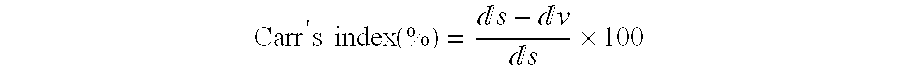Patents
Literature
321 results about "Dry-powder inhaler" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A dry-powder inhaler (DPI) is a device that delivers medication to the lungs in the form of a dry powder. DPIs are commonly used to treat respiratory diseases such as asthma, bronchitis, emphysema and COPD although DPIs (such as inhalable insulin Afrezza) have also been used in the treatment of diabetes mellitus.
Dry powder inhaler
ActiveUS20070235029A1Small accurate volumePrecise deliveryRespiratorsLiquid surface applicatorsMicroDoseAerosolize
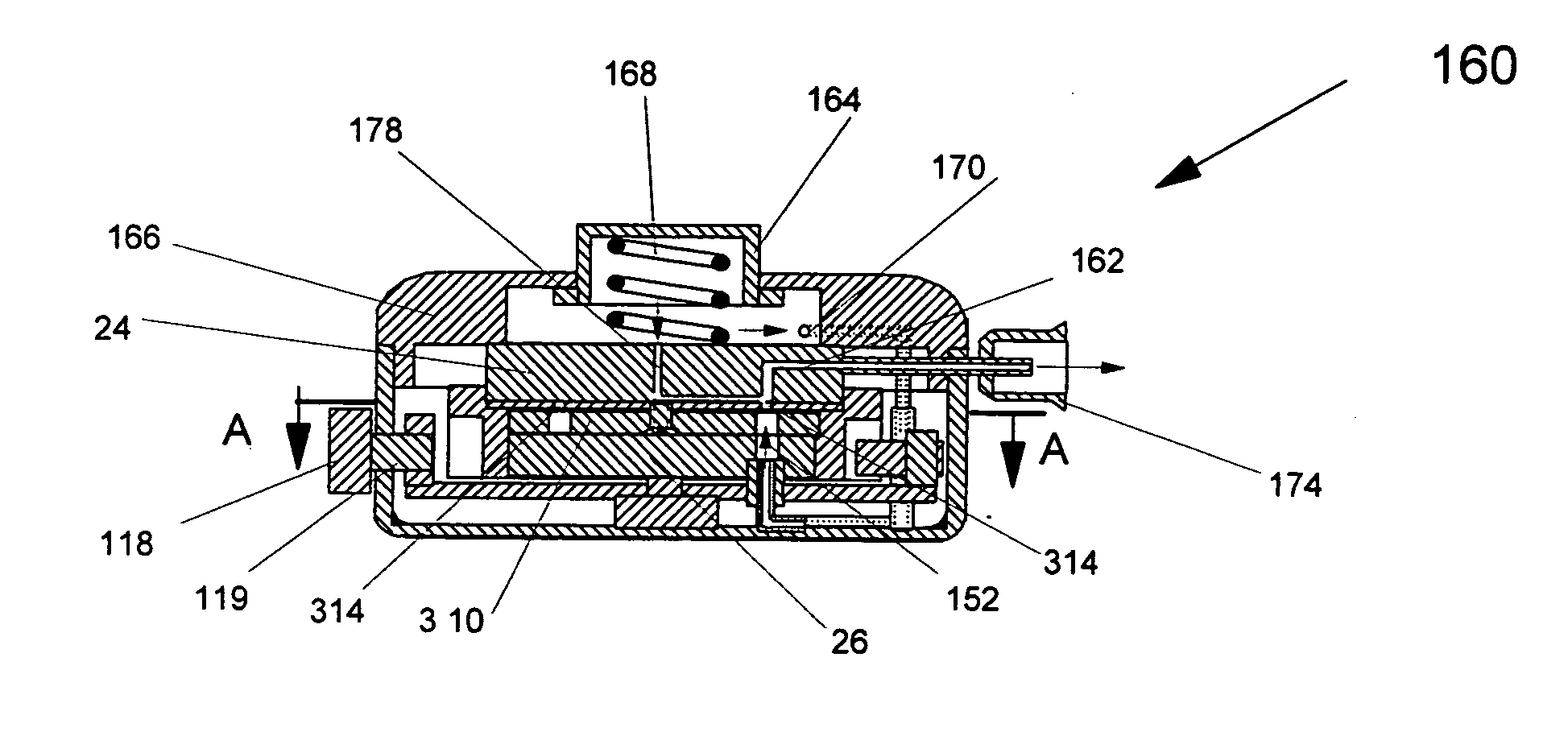
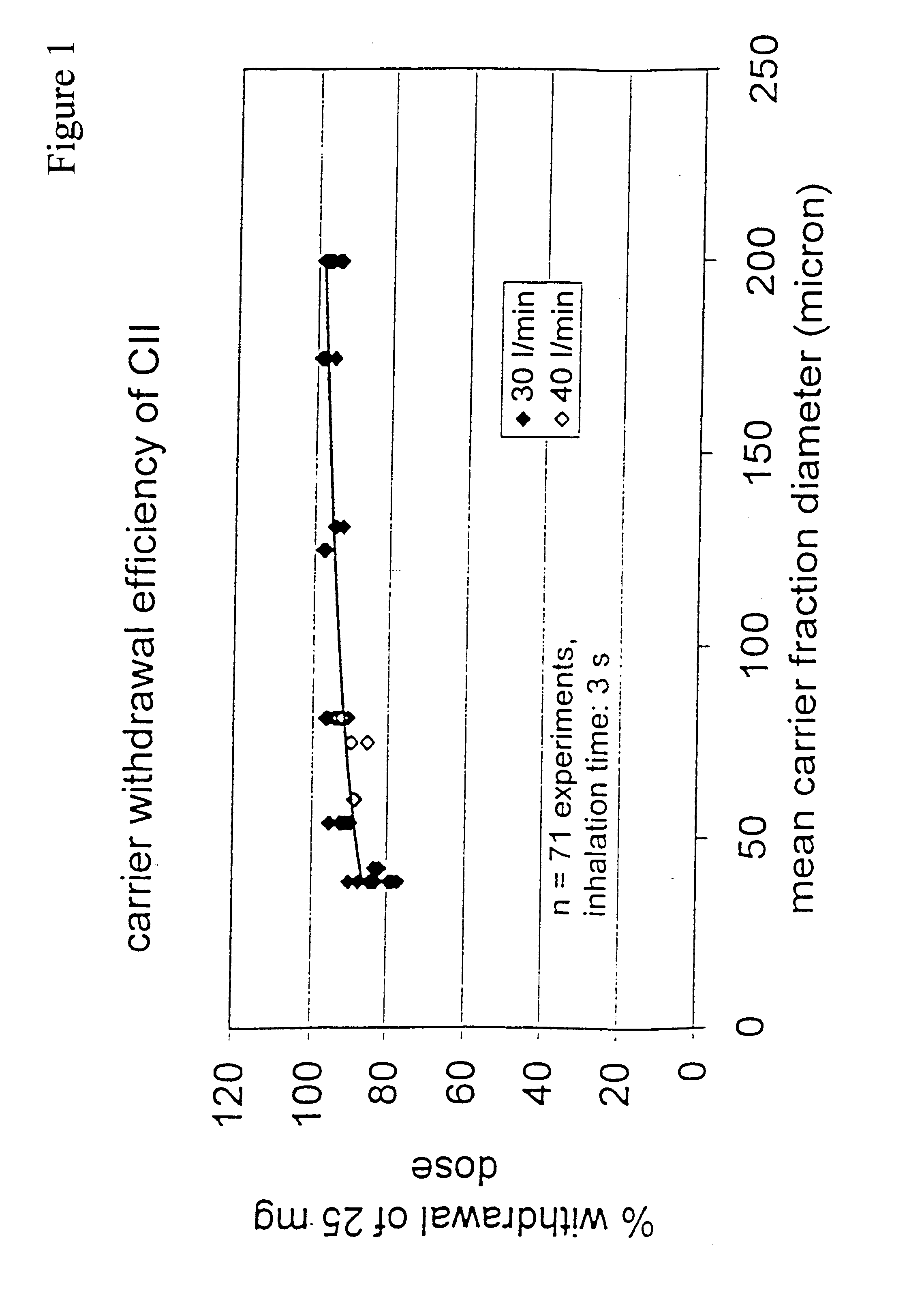
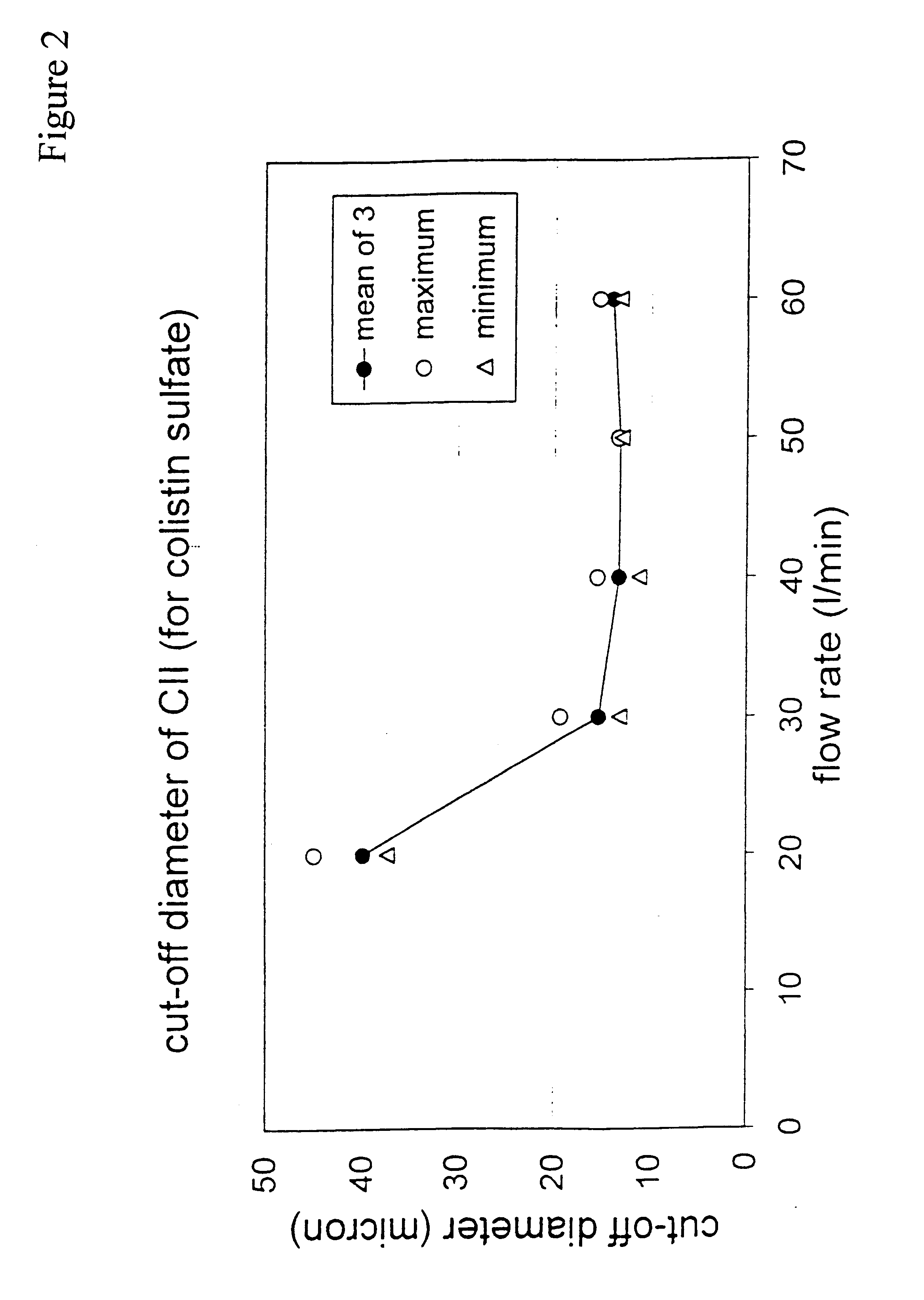
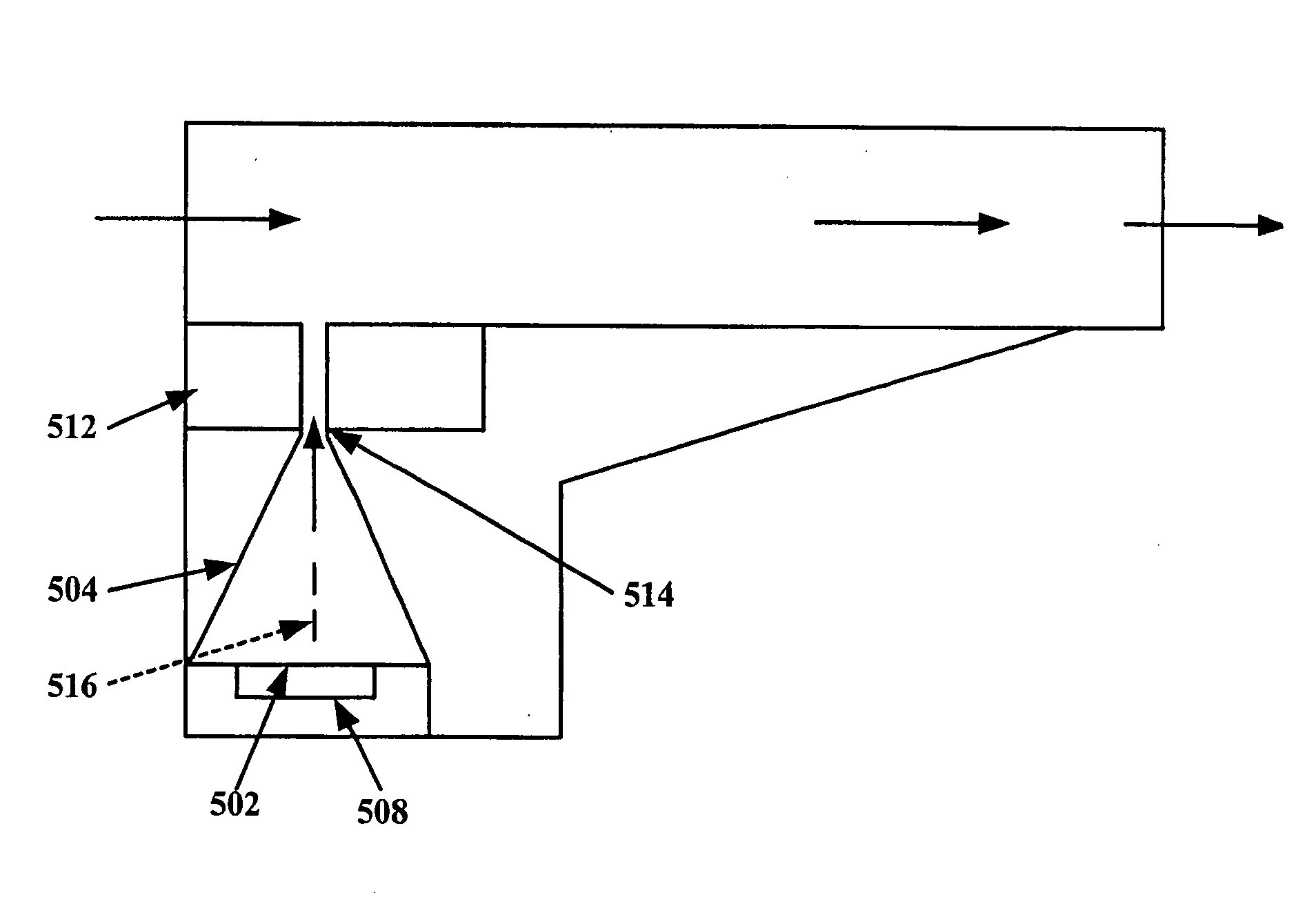
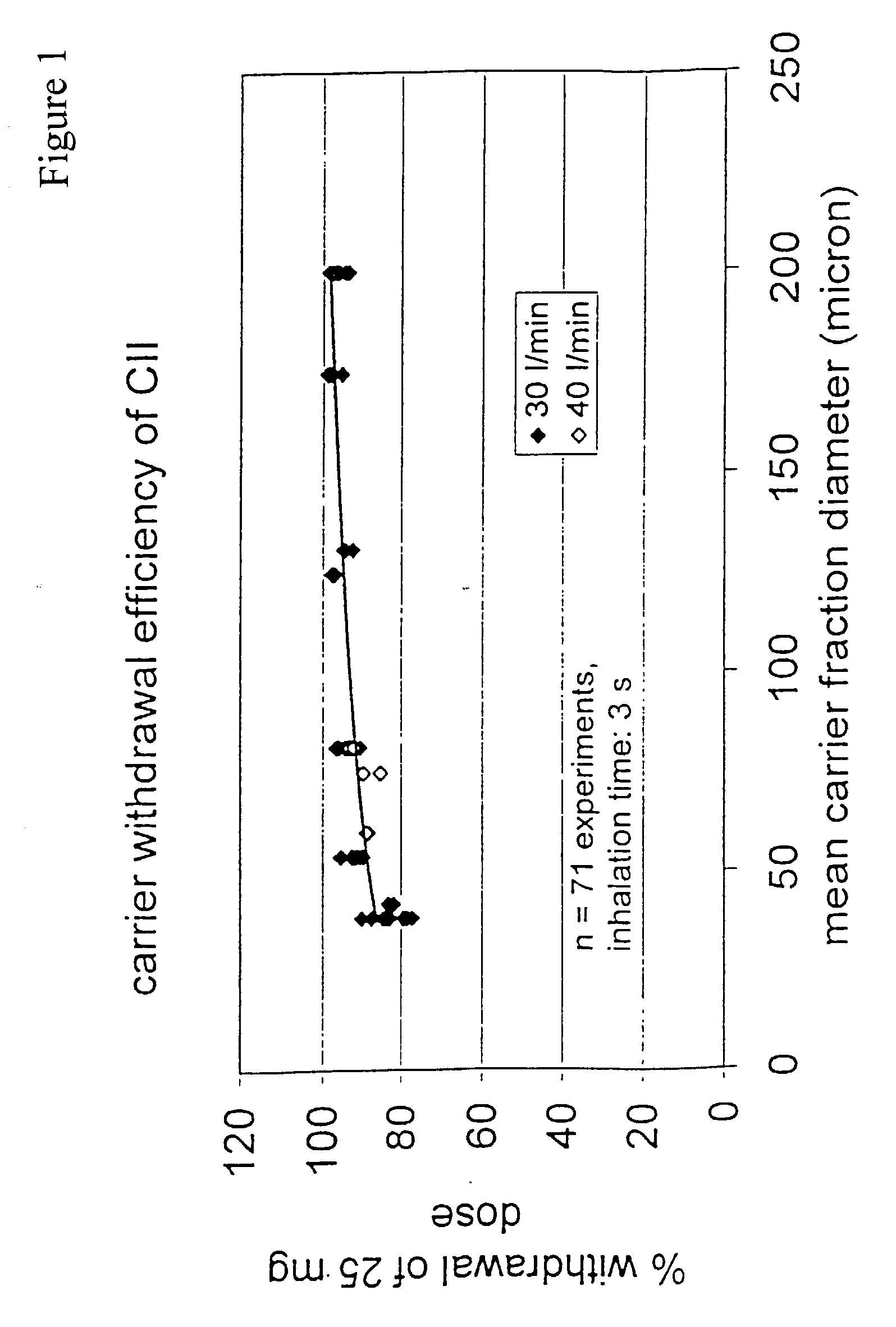
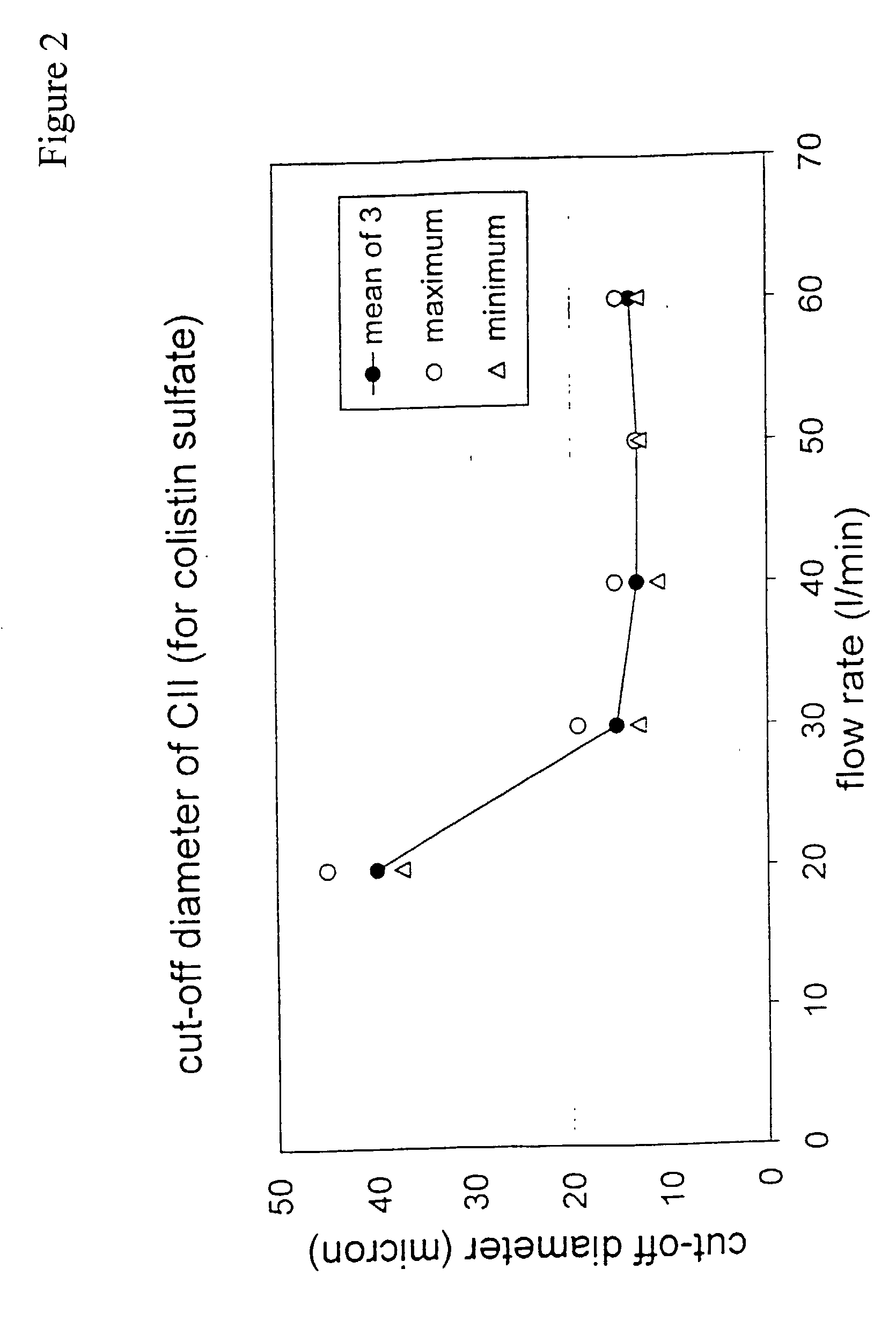
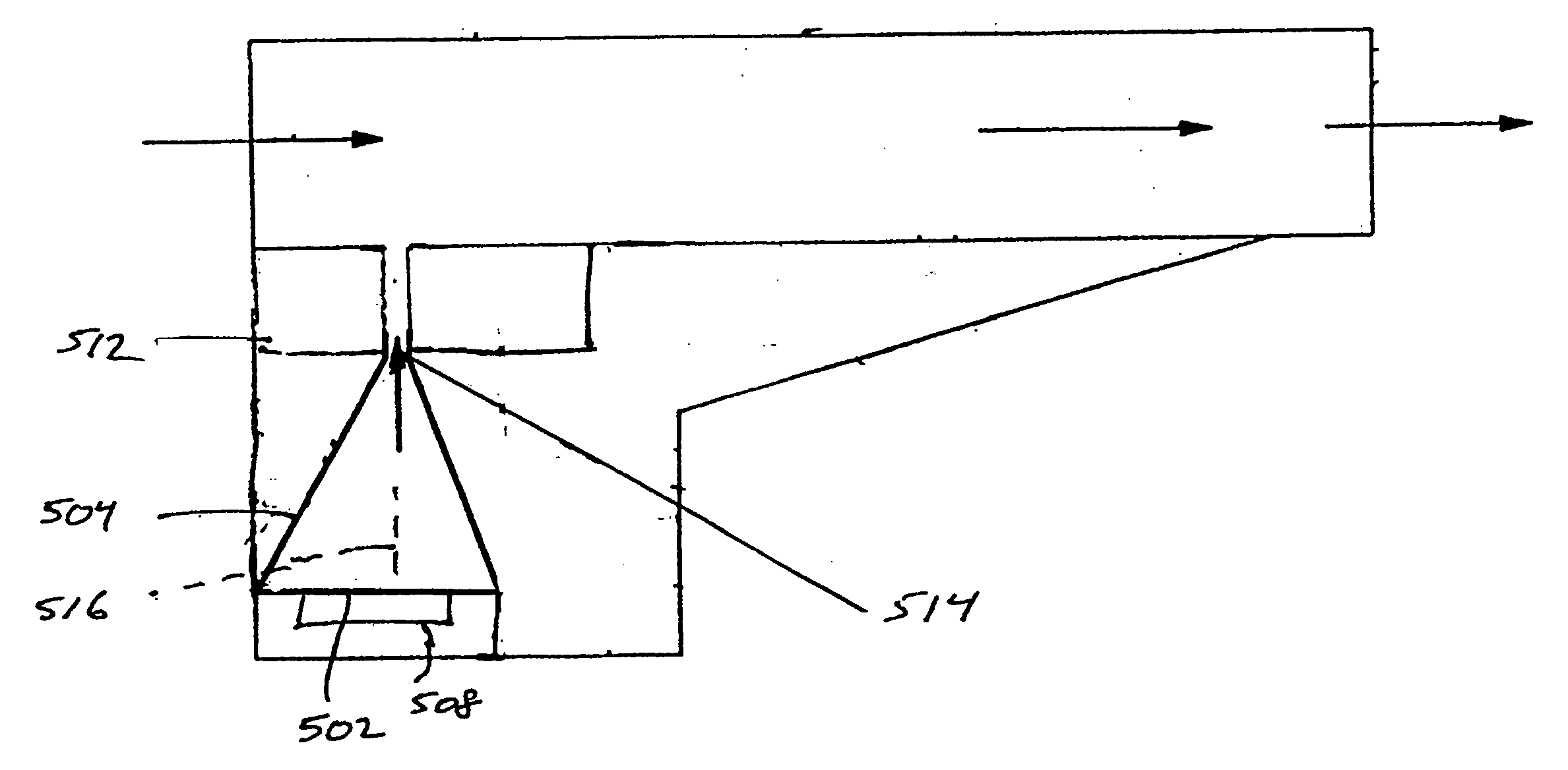
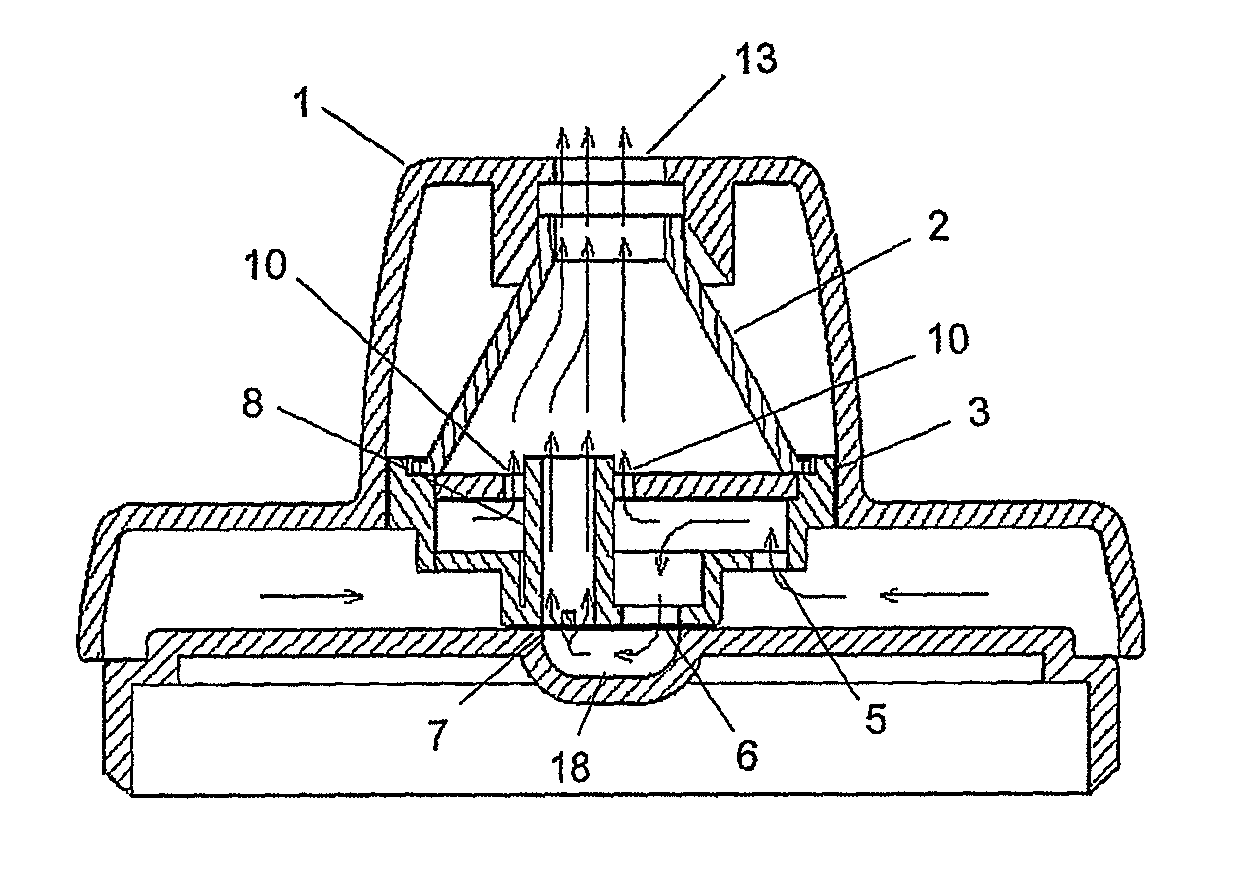
A new dry powder inhaler is developed as a pulmonary medicine delivery device for dispersing precise tiny dosages (10 μg-50 mg) of pure carrier-free ultra-fine powdered medicament (<5 μm aerodynamics particle size) into a patient's lung. The powder is drawn from the blister cell and dispersed through an outlet tube assisted by two air streams. The first air stream goes through a the blister cell from its upstream side, to significantly fluidize the medicament in the dose to flow upward. The second one extracts the fluidized powder from downstream of the blister cell for further deagglomeration and dispersion of the medicament powder by shear force. The rotating multi-dose blister can hold up to 60 doses, which are pre-metered with pure ultra-fine powdered medicament. So that it has higher drug loading capability in small volumes, compared to most current dry powder inhalers, which usually use some excipient. The inhaler efficiently disperse the aerosolized medicament in the air stream to the deep interior of patient's lung. The fine particle fraction (<4.7 μm) is reported to reach as high as 80% using this inhaler.
Owner:NINGBO INHAL PHARMA CO LTD
Powder formulation disintegrating system and method for dry powder inhalers
