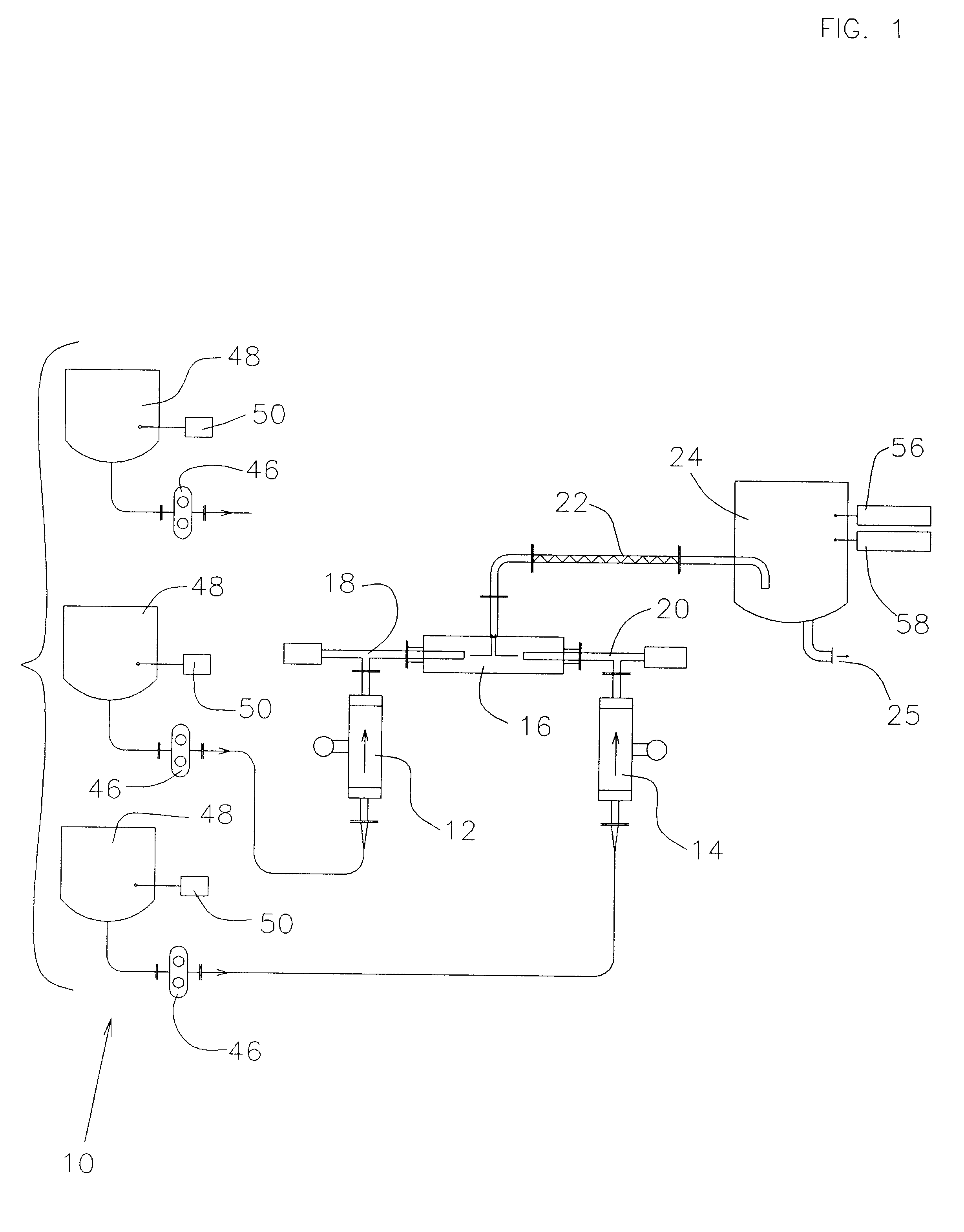Patents
Literature
16219results about "Solid material" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
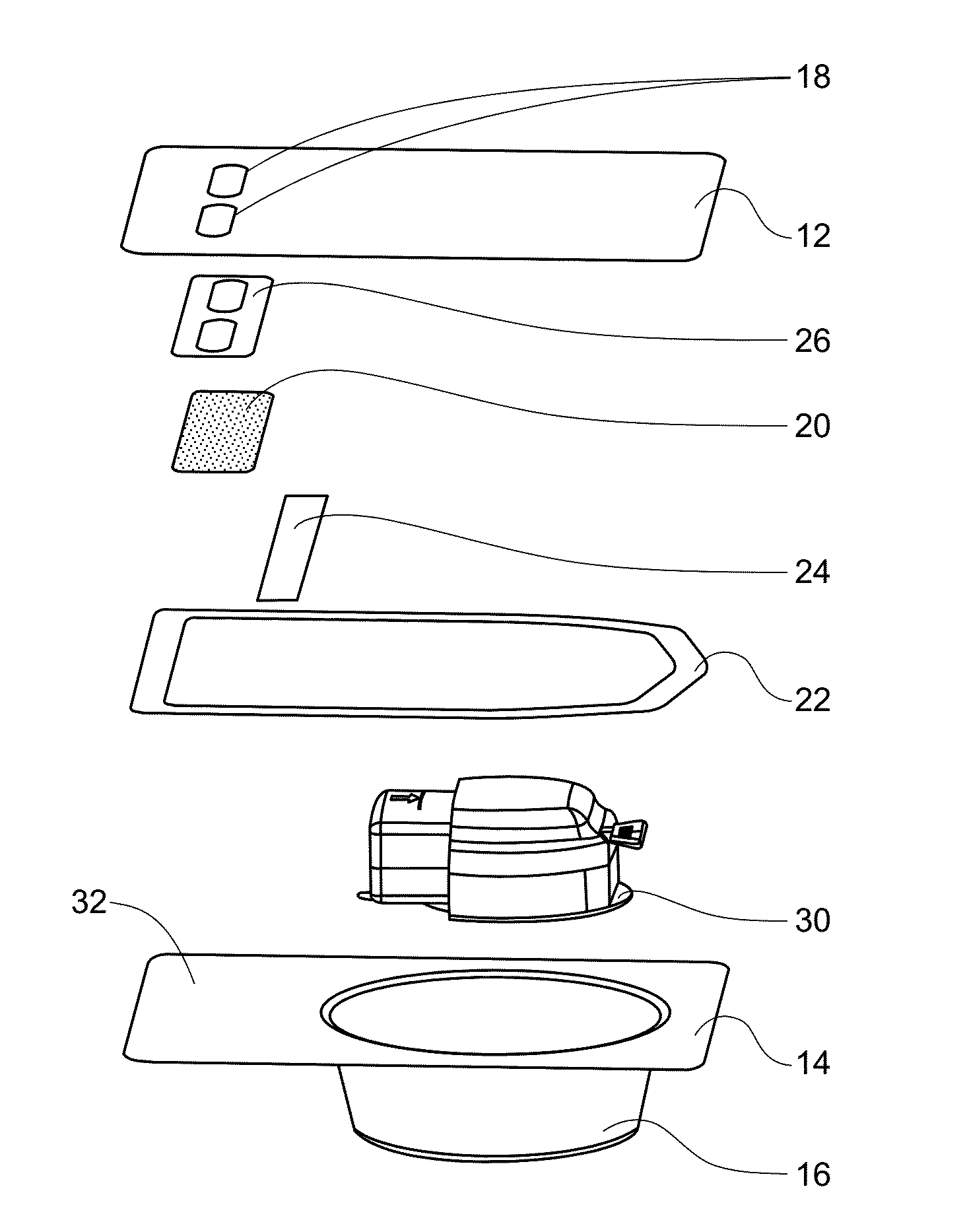
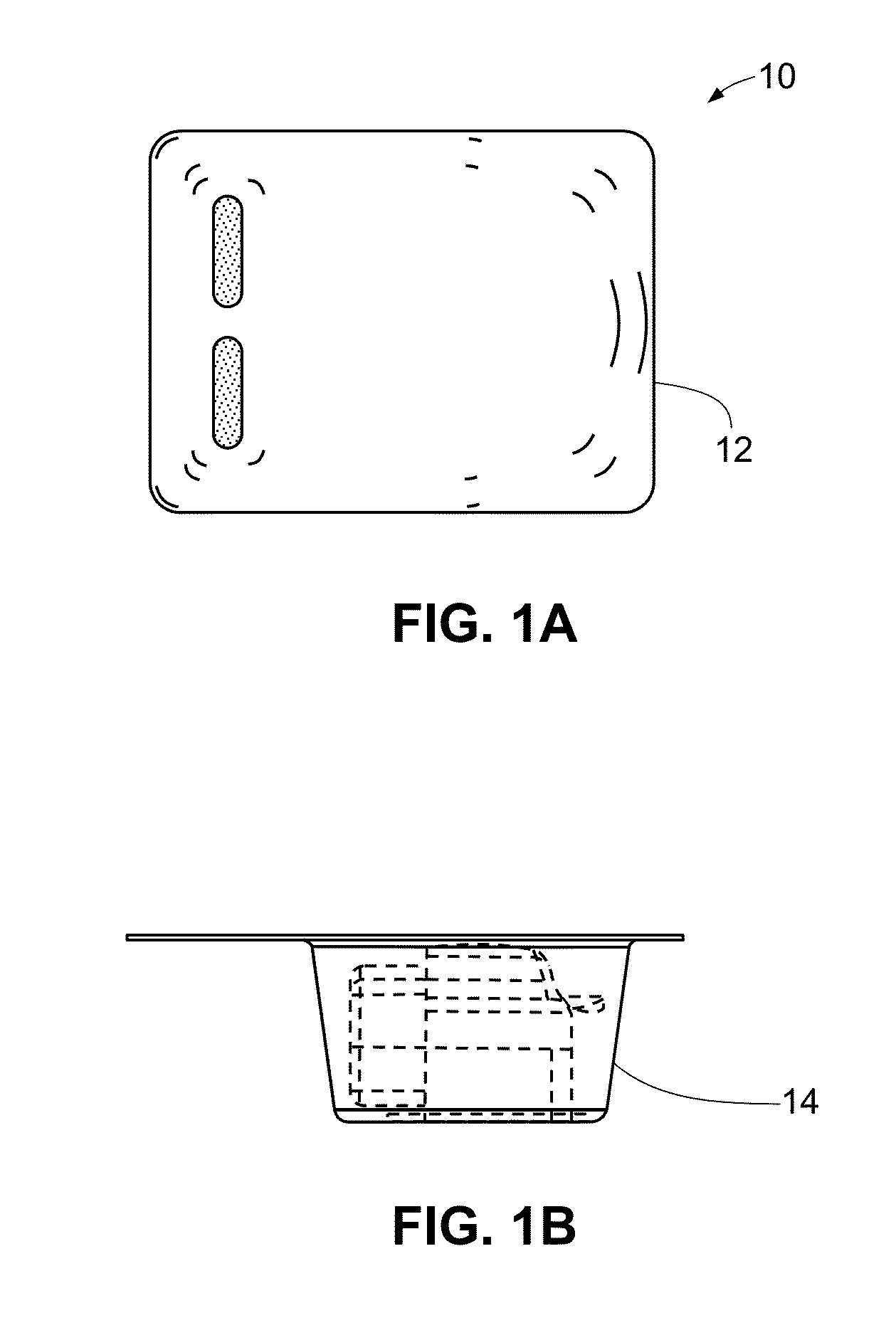
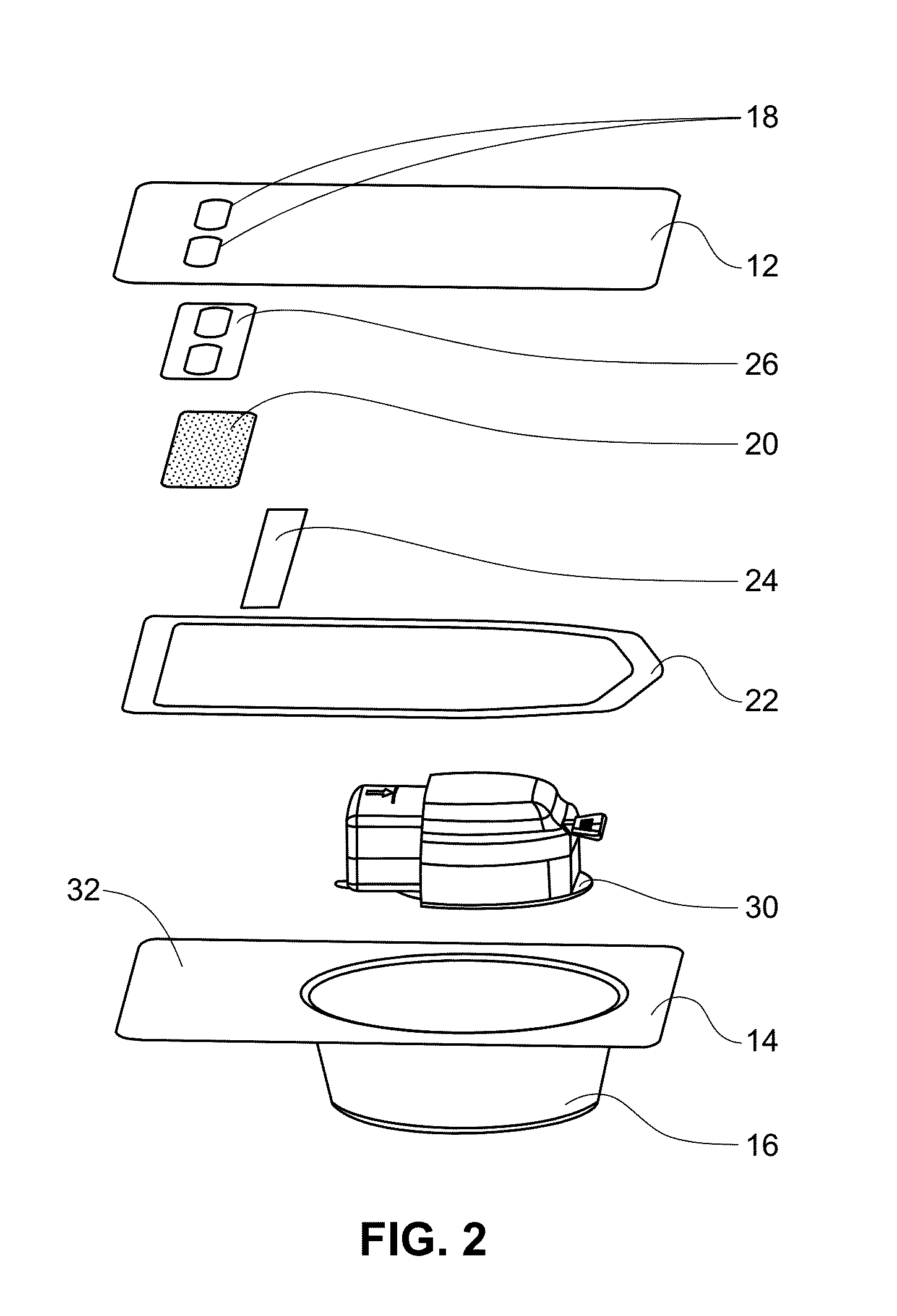
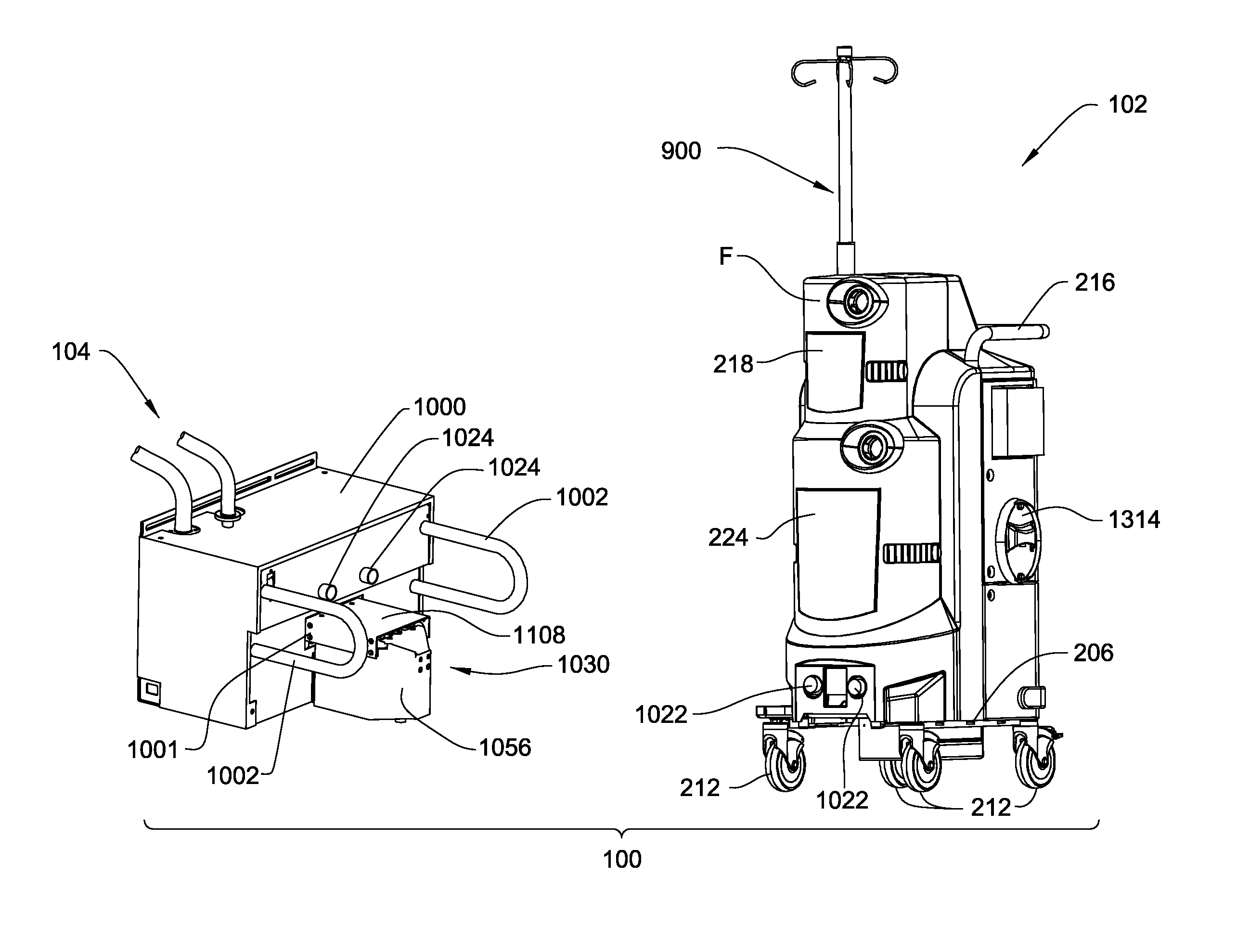
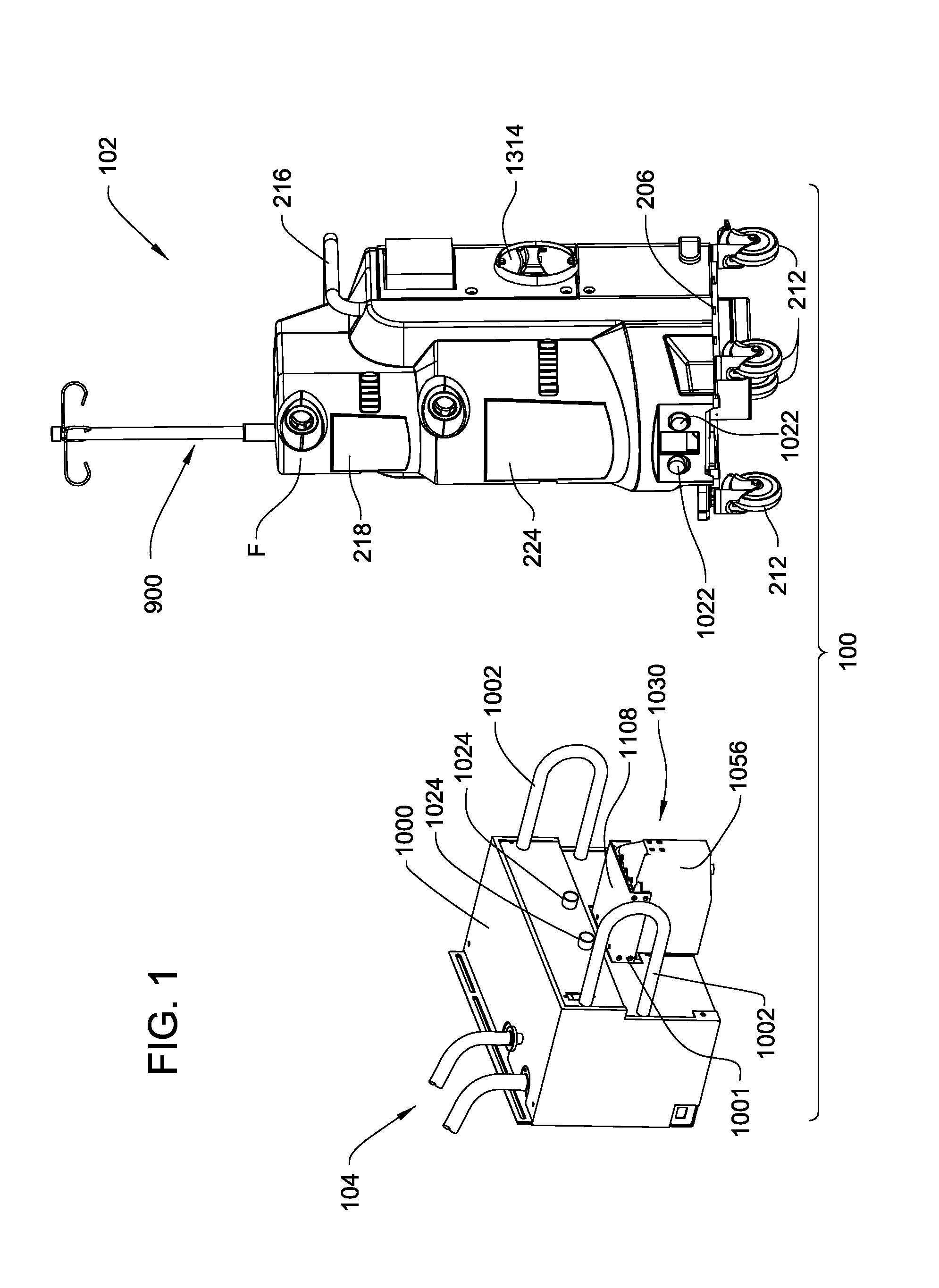
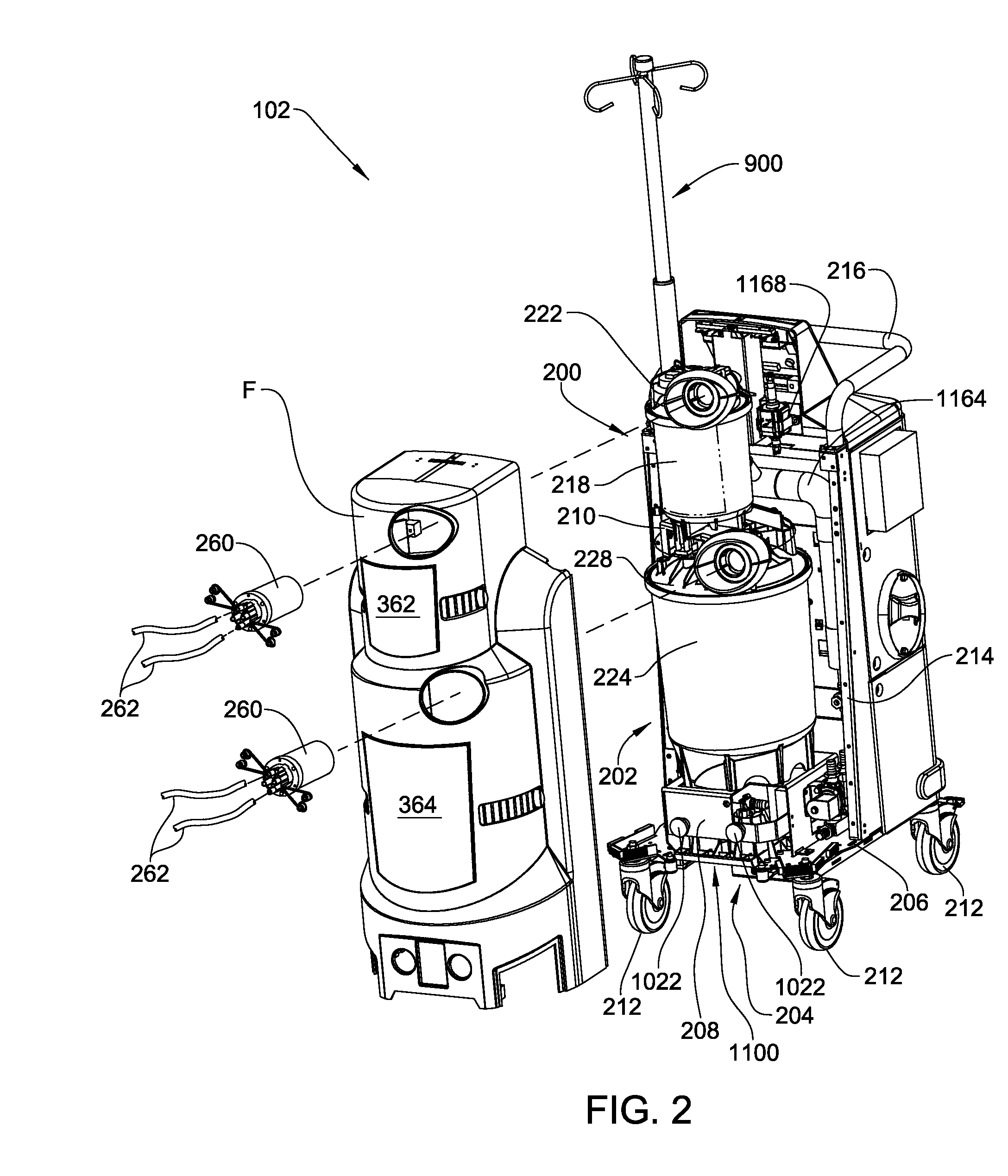
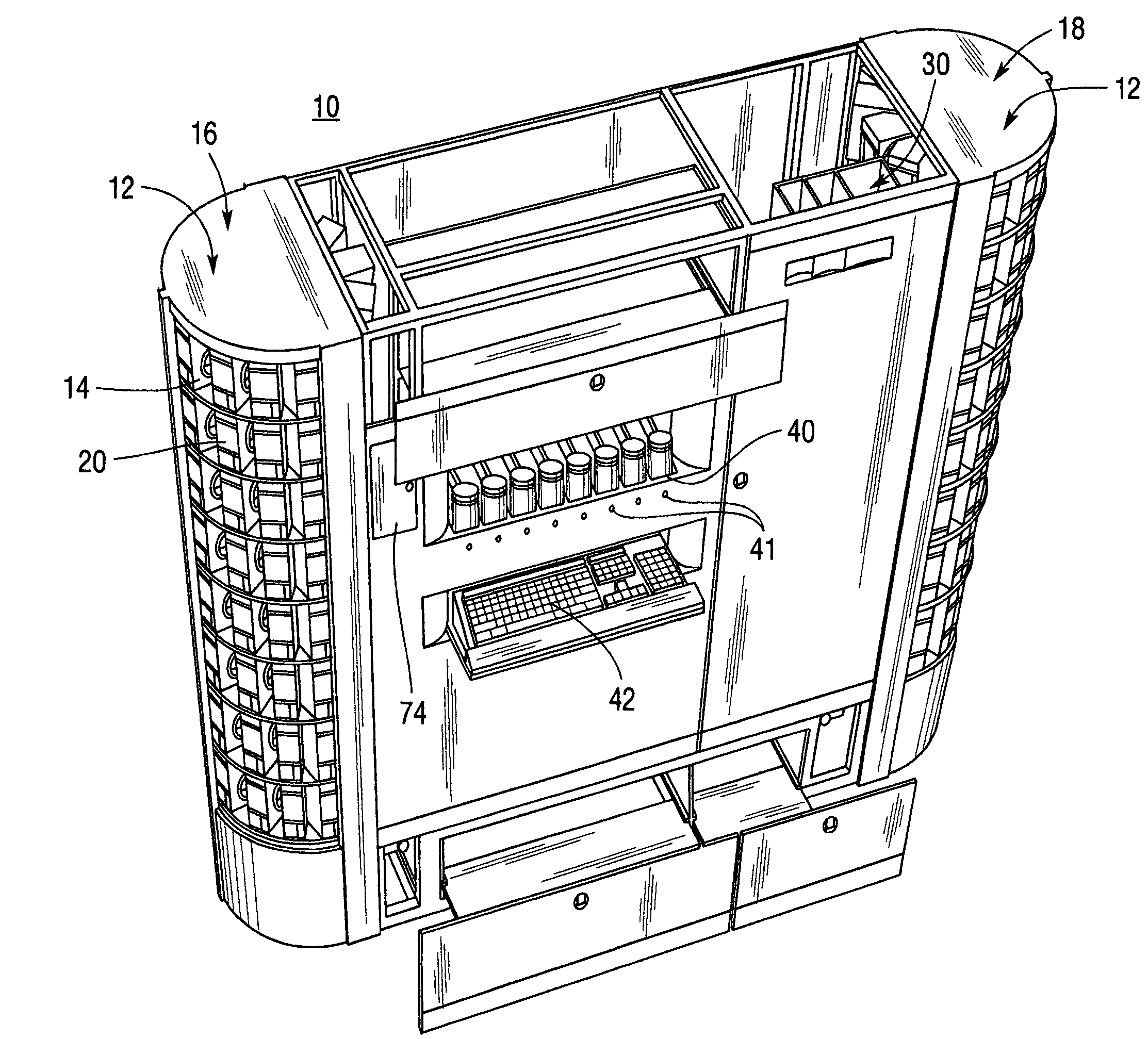
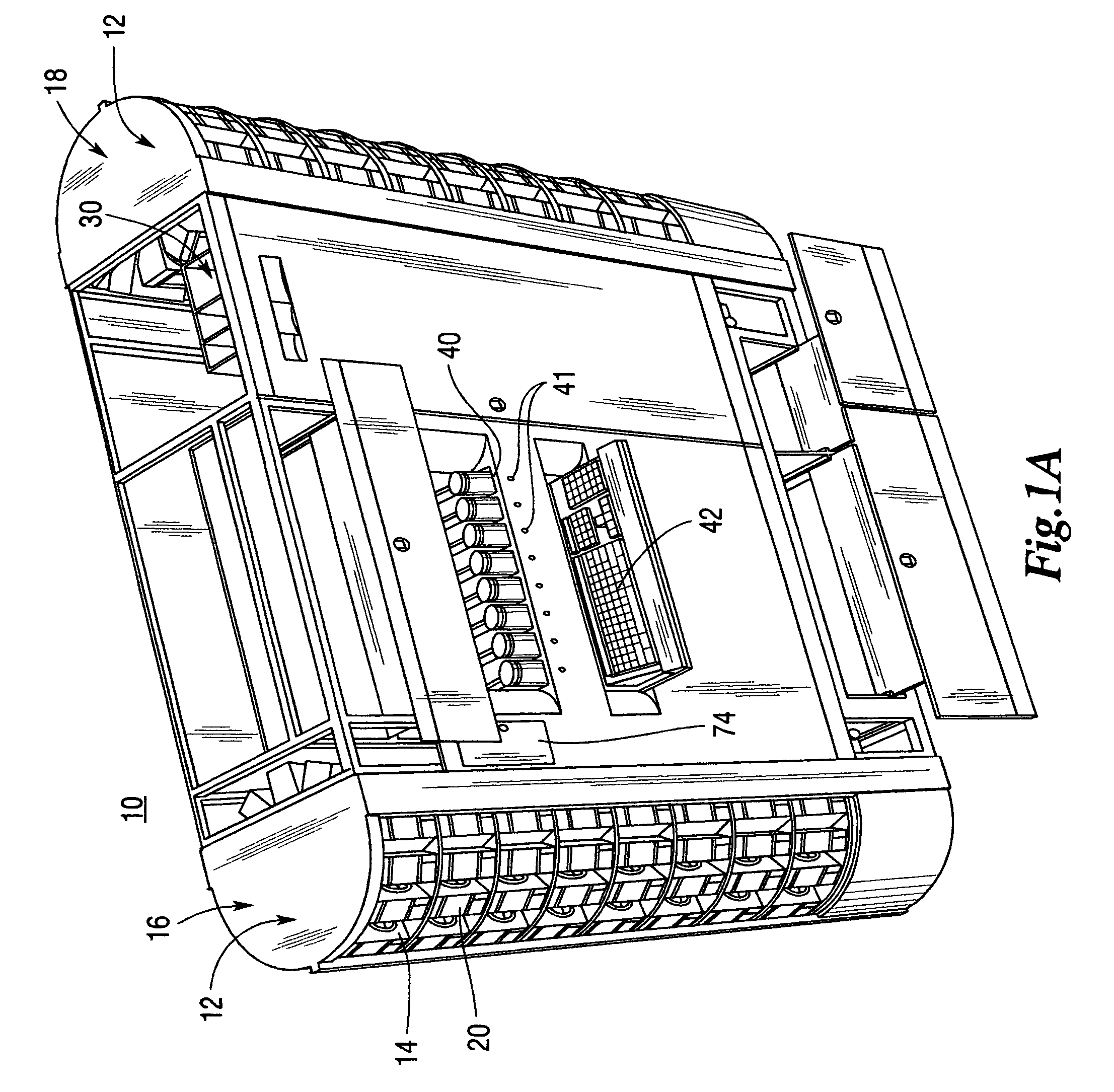
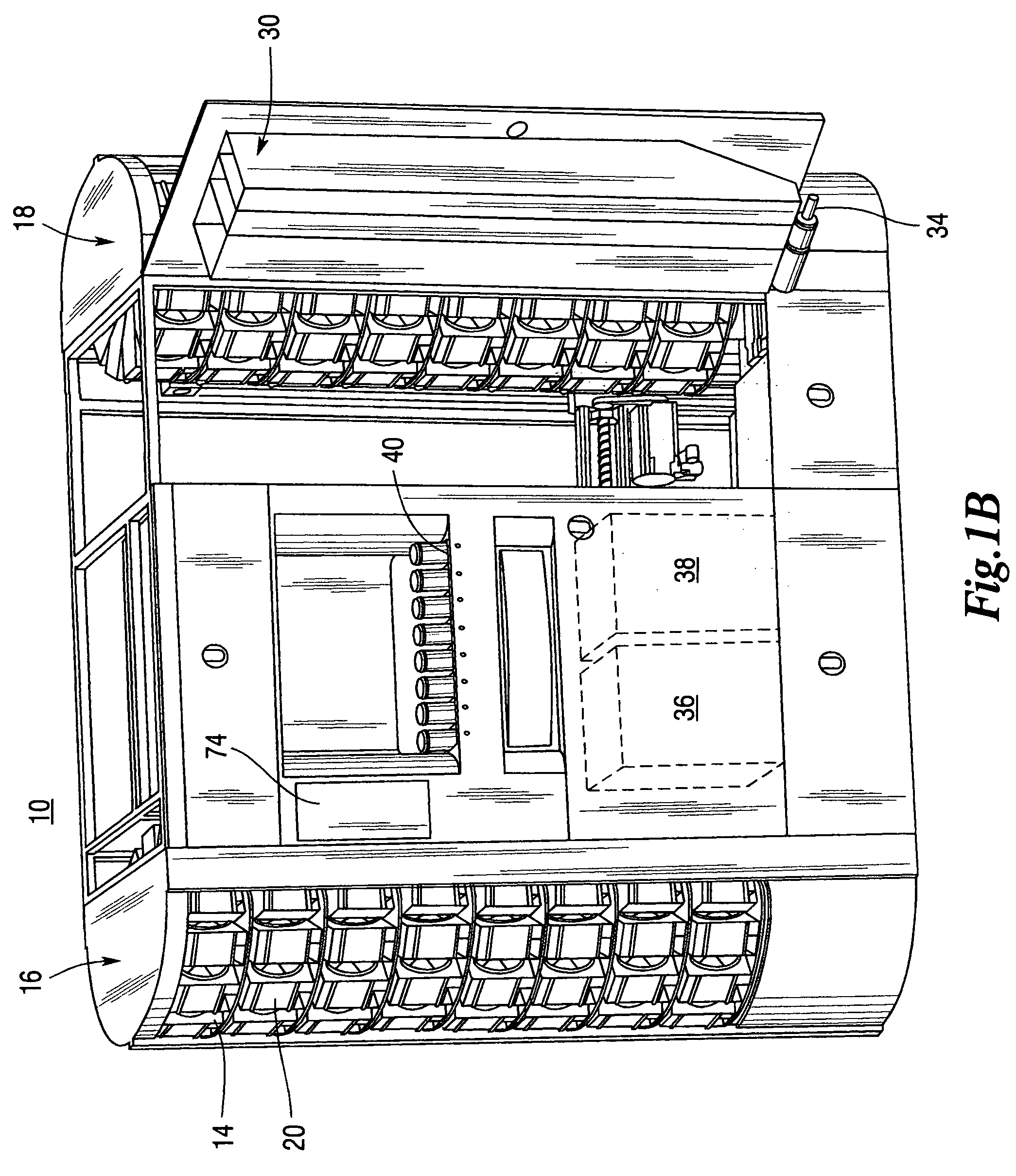
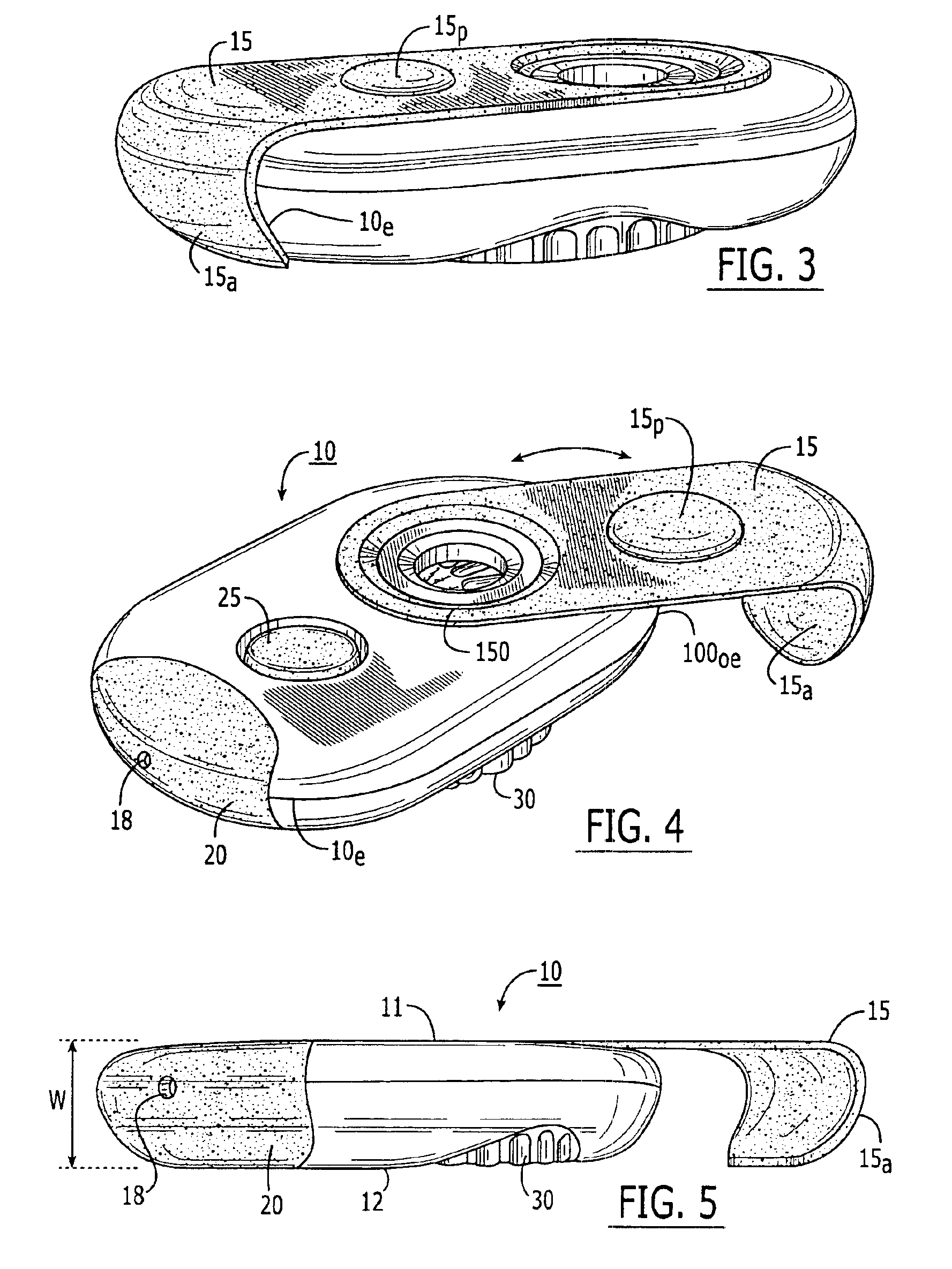
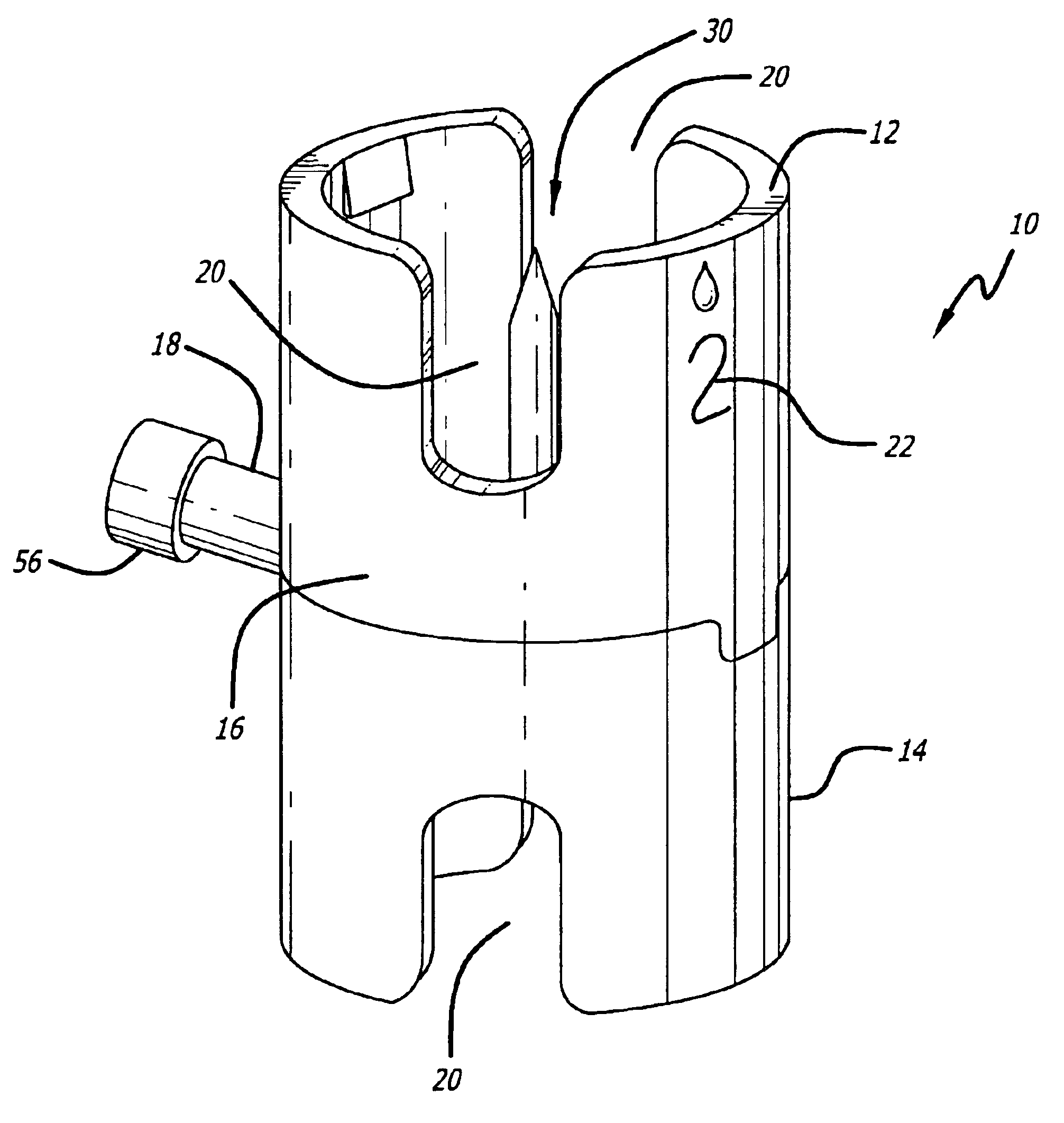
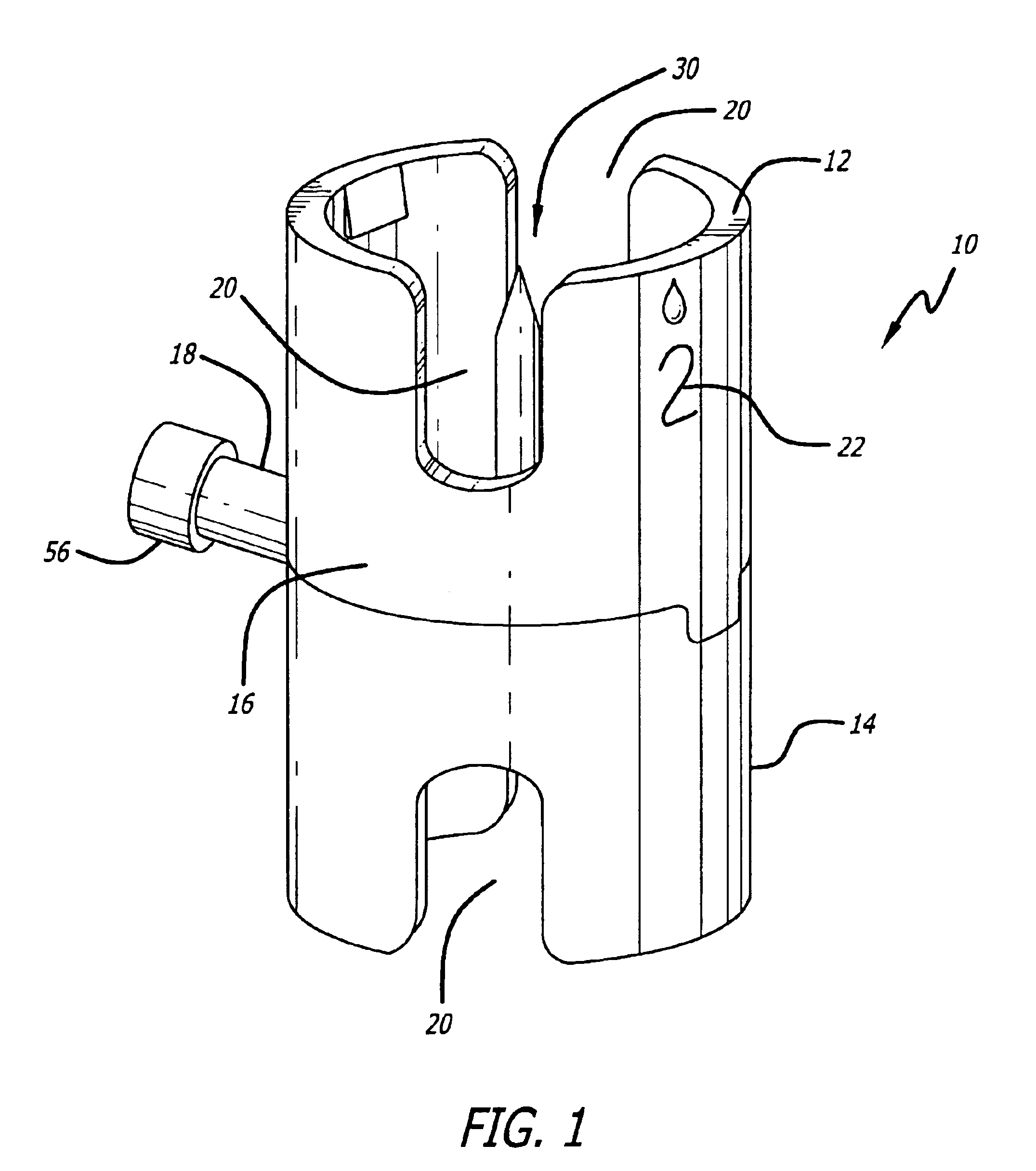
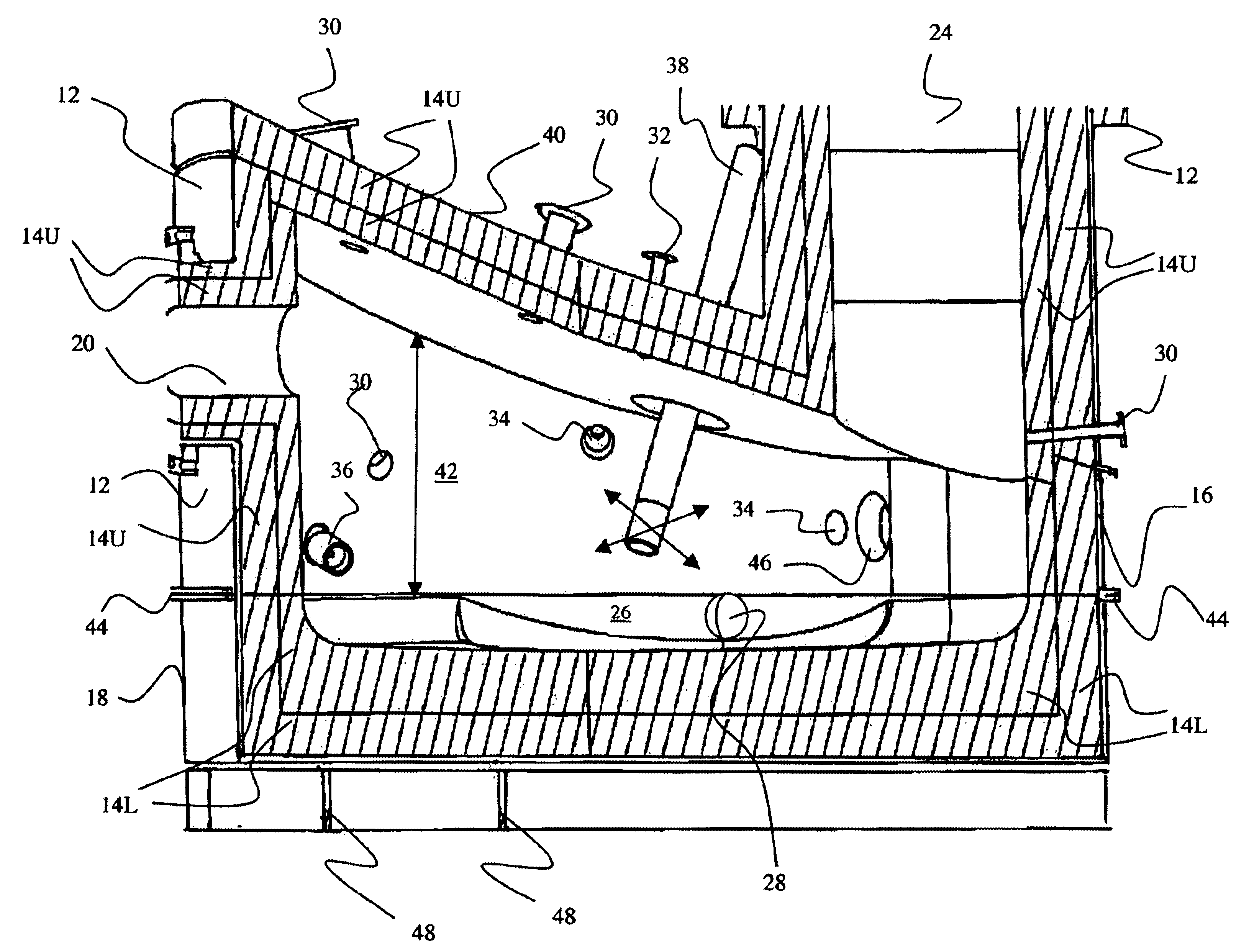
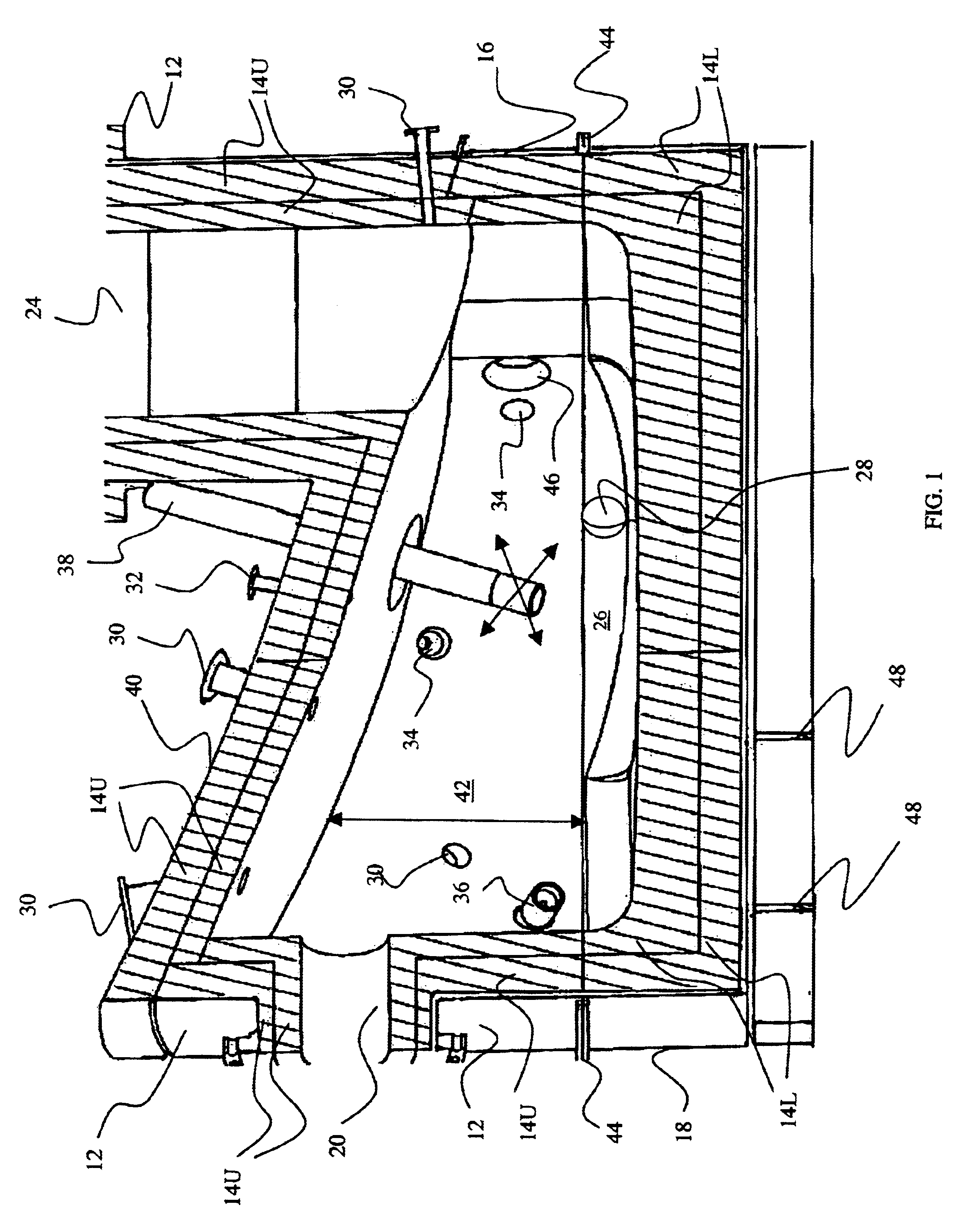
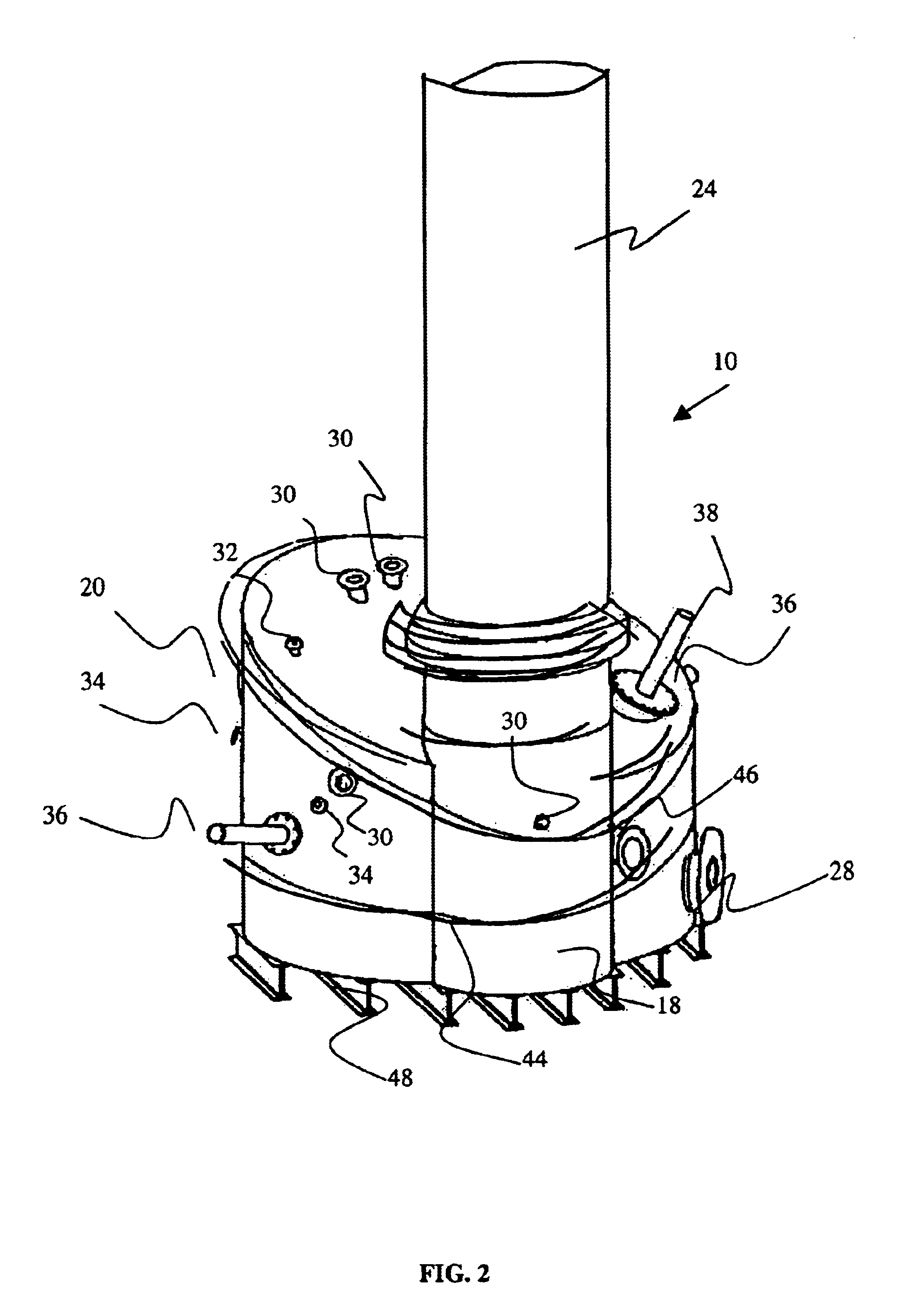
Medical/surgical waste collection unit including waste containers of different storage volumes with inter-container transfer valve and independently controlled vacuum levels
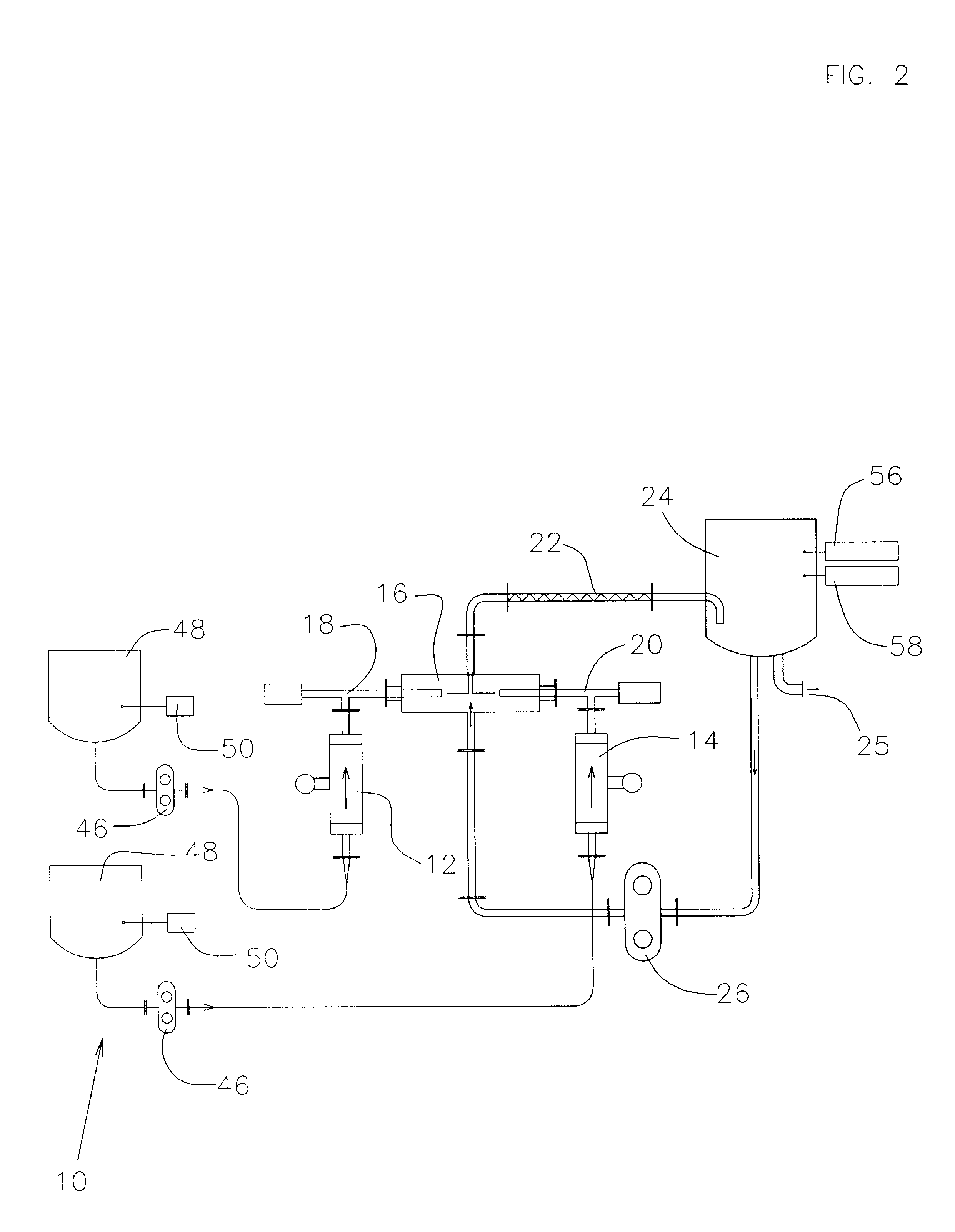
ActiveUS7621898B2Reduce in quantityLarge storage capacityMechanical apparatusDispersed particle filtrationDocking stationVacuum level
Owner:STRYKER CORP
Isolation system for a mobile computing device
InactiveUS20120008880A1Maintaining visual acuityReduce glareSurgical furnitureDiagnosticsEngineeringEmbedded system
An isolation system for isolating a mobile computing device from an environment while retaining the functionality of the mobile computing device that includes at least one sheet and a sealable region. The sheet may include one or more layer to maintain functionality of the enclosed mobile computing device during use. The isolation system may include a sterilizable outer surface for rendering an enclosed mobile computing device usable in a surgical environment. The isolation system may include a pump and channels or valves to allow for fluid removal or addition to the interior of the isolation system. The sheet of the isolation system may further comprise microtextured inner layer so as to maintain functionality of the enclosed mobile computing device in an aqueous environment or in a soiled condition.
Owner:TOTH LANDY
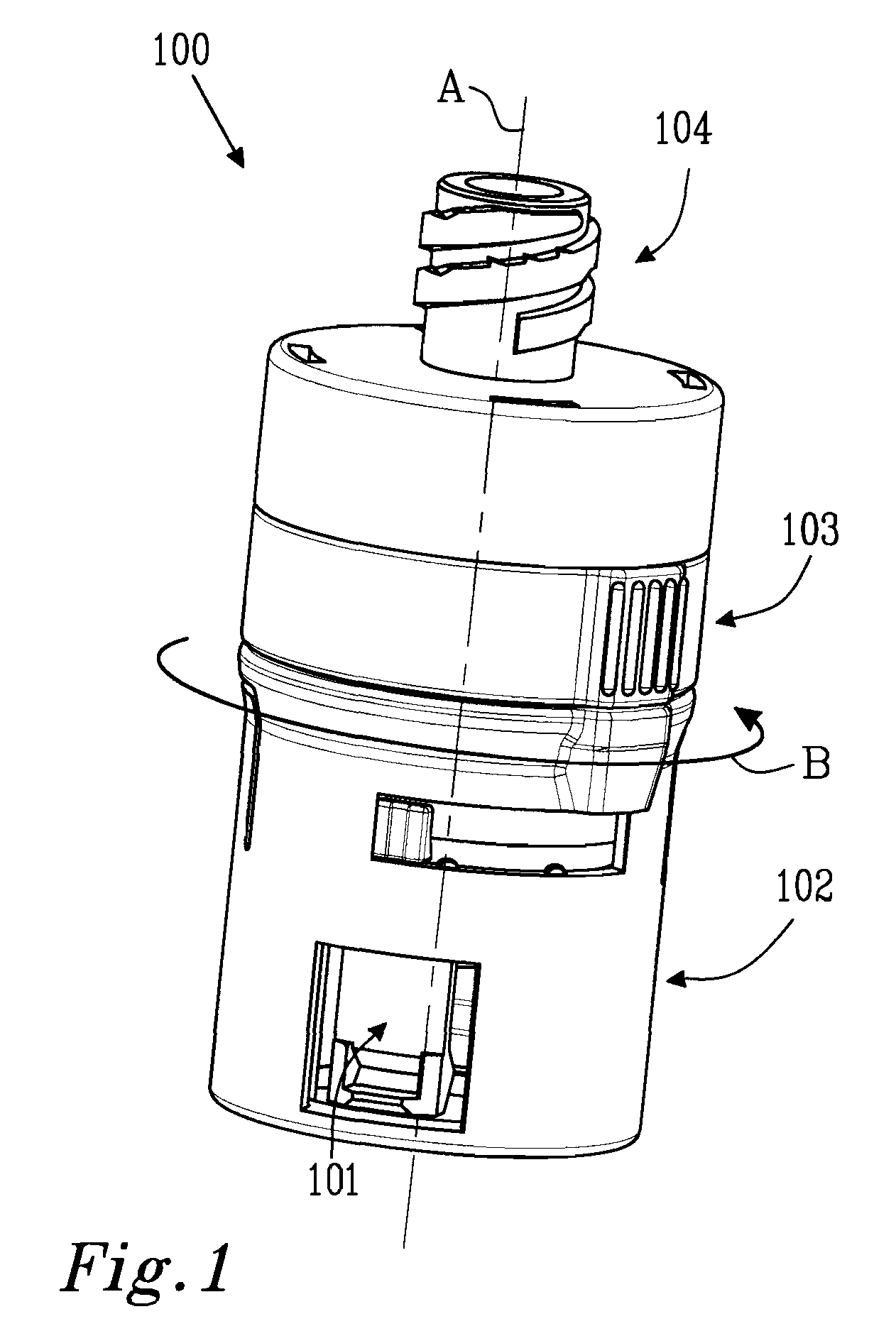
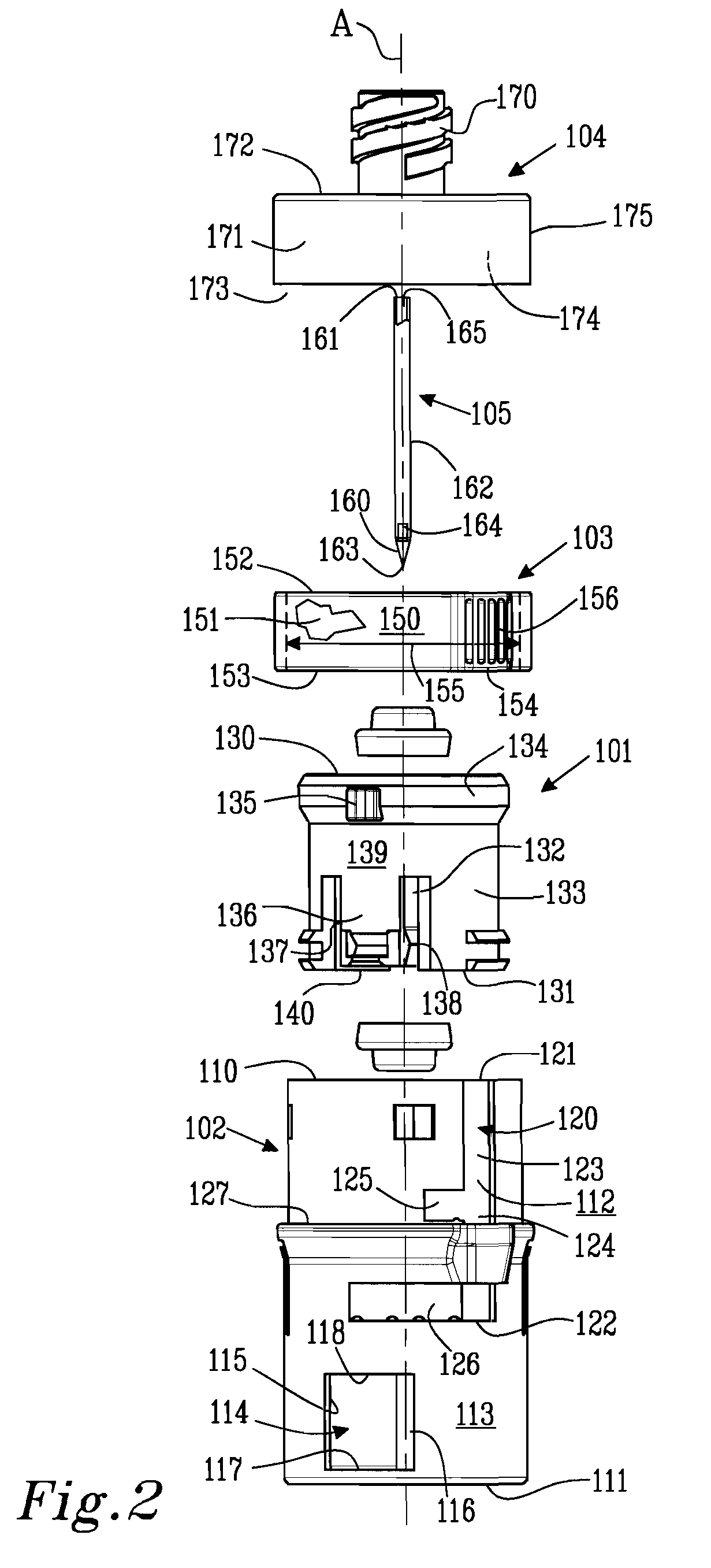
Fuel cartridge with connecting valve
