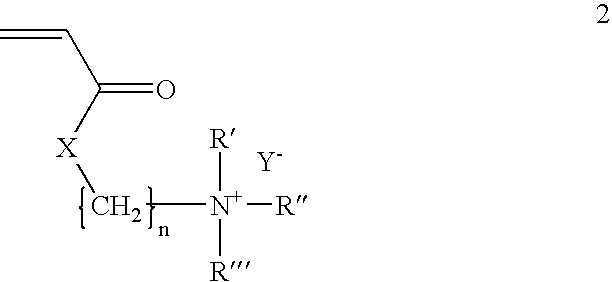Patents
Literature
763 results about "Sterile environment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In horticulture, a sterile environment often refers to an indoor grow facility that is free from plant pathogens like bacteria, pest insects, molds, mildews, and weeds.
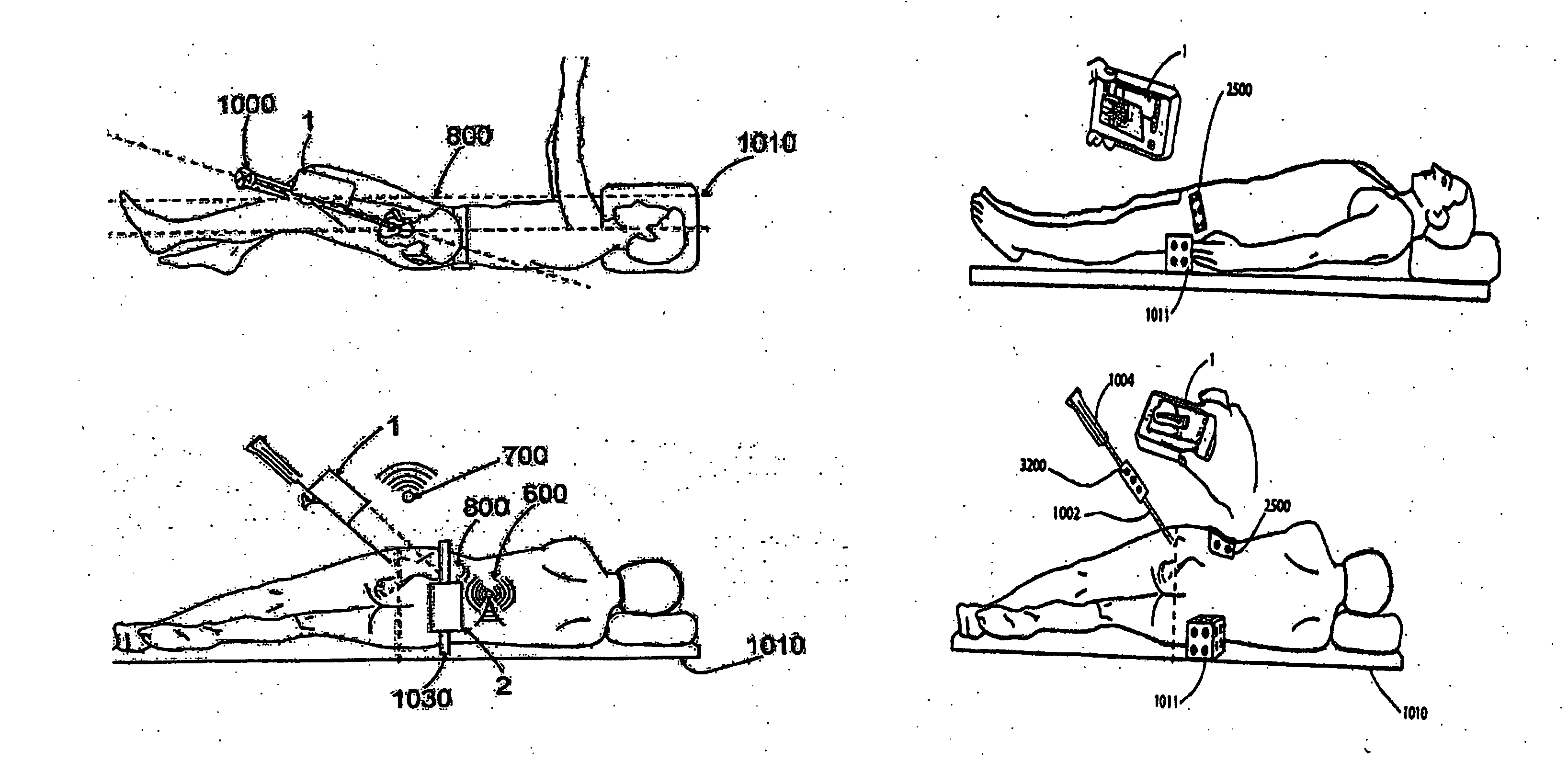
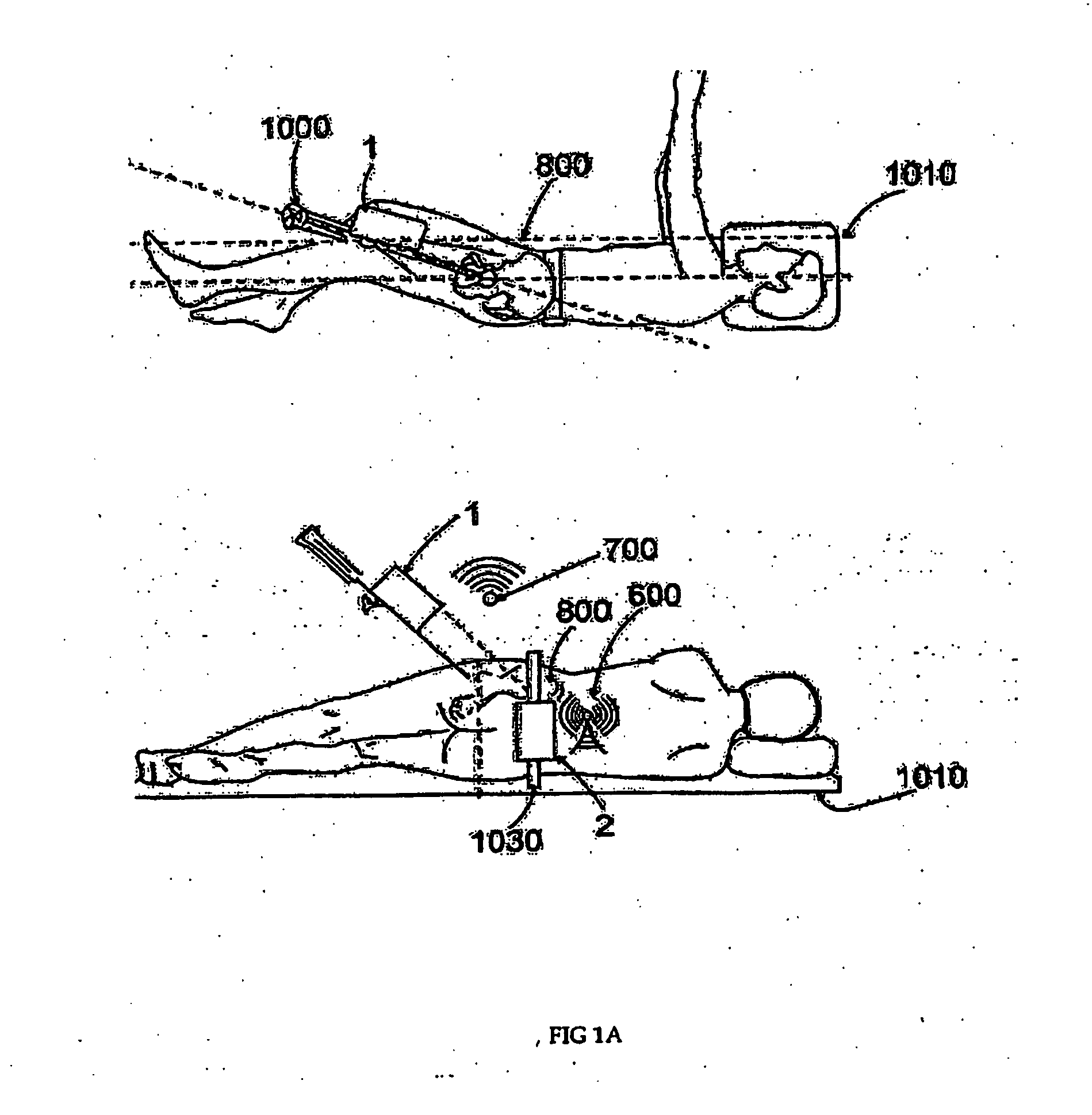
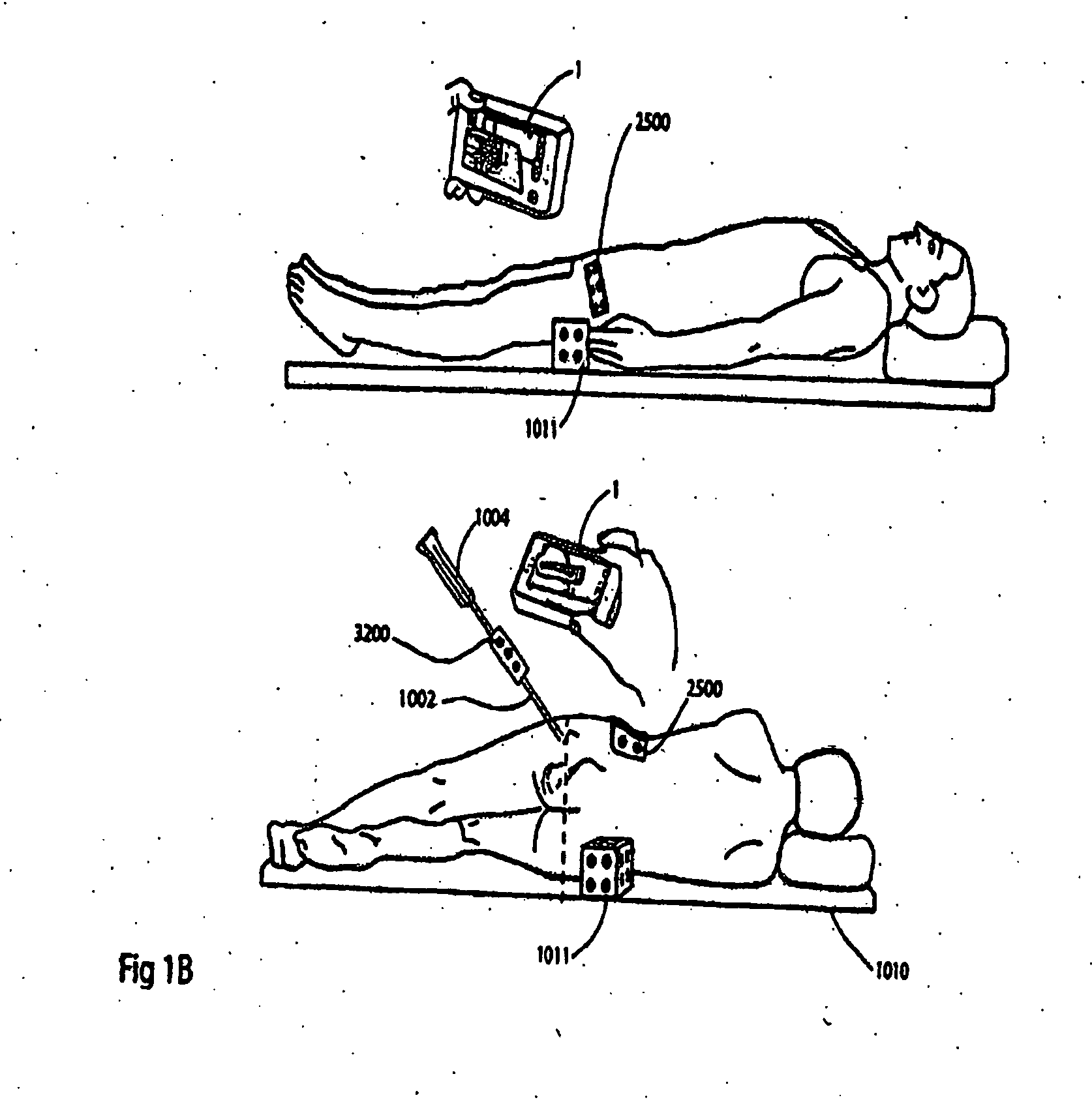
System, method and apparatus for tableside remote connections of medical instruments and systems using wireless communications
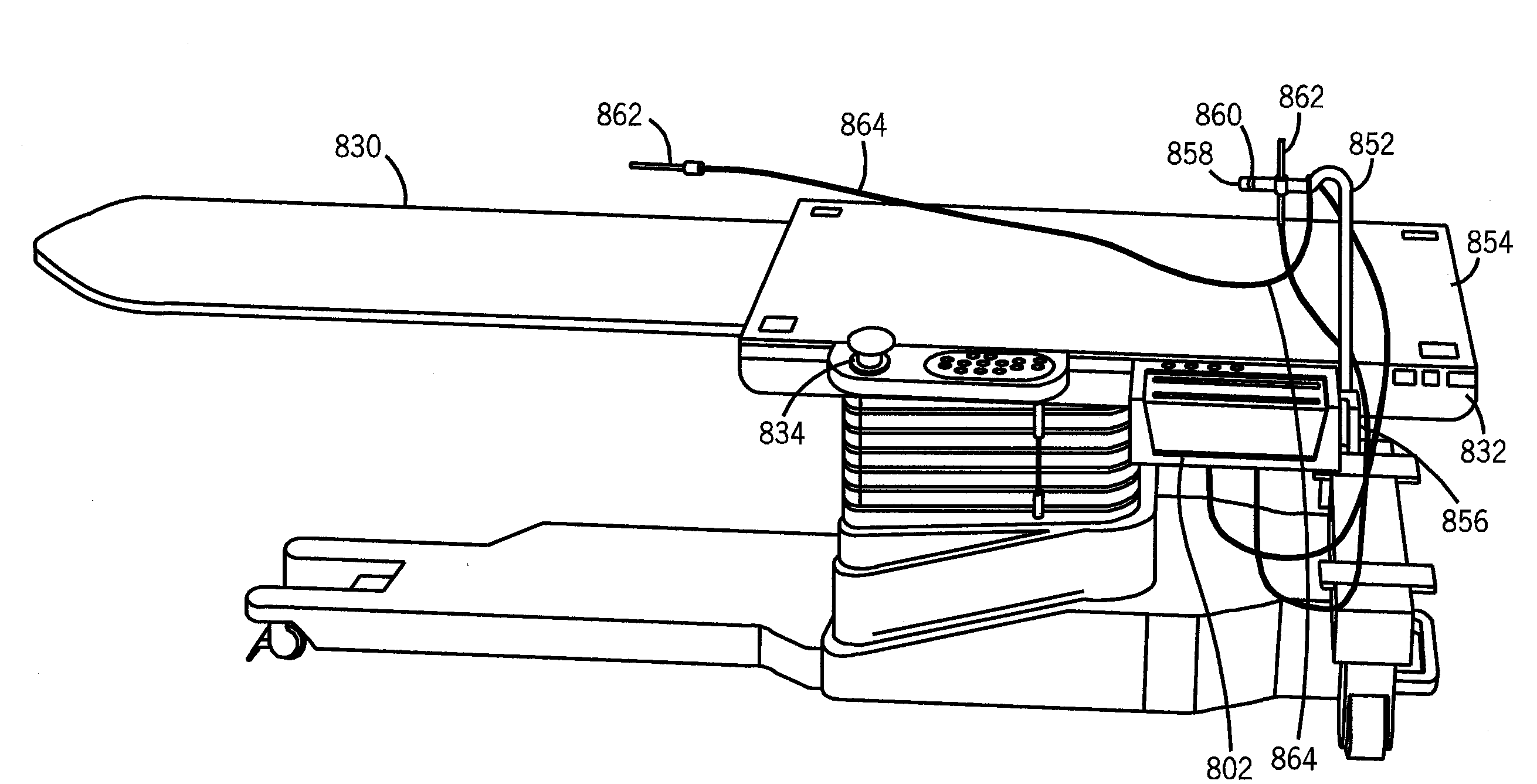
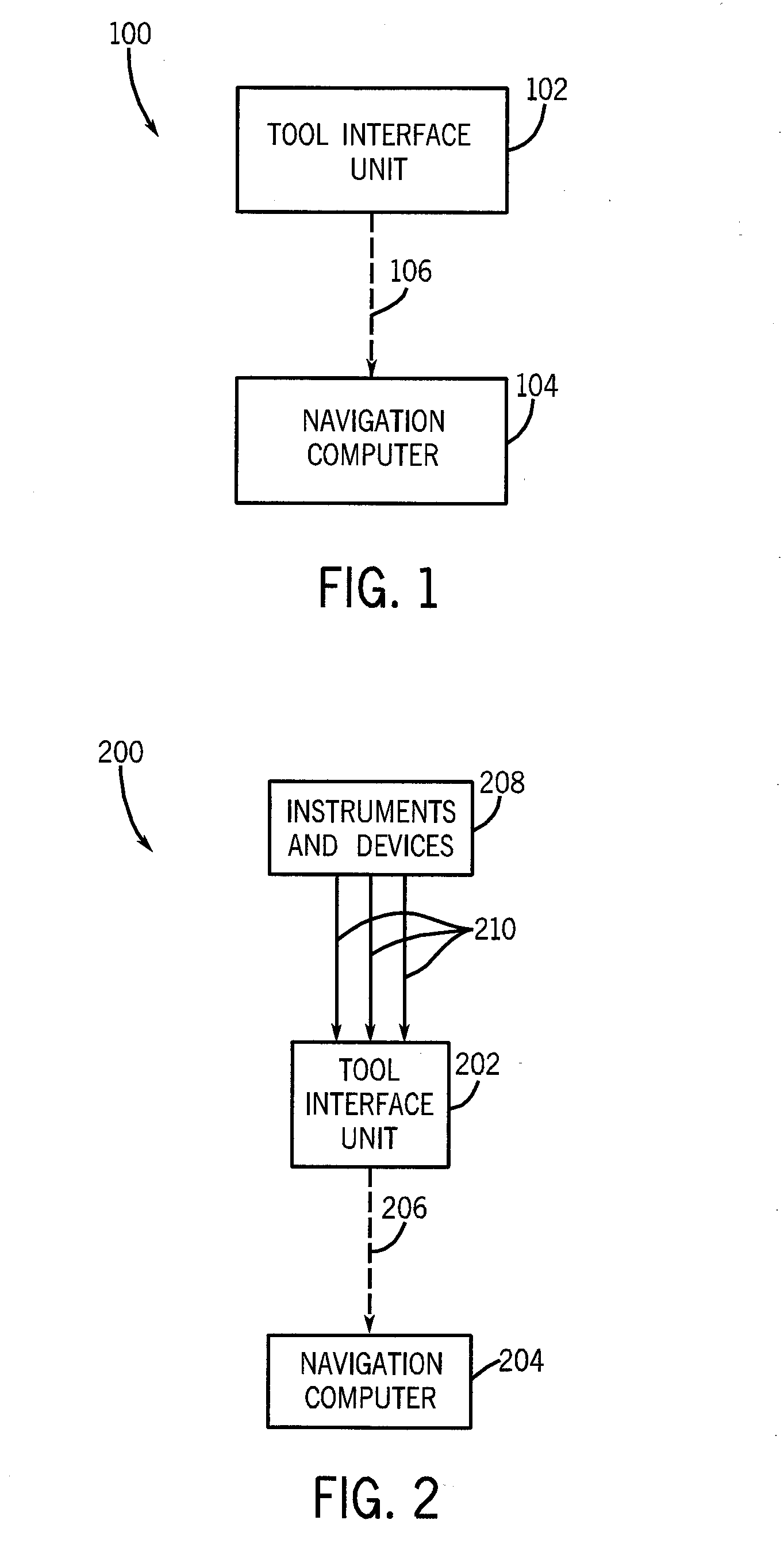
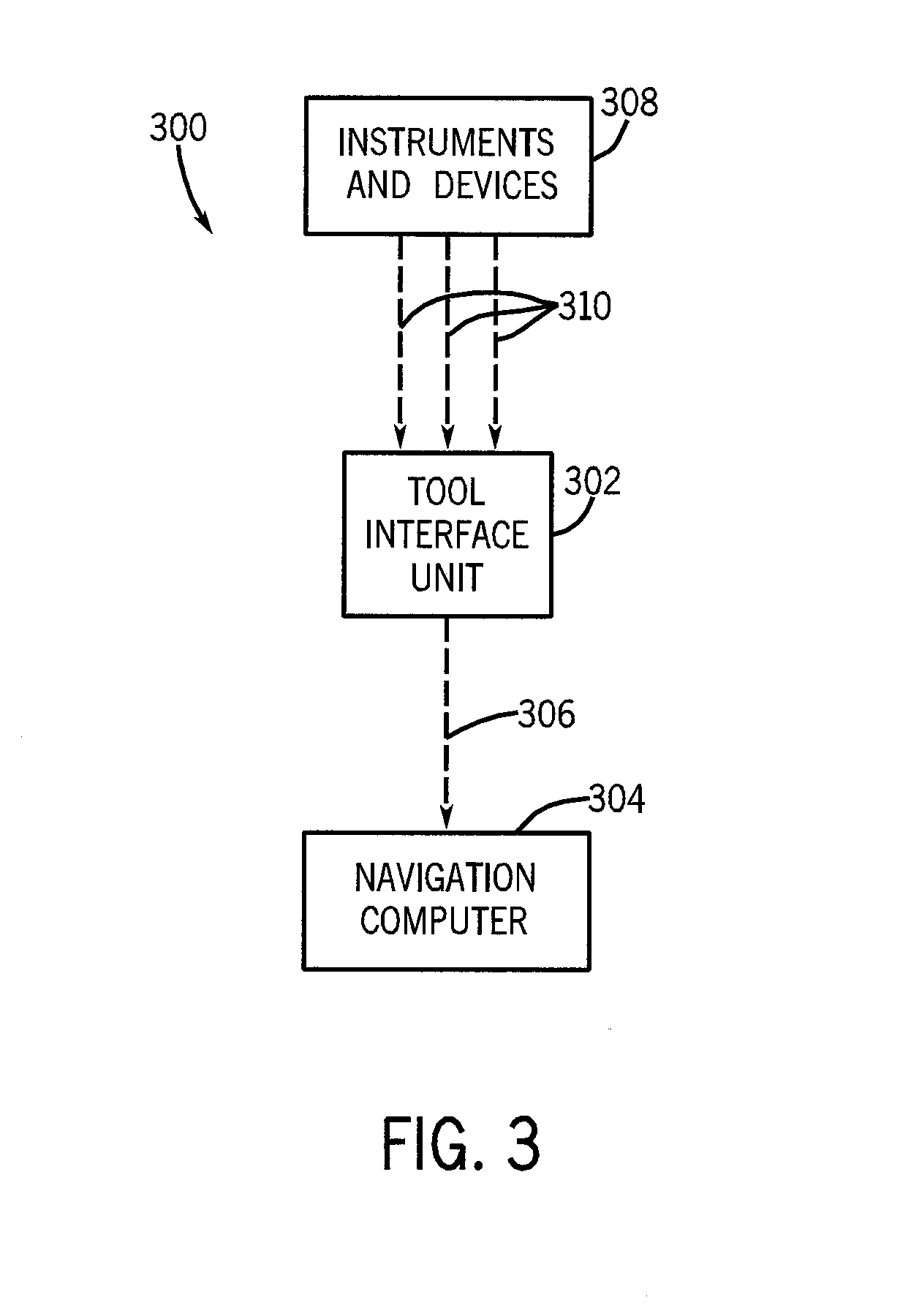
InactiveUS20080109012A1Surgical navigation systemsPatient positioning for diagnosticsSterile environmentTransceiver
System, method and apparatus are provided through which in some embodiments, a tool interface unit (TIU) of a medical navigation system or an integrated medical imaging and navigation system includes a wireless transceiver to communicate with a navigation computer in order to reduce or eliminate cabling between the TIU and the navigation computer. In some embodiments, the TIU is mounted to a side of a surgical table in order to reduce the possibility of contamination of the cables from a non-sterile environment. In some embodiments, the TIU is battery powered and includes a battery and a battery charger.
