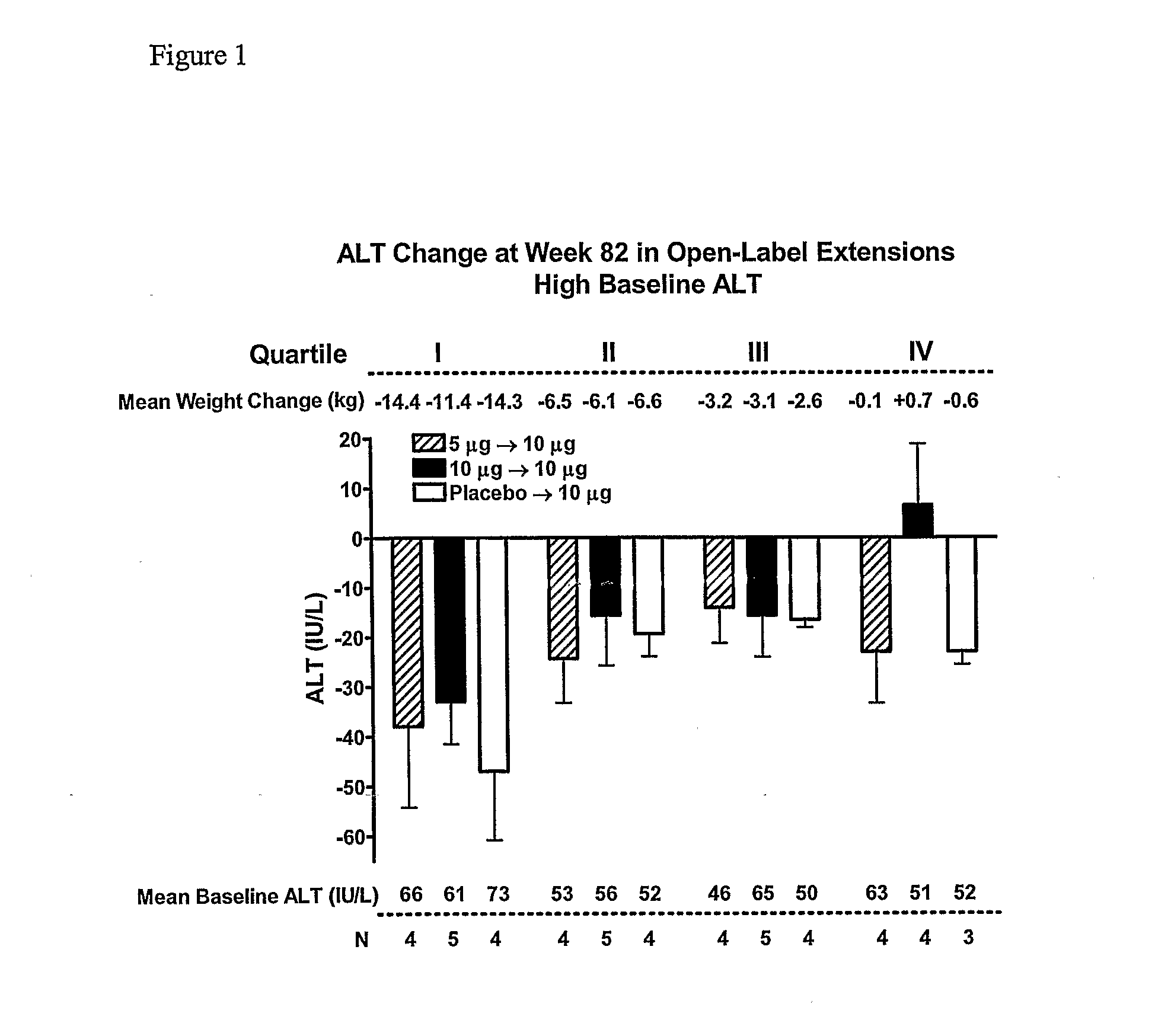
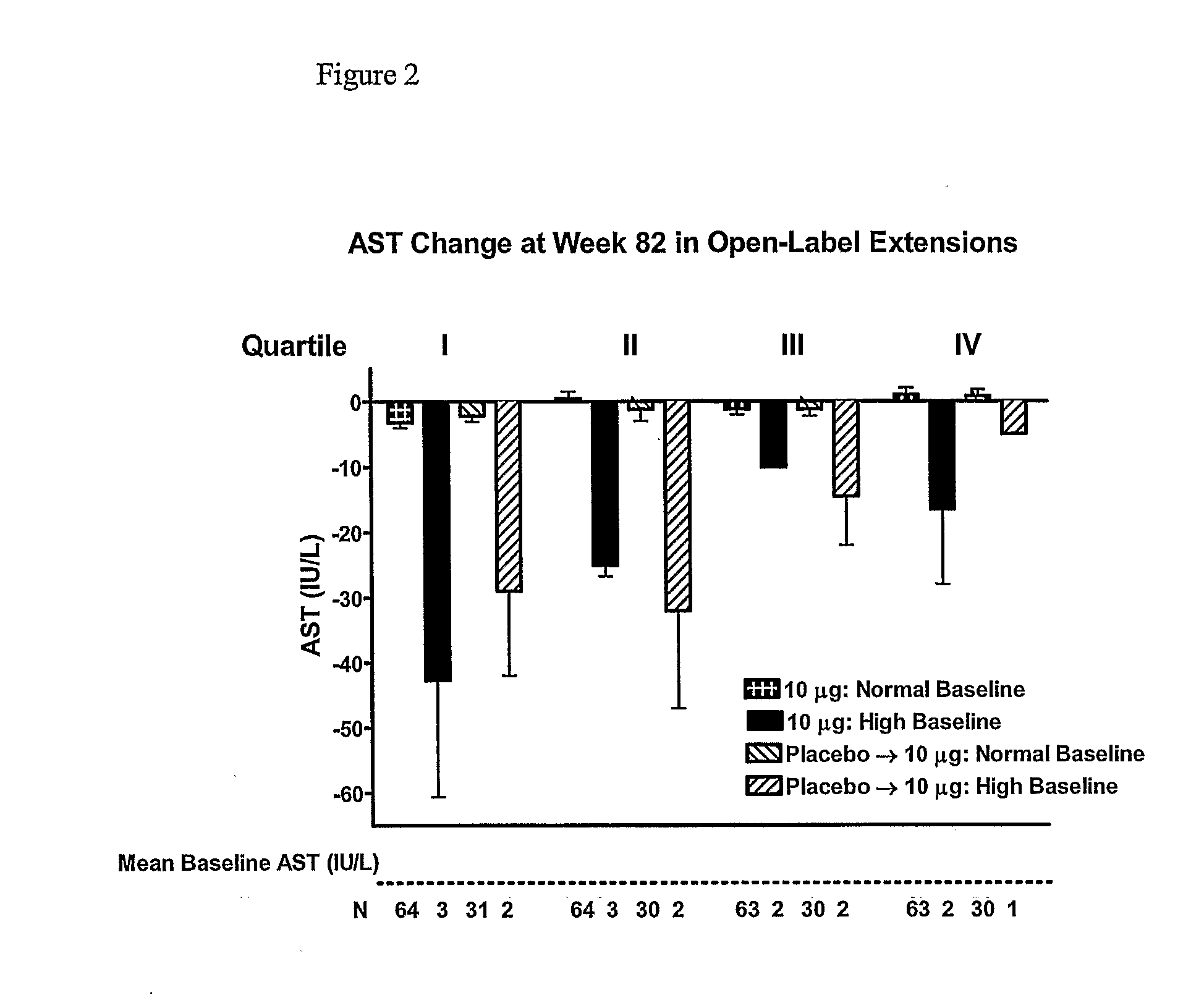
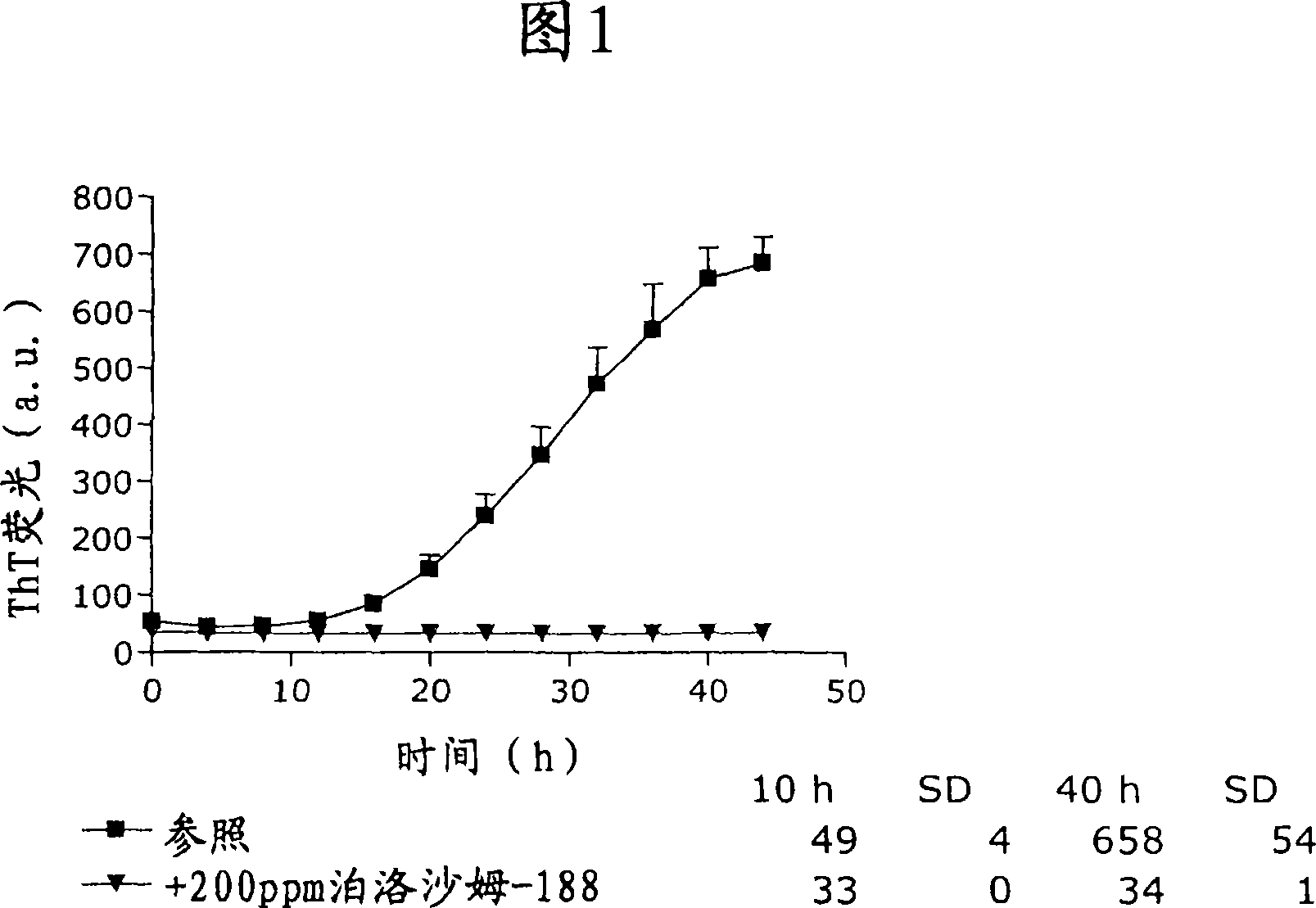
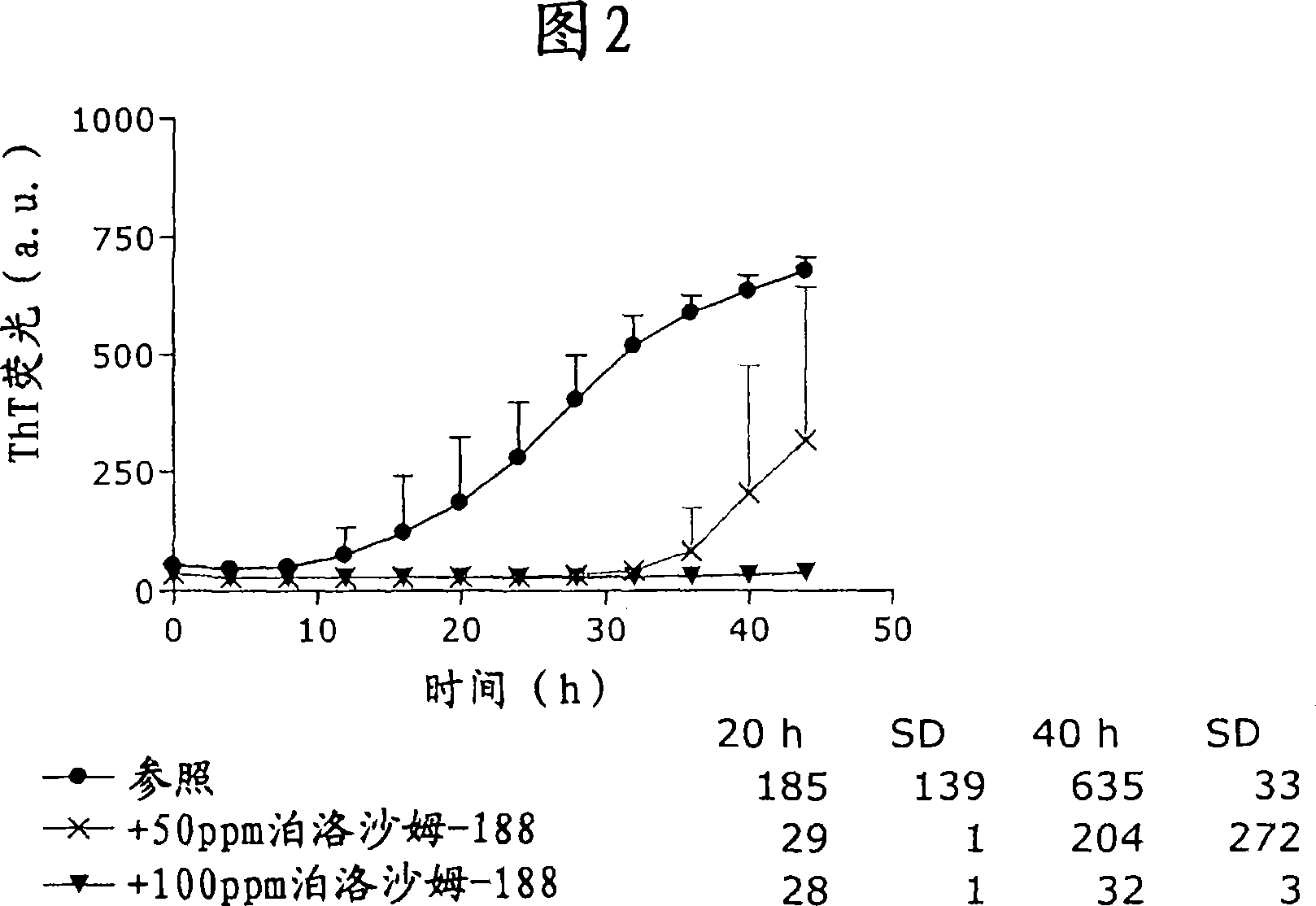
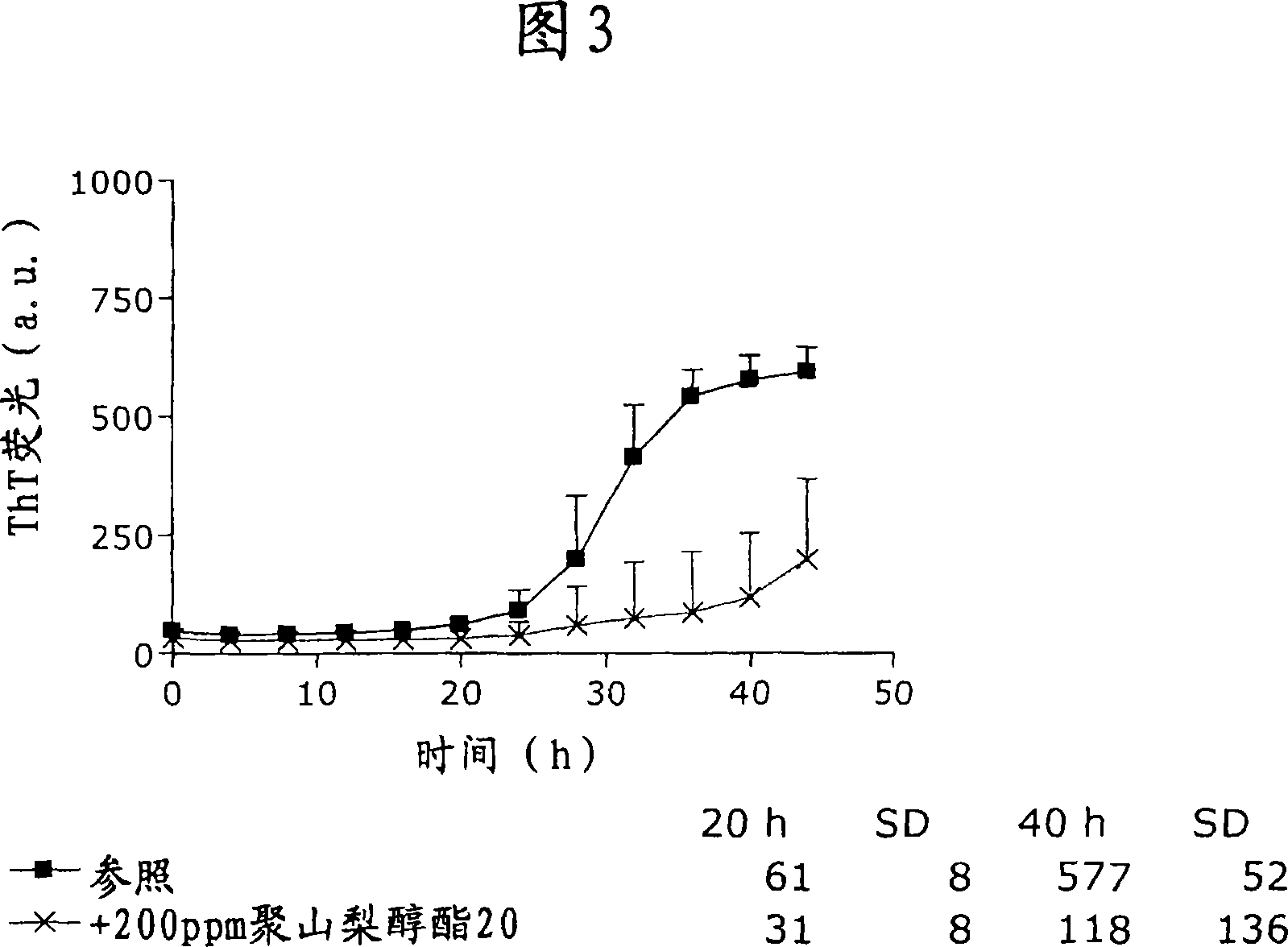
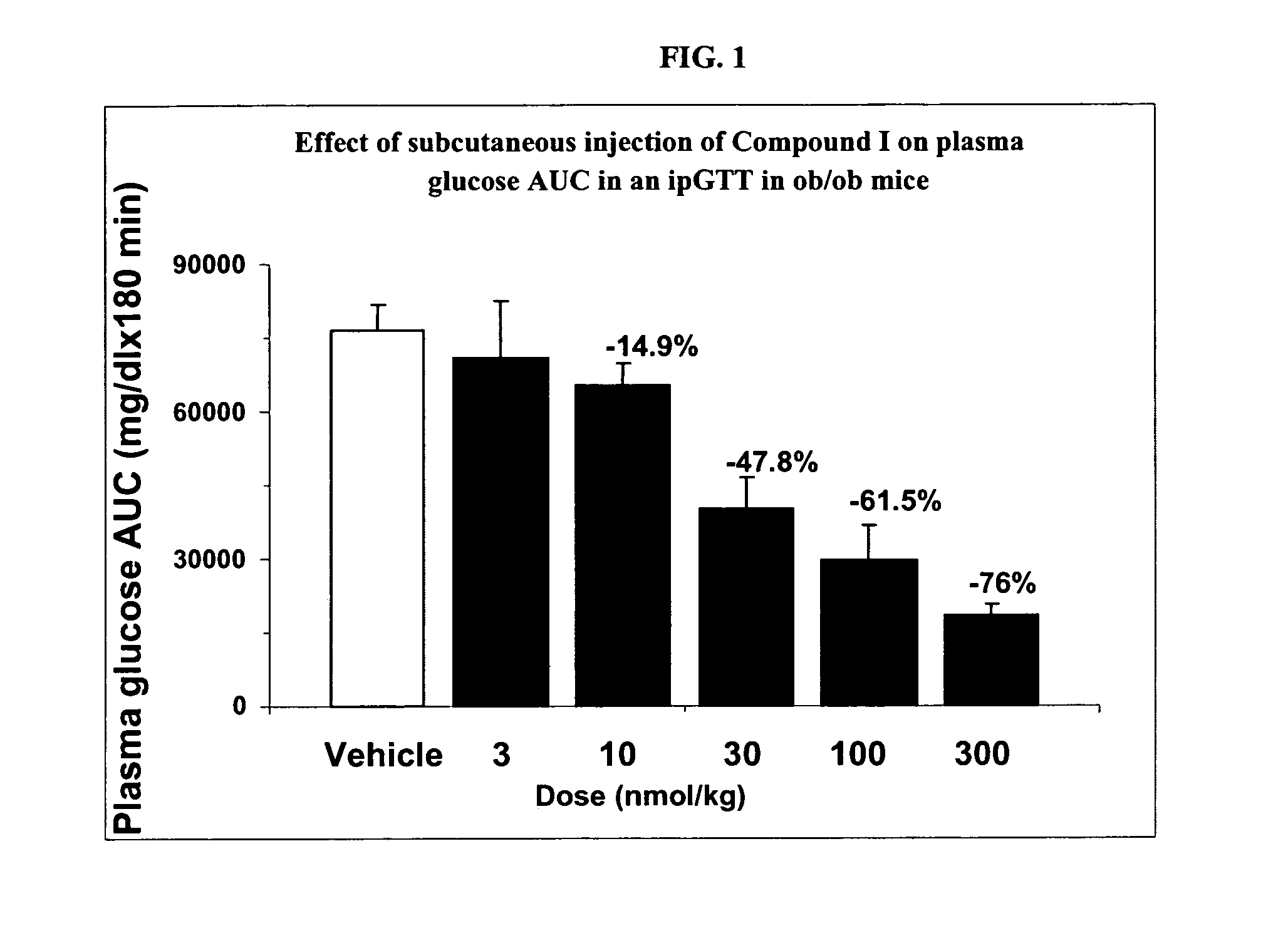
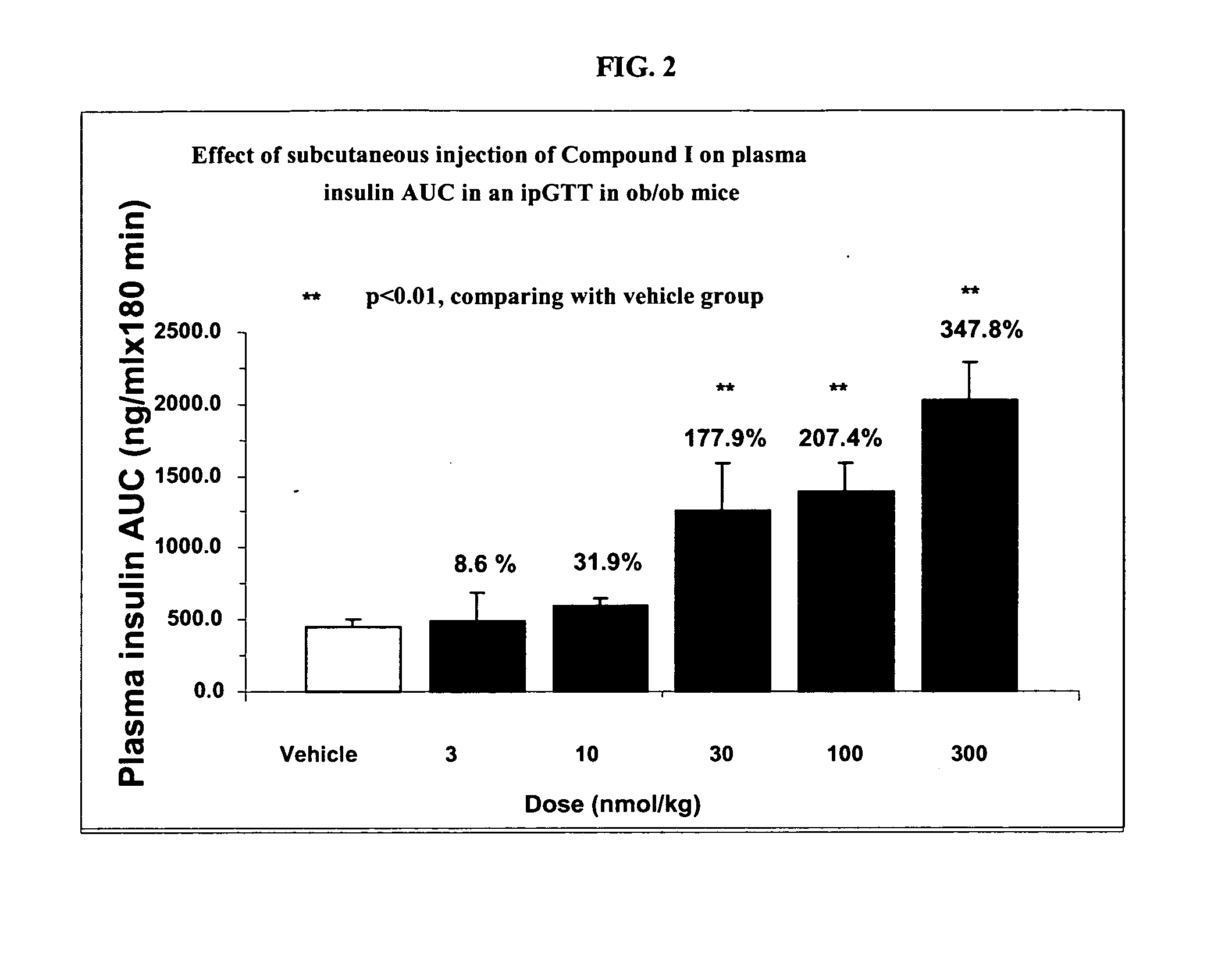
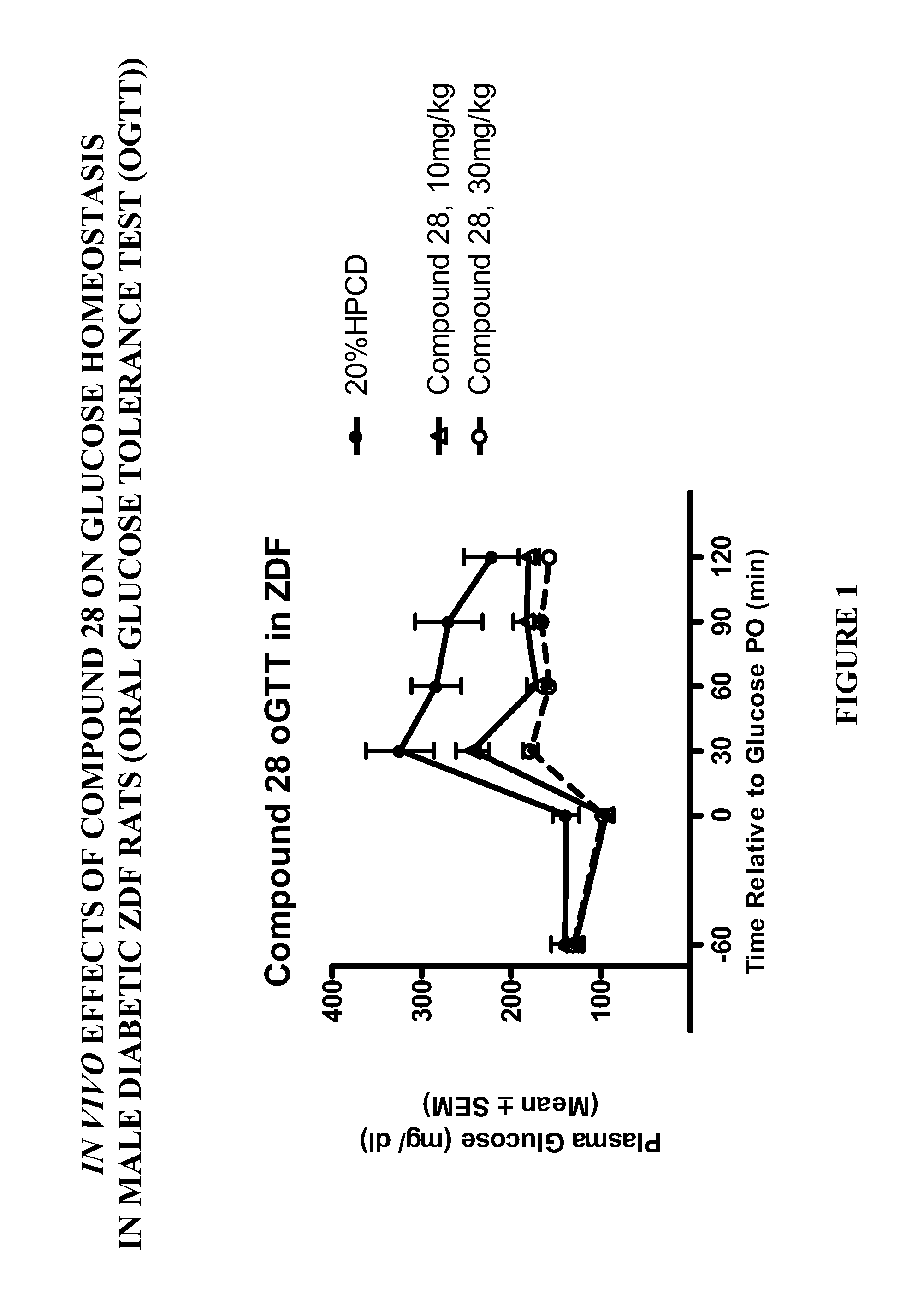
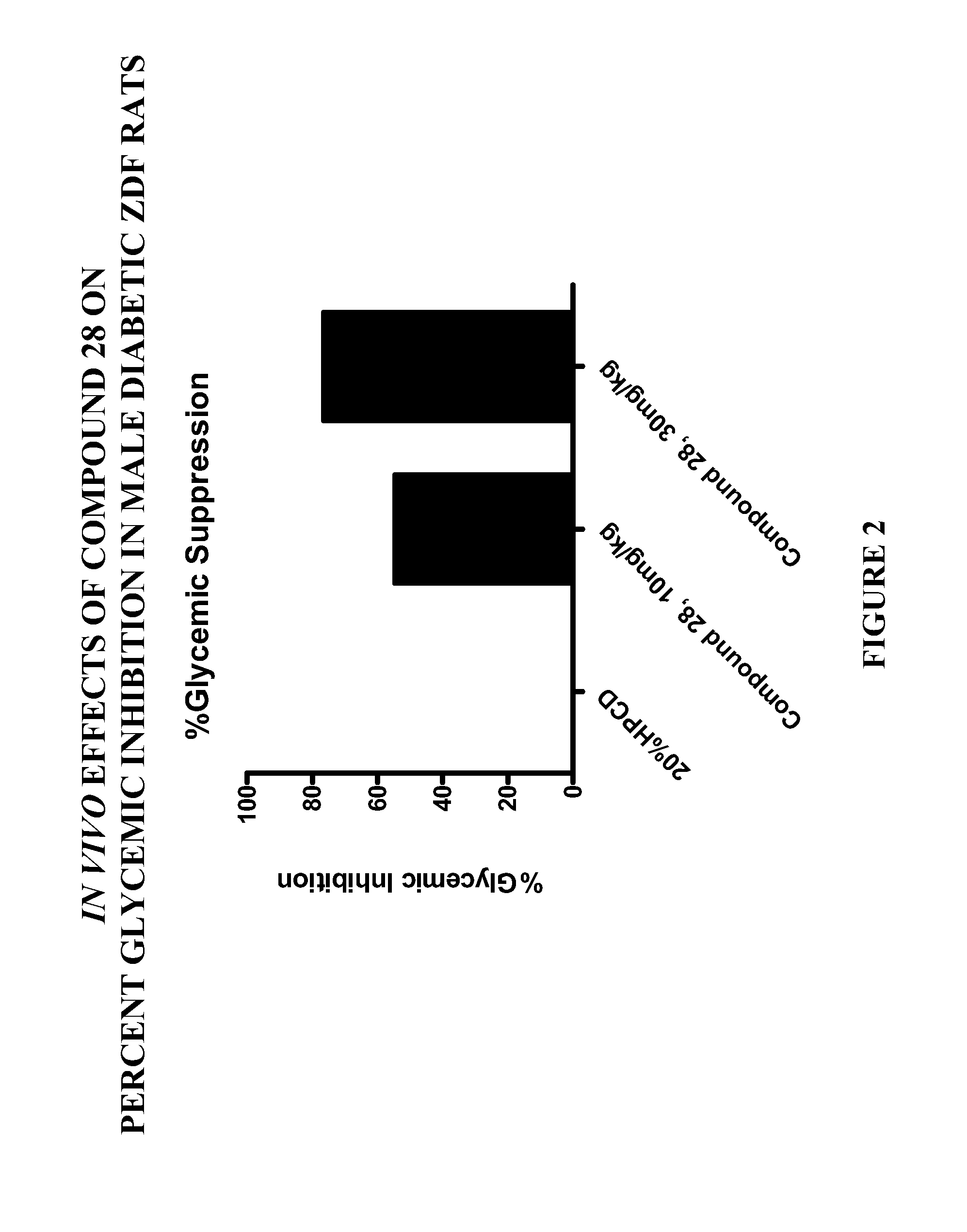
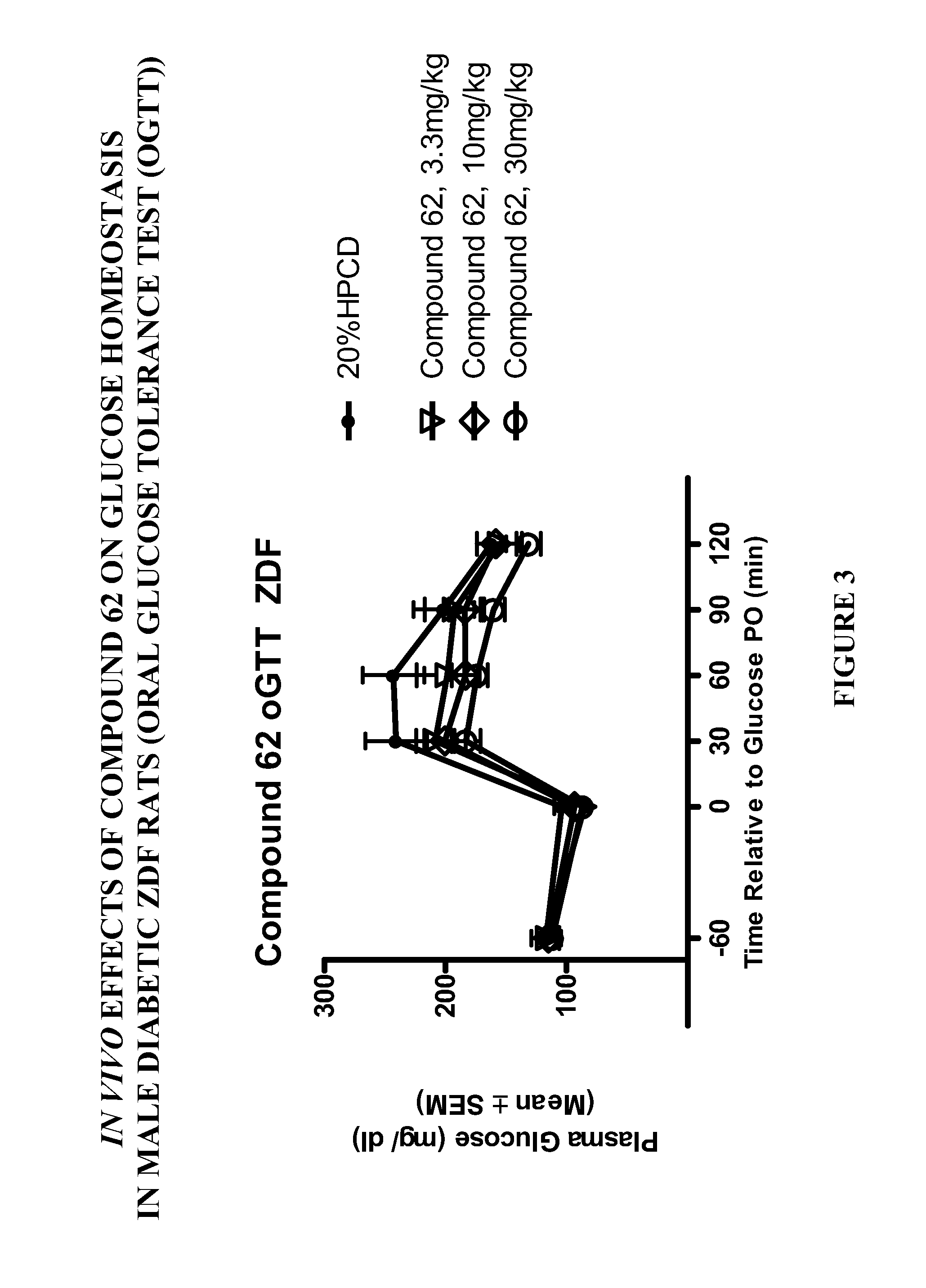
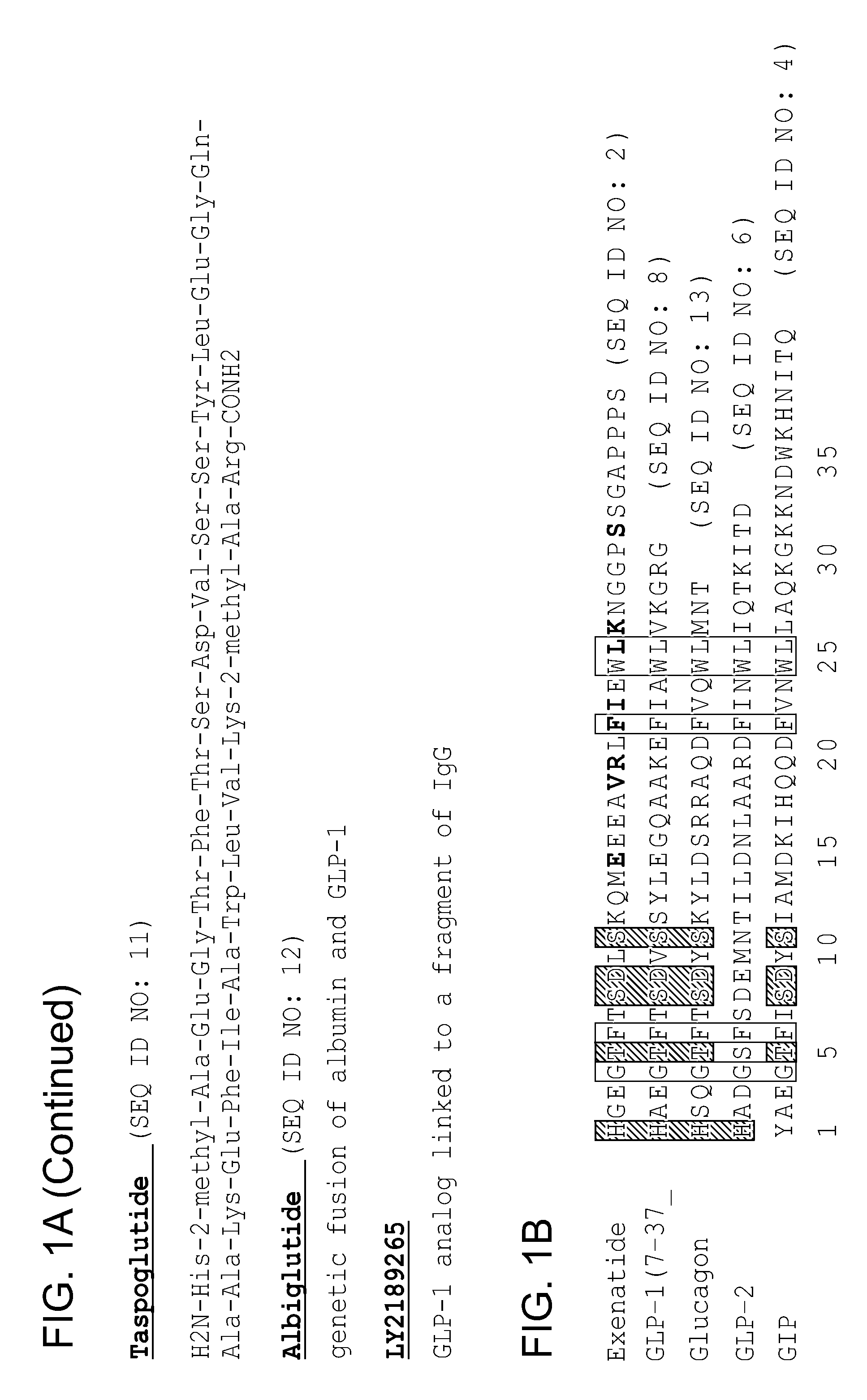
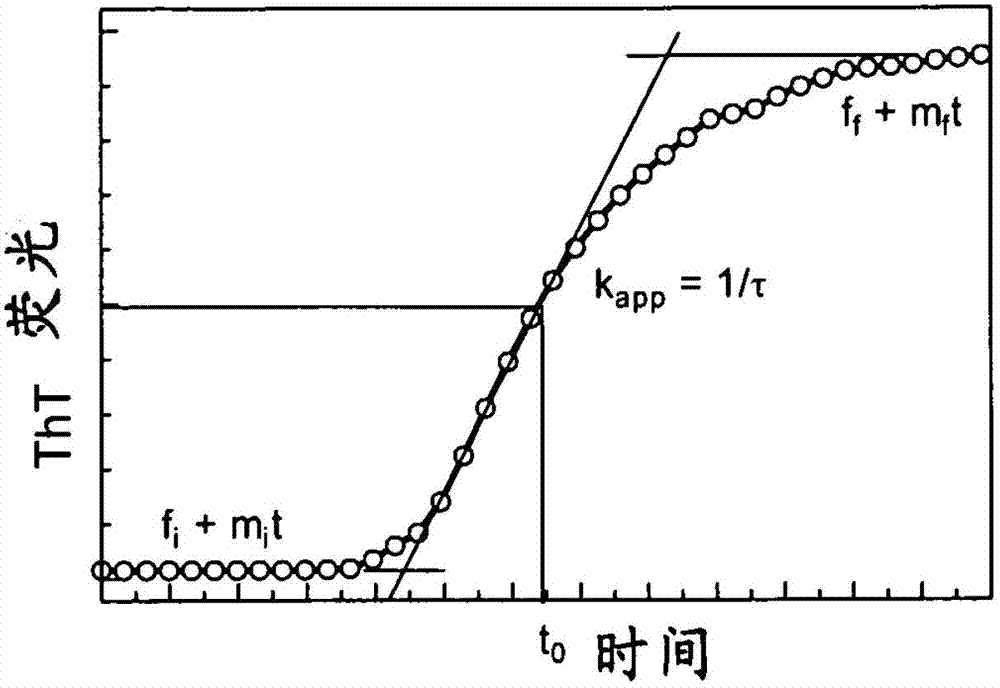
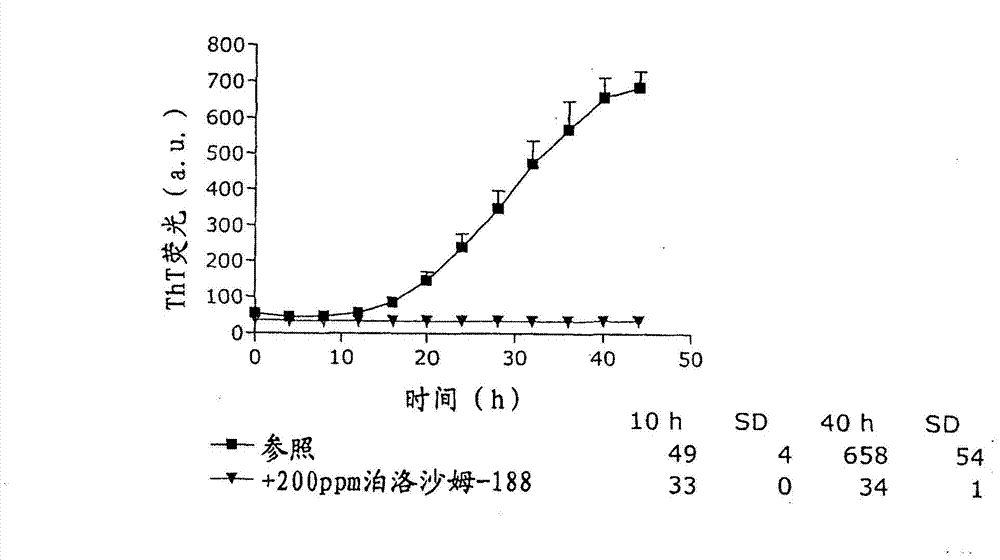
