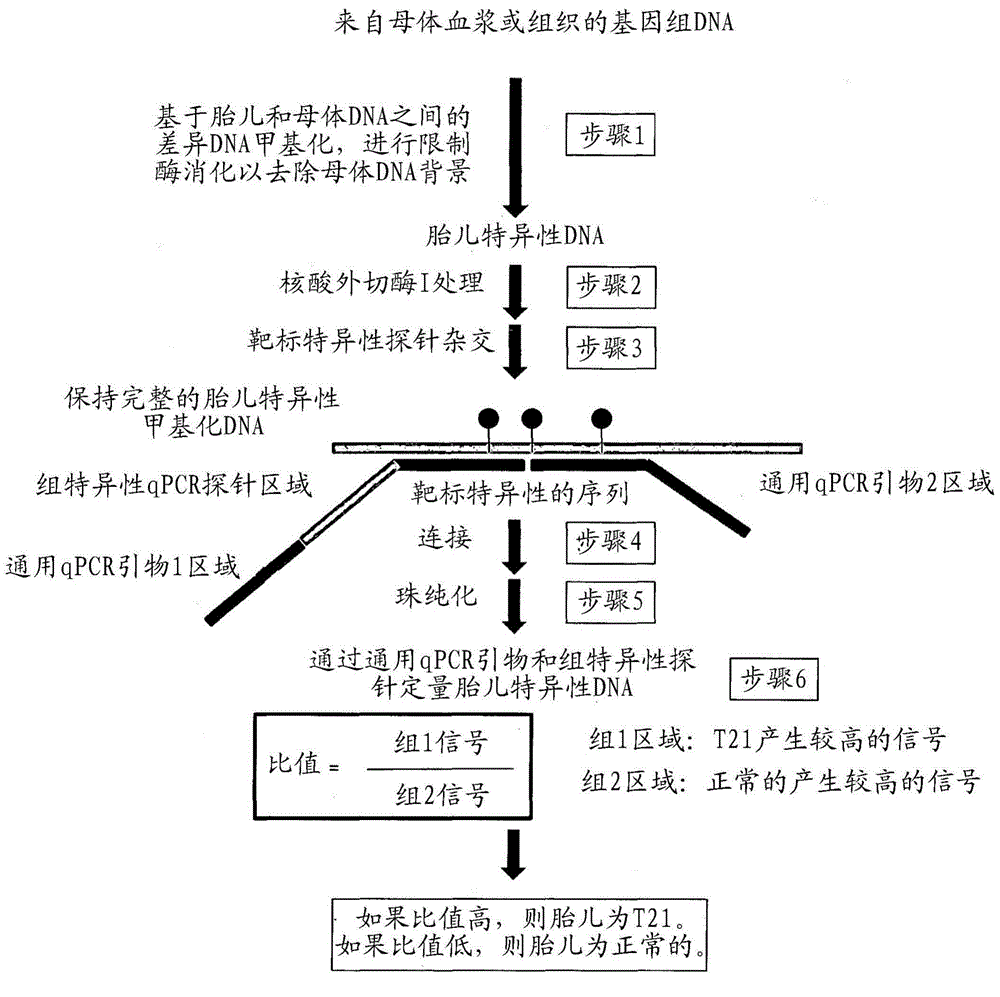
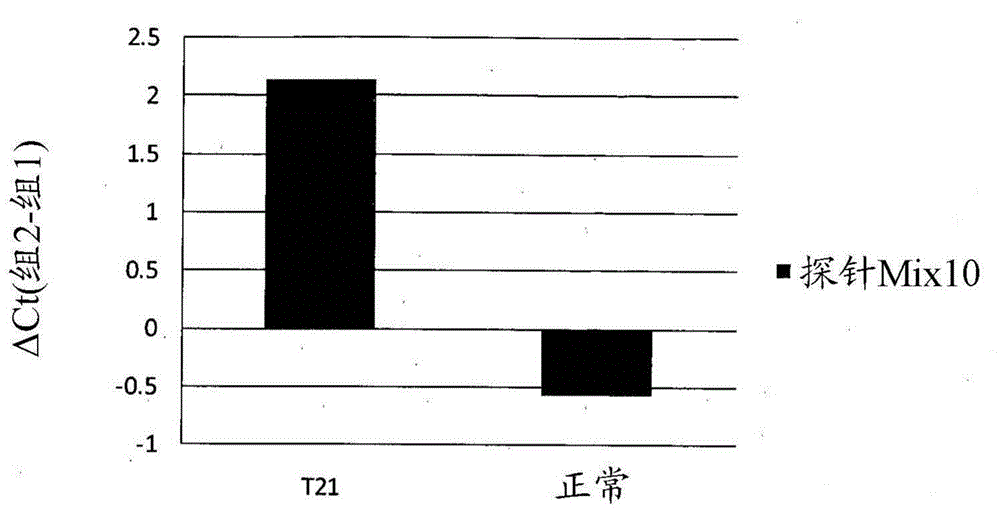
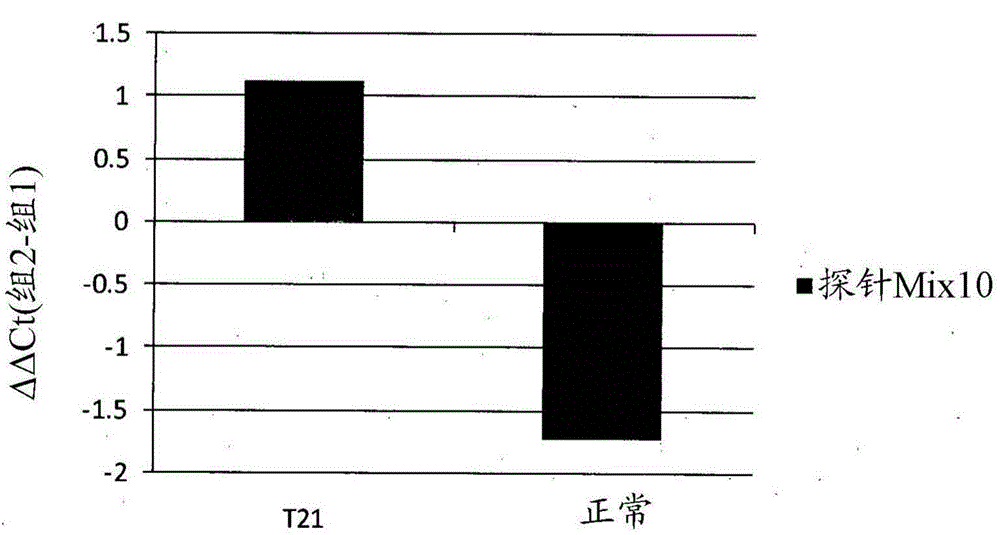
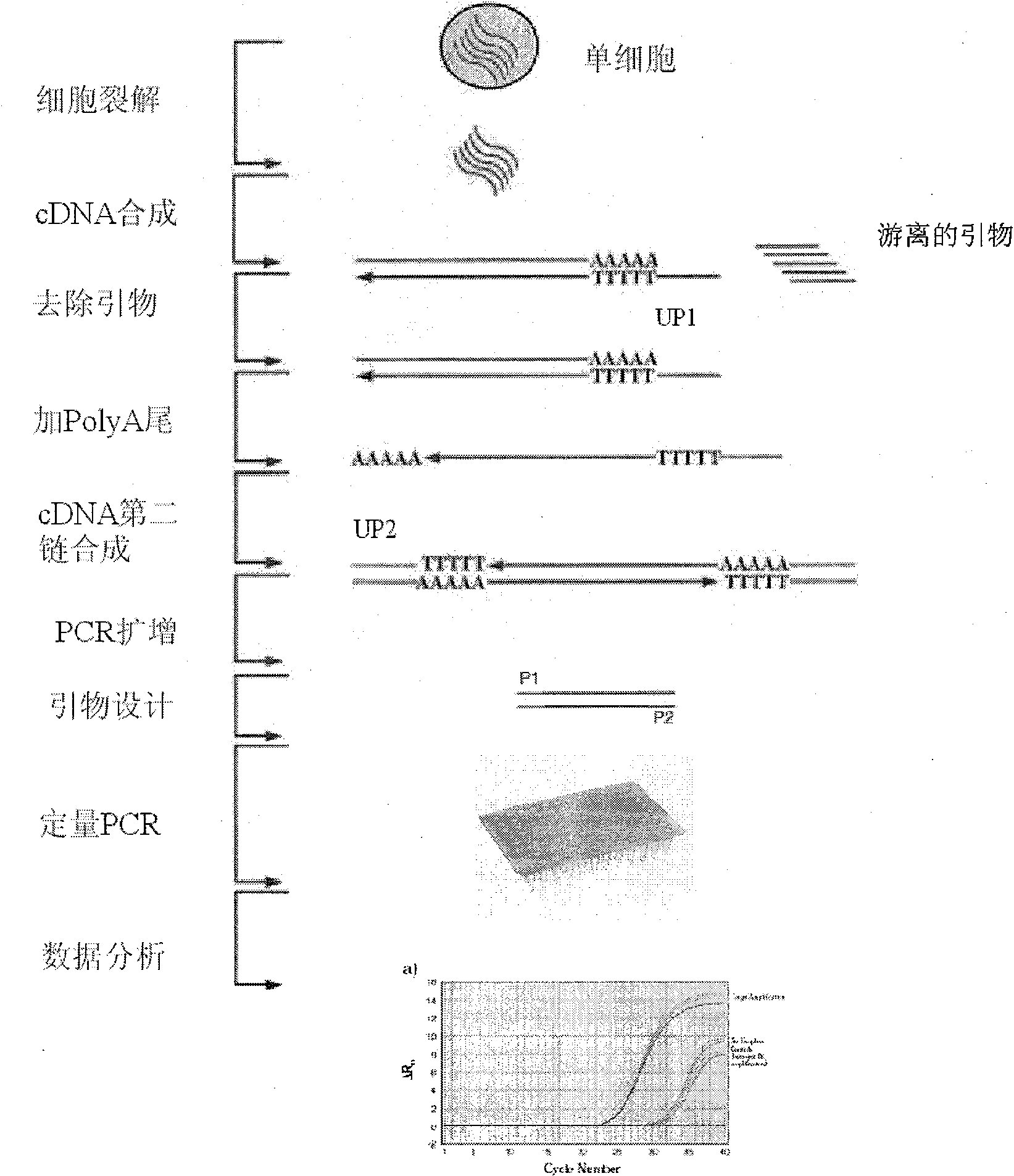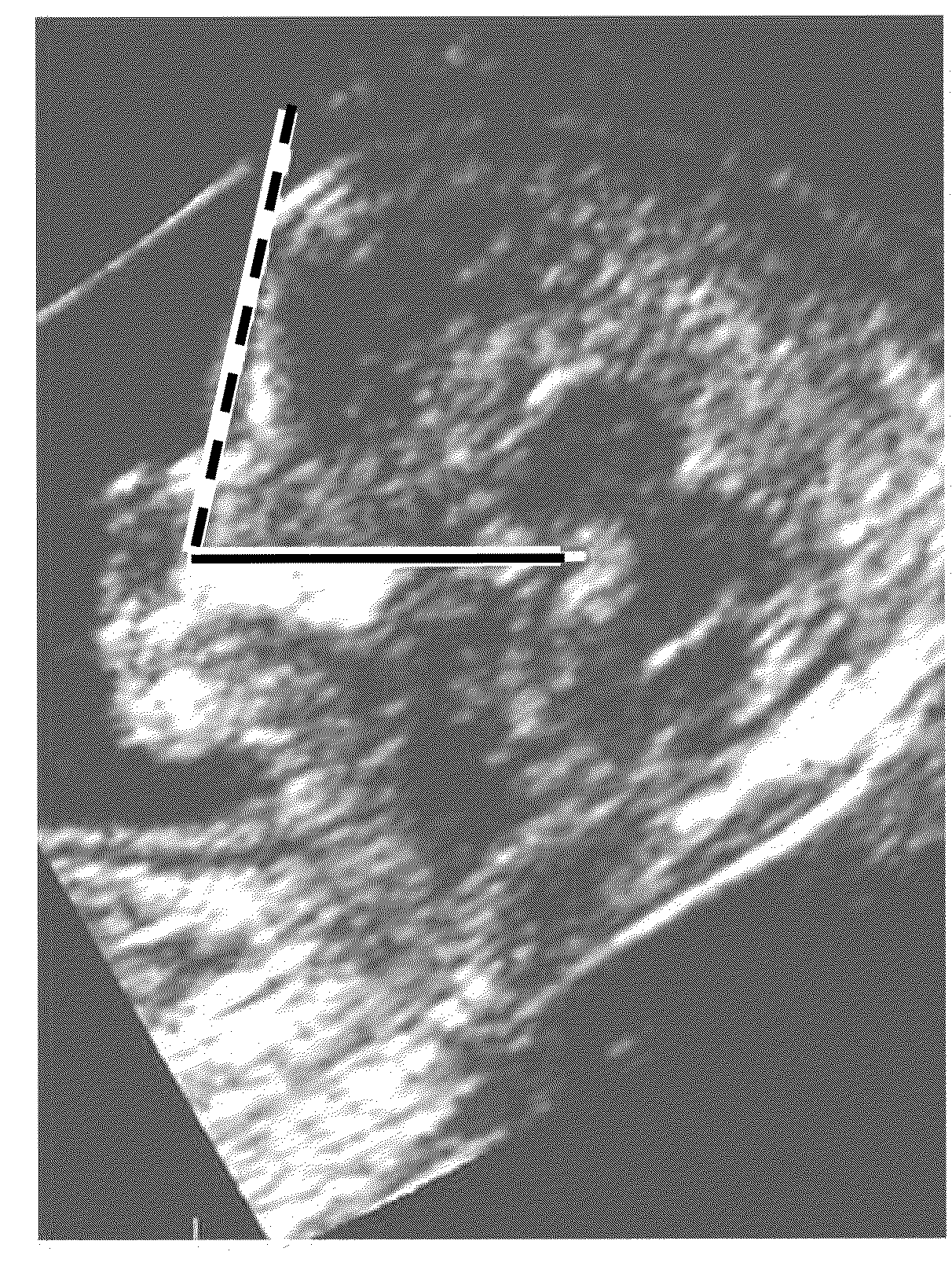Patents
Literature
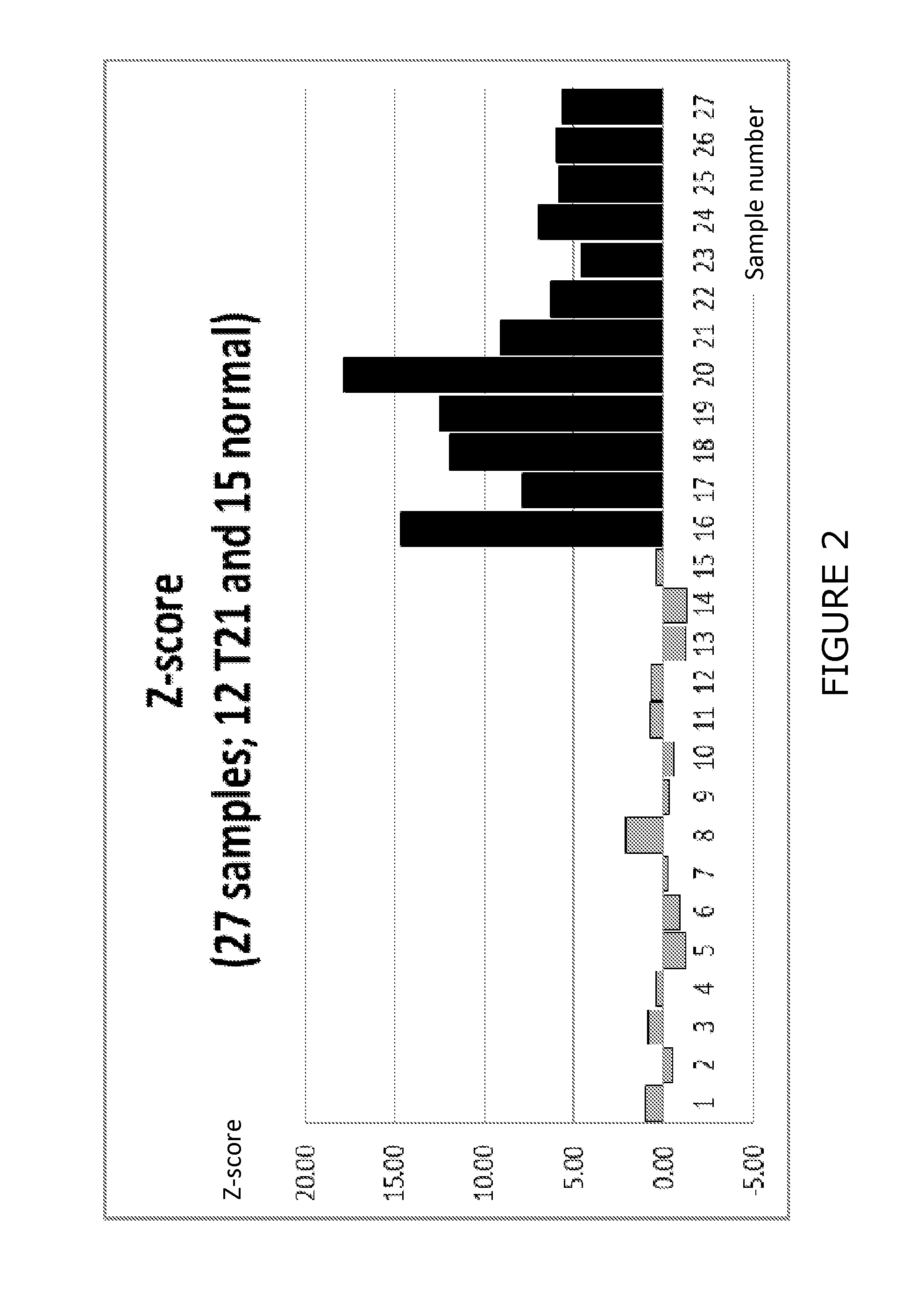
77 results about "Trisomy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A trisomy is a type of polysomy in which there are three instances of a particular chromosome, instead of the normal two. A trisomy is a type of aneuploidy (an abnormal number of chromosomes).
