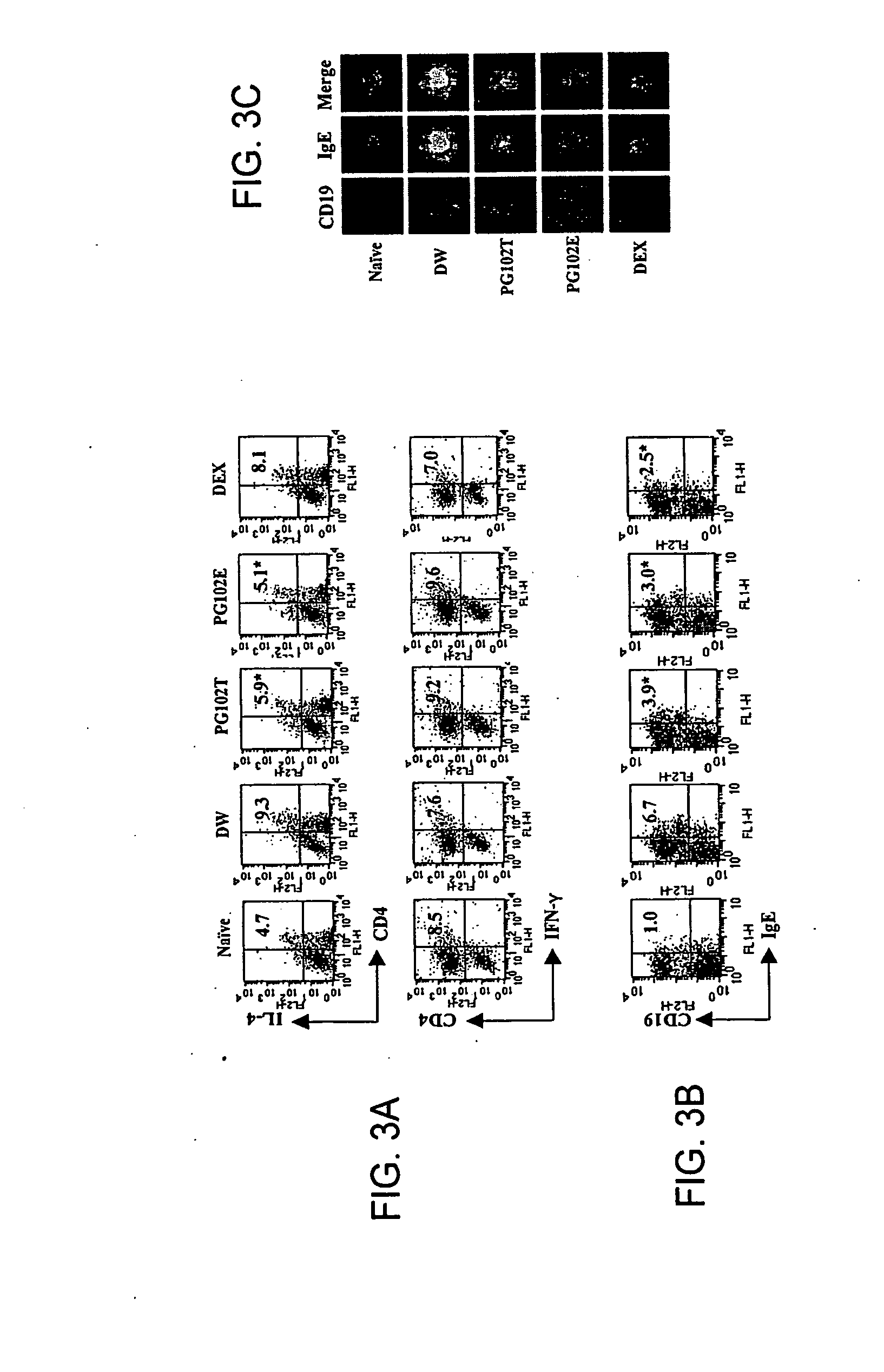
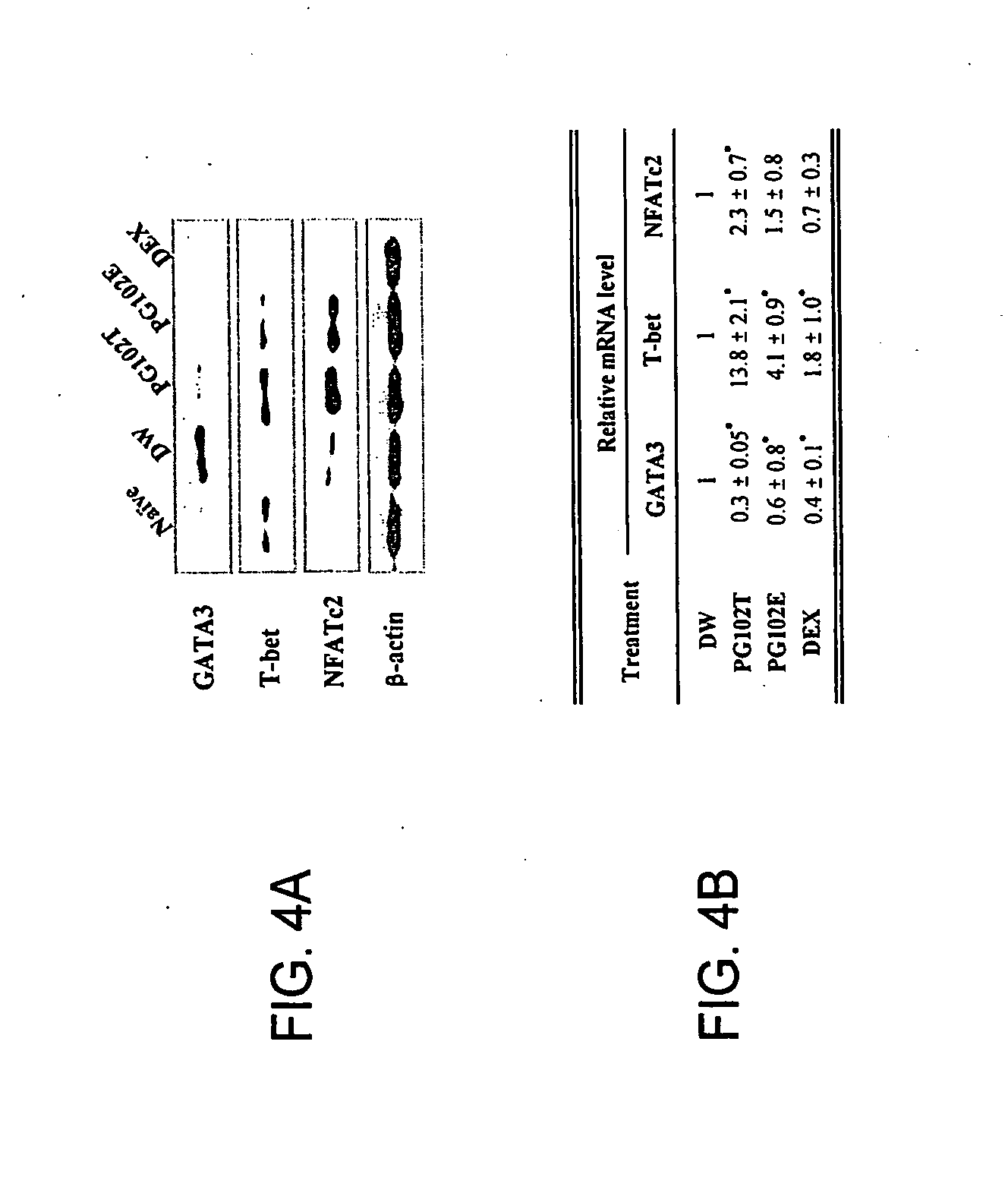
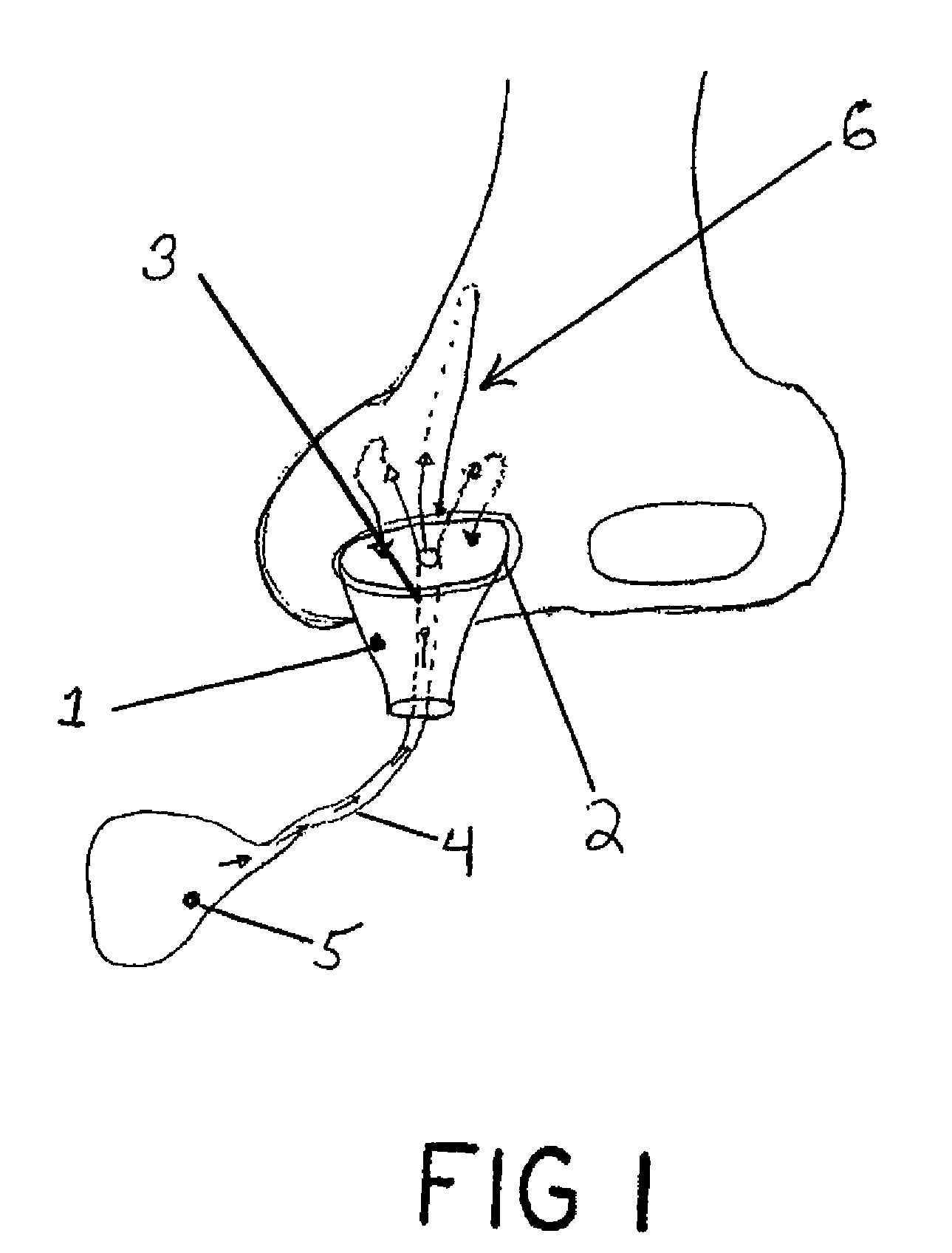
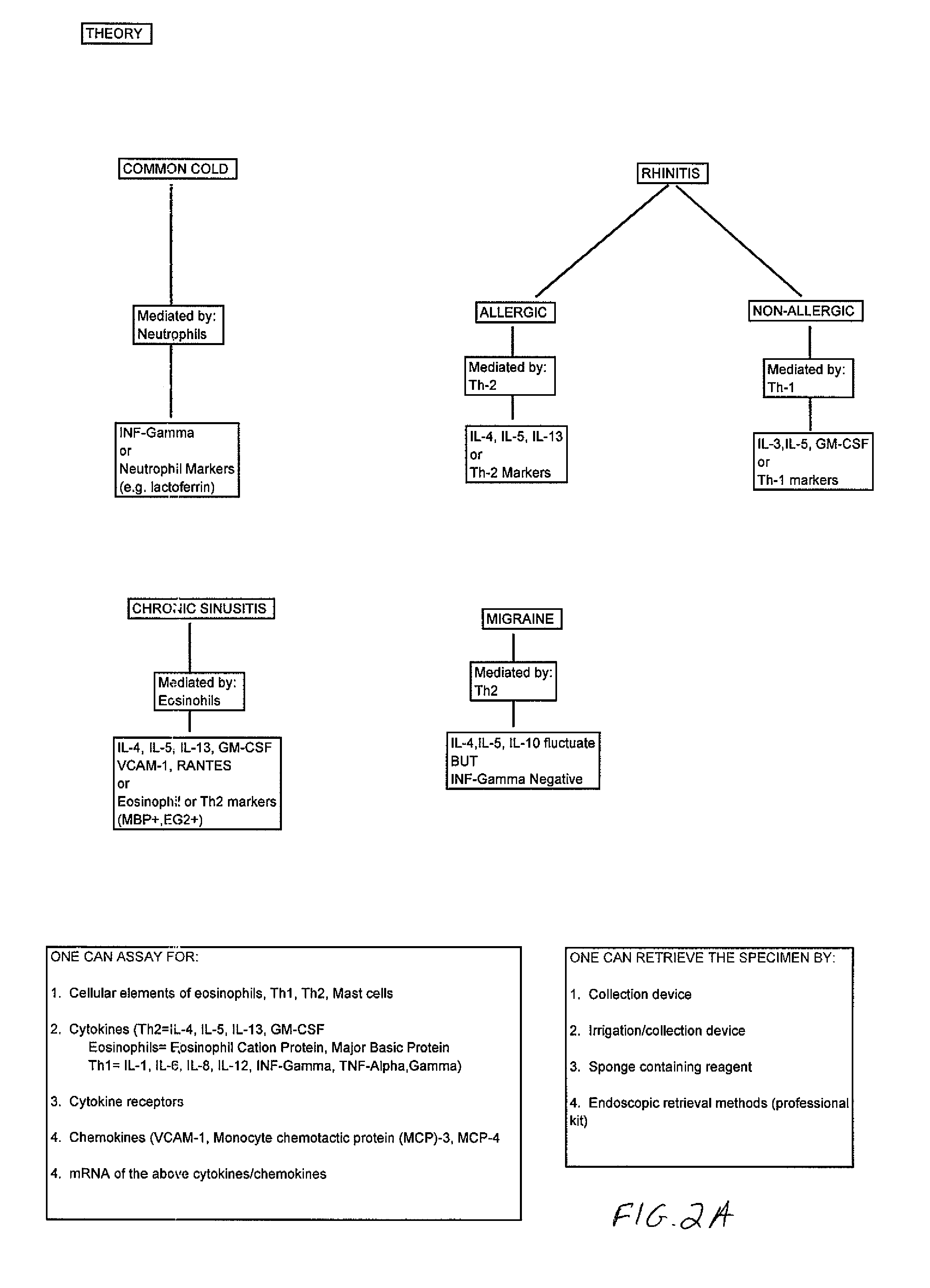
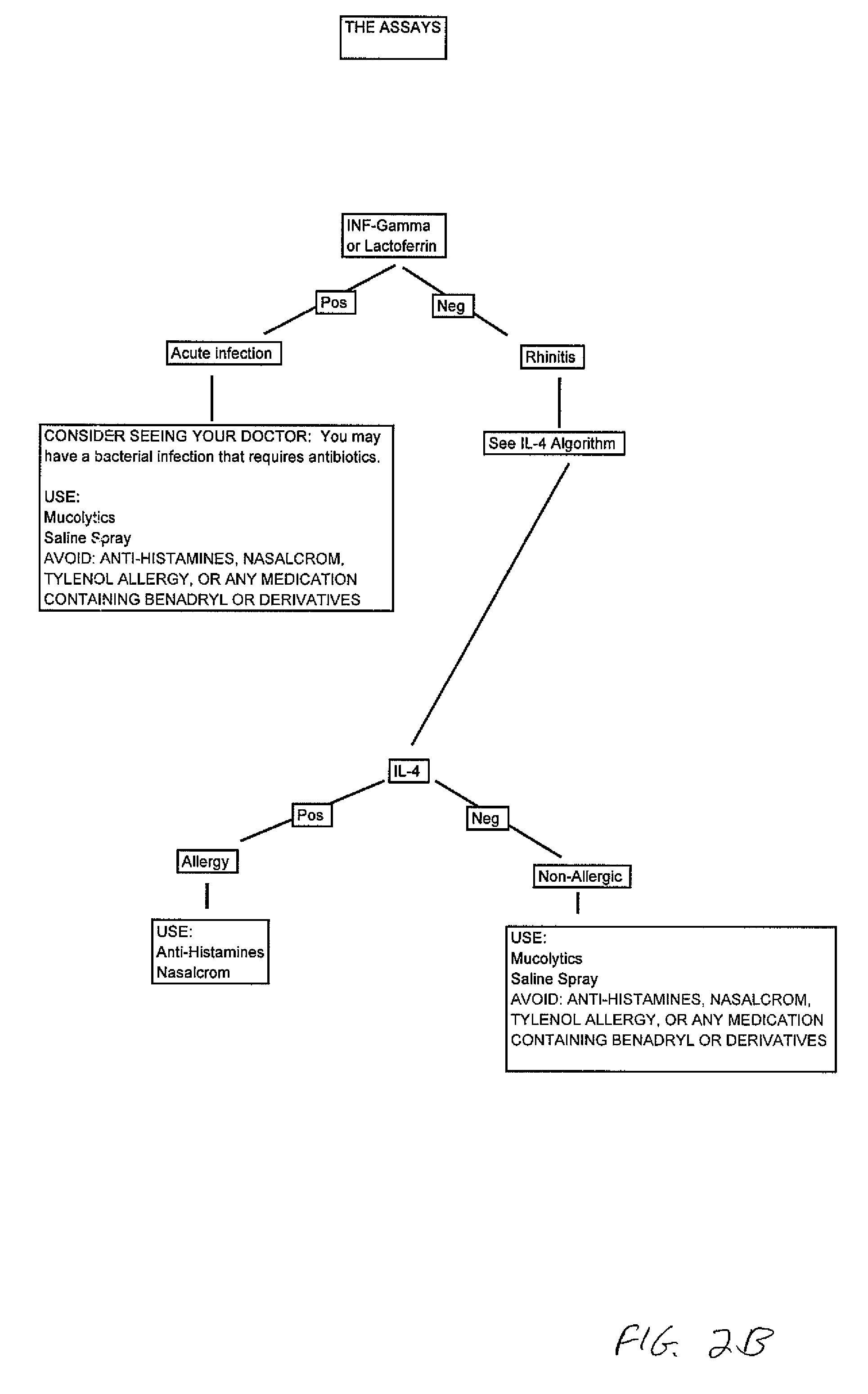
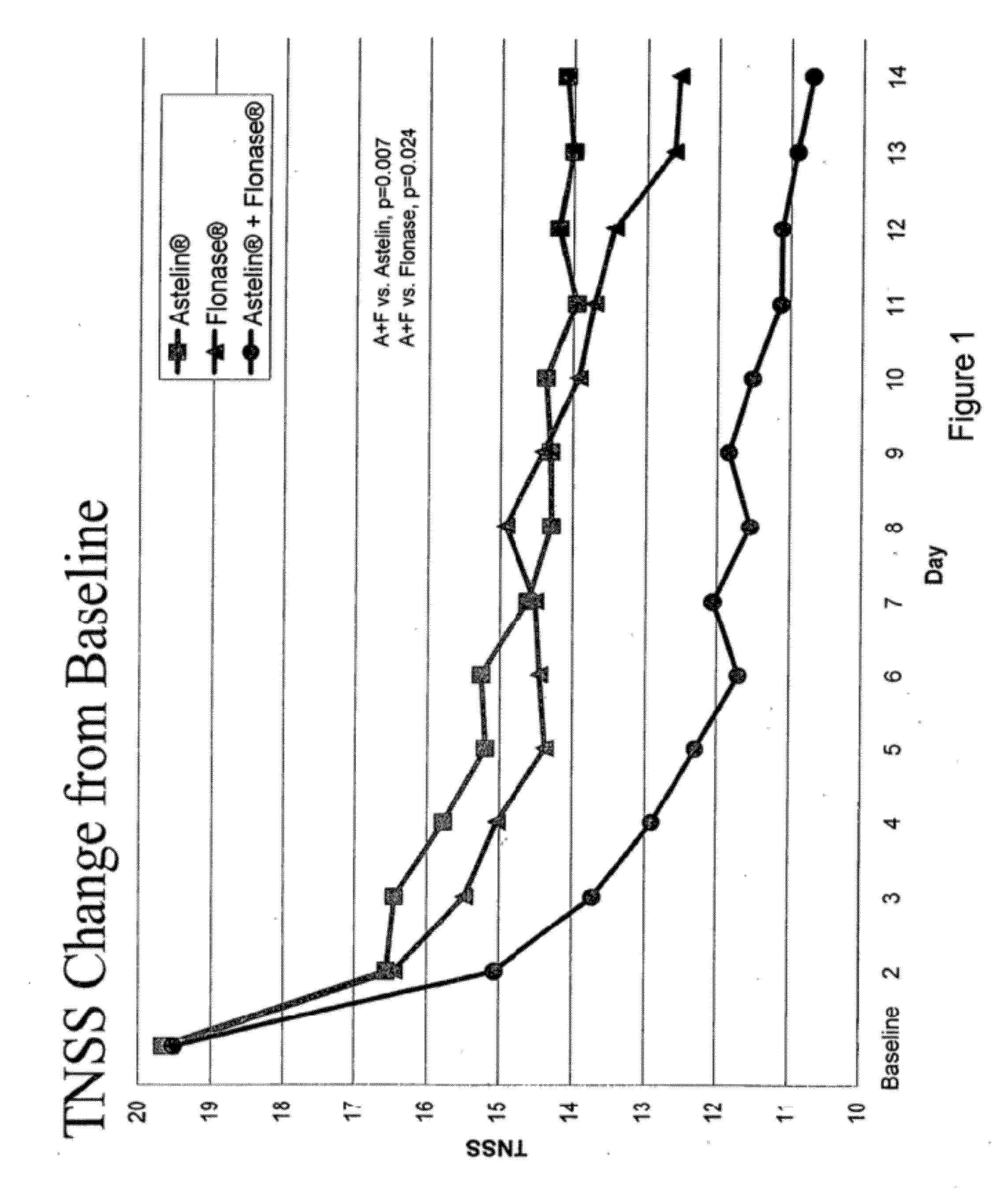
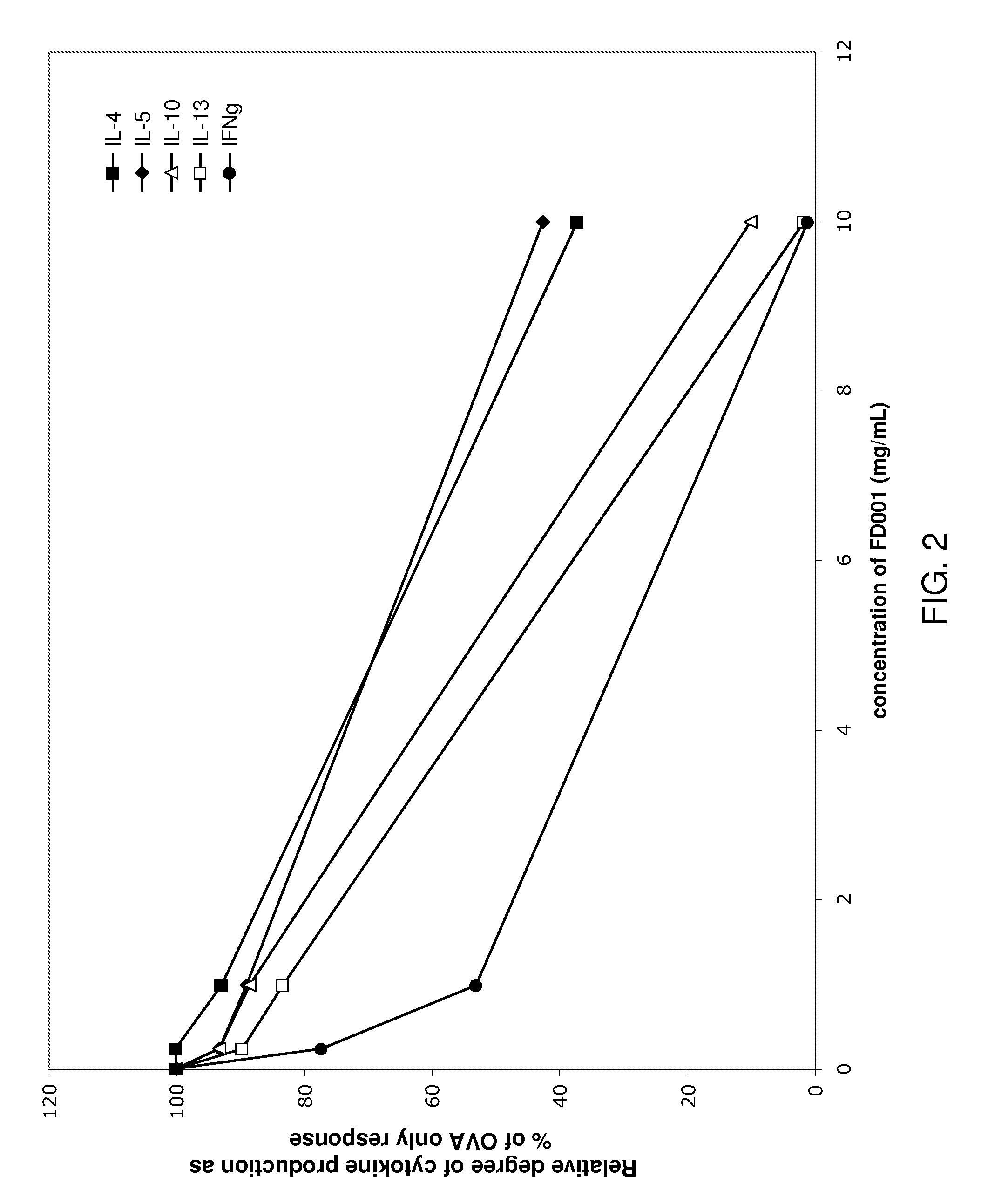
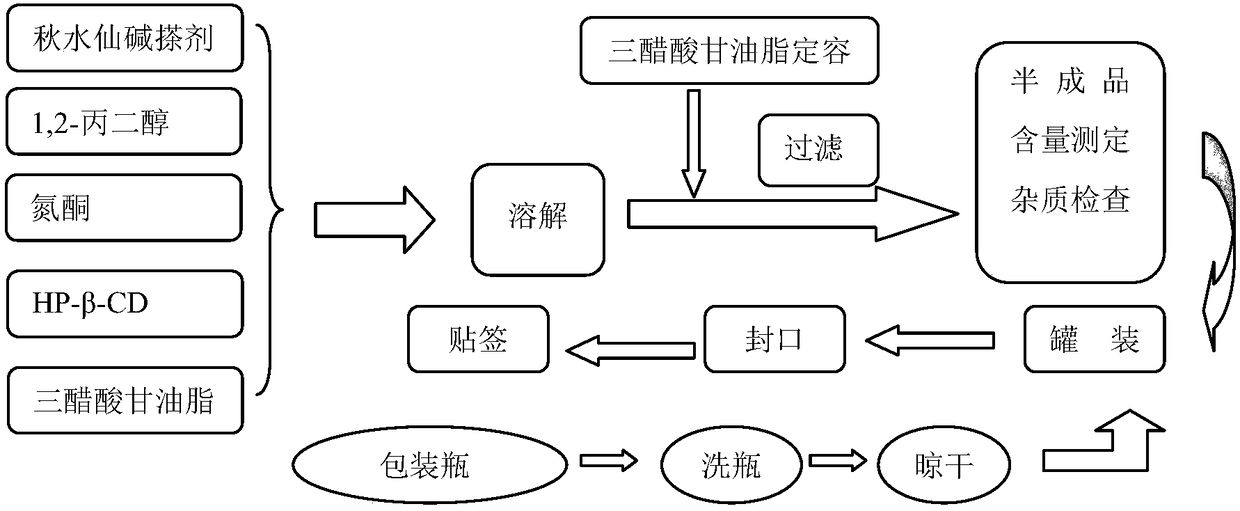
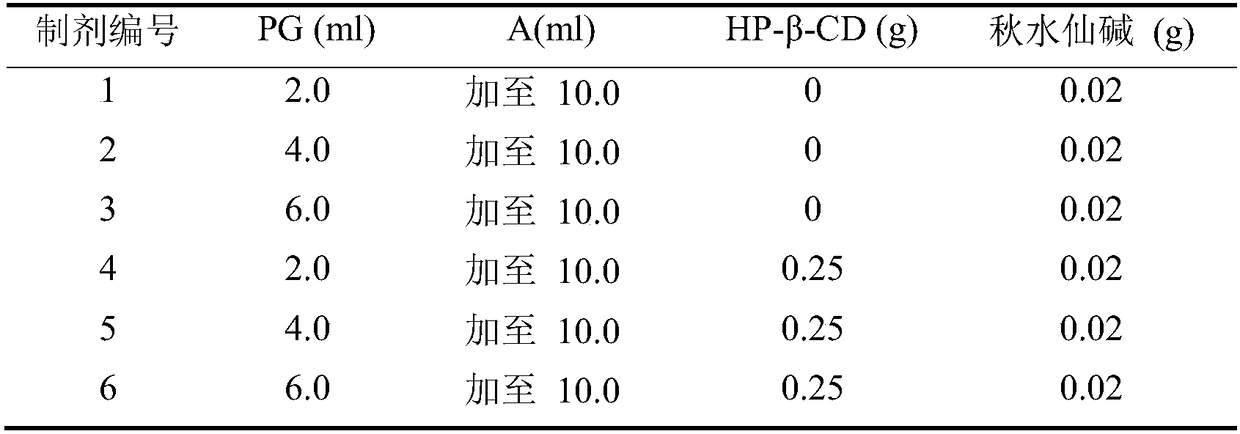
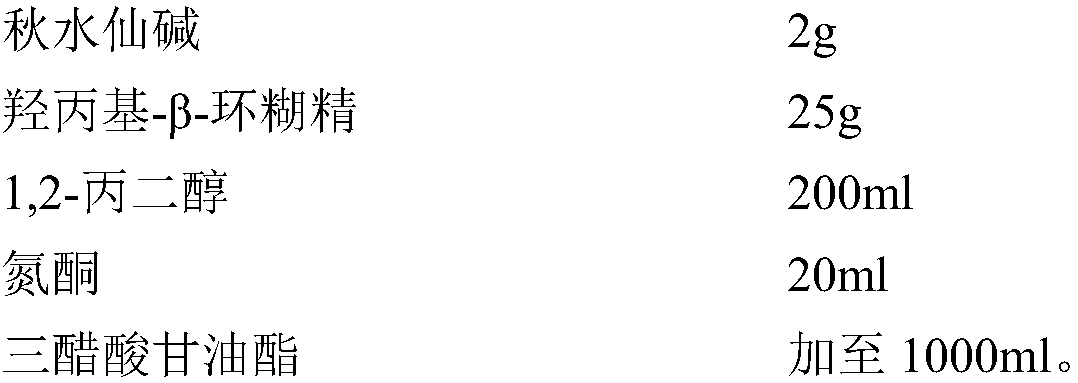Patents
