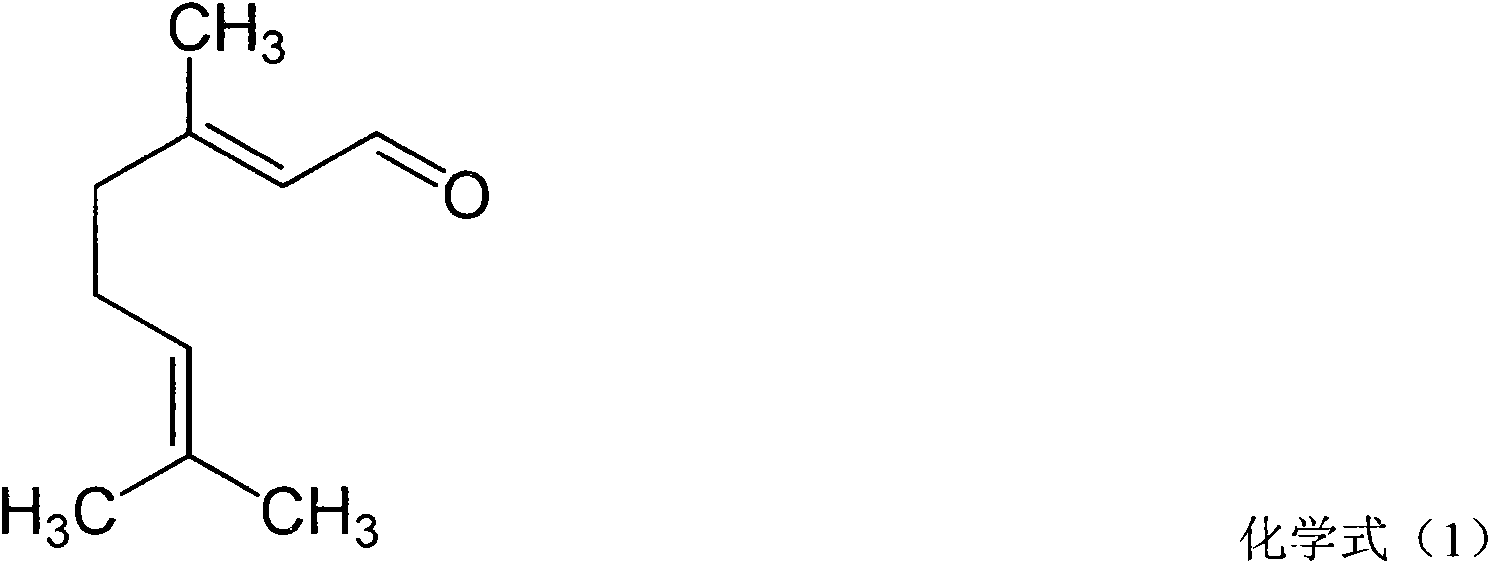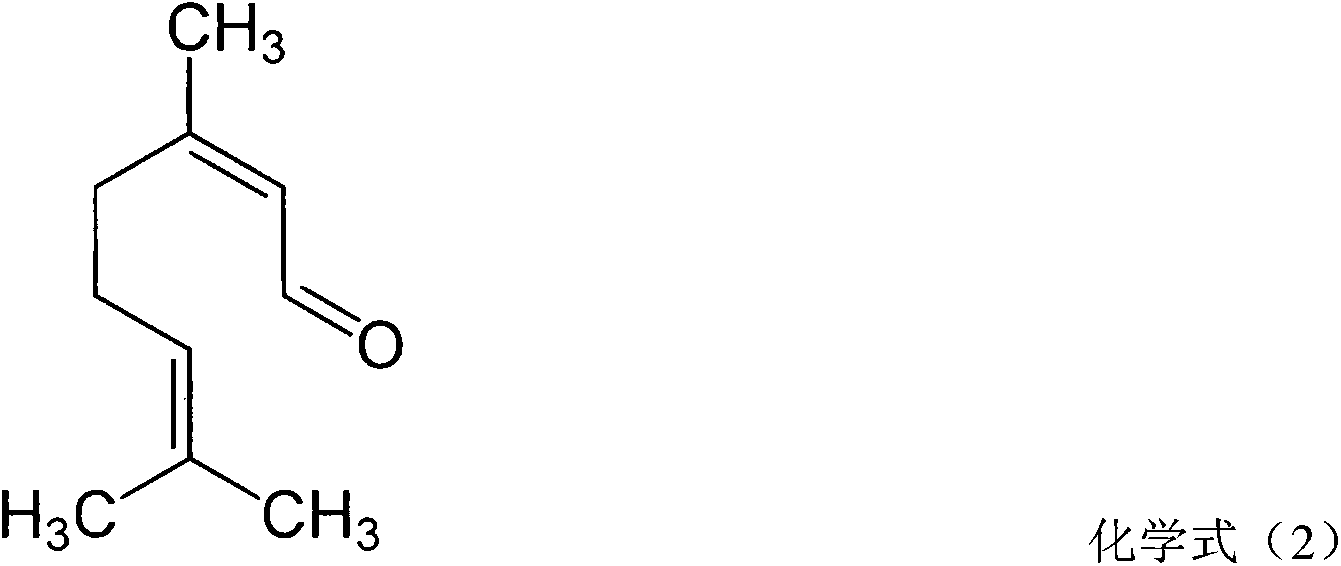Patents
Literature
44 results about "Azelastine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to relieve nasal symptoms such as runny/itching/stuffy nose, sneezing, and post-nasal drip caused by allergies or other conditions.
