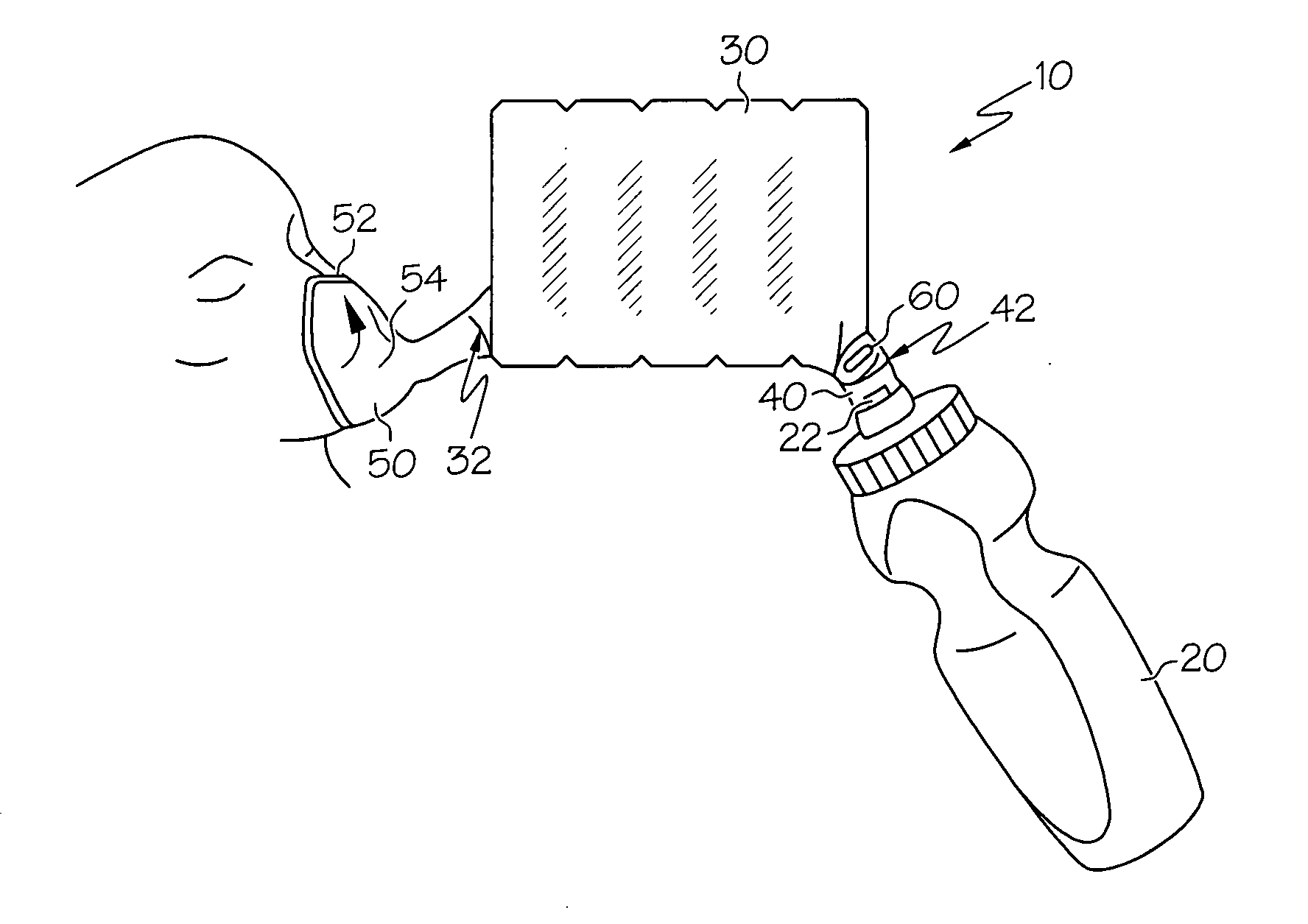
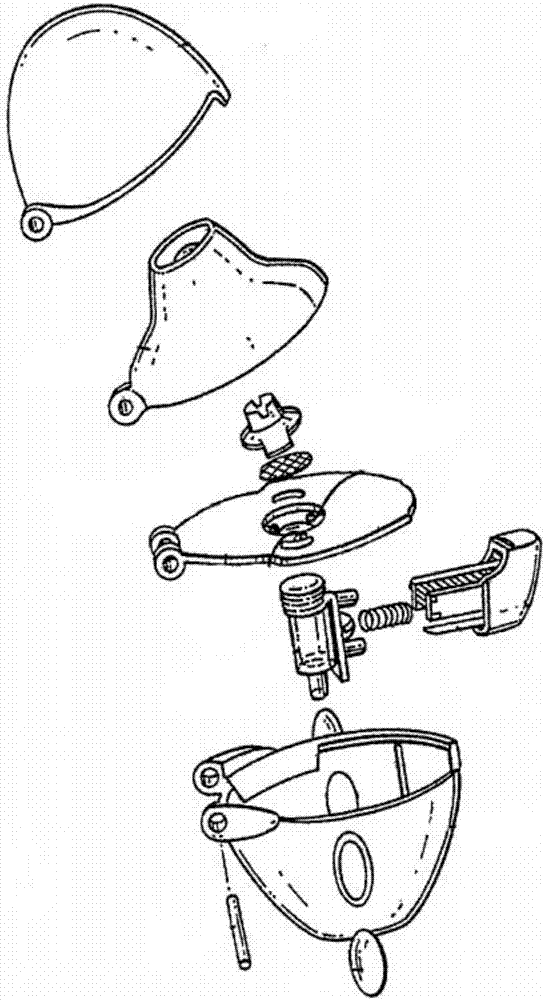
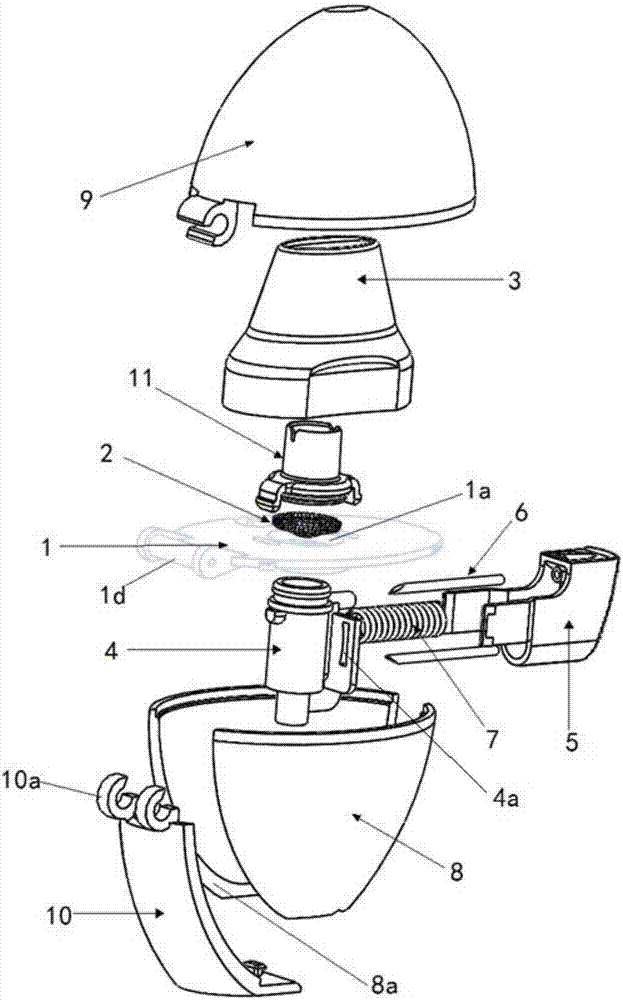
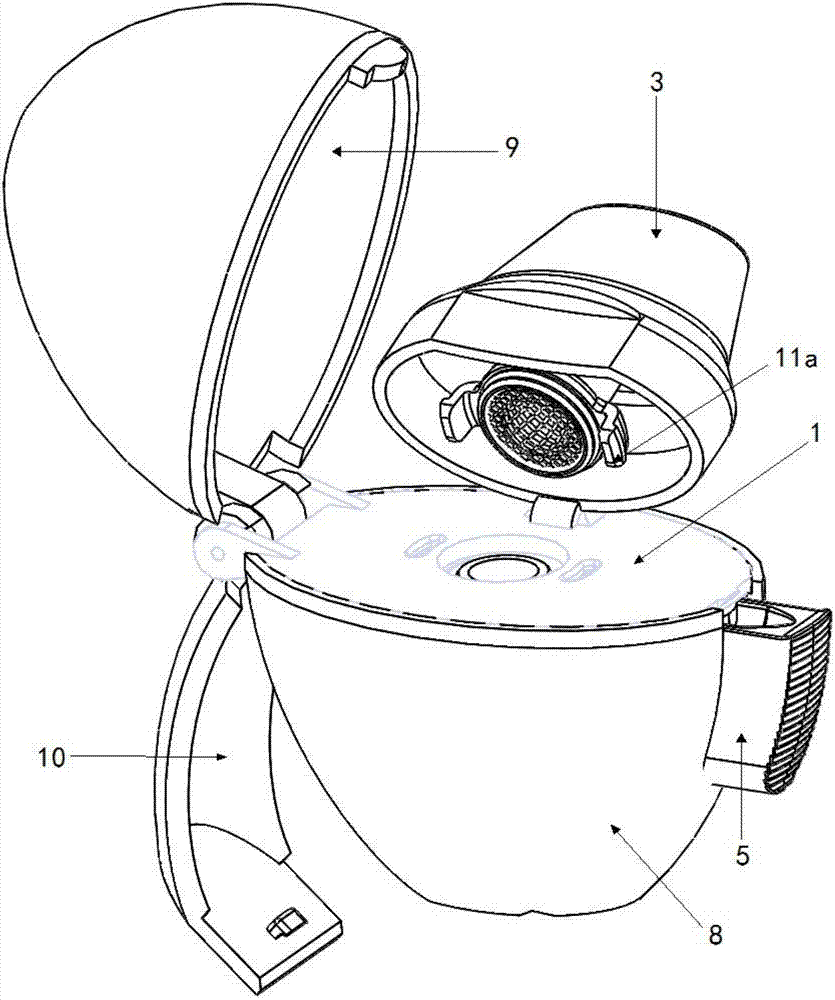
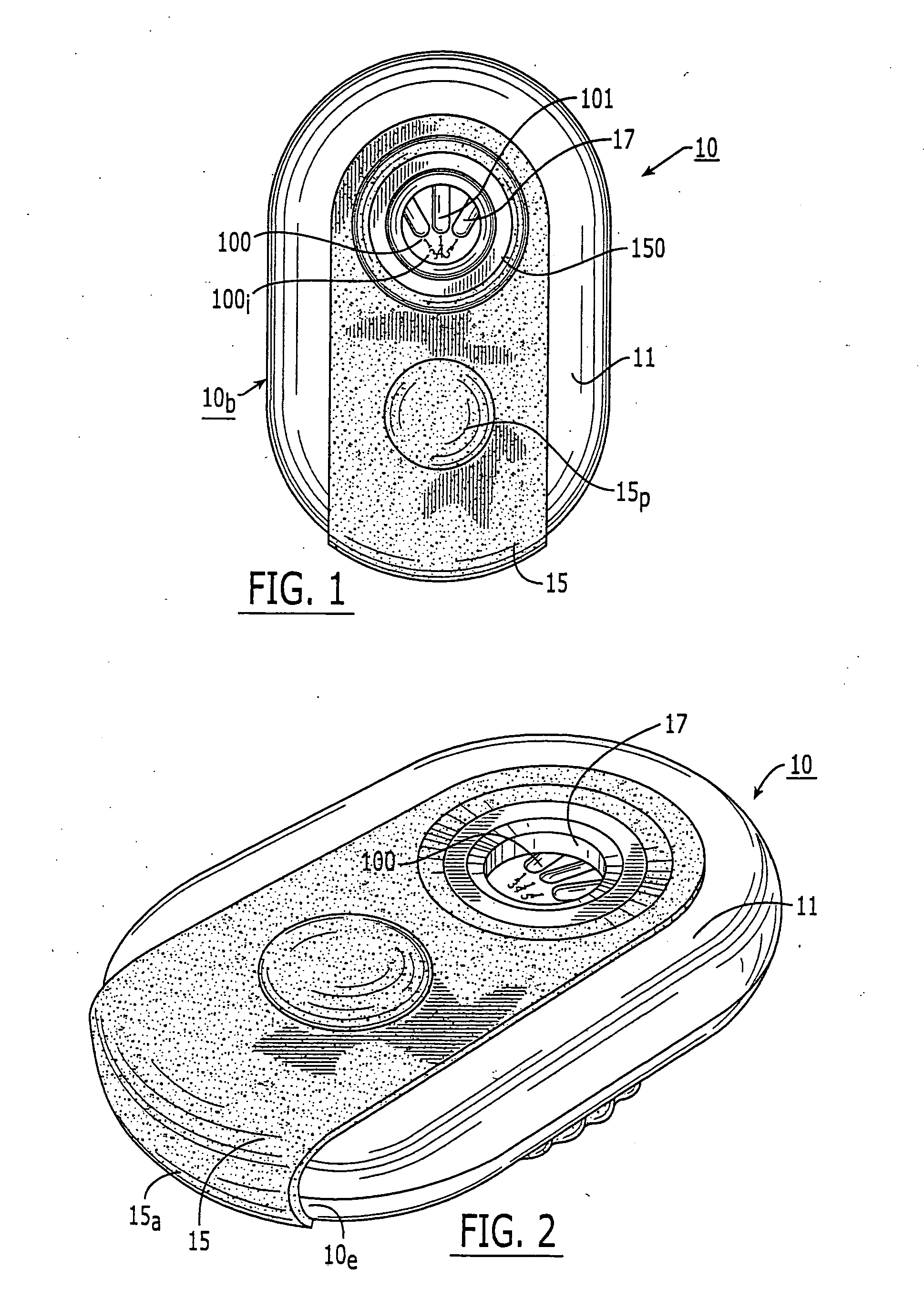
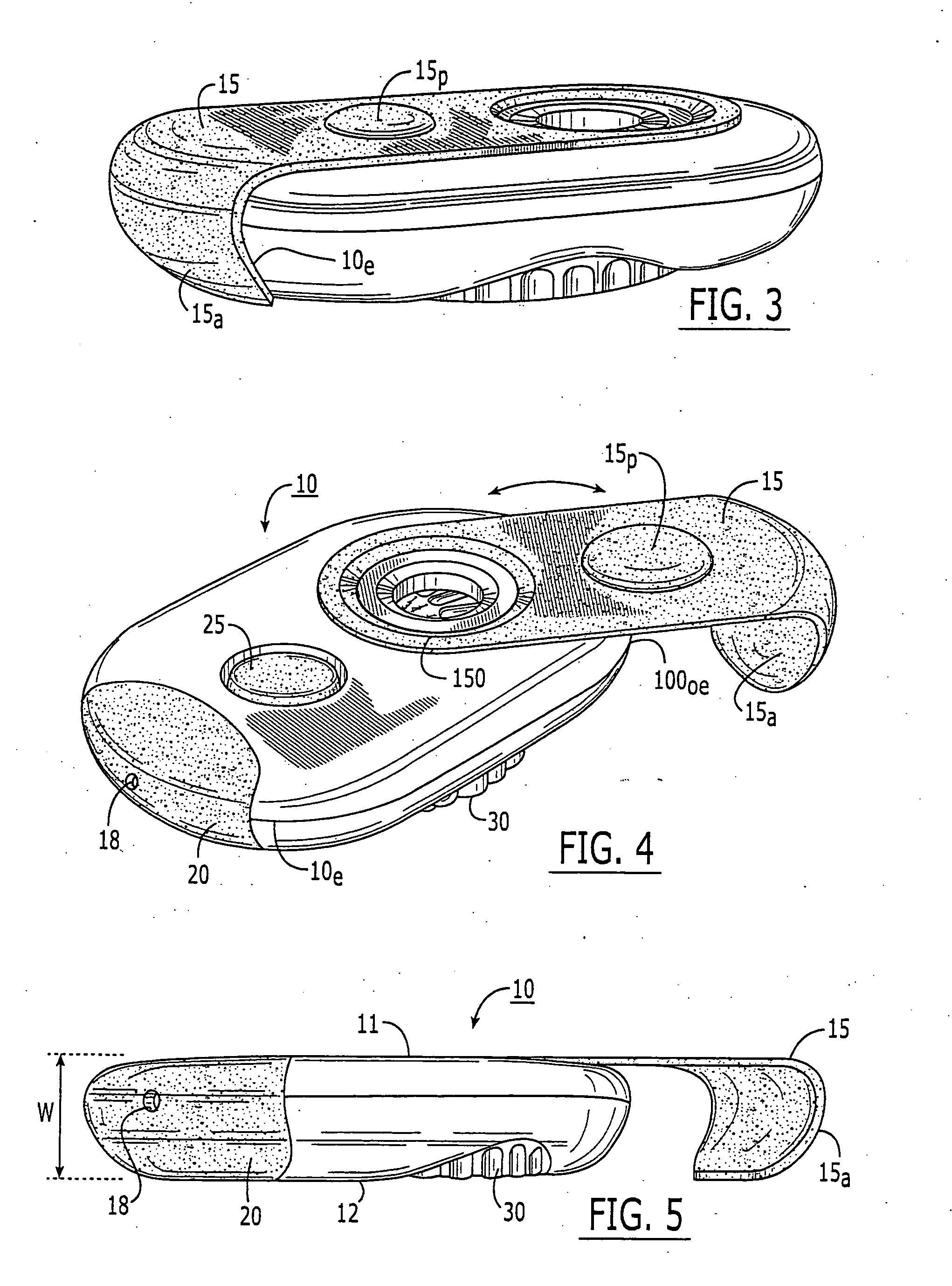
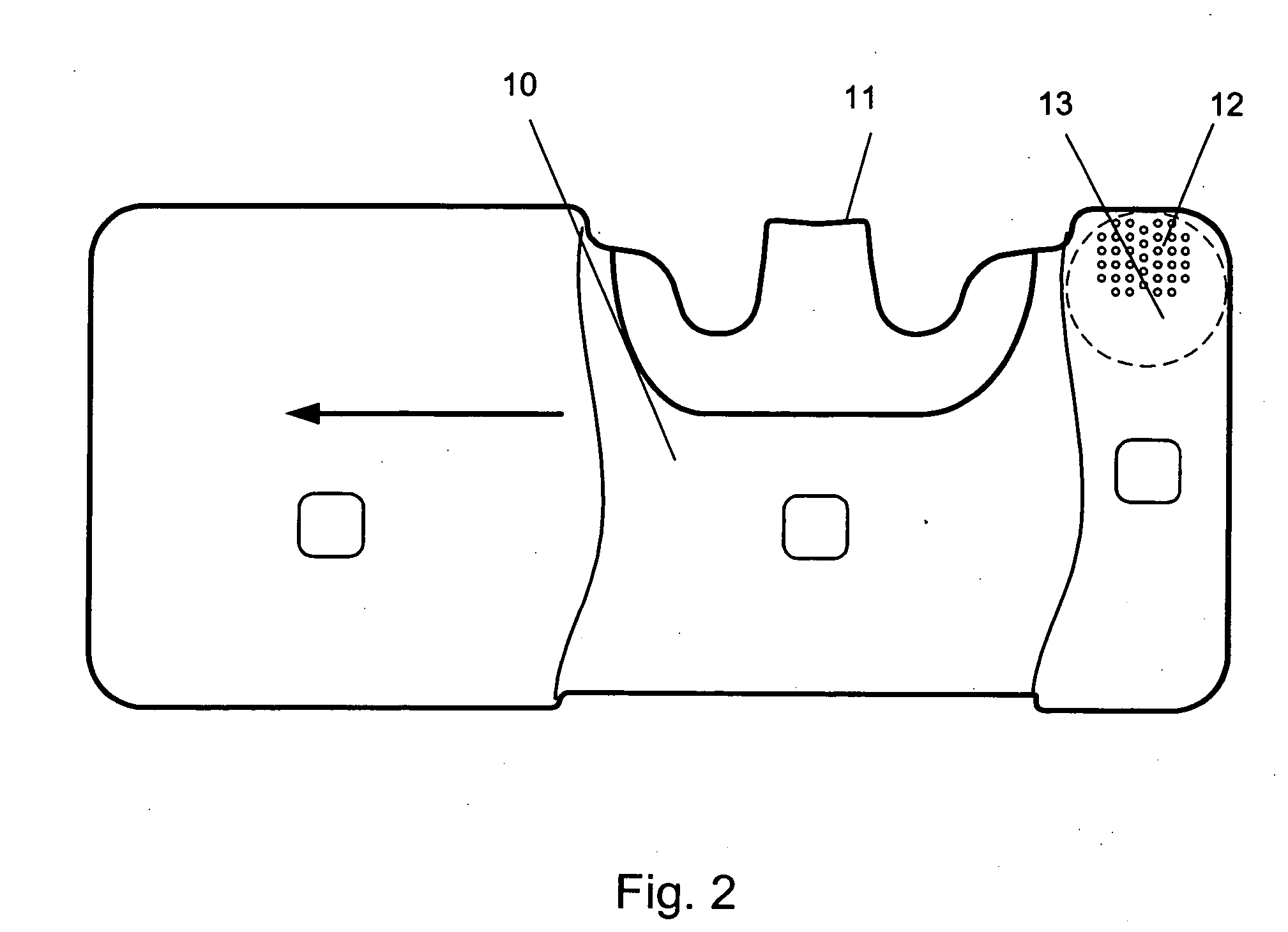
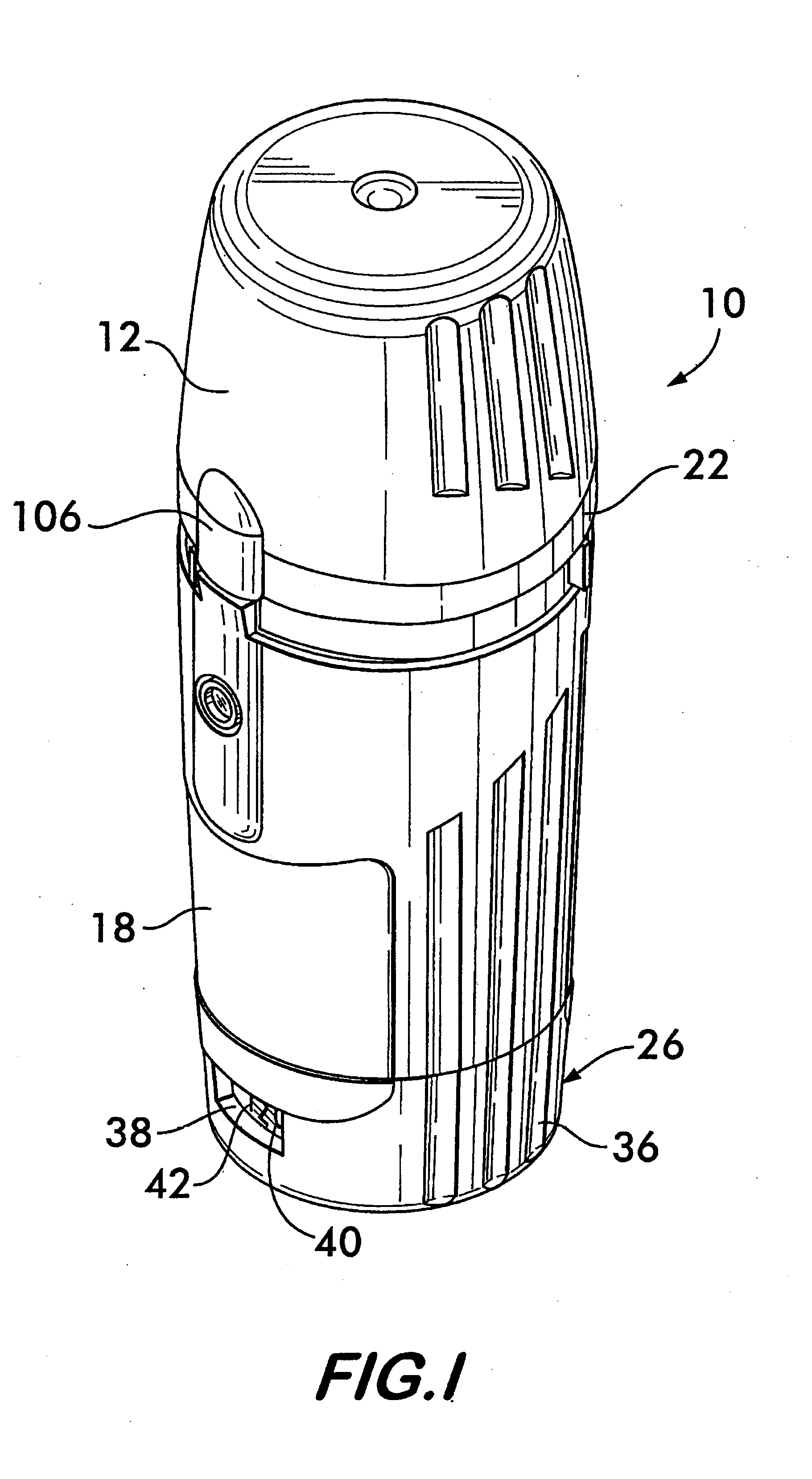
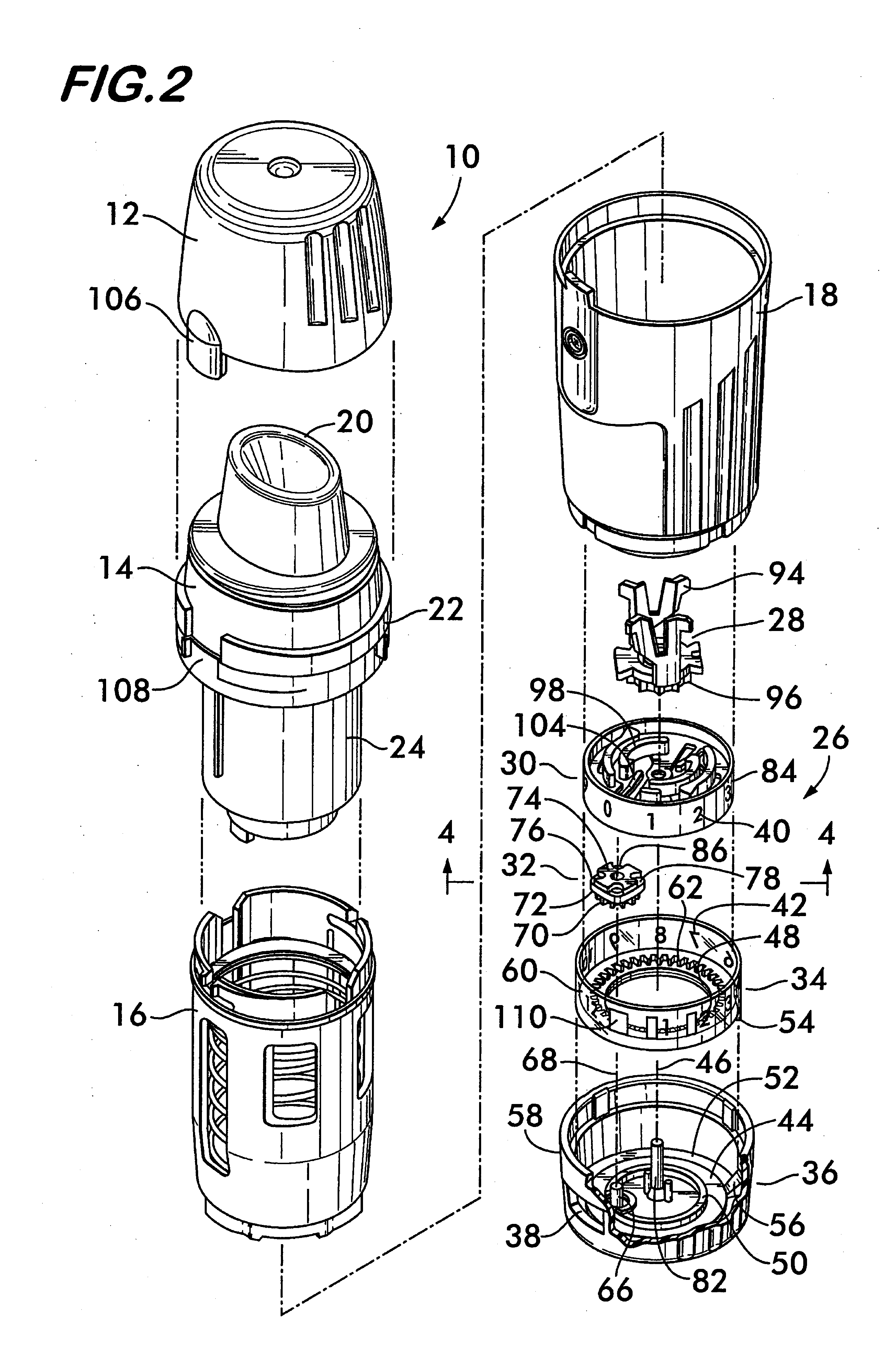
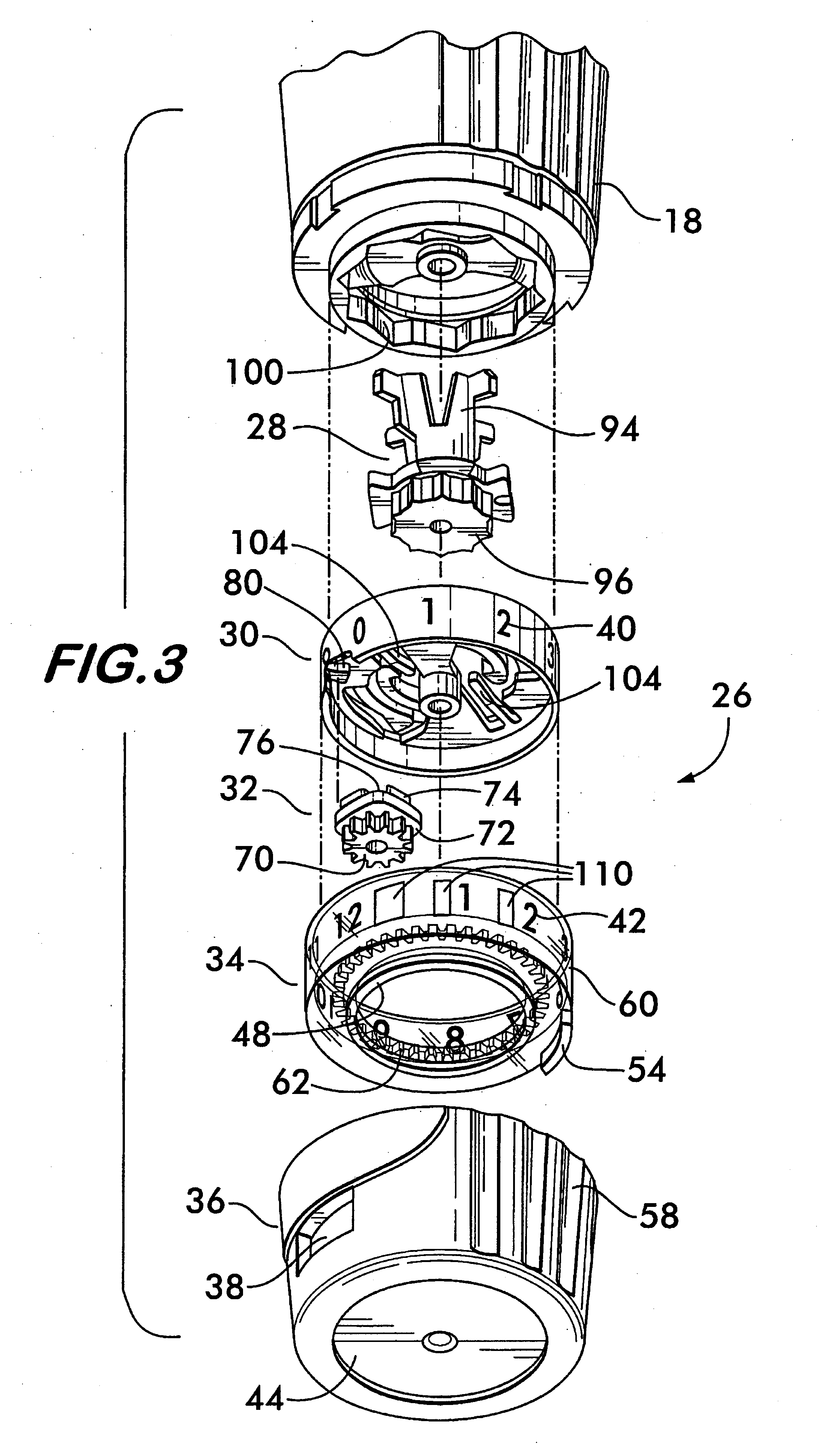
Patents
Literature
60 results about "Dry Powder Inhaler (device)" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
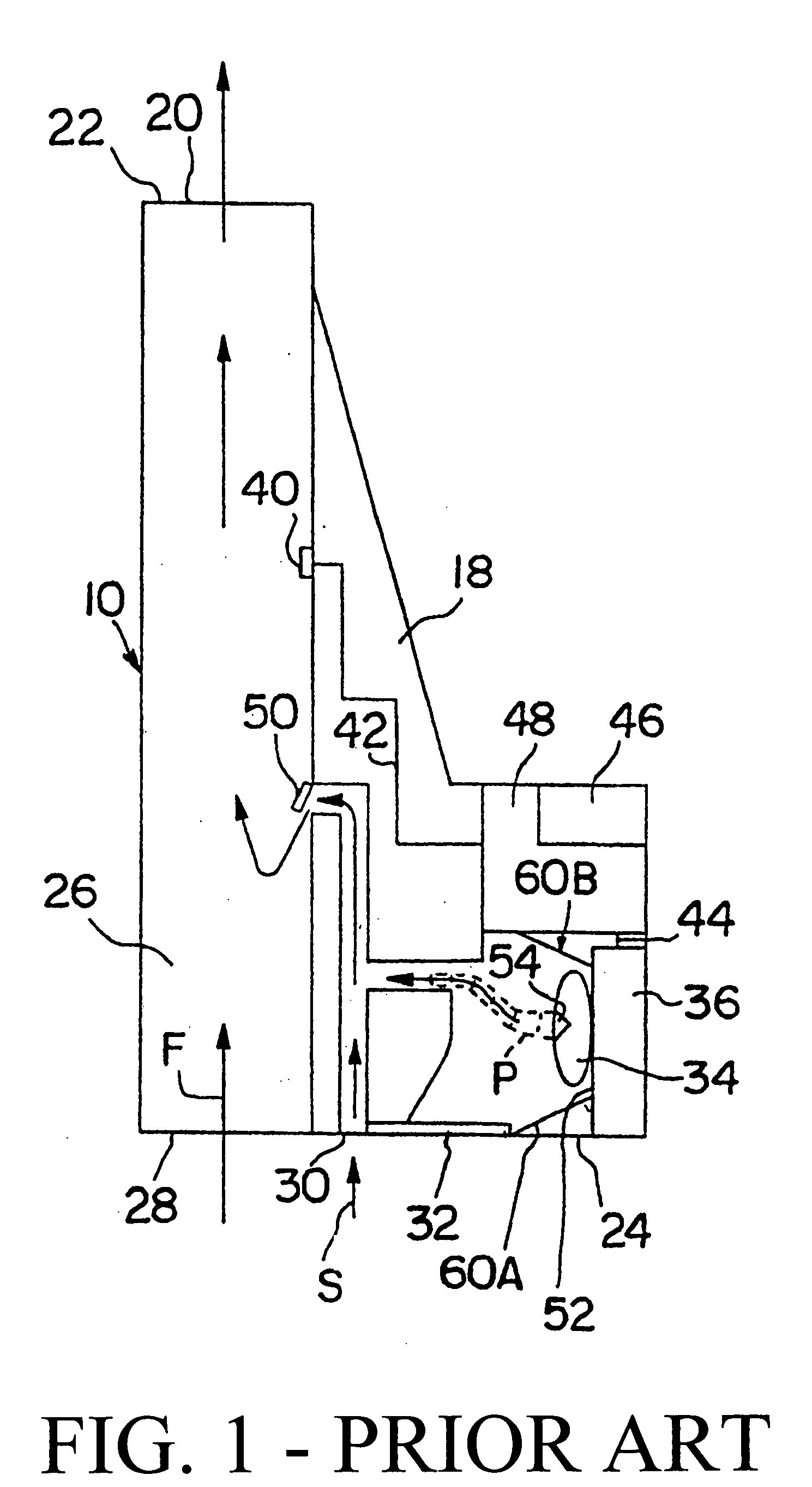
A dry-powder inhaler (DPI) is a device that delivers medication to the lungs in the form of a dry