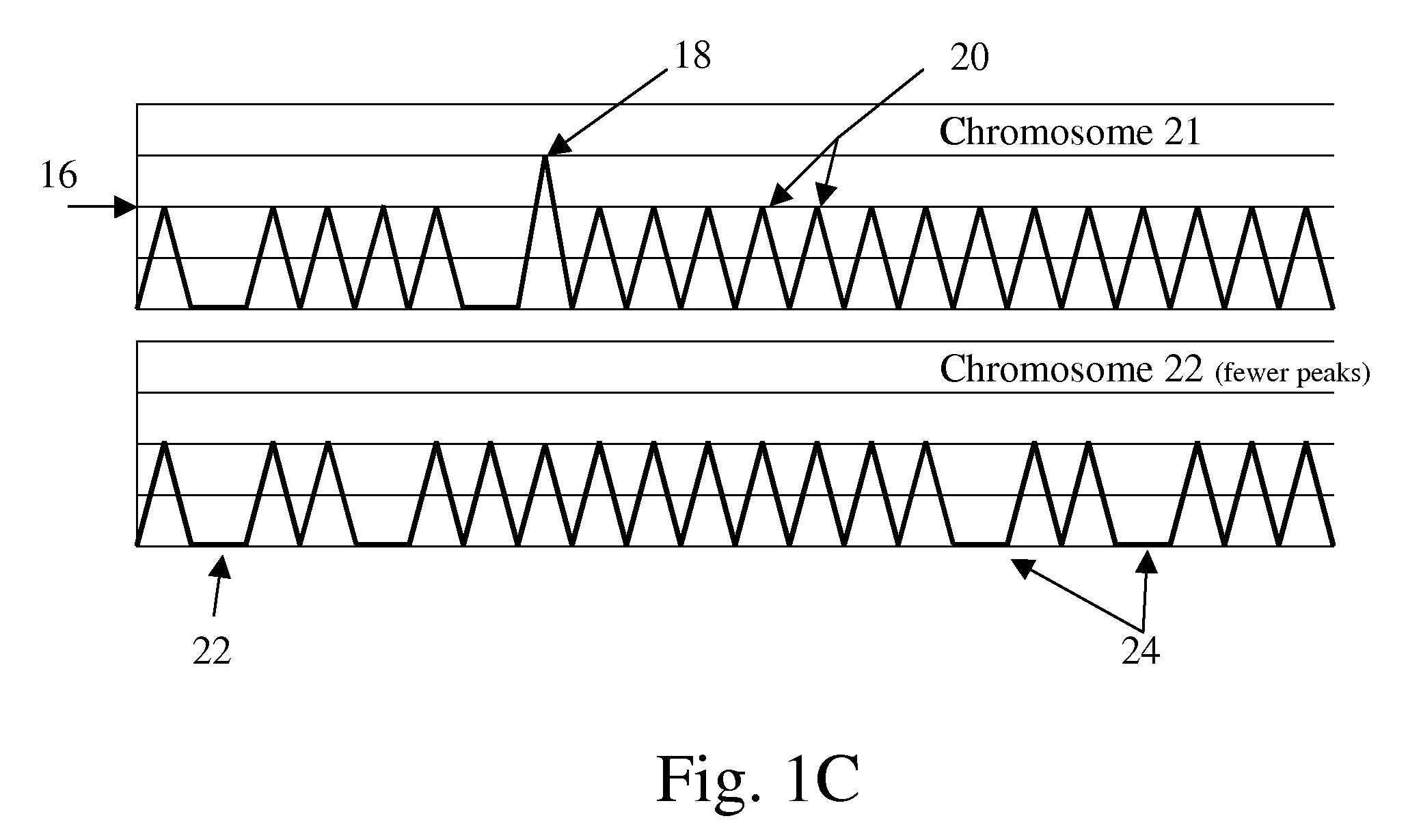
Non-invasive fetal genetic screening by digital analysis
ActiveUS20070202525A1Enriching fetal DNAMicrobiological testing/measurementFermentationChorionic villiMassively parallel
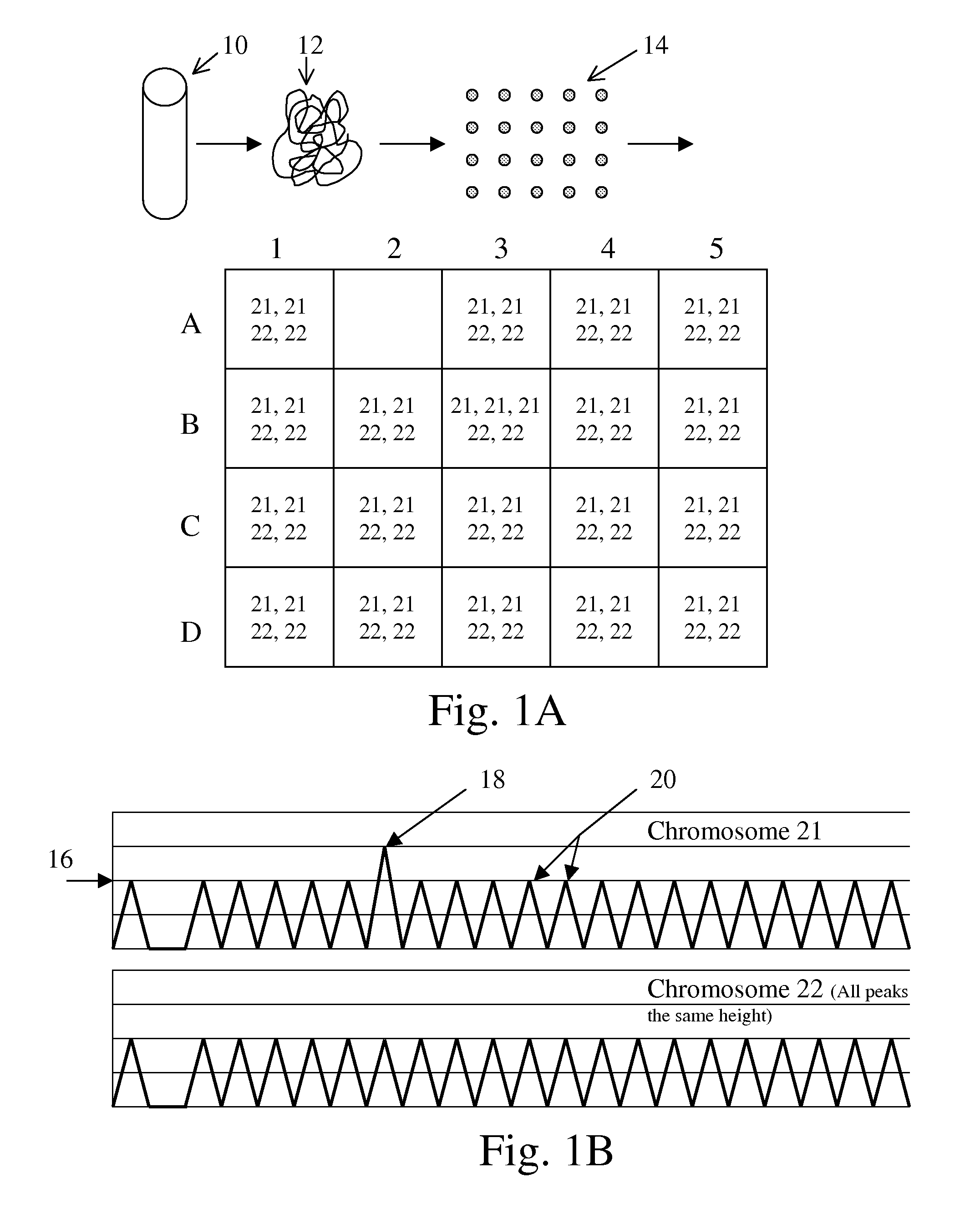
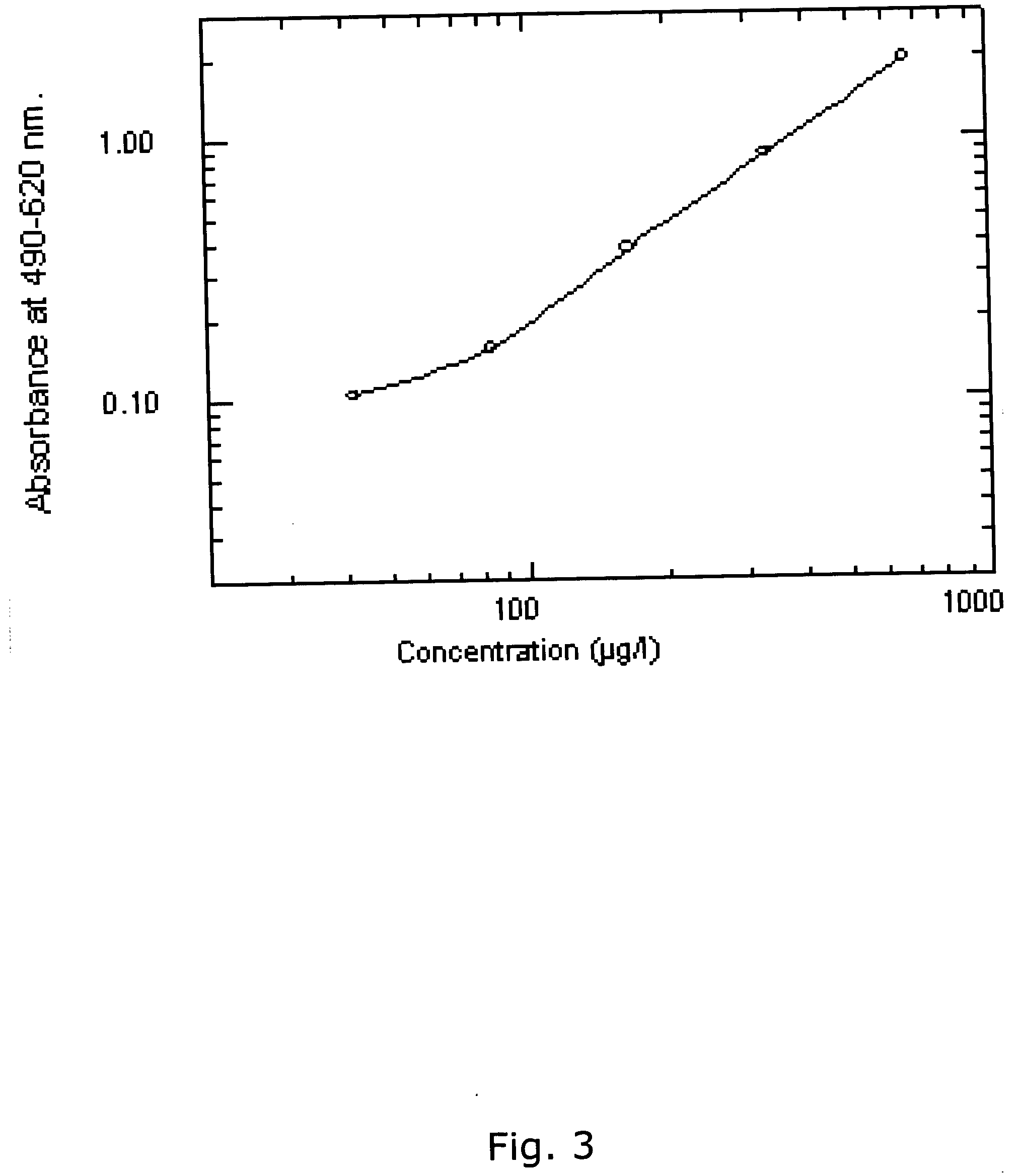
The present methods are exemplified by a process in which maternal blood containing fetal DNA is diluted to a nominal value of approximately 0.5 genome equivalent of DNA per reaction sample. Digital PCR is then be used to detect aneuploidy, such as the trisomy that causes Down Syndrome. Since aneuploidies do not present a mutational change in sequence, and are merely a change in the number of chromosomes, it has not been possible to detect them in a fetus without resorting to invasive techniques such as amniocentesis or chorionic villi sampling. Digital amplification allows the detection of aneuploidy using massively parallel amplification and detection methods, examining, e.g., 10,000 genome equivalents.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Rapid aneuploidy detection
ActiveUS20150051085A1Assessing genomic copy number sensitively and rapidlyNucleotide librariesMicrobiological testing/measurementCell freeTrisomy
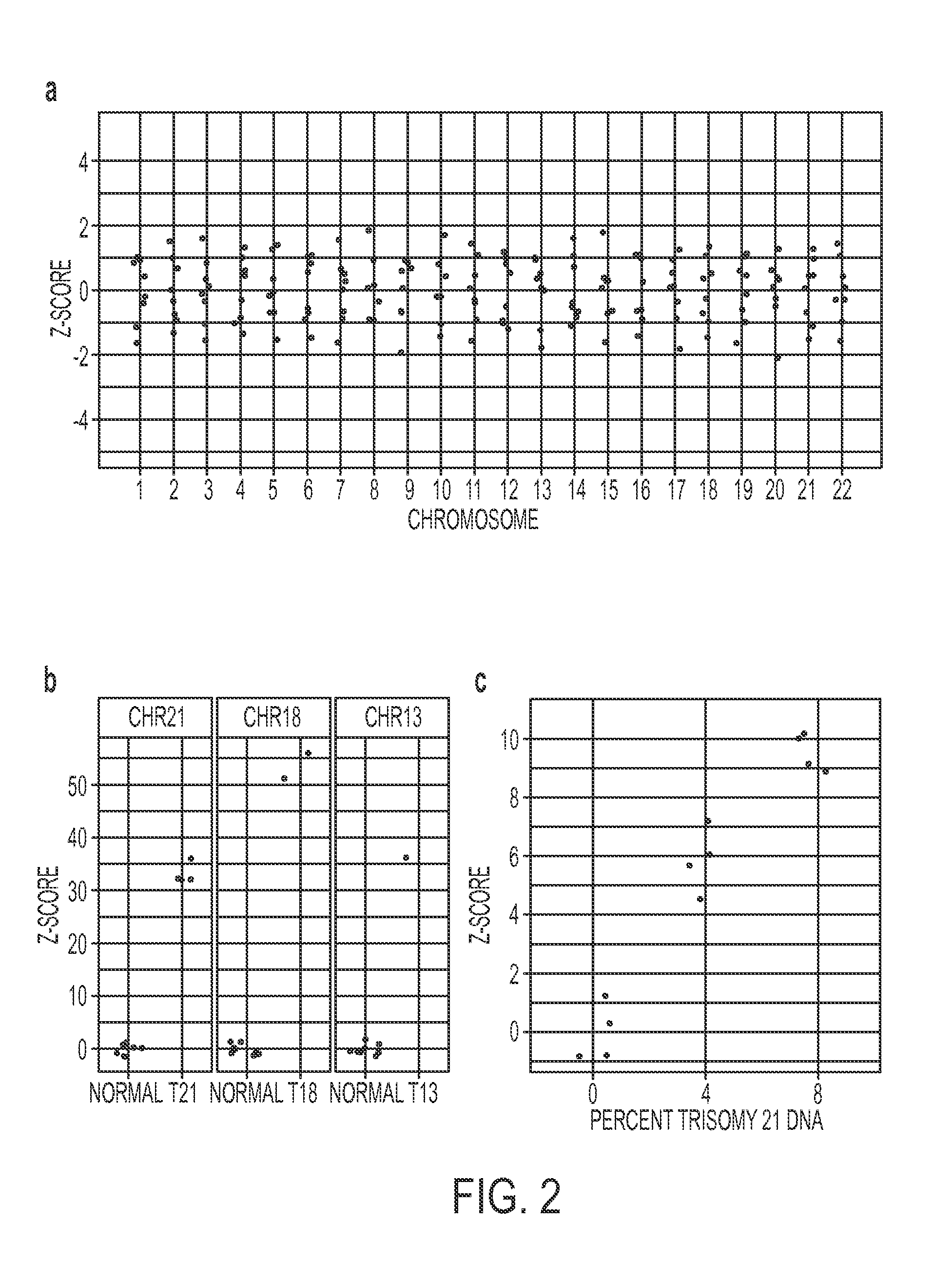
Massively parallel sequencing of cell-free, maternal plasma DNA was recently demonstrated to be a safe and effective screening method for fetal chromosomal aneuploidies. Here, we report an improved sequencing method achieving significantly increased throughput and decreased cost by replacing laborious sequencing library preparation steps with PCR employing a single primer pair. Using this approach, samples containing as little as 4% trisomy 21 DNA could be readily distinguished from euploid samples.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Markers for prenatal diagnosis and monitoring
Methods and kits are provided for diagnosing, monitoring, or predicting preeclaimpsia in a pregnant woman, trisomy 18 and trisomy 21 in a fetus, as well as for detecting pregnancy in a woman, by quantitatively measuring in the maternal blood the amount of one or more RNA species derived from a set of genetic loci and comparing the amount of the RNA species with a standard control.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Markers for prenatal diagnosis and monitoring
Methods and kits are provided for diagnosing, monitoring, or predicting preeclaimpsia in a pregnant woman, trisomy 18 and trisomy 21 in a fetus, as well as for detecting pregnancy in a woman, by quantitatively measuring in the maternal blood the amount of one or more RNA species derived from a set of genetic loci and comparing the amount of the RNA species with a standard control.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Methods and reagents for identifying rare fetal cells in the maternal circulation
Owner:SCHUELER PAULA A +6
Non-Invasive Fetal Genetic Screening by Digital Analysis
The present methods are exemplified by a process in which maternal blood containing fetal DNA is diluted to a nominal value of approximately 0.5 genome equivalent of DNA per reaction sample. Digital PCR is then be used to detect aneuploidy, such as the trisomy that causes Down Syndrome. Since aneuploidies do not present a mutational change in sequence, and are merely a change in the number of chromosomes, it has not been possible to detect them in a fetus without resorting to invasive techniques such as amniocentesis or chorionic villi sampling. Digital amplification allows the detection of aneuploidy using massively parallel amplification and detection methods, examining, e.g., 10,000 genome equivalents.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
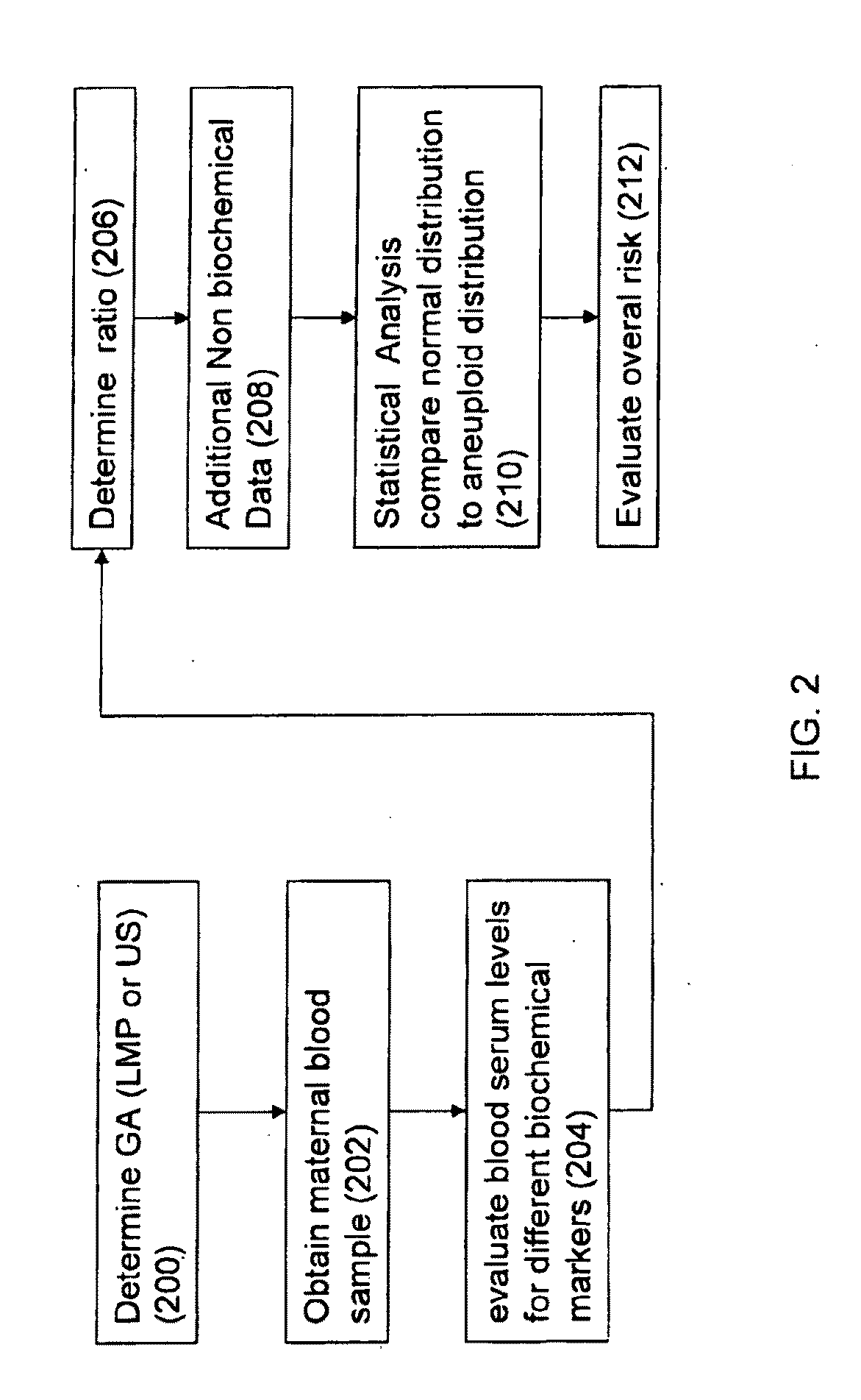
Method for antenatal estimation of risk of aneuploidy
InactiveUS20100120076A1Reducing false positive detectionImprove the detection rateMicrobiological testing/measurementBiological material analysisGenetic AnomalyTrisomy
The present invention relates to a system and a method for evaluating the risk of carrying a fetus with genetic anomalies such as aneuploidy and in particular, to such a system and method where a screening system and method is provided to identify fetus' having trisomy-21 (Down's syndrome) with the use of biochemical marker concentrations evaluated from the maternal blood serum.