Owner:GENERAL ELECTRIC CO
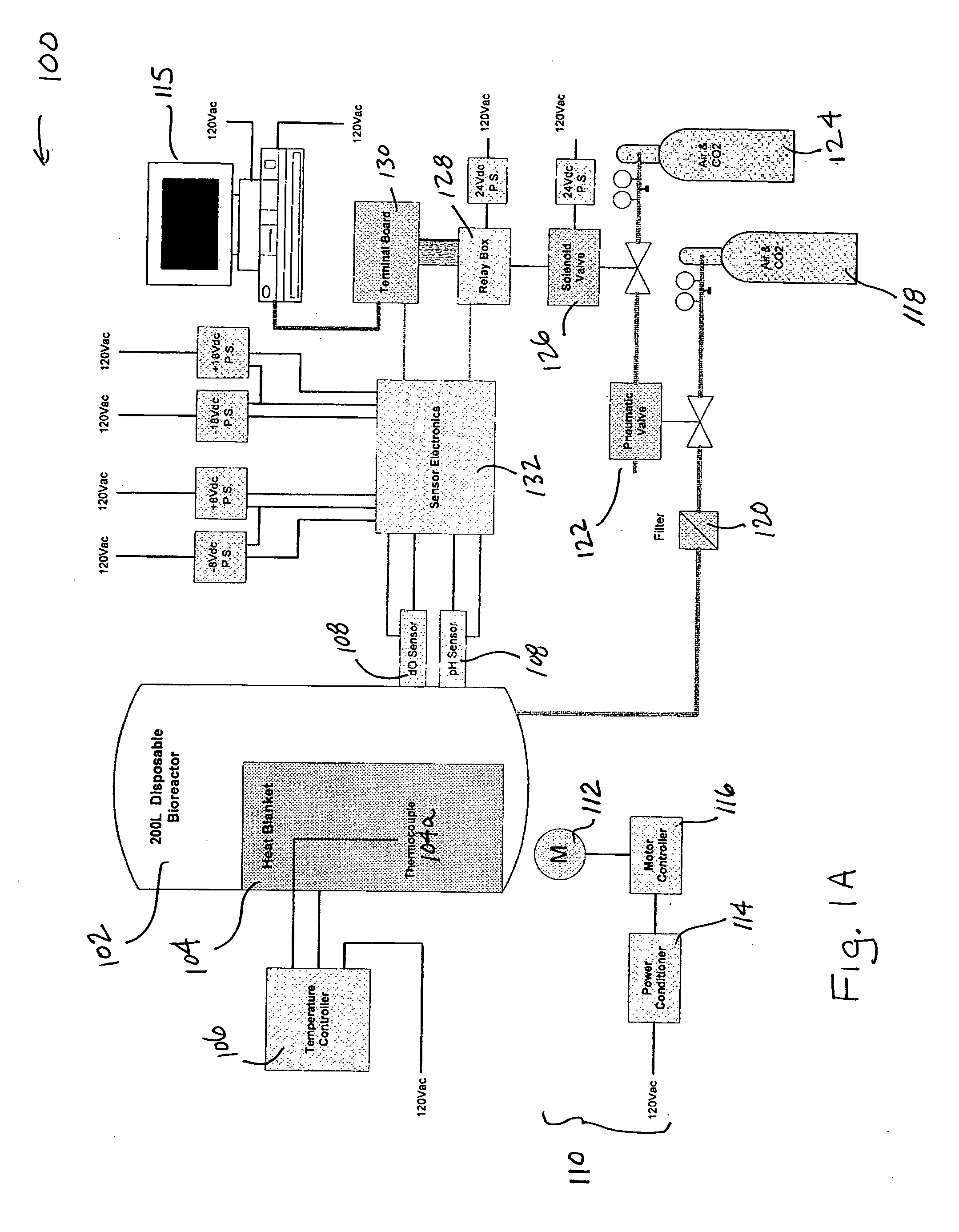
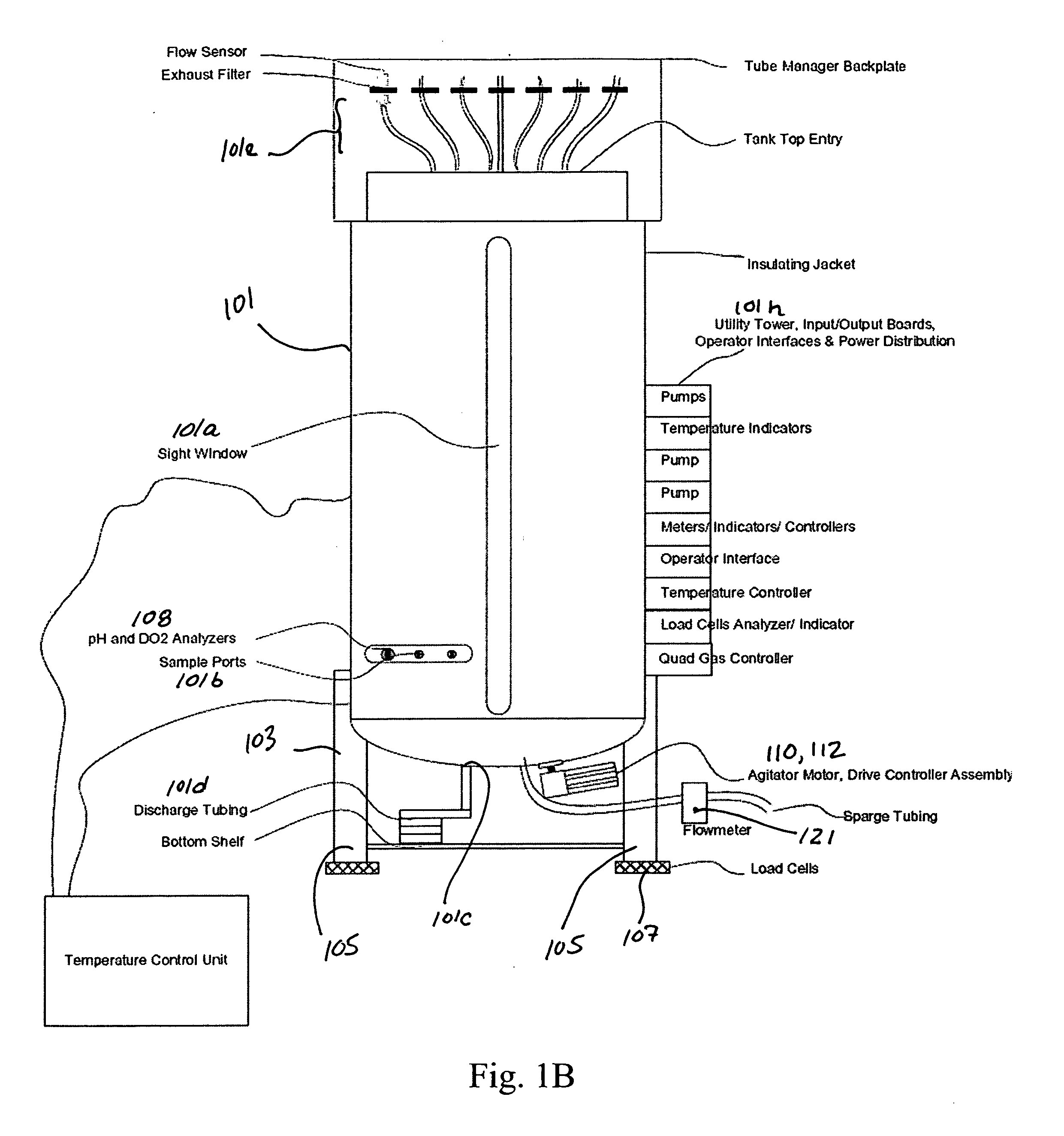
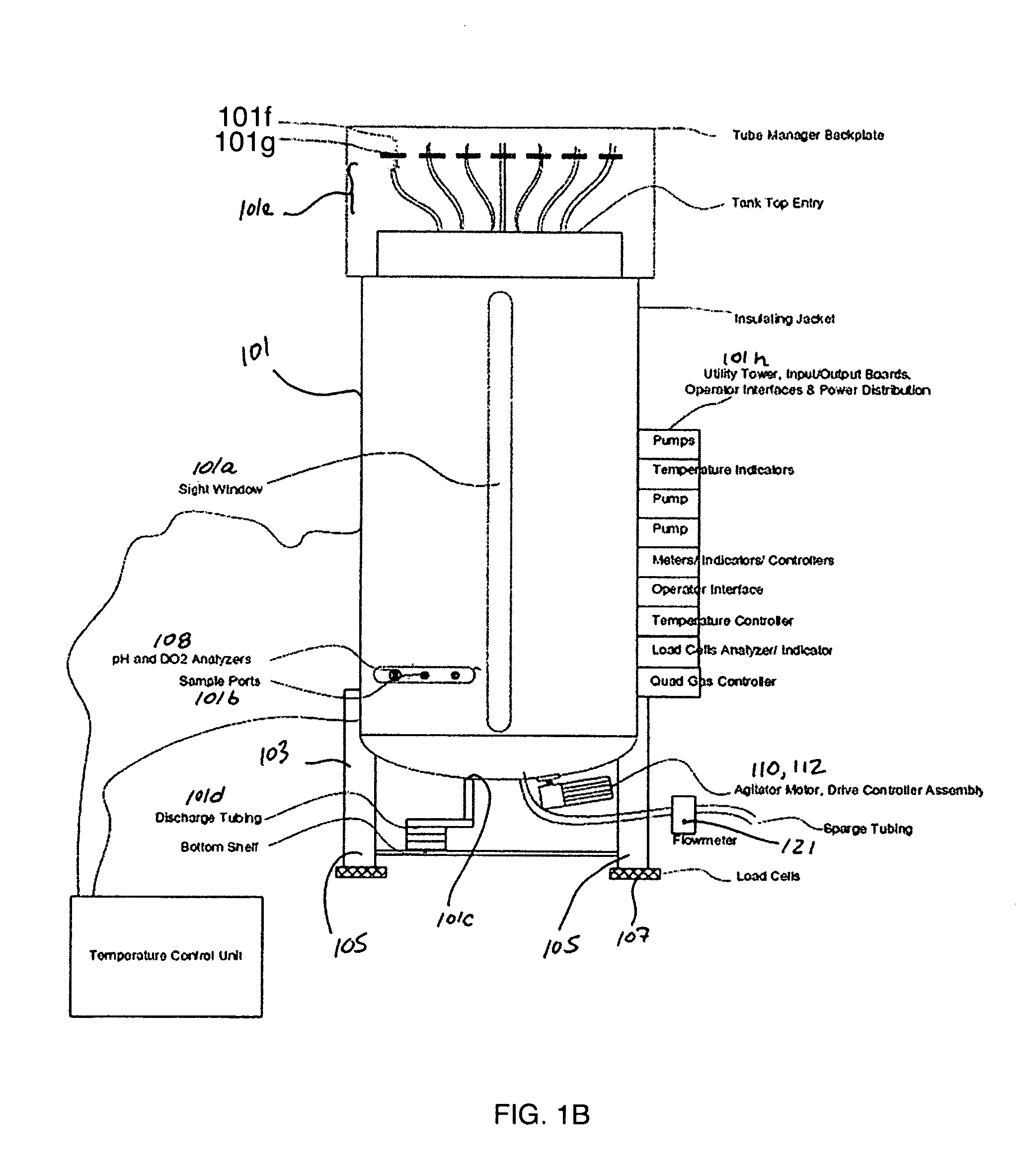
Disposable bioreactor systems and methods
ActiveUS20050272146A1Reduce wearReduce tearingBioreactor/fermenter combinationsBiological substance pretreatmentsOpen portSterile environment
Accordingly, in one embodiment of the invention, a bioreactor system is presented and includes a disposable container for housing biomaterials for processing, the disposable container including at least one input port, at least one exhaust port, at least one harvest port, and the integrity of the sterile environment is protected with sterile filters attached to all external open ports a structure for supporting the disposable container, one or more sensors for sensing one or more parameters of the biomaterials in the container, a heater for heating the contents of the container, the heater having a thermostat and mixing system arranged with the system such that biomaterials contained in the disposable container are mixed.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
Disposable bioreactor systems and methods
ActiveUS7629167B2Reduce wearReduce chanceBioreactor/fermenter combinationsBiological substance pretreatmentsSterile environmentHybrid system
Accordingly, in one embodiment of the invention, a bioreactor system is presented and includes a disposable container for housing biomaterials for processing, the disposable container including at least one input port, at least one exhaust port, at least one harvest port, and the integrity of the sterile environment is protected with sterile filters attached to all external open ports a structure for supporting the disposable container, one or more sensors for sensing one or more parameters of the biomaterials in the container, a heater for heating the contents of the container, the heater having a thermostat and mixing system arranged with the system such that biomaterials contained in the disposable container are mixed.
Owner:GLOBAL LIFE SCI SOLUTIONS USA LLC
Handheld tracking system and devices for aligning implant systems during surgery
InactiveUS20150133945A1Accurate placementPrevent any length discrepancy in leg lengthSurgical navigation systemsJoint implantsSterile environmentScheie operation
The present invention discloses handheld tracking systems and devices comprising of at least one handheld tracking device for intra-operative aligning or positioning of surgical implant systems and instruments with reference to the anatomy of a patient. The handheld tracking system further comprises of at least one trackable element. The handheld device is mounted at the proposed implantation site using the holding means while trackable element(s) is / are mounted at predetermined location(s) such that data from said deployed trackable element(s) relating to the position of the patient and the surgical instruments are input continuously into the handheld devices. The handheld device then processes the data on the basis of pre-loaded preoperative scanned images in the processing means to monitor the accurate placement of said implant system onsite in sterile environment.