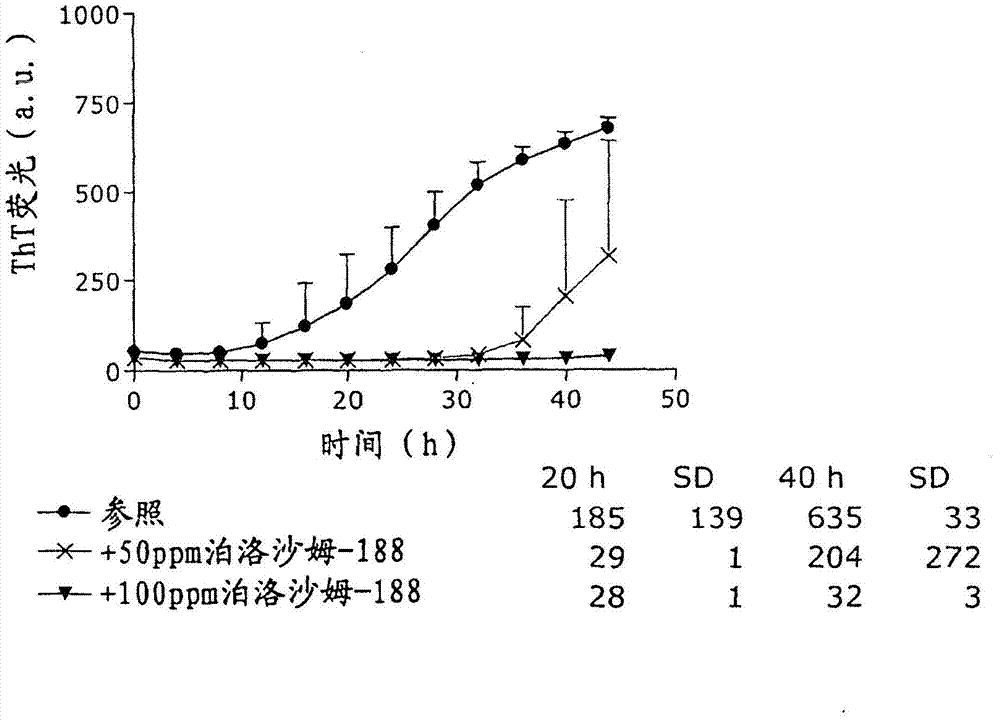
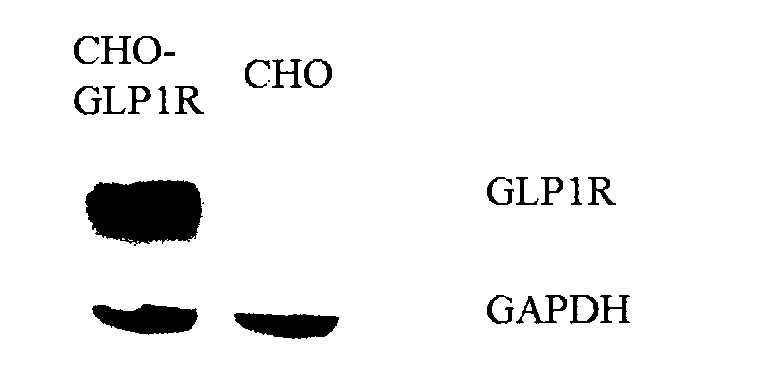
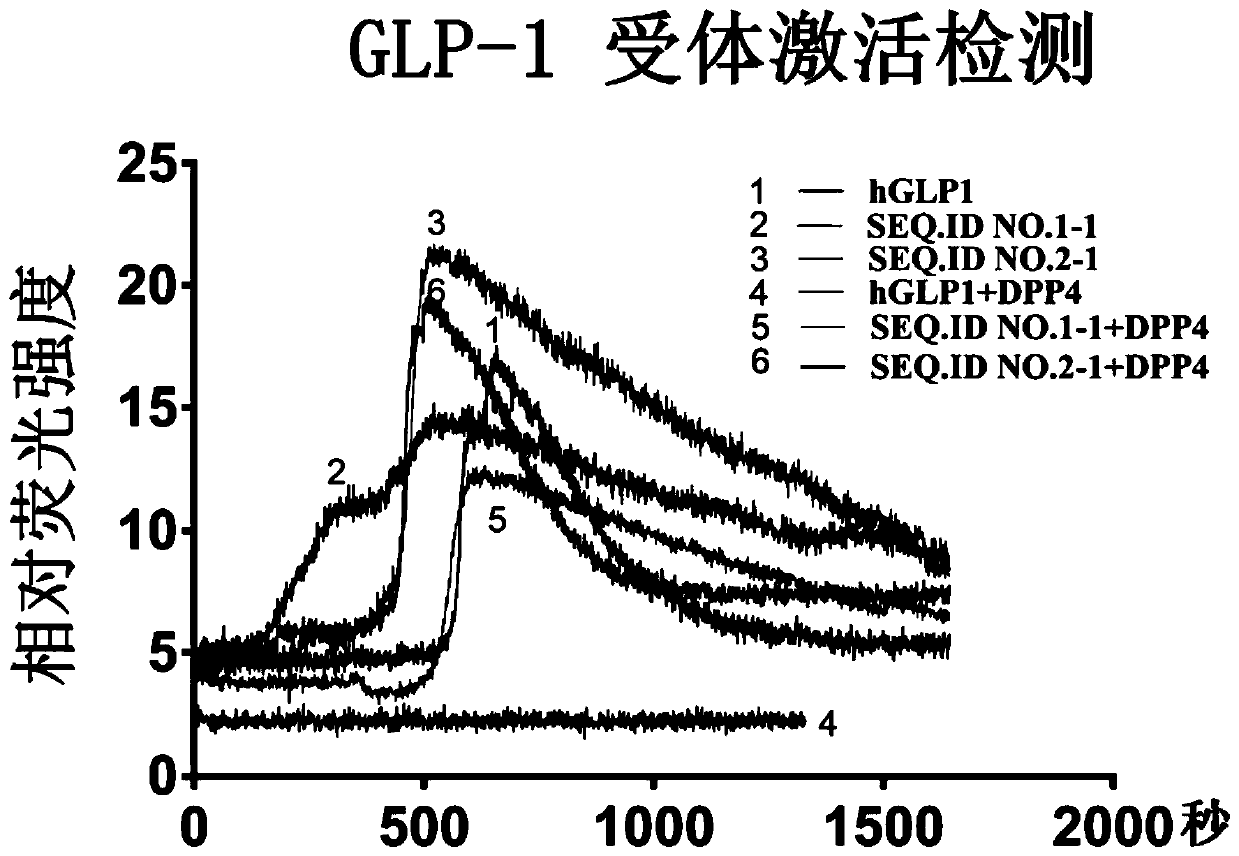
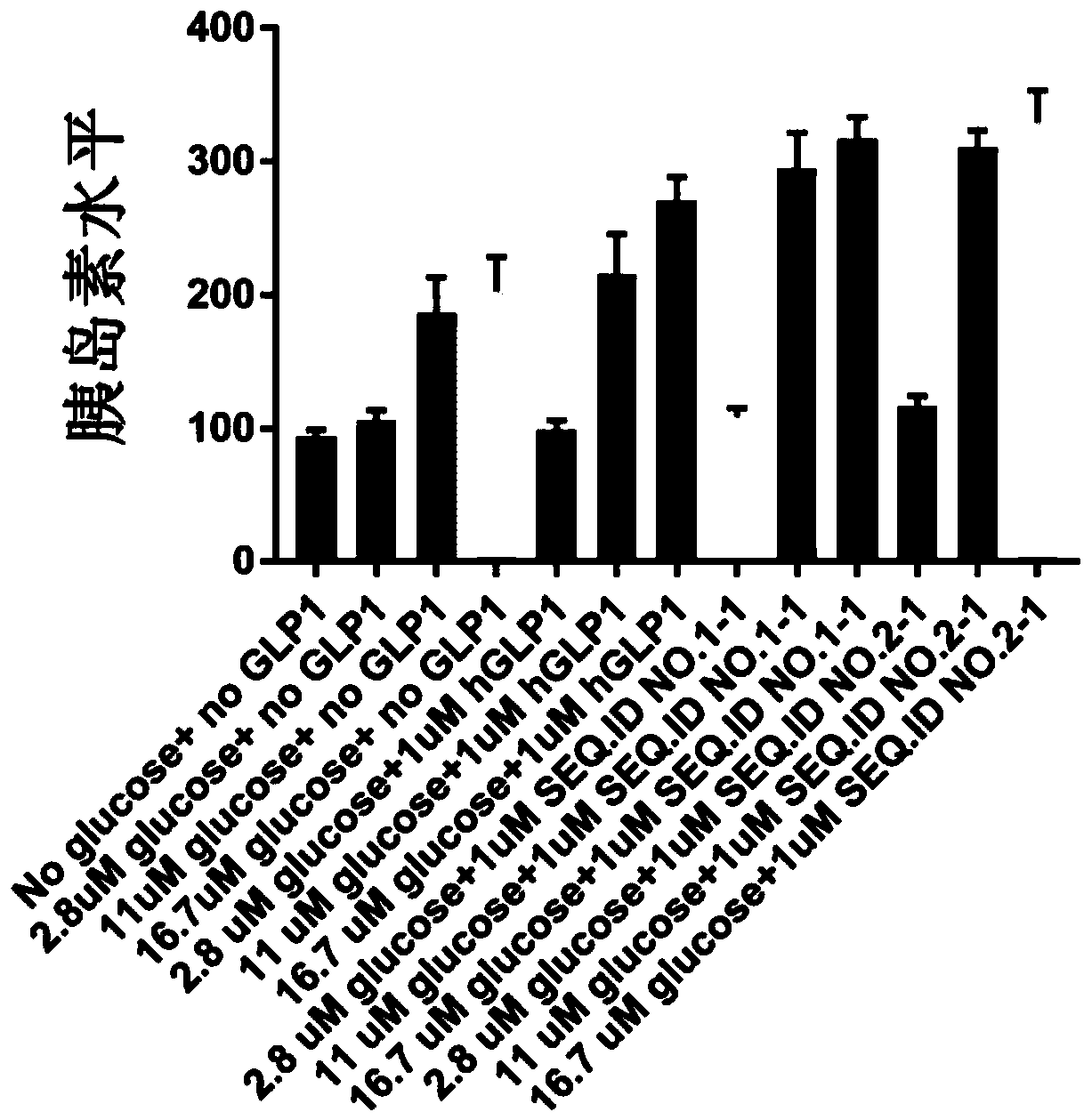
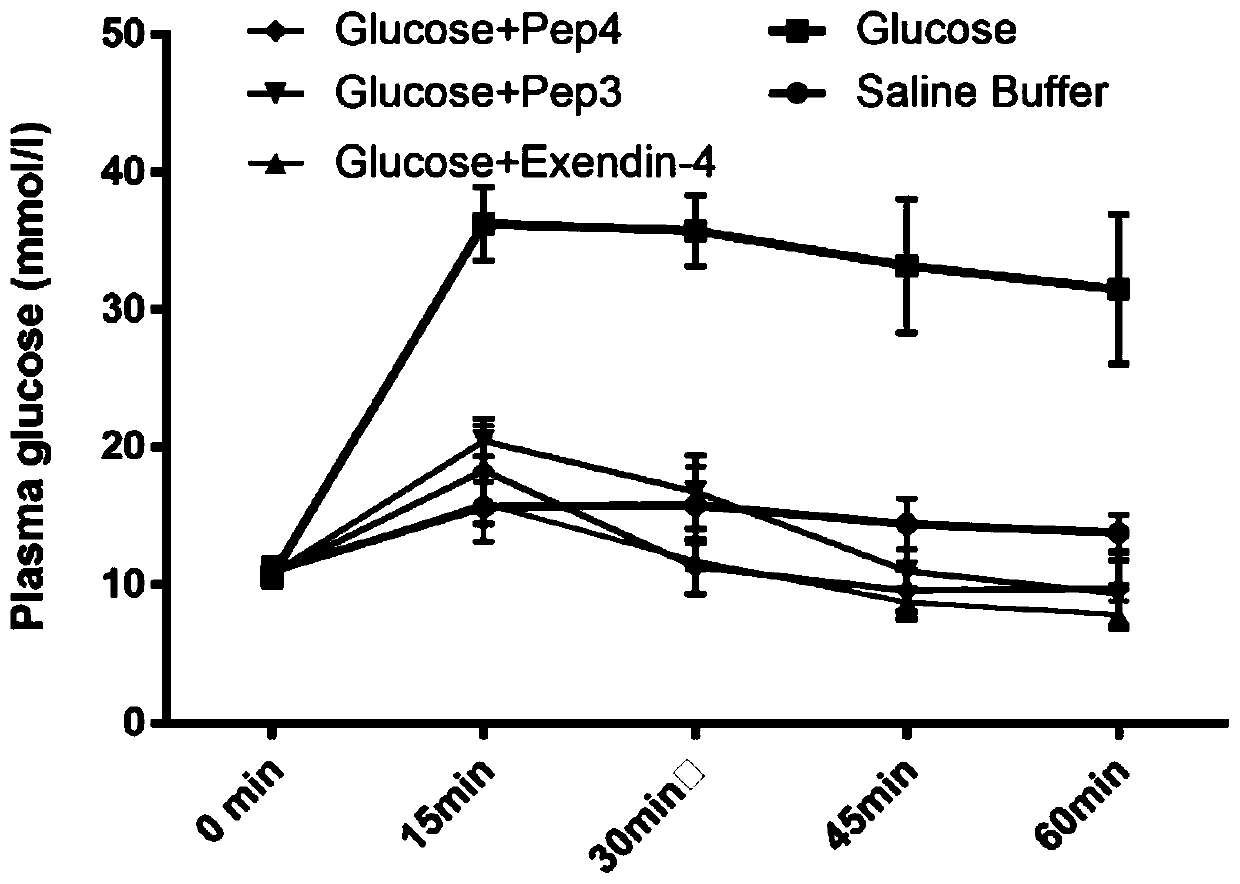
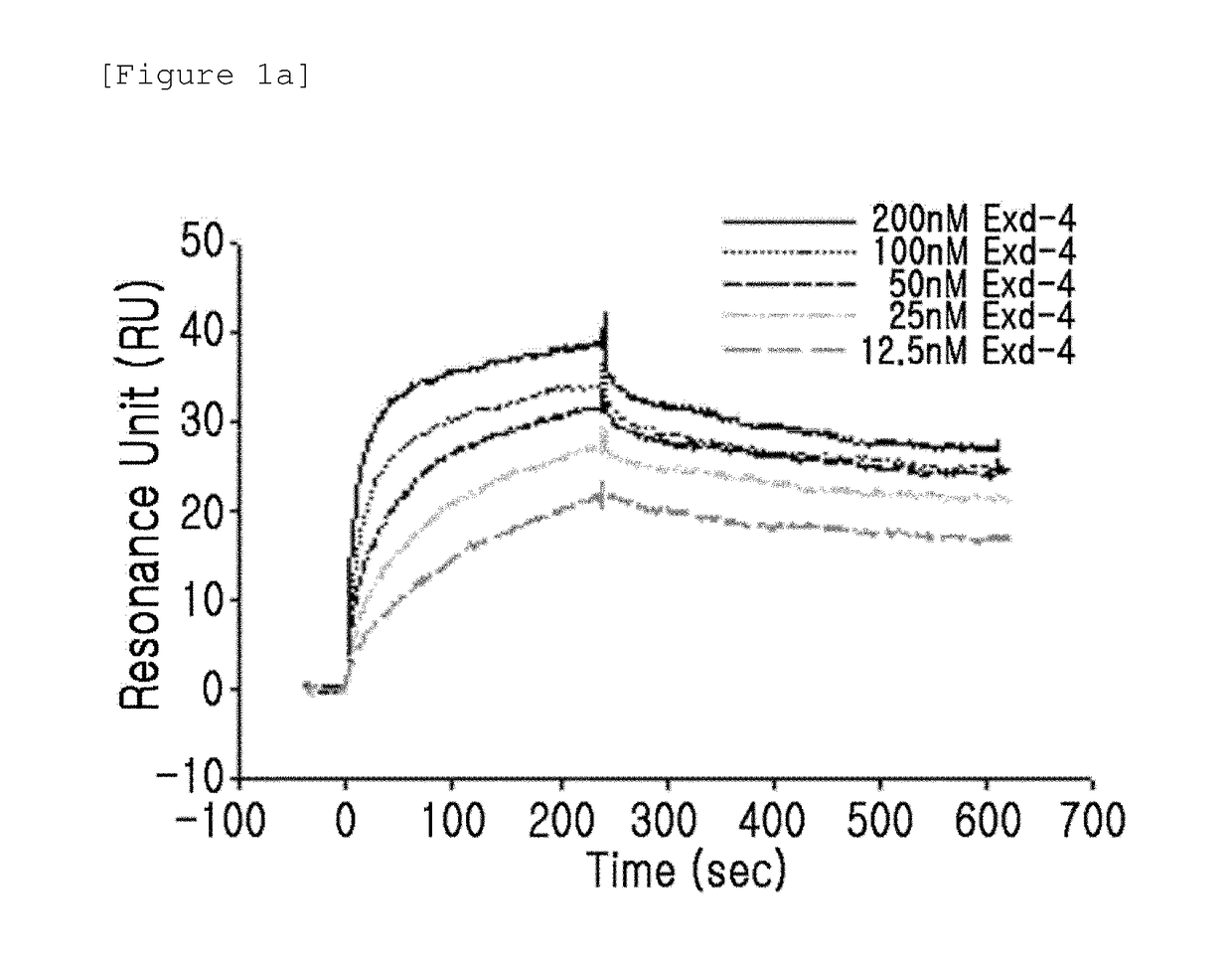
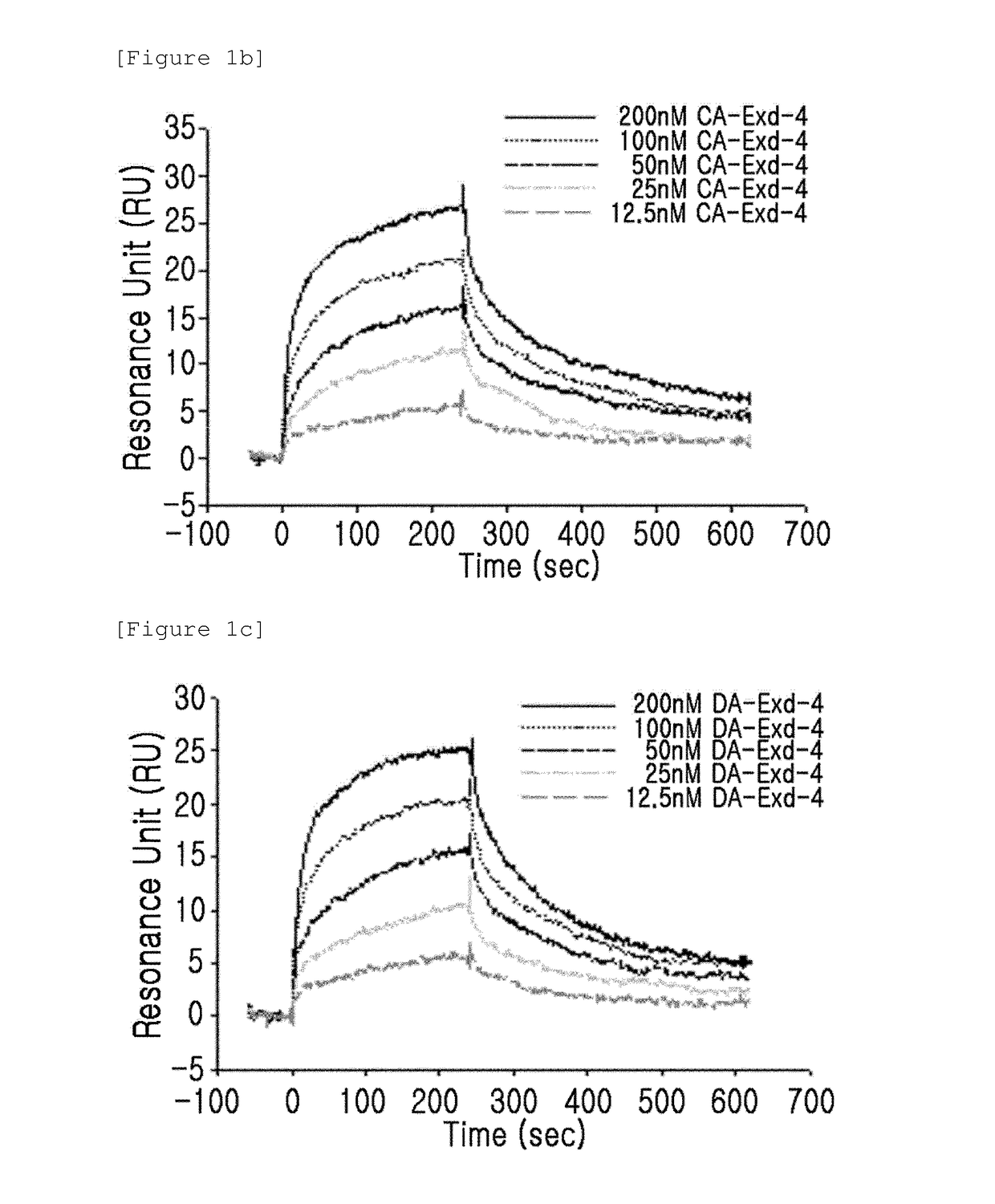
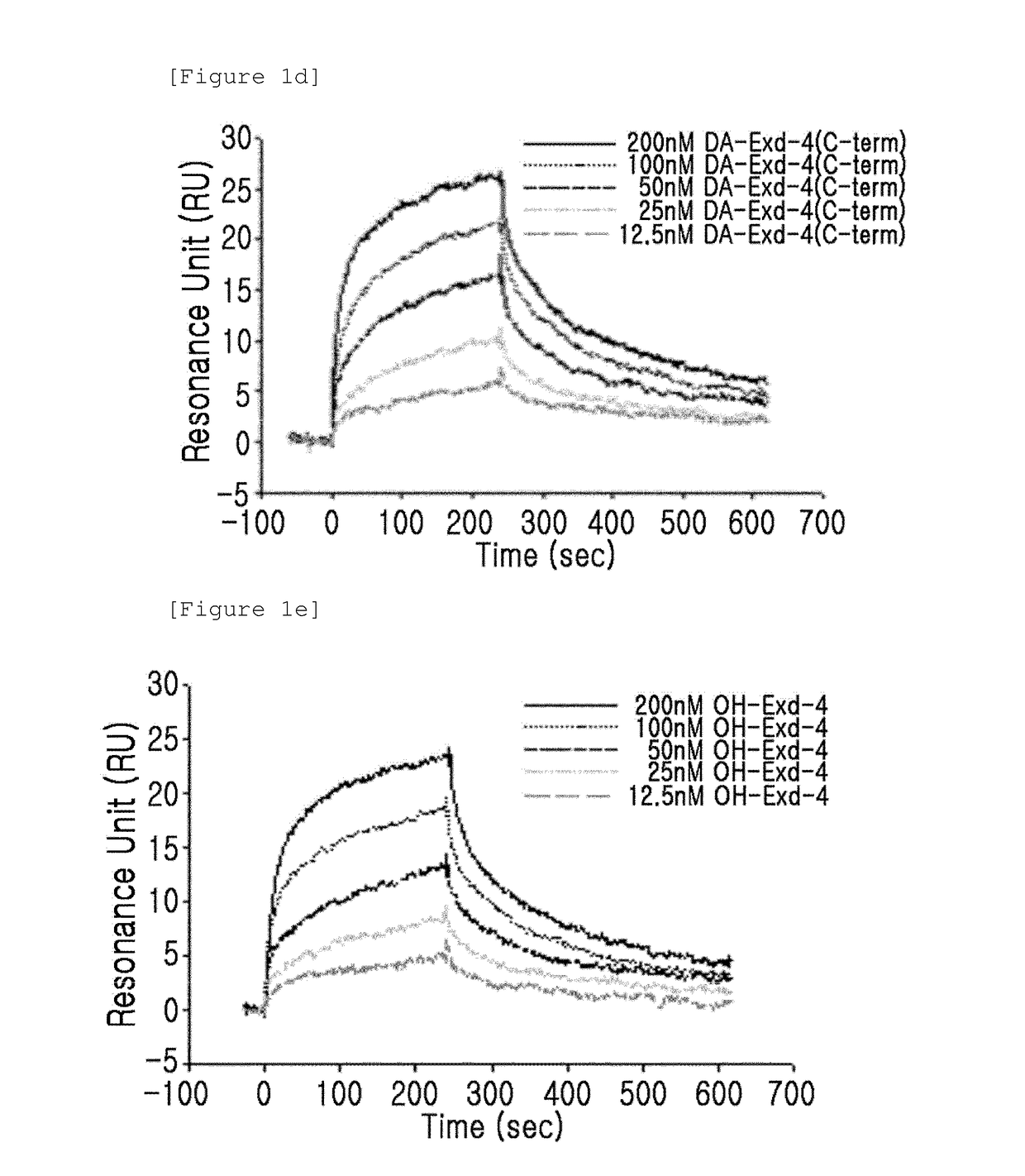
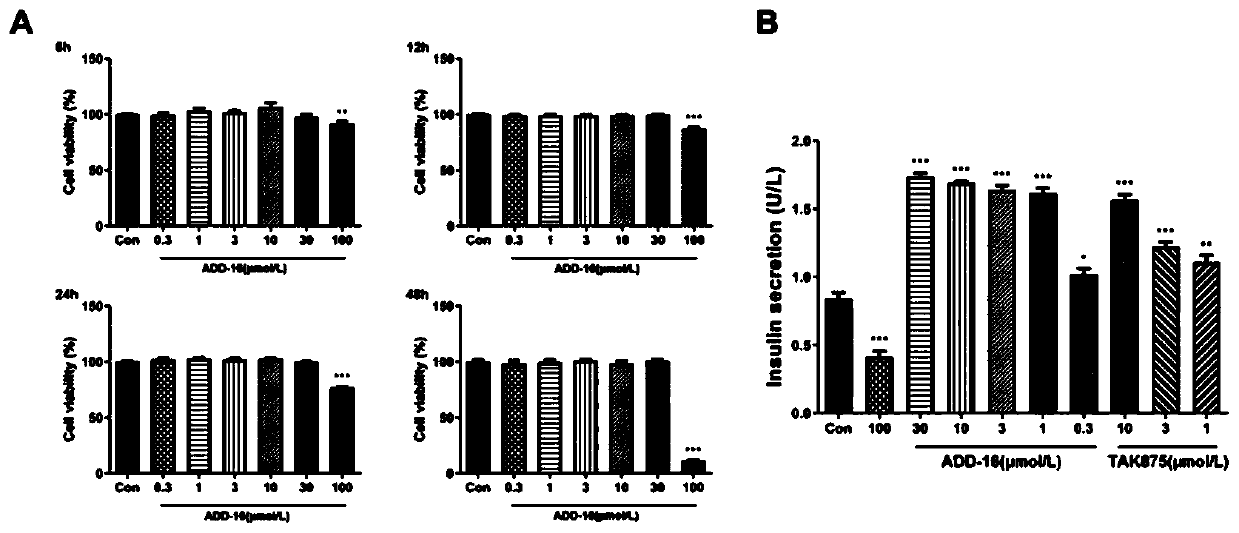
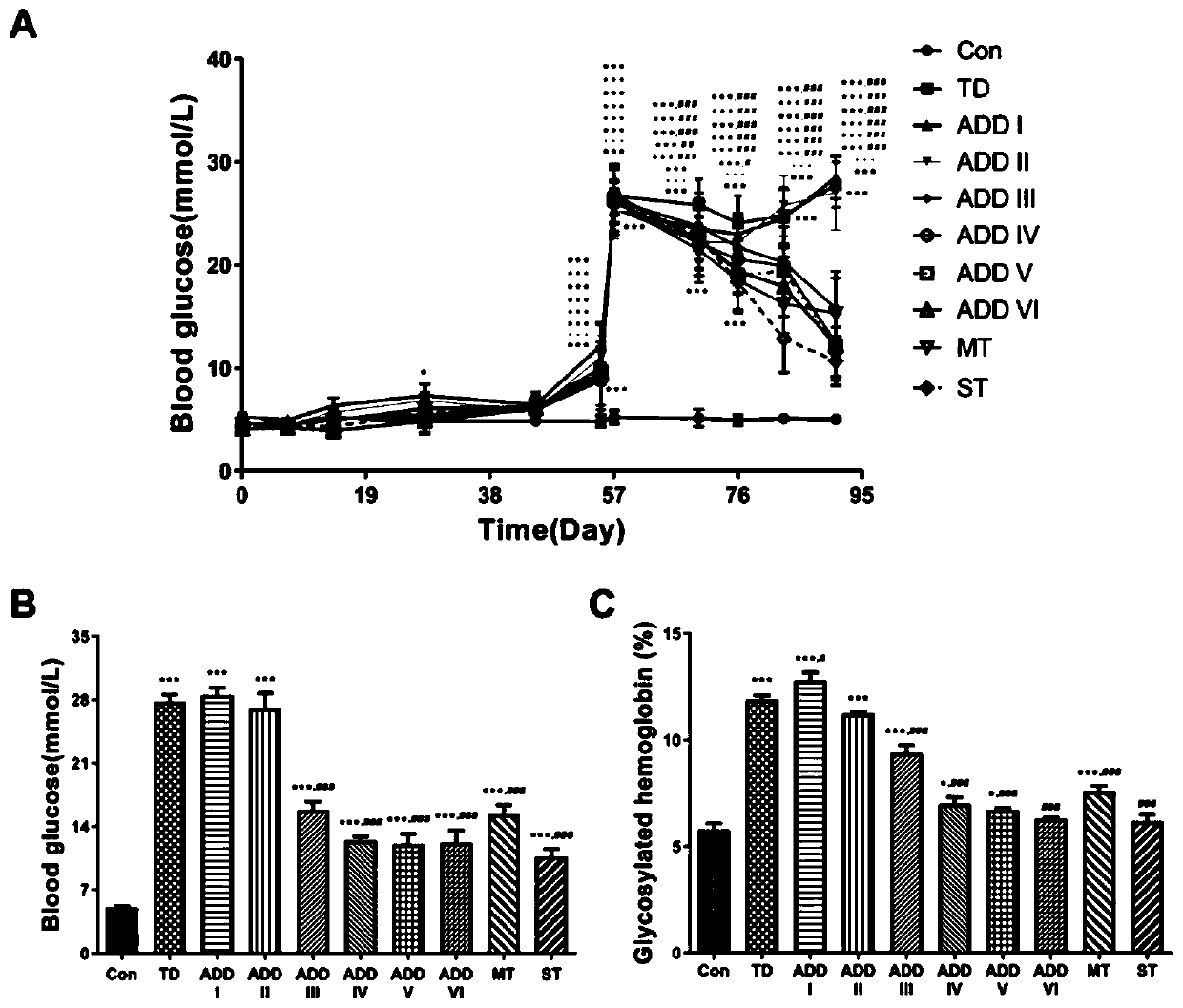
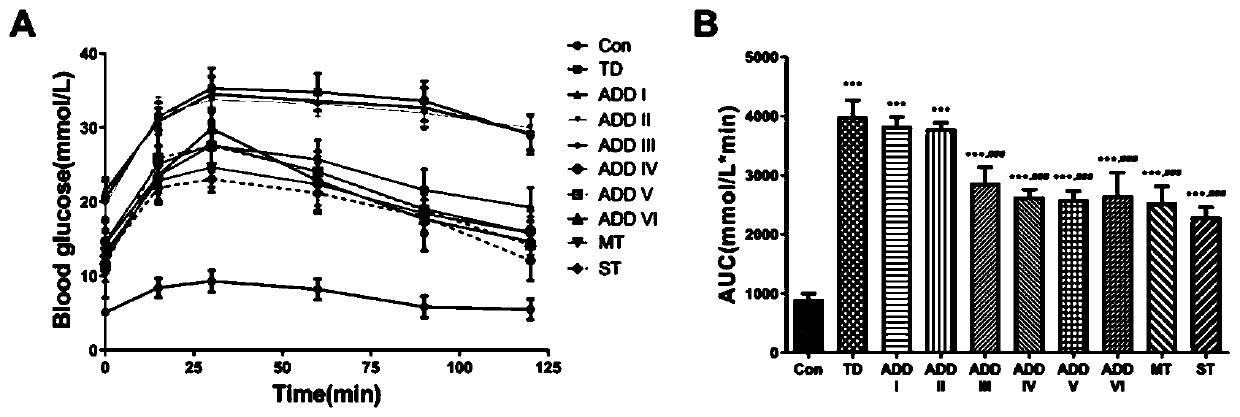
Patents
Literature
