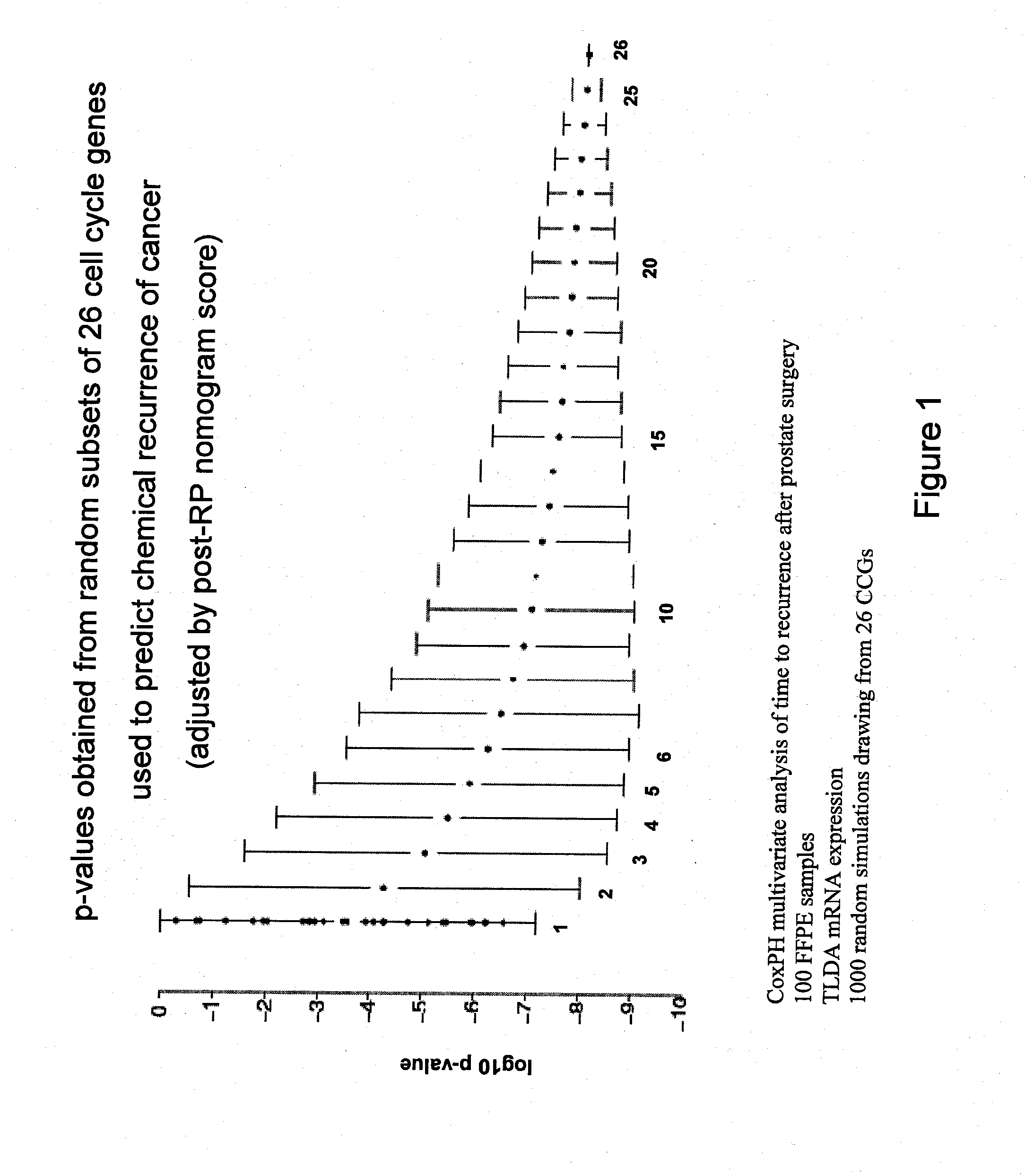
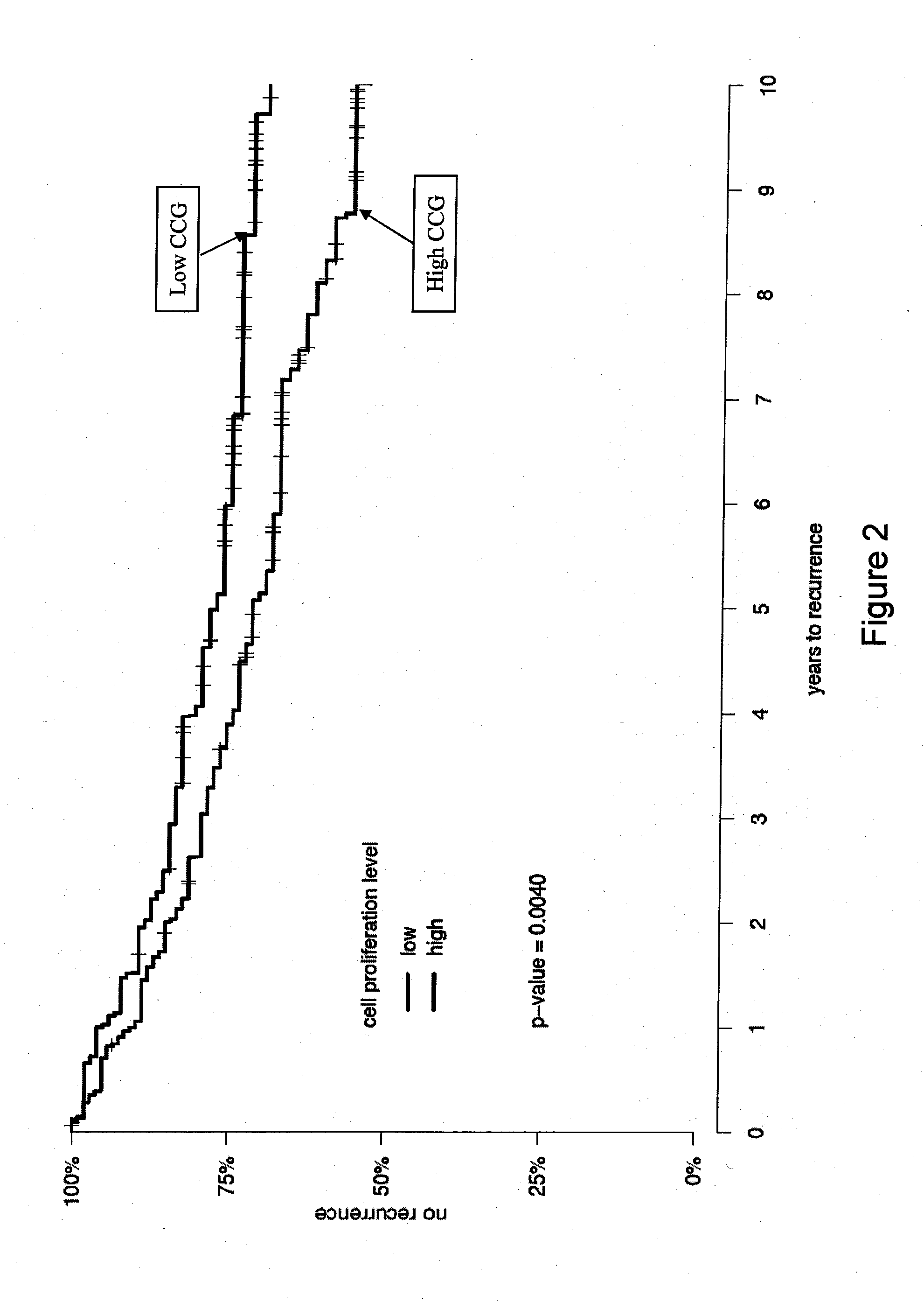
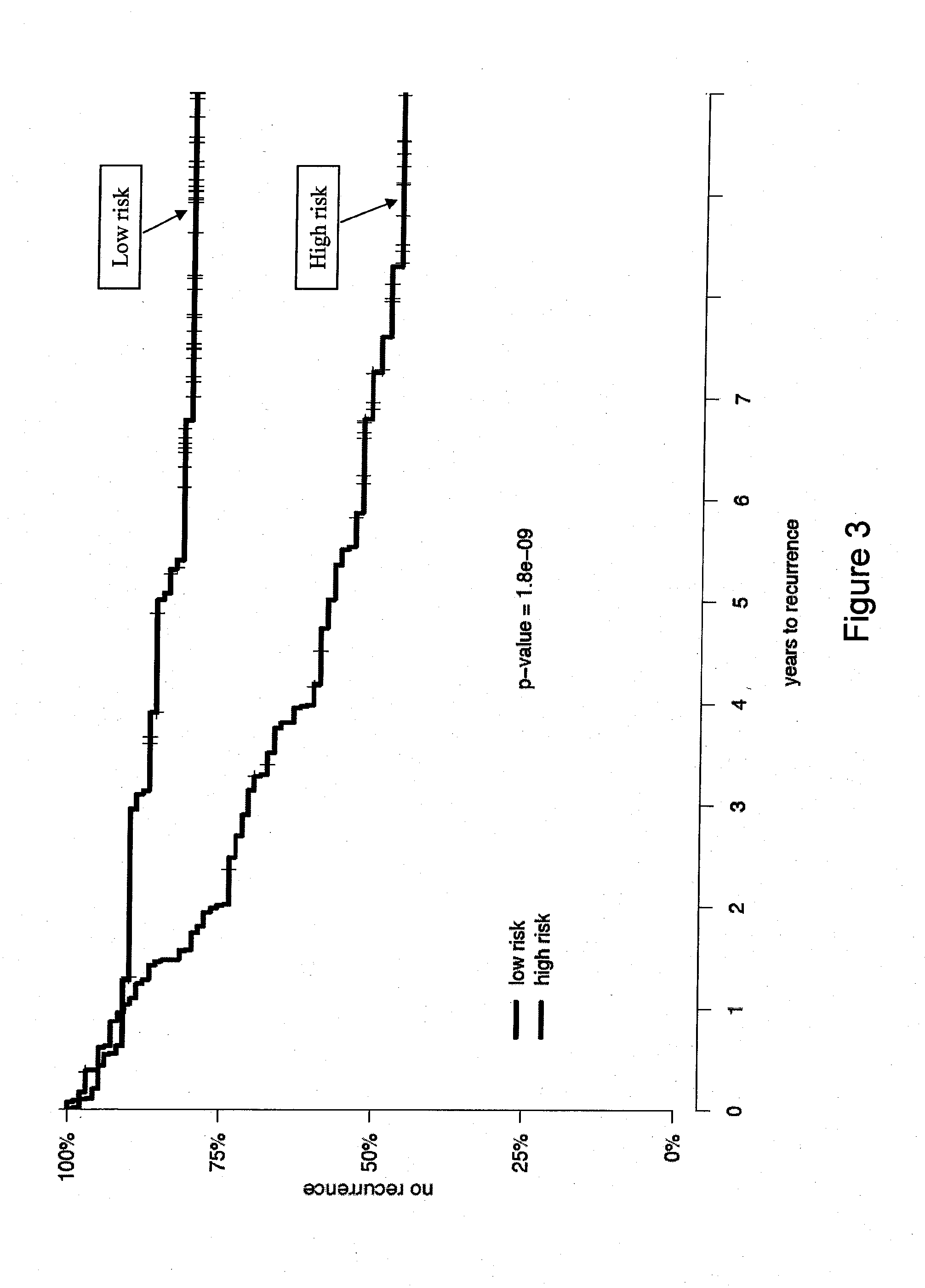
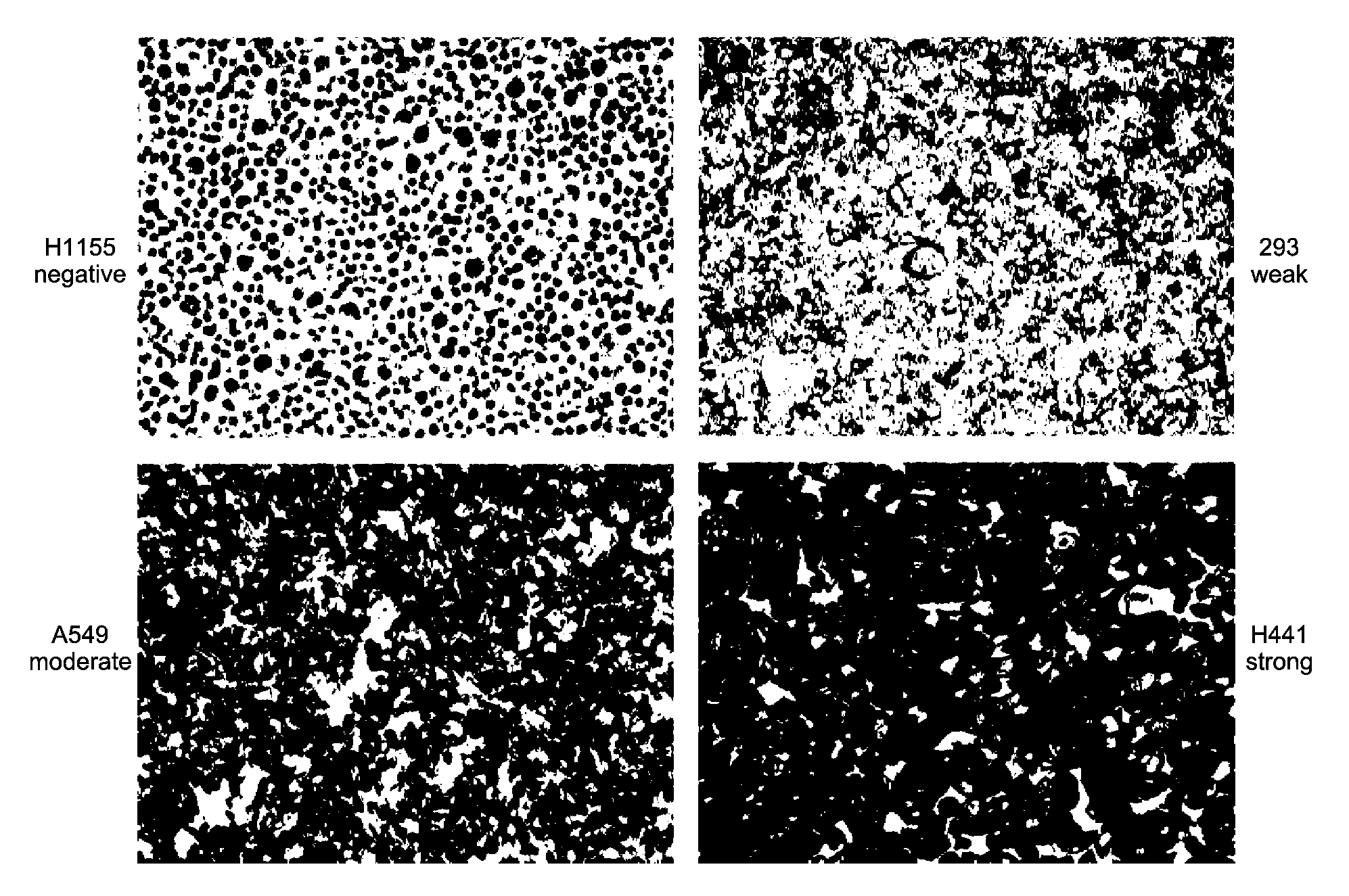
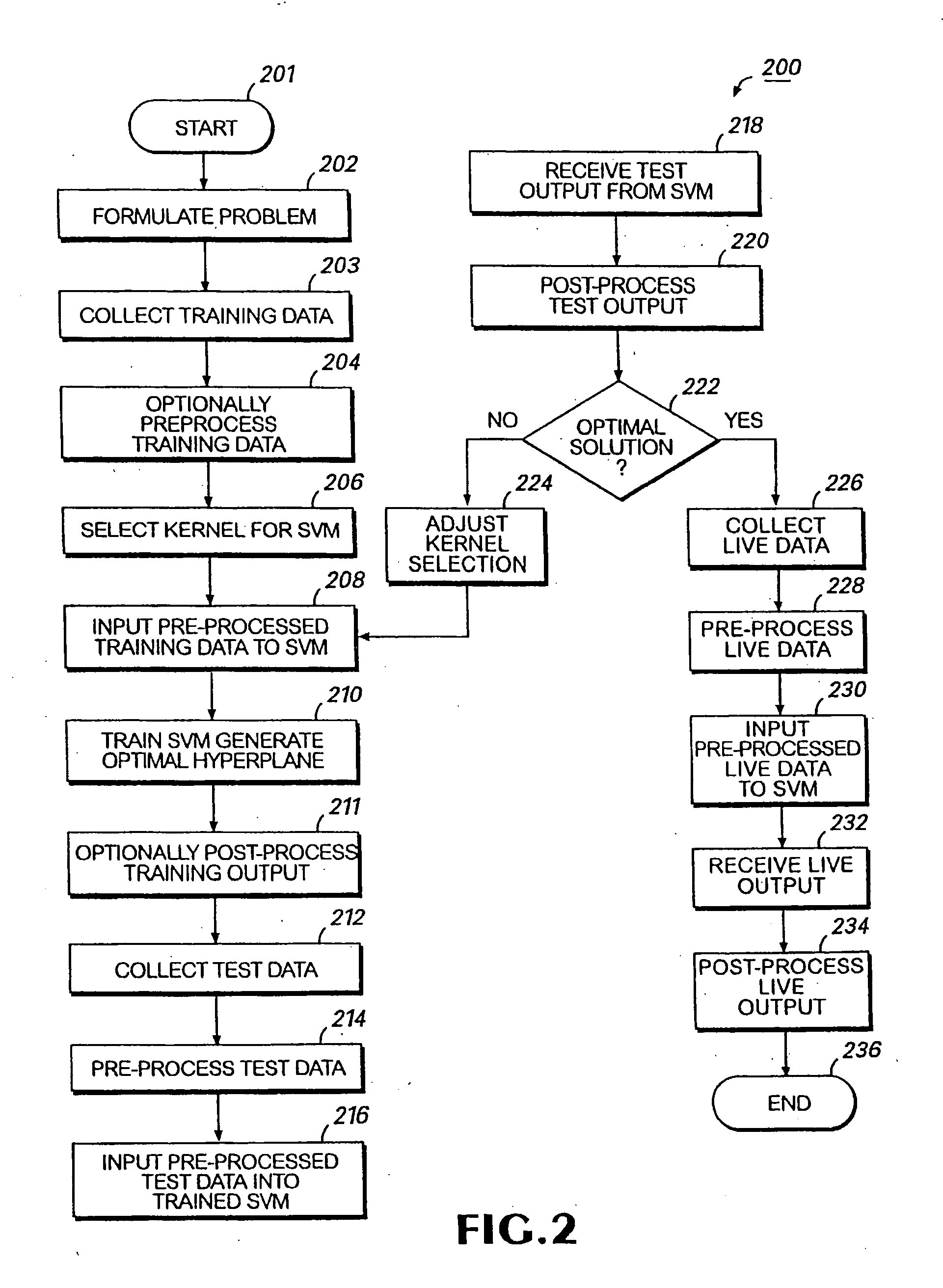
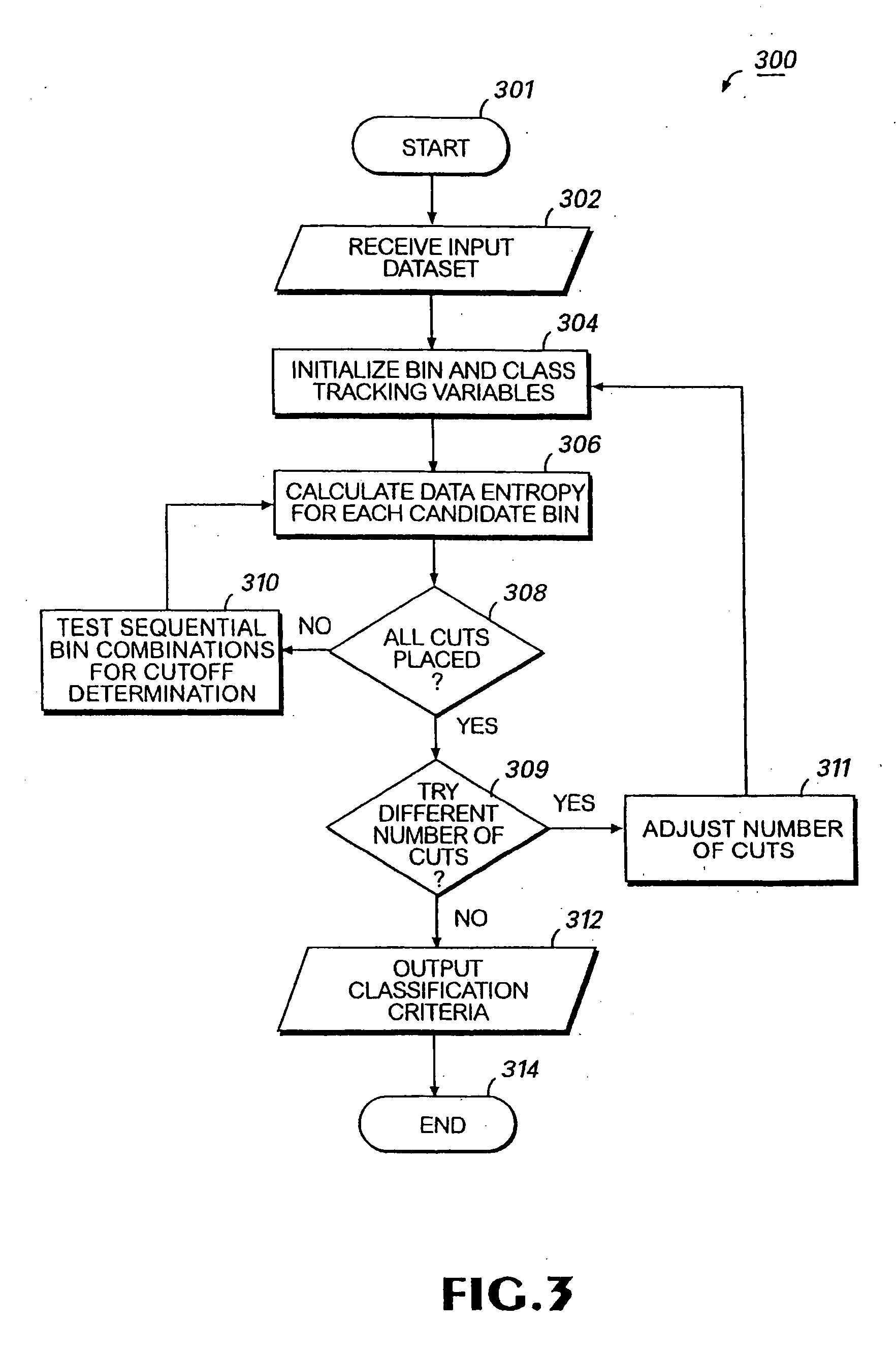
Patents
Literature
182 results about "Tumor Biomarkers" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A cancer biomarker refers to a substance or process that is indicative of the presence of cancer in the body. A biomarker may be a molecule secreted by a tumor or a specific response of the body to the presence of cancer.