A disperser for dry powders which can be used with different dose systems, dose weights ranging from 2 to 25 mg and different types of powder formulation. In one embodiment, the disperser acts both as a de-agglomeration (disintegration; aerosolization) means and as an air classifier for especially adhesive mixtures. Only fine drug particles are emitted whereas the larger agglomerates and carrier crystals are retained by the disperser. Another embodiment enables time controlled release of carrier crystals in these mixtures. Yet another embodiment has optimized performance with spherical pellets, containing no carrier crystals. Other possible embodiments of the invention make it possible to control the total inhaler resistance and the powder deposition in the upper respiratory tract by means of the addition of a so-called sheath flow of clean air. Modifications also enable carrier retainment in the mouthpiece and elimination of the tangential flow component of the discharge cloud.
Owner:ASTRAZENECA AB
Unit dose cartridge and dry powder inhaler
InactiveUS7464706B2Broad inhalation tidal volume range of human breathMaintain good propertiesRespiratorsLiquid surface applicatorsMedicineCheck valve
A dry powder inhaler having improved aerodynamic properties for diluting, dispersing, and metering drug particles for increasing the efficiency of pulmonary drug delivery to a patient is described. The inhaler comprises, in general, a housing having an air intake, an air flow-control / check-valve, a mixing section and a mouthpiece. A cartridge loaded with a single dose of medicament can be installed in the mixing section.
Owner:MANNKIND CORP
Engineered particles and methods of use
InactiveUS7306787B2Reduce deliveryLess attractivePowder deliveryOrganic active ingredientsNebulizerActive agent
Engineered particles are provided may be used for the delivery of a bioactive agent to the respiratory tract of a patient. The particles may be used in the form of dry powders or in the form of stabilized dispersions comprising a nonaqueous continuous phase. In particularly preferred embodiments the particles may be used in conjunction with an inhalation device such as a dry powder inhaler, metered dose inhaler or a nebulizer.
Owner:NOVARTIS AG
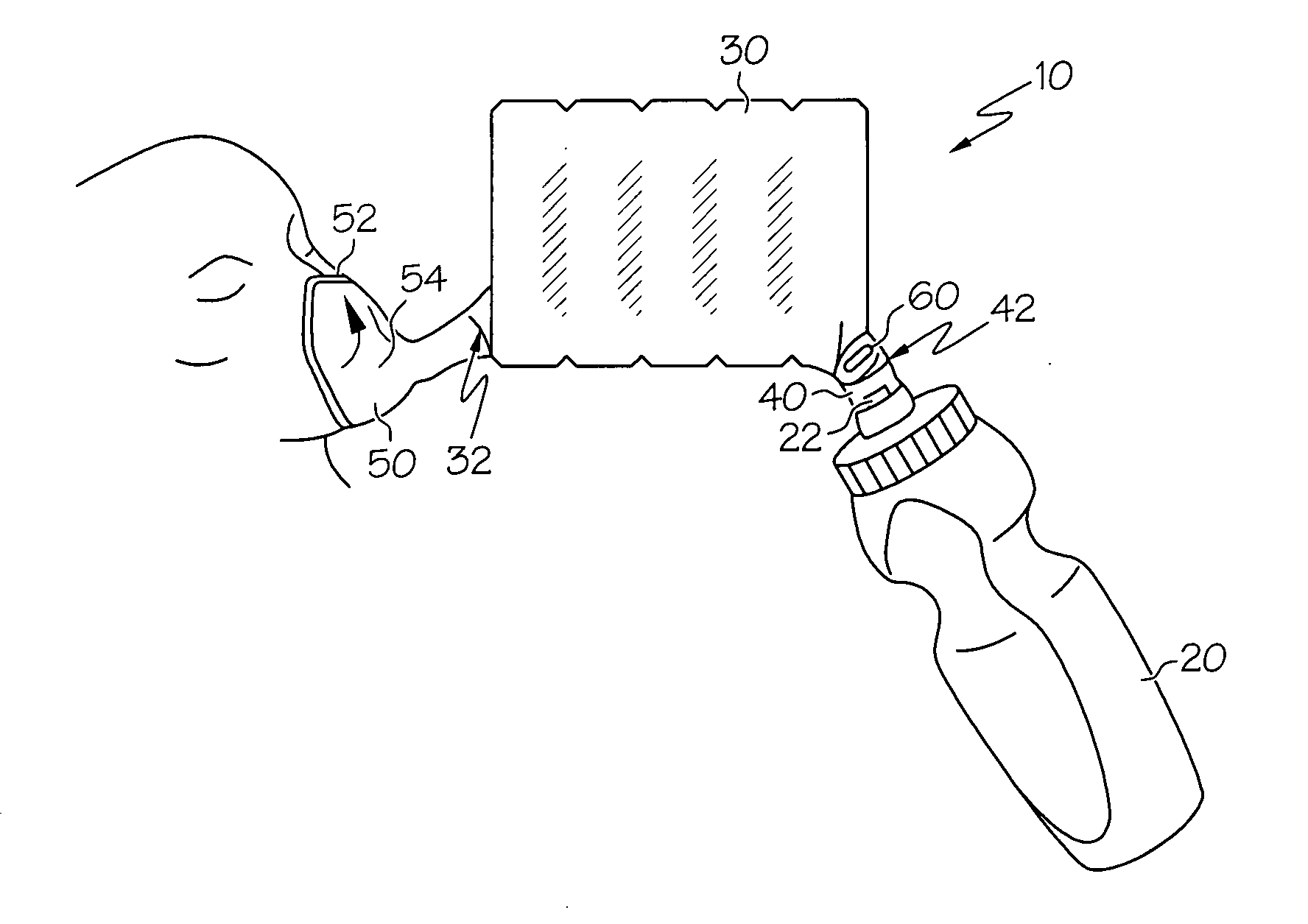
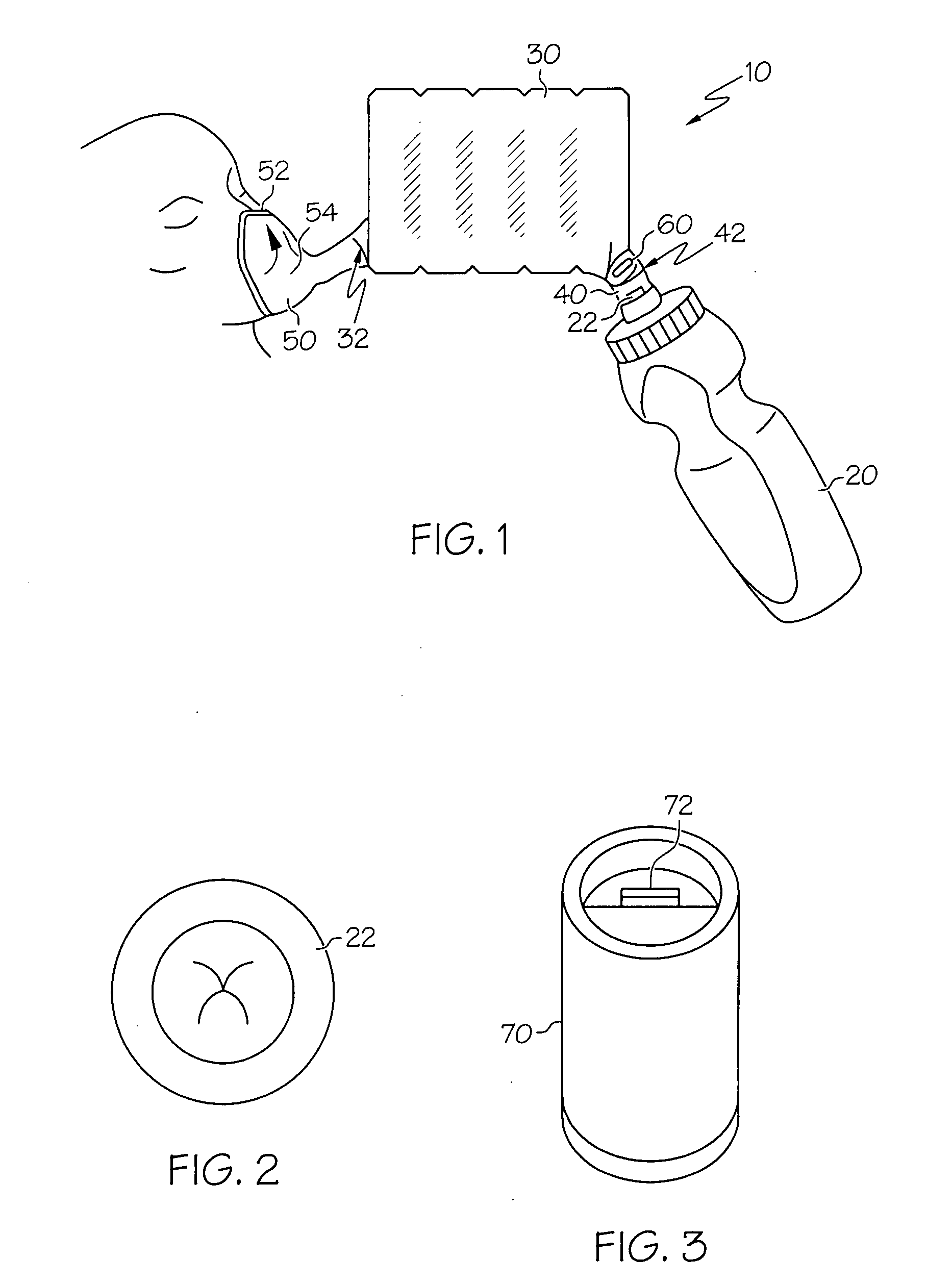
Synthetic jet based medicament delivery method and apparatus
A dry powder inhaler consisting of first chamber having an orifice for holding a dry powder and a gas, and a second chamber for receiving a deaggregated form of the dry powder and for communicating the deaggregated dry powder to a user. A synthetic jet drives the dry powder from the first chamber to the second chamber.
