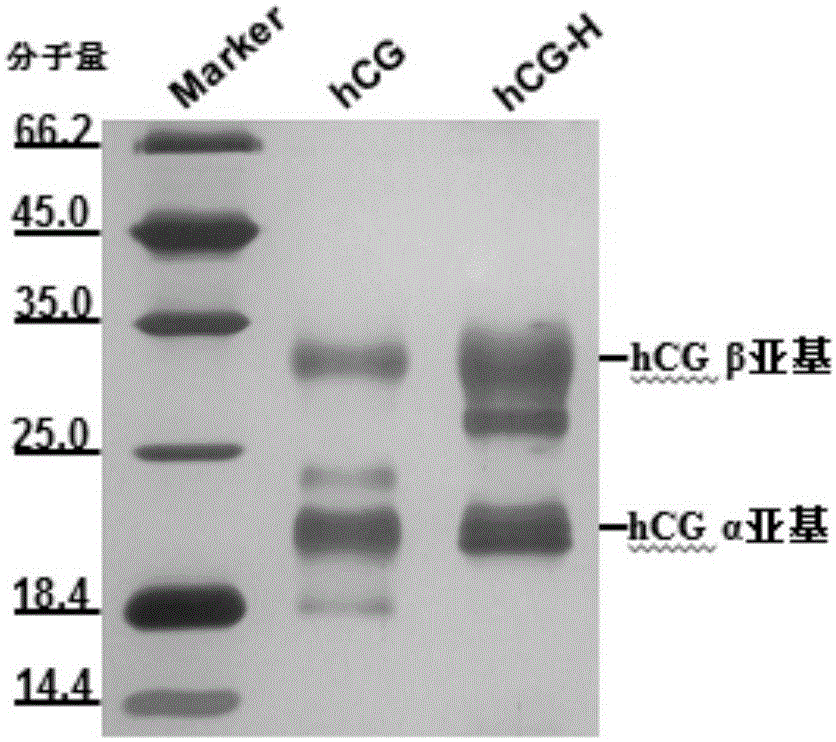
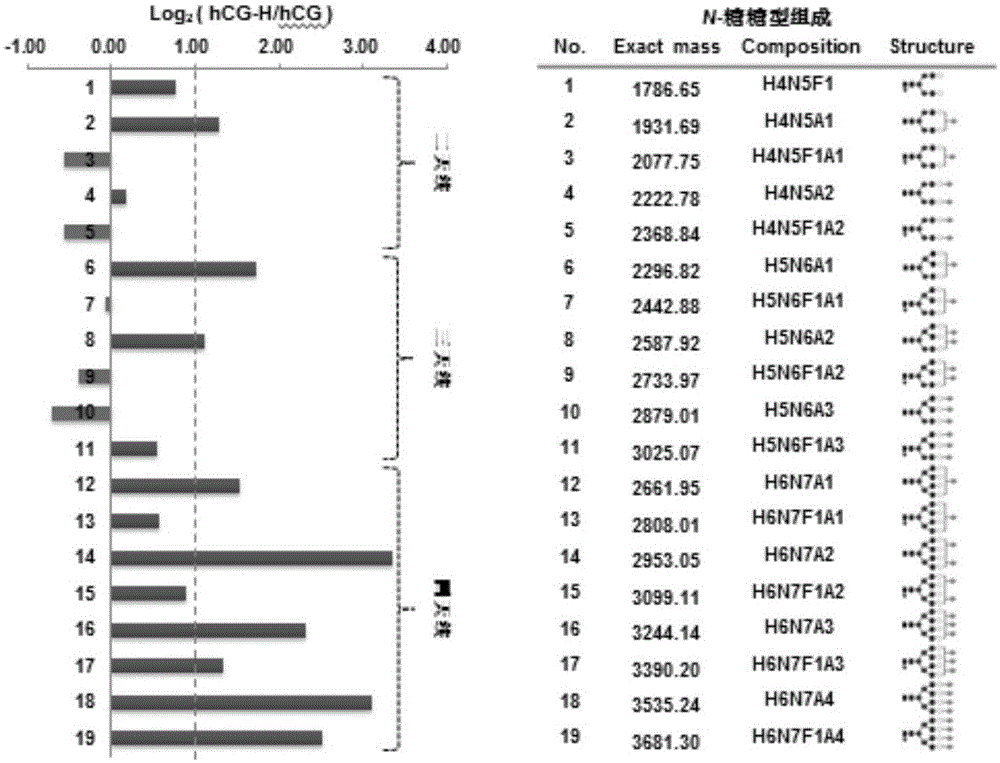
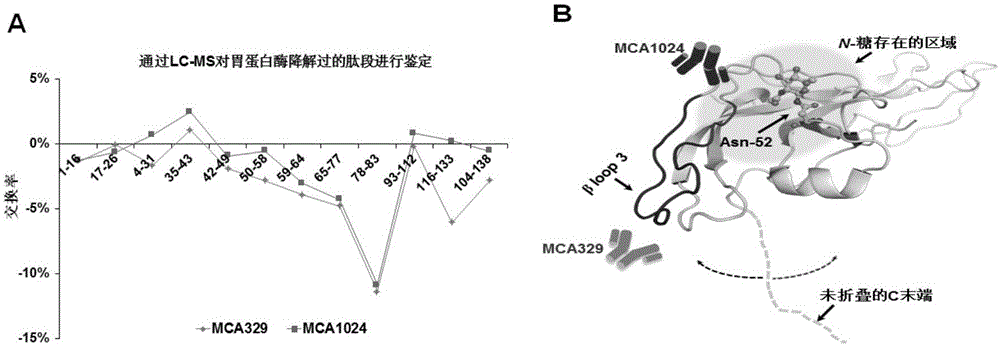
Patents
Literature
109 results about "Chorionic gonadotrophin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
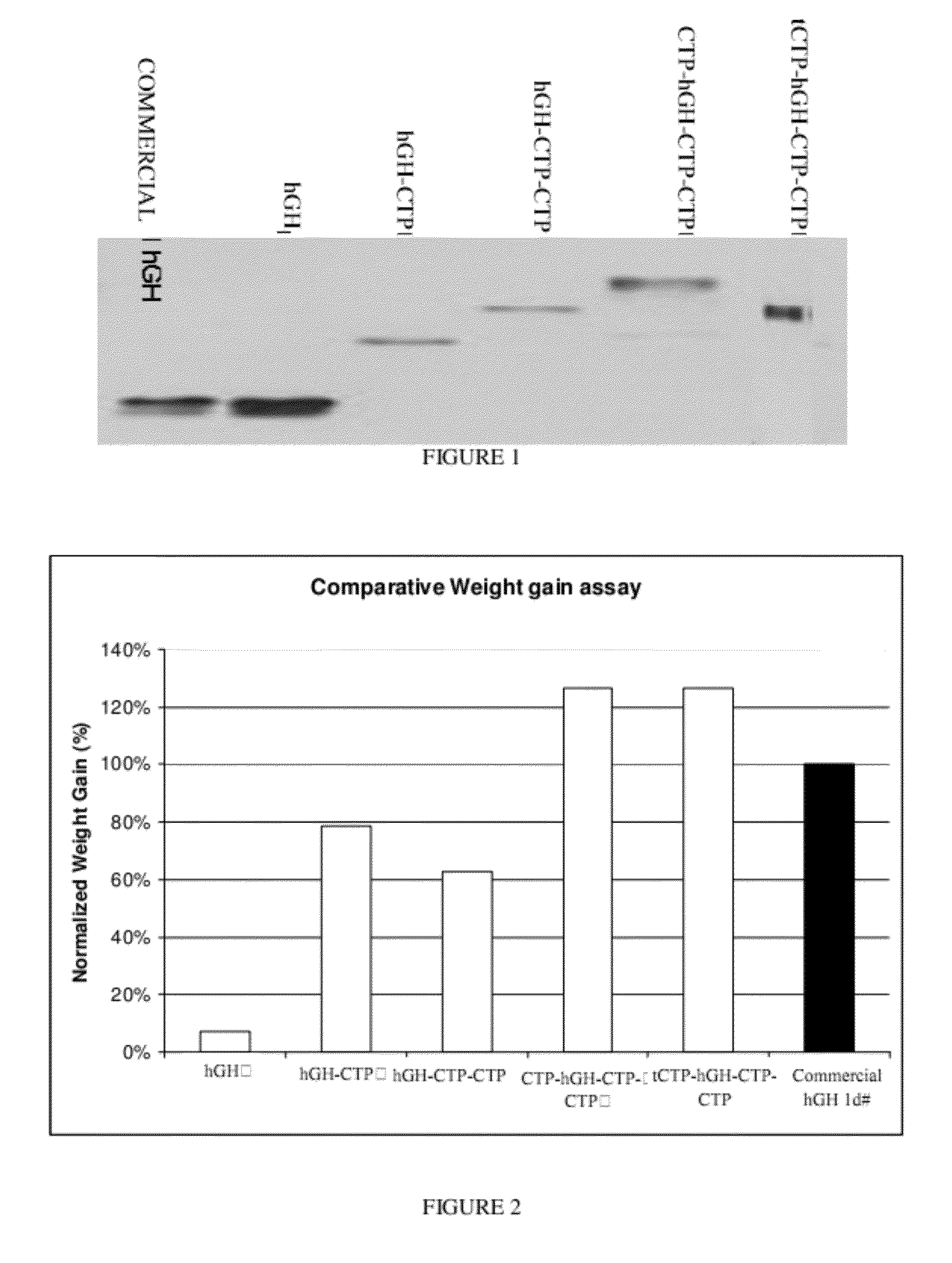
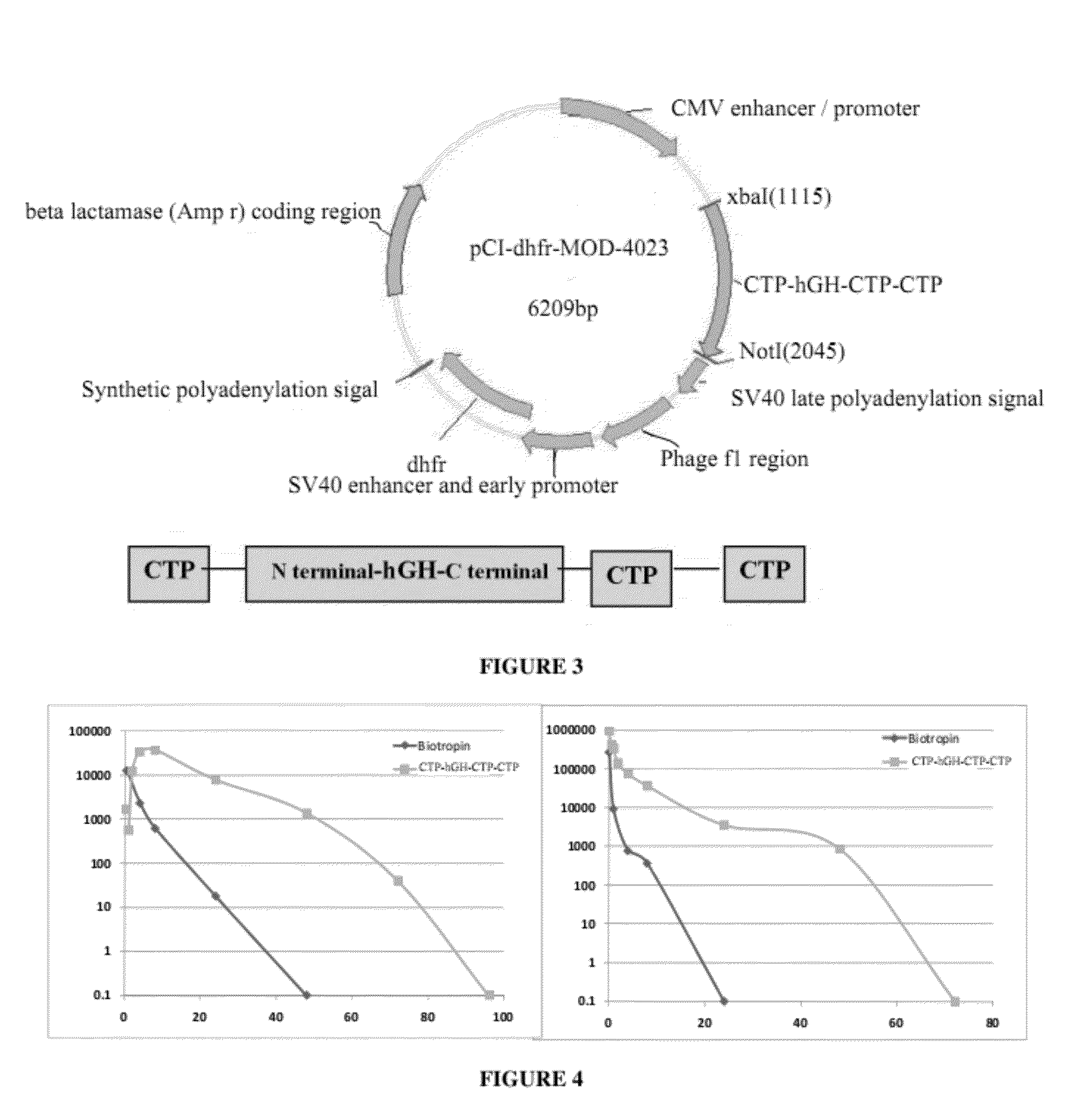
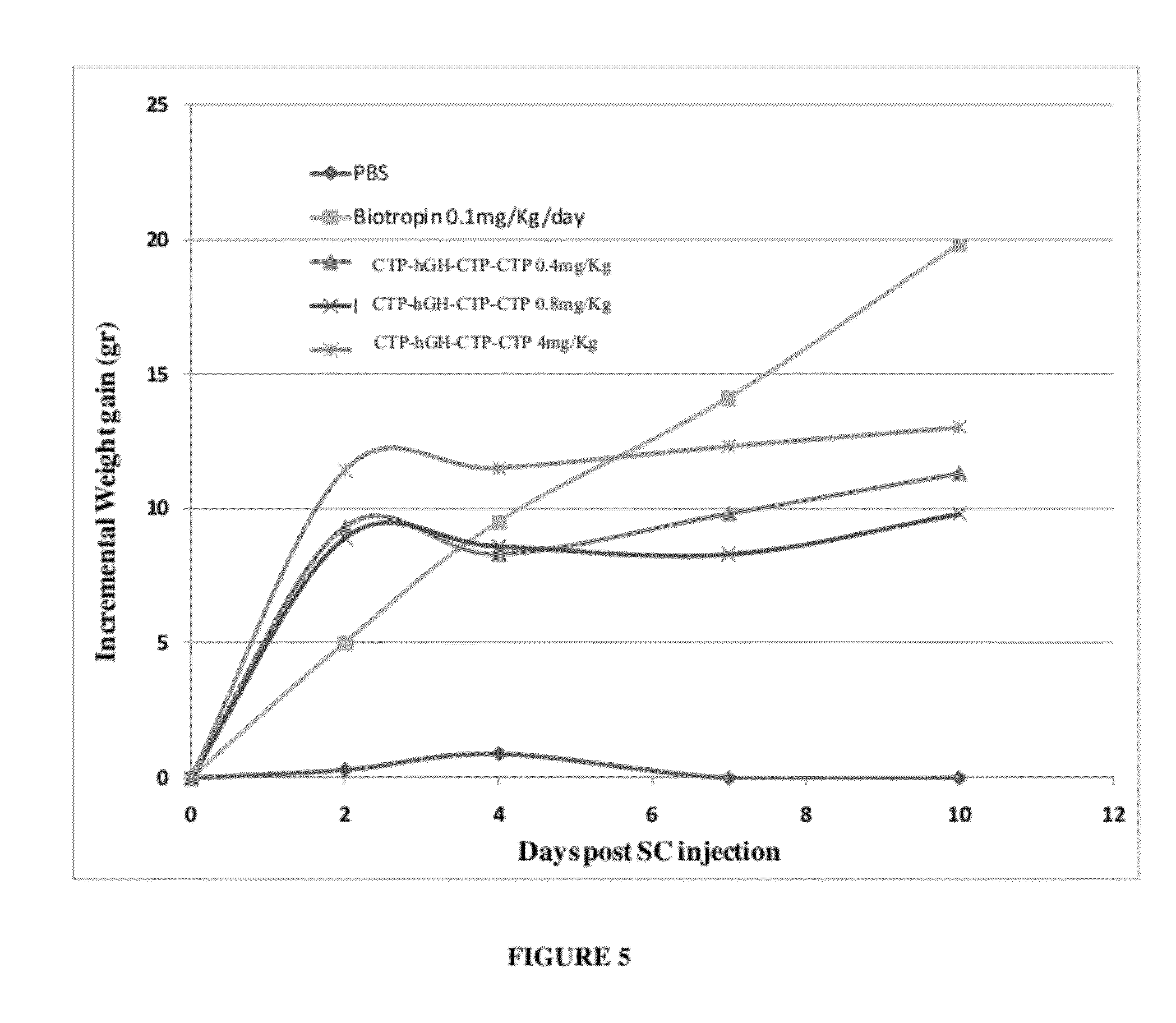
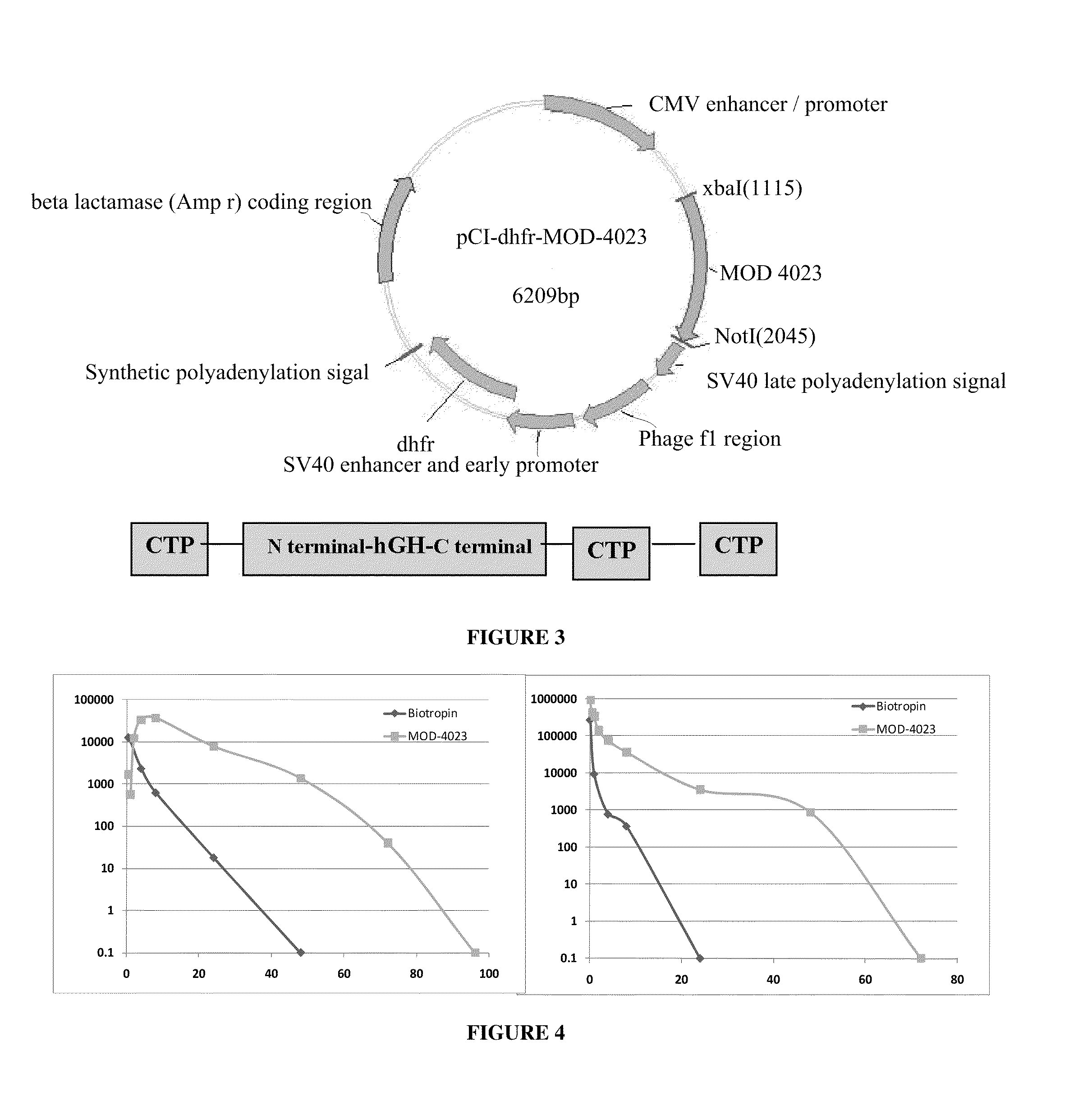
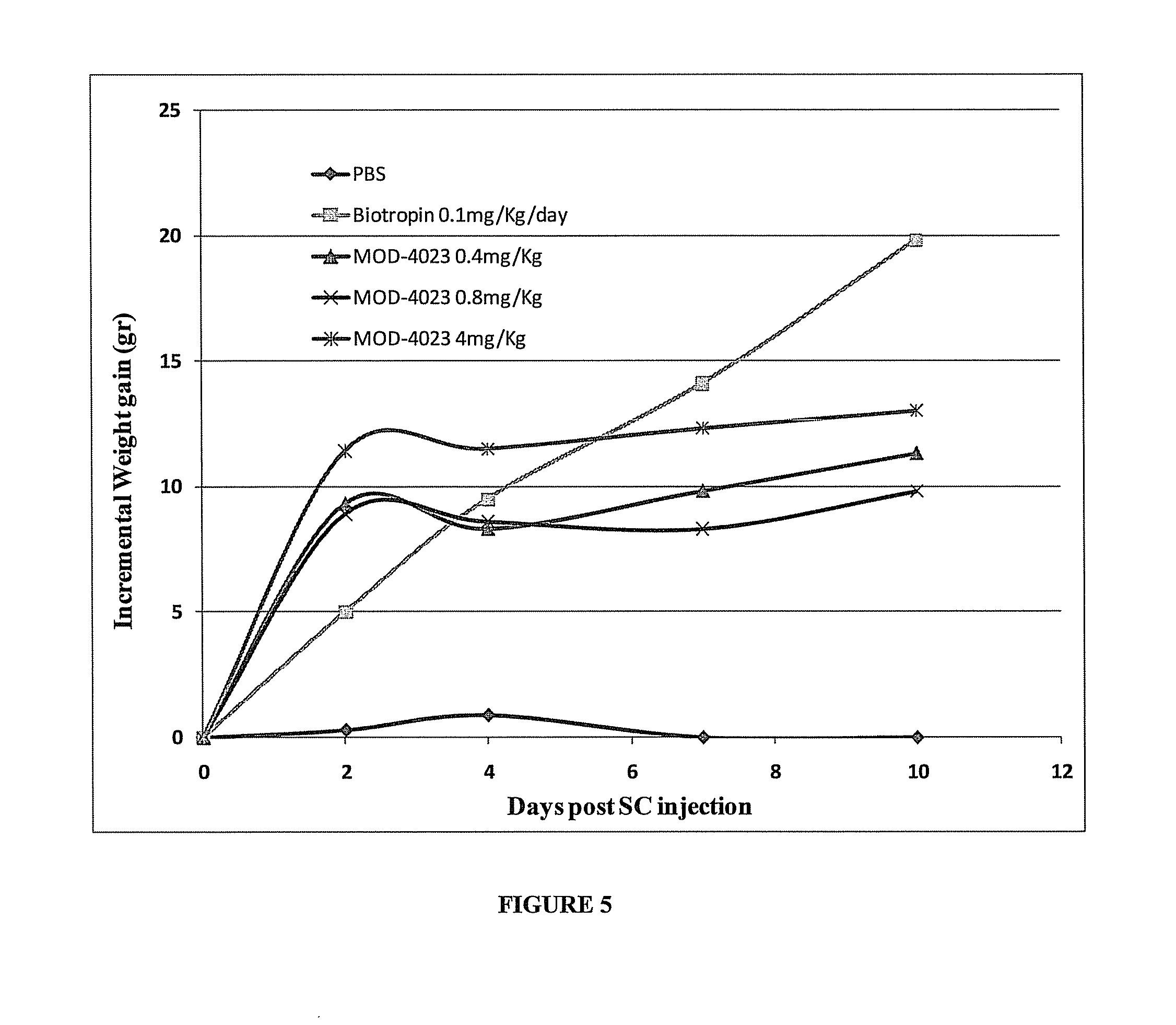
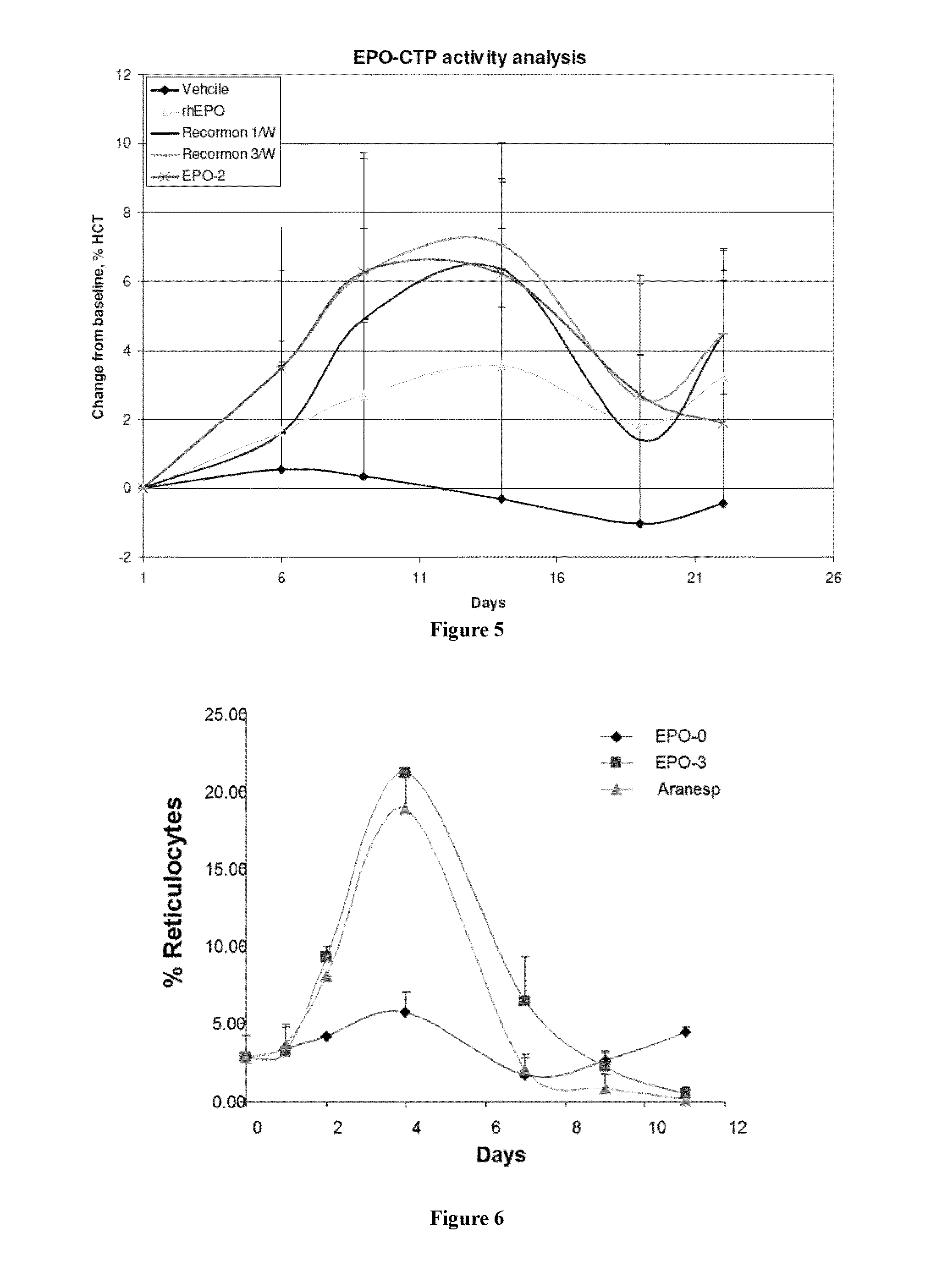
Long-acting growth hormone and methods of producing same
ActiveUS20120035101A1Decreasing body fatReduce weight lossPeptide/protein ingredientsMetabolism disorderSomatotropic hormoneNucleotide
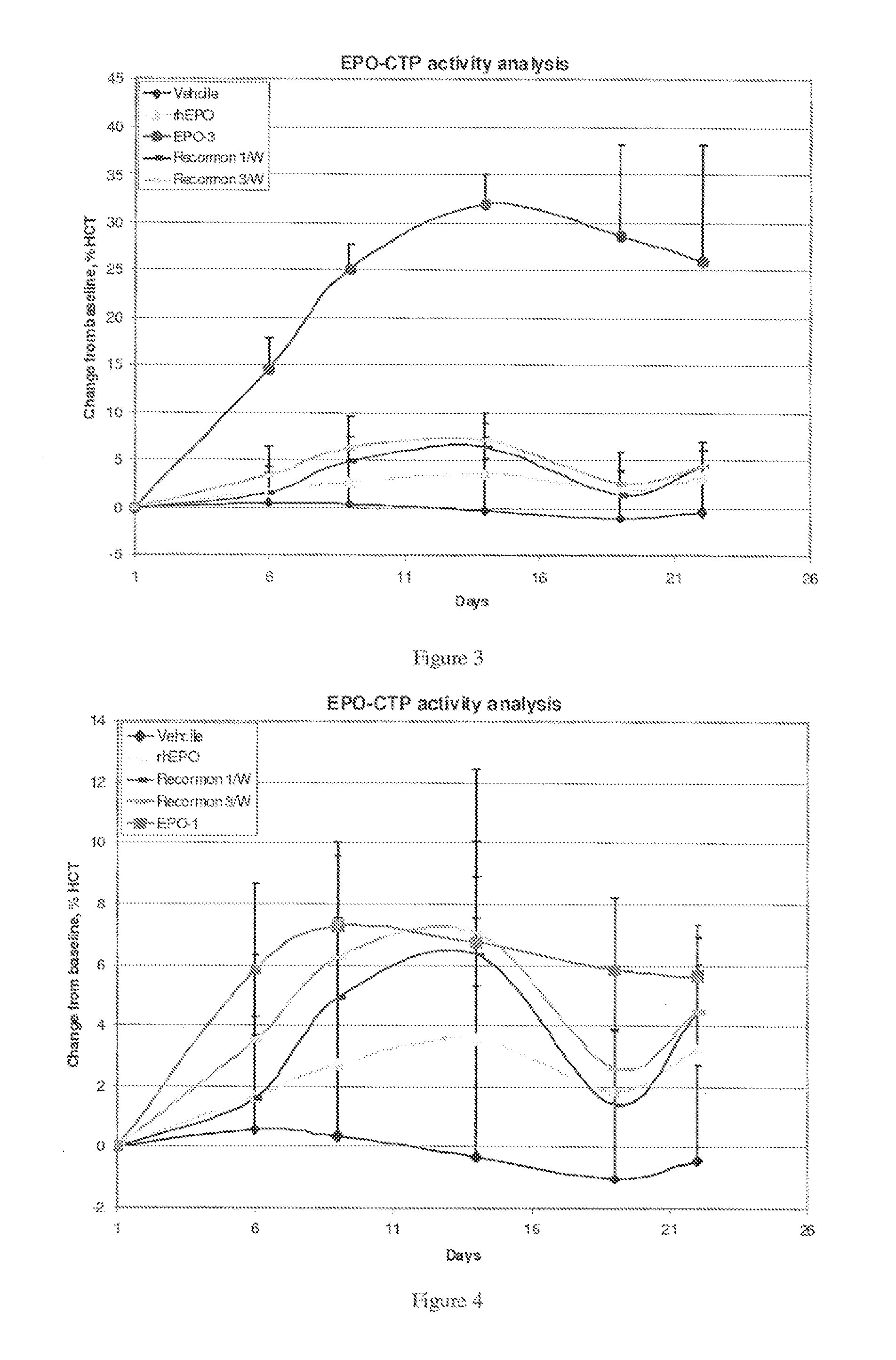
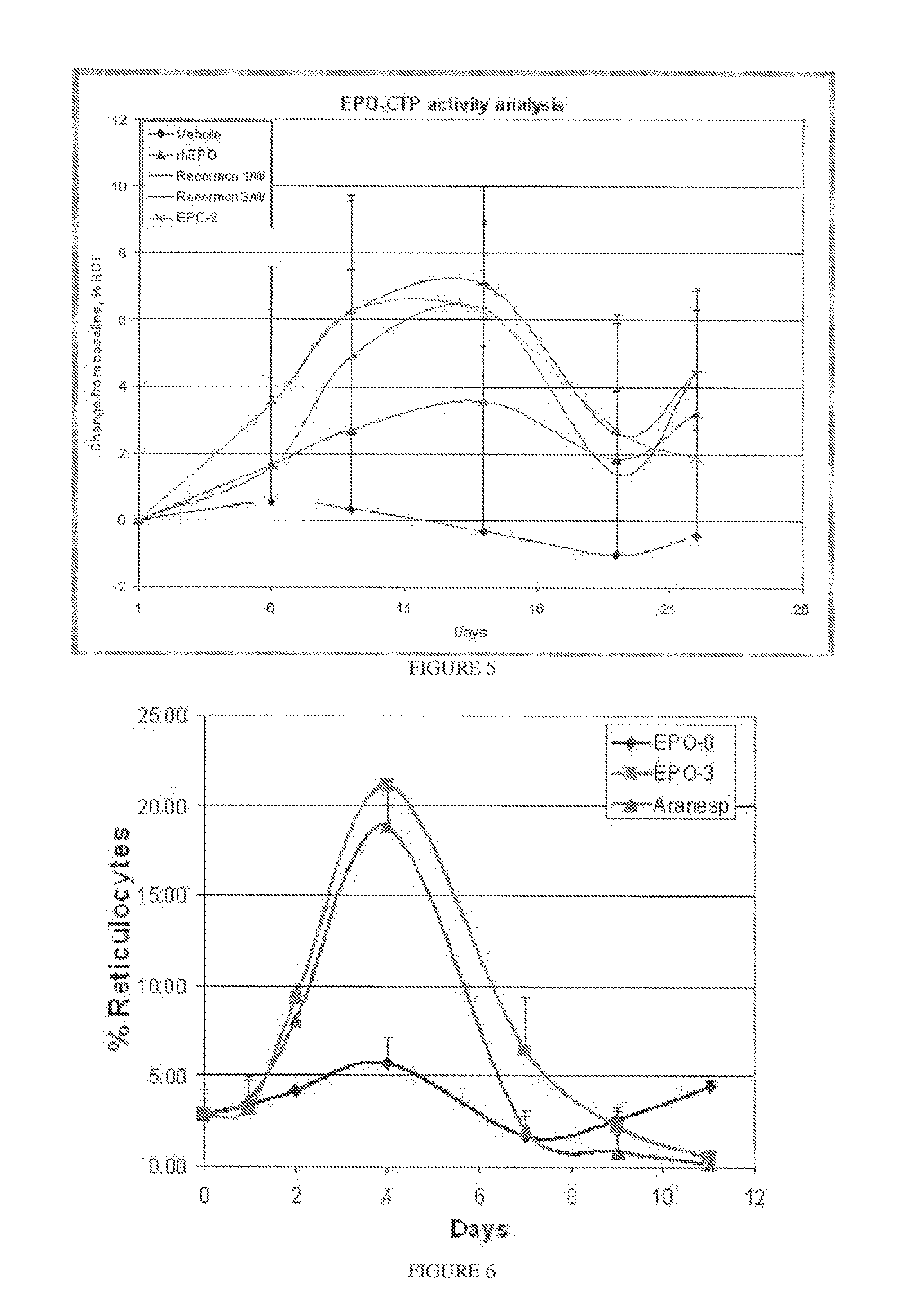
