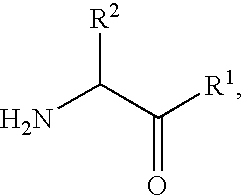
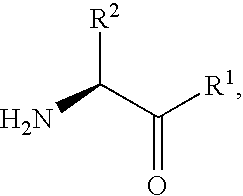
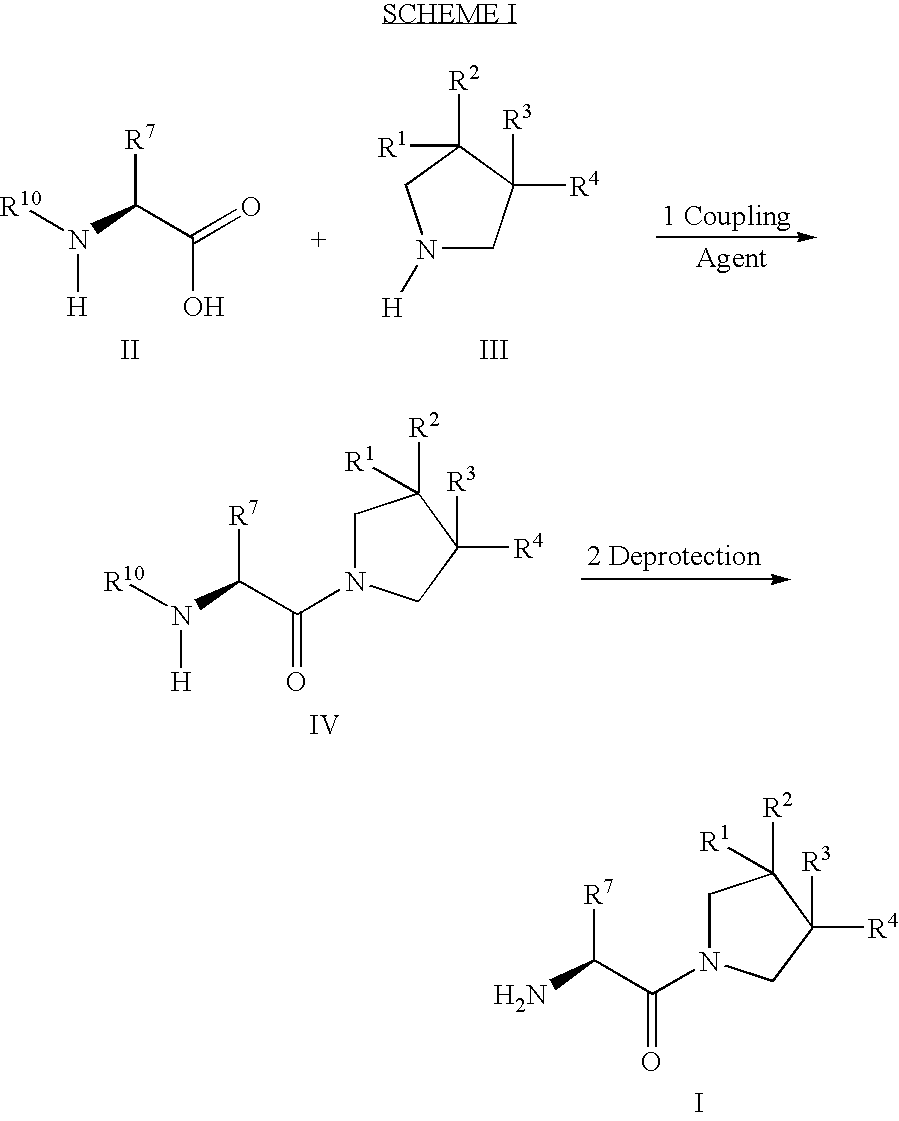
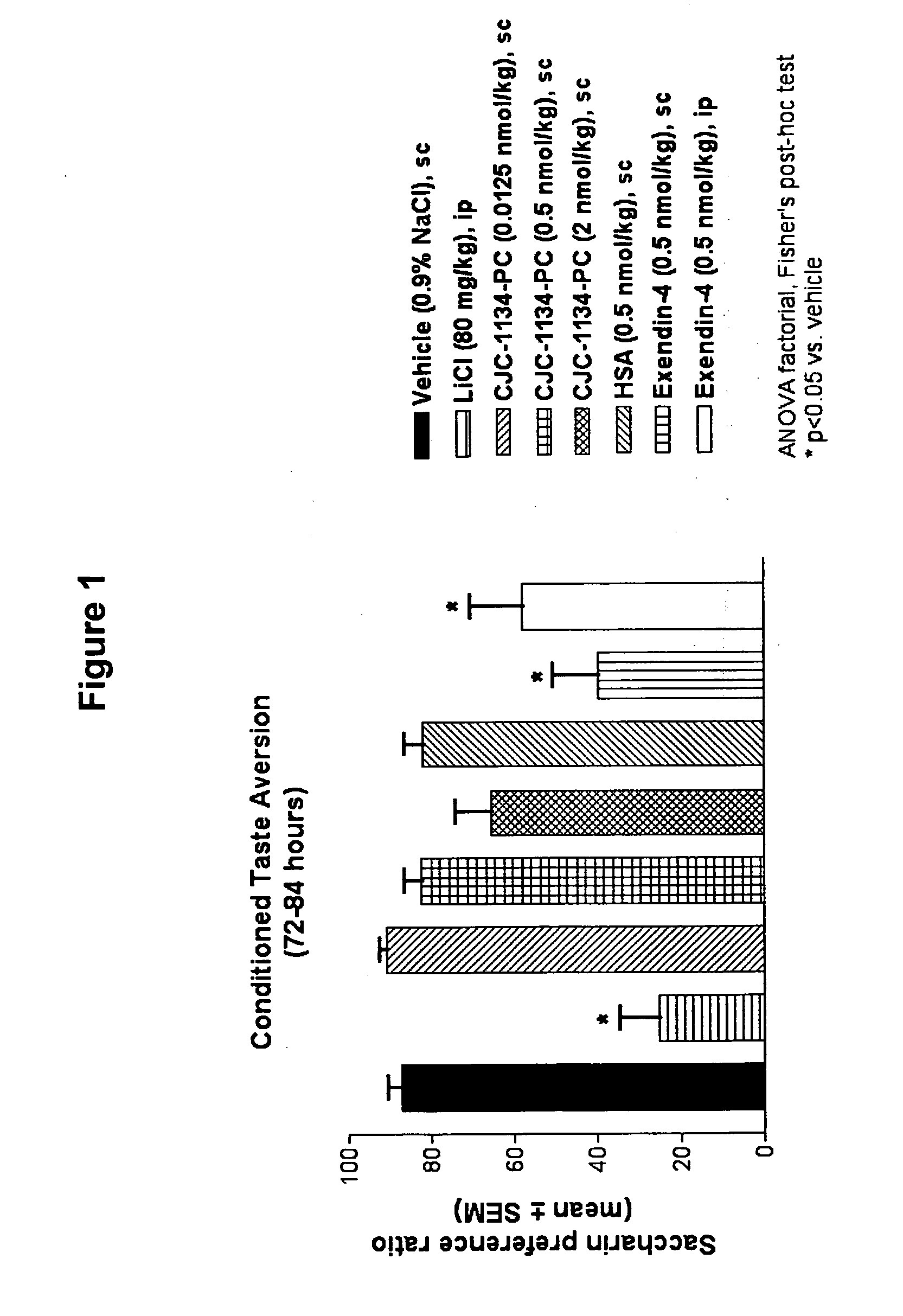
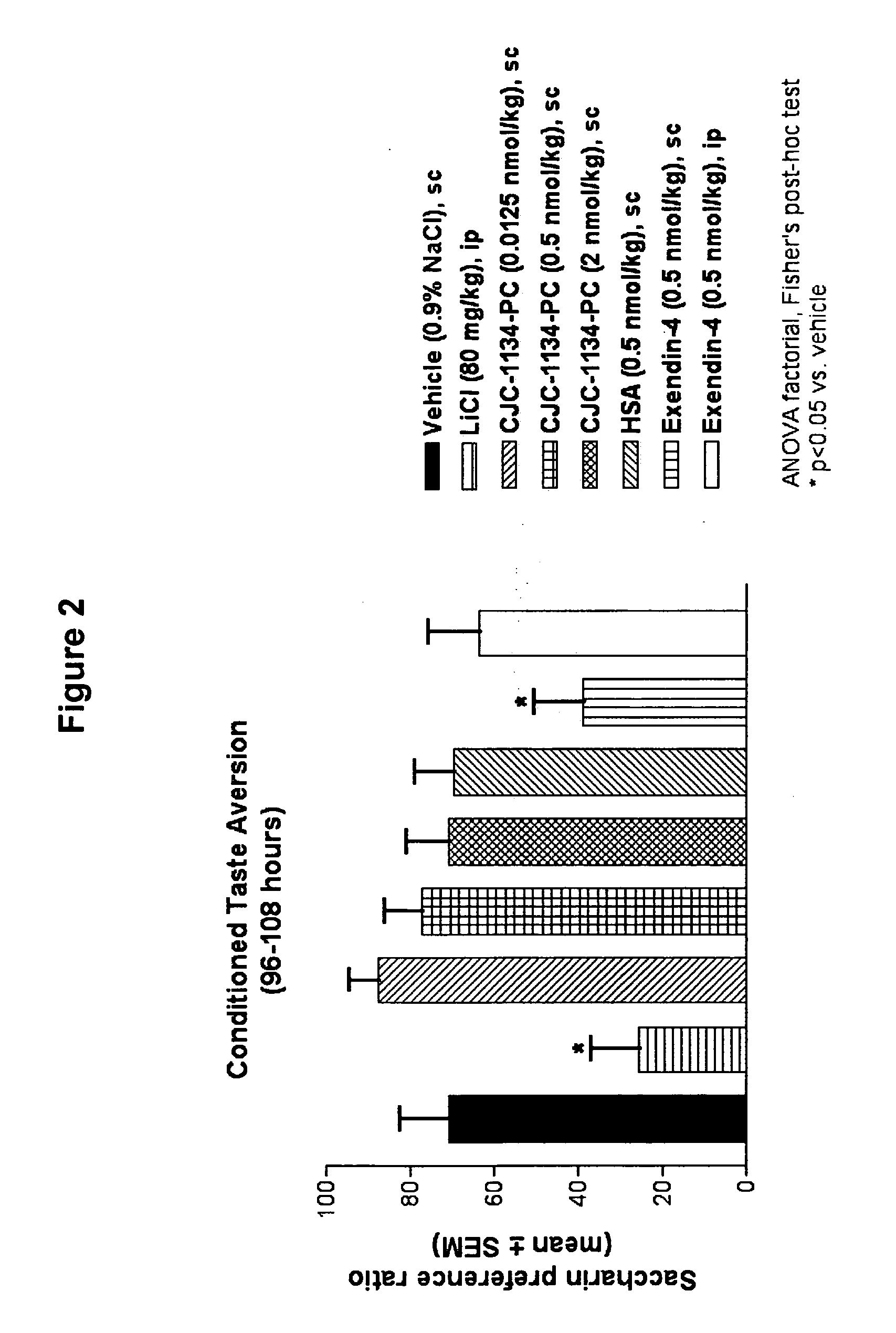
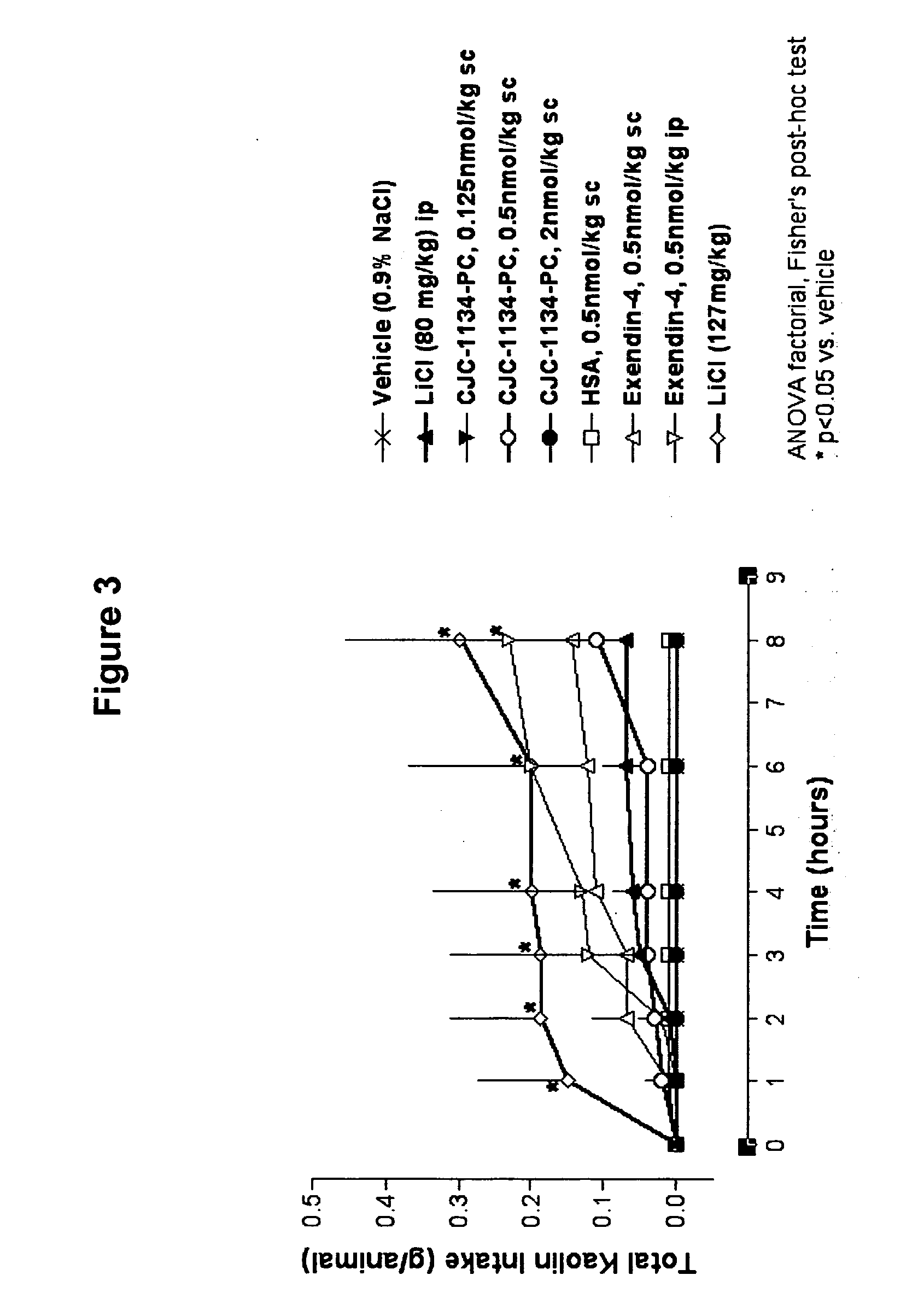
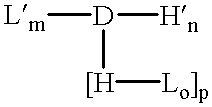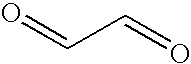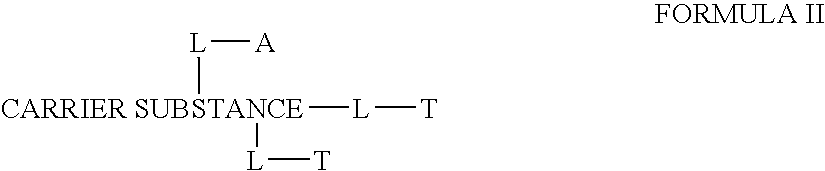Patents
Literature
2572 results about "Pancreatic hormone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A pancreatic hormone is any of various hormones produced by the pancreas. These include glucagon, insulin, pancreatic polypeptide, preproinsulin, proglucagon, and somatostatin.
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
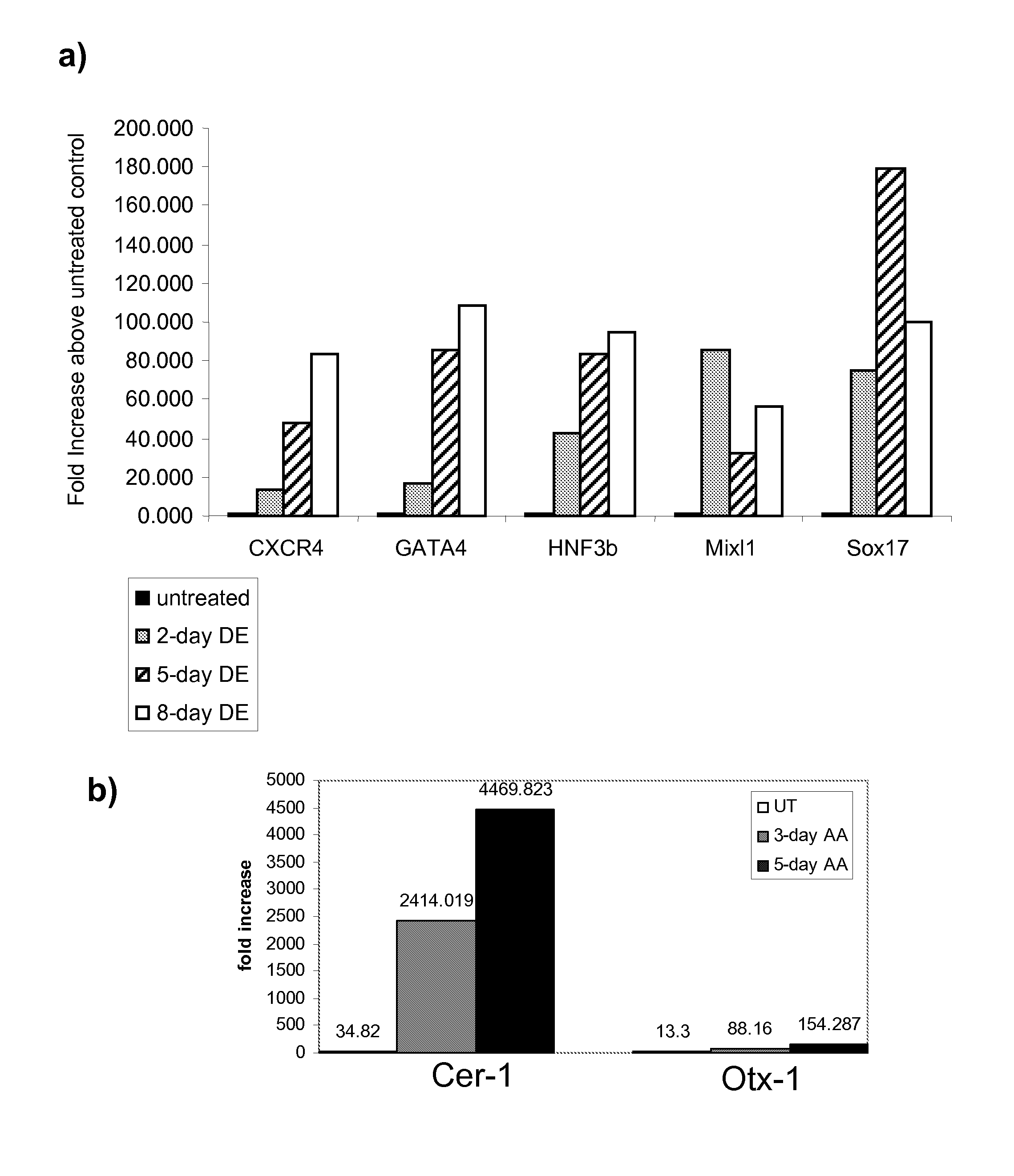
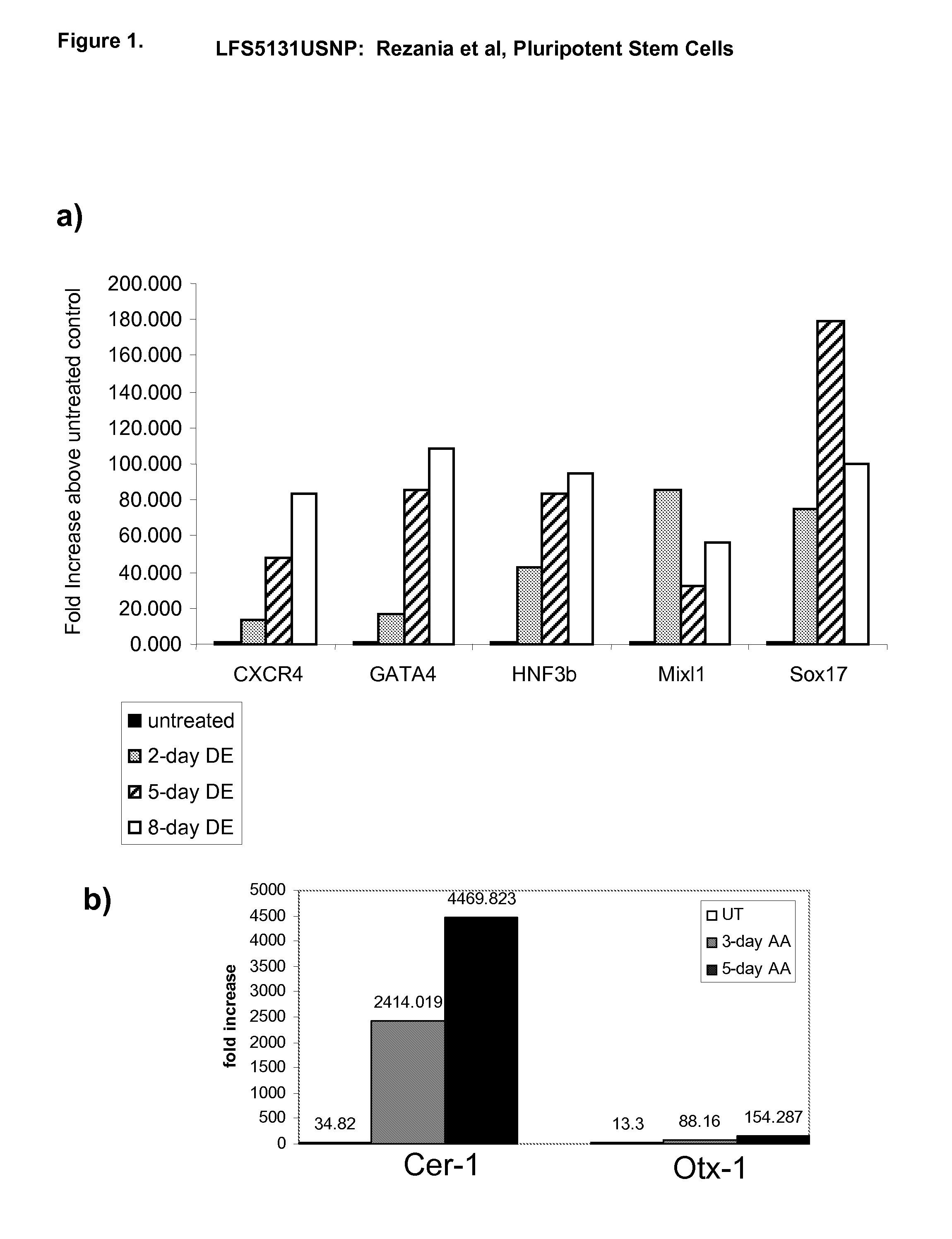
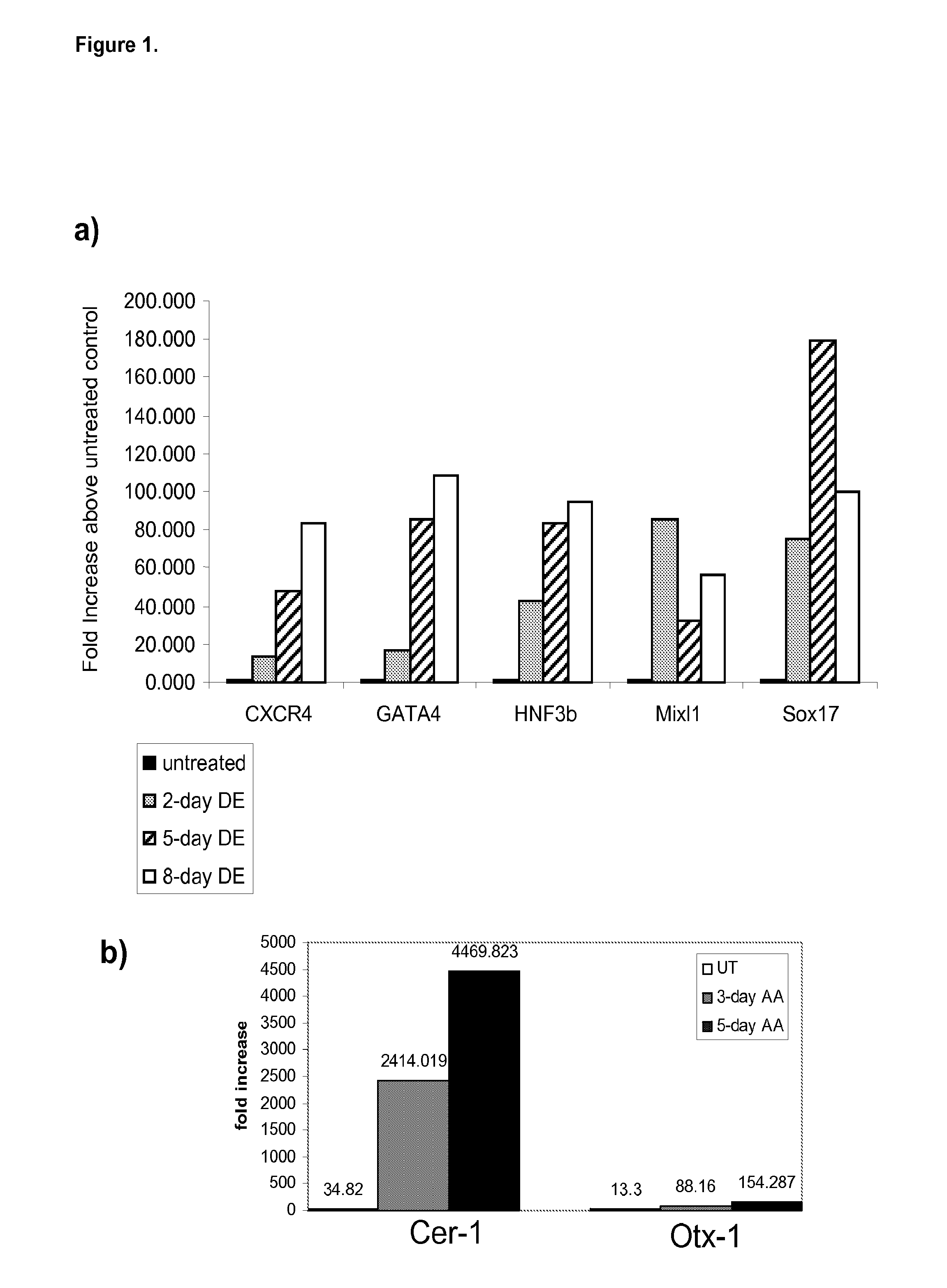
Differentiation of human embryonic stem cells
ActiveUS20070254359A1Inhibits Notch signalingPancreatic cellsArtificial cell constructsGerm layerPluripotential stem cell
The present invention provides methods to promote the differentiation of pluripotent stem cells. In particular, the present invention provides an improved method for the formation of pancreatic endoderm, pancreatic hormone expressing cells and pancreatic hormone secreting cells. The present invention also provides methods to promote the differentiation of pluripotent stem cells without the use of a feeder cell layer.