63 results about "Insulinotropin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Insulinotropic hormone derivatives and uses thereof
InactiveUS7138486B2Promote insulin secretionIncrease insulin levelsBiocidePeptide/protein ingredientsInsulinotropinPancreatic hormone
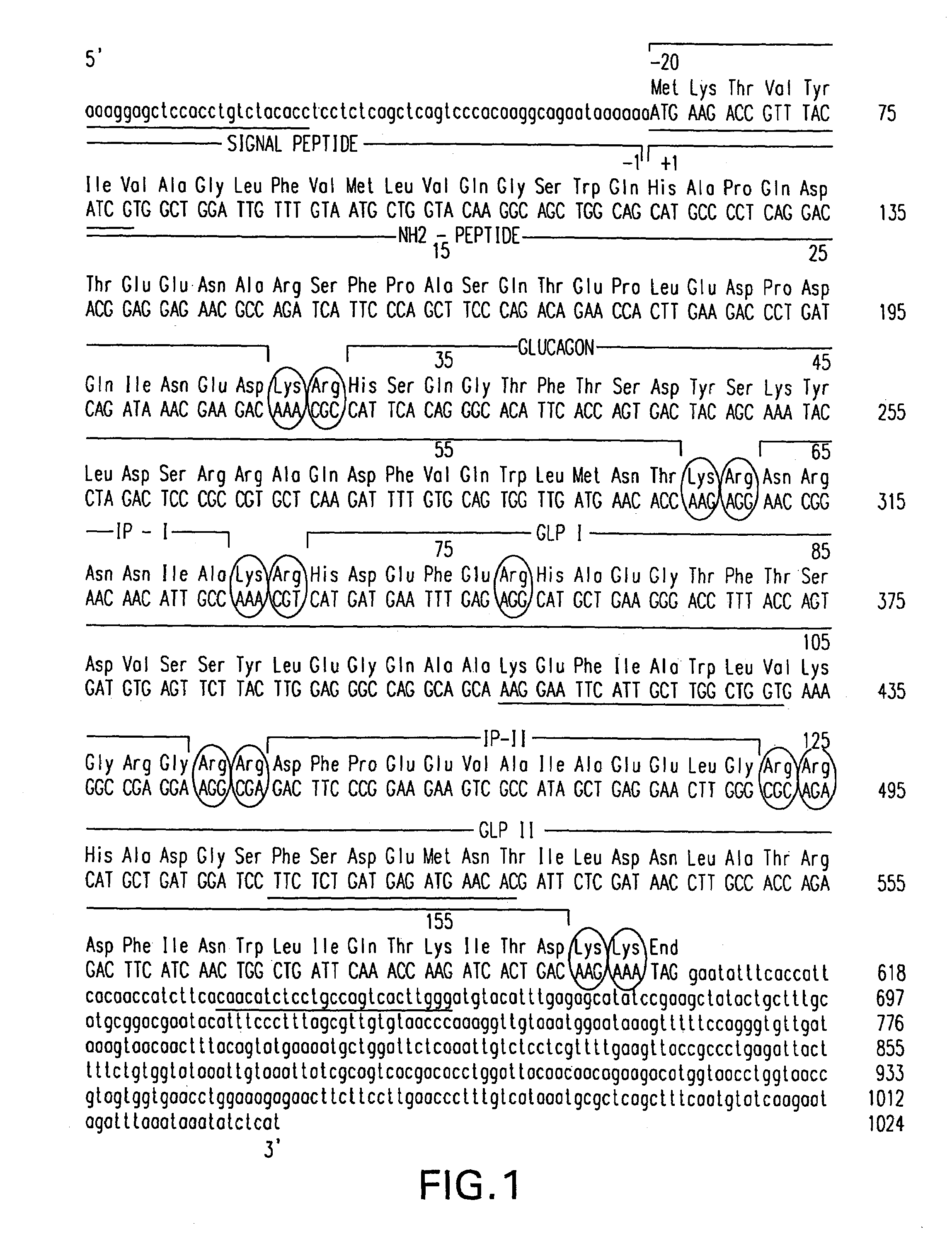
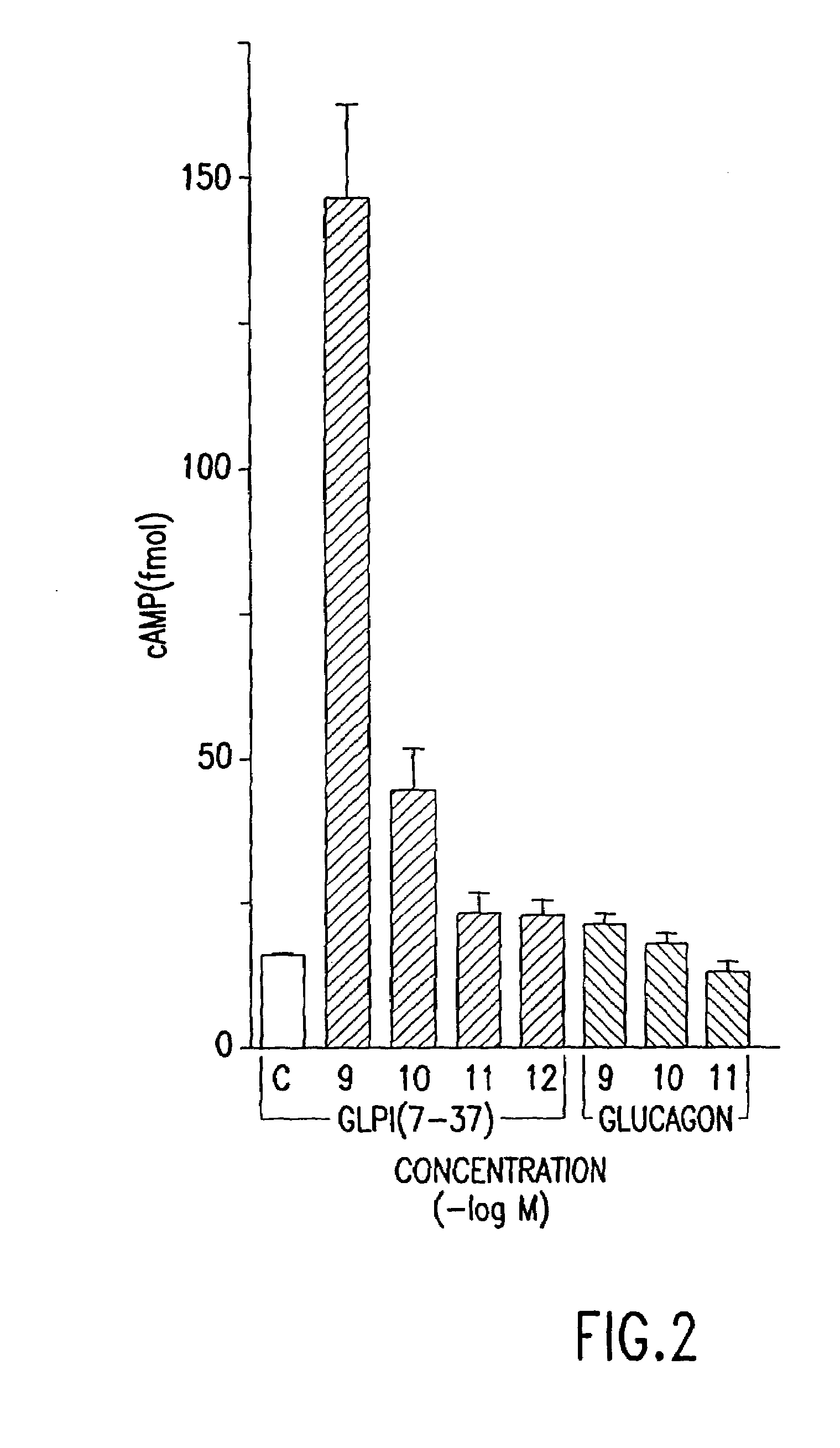
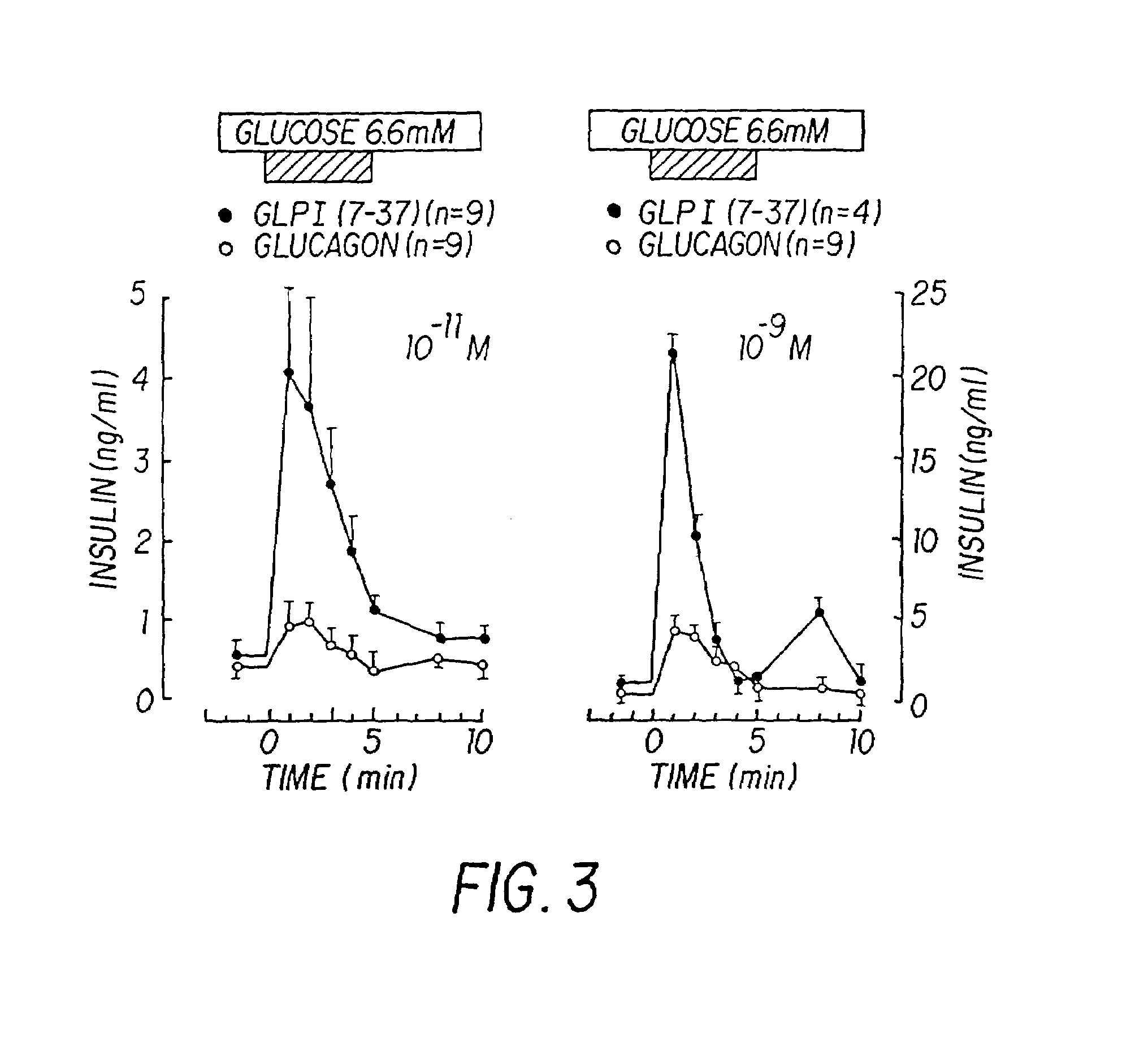
Derivatives of glucagon-like peptide I (GLP-1) and especially GLP-1 (7-37) have been found to have insulinotropic activity. The invention pertains to a composition comprising an acid addition