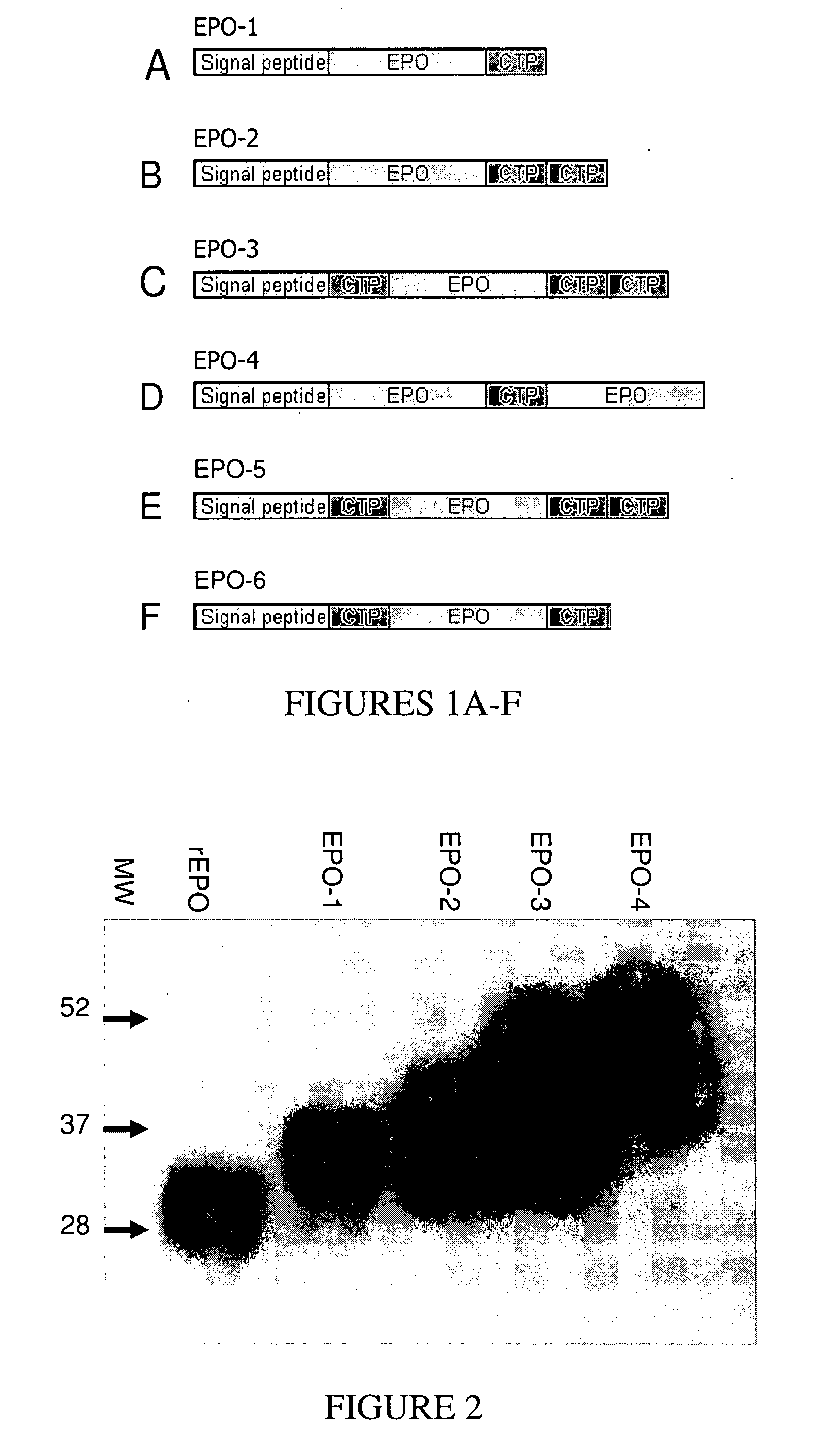
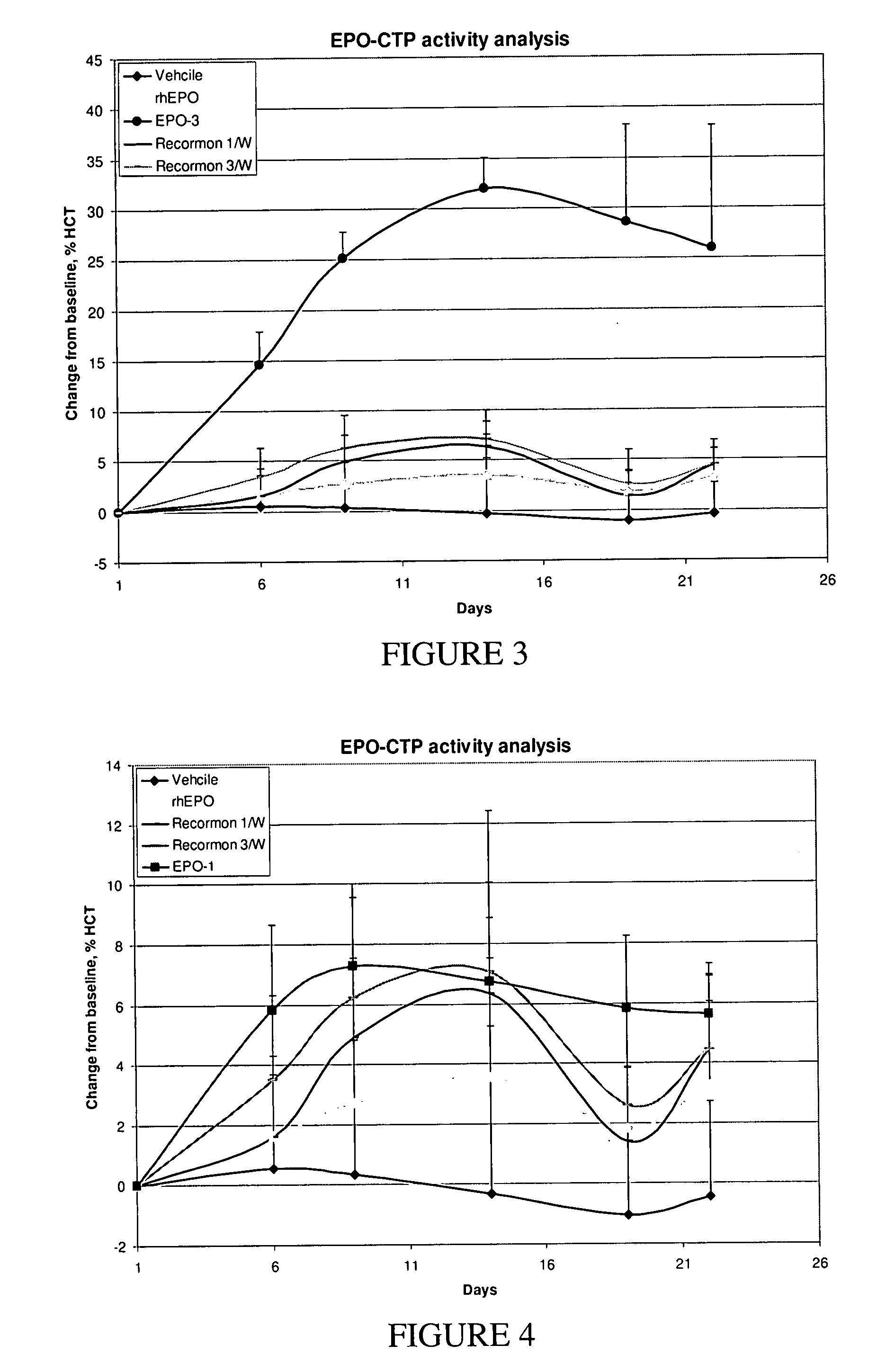
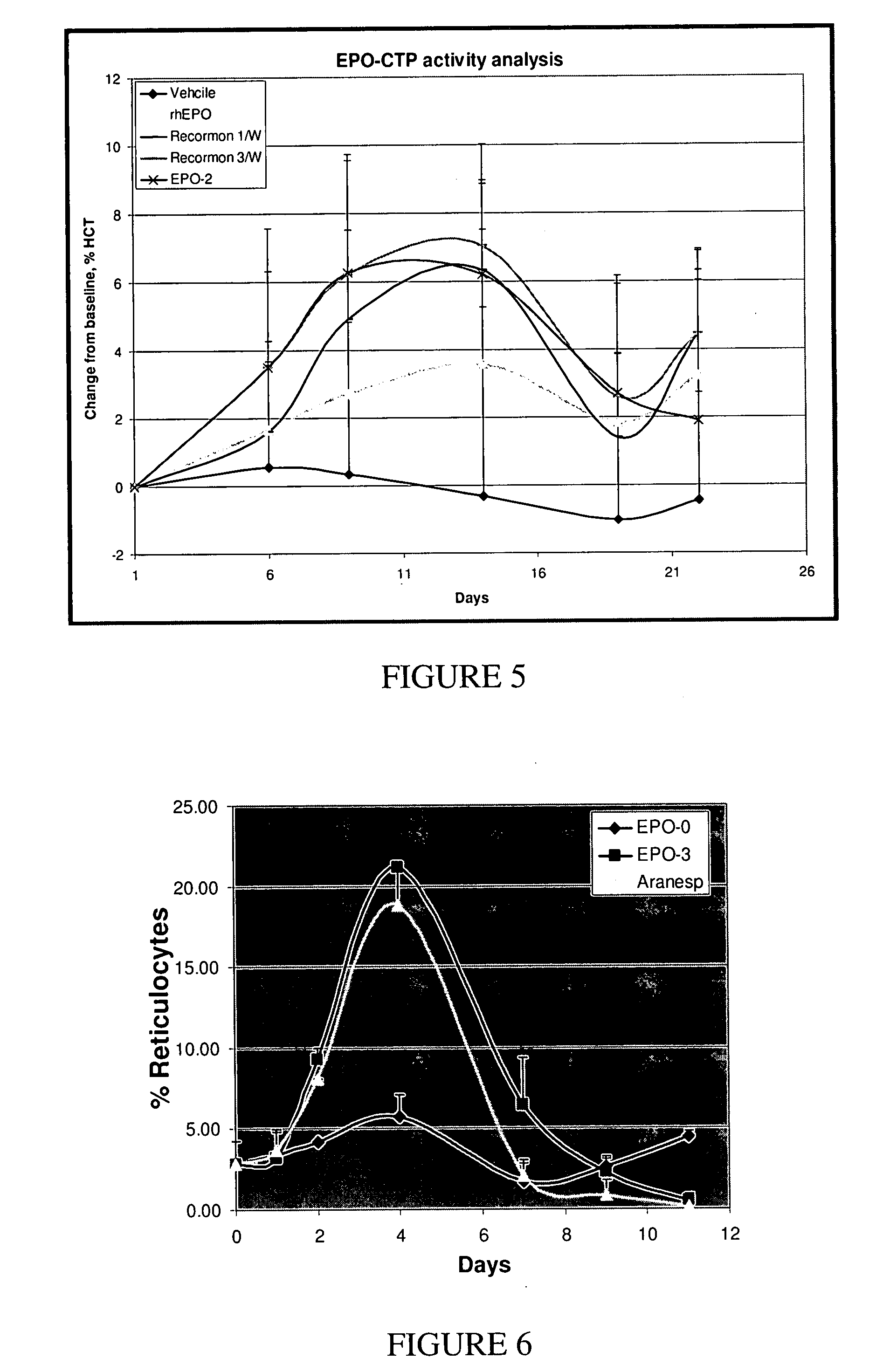
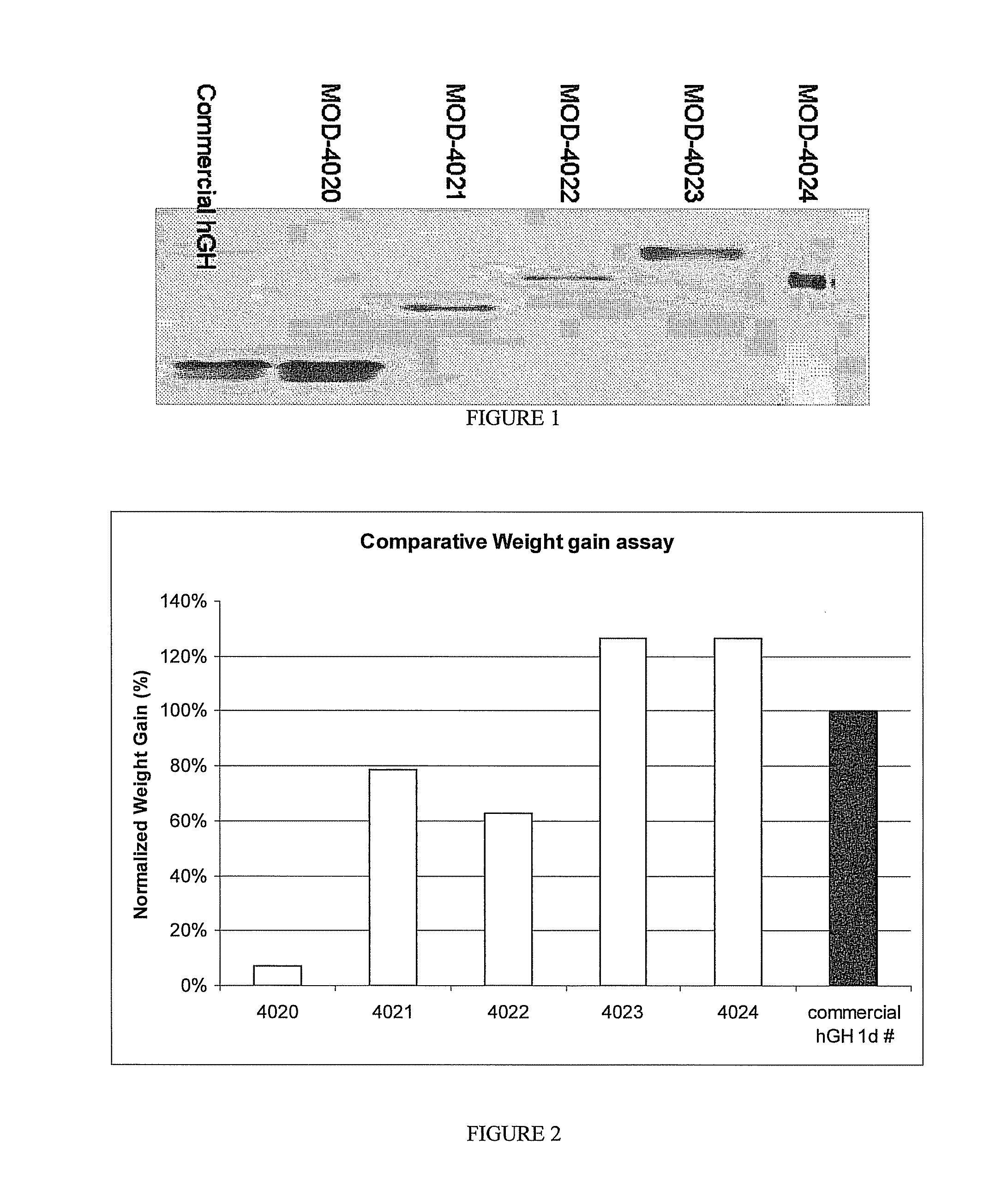
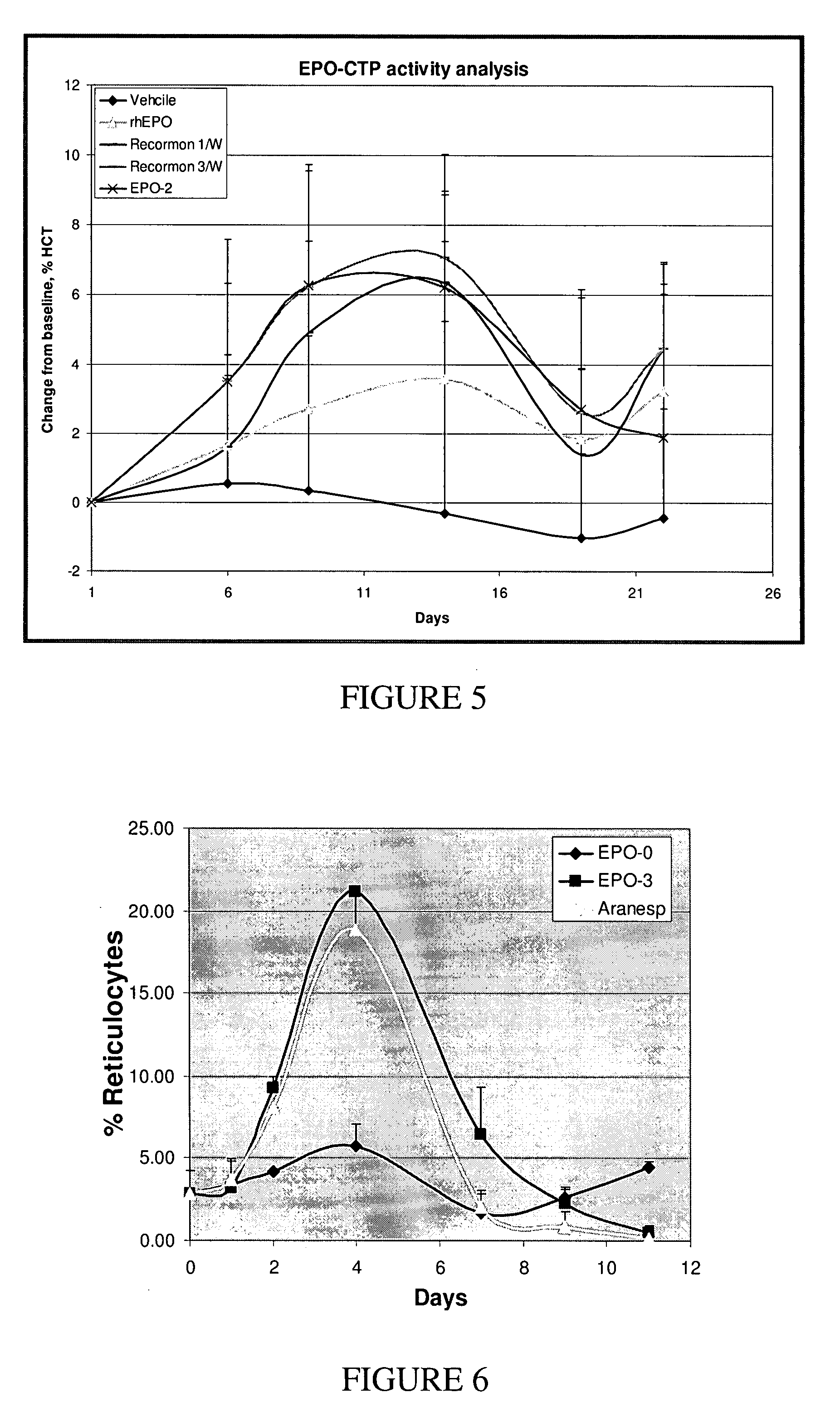
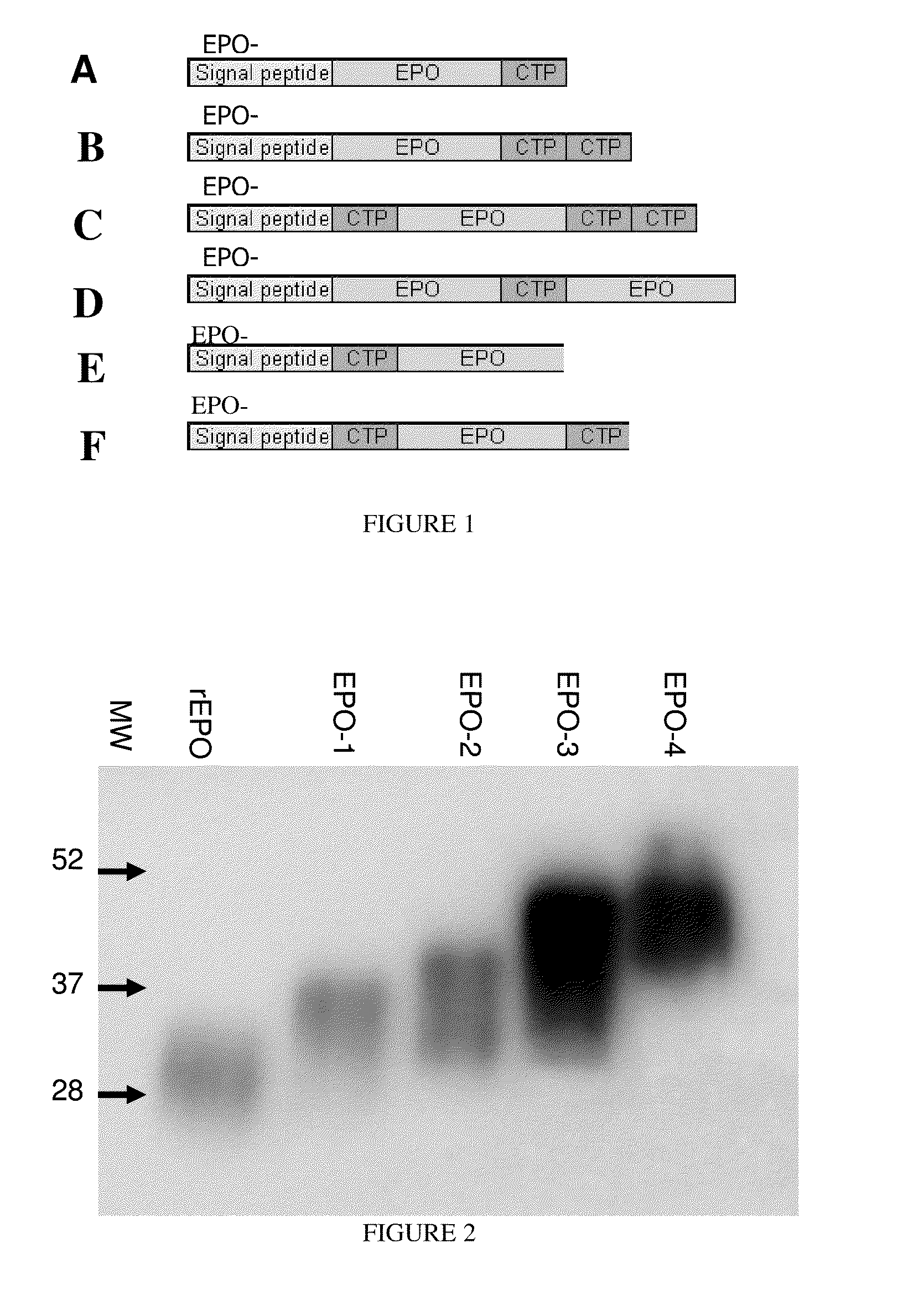
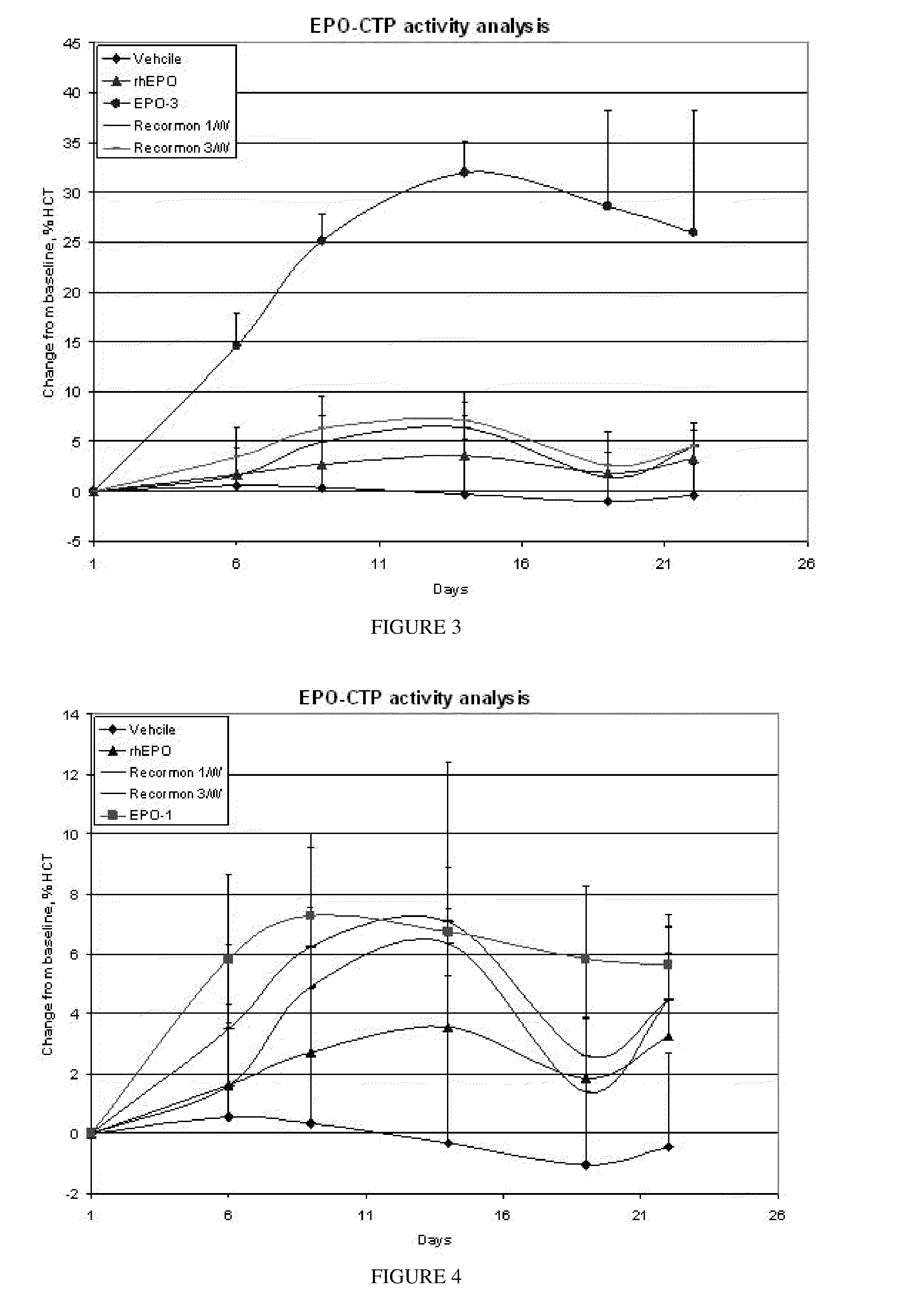
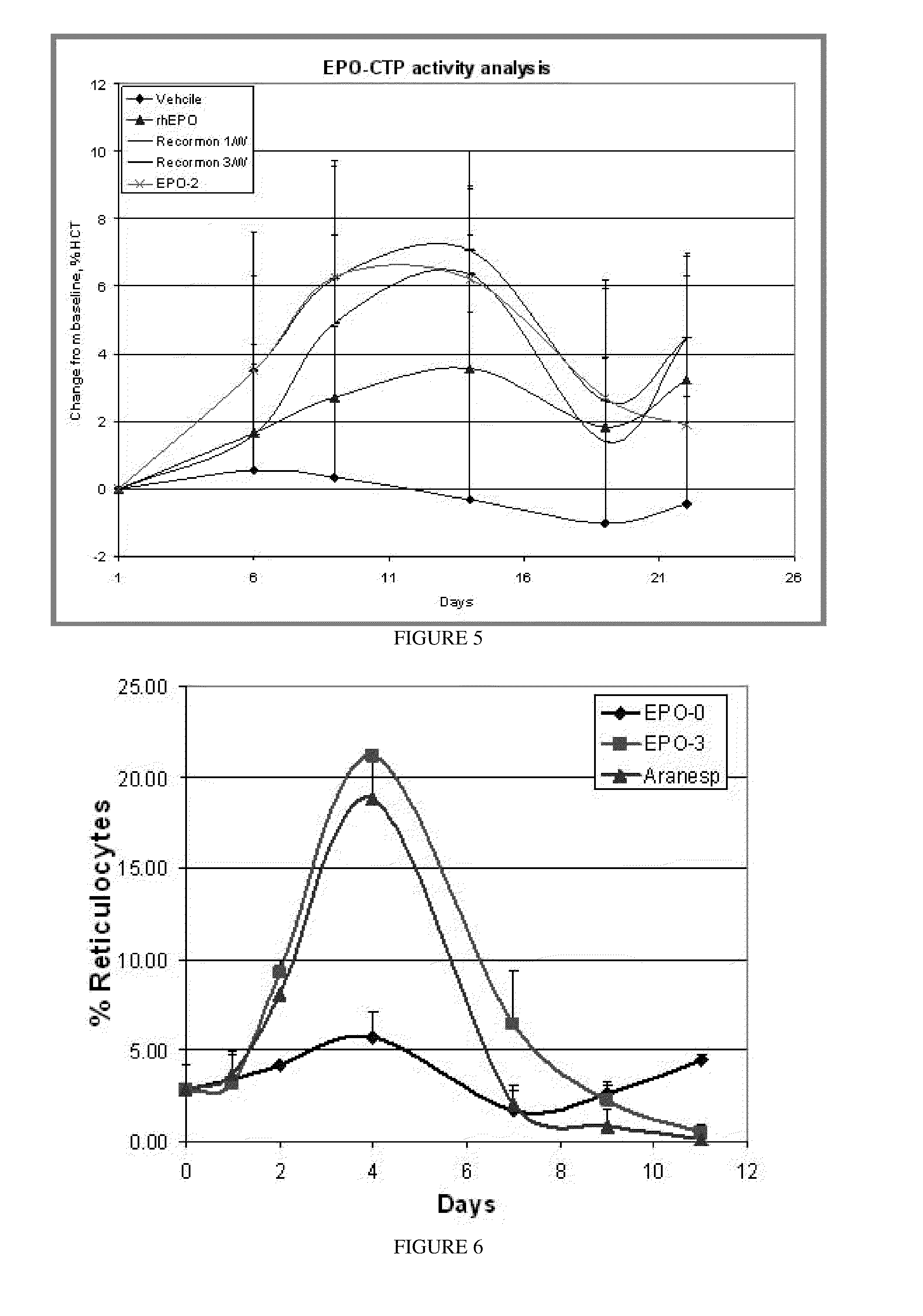
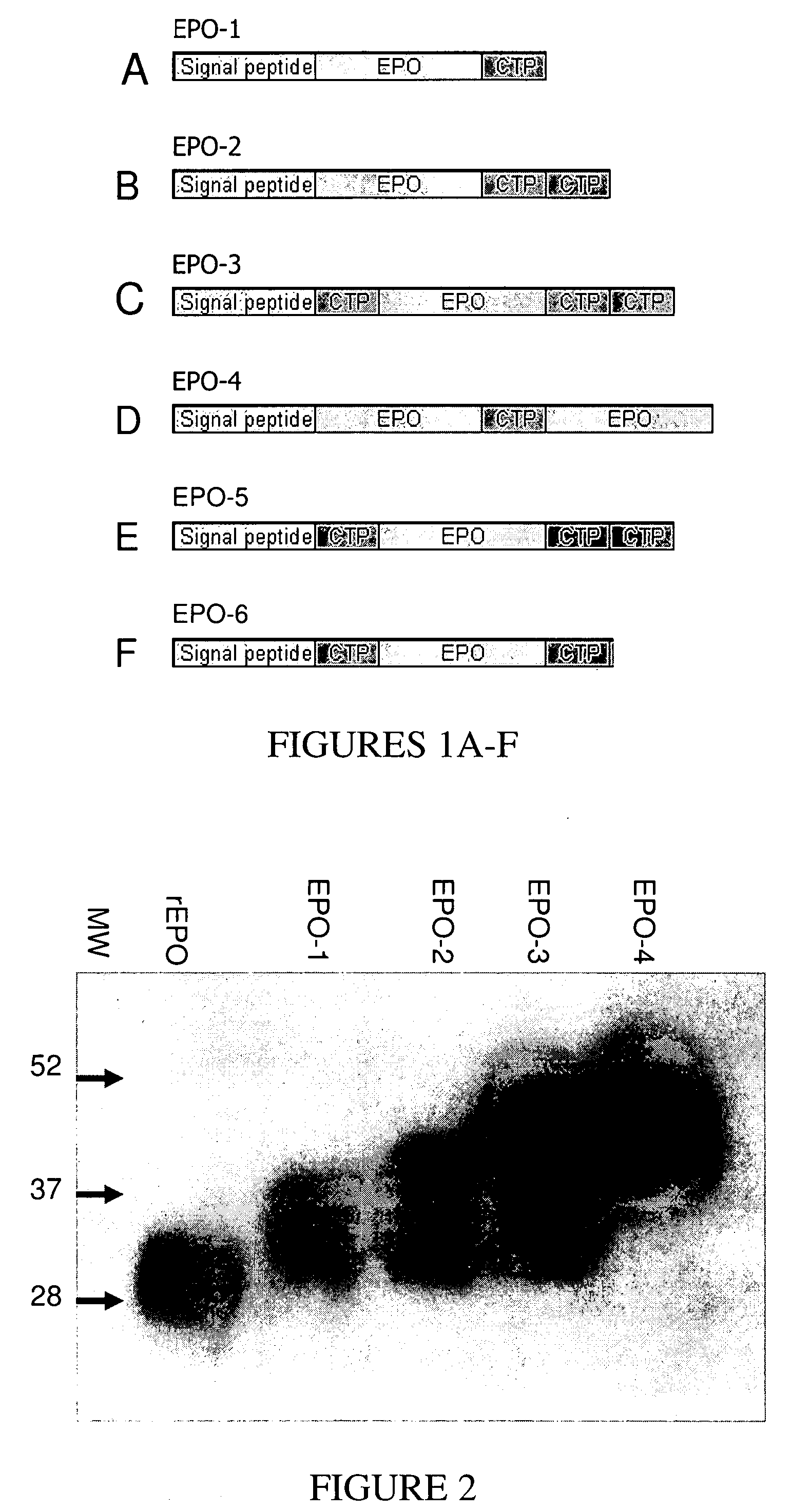
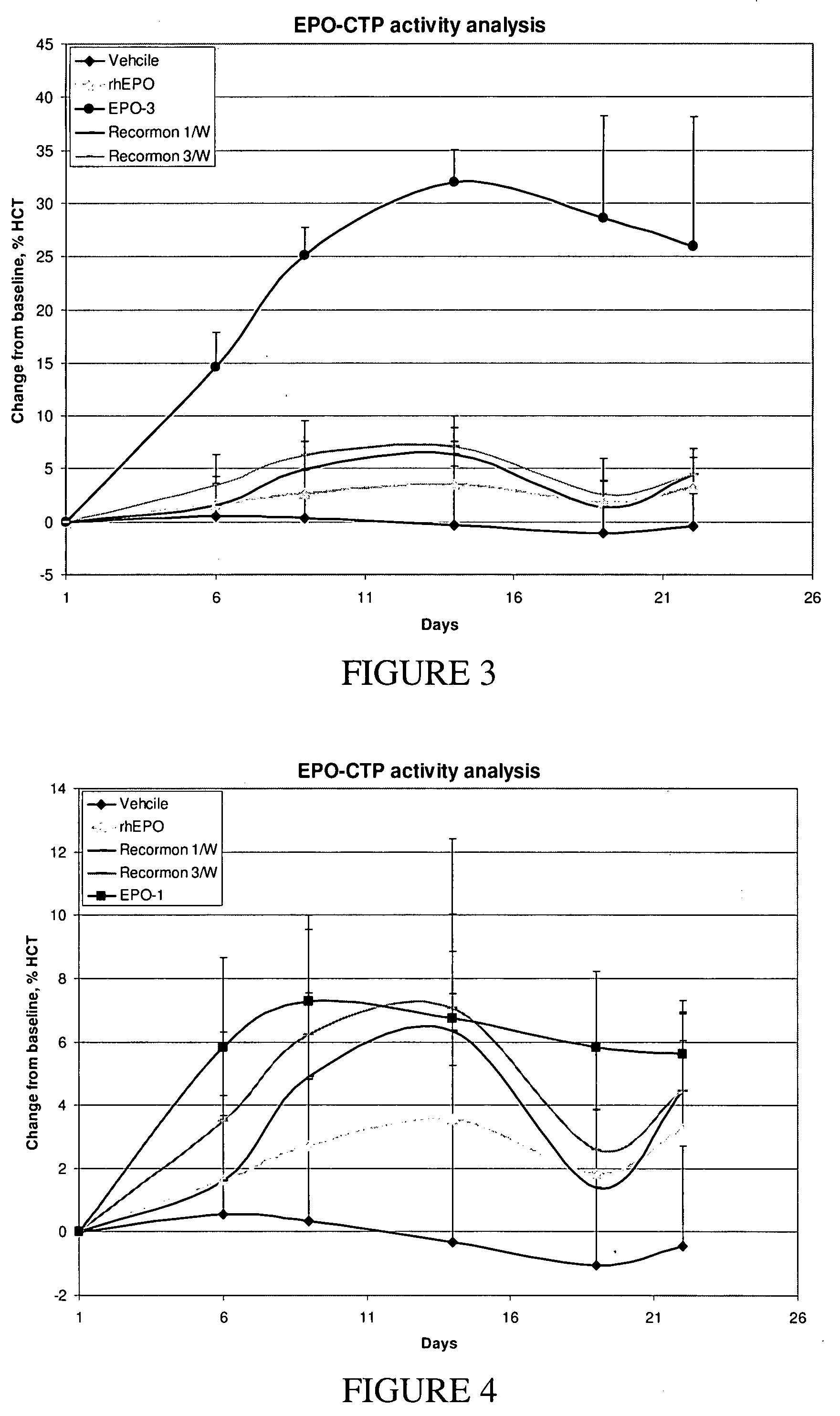
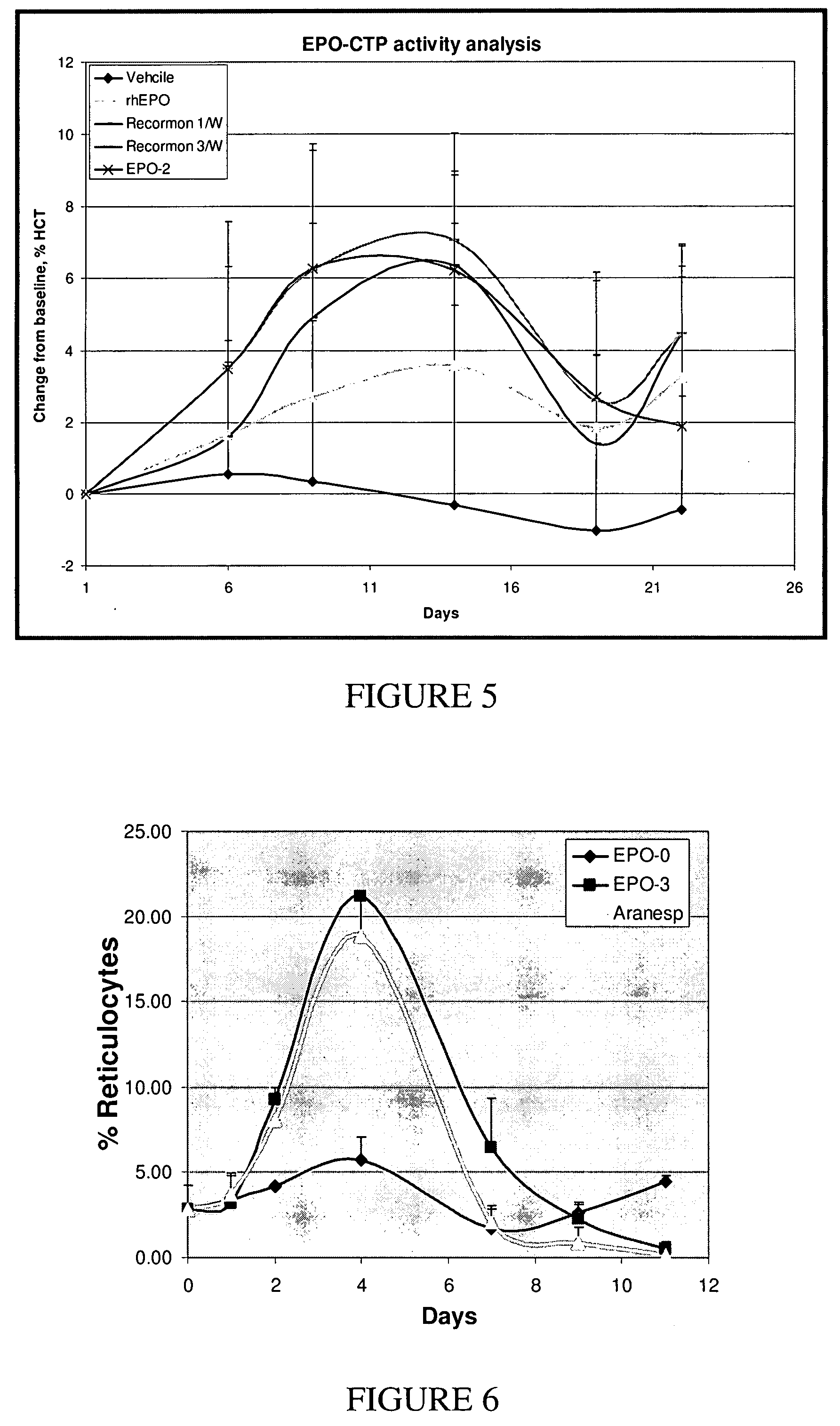
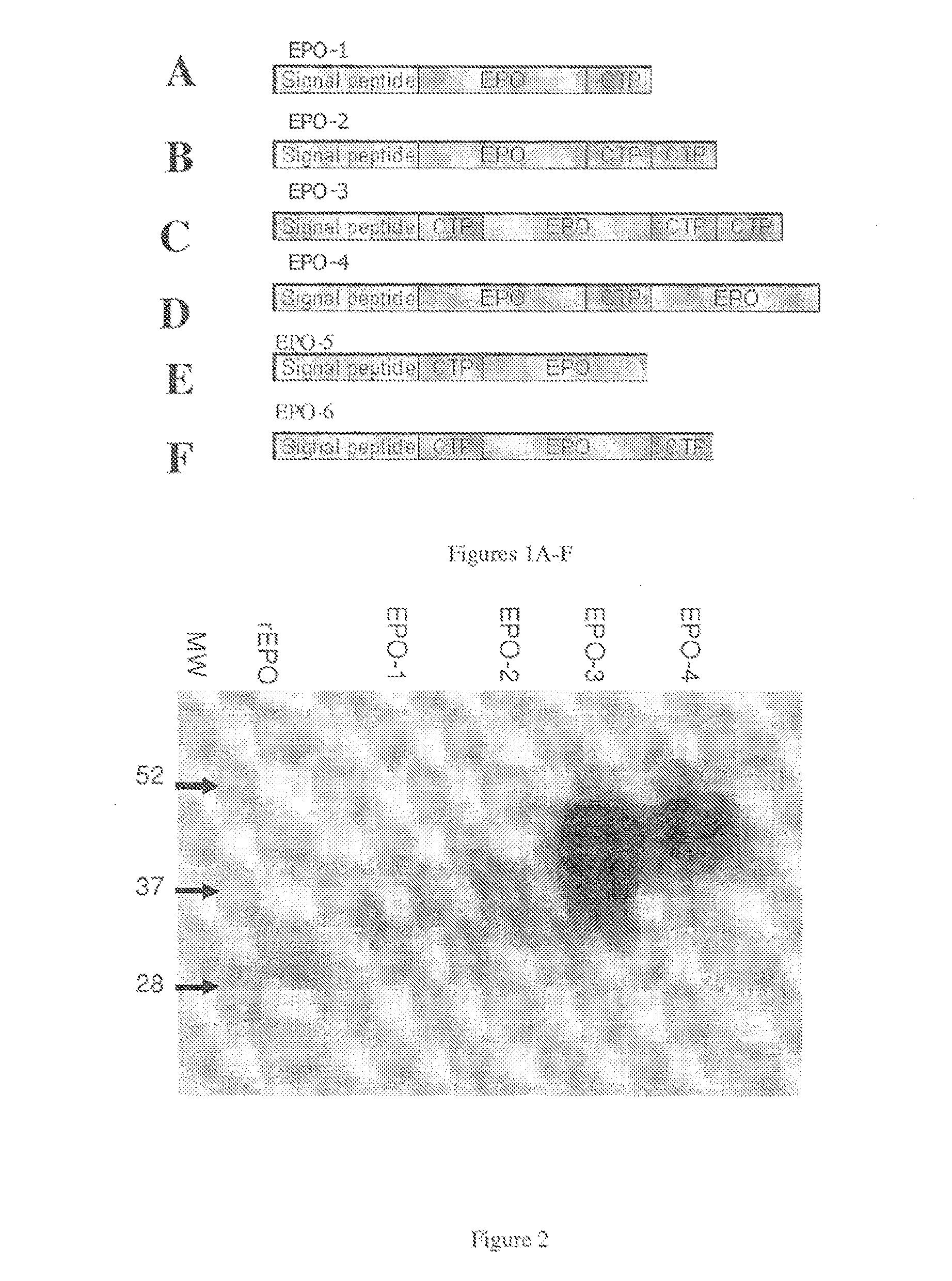
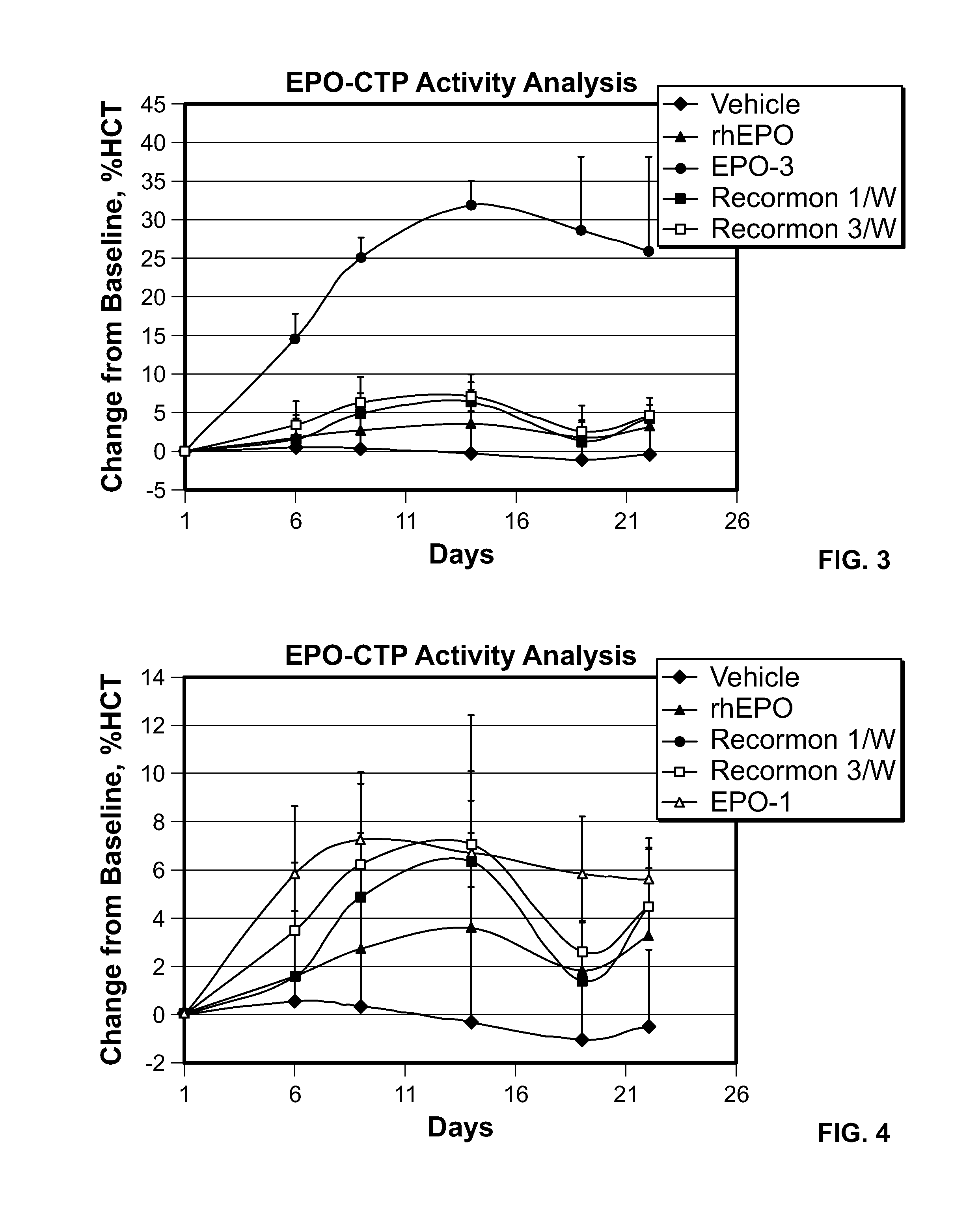
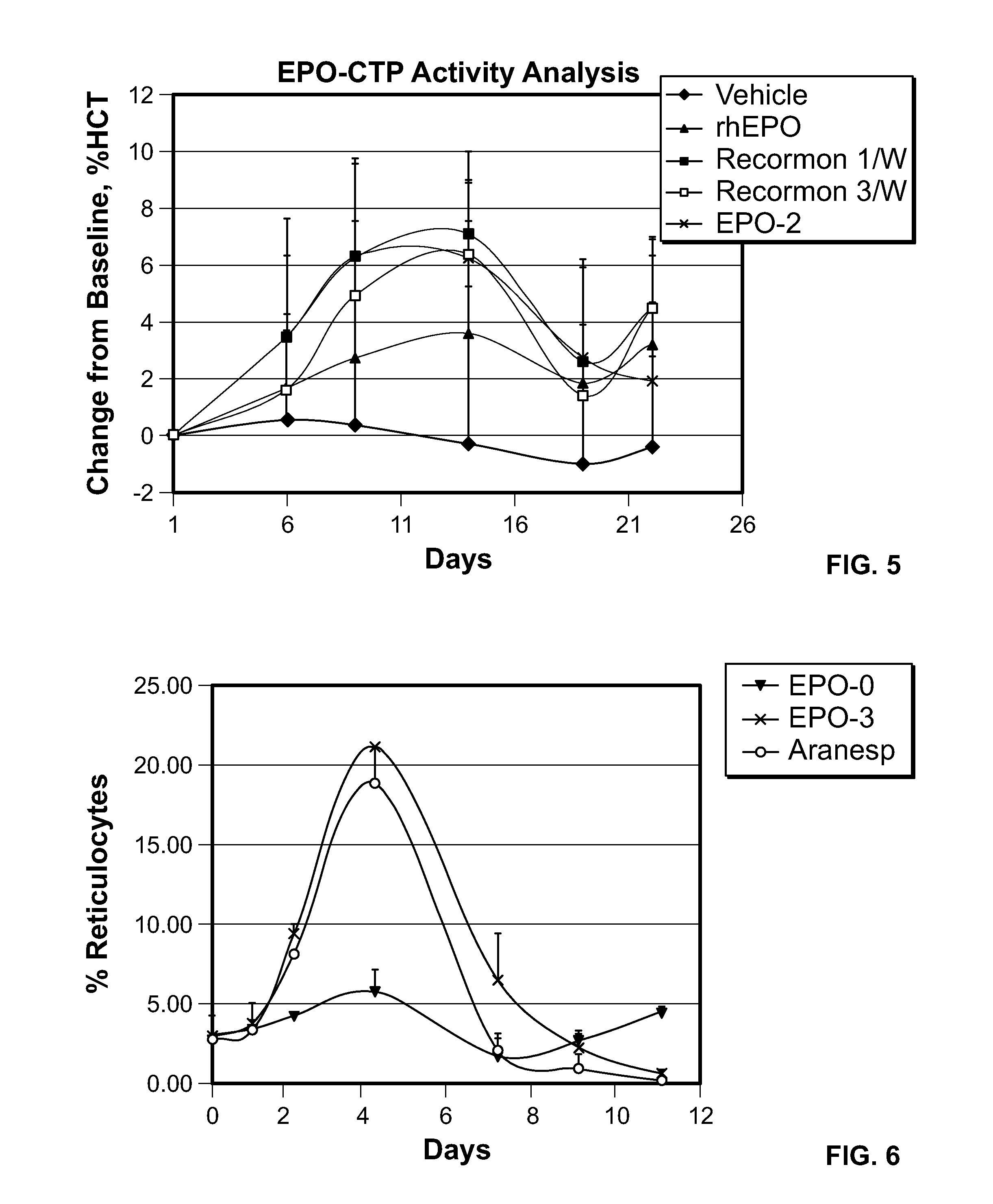
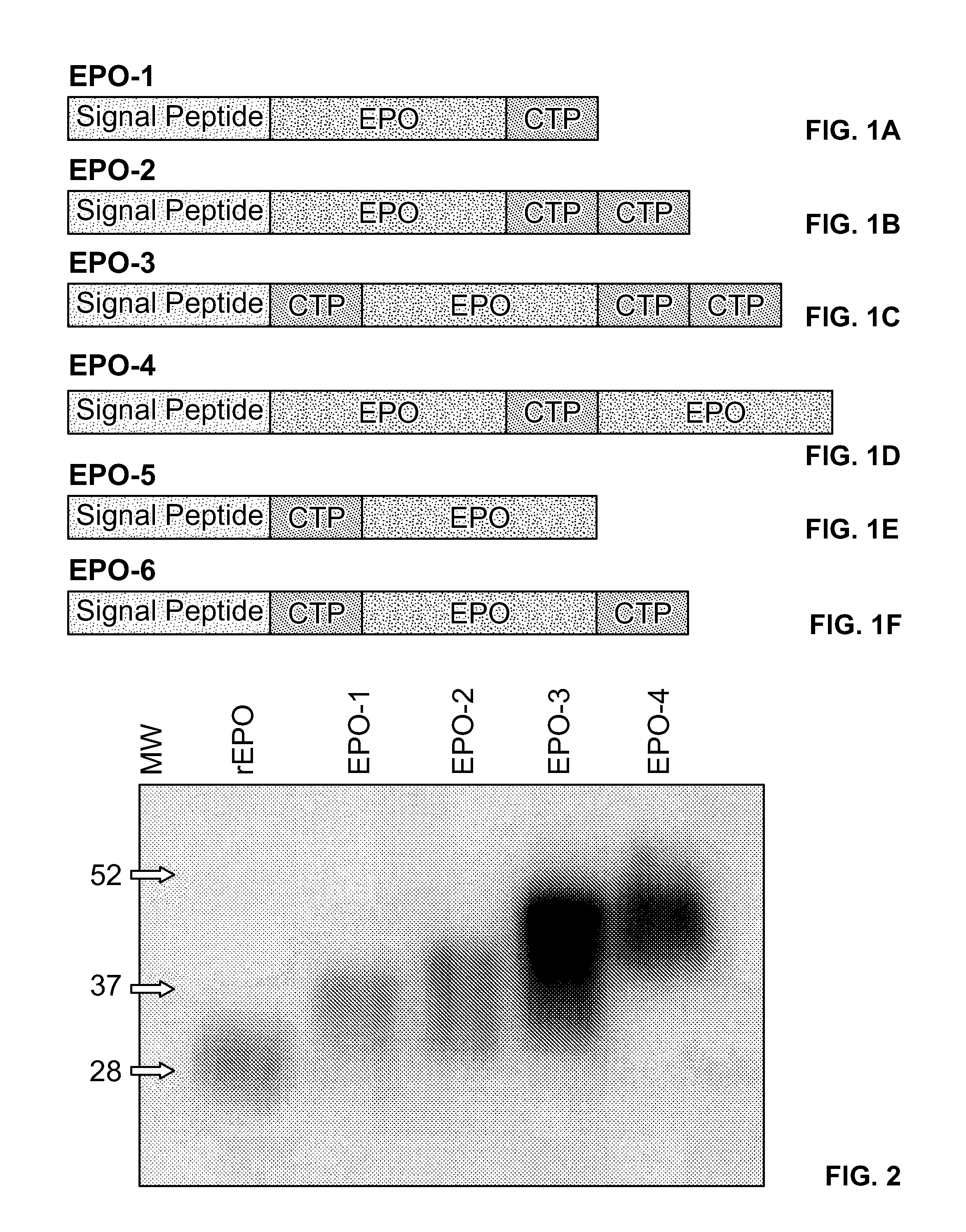
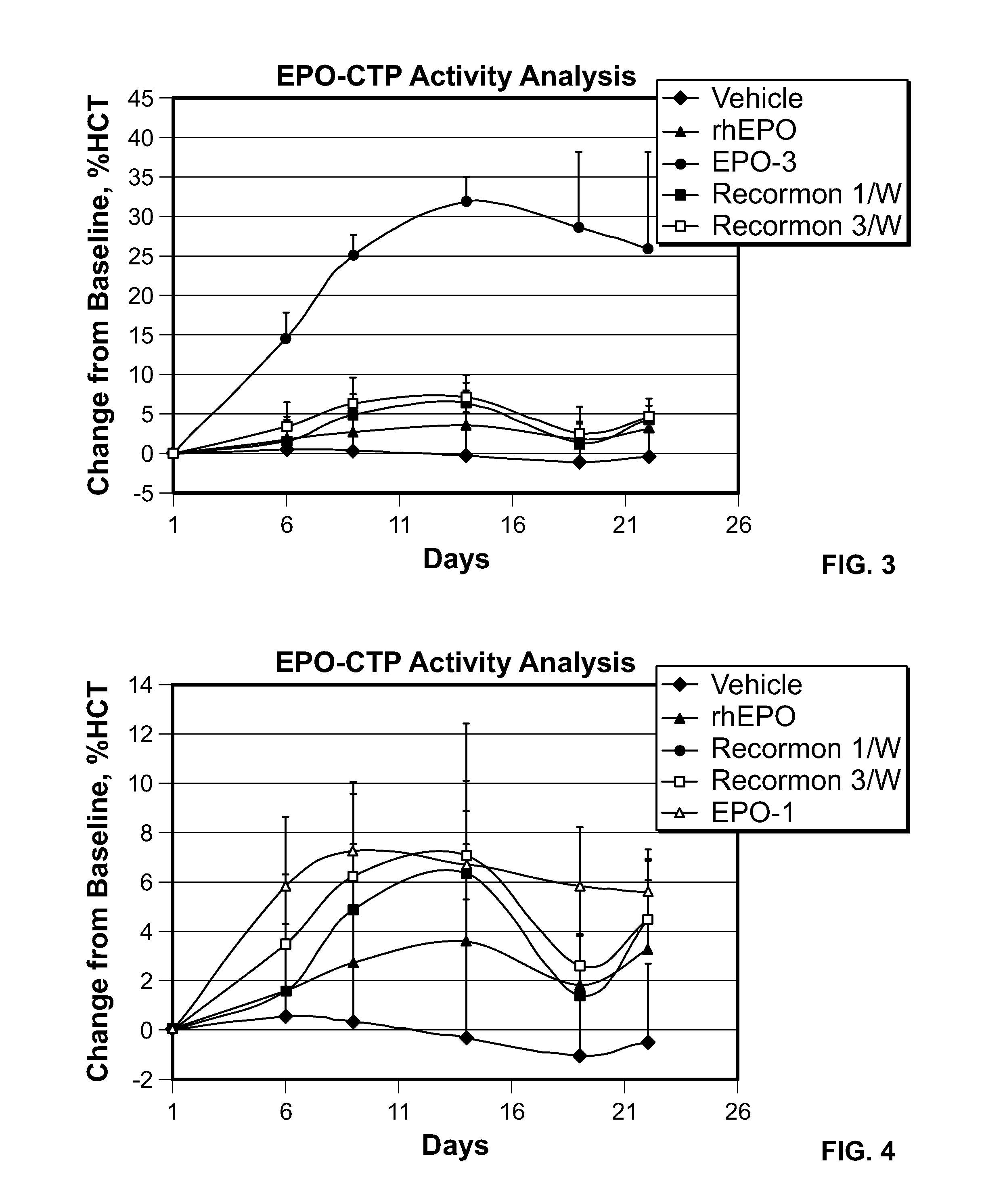
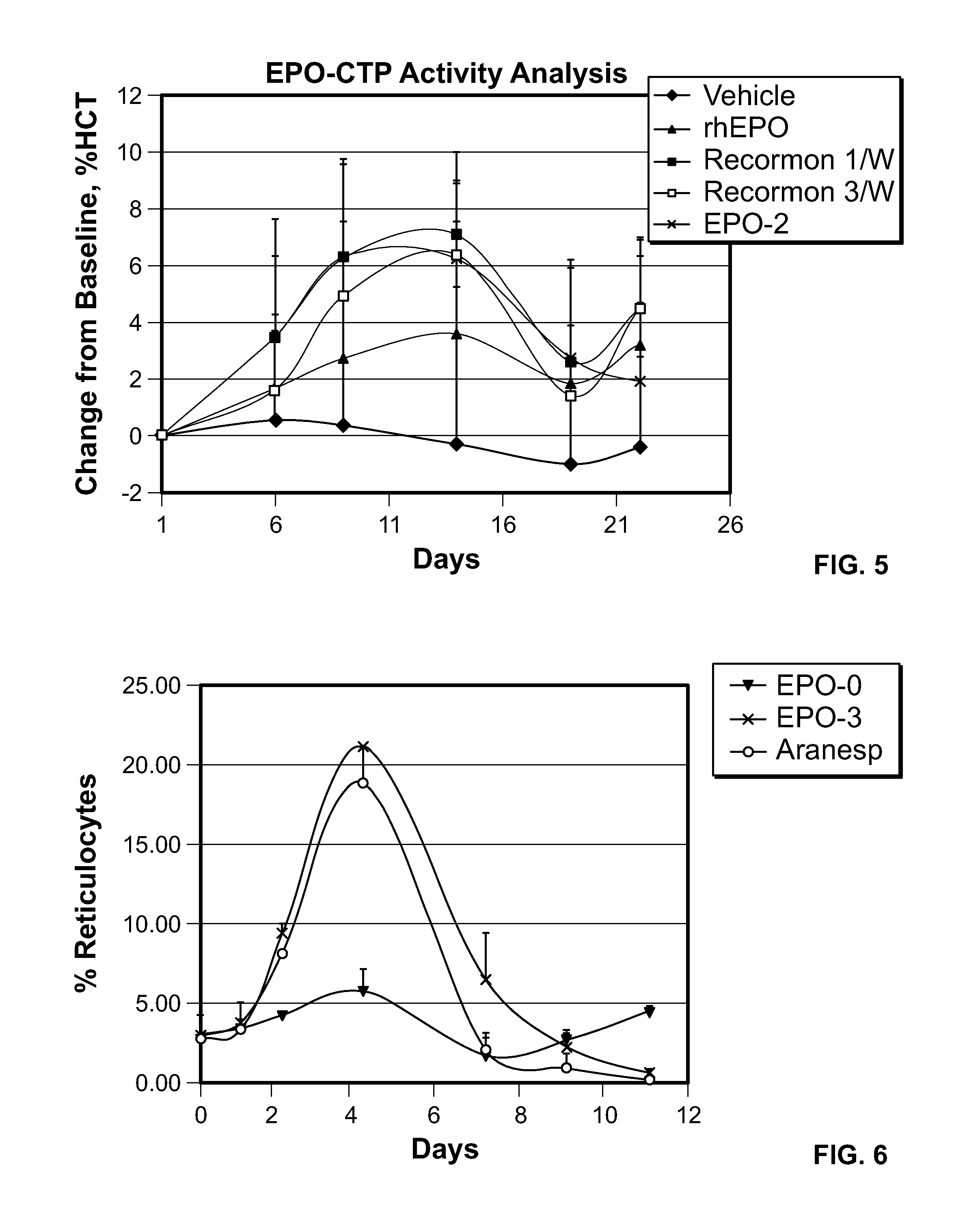
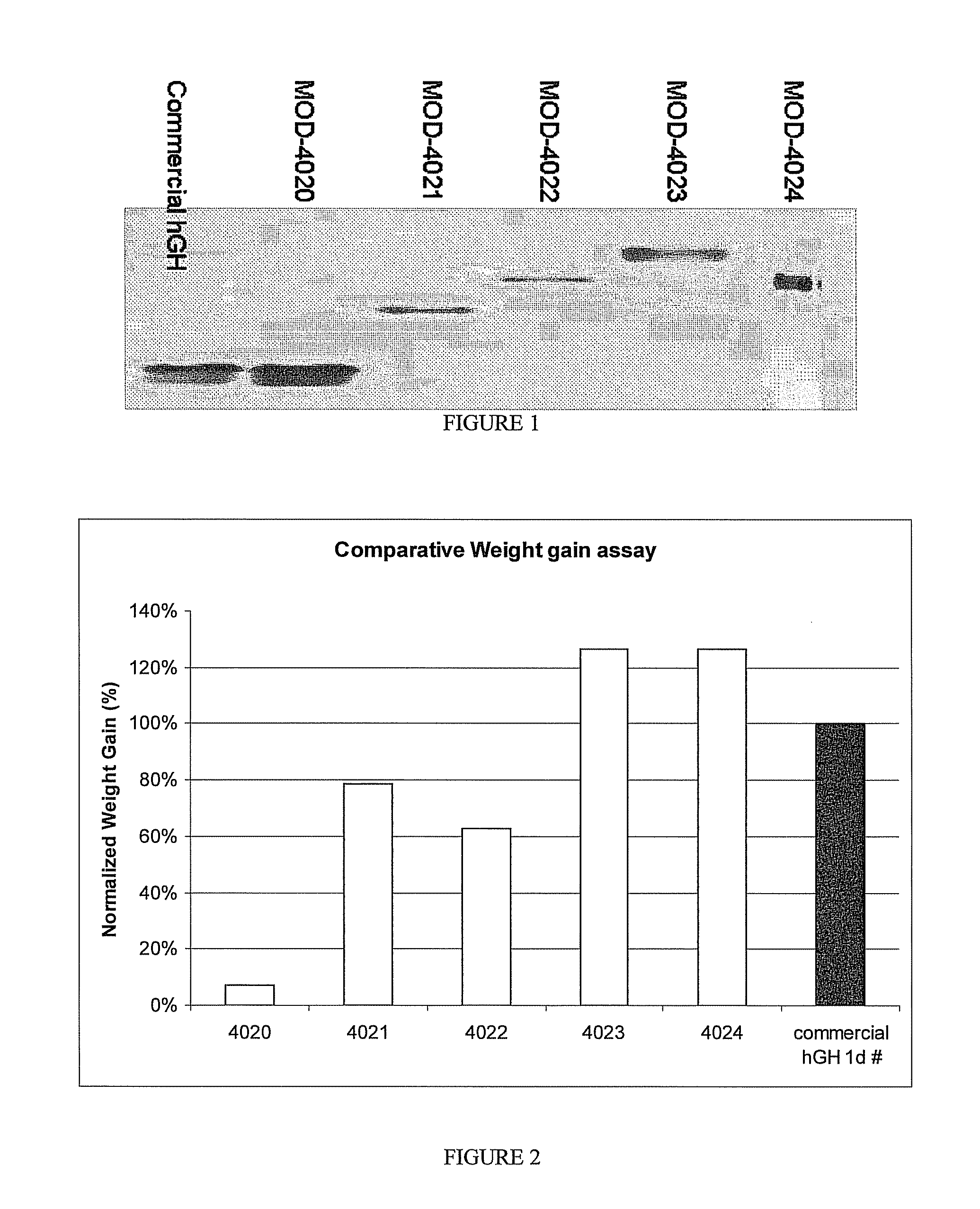