Personalized Tumor Biomarkers
ActiveUS20150344970A1Microbiological testing/measurementLibrary member identificationBlood plasmaWilms' tumor
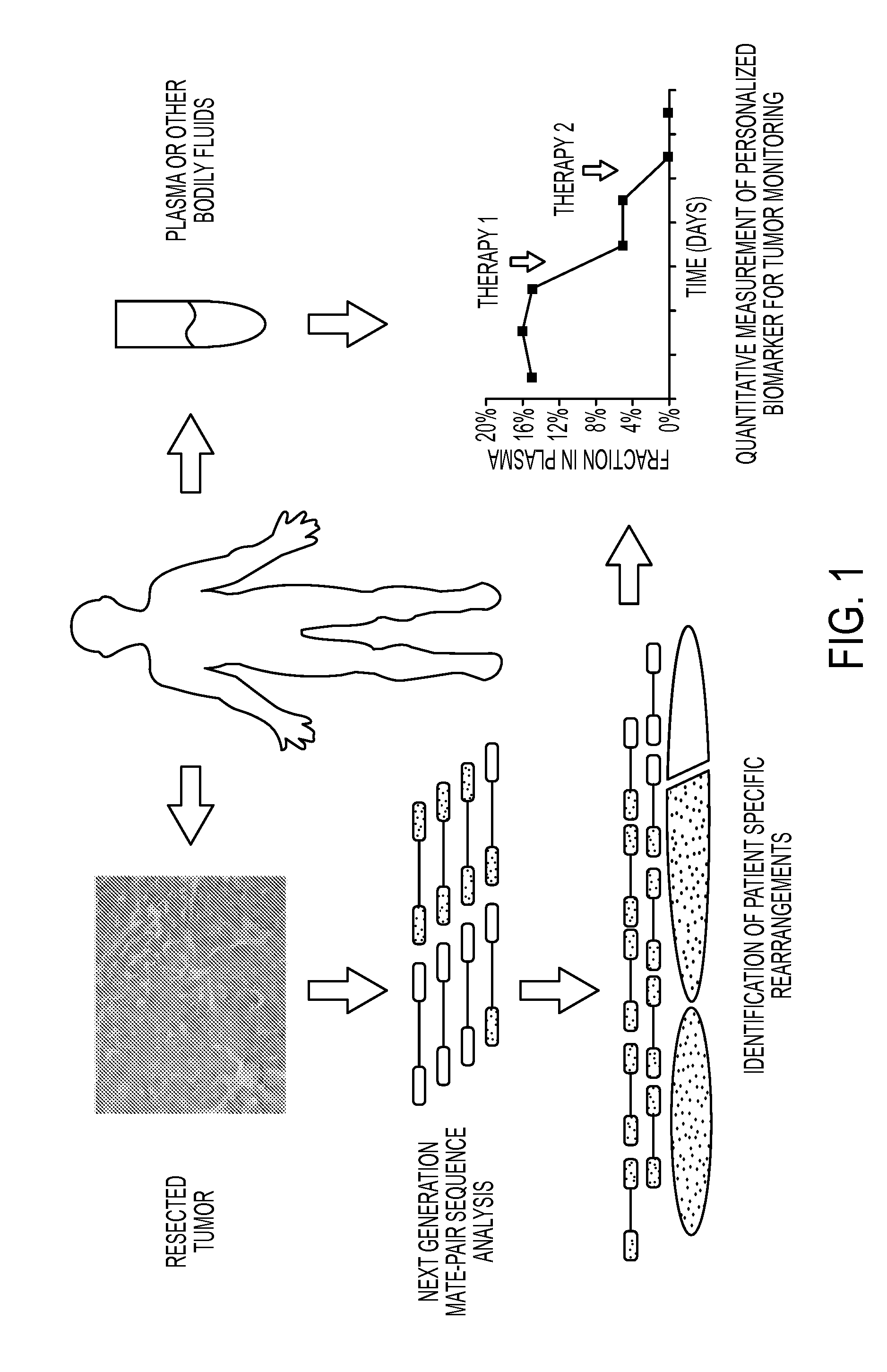
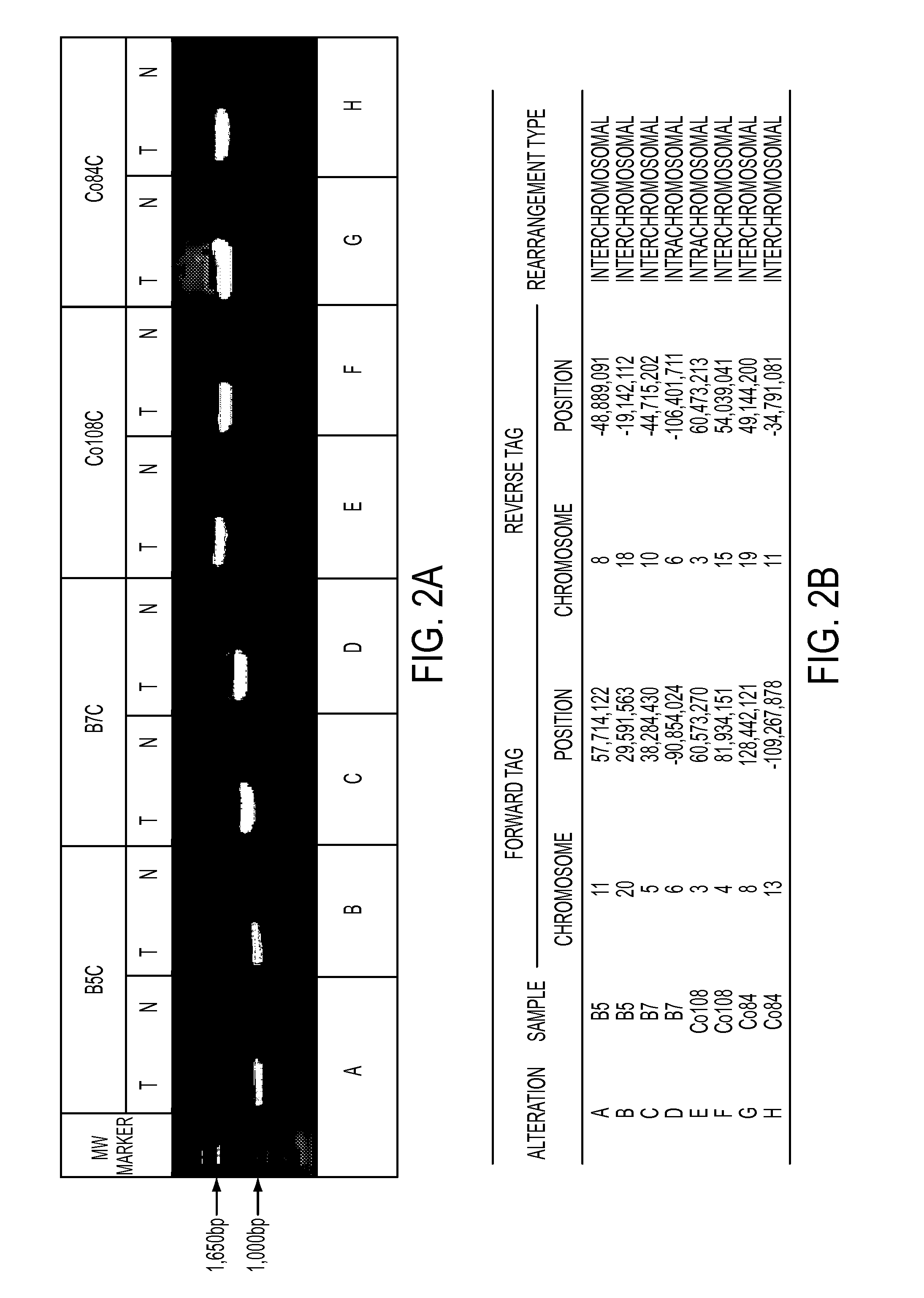
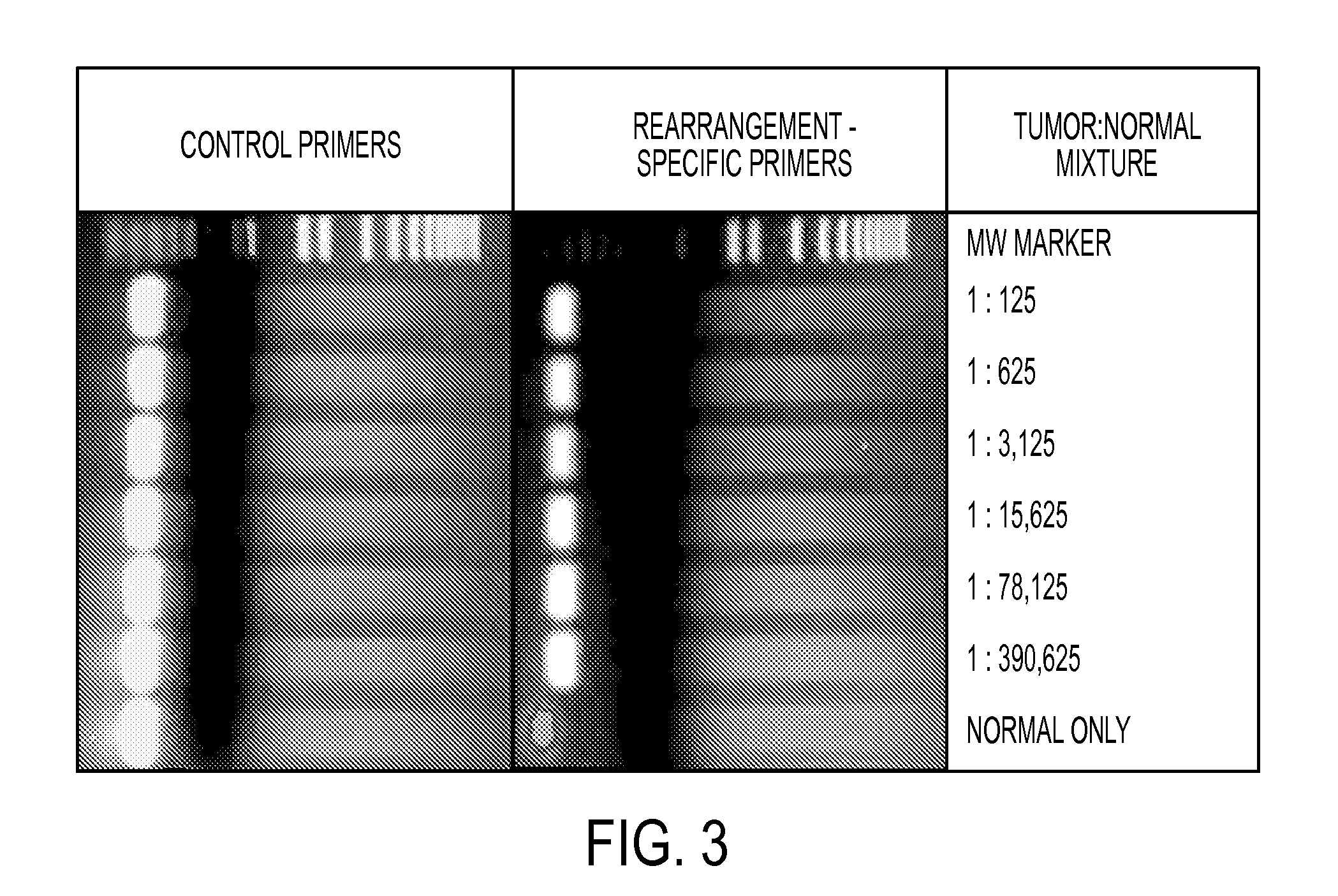
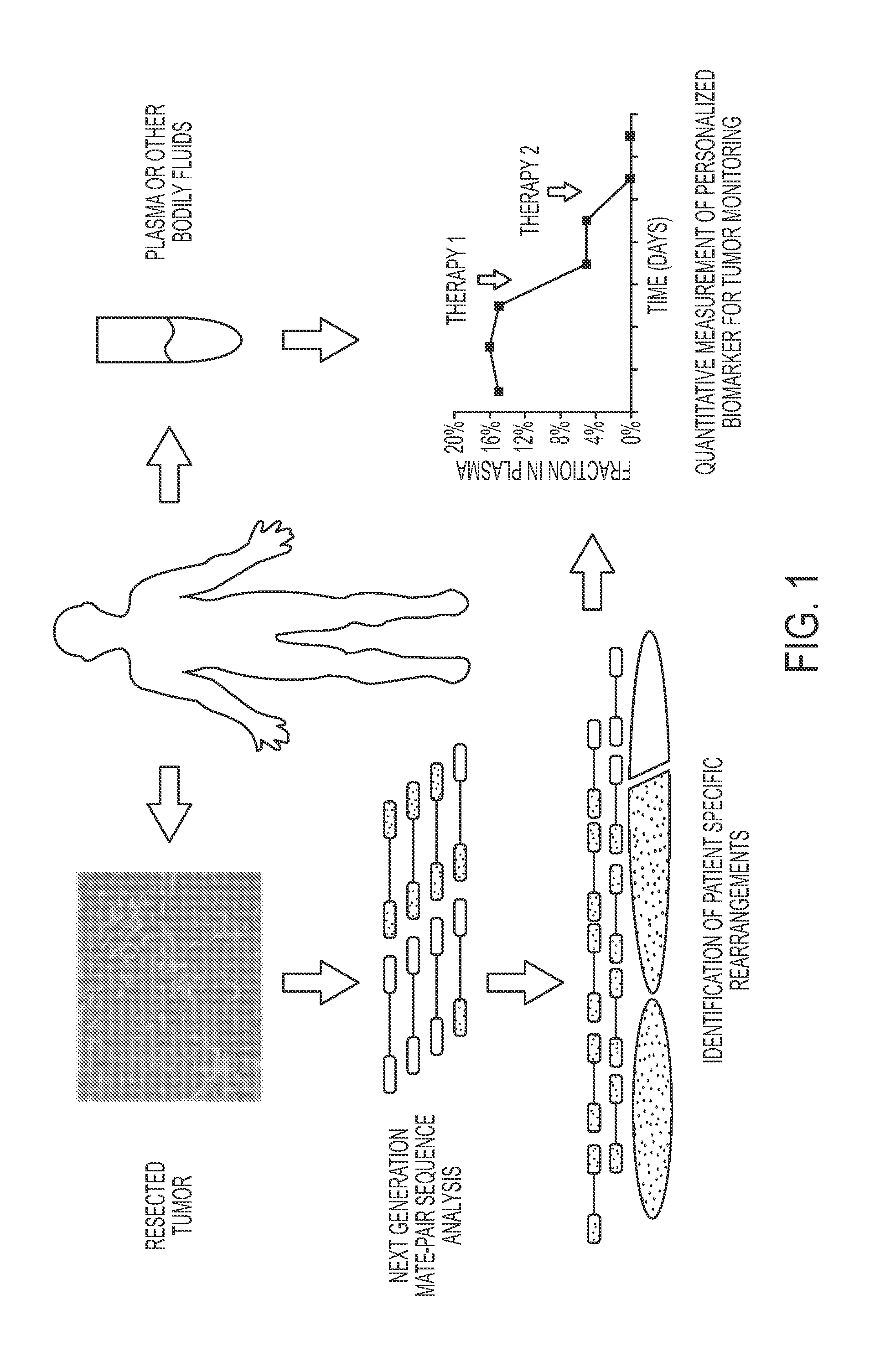
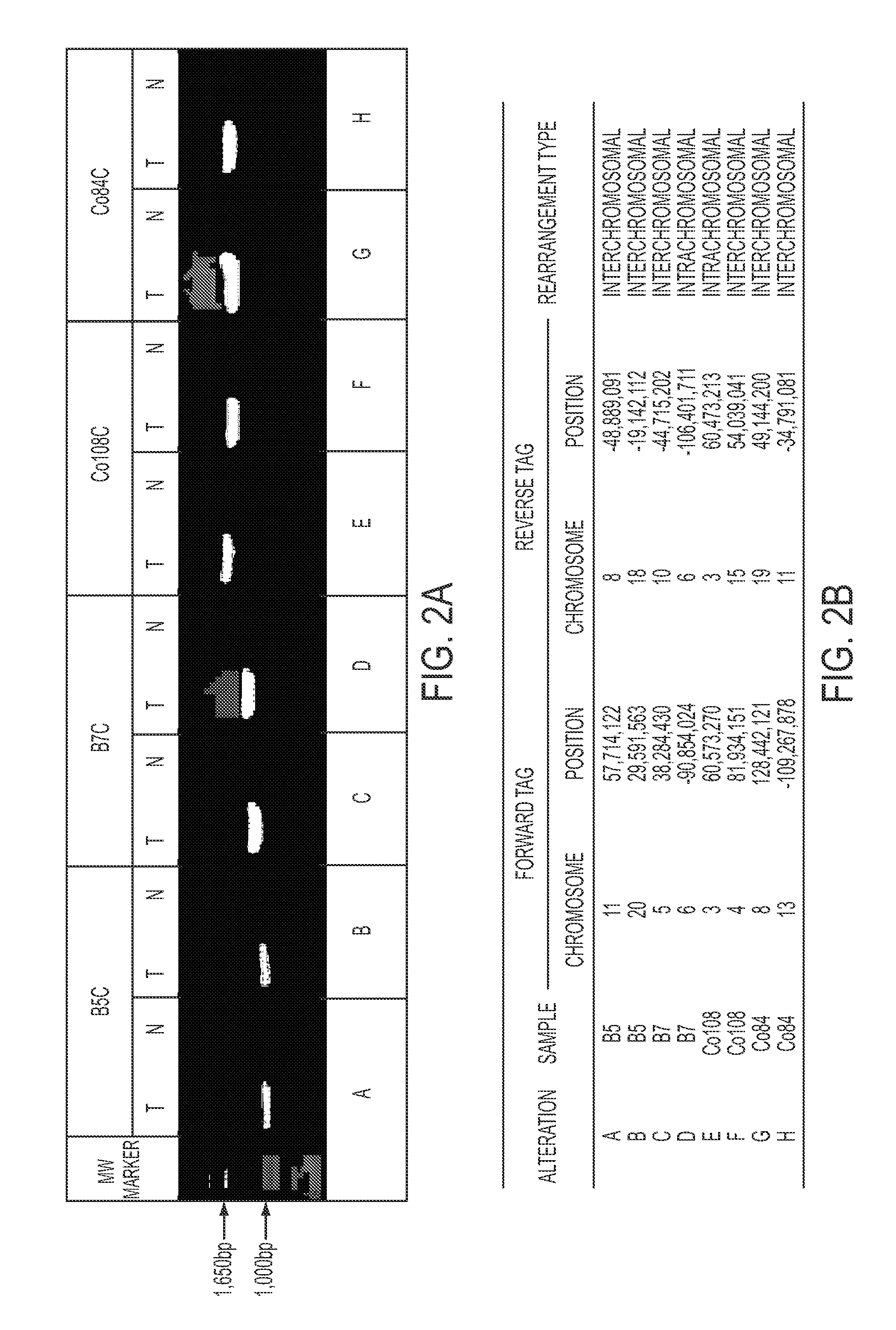
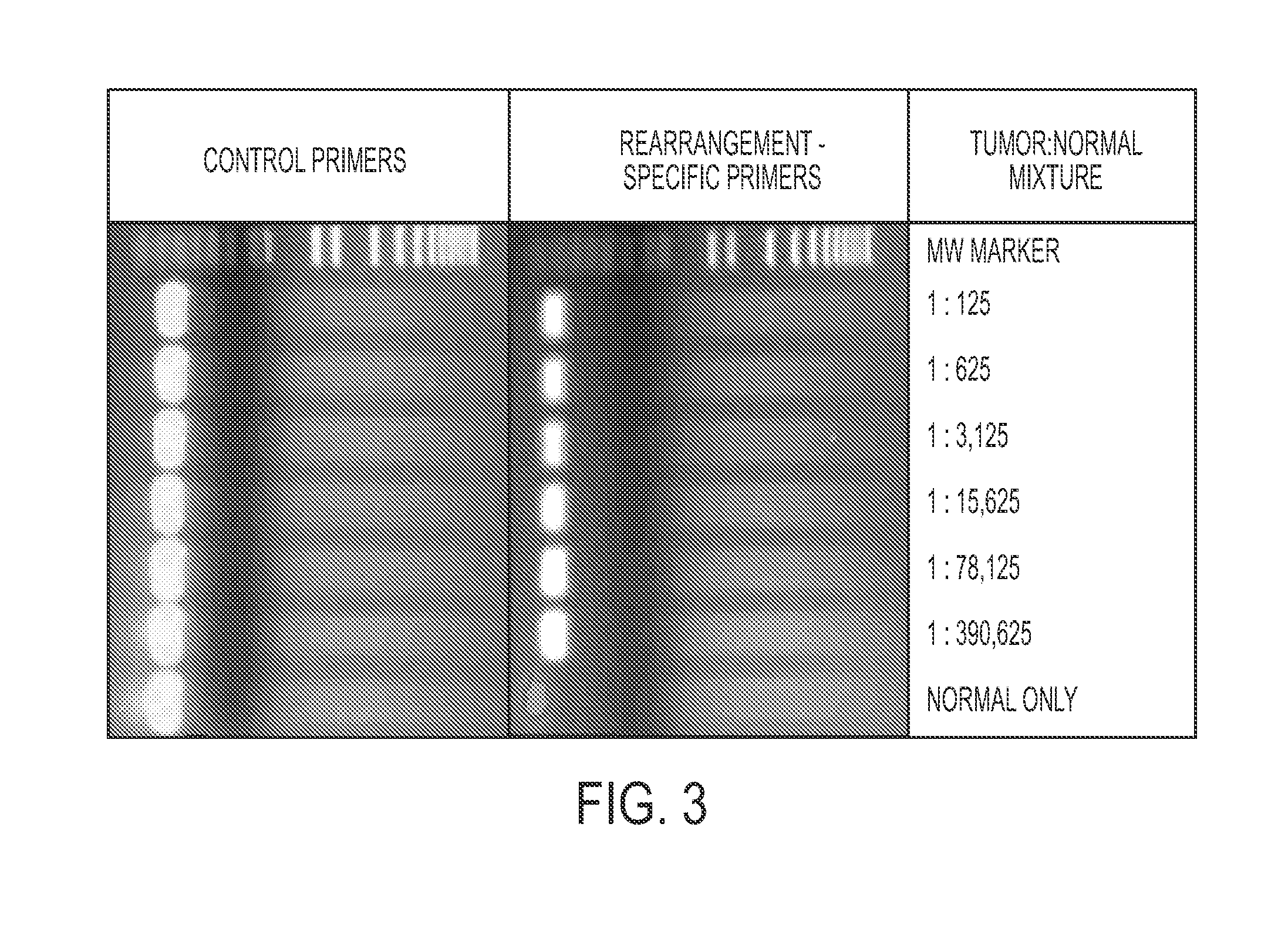
Clinical management of human cancer is dependent on the accurate monitoring of residual and recurrent tumors. We have developed a method, called personalized analysis of rearranged ends (PARE), which can identify translocations in solid tumors. Analysis of four colorectal and two breast cancers revealed an average of nine rearranged sequences (range 4 to 15) per tumor. Polymerase chain reaction with primers spanning the breakpoints were able to detect mutant DNA molecules present at levels lower than 0.001% and readily identified mutated circulating DNA in patient plasma samples. This approach provides an exquisitely sensitive and broadly applicable approach for the development of personalized biomarkers to enhance the clinical management of cancer patients.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Detecting tumor biomarker in oral cancer
InactiveUS20080038738A1Rapid and non-invasive analysisHigh sensitivityDiagnostics using lightMicrobiological testing/measurementSquamous cancerTumor Biomarkers
Methods and device for detecting the presence of tumor biomarkers in oral squamous cell carcinoma. A membrane based cell capture device allows deliver of cell samples and reagents to the membrane
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Reagents and Methods for miRNA Expression Analysis and Identification of Cancer Biomarkers
InactiveUS20090099034A1Microbiological testing/measurementLibrary screeningTumor BiomarkersTumor Sample
This invention provides methods for amplifying, detecting, measuring, and identifying miRNAs from biological samples, particularly limited amounts of a biological sample. miRNAs that are differentially expressed in tumor samples and normal tissues are useful as cancer biomarkers for cancer diagnostics.