A shut-off valve or connecting valve capable of connecting a fuel supply to a fuel cell is disclosed. The valve comprises a first valve component and a second valve component. Each valve component has an outer housing and a biased slidable member disposed inside the housing forming an internal seal. During the connection process, the two valve components establish an inter-component seal. Afterward, in one suitable embodiment the slidable member moves inward and opens the internal seal in the valve component to establish a flow path. In another embodiment, the slidable member moves inward and exposes a first filler and the first filler abuts a second filler in the other valve component to establish a flow path. In other embodiments, at least one valve component is sized and dimensioned to limit access to the internal seal.
Owner:INTELLIGENT ENERGY LTD
Apparatus for applying tissue sealant
InactiveUS6132396AEasy to fillEasy to assembleLiquid surface applicatorsSurgeryTissue sealantGear wheel
A device and method for applying a fibrinogen-based tissue sealant to seamlessly connect human or animal tissues or organ parts, to seal wounds, stop bleeding and the like by mixing fibrin or fibrinogen with blood clot-promoting coagulation factors are disclosed. The device includes two cylindrical compartments for separately containing the separate fluid components of the sealant preparation, which are simultaneously displaced from the respective compartments by plungers commonly depressable with the same effective strokes. The plungers may be depressed directly or by a common mechanism (e.g., rack and pinion) for accurately controlling the rate of dispensing fluid. The cylindrical compartments are of the same or different cross-sectional area and are arranged either concentrically or side-by-side. The device further includes structure for merging the two fluid components within an outer sleeve housing an inner needle. The sleeve and needle contain conduits for the flow of the two fluid sealant components as they are expressed from the respective compartments. Also disclosed are a convenient device for filling the two compartments, structure for mixing the fluid components, and for atomizing the effluent sealant fluid stream (i.e., spraying).
