Patents
Literature
50 results about "Eosinophilia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Eosinophilia is a condition in which the eosinophil count in the peripheral blood exceeds 5.0×10⁸/l (500/μL). Eosinophils usually account for less than 7% of the circulating leukocytes. A marked increase in non-blood tissue eosinophil count noticed upon histopathologic examination is diagnostic for tissue eosinophilia. Several causes are known, with the most common being some form of allergic reaction or parasitic infection. Diagnosis of eosinophilia is via a complete blood count (CBC), but diagnostic procedures directed at the underlying cause vary depending on the suspected condition(s). An absolute eosinophil count is not generally needed if the CBC shows marked eosinophilia. The location of the causal factor can be used to classify eosinophilia into two general types: extrinsic, in which the factor lies outside the eosinophil cell lineage; and intrinsic eosinophilia, which denotes etiologies within the eosiniphil cell line. Specific treatments are dictated by the causative condition, though in idiopathic eosinophilia, the disease may be controlled with corticosteroids. Eosinophilia is not a disorder (rather, only a sign) unless it is idiopathic.
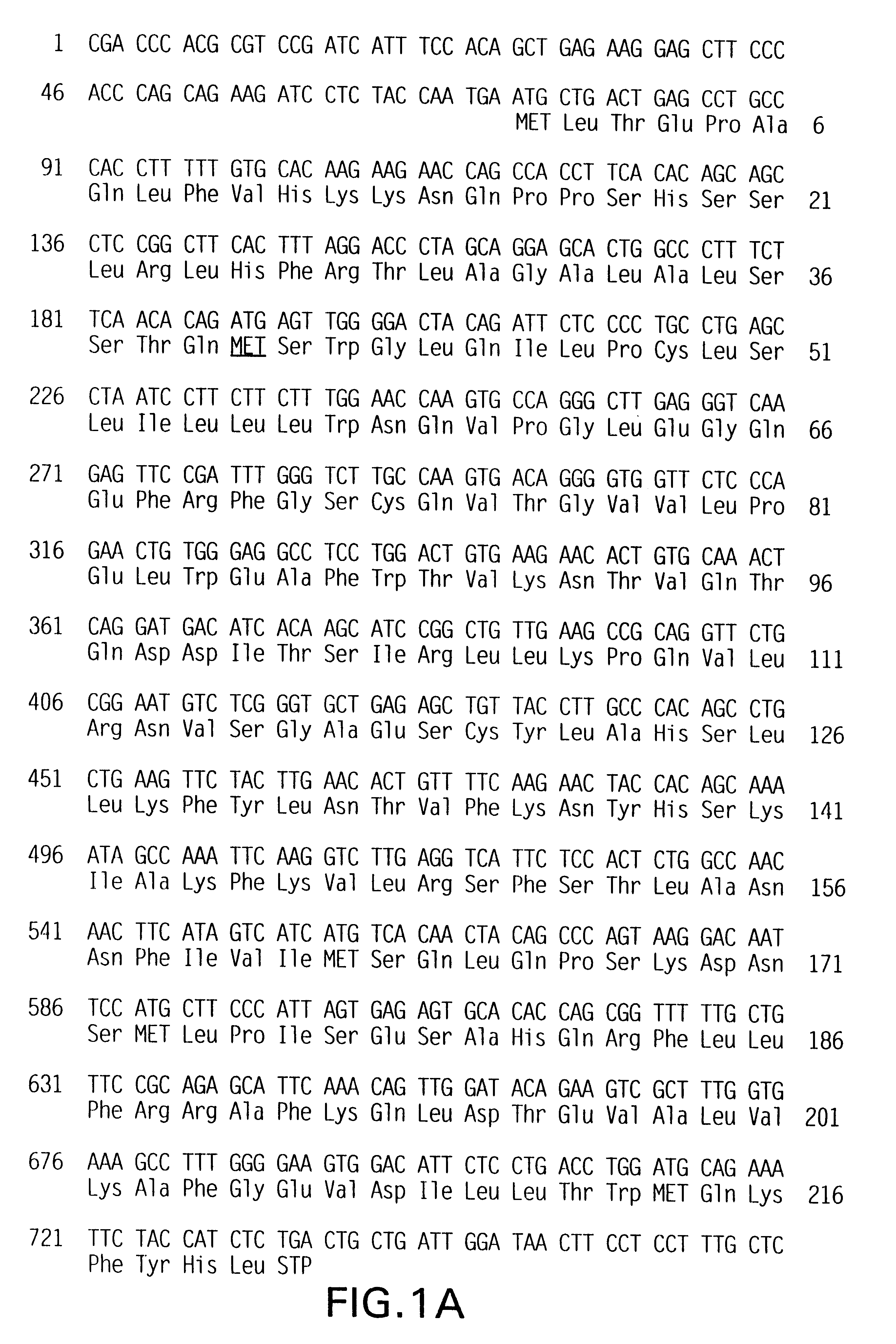
TH2-specific gene
InactiveUS6190909B1Increase the number of cellsEffective in number of cellOrganic active ingredientsFungiContact dermatitisTransgene
The present invention relates to the discovery, identification and characterization of nucleic acids that encode a novel protein differentially expressed within the TH2 cell subpopulation (hereinafter referred to as STIF). The invention encompasses STIF nucleotides, host cell expression systems, STIF proteins, fusion proteins, polypeptides and peptides, antibodies to the STIF protein, transgenic animals that express a STIF transgene, or recombinant knock-out animals that do not express the STIF protein, and compounds that modulate STIF gene expression or STIF activity that can be used for diagnosis, drug screening, clinical trial monitoring, and / or used to treat STIF based disorders, such as proliferative disorders and T-lymphocyte-related disorders including, but not limited to, chronic inflammatory diseases and disorders, such as Crohn's disease, reactive arthritis, including Lyme disease, insulin-dependent diabetes, organ-specific autoimmunity, including multiple sclerosis, Hashimoto's thyroiditis and Grave's disease, contact dermatitis, psoriasis, graft rejection, graft versus host disease, sarcoidosis, atopic conditions, such as asthma and allergy, including allergic rhinitis, gastrointestinal allergies, including food allergies, eosinophilia, conjunctivitis, glomerular nephritis, certain pathogen susceptibilities such as helminthic (e.g., leishmaniasis) and certain viral infections, including HIV, and bacterial infections, including tuberculosis and lepromatous leprosy.
Owner:MILLENNIUM PHARMA INC
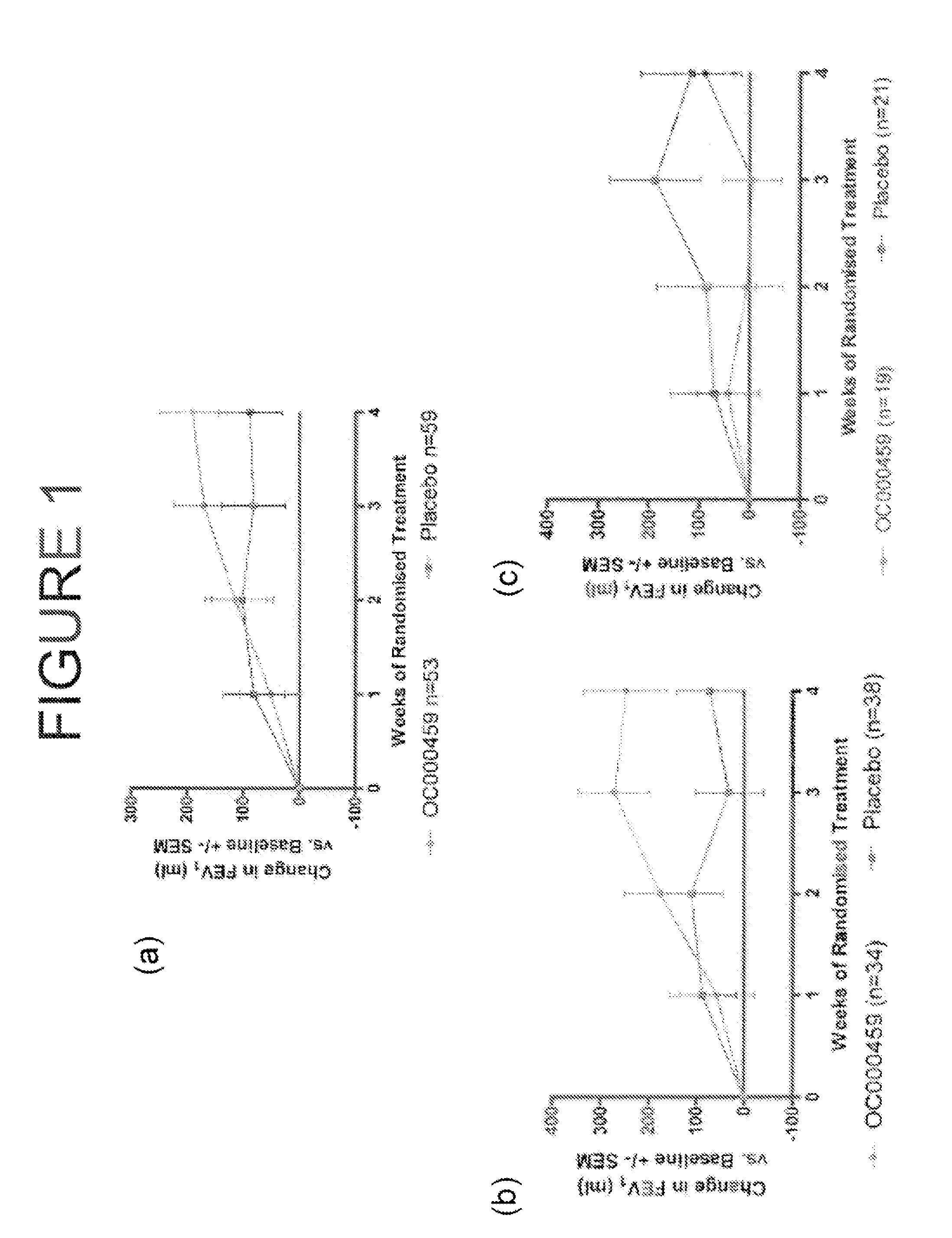
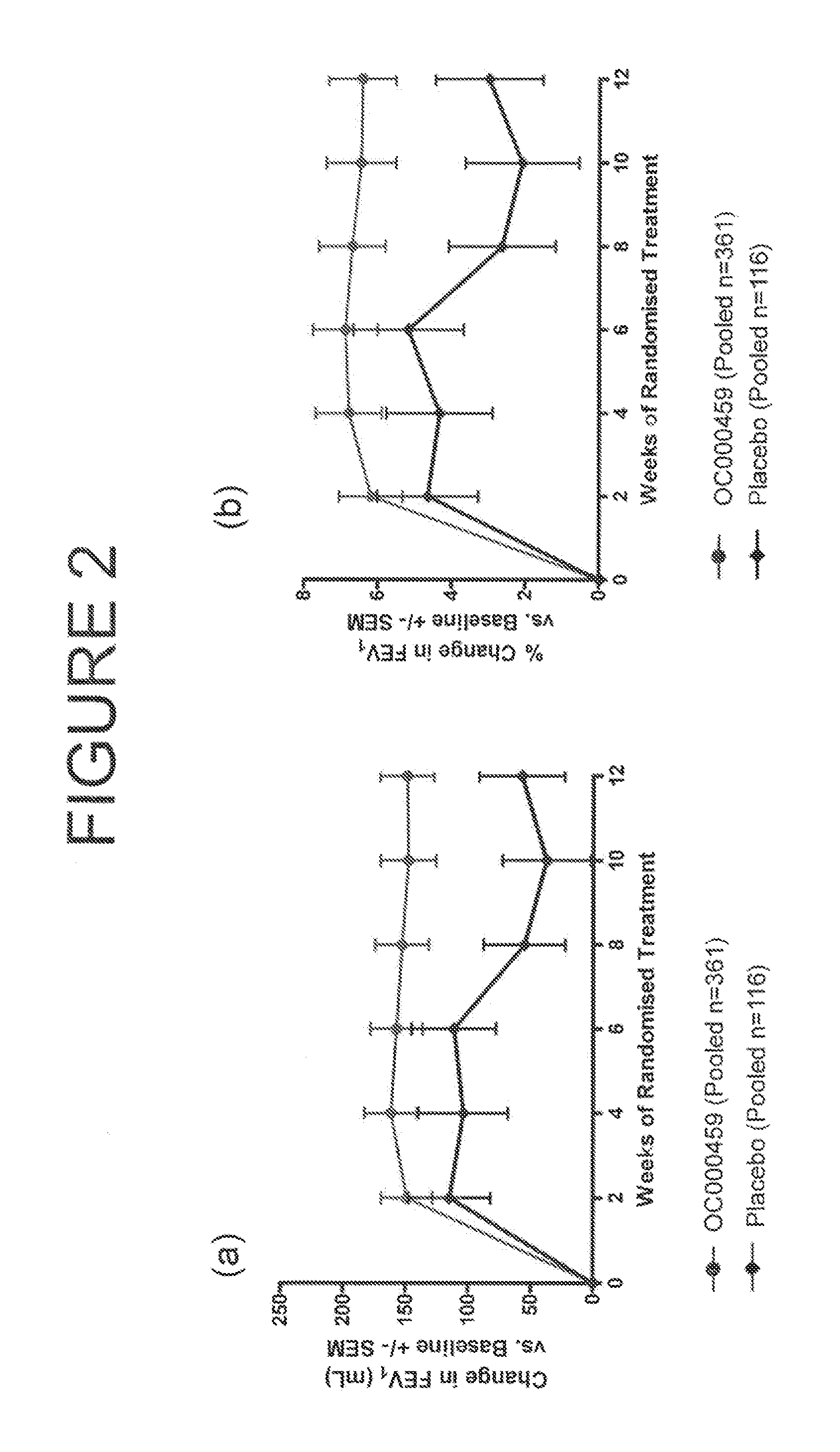
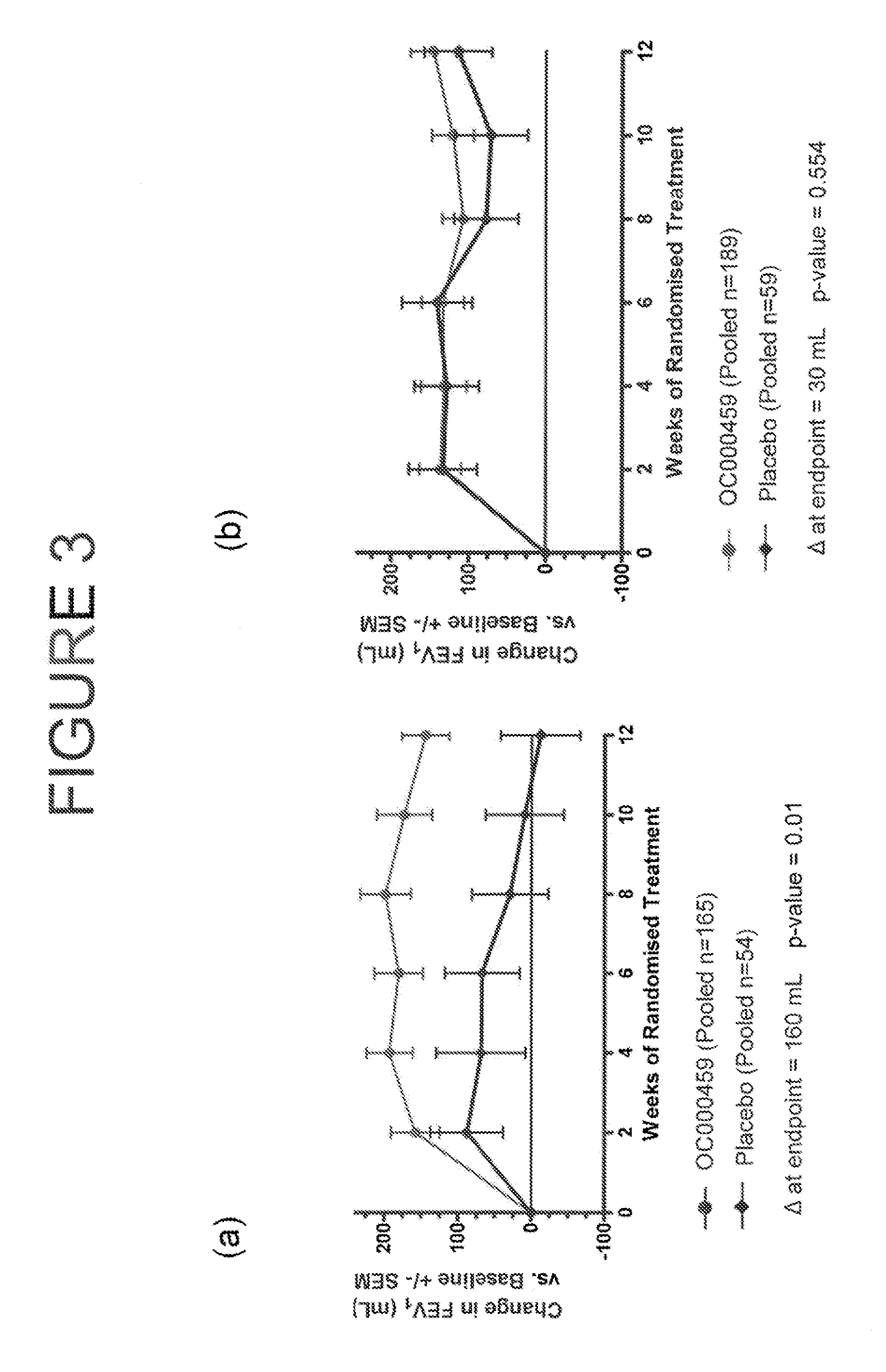
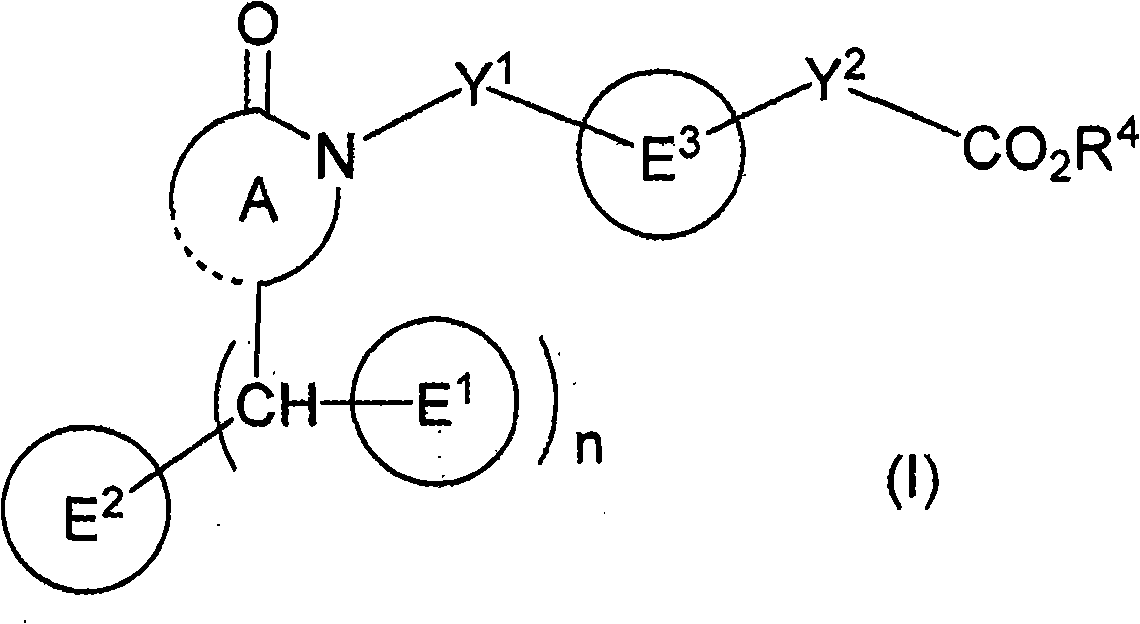
