Patents
Literature
2890 results about "Bitter taste" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
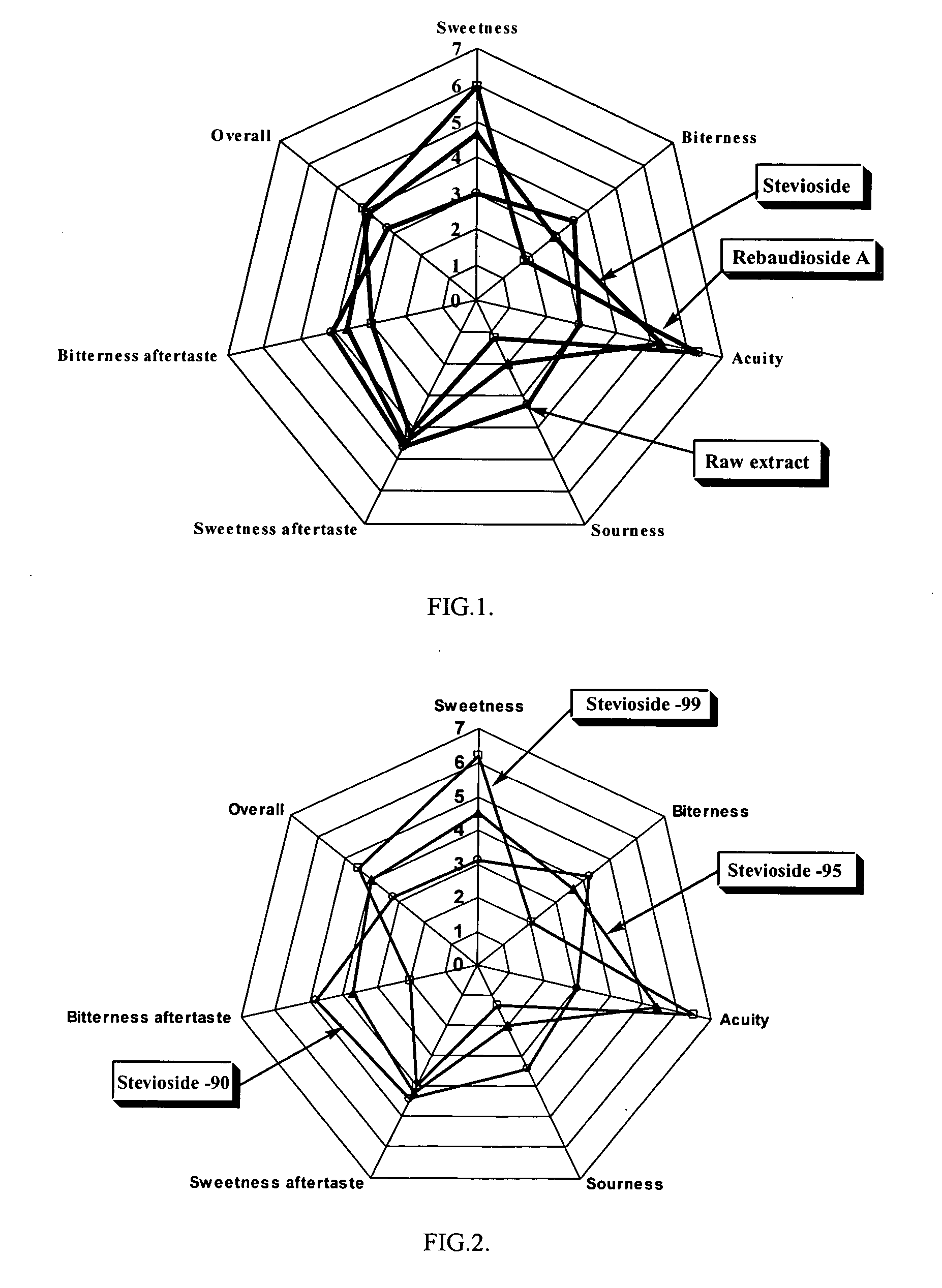
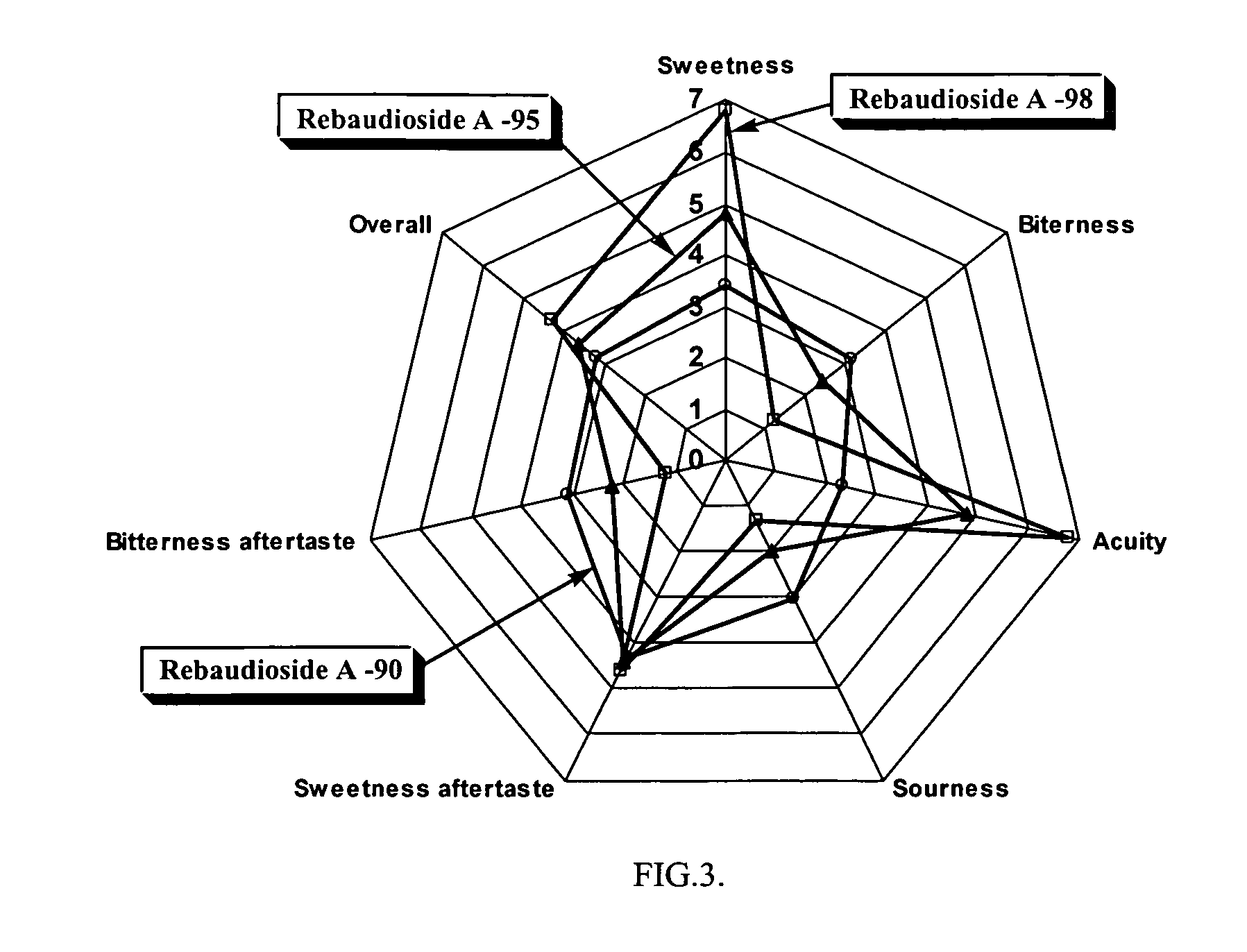
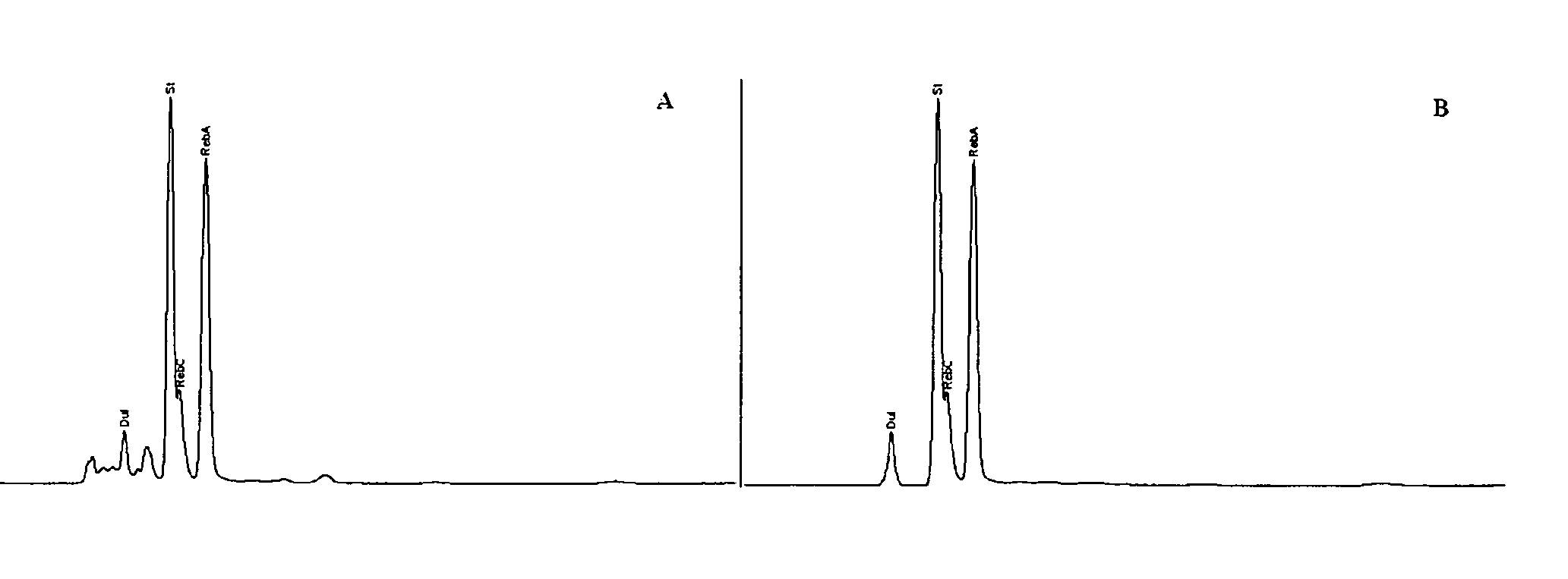
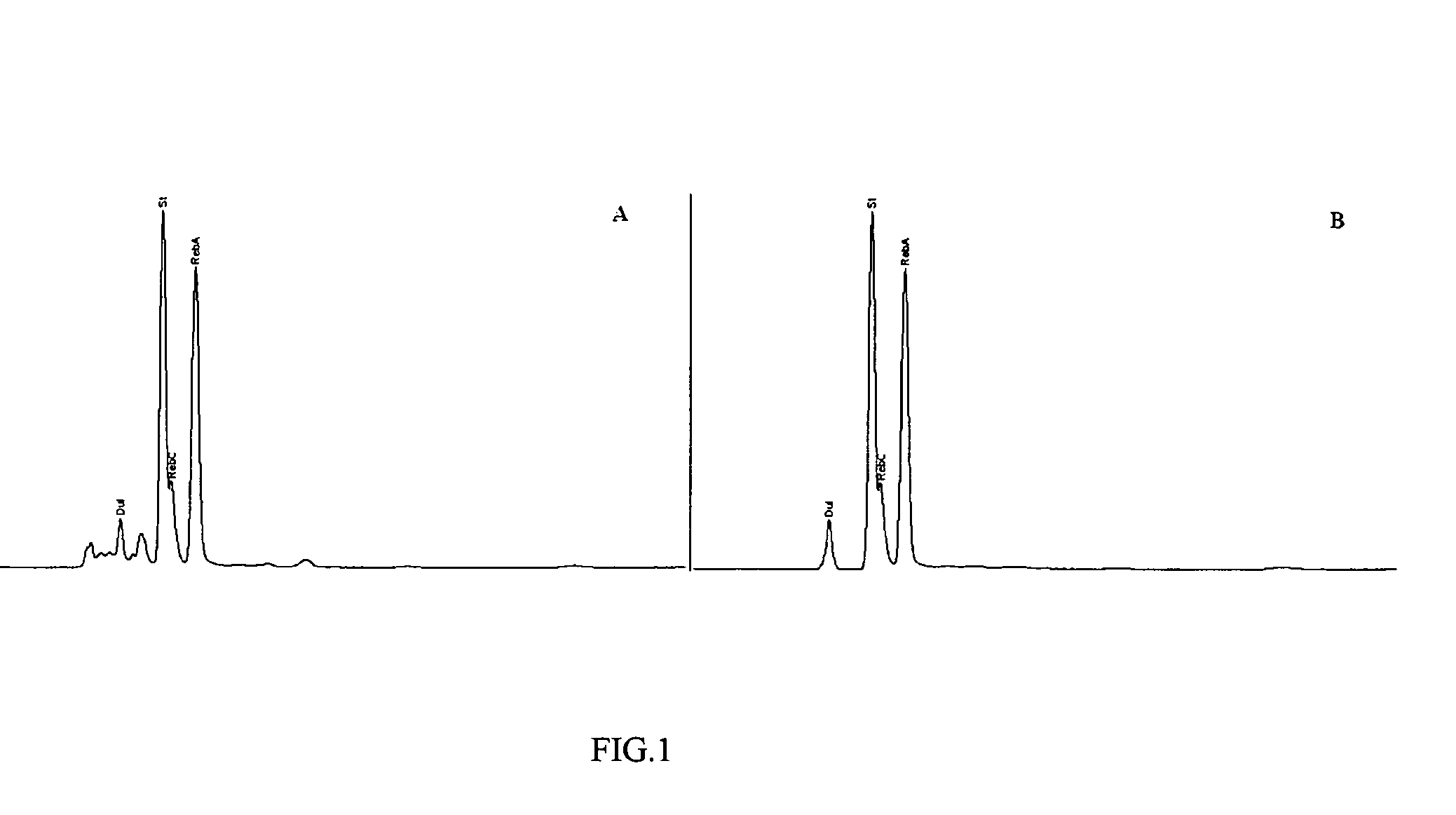
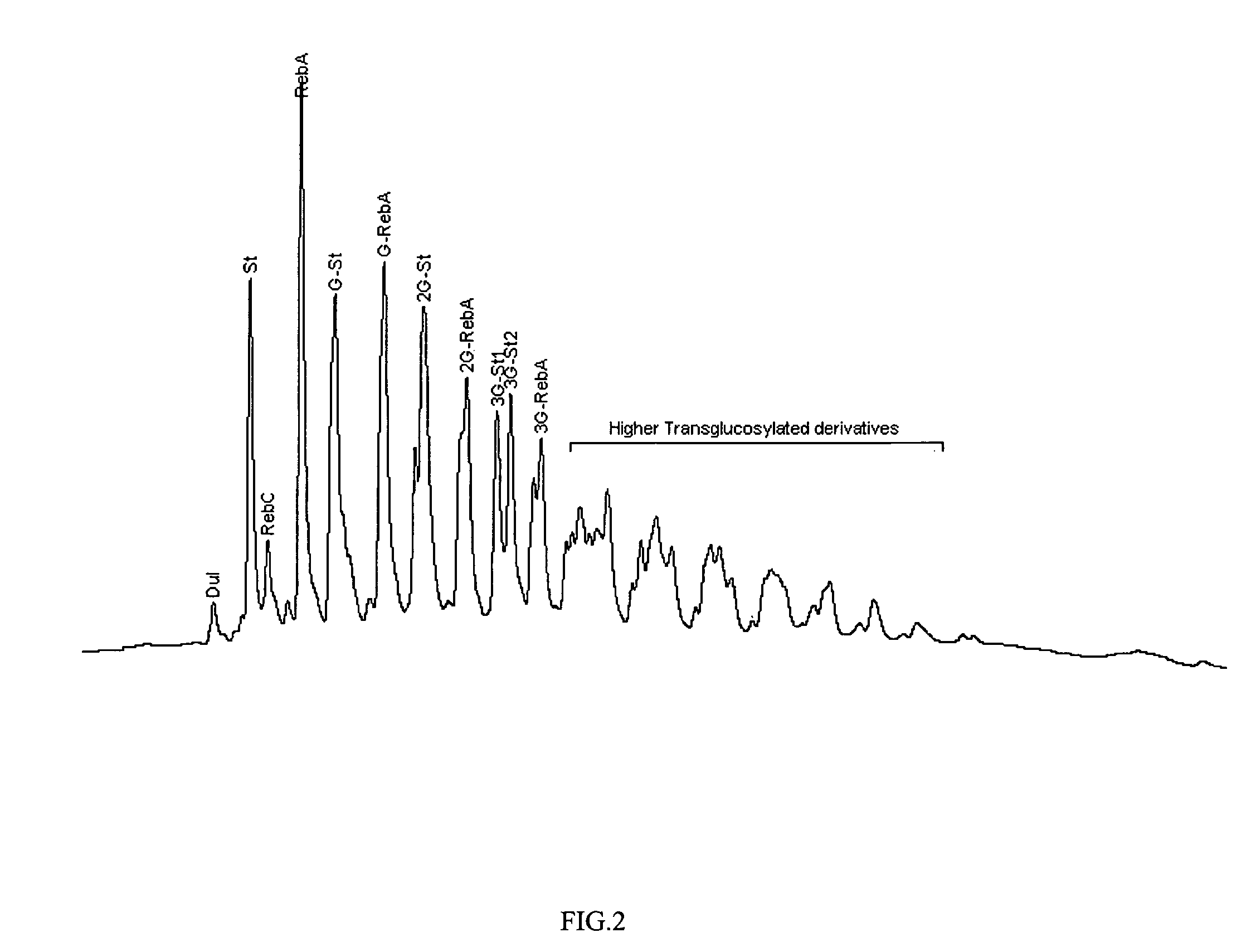
High yield method of producing pure rebaudioside A
ActiveUS20060083838A1Reduction in yieldQuality improvementSugar derivativesMetabolism disorderSolubilityAdditive ingredient
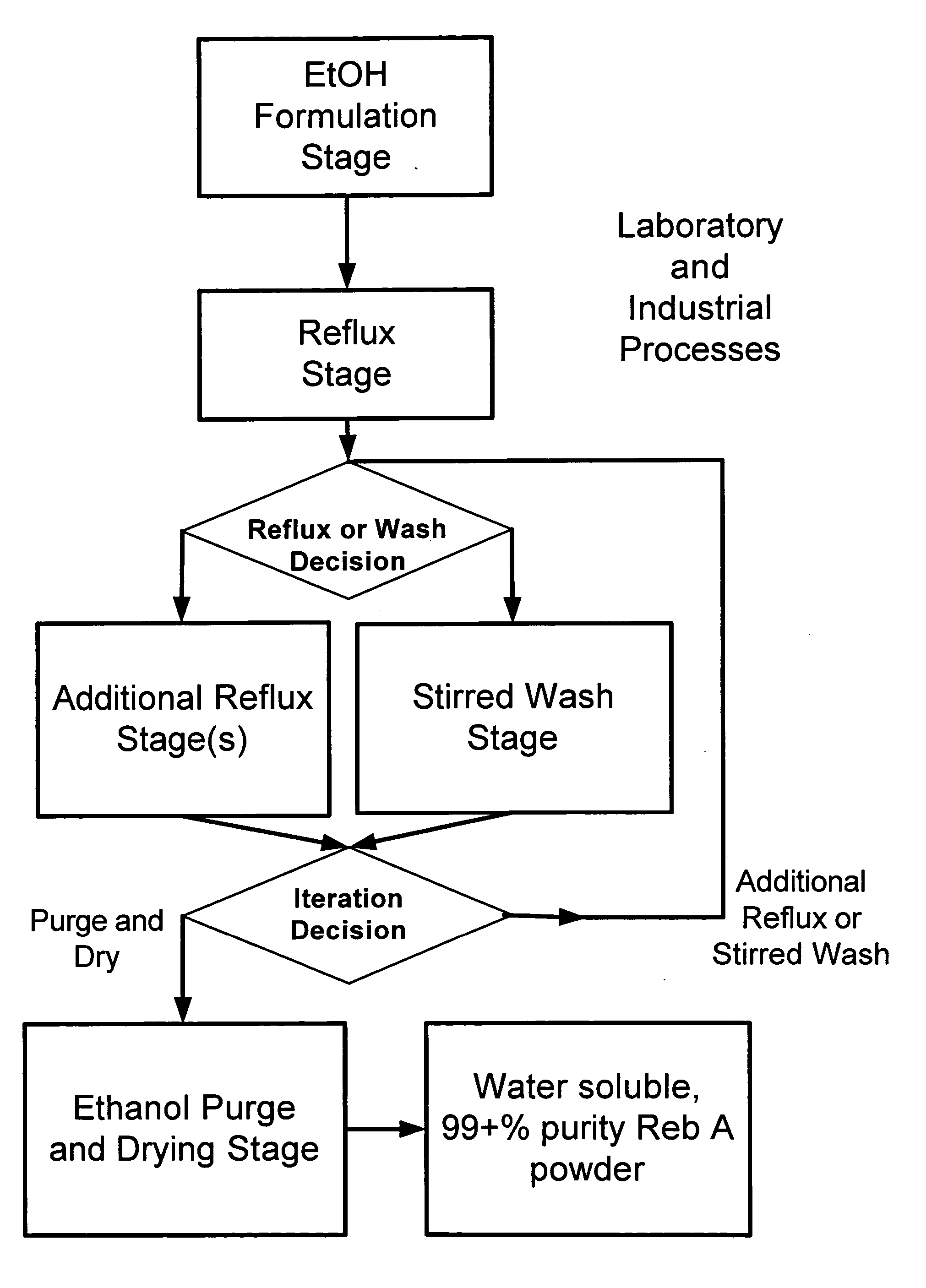
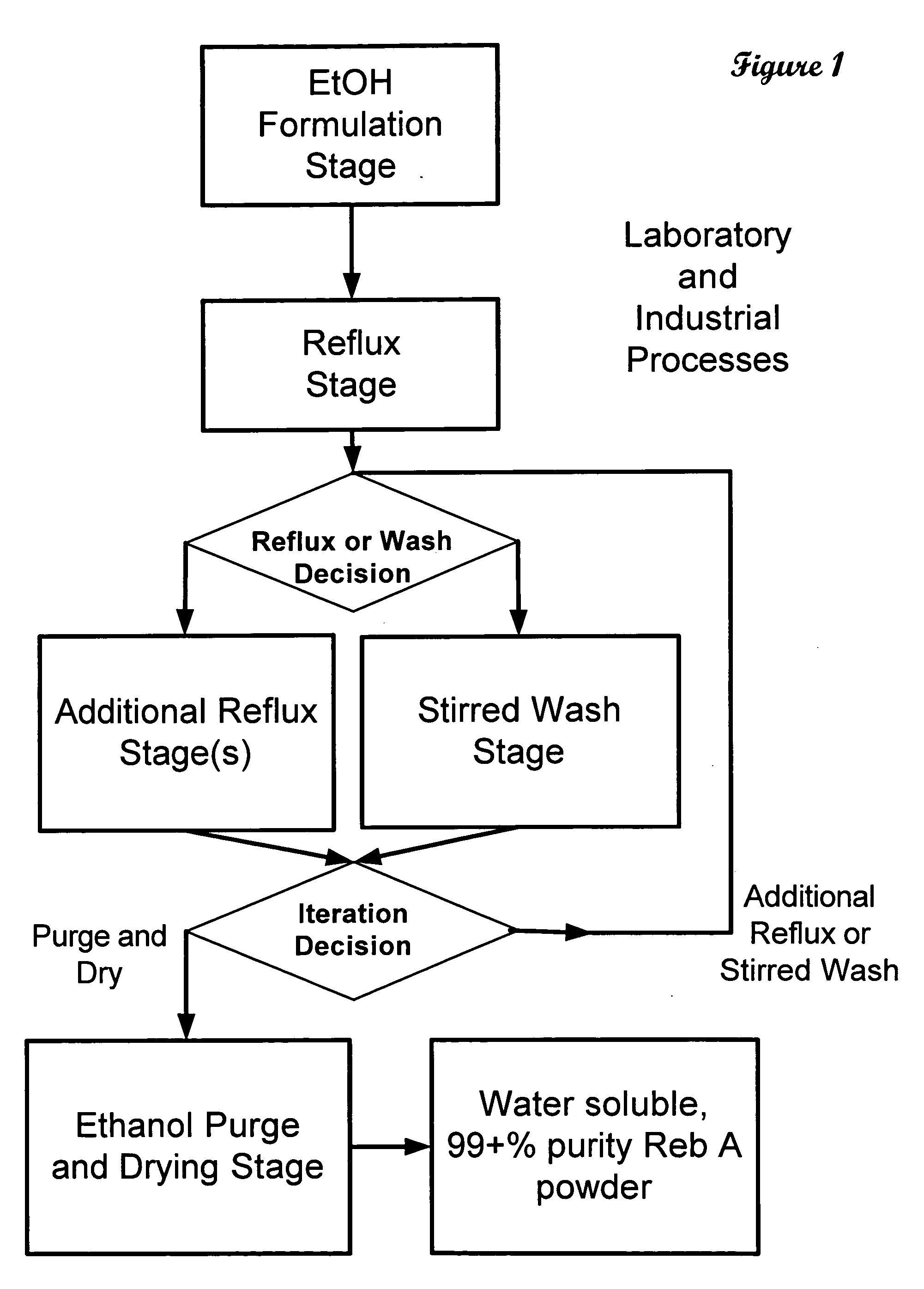
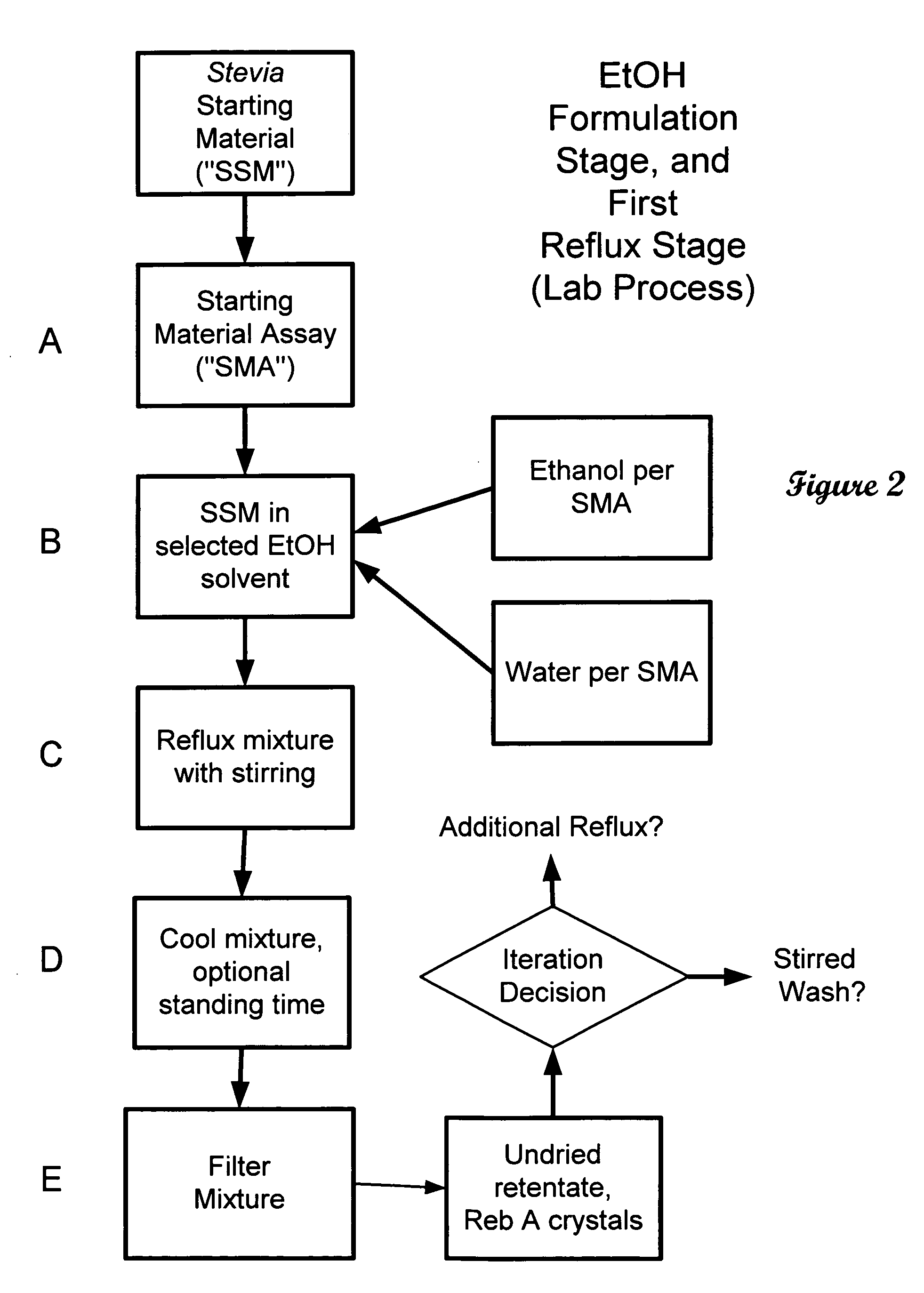
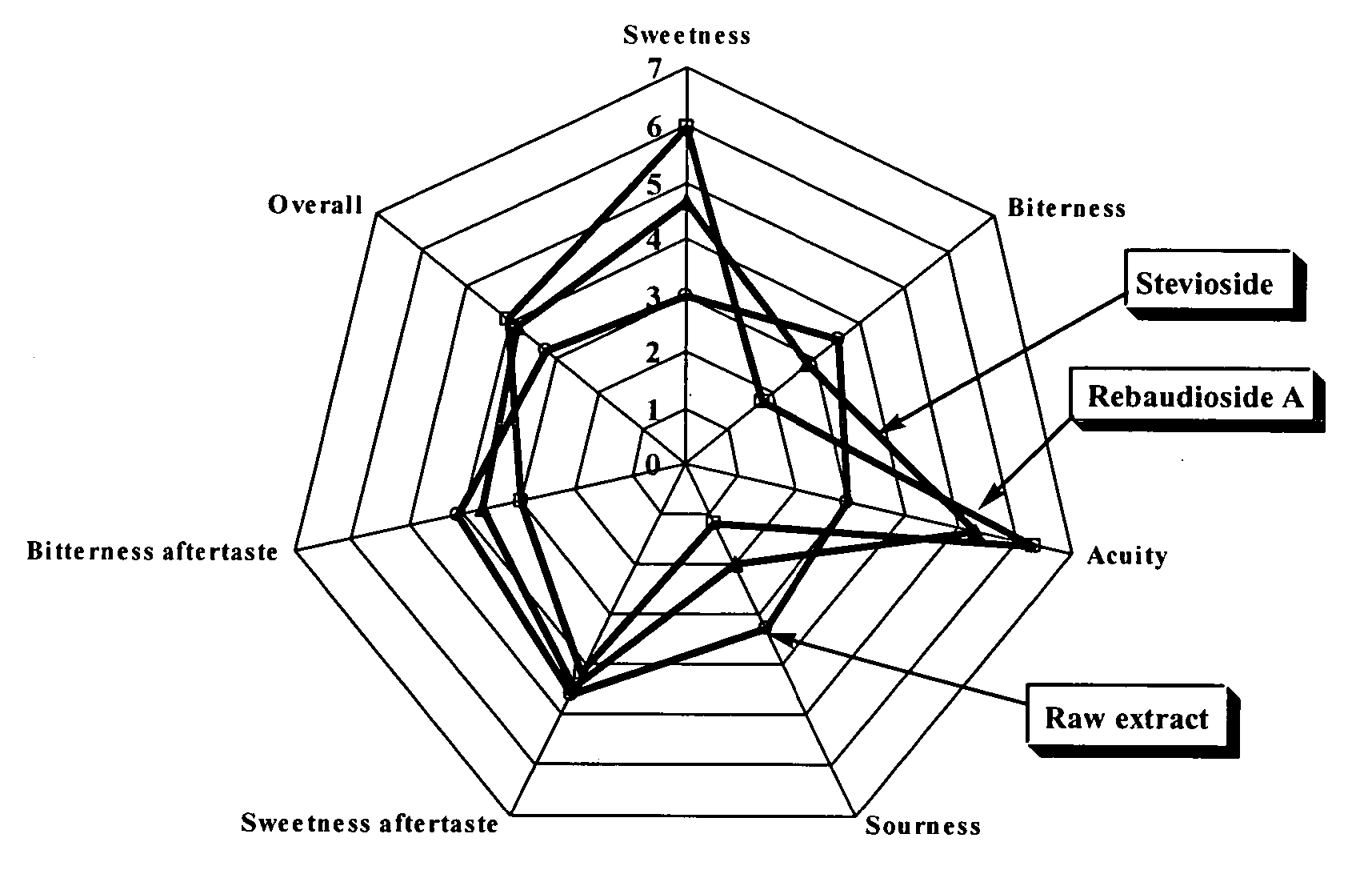
The invention provides a high throughput, high purity, high yield system and method of isolating and purifying rebaudioside A (“Reb A”), with acceptable water solubility for all commercial uses, from commercially available Stevia rebaudiana starting material. The invention also provides a means of maximizing yields of 99+% purity Reb A based on the attributes of a given batch of Stevia starting material. The Reb A produced by the invention is water soluble, devoid of bitterness heretofore associated with rebaudioside sweeteners, non-caloric, and suitable for use as a reagent and as an ingredient in orally consumed products, e.g., as a sweetener, flavor enhancer, and flavor modifier.