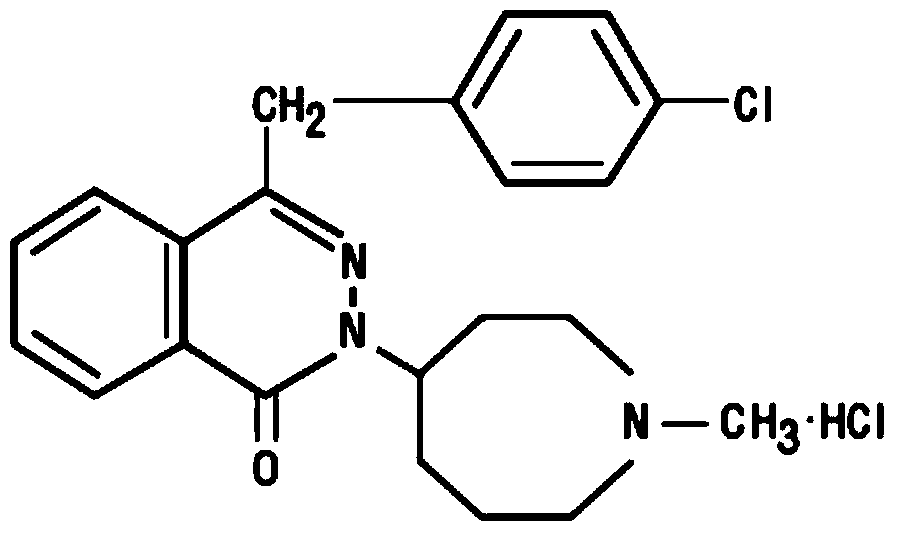
Compositions comprising azelastine and methods of use thereof
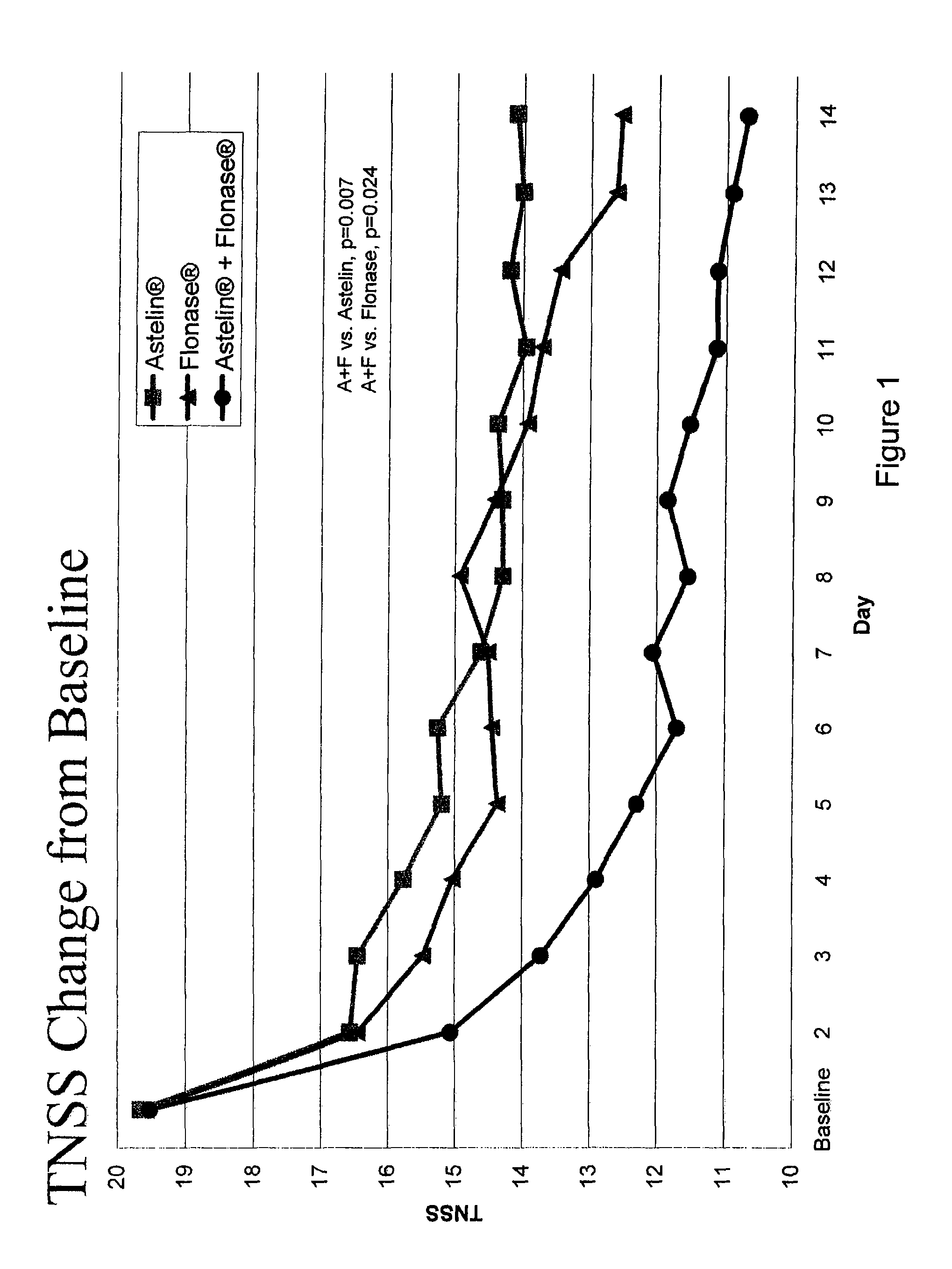
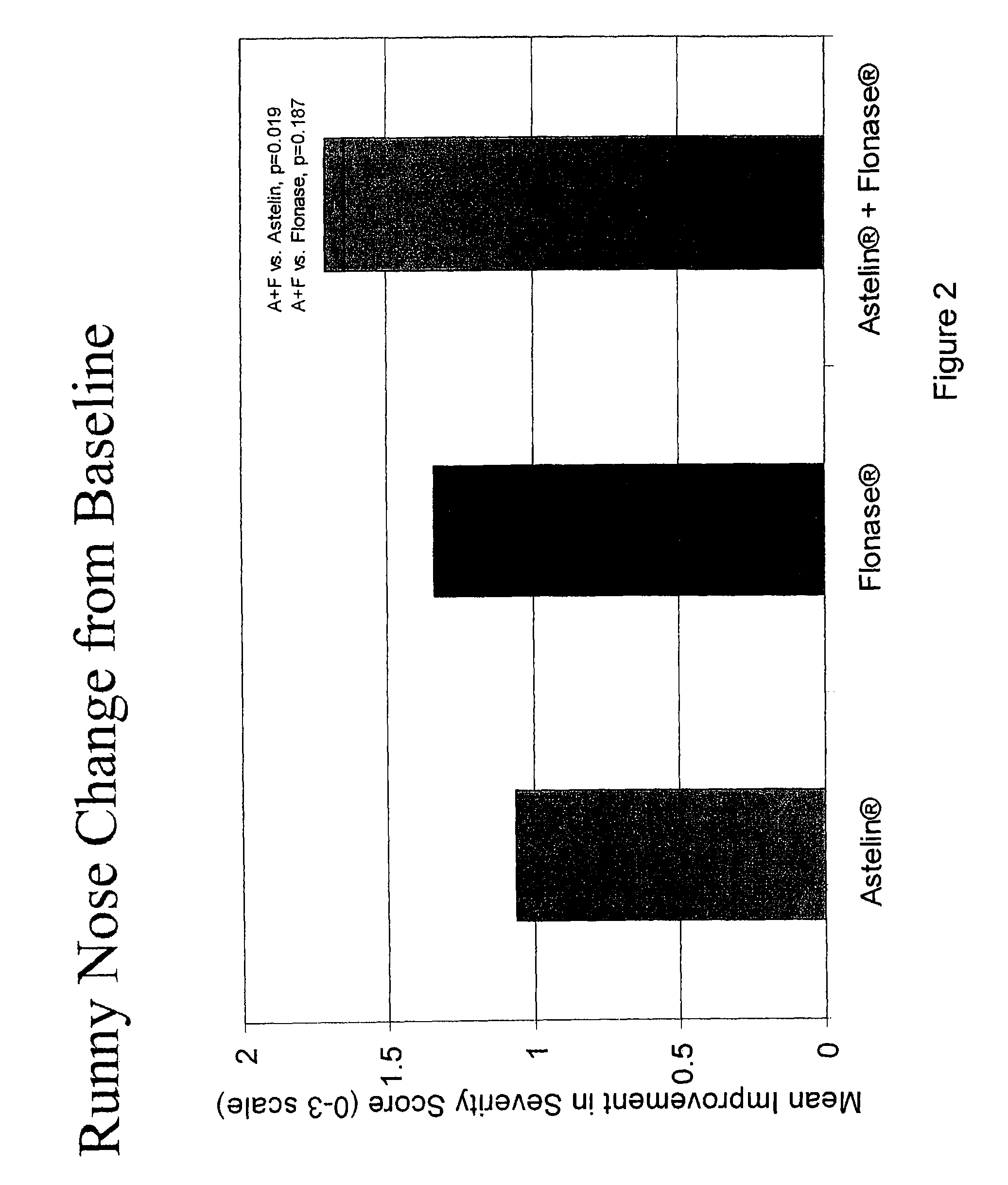
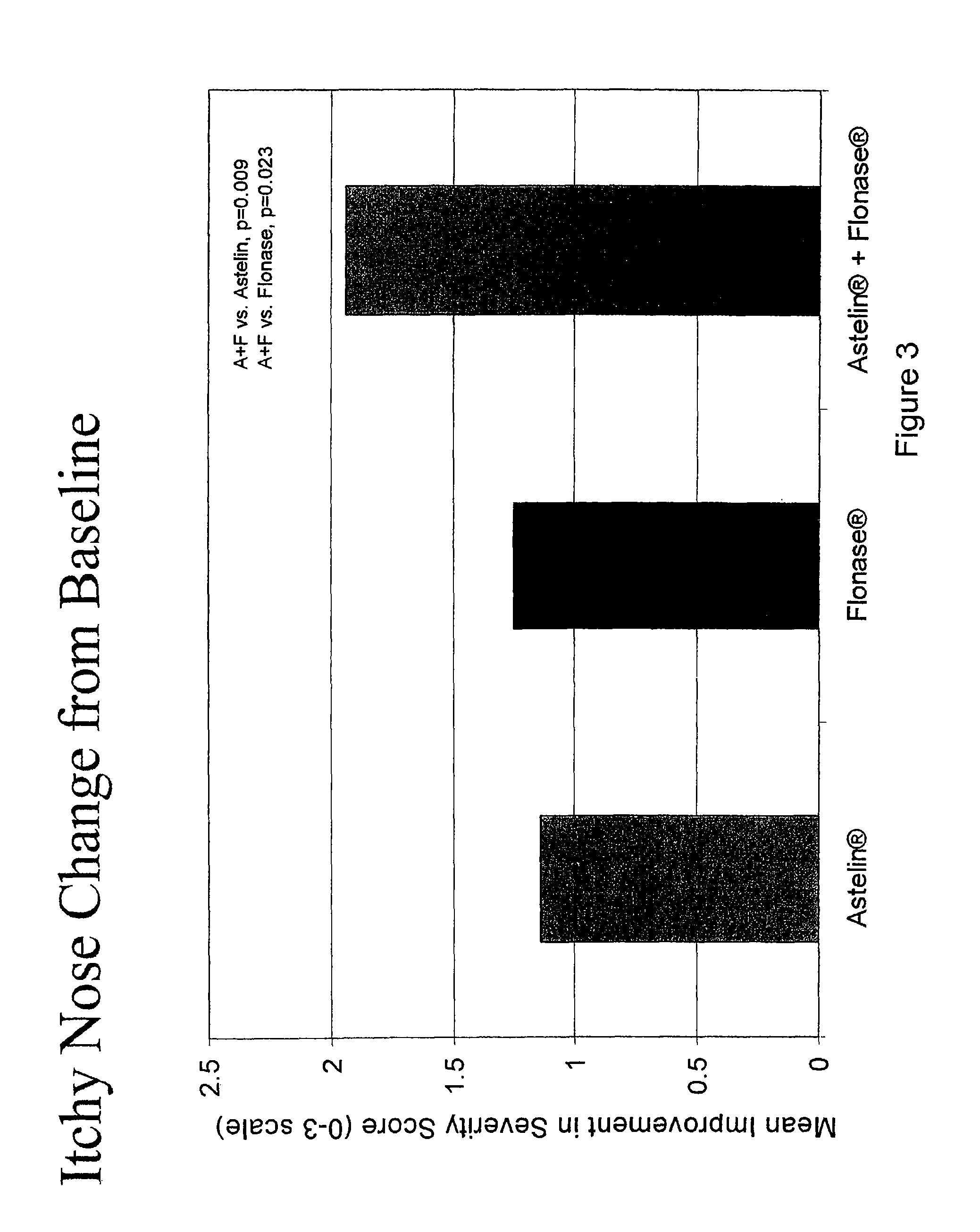
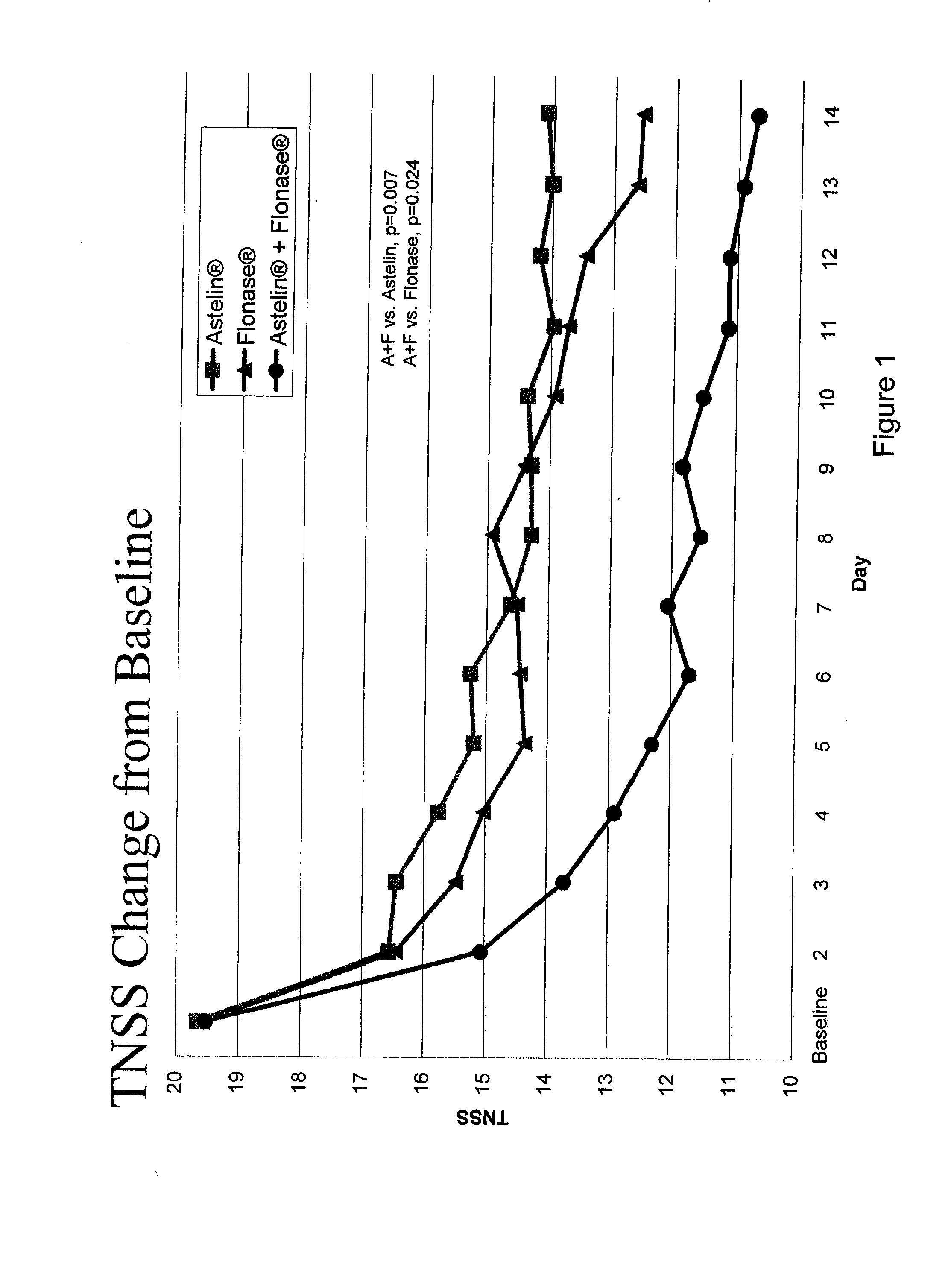
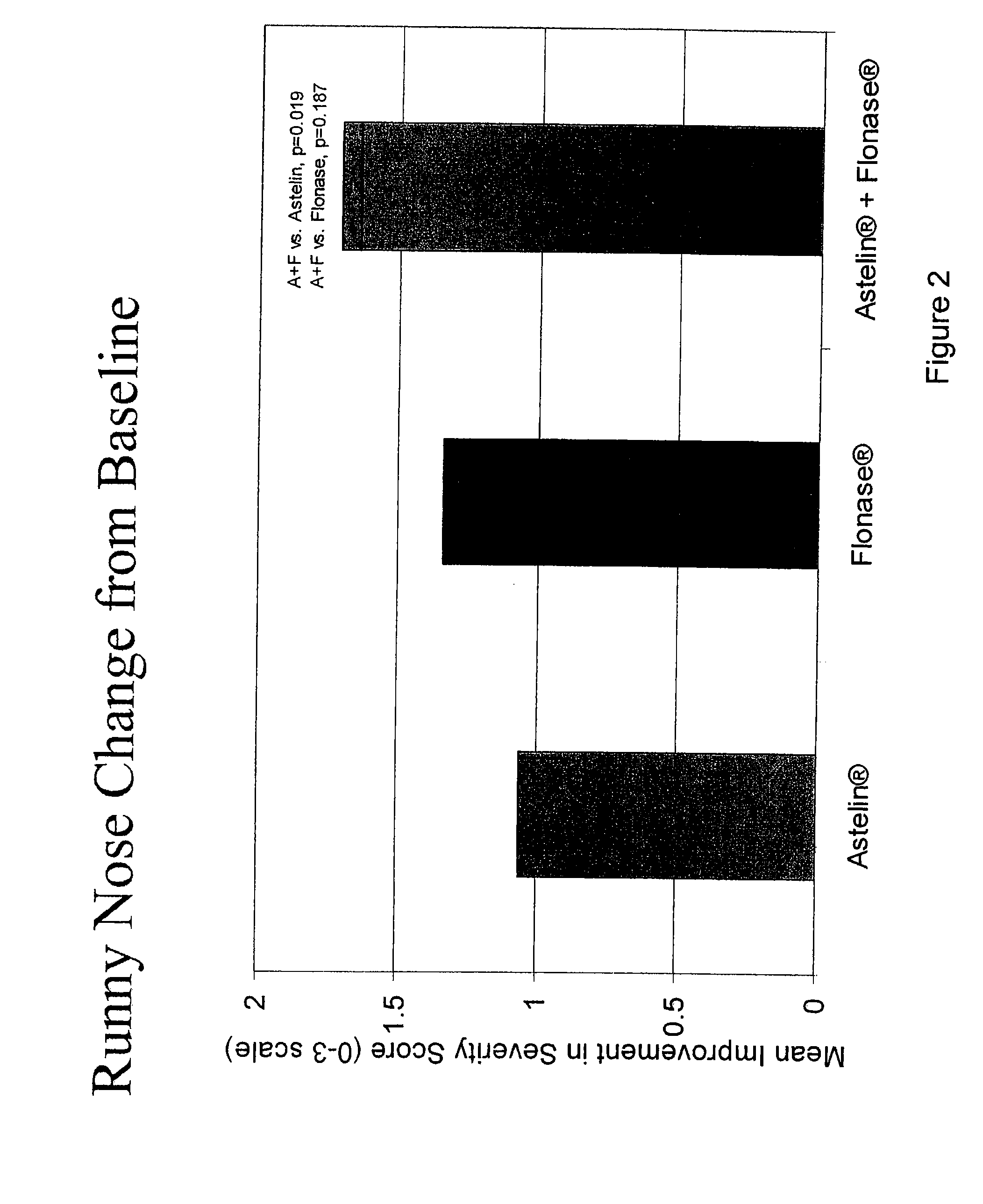
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833550B2Reduce morbidityNot impart bitter tastePowder deliverySenses disorderNasal cavityAzelastine
Dry powder formulations of drugs such as antihistamine for nasal administration are provided where the drug is retained in the nasal cavity, and systemic side effects minimized or eliminated, through the selection of a narrow particle size range, between approximately 10 and 20 microns in diameter. In a preferred embodiment wherein the drug is an antihistamine, retention of the antihistamine at the nasal mucosa is improved and the bitter aftertaste associated with liquid antihistamine formulations significantly reduced. By making a dry powder formulation of an antihistamine (e.g., azelastine) having an average particle size of between 10 and 20 microns, the antihistamine is restricted primarily to the desired target organ, the nasal mucosa. Because the active ingredient stays in the nasal region, a lower dose can be used to achieve the same desired effect. As demonstrated by the examples, this lower dose reduces the incidence of somnolence, and because the active ingredient remains at the target organ and does not accumulate in the back of the throat and mouth, this formulation does not impart a bitter taste.