CRTH2 Antagonists for Treatment of Eosinophilic Diseases and Conditions
The present invention provides a method for the treatment of allergic and inflammatory diseases or conditions by administering a compound of Formula (I). The invention provides a method of treatment that is particularly suited for patients with a high degree of airway eosinophilia in contrast to those with a lower degree of airway eosinophilia. The invention also provides a method of treatment that is particularly suited for patients with a high atopic status in contrast to those patients with a lower atopic status.
Owner:ATOPIX THERAPEUTICS
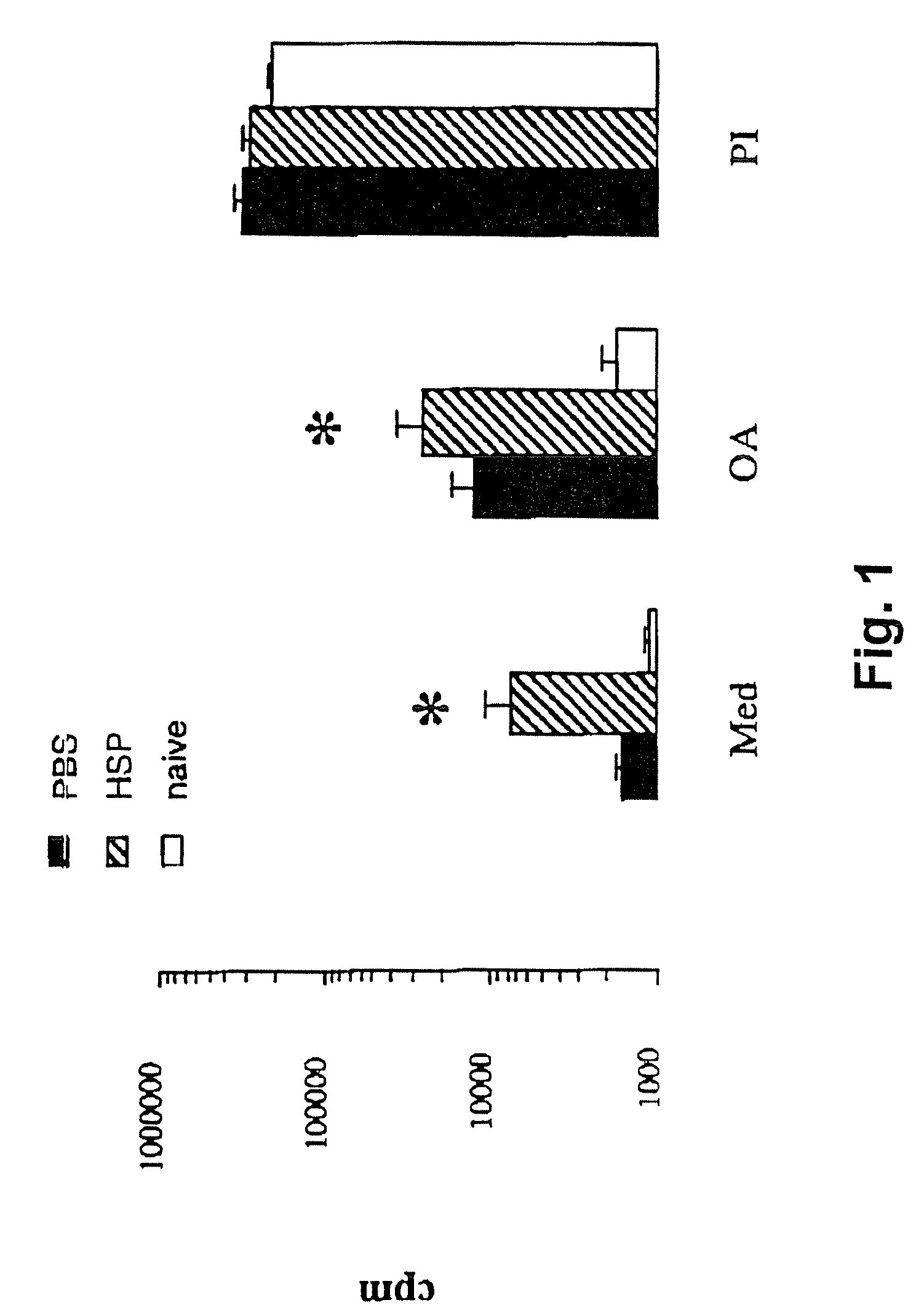
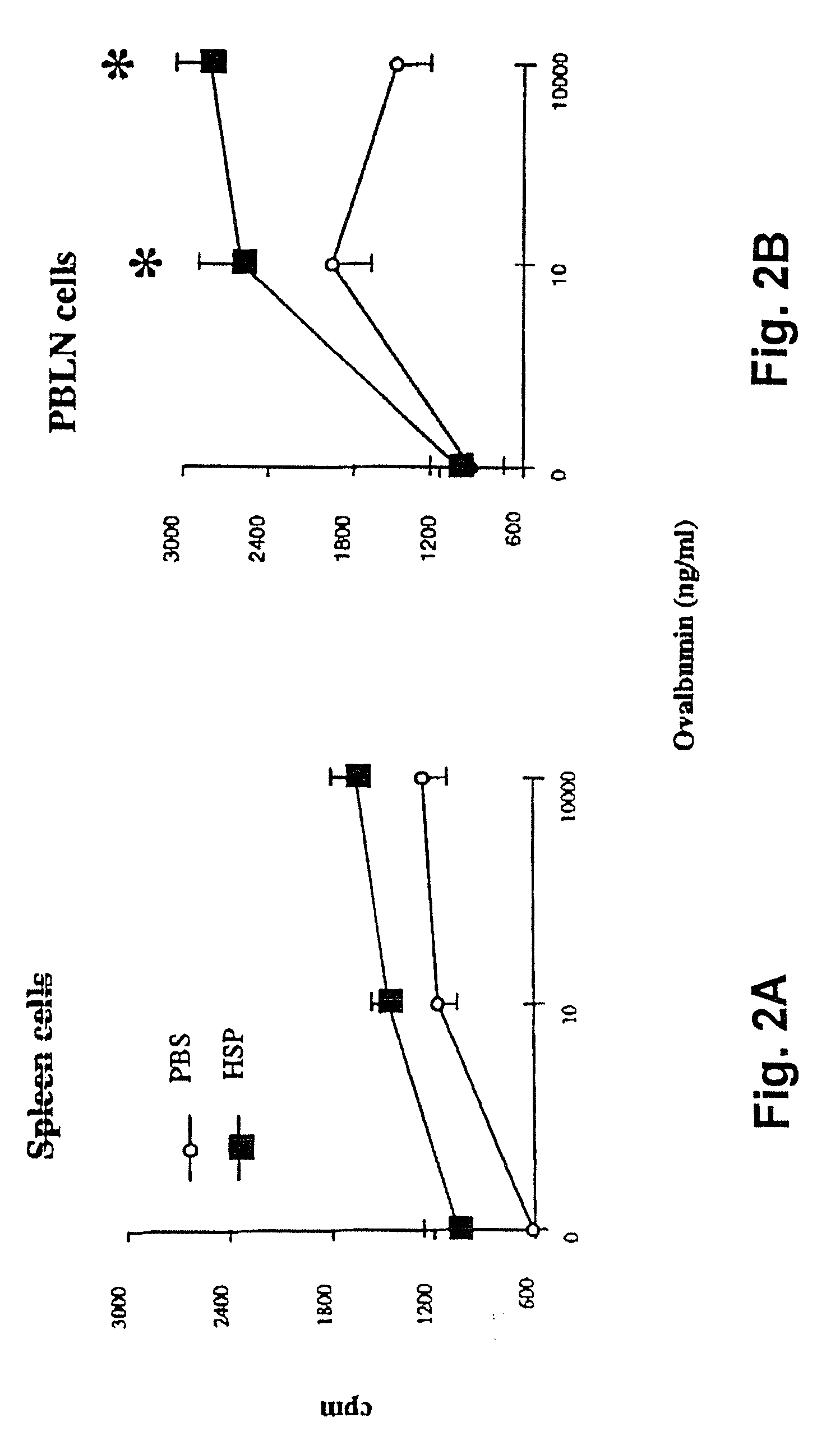
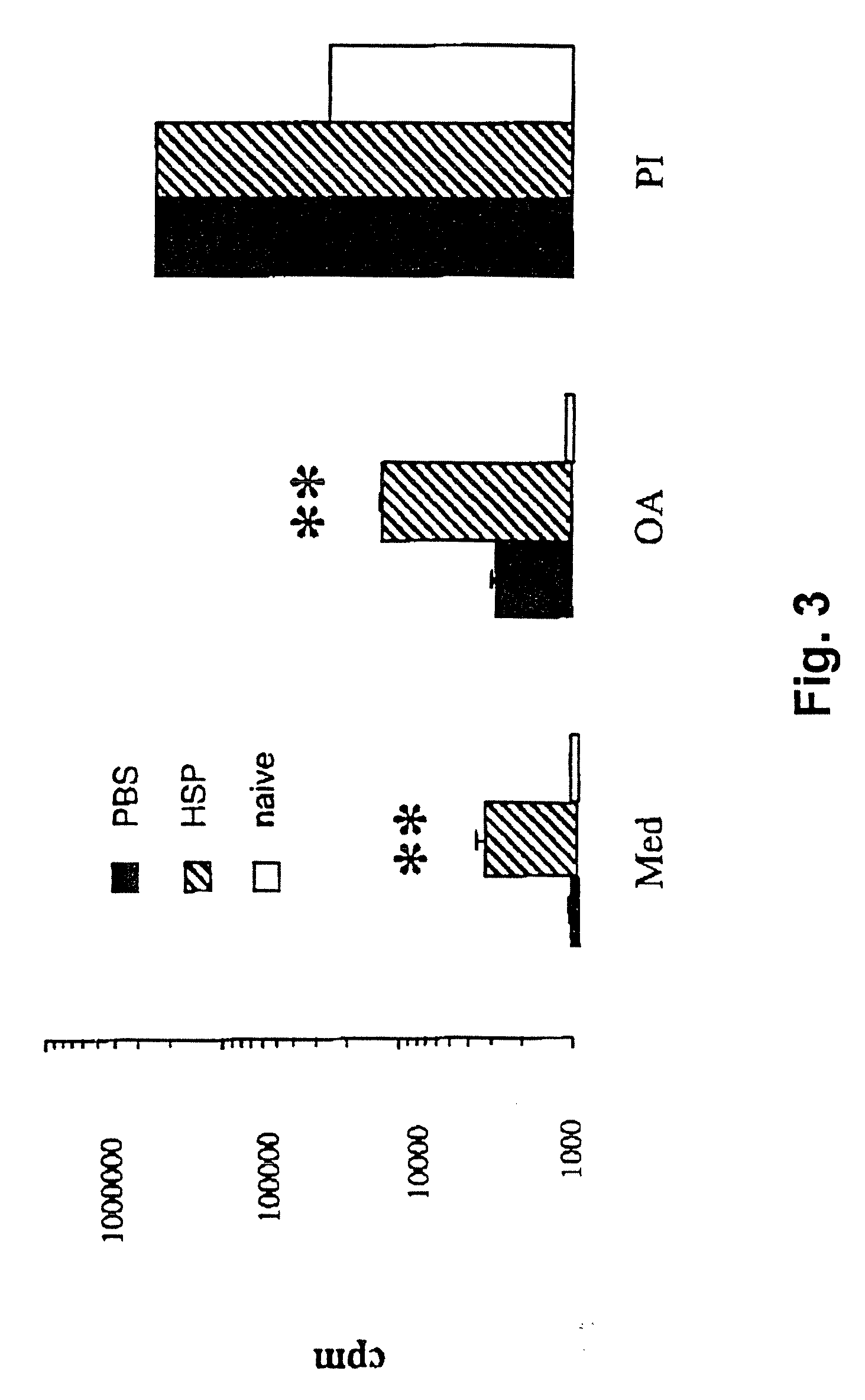
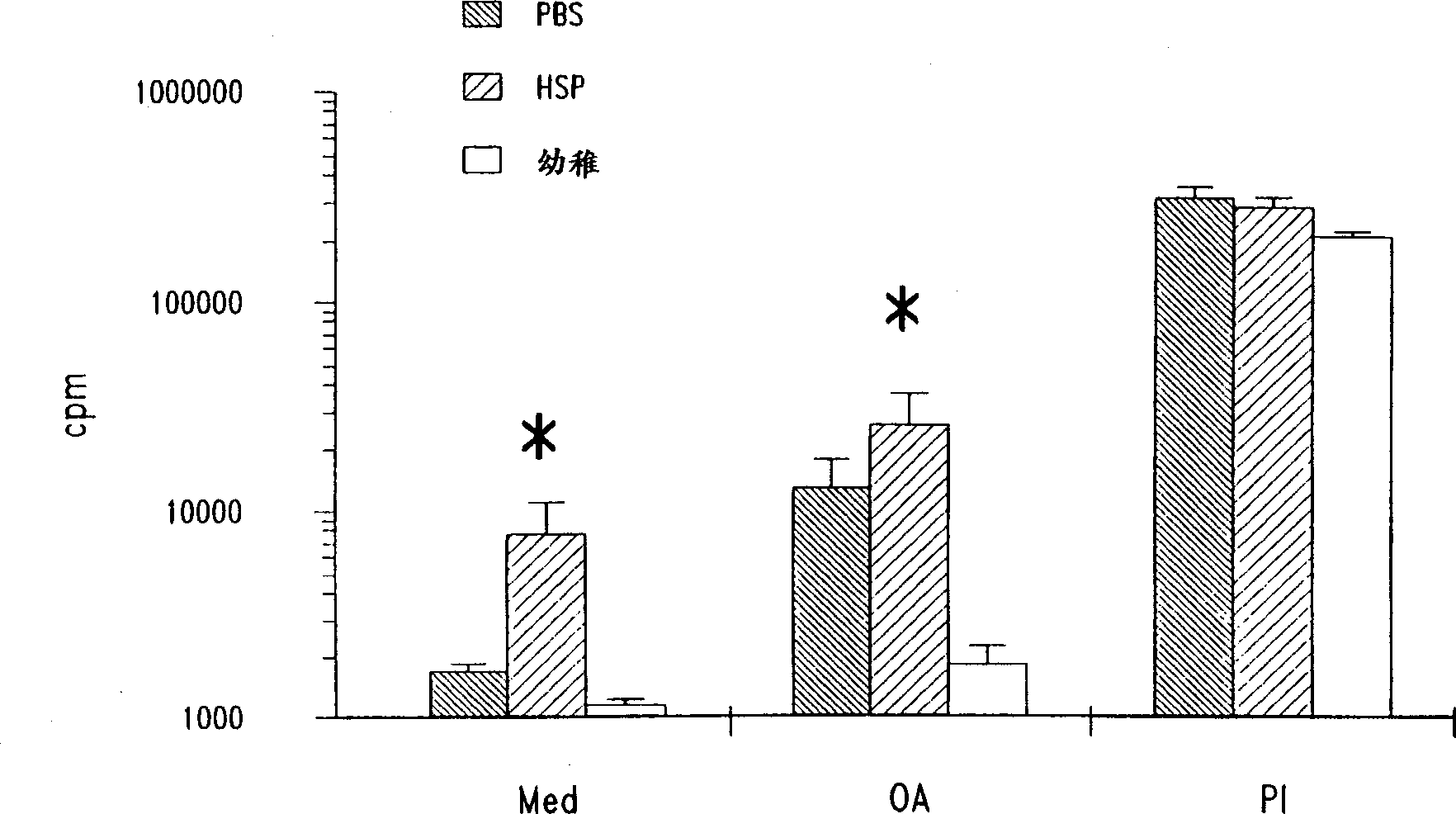
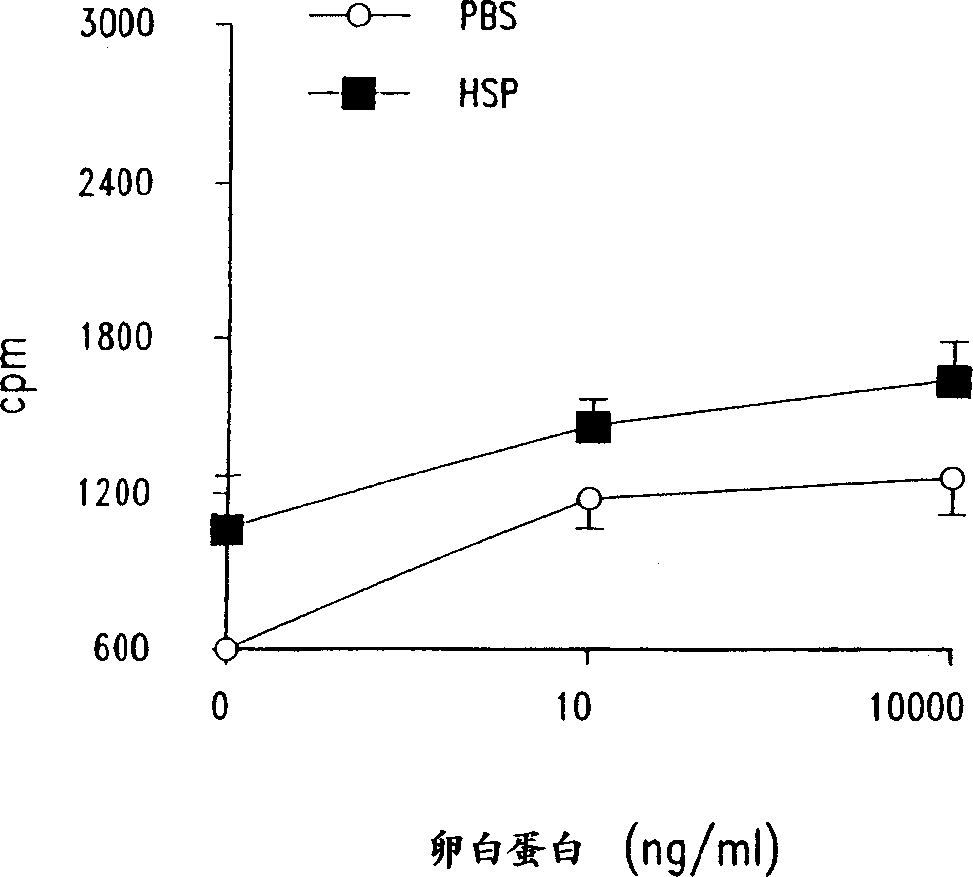
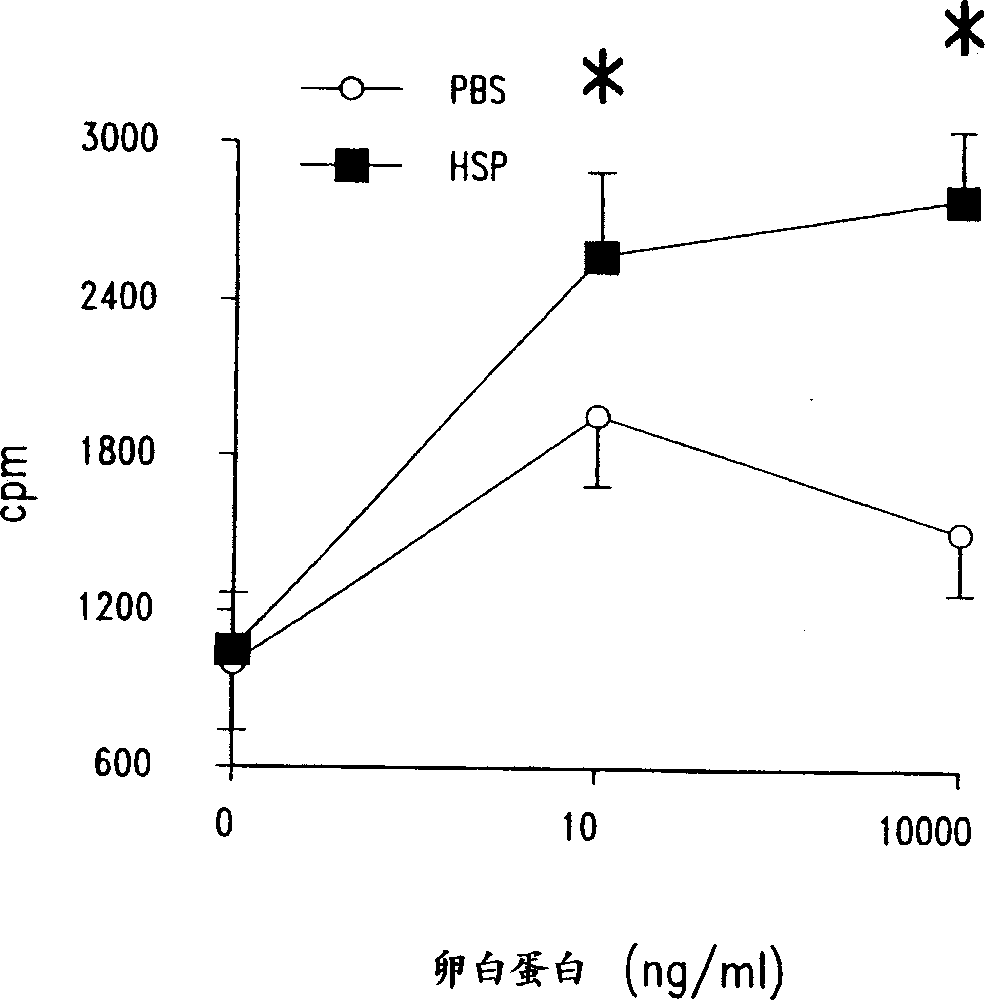
Method for treating inflammatory diseases using heat shock proteins
InactiveUS20070179087A1Decrease airway methacholine responsivenessReduces airflow limitationPowder deliverySenses disorderHeat shockPresent method
This invention relates to a method to protect a mammal from a disease associated with an inflammatory response, and in particular, from an inflammatory disease characterized by eosinophilia, airway hyperresponsiveness and / or a Th2-type immune response. The method includes administration of a heat shock protein to a mammal having such a disease. Formulations useful in the present method are also disclosed.
Owner:NAT JEWISH MEDICAL & RES CENT
Polycyclic acid compounds useful as crth2 antagonists and antiallergic agents
InactiveCN101636386AHas CRTH2 binding activityOrganic active ingredientsSenses disorderAllergic dermatitisDisease
The present invention relates to a novel compound or a salt thereof, which is useful as a CRTH2 antagonist, especially as a medicament for disorder that participates eosinophil, for example, allergic disorder such as asthma, allergic rhinitis, allergic dermatitis, conjunctival inflammation, hives, eosinophilic bronchitis, food allergy, inflammation of the nasal sinuses, multiple sclerosis, angiitis, or chronic obstructive pulmonary disease (COPD) and the like.
Owner:ASTELLAS PHARMA INC
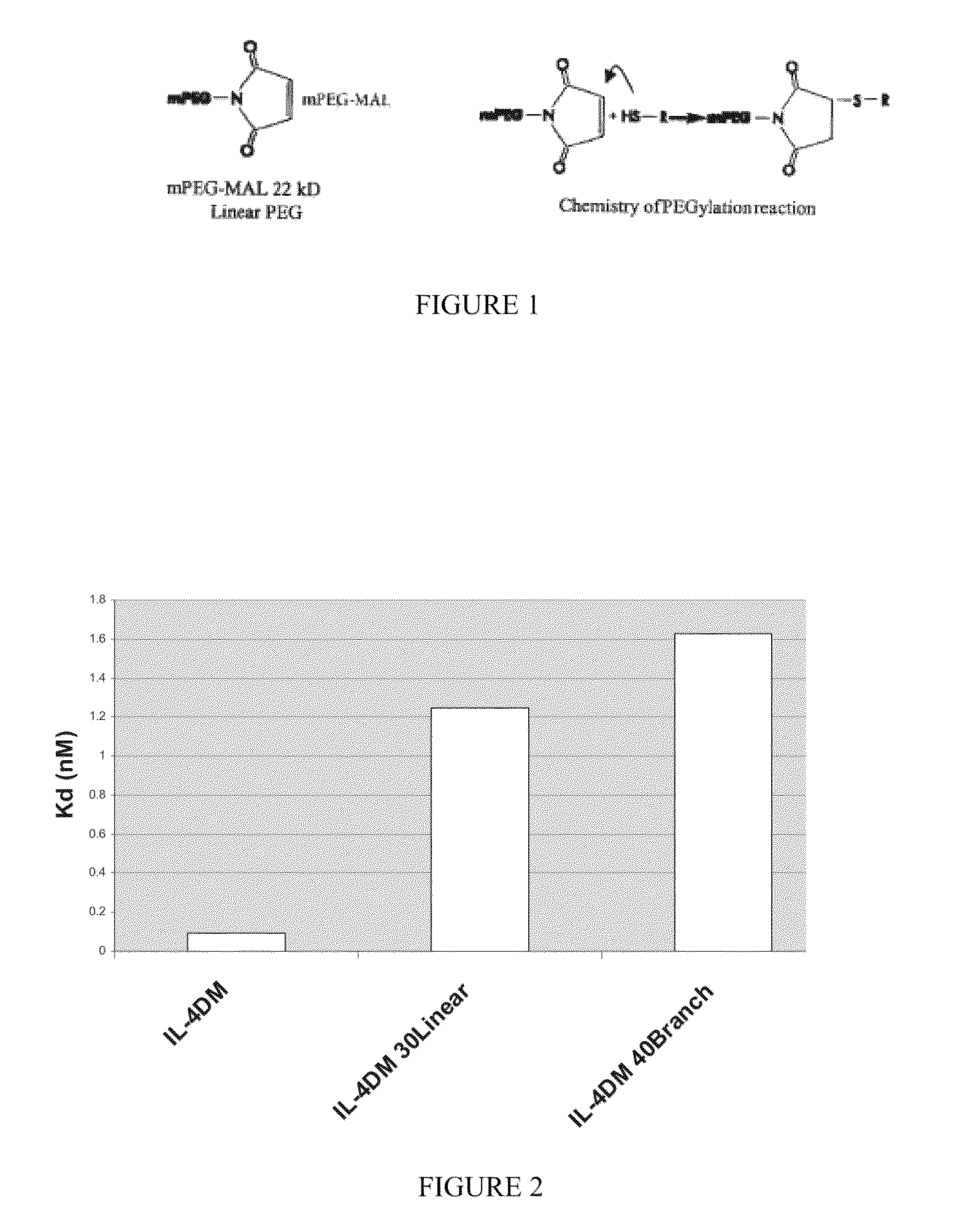
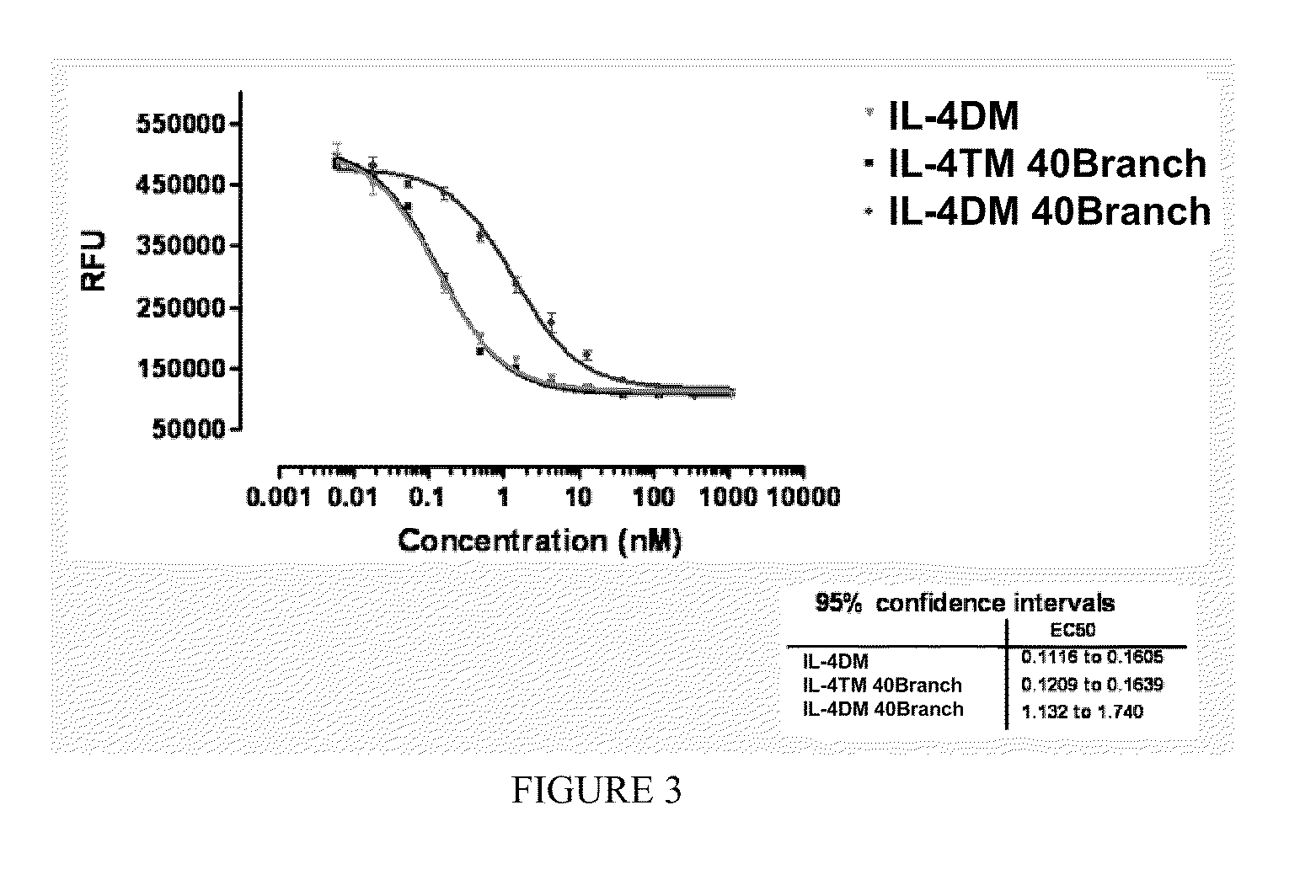
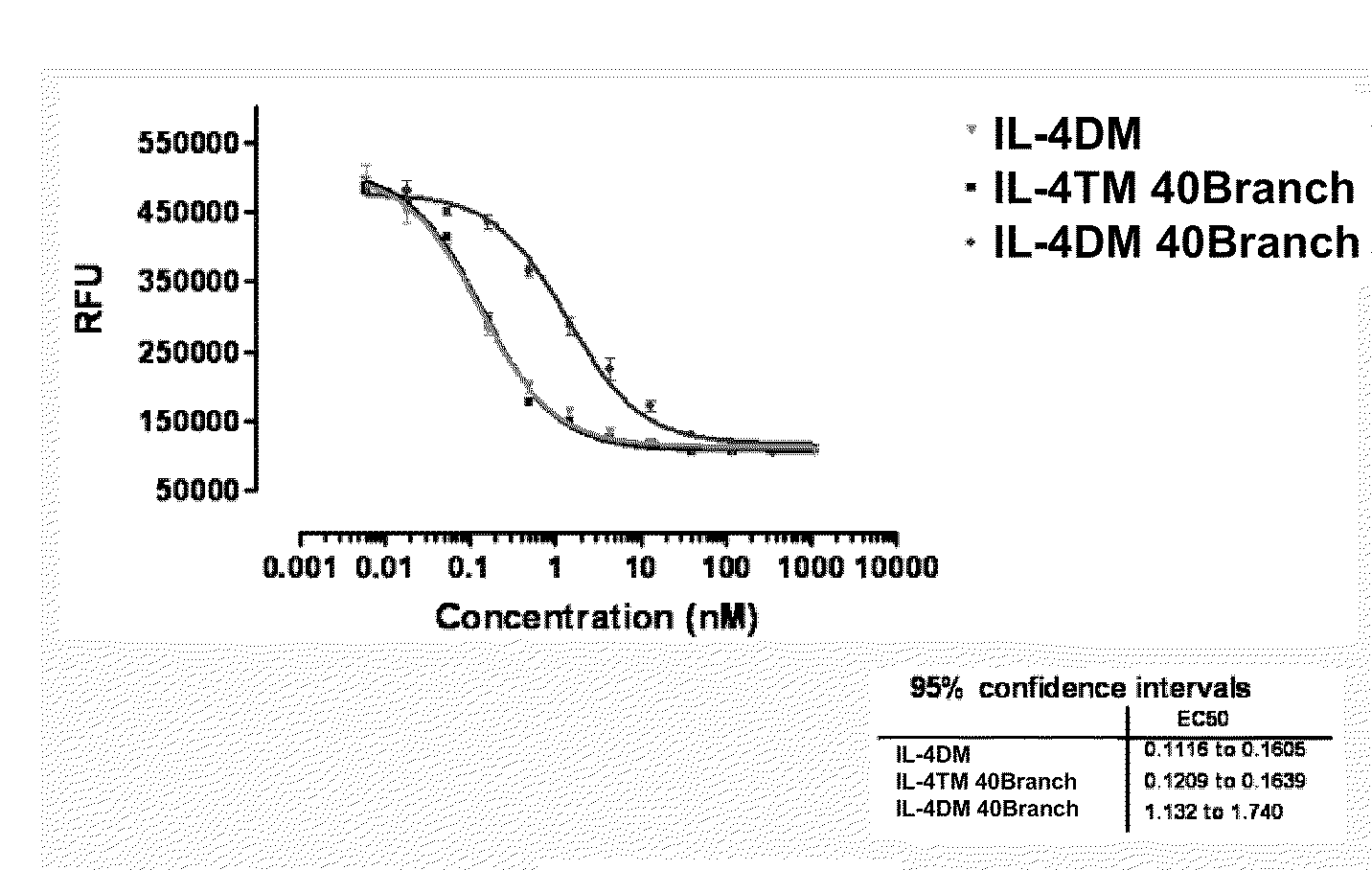
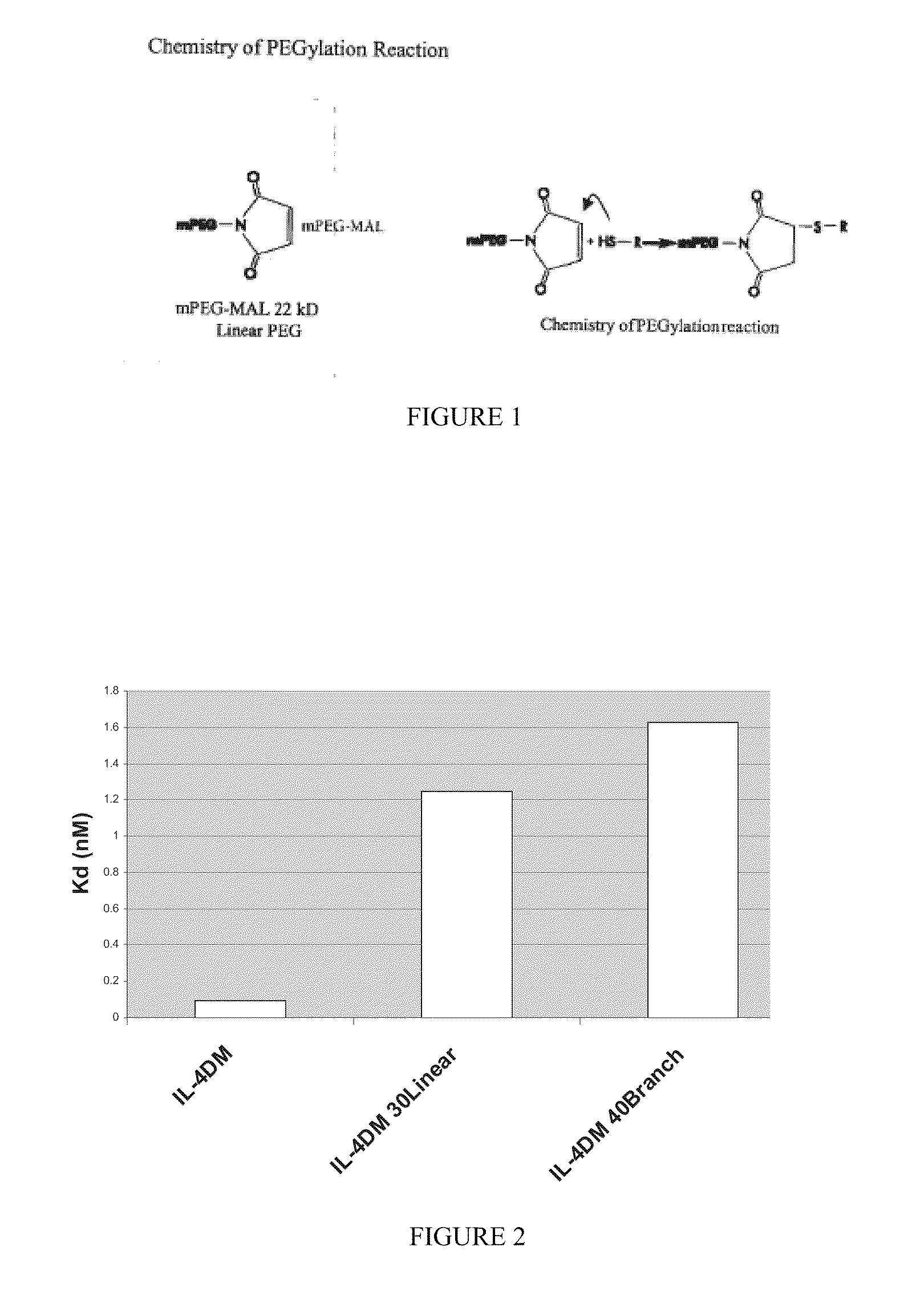
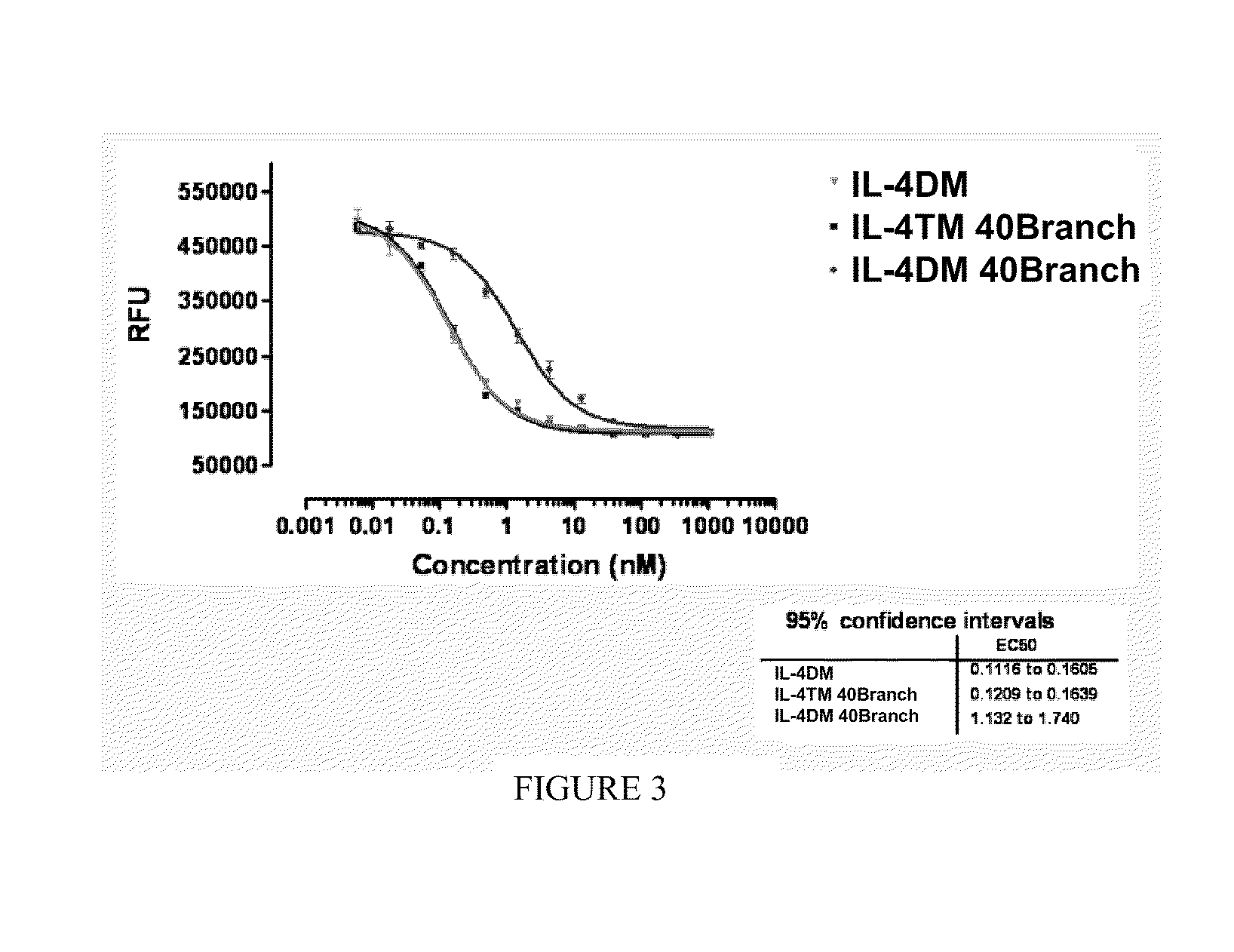
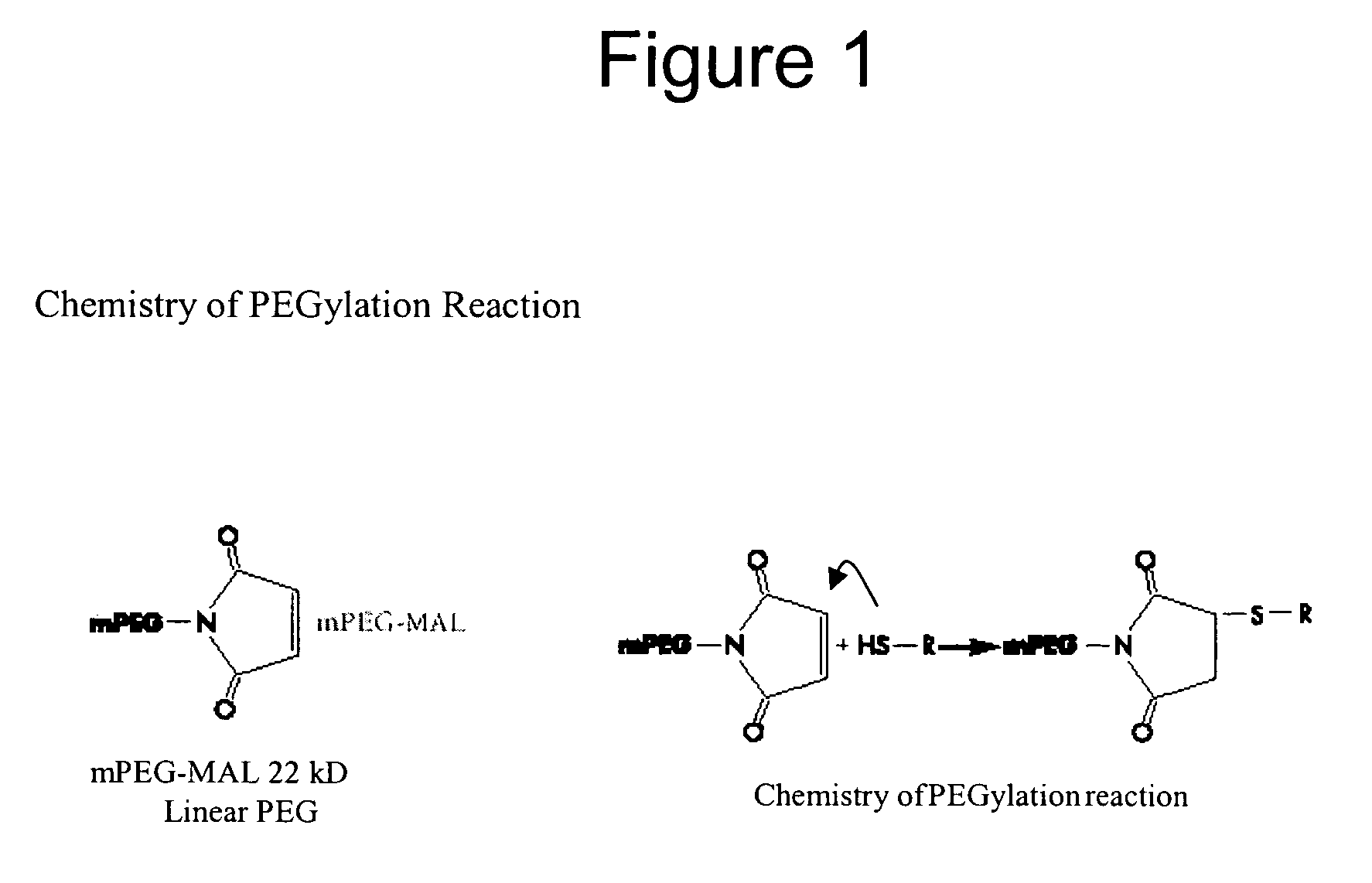
Modified IL-4 mutein receptor antagonists
This invention relates to modified IL-4 mutein receptor antagonists comprising an IL-4 mutein receptor antagonist coupled to polyethylene glycol. Related formulations and dosages and methods of administration thereof for therapeutic purposes are also provided. These modified IL-4 mutein receptor antagonists, compositions and methods provide a treatment option for those individuals afflicted with a respiratory disorder such as asthma by inhibiting IL-4 and IL-13-mediated airway hyperresponsiveness and eosinophilia. More particularly, these antagonists have an increased duration of effect versus unmodified IL-4RA by virtue of a greater plasma half-life.