Literature
80 results about "Non allergic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
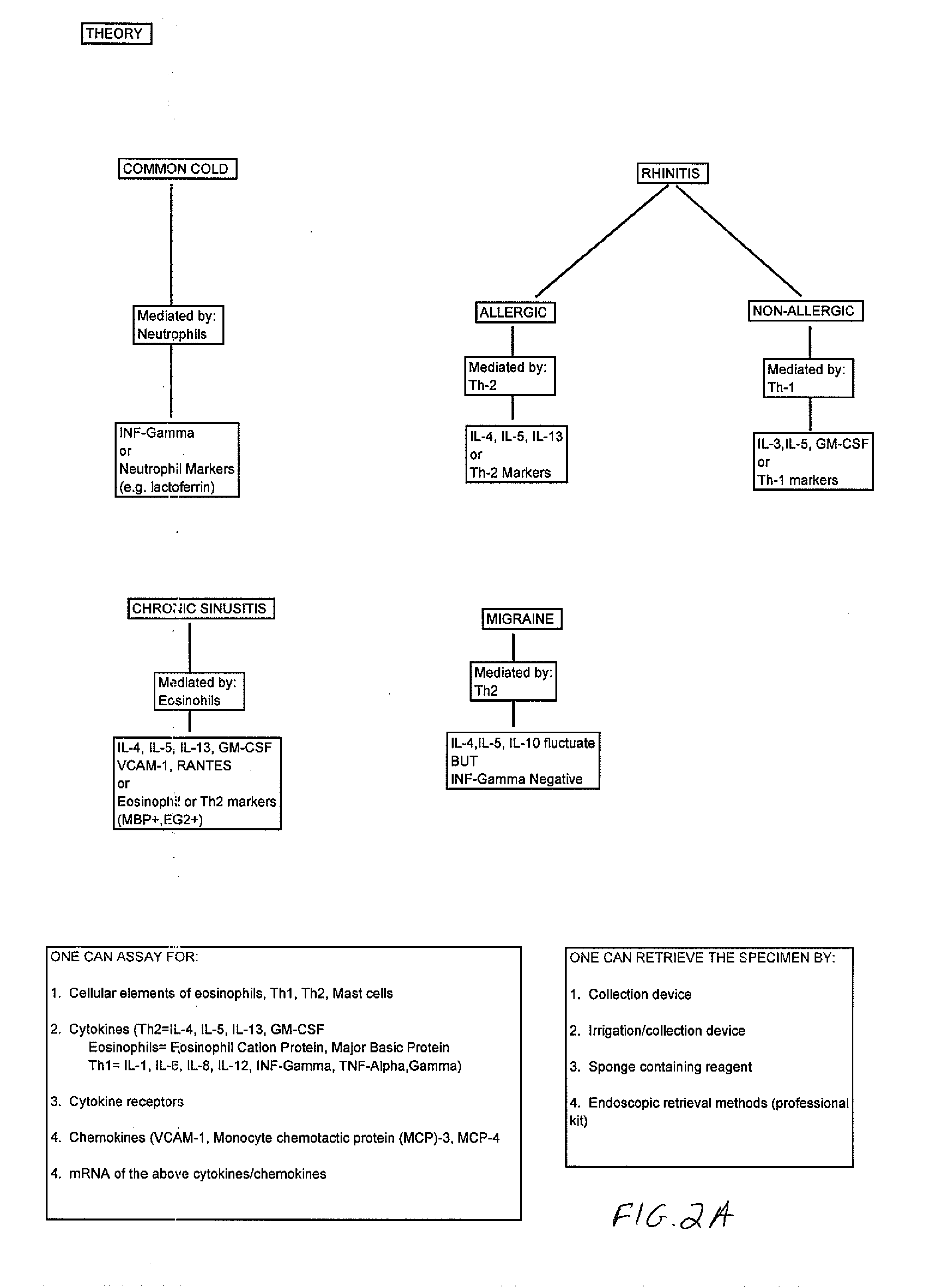
Non-allergic rhinitis is a medical condition of unknown cause, leading to symptoms very similar to allergic rhinitis, or hay-fever. Approximately half of the people suffering from allergies also have a non-allergic component to their symptoms.
