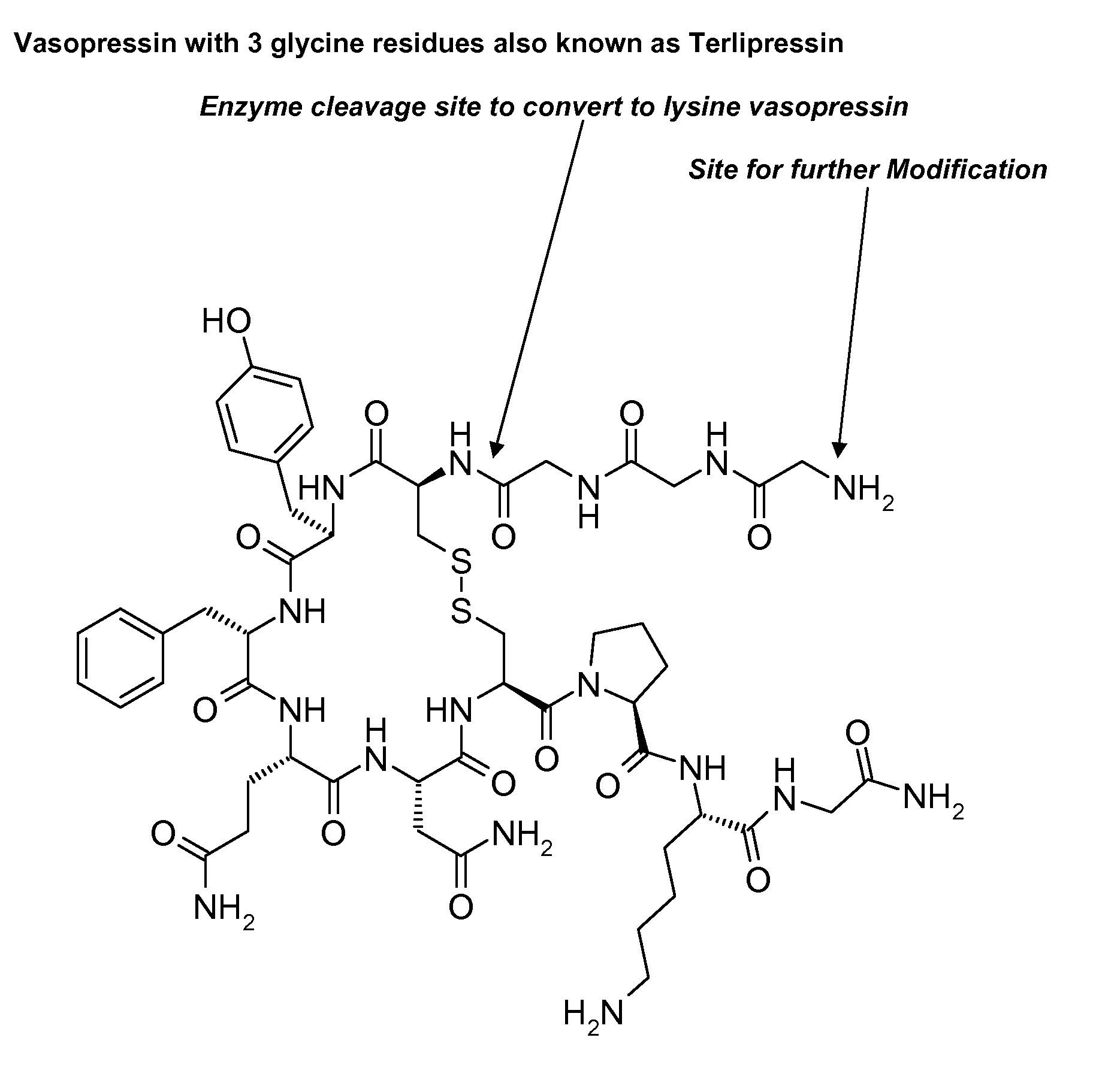
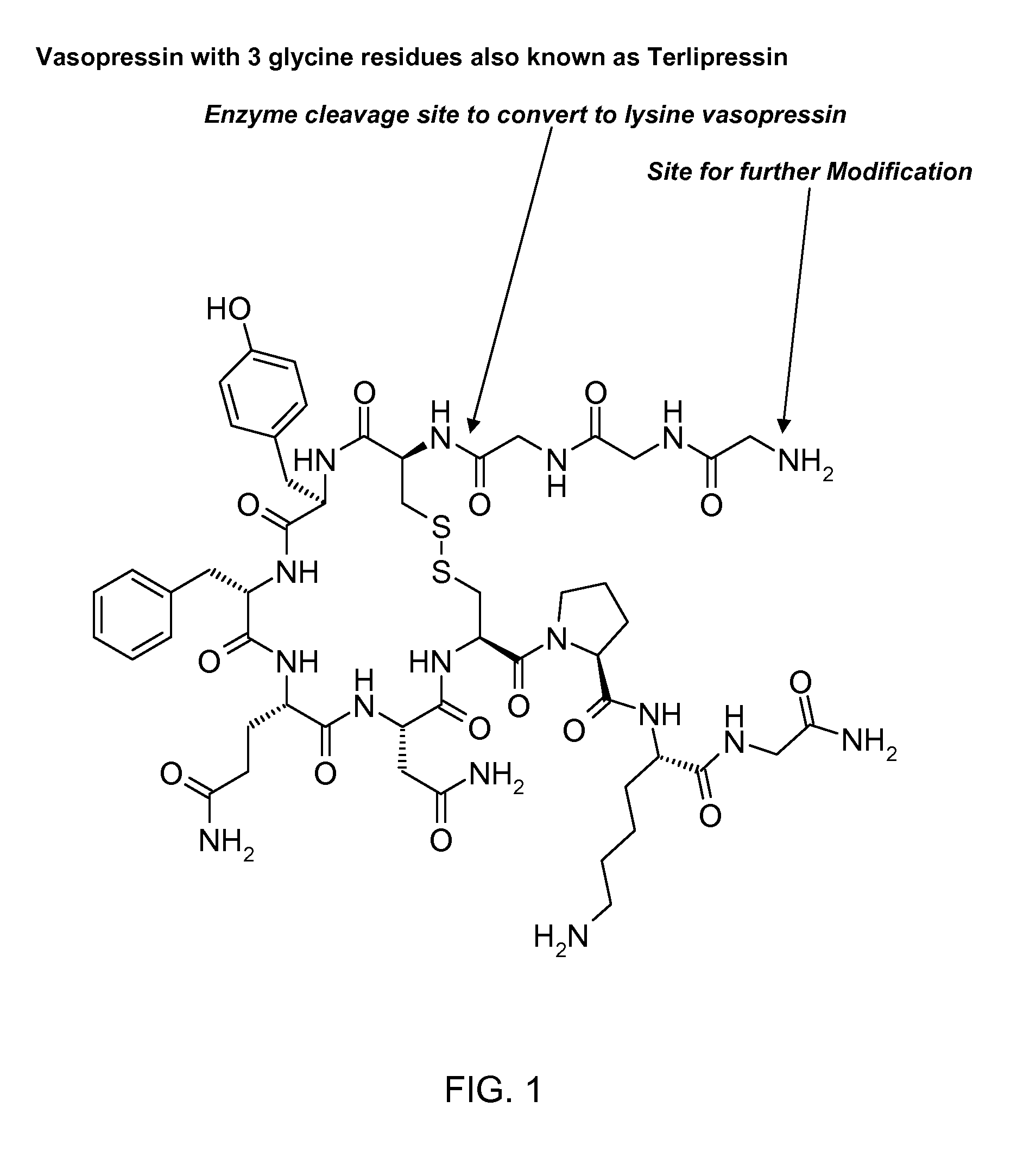
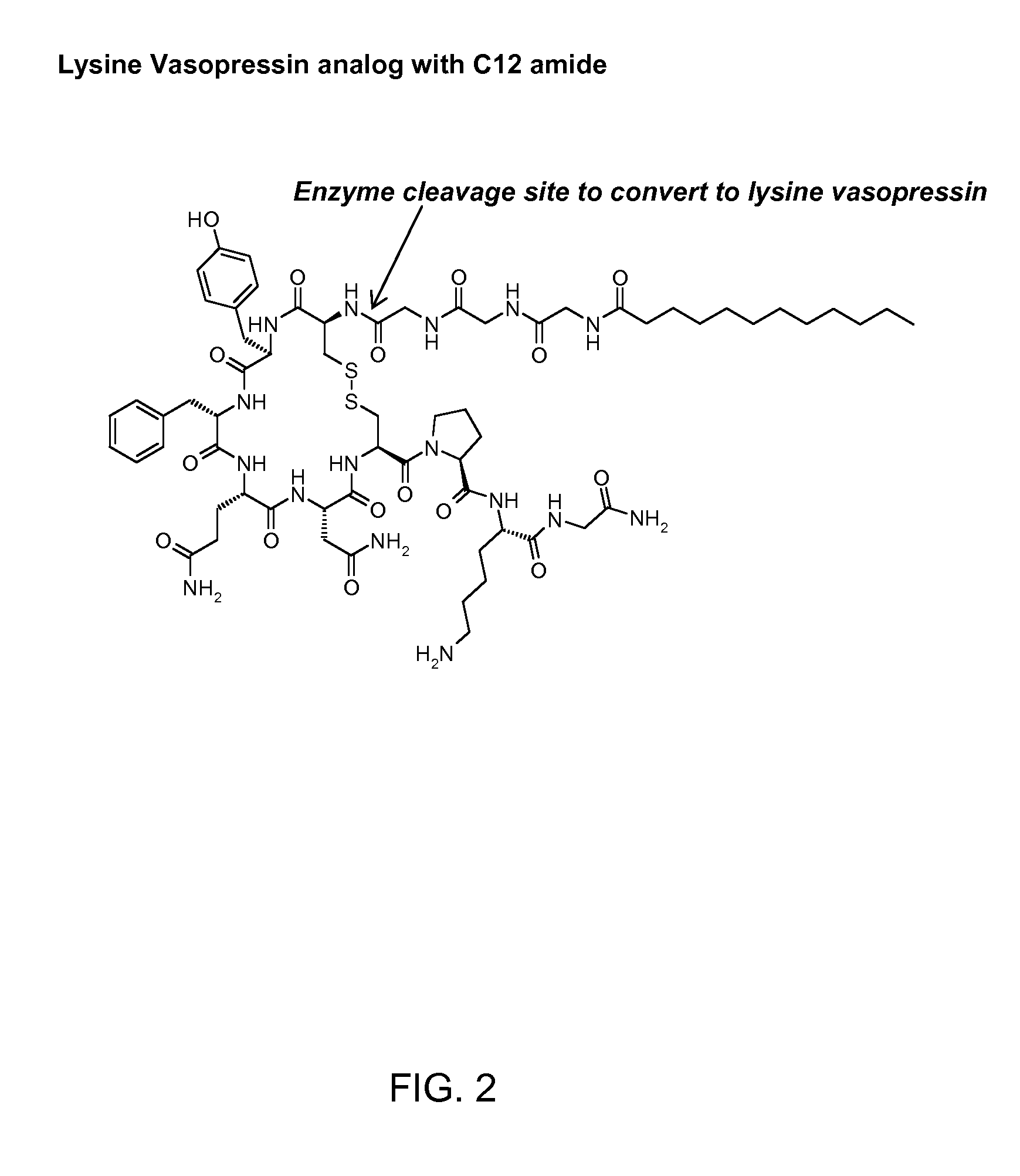
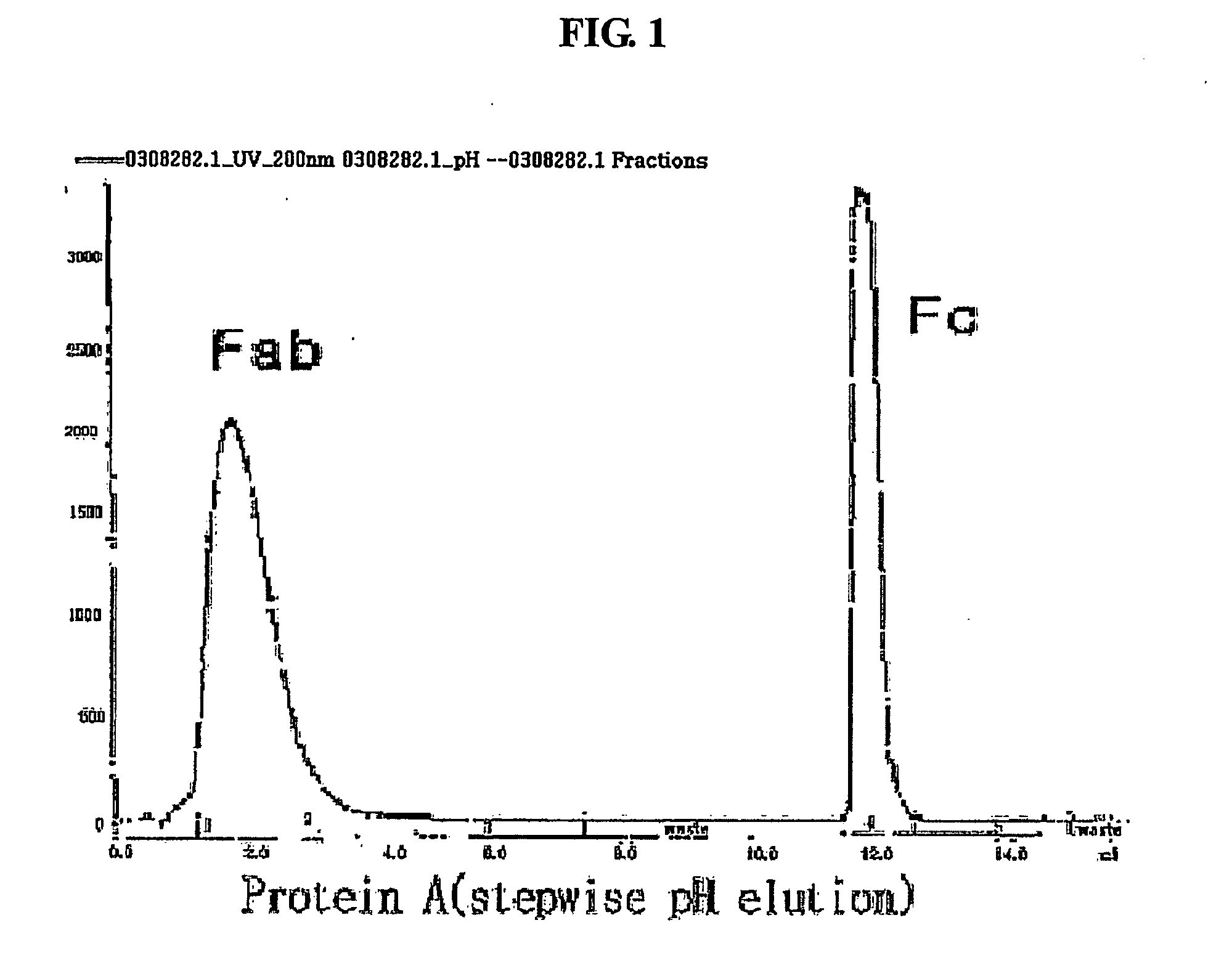
Patents
Literature
4334 results about "Long acting" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
(lɔŋ æktɪŋ) adjective. (Pharmaceutical: Administration) A long-acting drug is slowly absorbed after being administered, and maintains its effects over a long period of time. Long-acting drugs, in which the active ingredients are released slowly, are often administered for the long-term relief of chronic pain.
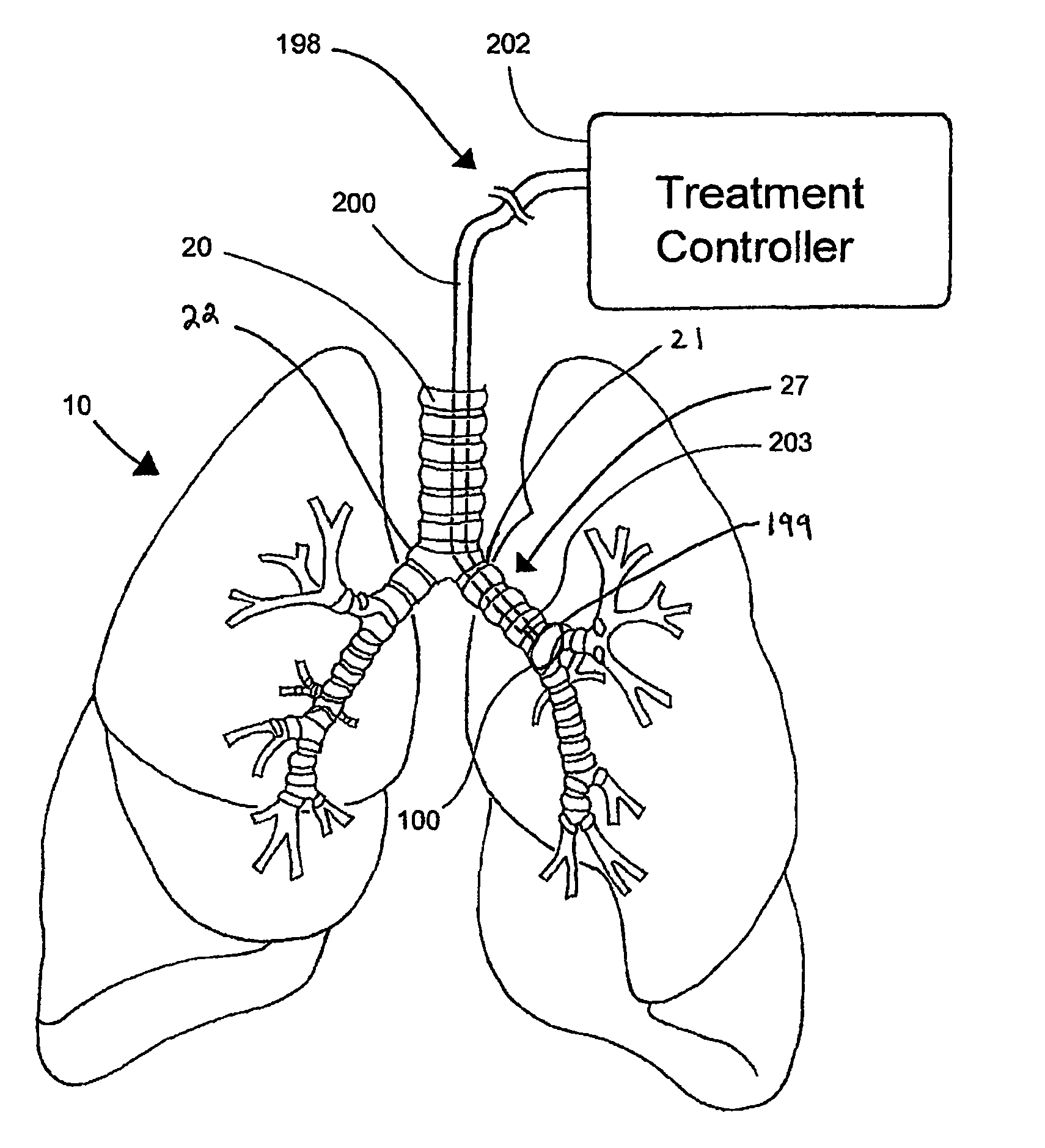
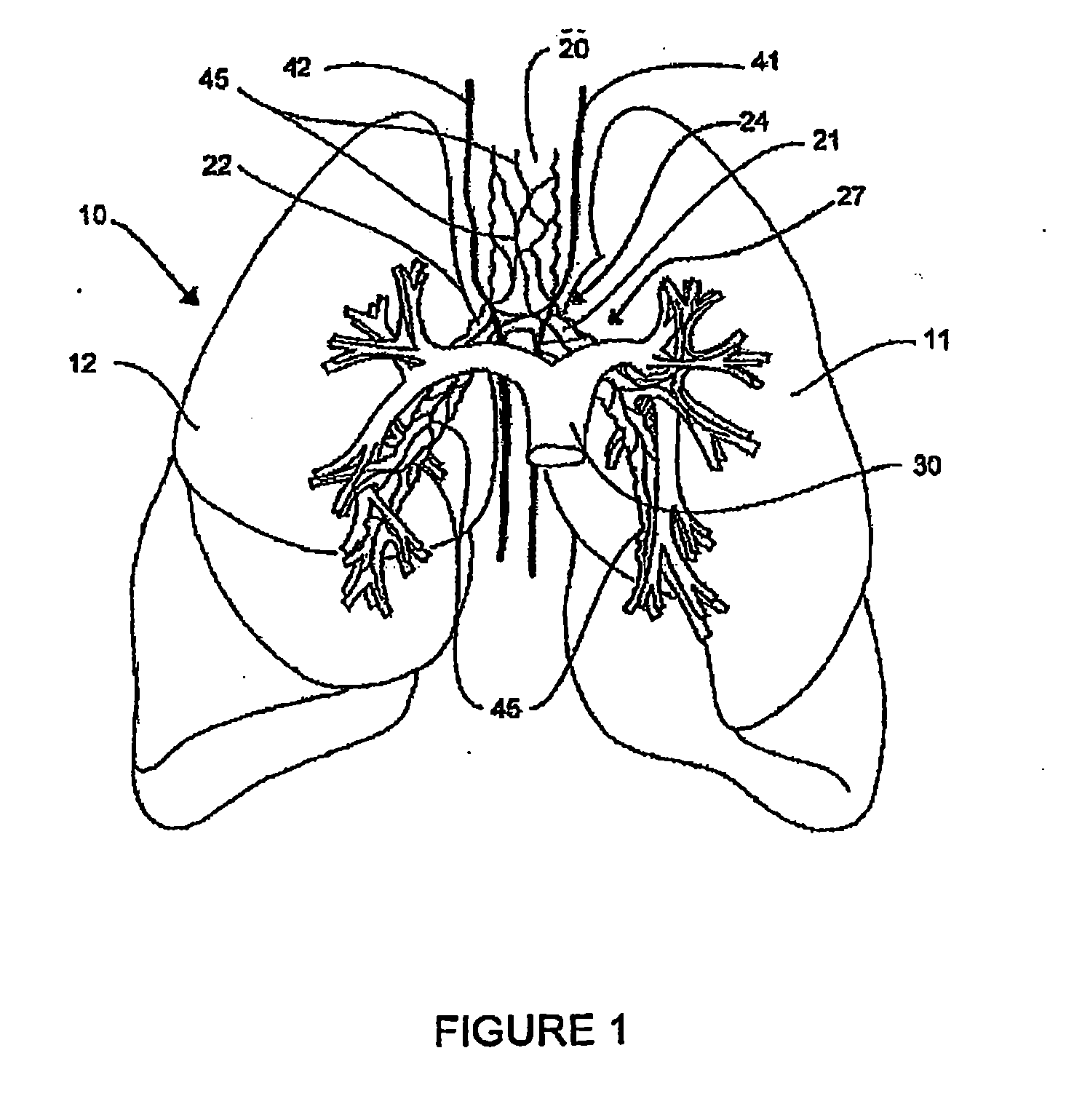
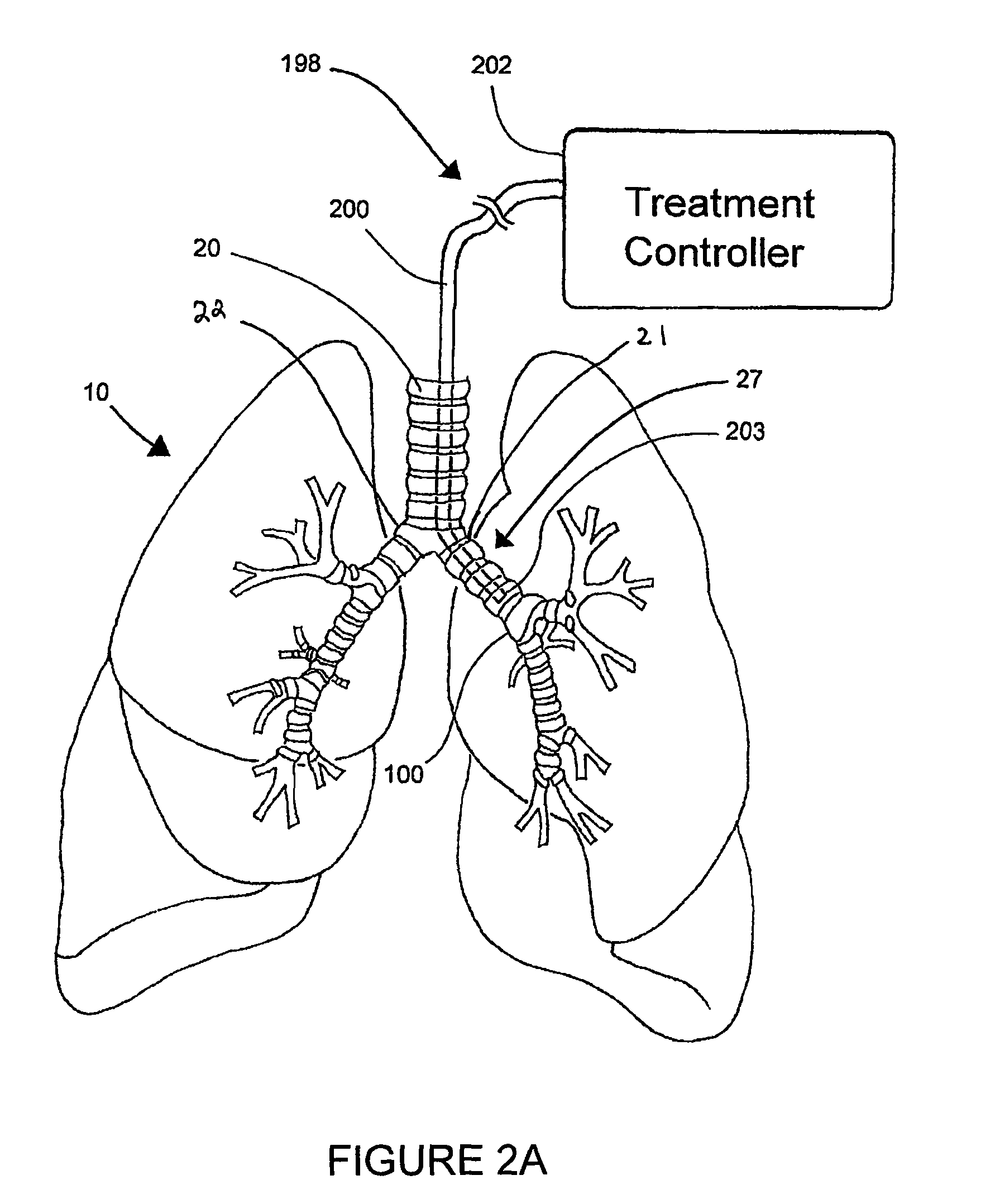
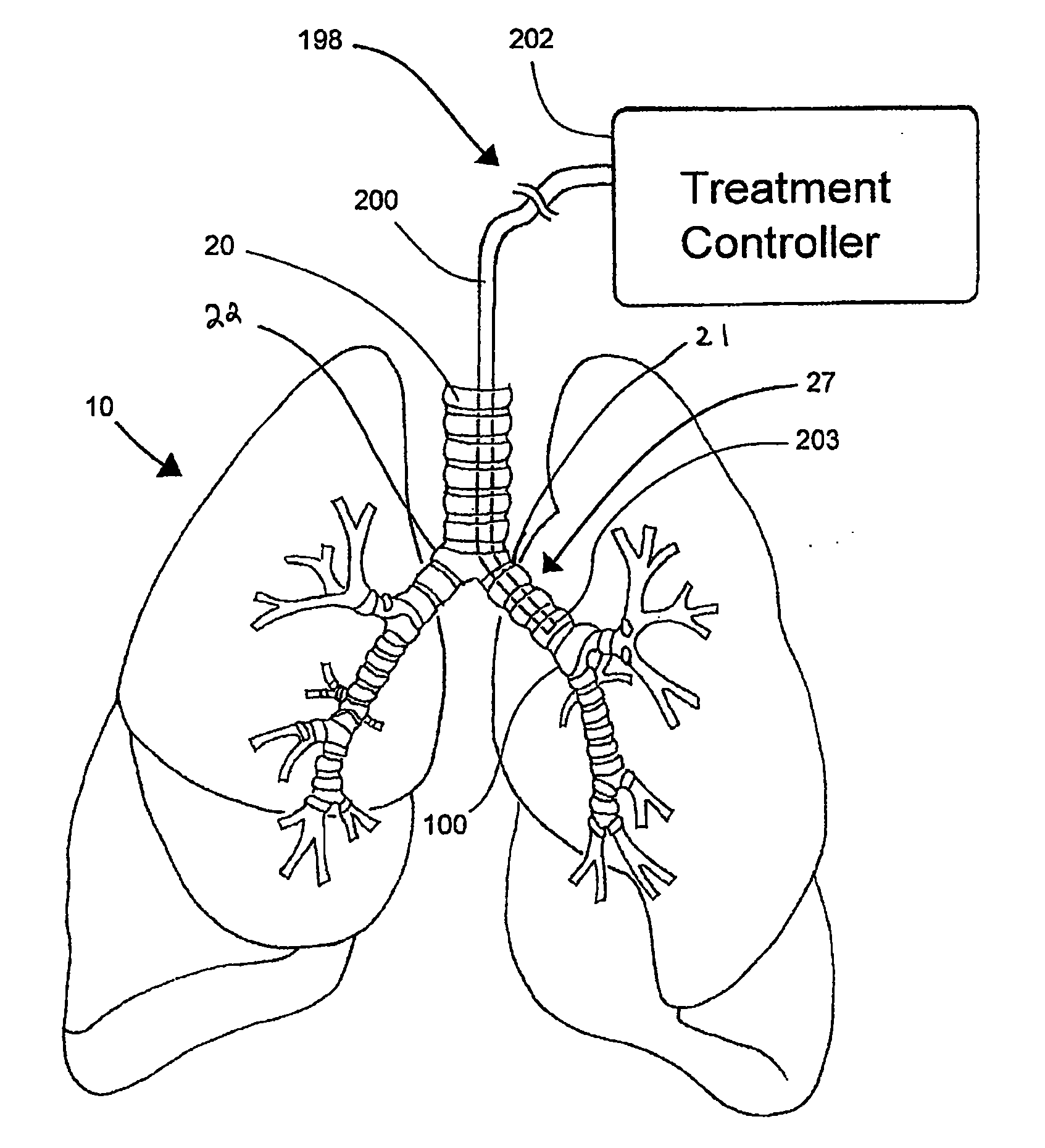
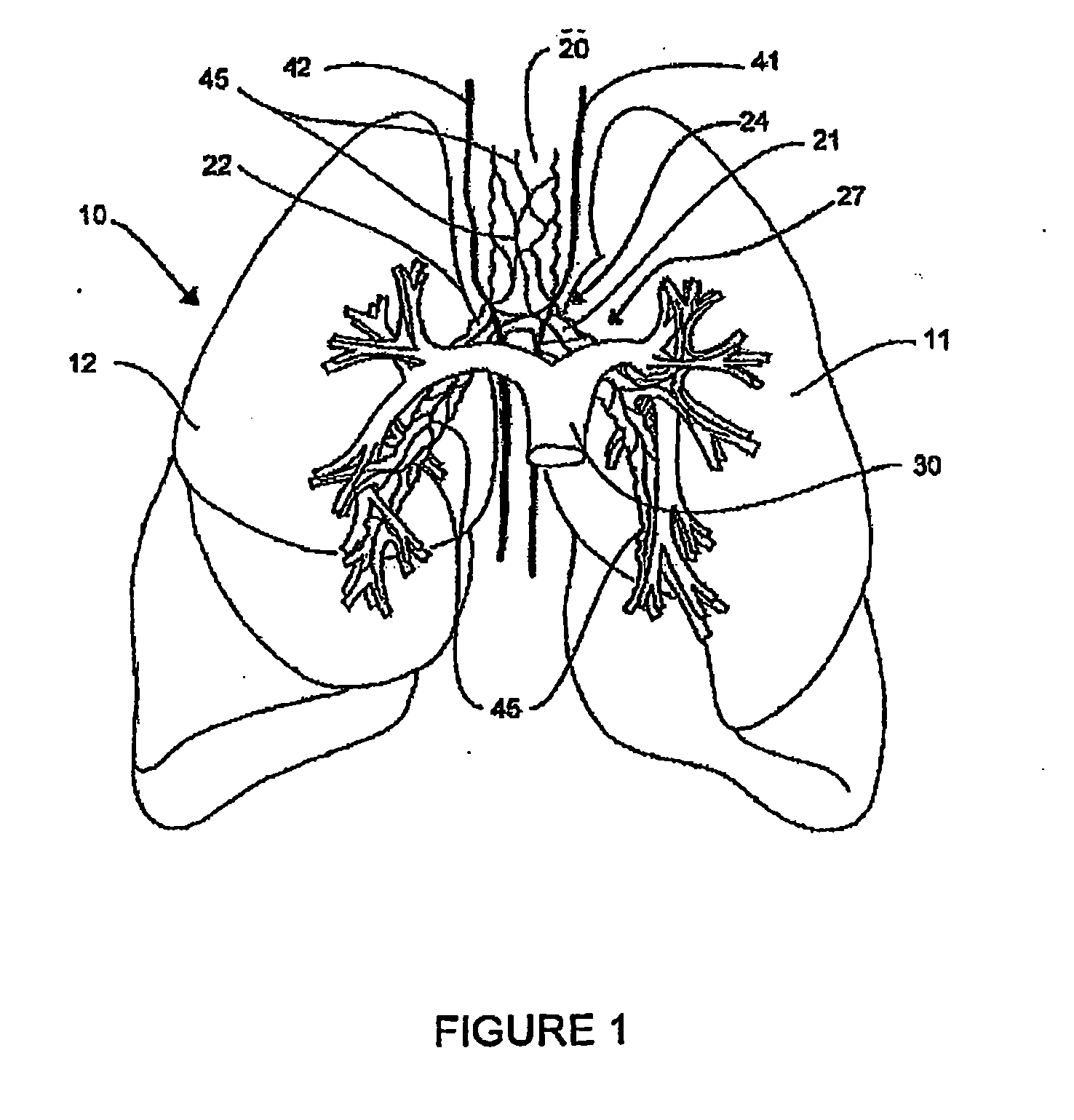
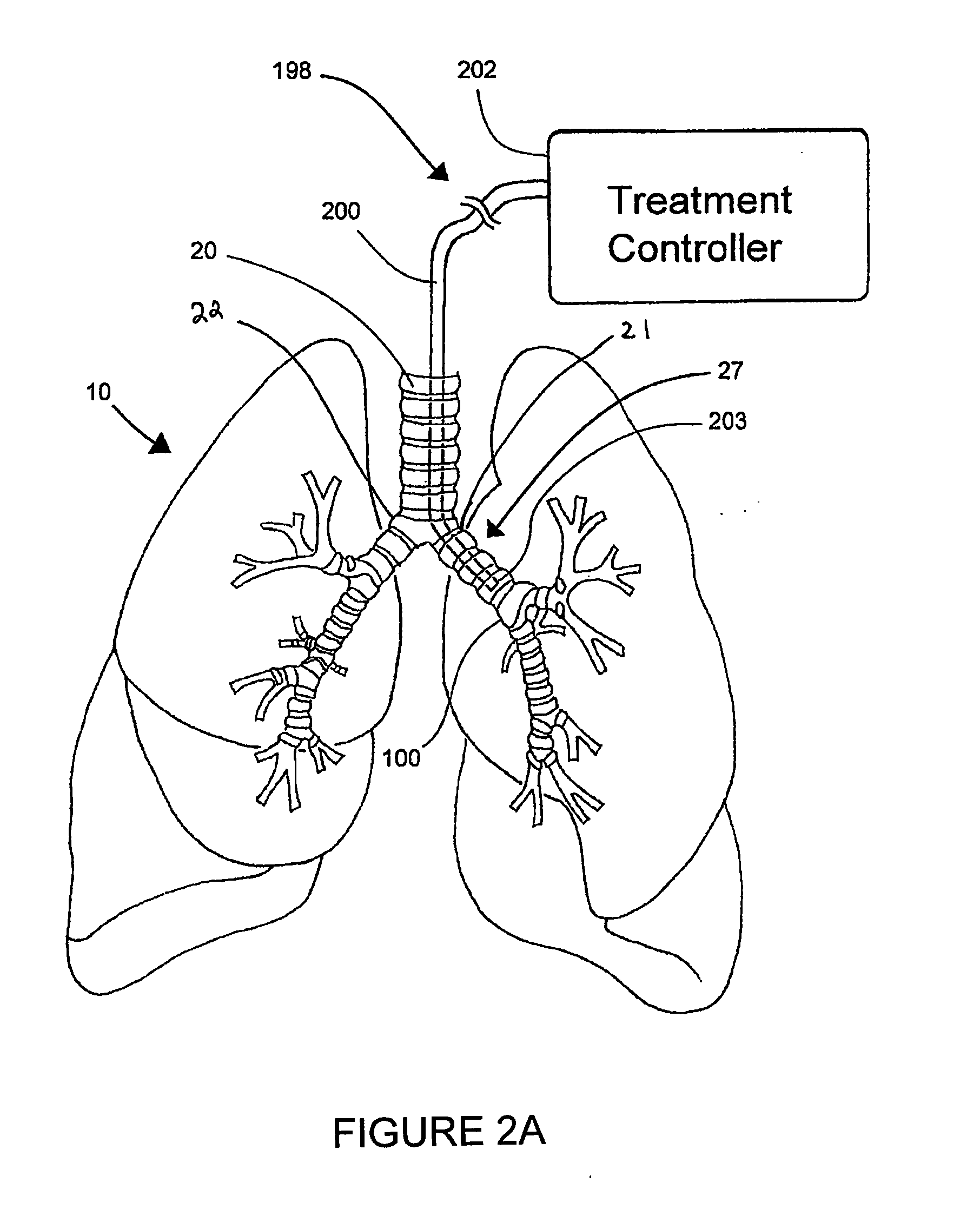
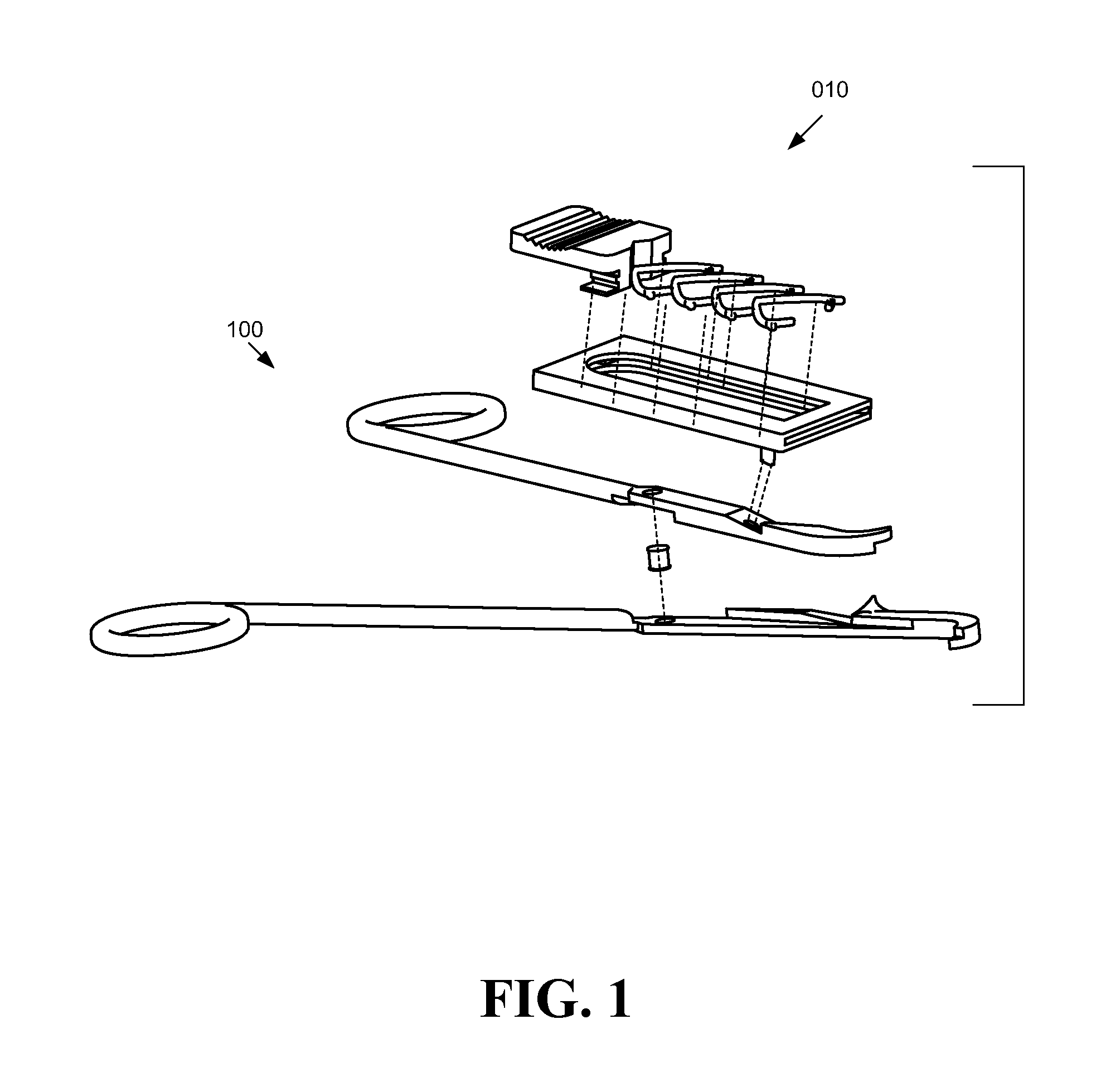
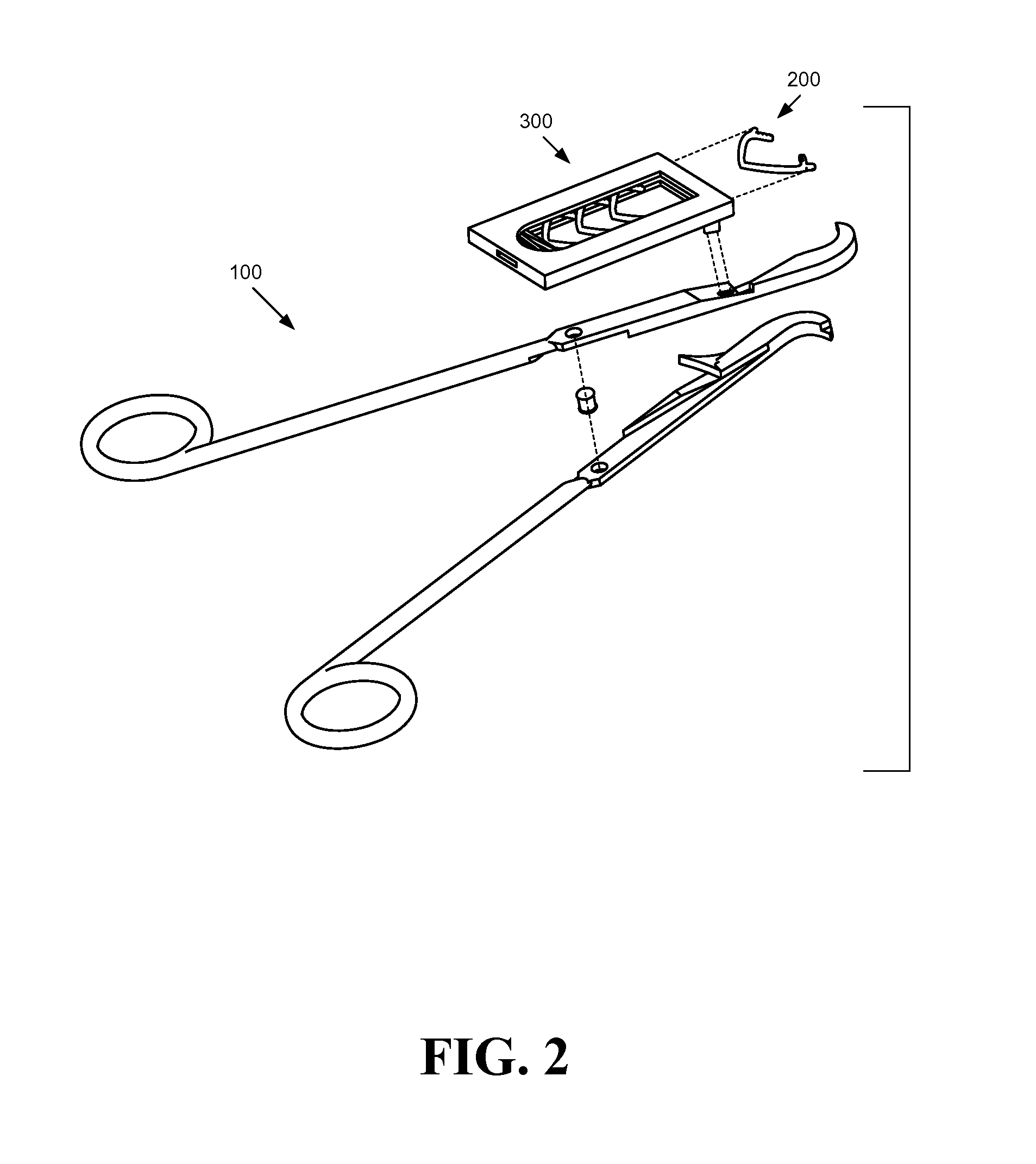
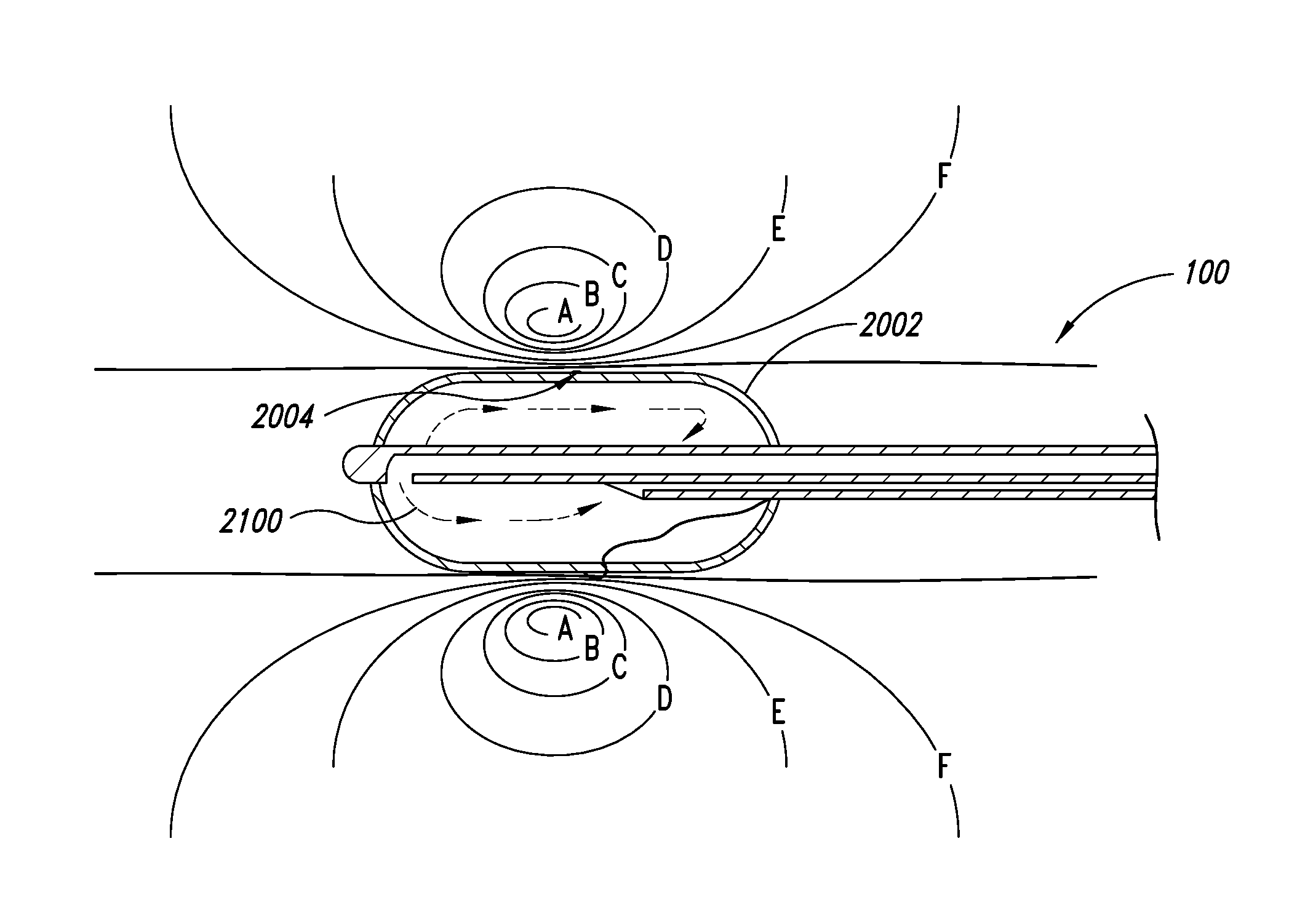
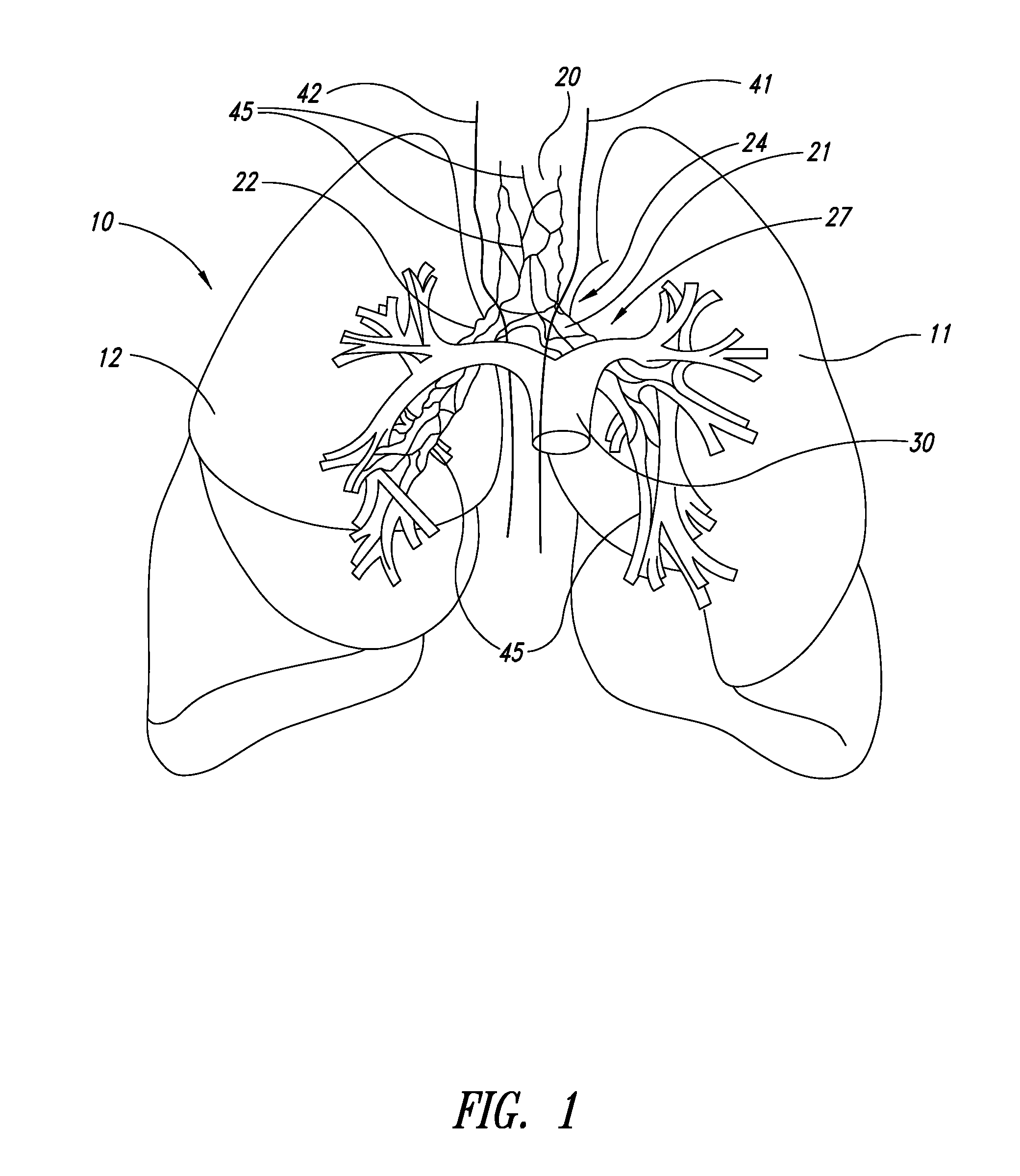
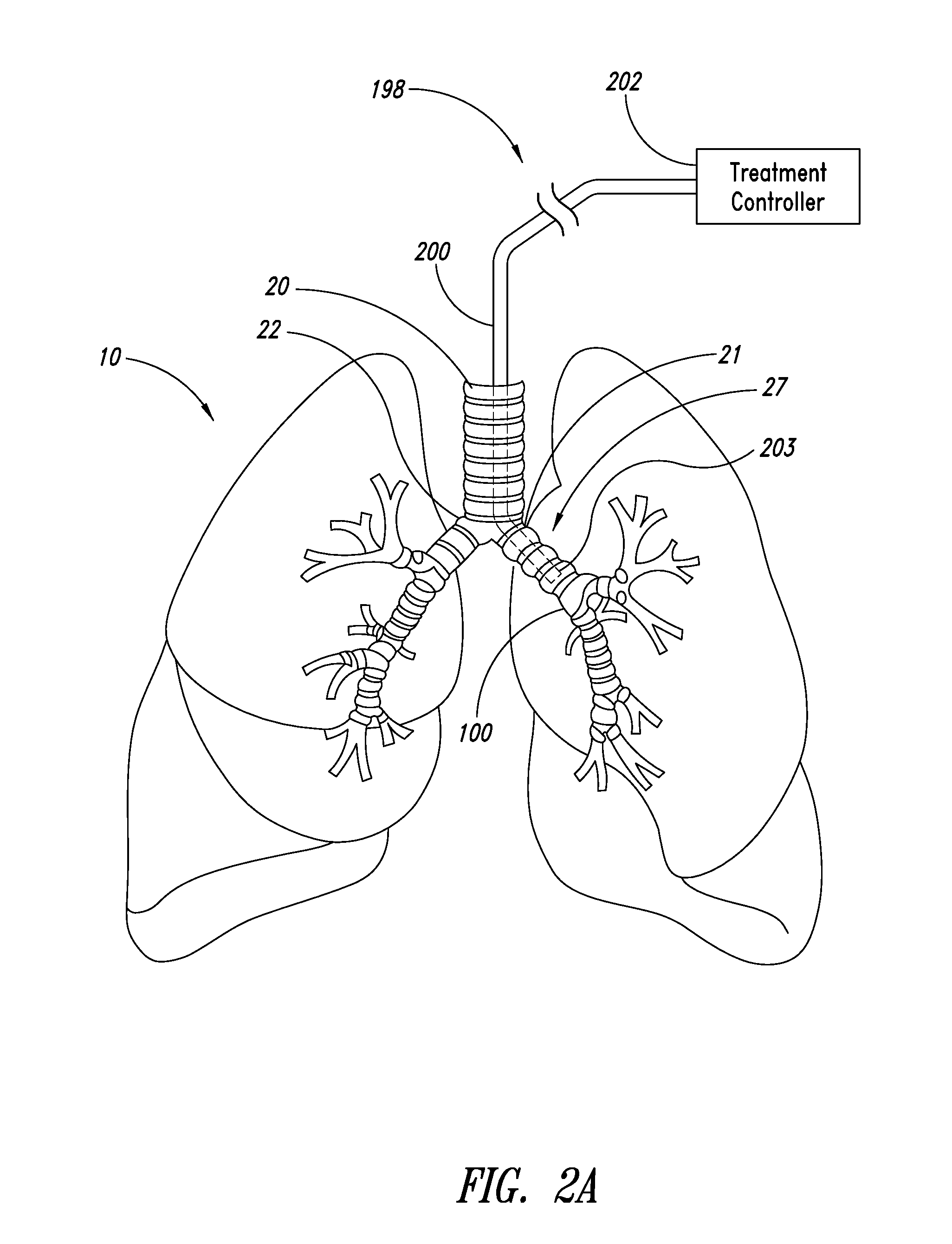
Systems, assemblies, and methods for treating a bronchial tree
ActiveUS8088127B2Improve the immunityWithout eliminating smooth muscle toneUltrasound therapySurgical needlesNervous systemCell membrane
Systems, assemblies, and methods to treat pulmonary diseases are used to decrease nervous system input to distal regions of the bronchial tree within the lungs. Treatment systems damage nerve tissue to temporarily or permanently decrease nervous system