Owner:LIFESCAN INC
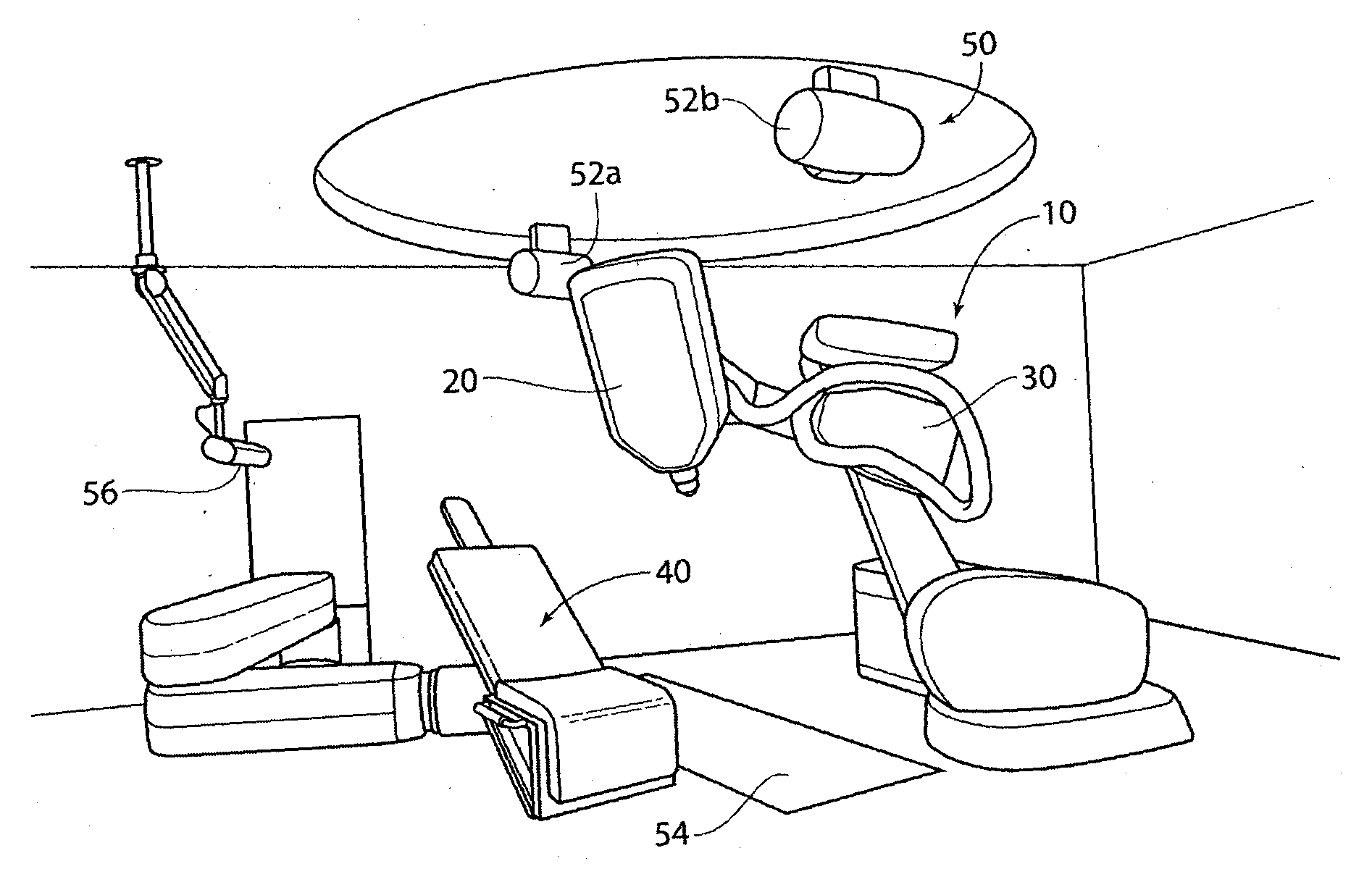
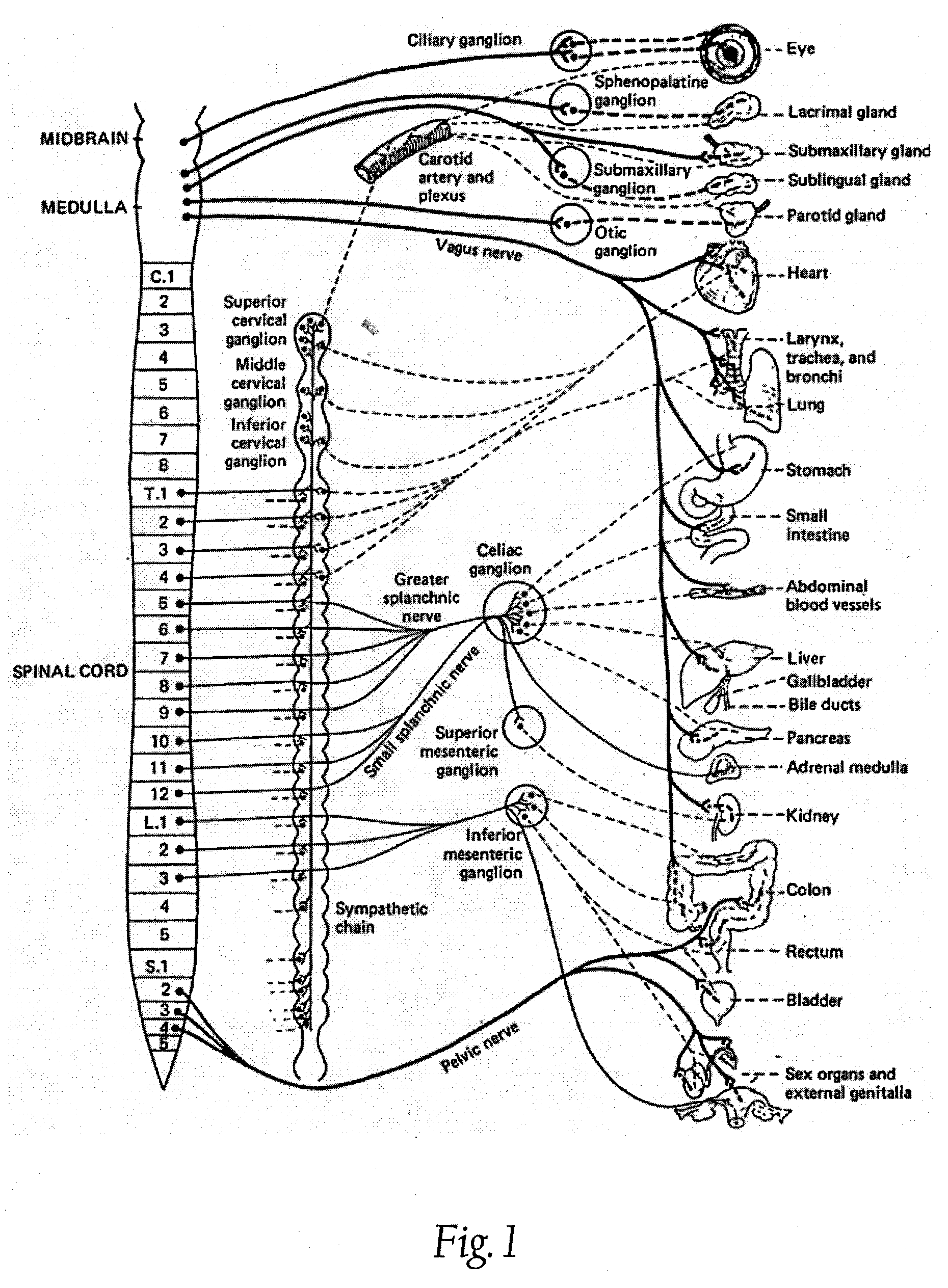
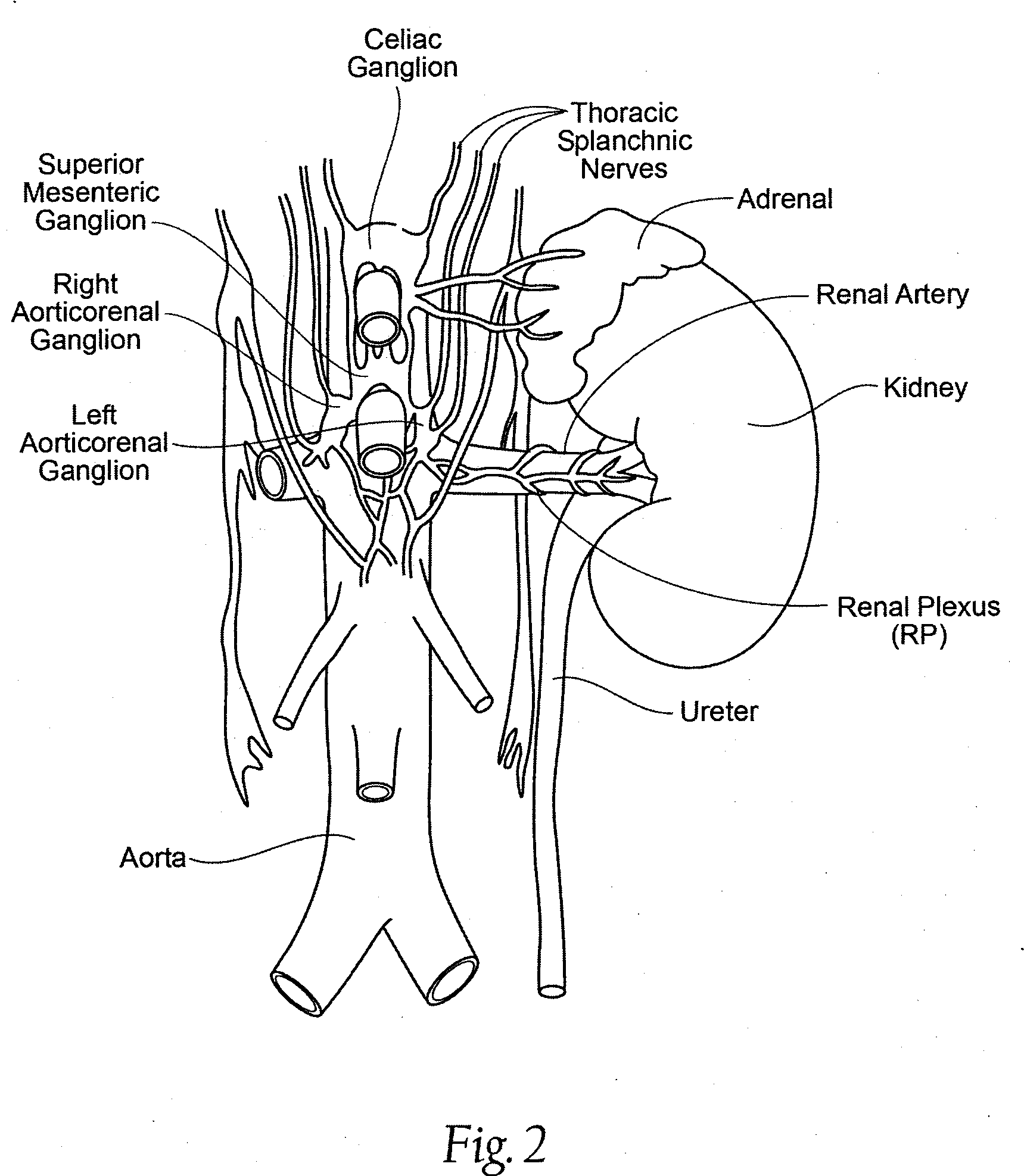
Methods and apparatus for renal neuromodulation via stereotactic radiotherapy
InactiveUS20110200171A1Precise positioningReduce and minimize exposureUltrasound therapySurgical instrument detailsDiseaseStereotactic radiotherapy
The present disclosure describes methods and apparatus for renal neuromodulation via stereotactic radiotherapy for the treatment of hypertension, heart failure, chronic kidney disease, diabetes, insulin resistance, metabolic disorder or other ailments. Renal neuromodulation may be achieved by locating renal nerves and then utilizing stereotactic radiotherapy to expose the renal nerves to a radiation dose sufficient to reduce neural activity. A neural location element may be provided for locating target renal nerves, and a stereotactic radiotherapy system may be provided for exposing the located renal nerves to a radiation dose sufficient to reduce the neural activity, with reduced or minimized radiation exposure in adjacent tissue. Renal nerves may be located and targeted at the level of the ganglion and / or at postganglionic positions, as well as at pre-ganglionic positions.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
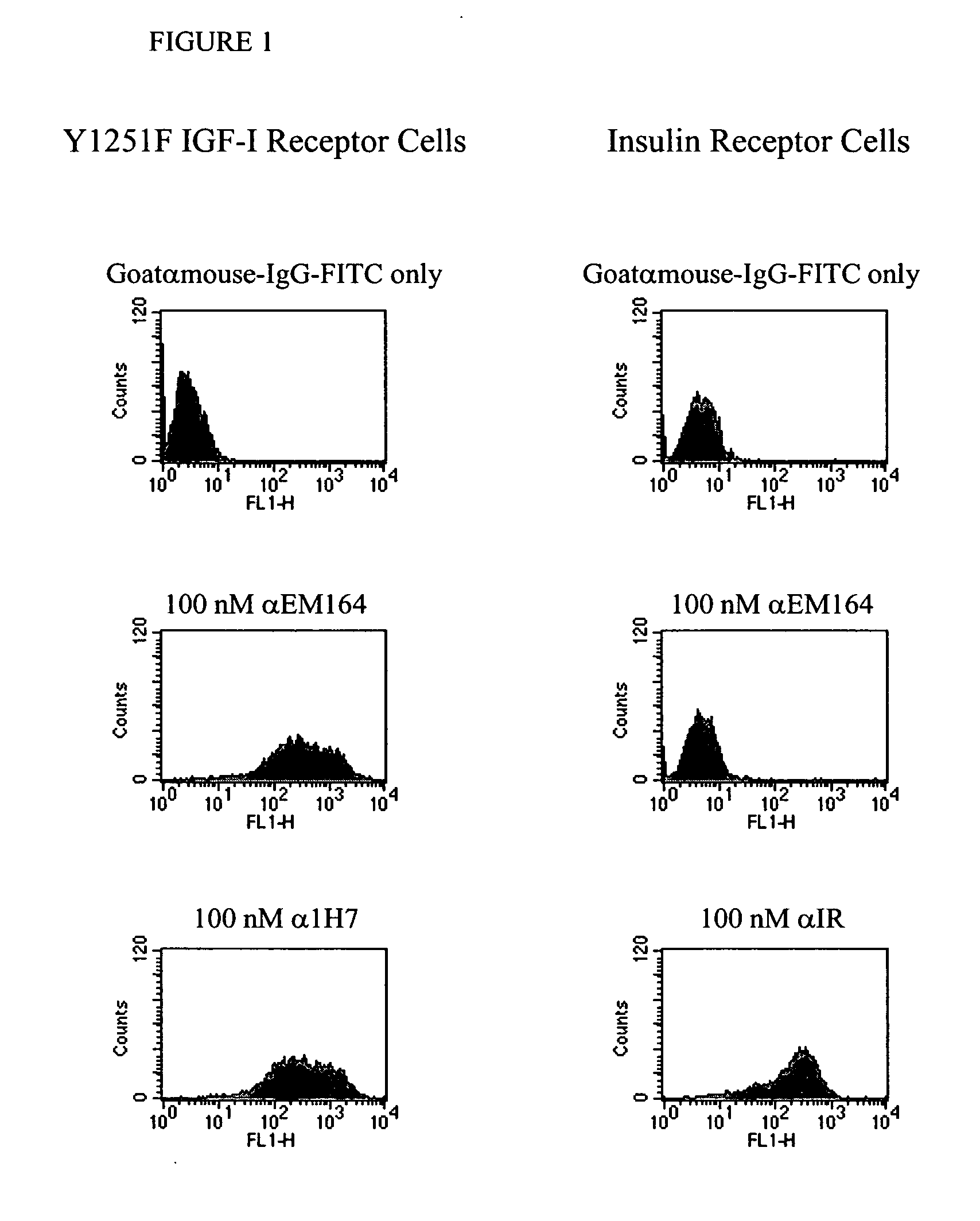
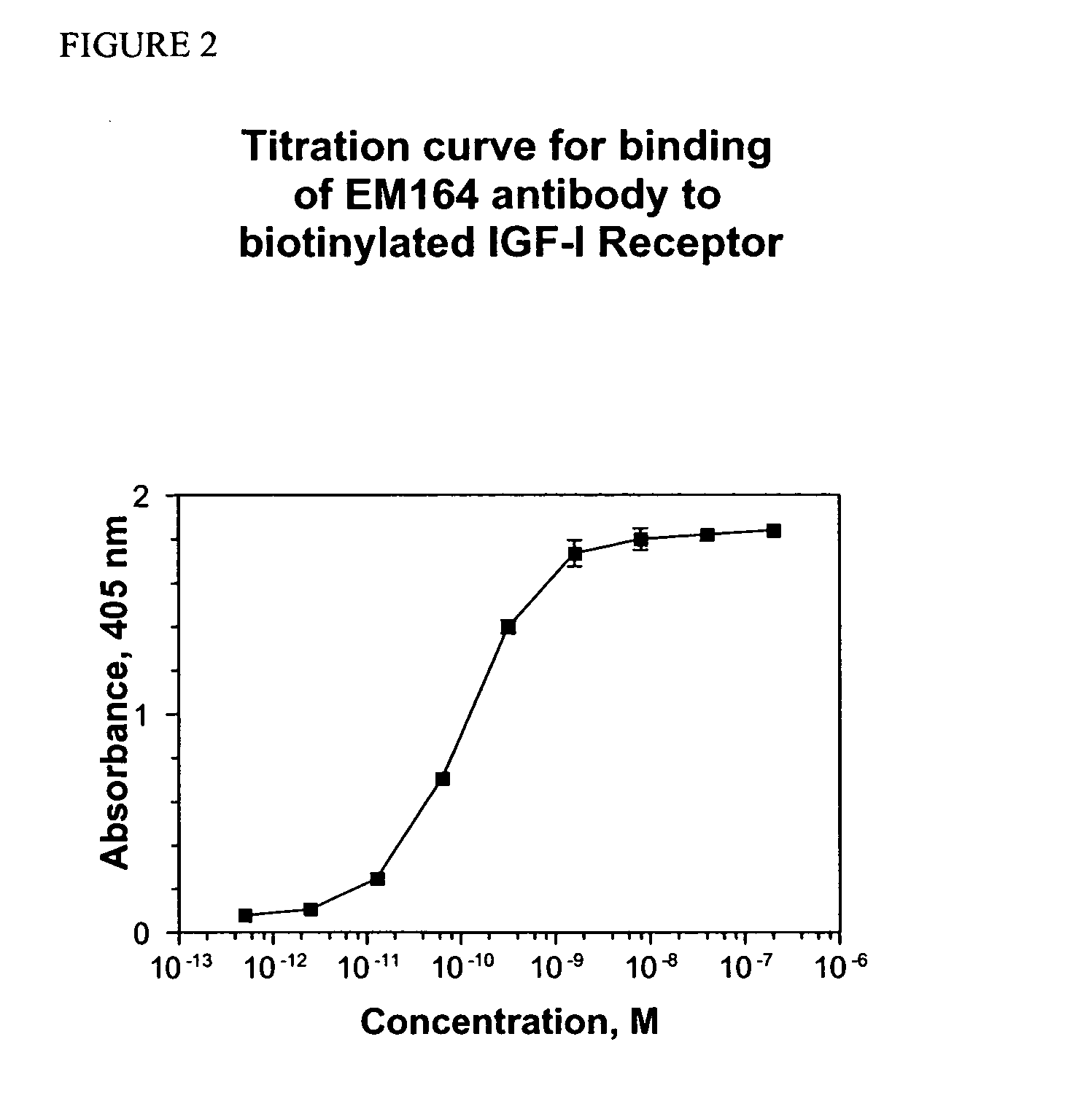
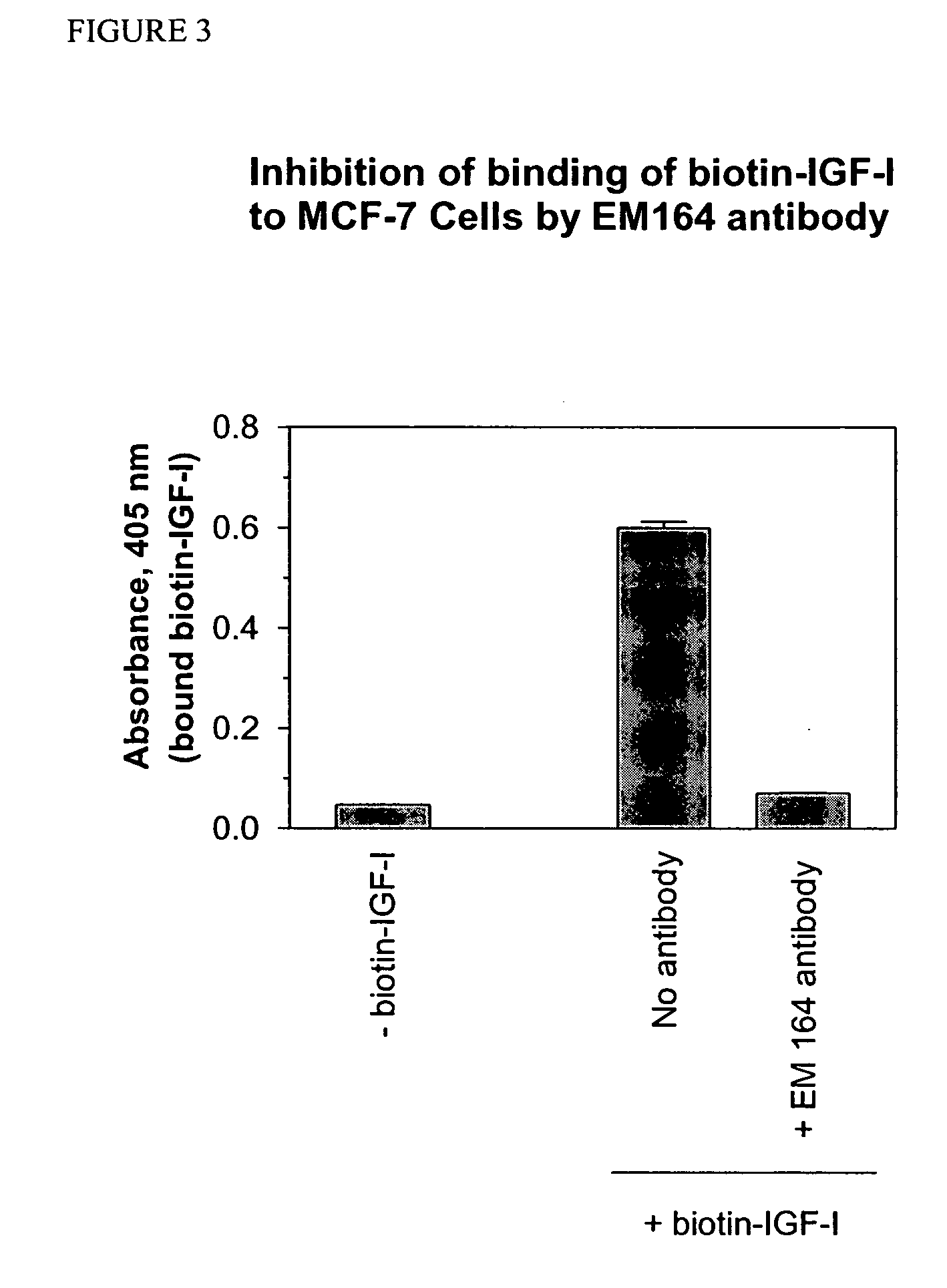
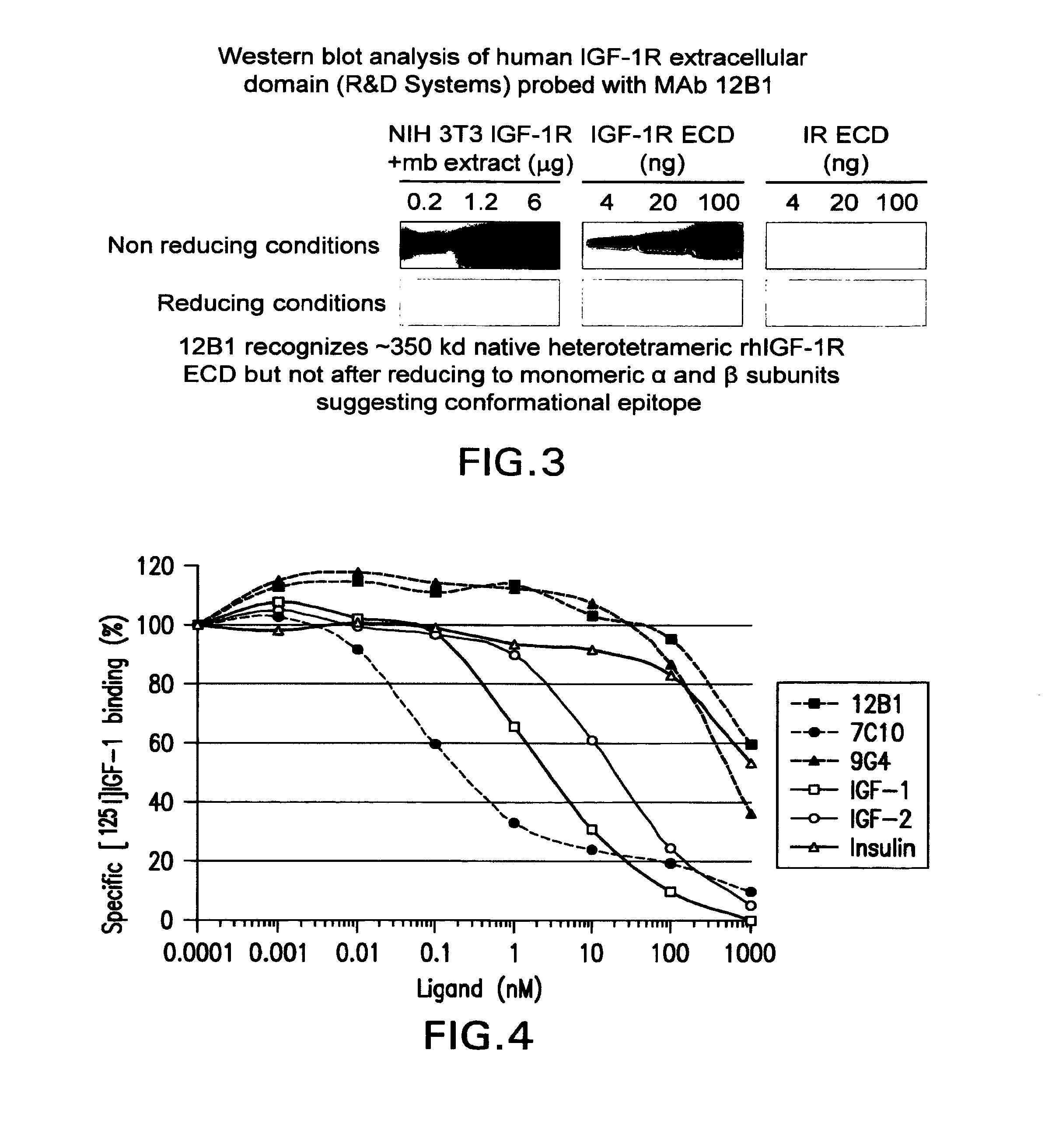
Anti-IGF-I receptor antibody
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to, and inhibit, insulin-like growth factor-I receptor, antagonize the effects of IGF-I, IGF-II and serum on the growth and survival of tumor cells, and which are substantially devoid of agonist activity. Said antibodies and fragments thereof may be used, optionally in conjunction with other therapeutic agents, in the treatment of tumors that express elevated levels of IGF-I receptor, such as breast cancer, colon cancer, lung cancer, ovarian carcinoma, synovial sarcoma, prostate cancer and pancreatic cancer, and said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of IGF-I receptor.
Owner:IMMUNOGEN INC
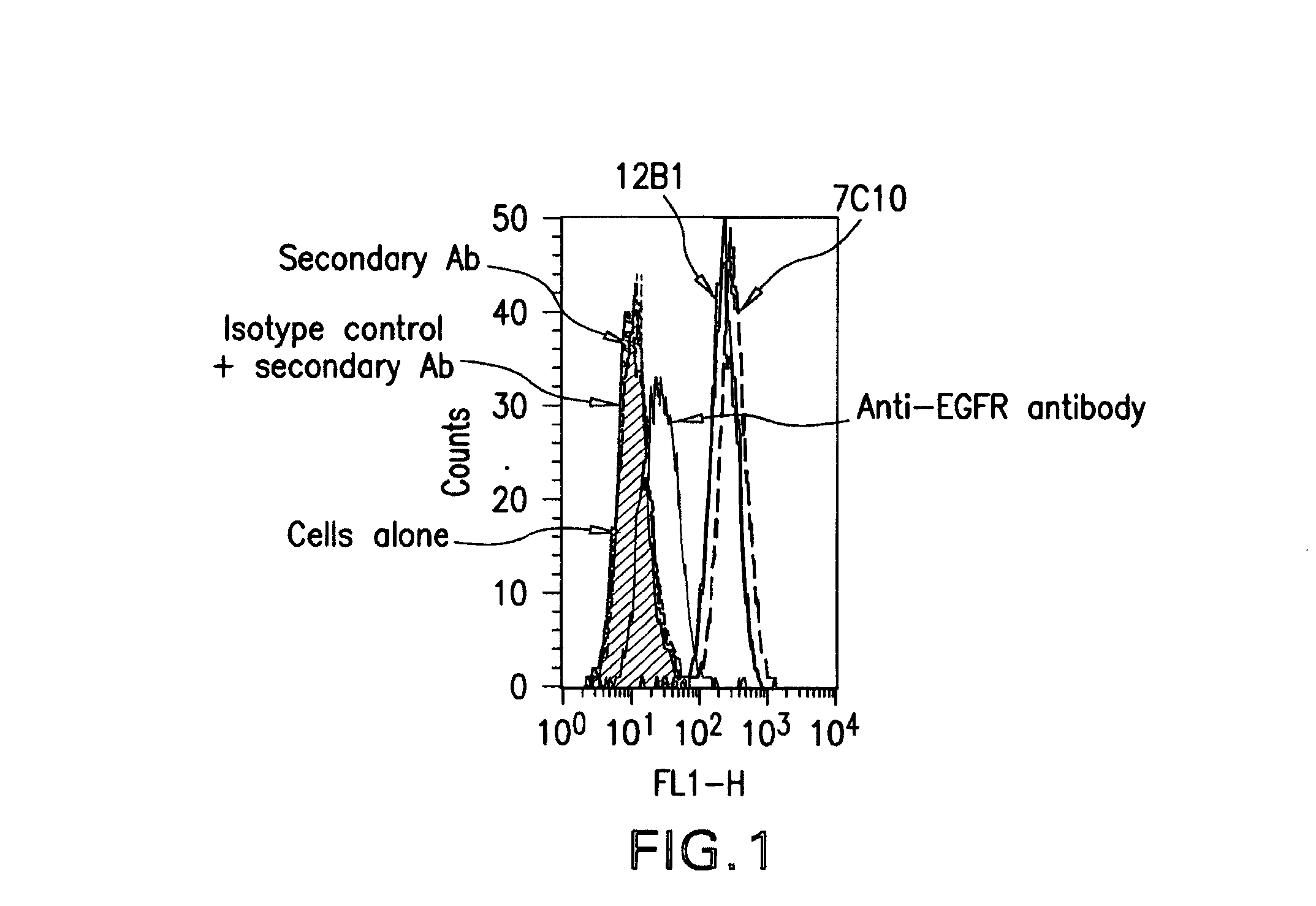
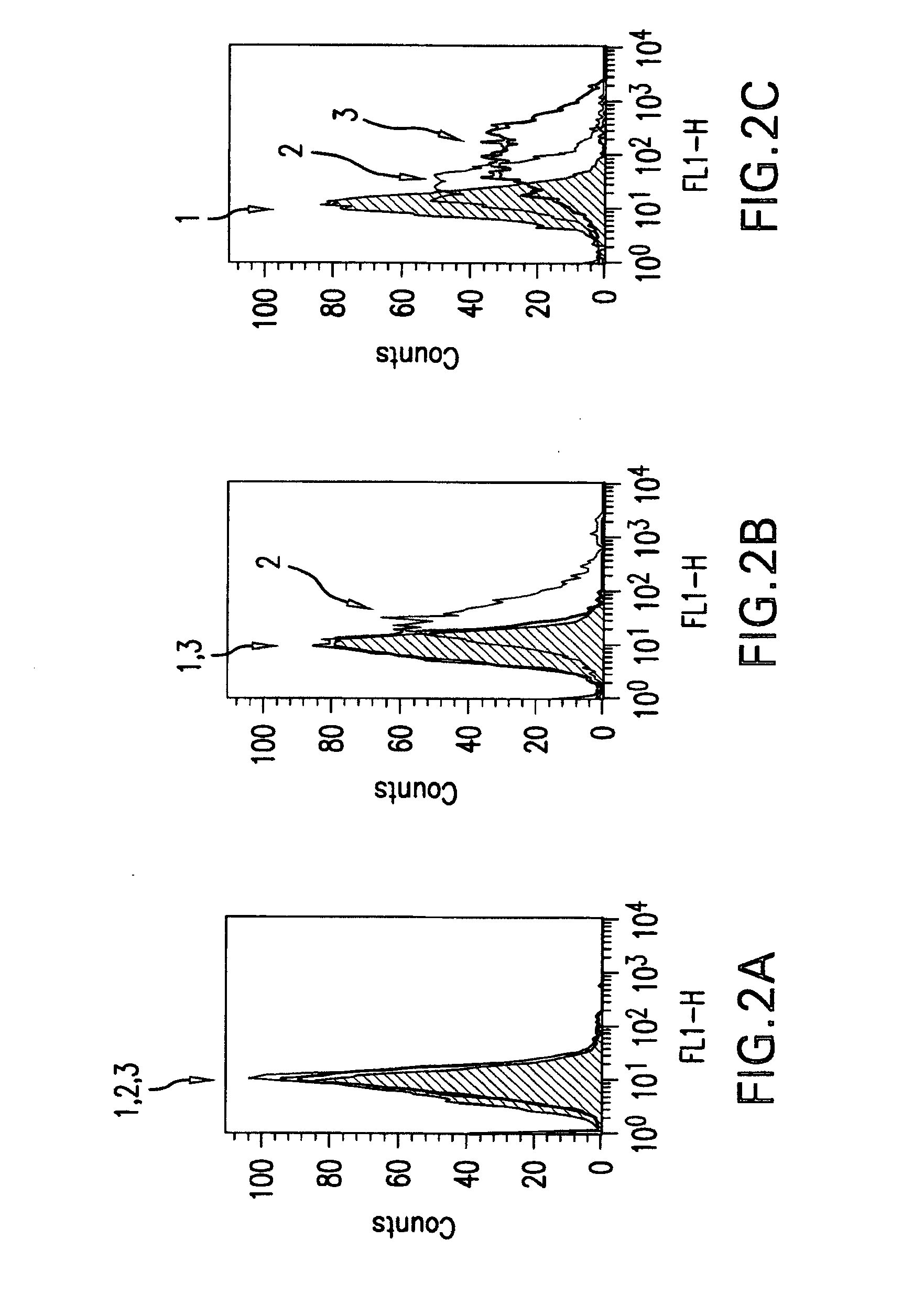
Igf-1r specific antibodies useful in the detection and diagnosis of cellular proliferative disorders
InactiveUS20130084243A1High affinityUseful in detectionAnimal cellsIn-vivo radioactive preparationsDiseaseSingle-Chain Antibodies
The present invention relates to mammalian antibodies, designated 12B1 and antigen-binding portions thereof that specifically bind to insulin-like growth factor I receptor (IGF-IR), preferably human IGF-IR. Also included are chimeric, bispecific, derivatized, single chain antibodies derived from the antibodies disclosed herein. Nucleic acid molecules encoding the mammalian antibodies as well as methods of use thereof are also disclosed. Also included are pharmaceutical compositions comprising these antibodies and methods of using the antibodies and compositions thereof for treatment and diagnosis of pathological hyperproliferative oncogenic disorders associated with expression of IGf-1R.
Owner:GOETSCH LILIANE +4
Autism treatment
InactiveUS20120128683A1Treat and prevent associated lossMinimally invasiveNervous disorderPeptide/protein ingredientsMedicineNose
A safe and effective treatment to curtail and cure autism spectrum disorders has been described in this invention using insulin, IGF-1, with multiple known adjuvant therapeutic agents, as well as other pharmaceutical, biochemical, nurticeuticals, and biological agents or compounds delivered through the olfactory mucosal region of the nose and external auditory meatus.
Owner:SHANTHA TOTADA R
Fluorinated cyclic amides as dipeptidyl peptidase IV inhibitors
InactiveUS6710040B1Easy to prepareEase of detectabilityBiocideOrganic chemistryAcute coronary syndromeDisease progression
