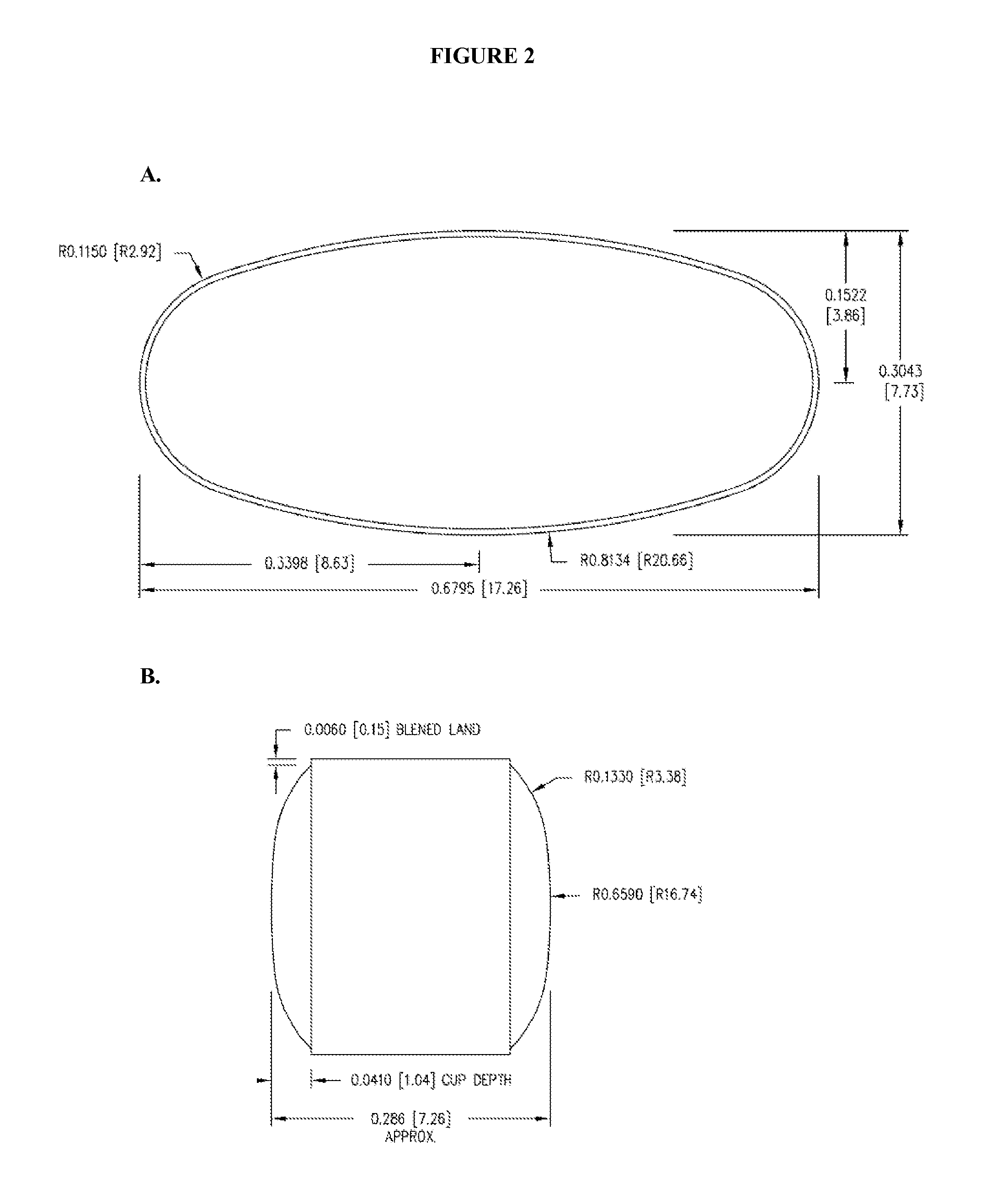
The preferred exemplary embodiments in the present application provide formulations and methods for the delivery of drugs, particularly drugs of abuse, having an abuse-relevant drug substantially confined in the core and a non-abuse relevant drug in a non-core region. These formulations have reduced potential for abuse. In the formulation, preferably the abuse relevant drug is an opioid and the non-abuse relevant drug is acetaminophen or ibuprofen. More preferably, the opioid is hydrocodone, and the non-abuse relevant analgesic is acetaminophen. In certain preferred embodiments, the dosage forms are characterized by resistance to solvent extraction; tampering, crushing or grinding. Certain embodiments of the inventions provide dosage forms that provide an initial burst of release of drug followed by a prolonged period of controllable drug release.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Dosage form containing multiple drugs
A pharmaceutical dosage form comprising a first drug and a second drug, both of which are selected from decongestants, antitussives, expectorants, analgesics and antihistamines. The dosage form provides a plasma concentration within a therapeutic range of the second drug over a period which is coextensive with at least about 70% of a period over which the dosage form provides a plasma concentration within a therapeutic range of the first drug. This Abstract is neither intended to define the invention disclosed in this specification nor intended to limit the scope of the invention in any way.
Owner:SOVEREIGN PHARMA
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Methods and related compositions for reduction of fat and skin tightening
InactiveUS20060127468A1Efficient tighteningTighten regionBiocideCosmetic preparationsCelluliteExcipient
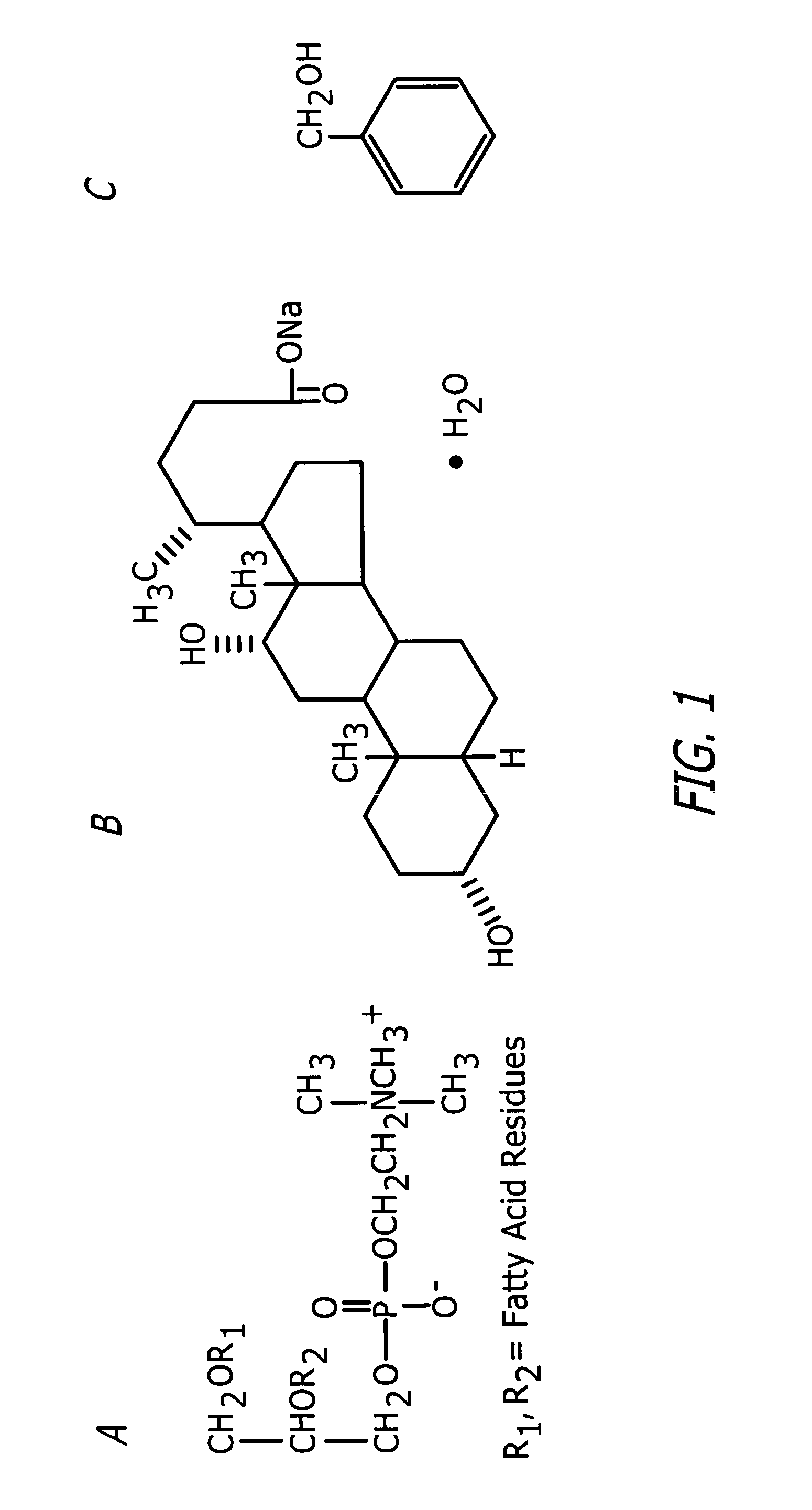
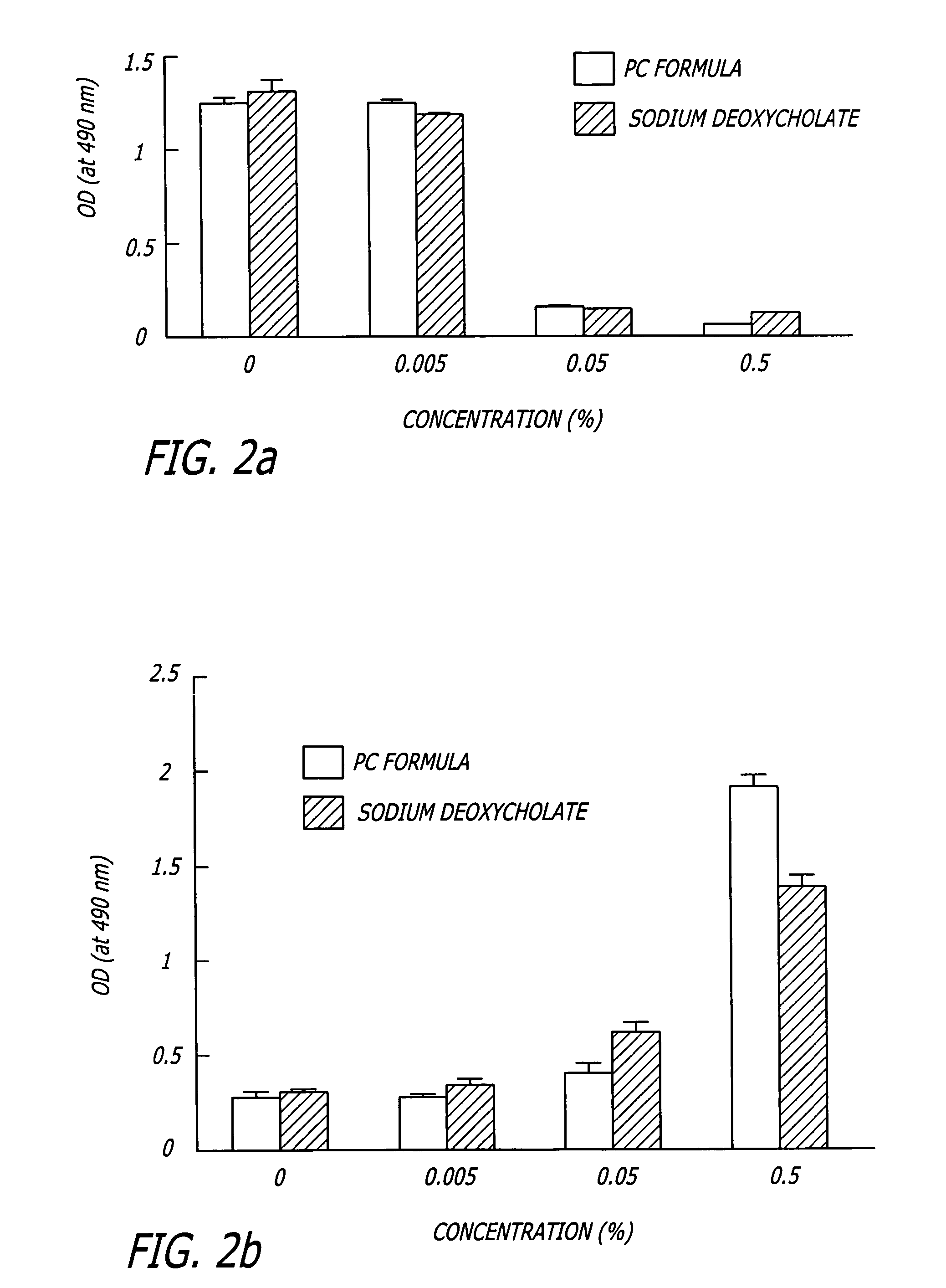
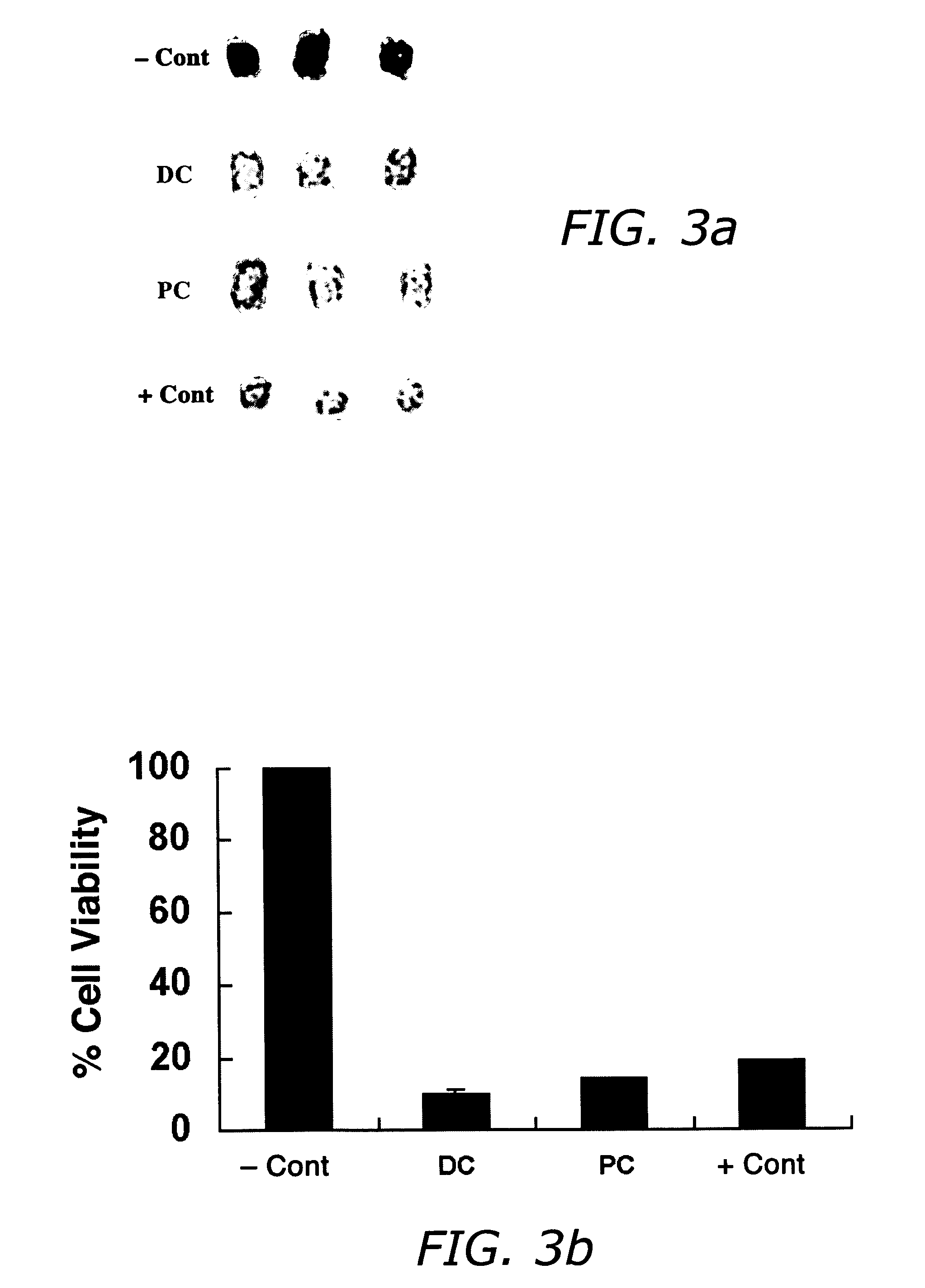
Compositions and methods useful in the reduction of localized fat deposits and tightening of loose skin in subjects in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion or anti-dispersion agents and pharmaceutically acceptable excipients. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including, for example, lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:RGT UNIV OF CALIFORNIA +1
Supplemented and unsupplemented tissue sealants, methods of their production and use
ActiveUS7189410B1Low antigenicityDecreasing thrombogenicityAntibacterial agentsOrganic active ingredientsTissue sealantVascular dilatation
This invention provides a fibrin sealant bandage, wherein said fibrin sealant may be supplemented with at least one composition selected from, for example, one or more regulatory compounds, antibody, antimicrobial compositions, analgesics, anticoagulants, antiproliferatives, anti-inflammatory compounds, cytokines, cytotoxins, drugs, growth factors, interferons, hormones, lipids, demineralized bone or bone morphogenetic proteins, cartilage inducing factors, oligonucleotides polymers, polysaccharides, polypeptides, protease inhibitors, vasoconstrictors or vasodilators, vitamins, minerals, stabilizers and the like. Also disclosed are methods of preparing and / or using the unsupplemented or supplemented fibrin sealant bandage.
