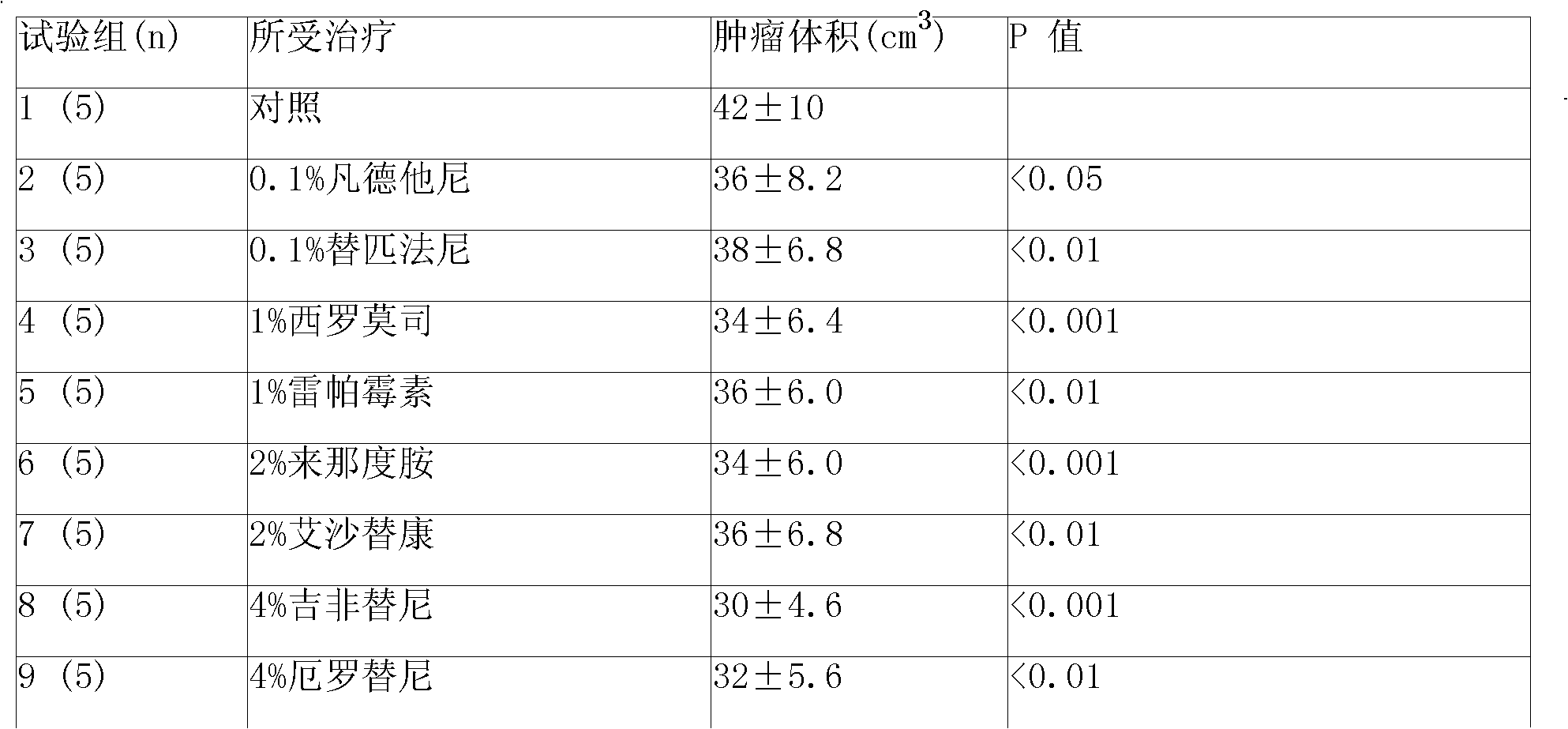
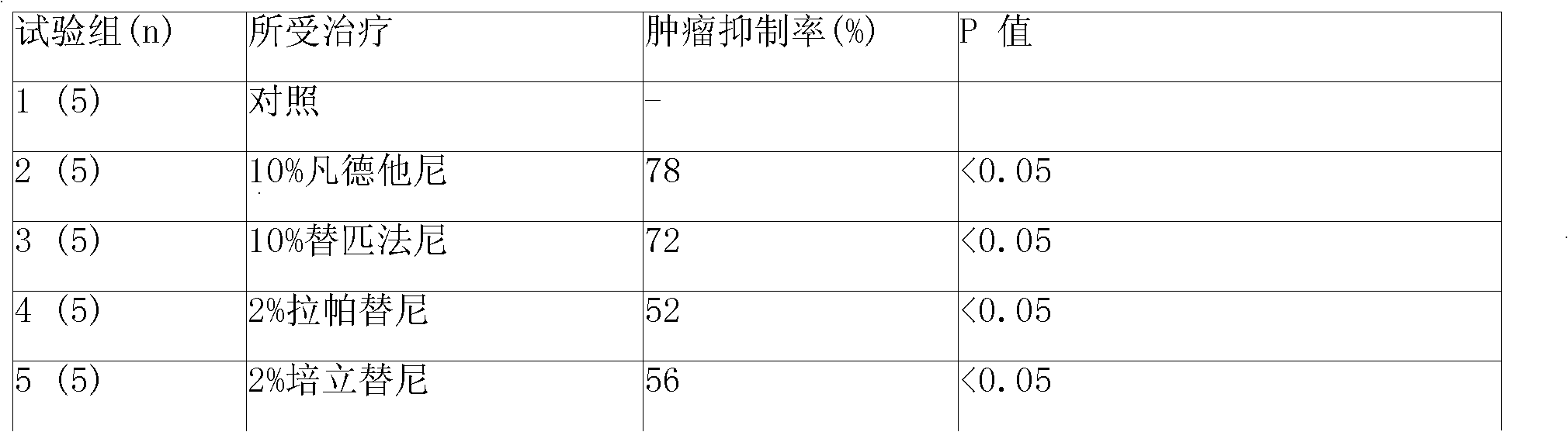
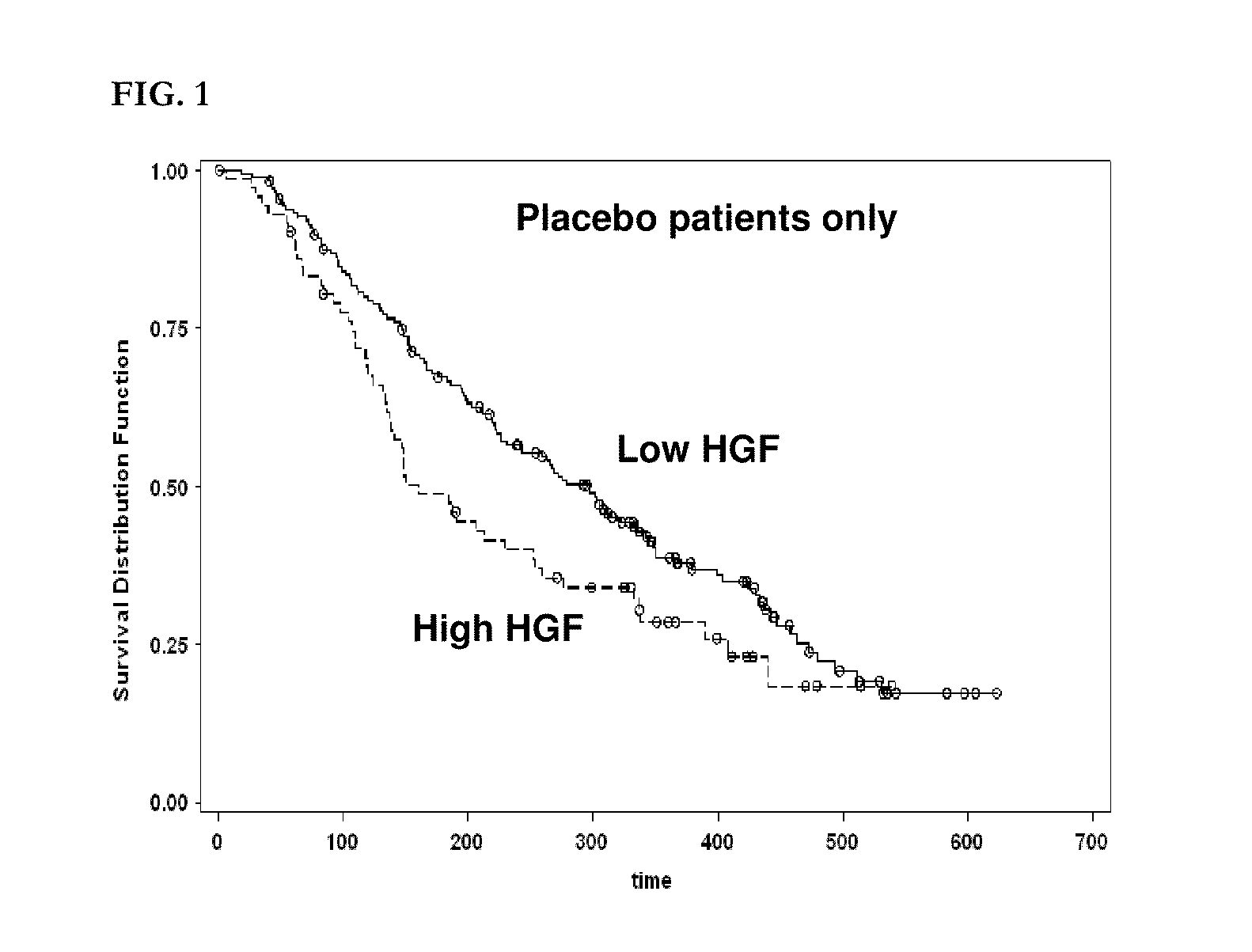
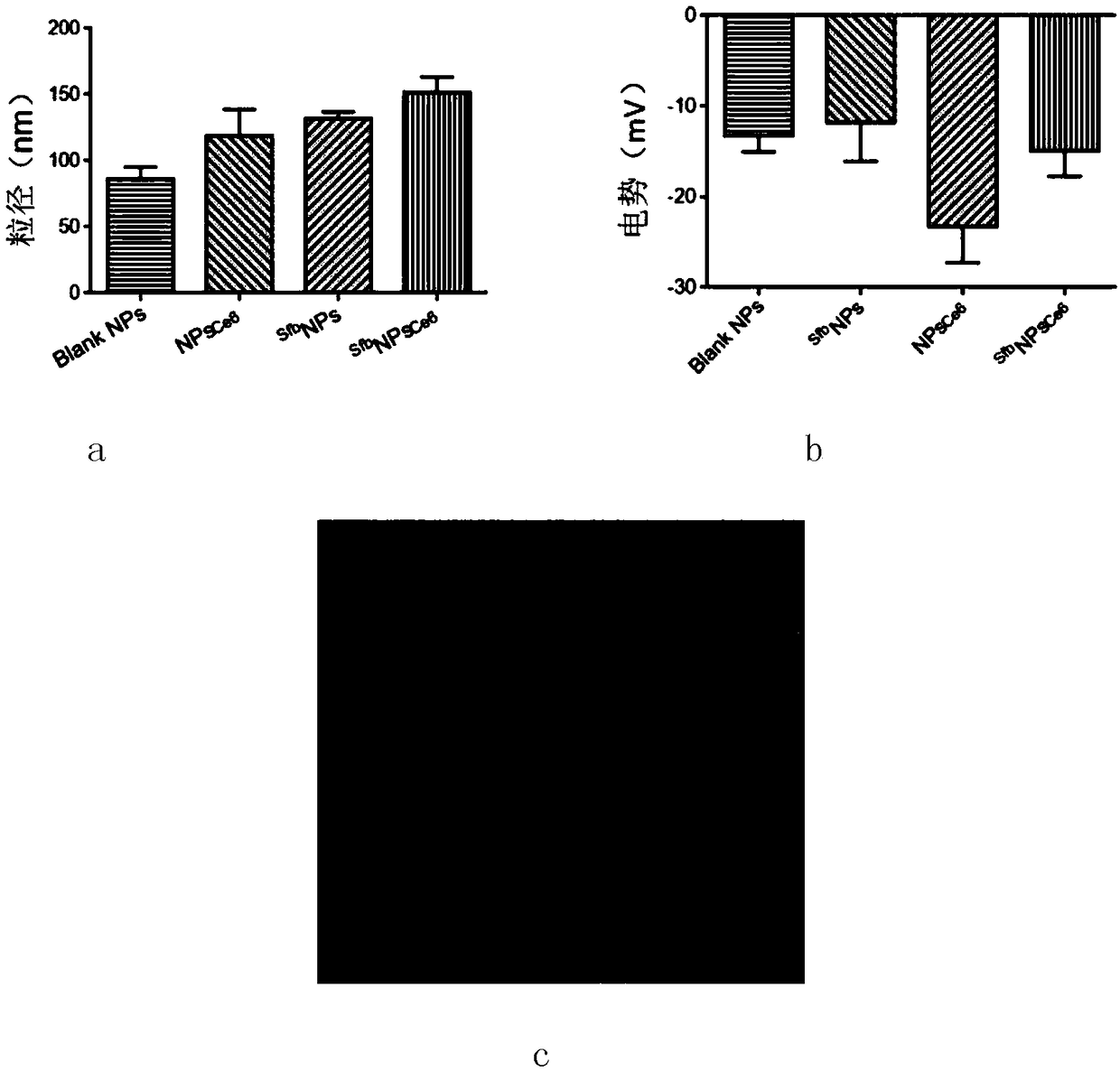
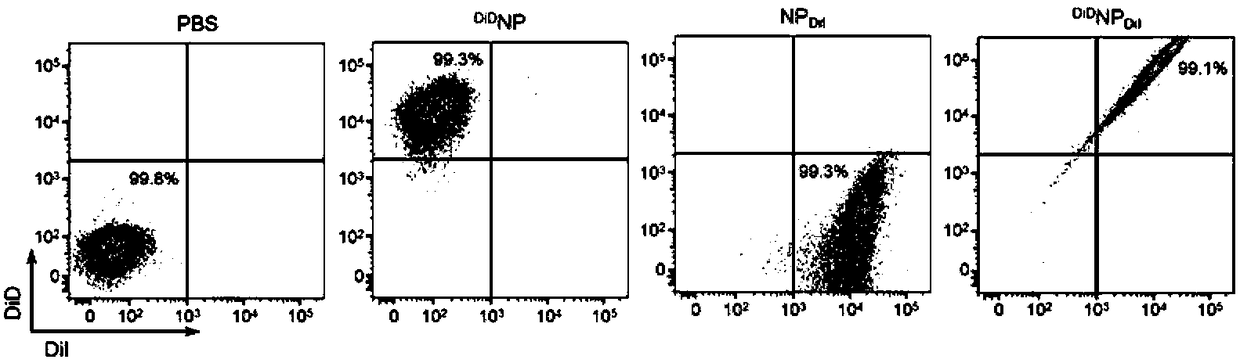
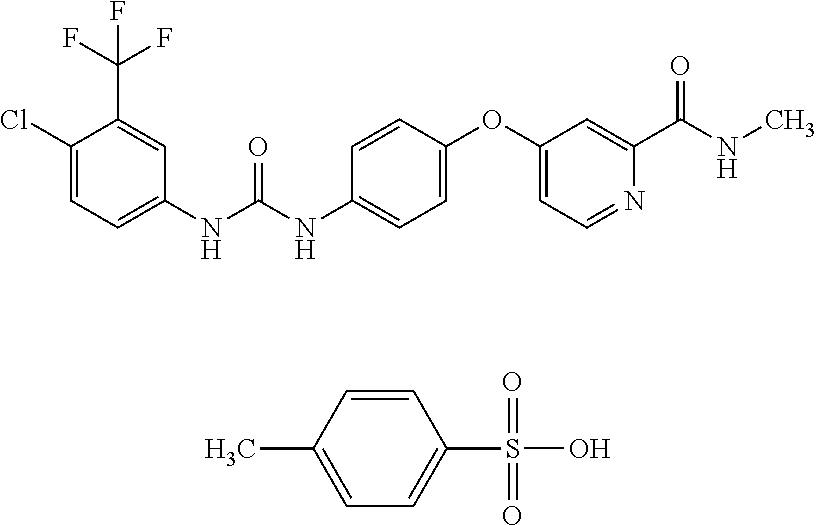
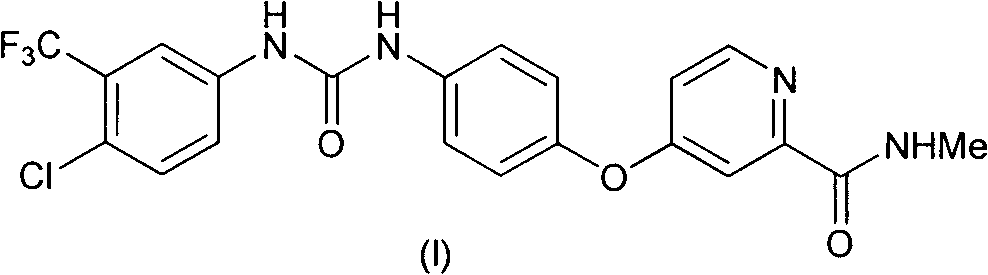
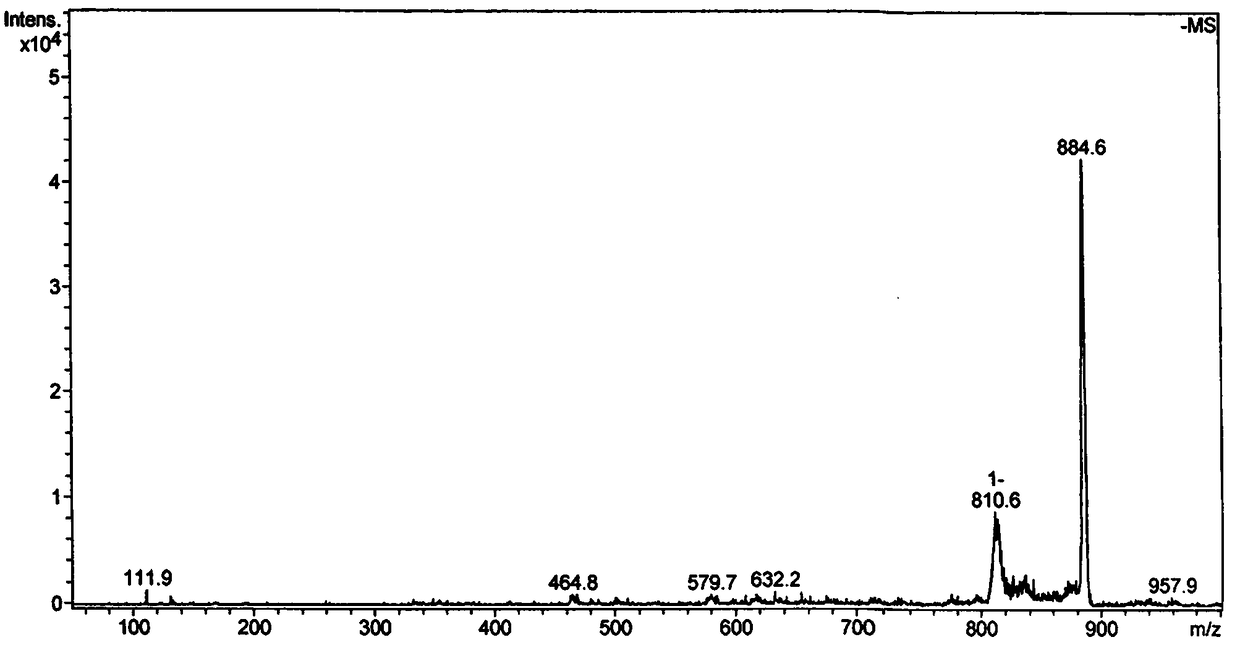
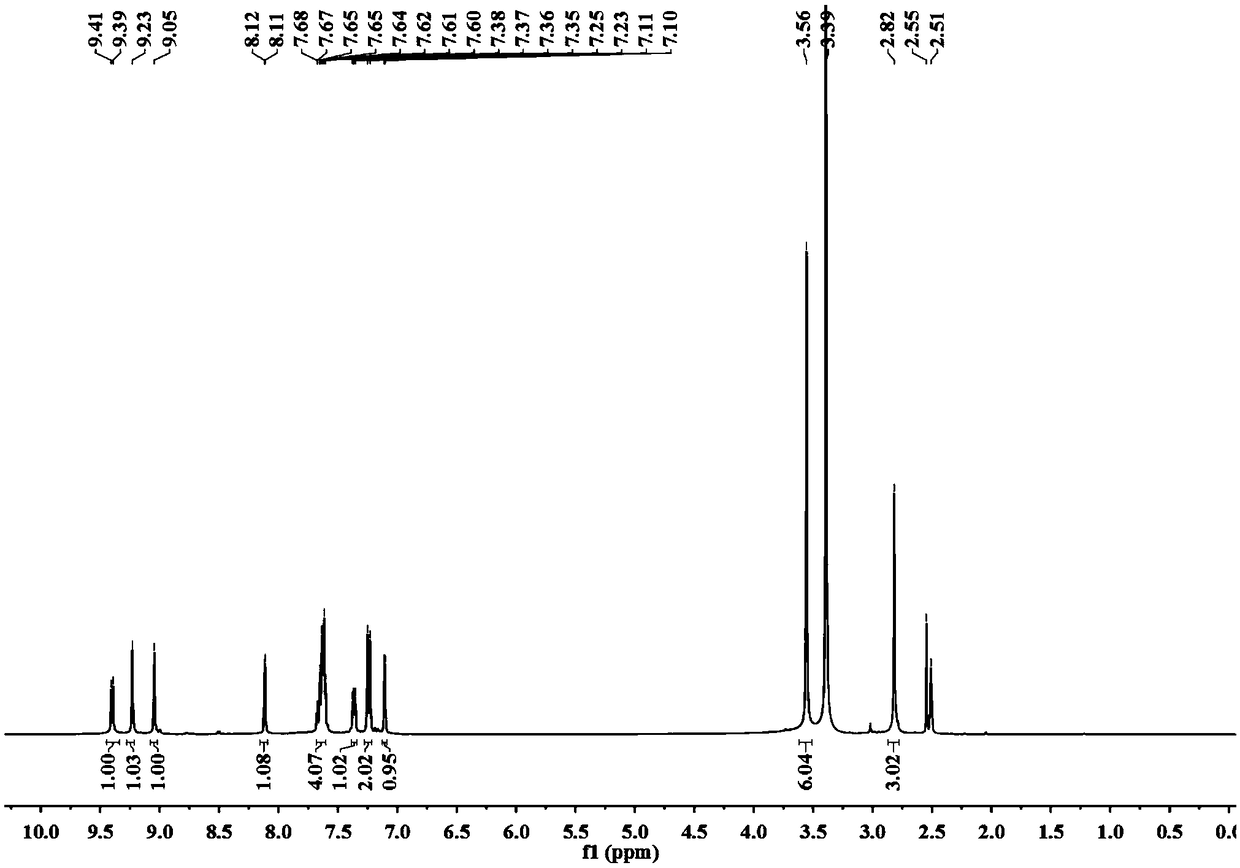
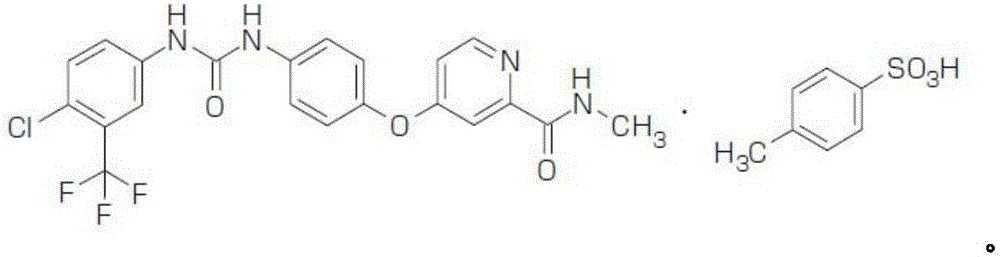
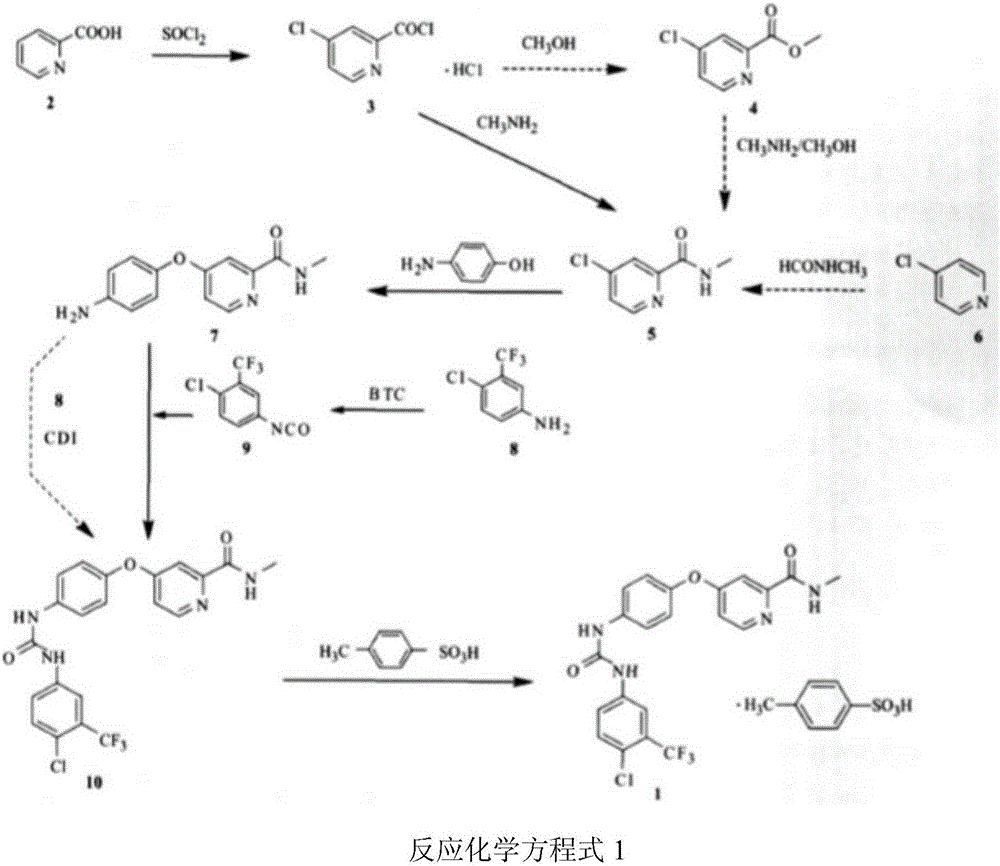
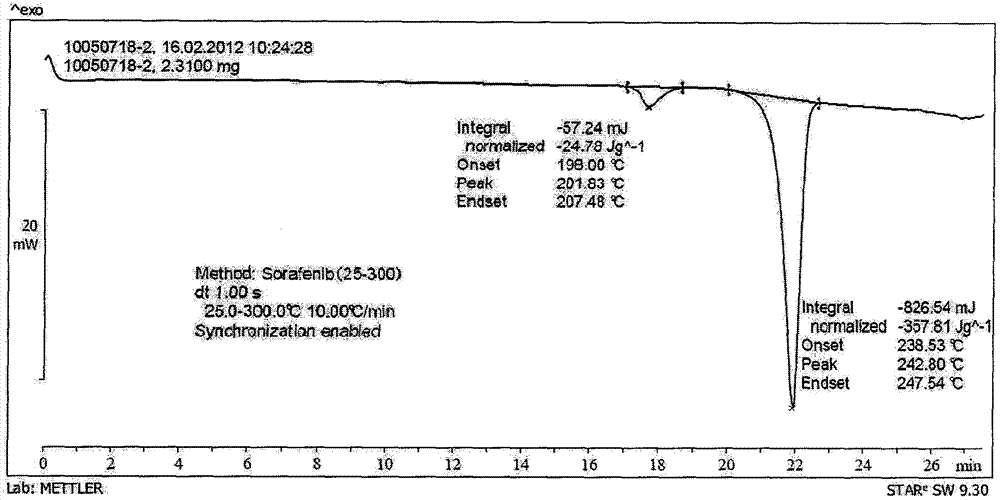
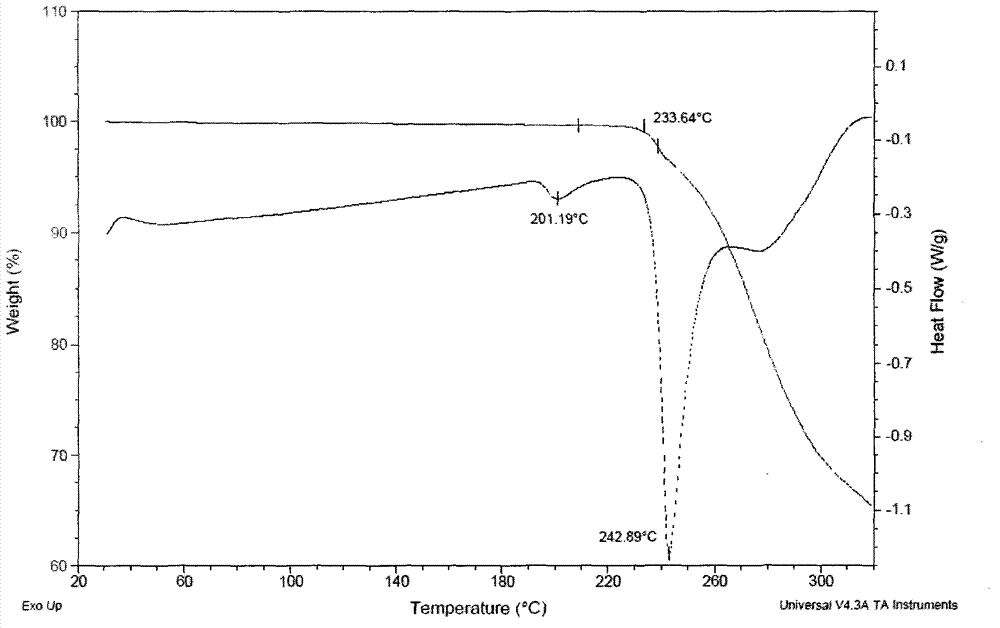
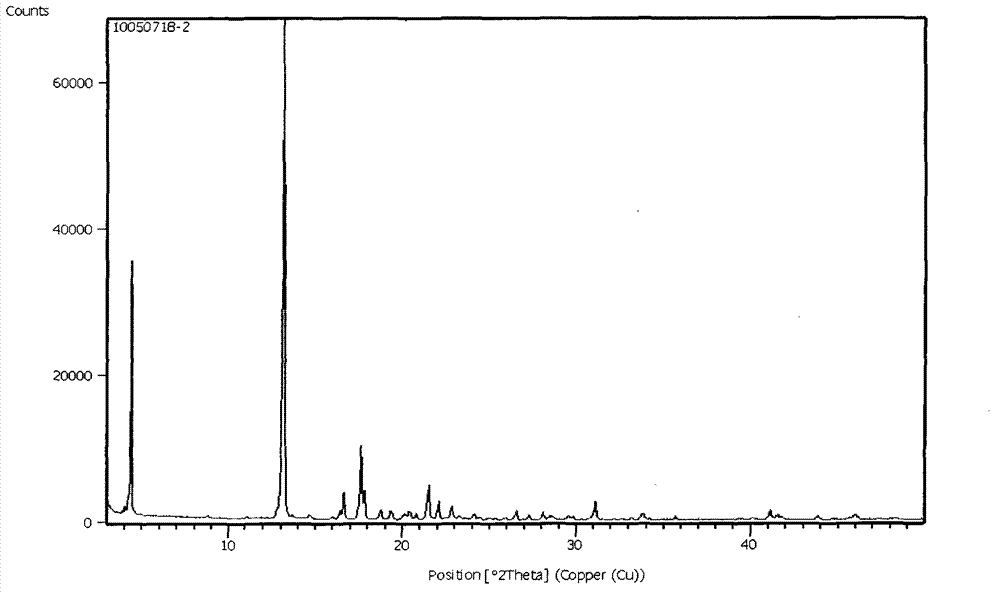
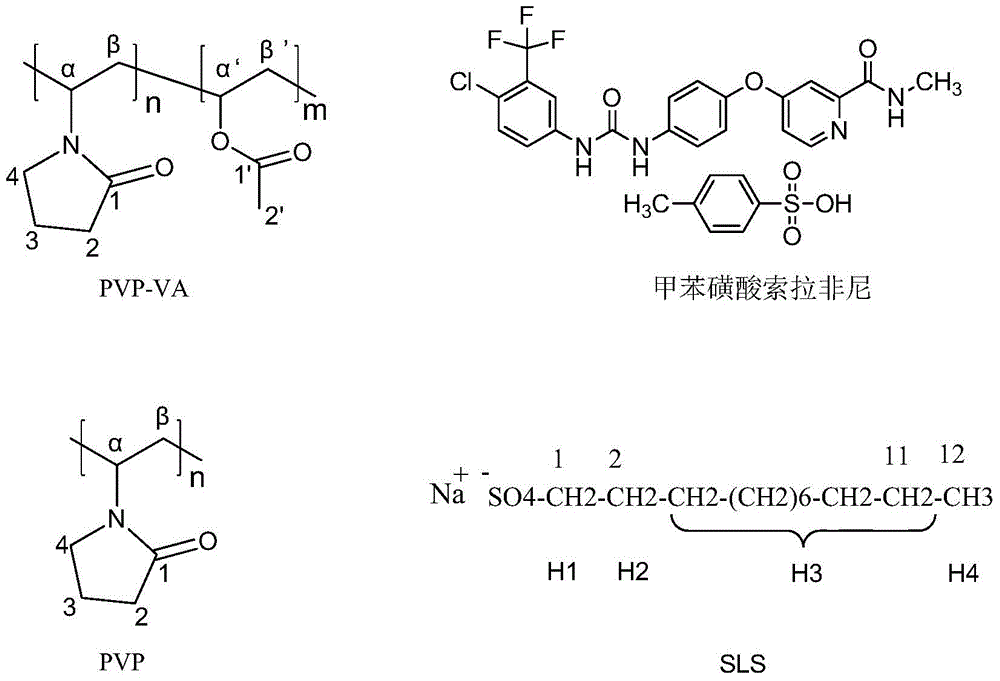
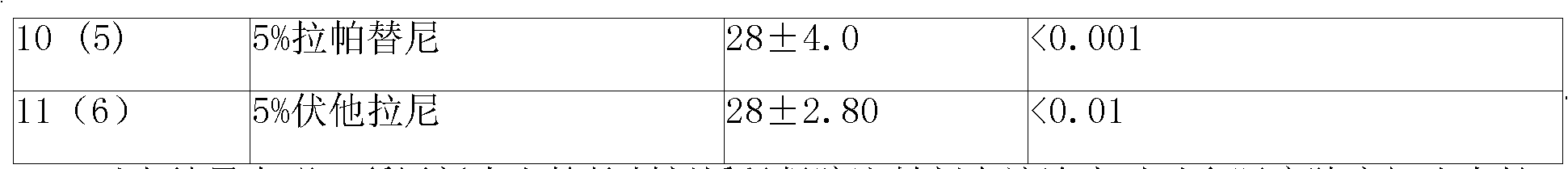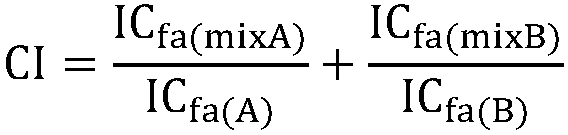Patents
Literature