Owner:AEROVANCE INC
Compositions of cis-9, trans-11 conjugated linoleic acid and vaccenic acid and uses thereof
InactiveUS20090048339A1Reducing and preventing degradationBiocideAnimal repellantsWhite blood cellMedicine
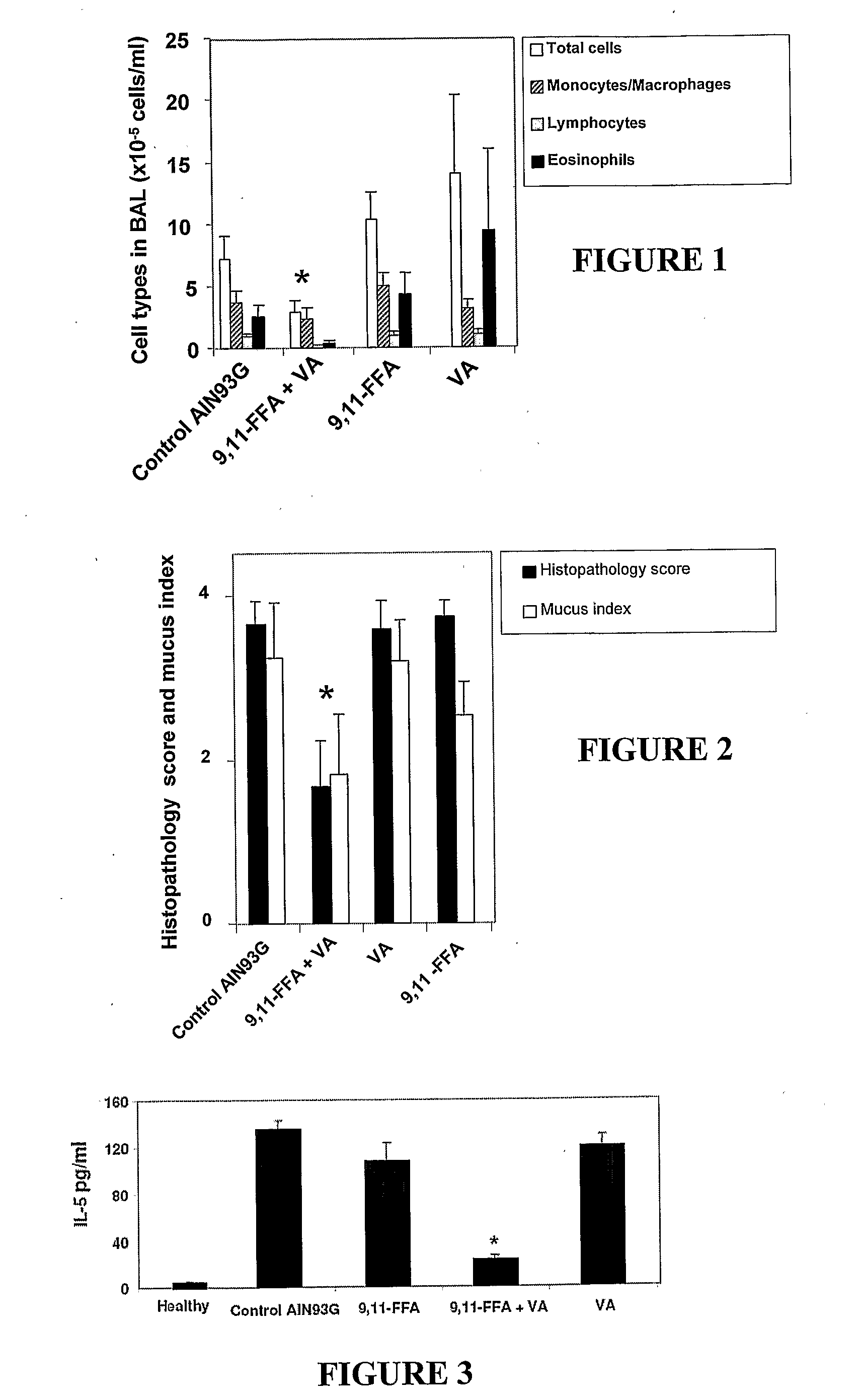
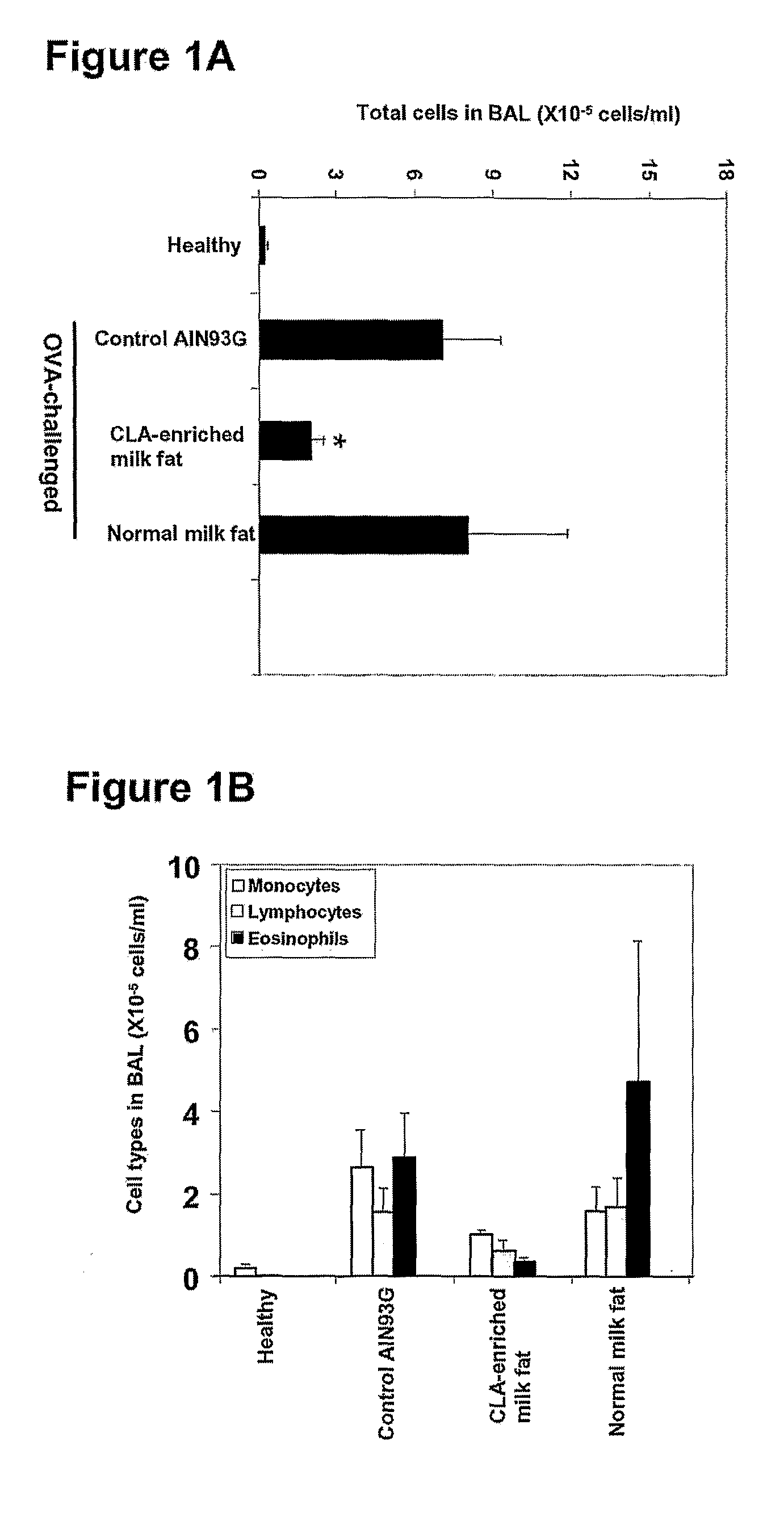
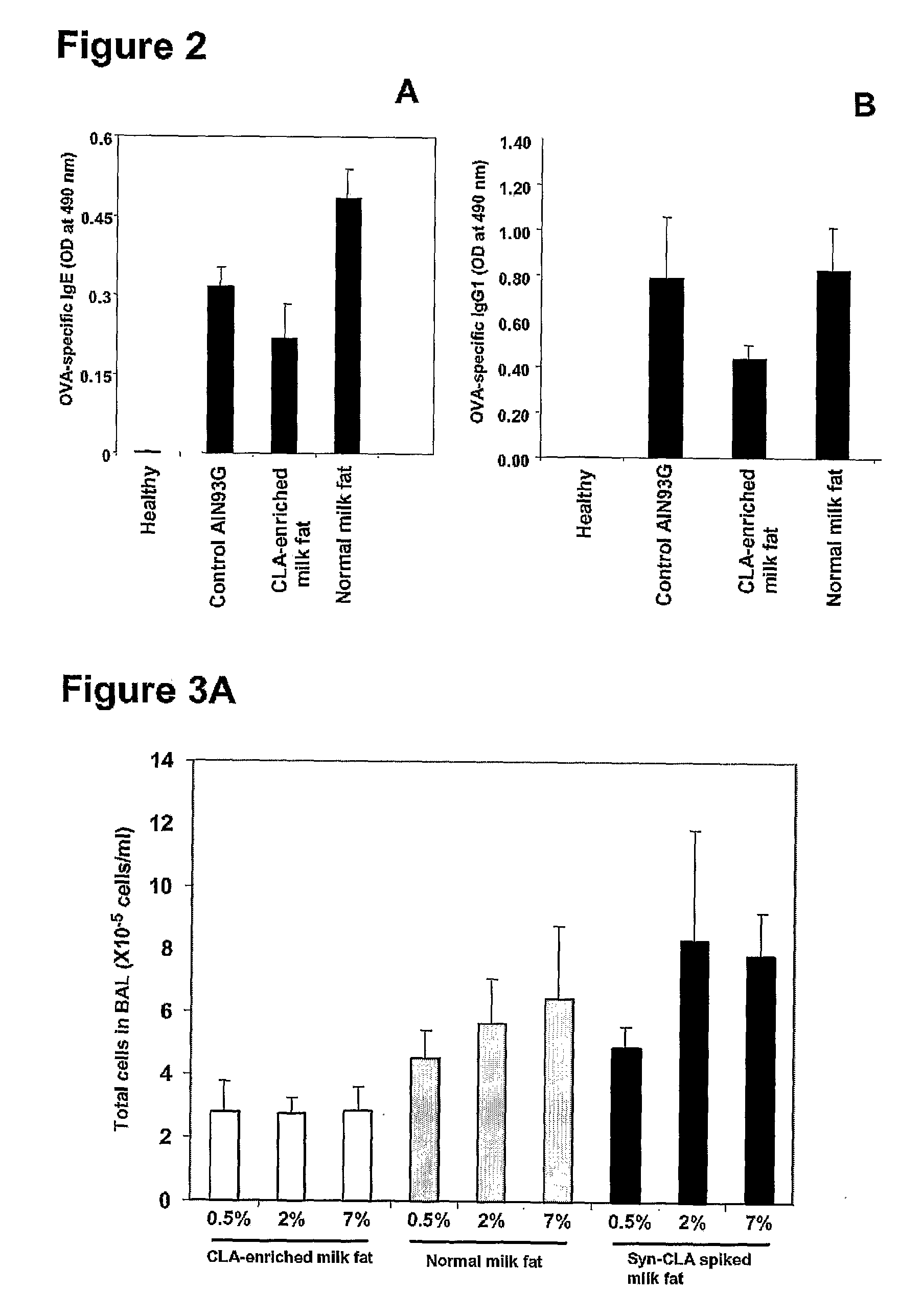
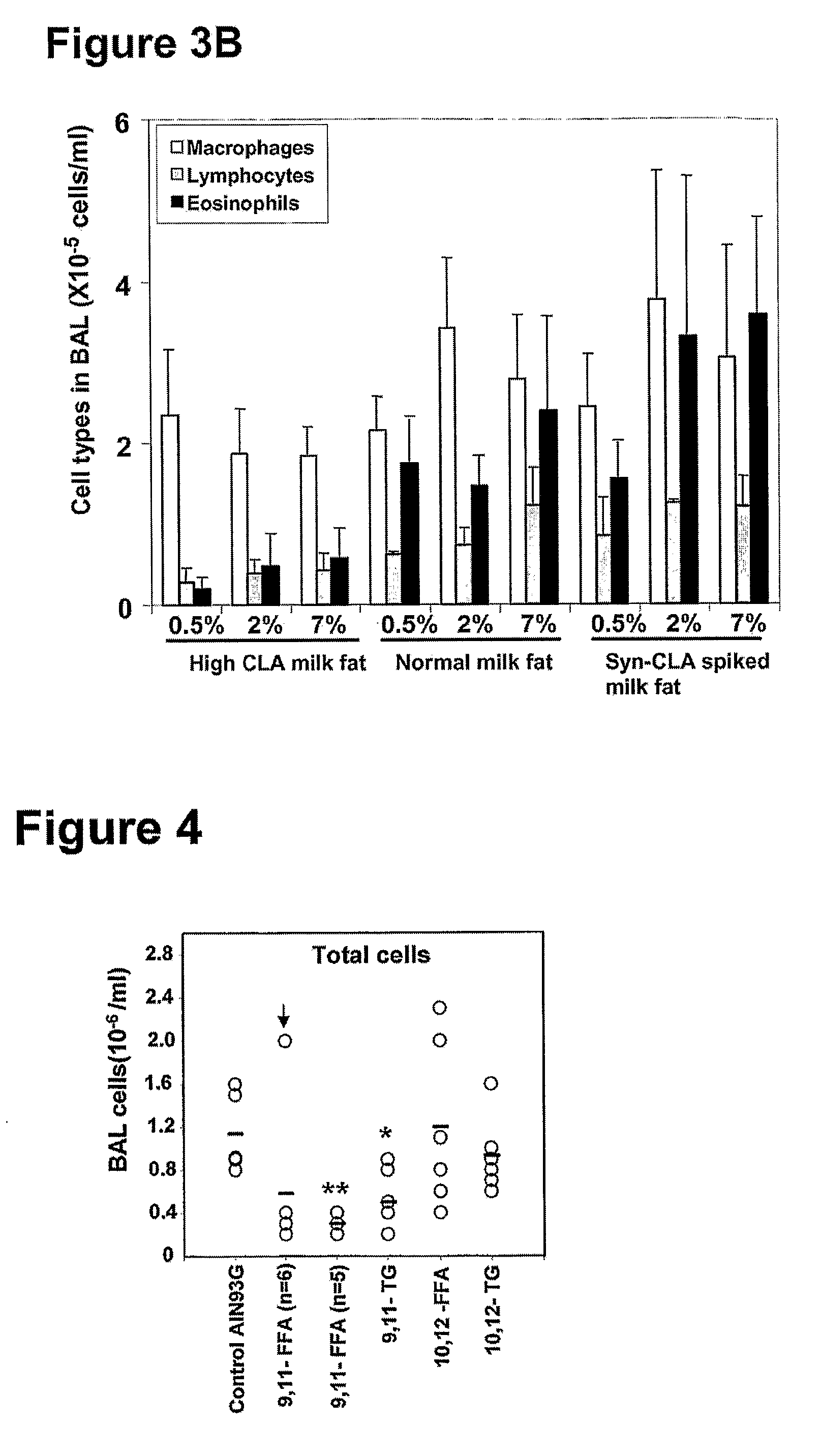
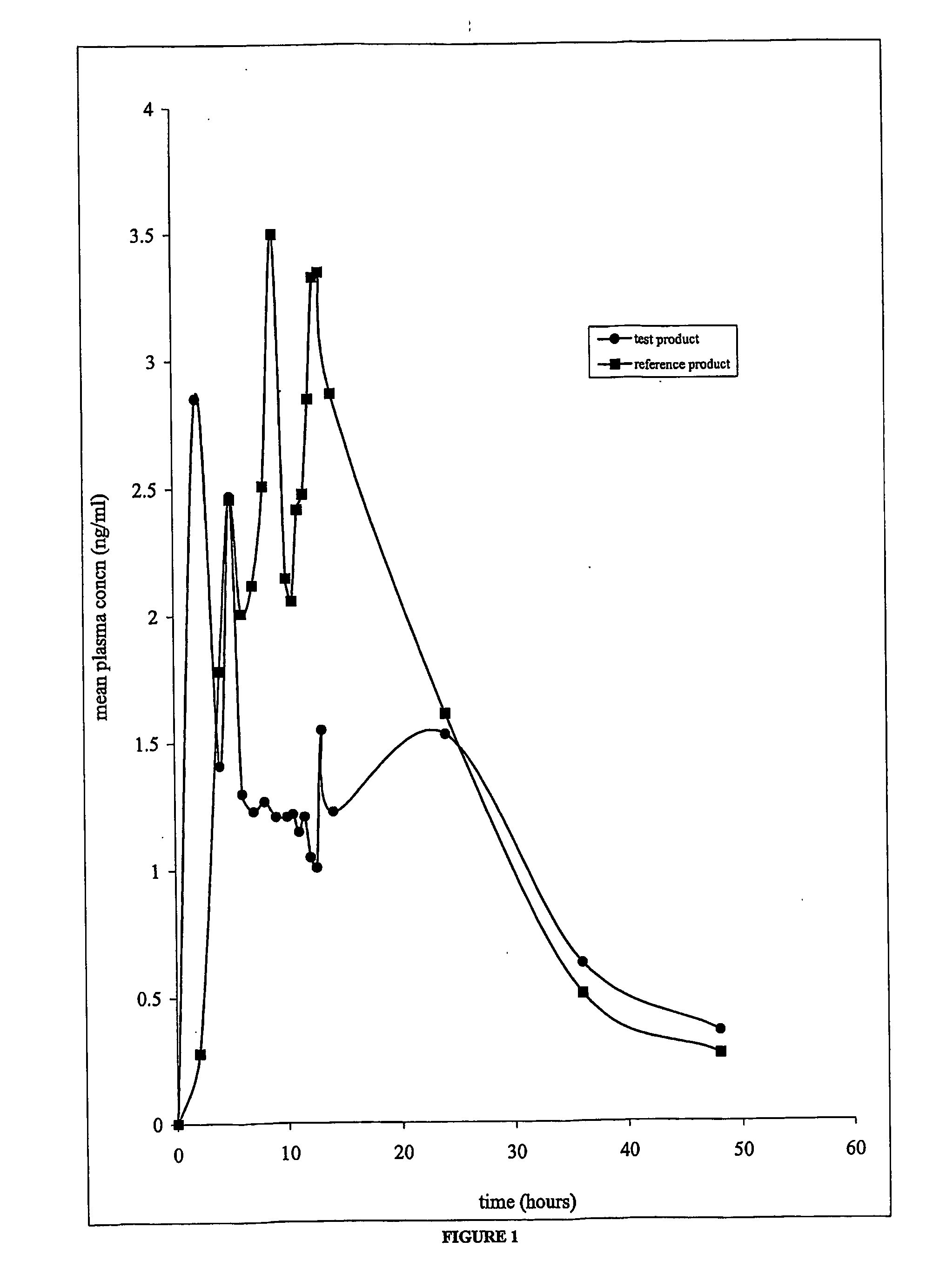
The invention relates to use of the cis-9, trans-11 isomer of conjugated linoleic acid or a salt or ester thereof (cis-9, trans-11 CLA) and vaccenic acid or a salt or ester thereof (VA) to treat or prevent conditions associated with one or more of leukocyte infiltration, eosinophilia, airway remodelling, bronchoconstriction, mucus hypersecretion, and lung and skin inflammation. The present invention also relates to a composition comprising cis-9, trans-11 CLA and VA and use of the composition to treat or pre-vent conditions associated with one or more of leukocyte infiltration, eosinophilia, airway remodelling, bronchoconstriction, mucus hypersecretion, and lung and skin inflammation. In particular, the medicinal uses, compositions and methods of the invention may be used to treat or prevent conditions such as asthma and dermatitis, and related disorders.
Owner:AUCKLAND UNISERVICES LTD +2
Pharmaceutical composition comprising an extract of pseudolysimachion longifolium and the catalpol derivatives isolated therefrom having antiinflammatory, antiallergic and antiasthmatic activity
ActiveCN101208084ATreat and prevent inflammationOrganic active ingredientsDrug compositionsDiseaseBlood plasma
The present invention relates to a composition comprising an extract of Pseudolysimachion genus plant, and the catalpol derivatives isolated therefrom having anti-inflammatory, antiallergic and anti-asthmatic activity. The extractof Pseudolysimachion genus plantand the catalpol derivatives isolated therefrom shows potent suppressing effect on elevated IgE, IL-4 and IL- 13 levels and eosinophilia in the plasma and BALF, and mucus overproduction in the lung tissues in an OVA-induced asthmatic mouse model. Therefore, it can be used as the therapeutics or functional health food for treating and preventing inflammatory, allergic and asthmatic disease.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Anaphylatoxins for detecting clinical conditions
InactiveUS20090226374A1Compounds screening/testingMicrobiological testing/measurementVasculitisBasophilia
Non-allergic hypersensitivity reactions can be observed in a sample of cells from a subject in response to anaphylatoxins. Accordingly, methods are provided for detecting clinical conditions such as cellular hyper-reactivity, non-allergic hypersensitivity, asthma, inflammation, chronic or acute infection, bacterial infection, viral infection, parasite infection, adverse drug reaction, organ rejection, vasculitis, mastocytosis, eosinophilia, basophilia, leukemia, and / or C3a or C5a receptor defects in a subject. Also provided are kits for detecting such clinical conditions in a subject.