Owner:STRYKER GLOBAL TECH CENT
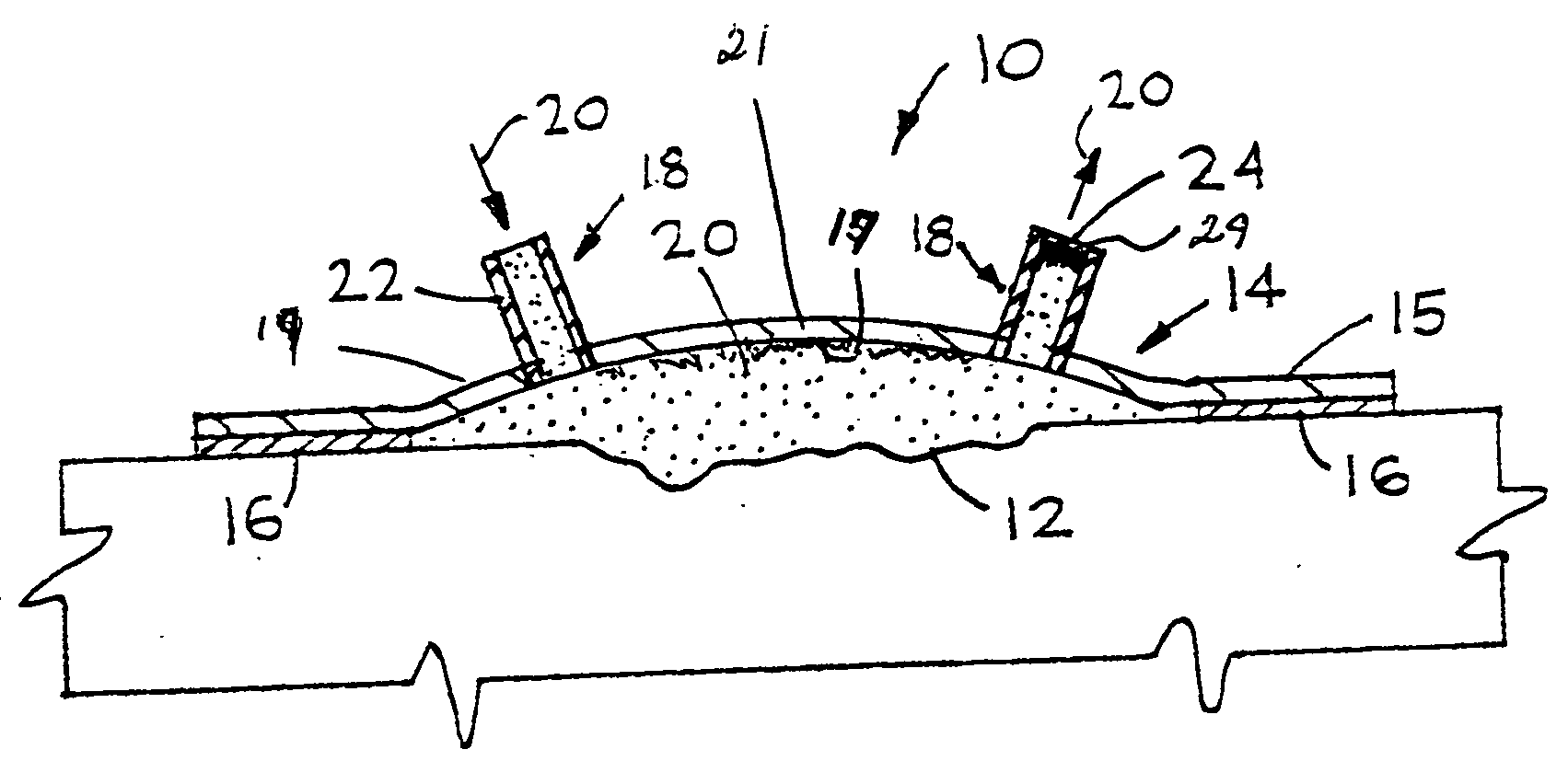
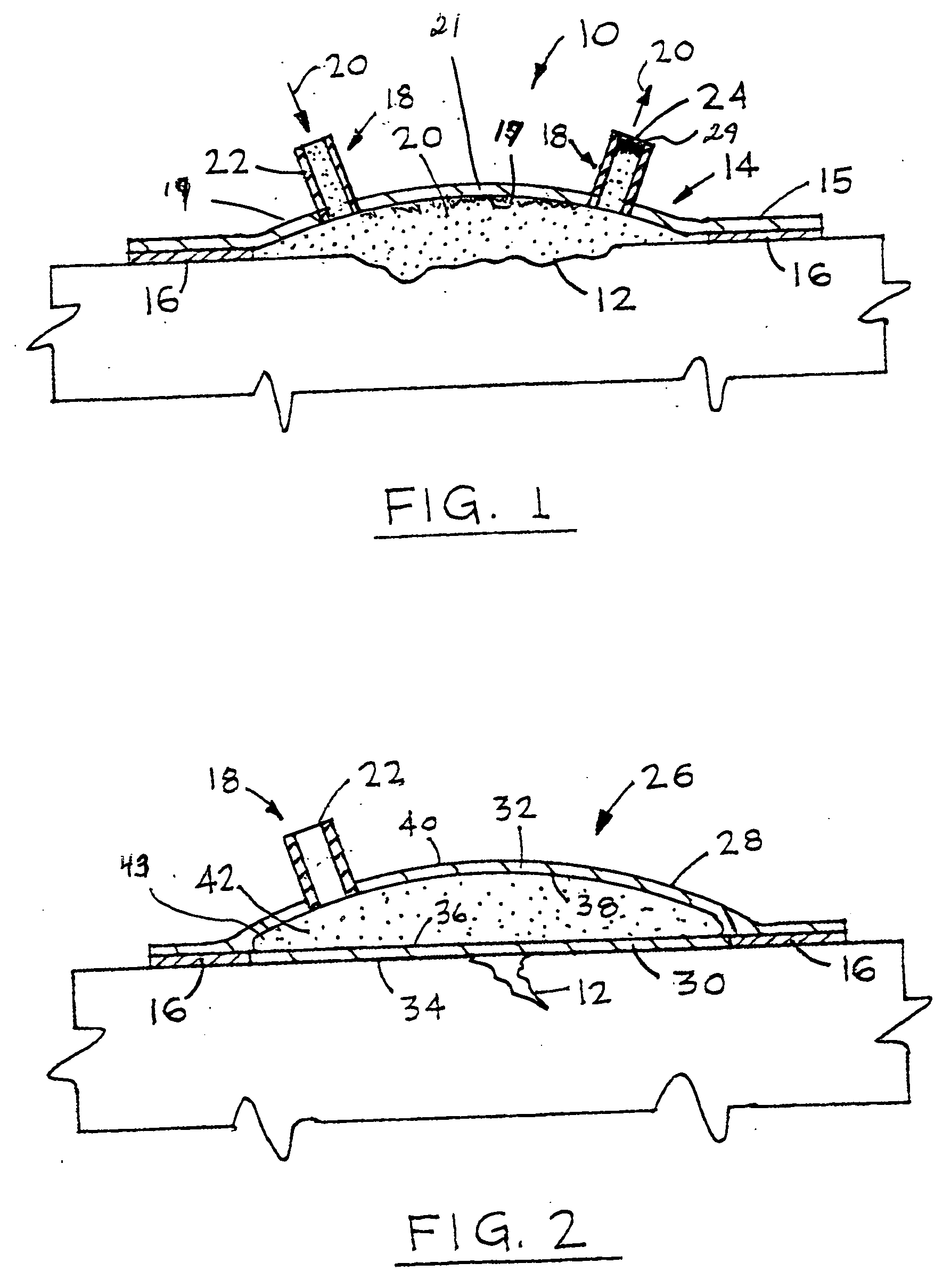
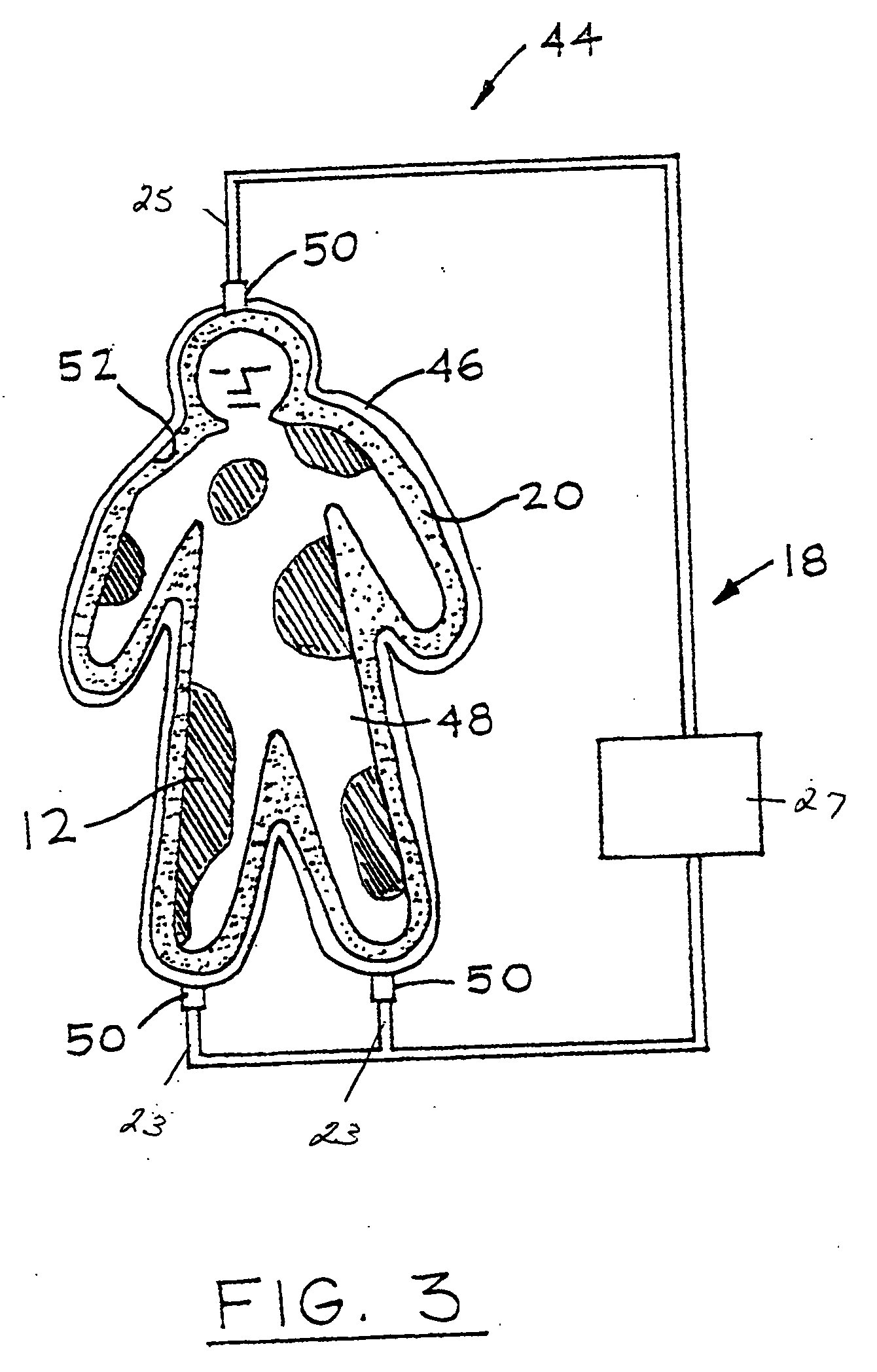
Wound alternative treatment system
InactiveUS20060116620A1Permit visual inspectionPrevent dehydrationPlastersAdhesive dressingsSterile environmentAlternative treatment
A wound treatment device for treating damaged body tissue comprising an encapsulating member having a size and shape capable of being attached to a patient to encapsulate a wound. A fluid communication member is provided for introducing treatment fluid within the encapsulating means for treatment of the wound. The fluid communication member is connected with a supply means for supplying treatment fluid thereto. The fluid communication member is capable of simultaneously transmitting multiple treatment fluids from the supply means into said encapsulating member. The inner surface of the wall of the encapsulating member is textured to allow fluid flow across the wound. The encapsulating member is also formed from a clear material to permit continuous visual inspection of the wound while maintaining a sterile environment.
Owner:OYASKI MICHAEL F
Inherently antimicrobial quaternary amine hydrogel wound dressings
InactiveUS6039940APrevent discolorationAvoid hydrolysisBiocideConductive materialSterile environmentAryl
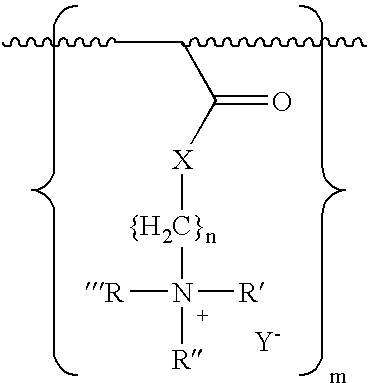
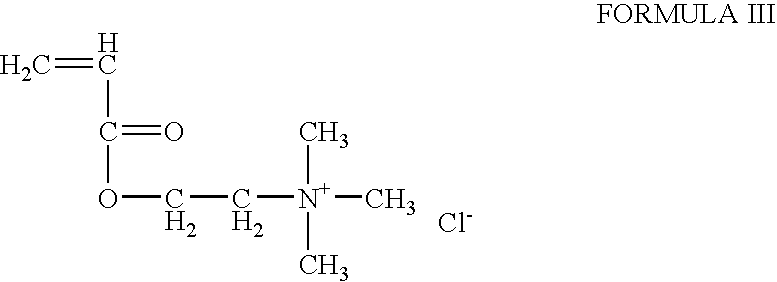
A composition and method for treating a wound with an inherently antimicrobial dressing. The dressing is a hydrogel containing from about 15 to 95 percent, and preferably from about 61 to 90 percent, by weight of a cationic quaternary amine acrylate polymer prepared by the polymerization of acryloyloxyethyl(or propyl)-trialkyl(or aryl)-substituted ammonium salts or acrylamidoethyl(or propyl)-trialkyl(or aryl)-substituted ammonium salts. The antimicrobial hydrogels are non-irritating to the wound, absorb wound exudate, and, due to the inherently antimicrobial properties, enhance the sterile environment around the wound. The hydrogels have sufficient adhesive properties that loose contact with the wound is assured but can also be removed without leaving any gel residue on the wound. The wound dressings are preferably formed on a substrate, such as a web or patch, for ease in application to and removal from the wound. If desired, additional antimicrobial or other pharmaceutically active agents can also be incorporated into the hydrogel structure.
Owner:AVENT INC
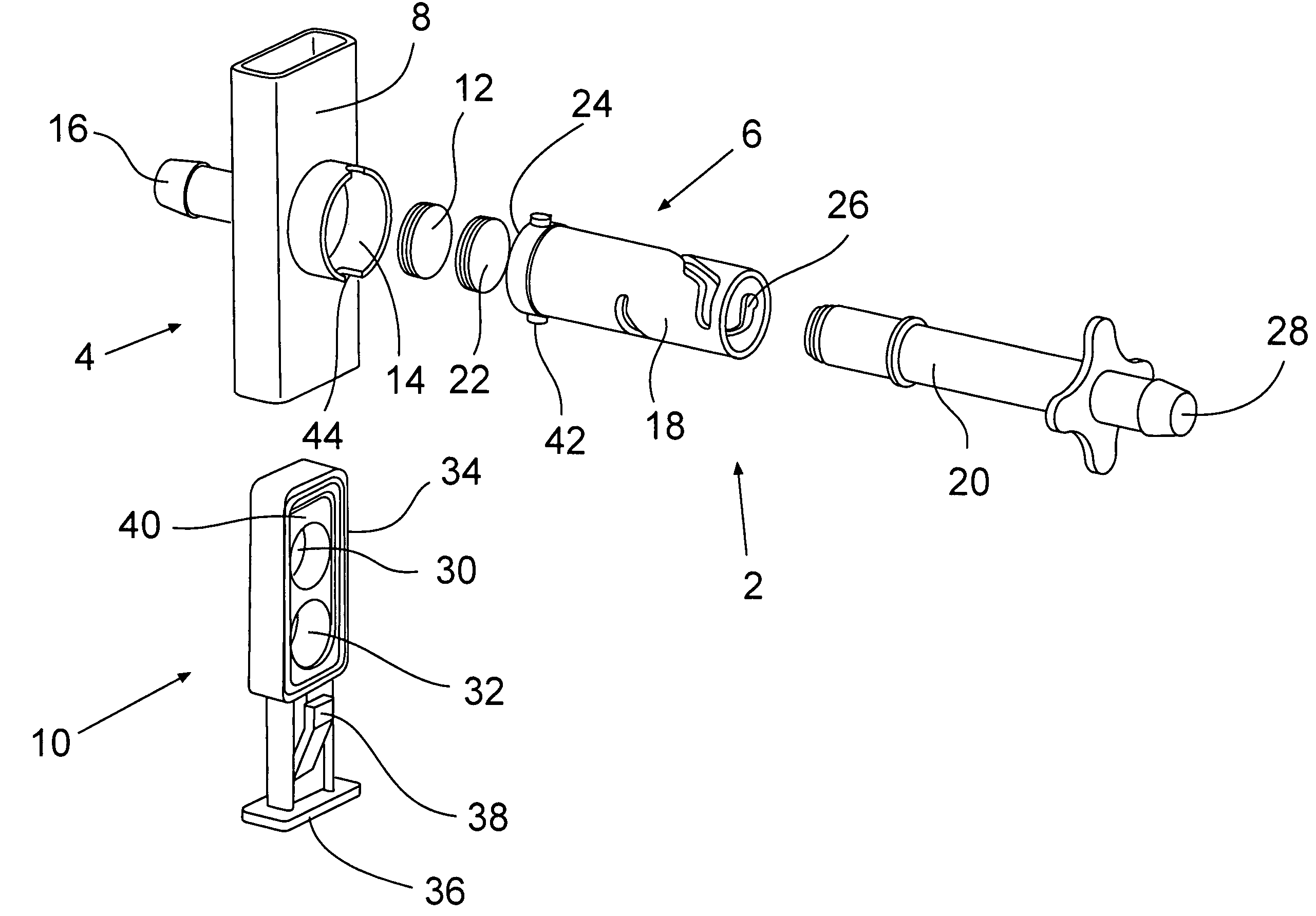
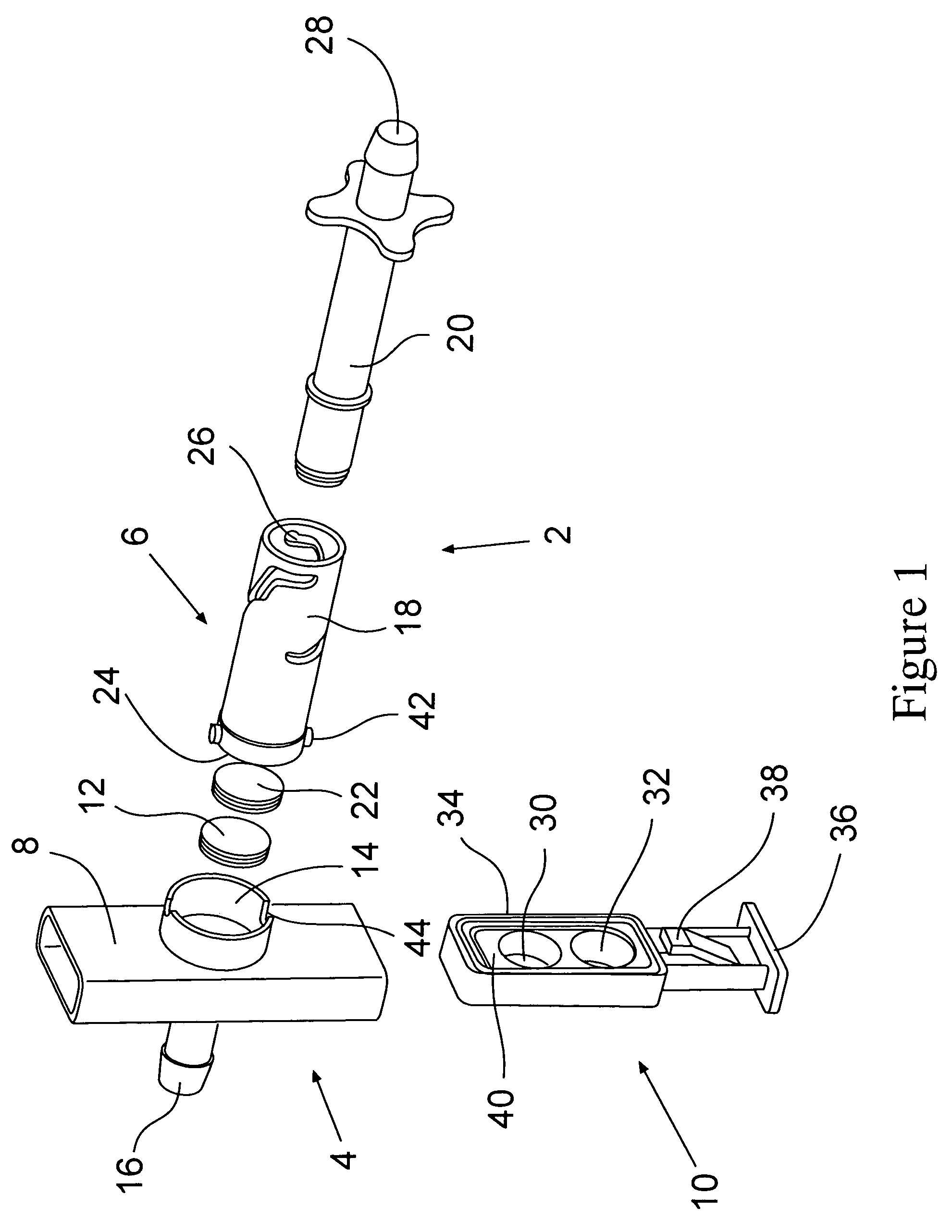
Continuous Feed Hypodermic Syringe with Self Contained Cartridge Dispenser
InactiveUS20080058732A1Provide impact resistancePrevented being caughtAmpoule syringesAutomatic syringesPatient needSterile environment
A hypodermic syringe and a plurality of single use cartridges able to be successively loaded into said syringe for providing rapid dispensing of a medicant to numerous users without possibility of contamination. The cartridges are continuously fed through the syringe for the quick and efficient inoculation of patients. An operator inserts a needle into a patient by pushing in a trigger. Prior to the needle being inserted into the patient, a disinfectant is dispersed from the cartridge to maintain sterility. An operator then pushes in a plunger forcing the medication through the needle into the patient. The plunger is pulled back into its original location after use. The needle is pulled back with the plunger out of the patient's skin and back into the cartridge. A new cartridge on a clip is then advanced into the syringe for use in inoculating the next patient needing medication. The syringe and cartridge dispenser further maintains a sterile environment during successive vaccinations reducing the transmission of any disease from patient to patient.