salt of GLP-I (7-37) and to a composition comprising a carboxylate salt of GLP-I (7-37). The invention also pertains to method of treating type II diabetes mellitus by providing derivatives of GLP-I (7-37) to the patient.
Owner:THE GENERAL HOSPITAL CORP
Method of treatment of diabetes and/or obesity with reduced nausea side effect
InactiveUS20070207958A1Prevent and reduce and eliminate nausea side effectReduces and eliminates side effectPeptide/protein ingredientsMetabolism disorderSide effectInsulinotropin
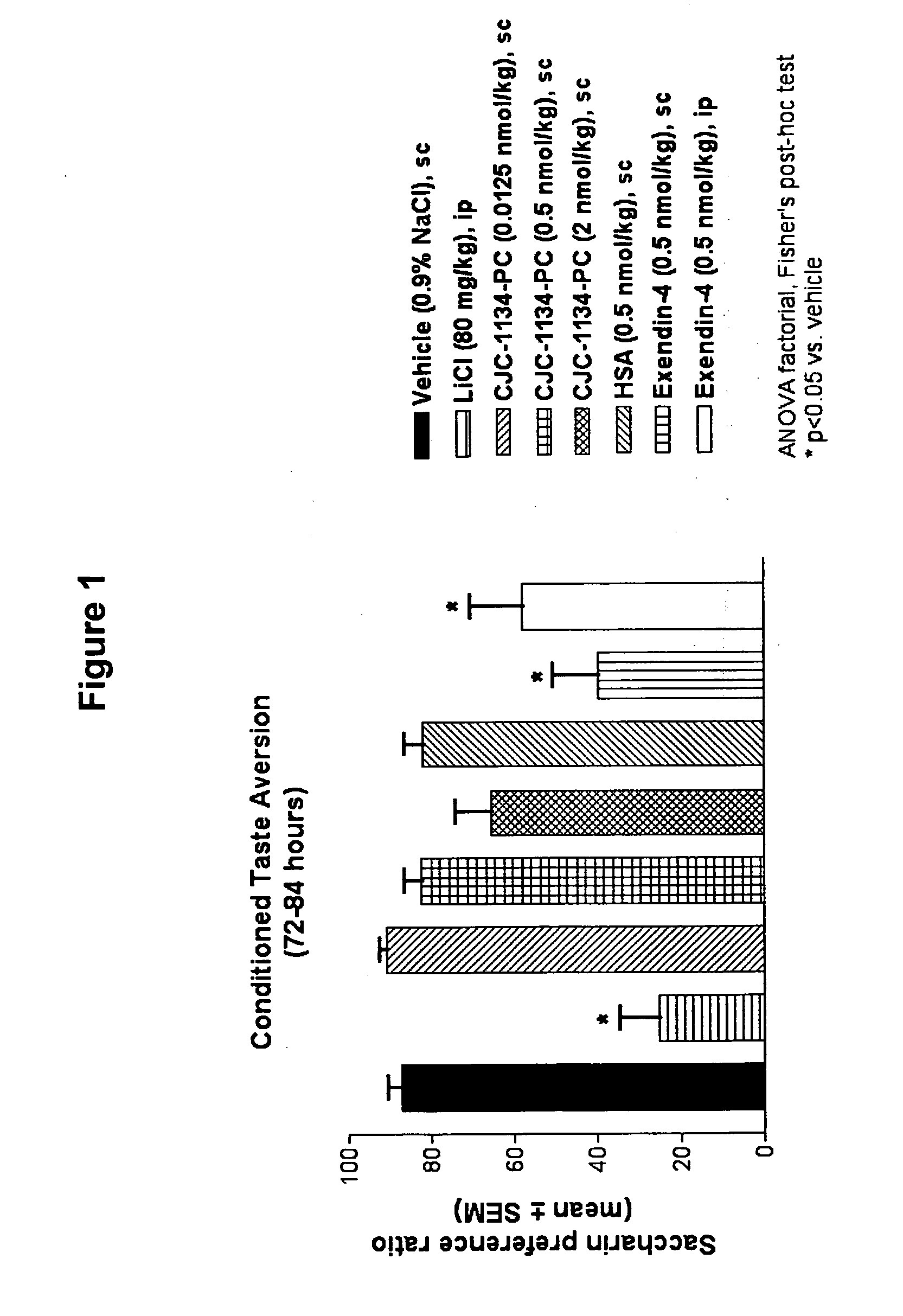
The present invention provides methods of administering an insulinotropic peptide in an amount effective to treat a disorder or condition while reducing nausea side effect by administering to a subject in need thereof an insulinotropic peptide conjugated to albumin. The present invention also provides methods of selecting a subject for administration of a conjugated insulinotropic peptide. Exemplary disorders or conditions treatable with an insulinotropic peptide include obesity and type II diabetes.