Owner:PLASMASEAL
Two-part package for medical implant
ActiveUS7712606B2Low costProcedure be minimizedDispensing apparatusHeart valvesBiomedical engineeringMedical treatment
Owner:BOSTON SCI SCIMED INC
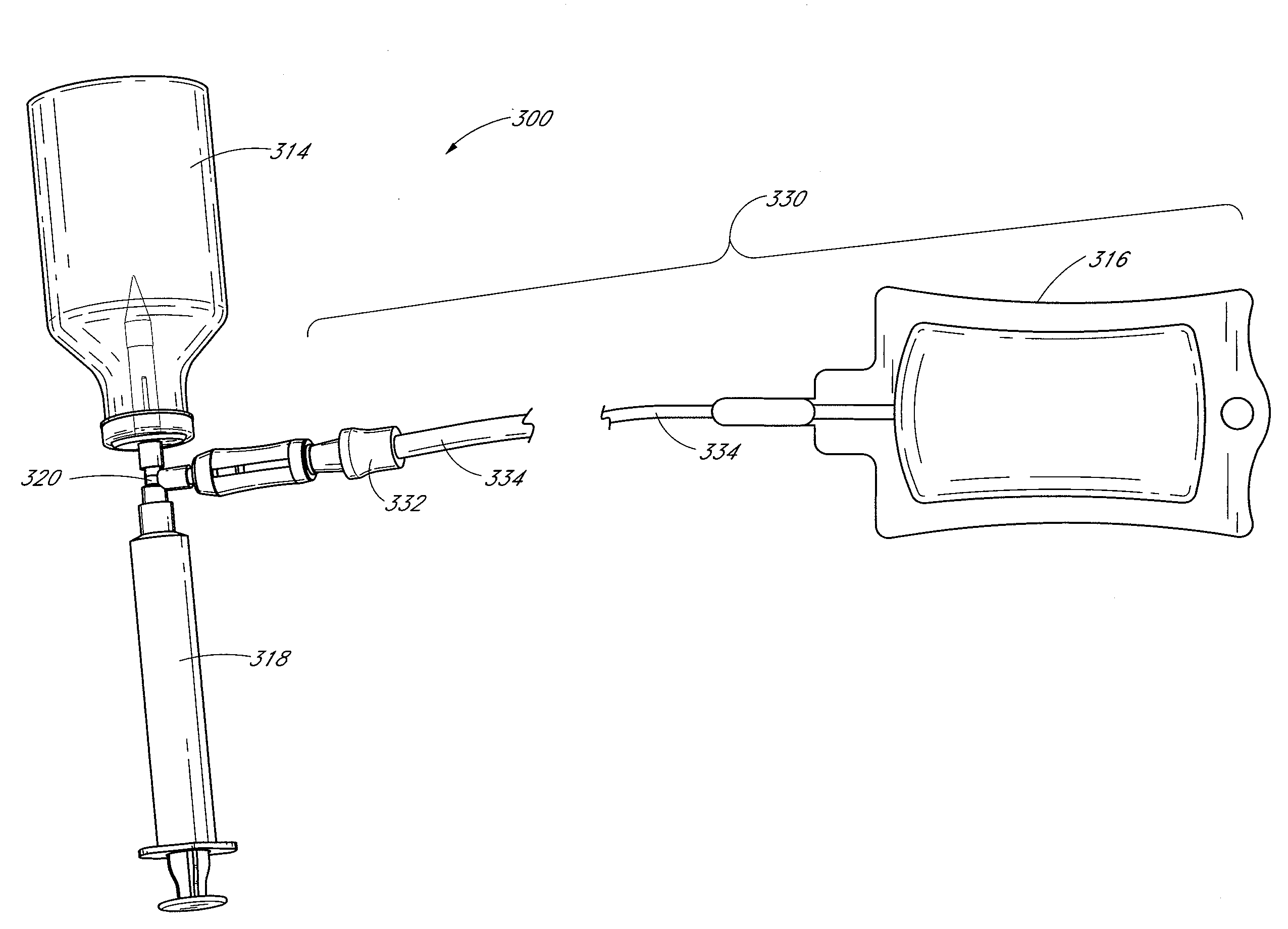
Fluid transfer devices and methods of use
Some embodiments disclosed herein related to a device for transferring precise amounts of fluid from at least one source container to a at least one target container. In some embodiments, the fluid is first transferred from the source container (e.g., a vial) through a connector to an intermediate measuring container (e.g., a syringe). In some embodiments air can pass through an air inlet and enter the vial to compensate for the volume of fluid withdrawn from the vial. An air check valve or a bag or a filter can prevent the fluid from escaping through the air inlet. The precisely measured amount of fluid can then be transferred from the intermediate measuring container to the target container (e.g., an IV bag). In some embodiments the connector can include a source check valve and a target check valve to direct fluid first from the source container to the intermediate measuring container and then from the intermediate measuring container to the target container. Some embodiments of the device can include a motor and a controller for automatically actuating a plunger of the syringe to transfer the desired amount of fluid.
Owner:ICU MEDICAL INC
Two-Part Package For Medical Implant
ActiveUS20070061008A1Low costMinimize preparation timeDispensing apparatusHeart valvesBiomedical engineeringMedical treatment
The invention provides a two-part package and method of use for a pre-attached medical implant and delivery tool system. The package includes a wet compartment and a dry compartment and allows a pre-attached implant and delivery tool system to be at least partially stored immersed in a fluid in the wet compartment and at least partially stored in the dry compartment. In one embodiment the implant comprises a replacement heart valve, and the heart valve is stored inside the wet compartment while the heart valve delivery tool remains dry in the dry compartment.