Owner:WISCONSIN ALUMNI RES FOUND
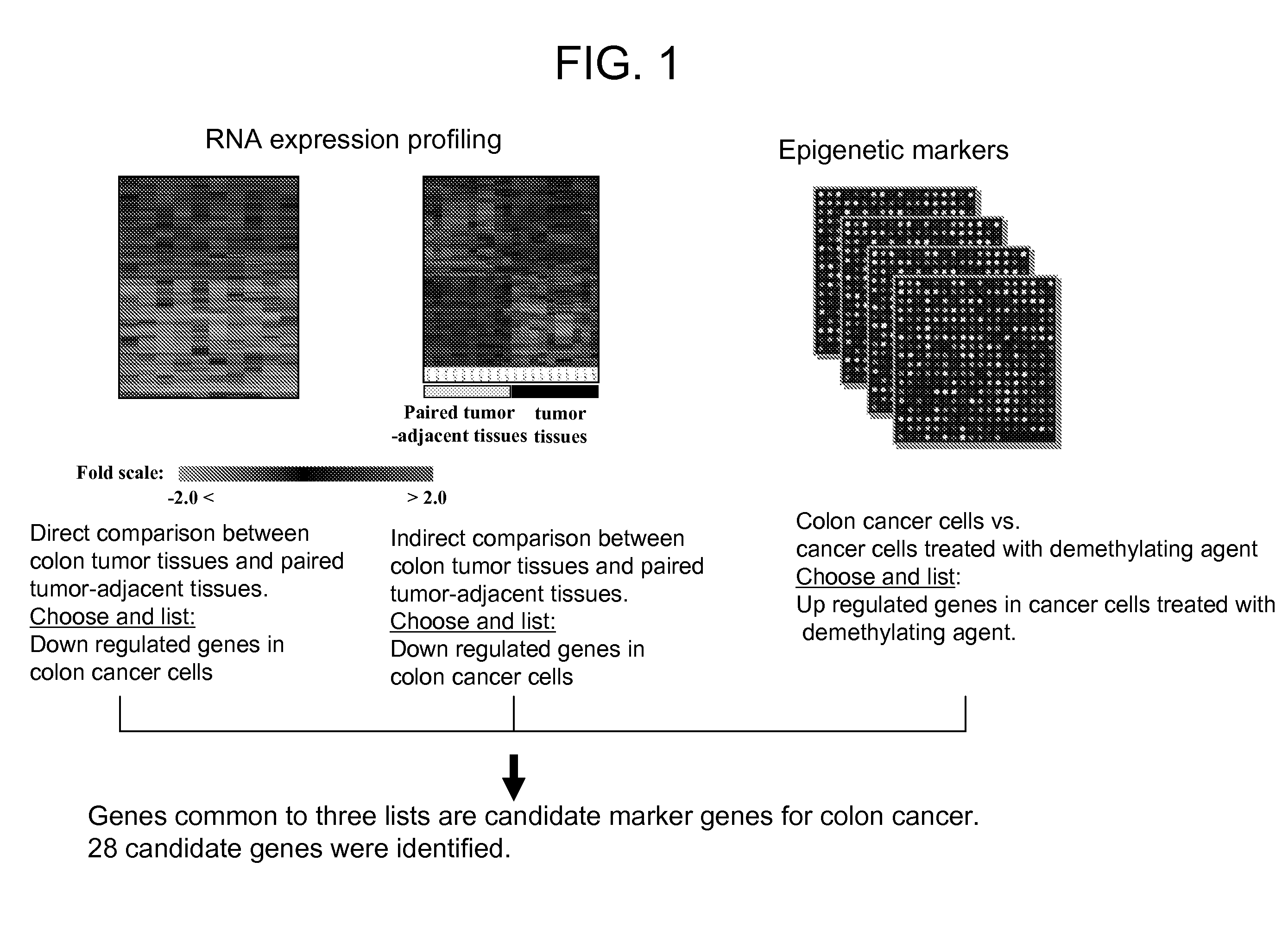
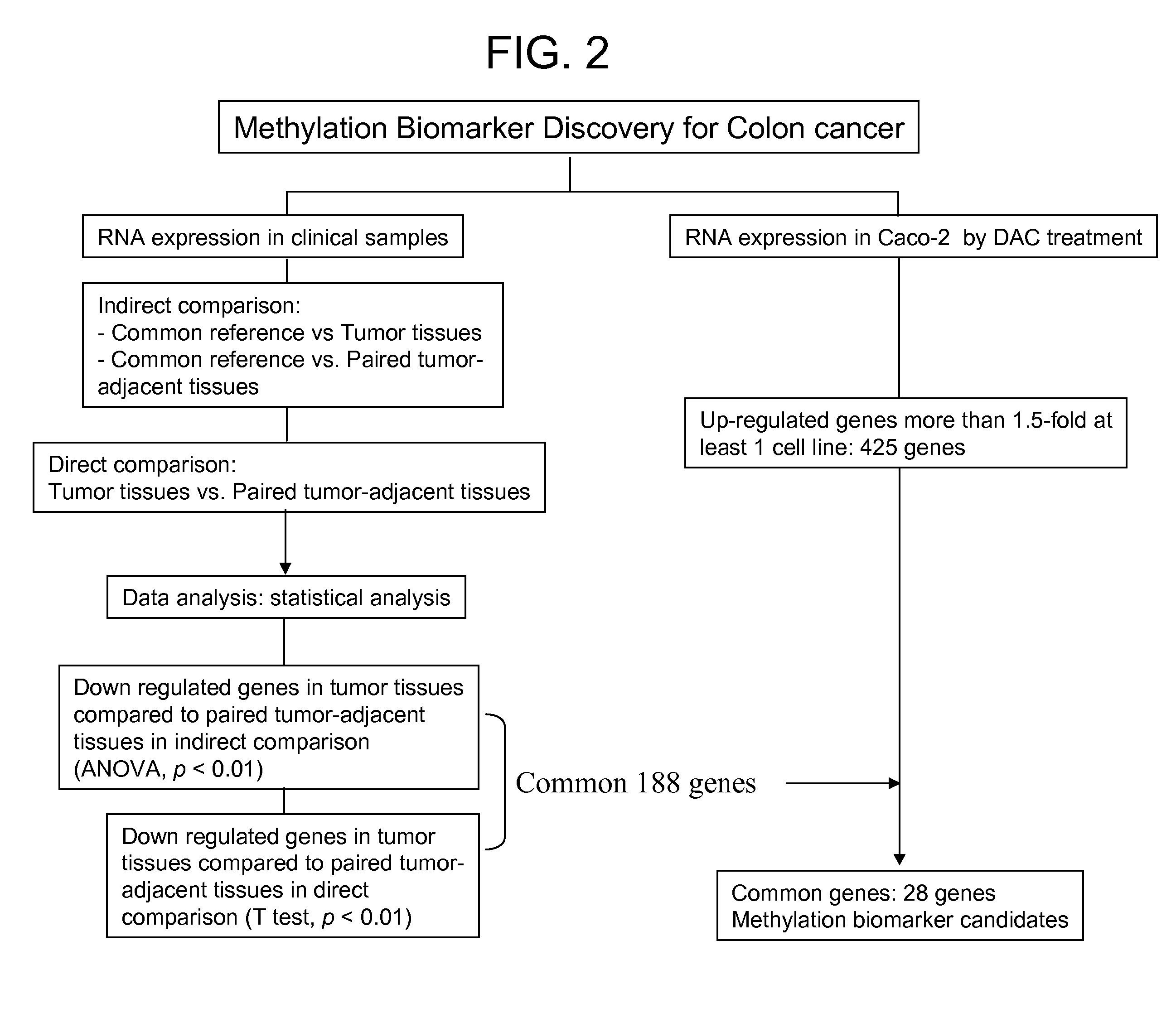
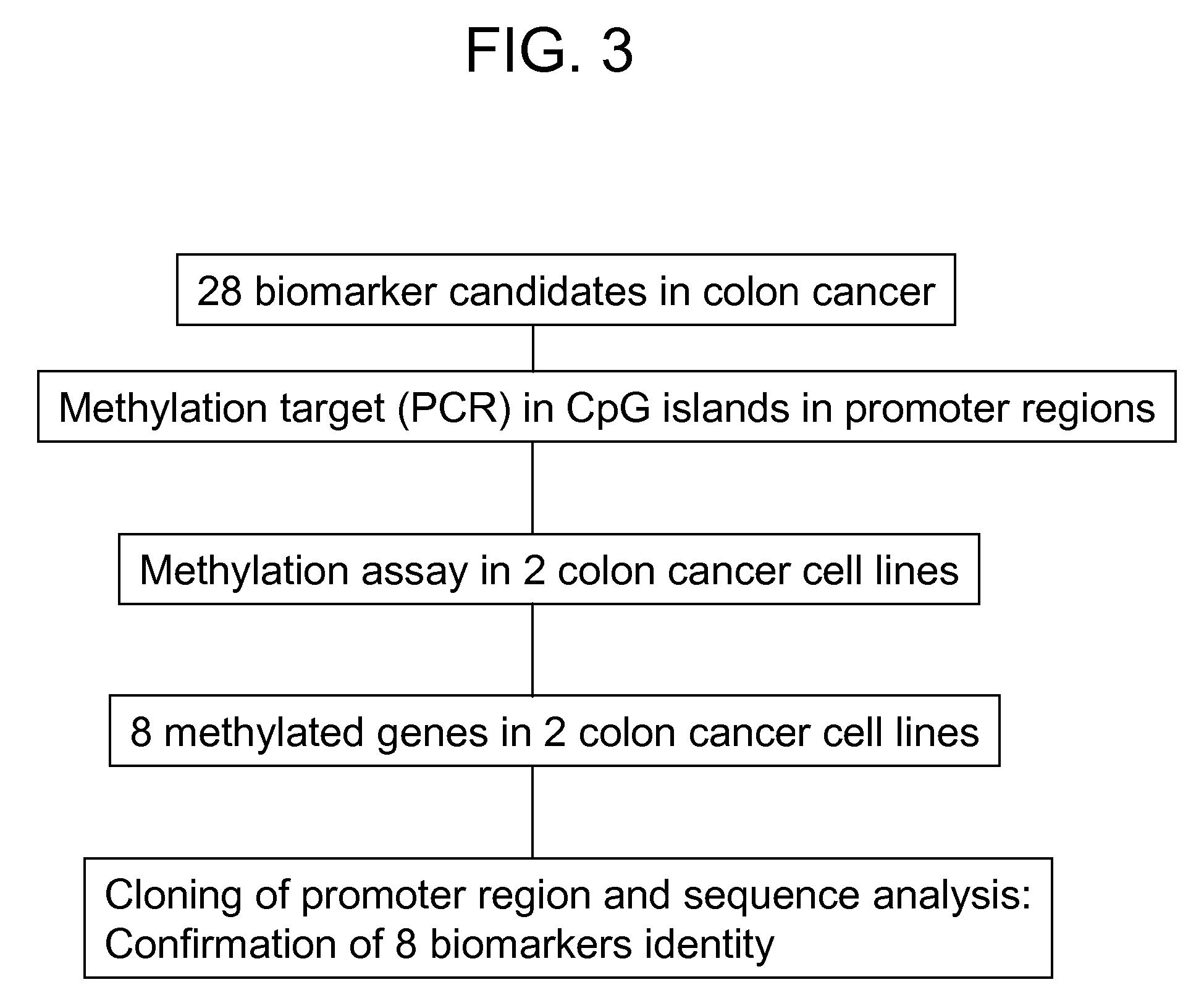
Colon cancer biomarker discovery
InactiveUS20060234254A1Early detectionAccurate diagnostic systemSugar derivativesMicrobiological testing/measurementTumor BiomarkersOncology
Owner:GENOMICTREE
Personalized tumor biomarkers
InactiveUS20130210645A1Sugar derivativesMicrobiological testing/measurementBlood plasmaRecurrent Tumor
Clinical management of human cancer is dependent on the accurate monitoring of residual and recurrent tumors. We have developed a method, called personalized analysis of rearranged ends (PARE), which can identify translocations in solid tumors. Analysis of four colorectal and two breast cancers revealed an average of nine rearranged sequences (range 4 to 15) per tumor. Polymerase chain reaction with primers spanning the breakpoints were able to detect mutant DNA molecules present at levels lower than 0.001% and readily identified mutated circulating DNA in patient plasma samples. This approach provides an exquisitely sensitive and broadly applicable approach for the development of personalized biomarkers to enhance the clinical management of cancer patients.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method, compositions and classification for tumor diagnostics and treatment
InactiveUS20060160157A1Quick and easy determinationImprove survivalCompound screeningApoptosis detectionAbnormal tissue growthSide effect
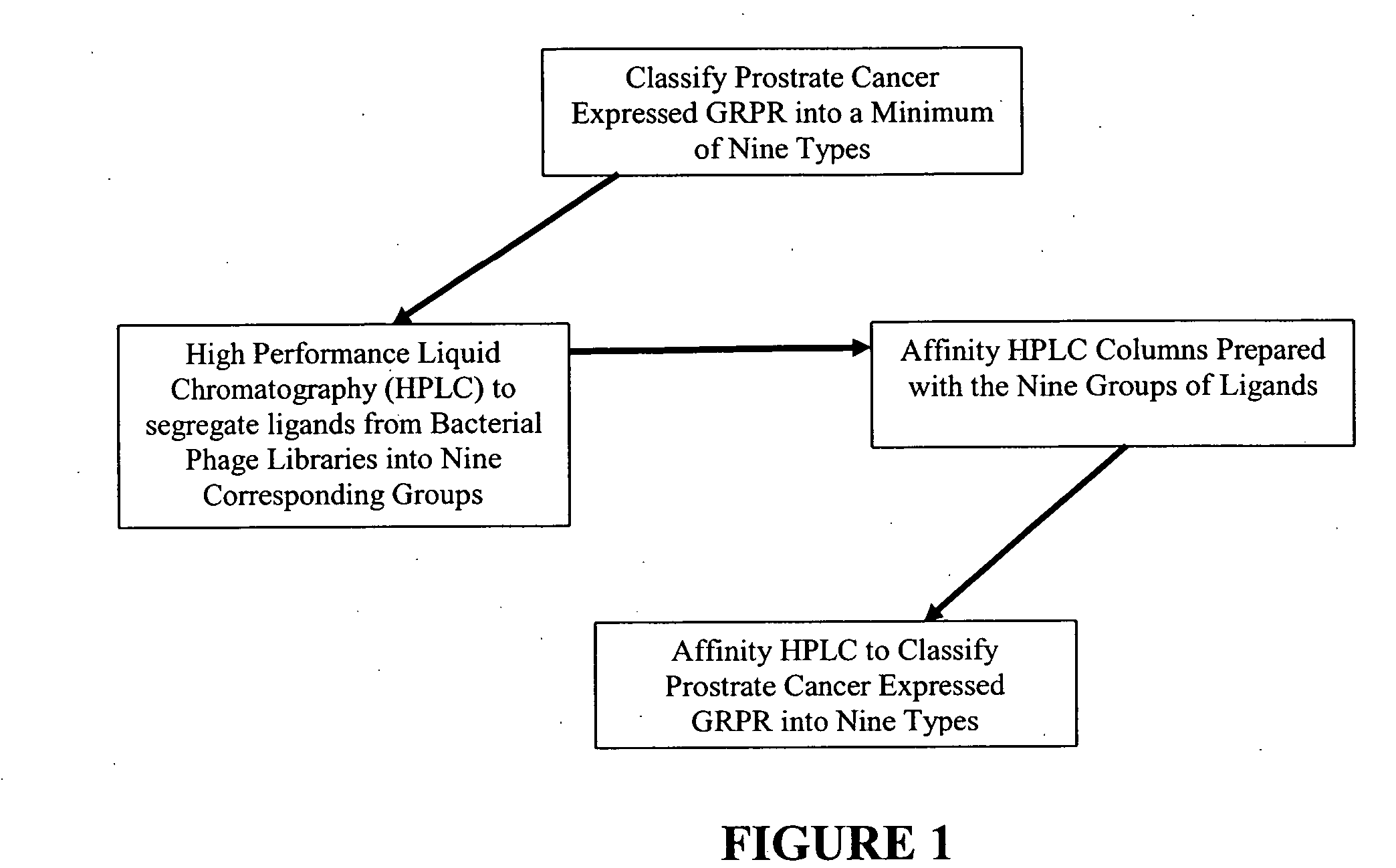
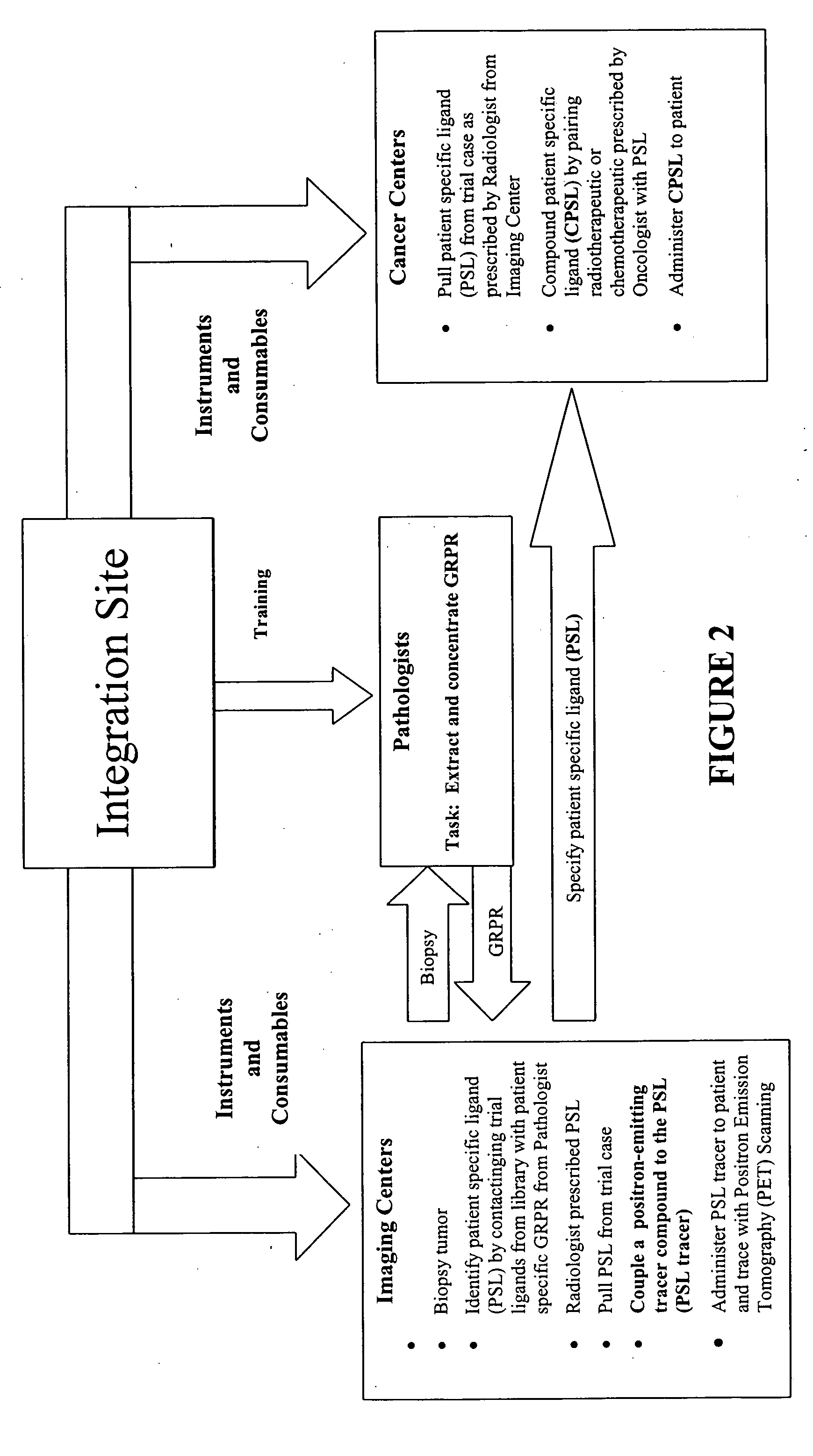
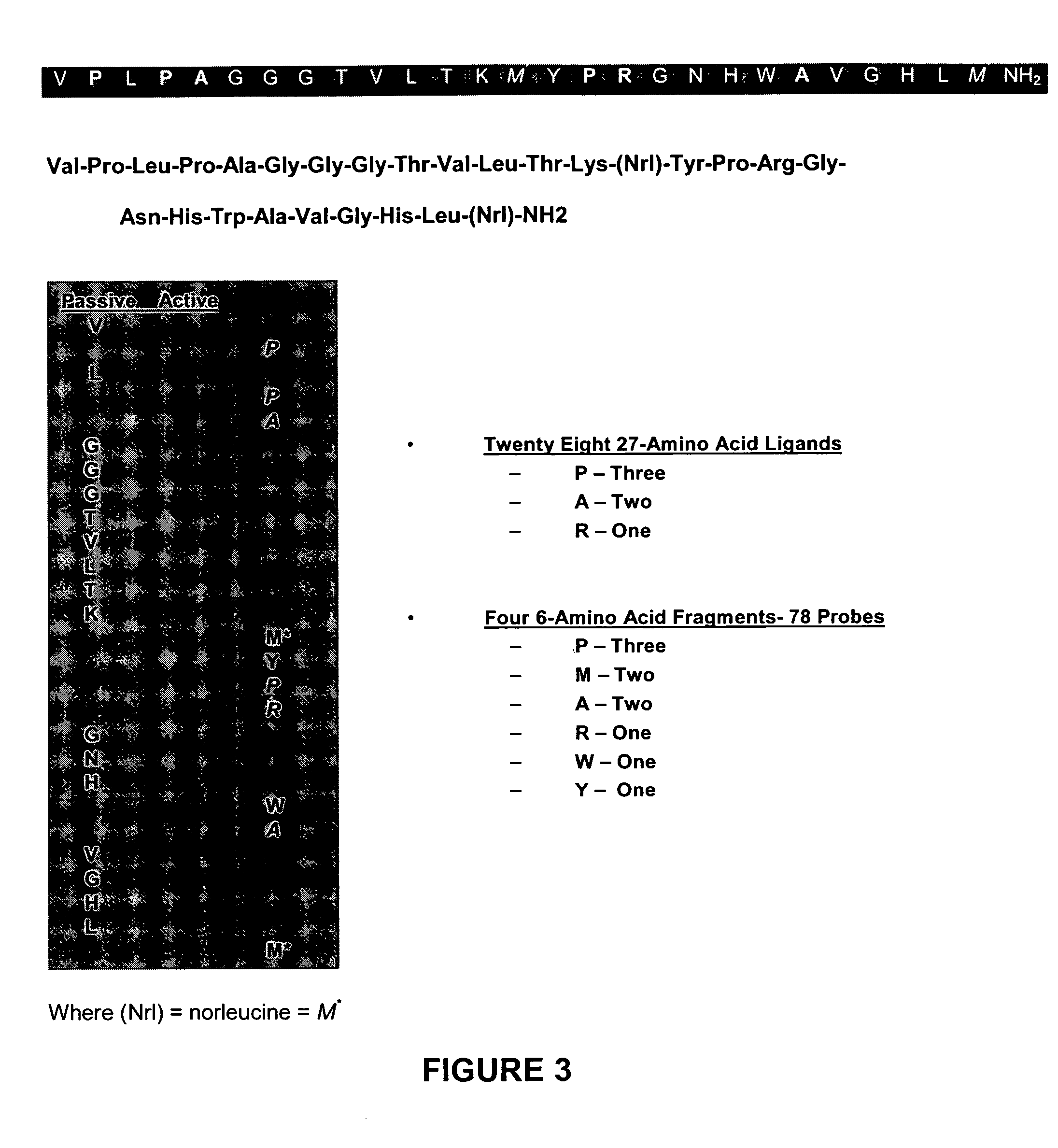
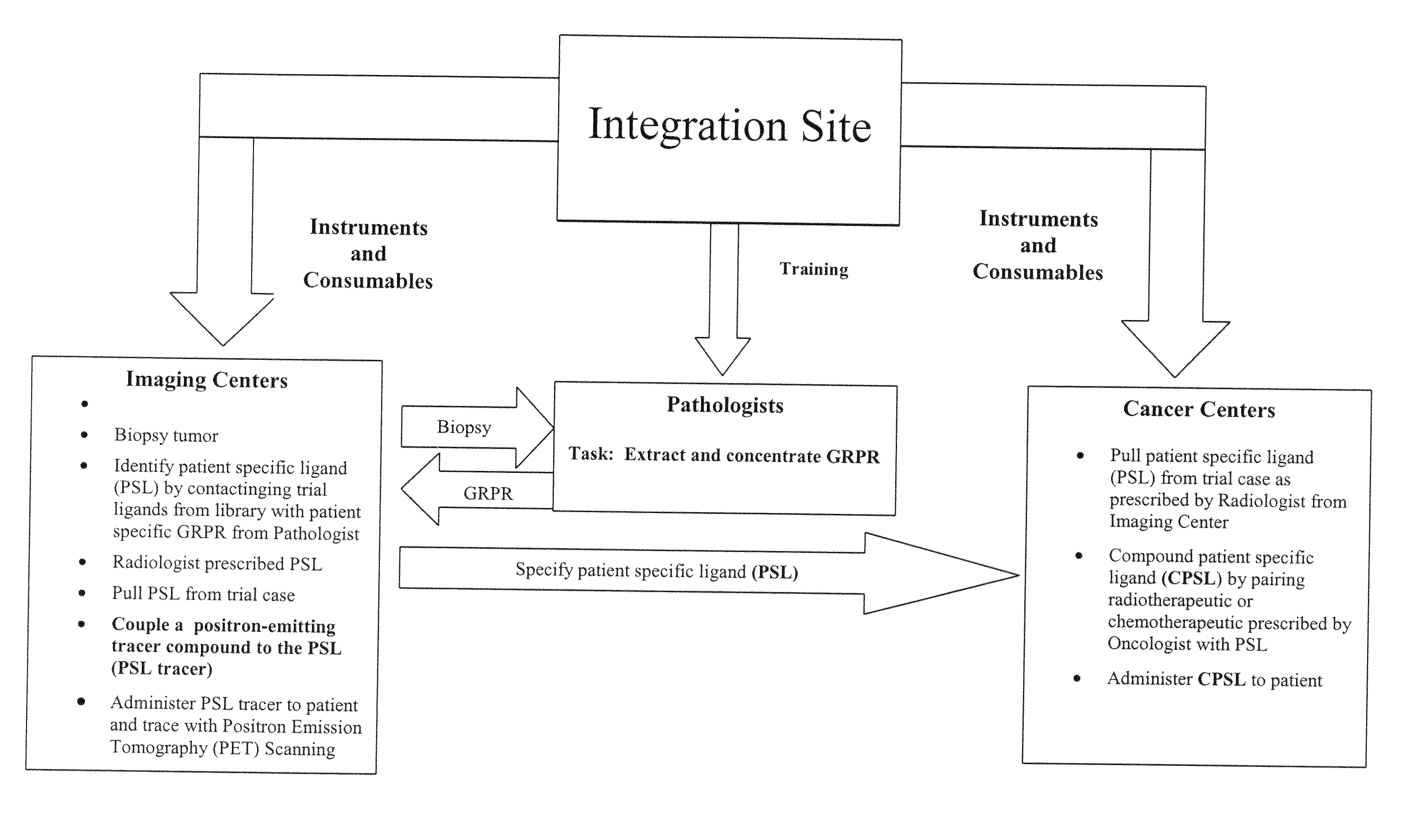
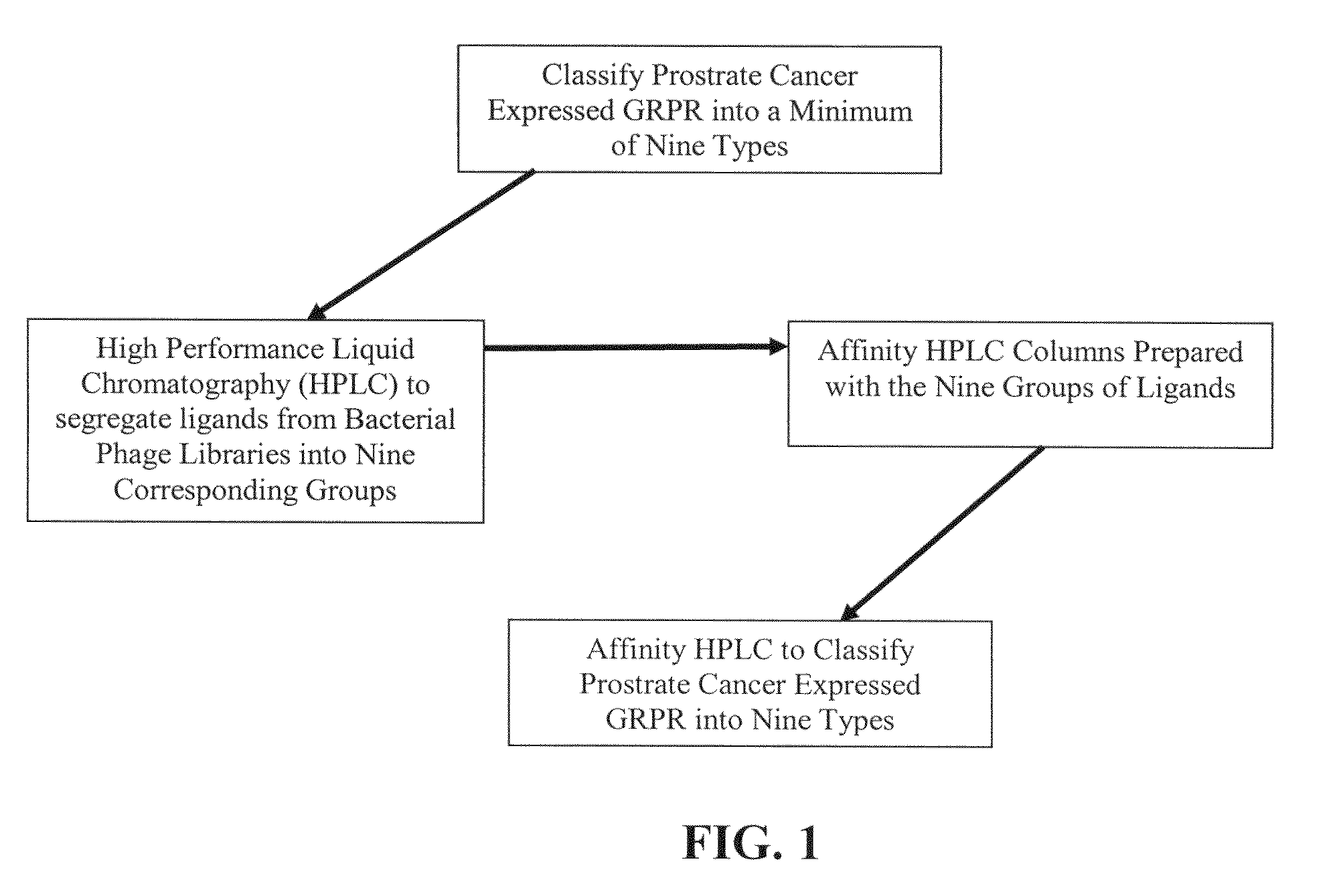
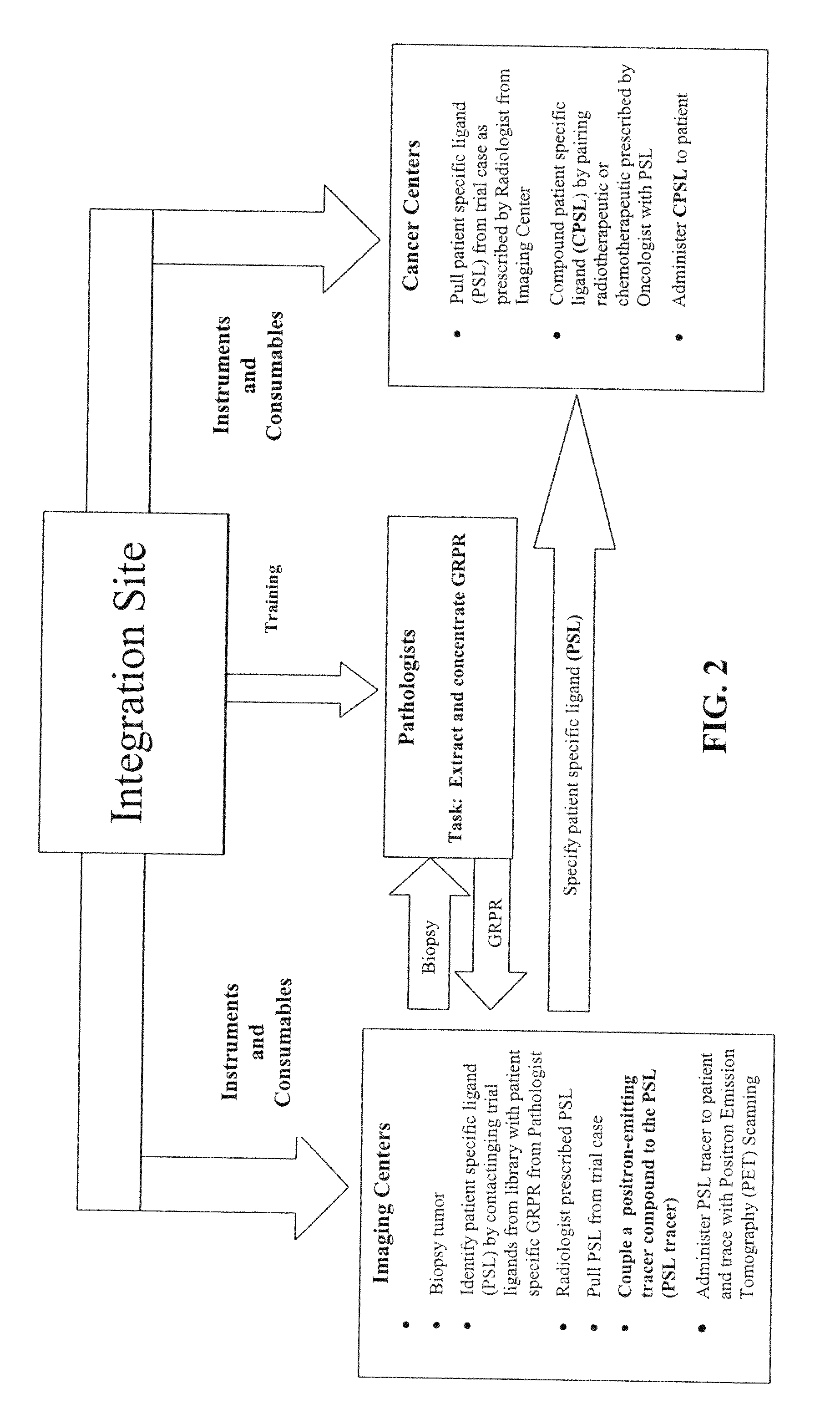
The present invention is directed towards classifying tumor biomarkers, particularly membrane receptors, and more particularly the gastrin-releasing peptide (GPR) receptors, identified in patient samples, then linking therapeutic agents (chemical, radiological, or biological) to patient-specific ligands that bind to such receptors, clinicians can produce diagnostic and treatment compositions and implement treatment regimens which, by using the classified and identified biomarkers, and due to their improved accuracy, increase success and decrease undesired side effects from such treatments.