Owner:AMERICAN NAT RED CROSS
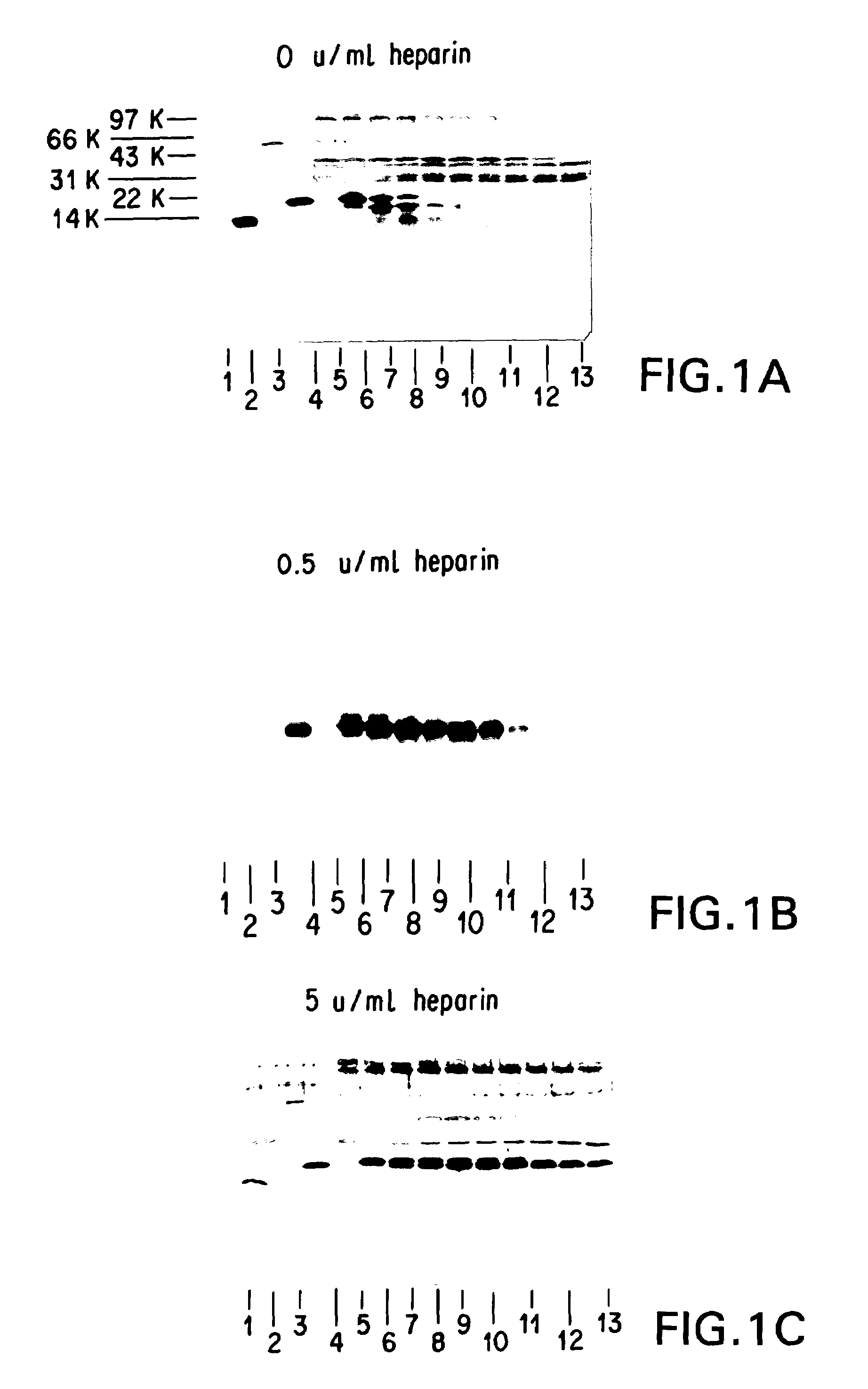
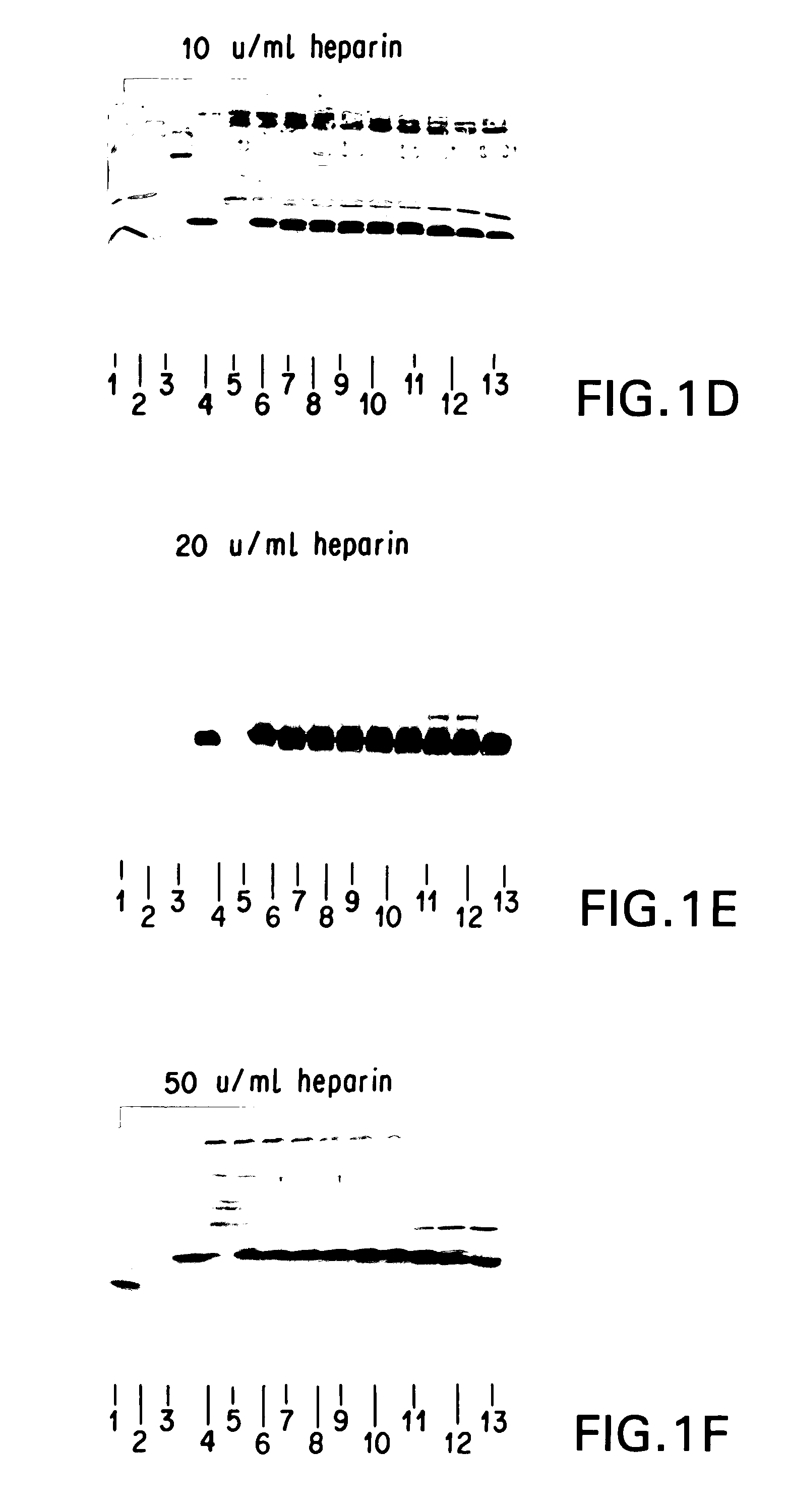
Encased Tamper Resistant Controlled Release Dosage Forms
ActiveUS20120164220A1Reducing abuse potential of dosage formBiocideNervous disorderGastric fluidEnzyme
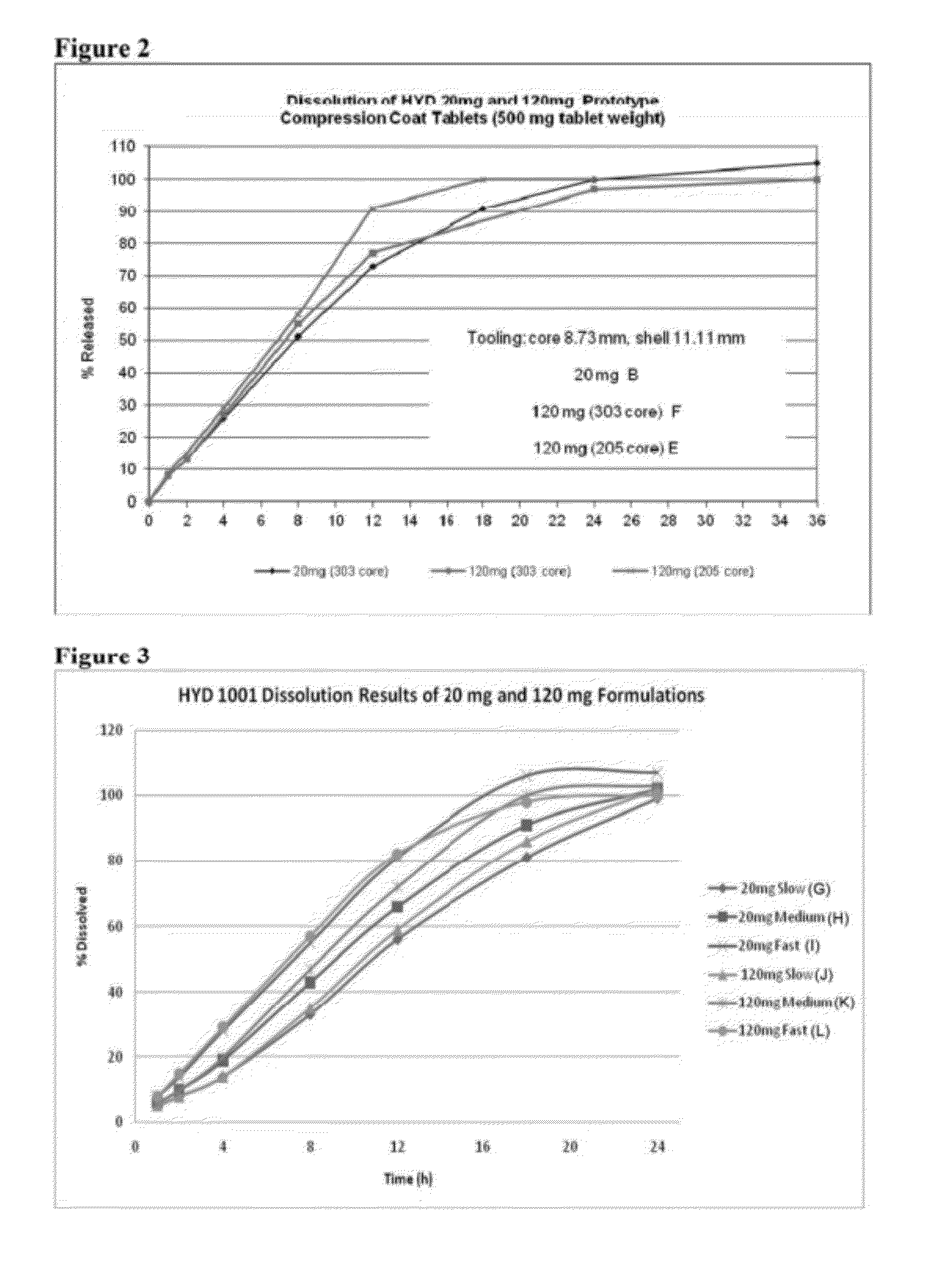
In certain embodiments, the present invention is directed to a solid controlled release dosage form comprising: a core comprising a first portion of an opioid analgesic dispersed in a first matrix material; and a shell encasing the core and comprising a second portion of the opioid analgesic dispersed in a second matrix material; wherein the amount of opioid analgesic released from the dosage form is proportional within 20% to elapsed time from 8 to 24 hours, as measured by an in-vitro dissolution in a USP Apparatus 1 (basket) at 100 rpm in 900 ml simulated gastric fluid without enzymes (SGF) at 37 C.