input. The treatment systems are capable of heating nerve tissue, cooling the nerve tissue, delivering a flowable substance that cause trauma to the nerve tissue, puncturing the nerve tissue, tearing the nerve tissue, cutting the nerve tissue, applying pressure to the nerve tissue, applying ultrasound to the nerve tissue, applying ionizing radiation to the nerve tissue, disrupting cell membranes of nerve tissue with electrical energy, or delivering long acting nerve blocking chemicals to the nerve tissue.
Owner:HOLAIRA INC
Systems, assemblies, and methods for treating a bronchial tree
ActiveUS20090306644A1Improve the immunityWithout eliminating smooth muscle toneUltrasound therapyDiagnosticsNervous systemCell membrane
Systems, assemblies, and methods to treat pulmonary diseases are used to decrease nervous system input to distal regions of the bronchial tree within the lungs. Treatment systems damage nerve tissue to temporarily or permanently decrease nervous system input. The treatment systems are capable of heating nerve tissue, cooling the nerve tissue, delivering a flowable substance that cause trauma to the nerve tissue, puncturing the nerve tissue, tearing the nerve tissue, cutting the nerve tissue, applying pressure to the nerve tissue, applying ultrasound to the nerve tissue, applying ionizing radiation to the nerve tissue, disrupting cell membranes of nerve tissue with electrical energy, or delivering long acting nerve blocking chemicals to the nerve tissue.
