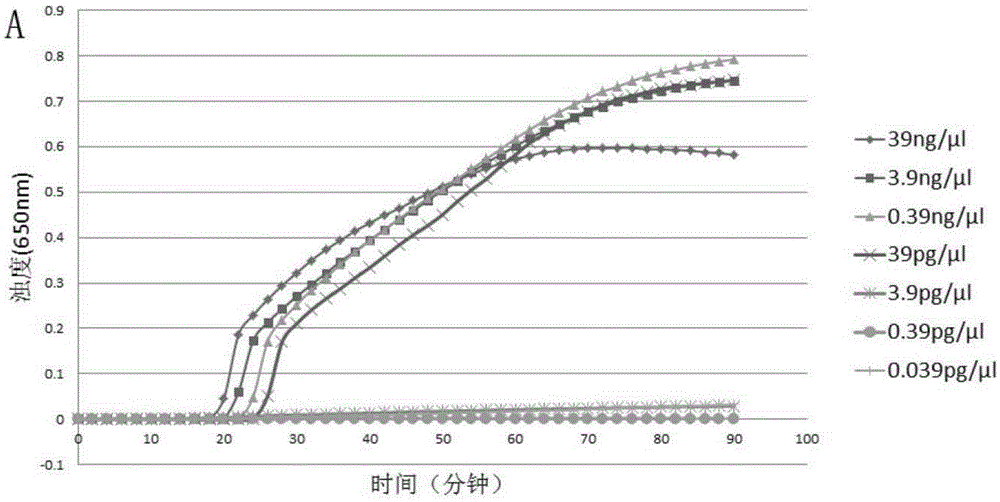
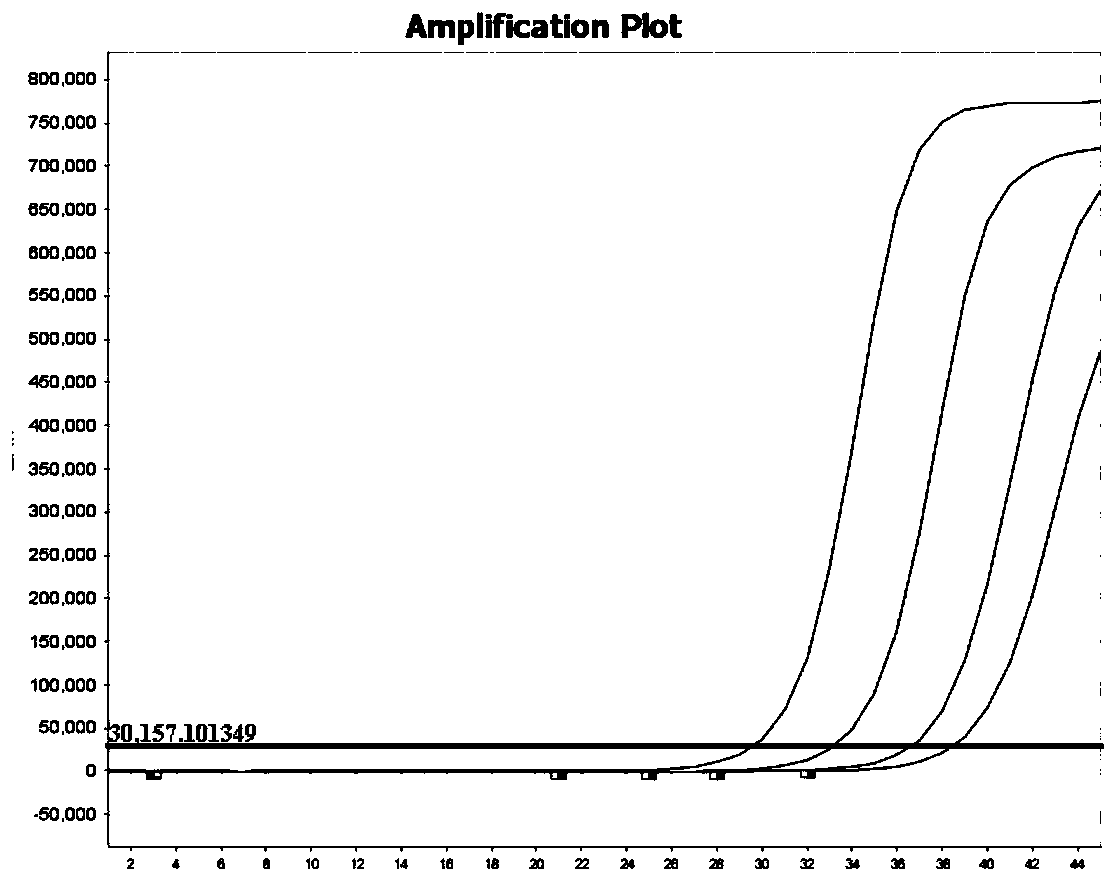
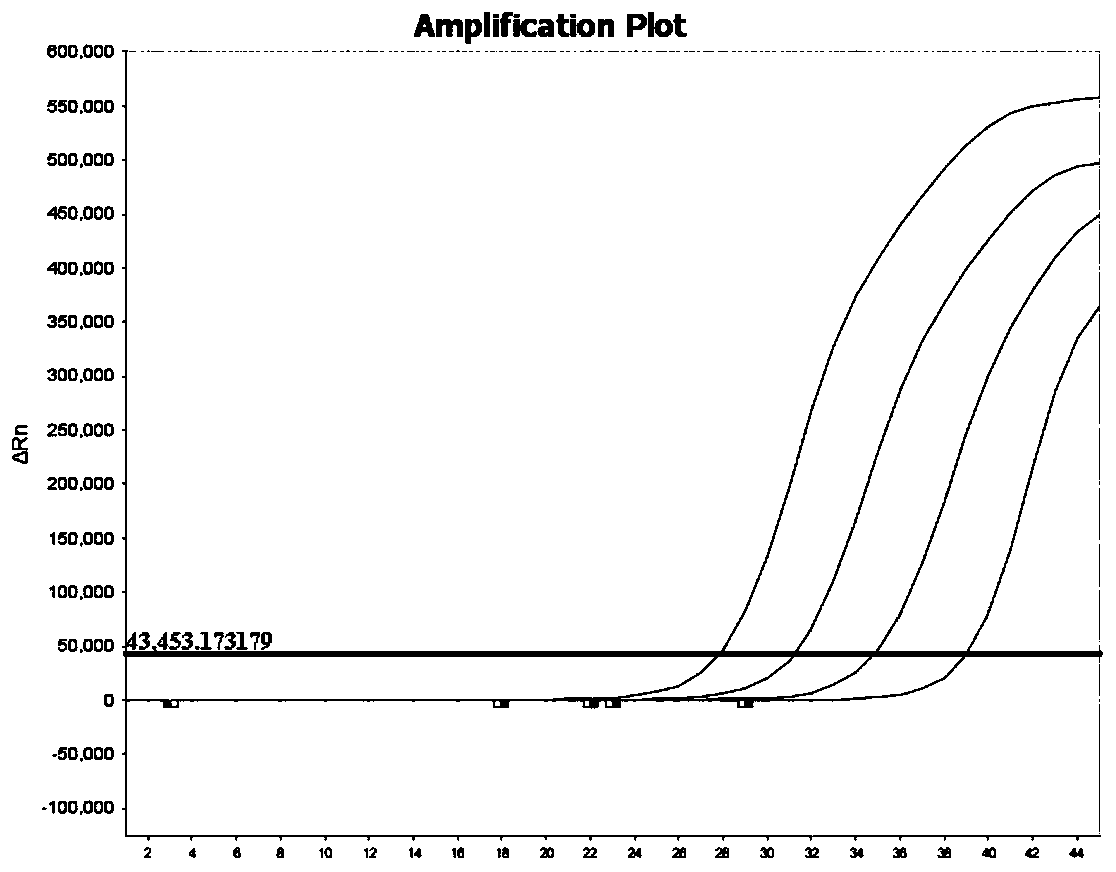
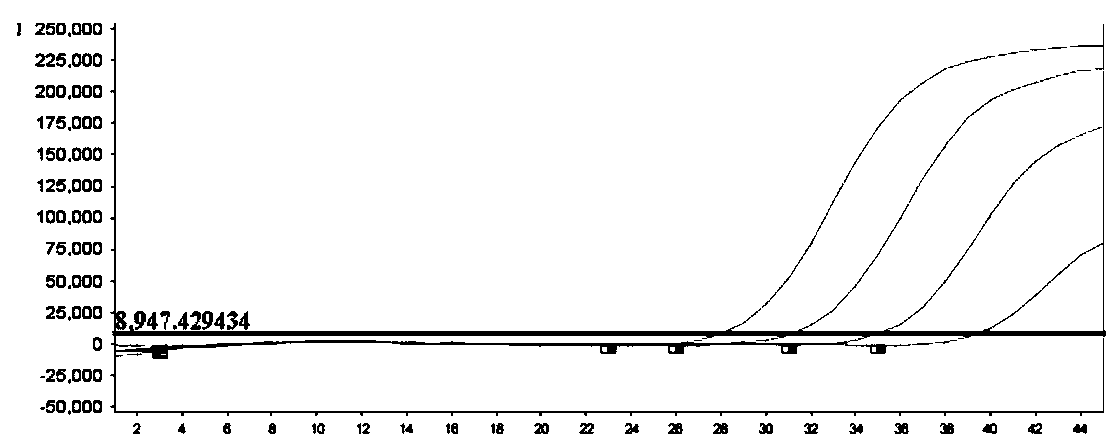
Patents
Literature
129 results about "Bronchoalveolar lavage" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
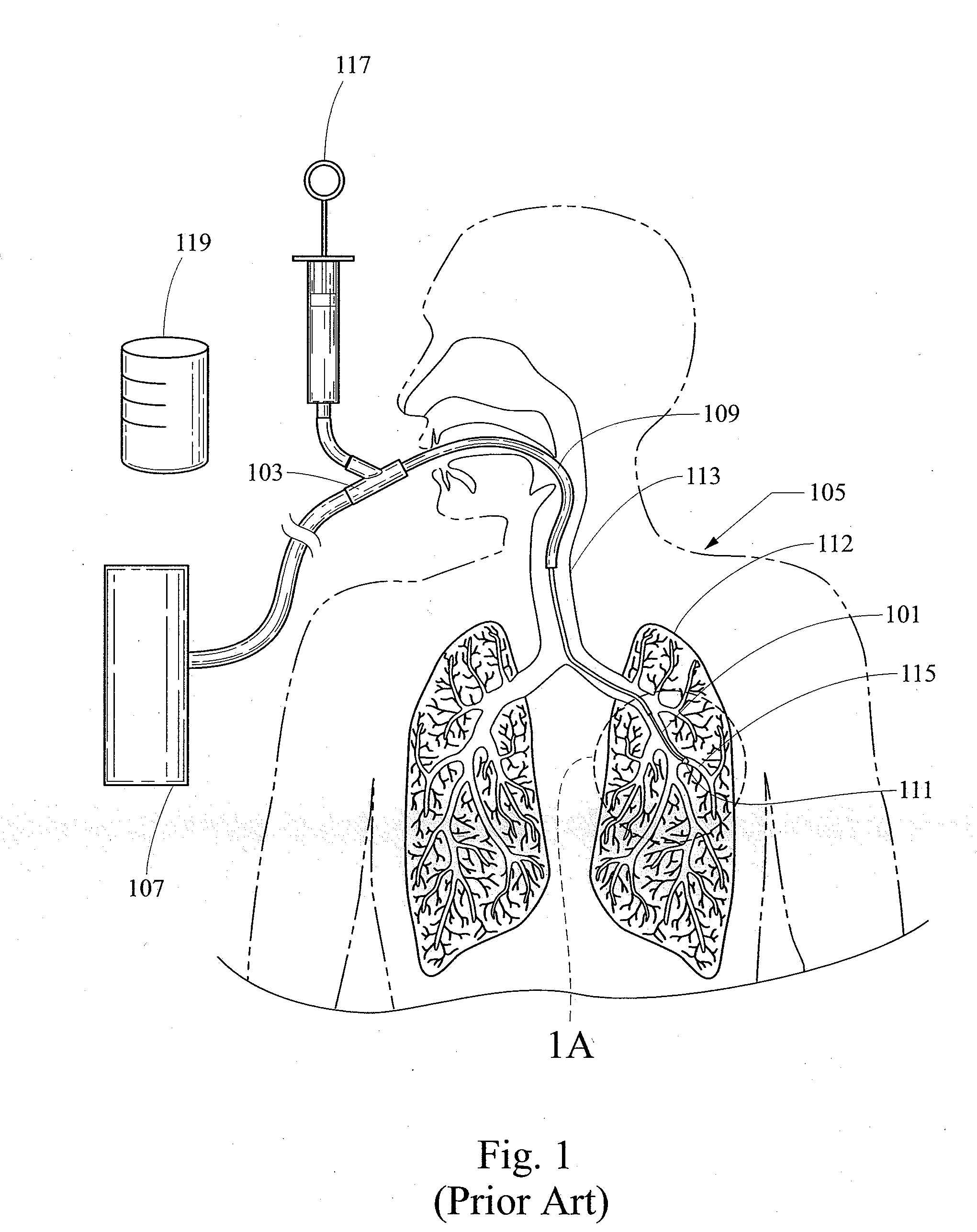
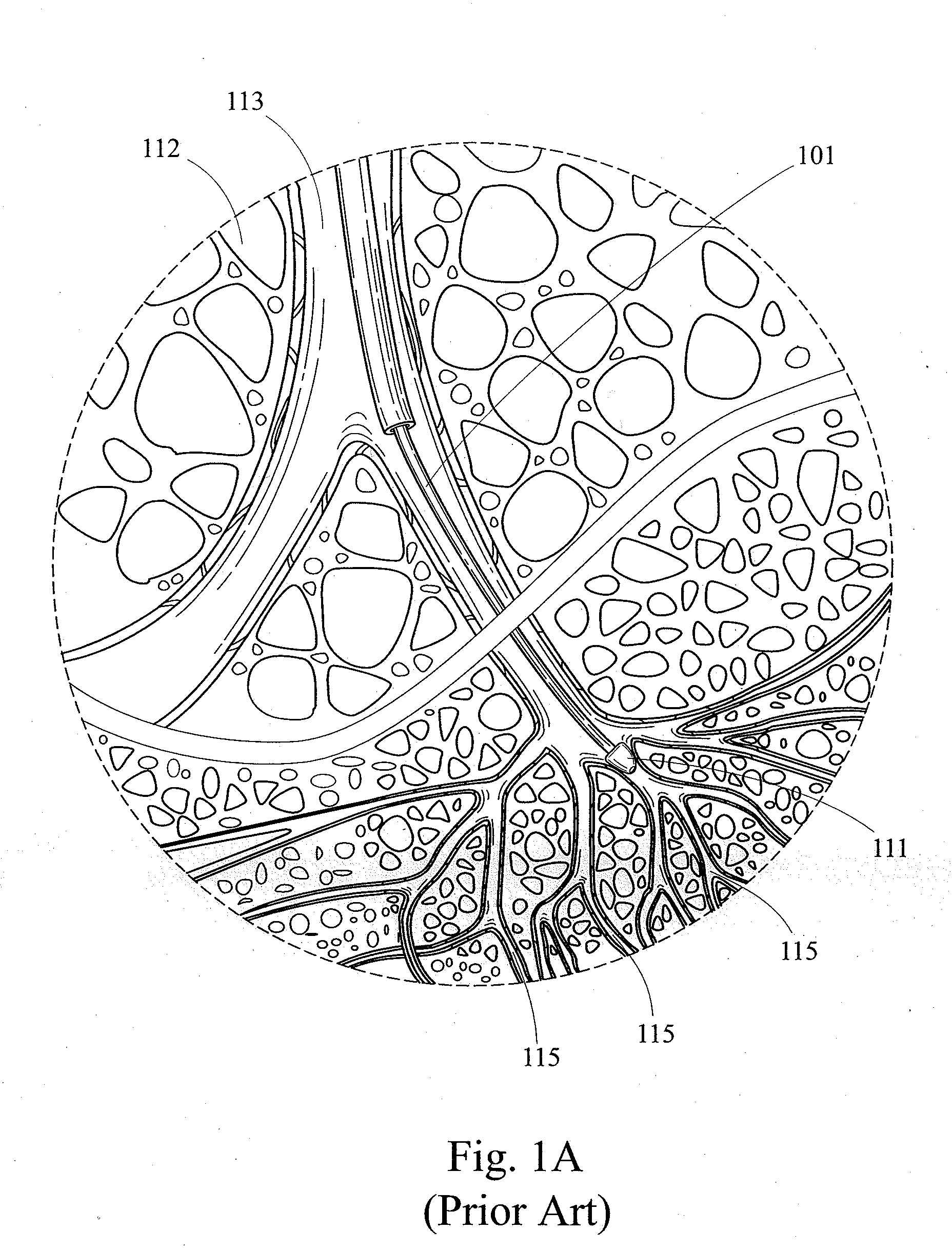
Bronchoalveolar lavage (BAL) [not to be confused with bronchial washing], is a medical procedure in which a bronchoscope is passed through the mouth or nose into the lungs and fluid is squirted into a small part of the lung and then collected for examination. It is typically performed to diagnose lung disease.
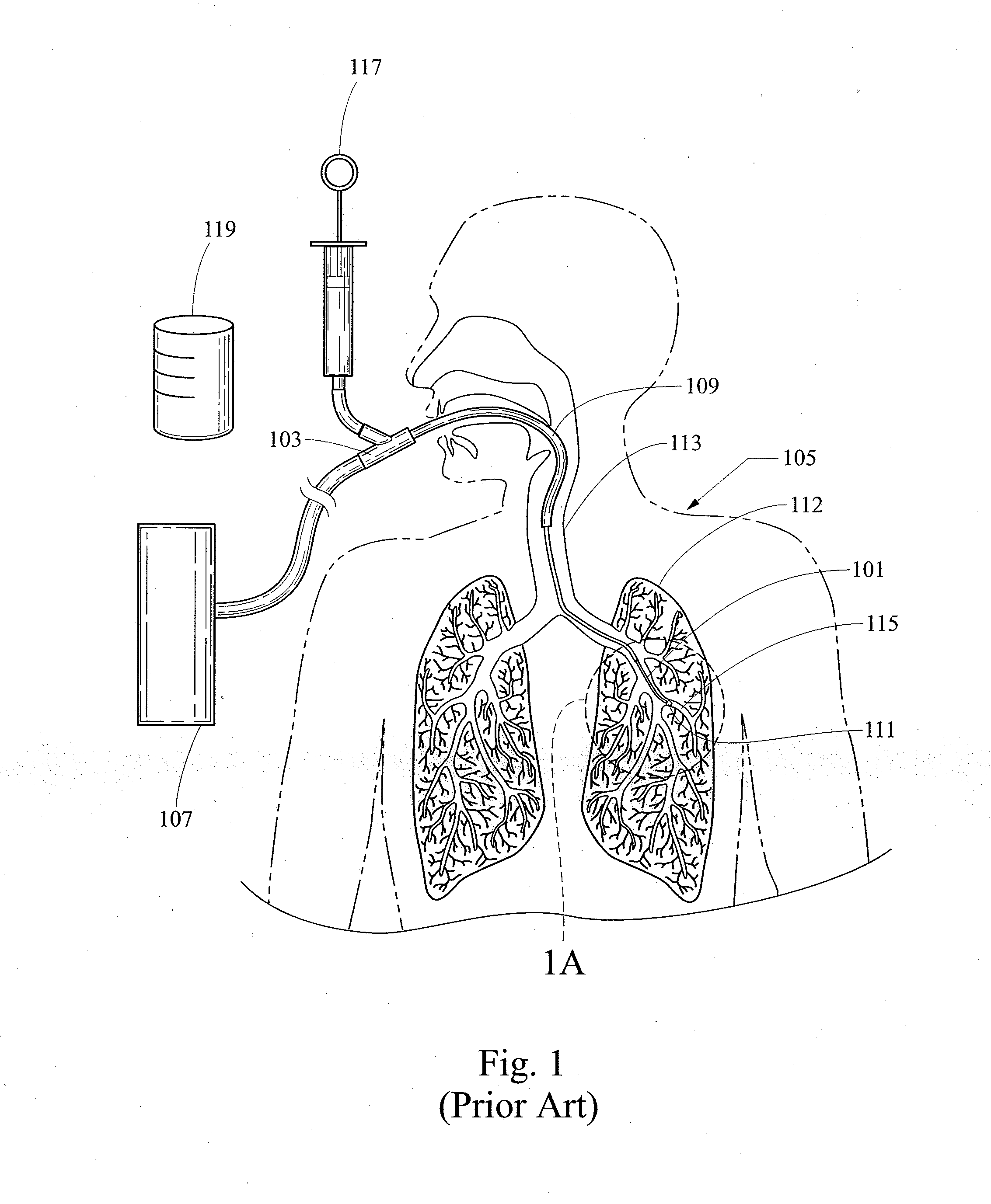
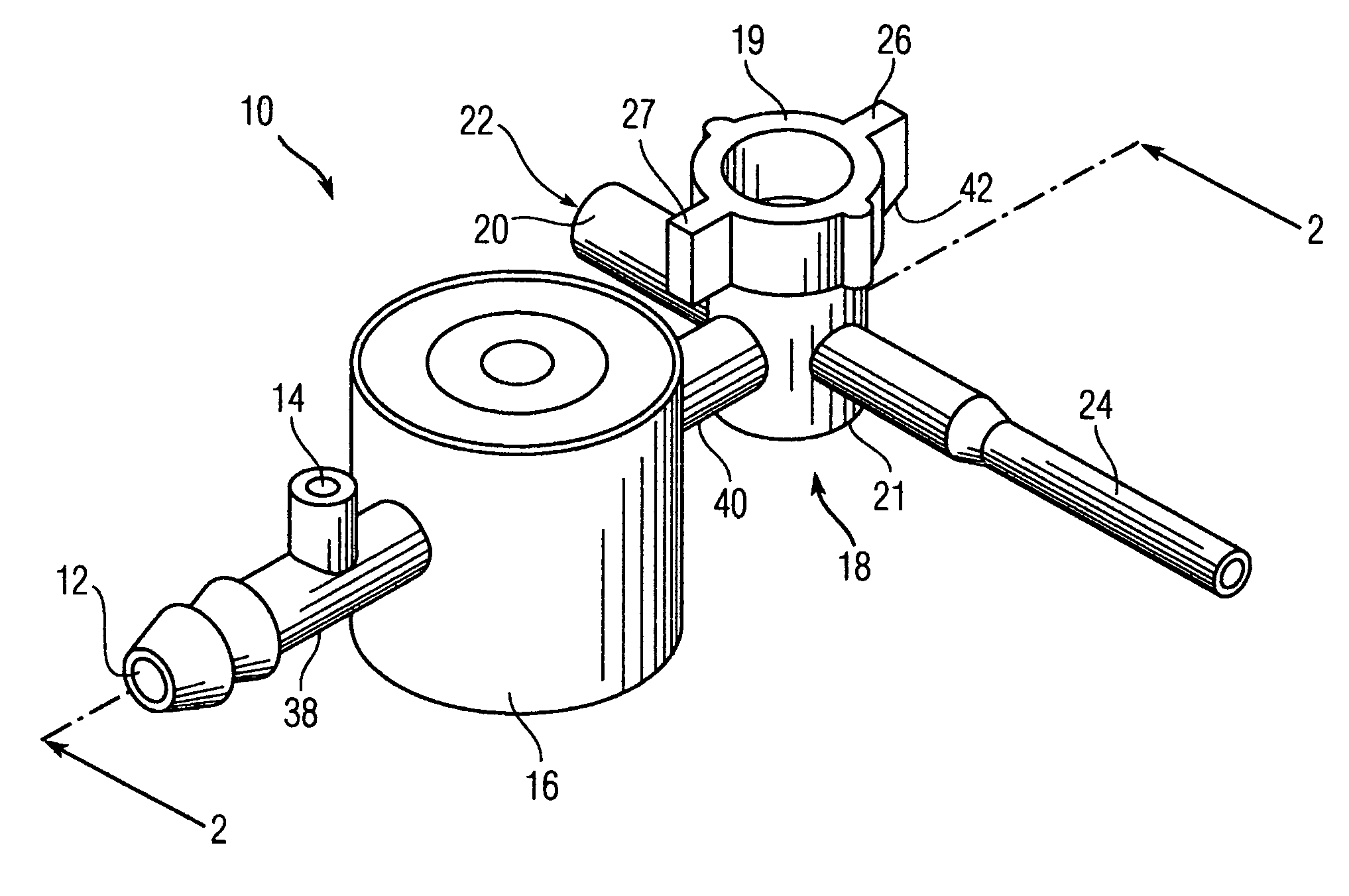
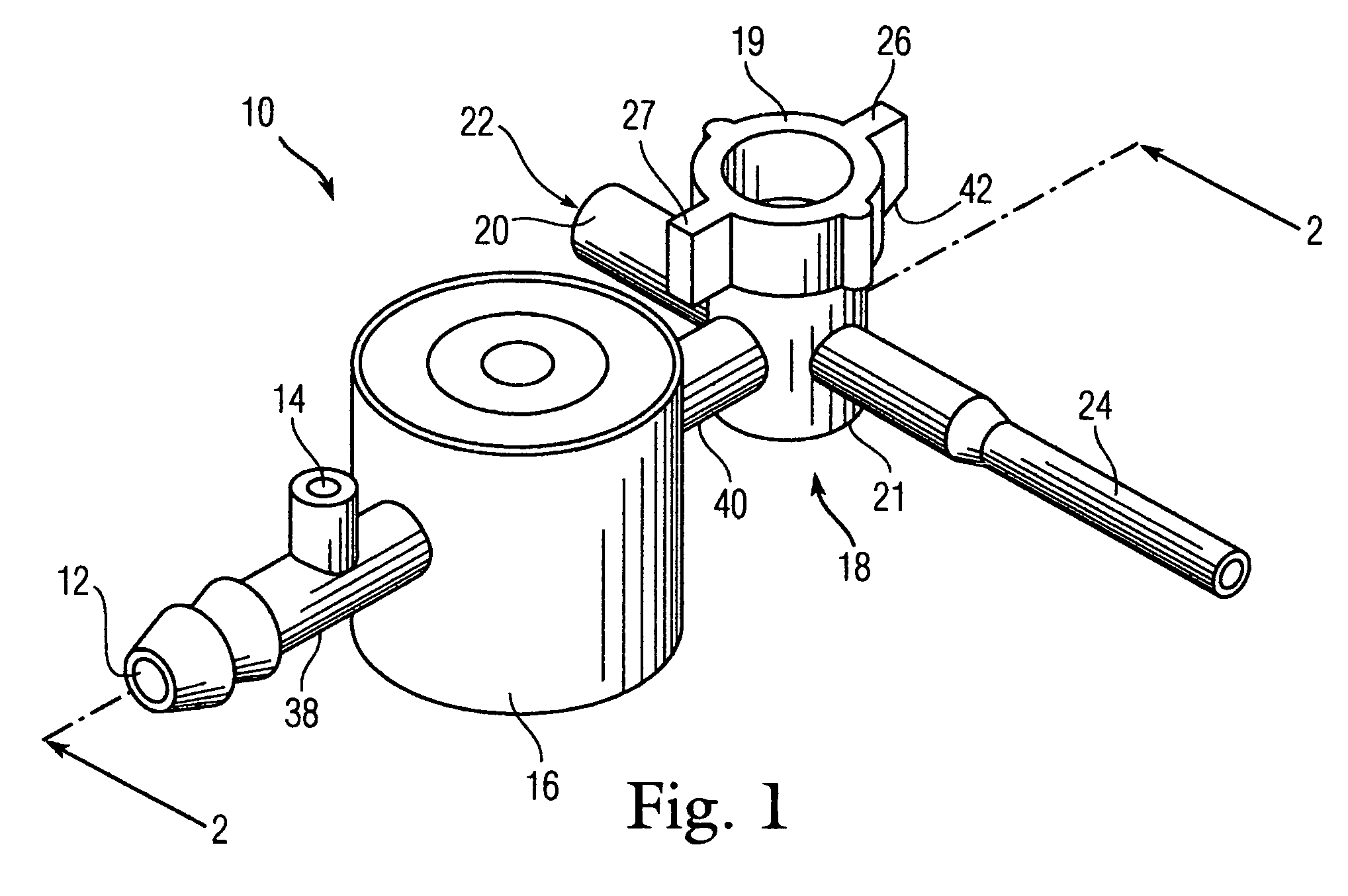
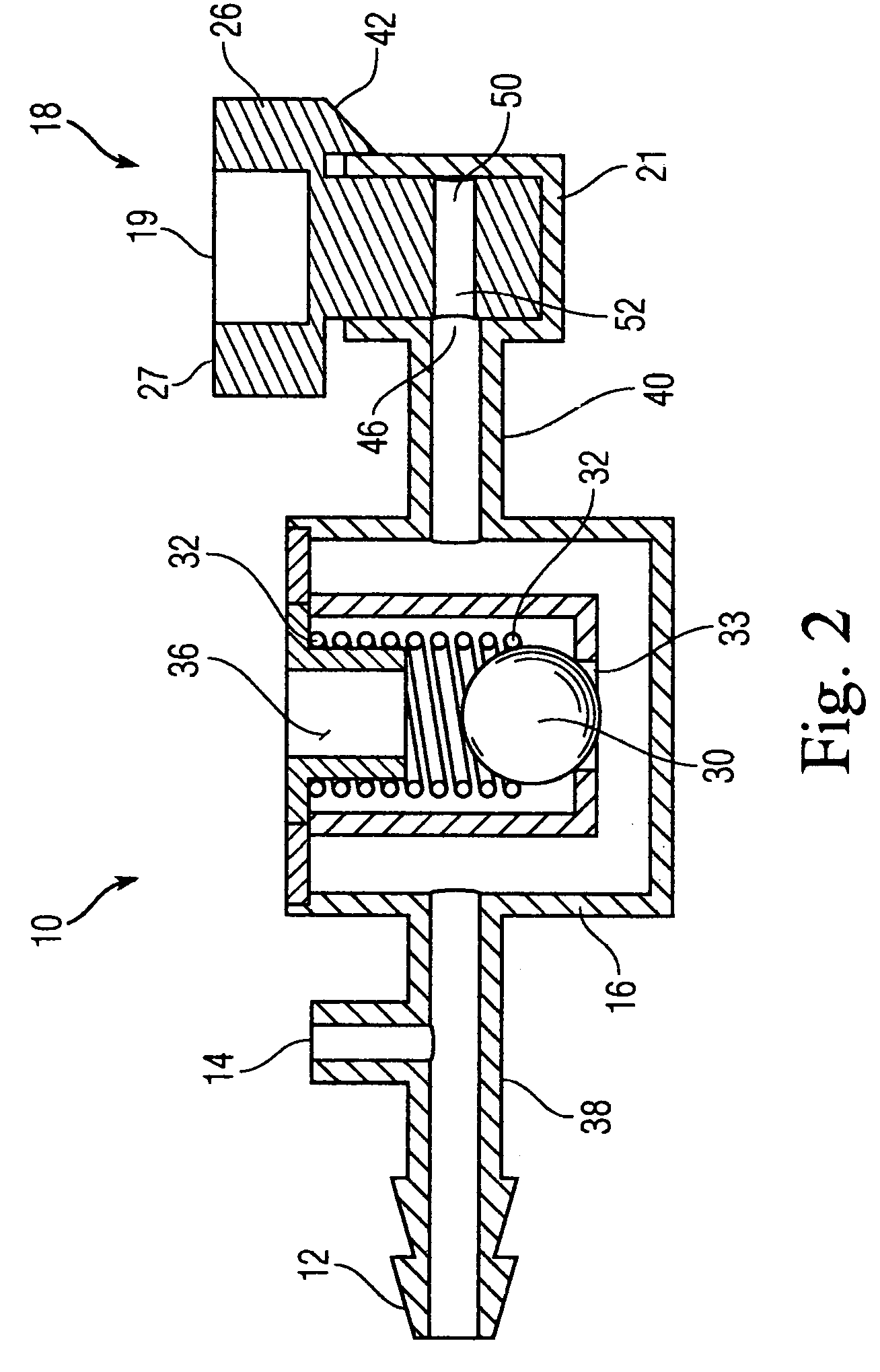
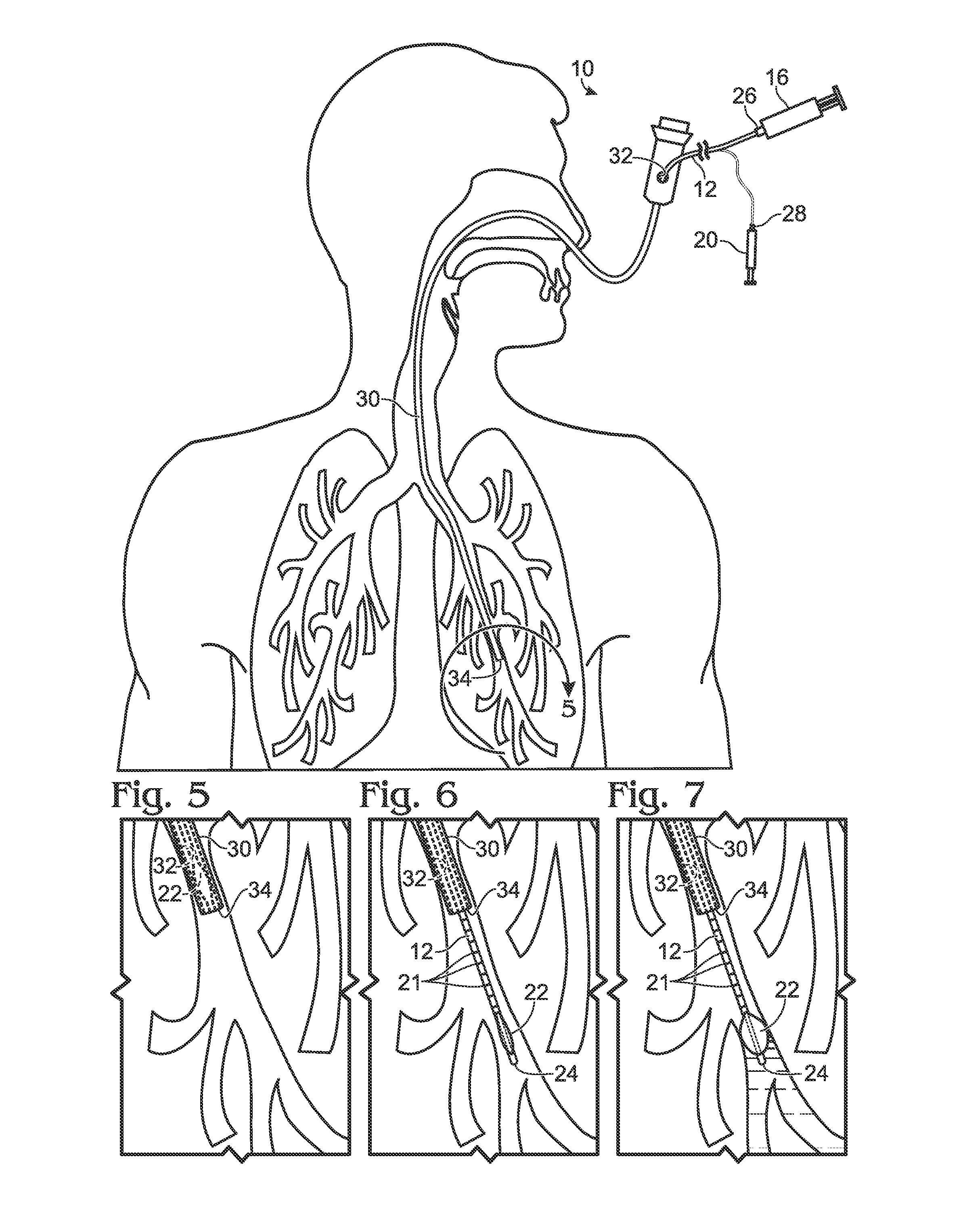
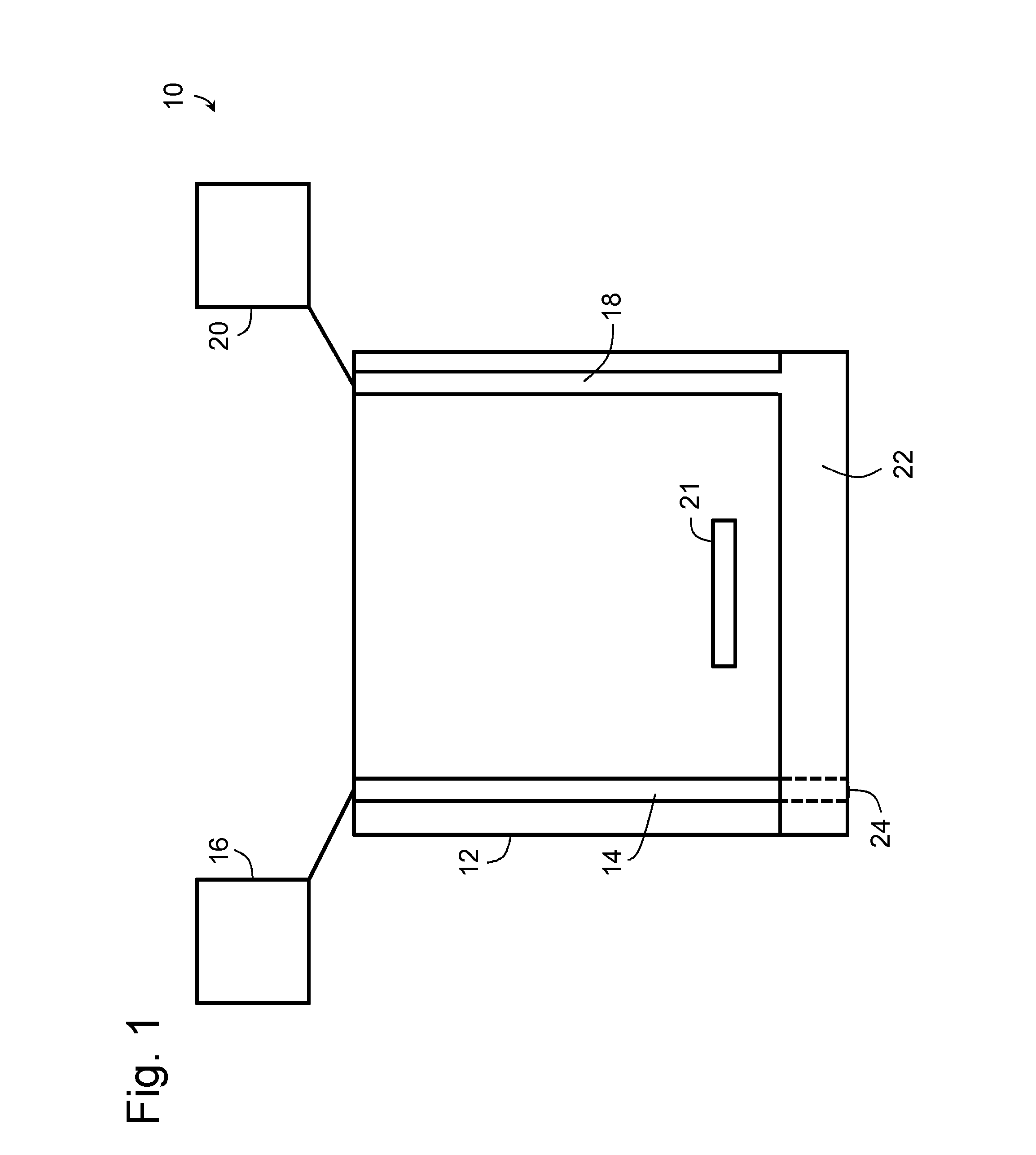
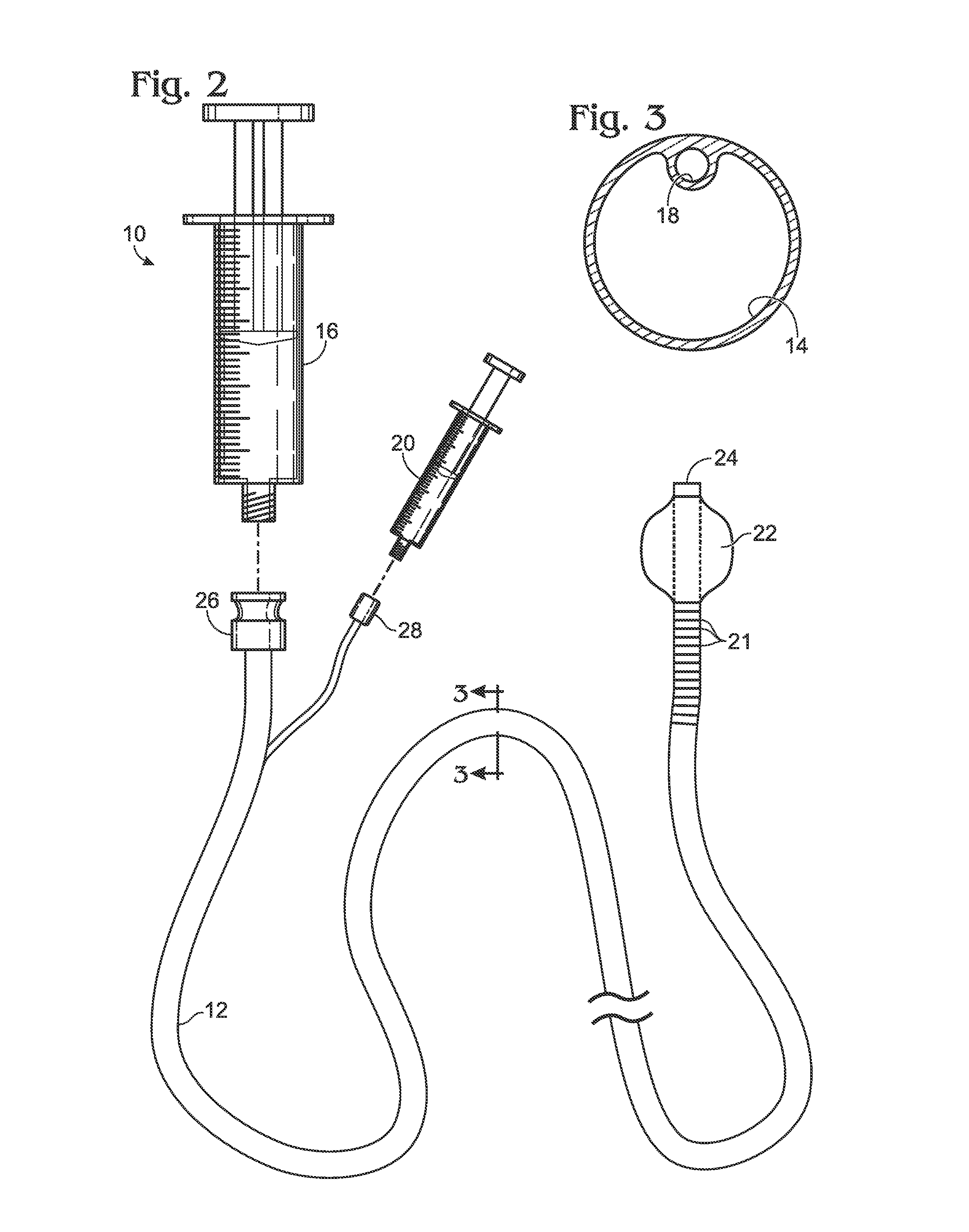
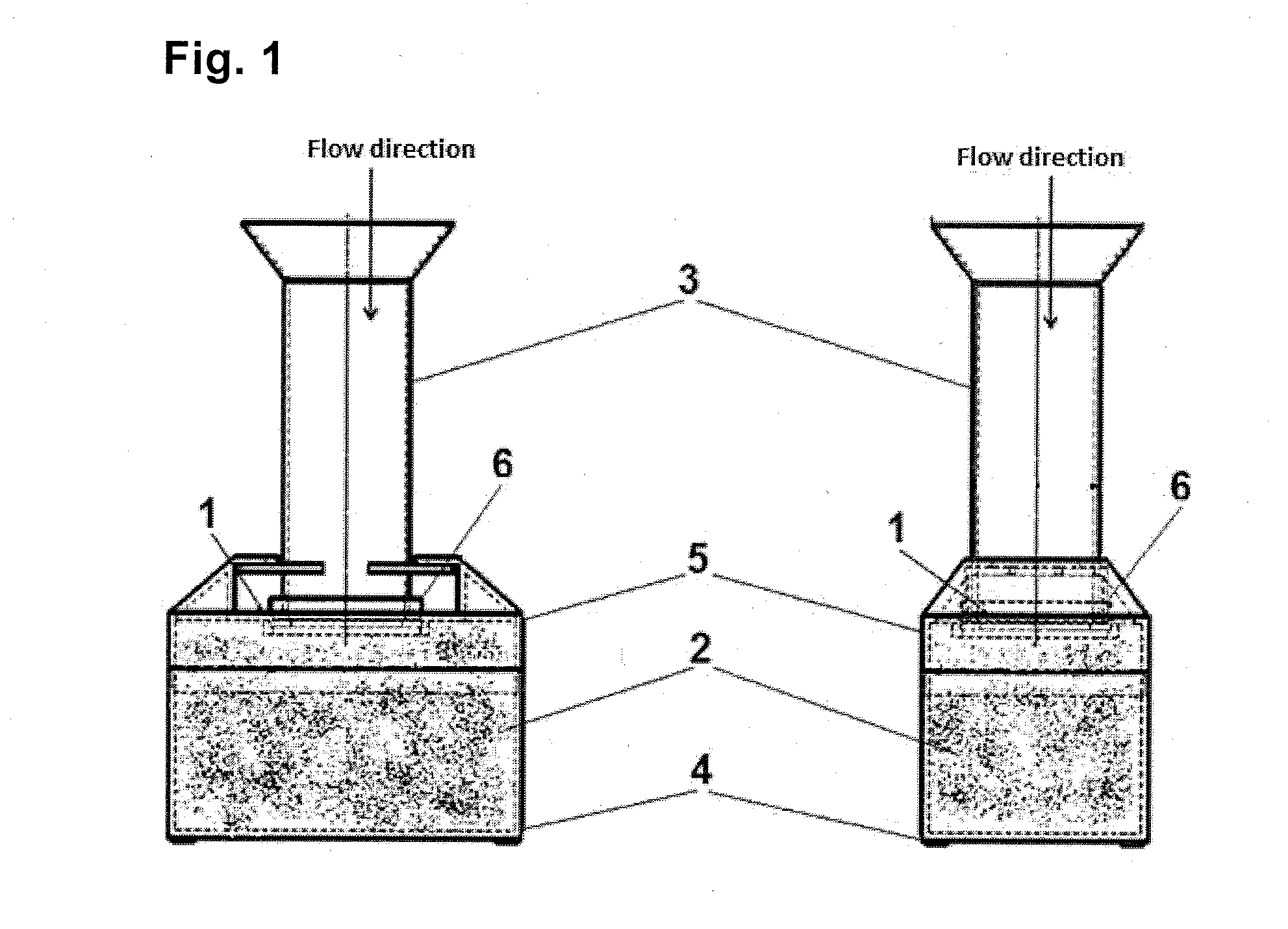
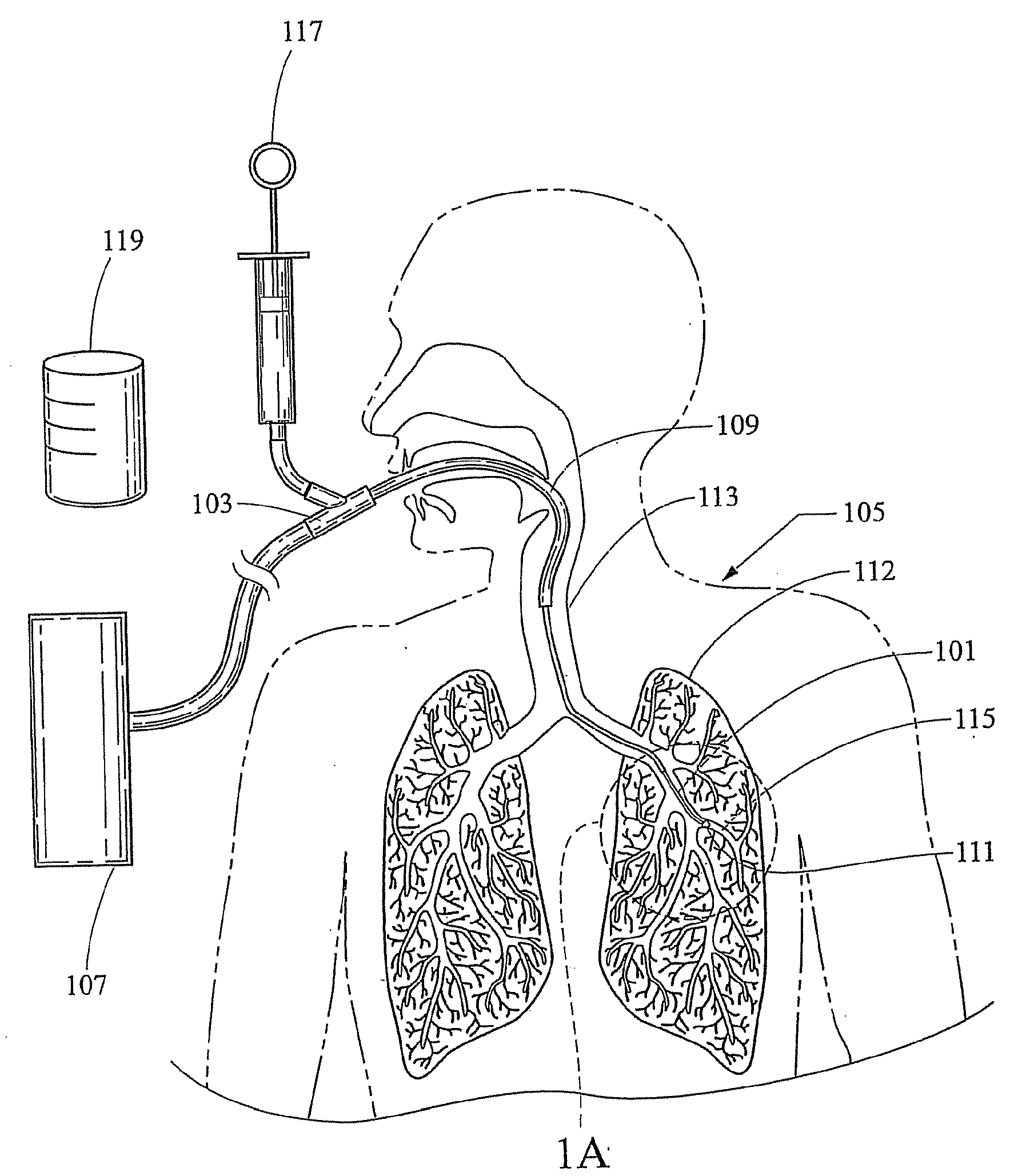
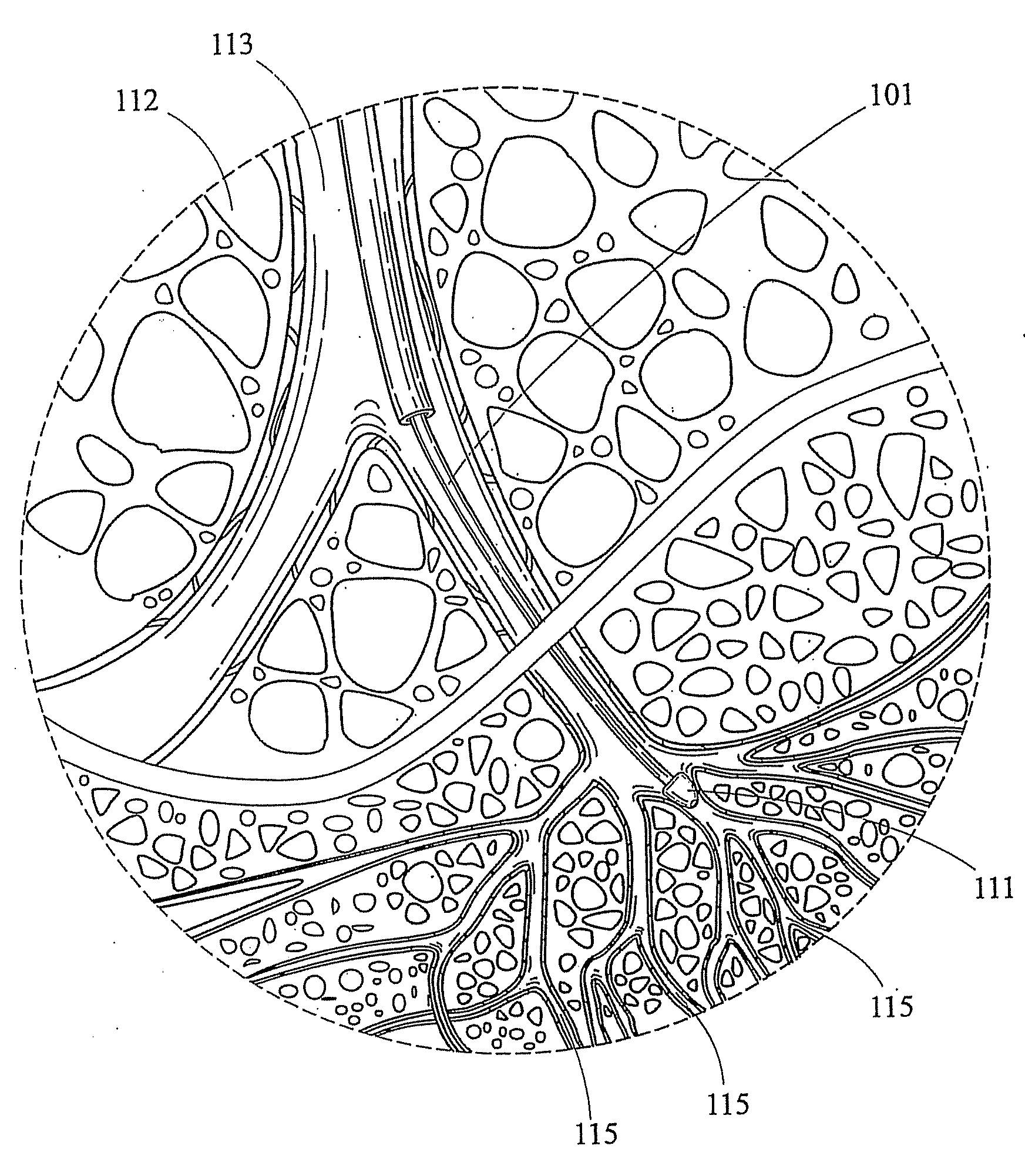
Bronchoalveolar lavage catheter assembly
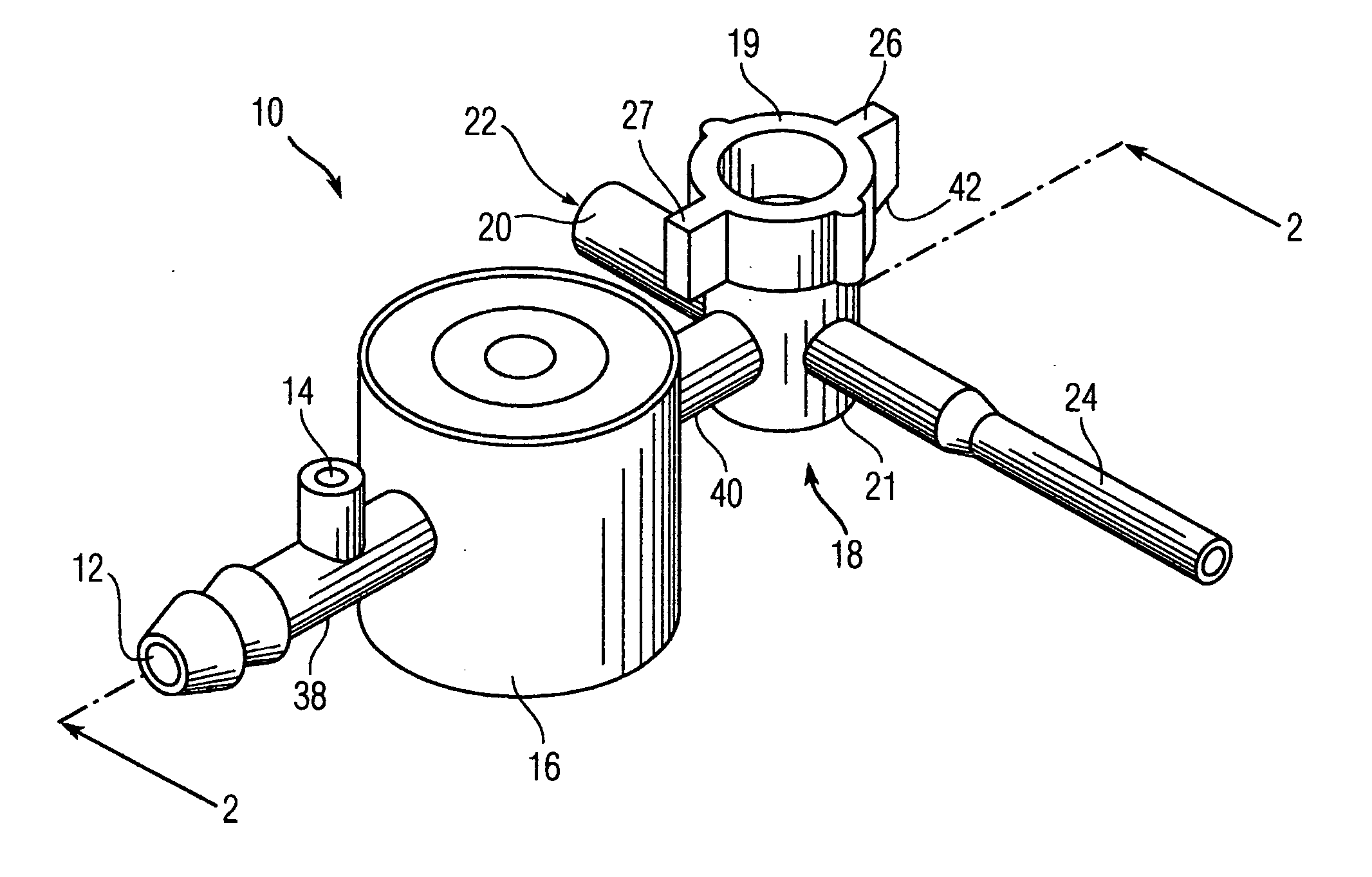
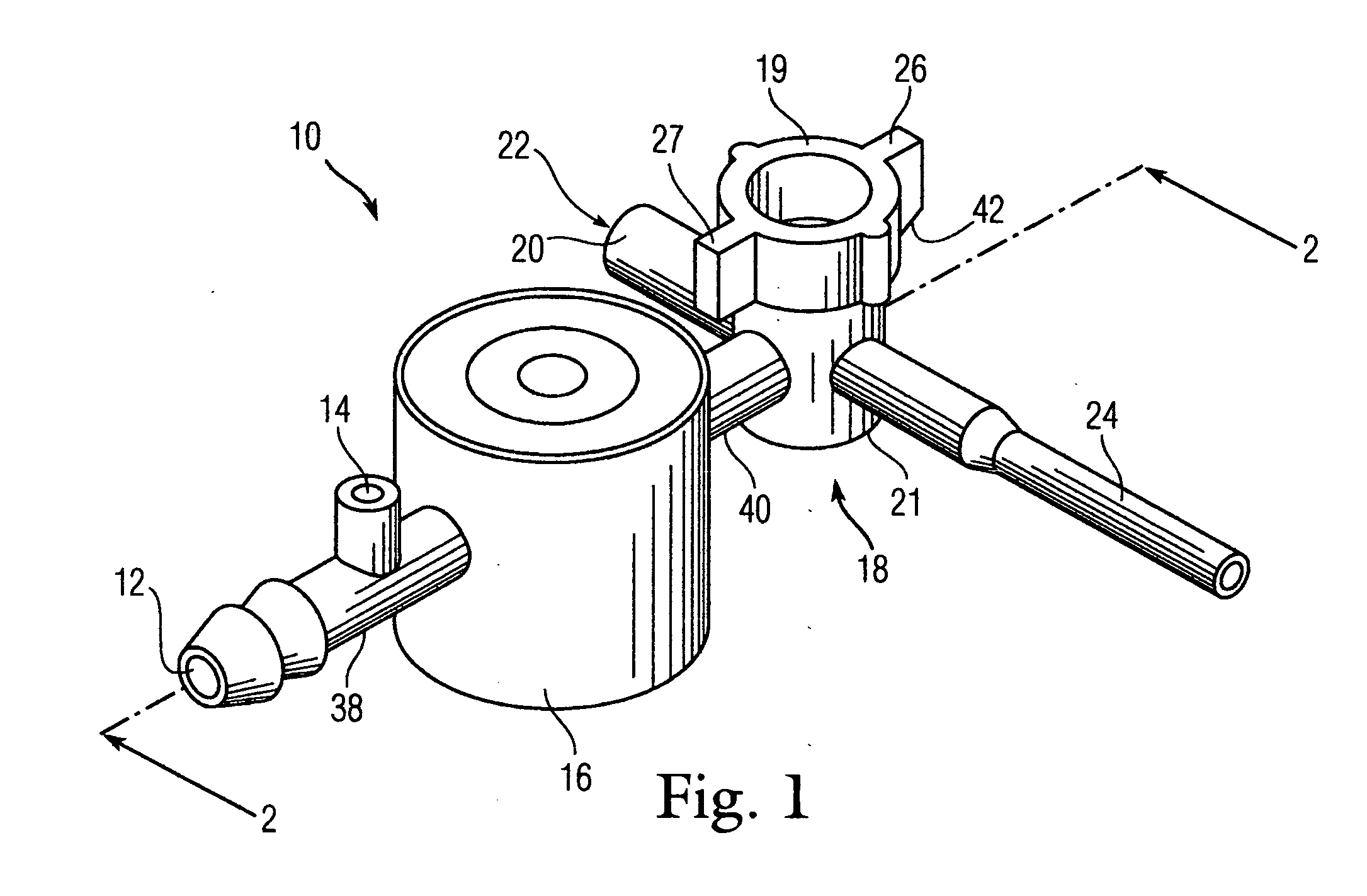
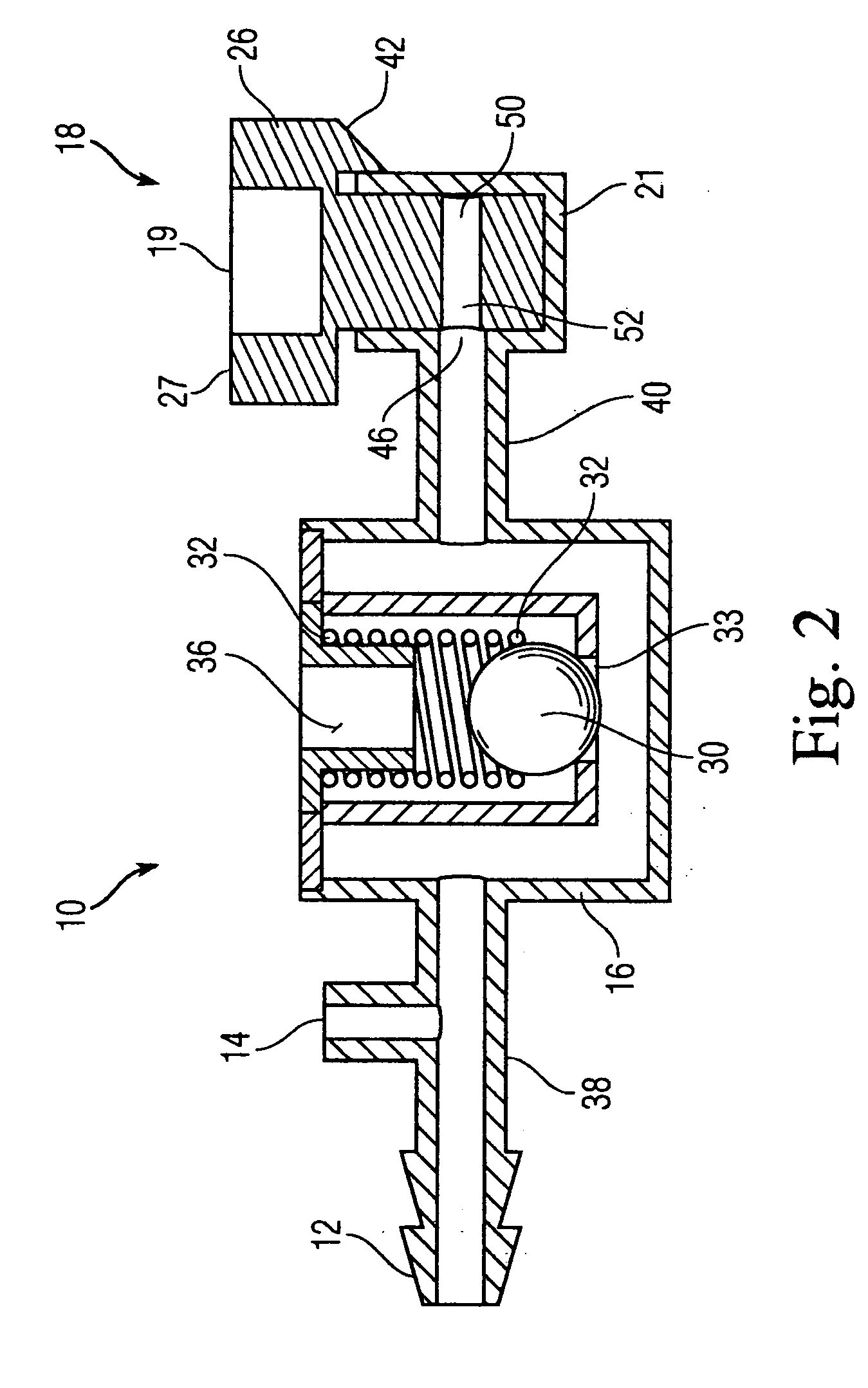
A catheter assembly configured for use in a non-bronchoscopic bronchoalveolar lavage procedure. The catheter assembly may include an inner catheter member having an inner catheter lumen and an outer catheter member having an outer catheter lumen, where the inner catheter member is disposed longitudinally and coaxially through at least a lengthwise portion of the outer catheter lumen, a distal end portion of the outer catheter includes an atraumatically-shaped disruptable seal—which seal includes