The invention relates to new therapeutically active and selective inhibitors of the enzyme dipeptidyl peptidase-IV, pharmaceutical compositions comprising the compounds and the use of such compounds for treating diseases that are associated with proteins that are subject to processing by DPP-IV, such as Type 2 diabetes mellitus, hyperglycemia, impaired glucose tolerance, metabolic syndrome (Syndrome X or insulin resistance syndrome), glucosuria, metabolic acidosis, cataracts, diabetic neuropathy, diabetic nephropathy, diabetic retinopathy, diabetic cardiomyopathy, Type 1 diabetes, obesity, conditions exacerbated by obesity, hypertension, hyperlipidemia, atherosclerosis, osteoporosis, osteopenia, frailty, bone loss, bone fracture, acute coronary syndrome, infertility due to polycystic ovary syndrome, short bowel syndrome, anxiety, depression, insomnia, chronic fatigue, epilepsy, eating disorders, chronic pain, alcohol addiction, diseases associated with intestinal motility, ulcers, irritable bowel syndrome, inflammatory bowel syndrome and to prevent disease progression in Type 2 diabetes. The invention also relates to a method of identifying an insulin secretagogue agent for diabetes.
Owner:PFIZER INC
Composition Containing Statins and Omega-3 Fatty Acids
InactiveUS20080089876A1Hydroxy compound active ingredientsPeptide/protein ingredientsFatty acidStatine
A combination is described comprising at least one omega-3 fatty acid, optionally esterified or salified, at least one statin, Coenzyme Q10, resveratrol, at least one policosanol, pantethine, selenium, and zinc. This combination is endowed with a synergistic effect and is useful in the treatment of disease forms due to insulin resistance and in cardiovascular diseases.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Insulin and IGF-1 receptor agonists and antagonists
Peptide sequences capable of binding to insulin and / or insulin-like growth factor receptors with either agonist or antagonist activity and identified from various peptide libraries are disclosed. This invention also identifies at least two different binding sites, which are present on insulin and insulin-like growth factor receptors, and which selectively bind the peptides of this invention. As agonists, the peptides of this invention may be useful for development as therapeutics to supplement or replace endogenous peptide hormones. The antagonist peptides may also be developed as therapeutics.
Owner:NOVO NORDISK AS +1
Chloroquine coupled antibodies and other proteins with methods for their synthesis
InactiveUS20070166281A1Improve efficacyImprove transportBiocidePeptide/protein ingredientsDrug conjugationTreatment effect
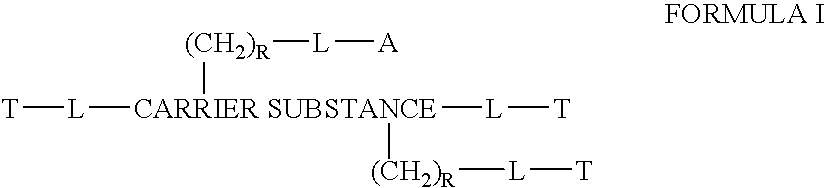
This invention discloses compositions of chloroquine-coupled active agents such as therapeutic antibodies or insulin, including methods for their preparation. The prior art has shown that chloroquines given as free drug in high enough concentration, enhances the release of various agents from cellular endosomes into the cytoplasm. The purpose of these compositions is to provide a controlled amount of chloroquine at the same site where the drug is delivered, thereby reducing the overall dosage needed. The compositions comprise a chloroquine substance coupled to a drug directly or through a variety of pharmaceutical carrier substances. The carrier substances include polysaccharides, synthetic polymers, proteins, micelles and other substances for carrying and releasing the chloroquine compositions in the body for therapeutic effect. The compositions can also include a biocleavable linkage for carrying and releasing the drug for therapeutic or other medical uses. The invention also discloses carrier compositions that are coupled to targeting molecules for targeting the delivery of chloroquine substances and antibody or insulin to their site of action.