Owner:BRAUN GUR +1
Non-Invasive Fetal Genetic Screening by Digital Analysis
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods for identifying chromosomal aneuploidy
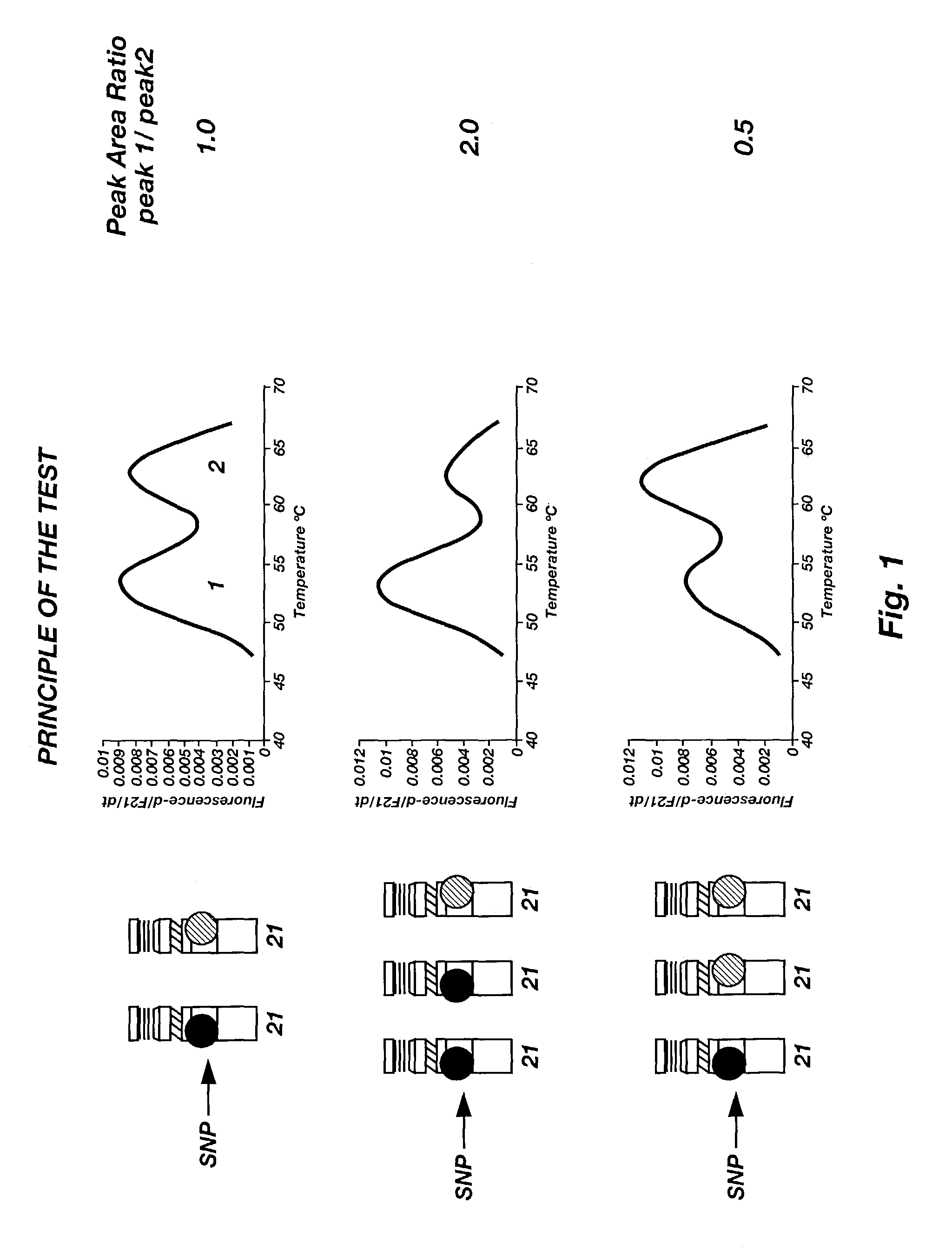
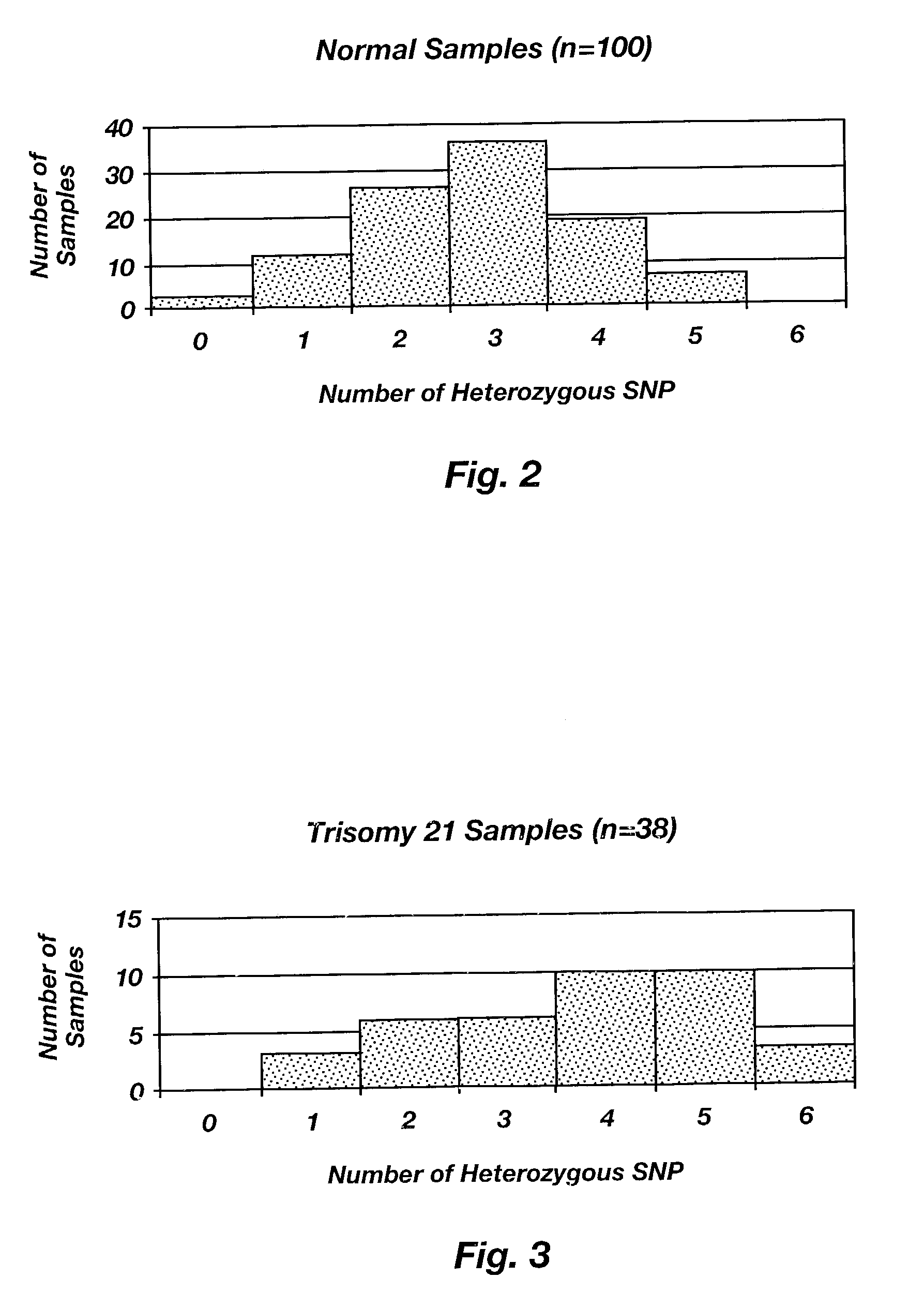
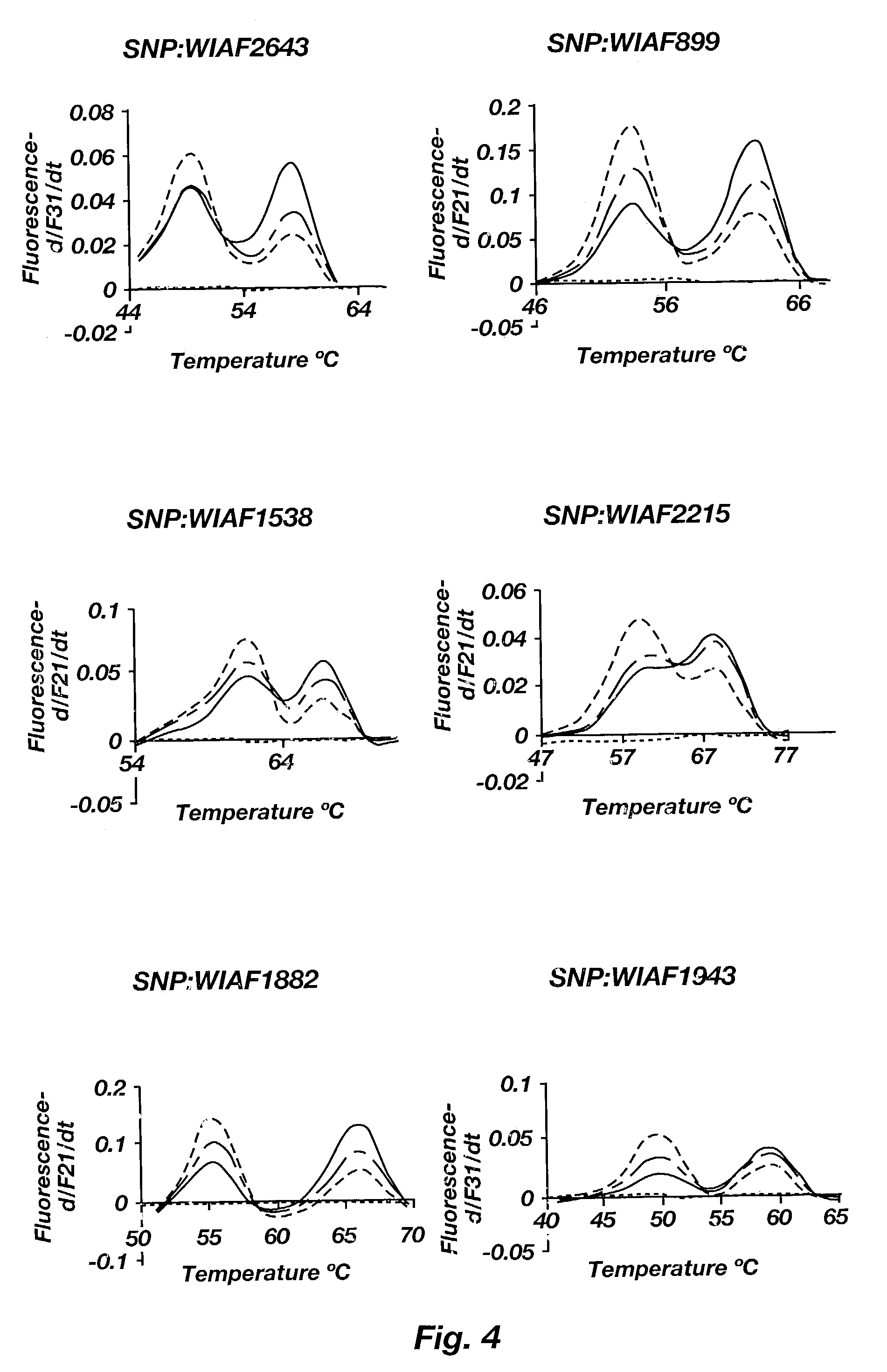
InactiveUS6979541B1Precise definitionSugar derivativesMicrobiological testing/measurementHeterozygous snpsTrisomy
Methods are disclosed for identifying chromosomal aneuploidy using heterozygous SNPs in a melting curve analysis. In one aspect, a panel of SNPs may be used. The heterozygous nature of the SNPs may in some aspects, act as an internal control for the melting curve analysis, and alleviate the need for external controls or competitors. In another aspect, each of the SNP in the panel may have a heterozygocity index of greater than about 30%. While a number of aneuploidies may be identified using the disclosed methods, in one aspect, the chromosomal aneuploidy identified may be trisomy 21.
Owner:UNIV OF UTAH RES FOUND
Methods of prenatal screening for trisomy 21
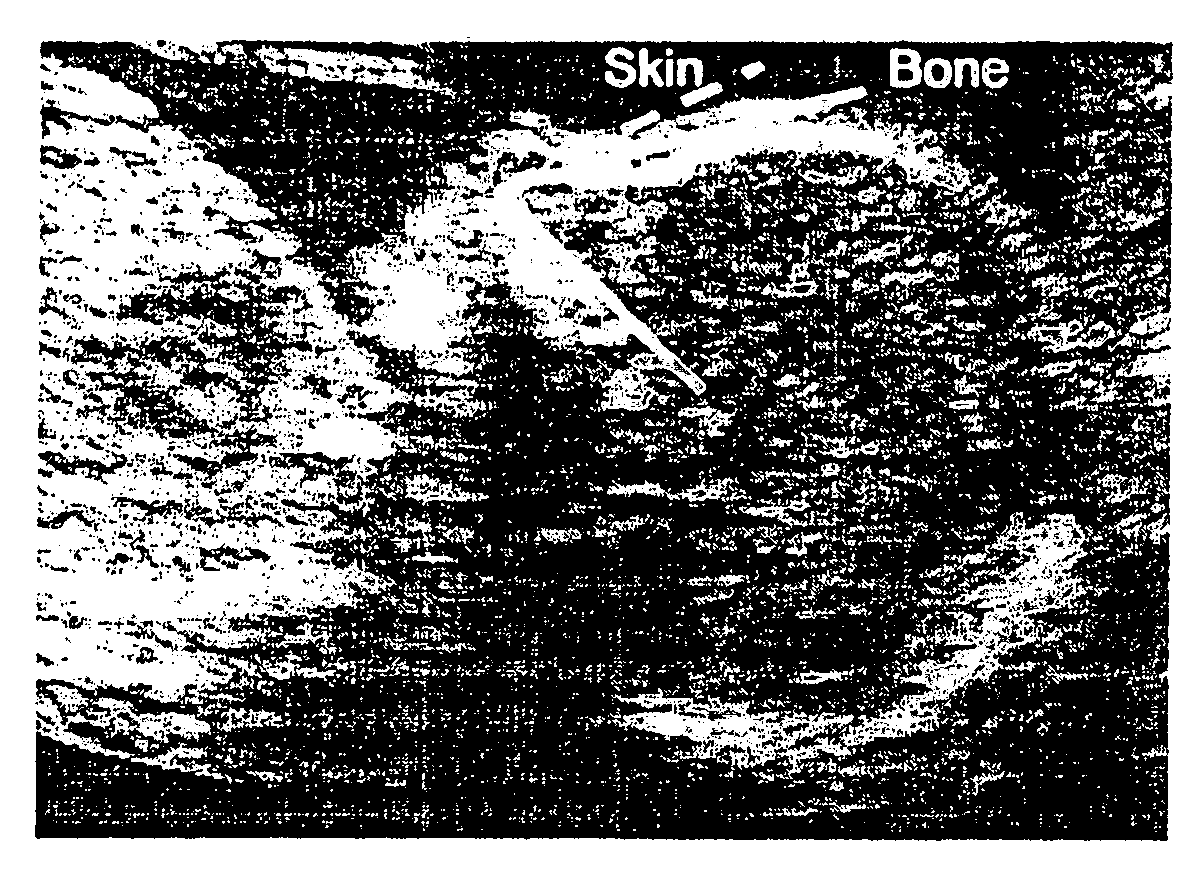
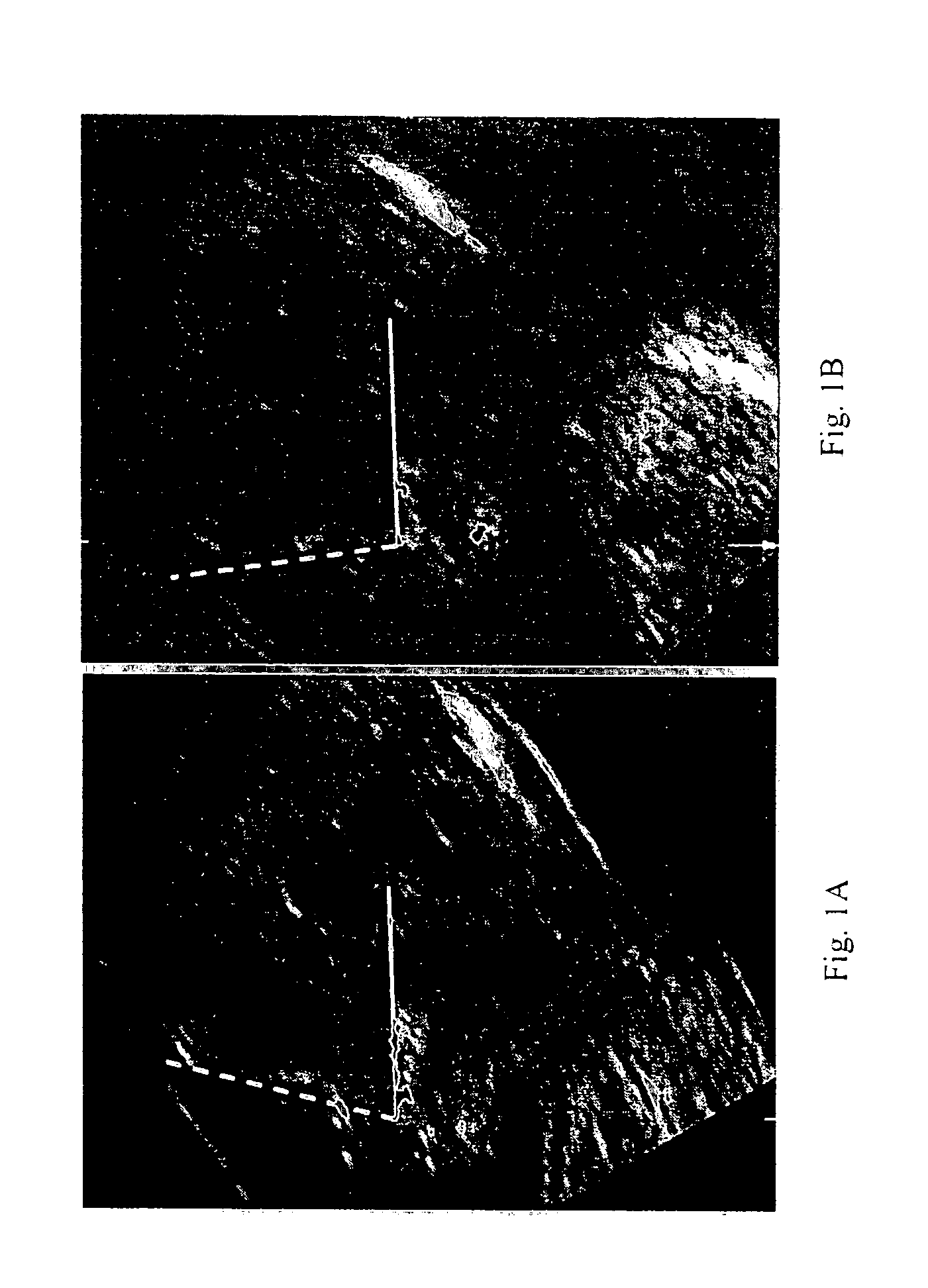
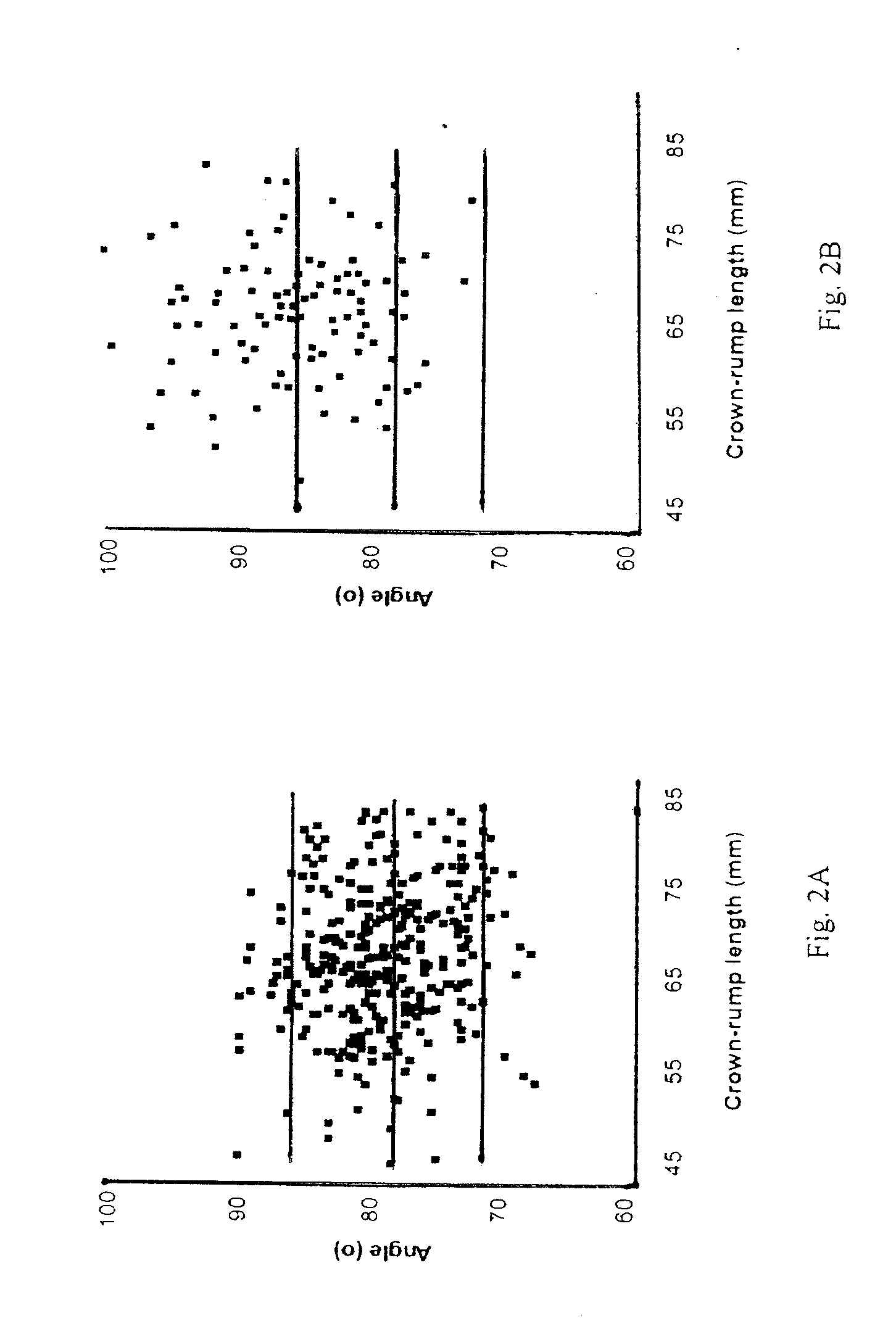
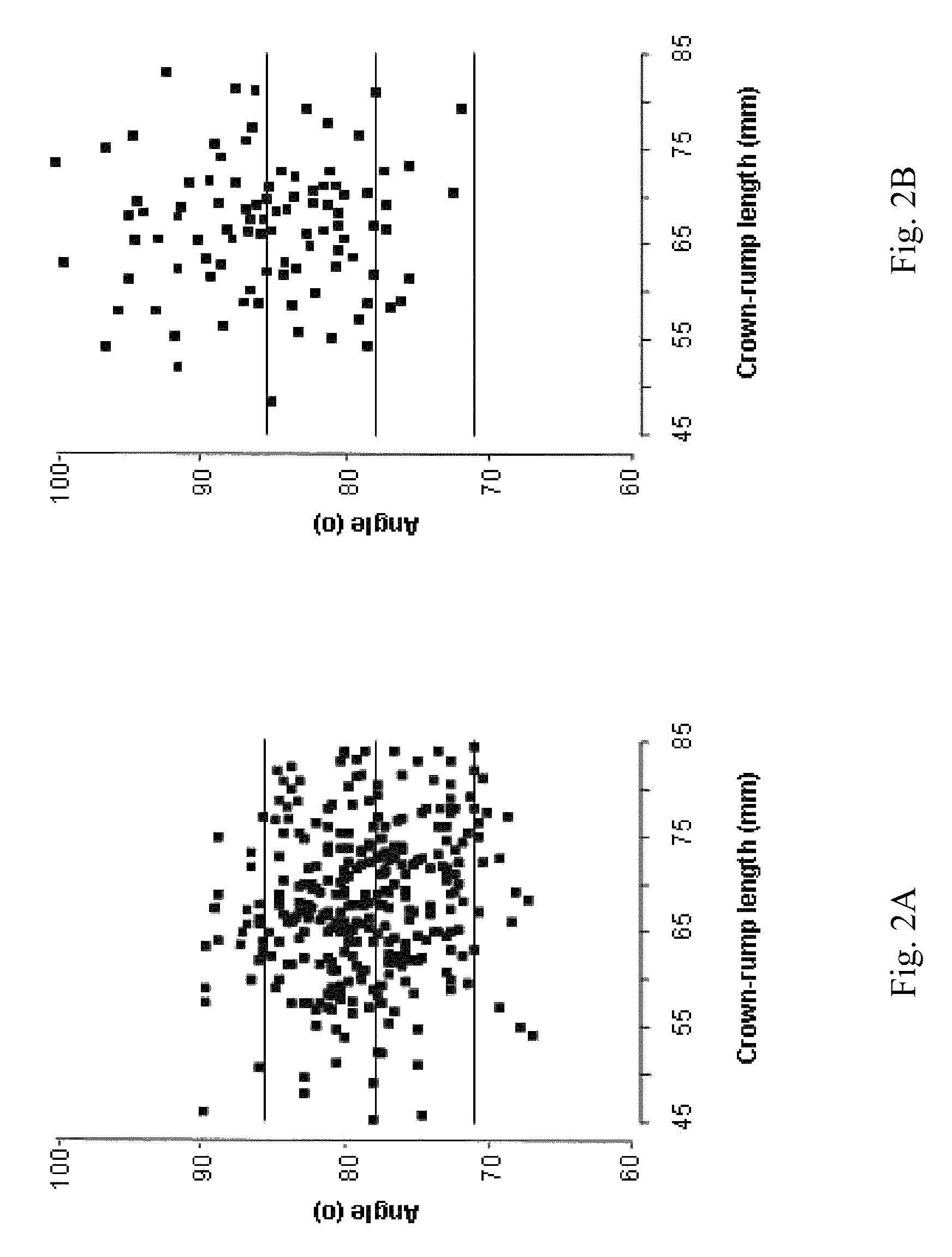
InactiveUS20080188748A1Raise the possibilityUltrasonic/sonic/infrasonic diagnosticsPerson identificationNormal fetusTrisomy
Methods for prenatal screening for trisomy 21 employ examination of the fronto-maxillary facial (FMF) angle of a fetus. In one embodiment, the methods comprise obtaining a two or three dimensional image of a fetal face, measuring the FMFbone angle on the image, and comparing the measured FMFbone angle with an FMFbone angle characteristic of chromosomally normal fetuses, wherein a measured FMFbone angle greater than the FMFbone angle characteristic of chromosomally normal fetuses provides an indication of an increased likelihood of the occurrence of trisomy 21 in the fetus. In another embodiment, the methods comprise obtaining a two or three dimensional image of a fetal face, measuring the FMFskin angle on the image, and comparing the measured FMFskin angle with an FMFskin angle characteristic of chromosomally normal fetuses, wherein a measured FMFskin angle greater than the FMFskin angle characteristic of chromosomally normal fetuses provides an indication of an increased likelihood of the occurrence of trisomy 21 in the fetus. In additional embodiments, both FMFbone angle and FMFskin angle are measured and compared with values characteristic of chromosomally normal fetuses.