Owner:NUVAIRA INC
Methods of treating headaches using 5-HT agonists in combination with long-acting NSAIDs
Owner:POZEN INC
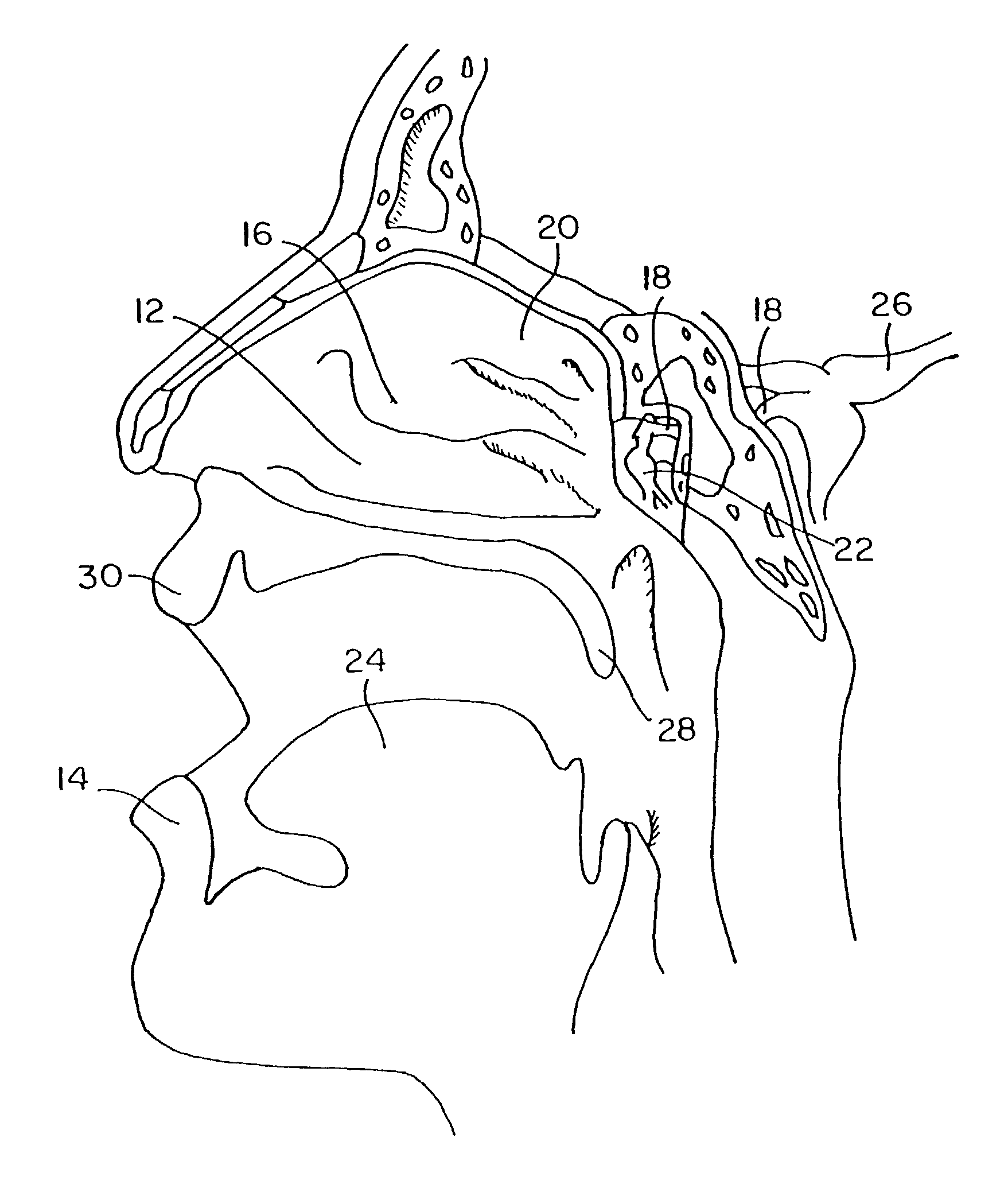
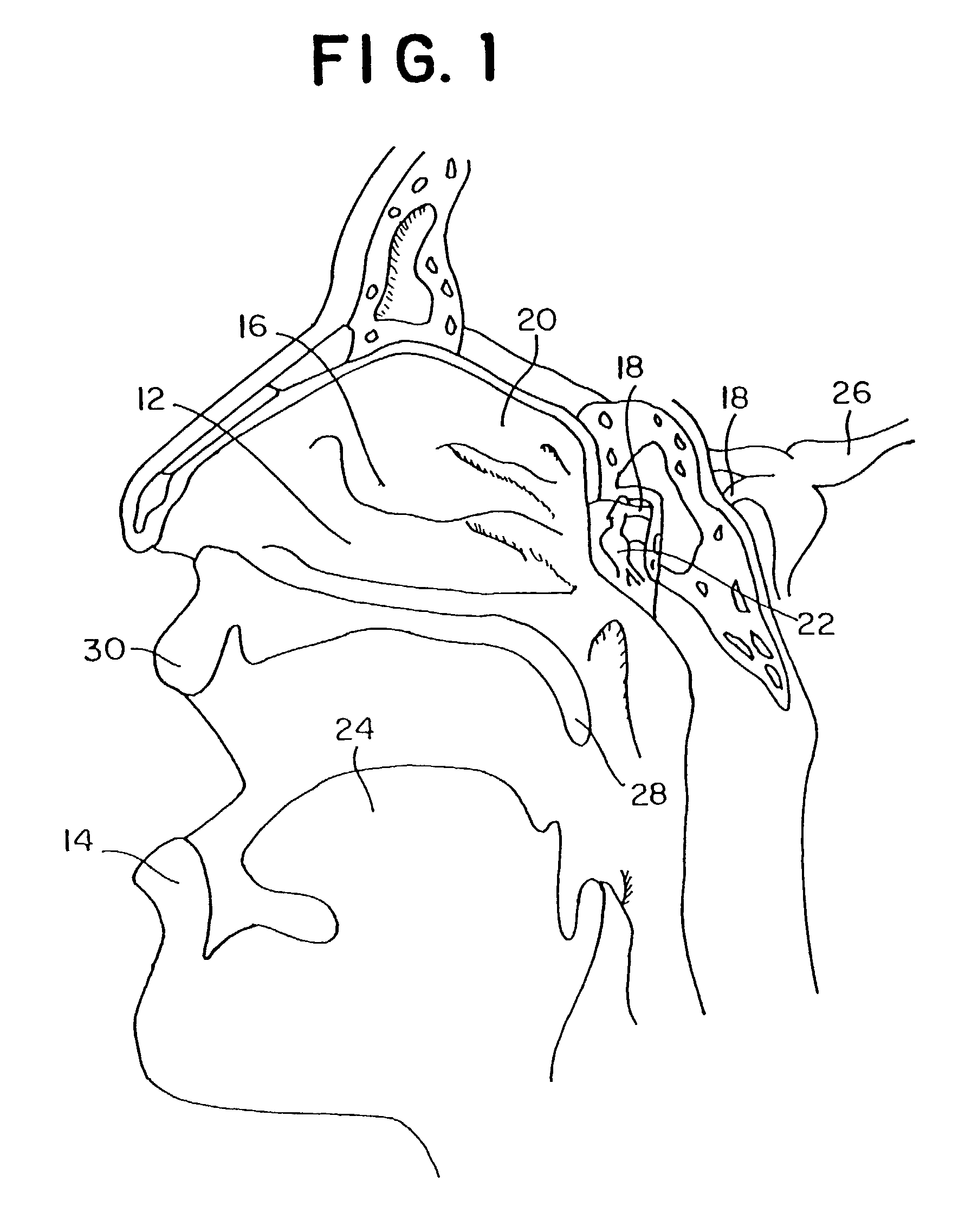
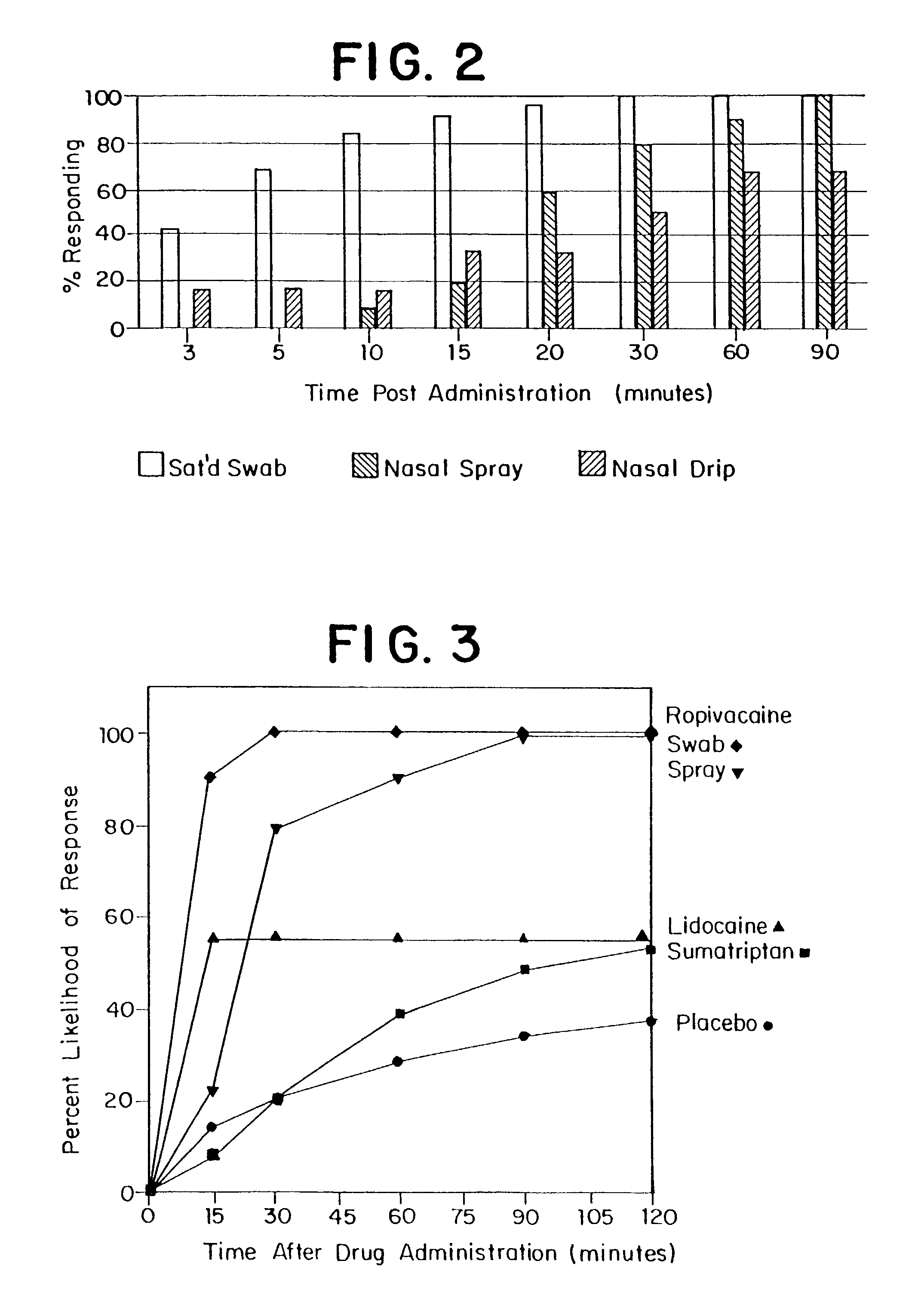
Method for directed intranasal administration of a composition
Methods, kits, apparatus, and compositions for inhibiting a cerebral neurovascular disorder, a muscular headache, or cerebral inflammation in a human patient are provided. The methods comprise intranasally administering to the patient a pharmaceutical composition comprising a local anesthetic, and preferably a long-acting local anesthetic ingredient. A composition useful for practicing the methods of the invention is described which comprises at least one local anesthetic in a pharmaceutically acceptable carrier, wherein the composition is formulated for intranasal delivery. Cerebral neurovascular disorders include migraine and cluster headache. Muscular headaches include tension headaches and muscle contraction headaches. A kit comprising the composition and an intranasal applicator and a method of systemically delivering a pharmaceutically active agent to an animal are also included in the invention. Apparatus for directed intranasal administration of the compositions of the invention and for performing the methods of the invention are also described.
Owner:BHL PATENT HLDG
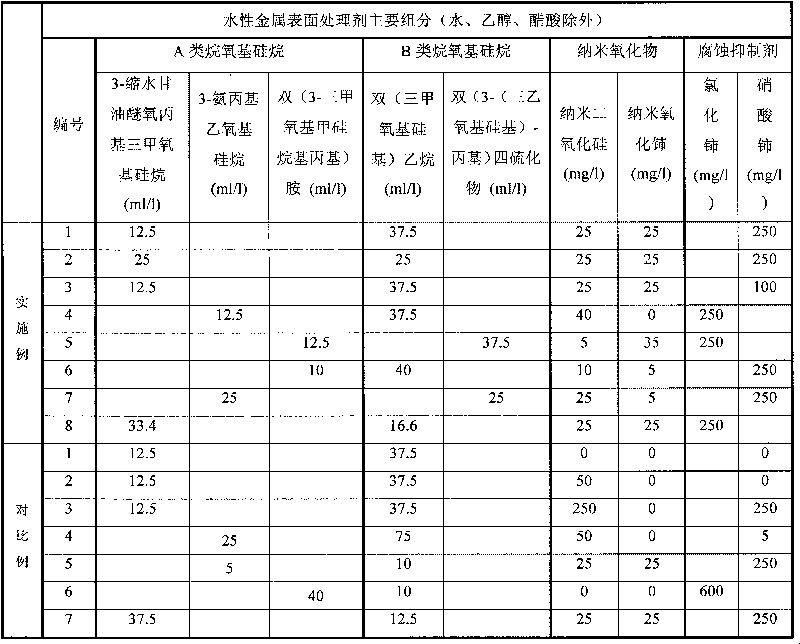
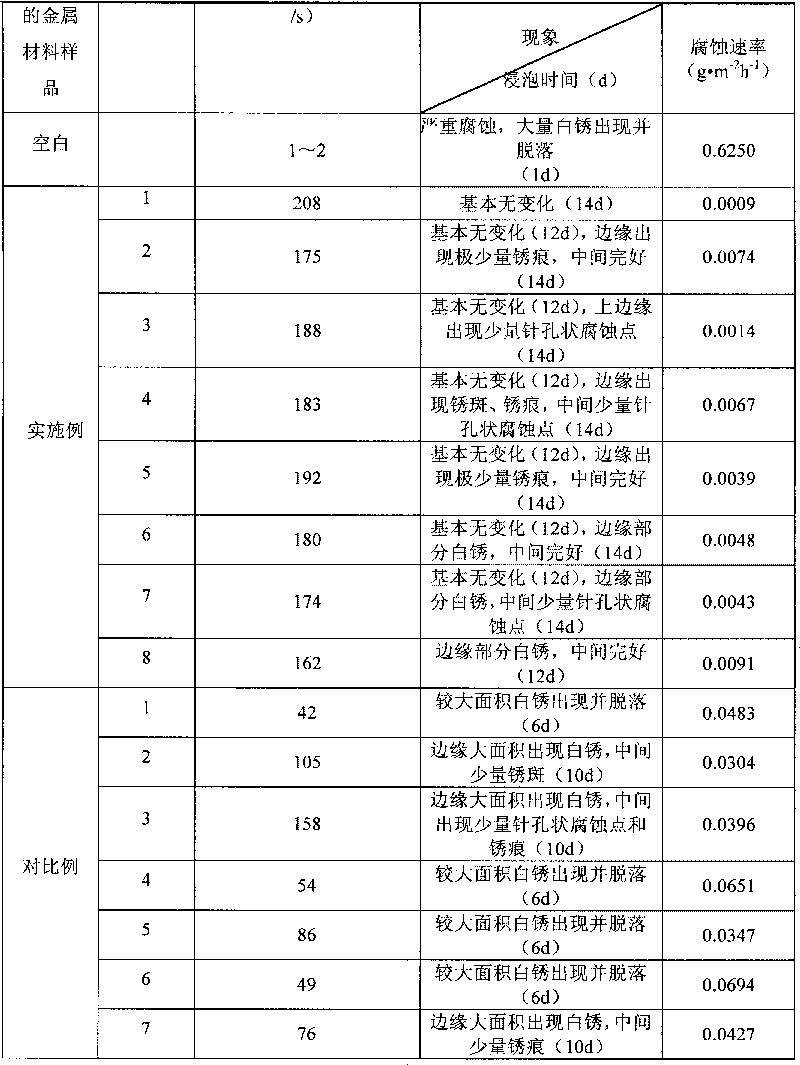
Environment-friendly nano water-based silane treatment agent capable of improving anti-corrosion performance of metal surface
ActiveCN101717930ASimple processApplicable industrial scaleMetallic material coating processesWater basedEpoxy
The invention discloses an environment-friendly nano water-based silane treatment agent capable of improving anti-corrosion performance of metal surface. The treatment agent is water-based silane solution which consists of at least one alkoxy silane containing epoxy or at least one alkoxy silane containing amino, at least one disilyl silane, nano-silicon dioxide, rare earth salt type corrosion inhibitor or rare earth salt type and rare earth nano oxide, water, or acetic acid and a small amount of ethanol. The metal material is coated by using the silane solution for impregnation, brushing, spraying or spin-coating, a siloxane layer is formed on the metal surface, then the long-acting corrosion resistance is formed by curing for 3 hours at the temperature of 100 DEG C, and a nano organic silane film which has close bonding force with a coating is formed. Nanoparticles can not only improve the corrosion resistance and enhance the mechanical strength of the silane film in a coating layer, but also realize the synergistic corrosion resistance with the corrosion inhibitor. The technology has the advantages of simple process, greenness, environmental protection and strong practicality.
Owner:HAISO TECH
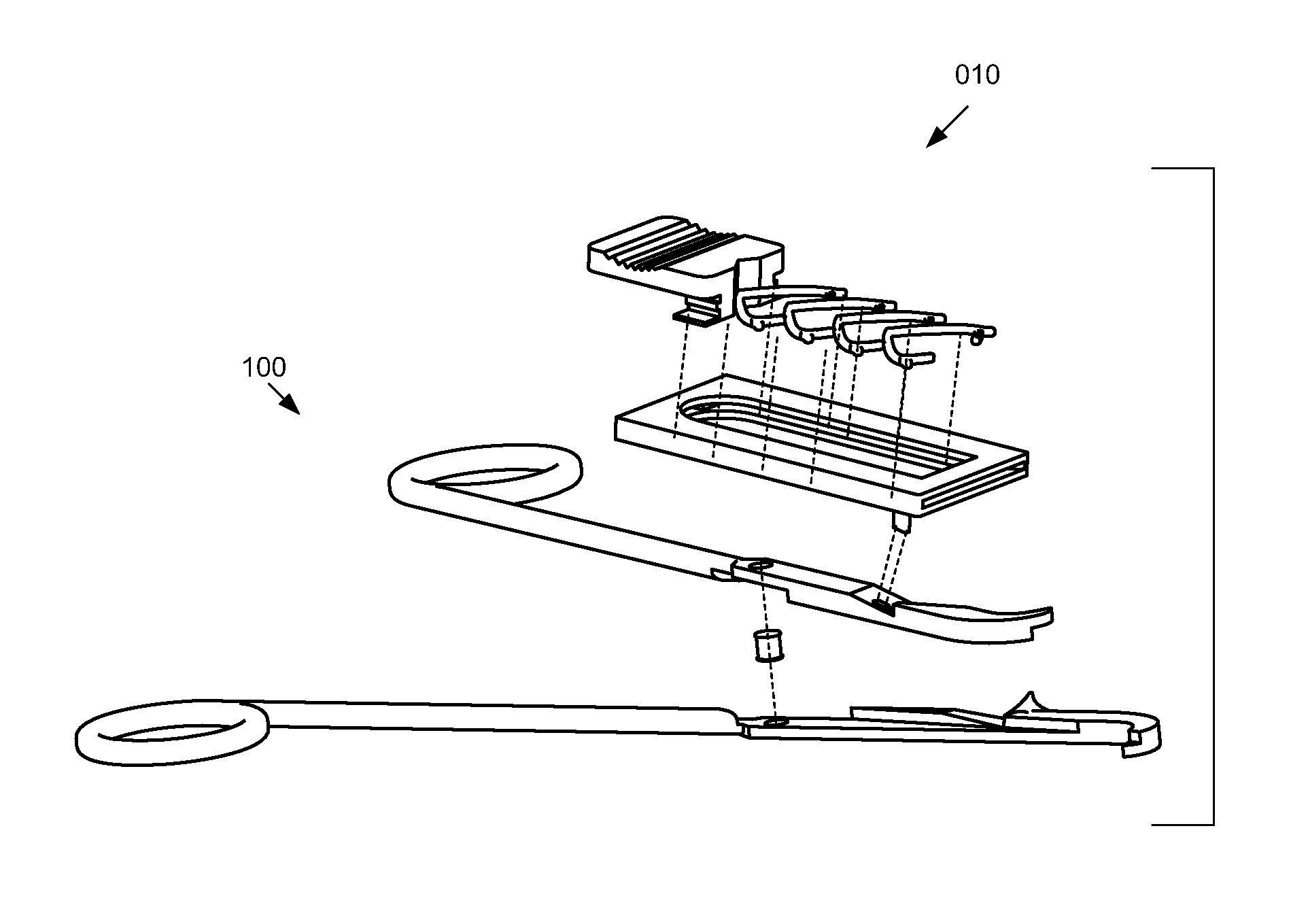
Bio-absorbable tissue closure system
InactiveUS20080103510A1Easy to closeFacilitates rapid closureStaplesNailsRadioactive agentInternal wounds