358 results about "Sorafenib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
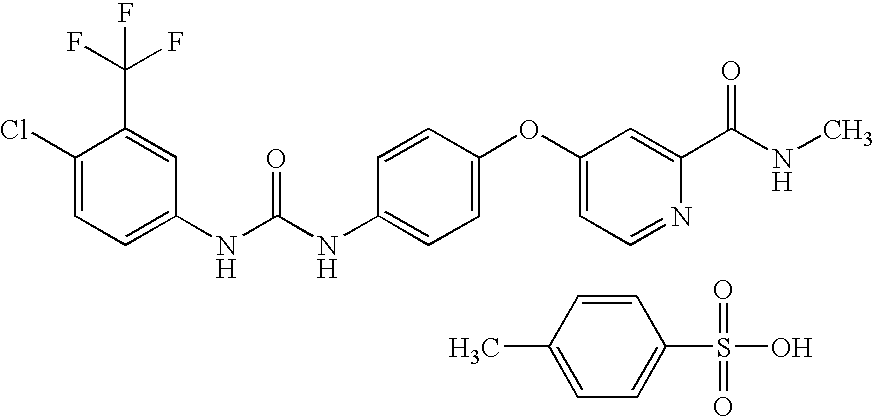
Sorafenib is used to treat kidney, liver, and thyroid cancer.
Nanoparticulate sorafenib formulations
InactiveUS20080213374A1Improve bioavailabilityHigh dissolution rateBiocidePowder deliveryDiseaseBioavailability
The present invention is directed to compositions comprising a nanoparticulate sorafenib, or a salt, such as a sorafenib tosylate, or derivative thereof, having improved bioavailability. The nanoparticulate sorafenib particles of the composition have an effective average particle size of less than about 2000 nm and are useful in the treatment of cancer, renal cancer, and related diseases.