Immunostimulatory nucleic acid for treatment of non-allergic inflammatory diseases
InactiveUS20050250726A1Reduces and prevents non-allergic inflammationOrganic active ingredientsGenetic material ingredientsInflammatory Bowel DiseasesNon allergic
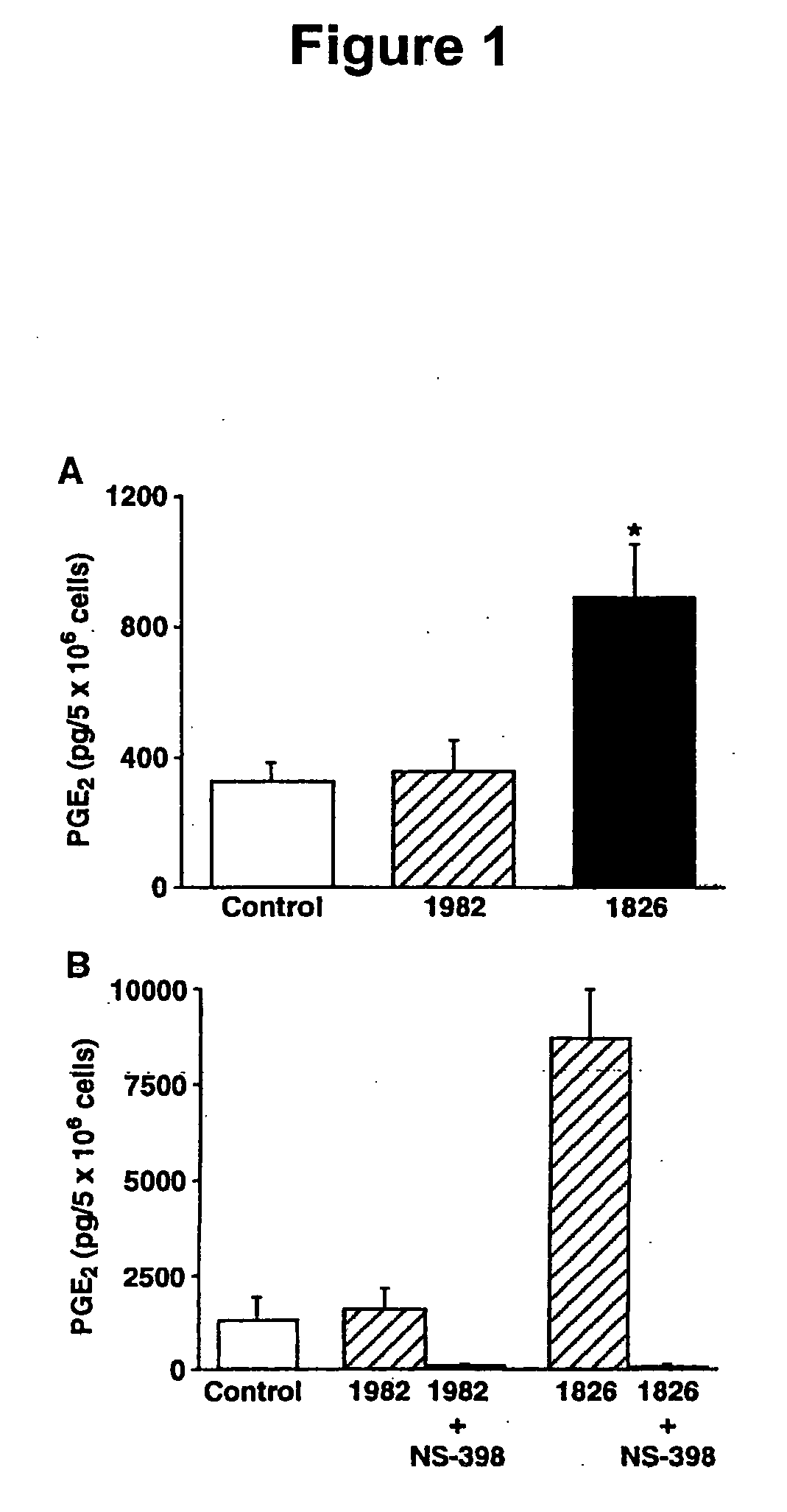
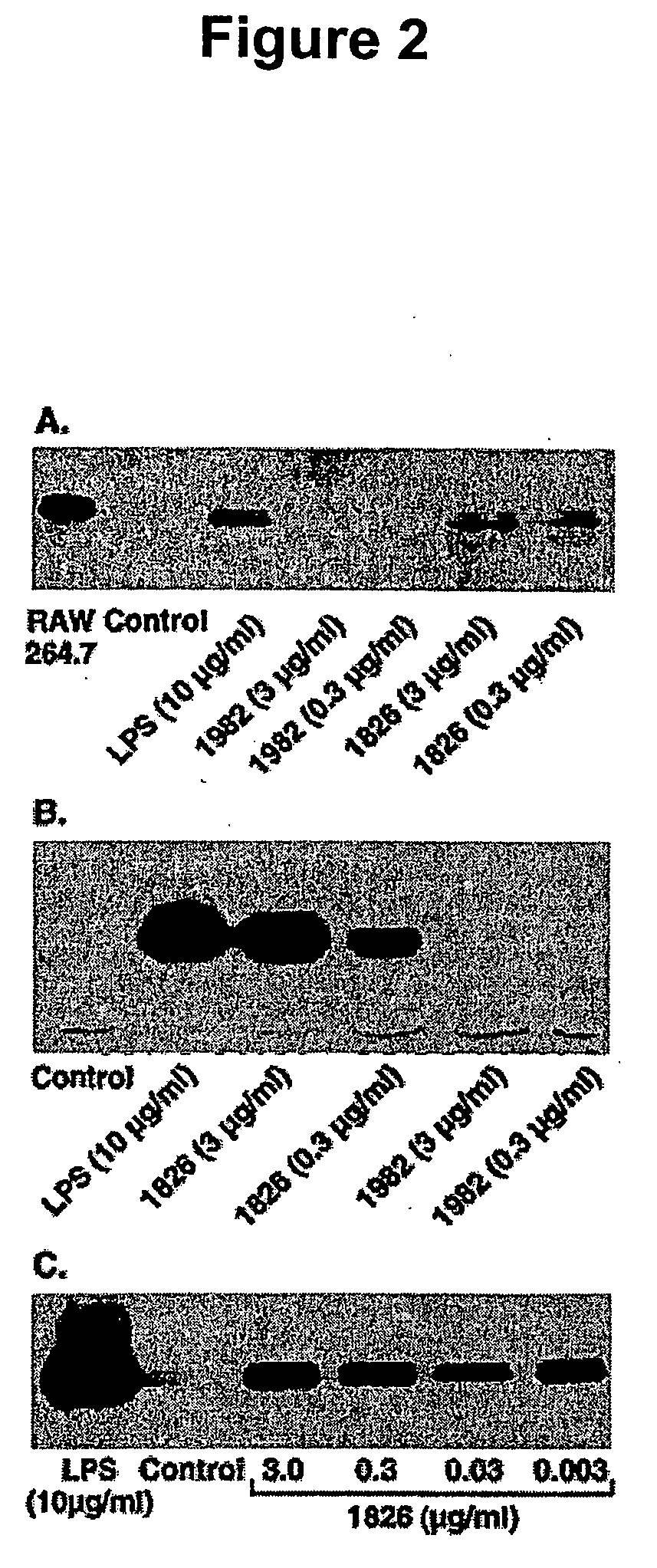
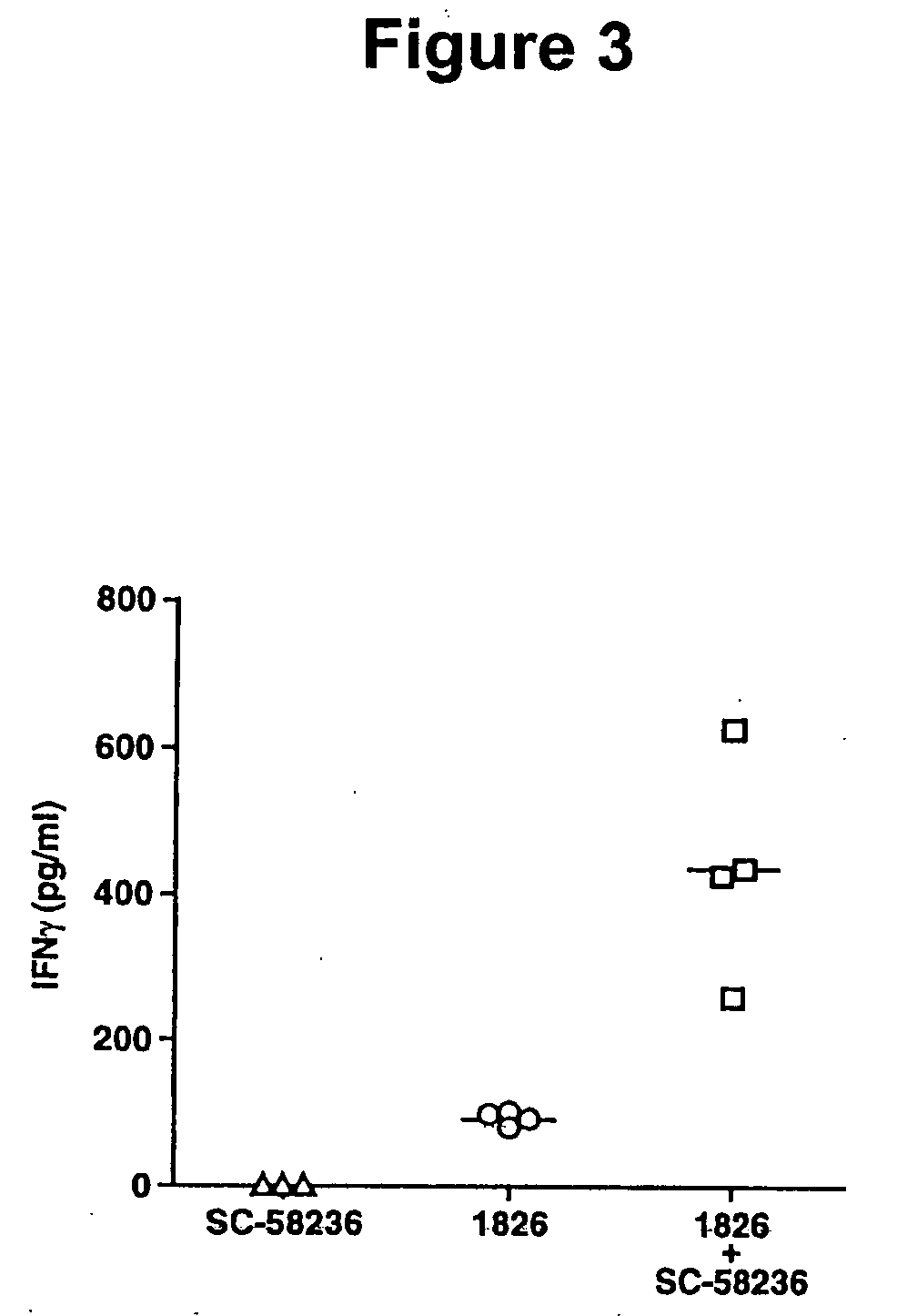
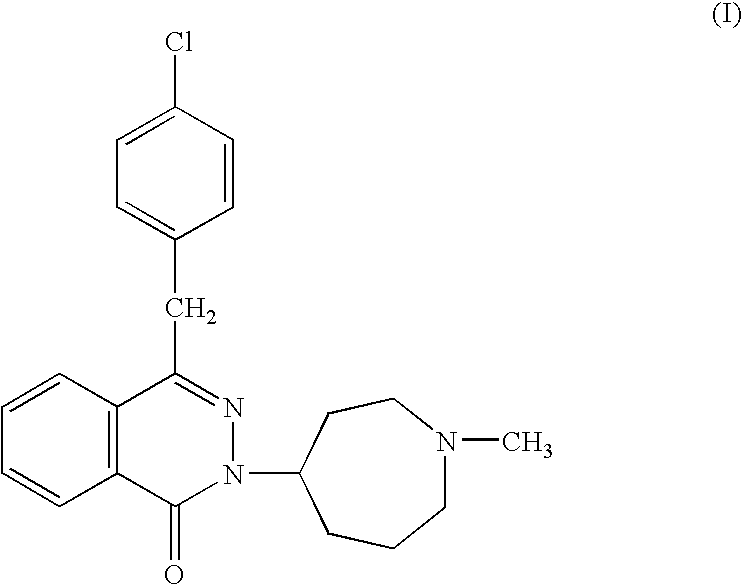
The invention provides methods and compositions for using immunostimulatory nucleic acids to treat non-allergic inflammatory diseases. Non-allergic inflammatory diseases that may be treated according to the methods and products of the invention include psoriasis and inflammatory bowel disease. The invention further provides methods for augmenting a Th1 response to immunostimulatory nucleic acid involving inhibition of prostaglandin-mediated counter-regulatory response.