powder. DPIs are commonly used to treat respiratory diseases such as asthma, bronchitis, emphysema and COPD although DPIs (such as inhalable insulin Afrezza) have also been used in the treatment of diabetes mellitus.
Synthetic jet based medicament delivery method and apparatus
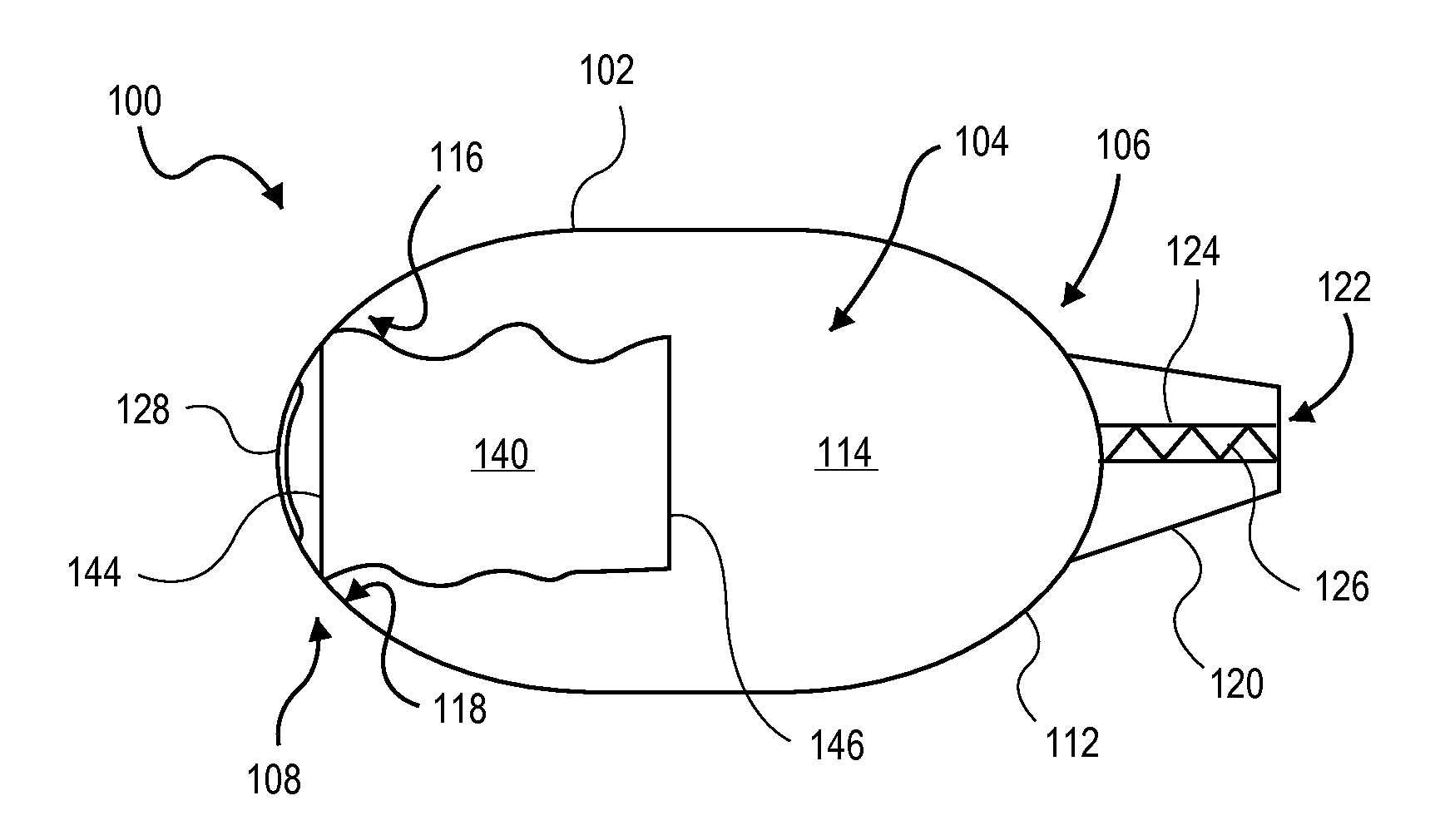
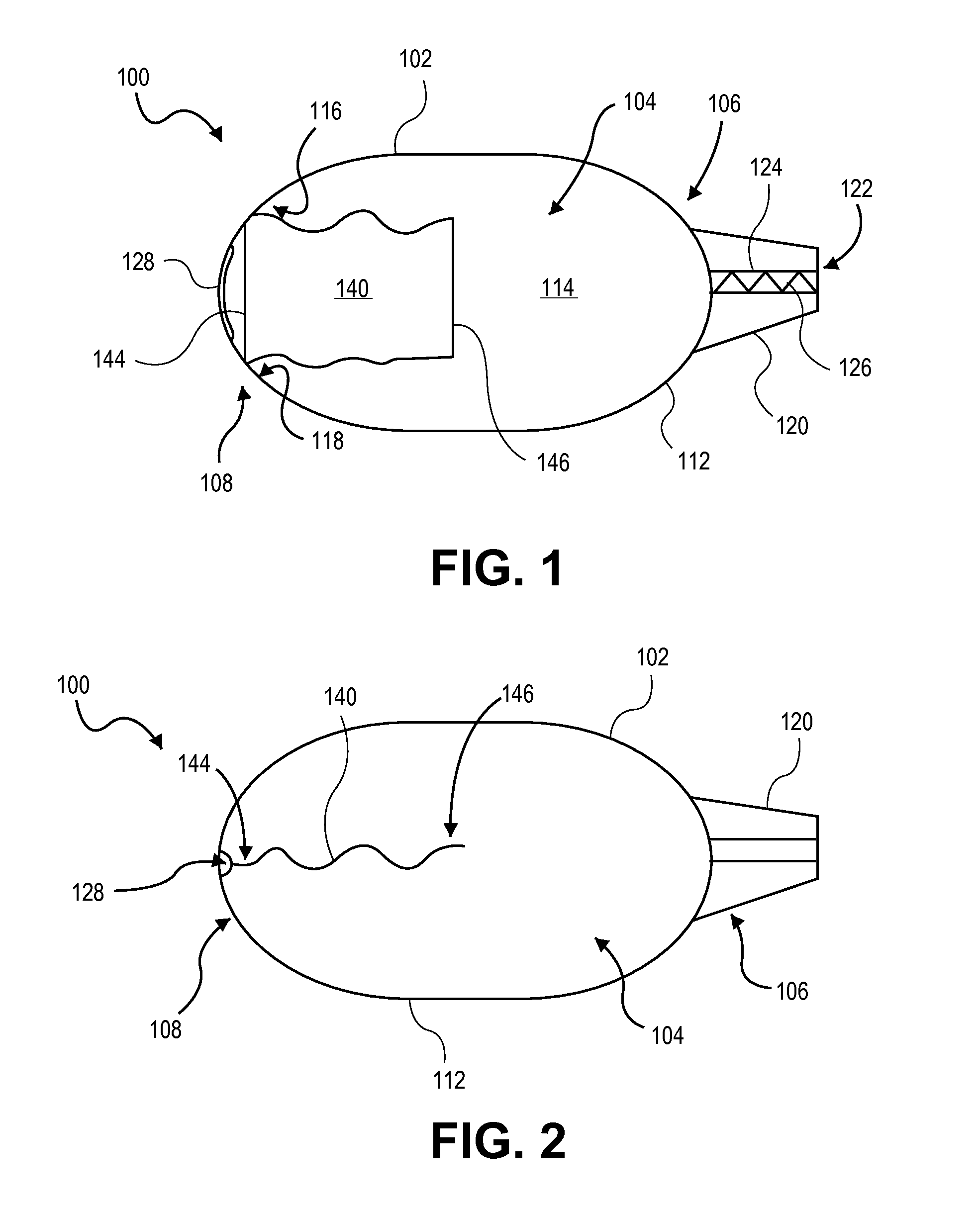
A dry powder inhaler consisting of first chamber having an orifice for holding a dry powder and a gas, and a second chamber for receiving a deaggregated form of the dry powder and for communicating the deaggregated dry powder to a user. A synthetic jet drives the dry powder from the first chamber to the second chamber.
