Nucleic acid detection kit for novel coronavirus COVID-19 and use method thereof
A technology of COVID-19 and detection kits, which is applied in the direction of microorganism-based methods, biochemical equipment and methods, and microorganism measurement/inspection, which can solve the problems of missed diagnosis, low accuracy of detection results, unfavorable epidemic situation, etc., and achieve improvement Accuracy, the effect of shortening the time of diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1. Experimental materials / equipment / reagents:
[0049] 1. Experimental materials:
[0050] Clinical sample RNA extraction kit: Qiagen RNeasy Mini Kit (50) 74104;
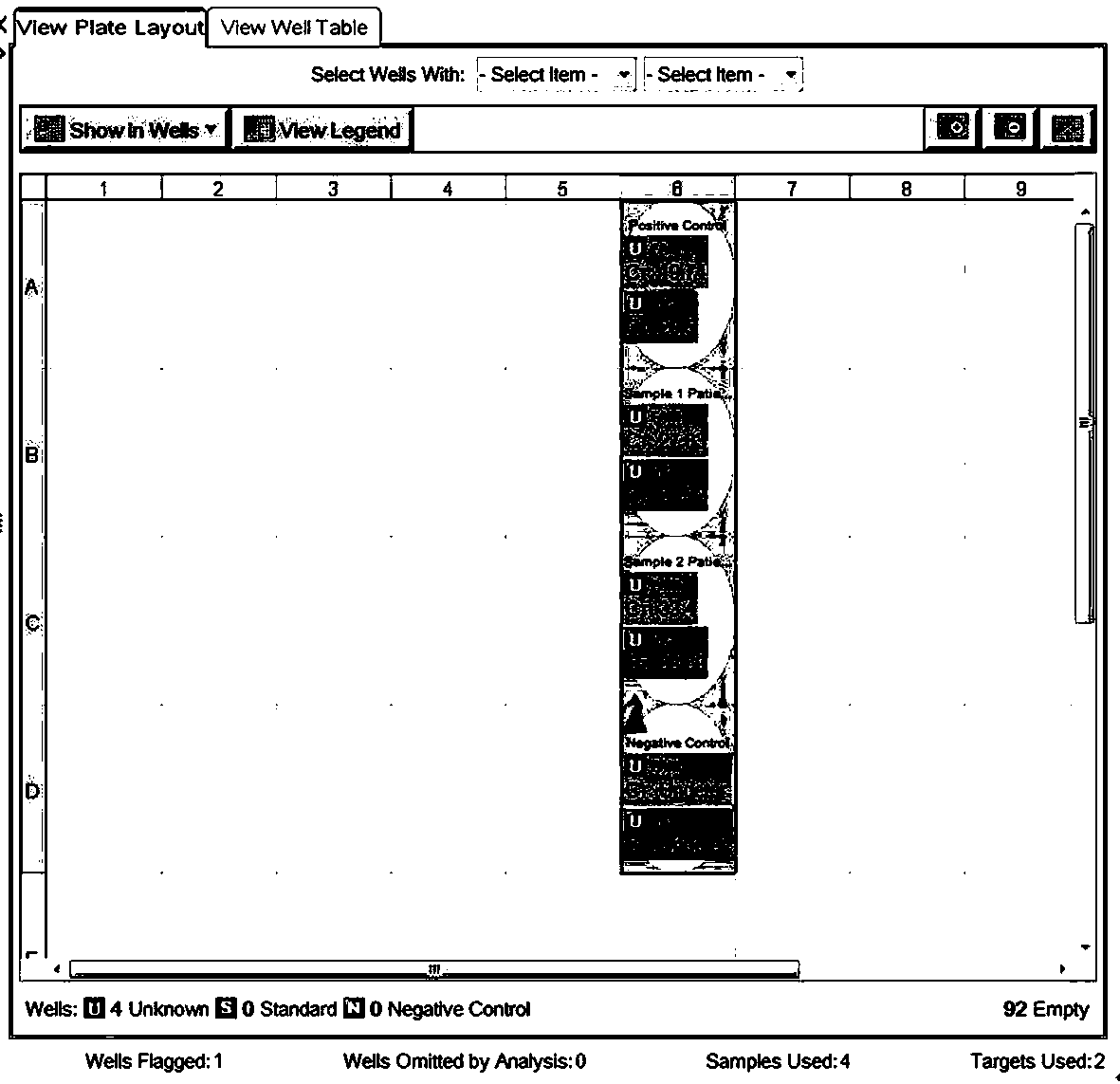
[0051] Novel coronavirus COVID-19 nucleic acid detection kit, the main components of the kit are shown in Table 1;
[0052]
[0053] Among them, the 2019 novel coronavirus negative quality control product is obtained by taking an appropriate amount of RNase-Free ddH 2 O is subpackaged into centrifuge tubes under a sterile environment, that is, a negative quality control product is obtained.