Owner:PURDUE PHARMA LP
Apparatus for the delivery to an animal of a beneficial agent
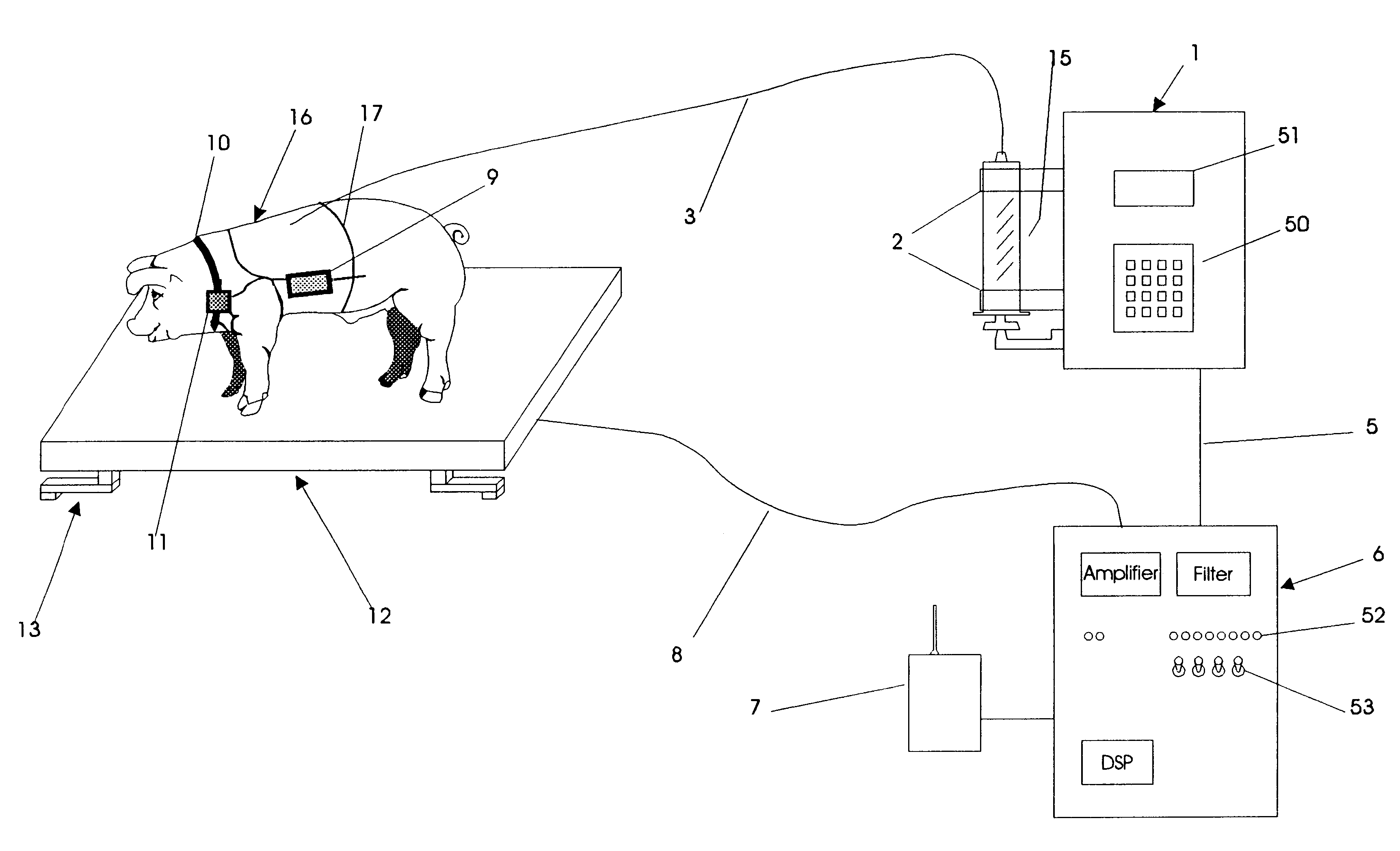
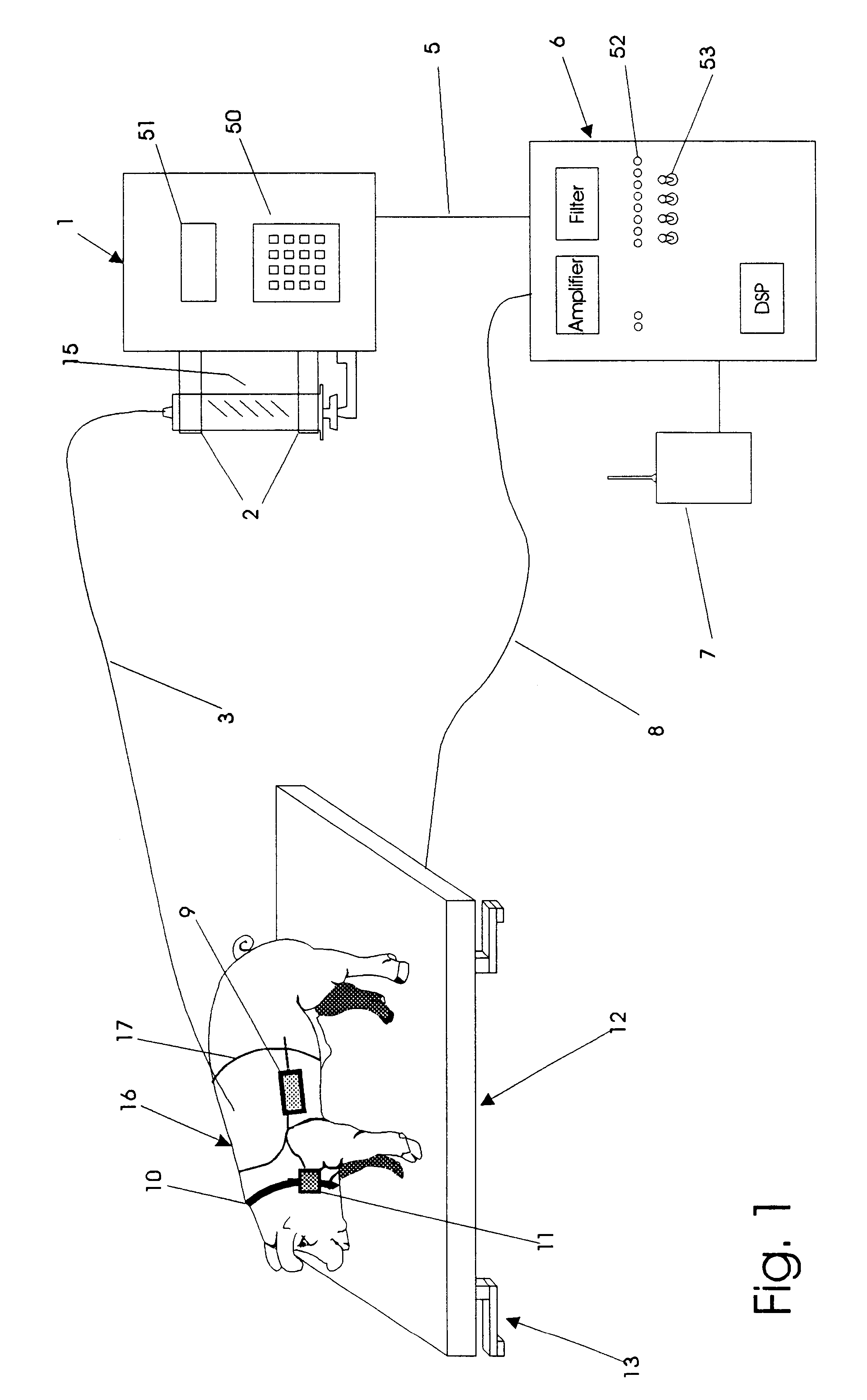
An apparatus for the automatic delivery to an animal of a beneficial agent such as analgesia by infusion with the pain of the animal being taken into account when determining the rate of dispensing the agent. As the pain experienced by an animal and therefore its distress is manifested in increased agitation, the apparatus includes elements for the measurement of the frequency and amplitude of an animal's movement and the generation of a trigger signal when the either the frequency or the amplitude exceeds a predetermined threshold. The trigger can be used to generate a visual or audible alarm. The apparatus also preferably includes a dispensing means for delivering doses of beneficial agent to the animal in response to the trigger signal. The apparatus serves primarily to reduce the post-operative distress experienced by an animal by providing for timely administration of analgesic compounds. The apparatus also aids the animal care-giver by reducing the amount of time spent assessing the animal and administering beneficial compounds.
Owner:SALASOFT
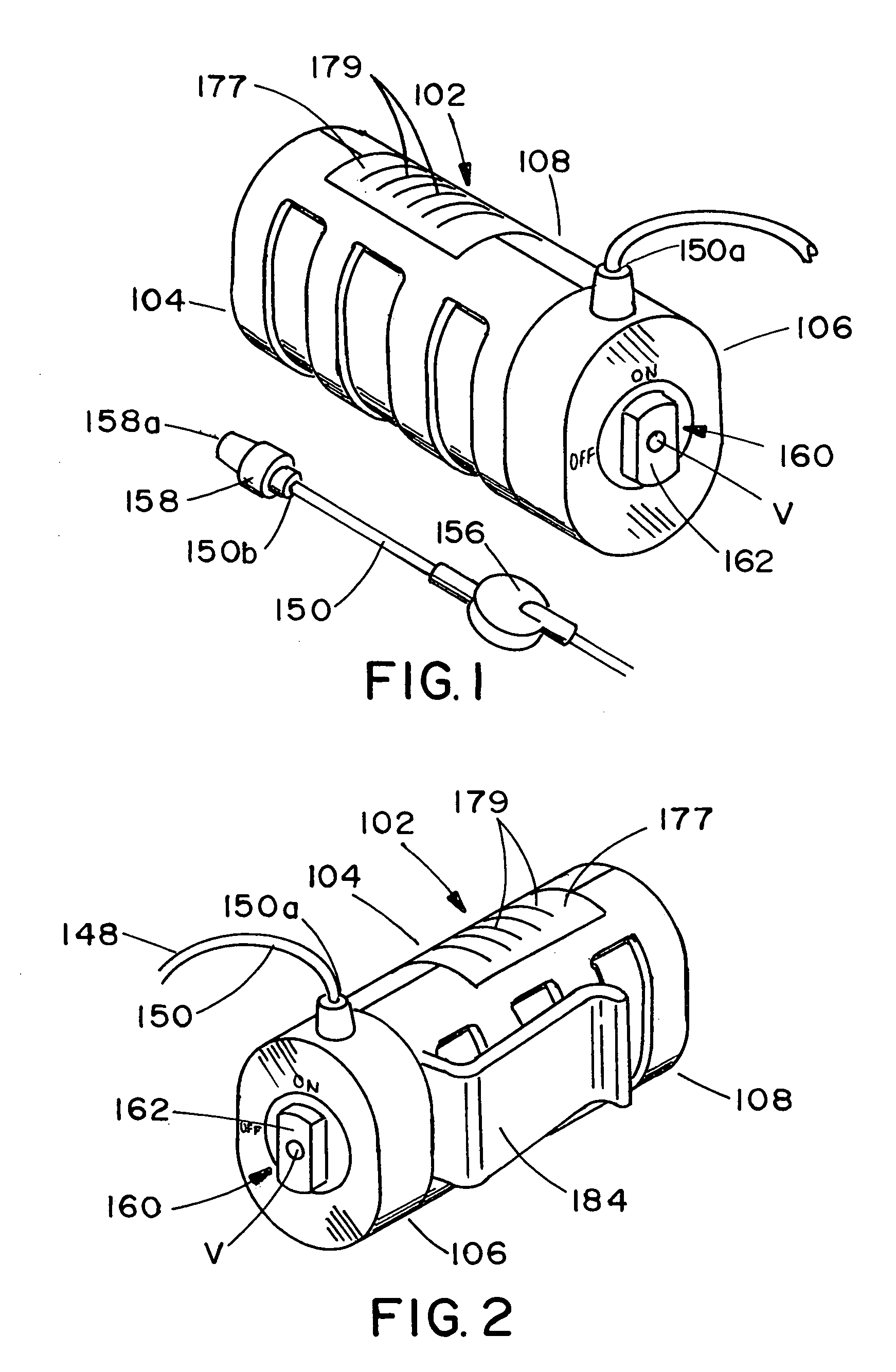
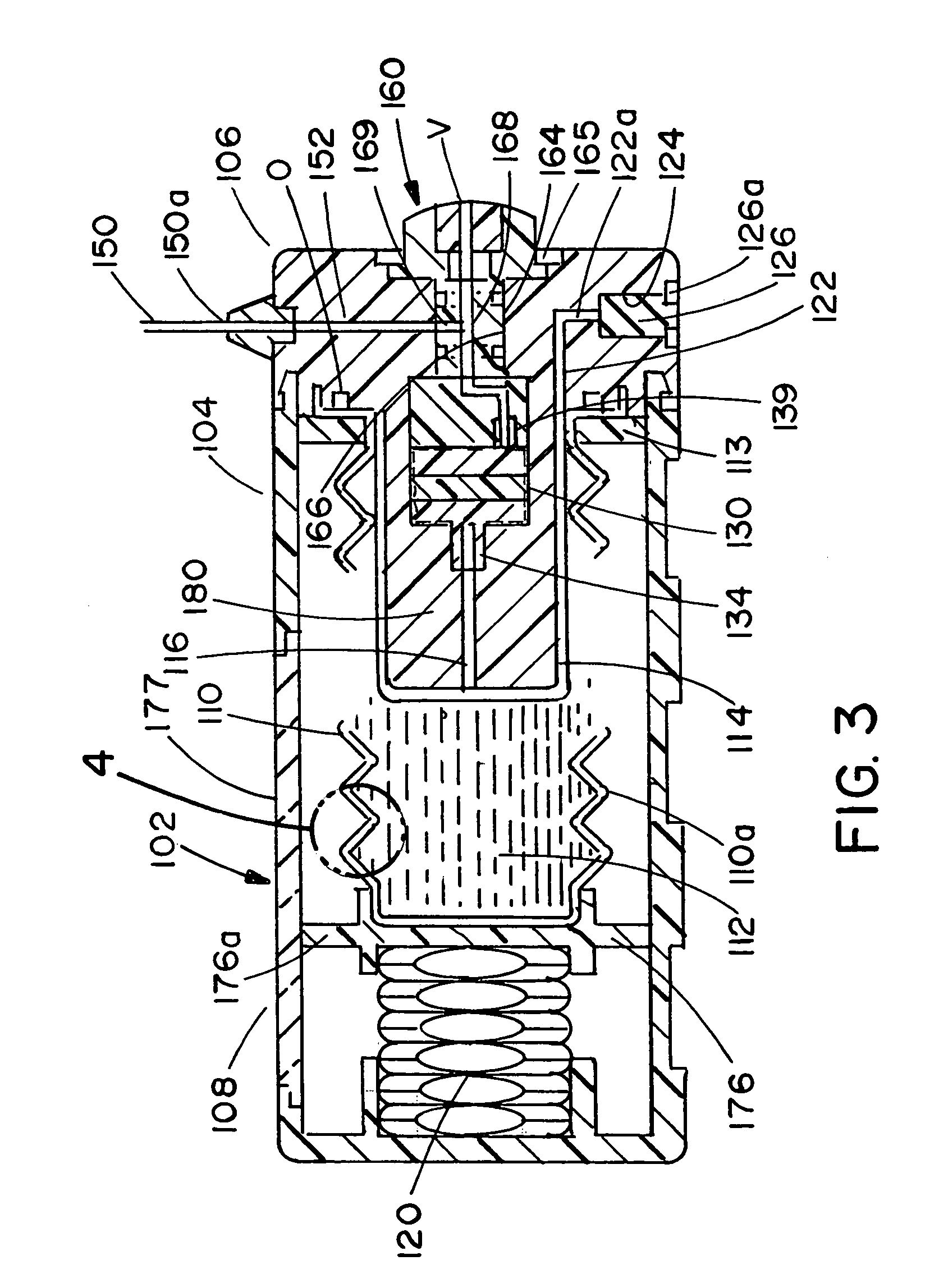
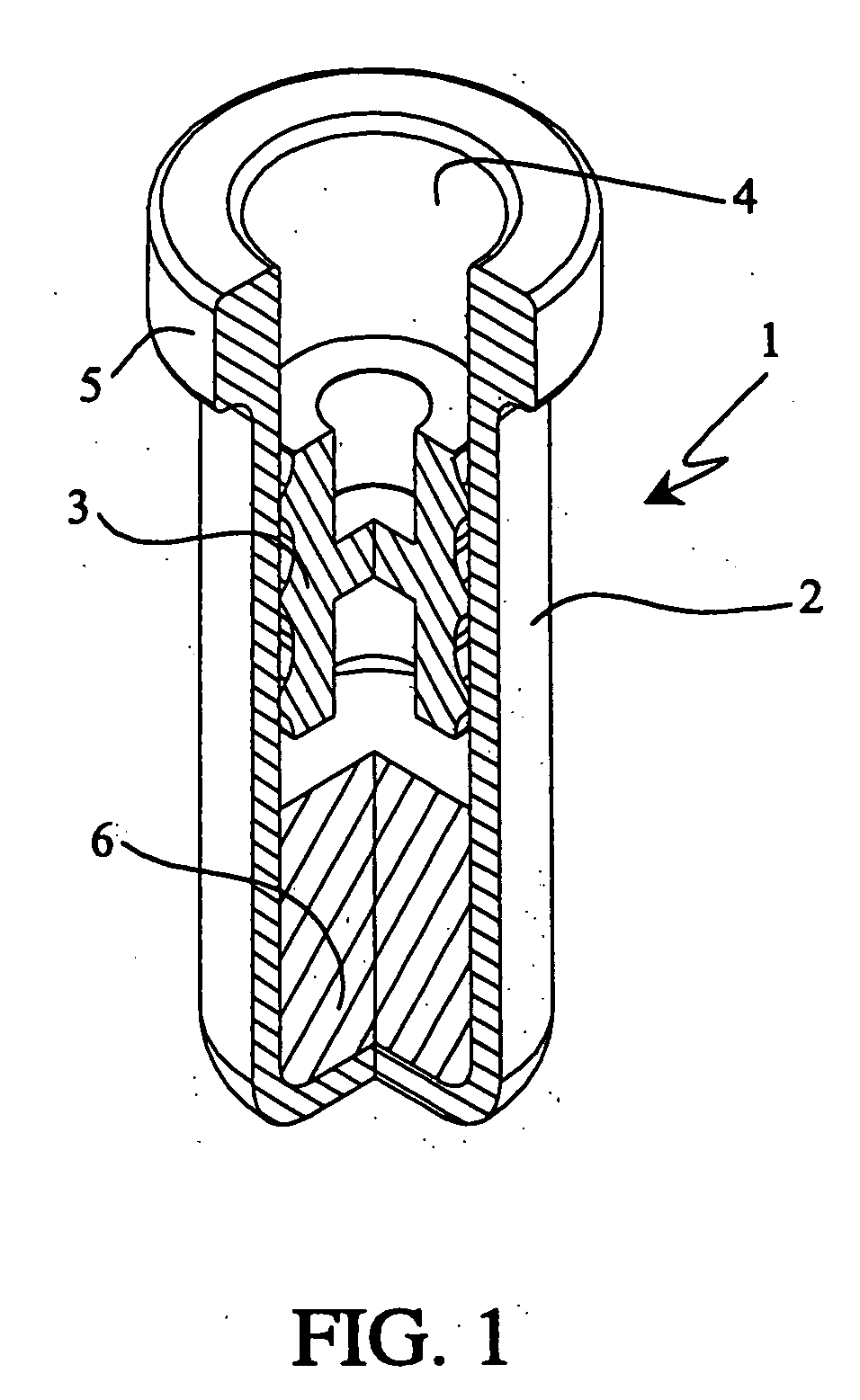
Infusion apparatus
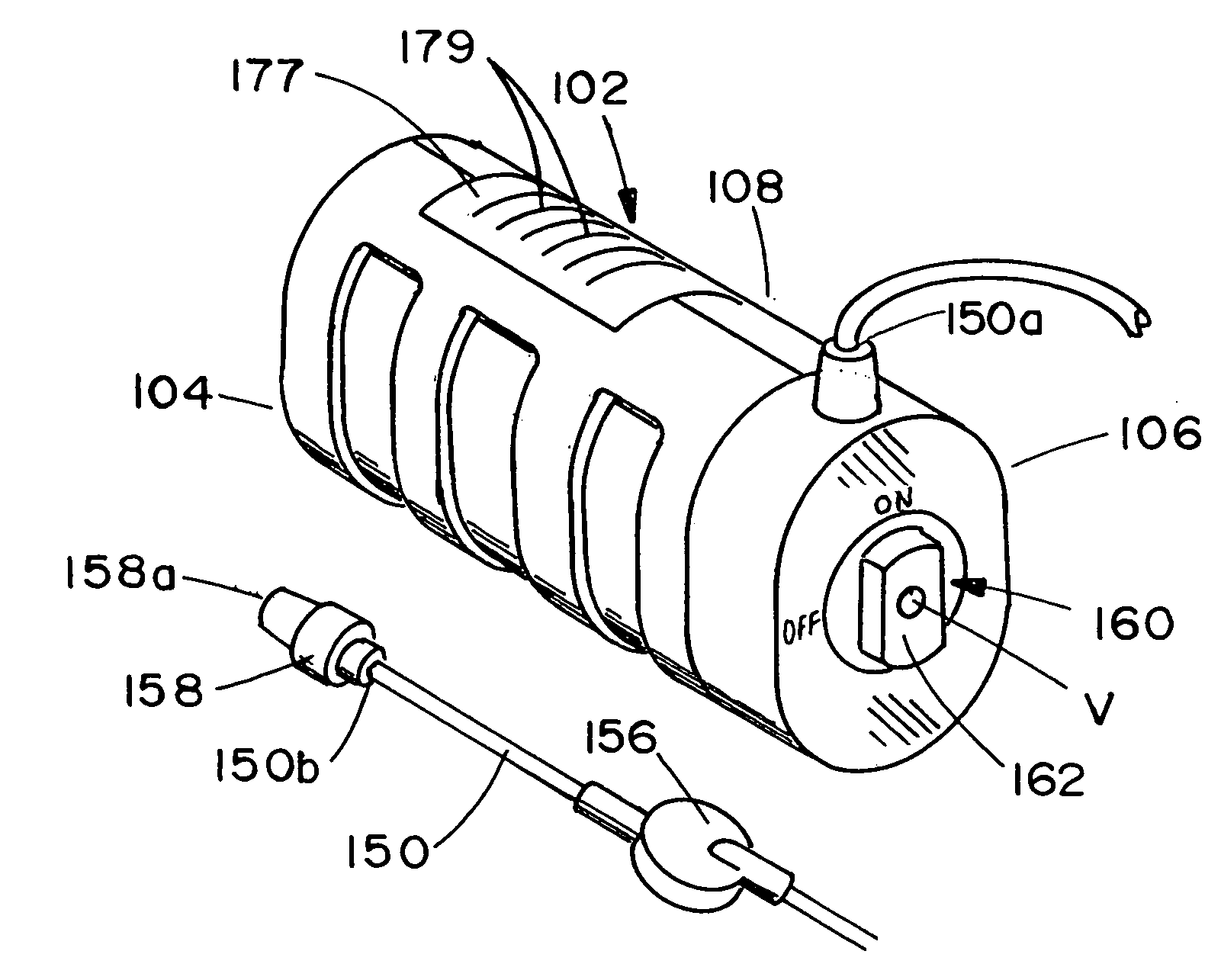
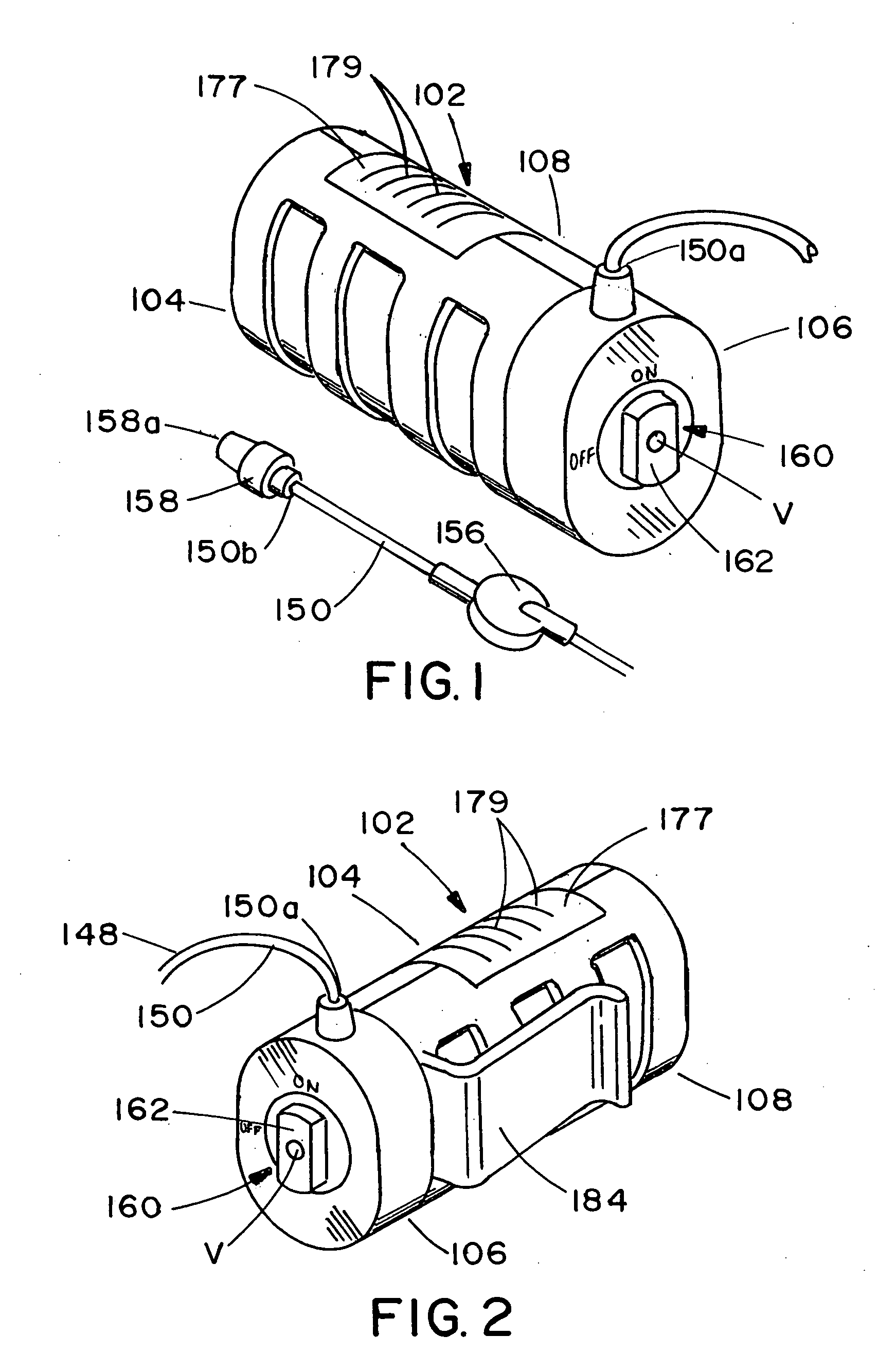
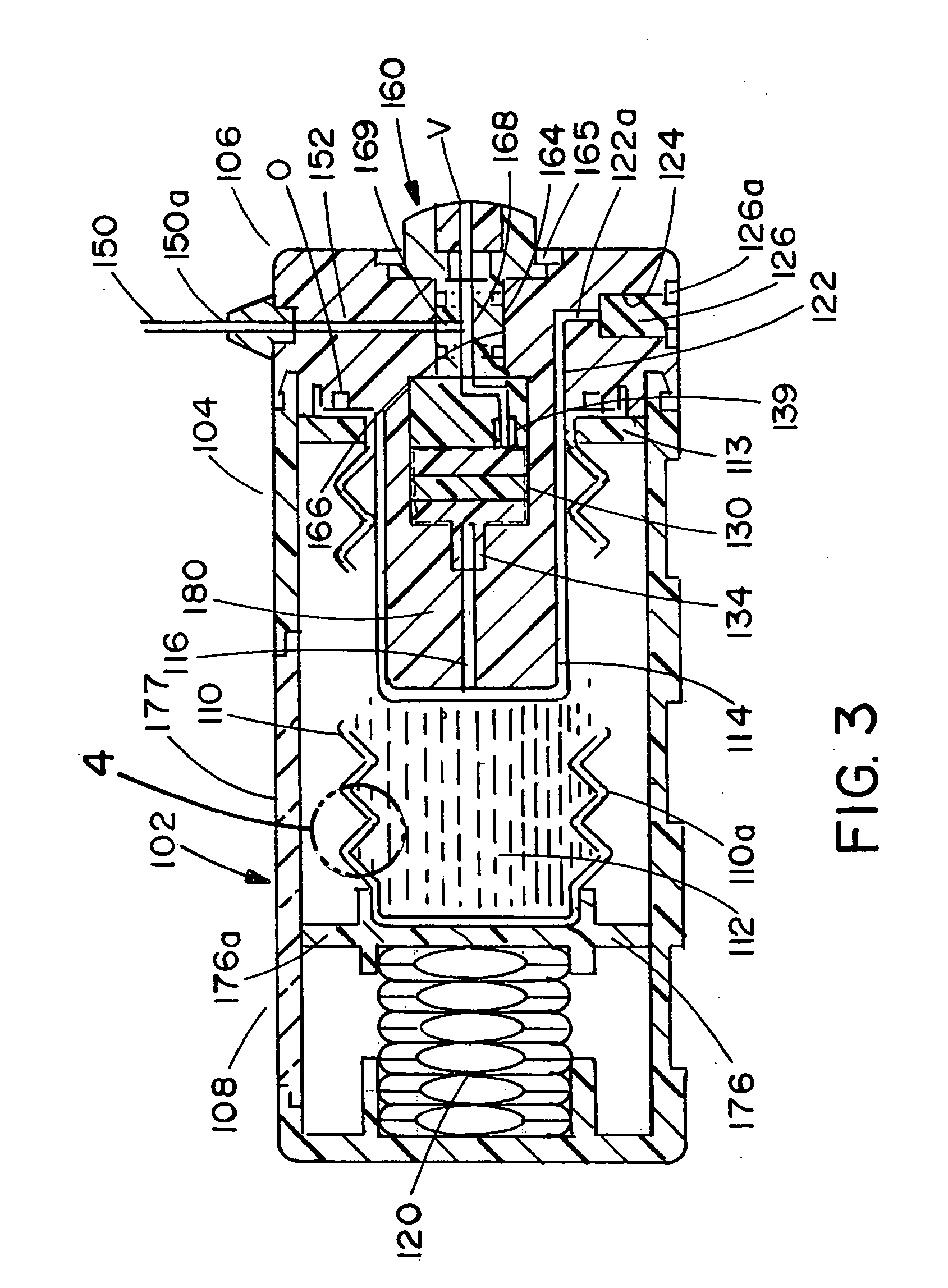