Owner:SONEK JIRI D +1
Reagent box for detecting No 21 chromosome and idiochromosome number abnormality
The invention relates to a kit used for screening the numerical abnormality of 21-trisomy and sex chromosome at early stage. The kit adopts quantitative fluorescence multiplex polymerase chain reaction technology, carries out the fluorescent primer sevenfold multiplex amplification respectively to differential genetic locus on the 21-trisomy and sex chromosome and analyzes the numerical abnormality of chromosome according to the difference of the dosage of amplified products, thus achieving the purpose of detecting the numerical abnormality of 21-trisomy and sex chromosome.
Owner:DAAN GENE CO LTD
Amplification composition for detecting abnormal number of chromosomal aneuploid and rapid detection kit
ActiveCN104651488AShort detection cycleHigh speedMicrobiological testing/measurementDNA/RNA fragmentationY chromosomeX chromosome
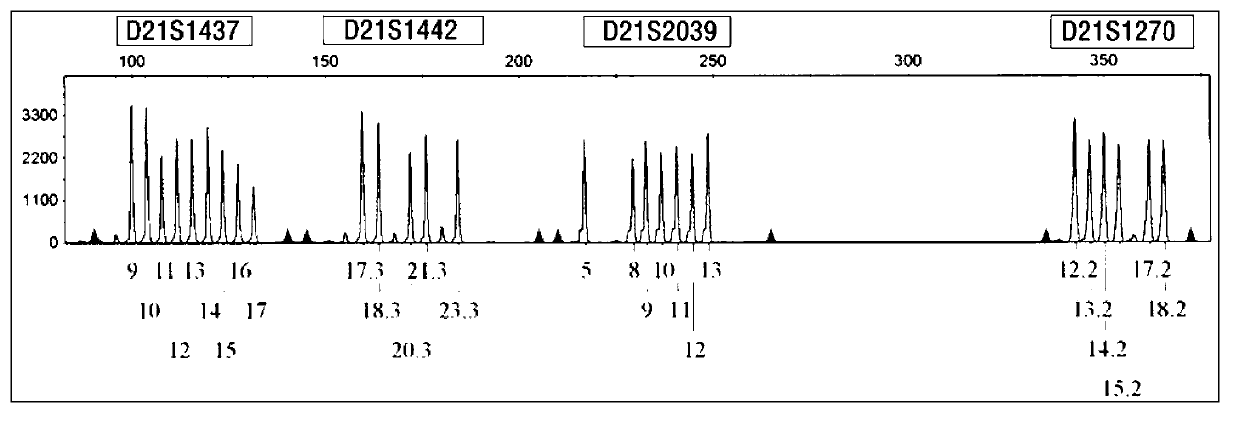
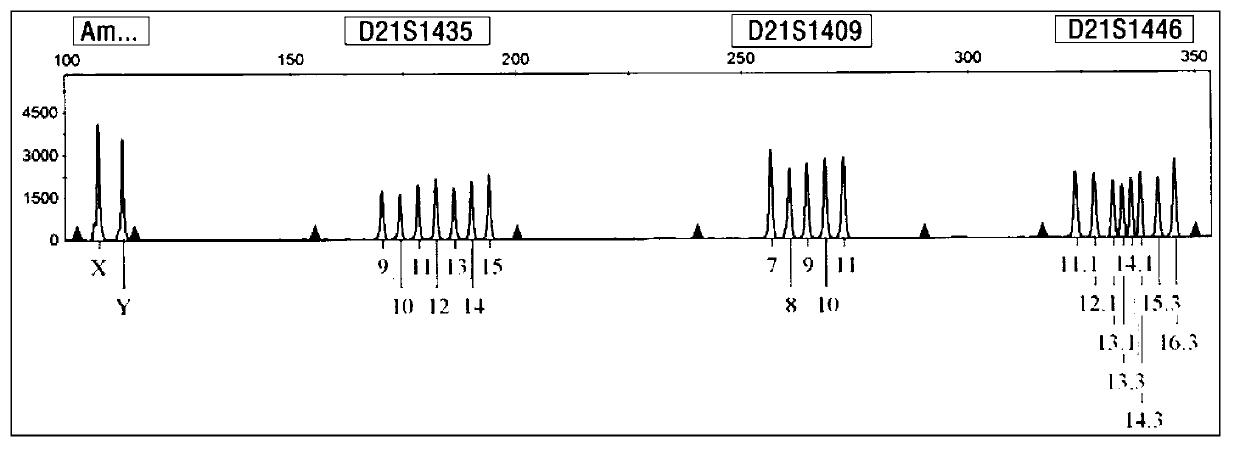
The invention relates to an amplification composition for detecting abnormal number of chromosomal aneuploid and a rapid detection kit, and belongs to the technical field of biology. The amplification composition can be used for amplification detection of STR sites related to 8 21# chromosomes, 6 18# chromosomes, 6 13# chromosomes and 3 X chromosomes and amplification detection of four amelo; all sites can be amplified by a quantitative fluorescent PCR (QF-PCR) technology, so as to detect the abnormal number of 21, 18, 13, X and Y chromosomal aneuploid. According to the kit, detection cycle is short, so the result can be obtained within 5 hours after obtaining the sample; the sensitivity is high; DNA at nanogram level, which is equal to DNA contained in 100 cells, is needed for each detection, so few samples are needed; 1 to 2ml of amniotic fluid can be collected to meet the demand; due to high sensitivity, a chimera containing more than 10% of trisomies can be detected.
Owner:BEIJING MICROREAD GENE TECH
Kit for detecting aneuploidy of five human chromosomes through monotube multiple amplification
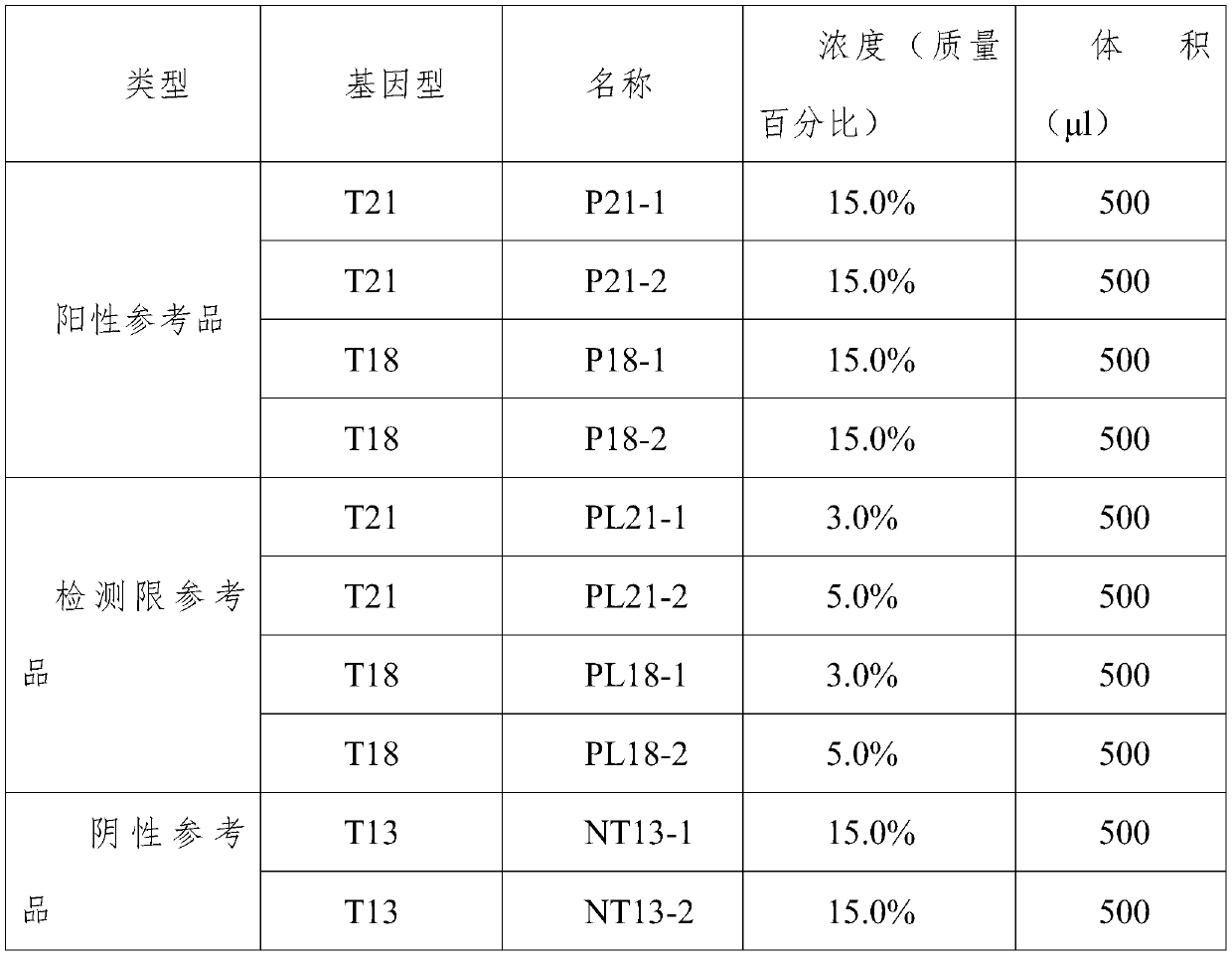
ActiveCN103555849AHigh detection sensitivityStrong specificityMicrobiological testing/measurementTrisomy 13 SyndromeReference product
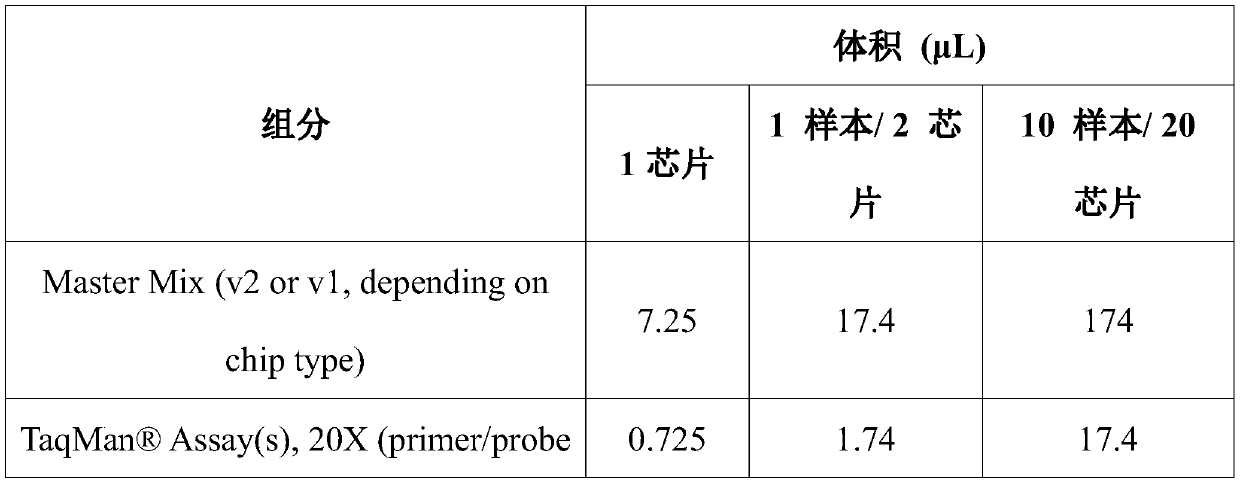
The invention relates to a kit for detecting the STR (Short Tandem Repeat) genetype of human chromosomes 21, 18 and 13 and sex chromosomes, and particularly relates to a QF-PCR (Quantitative Fluorescence-Polymerase Chain Reaction) kit for detecting the number of the chromosomes 21, 18 and 13 and the sex chromosomes by adopting five-color fluorescence labeling monotube fast multiple amplification and mainly for diagnosing 21 trisomy syndrome, 18 trisomy syndrome, 13 trisomy syndrome and the aneuploid abnormality of the sex chromosomes. The kit comprises a primer mixture, a hot start C-Taq enzyme, an amplification reaction solution, a positive quality control product, a negative reference product, a fluorescence interior label Siz-500 and an allelic gene typing standard substance. Compared with the traditional antenatal diagnosis method, the kit disclosed by the invention can realize high-flux, fast, reliable and standardized detection.
Owner:AGCU SCIENTECH +1
Preimplantation genetic diagnosis on embryo by using new single cell nucleic acid amplification technology
InactiveCN102094083APrevent birthBirth to avoidMicrobiological testing/measurementRecessive inheritanceCentral dogma of molecular biology
The invention relates to preimplantation genetic diagnosis on an embryo by using new single cell nucleic acid amplification technology, which mainly utilizes the signal amplification action of the mRNA (messenger Ribose Nucleic Acid) to detect the multiplication or deletion of DNAs (Deoxyribonucleic Acids) of certain chromosome segments. According to the central dogma, the mRNA is transcribed by using the DNA as the template, the abnormity of the number of copies of the DNA template can cause the change of the quantity of the mRNAs; and in the transcription process, the multiplication or deletion of the DNA template can be amplified on the mRNA level, and can be easily detected. The main technical method is as follows: the single cell mRNA of a human embryo is subjected to PCR (Polymerase Chain Reaction) amplification after being subjected to reverse transcription and addition of a common primer; by using the amplification product as the template, quantitative PCR with a 96-pore plate is used for detecting the expression level of 8 genes of a single cell or a small amount of cells; and the detection result can be compared with a normal diploid embryo, so as to distinguish the embryo sex of X chromosome recessive inheritance family history, and the multiplication and deletion of Trisomy 21, Trisomy 18, Trisomy 13 and sex chromosome and carry out genetic diagnosis on some common chromosome anomalies.
Owner:PEKING UNIV
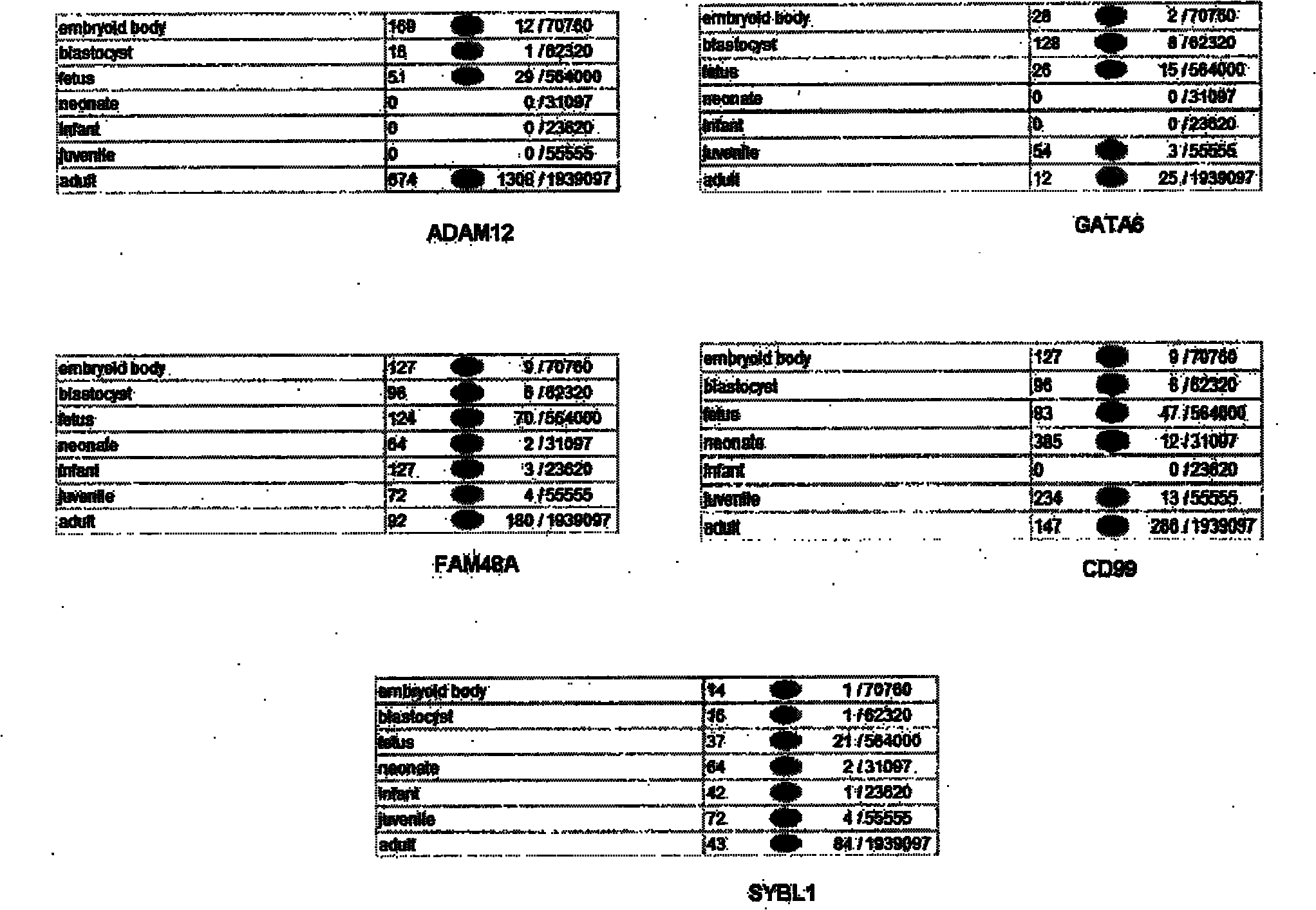
ADAM12, a novel marker for abnormal cell function
InactiveUS20060134654A1Improve the detection rateReduce false alarm rateMicrobiological testing/measurementPeptide preparation methodsDiseaseADAM12
The present invention provides a method, an assay and a kit for providing an indication of abnormal cell function. It was surprisingly found that the change in the serum ADAM12 concentration in individuals was useful as a prognostic tool to predict the clinical outcome, complications and mortality following an abnormal cell function. The present inventors describes ADAM12 as a overall general marker for abnormal cell function, and the present inventor for the first time demonstrate that ADAM12 is an important indicator of fetal chromosomal disease and placenta function. Specifically ADAM12 is a good marker for e.g. Downs's syndrome, trisomy 18, preeclampsia, Turner syndrome in both first and second trimester. The present inventors developed an enzyme-linked immunosorbent assay (ELISA) and a time-resolved immunofluorometric assay for the quantification of ADAM12 in serum. The present application demonstrates in several examples the variation of the ADAM12 level in fetal abnormality and / or adverse pregnancy outcomes correlated gestational age when compared to normal controls. It is an object of the invention to provide an improvement of the existing marker tests that exhibits a decreased false positive rate.