Owner:BOSTON SCI SCIMED INC
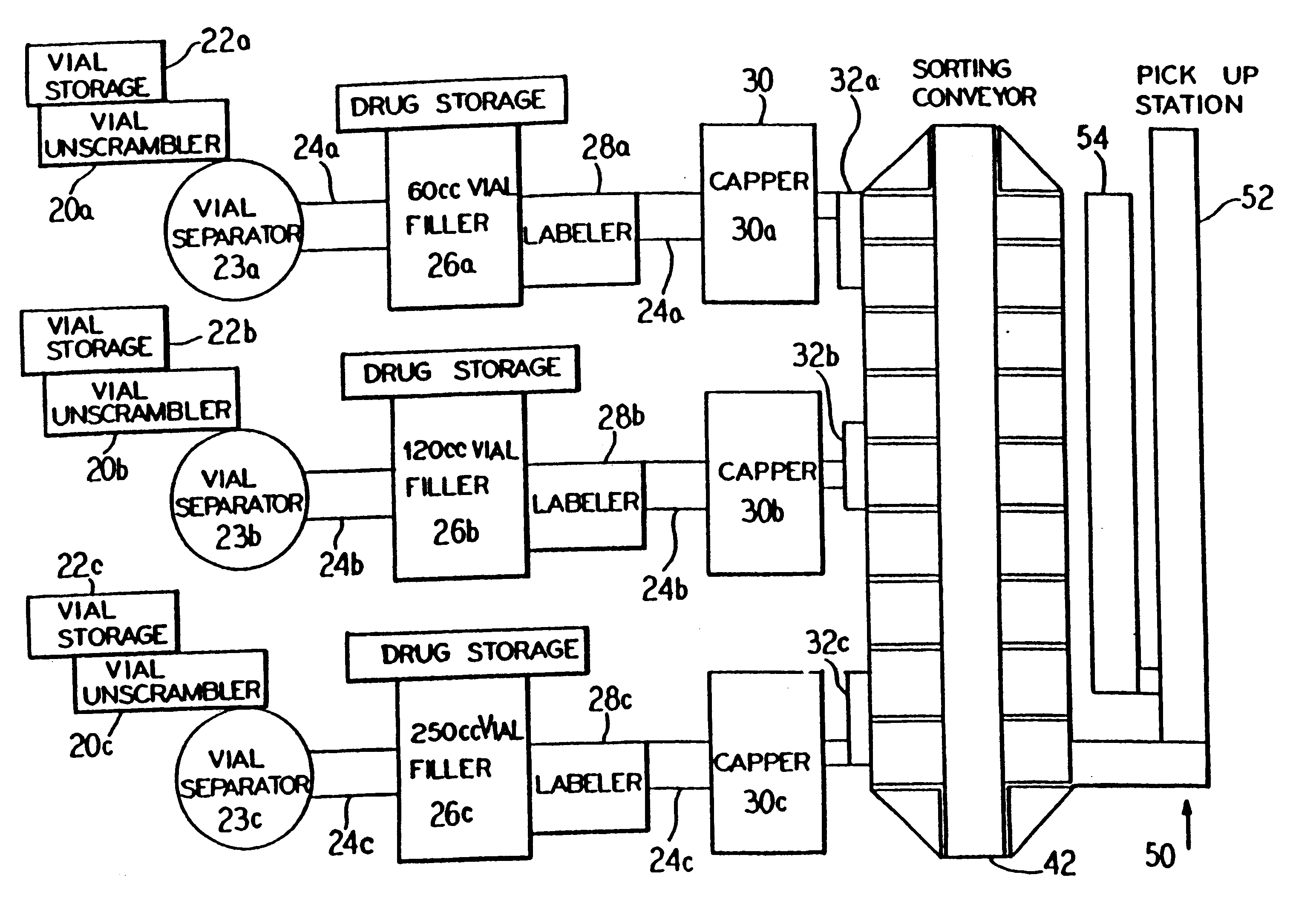
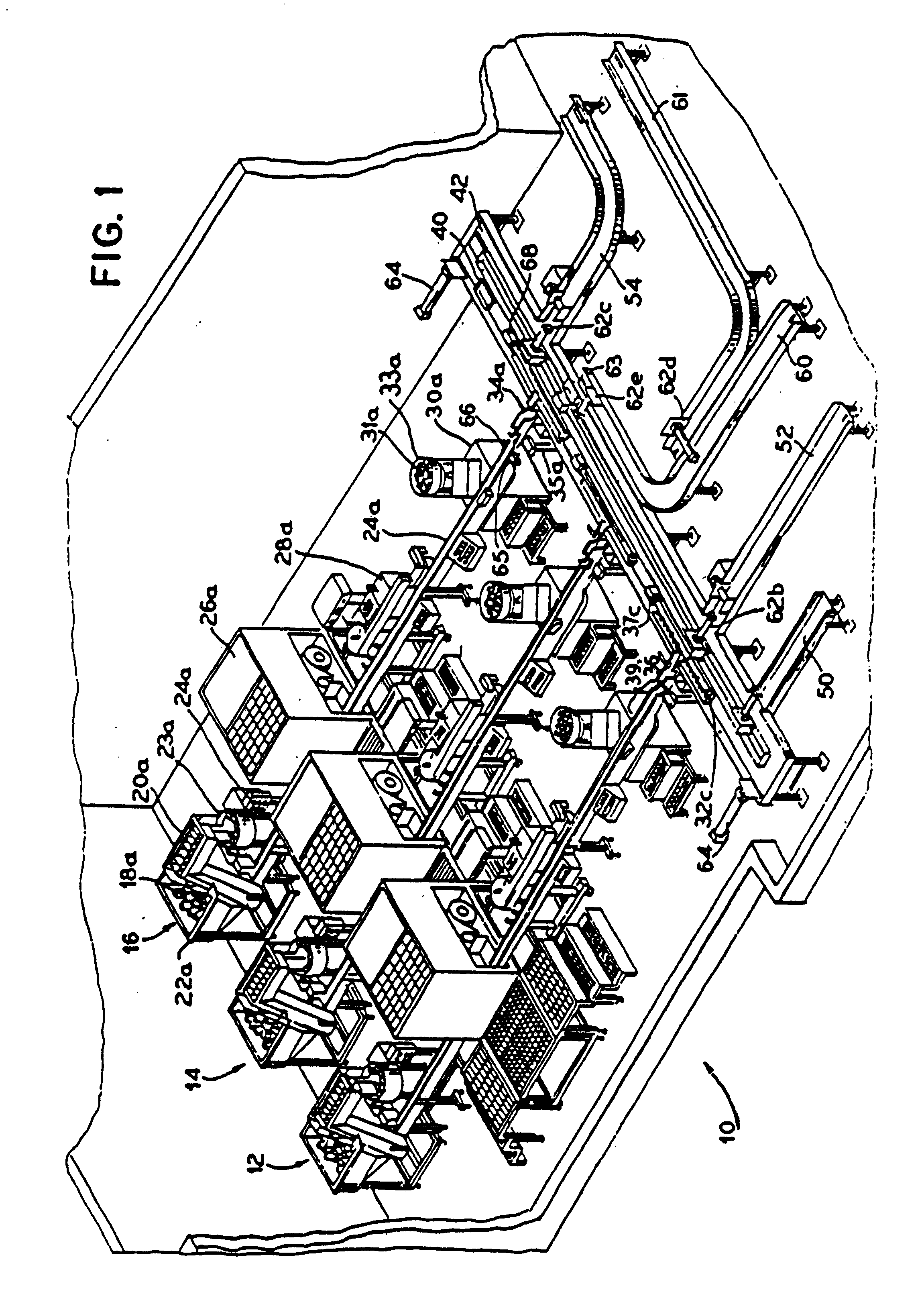
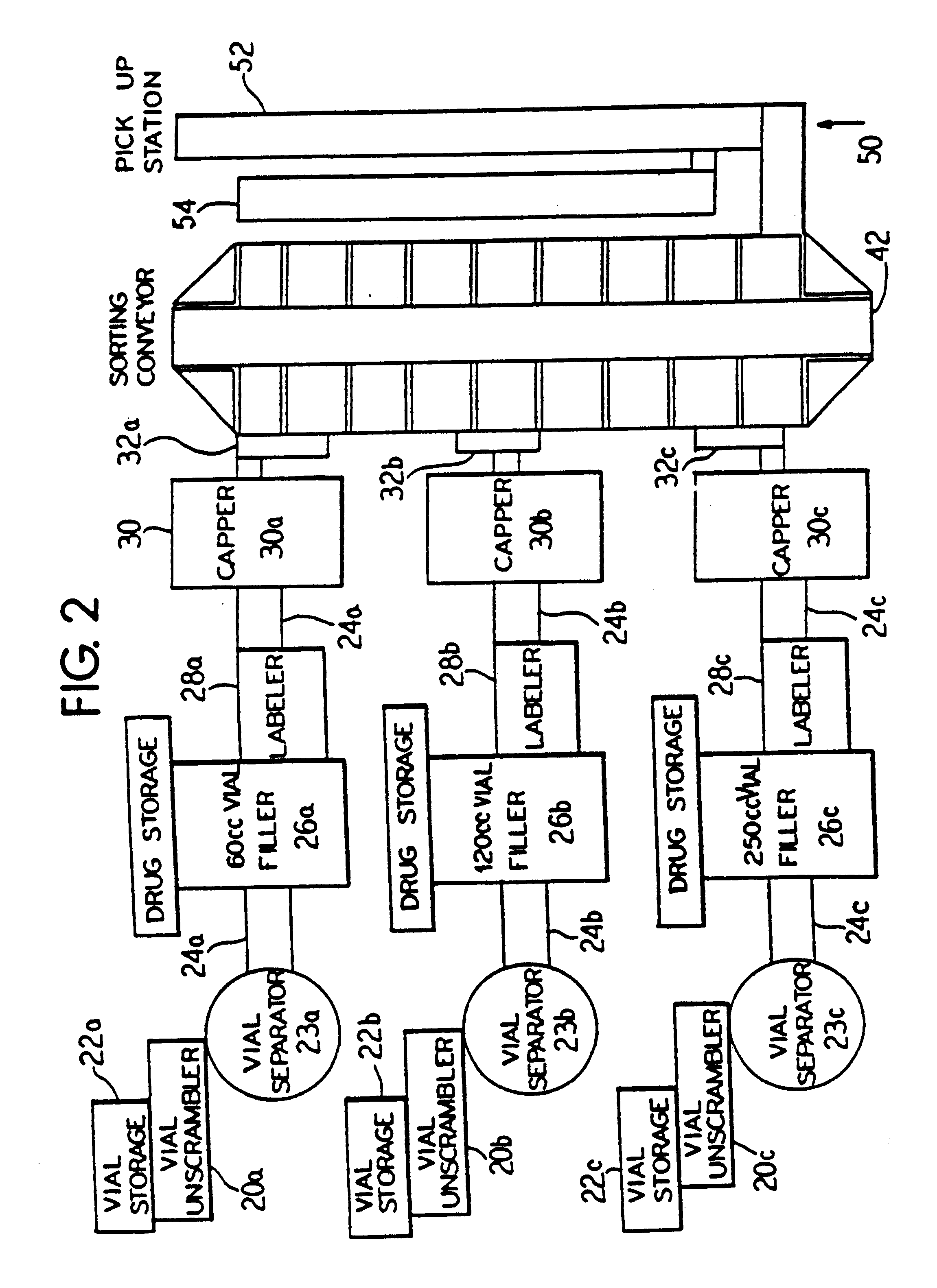
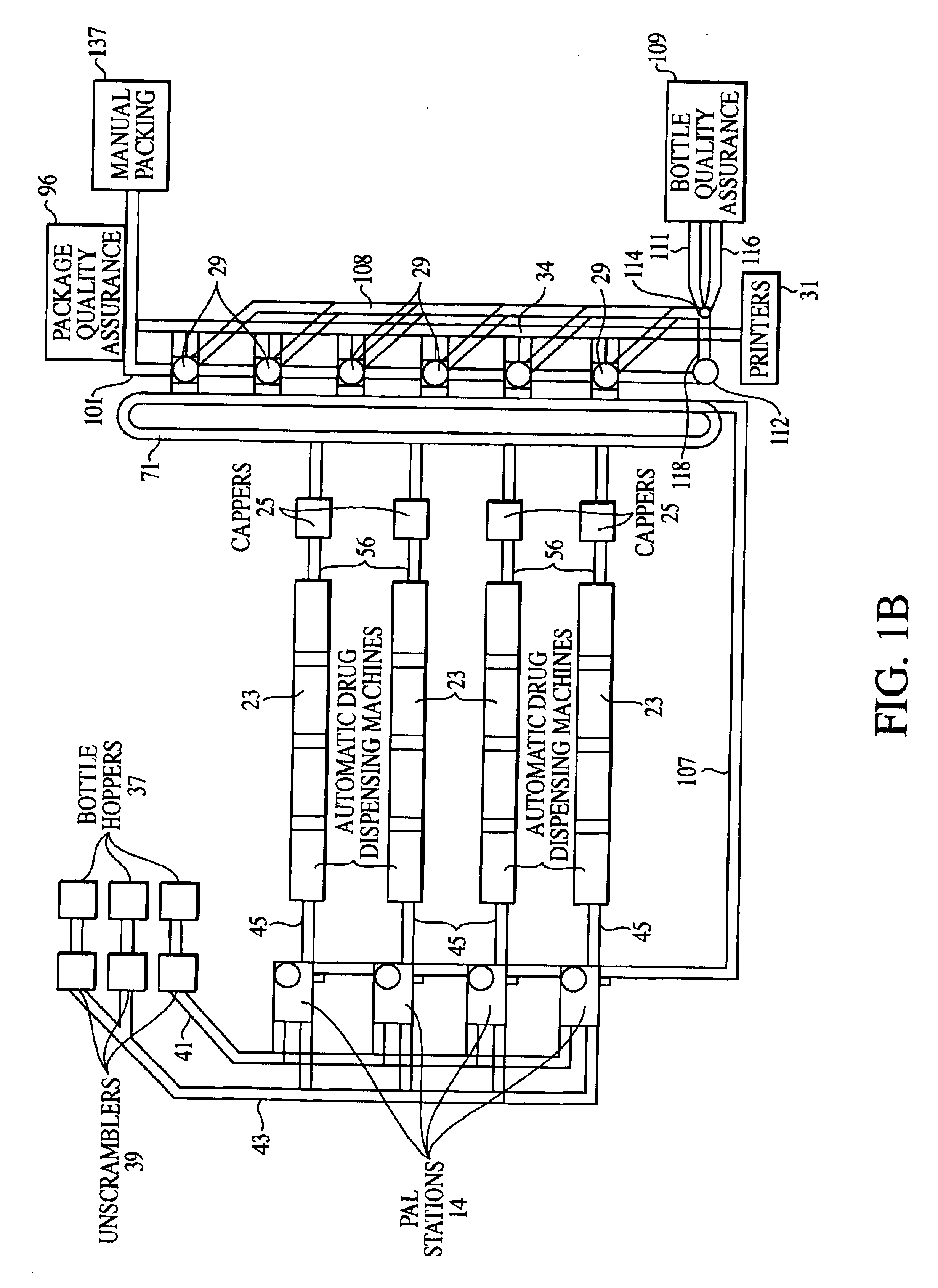
Automated prescription vial filling system
InactiveUSRE37829E1Reduction and inventoryLow costDrug and medicationsDigital data processing detailsMedical prescriptionBiomedical engineering
A method and apparatus for dispensing drugs, wherein a patient's order of one or more prescriptions is automatically filled. Various drugs are stored in three or more filler lines. A vial size is assigned to each line. When a prescription is filled, it is automatically assigned to a line in view of the vial size requirements and processed accordingly. Provisions are made for the inability to fill a prescription or order. Subsequently, all of the patient's prescriptions are collected and made available as a single order.
Owner:AUTOMED TECH
Pressure equalizing device for vial access
ActiveUS7900659B2Prevent buildupPrevent escapeDiagnosticsSurgeryPressure balanceAtmospheric pressure
A pressure-equalizing vial access device and method providing closed and sealed reconstitution of vial contents. A rigid container with a fixed internal volume is connected with a vent lumen extending into the vial. As pressure in the vial increases, the pressure is equalized with atmospheric pressure by varying the volume of a compartment within the rigid container. The compartment is formed with a volume control device that automatically varies the volume of the compartment in the rigid container to accommodate and equalize the pressure in the vial by increasing or decreasing the volume of the compartment. In one case the volume control device comprises a sliding disk and in another, a bladder that compresses with an increase in volume in the container and expands with a decrease.
Owner:CAREFUSION 303 INC
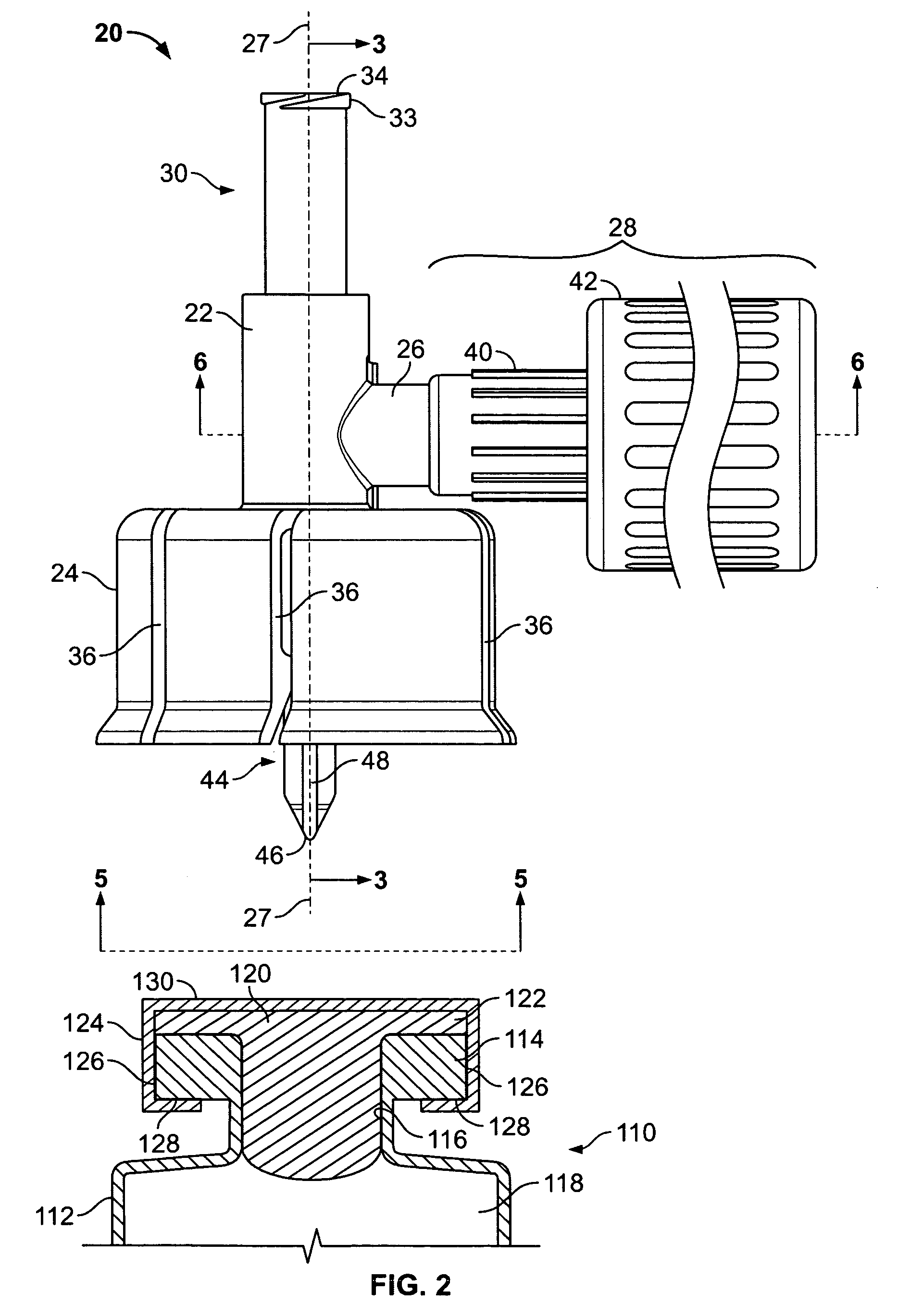
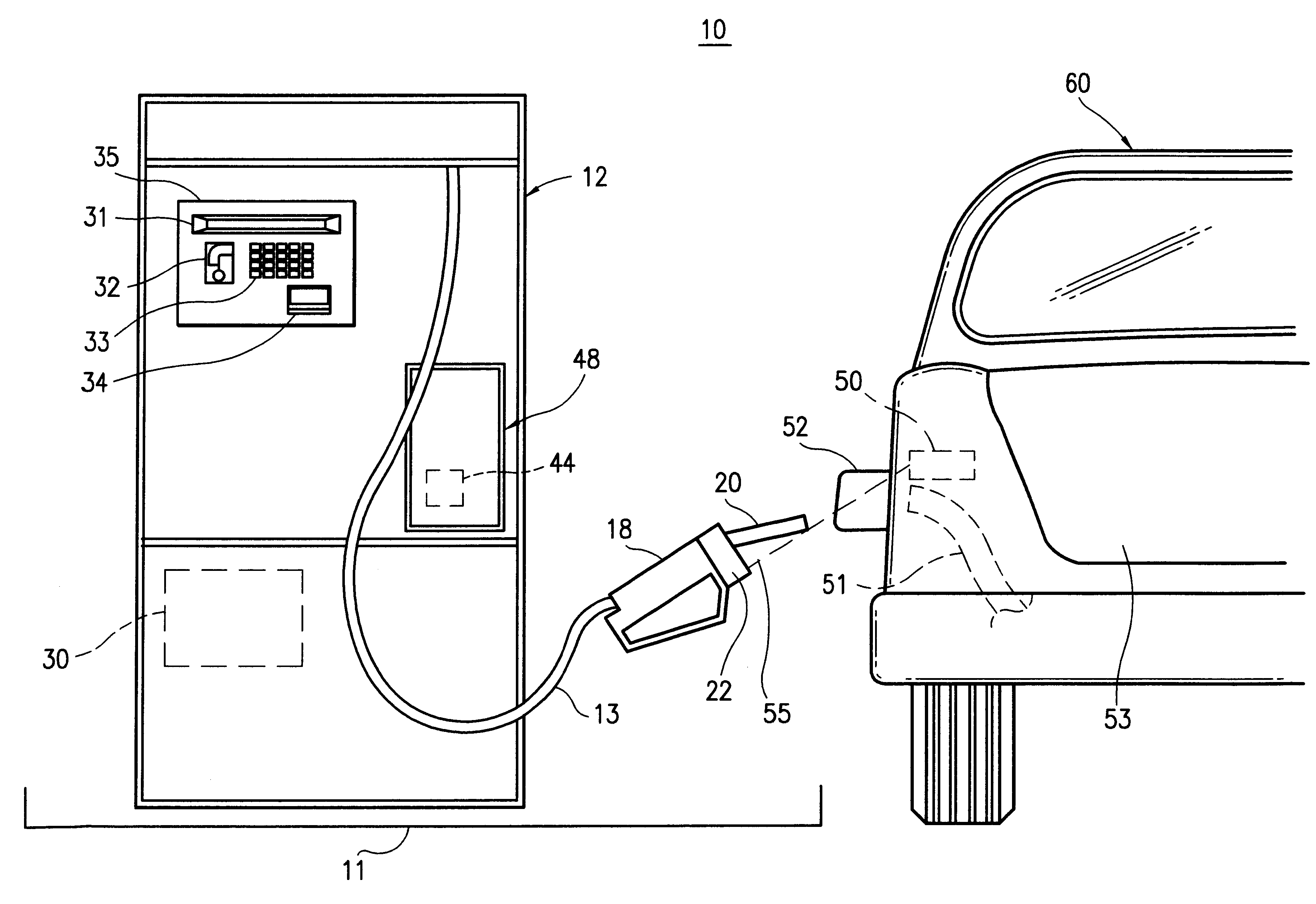
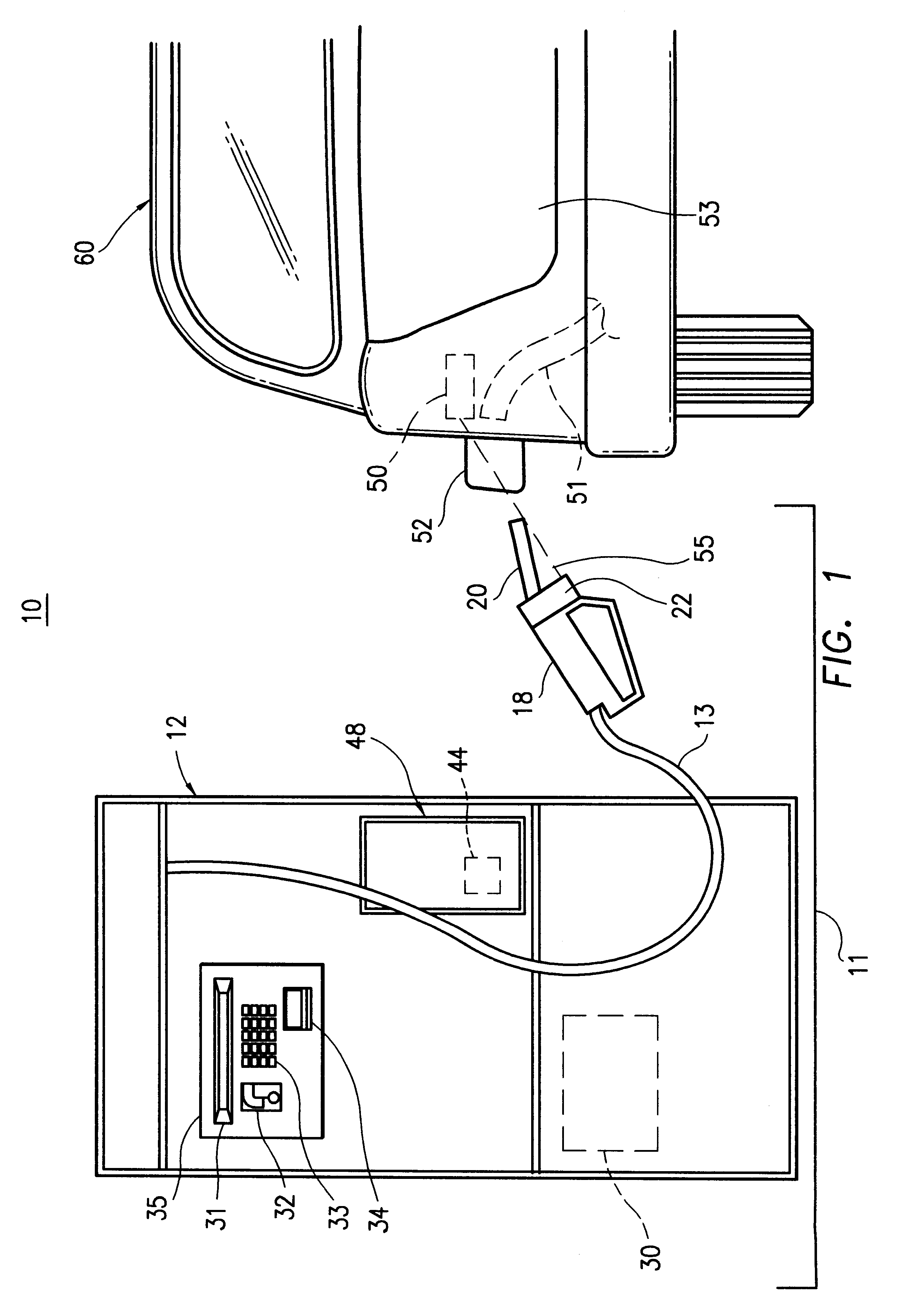
