Patents
Literature
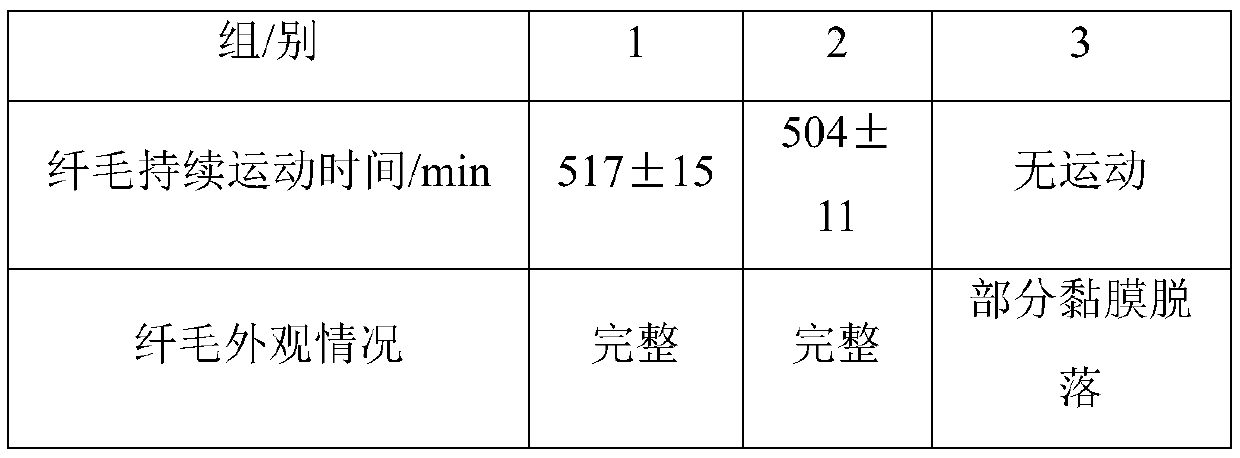
66 results about "Inhalation powder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
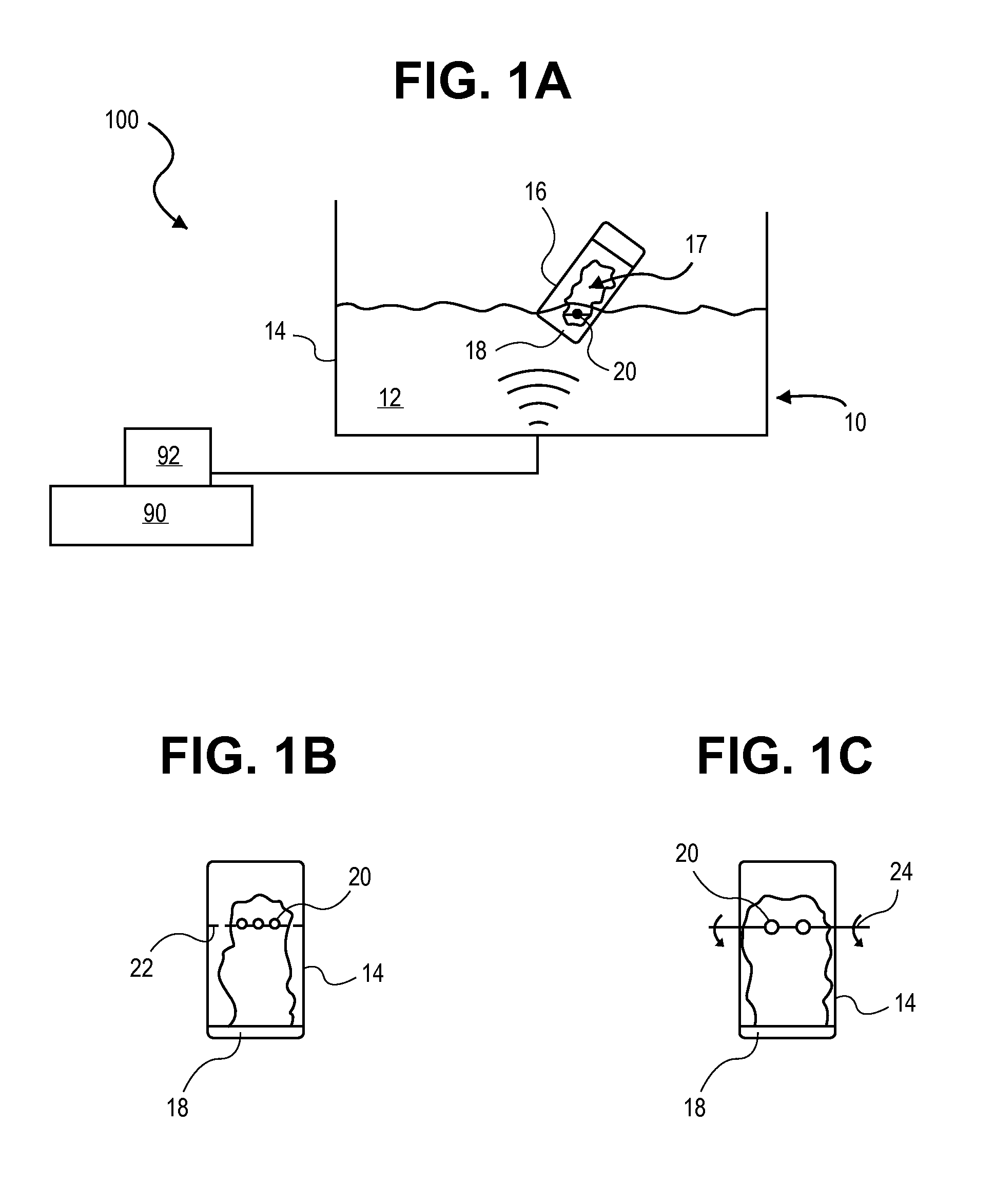
Methods and systems for dosing and coating inhalation powders onto carrier particles
A method of method of coating powdered medical agent onto a carrier particle for use in a dry powder inhaler may include applying ultrasonic energy to agglomerated powdered medical agent to deaggregate and aerosolize particles of the medical agent into particles having a desired average particle size, and coating at least one carrier particle with a desired amount of the deaggregated and aerosolized particles of the medical agent.
Owner:STC UNM
Salts of the CGRP antagonist BIBN4096 and inhalable powdered medicaments containing them
InactiveUS6900317B2Improve solubilityHigh activityPeptide/protein ingredientsDipeptidesBrominePiperazine
The invention relates to salts of the active substance base 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine [BIBN4096] of formula I, the preparation thereof, a process for preparing an inhalation powder containing a salt of the active substance BIBN4096 as well as the inhalation powders which can be obtained by the process.
Owner:BOEHRINGER INGELHEIM PHARMA KG
Salts of the CGRP antagonist BIBN4096 and inhalable powdered medicaments containing them
InactiveUS20030191068A1Hormone peptidesOrganic active ingredientsPyridinylpiperazinePharmaceutical Substances
The invention relates to salts of the active substance base 1-[N<2>-[3,5-dibromo-N-[[4-(3,4-dihydro-2(1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine[BIBN4096] of formula I, the preparation thereof, a process for preparing an inhalation powder containing a salt of the active substance BIBN4096 as well as the inhalation powders which can be obtained by the process.
Owner:BOEHRINGER INGELHEIM PHARM KG
Inhalation powder containing the CGRP antagonist BIBN4096 and process for the preparation thereof
The invention relates to an inhalation powder for treating migraine, containing the CGRP antagonist 1-[N<2>-[3,5-dibromo-N-[[4-(3,4-dihydro-2(1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine [BIBN4096] of formula I as the active substance base in the form of spherically nanostructured microparticles, and a process for the manufacture thereof.
Owner:BOEHRINGER INGELHEIM PHARM KG
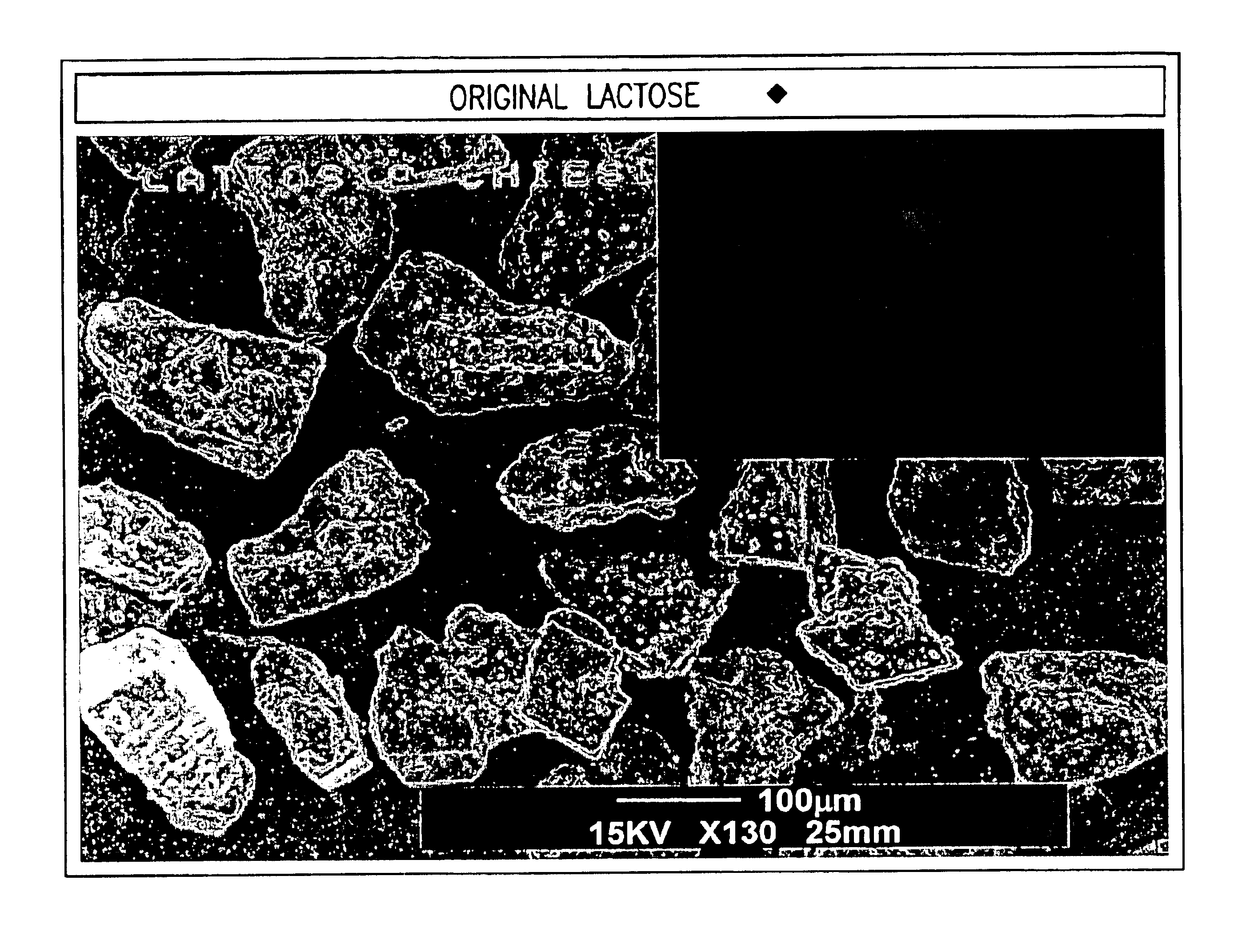
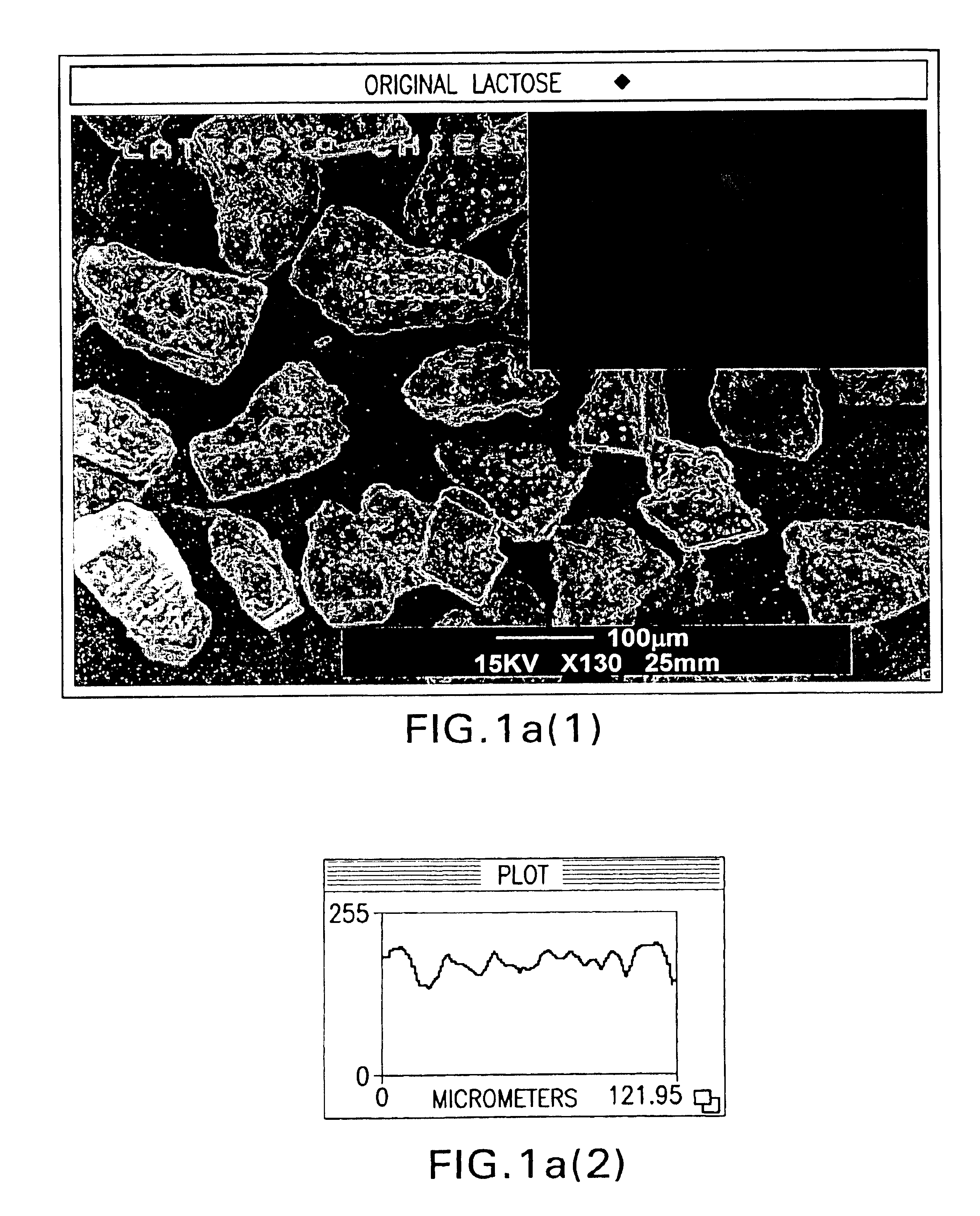
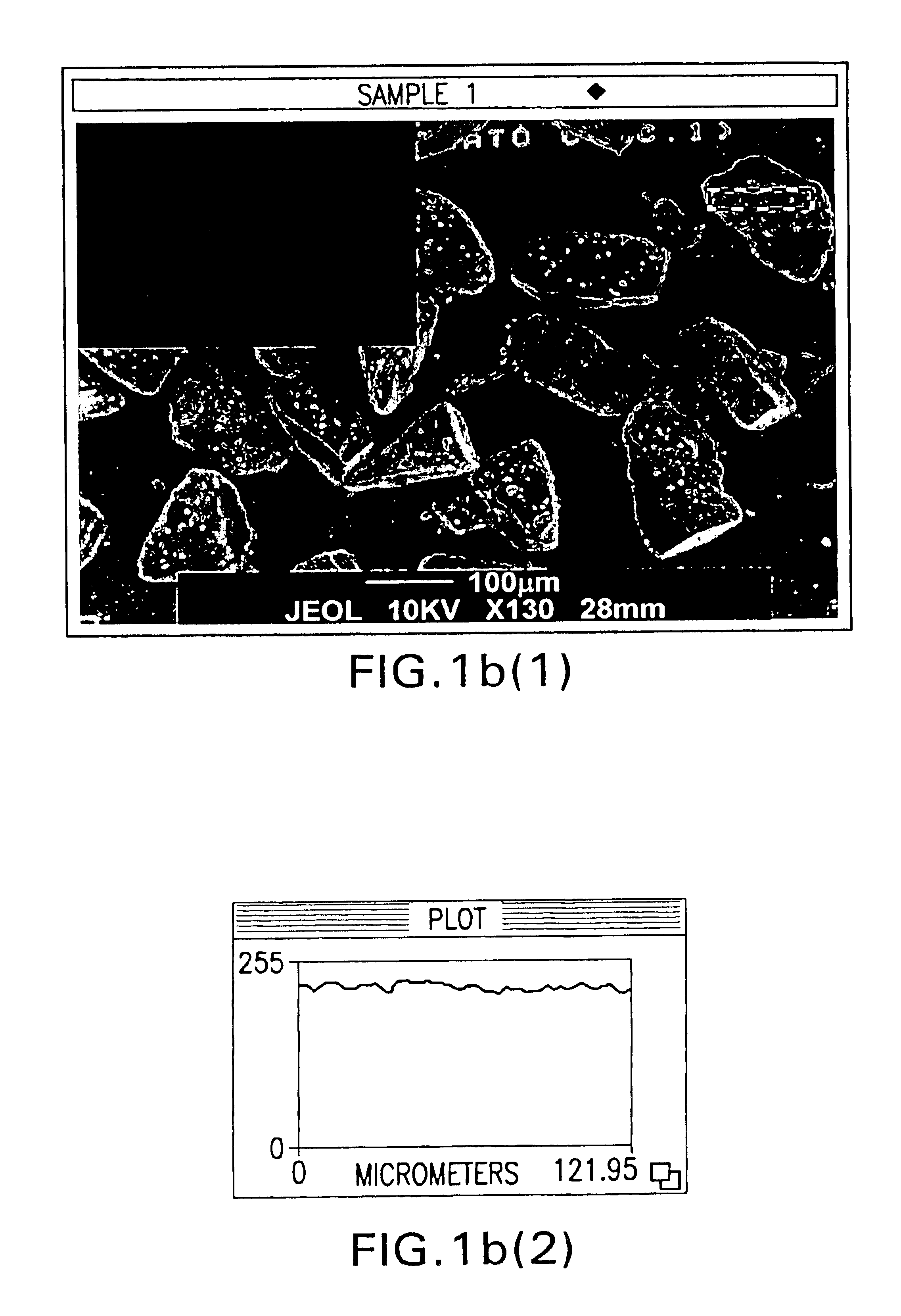
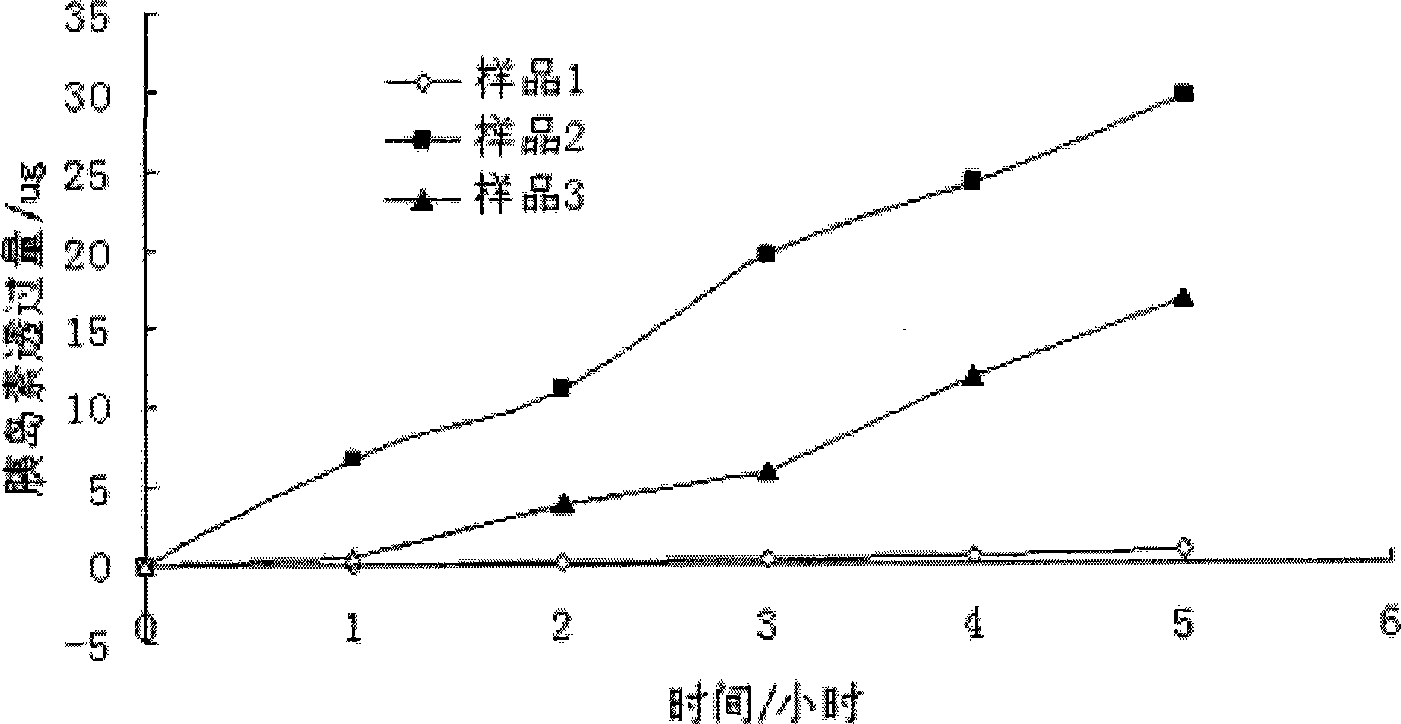
Powder particles with smooth surface for use in inhalation therapy
InactiveUS6780508B1Change surface propertiesChange propertiesPowder deliveryBiocidePowder InhalerInhalation powder
Carriers for use in the preparation of mixtures for inhalation powders intended for pulmonary administration of micronized drugs by means of a dry powder inhaler and the method for their preparation are described.