Owner:MANNKIND CORP
Dry powder formulations of antihistamine for nasal administration
InactiveUS7833549B2Reduce morbidityNot impart bitter tasteBiocidePowder deliveryNasal cavityAzelastine
Owner:MANNKIND CORP
Compositions Comprising Azelastine and Methods of Use Thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Combination of loteprednol and antihistamines
The present invention relates to a novel combination of a soft steroid, in particular loteprednol, and at least one antihistamine, such as, for example, azelastine and / or levocabastine, for simultaneous, sequential or separate administration in the local treatment of allergies and airway disorders, for example of allergic rhinitis (rhinoconjunctivitis).
Owner:VIATRIS GMBH & CO KG
Method of treating Alzheimer's disease
Methods of treating patients suffering from or exhibiting symptoms of mental, behavioral, and / or cognitive disorders with azelastine or a pharmaceutically acceptable salt of azelastine is disclosed.
Owner:LA PHARMATECH INC
Pharmaceutical compositions and methods for treating Alzheimer's disease
A pharmaceutical composition containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and donepezil or rivastigmine or galantamine or a pharmaceutically acceptable salt of thereof is disclosed. A method of using the pharmaceutical composition for treating patients suffering from mental, behavioral, cognitive disorders is also disclosed.
Owner:LA PHARMATECH INC
Novel pharmaceutical compositions and methods for anxiety, depression and other psychiatric disorders