Owner:ZUCKERMAN MATHEW MARK
Detection And Quantification Of Biomarkers Via A Piezoelectric Cantilever Sensor
InactiveUS20090078023A1High sensitivityShort timeMaterial analysis using sonic/ultrasonic/infrasonic wavesMicrobiological testing/measurementEscherichia coliMultiple sensor
Quantification of a target analyte is performed using a single sample to which amounts of the target analyte are added. Calibration is performed as part of quantification on the same sample. The target analyte is detectable and quantifiable using label free reagents and requiring no sample preparation. Target analytes include biomarkers such as cancer biomarkers, pathogenic Escherichia coli, single stranded DNA, and staphylococcal enterotoxin. The quantification process includes determining a sensor response of a sensor exposed to the sample and configured to detect the target analyte. Sensor responses are determined after sequential additions of the target analyte to the sample. The amount of target analyte detected by the sensor when first exposed to the sample is determined in accordance with the multiple sensor responses.
Owner:DREXEL UNIV
Combination methods of diagnosing cancer in a patient
InactiveUS20120231479A1Microbiological testing/measurementBiological material analysisTumor BiomarkersOncology
Owner:TRAXXSSON
Cancer biomarkers
InactiveUS20120041274A1Improve predictive performanceIncreased likelihood of recurrenceBioreactor/fermenter combinationsBiological substance pretreatmentsTumor BiomarkersBiologic marker
Biomarkers and methods using the biomarkers for the prediction of the recurrence risk of cancer in a patient are provided.
Owner:MYRIAD GENETICS
Biomarkers and methods of treatment
InactiveUS20120089541A1Effective treatmentIncreased riskOrganic active ingredientsNervous disorderTumor BiomarkersBiologic marker
The present invention concerns cancer biomarkers. In particular, the invention concerns c-met as biomarkers for patient selection and patient prognosis in cancer, as well as methods of therapeutic treatment, articles of manufacture and methods for making them, diagnostic kits, methods of detection and methods of advertising related thereto.
Owner:GENENTECH INC
Early-stage lung adenocarcinoma miRNA (micro ribonucleic acid) specific expression profile and reverse transcription primer and application thereof
InactiveCN103173448AAccurate diagnosisEasy to distinguishMicrobiological testing/measurementDNA/RNA fragmentationTumor BiomarkersAdenocarcinoma
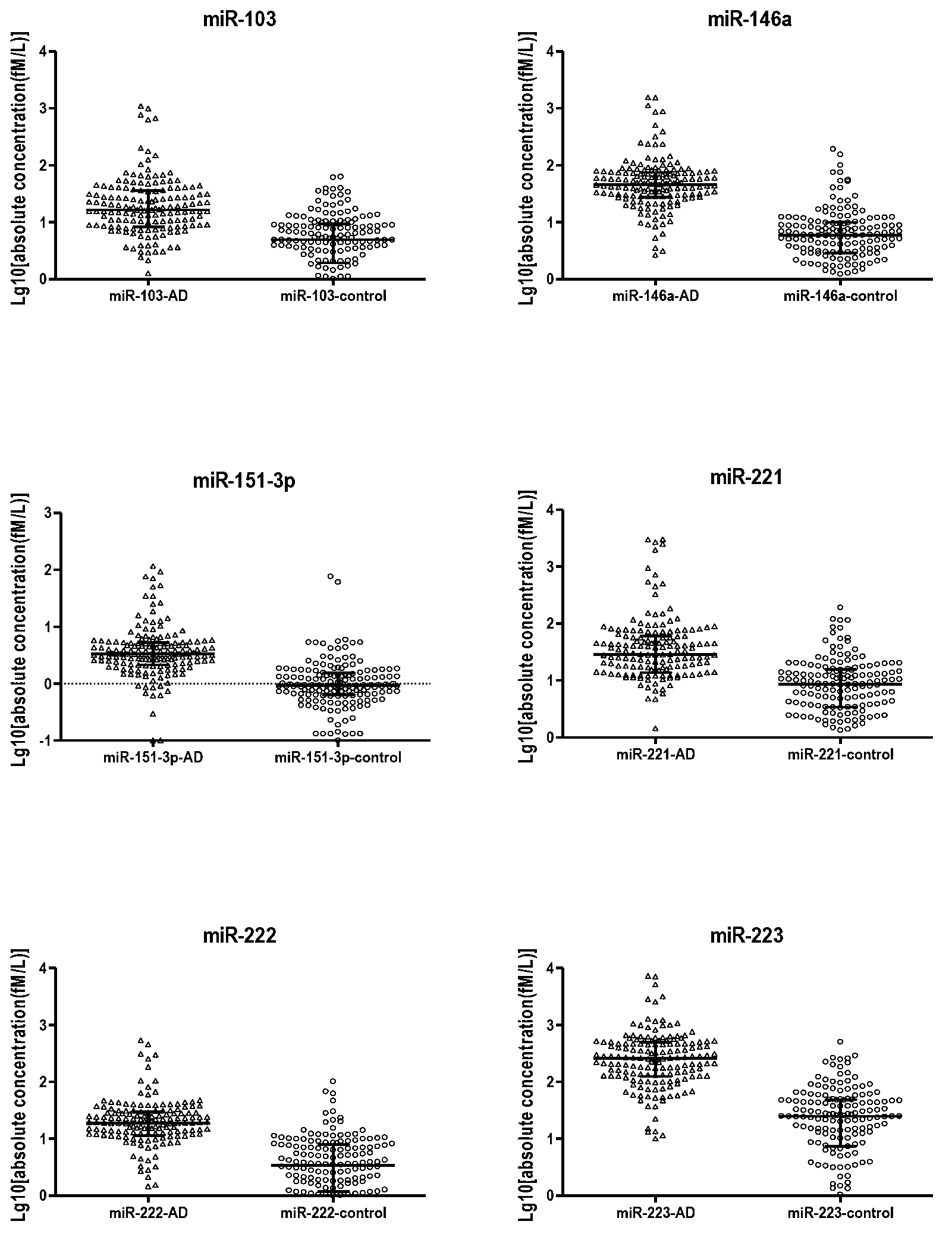
The invention belongs to the field of biological and medicine examination, relates to a tumor biomarker, and in particular relates to an early-stage lung adenocarcinoma miRNA (micro ribonucleic acid) specific expression profile and a reverse transcription primer and an application thereof. The early-stage lung adenocarcinoma miRNA specific expression profile comprises more than one of up-regulated expression miR-103, miR-146a, miR-151, miR-221, miR-222 and miR-223. The invention provides an early-stage lung adenocarcinoma diagnosis model, a reverse transcription primer of specific expression miRNA and a kit or biochip for early-stage lung adenocarcinoma diagnosis based on the specific expression profile. The technical scheme provided by the invention can realize accurate diagnosis of early-stage lung adenocarcinoma by detecting a small amount of miRNA, and provides possibility to the production of accurate, efficient and economical detection products. The established lung adenocarcinoma prediction model can distinguish lung adenocarcinoma from a healthy individual, and has great practical significance in clinic.
Owner:SHANGHAI CHEST HOSPITAL
Early detection and prognosis of colon cancers
InactiveCN101688239ASugar derivativesMicrobiological testing/measurementGenes mutationTumor specific
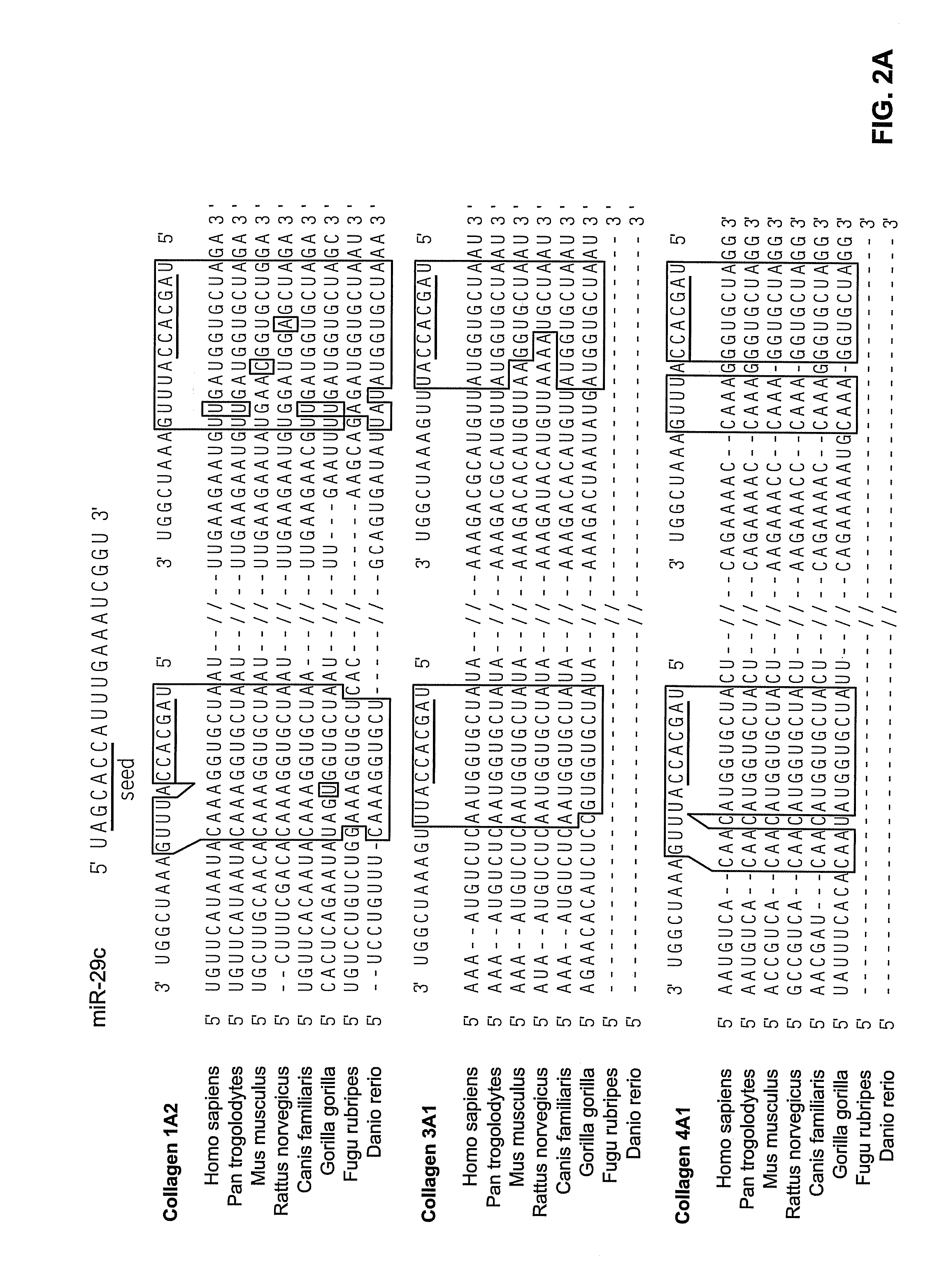
We have developed a transcriptome-wide approach to identify genes affected by promoter CpG island hypermethylation and transcriptional silencing in colorectal cancer (CRC). By screening cell lines andvalidating tumor specific hypermethylation in a panel of primary human CRC samples, we estimate that nearly 5% of all known genes may be promoter methylated in an individual tumor. When directly compared to gene mutations, we find a much larger number of genes hypermethylated in individual tumors, and much higher frequency of hypermethylation within individual genes harboring either genetic or epigenetic changes. Thus, to enumerate the full spectrum of alterations in the human cancer genome, and facilitate the most efficacious grouping of tumors to identify cancer biomarkers and tailor therapeutic approaches, both genetic and epigenetic screens should be undertaken. The genes we identified can be used inter alia diagnostically to detect cancer, pre-cancer, and likelihood of developing cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
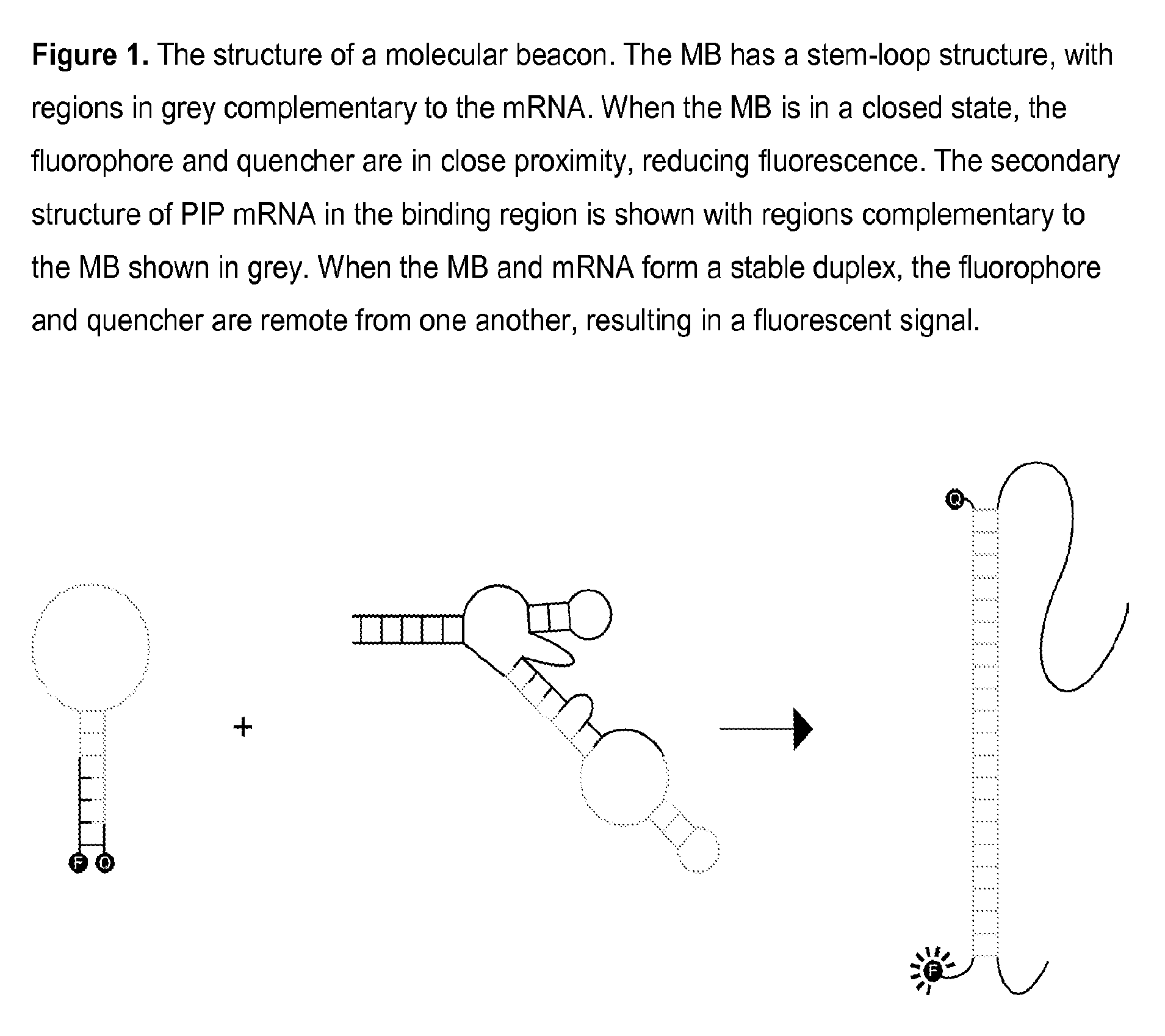
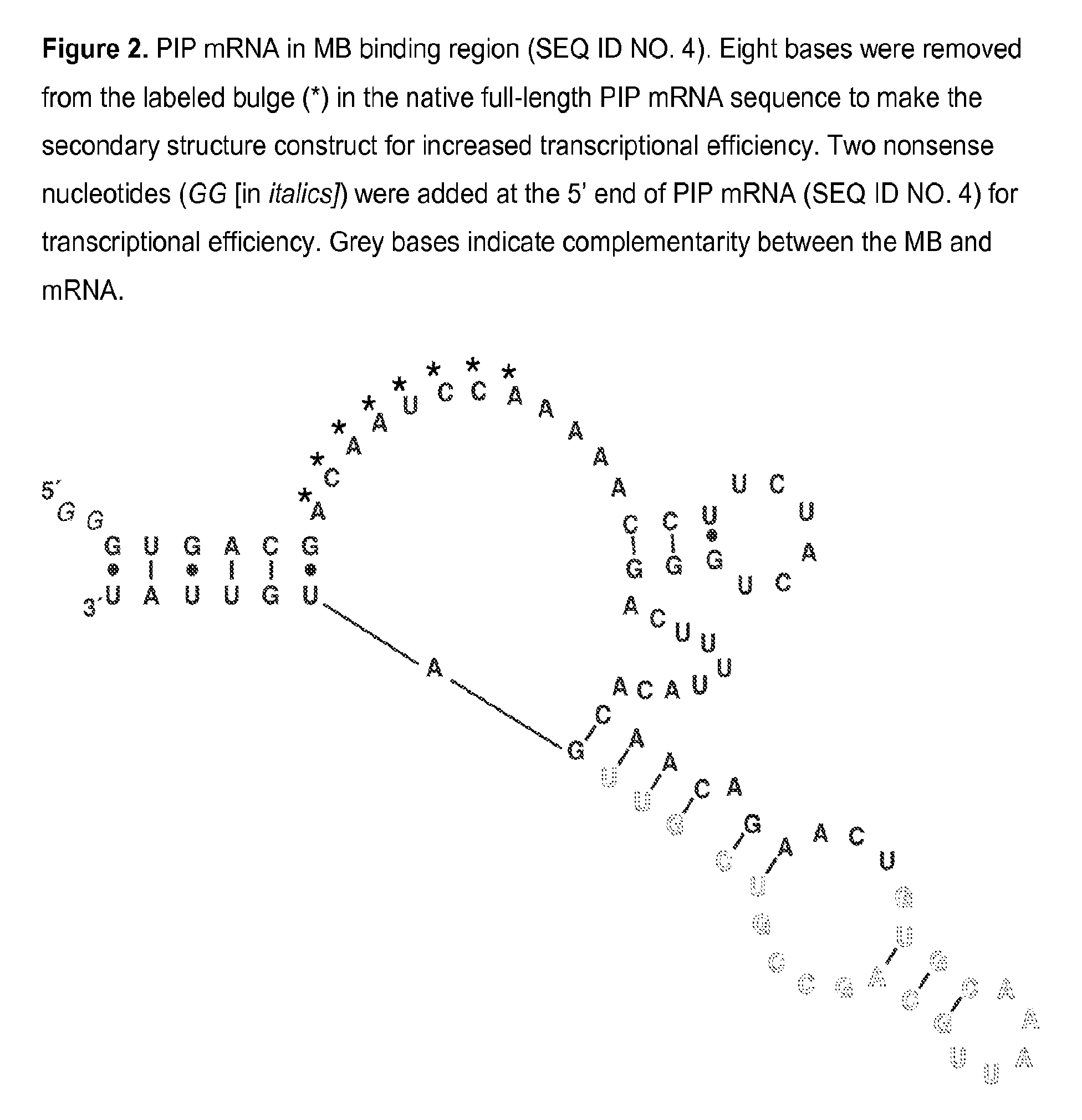
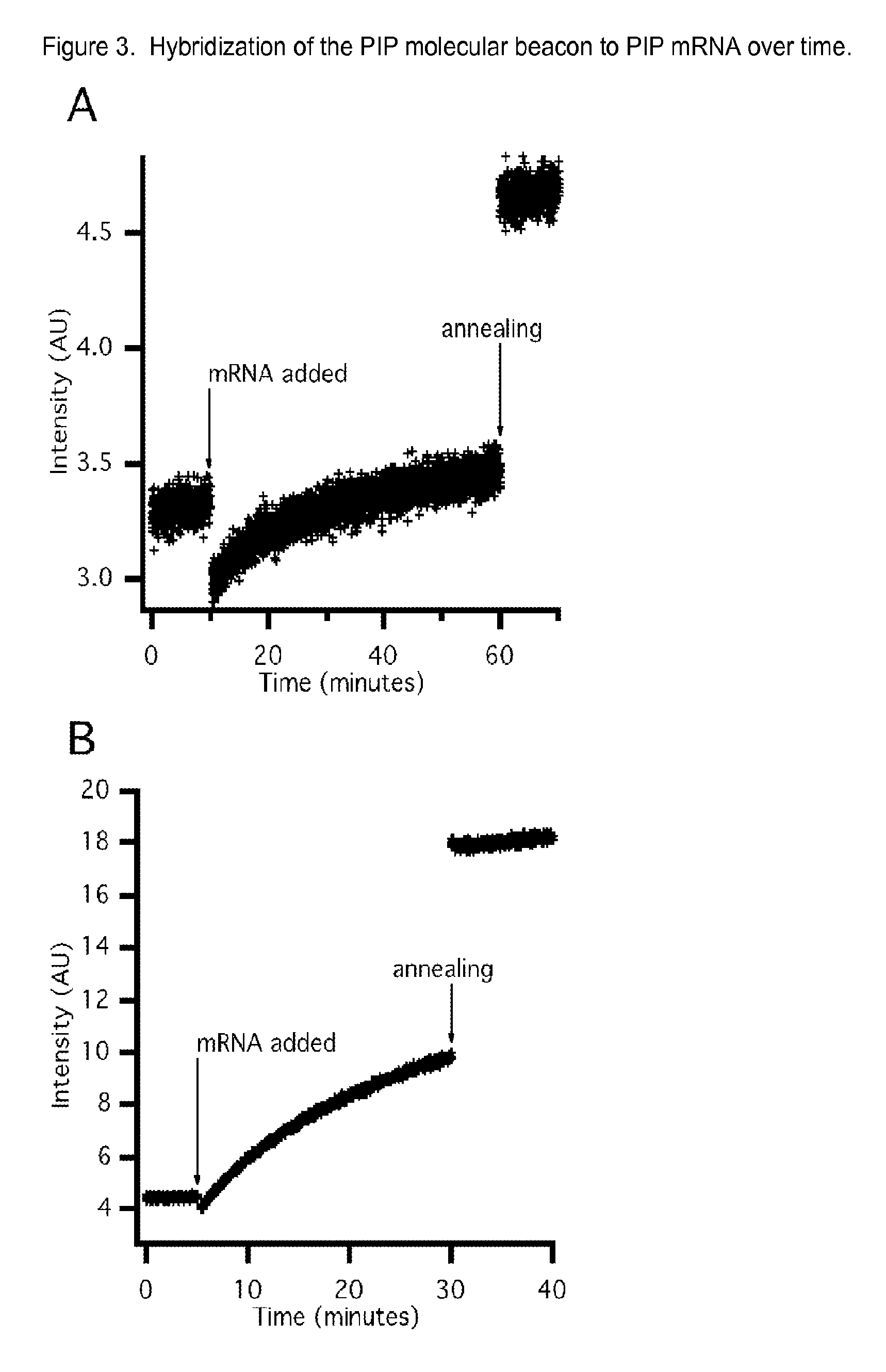
Molecular beacon based assay for the detection of biomarkers for breast cancer metastasis
InactiveUS9297047B2RobustnessShort analysis timeSugar derivativesMicrobiological testing/measurementBreast cancer metastasisSerum ige
The invention encompasses molecular beacon (MB) probes for monitoring the presence of human breast cancer biomarkers and for the analysis of breast cancer metastasis. The molecular beacon is an oligonucleotide probe which sensitively and specifically identifies biomarker mRNA in samples, in the presence of serum, in minimal time using fluorescence detection. The molecular beacons may be comprised in kits for the detection / quantitation of cancer biomarkers in clinical samples. The invention provides improvements in simplicity, accuracy, and speed over current methods, which could allow for improved patient treatment and prognoses.