[0054] Inspection method: Visual inspection of product appearance, colorless and clear liquid, no sediment, no suspended matter, no floc.
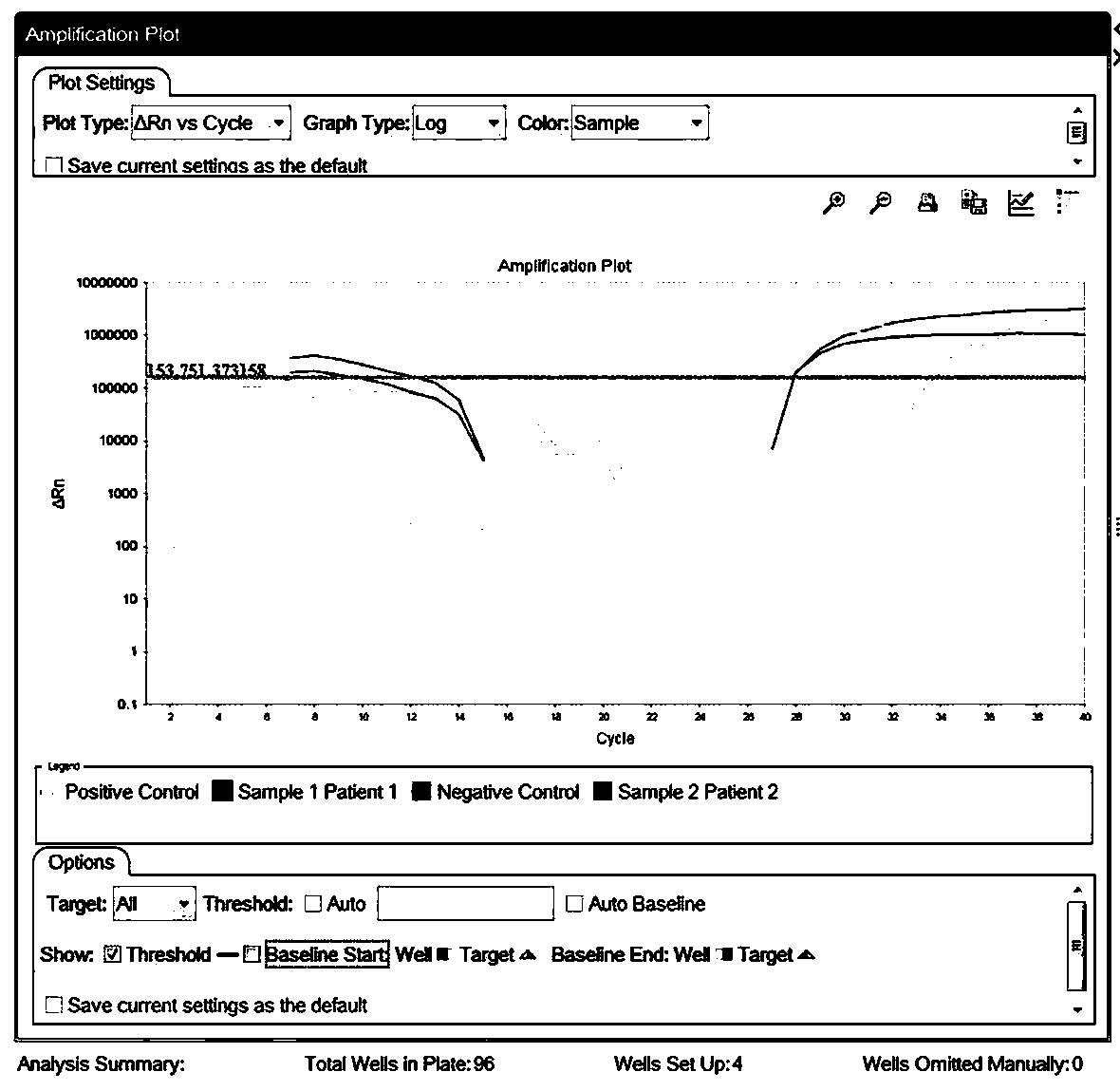
[0055] For the preparation process of the positive quality control product of the novel coronavirus image 3 .
[0056] Production Process:
[0057] (1) Centrifuge 1 μg of dry plasmid powder at 12,000 rpm for 2 min. Add 50 μL ddH 2 O, briefly centrifuge after shaking for 2...