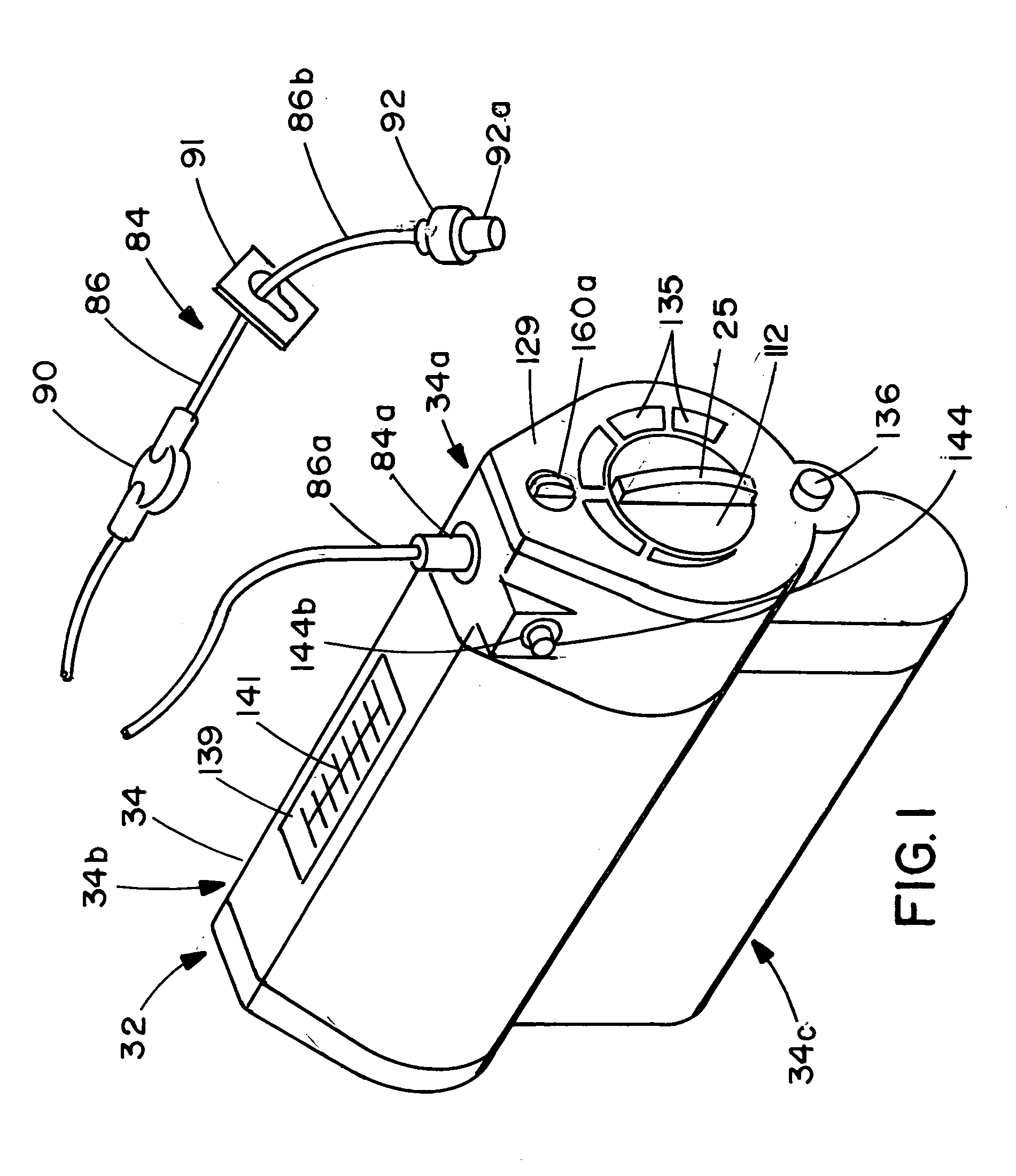
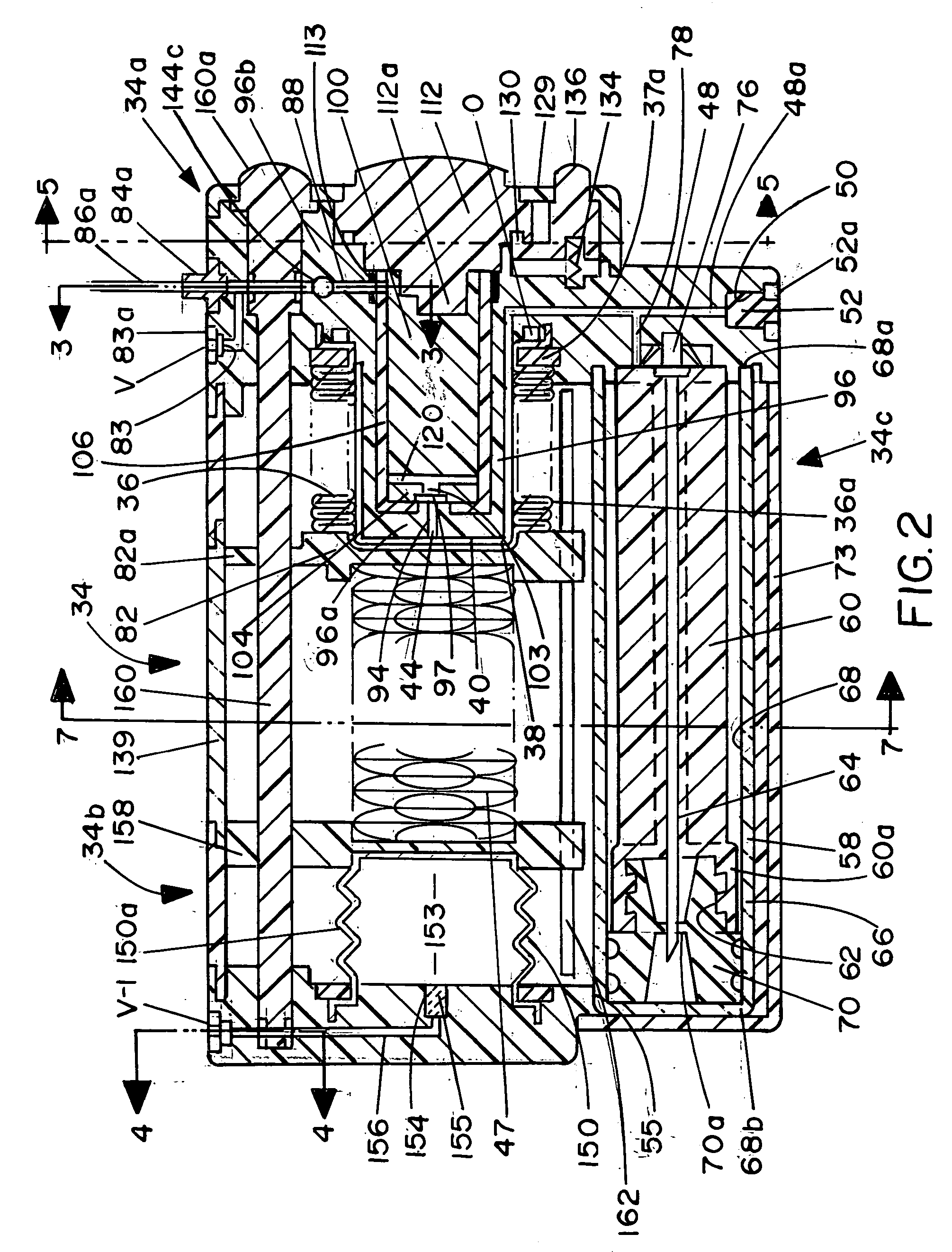
ActiveUS20050277882A1Easy to useEasy constructionAmpoule syringesAutomatic syringesDrugs solutionStored energy
A compact fluid dispenser for use in controllably dispensing fluid medicaments, such as, antibiotics, oncolytics, hormones, steroids, blood clotting agents, analgesics, and like medicinal agents from prefilled containers at a uniform rate. The dispenser uniquely includes a stored energy source that is provided in the form of a substantially constant-force, compressible-expandable wave spring that provides the force necessary to continuously and uniformly expel fluid from the device reservoir. The device further includes a fluid flow control assembly that precisely controls the flow of medicament solution to the patient.
Owner:MARSHALL S KRIESEL REVOCABLE TRUST
Pharmaceutical compositions
Methods and compositions are provided which comprise effective amounts of analgesic to treat a subject, including reducing or eliminating an adverse effect associated with the analgesic.
Owner:LOCL PHARMA
Infusion apparatus
A compact fluid dispenser for use in controllably dispensing fluid medicaments, such as, antibiotics, oncolytics, hormones, steroids, blood clotting agents, analgesics, and like medicinal agents from prefilled containers at a uniform rate. The dispenser uniquely includes a stored energy source that is provided in the form of a substantially constant-force, compressible-expandable