Owner:HARRIS ARTHUR
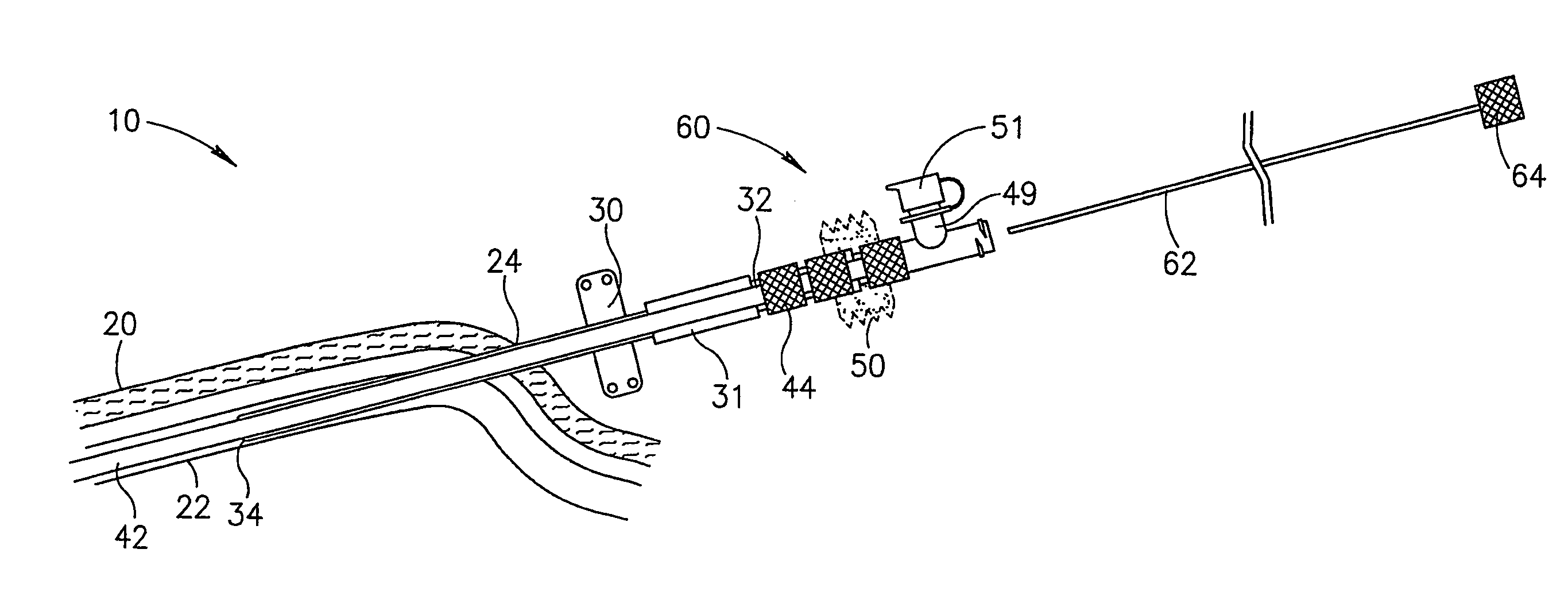
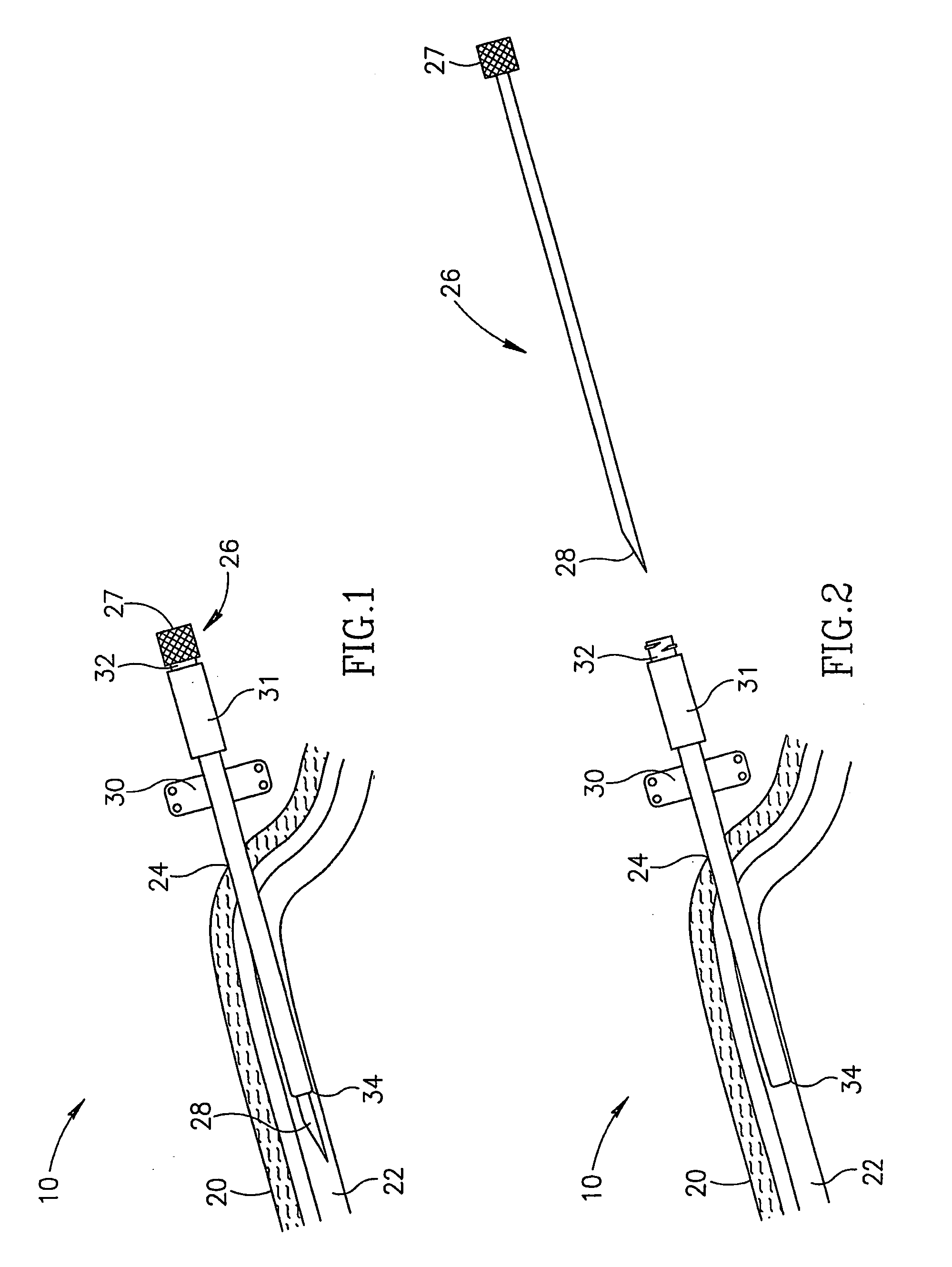
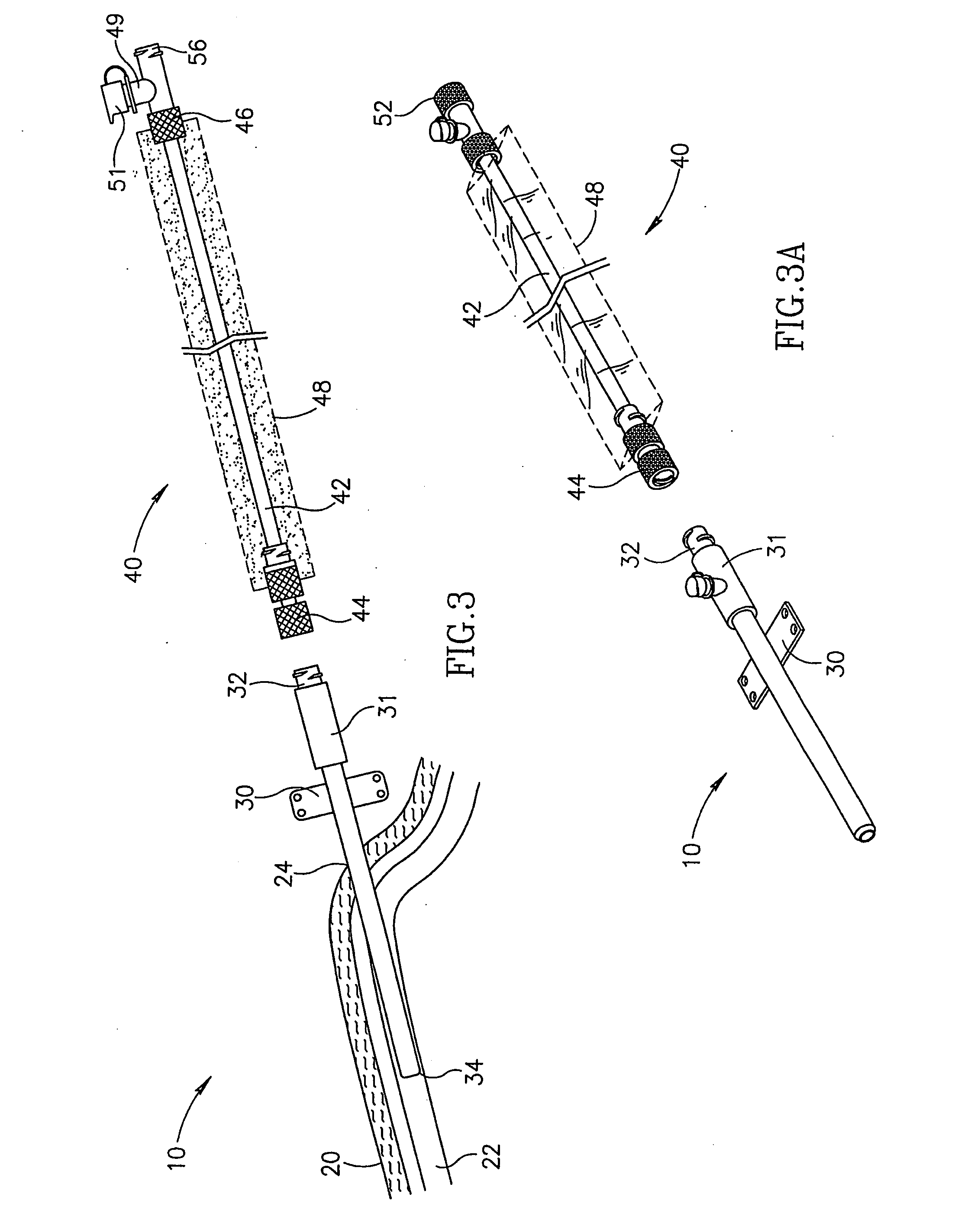
Method and apparatus for penetrating tissue
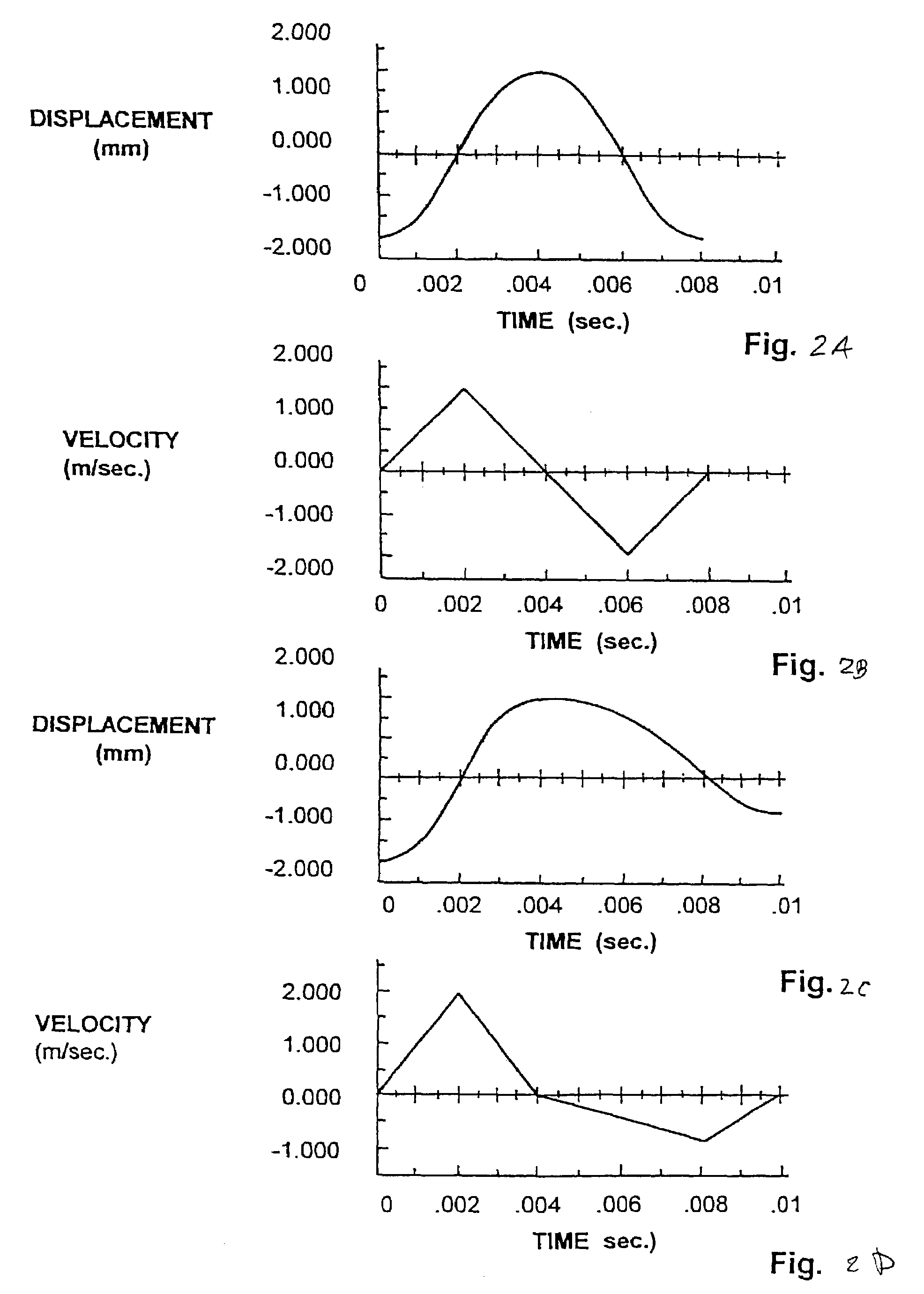
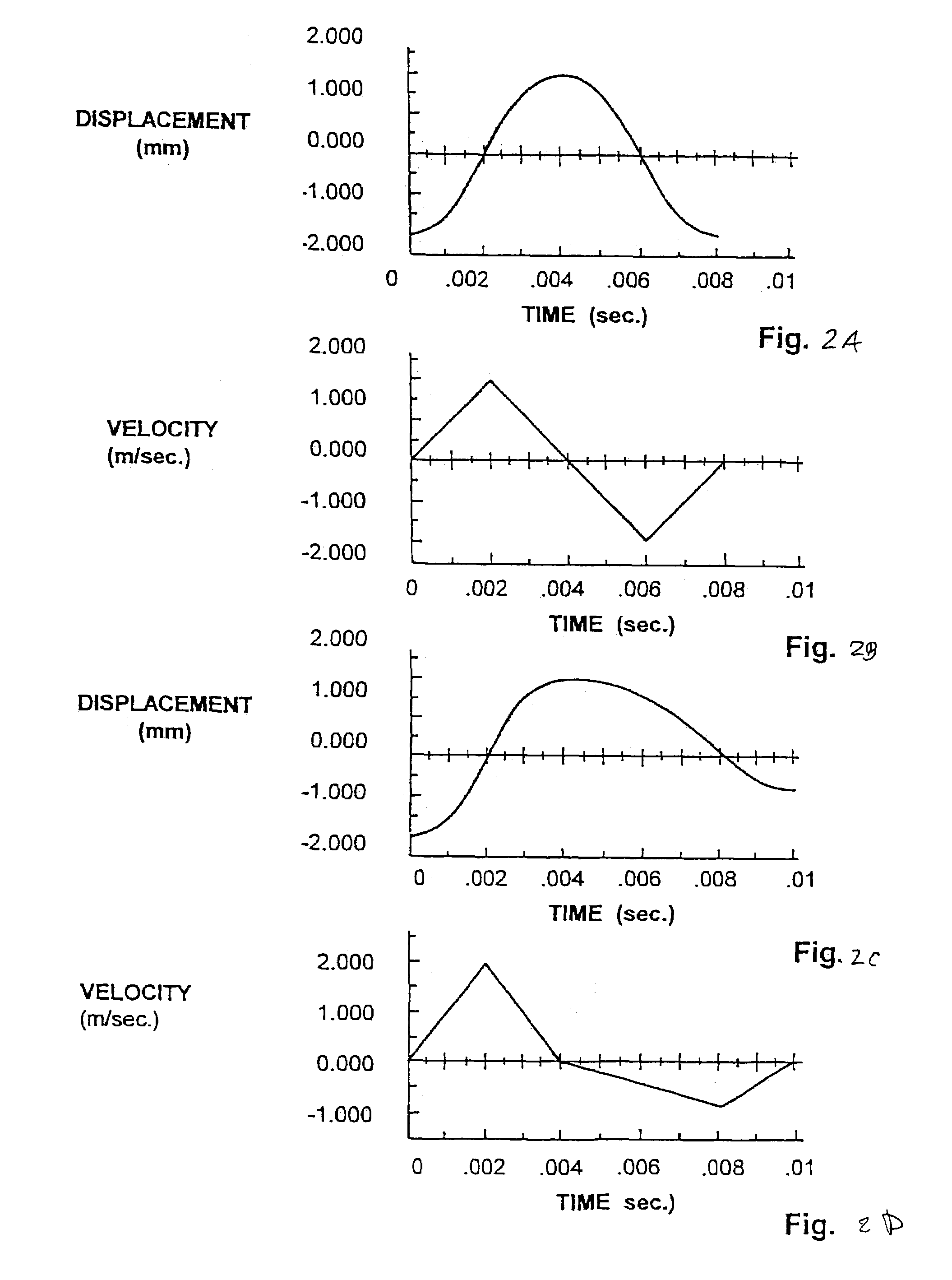
InactiveUS7648468B2Relieve painIncision instrumentsLaboratory glasswaresSterile environmentEngineering
A body fluid sampling system for use on a tissue site includes an electrically powered drive force generator. A penetrating member is operatively coupled to the force generator. The force generator moves the member along a path out of a housing having a penetrating member exit, into the tissue site, stops in the tissue site, and withdraws out of the tissue site. A cartridge houses the penetrating member. The cartridge has first and second seals coupled to the penetrating member to maintain a sterile environment around a portion of the penetration member prior to penetrating member actuation.
Owner:SANOFI AVENTIS DEUT GMBH
Method and apparatus for penetrating tissue
InactiveUS7331931B2Relieve painIncision instrumentsLaboratory glasswaresSterile environmentEngineering
These and other objects of the present invention are achieved in a body fluid sampling system for use on a tissue site that includes an electrically powered drive force generator. A penetrating member is operatively coupled to the force generator. The force generator moves the member along a path out of a housing having a penetrating member exit, into the tissue site, stops in the tissue site, and withdraws out of the tissue site. A cartridge houses the penetrating member. The cartridge has first and second seals coupled to the penetrating member to maintain a sterile environment around a portion of the penetration member prior to penetrating member actuation. A user interface is configured to relay at least one of, penetrating member performance or a penetrating member setting.