A surgical fastening system, utilizing surgical clips made from bio-absorbable materials, for use in the rapid closing of deep internal wounds in humans or animals is disclosed. Elements of the system include surgical clips, applicators of surgical clips and dispensers of surgical clips. The surgical clips may contain small amounts of prophylactic antibiotic medication, long-acting time-release pain medication, radio-opaque markers, small amounts of radioactive material, colors, and / or patterns.
Owner:APOGEE SURGICAL INSTR
Systems, assemblies, and methods for treating a bronchial tree
InactiveUS20110257647A1Maintaining the airways ability to moveWithout eliminating smooth muscle toneUltrasound therapySurgical needlesNervous systemCell membrane
Owner:INNOVATIVE PULMONARY SOLUTIONS INC
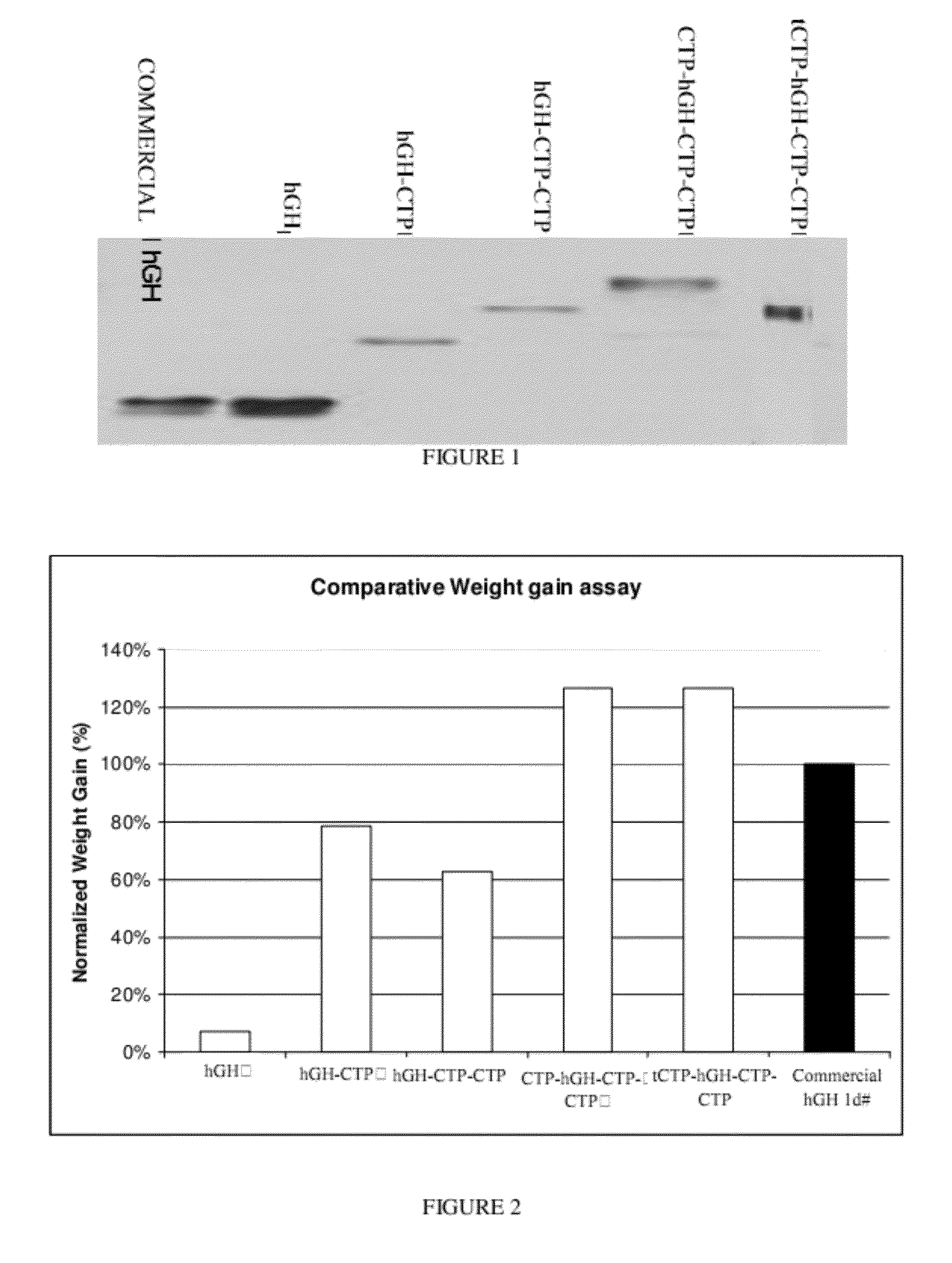
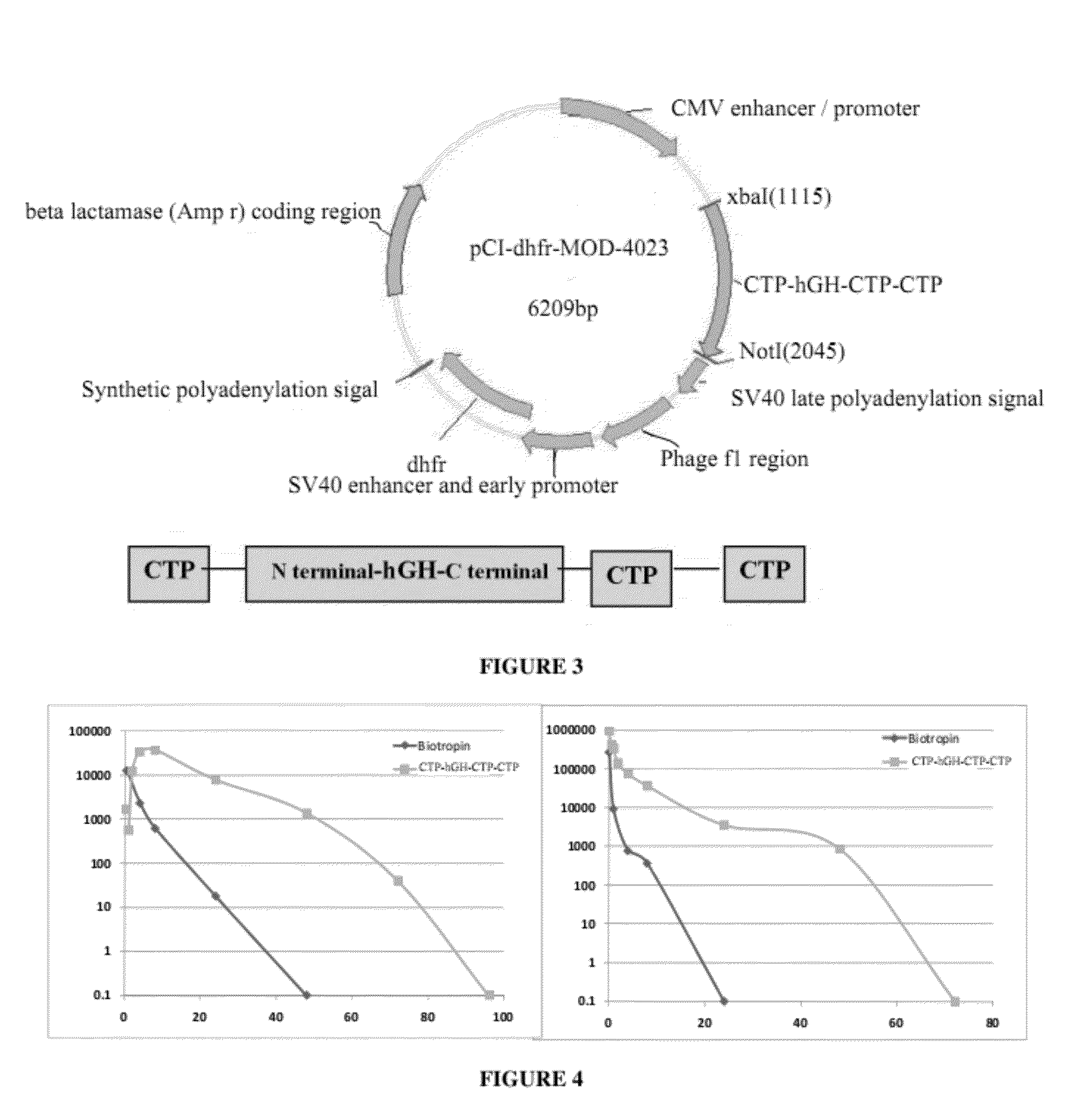
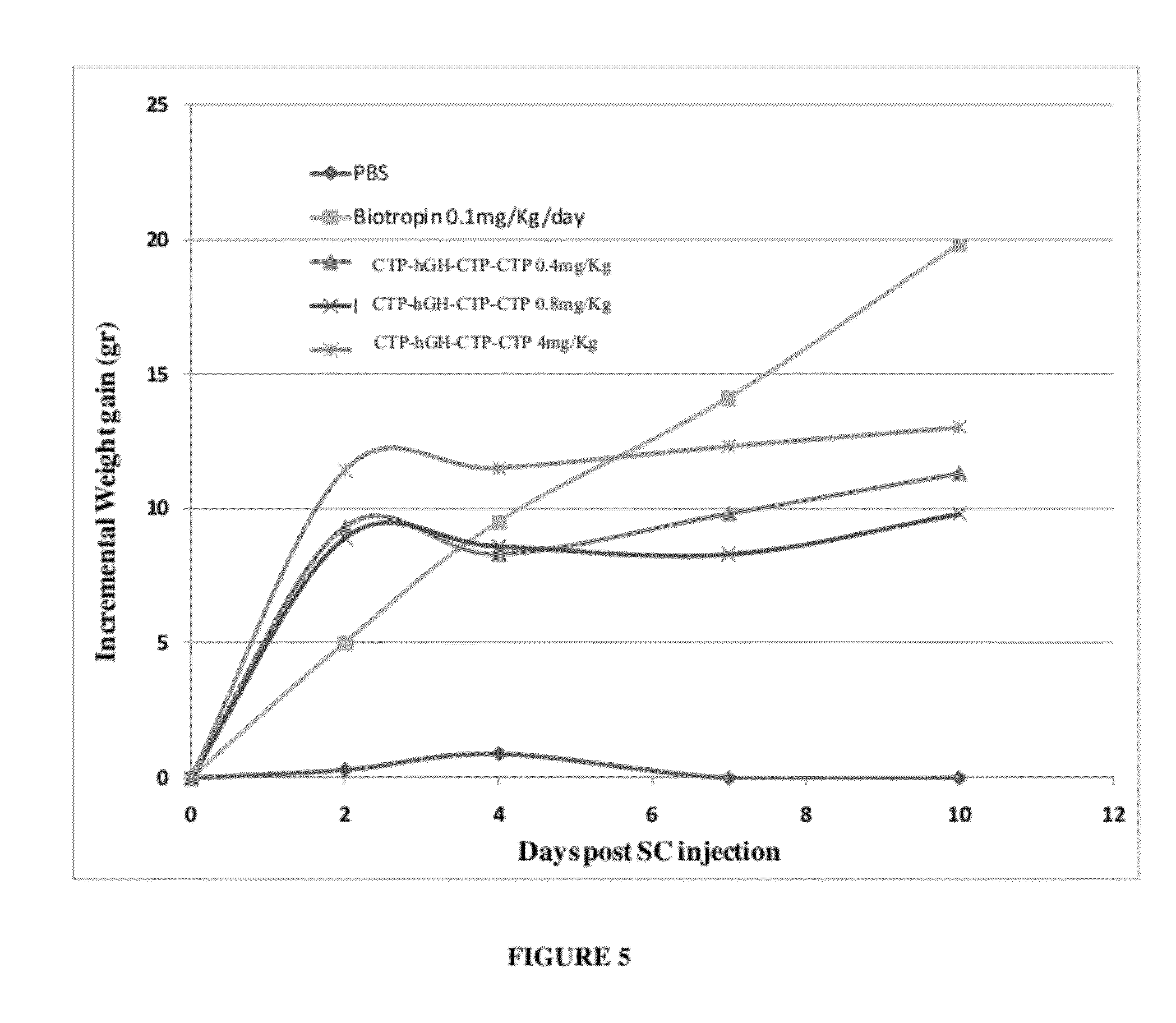
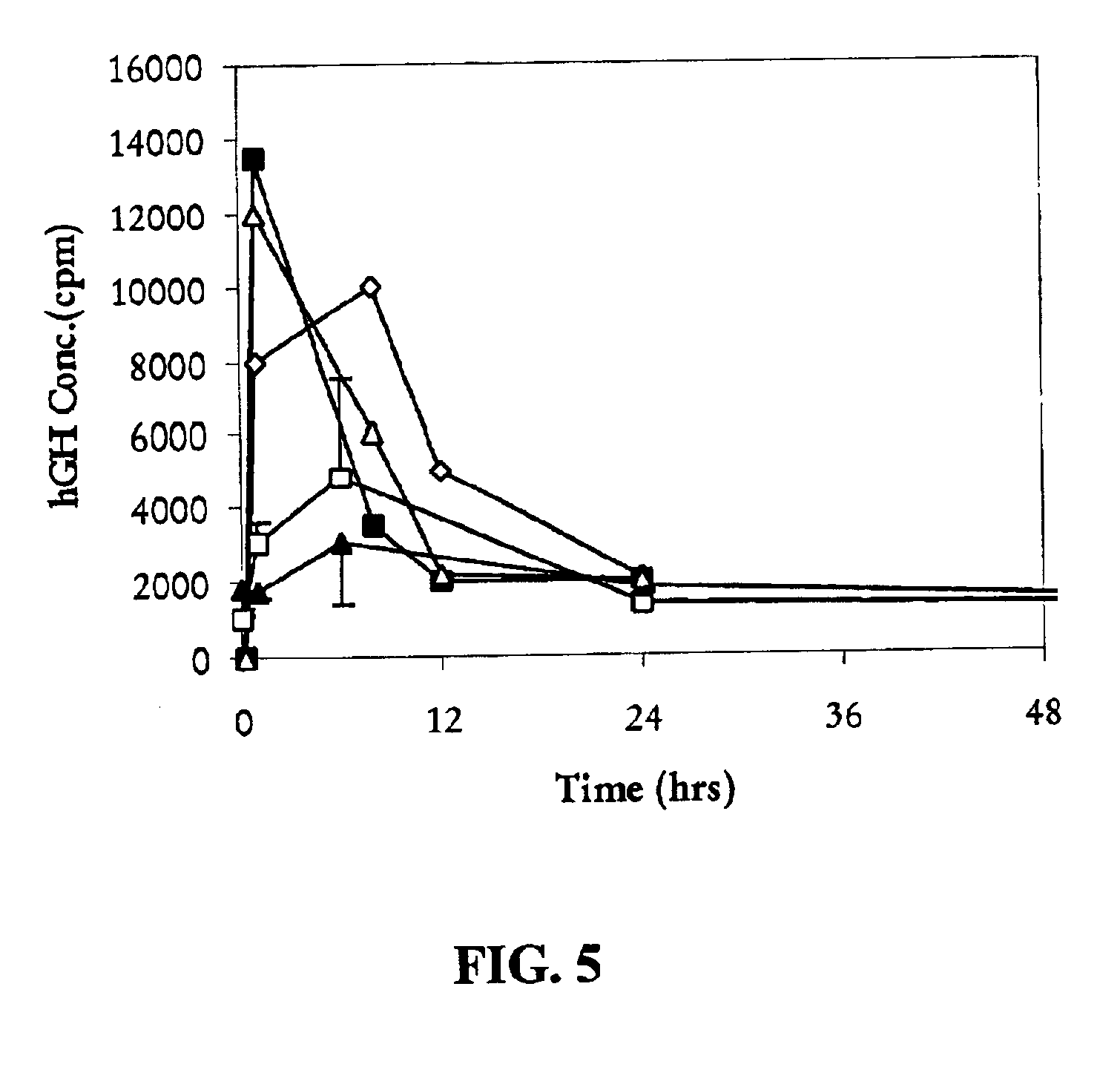
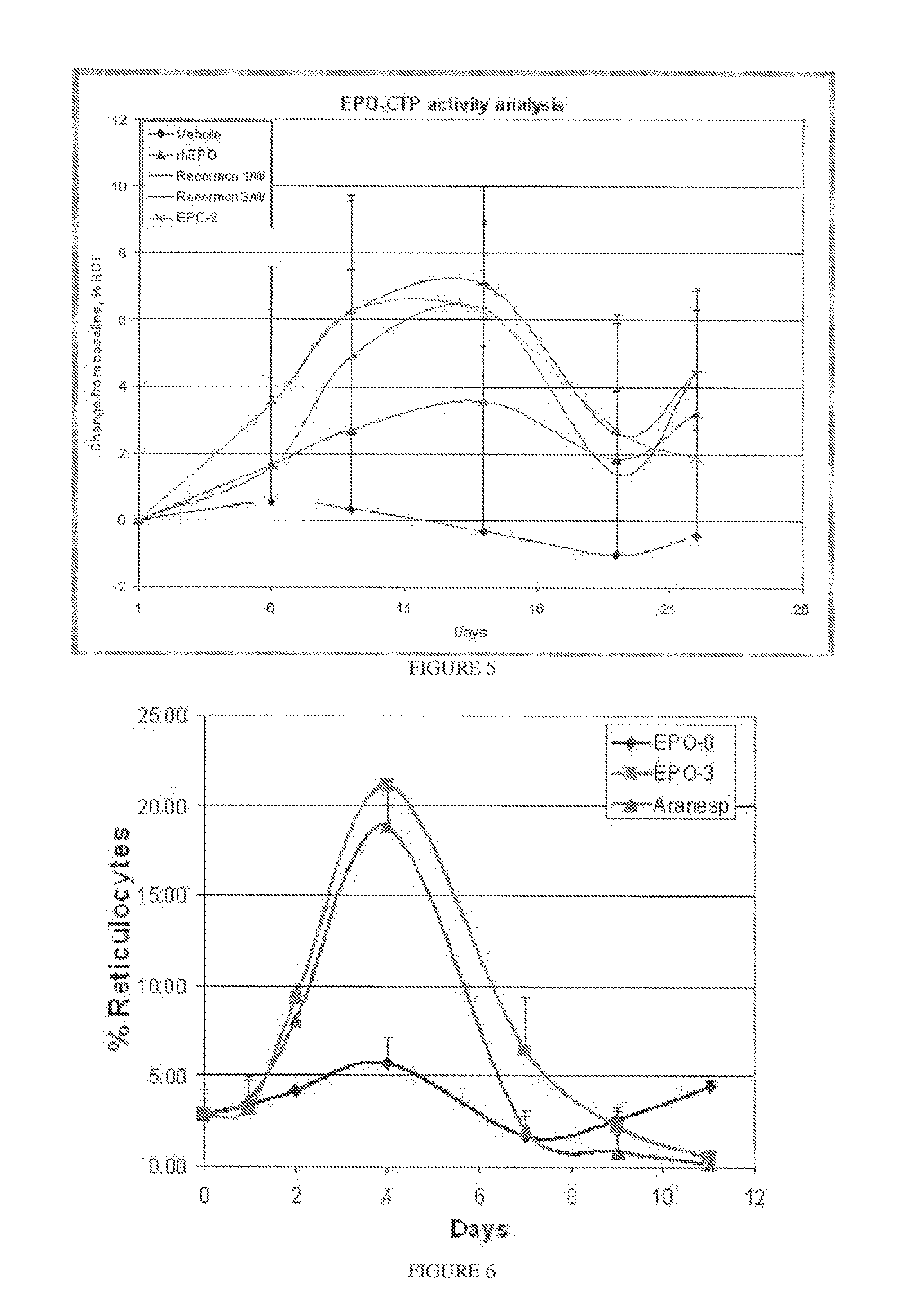
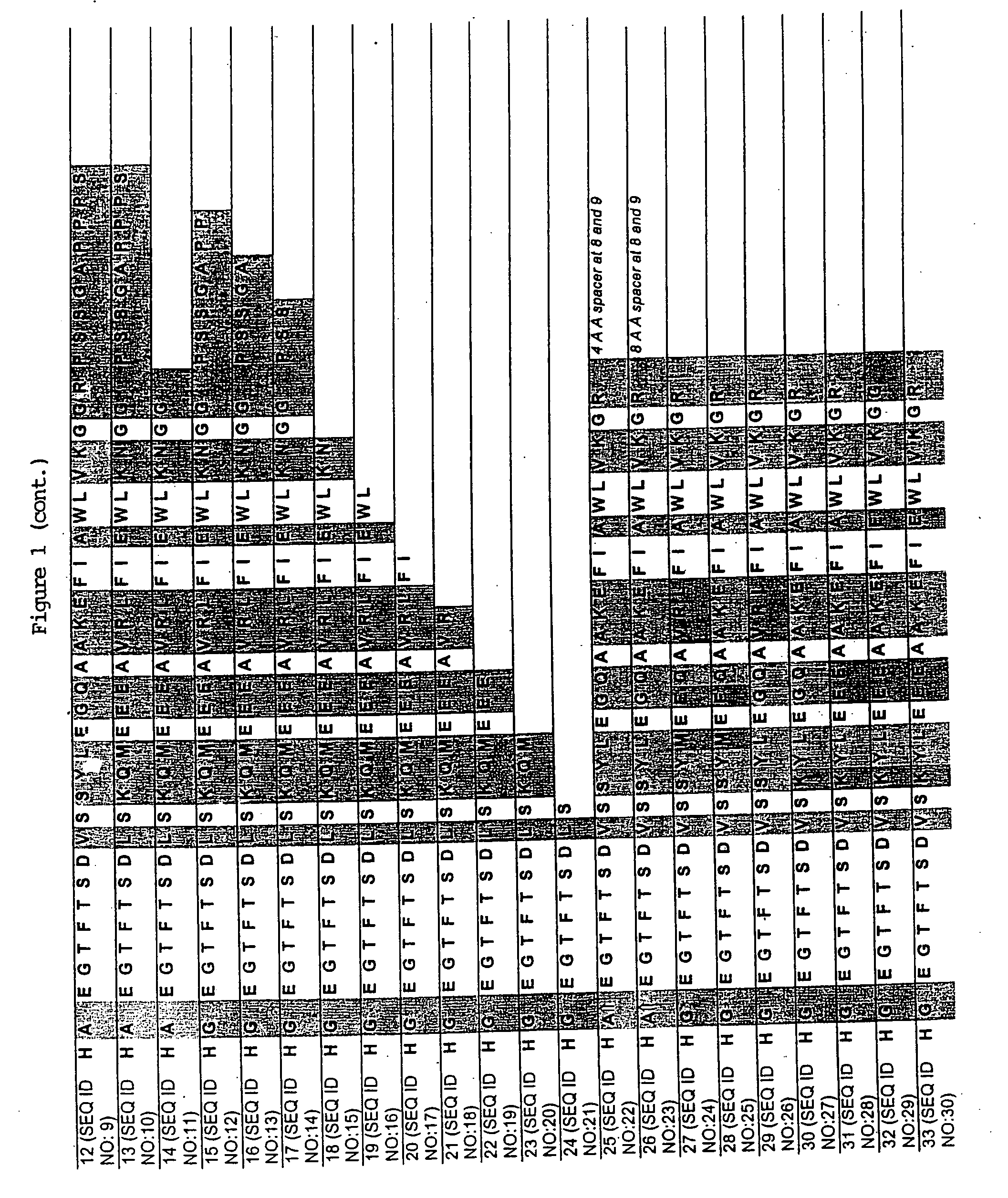
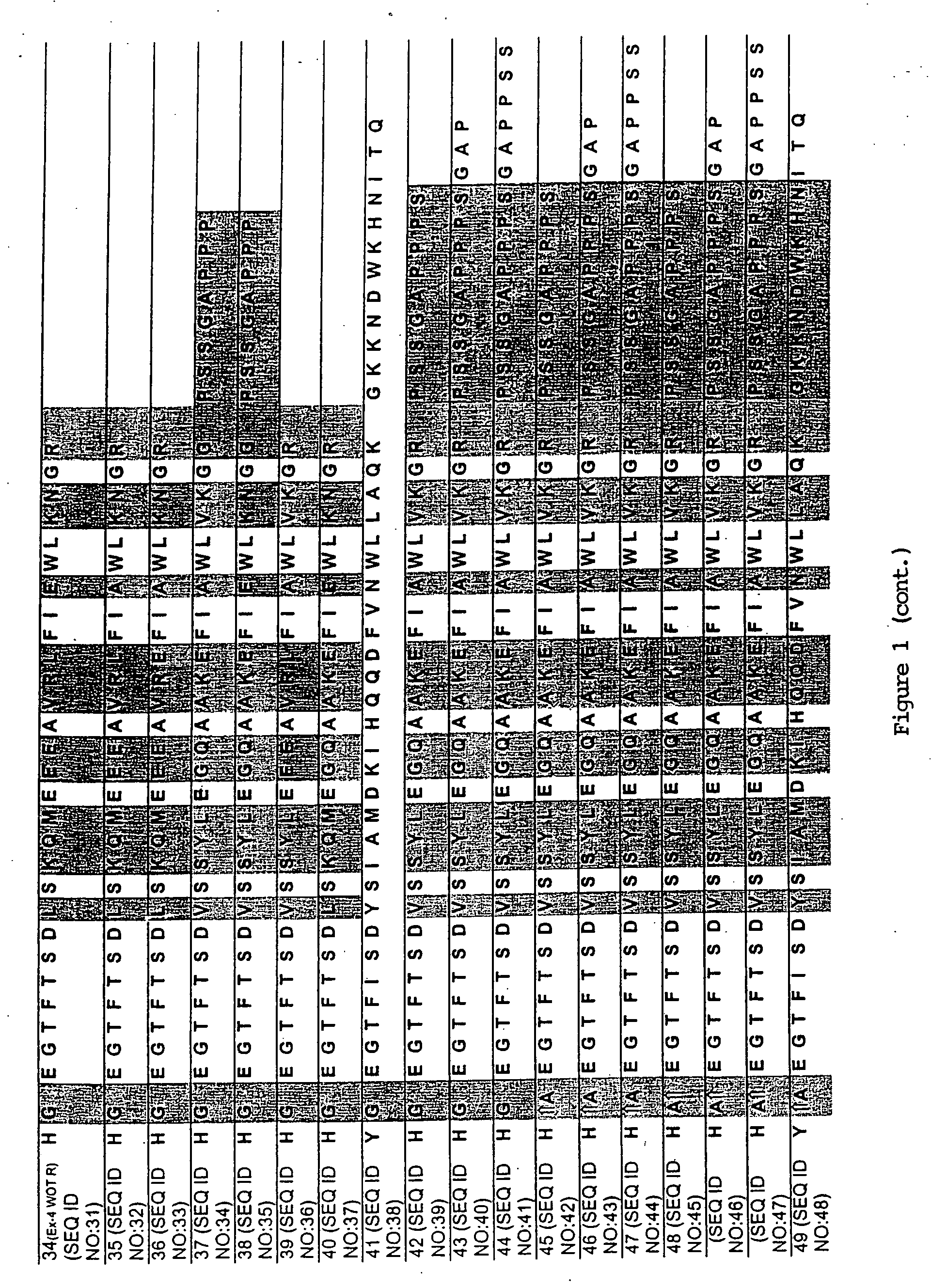
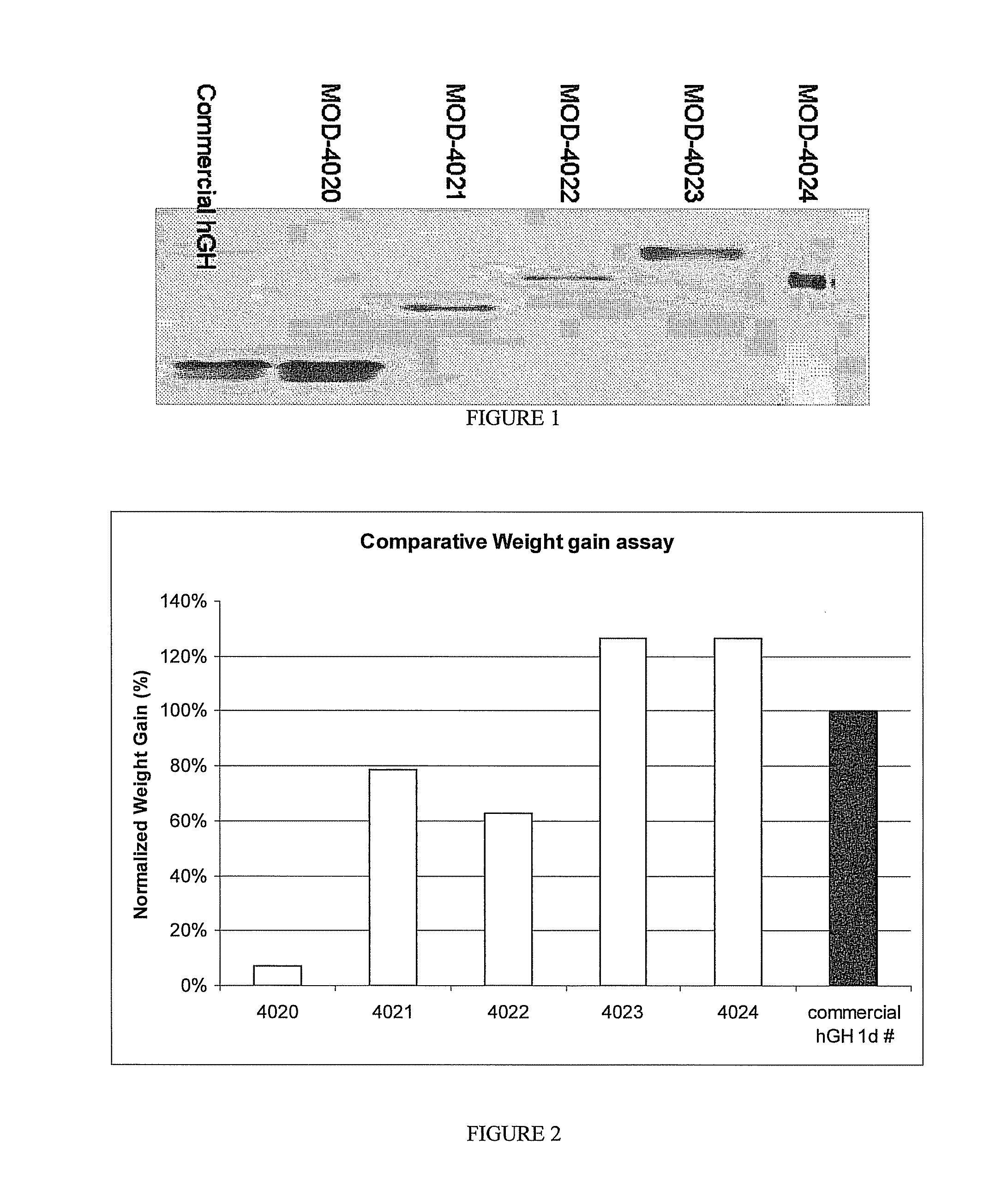
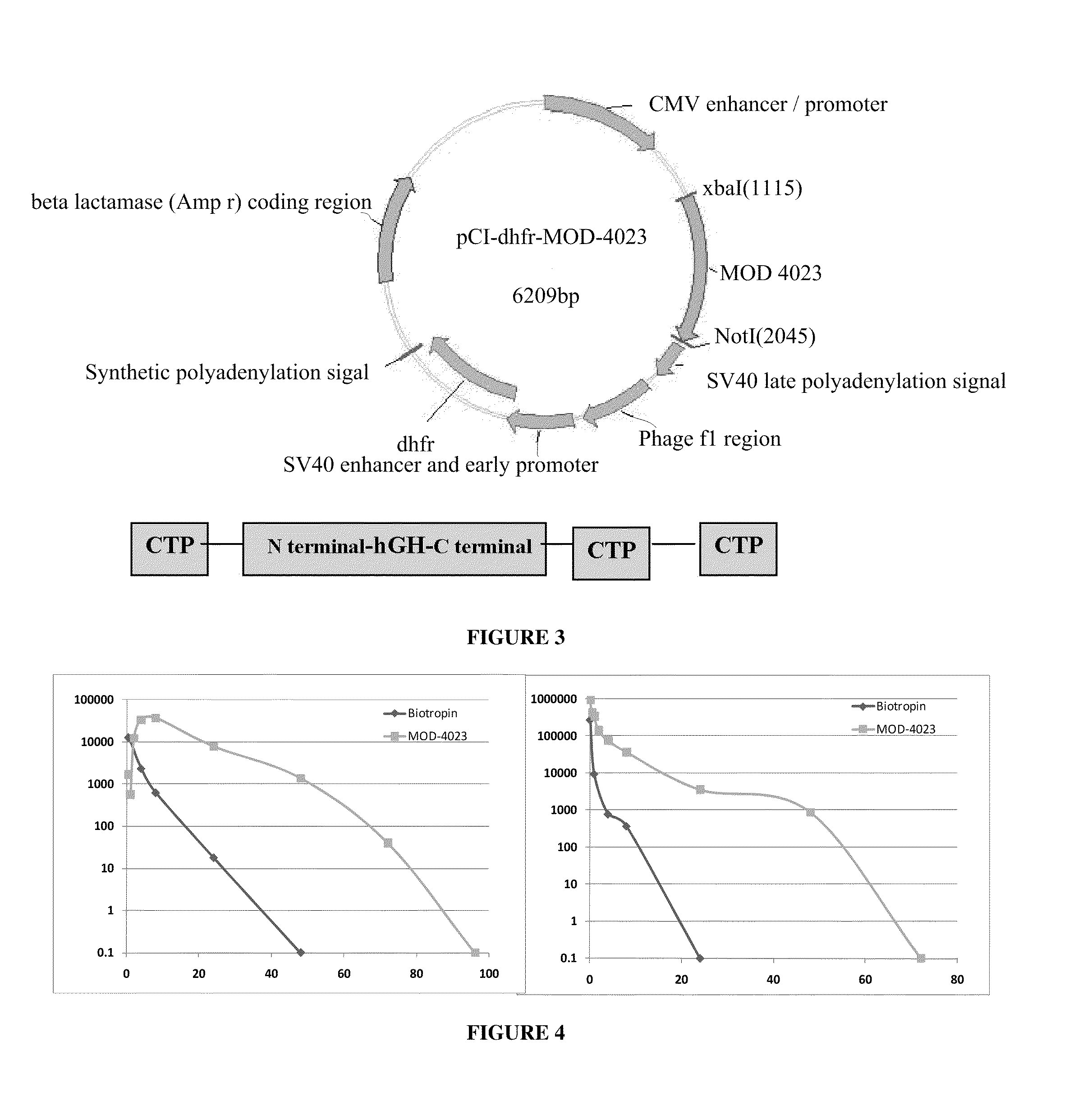
Long-acting growth hormone and methods of producing same
ActiveUS20120035101A1Decreasing body fatReduce weight lossPeptide/protein ingredientsMetabolism disorderSomatotropic hormoneNucleotide
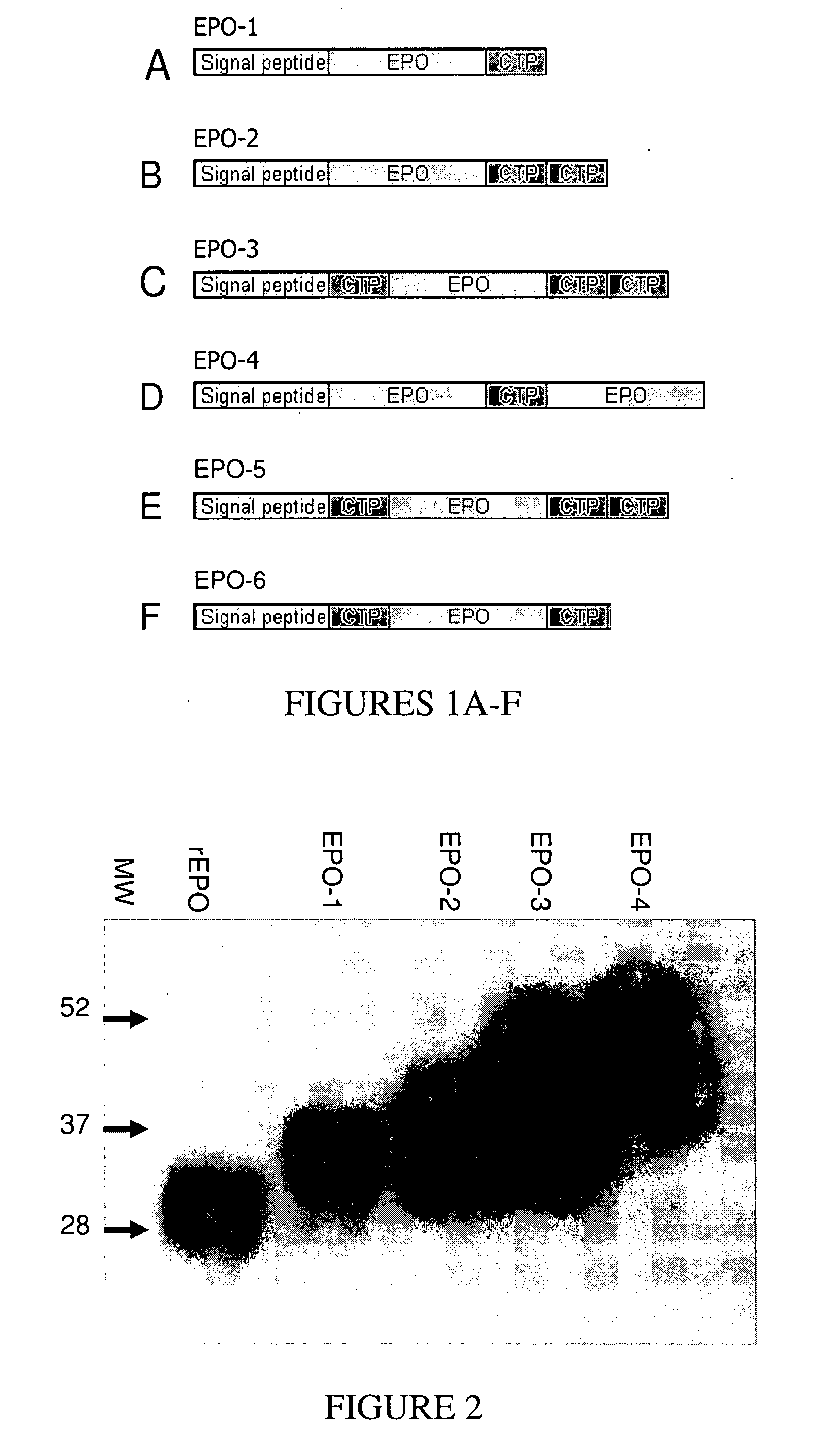
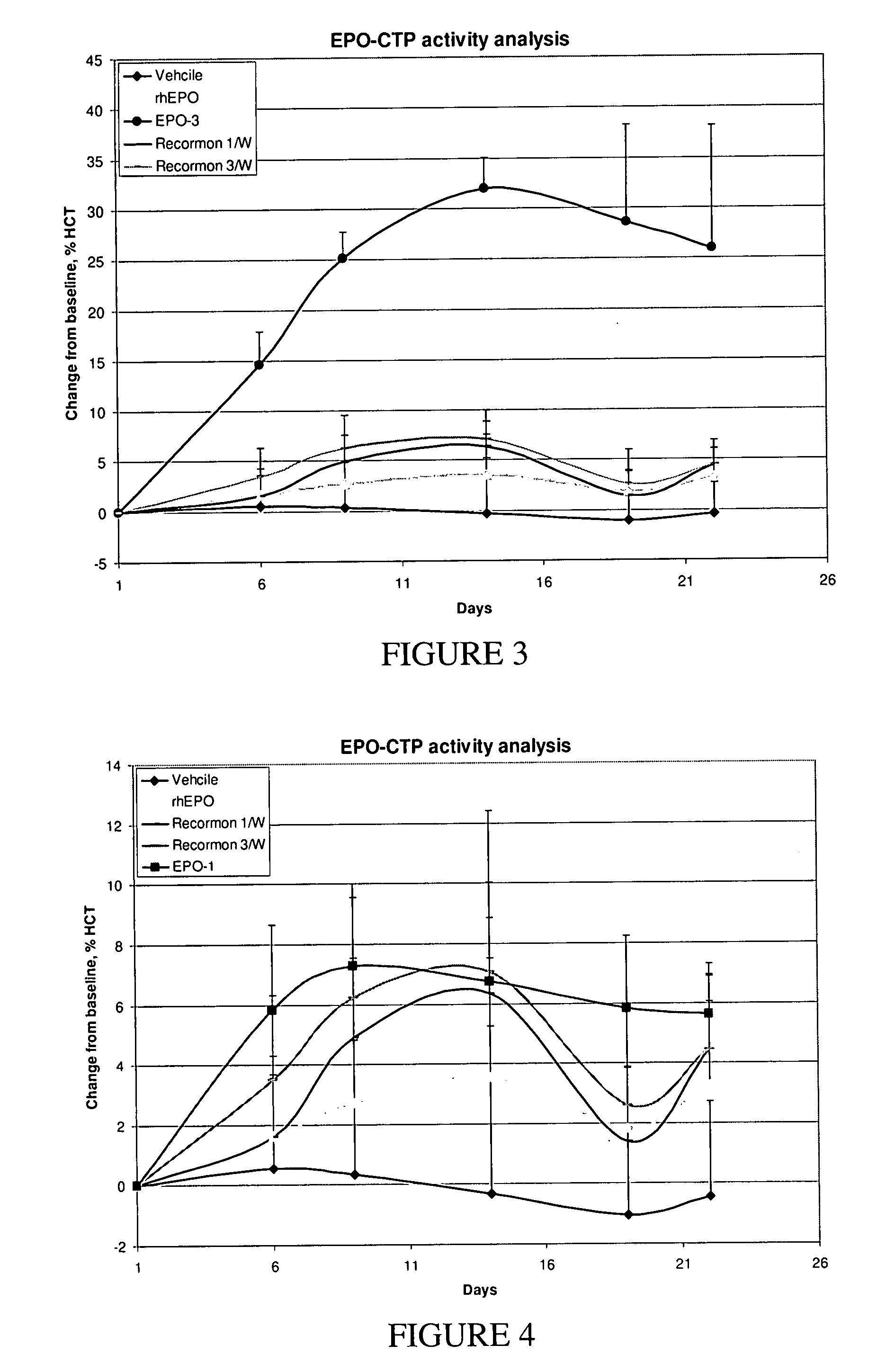
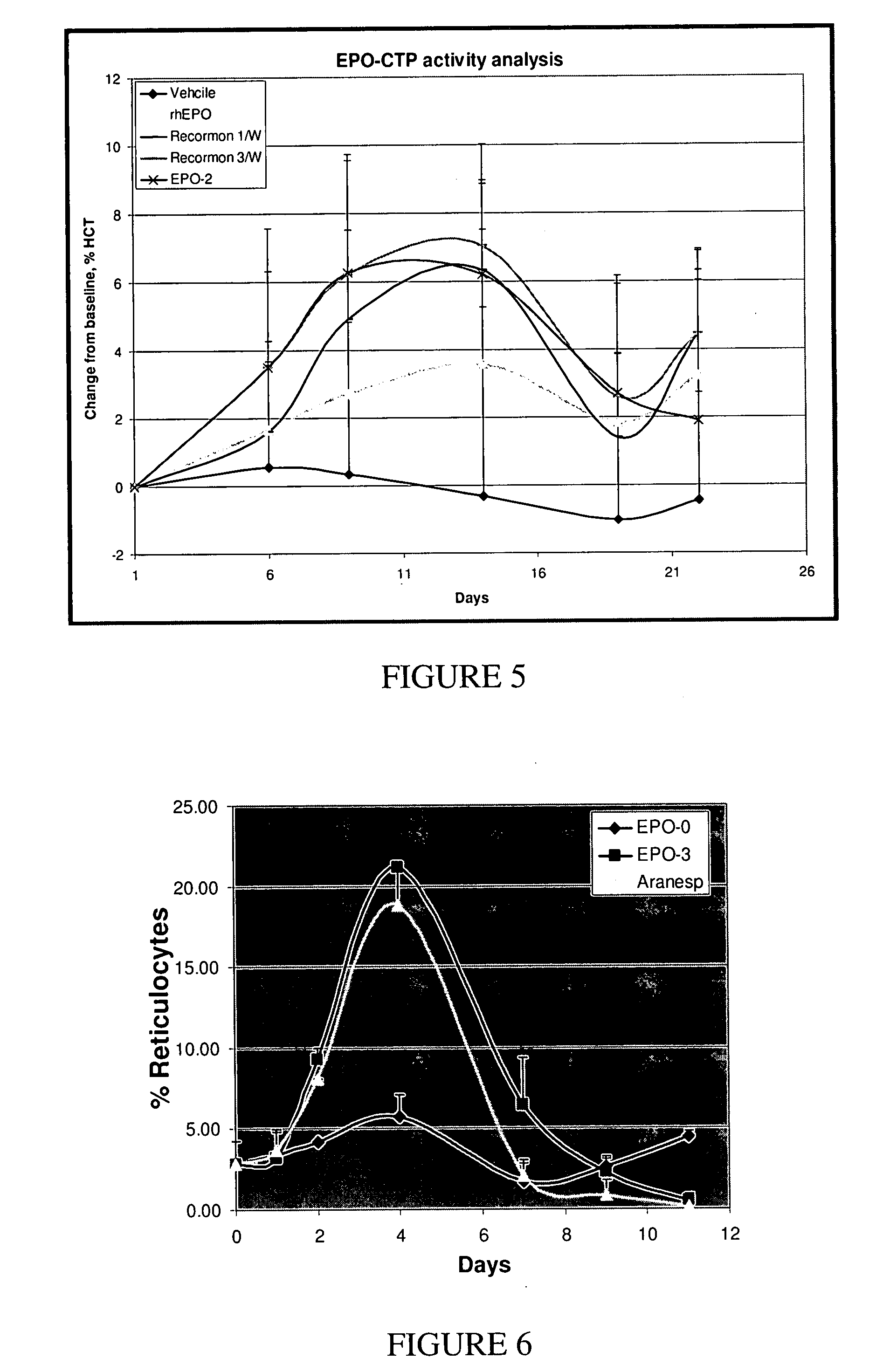
Use of a growth hormone protein and polynucleotides encoding same comprising an amino-terminal carboxy-terminal peptide (CTP) of chorionic gonadotrophin and two carboxy-terminal chorionic gonadotrophin CTPs attached to the growth hormone in methods of inducing growth or weight gain, method of increasing insulin-like growth factor (IGF-1) levels, and methods of reducing the dosing frequency of a growth hormone in a human subject are disclosed. Pharmaceutical compositions comprising the growth hormone and polynucleotides encoding the growth hormone of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Composition for long-acting peptide analogs
ActiveUS20090088387A1Increase perfusionImprove the level ofAntibacterial agentsPeptide/protein ingredientsHalf-lifeArginine
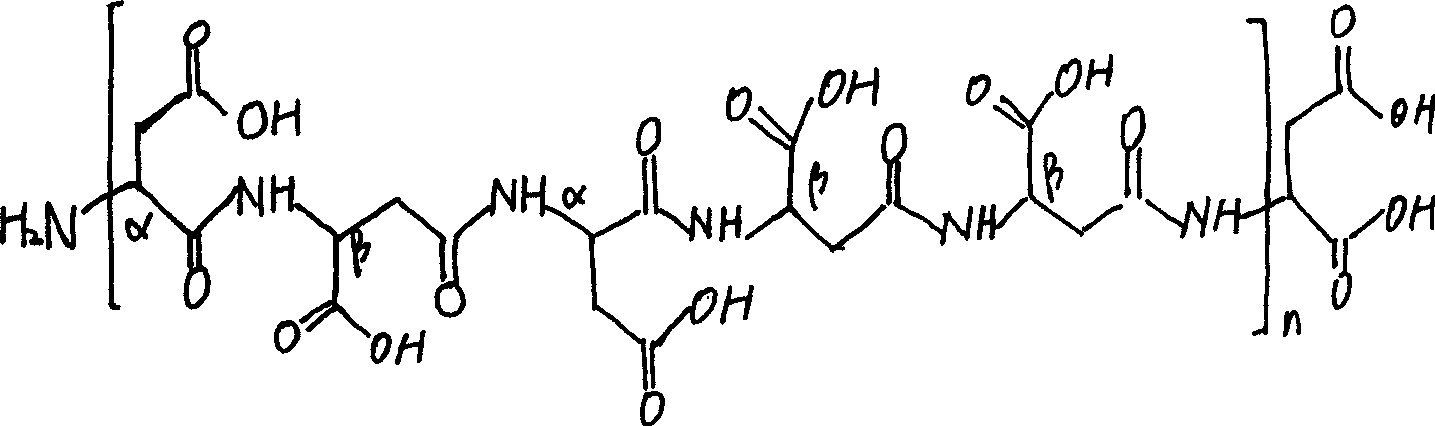
The invention describes compositions of peptide analogs that are active in blood or cleavable in blood to release an active peptide. The peptide analogs have a general formula: A-(Cm)x-Peptide, wherein A is hydrophobic moiety or a metal binding moiety, e.g., a chemical group or moiety containing 1) an alkyl group having 6 to 36 carbon units, 2) a nitrilotriacetic acid group, 3) an imidodiacetic acid group, or 4) a moiety of formula (ZyHisw)p, wherein Z is any amino acid residue other than histidine, His is histidine, y is an integer from 0-6; w is an integer from 1-6; and p is an integer from 1-6; wherein if A has alkyl group with 6 to 36 carbon units x is greater than 0; and Cm is a cleavable moiety consisting of glycine or alanine or lysine or arginine or N-Arginine or N-lysine, wherein x is an integer between 0-6 and N may be any amino acid or none. The peptide analogs are complexed with polymeric carrier to provide enhanced half-life.