Owner:HEALTH AIRE
Immune Function Modulating Agents
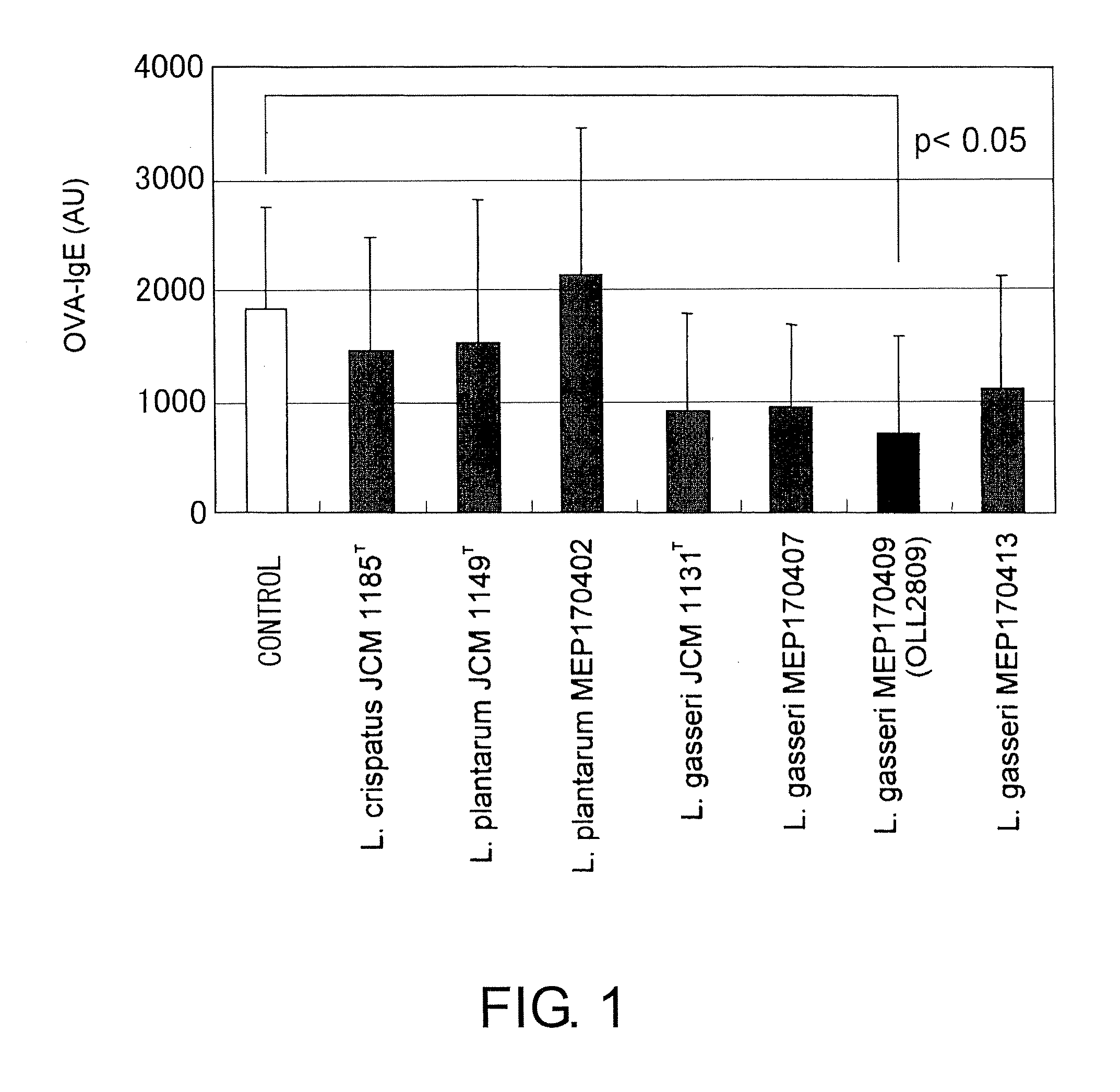
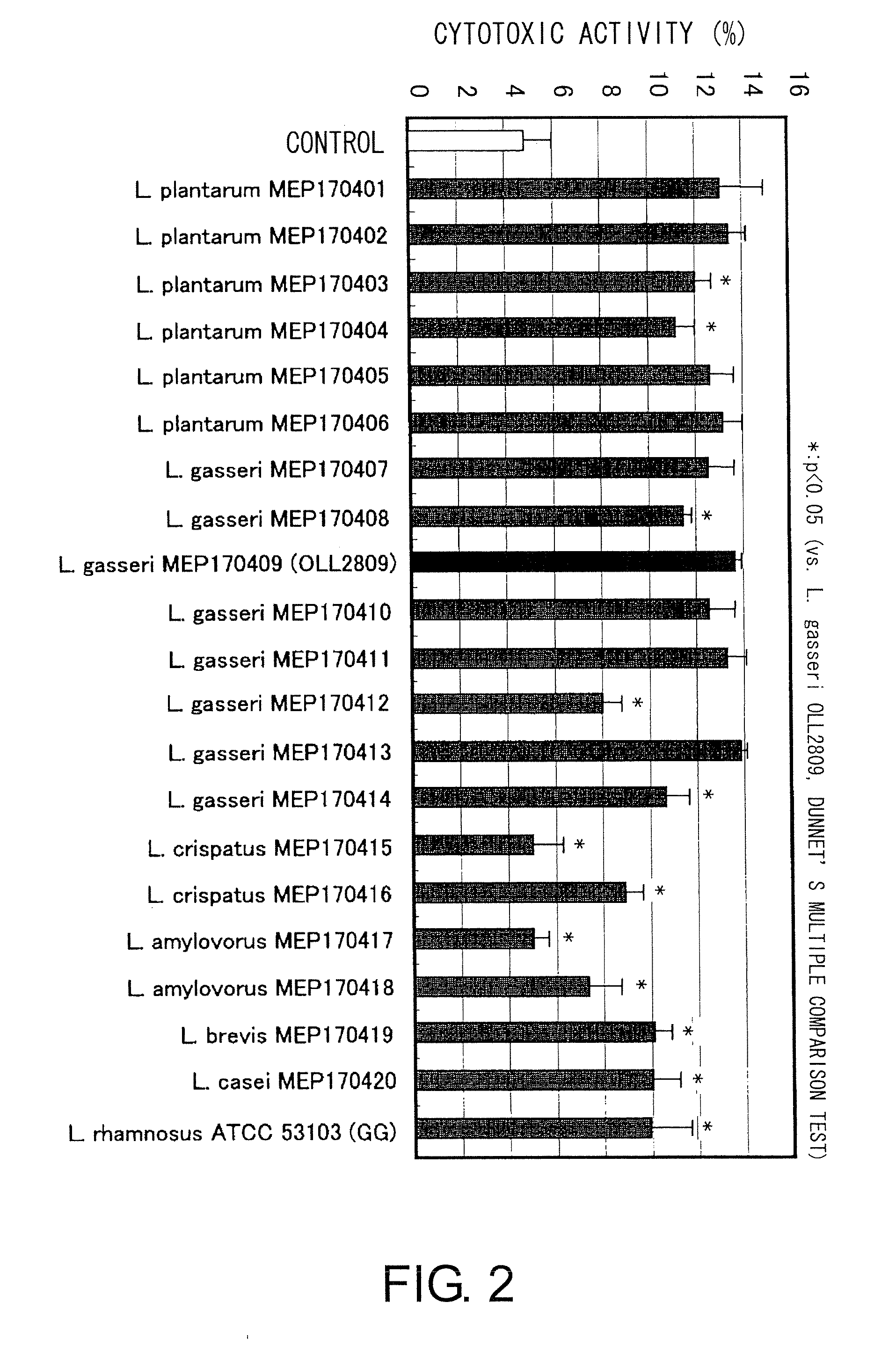
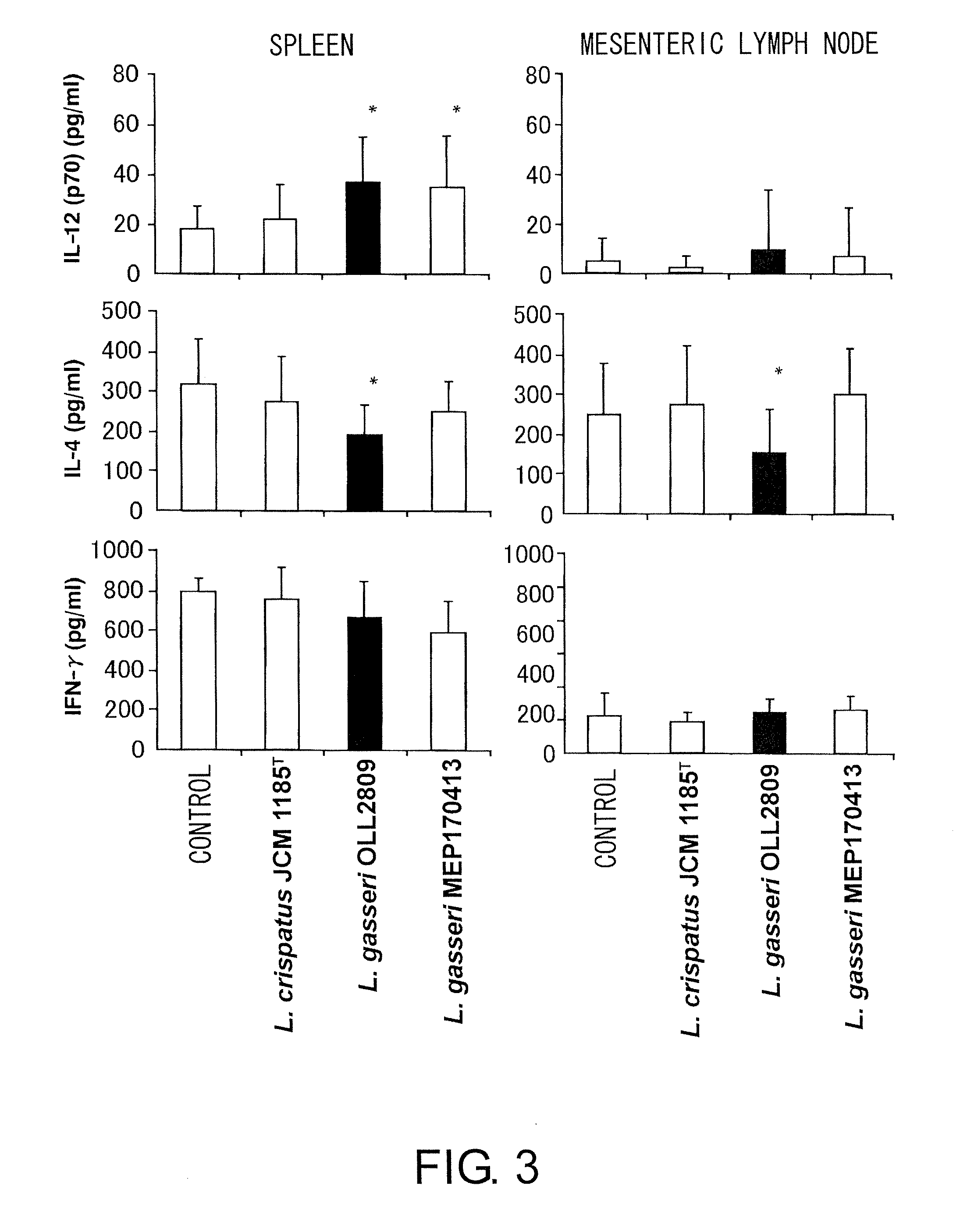
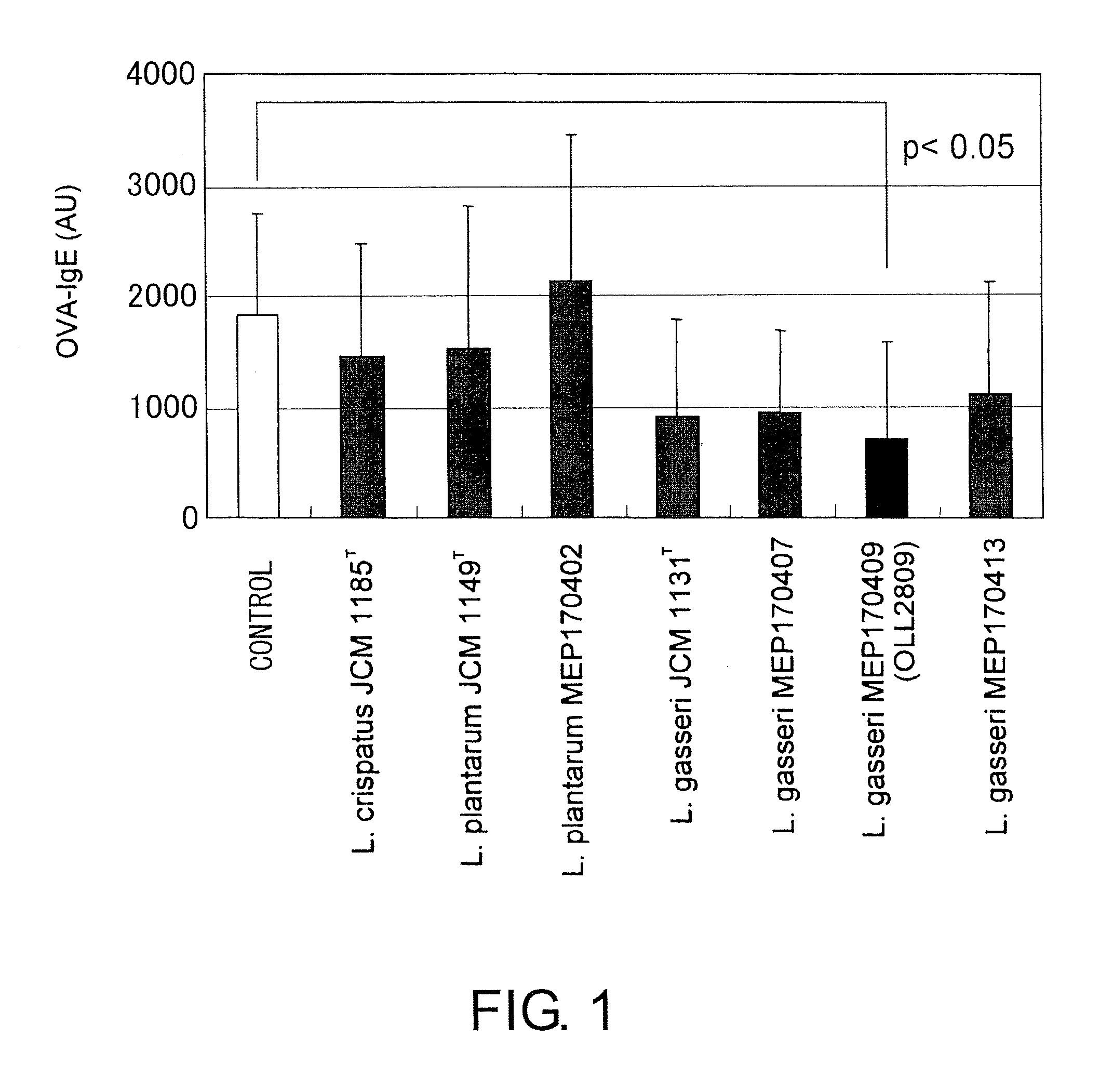
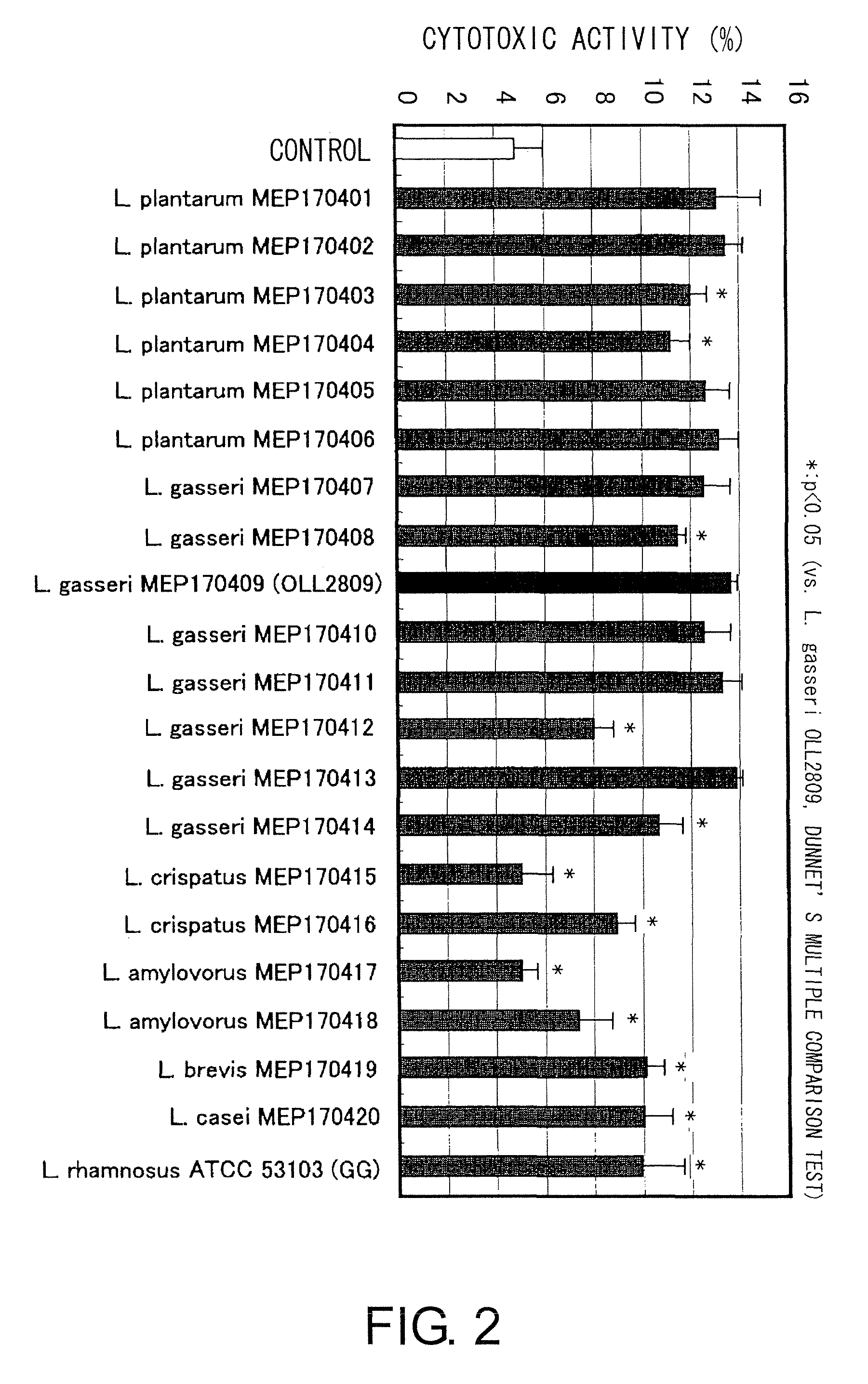
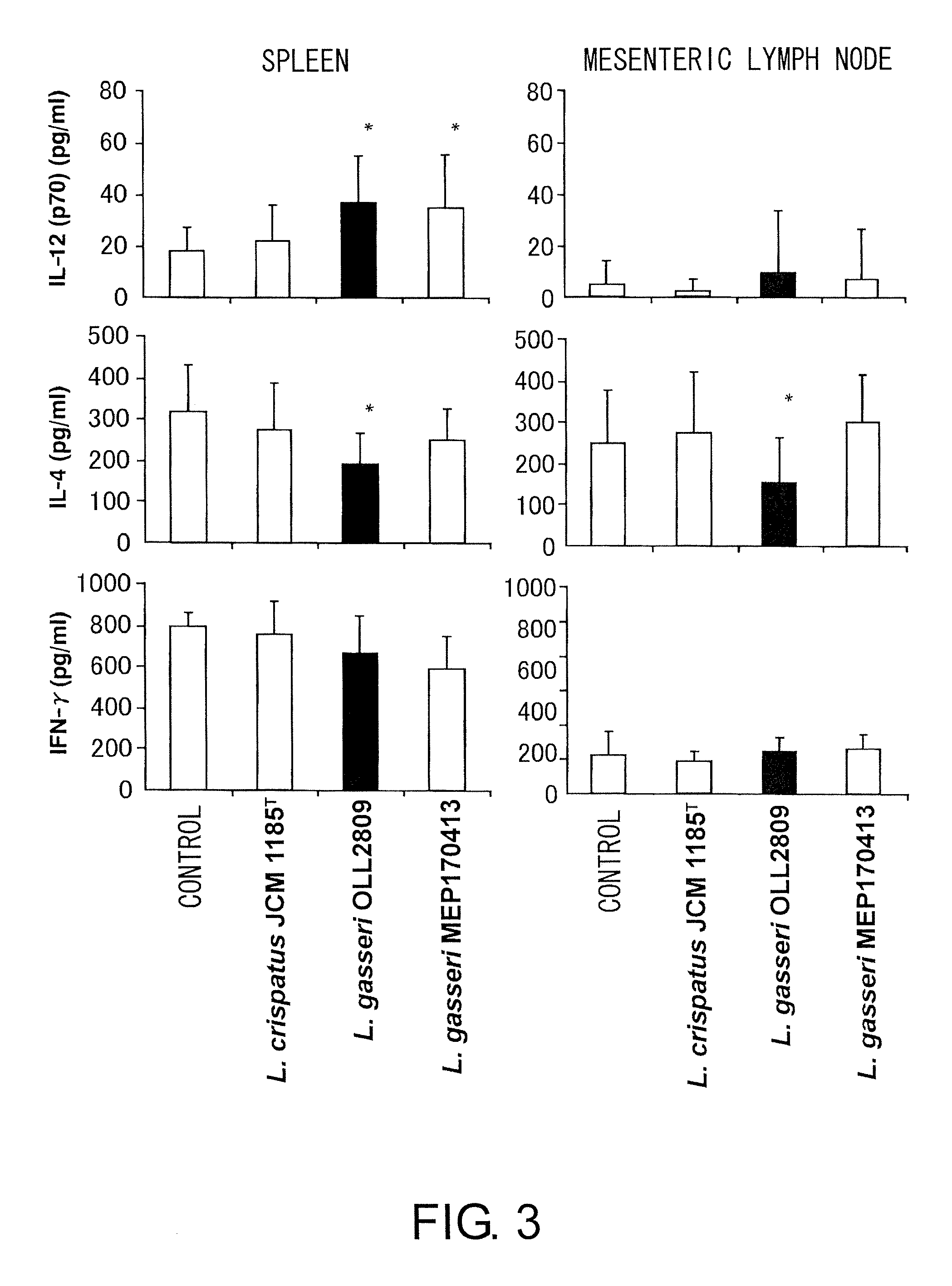
A probiotic lactobacillus was discovered from lactobacilli of the Lactobacillus genus independently isolated from human adult feces. The probiotic lactobacillus was selected from other bacterial strains for: (1) being highly resistant to gastric acid / bile acid; (2) having a high promoting activity on IL-12 production from mouse derived spleen cells and a high Th1 / Th2 balance-improving effect; (3) having a high ability to inhibit the production of antigen-specific IgE induced by intraperitoneally administering ovalbumin to BALB / c mice; (4) having a high ability to inhibit the production of antigen-specific IgE induced by orally administering a food antigen to C57BL / 6N mice; (5) having a high Natural Killer cell-activating ability; (6) having a high IL-12 production-promoting activity on spleen cells and mesenteric lymph node cells derived from mice immunized with ovalbumin and a high Th1 / Th2 balance-improving effect; and (7) having a high ability to suppress eosinophilia induced by a cedar pollen-extracted antigen. This discovery led to the completion of the present invention.
Owner:MEIJI CO LTD
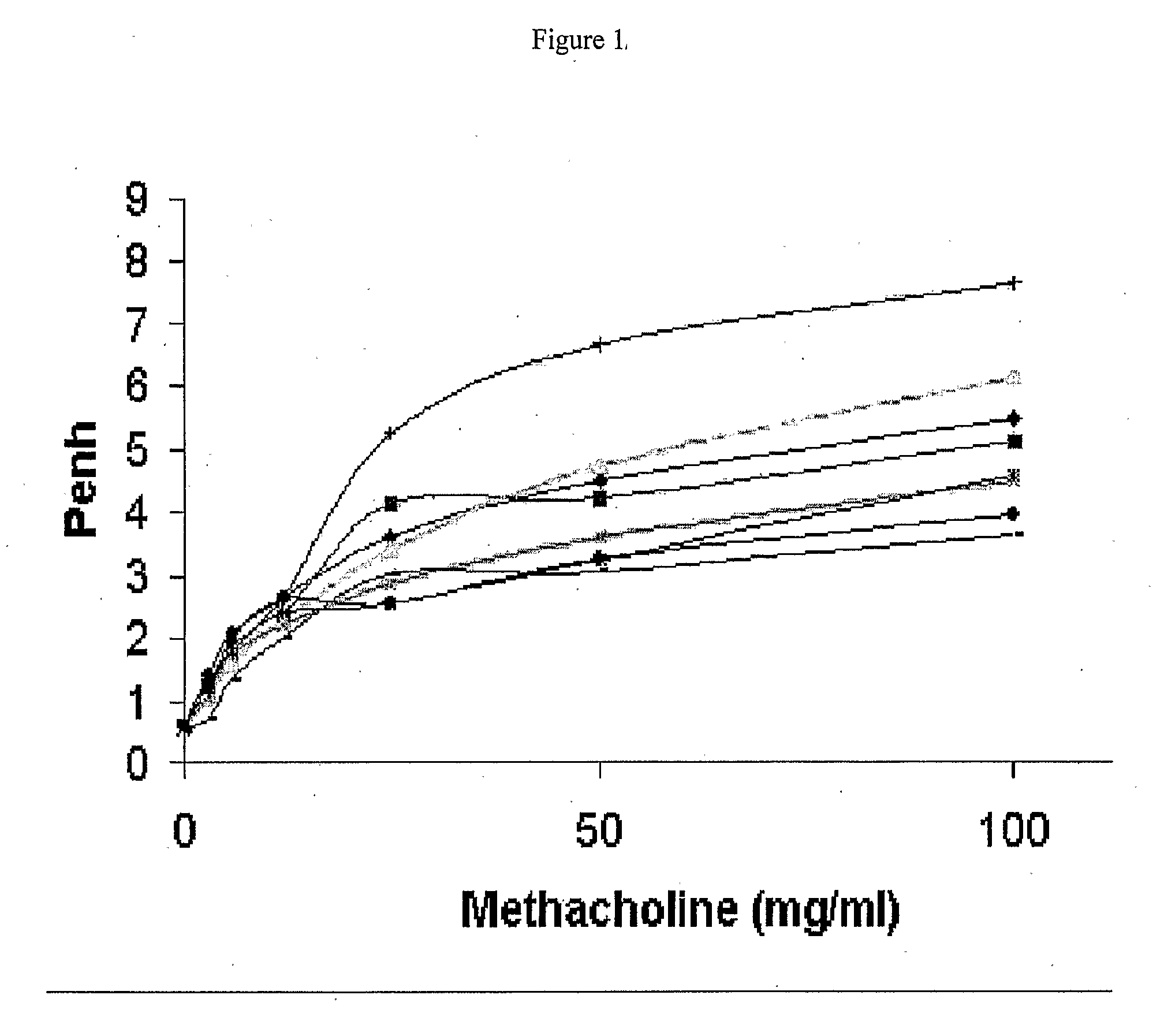
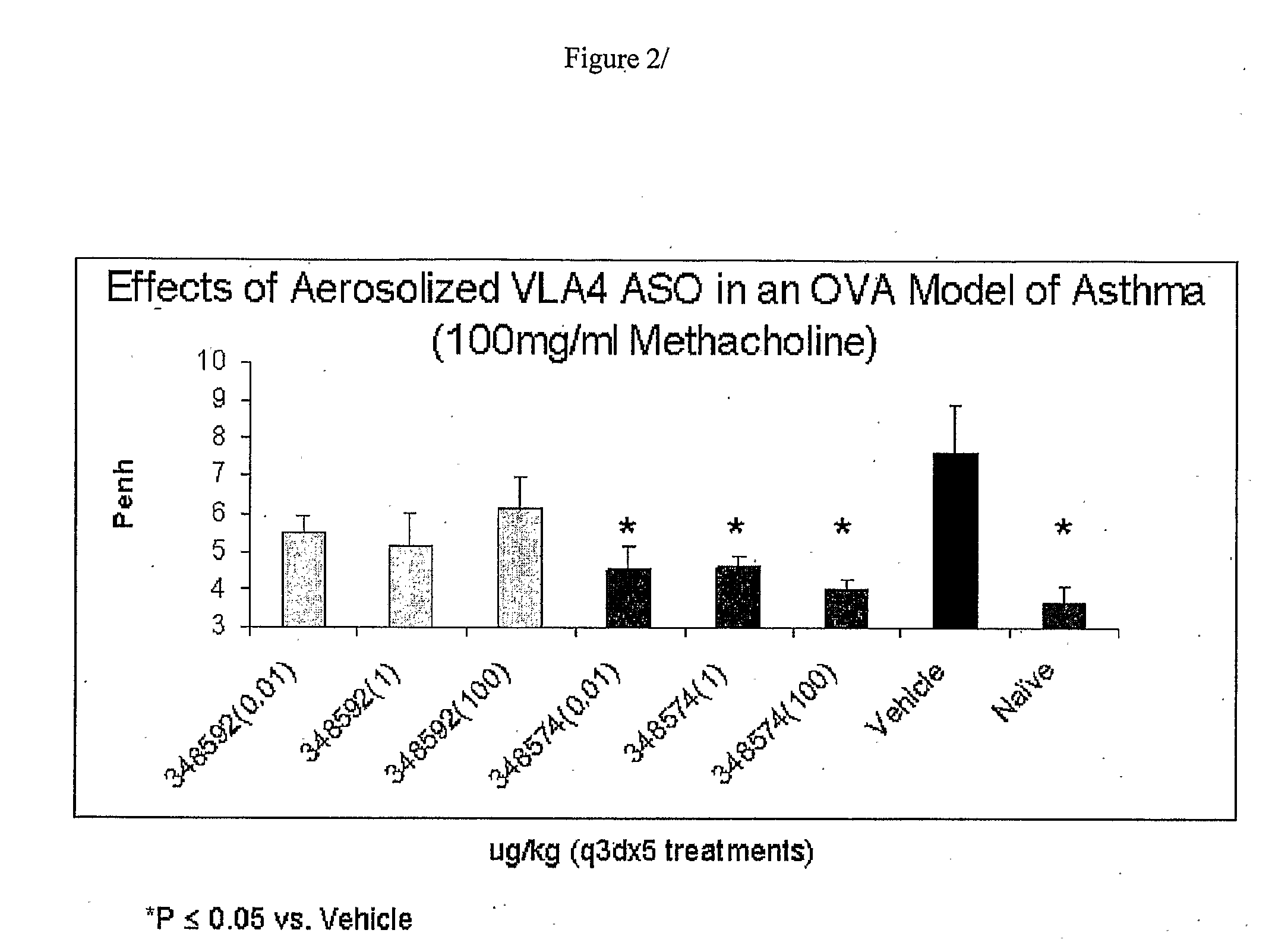
Topical administrations of antisense compounds to vla-4 for the treatment of respiratory conditions
InactiveUS20090029931A1Avoid successionGood flexibilityOrganic active ingredientsGenetic material ingredientsDiseaseRespiratory disease
A method for the treatment and / or prophylaxis of an animal having a respiratory disease or condition associated with airway hyperresponsiveness, eosinophilia, neutrophilia, leukocytes or overproduction of mucus and / or with the expression of integrin α4 comprising administering to the animal a composition comprising from. 0.001 to 1000 μg per kg body weight of the animal of an antisense compound targeted to a nucleic acid molecule encoding integrin α4.
