Novel glucagon analogue and application thereof
A technology of glucagon and analogues, which is applied in the field of polypeptide drugs, can solve the problems of no clinical use value, etc., and achieve the effect of enhancing biological stability, good stability and long-term activity in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
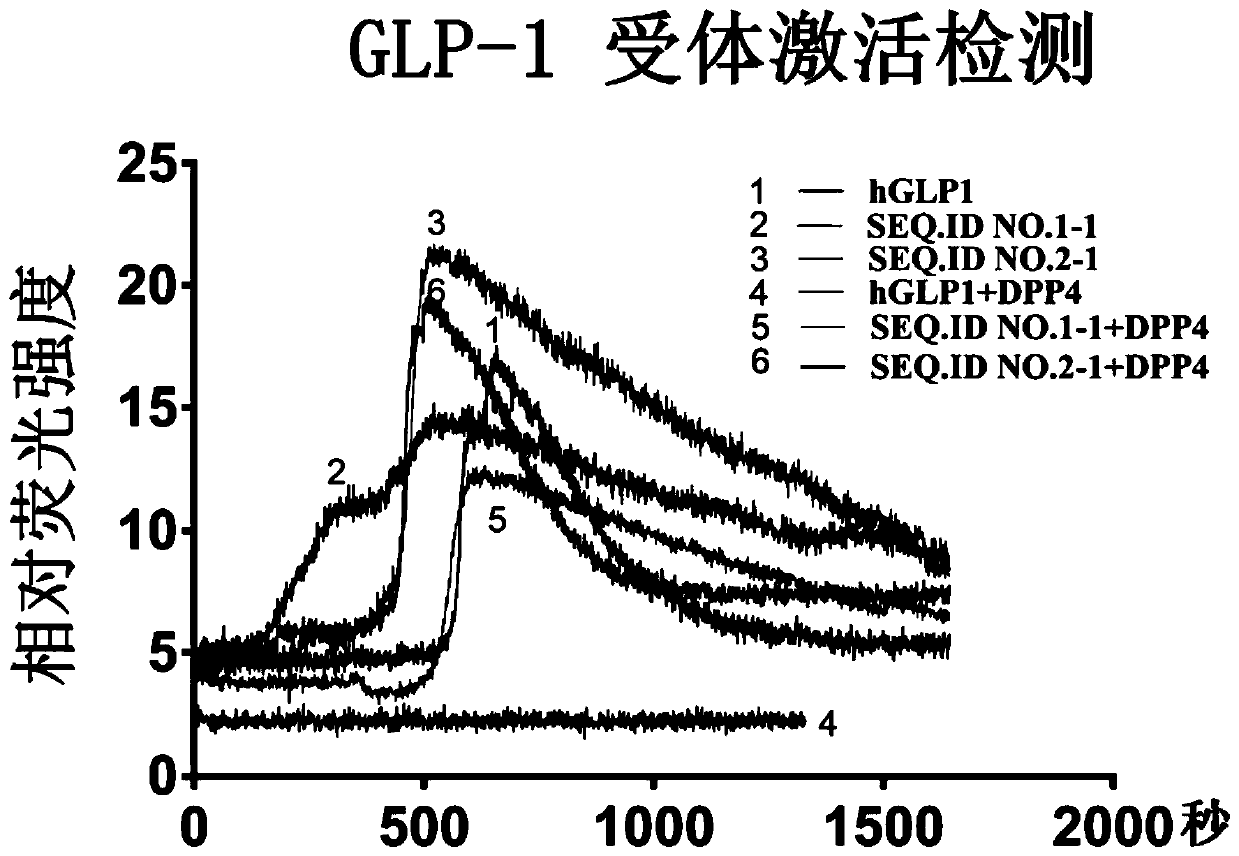
[0146] Example 1. Newly discovered peptide SEQ.ID NO.1 (His-Ser-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Ser-Tyr-Leu-Glu-Gly-Lys-Ala -Thr-Lys-Glu-Phe-Ile-Ala-Trp-Leu-Val-Lys-Gly-Leu-Glu) and SEQ.ID NO.2 (His-Ser-Glu-Gly-Thr-Phe-Thr-Ser -Asp-Phe-Ser-Ser-Tyr-Leu-Asp-Gly-Lys-Ala-Ala-Lys-Glu-Phe-Val-Ala-Trp-Leu-Val-Lys-Ser-Leu-Glu) can effectively stimulate GLP-1 receptor and can resist the hydrolysis of DPP4 in humans
[0147] In order to verify whether the sequences SEQ.ID NO.1 and SEQ.ID NO.2 can act as GLP-1 receptor agonists, we chemically synthesized polypeptides with a purity greater than 95%. The polypeptide was diluted to a concentration of 100 nM in Hank's Balanced Salt Solution (HBSS, pH 7.4) containing 20 mM HEPES and 2.5 mM Sulfamide. In order to detect whether the synthesized GLP-1 polypeptide can activate the GLP-1 receptor, Millipore purchased ready-to-use cells for GLP-1 detection (product number HTS163RTA) and corresponding complete medium. The cells are derived fr...