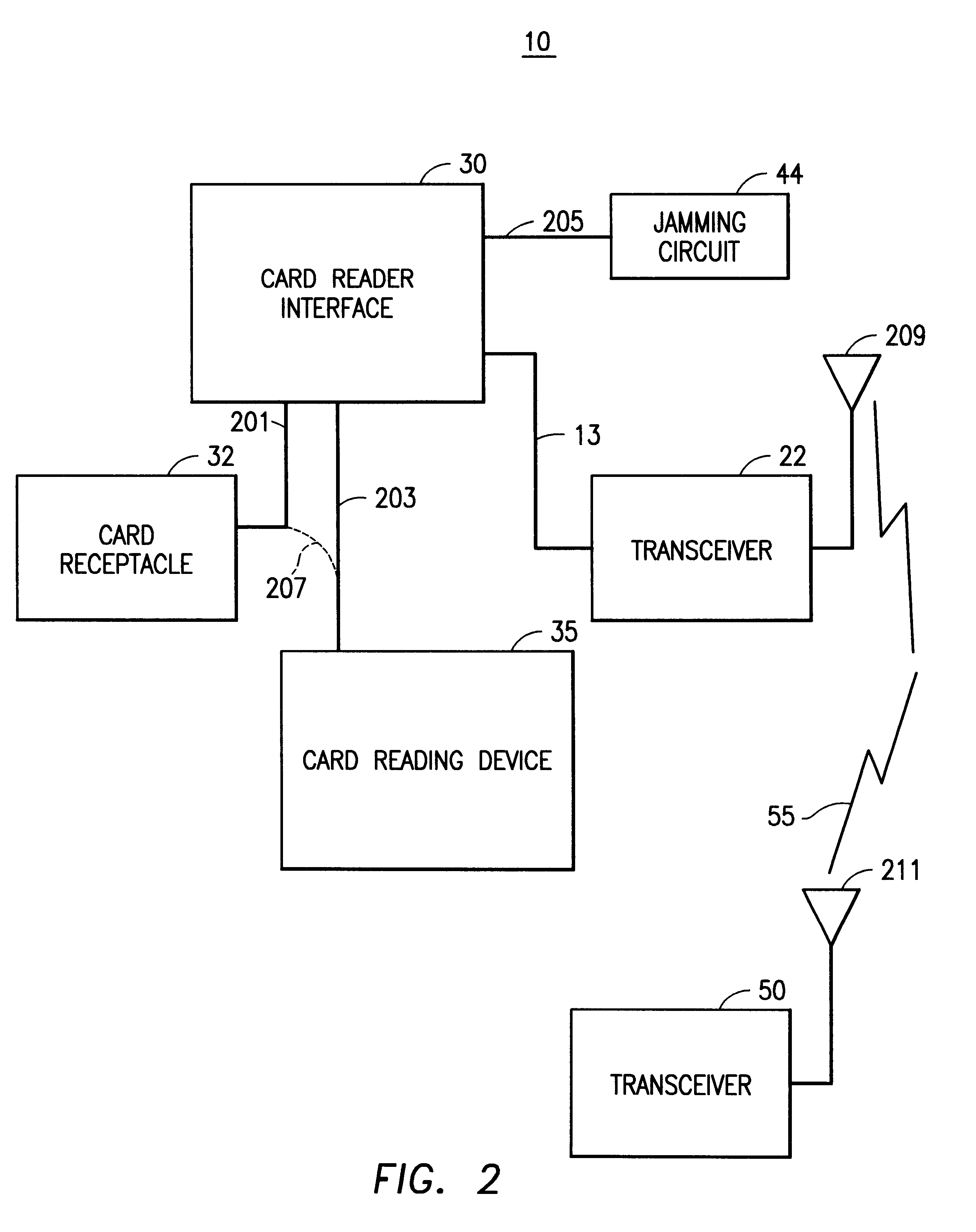
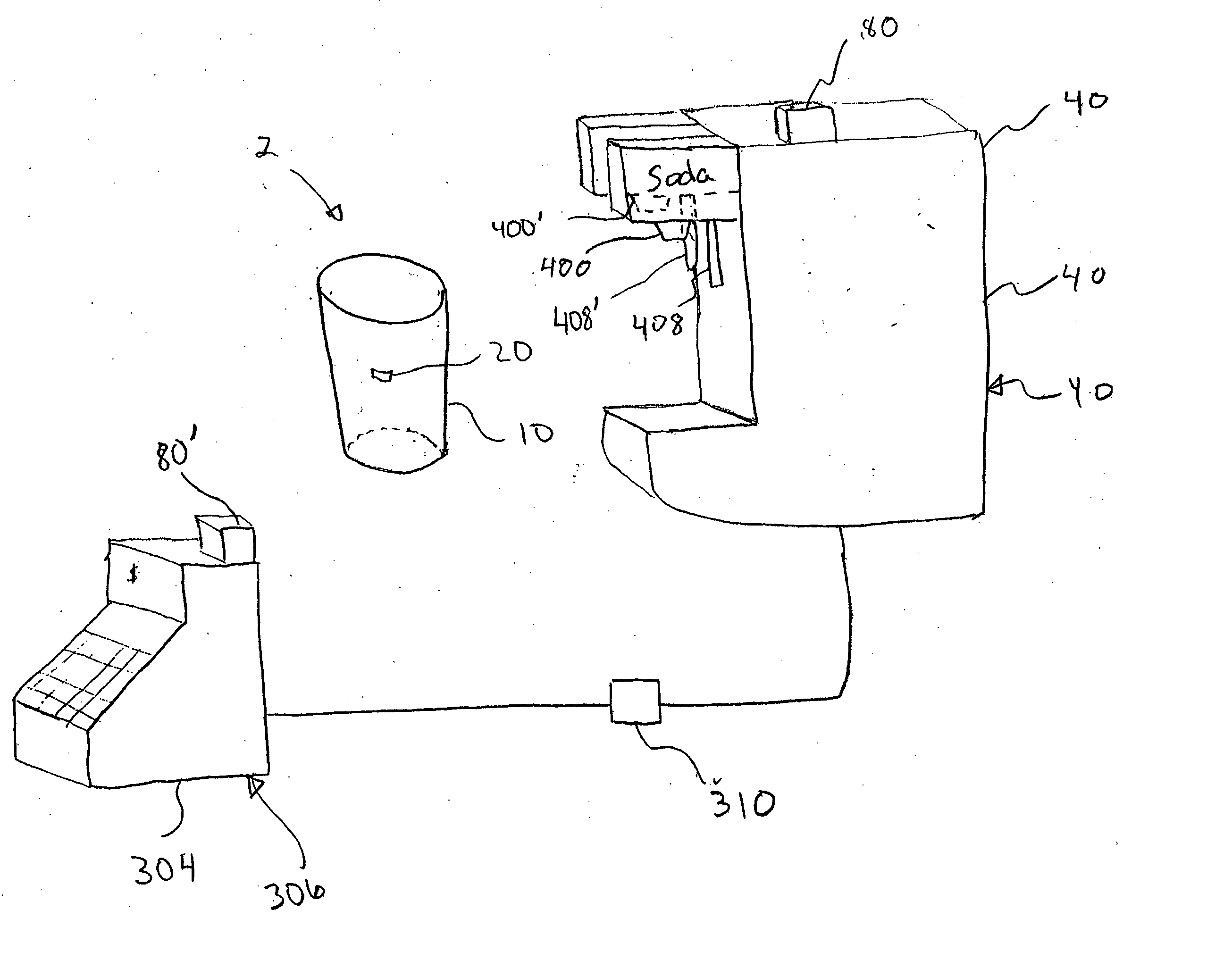
Method and apparatus for transmitting a digital information signal and vending system incorporating same
A cashless business transaction system (e.g., a vending system, a material tracking system, or a highway toll system) incorporates a method and apparatus for transmitting a digital information signal. A signal generator (311) generates a constant frequency signal. A phase modulator (305) varies the instantaneous phase of the constant frequency signal to represent digital information, thereby producing a phase modulated signal (325). A tuned resonant circuit (307) filters and averages the phase modulated signal to produce a simulated FM signal, and transmits the simulated FM signal via its antenna (309). One such business transaction system (e.g., a vending system) incorporates such a transmitter to facilitate transmission of billing information from a device located within a substantially electrically shielded environment. Another such business transaction system preferably incorporates such a transmitter to facilitate half-duplex transmission of digital information regardless of whether or not the digital information is transmitted from a device located within a substantially electrically shielded environment.
Owner:POLE ZERO ACQUISITION
Preparation of capsules
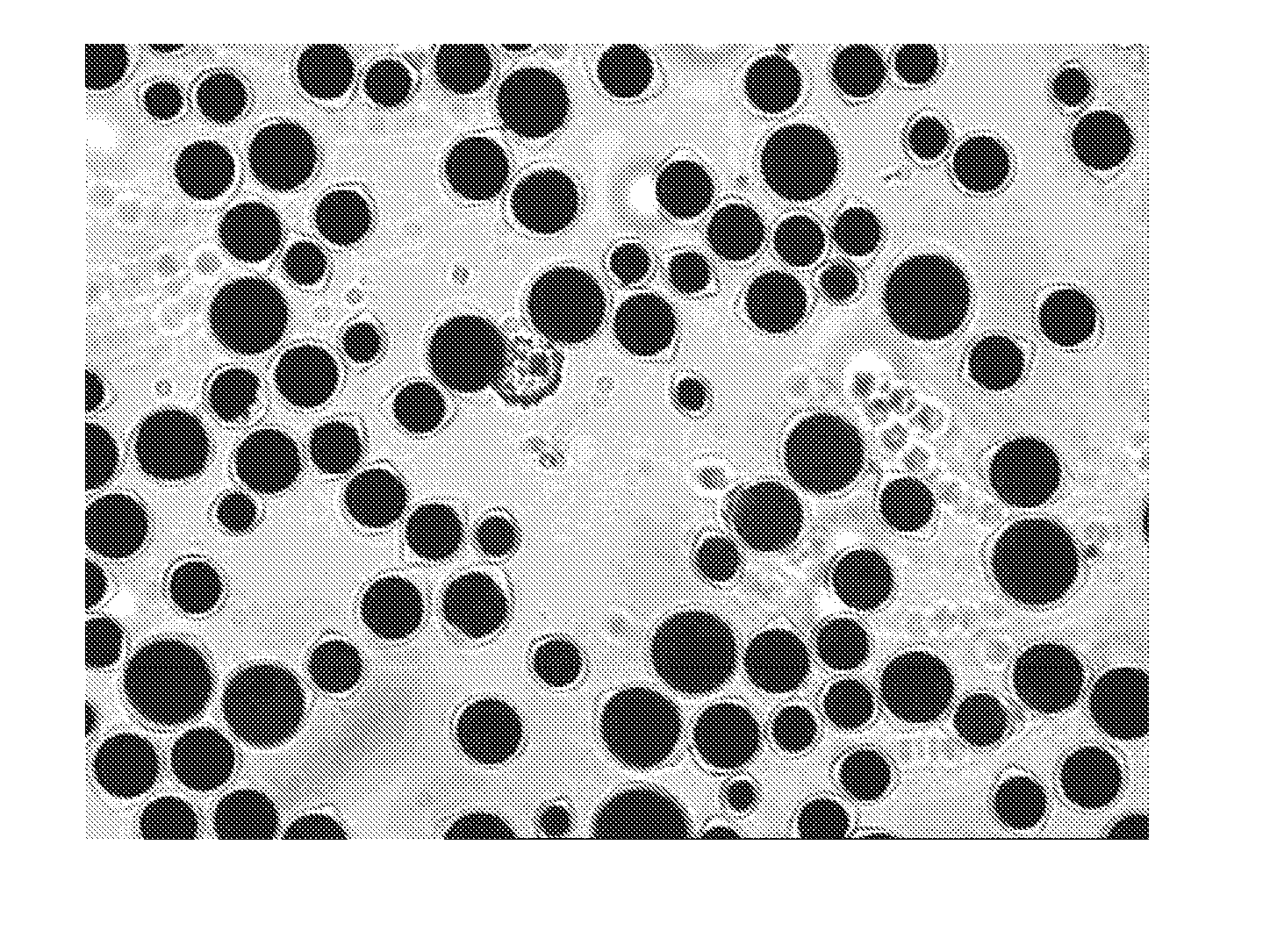
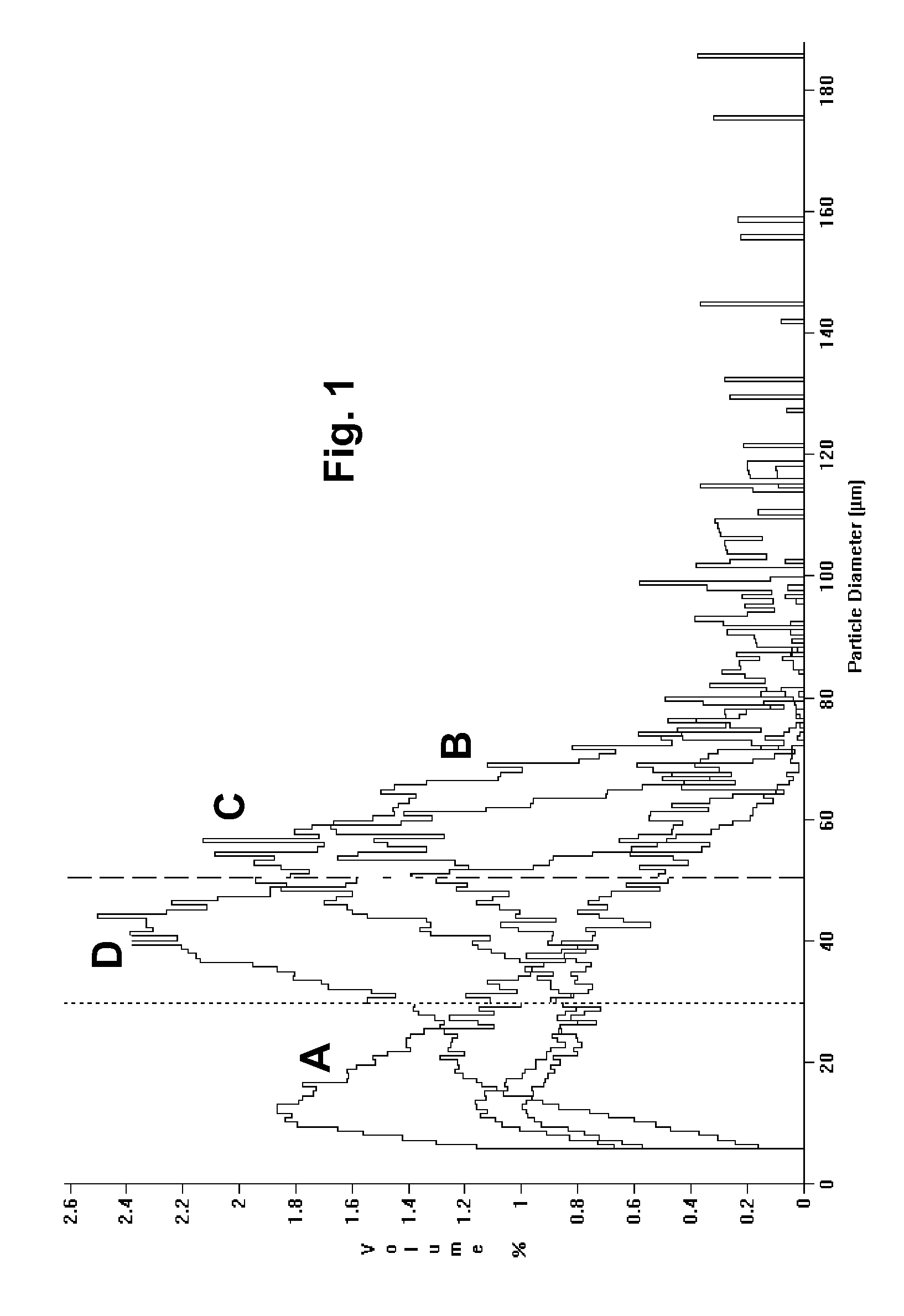
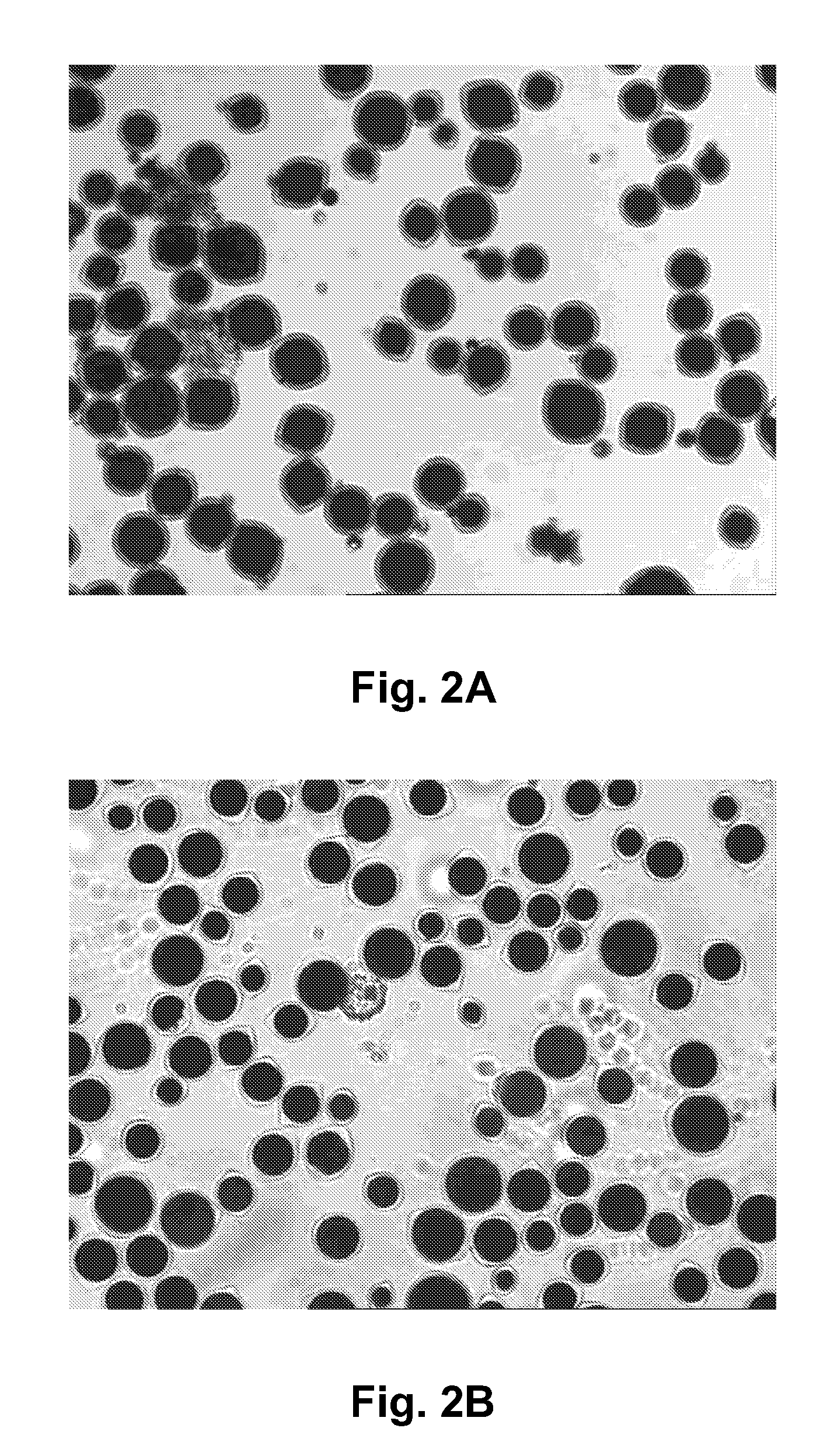
Prior art processes for producing protein-based capsules (for example, capsules for use in electrophoretic media) tend to be wasteful because they produce many capsules outside the desired size range, which is typically about 20 to 50 μm. Capsule size distribution and yields can be improved by either (a) emulsifying a water-immiscible phase in a preformed coacervate of the protein; or (b) using a limited coalescence process with colloidal alumina as the surface-active particulate material.