Owner:ALKERMES PHARMA IRELAND LTD
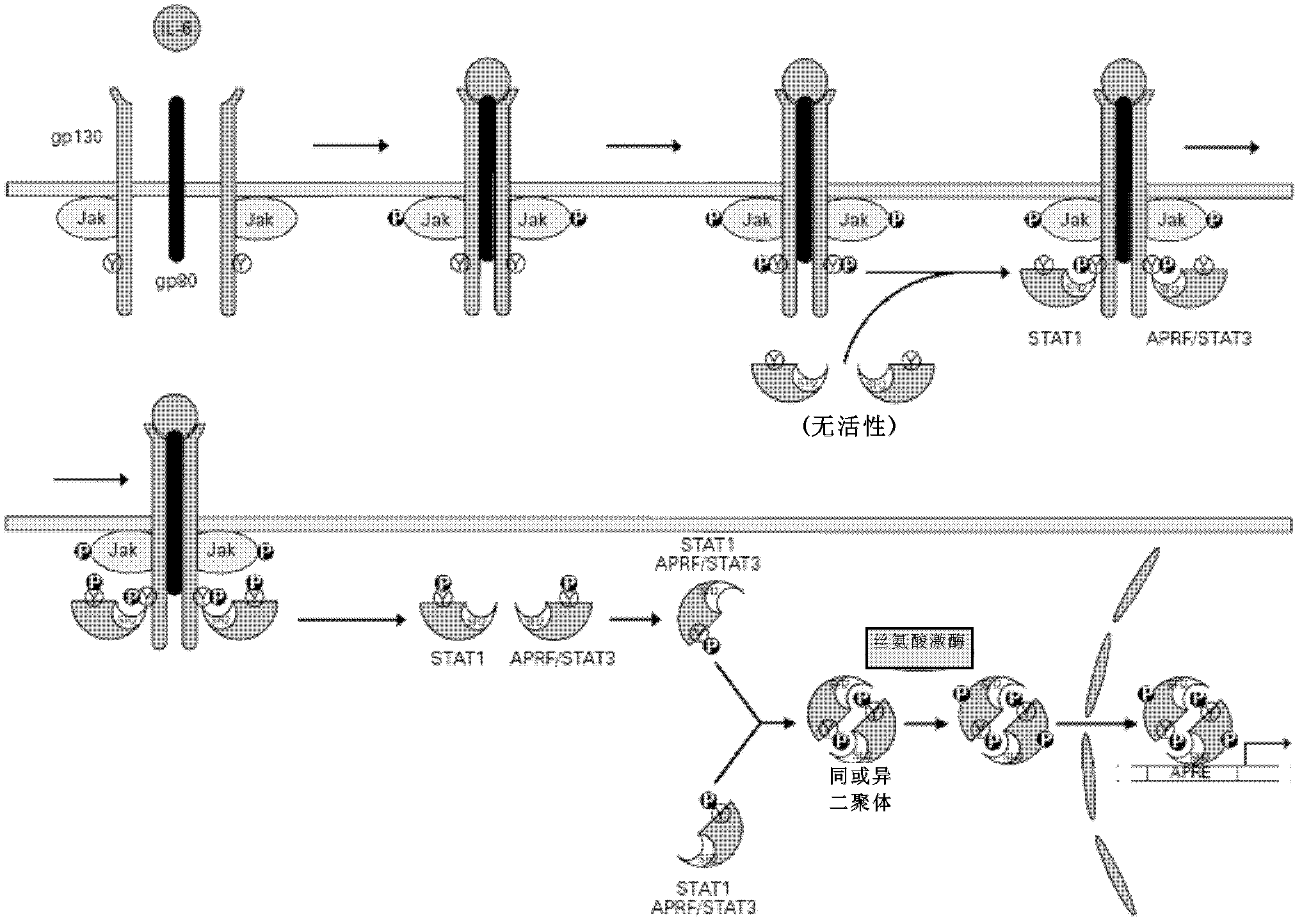
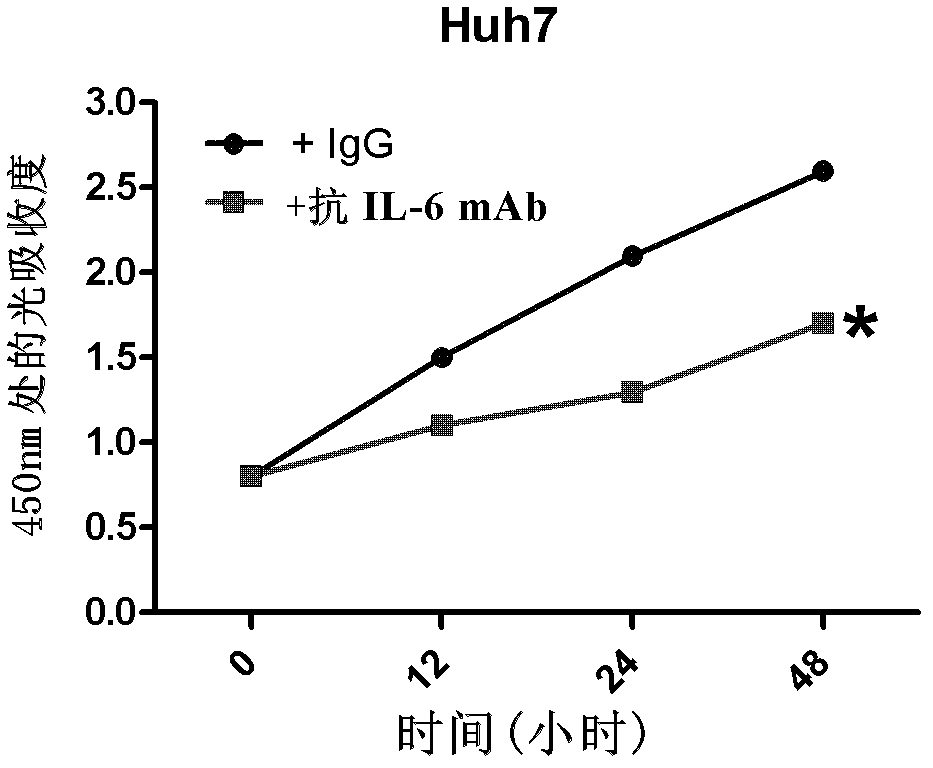
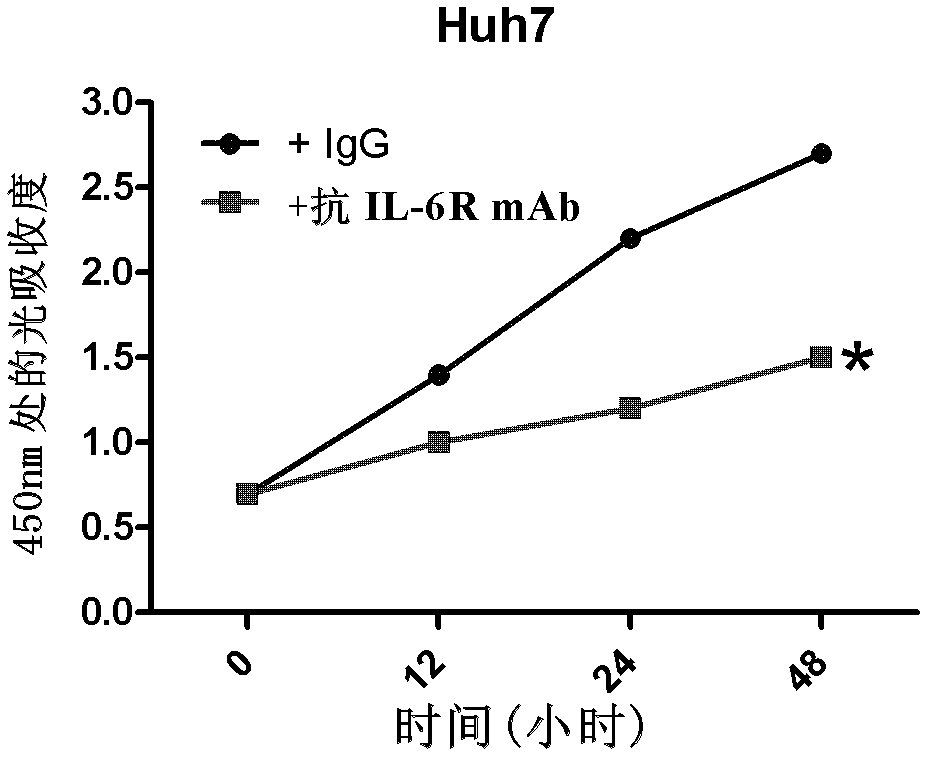
Application of antagonizing and/or blocking IL-6/IL-6R/gp130 signaling pathway in treatment of anti-hepatoma
The invention relates to an application of antagonizing and / or blocking IL-6 / IL-6R / gp130 signaling pathway in treatment of anti-hepatoma, and specifically relates to an application of an antagonist and / or a blocking agent of the IL-6 / IL-6R / gp130 signaling pathway and conventional liver cancer treatment drugs (such as cisplatin, doxorubicin and sorafenib) in preparation of anti-hepatoma drug compositions. The composition provided by the invention has significant curative effect for the liver cancers and has the advantages of small toxic and side effects, capability for synergizing with the conventional liver cancer treatment drugs and increasing the effect of the conventional anti-hepatoma treatment, and the like.
Owner:SHANGHAIMED
Anticancer sustained-release gel injection
InactiveCN101336890APharmaceutical delivery mechanismPharmaceutical non-active ingredientsLestaurtinibSolvent
The invention relates to an anticancer slow-release gel injection which contains slow-release microspheres containing an angiogenesis inhibitor, an amphiphilic block polymer, a solvent and a slow-release agent, wherein the composition of the amphiphilic block polymer and the non-organic solvent exhibit the property of temperature-sensitive gelation. After the in vivo injection, the injection turns into a stagnant and biodegradable water-insoluble gel and the gel slowly releases the drug contained therein for a plurality of weeks to a plurality of months. After the intratumoral or peritumoral injection, the anticancer slow-release gel injection can significantly reduce the general drug reactions and is used for treating tumors of different stages. The angiogenesis inhibitor is selected from SU5416, SU6668, bosutinib, sprycel, erlotinib, vandetanib, gefitnib, canertinib, lapatinib, lestaurtinib, masitinib, vatalanib, mubritinib, tandutinib, nilotinib, marimastat, nilotinib, pelitinib, telatinib, sunitinib, sorafenib, zarnestra, sirolimus, imatinfb, lenalidomide and thalidomide.
Owner:济南基福医药科技有限公司
Identification of signature genes associated with hepatocellular carcinoma
InactiveUS20110257035A1Lower levelImprove survivalPeptide librariesNucleotide librariesRegimenTumor Biomarkers
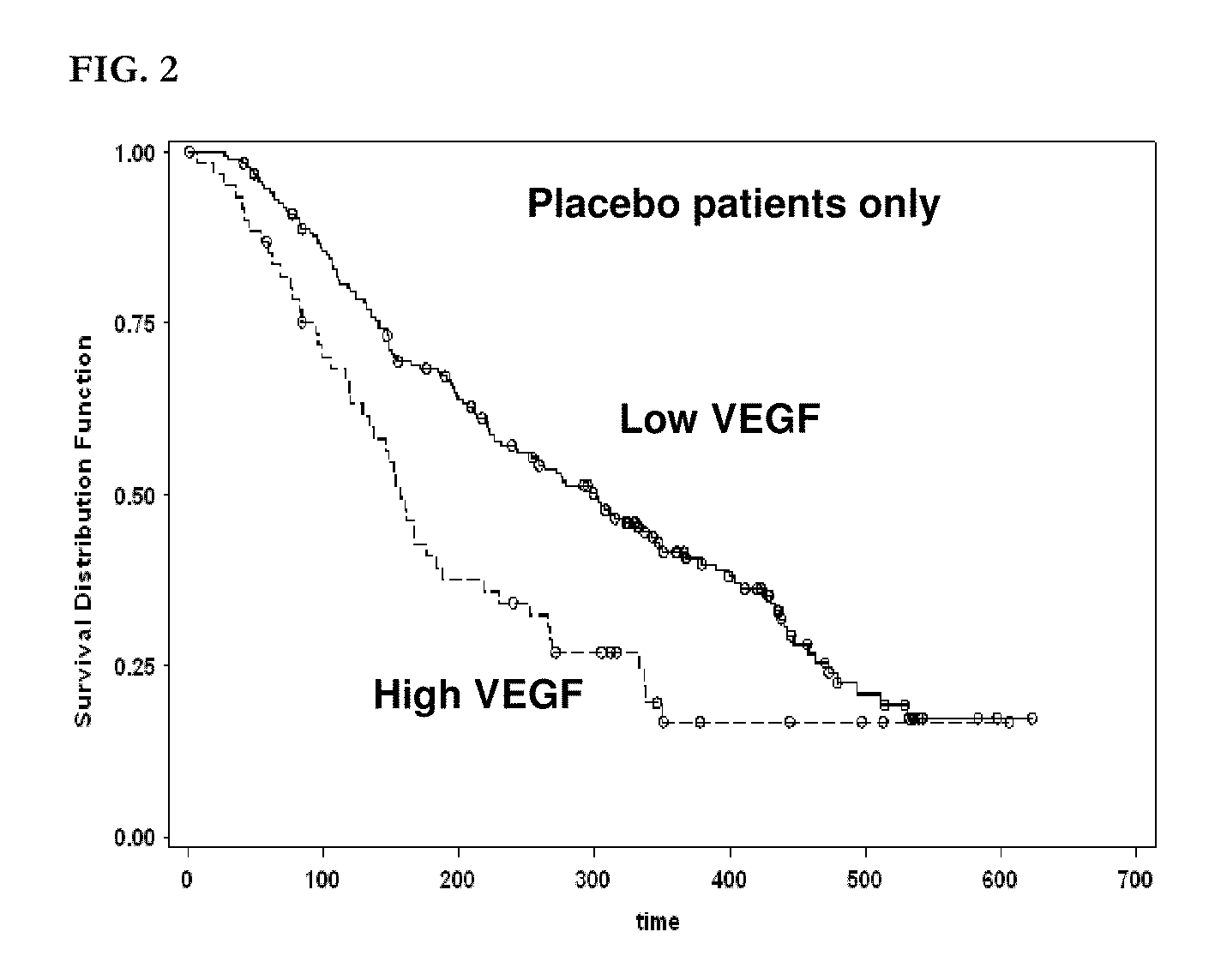