Owner:CHIESI FARM SPA
Insulin intranasal inhalation powder spray
InactiveCN101428009AImprove stabilityImprove bioavailabilityPeptide/protein ingredientsMetabolism disorderNasal cavityLymphatic vessel
The invention provides insulin nasal dry powder inhalation which comprises the following components by contents (weight percentages): 1% to 100% of insulin freeze-dry powder with self emulsifying effect and 0% to 99% of carrier. In the insulin nasal dry powder inhalation, the dosage of grease is determined according to the surface area and the grain size of grease drops, and a large quantity of animal experiments prove that under the condition and in the proportion, the optimal drug treatment effect can be achieved. Compared with the liquid preparation, the stability of the dry powder is increased, and the dry powder can be automatically re-dissolved into nano-sized emulsion after being in contact with water; after the drug-containing compound enters the nasal cavity, the nano-size emulsion easily passes by the barrier of the nasal mucosa and enters the body via the rich capillaries and lymphatic vessels in the nasal mucosa to exert the efficacy, thereby remarkably improving the bioavailability of the drug and being rapidly absorbed, without stimulation to the nasal mucosa; in addition, the adoption of bio-adhesive increases the retention time of the drug-containing powder on the nasal mucosa, so that the absorption and the utilization of the drug are more complete.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Method for treating carrier particles and its use
InactiveUS20060025326A1Easy to separateEfficiently deliberatePowder deliveryPeptide/protein ingredientsParticulatesLiquid medium
A method for treating a particulate carrier for an inhalation powder improving the stability and flowing properties of the carrier. The carrier is abraded suspended in a liquid medium, in which the carrier is essentially insoluble, the liquid medium is evaporated and the carrier recovered.
Owner:LAB PHARMA LTD
Salts of the CGRP antagonist BIBN4096 and inhalable powdered medicaments containing them