at least a pair of overlapping slits that extend at least partially through an internal distal end wall portion of the outer catheter—and where a distal end portion of the inner catheter includes a wedging structure configured to at least partially sealingly contact an inner circumference of a passage in a lower portion of a patient lung.
Owner:CAREFUSION 2200 INC
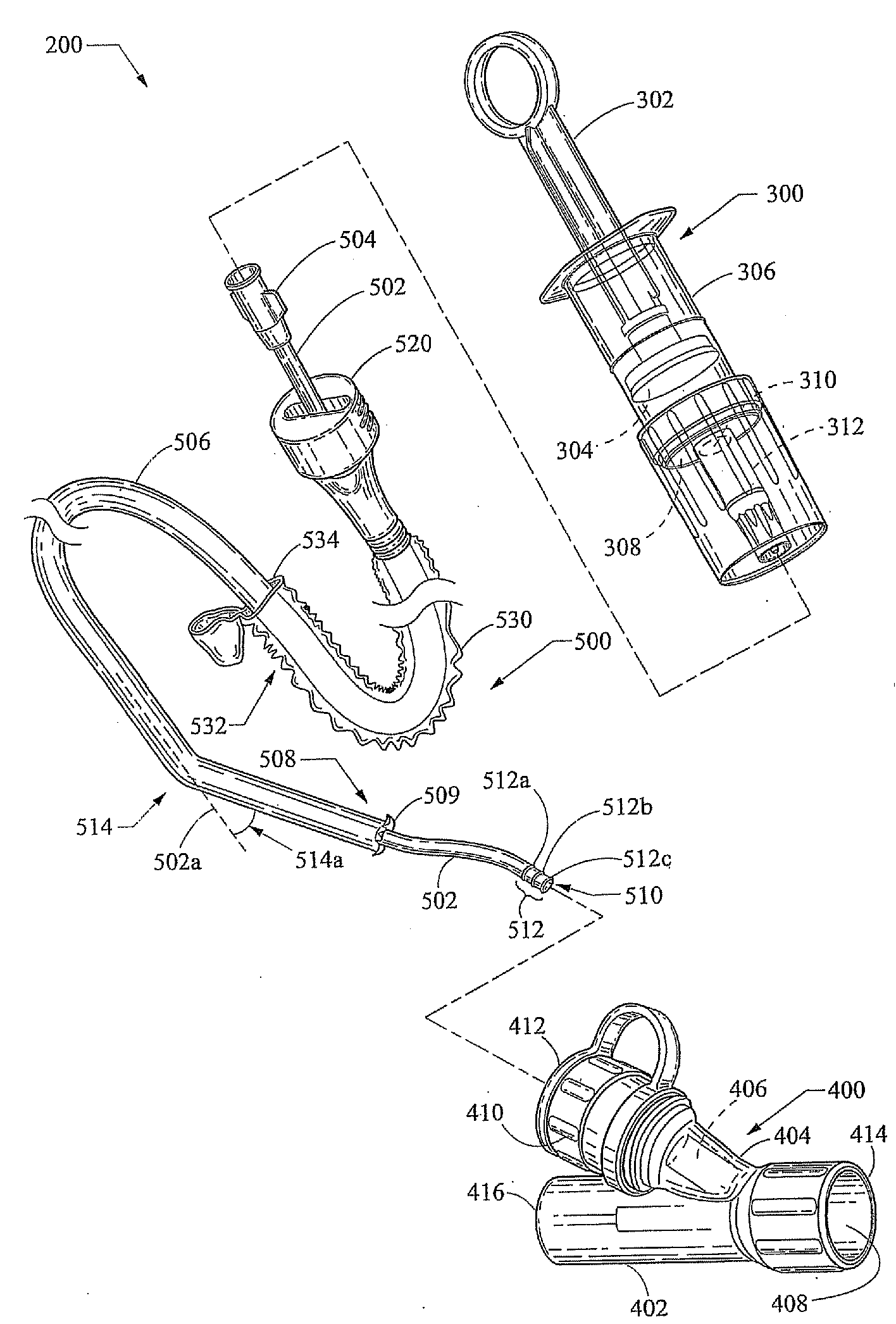
Diagnostic sample collection system and method of use
A bronchoalveolar lavage instillation / aspiration system which includes (i) a needleless bronchoalveolar lavage instillation / aspiration device with a barrel member including a barrel lumen having a distal aperture and a self-sealing member configured for maintaining a fluid-disruptable, resealable barrier to the distal aperture; and (ii) a catheter assembly configured to provide a patent path of fluid communication between the instillation / aspiration device and a patient bronchial passage, where the catheter includes a wedging structure configured to engage a patient bronchial passage. In another aspect, embodiments of the present invention may include one or more methods of making and using a system or component described herein.
Owner:CAREFUSION 2200 INC
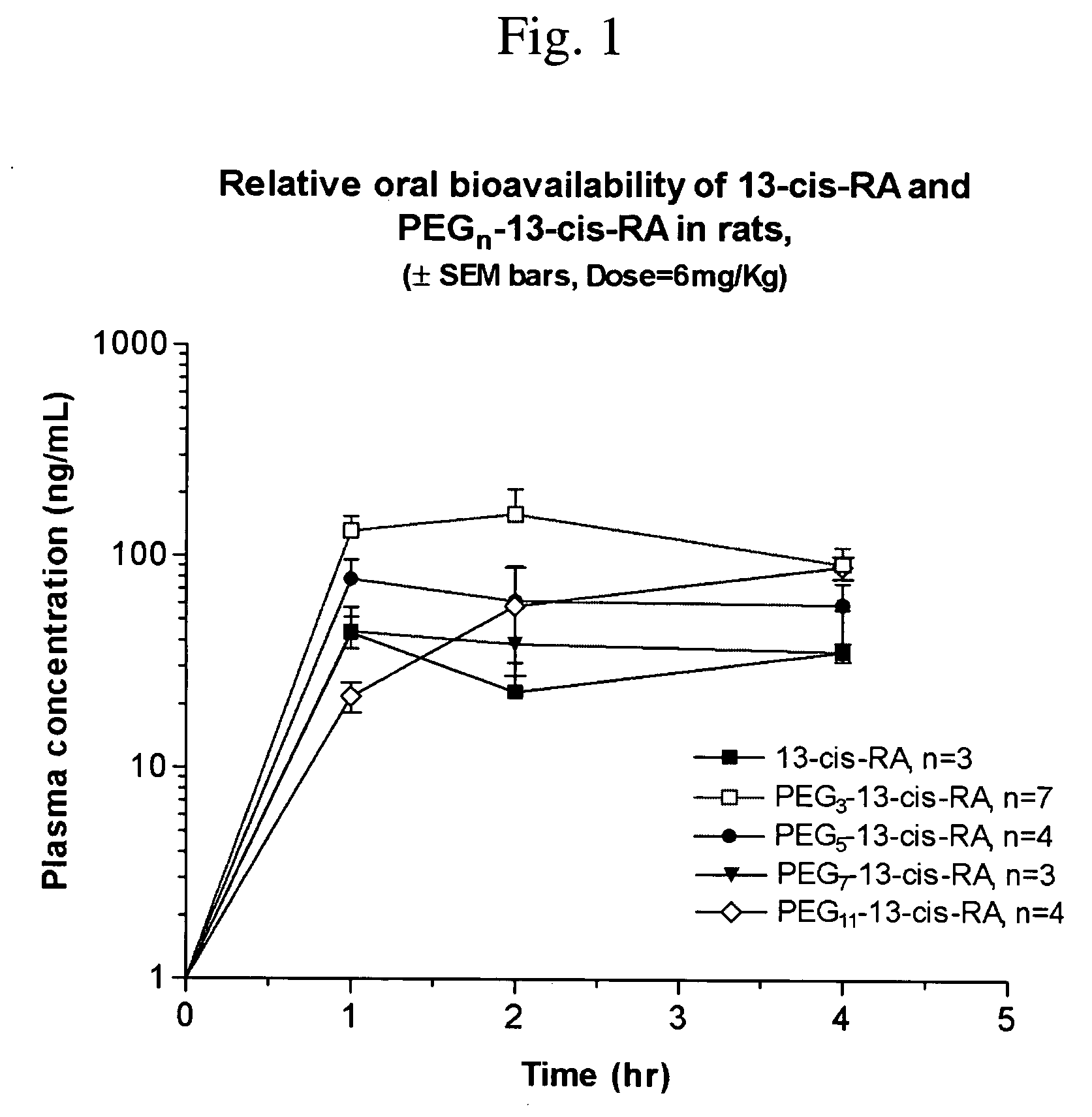
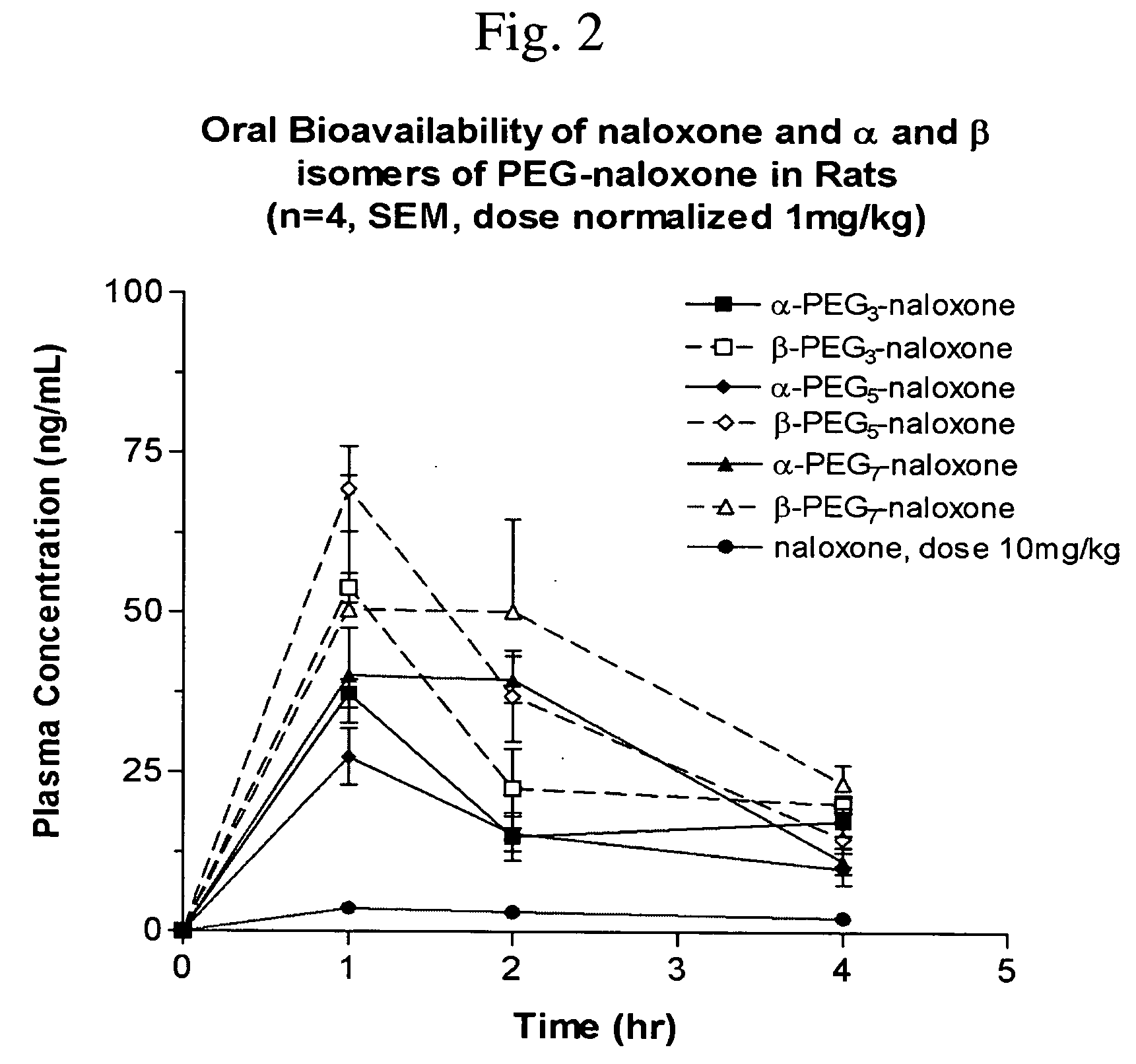
Chemically modified small molecules
Methods of modifying the rate of systemic absorption of a drug administered to a subject by a pulmonary route, the method comprising covalently conjugating a hydrophilic polymer to a drug, wherein the drug has a half-life of elimination from the lung of less than about 180 minutes, to form a drug-polymer conjugate, wherein the drug-polymer conjugate has a net hydrophilic character and a