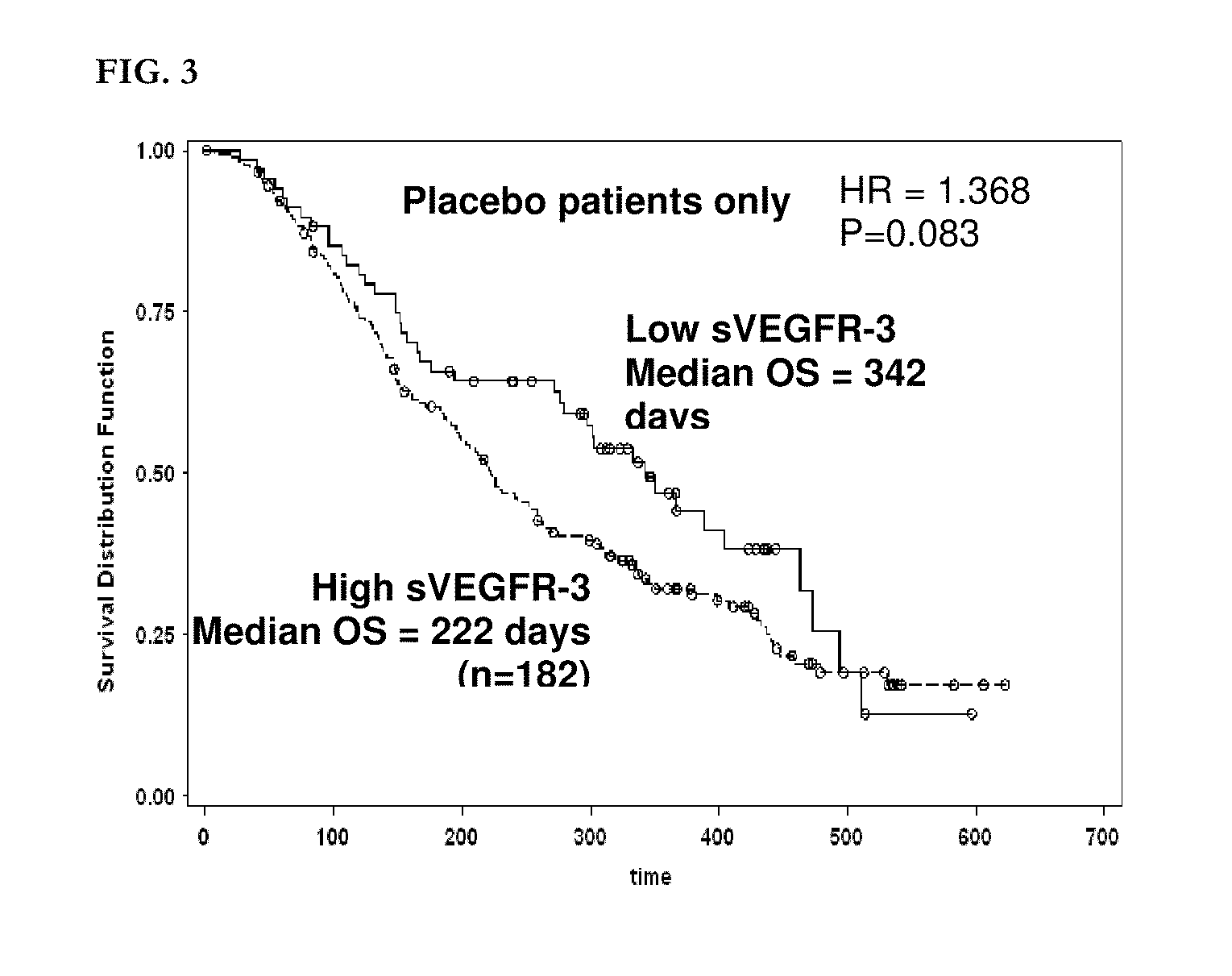
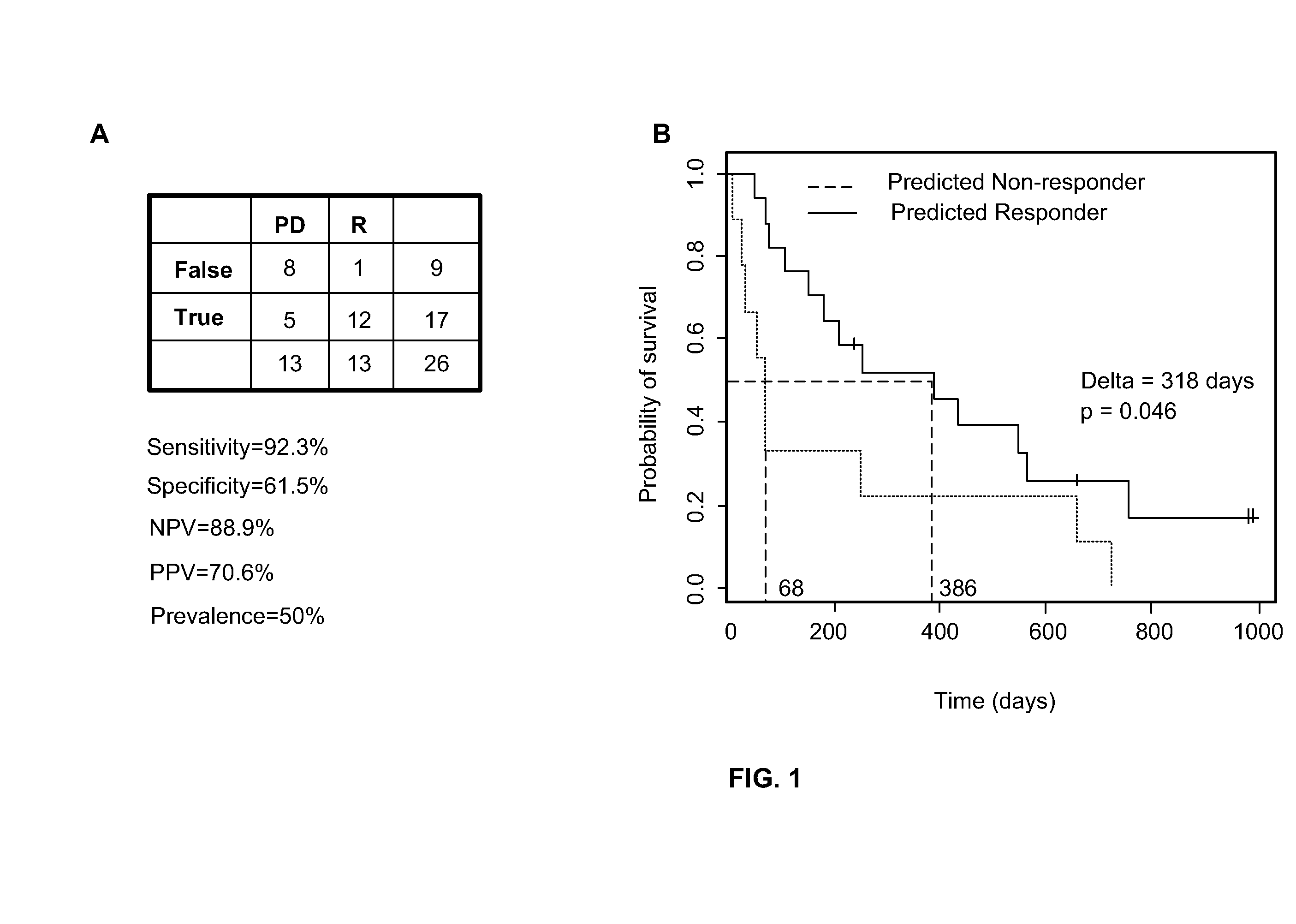
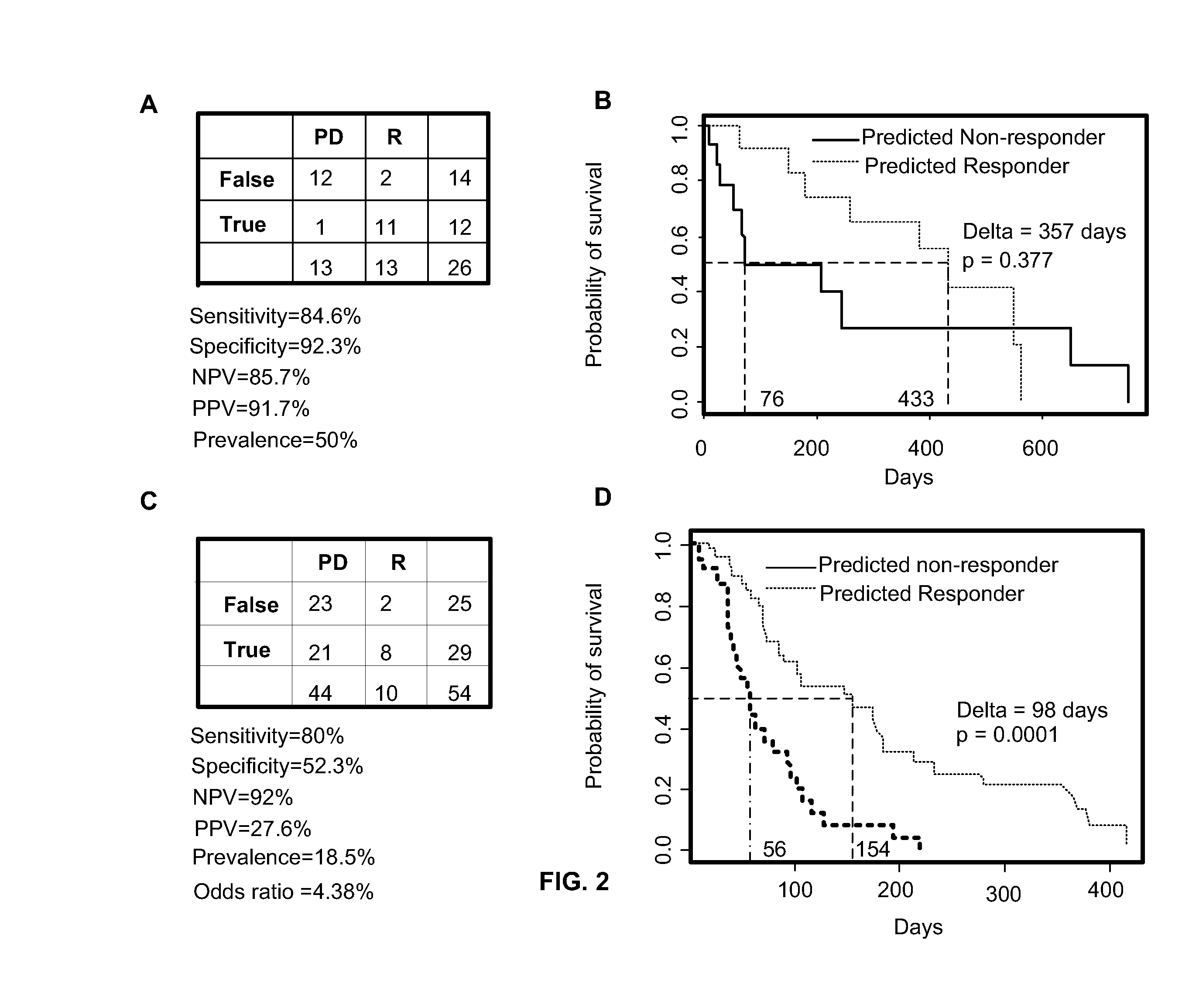
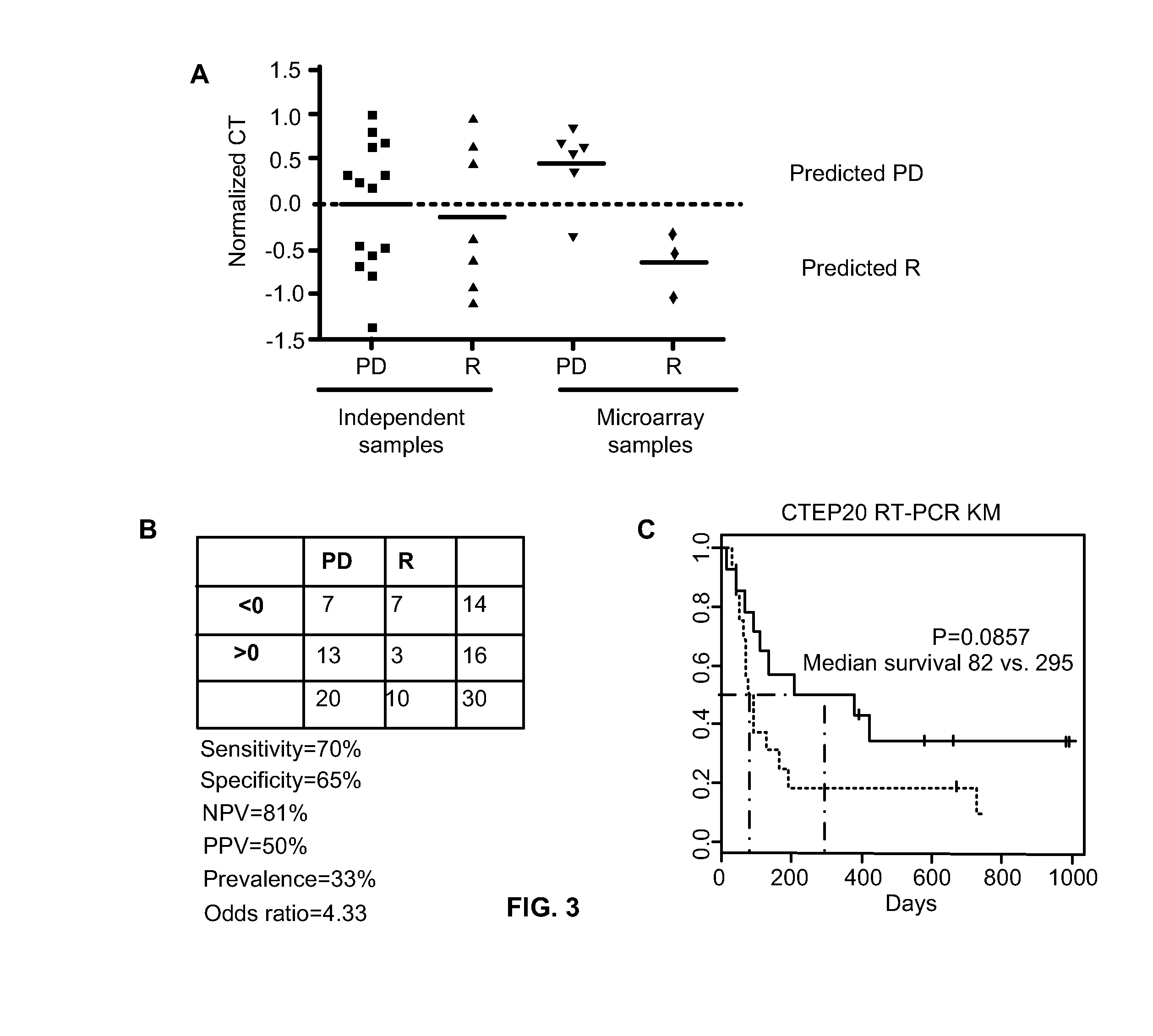
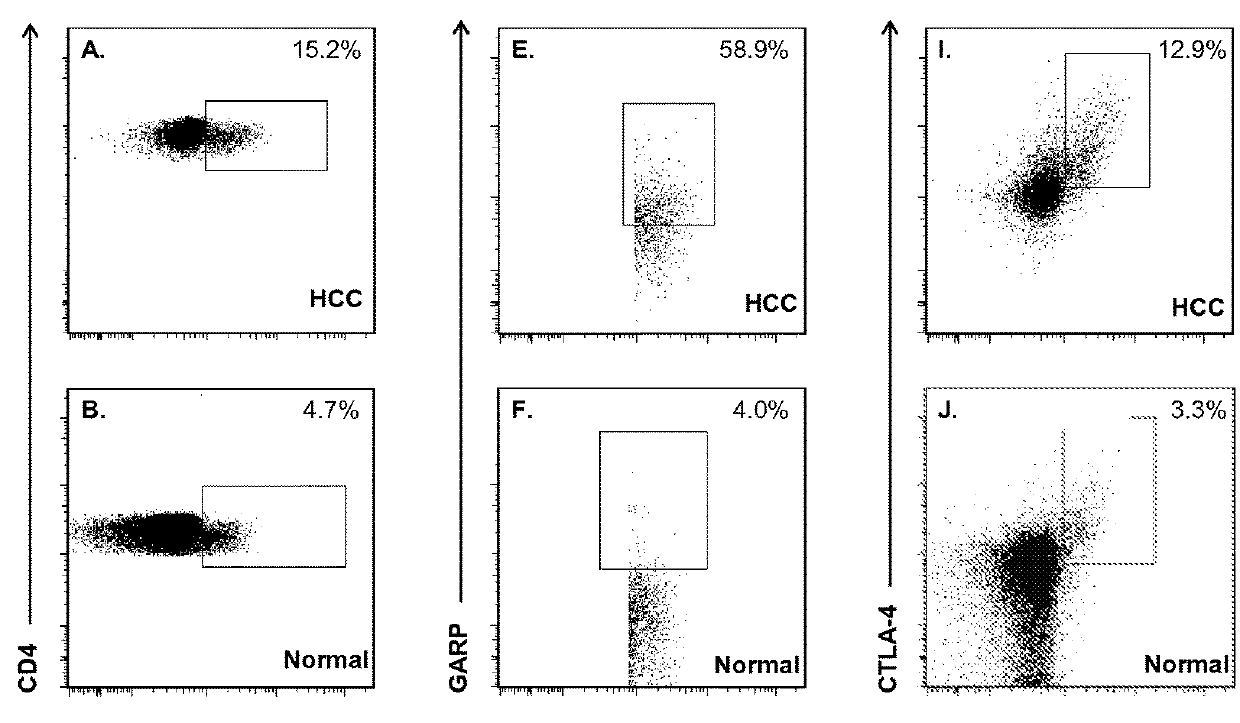
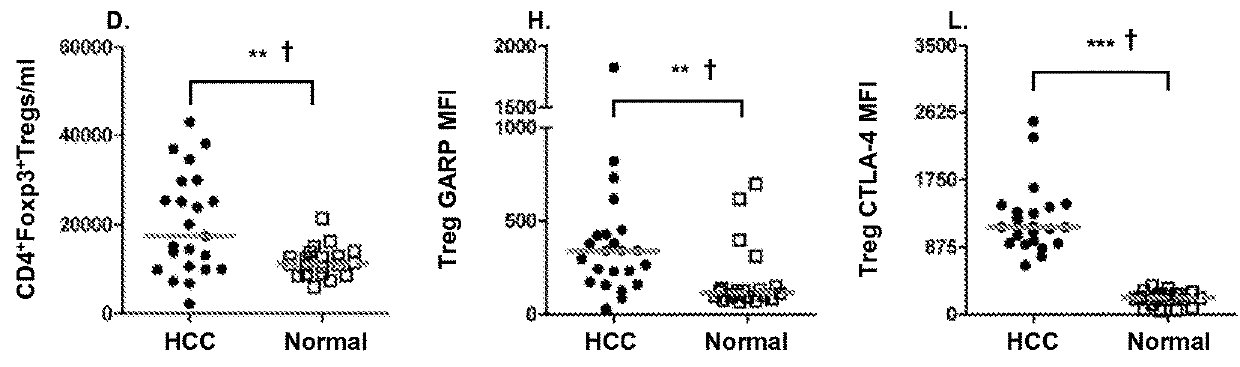
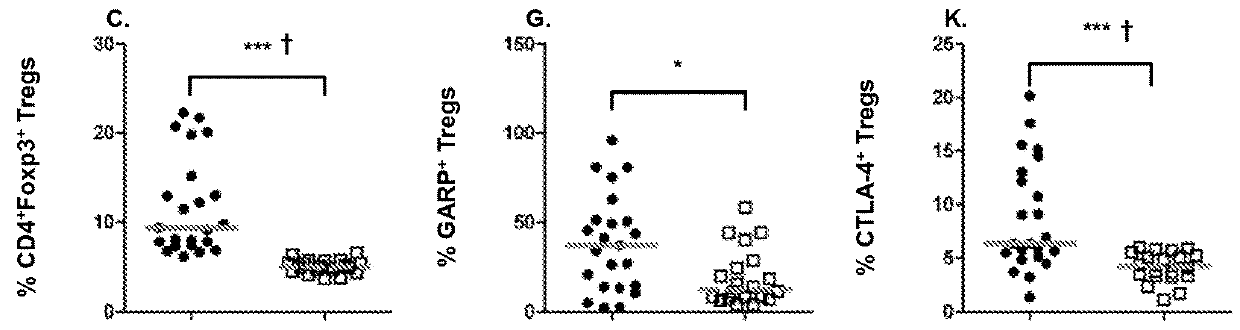
The present invention relates to, for example, (1) a novel method for identification of clinically useful serum and / or tumor biomarkers and expression signatures that can be used for detection, prognostication and guidance for the treatment of patients with hepatocellular carcinoma (HCC); and (2) discovery of an expression signature that can be used to monitor and / or study the efficacy of a chemotherapeutic regimen, such as, for example, sorafenib (solely or in combination with other agents). The present invention also provides a method for predicting clinical outcomes, such as, for example, overall survival (OS), time to progression (TTP) and / or likelihood of benefitting from a chemotherapeutic treatment (i.e., sorafenib) in HCC patients based on the analysis of such biomarkers.
Owner:BAYER HEALTHCARE LLC
Liver targeted medicine
InactiveCN107929273AImproved Liver TargetingGood curative effectDigestive systemPharmaceutical non-active ingredientsDiseaseCitrulline
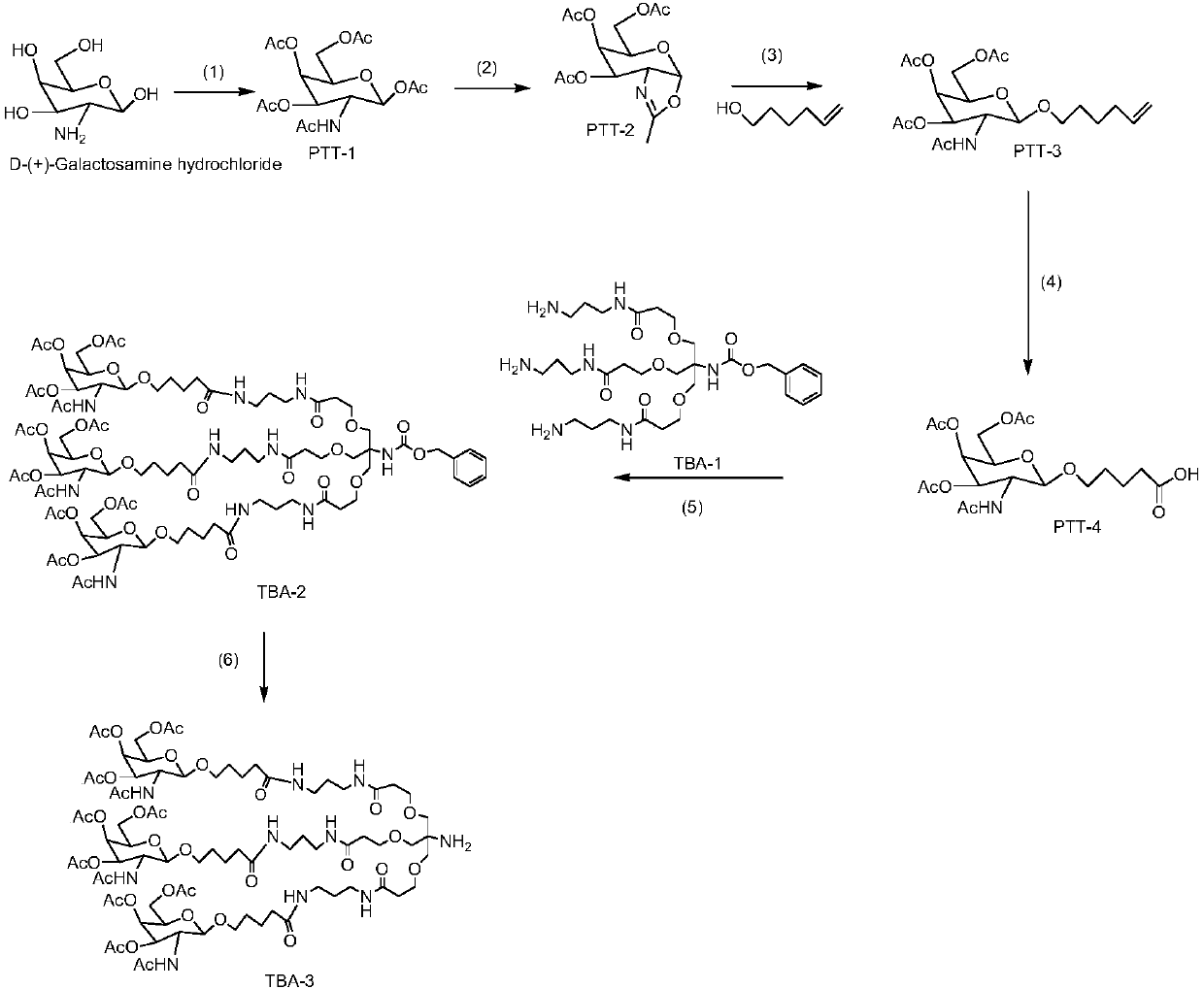
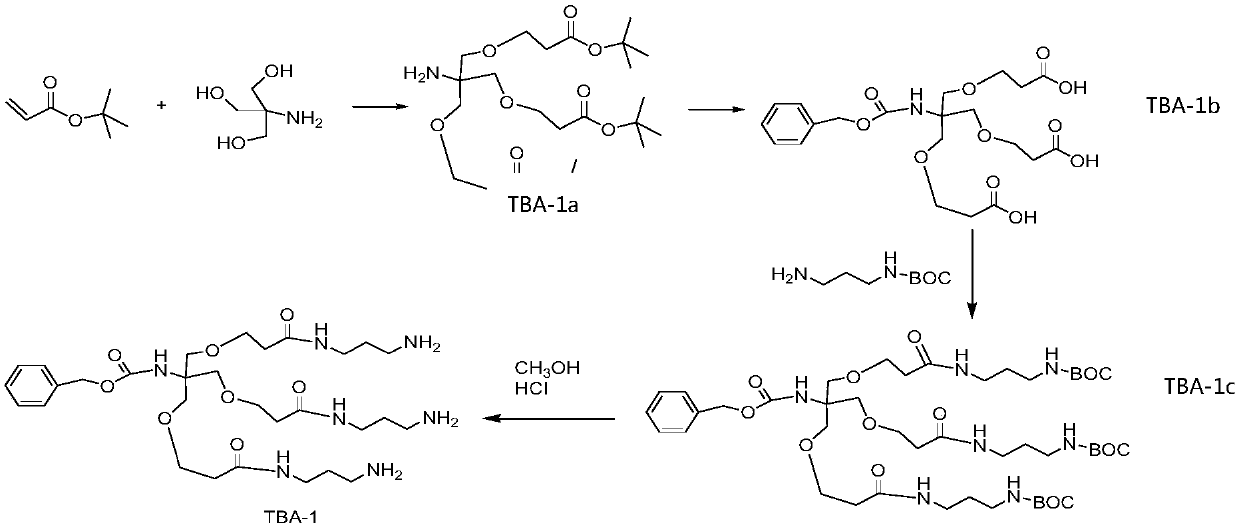
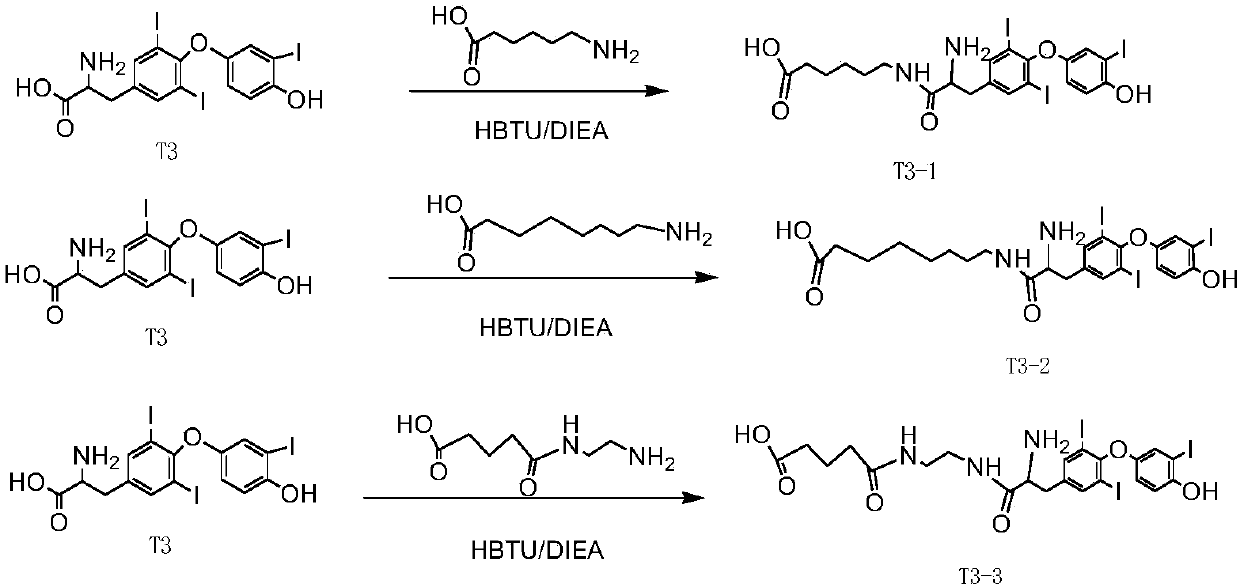
The invention relates to the field of biological medicine, and in particular relates to liver targeted medicine. The medicine is chemical micromolecular medicine connecting galactosamine. The chemicalmicromolecular medicine is medicine for treating liver diseases or liver-related diseases. The chemical micromolecular medicine is prepared from but not limited to thyroxine T3, sorafenib, taxol, regorafenib, lamivudine, entecavir, telbivudine, statins, fibrates, niacin, bile acid sequestrants, other hepatitis virus DNA (RNA) polymerase inhibition compounds and the like. The galactosamine is tervalent acetylgalactosamine. The connection is the direct connection of the galactosamine and the chemical micromolecular medicine or the connection through linking fragments; the linking fragments comprise but not limited to carbon chains, disulfide bonds, pyrophosphate diester, cysteic acid, polypeptide and thioether or valine-citrulline. The medicine provided by the invention has the advantages that the liver targeted performance is improved; the medicine curative effect is enhanced; the toxic and side reactions on other non-targeted tissues are few.