Owner:E INK CORPORATION
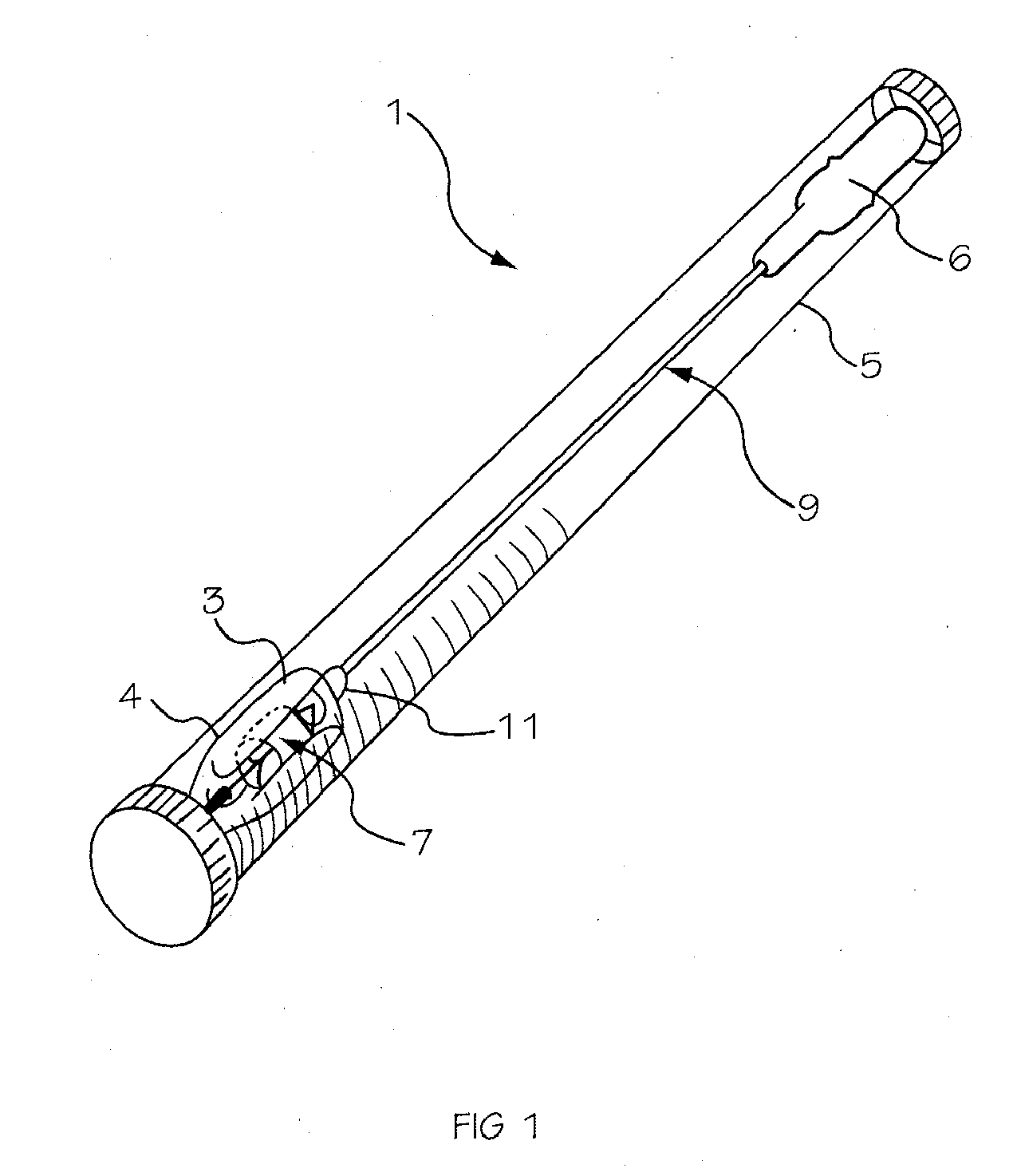
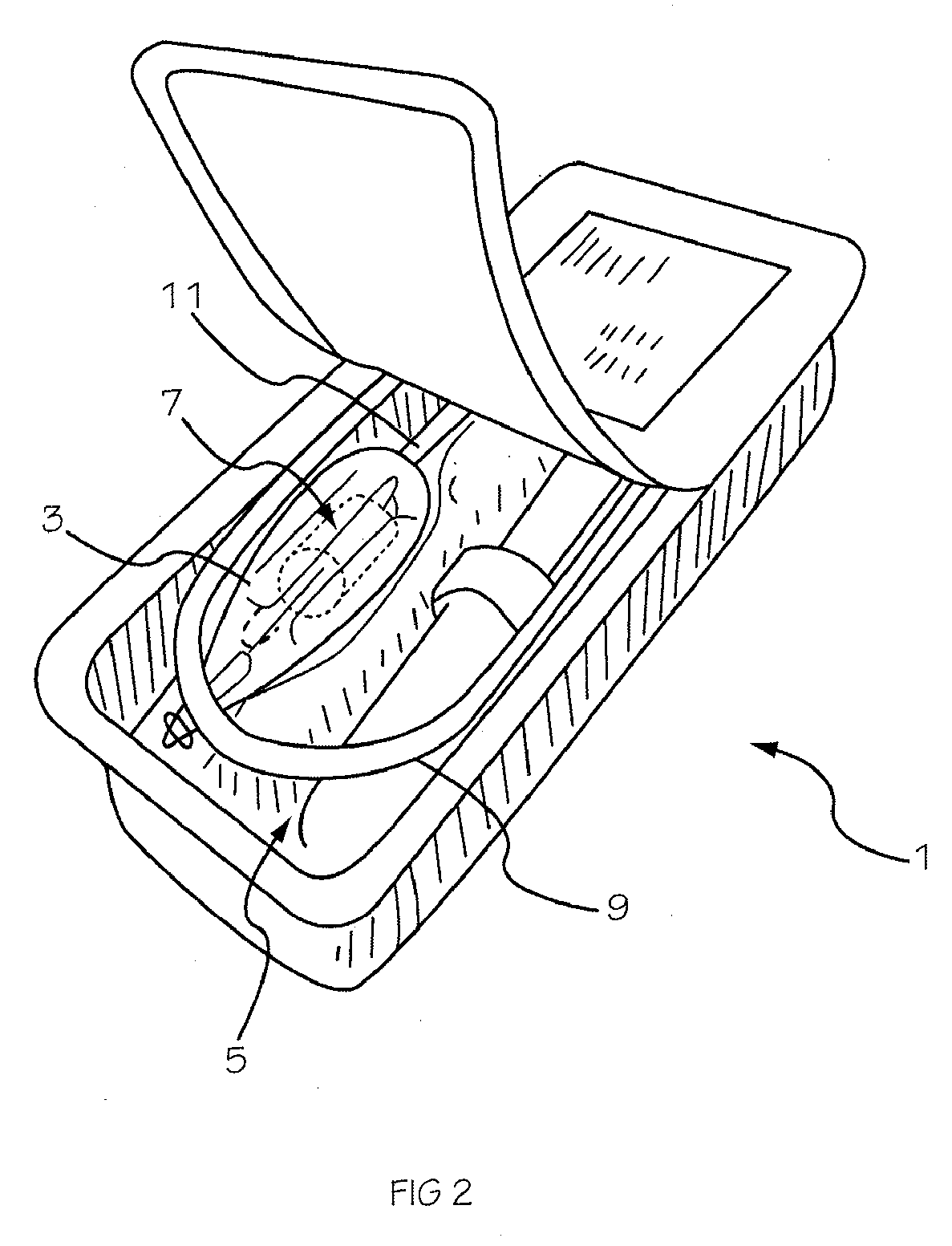
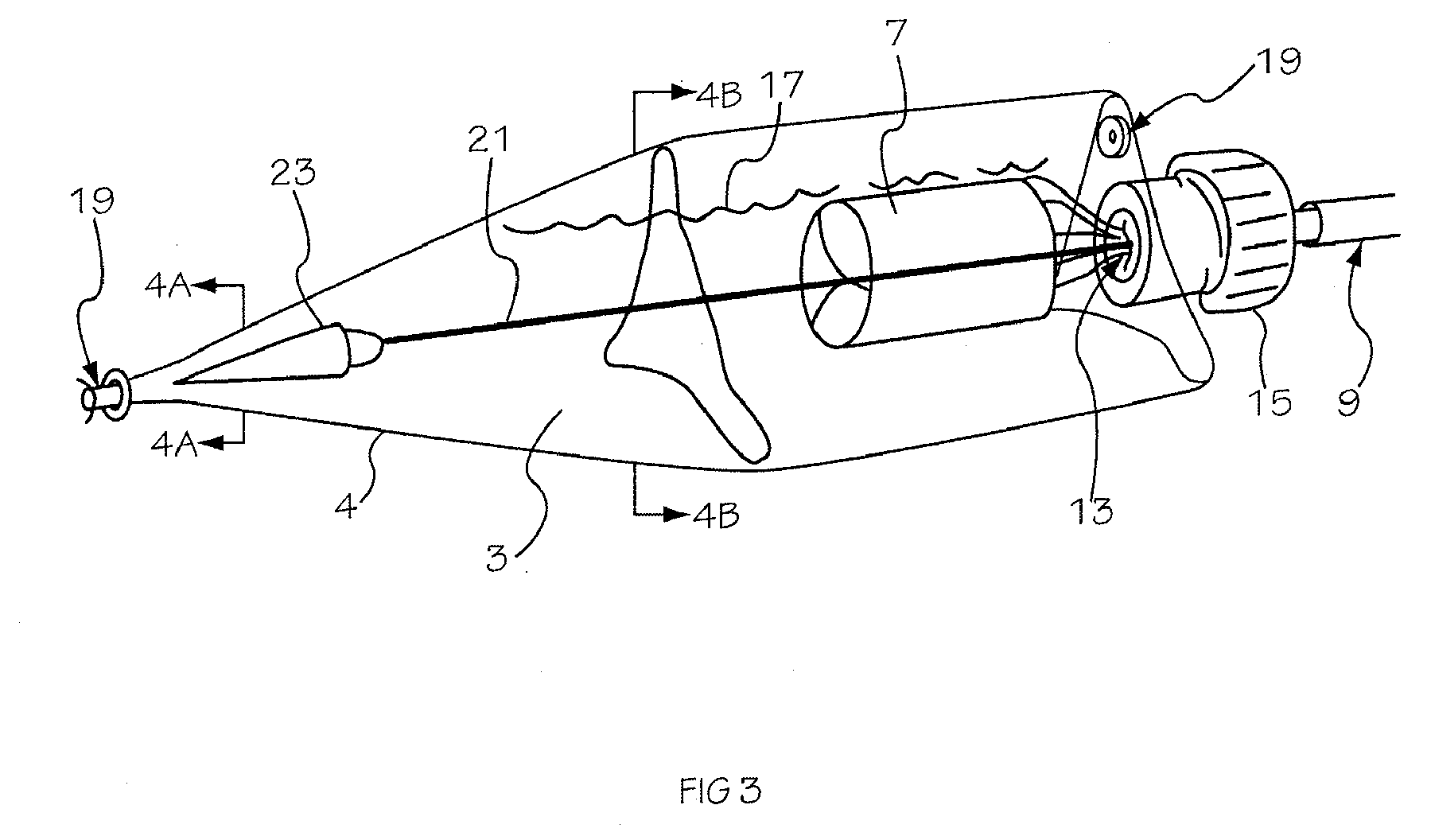
Prescription filling apparatus implementing a pick and place method
ActiveUS7228198B2Easy to scaleSmall footprintDigital data processing detailsSolid materialComputer control systemOutput device
An apparatus for filling vials comprises a shelving unit defining an array of storage locations. The shelving unit may be an array in an XY plane or one or more carousels. A plurality of storage containers are provided, each removably carried by one of the storage locations. A counting and dispensing unit, a source of vials, a label printer and application unit or units, and an output device are also provided. The output device may take a variety of forms such as an output chute, which is preferably used when a capping unit is provided, an output conveyor, a plurality of output lanes, and an output carousel, which may be a dedicated carousel or a portion of the carousel providing the plurality of storage locations. A computer controlled engagement device provides motion in a Z direction. The engagement device may be comprised of a first stage for engaging the storage containers and a second stage for engaging the vials. A computer controlled system carries the engagement device and moves the engagement device in XY directions among the plurality of storage locations, counting and dispensing unit, source of vials, label printer and application unit, and output device. Methods of operating and refilling the vial filling apparatus are also disclosed.
Owner:MCKESSON AUTOMATION SYST
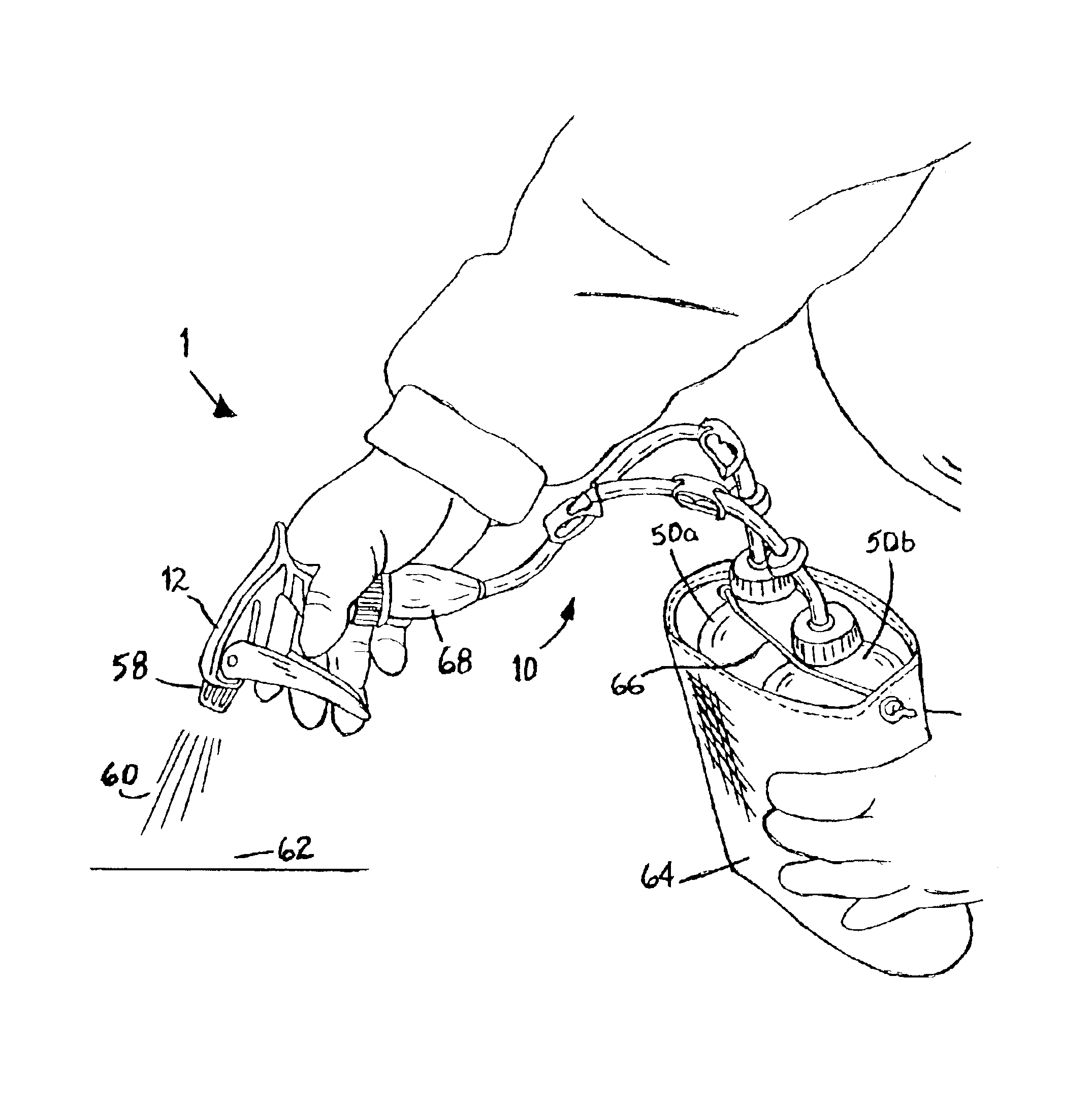
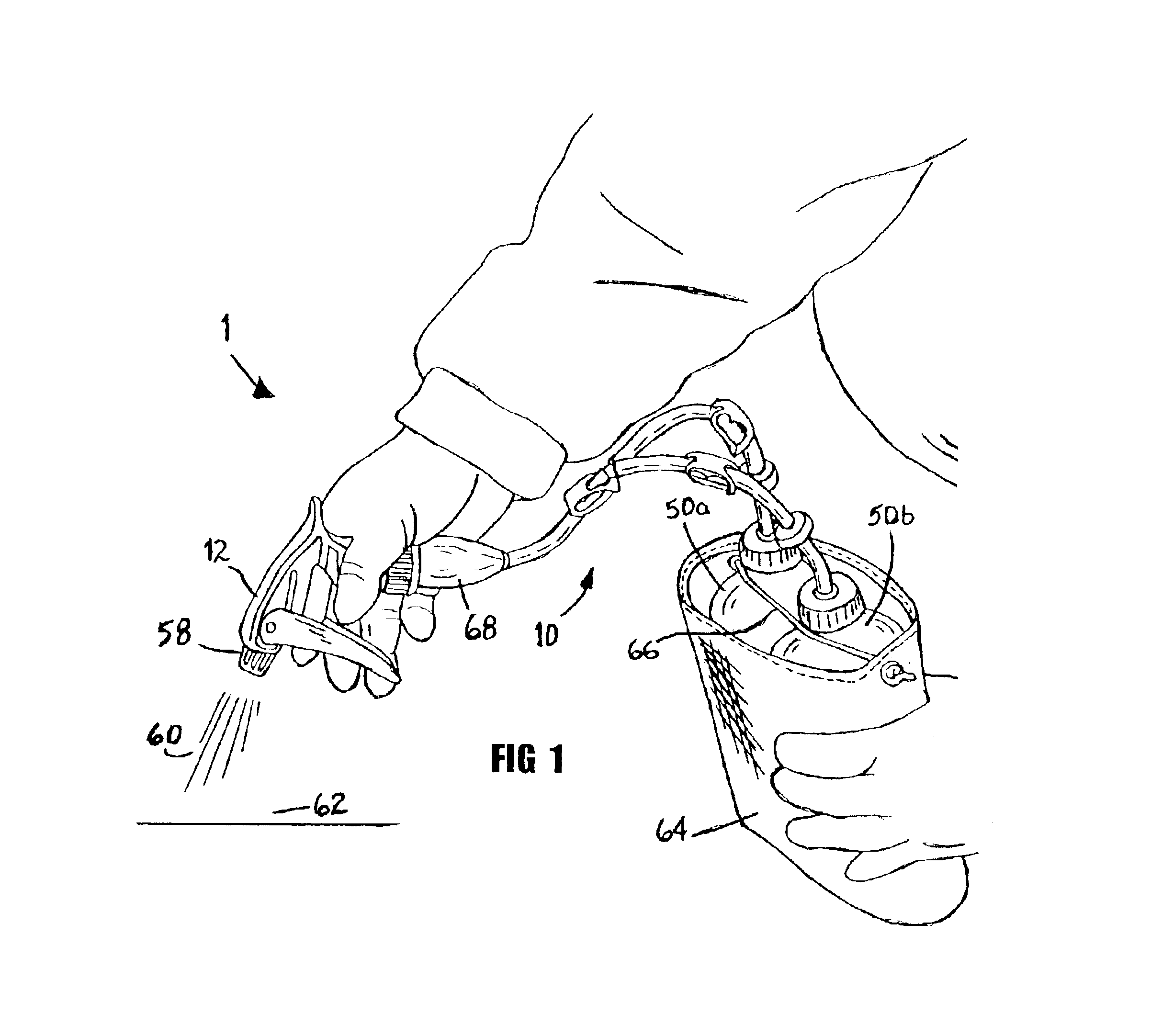
Multiple fluid closed system dispensing device
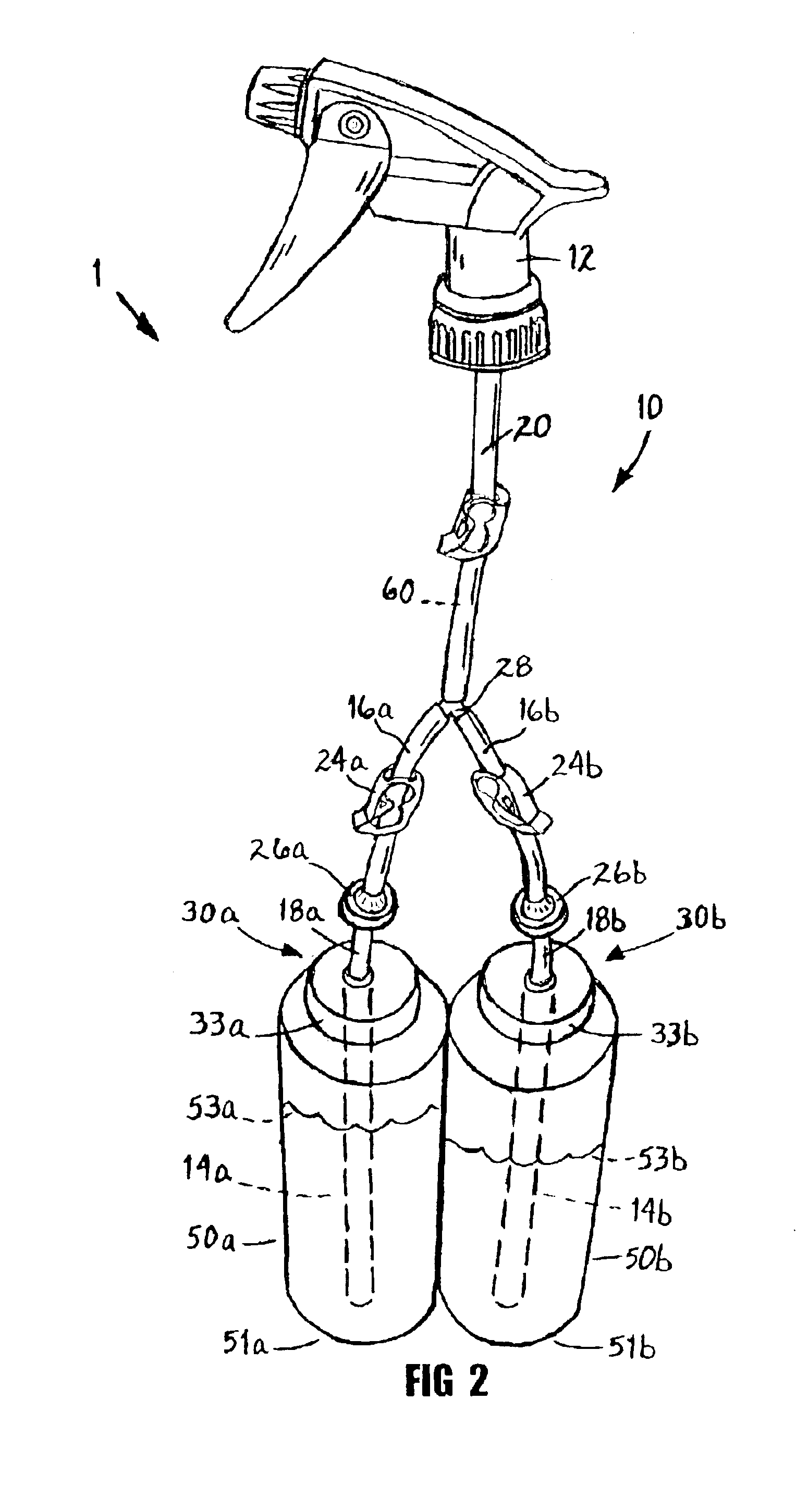
InactiveUS6843390B1Improve efficiencyEffective alternativeLiquid transferring devicesSolid materialTarget surfaceEngineering
A dispensing device (1) with multi-arm tubing assembly (10) connected to a single source pumping means (12) draws and mixes multiple fluids from plurality of flexible walled sealed supply containers (50a,b) then expels the mixture (60) through nozzle (58) to a target surface (62). Dispensing device (1) provides a closed system whereby no venting occurs, rather supply containers (50a,b) contract in size equal to the volume of fluid expelled. Unstable fluids thus remain protected from exposure to outside air. Additionally, a new use of a repressurization device is disclosed for maintaining the potency of unstable fluids like hydrogen peroxide and a kit is provided which allows user to choose from various components and accessories as needed to suit their multi-chemical dispensing needs.
Owner:WANDERS INC
Apparatus and method for supporting, positioning and rotating a substrate in a processing chamber
ActiveUS20080276864A1Increase pressureReduce stressLiquid surface applicatorsSemiconductor/solid-state device manufacturingAir bearingThin layer
An apparatus and method for supporting, positioning and rotating a substrate are provided. In one embodiment, a support assembly for supporting a substrate includes an upper base plate and a lower base plate. The substrate is floated on a thin layer of air over the upper base plate. A positioning assembly includes a plurality of air bearing edge rollers or air flow pockets used to position the substrate in a desired orientation inside above the upper base plate. A plurality of slanted apertures or air flow pockets are configured in the upper base plate for flowing gas therethrough to rotate the substrate to ensure uniform heating during processing.
Owner:APPLIED MATERIALS INC
Method and System for Providing An Integrated Analyte Sensor Insertion Device and Data Processing Unit
ActiveUS20080097246A1Simple and accurate sensor deploymentEasy to operateSurgeryVaccination/ovulation diagnosticsAnalyteComputer science
Method and apparatus for providing an integrated analyte sensor and data processing unit assembly is provided.
Owner:ABBOTT DIABETES CARE INC
Transportable hydrogen refueling station
InactiveUS6755225B1Easy to monitorReduce riskTank vehiclesGas handling applicationsHigh pressureGaseous hydrogen
A portable hydrogen refueling stations which can dispense gaseous hydrogen from one or more internal high pressure tanks. The refueling station can be refilled with a lower pressure hydrogen gas feed and then compressed for storage within the refueling station.
Owner:QUANTUM FUEL SYST TECH WORLDWIDE INC
Dry powder inhalers, related blister devices, and associated methods of dispensing dry powder substances and fabricating blister packages
InactiveUS6889690B2Easy to optimizeLimit amount of resistanceSmall article dispensingLiquid surface applicatorsPowder InhalerInhalation
The present invention includes dry powder inhalers and associated multi-dose dry powder packages for holding inhalant formulated dry powder substances and associated fabrication and dispensing methods. The multi-dose package can include a platform body comprising at least one thin piezoelectric polymer material layer defining at least a portion of a plurality of spatially separated discrete elongate dry powder channels having an associated length, width and height; and a metallic material attached to selected portions of the piezoelectric polymer material including each of the regions corresponding to the elongate dry powder channels to, in operation, define active energy releasing vibratory channels. In operation, the elongate channels can be selectively individually activated to vibrate upon exposure to an electrical input.The dry powder inhaler includes an elongate body having opposing first and second outer primary surfaces with a cavity therebetween and having opposing top and bottom end portions and a multi-dose sealed blister package holding a plurality of discrete meted doses of a dry powder inhalable product located in the cavity of the elongate body. The inhaler also includes an inhalation port formed in the bottom end portion of the elongate body, the inhalation port configured to be in fluid communication with at least one of the discrete meted doses during use and a cover member that is pivotably attached to the elongate body so that it remains attached to the body during normal operational periods of use and moves to a first closed position to overlie the inhalation port at the bottom end portion of the body during periods of non-use and moves to a second open position away from the inhalation port during periods of use to allow a user to access the inhalation port.