Owner:UNIV OF IOWA RES FOUND
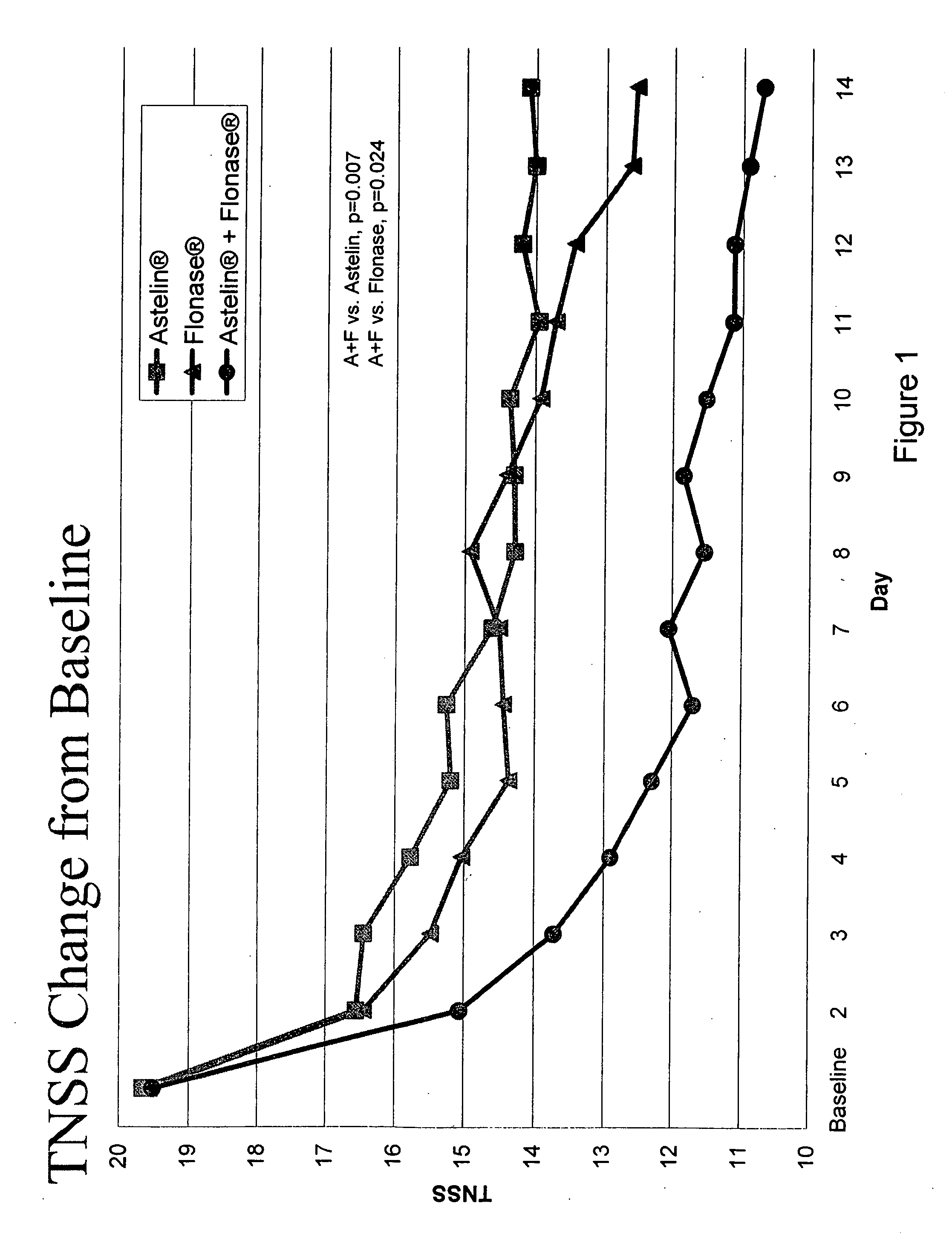
Compositions comprising azelastine and methods of use thereof
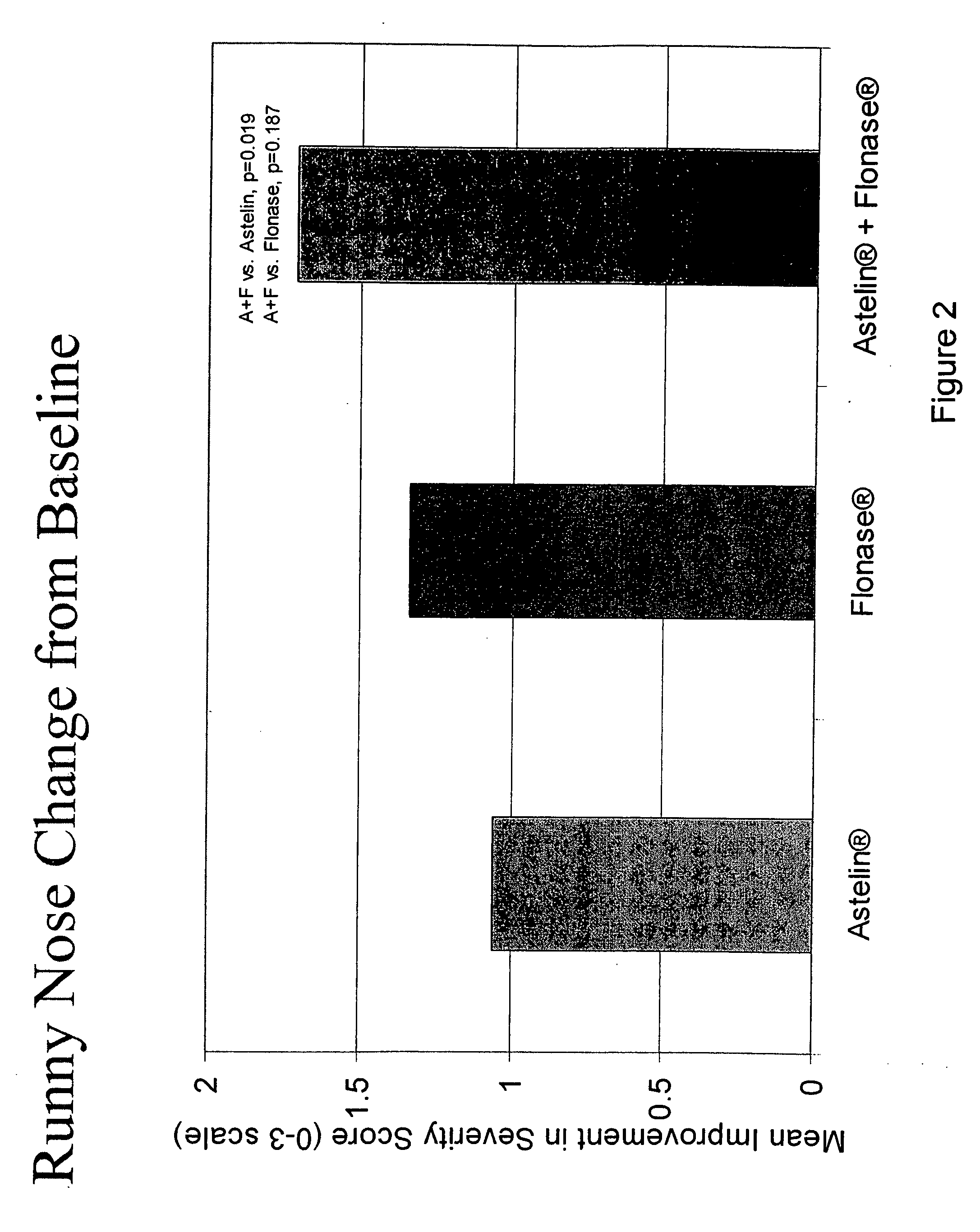
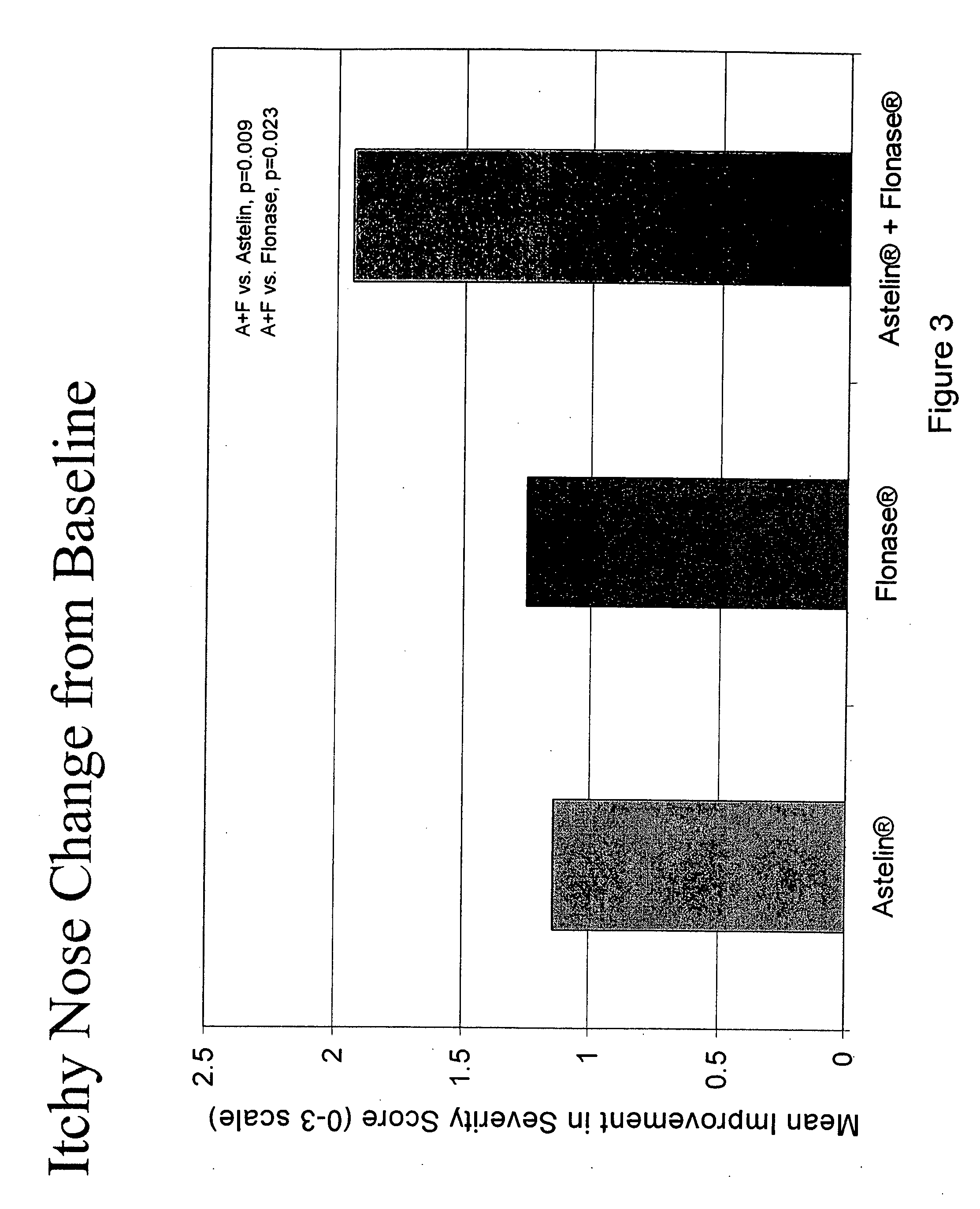
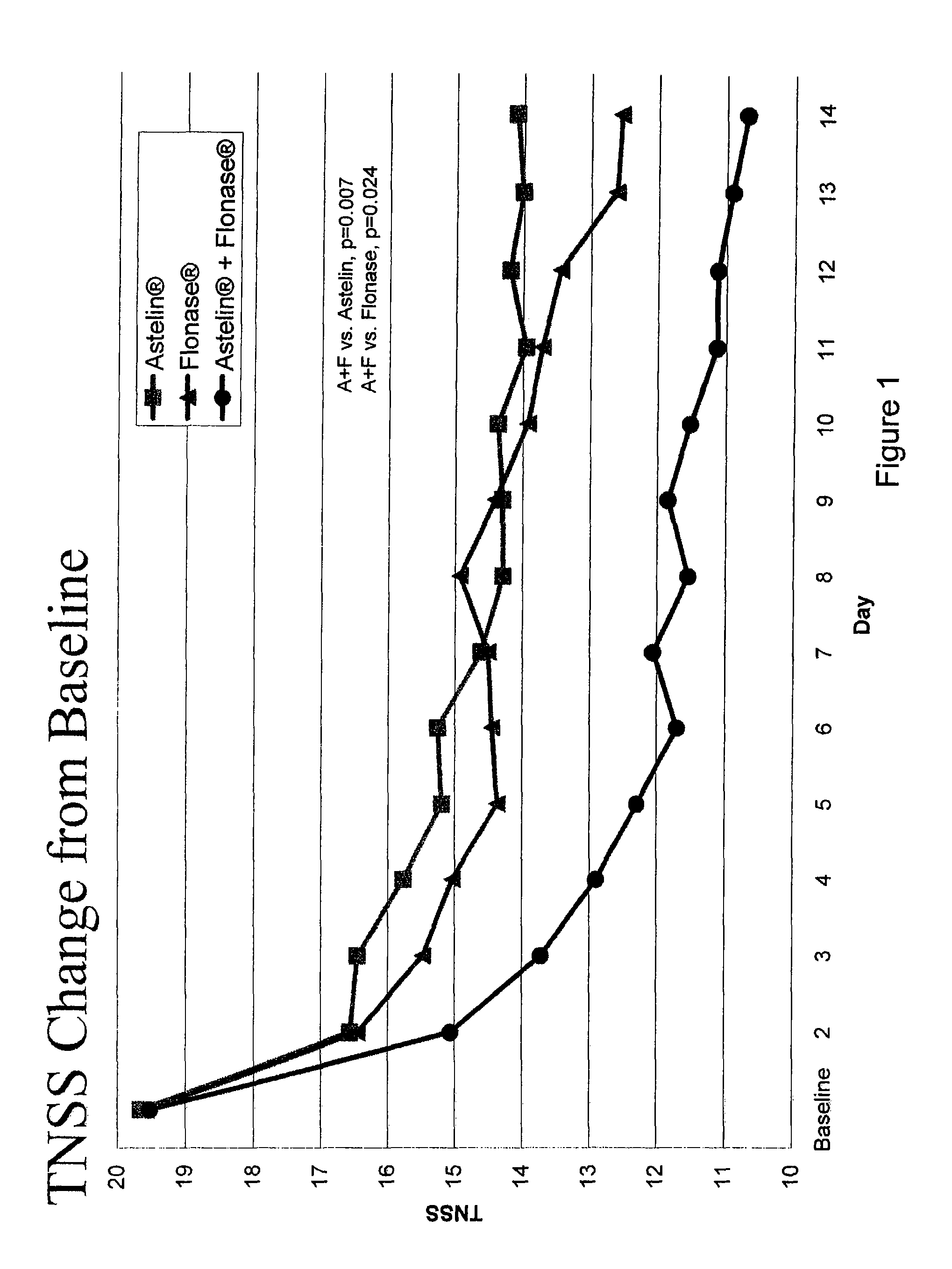
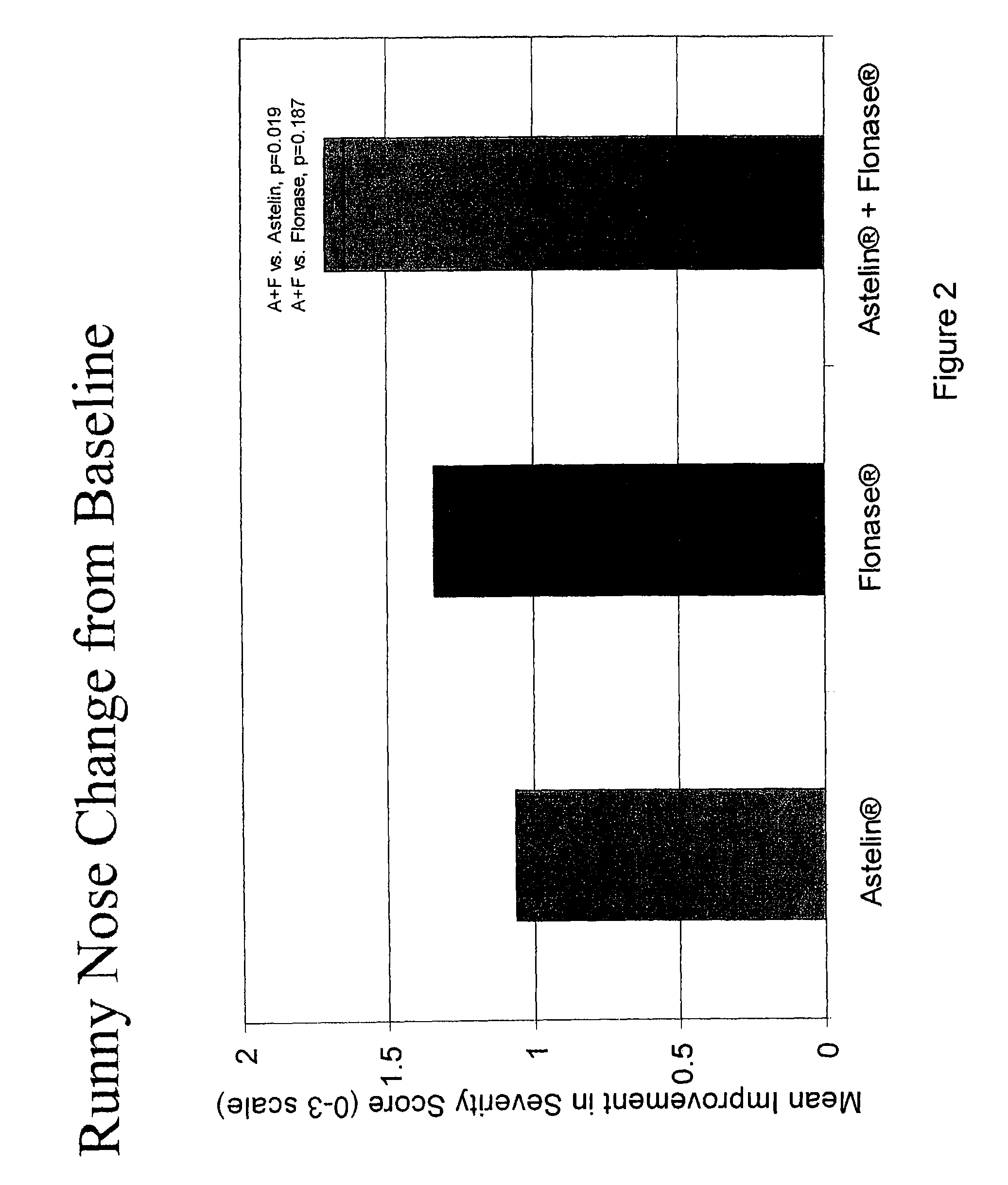
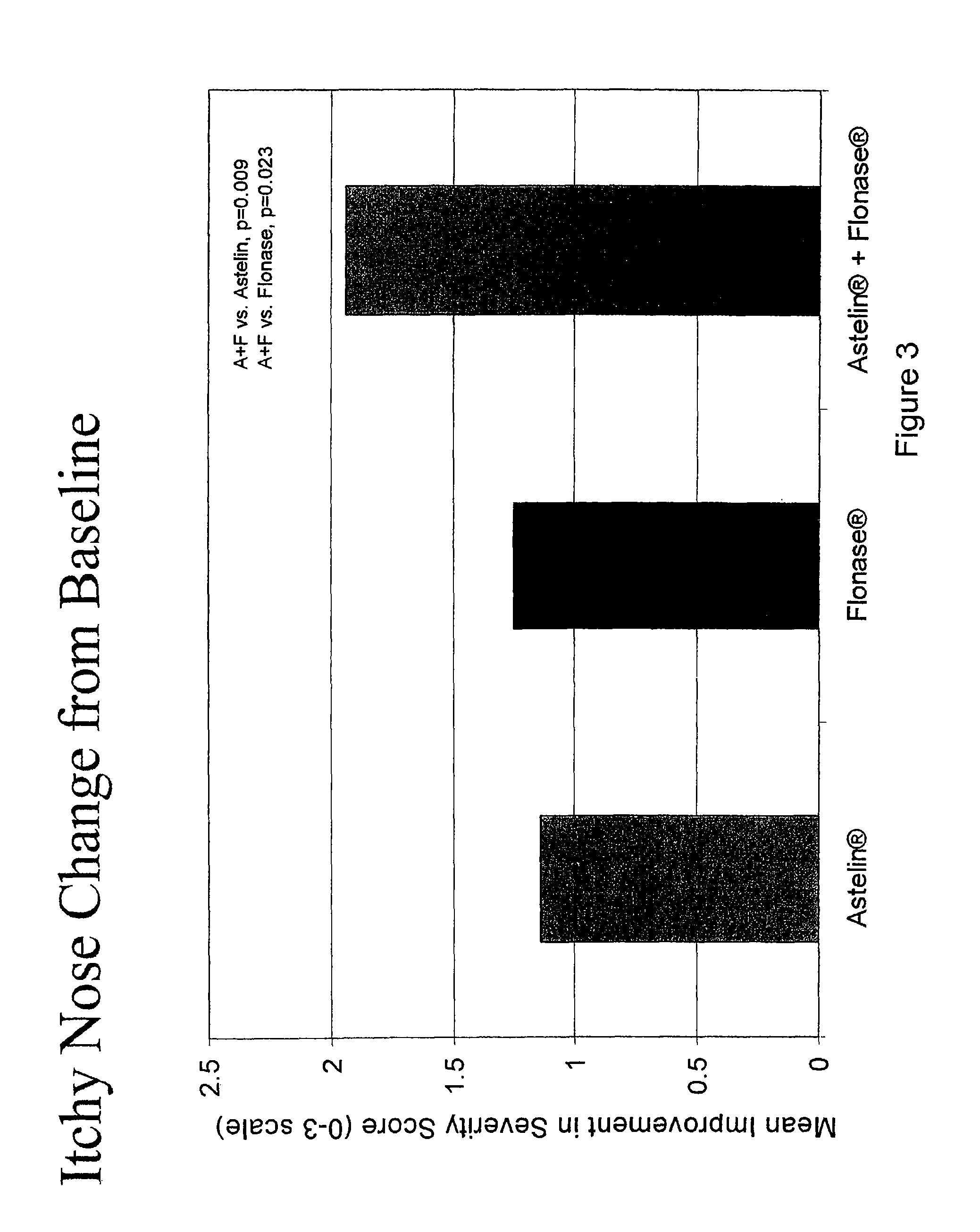
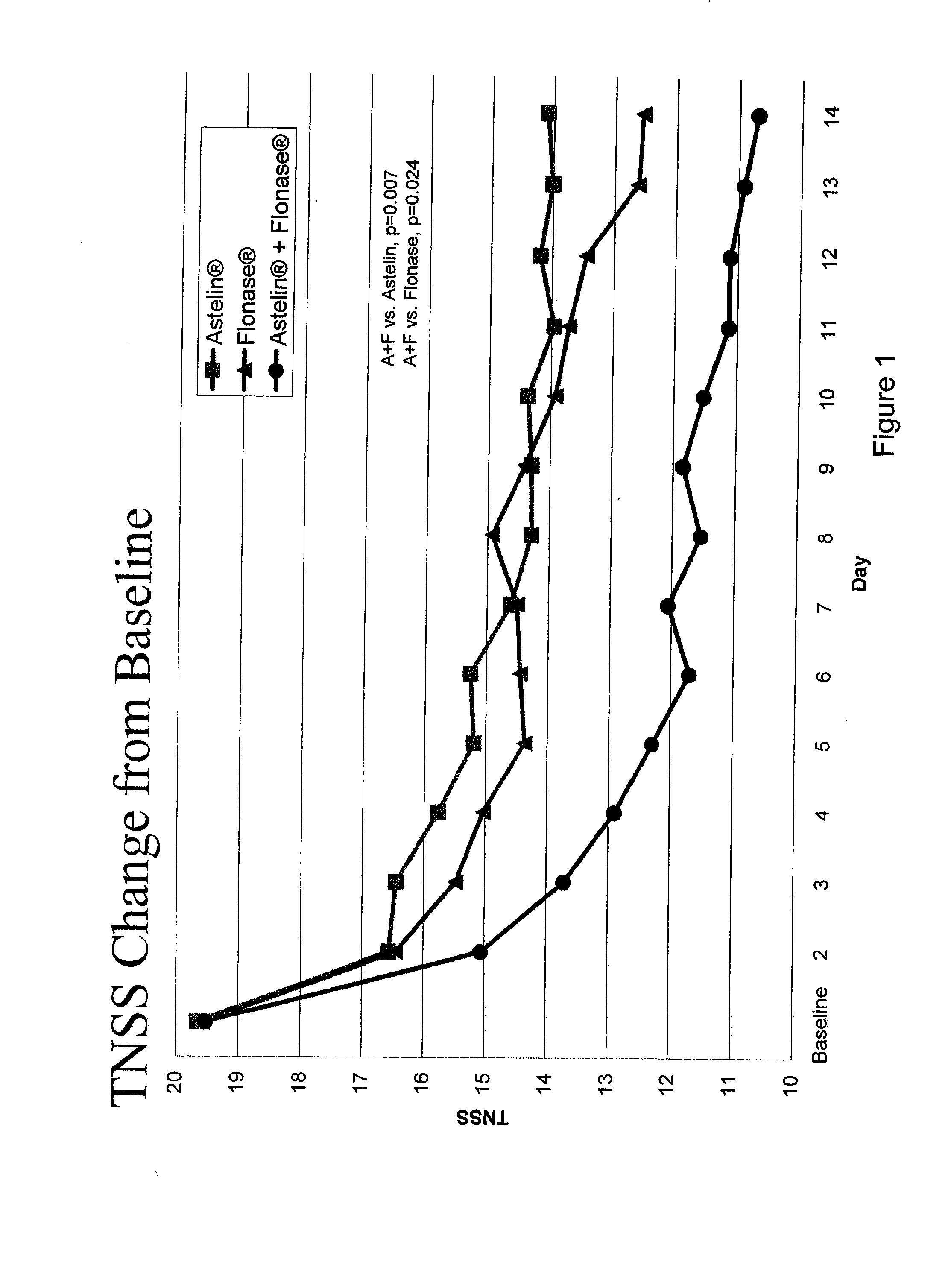
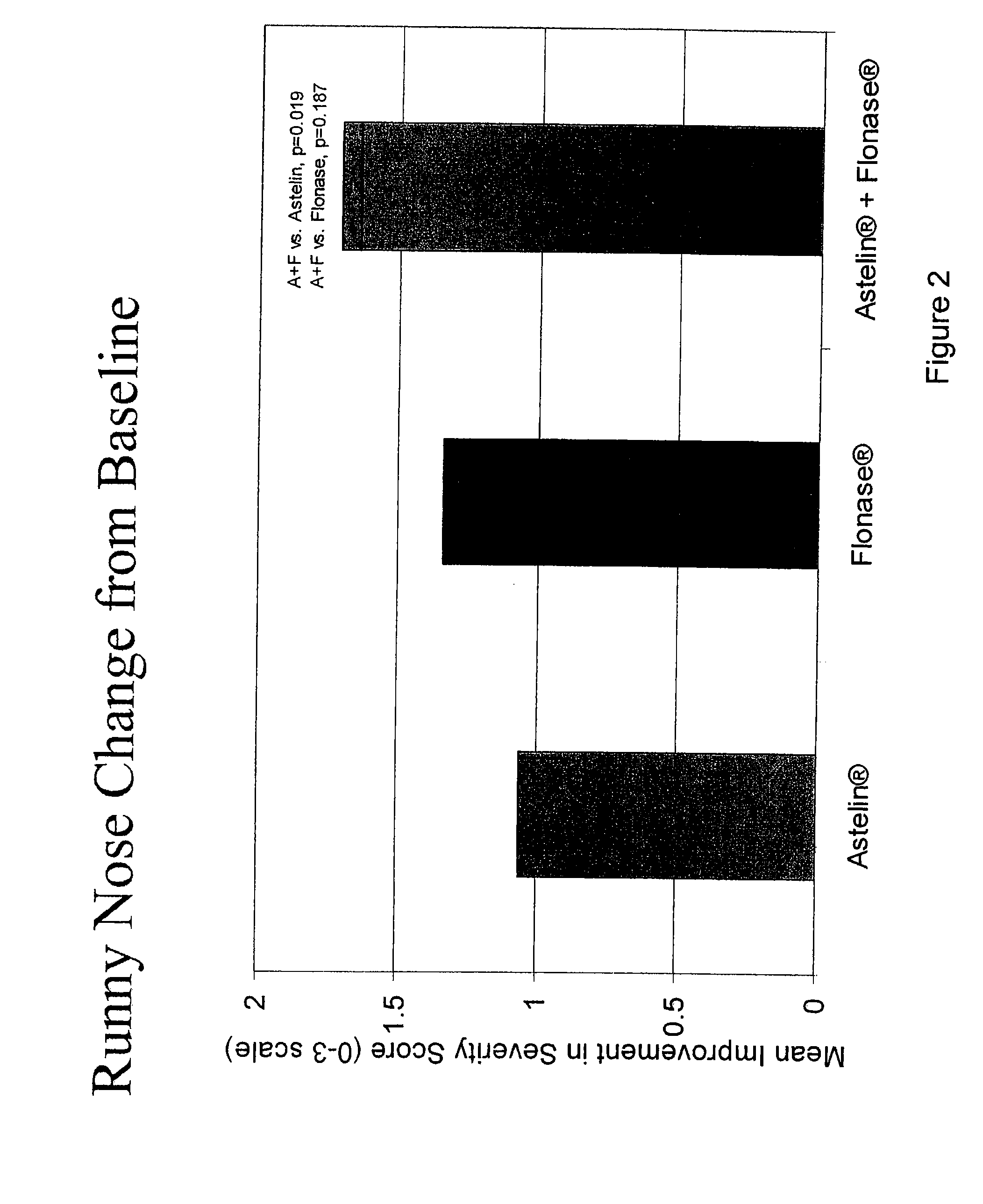
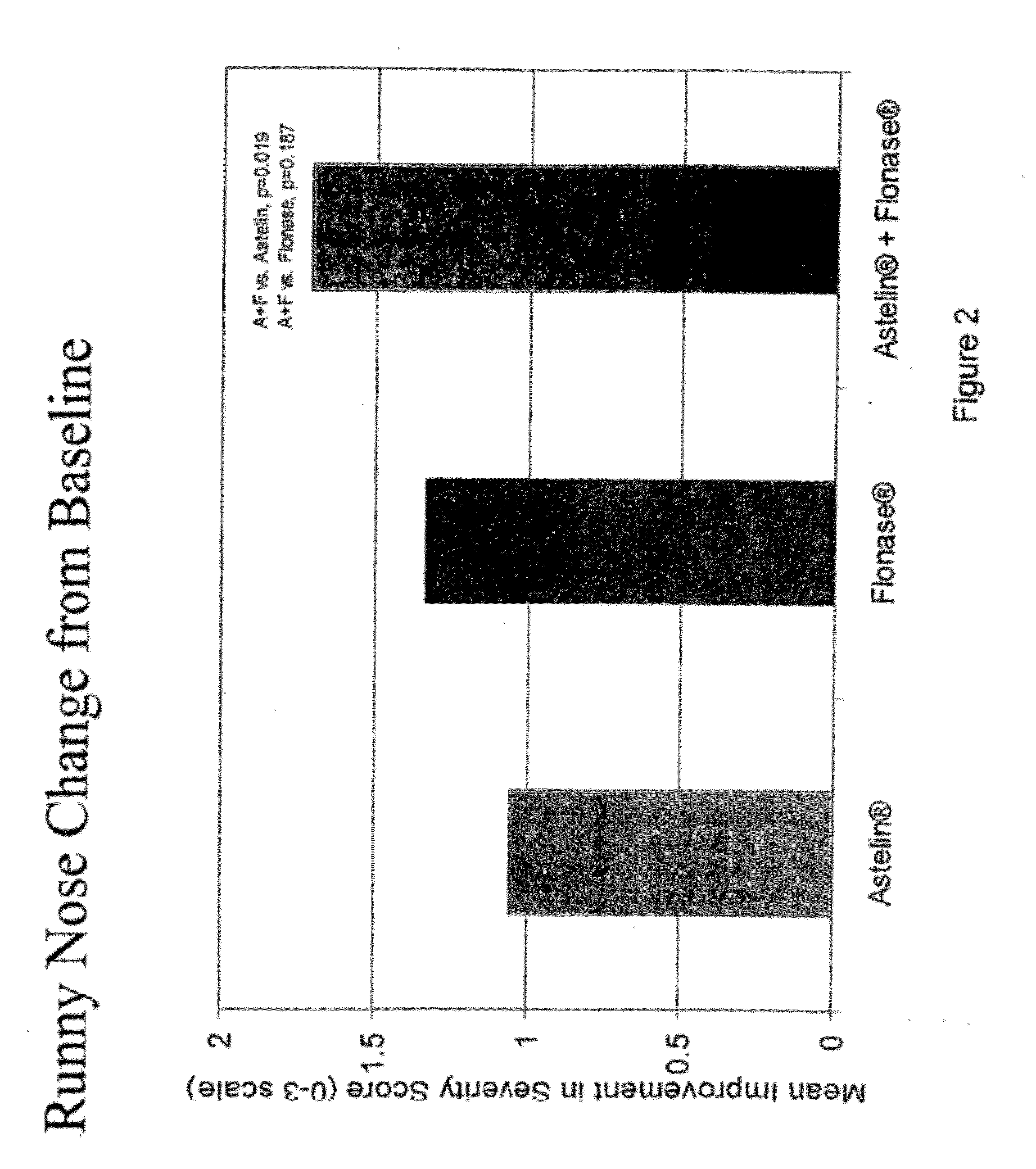
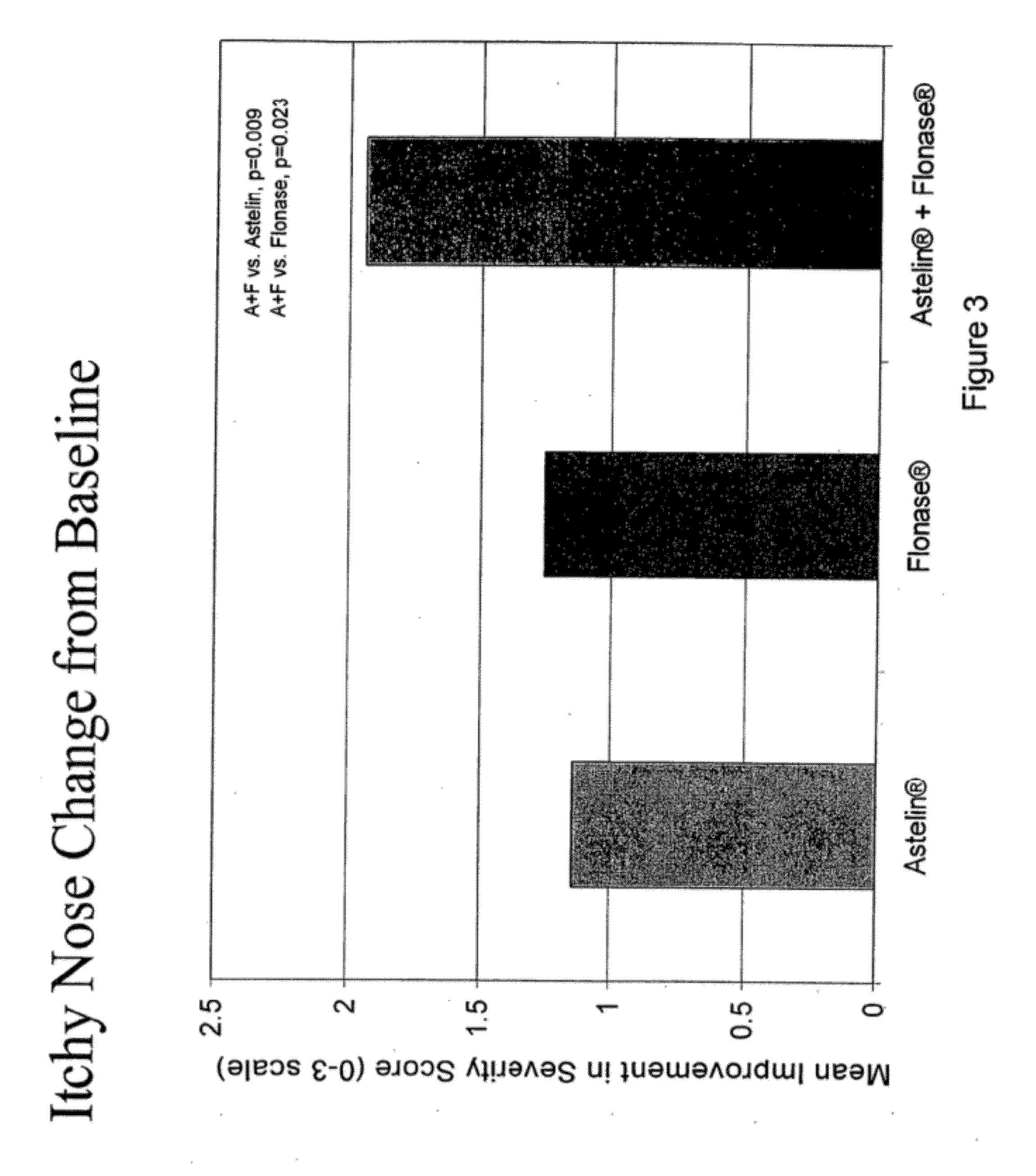
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
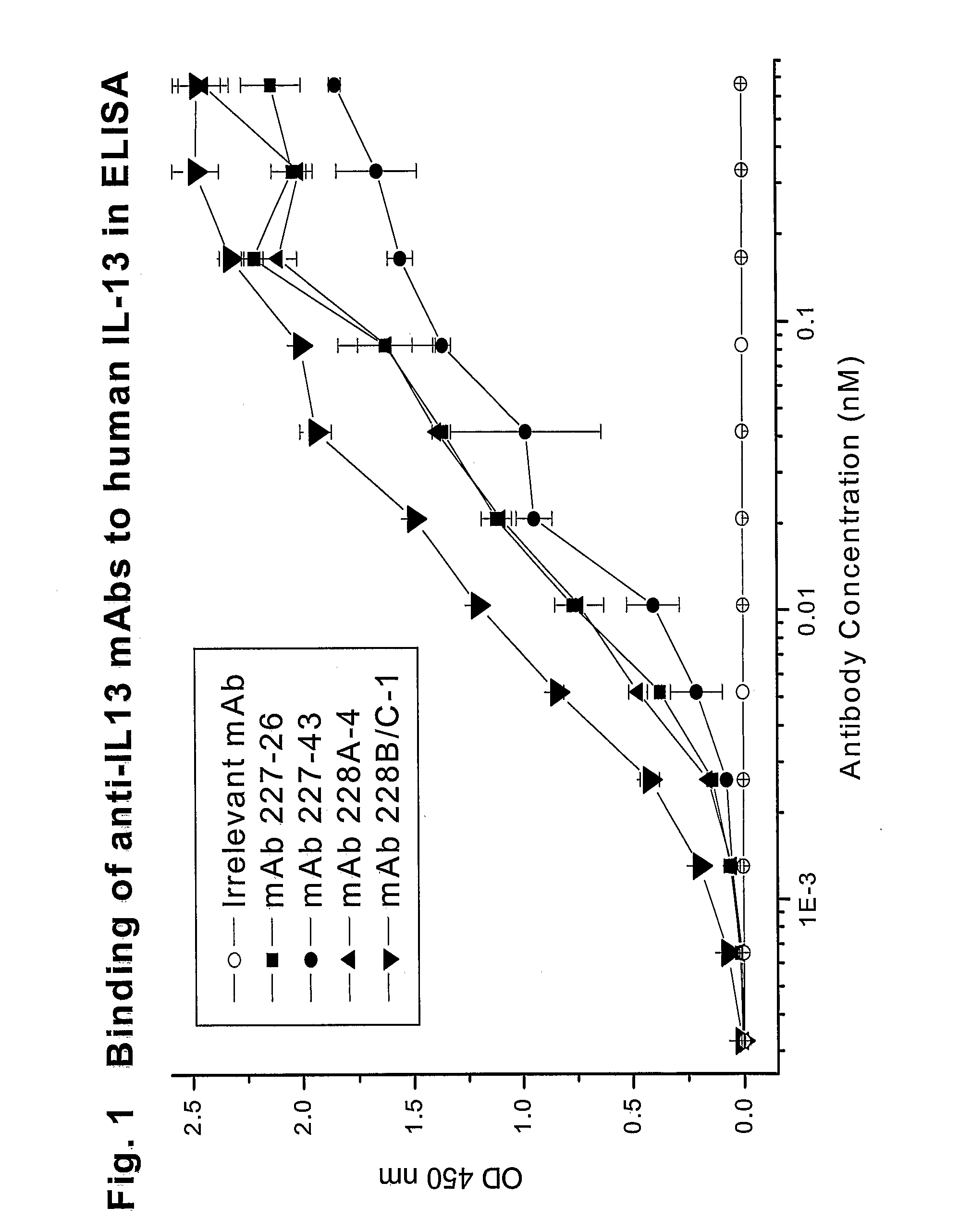
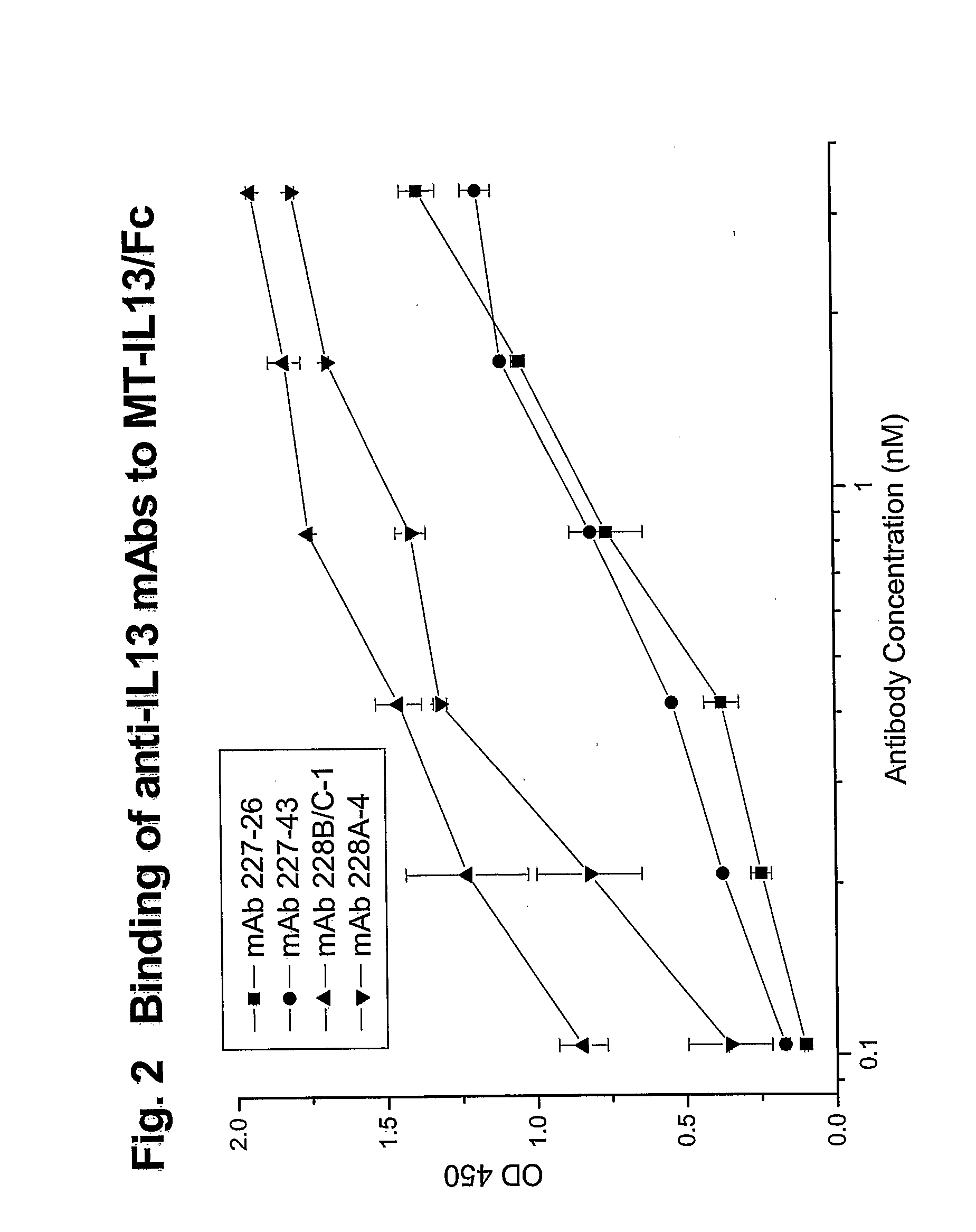
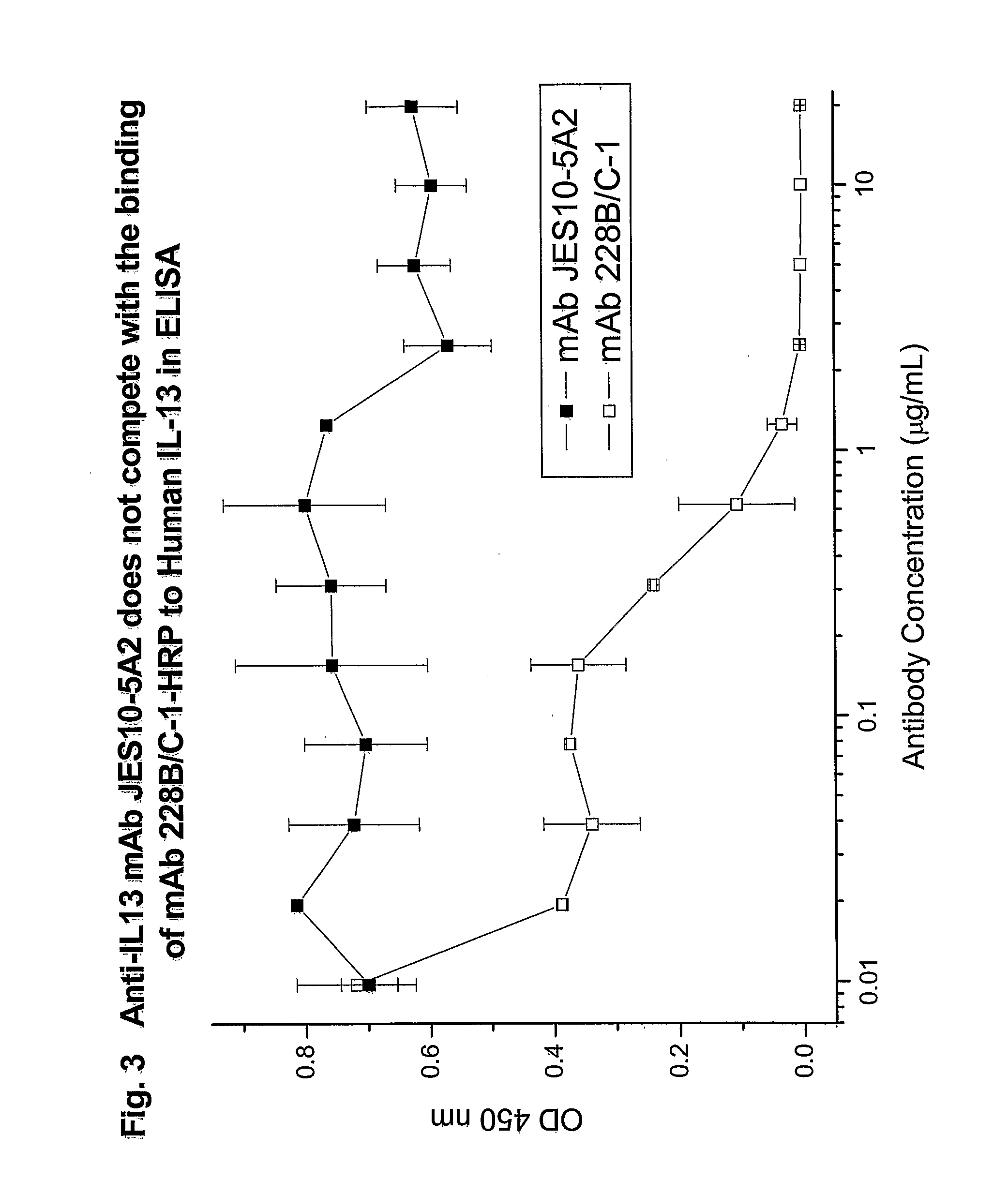
Novel anti-IL13 antibodies and uses thereof
ActiveUS20090214523A1Inhibiting antibody productionRelieve symptomsSenses disorderAntipyreticUveitisNonallergic rhinitis
The present invention relates to anti-IL13 antibodies that bind specifically and with high affinity to both glycosylated and non-glycosylated human IL13, does not bind mouse IL13, and neutralize human IL13 activity at an approximate molar ratio of 1:2 (MAb:IL13). The invention also relates to the use of these antibodies in the treatment of IL13-mediated diseases, such as allergic disease, including asthma, allergic asthma, non-allergic (intrinsic) asthma, allergic rhinitis, atopic dermatitis, allergic conjunctivitis, eczema, urticaria, food allergies, chronic obstructive pulmonary disease, ulcerative colitis, RSV infection, uveitis, scleroderma, and osteoporosis.