Owner:MICRODOSE THERAPEUTX INC
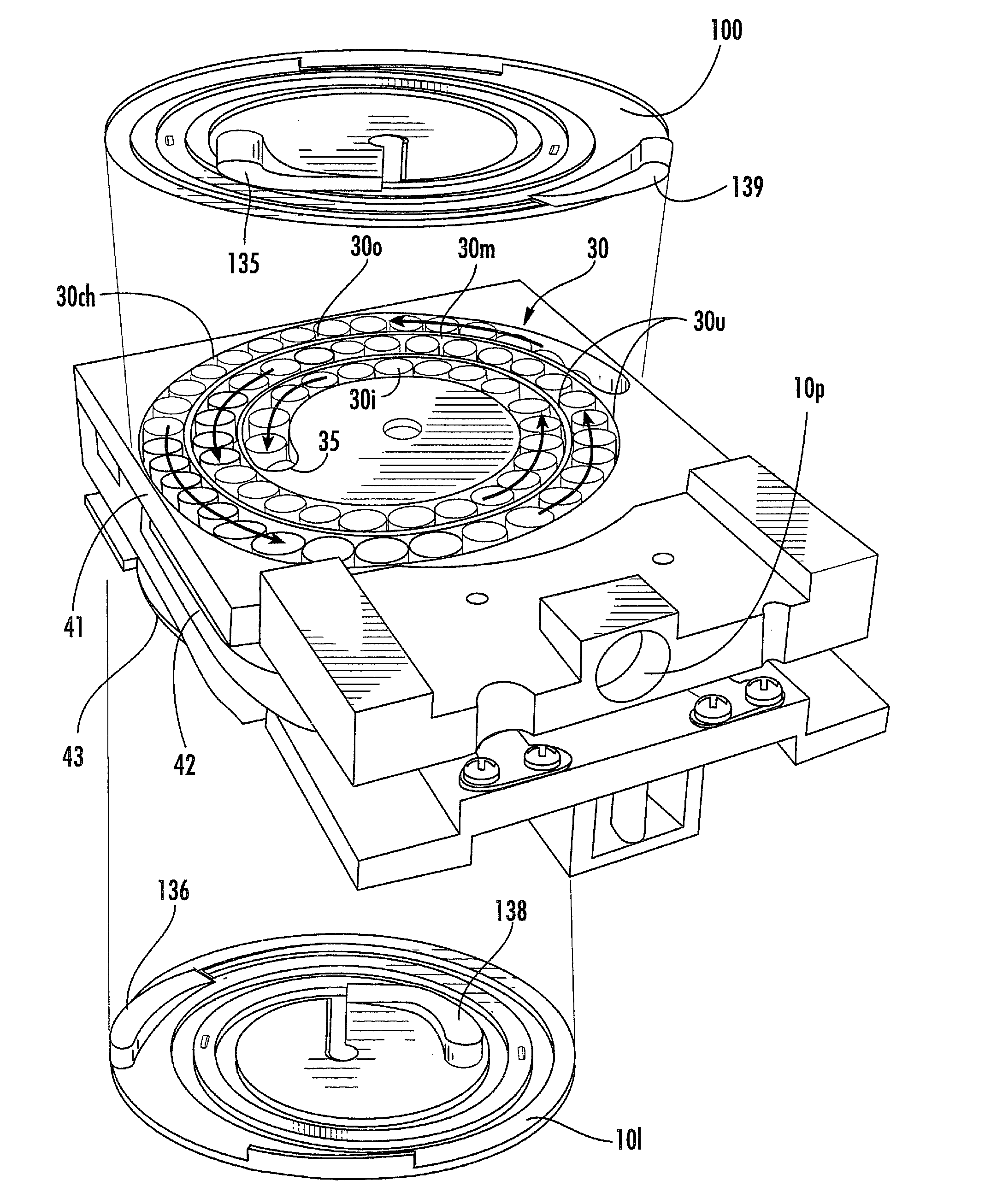
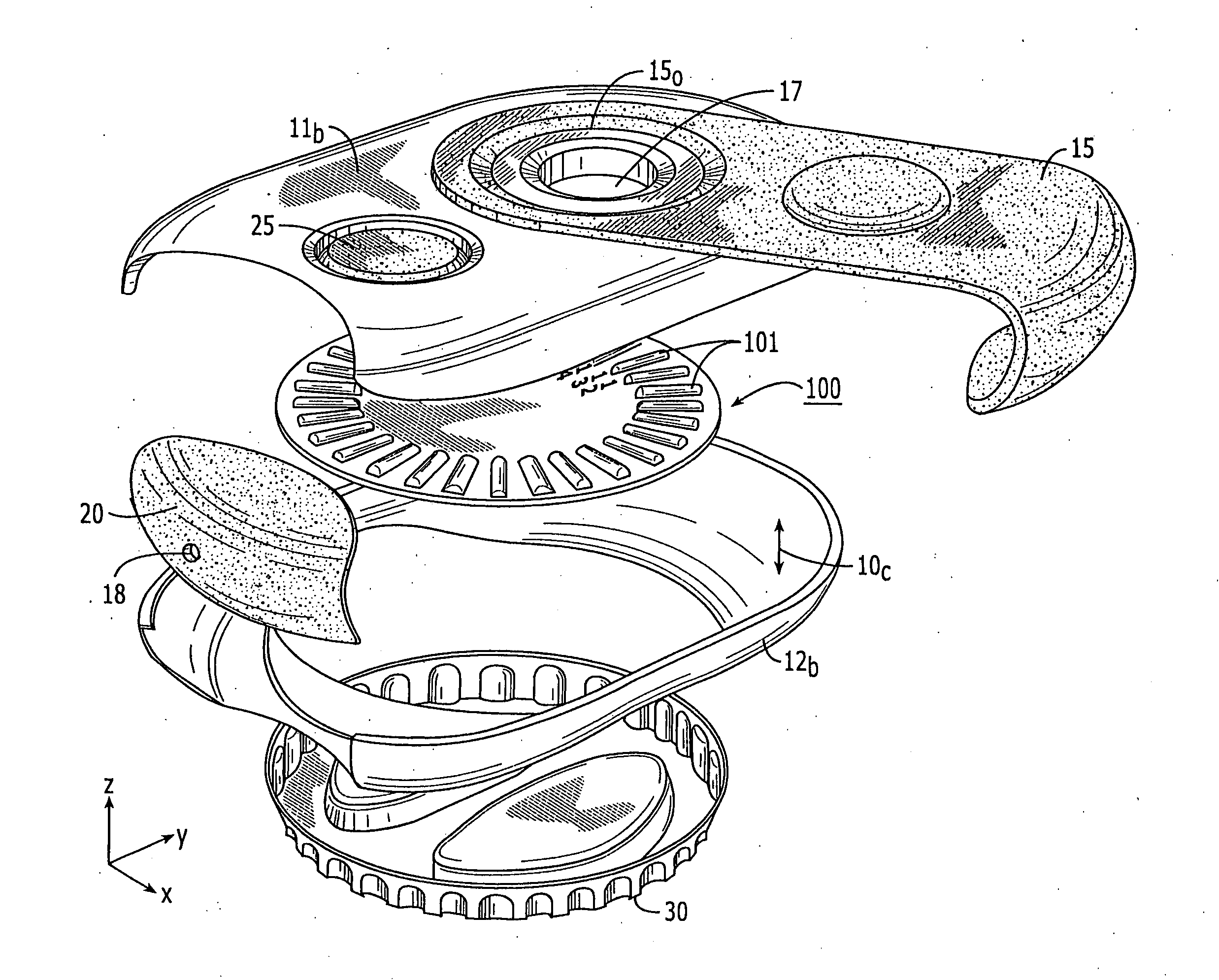
Dry powder inhalers having spiral travel paths, unit dose microcartridges with dry powder, related devices and methods
Dry powder inhalers include: (a) a first generally planar spiral travel path in an inhaler body, wherein the first spiral travel path has a plurality of adjacent curvilinear channels forming lanes with upstanding sidewalls, including an inner lane and an outer lane; and (b) a plurality of discrete sealed microcartridges with substantially rigid bodies disposed in the first travel path, each comprising a pre-metered (typically dose) amount of dry powder, the microcartridges being configured to slidably advance along the first travel path toward an inhalation chamber that merges into an inhalation output port. In operation, at least one microcartridge is held in the inhalation chamber to release the dry powder therein during inhalation.
Owner:ORIEL THERAPEUTICS INC
Inhaler for the administration of powdered pharmaceuticals, and a powder cartridge system for use with this inhaler
ActiveUS20060037612A1Cost-effective medical treatmentIncrease costRespiratorsLiquid surface applicatorsLocking mechanismSurgery
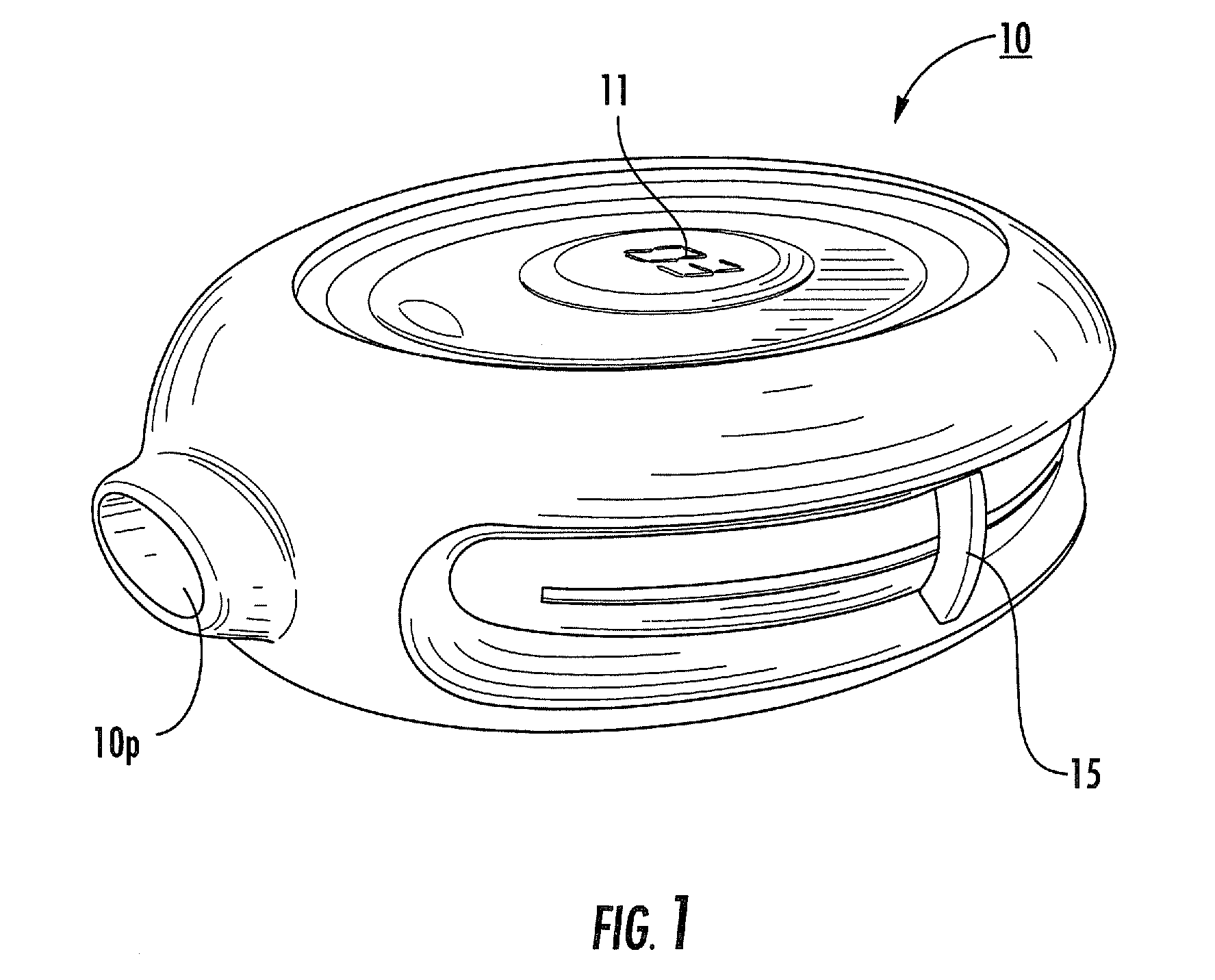
In order to improve the security and reliability of administration of powdered pharmaceuticals through dry powder inhalers, the invention proposes an inhaler (1) for powdered medicaments, comprising an activating device (4) for manual engagement by the patient for repeatedly metering a dose of medicament to be administered to the patient, and further comprising an advancing mechanism (25) for advancing a counter or indexing means (8) each time the activating device (4) has been engaged by the patient so that a dose of medicament has been released for administration to the patient, wherein the counter or indexing means (8) comprises an index (9), the index (9) being detectable by a detection means (10) of the inhaler, and the detection means (10) being coupled to a locking mechanism (12), the locking mechanism (12) blocking the activating device (4) and / or any transportation mechanism (5) of the inhaler (1) delayed by a predetermined number of metering cycles since detection of the index (9), and a cartridge (3).
Owner:ASTRAZENECA AB
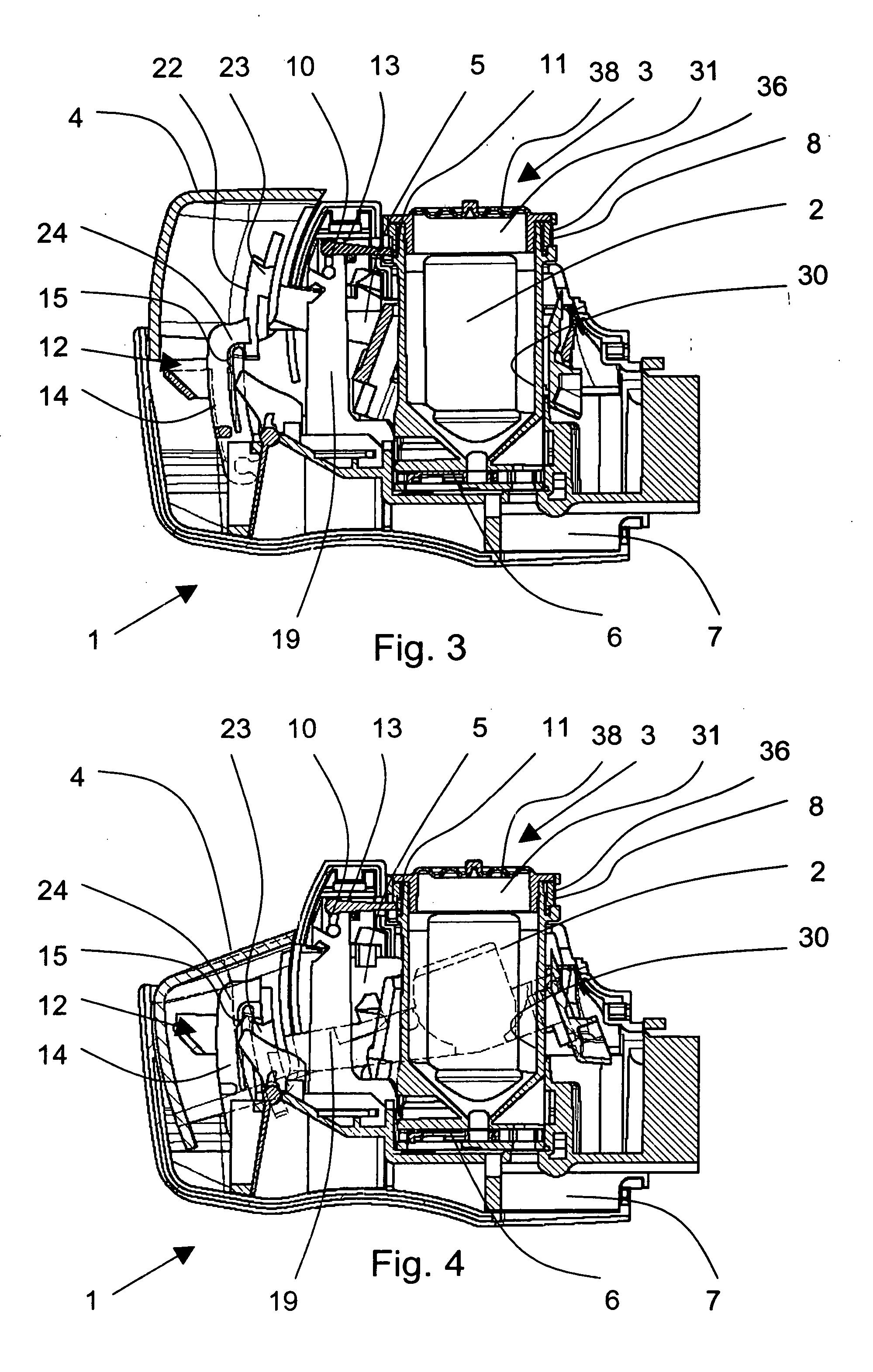
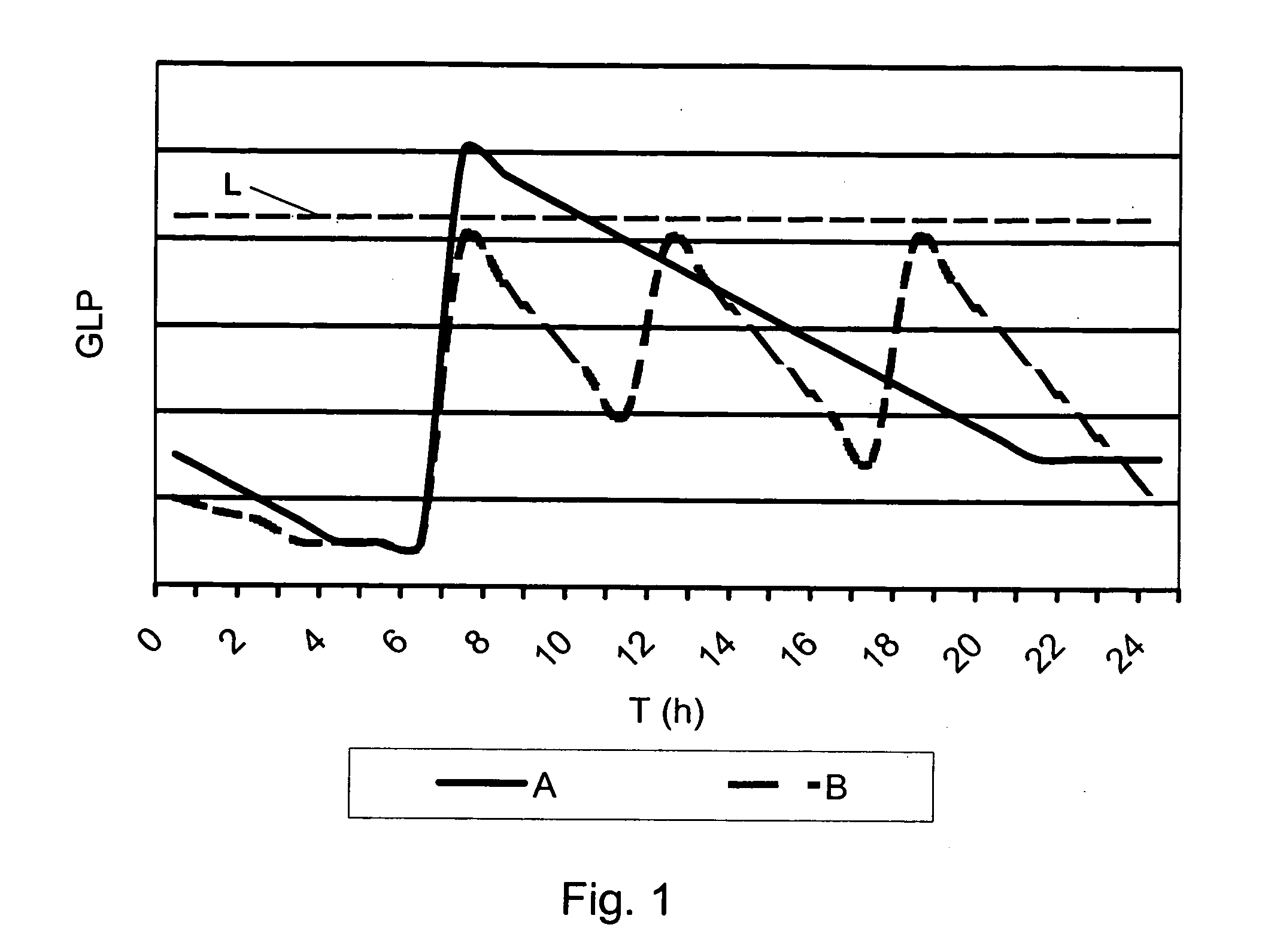
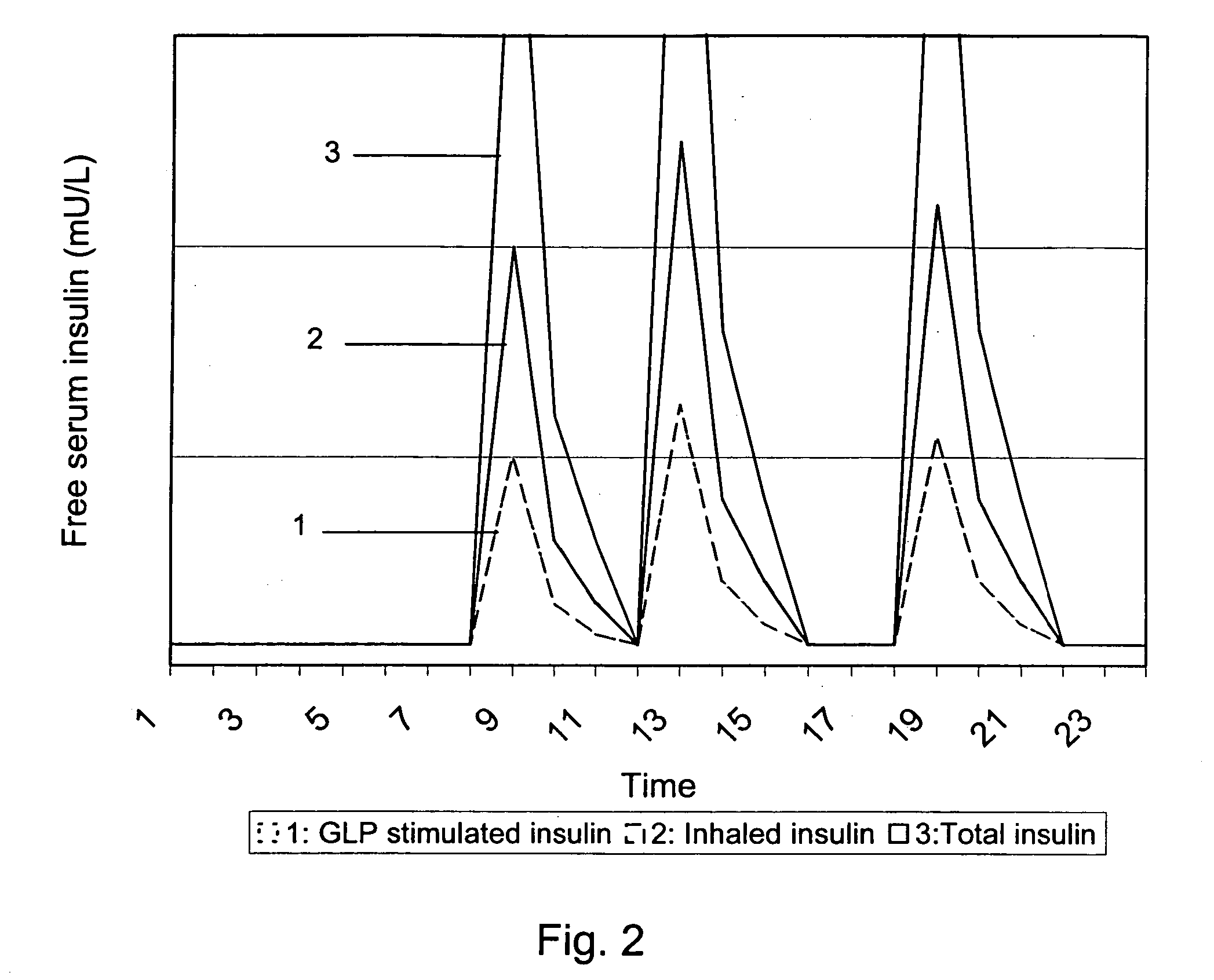
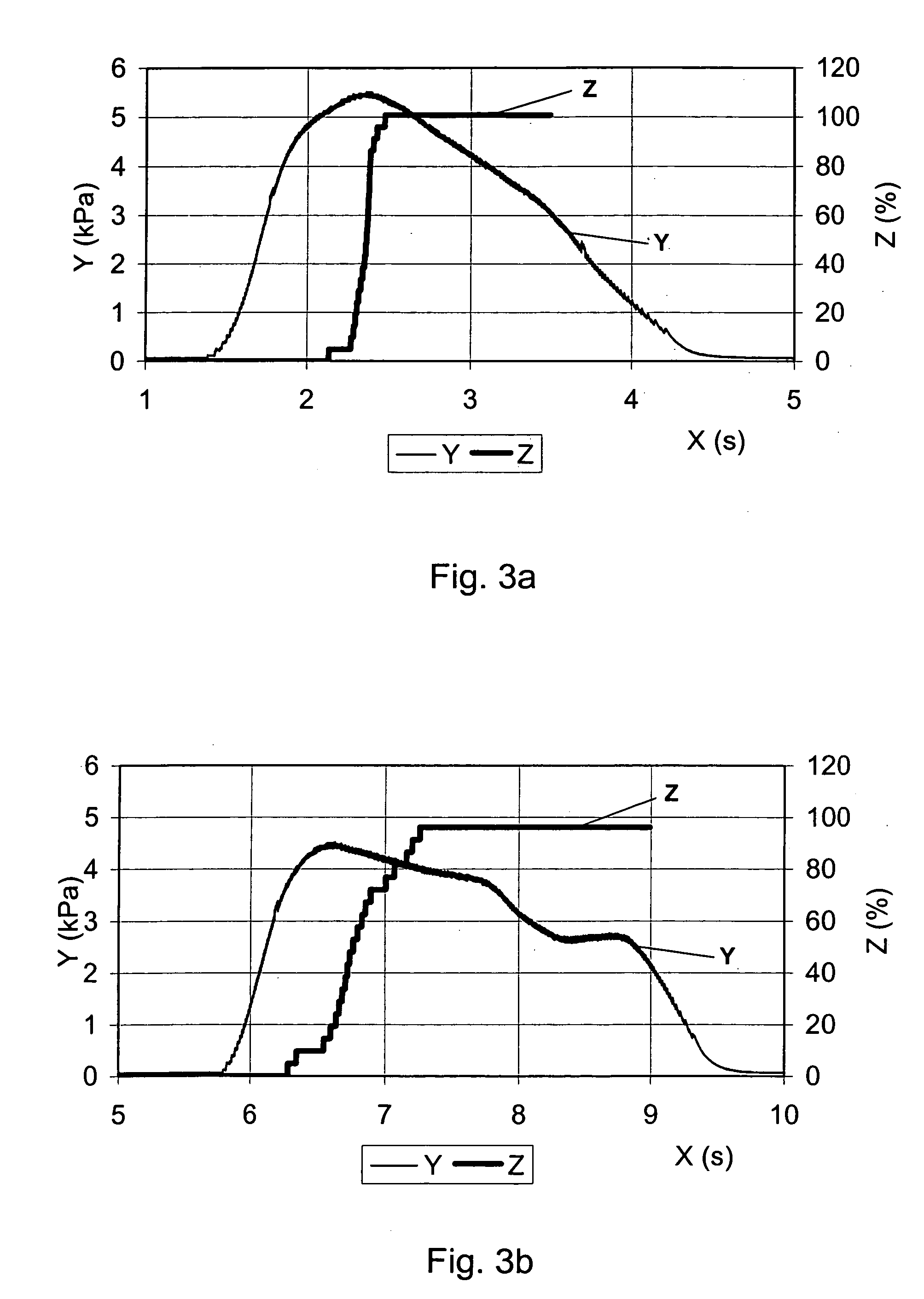
Medical product
InactiveUS20060239933A1Powder deliveryPeptide/protein ingredientsPulmonary inhalationMedical product
A medical product is disclosed. The medical product contains an accurately metered dose of at least one GLP medicament intended for pulmonary inhalation put into a moisture-tight, high barrier seal container. The medical product optionally also contains a dose of insulin. The container is adapted for application into a dry powder inhaler. The dose loaded in the container is intended for a prolonged delivery by inhalation to the deep lung where the active ingredients are absorbed into the system. Optionally the medical product also may comprise at least one biologically acceptable excipient.