Owner:STATENS SERUM INST +2
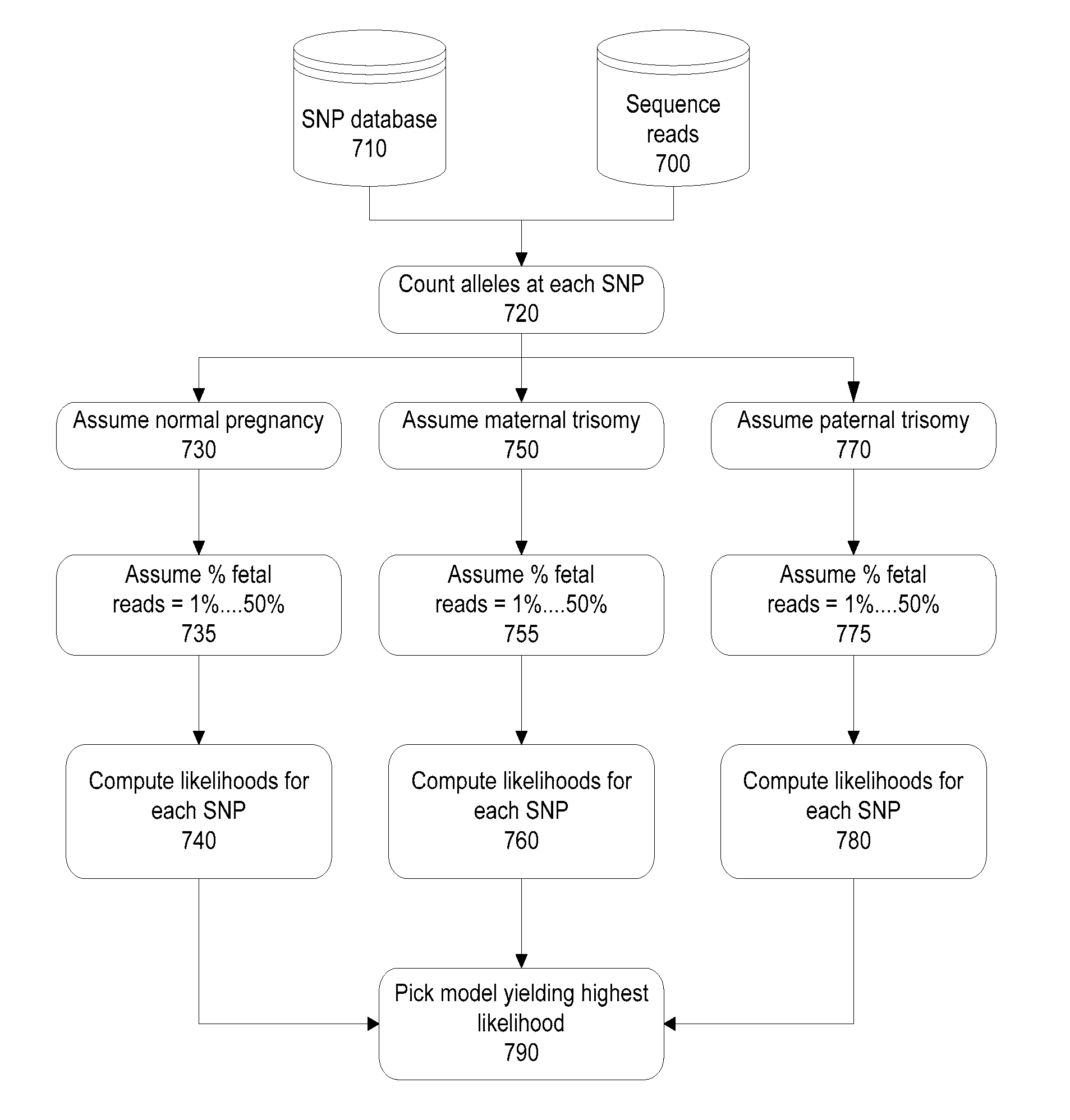
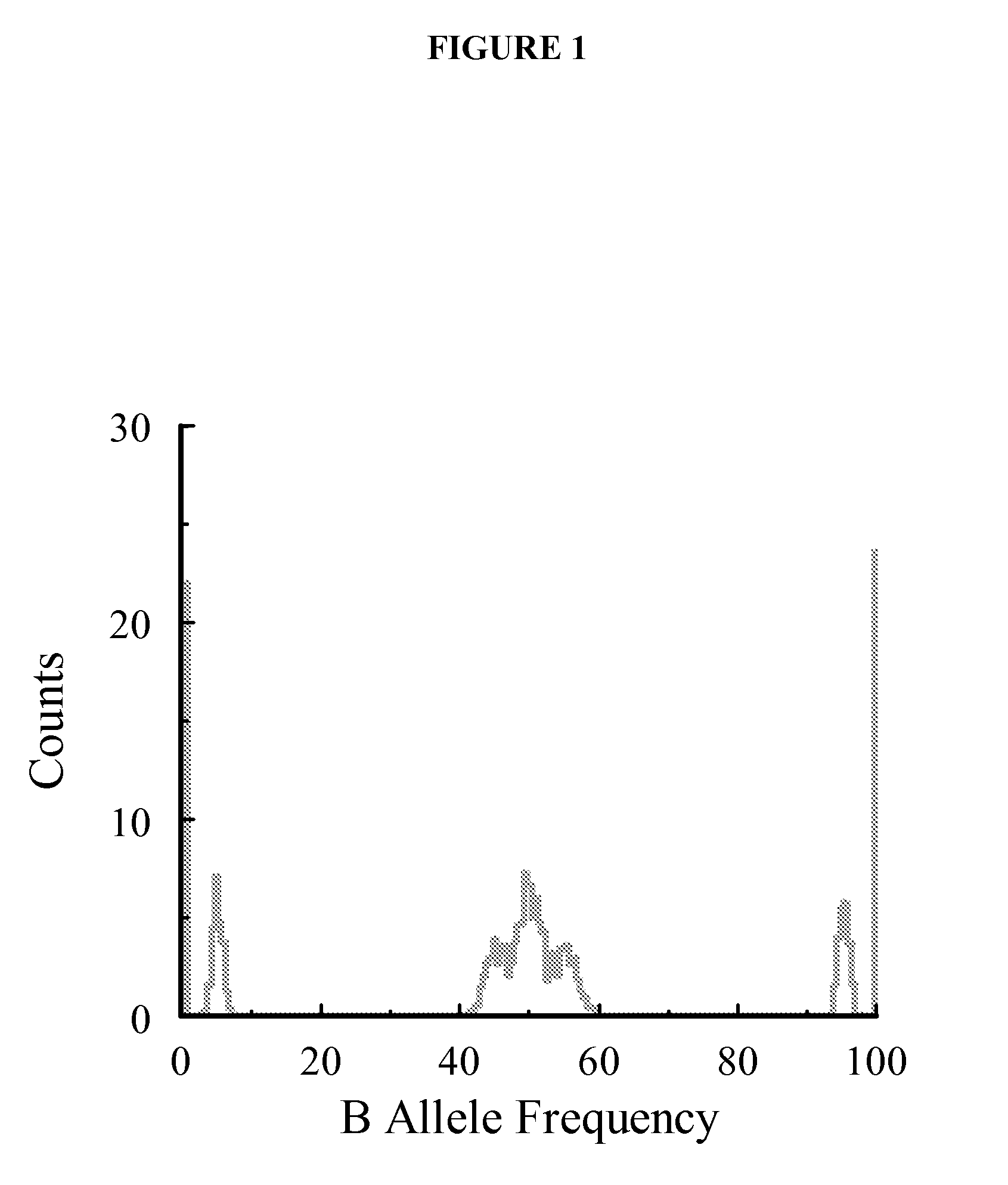
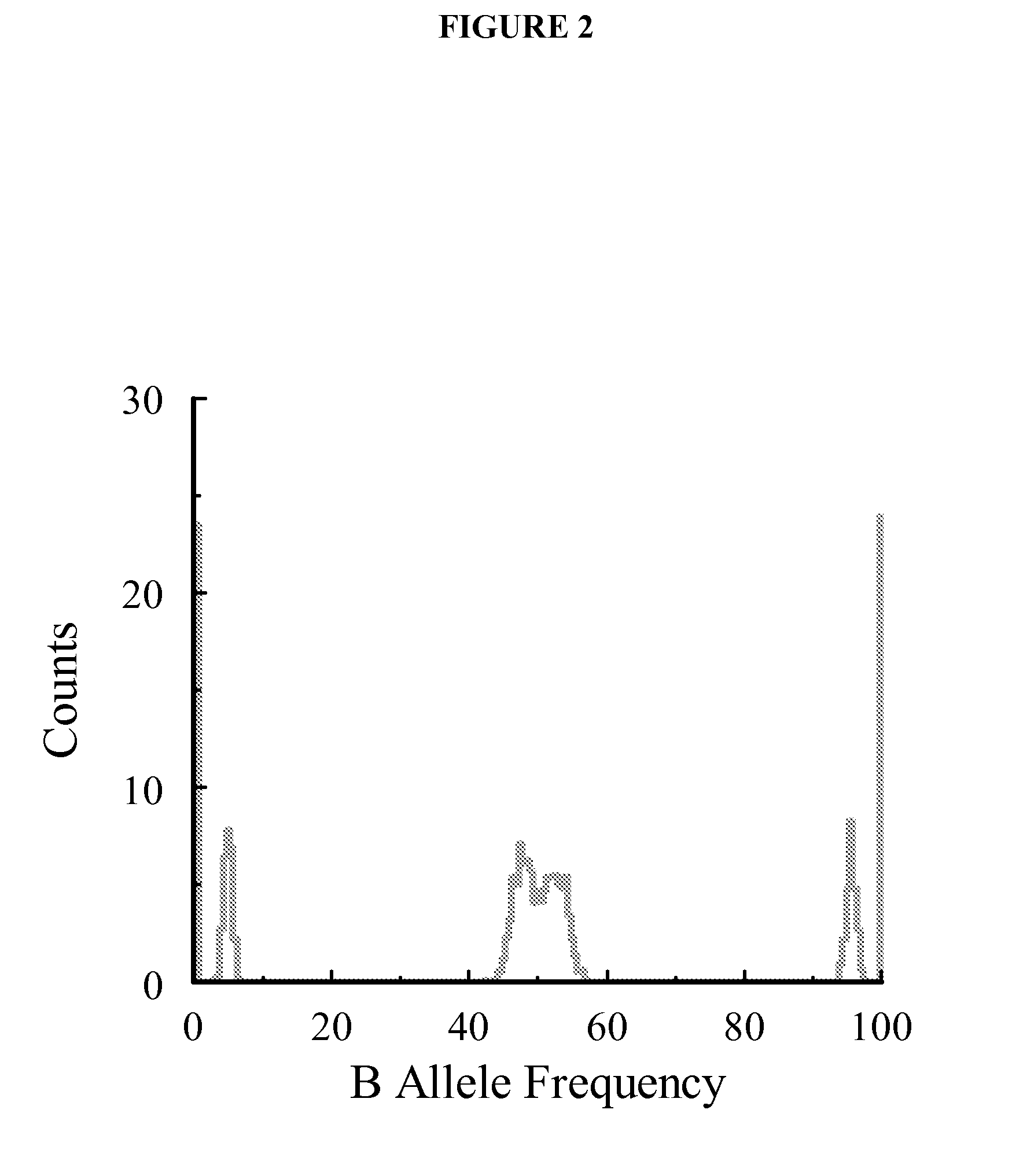
Methods and systems for determining fetal chromosomal abnormalities
InactiveUS20130261984A1Minimize or eliminate false negativesMicrobiological testing/measurementMedical automated diagnosisTrisomyGenotype
The present disclosure provides methods and systems for determining the presence or absence of aneuploidy in a fetus. In particular, the present disclosure provides noninvasive methods and systems for detecting the presence of fetal trisomy and other fetal chromosomal anomalies, paternity of a fetus and fetal genotype.
Owner:ILLUMINA INC
Biomarkers for the detection and screening of down syndrome
InactiveUS20120288873A1Nervous disorderMaterial analysis by observing effect on chemical indicatorBile fluidBiology
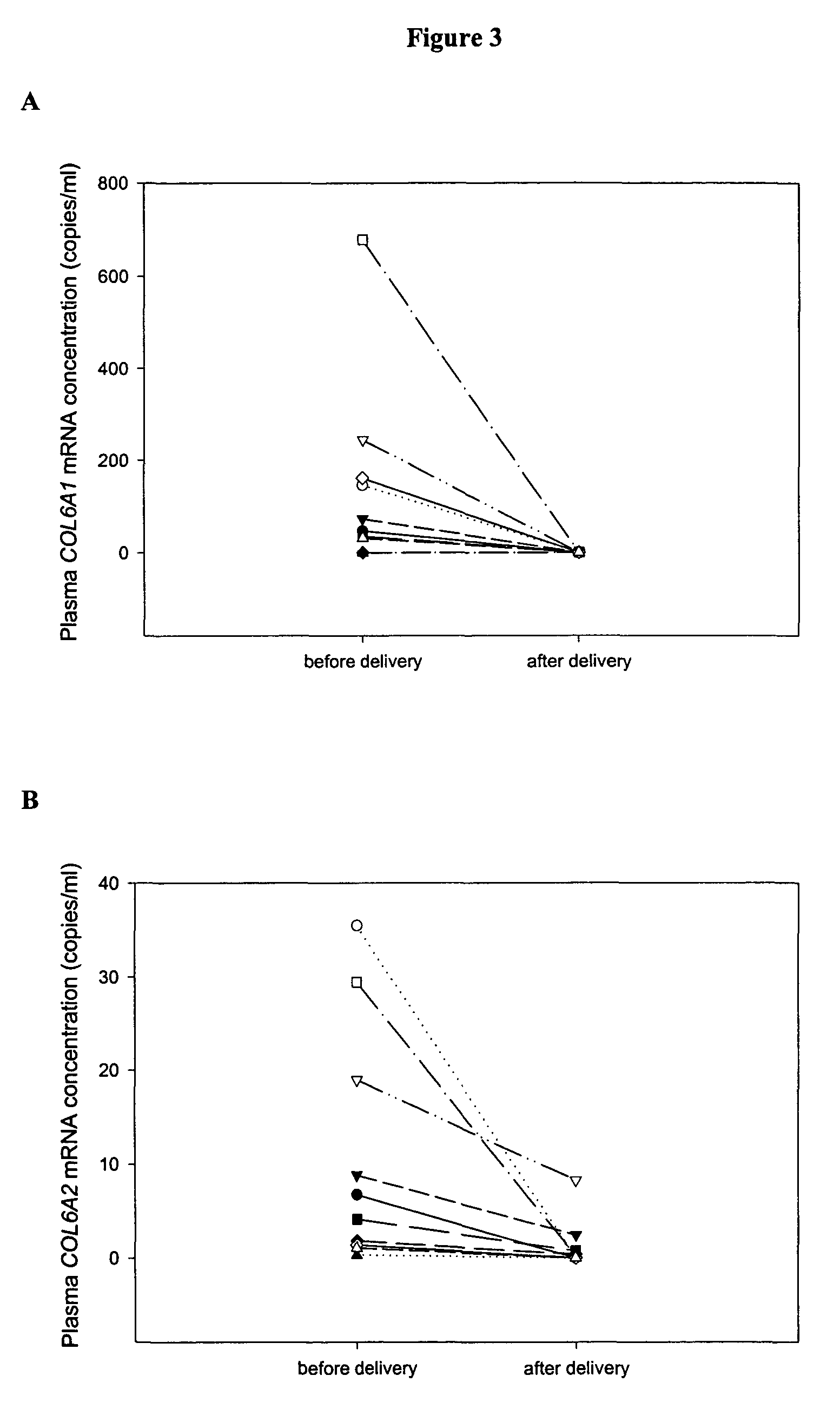
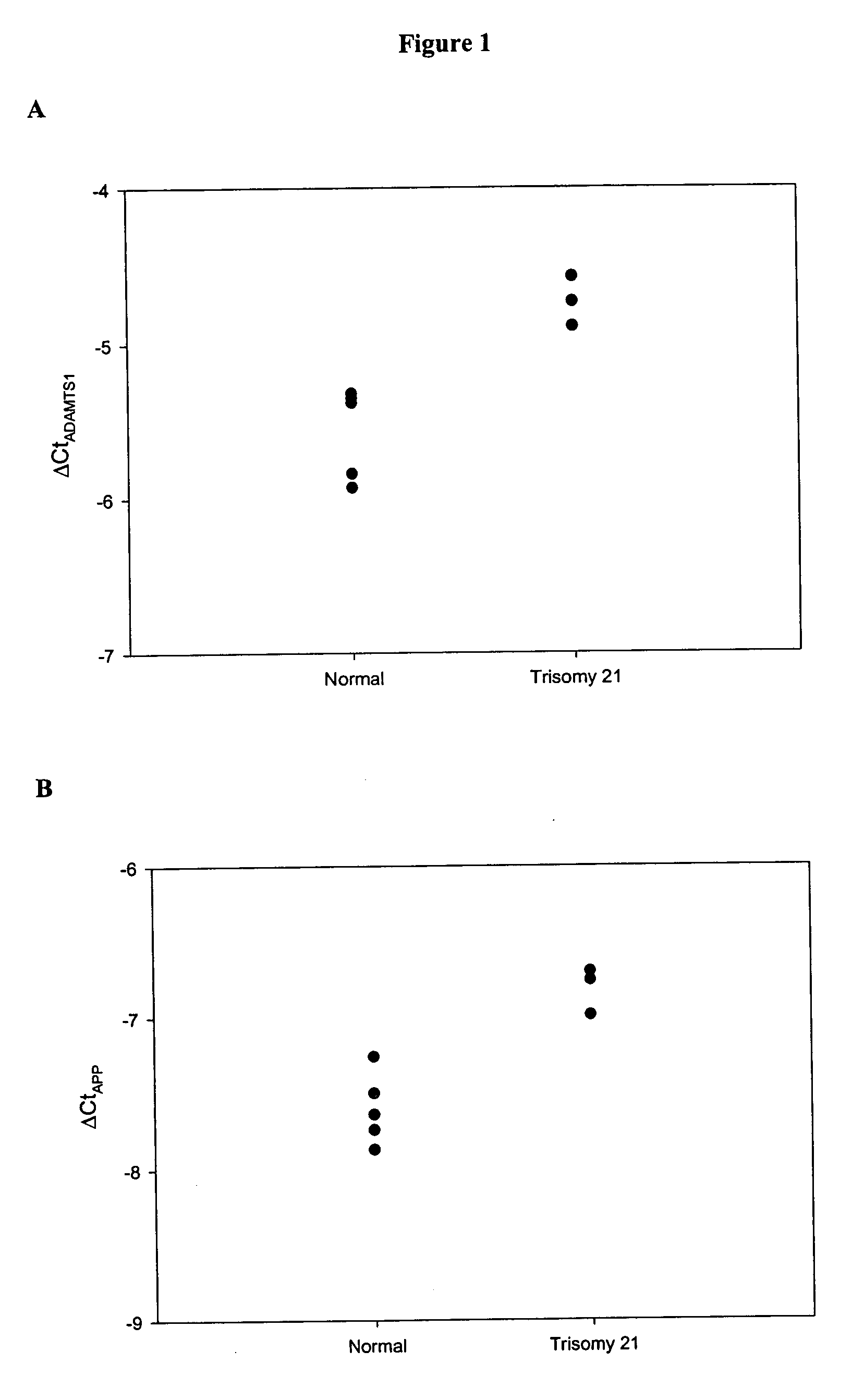
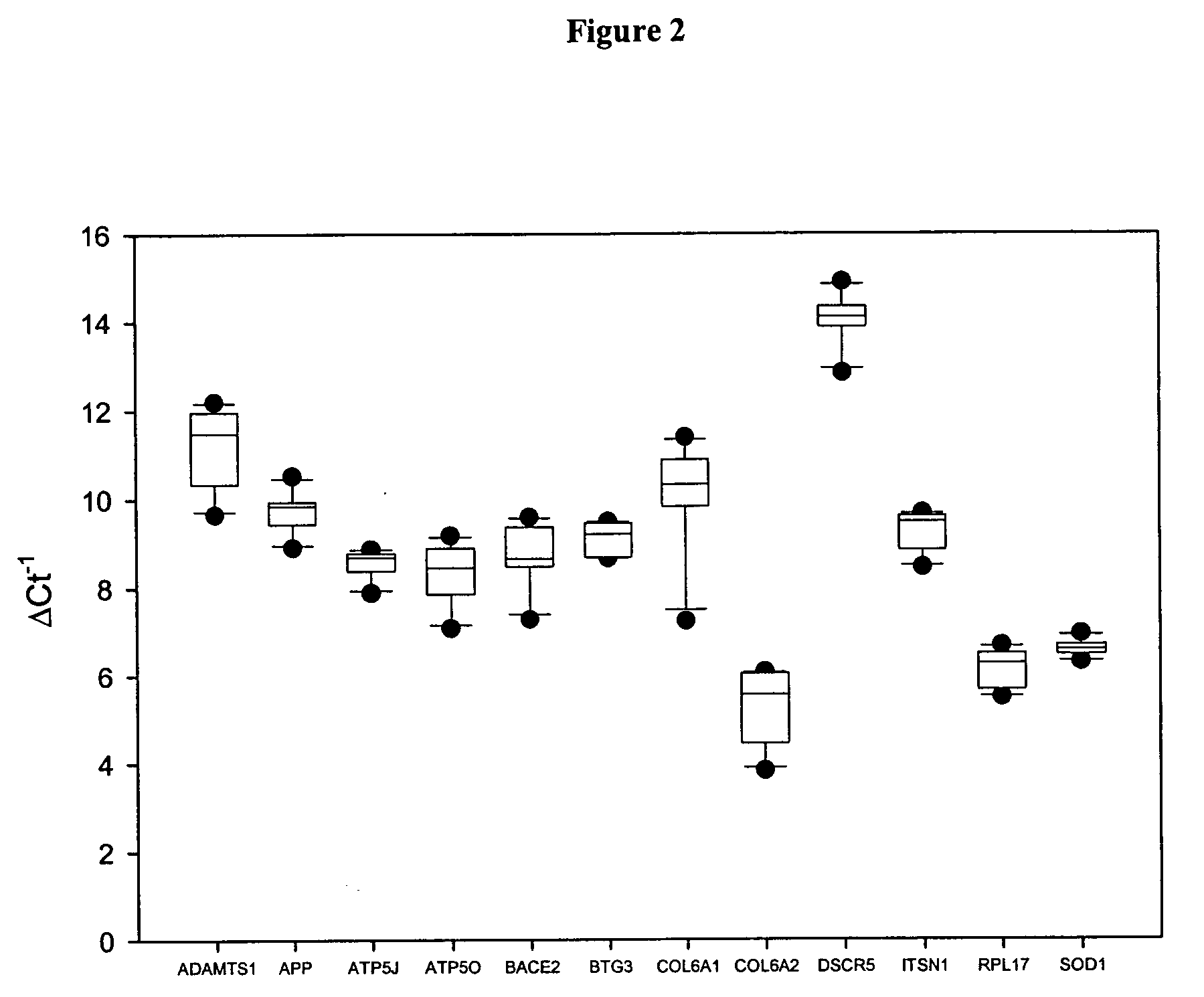