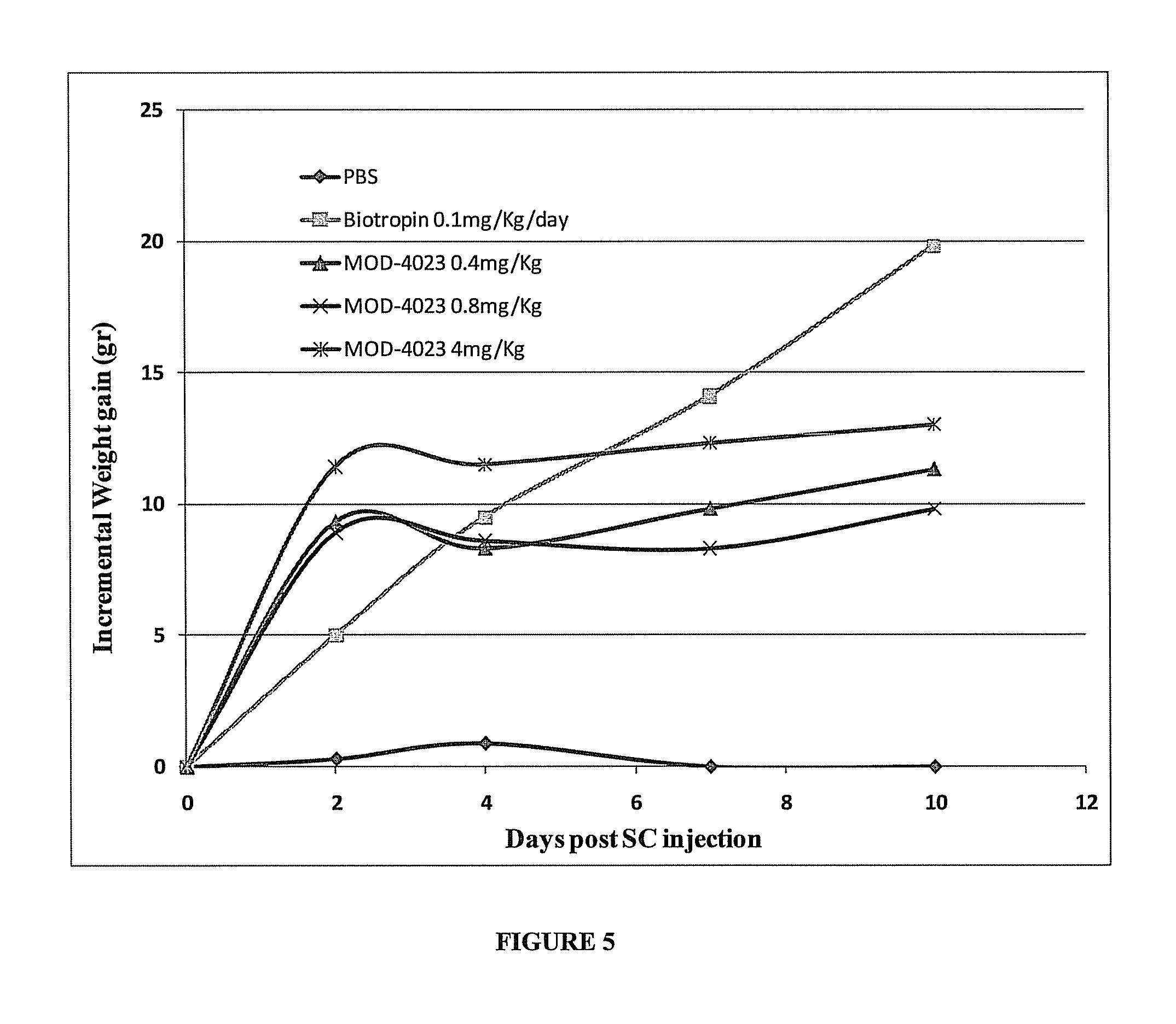
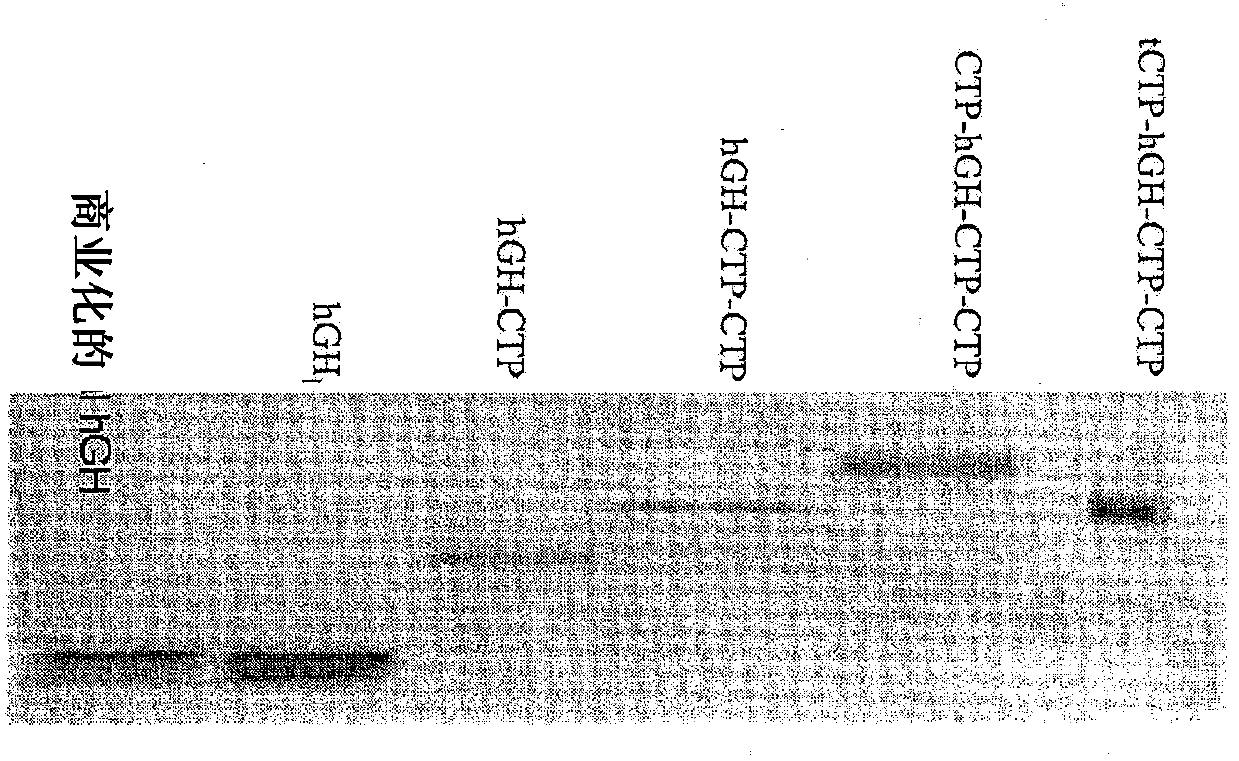
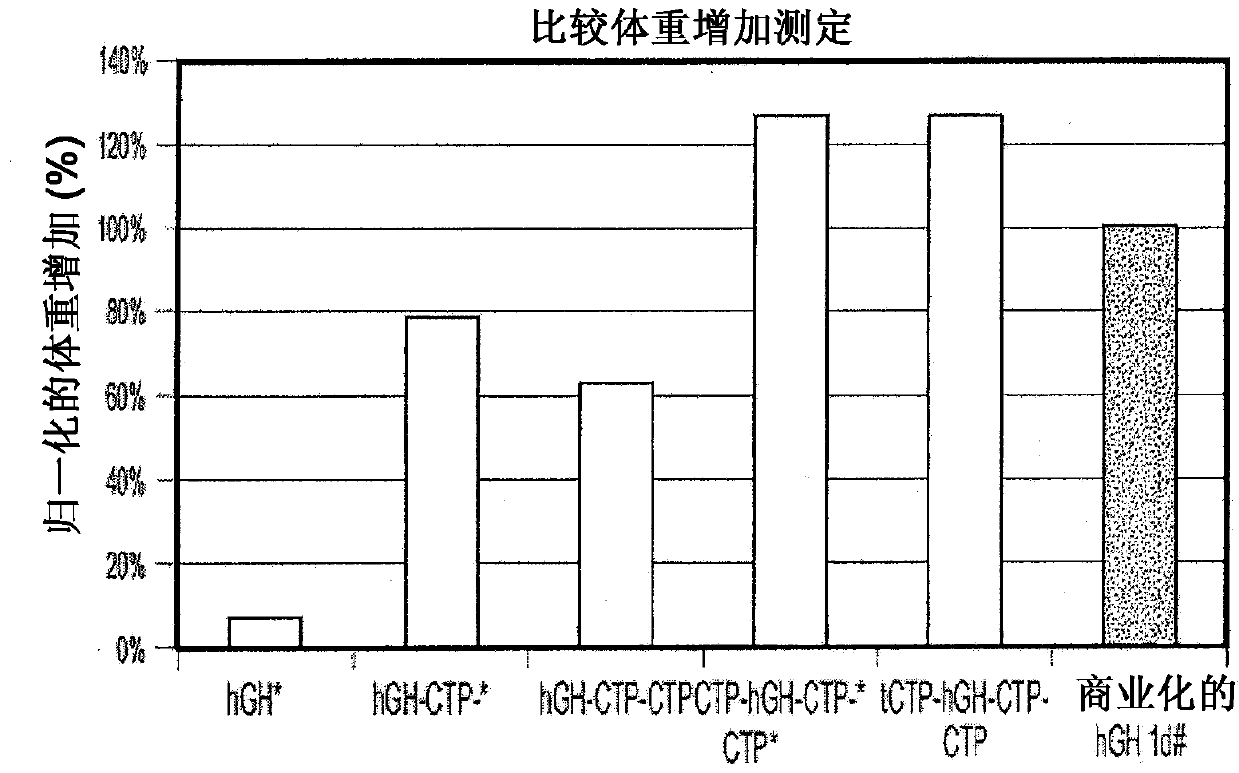
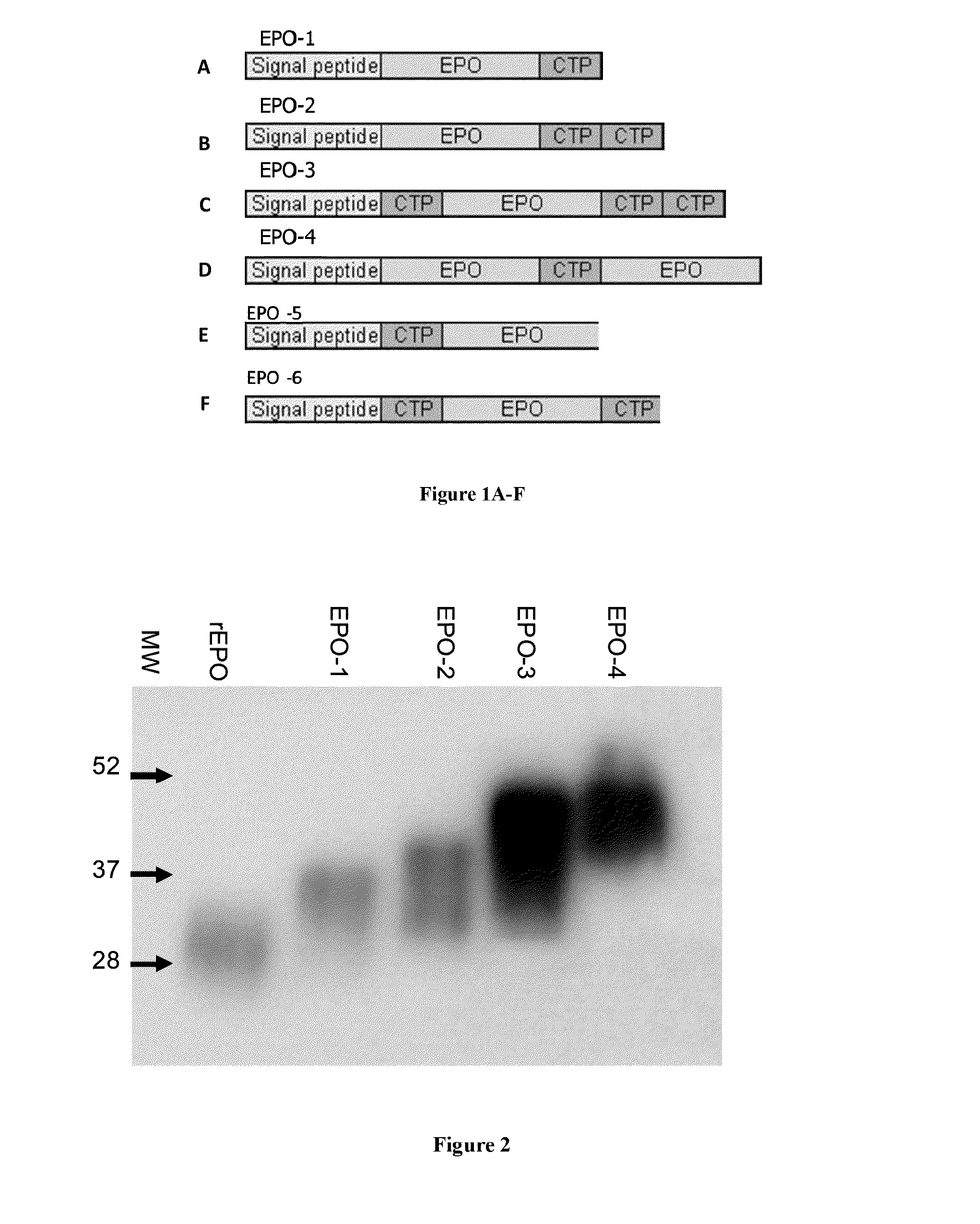
Use of a growth hormone protein and polynucleotides encoding same comprising an amino-terminal carboxy-terminal peptide (CTP) of chorionic gonadotrophin and two carboxy-terminal chorionic gonadotrophin CTPs attached to the growth hormone in methods of inducing growth or weight gain, method of increasing insulin-like growth factor (IGF-1) levels, and methods of reducing the dosing frequency of a growth hormone in a human subject are disclosed. Pharmaceutical compositions comprising the growth hormone and polynucleotides encoding the growth hormone of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Long-acting polypeptides and methods of producing and administering same