Owner:MICRODOSE THERAPEUTX INC
Powder formulation disintegrating system and method for dry powder inhalers
A disperser for dry powders which can be used with different dose systems, dose weights ranging from 2 to 25 mg and different types of powder formulation. In one embodiment, the disperser acts both as a de-agglomeration (disintegration; aerosolization) means and as an air classifier for especially adhesive mixtures. Only fine drug particles are emitted whereas the larger agglomerates and carrier crystals are retained by the disperser. Another embodiment enables time controlled release of carrier crystals in these mixtures. Yet another embodiment has optimized performance with spherical pellets, containing no carrier crystals. Other possible embodiments of the invention make it possible to control the total inhaler resistance and the powder deposition in the upper respiratory tract by means of the addition of a so-called sheath flow of clean air. Modifications also enable carrier retainment in the mouthpiece and elimination of the tangential flow component of the discharge cloud.
Owner:ASTRAZENECA AB
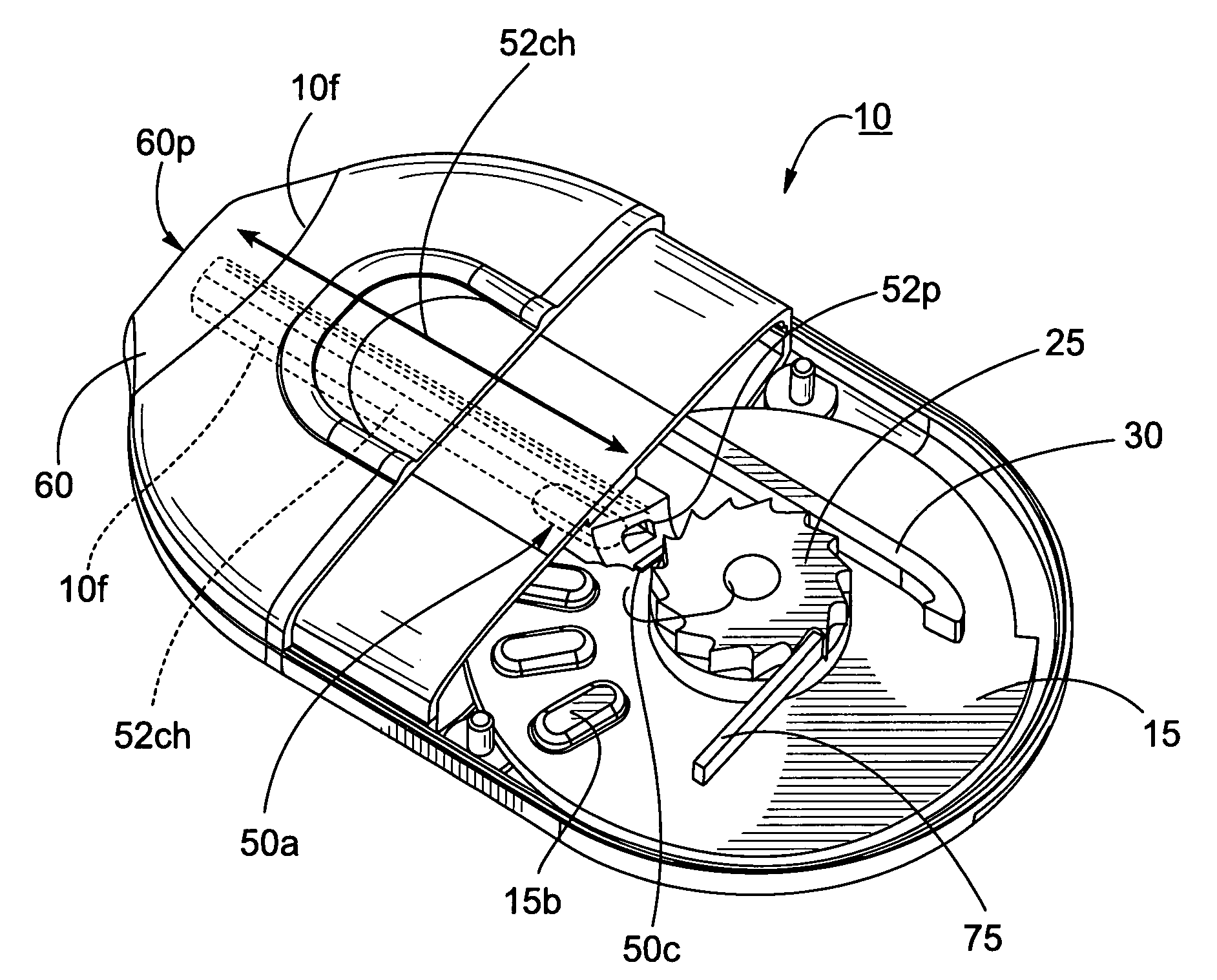
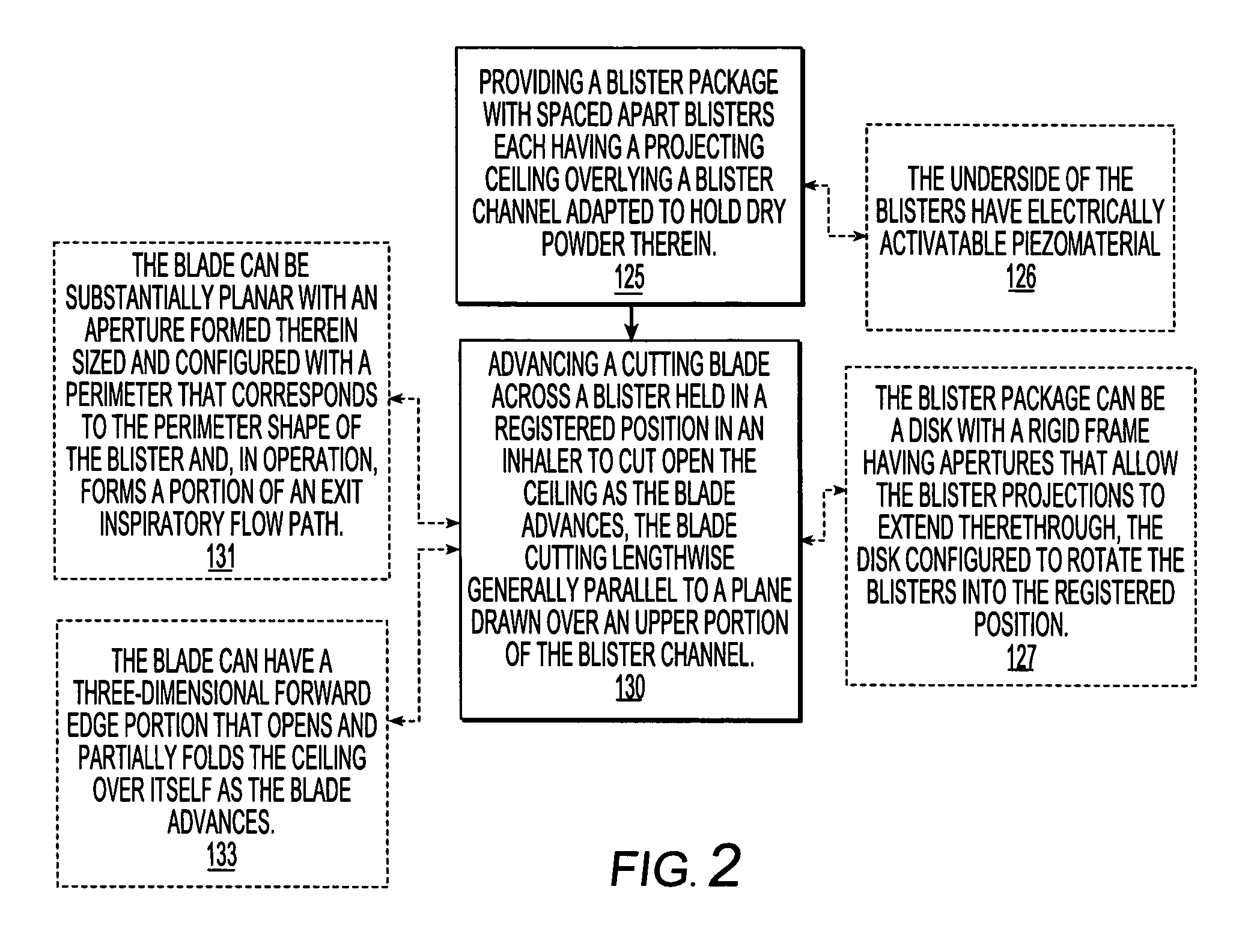
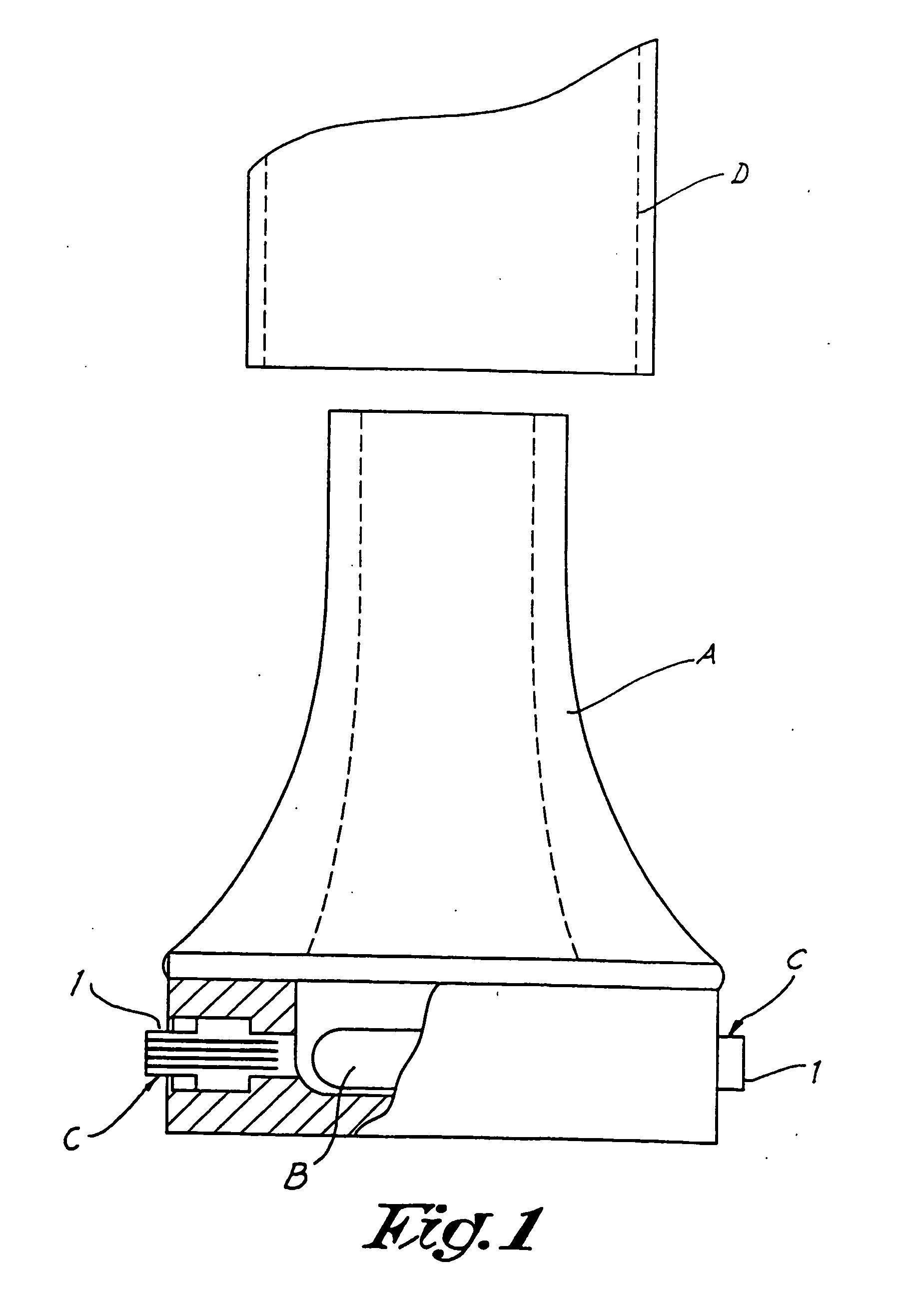
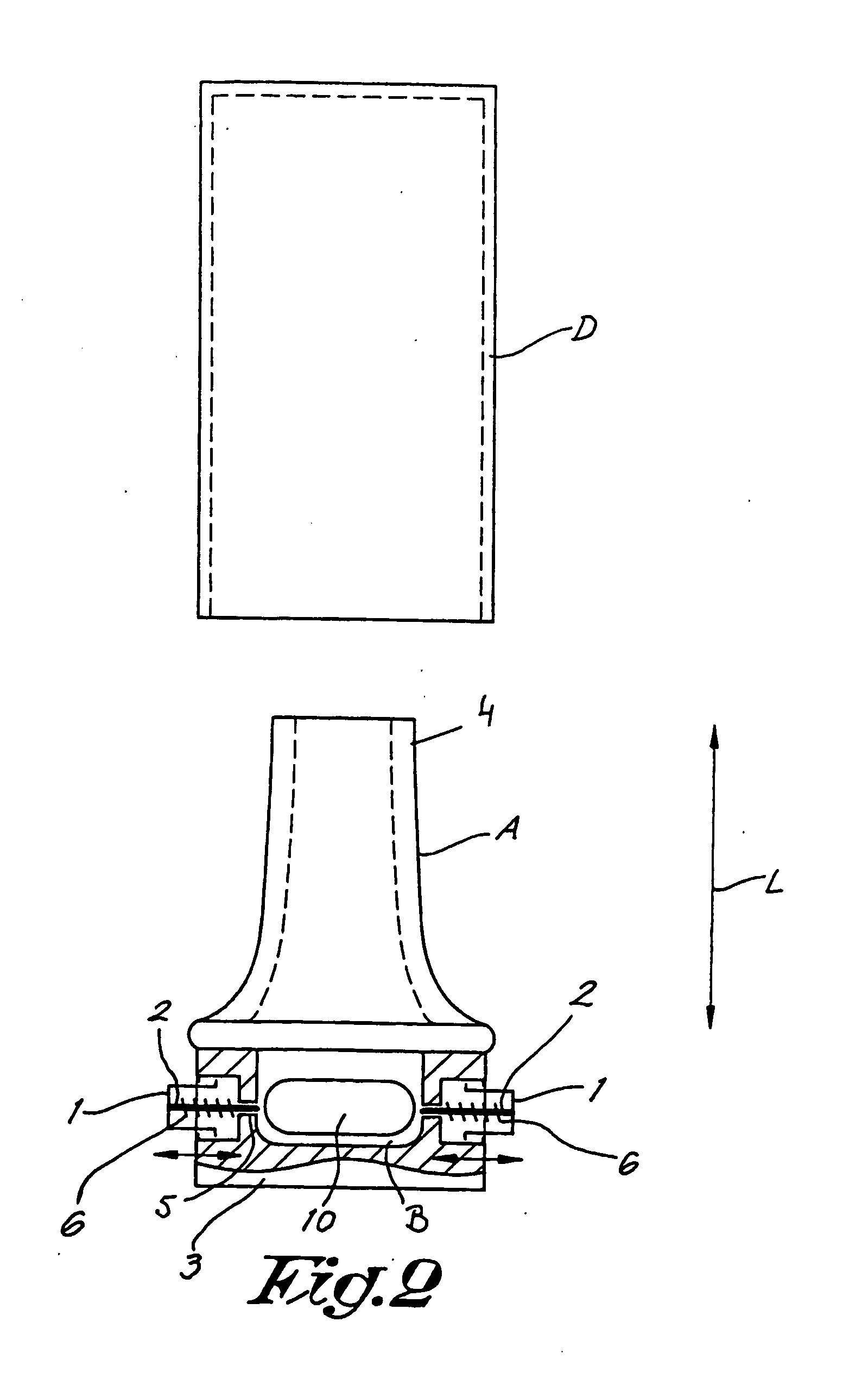
Dry powder inhalers, related blister package indexing and opening mechanisms, and associated methods of dispensing dry powder substances
Dry powder inhalers with a multi-dose dry powder package for dispensing pharmaceutical grade formulations of inhalable dry powder, include: (a) a blister package comprising a plurality of spaced apart sealed blisters thereon, each blister having a projecting ceiling and a floor defining a blister channel therebetween, the blister channel comprising a dry powder therein; (b) a movable blade cartridge holding a blade at a forward portion thereof; and (c) an extendable mouthpiece attached to the movable blade cartridge. In operation, a user pulls the mouthpiece outward and then pushes the mouthpiece inward to cause the blister package to advance to position a blister in a selected dispensing position in the inhaler and to cause the blade cartridge to move the blade across a blister ceiling held in the dispensing position in the inhaler to thereby open the blister held in the dispensing position.
Owner:ORIEL THERAPEUTICS INC
De-agglomerator for breath-actuated dry powder inhaler
A de-agglomerator is provided for use with a breath-actuated dry powder inhaler for breaking up aggregates and micronizing particles of dry powder prior to inhalation of the powder by a patient using the inhaler. The de-agglomerator includes an inner wall defining a swirl chamber extending along an axis from a first end to a second end, a dry powder supply port, an inlet port, and an outlet port. The supply port is in the first end of the swirl chamber for providing fluid communication between a dry powder delivery passageway of an inhaler and the first end of the swirl chamber. The inlet port is in the inner wall of the swirl chamber adjacent to the first end of the swirl chamber and provides fluid communication between a region exterior to the de-agglomerator and the swirl chamber. The outlet port provides fluid communication between the second end of the swirl chamber and a region exterior to the de-agglomerator, whereby a breath induced low pressure at the outlet port causes air flows into the swirl chamber through the dry powder supply port and the inlet port. The air flows collide with each other and with the wall of the swirl chamber prior to exiting through the outlet port, such that any powder entrained in the air flows is broken down and micronized. The de-agglomerator further includes vanes at the first end of the swirl chamber for creating additional collisions and impacts of entrained powder.
Owner:NORTON HEALTHCARE
Synthetic jet based medicament delivery method and apparatus
A dry powder inhaler consisting of first chamber having an orifice for holding a dry powder and a gas, and a second chamber for receiving a deaggregated form of the dry powder and for communicating the deaggregated dry powder to a user. A synthetic jet drives the dry powder from the first chamber to the second chamber.
Owner:MICRODOSE THERAPEUTX INC
Pharmaceutical formulations for dry powder inhalers in the form of hard-pellets
InactiveUS20030180227A1Reduce intensityEfficient deliveryPowder deliveryDispersion deliveryPrillInhalation
The invention provides a formulation to be administered as dry powder for inhalation suitable for efficacious delivery of active ingredients into the low respiratory tract of patients suffering of pulmonary diseases such as asthma. In particular, the invention provides a formulation to be administered as dry powder for inhalation freely flowable, which can be produced in a simple way, physically and chemically stable and able of delivering either accurate doses and high fine particle fraction of low strength active ingredients by using a high- or medium resistance device.