weight average molecular weight of from about 50 to about 20,000 Daltons, and wherein the half-life of elimination from the lung of the drug-polymer conjugate is at least about 1.5-fold greater than the half-life of elimination from the lung of the drug, wherein the half-life of elimination from the lung is measured by bronchoalveolar lavage followed by assaying residual lung material.
Owner:NEKTAR THERAPEUTICS INC
Bronchoscopy oxygenation system
A bronchoscopy oxygenation system having a channel for inserting alternately an instrument or fluids and for delivering oxygen to a patient. The system being provided with pressure relief vent and a pressure relief valve for the relief of excessive oxygen pressure. The bronchoscopy oxygenation system may be used during bronchoscopy and with patient suctioning, bronchoalveolar lavage or biopsy. The bronchoscopy oxygenation system is intended to be used with a conventional bronchoscope.
Owner:WILLEFORD KENNETH L
Method and kit for detecting mycobacterium tuberculosis and drug-resistant gene mutation thereof
ActiveCN101144099AImprove diffusivityIncrease concentrationMicrobiological testing/measurementFermentationResistant genesMicrobiology
The present invention relates to a method and a reagent kit which are used for detecting the existence of the mycobacterium tuberculosis in the clinical biological sample and the mutations of the drug-resistant genes, in particular to a method and a reagent kit which are used for quickly detecting the existence of the mycobacterium tuberculosis in the biological samples such as the clinical sputamentum, the bronchoalveolar lavage fluid, the blood, the ascites, and the cerebrospinal fluid, etc. with the reverse dot-blot hybridization technology.