ActiveUS20130184207A1Reduce dosing frequencyIncrease the areaPeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides comprising at least two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a non-human peptide-of-interest are disclosed. Pharmaceutical compositions comprising the non-human polypeptides and polynucleotides of the invention and methods of using both human and non-human polypeptides and polynucleotides are also disclosed.
Owner:OPKO BIOLOGICS
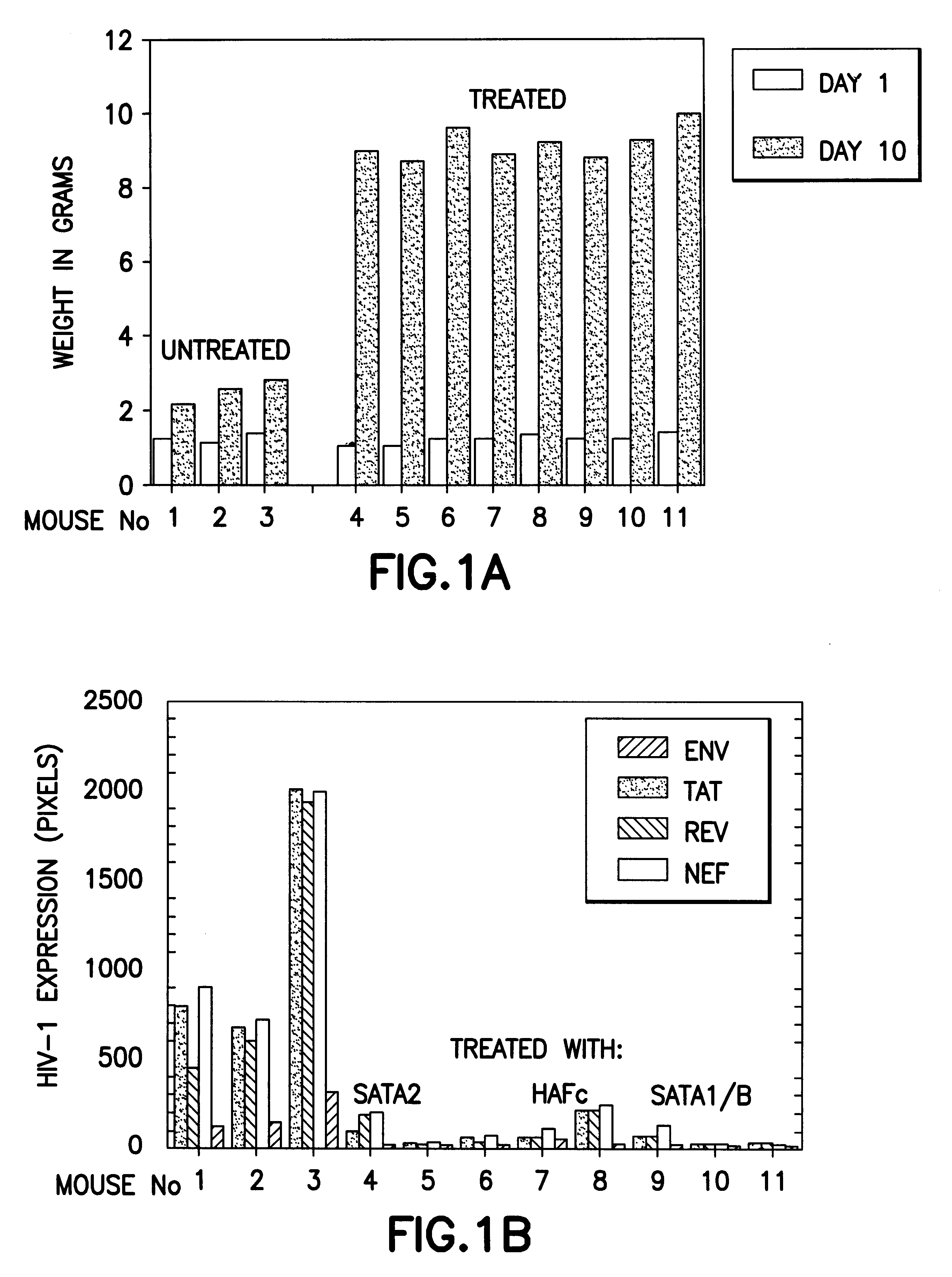
Method for treating HIV
InactiveUS6620416B1Lower Level RequirementsIncrease CD4.sup.+ T cellsBiocidePeptide/protein ingredientsDiseaseHCG - Human chorionic gonadotropin
The present invention relates to peptides of one or more portions of the human chorionic gonadotropin beta-chain as well as methods for treatment and prevention of diseases, including HIV infection, using human chorionic gonadotropin, employing the beta-chain of human chorionic gonadotropin, peptides containing a sequence of one or more portions of the beta-chain of human chorionic gonadotropin and derivatives and analogues thereof. The invention further relates to fractions of sources and or preparations of human chorionic gonadotropin, such as fractions of human early pregnancy urine, which fractions have anti-HIV activity. The present invention further relates to pharmaceutical compositions for treating and / or preventing HIV infection.