Owner:CHIESI FARM SPA
Inhaler
A dry powder inhaler has a vibrator coupled to a blister filled with a dry powder drug substance. One or more of drug ejection apertures in the blister are substantially opposite the vibrator. One or more air intake apertures in the blister are not opposite the vibrator. Upon vibration of the vibrator, the drug substance is deaggregated, aerosolized, and ejected from the drug ejection apertures for inhalation by a patient.
Owner:MICRODOSE THERAPEUTX INC
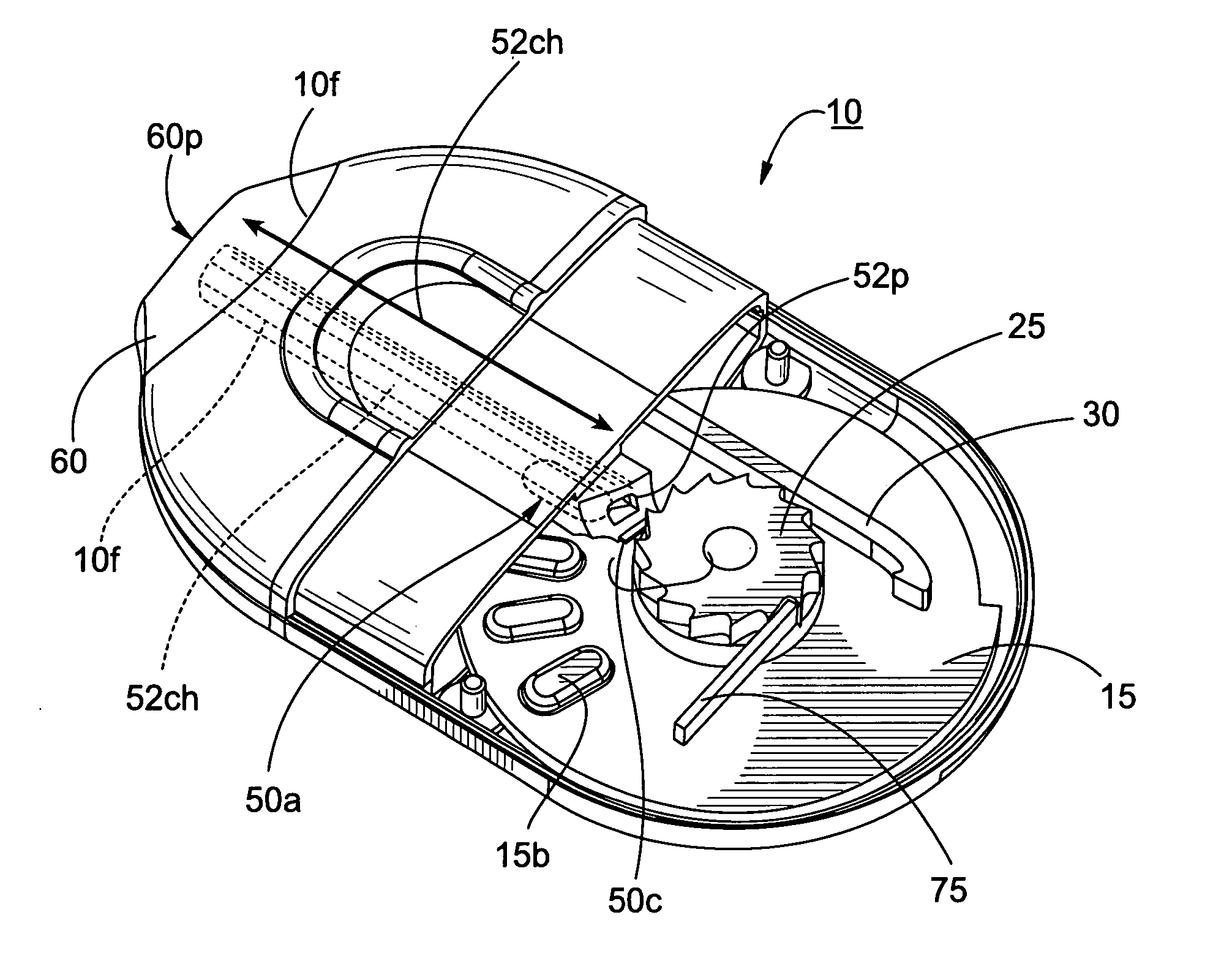
Dry powder inhalers having spiral travel paths, unit dose microcartridges with dry powder, related devices and methods
Dry powder inhalers include: (a) a first generally planar spiral travel path in an inhaler body, wherein the first spiral travel path has a plurality of adjacent curvilinear channels forming lanes with upstanding sidewalls, including an inner lane and an outer lane; and (b) a plurality of discrete sealed microcartridges with substantially rigid bodies disposed in the first travel path, each comprising a pre-metered (typically dose) amount of dry powder, the microcartridges being configured to slidably advance along the first travel path toward an inhalation chamber that merges into an inhalation output port. In operation, at least one microcartridge is held in the inhalation chamber to release the dry powder therein during inhalation.
Owner:ORIEL THERAPEUTICS INC
Rotary cassette system for dry powder inhaler
InactiveUS20100294278A1Simpler and more compact assemblyRespiratorsLiquid surface applicatorsEngineeringBlister pack
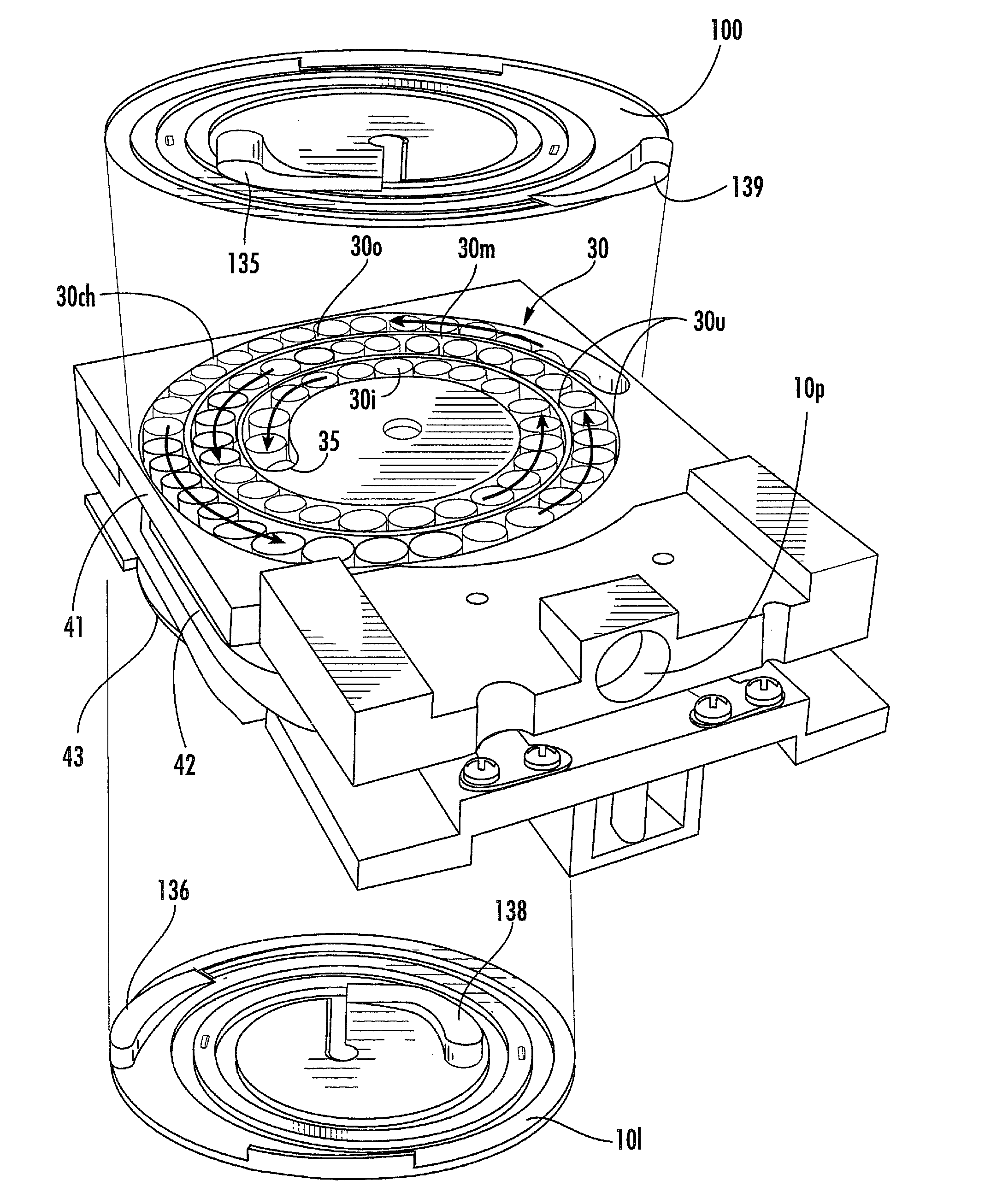
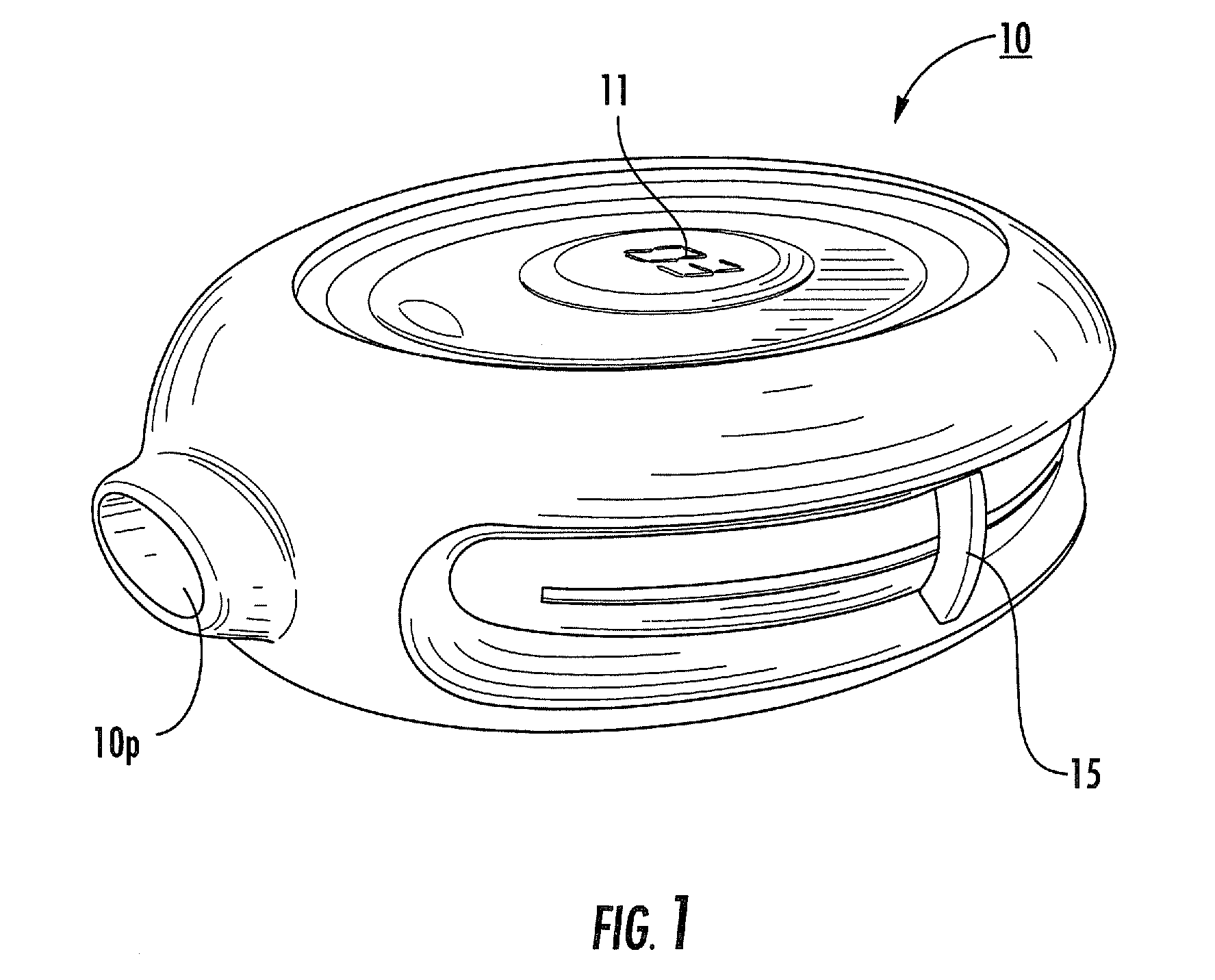
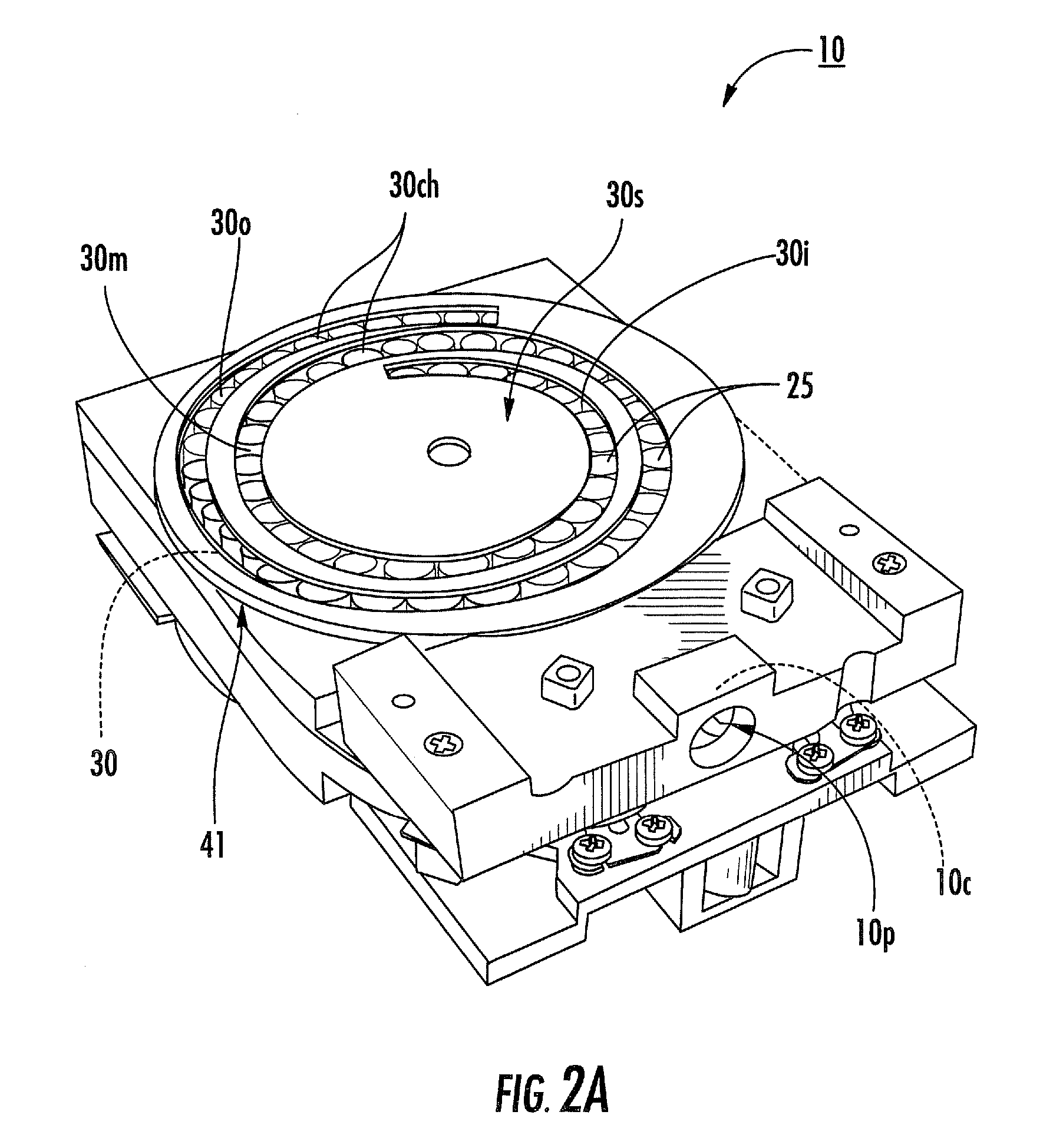
The present disclosure provides an inhaler having a vibration element for aerosolizing medicament contained in a blister pack, wherein a plurality of individual blister packs are arranged in a rotary cassette that fits within a housing, and wherein the individual blister packs are dragged up into a clamping position between the vibration element and a piercing element. The motion of the blister pack is controlled by a rotary disk within the housing which further coordinates the movement of the piercing and vibrating elements for the piercing and deaggregation, respectively, of the individual blister packs.
Owner:MICRODOSE THERAPEUTX INC
Dry powder inhaler
ActiveUS8037880B2Small accurate volumePrecise deliveryRespiratorsLiquid surface applicatorsUltra fineExcipient
A new dry powder inhaler is developed as a pulmonary medicine delivery device for dispersing precise tiny dosages (10 μg-50 mg) of pure carrier-free ultra-fine powdered medicament (<5 μm aerodynamics particle size) into a patient's lung. The powder is drawn from the blister cell and dispersed through an outlet tube assisted by two air streams. The first air stream goes through a the blister cell from its upstream side, to significantly fluidize the medicament in the dose to flow upward. The second one extracts the fluidized powder from downstream of the blister cell for further deagglomeration and dispersion of the medicament powder by shear force. The rotating multi-dose blister can hold up to 60 doses, which are pre-metered with pure ultra-fine powdered medicament. So that it has higher drug loading capability in small volumes, compared to most current dry powder inhalers, which usually use some excipient. The inhaler efficiently disperse the aerosolized medicament in the air stream to the deep interior of patient's lung. The fine particle fraction (<4.7 μm) is reported to reach as high as 80% using this inhaler.