Owner:GUANGZHOU DARUI BIOTECH
Leukocyte detecting method aiming at bronchoalveolar lavage smear
ActiveCN104156951ASimple and fast operationImprove detection efficiencyImage analysisSurgical needlesMicroscopic imageWhite blood cell
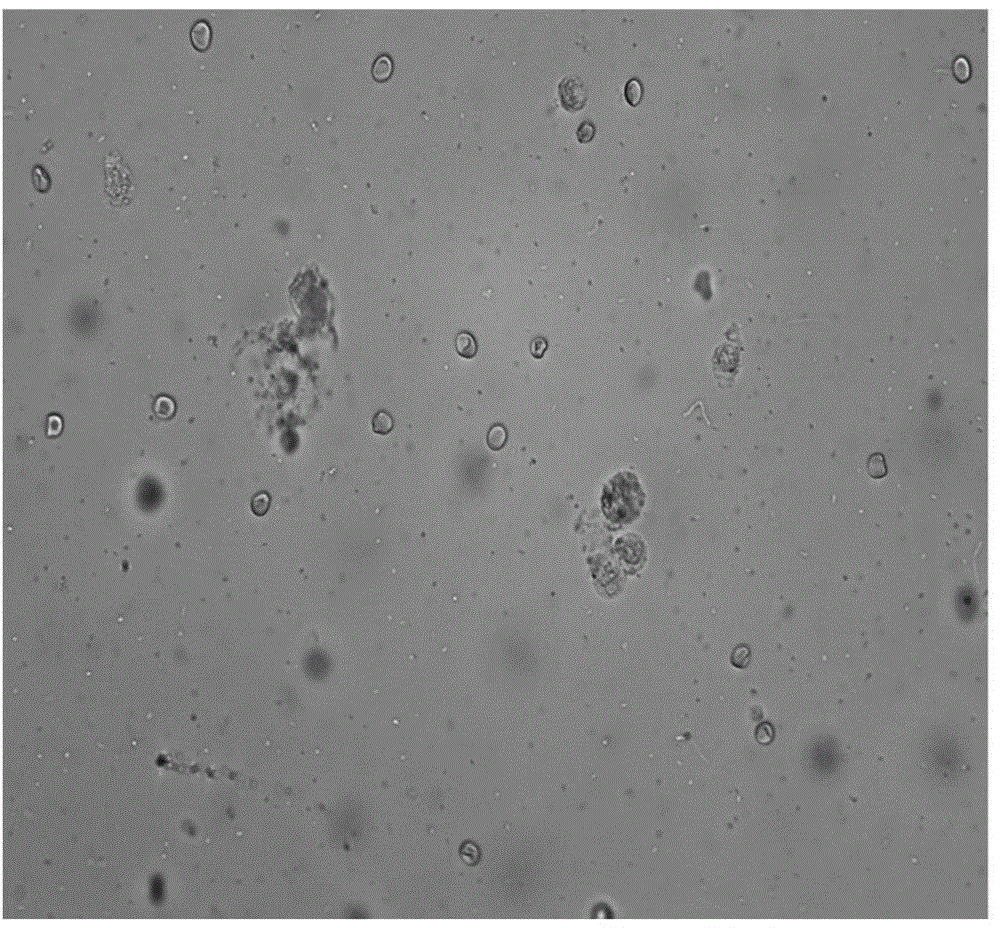
The invention discloses a leukocyte detecting method aiming at a bronchoalveolar lavage smear, and relates to an automatic detecting method aiming at leukocytes in the bronchoalveolar lavage smear, in particular to an automatic detecting method for leukocytes in the bronchoalveolar lavage smear on the basis of a digital image processing technique. According to the method, a bronchoalveolar lavage smear microscopic image collected by a microscope is subjected to graying and binarization processing, screening is performed by utilizing the profile features and internal features of leukocytes, and leukocytes are identified finally. Therefore, the method has the effects that the operation is simple and convenient, the detecting efficiency is high, the precision is high, the omission ratio and false-detection ratio are low, and the cost is low.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Bronchoscopy oxygenation system
A bronchoscopy oxygenation system having a channel for inserting alternately an instrument or fluids and for delivering oxygen to a patient. The system being provided with pressure relief vent and a pressure relief valve for the relief of excessive oxygen pressure. The bronchoscopy oxygenation system may be used during bronchoscopy and with patient suctioning, bronchoalveolar lavage or biopsy. The bronchoscopy oxygenation system is intended to be used with a conventional bronchoscope.
Owner:WILLEFORD KENNETH L
Nucleic acid detection kit for novel coronavirus COVID-19 and use method thereof
ActiveCN111118228AImprove accuracyShorten the timeMicrobiological testing/measurementMicroorganism based processesDifferential diagnosisCoronavirus
The invention relates to a nucleic acid detection kit for novel coronavirus COVID-19. The kit comprises a first primer pair and a first probe which correspond to Cov-n, and a second primer pair and asecond probe which correspond to Cov-ORF1ab. The kit disclosed by the invention can be used for simultaneously carry out multiple-gene and multiple-site detection on the novel coronavirus, so that thedetection accuracy is improved; and in addition, when the multiple fluorescence PCR reaction is carried out, novel coronavirus detection information can be provided through one-time detection within70 minutes, so that the diagnosis time of the novel coronavirus COVID-19 is shortened. The kit can be used for in vitro qualitatively detecting throat swabs, sputum and bronchoalveolar lavage fluid samples of suspected pneumonia cases infected by the novel coronavirus, suspected aggregated case patients, and other diagnosis people who are required to perform novel coronavirus infection diagnosis or identification.
Owner:HEMOSMART MEDICAL TECH LTD
Medical systems and methods
A medical device for performing a bronchoalveolar lavage (BAL) that includes a tubing having a first lumen configured for communication with a first reservoir, the first lumen having an open distal end configured for communication with the first reservoir, and a second lumen that is separate from the first lumen and configured for communication with a second reservoir. The tubing also includes an inflatable cuff that is disposed proximate the open distal end and configured for communication with the second reservoir via the second lumen, wherein the open distal end and the inflatable cuff in a deflated position are both configured for receipt within a working channel of a bronchoscope.
Owner:GHOSH SOMNATH
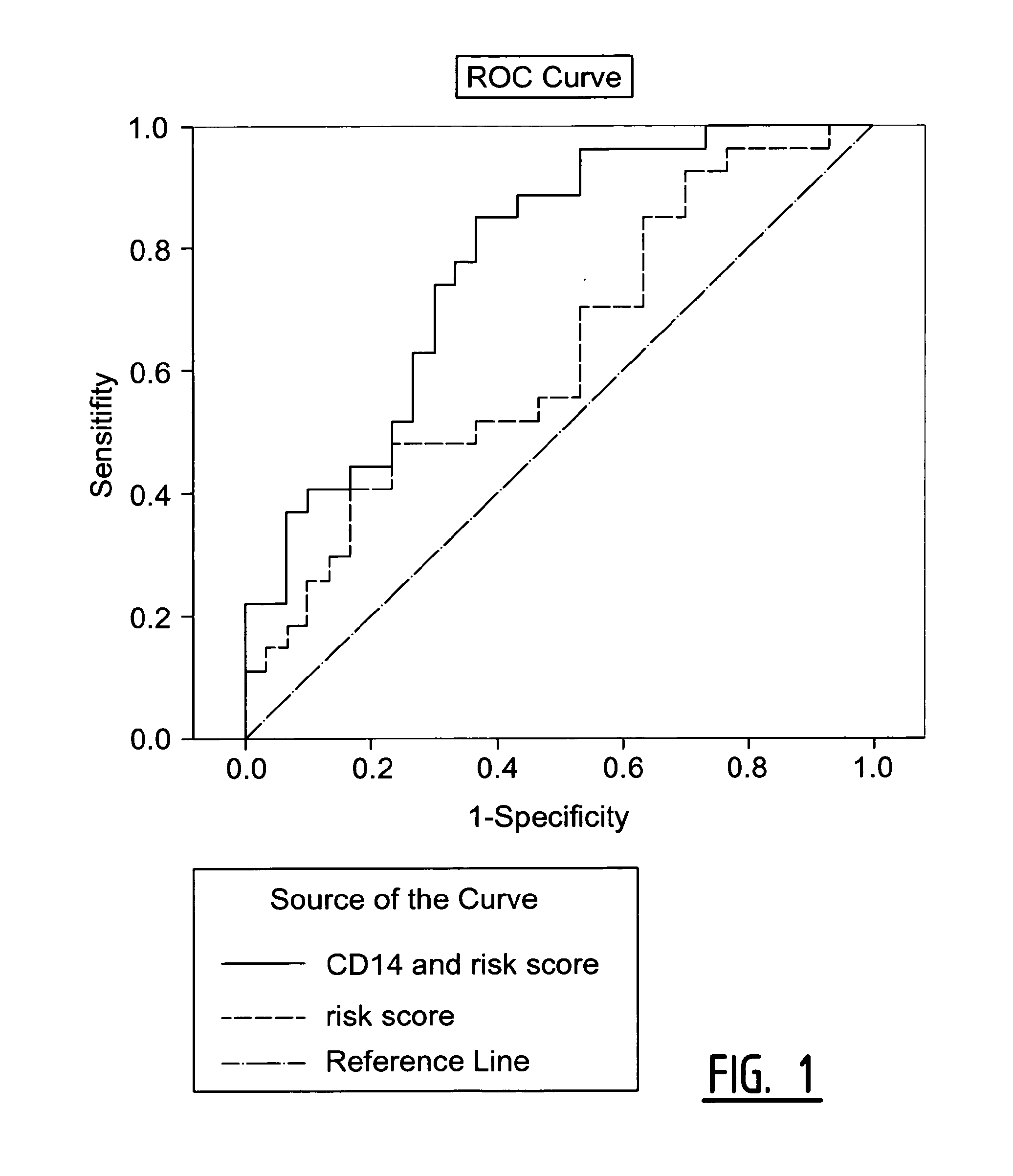
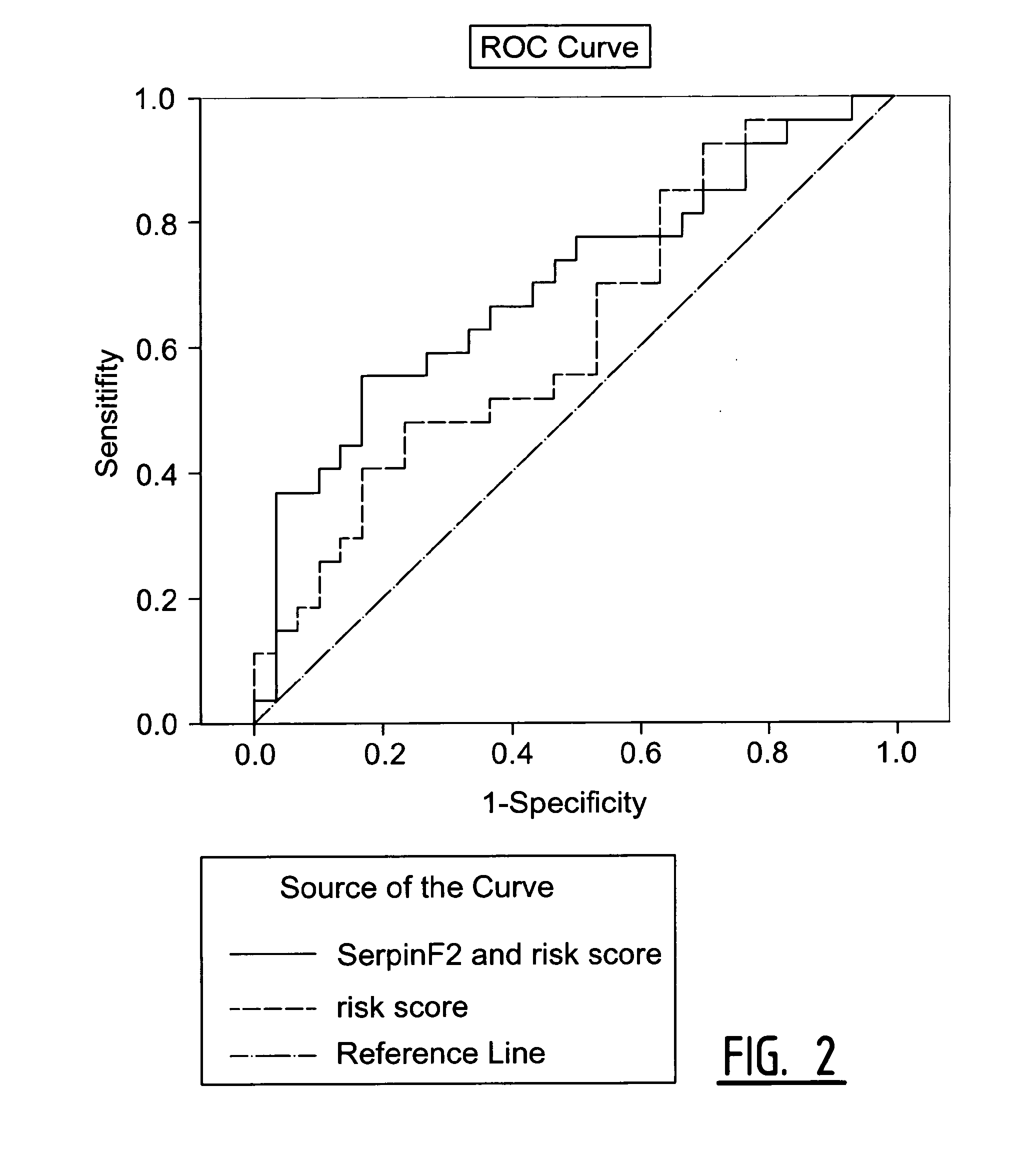
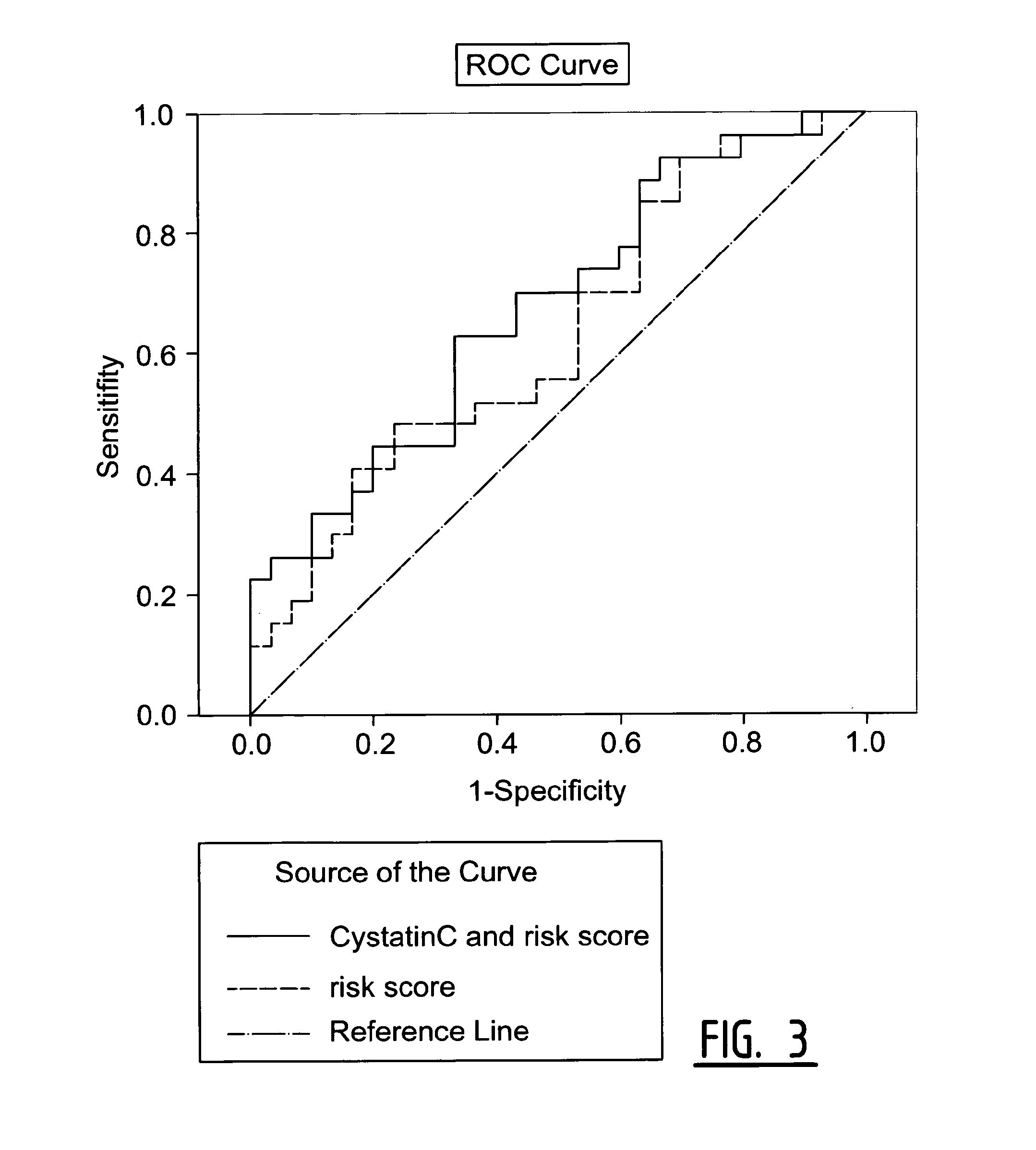
Determination of Exosomel Biomarkers for Predicting Cardiovascular Events
InactiveUS20120309041A1Reduce stepsComplicate to detectMicrobiological testing/measurementImmunoglobulins against animals/humansAmniotic fluidNGAL Protein
The present invention relates to a method of predicting the risk of a subject developing a cardiovascular event, comprising determining the presence of a biomarker that is indicative of the risk of developing a cardiovascular event in exosomes from the subject. The exosomes are suitably isolated from a body fluid selected from serum, plasma, blood, urine, amniotic fluid, malignant ascites, bronchoalveolar lavage fluid, synovial fluid, breast milk, saliva, in particular serum. The biomarker is selected from the proteins Vitronectin, Serpin F2, CD14, Cystatin C, Plasminogen, Nidogen 2 or any combination of two or more of these proteins.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
LAMP (loop-mediated isothermal amplification) kit for detection of mycoplasma pneumoniae and special LAMP primer for detection of mycoplasma pneumoniae
InactiveCN105238860AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesLoop-mediated isothermal amplificationPneumonitis
The invention discloses a LAMP (loop-mediated isothermal amplification) kit for detection of mycoplasma pneumoniae and a special LAMP primer for detection of mycoplasma pneumoniae. The special LAMP primer for detection of mycoplasma pneumoniae is designed according to a specificity conservative target sequence of a mycoplasma pneumoniae P1 gene (GenBank number: CP002077.1). The LAMP primer is formed by six primers including outer primers MP-16F3 and MP-16B3, inner primers MP-16FIP and MP-16BIP and loop primers MP-16LF and MP-16LB. By the aid of the LAMP kit and the special LAMP primer for detection of mycoplasma pneumoniae, quickness, convenience, high efficiency, high specificity and high sensitivity in qualitative detection of the mycoplasma pneumoniae in samples of pure bacteria, sputum, bronchoalveolar lavage fluid, throat swabs and the like can be realized without complicated instruments, and a new technical platform is provided for detection of the mycoplasma pneumoniae.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION +1
Application of sinomenine or pharmaceutically acceptable salt thereof as medicament for preventing and treating pulmonary interstitial fibrosis
ActiveCN102579445AAvoid generatingAvoid developmentOrganic active ingredientsAntipyreticHydroxyprolineAdjuvant
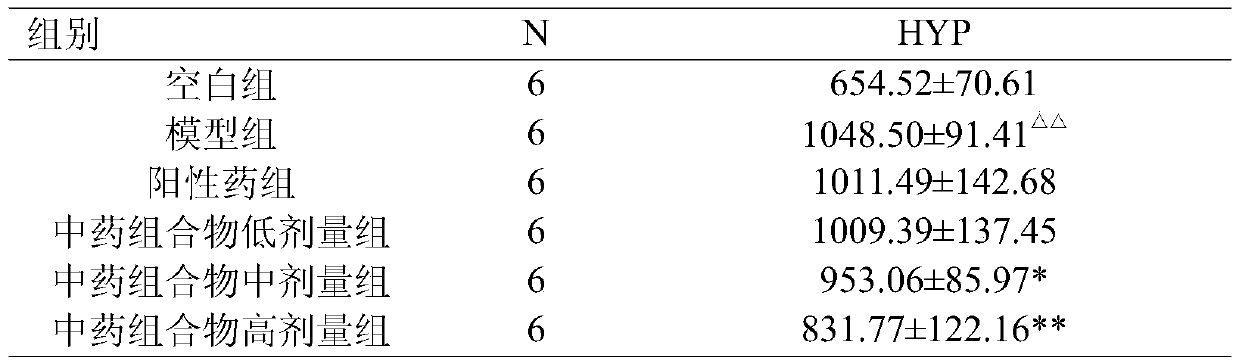
The invention relates to application of sinomenine as a medicament for preventing and treating pulmonary interstitial fibrosis. The medicament is prepared by sinomenine or pharmaceutically acceptable salt thereof and other adjuvants, wherein sinomenine or pharmaceutically acceptable salt thereof serves as an activating agent. The routes of administration of the medicament include intravenous drip, intramuscular injection, oral administration, transdermal absorption, atomization inhalation and bronchoalveolar lavage. Experiments show that sinomenine does not have obvious difference with the dexamethasone control group in the effect on inhibiting formation of bleomycin-induced pulmonary fibrosis in mice and prove that sinomenine can alleviate pulmonary alveolitis and fibrosis degrees of themice with bleomycin-induced pulmonary fibrosis and the mechanisms are probably realized by inhibiting expressions of TGF (transforming growth factor)-beta1 and alpha-SMA (smooth muscle actin) and thecontent of HYP (hydroxyproline).