InactiveUS20050147568A1Improve solubilityHigh activityHormone peptidesOrganic active ingredientsPyridinylpiperazinePiperazine
The invention relates to salts of the active substance base 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2-(1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine [BIBN4096] of formula I, the preparation thereof, a process for preparing an inhalation powder containing a salt of the active substance BIBN4096 as well as the inhalation powders which can be obtained by the process.
Owner:BOEHRINGER INGELHEIM PHARMA GMBH & CO KG
Ribavirin inhalation powder atomizing agent and its preparing process and anti-virus infection use
The Ribavirin inhalation is a kind of respiratory tract mucous membrane absorbed preparation comprising superfine Ribavirin powder and fine medicinal carrier powder and is administrated with special administrating device. It has high and fast target effect, safe antiviral function, no stimulation to mucous membrane and other advantages. The present invention further expands the clinical application range of Ribavirin.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Antibiotic microparticles for inhalation
Powder formulations for inhalation which comprise microparticles containing an antibiotic and magnesium stearate are useful for the treatment of bacterial infections associated with certain pulmonary diseases.
Owner:CHIESI FARM SPA
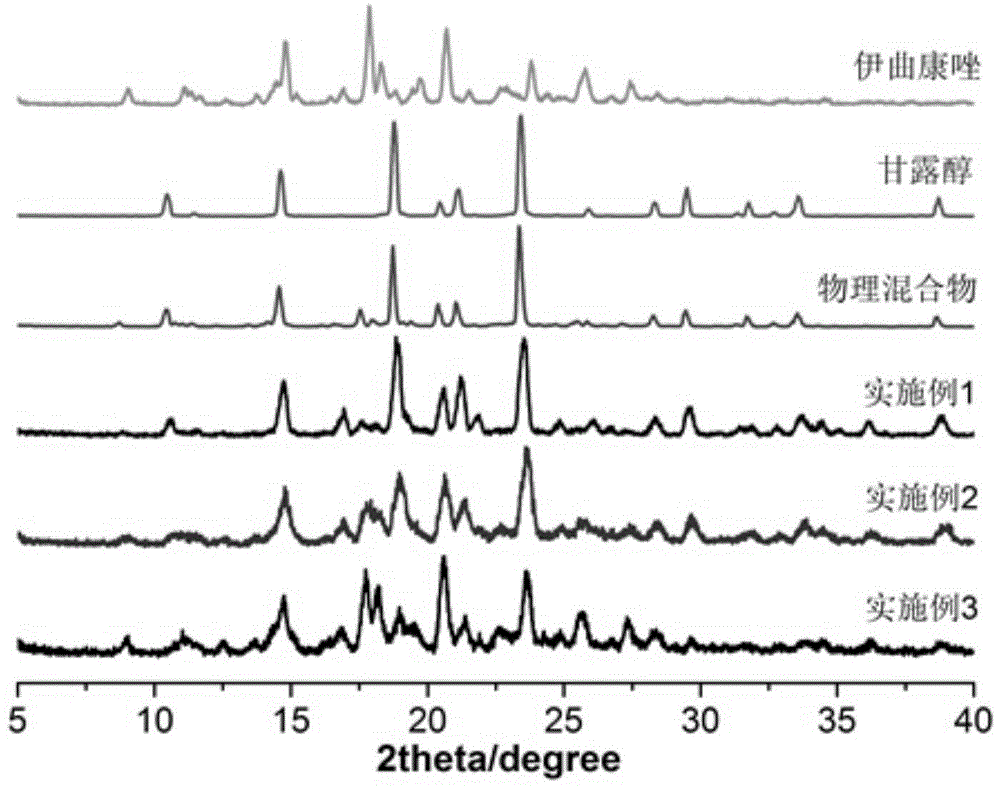
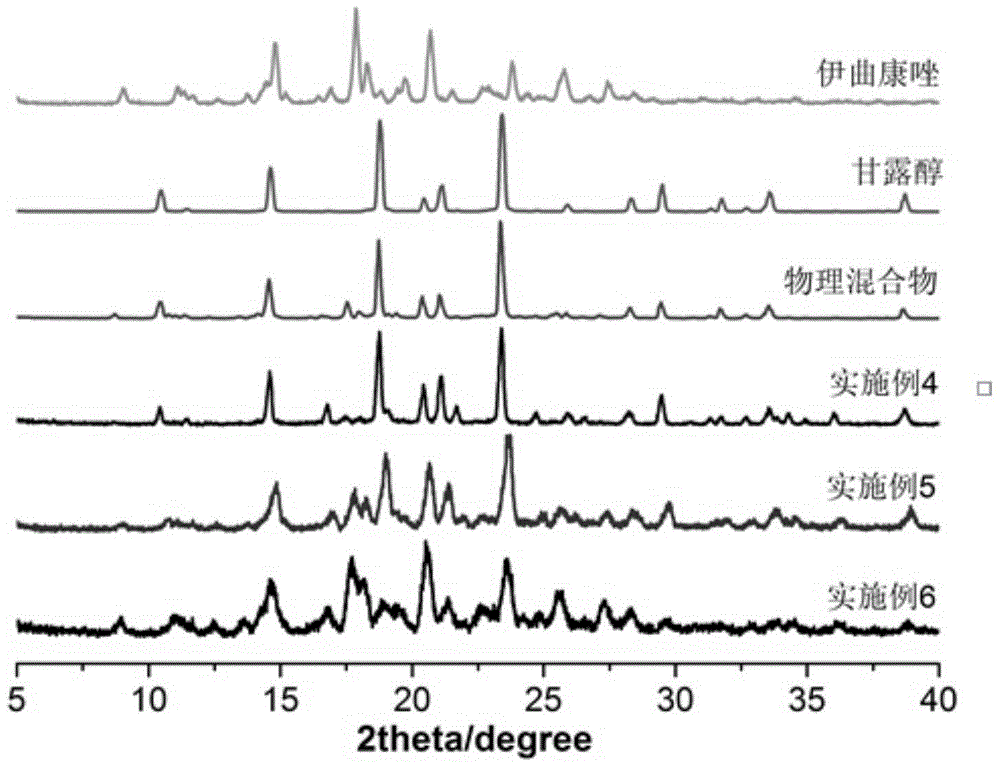
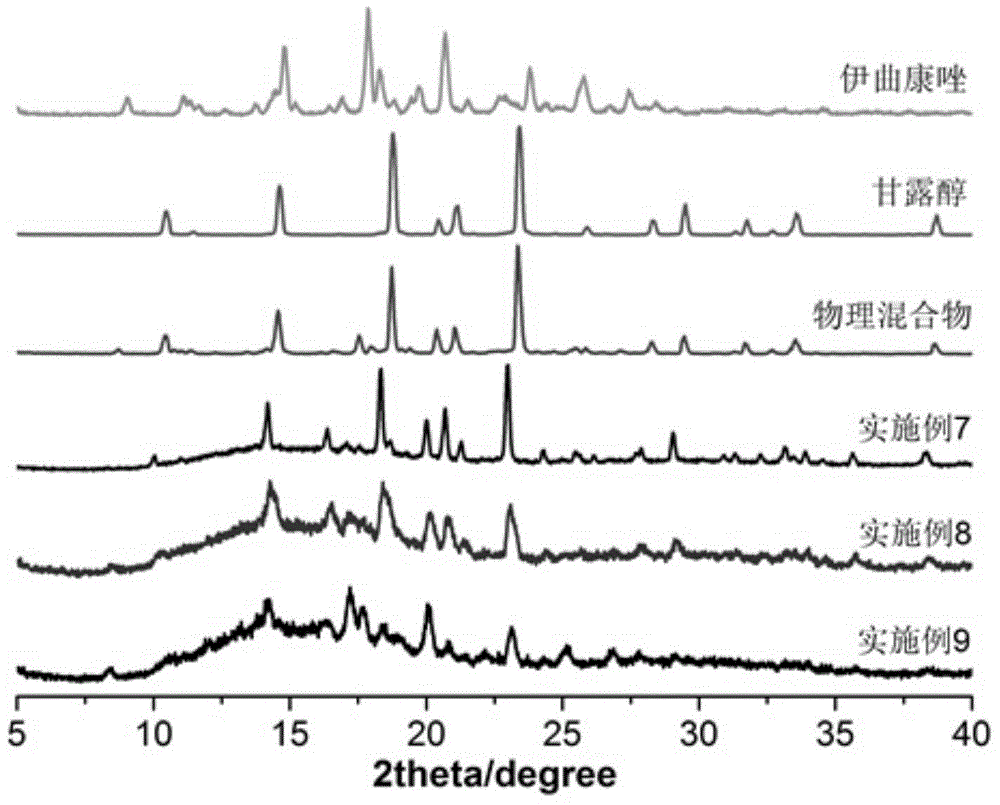
Itraconazole inhalation powder aerosol and its preparation method
ActiveCN104398497AImprove physical stabilityGood fluidity of powderOrganic active ingredientsAntimycoticsSide effectItraconazole
The invention discloses an itraconazole inhalation powder aerosol and its preparation method. The inhalation powder aerosol is prepared by using itraconazole and 20-80mass% of a carrier, and the carrier is at least one of lactose, mannitol, inulin and amino acids. The method comprises the following steps: mixing itraconazole with the carrier, adding the obtained mixture into a