Owner:NINGBO INHAL PHARMA CO LTD
Inhaler for the administration of powdered pharmaceuticals, and a powder cartridge system for use with this inhaler
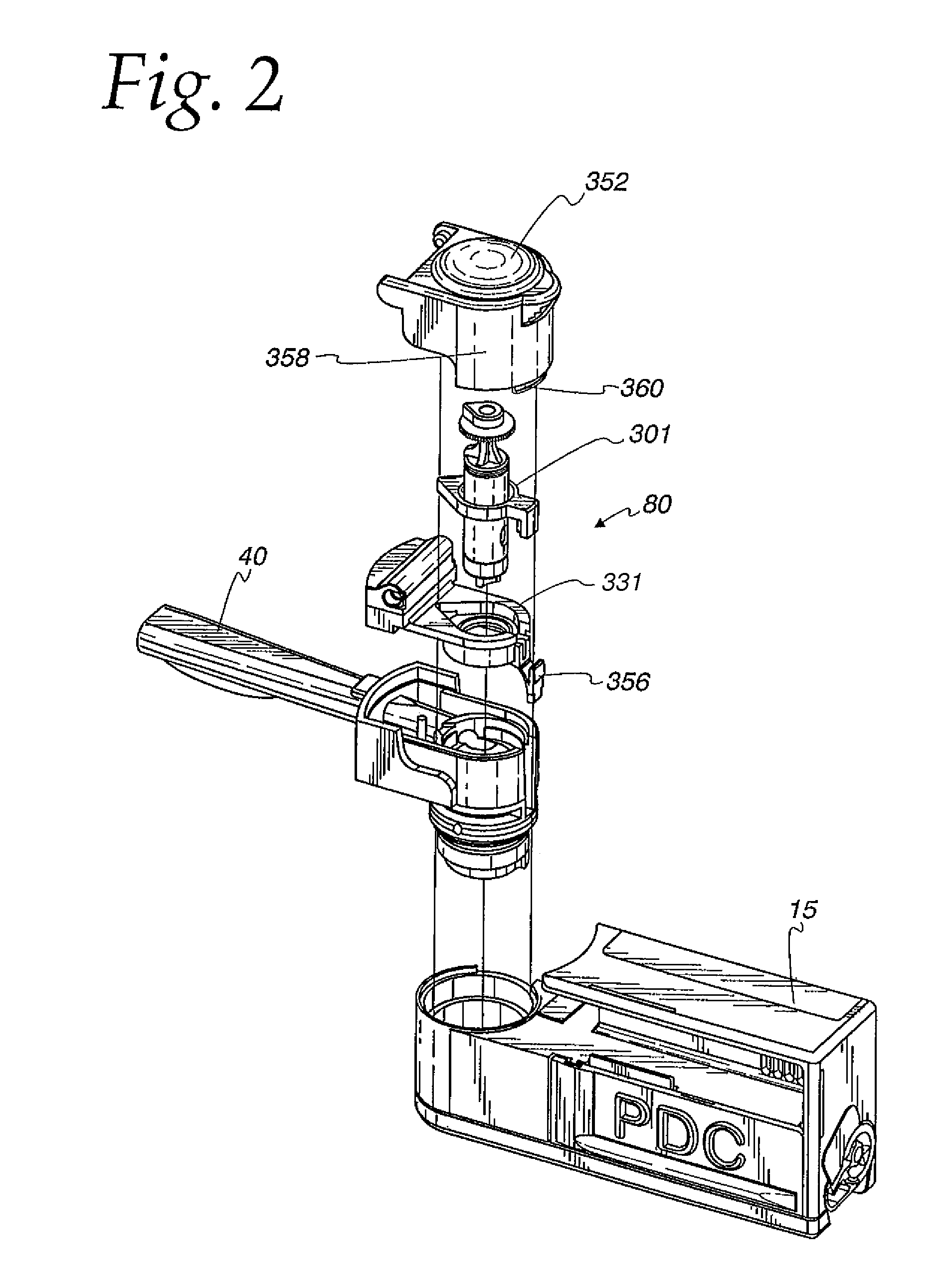
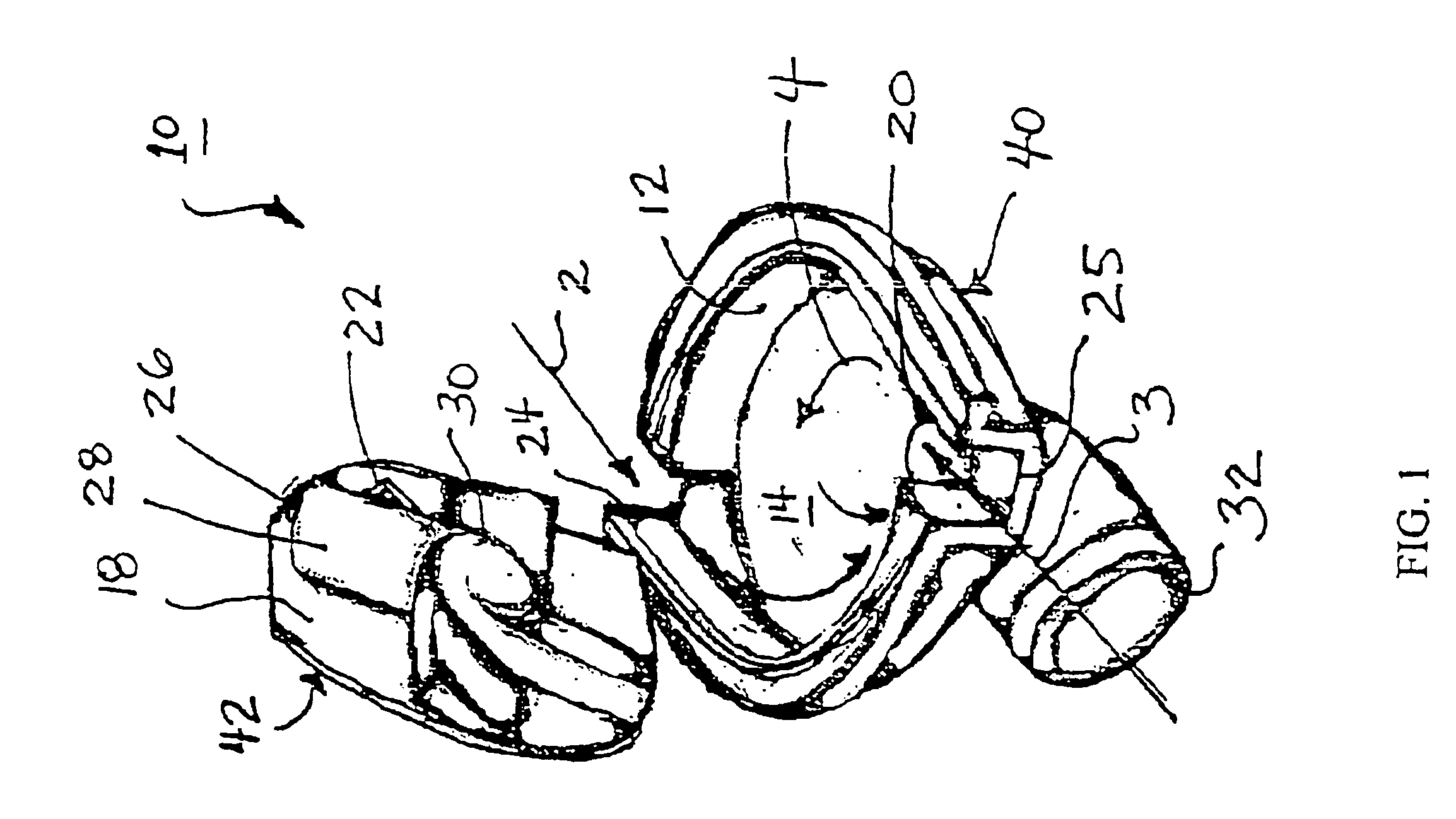
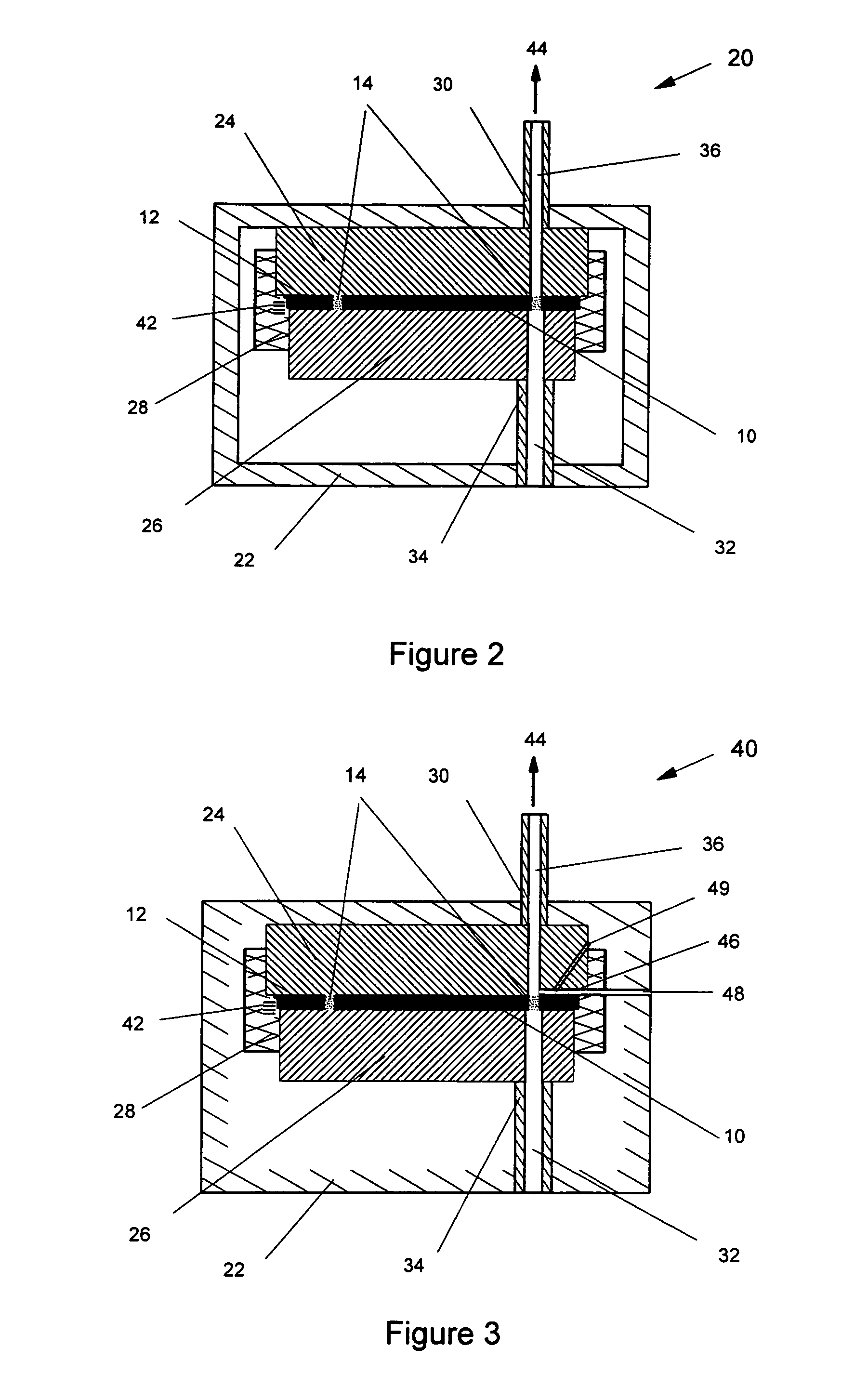
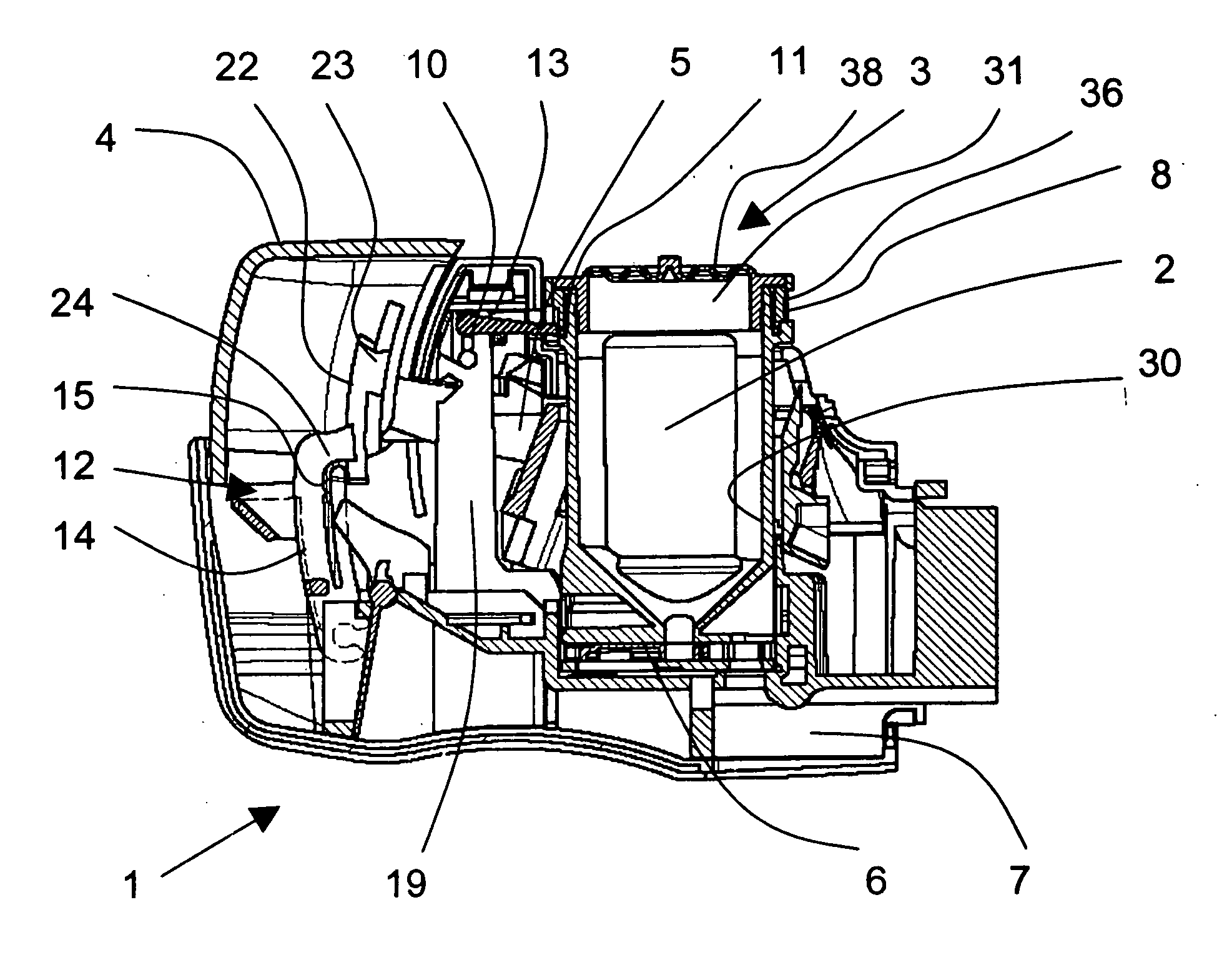
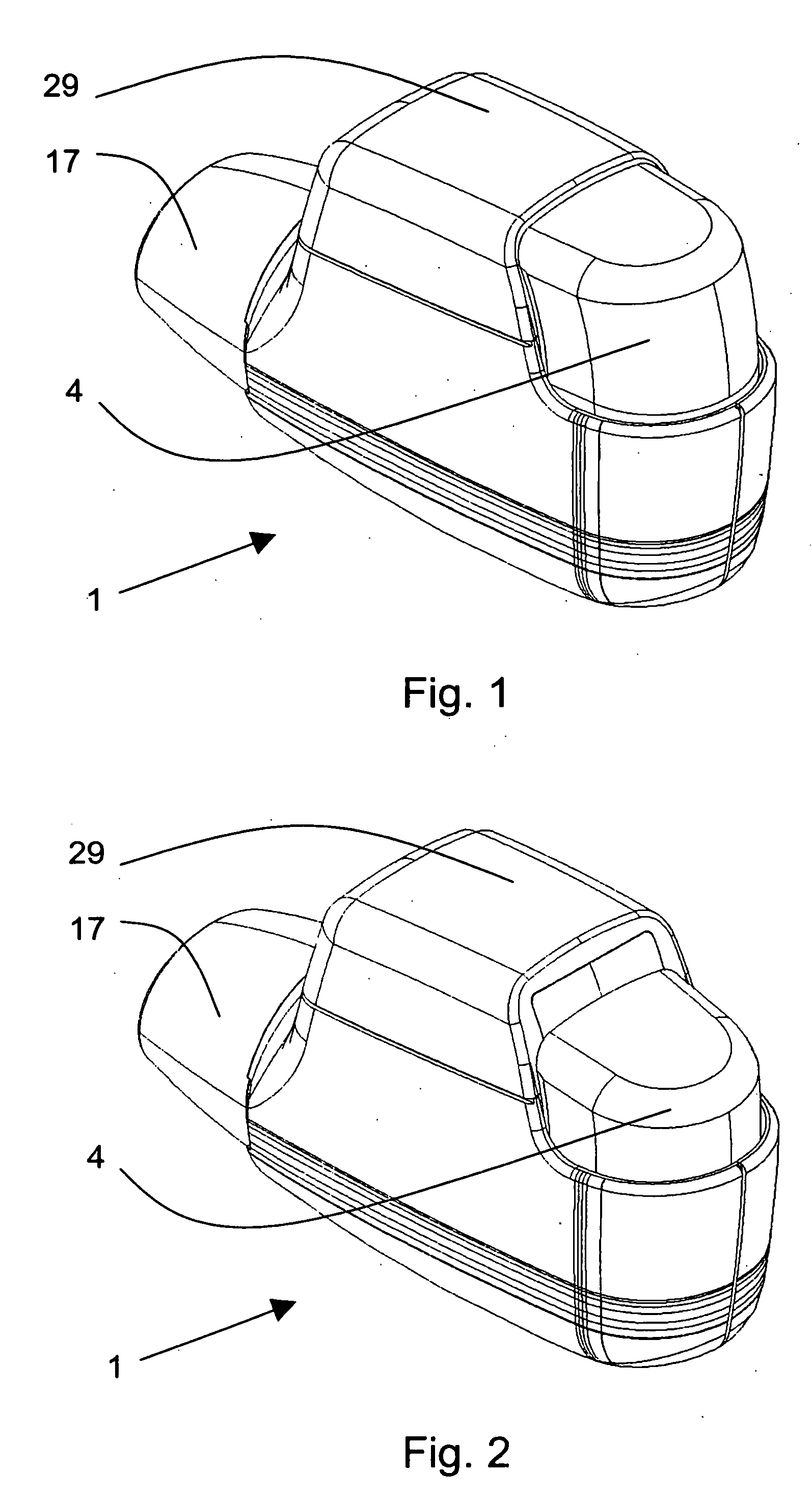
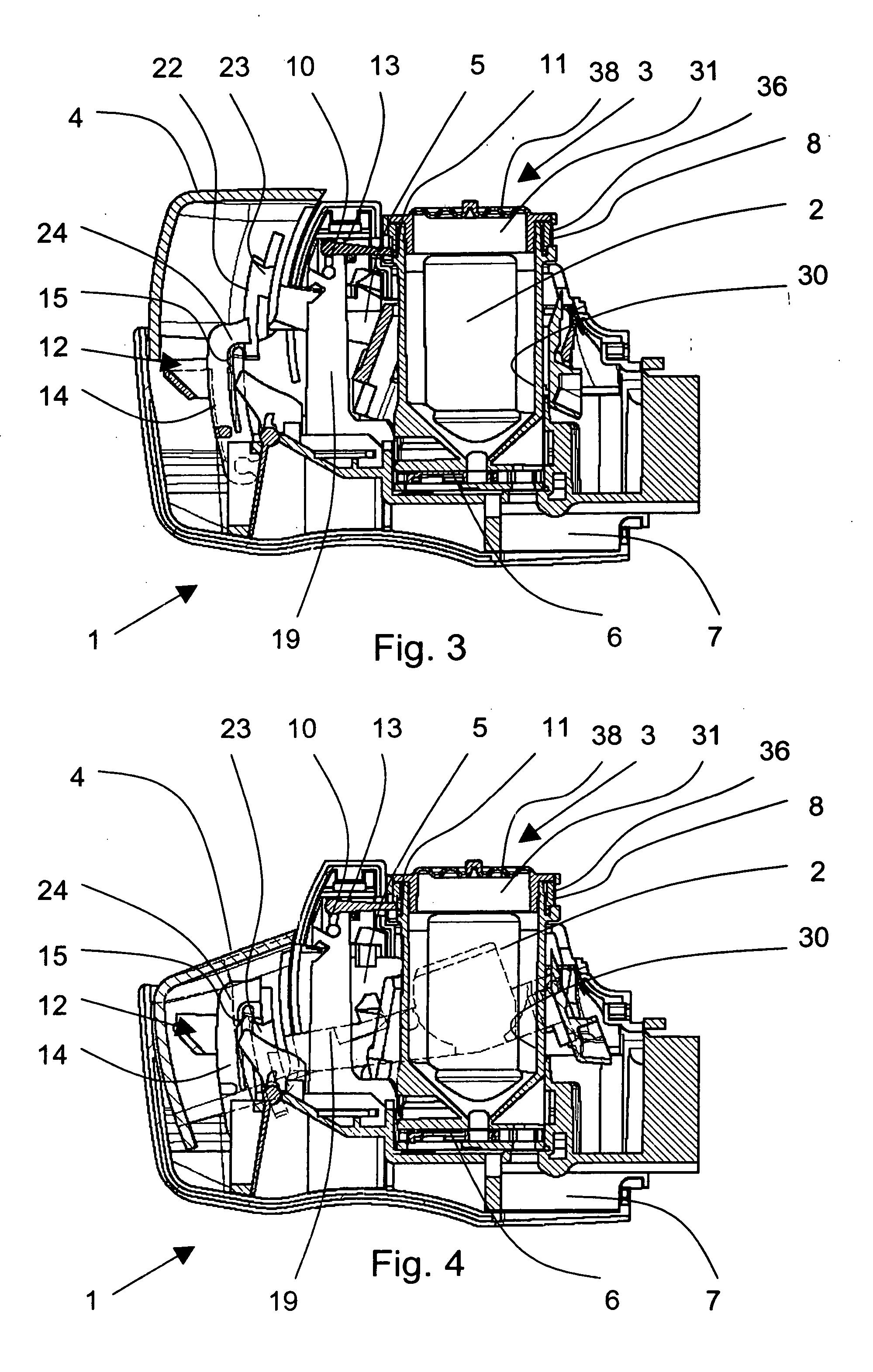
ActiveUS20060037612A1Cost-effective medical treatmentIncrease costRespiratorsLiquid surface applicatorsLocking mechanismSurgery
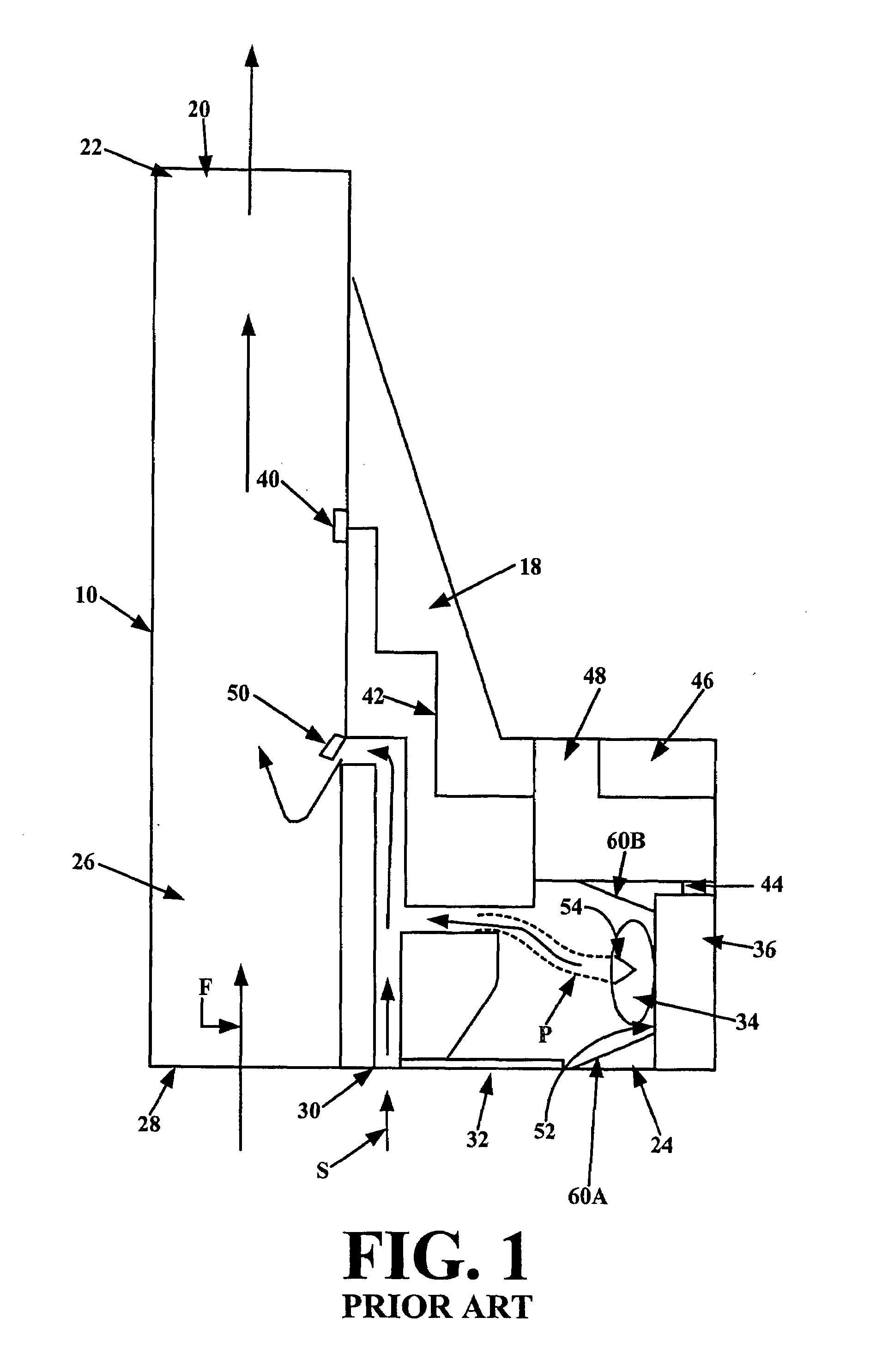
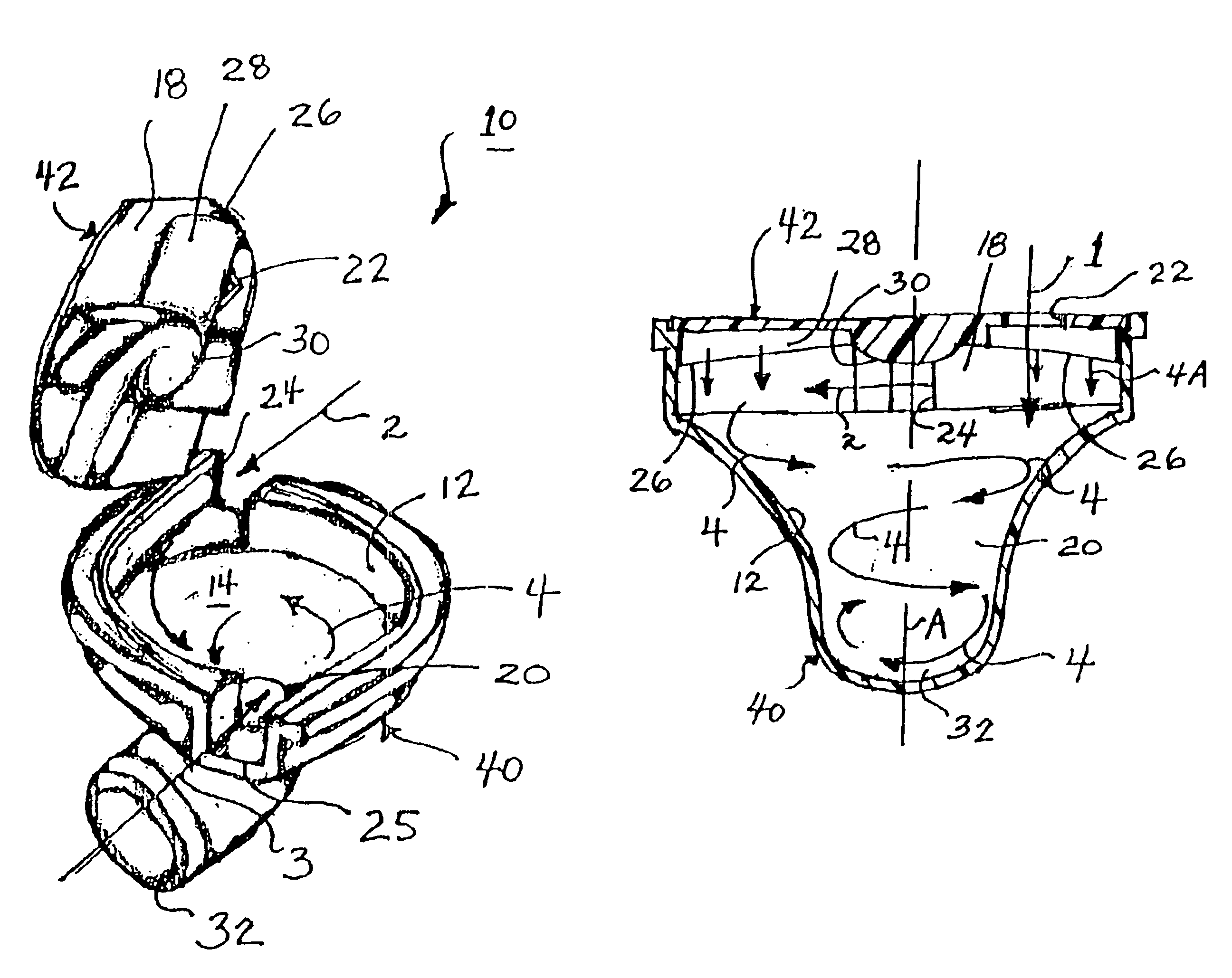
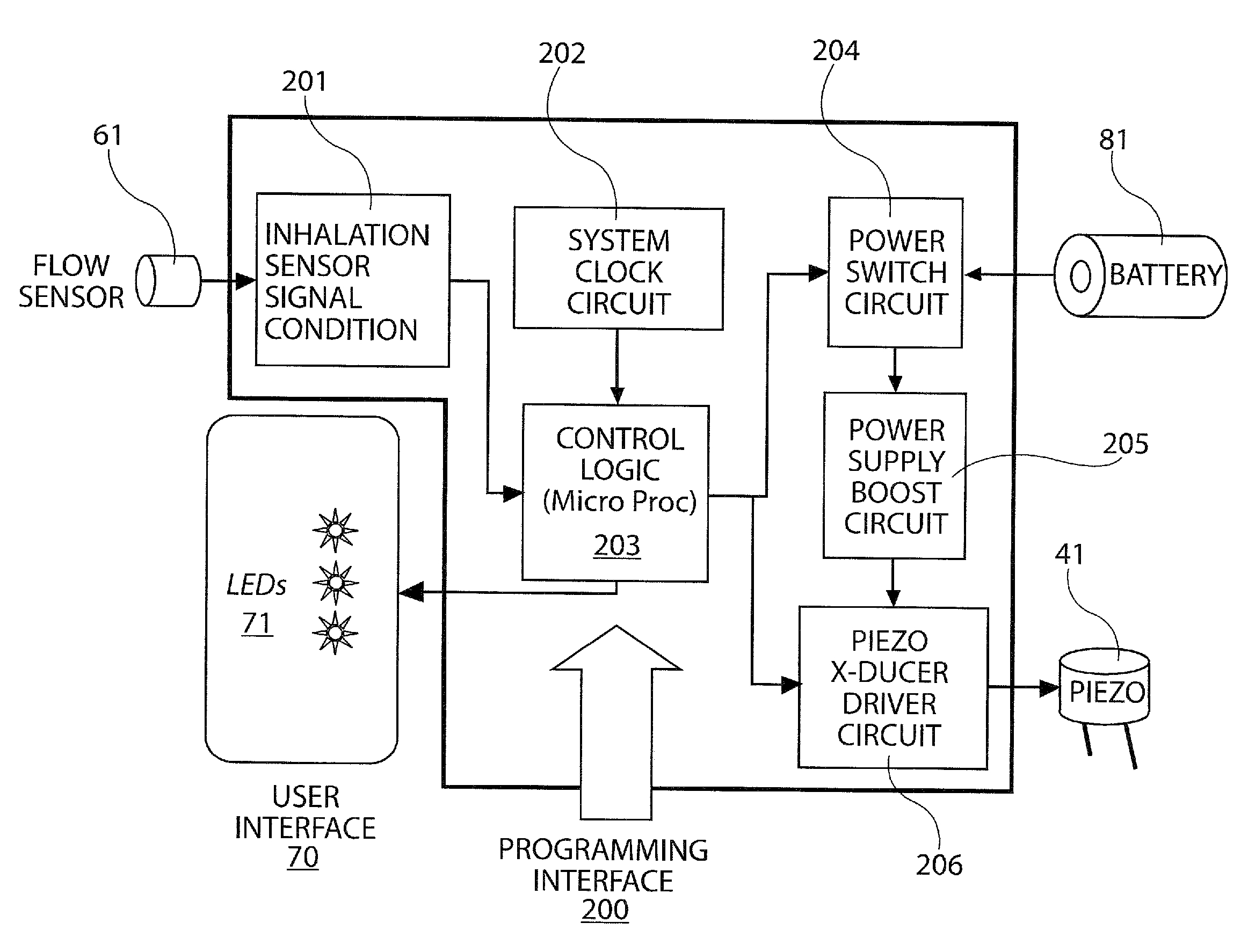
In order to improve the security and reliability of administration of powdered pharmaceuticals through dry powder inhalers, the invention proposes an inhaler (1) for powdered medicaments, comprising an activating device (4) for manual engagement by the patient for repeatedly metering a dose of medicament to be administered to the patient, and further comprising an advancing mechanism (25) for advancing a counter or indexing means (8) each time the activating device (4) has been engaged by the patient so that a dose of medicament has been released for administration to the patient, wherein the