Owner:李蕴麟
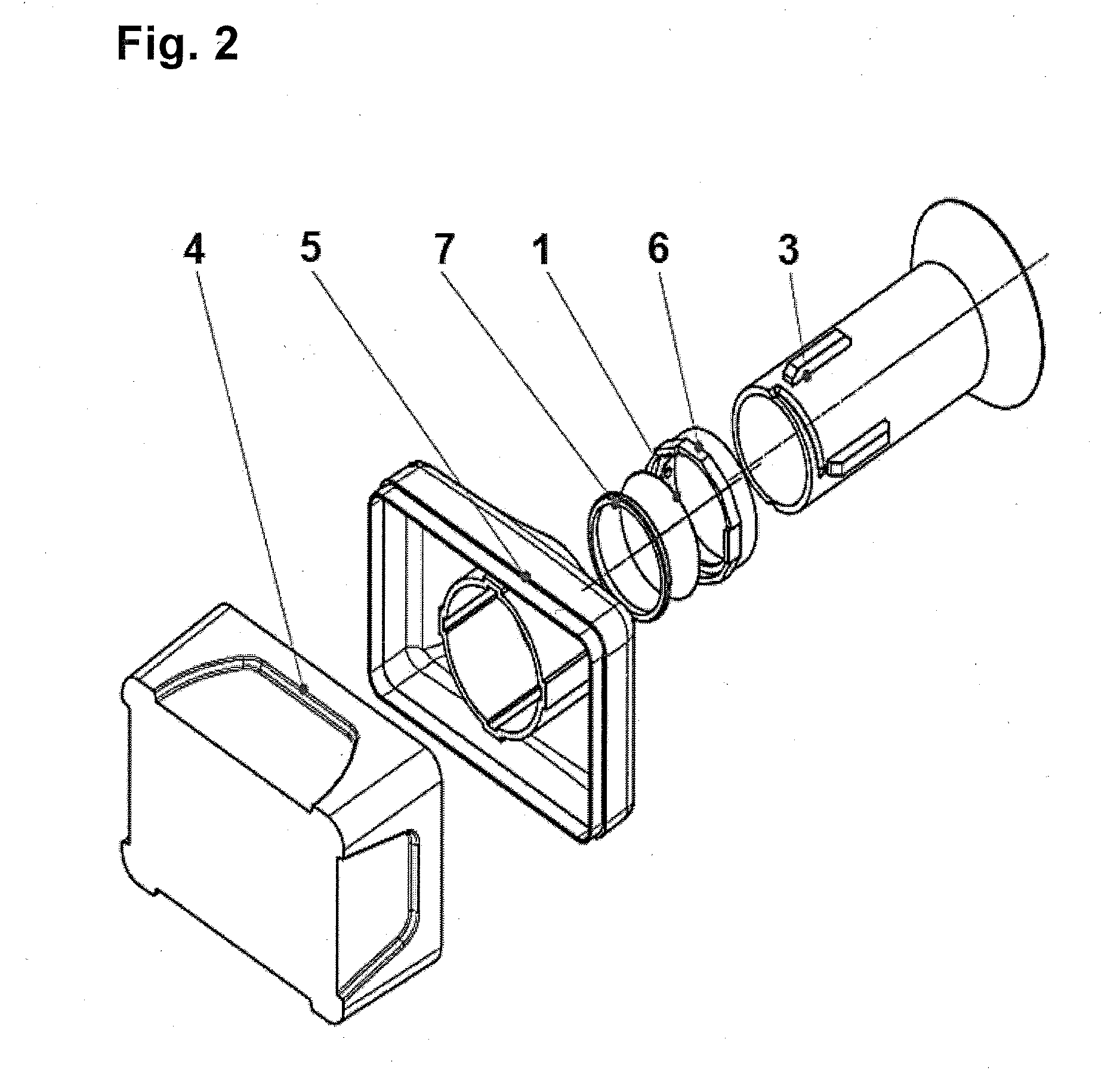
Diagnostic sample collection system
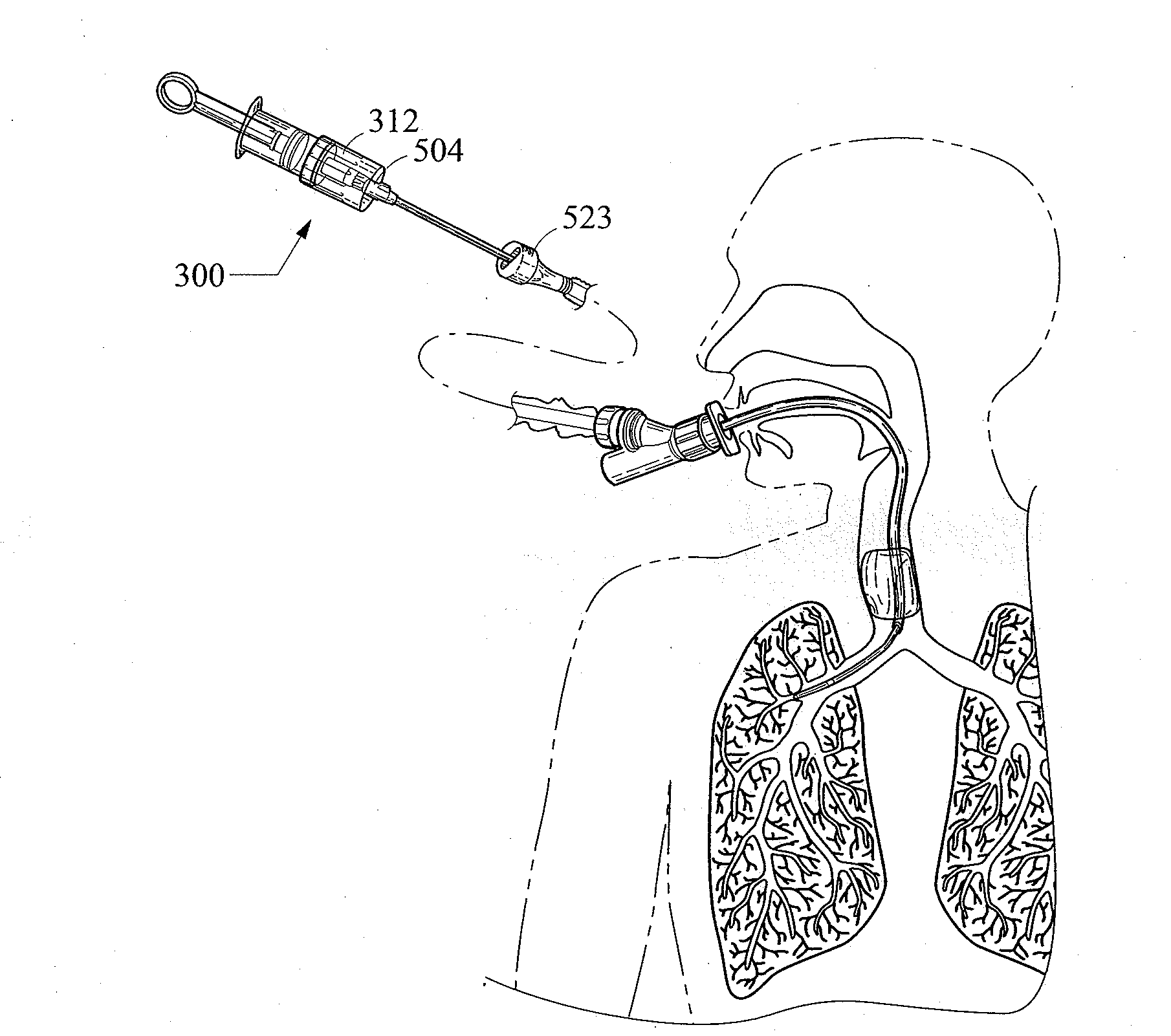
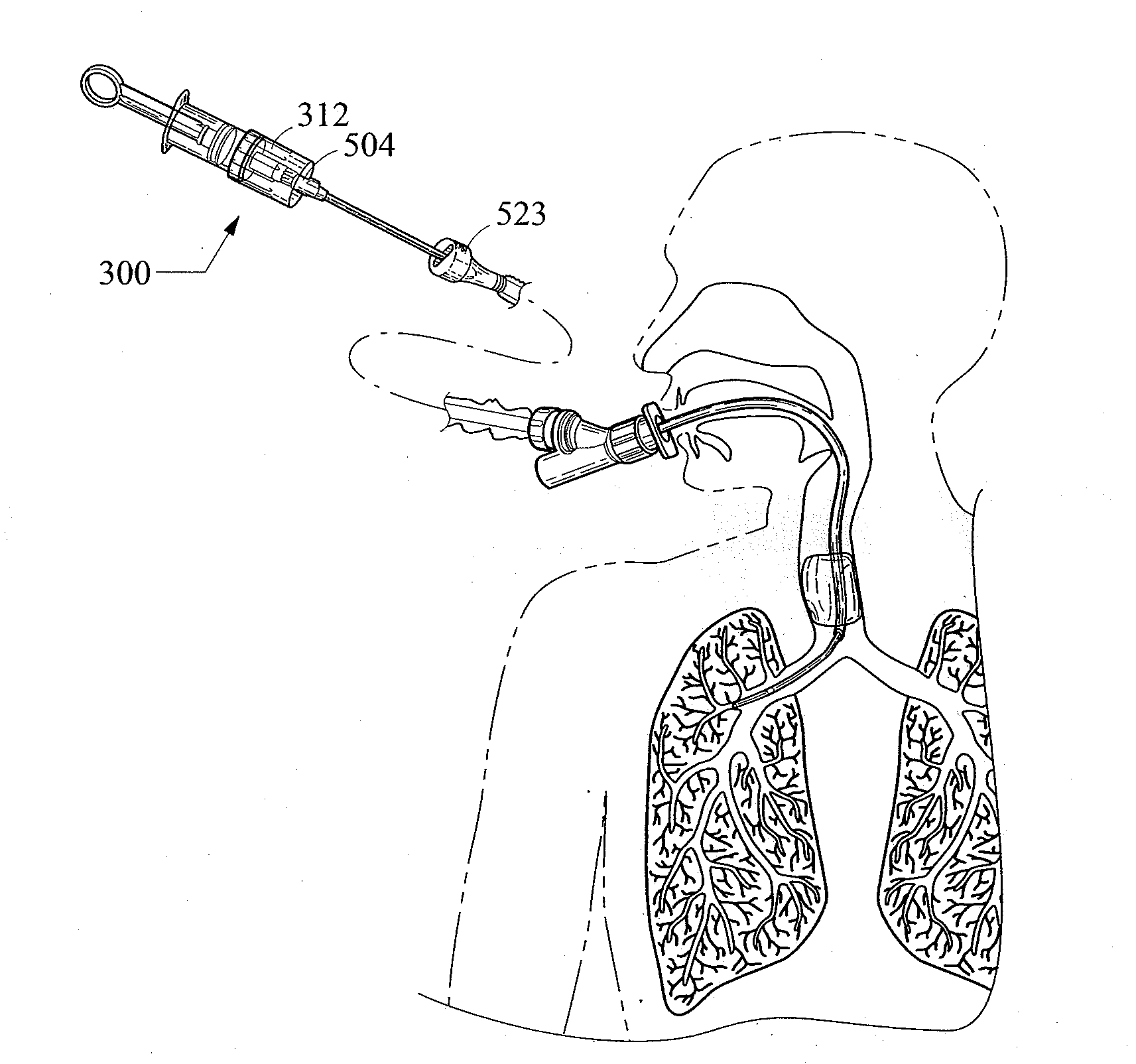
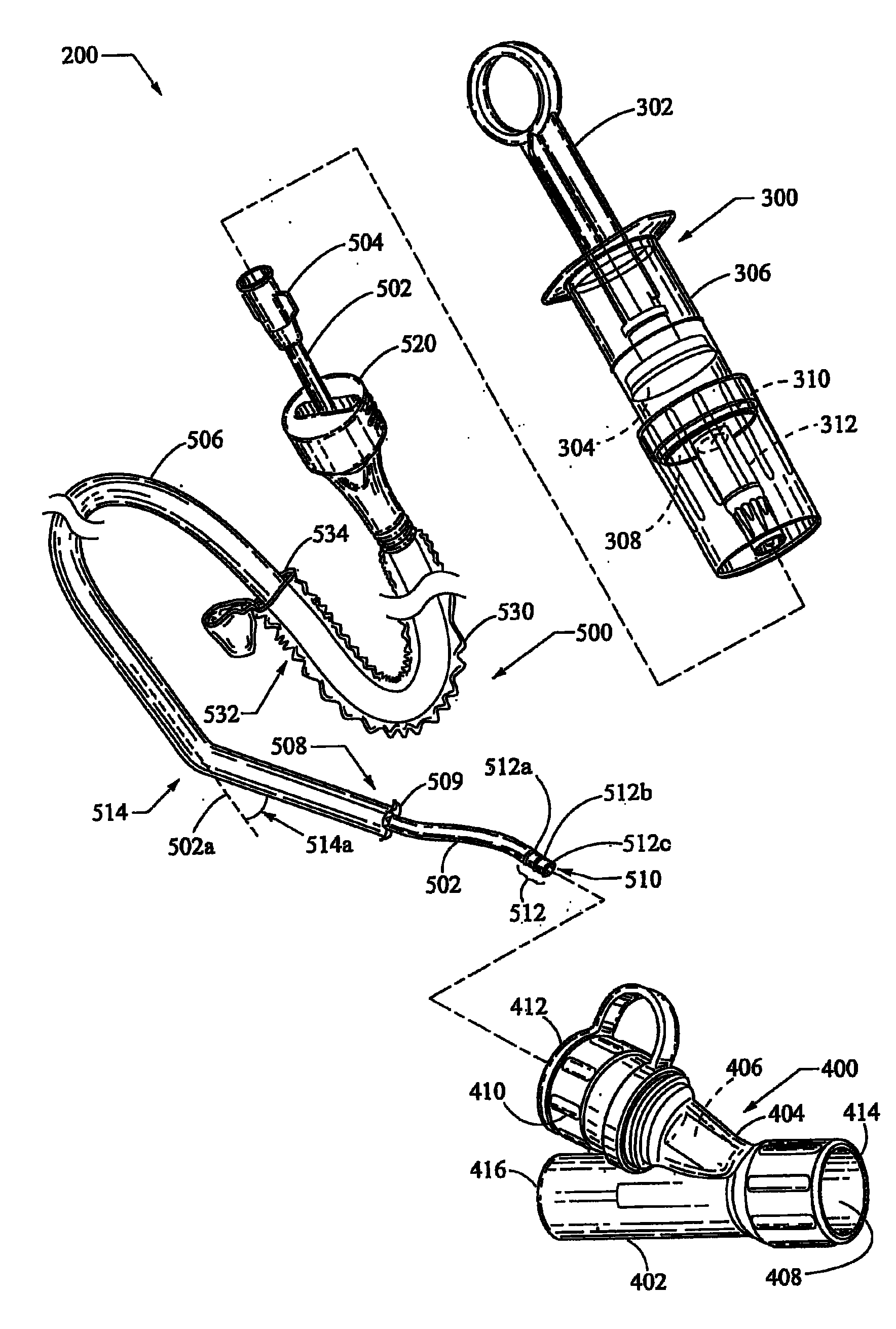
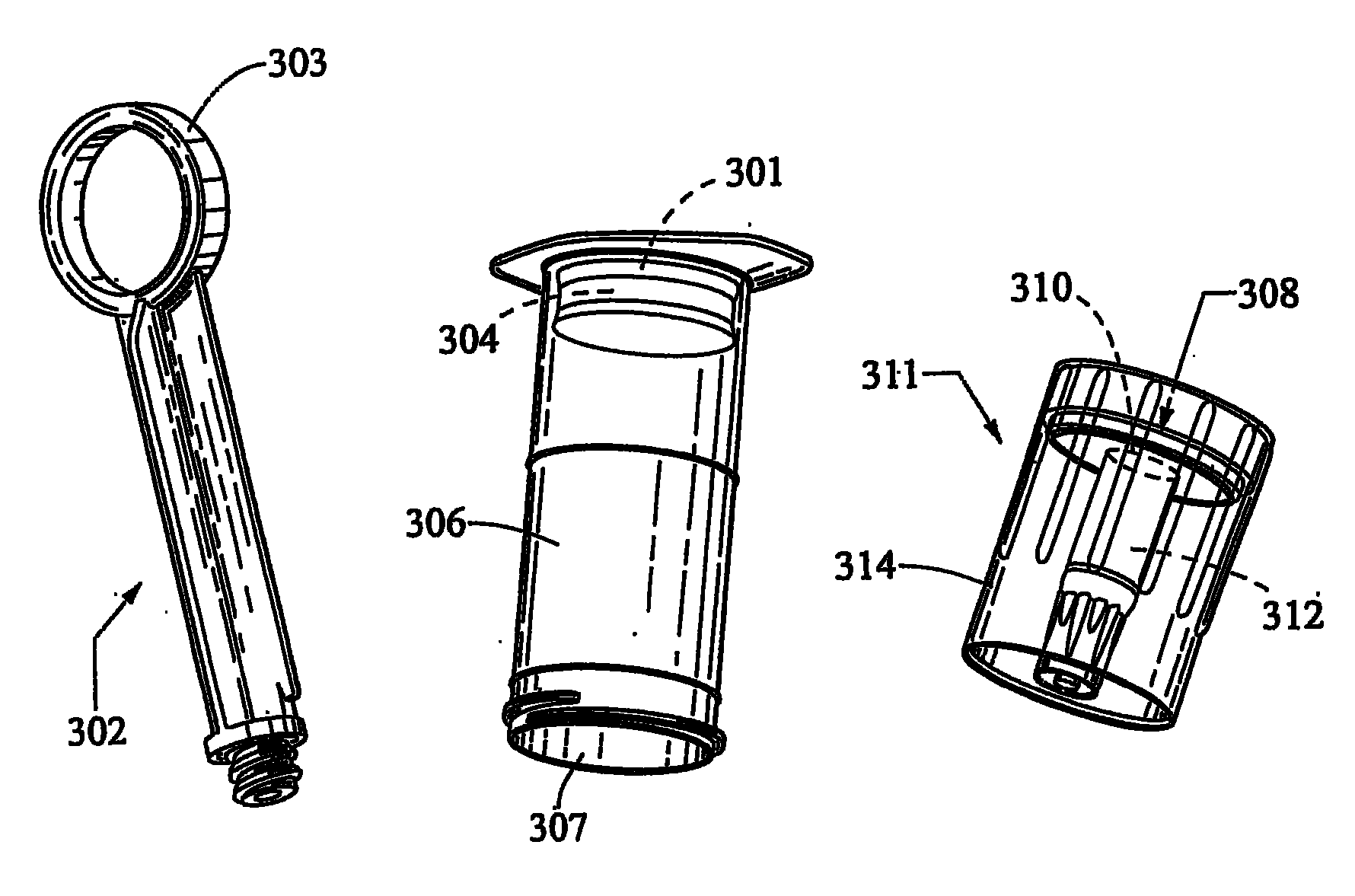
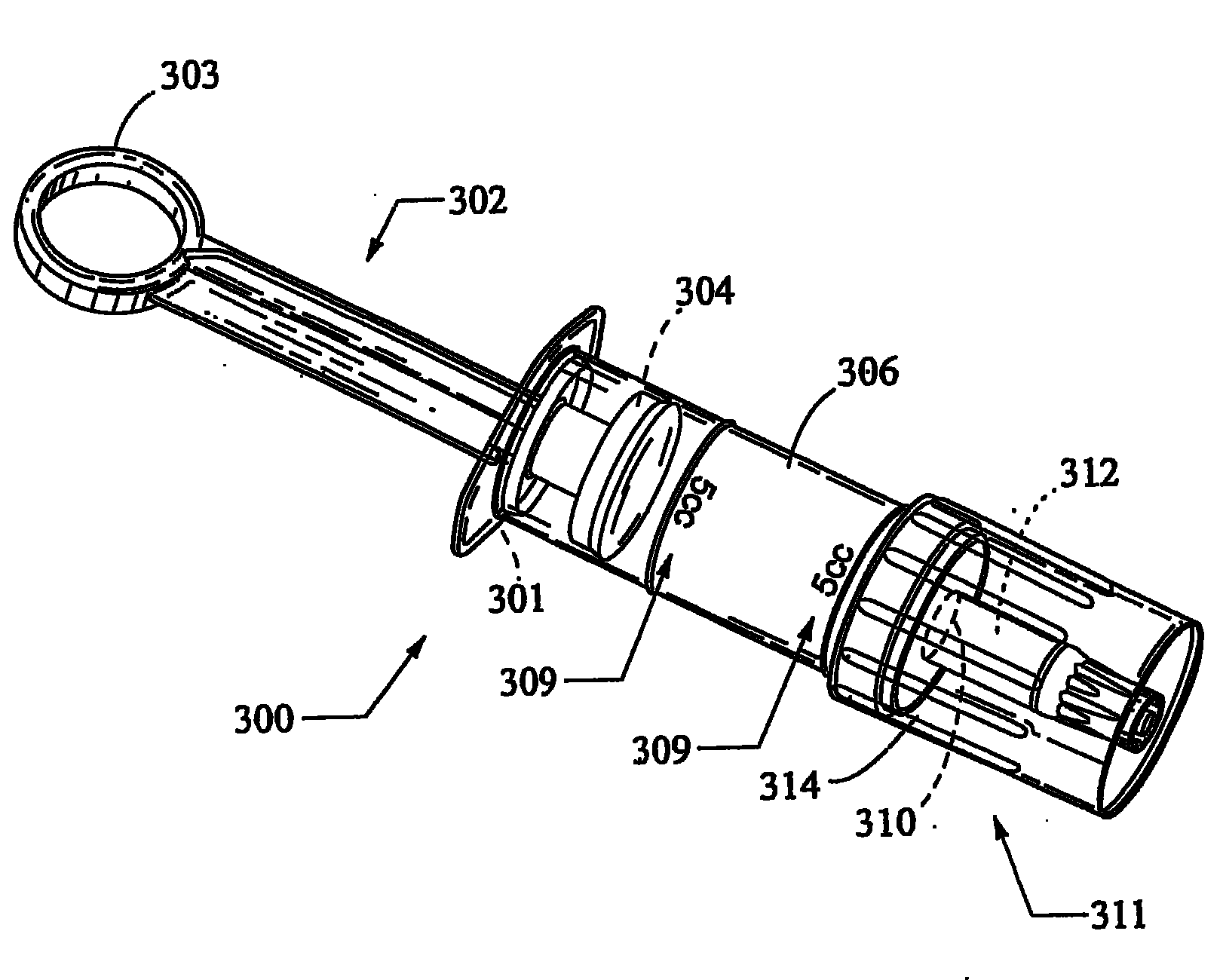
A bronchoalveolar lavage instillation / aspiration system (200) which includes (i) a needleless bronchoalveolar lavage instillation / aspiration device (300) with a barrel member (306) including a barrel lumen (307) having a distal aperture (310) and a self-sealing member (312) configured for maintaining a f luid-disruptable, resealable barrier to the distal aperture (310); and (ii) a catheter asssembly (500) configured to provide a patent path of fluid communication between the instillation / aspiration device (300) and a patient bronchial passage, where the catheter (502) includes a wedging structure (512) configured to engage a patient bronchial passage. In another aspect, embodiments of the present invention may include one or more methods of making and using a system or component described herein.
Owner:CAREFUSION 2200 INC
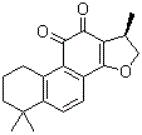
Cryptotanshinone for preventing and treating pulmonary fibrosis and application thereof
InactiveCN106798737AAntibacterialAnti-inflammatoryOrganic active ingredientsRespiratory disorderDiseaseSalvia miltiorrhiza
The invention relates to the technical field of medicines, and particularly relates to cryptotanshinone for preventing and treating pulmonary fibrosis and an application thereof. The cryptotanshinone (I) is quinone diterpene extracted from the root part of salviae miltiorrhizae; the modern pharmacological research shows that the cryptotanshinone has a bacteriostatic effect, an anti-inflammatory effect and a hormone-like pharmacologic effect, and is clinically used for treating myocardial fibrosis, lung acute injury and arthritis, can be used for preventing and / or treating a pulmonary fibrosis disease, can also be used for preparing medicines for reducing body weight of a pulmonary fibrosis rat, a lung coefficient and the content of hydroxyproline in tissue and can be used for preparing the medicines for reducing the content of IL-1beta, IL-6 and TNF-alpha in a bronchoalveolar lavage liquid and the medicines of effecting the pathogeny structure of pulmonary fibrosis tissue of the rat. The formula (I) is as shown in the specification.
Owner:SUN YAT SEN UNIV +1
Application of eupatorium sesquiterpene component in preparing drug for resisting acute lung injury
ActiveCN104161783AOrganic active ingredientsRespiratory disorderInterleukin 6Tumor necrosis factor alpha
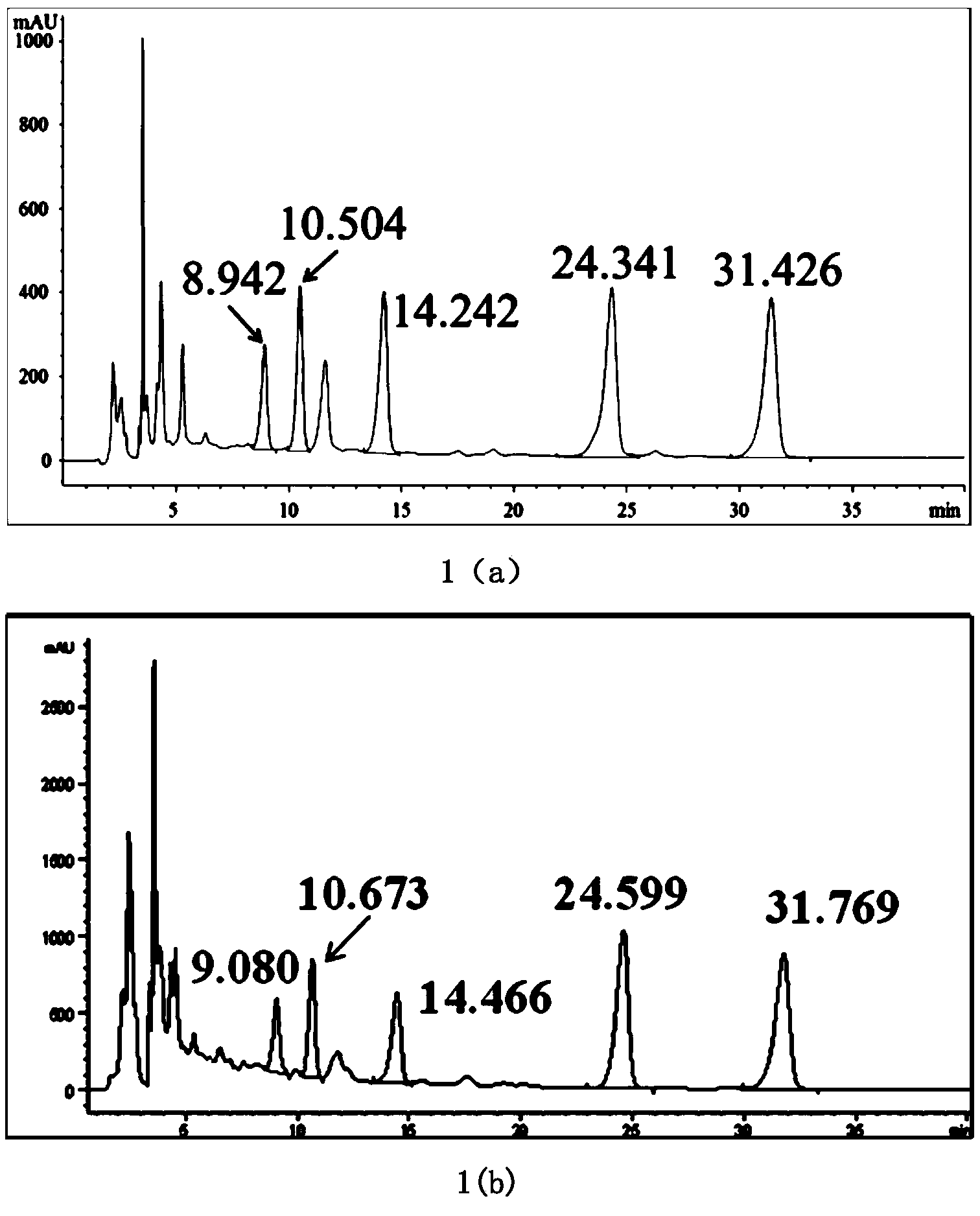
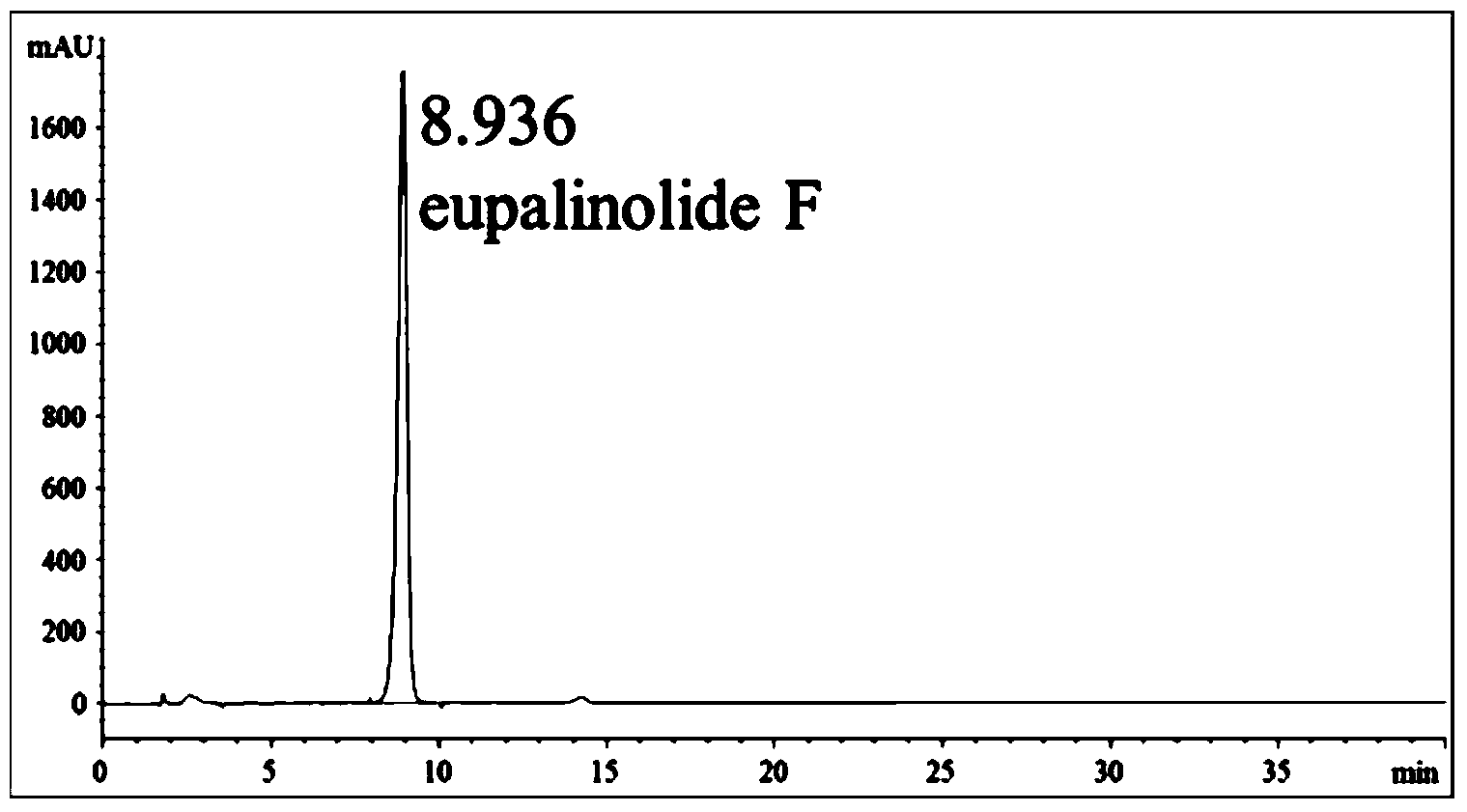
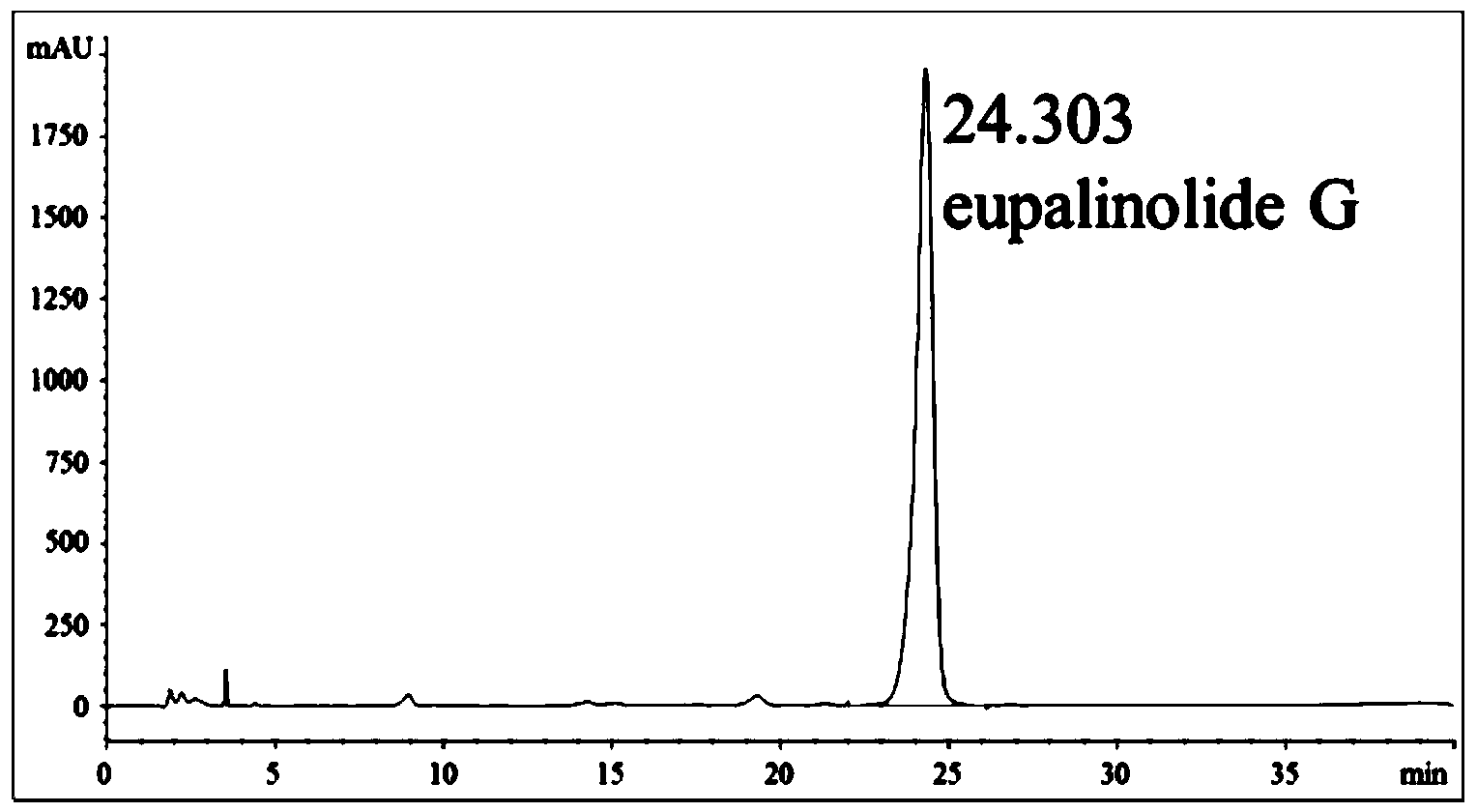
The invention discloses an application of eupatorium sesquiterpene components in preparing a drug for resisting acute lung injury. Eupatorium sesquiterpene components are made into a veterinary drug. A technical solution provided by the invention comprises the steps of making the eupatorium sesquiterpene componentsinto a veterinary drug; inducing acute lung injury in mice by using lipopolysaccharide (LPS) in a manner of in-vivo dosing to the mice; then determining a lung wet / dry ratio of the mice with the acute lung injury, the content of nitric oxide (NO) and protein in a bronchoalveolar lavage fluid (BALF); detecting activities of myeloperoxidase (MPO) and superoxide dismutase (SOD) in lung tissue homogenate, the content of tumor necrosis factor-alpha (TN-alpha), interleukin-6 (IL-6) and interleukin-1[beta] (IL-1[beta]) in the BALF and the content of complement C3 and C3c in blood serum; and observing pathological changes of lung tissues of the mice after H&E staining. Results show that eupatorium sesquiterpene components of eupalinolide F, eupalinolide G, eupalinolide H, eupalinolide I and eupalinolide K have activity for resisting the acute lung injury.