Owner:NOBEL BIOSCI
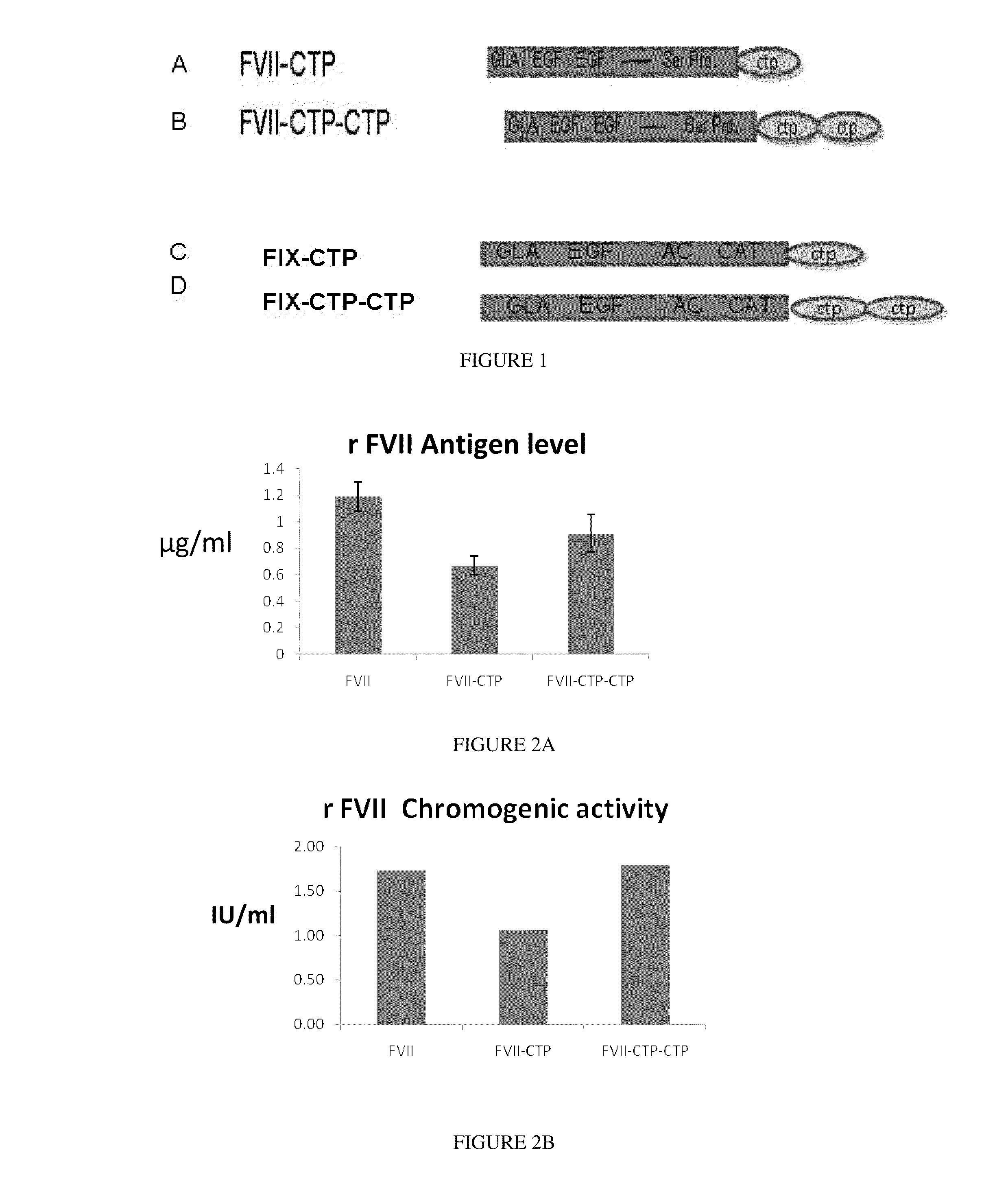
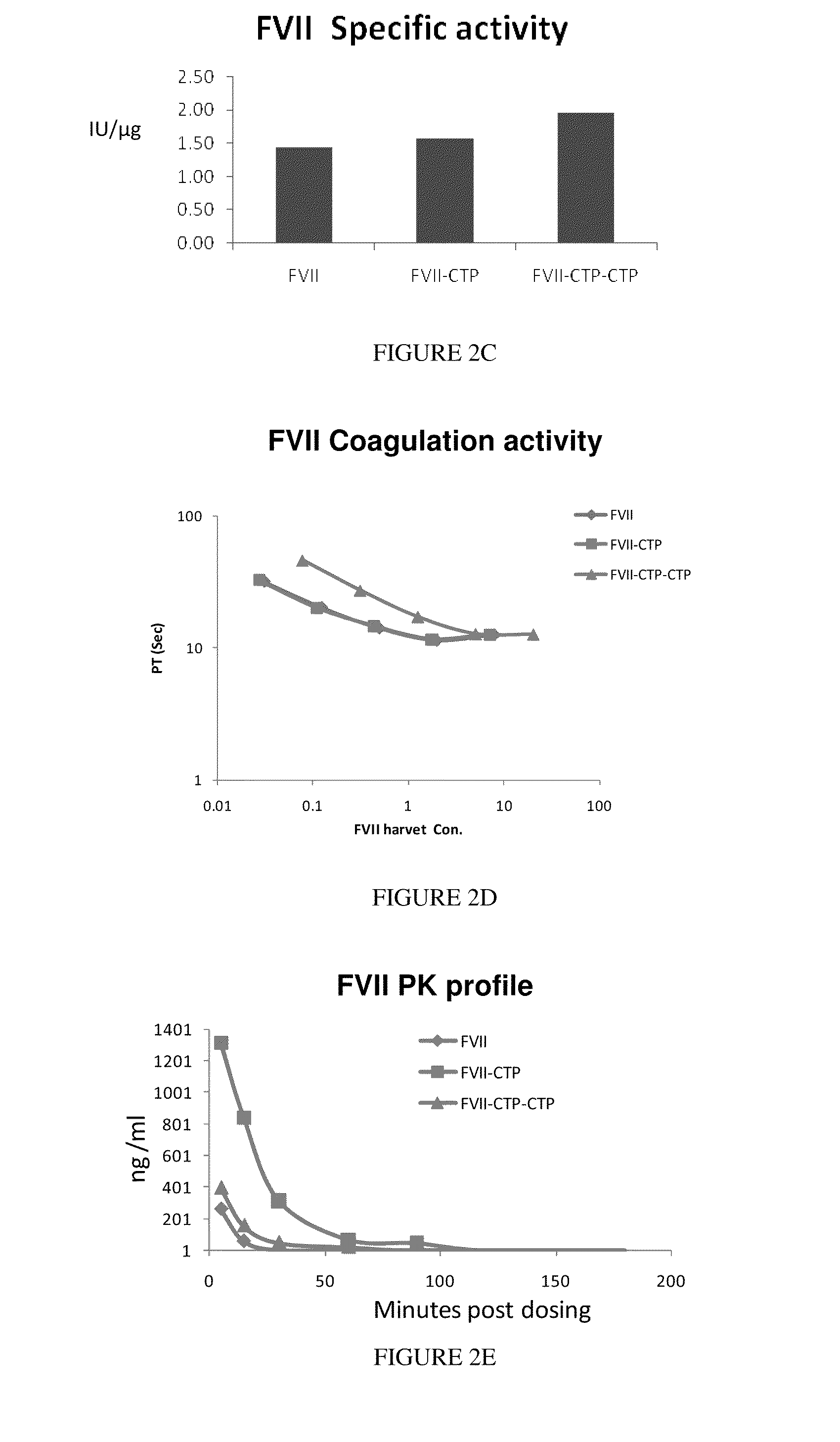
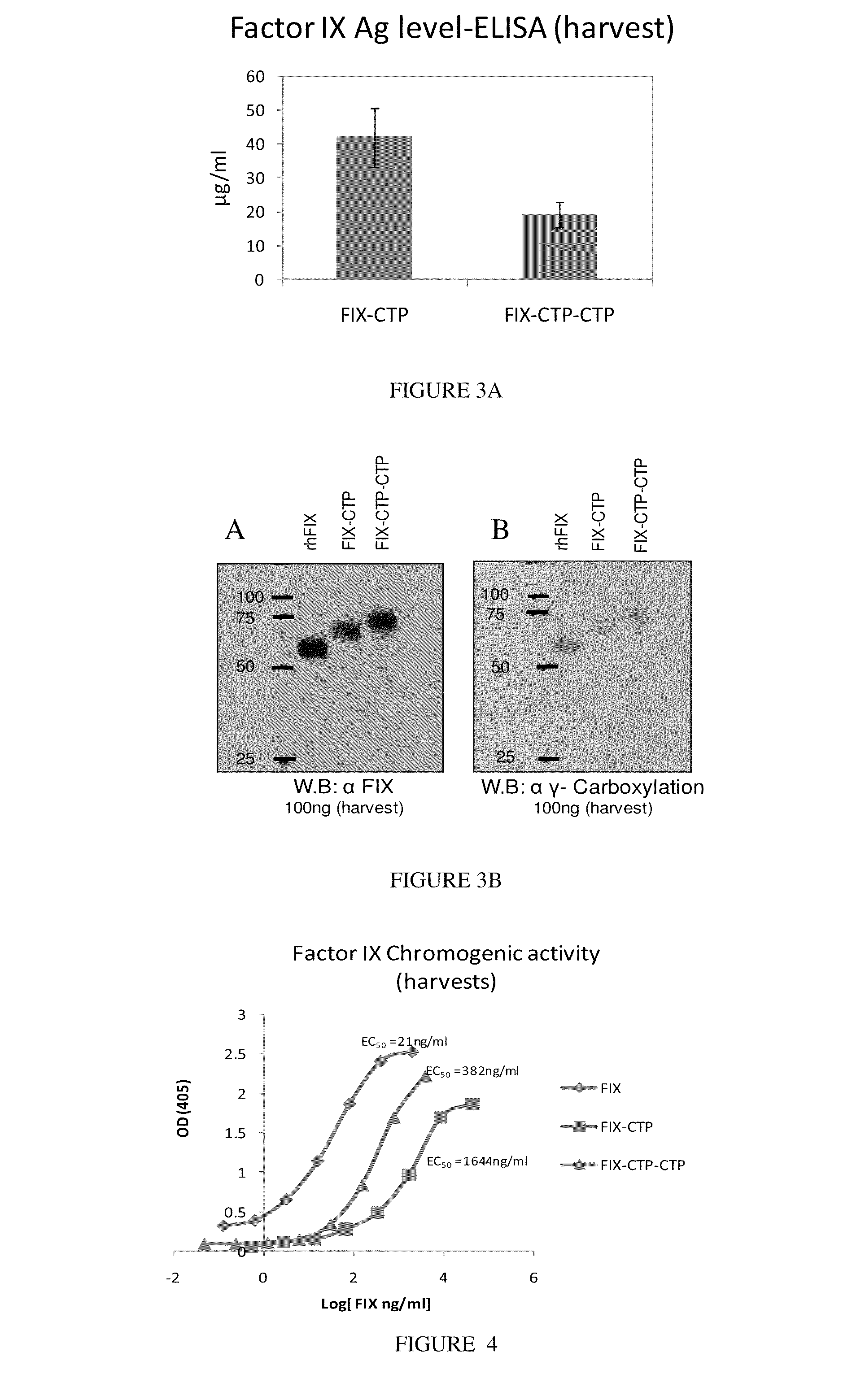
Long-acting coagulation factors and methods of producing same
ActiveUS20100317585A1Improving biological half lifeImproving area under curve (AUC)Peptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
Polypeptides and polynucleotides encoding same comprising at least one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to a carboxy terminus of a coagulation factor and not to an amino terminus are disclosed. Pharmaceutical compositions comprising the polypeptides and polynucleotides of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Long-acting veterinary polypeptides and methods of producing and administering same
InactiveUS20070184530A1Improving biological half lifeProlong lifePeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides comprising at least two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a non-human peptide-of-interest are disclosed. Pharmaceutical compositions comprising the non-human polypeptides and polynucleotides of the invention and methods of using both human and non-human polypeptides and polynucleotides are also disclosed.
Owner:MODIGENE INC
Long-acting growth hormone and methods of producing same
ActiveUS20100081614A1Increase the areaReduce dosing frequencyNervous disorderPeptide/protein ingredientsNucleotideGrowth hormone
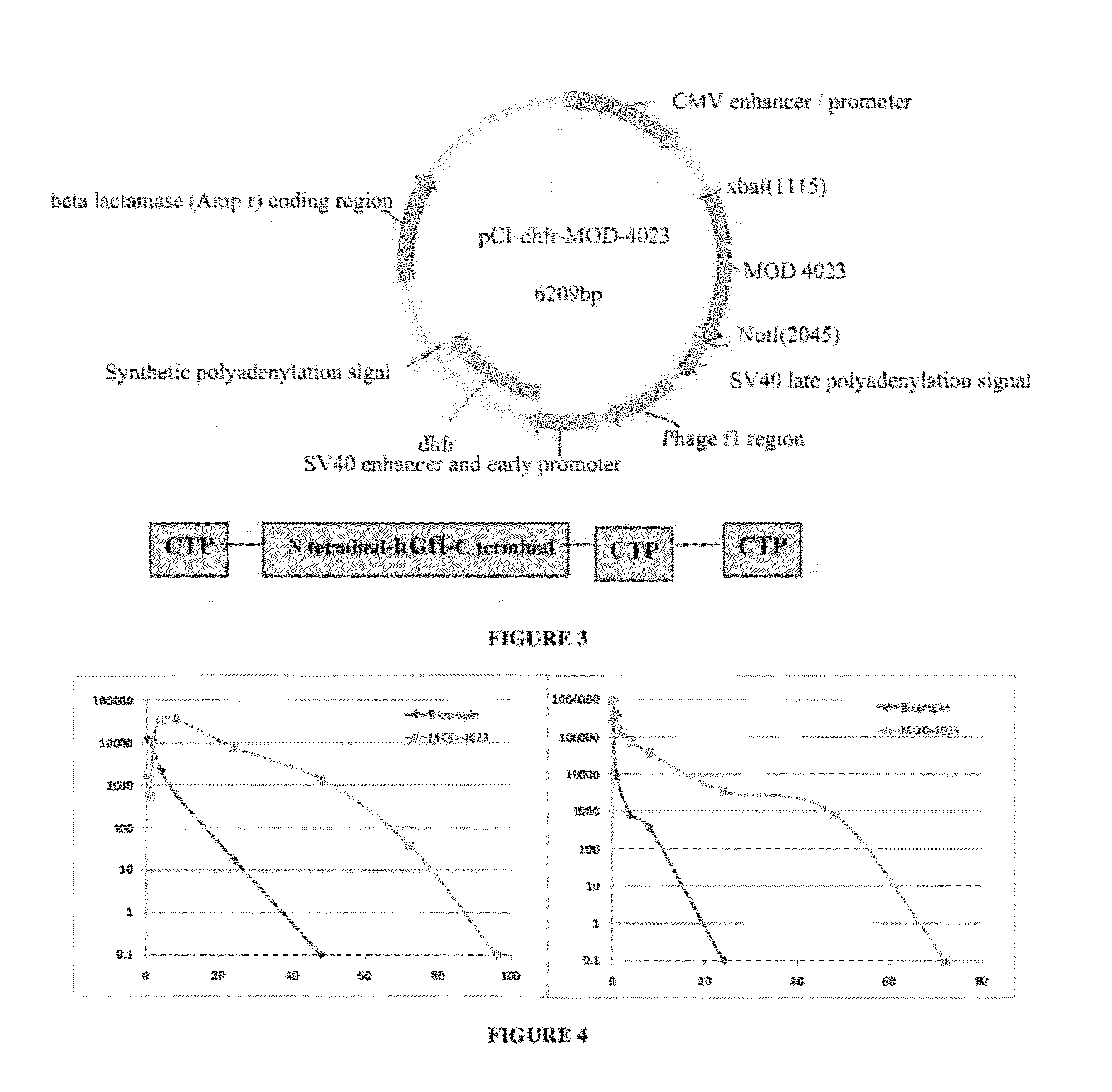
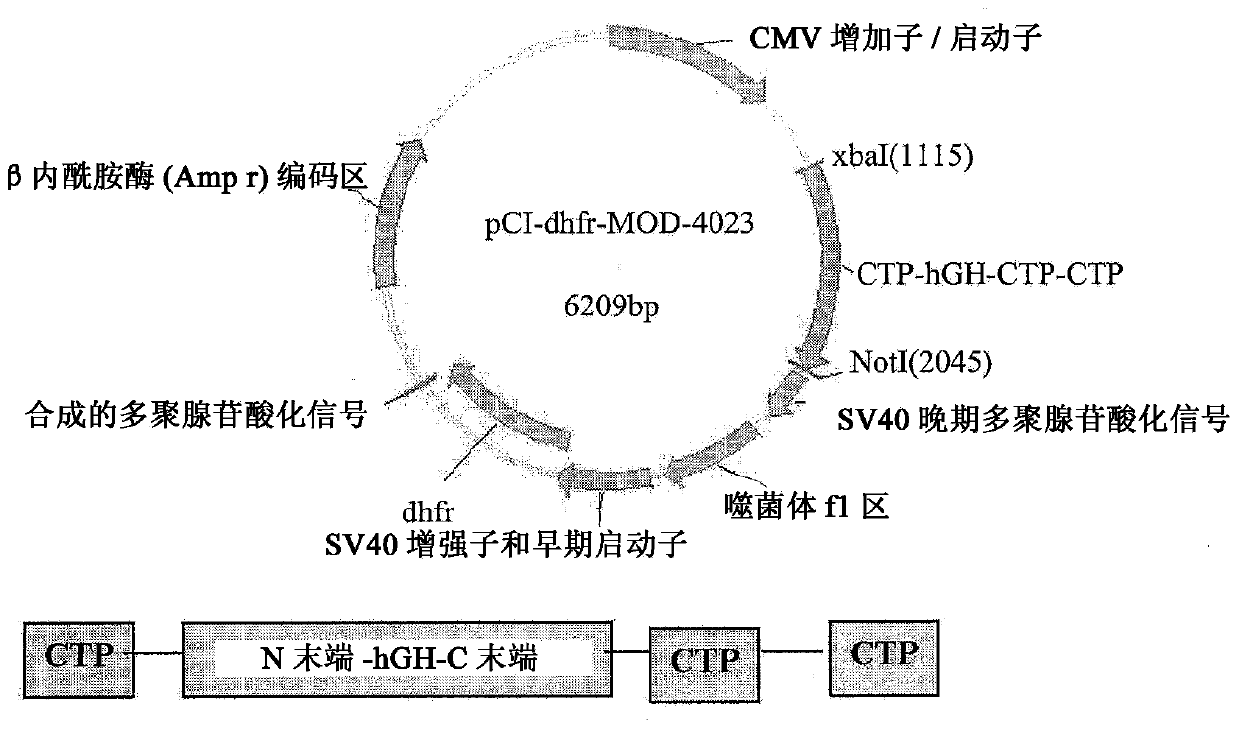
A polypeptide and polynucleotides encoding same comprising one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to an amino terminus of a growth hormone and two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a carboxy terminus of a growth hormone are disclosed. Pharmaceutical compositions comprising the polypeptide and polynucleotides of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Long-acting polypeptides and methods of producing same
ActiveUS20070190611A1Reduce morbidityProlong lifePeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides encoding same comprising at least two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a peptide-of-interest are disclosed. Pharmaceutical compositions comprising the polypeptide and polynucleotides of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Long-acting polypeptides and methods of producing same
ActiveUS20090312254A1Reduce dosing frequencyImprove compliancePeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides encoding same comprising one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to an amino terminus of a cytokine and two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a carboxy terminus of a cytokine are disclosed. Pharmaceutical compositions comprising the polypeptide and polynucleotides of the invention and methods of using same are also disclosed.
Owner:MODIGENE LLC +1
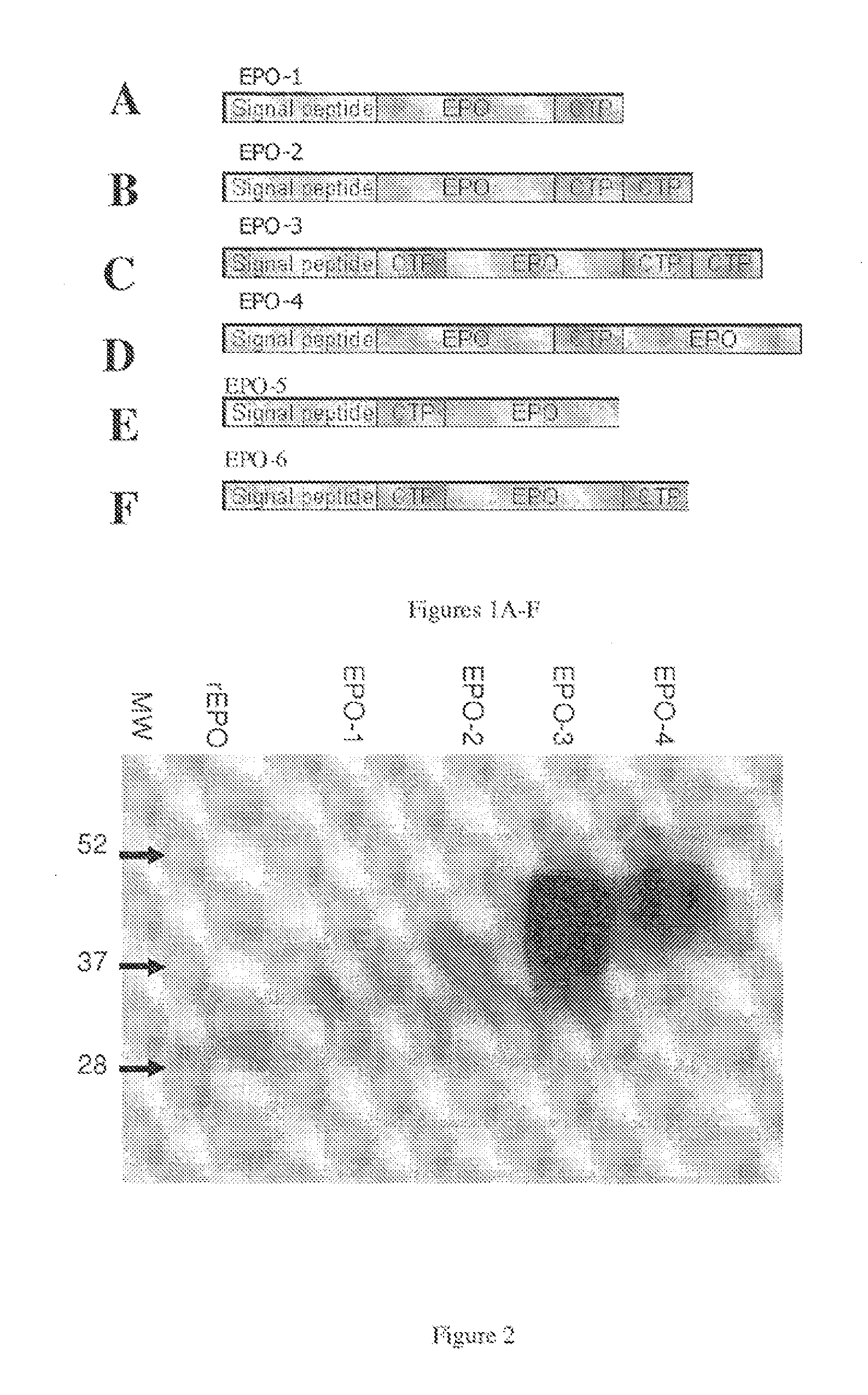
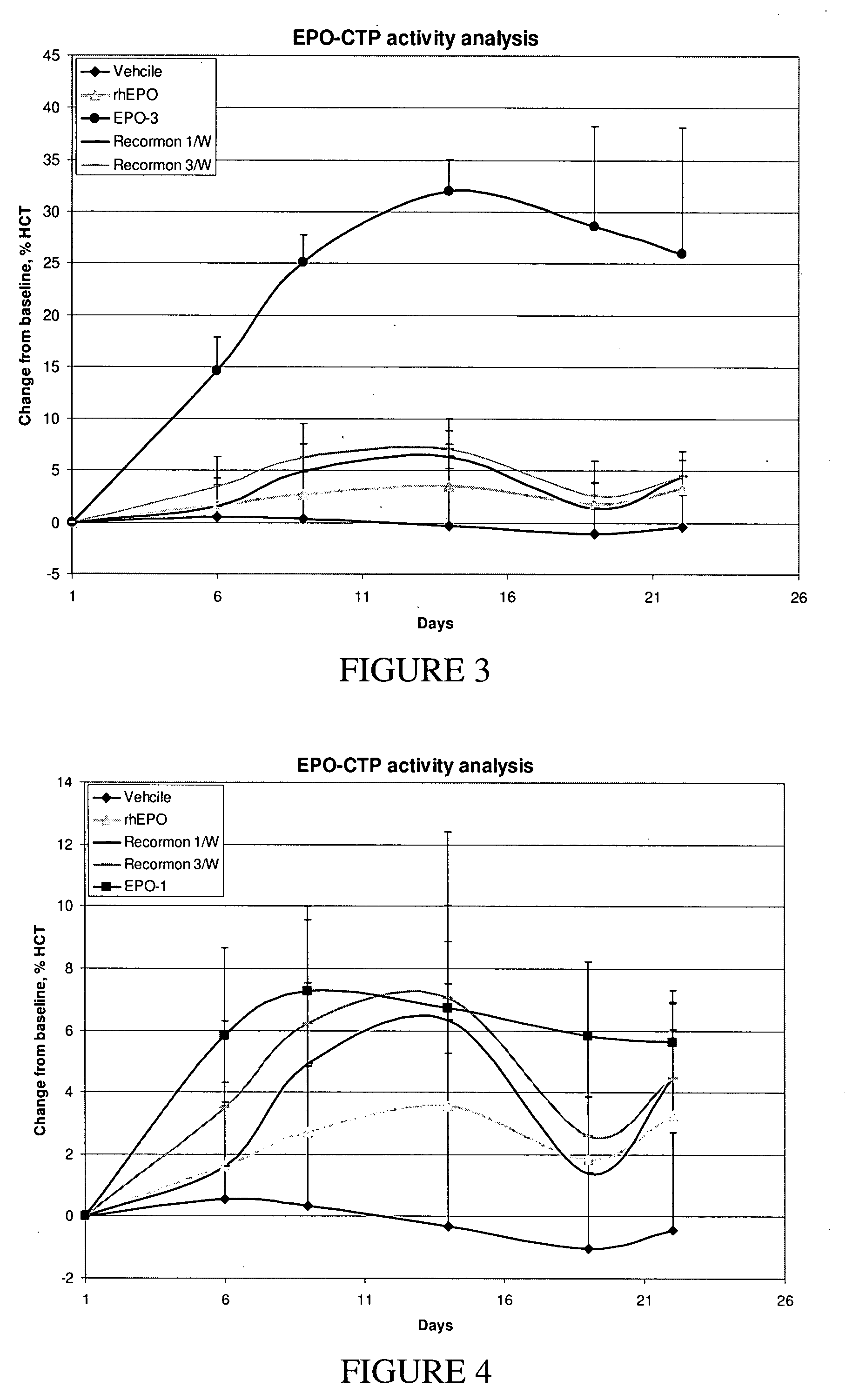
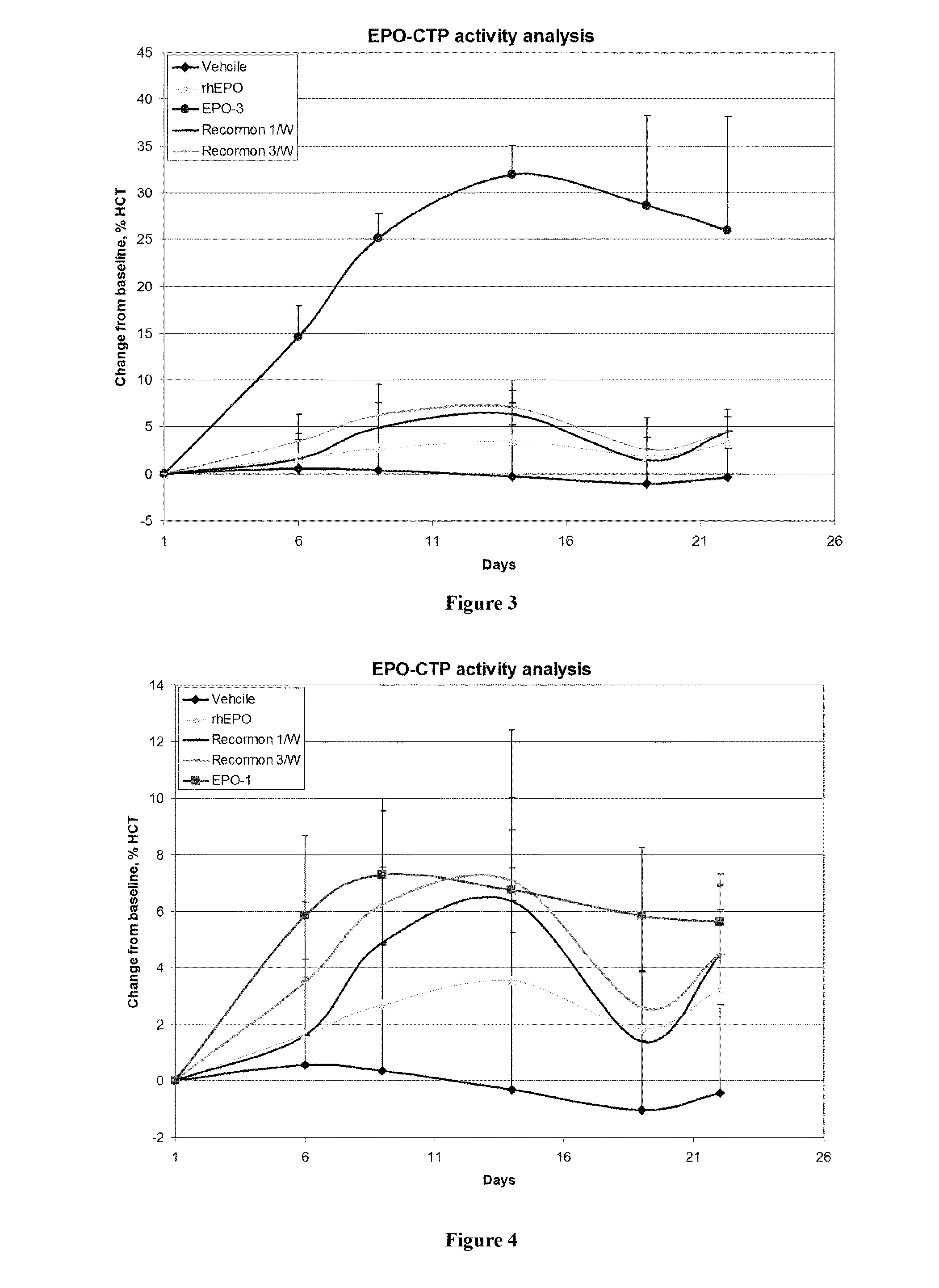
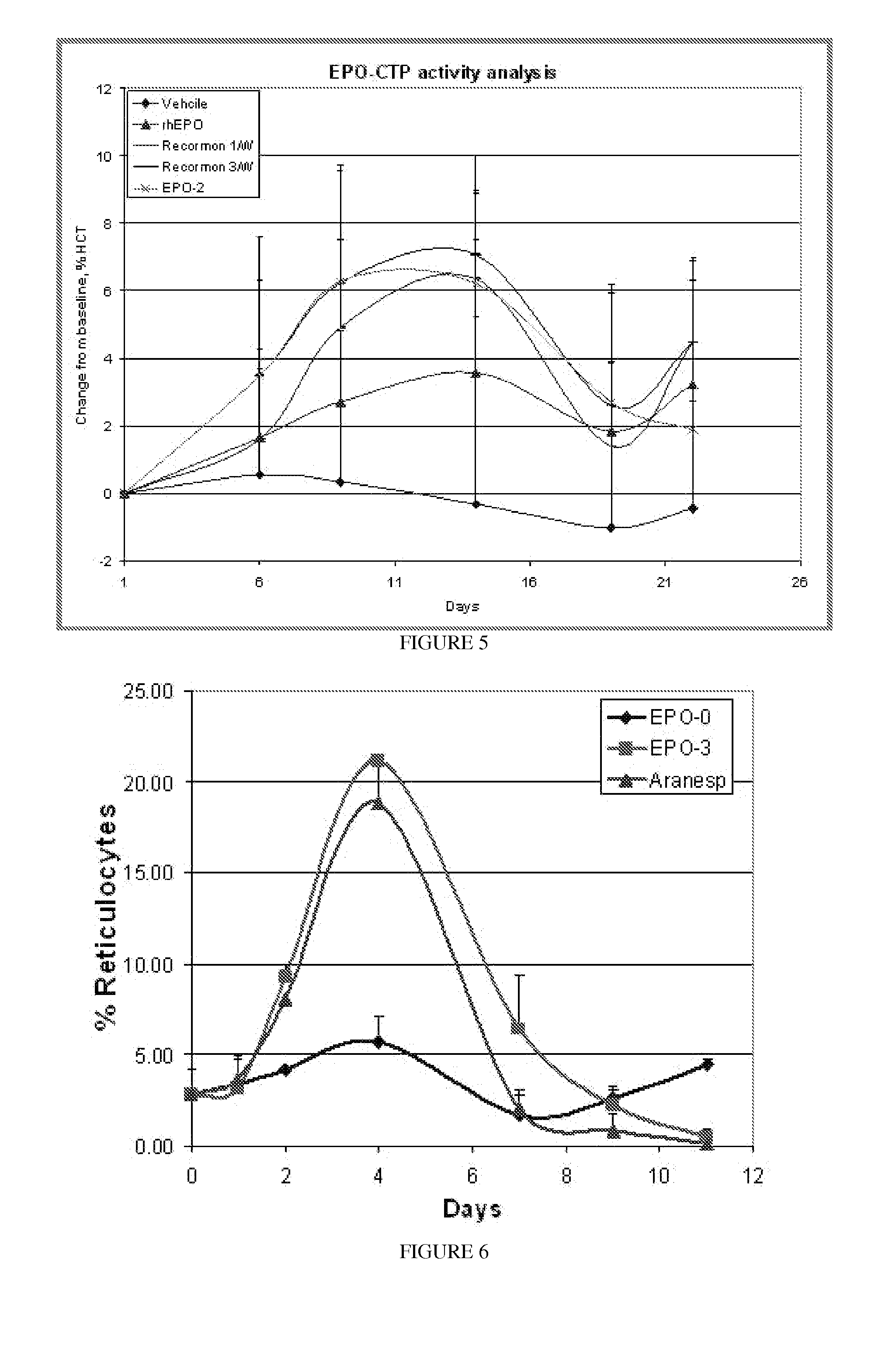
Long-acting EPO polypeptides and derivatives thereof and methods thereof
ActiveUS20070190610A1Prolong lifeReduce morbidityPeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides encoding same comprising at least two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to an EPO peptide are disclosed. Pharmaceutical compositions comprising the polypeptide and polynucleotides of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Linker peptide for constructing fusion protein
ActiveCN106317226AWide applicabilityMeet different requirements of rigidityPeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeSide chain
The invention provides a linker peptide for constructing a fusion protein, comprising a flexible peptide and a rigid peptide; the flexible peptide is composed of one or more flexible units, and the rigid peptide is composed of one or more rigid units, wherein each flexible unit comprises two or more amino acid residues selected from Gly, Ser, Ala and Thr, and each ridge unit comprises a plurality of glycosylation-sited carboxyl-terminal peptides (CTP) of human chorionic gonadotrophin beta-subunit. The linker peptide herein is more efficient in eliminating steric-hindrance effect between two fusion molecules, and reducing polymerization or activity decrease or loss due to misfolding or comformational change of active proteins; in addition, negatively-charged high-sialyl CTPs can resist removal by kidney, half-life period of the fusion molecules is further extended, bioavailability of the fusion protein is improved; more additionally, protection from glycosyl side chains of the CTPs enables reduced sensitivity of the linker peptide to protease such that the fusion protein is rarely degraded in a linker region.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
Long-acting interferons and derivatives thereof and methods thereof
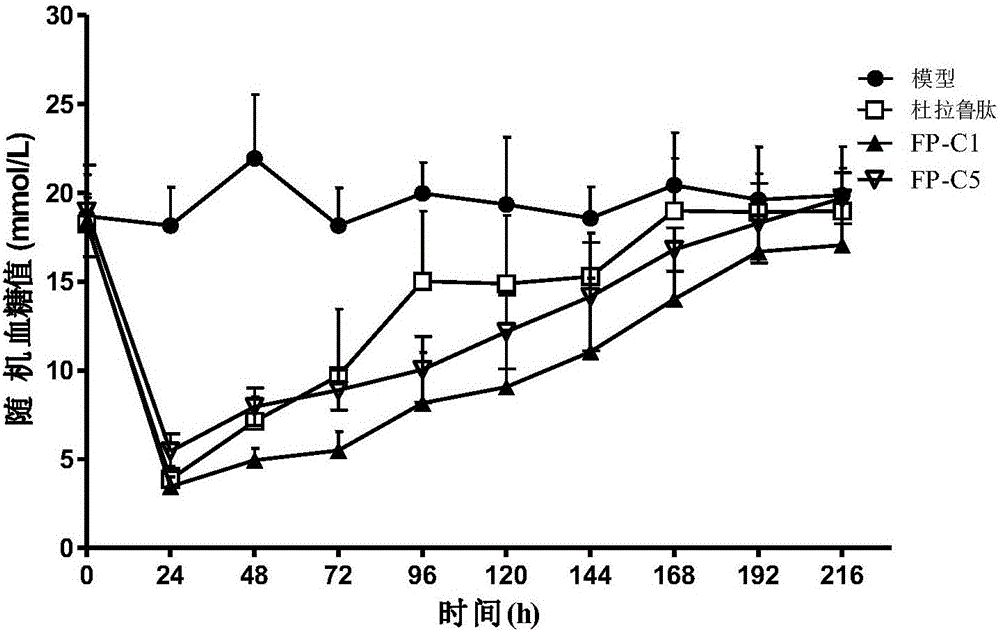
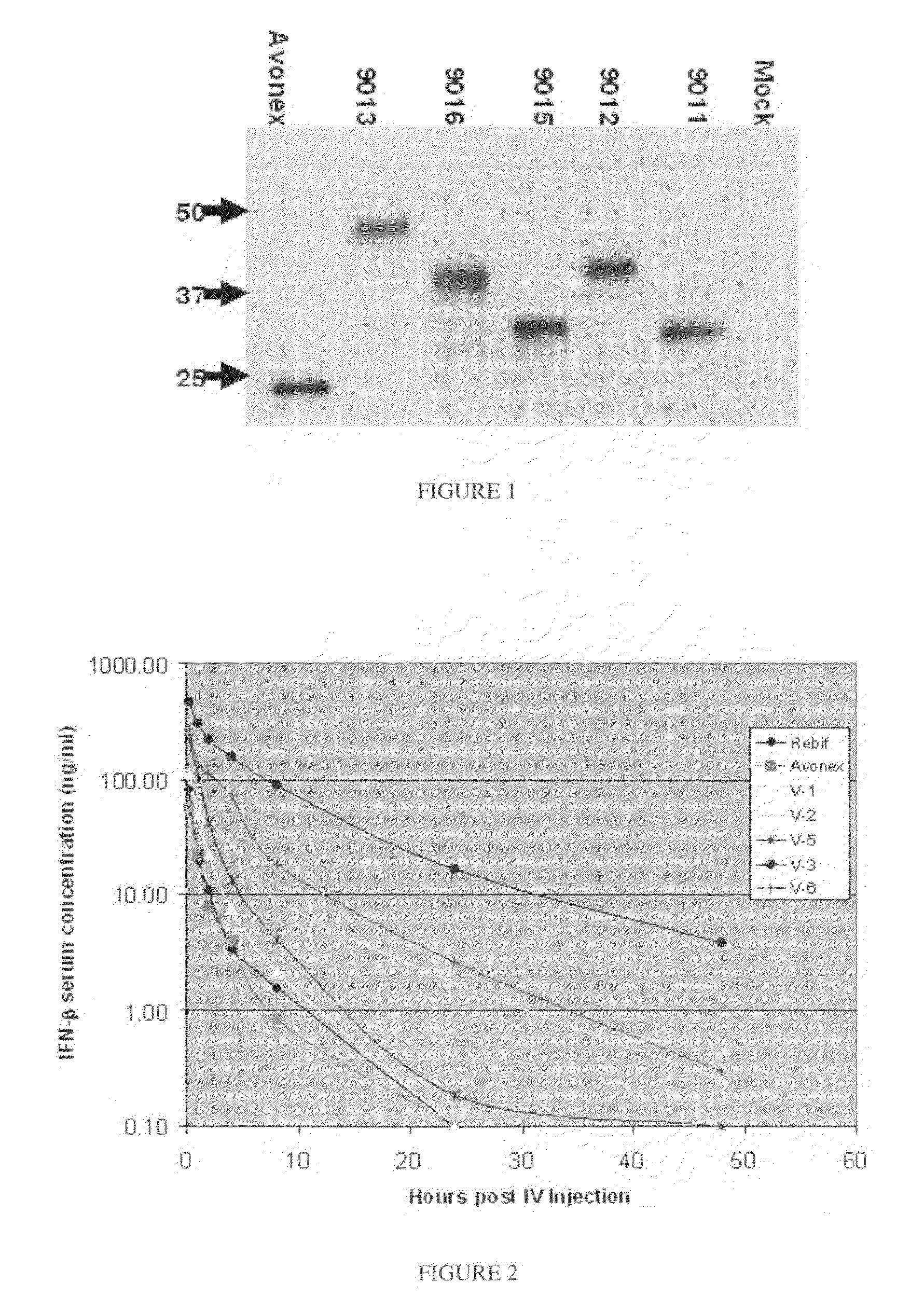
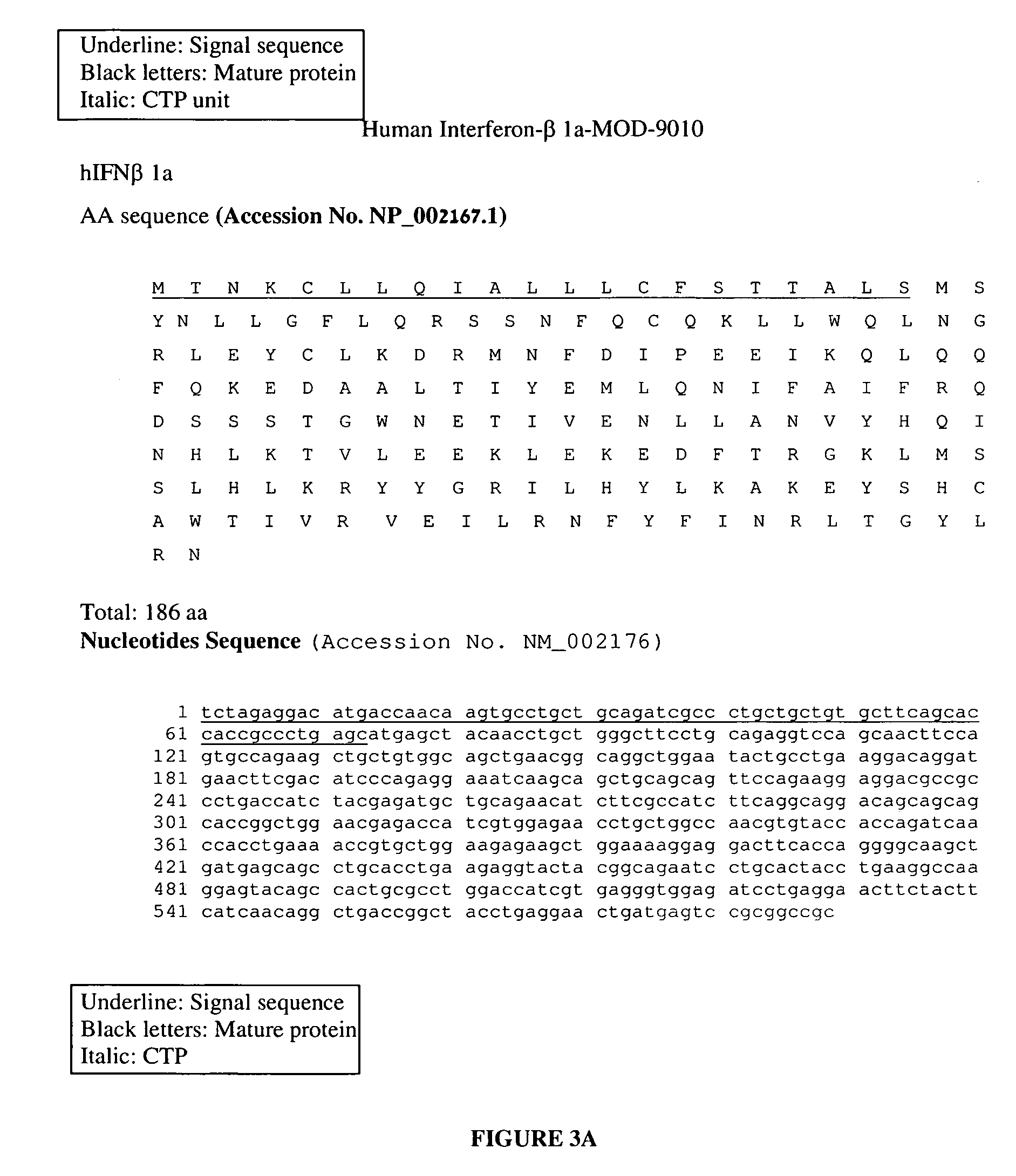
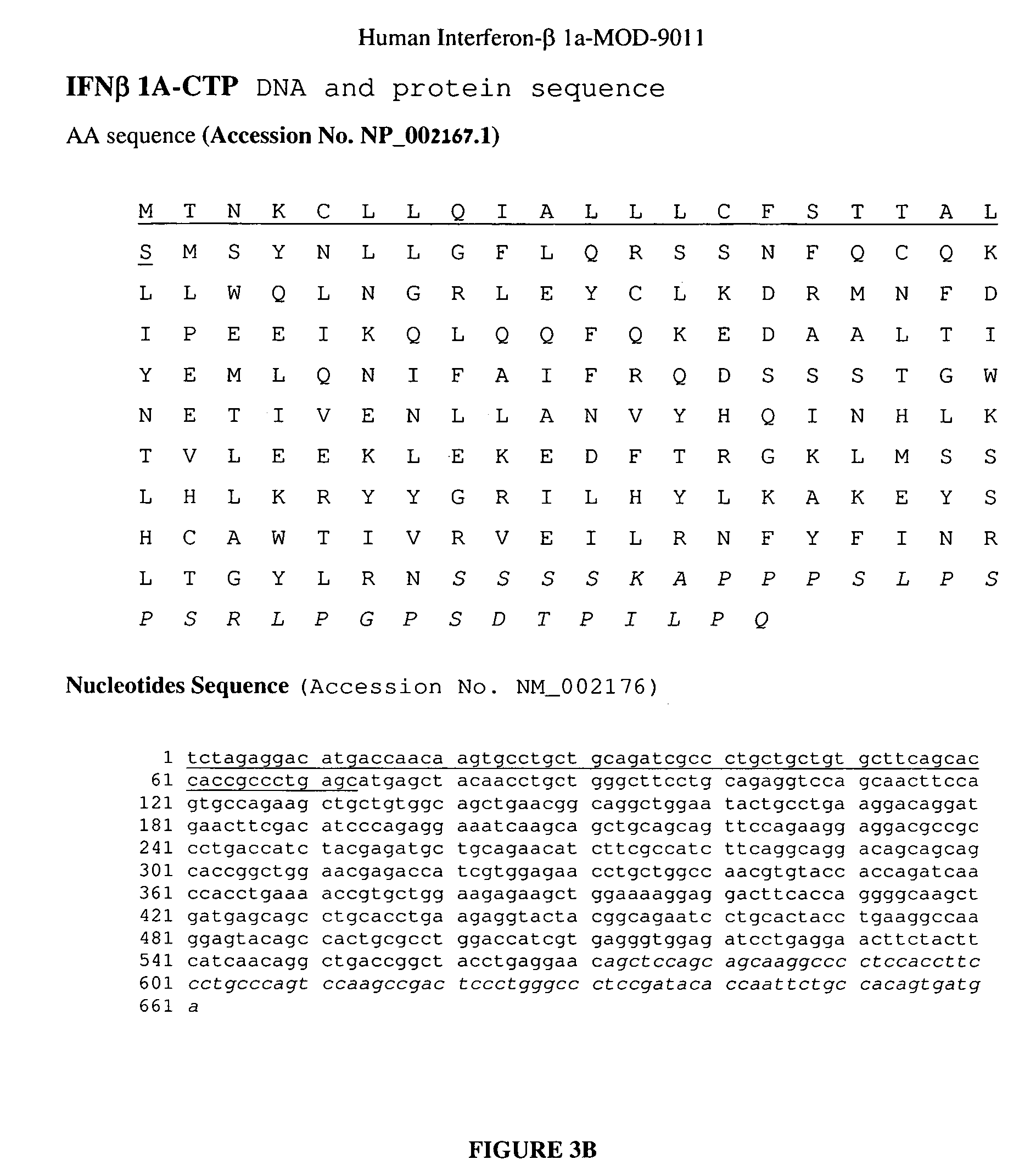
ActiveUS8048848B2Prolong lifeAntibacterial agentsNervous disorderChorionic gonadotrophinPolynucleotide
A polypeptide and polynucleotides encoding same comprising carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to an IFN protein are disclosed. Pharmaceutical compositions comprising the polypeptide and polynucleotides of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Long-acting polypeptides and methods of producing and administering same
InactiveUS20140113860A1Increase the areaReduce dosing frequencyPeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides comprising at least two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a non-human peptide-of-interest are disclosed. Pharmaceutical compositions comprising the non-human polypeptides and polynucleotides of the invention and methods of using both human and non-human polypeptides and polynucleotides are also disclosed.
Owner:OPKO BIOLOGICS
Long-acting polypeptides and methods of producing and administering same