Owner:GENENTECH INC
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Composition comprising the extract of Actinidia arguta and related species for the prevention and treatment of allergic disease and non-allergic inflammatory disease
ActiveUS9131722B2Reduce actionTreat and prevent allergic diseaseBiocideSenses disorderFood additiveDisease
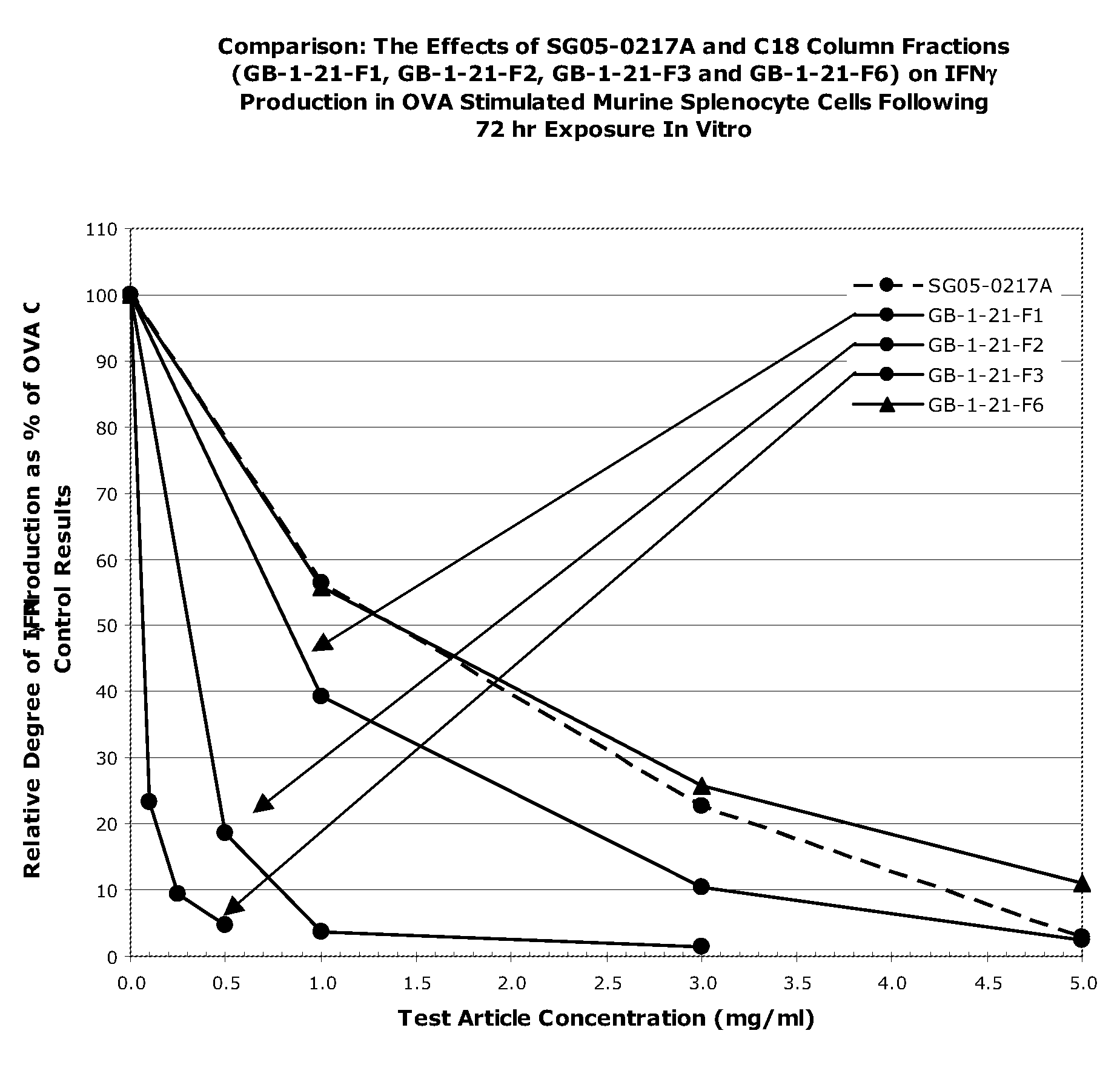
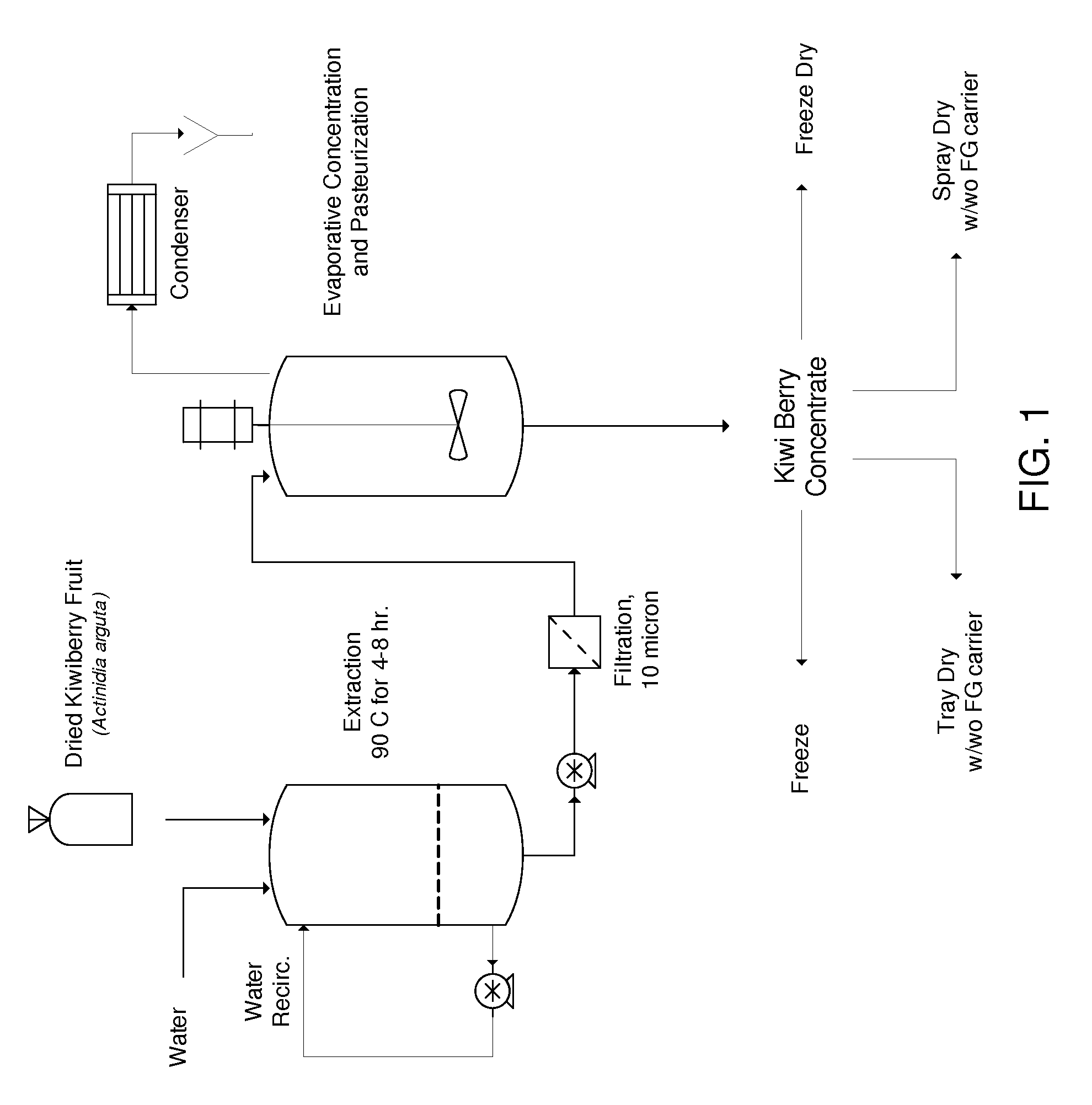
The present invention provides a pharmaceutical composition comprising the extract of hardy kiwifruit as an active ingredient in an effective amount to treat and prevent allergic disease and non-allergic inflammatory disease by reducing inflammation action, by inhibiting histamine release from mast cell, and by increasing the level of Th1 cytokines, IgG2a in serum and reducing the level of Th2 cytokines and IgE in serum. The present invention also provides a use of above extract for the preparation of pharmaceutical composition. The present invention also provides a health food or food additives, a cosmetic composition, a feed or feed additives comprising above extract for prevention or alleviation of allergic disease and non-allergic inflammatory disease by reducing inflammation action, by inhibiting histamine release from mast cell, and by increasing the level of Th1 cytokines, IgG2a in serum, and reducing the level of Th2 cytokines and IgE in serum.
Owner:VIROMED CO LTD
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Compositions Comprising Azelastine and Methods of Use Thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also generally relates to pharmaceutical compositions comprising one or more active pharmaceutical ingredients, such as azelastine or pharmaceutically acceptable salts or esters thereof including azelastine hydrochloride, particularly wherein the compositions are provided in unit dosage form. In certain embodiments, the invention provides such unit dosage pharmaceutical compositions comprising azelastine hydrochloride formulated for use as nasal sprays and / or ocular solutions or drops. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of a variety of allergic and non-allergic conditions, particularly conjunctivitis, sinusitis, rhinitis and rhinosinusitis. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Compositions comprising azelastine and methods of use thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders. The compositions and methods of the present invention provide significant value in terms of patient acceptability, convenience, and compliance.