Owner:MEDERIO AG
Dry powder inhaler system
InactiveUS20060254583A1Easy piercingRespiratorsPowder deliveryBULK ACTIVE INGREDIENTActive ingredient
An improved dry powder inhalation system comprising: at least one micronized active ingredient in an hydroxypropylmethylcellulose capsule (10), and an dry powder inhaler device equipped with piercing systems (12) having an equivalent diameter of not less than 0.8 mm.
Owner:GALEPHAR PHARMA RES
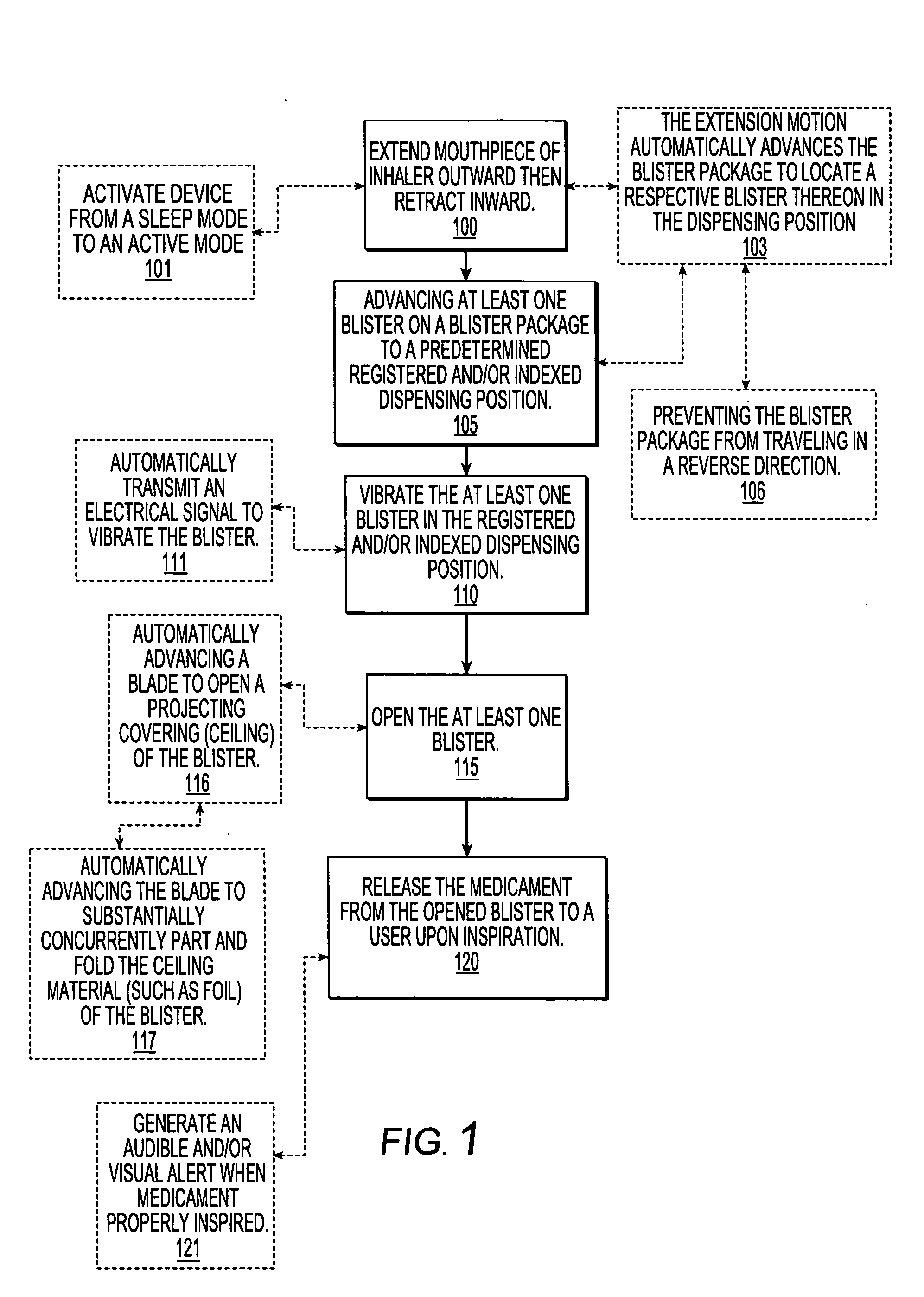
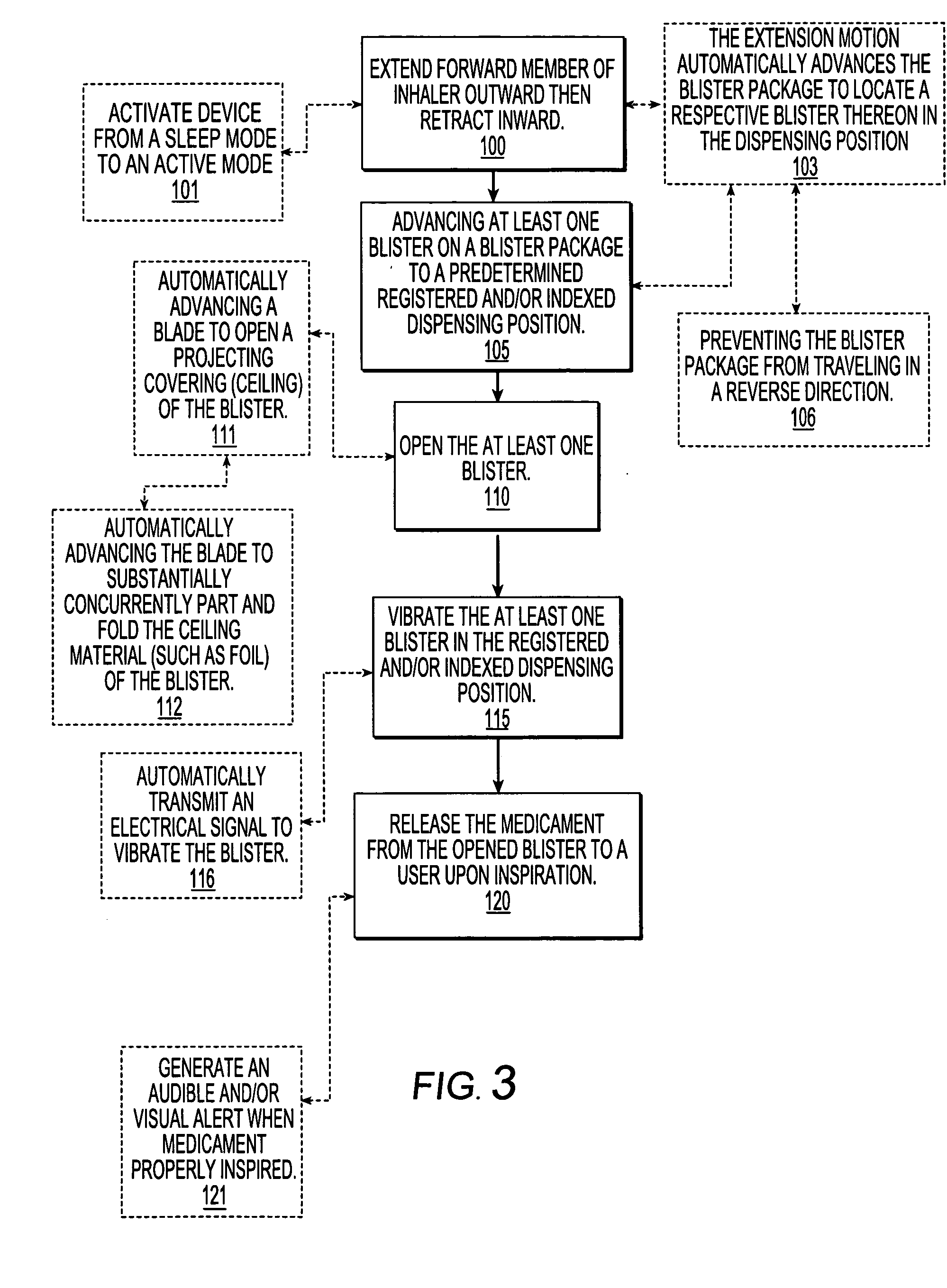
Dry powder inhalers, related blister package indexing and opening mechanisms, and associated methods of dispensing dry powder substances
Dry powder inhalers with a multi-dose dry powder package for dispensing pharmaceutical grade formulations of inhalable dry powder, include: (a) a blister package comprising a plurality of spaced apart sealed blisters thereon, each blister having a projecting ceiling and a floor defining a blister channel therebetween, the blister channel comprising a dry powder therein; (b) a movable blade cartridge holding a blade at a forward portion thereof; and (c) an extendable mouthpiece attached to the movable blade cartridge. In operation, a user pulls the mouthpiece outward and then pushes the mouthpiece inward to cause the blister package to advance to position a blister in a selected dispensing position in the inhaler and to cause the blade cartridge to move the blade across a blister ceiling held in the dispensing position in the inhaler to thereby open the blister held in the dispensing position.
Owner:ORIEL THERAPEUTICS INC
Human-powered dry powder inhaler and dry powder inhaler compositions
InactiveUS20080035143A1Easy to useEfficient deliveryRespiratorsPowder deliveryNoseBULK ACTIVE INGREDIENT
In one embodiment, a human-powered dry powder inhaler comprises a human-powered compressible component operable to discharge an air pulse at an outlet at a pressure of about 1-40 psi; an inflatable reservoir operable to receive an air pulse discharged from the human-powered compressible component to provide an aerosol of a dry powder pharmaceutical formulation in the reservoir, the reservoir including an outlet valve; and a receiving mask in communication with the outlet valve and operable to receive an aerosol of dry powder from the reservoir and to deliver the aerosol to at least a mouth or nose of a patient. In another embodiment, the inhaler comprises a human-powered compressible component operable to discharge an air pulse at an outlet of a polymeric pressure release valve at a pressure of about 1-40 psi; and a receiving mask in communication with the outlet of the compressible component and operable to deliver an aerosol of dry powder to at least a mouth or nose of a patient. Methods for delivery of a dry powder pharmaceutical formulation to a patient are conducted in the absence of electrical power and circuitry and pre-pressurized propellant gas. Suitable dry powder pharmaceutical formulations may include myo-inositol and / or maltodextrin as a carrier and active ingredients such as vaccines or siRNA.