PendingUS20150038413A1Increase the areaReduce dosing frequencyPeptide/protein ingredientsAntibody mimetics/scaffoldsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides comprising at least two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a non-human peptide-of-interest are disclosed. Pharmaceutical compositions comprising the non-human polypeptides and polynucleotides of the invention and methods of using both human and non-human polypeptides and polynucleotides are also disclosed.
Owner:OPKO BIOLOGICS
Process for the synchronization of ovulation for timed breeding without heat detection
ActiveUS20050130894A1Efficient methodAnimal reproductionPeptide/protein ingredientsOvulation timesSingle injection
A method for synchronizing ovulation in sows and gilts by a single injection of hormones is disclosed. A hormone, gonadotropin releasing hormone (GnRH), luteinizing hormone (LH), follicle stimulating hormone (FSH), human chorionic gonadotropin (hCG), analogues, derivatives, agonists or combinations thereof is administered to an open sow post weaning at a specific time to stimulate ovulation of mature responsive follicles. The sow is then bred, without heat detection, at a specific subsequent timed interval after injection with hormone, with one or two artificial or natural breedings. In gilts, the hormone is injected at a timed interval from onset of estrus or at a specific timed interval following Prostaglandin F2a for those gilts which have been held in a state of pseudopregnancy.
Owner:THORN BIOSCI
Long-acting coagulation factors and methods of producing same
Polypeptides comprising at least one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to the carboxy terminus but not to the amino terminus of a coagulation factor and polynucleotides encoding the same are disclosed. Pharmaceutical compositions comprising the polypeptides and polynucleotides of the invention and methods of using and producing same are also disclosed.
Owner:OPKO BIOLOGICS
Long-acting growth hormone and methods of producing same
ActiveUS8304386B2Increase the areaReduce dosing frequencyNervous disorderPeptide/protein ingredientsNucleotideChorionic gonadotrophin
A polypeptide and polynucleotides encoding same comprising one carboxy-terminal peptide (CTP) of chorionic gonadotrophin attached to an amino terminus of a growth hormone and two carboxy-terminal peptides (CTP) of chorionic gonadotrophin attached to a carboxy terminus of a growth hormone are disclosed. Pharmaceutical compositions comprising the polypeptide and polynucleotides of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Dissociate human chorionic gonadotrophin beta-subunit magnetic particle chemiluminescence quantitative assay kit and its preparation method
InactiveCN103076458AGuaranteed SensitivityHigh sensitivityChemiluminescene/bioluminescenceBiological testingImmune profilingChromogenic Substrates
The invention relates to the medical field of immunoassay, and concretely provides a dissociate human chorionic gonadotrophin beta-subunit magnetic particle chemiluminescence quantitative assay kit having the advantages of simplicity, rapidness, high sensitivity, wide linear range and good stability, and its preparation method. The method combines a magnetic particle immune separating technology to apply an enzymatic chemiluminescence substrate on the basis of enzyme-linked immunosorbent assay, and allows an optical signal generated by the detection of the luminescence substrate to substitute a chromogenic substrate in enzyme immune assay, so the method has the advantages of substantially improved sensitivity, simple operation and wide practicality, can be applied to open semi-automatic chemiluminescence detectors, can also be applied to full-automatic measure systems, and can realize batch and fast detection, low use cost, and easy popularization and application.
Owner:BEIJING LEADMAN BIOCHEM
Long-acting growth hormone and methods of producing same
InactiveCN104010650AIncrease weightPeptide/protein ingredientsMetabolism disorderNucleotidePolynucleotide
Use of a growth hormone protein and polynucleotides encoding the same comprising an amino-terminal carboxy-terminal peptide (CTP) of chorionic gonadotrophin and two carboxy-terminal chorionic gonadotrophin CTPs attached to the growth hormone in methods of inducing weight loss or body fat reduction, methods of increasing insulin-like growth factor (IGF-1) levels, and methods of reducing the dosing frequency of a growth hormone in a human subject are disclosed. Pharmaceutical compositions comprising the growth hormone and polynucleotides encoding the growth hormone of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Method for promoting female and male parent giant salamanders to synchronously mature
InactiveCN103238563APromote maturationAvoid physical damageAnimal husbandryBroodstockArtificial rearing
The invention belongs to the field of animal husbandry, and particularly relates to a method for promoting female and male parent giant salamanders to synchronously mature. The method is suitable for artificial rearing of giant salamanders. The method is characterized by being realized through the following mode: firstly, selecting sexually-mature parent giant salamanders, and artificially domesticating the parent giant salamanders to enable the same to be capable of taking in food initiatively; and secondly, feeding spawning-inducing medicine to the artificially-domesticated parent giant salamanders from the middle of August to the beginning of September in each year, wherein the spawning-inducing medicine is prepared by mixing luteinizing hormone releasing hormone analogs (LHR-A), human chorionic gonadotrophin (HCG) for fishes and carp pituitary glands. The method of feeding the spawning-inducing medicine is adopted to promote sexual glands of the parent giant salamanders to mature, so that sperm producing amount of male parent giant salamanders is increased by at least 35%, sperm activity is improved by more than 40%, egg laying amount of female parent giant salamanders is increased, and success rate of artificial fertilization is increased by more than 85%.