Owner:崔坤元
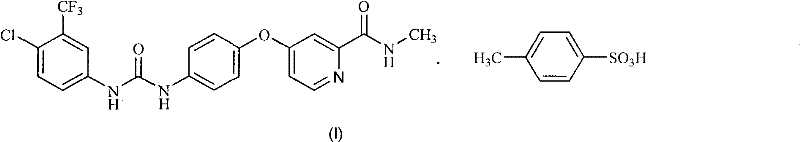
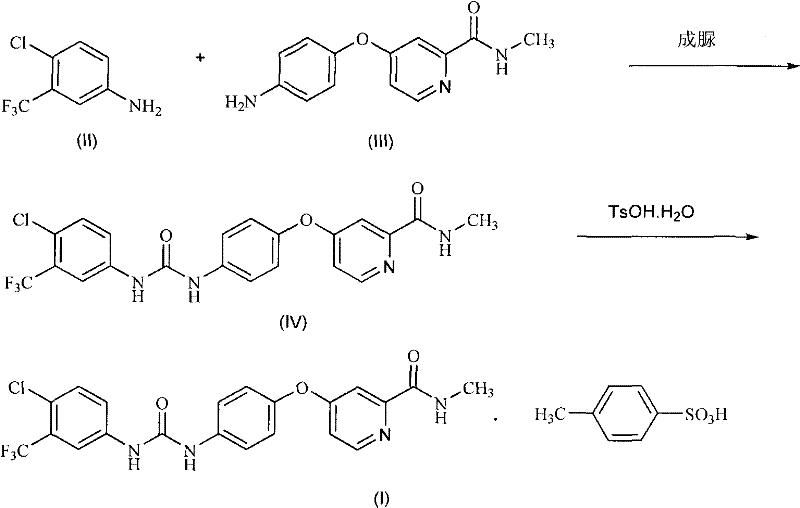
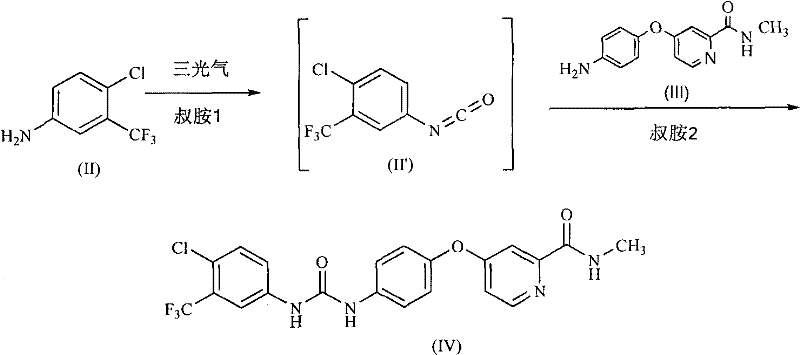
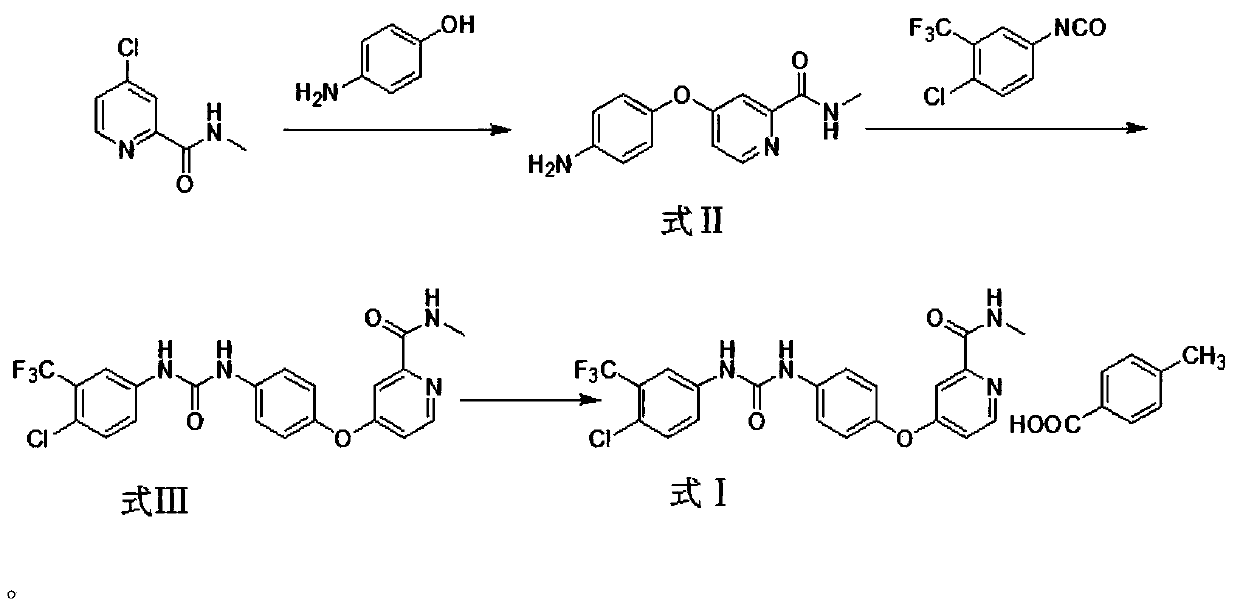
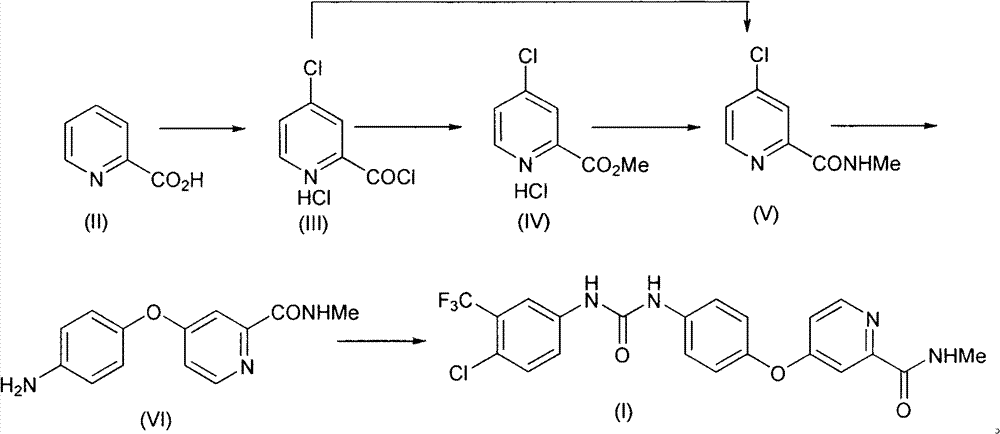
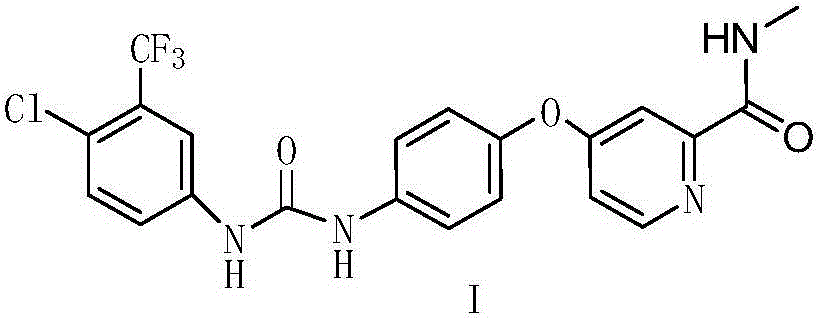
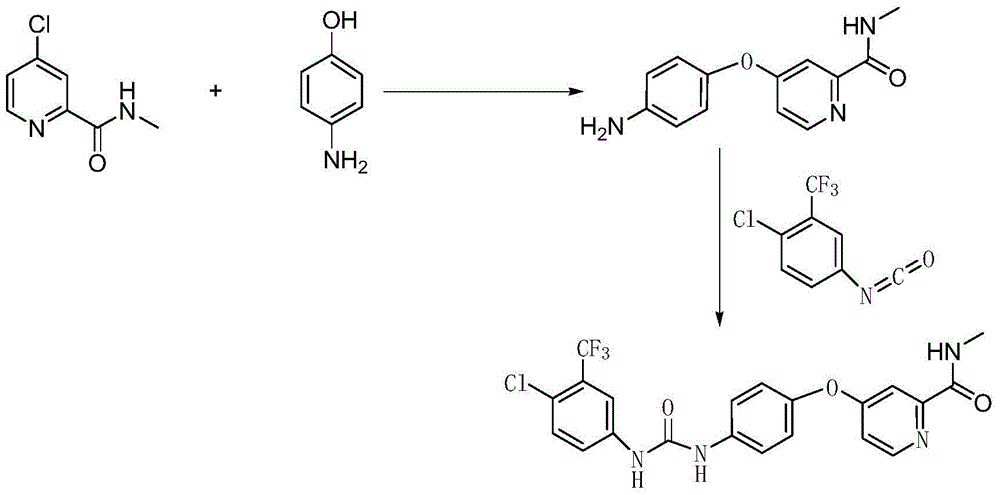
Method for preparing sorafenib
The invention discloses a method for preparing sorafenib, which comprises the steps of: 1) reacting triphosgene with 3-trimethyl fluoride-4-chloroaniline (II) in an inert solvent 1 under the existence of tertiary amine 1, thus obtaining a solution containing 3-trimethyl fluoride-4-chlorphenyl isocyanate (II'), wherein isolation and purification are not needed; and 2) reacting the 3-trimethyl fluoride-4-chlorphenyl isocyanate (II') with 4-(4-aminophenoxy)-N-methyl-2-pyridine carbonxamide (III) in an inert solvent 2 under the existence of tertiary amine 2, thus obtaining sorafenib (IV). The tertiary amine 1 and the tertiary amine 2 are the same or different, the inert solvent 1 and the inert solvent 2 are the same or different, and the feeding sequence of the 3-trimethyl fluoride-4-chloroaniline (II) and the 4-(4-aminophenoxy)-N-methyl-2-pyridine carbonxamide (III) can be interchangeable. The method is simple and convenient to operate, short for reaction time free from separation of intermediates with high reactivity, as well as is free from special equipment and conditions, and high in yield.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
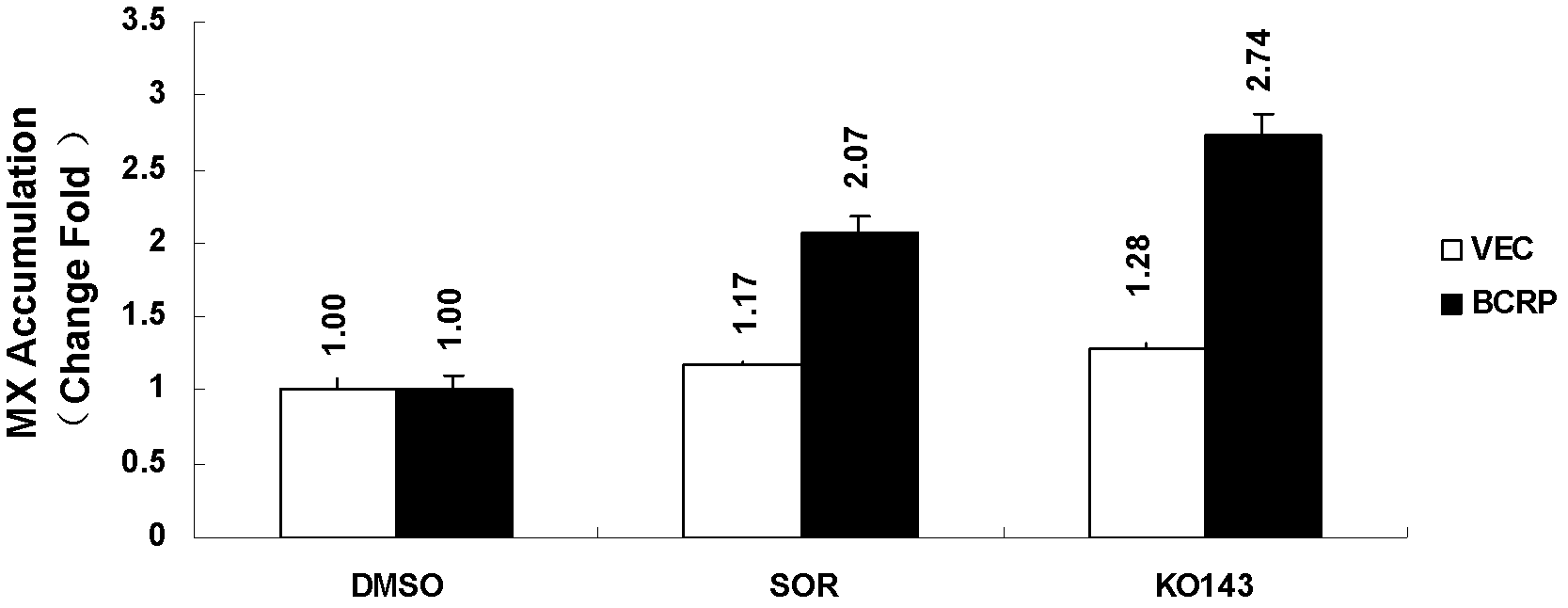
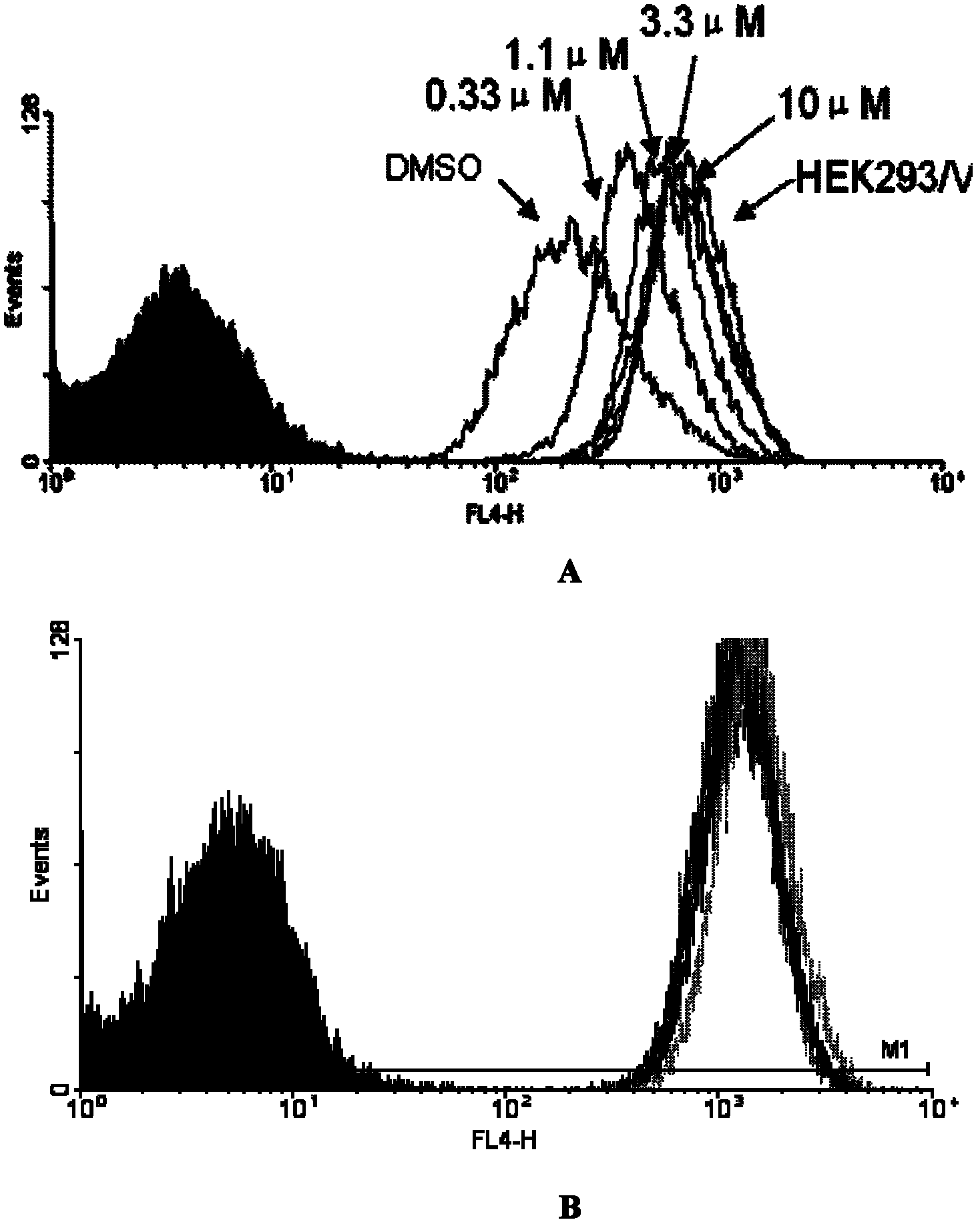
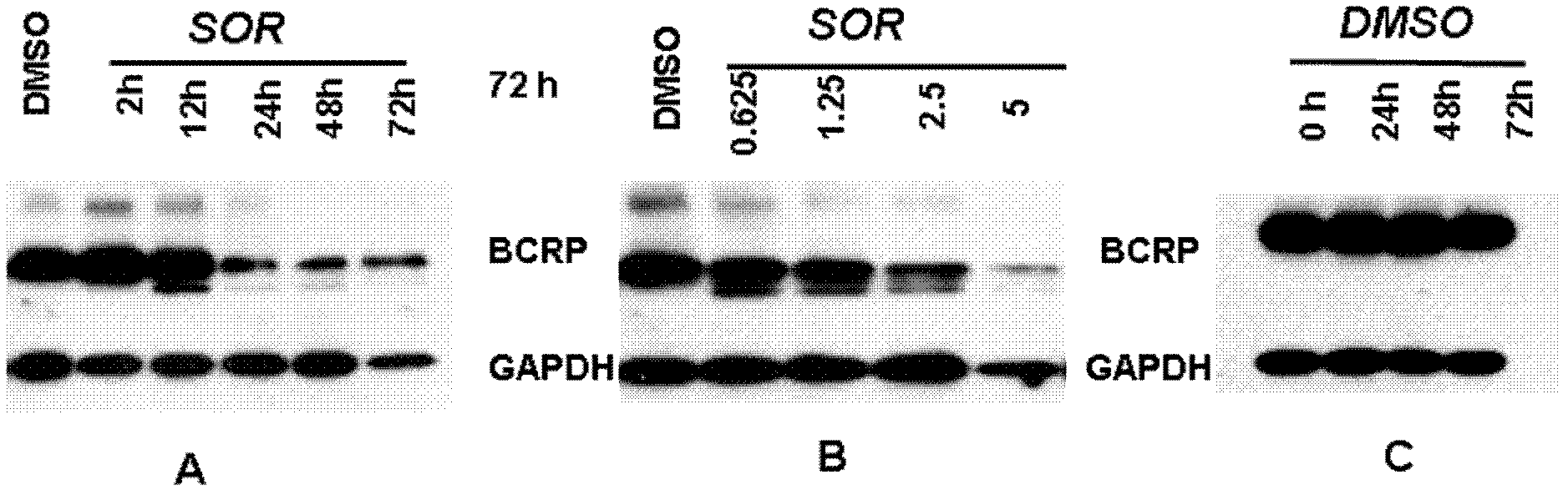
Application of sorafenib in the preparation of drugs for reversing tumor multidrug resistance
InactiveCN102274220APromote accumulationRestore sensitivityAntineoplastic agentsHeterocyclic compound active ingredientsMedicineChemotherapy resistant
The invention belongs to the production technology for antitumor drugs, and discloses uses of sorafenib and sorafenib salts in preparing drugs for reversing BCRP protein-mediated multidrug resistance of tumors. According to the present invention, the sorafenib and the sorafenib salts are combined with antitumor drugs, such that the sensitivity of multidrug resistant tumor cells to the antitumor drugs are recovered; the prepared drugs are applicable for providing combination chemotherapy for the clinical chemotherapy resistant patients or preventing the drug resistance of the tumor cells from generation.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
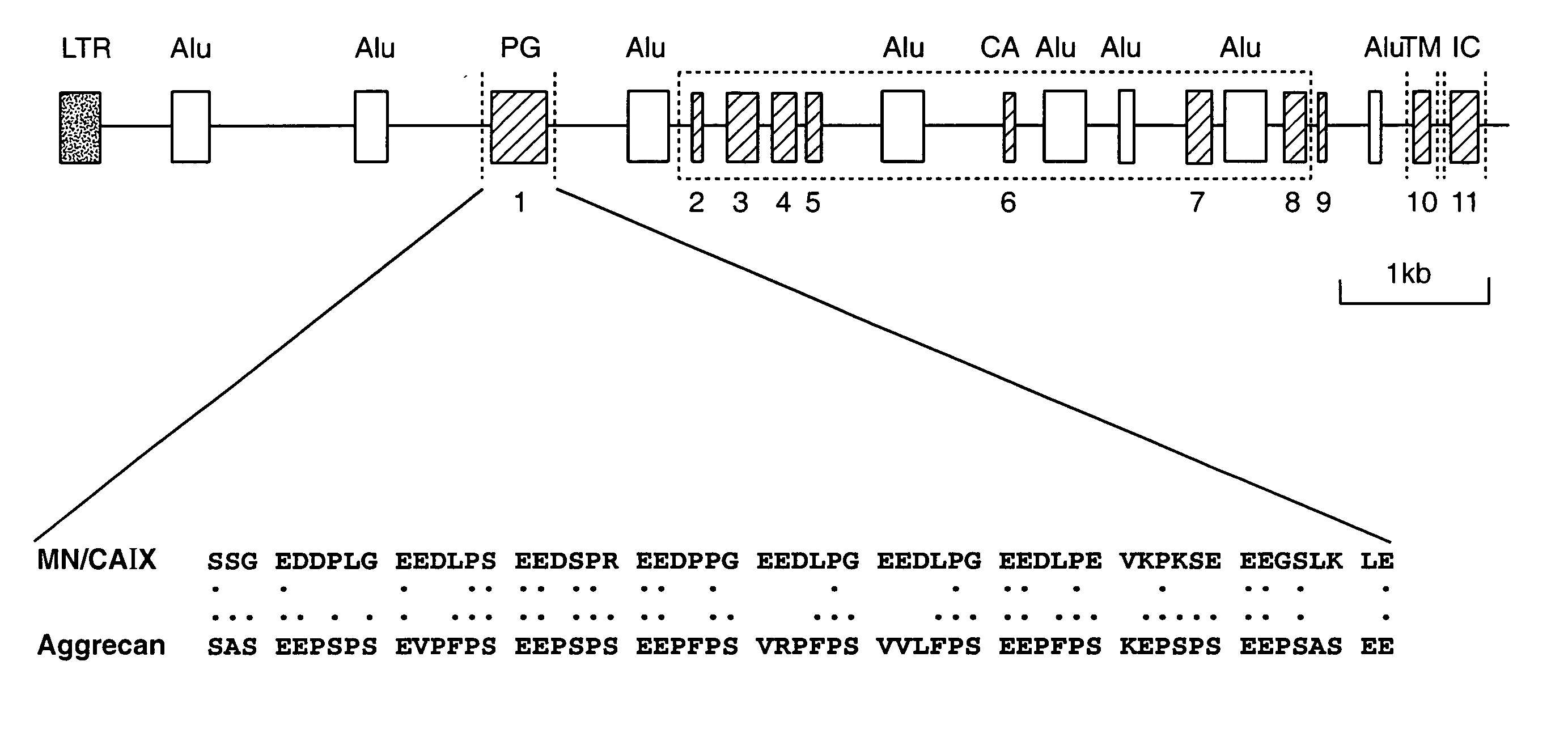
MN/CA IX and MAPK inhibition
InactiveUS20080038251A1High expressionIncrease cell densityBiocidePeptide/protein ingredientsDiseaseMitogen-activated protein
The invention is based upon the discovery that the mitogen-activated protein kinase (MAPK) pathway can increase CA9 expression independently of HIF-1, as well as increasing CA9 expression under HIF-1-dependent pathways initiated by hypoxia or high cell density. Disclosed herein are novel therapeutic methods for treating preneoplastic / neoplastic diseases associated with abnormal MN / CA IX expression, using MAPK pathway inhibitors. Preferably, the MAPK pathway inhibitors are raf kinase inhibitors, particularly the raf kinase inhibitor Sorafenib. Further disclosed are methods for patient therapy selection for MAPK pathway inhibitors, preferably in combination with other cancer therapies, based on detection of abnormal MN / CA9 gene expression in preneoplastic / neoplastic tissues.