Owner:KOSAK KENNETH M
Synthetic triterpenoids and methods of use in the treatment of disease
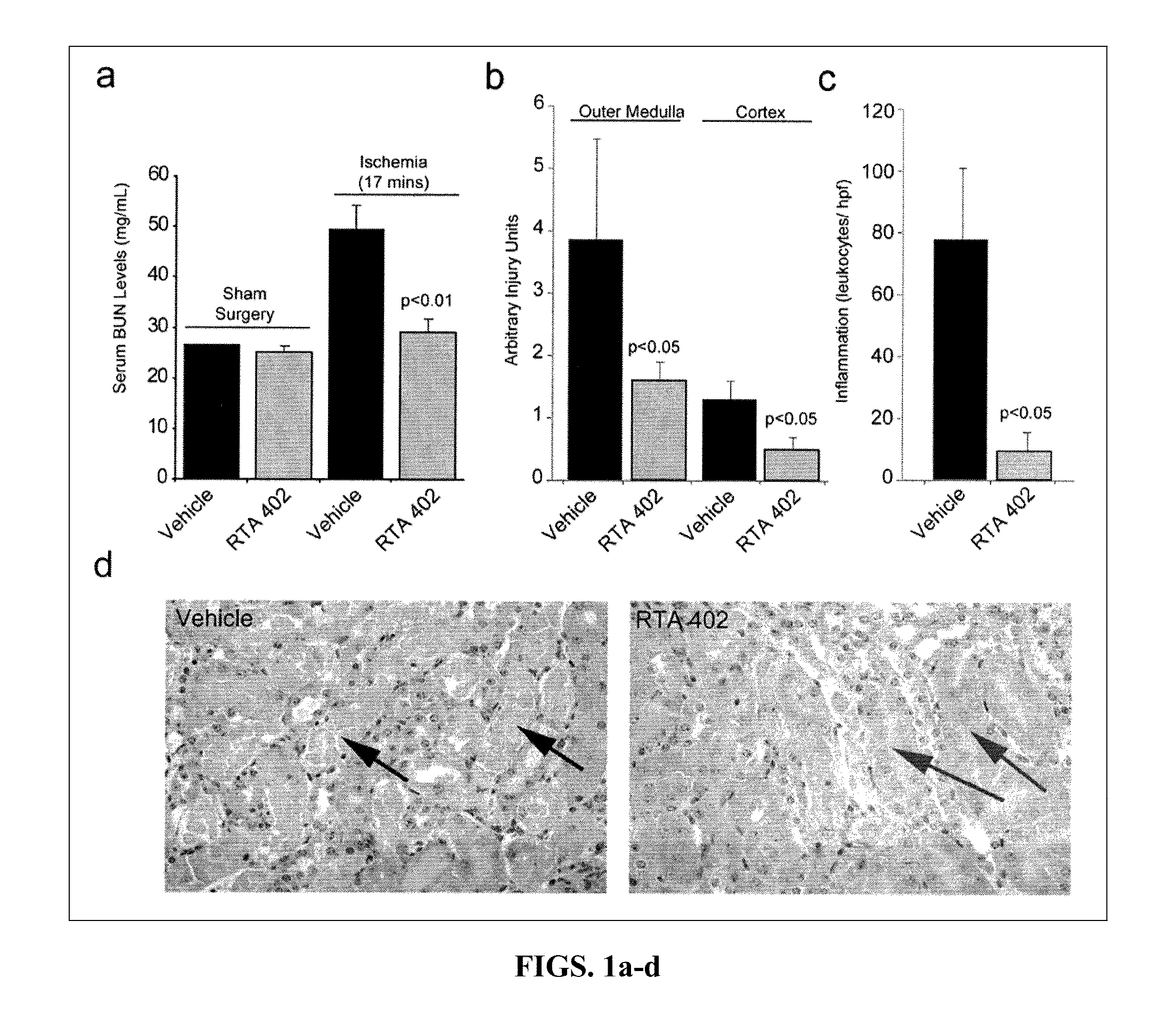
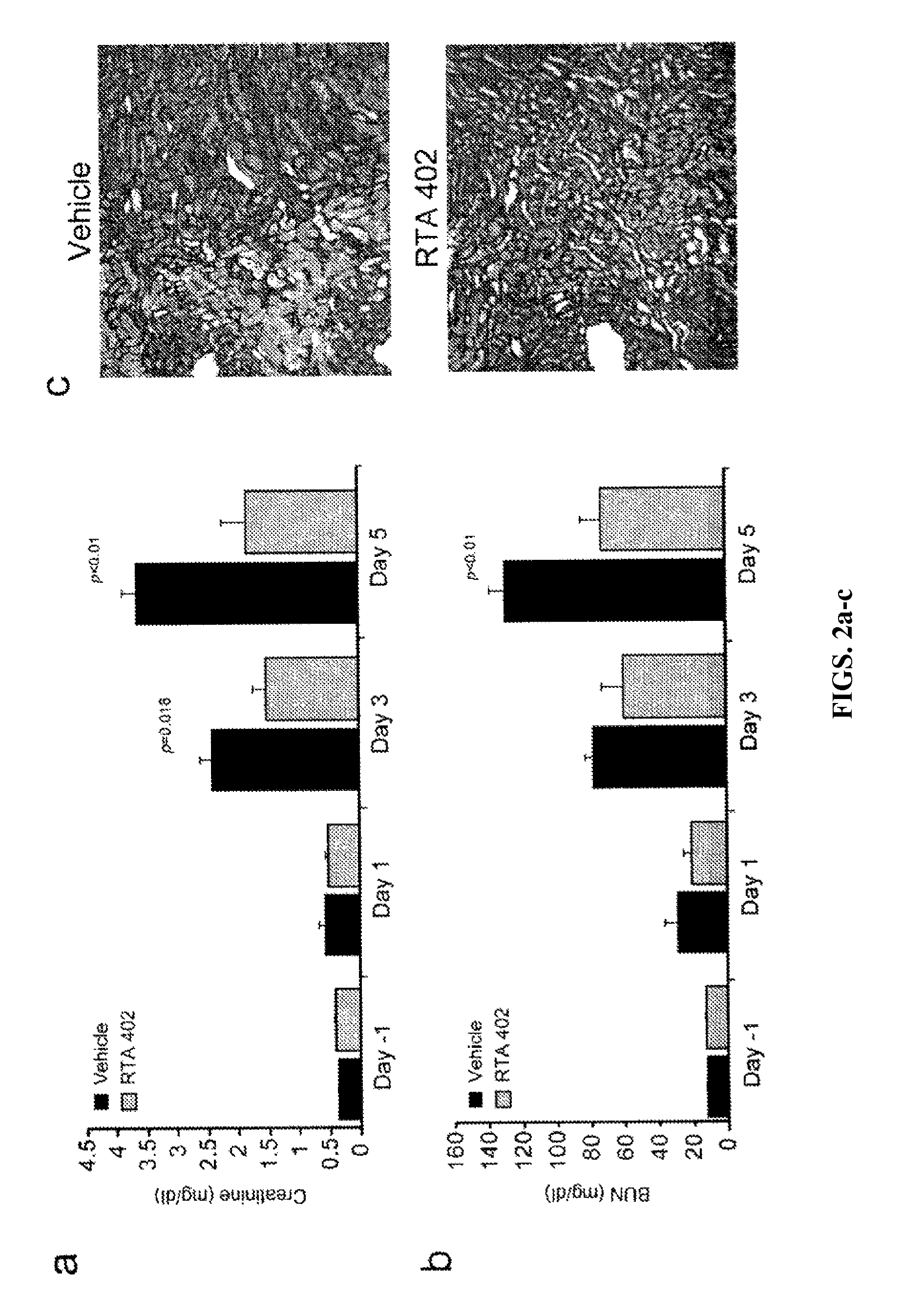
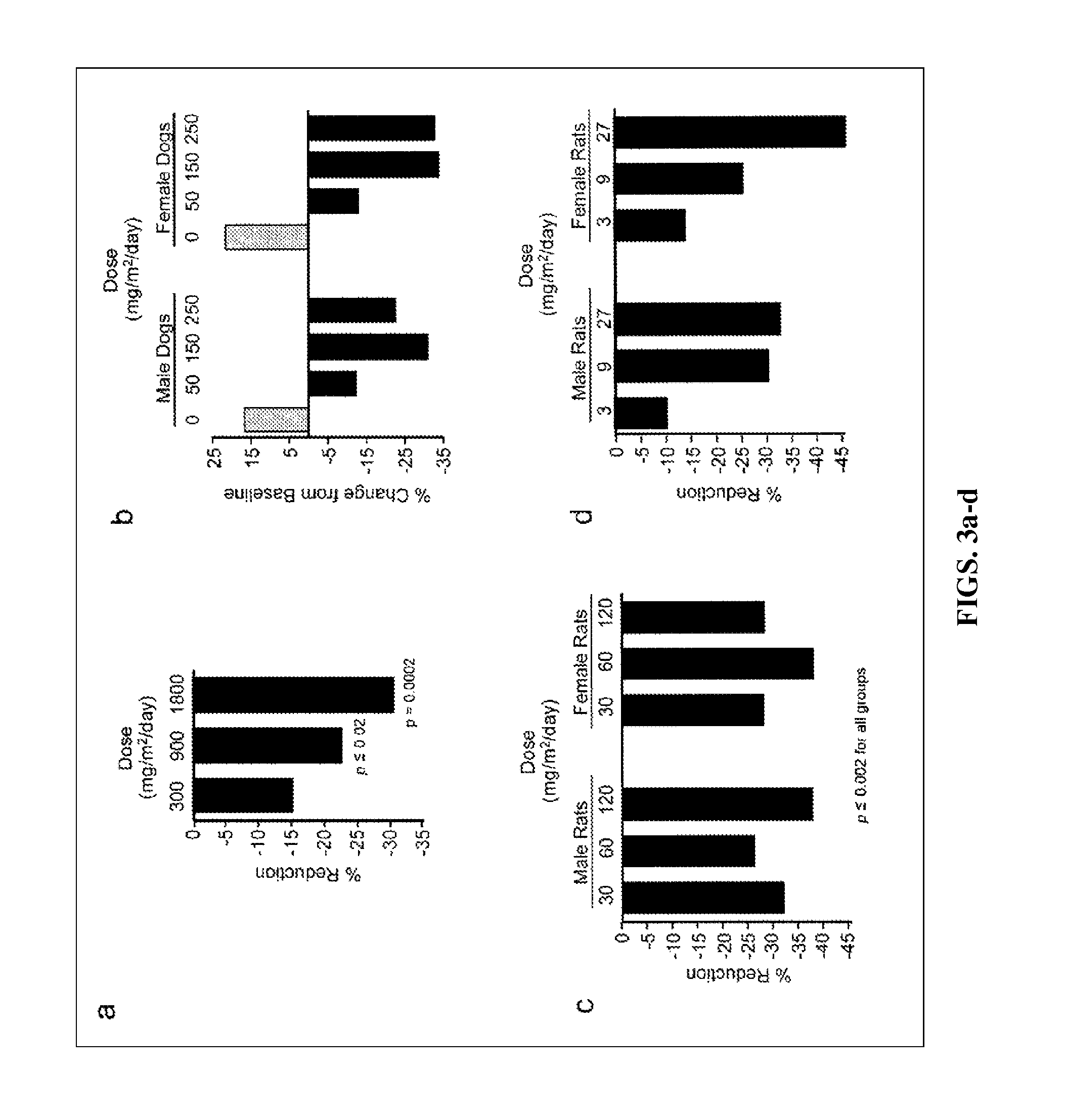
ActiveUS8129429B2Improve clearanceIncrease ratingsBiocideSenses disorderFatty liverEndothelial dysfunction
The present invention concerns methods for treating and preventing renal / kidney disease, insulin resistance / diabetes, fatty liver disease, and / or endothelial dysfunction / cardiovascular disease using synthetic triterpenoids, optionally in combination with a second treatment or prophylaxis.
Owner:REATA PHARM HLDG LLC +1
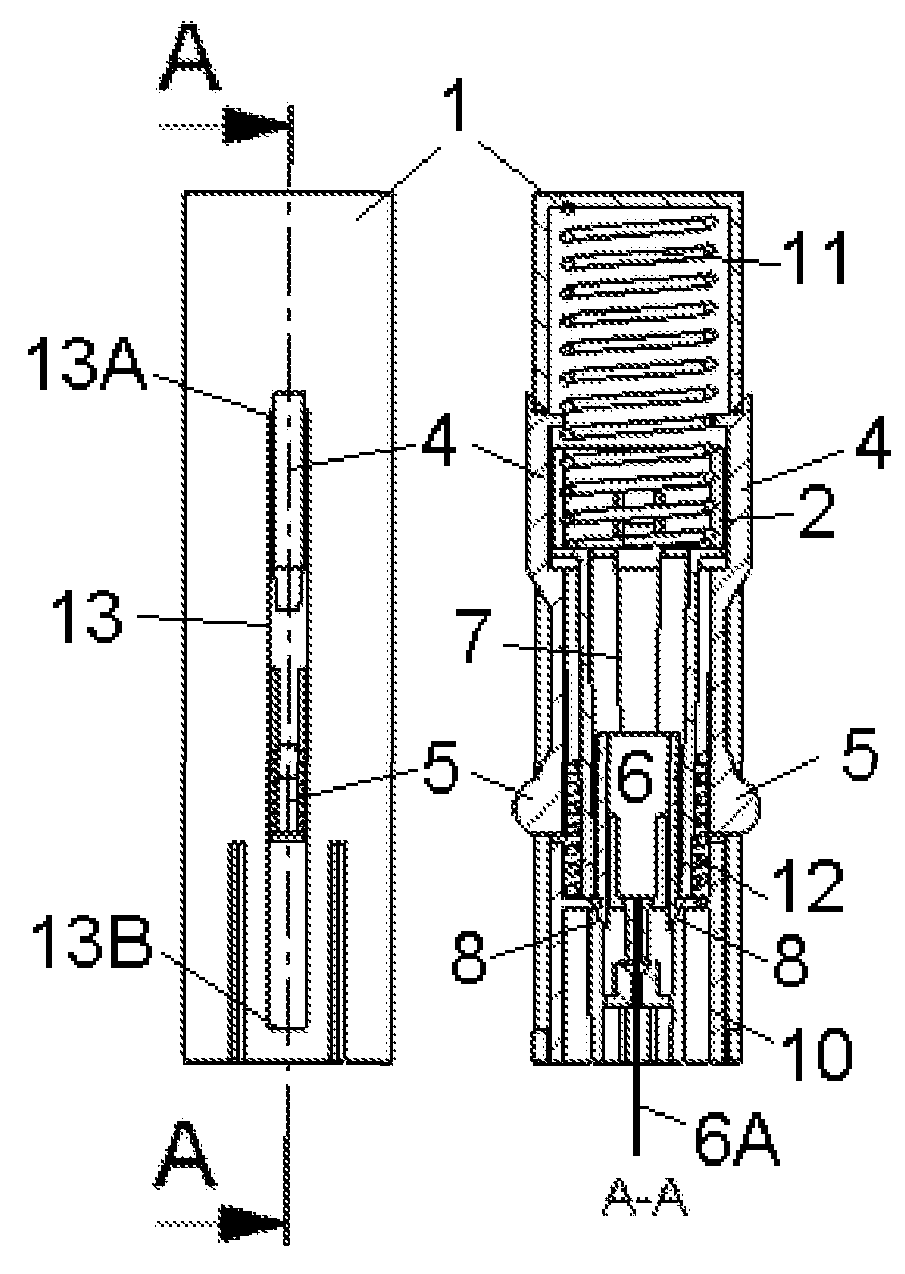
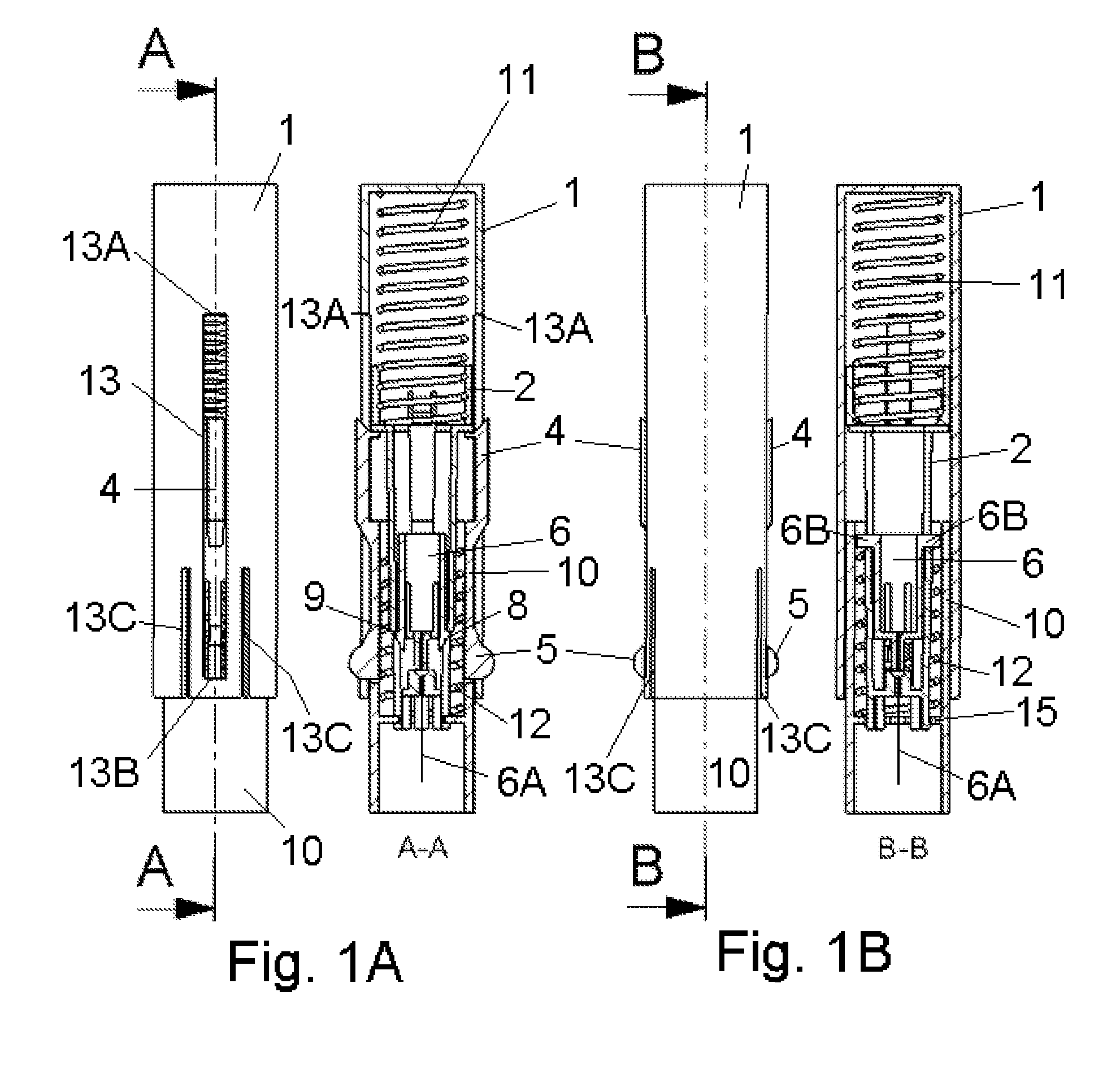
Inserter Having Two Springs
ActiveUS20100228226A1Simple and safe processNon-expensive inserterAutomatic syringesMedical devicesInfusion setBiological activation
The invention relates to an inserter for a medical device e.g. an infusion set or the like for intermittent or continuous administration of a therapeutical substance, such as e.g. insulin. The inserter comprises a needle hub comprising an insertion needle and two elastic elements assuring automatic insertion and automatic retraction of the insertion needle. Activation of the first elastic element (11) cause a penetrating member (6A) to be inserted sub-or transcutaneously into the skin of a patient, and the second elastic element (12) cause the penetrating member (6A) to be retracted from the skin of the patient. The first elastic element (11) is in an unloaded state before activation and upon activation the first elastic element (11) energizes the second elastic element (12).
Owner:UNOMEDICAL AS
Bioeffective krill oil compositions
InactiveUS20080274203A1Increasing flesh colorationPromote growthBiocideMetabolism disorderInsulin resistanceAnti oxidant
This invention discloses new krill oil compositions characterized by having high amounts of phospholipids, astaxanthin esters and / or omega-3 contents. The krill oils are obtained from krill meal using supercritical fluid extraction in a two stage process. Stage 1 removes the neutral lipid by extracting with neat supercritical CO2 or CO2 plus approximately 5% of a co-solvent. Stage 2 extracts the actual krill oils by using supercritical CO2 in combination with approximately 20% ethanol. The krill oil materials obtained are compared with commercially available krill oil and found to be more bioeffective in a number of areas such as anti-inflammation, anti-oxidant effects, improving insulin resistances and improving blood lipid profile.
Owner:AKER BIOMARINE ANTARCTIC
Compositions and methods for treating insulin resistance and diabetes mellitus
Provided are electrokinetically-altered fluids (gas-enriched electrokinetic fluids) comprising an ionic aqueous solution of charge-stabilized oxygen-containing nanostructures in an amount sufficient to provide modulation of at least one of cellular membrane potential and cellular membrane conductivity, and therapeutic compositions and methods for use in treating diabetes and diabetes-associated conditions or disorders (e.g., insulin resistance), or symptoms thereof. Provided are electrokinetically-altered ioinic aqueous fluids optionally in combination with other therapeutic agents. Particular aspects provide for regulating or modulating intracellular signal transduction associated with said inflammatory responses by modulation of at least one of cellular membranes, membrane potential, membrane proteins such as membrane receptors, including but not limited to G-Protein Coupled Receptors (GPCR), and intercellular junctions (e.g., tight junctions, gap junctions, zona adherins and desmasomes). Other embodiments include particular routes of administration or formulations for the electrokinetically-altered fluids (e.g., electrokinetically-altered gas-enriched fluids and solutions) and therapeutic compositions.