counter or indexing means (8) comprises an index (9), the index (9) being detectable by a detection means (10) of the inhaler, and the detection means (10) being coupled to a locking mechanism (12), the locking mechanism (12) blocking the activating device (4) and / or any transportation mechanism (5) of the inhaler (1) delayed by a predetermined number of metering cycles since detection of the index (9), and a cartridge (3).
Owner:ASTRAZENECA AB
Medical product
InactiveUS20060239933A1Powder deliveryPeptide/protein ingredientsPulmonary inhalationMedical product
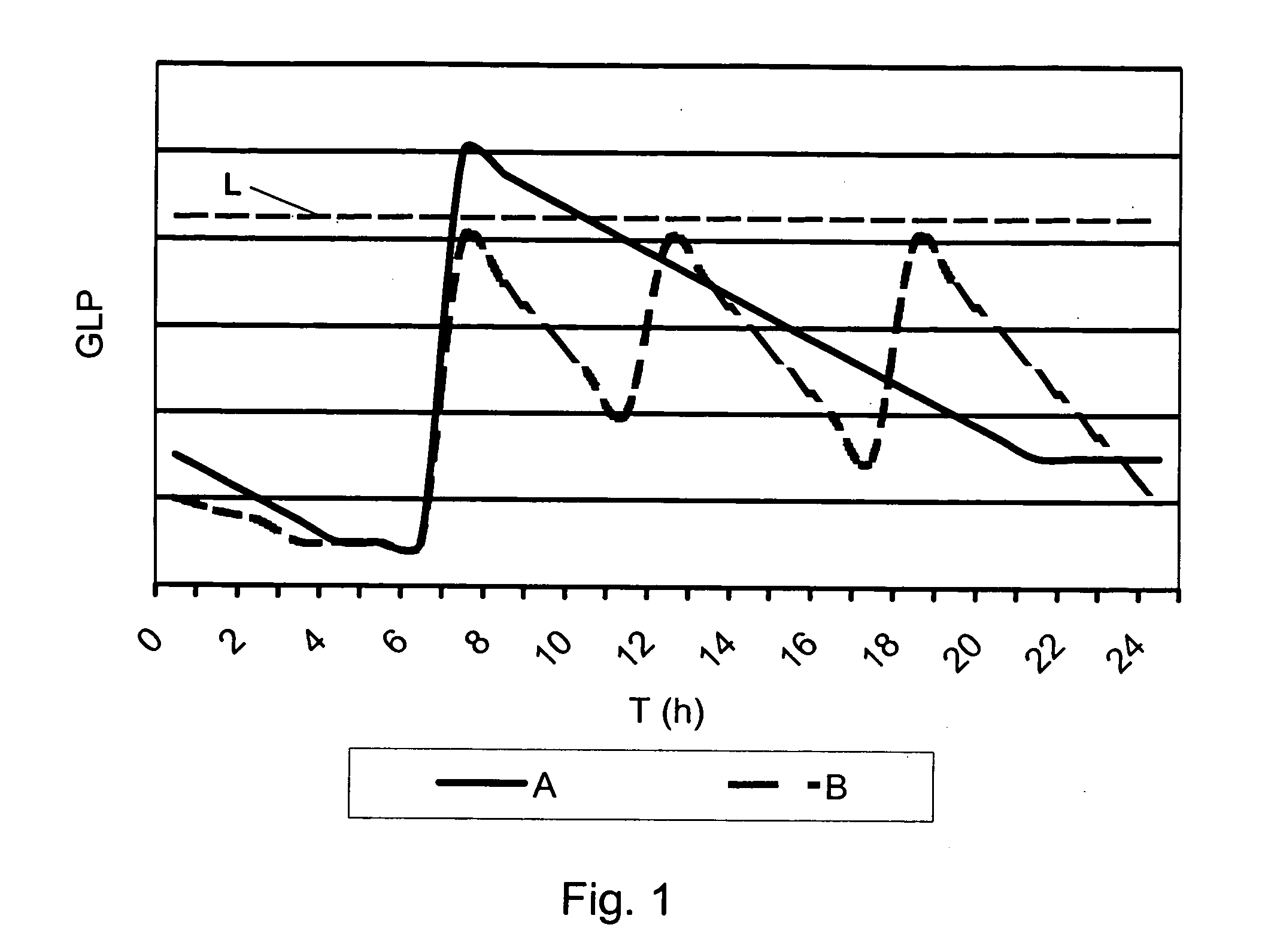
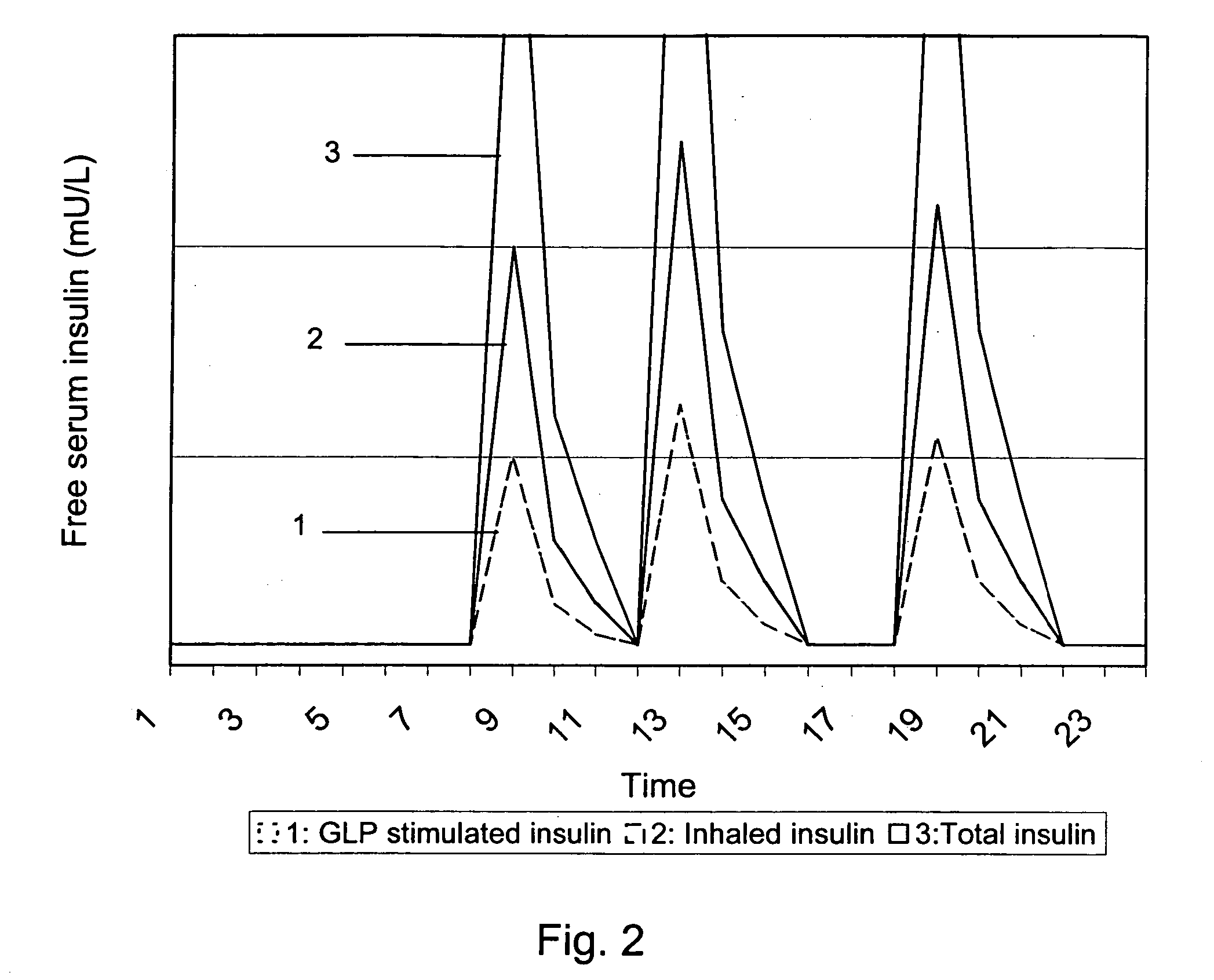
A medical product is disclosed. The medical product contains an accurately metered dose of at least one GLP medicament intended for pulmonary inhalation put into a moisture-tight, high barrier seal container. The medical product optionally also contains a dose of insulin. The container is adapted for application into a dry powder inhaler. The dose loaded in the container is intended for a prolonged delivery by inhalation to the deep lung where the active ingredients are absorbed into the system. Optionally the medical product also may comprise at least one biologically acceptable excipient.