Owner:INST OF VIROLOGY SLOVAK ACAD OF SCI +1
Ophthalmic pharmaceutical compositions for the treatment of neoangiogenic pathologies of the eye
The present invention relates to ophthalmic pharmaceutical compositions comprising Sorafenib, its derivatives or active metabolites, for the treatment and prevention of ocular neoangiogenic pathologies.
Owner:S I F I SPA
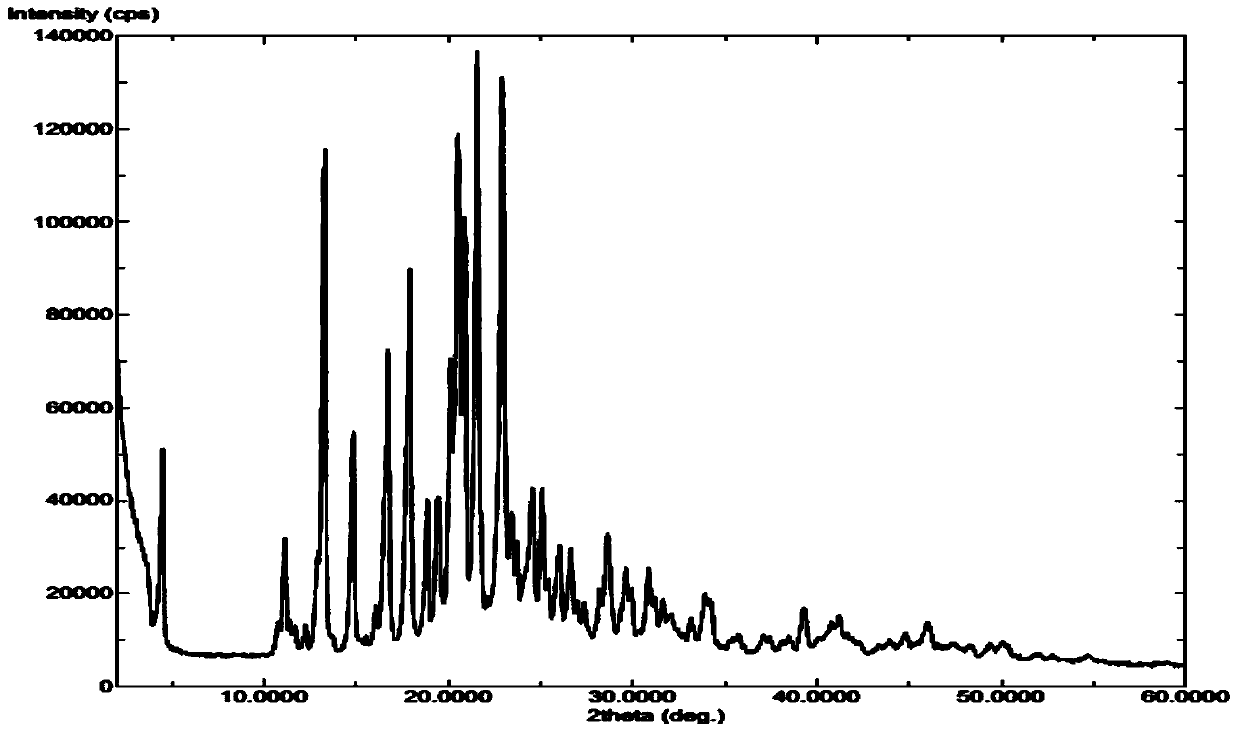
Method for industrial production of sorafenib tosylate polymorphic form I
InactiveCN104177292ASimple methodEasy to handleOrganic chemistryOrganic compound preparationTrifluorideP-Aminophenol
The invention discloses a method for industrial production of a sorafenib tosylate polymorphic substance I. The method comprises the following steps: by adopting N-methyl-4-chloro-pyridine carboxamide and p-aminophenol as raw materials, adding a proper amount of alkali to obtain an intermediate 4-(4-aminophenoxy)-N-methyl-2-pyridine carboxamide by virtue of nucleophilic substitution reaction in an organic solvent, performing condensation on 4-(4-aminophenoxy)-N-methyl-2-pyridine carboxamide and 4-chloro-3-benzenyl trifluoride isocyanate to obtain free alkali of sorafenib, finally performing back-flow and salification with p-toluenesulfonic acid in a mixed solvent of acetone and acetonitrile, and cooling and crystallizing to obtain sorafenib tosylate of the polymorphic substance I. Compared with the prior art, the scheme of the method disclosed by the invention is simple and safe to operate, is mild in reaction condition, and does not need crystal form transformation steps; an obtained product is single and stable in crystal form and high in purity, and is suitable for large-scale industrial production.
Owner:TAIZHOU EOC PHARMA CO LTD
Preparation method of sorafenib
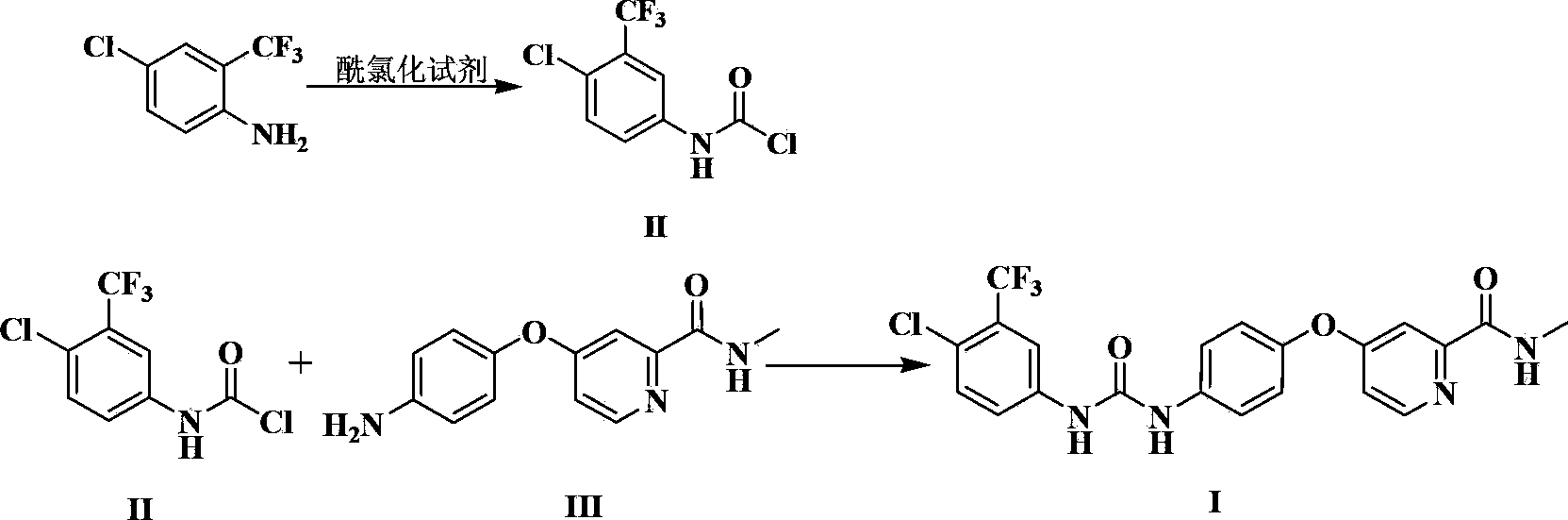
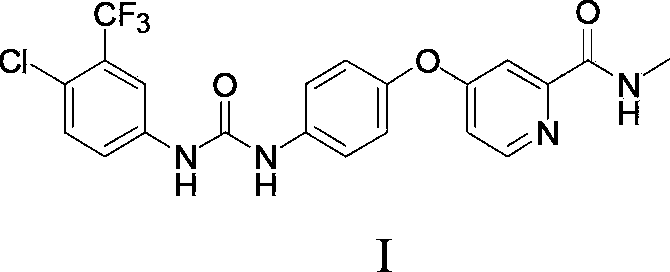
The invention relates to a preparation method of sorafenib. The method of the invention comprises the following steps: reacting 4-chloro-3-trifluoromethyl-aniline or acid addition salts of 4-chloro-3-trifluoromethyl-aniline with an acylating chlorination reagent by a one-pot method in the presence of alkali at -10 DEG C-35 DEG C to obtain an N-chloroformyl-4-chloro-3-trifluoromethyl-aniline intermediate, and then directly allowing the intermediate to carry out an ammonolysis reaction with 4-(4-amino phenoxyl)-N-methyl-2-pyridine carboxamide to obtain sorafenib with high yield. The process is simple in operation, short in production period, and high in yield, and the obtained product has a purity of more than 98%.
Owner:QILU PHARMA +1
Application of compound containing tyrosine kinase inhibitor and resveratrol in preparing antitumor drugs
InactiveCN108939080AGood synergyHydroxy compound active ingredientsAntineoplastic agentsTyrosine-kinase inhibitorDasatinib
The invention provides an application of a compound containing tyrosine kinase inhibitor and resveratrol in preparing antitumor drugs. The application is characterized in that the tyrosine kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib hydrochloride tablets, sorafenib, dasatinib tablets and tasigna or pharmaceutically acceptable salts or solvates or pharmaceutically acceptable salt solvents thereof; the tumor is selected from one of gastric cancer, liver cancer, lung cancer, kidney cancer, cervical cancer, pancreatic cancer, breast cancer, esophagus cancer, nasopharynxcancer and ovarian cancer; the mole ratio of resveratrol to tyrosine kinase inhibitor is (1-100):1.
Owner:黄泳华
Materials and methods for preventing or treating neurodegenerative conditions associated with abeta peptide accumulation
The subject invention concerns methods for preventing and / or treating neurodegenerative conditions associated with Abeta peptide accumulation in neural tissue in a human or animal. The subject invention also concerns methods for preventing or treating Alzheimer's disease-like neuropathology in a person or animal having trisomy 21 (Down's syndrome). In one embodiment, a method of the invention comprises administering a therapeutically effective amount of a compound that inhibits function or activity of a Raf protein to a person or animal in need of treatment. In a specific embodiment, the Raf inhibitor is Sorafenib (NEXAVAR). Neurodegenerative conditions contemplated within the scope of the present invention include, for example, Alzheimer's disease and Parkinson's disease. The subject invention also concerns methods for preventing or inhibiting neuronal cell death and / or improving cell viability.
Owner:UNIV OF SOUTH FLORIDA +1
Method of determining acute myeloid leukemia response to treatment with farnesyltransferase inhibitors
ActiveUS20130130999A1Faster assayQuick forecastBiocideMicrobiological testing/measurementMyeloid leukemiaAptX
The disclosed method rapidly identifies with desired accuracy AML patients, including elderly AML patients, likely to respond to treatment with a combination of a farnesyltransferase inhibitor and one or more of etoposide, teniposide, tamoxifen, sorafenib, paclitaxel, temozolomide, topotecan, trastuzumab and cisplatinum. In an embodiment, the improvements include the use of whole blood rather than the customary bone marrow sample, thus making the assay more accurate, rapid, less intrusive, less expensive as well as less painful. The method includes evaluation of a two-gene expression ratio (RASGRP1:APTX), which with a corresponding threshold, provides sufficient accuracy for predicting the response to the combination treatment. In the preferred embodiment the combination treatment combines tipifarnib (R115777, ZARNESTRA®) with etoposide. Further, the elderly AML patients identified as being likely responsive to the combination treatment with tipinifarb and etoposide have a complete recovery rate comparable to the best therapy available for younger patients.
Owner:JANSSEN PHARMA NV
Application of nanometer drug-loading system in preparation of medicines for curing refractory thyroid cancer
InactiveCN108354913AReduce dosageEliminate side effectsEnergy modified materialsPharmaceutical non-active ingredientsNanocarriersRos responsive
The invention provides application of a nanometer drug-loading system in the preparation of medicines for curing refractory thyroid cancer, and belongs to the field of biotechnology. The nanometer drug-loading system comprises an ROS responsive hyperbranched macromolecule, a photosensitizer and a chemotherapy drug, wherein the structural formula of the hyperbranched macromolecule is shown as a formula I; the photosensitizer is chlorines e6; and the chemotherapy drug is Sorafenib. The nanometer drug-loading system can be enriched in tumor tissue, the ROS level inside the tumor tissue further can be increased under the laser radiation of 660 nm, a nano-carrier inside the tumor tissue is disintegrated, and the chemotherapy drug is quickly released, so that the cure of a tumor is synergistically interacted, simultaneously the using amount of the chemotherapy drug is reduced, and toxic and side effects of the chemotherapy drug are alleviated.
Owner:JILIN UNIV
Targeted sustained-release microsphere of vascular occlusive agent containing sodium alginate and sorafenib, production method and use thereof
InactiveUS20120093932A1Rapid and long and effectTargeted optimizationPowder deliveryBiocideSide effectMicrosphere
A targeted sustained-release microsphere vascular embolizing agent, the production method and the use thereof are disclosed. The microsphere comprises sodium alginate as the carrier and sorafenib as the targeted anti-tumor medicine and sorafenib is encapsulated by sodium alginate. The weight ratio of sorafenib to sodium alginate is 1:1˜1:30. The microspheres are used for manufacturing medicament for the treatment of solid tumors with advantages including high medicine concentration in the target regions with reduced systemic dosage and toxic and side effects.
Owner:BEIJING SHENGYIYAO SCI & TECH DEV
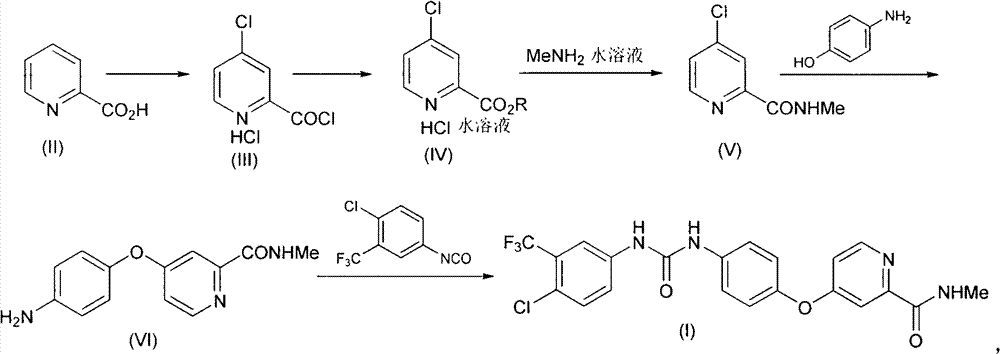
Method for preparing sorafenib
The invention discloses a method for preparing sorafenib. 2-picolinic acid is subjected to chlorination, esterification and methylamination to form a key intermediate, namely 4-chloropyridine-N-methyl-2-formamide, the intermediate and p-aminophenol are subjected to coupled reaction under alkaline condition, and a reaction product is reacted with 4-chloro-3-trifluoromethyl phenyl isocyanate to form a target compound.