PendingUS20200323876A1Organic active ingredientsPharmaceutical delivery mechanismSeasonal Affective DisordersCompulsive disorders
Pharmaceutical compositions comprising azelastine or a pharmaceutically acceptable salt of azelastine and alprazolam are disclosed. Methods of using the pharmaceutical compositions for treating patients suffering from one or more psychiatric disorders such as major depressive disorder, generalized anxiety disorder, panic disorder, agitation, social anxiety disorder, mild chronic depression, obsessive-compulsive disorder, premenstrual dysphoric disorder, seasonal affective disorder, dysthymia, childhood enuresis, bipolar disorder, posttraumatic stress disorder, sleep disorder related to anxiety, are also disclosed.
Owner:LA PHARMATECH INC
Method of treating Parkinson's disease
Methods of treating patients suffering from or exhibiting symptoms of mental, behavioral, and / or cognitive disorders with azelastine or a pharmaceutically acceptable salt of azelastine is disclosed.
Owner:LA PHARMATECH INC
Compositions Comprising Azelastine and Methods of Use Thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders.
Owner:MEDA PHARMA INC
Method of treating dementia
Methods of treating patients suffering from or exhibiting symptoms of mental, behavioral, and / or cognitive disorders with azelastine or a pharmaceutically acceptable salt of azelastine are disclosed.
Owner:LA PHARMATECH INC
Novel pharmaceutical compositions and methods for psychiatric symptoms of patients with alzheimer's disease