Owner:MEDERIO AG
Inhaler
A dry powder inhaler has a vibrator coupled to a blister filled with a dry powder drug substance. One or more of drug ejection apertures in the blister are substantially opposite the vibrator. One or more air intake apertures in the blister are not opposite the vibrator. Upon vibration of the vibrator, the drug substance is deaggregated, aerosolized, and ejected from the drug ejection apertures for inhalation by a patient.
Owner:MICRODOSE THERAPEUTX INC
Pharmaceutical formulations for dry powder inhalers
InactiveUS7541022B2Powder deliveryOrganic active ingredientsBULK ACTIVE INGREDIENTPharmaceutical formulation
A powder for use in a dry powder inhaler comprises: i) a fraction n of fine particle size constituted by a mixture of physiologically acceptable excipient and an additive; ii) a fraction of coarse particles; and iii) at least one active ingredient. The powder is suitable for efficacious delivery of active ingredients into the low respiratory tract of patients suffering from pulmonary diseases such as asthma. In particular, the invention provides a formulation to be administered as dry powder for inhalation which is freely flowable, can be produced in a simple way, is physically and chemically stable and capable of delivering accurate doses and / or high fine particle fraction of low strength active ingredients by using a high- or medium resistance device.
Owner:VECTURA LTD
Pharmaceutical formulations for dry powder inhalers
InactiveUS20030185764A1Powder deliveryOrganic active ingredientsBULK ACTIVE INGREDIENTPharmaceutical formulation
A powder for use in a dry powder inhaler comprises: i) a fraction of fine particle size constituted by a mixture of physiologically acceptable excipient and an additive; ii) a fraction of coarse particles; and iii) at least one active ingredient. The powder is suitable for efficacious delivery of active ingredients into the low respiratory tract of patients suffering from pulmonary diseases such as asthma. In particular, the invention provides a formulation to be administered as dry powder for inhalation which is freely flowable, can be produced in a simple way, is physically and chemically stable and capable of delivering accurate doses and / or high fine particle fraction of low strength active ingredients by using a high- or medium resistance device.
Owner:VECTURA LTD
Dry powder inhaler
ActiveUS20120145150A1Improve performanceReduce flow rateRespiratorsLiquid surface applicatorsMedicineActuator
A dry powder inhaler includes a chamber holding an actuator to which a powdered medicament is adhered. Air is drawn into the chamber through an inlet flow channel and exits through an outlet flow channel. The actuator oscillates in response to the air flow, dislodging powdered medicament to be entrained in the air flow and delivered to the patient. A retaining member prevents the actuator from exiting the chamber. Thus, the medicament may be delivered to the patient without the use of carrier particles.
Owner:RESPIRA THERAPEUTICS INC
Dry powder inhaler system
InactiveUS20060254583A1Easy piercingRespiratorsPowder deliveryBULK ACTIVE INGREDIENTActive ingredient
An improved dry powder inhalation system comprising: at least one micronized active ingredient in an hydroxypropylmethylcellulose capsule (10), and an dry powder inhaler device equipped with piercing systems (12) having an equivalent diameter of not less than 0.8 mm.
Owner:GALEPHAR PHARMA RES
Dry powder inhalers, related blister package indexing and opening mechanisms, and associated methods of dispensing dry powder substances
Dry powder inhalers with a multi-dose dry powder package for dispensing pharmaceutical grade formulations of inhalable dry powder, include: (a) a blister package comprising a plurality of spaced apart sealed blisters thereon, each blister having a projecting ceiling and a floor defining a blister channel therebetween, the blister channel comprising a dry powder therein; (b) a movable blade cartridge holding a blade at a forward portion thereof; and (c) an extendable mouthpiece attached to the movable blade cartridge. In operation, a user pulls the mouthpiece outward and then pushes the mouthpiece inward to cause the blister package to advance to position a blister in a selected dispensing position in the inhaler and to cause the blade cartridge to move the blade across a blister ceiling held in the dispensing position in the inhaler to thereby open the blister held in the dispensing position.
Owner:ORIEL THERAPEUTICS INC
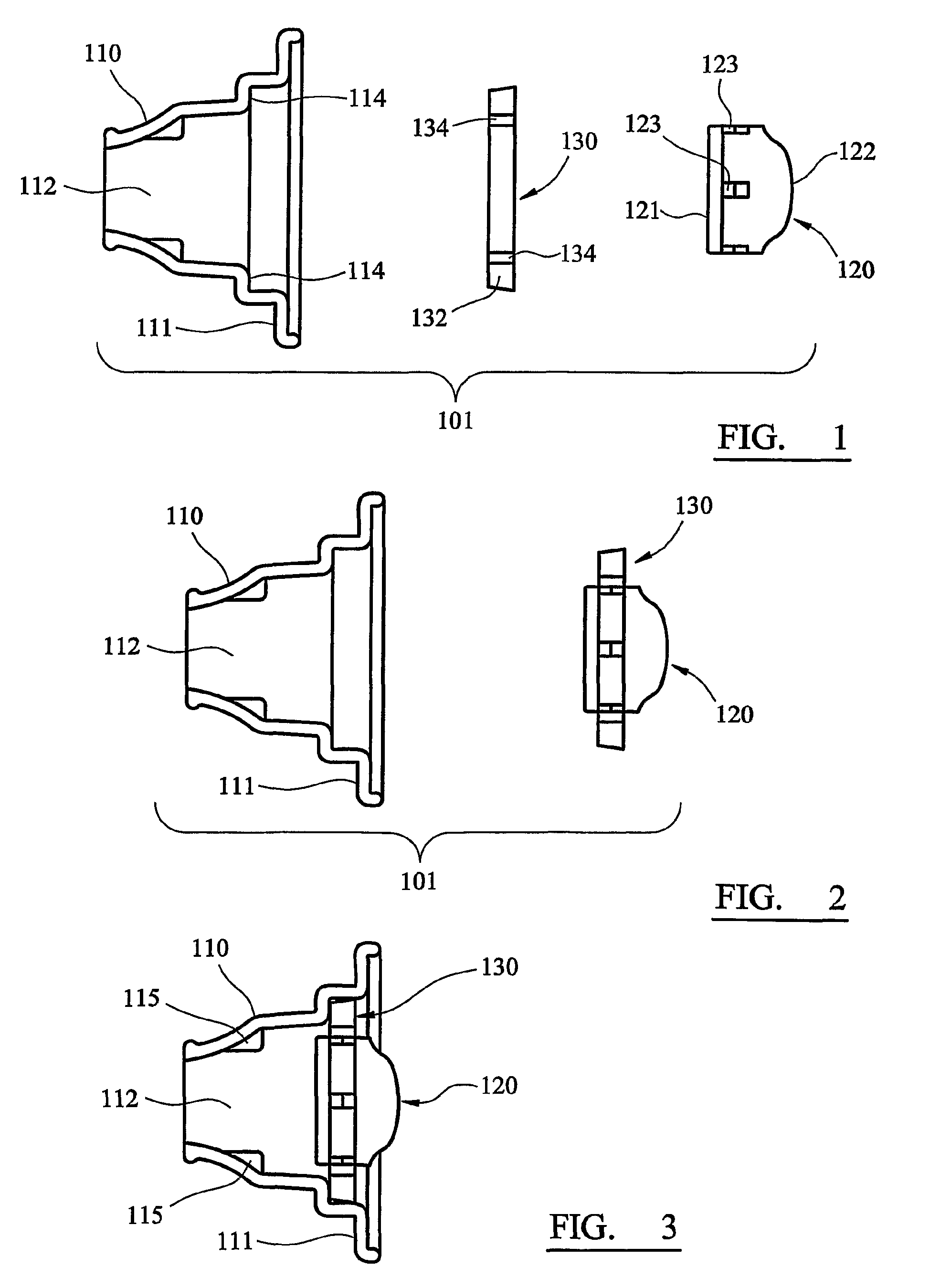
Inhaler
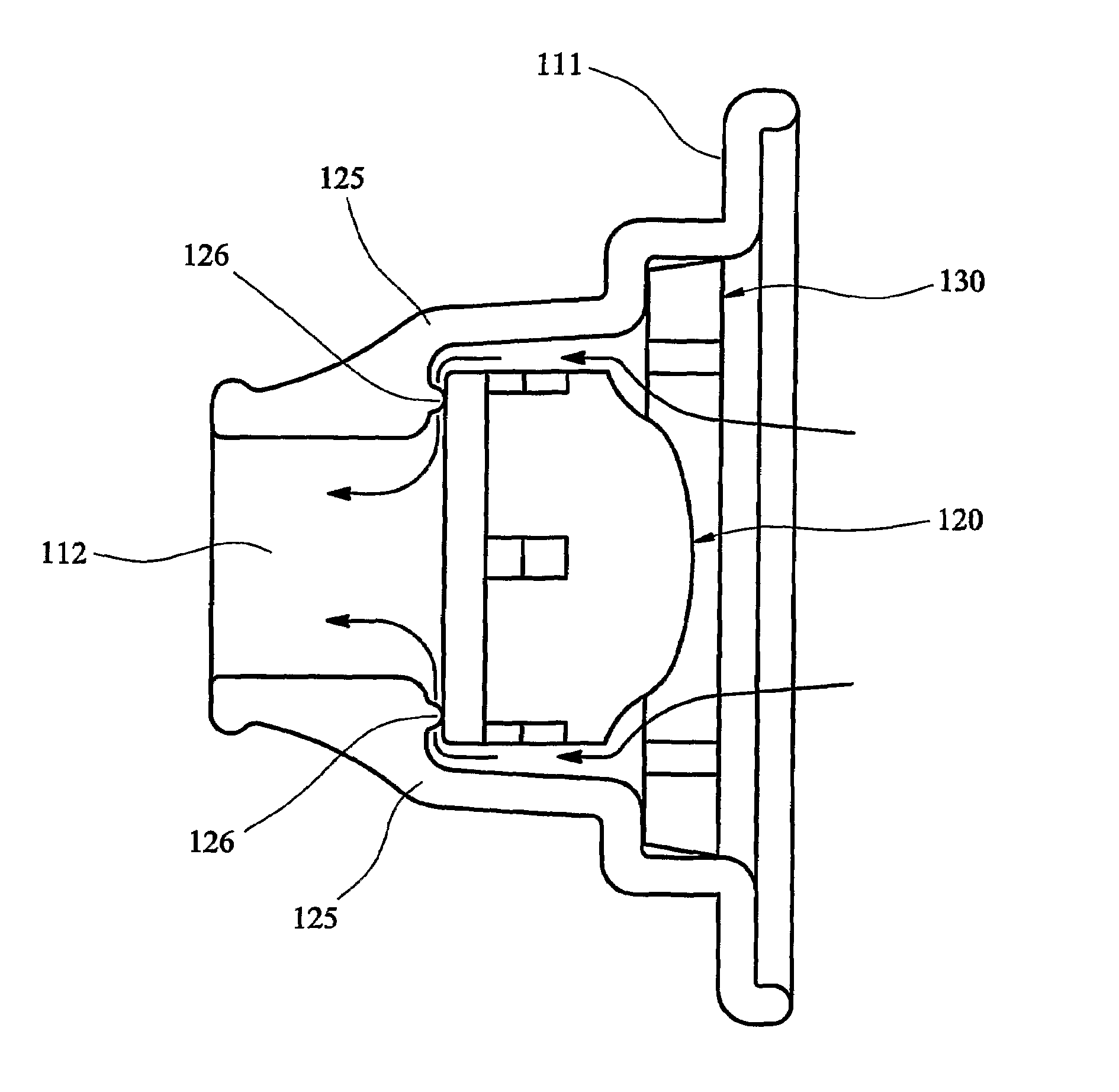
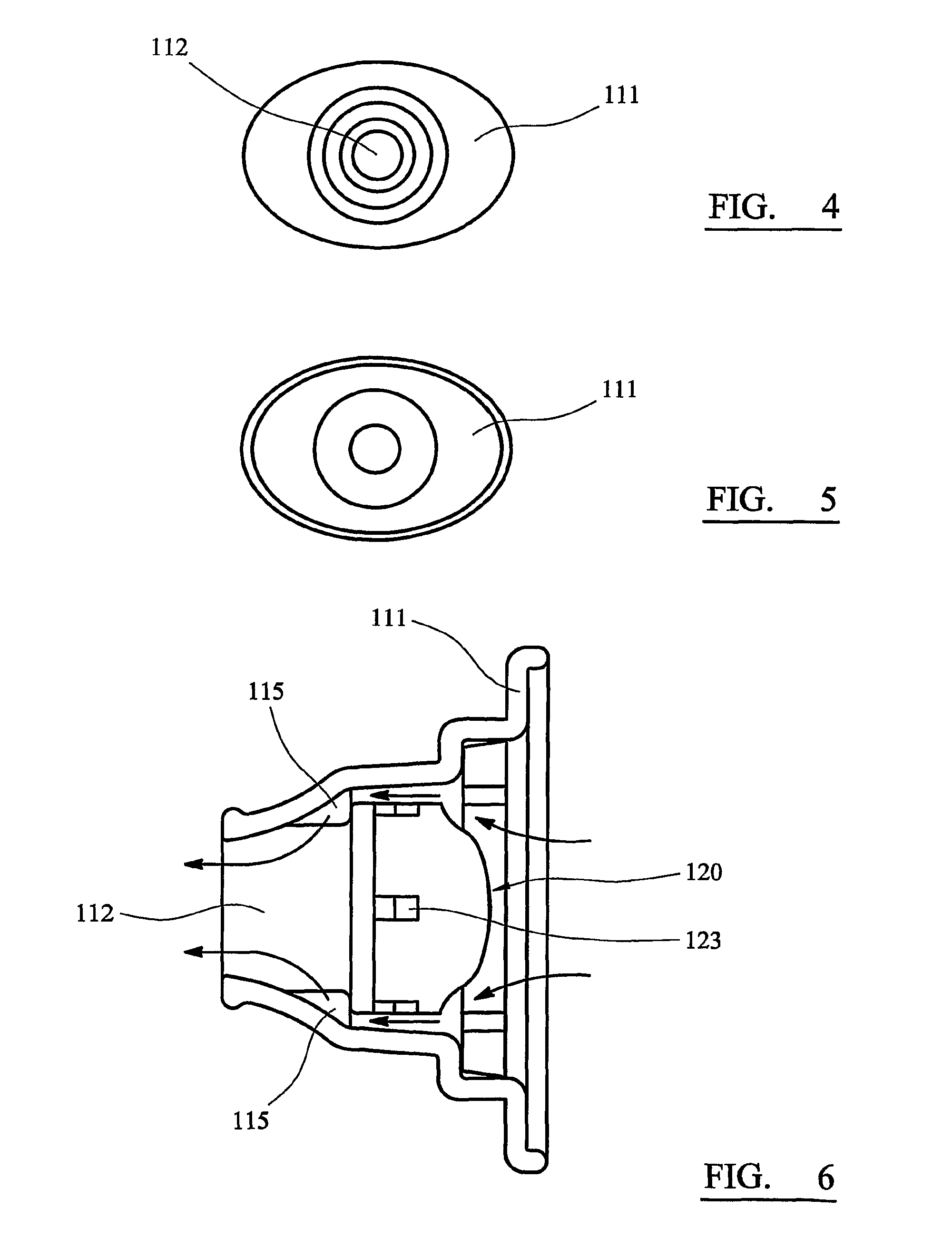
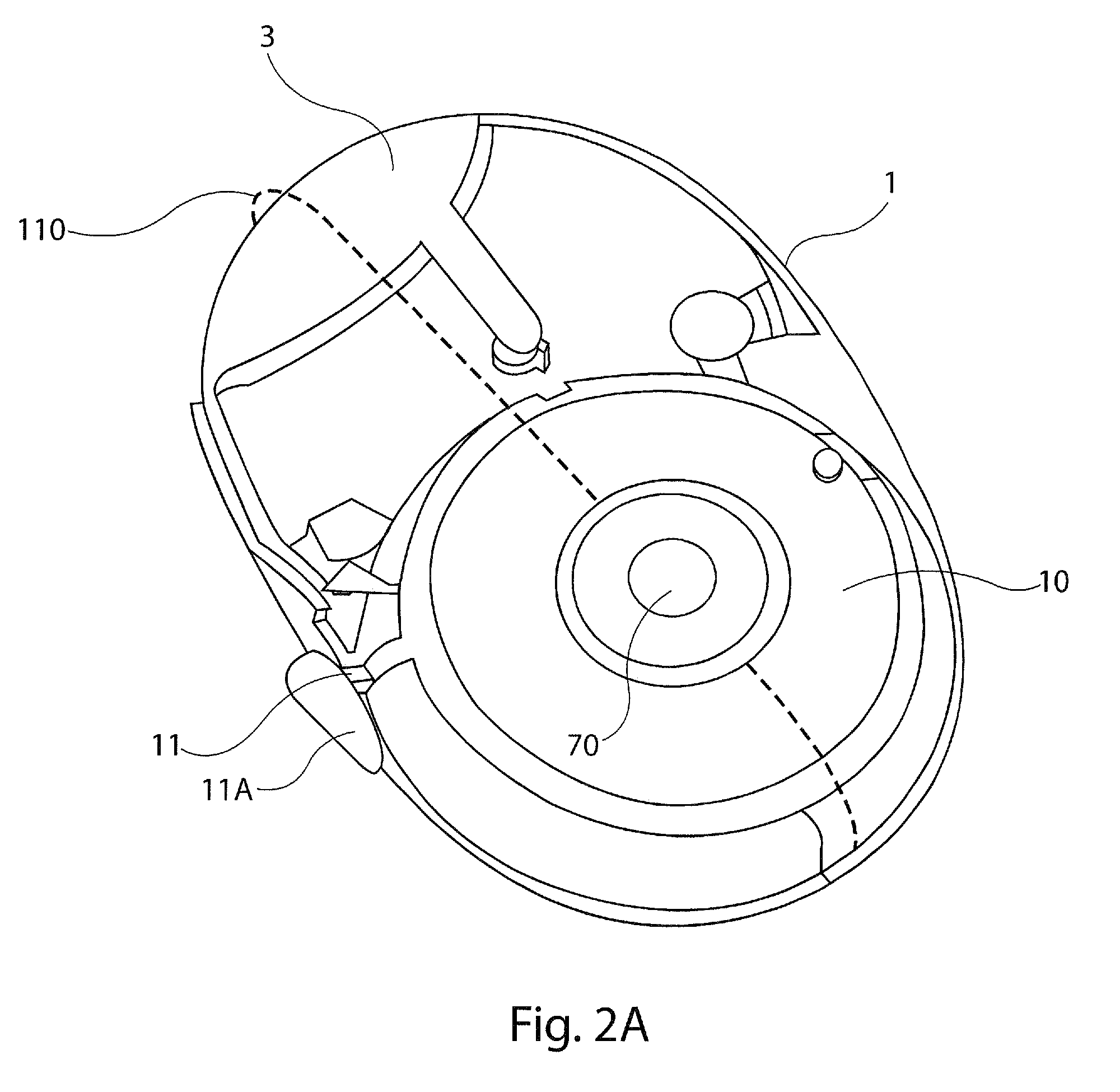
InactiveUS8201555B2Overcomes and substantially mitigates disadvantageIncreasing the fine particle fraction of the medicamentRespiratorsLiquid surface applicatorsBiomedical engineeringDry-powder inhaler
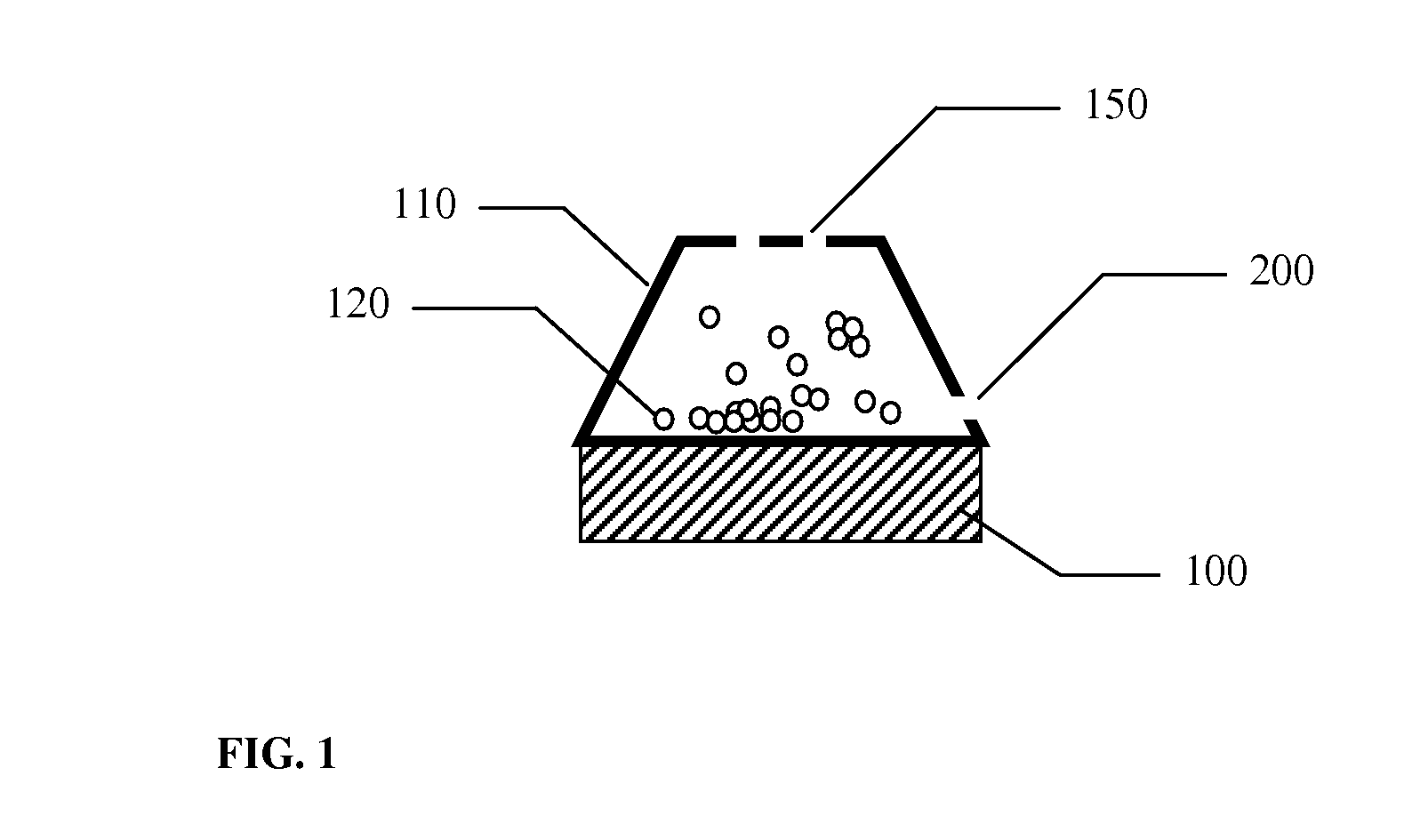
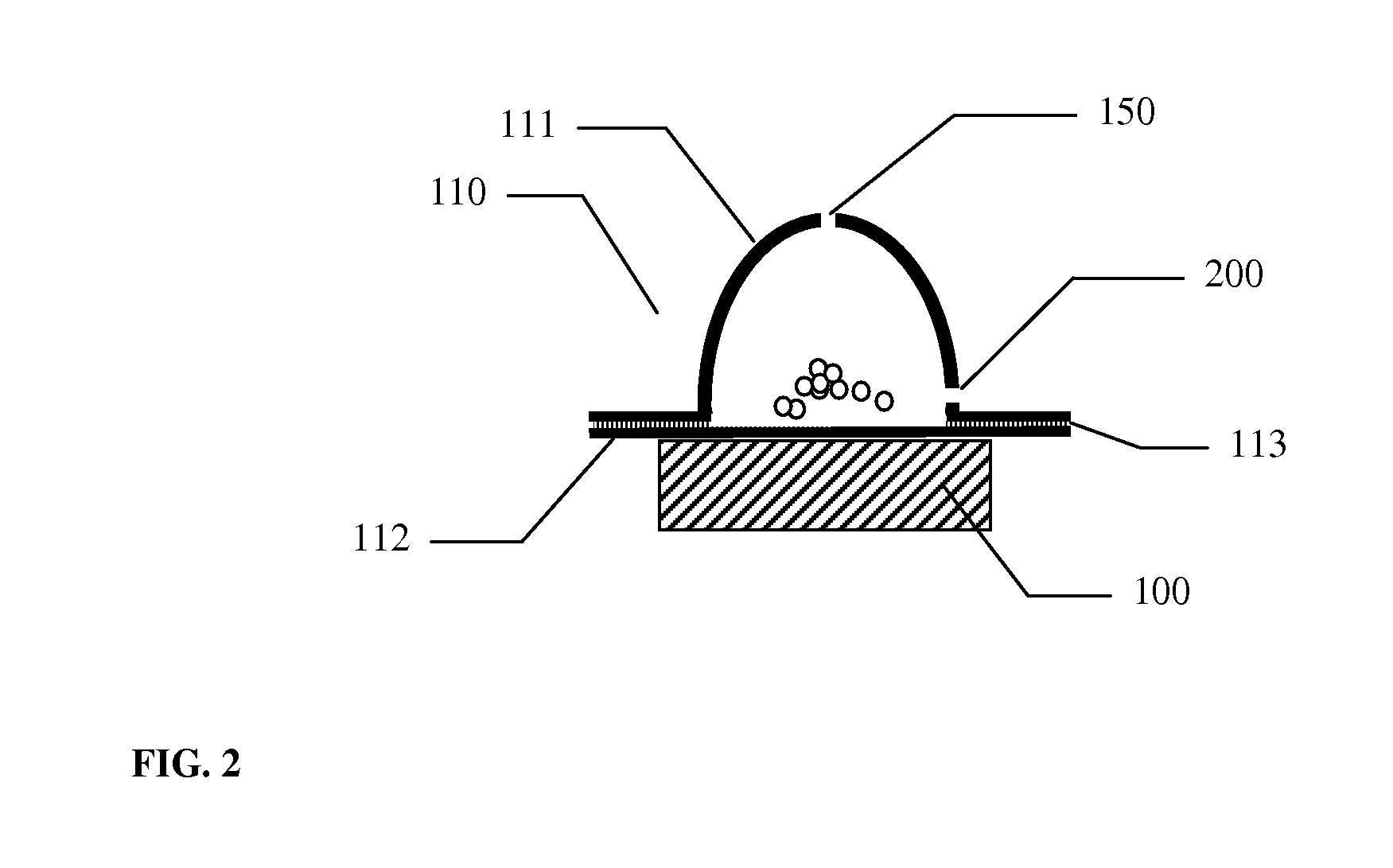
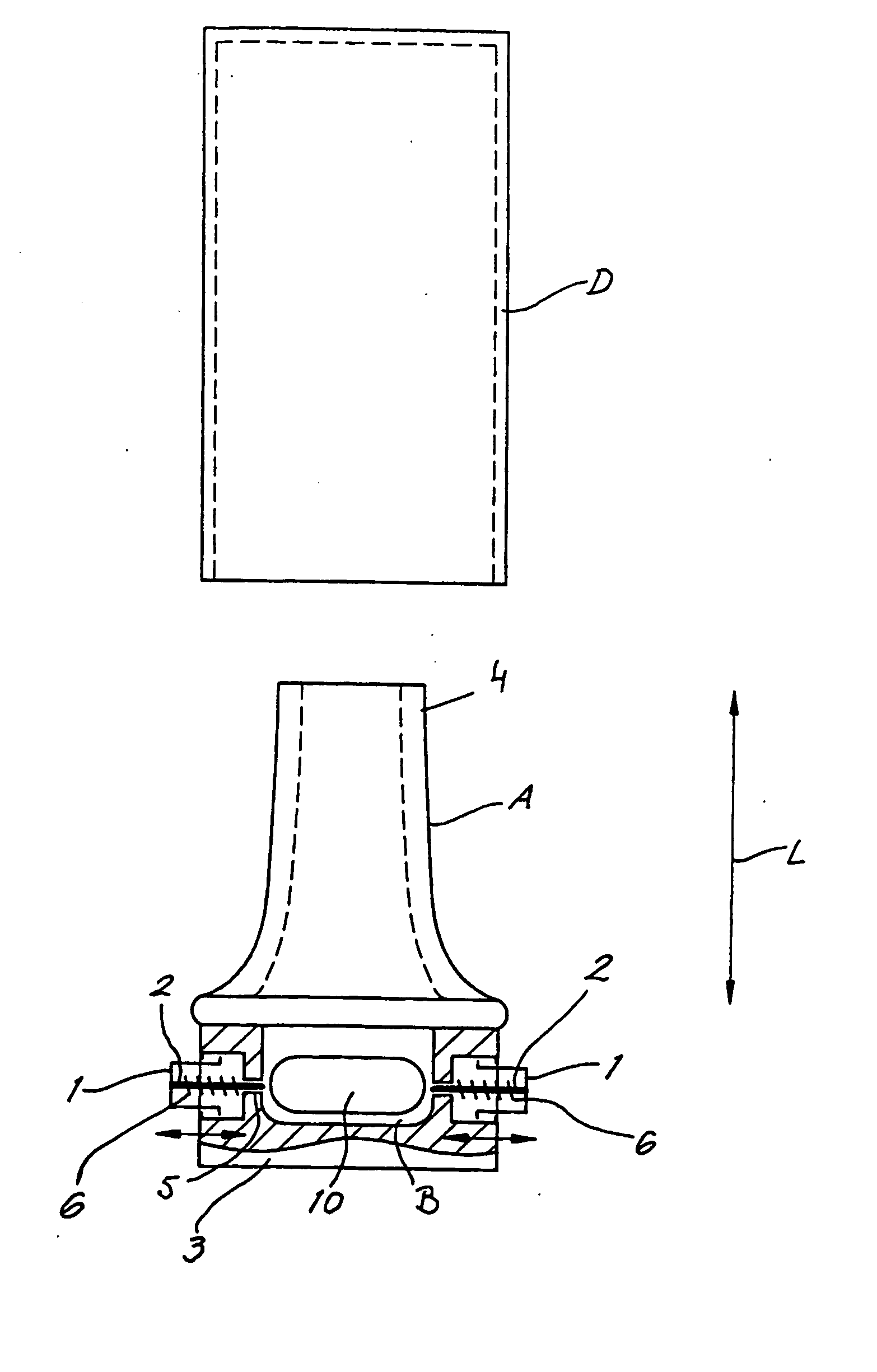
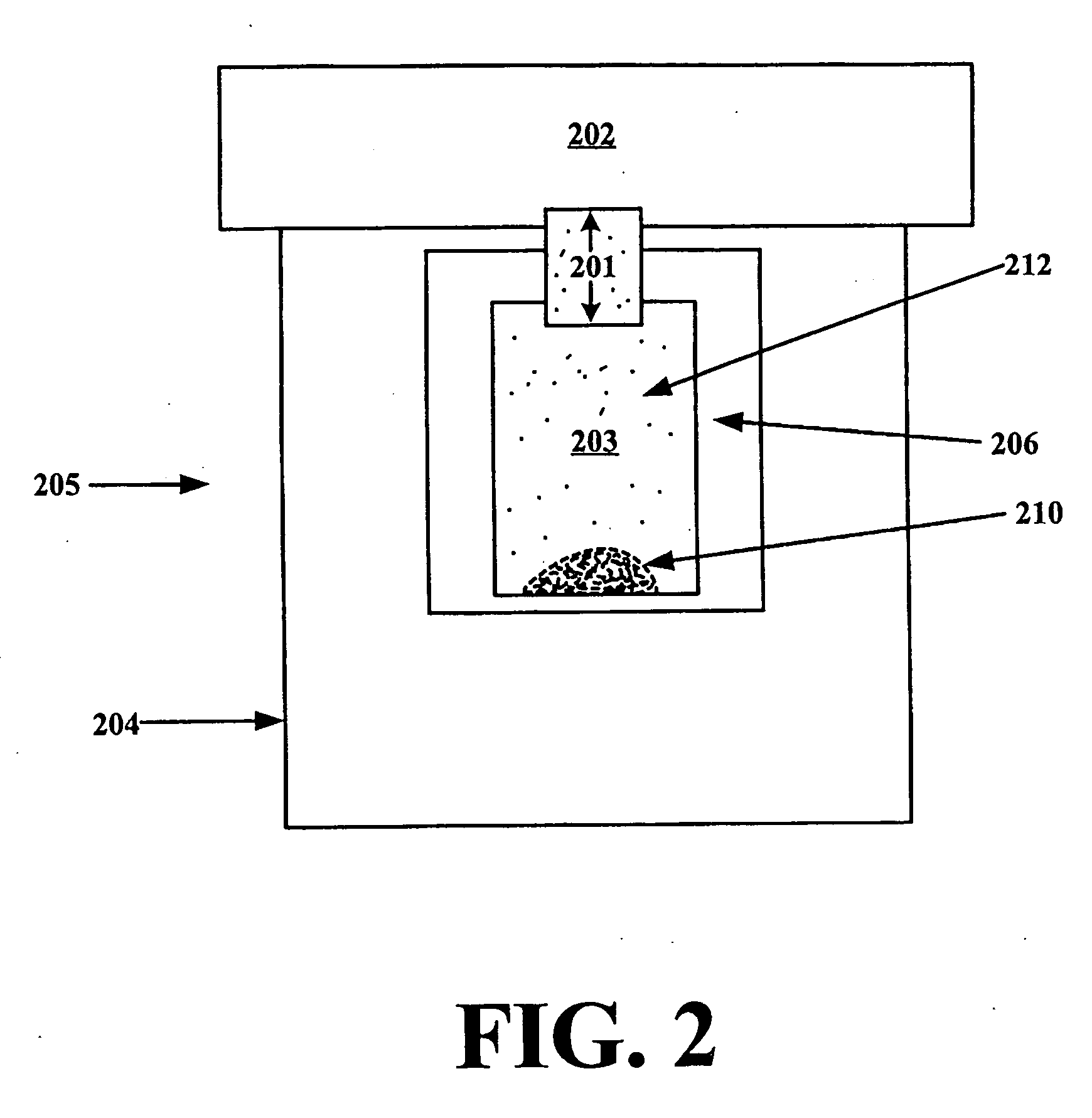