Owner:SHANGHAI AOBO PHARMTECH INC LTD
Synthesis method for sorafenib
InactiveCN103724259AImprove protectionEasy to operateOrganic chemistryOrganic solventSynthesis methods
The invention provides a synthesis method for sorafenib. The synthesis method is characterized by comprising the following steps: (1) dissolving the raw materials of 4-chlorine-3-trifluoromethylaniline and CDI into an organic solvent, reacting for 2 hours under theat a reaction temperature of 15-40 DEG C, and obtaining the intermediate N-(4-chlorine-3-trifluoromethylaniline)-1H-glyoxaline-1-acid amide; (2) adding a raw material of 4-(4-aminophenoxy)-N-methyl picolinamide, continuously reacting for 1 hour under the same condition, and post-processing, so thatso as to obtain the sorafenib is obtained. Compared with the known synthesis process, the synthesis method provided by the invention is simple to operate, the nitrogen protection is eliminated, and the post-processing in the first step is eliminated; by changing the solvent types, the reaction time is shortened greatly, and the yield is increased obviously, so that industrialized production is more facilitated.
Owner:JIANGSU JIXIAN GREEN CHEM TECH RESINST
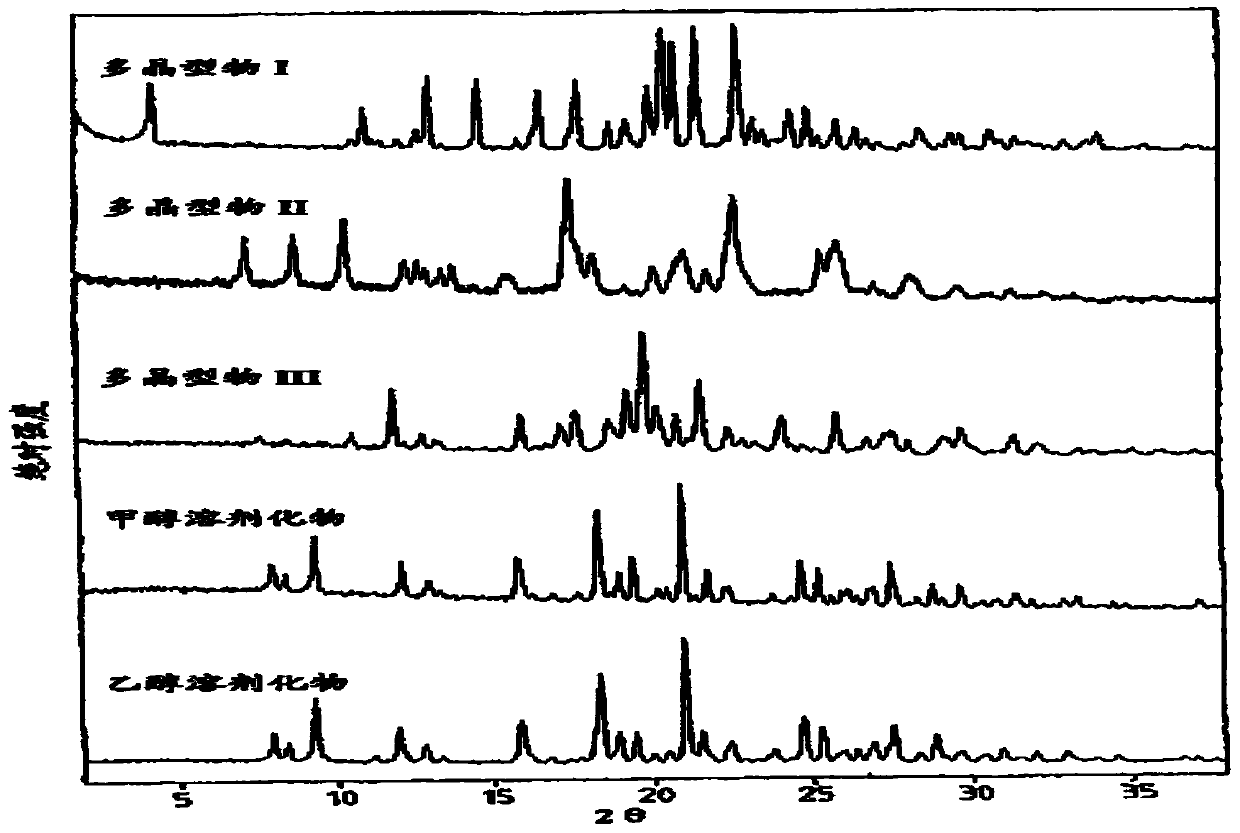
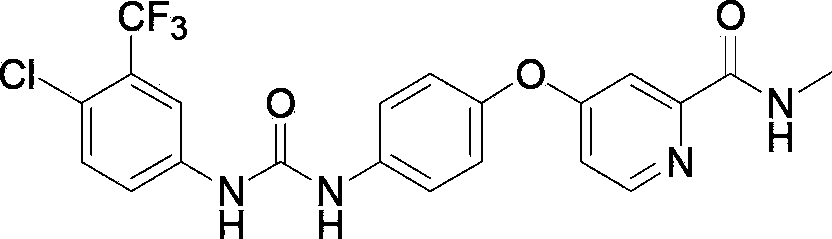
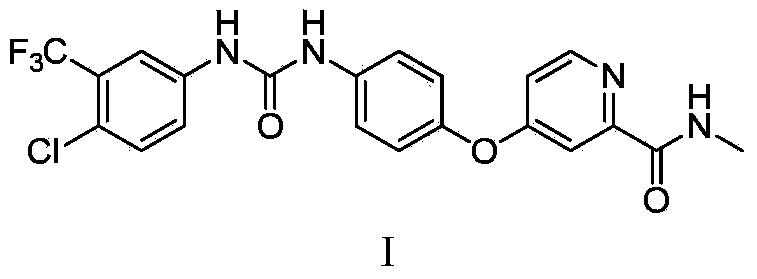
Crystal form A of Sorafenib and preparation method thereof
The invention belongs to the field of pharmaceutical chemicals, and particularly relates to a crystal form A of 4-[4-[3-(4-chloro-3-trifluoromethyl phenyl) ureide) phenoxy]-pyridine-2-formamide (sorafenib) with antitumor activity and a preparation method thereof. The purity of a crystal form A of Sorafenib prepared according to the invention is high, and is over 99.0%, an X-ray powder diffraction of the crystal form expressed by a 2theta angle has characteristic peaks at angles of 11.2-11.6 degrees, 11.4-11.8 degrees, 18.4-18.8 degrees, 22.3-22.7 degrees, 24.6-25.0 degrees and 29.5-29.9 degrees, and the crystal form is uneasy to decompose at a temperature of 25-50 DEG C, so that the crystal form shows good stability.
Quinazolinyl-aryl urea derivatives with antitumor function and application thereof
ActiveCN104292170AStrong antiproliferative activityOrganic active ingredientsOrganic chemistryUrea derivativesAryl
The invention relates to quinazolinyl-aryl urea derivatives with antitumor function disclosed as general formula (II) and application thereof. The substituent group in the general formula (II) is defined in the specification. By using sorafenib and gefitinib as lead compounds, the pharmacophore-uramido group of the sorafenib is retained; and meanwhile, the quinazoline rings in the gefitinib and other EGFR-TKIs are retained to synthesize a series of quinazolinyl-aryl urea derivatives. The in-vitro activity test proves that parts of the compounds have excellent antitumor activity, and the compounds have higher research and practical values. (II).
Owner:GUANGXI NORMAL UNIV
Sorafenib antitumor platinum (II) complex targeted at human lung cancer drug-resistant cells and preparation method and application thereof
ActiveCN108774270AGood antitumor activityEnhanced inhibitory effectOrganic active ingredientsPlatinum organic compoundsChemical structurePlatinum
A sorafenib antitumor platinum (II) complex targeted at human lung cancer drug-resistant cells is disclosed. The chemical structure of the complex is shown as follows in the description. The complex shows excellent antitumor activity in vitro, has potential medicine value and can be used for preparing various antitumor medicines.
Owner:YULIN NORMAL UNIVERSITY
Method for preparing sorafenib tosylate
ActiveCN105801475AHigh yieldThe reaction steps are simpleOrganic chemistry methodsP-AminophenolP-Toluenesulfonic acid
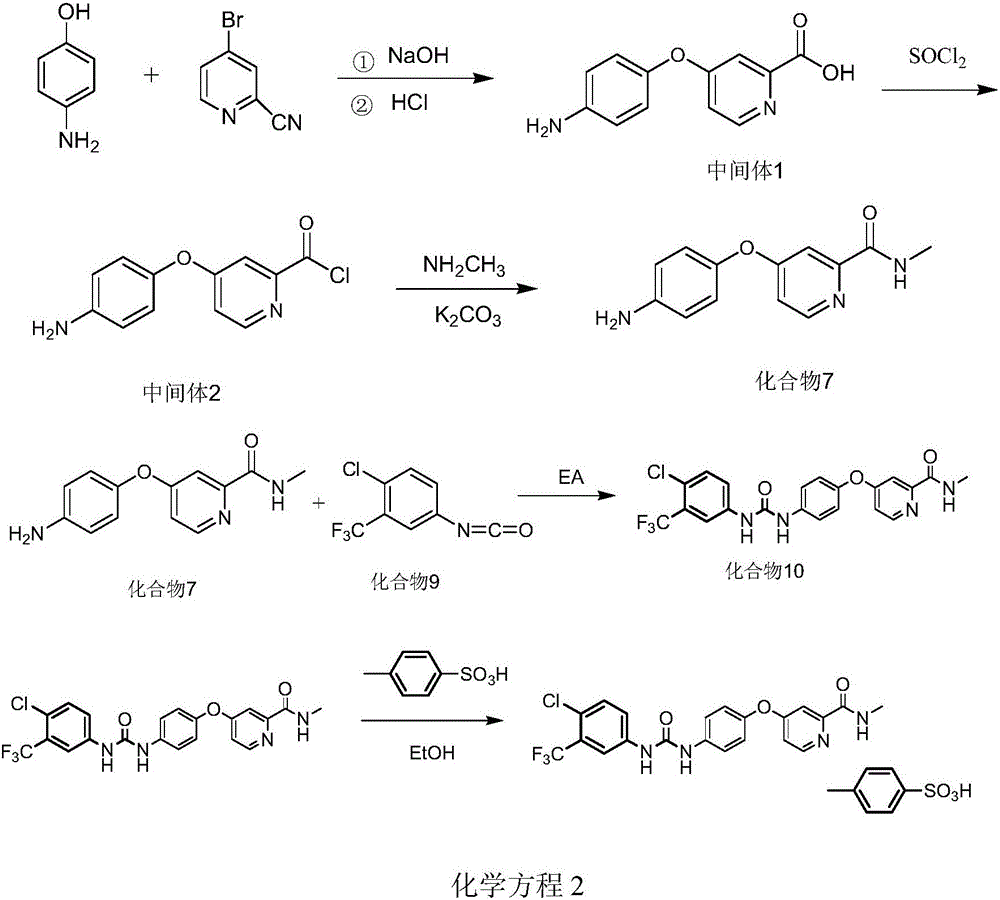
The invention discloses a method for preparing sorafenib tosylate. The method comprises the following steps: reacting p-aminophenol with 4-bromo-2-cyanopyridine in the presence of sodium hydroxide, and then obtaining 4-(4-aminophenoxy)-2-(formic acid) pyridine after acidification; after carrying out a substitution reaction with thionyl chloride, reacting with methylamine in the presence of potassium carbonate to obtain 4-(4-aminophenoxy)-2-(methyl carbamyl) pyridine; after carrying out direct condensation with a 4-chlorine-3-(trifluoromethyl) phenyl isocyanate compound, salifying with p-toluenesulfonic acid to obtain sorafenib tosylate. The 4-bromo-2-cyanopyridine is used as a starting raw material to prepare the 4-(4-aminophenoxy)-2-(formic acid) pyridine, the steps of subsequent reaction are simplified, the steps of whole reaction are simple, and the yield of product is high.
Owner:JINAN LIMIN PHARMA
Medicine composition containing tyrosine kinase restraining agent
InactiveCN101081207APharmaceutical delivery mechanismPharmaceutical non-active ingredientsDocetaxelWhole body
The anticancer medicine composition containing tyrosine kinase inhibitor is slow released injection and slow released implant. The effective anticancer components include tyrosine kinase inhibitor selected from Erbitux, Iressa, Tarceva, Sunitinib, Trastuzumab, etc, and / or composition selected from Docetaxel, deacetyl taxol, taxol, etc. The slow releasing supplementary material is selected from p(LAEG-EOP), p(DAPG-EOP), etc. The released injection and slow released implant may be injected or set in tumor for slow releasing to maintain effective medicine concentration for over 50 days, and has obviously lowered systemic reaction on the medicine and capacity of enhancing the chemotherapeutic and radiotherapeutic effect.
Owner:JINAN KANGQUAN PHARMA TECH
Combination therapy for hepatocellular carcinoma
ActiveUS20180162941A1Resume normal productionLevelBiological material analysisMammal material medical ingredientsLiver disorderTyrosine-kinase inhibitor
Provided are methods and compositions for a combination therapy for liver disorders such as hepatocellular carcinoma. Also provided is a method for determining the effectiveness of therapy involving tyrosine kinase inhibitors such as sorafenib. The method comprises determining the status of PD-1 on T cells, and based on a change in the level of PD-1 on certain cells, a determination of the effectiveness of the tyrosine kinase, and an indication for a combination therapy comprising a lower dose of tyrosine kinase inhibitor and a PD-1 inhibitor can be made.
Owner:HEALTH RES INC
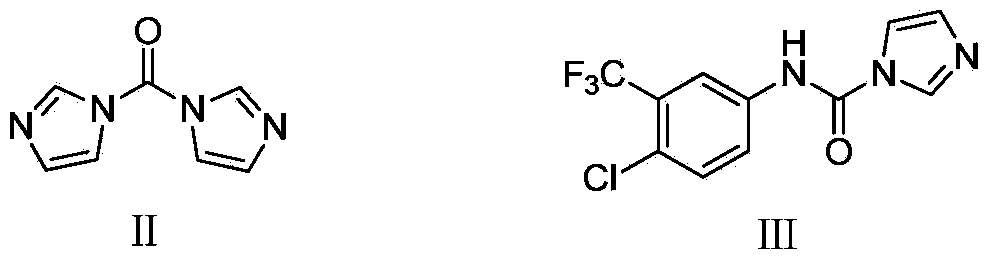
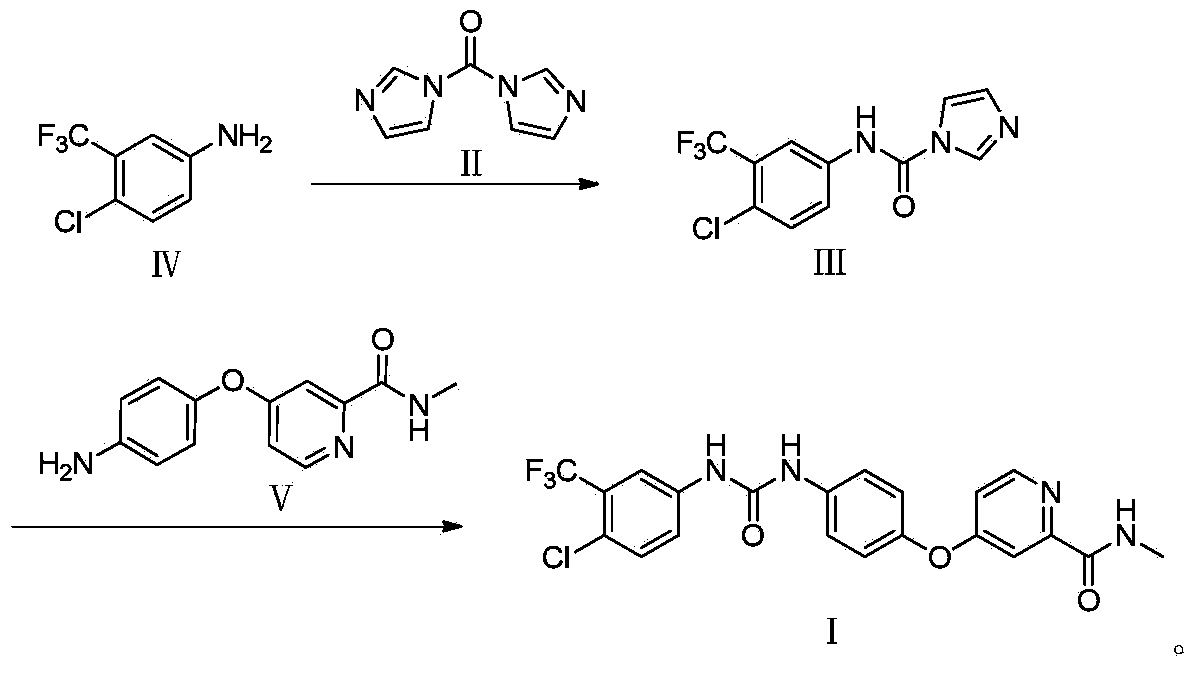
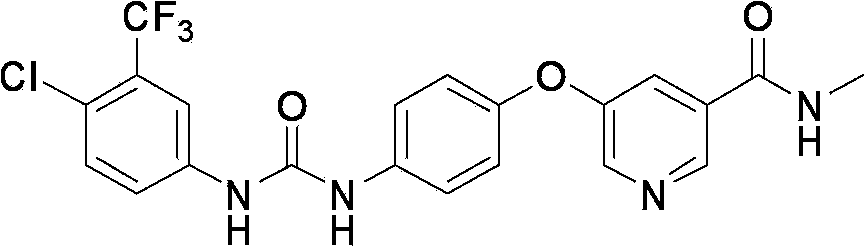
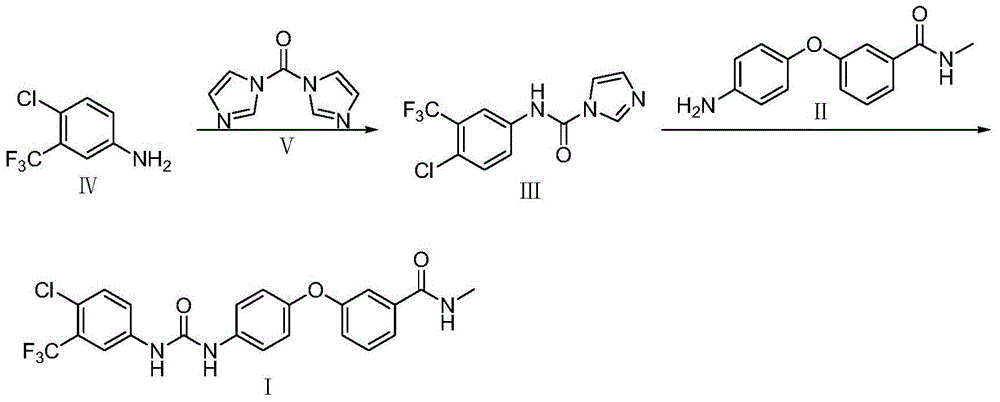
Novel method for synthesizing sorafenib
ActiveCN104402813ASimple processNo high temperature and high pressure reactionOrganic chemistryOrganic solventFormamide
The invention discloses a novel method for synthesizing sorafenib. The method comprises the following steps: using a (4-(4-aminophenoxy)-N-methylpyridine-2-carboxamide) compound II and a (4-chloro-3-trifluoromethylphenyl-carbamoyl-imidazole) compound III as raw materials, and reacting in an organic solvent inert to the compound III to produce a sorafenib crude product; then, dissolving the sorafenib crude product by the organic solvent and an organic or inorganic acid, and adding a solvent into which sorafenib is difficult to dissolve to enable the sorafenib to be separated out, so as to obtain a sorafenib pure product. The novel method is simple in technical process, ensures high product purity, facilitates operation and is suitable for large-scale industrial production.