Owner:SANOFI AVENTIS DEUT GMBH
Partially disposable endoscopic device
An endoscopic device having a one-handed, either-handed steering mechanism is presented. The endoscopic device includes some components that are sterile and used on only a single patient. The single-use components are medically disposed of after exposure to a non-sterile environment such as an internal body cavity of a patient. The endoscopic device also includes some reusable components that are not exposed to a non-sterile environment.
Owner:LUCENT MEDICAL SYST
Method and apparatus for loading penetrating members
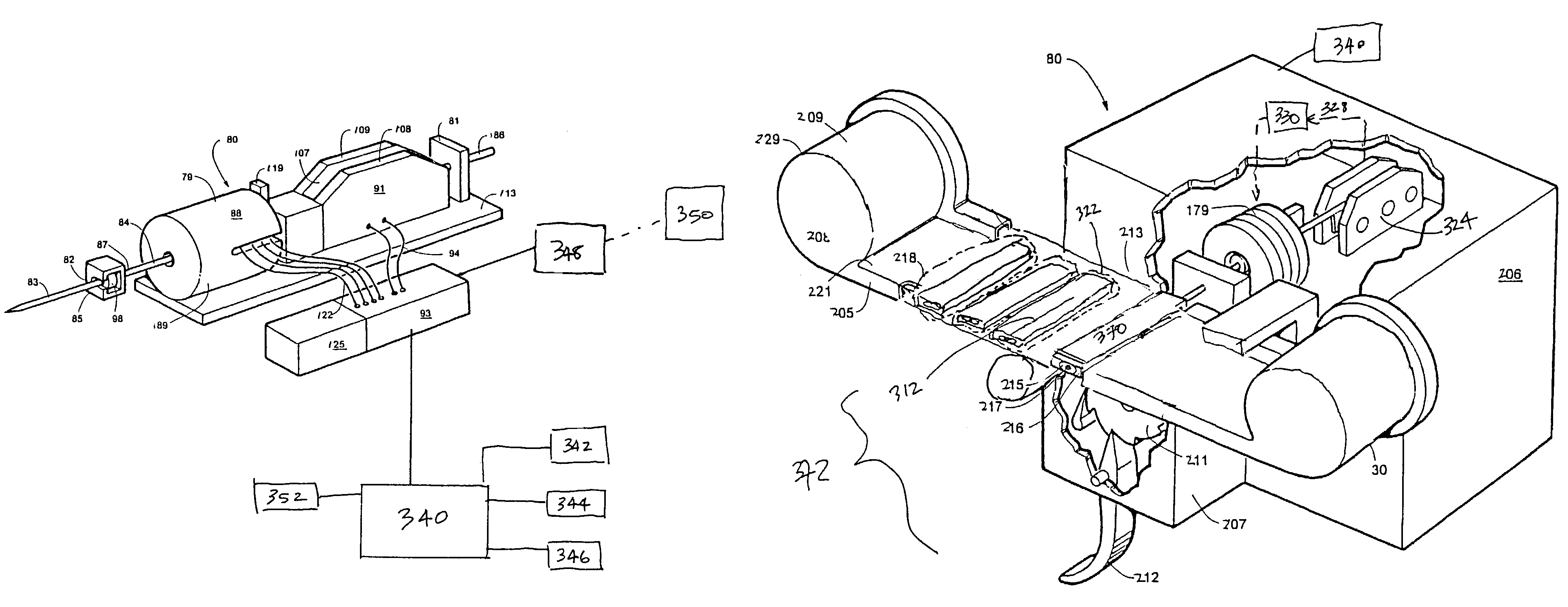
InactiveUS20060195128A1Minimizes user handlingSensorsBlood sampling devicesSterile environmentHuman–machine interface
A body fluid sampling system is provided. In one embodiment, the system may comprise a penetrating member driver, a plurality of penetrating members sufficient for penetrating tissue; a tape coupling together at least two of the penetrating members; and a penetrating member release device removing the penetrating member from a sterile environment prior to use and moving the penetrating member into position to be operatively coupled to the penetrating member driver. The driver may comprises of a drive force generator for advancing the penetrating member and a processor coupled to the drive force generator capable of changing the direction and magnitude of force exerted on the penetrating member during the lancing cycle. The driver may further include a human interface on the housing providing at least one output for communicating with the patient.
Owner:PELIKAN TECH INC
Apparatus and method for deployment of a bronchial obstruction device
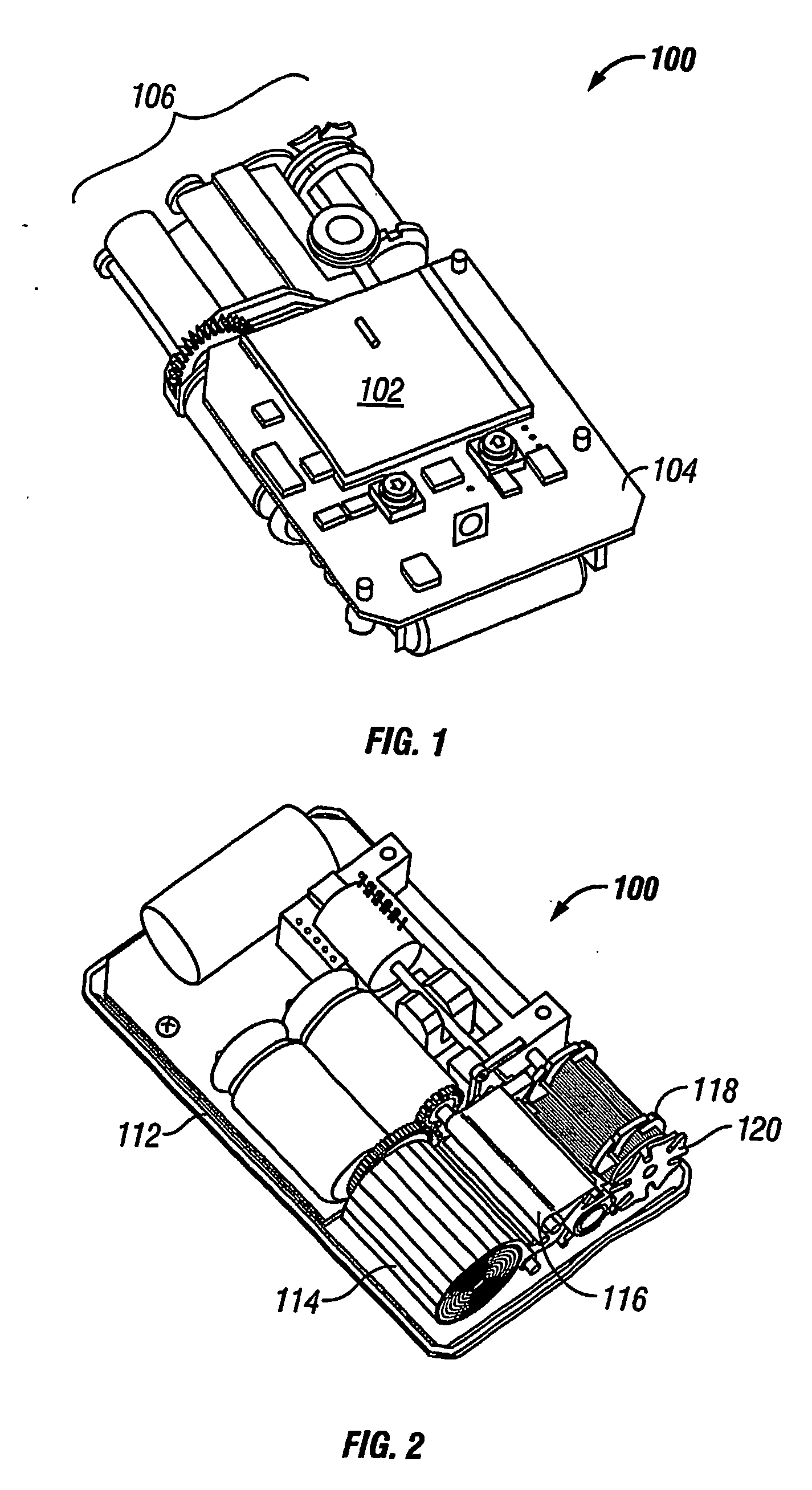
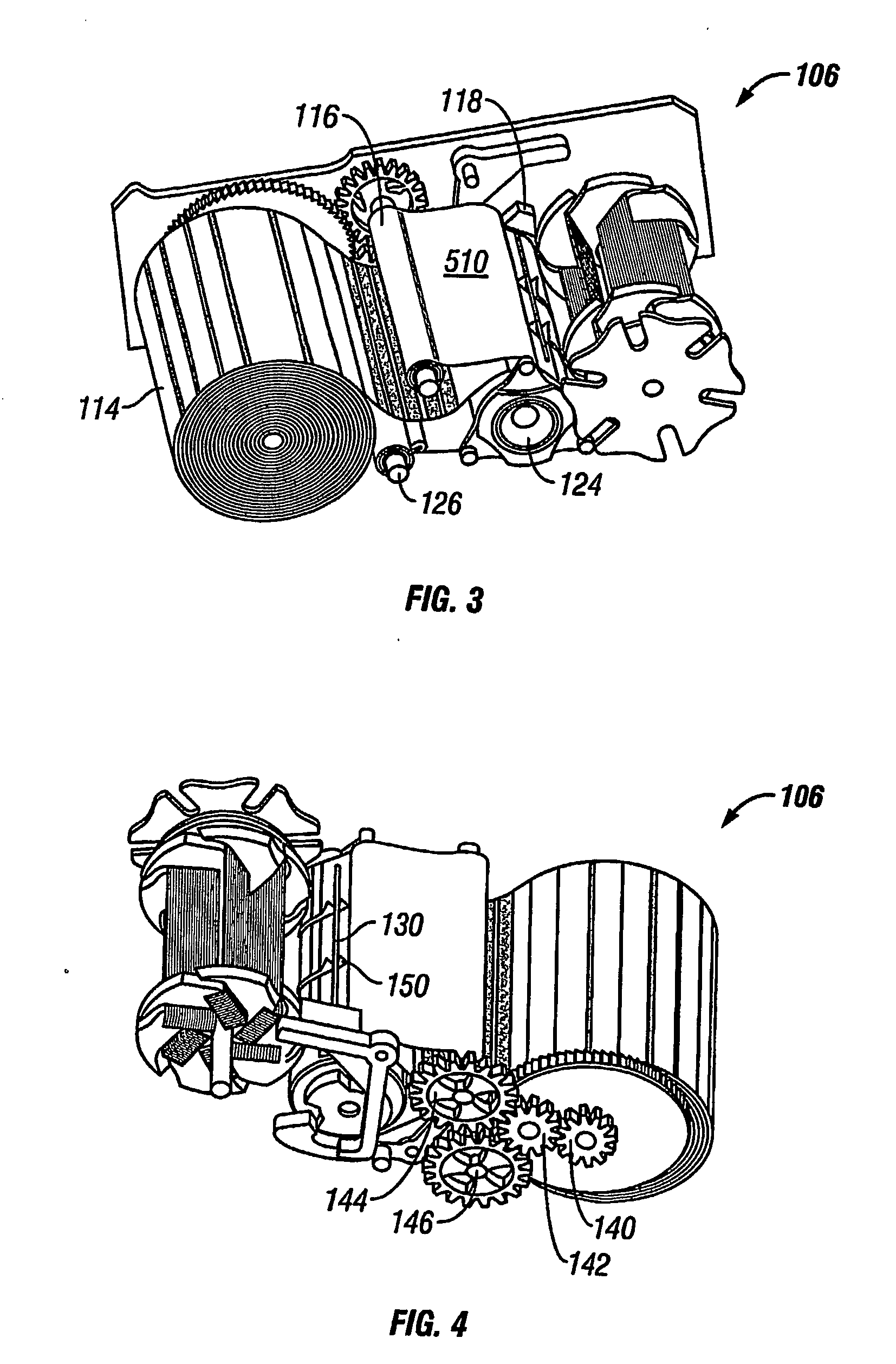
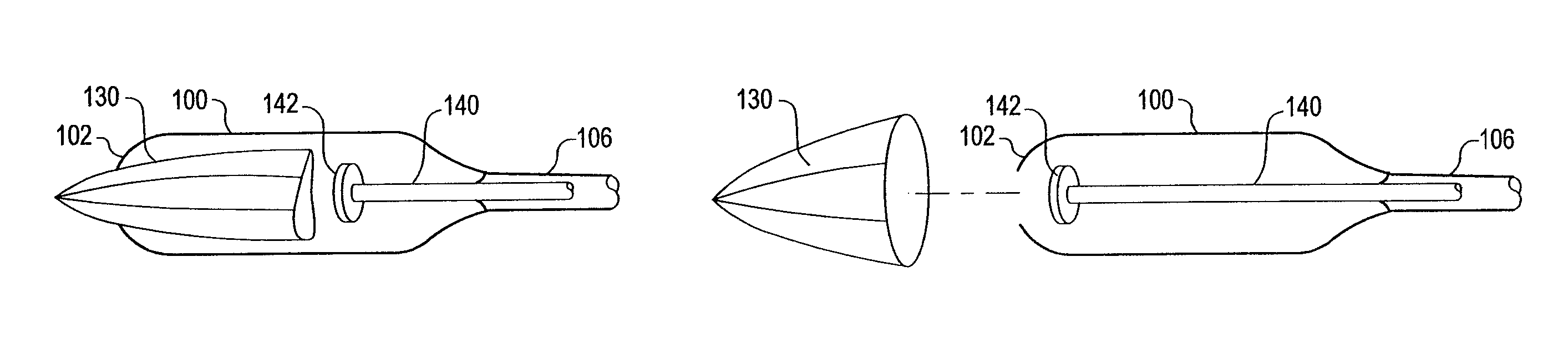
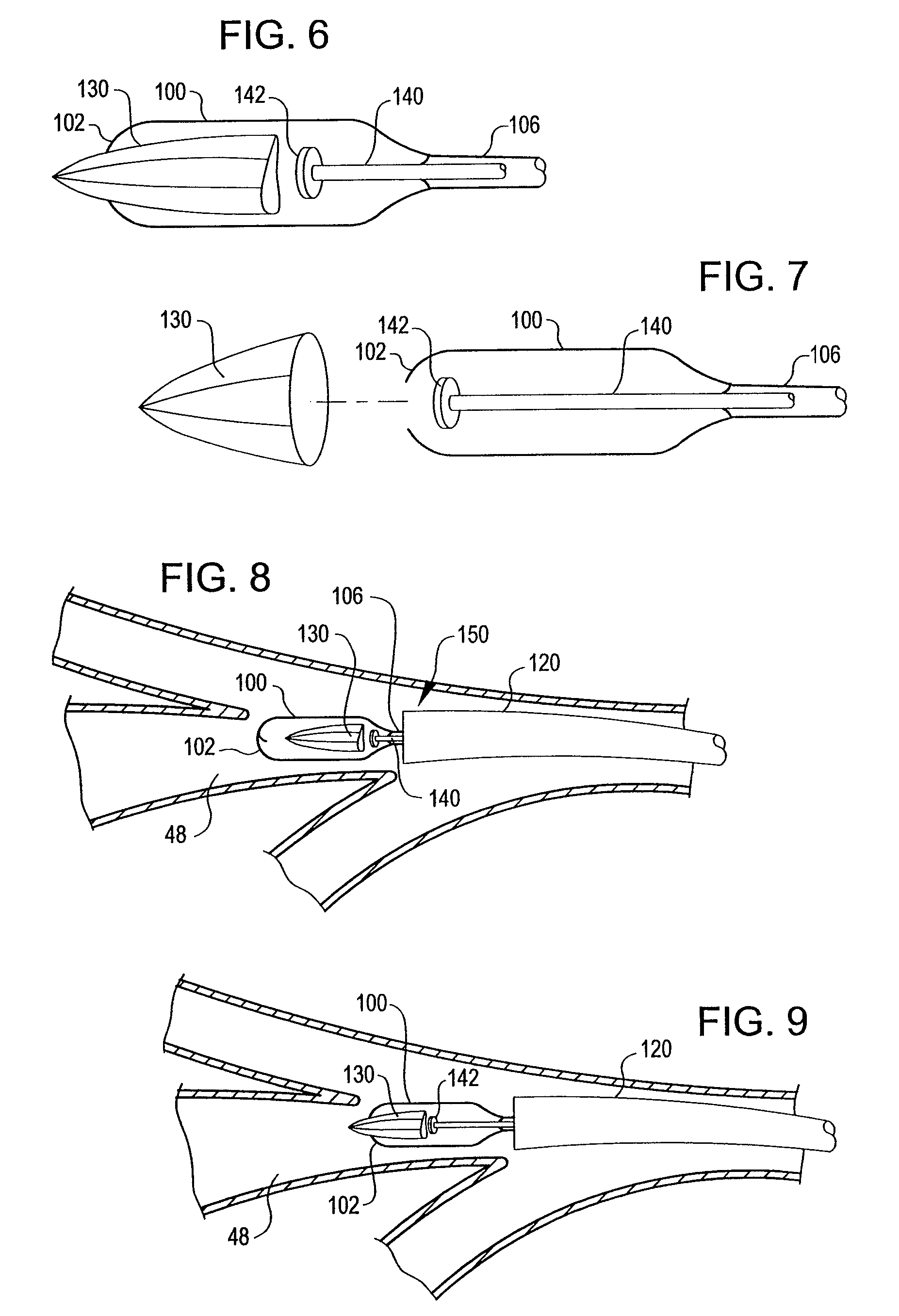
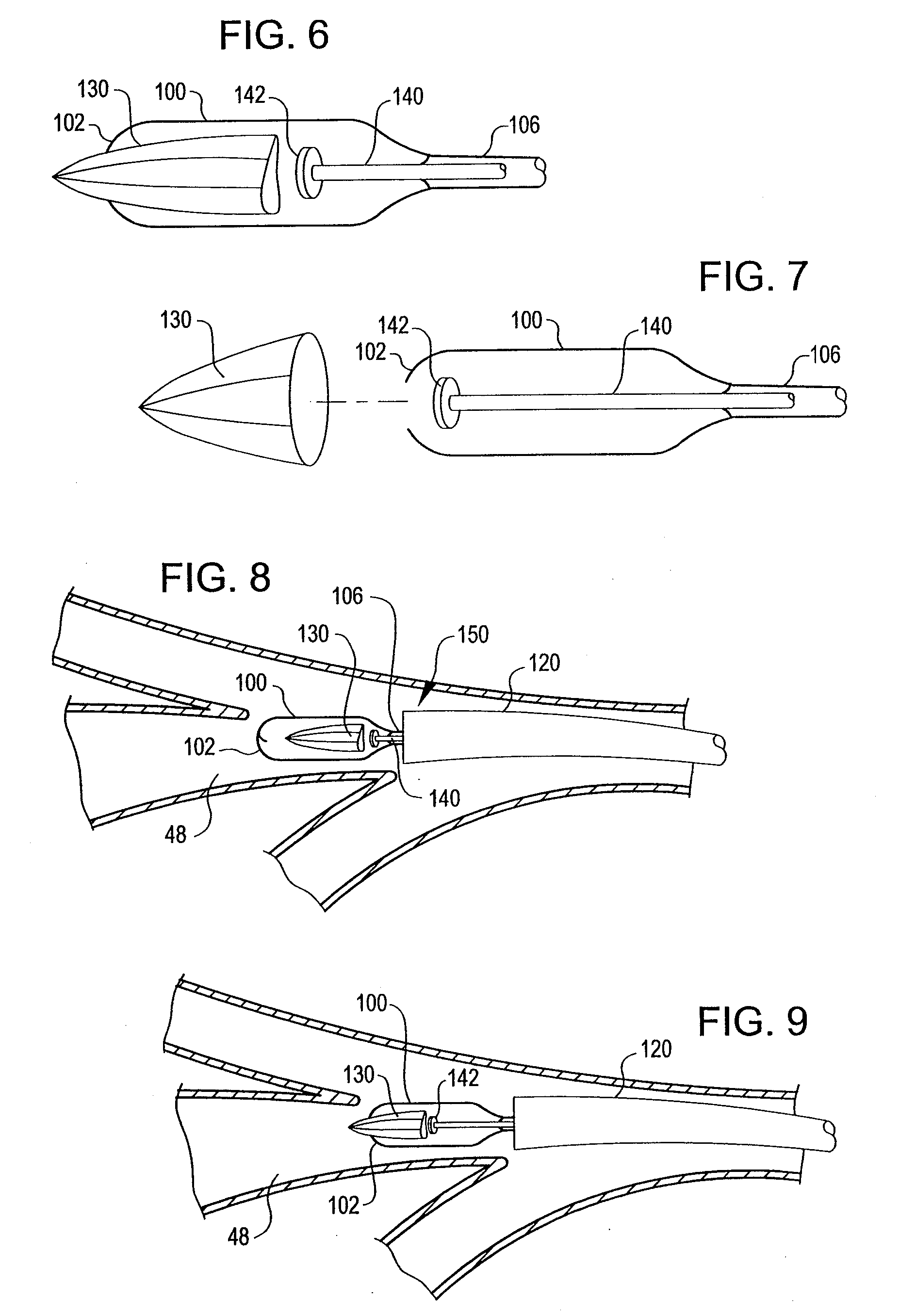
An apparatus and method deploy a self-expandable bronchial obstruction device in an air passageway. The apparatus includes a catheter configured to be passed down the trachea. The apparatus further includes a capsule for housing the self-expandable bronchial obstruction device in a sterile environment. The capsule is configured to be advanced down the catheter. The capsule further includes a tubular extension. The capsule has a breakable seam so as to release the bronchial obstruction device in the air passageway upon a proximal force being exerted upon the bronchial obstruction device. The method includes guiding a conduit down a trachea into the air passageway. The method further includes advancing a capsule having a bronchial device therein down an internal lumen of the conduit into the air passageway. The method further includes releasing the bronchial device from the capsule. The method further includes deploying the bronchial device into the air passageway.