Owner:SWEET GREEN FIELDS INT CO LTD
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS7144587B2Reducing abuse potential of dosage formLower potentialPowder deliveryPill deliveryOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
Process for manufacturing a sweetner and use thereof
Highly purified Stevioside and Rebaudioside A were prepared from sweet glycoside extracts obtained from Stevia rebaudiana Bertoni leaves. The resulting sweeteners are suitable as non-calorie, non-cariogenic, non-bitter, non-lingering sweeteners, which may be advantageously applied in foods, beverages, and milk products.
Owner:PURECIRCLE SDN BHD
Sweetner and use
Sweeteners on the basis of a simultaneously transglucosylated sweet glycoside mixture of Stevia rebaudiana Bertoni are prepared. The transglycosylation was developed in the presence of starch under the action of cyclodextrin glucanotransferase. The remaining maltodextrins are transferred to the fructose-terminated oligosaccharides. The sweeteners are purified to not less than 98% content of sweet glycosides and derivatives. The preparations are almost non-caloric, non-cariogenic, non-bitter, non-lingering sweeteners, which may be advantageously applied in foods, beverages, cosmetics and milk products.
Owner:PURECIRCLE SDN BHD
Sweetener comprising a stevia-derived sweet substance
InactiveUS20070003679A1Bitter taste inherentEasy to useFood ingredientsFood preparationCyclodextrinSweetness
A sweetener containing a Stevia-derived sweet substance is provided that, while containing cyclodextrin only in small amounts, can mask the bitter taste inherent in the Stevia-derived sweet substance. Cyclodextrin with a particle size of less than or equal to 30 μm is blended into the Stevia-derived sweet substance at 0.5 to 20 mass % with respect to the mass of the Stevia-derived sweet substance.
Owner:SATO PHARMA
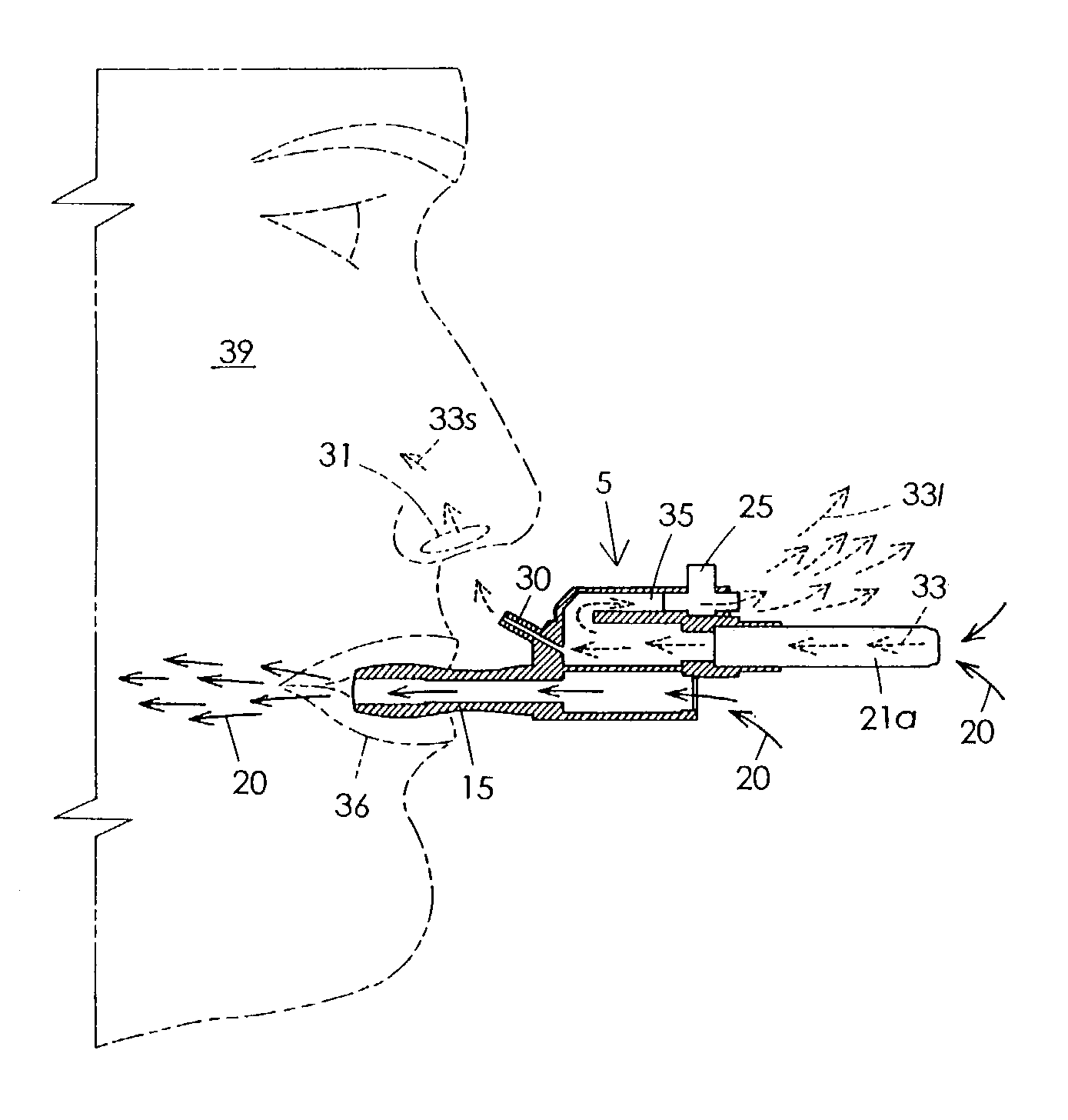
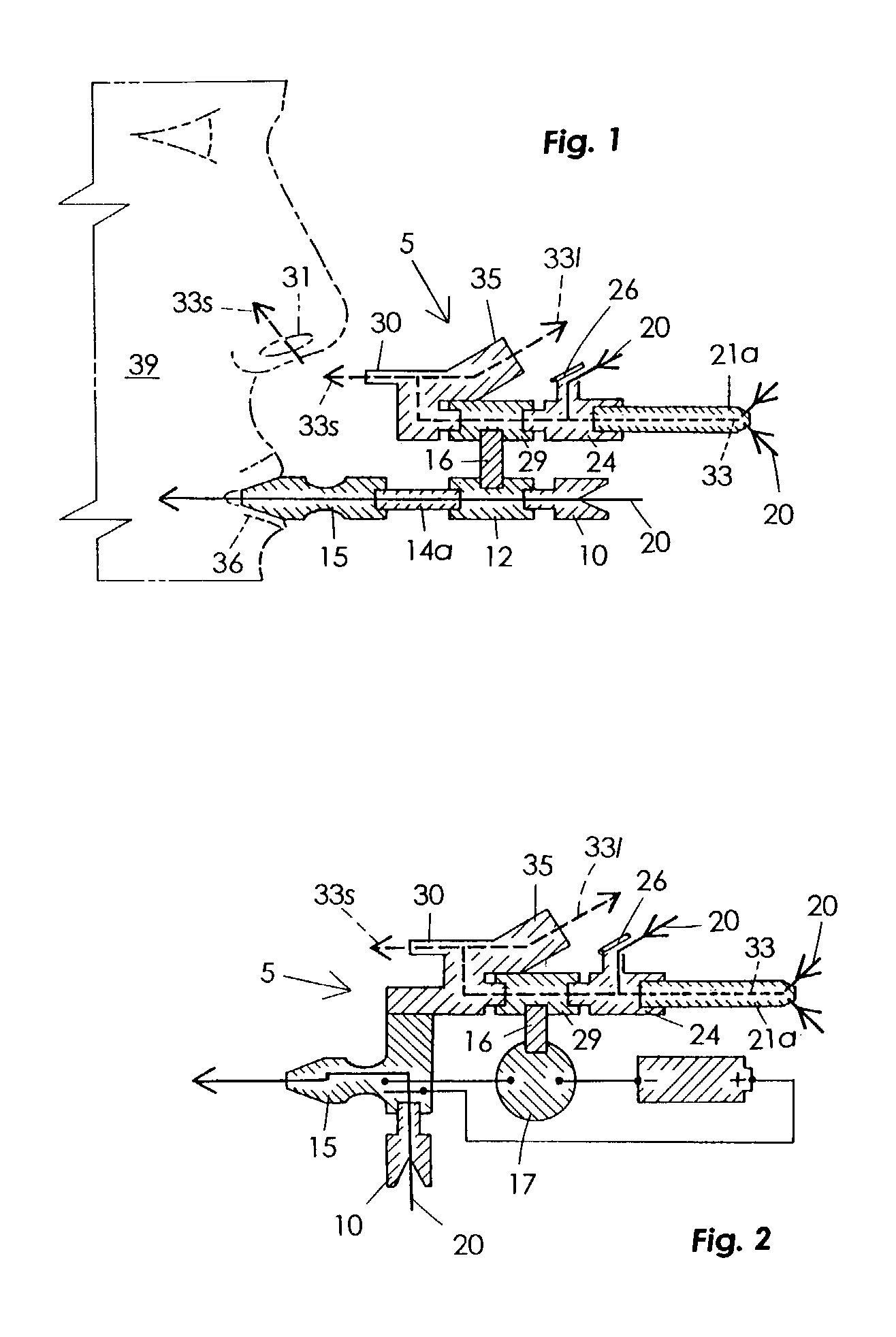
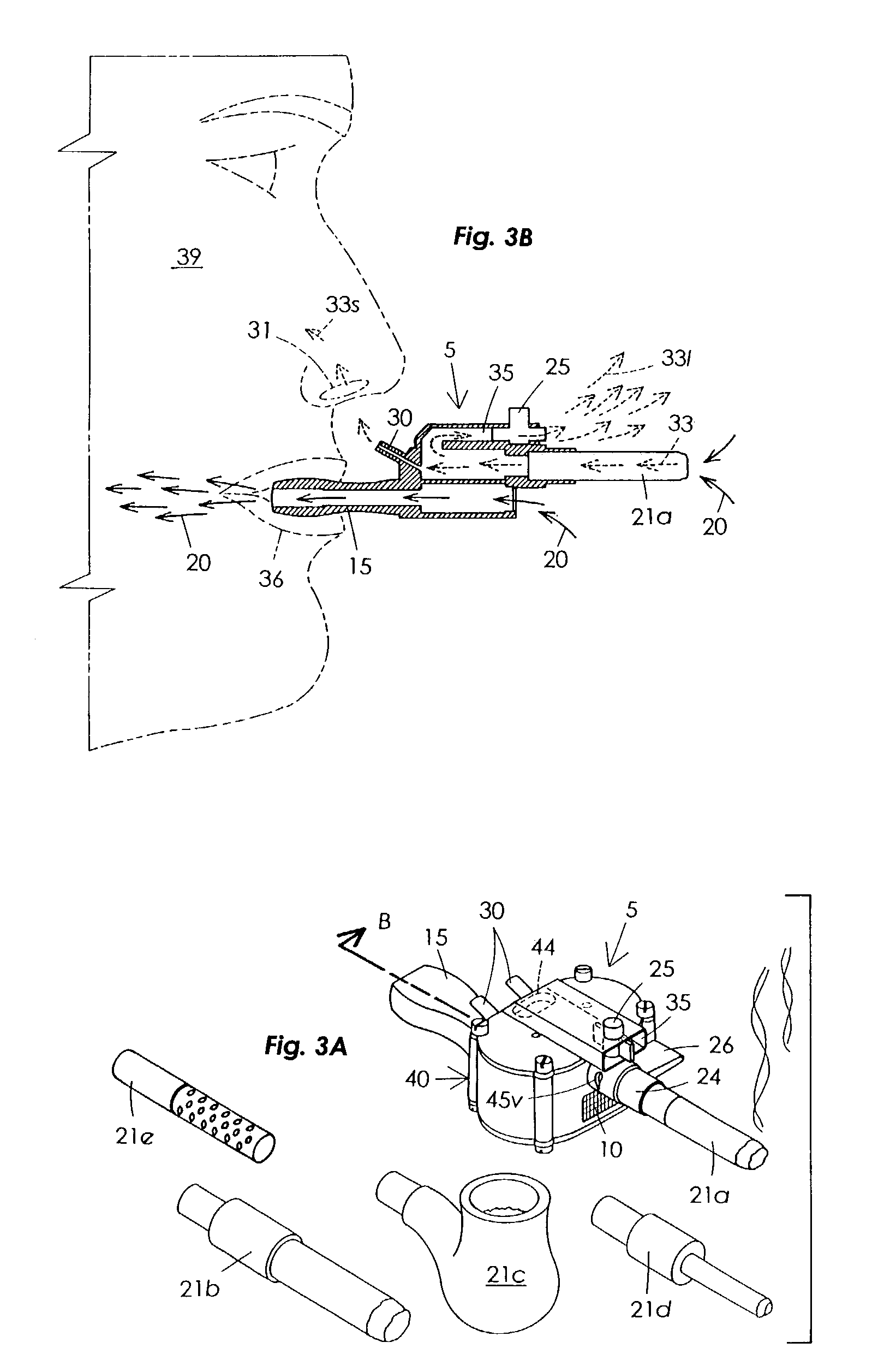
Sensory smoking simulator
The Sensory Smoking Simulator (5) performs by means of suction to draw smoke (33) from a cigarette (21a), only a small amount of which is smelled by the user (39). It is a main body (40) that includes an air motor (12) and a vacuum pump impellers (29) operating in two separate sections (41 and 42). The lower section (42) has a window (10) to connect with the ambient air and a mouthpiece (15) that yields a bitter taste. Means is provided so that when a person (39) inhales, air is draw into the lower section (42) and through the mouthpiece (15). The upper section chamber (41) has a cigarette receiver (24) and two passageways attached, one of them (35) to exhaust the cigarette smoke to ambient air, the other one (30) to let exhaust a lesser amount of smoke (33s) near the user's nose (31). The smell from cigarette smoke (33s) and the taste secreted from the mouthpiece (15) create a mental effect similar to that of smoking, but far less harmful to a person's health.
Owner:DOMINGUEZ ARMANDO
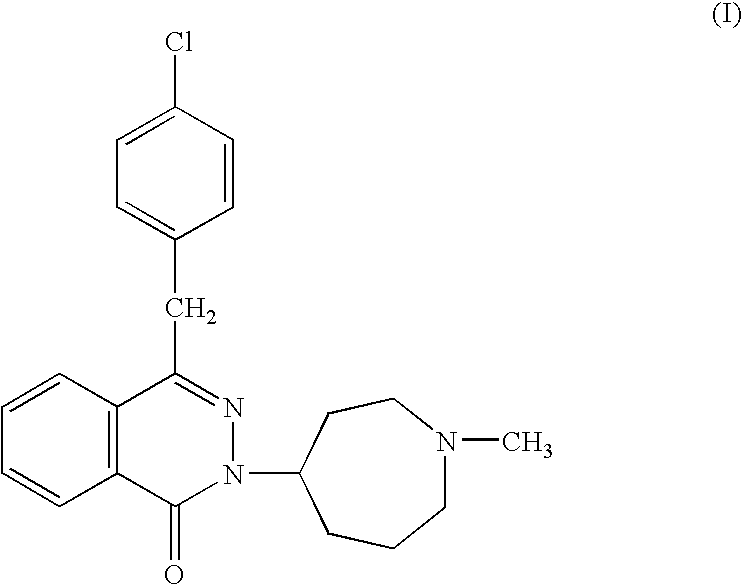
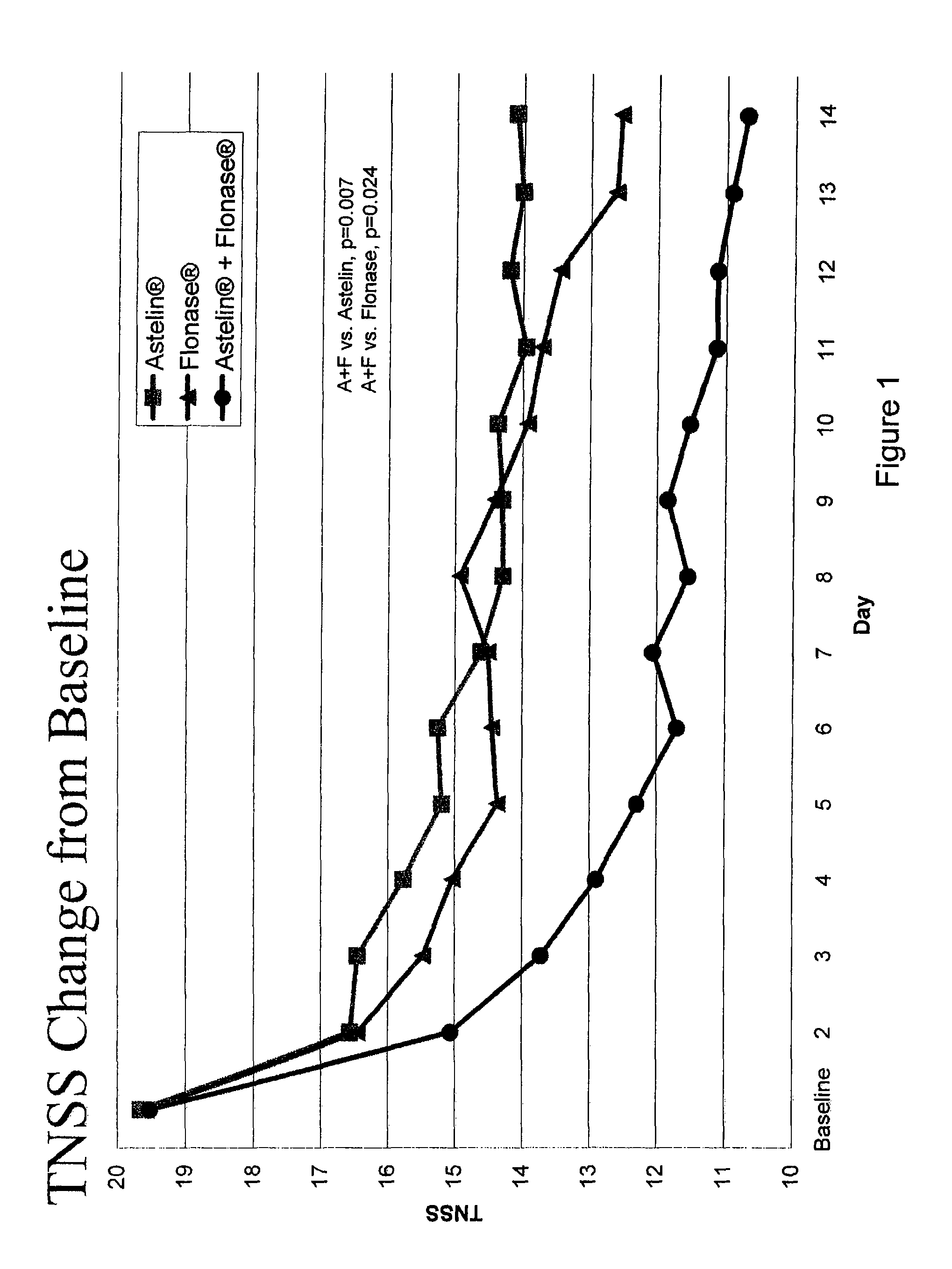
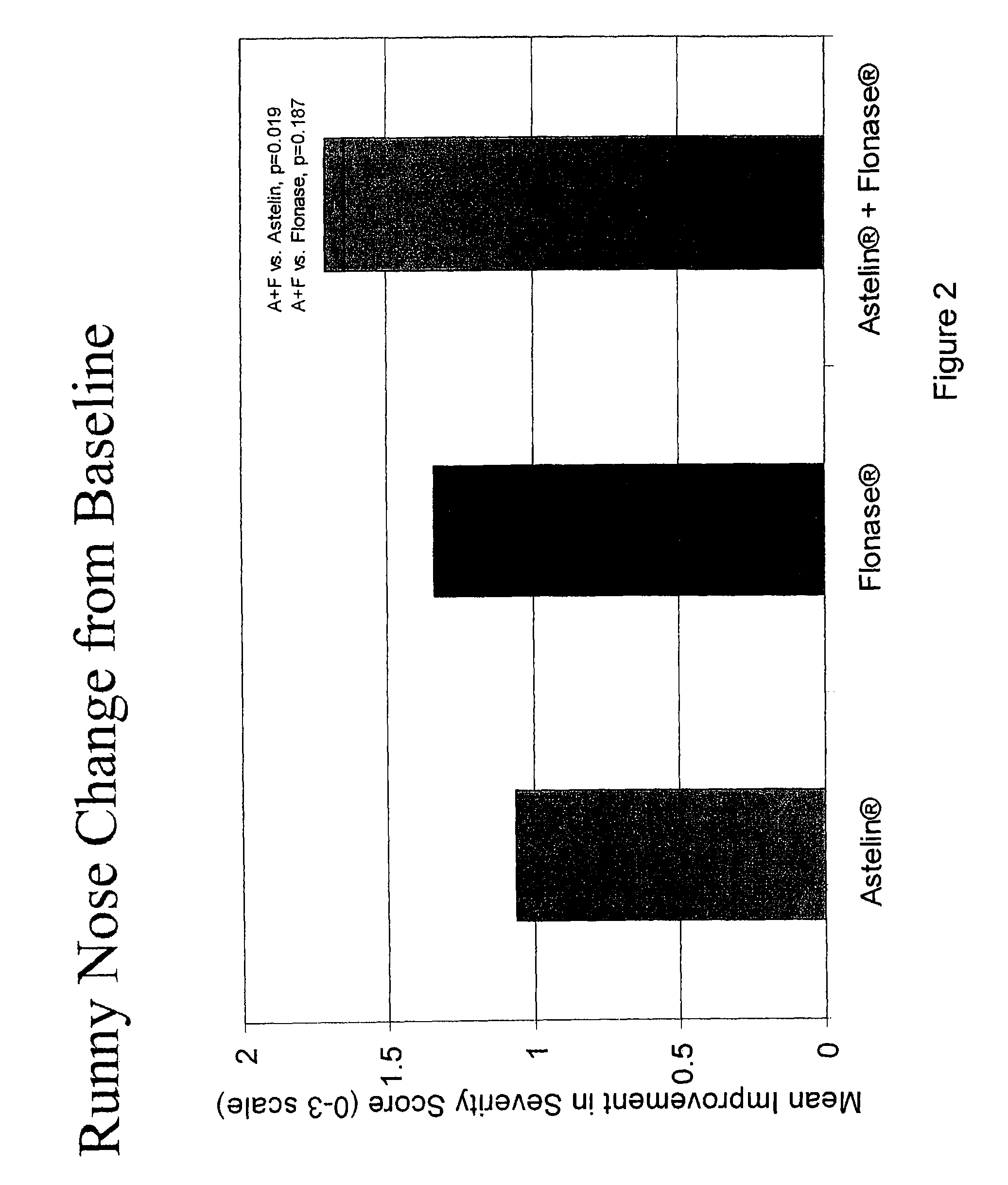
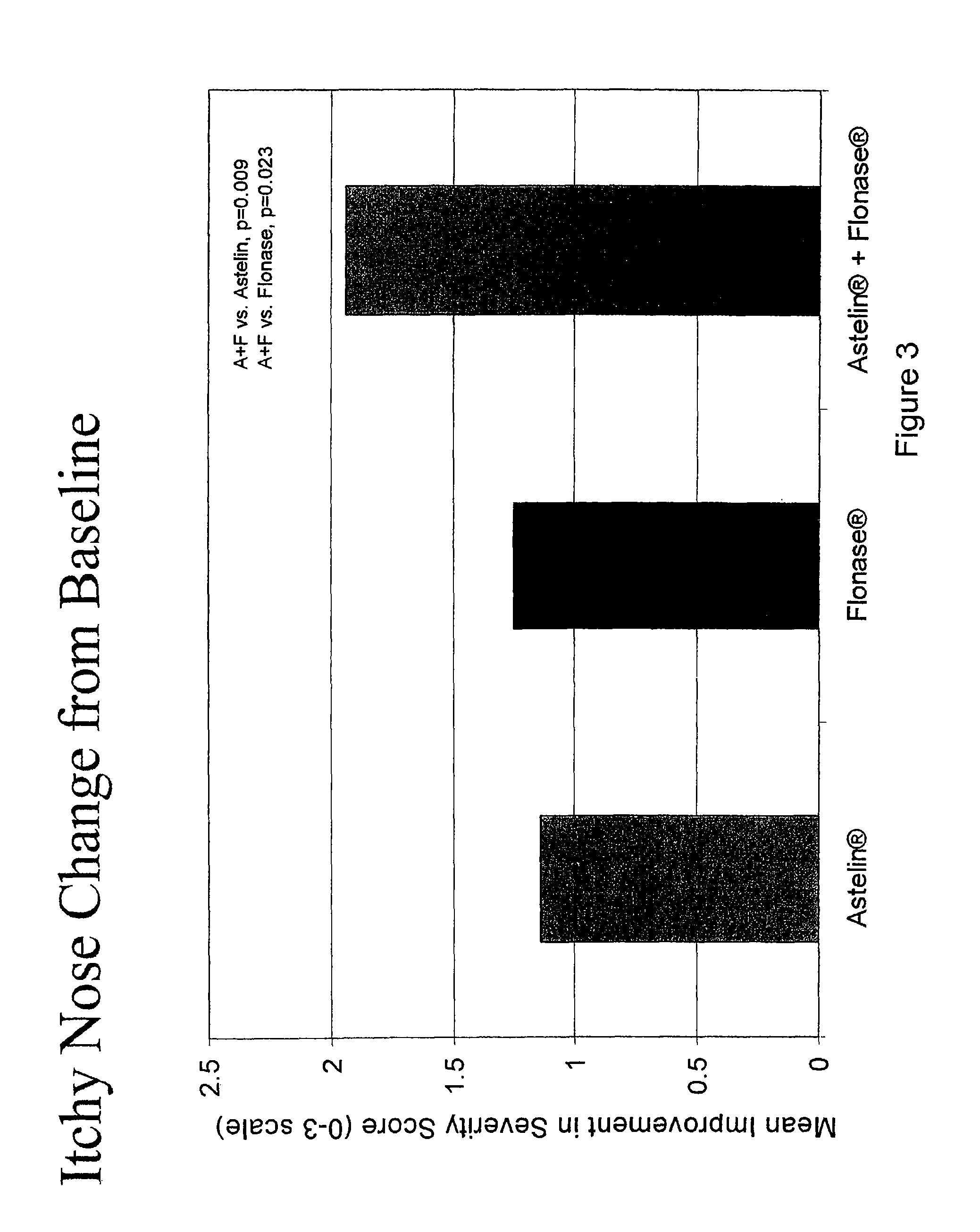
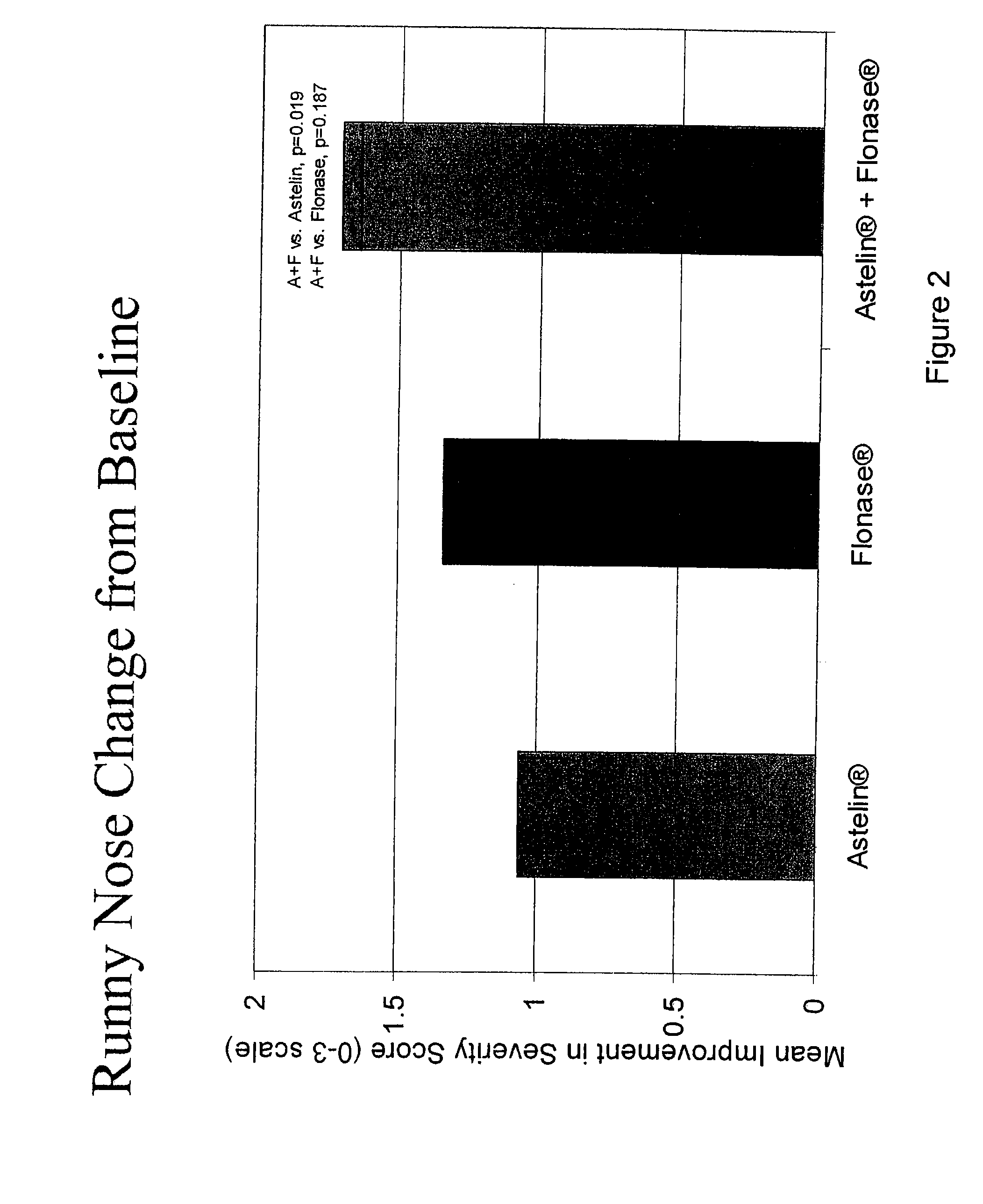
Compositions comprising azelastine and methods of use thereof
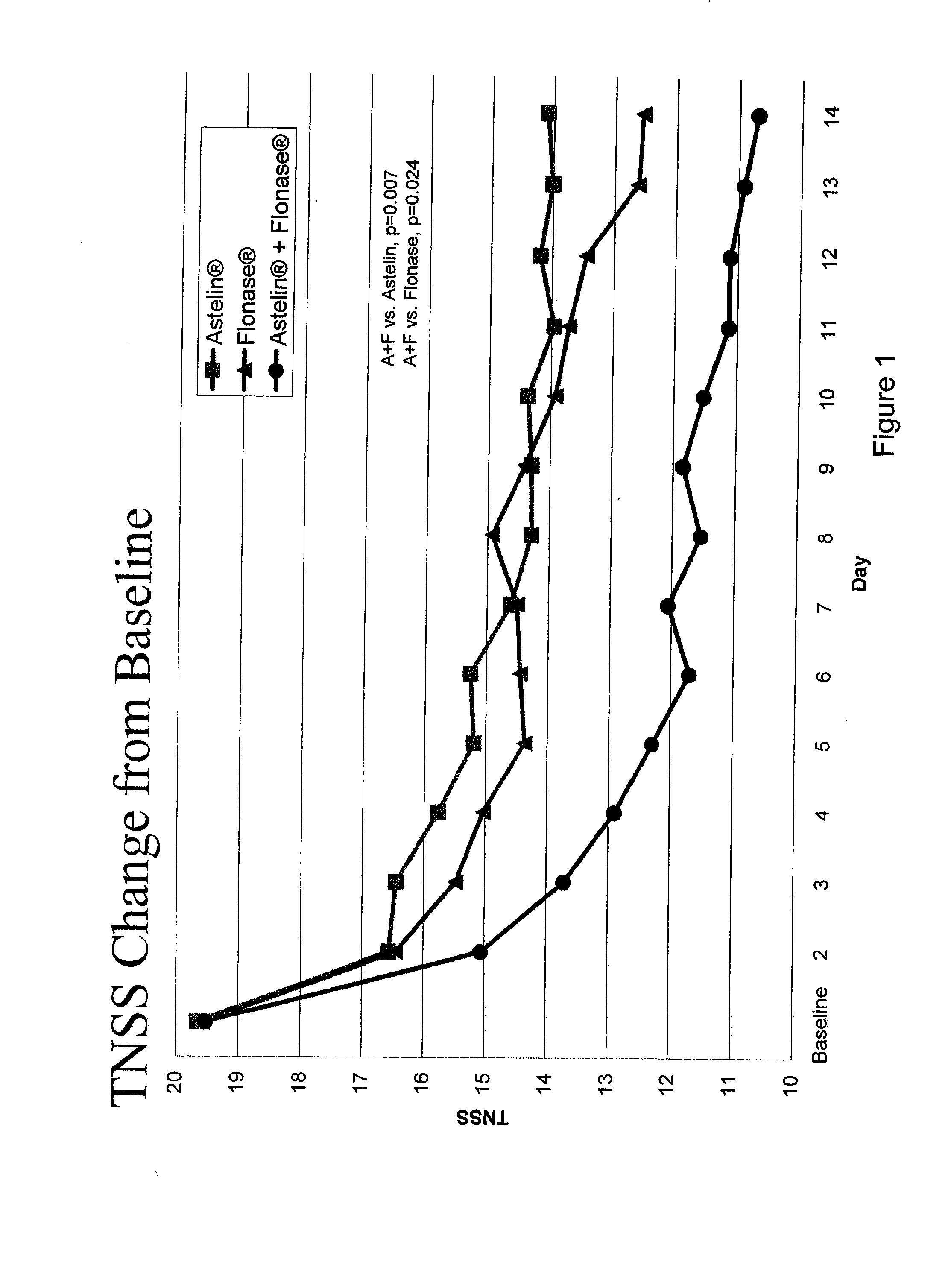
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Whole grain non-dairy milk production, products and use
ActiveUS20070014892A1Increase coverageReserved functionDough treatmentWort preparationSlurryWhole milk
A method comprising selection of unbroken whole grain rice that are first washed, or whole grain corn that is first reduced in size, and then making an aqueous slurry that is subsequently wet milled to release all the protein, fat, fiber, and starch components normally held in the structure of the grain. The resulting slurry can be reacted with heat to gelatinize the starch and the subsequent product dried. Also, the heated slurry containing the liberated components can be treated to enzymatic hydrolysis via the process of liquefaction and optionally saccharification, producing whole grain rice milk products having diverse carbohydrate compositions. The whole grain milk products are characterized by a nutritional composition containing substantially all the nutritional components of the whole grain, being an opaque whole milk colloid, having smooth texture versus pulpiness, lacking in all bitterness normally associated with whole grain products, and having a variety of sweetness levels from non-sweet to very sweet.