Owner:ANTISENSE THERAPEUTICS LTD
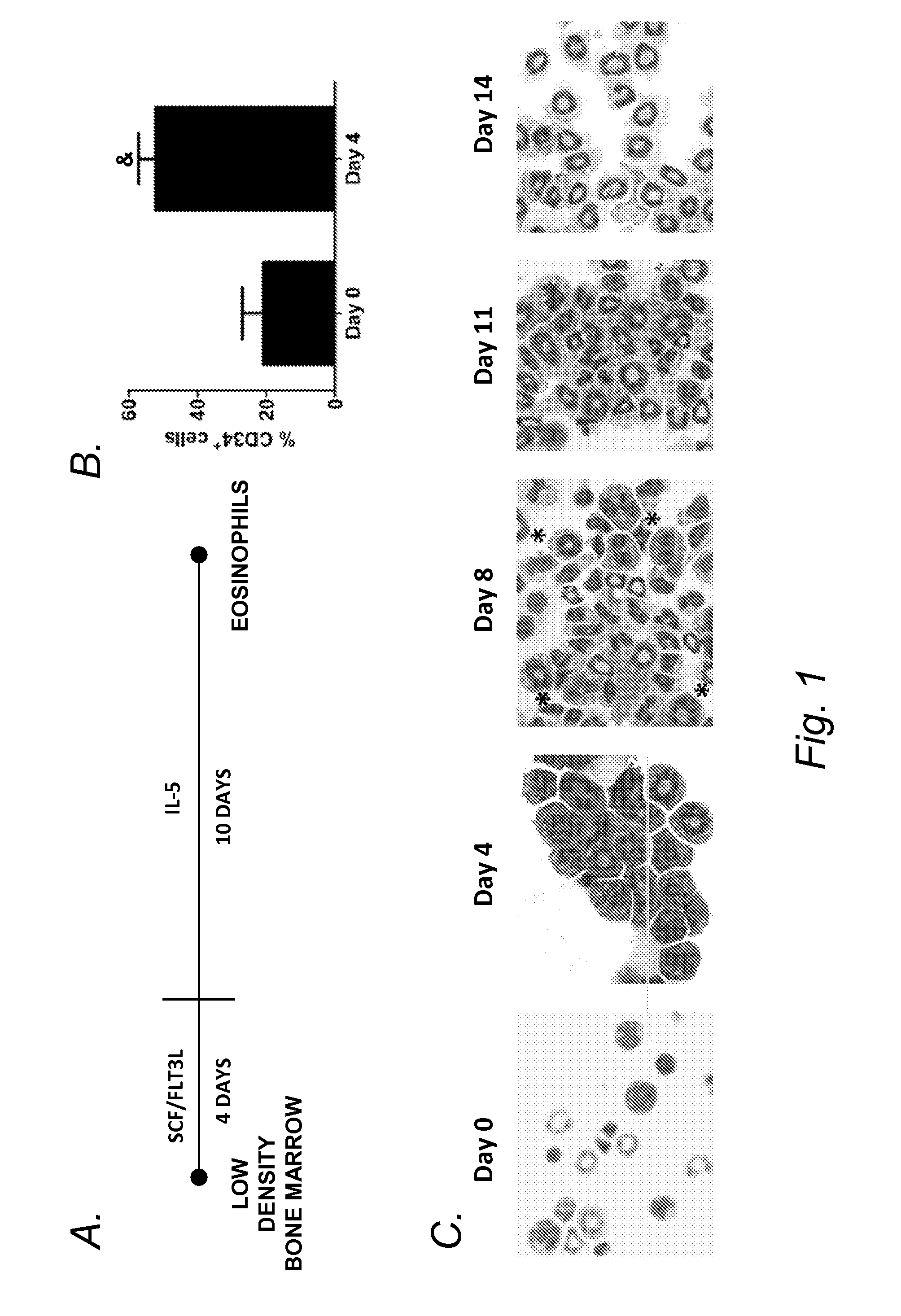
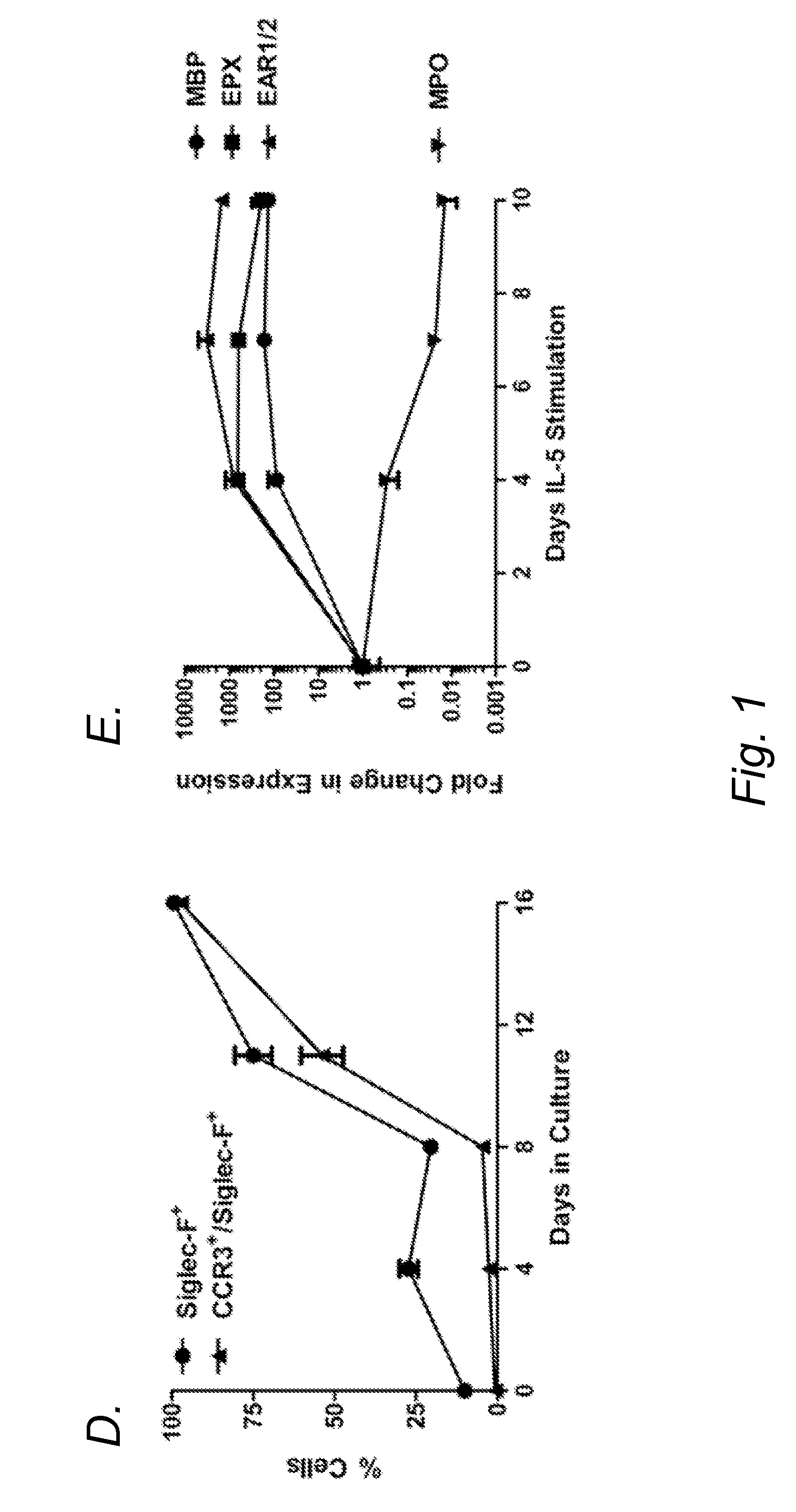
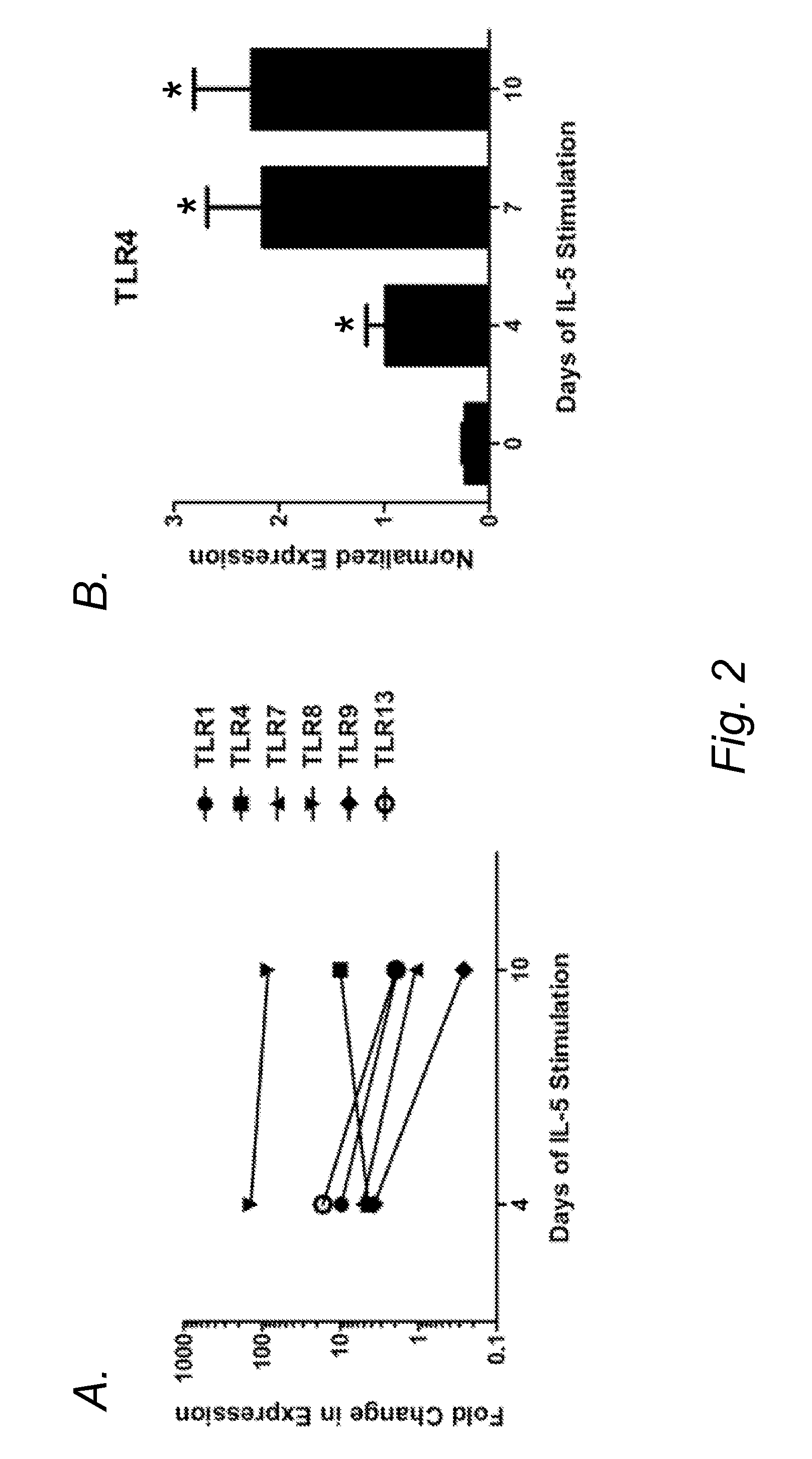
Blockade of eosinophil production by toll-like receptors
InactiveUS20140228315A1Reduce productionReduce in quantityBiocideOrganic active ingredientsProgenitorDisease
It has long been known that eosinopenia is observed during acute bacterial infection yet the mechanism remains undefined. Herein, we investigated the consequence of exposure to microbial products, specfically bacterial lipopolysaccharide (LPS), on eosinophil production. We demonstrate that developing murine eosinophils transiently express mRNA for six Toll-like receptors (TLR5) with highest expression of TLR2 and TLR4 throughout eosinophil development and nearly undetectable levels on mature eosinophils. LPS stimulation of eosinophil progenitors ex vivo markedly inhibited IL-5- mediated cellular proliferation and expansion Further LPS adrninistratwn in vivo reduced numbers of eosinophil progenitors in the bone marrow and blood in mice. Notably, LPS effectively reduced eosinophilia even in hypereosinophilic mice induced by the IL-S transgene. Taken together, these findings identify a mechanistic explanation for eosinopenia following bacterial infections and a novel therapeutic strategy for depleting eosinophil progenitors and inhibiting peripheral eosinophilia in eosinophil associated diseases.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Immune function modulating agents
A probiotic lactobacillus was discovered from lactobacilli of the Lactobacillus genus independently isolated from human adult feces. The probiotic lactobacillus was selected from other bacterial strains for: (1) being highly resistant to gastric acid / bile acid; (2) having a high promoting activity on IL-12 production from mouse derived spleen cells and a high Th1 / Th2 balance-improving effect; (3) having a high ability to inhibit the production of antigen-specific IgE induced by intraperitoneally administering ovalbumin to BALB / c mice; (4) having a high ability to inhibit the production of antigen-specific IgE induced by orally administering a food antigen to C57BL / 6N mice; (5) having a high Natural Killer cell-activating ability; (6) having a high IL-12 production-promoting activity on spleen cells and mesenteric lymph node cells derived from mice immunized with ovalbumin and a high Th1 / Th2 balance-improving effect; and (7) having a high ability to suppress eosinophilia induced by a cedar pollen-extracted antigen. This discovery led to the completion of the present invention.
Owner:MEIJI CO LTD
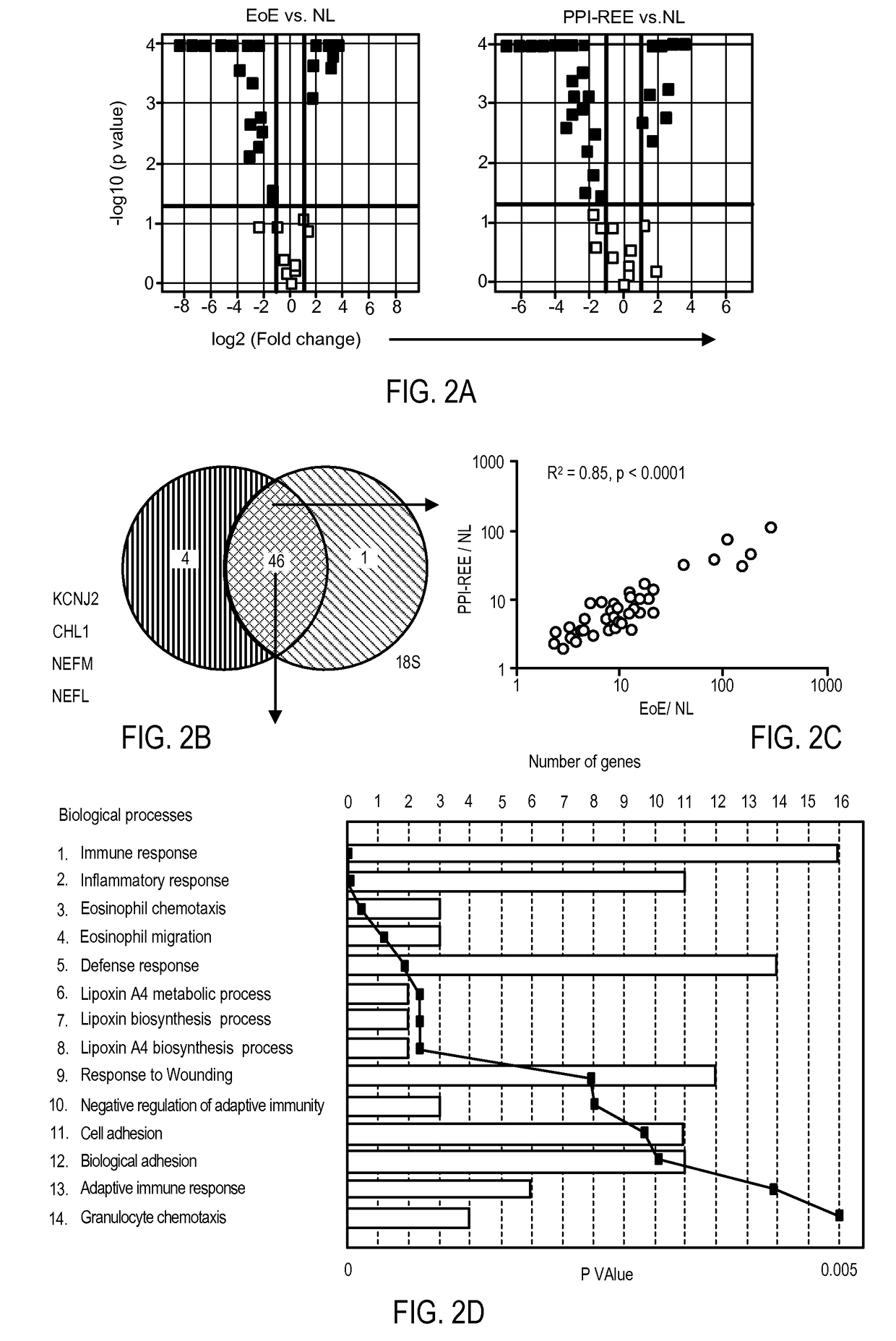
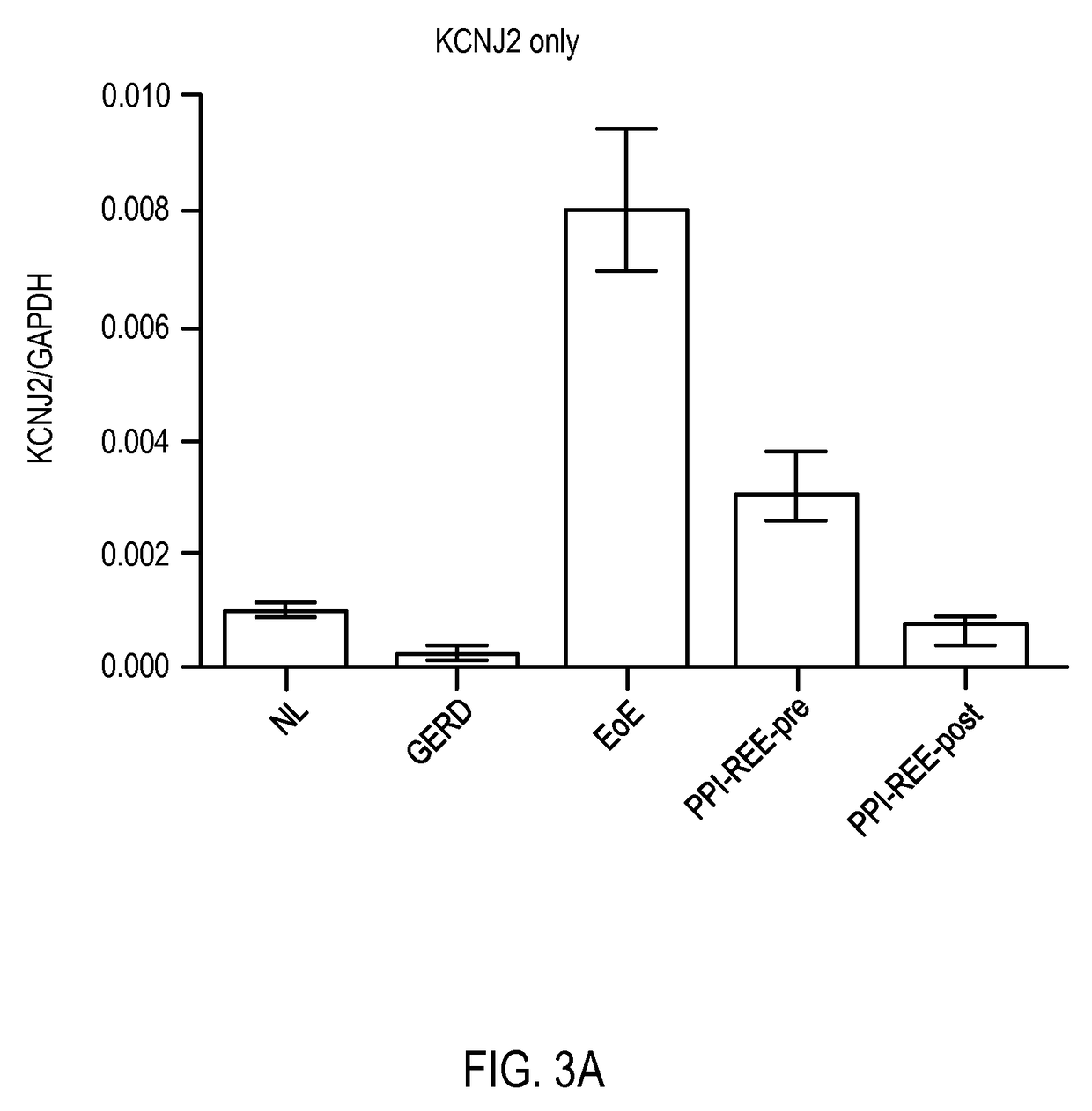
Diagnostic method for distinguishing forms of esophageal eosinophilia
ActiveUS20170233813A1Improves care for EoE patientsOrganic active ingredientsMicrobiological testing/measurementCytosisOncology
The invention provides methods for diagnosing eosinophilic esophagitis in a patient using a biomarker based assay directed to KCNJ2 / Kir2.1 and related compositions, kits, and computer program products.
Owner:RGT UNIV OF CALIFORNIA +1
Method for treating inflammatory inflammatory diseases using heat shock proteins
This invention relates to a method to protect a mammal from a disease associated with an inflammatory response, and in particular, from an inflammatory disease characterized by eosinophilia, airway hyperresponsiveness and / or a Th2-type immune response. The method includes administration of a heat shock protein to a mammal having such a disease. Formulations useful in the present method are also disclosed.
Owner:NAT JEWISH MEDICAL & RES CENT
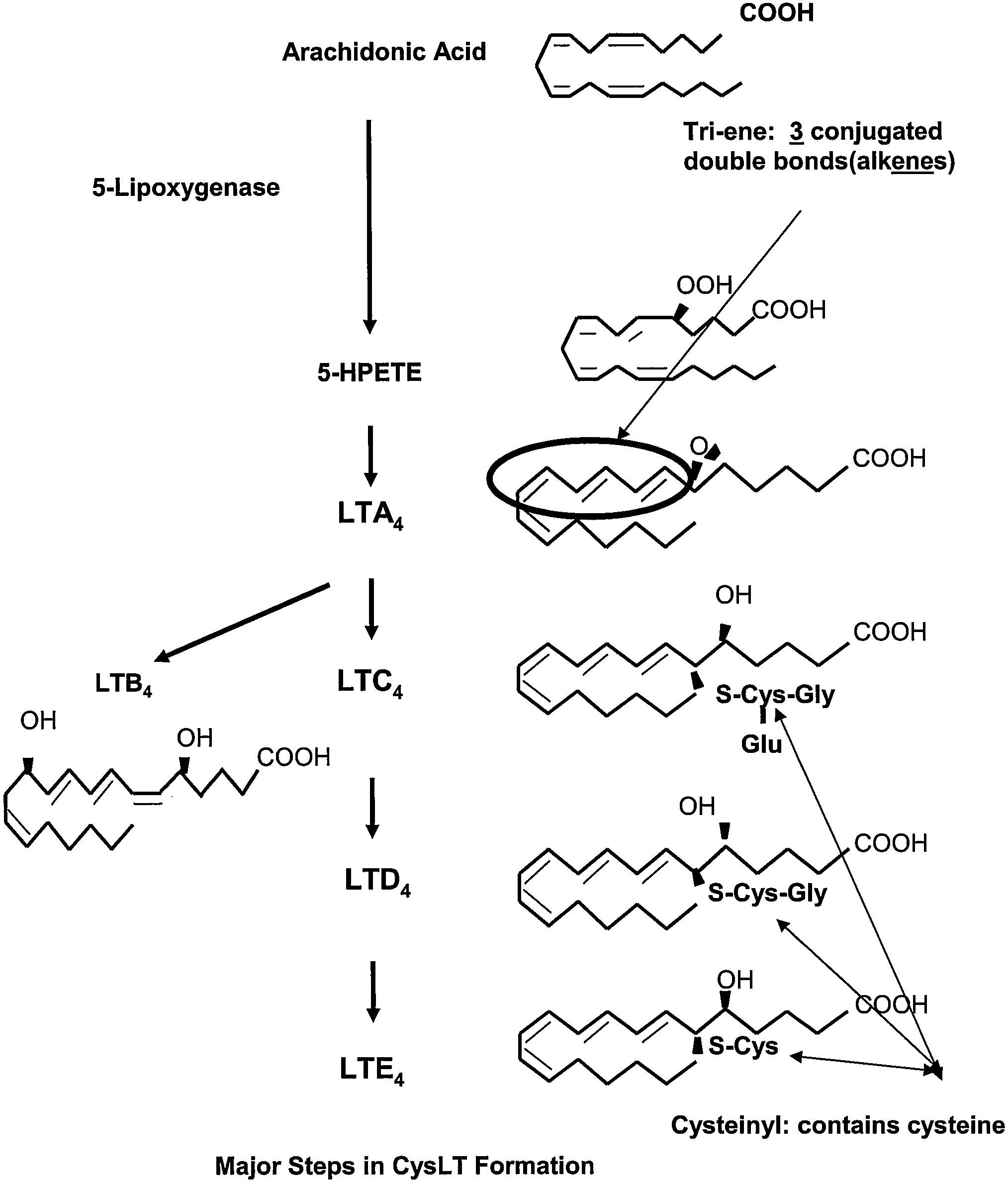
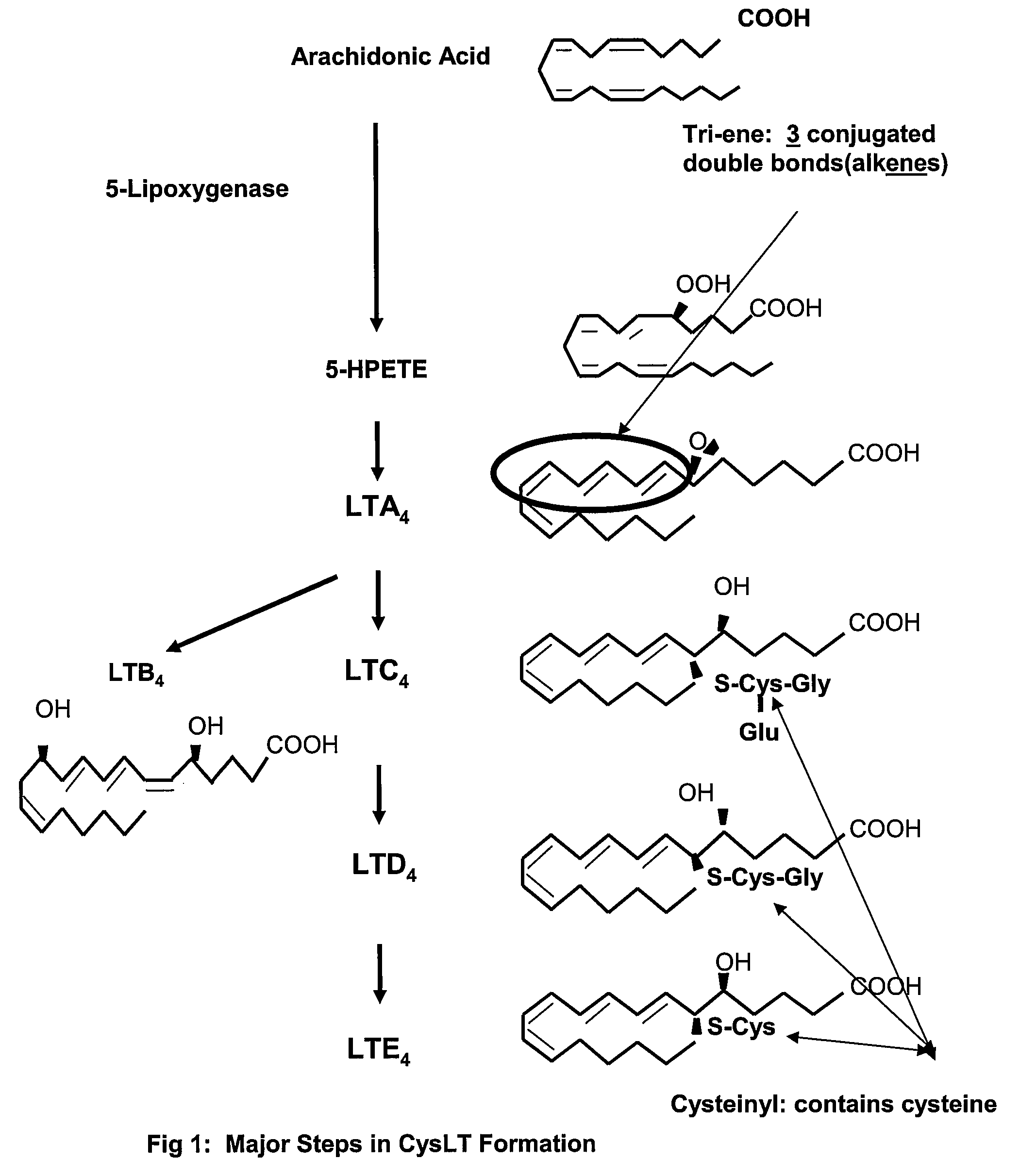
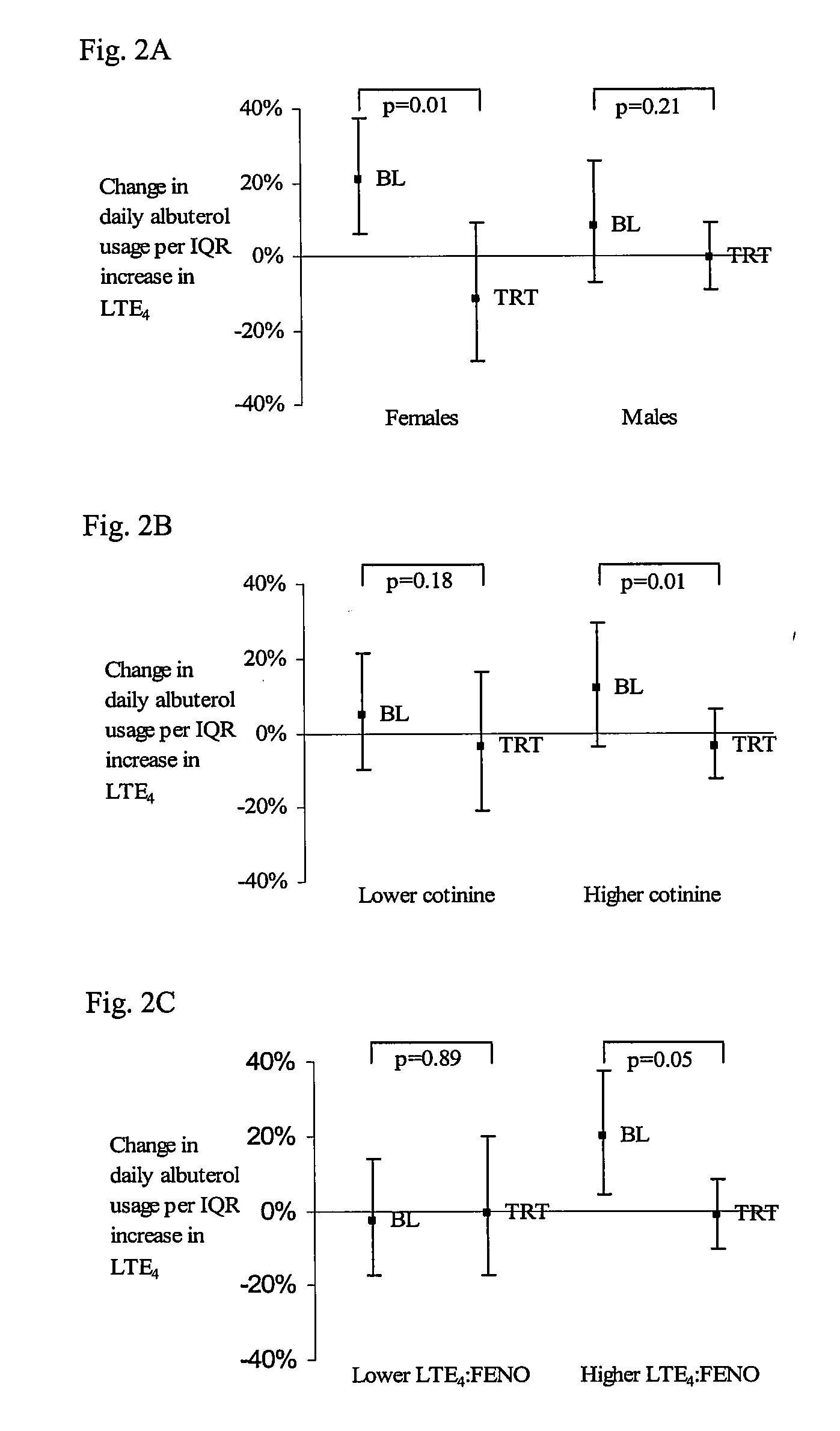
Methods to determine suceptibility to treatment with leukotriene modifiers
ActiveUS20090233963A1Determine of subjectBiocideDisease diagnosisCysteinyl leukotrienesCysteinyl-leukotriene
The present invention provides a method of determining the susceptibility of a subject to treatment with a leukotriene modifier by determining the subject's cysteinyl leukotriene (CysLT) level and the subject's level of eosinophilic airway inflammation and identifying a subject with a high ratio of CysLT levels to eosinophilic airway inflammation as susceptible to treatment with the leukotriene modifier. Also discussed is a method of treatment of subjects who are susceptible to treatment that includes administering a leukotriene modifier to such a subject.