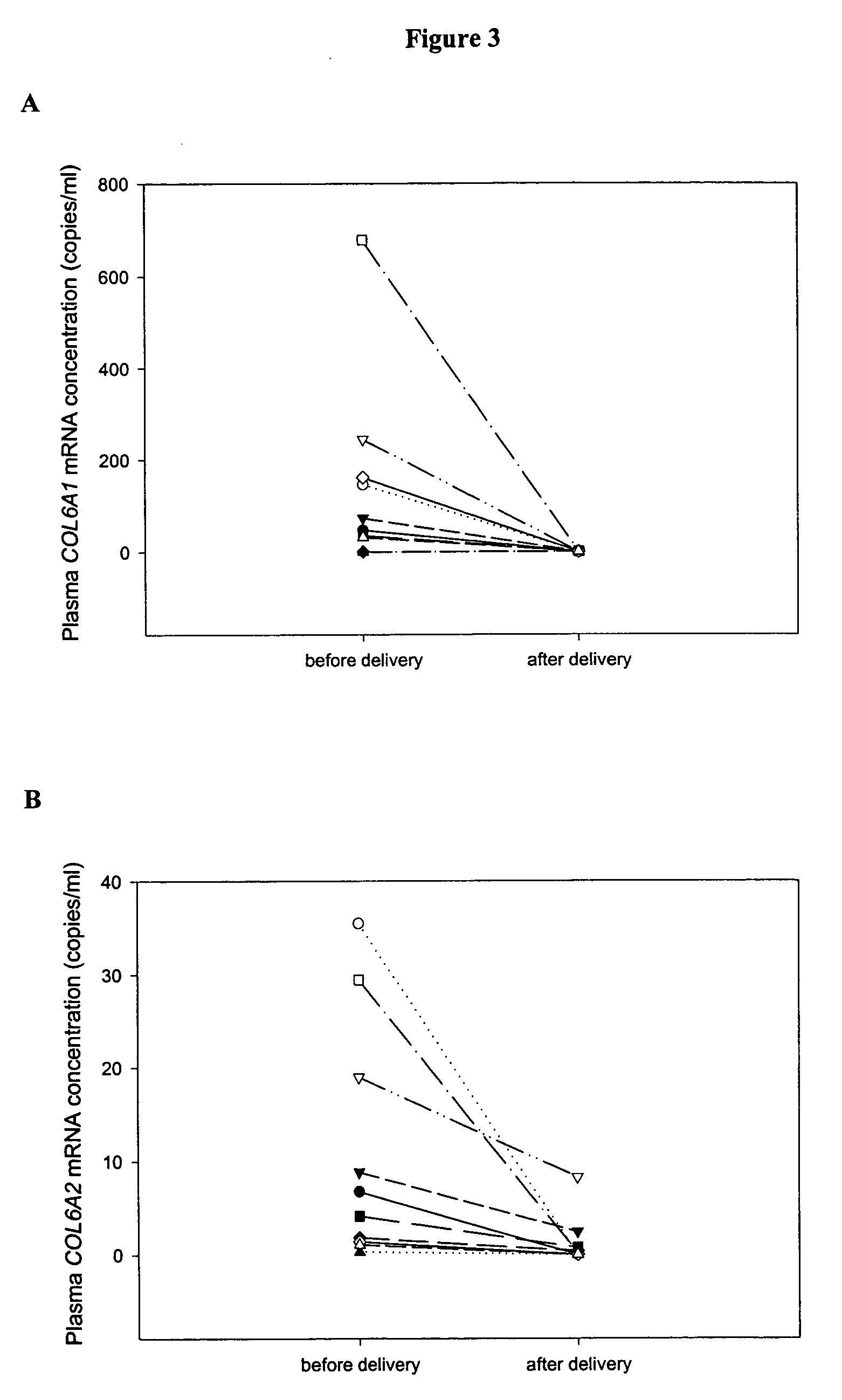
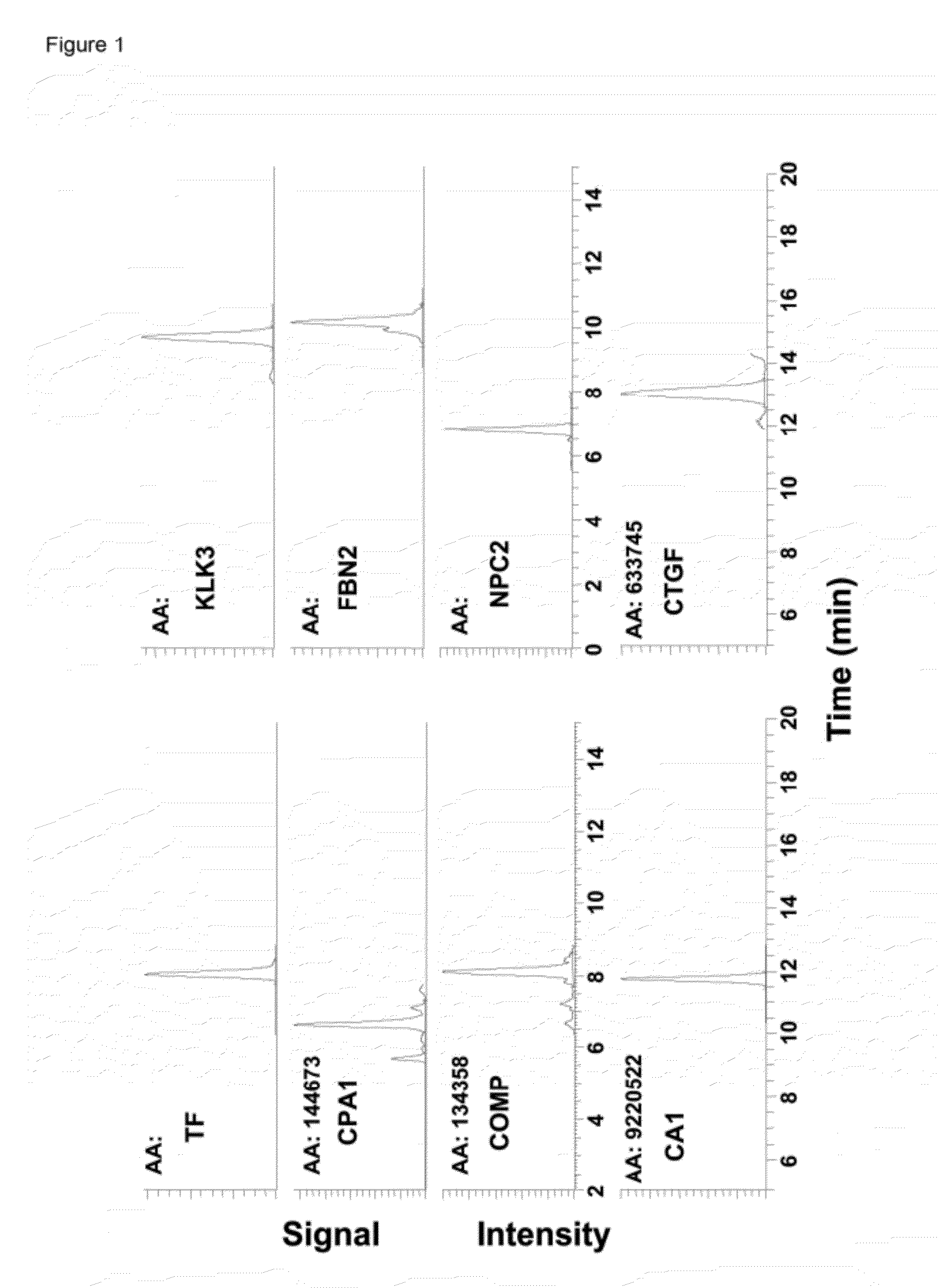
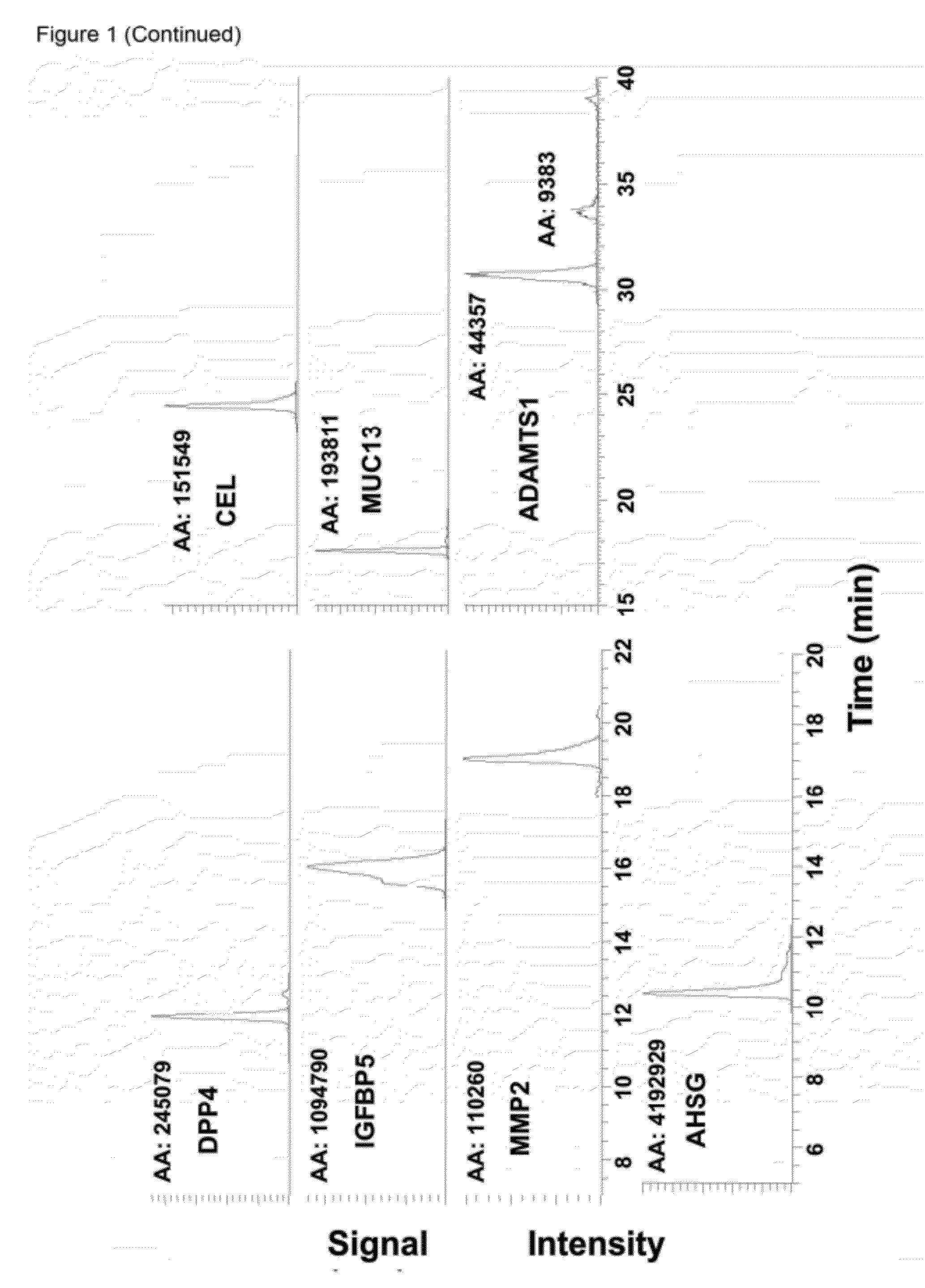
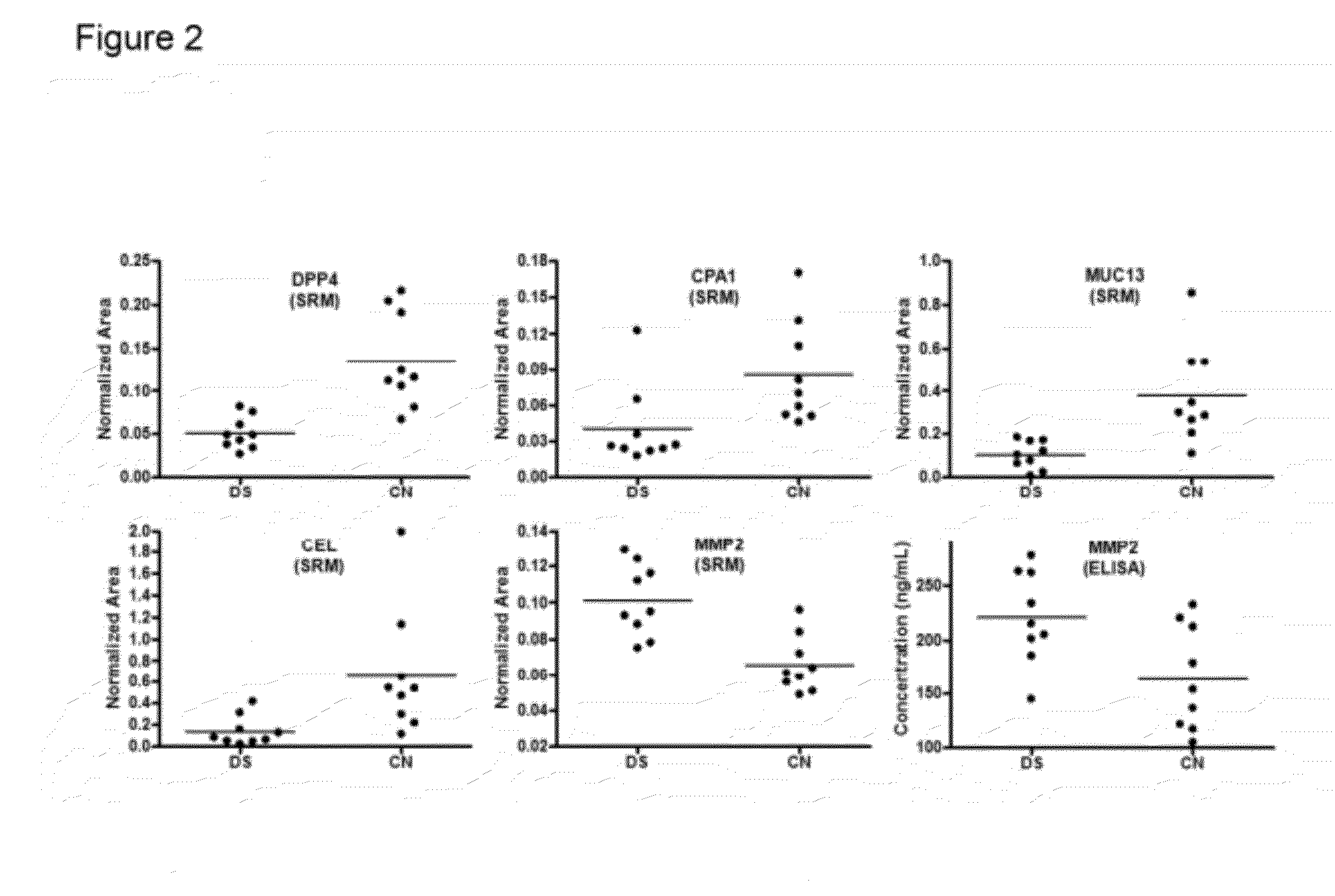
The disclosure includes assays and methods for screening for risk of Down syndrome and / or trisomy 21 in a fetus. The assays and methods comprise determining the level of at least one biomarker selected from mucin 13 (MUC13), bile salt-activated lipase (CEL), dipeptidyl peptidase 4 (DPP4), carboxypeptidase A1 (CPA1), amyloid precursor protein (APP) and tenascin-C (TNC-C) polypeptides in a test biological sample from a pregnant subject, wherein a decreased level of MUC13, CEL, DPP4, and / or CPA1 polypeptide and / or an increased level of APP and / or TNC-C polypeptide in the test biological sample compared to a corresponding reference biomarker polypeptide level indicates an increased risk of Down syndrome or trisomy 21 in the fetus. The disclosure also includes assays, compositions, immunoassays, and kits for performing the methods disclosed herein.