Owner:哈药集团股份有限公司 +1
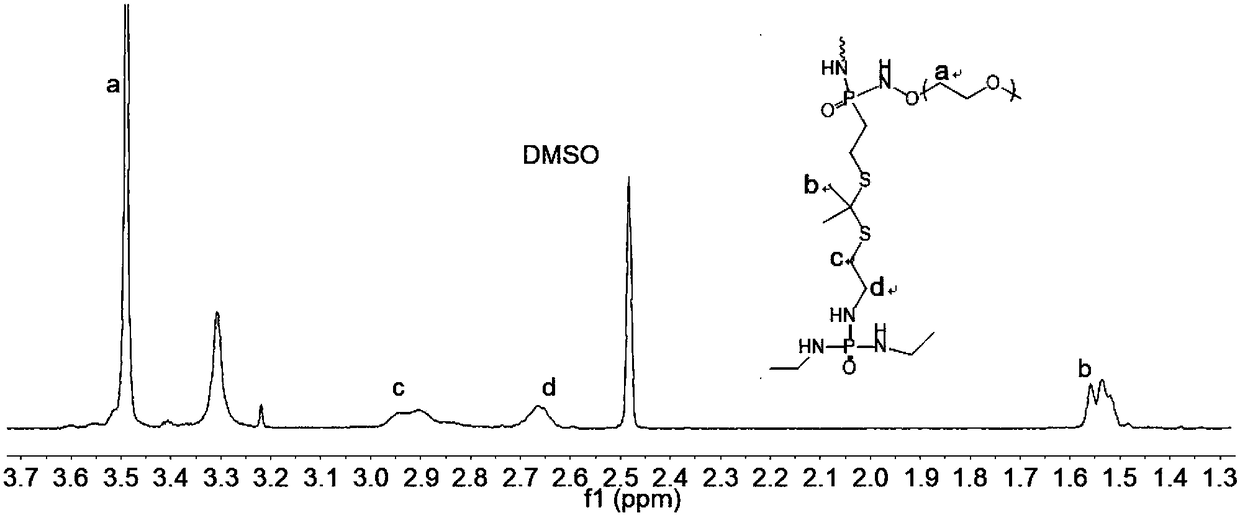
Sorafenib lipidosome freeze-dried injection for injection and preparation method thereof
InactiveCN104523607AImprove bioavailabilitySmall dosePowder deliveryLyophilised deliveryCholesterolFreeze-drying
The invention discloses a sorafenib lipidosome freeze-dried injection for injection and a preparation method thereof. The freeze-dried injection is prepared from the following components in molar ratio: 1 mol of sorafenib, 5-60 mol of phospholipid, 1-30 mol of cholesterol, 1-60 mol of phosphatidyl glycerol, 1-10 mol of a polyethylene glycol surfactant and 50-400 mol of a freeze-drying protecting agent. The preparation method comprises the following steps: sequentially dissolving phospholipid, cholesterol, phosphatidyl glycerol, polyethylene glycol surfactant and sorafenib in an organic solvent in proportion; then, carrying out rotary and vacuum evaporation on the obtained organic solution in a round bottom glass bottle, so that a uniform thin film is formed on the inner wall of the glass bottle; then, adding a buffer liquid with the pH of 7-10 into the glass bottle to be continuously stirred to form a suspension; reducing the grain size of the suspension by an ultrasonic or high-pressure homogenizing method; and adding the freeze-drying protecting agent and freeze-drying. The sorafenib lipidosome disclosed by the invention remarkably improves the bioavailability and is stable in property, and the encapsulation efficiency can reach over 96%.
Owner:TIANJIN ZHONGXI MEIHUA BIOMEDICAL TECH
Novel sorafenib tosylate polycrystalline form and preparation method thereof
InactiveCN102850267AGood repeatabilitySimple and fast operationOrganic chemistryAlcoholDecomposition
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
Preparation for improving bioavailability of sorafenib
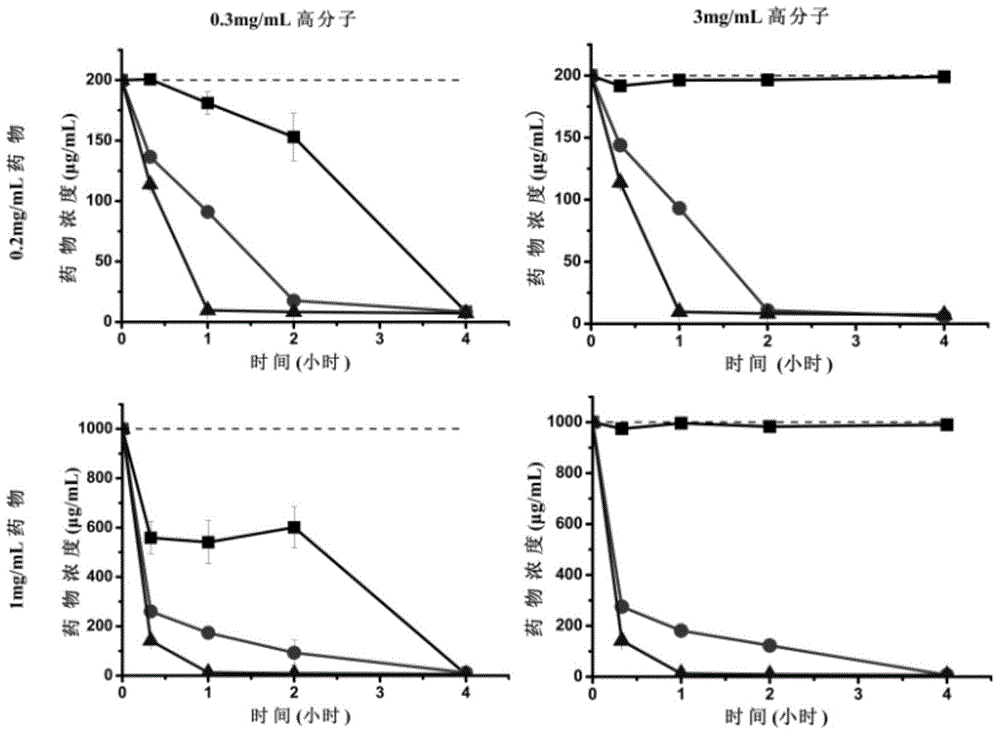
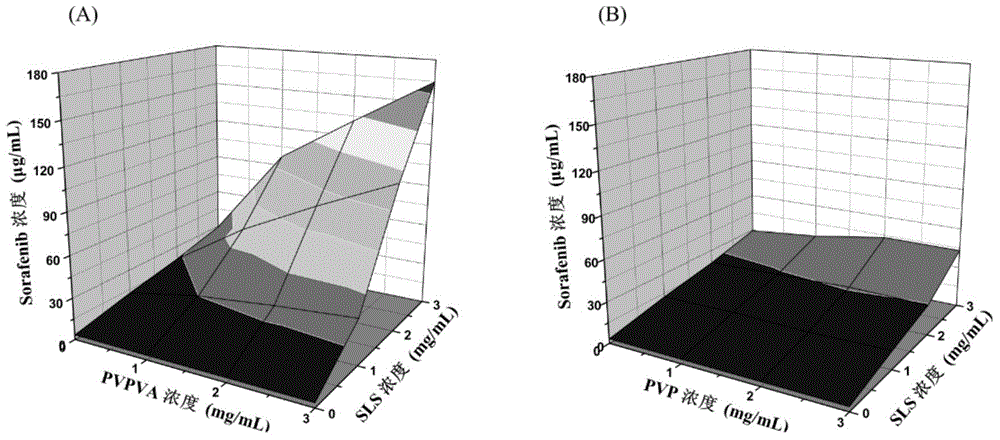
InactiveCN105126111AIncrease dissolution rateExtension of timePharmaceutical non-active ingredientsPill deliveryAcetic acidBioavailability
The invention provides a sorafenib preparation. The sorafenib preparation includes active ingredients and macromolecular cosolvent. The active ingredients are selected from at least one of sorafenib, sorafenib salt, a sorafenib derivative or a prodrug of the sorafenib derivative. The macromolecular cosolvent is provided with vinyl acetate radical groups. The sorafenib preparation has the advantage that the bioavailability of the drug active ingredients is high.
Owner:TSINGHUA UNIV
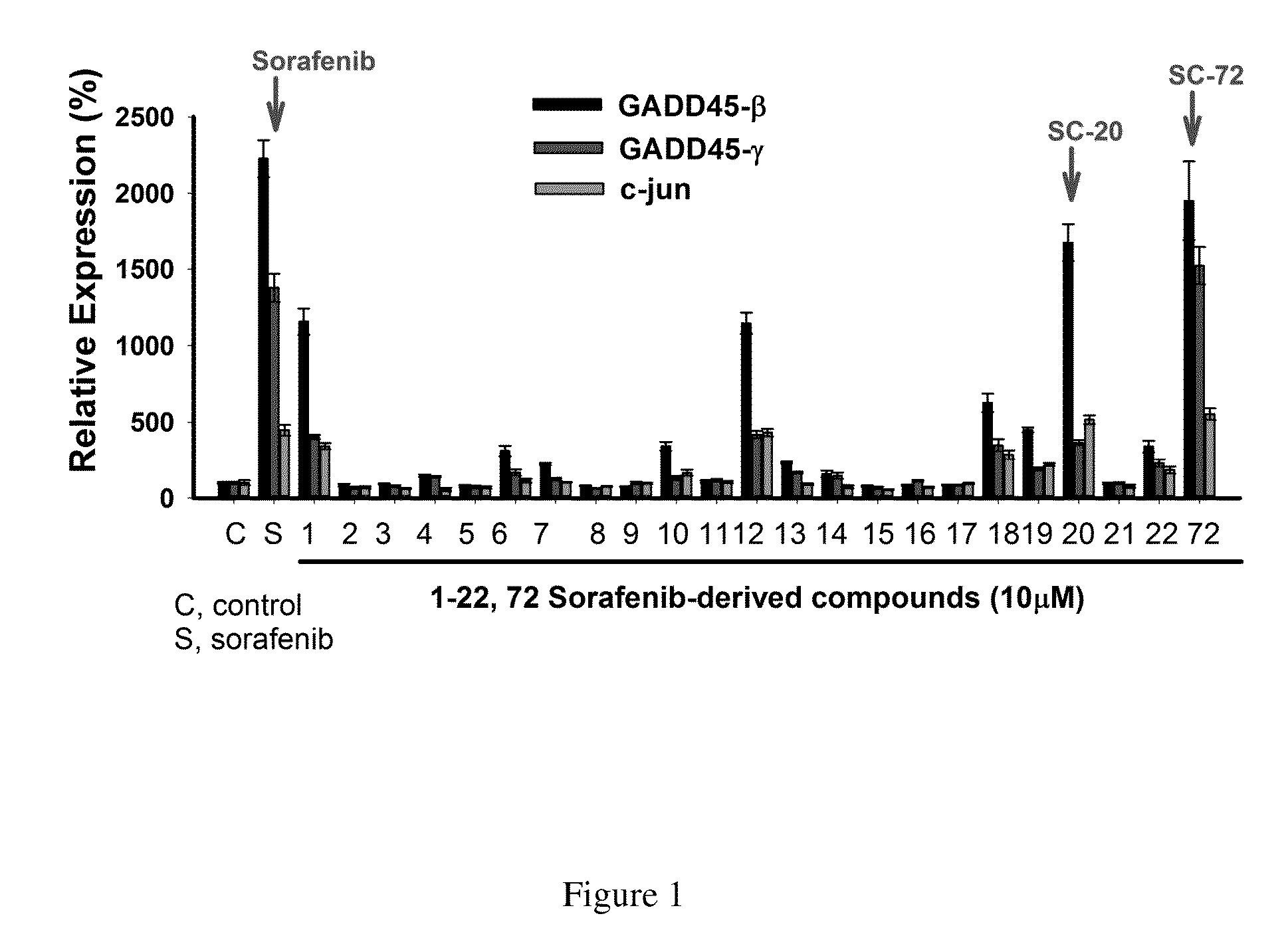
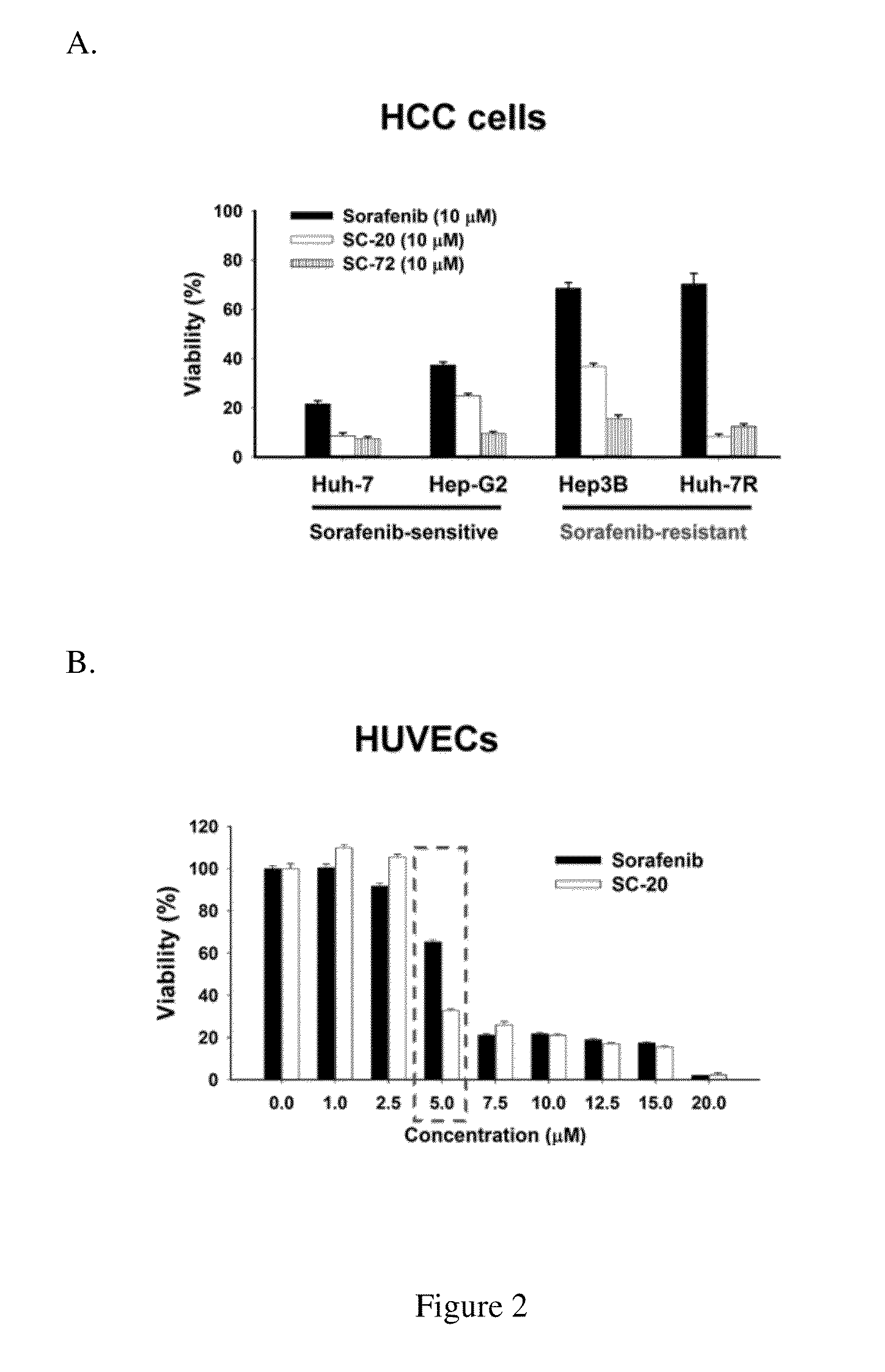
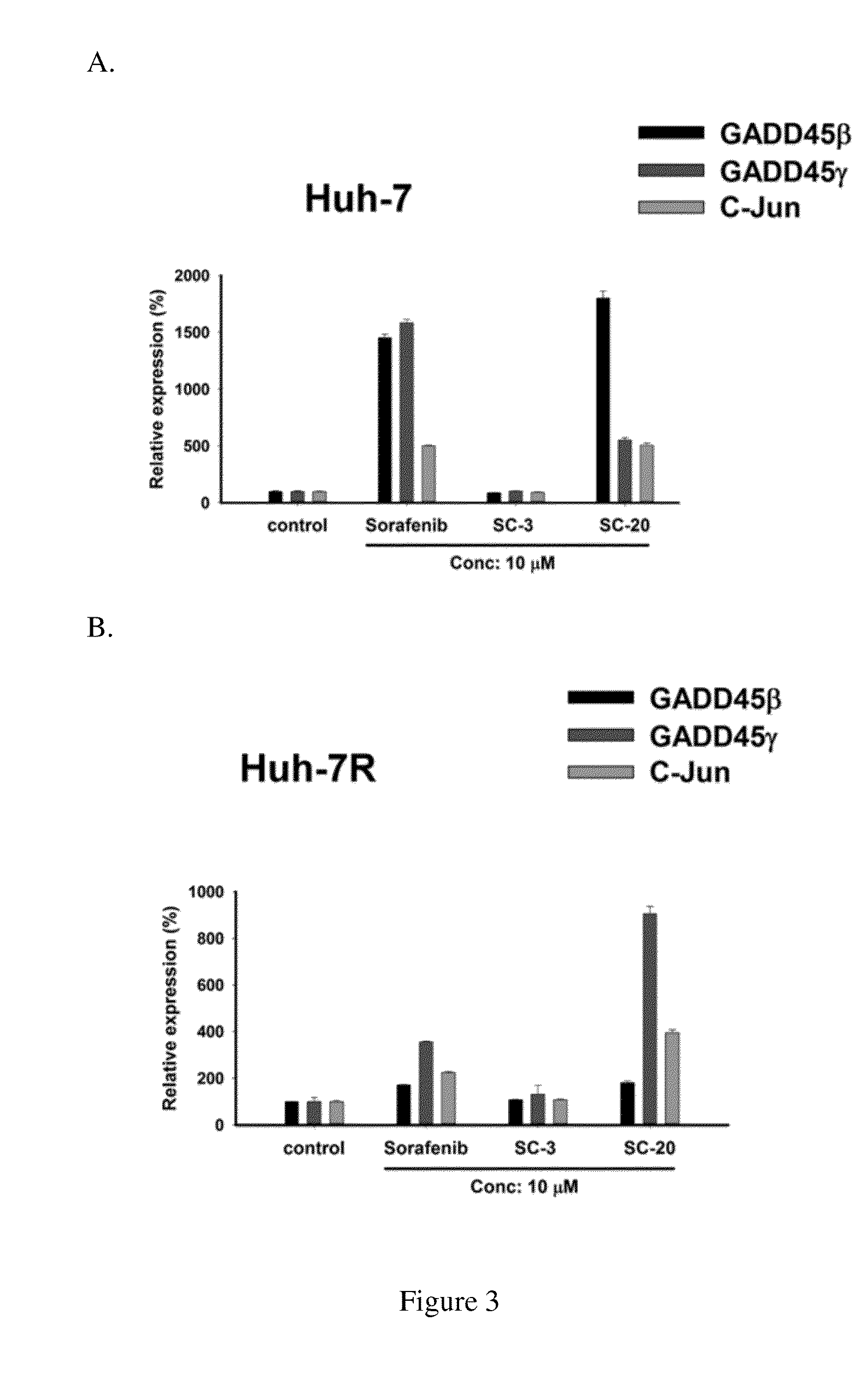
Method for screening Anti-cancer drugs and method of cancer treatment
The present application provides a method for screening compounds for cancer treatment comprising treating cancer cells with a candidate compound for sufficient time for detectable expression of a gene selected from GADD45 family; and detecting the level of expression of the gene as compared to the level of expression in the absence of the candidate compound; wherein an increased level of expression in the presence of the candidate compound as compared to expression in the absence of the candidate compound indicates that the candidate compound acts as an inhibitor of the cancer cells. In some embodiments, the method can be used for screening compounds for treatment of sorafenib-resistant hepatocellular carcinoma. The present application further provides a method for treating hepatocellular carcinoma comprising administering to a subject having hepatocellular carcinoma a therapeutic effective amount of an agonist of GADD45 family.
Owner:NAT TAIWAN UNIV
Biaryl amide structure containing sorafenib derivative as well as preparation method and applications thereof
InactiveCN105153026AEnhanced inhibitory effectVEGFR kinase activity inhibitionOrganic active ingredientsOrganic chemistryCancer preventionDisease
The invention discloses a biaryl amide structure containing sorafenib derivative as well as a geometrical isomer, a pharmaceutically acceptable salt, a hydrate, a solvate or a prodrug and a preparation method of the sorafenib derivative. The biaryl amide structure containing sorafenib derivative as well as the pharmaceutically acceptable salt, the hydrate or the solvate of the sorafenib derivative are taken as active components and mixed with a pharmaceutically acceptable carrier or excipient for preparation of composition and clinically acceptable dosage forms. The invention further discloses applications of the compound to preparation of drugs for treating and / or preventing hyperplastic diseases, preparation of drugs for treating and / or preventing cancer and preparation of drugs for treating and / or preventing prostate cancer and lung cancer.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com