Owner:GYRUS ACMI INC (D B A OLYMPUS SURGICAL TECH AMERICA)
Surgical system with medical manipulator and sterile barrier
A surgical system for use in performing medical procedures on a body of a patient is provided. The system can include a manipulator having a tool mounting arrangement including a modulated mechanical energy transmitter capable of transferring power. The manipulator is capable of moving the tool mounting arrangement with at least one degree of freedom. The system also includes a tool support including a modulated mechanical energy receiver capable of receiving power. A sterile barrier is arranged between the robotic mechanism and the tool support to isolate the robotic mechanism from the sterile environment. The tool support is engageable with the tool mounting arrangement with the sterile barrier therebetween and with the sterile barrier extending between the modulated mechanical energy transmitter and receiver. The modulated mechanical energy transmitter and the modulated mechanical energy receiver can transmit power across the sterile barrier between the manipulator and the tool support when the tool support is engaged with the tool mounting arrangement. The system can further include a retention mechanism configured for engaging the tool support with the tool mounting arrangement with the sterile barrier therebetween only when the tool support and tool mounting arrangement are in at least one desired orientation relative to each other.
Owner:MICRODEXTERITY SYST
Intravenous catheter assembly
A self-contained sterile catheter apparatus for use with an intravenous cannula element. The cannula element has first and second ends and a bore formed therebetween, and is configured for transcutaneous positioning such that the first end is adapted to protrude from a limb of a subject and the second end is brought into communication with an interior of a body organ of a subject. The self-contained sterile catheter apparatus includes first and second ends and a flexible catheter tube therebetween, the catheter tube having a predetermined length and a diameter adapted for slidable insertion through the bore of the intravenous cannula element into the subject, and an integral sterile environment containment element thereby to allow insertion of the catheter tube through the cannula element into the subject in a generally non-sterile environment.
Owner:AMISAR SHAI +2
Method and apparatus for penetrating tissue
InactiveUS20070167871A1Relieve painIncision instrumentsLaboratory glasswaresSterile environmentEngineering
A body fluid sampling system for use on a tissue site includes an electrically powered drive force generator. A penetrating member is operatively coupled to the force generator. The force generator moves the member along a path out of a housing having a penetrating member exit, into the tissue site, stops in the tissue site, and withdraws out of the tissue site. A cartridge houses the penetrating member. The cartridge has first and second seals coupled to the penetrating member to maintain a sterile environment around a portion of the penetration member prior to penetrating member actuation.
Owner:SANOFI AVENTIS DEUT GMBH
Inherently antimicrobial quaternary amine hydrogel wound dressings
InactiveUS6800278B1Prevent discolorationAvoid hydrolysisPowder deliveryBiocideSterile environmentAryl
A composition and method for treating a wound with an inherently antimicrobial dressing. The dressing is a hydrogel containing from about 15 to 95 percent, and preferably from about 61 to 90 percent, by weight of a cationic quaternary amine acrylate polymer prepared by the polymerization of acryloyloxyethyl(or propyl)-trialkyl(or aryl)-substituted ammonium salts or acrylamidoethyl(or propyl)-trialkyl(or aryl)-substituted ammonium salts. The antimicrobial hydrogels are non-irritating to the wound, absorb wound exudate, and, due to the inherently antimicrobial properties, enhance the sterile environment around the wound. The hydrogels have sufficient adhesive properties that loose contact with the wound is assured but can also be removed without leaving any gel residue on the wound. The wound dressings are preferably formed on a substrate, such as a web or patch, for ease in application to and removal from the wound. If desired, additional antimicrobial or other pharmaceutically active agents can also be incorporated into the hydrogel structure.
Owner:AVENT INC
Methods and apparatus for implanting devices into non-sterile body lumens or organs
InactiveUS20050197715A1Minimize infectionImprove sealingSuture equipmentsMammary implantsSterile environmentDevice implant
Implantable devices for use in a non-sterile environment of a patient's anatomy are medicated or include medication. For example, the housing of the device and / or members for securing the device to the patient's anatomy (e.g., the muscular esophageal wall) may be medicated to, among other things, prevent a rejection mechanism from being triggered, to prevent or reduce bacterial infection, or to promote tissue ingrowth. The medication may be for medicating tissue at the implant site, or for medicating some other portion of the patient's anatomy.
Owner:TORAX MEDICAL
Sterile connector
A sterile to sterile connection device comprising a connector and one or more coupling devices. The connector has body portion which has two openings sealed from the environment so as to form a sterile environment within the connector. At least one of the openings being sealed from the environment by a sterile barrier plug. The connector also has a port capable movement within the body of the connector to at least an open and a closed position. The coupling device is formed of a body having two openings and a stem having a bore through at least a portion of the stem. The stem is contained within the body and capable of moving at least linearly through the body between a first and second stem position. One of the openings of the stem is sealed from the environment by a sterile barrier plug and the other is sealed to a presterilized component. The coupling device opening containing the sterile barrier plug is attached to either the inlet or outlet of the connector.
Owner:MILLIPORE CORP
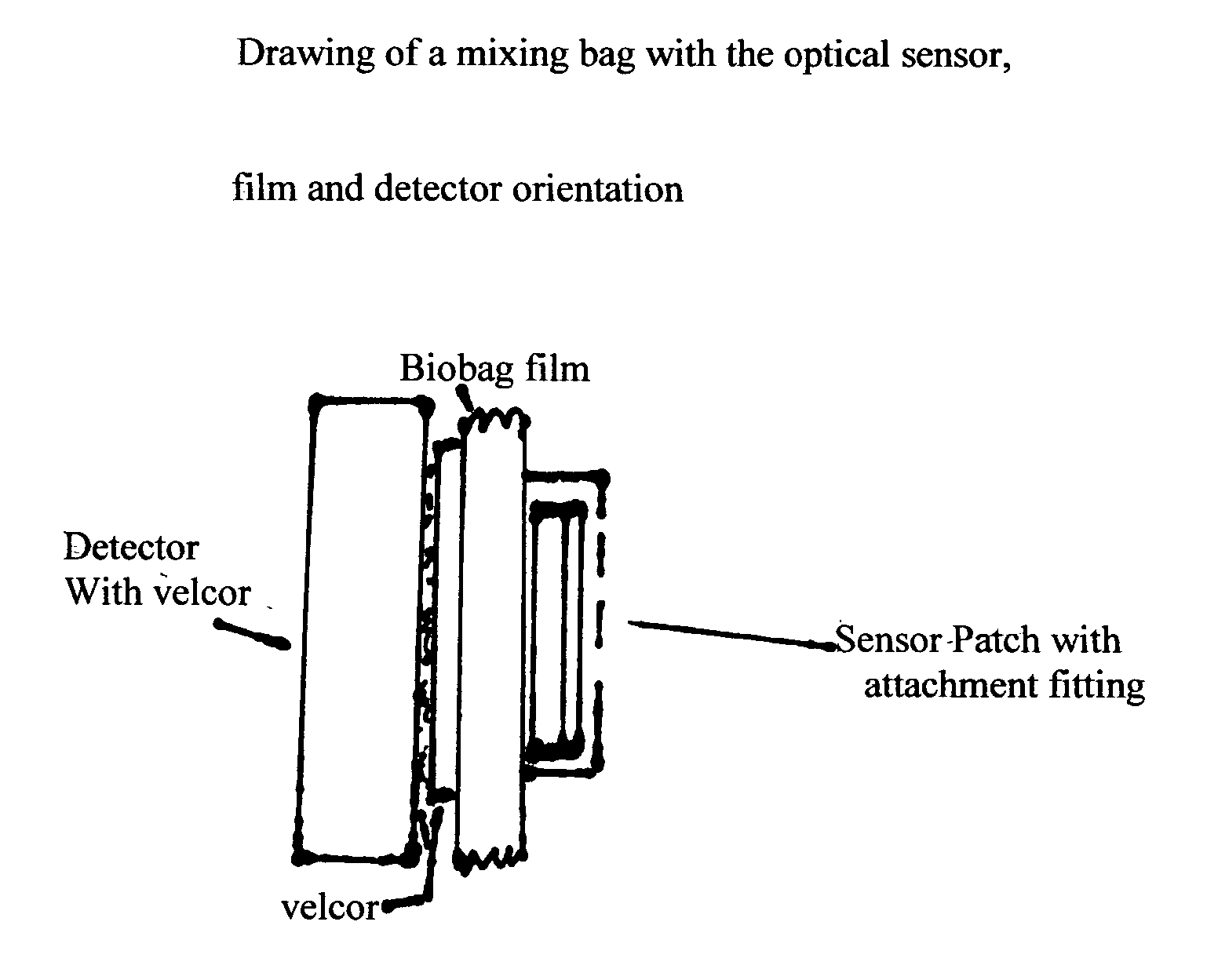
Single-use biobags with sendors: DO, pH, CO2 and temperature
InactiveUS20050163667A1Low costAnalysis using chemical indicatorsBiochemistry apparatusSterile environmentFluorescence
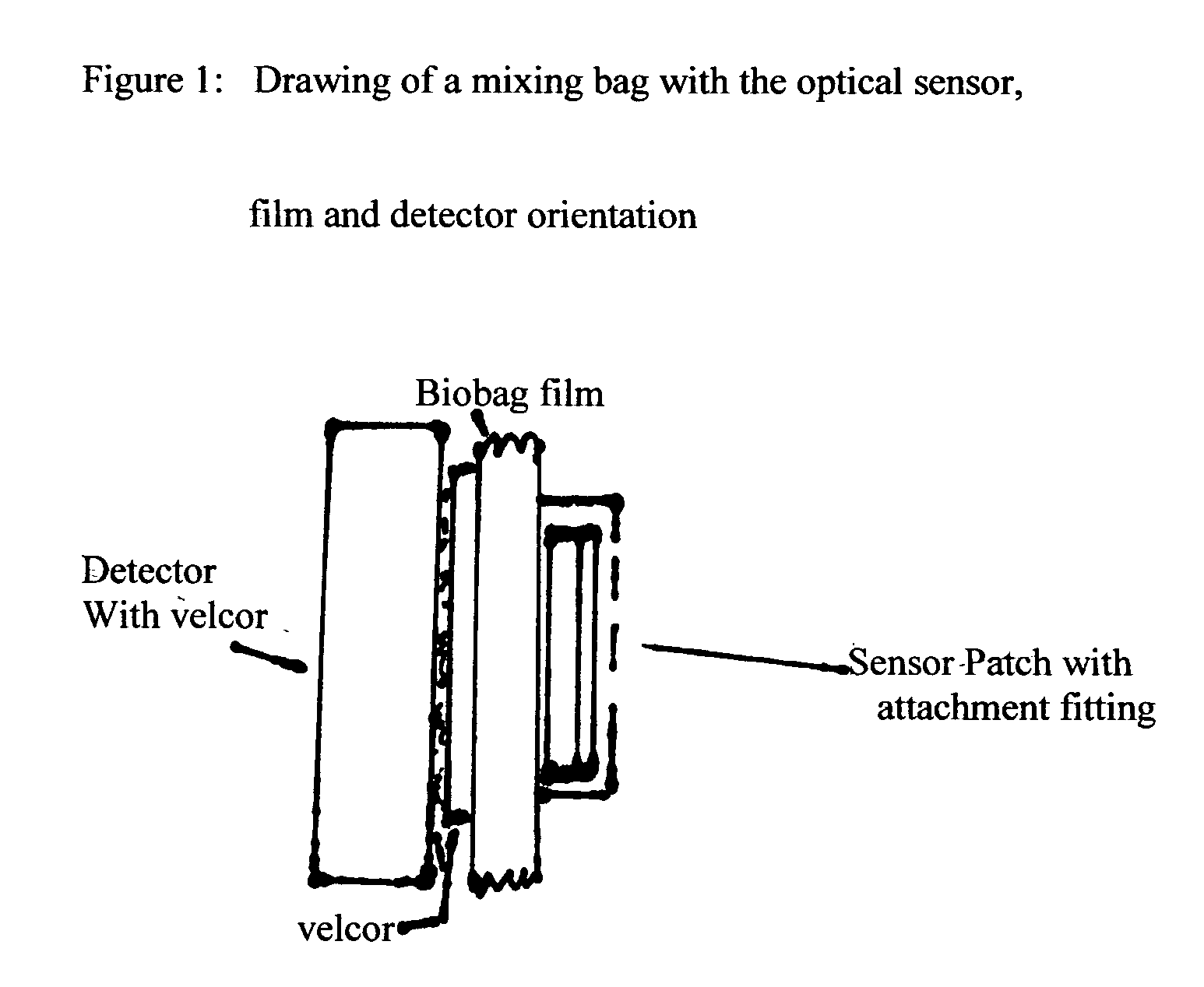
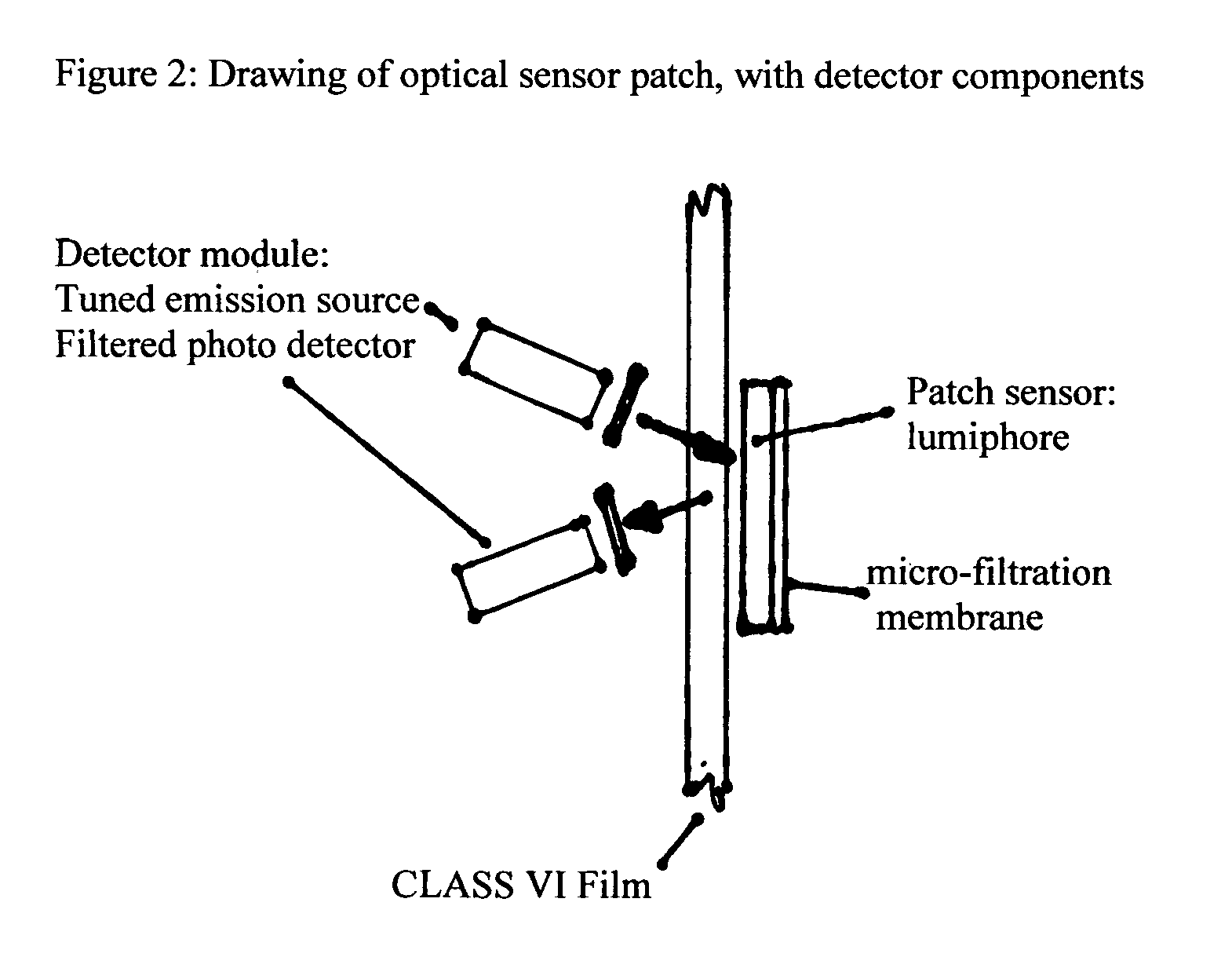
A single-use biobag provided with at least two none invasive florescent-optical sensors. The sterile sensors inside the biobag secured by an adhesive backing or secured by a CLASS VI fitting assures the sterile environment of the fluids in the bags. The sensors detectors are located outside the bag and secured in a position to align the sensors with the detectors. Either Velcro on the detector and outside of the bag has been used for sensor / detector alignment or the detector is mounted on the containment vessel and biobag straps are used to assure position of the bag for sensor / detector alignment. The process variables to be measured by the fluorescent optical disposable sensors are: DO, pH, CO2 and temperature. The biobag film is CLASS VI material and should be optically clear and free of interfering florescent compounds.
Owner:KRAUSE RICHARD J
Wound alternative treatment system
InactiveUS7612247B2Improve fluid flowLow production costPlastersAdhesive dressingsSterile environmentAlternative treatment
A wound treatment device for treating damaged body tissue comprising an encapsulating member having a size and shape capable of being attached to a patient to encapsulate a wound. A fluid communication member is provided for introducing treatment fluid within the encapsulating means for treatment of the wound. The fluid communication member is connected with a supply means for supplying treatment fluid thereto. The fluid communication member is capable of simultaneously transmitting multiple treatment fluids from the supply means into said encapsulating member. The inner surface of the wall of the encapsulating member is textured to allow fluid flow across the wound. The encapsulating member is also formed from a clear material to permit continuous visual inspection of the wound while maintaining a sterile environment.
Owner:OYASKI MICHAEL F
Catheter assemblies with sized sheaths
InactiveUS20080091145A1Increase efficiency and sterilityAvoid communicationCatheterIntravenous devicesSterile environmentCatheter device
A grippable sheath for a catheter assembly is disclosed providing a sterile environment for a catheter. The grippable sheath may have a flattened diameter ranging from about 10 mm to about 50 mm. More specifically, the grippable sheath may have a flattened diameter ranging from about 15 mm to about 40 mm to provide improved usability and manipulability.