Owner:ORIEL THERAPEUTICS INC
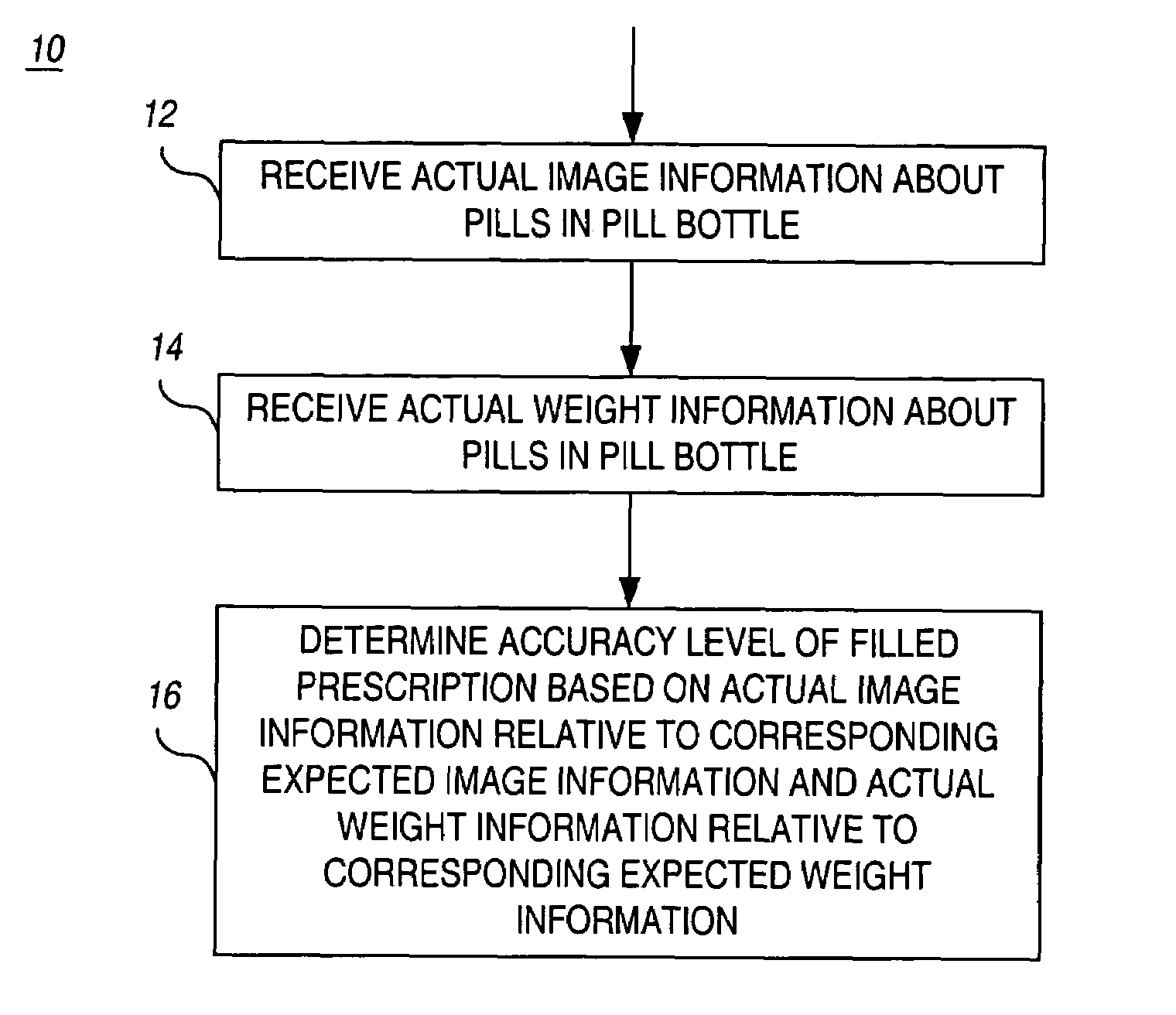
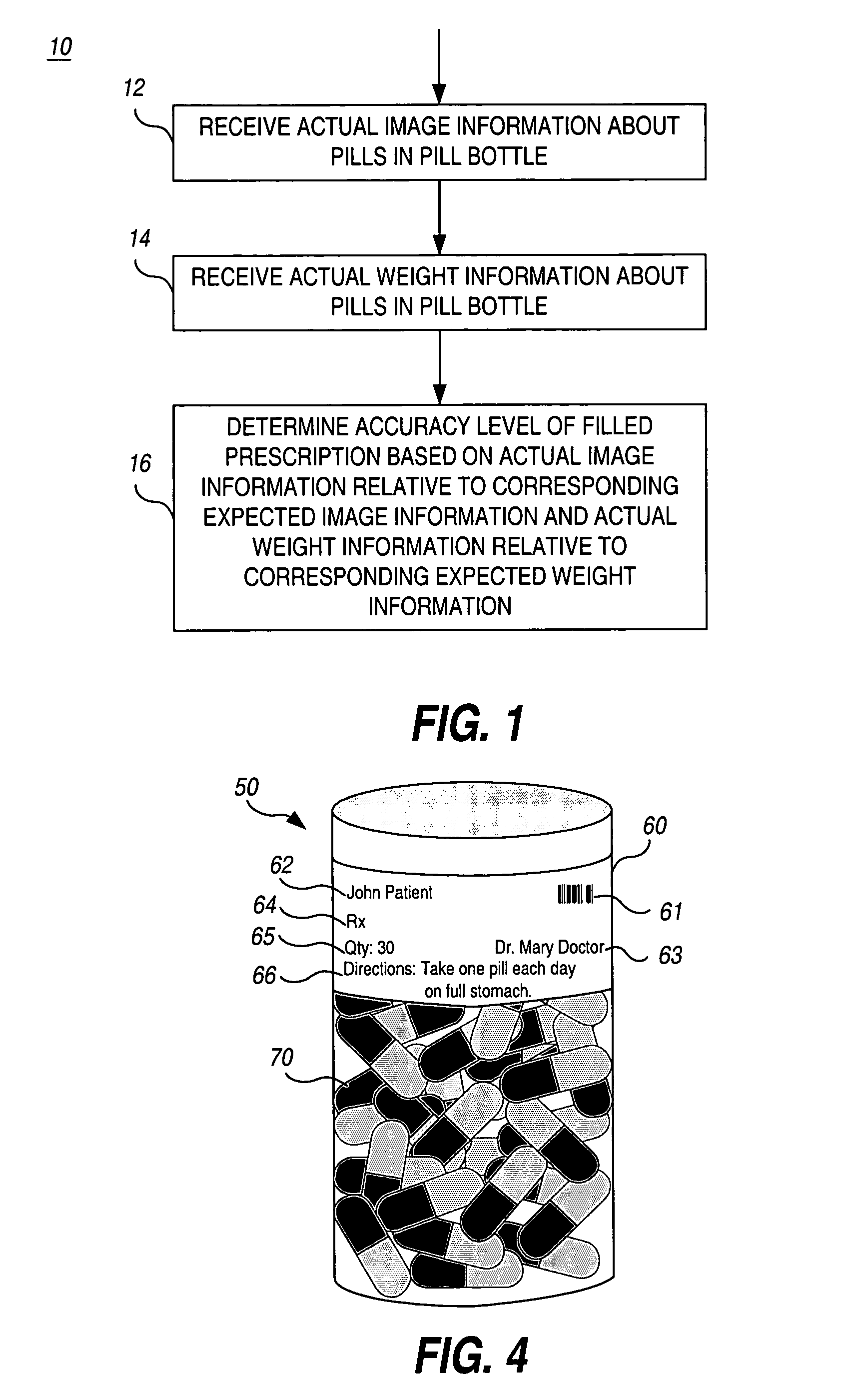
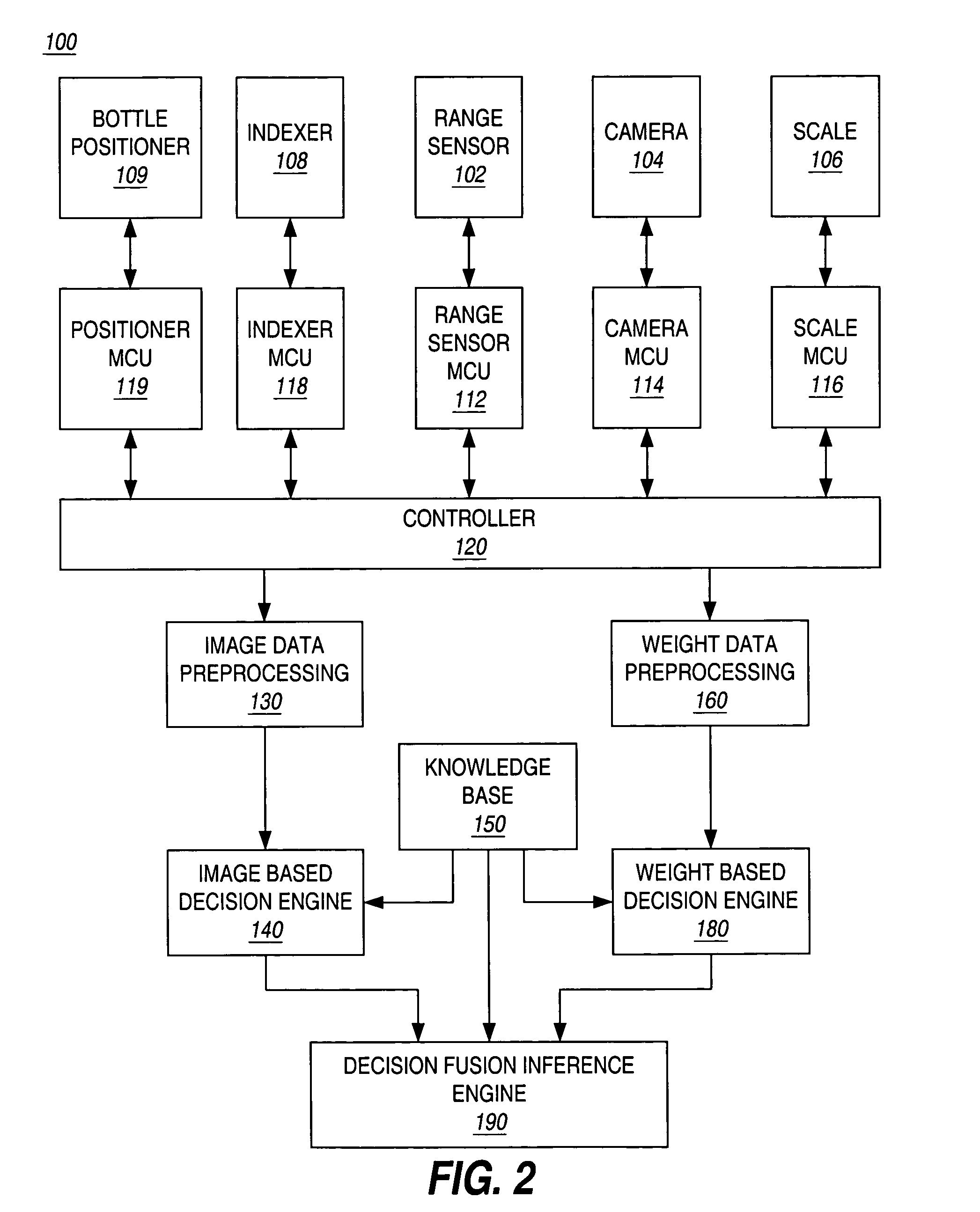
Apparatus and method for automatic prescription verification
InactiveUS7028723B1Eliminate unnecessary idle timeLiquid fillingDrug and medicationsThe InternetOrder form
An apparatus and method are used to perform the verification of a pharmaceutical prescription after it is filled but prior to shipment. Due to the high volume of mail / internet orders, the analysis is to be performed on the whole prescription, not on each individual pill of the prescription. The apparatus automatically gathers information about the pills contained in a pill bottle, including image and weight information, and incorporates a decision-making engine to decide whether the content of the pill bottle matches the prescription order.
Owner:ALOUANI ALI TAHOR +1
Reconstitution device and method of use
A reconstitution device is disclosed and includes a first container receiver having a first component cannula disposed therein, the first component cannula having a withdrawal port and a first transfer port formed thereon, a second container receiver having a second component cannula disposed thereon, the second component cannula having a vent port and a second transfer port formed thereon, a device body coupling the first container receiver to the second container receiver and having a transfer lumen formed therein, the transfer lumen in fluid communication with the first and second transfer ports, a selectively sealing interface secured to the device body and in fluid communication with the withdrawal port, and a venting member in communication with the vent port through a vent lumen.
Owner:TAKEDA PHARMA CO LTD
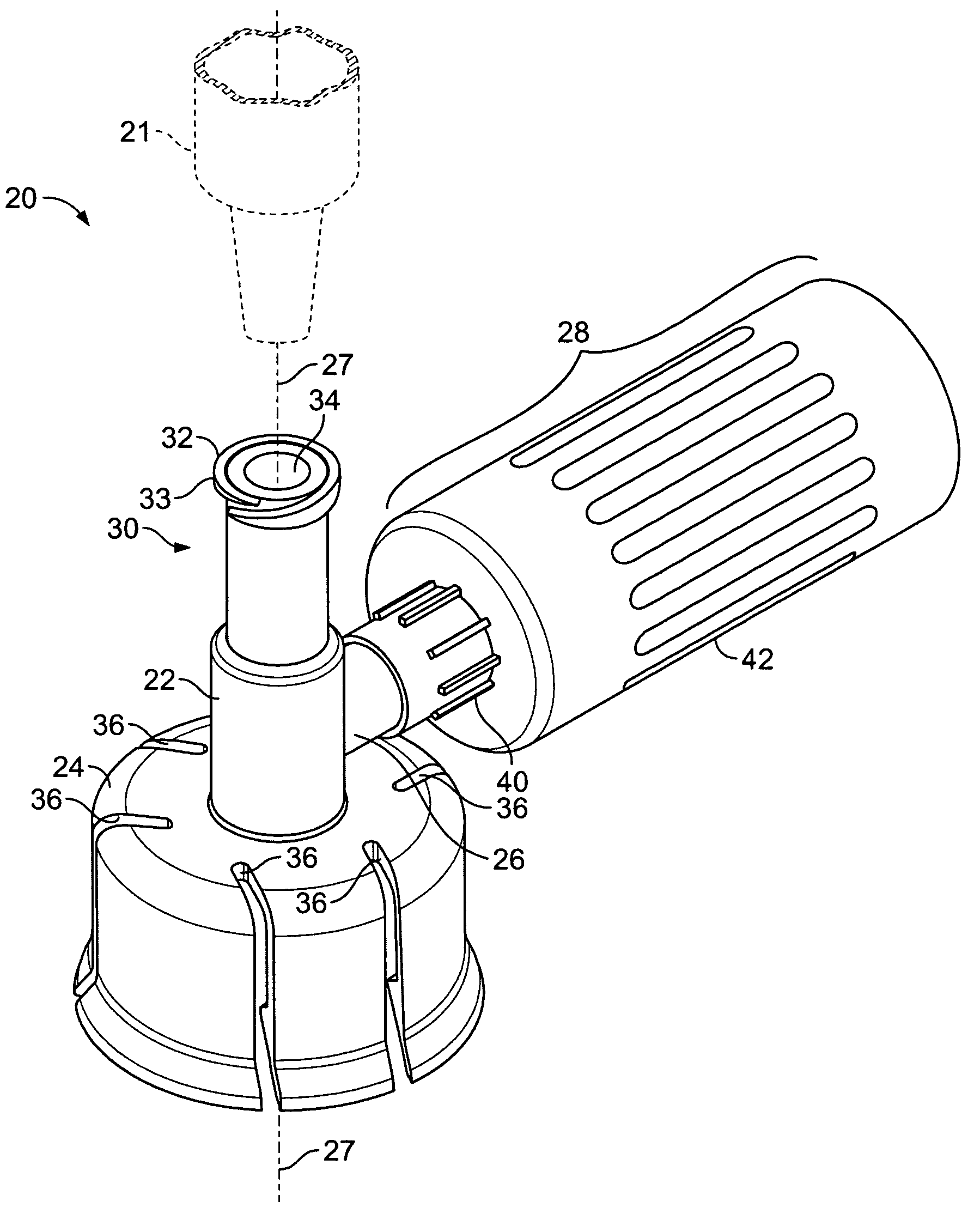
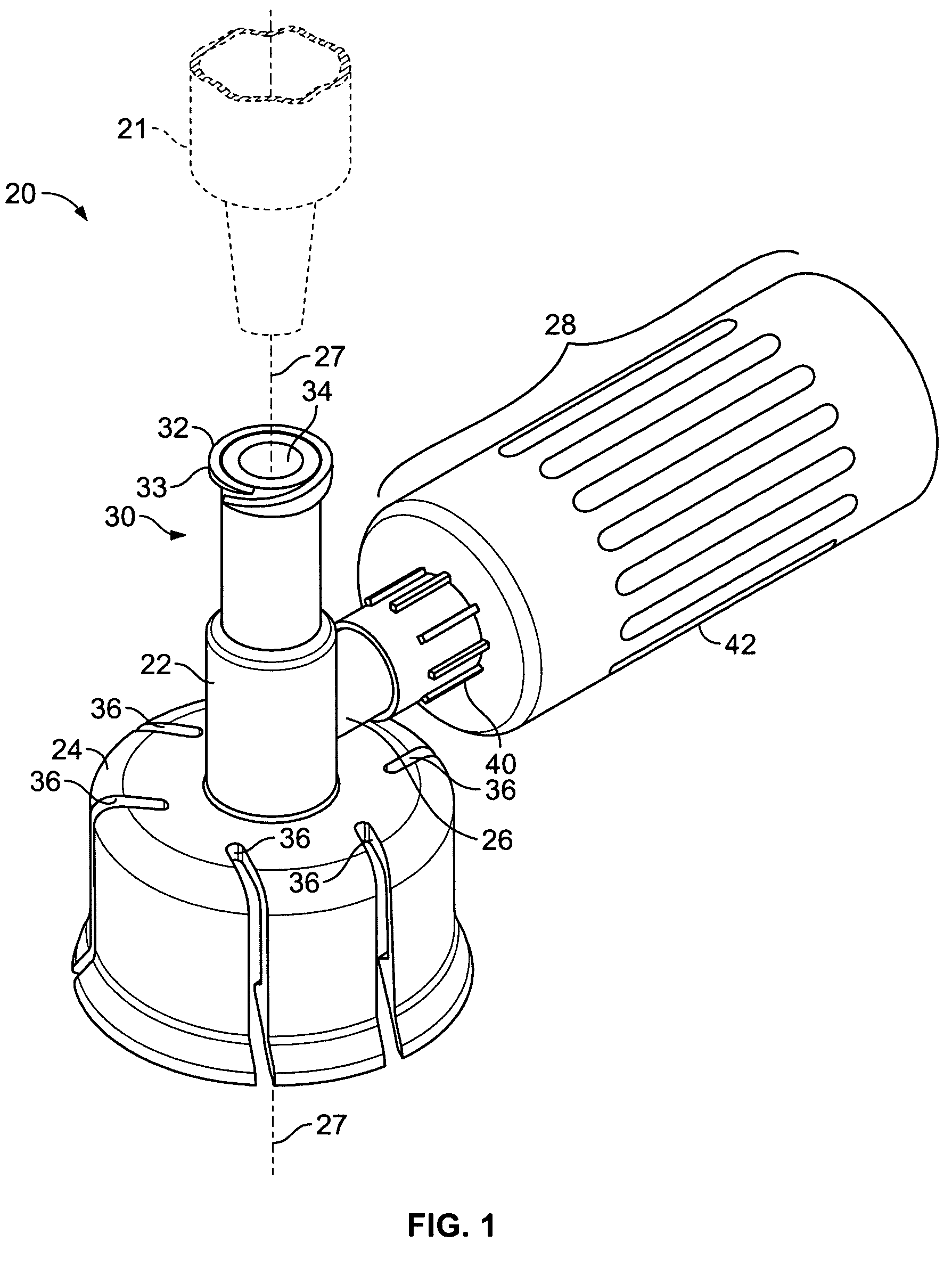
Piercing member protection device
ActiveUS8287513B2Avoid connectionPrevent disengagementCapsLiquid fillingEngineeringElectrical and Electronics engineering
Owner:CARMEL PHARMA
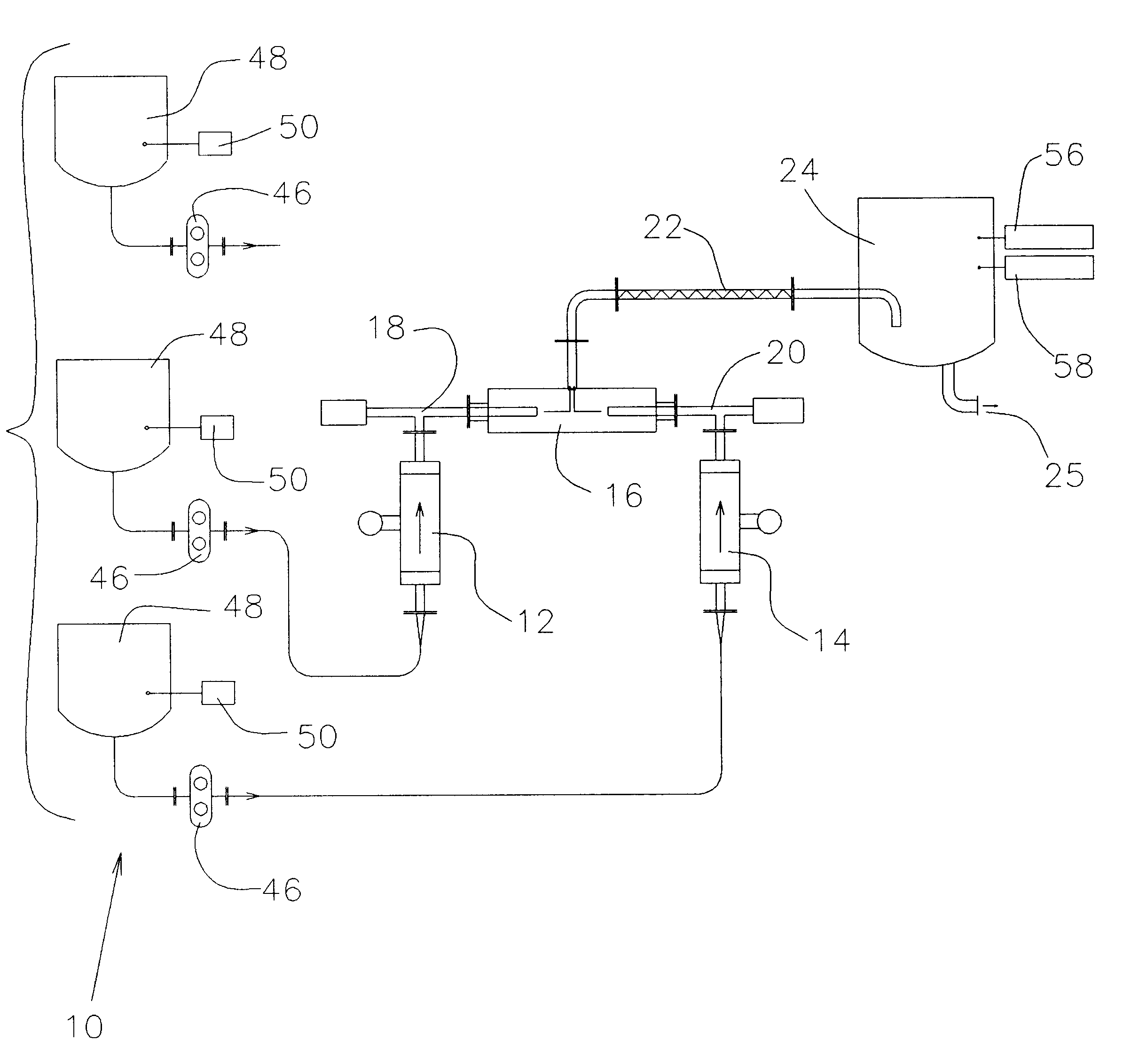
Continuous liquid stream digital blending system
InactiveUS6186193B1More disadvantageSimplifies software and set-up computationLiquid fillingControlling ratio of multiple fluid flowsHybrid systemDigital clock
The present invention consists of a method and apparatus providing for the continuous stream blending, preferably on a mass ratio basis, of two or more liquids. Each individual liquid stream is synchronously dosed in precise mass ratio to a common mixing point. The flow of each stream is on-off or digital. Repeated mass ratio doses of defined and matching flow interval, referred to as synchronous digital flow, interspersed with a defined interval of no flow, constitutes digital flow at a net rate sufficient to meet or exceed some required take-away of the blended liquids. In one preferred embodiment, each dose stream flow is produced and measured by a four element apparatus preferably consisting of a servo motor and controller, a precision positive displacement pump, a Coriolis mass meter and a precision flow stream shut-off device. The servo motor and controller establish and control a periodic and intermittent flow rate necessary to displace a defined mass dose in a precisely defined flow interval. The flow interval is measured against a precision millisecond digital clock. The Coriolis mass meter is used only to totalize mass flow to define the desired mass dose during the defined digital flow interval. The flow stream shut-off device ensures precise delivery of the mass dose to the common mixing point. The flow rate of a stream is automatically adjusted by the control electronics until the required mass dose is delivered in the defined flow interval.