Owner:SUZHOU UNIV
Method for separation of sporadic cells from body fluids, and apparatus for carrying out said method
InactiveUS20160122704A1Increase success rateAvoid clotsBioreactor/fermenter combinationsBiological substance pretreatmentsPresent methodAmniotic fluid
Method for gentle separation of viable sporadic cells from body fluids such as blood, from malignant effusions, bronchoalveolar lavage fluid, peritoneal lavage fluid and amniotic fluid, based on a filter membrane which is in an intimate contact with an absorbent material. Using the present method it is possible to isolate for example circulating and disseminated tumor cells, endometrial cells and circulating trophoblast cells, allowing subsequent detection, quantification, characterization and especially culturing of said cells. An apparatus for carrying out the method is further disclosed.
Owner:METACELL
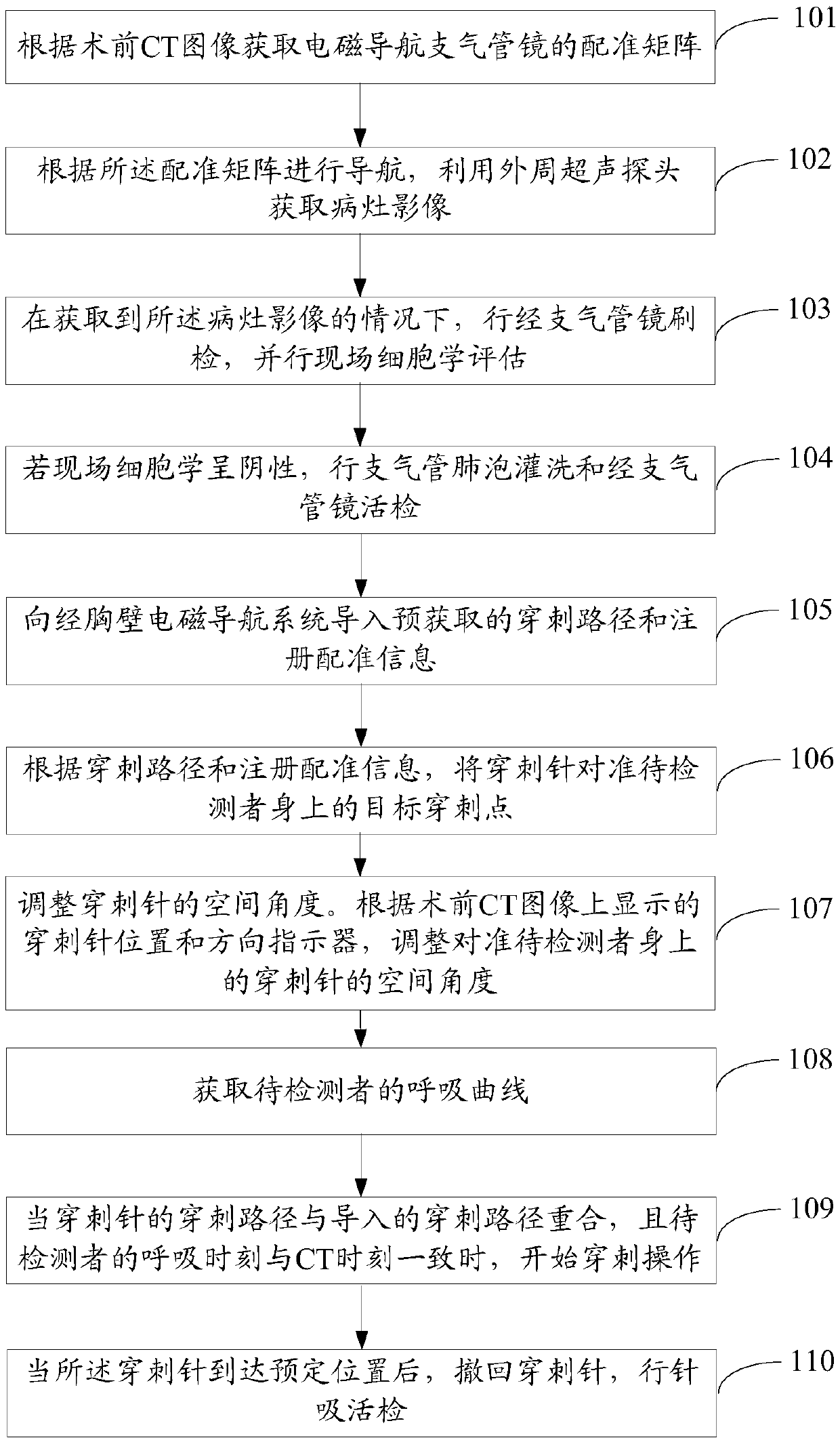
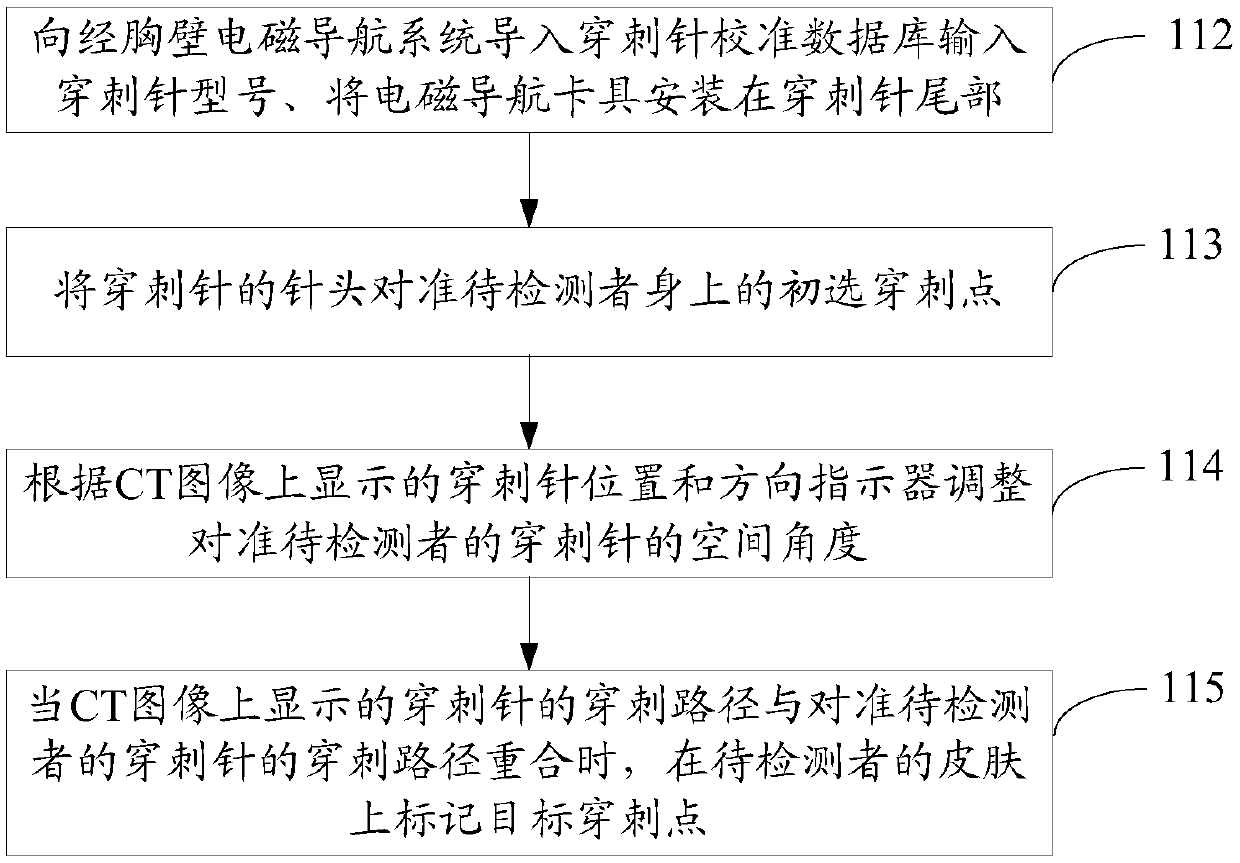
Auxiliary lung diagnosis method and device
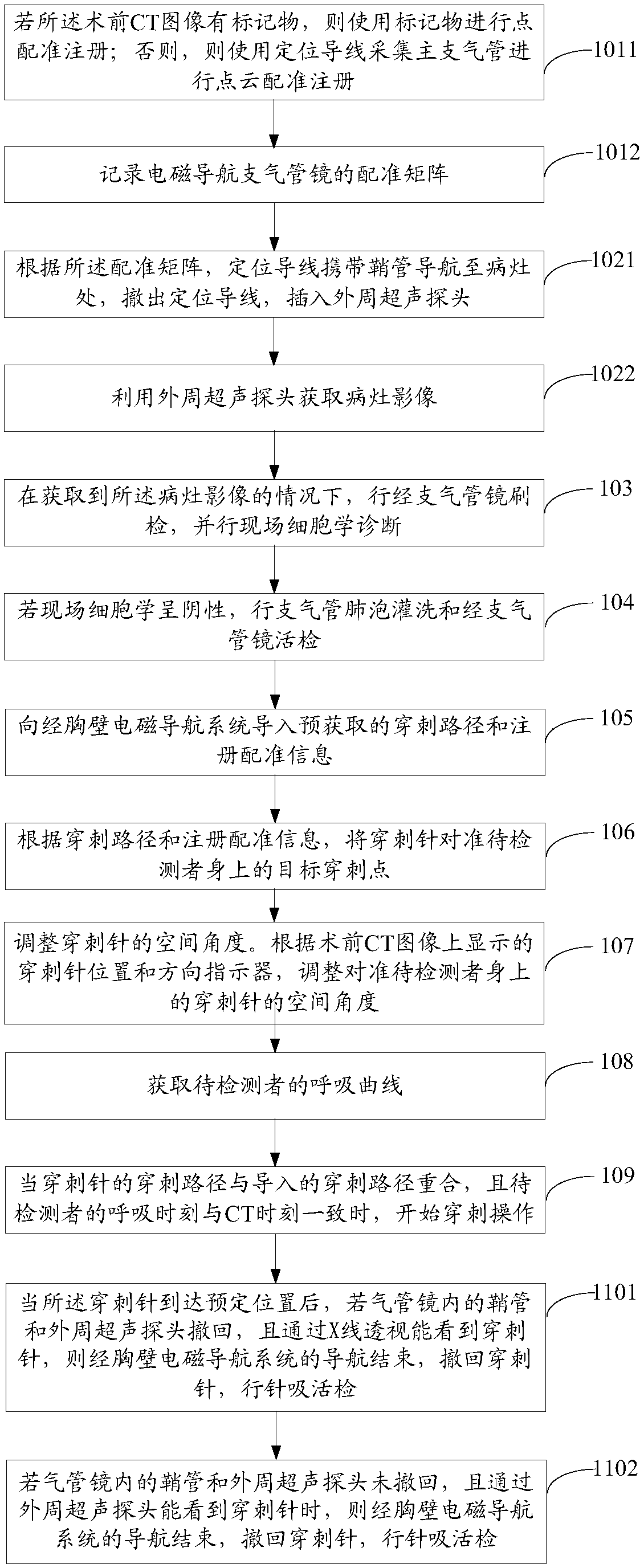
ActiveCN109620303AShorten the timeReduce riskSurgical needlesVaccination/ovulation diagnosticsPuncture BiopsyBronchial tube
The embodiment of the invention provides an auxiliary lung diagnosis method and device. The method comprises the following steps of acquiring a rectification matrix of an electromagnetic navigation bronchoscope according to a preoperative CT image; using a peripheral ultrasonic probe for acquiring a lesion image; performing on-site cytological evaluation under the condition that the lesion image is acquired; if an on-site cytology result is negative, conducting bronchoalveolar lavage and a trans-bronchoscope biopsy; inputting a puncture path and registration information to a trans-chest-wall electromagnetic navigation system; directing a puncture needle at a target puncture point on a person to be detected; adjusting the spatial angle of the puncture needle; acquiring a respiration curve of the person to be detected; when the puncture path of the puncture needle is overlapped with the input puncture path and the breathing time of the person to be detected is consistent with CT time, starting the puncture operation; after the puncture needle reaches a predetermined position, carrying out needle suction biopsy. Therefore, when an electromagnetic navigation bronchoscope biopsy is notideal, a trans-chest-wall puncture biopsy guided by electromagnetic navigation is performed, so that the diagnosis transit time of a patient is shortened to the maximum extent, and the puncture risk of the patient is reduced to the maximum extent.
Owner:SUZHOU LANGKAI MEDICAL TECH
Nano-particles capable of improving gene transfection efficiency and preparation method of gene transfection reagent based on particles
InactiveCN103865942AOvercoming the deficiency of low transfection rateHigh transfection rateVector-based foreign material introductionCytotoxicityDna load
The invention belongs to the technical field of biological medicines and aims at improving the gene transfection efficiency by adopting nano-particles NoNPs released through cells. The nano-particles exist extensively in a biosystem and an extracellular circulatory system, such as bronchoalveolar lavage fluid, body fluid, blood, saliva, cow milk or urine. A non-virus gene transfection vector is doped with the nano-particles so that the DNA (Desoxvribose Nucleic Acid) carrying capacity of the gene transfection vector can be improved remarkably without increase of cytotoxicity. Experimental results indicate that the NONPs, which are extracted from the cow milk and added to the PEI gene transfection vector, are capable of remarkably improving the gene transfection efficiency under the circumstance of hardly increasing the cytotoxicity. The major use of the nano-particles is to serve as the gene transfection reagent which can be used for cell biology research, gene transfection preparation production, gene therapy and the like.
Owner:常州碳宇纳米科技有限公司
Chemically Modified Small Molecules
Methods of modifying the rate of systemic absorption of a drug administered to a subject by a pulmonary route, the method comprising covalently conjugating a hydrophilic polymer to a drug, wherein the drug has a half-life of elimination from the lung of less than about 180 minutes, to form a drug-polymer conjugate, wherein the drug-polymer conjugate has a net hydrophilic character and a weight average molecular weight of from about 50 to about 20,000 Daltons, and wherein the half-life of elimination from the lung of the drug-polymer conjugate is at least about 1.5-fold greater than the half-life of elimination from the lung of the drug, wherein the half-life of elimination from the lung is measured by bronchoalveolar lavage followed by assaying residual lung material.
Owner:NEKTAR THERAPEUTICS INC
Method for preparing pneumonia rat model and model evaluation method
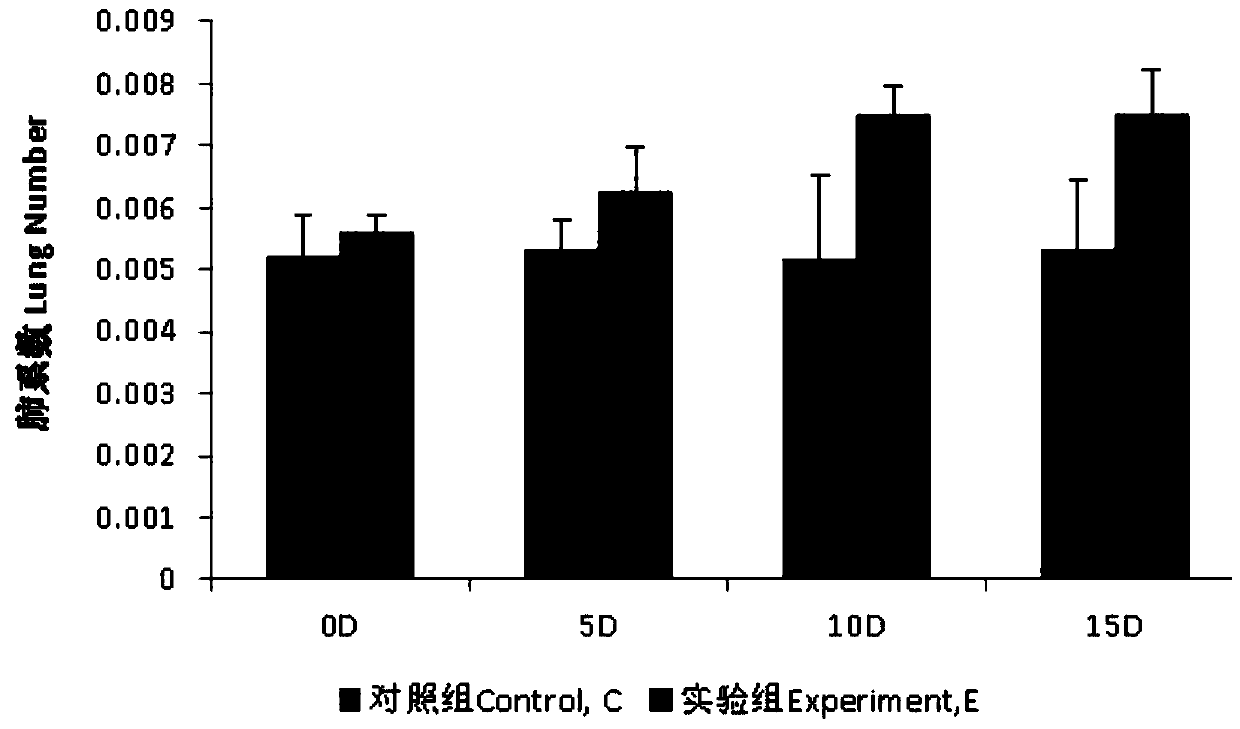
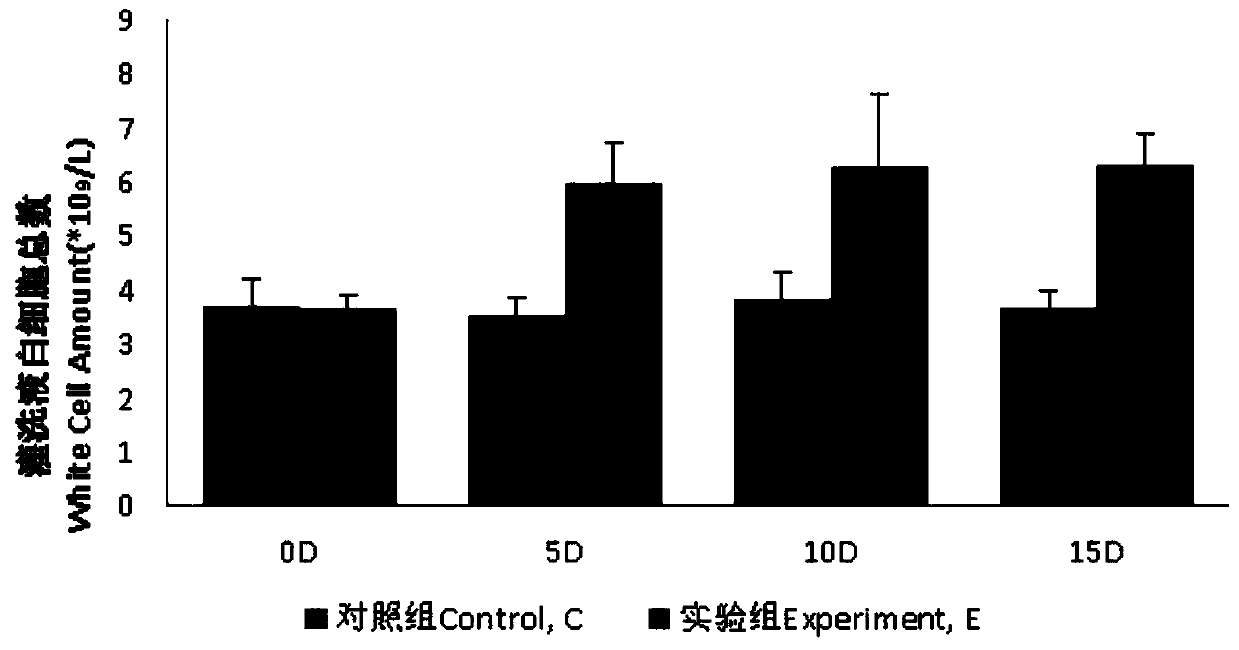
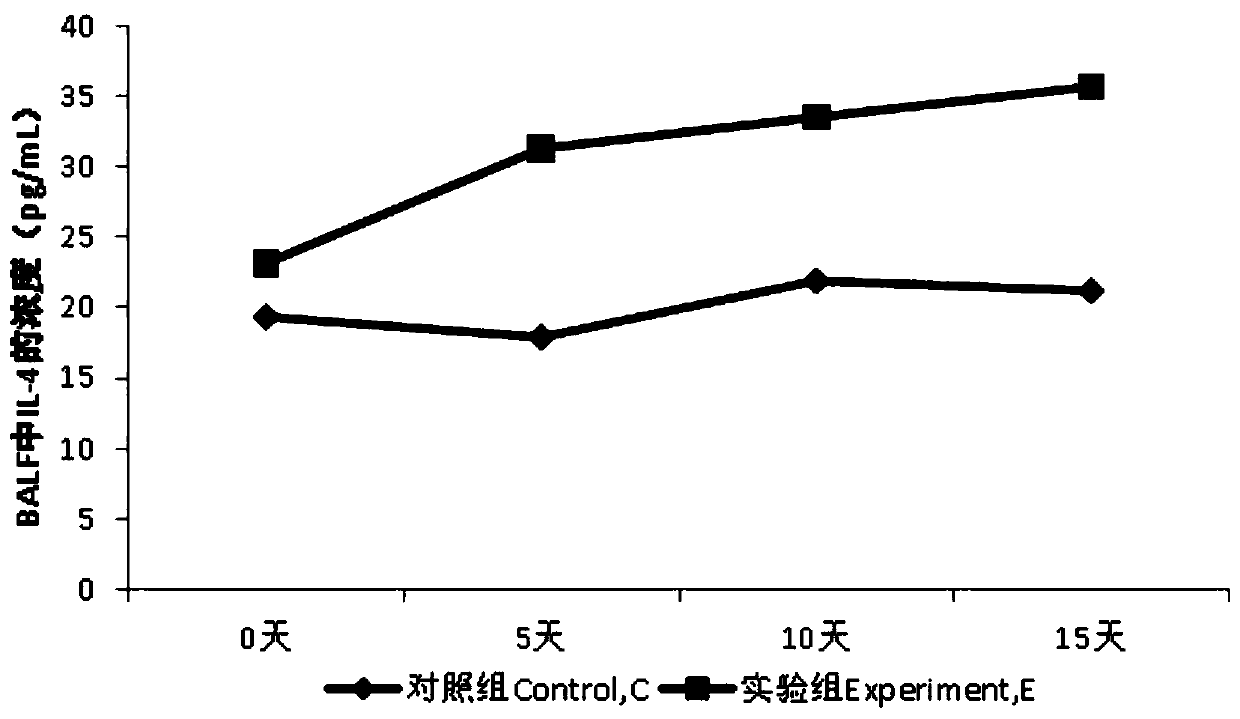
InactiveCN109833333AAvoid deathWon't hurtBacteria material medical ingredientsColor/spectral properties measurementsInflammatory factorsAlveolar lavage fluid
The invention discloses a method for preparing a pneumonia rat model and a model evaluation method, and belongs to the technical field of biology. The method for establishing the pneumonia rat model is non-invasive and safe to rats, does not damage other organs of rats in the whole process, and effectively avoids the death of the rats. Besides, the method has simple operation and high success rate. According to the pneumonia rat model established by the method, pseudomonas aeruginosa in bronchoalveolar lavage fluid is obviously increased, inflammatory factors IL-4 and TNF-alpha are obviously increased, the level of adhesion factor IFN-gamma is obviously reduced, and the number of leukocytes is obviously increased. Meanwhile, the lung index is also obviously increased, which indicates thatthe model is established well.
Owner:BEIJING CHINESE MEDICINE HOSPITAL AFFILIATED CAPITAL MEDICAL UNIV
Allergen protein BA2 of Periplaneta americana and expression method thereof
ActiveCN107759677AIncreased IgE contentIncreased levels of inflammatory factorsPeptide preparation methodsFermentationBronchoalveolar lavageBiology
The invention discloses an allergen protein BA2 of Periplaneta americana and an expression method thereof. The expression method comprises the following steps: (1) extracting the RNAs of Periplaneta americana, subjecting the RNAs to inverse transcription to obtain cDNAs and carrying out PCR amplification with the cDNAs as a template so as to obtain a coding gene fragment of the allergen protein ofPeriplaneta americana; and (2) cloning an allergen gene into a prokaryotic expression vector to construct recombinant expression plasmid pET28a(+)-BA2, transferring the recombinant expression plasmidinto the host bacterium Escherichia coli BL21, inducing the expression of the recombinant expression plasmid with IPTG and carrying out purifying by using affinity chromatography so as to obtain therecombinant allergen protein BA2 of Periplaneta americana. The results of pharmacological experiments show that the allergen protein BA2 can obviously increase the IgE content (wherein P is less than0.05) of mouse serum, substantially increase the contents of IL-4, IL-5 and IL-13 inflammatory factors in mouse bronchoalveolar lavage fluid and mouse splenocyte supernatant, and result in obvious pathological changes of mouse lung tissue. The method provided by the invention can prepare the allergen protein with high purity and provides referential bases for subsequent research on the allergens of Periplaneta americana and potential allergen substances in other animals.