Owner:NAT JEWISH HEALTH
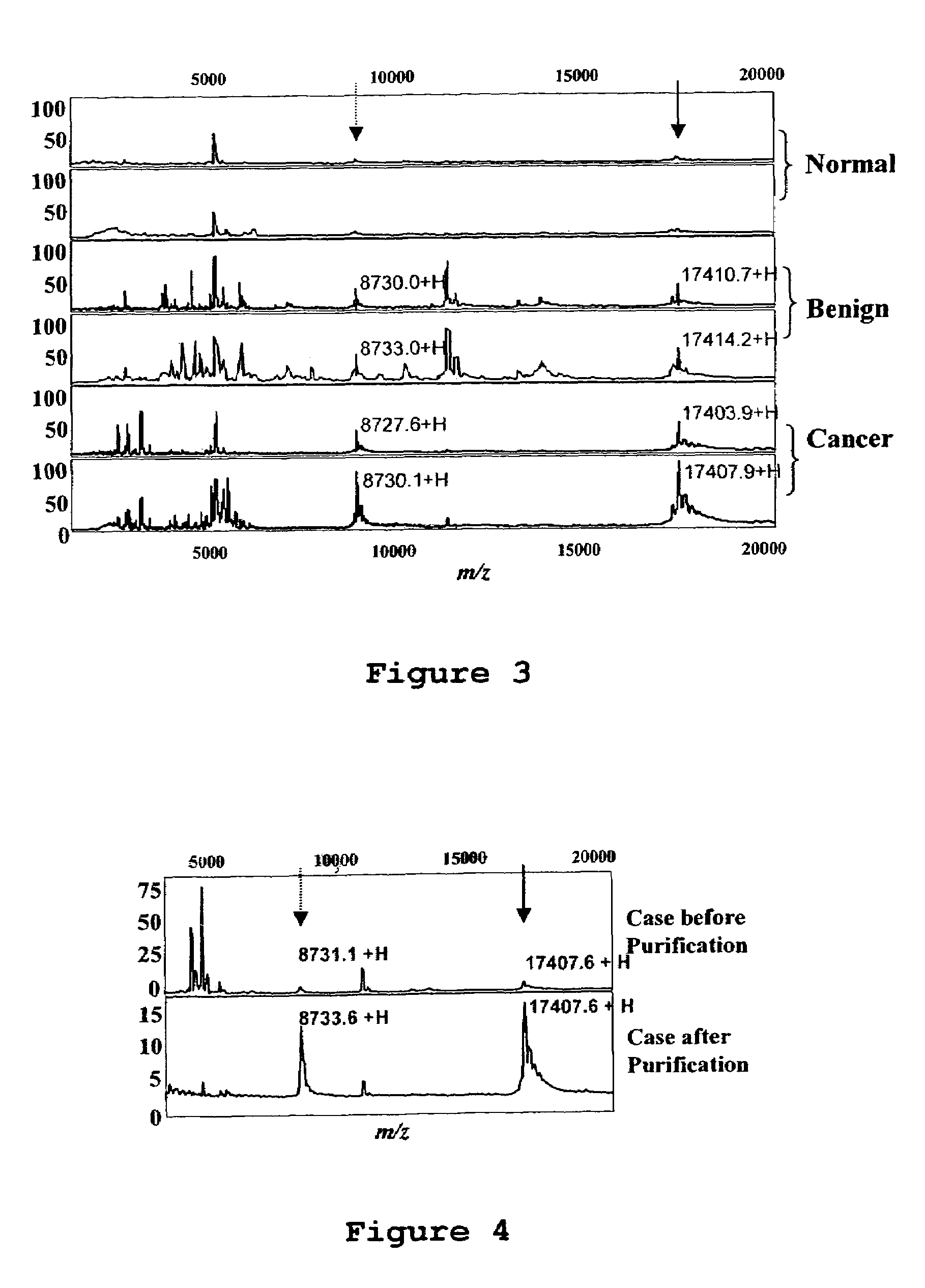
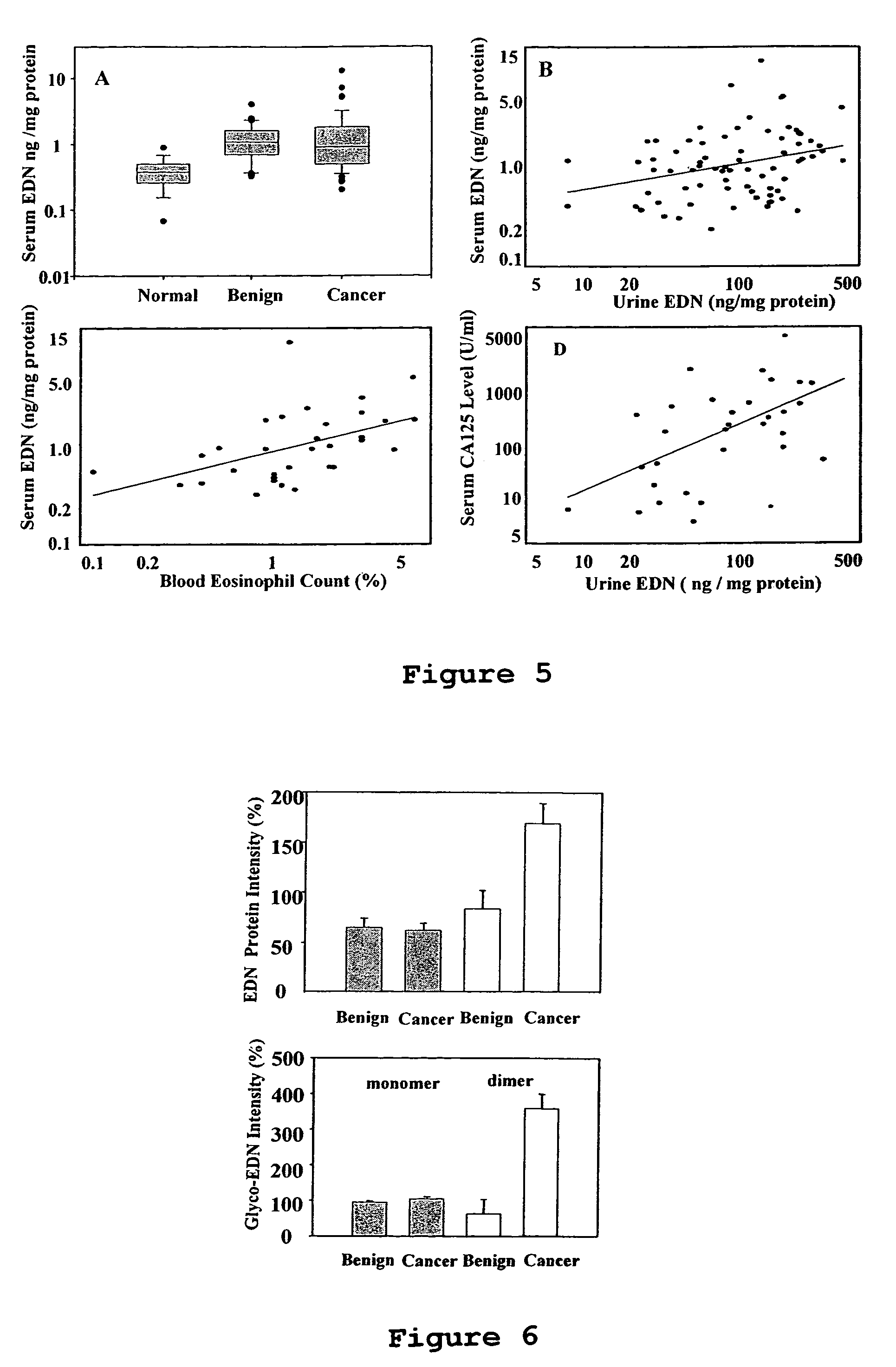
Eosinophil-derived neurotoxin as a marker for ovarian cancer
InactiveUS7288383B2Eliminate needMicrobiological testing/measurementBiological material analysisIncreased riskNeurexin
The present invention is directed to methods of identifying women at increased risk of having ovarian cancer. Identification is based upon the extent to which biological samples derived from the women exhibit elevated levels of eosinophil-derived neurotoxin (EDN).
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
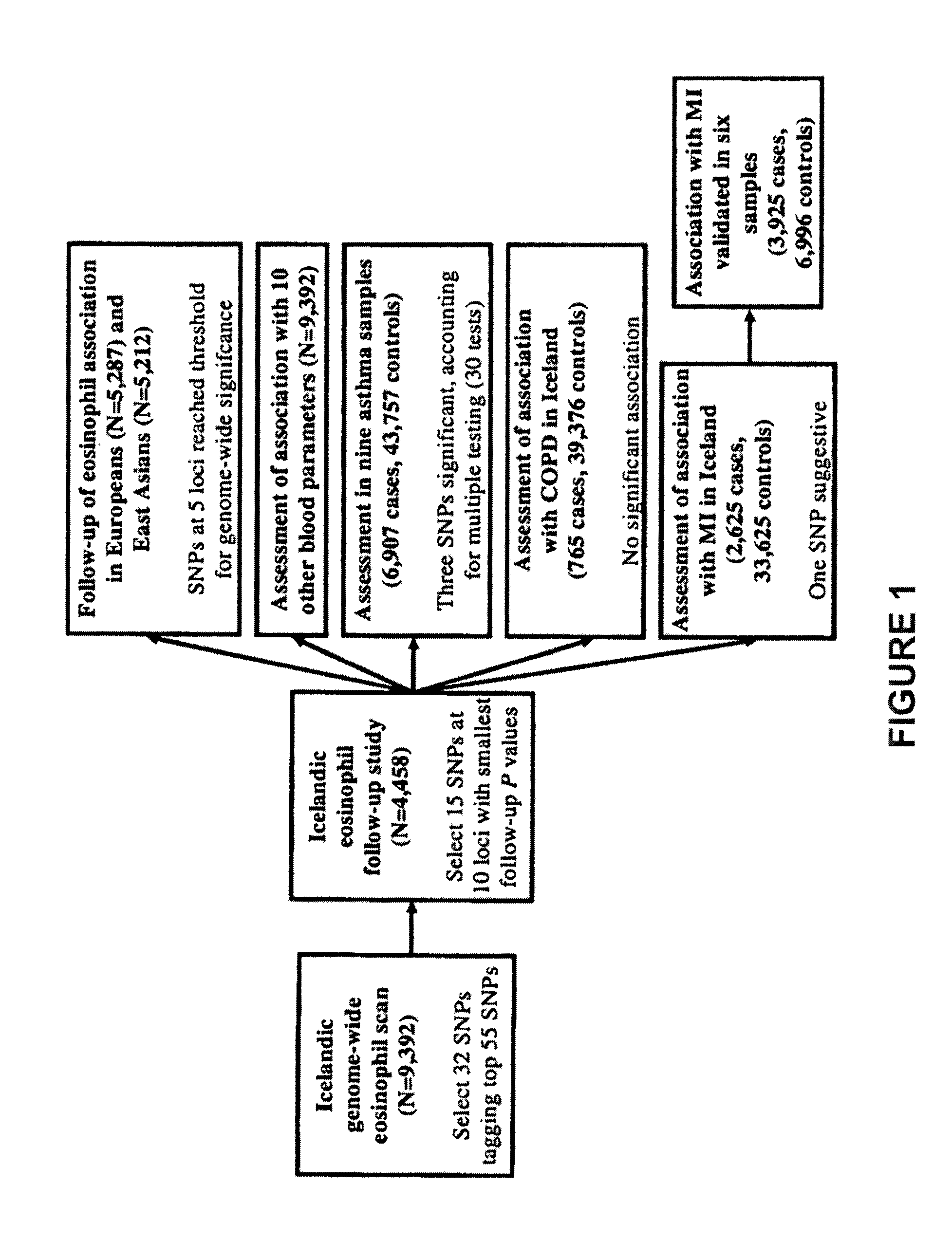
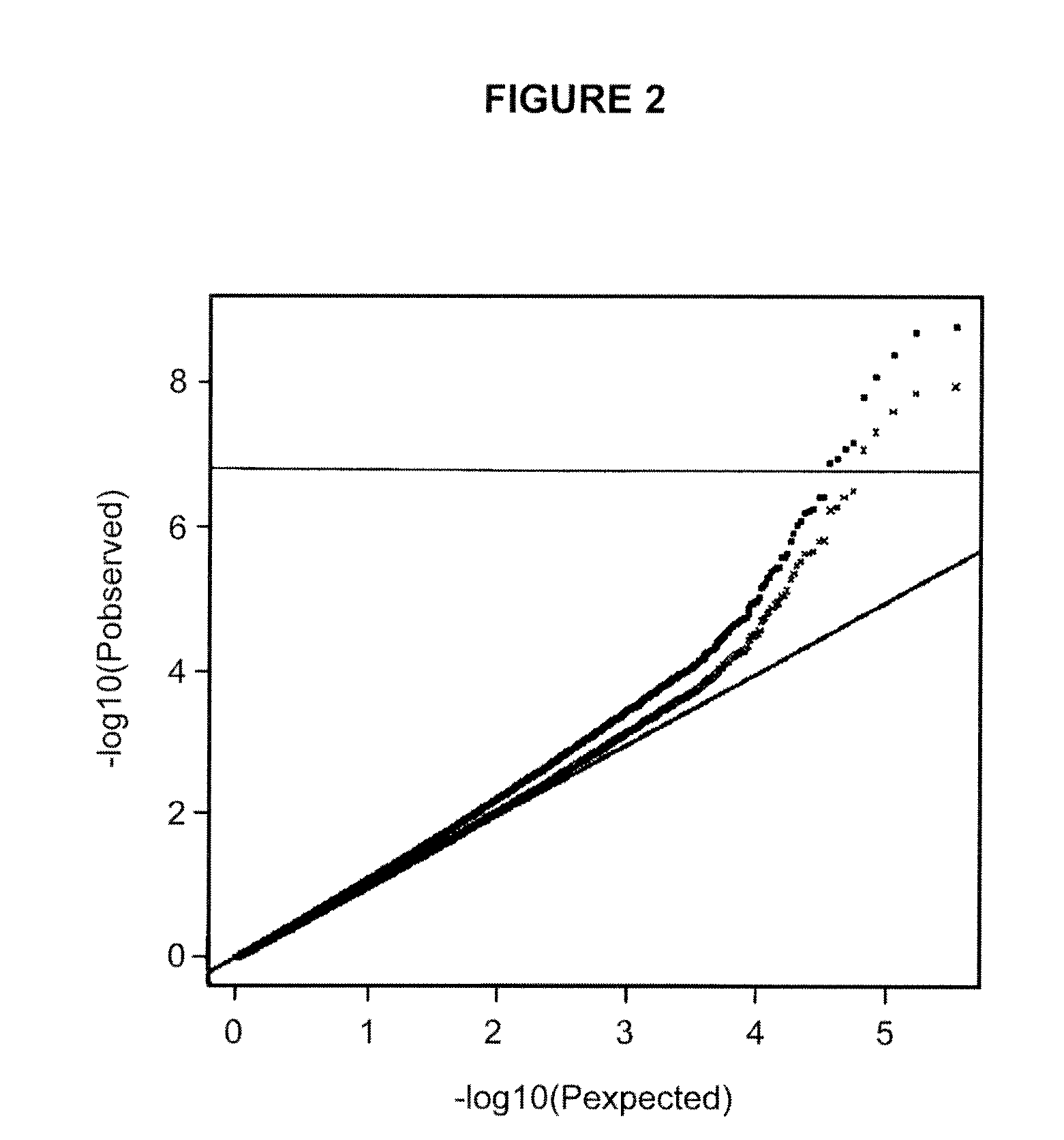
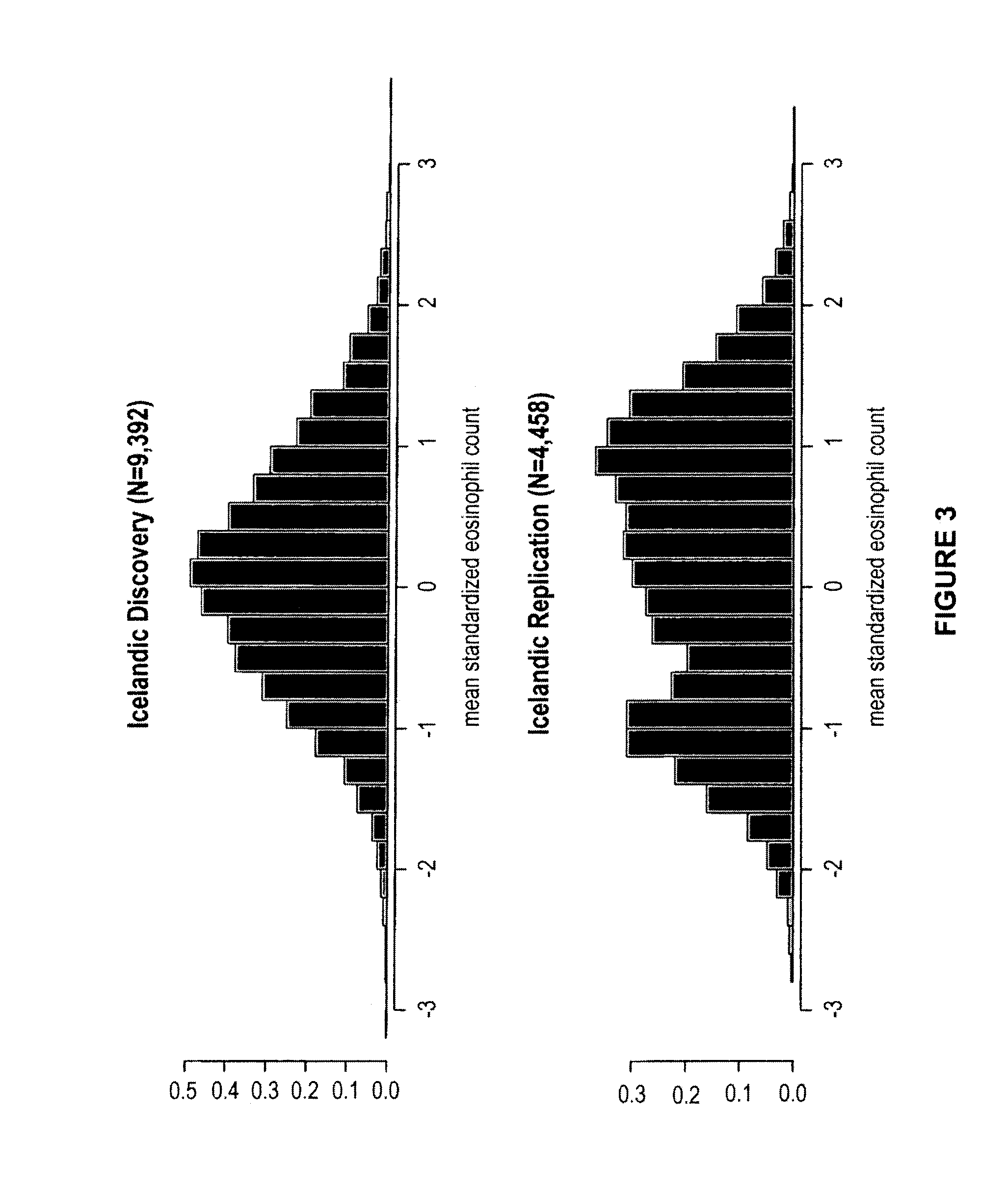
Genetic variants as markers for use in diagnosis, prognosis and treatment of eosinophilia, asthma, and myocardial infarction
Polymorphic variants (e.g., certain alleles of polymorphic markers) that have been found to be associated with high blood eosinophil counts, conditions causative of eosinophilia (e.g., asthma, myocardial infarction), and / or hypertension are provided herein. Such polymorphic markers are useful for diagnostic purposes, such as in methods of determining a susceptibility, and for prognostic purposes, including methods of predicting prognosis and methods of assessing an individual for probability of a response to a therapeutic agent, as further described herein. Further applications utilize the polymorphic markers of the invention include, screening methods and genotyping methods. The invention furthermore provides related kits, computer-readable medium, and apparatus.
Owner:DECODE GENETICS EHF
Modified il-4 mutein receptor antagonists
InactiveUS20090010874A1Peptide/protein ingredientsPeptide preparation methodsDiseaseTreatment choices
This invention relates to modified IL-4 mutein receptor antagonists comprising an IL-4 mutein receptor antagonist coupled to polyethylene glycol. Related formulations and dosages and methods of administration thereof for therapeutic purposes are also provided. These modified IL-4 mutein receptor antagonists, compositions and methods provide a treatment option for those individuals afflicted with a respiratory disorder such as asthma by inhibiting IL-4 and IL-13-mediated airway hyperresponsiveness and eosinophilia. More particularly, these antagonists have an increased duration of effect versus unmodified IL-4RA by virtue of a greater plasma half-life.
Owner:AEROVANCE INC
Modified IL-4 mutein receptor antagonists
This invention relates to modified IL-4 mutein receptor antagonists comprising an IL-4 mutein receptor antagonist coupled to polyethylene glycol. Related formulations and dosages and methods of administration thereof for therapeutic purposes are also provided. These modified IL-4 mutein receptor antagonists, compositions and methods provide a treatment option for those individuals afflicted with a respiratory disorder such as asthma by inhibiting IL-4 and IL-13-mediated airway hyperresponsiveness and eosinophilia. More particularly, these antagonists have an increased duration of effect versus unmodified IL-4RA by virtue of a greater plasma half-life.
Owner:AEROVANCE INC
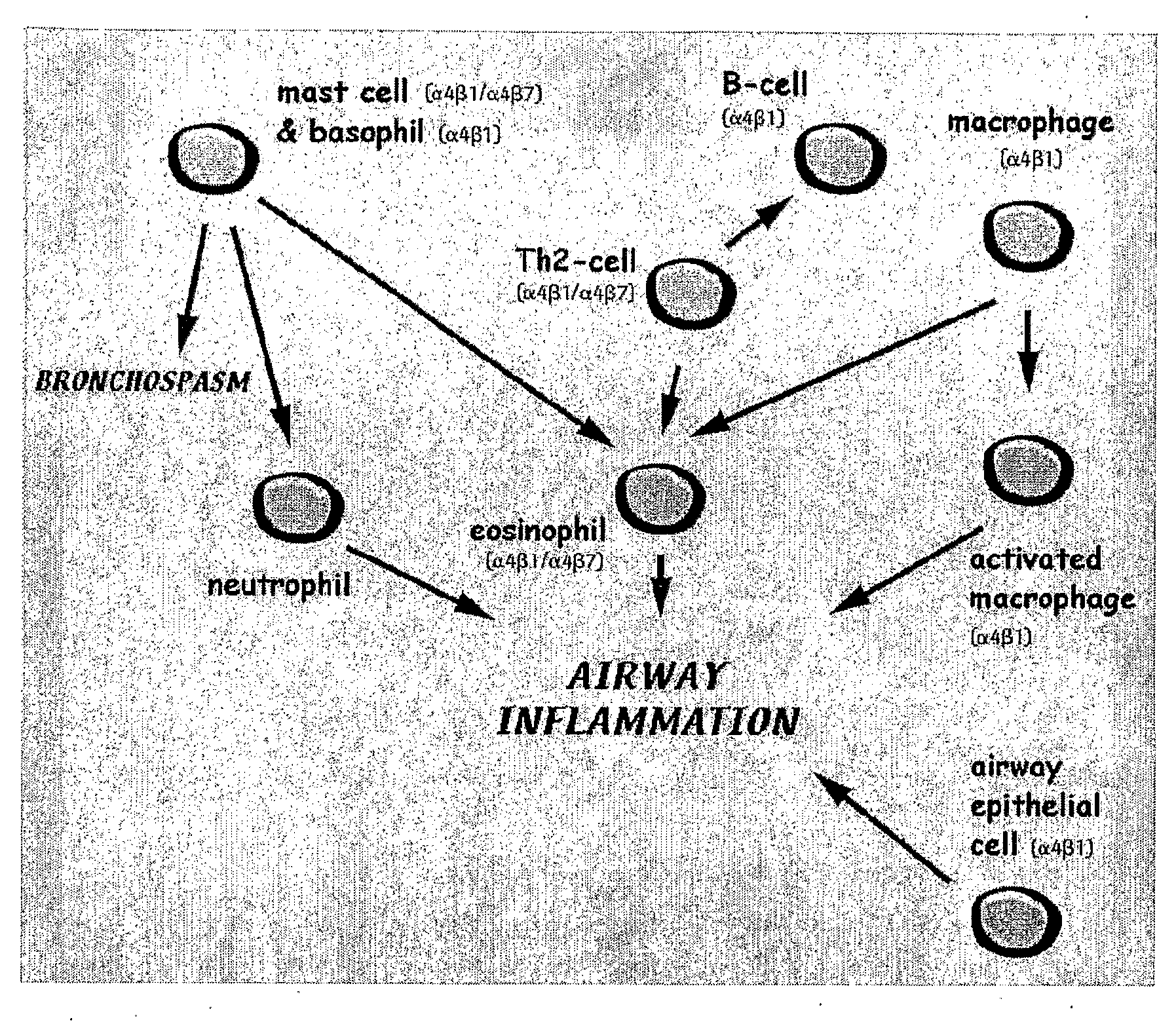
Asthma/allergy therapy that targets T-lymphocytes and/or eosinophils
InactiveUS7592327B2Relieve symptomsBiocidePeptide/protein ingredientsUpper urinary tract infectionAllergy
A pharmaceutical composition for the treatment and / or prophylaxis of diseases caused by type I hypersensitivity reactions consisting essentially of Glicophosphopeptical, or pure Nigella Sativa seeds, in a concentration which stimulate Th1 lymphocytes and selectively switch-off the eosinophilic airway inflammationA method of treatment of allergy using Th1 stimulating agents, to be administered to a mammal such as human in need of such treatment in a shot of 5 days only, resulted in significant decrease in symptom score started day 3, and in sputum eosinophils by day 14, followed by long-term clinical remission of a mean of 6 months.The BCG-like Th1 stimulation is also used in treating diseases in which the body defensive mechanism is a Cell Mediated Immunity, including viral infections, as but not limited to influenza and common cold, Chronic and recurrent urinary tract infection, pelvic inflammatory diseases as neuroimmune appendicitis, cancer, crohns disease and facial palsy.