Owner:REVALESIO CORP
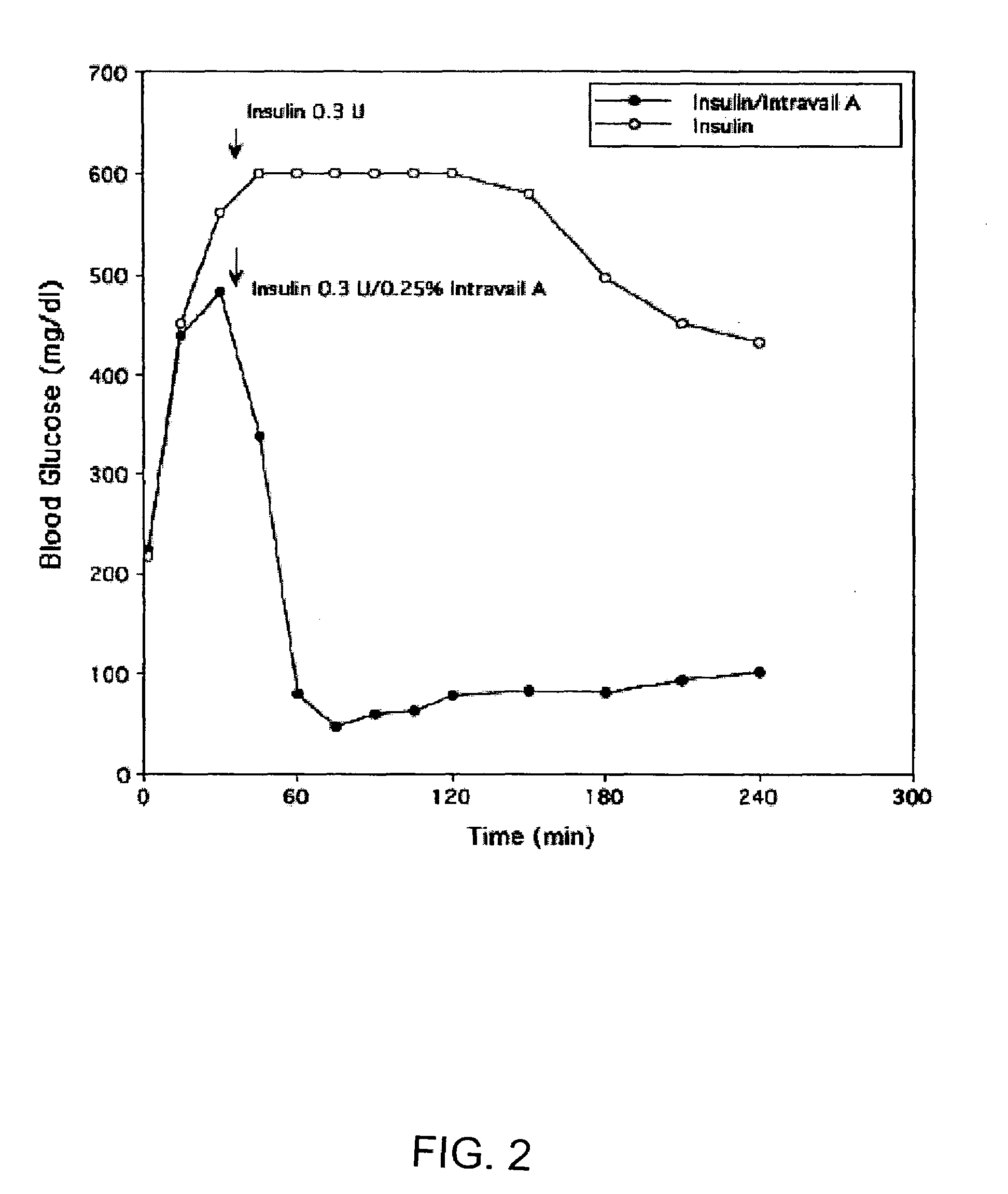
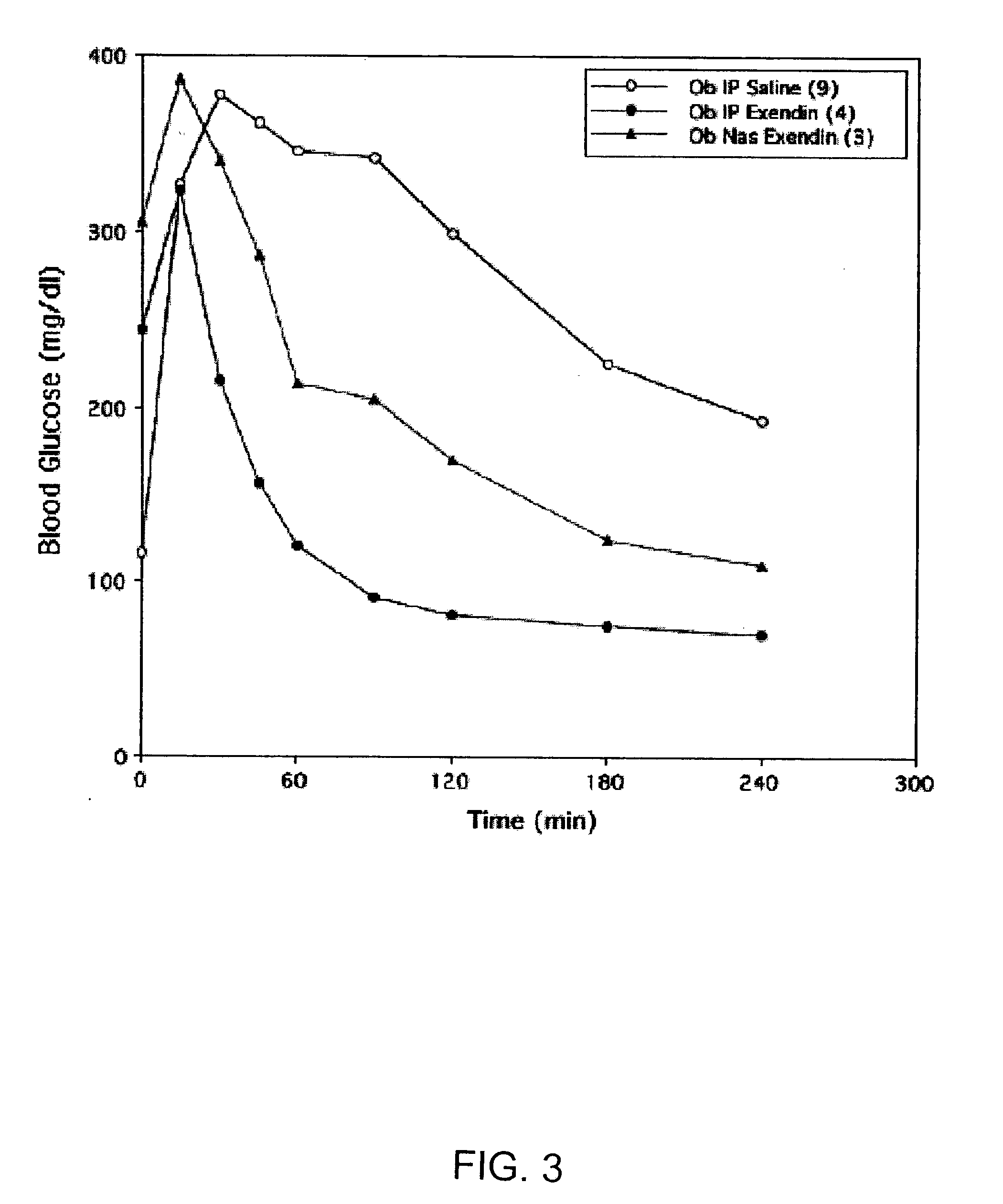
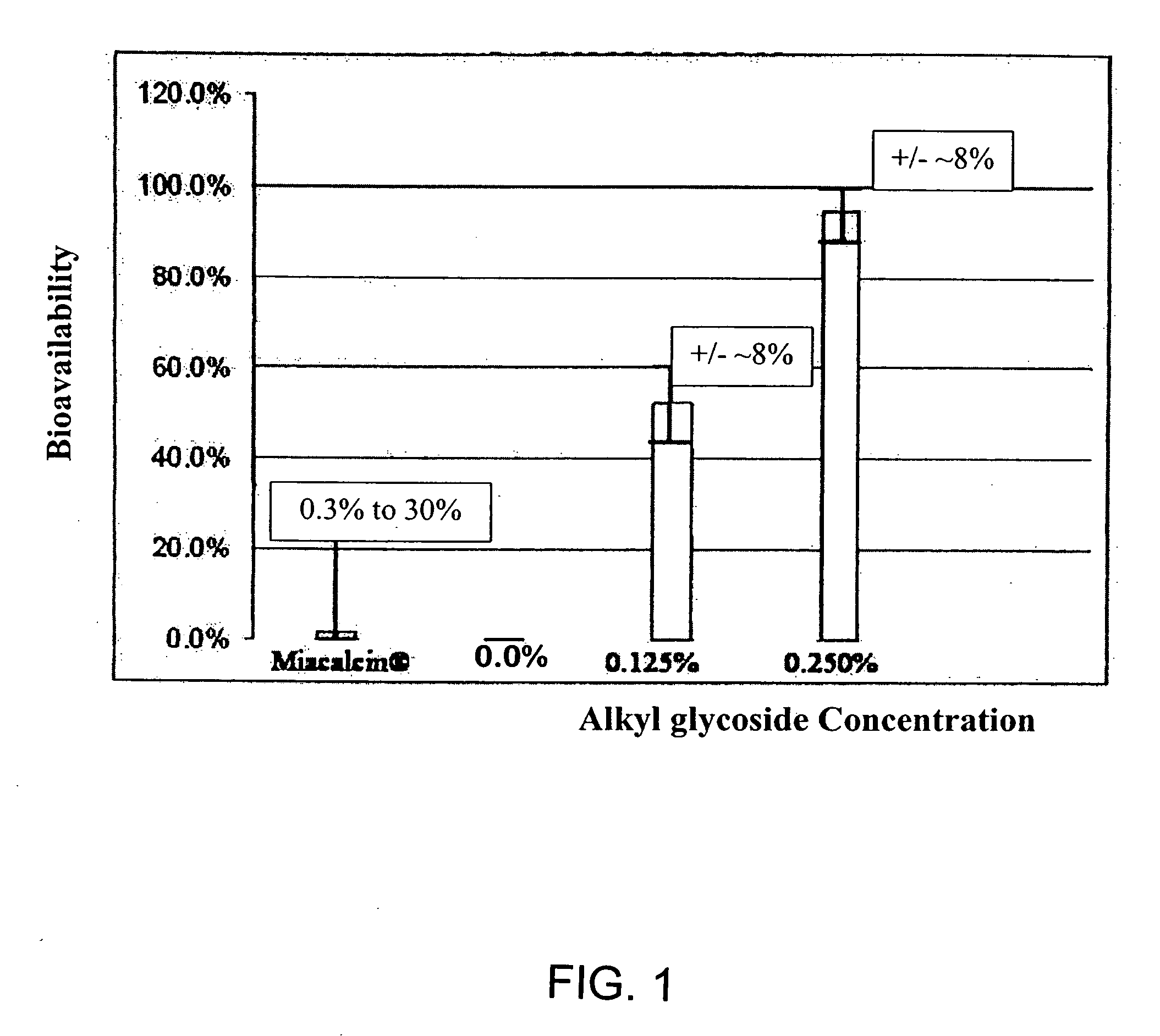
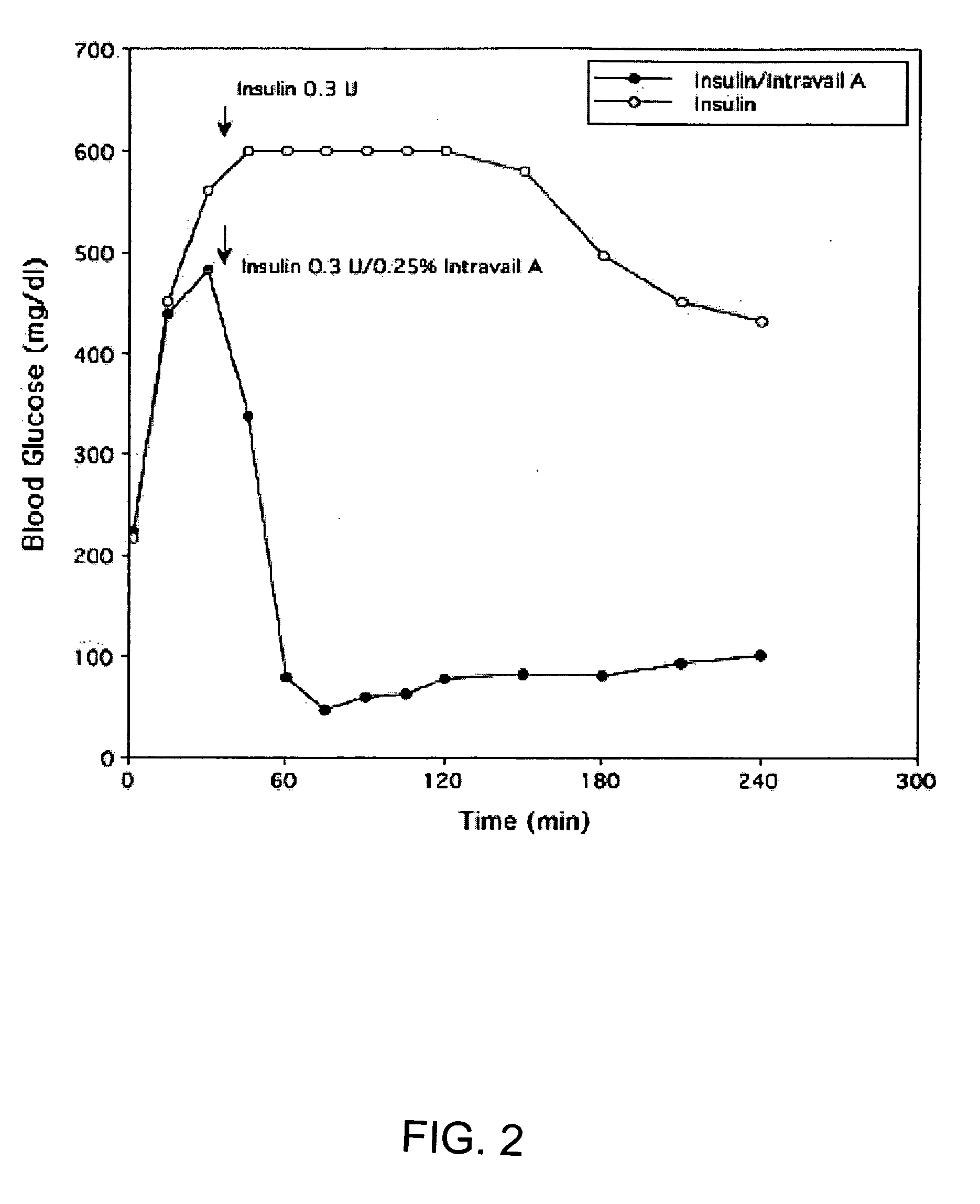
Absorption Enhancers for Drug Administration
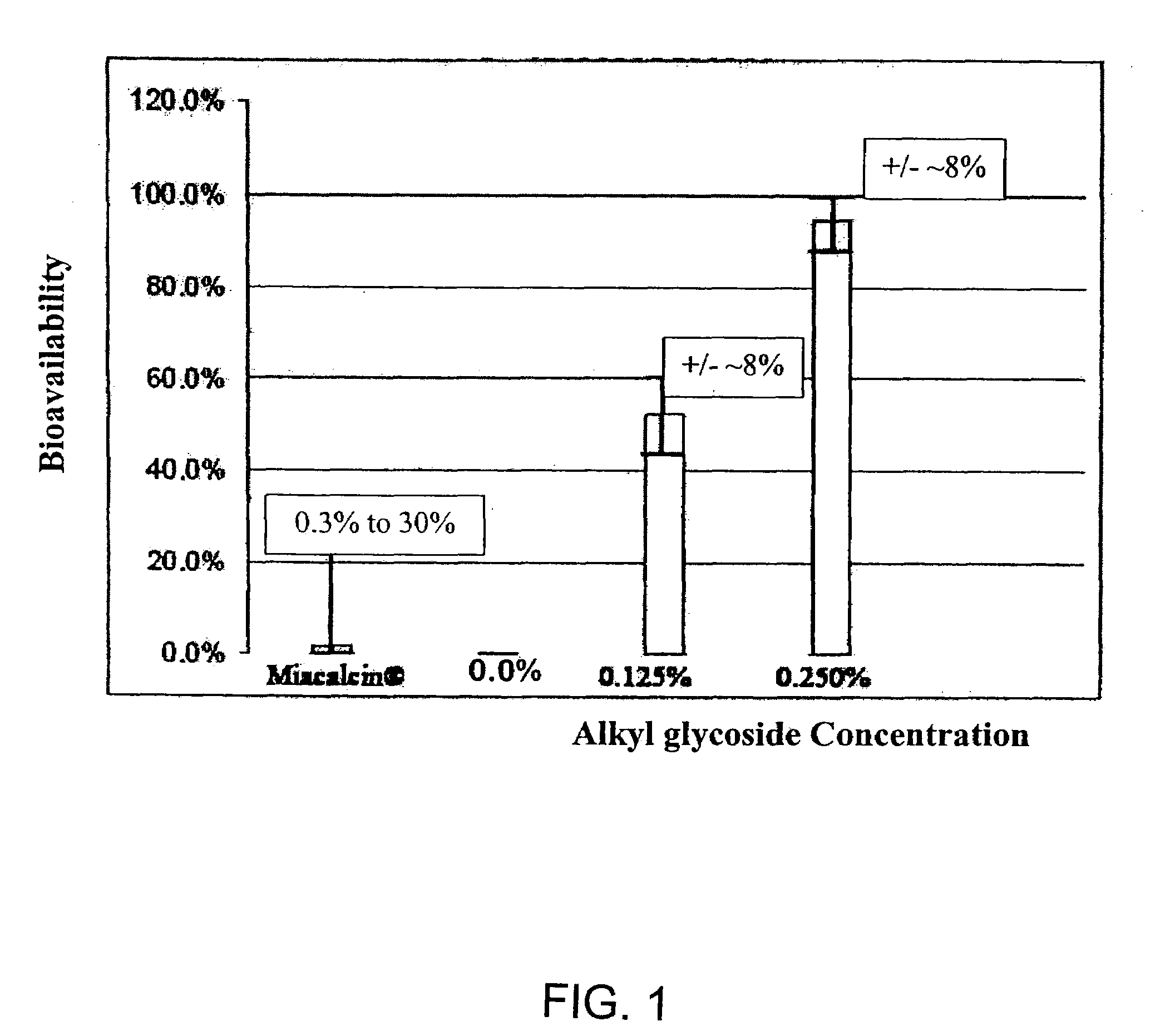
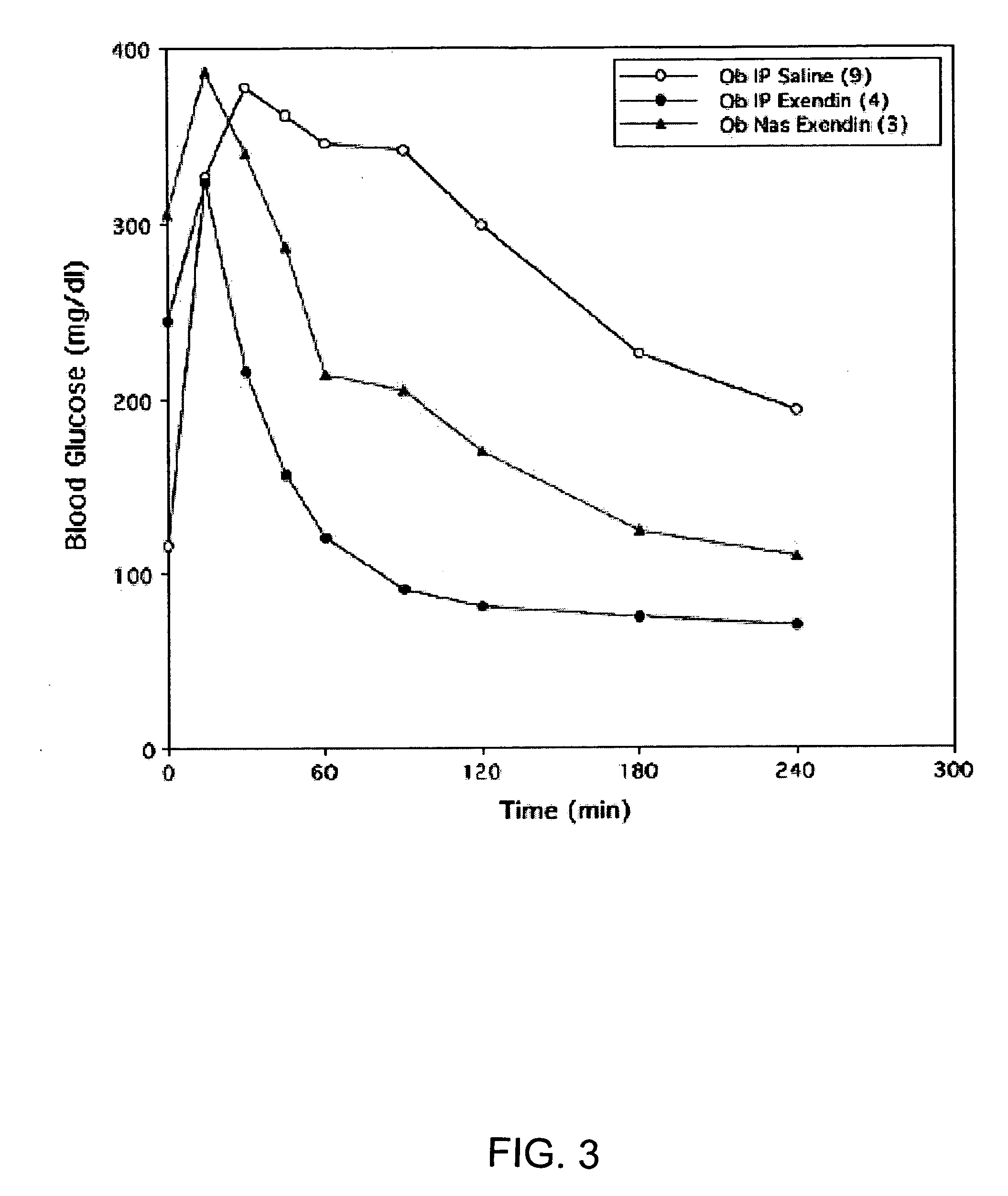
ActiveUS20080299079A1Improve absorption and bioavailabilityToxic effectsBiocideNervous disorderActive agentPancreatic hormone
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC
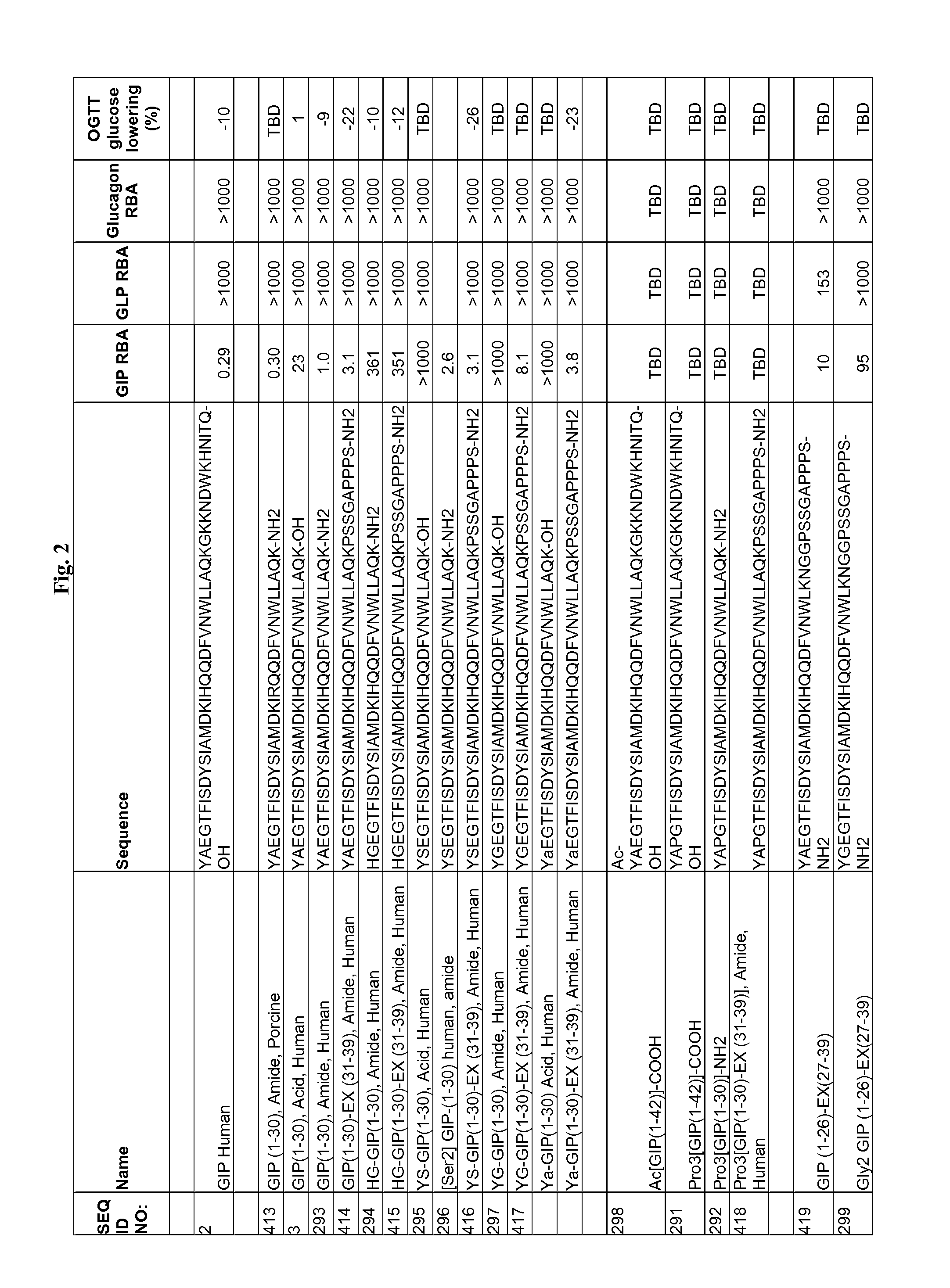
Gip analog and hybrid polypeptides with selectable properties
InactiveUS20090036364A1Increased insulin secretionDecreasing bone loss boneSenses disorderNervous disorderDyslipidemiaPhysiology
The present invention relates generally to novel GIP analogs and GIP hybrid polypeptides with selectable properties, useful as agents for the treatment and prevention of metabolic diseases and disorders, for example those which can be alleviated by control plasma glucose levels, insulin levels, and / or insulin secretion, positive inotropic effects, reduction of catabolic effects, slowing of gastric emptying. Such conditions and disorders include, but are not limited to, hypertension, dyslipidemia, cardiovascular disease, eating disorders, critical care, insulin-resistance, obesity, and diabetes mellitus of any kind, including type 1, type 2, and gestational diabetes.
Owner:ASTRAZENECA PHARMA LP
Neutralizing human anti-IGFR antibody
The present invention includes fully human, neutralizing, monoclonal antibodies against human Insulin-like Growth Factor Receptor-I (IGFR1). The antibodies are useful for treating or preventing cancer in a subject. Also included are methods of using and producing the antibodies of the invention.
Owner:MERCK SHARP & DOHME CORP
Inhibitors of the 11-beta-hydroxysteroid dehydrogenase type 1 enzyme
ActiveUS20060149070A1Improve throughputLimit deliveryOrganic active ingredientsOrganic chemistryInsulin dependentEnzyme inhibitor
The present invention relates to compounds that are inhibitors of the 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme. The present invention further relates to the use of inhibitors of 11-beta-hydroxysteroid dehydrogenase Type 1 enzyme for the treatment of non-insulin dependent type 2 diabetes, insulin resistance, obesity, lipid disorders, metabolic syndrome and other diseases and conditions that are mediated by excessive glucocorticoid action.