Pharmaceutical compositions containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and alprazolam are disclosed. Methods of using the pharmaceutical compositions for treating patients with Alzheimer's disease for symptoms of depression, anxiety, agitation, delusions, hallucination, irritability and sleeping disorder, are also disclosed.
Owner:LA PHARMATECH INC
Pharmaceutical compositions and methods for psychiatric symptoms of patients with Alzheimer's disease
Pharmaceutical compositions containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and alprazolam are disclosed. Methods of using the pharmaceutical compositions for treating patients with Alzheimer's disease for symptoms of depression, anxiety, agitation, delusions, hallucination, irritability and sleeping disorder, are also disclosed.
Owner:LA PHARMATECH INC
Pharmaceutical compositions and methods for treating mental, behavioral, cognitive disorders
A pharmaceutical composition containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and memantine or a pharmaceutically acceptable salt of memantine is disclosed. A method of using the pharmaceutical composition for treating patients suffering from mental, behavioral, cognitive disorders is also disclosed.
Owner:LA PHARMATECH INC
Pharmaceutical compositions and methods for treating mental, behavioral, cognitive disorders
A pharmaceutical composition containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and memantine or a pharmaceutically acceptable salt of memantine is disclosed. A method of using the pharmaceutical composition for treating patients suffering from mental, behavioral, cognitive disorders is also disclosed.