Owner:STEUBEN FOODS
Medicinal compositions with relieved bitterness
InactiveUS6576677B1Alleviate bad tasteGrowth inhibitionPowder deliveryDispersion deliveryD-SorbitolAntioxidant
The present invention provides a composition alleviated in a bitter taste or the like of a medicament. The present invention relates to a composition comprising a basic medicament having an unpleasant taste and polyvinylpyrrolidone and / or copolyvidone; or a method for alleviating an unpleasant taste of a basic medicament having the unpleasant taste by adding polyvinylpyrrolidone and / or copolyvidone. The present invention further provides a composition comprising (1) a basic medicament, (2) polyvinylpyrrolidone and / or copolyvidone, and (3) propylene glycol and / or D-sorbitol; a composition comprising (1) a basic medicament, (2) polyvinylpyrrolidone and / or copolyvidone, and (4) an antioxidant; and a composition comprising (1) a basic medicament, (2) polyvinylpyrrolidone and / or copolyvidone, and (5) a colorant or flavor containing a sulfuric acid or sulfurous acid group.
Owner:EISAI CO LTD
Lhg compositions for reducing lingering bitter taste of steviol glycosides
InactiveUS20080226788A1Reduce odorMask lingering bitter taste(s)Food ingredient as taste affecting agentFood preparationSweetnessMogroside V
Aspects of the invention relate to beverage compositions, including, for example, concentrated and ready-to-drink formulations sweetened with at least a steviol glycoside and further including an LHG juice, extract or combinations thereof in an amount sufficient to reduce the lingering bitter taste of the steviol glycoside and improve the mouthfeel of the beverage. In exemplary embodiments, additional sweeteners are utilized in the beverage in addition to the LHG juice and the steviol glycoside. In other exemplary aspects, LHG powder of mogroside v content from 2 to 99% by weight may be utilized in lieu of or in addition to the LHG juice concentrate. In certain exemplary embodiments having additional non-nutritive sweeteners, the amount of LHG composition is sufficient to reduce one or more off-note tastes of one or more of the additional sweeteners.
Owner:CONCENTRATE MFG OF IRELAND
Solvent free taste masked pharmaceutical compositions
A taste masked pharmaceutical composition comprising: (a) a core comprising a bitter tasting drug, such as cetirizine dihydrochloride; and (b) a coating comprising a pharmaceutically acceptable cationic co-polymer based on mono- or dialkylaminoalkyl methacrylate and neutral acrylic or methacrylic esters, wherein the alkyl group independently has 1 to 6 carbon atoms, wherein said coating is applied to the surface of said core. The taste masked pharmaceutical compositions of the invention may be prepared without using an organic solvent or a cyclodextrin.
Owner:KUMARAPERUMAL NATRAJAN +2
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
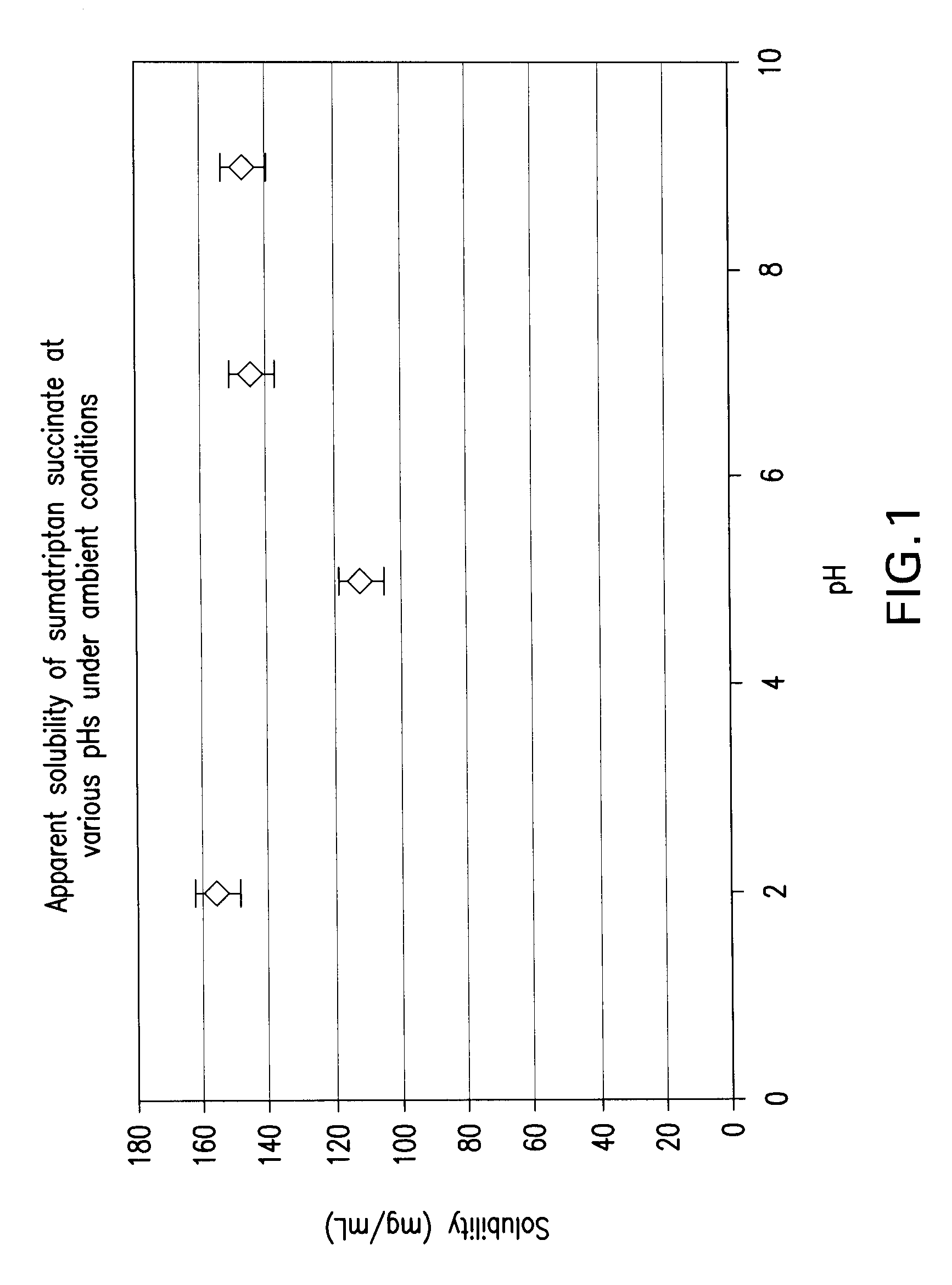
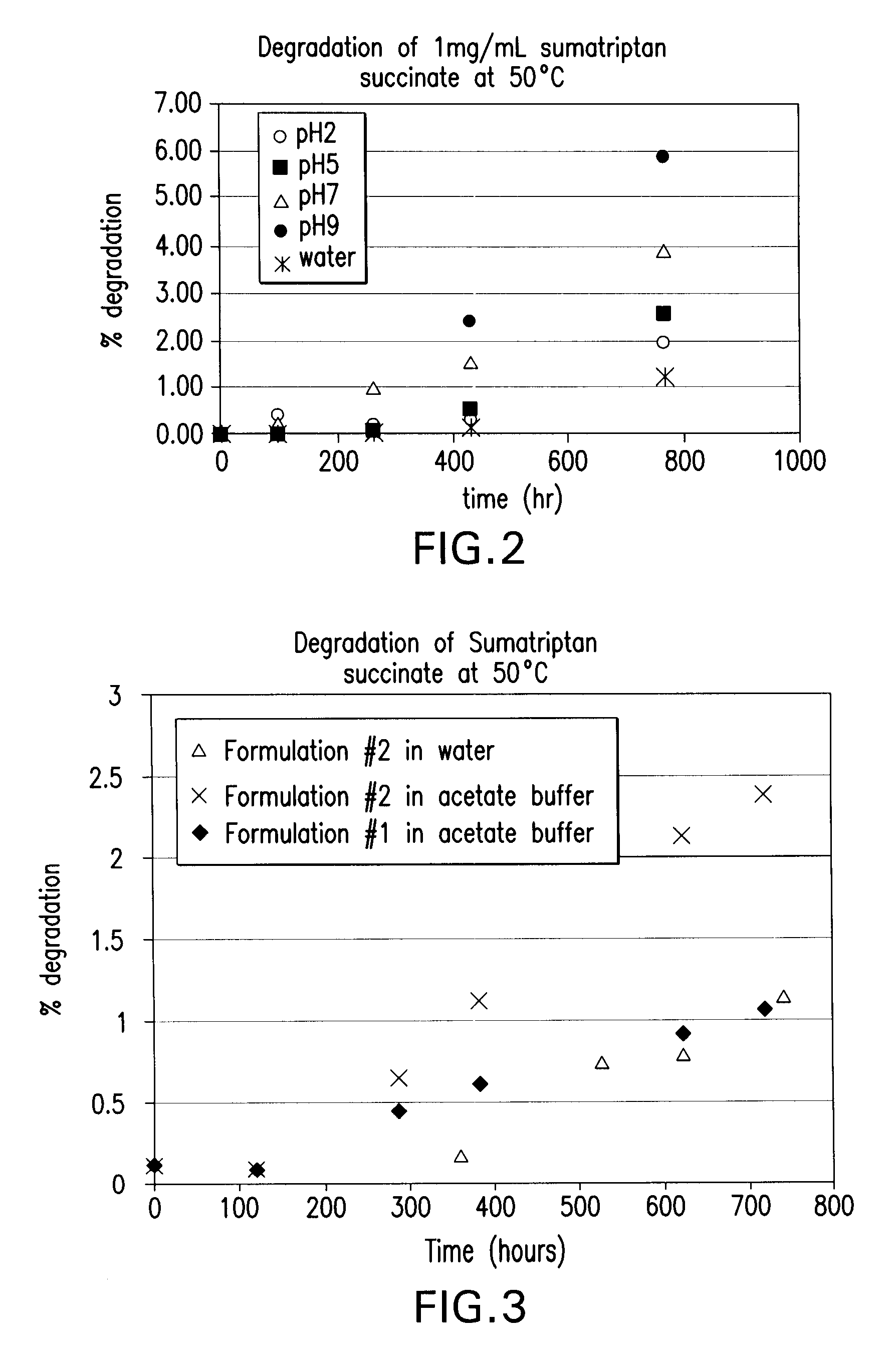
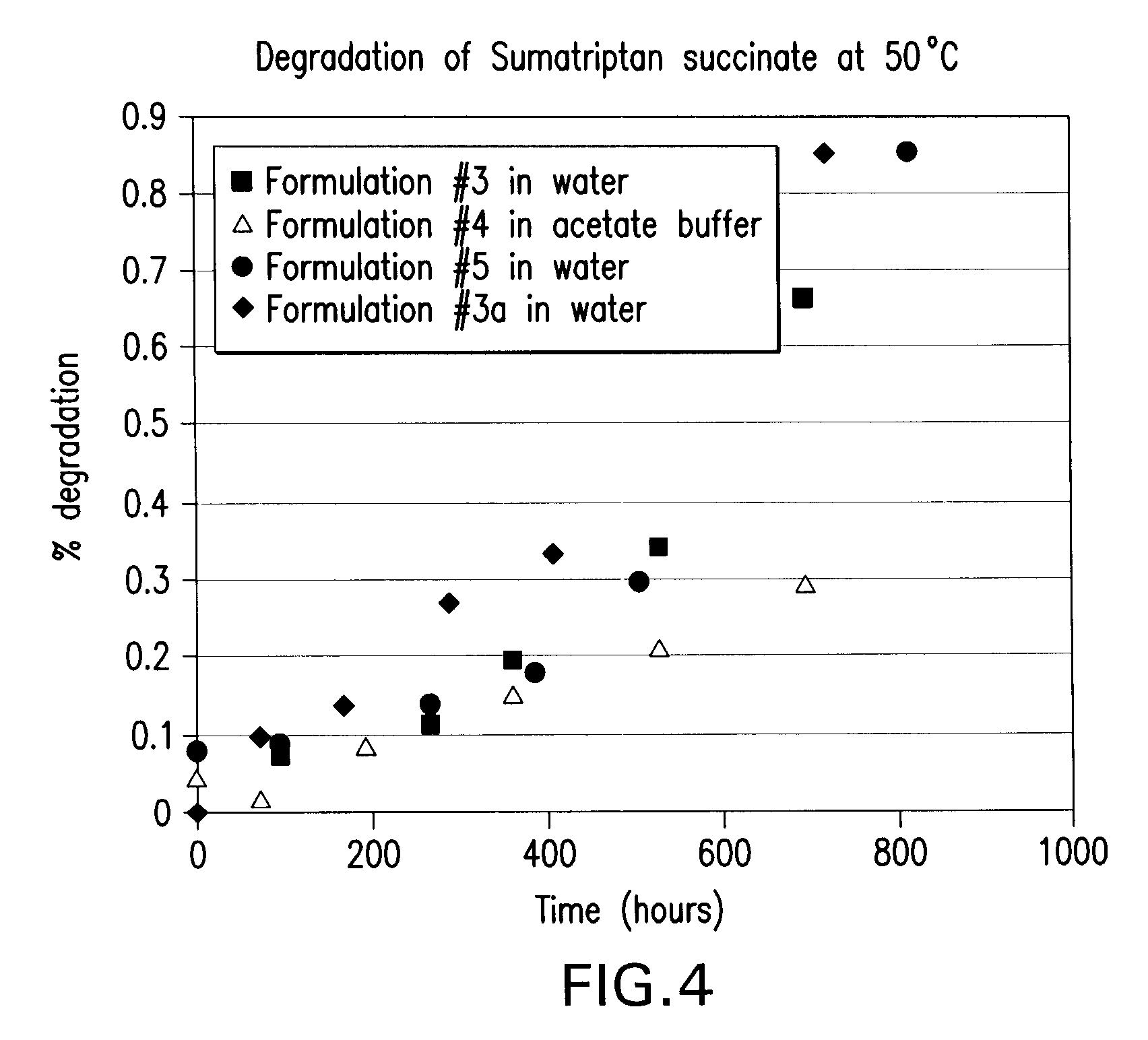
Stable and palatable oral liquid sumatriptan compositions
InactiveUS20070166336A1Minimize and mask bitternessReduce bitternessBiocideNervous disorderMetaboliteSumatriptan
The present invention is directed to improved oral liquid compositions that include sumatriptan, or a pharmaceutically acceptable salt or metabolite thereof, and a pharmaceutically acceptable carrier that includes a liquid portion of the composition. The compositions are substantially free of oxidation impurities. Typically, the compositions include a sweetening agent and a flavoring agent, or a bitterness-reducing agent and flavoring agent. Processes of preparing such compositions and methods of administering such compositions are also included.