double screw hot melt extruder, setting the extrusion temperature at 150-170DEG C, starting screws after the preset temperature is reached, and carrying out strip extrusion under a screw rotating speed of 150-200r / min, cooling obtained strips in a dryer to room temperature, crushing by using a micro pulverization machine, and micronizing by using an airflow crusher to obtain the above powder. The inhalation powder aerosol has the advantages of good physical stability, good powder fluidity, high deposition amount at effective positions, high dissolution rate, fast dissolution speed, small drug dose, and low incidence rate of toxic side effects; and the method has the advantages of simple technology, no organic solvents, antiseptics or other impurities in the process, good reappearance and easy industrial production.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
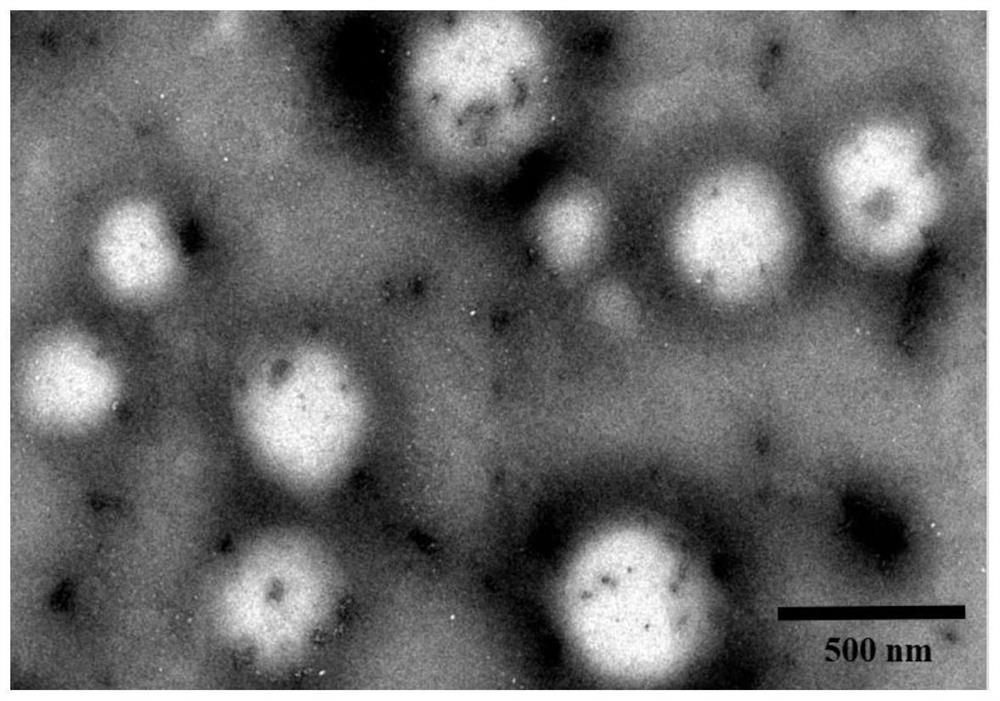
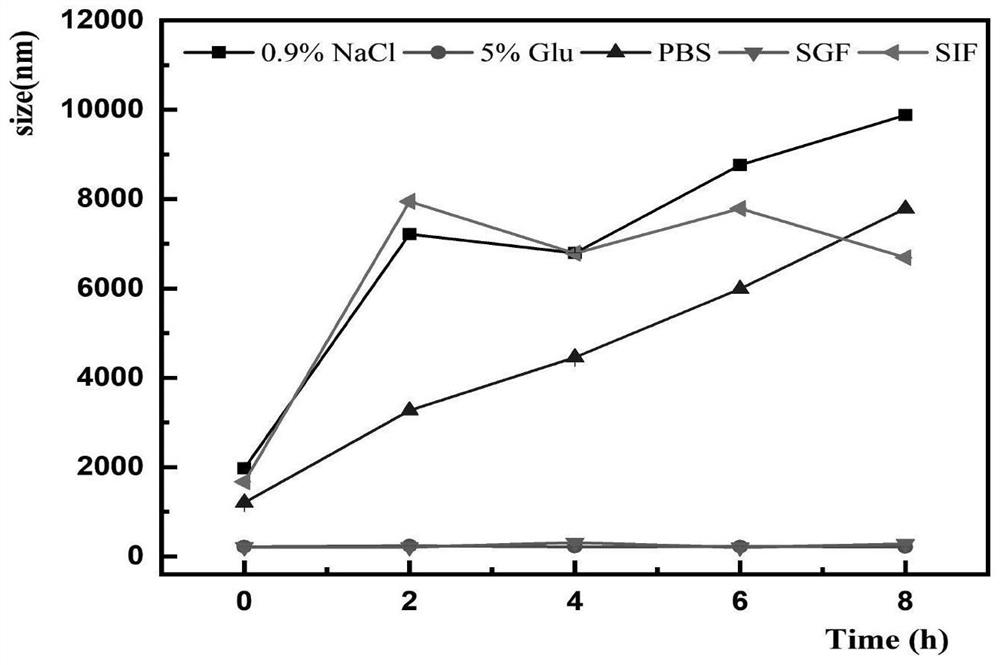
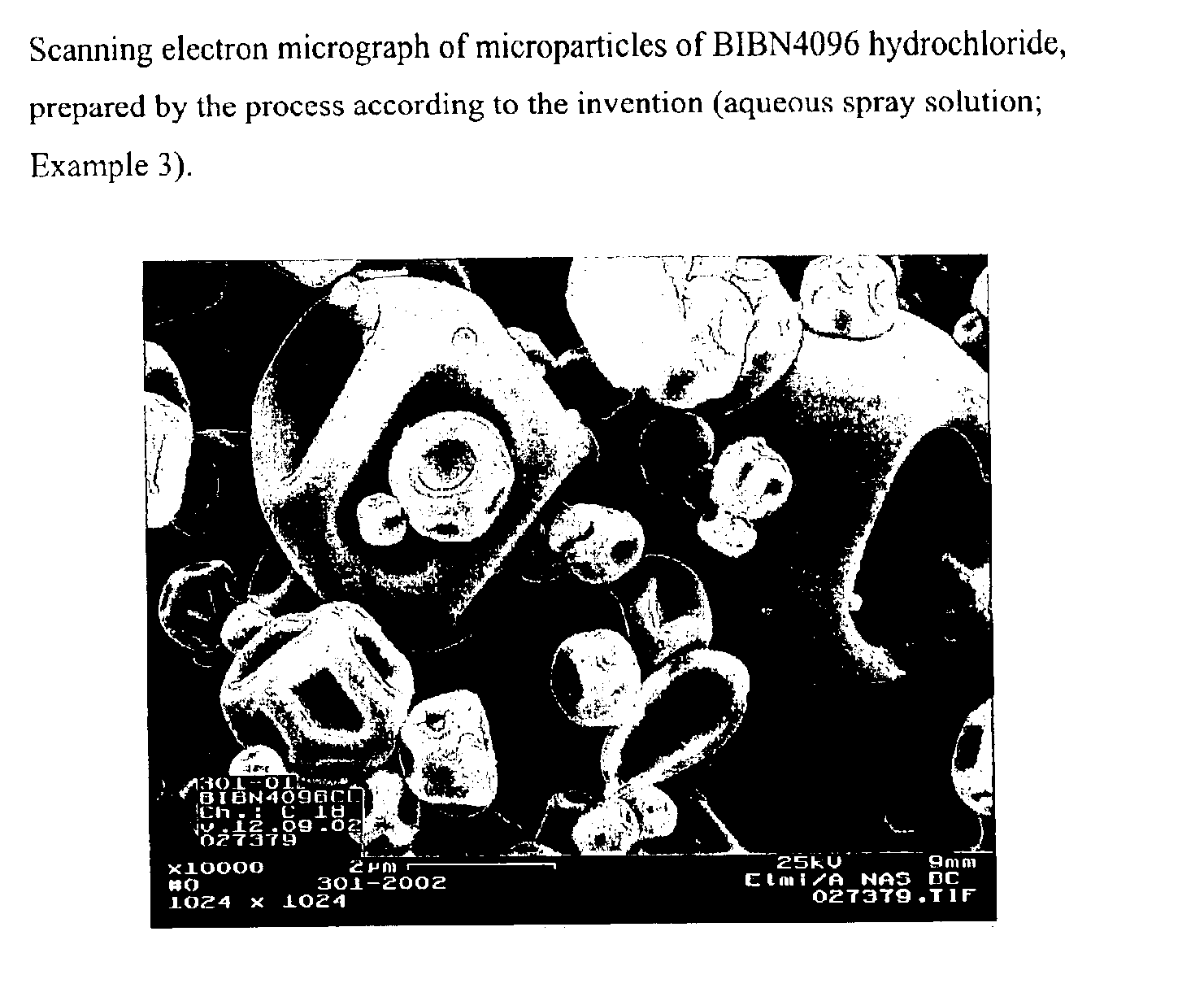
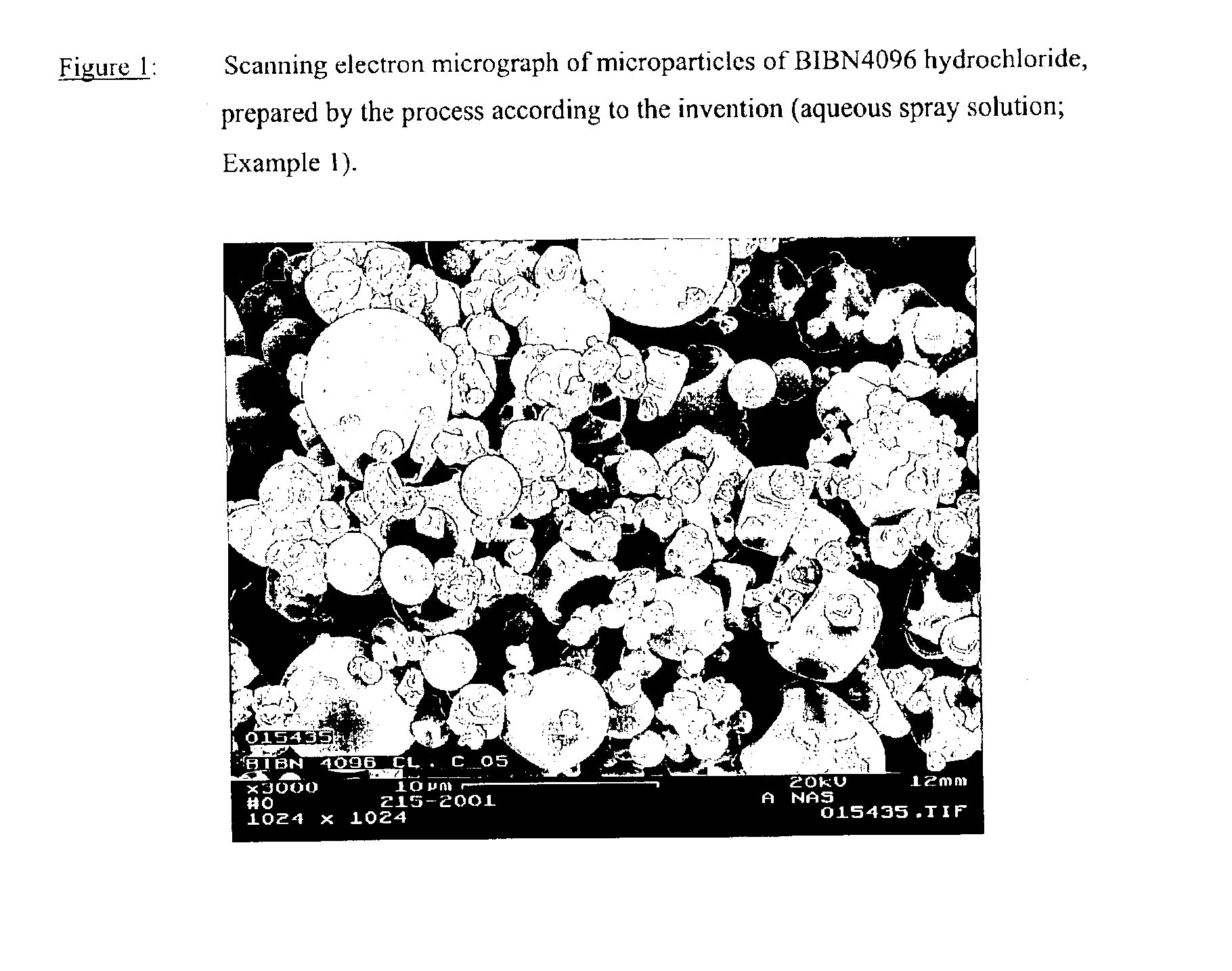
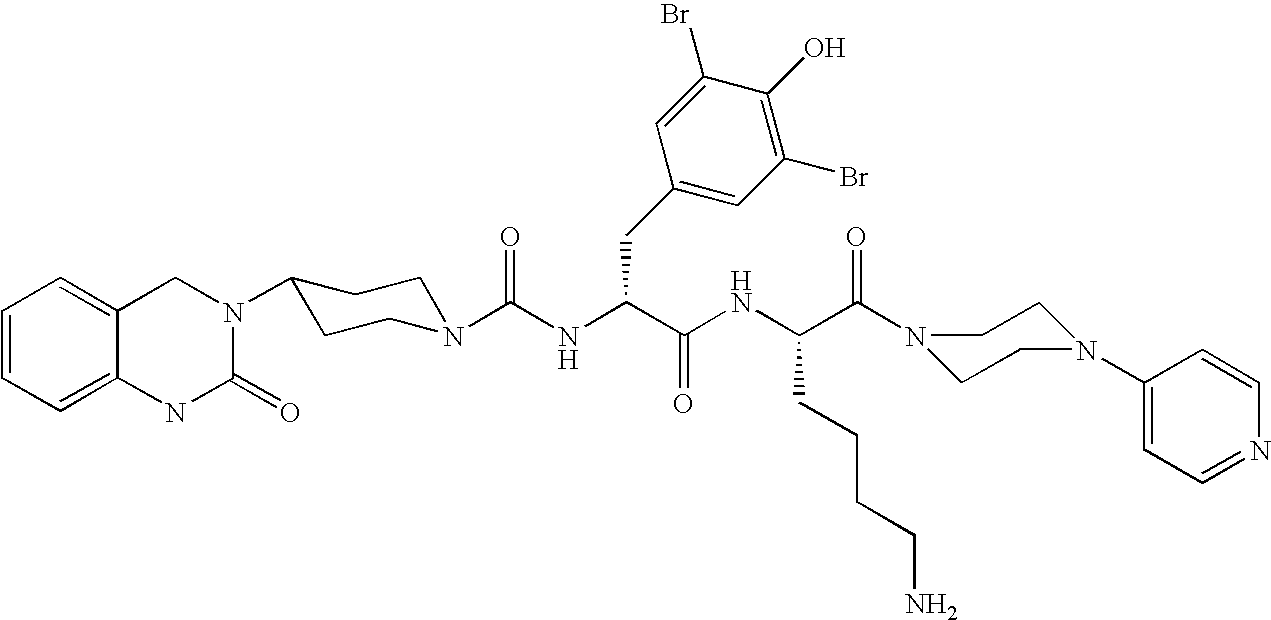
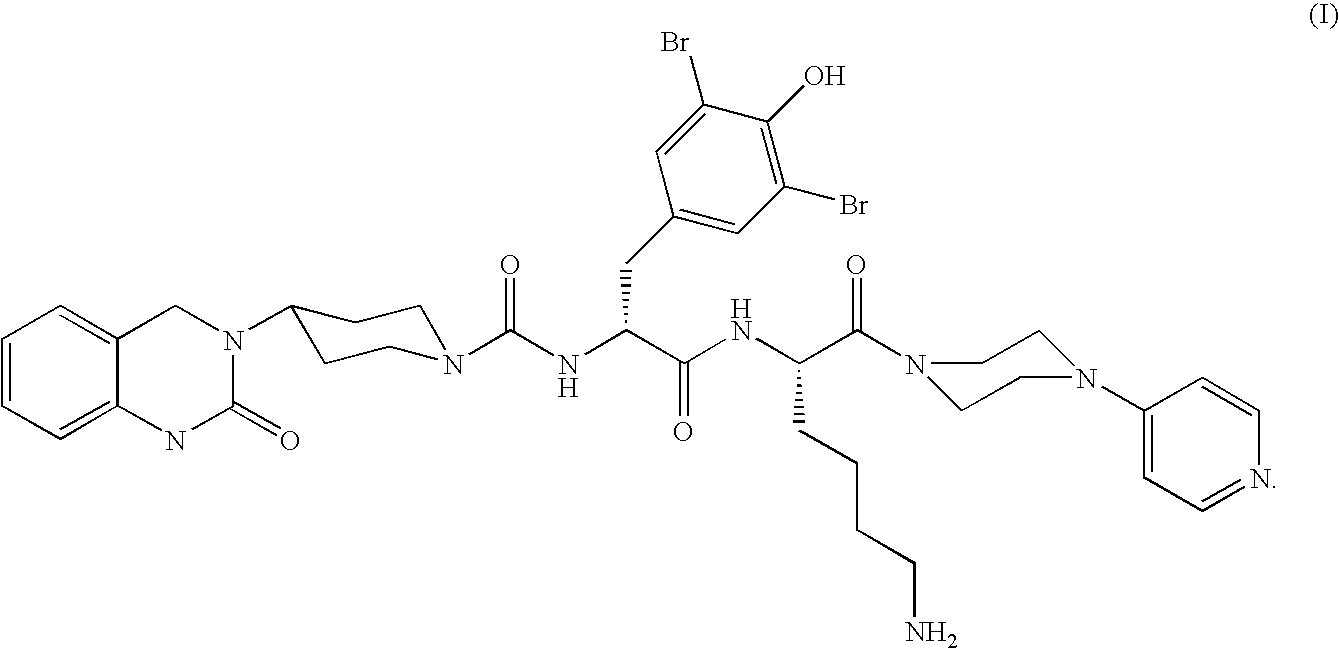
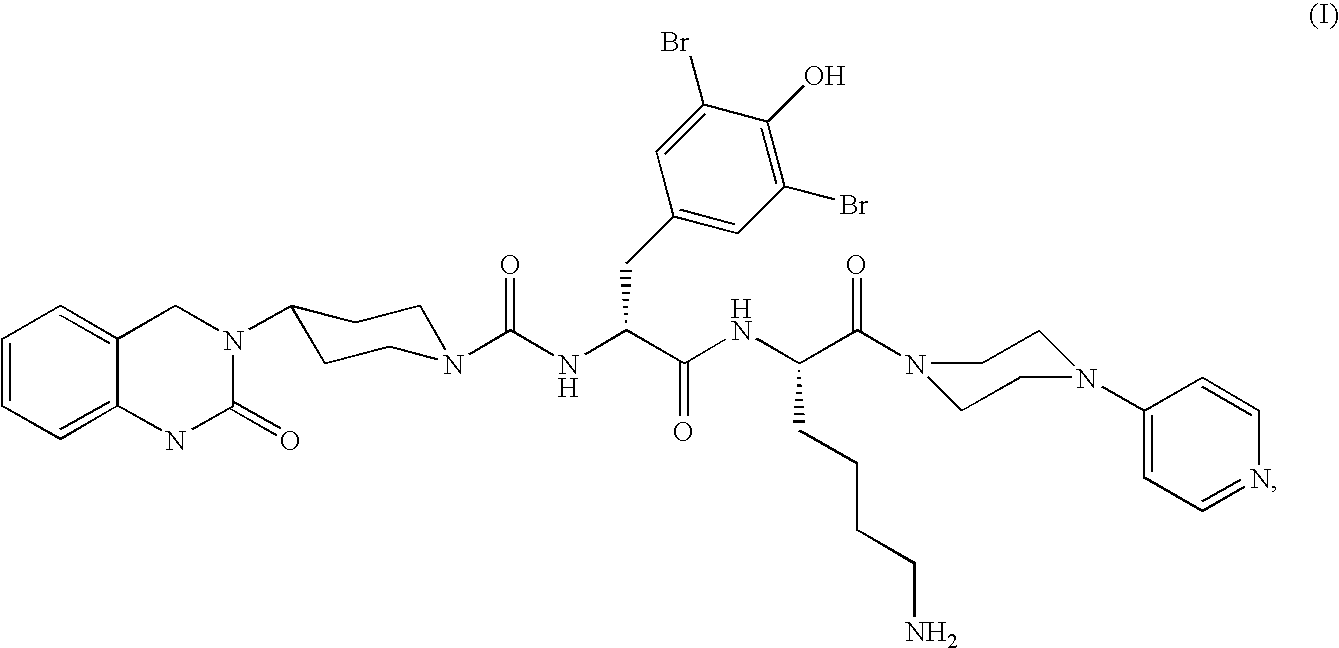
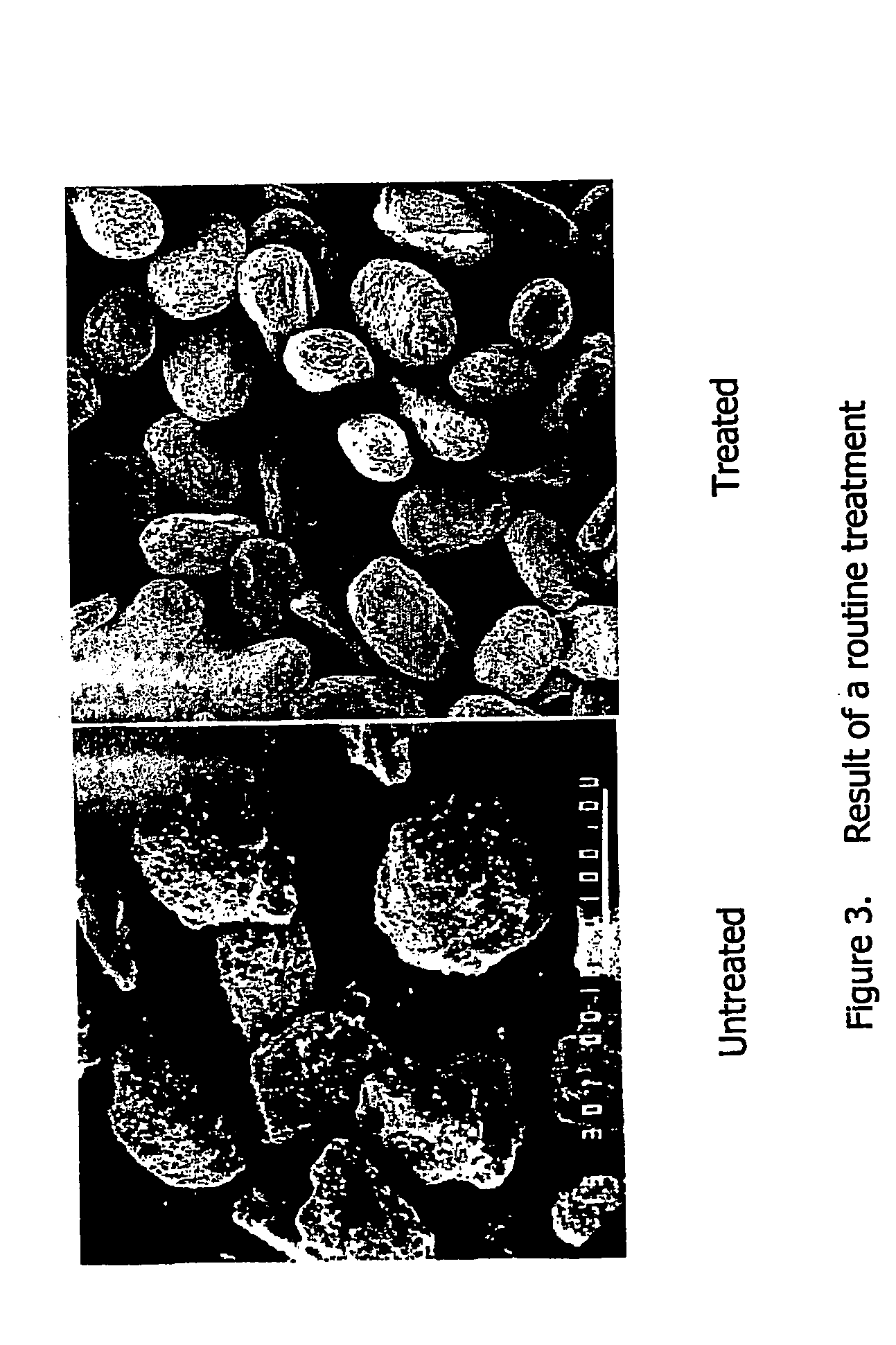
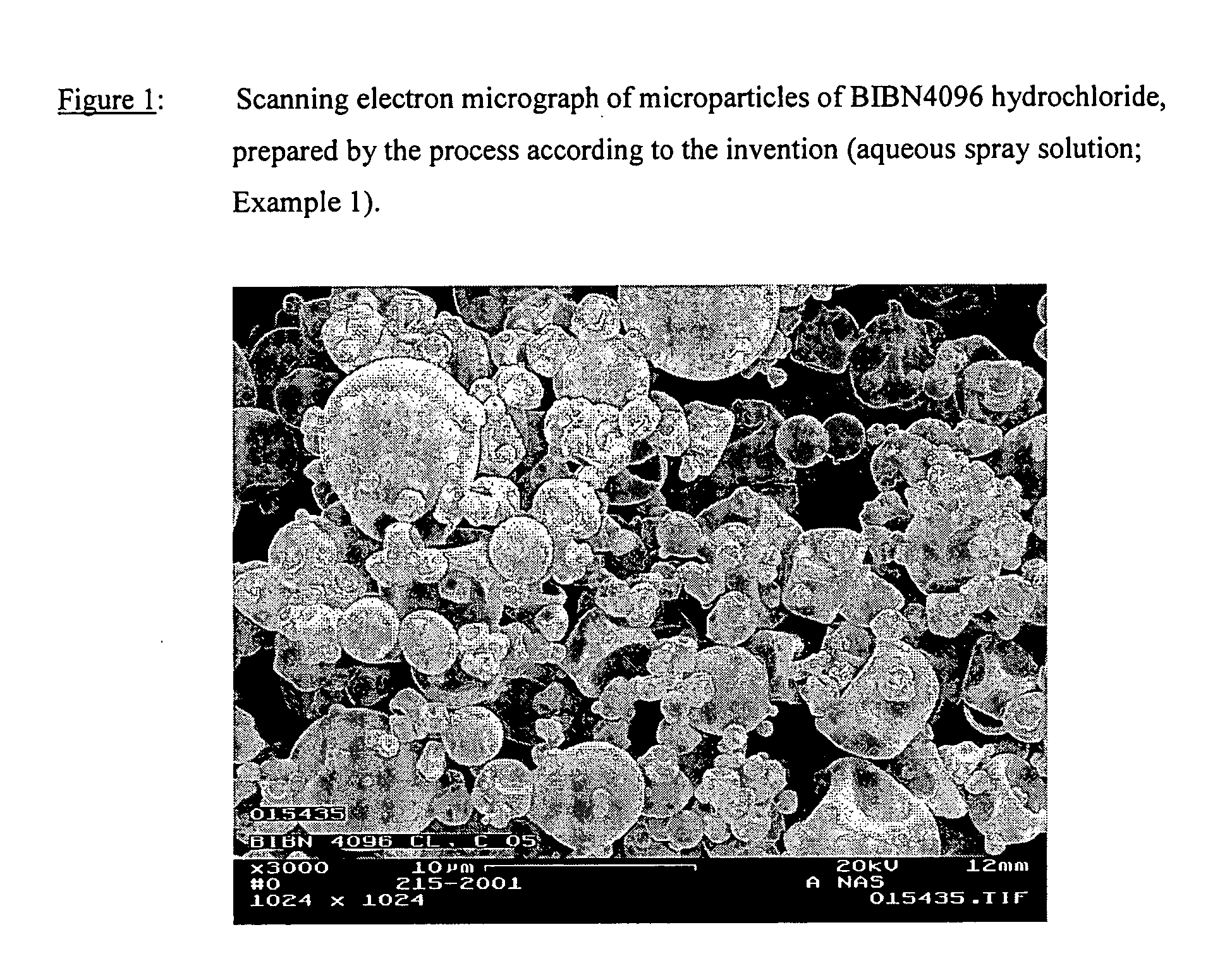
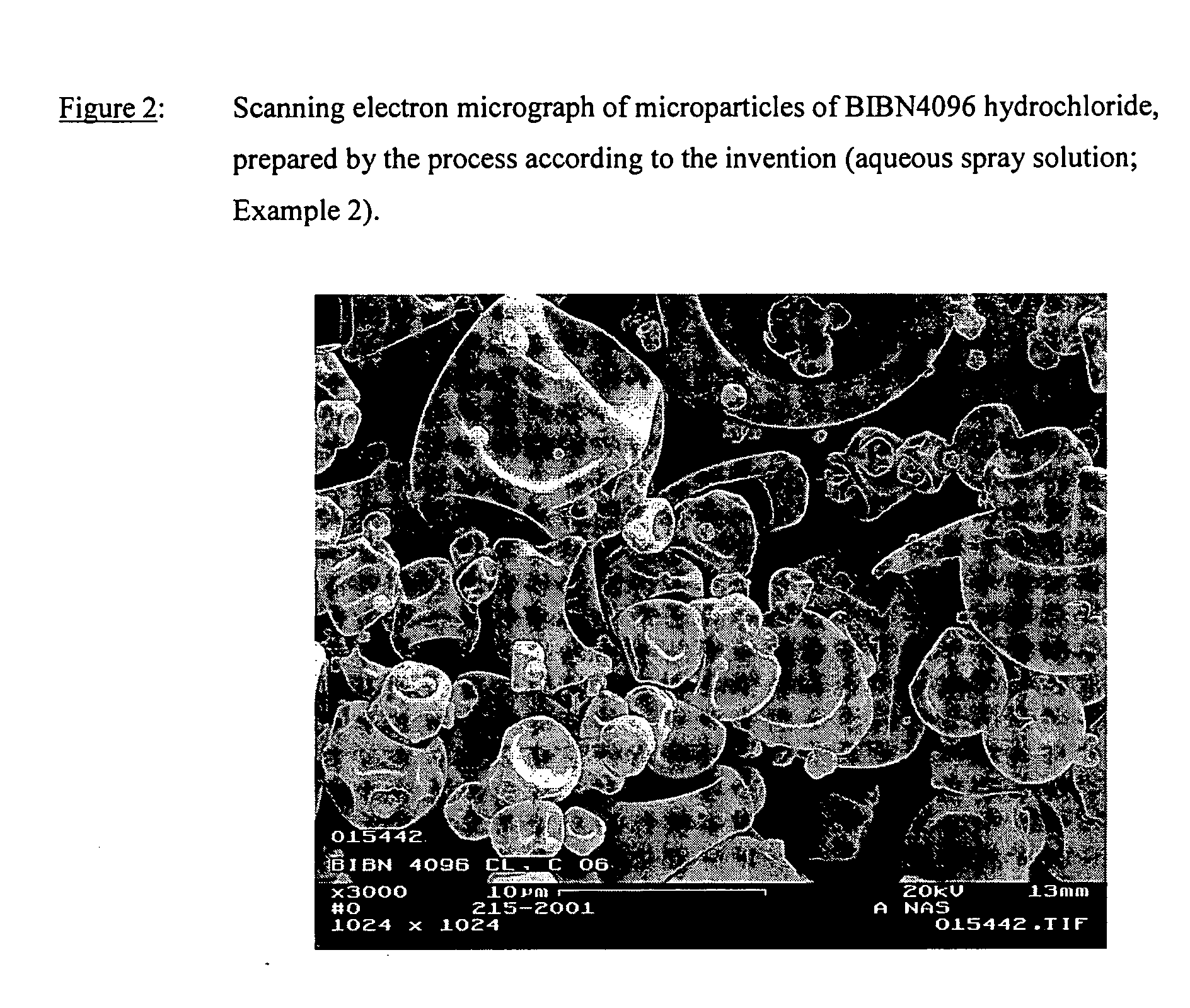
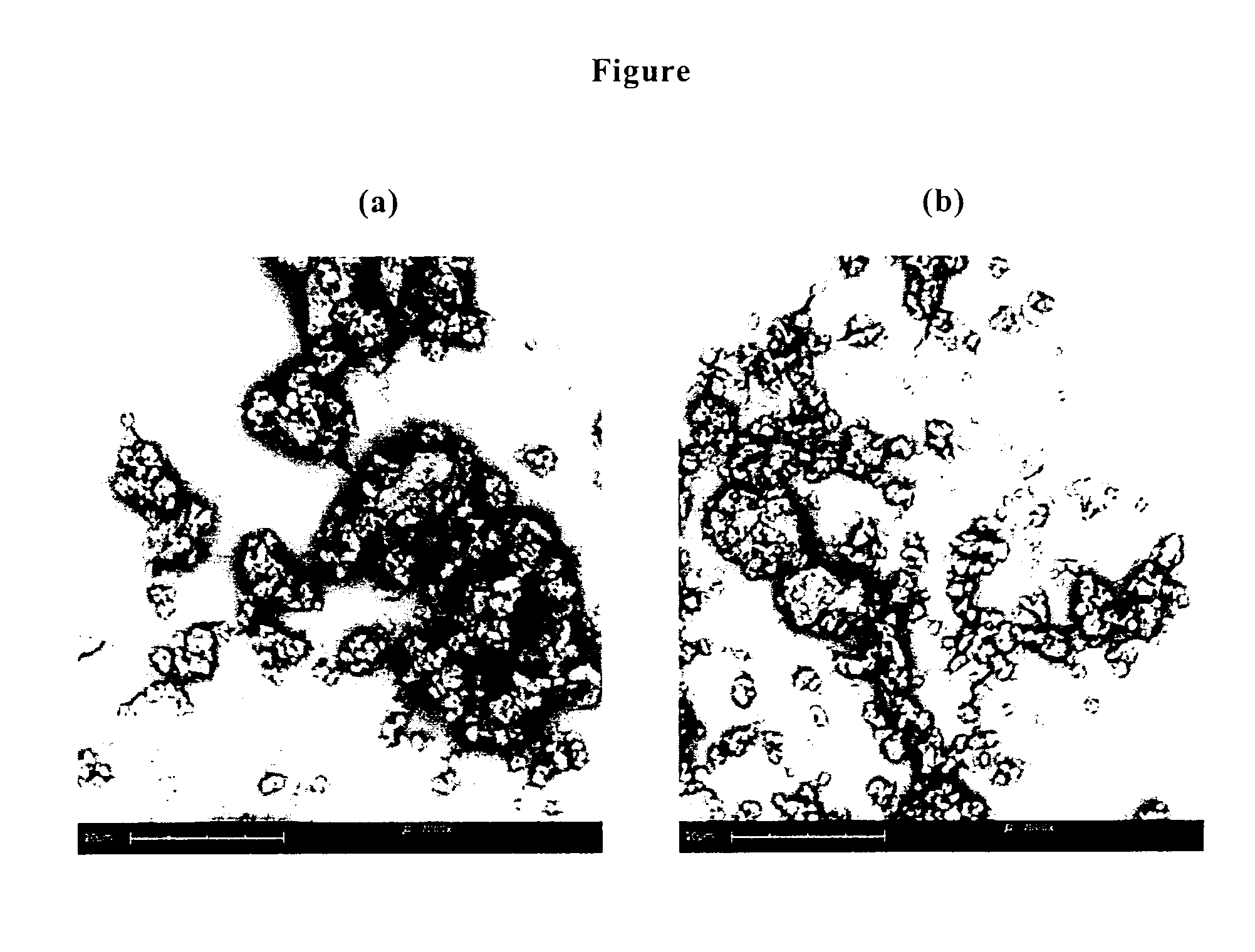
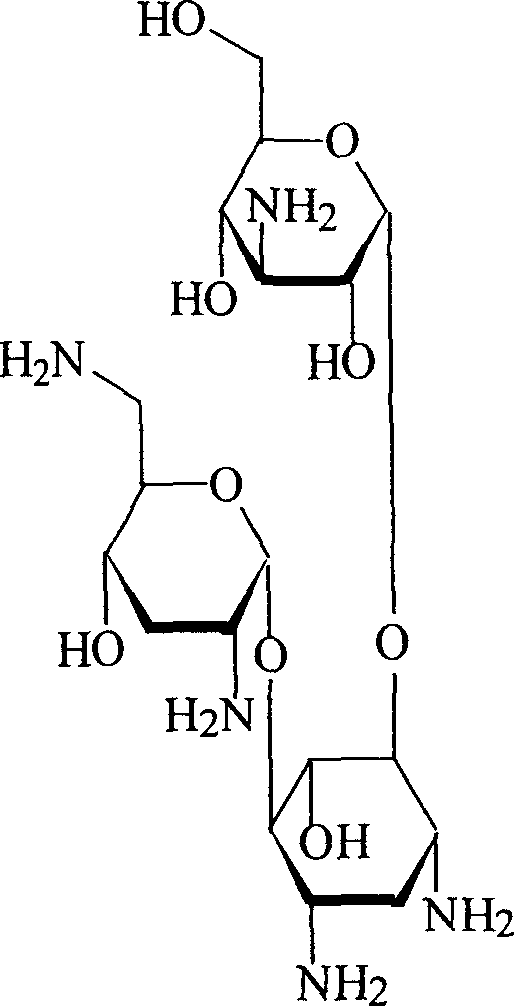
Microparticles containing the CGRP-antagonist 1-[N2-[3,5-dibrom-N-[[4-(3,4-dihydro-2(1H)-oxoquinazoline-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine, process for preparing and the use thereof as inhalation powder
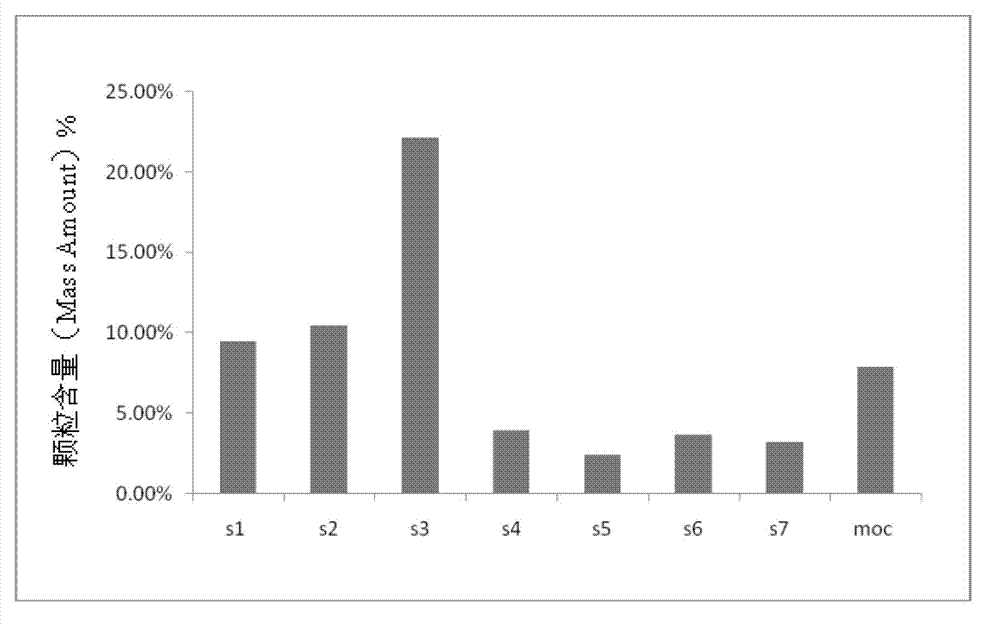
The invention relates to inhalable powders in the form of stable, spray-dried microparticles (embedding particles) for pulmonary or nasal inhalation, containing the CGRP antagonist 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine (A) or a physiologically acceptable salt thereof and one or more excipients, processes for preparing such microparticles and the use thereof for preparing a powder inhalant for the treatment of headaches, migraine and cluster headache.