A dry powder inhaler (101) comprises an inhaler body (110), including an air passageway, and a medicament container (120). The medicament container (120) holds a dose of medicament and is provided with at least one dispensing aperture (123) through which the medicament may be drawn from the medicament container (120). The medicament container (120) is displaceable from a first position, in which the dispensing aperture (123) is occluded, to a second position, in which the dispensing aperture (123) is open and air is able to flow along the air passageway. When the medicament container (120) is in the first position, the air passageway is occluded by the medicament container (120).
Owner:BRIN TECH INT
Human-powered dry powder inhaler and dry powder inhaler compositions
InactiveUS20080035143A1Easy to useEfficient deliveryRespiratorsPowder deliveryNoseBULK ACTIVE INGREDIENT
In one embodiment, a human-powered dry powder inhaler comprises a human-powered compressible component operable to discharge an air pulse at an outlet at a pressure of about 1-40 psi; an inflatable reservoir operable to receive an air pulse discharged from the human-powered compressible component to provide an aerosol of a dry powder pharmaceutical formulation in the reservoir, the reservoir including an outlet valve; and a receiving mask in communication with the outlet valve and operable to receive an aerosol of dry powder from the reservoir and to deliver the aerosol to at least a mouth or nose of a patient. In another embodiment, the inhaler comprises a human-powered compressible component operable to discharge an air pulse at an outlet of a polymeric pressure release valve at a pressure of about 1-40 psi; and a receiving mask in communication with the outlet of the compressible component and operable to deliver an aerosol of dry powder to at least a mouth or nose of a patient. Methods for delivery of a dry powder pharmaceutical formulation to a patient are conducted in the absence of electrical power and circuitry and pre-pressurized propellant gas. Suitable dry powder pharmaceutical formulations may include myo-inositol and / or maltodextrin as a carrier and active ingredients such as vaccines or siRNA.
Owner:UNIV OF COLORADO THE REGENTS OF
Formulation of powder containing nanoparticles for aerosol delivery to the lungs
Respirable particles carrying active principles or diagnostics in nanoparticle form are created by mixing the nanoparticles with liquid carrier, then forming the resultant mixture into respirable particles. Spray-drying, freeze spray drying and drying followed by comminution may be used to create the respirable particles, which may be delivered to the lung via a dry powder inhaler. In one example, lactose was used as the excipient and spray-dried with two different types of nanoparticle: gelatin and poly butylcyanoacrylate nanoparticles. The incorporation of nanoparticles did not affect the respirable fraction of the carrier powders.
Owner:FINLAY WARREN HUGH +2
Rotary cassette system for dry powder inhaler
InactiveUS8763606B2Simpler and more compact assemblyRespiratorsLiquid surface applicatorsEngineeringBlister pack
Owner:MICRODOSE THERAPEUTX INC
Dry powder inhaler
InactiveUS8037881B2Increase resistanceReduce resistanceRespiratorsLiquid surface applicatorsMedicineBlisters
Owner:PENTAFRAGAS DIMITRIOS
Synthetic jet based medicament delivery method and apparatus
A dry powder inhaler consisting of first chamber having an orifice for holding a dry powder and a gas, and a second chamber for receiving a deaggregated form of the dry powder and for communicating the deaggregated dry powder to a user. A synthetic jet drives the dry powder from the first chamber to the second chamber.
Owner:MICRODOSE THERAPEUTX INC
Particulate drug-containing products and method of manufacture
InactiveUS7125566B2Improve featuresEfficiently aerosolizedBiocidePowder deliveryParticulatesOrganic solvent
Provided is a compressed anti-solvent technique for manufacture of drug-containing powders for pulmonary delivery. The drug is processed in a cosolvent system including two or more mutually soluble organic solvents. Also provided are powders manufacturable by the manufacture method, including powders of substantially pure drug and powders including a biocompatible polymer for pulmonary sustained drug release applications. Also provided are packaged products including drug-containing powder in a container that is receivable by and operable with a dry powder inhaler to produce an aerosol including dispersed drug-containing particles when the inhaler is actuated.
Owner:ENDO PHARMA COLORADO
Methods and systems for dosing and coating inhalation powders onto carrier particles
A method of method of coating powdered medical agent onto a carrier particle for use in a dry powder inhaler may include applying ultrasonic energy to agglomerated powdered medical agent to deaggregate and aerosolize particles of the medical agent into particles having a desired average particle size, and coating at least one carrier particle with a desired amount of the deaggregated and aerosolized particles of the medical agent.
Owner:STC UNM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com