Owner:PHARMAIN CORP
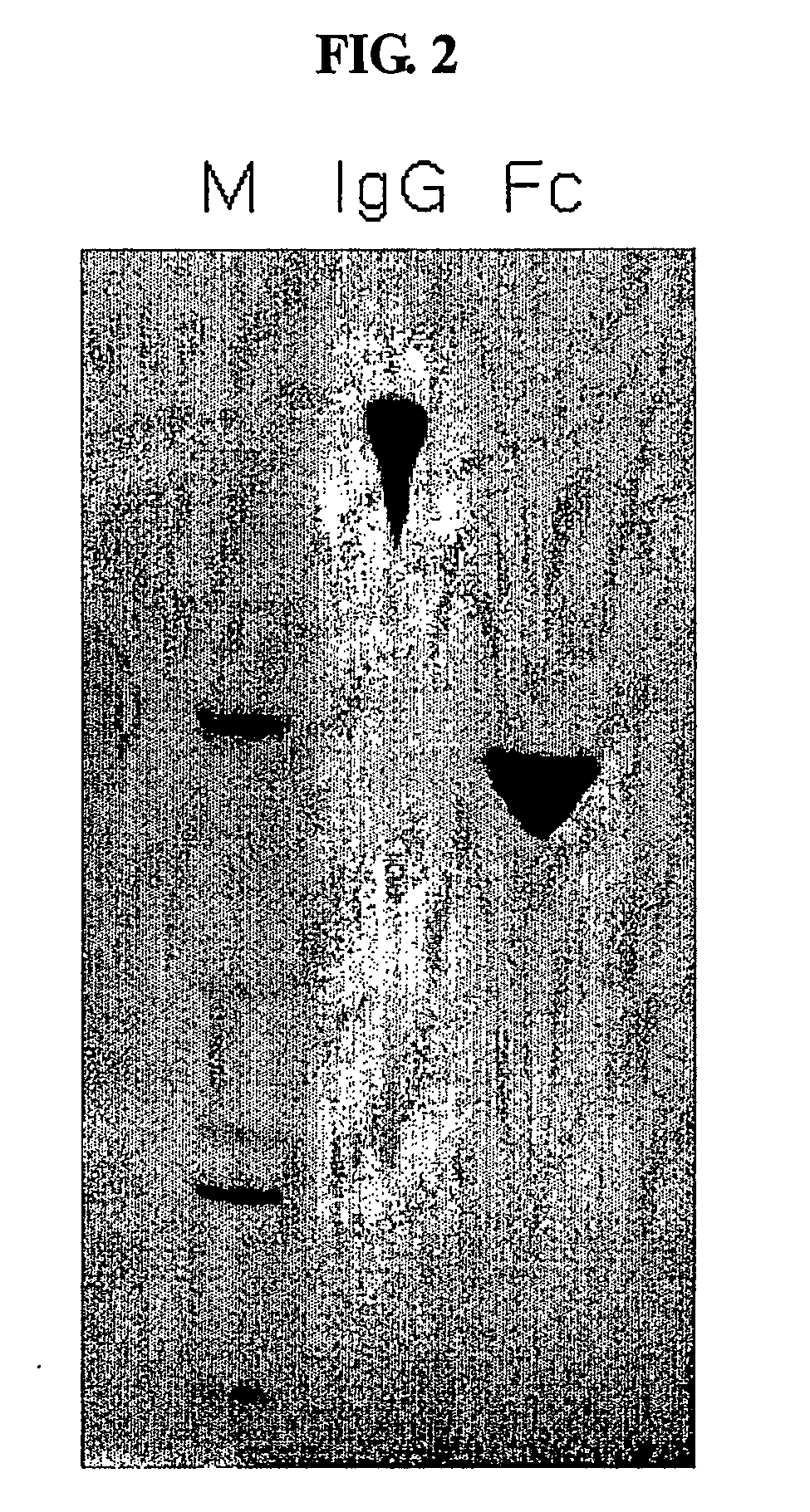
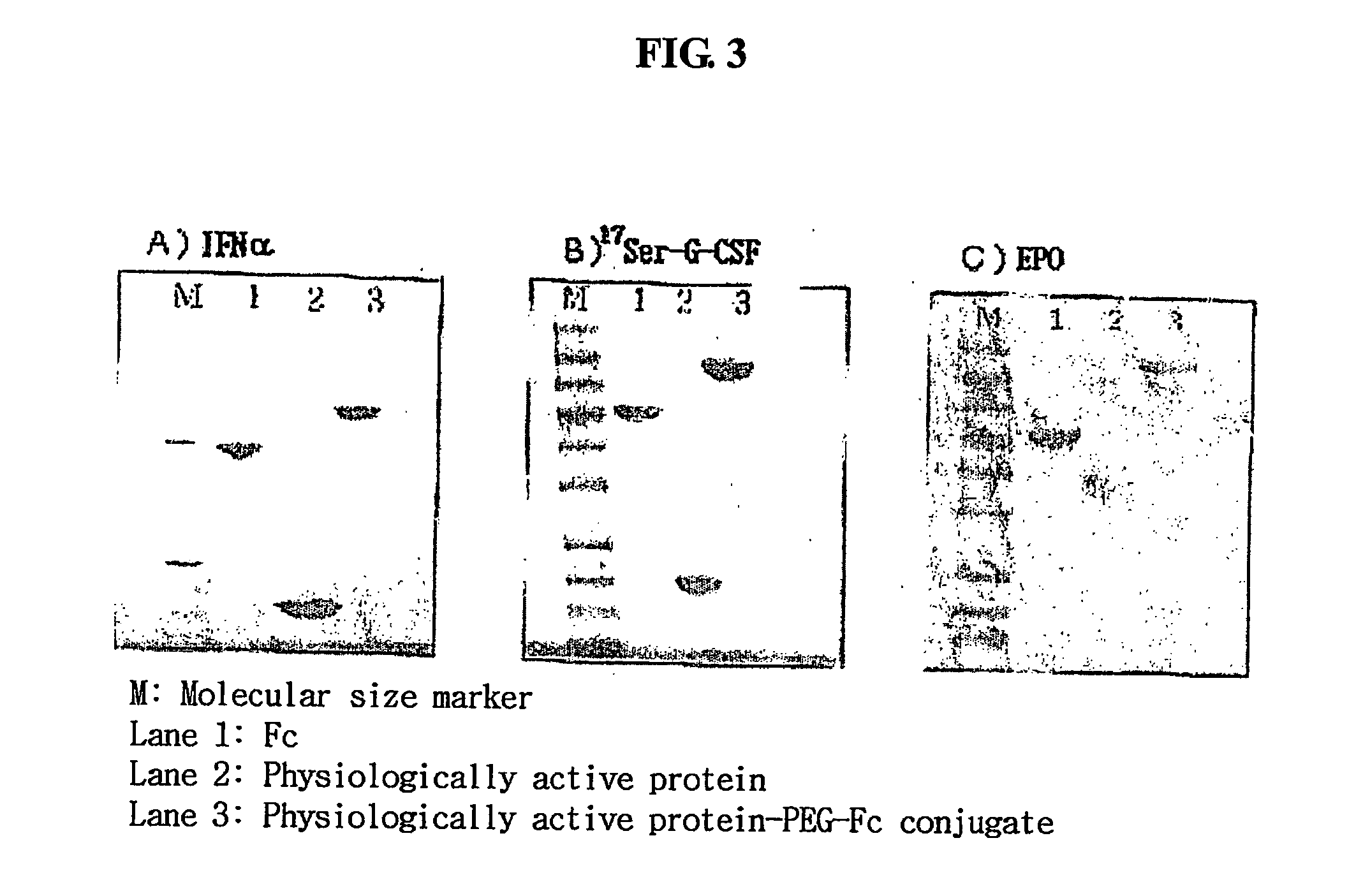
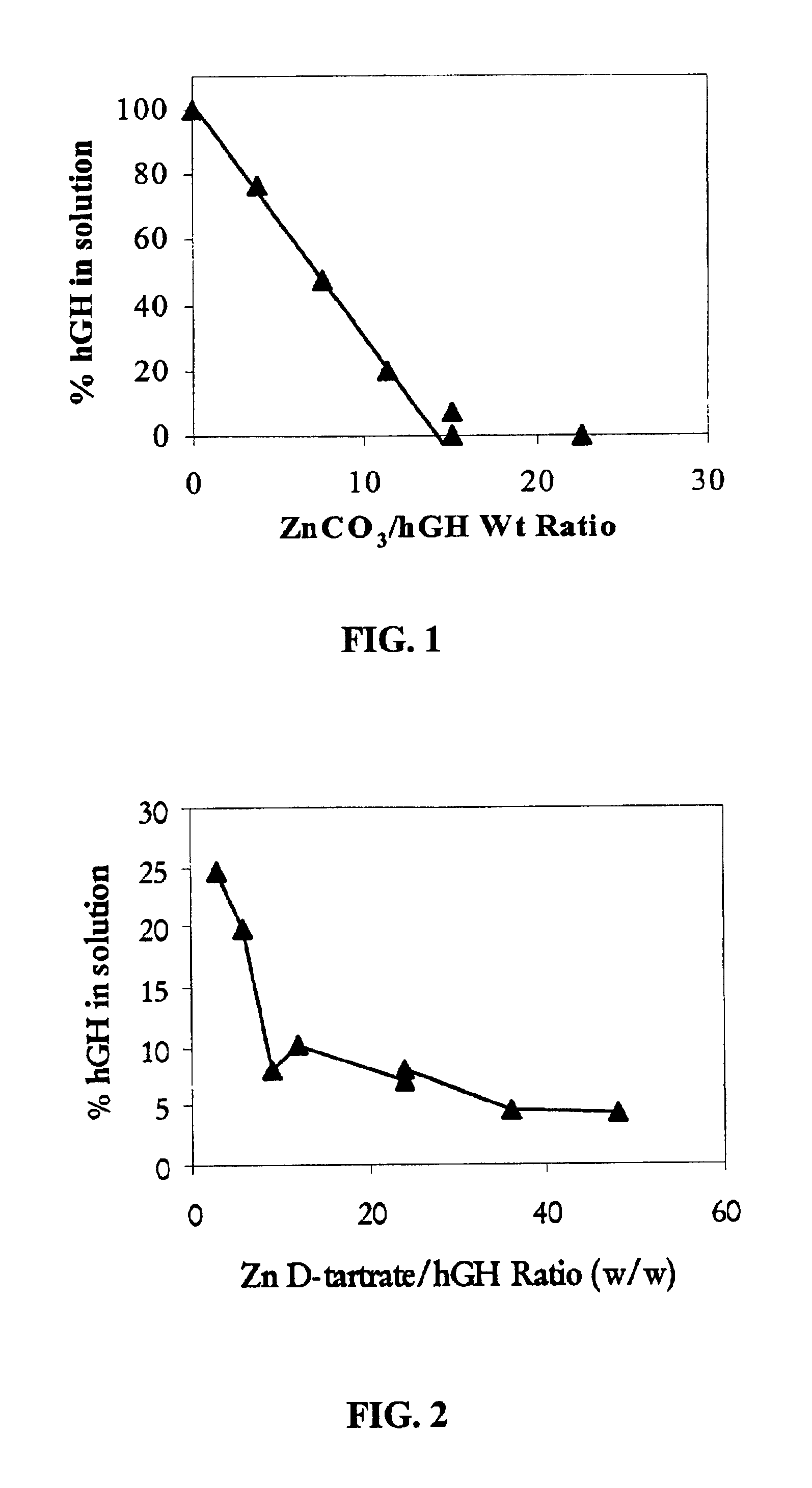
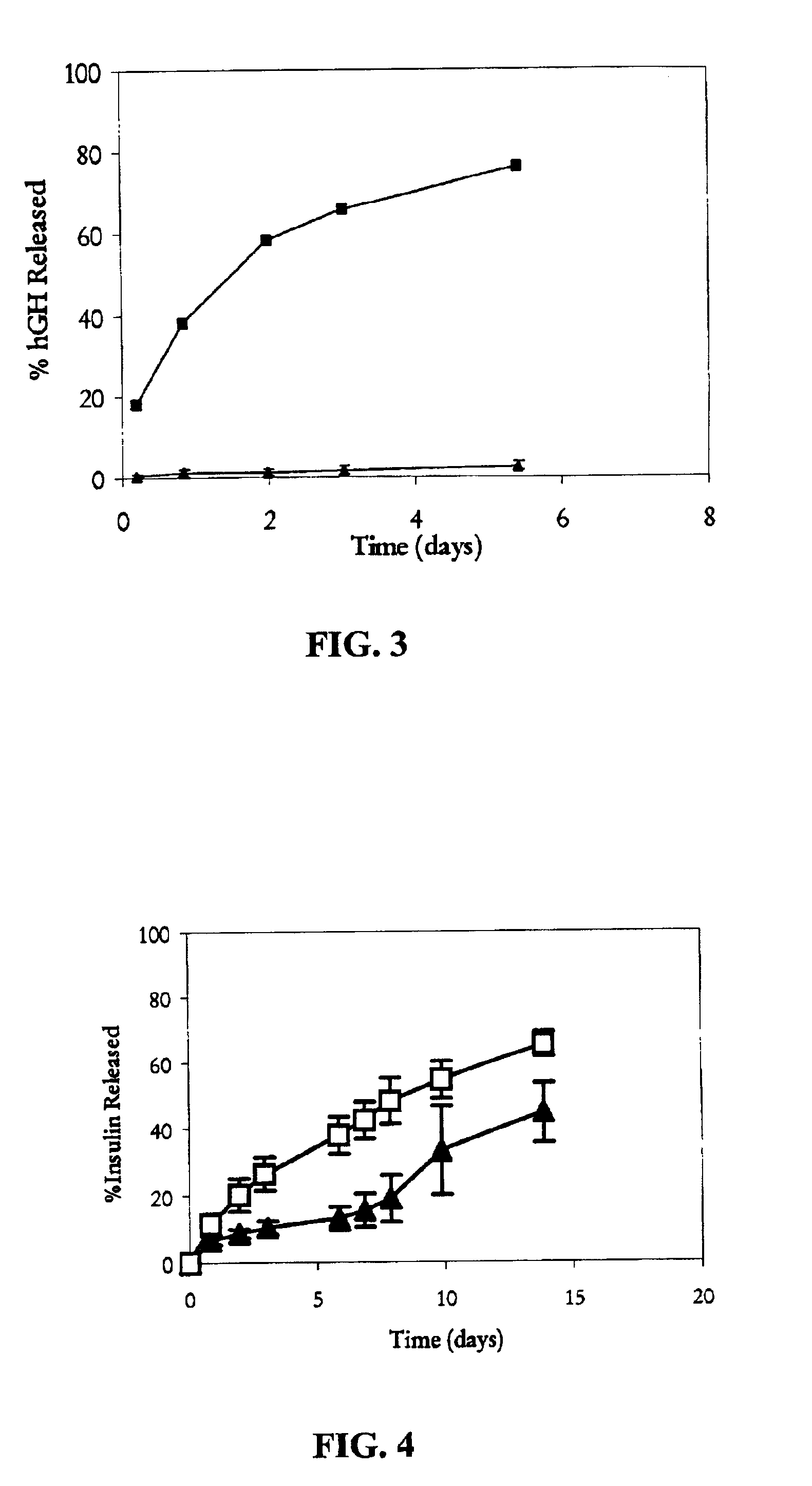
Pharmaceutical composition comprising an immunoglobulin fc region as a carrier
ActiveUS20060275254A1Prolong the action timePeptide/protein ingredientsAntibody mimetics/scaffoldsProtein targetHalf-life
Disclosed is a novel use of an immunoglobulin Fe fragment, and more particularly, a pharmaceutical composition comprising an immunoglobulin Fe fragment as a carrier. The pharmaceutical composition comprising an immunoglobulin Fe fragment as a carrier remarkably extends the serum half-life of a drug while maintaining the in vivo activity of the drug at relatively high levels. Also, when the drug is a polypeptide drug, the pharmaceutical composition has less risk of inducing immune responses compared to a fusion protein of the immunoglobulin Fe fragment and a target protein, and is thus useful for developing long-acting formulations of various polypeptide drugs.
Owner:HANMI SCI CO LTD
Proteins deposited onto sparingly soluble biocompatible particles for controlled protein release into a biological environment from a polymer matrix
The present invention relates to compositions and methods for the modulated release of one or more proteins or peptides. The composition is comprised of a biocompatible polymeric matrix, a protein and / or peptide, and a sparingly water-soluble or essentially insoluble particle. The protein is deposited by adsorption or some other mechanism onto the sparingly water-soluble biocompatible particle wherein the protein-particle combination is dispersed within the polymeric matrix. The deposition of the protein onto the particle acts to modulate the release of the protein or peptide from dosage forms including long-acting dosage systems.
Owner:BTG INT LTD
Dosing regimen associated with long acting injectable paliperidone esters
The present invention provides a method of treating patients in need of treatment with long acting injectable paliperidone palmitate formulations.
Owner:JANSSEN PHARMA NV
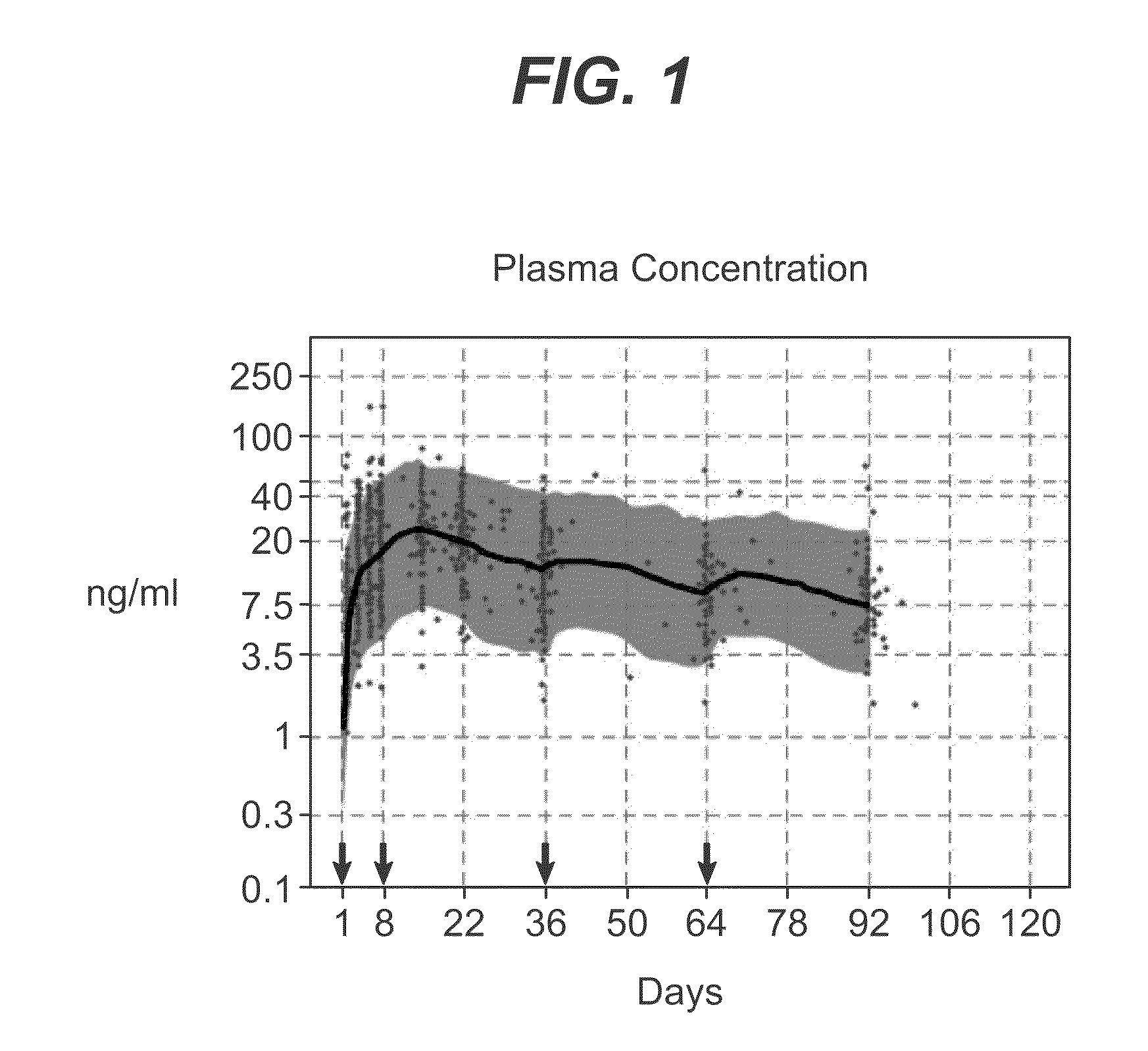
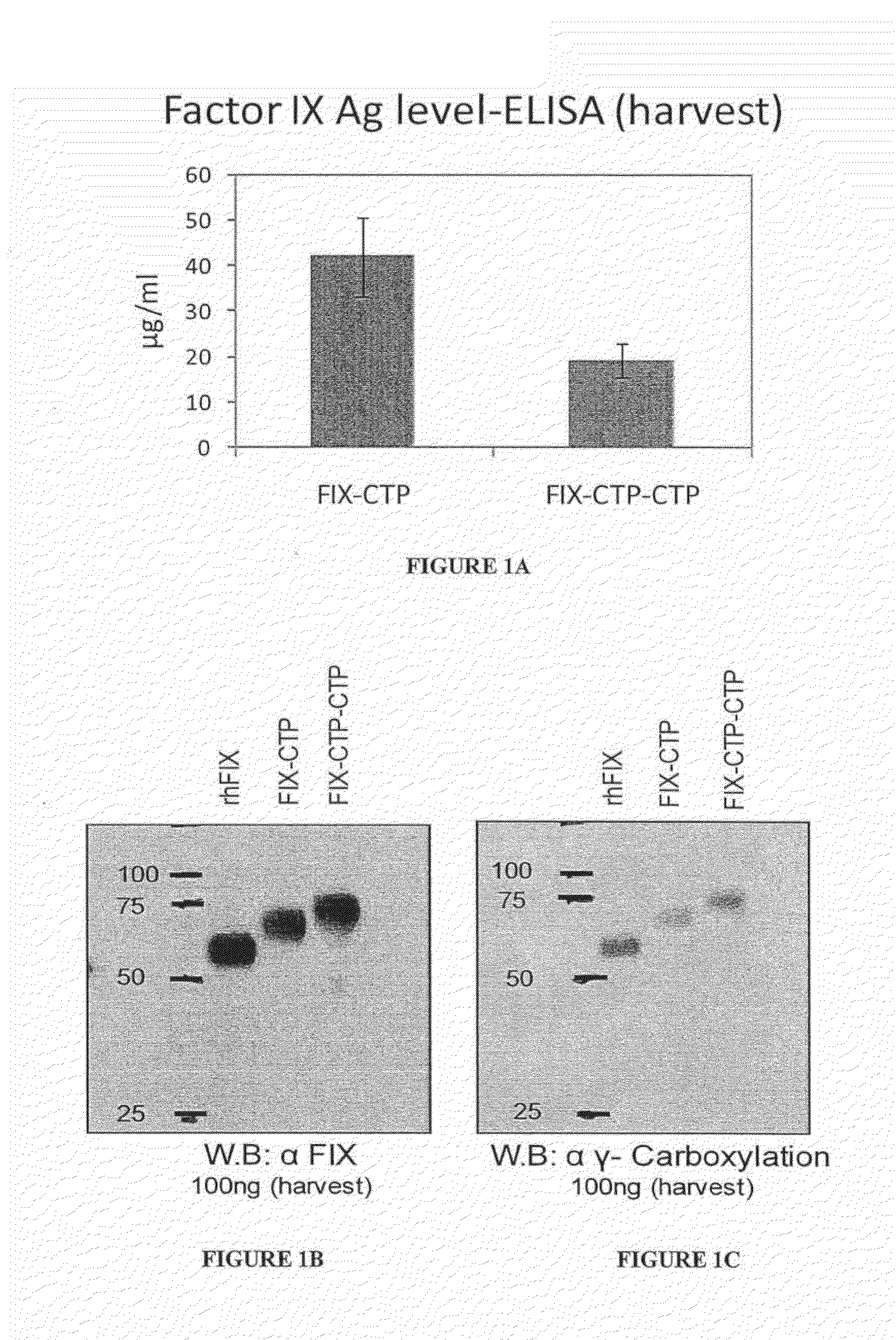
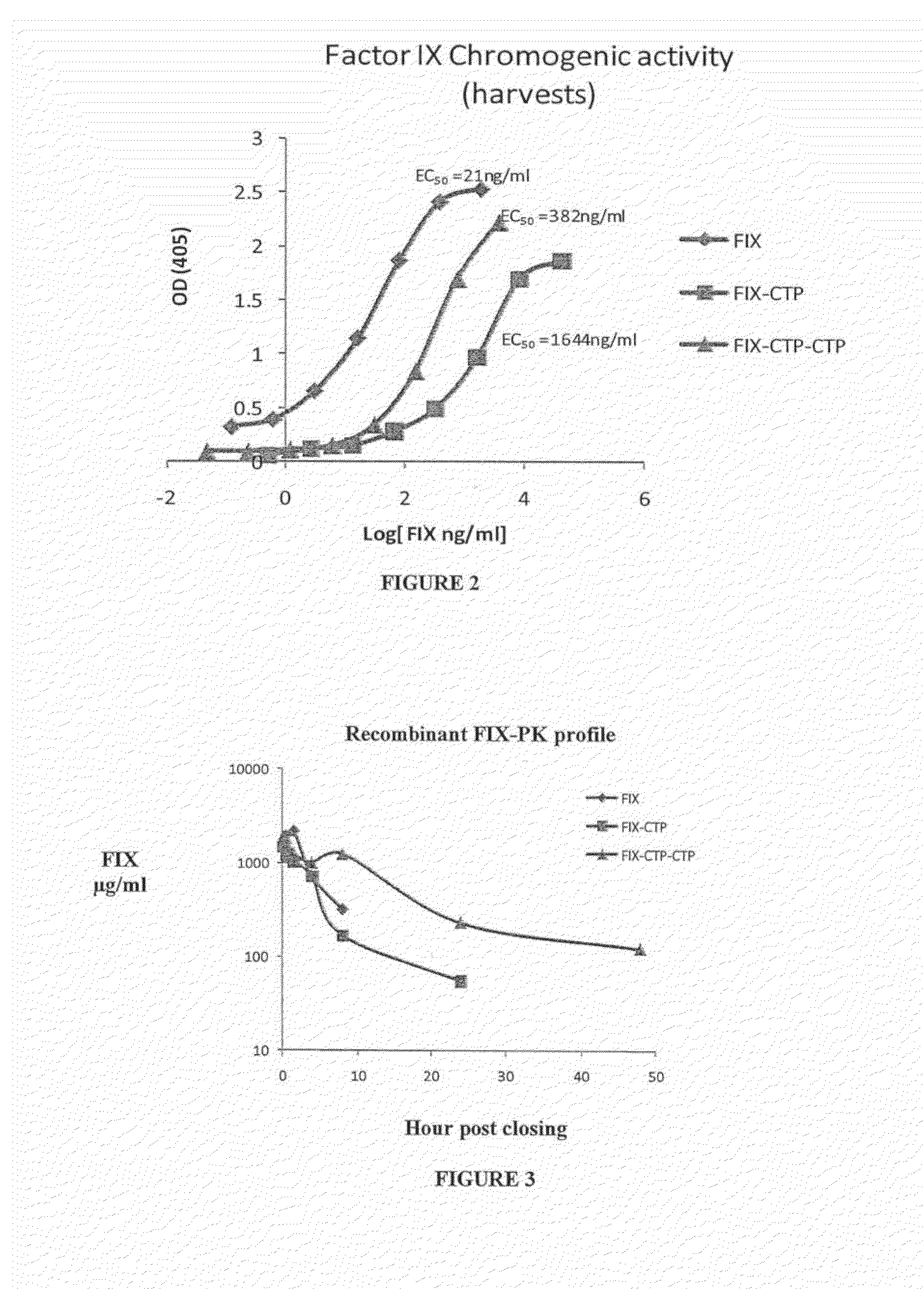
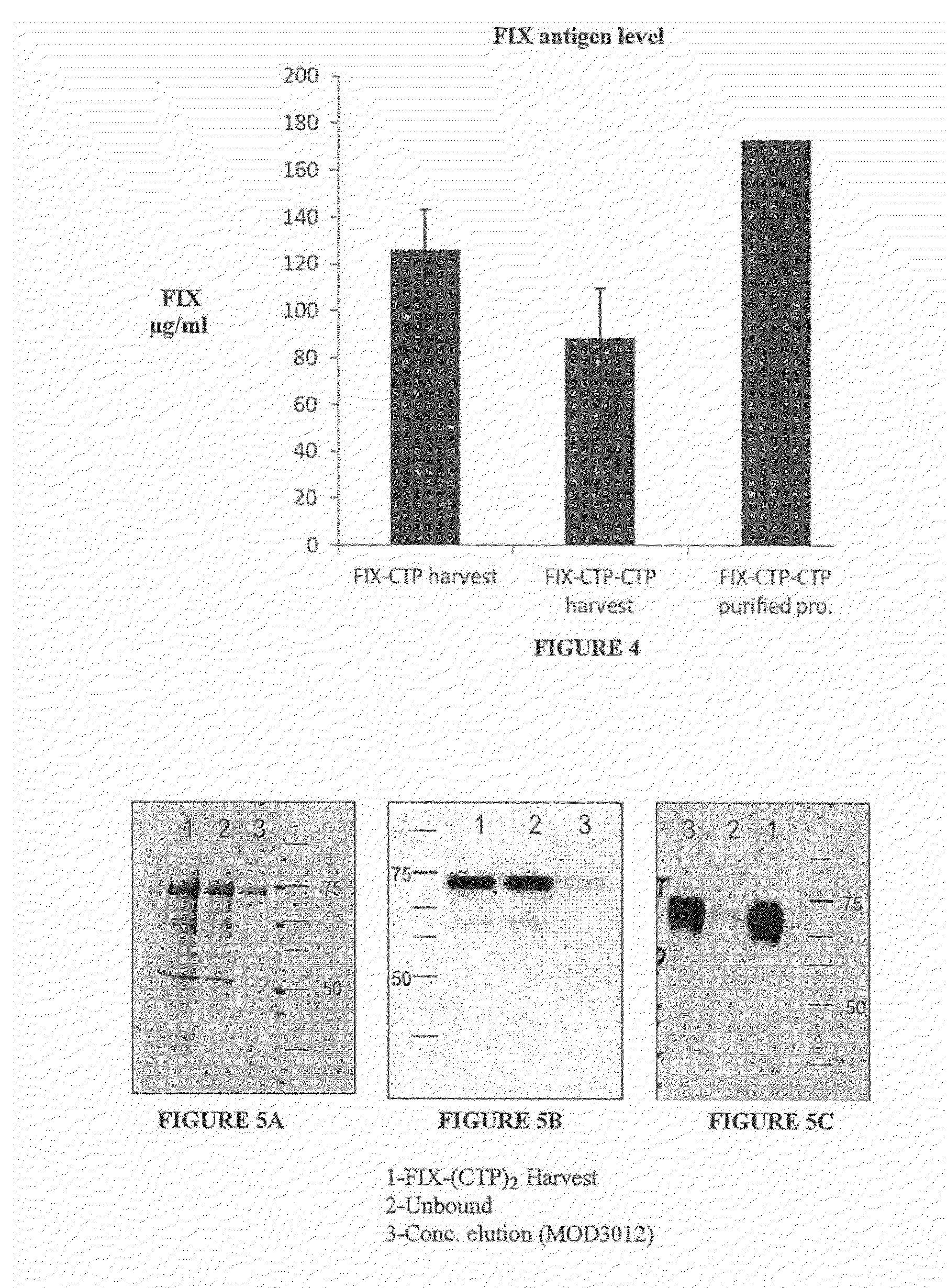
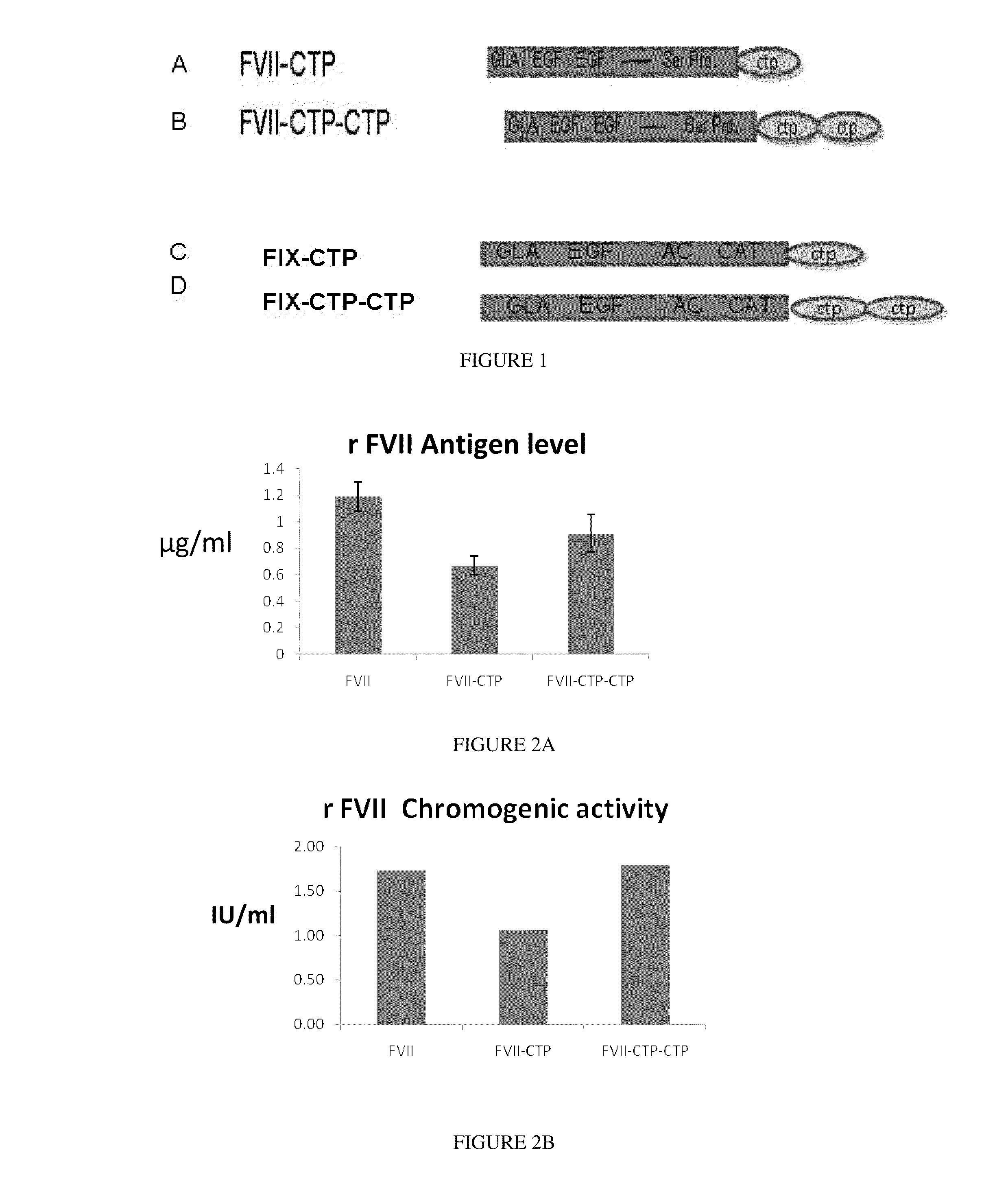
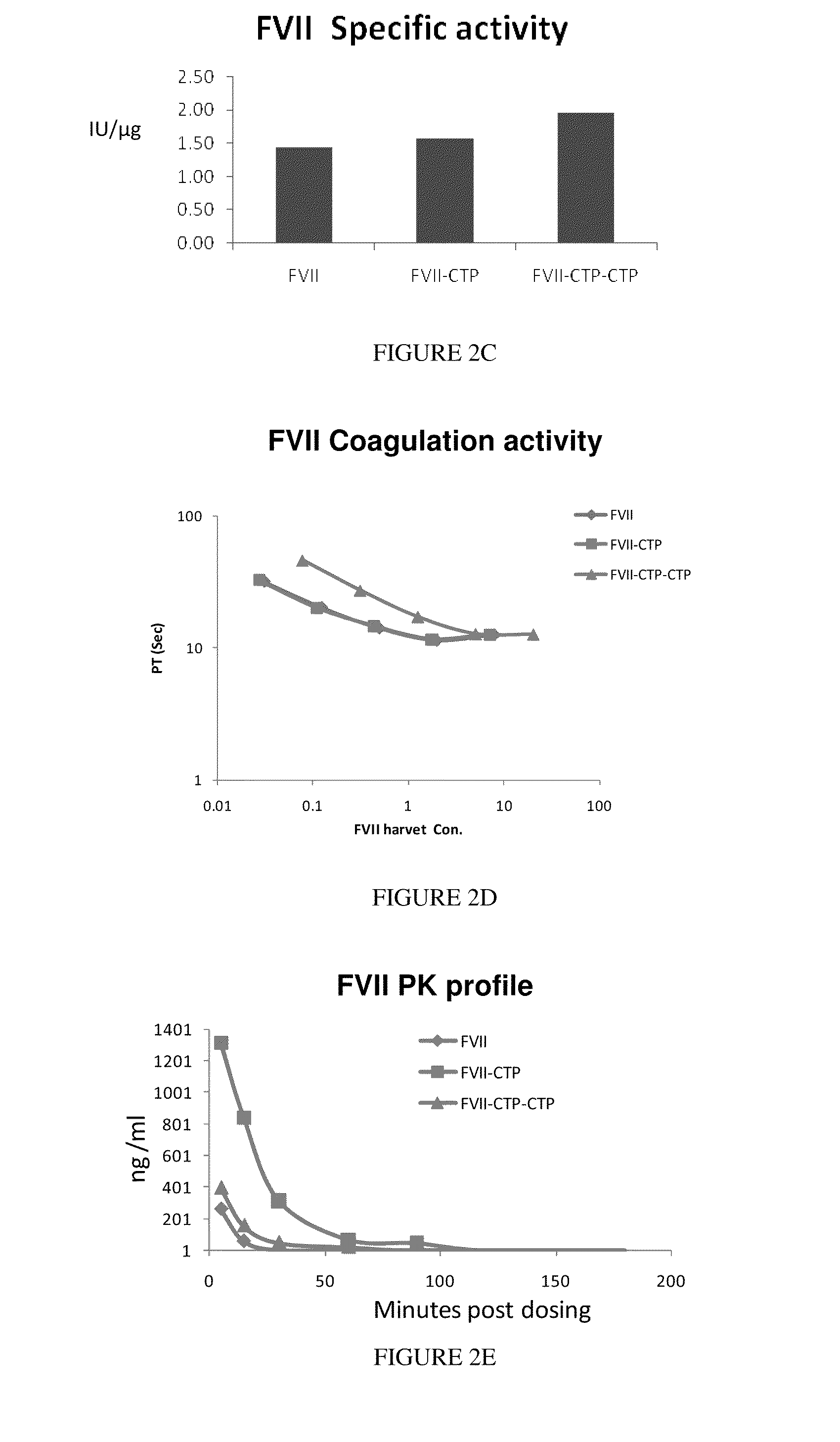
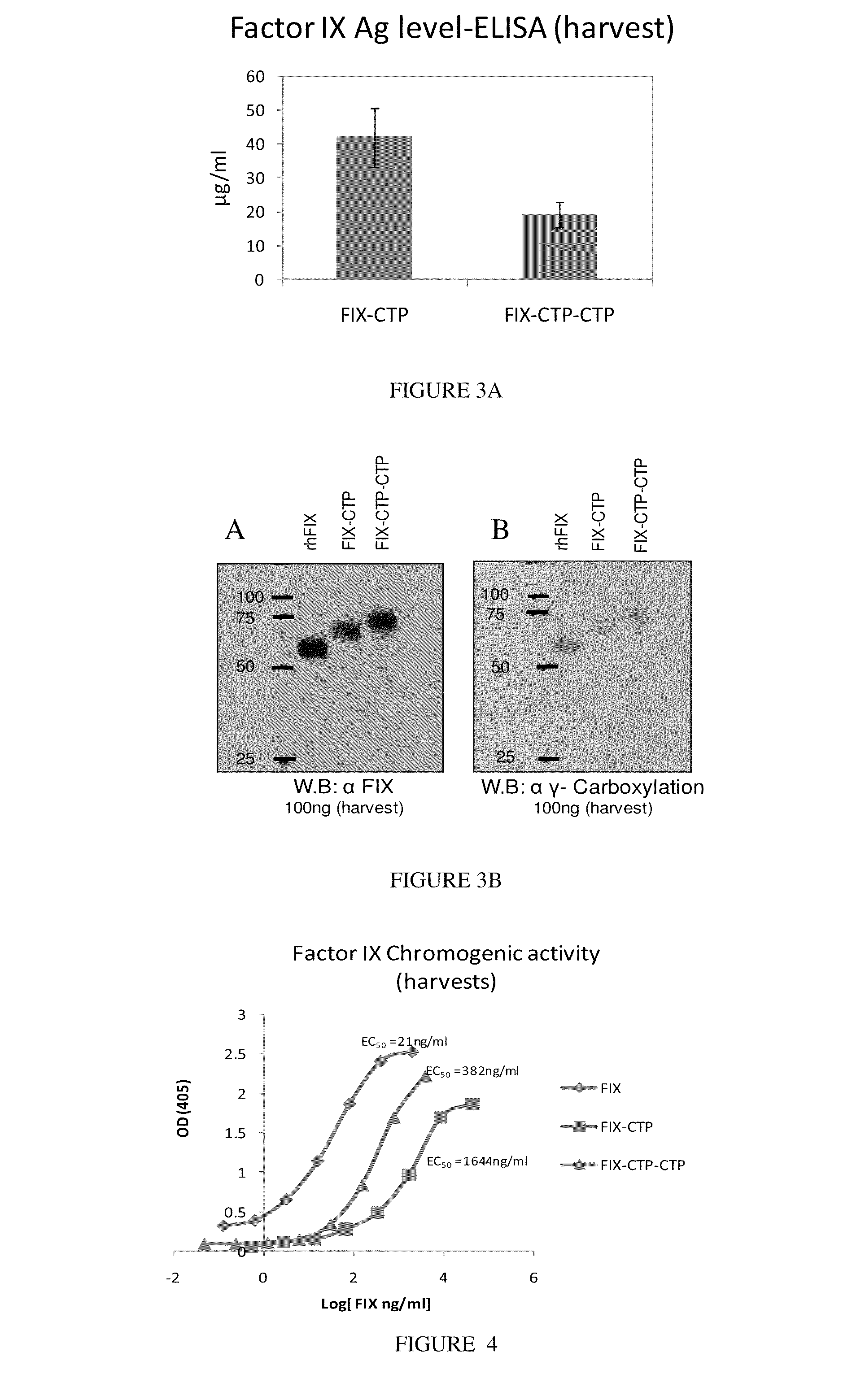
Long-acting coagulation factors and methods of producing same
ActiveUS20130243747A1Prevent coagulationPreventing hemophiliaBacteriaPeptide/protein ingredientsNucleotidePolynucleotide
Polypeptides comprising at least one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to the carboxy terminus but not to the amino terminus of a coagulation factor and polynucleotides encoding the same are disclosed. Pharmaceutical compositions comprising the polypeptides and polynucleotides of the invention and methods of using and producing same are also disclosed.
Owner:OPKO BIOLOGICS
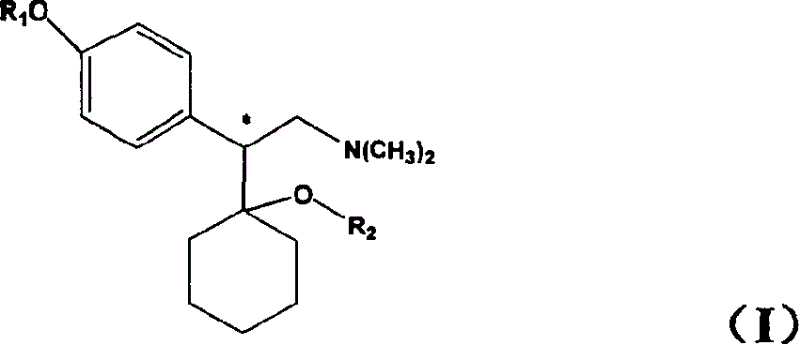
Medicine precursor containing long chain fatty acyl group substituted venlafaxine and its prepn and use
The present invention discloses one kind of medicine precursors containing long chain fatty acyl group substituted venlafaxine in the structure as shown in general expression I and its medicinal salt and hydrate. The present invention also discloses the preparation process of the medicine precursors and their application in preventing and treating diseases of central nerve system. The medicine precursors of the present invention has half life near or over 10 hr, and compared with venlafaxine, they has excellent long-acting effect.