Embodiment 2
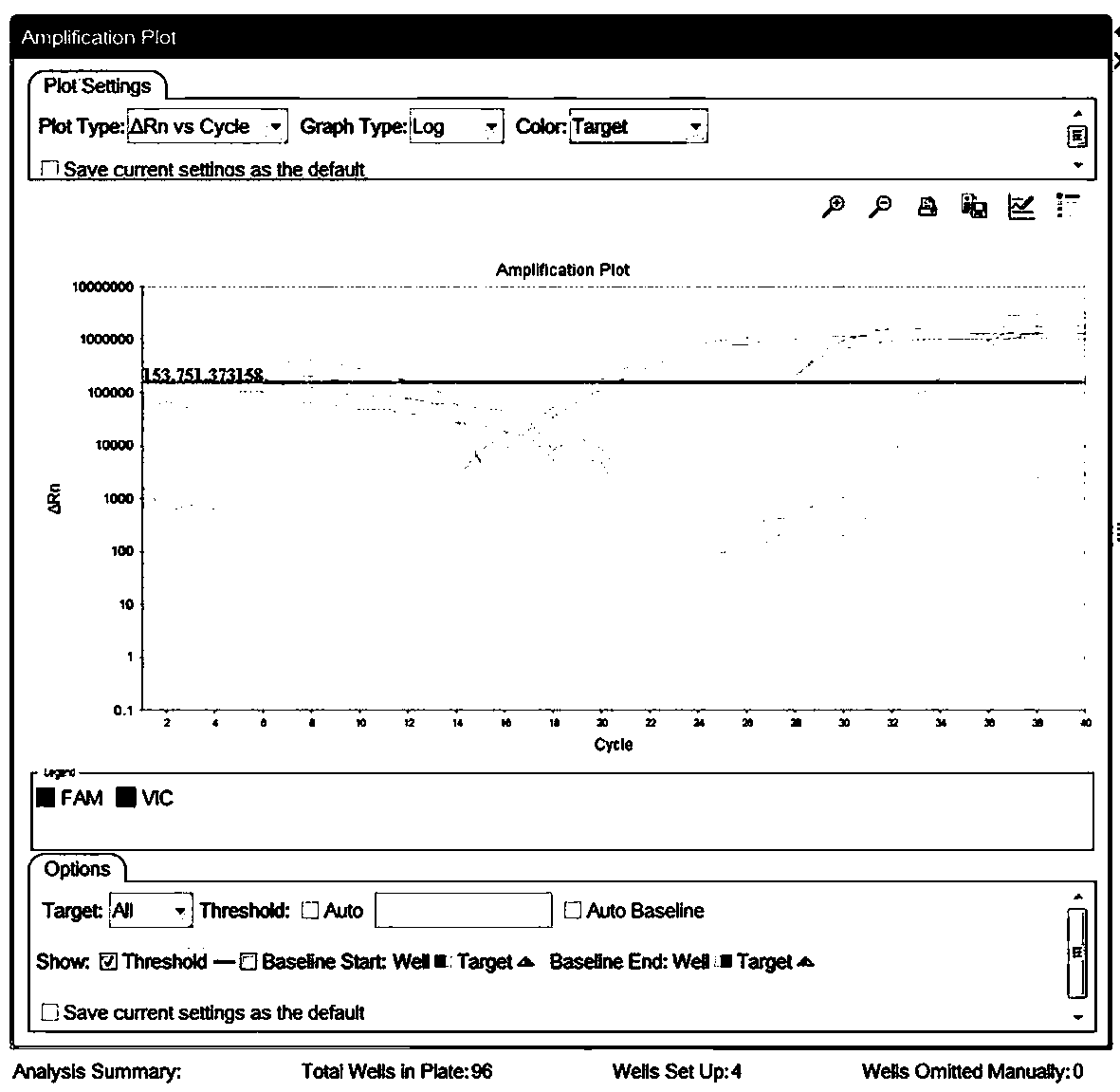
[0091] The sequence of the first positive quality control product as shown in SEQ ID NO.10, the second positive quality control product as shown in SEQ ID NO.11, the third positive quality control product as shown in SEQ ID NO.12 Respectively replace the samples in Example 1, detect according to the method of Example 1, each positive quality control product is repeated three times, and the detection results are as follows: Figure 4As shown, where gene E is the third positive quality control product, gene N is the first positive quality control product, and gene ORFlab is the second positive quality control product.
Embodiment 3
[0093] Get 10 negative throat swab samples to detect according to the method of embodiment 1, test result is as follows: Figure 5A with 5B As shown, all 10 samples were negative.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com