Owner:ABBVIE INC
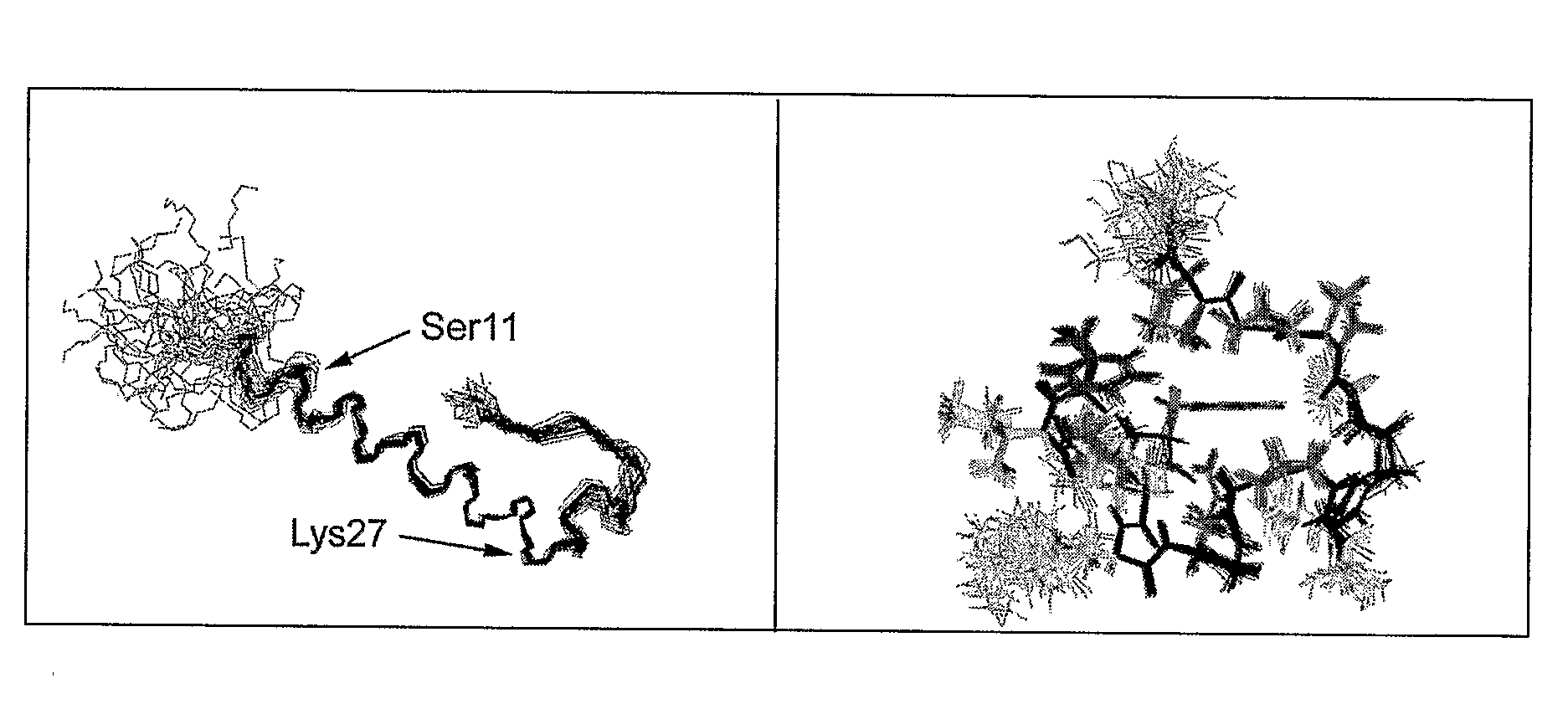
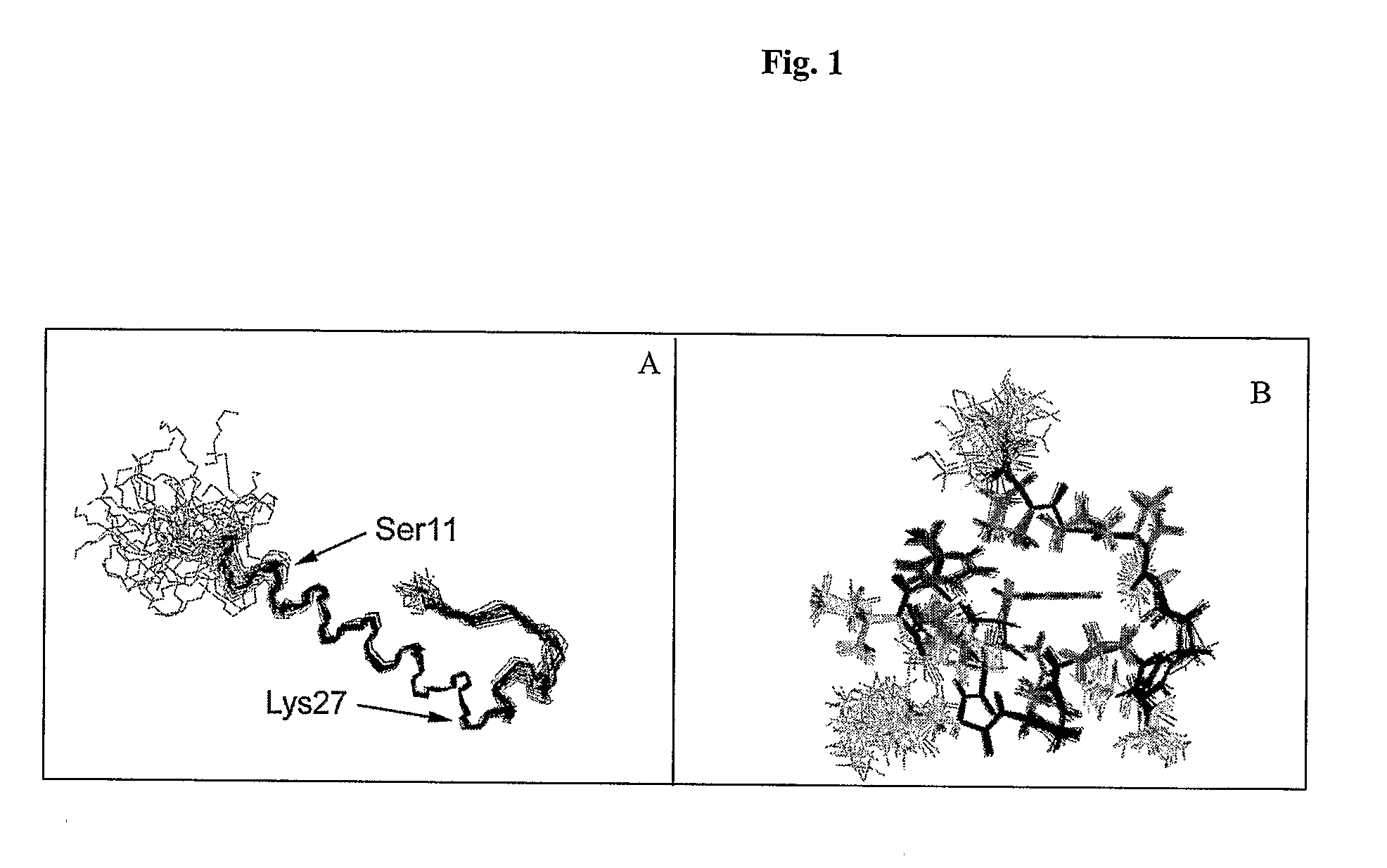
Insulinotropic hormone derivatives and uses thereof
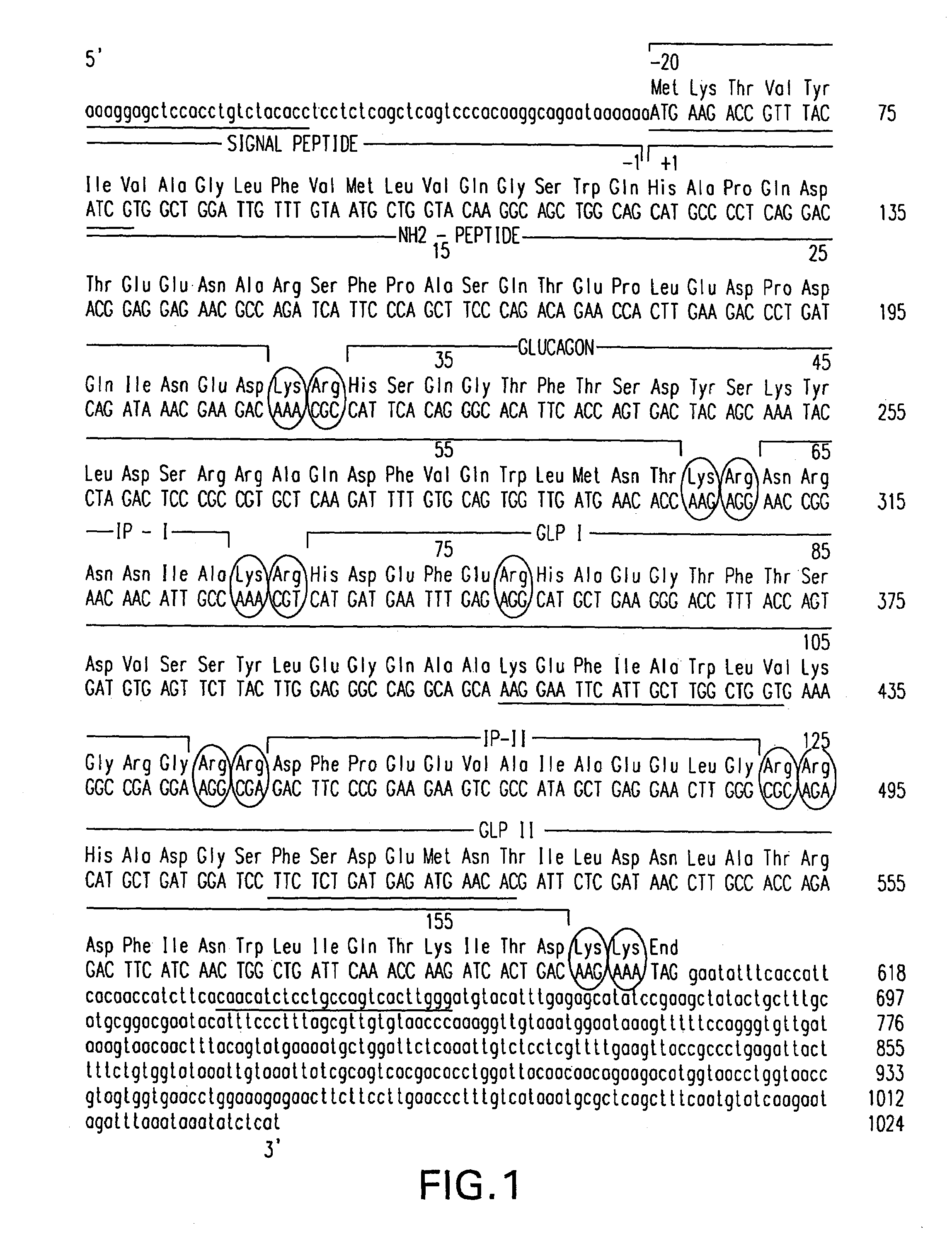
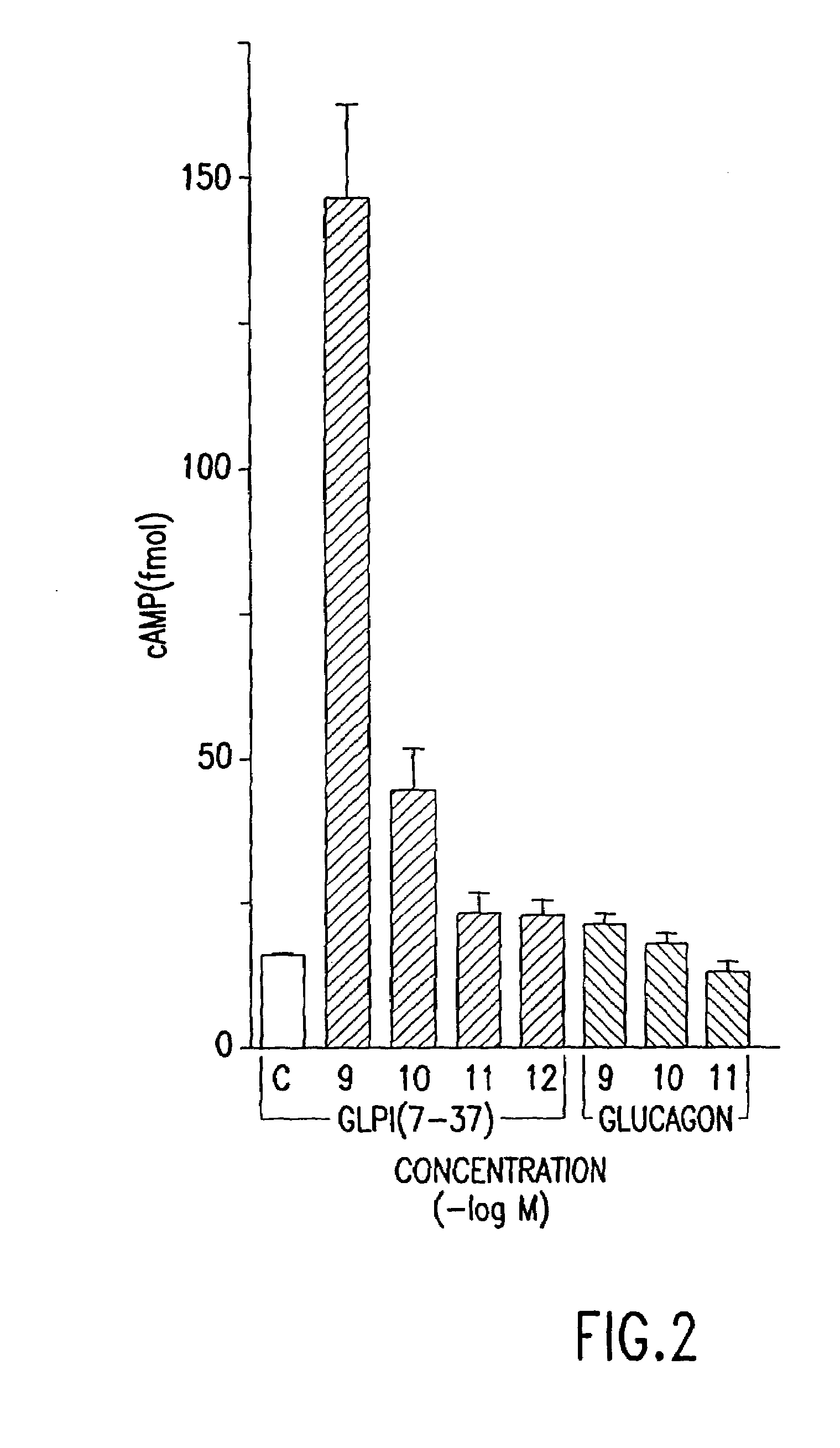
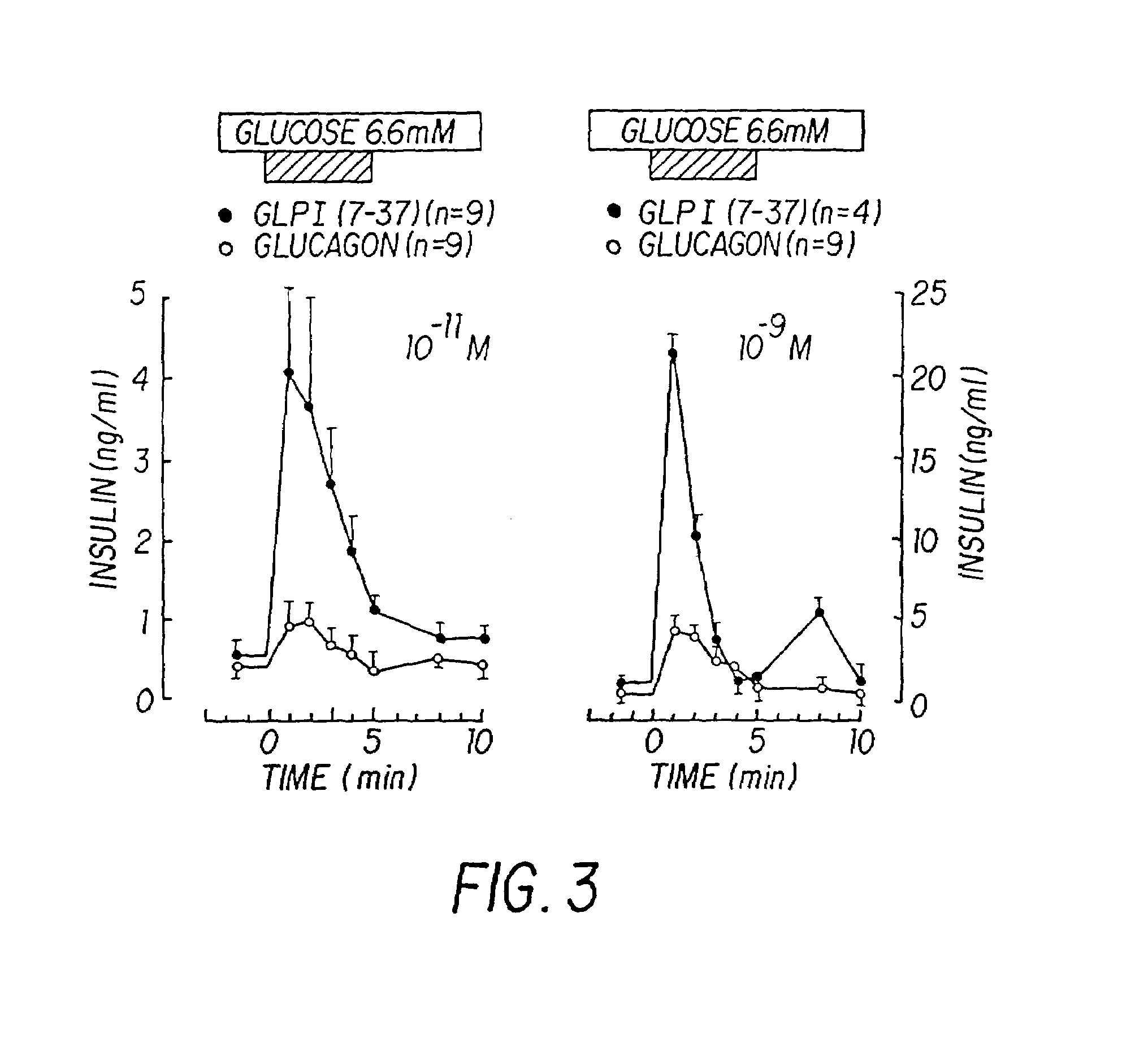
InactiveUS7138486B2Promote insulin secretionIncrease insulin levelsBiocidePeptide/protein ingredientsInsulinotropinPancreatic hormone
Derivatives of glucagon-like peptide I (GLP-1) and especially GLP-1 (7-37) have been found to have insulinotropic activity. The invention pertains to a composition comprising an acid addition salt of GLP-I (7-37) and to a composition comprising a carboxylate salt of GLP-I (7-37). The invention also pertains to method of treating type II diabetes mellitus by providing derivatives of GLP-I (7-37) to the patient.
Owner:THE GENERAL HOSPITAL CORP
Anti-IGF-I receptor antibody
InactiveUS7538195B2Increase profitOrganic active ingredientsFungiInsulin-like growth factorSynovial sarcoma
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of these molecules with cytotoxic agents, which specifically bind to and inhibit insulin-like growth factor-I receptor, antagonize the effects of IGF-I and are substantially devoid of agonist activity toward the insulin-like growth factor-I receptor. These molecules can be conjugated to cytotoxic agents for use in the treatment of tumors that express elevated levels of IGF-I receptor, such as breast cancer, colon cancer, lung cancer, ovarian carcinoma, synovial sarcoma and pancreatic cancer. These molecules can also be labeled for in vitro and in vivo diagnostic uses, such as in the diagnosis and imaging of tumors that express elevated levels of IGF-I receptor.