wave spring that provides the force necessary to continuously and uniformly expel fluid from the device reservoir. The device further includes a fluid flow control assembly that precisely controls the flow of medicament solution to the patient.
Owner:MARSHALL S KRIESEL REVOCABLE TRUST
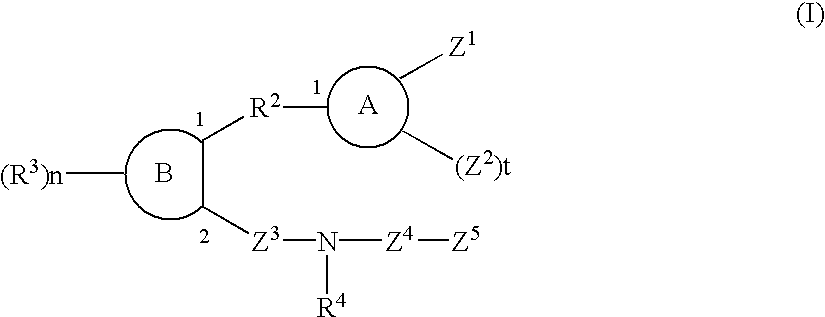
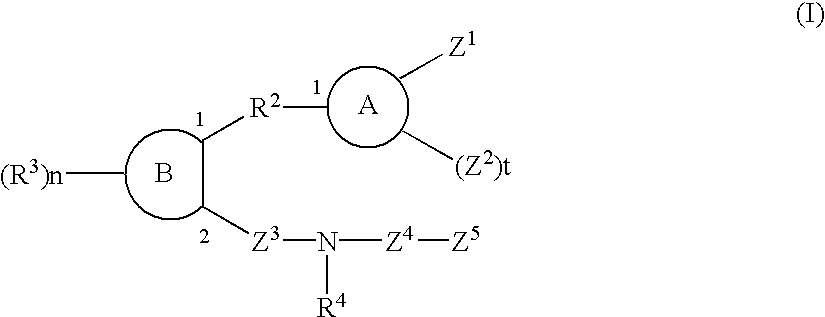
Sulfonamide and carboxamide derivatives and drugs containing the same as the active ingredient
InactiveUS6448290B1Low toxicityEasy to useBiocideOrganic chemistryBULK ACTIVE INGREDIENTAnalgesic agents
The sulfonamide or carboamide derivatives of the formula (I) and a pharmaceutical composition which comprise them as an active ingredient:(wherein A ring, B ring is carbocyclic ring, heterocyclic ring; Z1 is -COR1, -CH=CH-COR1 etc.; Z2 is H, alkyl etc.; Z3 is single bond, alkylene; Z4 is SO2, CO; Z5 is alkyl, phenyl, heterocyclic ring etc.; R2 is CONR8, O, S, NZ6, Z7-alkylene, alkylene etc.; R3 is H, alkyl, halogen, CF3 etc.; R4 is H, (substituted) alkyl etc.; n, t is 1-4).The compounds of the formula (I) can bind to receptors of PGE2 and show antagonistic activity against the action thereof or agonistic activity. Therefore, they are considered to be useful as medicine for inhibition of uterine contraction, analgesics, antidiarrheals, sleep inducers, medicine for increase of vesical capacity or medicine for uterine contraction, cathartic, suppression of gastric acid secretion, antihypertensive or diuretic agents.