Owner:COLORADO CATHETER
Method and Machine for Closing Bottles with Sterile Caps
ActiveUS20070006550A1Improve throughputImprove protectionCapsThreaded caps applicationSterile environmentLinear machine
For closing bottles with sterile caps, the caps are placed in a non-sterile environment in a vertical arrangement and are supplied vertically to a first sterile area. The interior of the caps is arranged to be accessible horizontally. The caps are sterilized in the first sterile area and transferred to a second sterile area where the caps are placed onto bottles. Subsequently, the bottle is closed with the cap. The machine for sterile closing of bottles with caps has a sterilization device with an individualization device for picking up caps and a vertical transport path feeding the caps to the sterilization chamber. A placing device receives the caps from the sterilization device and places the caps onto bottles. A closing device closes the bottles with the cap placed thereon. The sterilization device, the placing device, and the closing device operate as synchronized modules of a linear machine.
Owner:KHS CORPOPLAST GMBH & CO KG
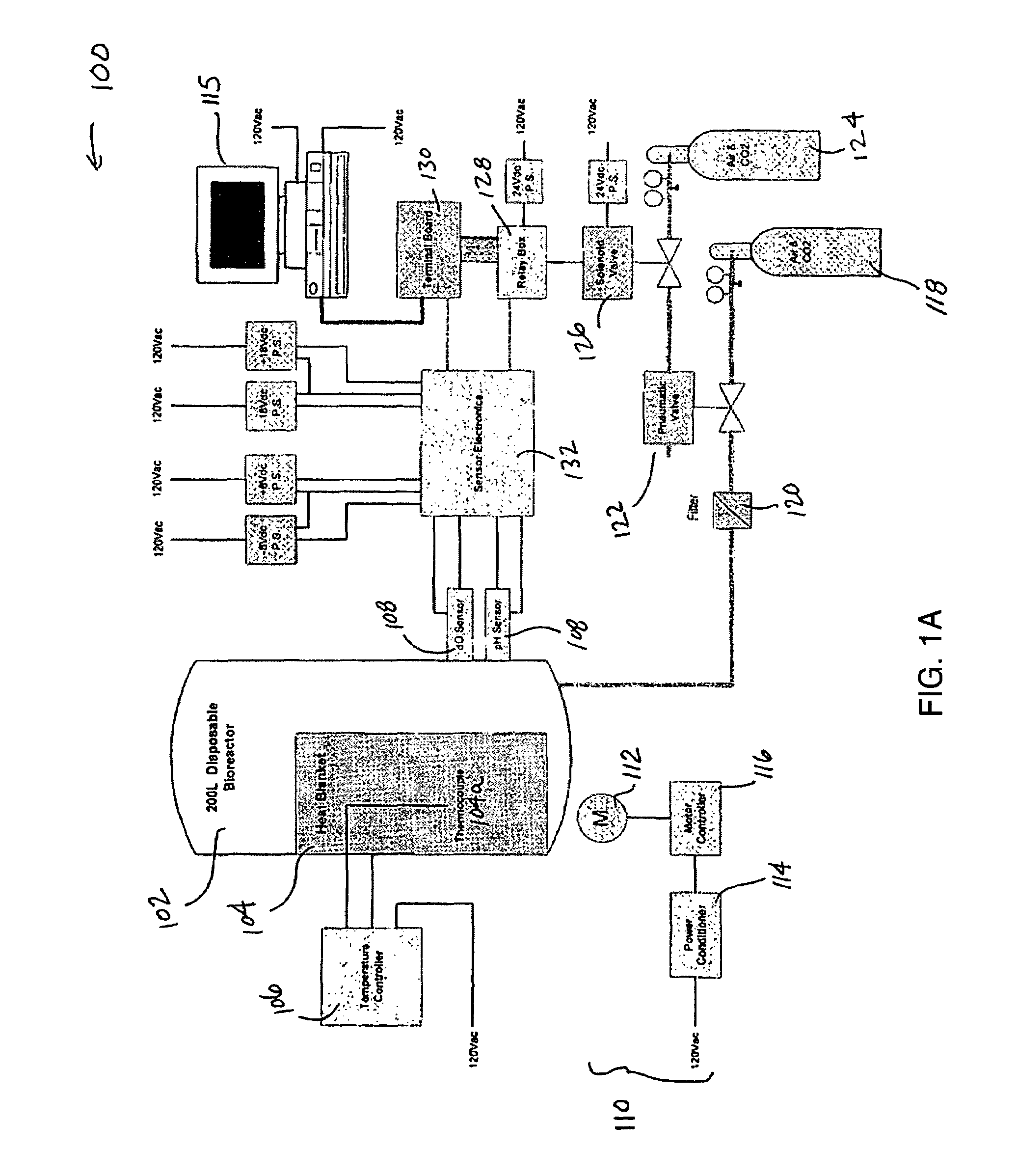
Automated cell culture system
ActiveUS20130210130A1Possible to moveMaximum flexibilityBioreactor/fermenter combinationsBiological substance pretreatmentsSterile environmentBiology
The automated cell culture arrangement according to the invention comprises at least one closed cell culture module with at least one bioreactor. The closed cell culture module is a closed system, which means that within the closed cell culture module a closed sterile environment can be maintained. The automated cell culture arrangement according to the invention, further comprises at least one pump for pumping liquids within the closed cell culture module and at least one additional tool module, which is configured or configurable to act upon or to monitor the contents of a bioreactor and is movable relative to the at least one closed cell culture module or it is movable relative to one or several components of the at least one closed cell culture module.
Owner:OCTANE BIOTECH
Apparatus and method for deployment of a bronchial obstruction device
An apparatus and method deploy a self-expandable bronchial obstruction device in an air passageway. The apparatus includes a catheter configured to be passed down the trachea. The apparatus further includes a capsule for housing the self-expandable bronchial obstruction device in a sterile environment. The capsule is configured to be advanced down the catheter. The capsule further includes a tubular extension. The capsule has a breakable seam so as to release the bronchial obstruction device in the air passageway upon a proximal force being exerted upon the bronchial obstruction device. The method includes guiding a conduit down a trachea into the air passageway. The method further includes advancing a capsule having a bronchial device therein down an internal lumen of the conduit into the air passageway. The method further includes releasing the bronchial device from the capsule. The method further includes deploying the bronchial device into the air passageway.
Owner:GYRUS ACMI INC (D B A OLYMPUS SURGICAL TECH AMERICA)
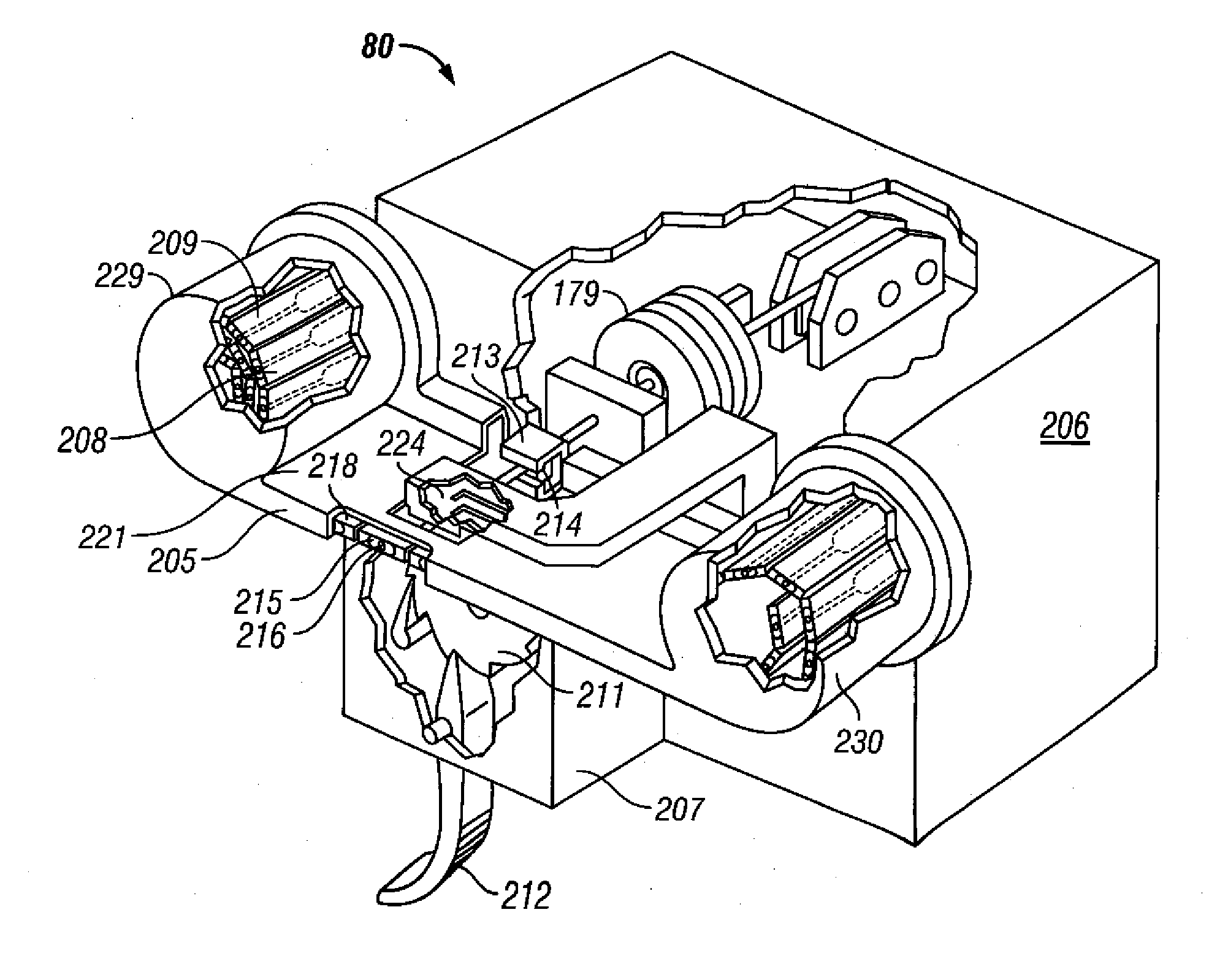
System and method for providing a single use imaging device for medical applications
A system and methods for providing and reclaiming a single use imaging device for sterile environments is disclosed and described. The system may include a single use high definition camera used for general purpose surgical procedures including, but not limited to: arthroscopic, laparoscopic, gynecologic, and urologic procedures, may comprise an imaging device that is a sterile and designed to ensure single use. The imaging device may have a single imaging sensor, either CCD or CMOS, encased in a housing.
Owner:DEPUY SYNTHES PROD INC
Components and preparation method of beta-lactam injection
ActiveCN101721366AReduce the number of dosesReduce stressAntibacterial agentsSolution deliverySterile environmentVegetable oil