Owner:ODEN MACHINERY
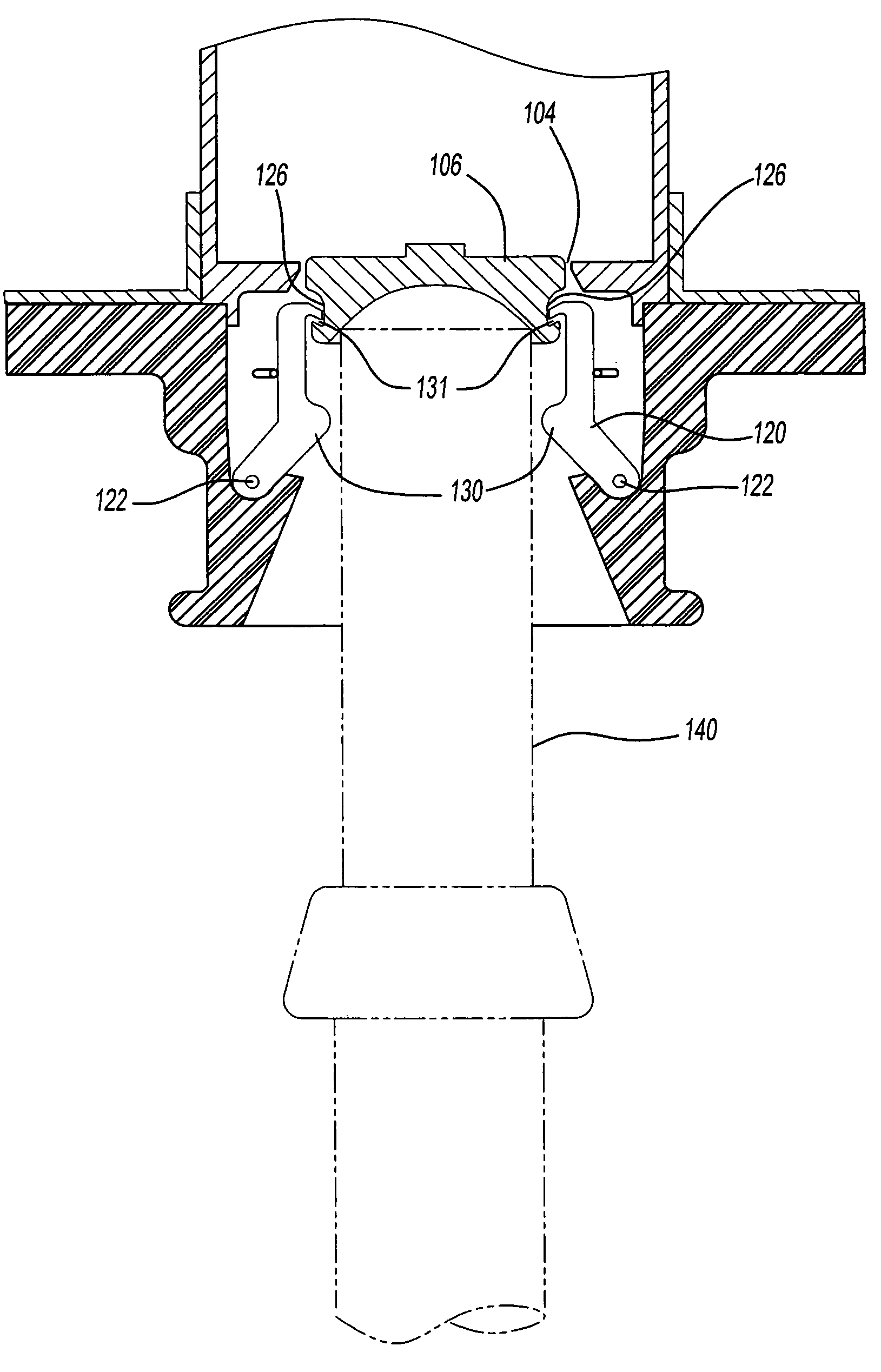
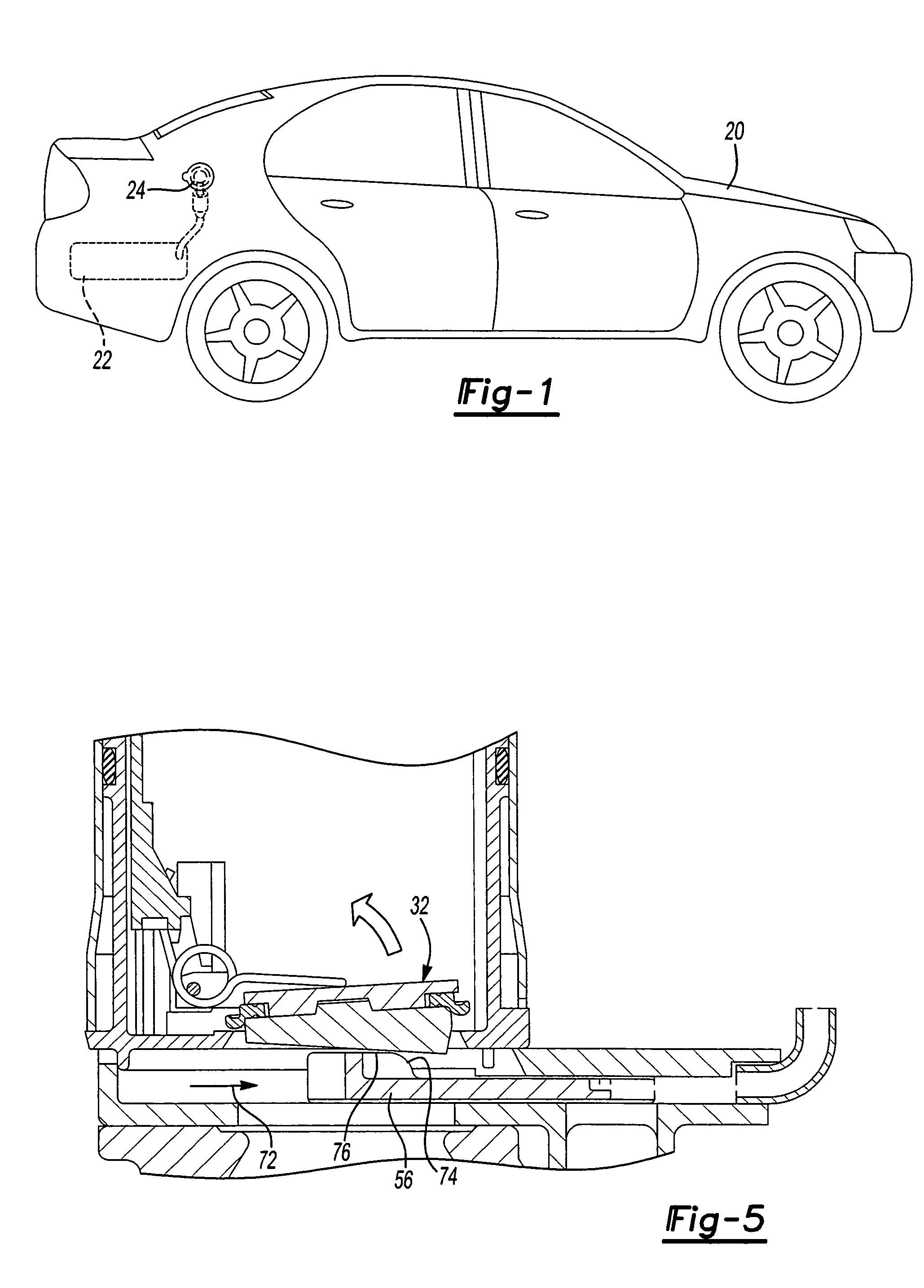
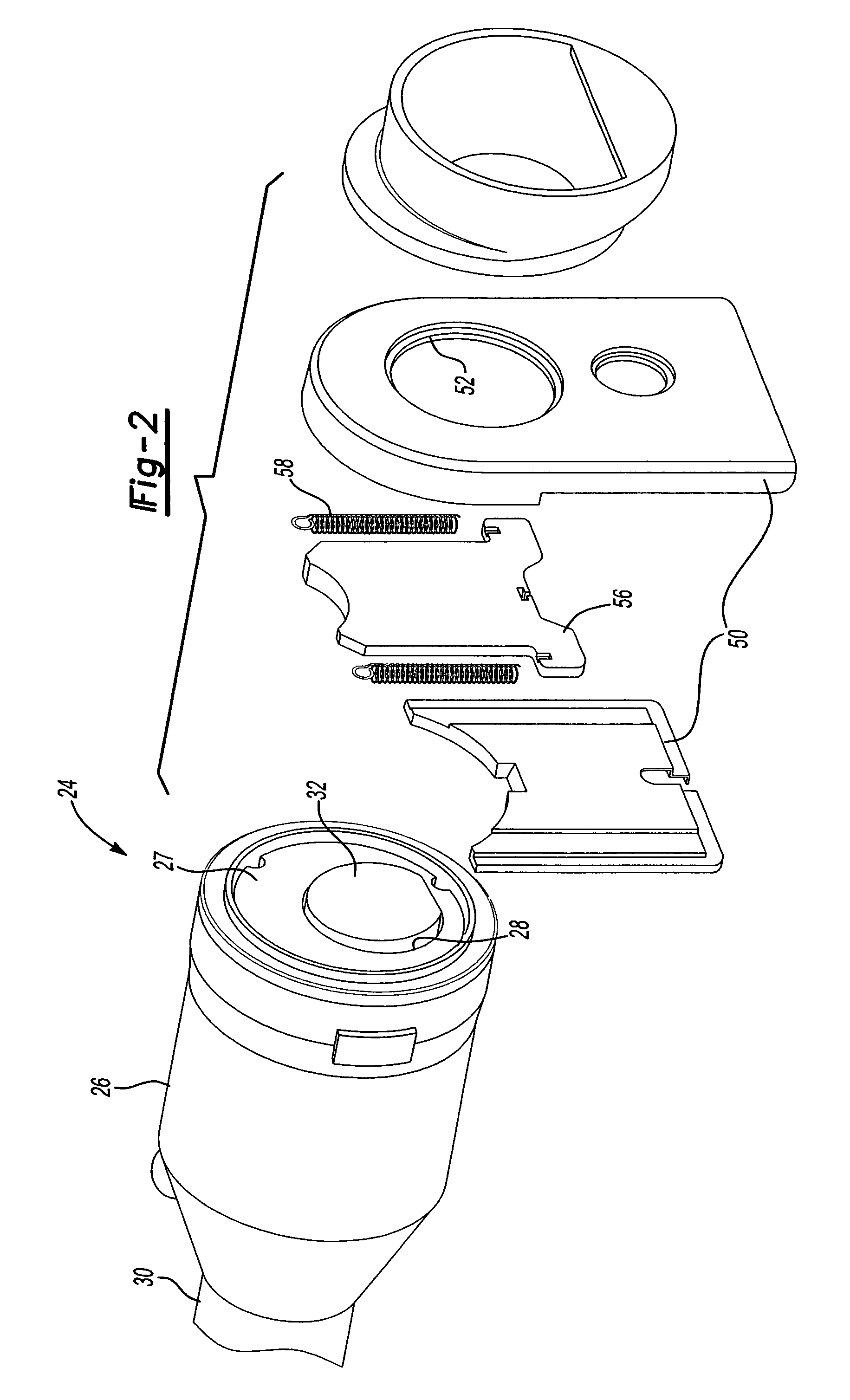
Capless automotive fueling system
A capless automotive fueling system having a funnel with a port open to receive a fuel filling nozzle. A spring loaded valve is movable between an open position in which the port is open and a closed position in which the fuel valve overlies and sealingly covers the port. A shield is movable between a first position in which the shield overlies and covers the valve, and a second position in which the shield is laterally disposed to one side of the valve to permit insertion of the fuel filling nozzle. A motor is drivingly connected to the shield and, on activation, moves the shield from its first and to its second position. Alternatively, two latches engage the valve to retain the valve in a closed position. Upon insertion of a properly sized fuel nozzle, the nozzle engages the latches and pivots them to an open position.
Owner:MARTINREA IND INC
Method and apparatus for refueling multiple vehicles
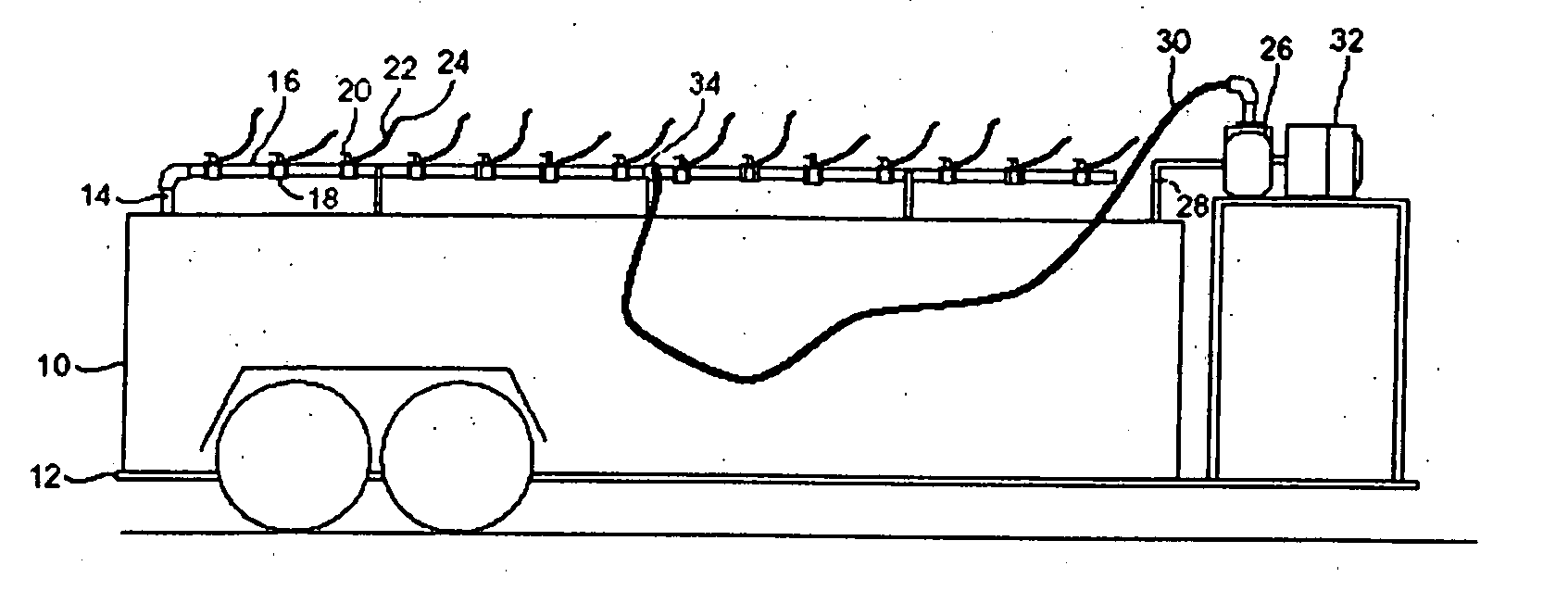
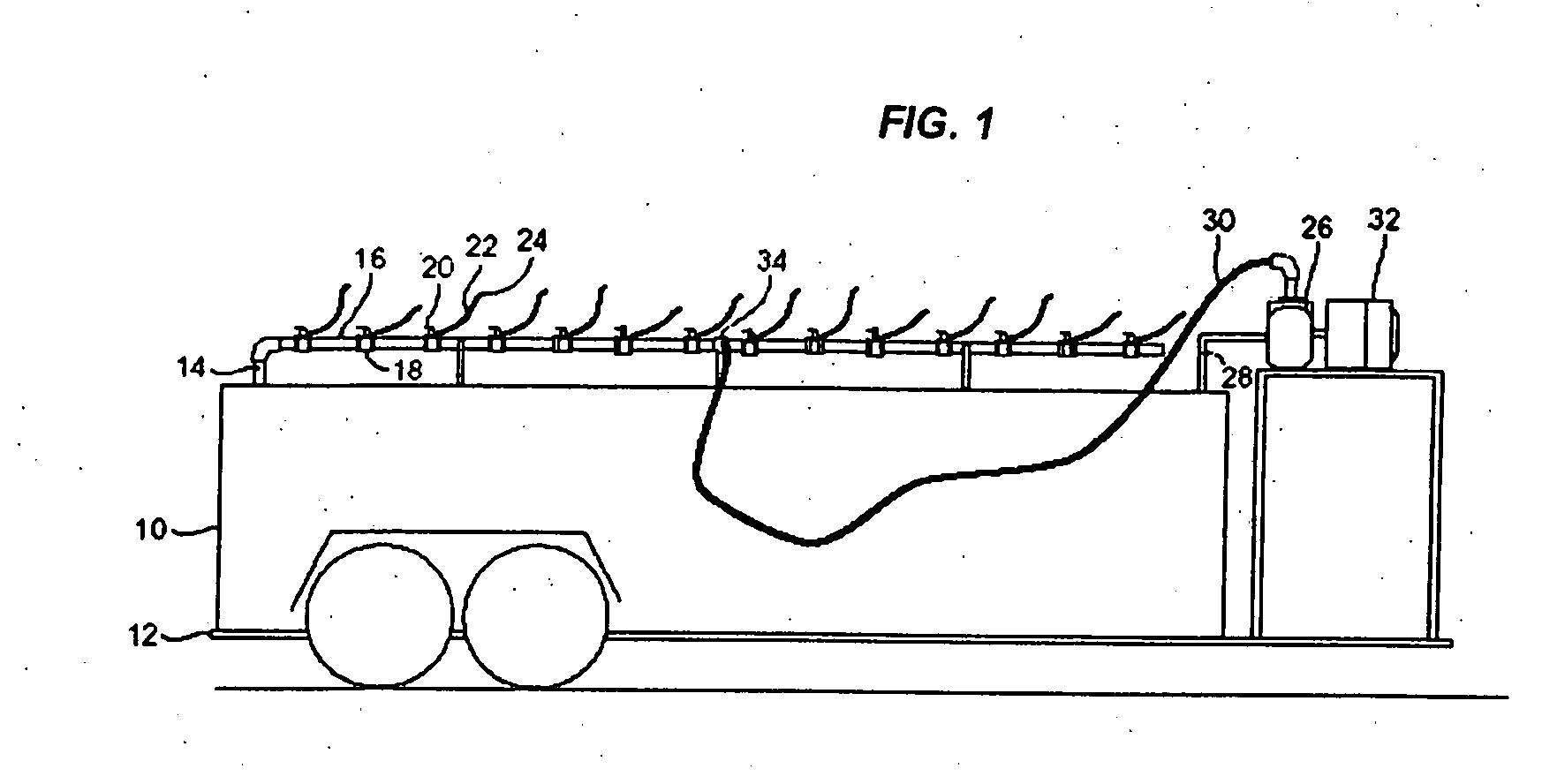
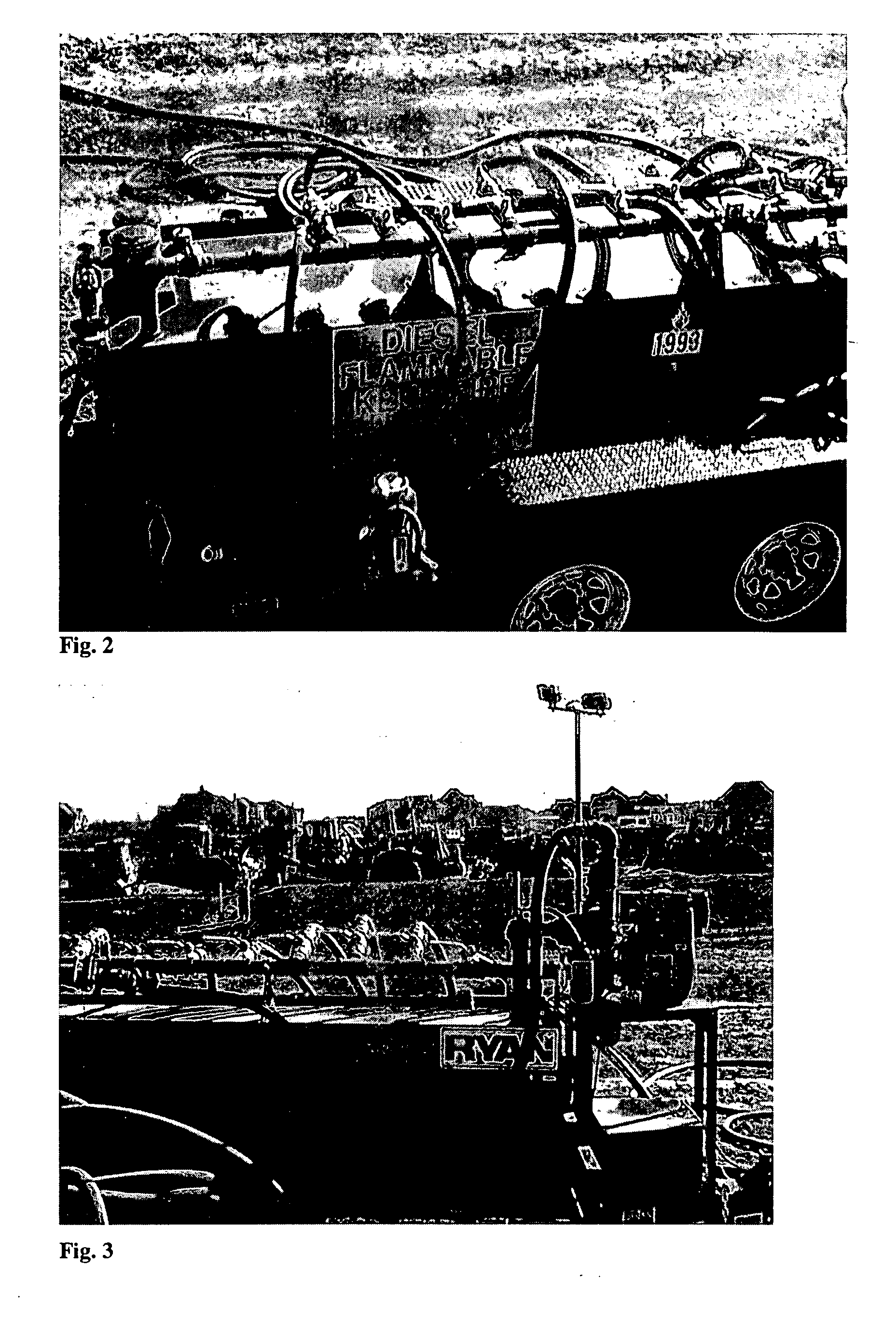
InactiveUS20070181212A1Easy accessReduces time and expenseSolid materialItem transportation vehiclesFuel tankTruck
A refueling dock for refueling multiple vehicles at one time is disclosed. The dock has a manifold that has an inlet that can be connected to a fuel tanker truck and a plurality of outlet openings. Each outlet opening has a valve that controls the flow of fuel through the hose. A nozzle is fitted to the hose and is located at the end of the hose distant from the outlet opening. Two or more hoses can be simultaneously connected to different equipment in need of refueling. A valved fuel tank inlet line enables fuel to flow from the manifold to a fuel tank on the dock. When needed, fuel from the tank can then be pumped to the manifold through a fuel tank outlet line,.which can be connected to the same inlet on the manifold that is used to receive fuel from the tanker trick.
Owner:RYAN CENT
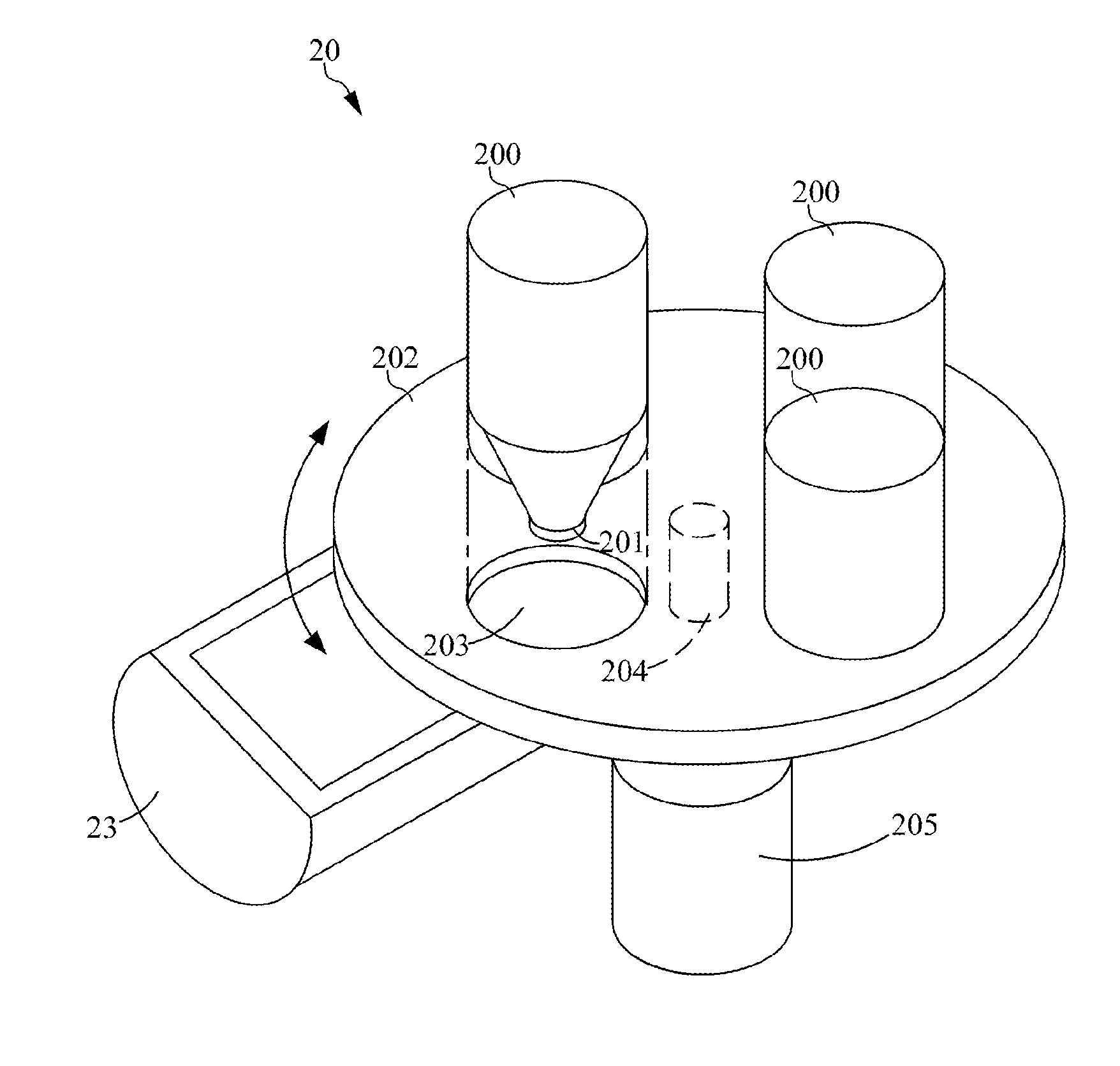
Device and method for powder distribution and additive manufacturing method using the same
ActiveUS20130186514A1Improve compactnessWell mixedLiquid surface applicatorsManufacturing driving meansMetallurgyThermal deformation
The present disclosure provides a device and method for powder distribution and an additive manufacturing method, wherein different size or kind of powders could be chosen to be accommodated within a receptacle. The receptacle can uniformly mix the powder by a rotation movement, pour out the powders by the rotation movement and distribute the powders for forming a layer by a translation movement. In another embodiment, the receptacle further comprises a heating element for preheating the powders. Not only can the present disclosure uniformly mix the powders so as to reduce the thermal deformation and distribute the powder layer compactly, but also can the present disclosure distribute different kinds of powder in different layer so as to increase the diversity in additive manufacturing.
Owner:IND TECH RES INST
Fluid dispenser
InactiveUS6192945B1Efficient and reliableEasy to manufacturePreparing sample for investigationLiquid flow controllersModularityReactive system
A method and apparatus for an automated biological reaction system is provided. In the processing of a biological reaction system, there is a need for consistently placing an amount of fluid on a slide. In order to accomplish this, several methods are used including a consistency pulse and a volume adjust means. Moreover, in order to reliably operate an automated biological reaction system, the dispenser must be reliable, easy to assemble and accurate. Among other things, in order to accomplish this, the dispense chamber is substantially in line with the reservoir chamber, the reservoir chamber piston is removed, and the flow of fluid through the dispenser is simplified. Further, in order to operate the automated biological reaction system more reliably, the system is designed in modular pieces with higher functions performed by a host device and the execution of the staining operations performed by remote devices. Also, to reliably catalog data which is used by the automated biological reaction system, data is loaded to a memory device, which in turn is used by the operator to update the operator's databases. The generation of the sequence of steps for the automated biological reaction device based on data loaded by the operator, including checks to determine the ability to complete the run, is provided.
Owner:VENTANA MEDICAL SYST INC
Pipetting station apparatus
InactiveUS6325114B1Withdrawing sample devicesMaterial analysis by optical meansPipetteMicrowell Plate
A pipette station is described for use in the field of sample analysis. The pipette station increases the rate and ease with which a liquid may be manipulated into and out of sample carriers such as microwell plates. The pipette station includes shafts in the X, Y, and Z direction which possess ball screws which are integrated with motor shafts thus improving accuracy and eliminating the need for a coupling apparatus thereby reducing the space required for the pipette station. The pipette station may be interfaced with an automated laboratory system.
Owner:VELOCITY11 +1
Multiple plasma generator hazardous waste processing system
A waste processing system is provided herein which entails the use of at least one fixed-position plasma arc generator for primary processing and at least one moveable plasma arc generator for secondary processing assistance and / or final conditioning of the slag prior to exit from the reactor vessel. This optimum processing environment is provided by control of reactor vessel configuration and real time control of processing characteristics to ensure maximum processing efficiency.
Owner:PLASCO ENERGY GROUP INC
RF device in drinkware to record data/initiate sequence of behavior
InactiveUS20050087255A1Easy and efficient to manufactureDurable and reliable constructionCredit registering devices actuationLiquid fillingEngineeringCommunication device
A drinkware is provided to provide information to a receiver. The drinkware is a RF device coupled to a handheld drinking container. The RF device can receive and send RF signals and communicate with a sensory device. A transmitter can send a signal to the communication device, which then activates sensory device. The RF device can communicate with the transmitter to control a fountain machine.
Owner:WHIRLEY IND
Automated prescription filling system/method with automated labeling and packaging system/method automated order consolidation system/method
Computer assisted systems, methods and mediums for filling one or more orders. One embodiment of the present invention is a system that includes an order consolidation station configured to receive at least one bottle containing pills individually counted and / or at least one package containing pharmaceutical products without having been designated for any of the orders when the package was created and / or at least one literature pack optionally including patient specific information. The order consolidation station is further configured to combine automatically the received bottle and / or package and / or literature pack into a container to be sent to a recipient including, for example, mail order pharmacies, wholesalers and / or central fill dealers for subsequent distribution or sale including retailer distribution or sale. The bottle is specifically designated for the order, and the order generally includes at least one prescription for the package.
Owner:EXPRESS SCRIPTS STRATEGIC DEV INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com