Owner:SICHUAN GOODDOCTOR PANXI PHARMA
Traditional Chinese medicine composition with function of pulmonary fibrosis resistance and preparation method and application of traditional Chinese medicine composition with function of pulmonary fibrosis resistance
ActiveCN105497469ARigorous and scientific medicationReduce lesionsPill deliveryGranular deliverySalvia miltiorrhizaSocial benefits
The invention discloses a traditional Chinese medicine composition with a function of pulmonary fibrosis resistance and a preparation method of the traditional Chinese medicine composition with the function of pulmonary fibrosis resistance. The traditional Chinese medicine composition with the function of pulmonary fibrosis resistance is prepared from, by weight, 2-10 parts of Radix Ginseng, 2-20 parts of Cortex Mori, 2-20 parts of Cortex Lycii, 1-10 parts of Radix Glycyrrhizae, 2-20 parts of Rhizoma Anemarrhenae, 2-20 parts of Exocarpium Citri Rubrum, 2-20 parts of Radix Asparagi and 2-20 parts of Radix Salviae Miltiorrhizae. The traditional Chinese medicine composition is prepared by matching of traditional Chinese medicines through a large amount of experiments according to syndrome differentiation and treatment theories and traditional Chinese medicine theories. According to experimental results, the traditional Chinese medicine composition has remarkable functions of alleviating pulmonary fibrosis and other pulmonary lesions caused by bleomycin and improving pulmonary functions and is capable of remarkably lowering pulmonary fibrosis closely related biological indicators such as pulmonary hydroxyproline content and bronchoalveolar lavage fluid PDGF, NF-kB, TNF-alph and IFNgamma levels and reducing pulmonary tissue TGFbeta, Smad3mRNA and protein expression. Furthermore, the traditional Chinese medicine composition with the function of pulmonary fibrosis resistance can be used for treatment of various idiopathic and secondary pulmonary fibrosis and has significant social benefits and economic benefits.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Respiratory tract infection multiple detection reagent kit and detection method
ActiveCN110273026AImprove featuresHigh sensitivityMicrobiological testing/measurementMicroorganism based processesAlveolar lavage fluidFluorescence
The invention provides a respiratory tract infection multiple detection reagent kit and detection method. Particularly, a multiplex respiratory tract pathogen PCR amplification system is designed and subjected to experimental verification, so that a multiplex fluorescent PCR amplification system for detecting various respiratory tract pathogens is obtained, and a single system can detect 3 kinds of respiratory tract pathogens at the same time. The reagent kit has high sensitivity and specificity. Through the reagent kit, quick detection and analysis of multiplex respiratory tract pathogens in samples of nasopharyngeal swabs, bronchoalveolar lavage fluid, phlegm and the like can be realized.
Owner:DAAN GENE CO LTD
Bronchoalveolar lavage catheter assembly
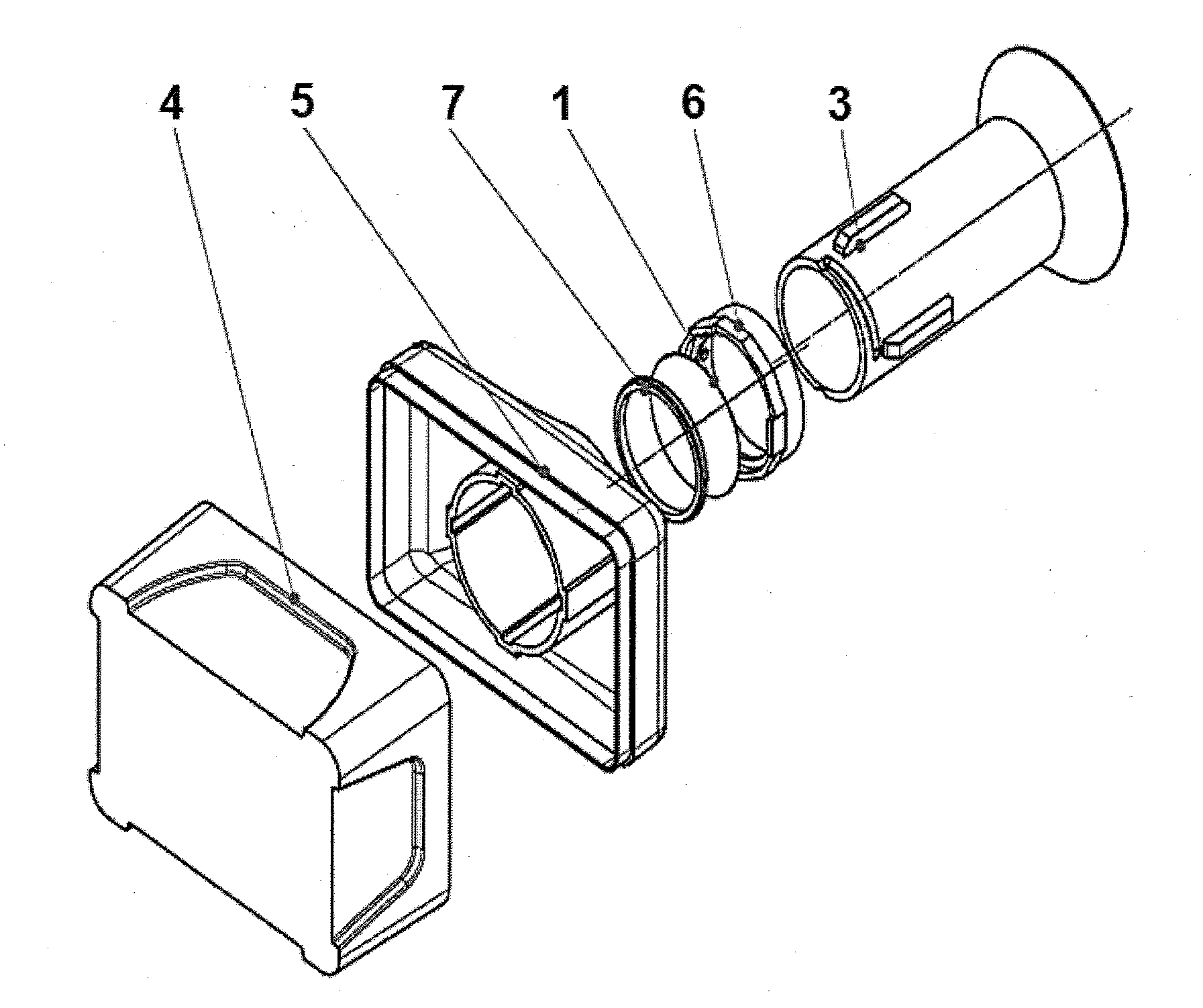
A catheter assembly configured for use in a non-bronchoscopic bronchoalveolar lavage procedure. The catheter assembly may include an inner catheter member (502) having an inner catheter lumen and an outer catheter member (506) having an outer catheter lumen, where the inner catheter member is disposed longitudinally and coaxially through at least a lengthwise portion of the outer catheter lumen, a distal end portion (508) of the outer catheter (506) includes an atraumatically-shaped disruptable seal (509) - which seal includes at least a pair of overlapping slits that extend at least partially through an internal distal end wall portion of the outer catheter - and where a distal end portion (510) of the inner catheter (502) includes a wedging structure (512) configured to at least partially sealingly contact an inner circumference of a passage in a lower portion of a patient lung.
Owner:CAREFUSION 2200 INC
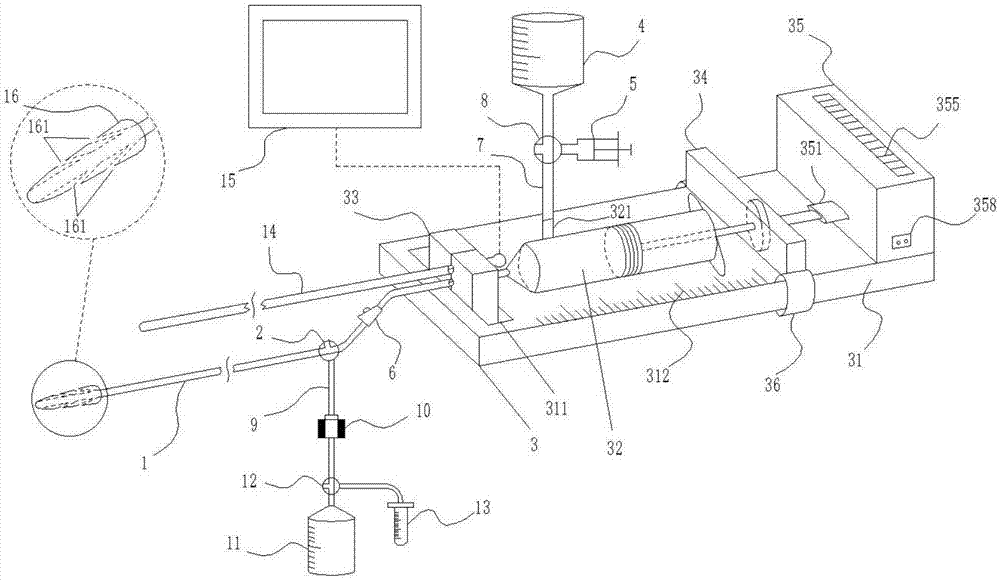
Bronchoalveolar lavage device for assisted treatment of children mycoplasma pneumonia
InactiveCN107261241AAvoid damageQuality assuranceEnemata/irrigatorsMedical devicesTreatment effectBottle
The invention discloses a bronchoalveolar lavage device for assisted treatment of children mycoplasma pneumonia. The bronchoalveolar lavage device comprises a perfusion part and a drainage part. The perfusion part comprises a perfusion pipe, a first three-way valve, a pressurized injection device, a liquid storage bottle and a medicine feeding pipe. The first three-way valve is arranged at the middle of the perfusion pipe, the pressurized injection device is connected to the upper end of the first three-way valve, and the pressurized injection device is connected with the liquid storage bottle and the medicine feeding pipe through a catheter and a second three-way valve on the catheter respectively. The drainage part comprises a drainage pipe, a negative pressure generator and a waste liquid measuring bottle. One end of the drainage pipe is connected with the first three-way valve, the other end of the drainage pipe is connected with the negative pressure generator, a third three-way valve is arranged below the negative pressure generator, the waste liquid measuring bottle is connected to the lower portion of the third three-way valve, and the side end of the third three-way valve is connected with a sampling bottle. In a word, the bronchoalveolar lavage device is reasonable in design, convenient to operate, high in practicality and good in diagnosis and treatment effect.
Owner:尹文艳
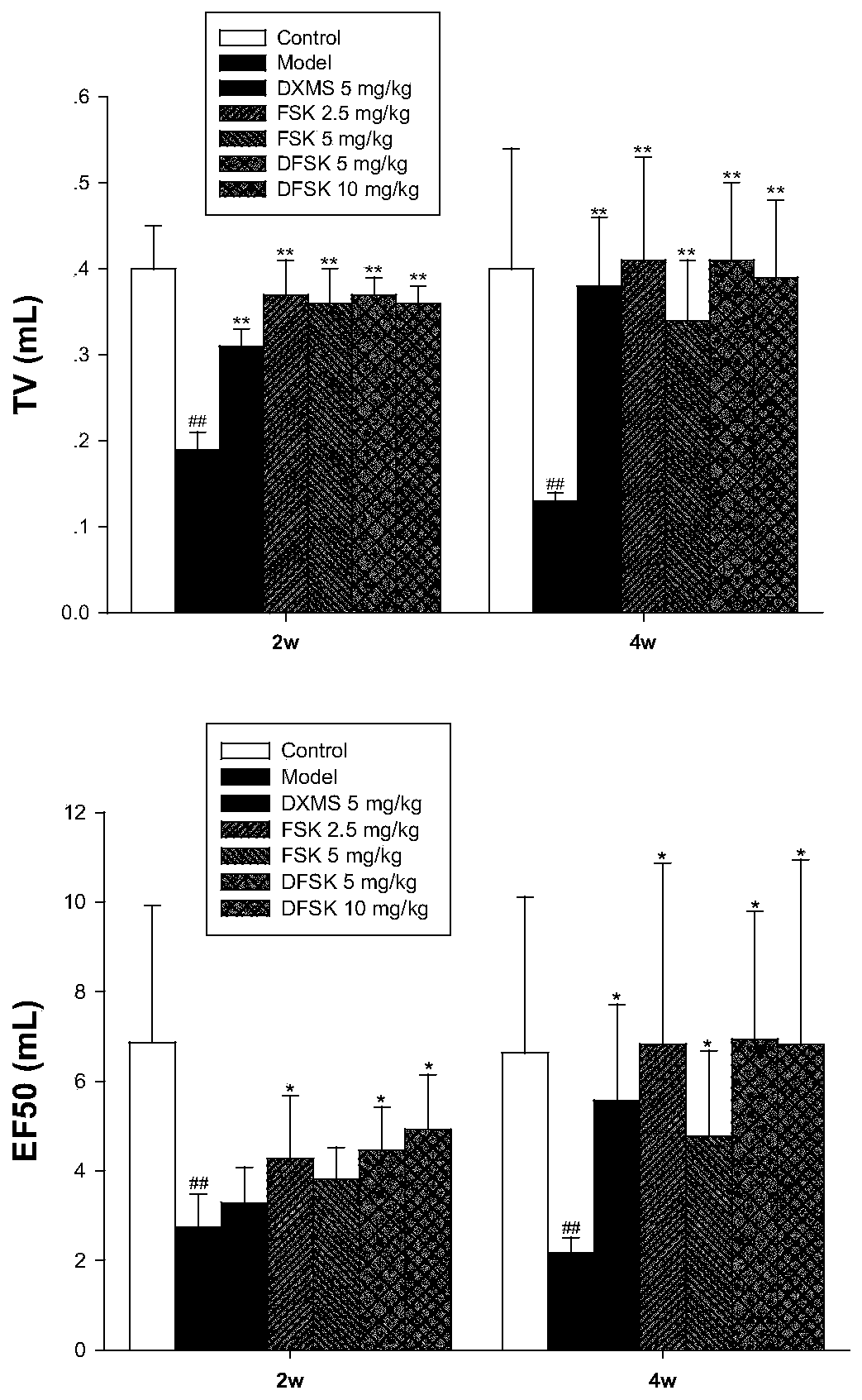
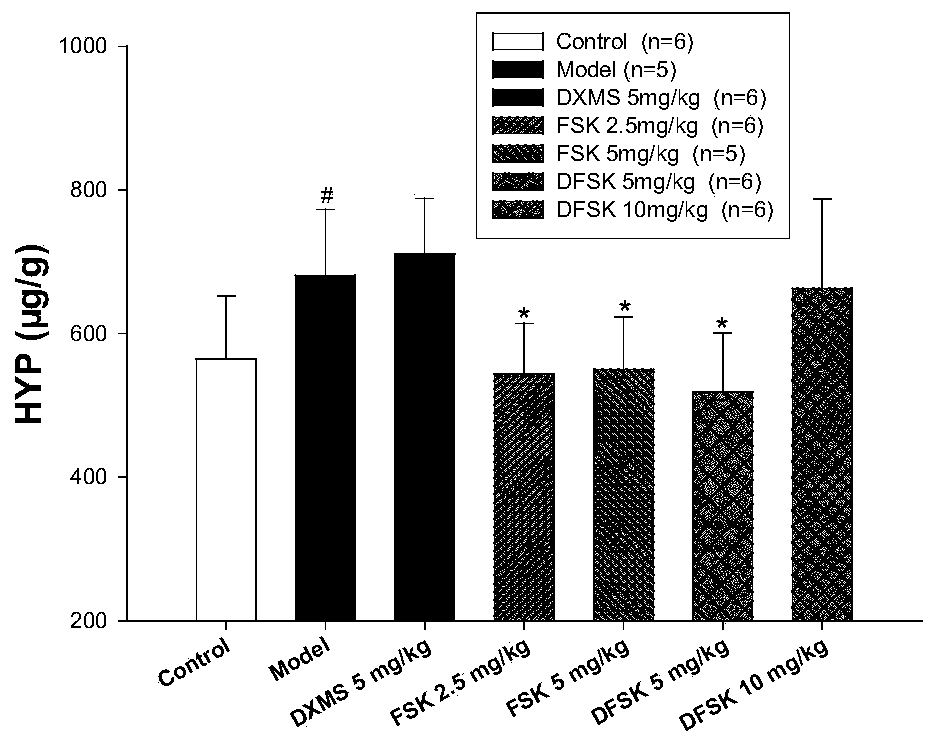
Application of forskolin and derivatives thereof in preparing anti-pulmonary fibrosis drug
ActiveCN109875992AGood interventionOrganic active ingredientsRespiratory disorderHydroxyprolineLung tissue
The invention discloses use of forskolin (FSK) and derivatives thereof, including isoforskolin (ISOF) and deacetylforskolin (DFSK), in preparing a drug or a health care product for preventing and treating pulmonary fibrosis. FSK, ISOF and DFSK are a diterpenoid compound isolated from a medicinal plant coleus forskohlii. The FSK and the DFSK can significantly reduce lung inflammation and fibrosis in mice induced by paraquat, reduce hydroxyproline content in lung tissues and improve the lung function in mice. The ISOF can significantly reduce the hydroxyproline content in the mice lung tissues of bleomycin-induced pulmonary fibrosis, reduce the deposition of collagen fibers in the lung tissues, down regulate the expression of fibrogenic factors and decrease the level of inflammatory cytokines in the bronchoalveolar lavage fluid. Experimental results prove that the FSK and the derivatives ISOF and DFSK have the new use as the drug for preventing and treating the pulmonary fibrosis.
Owner:KUNMING MEDICAL UNIVERSITY
Application of euphorbia jolkini lactone B derivative in drug for treating chronic obstructive pulmonary disease
InactiveCN107998124AImprove lesionReduce the number of inflammatory cellsOrganic active ingredientsRespiratory disorderAlveolar lavage fluidObstructive Pulmonary Diseases
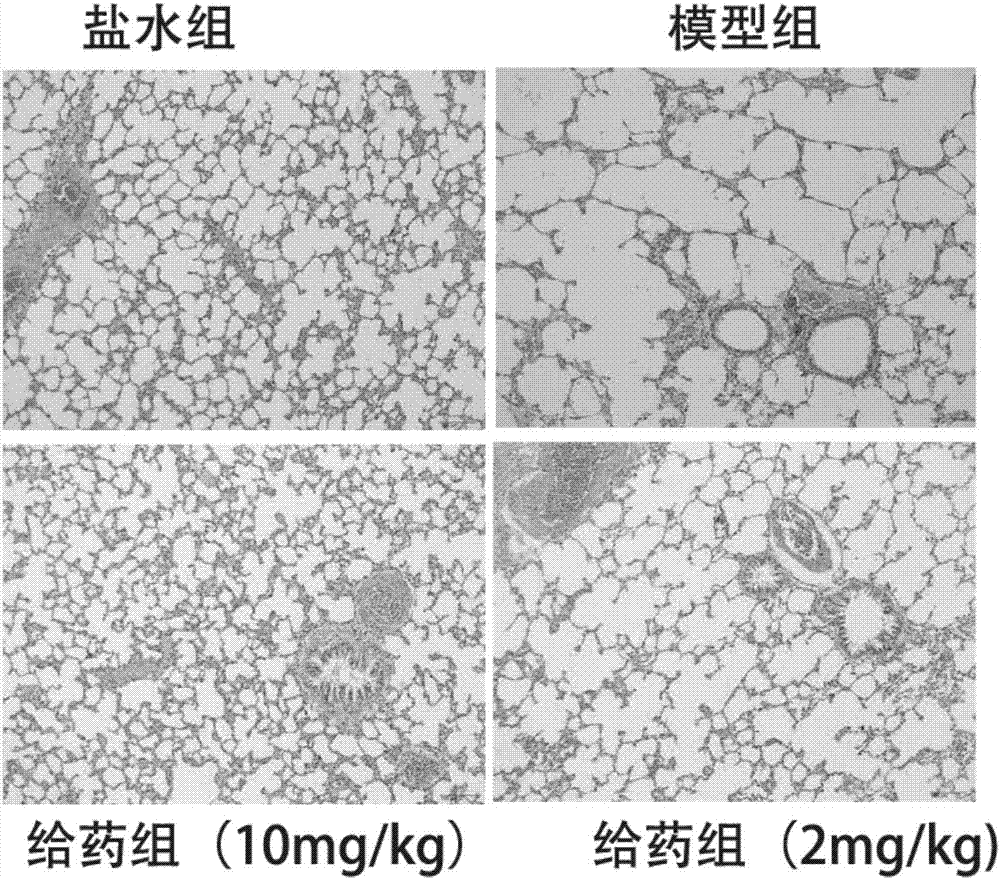
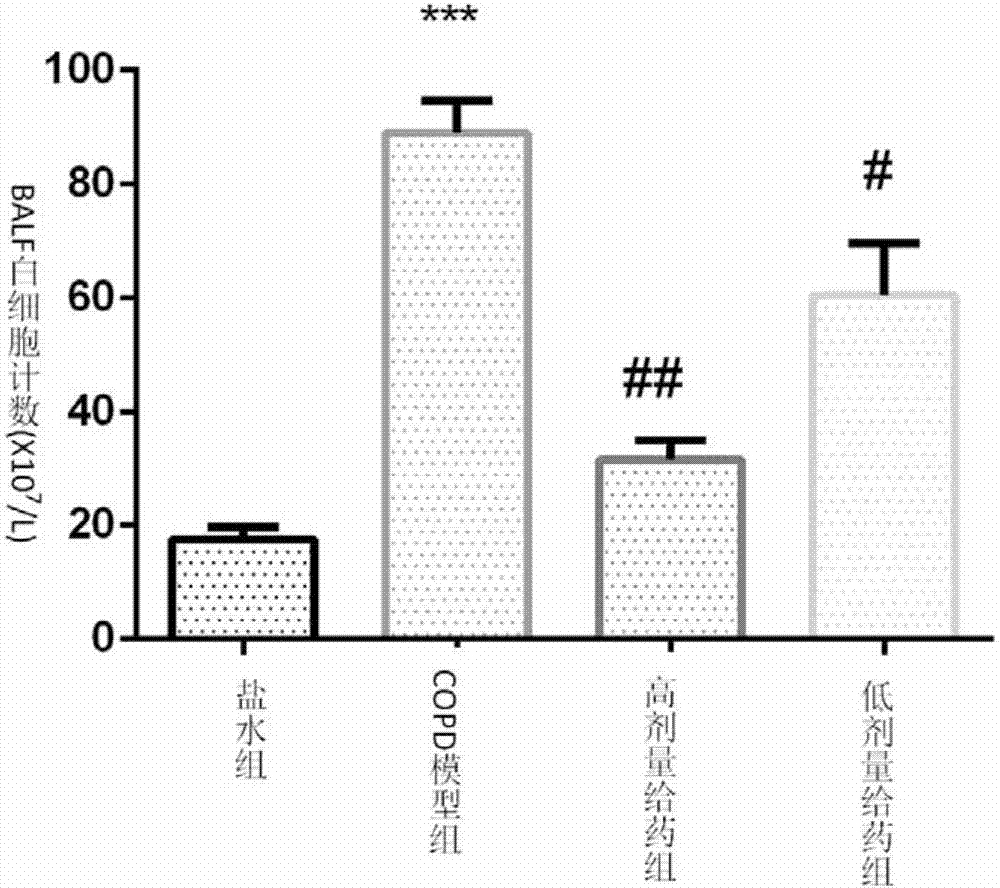
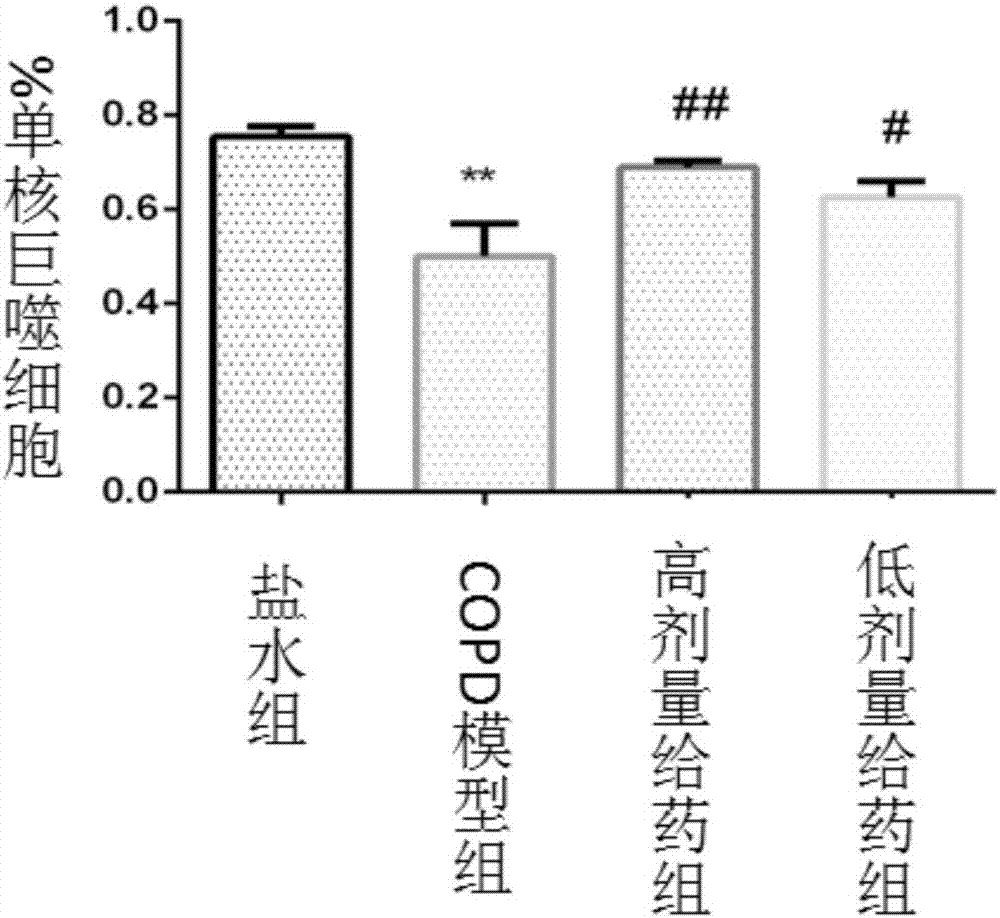
The invention relates to application of a euphorbia jolkini lactone B derivative in a drug for treating a chronic obstructive pulmonary disease, and belongs to the field of medicines. In the application, a pharmacodynamic experiment is performed on a mouse model with the chronic obstructive pulmonary disease by adopting a compound; a result shows that HJB-1 has an obvious effect of improving lungtissue lesions of the mouse with the chronic obstructive pulmonary disease and a function of decreasing the number of inflammatory cells in bronchoalveolar lavage fluid of the mouse with the chronic obstructive pulmonary disease; the euphorbia jolkini lactone B derivative is used for developing the drug for treating the chronic obstructive pulmonary disease.