Owner:LA PHARMATECH INC
Novel pharmaceutical compositions and methods for menopause related anxiety and depression
InactiveUS20210069209A1Lower estrogen levelsNervous disorderPharmaceutical delivery mechanismDuring menopauseAzelastine
Pharmaceutical compositions comprising azelastine or a pharmaceutically acceptable salt of azelastine and alprazolam are disclosed. Methods of using the pharmaceutical compositions for treating perimenopausal or menopausal patients, such as patients suffering from, experiencing, exhibiting and / or having one or more symptoms of anxiety or depression, are also disclosed.
Owner:LA PHARMATECH INC
Pharmaceutical compositions and methods for treating mental, behavioral, cognitive disorders
A pharmaceutical composition containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and memantine or a pharmaceutically acceptable salt of memantine is disclosed. A method of using the pharmaceutical composition for treating patients suffering from mental, behavioral, cognitive disorders is also disclosed.
Owner:LA PHARMATECH INC
Dry powder formulations of antihistamine for nasal administration
InactiveUS20080038358A1Reduce morbidityNot impart bitter tastePowder deliveryBiocideSide effectAzelastine
Dry powder formulations of drugs such as antihistamine for nasal administration are provided where the drug is retained in the nasal cavity, and systemic side effects minimized or eliminated, through the selection of a narrow particle size range, between approximately 10 and 20 microns in diameter. In a preferred embodiment wherein the drug is an antihistamine, retention of the antihistamine at the nasal mucosa is improved and the bitter aftertaste associated with liquid antihistamine formulations significantly reduced. By making a dry powder formulation of an antihistamine (e.g., azelastine) having an average particle size of between 10 and 20 microns, the antihistamine is restricted primarily to the desired target organ, the nasal mucosa. Because the active ingredient stays in the nasal region, a lower dose can be used to achieve the same desired effect. As demonstrated by the examples, this lower dose reduces the incidence of somnolence, and because the active ingredient remains at the target organ and does not accumulate in the back of the throat and mouth, this formulation does not impart a bitter taste.
Owner:MANNKIND CORP
Novel pharmaceutical compositions and methods for treating mental, behavioral, cognitive disorders
A pharmaceutical composition containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and donepezil or rivastigmine or galantamine or a pharmaceutically acceptable salt of thereof is disclosed. A method of using the pharmaceutical composition for treating patients suffering from mental, behavioral, cognitive disorders is also disclosed.
Owner:LA PHARMA TECH INC
Azelastine composition and use
ActiveCN104173286ASkin irritationIn line with the trend of inputAerosol deliveryPharmaceutical non-active ingredientsDiseaseAzelastine
