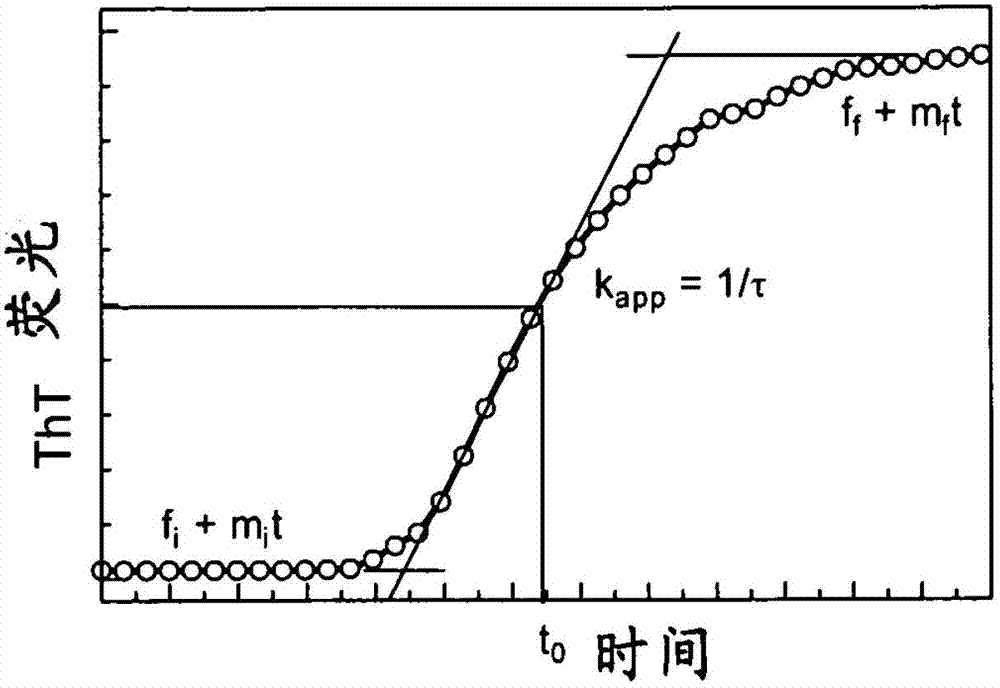
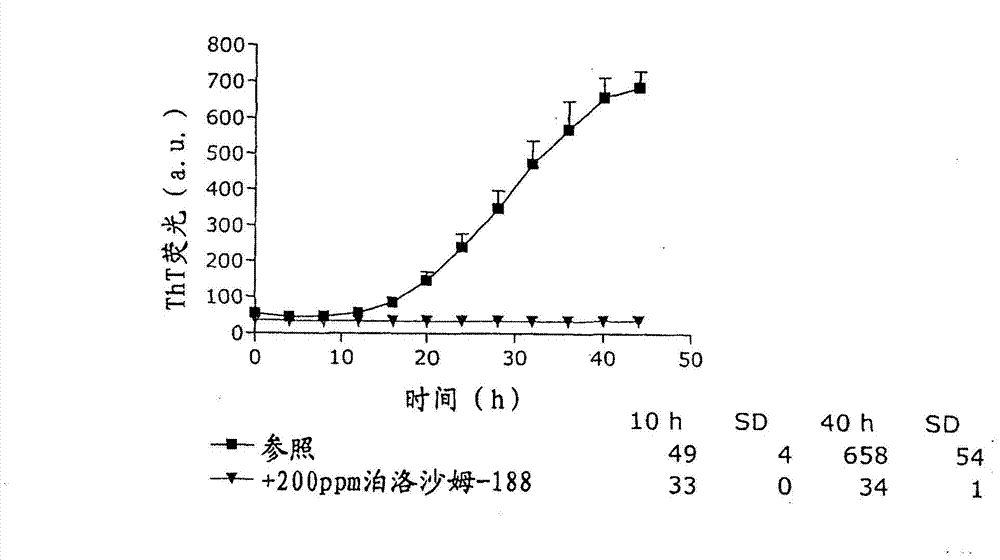
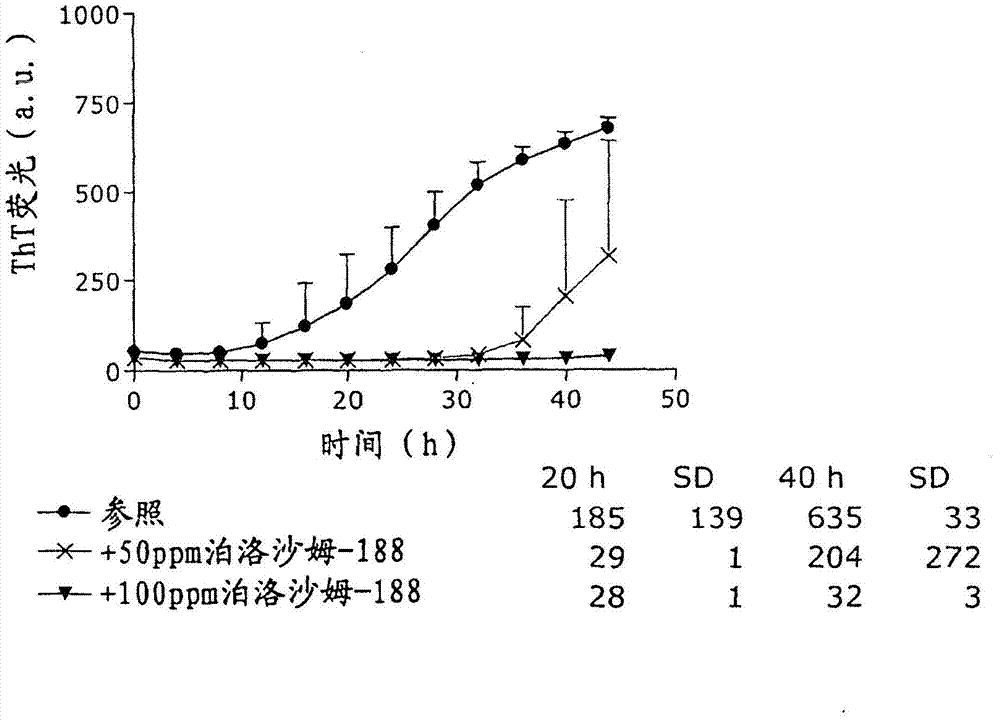
Stable formulations of insulinoptropic peptides
A stable and compound technology, applied in the direction of hormone peptides, glucagon, medical preparations of non-active ingredients, etc.
Inactive Publication Date: 2012-11-14
NOVO NORDISK AS
View PDF5 Cites 7 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
GLP-2 may be effective in the treatment of gastrointestinal diseases
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0165] Example 2
Embodiment 2
[0167] Example 3
Embodiment 3
[0169] Example 4
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention provides a stable formulations of insulinoptropic peptides, comprising a stable drug composite for insulinoptropic peptides.
Description
[0001] This divisional application is based on the divisional application of the Chinese patent application with the application number 200580038571.3 and the application date as November 14, 2005, and the invention name is a stable preparation of insulin-stimulating peptide. technical field [0002] The invention relates to the field of pharmaceutical preparations. More specifically, the present invention relates to shelf-stable pharmaceutical formulations comprising insulinotropic peptides. Background technique [0003] Therapeutic peptides are widely used in medical practice. Pharmaceutical compositions of such therapeutic peptides are required to have a shelf life of several years for common use. However, peptide compositions are inherently unstable due to susceptibility to chemical and physical degradation. Chemical degradation involves changes in covalent bonds such as oxidation, hydrolysis, racemization or cross-linking. Physical degradation involves conformational...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K38/26A61K9/19A61K47/34A61K47/26A61K47/10A61P3/10A61P3/04A61P3/06
CPCA61K9/08A61K9/19A61K38/26A61K9/0019A61K47/10A61K47/26A61P3/10A61P3/04A61P3/06A61P43/00A61K38/22A61K47/18C07K14/605
Inventor S·路德维希森M·施莱因T·E·G·博芬C·邦德A-M·利莱奥雷D·K·恩格伦德B·R·尼尔森
Owner NOVO NORDISK AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com