Owner:IMMUNOGEN INC
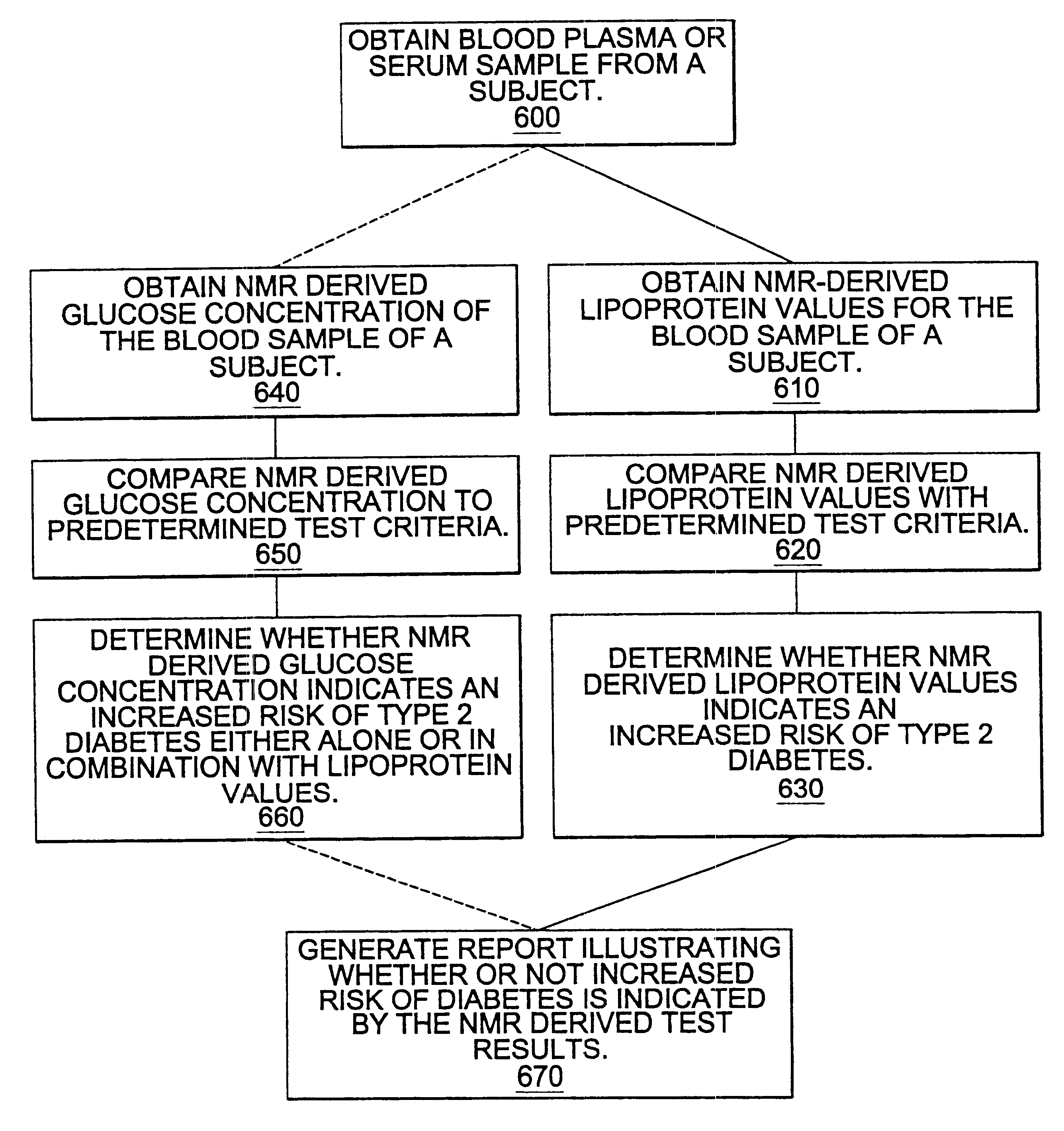
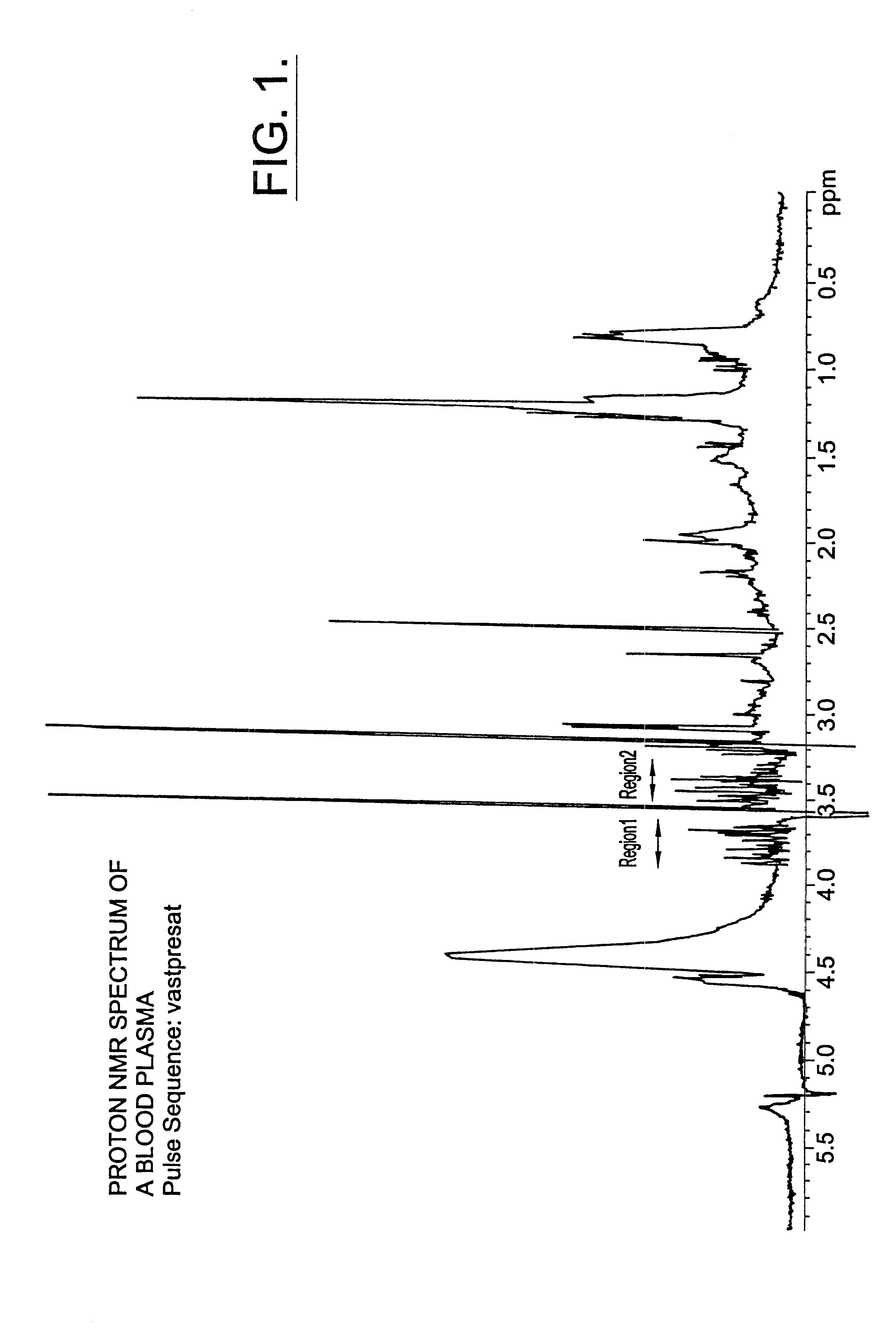
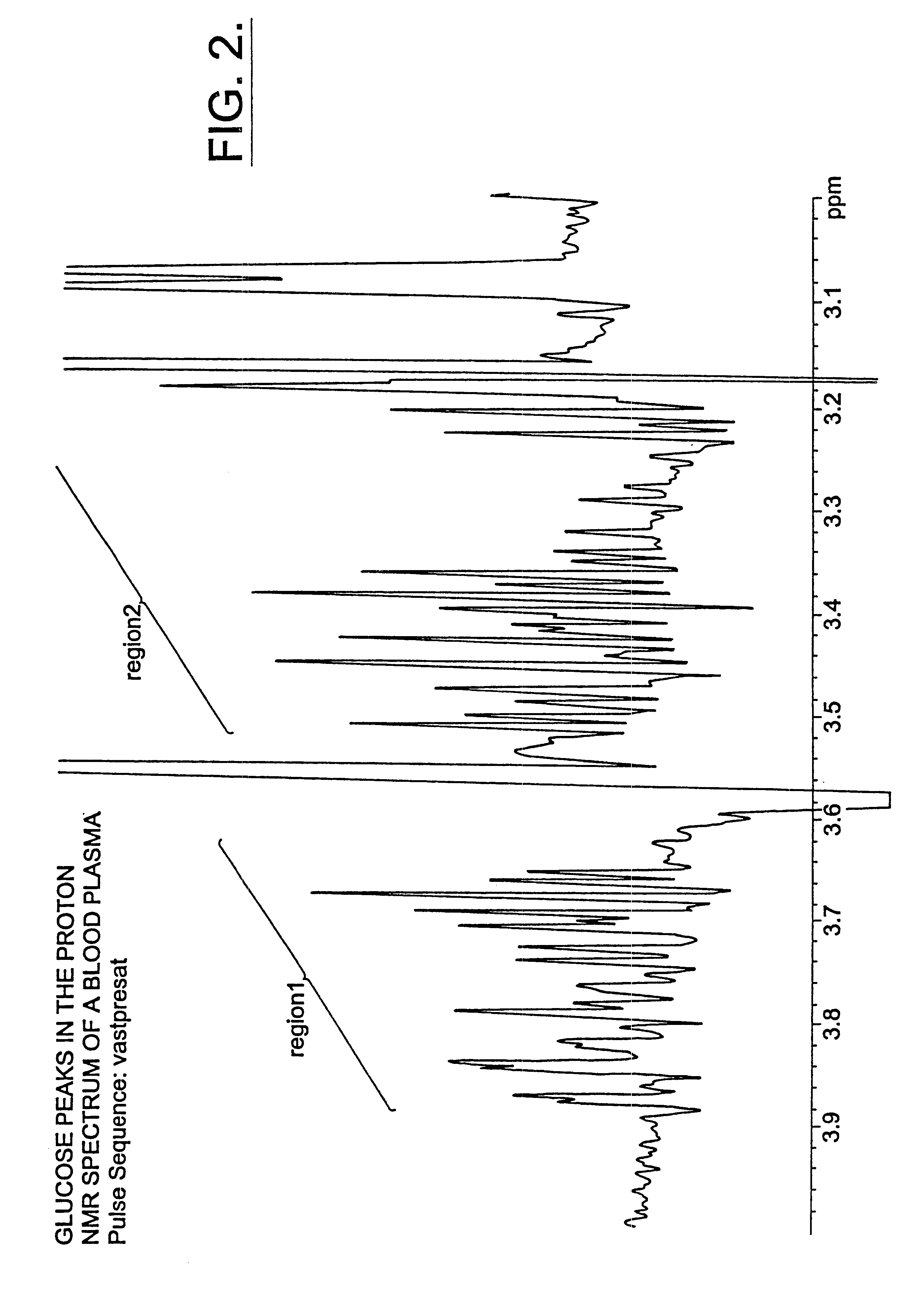
Methods and computer program products for determining risk of developing type 2 diabetes and other insulin resistance related disorders
InactiveUS6518069B1Facilitate early detectionAvoid spendingDisease diagnosisAnalysis using nuclear magnetic resonanceDiseaseData set
Methods for assessing the risk of developing Type 2 diabetes and other related disorders include obtaining an NMR derived reference spectrum for a known glucose concentration sample and storing this information as a reference standard. A patient blood sample is collected and NMR derived patient spectrums for the blood sample are obtained. The two NMR data sets (the reference and the patient) are compared and a glucose concentration is determined for the patient sample. The glucose concentration can be evaluated with a blood sample undergoing lipoprotein cholesterol evaluation. The NMR based test can be used to concurrently provide a glucose concentration and lipoprotein constituent values based on a single testing event. The disclosure also includes a multi-purpose test, i.e., a test which concurrently provides lipoprotein screening and coronary heart disease risk evaluation along with a diabetes screening and risk assessment for developing Type 2 diabetes. A method for assessing diabetes includes identifying the presence of diabetic dyslipidemia based on the values of predetermined NMR measured lipoprotein constituents.
Owner:LIPOSCI +1
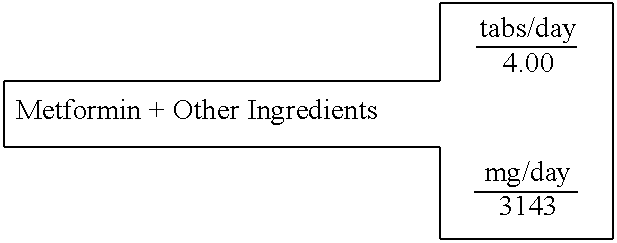
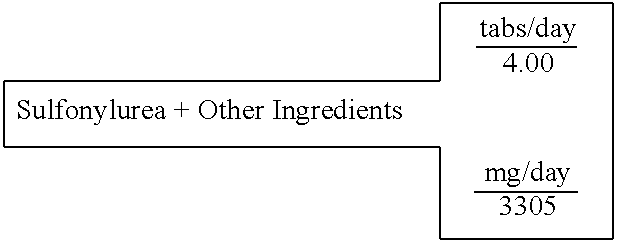
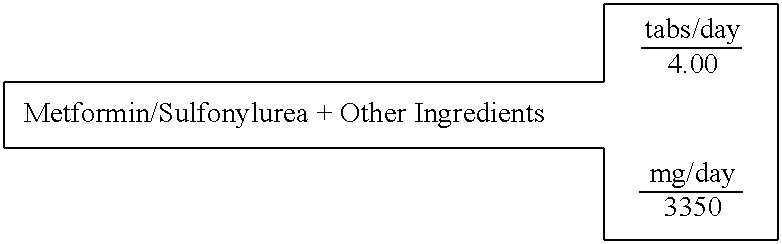
Biguanide and sulfonylurea formulations for the prevention and treatment of insulin resistance and type 2 diabetes mellitus
InactiveUS20030078269A1Maximum complementarityImprove effectivenessBiocidePeptide/protein ingredientsSulfonylureaTreatment level
The invention describes formulations that include either metformin, sulfonylurea or a biguanide-sulfonylurea combination as one active ingredient in addition to specific, other active ingredients. The compositions and dosage forms of the invention are clinically useful as methods for increasing the effectiveness, efficiency and safety of the included biguanide (metformin) and / or sulfonylurea in the prevention and treatment of insulin resistance and diabetes mellitus. The carefully chosen additional active ingredients of the invention are designed in a modular fashion to prevent and rectify adverse events associated with insulin resistance syndrome and diabetes mellitus, and those adverse incidences associated with the concurrent use of metformin and / or the sulfonylureas. When clinically administered, the invention will provide therapeutic levels of metformin and of a sulfonylurea, alone or in combination, and broaden their usefulness. The invention will retard the progression of insulin resistance to type 2 diabetes, and reduce the serious microvascular and macrovascular complications commonly associated with insulin resistance syndrome and diabetes mellitus.
Owner:CHRONORX
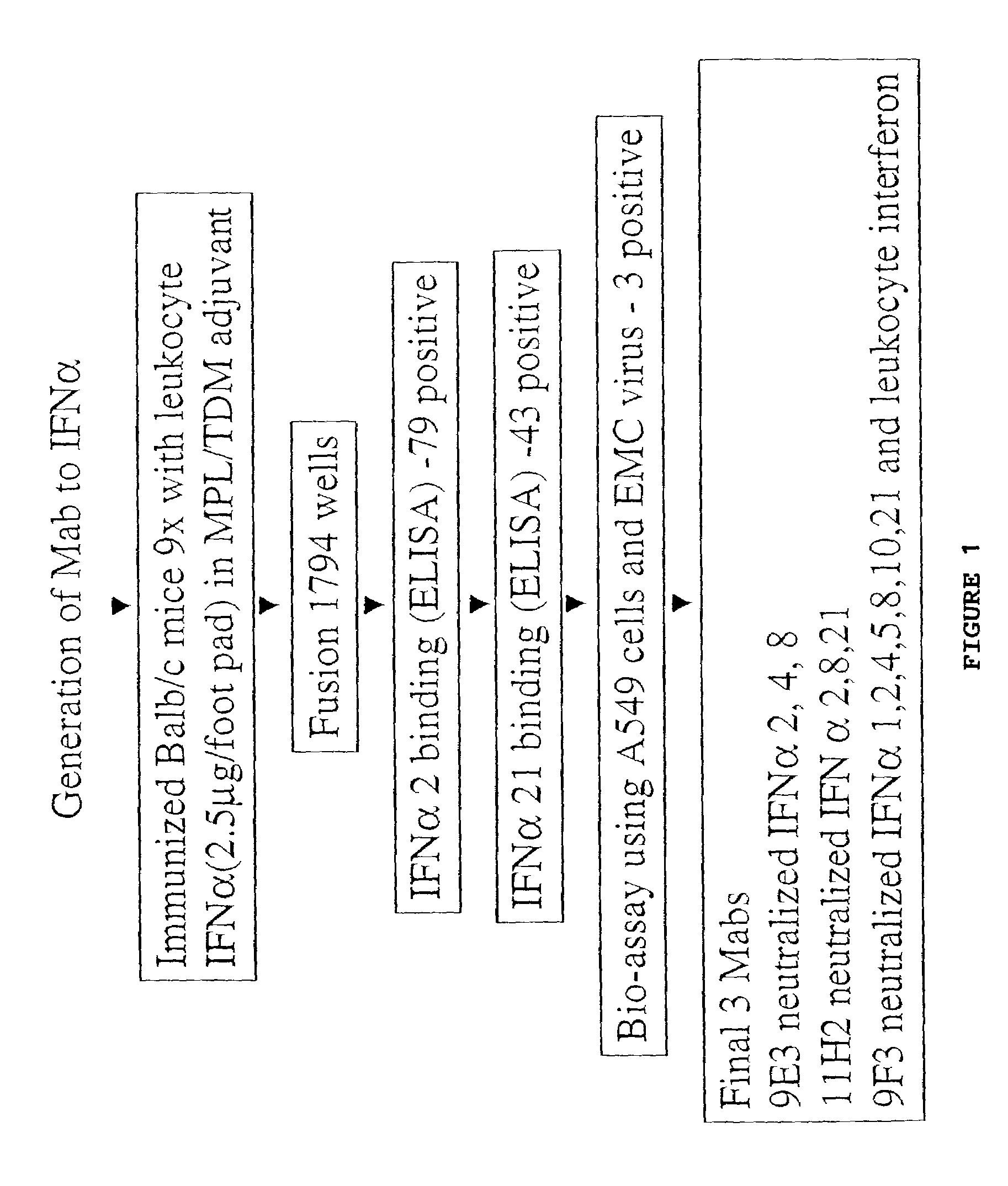
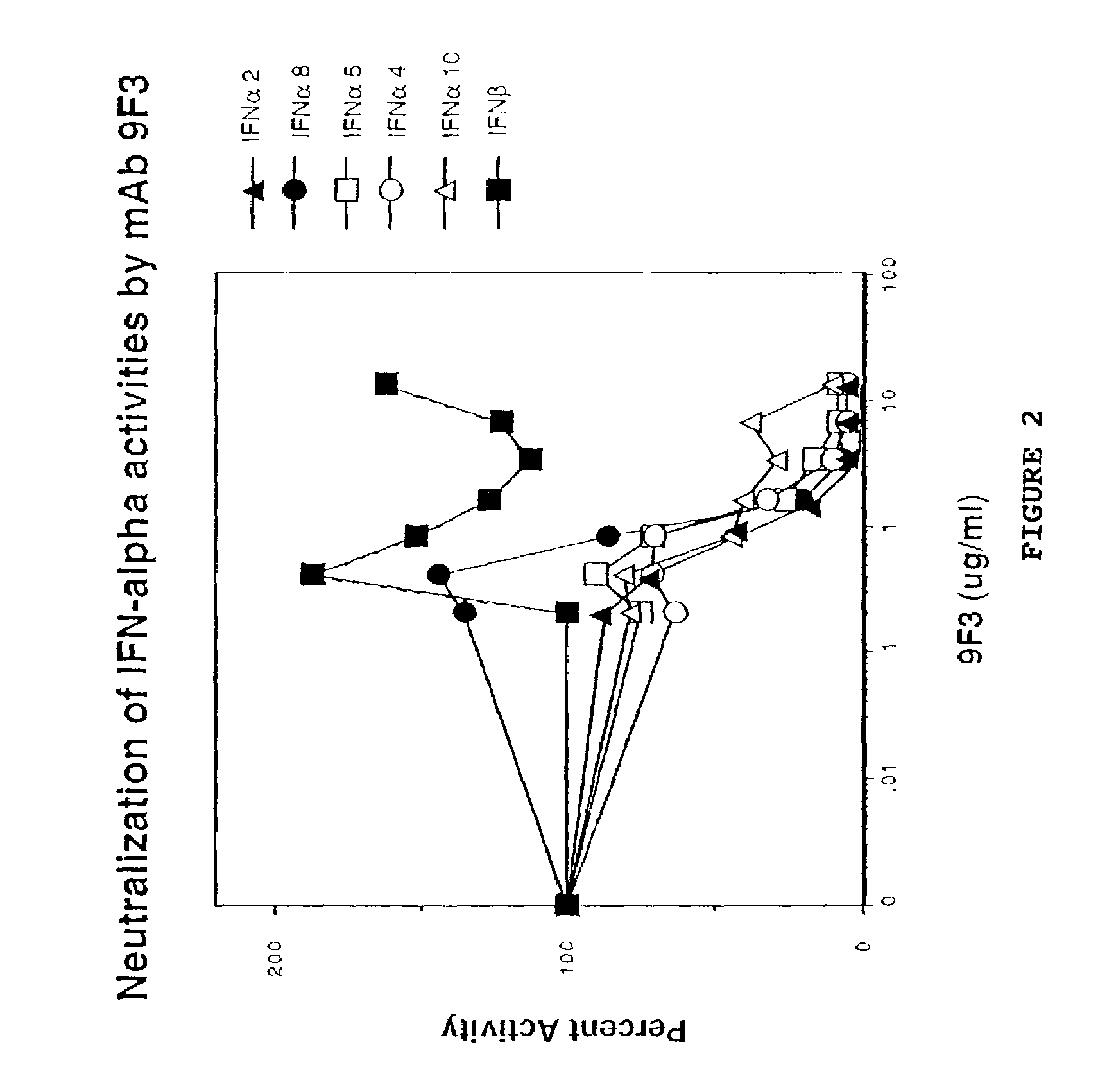
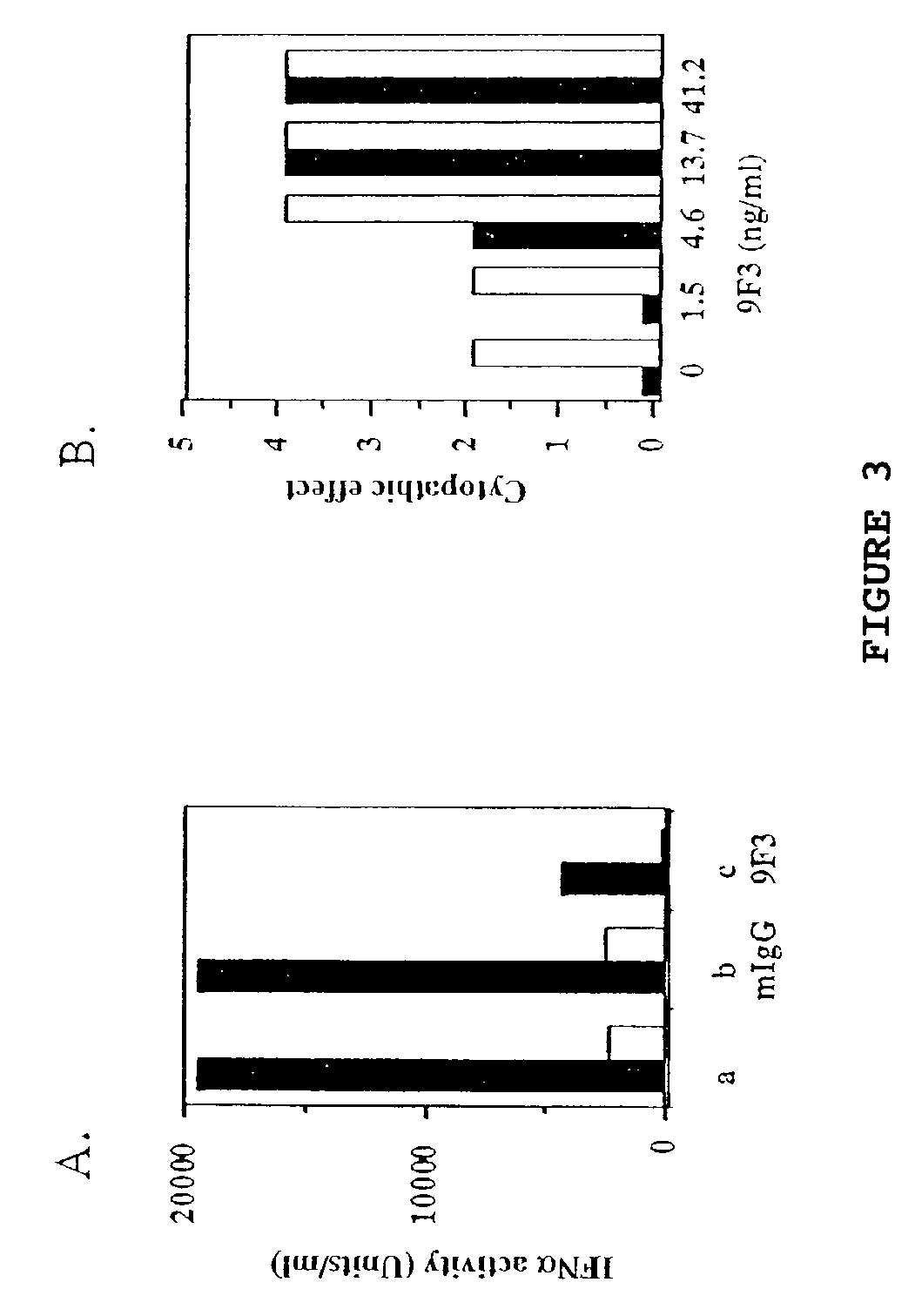
Anti-interferon-α antibodies
InactiveUS7087726B2Reduce and eliminate biological activityFungiBacteriaDiseaseAntiendomysial antibodies
The present invention relates generally to the generation and characterization of neutralizing anti-IFN-α monoclonal antibodies with broad reactivity against various IFN-α subtypes. The invention further relates to the use of such anti-IFN-α antibodies in the diagnosis and treatment of disorders associated with increased expression of IFN-α, in particular, autoimmune disorders such as insulin-dependent diabetes mellitus (IDDM) and systemic lupus erythematosus (SLE).