Owner:FURCHAK JENNIFER +2
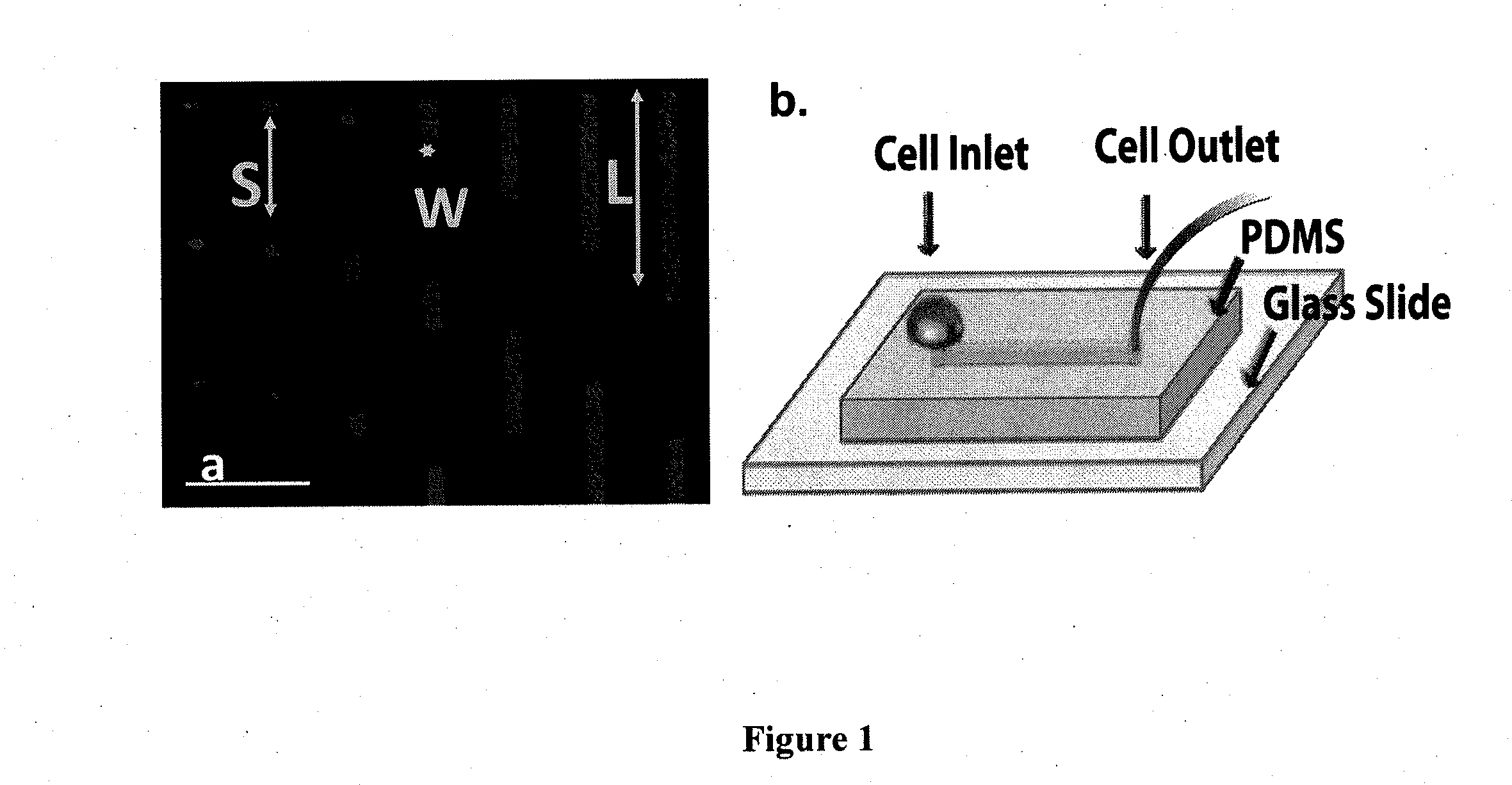
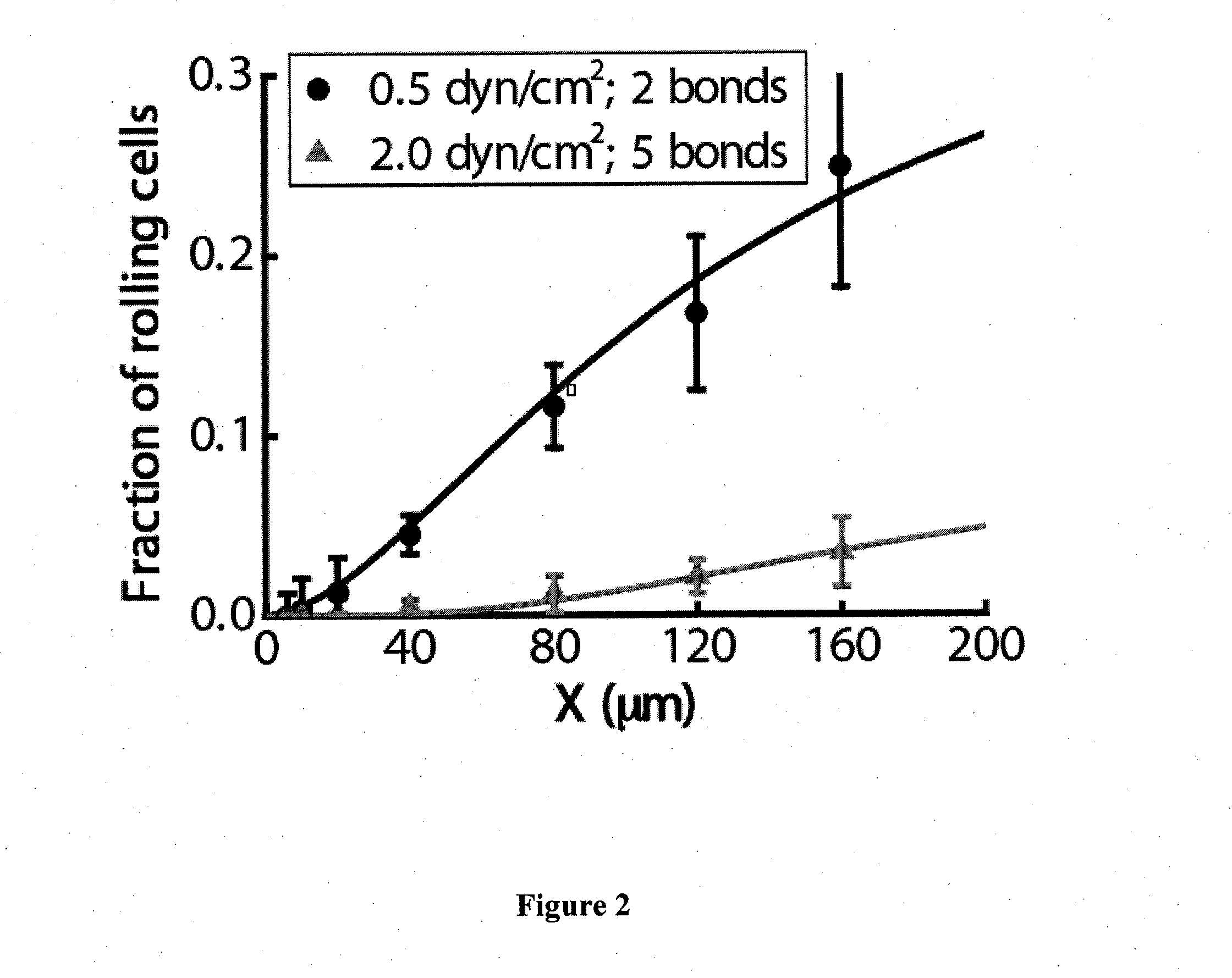
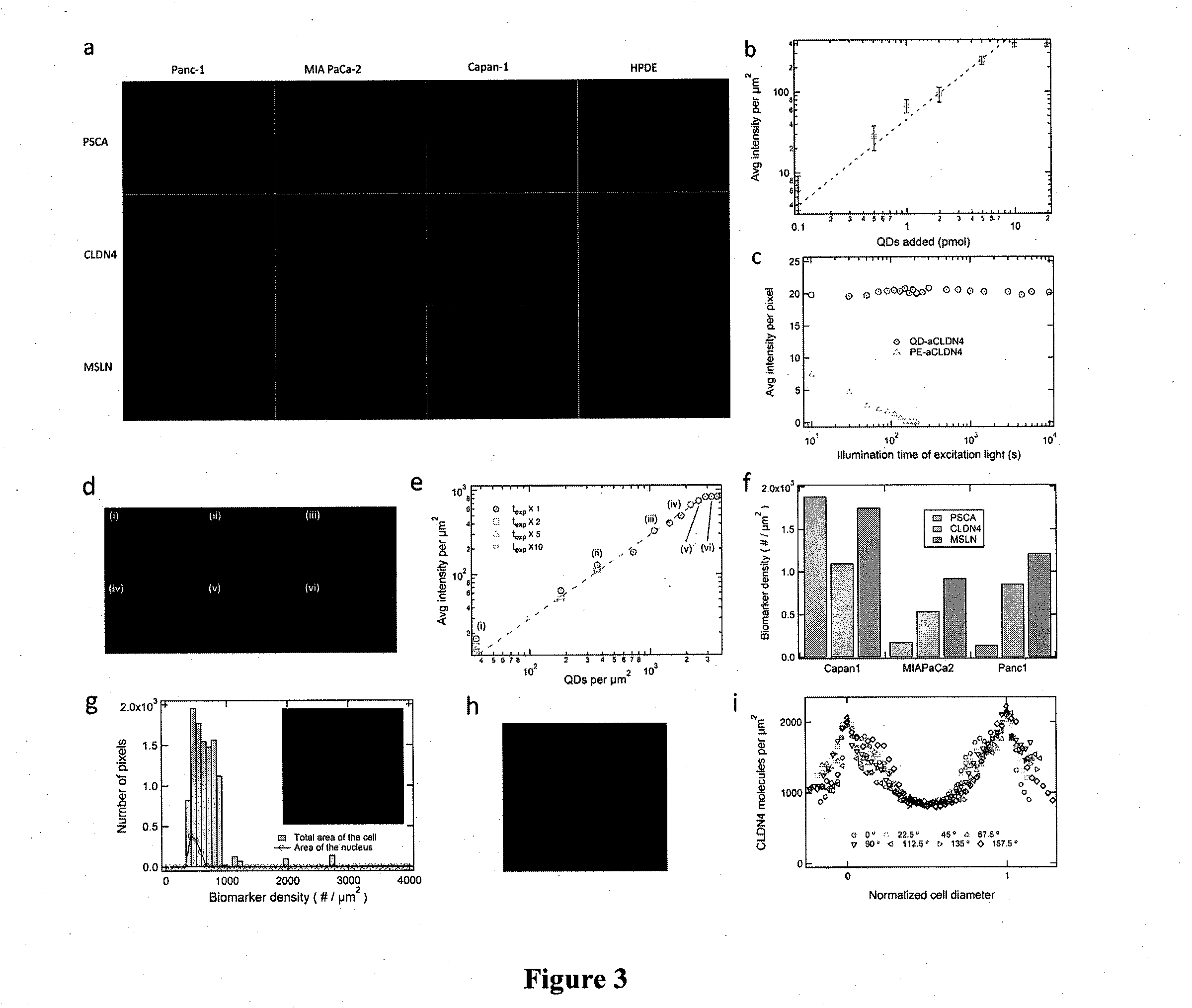
Device for capture, enumeration, and profiling of circulating tumor cells
InactiveUS20120100560A1Bioreactor/fermenter combinationsBiological substance pretreatmentsSpatial mappingBiology
Applications in nanomedicine, such as diagnostics and targeted therapeutics, rely on the detection and targeting of membrane biomarkers. The present invention, in one embodiment, utilizes quantitative profiling, spatial mapping, and multiplexing of cancer biomarkers using functionalized quantum dots. This approach provides highly selective targeting molecular markers for pancreatic cancer with extremely low levels of non-specific binding and provides quantitative spatial information of biomarker distribution on a single cell, which is important since tumors cell populations are inherently heterogeneous. The quantitative measurements (number of molecules per square micron) is validated using flow cytometry and demonstrated using multiplexed quantitative profiling using color-coded quantum dots.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
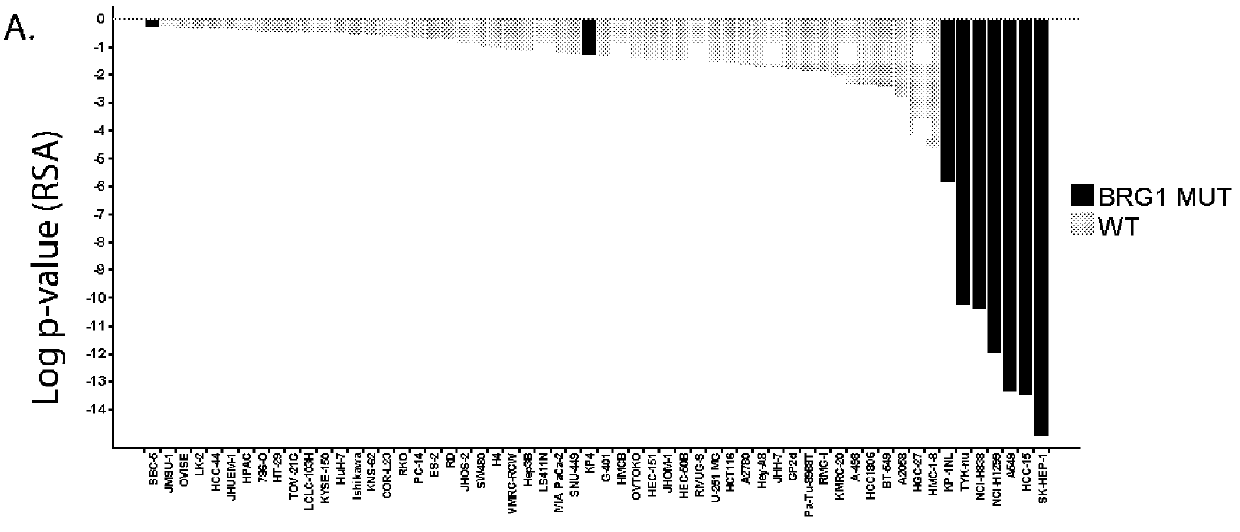
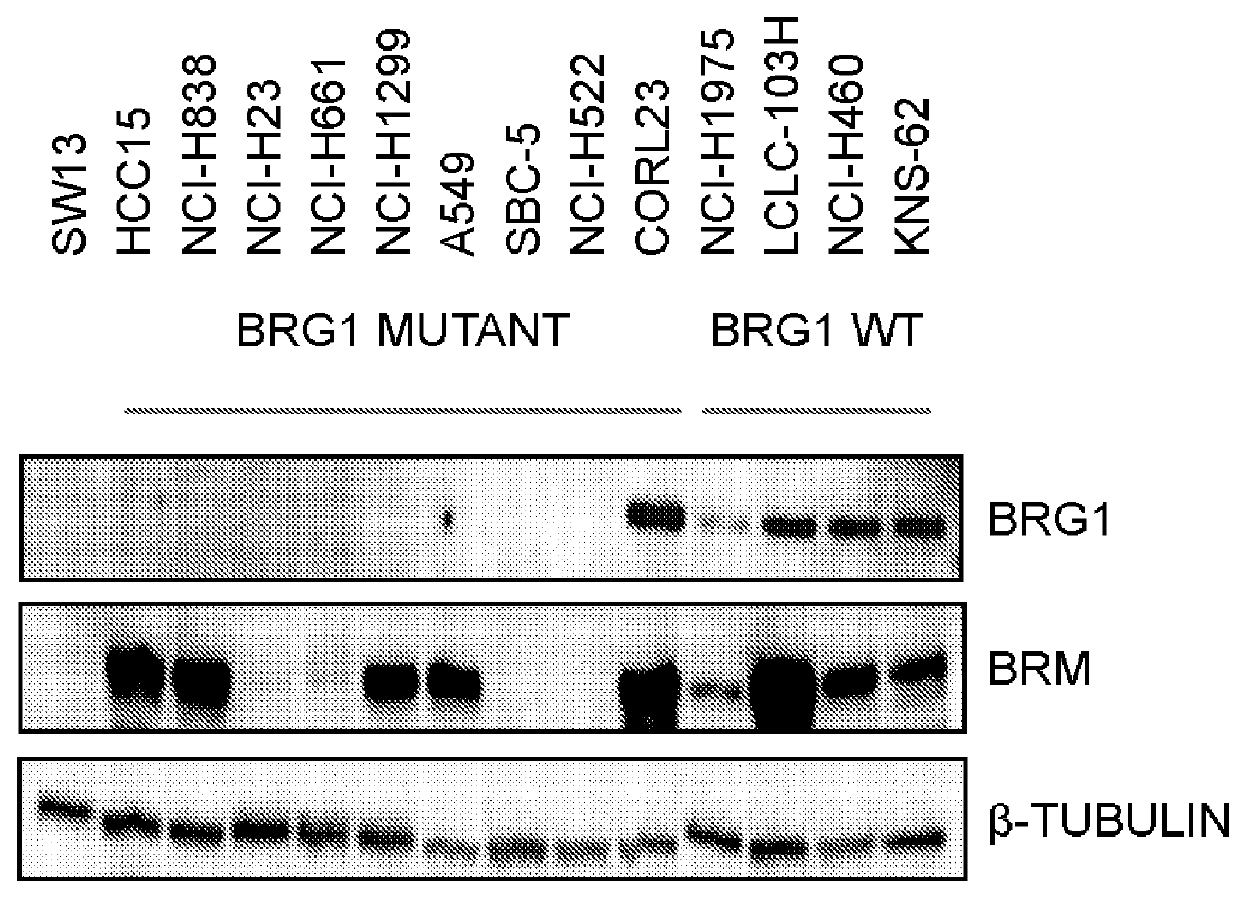
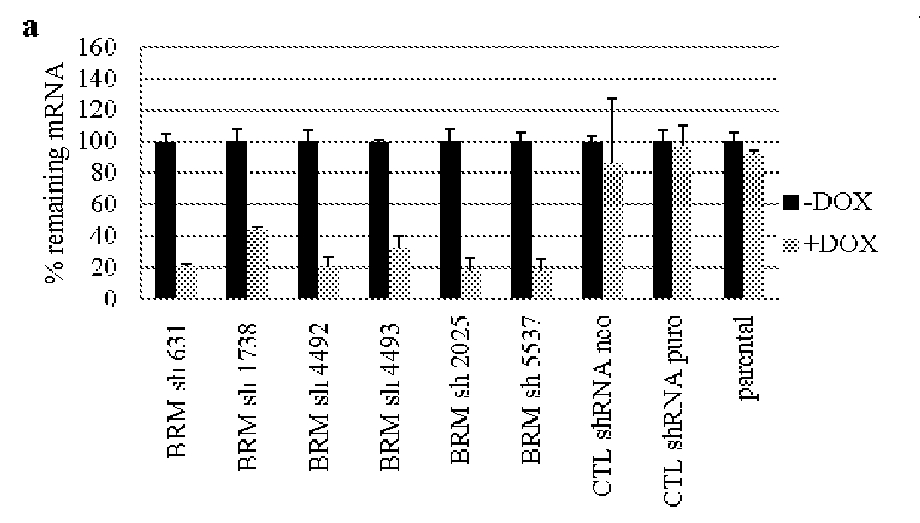
Biomarkers associated with brm inhibition
ActiveUS20160032402A1Simple designMicrobiological testing/measurementLibrary screeningTumor BiomarkersOncology
The invention provides methods of detecting cancer biomarkers, such as one or more SWI / SNF complex mutations, in order to determine a cancer subject's amenability to therapeutic treatment with a BRM inhibitor. Kits, methods of screening for candidate BRM inhibitors, and associated methods of treatment are also provided.
Owner:NOVARTIS AG
Colon cancer biomarkers
InactiveUS20050165556A1Improve abilitiesGreat dimensionalityImage analysisKernel methodsLearning machineFuzzy support vector machine
Owner:HEALTH DISCOVERY CORP +1
Identification of signature genes associated with hepatocellular carcinoma
InactiveUS20110257035A1Lower levelImprove survivalPeptide librariesNucleotide librariesRegimenTumor Biomarkers
The present invention relates to, for example, (1) a novel method for identification of clinically useful serum and / or tumor biomarkers and expression signatures that can be used for detection, prognostication and guidance for the treatment of patients with hepatocellular carcinoma (HCC); and (2) discovery of an expression signature that can be used to monitor and / or study the efficacy of a chemotherapeutic regimen, such as, for example, sorafenib (solely or in combination with other agents). The present invention also provides a method for predicting clinical outcomes, such as, for example, overall survival (OS), time to progression (TTP) and / or likelihood of benefitting from a chemotherapeutic treatment (i.e., sorafenib) in HCC patients based on the analysis of such biomarkers.