Owner:RADIOLOGY INST ACAD OF MILITARY MEDICINE SCI PLA
Long-acting sour milk containing beneficial bacteria factor and its producing method
The invention discloses a long-effect yoghourt and process for preparation, wherein the yoghourt is prepared from the following raw materials (by weight portions): milk 50-100, isomaltose hypgather 0. 01-20. 00 and stabilizing agent 0. 2-8. 0. The nourishing constituents in the yoghourt can be preserved, and the storage period of the product can reach longer than six months.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Long-acting polypeptides and methods of producing and administering same
ActiveUS20130184207A1Reduce dosing frequencyIncrease the areaPeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides comprising at least two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a non-human peptide-of-interest are disclosed. Pharmaceutical compositions comprising the non-human polypeptides and polynucleotides of the invention and methods of using both human and non-human polypeptides and polynucleotides are also disclosed.
Owner:OPKO BIOLOGICS
Immune enhancing compositions and methods of use thereof
InactiveUS20050271726A1Easy to synthesizeEffective absorptionPowder deliveryOrganic active ingredientsGlycineBlood level
A method of administering parenterally, particularly intramuscularly, glutamine and cystine and glycine plus selenium; or lactalbumin plus selenium; or lactalbumin and glutamine and cystine and glycine plus selenium, through a long-acting pharmaceutically acceptable carrier to a patient. The method comprises injecting a mixture of glutamine, cystine, glycine, lactalbumin and selenium in order to maintain the mixture systemically or locally for a sufficient time period so as to maintain blood levels of glutathione within an improved therapeutic range.
Owner:CRUM ALBERT
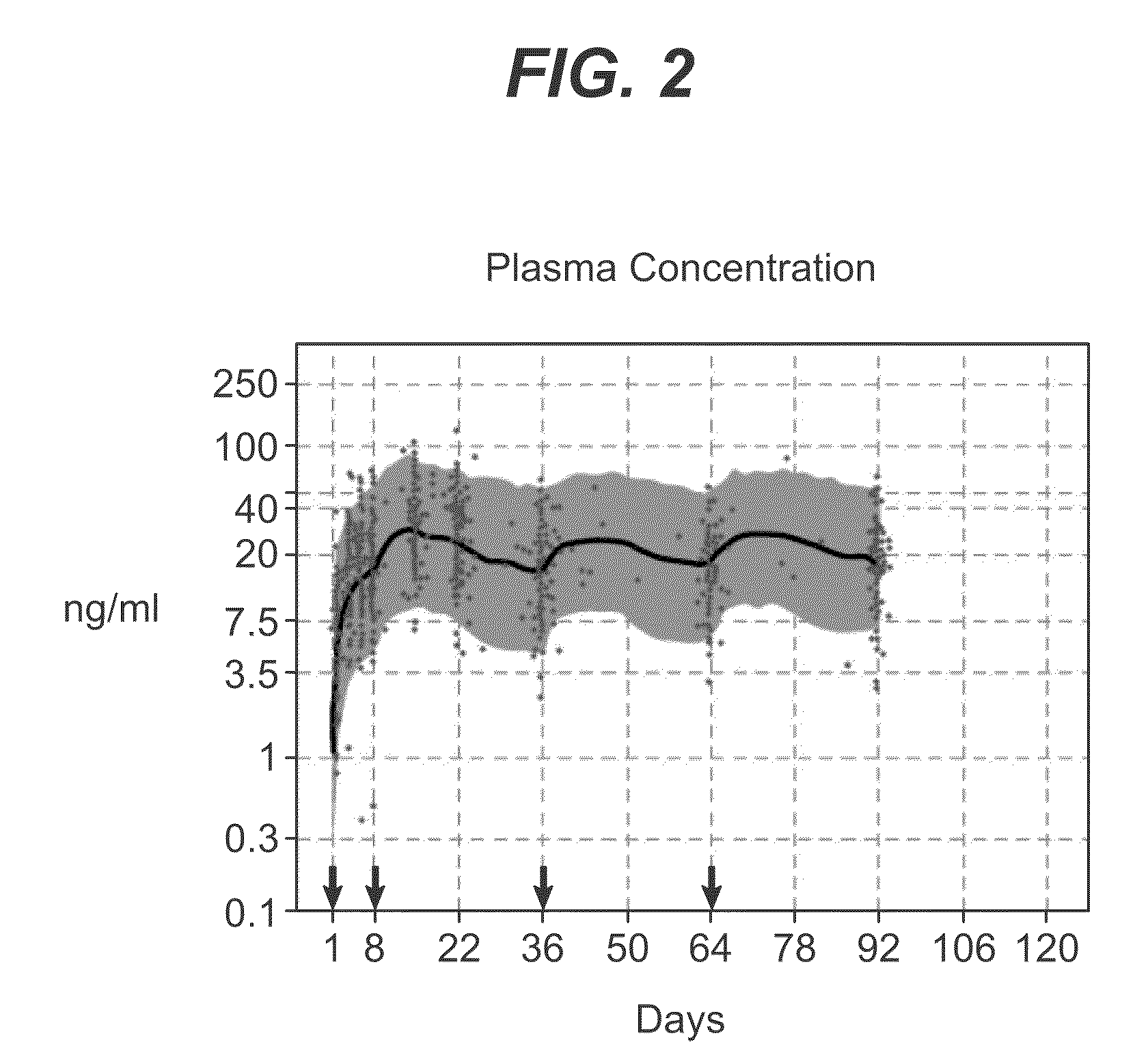
Long-acting coagulation factors and methods of producing same
ActiveUS20100317585A1Improving biological half lifeImproving area under curve (AUC)Peptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
Polypeptides and polynucleotides encoding same comprising at least one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to a carboxy terminus of a coagulation factor and not to an amino terminus are disclosed. Pharmaceutical compositions comprising the polypeptides and polynucleotides of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
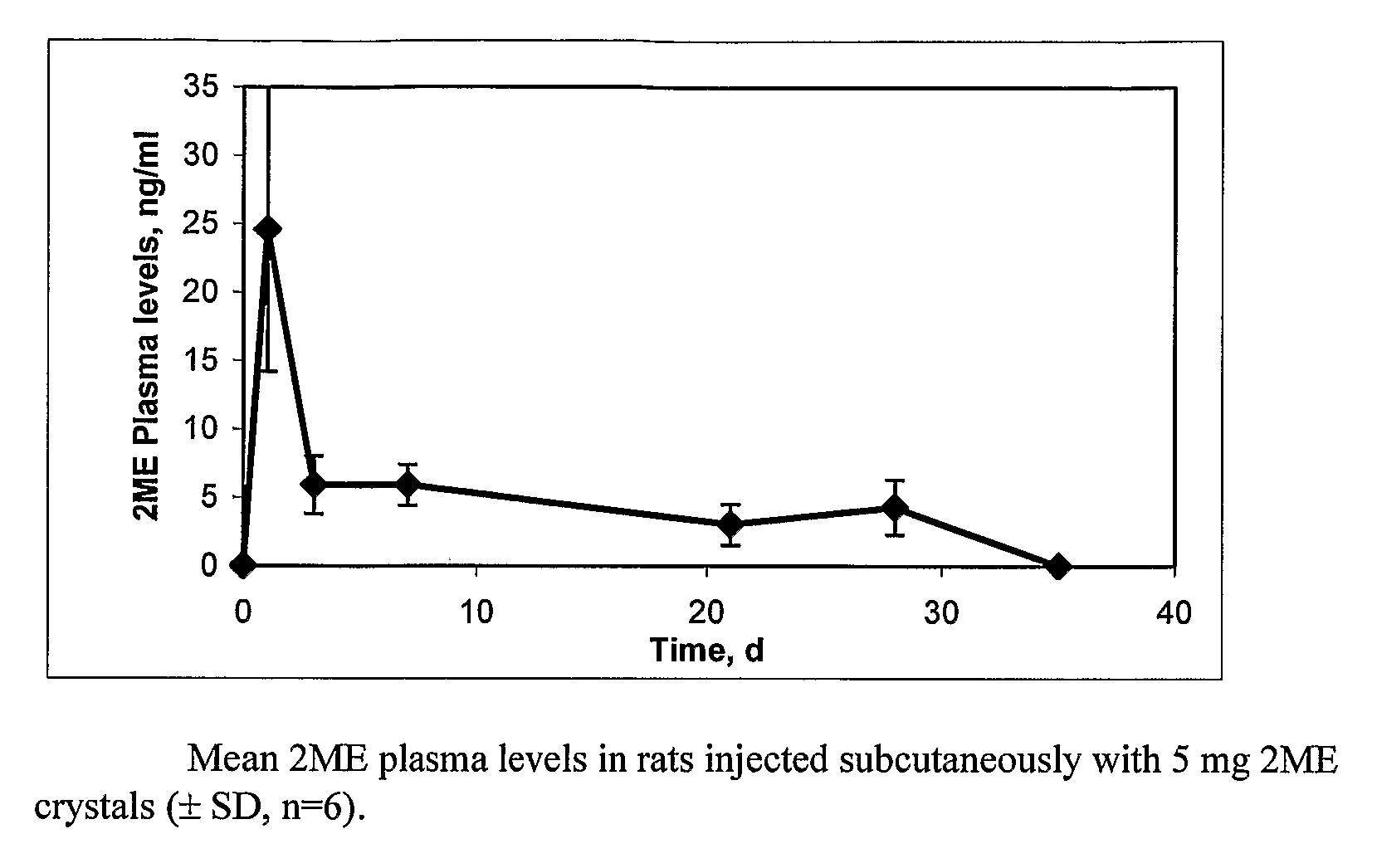
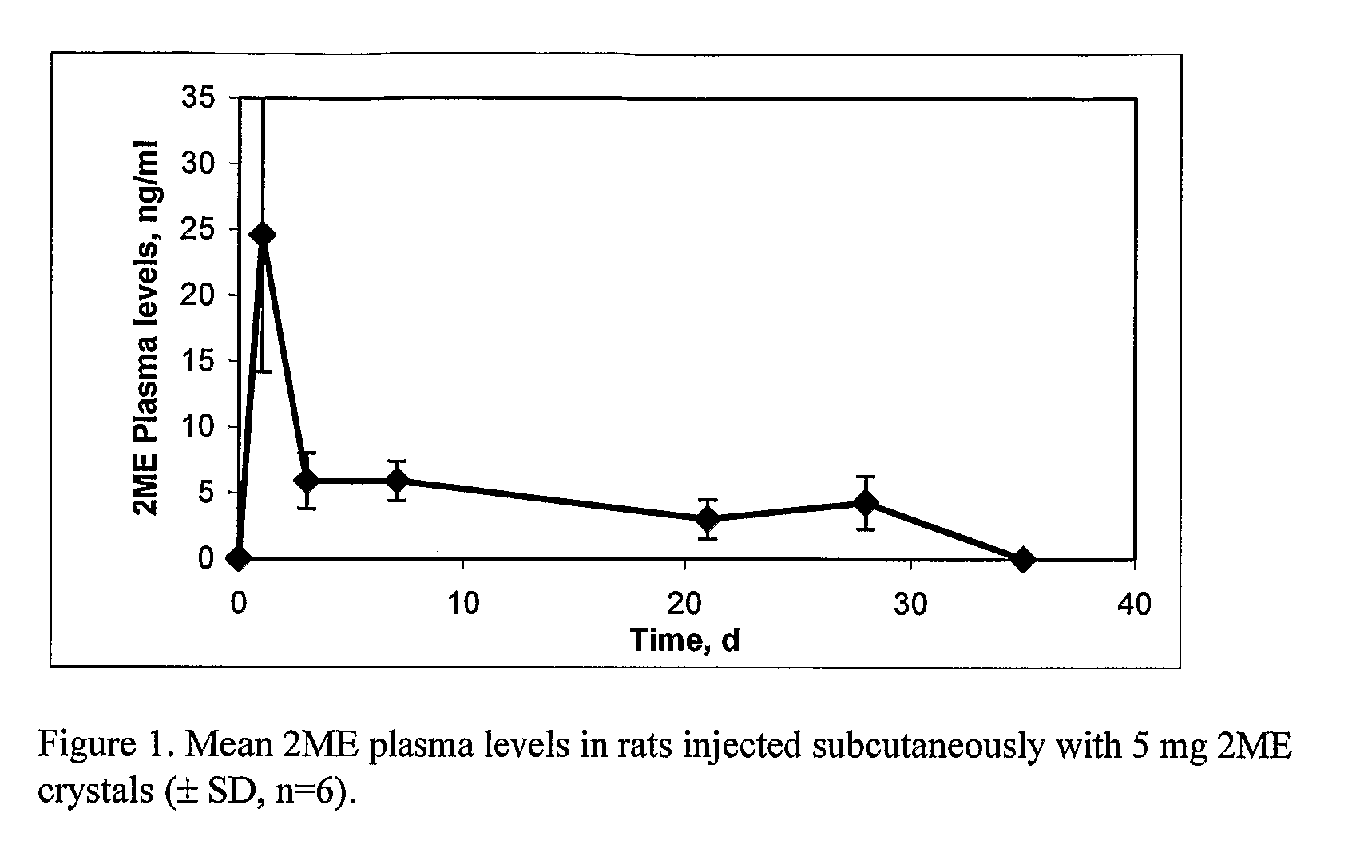
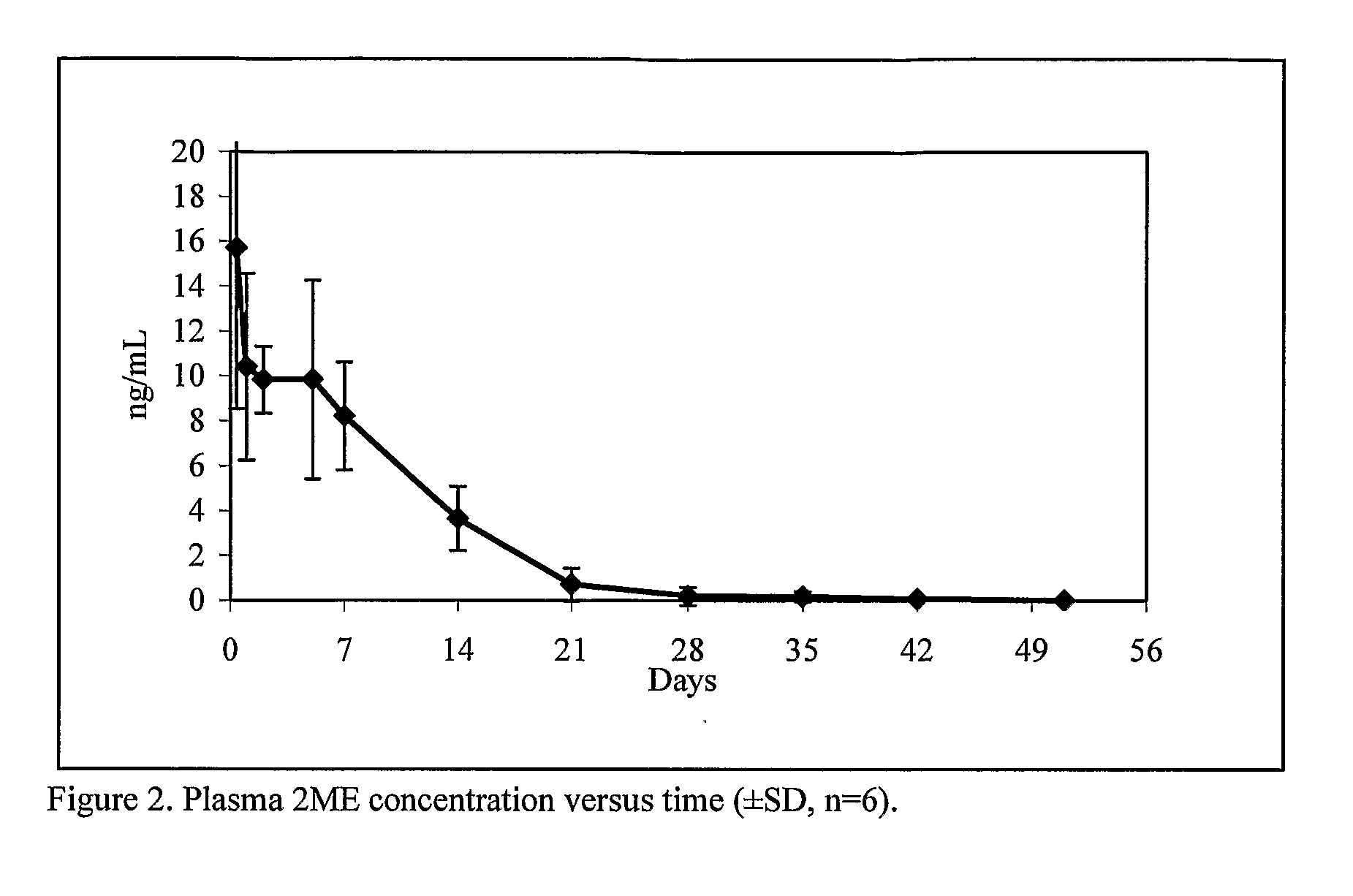
Long Acting Injectable Crystal Formulations of Estradiol Metabolites and Methods of Using Same
InactiveUS20080220069A1Simpler and less-expensive to manufactureEasy to managePowder deliveryOrganic active ingredientsDiseaseMetabolite
The present invention provides sustained release formulations of estradiol metabolites whereby the in vivo pharmacokinetics are manipulated by a method selected from the group consisting of chemical modification, crystal packing formation, particle size or a combination thereof. Such compositions are useful in the long-term treatment of a wide variety of diseases.