Owner:CONJUCHEM
Glucagon-like insulinotropic peptides, compositions and methods
The present invention provides novel complexes consisting of certain GLP-1 molecules associated with a divalent metal cation that is capable of co-precipitating with a GLP-1 molecule. Pharmaceutical compositions and methods of using such complexes for enhancing the expression of insulin in B-type islet cells is claimed, as is a method for treating maturity onset diabetes mellitus in mammals, particularly humans.
Owner:ELI LILLY & CO
An insulinotropic complex using an immunoglobulin fragment
ActiveUS20100105877A1Improve efficacyProlong lifePeptide/protein ingredientsVasoactive intestinal peptidePeptide drugHalf-life
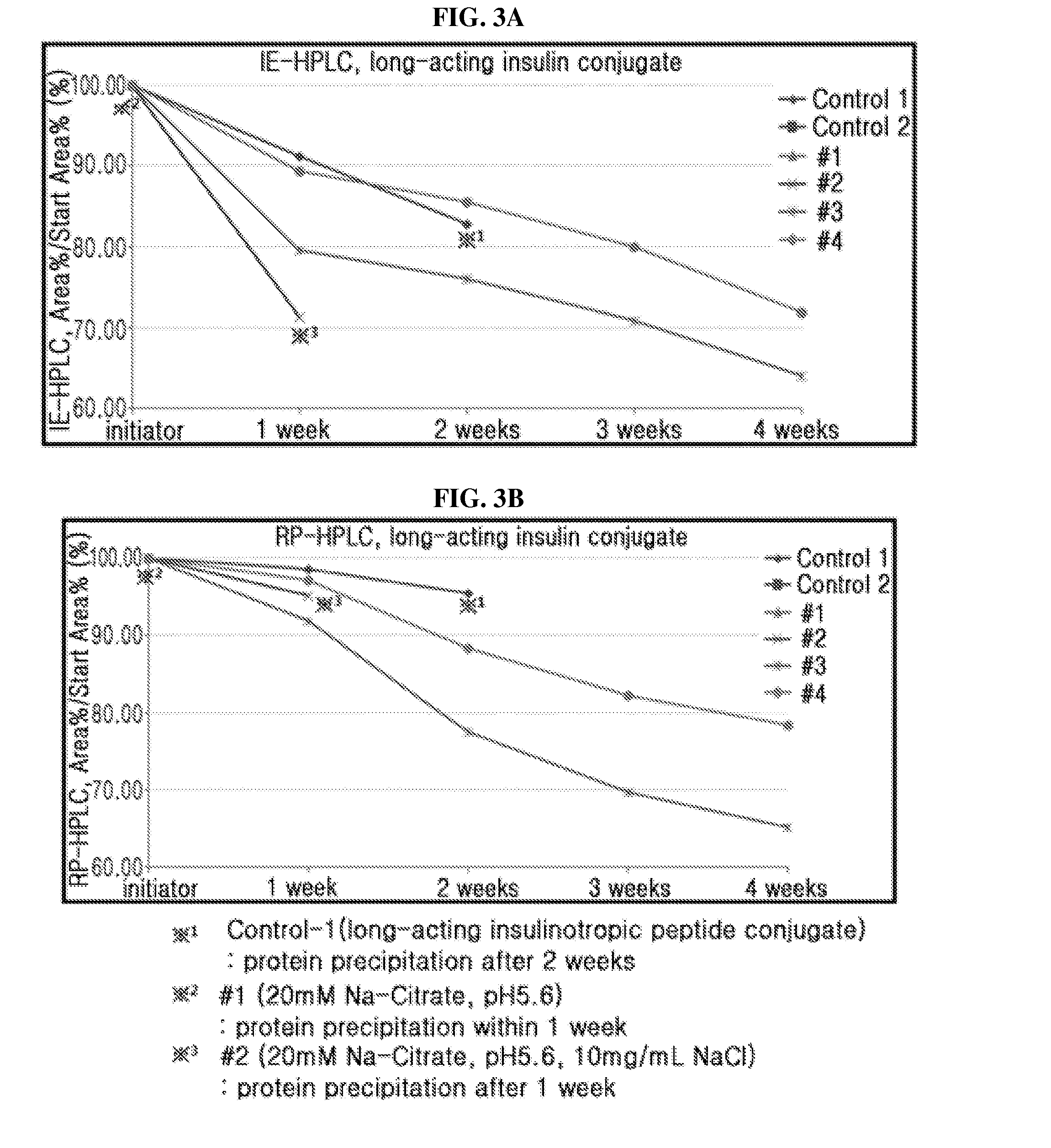
The present invention relates to an insulinotropic peptide conjugate having improved in-vivo duration of efficacy and stability, comprising an insulinotropic peptide, a non-peptide polymer and an immunoglobulin Fc region, which are covalently linked to each other, and a use of the same. The insulinotropic peptide conjugate of the present invention has the in-vivo activity which is maintained relatively high, and has remarkably increased blood half-life, and thus it can be desirably employed in the development of long acting formulations of various peptide drugs.
Owner:HANMI SCI CO LTD
Stable Formulations Of Peptides
Owner:NOVO NORDISK AS
Insulinotropic peptide conjugate using an immunoglobulin fc
The present invention relates to an insulinotropic peptide conjugate having improved in-vivo duration of efficacy and stability, comprising an insulinotropic peptide, a non-peptide polymer and a carrier substance, which are covalently linked to each other, and a use of the same. The insulinotropic peptide conjugate of the present invention has the in-vivo activity which is maintained relatively high, and has remarkably increased blood half-life, and thus it can be desirably employed in the development of long acting formulations of various peptide drugs.
Owner:HANMI PHARMA
Uses of Glucoregulatory Proteins
ActiveUS20090312246A1Facilitated releasePromote regenerationPeptide/protein ingredientsMetabolism disorderDiseaseInsulinotropin
The present invention relates generally to the novel use of incretin compounds (ICs) and amylinomimetic compounds to treat, prevent, or ameliorate a variety of metabolic conditions or diseases.
Owner:ASTRAZENECA PHARMA LP
Pharmaceutical compositions and methods for fabrication of solid masses comprising glucose regulating proteins
ActiveUS20150328287A1Minimize adverse effectsHigh treatment ratePeptide/protein ingredientsMetabolism disorderIntestinal wallsWhite blood cell
Embodiments of the invention provide shaped masses comprising one or more drugs such as proteins or polypeptides and methods for forming such shaped masses. One embodiment provides a shaped mass comprising a drug such as a protein or polypeptide having a biological activity in the body of a mammal. The shaped mass is formed by compression of a precursor material comprising the drug wherein an amount of biologically active drug in the mass is a preserved above a minimum level. Drugs which may be incorporated into the shaped mass may include one or more glucose regulating proteins such as insulin, incretins; and immunoglobulins such as TNF-inhibiting antibodies or interleukin neutralizing antibodies. Embodiments of the shaped mass may be incorporated into a tissue penetrating member which is inserted into the intestinal wall allowing for the oral delivery of proteins and peptides which would otherwise be degraded in the intestinal tract.
Owner:RANI THERAPEUTICS
Insulinotropic complex using an immunoglobulin fragment
ActiveUS8476230B2Improve efficacyProlong lifePeptide/protein ingredientsVasoactive intestinal peptidePeptide drugHalf-life
The present invention relates to an insulinotropic peptide conjugate having improved in-vivo duration of efficacy and stability, comprising an insulinotropic peptide, a non-peptide polymer and an immunoglobulin Fc region, which are covalently linked to each other, and a use of the same. The insulinotropic peptide conjugate of the present invention has the in-vivo activity which is maintained relatively high, and has remarkably increased blood half-life, and thus it can be desirably employed in the development of long acting formulations of various peptide drugs.
Owner:HANMI SCI CO LTD
Drug fusions and conjugates with extended half life
The present invention relates to drug fusions and conjugates that have improved serum half lives. These fusions and conjugates comprise immunoglobulin (antibody) single variable domains and insulinotropic and / or incretin and / or gut peptide molecules. The invention further relates to uses, formulations, compositions and devices comprising such drug fusions and conjugates. The invention also relates to compositions which comprise more than one insulinotropic and / or incretin and / or gut peptide molecules present as part of a fusion or conjugate and to uses and formulations thereof.
Owner:GLAXO GROUP LTD
Stable formulations of insulinoptropic peptides
The present invention provides a stable pharmaceutical composition comprising insulinotropic peptide.
Owner:NOVO NORDISK AS
N-terminally modified GLP-1 receptor modulators
InactiveUS20070021346A1Improve efficacyMaintain good propertiesSenses disorderNervous disorderGlucagon-like peptide-1Drug biological activity
The subject matter described herein provides novel human glucagon-like peptide-1 (GLP-1) receptor modulators that have biological activity similar or superior to native GLP-1 peptide and thus are useful for the treatment or prevention of diseases or disorders associated with GLP activity. The described compounds include chemically modified peptides that not only stimulate insulin secretion in type II diabetics, but also produce other beneficial insulinotropic responses. These synthetic peptide GLP-1 receptor modulators exhibit increased stability to proteolytic cleavage making them ideal therapeutic candidates for oral or parenteral administration. The disclosed and claimed peptides show desirable pharmacokinetic properties and desirable potency in efficacy models of diabetes.
Owner:BRISTOL MYERS SQUIBB CO
GLP-1 as a diagnostic test to determine β-cell function and the presence of the condition of IGT and type-II diabetes
InactiveUS6884579B2Promote insulin secretionTest β-cell functionCompounds screening/testingPeptide/protein ingredientsDiagnostic testSide effect
Since glucagon-like peptide-1 (GLP-1) is the most potent insulinotropic hormone known and has been shown to stimulate insulin secretion strongly in patients with type II diabetes, this invention uses GLP-1 or its biologically active analogues in β-cell stimulatory tests in order to test β-cell function in a simple way. The test provides information about insulin secretory capacity, is easy and reproducible and has insignificant side effects.
Owner:AMYLIN PHARMA INC
A pharmaceutical composition for treating obesity-related disease comprising insulinotropic peptide conjugate
ActiveCN101878036ALong lasting effectInhibition of uptakePeptide/protein ingredientsMetabolism disorderDiseaseEfficacy
The present invention relates to a composition for treating obesity-related diseases comprising an insulinotropic peptide conjugate, more particularly, to a composition for treating obesity-related diseases comprising a conjugate prepared by covalently linking the insulinotropic peptide with a carrier substance via a non-peptidyl linker, and a method for treating obesity-related diseases by using the same. In particular, the composition for treating obesity-related diseases according to the present invention remarkably improves the efficacy of suppressing food intake and its duration to reduce body weight and body fat, thereby being useful for the treatment of obesity-related diseases.
Owner:HANMI SCI CO LTD
Liquid formulation of long-acting insulin and insulinotropic peptide
ActiveUS20150190528A1Good storage stabilityMaintain activityPeptide/protein ingredientsMetabolism disorderHigh concentrationAlcohol sugars
The present invention relates to a liquid formulation of a combination of long-acting insulin and insulinotropic peptide, comprising insulin which is a physiologically active peptide, insulinotropic peptide, and albumin-free stabilizer, wherein the stabilizer comprises a buffer, a sugar alcohol, a non-ionic surfactant, and an isotonic agent; and a method for preparing the liquid formulation. The liquid formulation of the present invention does not contain a human serum albumin and potentially toxic factors to the body, and thus it has excellent storage stability for insulin conjugate and insulinotropic peptide conjugate at high concentration, without a risk of viral contamination.
Owner:HANMI PHARMA
Fusion protein, preparation method thereof, DNA sequence for coding protein, expression vector, host cell and protein-containing medicinal compoisition
ActiveCN102070717AExtended half-lifeAvoid side effectsPeptide/protein ingredientsMetabolism disorderHalf-lifeProtein C
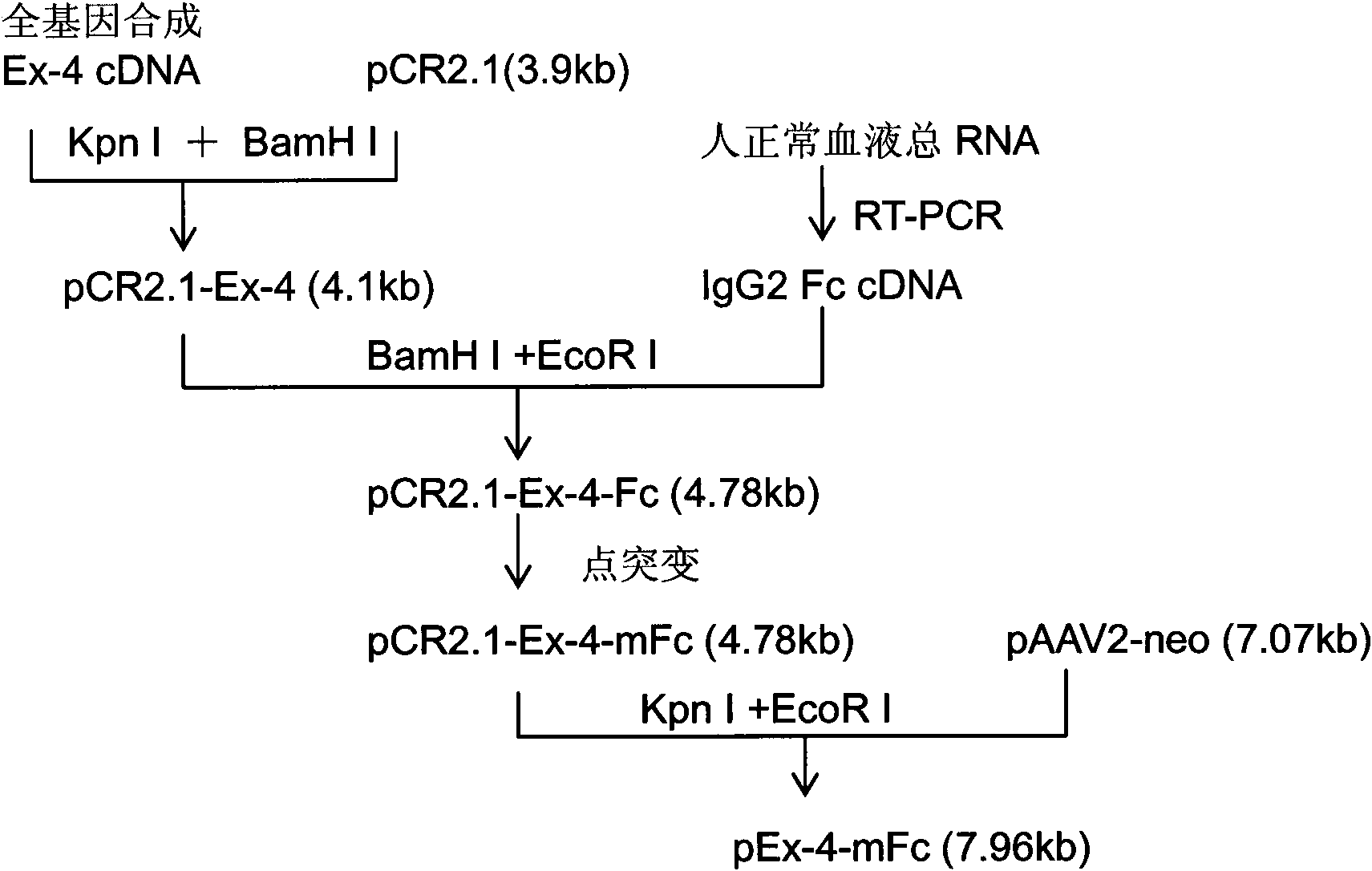
The invention relates to a fusion protein. The fusion protein sequentially contains exendin-4, a connecting peptide and a human IgG2 Fc mutant from an N end to a C end. The invention also relates to a preparation method for the fusion protein. The fusion protein can be highly expressed in a mannalian cell, and a purification process is simple and favorable for further preparing the fusion proteins in large scale. The fusion protein provided by the invention has the bioactivity of natural exendin-4 and the advantage of longer serum half-life period, and can stimulate the secretion of insulin, suppress the release of glucagons after food intake and be used for treating various diabete.
Owner:SHENZHEN TAILI BIOTECHNOLOGY CO LTD
Modulators of the gpr119 receptor and the treatment of disorders related thereto
InactiveUS20130023494A1Increase secretionIncreasing blood incretin levelBiocideNervous disorderBiguanideSGLT2 Inhibitor
The present invention relates to compounds of Formula (Ia) and pharmaceutically acceptable salts, solvates, and hydrates thereof, that are useful as a single agent or in combination with one or more additional pharmaceutical agents, such as, an inhibitor of DPP-IV, a biguanide, an SGLT2 inhibitor, or an alpha-glucosidase inhibitor, in the treatment of, for example, a disorder selected from: a GPR119-receptor-related disorder; a condition ameliorated by increasing a blood incretin level; a metabolic-related disorder; type 2 diabetes; obesity; and complications related thereto.
Owner:ARENA PHARMA
Marine oligosaccharide compound with type II diabetes resisting activity
ActiveCN101649004AGood anti-type II diabetes activityImprove securityOrganic active ingredientsSugar derivativesPolymannuronic acidCarboxyl radical
The invention relates to a marine oligosaccharide compound with type II diabetes resisting activity, which is a compound which is formed by following steps: taking D-polymannose aldehydic oligosaccharide which comes from sea and contains carboxyl in each oligosaccharide ring, reacting with dilute alkali and chromium salt solution and introducing trivalent chromium ions which is closely relative tothe generation and the development of diabetes into oligosaccharide molecules. A pharmacological experiment proves that the product has remarkable effect on accelerating insulin secretion, is not influenced by amylin and has effects on lightening glucose load, improving blood-lipoid metabolism, insulin sensitivity and certain kidney protection and lightening pancreatic injury for type II diabetesrats and mouse. The product of the invention comes from marine natural species, has the advantages of good security, unique structure, low molecular weight, high chromium binding ratio, good oral absorption effect, and the like, can play a hypoglycemic role in a plurality of links and has favorable market application prospect on the aspect of preventing and treating type II diabetes.
Owner:OCEAN UNIV OF CHINA
Stabilized insulinotropic peptides and methods of use
ActiveUS9296805B2Superior and unexpected benefitEnhanced alpha-helicityPeptide/protein ingredientsMetabolism disorderCross-linkAbnormal glucose homeostasis
The present invention provides stably cross-linked insulionotropic polypeptides having superior and unexpected benefits in the treatment of conditions involving abnormal glucose homeostasis, e.g., type 2 diabetes and conditions relating to type 2 diabetes. Such benefits include, but are not limited to, extended polypeptide half-life, enhanced alpha-helicity, improved thermal stability and protease resistance, increased functional activity and pharmacologic properties, improved bioavailability when administered by any route, and improved bioavailability and gastrointestinal absorption when delivered orally, as compared to the corresponding unmodified polypeptides. The invention also provides compositions for administering the polypeptides of the invention, as well as methods for preparing and evaluating the polypeptides of the invention.