Owner:BAYER HEALTHCARE LLC
Cancer Biomarkers and Methods of Use
InactiveUS20150072349A1Increase probabilityMicrobiological testing/measurementBiological material analysisPancreas CancersTumor Biomarkers
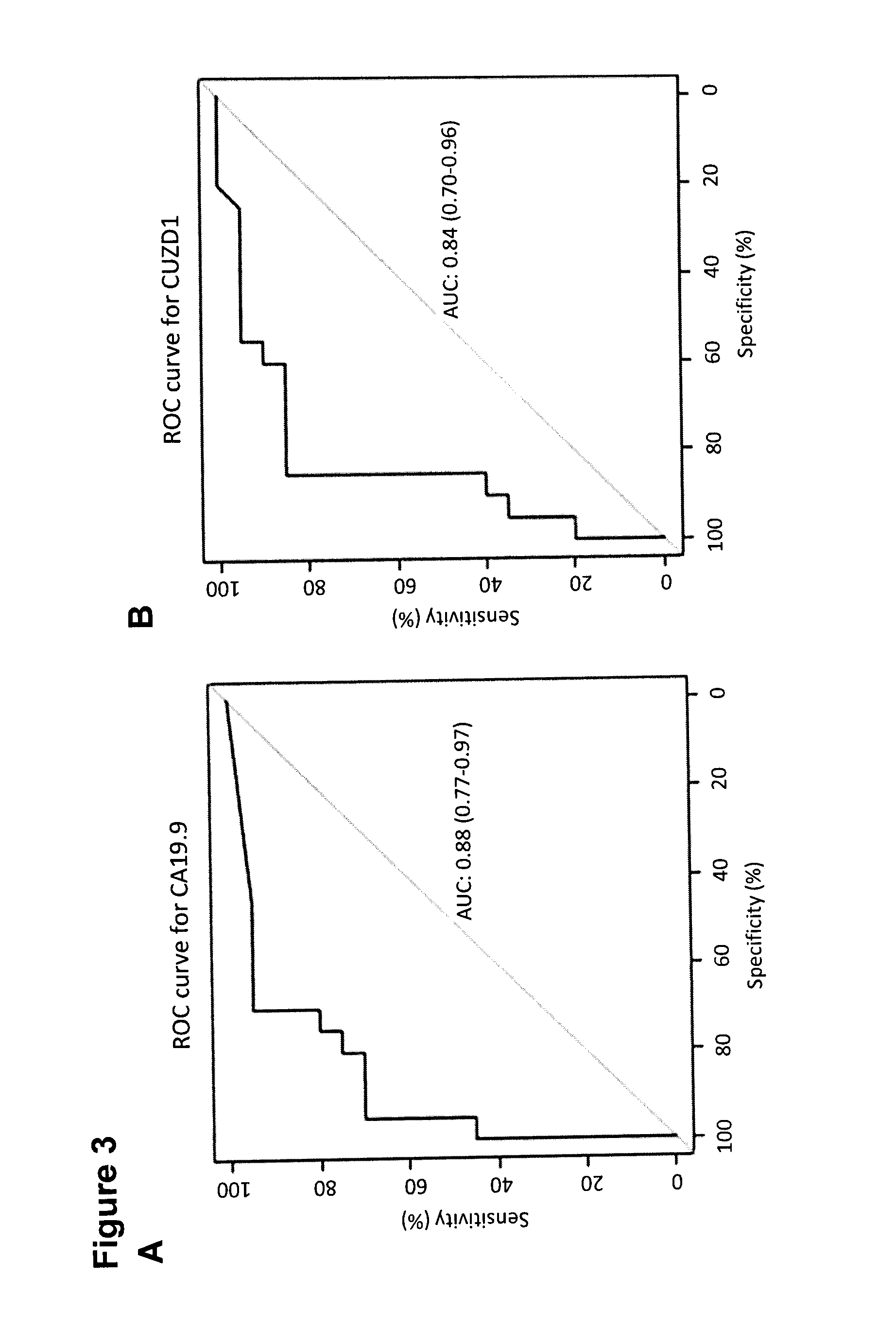
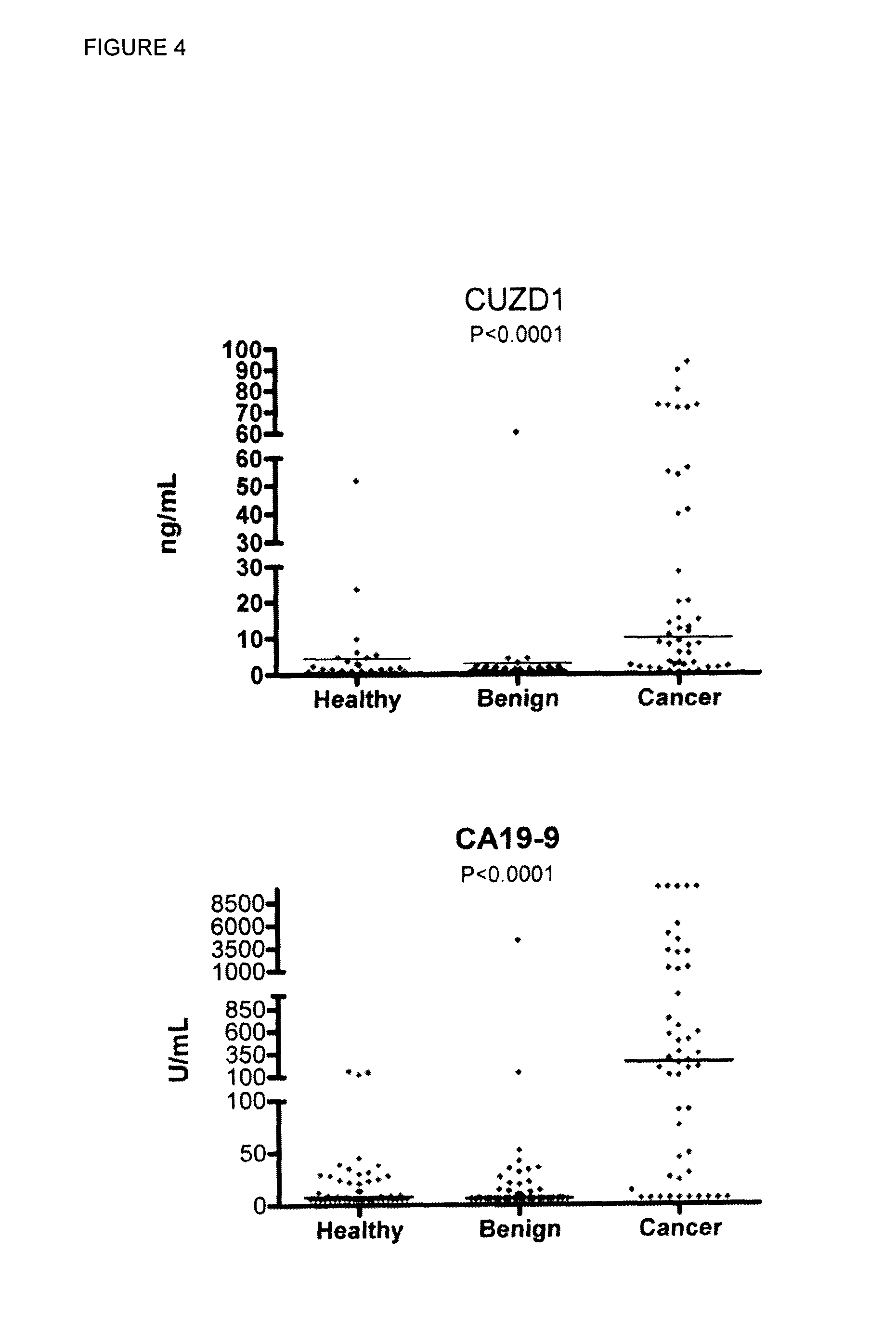
A method of evaluating a probability a subject has a cancer, diagnosing a cancer and / or monitoring cancer progression comprising: a. measuring an amount of a biomarker selected from the group consisting of CUZD1 and / or LAMC2 and / or the group CUZD1, LAMC2, AQP8, CELA2B, CELA3B, CTRB1, CTRB2, GCG, IAPP, INS, KLK1, PNLIPRP1, PNLIPRP2, PPY, PRSS3, REG3G, SLC30A8, KLK3, NPY, PSCA, RLN1, SLC45A3, DSP, GP73, DSG2, CEACAM7, CLCA1, GPA33, LEFTY1, ZG16, IRX5, LAMP3, MFAP4, SCGB1A1, SFTPC, TMEM100, NPY, PSCA, RLN1 and / or SLC45A3 in a test sample from a subject with cancer; wherein the cancer is pancreas cancer if CUZD1, LAMC2, AQP8, CELA2B, CELA3B, CTRB1, CTRB2, GCG, LAPP, INS, KLK1, PNLIPRP1, PNLIPRP2, PPY, PRSS3, REG3G, SLC30A8, DSP, GP73 and / or DSG2 is selected; the cancer is colon cancer if CEACAM7, CLCA1, GPA33, LEFTY1 and / or ZG16 is selected, the cancer is lung cancer if IRX5, LAMP3, MFAP4, SCGB1A1, SFTPC and / or TMEM100 is selected; or the cancer is prostate cancer if NPY, PSCA, RLN1 and / or SLC45A3 is selected; b. comparing the measured amount to a control and detecting an increase in the amount of the biomarker compared to control; and c. identifying the subject as having or having an increased probability of having the cancer when an increase in the biomarker compared to control is detected.
Owner:UNIV HEALTH NETWORK
Cancer medical image data processing method, system and device and storage medium
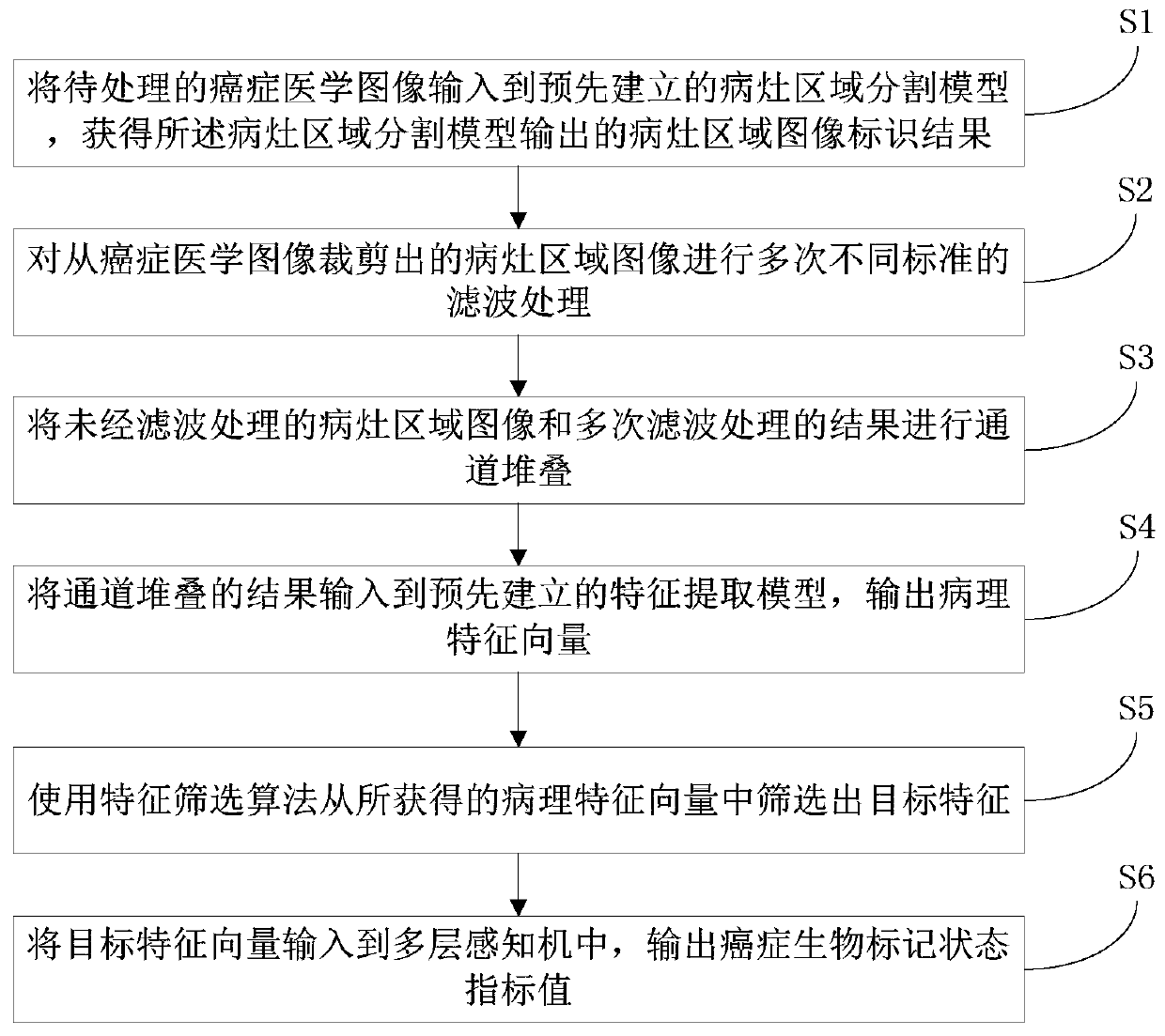
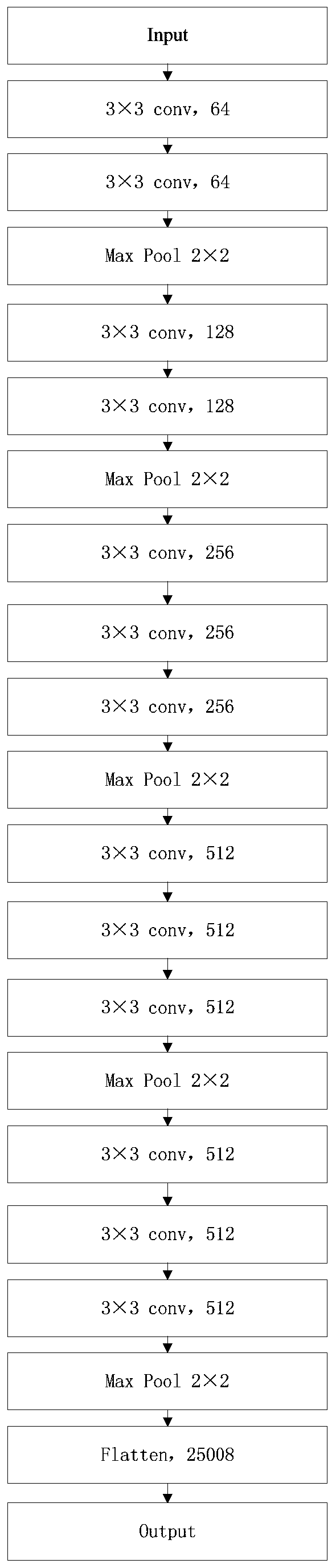
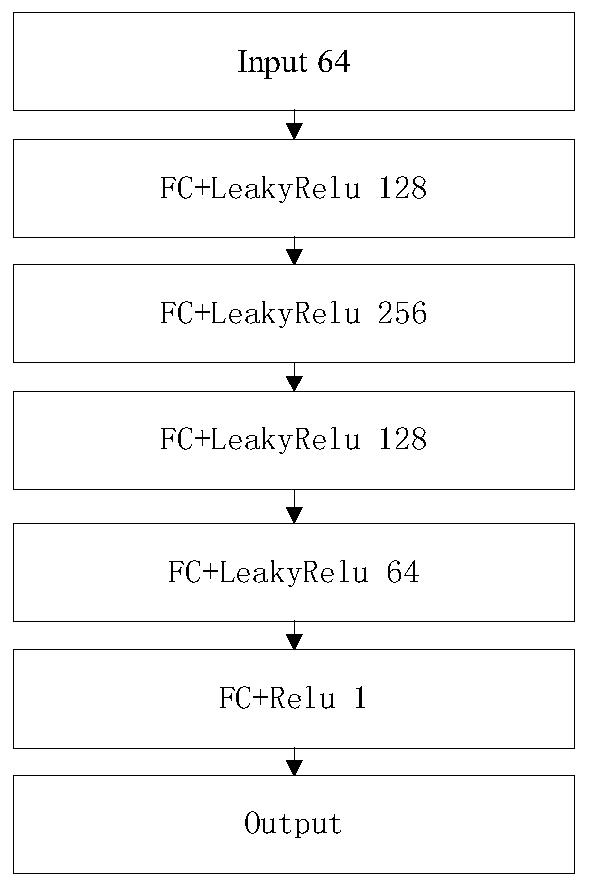
InactiveCN109785300AImprove recognition efficiencyImprove cutting efficiencyImage analysisNeural architecturesFeature vectorImaging processing
The invention discloses a cancer medical image data processing method, system, device and a storage medium. The method comprises the following steps: inputting a to-be-processed cancer medical image into a pre-established focus area segmentation model, obtaining a focus area image identification result output by the focus area segmentation model, filtering processing of different standards is carried out on the focus area image cut from the cancer medical image for multiple times, channel stacking is carried out, a channel stacking result is input into a pre-established feature extraction model, and a feature screening algorithm is used for screening out target features from the obtained pathological feature vectors; inputting the target characteristics into a multilayer perceptron to obtain cancer biomarker state index values output by the multilayer perceptron, and the like. According to the method, the focus area on the cancer medical image can be automatically identified, and the focus area image is cut out, so that the identification and cutting efficiency and accuracy are greatly improved. The method is widely applied to the technical field of image processing.