Owner:PR PHARMA
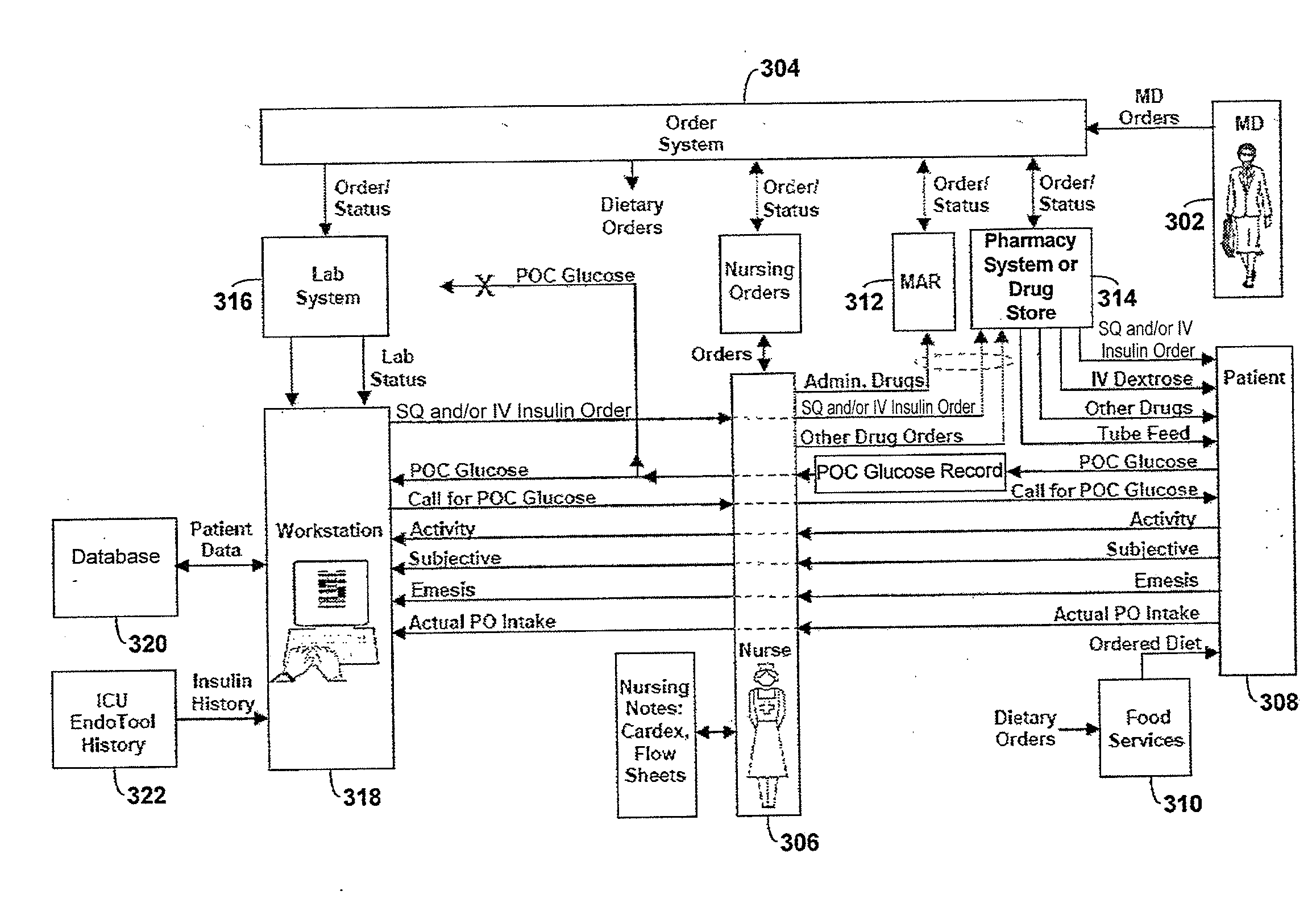
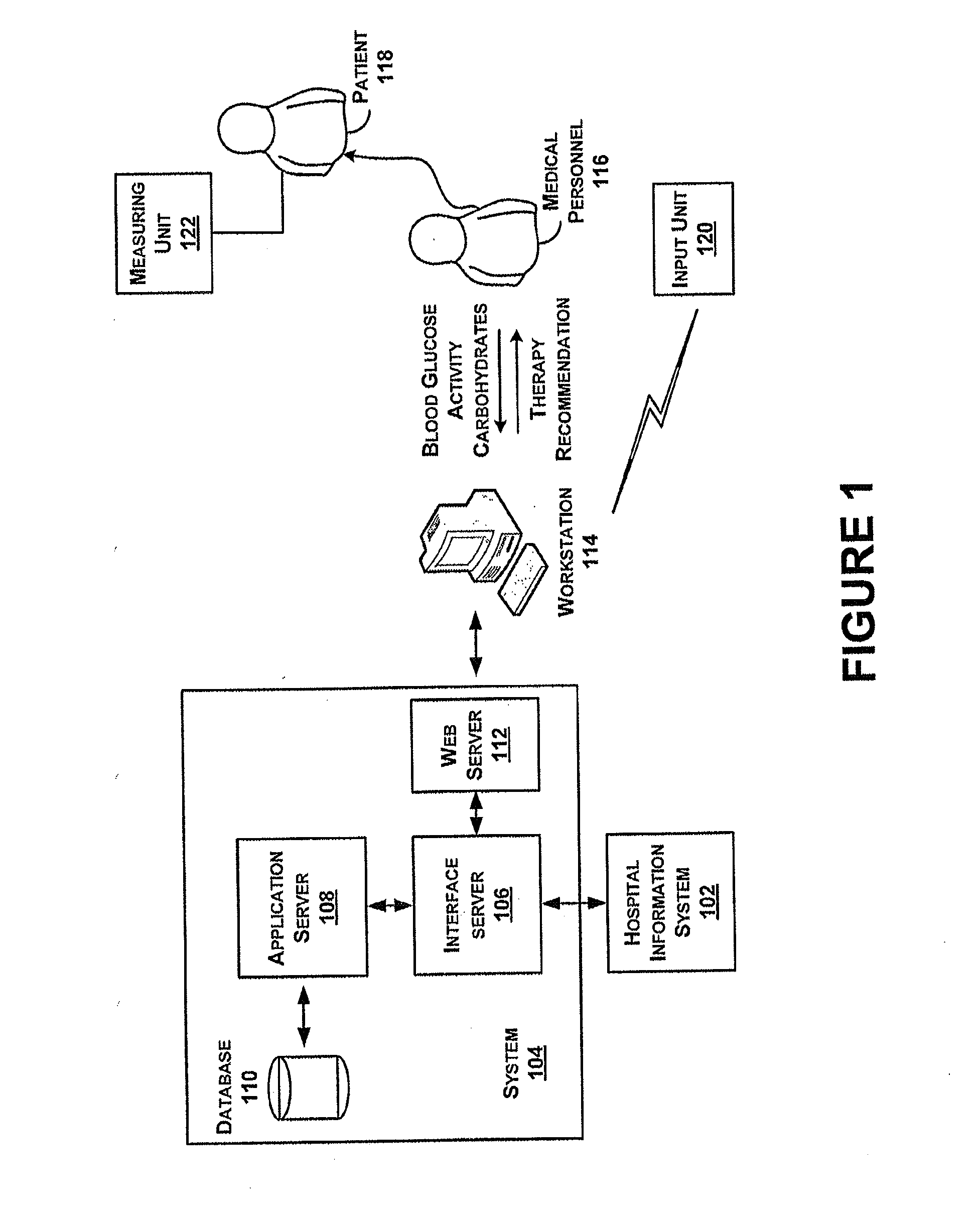
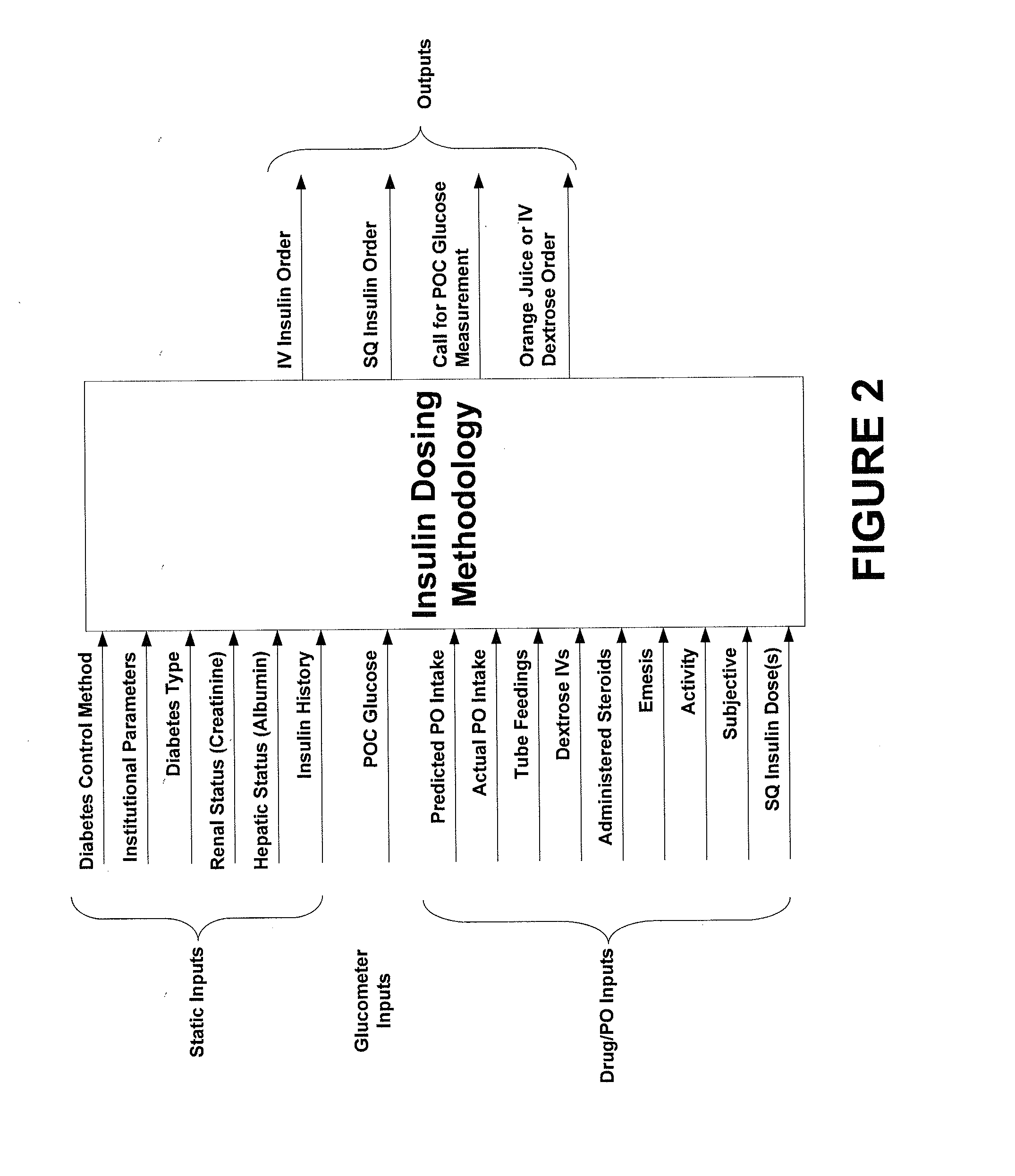
Systems and methods for determining insulin therapy for a patient
ActiveUS20130165901A1Data processing applicationsDrug and medicationsSubcutaneous insulinLong acting insulin
In example methods and systems described, insulin therapy for a patient can be determined. At least one of a short-acting subcutaneous insulin dosage recommendation, a correction subcutaneous insulin dosage recommendation, an intravenous insulin dosage recommendation, a recommended amount of carbohydrates to be administered to the patient, or combinations thereof, can be determined. In addition, information indicating a confirmation of a nutrition intake for the patient, and a long-acting insulin-on-board for the patient can be received, and based on this information, a required long-acting subcutaneous or intravenous insulin dosage for the patient can be determined. The short-acting subcutaneous or intravenous insulin dosage recommendation can be adjusted based, at least in part, on a difference between the long-acting insulin-on-board and the required long-acting subcutaneous or intravenous insulin dosage.
Owner:MONARCH MEDICAL TECH
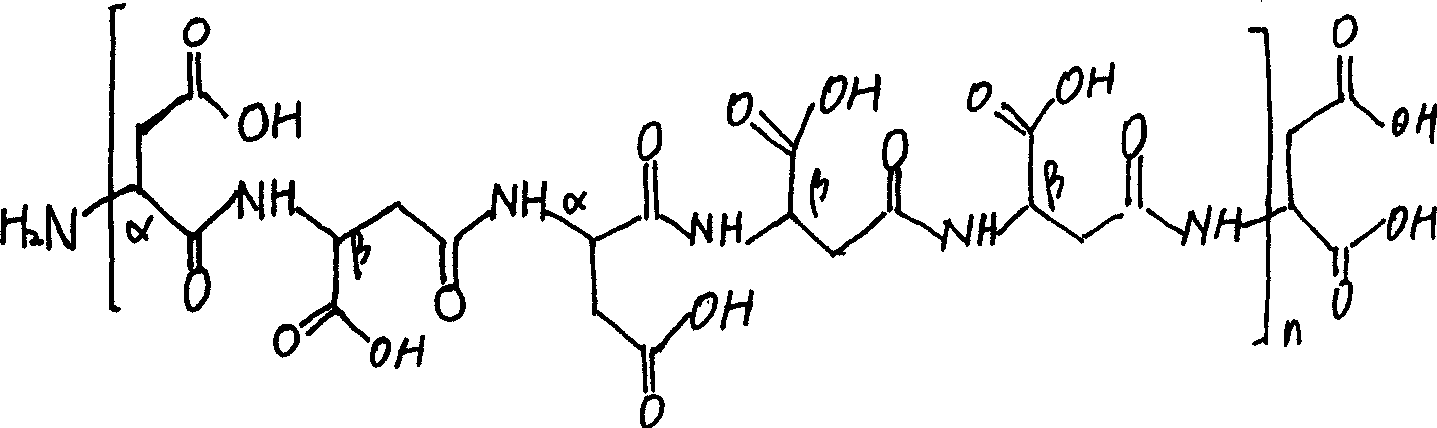
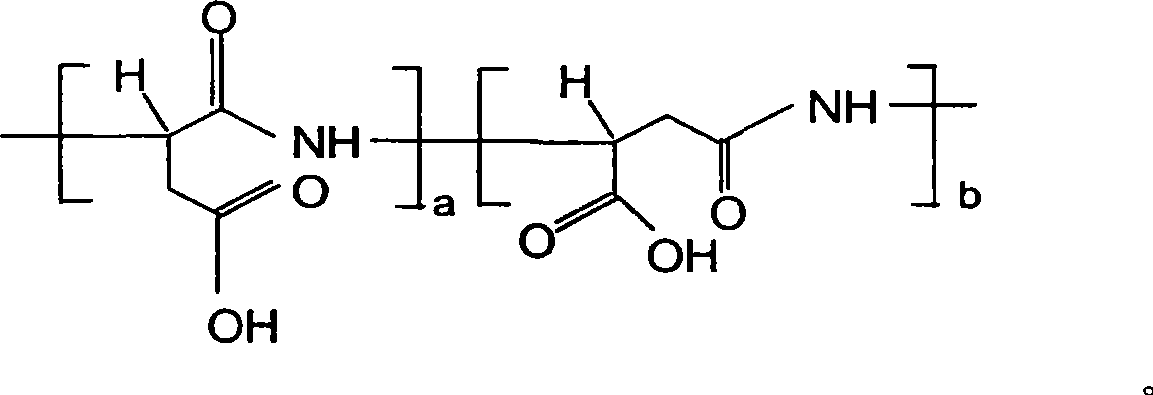
Nitrogen fertilizer compound synergist and preparation method
ActiveCN101450880AImprove the absorption and utilization effectIncrease profitAgriculture gas emission reductionFertilizer mixturesTrace element compositionNitrification inhibitors
The invention relates to nitrogen element fertilizer, in particular to a nitrogen element fertilizer composite synergist and a preparation method thereof. The composite synergist consists of a biochemical inhibitor, a synergist, a nitrogen element stabilizer, a carrier and microelements, the weight portion ratio of the materials is 1:0.01-1: 0.01-0.5: 0.5-1: 0-0.05, wherein the biochemical inhibitor can be a nitrification inhibitor and a urease inhibitor, the synergist can be poly-aspartic acid, and the nitrogen element stabilizer can be hydrolyzed amino acid, humic acid, alginic acid or alginic acid water soluble salt. The preparation method comprises the following steps: crushing the raw materials, screening the raw materials with a sieve of 30 to 100 meshes, and stirring and mixing the raw materials according to the proportion. The nitrogen element fertilizer composite synergist is suitable for growing long acting nitrogenous fertilizer taking urea or ammonium nitrogen fertilizer as raw materials and can be applied to various soils together with urea or ammonium nitrogen fertilizer so as to effectively prolong the fertilizer efficiency period of the urea or the ammonium nitrogen fertilizer. The fertilizer efficiency period can reach 100 to 120 days. The nitrogen element fertilizer composite synergist has remarkable resistance to diseases, drought and lodging, effectively improves the capability of soil to reserve nutrients, and has good effects for off-season crops.
Owner:SHENYANG INST OF APPLIED ECOLOGY - CHINESE ACAD OF SCI
Anti-bacterial water-based paint and preparation method thereof
InactiveCN102702889AImprove aging resistanceImprove the pulverization performanceBiocideAntifouling/underwater paintsWater basedEscherichia coli
The invention relates to anti-bacterial water-based paint and a preparation method thereof. The paint comprises the following components in parts by weight: 0.2-11 parts of anti-bacterial agent, 8-33 parts of nano material, 23-64 parts of water-based resin dispersoid and 0.75-18 parts of adhesive resin or plasticizer. The preparation method comprises the following steps: firstly preparing a nano silver anti-bacterial agent; mixing deionized water, the anti-bacterial agent, a wetting agent, a dispersing agent and a defoaming agent and uniformly mixing, adding the nano material, uniformly dispersing to obtain the water-based dispersoid; adding the obtained water-based dispersoid to the mixed emulsion or water-based resin dispersoid, then adding the adhesive resin or plasticizer and various conventional assistants, stirring and dispersing evenly; adding pigments or colorant; and supplementing water to obtain the anti-bacterial water-based paint. The long-acting broad-spectrum antibacterial water-based paint has high fungicidal efficiency (more than 99%) on escherichia coli, staphylococcus aureus, black varietas of bacillus subtilis and the like and can reduce the high concentrate of organic matters of formaldehyde to the range of specified concentration index.
Owner:ANHUI JINDUN PAINT
Long-acting veterinary polypeptides and methods of producing and administering same
InactiveUS20070184530A1Improving biological half lifeProlong lifePeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides comprising at least two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a non-human peptide-of-interest are disclosed. Pharmaceutical compositions comprising the non-human polypeptides and polynucleotides of the invention and methods of using both human and non-human polypeptides and polynucleotides are also disclosed.
Owner:MODIGENE INC
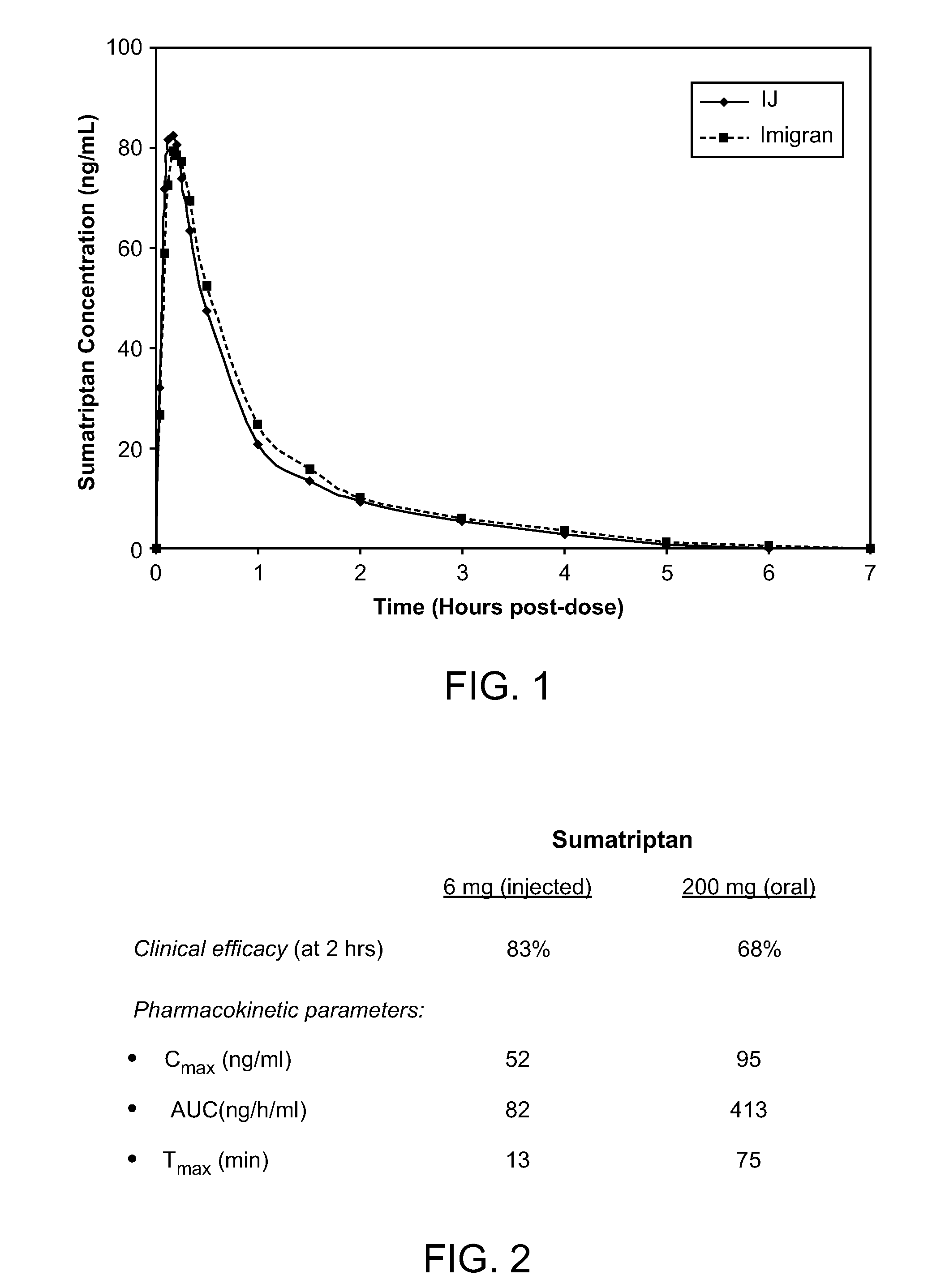
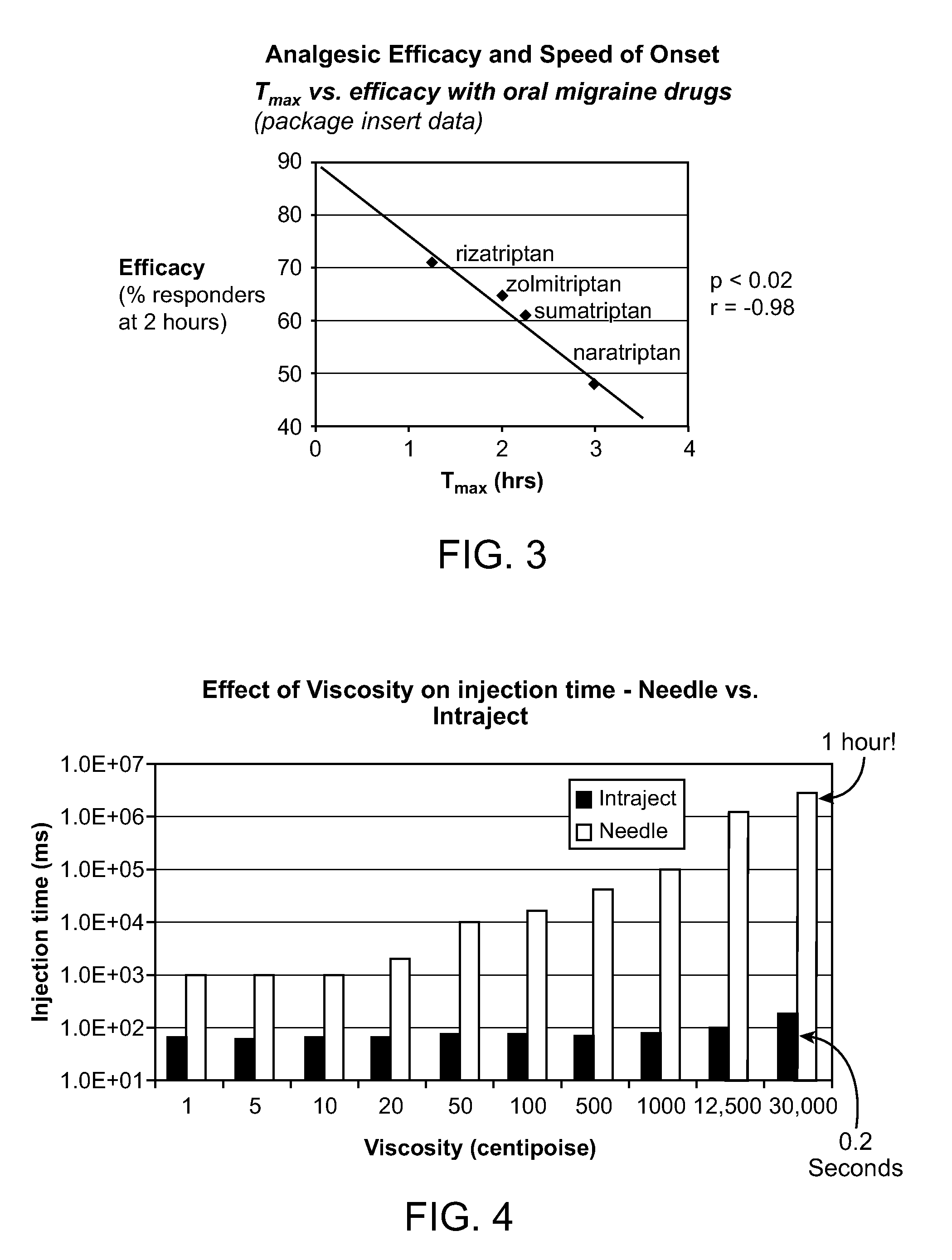
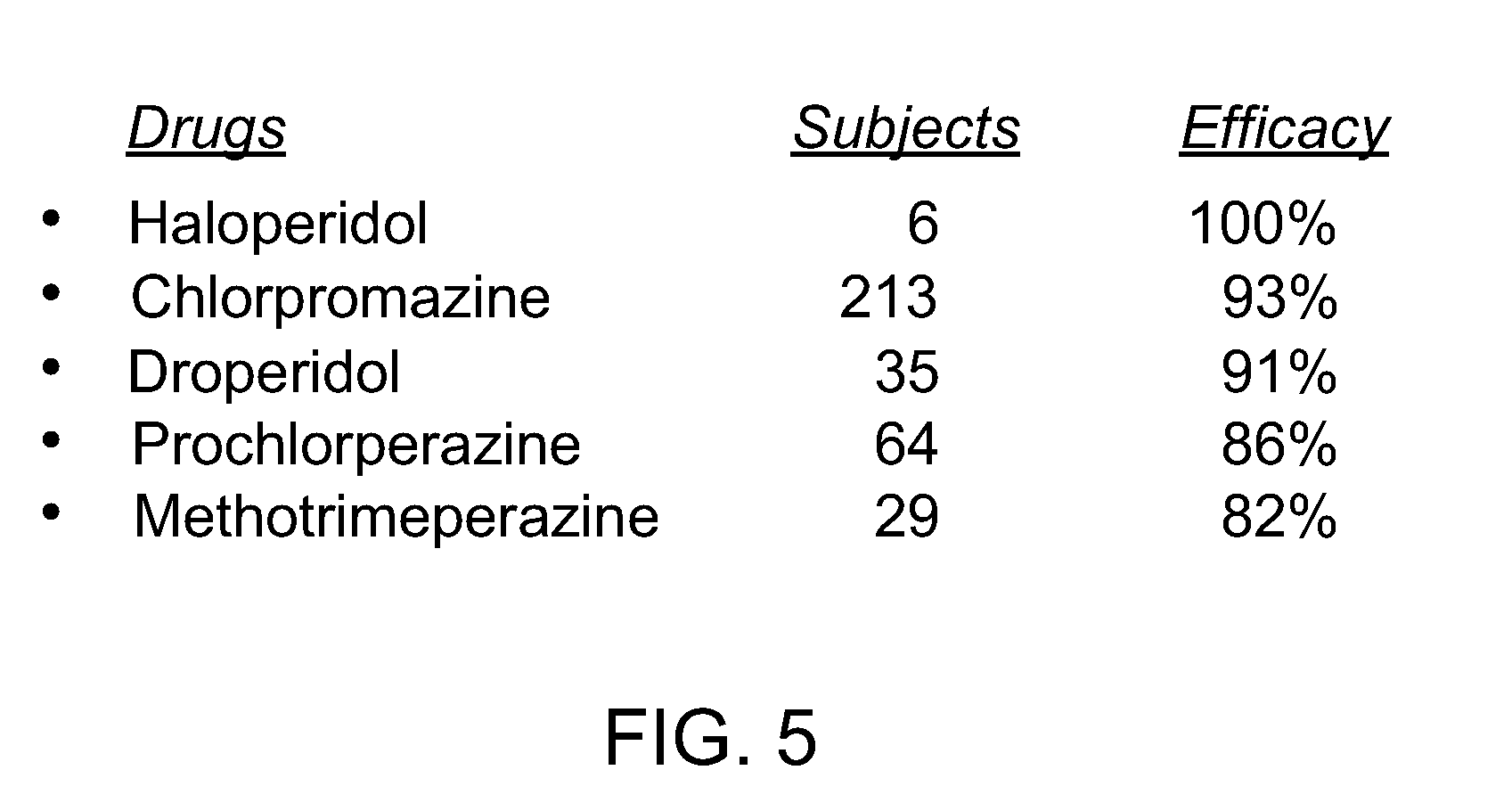
Novel formulations for treatment of migraine
InactiveUS20110118189A1Immediate pharmacological effectLonger effectBiocideSenses disorderNeedle freeHeadaches
Systems and methods are described for treating un-met medical needs in migraine and related conditions such as cluster headache. Included are treatments that are both rapid onset and long acting, which include sustained release formulations, and combination products. Also included are treatments for multiple symptoms of migraine, especially headache and nausea and vomiting. Systems that are self contained, portable, prefilled, and simple to self administer at the onset of a migraine attack are disclosed, and preferably include a needle-free injector and a high viscosity formulation, to eliminate such issues as fear of self administration with needles, and needle stick and cross contamination.