The invention relates to an azelastine composition and use, specifically relates to liquid compositions of azelastine, particularly relates to liquid compositions of azelastine hydrochloride and more particularly relates to a nonaqueous liquid composition. In one embodiment, the pharmaceutical composition contains azelastine or pharmaceutical salt thereof, polyhydric alcohol, polyethylene glycol and polyhydric alcohol fatty acid ester. The invention further relates to a preparation method of the liquid composition and pharmaceutical use of the liquid composition, for example, the invention further relates to the use of the composition in the treatment, relief or prevention of symptoms related to allergic and non-allergic diseases and the use in the treatment of dermatitis, such as allergic and / or contact dermatitis. The pharmaceutical composition disclosed by the invention can be in the dosage form of liniment or spray, so as to facilitate clinical use.
Owner:GUIZHOU YUNFENG PHARMA
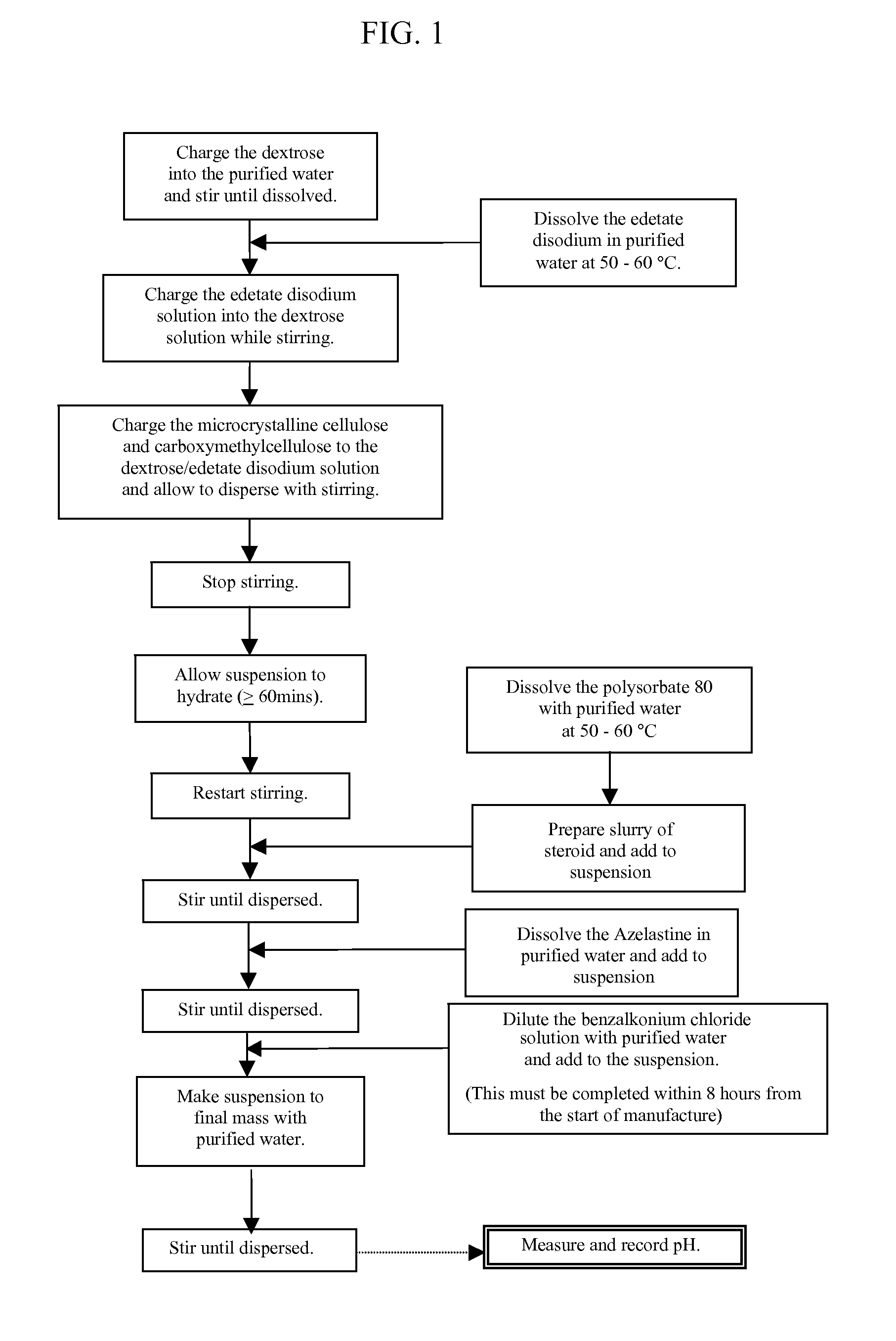
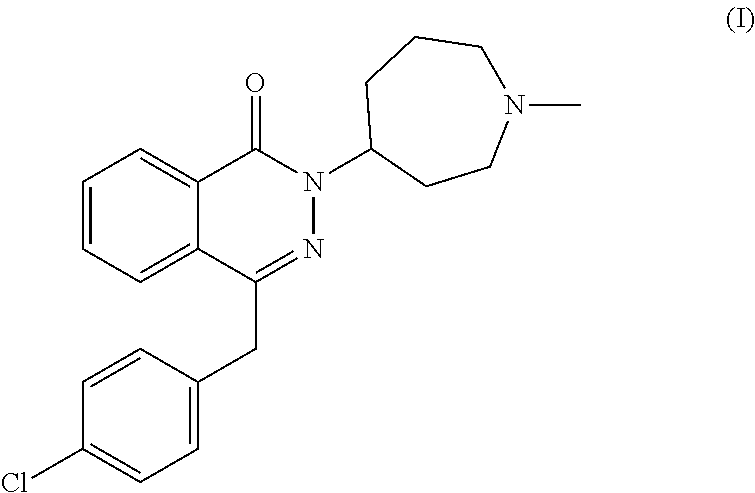
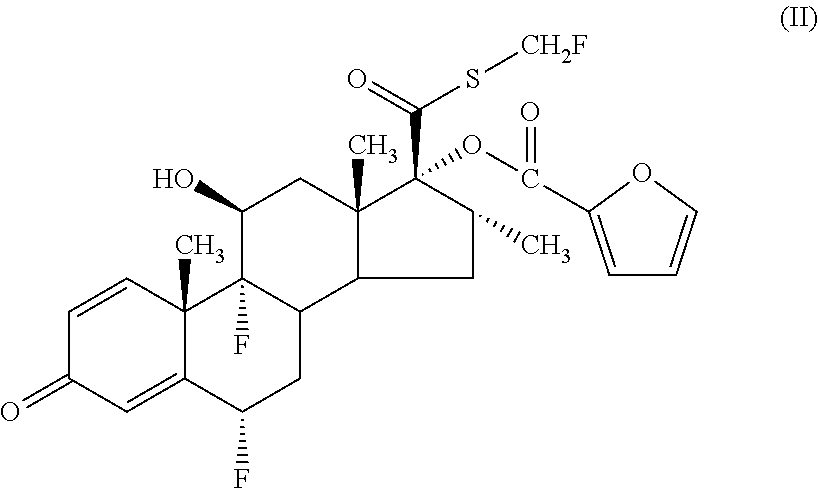
Pharmaceutical formulations comprising azelastine and a corticosteroid for the treatment of inflammatory or allergic conditions
InactiveUS20120065177A1Satisfied with stabilitySatisfactory shelf-life propertyAntipyreticAnalgesicsAllergic conditionAzelastine
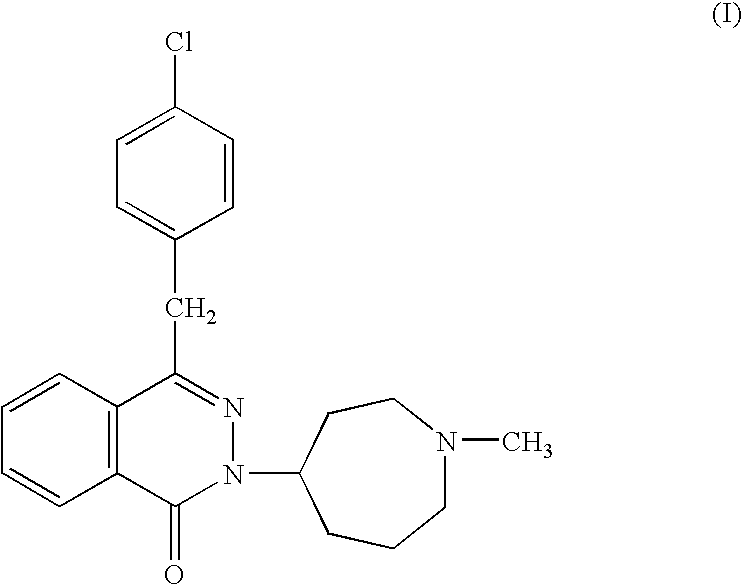
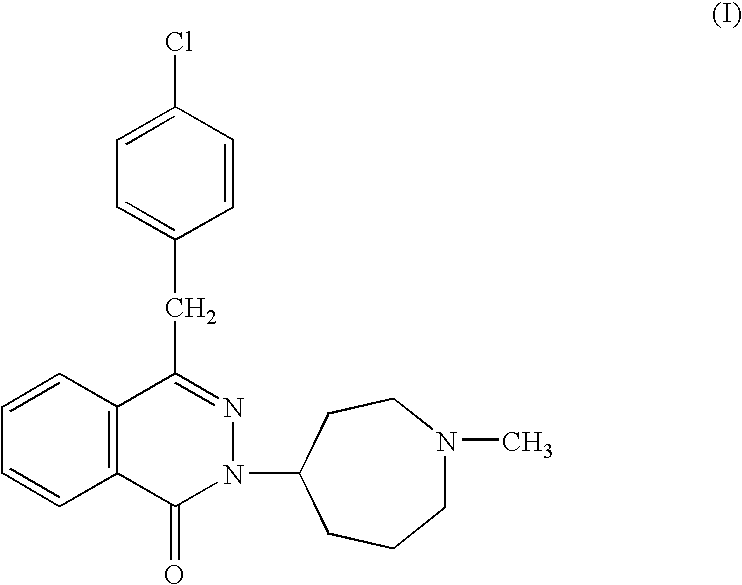
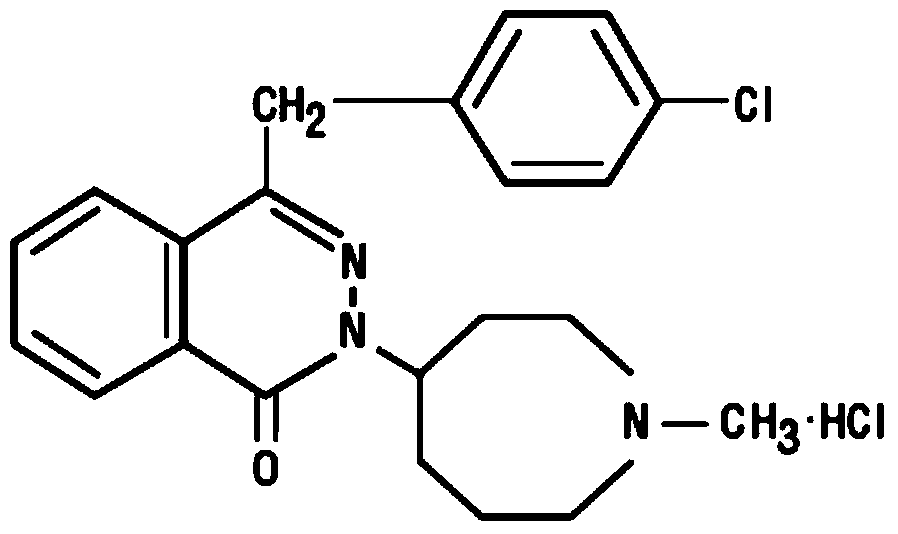
The present invention relates to method for the treatment of allergic rhinitis comprising: administrating to a patient in need thereof a pharmaceutical formulation comprising a compound for formula (I)or a salt thereof, and an anti-inflammatory glucocorticoid compound of formula (II)or a solvate thereof.
Owner:GLAXO GROUP LTD
Method of treating dementia
Methods of treating patients suffering from or exhibiting symptoms of mental, behavioral, and / or cognitive disorders with azelastine or a pharmaceutically acceptable salt of azelastine are disclosed.
Owner:LA PHARMATECH INC
Pharmaceutical compositions and methods for treating parkinson's and huntington's disease
A pharmaceutical composition containing the therapeutically active ingredients of azelastine or a pharmaceutically acceptable salt of azelastine and donepezil or rivastigmine or galantamine or a pharmaceutically acceptable salt of thereof is disclosed. A method of using the pharmaceutical composition for treating patients suffering from mental, behavioral, cognitive disorders is also disclosed.
Owner:LA PHARMATECH INC
Compositions and methods for treating alzheimer's disease and parkinson's disease
PendingUS20220096491A1Alleviate, reduce, prevent and/or eliminate one or more symptomsPharmaceutical delivery mechanismHeterocyclic compound active ingredientsMethylcobalaminAzelastine
Pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt of azelastine, and methylcobalamin are disclosed. Methods of using the pharmaceutical compositions for treating patients with Alzheimer's disease or Parkinson's disease are also disclosed.
Owner:LA PHARMATECH INC