Embodiment 2
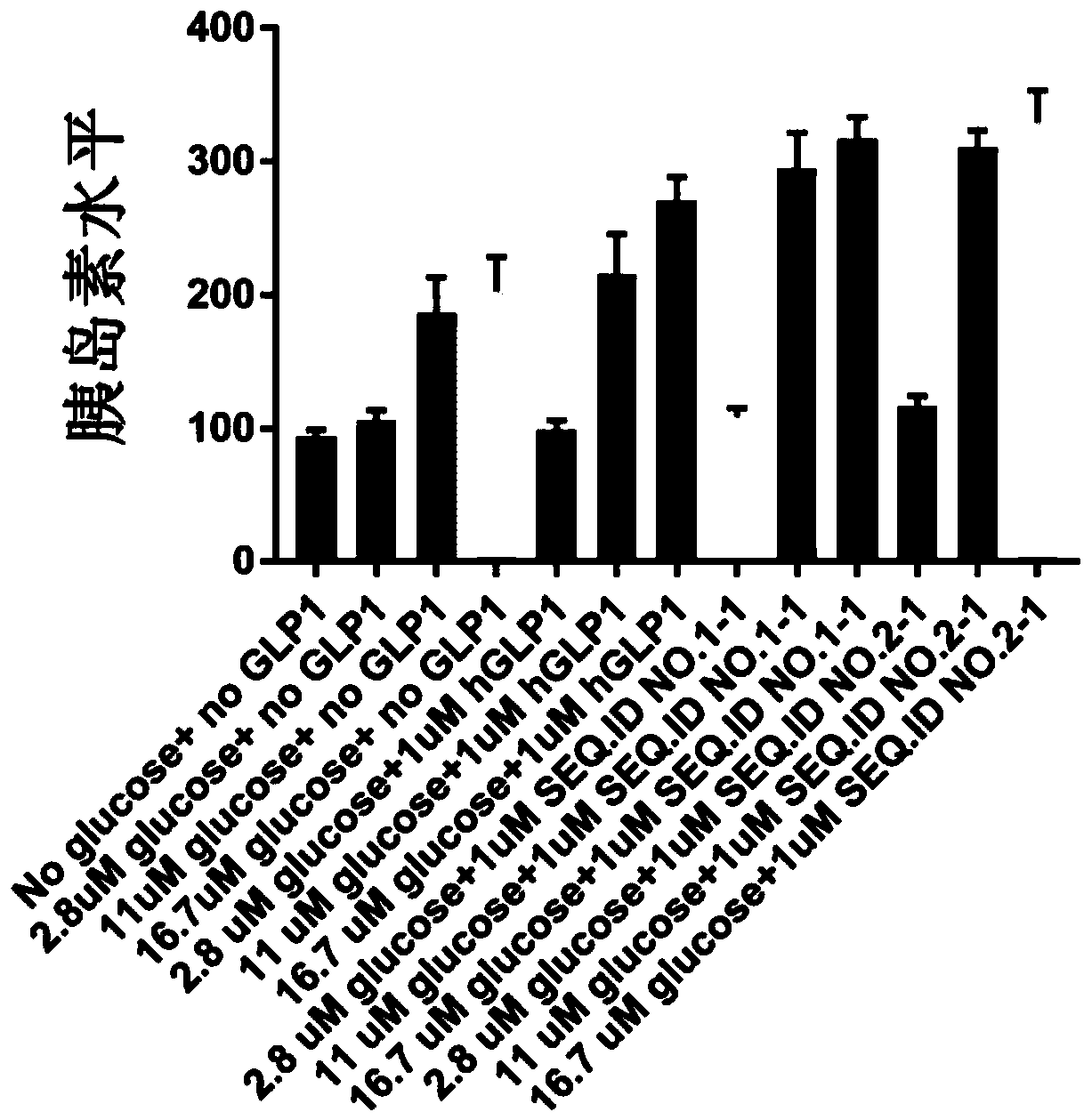
[0153] Example 2, SEQ.ID NO.1 and SEQ.ID NO.2 can regulate insulin secretion
[0154] The INS-1 832 / 13 cell line is derived from rat β cells transformed with the human proinsulin gene. Expression of GLP1 receptors, after being stimulated by active GLP1 or its analogues, and under high concentration glucose conditions, insulin secretion is increased. We have demonstrated that the GLP1 analogs to be tested can activate GLP-1R through calcium influx experiments. In this experiment, the INS-1 832 / 13 cell line was used to detect whether the GLP1 analogue to be tested could promote insulin release by activating GLP-1R.
[0155] The complete medium of the INS-1 832 / 13 cell line is RPMI 1640 supplemented with 10% fetal bovine serum, 50IU / ml penicillin, 50mg / L streptomycin, 10mM HEPES, 2mM L-glutamine, 1mM sodium pyruvate , and 50uM β-mercaptoethanol. INS-1 832 / 13 cells were passaged twice a week and cultured in a 5% CO2, 37°C incubator.