Owner:UNIV OF COLORADO THE REGENTS OF
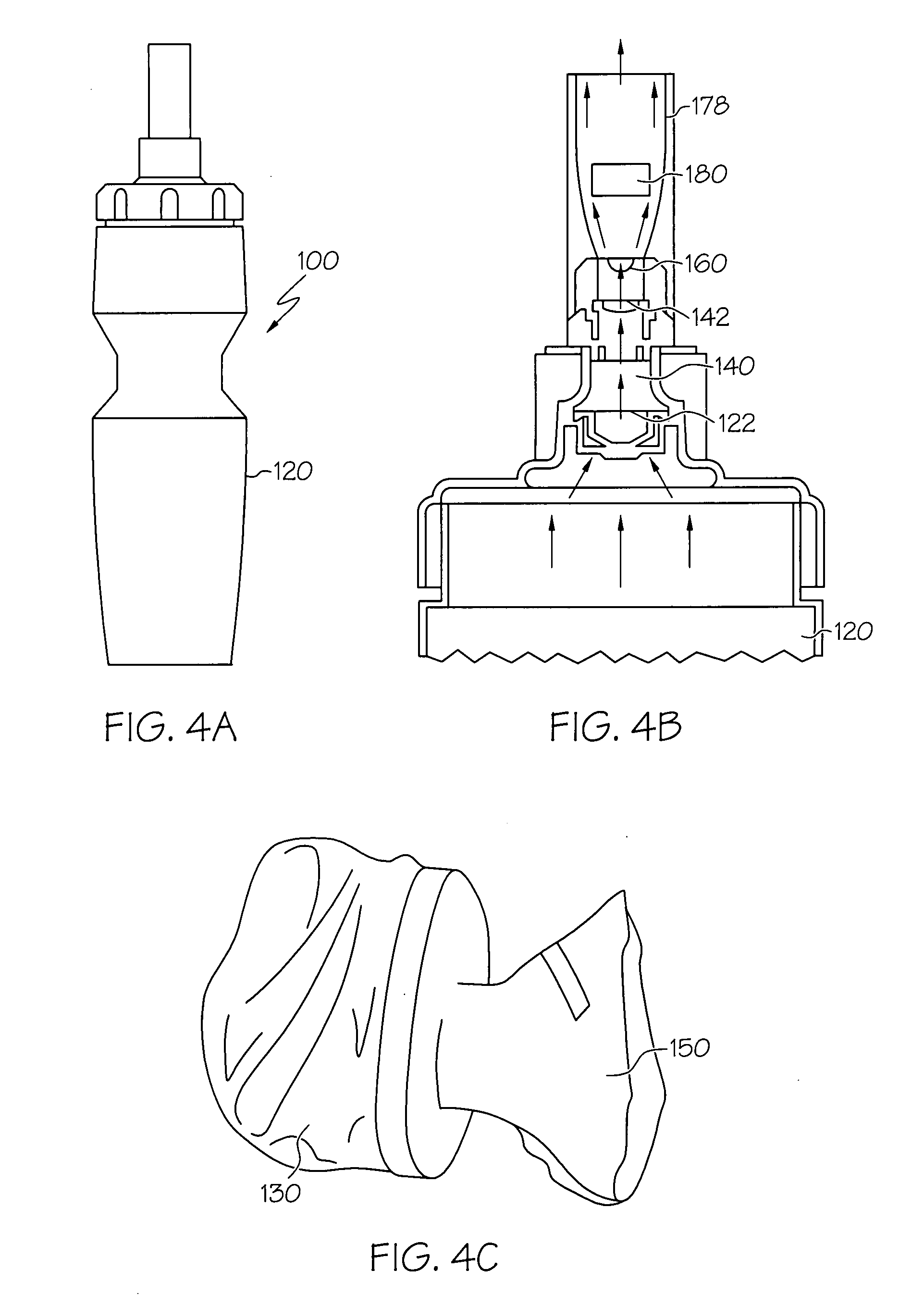
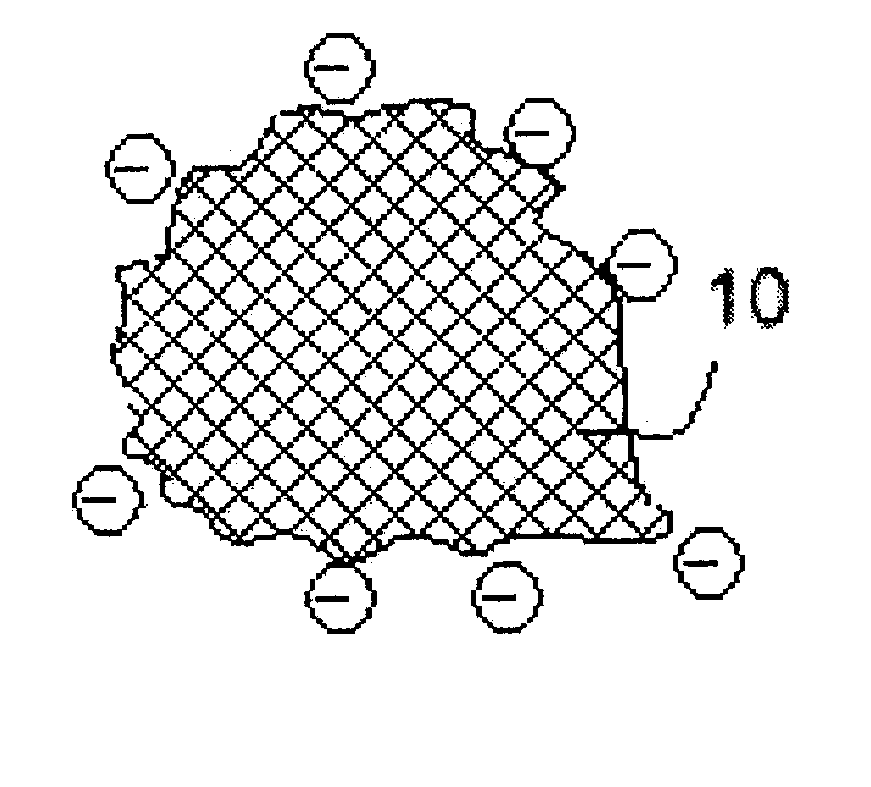
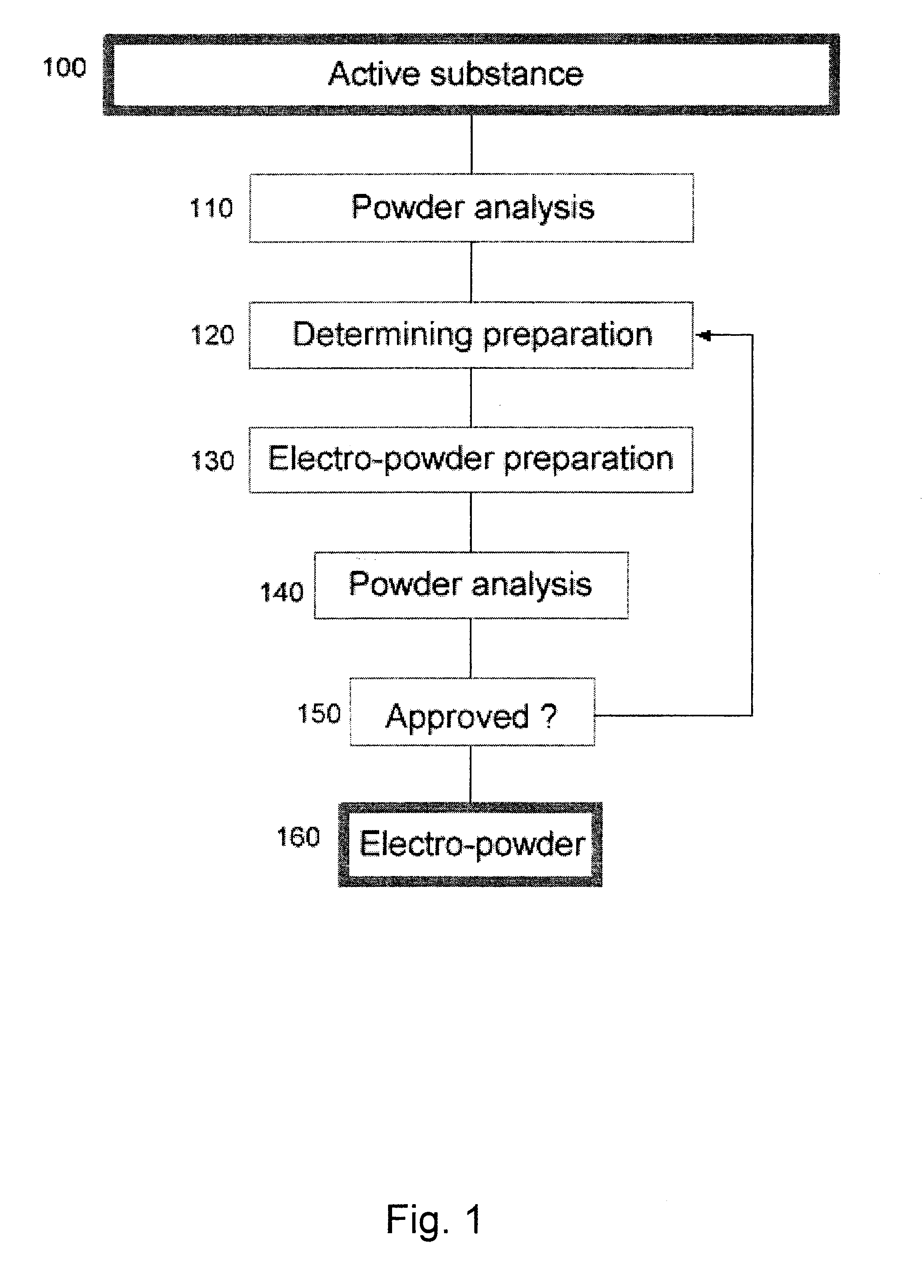
Electro-powder
A method and a process are disclosed for preparation of medical electro-powders. The electro-powder results from preparations of chemical and biological substances to form electro-powders suitable for electrostatic charging and dosing for functionality in a dry powder inhaler device. The electro-powder resulting from the method and process forms an active powder substance or a dry powder medical formulation with a fine particle fraction representing of the order 50% or more of the content having a size ranging between 0.5-5 mum and provides electrostatic properties with an absolute specific charge per mass after charging of the order 0.1x10<-6 >to 25x10<-6 >C / g and presenting a charge decay rate constant Q50>0.1 sec with a tap density of less than 0.8 g / ml and a water activity aw of less than 0.5. In the processing the active substance is a generally pharmaceutical active chemical or biological substance, for instance a polyeptide or any other corresponding substance selected alone or mixed or blended together with one or more excipients being a compound to improve electrostatic properties of the medical dry powder substance or dry powder medical formulation. Further the electro-powder may even be formed as a micro-encapsulation by coating micronized powder with the excipient in such a way that the active substance is capsulated, whereby the powder electrostatic properties mainly comes from the excipient.
Owner:MEDERIO AG
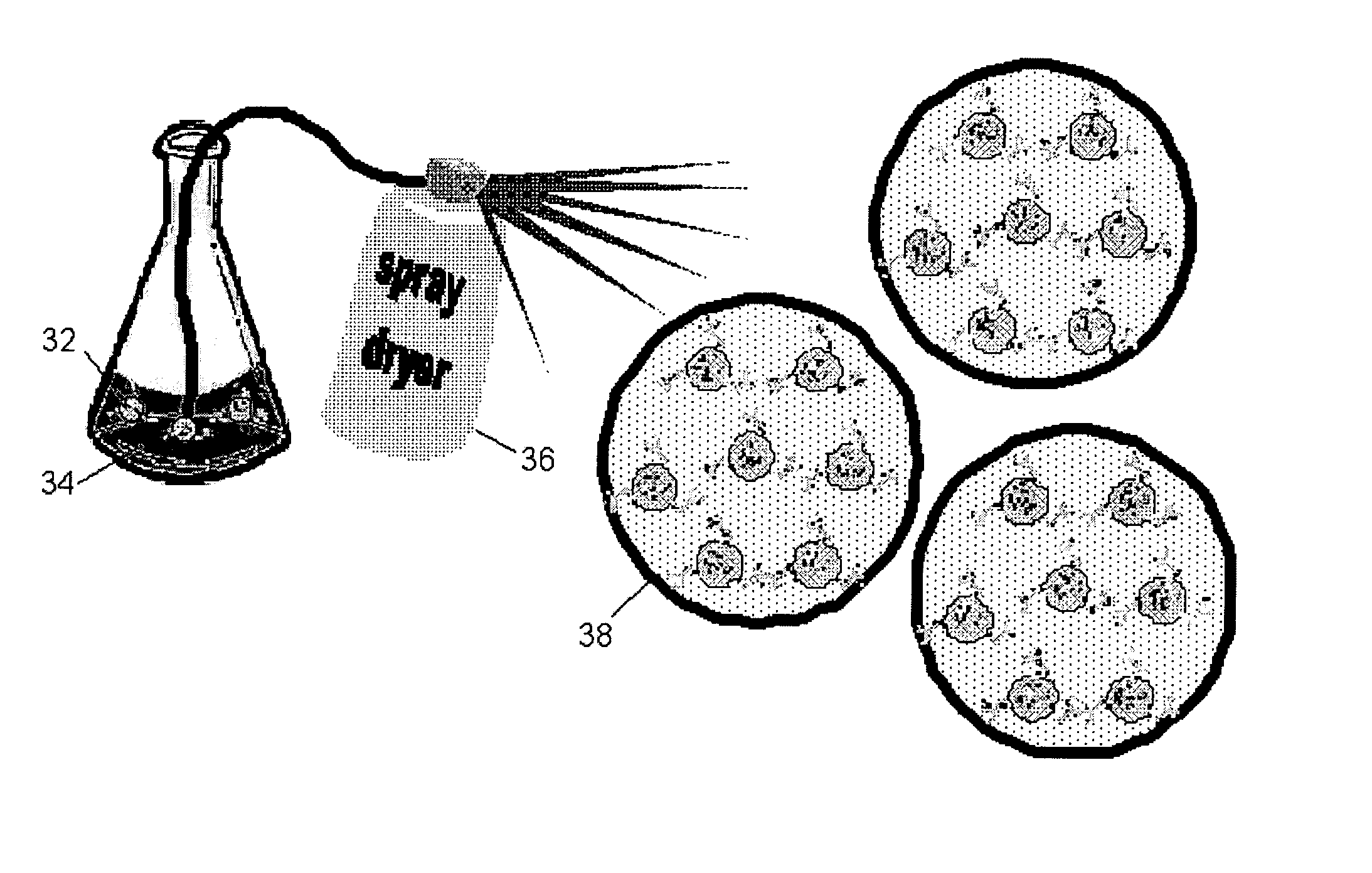
Formulation of powder containing nanoparticles for aerosol delivery to the lungs
Respirable particles carrying active principles or diagnostics in nanoparticle form are created by mixing the nanoparticles with liquid carrier, then forming the resultant mixture into respirable particles. Spray-drying, freeze spray drying and drying followed by comminution may be used to create the respirable particles, which may be delivered to the lung via a dry powder inhaler. In one example, lactose was used as the excipient and spray-dried with two different types of nanoparticle: gelatin and poly butylcyanoacrylate nanoparticles. The incorporation of nanoparticles did not affect the respirable fraction of the carrier powders.
Owner:FINLAY WARREN HUGH +2
Synthetic jet based medicament delivery method and apparatus
A dry powder inhaler consisting of first chamber having an orifice for holding a dry powder and a gas, and a second chamber for receiving a deaggregated form of the dry powder and for communicating the deaggregated dry powder to a user. A synthetic jet drives the dry powder from the first chamber to the second chamber.
Owner:MICRODOSE THERAPEUTX INC
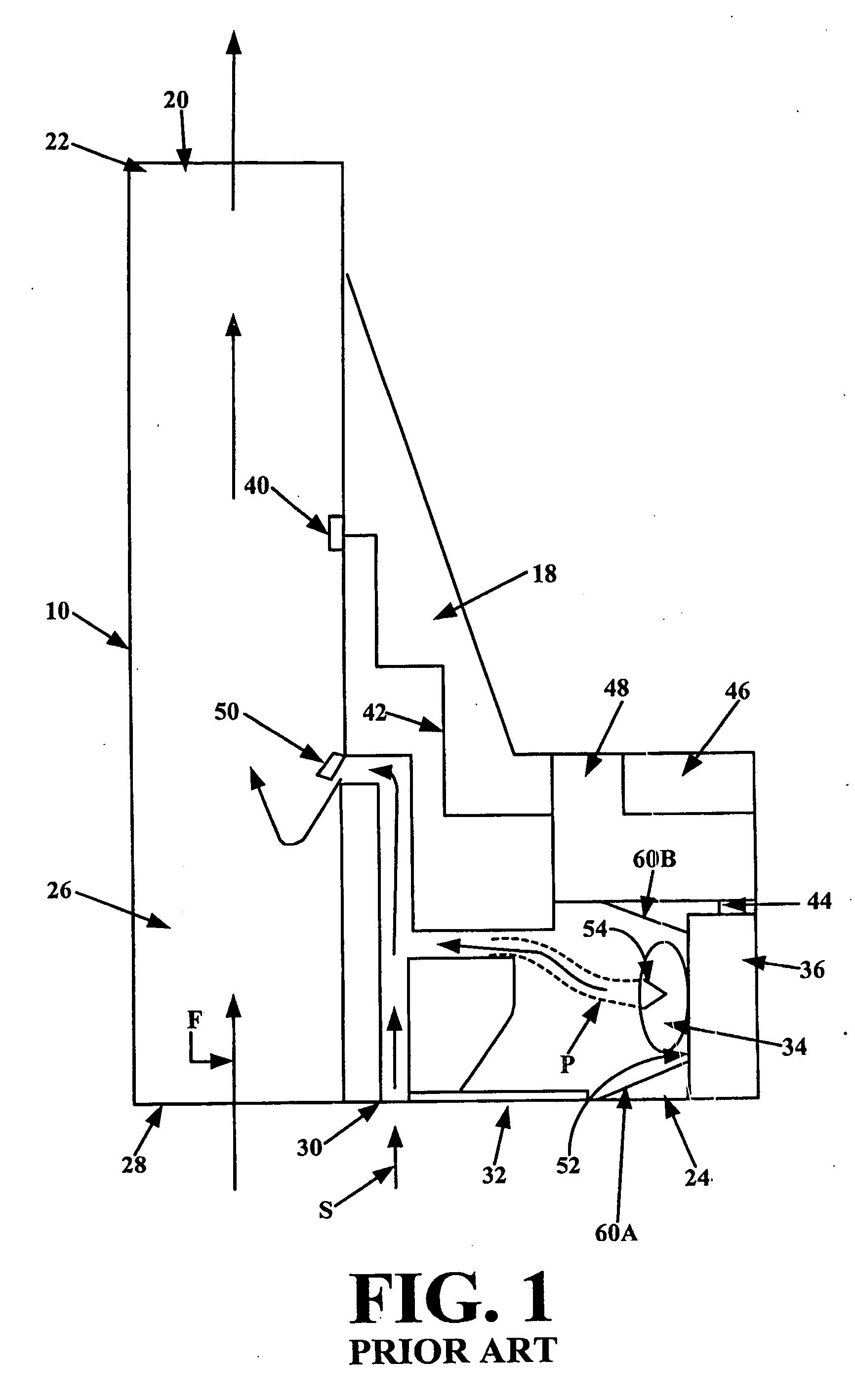
Dry Powder Inhalers that Inhibit Agglomeration, Related Devices and Methods
ActiveUS20080127971A1Promote depolymerizationLiquid surface applicatorsPowdered material dispensingInspiratory flowInhalation
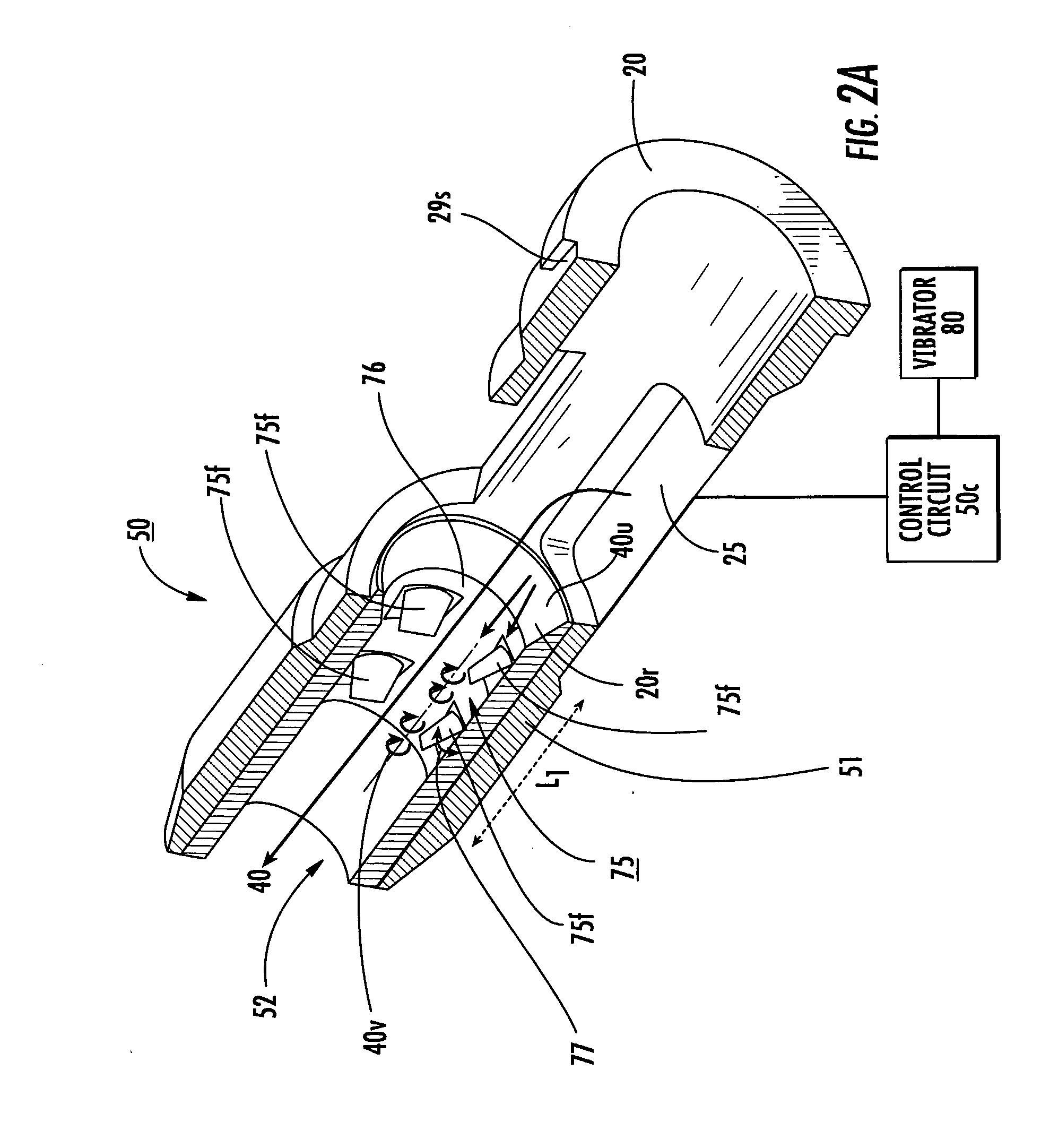
The disclosure describes methods and inhalers that deagglomerate dry powder using inspiratory effort of a user of an inhaler. Inhaler fin and mesh configurations are described that facilitate deagglomeration. At least in steady state conditions, a dry powder and airflow pattern can be generated having turbulence with flow vortices, some of which may have a vortex having an axis of rotation that extends in an inspiratory flow direction while others may have a vortex that is substantially orthogonal to the inspiratory flow direction in an inspiratory airflow path, as an amount of dry powder travels through the inhaler to thereby deagglomerate the dry powder without trapping undue amounts of the dry powder during inhalation.
Owner:ORIEL THERAPEUTICS INC
Methods and systems for dosing and coating inhalation powders onto carrier particles
A method of method of coating powdered medical agent onto a carrier particle for use in a dry powder inhaler may include applying ultrasonic energy to agglomerated powdered medical agent to deaggregate and aerosolize particles of the medical agent into particles having a desired average particle size, and coating at least one carrier particle with a desired amount of the deaggregated and aerosolized particles of the medical agent.