Owner:ONO PHARMA CO LTD
Apparatus and method for human algometry
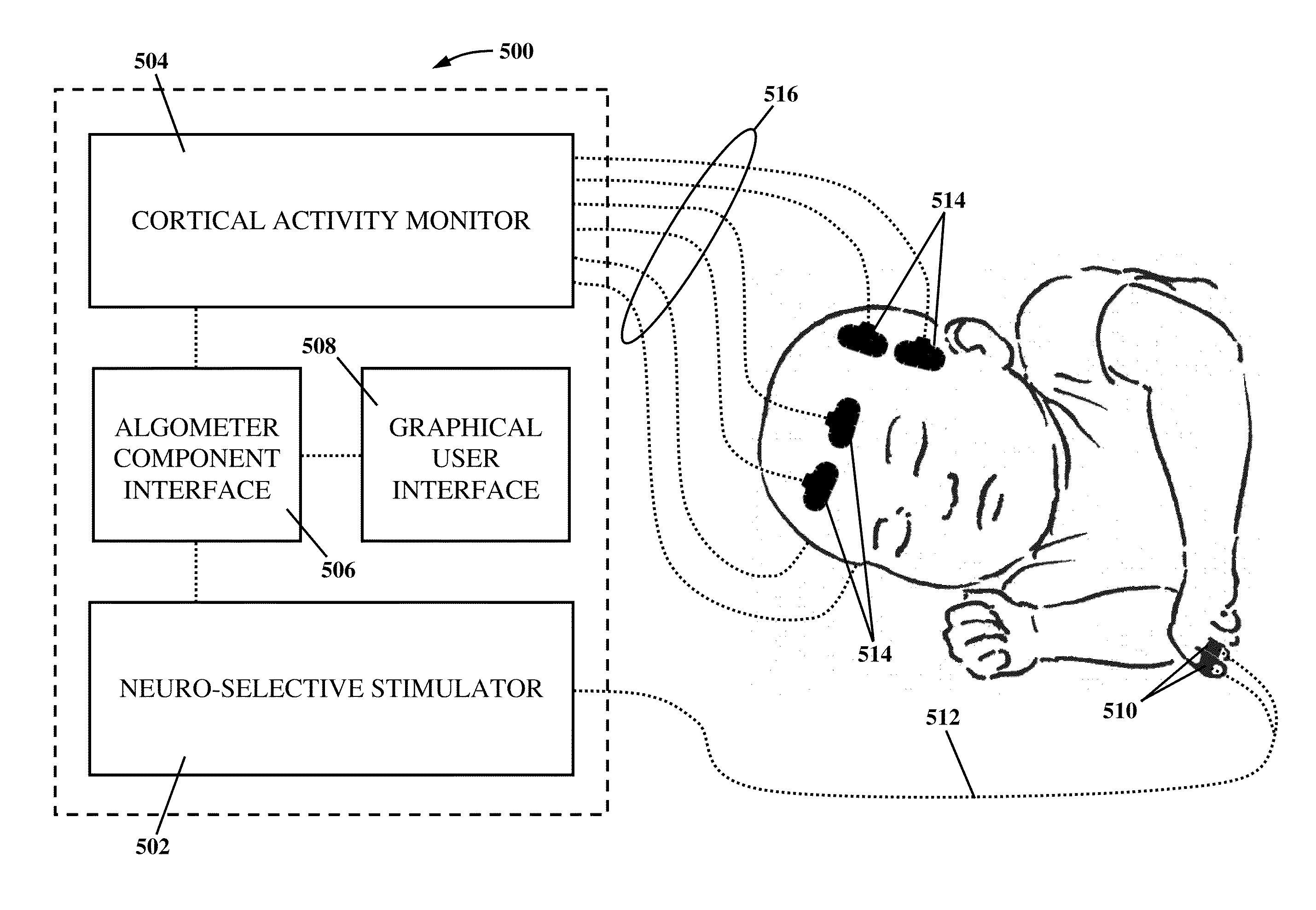
An apparatus and method for performing human algometry are disclosed. They include a stimulator configured to apply electrical stimulation of variable intensity to an area of a patient's body, a monitoring device configured to measure a. level of cortical activity in one or more regions of the patient's brain, and a microprocessor connected to the stimulator and the monitoring device that is configured to correlate the intensity of the electrical stimulation with the level of activity in the one or more regions of the patient's brain and to determine at least one of a measurement of pain intensity, a measurement of a sensory detection threshold (SDT), a measurement of a drug's analgesic impact, an indication of an onset of tolerance to a drug, an indication of an onset of analgesic-induced hyperalgesia, an indication of conditions of allodynia, a measurement of dose-response characteristics of pain management drugs, and a characterization of a pain condition.
Owner:CHILDRENS NAT MEDICAL CENT
Nerve cell protective agents
PCT No. PCT / JP97 / 01828 Sec. 371 Date Jan. 30, 1998 Sec. 102(e) Date Jan. 30, 1998 PCT Filed May 29, 1997 PCT Pub. No. WO97 / 45410 PCT Pub. Date Dec. 4, 1997The invention provides novel benzindole derivatives, processes for producing them, as well as a neuroprotective agent, an agent to prevent or treat diseases involving the degeneration, retraction or death of neurons, and an analgesic, each containing the benzindole derivatives as an active ingredient.
Owner:MOCHIDA PHARM CO LTD
Infusion apparatus with modulated flow control
InactiveUS20050033232A1Easy to useEasy constructionFlexible member pumpsMedical devicesStored energyConstant force
A compact fluid dispenser for use in controllably dispensing fluid medicaments, such as antibiotics, oncolytics, hormones, steroids, blood clotting agents, analgesics, and like medicinal agents from prefilled containers at a uniform rate. The dispenser uniquely includes a stored energy source that is provided in the form of a substantially constant-force, compressible-expandable wave spring that provides the force necessary to continuously and uniformly expel fluid from the device reservoir. The device further includes a fluid flow control assembly that precisely controls the flow of medicament solution to the patient. Additionally, the device includes a novel modulating assembly for controllably modulating the force exerted by the wave spring tending to expel the fluid from the device reservoir.
Owner:BIOQUIDDITY
Programmable multi-dose intranasal drug delivery service
InactiveUS20060021614A1InterestingEasily interfaceRespiratorsLiquid surface applicatorsMedicineNasal spray
An apparatus and method for the self-administration of a plurality of doses of an intranasal liquid pharmaceutical composition, including opioid analgesics, that includes a drug delivery device containing a plurality of sealed vials, each vial containing a predetermined volume of the pharmaceutical composition, a pump assembly for conveying the liquid pharmaceutical composition from the interior of the vial and discharging it as a nasal spray in response to manual activation by the patient, and programmable means for sequentially advancing a vial to the ready position after passage of a prescribed time interval following the last activation of the delivery device.
Owner:UNIV OF KENTUCKY RES FOUND