The invention discloses the components and preparation method of beta-lactam injection. The beta-lactam injection comprises 5 to 20 percent of beta-lactam antibiotics, 0.05 to 5 percent of suspending agent, 0.005 to 0.3 percent of antioxygen, 0.1 to 0.2 percent of nonionic surfactant and the balance of vegetable oil or grease for injection. The injection can be used for preventing and curing animal bacterial infectious diseases and can be injected hypodermically or in muscle and be applied through breast for a few times. The preparation method provided by the invention comprises: firstly, making the antibiotics and the antioxygen into micro powder and making the suspending agent into fine powder; secondly, adding the vegetable oil or grease for injection, which is sterilized at high temperature, into the fine powder of the suspending agent, heating the mixture, uniformly mixing the mixture and keeping the mixture in a sterile environment to cool the mixture to room temperature for later use; and finally, transferring the prepared oil or grease added with the suspending agent to a colloid mill, adding medicament micro powder, the antioxygen and the non-ionic surfactant with stirring, and performing uniform mixing and sterilization to obtain the beta-lactam injection.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Process and device for the dose dispensing of a radioactive solution
An apparatus and method for automatically dispensing a radioactive dose by filling a container, such as a vial or disposable syringe, with a required radioactive dose in a sterile environment; the apparatus being stand alone and radioactive-shielded. The apparatus further includes a control device for accurately dispensing and diluting the required radioactive dose using an online radioactivity measurement, which does not require knowledge of the volumetric radioactivity of the stock solution.
Owner:TOCHON DANGUY HENRI JAQUES +1
Sterile connector
A sterile to sterile connection device comprising a connector and one or more coupling devices. The connector has body portion which has two openings sealed from the environment so as to form a sterile environment within the connector. At least one of the openings being sealed from the environment by a sterile barrier plug. The connector also has a port capable movement within the body of the connector to at least an open and a closed position. The coupling device is formed of a body having two openings and a stem having a bore through at least a portion of the stem. The stem is contained within the body and capable of moving at least linearly through the body between a first and second stem position. One of the openings of the stem is sealed from the environment by a sterile barrier plug and the other is sealed to a presterilized component. The coupling device opening containing the sterile barrier plug is attached to either the inlet or outlet of the connector.
Owner:MILLIPORE CORP
Tool and tray sanitation
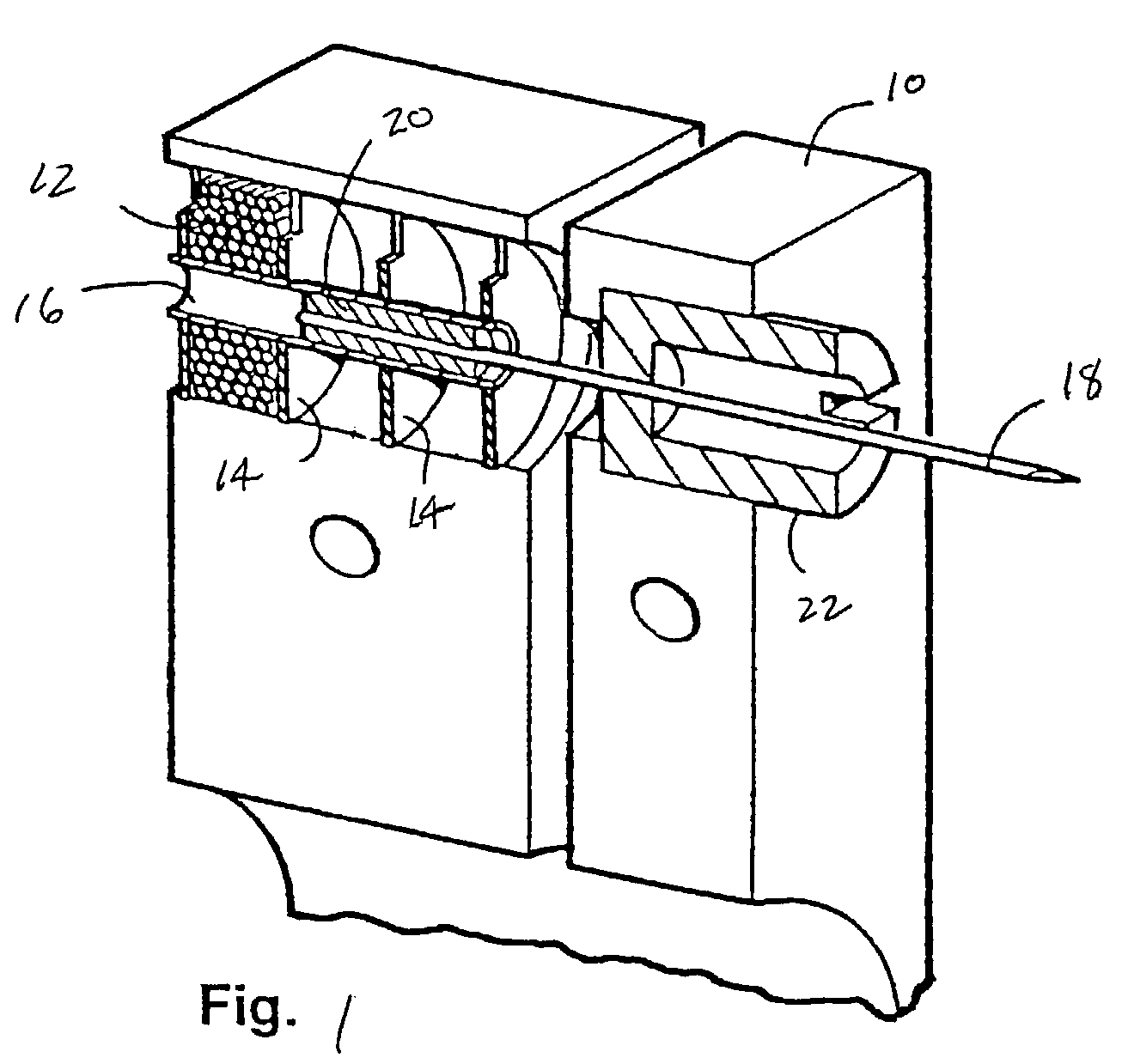
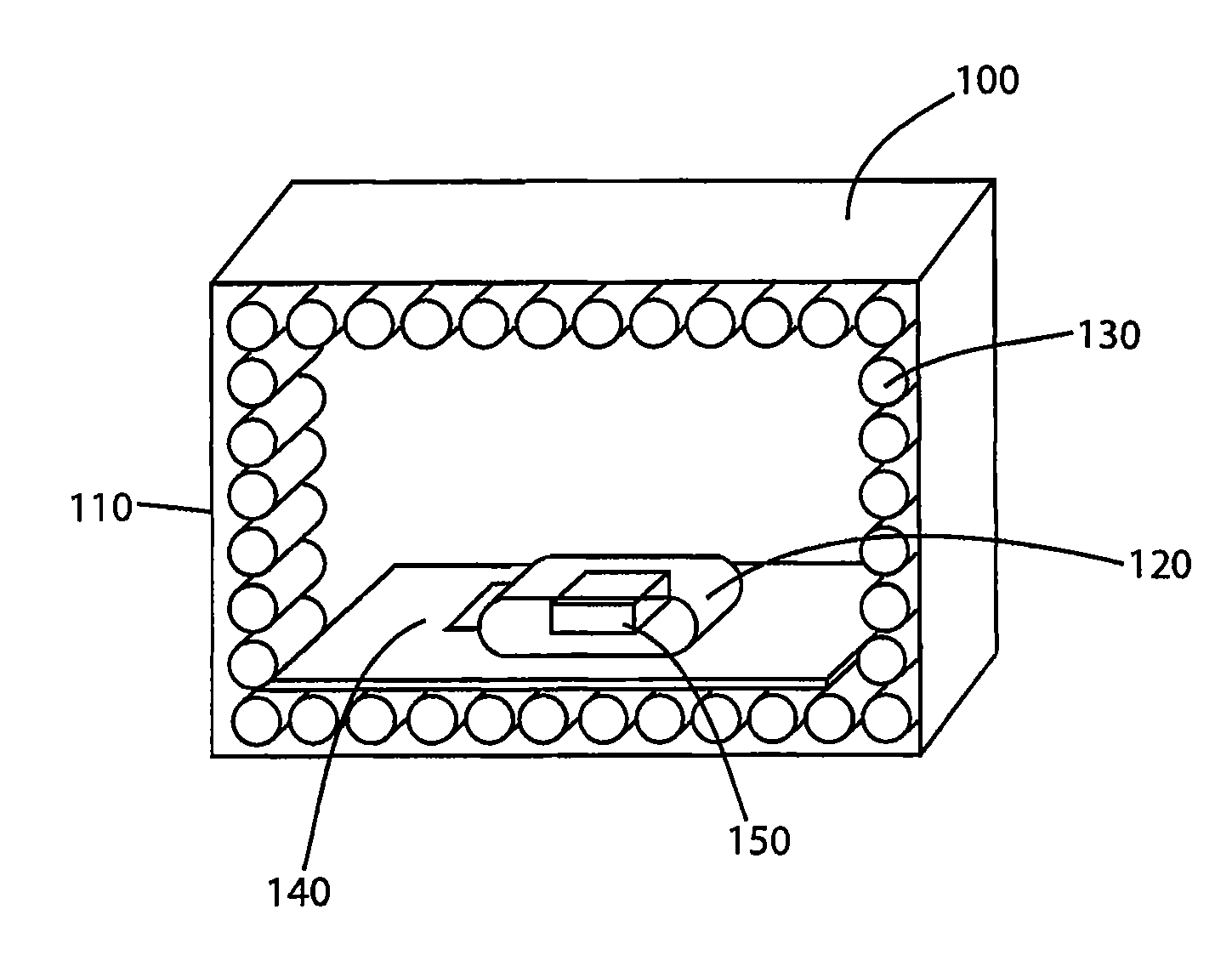
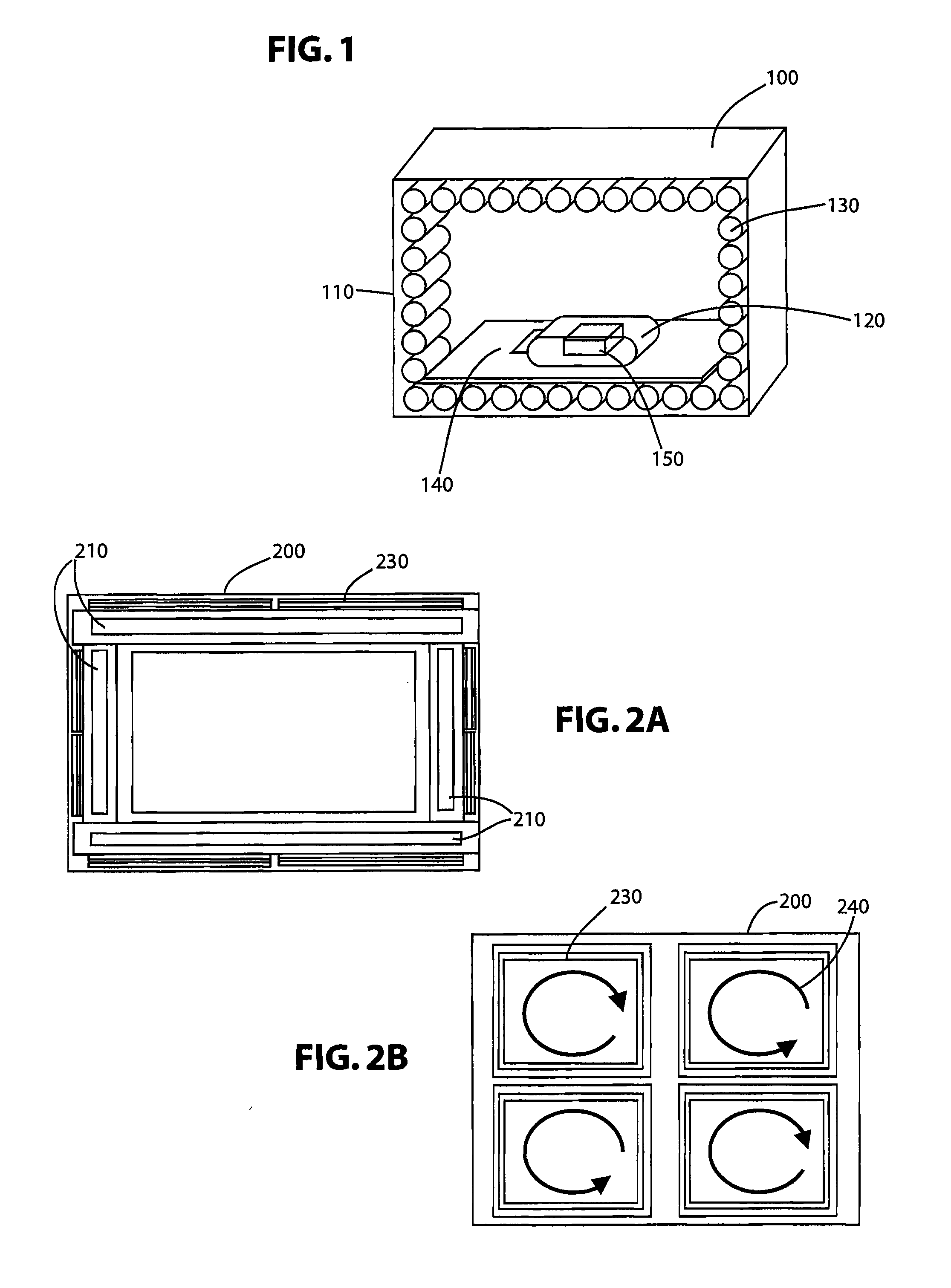
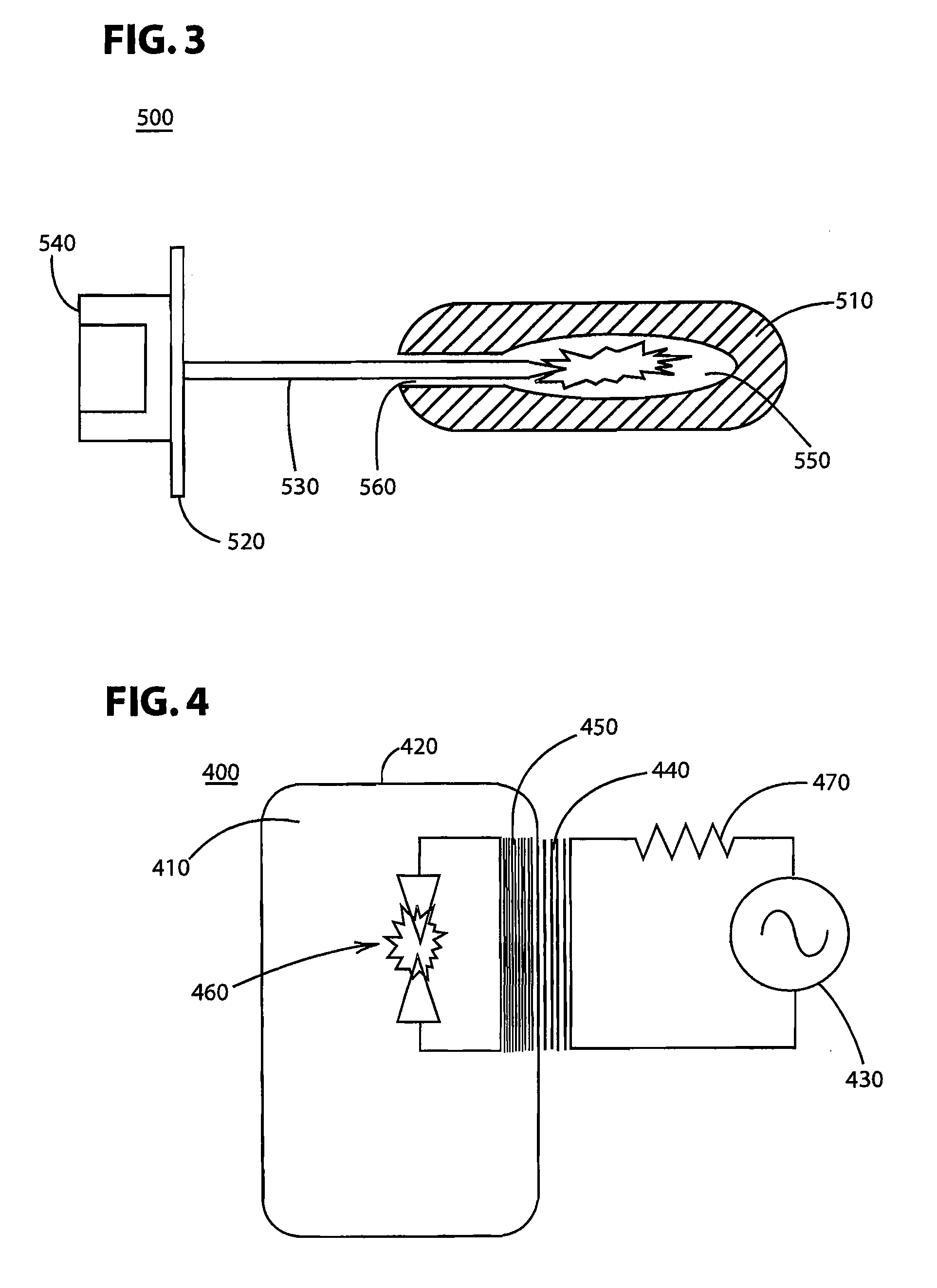
A system for sanitizing or sterilizing an item and storing the item in a sanitized or sterile environment. The system includes an enclosure into which a sealable package containing the item is placed. At least one ozone source is configured to introduce ozone inside the package. The ozone sterilizes the interior of the package and the item. The ozone source can include an electron beam which ionizes the oxygen, a corona discharge generated within the package, or Vacuum Ultra-Violet (“VUV”) radiation emitted into the package. A germicidal radiation source can be used to sanitize the item and generate ozone. The package containing the item is substantially transparent to germicidal radiation so that the germicidal radiation sanitizes the item as well as the interior of the package. Ultrasonic transducers can introduce ultrasonic energy into the item being sanitized, to break apart groups of clumped pathogens resident on the item.
Owner:GERMGARD LIGHTING
Mammalian cell culture chamber
InactiveUS6852525B1Immobilised enzymesBioreactor/fermenter combinationsSensor arraySterile environment
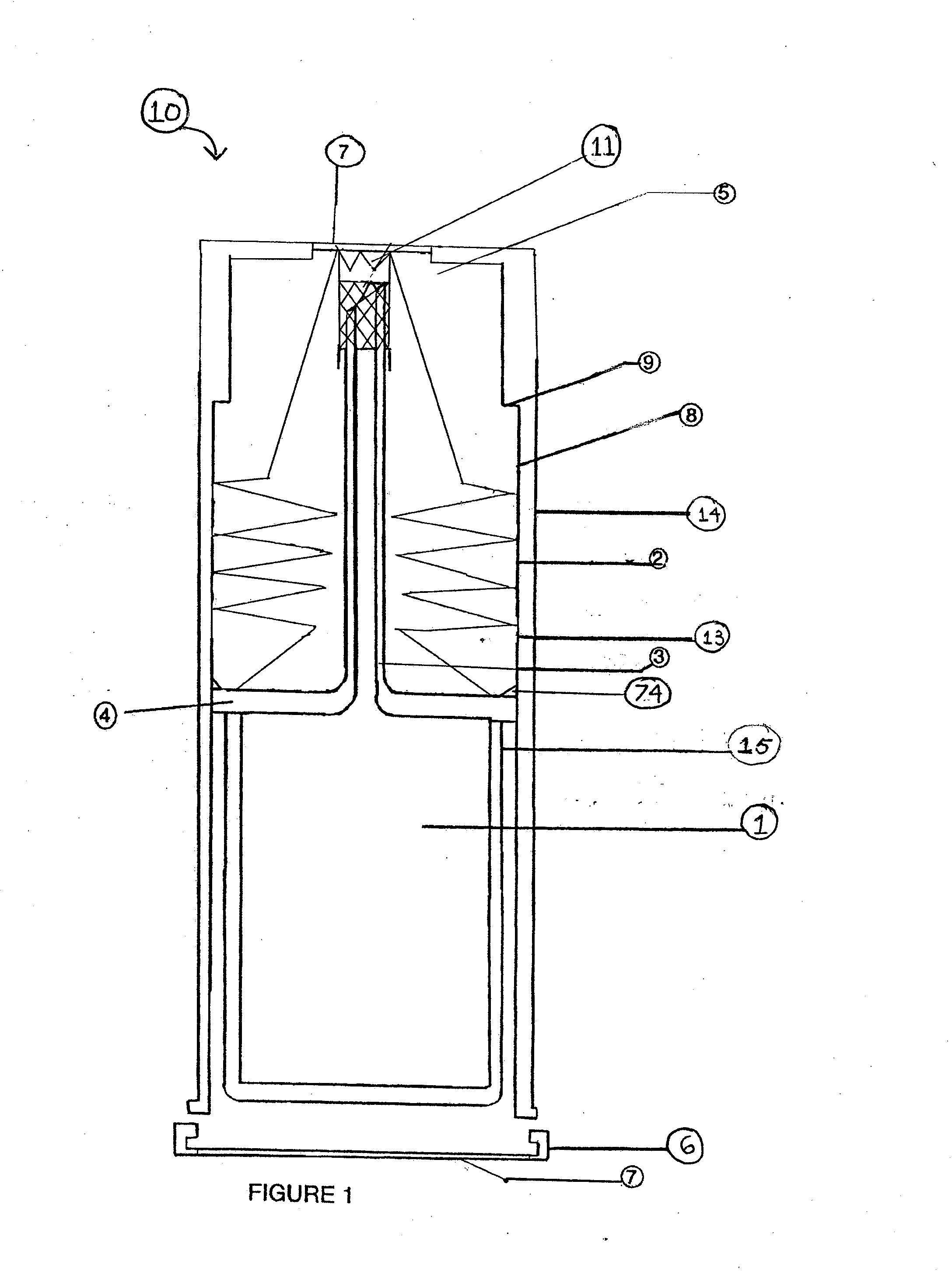
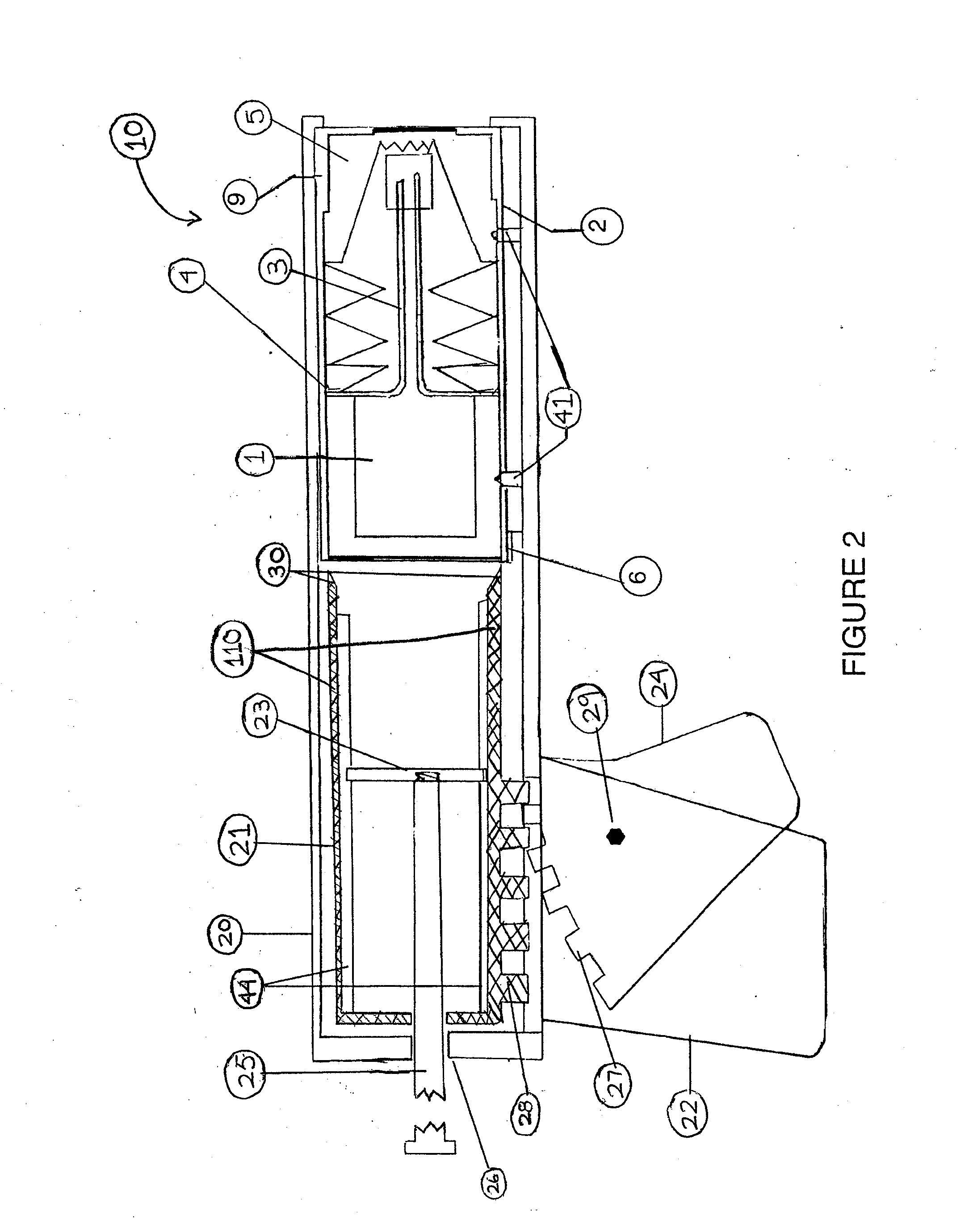
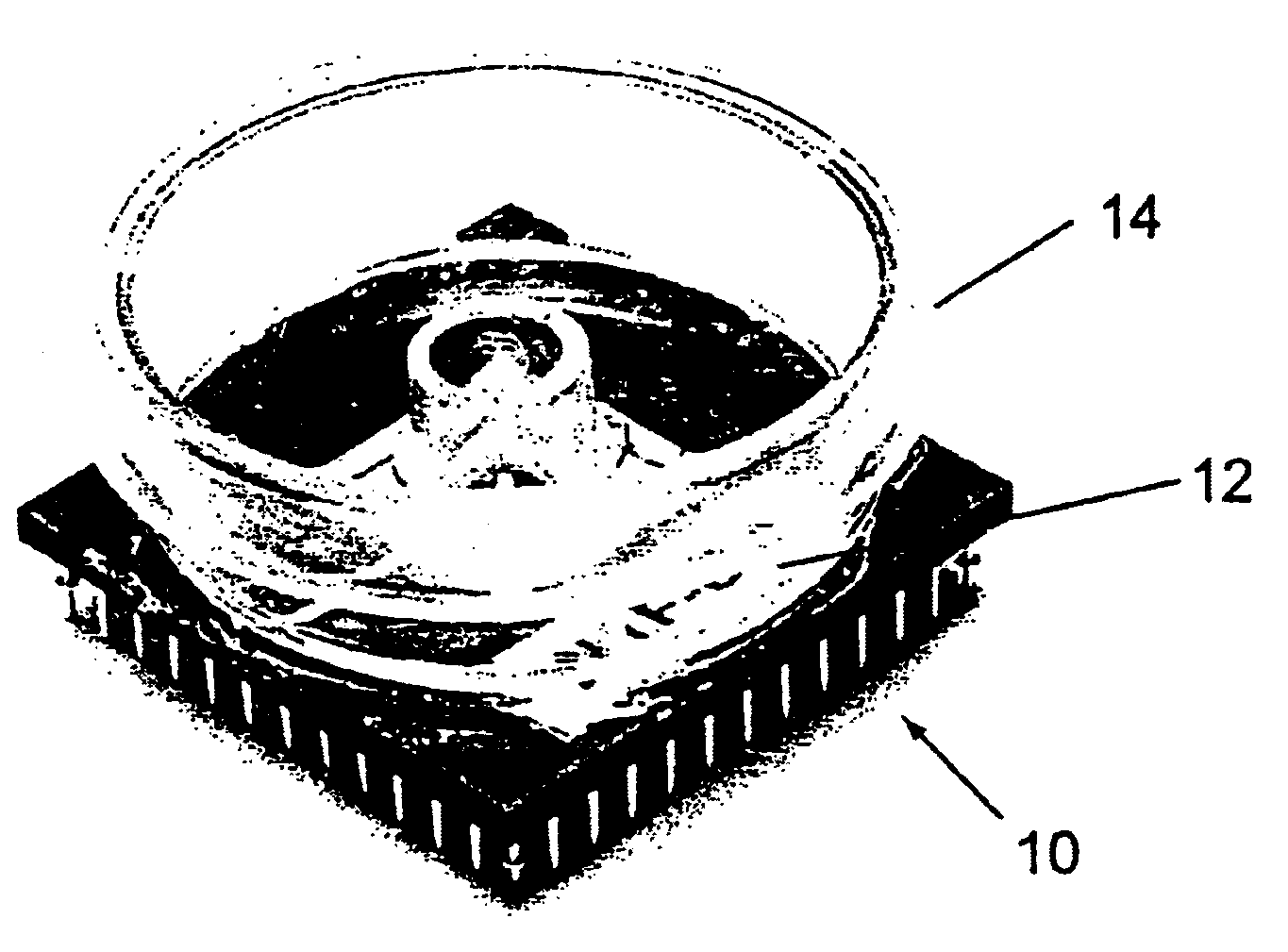
The present invention relates to a cell culture chamber (10) having a sterile environment (12) for the culture of cells, a cover (14) for allowing for a transfer of pits and a sensor array.
Owner:ADVANCED SENSOR TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com