Owner:BOEHRINGER INGELHEIM INT GMBH
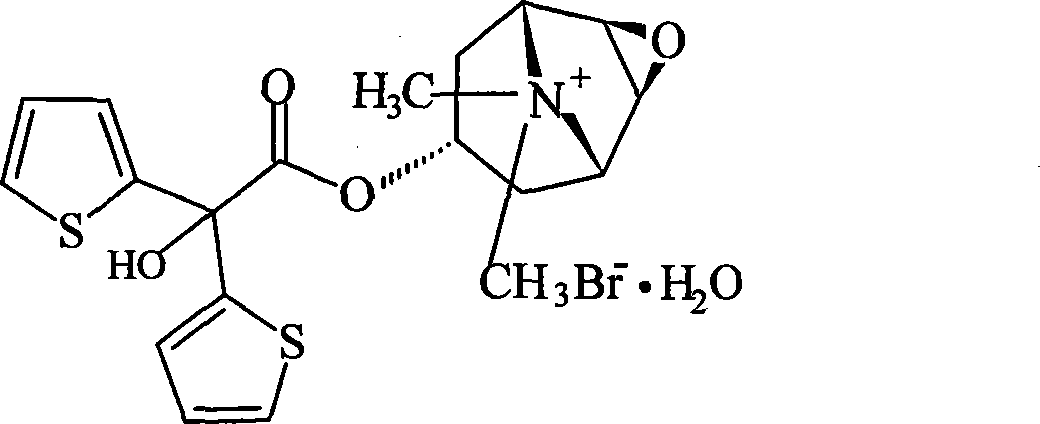
Capsule type tiotropium bromide inhalation powder
ActiveCN101032484AGuaranteed content uniformitySimple processPowder deliveryAerosol deliveryActive componentTIOTROPIUM BROMIDE MONOHYDRATE
The present invention discloses one kind of tiotropium bromide capsule atomized powder preparation, which includes tiotropium bromide or tiotropium bromide monohydrate in 0.04-1.5 wt% and fine lactose powder of size smaller than 15 microns for adsorbing tiotropium bromide or tiotropium bromide monohydrate. The present invention also provides the preparation process of the atomized powder preparation. The tiotropium bromide capsule atomized powder preparation has simple preparation process, excellent flowability and homogeneously distributed active component.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Netilmicin sulfate inhalation powder and preparation method thereof
ActiveCN102949379ANo first pass effectPromote absorptionAntibacterial agentsOrganic active ingredientsSolubilitySide effect
The invention discloses netilmicin sulfate inhalation powder and a preparation method thereof. The powder consists of the following raw materials:, namely netilmicin sulfate or a mixture of the netilmicin sulfate and an amino acid glidant, wherein the weight ratio of the netilmicin sulfate to the amino acid glidant is 10-30:1. The method has the advantages that the preparation technology is simple, no organic solvent or preservative is used in the preparation process, the flowability of the prepared powder is greatgood, the deposit rate at effective parts is high, and the problems that the netilmicin sulfate has high water solubility and cannot be absorbed when isbeing orally taken are solved; and meanwhile, as the inhalation powder is used as a medicine performing a local function, the dosage of administration is obviously reduced, and the occurrence rate of side effects related to an aminoglycoside dosage, such as ototoxicity and renal toxicity is reduced.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
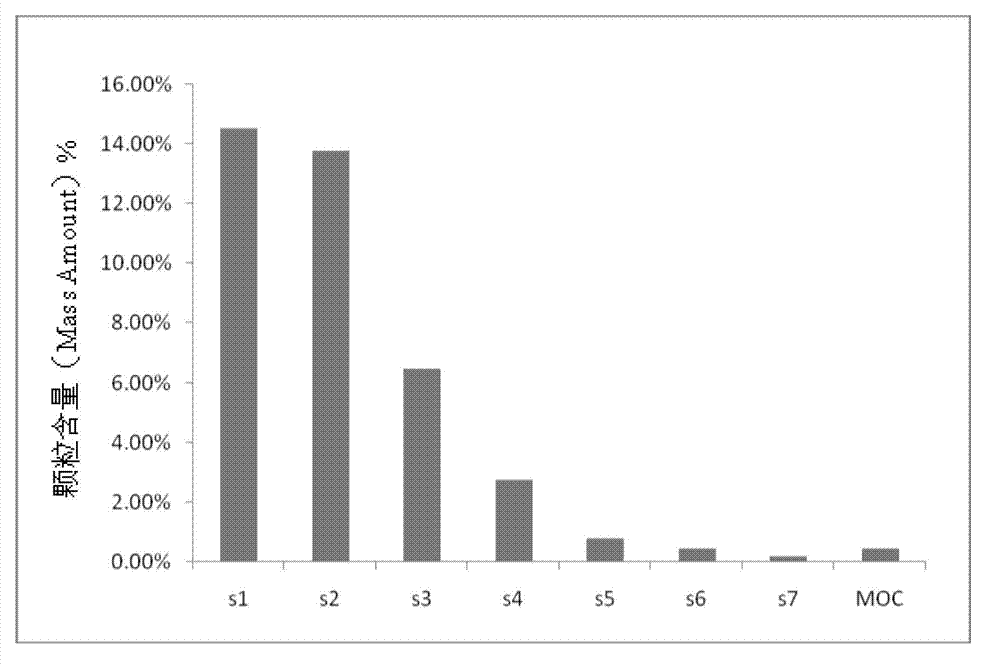
Quality control method for insulin inhalation powder
InactiveCN102087249AImprove securityGood curative effectPeptide/protein ingredientsComponent separationDrug contentRetention time
The invention provides a quality control method for insulin inhalation powder and the method comprises steps of: (1) determining the property of insulin inhalation powder contents; (2) discriminating with High Performance Liquid Chromatography (HPLC): in the chromatogram recorded according to a potency determination item, the retention time of the main peak of a tested object should be is consistent with the retention time of the main peak of a recombinant human insulin conference object and blank accessories do not interfere determination; (3) inspecting associated protein, content uniformity, the total inhalation times of each bottle, the main drug contents of each bottle, evacuation rates and the distribution of powder granules and determining that products are in accord with rules; (4) performing potency determinations on the products and determining the qualities of the products. According to the invention, a research on a quality control method for insulin inhalation powder is conducted, and effectively guarantees the security and curative effects of medicaments; the quality control method is advantageous with respect to a potency determination method with high precision, good reappearance and high stability; and moreover the inspection and discrimination methods can ensure the curative effects of the medicaments and realize the purpose of effectively controlling the qualities of the medicaments.
Owner:CHINA NAT ACAD NANOTECH & ENG
Nebcin inhalation powder mist agent and its preparation method
A powder inhalant of gernebcin for treating the pseudomonads infection to lung of the cystic fibrosis patient is prepared from the superfine gernebcin powder and superfine medical carrier through proportionally mixing and loading in container. Its advantage is high safety and curative effect.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
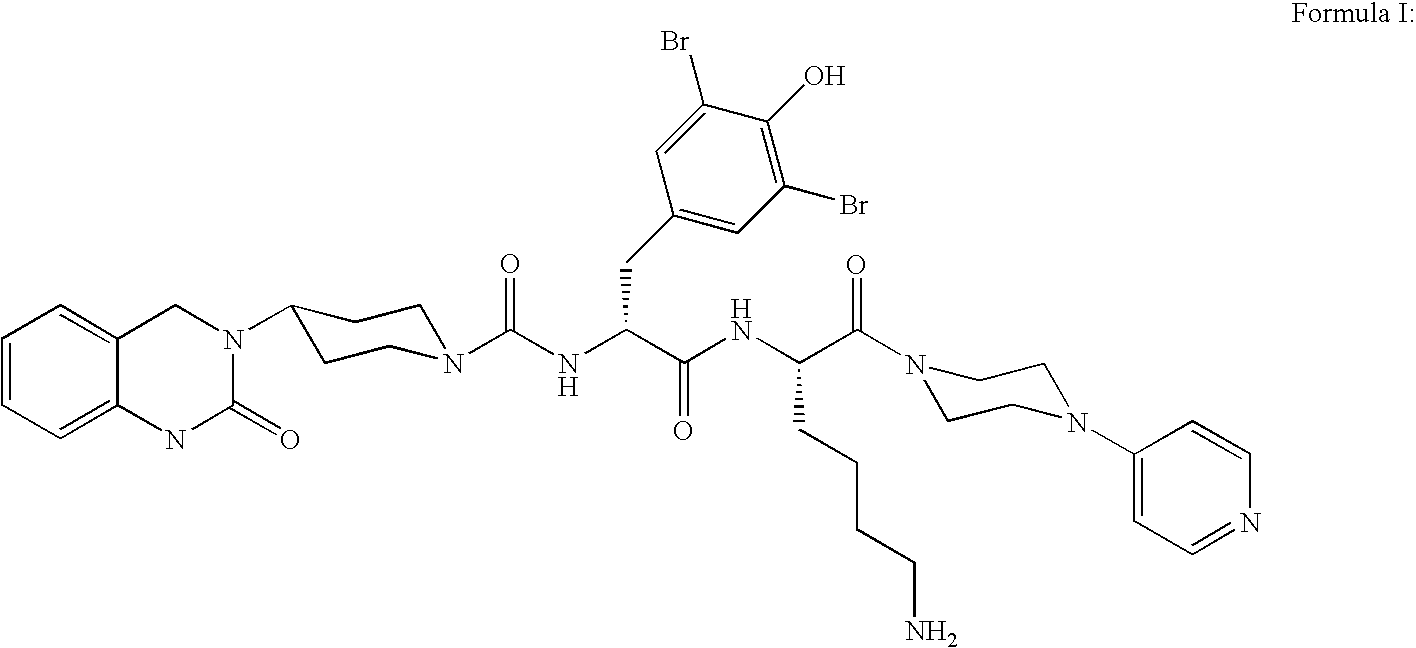
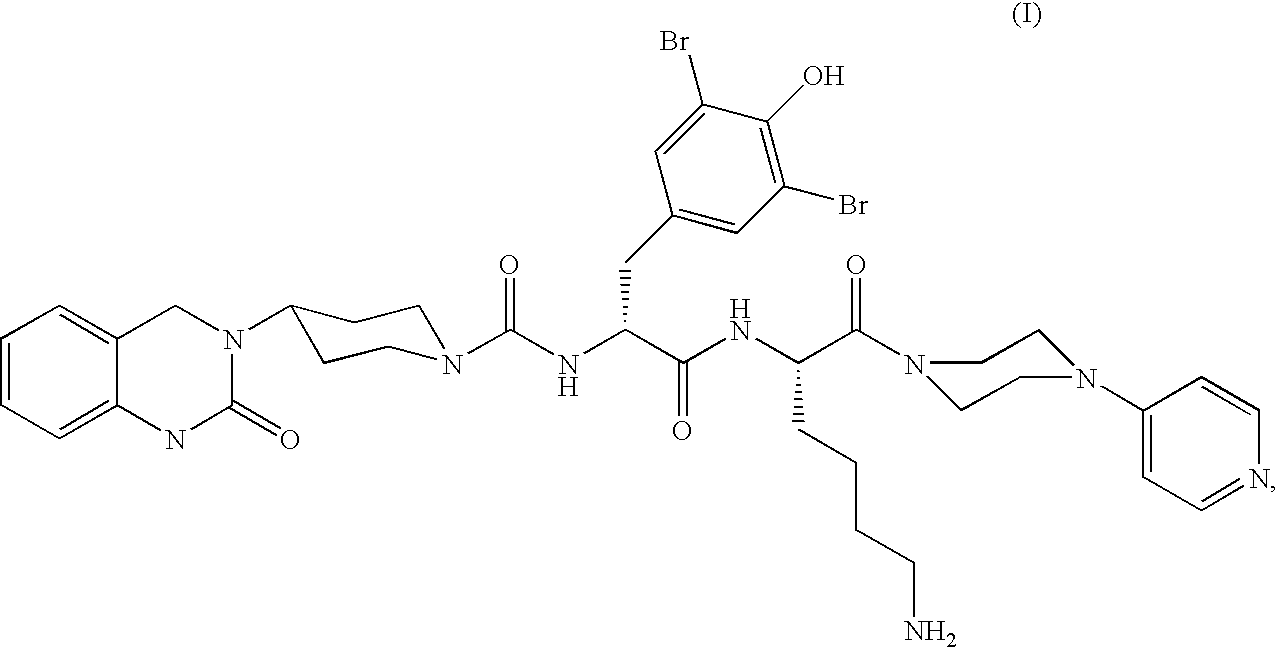
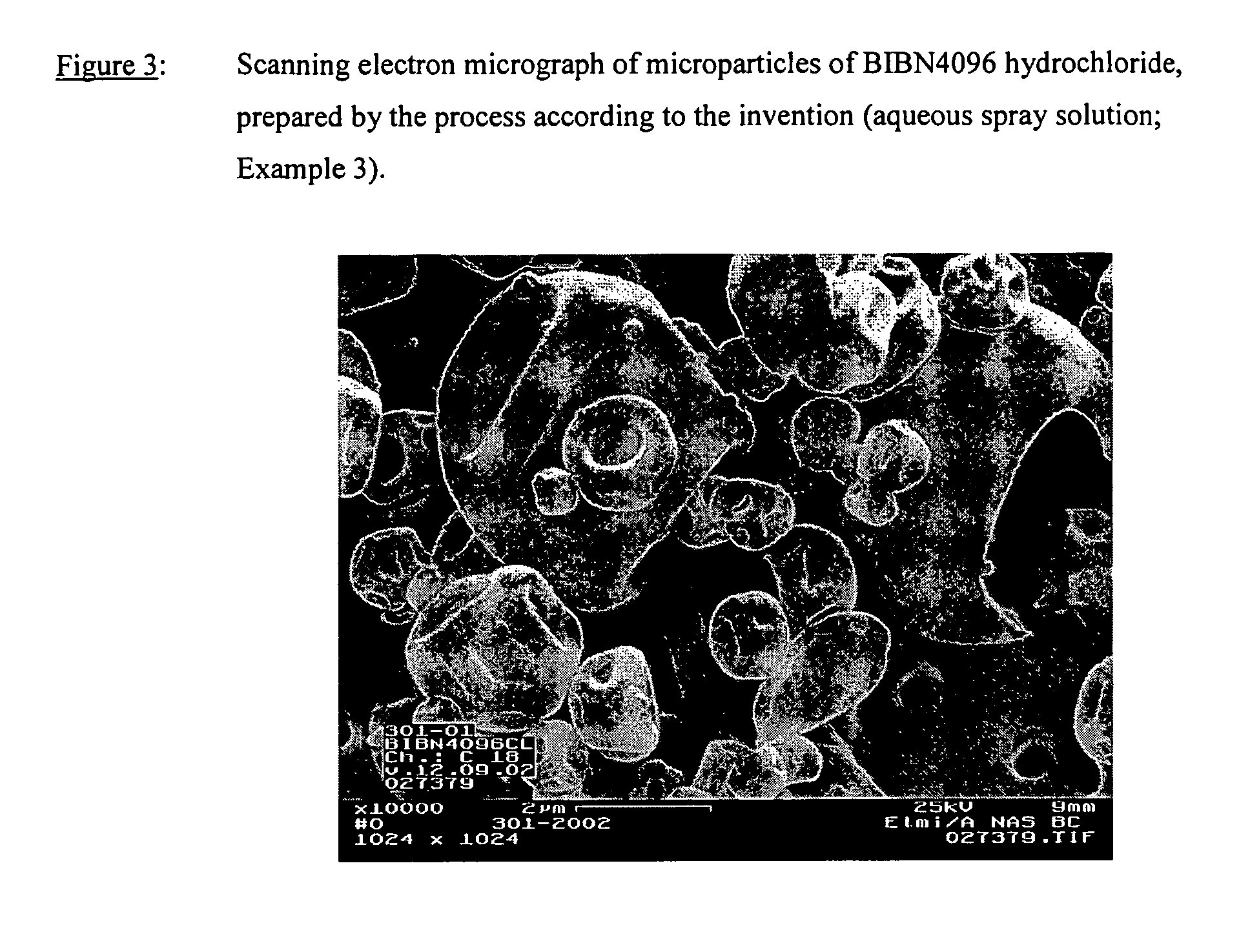
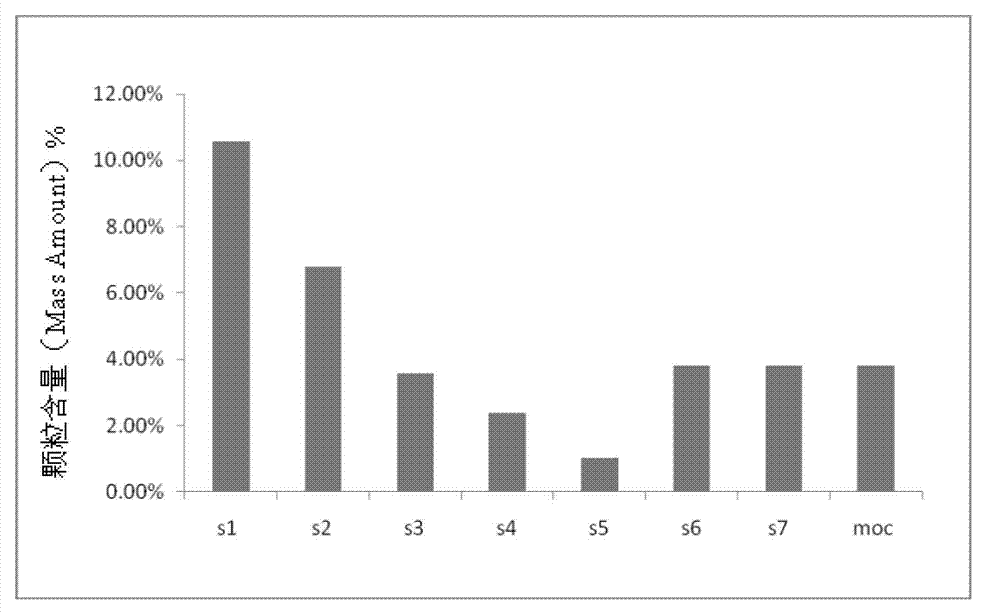
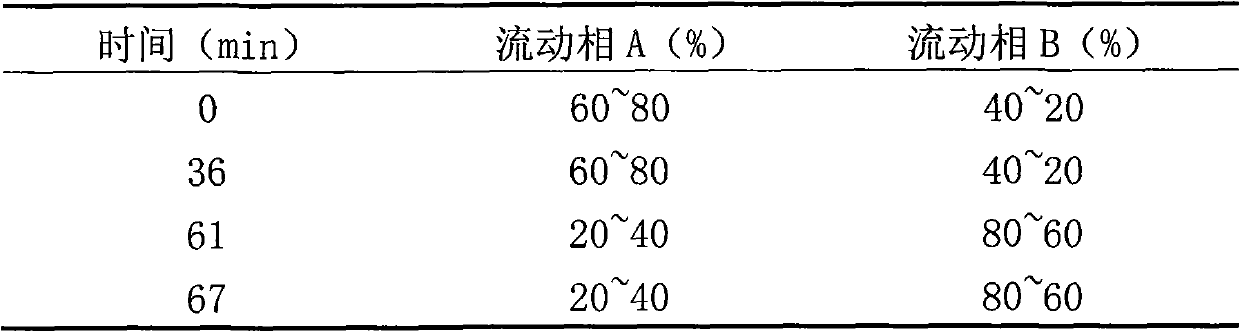
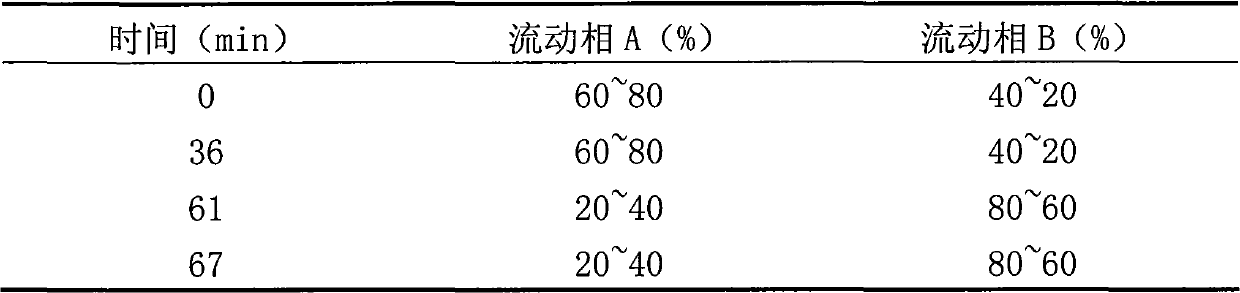
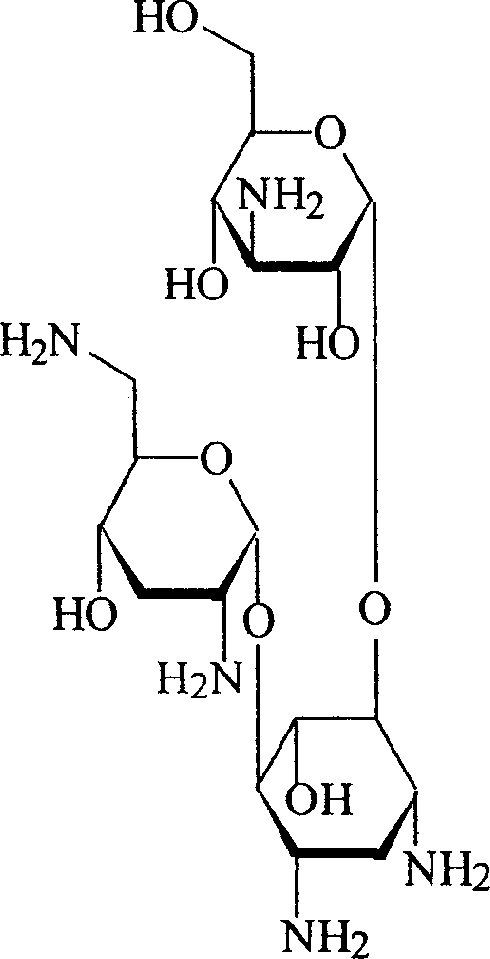
Powder formulation containing the CGRP antagonist 1 [N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2 (1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazin, process for preparing and the use thereof as inhalation powder
A powder inhalant for pulmonary or nasal inhalation, containing the CGRP antagonist 1-[N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2(1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine (A) in the form of spherically nanostructured microparticles, which are stable in their amorphous state under normal conditions (T<50° C., relative humidity <75%), a process for preparing these microparticles as well as the use thereof for preparing the powder inhalant for the treatment of headaches, migraine and cluster headache.