Orally administrable opioid formulations having extended duration of effect
InactiveUS20020081333A1Effective steady-state blood levelPowder deliveryBiocideBlood levelOral medication
Owner:PURDUE PHARMA LP
Methods and compositions for treating distress dysfunction and enhancing safety and efficacy of specific medications
InactiveUS20110159048A1Good treatment effectEliminate side effectsBiocideNervous disorderDiseaseNeurotransmitter systems
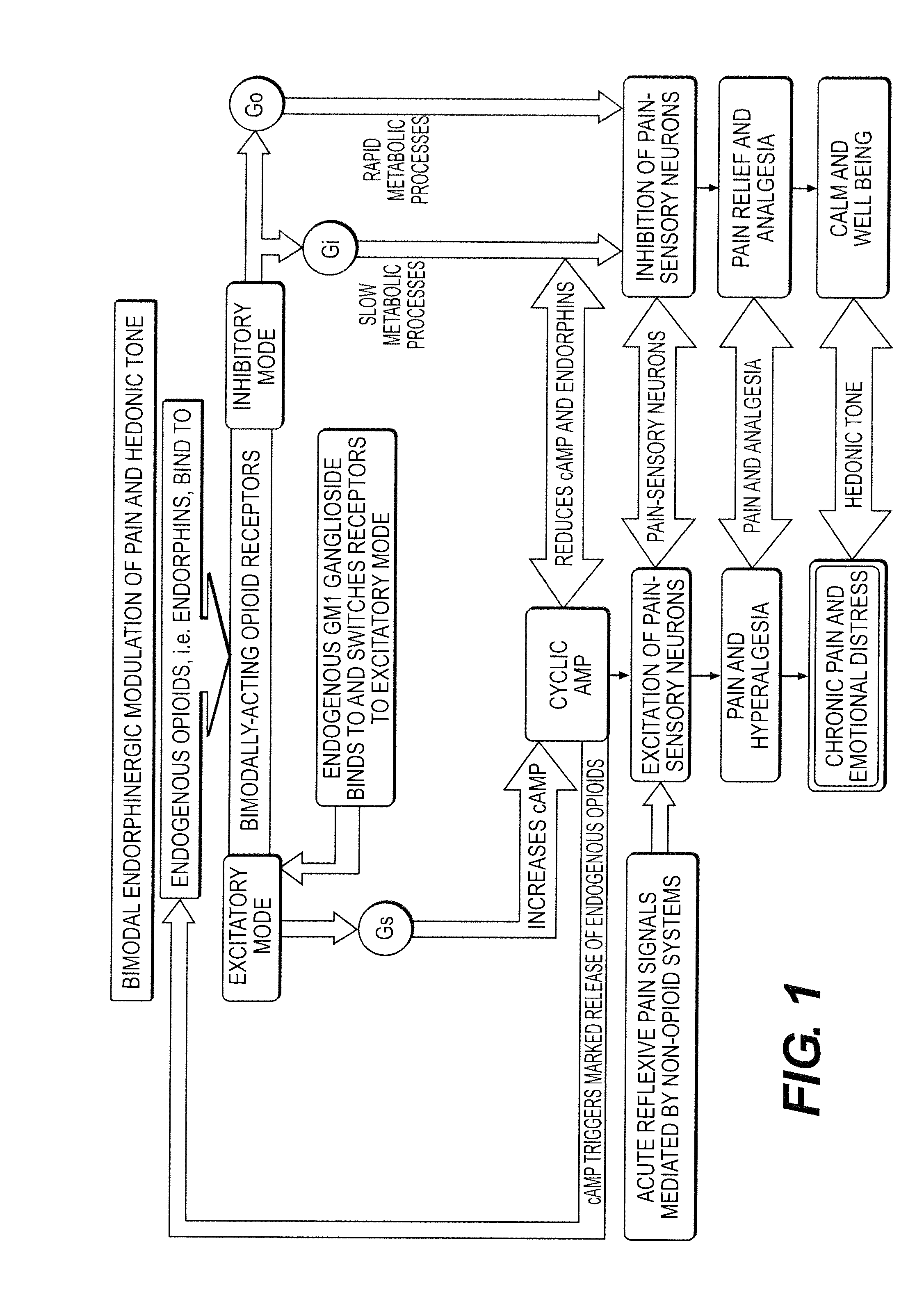
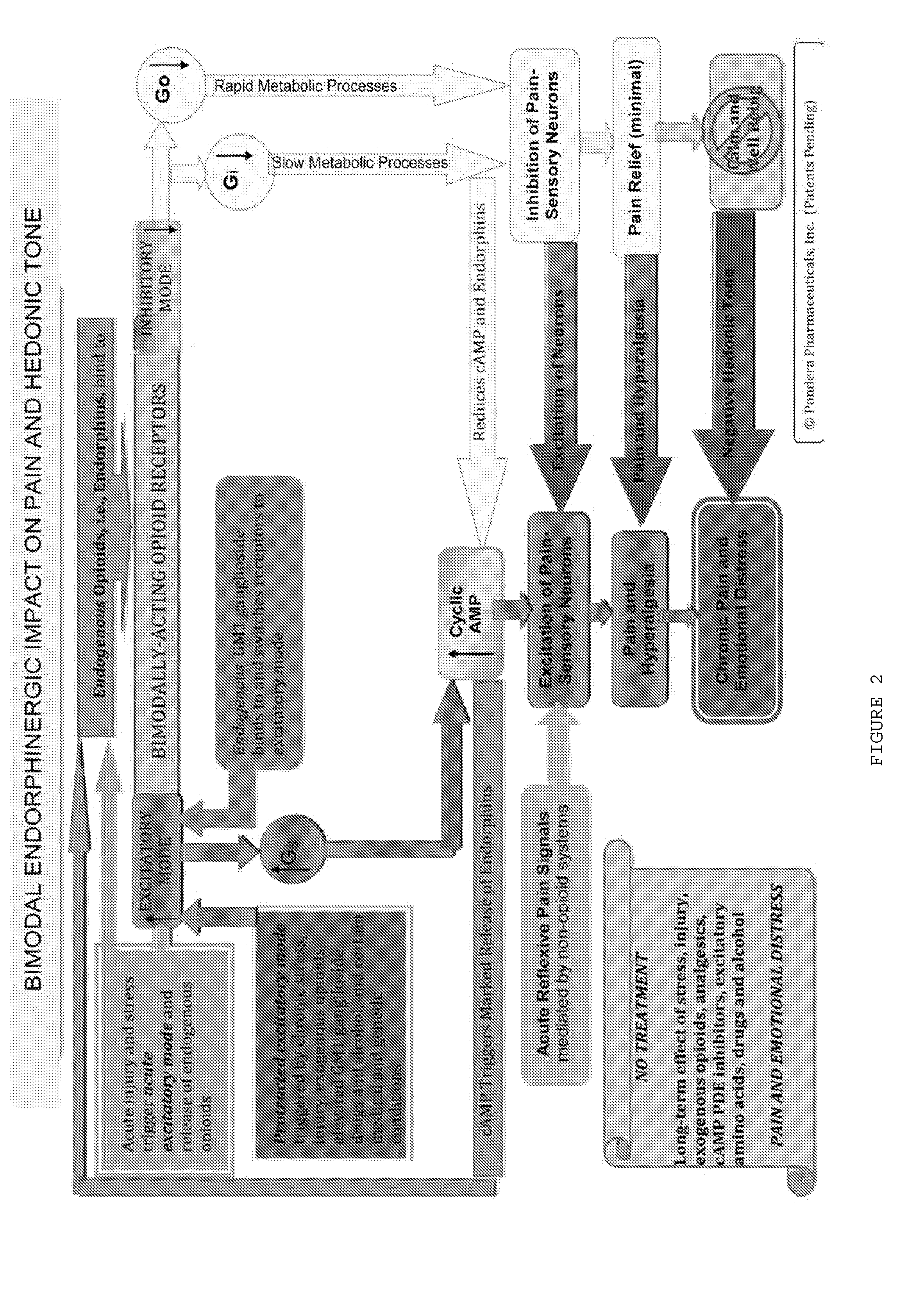
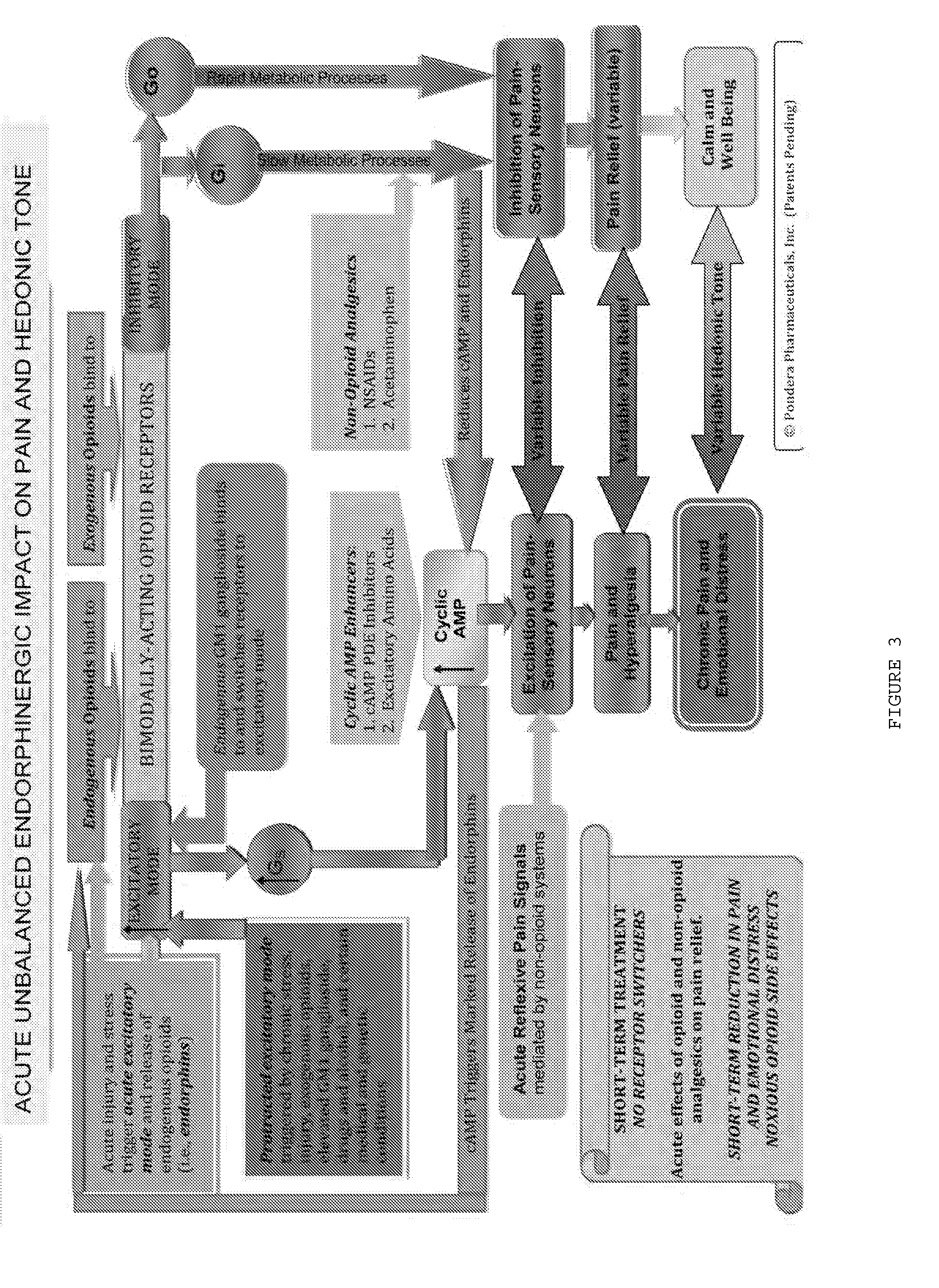
The present invention relates to methods and compositions for reducing Distress Dysfunction by restoring and maintaining homeostatic balance in the neurotransmitter systems underlying the Stress Response and the experience of distress and hedonic tone. Distress Dysfunction refers to the experience of dysfunctional emotional and physical distress that interferes with the individual's quality of life and functioning. A novel understanding of the bimodal opioid modulation of pain, and its impact, through serotonergic, dopaminergic, epinephrinergic, and norepinephrinergic processes, on hedonic tone, leads directly to new generation pharmaceutical formulations that are remarkably safe and effective for the treatment of a wide variety of Distress Dysfunctions, including anxiety, depression, anger, insomnia, mood disorders, eating disorders, sexual problems, pain, substance and behavioral addictions, gastrointestinal disorders, autistic spectrum disorders, attention-deficit and hyperactivity disorders, and other emotional and physical distress disorders. The foundation of this discovery is the power of Receptor Switchers, such as ultra-low-dose and very-low-dose opioid antagonists and GM1 ganglioside attenuators, in blocking acute and protracted excitatory opioid receptor signaling. Co-administration of Receptor Switchers with Endorphin Enhancers, such as specific cAMP PDE inhibitors and excitatory amino acids, is an excellent formulation for restoring healthy homeostatic balance to the endogenous opioid system, using the body's endorphins to reduce emotional and physical distress, and through synergistic and homeostatic processes, restoring positive hedonic tone. The addition of Synergistic Enhancers, such as amino acids, SSRI and SNRI agents, and non-opioid analgesics, as well as Exogenous Opioids, enhances and prolongs these therapeutic benefits. The novel principles discovered by this invention also teach a new generation of safe and effective formulations for the treatment of respiratory conditions, neuropathy, and nociceptive pain.
Owner:PONDERA BIOTECH
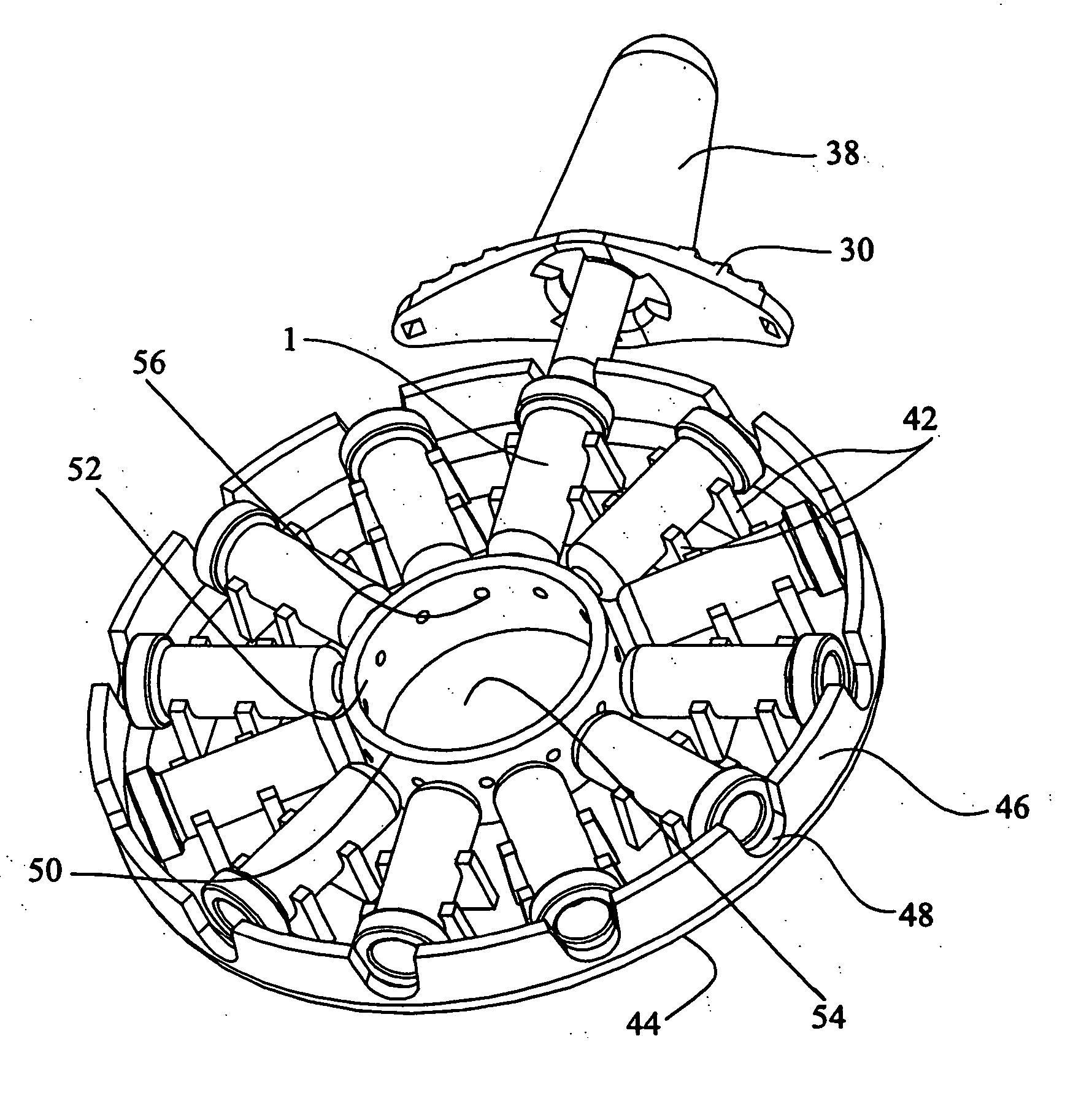
Multichannel fluid delivery device
ActiveUS7169128B2Uniform ratePharmaceutical delivery mechanismMedical devicesStored energyAntibiotic Y
A compact fluid dispenser for use in controllably dispensing fluid medicaments, such as antibiotics, oncolytics, hormones, steroids, blood clotting agents, analgesics, and like medicinal agents from a plurality of prefilled containers at a uniform rate. The dispenser uniquely includes a plurality of stored energy source that are provided in the form of compressible-expandable members of novel construction that provide the force necessary to continuously and uniformly expel fluid from a plurality of device reservoirs. The apparatus further includes a plurality of fluid flow control assemblies that precisely control the flow of the medicament solutions from the plurality of device reservoirs to the patient.