Owner:NASSIEF NIDA ABDUL GHANI
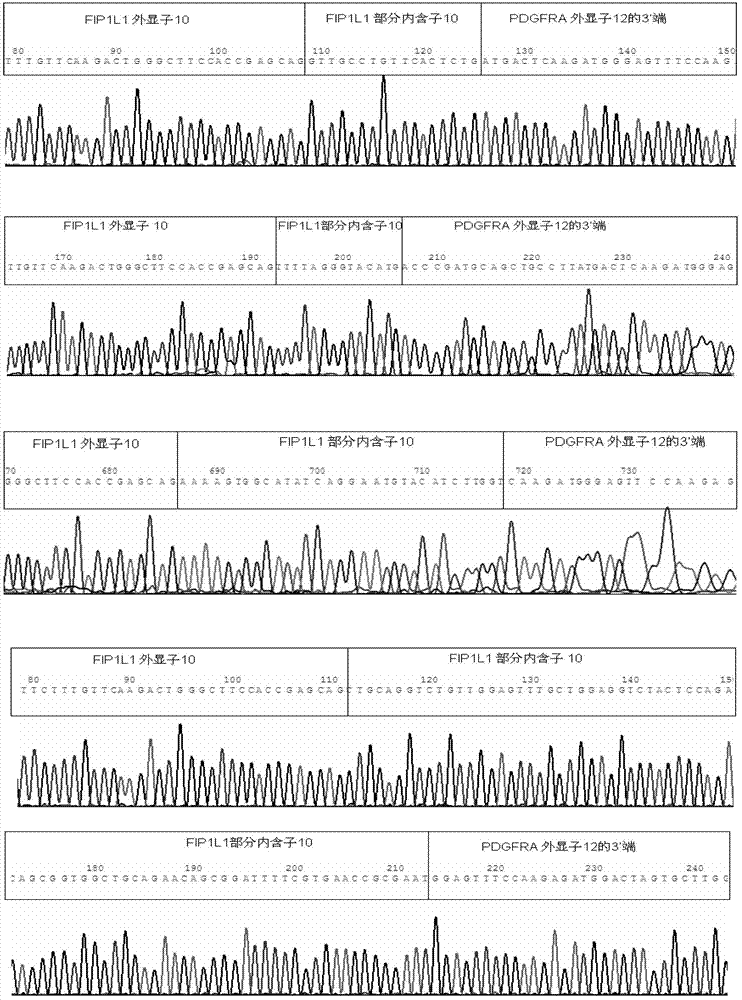
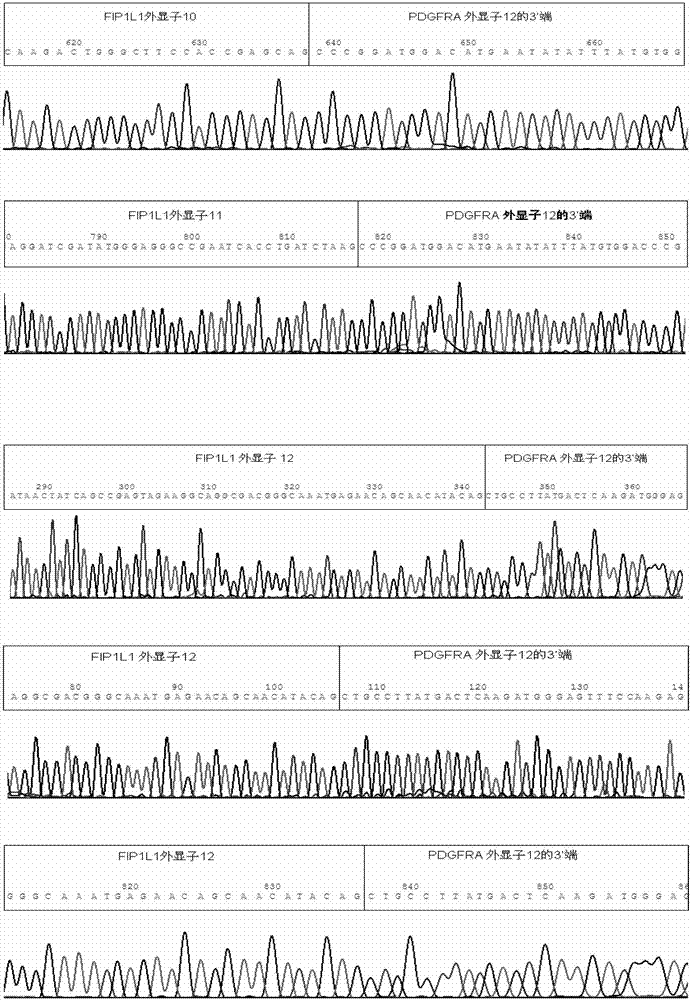
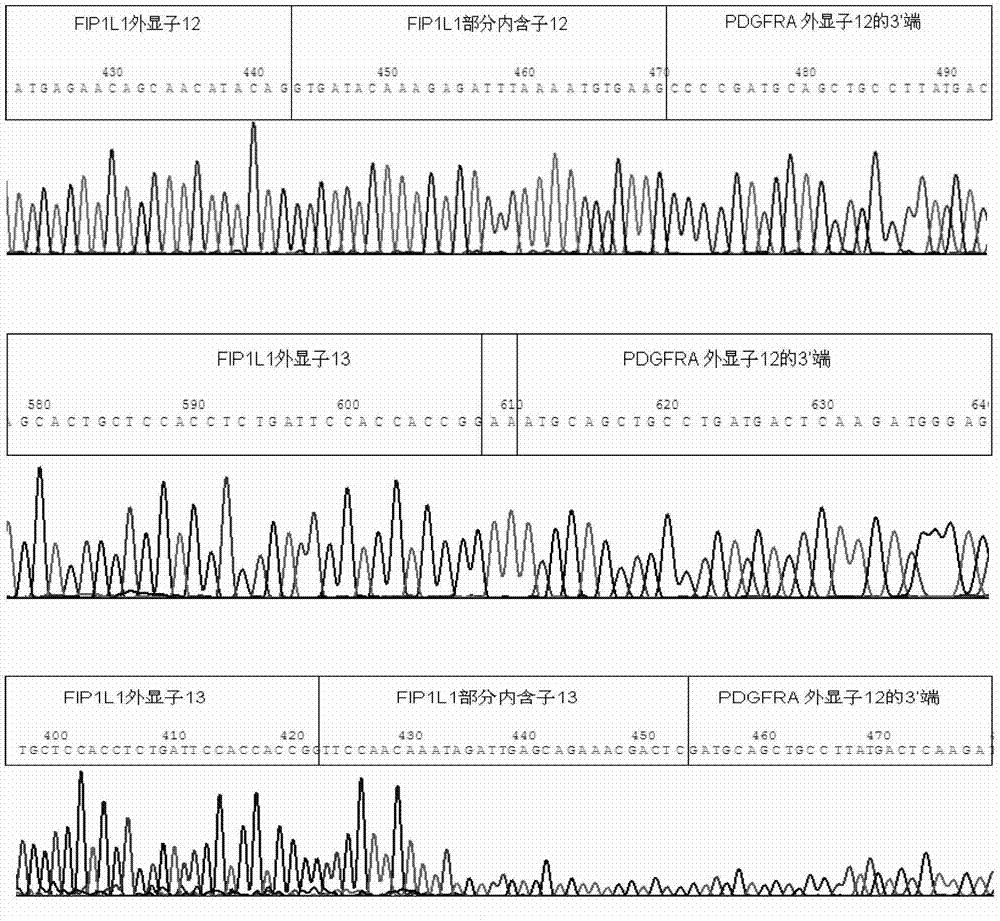
Reagent kid for quantitatively testing mRNA (messenger ribonucleic acid) level of FIP1L1-PDGFRA (feline infectious peritonitis 1 like 1-platelet-derived growth factor receptor alpha) fusion genes
InactiveCN102827935AGuaranteed specific amplificationAccurately reflect tumor burdenMicrobiological testing/measurementFluorescence/phosphorescenceFip1l1 pdgfraPlatelet-Derived Growth Factor Receptor Alpha
The invention discloses a reagent kit for quantitatively testing mRNA (messenger ribonucleic acid) level of FIP1L1-PDGFRA (feline infectious peritonitis 1 like 1-platelet-derived growth factor receptor alpha) fusion genes. The test reagent contains upstream primers I, downstream primers I and TaqMan probes I which are used for real-time quantitative PCR (polymerase chain reaction) testing for mRNA of the FIP1L1-PDGFRA fusion genes. The upstream primers I include at least one of single-chain DNA (deoxyribose nucleic acid) shown as sequences 1, 2, 3, 4 and 5 in a sequence table, the downstream primers I include at least one of two single-chain DNA as shown in sequence 9 and 10 in the sequence table, and the TaqMan probes I include at least one of two single-chain DNA as shown in sequences 9 and 10 in the sequence table. The reagent kit has the advantages of speediness, simplicity, convenience and the like in testing, common types of FIP1L1-PDGFRA fusion genes can be covered in one experiment, and the mRNA level of the FIP1L1-PDGFRA fusion genes can be tested. The reagent kit can be used for FIP1L1-PDGFRA fusion gene screening and therapeutic evaluation for eosinophilia patients, and further can be used for monitoring minimal residual diseases.
Owner:PEOPLES HOSPITAL PEKING UNIV
Application of tetrahydrocurcumin in improving allergic asthma
ActiveCN108771664AAlleviate itchingInhibitionKetone active ingredientsRespiratory disorderDiseaseGoblet cell
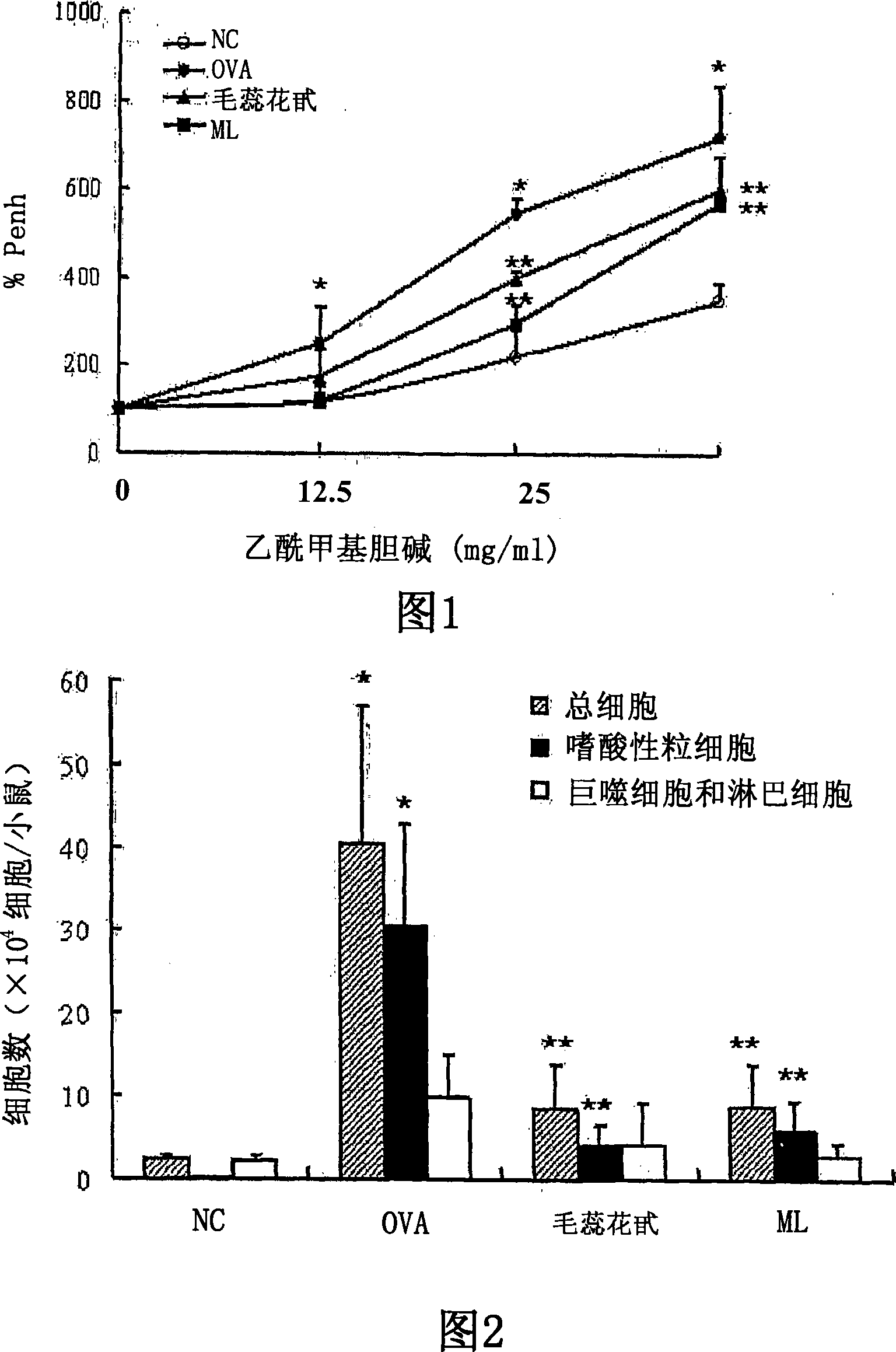
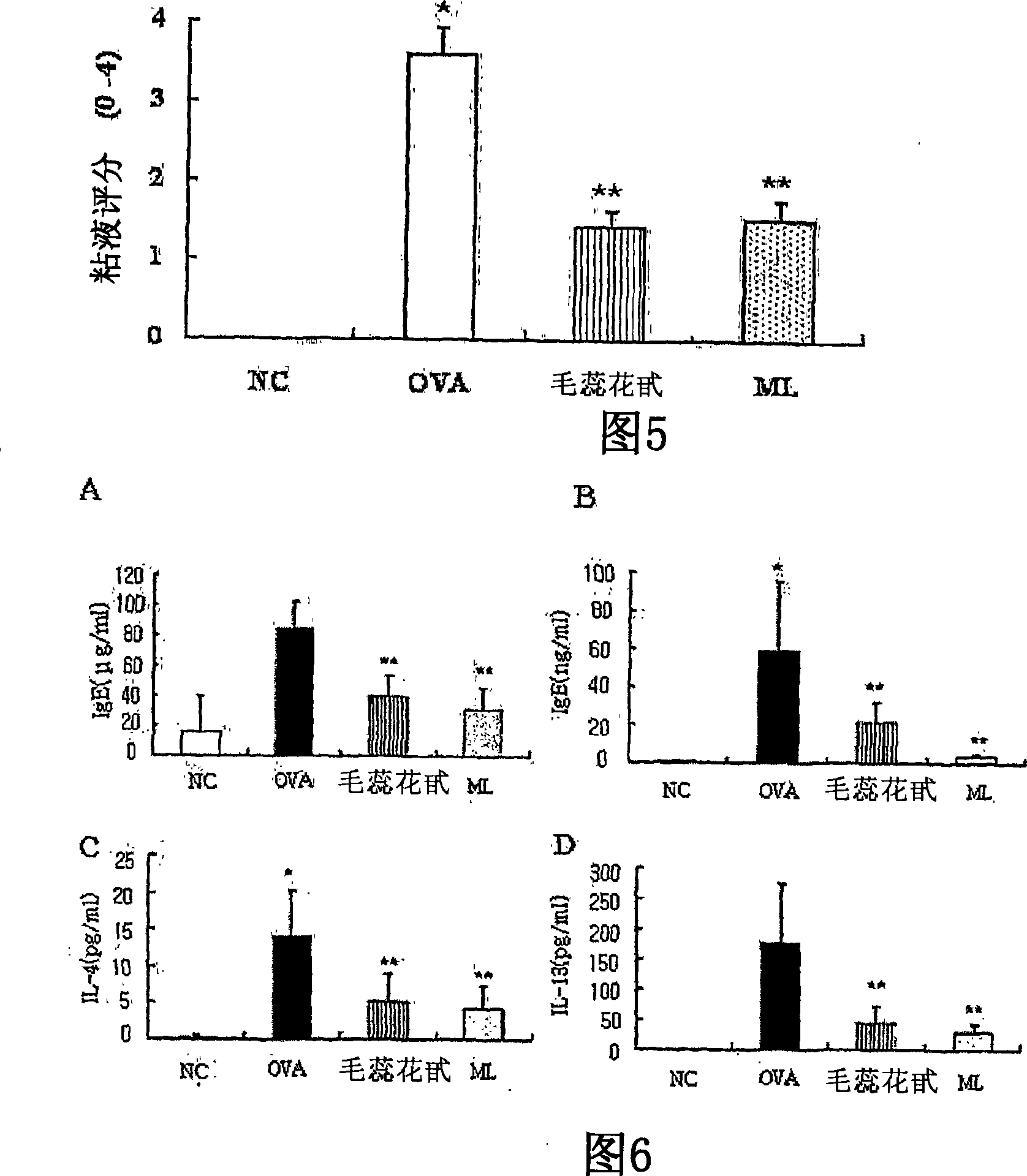
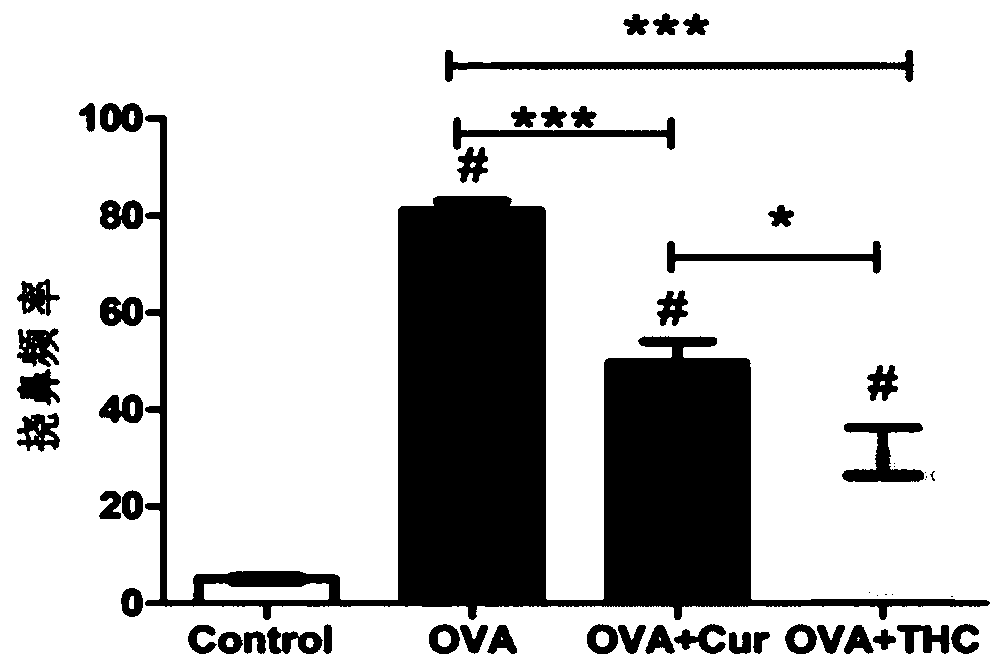
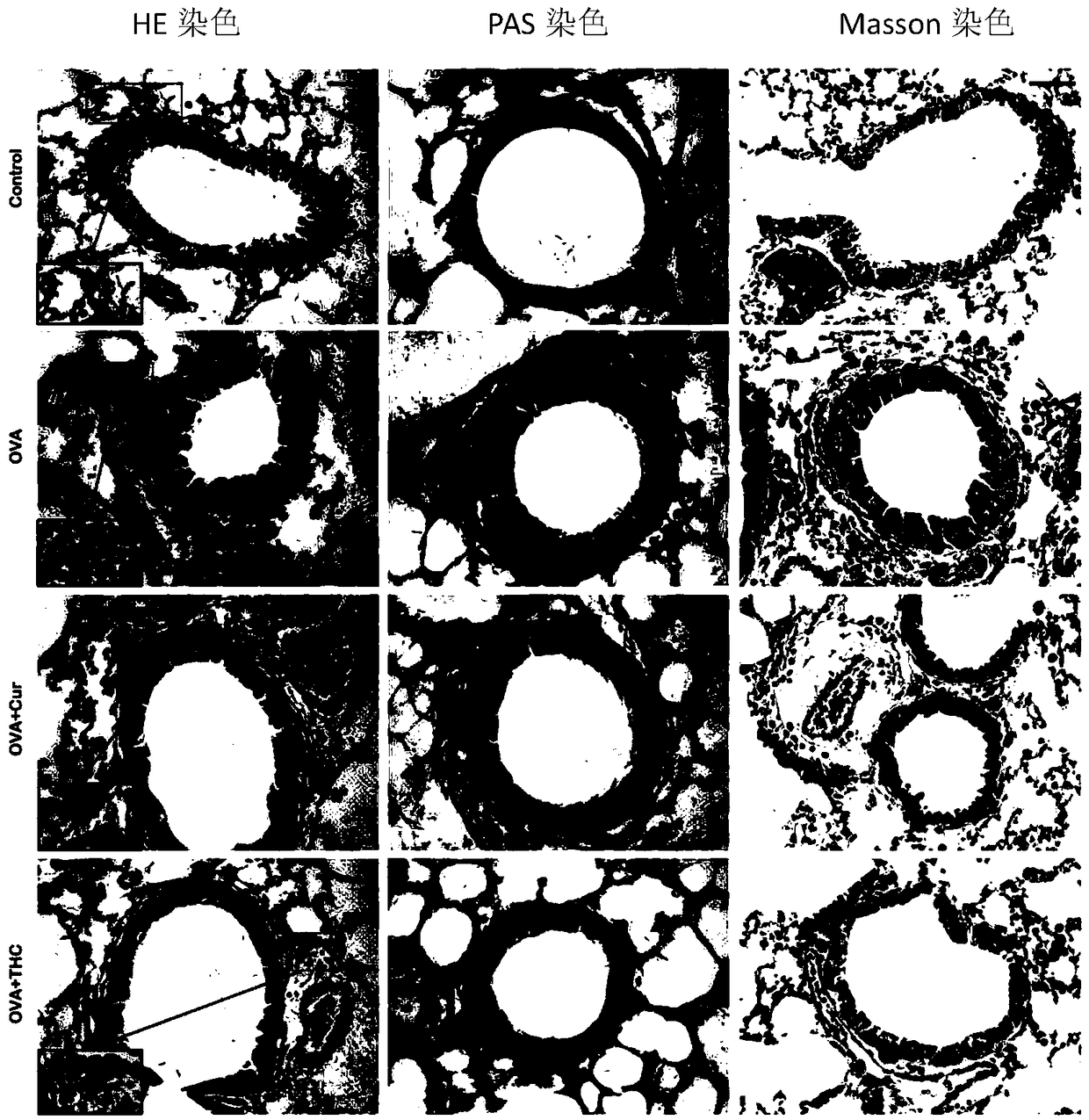
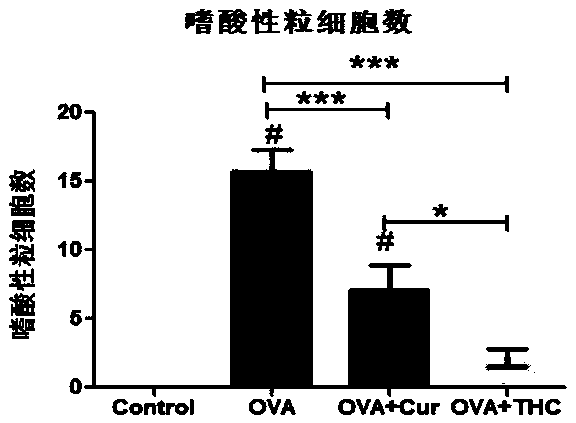
The invention discloses application of tetrahydrocurcumin in treating allergic asthma. The therapeutic action of the tetrahydrocurcumin (THC) on allergic asthma is firstly found and proved; from the perspective of immunity, the THC can be beneficial to alleviating nose allergy and itching, inhibiting lung eosinophilic granulocyte infiltration, reducing lung goblet cell grume generation, reducing lung collagen deposition, and inhibiting Th2 cell factor production, can provide beneficial information for clinic disease evaluation, and has a favorable application prospect in asthma treatment.
Owner:SUN YAT SEN UNIV
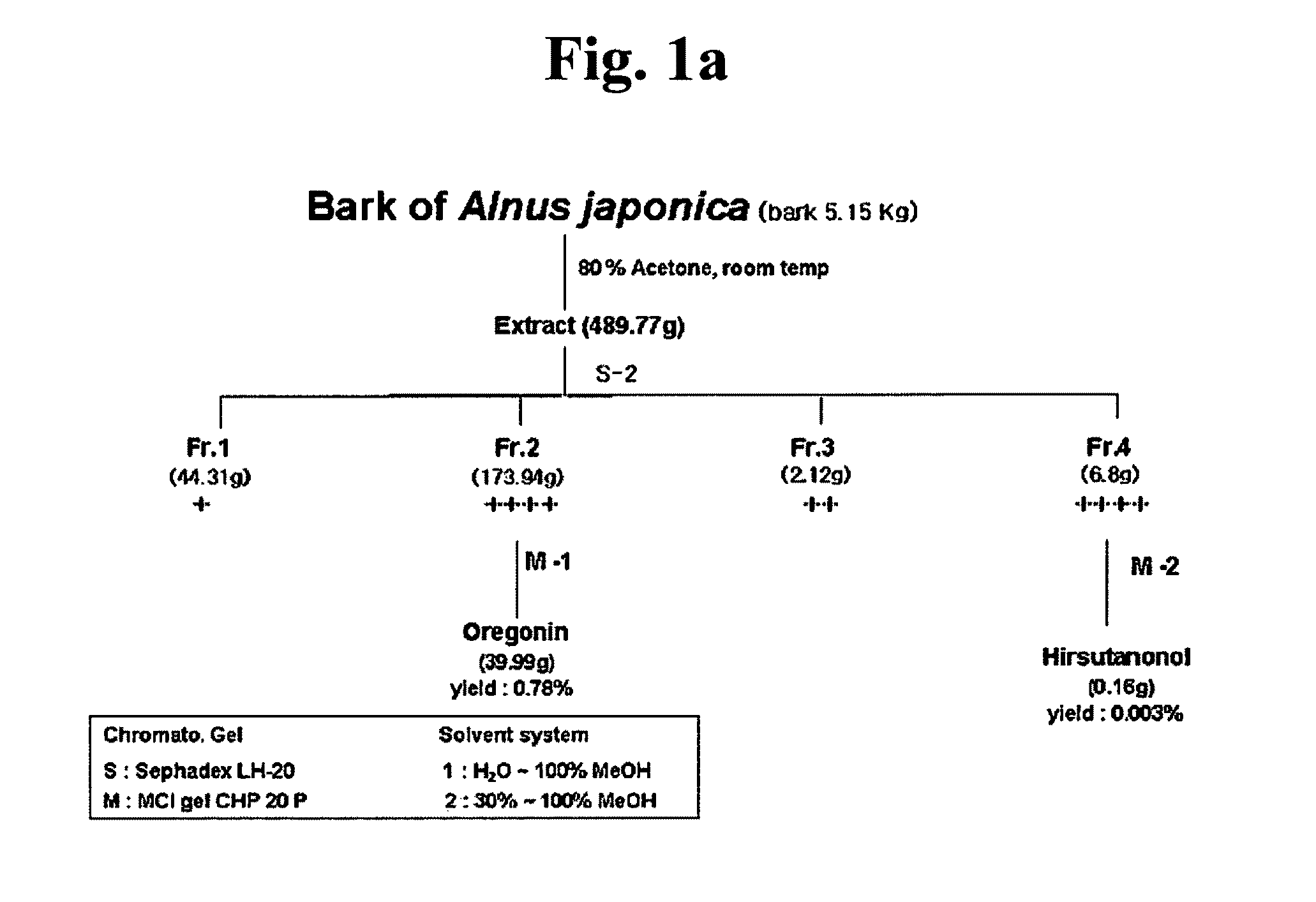
Composition for treating atopic dermatitis comprising hirsutenone as an active ingredient
InactiveUS8012486B2Reduce in quantityReduced expression levelBiocideCosmetic preparationsAtopic dermatitisBULK ACTIVE INGREDIENT
The present invention relates to a composition for treating atopic dermatitis comprising hirsutenone as an active ingredient. Hirsutenone as the active ingredient of the present composition decreases the number of eosinophil and the level of IgE increased in atopic dermatitis and remarkably reduces expression amounts of immune regulatory cytokine (e.g., IL-4, IL-5 and IL-13) associated with atopic dermatitis. In addition, hirsutenone decreases COX-2 and iNOS expression. Hirsutenone as the active ingredient of the present composition could be effectively used in drugs, cosmetics and foods for treating atopic dermatitis or relieving a symptom of atopic dermatitis.