Owner:ZOGENIX INC
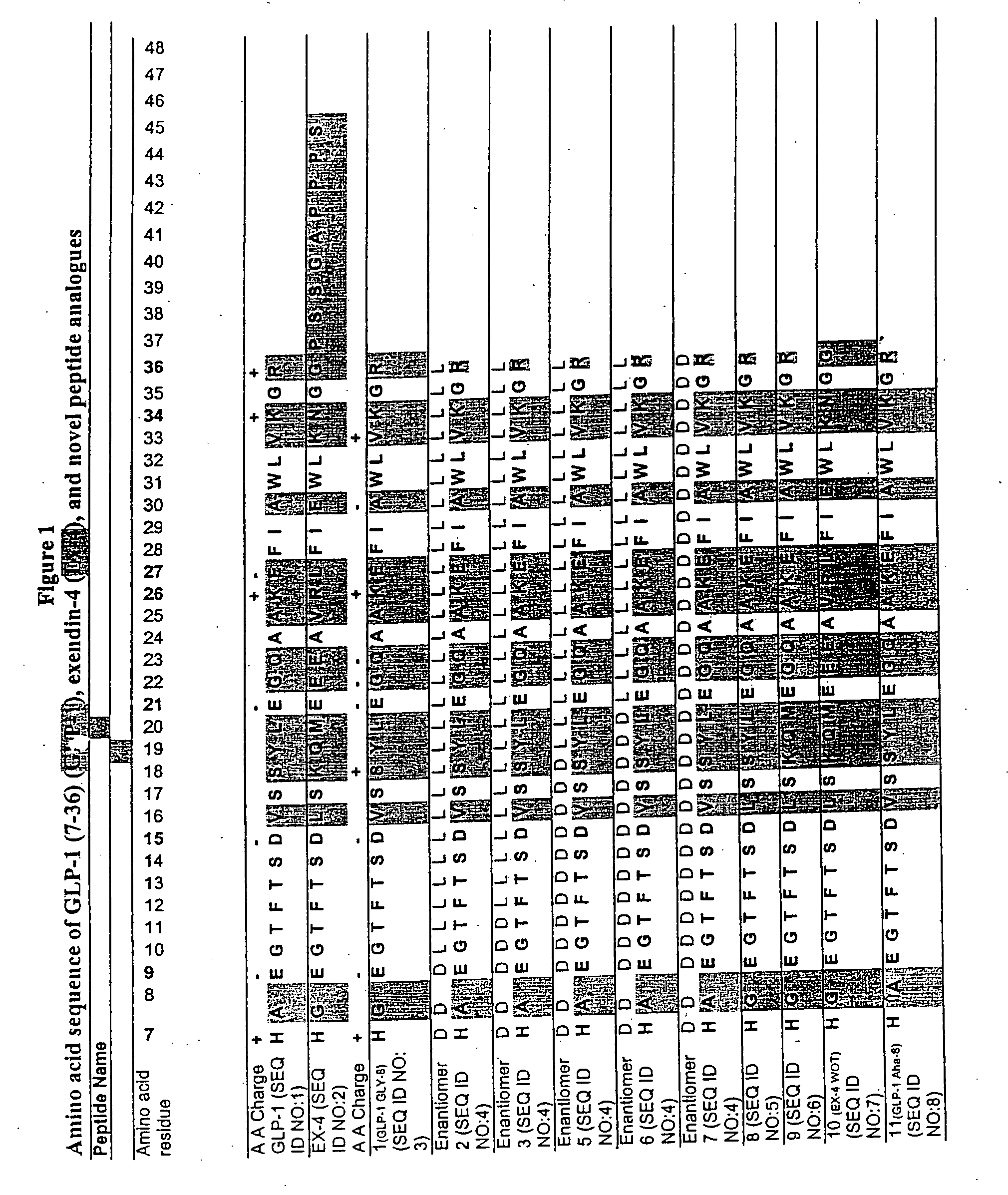
Glp-1 exendin-4 peptide analogs and uses thereof
Owner:UNITED STATES OF AMERICA
Long-acting growth hormone and methods of producing same
ActiveUS20100081614A1Increase the areaReduce dosing frequencyNervous disorderPeptide/protein ingredientsNucleotideGrowth hormone
A polypeptide and polynucleotides encoding same comprising one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to an amino terminus of a growth hormone and two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a carboxy terminus of a growth hormone are disclosed. Pharmaceutical compositions comprising the polypeptide and polynucleotides of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
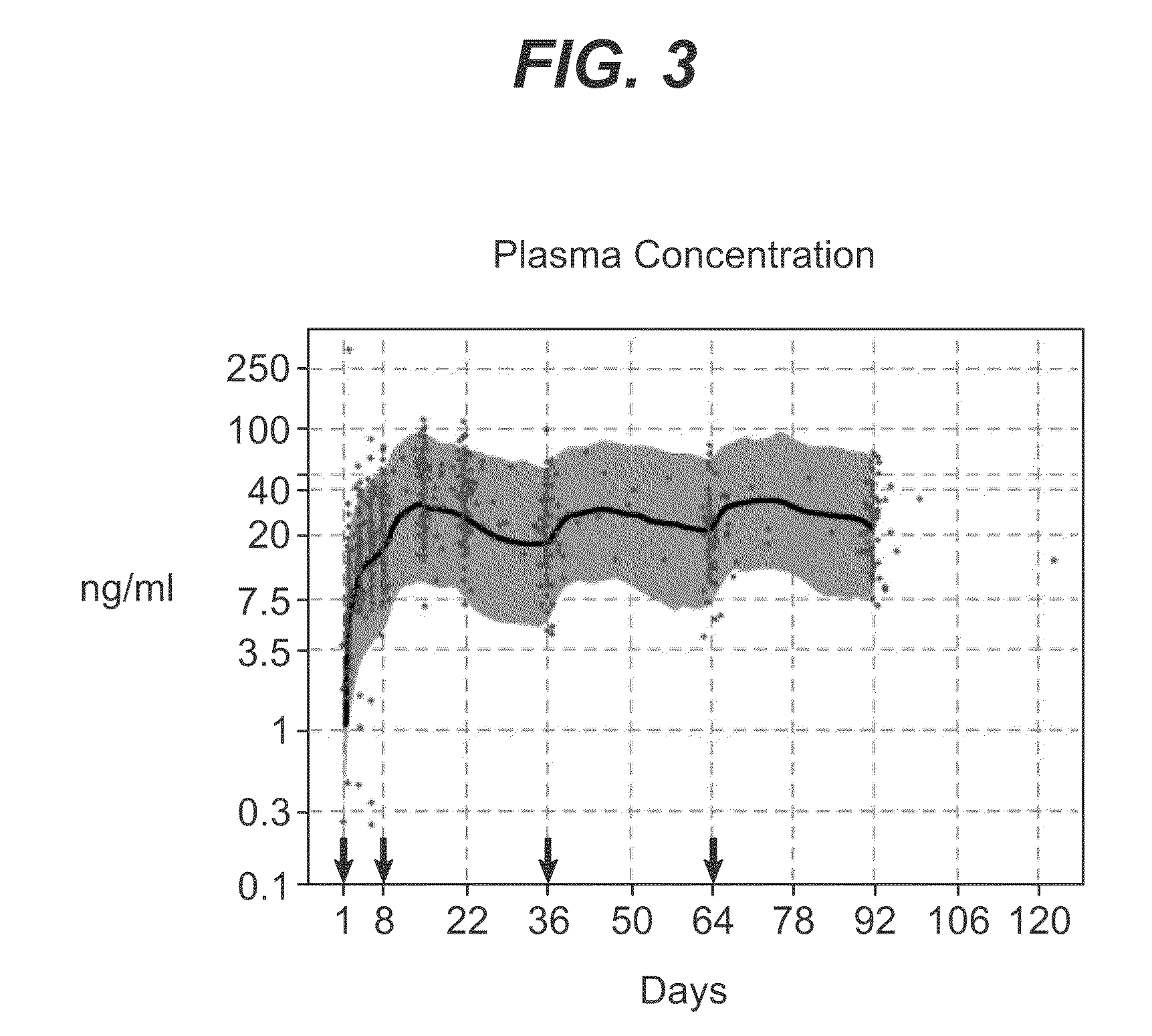
Long-acting coagulation factors and methods of producing same
Polypeptides comprising at least one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to the carboxy terminus but not to the amino terminus of a coagulation factor and polynucleotides encoding the same are disclosed. Pharmaceutical compositions comprising the polypeptides and polynucleotides of the invention and methods of using and producing same are also disclosed.
Owner:OPKO BIOLOGICS
Long Acting Biologically Active Conjugates
InactiveUS20070207952A1High level of drugPoor adhesionBiocidePeptide/protein ingredientsImmunodeficiency virusIn vivo
The invention provides biologically active compounds that may be reacted with macromolecules, such as albumin, to form covalent linked complexes wherein the resulting complexes exhibit a desired biological activity in vivo. More specifically, the complexes are isolated complexes comprising a biologically active moiety covalently bound to a linking group and a protein. The complexes are prepared by conjugating a biologically active moiety, for example, a renin inhibitor or a viral fusion inhibitor peptide, with purified and isolated protein. The complexes have extended lifetimes in the bloodstream as compared to the unconjugated molecule, and exhibit biological activity for extended periods of time as compared to the unconjugated molecule. The invention also provides anti-viral compounds that are inhibitors of viral infection and / or exhibit anti-fusiogenic properties. In particular, this invention provides compounds having inhibiting activity against viruses such as human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), human parainfluenza virus (HPV), measles virus (MeV), and simian immunodeficiency virus (SIV) and that have extended duration of action for the treatment of viral infections.
Owner:SEQUOIA PHARMACEUTICALS INC
Rapid acting and long acting insulin combination formulations
ActiveUS20080039368A1Increase speedReduce the amount of solutionPeptide/protein ingredientsMetabolism disorderBefore BreakfastIntensive insulinotherapy
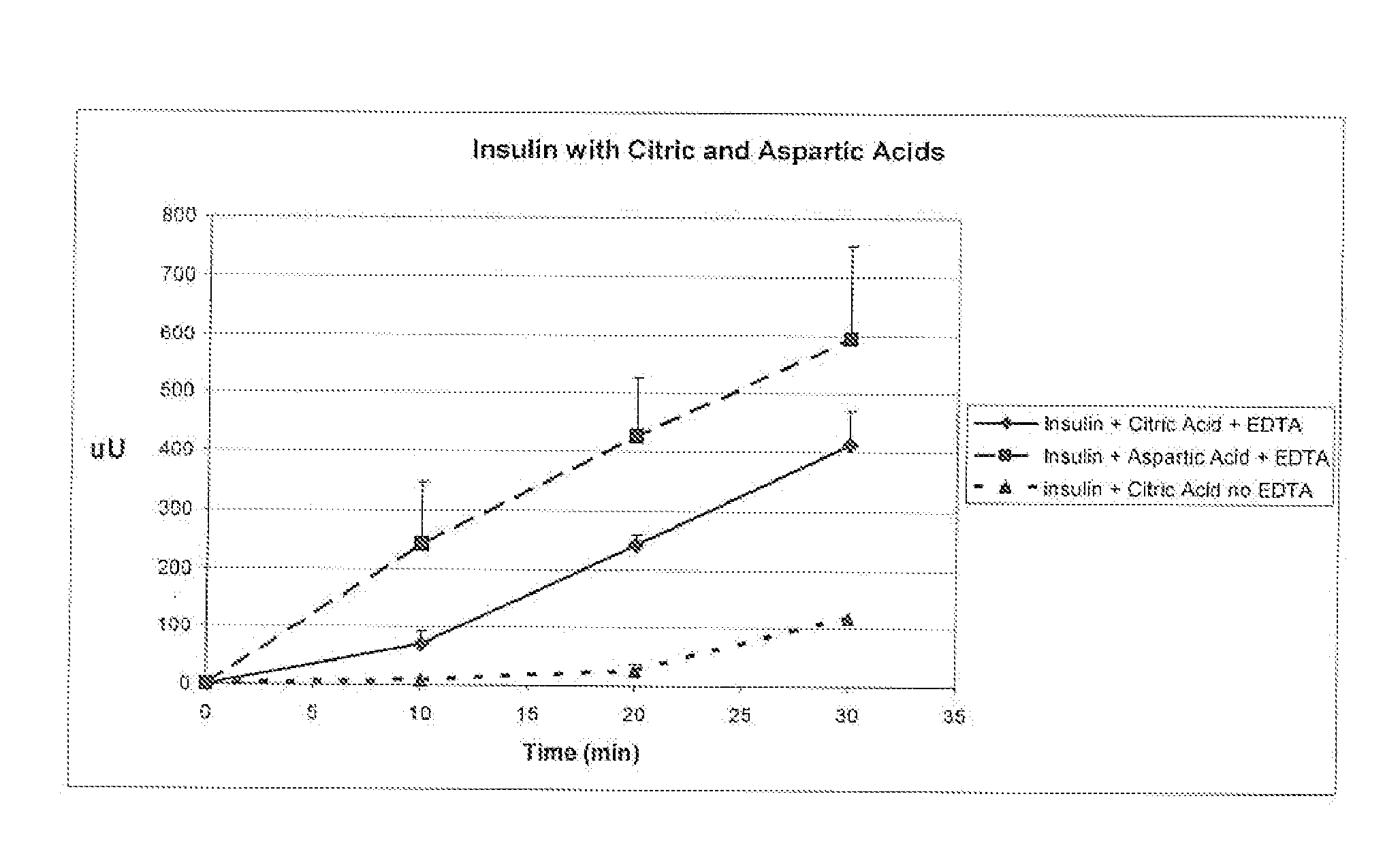
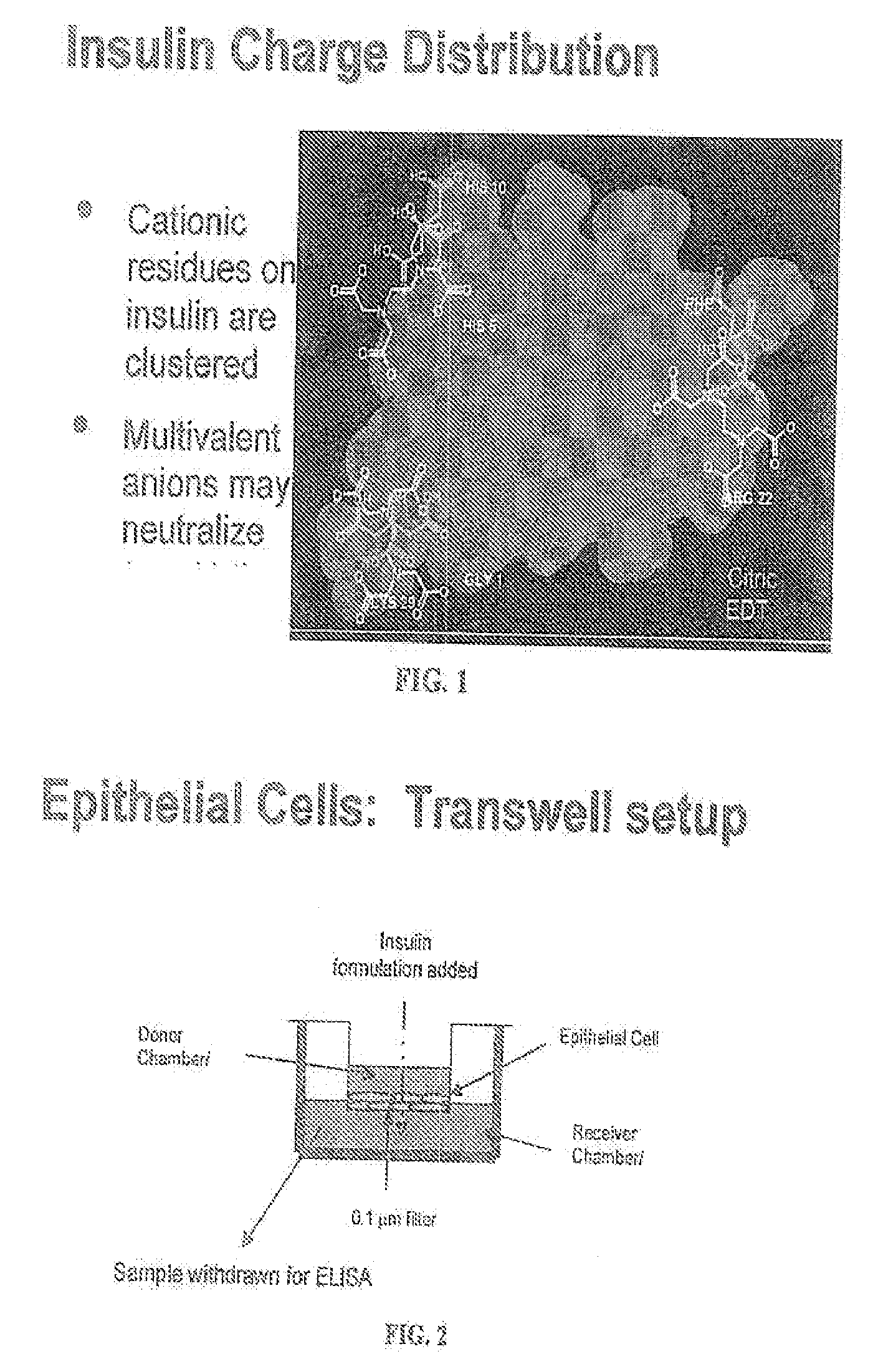
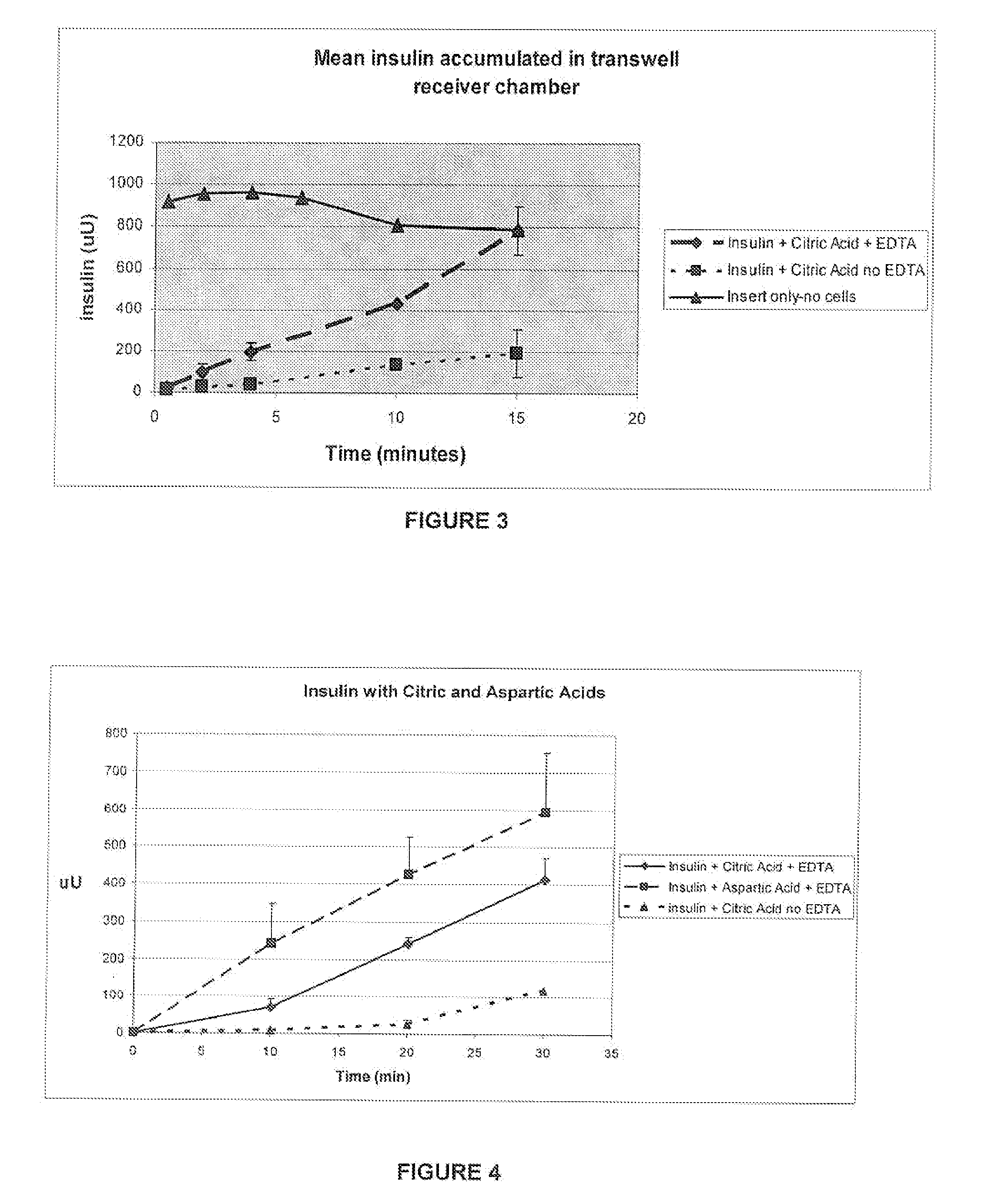
A combined rapid acting-long acting insulin formulation has been developed wherein the pH of the rapid acting insulin is adjusted so that the long acting glargine remains soluble when they are mixed together. In the preferred embodiment, this injectable basal bolus insulin is administered before breakfast, provides adequate bolus insulin levels to cover the meal, does not produce hypoglycemia after the meal and provides adequate basal insulin for 24 hours. Lunch and dinner can be covered by two bolus injections of a fast acting, or a rapid acting or a very rapid acting insulin. As a result, a patient using intensive insulin therapy should only inject three, rather than four, times a day. Experiments have been performed to demonstrate, the importance of the addition of specific acids to hexameric insulin to enhance speed and amount of absorption and preserve bioactivity following dissociation into the monomeric form by addition of a chelator such as EDTA. As shown by the examples, the preferred acids are aspartic, maleic, succinic, glutamic and citric acid. These are added in addition to a chelator, preferably ethylenediaminetetraacetic acid (EDTA). The results show that the citric acid formulation was more effective at dropping the blood glucose rapidly than the identical rapid acting formulation prepared with HCl in swine. Charge masking by the polyacid appears to be responsible for rapid insulin absorption. EDTA was not effective when used with adipic acid, oxalic acid or HCl at hastening the absorption of insulin. These results confirm the results seen in clinical subjects and patients with diabetes treated with the rapid acting insulin in combination with citric acid and EDTA.
Owner:ELI LILLY & CO
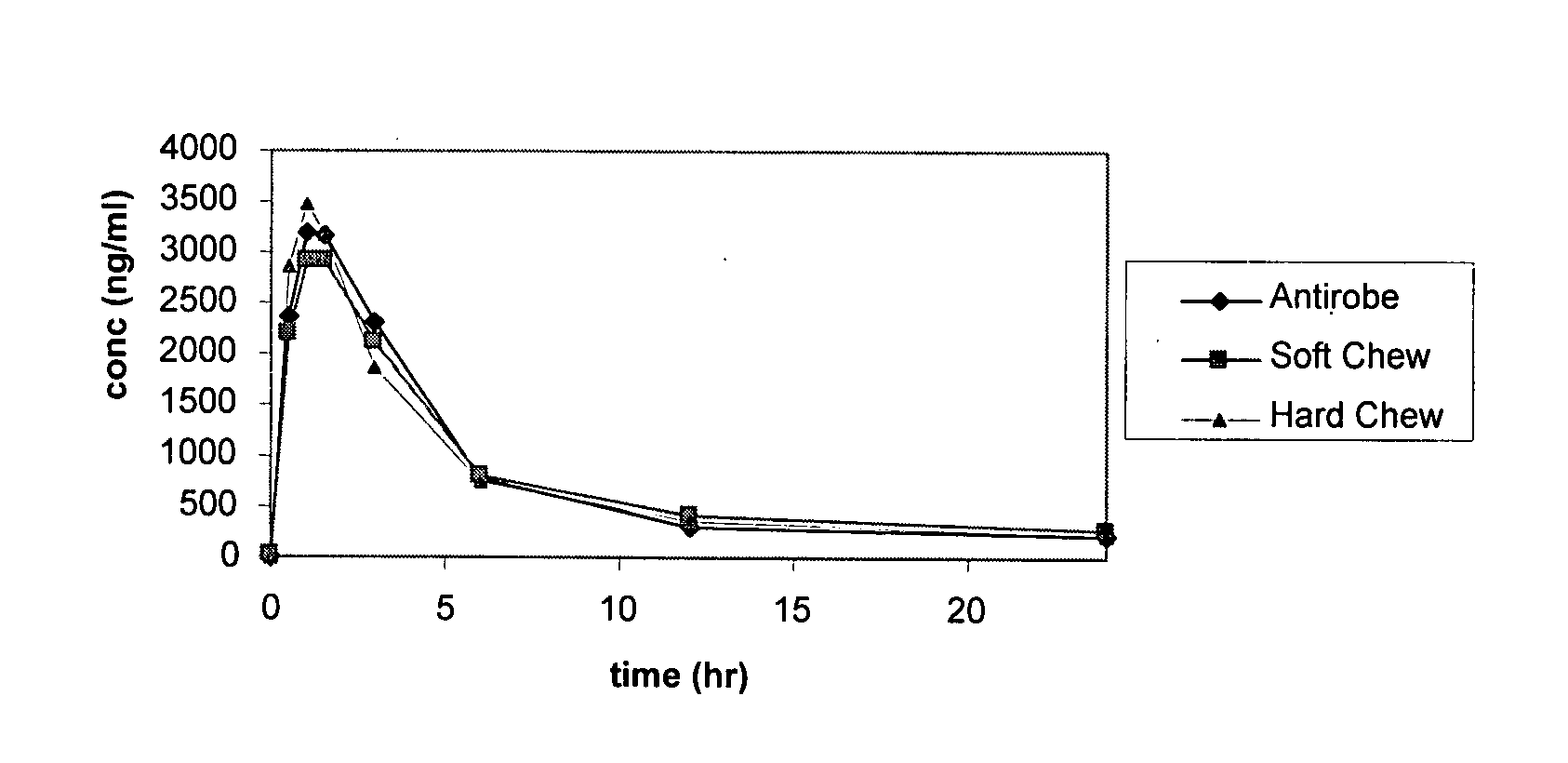
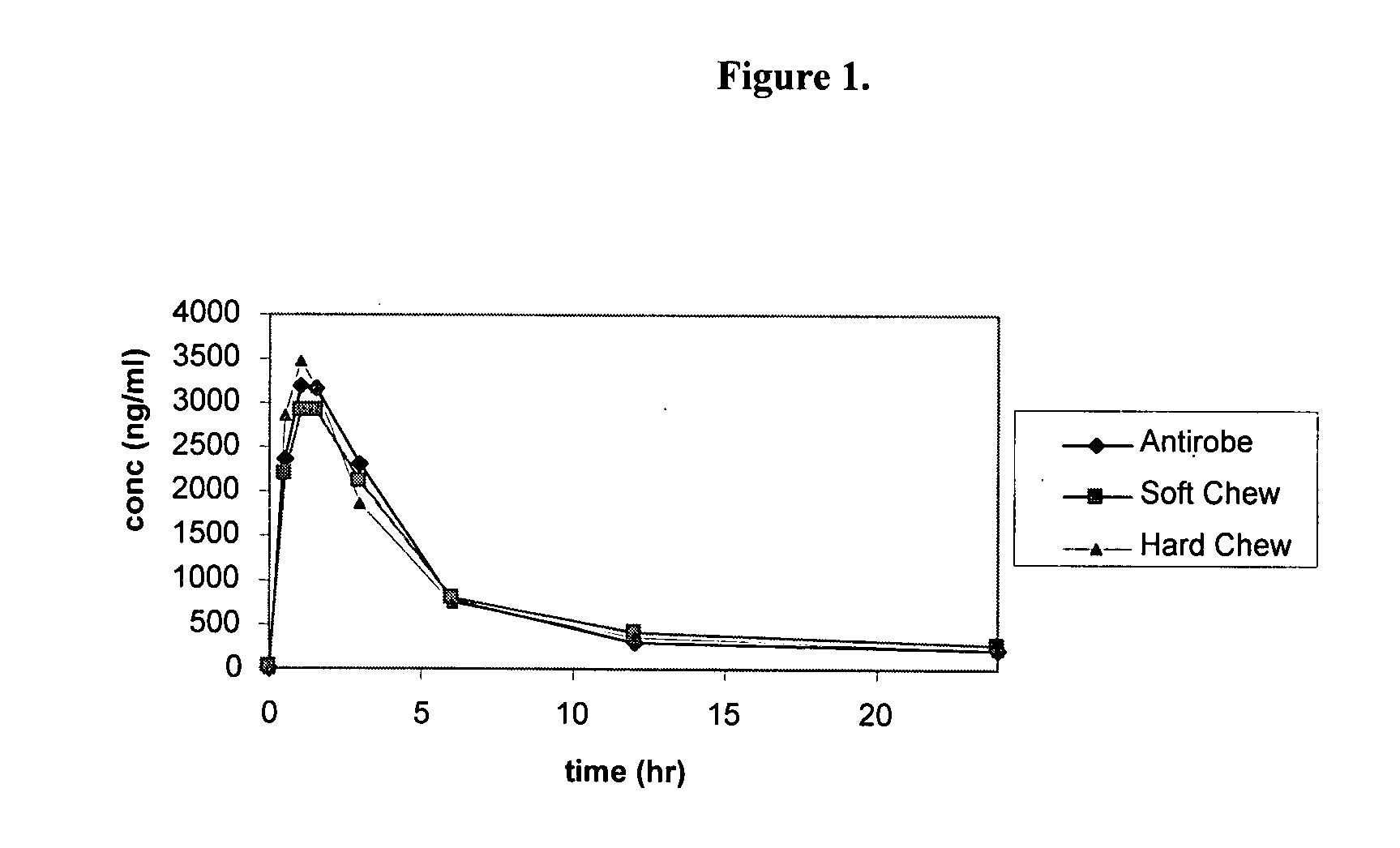
Novel soft chewable, tablet, and long-acting injectable veterinary antibiotic formulations
InactiveUS20080160067A1Effective treatmentReduce releaseBiocidePharmaceutical non-active ingredientsWhole bodyAntibiotic Y
This document relates to formulations for combating bacterial infections in animals which provide for improved long-acting oral and injectable formulations for systemic delivery of antibiotics, which are designed to achieve high bioavailability.
Owner:MERIAL LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com