Owner:SOUTH CHINA UNIV OF TECH
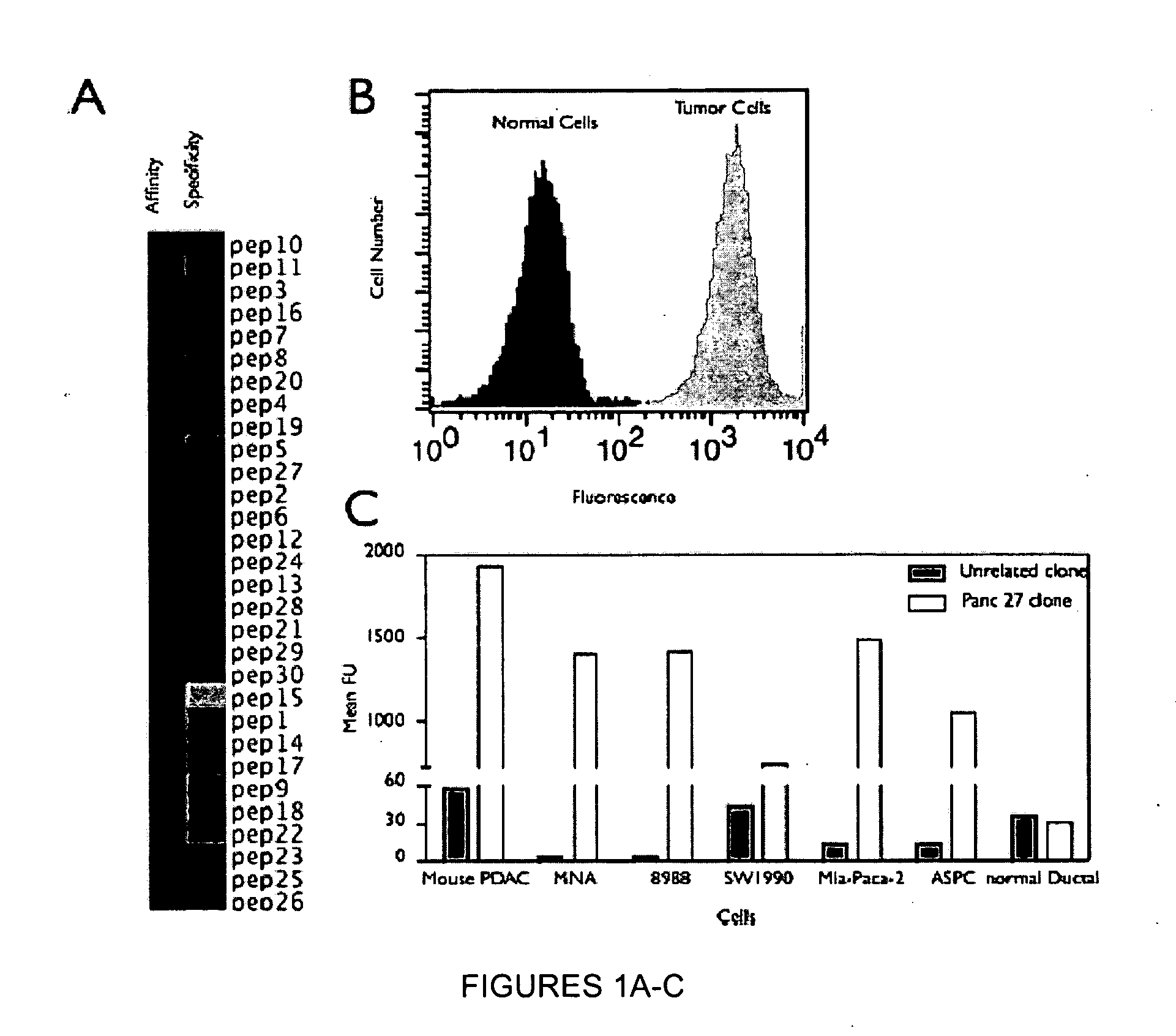
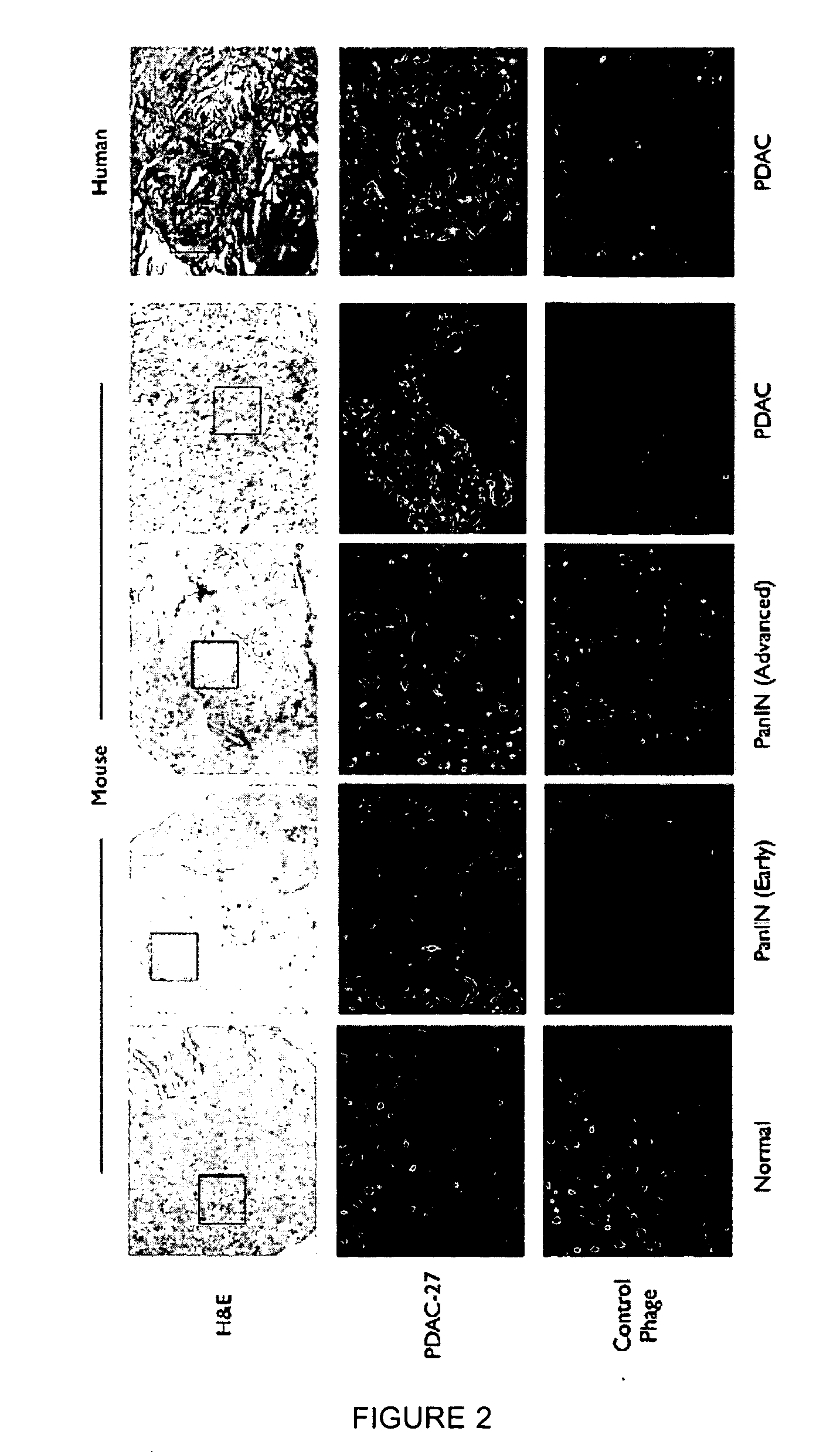
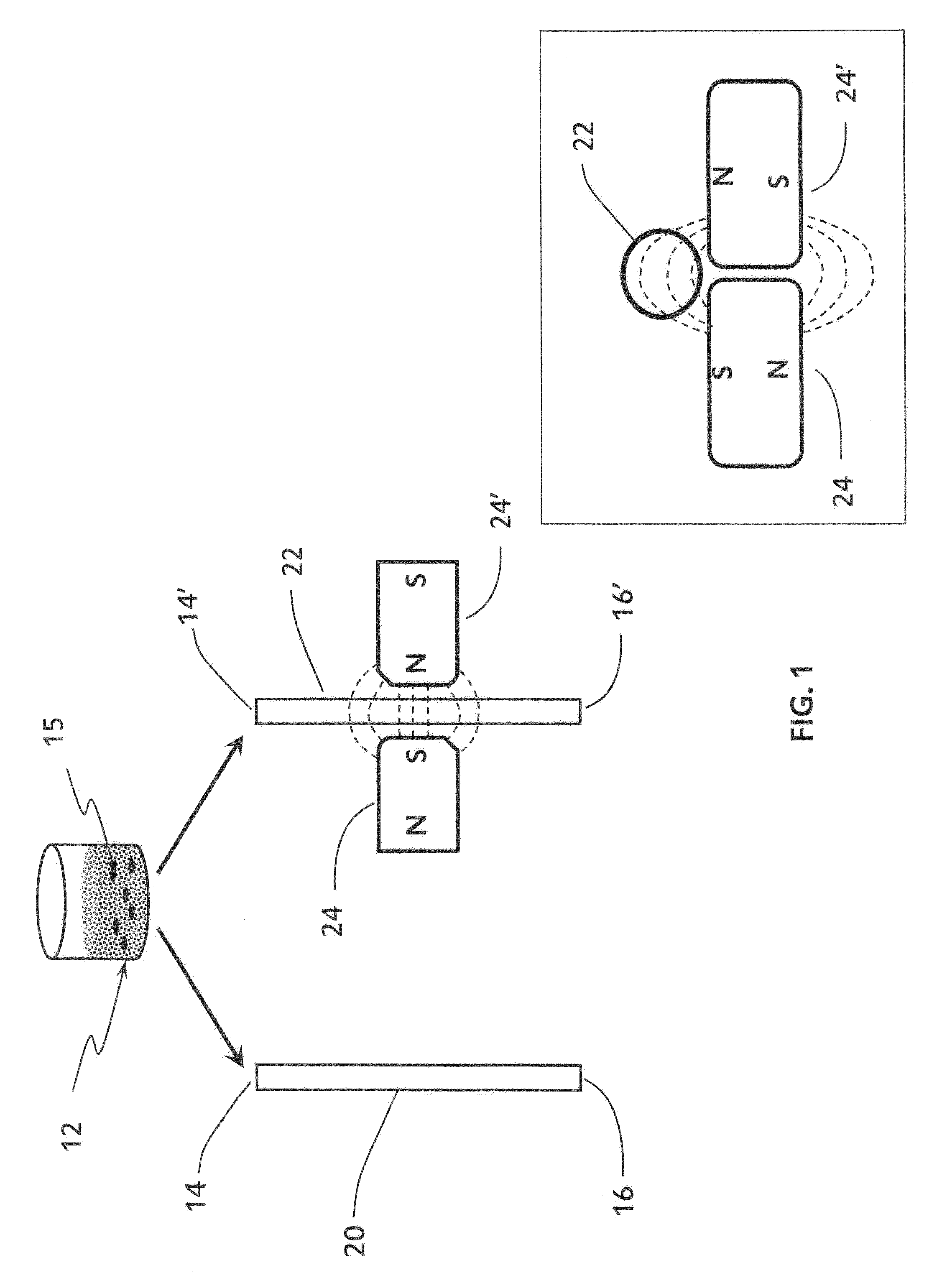
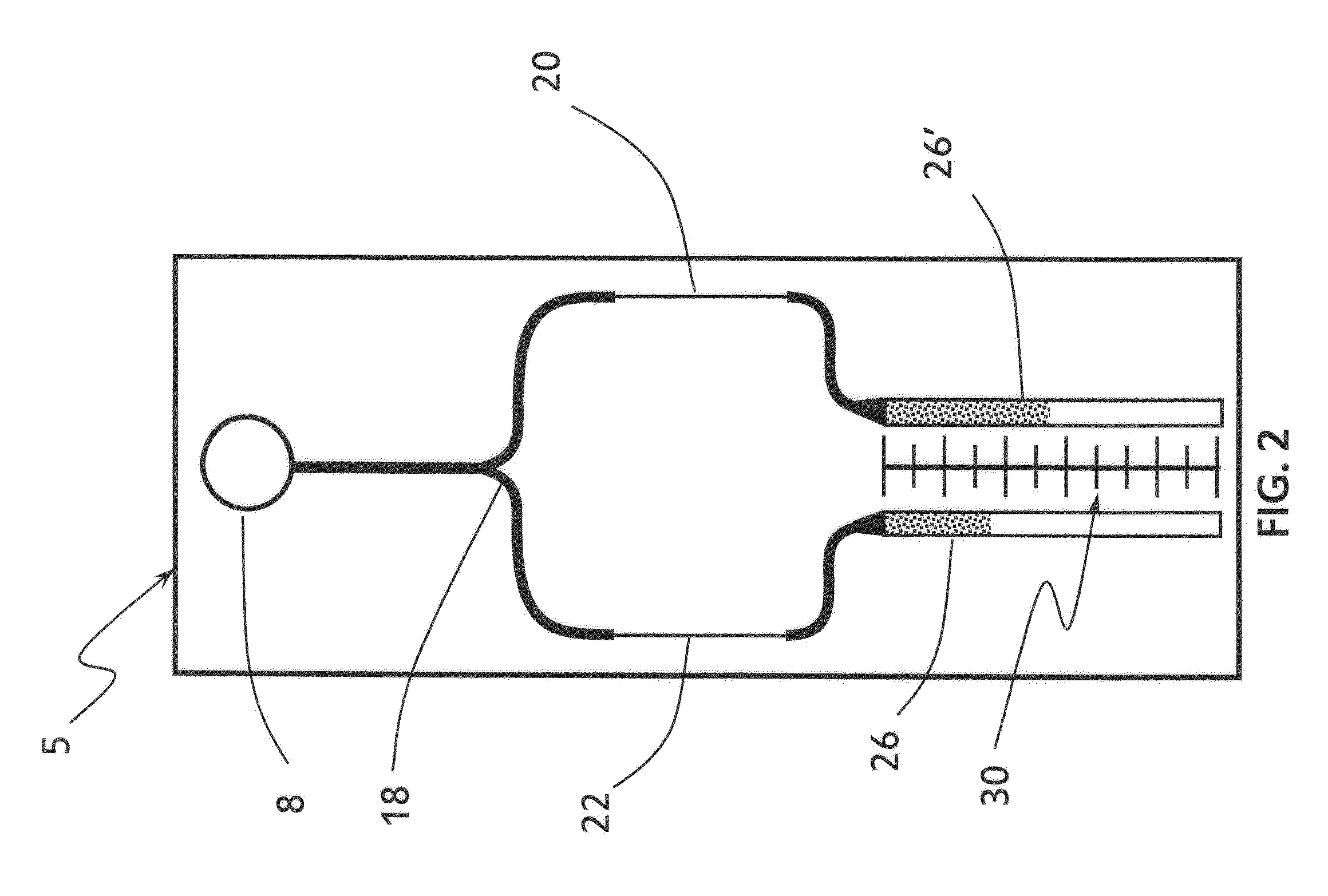
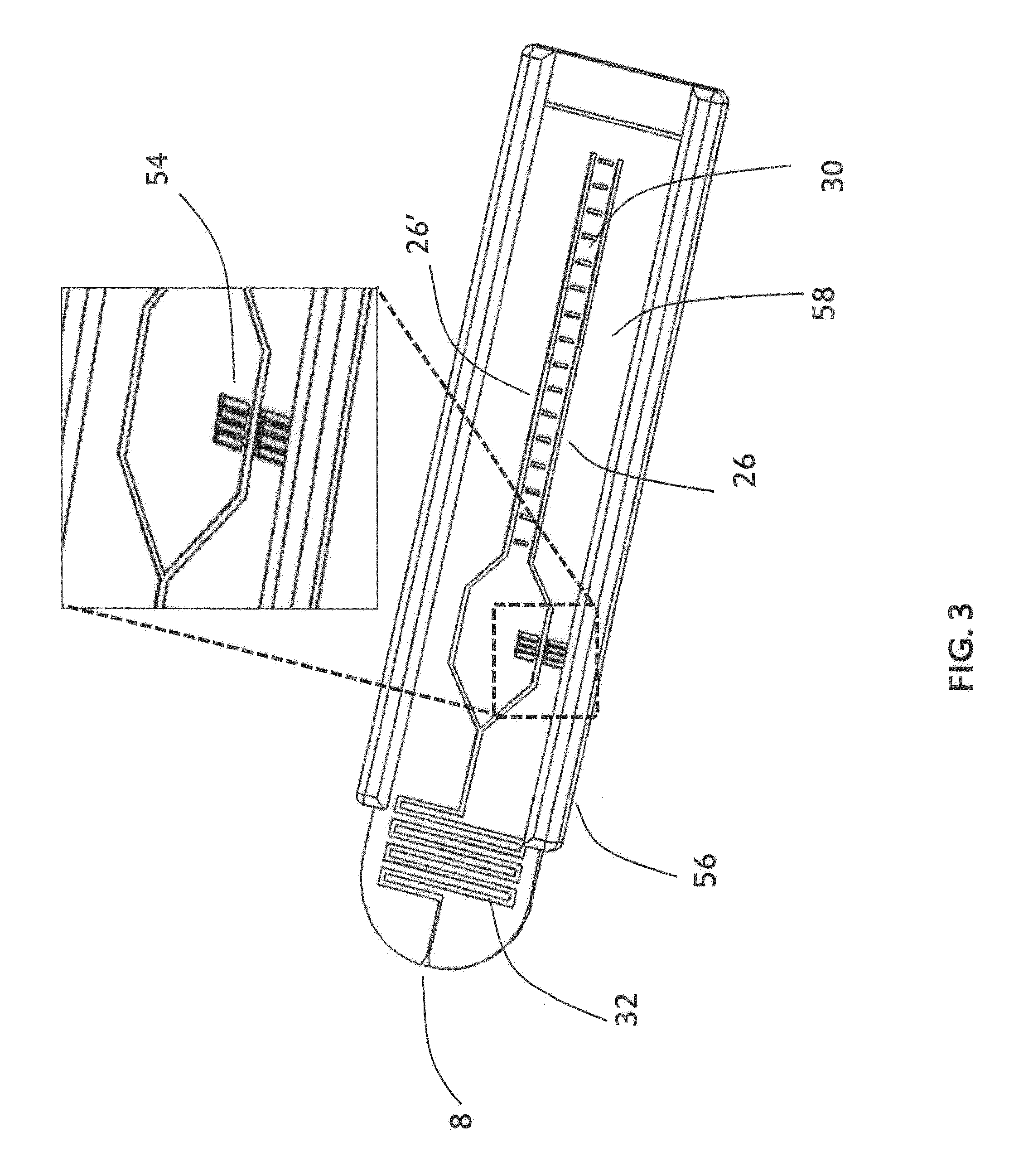
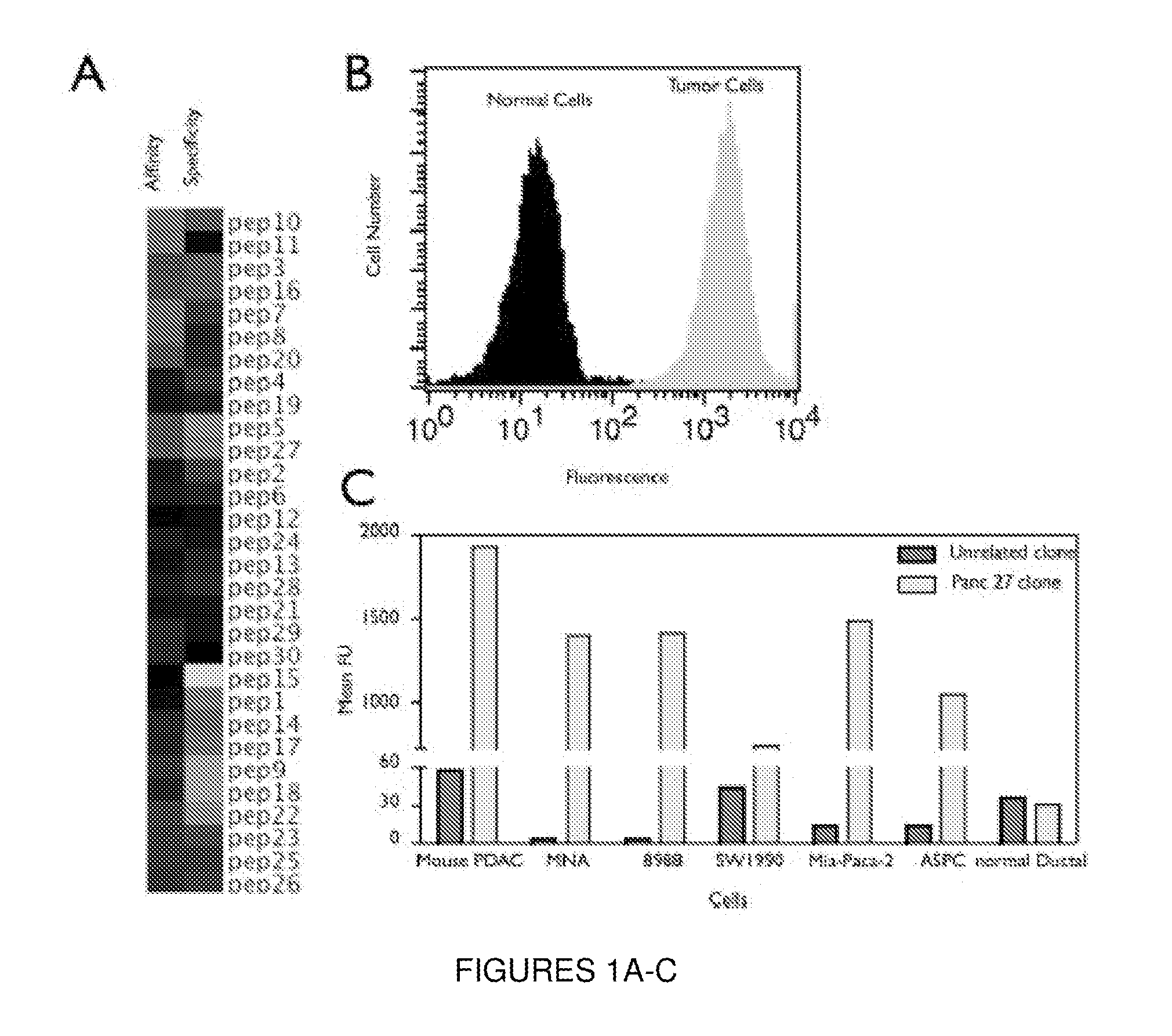
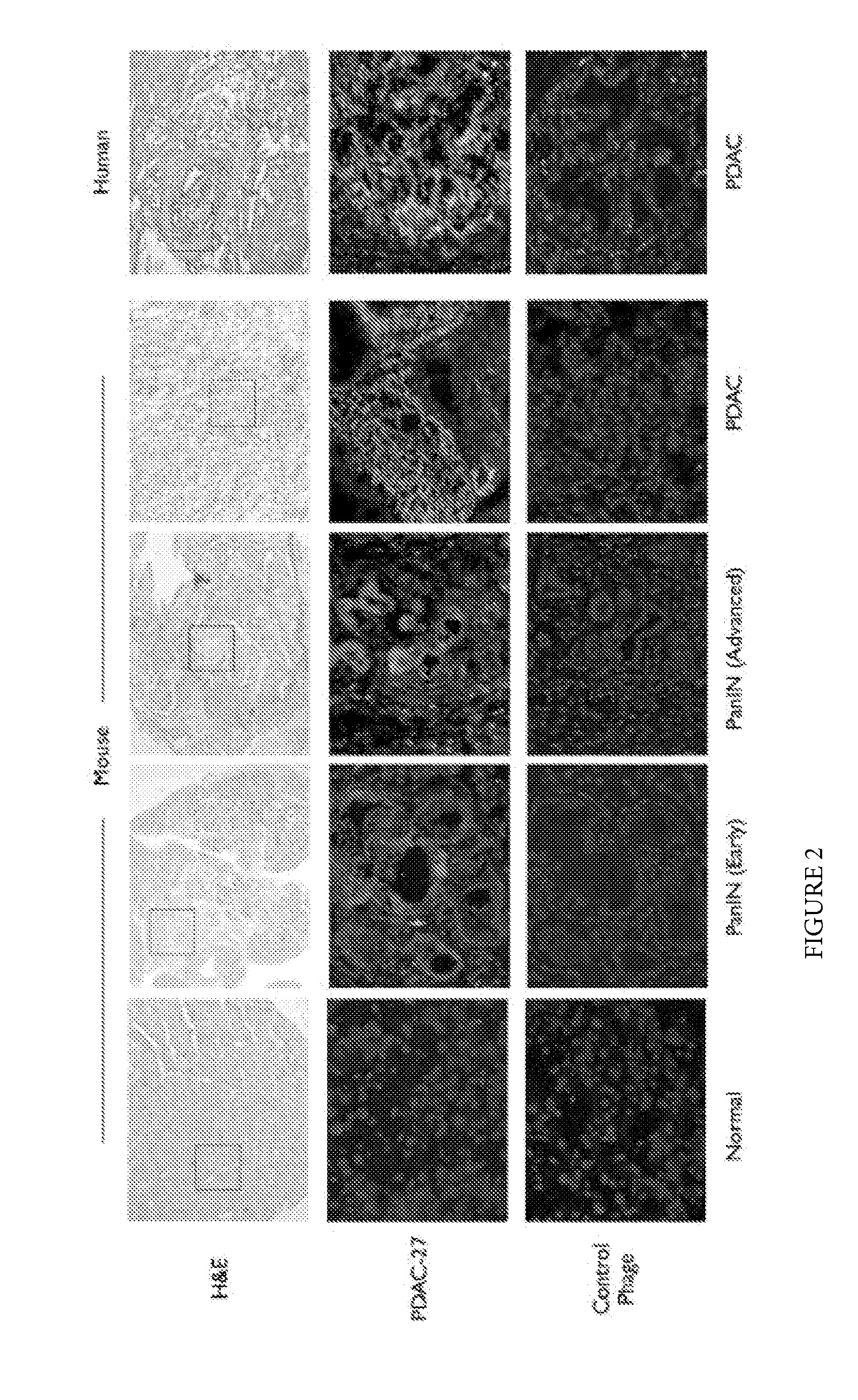
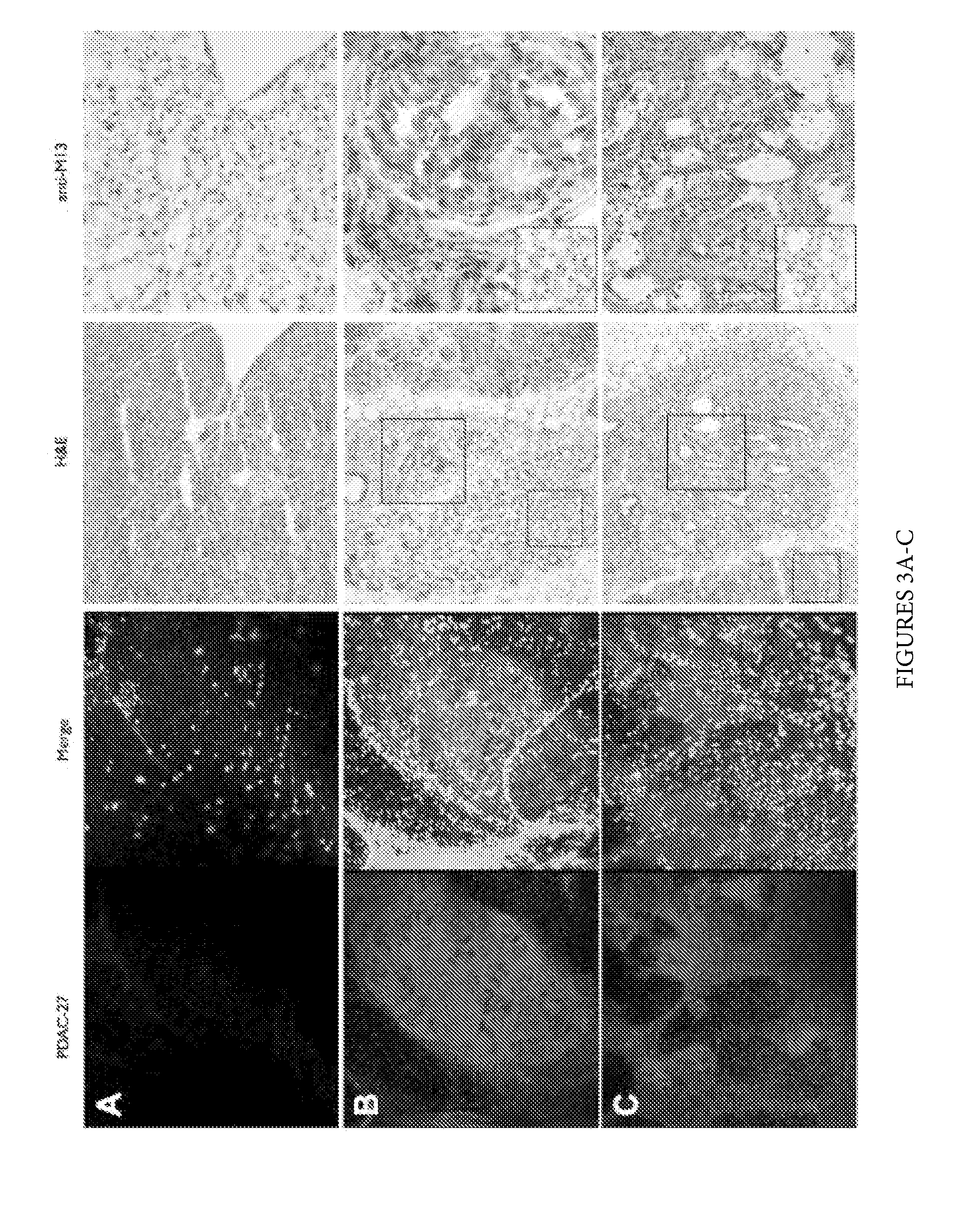
Plectin-1 targeted agents for detection and treatment of pancreatic ductal adenocarcinoma
ActiveUS20110182814A1Fast wayQuick identificationPeptide/protein ingredientsGeneral/multifunctional contrast agentsPancreas Ductal AdenocarcinomaCancer cell
Described herein are compositions and methods for cancer cell biomarkers, such as pancreatic ductal adenocarcinoma (PDAC) cell biomarkers, and binding molecules for diagnosis and treatment of cancer, e.g., PDAC. Methods of identifying “accessible” proteomes are disclosed for identifying cancer biomarkers, such as plectin-1, a PDAC biomarker. Additionally, imaging compositions are provided comprising magnetofluorescent nanoparticles conjugated to peptide ligands for identifying PDACs.
Owner:THE GENERAL HOSPITAL CORP
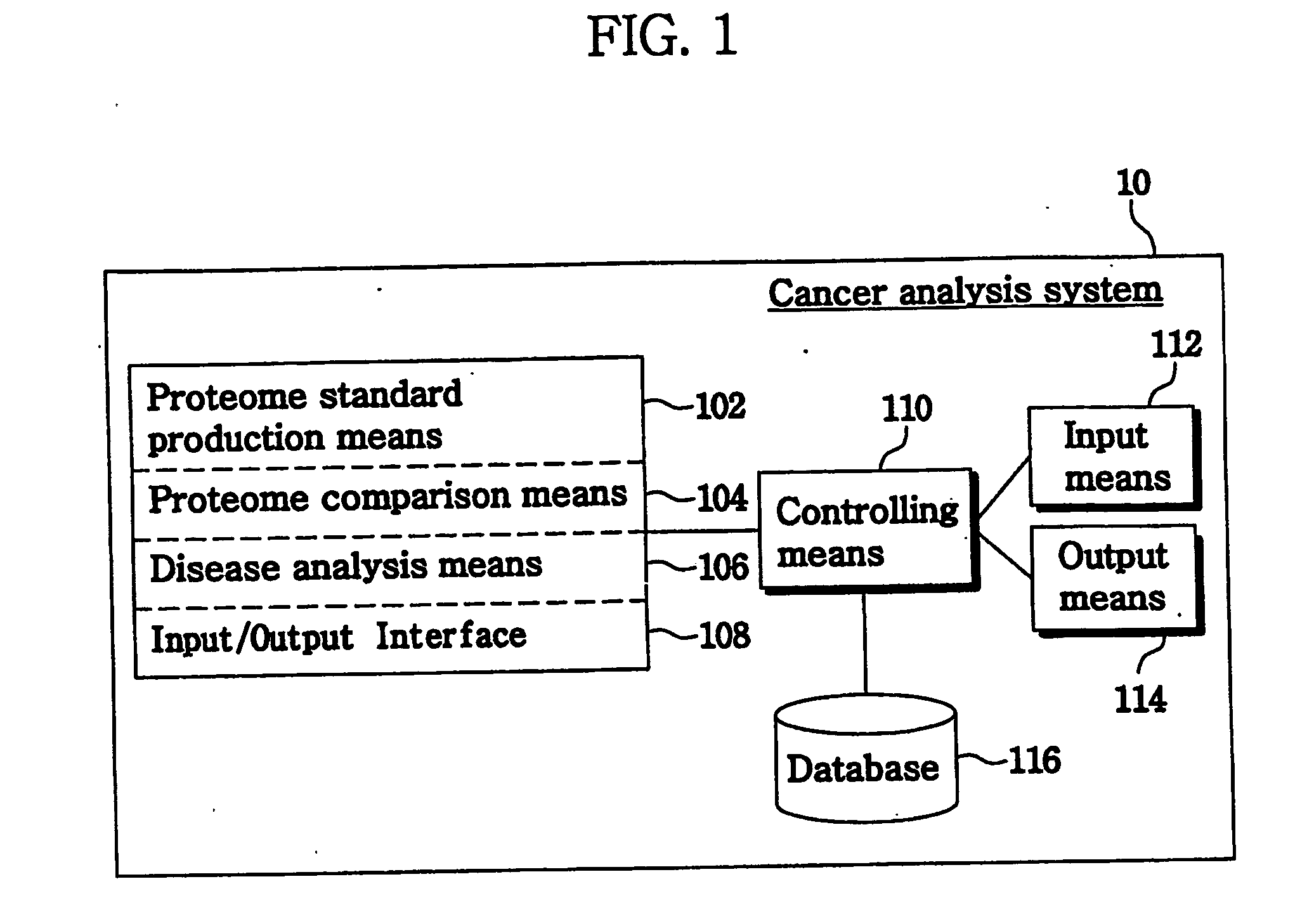
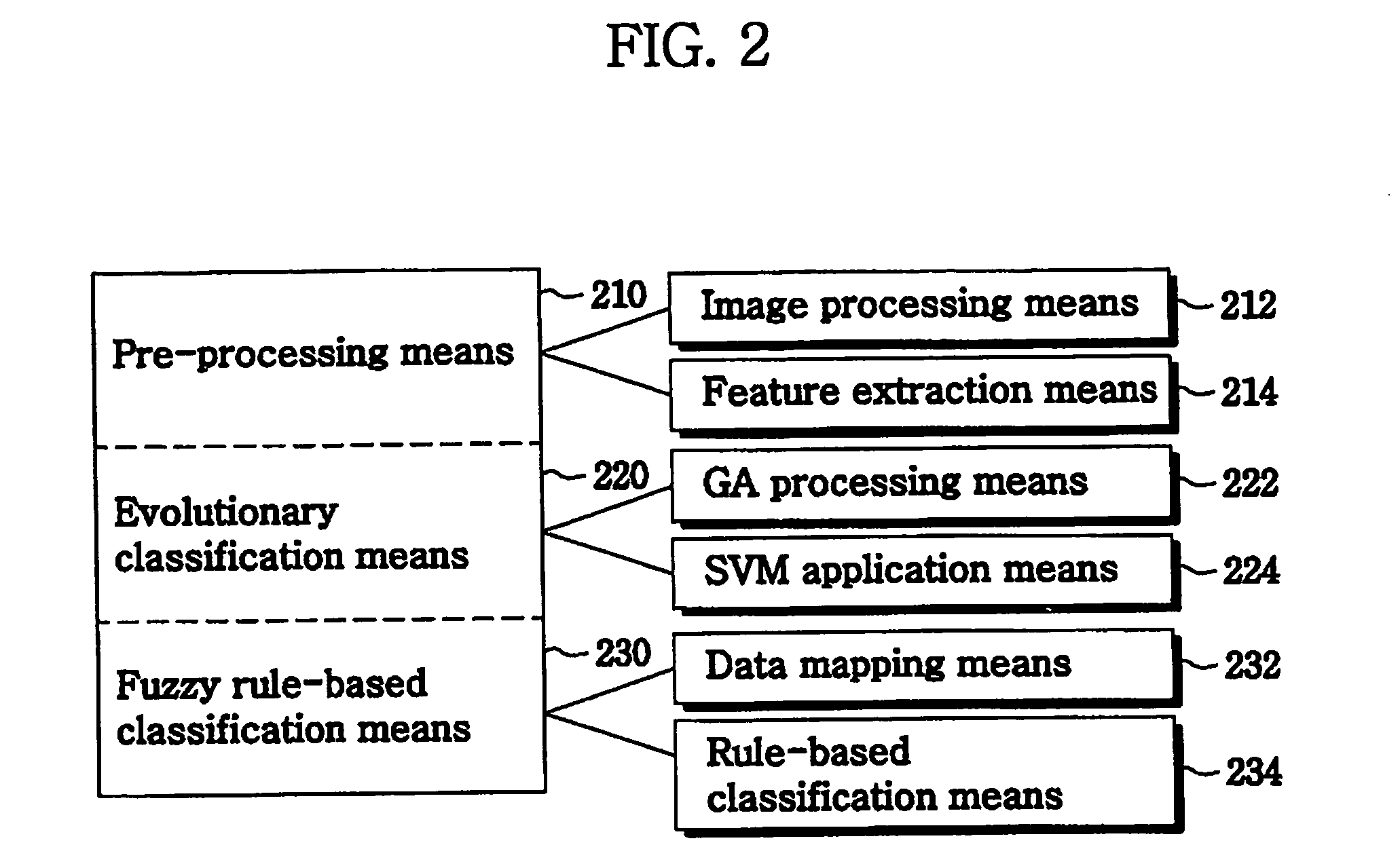
Method and system for analysis of cancer biomarkers using proteome image mining
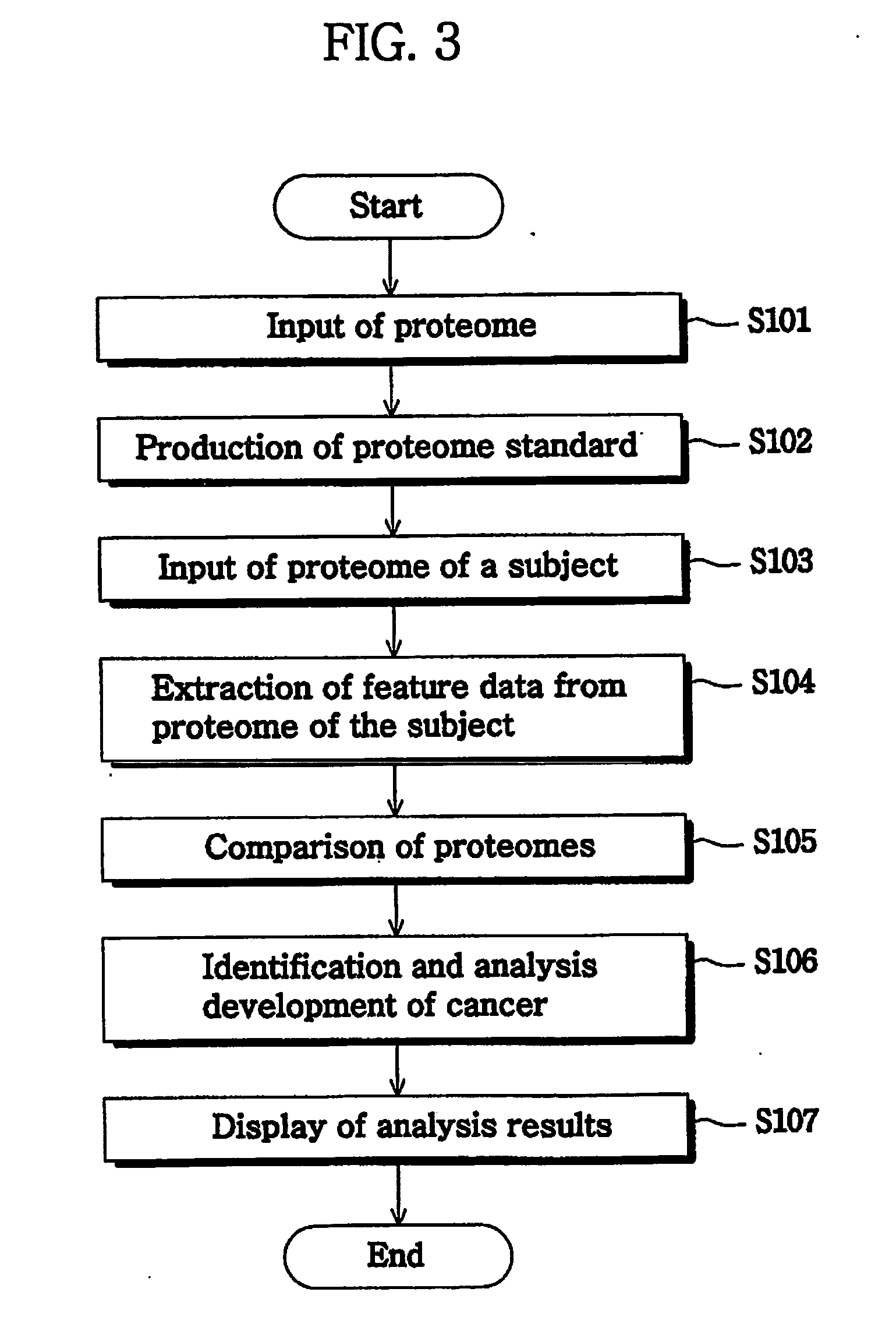
InactiveUS20070072250A1The analysis result is accurateEasy to distinguishBiostatisticsProteomicsTumor BiomarkersImaging technique
The present invention provide a system and a method for detection of cancer, by producing a serum proteome standard by an image mining technique, and provide cancer-specific biomarkers. Disclosed is a method of analyzing cancer, comprising the steps of transforming serum proteomes from normal individuals and individuals having cancer into two-dimensional images, and constructing a database consisting of the proteome standard by an image mining technique; inputting a serum proteome from a subject of interest, transforming the serum proteome into a image and comparing the structure of the serum proteome pattern of the subject with the proteome standard and determining whether the serum proteome of the subject is normal or abnormal. The system and method for cancer analysis facilitates detection of cancer by constructing a database from a plurality of serum proteome using an image technique and comparing serum proteome of a subject with a proteome standard.