Owner:STC UNM
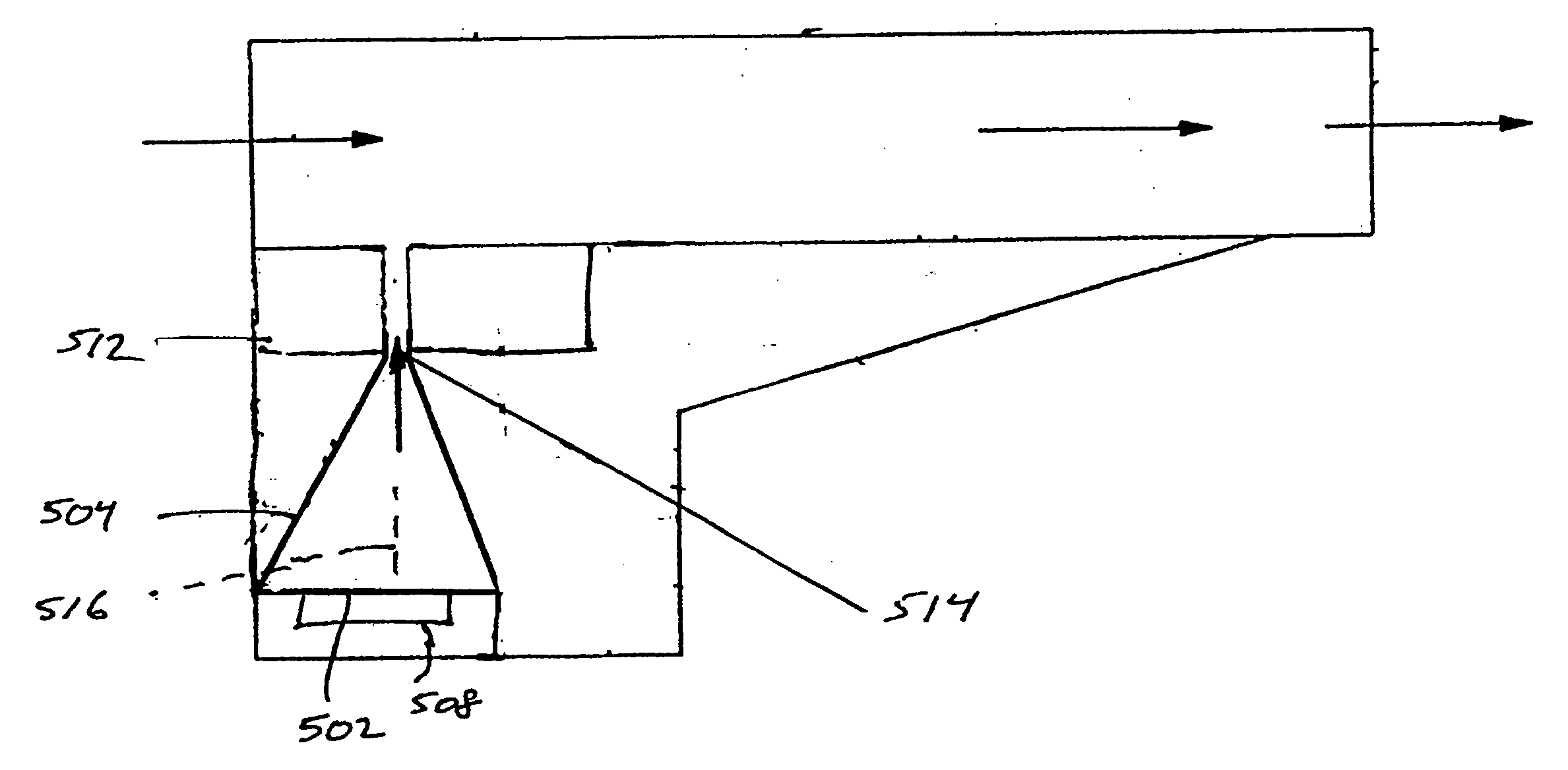
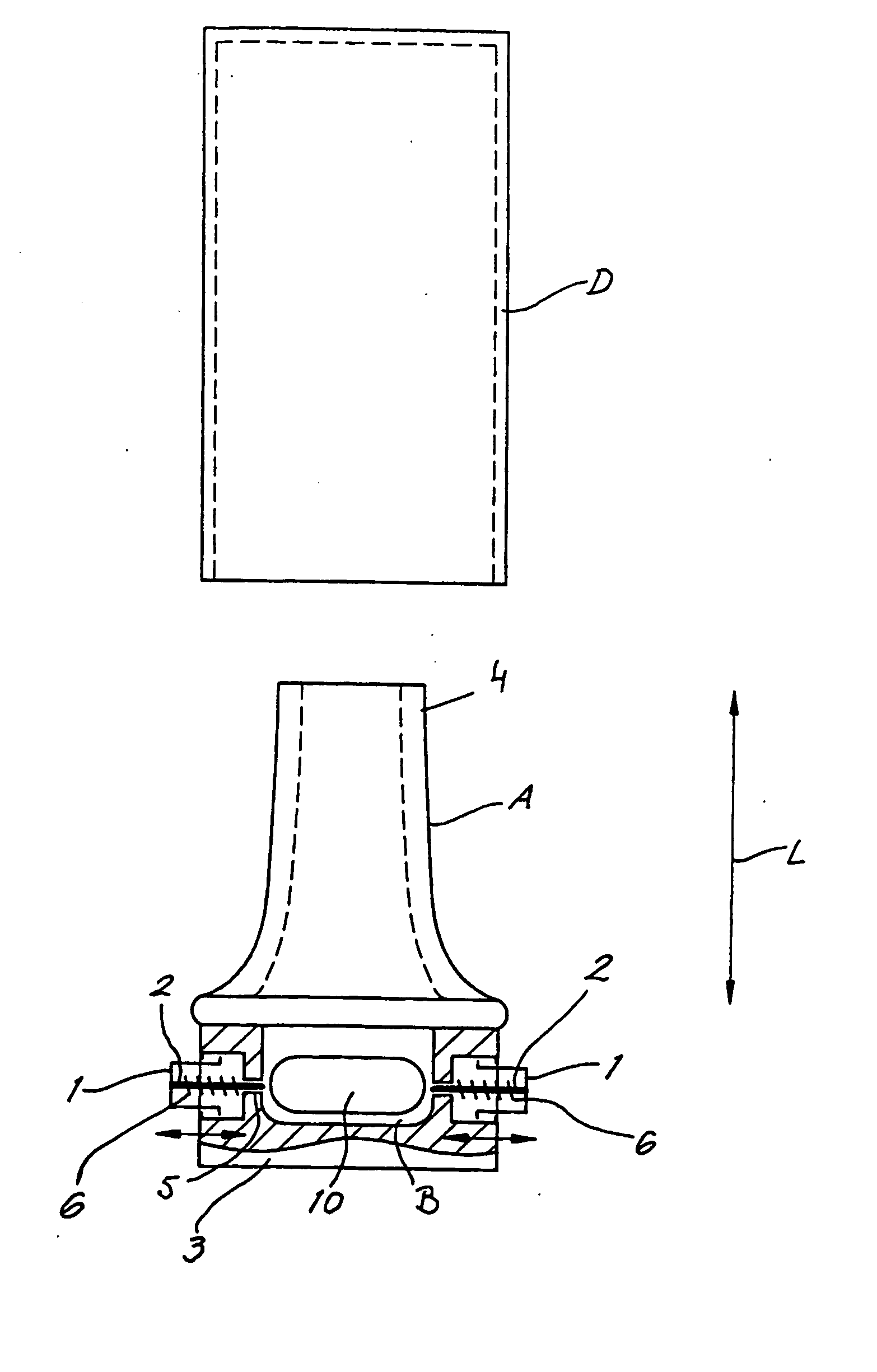
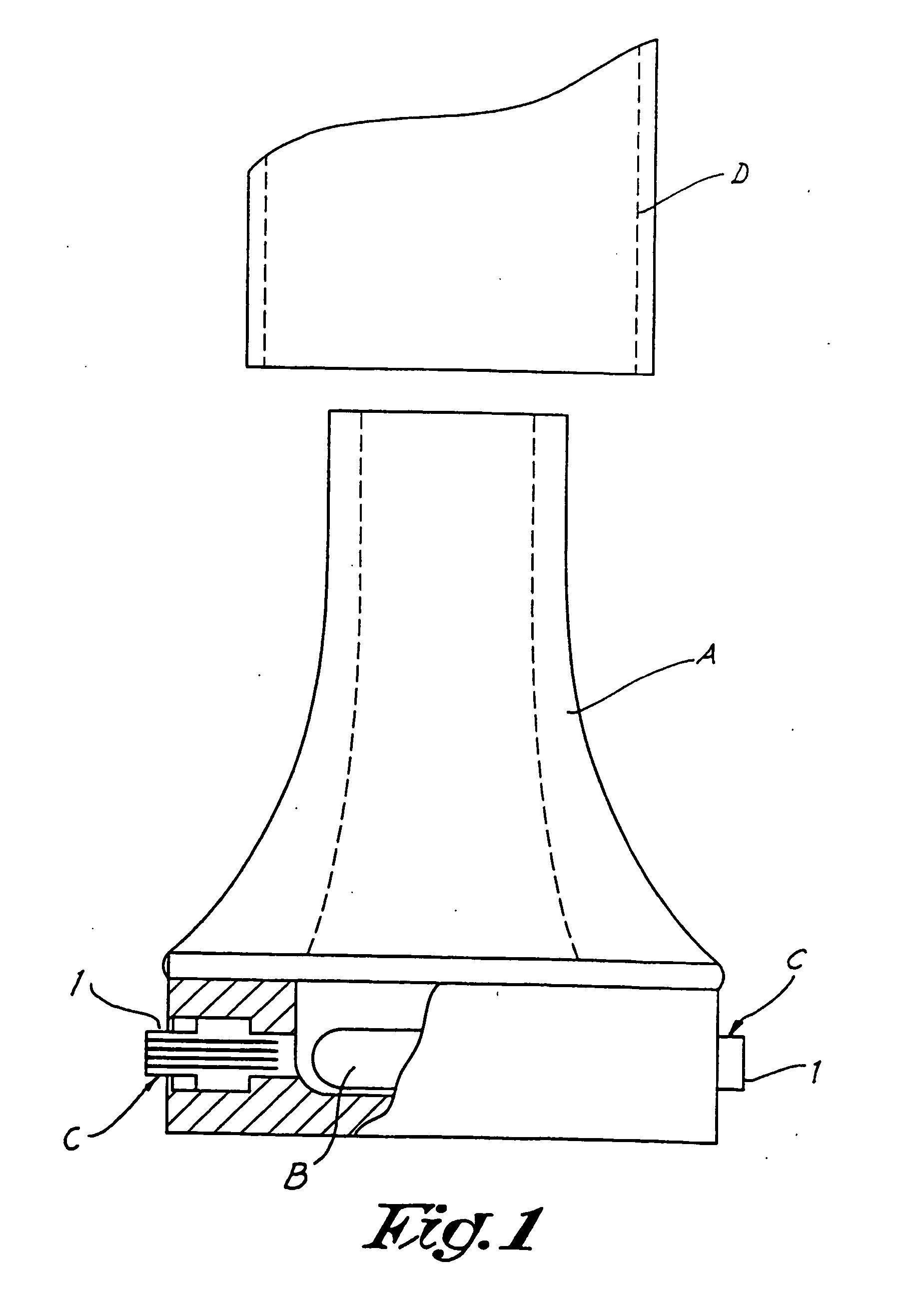
Add-on spacer design concept for dry-powder inhalers
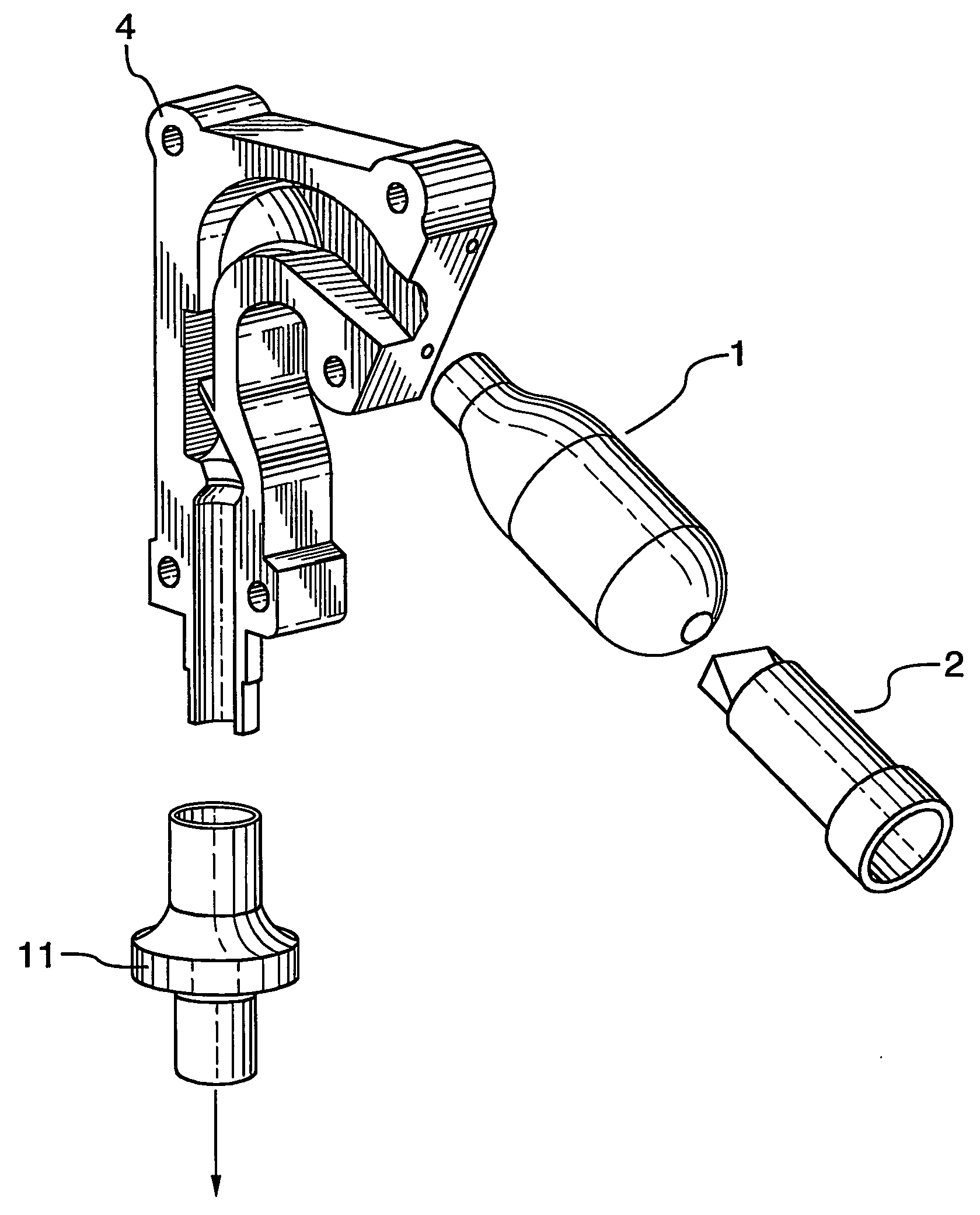
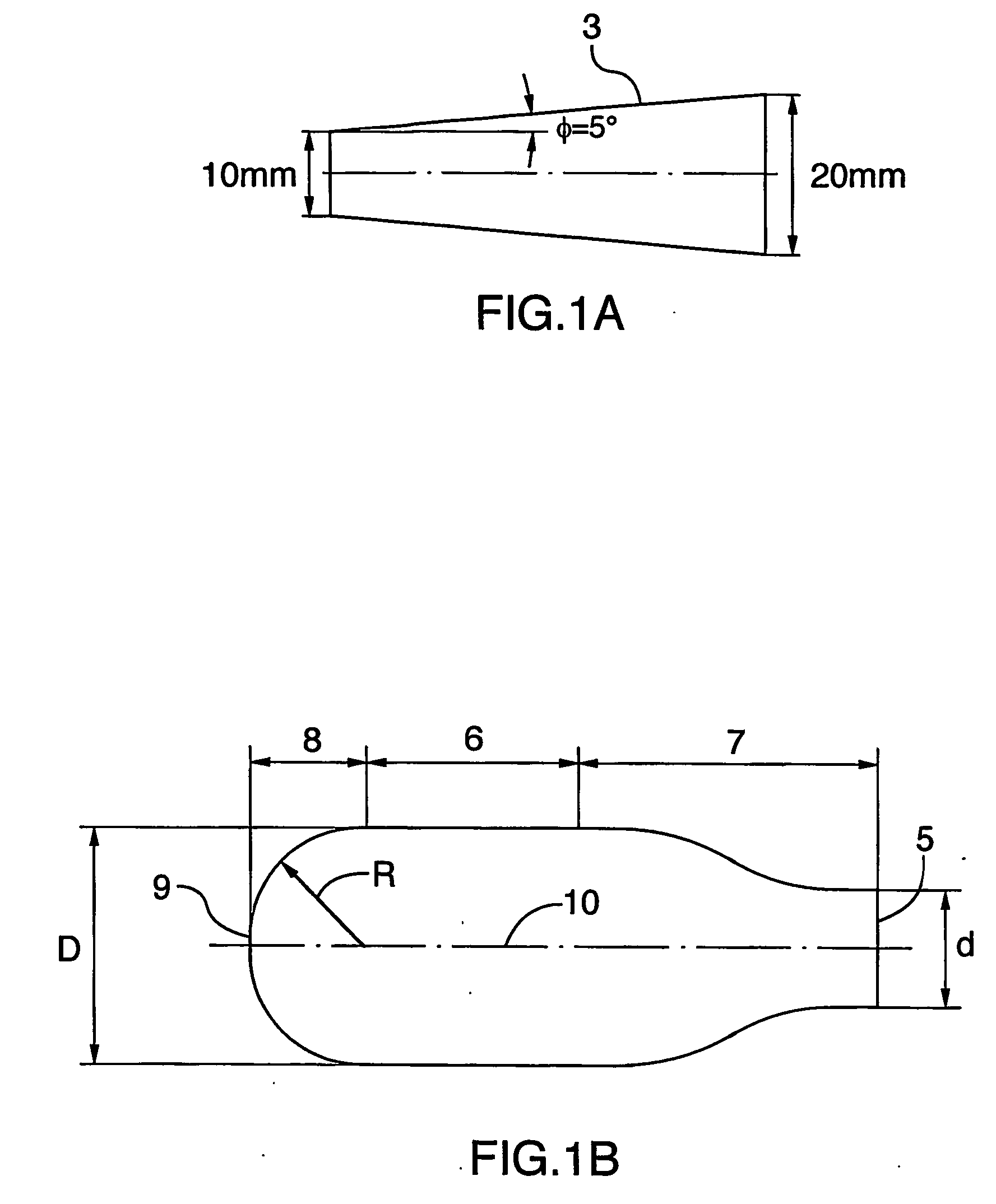
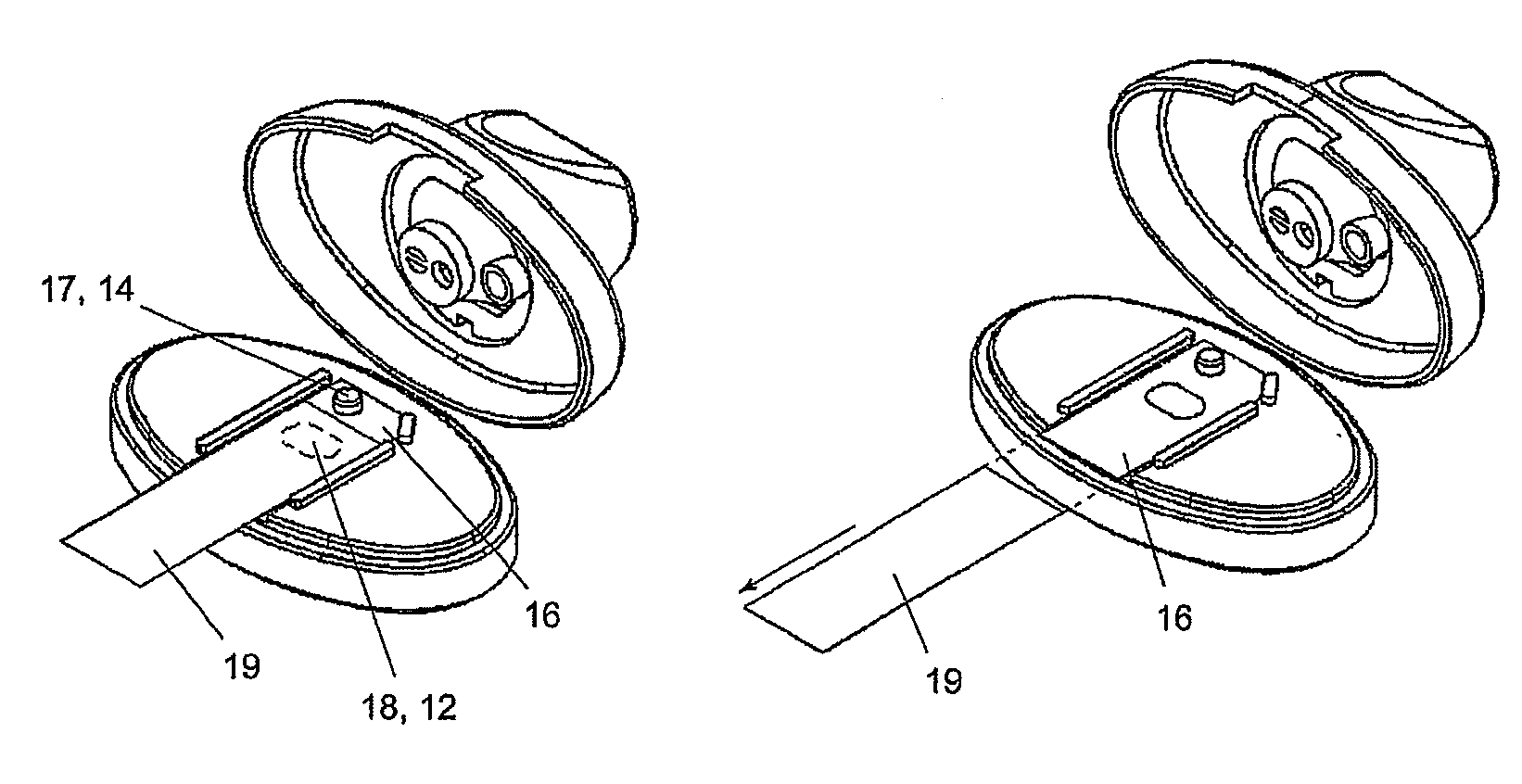
A spacer, for disposition between a user's mouth and a medicament inhaler outlet, has a hollow body defining an elongate internal chamber (10) with a diffuser portion (8) having a spacer inlet (9) adapted to engage the inhaler outlet in communication with the internal chamber, the diffuser portion extending axially outwardly from the spacer inlet; a buffer portion (6) extending axially from the diffuser portion; and a nozzle portion (7) having a spacer outlet (5) adapted to engage the user's mouth in communication with the internal chamber, the nozzle portion extending axially inwardly from the buffer portion.