Owner:赵道全
Recombinant pig FSH-CTP fusion protein as well as preparation method and application thereof
ActiveCN108676096AHigh purityImprove securityPeptide/protein ingredientsAntibody mimetics/scaffoldsAnimal scienceHalf-life
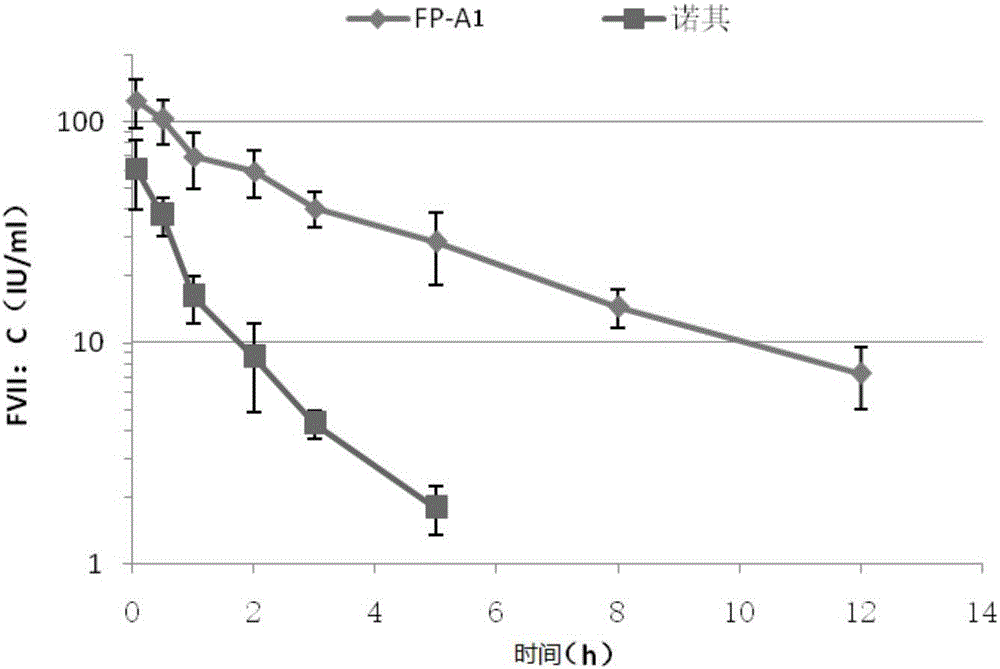
The invention provides a recombinant pig FSH-CTP fusion protein. The fusion protein means that a beta subunit of pig FSH is directly or indirectly linked to a beta subunit carboxy terminal peptide CTPof human, primate or equine mammalian chorionic gonadotropin, and an alpha subunit of pig FSH is bound to the beta subunit of pig FSH through Van der Waals force. The fusion protein can be prepared by using a eukaryotic expression system on the basis of a genetic engineering technology. Compared with pig pituitary FSH, the pig FSH-CTP fusion protein provided by the invention has a longer half-life and a better drug effect, and can replace pig pituitary FSH and pregnant horse serum gonadotropin in the current market in the field of animal propagation.
Owner:BEIJING VJT BIO CO LTD
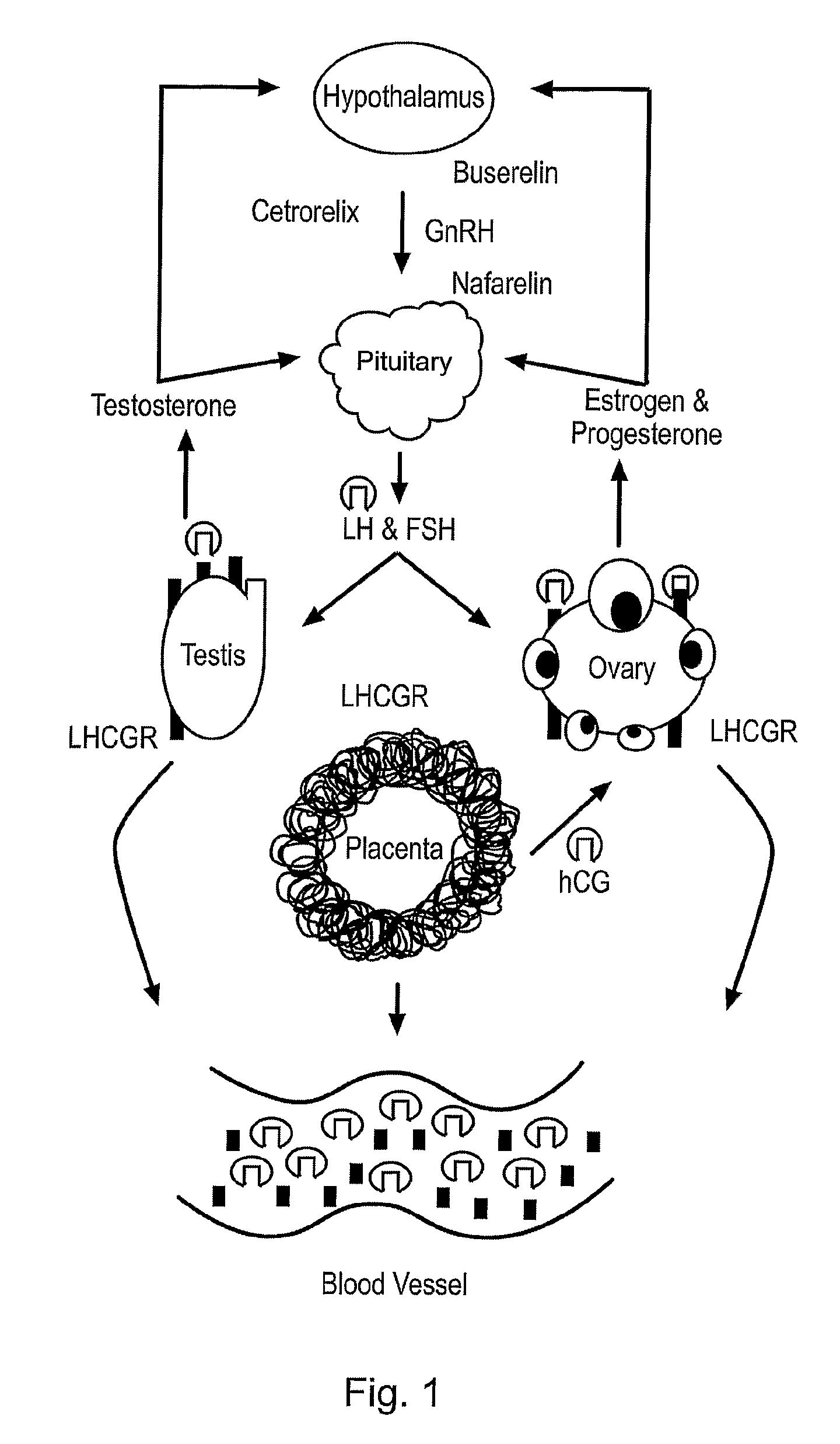
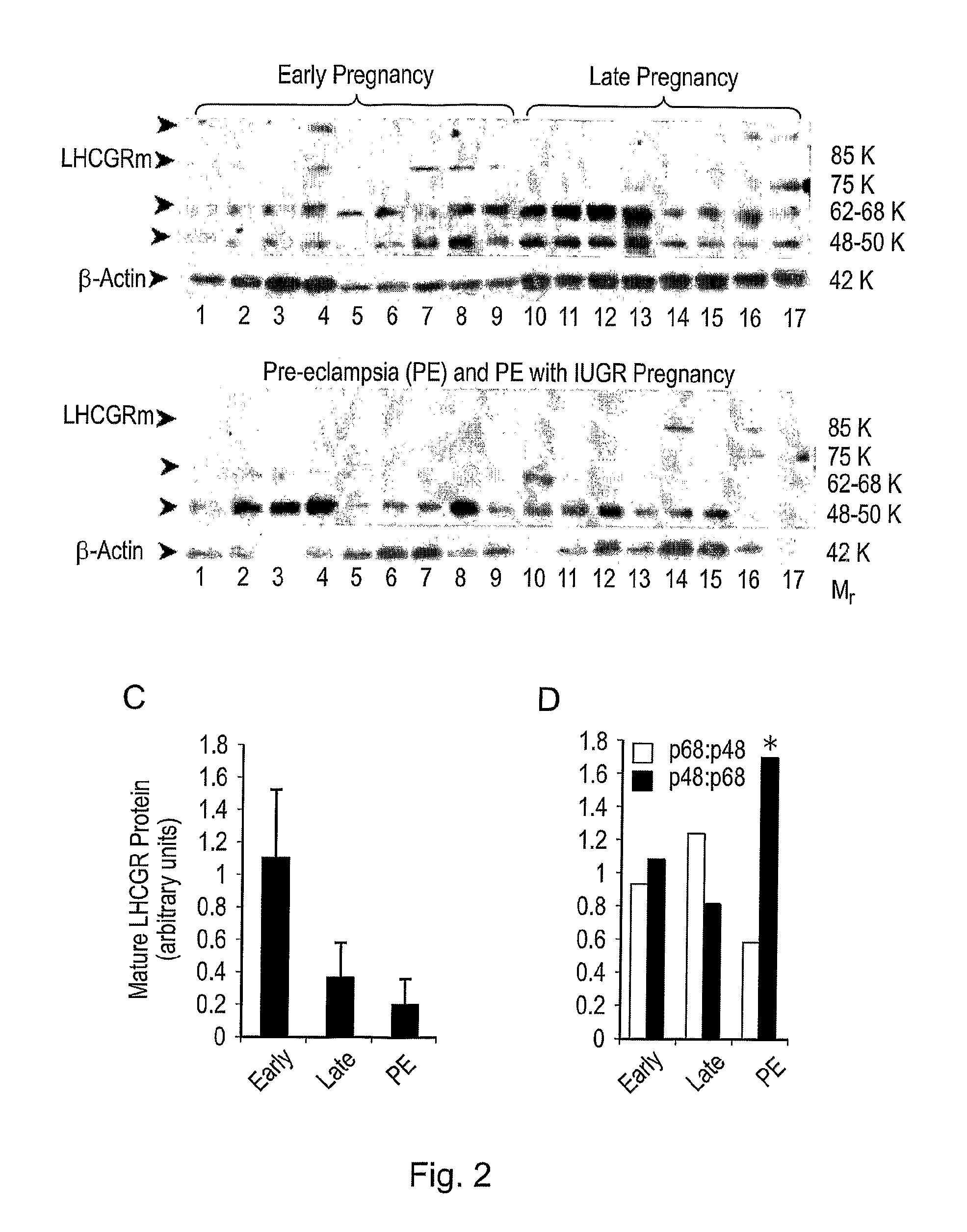
Diagnostic and therapeutic applications of soluble LHCGR protein
InactiveUS7892753B1Easy to measureImprove survival rateReceptors for hormonesBiological testingDiseasePhysiology
The invention relates to a soluble luteinising hormone / chorionic gonadotropin receptor (LHCGR) protein and its use in diagnosing, treating and preventing conditions associated with over- and under-production of the said receptor, with over- and under-production of luteinising hormone, with over- and under-production of chorionic gonadotropin, with reproductive failure, with gonadal cancer and metastases, and Alzheimer's disease.
Owner:BANERJEE SUBHASIS
Long-acting polypeptides and methods of producing and administering same
ActiveUS20150148291A1Reduce dosing frequencyIncrease the areaBacteriaPeptide/protein ingredientsPolynucleotideBiology
Owner:OPKO BIOLOGICS
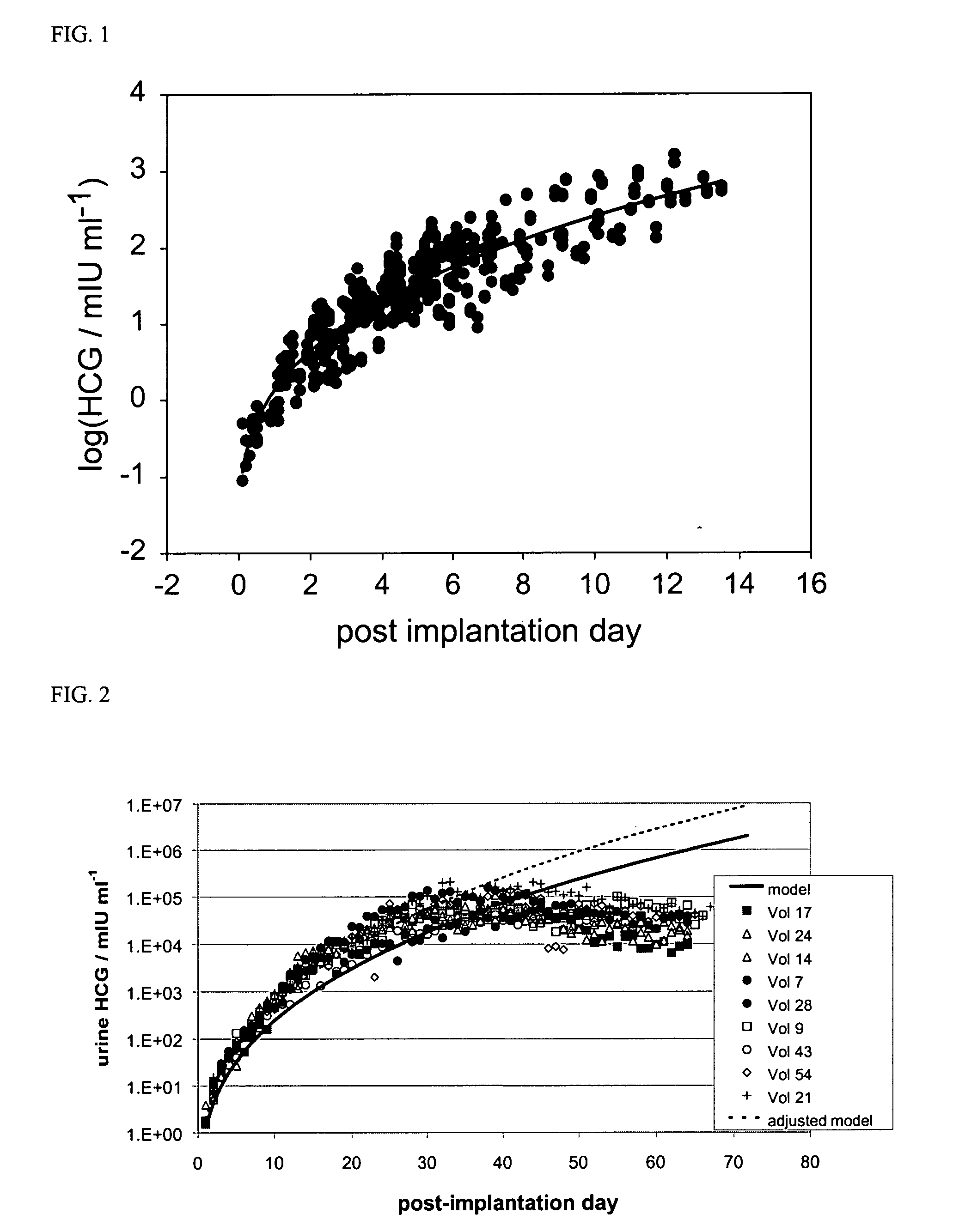
Determining the estimated date of embryo implantation and related dates
InactiveUS20050196812A1Biological testingSpecial data processing applicationsHuman FemalesChorionic gonadotrophin
A method of determining the estimated date of implantation of an embryo may include testing a sample of a body fluid from a pregnant human female subject so as to obtain data indicative of the concentration of human chorionic gonadotrophin (hCG) in the sample, and calculating an estimated date of implantation from the hCG concentration data.
Owner:INVERNESS MEDICAL SWITZERLAND GMBH
Hyperglycosylated-human chorionic gonadotrophin (H-HCG) rapid immune diagnosis chromatography test paper and preparation method thereof
InactiveCN106248975AStrong specificityIncreased sensitivityBiological testingChorionic gonadotrophinQuality control
The invention relates to hyperglycosylated-human chorionic gonadotrophin (H-HCG) rapid immune diagnosis chromatography test paper, characterized by comprising a sample pad, a nitrocellulose membrane enveloped with a detection line T and a quality control line C, and a sample absorption pad which are sequentially connected, wherein the sample pad, the nitrocellulose membrane and the sample absorption pad are stuck on a support plate. The H-HCG rapid immune diagnosis chromatography test paper has the advantages that (1) due to the design that a conjugate pad and the nitrocellulose membrane are separately enveloped with an H-HCG specific antibody B207 or B152, an antibody for resisting alpha-HCG or beta-HCG, or an antibody for resisting intact HCG, by detecting the embodiments such as blood, urine, saliva and other samples, of H-HCG content of a human body, whether a person to be tested is pregnant or not and whether the placental function of a pregnant woman is good or not can be known, abortion can be predicted, and the like; (2) the instant diagnostic reagent use an immune chromatography test paper method for rapid detectiong, which is suitable for diagnosis and differential diagnosis of normal first trimester pregnancy and abnormal first trimester pregnancy, and has the advantages of being high in specificity, high in sensibility, safe, easy to store, good in stability, convenient in application, and the like.
Owner:武汉百美生物科技有限公司
Long-acting polypeptides and methods of producing and administering same
ActiveUS20150126445A1Reduce dosing frequencyIncrease the areaBacteriaPeptide/protein ingredientsNucleotideChorionic gonadotrophin
Owner:OPKO BIOLOGICS
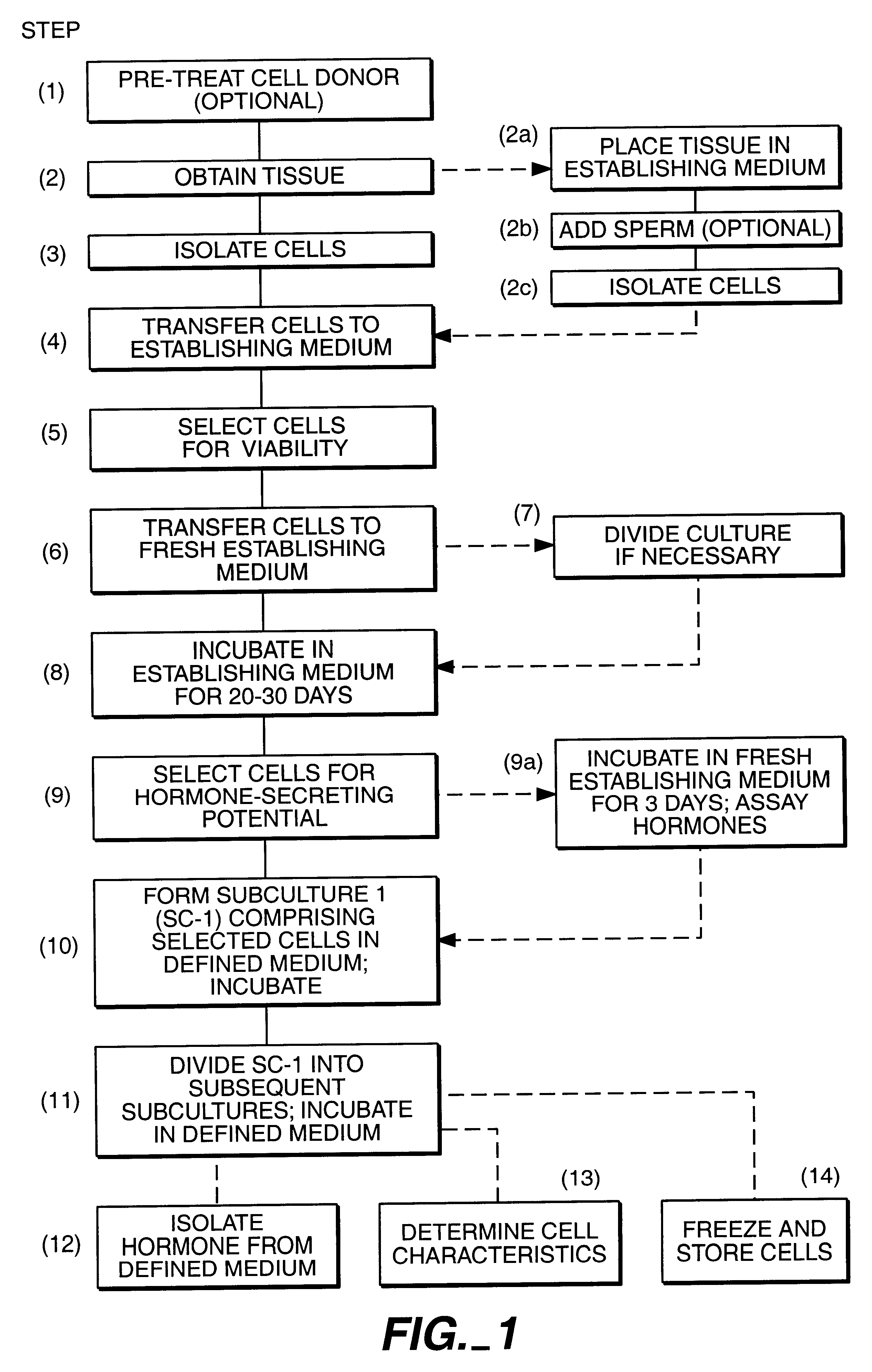
Hormone-secreting cells maintained in long-term culture
InactiveUS6372493B1Increased insulin secretionHigh glucose concentrationPeptide/protein ingredientsDiagnosticsCulture mediumsHuman chorionic gonadotropin
Methods are provided for the establishment and maintenance in long term culture of hormone secreting cells. Cells are derived from tumorous or non-tumorous animal or human tissues, including ovary, endometrium, trophoblast, pituitary, thyroid, and pancreas. The cells secrete into the culture medium hormones such as estrogens, progestins, follicle-stimulating hormone, luteinizing hormone, human chorionic gonadotrophin, thyroxin, glucagon, and insulin, depending on the tissue of origin of individual cell cultures. Contact with an appropriate secretogogue causes the cells to respond with increased hormone secretion. For instance, ovarian follicular cells respond to follicle-stimulating hormone with increased estrogen and progesterone secretion. Pancreatic cells respond to elevated glucose with increased insulin secretion. The cells proliferate in in vitro for up to one year or longer, during which time they retain their hormone-secretion profile. The cells may be frozen for storage, and retain their hormone-secretion profile after thawing. The cell cultures are useful for the production of human hormones, for the bio-assay of drugs such as therapeutic gonadotrophin, for the testing of drug efficacy and design, and for toxicity testing of drugs and chemicals. The cells may also be implanted in an individual to replace deficient hormone secretion. For instance, insulin secreting pancreatic cells may be implanted in a diabetic individual as an adjunct or replacement therapy for exogenously administered insulin.
Owner:PACIFIC BIOMEDICAL RES INC
Artificial breeding method for silurus meridionalis
InactiveCN104705225AImprove hatchabilityEasy to useClimate change adaptationPisciculture and aquariaChorionic gonadotrophinZoology
The invention provides an artificial breeding method for silurus meridionalis. The method includes the steps of selecting parent fish, cultivating the parent fish and performing artificial spawning induction, fertilization and hatch, wherein the weight of the selected parent fish ranges from 8 kg to 10 kg, the selected parent fish is more than three years old and is healthy and free of disease, the parent fish is introduced into a pond with the area being 120 square meter and water depth being 1.5-2.0 m to be raised, oxytocin is chorionic gonadotrophin and carp pituitary gland, water temperature for spawning induction is 25.5-29.8 DEG C, the effect time lasts for 10-12 hours, artificial dry fertilization is performed with the female-male ratio being 2:1, a hatching pond is 3.8 m long, 3.1 m wide and 1.2 m high, and water depth is 0.4 m; strict disinfection is performed in the mode that 0.3 kg of quicklime is spread on each square meter of the hatching pond; after toxicity disappears, water for hatching is placed in the hatching pond, and polyethylene net sheets with fertilized eggs are directly placed into the hatching pond for lentic hatching. The method is convenient to use, and the hatching rate can be increased by 15% when the silurus meridionalis is bred.
Owner:天津市宽余水产养殖专业合作社
Artificial-inducing mixing agent and artificial breeding method for largemouth bass
ActiveCN111956783AImprove breeding efficiencyPromote maturityOrganic active ingredientsPeptide/protein ingredientsAnimal scienceAquatic animal
The invention discloses an artificial-inducing mixing agent and artificial breeding method for largemouth bass, and belongs to the technical field of aquatic animal breeding. The provided mixing agentincludes chorionic gonadotropin, luteinizing hormone releasing hormone analogue No.2 and DOM. Through the adoption of the mixing agent to artificially breed largemouth bass, bred females can have high brood amount, high egg maturity can be achieved, fertilization rate and hatchability can be significantly increased, the breeding efficiency of the largemouth bass can be effectively enhanced, and time can be saved.
Owner:FRESHWATER FISHERIES RES CENT OF CHINESE ACAD OF FISHERY SCI
Beta-human chorionic gonadotrophin test kit (time-resolved fluoroimmunoassay) for prenatal screening and preparation method thereof
The invention discloses a beta-human chorionic gonadotrophin test kit (time-resolved fluoroimmunoassay) applicable for early-pregnancy and mid-pregnancy prenatal screening and a preparation method thereof. The kit comprises the following main components of an experimental buffer solution, a concentrated washing solution, an enhancement solution, a reaction plate, a standard product and a quality control product of filter paper dried blood spots and a europium marker. The method for preparing the kit according to the invention comprises the following steps of: 1. preparing the experimental buffer solution, the concentrated washing solution and the enhancement solution; 2. coating the reaction plate; 3. preparing the standard product and the quality control product; 4. preparing the europium marker; 5. separately loading; 6. sticking labels; and 7. assembling into a finished product. The invention has the characteristics that the accuracy and the sensitivity of a detected result are high, the stability is good, and a detecting method is economical, convenient and safe and has no traumatic property and the like. A filter paper dried blood spot technology is applied to prenatal screening work, which is beneficial to blood specimen collection, storage and transportation and ensures the experimental reliability.
Owner:广州市丰华生物股份有限公司
Detection kit for hyperglycosylated modification of hCG (human chorionic gonadotropin) tumor marker
InactiveCN105158485AHigh sensitivityImprove featuresDisease diagnosisBiological testingTumor BiomarkersImmunodiagnostics
The invention relates to a detection kit for hyperglycosylated modification of an hCG (human chorionic gonadotropin) tumor marker. According to the detection kit, magnetic beads are combined with an antibody (MCA-A) to serve as a capture antibody to capture total hCG in a to-be-detected sample, wherein the affinity of the antibody (MCA-A) is least influenced by glycosylation change; an antibody (MCA-B) most influenced by glycosylation change and another antibody (MCA-C) less influenced by glycosylation are used for detecting the total protein content and the N-glycosylation modification degree of hCG in the sample simultaneously, so that a tumor biomarker is identified. The detection kit improves the accuracy, the sensitivity and the specificity of an immunodiagnosis method greatly and has great practical value for detection of hyperglycosylated modification of the tumor marker in the actual sample.
Owner:SHANDONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com