Owner:BIOINFRA
Novel method for the detection of cancer biomarkers in cervical specimens
InactiveUS20050136405A1Microbiological testing/measurementMaterial analysisTumor BiomarkersProper treatment
Owner:CYTYC CORP
Analysis of exosomes and methods of diagnosing cancer
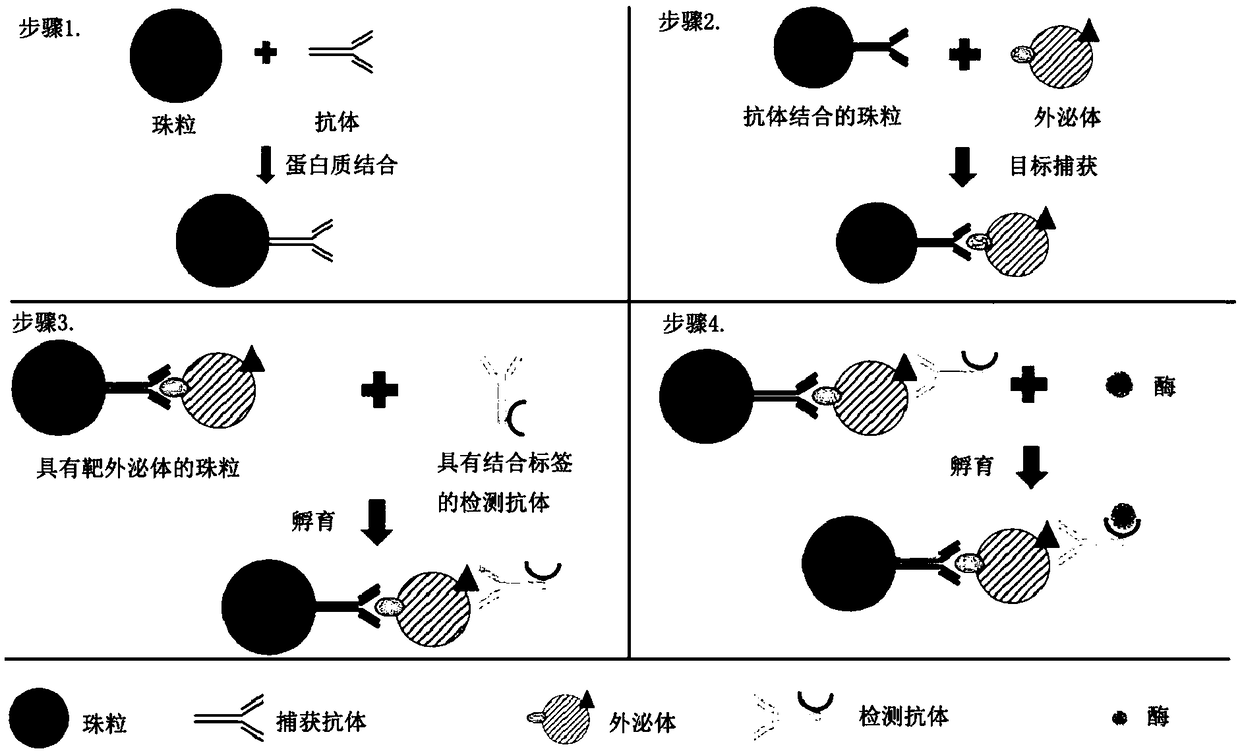
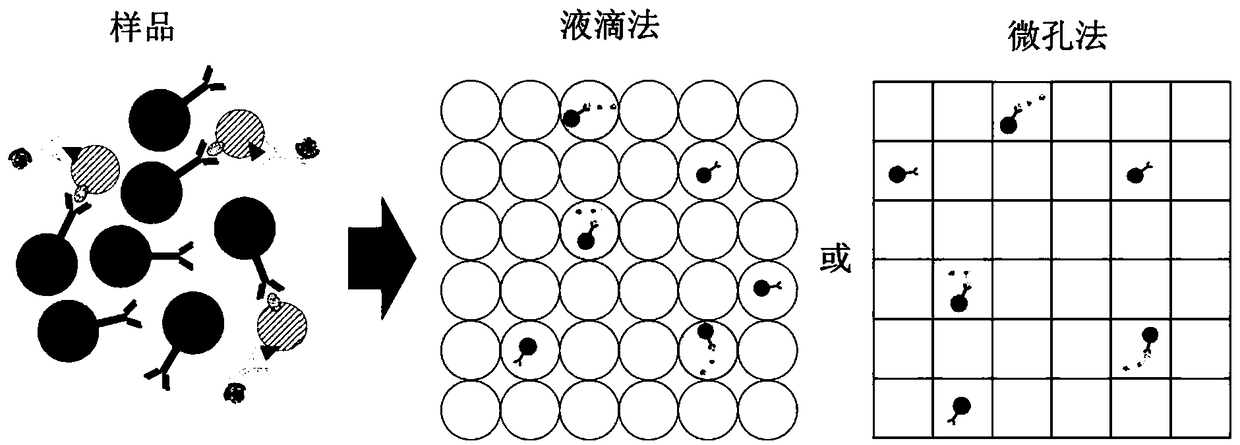
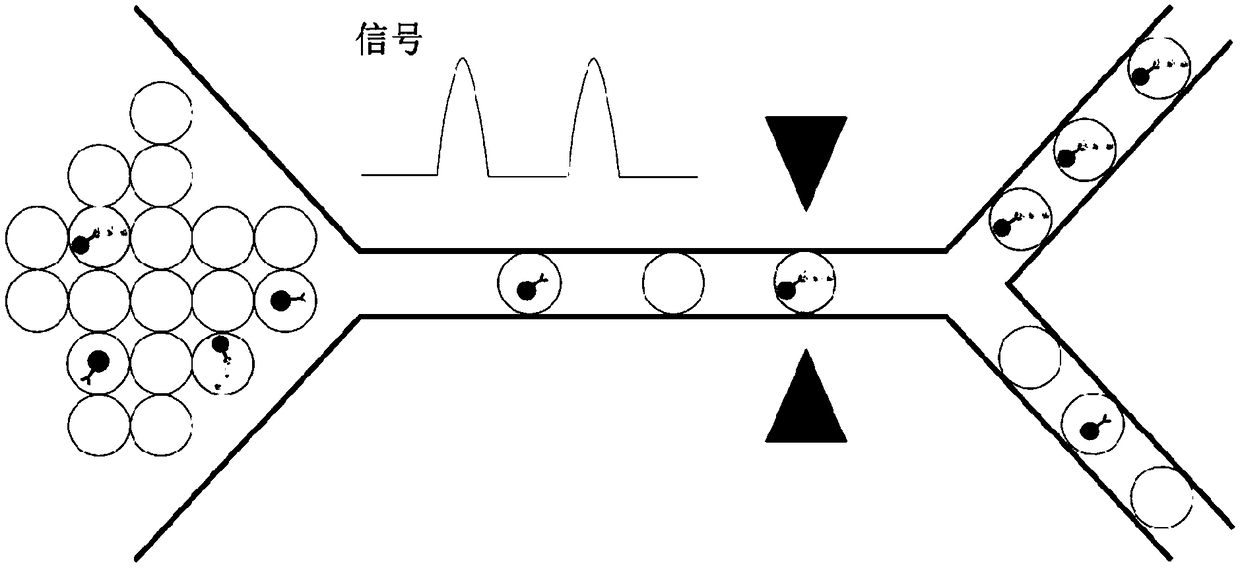
InactiveCN109490528ABiological material analysisMedical automated diagnosisTumor BiomarkersCancers diagnosis
The invention relates to quantification, isolation, and characterization of exosomes. In some embodiments, exosomes can be quantified by contacting a sample with a capture bead comprising a bead and afirst binding agent, and a second binding agent. The first binding agent binds to a first biomolecule in the exosomes to produce a first complex and the second binding agent binds to a second biomolecule in the exosomes of the first complex to produce a second complex. The first complexes and the second complexes are quantified based on a detectable signal conjugated to the second binding agent.A microwell or a droplet generation is utilized to quantify the first complexes and the second complexes. In optional embodiments, quantification of the exosomes is used to diagnose a cancer in a subject. In such methods, the first and the second binding agents bind to cancer biomarkers present in the exosomes.
Owner:THE HONG KONG UNIV OF SCI & TECH
Method, compositions and classification for tumor diagnostics and treatment
InactiveUS20090082551A1Quick and easy determinationImprove survivalCompound screeningIn-vivo radioactive preparationsTumor BiomarkersSide effect
The present invention is directed towards classifying tumor biomarkers, particularly membrane receptors, and more particularly the gastrin-releasing peptide (GPR) receptors, identified in patient samples, then linking therapeutic agents (chemical, radiological, or biological) to patient-specific ligands that bind to such receptors, clinicians can produce diagnostic and treatment compositions and implement treatment regimens which, by using the classified and identified biomarkers, and due to their improved accuracy, increase success and decrease undesired side effects from such treatments.
Owner:ZUCKERMAN MATHEW MARK
Early Detection and Prognosis of Colon Cancers
InactiveUS20080221056A1Organic active ingredientsGenetic material ingredientsGenes mutationBiomarker (petroleum)
We have developed a transcriptome-wide approach to identify genes affected by promoter CpG island hypermethylation and transcriptional silencing in colorectal cancer (CRC). By screening cell lines and validating tumor specific hypermethylation in a panel of primary human CRC samples, we estimate that nearly 5% of all known genes may be promoter methylated in an individual tumor. When directly compared to gene mutations, we find a much larger number of genes hypermethylated in individual tumors, and much higher frequency of hypermethylation within individual genes harboring either genetic or epigenetic changes. Thus, to enumerate the full spectrum of alterations in the human cancer genome, and facilitate the most efficacious grouping of tumors to identify cancer biomarkers and tailor therapeutic approaches, both genetic and epigenetic screens should be undertaken. The genes we identified can be used inter alia diagnostically to detect cancer, pre-cancer, and likelihood of developing cancer.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
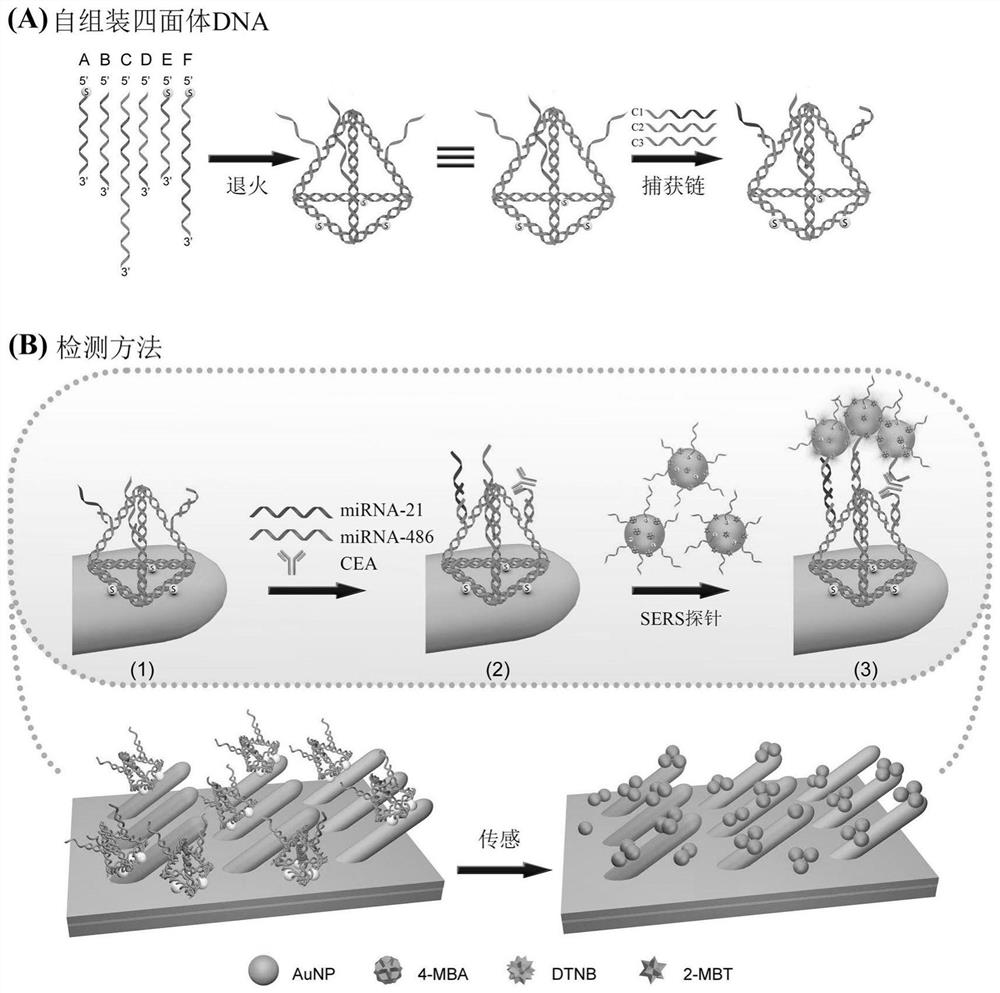
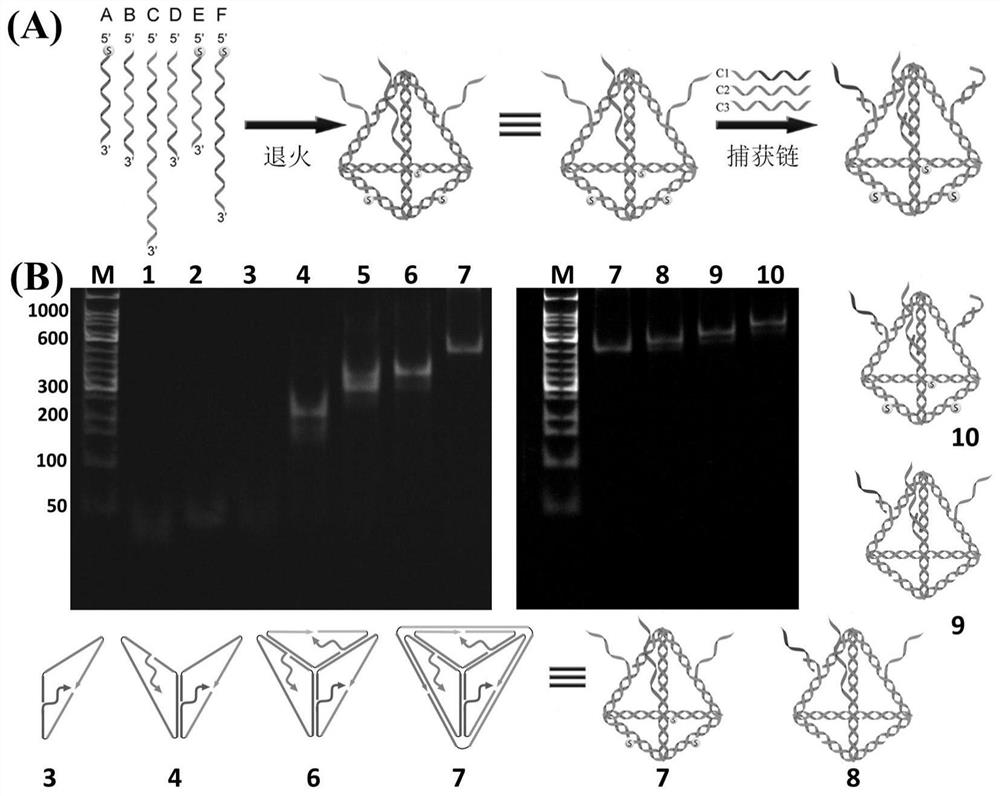
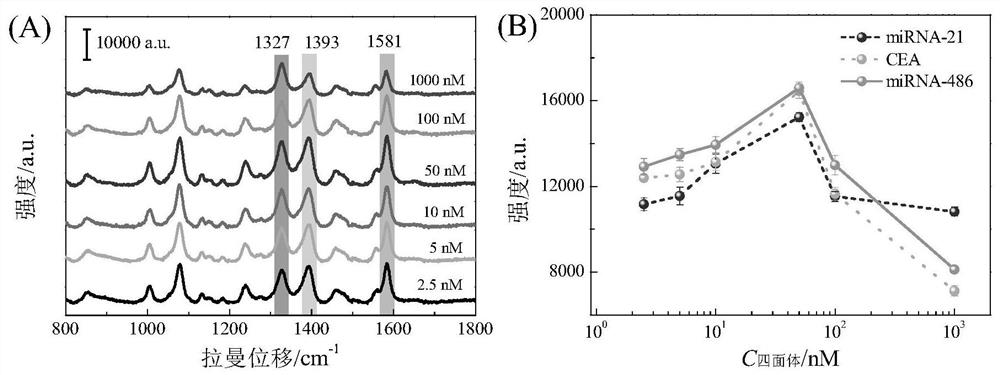
SERS sensor for integrated detection of tumor protein and nucleic acid markers and preparation method thereof
ActiveCN111781186AImprove structural rigidityImprove structural stabilityRaman scatteringLung cancerNucleic acid
The invention discloses an SERS sensor for integrated detection of tumor protein and nucleic acid markers and a preparation method thereof. The SERS sensor comprises a silver nanorod array substrate and an SERS probe, wherein the surface of the silver nanorod array substrate is modified with a tetrahedral DNA structure; the tetrahedral DNA structure is formed by self-assembling six DNA single chains, and three DNA single chains are arranged on three edges of the tetrahedral DNA structure, are respectively connected with three capture chains and are used for respectively and specifically binding nucleic acid and a protein marker. The detection limits of the SERS sensor for detecting nucleic acid and protein respectively reach the Amol / L magnitude and the fg / mL magnitude; the integrated detection of various nucleic acid and protein biomarkers in complex environments such as serum can be realized. The sensor disclosed by the invention is simple to prepare, high in detection sensitivity and good in reliability, can realize high-sensitivity detection of biomarkers with extremely low abundance in the early stage of lung cancer, and has good universality for various tumor biomarkers.
Owner:NANJING UNIV OF POSTS & TELECOMM
Glycoprotein cancer biomarker
Owner:UNIV OF GEORGIA RES FOUND INC
Device and method for detection and identification of immunological proteins, pathogenic and microbial agents and cells
ActiveUS20130078620A1Low costReduce complexityMicrobiological testing/measurementFluid pressure measurementPsa antigenCancer cell
The present invention provides a method and device for detecting and quantifying the concentration of magnetic-responsive micro-beads dispersed in a liquid sample. Also provided is a method and microfluidic immunoassay pScreen™ device for detecting and quantifying the concentration of an analyte in a sample medium by using antigen-specific antibody-coated magnetic-responsive micro-beads. The methods and devices of the present invention have broad applications for point-of-care diagnostics by allowing quantification of a large variety of analytes, such as proteins, protein fragments, antigens, antibodies, antibody fragments, peptides, RNA, RNA fragments, functionalized magnetic micro-beads specific to CD4+, CD8+ cells, malaria-infected red blood cells, cancer cells, cancer biomarkers such as prostate specific antigen and other cancer biomarkers, viruses, bacteria, and other pathogenic agents, with the sensitivity, specificity and accuracy of bench-top laboratory-based assays.
Owner:CARNEGIE MELLON UNIV
Plectin-1 Targeted Agents for Detection and Treatment of Pancreatic Ductal Adenocarcinoma
ActiveUS20150151010A1Fast wayQuick identificationPeptide/protein ingredientsGeneral/multifunctional contrast agentsCancer cellTumor Biomarkers
Described herein are compositions and methods for cancer cell biomarkers, such as pancreatic ductal adenocarcinoma (PDAC) cell biomarkers, and binding molecules for diagnosis and treatment of cancer, e.g., PDAC. Methods of identifying “accessible” proteomes are disclosed for identifying cancer biomarkers, such as plectin-1, a PDAC biomarker. Additionally, imaging compositions are provided comprising magnetofluorescent nanoparticles conjugated to peptide ligands for identifying PDACs.
Owner:THE GENERAL HOSPITAL CORP
Nitroreductase-specific fluorescent probe and preparation thereof and application in tumor-targeted fluorescence imaging and monitoring of tumor hypoxia
InactiveCN106749153AFluorescence enhancementHigh enhancement factorPeptidesIn-vivo testing preparationsTumor targetNitroreductase
The invention provides a nitroreductase-specific fluorescent probe and the preparation thereof and a reagent for applying the nitroreductase-specific fluorescent probe to tumor-targeted fluorescence imaging and monitoring of tumor hypoxia. The reagent is connected by tumor biomarker identifying groups (sensor), tumor targeted groups (target) and fluorescent groups (dye) via chemical bonds. The reagent can be used in the tumors with hypoxic microenvironment and high expression of nitroreductase based on tumor hypoxia and the high expression of nitroreductase in hypoxic tumors. Combined with a fluorescence imager, the reagent can be used in tumor targeted fluorescence imaging, tumor hypoxia monitoring and the monitoring of tumor metastasis, etc. The reagent realizes high sensitivity and high specificity in application and provides an effective tool for medical study of cancer and clinical monitoring and treatment of tumor metastasis, thereby having a good application prospect in tumor-targeted fluorescence imaging, tumor hypoxia monitoring and monitoring of tumor metastasis.
Owner:EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com