Linker peptide for constructing fusion protein
A fusion protein and linking peptide technology, which is applied in the field of building linking peptides of fusion proteins, can solve problems such as loss of function, shielding, and protease degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1, connecting peptide is used for the construction of fusion protein
[0083]The inventors constructed a series of fusion proteins K1-L-K2 containing connecting peptides. The composition of each fusion protein is shown in Table 1. The DNA sequences encoding the active molecule K1 and the active molecule K2 of the fusion protein are connected through the DNA sequence of the connecting peptide L to form a fusion gene sequence. Preferably, they are artificially optimized codons favored by CHO cells. It is preferably obtained using chemical synthesis methods. In order to facilitate the insertion of the target fragment of the fusion gene obtained above into the specific site of the expression vector, a restriction enzyme site was inserted at the 5' and 3' ends of the synthesized fragment, respectively SpeI and EcoRI. The fusion protein gene verified by sequencing was digested with the corresponding restriction endonuclease, and then inserted into the correspond...
Embodiment 2
[0087] Embodiment 2, coagulation factor FVII-Fc fusion protein preparation, biological activity and in vivo active half-life assay
[0088] 2.1 Preparation and identification of coagulation factor FVII-Fc fusion protein
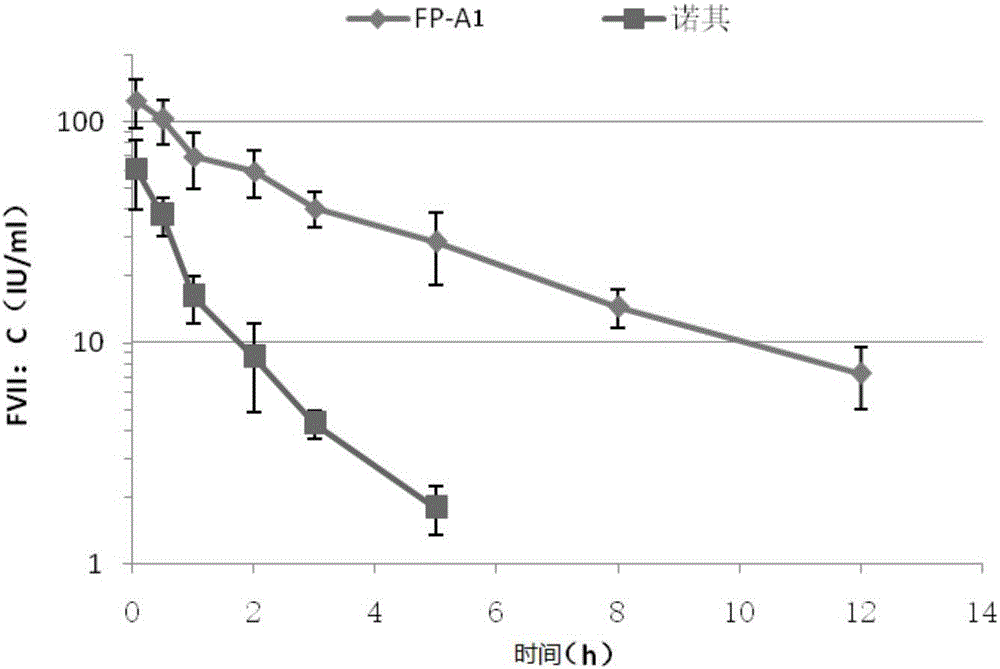
[0089] The FP-A1, FP-A2 and FP-A3CHO stably expressing cell lines obtained in Example 1 were cultured in shake flasks for 10-14 days, and were subjected to Protein A affinity chromatography, multidimensional mode chromatography, and anion exchange layer Four steps of purification and molecular sieve chromatography are carried out, and then the fusion protein is activated by solution incubation and self-activation method. SDS-PAGE protein electrophoresis detection shows that under reducing conditions, the unactivated FP-A2 single-chain molecule has two obvious bands around 70-85kDa and 40kDa, indicating the existence of degradation fragments, accounting for about 20-30% ;Under non-reducing conditions, the purified protein migrated to about 130kDa, accompanied...
Embodiment 3
[0099] Embodiment 3, coagulation factor FVIII-Fc fusion protein production, biological activity assay
[0100] 3.1 Production and identification of coagulation factor FVIII-Fc fusion protein
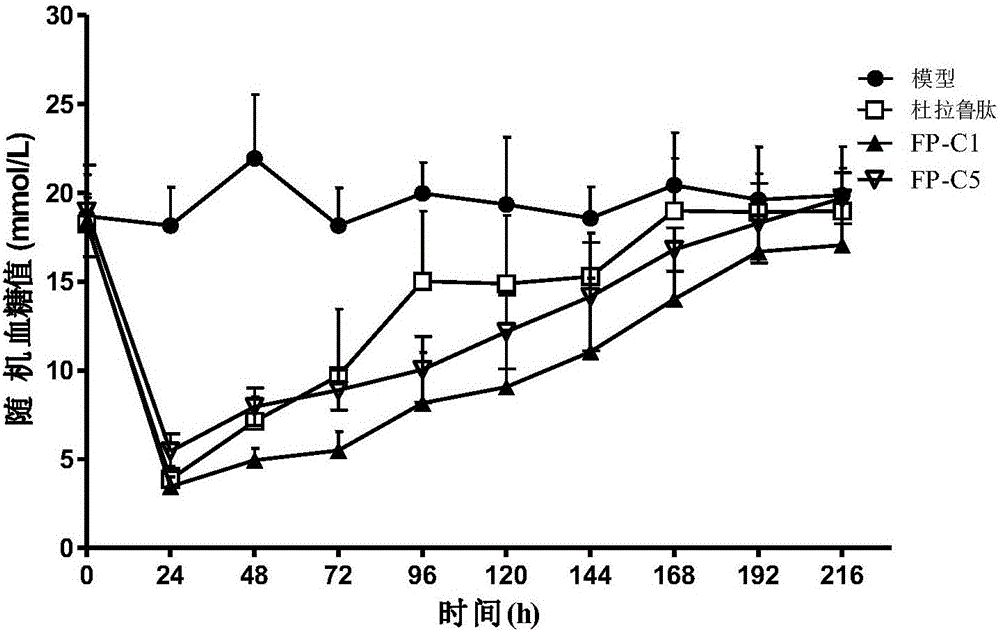
[0101] The FP-B1, FP-B2 and FP-B3 CHO stable expression cell lines obtained in Example 1, after 7-12 days of fed culture or semi-continuous tank flow culture, harvest the supernatant immediately for Protein A and / or VIII -select (GE) affinity chromatography purification. Under optimal culture conditions, the supernatant harvested from FP-B2 was purified by Protein A and VIII-select (GE) two-step affinity chromatography, and still contained various components. SDS-PAGE protein electrophoresis detection showed that under reducing conditions, a main band of 180kDa and multiple fragments of 40-100kDa appeared; under non-reducing conditions, most of the purified proteins migrated to >300kDa. It shows that FP-B2 products mostly exist in the form of polymers, which are unstable and easy to degr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com