Owner:MEDA PHARMA INC
Method for dyeing silk broadcloth by using plant dye solution extracted from lily
InactiveCN102587153ASafe to takeIncrease added valueDry-cleaning apparatus for textilesNatural dyesFiltrationPollen
The invention discloses a method for dyeing silk broadcloth by using the pollen of lily as a natural dye and belongs to the technical field of textile dyeing and finishing. The method comprises the following steps of: soaking fresh or dried dark-red lily pistil, heating and performing suction-filtration to obtain a dye solution; adding silk broadcloth fabric which is refined, and dyeing the silk broadcloth fabric; and adding different mordants for mordanting and thus obtaining silk fabric with different colors. The method has the advantages of low cost, simple process, high performance, high additional value, non-allergic and non-carcinogenic to skin, and the like; raw materials are inexpensive, easy to get, and reproducible; and taking of dyeing products is safe. Particularly, by taking plant ash as a mordant, pollution is avoided. The method is environment-friendly and energy-saving, and clothes which are made of fabric dyed by using the method are natural, environment-friendly and nonirritating to the skin.
Owner:ZHEJIANG IND POLYTECHNIC COLLEGE
Compositions Comprising Actinidia and Methods of Use Thereof
InactiveUS20100111927A1Regulate the amount of an antibody isotypeLower Level RequirementsBiocideAntipyreticDiseaseEnvironmental health
Disclosed are novel preparations of Actinidia, and particularly species of hardy kiwifruit, as well as compositions comprising the same. Also disclosed is the use of these preparations of Actinidia to prevent and / or treat a variety of diseases in which regulation of the immune response is effective, including both allergic and non-allergic inflammatory disease, viral infection, and cancer. Methods related to making and using these compositions are also described.
Owner:KIM SUNYOUNG
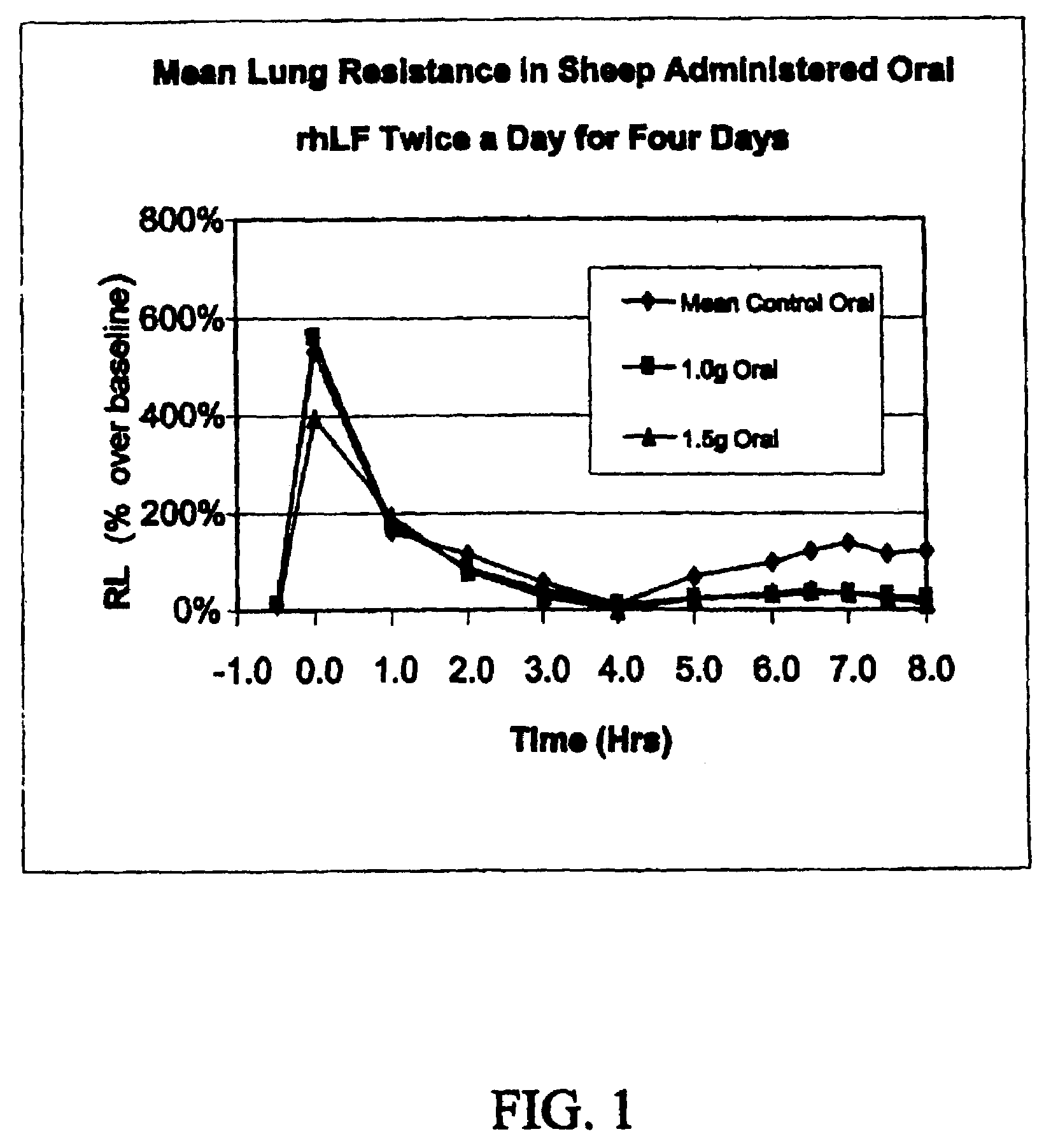
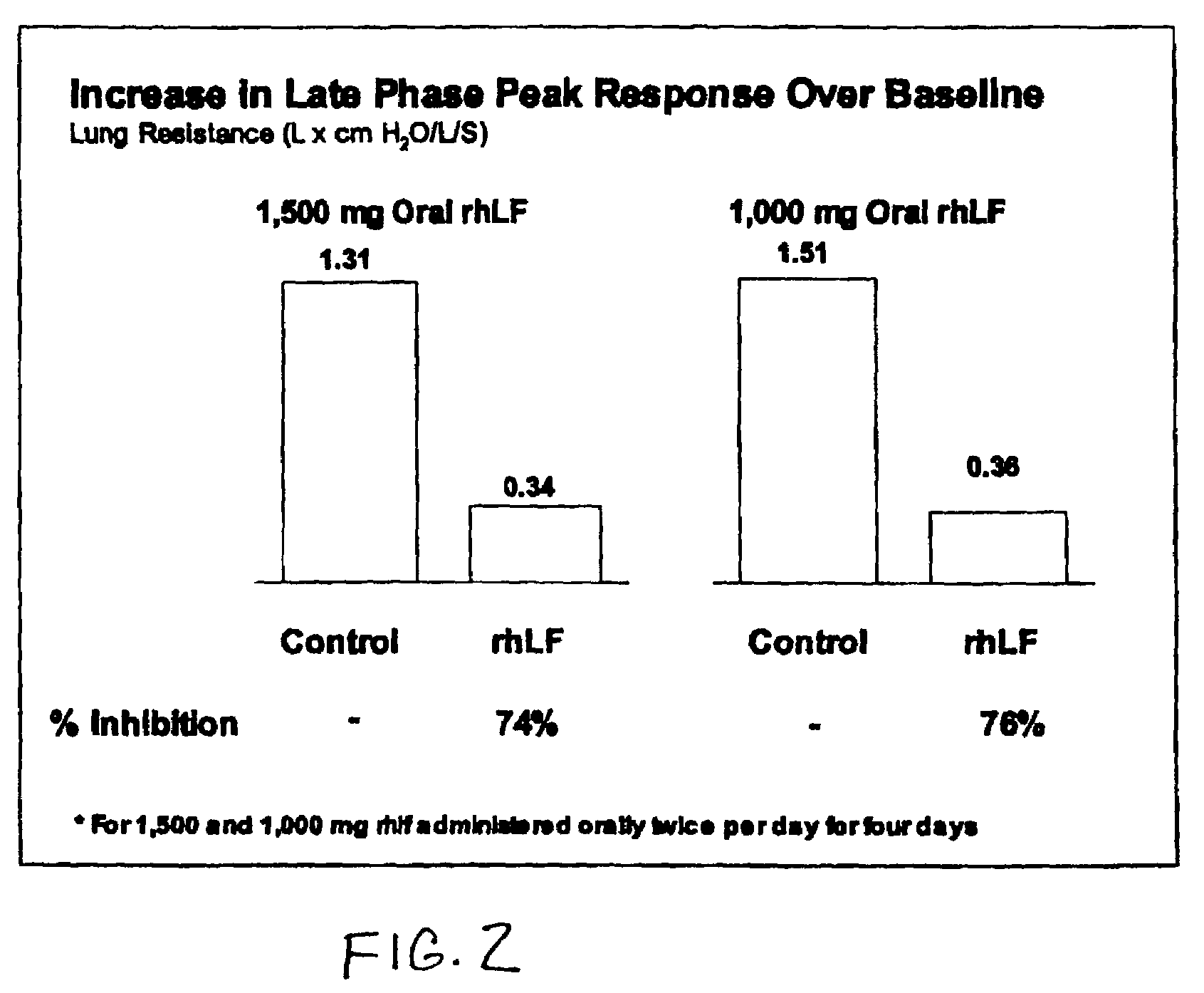
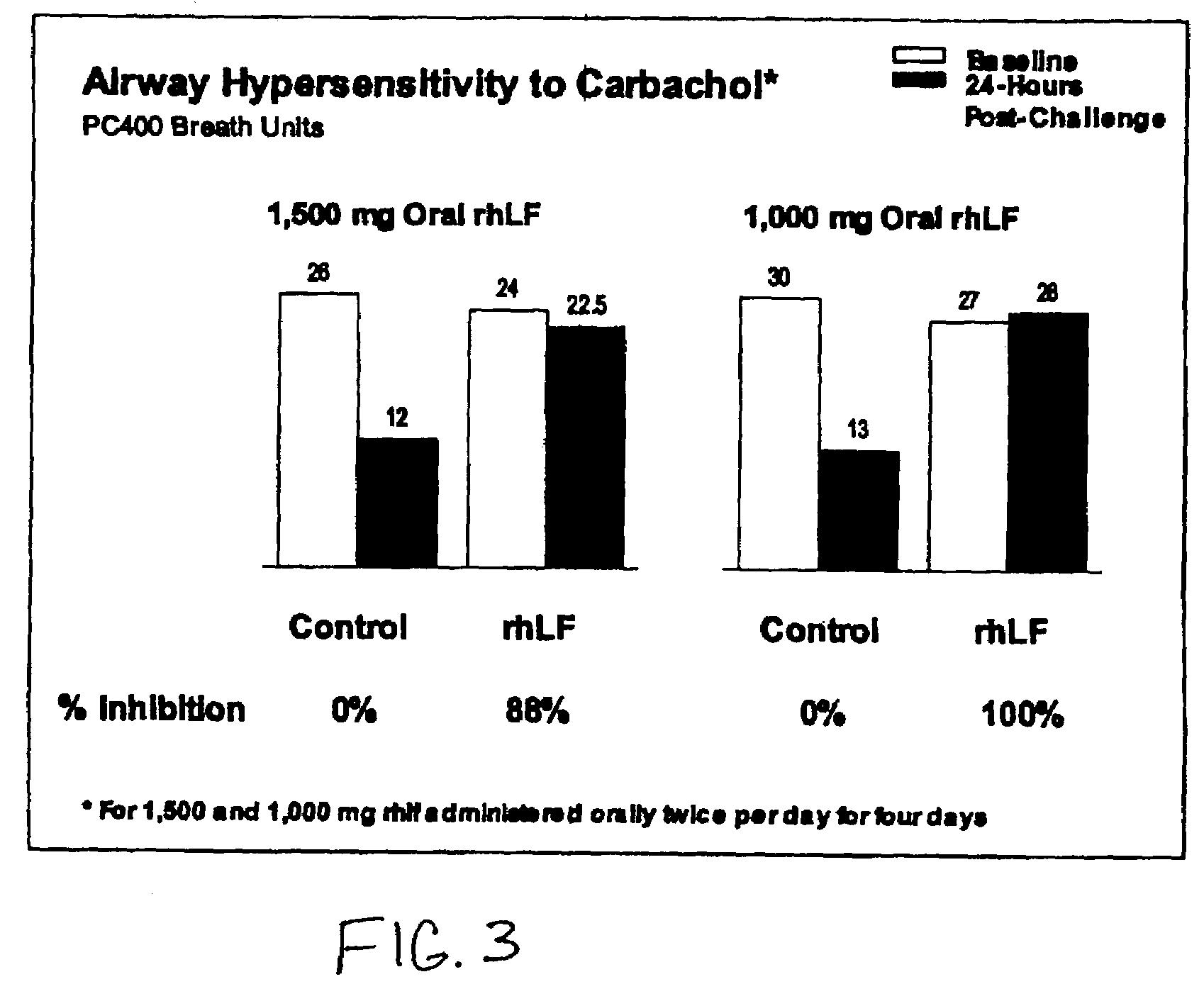
Oral lactoferrin in the treatment of respiratory disorders
InactiveUS7238661B2Increase productionEnhance immune responseOrganic active ingredientsBiocideDiseaseNon allergic
The present invention relates to methods of treating an allergic or non-allergic respiratory disorder by administering orally a composition of lactoferrin alone or in combination with metal chelators to treat respiratory disorders.
Owner:AGENNIX
Method and composition for dermatoses
The invention provides a method and composition for treating dermatoses by topical application of a composition containing an antihistamine, an NSAID and optionally a botanical medicinal compound. The compositions are useful for skin conditions such as inflammatory skin conditions, including eczema, atopic dermatitis, non-allergic dermatitis, psoriasis and rosacea, or any inflammation of the skin.
Owner:FAIRFIELD CLINICAL TRIALS
Anaphylatoxins for detecting clinical conditions
InactiveUS20090226374A1Compounds screening/testingMicrobiological testing/measurementVasculitisBasophilia
Non-allergic hypersensitivity reactions can be observed in a sample of cells from a subject in response to anaphylatoxins. Accordingly, methods are provided for detecting clinical conditions such as cellular hyper-reactivity, non-allergic hypersensitivity, asthma, inflammation, chronic or acute infection, bacterial infection, viral infection, parasite infection, adverse drug reaction, organ rejection, vasculitis, mastocytosis, eosinophilia, basophilia, leukemia, and / or C3a or C5a receptor defects in a subject. Also provided are kits for detecting such clinical conditions in a subject.
Owner:HEALTH AIRE
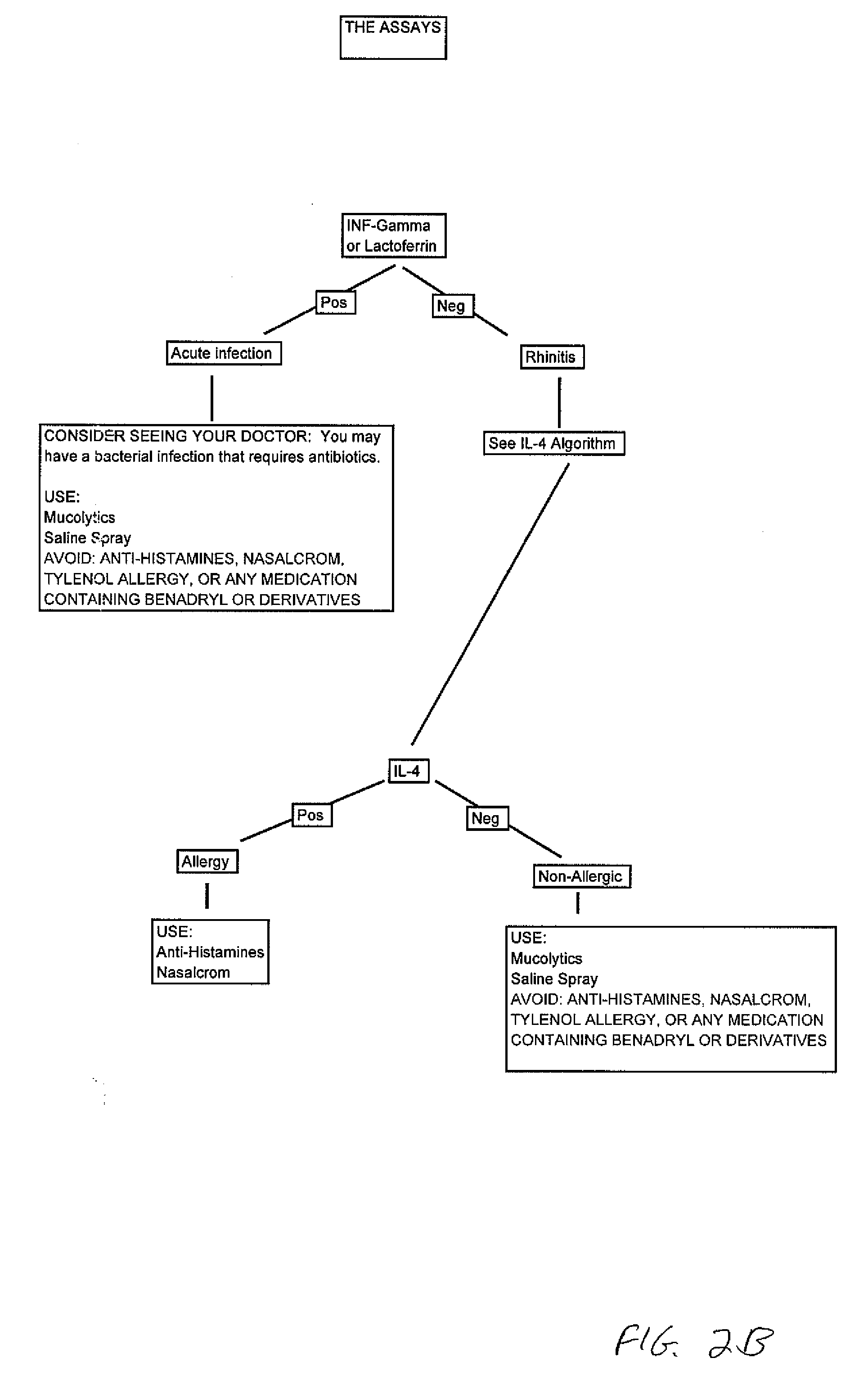
Rapid nasal assay kit
InactiveUS20070184495A1Peptide/protein ingredientsMammal material medical ingredientsNasal dischargeNon allergic
The present invention relates to an assay which can be used on nasal secretions. The assay is used to determine the cause of nasal secretions, for example whether the secretions are due to an allergic reaction or a non-allergic reaction.