[0156] 1x105 INS-1 832 / 13 cells were seede...
Embodiment 3
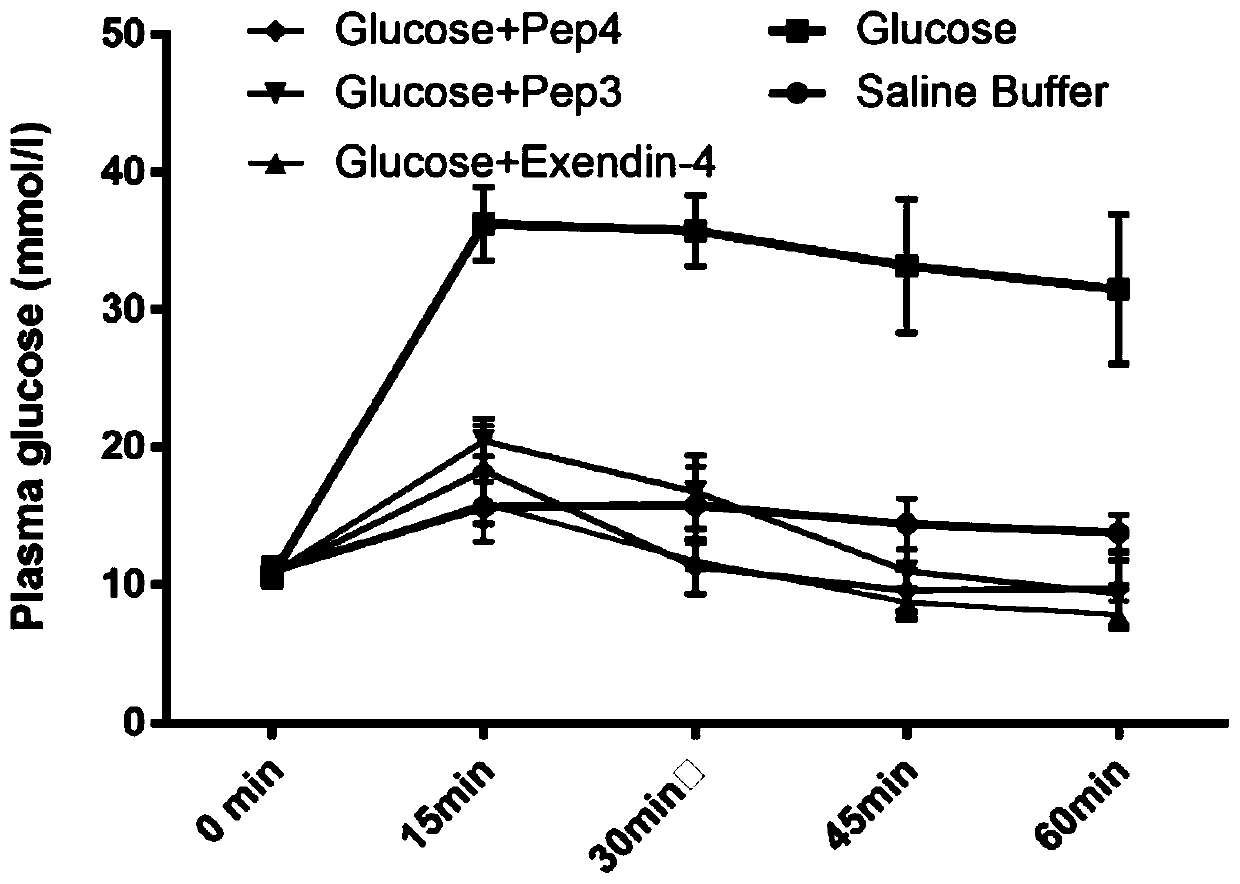
[0160] Example 3, SEQ.ID NO.1 and SEQ.ID NO.2 can regulate the insulin level of obese mice (BKS db) and reduce blood sugar.
[0161] In order to verify whether the peptides SEQ.ID NO.1 and SEQ.ID NO.2 can promote insulin secretion and reduce blood sugar concentration in type 2 diabetes model animals, we choose BKS.Cg-Dock7 + / +Lepr / J(db / db) male obese mice were used as experimental subjects (The Jackson Laboratory). Firstly, the experimental animals were starved for 18 hours, and then intraperitoneally injected with 25nmol / kg polypeptide concentration (SEQ.ID NO.1, SEQ.ID NO.2, Exendin-4) and 18mmol / kg glucose concentration. The control group was injected with normal saline. Blood glucose concentrations were measured before injection and then at 15-minute intervals (0 min, 15 min, 30 min, 45 min, 60 min). Rat tail blood was collected and analyzed by enzyme-linked immunosorbent assay (ELISA) to determine the concentration of insulin, and a blood glucose tester was used to me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com