Owner:CHUNG ANG UNIV IND ACADEMIC COOP FOUND
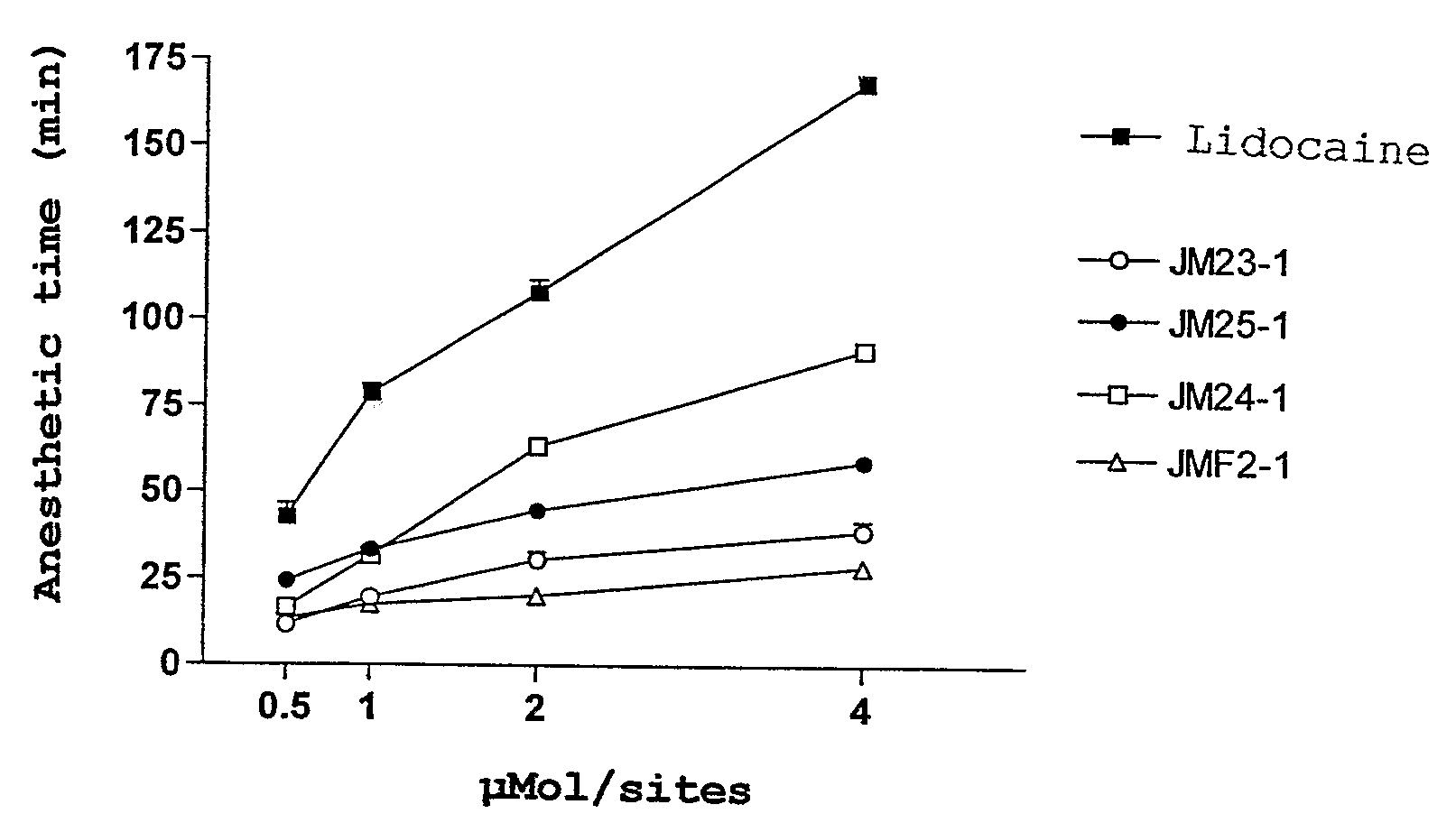
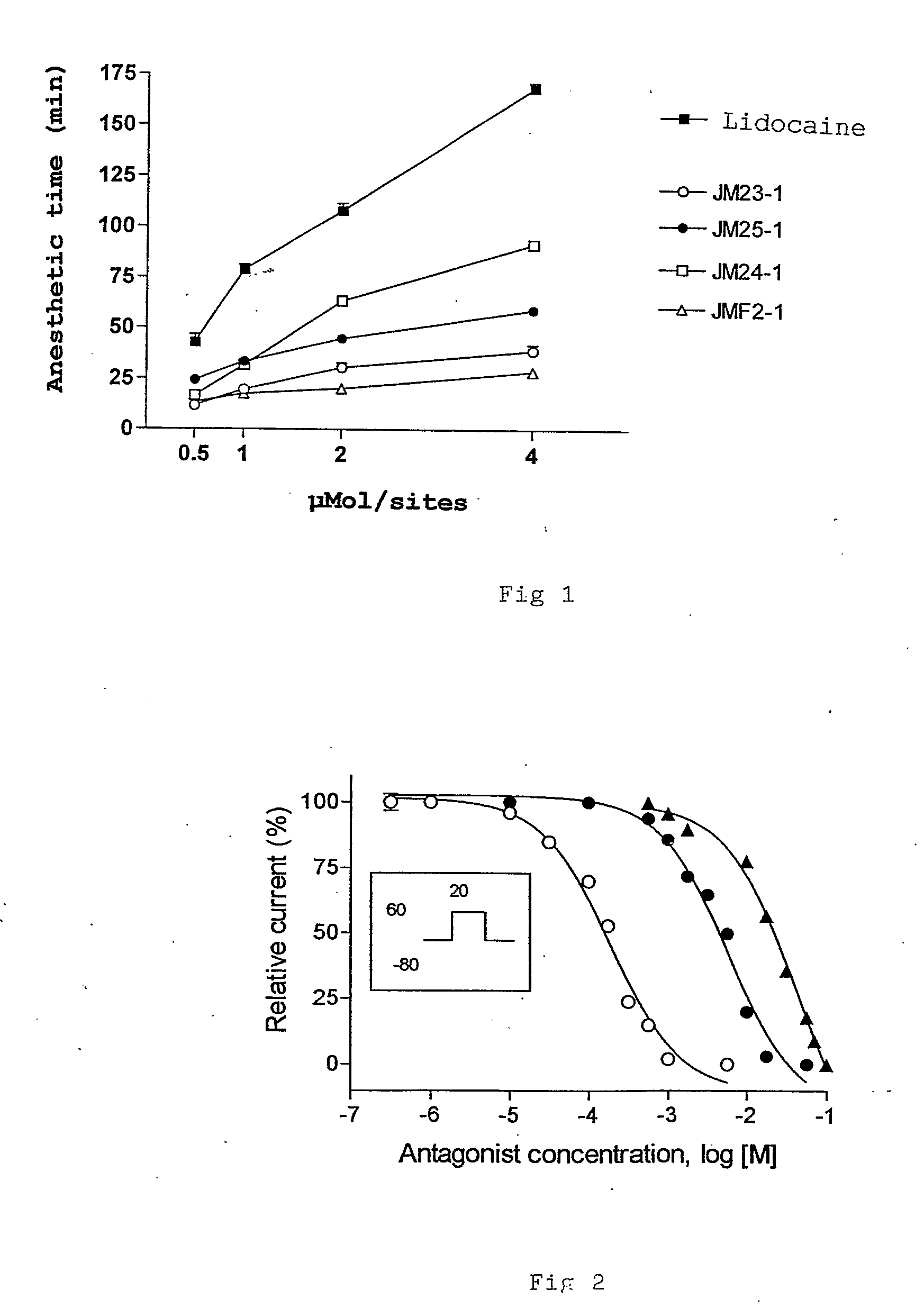
Compounds Derived From Lidocaine, Pharmaceutical Compositions, Use And Method Of Treatment, Prevention Or Inhibition Of Disease
InactiveUS20080221206A1Minimize side effectsBiocideNervous disorderIntestinal inflammationAllergic urticaria
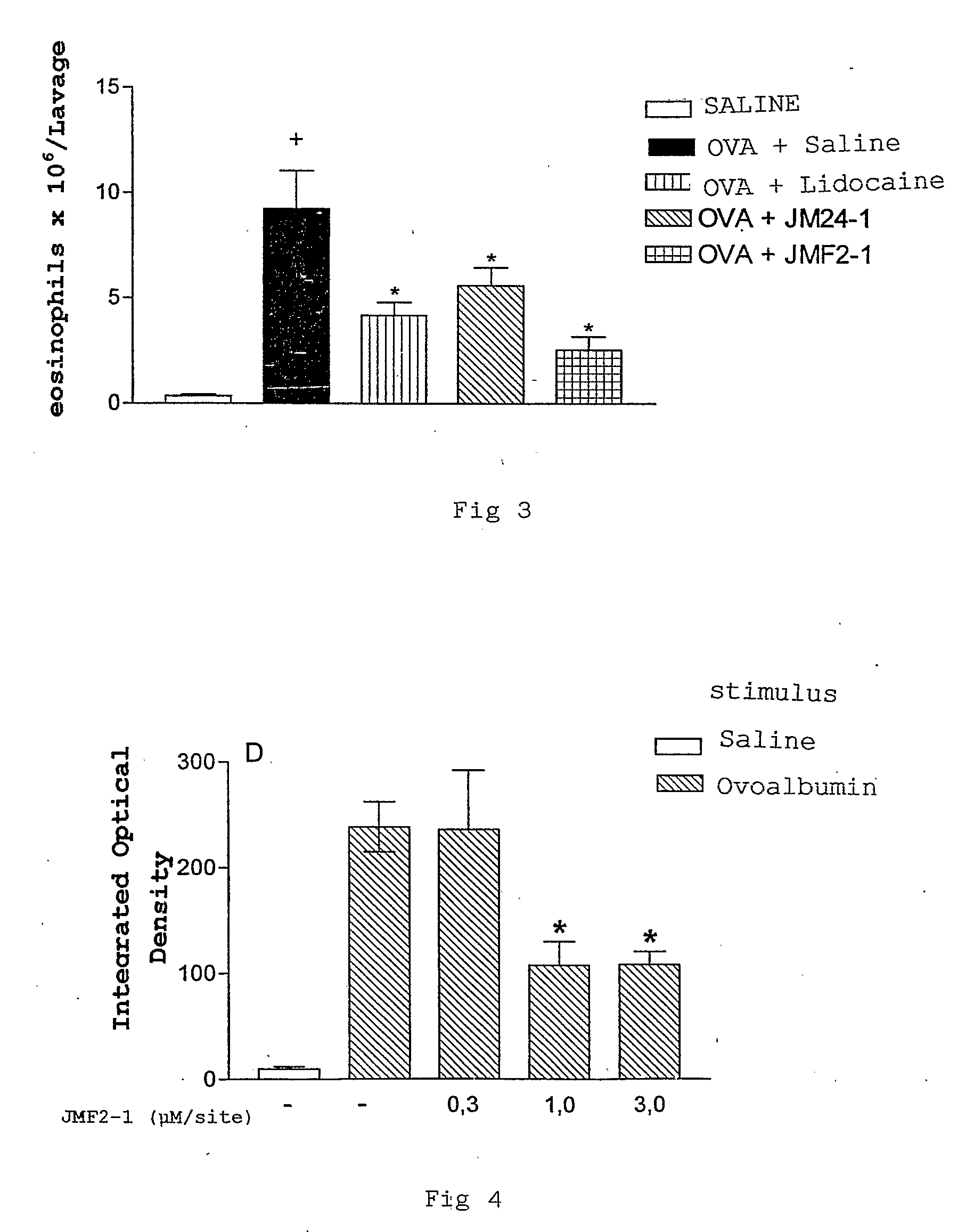
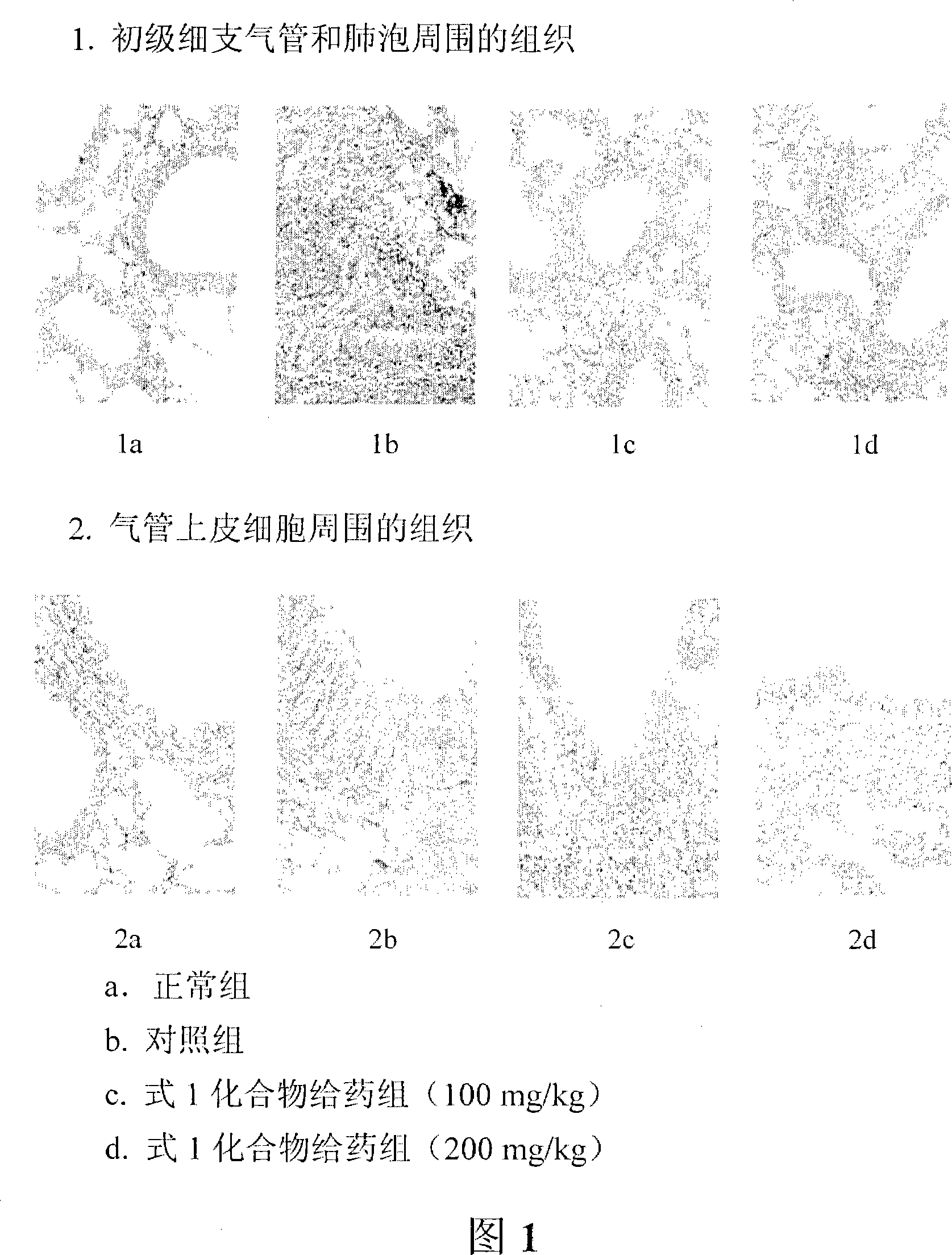
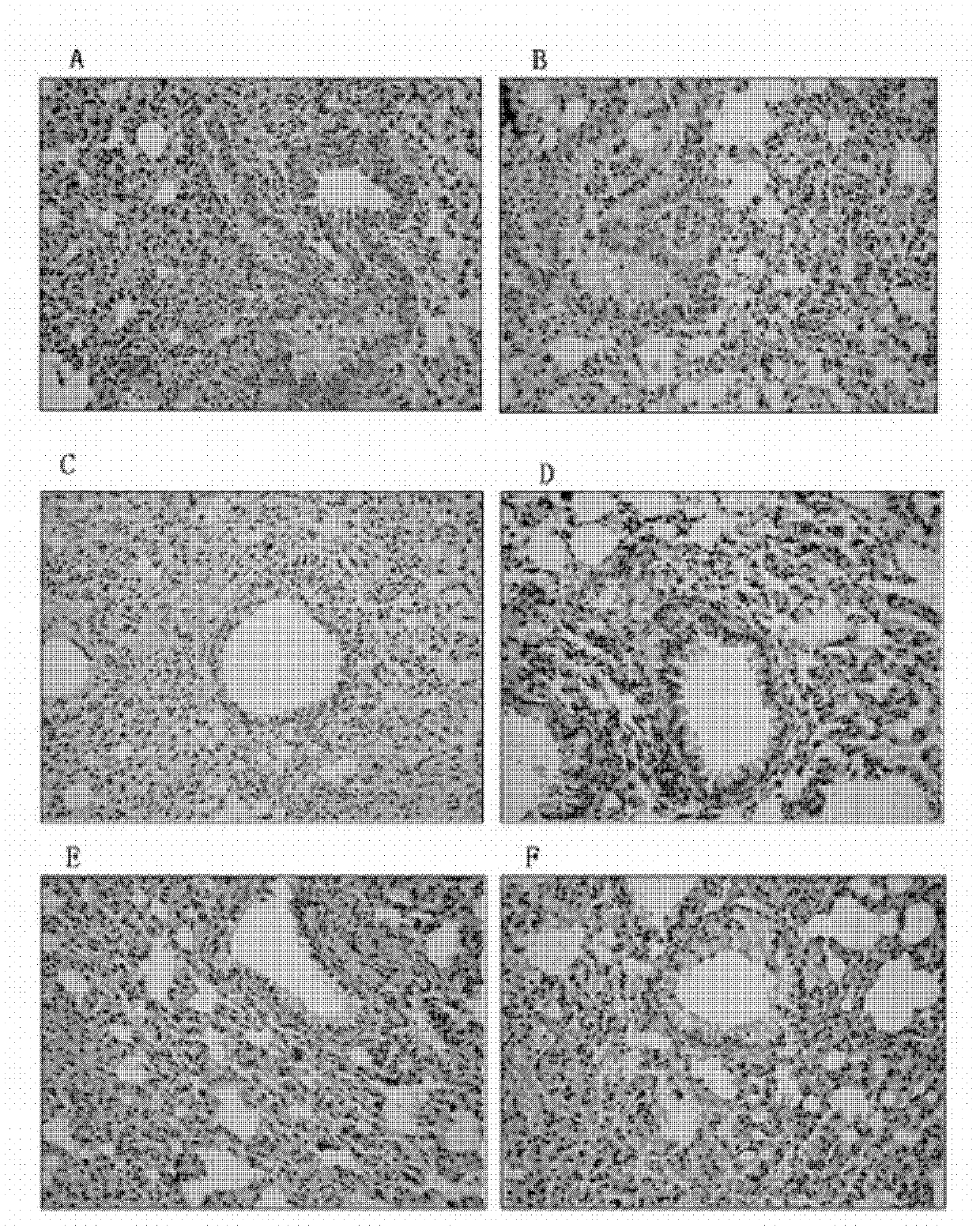
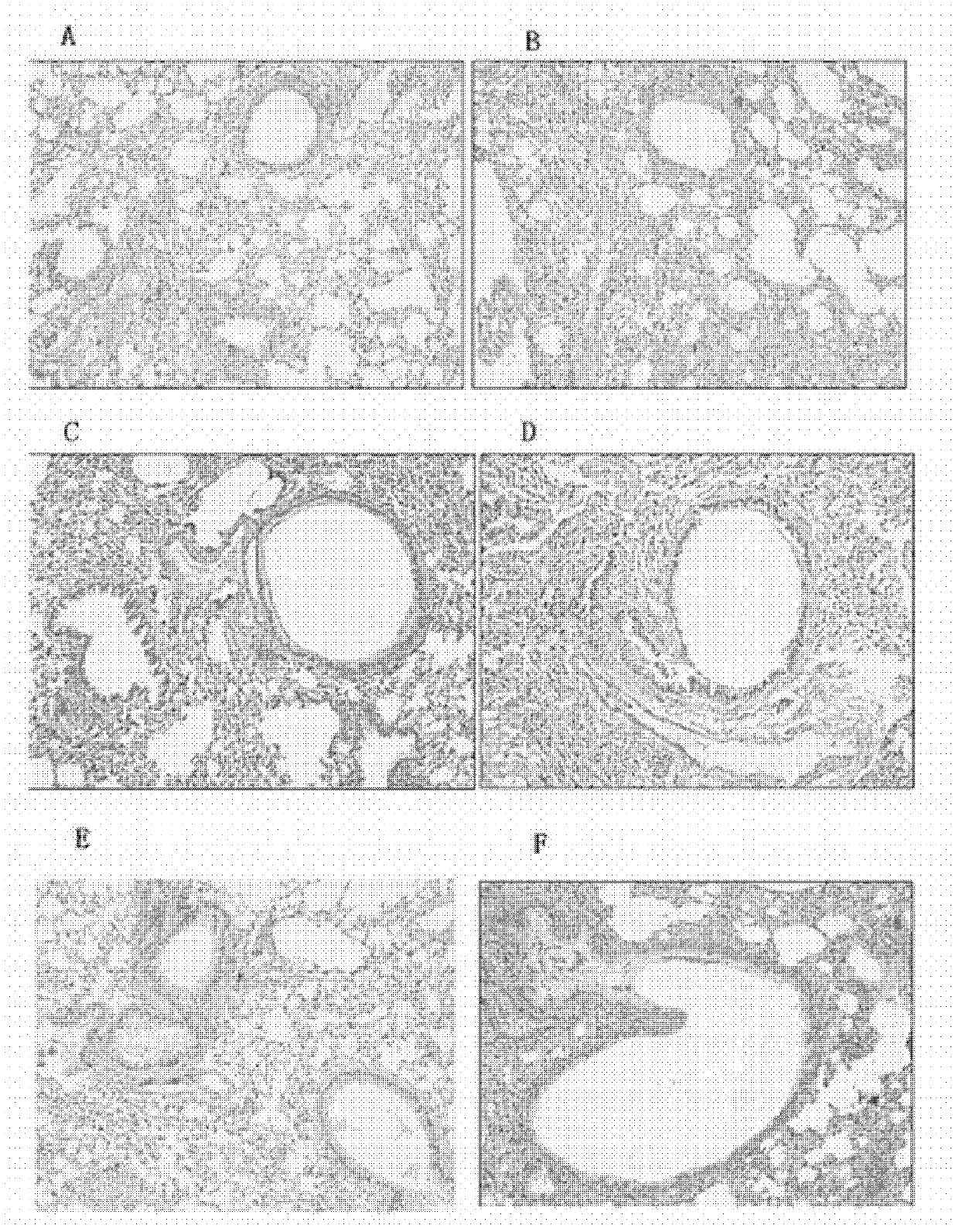
The present invention relates to lidocaine derived compounds, which present less anesthetic activity than lidocaine itself, but with more anti-inflammatory and spasmolytic activity than said lidocaine as well as pharmaceutical compositions with at least one of these compounds or a salt of those as active principle and to the use of such compositions to treat, prevent or inhibit atopic diseases including asthma, rhinitis, allergic urticaria, chronic lung inflammation associated with eosinophilia, following the example of atopic asthma and chronic intestinal inflammation, as colitis for instance. The pharmaceutical composition may be available in spray form, solution, suspension, emulsion destinated to be applied by nebulization, or in any of the pharmaceutical available forms for oral or injectable use.
Owner:FUNDACAO OSWALDO CRUZ FIOCRUZ
Composition for the prevention and treatment of allergic inflammatory disease
Disclosed herein is a composition for the treatment and prevention of allergic inflammatory diseases comprising N-hydroxy-4-{5- [4- (5-isopropyl-2-methyl-l, 3-thiazol-4- yl)phenoxy]pentoxy}-benzamidine, 4-{5- [4- (5-isopropyl-2- methyl-1, 3-thiazol-4-yl)phenoxy]pentoxy}-benzamidine or pharmaceutically acceptable salts. The composition exhibits excellent medicinal effects on allergic inflammatory diseases, with a great reduction in typical chronic inflammation symptoms, such as an increase of eosinophil level in bronchoalveolar lavage fluid, total leukocyte level and eosinophil level in blood, the hypertrophy or hyperplasia of bronchial epithelium due to an increase in the number of mucus cells, a reduction in alveolar surface area resulting from the hypertrophy of alveolar walls, and the infiltration of inflammatory cells.
Owner:DONG WHA PHARM CO LTD
Application of icariin to preparation of medicament for treating bronchial asthma
The invention belongs to the field of Chinese medicine pharmacy, and relates to an application of icariin to preparation of a medicament for treating bronchial asthma, in particular to treatment of bronchial asthma with icariin by prompting eosinophilic granulocyte apoptosis. As proved by the result of an influence experiment of eosinophilic granulocyte apoptosis of a mouse suffering from bronchial asthma, the icariin influences intrapulmonary eosinophilic granulocyte apoptosis of the mouse suffering from bronchial asthma and expression of relevant gene proteins Bc1-2 and Bax, and can be used for promoting asthmatic eosinophilic granulocyte apoptosis, reducing asthmatic air channel eosinophilic granulocyte infiltration and influencing the expression of apoptosis-relevant gene proteins Bcl-2 and Bax to achieve the effect of treating bronchial asthma. The icariin can be a medicinal composition for treating bronchial asthma prepared from active ingredients and pharmaceutically-acceptable carriers.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
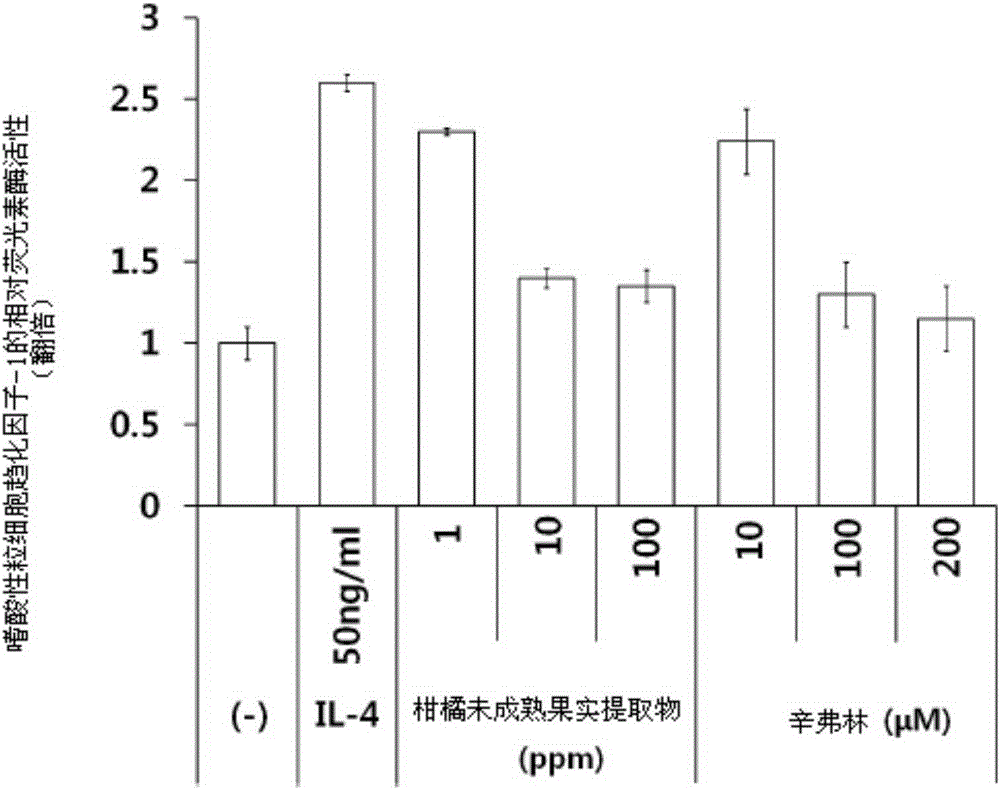
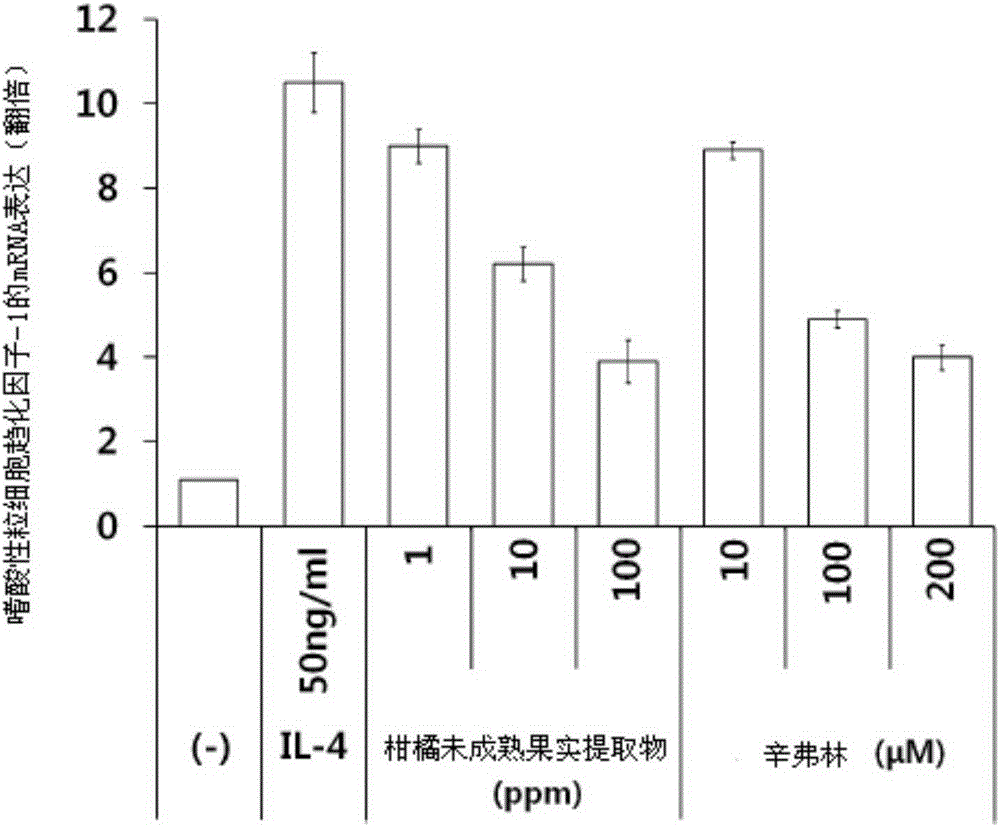
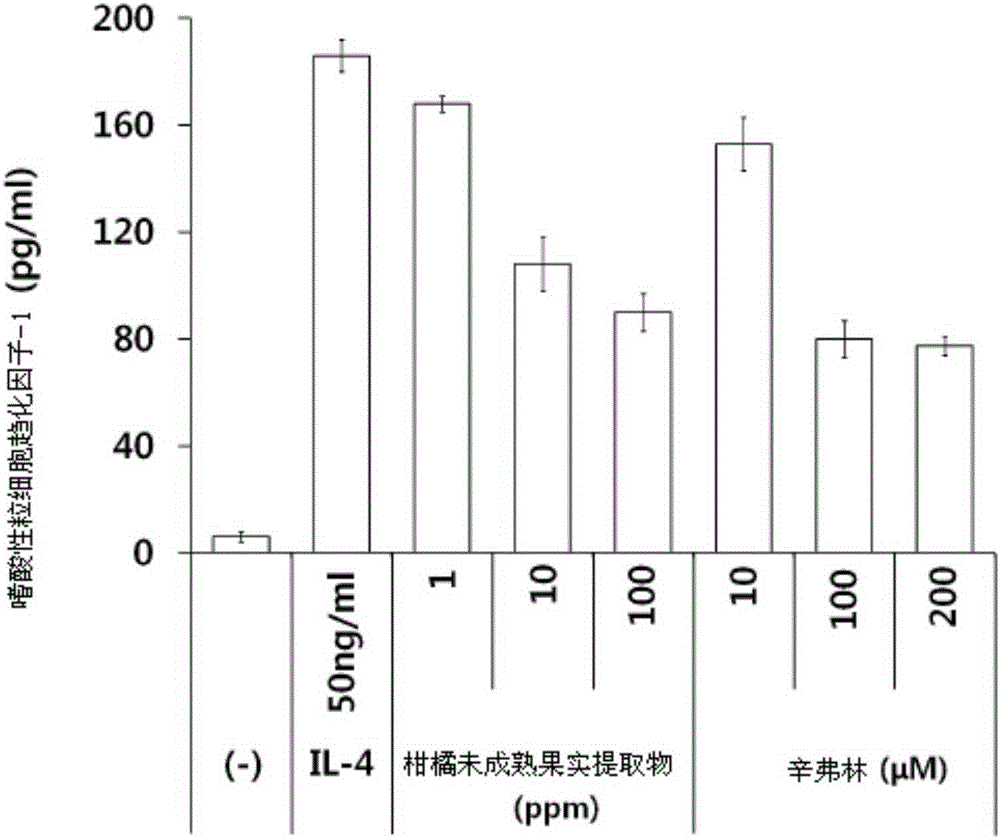
Composition for treating or preventing inflammatory skin disease, comprising, as active ingredient, immature citrus fruit extract, or synephrine or salt thereof
ActiveCN105358170APrevent agglutinationInhibit the inflammatory responsePowder deliveryOrganic active ingredientsSide effectCytotoxicity
The present invention relates to a composition for treating or preventing an inflammatory skin disease, comprising, as active ingredients, an immature citrus fruit extract and / or synephrine or salt thereof. The composition of the present invention prevents an inflammatory reaction by inhibiting activity of STAT6, expression of eotaxin-1 and an agglomeration function of eosinophil, and thus, shows effects of preventing and treating an allergic inflammatory disease, atopic dermatitis, eczema, psoriasis, etc. Also, the composition of the present invention does not have cytotoxicity and a skin side effect, and thus, can be safely applied to pharmaceutical products and cosmetics.
Owner:KOREA BIOSITE CO LTD
Methods and compositions for treating and preventing inflammatory conditions
Methods of treating eosinophilia by down regulating eotaxin and at least one other Th-2 related cytokine are dislosed as are multivalent immunogenic compositions that generate an active immune response in a subjet comprising autoantibodies to eotaxin-1, eotaxin-2, IL-4, IL-5, IL-9, and IL-13 and treatment methods using such compositions.
Owner:DRIVAS DIMITRIOS +1
Cla-Enriched Milkfat and Uses Thereof
InactiveUS20080193550A1Reducing and preventing degradationOrganic active ingredientsUnknown materialsButterfatWhite blood cell
The present invention relates to use of c-9, t-11 CLA or a salt, ester or precursor thereof or CLA-enriched milk fat comprising milk fat enriched with c-9, t-11 CLA or a salt, ester or precursor thereof for treating or preventing conditions such as those associated with one or more of leukocyte infiltration, eosinophilia, IgE secretion, airway remodelling, bronchoconstriction and mucus hypersecretion. The invention also relates to a pharmaceutical composition comprising CLA-enriched milk fat.
Owner:FONTERRA RES & DEV +2
Anti-asthmatic drug (asmakure) from indigenous herbs to cure the disease asthma
Asthma is defined as a chronic inflammatory disorder of the airways of the respiratory organ. It is characterized by reversible airflow obstruction causing cough, wheeze, chest lightness and shortness of breath. The inflammation of bronchial wall together with increased eosinophilis and other inflammatory products of the mast cells and lymphocytes further induce the hyper responsiveness of the bronchi so that it in turn, narrows more rapidly in response to a wide range of stimuli. Asmakure the anti-asthma drug has properties with proven pharmacological use for alleviating comman cold and persistent cough and finally building up of immunity against recurrence of asthma. One of the ingredients Adhatoda Vasica Nees (Basak) has a definite expectorant action. In acute bronchitis, it is found to afford immediate relief especially when the sputum is thick and tenacious. The depression of the Vagal terminals further relieves irritation and spasm of the bronchioles.
Owner:SUN PHARMA INDS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com