Owner:UNIV HEALTH NETWORK
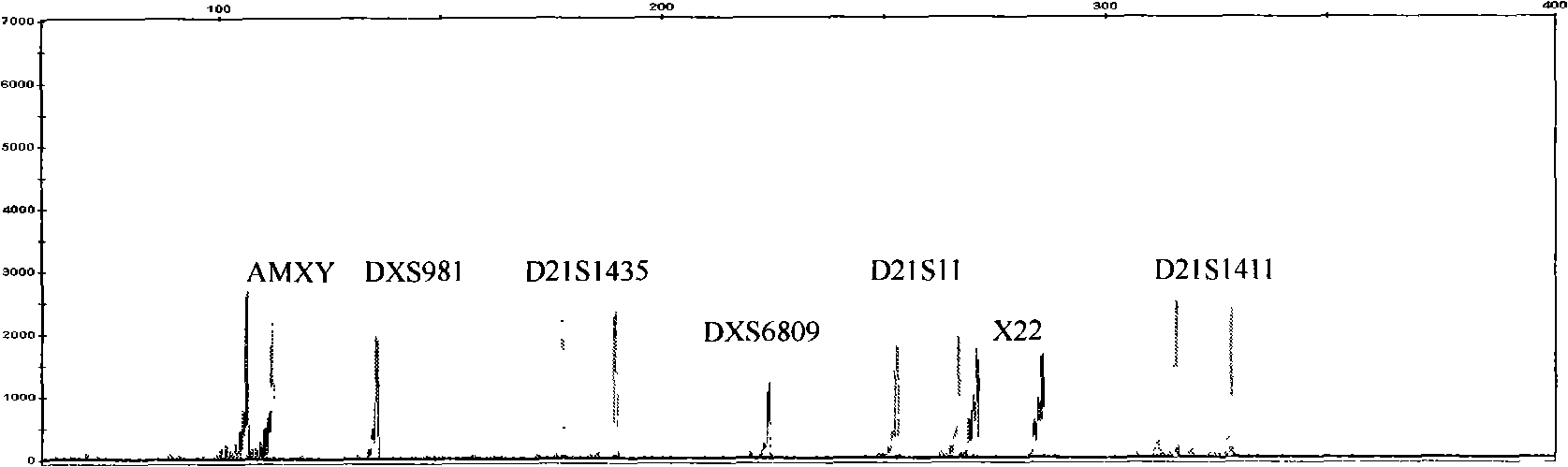
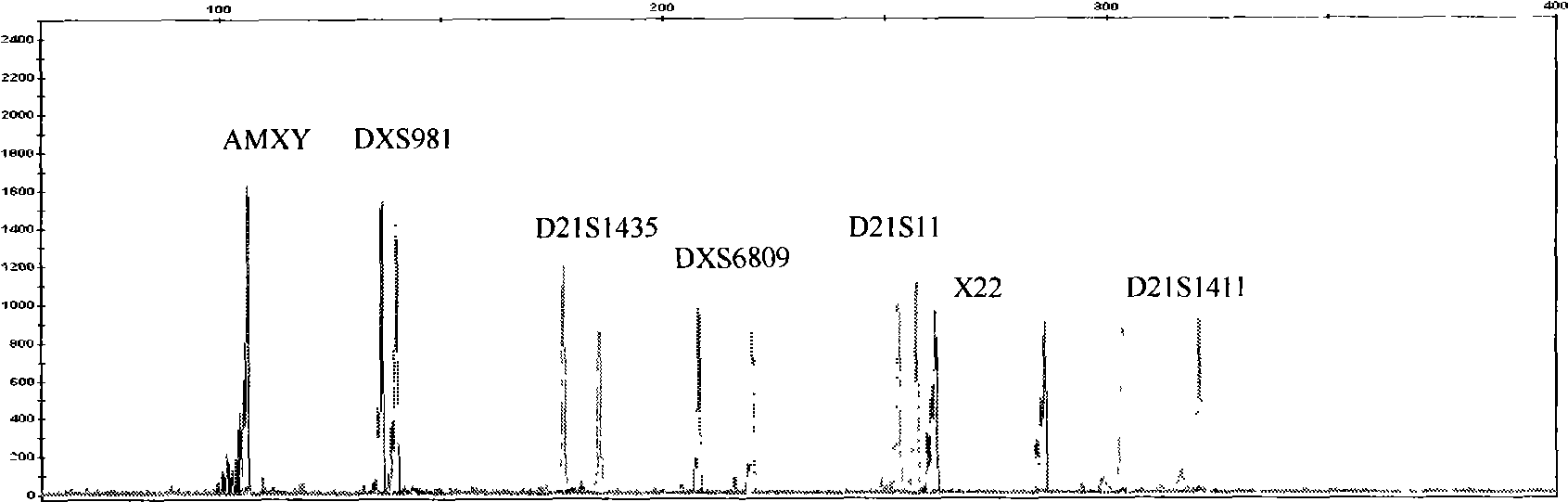
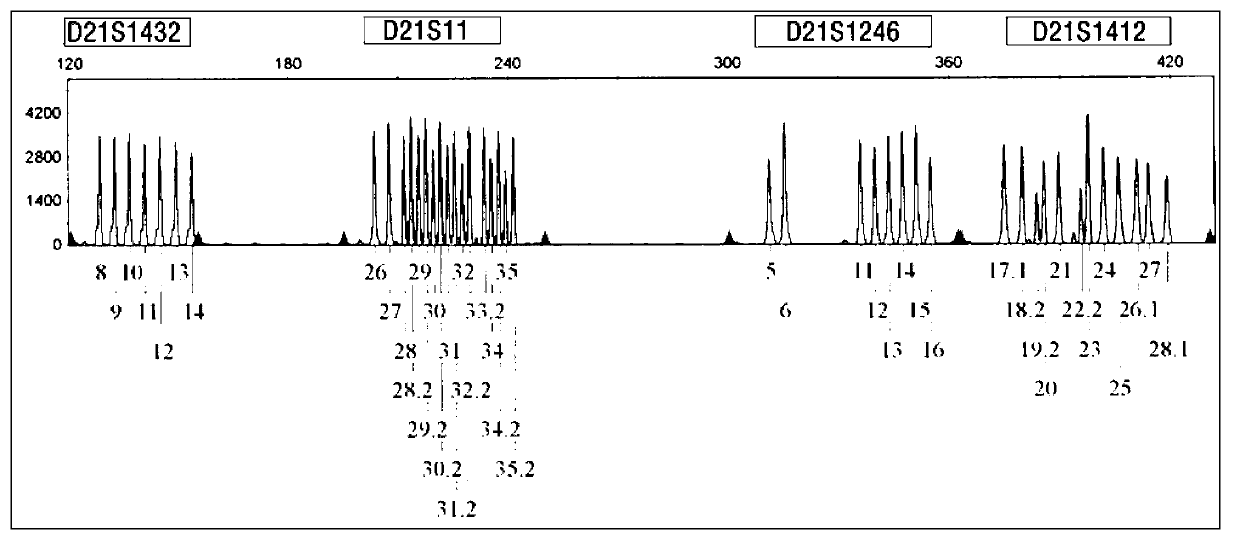
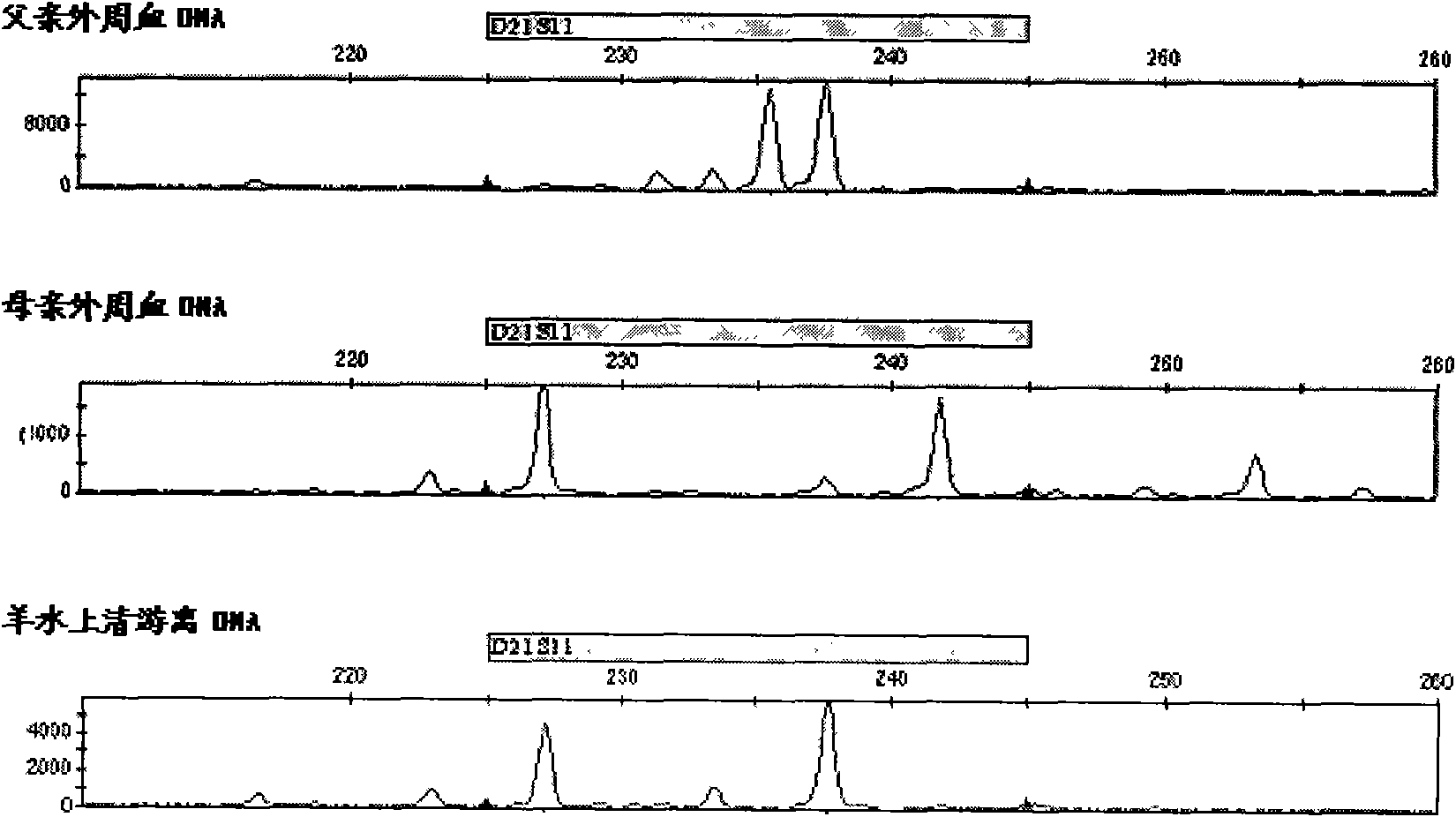
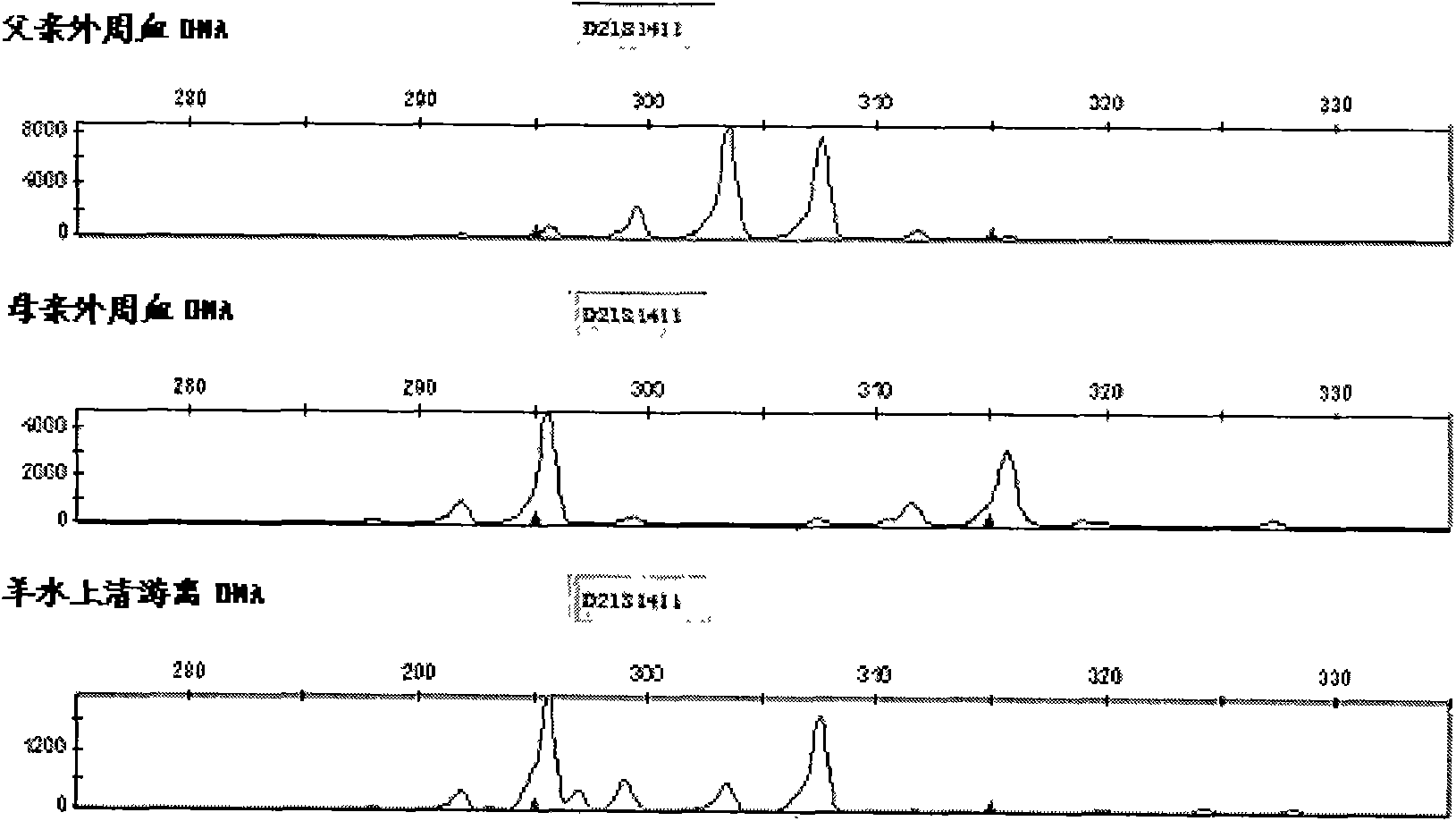
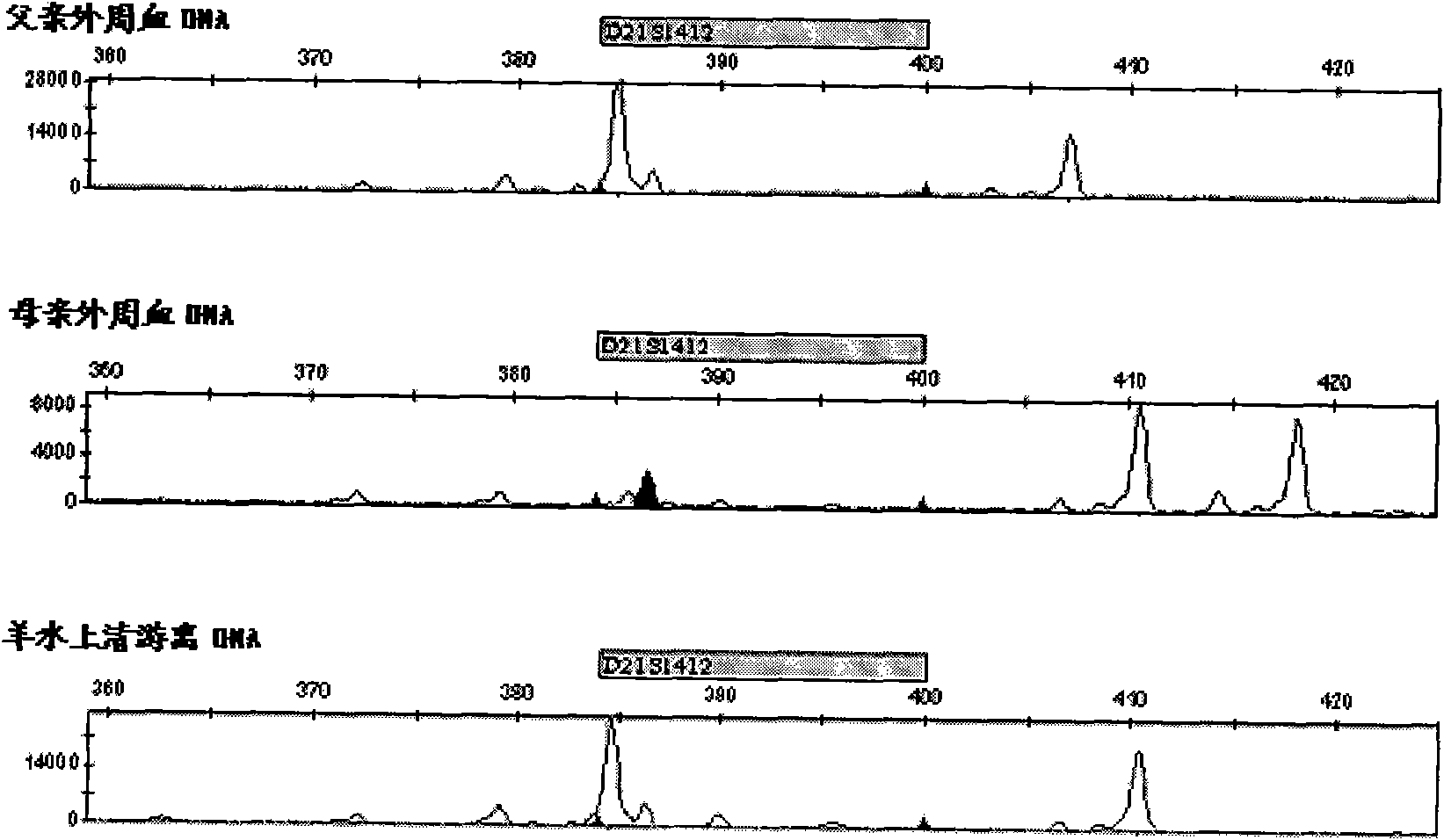
Kit for detecting genotype of human chromosome 21 STR (short tandem repeat)
ActiveCN103103267AHigh polymorphismImprove diagnostic capabilitiesMicrobiological testing/measurementPrenatal diagnosisGenetics
The invention provides a kit for detecting genotype of human chromosome 21 STR (short tandem repeat). The kit comprises an amplification reagent and an amplification product detection reagent and mainly contains primers of 12 pairs of fluorescent markers, a molecular weight internal label containing the size of 14 fragments, an allele typing standard product and a quality control product. According to the kit disclosed by the invention, when the kit is used, the required quantity of specimens is small, the diagnosis is fast, and the kit is suitable for villi, amniotic fluid, blood and other tissues, and can be used for not only prenatal diagnosis, but also source analysis of extra chromosomes of a 21-trisomy aborted fetus; the kit can precisely diagnose the genotype and is conductive to presentation of diagnosis result and laboratory quality evaluation; and the kit can further realize fast, accurate, automatic and high-throughput detection of the number of chromosomes 21 and realize quality controllability and standardization of a detection result, and thus has clinical application prospects.
Owner:ATTACHED OBSTETRICS & GYNECOLOGY OSPITAL MEDICALCOLLEGE ZHEJIANG UNIV +2
Quality control product, preparation method thereof, kit and method for detecting trisomy 21 and 18 syndromes of fetuses
PendingCN110106247AGuaranteed detection sensitivityShort detection cycleMicrobiological testing/measurementTrisomyQuality control
The invention relates to a quality control product, a preparation method thereof, a kit and a method for detecting trisomy 21 and 18 syndromes of fetuses. The digital PCR technology with single-molecule detection sensitivity and absolute quantitative capability is used, and a new simple and effective chromosome aneuploid non-invasive prenatal detection method is developed. By using peripheral blood of a pregnant woman, non-invasive prenatal detection can be completed on one sample within about 5 hours. By adopting the non-invasive prenatal detection technology based on the digital PCR, accurate, rapid and simple prenatal screening can be realized. The accuracy of the detection kit reaches 99%, the cost is only 1 / 5 that of the NIPT technology, and the detection time is 5 hours.
Owner:苏州行知康众生物科技有限公司
Biomarkers for down syndrome prenatal diagnosis
Disclosed is an isolated biomarker / biomarker region. Also disclosed are isolated biomarker / biomarker regions for detecting trisomy 21, methods of determining the likelihood of a foetus to suffer from a specific disease using the biomarker / biomarker region, a kit and a method of determining the methylation levels of a biomarker / biomarker region.
Owner:AGENCY FOR SCI TECH & RES +1
Method of detecting chromosomal abnormalities
InactiveUS20150267255A1Microbiological testing/measurementLibrary member identificationGestationSequence analysis
The invention relates to a method of detecting chromosomal abnormalities, in particular, the invention relates to the diagnosis of fetal chromosomal abnormalities such as trisomy 21 (Down's syndrome) which comprises sequence analysis of cell-free DNA molecules in plasma samples obtained from maternal blood during gestation of the fetus.