Owner:DANA FARBER CANCER INST INC
Stable formulations of insulinoptropic peptides
The invention provides a stable formulations of insulinoptropic peptides, comprising a stable drug composite for insulinoptropic peptides.
Owner:NOVO NORDISK AS
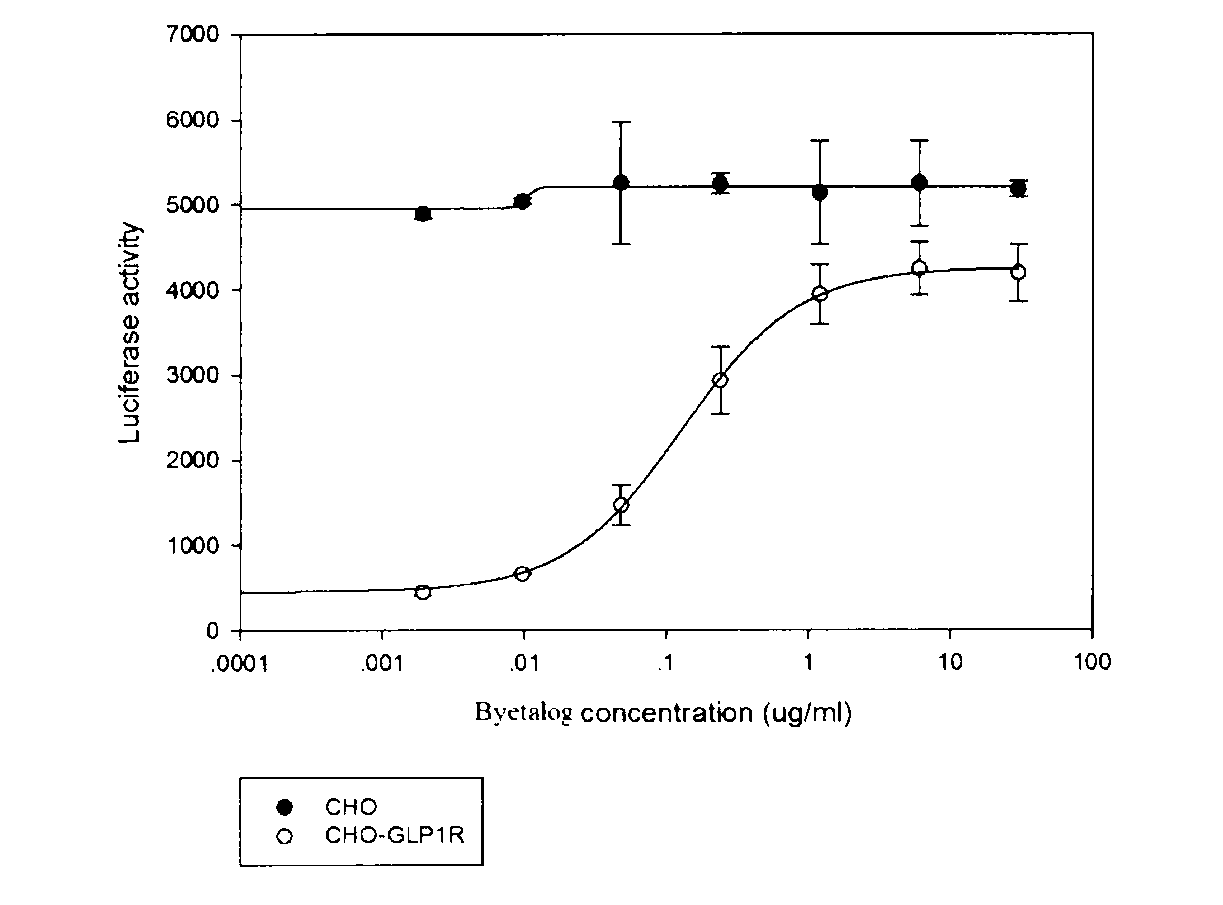
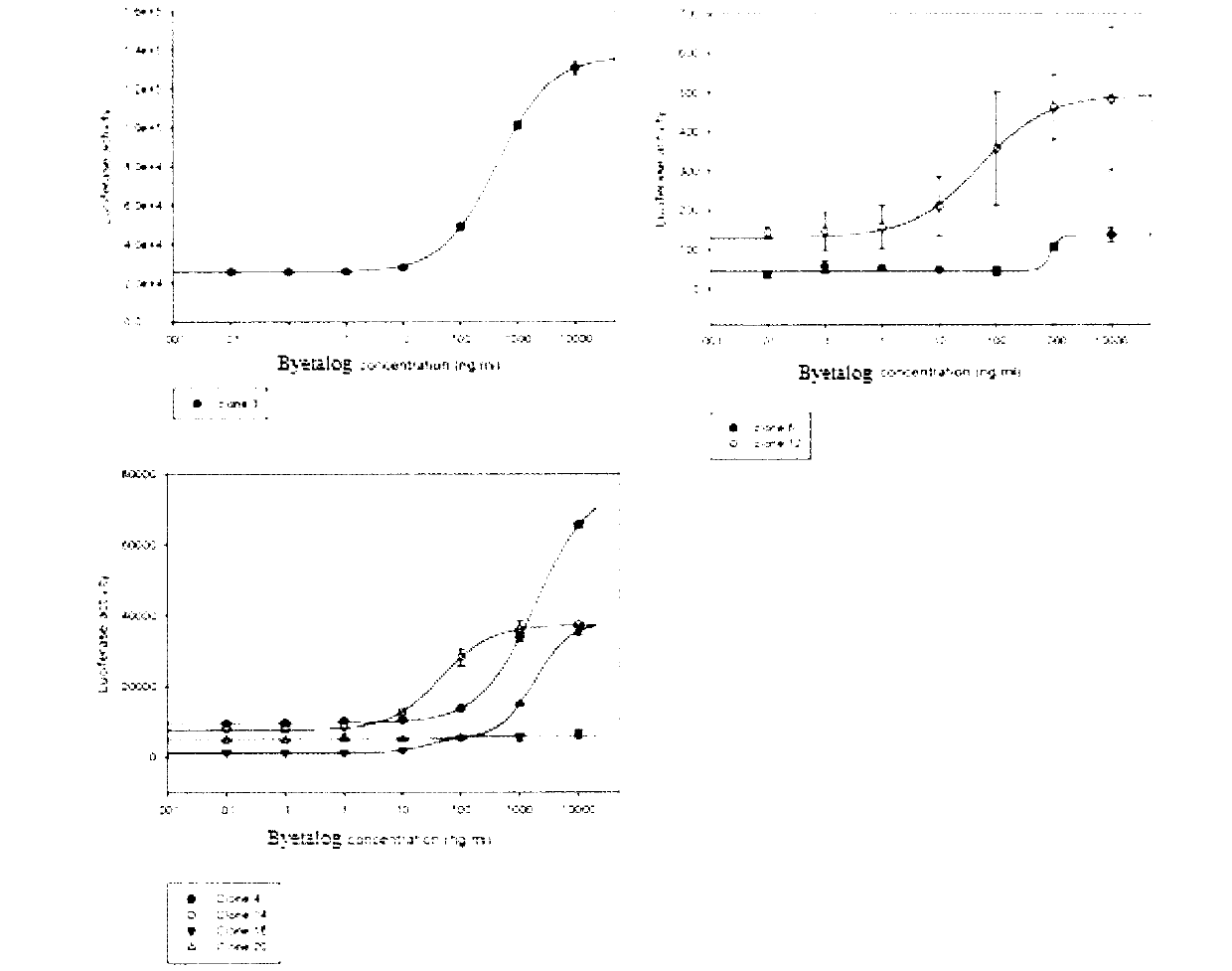
RGA (Report Gene Assay) method for detecting biological activity of exendin-4-HAS Byetalog
ActiveCN103293315APromote research and developmentQuality is easy to controlBiological testingForeign genetic material cellsDrug biological activityGene carrier
The invention provides a novel biological activity determination method of exendin-4-HAS Byetalog. The basic principle of the method is that a GLP-1R (Glucagon-Like Peptide-1R) and a cAMP (cyclic Adenosine Monophosphate) response element (CER) report gene carrier are used for co-transfection of a CHO-K1 cell, and the steps of pressurization, sieving and monoclonal separation and cultivation are carried out to obtain a stable monoclonal cell strain CHOglplr / crec14. After the GLP-1R is combined with a ligand, a series of signal transduction is carried out to stimulate the expression of CRE reporter gene luciferase, and the activity of the Byetalog is quantified by detecting the change of the expression of the luciferase after the Byetalog stimulates. The specific detection steps are as follows: 6000 CHOglplr / crec14 cells are placed on each pore of a 96-pore plate to be cultured for 16-18 hours; the Byetalog with different concentrations is added to stimulate the cells for 6 hours; the activity of the luciferase is detected. The method is simple to operate, sensitive in reaction and small in variability, and has very important meaning to quality control and clinical application of the Byetalog.
Owner:NAT INST FOR FOOD & DRUG CONTROL
Modulators of the gpr119 receptor and the treatment of disorders related thereto
The present invention relates to the GPR119 receptor agonists: 3-fluoro-4-(5-fluoro-6-(4-(3-(2-fluoropropan-2-yl)-1,2,4-oxadiazol-5-yl)piperidin-1-yl)pyrimidin-4-ylamino)-N,N-dimethylbenzamide; -fluoro-4-(5-fluoro-6-(4-(3-(2-fluoro-propan-2-yl)-1,2,4-oxadiazol-5-yl)piperidin-1-yl)pyrimidin-4-ylamino)-N-methylbenzamide; and 3-fluoro-4-(5-fluoro-6-(4-(3-(2-fluoropropan-2-yl)-1,2,4-oxadiazol-5-yl)piperidin-1-yl)pyrimidin-4-ylamino)benzamide, and pharmaceutically acceptable salts, solvates, and hydrates thereof, that are useful as a single pharmaceutical agent or in combination with one or more additional pharmaceutical agents, such as, a DPP-IV inhibitor, a biguanide, an alpha-glucosidase inhibitor, an insulin analogue, a sulfonylurea, an SGLT2 inhibitor, a meglitinide, a thiazolidinedione, or an anti-diabetic peptide analogue, in the treatment of for example, a disorder selected from: a GPR119-receptor-related disorder; a condition ameliorated by increasing secretion of an incretin; a condition ameliorated by increasing a blood incretin level; a condition characterized by low bone mass; a neurological disorder; a metabolic-related disorder; type 2 diabetes; obesity; and complications related thereto.
Owner:ARENA PHARMA
Fusion protein of Exendin-4 and mutational human serum albumin, and preparation method of fusion protein
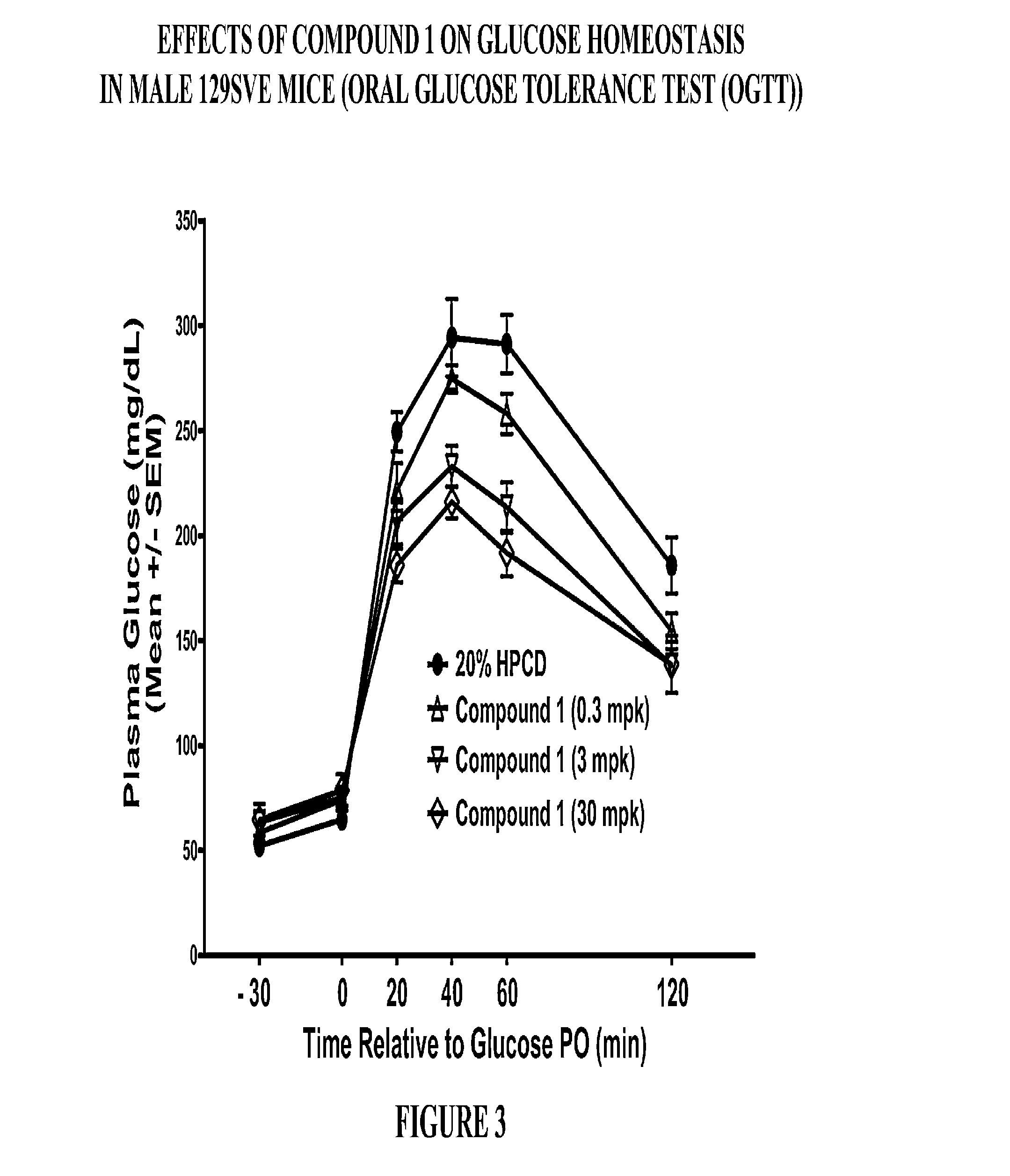
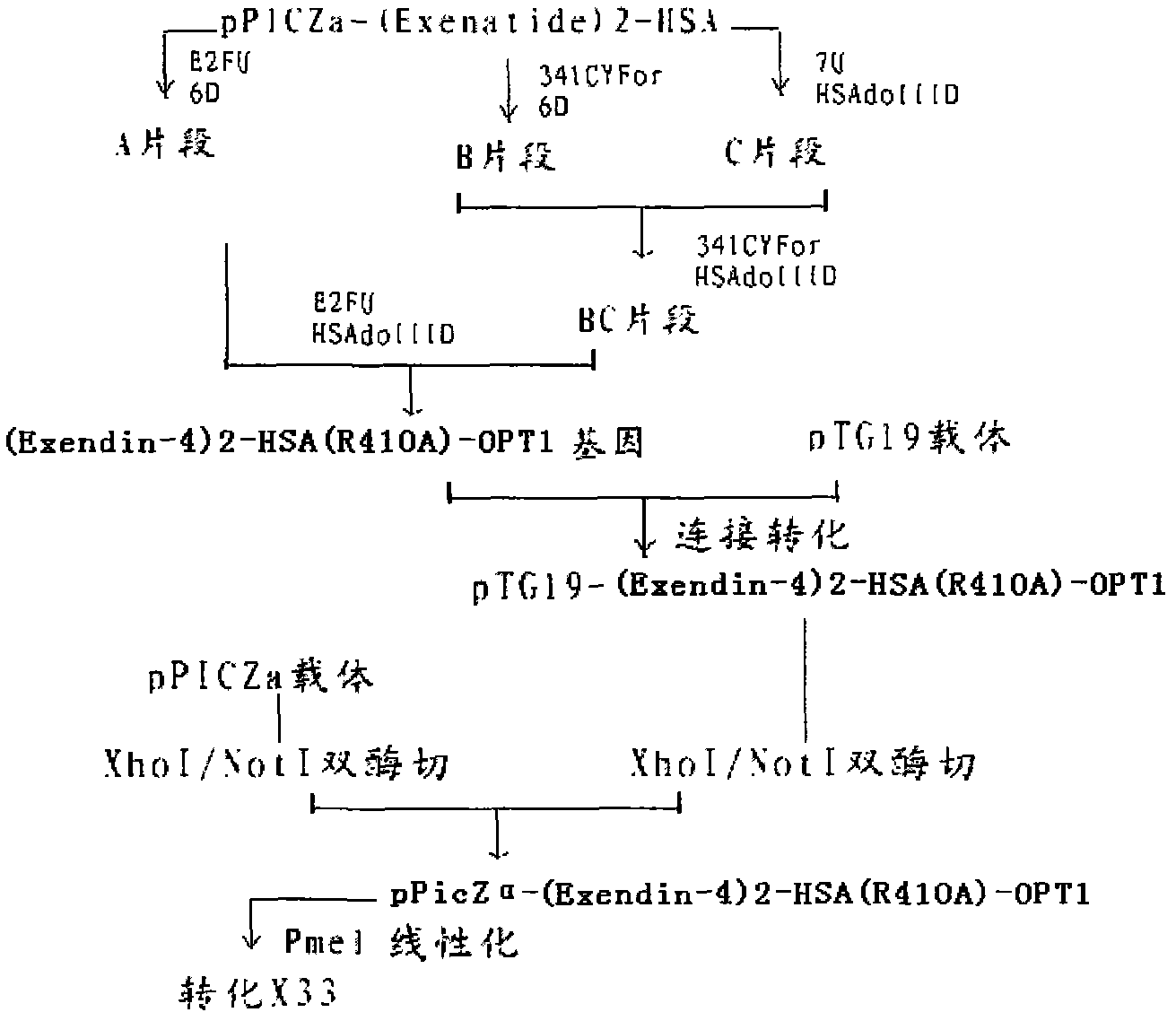
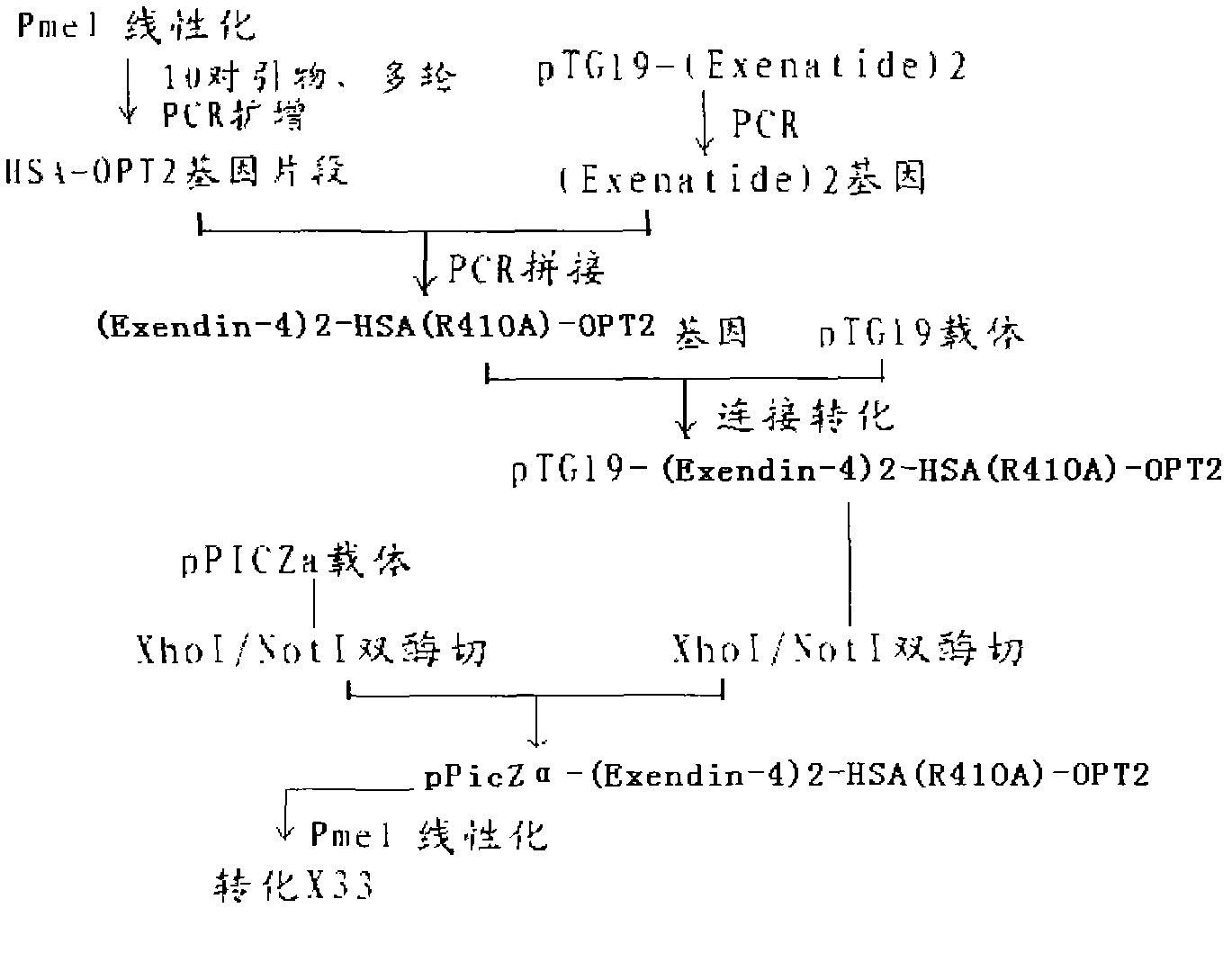
InactiveCN102827286AProlong the action timeFungiPeptide/protein ingredientsInsulin dependent diabetesArginine
The invention provides a fusion protein of Exendin-4 and mutational human serum albumin and a preparation method of the fusion protein, belonging to the technical field of long-acting recombined fusion protein drugs. The invention relates to the fusion protein of Exendin-4 and human serum albumin (HSA) in which the 410-site is mutated to A (Ala alanine) from R (Arg arginine). The fusion protein comprises two polypeptide zones, wherein zone I is composed of two Exendin-4 repetitive sequences, and zone II is site-mutation human serum albumin (HSA); the zone I is directly connected with the N-terminal of the zone II by the C-terminal of the zone I without adding any connecting peptides therebetween; and the structure of the fusion protein is: (Exendin-4)2-HSA (R410A). Compared with Exendin-4 and (Exendin-4)2-HAS, (Exendin-4)2-HSA (R410A) fusion protein provided by the invention has the characteristic of longer-lasting effect in vivo, and can be used for preparing long-lasting preparation of non-insulin-dependent diabetes.