Owner:WOCKHARDT EU OPERATIONS SWISS
Mouth cavity quick dissolving quick disintegrating freeze-dried tablet and its preparing method
InactiveCN1473562AFast disintegrationPrevent "throat stuck" phenomenonAntibacterial agentsPill deliveryThroatFreeze-drying
The oral cavity quick dissolving and quick disintegrating freeze dried tablet for children includes at least one medicinal active component and at least one medicinal stuffing, adhesive and other supplementary material. It is loose and porous tablet in network structure and prepared through common freeze drying process. The said medicinal active component may be different children's medicines, such as antibiotic, antipyretic, analgesic, cough stopping and phlegm eliminating medicine, cold medicine, etc. Bitter or excitant medicine may be coated and water insoluble medicine is prepared intofine powder of 50 micron below size for stable dispersion in liquid. The present invention has fast disintegration, no jamming in throat, simple preparation process and low cost.
Owner:刘辉
Low-salt liquid state fermented soy sauce production process
The invention discloses a low-salt liquid state fermented soy sauce production process, comprising the following main processes: preparing koji in a koji-maker machine; mixing the wheat which is fried and smashed with koji, inoculating the mixture in soybean meal and bran which are cooked; preparing finished koji in a koji making disc machine; mixing the finished koji with brine, sending the mixture in a fermenting vat for fermentation; preparing the finished soy sauce through soaking, pouring oil, filtrating, mixing, sterilizing and filling to obtain the finished soy sauce. The soy sauce is characterized in that materials containing zygosaccharomyces Rouxii 2.180 accounting for 1.5-2.5% of the total weight of materials and torulopsis candida 2.202 accounting for 1.5-2.5% of the total weight of materials are added during the medium term of the fermentation process and the raw materials--soybean meal and bran are cooked by high temperature short-time continuous cooking process. Compared with the prior art, the raw material digestion rate can reach 88-90%, the protein utilization rate can reach 83%, the activity of neutral protease of the finished koji can reach 2000mu / g, the enzymesystem is complete, the defects such as long period of high-salt liquid state fermentation (180 days), burnt flavour and heavy bitter taste of low-salt solid state fermentation and the like are overcame and the produced soy sauce is characterized by strong soysauce-like aroma, alcohol-like aroma and ester-like aroma and the like.
Owner:QIANHE CONDIMENT & FOOD CO LTD
Taste-masked oral formulations of influenza antivirals
The present invention relates to taste-masked oral formulations of influenza antivirals. The taste-masked pharmaceutical formulations for oral administration comprise one or more influenza antivirals, at least one taste-masking agent and at least one pharmaceutically acceptable excipient. Further, the taste-masked influenza antiviral formulations of the present invention are provided in the form of dispersible tablets, effervescent tablets, orally disintegrating tablets, chewable tablets, bite-dispersion tablets or the like, wherein the bitter taste of influenza antivirals is masked thereby providing palatable formulations.
Owner:RUBICON RES PTY LTD
Taste masked pharmaceutical compositions comprising bitter drug and pH sensitive polymer
InactiveUS20050136114A1Improve palatabilityA large amountPowder deliveryDispersion deliveryPH-sensitive polymersLiquid oral
The present invention discloses pharmaceutical compositions comprising of pH sensitive polymers used for taste masking highly bitter drugs. The pH sensitive polymer acts as a reverse enteric coating, which is soluble in the acidic pH range 1.0 to 3.0 normally found in the stomach but is insoluble in the pH range 3.5 to 7 thus inhibiting the release of the bitter drug at the pH of saliva and also at the pH of reconstitution medium in case of liquid orals.
Owner:COUNCIL OF SCI & IND RES
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833550B2Reduce morbidityNot impart bitter tastePowder deliverySenses disorderNasal cavityAzelastine
Dry powder formulations of drugs such as antihistamine for nasal administration are provided where the drug is retained in the nasal cavity, and systemic side effects minimized or eliminated, through the selection of a narrow particle size range, between approximately 10 and 20 microns in diameter. In a preferred embodiment wherein the drug is an antihistamine, retention of the antihistamine at the nasal mucosa is improved and the bitter aftertaste associated with liquid antihistamine formulations significantly reduced. By making a dry powder formulation of an antihistamine (e.g., azelastine) having an average particle size of between 10 and 20 microns, the antihistamine is restricted primarily to the desired target organ, the nasal mucosa. Because the active ingredient stays in the nasal region, a lower dose can be used to achieve the same desired effect. As demonstrated by the examples, this lower dose reduces the incidence of somnolence, and because the active ingredient remains at the target organ and does not accumulate in the back of the throat and mouth, this formulation does not impart a bitter taste.
Owner:MANNKIND CORP
Method for preparing collagen peptide from fish scales
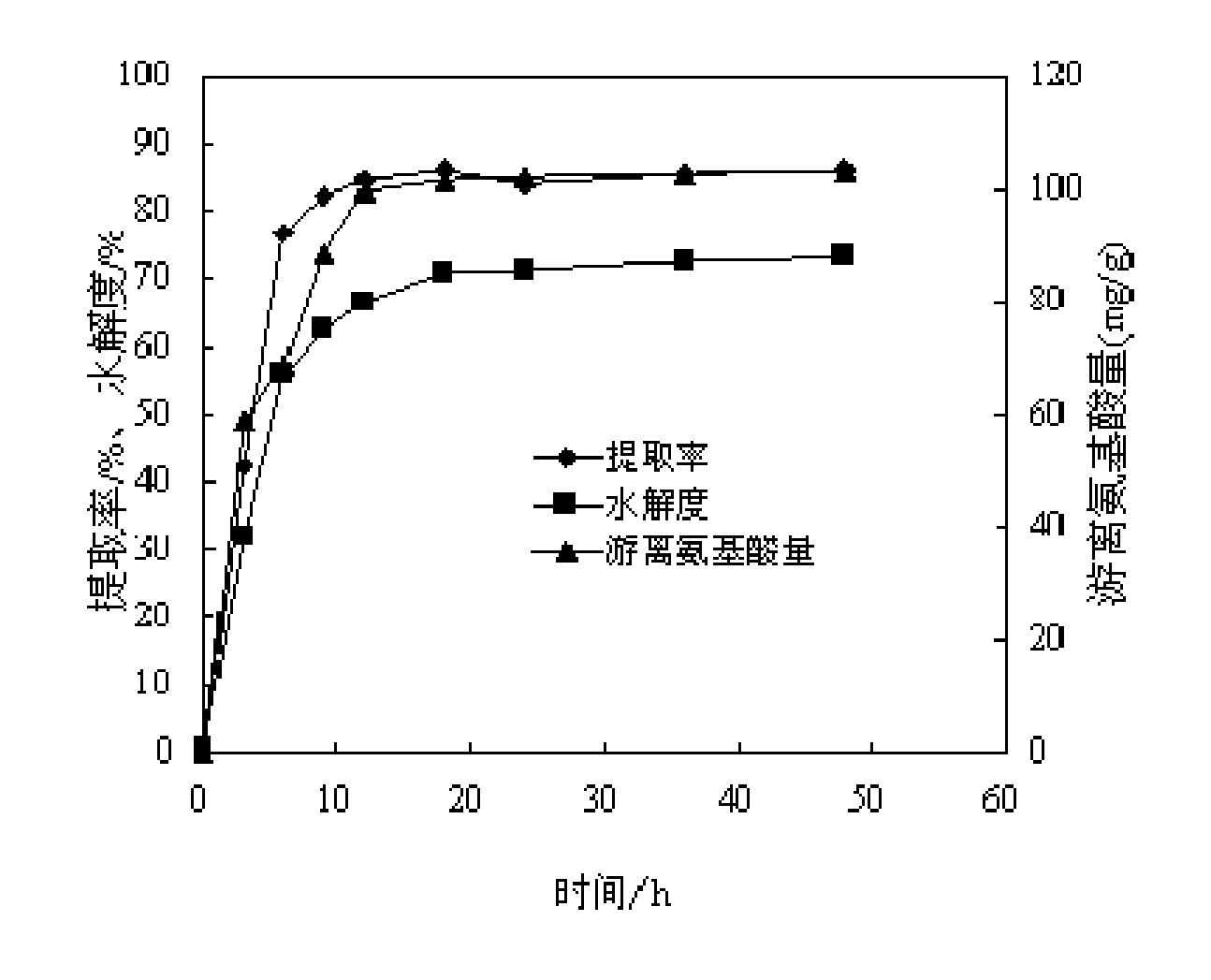
The invention discloses a method for preparing collagen peptide from fish scales, which is characterized by comprising the following steps of: 1, taking the fish scales and immersing hydrochloric acid to remove calcium; washing to be neutral, drying and crushing; 2, adding the calcium-removed, dried and crushed fish scales obtained by the step 1 into an enzyme reactor; adding water and mixed protease to extract the collagen peptide; after the extraction, carrying out enzyme deactivation and centrifuging to obtain an enzymolysis solution, wherein the mixed protease is a mixture of neutral protease, papain and flavored protease; and 3, carrying out film concentration on the enzymolysis solution obtained by the step 2; and freezing and drying a concentrated solution to obtain fish scale collagen peptide powder. According to the fish scale collagen peptide obtained by the invention, the neutral protease, the papain and the flavored protease are used for extracting the fish scale collagen peptide by an enzymic method at one step, so that not only can the enzymolysis time (9 hours)be reduced, but also the extracting rate (72.72%) of the collagen peptide is improved, and solves the special flavor problem of bitter taste of the collagen peptide and the like.
Owner:WENZHOU UNIVERSITY
Preparation method of freeze-drying ginkgo fruit
ActiveCN102138681ADip sugar evenlyModerate sweetnessFood preparationFreeze-dryingAdditive ingredient
The invention relates to a preparation method of freeze-drying ginkgo fruit, which mainly comprises the following steps of: scalding gingkoes with testa removed; removing endotesta; subjecting the gingkoes to treatments of debitterizing, curing, secondary cooking in sugar, and sugaring; freezing and drying the gingkoes in vacuum to reach the water content of less than 3% and the sugar content of 9-15% in the product; and finally adopting deoxidized packaging to obtain a freeze-drying ginkgo fruit product. As the raw materials are subjected to the debitterizing, curing and color protection treatments in the invention, the bad bitter taste of internal buds in the gingkoes is overcome, the appearance, shape and color of gingko nutlet are kept unchanged, and the crispness and hardness of the product are increased. As the secondary cooking in sugar and the sugaring are performed, nutrient components of the product are increased, and the palatability of the product is improved; the water content is lower than 3% at the same time as the biological active components in the gingko nutlet are preserved to the maximum extent due to the freezing and drying of the gingkoes in vacuum; therefore, the preparation method is suitable for preserving the product; moreover, the freeze-drying ginkgo fruit is suitable for people to carry and have in leisure and a trip.