Owner:PREMAITHA LTD
21-trisomy syndrome prenatal screening kit
InactiveCN101560565AEasy to getEasy to cultivate successMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceTrisomy
The invention discloses a 21-trisomy syndrome prenatal screening kit. The kit provided by the invention comprises a primer pair A, a primer pair B, a primer pair C and a primer pair D; the primer A consists of nucleotides represented by a sequence 1 in a sequence table and nucleotides represented by a sequence 2 in the sequence table; the primer pair B consists of nucleotides represented by a sequence 3 in the sequence table and nucleotides represented by a sequence 4 in the sequence table; the primer pair C consists of nucleotides represented by a sequence 5 in the sequence table and nucleotides represented by a sequence 6 in the sequence table; and the primer pair D consists of nucleotides represented by a sequence 7 in the sequence table and the nucleotides represented by a sequence 8 in the sequence table. The kit of the invention applied to 21-trisomy syndrome prenatal screening has the advantages of innovating specimen material, making prenatal screening earlier, reducing specimen acquisition amount, reducing diagnosis time, and judging extra chromosome resources. The kit has far-reaching significance for finding the causes of chromosomal disorder and in-time prevention and control of the chromosomal disorder.
Owner:PEKING UNIV THIRD HOSPITAL
Methods of prenatal screening for trisomy 21
InactiveUS7922660B2Raise the possibilityUltrasonic/sonic/infrasonic diagnosticsPerson identificationTrisomyPrenatal screening
Owner:SONEK JIRI D +1
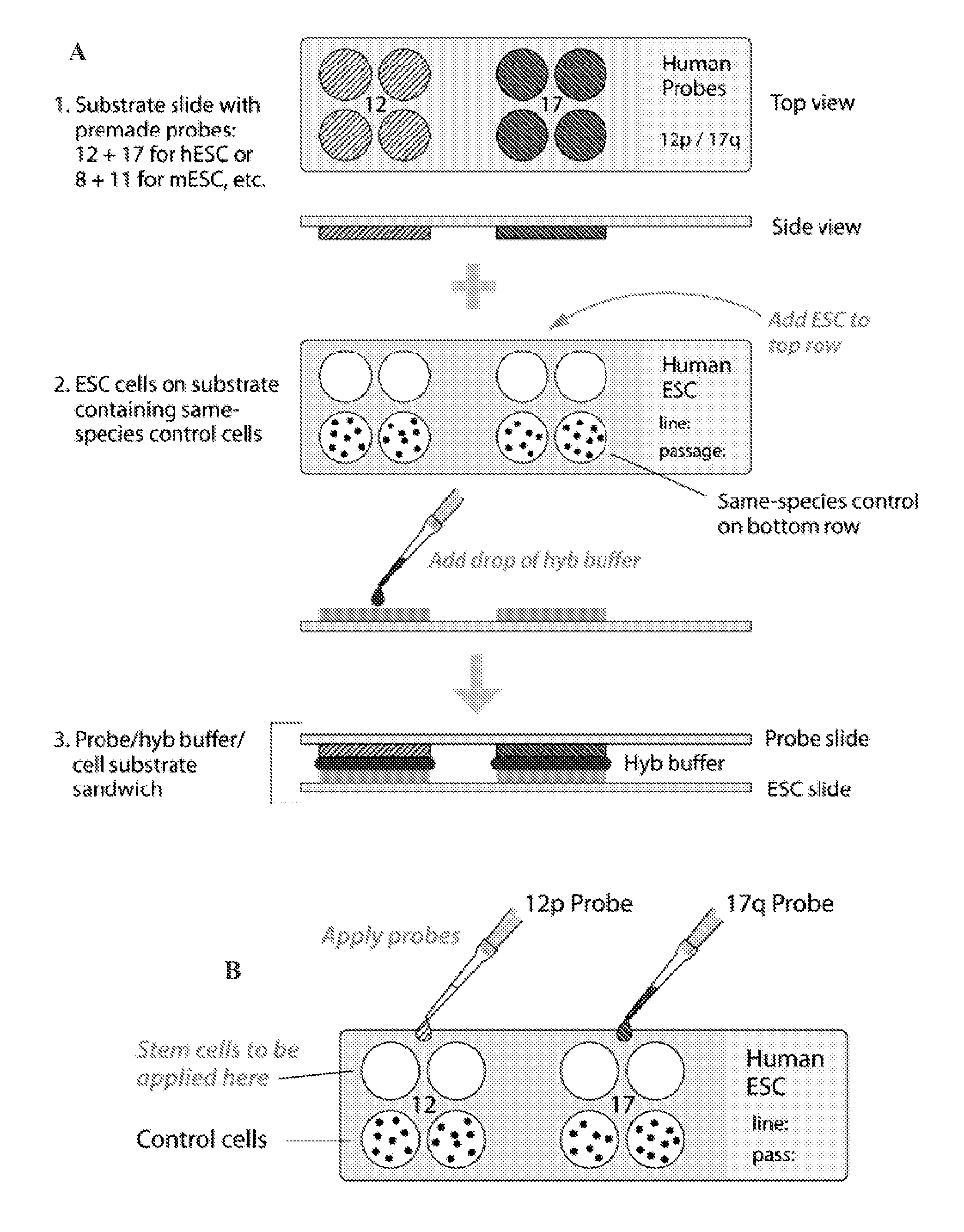
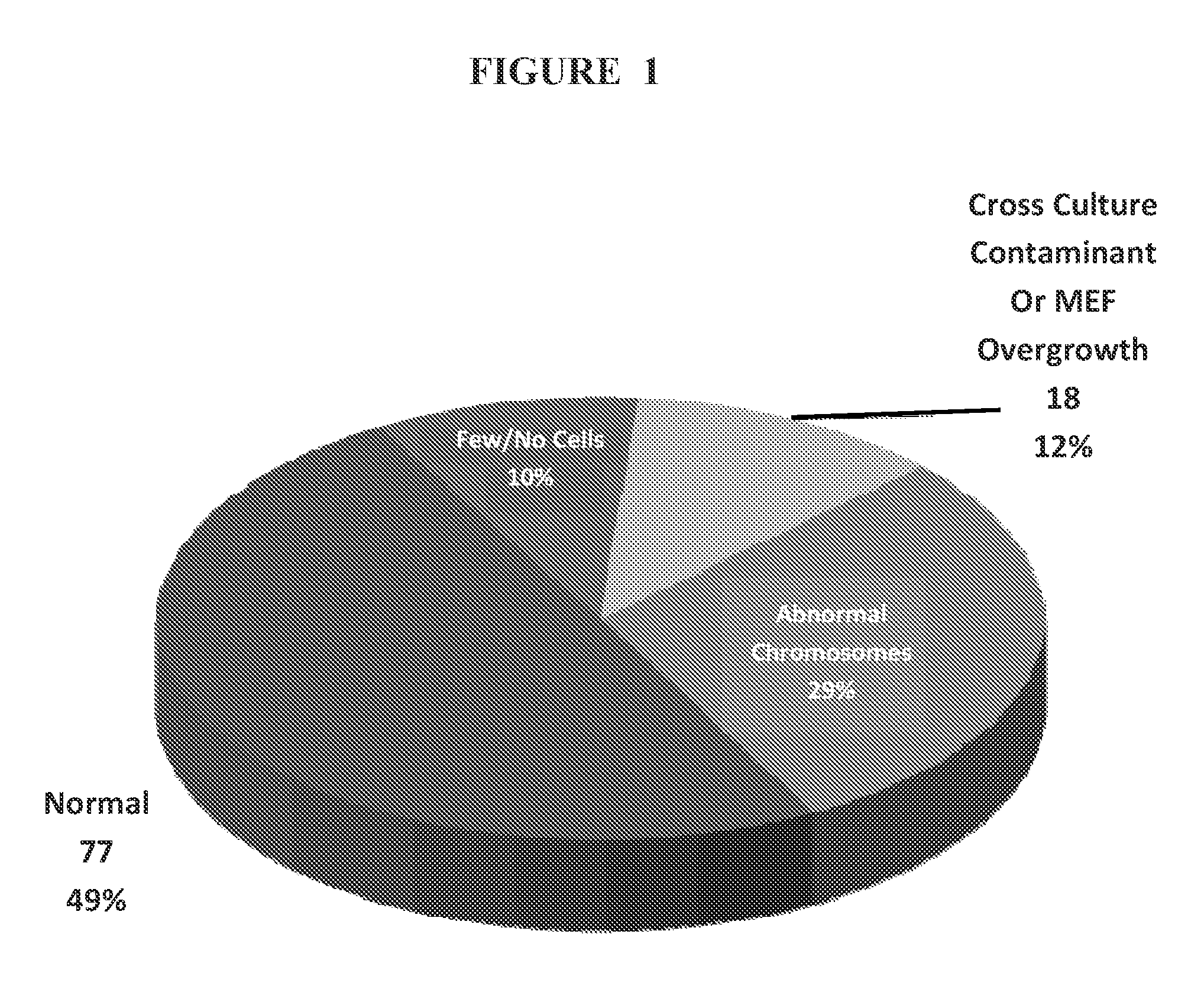
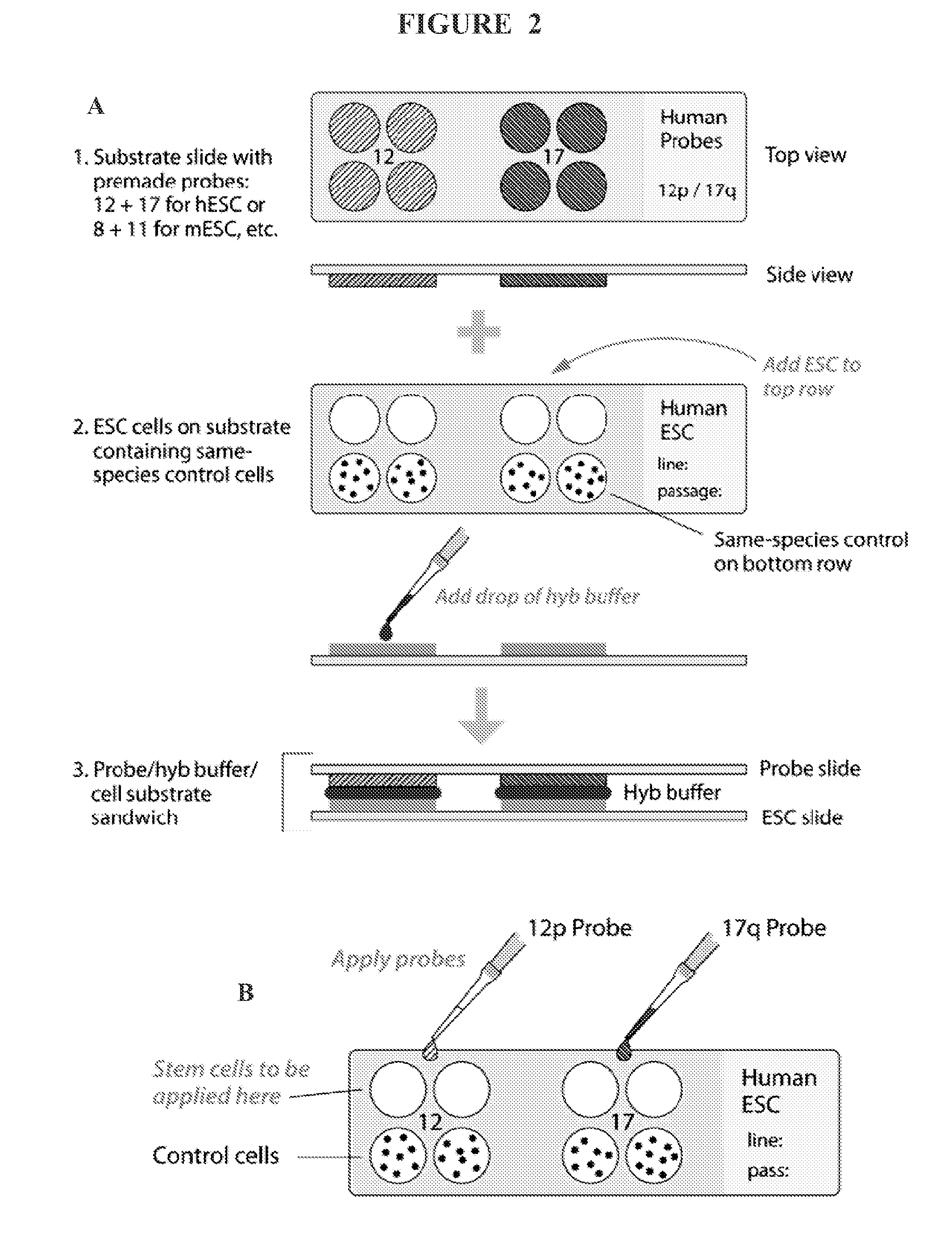
Methods and assays for screening stem cells
InactiveUS20090068667A1Space maximizationClear signalMicrobiological testing/measurementMammal material medical ingredientsTrisomyAbnormal cell
The present invention provides methods and assays for screening cells, such as stem cells, for chromosomal aberrations. In particular, the present invention provides a rapid, sensitive assay platform for detecting high and low levels of chromosomal aberrations present in a cell population. This includes, but is not limited to, detection of extra chromosomes (trisomies) as well as insertions of small segments that are undetectable using standard cytogenetic studies, wherein the abnormal cells comprise a low percentage of the total cell population.
Owner:CELL LINE GENETICS
Noninvasive prenatal screening 2, 1-trisomy syndrome kit
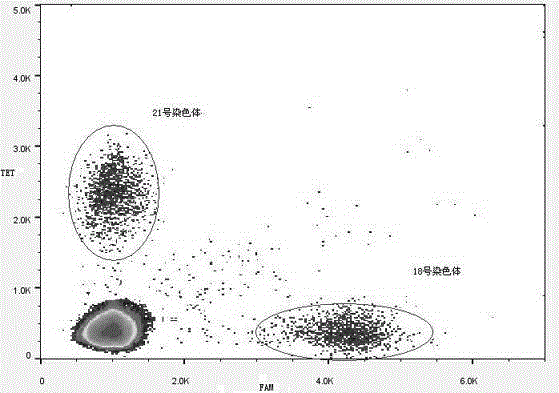
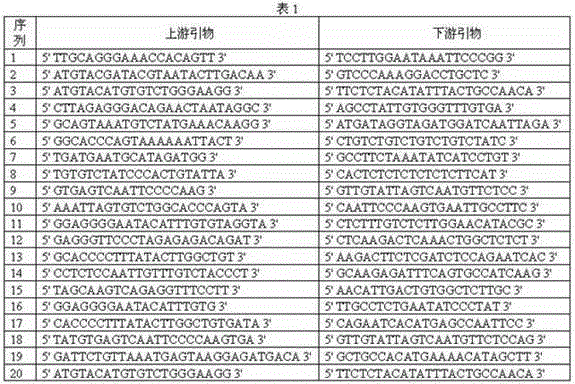
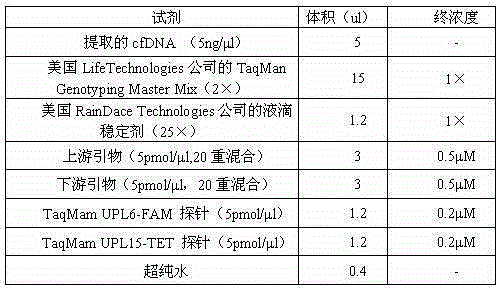
ActiveCN105132572AReasonable primer designPrevent birthMicrobiological testing/measurementTrisomyTrisomy X syndrome
The invention discloses a noninvasive prenatal screening 2, 1-trisomy syndrome kit, and relates to the technical field of a nucleic acid determination or inspection method. The noninvasive prenatal screening 2, 1-trisomy syndrome kit comprises a PCR reaction solution, wherein the PCR reaction solution contains a TaqMam UPL6-FAM probe and a TaqMam UPL15-TET probe, the TaqMam UPL6-FAM probe and the TaqMam UPL15-TET probe are respectively used for detecting No.18 and No.21 chromosomes, 20 pairs of primers are respectively designed for the No.21 and No.18 chromosomes, gene sequences of the primers are SEQ ID NO.1 to 40 and SEQ ID NO.41-80; and gene sequences of the TaqMam UPL6-FAM probe and the TaqMam UPL15-TET probe are SEQ ID NO.81 and SEQ ID NO.82. By adopting the noninvasive prenatal screening 2, 1-trisomy syndrome kit, the noninvasive prenatal screening of 21-trisomy syndrome, and the accuracy is high.
Owner:邯郸市康业生物科技有限公司
Method for detecting trisomy 13 and down syndrome by non-invasive maternal blood screening
InactiveCN101329352AImprove discovery rateIncrease percentageBiological testingSpecial data processing applicationsNormal densityObstetrics
A method and apparatus for determining if a pregnant woman is at significant risk of carrying a fetus with Down syndrome. The method comprises measuring the pregnant woman's maternal blood levels of the free beta subunit of human chorionic gonadotropin. The level of free beta subunit of human chorionic gonadotropin individually or with other markers may be compared to reference data. A computerized apparatus for making the determination preferably using a probability density function generated from a set of reference data by a linear discriminant analysis procedure is disclosed.
Owner:JN马克里技术有限责任公司
Prolactin regulatory element binding protein and uses thereof
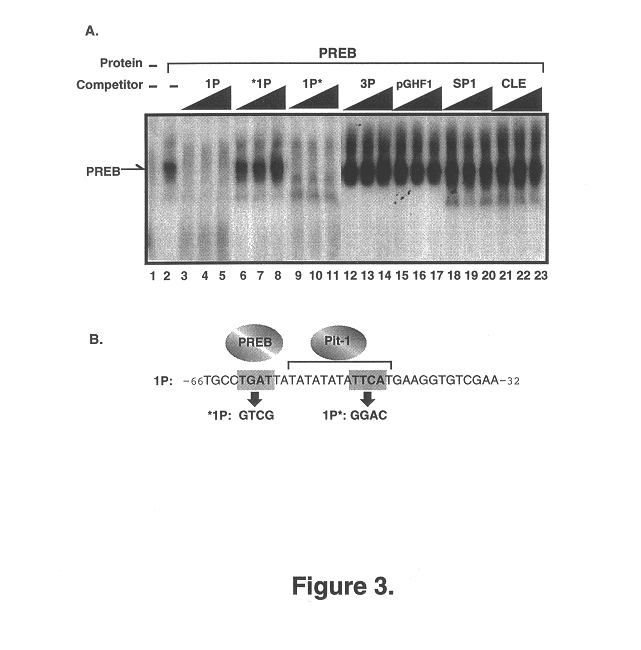
InactiveUS6586581B1Enhance immune responseIncrease gene expressionSugar derivativesPeptide/protein ingredientsTrisomyNucleic acid sequencing
The present invention relates to isolated nucleic acid molecules encoding Prolactin Regulatory Element Binding protein (PREB) and recombinant proteins encoded thereby. The nucleic acid sequences are useful in the production of recombinant PREB, as probes, and in the control of gene expression, and in particular, in the control of prolactin gene expression. In particular embodiments of the invention, PREB nucleic acid sequences are used to detect transcripts of the gene in astrocytomas, to detect trisomy and to detect a propensity of a subject to develop osteoporosis. In other embodiments of the invention, the PREB nucleic acid sequences, or the products thereof, are used for preventing or controlling osteoporosis in a subject.
Owner:MT SINAI SCHOOL OF MEDICINE
Detection method of genes in chromosome 21, correlated detection probe combination, detection kit and correlated application
ActiveCN103820565AAvoid pollutionIngenious designMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceTrisomy
The invention provides a detection method of genes in chromosome 21. The method comprises the steps as follows: genomic DNA (deoxyribonucleic acid) of a detected object is extracted; two sets of PCR (polymerase chain reaction) primer pairs and Taqman MGB (minor groove binder) fluorescence probes are combined to perform fluorescent quantitative PCR on the genomic DNA respectively, wherein the PCR primer pairs are nucleotide sequences represented by SEQ ID NO: 4 and SEQ ID NO: 5 as well as SEQ ID NO: 7 and SEQ ID NO: 8 respectively, and nucleotide sequences of the Taqman MGB fluorescence probes are represented by SEQ ID NO: 6 and SEQ ID NO: 9 respectively; and according to a real-time amplification curve formed by fluorescence signals collected in the fluorescent quantitative PCR, whether trisomy probability exists in the chromosome 21 of genomic DNA is analyzed. The invention further relates to correlated detection probe combination, a detection kit and a correlated application. According to the invention, the design is ingenious, sample contamination can be effectively prevented through closed tube detection, the detection is rapid, accurate, simple and convenient, and the method can be taken as reference for doctor diagnosis and medication and is suitable for large-scale popularization and application.
Owner:上海春夏正像生物科技有限公司
Reagent box for detecting No 21 chromosome and idiochromosome number abnormality
The invention relates to a kit used for screening the numerical abnormality of 21-trisomy and sex chromosome at early stage. The kit adopts quantitative fluorescence multiplex polymerase chain reaction technology, carries out the fluorescent primer sevenfold multiplex amplification respectively to differential genetic locus on the 21-trisomy and sex chromosome and analyzes the numerical abnormality of chromosome according to the difference of the dosage of amplified products, thus achieving the purpose of detecting the numerical abnormality of 21-trisomy and sex chromosome.
Owner:DAAN GENE CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com