Owner:ALBERTA UNIV OF
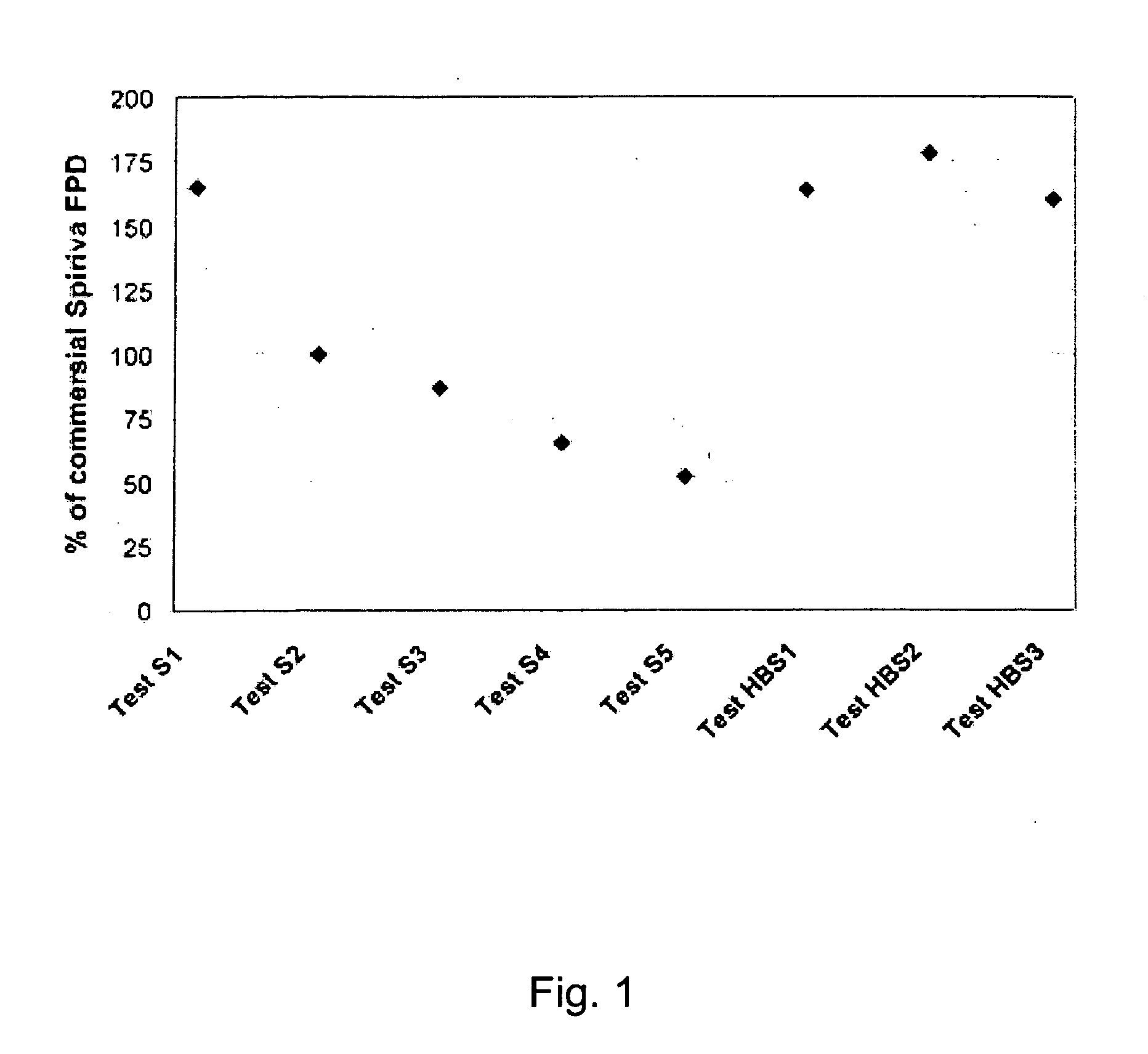
Pre-metered dry powder inhaler for moisture-sensitive medicaments
Owner:BOEHRINGER INGELHEIM INT GMBH
Engineered particles and methods of use
InactiveUS20050074498A1Reduce deliveryLess attractivePowder deliveryOrganic active ingredientsNebulizerActive agent
Engineered particles are provided may be used for the delivery of a bioactive agent to the respiratory tract of a patient. The particles may be used in the form of dry powders or in the form of stabilized dispersions comprising a nonaqueous continuous phase. In particularly preferred embodiments the particles may be used in conjunction with an inhalation device such as a dry powder inhaler, metered dose inhaler or a nebulizer.
Owner:NOVARTIS FARMA
Dry powder inhaler
InactiveUS20100154795A1Increase resistanceReduce resistanceRespiratorsLiquid surface applicatorsPhysical therapySurgery
The present invention relates to an improvement of the mouthpiece of a dry powder inhalation device wherein the medicament is packed in the blisters of single dose blister strips. According to the invention a portion of the air which enters the mouthpiece does not pass through the powder containing blister, but follows an alternative path through the mouthpiece, enabling therefore the modification of the resistance of the device in a simple and cost effective manner.
Owner:PENTAFRAGAS DIMITRIOS
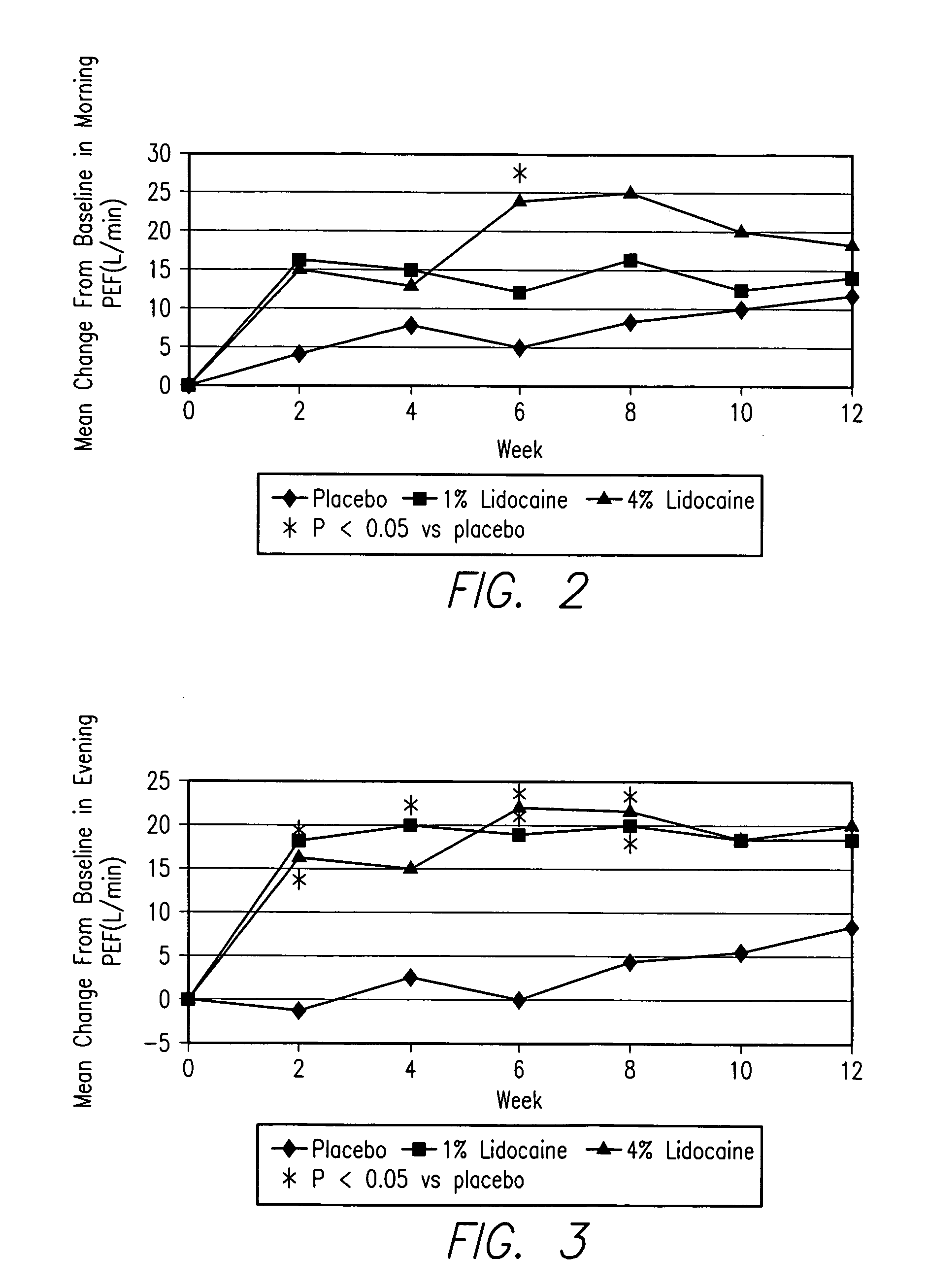
Inhalable lidocaine formulation for treatment of asthma and for reducing the need for corticosteroids in asthmatic patients
An inhalable lidocaine solution for treatment of asthma and a method for treatment of asthma with the reduced need for concurrent administration of oral corticosteroids in asthmatic patients. The lidocaine solution or lidocaine dry powder is delivered by an electronic nebulizer or by dry powder inhaler or dose meter one to several times a day.
Owner:CORUS PHARMA
Inhalers with extendable/retractable forward member and associated methods of dispensing inhalant substances
Dry powder inhalers for dispensing pharmaceutical grade formulations of inhalable dry powder include: an inhaler housing having a mouthpiece associated therewith; and a slidably extendable forward member that is movable between retracted and extended positions, held by the inhaler housing adjacent the mouthpiece. In the extended position, the forward member extends outward a distance beyond a forwardmost portion of the mouthpiece, and in the retracted position, a forwardmost portion of the forward member is positioned rearward of the forwardmost portion of the mouthpiece with an access portion of the mouthpiece accessible by a user.
Owner:ORIEL THERAPEUTICS INC
Dry powder inhaler
This invention provides a dry powder inhaler comprising: a dry powder medicament comprising fluticasone propionate, salmeterol xinafoate and a lactose carrier; wherein, the delivered dose of salmeterol per actuation is less than 50 μg; and wherein the dose provides a baseline-adjusted FEV1 in a patient of more than 150 mL within 30 minutes of receiving the dose. A method of treating a patient includes administering to a patient a dry powder medicament having fluticasone propionate, salmeterol xinafoate and a lactose carrier; wherein, the delivered dose of salmeterol per actuation is less than 50 μg; and wherein the dose provides a baseline-adjusted FEV1 in a patient of more than 150 mL within 30 minutes of receiving the dose.
Owner:TEVA BRANDED PHARMA PROD R & D
Dry powder inhaler
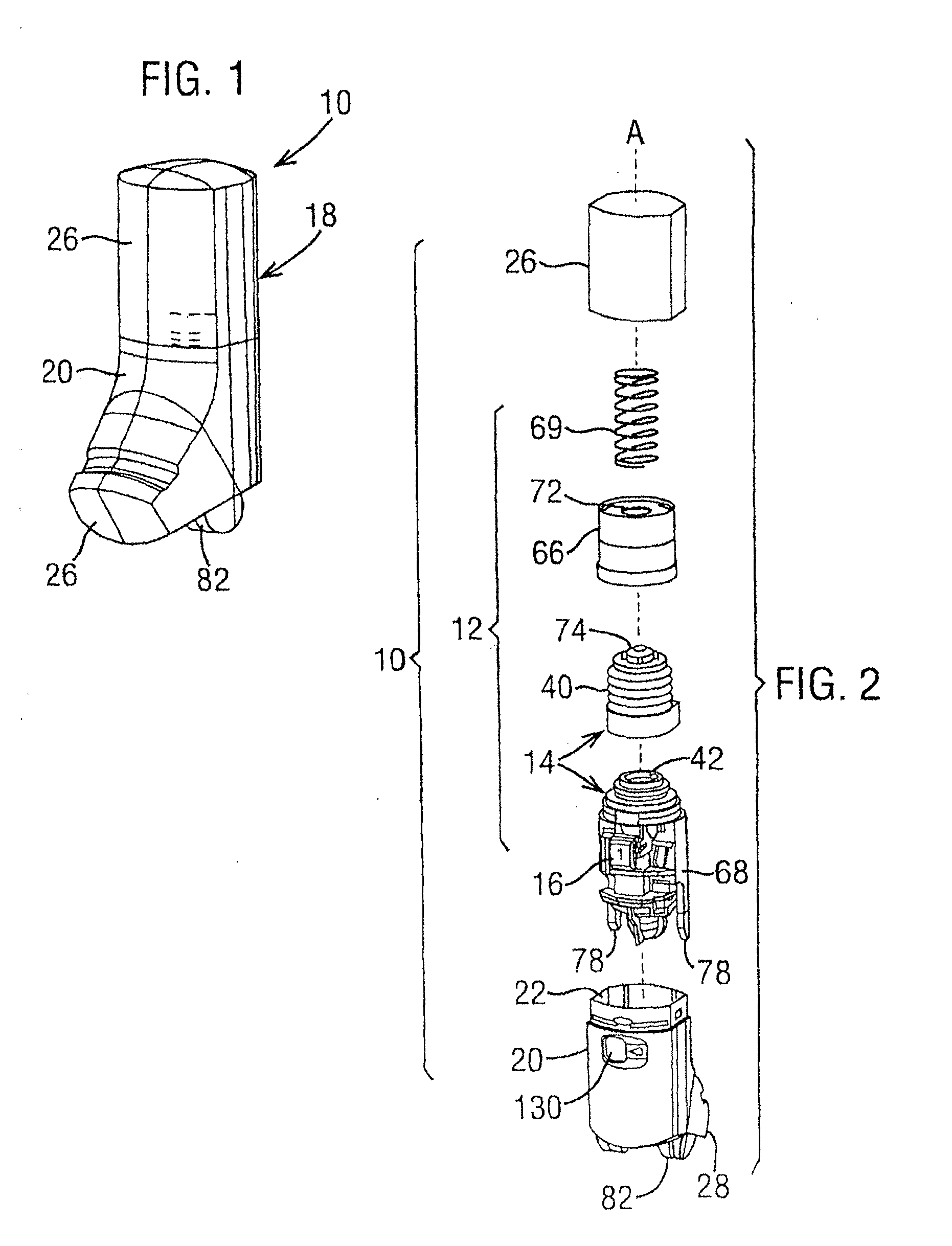
PendingCN106924845AEasy to assembleReduce manufacturing costMedical devicesInhalatorsBiomedical engineeringDry-powder inhaler
The invention discloses a dry powder inhaler, which comprises a bearing plate, a suction nozzle, a capsule chamber, a button, a spring and a lower shell, wherein through holes are formed in the bearing plate in a going-through mode; the suction nozzle is connected to the upper side of the bearing plate and is arranged on the through holes; the capsule chamber is connected to the lower side of the bearing plate and is located on the through holes; tip pins, which are made from metal, are connected to the button, and the tip pins, through a pressing action of the button, can extend into the capsule chamber; the spring is clamped between the button and the capsule chamber, and by virtue of the spring, elasticity away from the direction of the capsule chamber is exerted; the upper side of the lower shell is of an opening structure; the bearing plate is connected to an opening of the upper side of the lower shell, and meanwhile, the capsule chamber is accommodated within the lower shell; the tip pins are fixedly connected to the button by virtue of a plastic tip pin seat; the tip pins and the tip pin seat are fixed in an injection-molding mode; and the tip pin seat and the button are fixed in a clamping mode. According to the dry powder inhaler, the tip pins, when used, are prevented from getting fallen or displaced easily; and moreover, the capsule chamber can be cleaned by a user more conveniently.
Owner:SUZHOU SINGMED MEDICAL DEVICE SCI & TECH LTD
Dry powder inhalers, related blister devices, and associated methods of dispensing dry powder substances and fabricating blister packages
InactiveUS20050126569A1Limit amount of resistanceMore hygienic productRespiratorsLiquid surface applicatorsElastomerBlisters
The present invention includes dry powder inhalers for dispensing and / or holding inhalant formulated dry powder substances and associated fabrication and dispensing methods that can employ an amplitude modulated non-linear signal comprising a plurality of superimposed frequencies, the frequencies corresponding to a priori flow characteristic frequencies of the dry powder being dispensed. The present invention also includes pocket-sized inhalers with an elastomeric flexible pivoting cover.
Owner:ORIEL THERAPEUTICS INC
Inhaler for moisture sensitive drugs
InactiveUS20070068524A1Boosting dose delivery performancePerformance deteriorationRespiratorsLiquid surface applicatorsInhaled airMoisture
A dry powder inhaler device (DPI) is disclosed. When a user activates the inhaler, the DPI is capable of delivering a dry powder dose directly from a medicament container, loaded into the DPI. A method is also disclosed for delivering a dry powder medicament dose directly from a container to a user of a DPI, whereby a sealing foil of the container is being slit open concurrently with aerosolizing and entraining of the powder in the dose into the inhaled air.
Owner:MEDERIO AG
Slave wheel counter mechanism useable with an inhaler
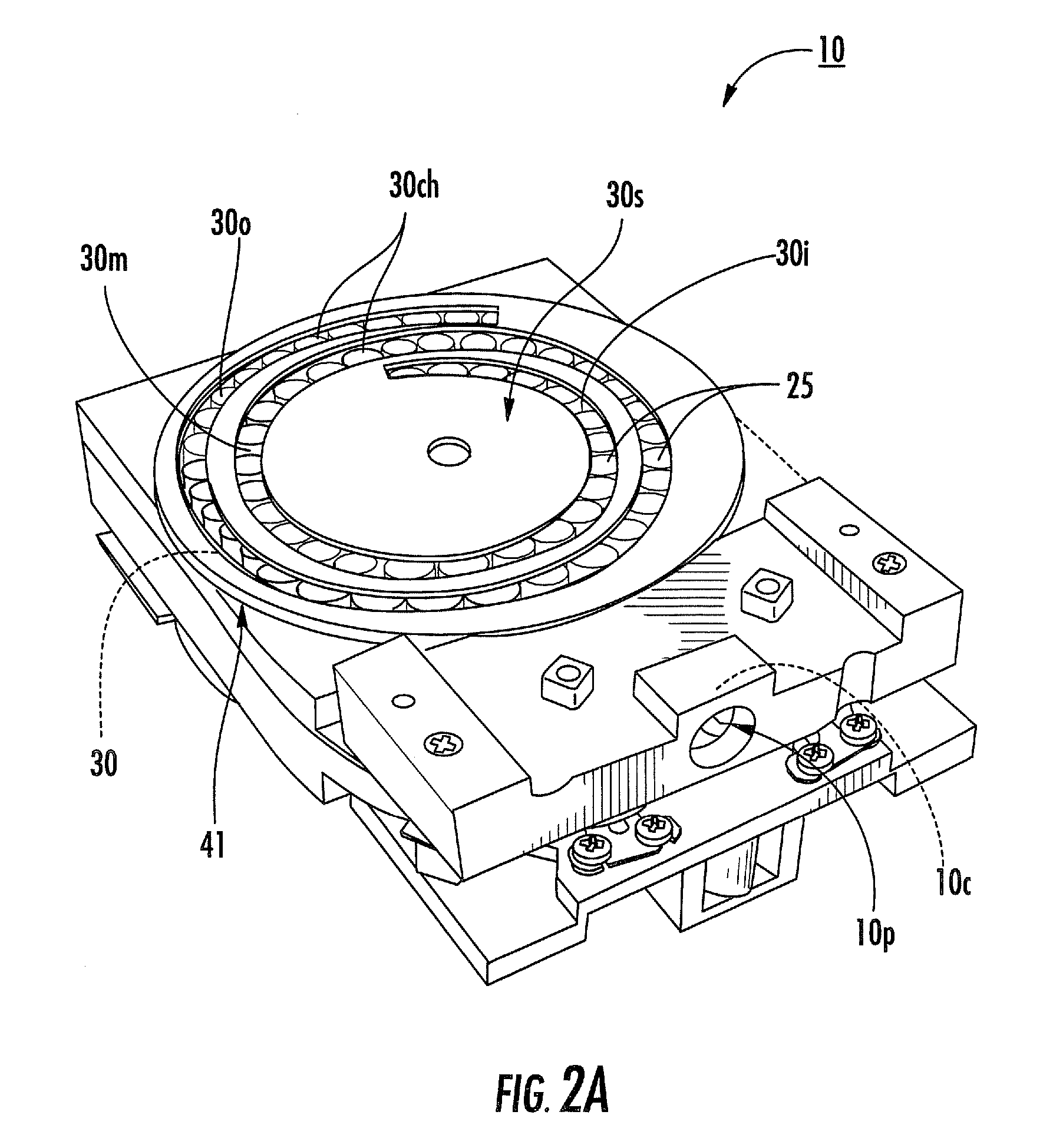
A counter mechanism useable with a dry powder inhaler is disclosed. The mechanism features first and second indicator members rotatable about a central axis. The indicator members have counting indicia visible to indicate inhaler doses remaining or dispensed. A coupling transmits rotary motion from the inhaler to the second indicator member when the inhaler is charged with a dose. A slave wheel, rotatable about an axis offset from the central axis, has a drive transfer wheel on one face and a gear on an opposite face. The gear engages the second indicator member, the drive transfer wheel intermittently engages and rotates the first indicator member in response to rotary motion of the second indicator member.
Owner:AVENTIS PHARMA LTD
Dry powder inhaler device
A DPI device comprising a dispensing chamber for receiving a discrete dose of medicament-containing powder and means for delivering said dose from the dispensing chamber to a patient in an air-flow that passes from the chamber to the patient via a mouth-piece along a first air passage that comprises de-agglomerating means, the device additionally comprises a second air passage in fluid communication with the dispensing chamber and the mouth-piece and which by-passes the de- agglomerating means and which is located such that it receives a portion of said air-flow that is free, or substantially free, of powder.
Owner:JAGOTEC AG
Dry powder inhalers comprising a carrier other than lactose
This invention relates to novel pharmaceutical compositions for inhalation comprising separately, sequentially or together, drugs having amine in the form of a dry powder in admixture with a pharmaceutically acceptable carrier, other than lactose, and its use in the treatment of respiratory condition selected from asthma and chronic obstructive pulmonary disease (COPD) and other obstructive airways diseases. In addition, the present invention relates to novel pharmaceutical composition for inhalation based on combinations of long acting muscarinic antagonists, long acting beta agonists, short acting beta-2 agonists, corticosteroids or a combination of two or more of them.
Owner:ARVEN ILAC SANAYI VE TICARET
Dry powder inhalers comprising a carrier other than lactose and a ternary component
This invention relates to novel pharmaceutical compositions for inhalation comprising separately, sequentially or together, drugs having amine in the form of a dry powder in admixture with a pharmaceutically acceptable carrier, other than lactose, and its use in the treatment of respiratory condition selected from asthma and chronic obstructive pulmonary disease (COPD) and other obstructive airways diseases. More particularly, the invention relates to pharmaceutical composition for inhalation further comprising a ternary component. In addition, the present invention relates to novel pharmaceutical composition for inhalation based on combinations of long acting muscarinic antagonists, long acting beta agonists, short acting beta-2 agonists, corticosteroids or a combination of two or more of them.