Ciclesonide azelastine compound composition
The invention relates to a ciclesonide azelastine compound composition. The ciclesonide azelastinenasal spray composition contains (A) ciclesonide monohydrate, and (B) azelastine or salt thereof and a pharmaceutically acceptable carrier, wherein the ciclesonide monohydrate exists in a crystal form.
Owner:TIANJIN JINYAO GRP
Pharmaceutical composition of azelastine hydrochloride
ActiveCN104173352ASkin irritationIn line with the trend of inputAntipyreticAnalgesicsDiseaseAzelastine
The invention relates to a pharmaceutical composition of azelastine hydrochloride, specifically relates to pharmaceutical compositions of azelastine, particularly relates to pharmaceutical compositions of azelastine hydrochloride and more particularly relates to a nonaqueous gelatinous pharmaceutical composition. In one embodiment, the pharmaceutical composition contains azelastine or pharmaceutical salt thereof, polyhydric alcohol, polyethylene glycol and polyhydric alcohol fatty acid ester. The invention further relates to a preparation method of the pharmaceutical composition and pharmaceutical use of the pharmaceutical composition, for example, the invention further relates to the use of the composition in the treatment, relief or prevention of symptoms related to allergic and non-allergic diseases and the use in the treatment of dermatitis, such as allergic and / or contact dermatitis.
Owner:GUIZHOU YUNFENG PHARMA
Pharmaceutical composition for treating rhinitis
InactiveCN104107178AQuick effectGood curative effectSenses disorderAntipyreticAzelastineActive component
The invention discloses a pharmaceutical composition for treating rhinitis. The pharmaceutical composition contains azelastine and citral as active components. The pharmaceutical composition has azelastine content of 0.001-0.5% and citral content of 0.01-5%. According to demands, a pharmaceutically acceptable amount of a carrier and / or a solubiliser and an excipient can be used.
Owner:田华
Inflammation-antagonistic agent containing lysophosphatidic acid
InactiveCN108096260AQuick and effective controlNo painAntipyreticAnalgesicsAzelastinePolyethylene glycol
The invention provides an inflammation-antagonistic agent containing lysophosphatidic acid. The inflammation-antagonistic agent is prepared from the following raw materials: sorbitol, hydroxypropyl methylcellulose, glycine, polyethylene glycol, zinc oxide, glycerinum, gentamicin, azelastine and lysophosphatidic acid. Compared with the prior art, the inflammation-antagonistic agent provided by theinvention has the following beneficial effects that multiple drugs are integrated to play a synergistic role, so that the drug effect is obvious, the disease control is rapid and effective, not only can the treating effect for skin inflammations be obvious, but also no pain is caused to a patient; simultaneously, no layering is caused in storage, and no agglomeration is caused at the bottom of a container.
Owner:GUANGDONG YIMING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com