Owner:SHANGHAI ALLIST PHARM CO LTD
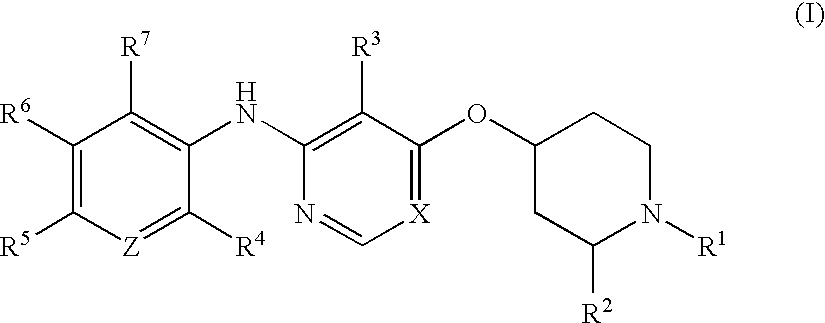
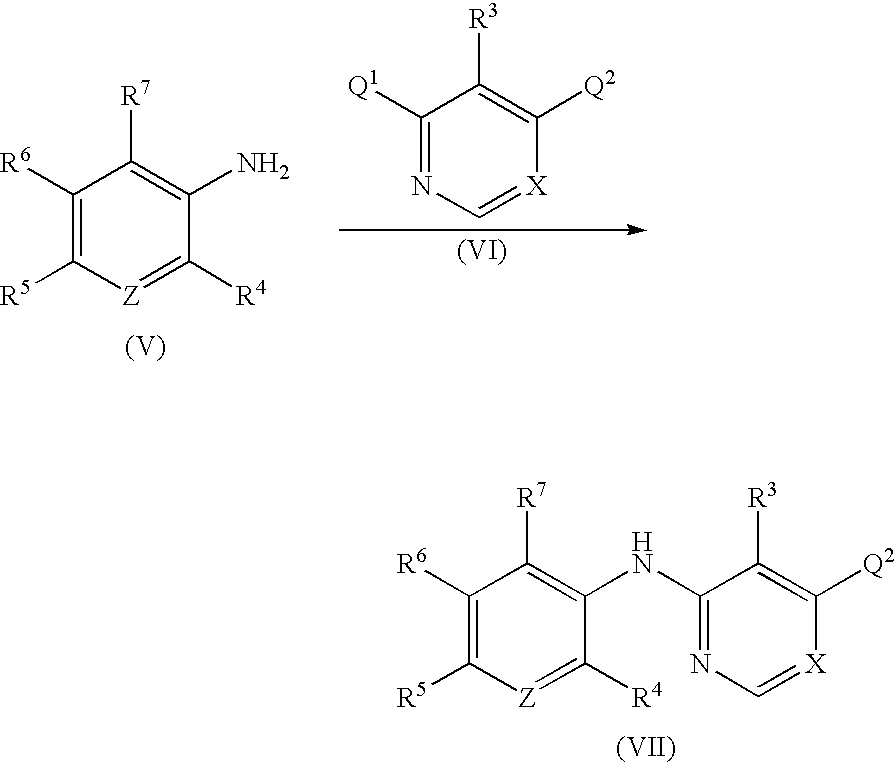
Process for the preparation of tri-substituted pyridine and tri-substituted pyrimidine derivatives useful as gdir agonists
InactiveUS20100113480A1Inadequate glucose toleranceBiocideOrganic active ingredientsDiabetes mellitusDisease
The present invention is directed to novel processes for the preparation of tri-substituted pyridine and tri-substituted pyrimidine derivatives, useful as glucose dependent insulinotropic receptor agonist, for the treatment of metabolic-related disorders and complications thereof, such as, diabetes and obesity.
Owner:ARENA PHARMA
GLP-1 and GIP co-agonist compound
PendingCN111825758AHormone peptidesPeptide/protein ingredientsInsulin dependent diabetesInsulinotropin
Owner:SHANGHAI HANSOH BIOMEDICAL +1
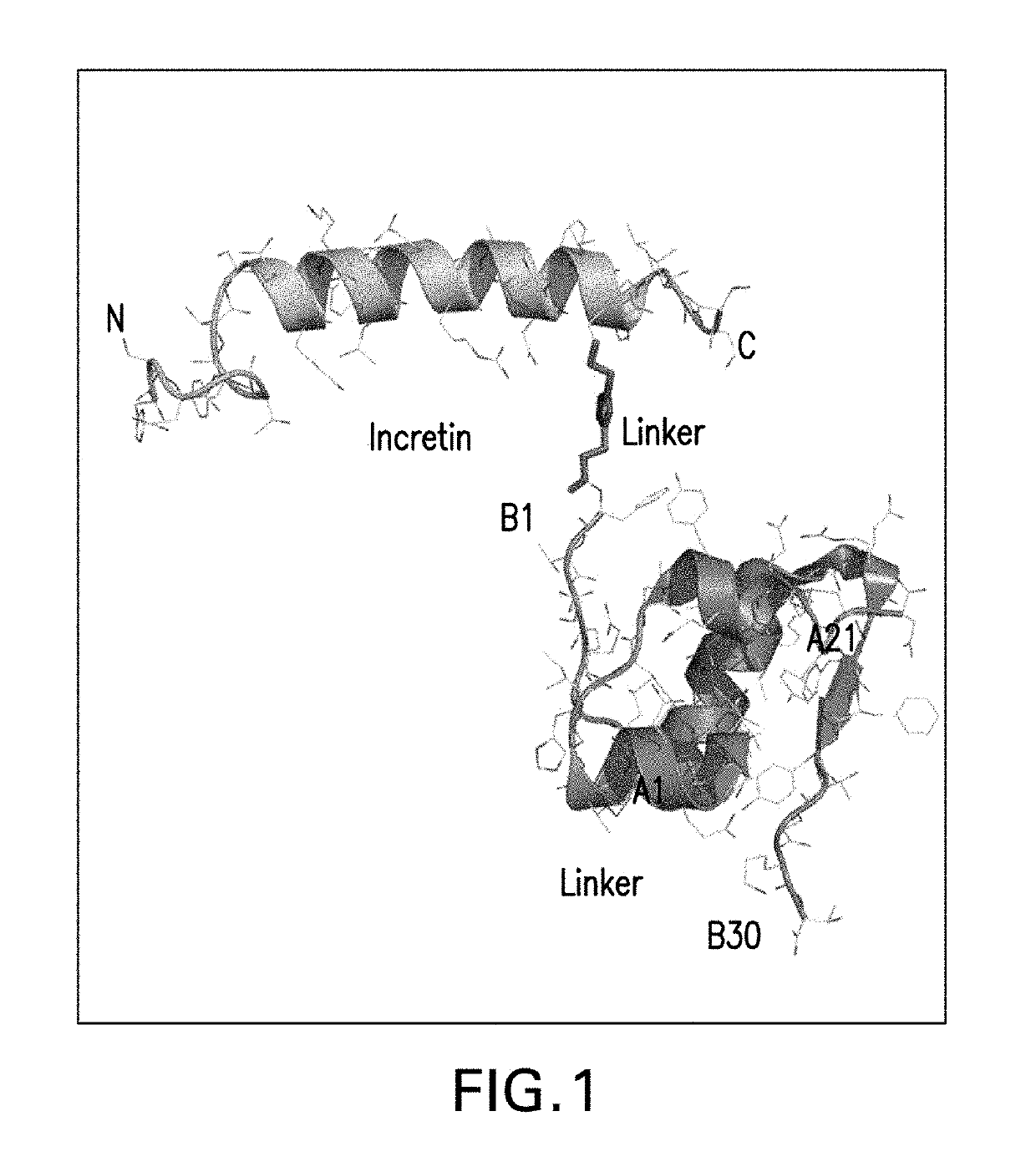
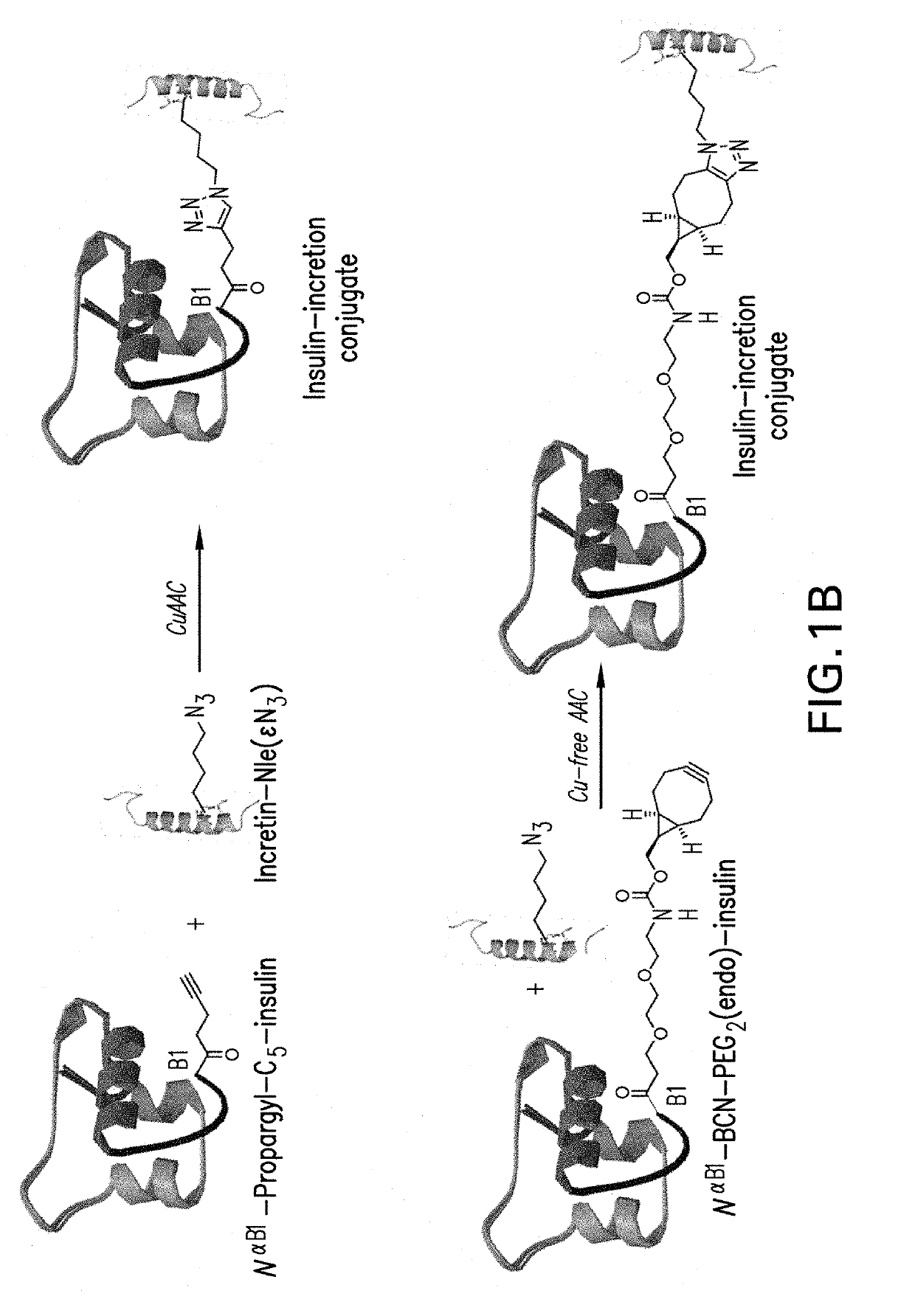
Insulin-incretin conjugates
InactiveUS20190175744A1Beneficial therapeutic additionHigh selectivityPeptide/protein ingredientsAntibody mimetics/scaffoldsPeptideChemistry
Insulin-incretin conjugates comprising a peptide having agonist activity at the glucagon-like 1 (GLP-1) receptor, the glucagon (GCG) receptor, and / or the gastric inhibitory protein (GIP) receptor conjugated to an insulin molecule having agonist activity at the insulin receptor and use of the conjugates for treatment of metabolic diseases, for example, Type 2 diabetes, are described.
Owner:MERCK SHARP & DOHME CORP
Novel glucagon analogue and application thereof
ActiveCN109762059AEasy to synthesizeEnhanced biological stability in vivoPeptide/protein ingredientsMetabolism disorderHalf-lifeWild type
Owner:清紫生物科技(深圳)有限公司 +1
Insulinotropic peptide derivative with modified n-terminal charge
InactiveUS20170226175A1Easy to useHigh activityPeptide/protein ingredientsMetabolism disorderReceptorPharmaceutical drug
The present invention relates to an insulinotropic peptide derivative with a modified N-terminal charge and a pharmaceutical composition including the same. Specifically, the insulinotropic peptide derivative is characterized in that the N-terminal positive charge of the insulinotropic peptide is modified to a neutral or net negative charge at neutral pH. The insulinotropic peptide derivative according to the present invention is rapidly dissociated from the GLP-1 receptor owing to the above modification in the N-terminal charge, and exhibits enhanced insulinotropic ability and blood glucose-lowering activity compared to the native insulinotropic peptide while maintaining its stability in blood. Accordingly, the insulinotropic peptide derivative of the present invention is very useful for the treatment of type 2 diabetes.
Owner:HANMI PHARMA
Application of acridinedione compound in preparation of anti-diabetic drug
ActiveCN111303030APromote secretionImprove the immunityOrganic active ingredientsOrganic chemistryAcridineReceptor
The invention provides an application of an acridinedione compound in preparation of an anti-diabetic medicine, and belongs to the technical field of biological medicines. According to the invention,the results prove that the acridinedione compound can participate in a GPR40-PPAR gamma-PI3K / Akt-GLUT4 signal path by activating and up-regulating GPR40 protein expression so as to promote insulin secretion, increase glucose consumption of liver and muscle tissues and improve insulin resistance; the action target of the acridinedione compound is a GPR40 receptor, the insulin secretion promoting effect of the acridinedione compound has glucose dependence, and when the peripheral blood glucose is lower than a certain degree, the blood glucose reducing effect of the acridinedione compound disappears; and the acridinedione compound is prepared into the anti-diabetic medicine, and brand-new selection and strategy are provided for treatment of diabetes mellitus.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Use of insulin promoting peptide and nutrient, parenteral nutrition composition and pharmaceuticals
The present invention discloses the use of one or more insulinotropic peptides and a nutritionally effective amount of at least one or more nutrients in the production of parenteral drugs for treating patients in need of parenteral nutrition, and also discloses a nutritionally effective amount of Parenteral nutrition compositions containing amounts of one or more nutrients and one or more insulinotropic peptides, and parenteral nutrition medicaments containing the compositions.
Owner:AMYLIN PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com