Owner:BOEHRINGER INGELHEIM INT GMBH
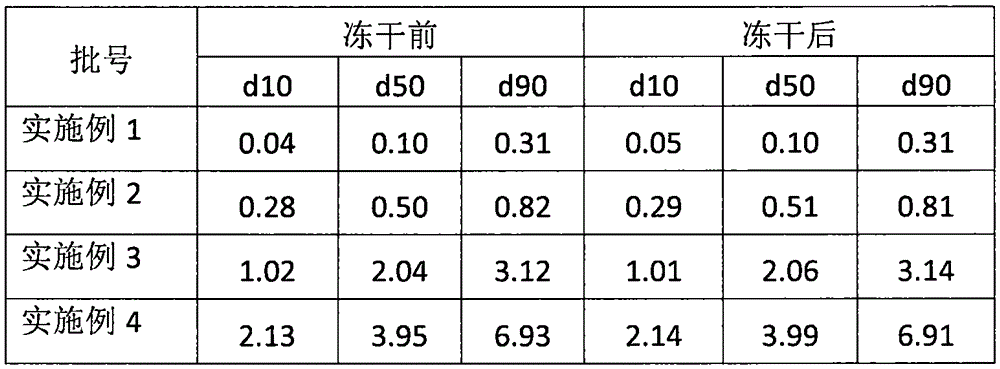
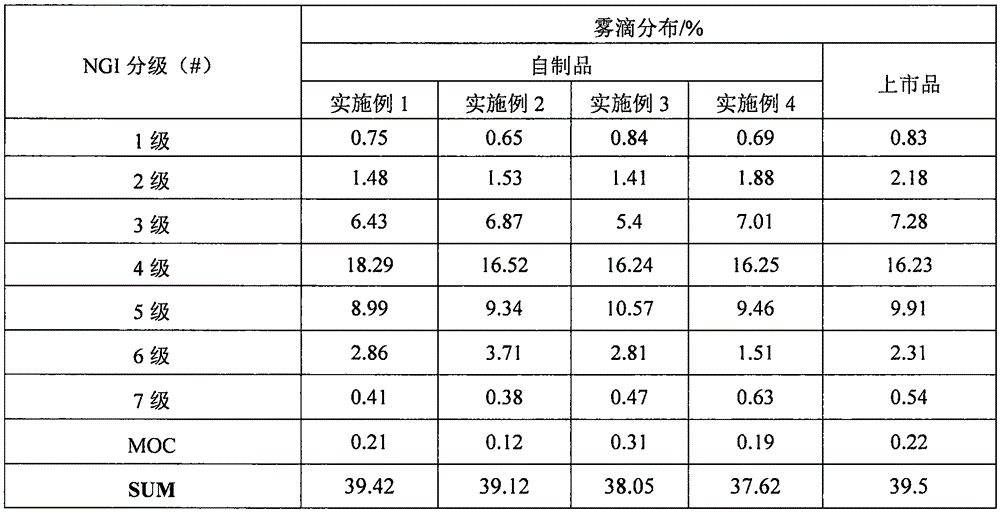
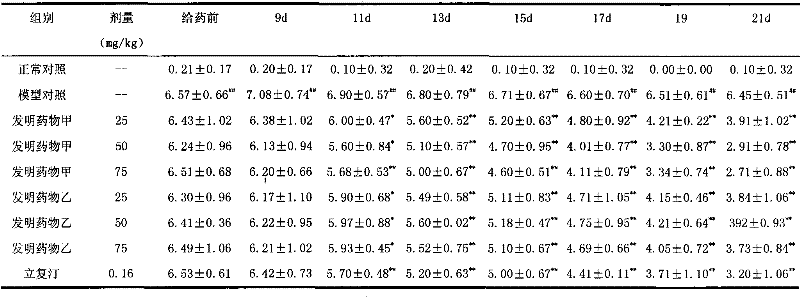
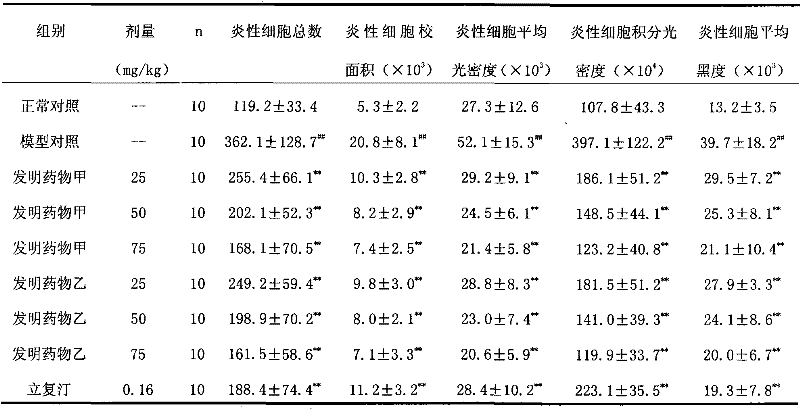
Nasal inhalation powder preparation and device thereof
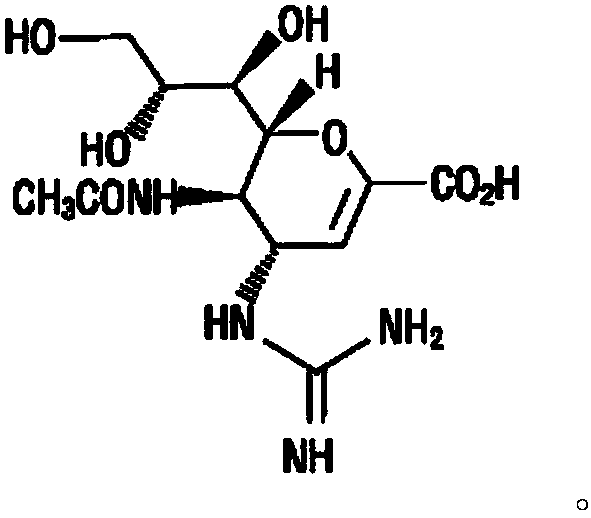
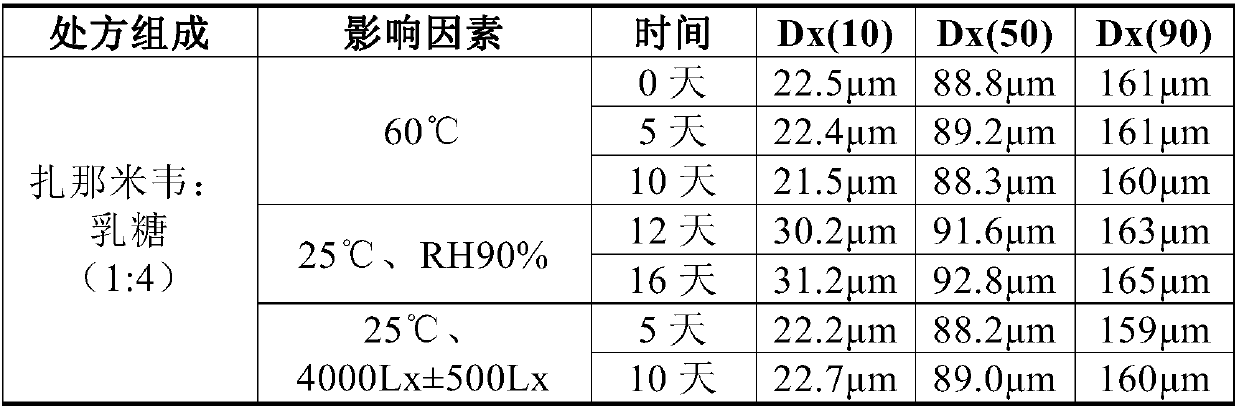
The present invention relates to a nasal inhalation powder preparation and a device thereof. The nasal inhalation powder preparation comprises the following materials in mass ratio: 1 part of zanamivir and 3-5 parts of a pharmaceutically acceptable adjuvant. The nasal inhalation powder preparation has a particle diameter of D10 > 5 [mu]m, D50 < 150 [mu]m and D90 < 300 [mu]m. The nasal inhalation powder preparation can be used for prevention of influenza viruses.
Owner:SHENZHEN HUALIKANG BIOLOGICAL MEDICINE +1
Inhalation powder spray for treating respiratory diseases such as chronic obstructive pulmonary disease and asthma
InactiveCN101428011ASignificant effectGood effectPharmaceutical delivery mechanismRespiratory disorderRespiratory tract diseaseObstructive Pulmonary Diseases
Owner:李虎山
Low temperature forming preparation method for getter
ActiveCN106571282AImprove production efficiencyHigh activityElectric discharge tubesElectric discharge lampsPorositySlurry
The invention belongs to the technical field of getter manufacturing, and particularly relates to a low temperature forming preparation method for a getter. Getter powder and a tert-butanol solvent are mixed to form inhalation powder slurry with certain flowability. The slurry is injected into a preform mold, and is rapidly frozen to realize low temperature forming. A formed blank undergoes vacuum low temperature de-alcohol treatment, and finally undergoes vacuum sintering to acquire an inhalation component. According to the preparation method provided by the invention, the preparation efficiency and the dimensional accuracy of the inhalation component are greatly improved; the prepared inhalation component is better than a product prepared by a traditional process; and the inhalation component has the advantages of large surface area, low activation temperature, high porosity, high inhalation rate, large inhalation capacity and the like.
Owner:GRIMAT ENG INST CO LTD
Novel inhalation preparation
ActiveCN106551919AParticle size unchangedReduce the overall heightPowder deliveryOrganic active ingredientsFreeze-dryingChemical stability
The invention relates to a novel inhalation preparation, in particular to a preparation suitable for inhalation drug administration of children ranging from 0 to 12 years. After medicine difficult to be dissolved in water and auxiliary materials are mixed, a wet grinding method is adopted for smashing, mixed suspension liquid containing particles with the particle size appropriate is obtained, the particle size is 0.1-7 microns, and preferentially, the particle size is 0.1-5 microns; and further, the preferential particle size is 0.1-3 microns. Solid powder is obtained through a freeze-drying method. Sealing is performed for obtaining the novel inhalation; and before use, the novel inhalation preparation is mixed with liquid suitable for atomization, re-dispersion is performed, and the mixed suspension liquid capable of being used for atomizing inhalation of children is obtained. The preparation is stored and transported in the form of the solid powder, the problem that when a mixed suspension liquid form is adopted, the physical stability and the chemical stability are poor is avoided, the medicine particle size hardly changes, impurity increase is little, and the content hardly changes. Compared with inhalation powder aerosol, the novel inhalation preparation is especially suitable for atomizing medicine administration of children, medicine smaller in particle size can be adopted, and even though under the situations that breathing of an infant is slight and the effective portion deposition rate is low, an ideal medicine effect can also be achieved.