Owner:TOXCURE
Bone fracture medicinal patch and preparation method thereof
InactiveCN102755595ANo allergiesNo pollutionHeavy metal active ingredientsAnthropod material medical ingredientsPoromaNon allergic
The invention discloses a bone fracture medicinal patch and a preparation method thereof, which belong to the field of external Chinese patent medicaments. The bone fracture medicinal patch comprises a Chinese medicinal formula, a substrate material, a back lining layer and a supporting layer, wherein the formula consists of the following components in parts by weight: 10 parts of Chinese angelica, 10 parts of szechuan lovage rhizome, 10 parts of common bletilla tuber, 10 parts of ground beetle, 20 parts of pseudo-ginseng root, 10 parts of cortex eucommiae, 10 parts of tree peony root-bark, 20 parts of sappan wood, 10 parts of suberect spatholobus stem, 20 parts of fortune's drynaria rhizome, 10 parts of himalayan teasel root, 20 parts of astragalus, 20 parts of sealwort, 10 parts of double-teeth pubescent angelica root, 20 parts of frankincense, 80 parts of myrrh, 20 parts of safflower, 20 parts of twotooth achyranthes root, 5 parts of native copper, 2 parts of dragon's blood, 20 parts of common selfheal fruit-spike, 20 parts of fenugreek seed, 20 parts of epimedium herb, 20 parts of south dodder seed, 10 parts of ainsliaea, 10 parts of corydalis tuber, 10 parts of peach kernel, 10 parts of common burreed rhizome and 10 parts of curcuma zedoary. The preparation method comprises the following steps of: preparing Chinese medicinal fine powder; preparing an extract; preparing a substrate material; preparing paste; and making a plaster. The bone fracture medicinal patch has the effects of relieving pain, stopping bleeding, diminishing swelling quickly and healing quickly, is free from harmful substances such as mercury, lead and the like, is nonirritant, is non-allergic to skin, does not pollute skin, is easy to strip, can be removed and stuck repeatedly, does not hurt hairs, and is mainly applied to patients with bone fractures and bone fracture injuries and whose tendons and bones are manually straightened or set, or people for which poroma is not formed a long time after bone fractures.
Owner:杨小平
Rapid nasal assay kit
InactiveUS7888049B2Peptide/protein ingredientsMammal material medical ingredientsNasal cavityNon allergic
The present invention relates to an assay which can be used on nasal secretions. The assay is used to determine the cause of nasal secretions, for example whether the secretions are due to an allergic reaction or a non-allergic reaction.
Owner:TOXCURE
Compositions Comprising Azelastine and Methods of Use Thereof
The present invention provides pharmaceutical compositions comprising azelastine, or a pharmaceutically acceptable salt or ester thereof including azelastine hydrochloride, and optionally one or more additional active agents. Preferred such compositions further comprise one or more pharmaceutically acceptable carriers or excipients that reduce the amount of post-nasal drip, and / or that minimize or mask the unpleasant bitter taste associated with post-nasal drip, of the compositions into the oral cavity, upon intranasal or ocular administration of the compositions. Especially effective excipients used in the compositions of the present invention are hypromellose as a viscosity modifier and sucralose as a taste-masking agent. The invention also provides methods of treating or preventing certain disorders, or symptomatic relief therefrom, by administering the compositions of the invention to a patient, e.g., for the symptomatic relief of allergic rhinitis, non-allergic vasomotor rhinitis, allergic conjunctivitis, as well as other disorders.
Owner:MEDA PHARMA INC
Multifunctional skin care product with effects of whitening skin, removing freckles, preserving moisture and removing wrinkle and preparation method of multifunctional skin care product
InactiveCN106420491AComprehensive and balanced nutritionCosmetic preparationsToilet preparationsWrinkle skinSide effect
The invention discloses a multifunctional skin care product with effects of whitening skin, removing freckles, preserving moisture and removing wrinkle and a preparation method of the multifunctional skin care product. The skin care product is prepared from food-grade or medical-grade skin nutrient composition, skin protection composition and a solvent, contains multiple nutrients such as hyaluronic acid, collagen, vitamins, amino acid and the like required by normal skin nutrition, and also contains healthcare components such as coenzyme I, coenzyme II, nicotinamide, coenzyme Q10 and the like required by skin health maintenance; the skin care product has remarkable effects of whitening skin, preserving moisture, removing wrinkle and removing freckles, and is anti-aging and anti-sunburn; the skin care product has the advantages of high safety, wide applicable range, good effects and the like, and is convenient to use, non-irritant, non-allergic and suitable for almost all people; all common skin abnormality problems can be solved with the product, besides, the skin care product takes effect rapidly, and can be used for a long time without producing toxic and side effects; the preparation method of the skin care product adopts mild conditions and a simple process, does not cause environmental pollution and is worthy of large-scale popularization.
Owner:ZHENGZHOU LANCI BIOENG CO LTD
Combination Therapy Comprising Actinidia and Steroids and Uses Thereof
Disclosed is a combination of an Actinidia preparation and one or more steroids, and the use of such a combination to prevent and / or treat allergic and non-allergic inflammatory conditions or diseases, to alleviate at least one symptom of such conditions or diseases, and / or to regulate an immune response in a mammal.
Owner:EFFICAS INC
Colchicine composition for external use and preparation method thereof
ActiveCN108057018ASeizure controlNo systemic side effectsOrganic active ingredientsSkeletal disorderSide effectOral medication
The invention relates to a colchicine composition for external use and a preparation method thereof. The colchicine composition is transdermally absorbed and comprises following components: colchicine, a solvent, a cosolvent, optional infiltration aid, and optional emulsifier. The colchicine composition can effectively prevent or treat gouty arthritis, is well transdermally absorbed, has a quick effect, and has prominent advantages in administration route and dosage form, compared with oral preparations. The colchicine composition is nonirritant and non-allergic to skin, barely has any toxic or side effect, and has the advantages of high stability, controllable quality, simple production technology, and suitability for industrial production.
Owner:BEIJING HUMANWELL JUNWEI PHARM TECH CO LTD
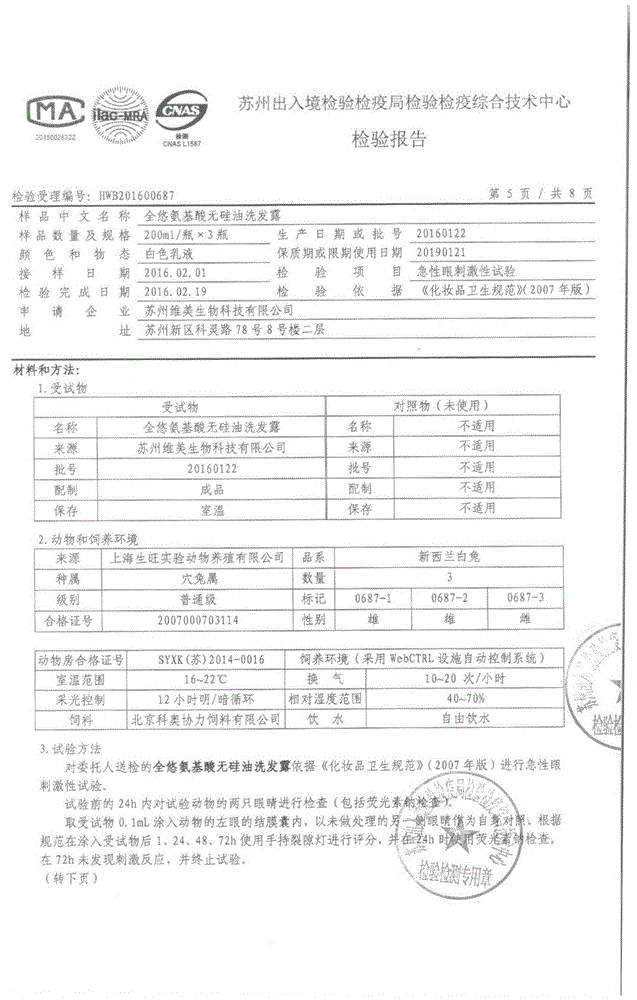
Dimethicone-free amino acid shampoo
The invention discloses dimethicone-free amino acid shampoo which comprises, by weight, 0.1-25% of amino acid surfactant as a main active ingredient, 60-90% of water, 0.5-5% of humectant, 0.5-5% of conditioner and 0.1-1% of preservative. The dimethicone-free amino acid shampoo has the advantages that the dimethicone-free amino acid shampoo is non-irritating and non-allergic, and injury to human bodies caused by residue of chemical detergent is avoided since common surfactants such as laureth sulfate and sodium dodecylbenzenesulfonate are replaced by amino acid surfactant; the dimethicone-free amino acid shampoo is environmentally friendly and easy to degrade, specifically, the biological degradability is equal to or higher than 99.9% under action of the amino acid surfactant, which is the highest value as compared with those of the similar substances, and thus, influence of emission of the shampoo to environments is lowered to the utmost extent, which accords with low-carbon environmental protection concept; the shampoo makes hair fluffy and flowing.
Owner:南通安佳家生物科技有限公司
Combination of H1, H3 and H4 receptor antagonists for treatment of allergic and non-allergic pulmonary inflammation, congestion and allergic rhinitis
Owner:SCHERING CORP
Composition comprising the extract of actinidia arguta and related species for the prevention and treatment of allergic disease and non-allergic inflammatory disease
InactiveUS20070122508A1Confirm inhibitory effectReduce actionBiocideSenses disorderFood additiveActinidia
The present invention provides a pharmaceutical composition comprising the extract of hardy kiwifruit as an active ingredient in an effective amount to treat and prevent allergic disease and non-allergic inflammatory disease by reducing inflammation action, by inhibiting histamine release from mast cell, and by increasing the level of Th1 cytokines, IgG2a in serum and reducing the level of Th2 cytokines and IgE in serum. The present invention also provides a use of above extract for the preparation of pharmaceutical composition. The present invention also provides a health food or food additives, a cosmetic composition, a feed or feed additives comprising above extract for prevention or alleviation of allergic disease and non-allergic inflammatory disease by reducing inflammation action, by inhibiting histamine release from mast cell, and by increasing the level of Th1 cytokines, IgG2a in serum, and reducing the level of Th2 cytokines and IgE in serum.
Owner:HELIXIR
Pharmaceutical composition for treating facial telangiectasis and preparation method and use thereof
ActiveCN104547064AHas multiple functionsEasy accessCosmetic preparationsToilet preparationsPhyllanthus urinariaAdditive ingredient
The invention discloses a pharmaceutical composition for treating facial telangiectasis and a preparation method and use thereof. Raw materials of the pharmaceutical composition are all natural soothing ingredients and are nonirritant to skin. The pharmaceutical composition with a facial telangiectasis treating effect is prepared from phyllanthus urinaria, polygala fallax hemsl, hypericum japonicum, gynostemma pentaphyllum, centella asiatica, kalimeris indica, mulberry leaves and euphorbia royleana, wherein the weight percent of any ingredient is not higher than 30% the overall weight. The raw materials of the pharmaceutical composition disclosed by the invention are simply obtained, and the preparation method is convenient. Proved by clinical trial results, the pharmaceutical composition disclosed by the invention has a good effect on the treatment of the facial telangiectasis, is nonirritant and non-allergic to the skin, is easily accepted by mass consumers, and has good application prospect and market prospect.
Owner:GUANGZHOU QINGLAN BIOTECHNOLOGY CO LTD
Pharmaceutical composition for treating dark eye circles and preparation method and use thereof
InactiveCN104546993AHas multiple functionsGood effectCosmetic preparationsToilet preparationsAdditive ingredientTrial drug
The invention discloses a pharmaceutical composition for treating dark eye circles and a preparation method and use thereof. Raw materials of the pharmaceutical composition are all natural active ingredients and are nonirritant to skin. The pharmaceutical composition with a dark eye circle treating effect is prepared from patrinia villosa, strobilanthes affinis, ilex pubescens, incarrillea sinensis, centella asiatica, saussurea involucrata, carpesium divaricatum and eupatorium odoratum, wherein the weight percent of any ingredient is not higher than 30% the overall weight. The raw materials of the pharmaceutical composition disclosed by the invention are simply obtained, and the preparation method is convenient. Proved by clinical trial results, the pharmaceutical composition disclosed by the invention has a good effect on the treatment of the dark eye circles, is nonirritant and non-allergic to the skin, is easily accepted by mass consumers, and has good application prospect and market prospect.
Owner:GUANGZHOU QINGLAN BIOTECHNOLOGY CO LTD
Multifunctional edible mask powder and preparation method thereof
InactiveCN104288020ABalance oil secretionRepair dark spotsCosmetic preparationsToilet preparationsNon allergicRaw material
The invention relates to the technical field of cosmetics beauty, and particularly relates to multifunctional edible mask powder and a preparation method thereof. The multifunctional edible mask powder is specifically prepared from the following raw materials in parts by weight: 60-100 parts of sargassum pallidum C.Ag, 60-100 parts of aloe, 50-100 parts of spirulina and 60-100 parts of cactus. The used raw materials are health food raw materials and generate no injury to body or skin, and the multifunctional edible mask powder is multifunctional / purely natural / additive-free / non-allergic, edible and is convenient to use, thus the mask powder can be used for comprehensively caring the skin.
Owner:商佩大 +1
High-elastic non-allergic antibacterial latex used for mattress
The invention discloses high-elastic non-allergic antibacterial latex used for mattress, belongs to the technical field of high polymer materials, and specifically relates to high-elastic non-allergicantibacterial latex used for the mattress. The high-elastic non-allergic antibacterial latex used for the mattress is prepared from the following raw materials in parts by weight: 50 to 80 parts of adandelion extract, 50 to 100 parts of guayule rubber, 10 to 20 parts of aloe vera gel, 2 to 5 parts of a tea-leaf extract, 20 to 30 parts of gutta-percha, 5 to 10 parts of cassawa starch, 1 to 3 parts of a cactus extract, 0.5 to 1 part of terpene resin, 1 to 2 parts of abietin, 1 to 2 parts of casein and 3 to 10 parts of starch. The high-elastic non-allergic antibacterial latex used for mattresses disclosed by the invention is high in elasticity, high in tensile strength, high in stain resistance, resistant to water, breathable, excellent in weather resistance, antibacterial and antifungal, high in stability and free of sensitivity or stimulation, attractive appearance and comfort of the mattress can be maintained, and long-term protection is provided.
Owner:安徽玉然经编科技有限公司
Gluten-free ready-to-eat food composition
InactiveUS20140030376A1Improve food valueHigh nutritional valueDough treatmentBakery productsBiotechnologyNutrition
A gluten free, ready-to-eat food product is provided comprising millet and a flavoring. In some embodiments, the gluten-free food product may also be dairy-free and / or allergen free. In further embodiments, the food products may further comprise one or more vitamins or cofactors therefore, minerals, non-allergenic proteins or amino acids, probiotics, botanicals or combinations of these and be utilized in methods of providing adequate nutrition to individuals diagnosed with an allergy or intolerance. Methods of making the food products, and packaged food products are also provided.
Owner:CAWS MICHELE ZEBERT
Special-effect deodorizing and sweat-removing powder
InactiveCN102048875AUnique curative effectImprove the bactericidal effectCosmetic preparationsSalicyclic acid active ingredientsBenzoic acidSide effect
The invention discloses special-effect deodorizing and sweat-removing powder and relates to the technical field of preparation of traditional Chinese medicines, in particular to materials and process for preparing special-effect deodorizing and sweat-removing powder. The special-effect deodorizing and sweat-removing powder is mainly refined by carrying out roasting, crushing, mixing and other processes on the following raw materials in parts by weight: 1000 parts of lightyellow sophora root, 500 parts of sandalwood, 500 parts of baical skullcap root, 500 parts of amur corktree bark, 500 parts of swordlike atractylodes rhizome, 500 parts of dried alum, 500 parts of clam shell powder, 500 parts of calcined oyster shell, 500 parts of sea-ear shell, 400 parts of camphor powder, 500 parts of amber, 300 parts of menthol, 500 parts of salicylic acid, 400 parts of benzoic acid, 1000 parts of boric acid, 500 parts of talc powder and 100 parts of neomycin powder. The product has special effect, can quickly sterilize, deodorize and remove sweat, is strong in efficacy, easy for absorption and non-allergic, has no toxic and side effects, and can be still used at ulcerated parts.
Owner:栗全正 +1
Azelastine composition and use
ActiveCN104173286ASkin irritationIn line with the trend of inputAerosol deliveryPharmaceutical non-active ingredientsDiseaseAzelastine
The invention relates to an azelastine composition and use, specifically relates to liquid compositions of azelastine, particularly relates to liquid compositions of azelastine hydrochloride and more particularly relates to a nonaqueous liquid composition. In one embodiment, the pharmaceutical composition contains azelastine or pharmaceutical salt thereof, polyhydric alcohol, polyethylene glycol and polyhydric alcohol fatty acid ester. The invention further relates to a preparation method of the liquid composition and pharmaceutical use of the liquid composition, for example, the invention further relates to the use of the composition in the treatment, relief or prevention of symptoms related to allergic and non-allergic diseases and the use in the treatment of dermatitis, such as allergic and / or contact dermatitis. The pharmaceutical composition disclosed by the invention can be in the dosage form of liniment or spray, so as to facilitate clinical use.
Owner:GUIZHOU YUNFENG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com