Owner:徐州银杏源生物工程有限公司
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833549B2Reduce morbidityNot impart bitter tasteBiocidePowder deliveryNasal cavityAzelastine
Owner:MANNKIND CORP
Pharmaceutical Composition
InactiveUS20030161888A1Great tasteImprove mouth "feelAntibacterial agentsPowder deliveryLipid formationOral medication
A composition comprising cefuroxime axetil in particulate form, the particles being coated with integral coatings of a lipid or mixture of lipids which are insoluble in water in which the composition further comprises a sweetener system and a texture modifier which serves to mask the bitter taste of cefuroxime axetil upon oral administration is disclosed.
Owner:FERNANDEZ MATILDE IBANEZ +1
Decolored and debitterized Momordica grosvenori extract and preparation method thereof
InactiveCN101327244AAvoid Yield ProblemsAvoid the disadvantages of multiple column chromatography proceduresMetabolism disorderDigestive systemFruit juiceMomordica
The invention discloses an extractive of discoloring, debitterizing corsvenor momordica fruit and a preparation method thereof, the method is as follows: the corsvenor momordica fruit is cleaned, crushed and extracted to juice, the fruit juice is centrifugated, the supernatant fluid enters into the macroporous absorption resin, the resin is washed by water, alkali solvent and water in order, till the effluent liquid is neutral; and then is eluted by 30 percent -65 percent ethanol, the ethanol eluent is collected, ethanol is recovered and dried, and then the extractive is obtained. The technology is simple, the production cost is low, and the production is easy to control; the traditional heating extracting method is replaced by extracting the juice, so the energy consumption is reduced, the color of the obtained extractive is light, the impurity is little, and the product quality is better; the adopted absorption resin achieves better discoloring, debitterizing effects at the same time of enriching the active components completely, the content of momordica glycosides is improved, the pigment and the impurity in the extractive are removed completely through eluting; the obtained product is white powder, the taste is sweet and pure, without traditional bitter taste, wherein, the gypenosides weight content of momordica glycosides can reach over 90 percent, and the V weight content of momordica glycosides can reach over 60 percent.
Owner:卢照凯 +1
Compositions Comprising Azelastine and Methods of Use Thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Honeysuckle tea and processing method thereof
InactiveCN103190498AOvercome the defect of long-term drinking injury to the stomachMaintain the efficacy of the drugPre-extraction tea treatmentBitter tasteTea leaf
The invention provides a processing method of the honeysuckle tea, and relates to the technical field of tea processing. The method mainly comprises the following steps of: picking up and treating the honeysuckle, picking up fresh tea, withering tea green, cutting up the honeysuckle, evenly mixing honeysuckle with the tea, rolling, fermenting, extracting fragrance in a drying way, carrying out vacuum package and the like. According to a 'curing' process, the honeysuckle tea provided by the invention is weak in irritation, more gentle and temperate, can play the functions of the red tea for effectively promoting the stomach digestion, promoting the appetite, carrying out detumescence and diuresis, strengthening the heart and the like, can keep the pesticide effect action of the honeysuckle, and keeps the health protection effect of the honeysuckle. The juice of the honeysuckle can be sufficiently absorbed by the tea according to the rolling and fermenting of both of the red tea and the honeysuckle, and the taste of the honeysuckle and that of the tea are mediated with each other, so that the mouth feel is more delicious, the bitter taste of the honeysuckle disappears, and the tea has better sweet aftertaste.
Owner:福建省柘荣县天康茶业有限公司
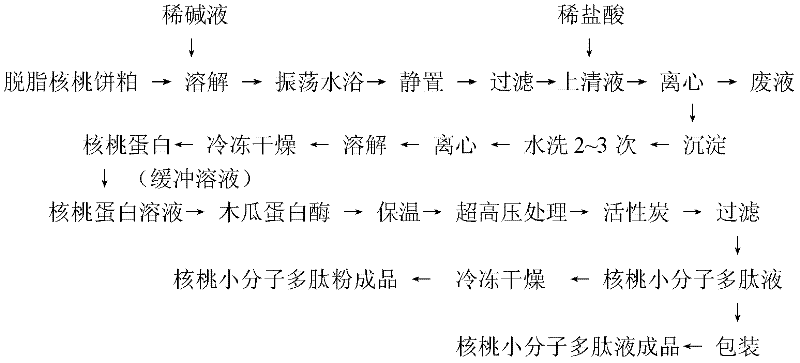
Walnut low molecular weight polypeptide and preparation method thereof
ActiveCN102406050ASimple compositionRaise the ratioProtein composition from vegetable seedsFood additiveFreeze-drying
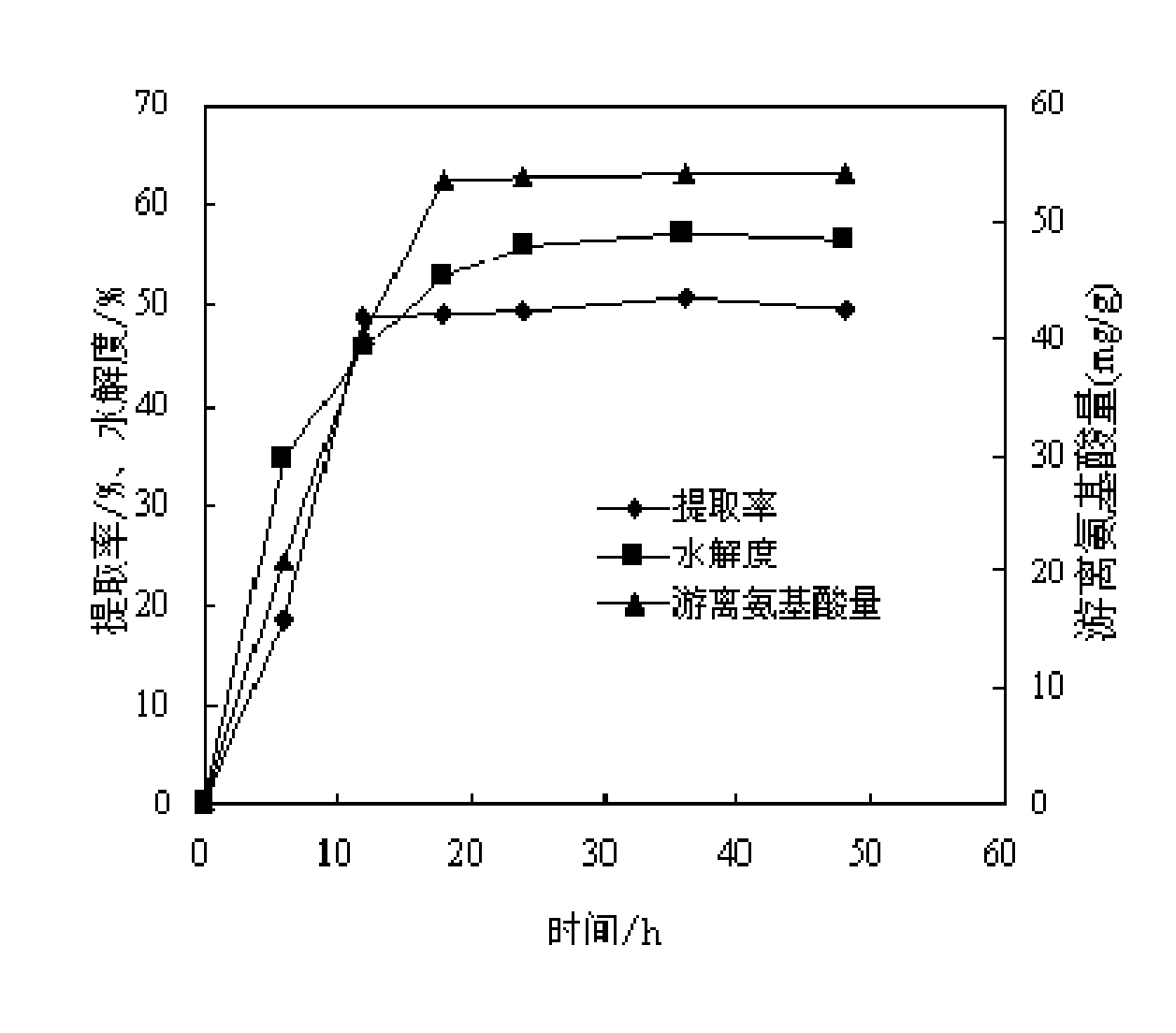
The invention discloses a walnut low molecular weight polypeptide and a preparation method thereof. The preparation method comprises the following steps: hydrolyzing walnut albumen powder through a high static pressure technique combined with a biological enzyme; decoloring and adsorbing to remove bitter substances in the walnut low molecular weight polypeptide, and then preparing the walnut low molecular weight polypeptide with high purity, good quality and high low molecular weight peptide proportion; and directly packaging or carrying out freeze drying to obtain the finished walnut low molecular weight polypeptide product. The walnut low molecular weight polypeptide disclosed by the invention can be widely applied to medicine raw materials, medicaments, food, health care products, foodadditives and the like. By using the method disclosed by the invention, the hydrolysis degree of protein is improved so that the proportion of the walnut low molecular weight polypeptide is improved,thereby being more beneficial to the absorption and utilization of walnut polypeptide, enhancing the nutrition and health care effects of walnut and improving the product additional value of walnut. Thus, the walnut low molecular weight polypeptide has a good market application prospect.
Owner:厦门元之道生物科技有限公司
Processing method for reducing bitter taste of green tea and product
The invention provides a processing method for reducing bitter taste of green tea and a product. The method comprises the following steps: 1), grading fresh tea leaves, removing non-tea impurities, and spreading the tea leaves to cool the tea leaves under a certain temperature and humidity conditions or a natural condition till the water content of the fresh tea leaves is about 70 percent; 2) subjecting graded fresh tea leaves to deactivation of enzymes to fully destruct the activity of enzymes and reduce the water content in the fresh leaves; 3) subjecting the tea leaves subjected to primarydeactivation of enzymes to secondary deactivation of enzymes, and re-softening the leaves; 4) re-moisturizing the leaves subjected to secondary deactivation of enzymes or sealing the tea leaves till the tea leaves turning yellow; 5) twisting at controlled temperature and humidity; 6) drying the twisted leaves, drying the twisted leaves over gross fire till the water content is 20 to 30 percent, and drying the twisted leaves over a high fire till the water content is about 5 percent; and 7) packaging. In the invention, the deactivation of enzymes is performed twice, the re-moisturizing and sealed yellowing are performed under certain temperature and humidity conditions, and twisting at controlled temperature and humidity is performed. Compared with the conventional processing technique, the method obviously relieves the bitter taste of green tea and makes the flavor of the product more mellow.
Owner:SHENZHEN SHENBAO HUACHENG TECH
Chinese medicinal composition for treating symptom of gallstones and preparation method thereof
InactiveCN101850086AAchieve standardizationAchieve scaleAnthropod material medical ingredientsDigestive systemBile ducts stonesMyrrh
The invention discloses a new Chinese medicinal composition for treating the symptom of gallstones and a preparation method thereof. The Chinese medicinal composition mainly comprises the following raw material medicaments: pangolin scales, immature bitter orange, curcuma aromatica, green tangerine peel, chicken's gizzard-membrane, peach seed, safflower, common burreed rhizome, zedoary, rhizoma corydalis, frankincense, myrrh, dragon's blood, Chinese honeylocust spine, root of red-rooted salvia, sappan wood, dried lacquer, turtle shell, rhubarb, Chinese dwarf cherry seed, golden thread, honeysuckle, artemisia capillaries, longhairy antenoron herb, pantain seed, talc and Japanese climbing fern spore. The Chinese medicinal composition of the invention can be prepared into any common oral administration preparation according to the conventional method of Chinese medicinal preparations. The Chinese medicinal composition can improve symptoms such as pain, paroxysmal aggravation, abdominal distension, bitter taste, constipation, yellow urine, ictericsclera, yellow body and the like which are caused by gallstones obviously, call away the gallstones, bile duct stones and stones in intrahepatic bile ducts, and has the definite clinical curative effect and obvious curative effect on the treatment of cholecystitis. The Chinese medicinal composition has the advantages of low cost, no toxic or side effect basically and the like.
Owner:TAIYI HEPU BEIJING RES INST OF TCM
Novel Process for the Preparation of Rebaudioside D and Other Related Naturally Occurring Sweetners
A novel process for preparation of Rebaudioside D (RD), and other related naturally occurring sweeteners is provided. RD is a natural sweetening agent which can decrease the bitter aftertaste of steviol glycosides. The said process is suitable for commercial manufacturing by using readily available natural products and nontoxic reagents.
Owner:PHARMA SHANGHAI
Micro ventilation lime wood antrodia camphorate cultivation method
The invention relates to a 'micro ventilation lime wood antrodia camphorate cultivation method', and belongs to the field of fungus cultivation. The method is characterized by comprising: inoculating a liquid strain onto a camphorwood section with the moisture content of 50 to 70 percent; and performing micro ventilation culture to culture fruit bodies at a temperature of 25 to 28 DEG C and a humidity of 85 to 95 percent. In the method, camphorwood is treated, the liquid strain obtained by liquid fermentation and reproduction is inoculated onto the surface of camphorwood, culture is performed in a micro ventilation mode by controlling culture temperature, humidity, light and other conditions, the growth period is 11 months, the biological conversion rate is 15 percent, the polysaccharide content is equal to that of wild antrodia camphorate, the antrodia camphorate cultivated on the lime wood has the same appearance, fragrance, bitter taste, medicinal effectiveness and the like as wild antrodia camphorate.
Owner:QINGDAO AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com