Owner:ARVEN ILAC SANAYI VE TICARET
Inhalation compositions with high drug ratios
InactiveUS20060292083A1Easy to handleAccurate measurementPowder deliveryOrganic active ingredientsParticulatesTherapeutic treatment
The invention provides a dry powder inhalation composition comprising, at least 0.25% by weight of the composition of an active ingredient with a particle size of less than 10 microns in diameter and a pharmaceutically acceptable particulate carrier with a particle size of less than 250 microns in diameter. Also disclosed are methods for use of the compositions of the invention with dry powder inhalers for therapeutic treatments.
Owner:NORTON HEALTHCARE
Dry powder inhaler with flutter dispersion member
A dry powder inhaler including a housing defining a chamber for receiving a dose of powdered medicament, an inhalation port in fluid communication with the chamber, at least one airflow inlet providing fluid communication between the chamber and an exterior of the housing, and a flutter element in the chamber and associated with a dose of powdered medicament. The flutter element has a tensioned distal end proximate the at least one airflow inlet and a free proximal end opposite to the distal end and downstream of the inlet. The flutter element is configured to vibrate in response to airflow through the chamber and aerosolize the dose of powdered medicament.
Owner:STC UNM
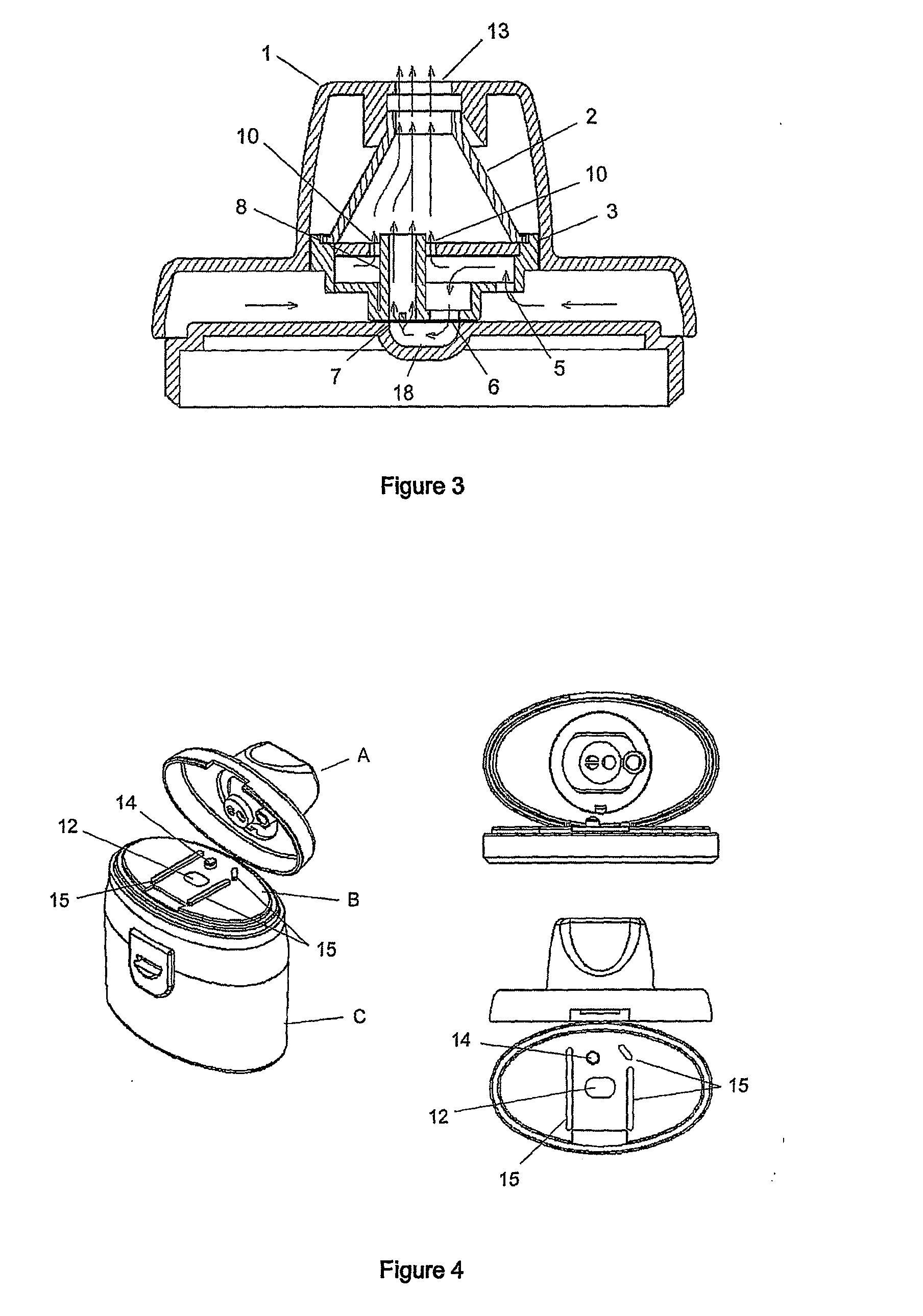
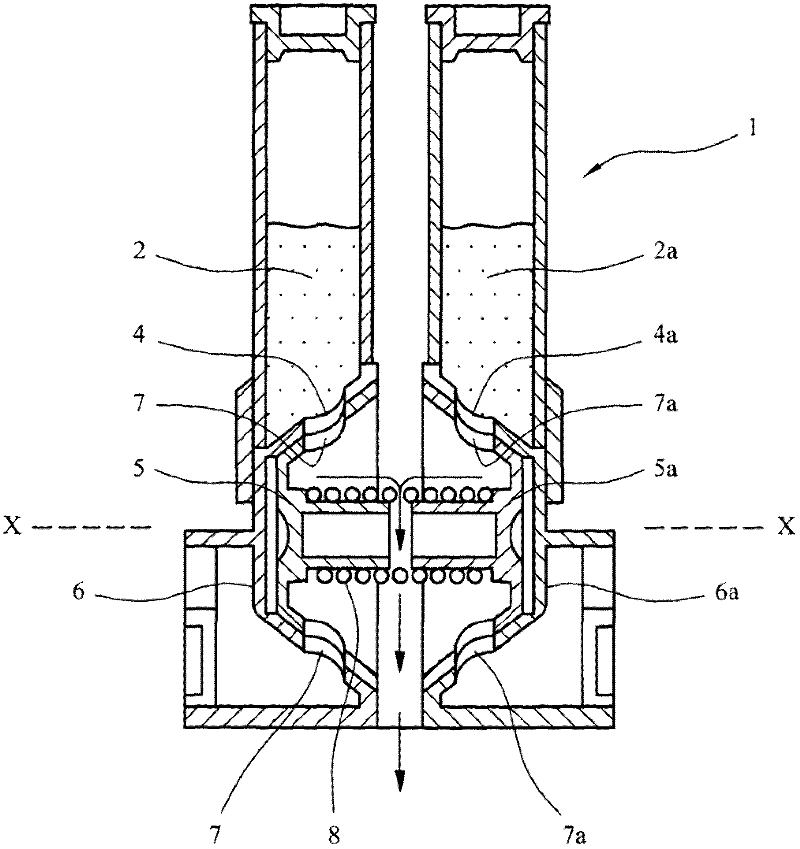
Improvements in or relating to dry powder inhalers
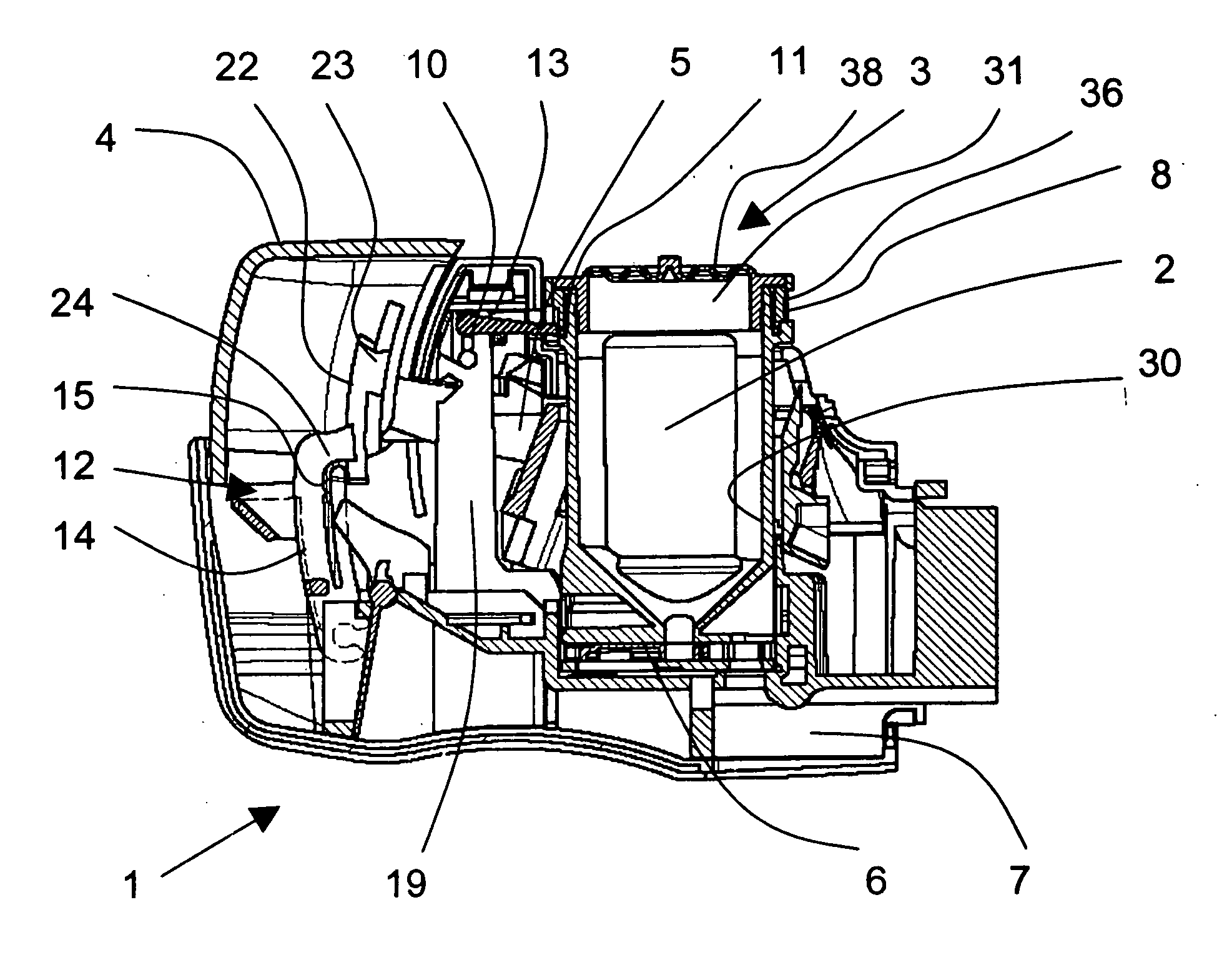
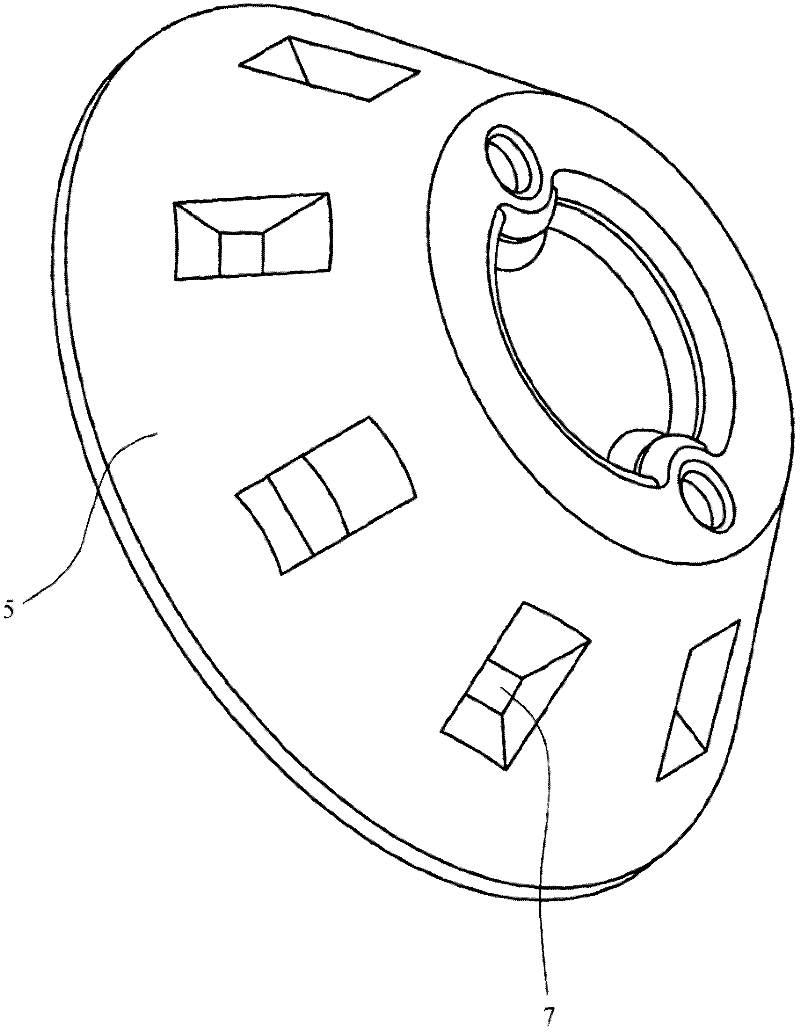
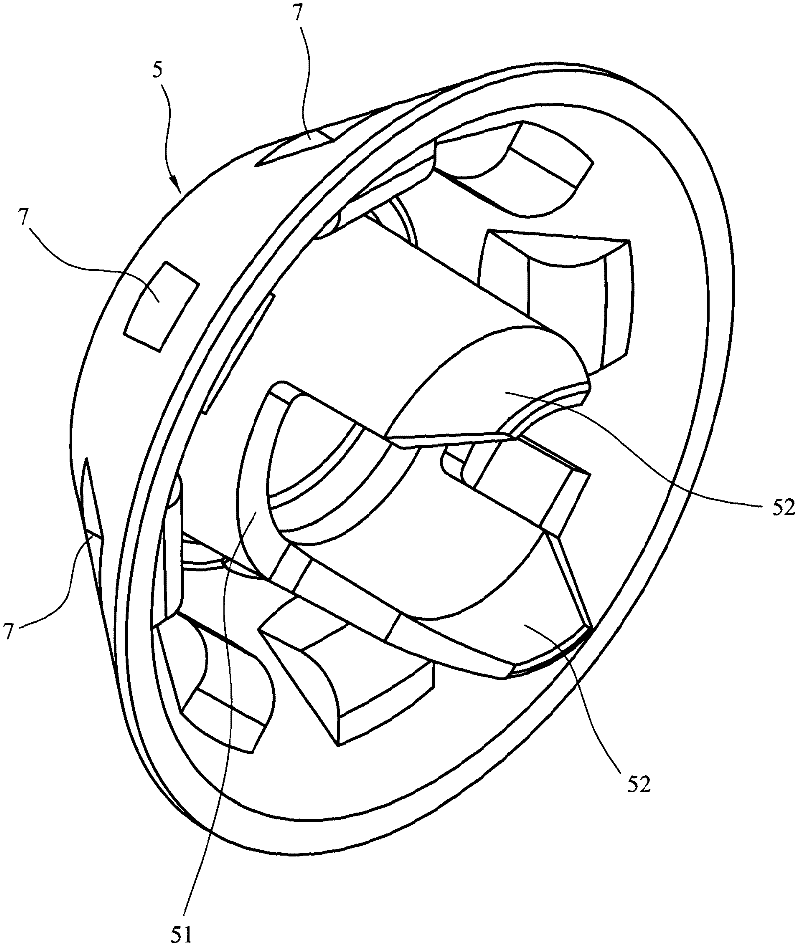
A dry powder inhaler (1) is disclosed comprising first and second medicament reservoirs (2, 2a) and respective rotatable first and second metering members (5, 5a), which are urged into sealing engagement with the respective medicament reservoir (2, 2a) by a compression spring (8) located between the metering members(5, 5a). The inhaler (1) includes an actuator by which the first and second metering members (5, 5a) are rotated in unison from a metering position to a dispensing position. The actuator acts upon the first and second metering members (5, 5a) via a wheel (9) mounted between the first and second metering members(5, 5a). The first and second metering members (5, 5a) are provided with sockets (51) that receive the respective ends of the compression spring (8). The sockets (51) have extensions (52) that project into an axial bushing (92) of the wheel (9), the bushing (92) being provided on its internal surface with formations (94a, 94b, 94c, 94d) that engage the extensions (52) to cause the metering members (5,5a) to rotate when the wheel (9) is rotated.
Owner:TIANJIN KINNOVATA PHARMA CO LTD
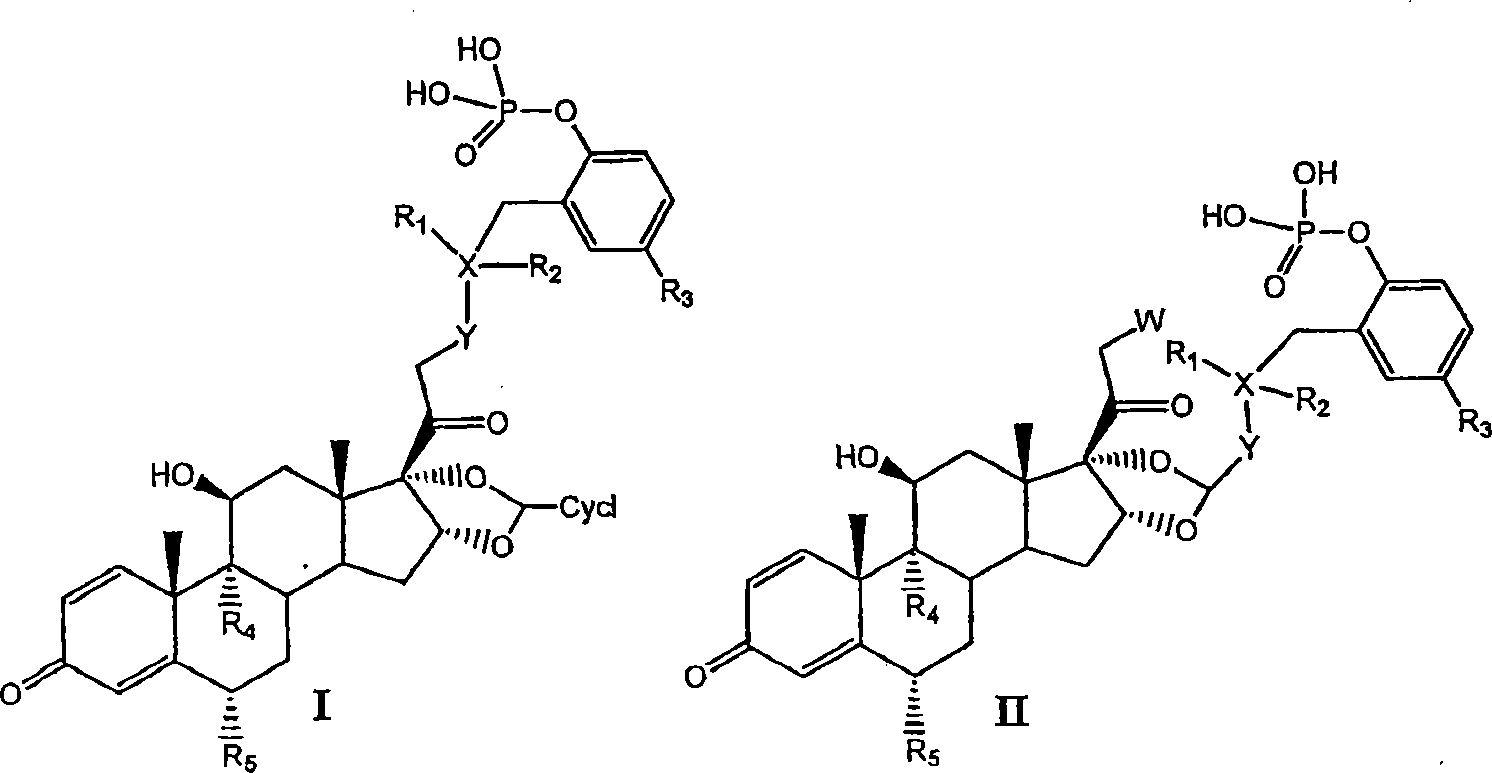
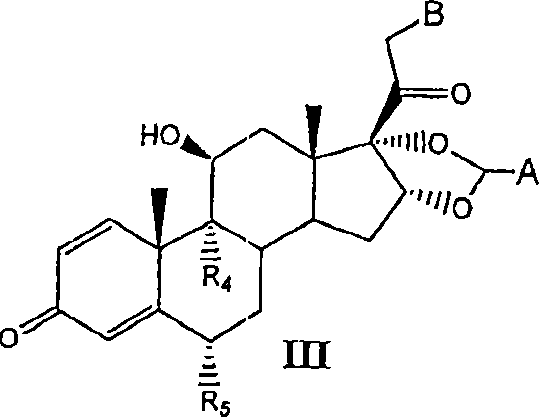
Substituted phenylphosphates as mutual prodrugs of steroids and beta-agonists for the treatment of pulmonary inflammation and bronchoconstriction
A mutual prodrug of a corticosteroid and a substituted phenylphosphate (ss-agonist derivative) for formulation for delivery by aerosolization to inhibit pulmonary inflammation and bronchoconstriction is described. The mutual prodrug is preferably formulated in a small volume solution (10-500 L) dissolved in a quarter normal saline having pH between 5.0 and 7.0 for the treatment of respiratory tract inflammation and bronchoconstriction by an aerosol having mass median average diameter predominantly between 1 to 5 , produced by nebulization or by dry powder inhaler.
Owner:GILEAD SCI INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com