Owner:北京天衡药物研究院有限公司
A pharmaceutical composition with the effect of treating rhinitis and its preparation method
The invention discloses a pharmaceutical composition for treating rhinitis, sinusitis and allergic rhinitis and a preparation method thereof. , myrobalan or licorice. The preparation method uses methods such as alcohol extraction and water precipitation or water extraction and alcohol precipitation to prepare clinically accepted conventional preparations according to conventional processes, including but not limited to sprays, powder mist, gels, granules and other dosage forms. A large number of experiments show that the pharmaceutical composition of the invention has better effects on treating rhinitis, sinusitis and allergic rhinitis.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
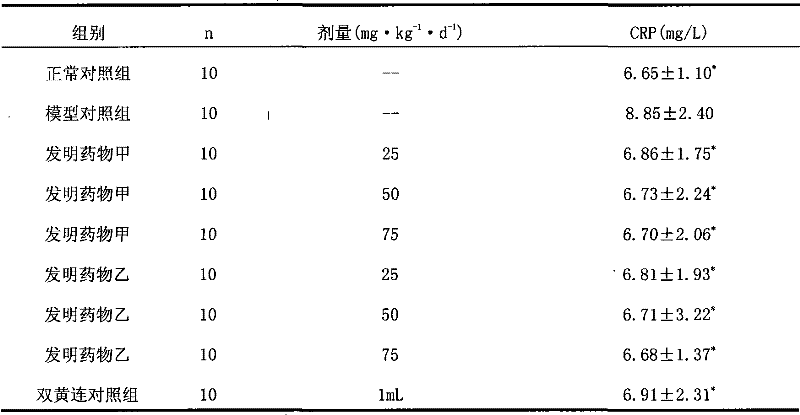
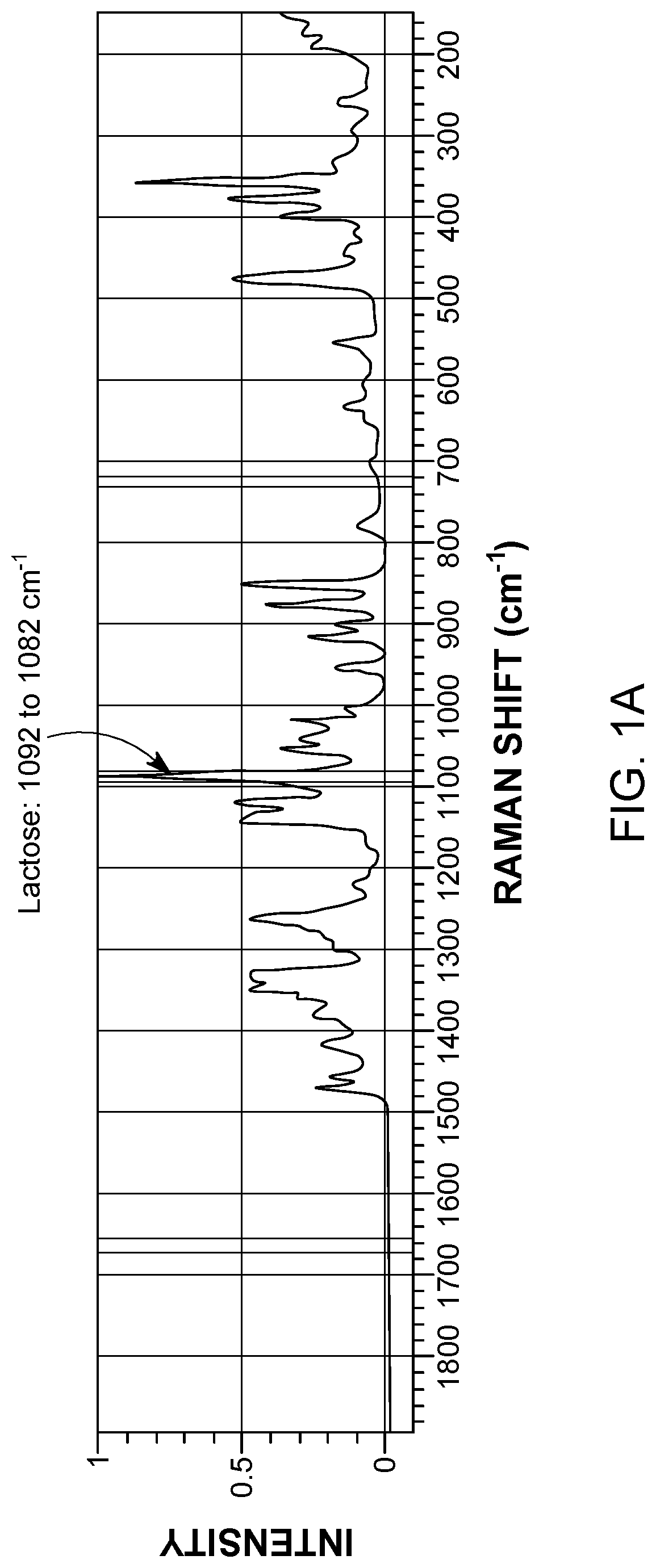
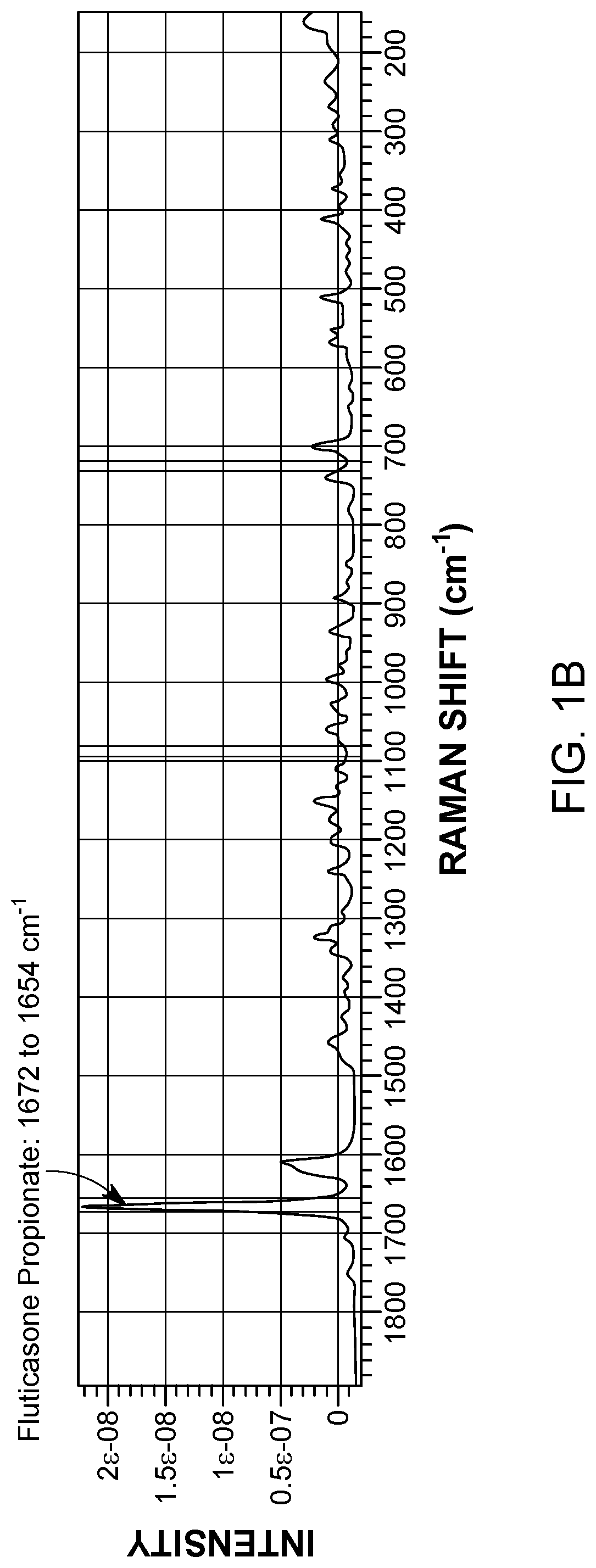
Application of raman spectroscopy for the manufacture of inhalation powders
The present invention generally relates to improved methods for the manufacture of inhalation powders. More particularly, aspects of the disclosure relate to methods for in-line monitoring of powder blending by Raman spectroscopy.
Owner:NORTON (WATERFORD) LTD
Inhalation powder mist preparation for preventing and treating respiratory tract infectious diseases
PendingCN113244262AEffective Lung Targeted DrugsAntibacterial agentsPowder deliveryPulmonary infectionDirect targeting
The invention relates to an inhalation powder mist preparation for preventing and treating respiratory tract infectious diseases. Specially, the invention relates to an inhalation preparation for preventing and treating respiratory tract infectious diseases, in particular to an inhalation powder mist preparation. The inhalation powder mist preparation comprises iodine molecules as an active substance and / or a pharmaceutically acceptable carrier. The medicine is inhaled into the respiratory tract or the lung through powder mist for preventing and treating infectious diseases of the respiratory tract and the lung, and has the advantages of direct targeting action on infected parts, quick response, small dosage and small side effect. Various pathogens in the respiratory tract can be rapidly killed, and the purposes of reducing infectivity, relieving pain of a patient, shortening the rehabilitation period, improving the cure rate, reducing complications and reducing the death rate are achieved.
Owner:BEIJING HUMANWELL JUNWEI PHARM TECH CO LTD
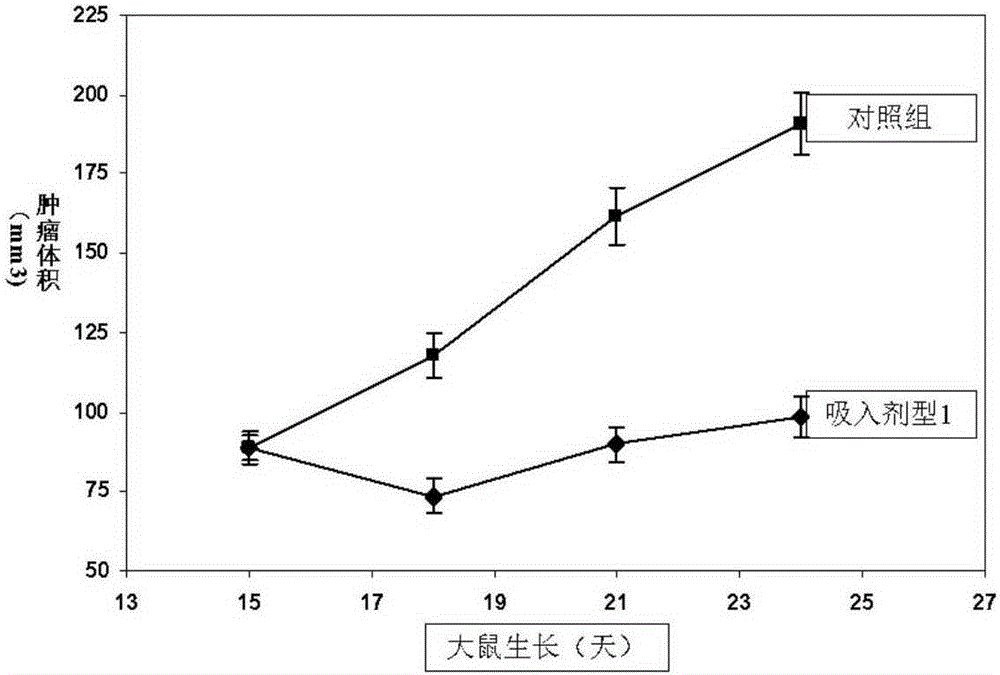
Chemotherapy-type anticancer drug inhalation powder preparation and preparing method and application thereof
InactiveCN106606482ALow toxicityGrowth inhibitionPowder deliveryElcosanoid active ingredientsDiseaseInhalation powder
The invention relates to a chemotherapy-type anticancer drug inhalation powder preparation and a preparing method and application thereof. According to the technical scheme, the chemotherapy-type anticancer drug inhalation powder preparation is obtained by mixing effective constituent powder with the grain diameter being 0.4-5 microns or 10-100 microns and carrier powder with the grain diameter being 10-100 microns. The effective constituent powder comprises a chemotherapy-type anticancer drug and Omega-3 fatty acid. The carrier powder comprises saccharides, an emulsifier and magnesium stearate. The application effect of the preparation in treatment of respiratory system cancers like a laryngeal cancer, a lung cancer and other tumor diseases is obvious. The inhalant form toxicity is small, the application is convenient, and the anticancer treatment effect has advantages compared with other dosage forms.
Owner:ZHOUSHAN SANHE BIOTECH CO LTD
Method for preparing throat coughing atomization inhalation powder
InactiveCN105250614AReduce cough reflex sensitivityReduce irritating coughPharmaceutical delivery mechanismRespiratory disorderUltrafiltrationSemen
The invention discloses a method for preparing throat coughing atomization inhalation powder to solve the problem of laryngeal cough treatment. The method is characterized by comprising the following steps: (1) extracting volatile oils from pawpaws, wintercreeper, ailanthus altissima swingle, pseudodrynaria coronans, semen aesculi, cinnamomum camphora, solanum lyratum thunb, semen lini and resina liquidambaris coarse powder to obtain mixed volatile oil and medicinal dregs for later use; (2) uniformly mixing pyrola rotundifolia, tricyrtis root and semen euryales with the medicinal dregs obtained in the step (1), performing water extraction and alcohol precipitation, filtering to remove alcohol, performing ultrafiltration by a microporous membrane, concentrating and drying the filtrate to obtain a primary material; and (3) spraying the volatile oil obtained in the step (1) into the primary material obtained in the step (2), uniformly mixing, grinding, screening by a 100-mesh screen, and sterilizing. Clinical experiments prove that the throat coughing atomization inhalation powder has the characteristics of good curative effect and relatively high safety when being used for treating laryngeal cough, and is worthy of clinical application and popularization.
Owner:刘庆华
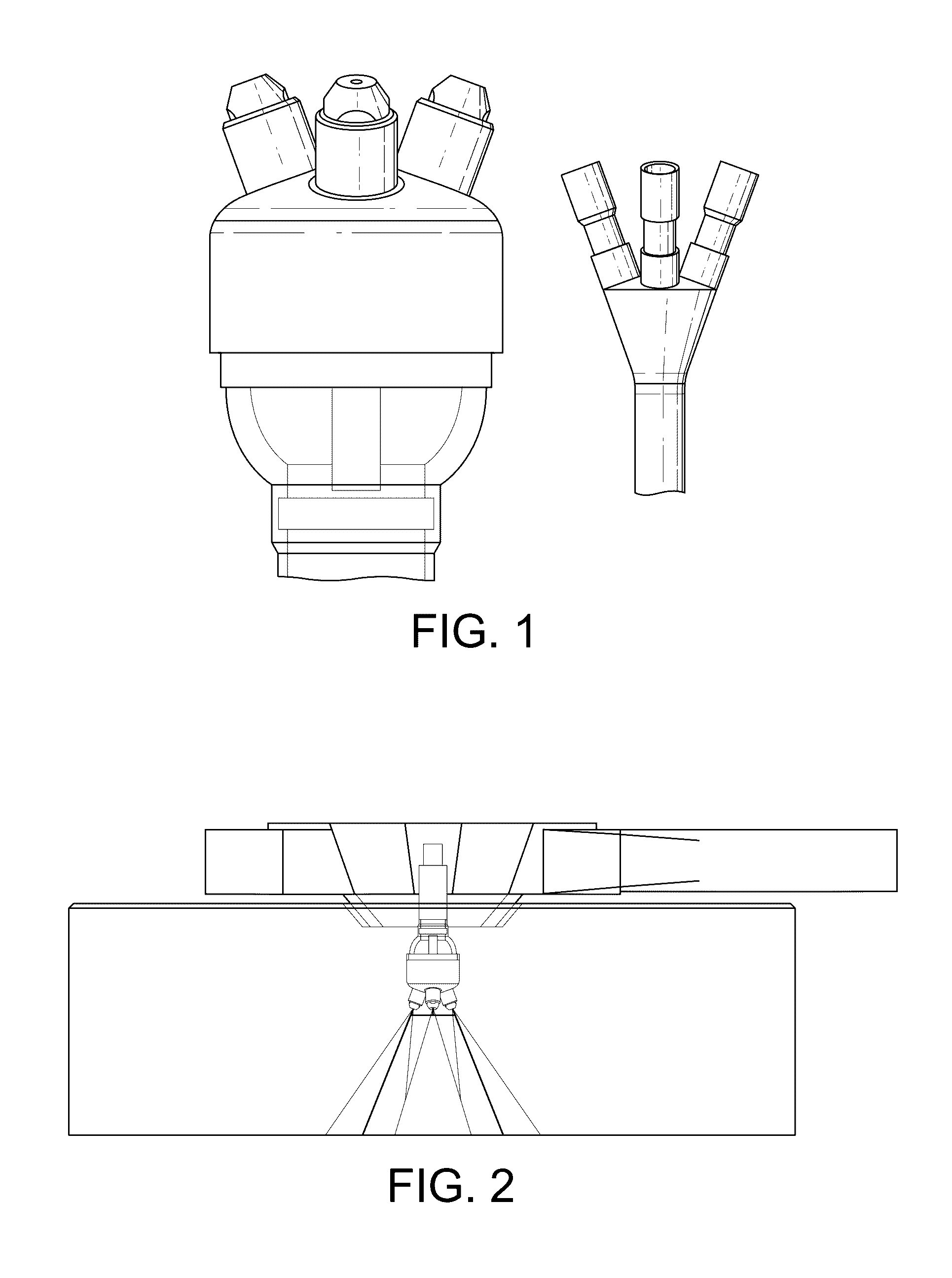
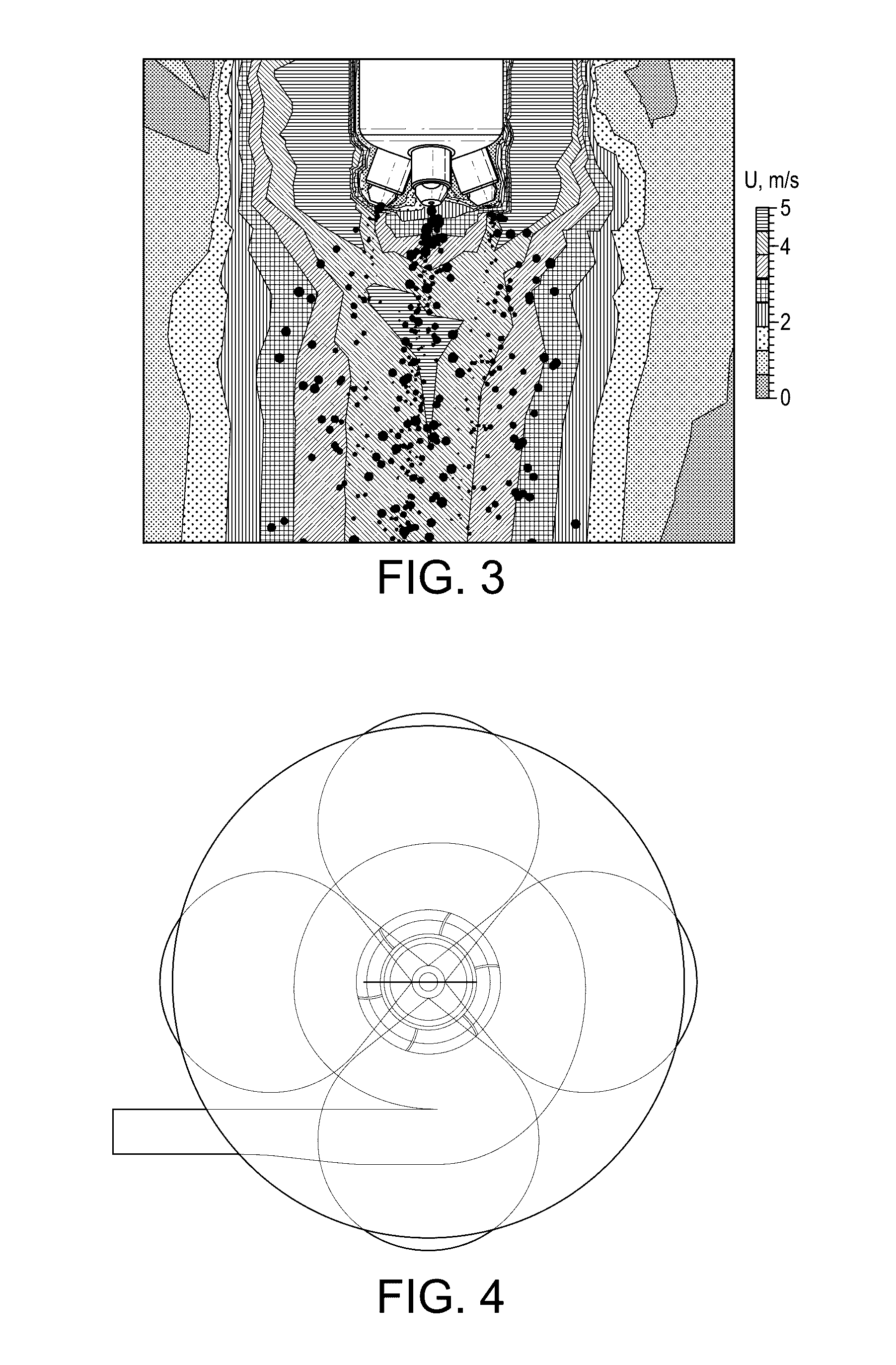
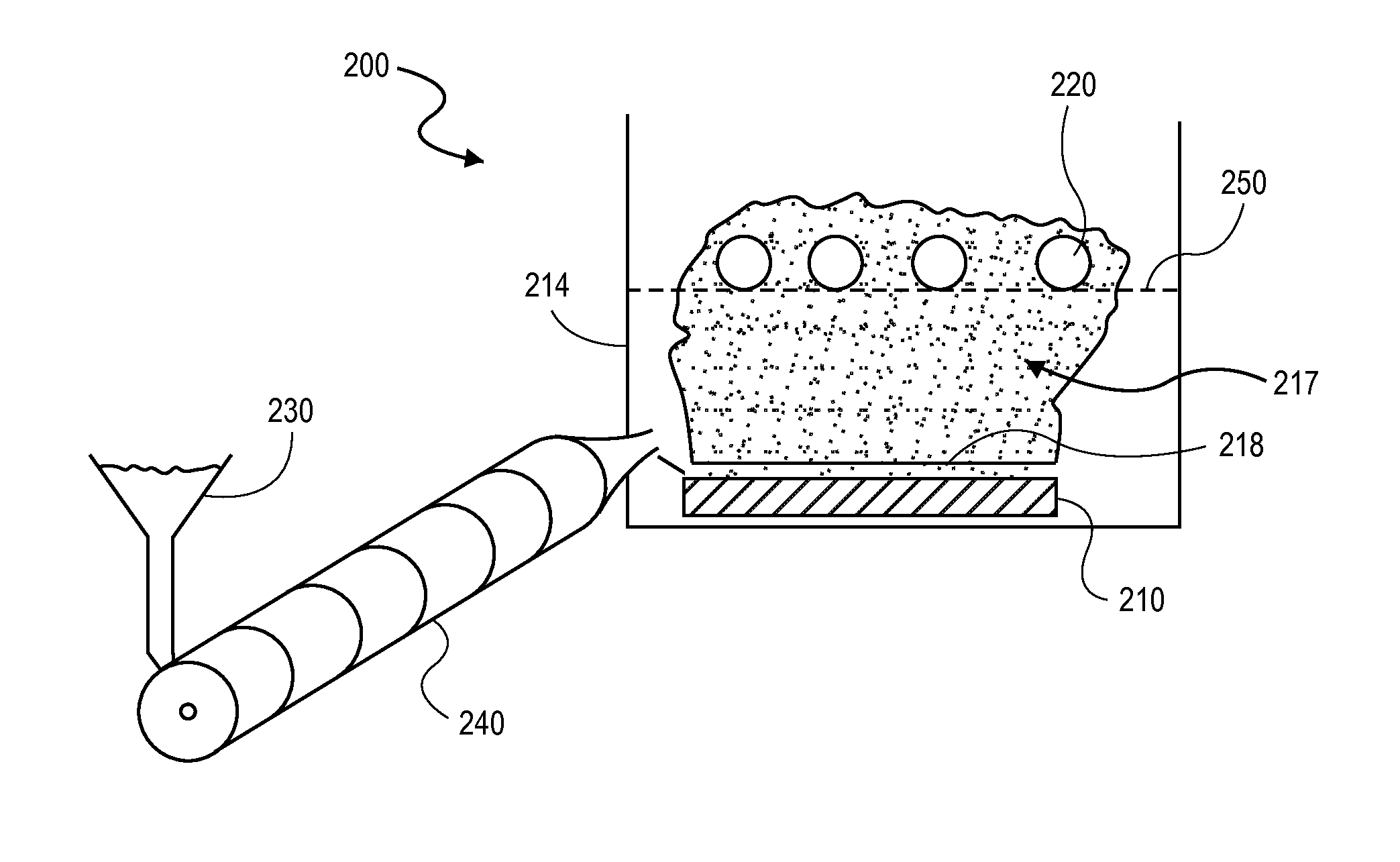
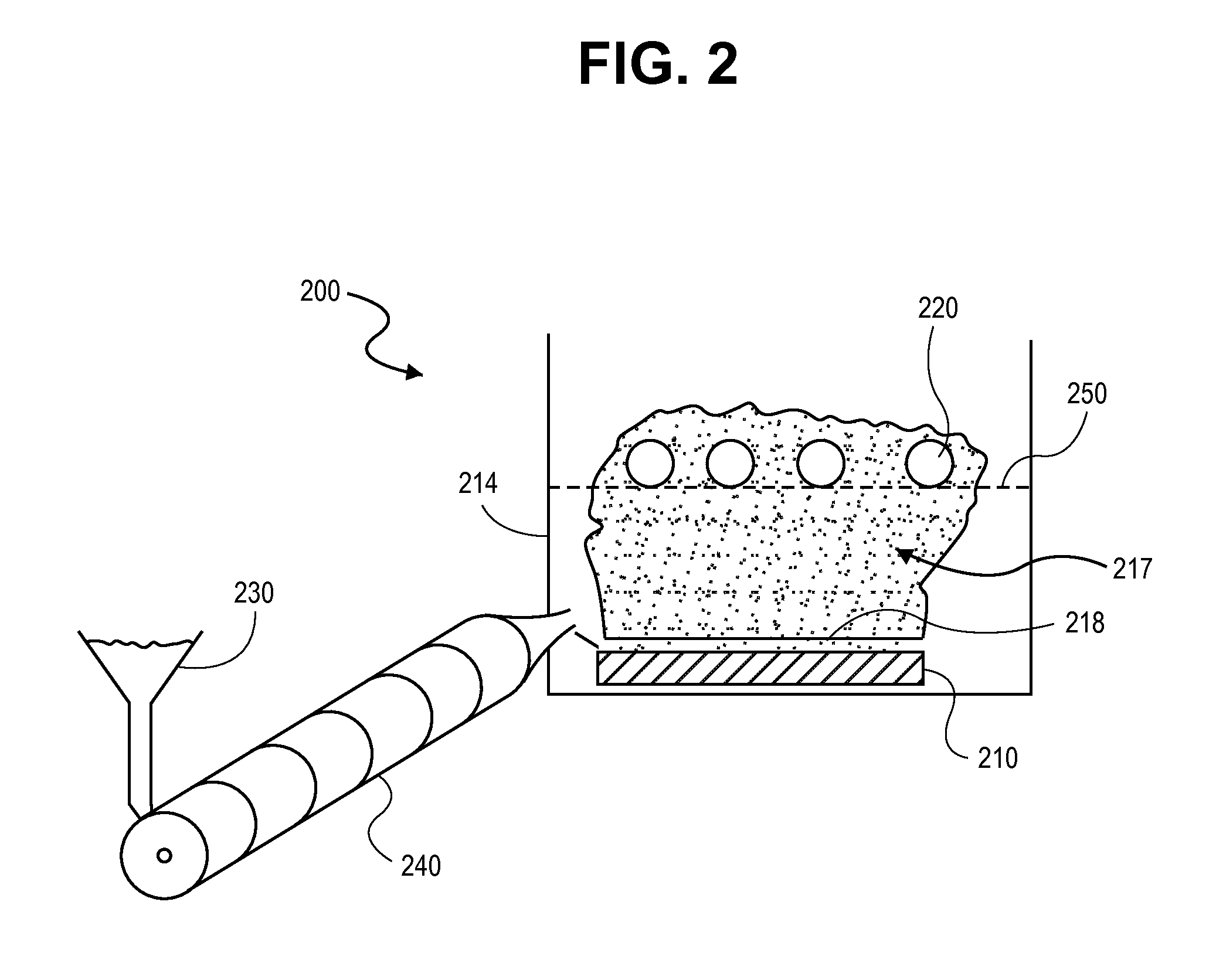
Multi-Nozzle Spray Dryer, Method for Scale-Up of Spray Dried Inhalation Powders, Multi-Nozzle Apparatus and Use of Multiple Nozzles in a Spray Dryer
ActiveUS20170014789A1Low applicabilitySimple processGranulation by liquid drop formationGranular deliverySpray nozzleEngineering
This present invention provides a spray dryer for use in preparing particles for inhalation, the spray dryer comprising a multi-nozzle apparatus comprising multiple single nozzles suitable for use in preparing inhalation powders and with a drying gas flow rate greater than about 80 kg / h. Also provided is a method for scaling-up a spray drying process for preparing particles for inhalation from a smaller scale spray dryer to a larger scale spray dryer, relative in size to each other, the method comprising the use in the larger scale spray dryer of a multi-nozzle apparatus comprising single nozzles suitable for use in preparing inhalation powders, wherein the number of nozzles in the larger spray dryer is determined by the ratio of the drying gas flow rate of the larger scale spray dryer to the drying gas flow rate of the smaller scale spray dryer. Also provided is a multi-nozzle apparatus produced from the method of the present invention, the multi-nozzle apparatus comprising multiple single nozzles suitable for use in preparing inhalation powders. Also provided is the use of multiple single nozzles suitable for use in preparing inhalation powders in a spray dryer with a drying gas flow rate greater than about 80 kg / h. The nozzles used in the present invention preferably prepare particles with a mean particle size less than about 5 microns.
Owner:HOVIONE HLDG LTD
Capsule type Eletriptan nose inhalation powder aerosols and preparation method and application thereof
InactiveCN110585176AImprove complianceLow priceOrganic active ingredientsNervous disorderSide effectNose