Owner:GENENTECH INC
Absorption enhancers for drug administration
InactiveUS20060045869A1Avoid effectIncrease absorption and bioavailability of drugBiocideNervous disorderDrugDrug administration
A composition including a surfactant and at least one alkyl glycoside and / or saccharide alkyl ester and a drug. The surfactant composition(s) when admixed with a drug is non-toxic and non-irritating, while stabilizing and increasing the bioavailability of the drug. The invention also provides compositions that enhance absorption of drugs via the oral, ocular, nasal, nasolacrimal, inhalation or pulmonary, oral cavity (sublingual or Buccal cell) or CSF delivery route of a patient, including but not limited to insulin, glucagon and exendin-4.
Owner:AEGIS THERAPEUTICS LLC +1
Insulin and IGF-1 receptor agonists and antagonists
Peptide sequences capable of binding to insulin and / or insulin-like growth factor receptors with either agonist or antagonist activity and identified from various peptide libraries are disclosed. This invention also identifies at least two different binding sites, which are present on insulin and insulin-like growth factor receptors, and which selectively bind the peptides of this invention. As agonists, certain of the peptides of this invention may be useful for development as therapeutics to supplement or replace endogenous peptide hormones. The antagonists may also be developed as therapeutics.
Owner:NOVO NORDISK AS +1
Differentiation of Human Embryonic Stem Cells
The present invention provides methods to promote the differentiation of pluripotent stem cells. In particular, the present invention provides an improved method for the formation of pancreatic endoderm, pancreatic hormone expressing cells and pancreatic hormone secreting cells. The present invention also provides methods to promote the differentiation of pluripotent stem cells without the use of a feeder cell layer.
Owner:LIFESCAN INC
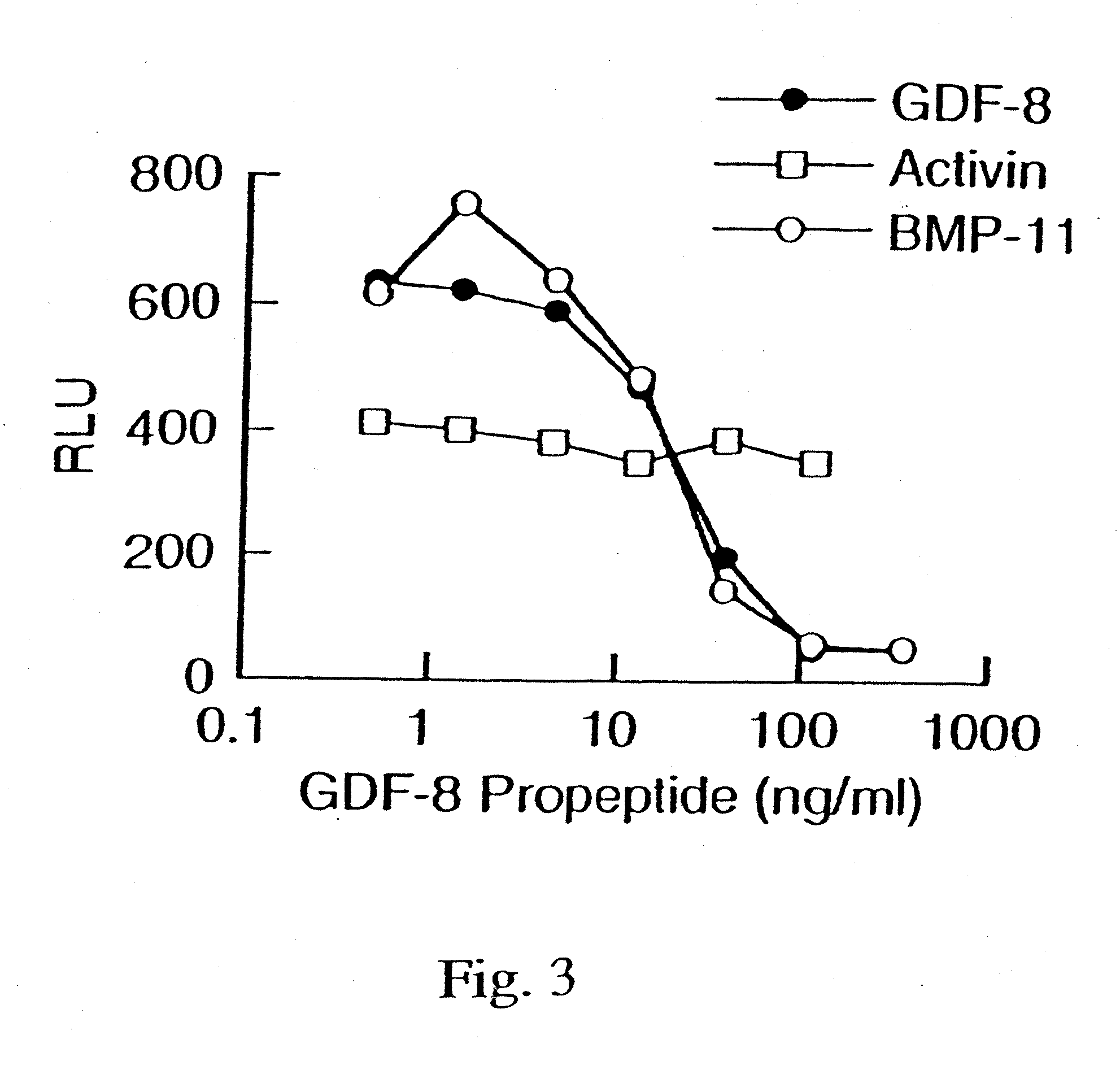
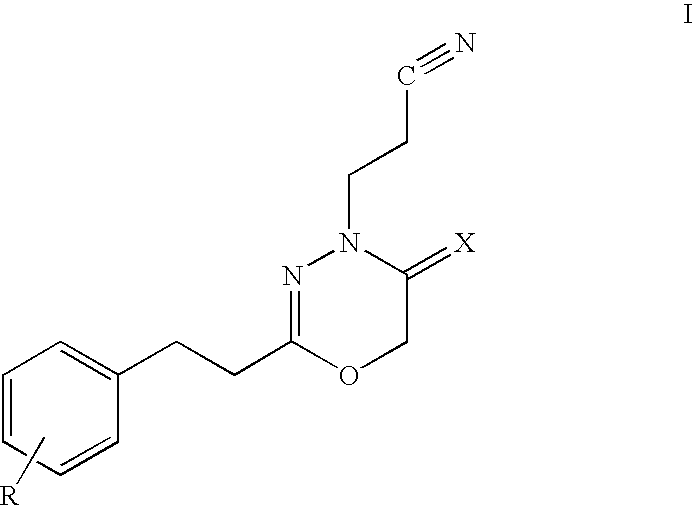
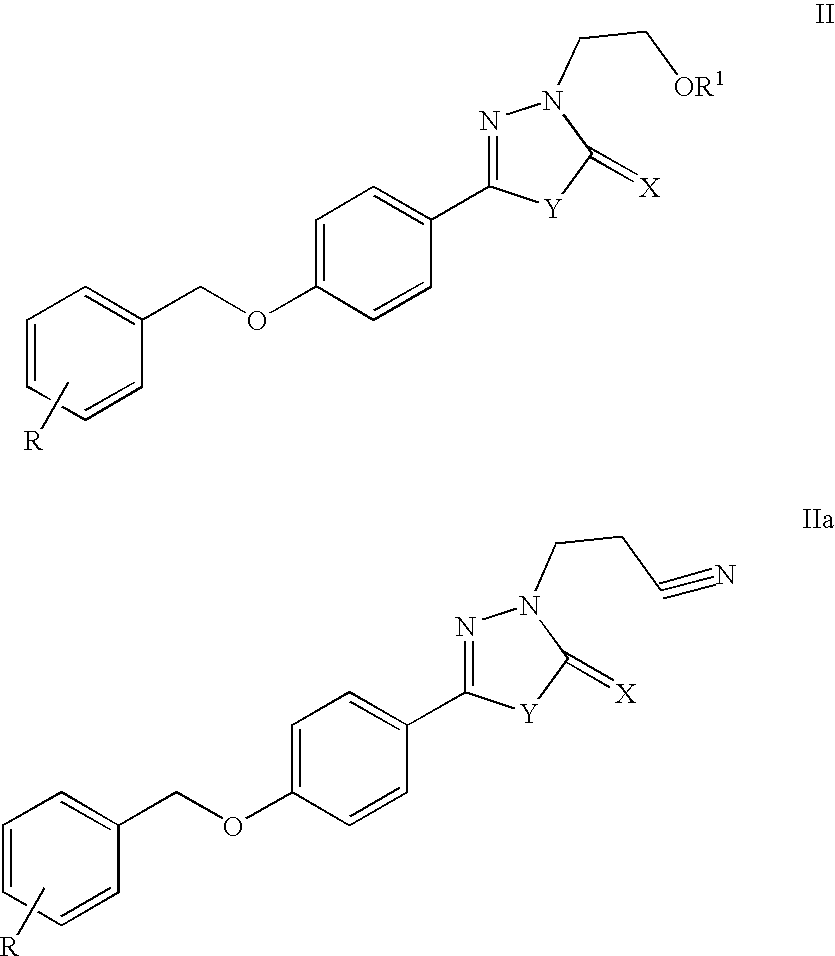
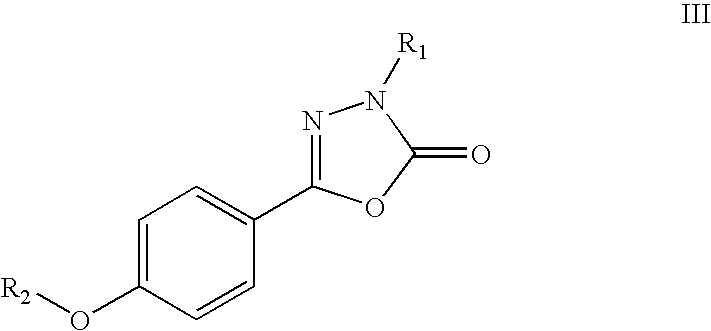
Modified and stabilized gdf propeptides and uses thereof
InactiveUS20070149455A1Avoid utilizationImproved pharmacokinetic propertiesFungiBacteriaMuscle tissueAmytrophic lateral sclerosis
Modified and stabilized propeptides of Growth Differentiation Factor proteins, such as GDF-8 and Bone Morphogenetic Protein-11, are disclosed. Also disclosed are methods for making and using the modified propeptides to prevent or treat human or animal disorders in which an increase in muscle tissue would be therapeutically beneficial. Such disorders include muscle or neuromuscular disorders (such as amyotrophic lateral sclerosis, muscular dystrophy, muscle atrophy, congestive obstructive pulmonary disease, muscle wasting syndrome, sarcopenia, or cachexia), metabolic diseases or disorders (such as such as type 2 diabetes, noninsulin-dependent diabetes mellitus, hyperglycemia, or obesity), adipose tissue disorders (such as obesity), and bone degenerative diseases (such as osteoporosis).
Owner:WYETH LLC
Mao-b inhibitors useful for treating obesity
InactiveUS20070078172A1Reduce the amount requiredEfficient reductionBiocideAnimal repellantsDyslipidemiaBlood lipids
The invention provides a method of treating obesity, diabetes, and / or cardiometabolic disorders (e.g., hypertension, dyslipidemias, high blood pressure, and insulin resistance) in a mammal by administering to the mammal a therapeutically effective amount of an irreversible MAO-B inhibitor.
Owner:JENRIN DISCOVERY
Method of treatment of diabetes and/or obesity with reduced nausea side effect
InactiveUS20070207958A1Prevent and reduce and eliminate nausea side effectReduces and eliminates side effectPeptide/protein ingredientsMetabolism disorderSide effectInsulinotropin
The present invention provides methods of administering an insulinotropic peptide in an amount effective to treat a disorder or condition while reducing nausea side effect by administering to a subject in need thereof an insulinotropic peptide conjugated to albumin. The present invention also provides methods of selecting a subject for administration of a conjugated insulinotropic peptide. Exemplary disorders or conditions treatable with an insulinotropic peptide include obesity and type II diabetes.
Owner:CONJUCHEM
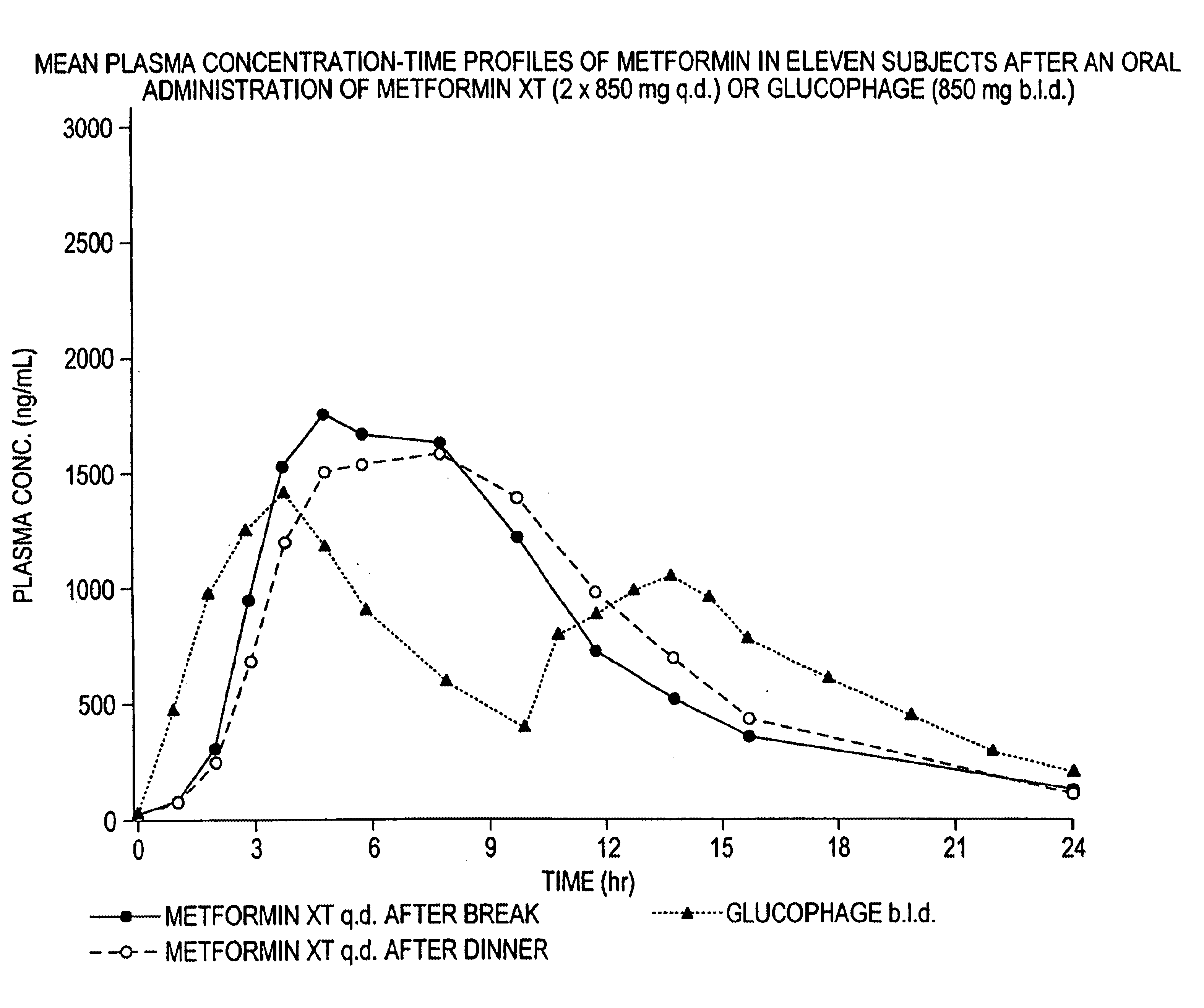
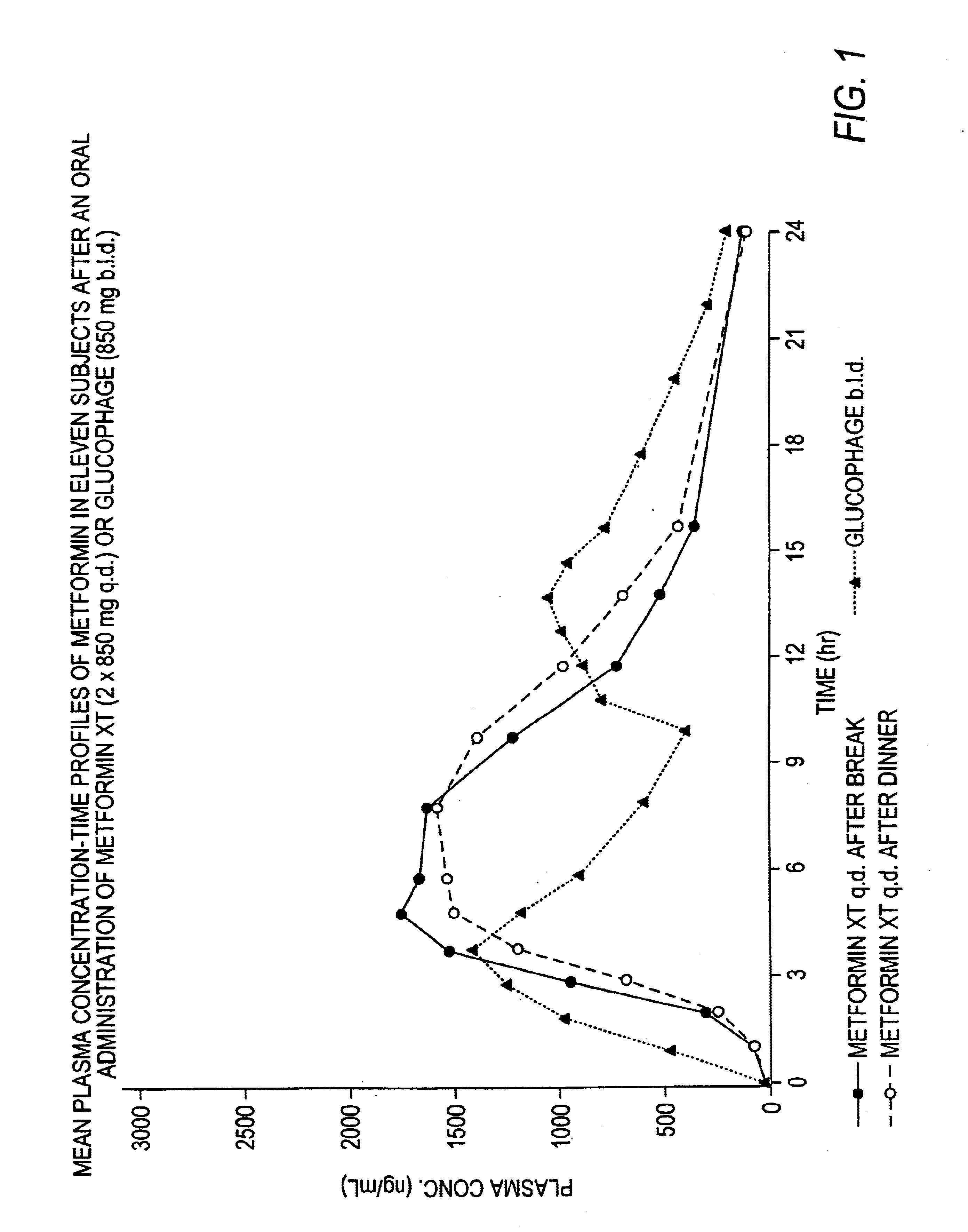
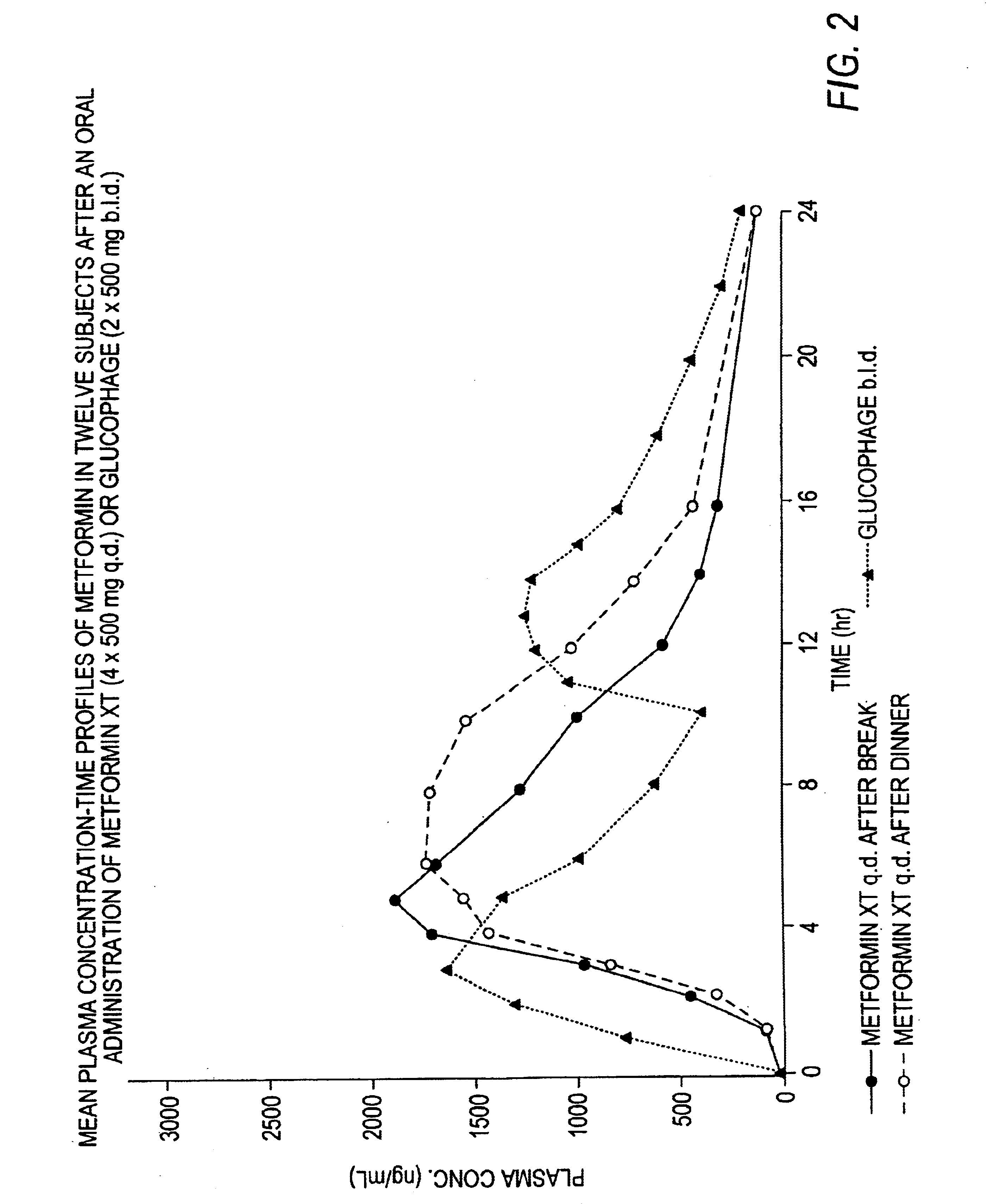
Controlled release metformin compositions
InactiveUS6866866B1Effective controlImprove bioavailabilityOrganic active ingredientsCoatingsCo administrationBlood plasma
A composition for treating patients having non-insulin-dependent diabetes mellitus (NIDDM) by administering a controlled release oral solid dosage form containing preferably a biguanide drug such as metformin, on a once-a-day basis. The dosage form provides a mean time to maximum plasma concentration (Tmax) of the drug which occurs at 5.5 to 7.5 hours after oral administration on a once-a-day basis to human patients. Preferably, the dose of drug is administered at dinnertime to a patient in the fed state.
Owner:ANDRX LABS
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com