Owner:JILIN UNIV
Application of PD-1H agonists to asthma treatment
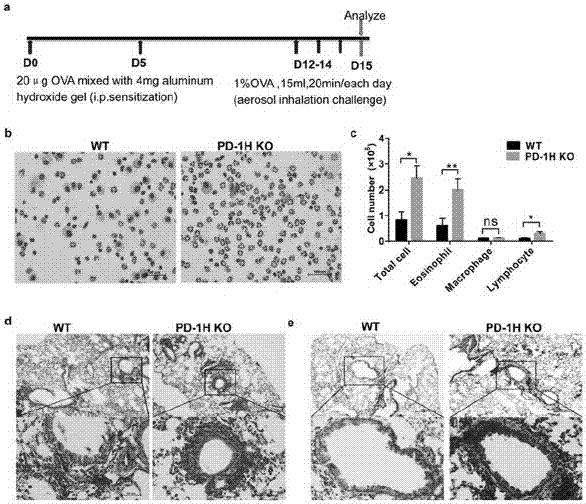
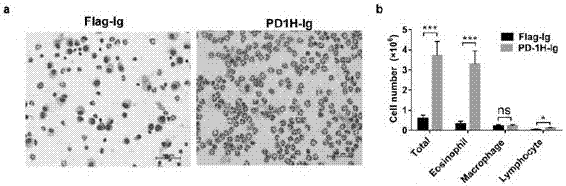
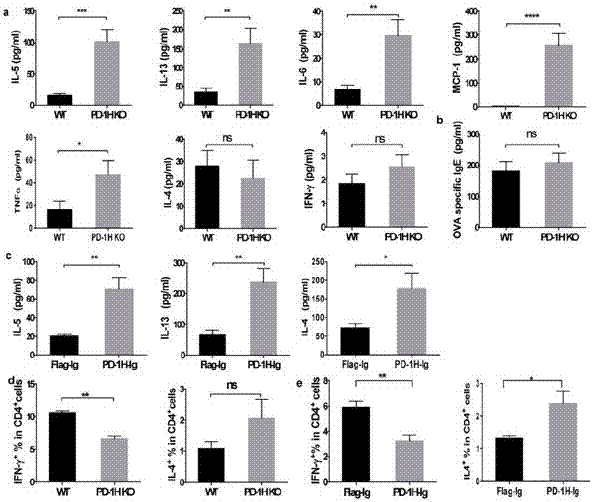
The invention discloses application of PD-1H agonists to preparing medicine compositions for preventing or treating asthma of objects. The application has the advantages that treatment is carried out by the aid of agonist type PD-1H monoclonal antibodies, accordingly, the quantities of inflammatory cells (eosinophil and lymphocyte in particular) in mouse BALF (bronchoalveolar lavage fluid) are obviously reduced, pulmonary inflammation and myxopoiesis are obviously reduced, and accordingly the fact that PD-1H can be targeted for treating allergic diseases is indicated by results.
Owner:SUN YAT SEN UNIV
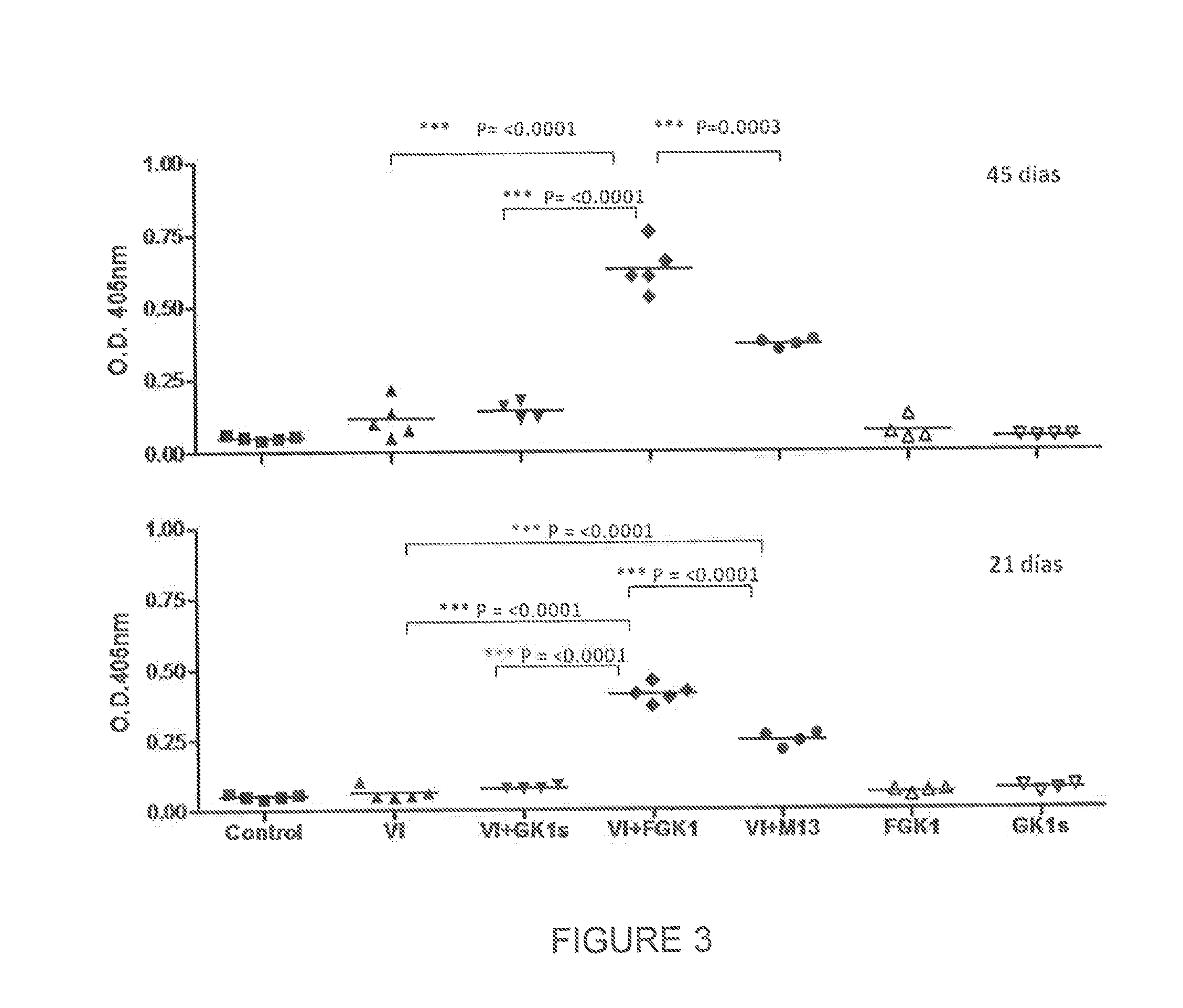
Use of gk-1 peptide expressed on m13 filamentous phage as pharmaceutical ingredient to enhance the efficiency of the immune response induced by vaccine or pathogen antigens
ActiveUS20140302085A1Enhance immune responsePotentiate antibody productionSsRNA viruses negative-sensePeptide/protein ingredientsAdditive ingredientVaccine antigen
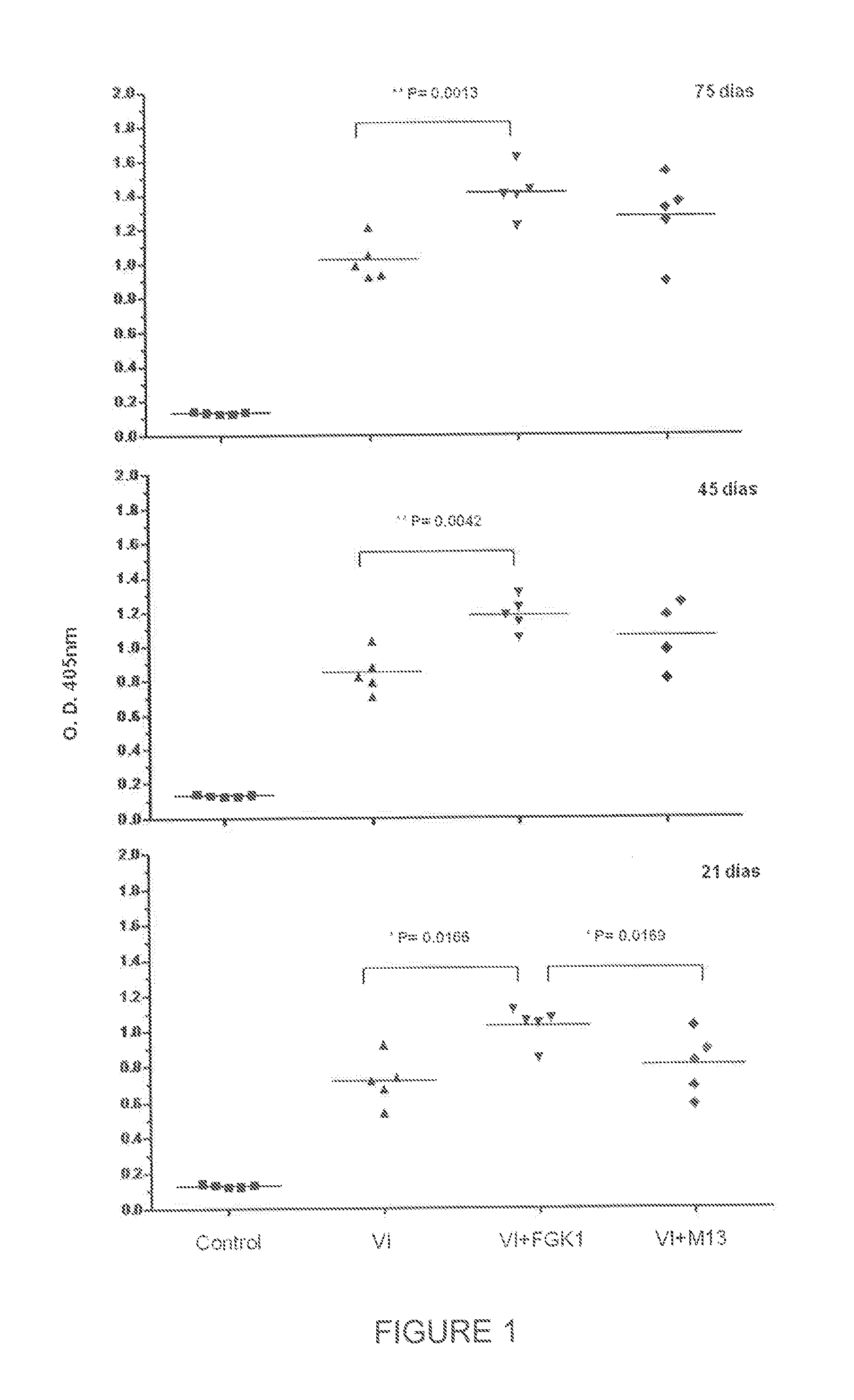
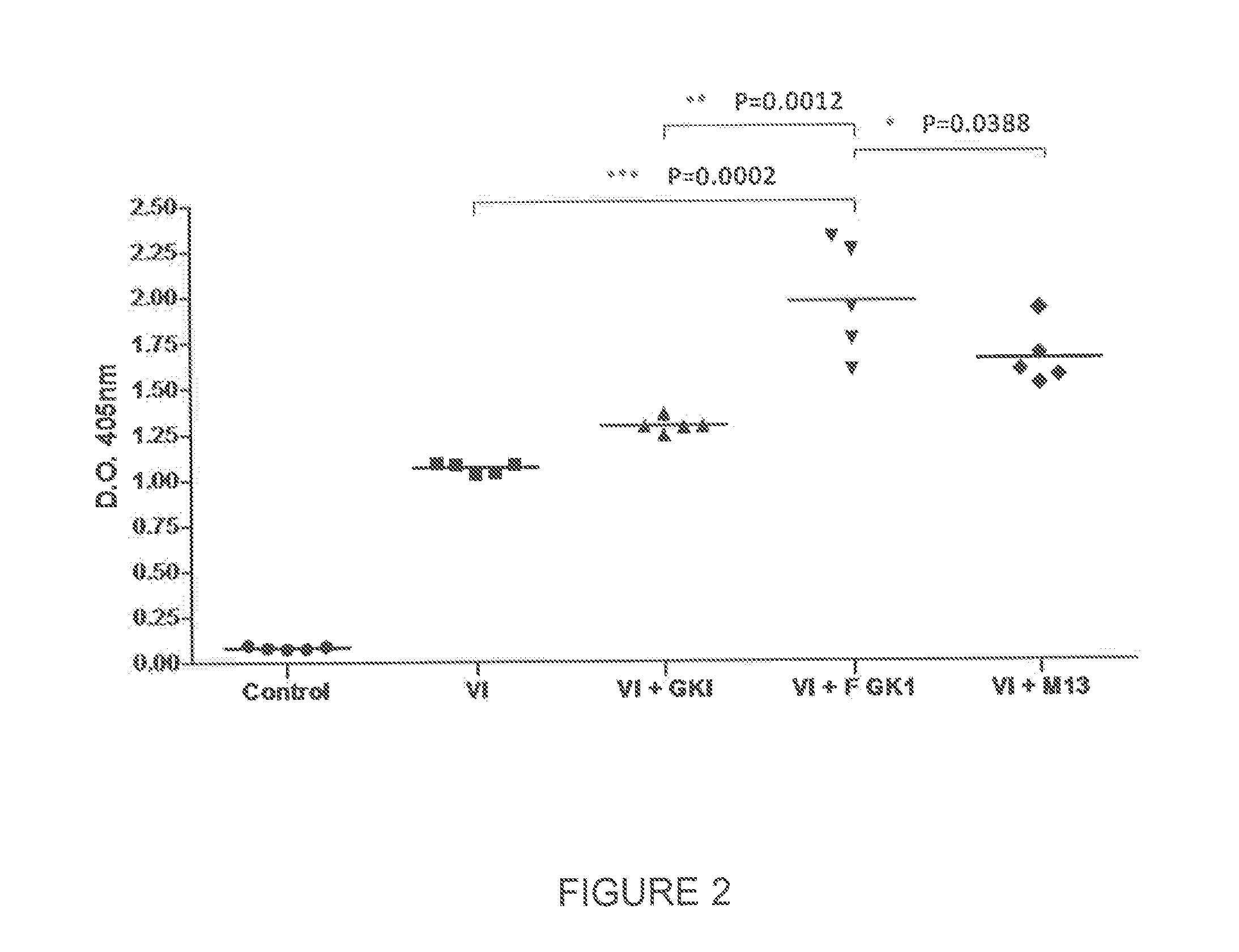
The present invention is directed to the use of FGK-1 immunopotentiator, composed by the peptide named GK-1, characterized by the sequence G-Y-Y-Y-P-S-D-P-N-T-F-Y-A-P-P-Y-S-A and linked to the pVIII surface protein of M13 filamentous phage, to prepare pharmaceutical products potentiating the protective immune response of vaccine antigens when used by itself or conjointly with these antigens administered either intranasally, subcutaneously, or intramuscularly, yielding an increase in the level of specific antibodies against vaccine antigens in serum and in bronchoalveolar lavages.
Owner:UNIV NAT AUTONOMA DE MEXICO
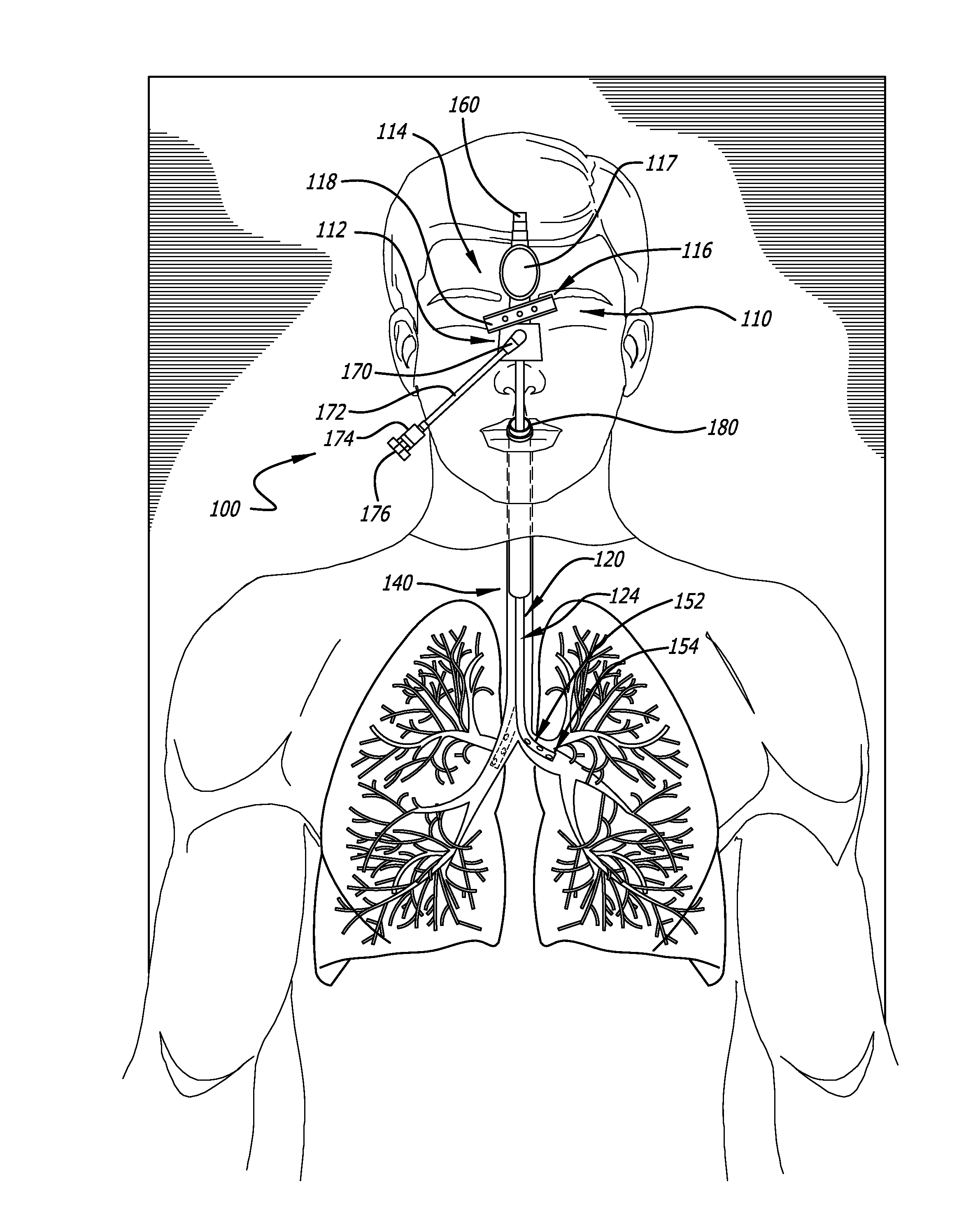
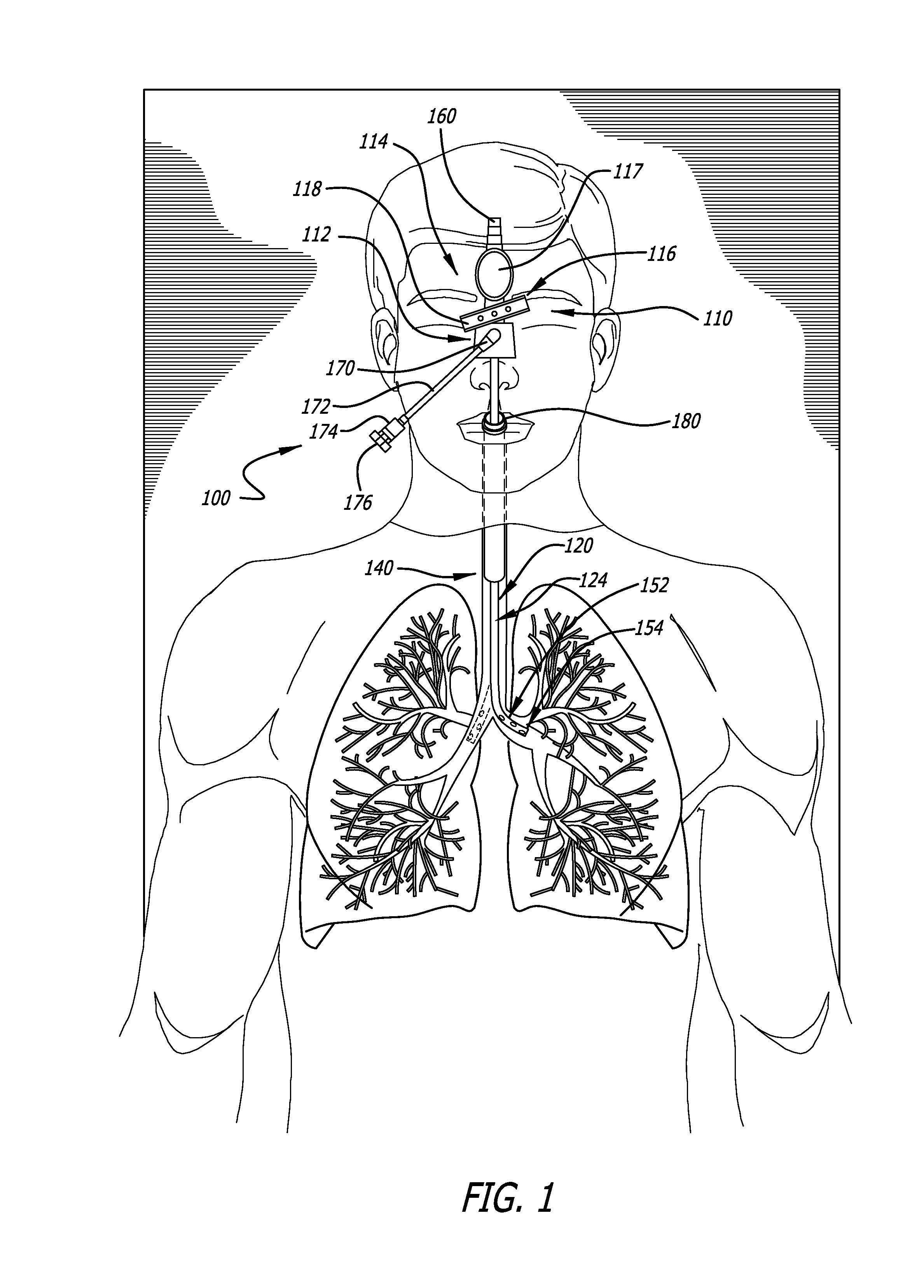
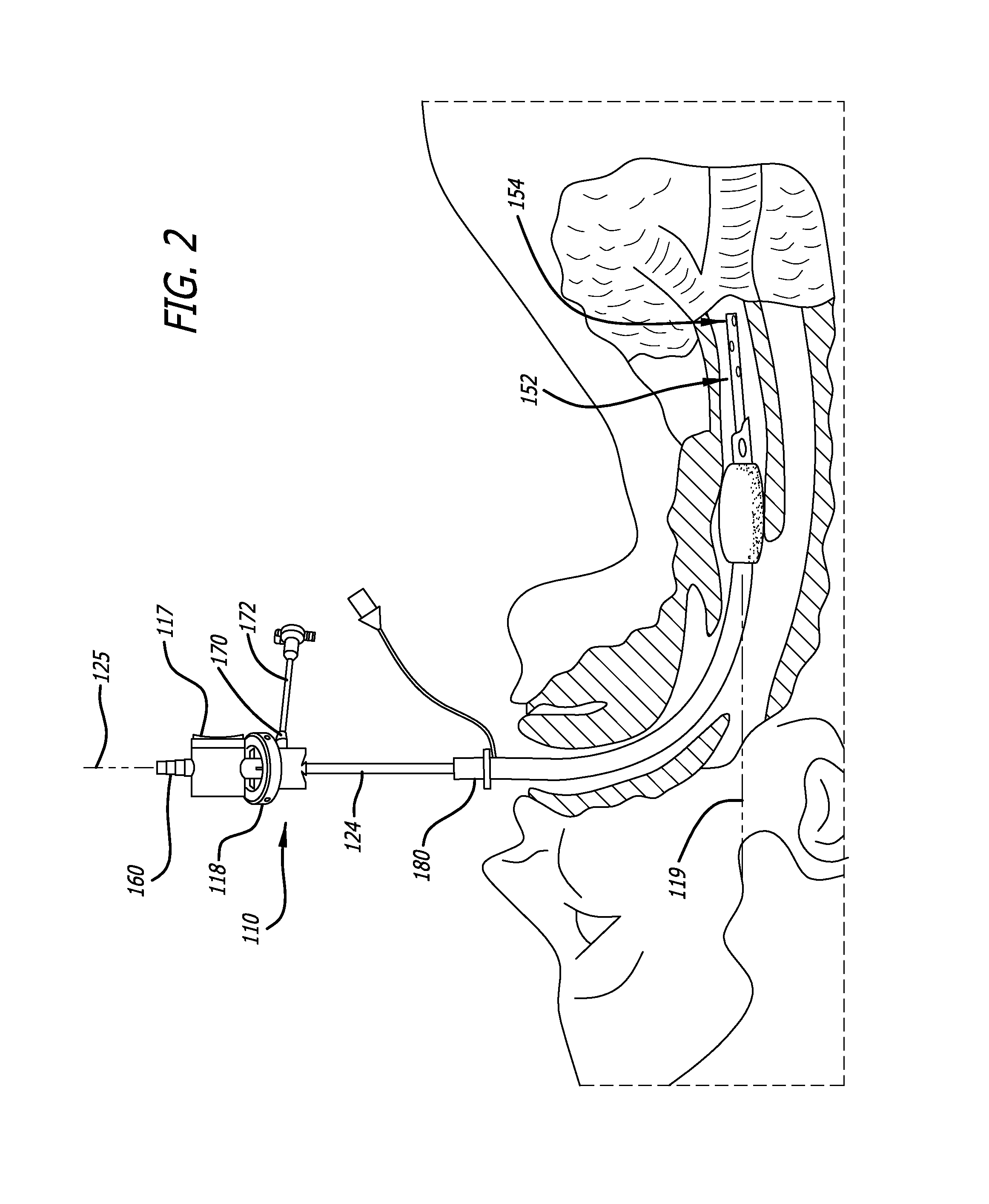
Bi-lateral endobronchial suctioning device and medical suctioning system for intubated patients
ActiveUS20160250430A1Direct controlPrecise suctionTracheal tubesBronchoscopesLung alveolusBronchial tube
A bi-lateral endobronchial suctioning device includes actuating and articulating components that allow a provider to accurately suction both right and left bronchi as well as the trachea in a controlled and safe manner. A control mechanism is used to manipulate the actuating components in tubes forming a suction catheter to flex the tip of the bi-lateral endobronchial suctioning device to the left and to the right when the device is inserted through an endotracheal tube or other similar device, to enable directional control of a catheter for suctioning the lungs or trachea. The device further includes a bronchoalveolar lavage (BAL) port allowing sterile saline or other liquids or fluids to travel down the catheter lumen to assist with breaking up thick secretions which collect in the airways.
Owner:URE JOHN P +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com