The invention discloses capsule type Eletriptan nose inhalation powder aerosols. The capsule type Eletriptan nose inhalation powder aerosols consist of Eletriptan micro powder and carrier micro powder. The capsule type Eletriptan nose inhalation powder aerosols are characterized in that the particle sizes of raw material micro powder of the Eletriptan are d10=5-30[mu]m, d50=10-100[mu]m and d90=30-150[mu]m, and the particle size of the carrier micro powder is d90=20-320[mu]m. The invention also provides a preparation method of the capsule type Eletriptan nose inhalation powder aerosols. The preparation method comprises the working procedures of performing Eletriptan micronizing, performing lactose particle preparation, performing mixing and performing subpackaging, wherein during mixing, the micronized Eletriptan and lactose particles are taken, mixing is performed in an equal progressively-increased manner, the mixing speed is 2-15 revolutions per minute, and the mixing time is 5-45 minutes. The capsule type Eletriptan nose inhalation powder aerosols disclosed by the invention comprise 2 kinds of inhalation manners of nose inhalation and oral inhalation, and the Eletriptan can alsoexist in the form of normal saline. The capsule type Eletriptan nose inhalation powder aerosols can reach pain locations in a nose and brain targeting administration manner and can take effect quickly, first-pass effects of the liver are avoided, the biological availability can be improved, the first-pass effects of the liver can be avoided through direct inhalation of the capsule type Eletriptannose inhalation powder aerosols, and consumption and side effects of the medicine are reduced.
Owner:ZHUHAI RESPROLY PHARM TECH CO LTD
Multi-nozzle spray dryer, method for scale-up of spray dried inhalation powders, multi-nozzle apparatus and use of multiple nozzles in a spray dryer
InactiveCN106457181AIncrease the number ofLow applicabilityGranulation by liquid drop formationGranular deliverySpray nozzleEngineering
This present invention provides a spray dryer for use in preparing particles for inhalation, the spray dryer comprising a multi-nozzle apparatus comprising multiple single nozzles suitable for use in preparing inhalation powders and with a drying gas flow rate greater than about 80 kg / h. Also provided is a method for scaling-up a spray drying process for preparing particles for inhalation from a smaller scale spray dryer to a larger scale spray dryer, relative in size to each other, the method comprising the use in the larger scale spray dryer of a multi-nozzle apparatus comprising single nozzles suitable for use in preparing inhalation powders, wherein the number of nozzles in the larger spray dryer is determined by the ratio of the drying gas flow rate of the larger scale spray dryer to the drying gas flow rate of the smaller scale spray dryer. Also provided is a multi-nozzle apparatus produced from the method of the present invention, the multi-nozzle apparatus comprising multiple single nozzles suitable for use in preparing inhalation powders. Also provided is the use of multiple single nozzles suitable for use in preparing inhalation powders in a spray dryer with a drying gas flow rate greater than about 80 kg / h. The nozzles used in the present invention preferably prepare particles with a mean particle size less than about 5 microns.
Owner:HOVIONE INTERNAT LTD
Naringin nano inhalation powder inhalation taking polylysine as carrier as well as preparation method and application of naringin nano inhalation powder inhalation
ActiveCN114191419ASimple preparation processHigh drug loadingAntibacterial agentsOrganic active ingredientsNaringinPharmaceutical drug
The invention relates to the field of nano-drug inhalation preparations, in particular to preparation of naringin nano-inhalation powder inhalation taking polylysine PL as a carrier and research on the treatment effect of the naringin nano-inhalation powder inhalation on cough after infection. The preparation method comprises the following steps: firstly, preparing a naringin nano suspension by adopting a medium grinding method based on a Top-down principle, and then further drying and curing by adopting a spray drying method to obtain the naringin nano inhalation powder aerosol. The naringin nano inhalation powder inhalation disclosed by the invention is simple in preparation process, high in drug loading capacity and good in in-vivo release effect; in-vivo pharmacodynamic experiment results show that the prepared naringin nano inhalation dry powder can significantly improve the cough relieving, anti-inflammatory and anti-oxidation curative effects of the medicine, and the naringin nano inhalation dry powder enables the medicine and a carrier to be absolutely safe due to the fact that no organic reagent is added in the whole preparation process, and has great market application and popularization prospects.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


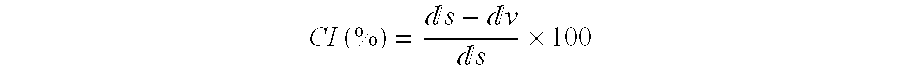
![Microparticles containing the CGRP-antagonist 1-[N2-[3,5-dibrom-N-[[4-(3,4-dihydro-2(1H)-oxoquinazoline-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine, process for preparing and the use thereof as inhalation powder Microparticles containing the CGRP-antagonist 1-[N2-[3,5-dibrom-N-[[4-(3,4-dihydro-2(1H)-oxoquinazoline-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine, process for preparing and the use thereof as inhalation powder](https://images-eureka.patsnap.com/patent_img/40c0f1df-a924-45b9-ac7b-8de44dfff593/US20050042178A1-20050224-C00001.png)
![Microparticles containing the CGRP-antagonist 1-[N2-[3,5-dibrom-N-[[4-(3,4-dihydro-2(1H)-oxoquinazoline-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine, process for preparing and the use thereof as inhalation powder Microparticles containing the CGRP-antagonist 1-[N2-[3,5-dibrom-N-[[4-(3,4-dihydro-2(1H)-oxoquinazoline-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine, process for preparing and the use thereof as inhalation powder](https://images-eureka.patsnap.com/patent_img/40c0f1df-a924-45b9-ac7b-8de44dfff593/US20050042178A1-20050224-C00002.png)
![Microparticles containing the CGRP-antagonist 1-[N2-[3,5-dibrom-N-[[4-(3,4-dihydro-2(1H)-oxoquinazoline-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine, process for preparing and the use thereof as inhalation powder Microparticles containing the CGRP-antagonist 1-[N2-[3,5-dibrom-N-[[4-(3,4-dihydro-2(1H)-oxoquinazoline-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazine, process for preparing and the use thereof as inhalation powder](https://images-eureka.patsnap.com/patent_img/40c0f1df-a924-45b9-ac7b-8de44dfff593/US20050042178A1-20050224-C00003.png)
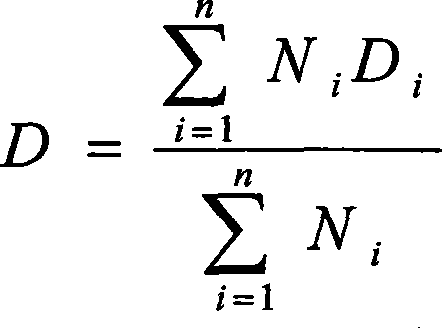

![Powder formulation containing the CGRP antagonist 1 [N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2 (1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazin, process for preparing and the use thereof as inhalation powder Powder formulation containing the CGRP antagonist 1 [N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2 (1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazin, process for preparing and the use thereof as inhalation powder](https://images-eureka.patsnap.com/patent_img/8a514af7-6298-45db-a789-9efa6e2ec9a2/US20050042180A1-20050224-C00001.png)
![Powder formulation containing the CGRP antagonist 1 [N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2 (1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazin, process for preparing and the use thereof as inhalation powder Powder formulation containing the CGRP antagonist 1 [N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2 (1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazin, process for preparing and the use thereof as inhalation powder](https://images-eureka.patsnap.com/patent_img/8a514af7-6298-45db-a789-9efa6e2ec9a2/US20050042180A1-20050224-C00002.png)
![Powder formulation containing the CGRP antagonist 1 [N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2 (1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazin, process for preparing and the use thereof as inhalation powder Powder formulation containing the CGRP antagonist 1 [N2-[3,5-dibromo-N-[[4-(3,4-dihydro-2 (1H)-oxoquinazolin-3-yl)-1-piperidinyl]carbonyl]-D-tyrosyl]-L-lysyl]-4-(4-pyridinyl)-piperazin, process for preparing and the use thereof as inhalation powder](https://images-eureka.patsnap.com/patent_img/8a514af7-6298-45db-a789-9efa6e2ec9a2/US20050042180A1-20050224-C00003.png)