Owner:BIOQ PHARMA
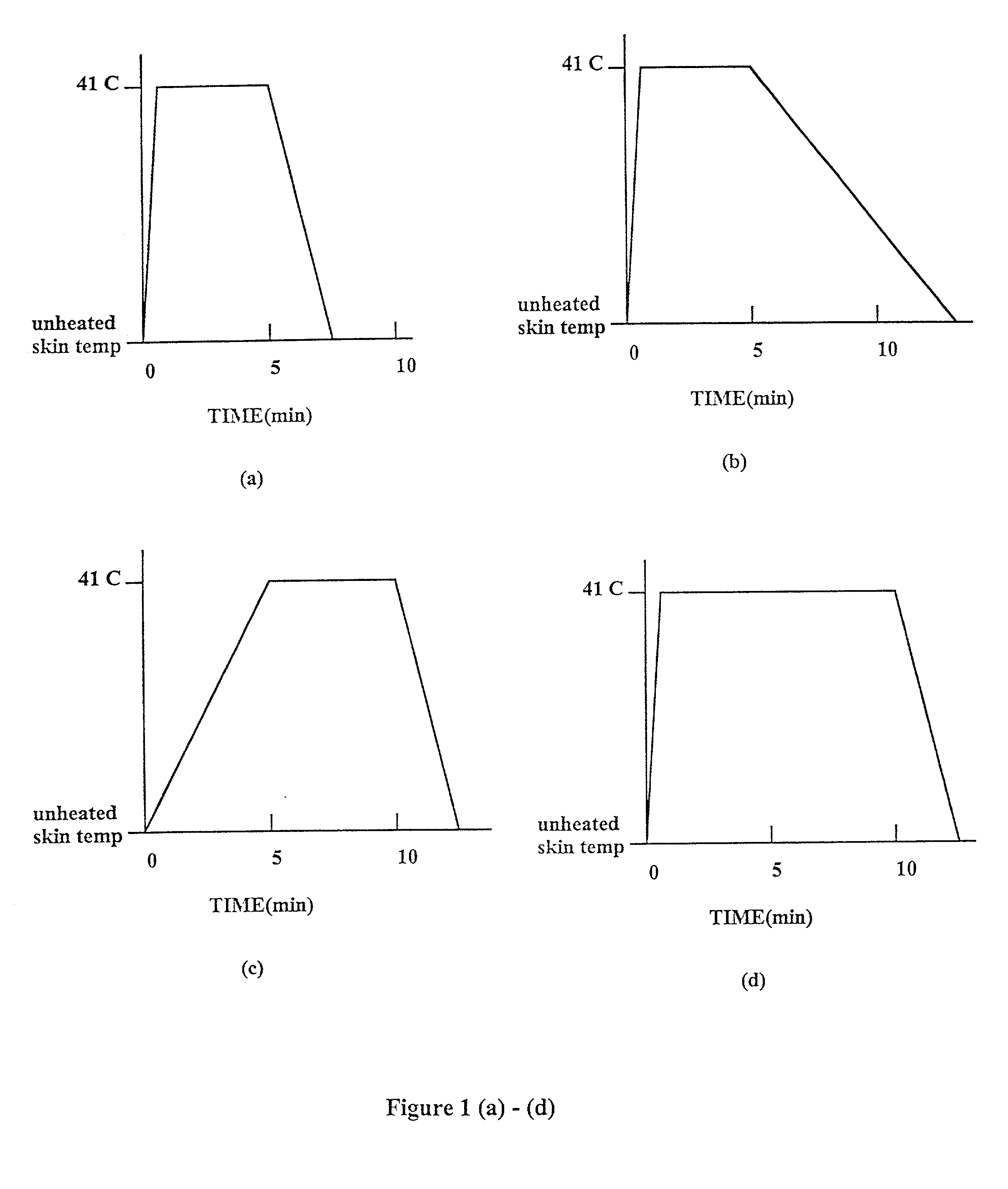
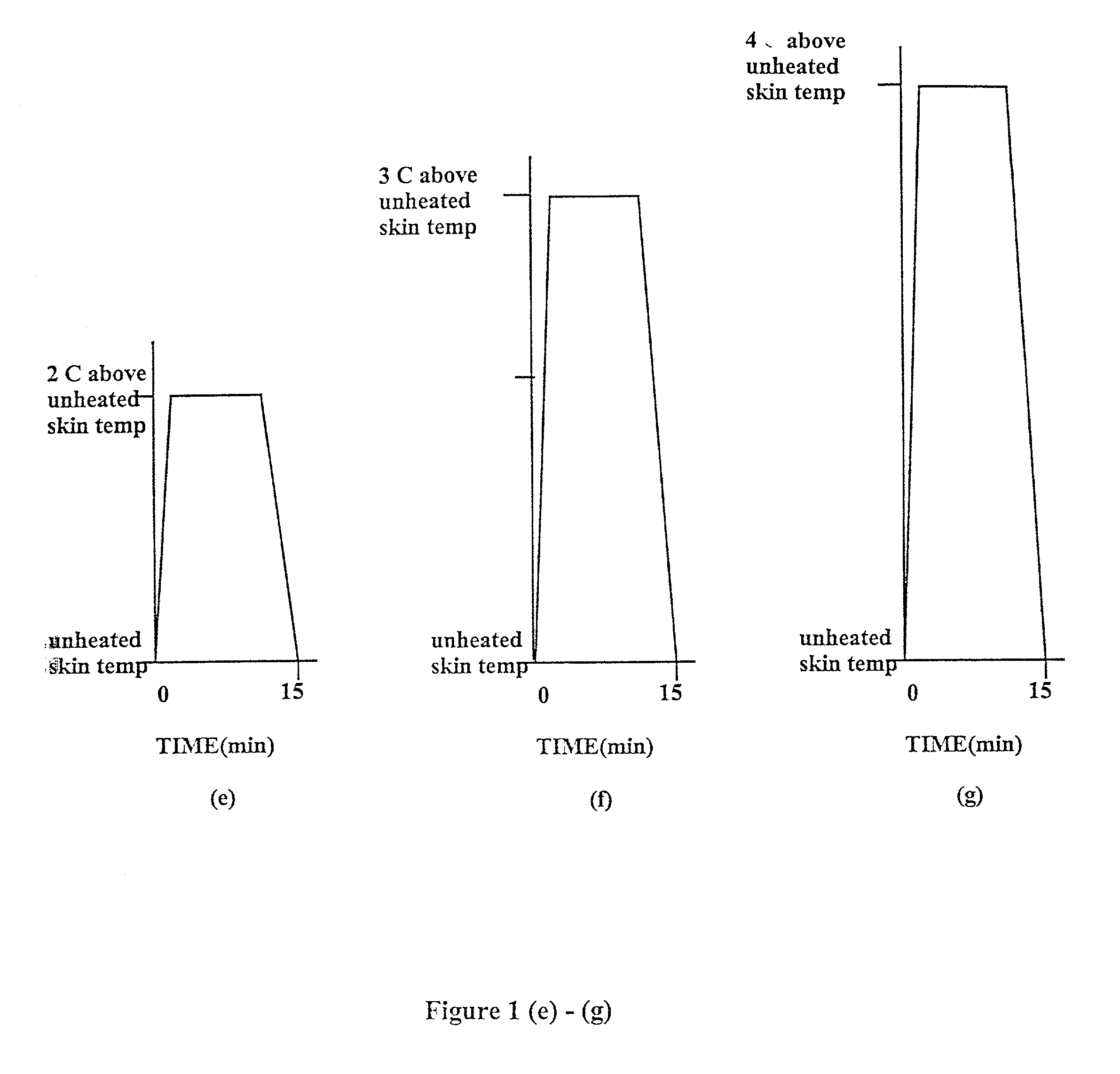
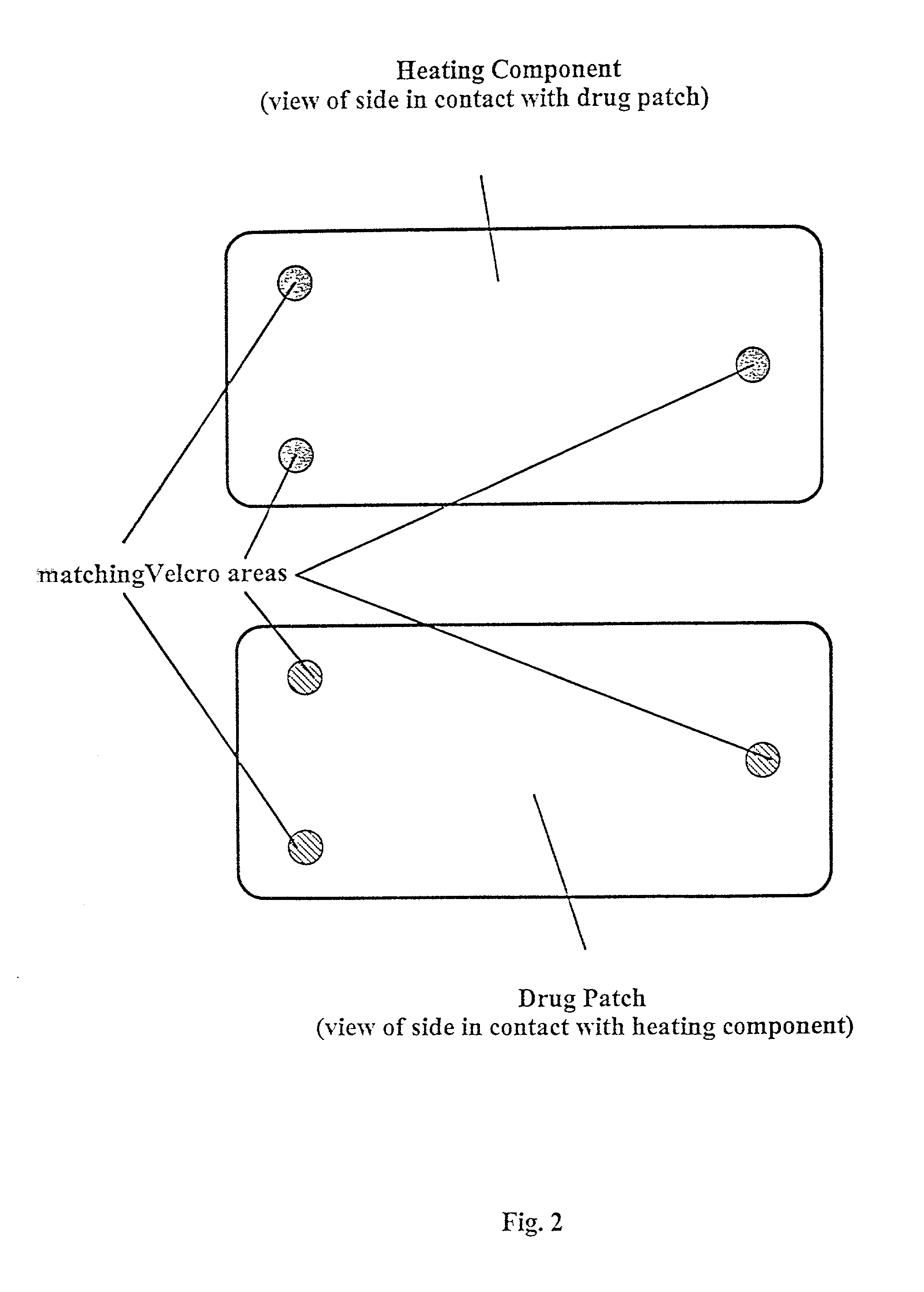
Methods and apparatus for using controlled heat to regulate transdermal and controlled release delivery of fentanyl, other analgesics, and other medical substances
InactiveUS20010037104A1Increase blood flowDrive fastCosmetic preparationsToilet preparationsControlled releaseMedicine
The present invention is directed toward an apparatus to rapidly deliver a drug to a patient The invention comprises a drug beneath a patient's skin in a drug depot site. A heating component is placed near the drug depot site and generates heat in and near the drug depot site. A control component connected to the heating component is used to control the magnitude and duration of heat generated by the heating component.
Owner:CRESCITA THERAPEUTICS INC
Pharmaceutical compositions
ActiveUS20110262539A1Fast dissolutionTreating and preventing painNervous disorderAntipyreticAdverse effectComposition methods
Methods and compositions are provided which comprise effective amounts of analgesic to treat a subject, including reducing or eliminating an adverse effect associated with the analgesic.
Owner:LOCL PHARMA
Sustained release opioid formulations and methods of use
The invention combines two different subunits with different release profiles in novel sustained-release oral dosage forms. In particular, the oral dosage forms include a subunit that comprises an opioid analgesic and a sustained-release material, wherein the dissolution rate in-vitro of the subunit, when measured by the standard USP Drug Release test of U.S. Pharmacopeia XXVI (2003) <724>, is less than about 10% within about 6 hours and at least about 60% within about 24 hours; less than about 10% within about 8 hours and at least about 60% within about 24 hours; or less than about 10% within about 12 hours and at least about 60% within about 24 hours; the dosage form providing a duration of therapeutic effect of about 24 hours.
Owner:ALPHARMA PHARMA
Phthalimide derivatives of non-steroidal Anti-inflammatory compounds and/or tnf-alpha modulators, method for producing same, pharmaceutical compositions containing same and uses thereof for the treatment of inflammatory diseases
InactiveUS20120115817A1Inhibit inflammationMinimizing major limitation and complicationBiocideMonoazo dyesNon steroidal anti inflammatoryRheumatoid arthritis
The present invention relates to phthalimide derivatives of non-steroidal and / or TNF-α modulating anti-inflammatory compounds as well as the process of obtaining the so-called derivatives, pharmaceutical compositions containing such derivatives and their uses, including use in the treatment of inflammatory diseases, especially those related to chronic inflammatory processes, such as rheumatoid arthritis and intestinal inflammatory diseases (for instance, Chron's disease) and the use of the referred to pharmaceutical compositions as antipyretic, analgesic and platelet antiaggregating medications.
Owner:EMS +1
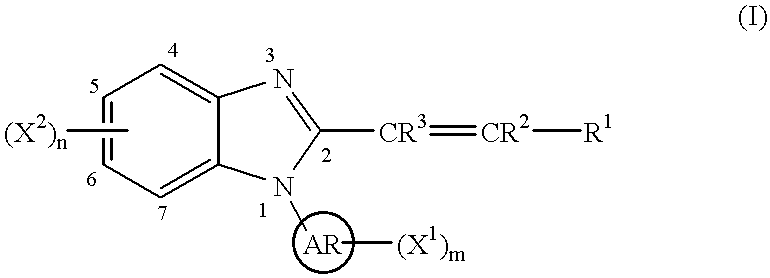
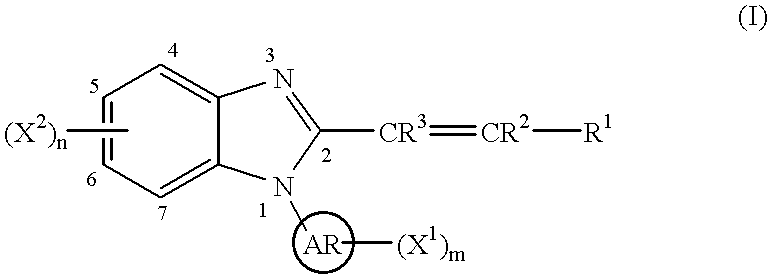
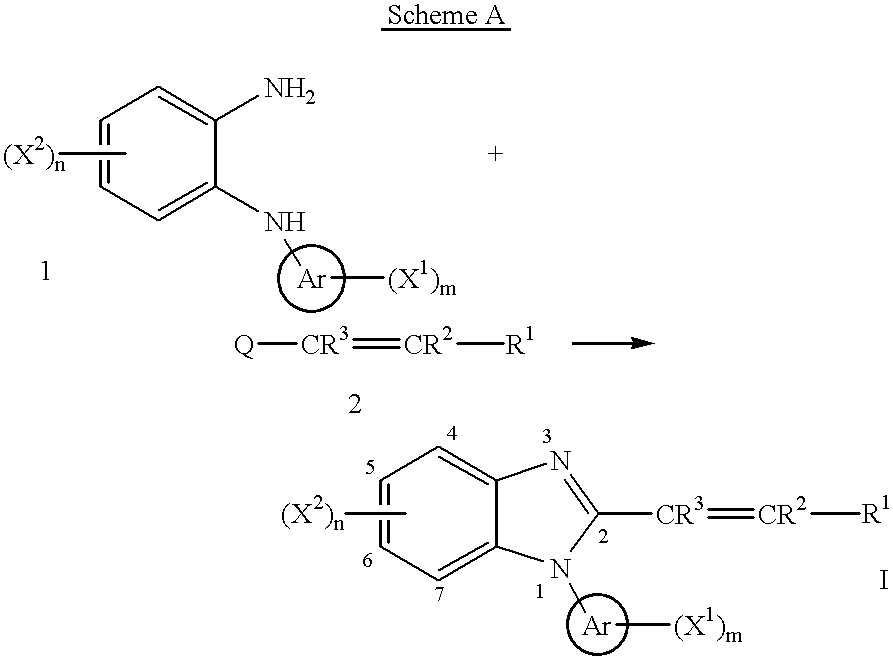
Benzimidazole cyclooxygenase-2 inhibitors
This invention provides a compound of the following formula:or the pharmaceutically acceptable salts thereof, whereinAr is heteroaryl; X1 and X2 are independently selected from halo, C1-C4 alkyl, hydroxy, C1-C4 alkoxy, amino, C1-C4 alkanoyl, carboxy, carbamoyl, cyano, nitro, mercapto, (C1-C4 alkyl)thio, (C1-C4 alkyl)sulfinyl, (C1-C4 alkyl)sulfonyl, aminosulfonyl, or the like; R1 is selected from hydrogen, straight or branched C1-C4 alkyl, C3-C8 cycloalkyl, C4-C8 cycloalkenyl, phenyl , heteroaryl and the like; R2 and R3 are independently selected from hydrogen, halo, C1-C4 alkyl, phenyl and the like; or R1 and R2 can form, together with the carbon atom to which they are attached, a C5-C7 cycloalkyl ring; and m and n are independently 0, 1, 2 or 3.These compounds and pharmaceutical compositions containing such compounds are useful as analgesics and anti-inflammatory agents.
Owner:PFIZER INC
Dimethicone-containing sustained release injection formulation
InactiveUS20070053943A1Prevention and treatment of problemBiocidePharmaceutical delivery mechanismDrugAnalgesic agents
A sustained release formulation by using dimeticone as the dispersion medium, which includes active ingredient (e.g., drugs against parasites, insecticides, NSAIDs, antibiotics, sex hormone like agents or oily soluble vitamins) and dimeticone as the medium. Suitable stabilizer, antioxidant, local analgesics and material for sustained release may be added. The formulation is bio-compatible, stable and injectable.
Owner:WANG YUWAN +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com