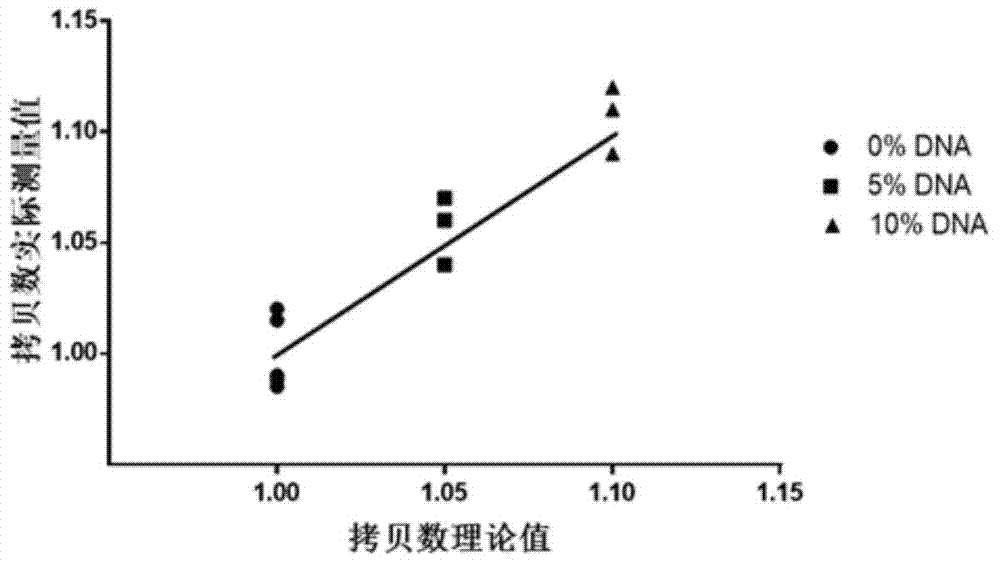
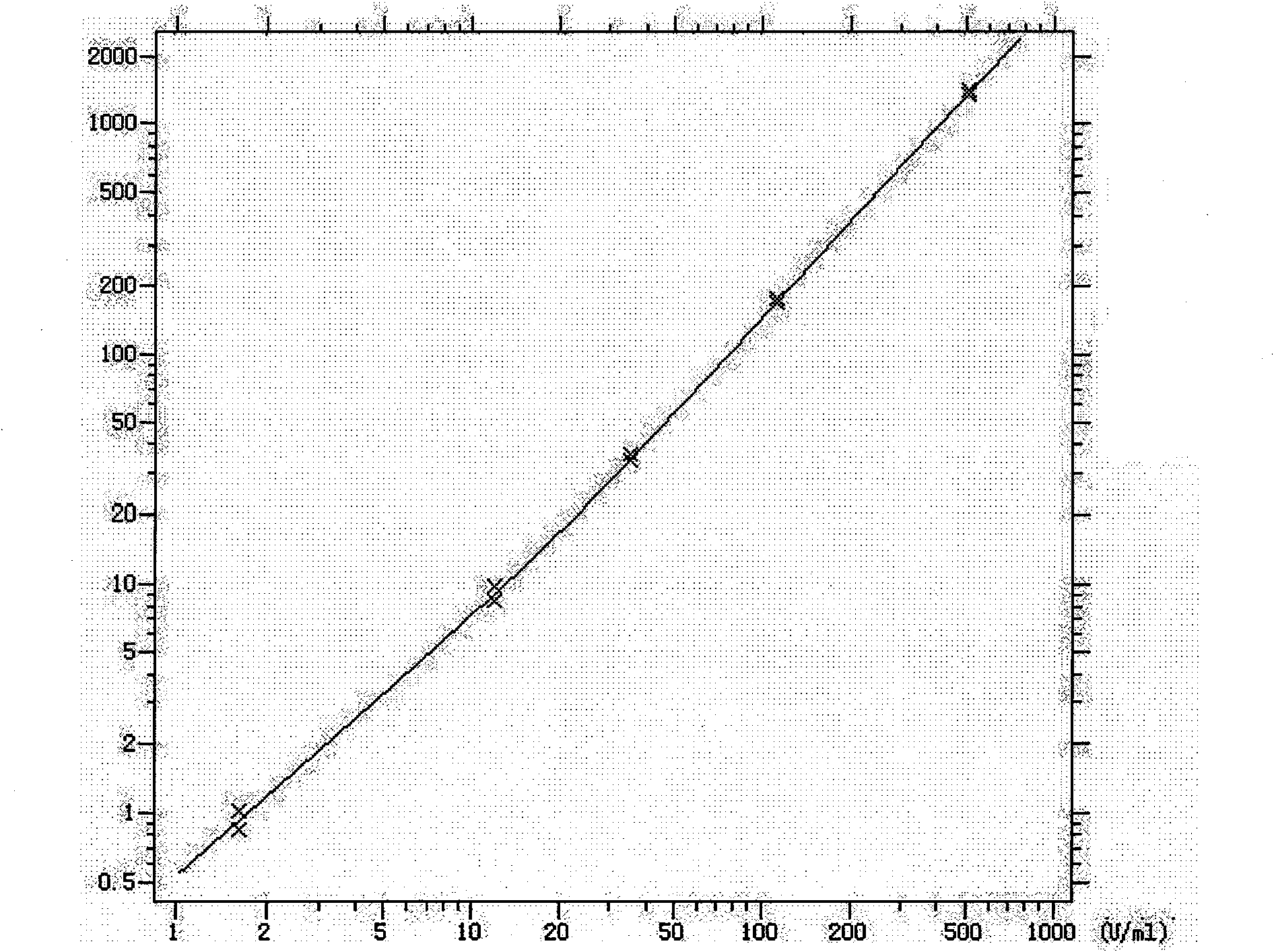
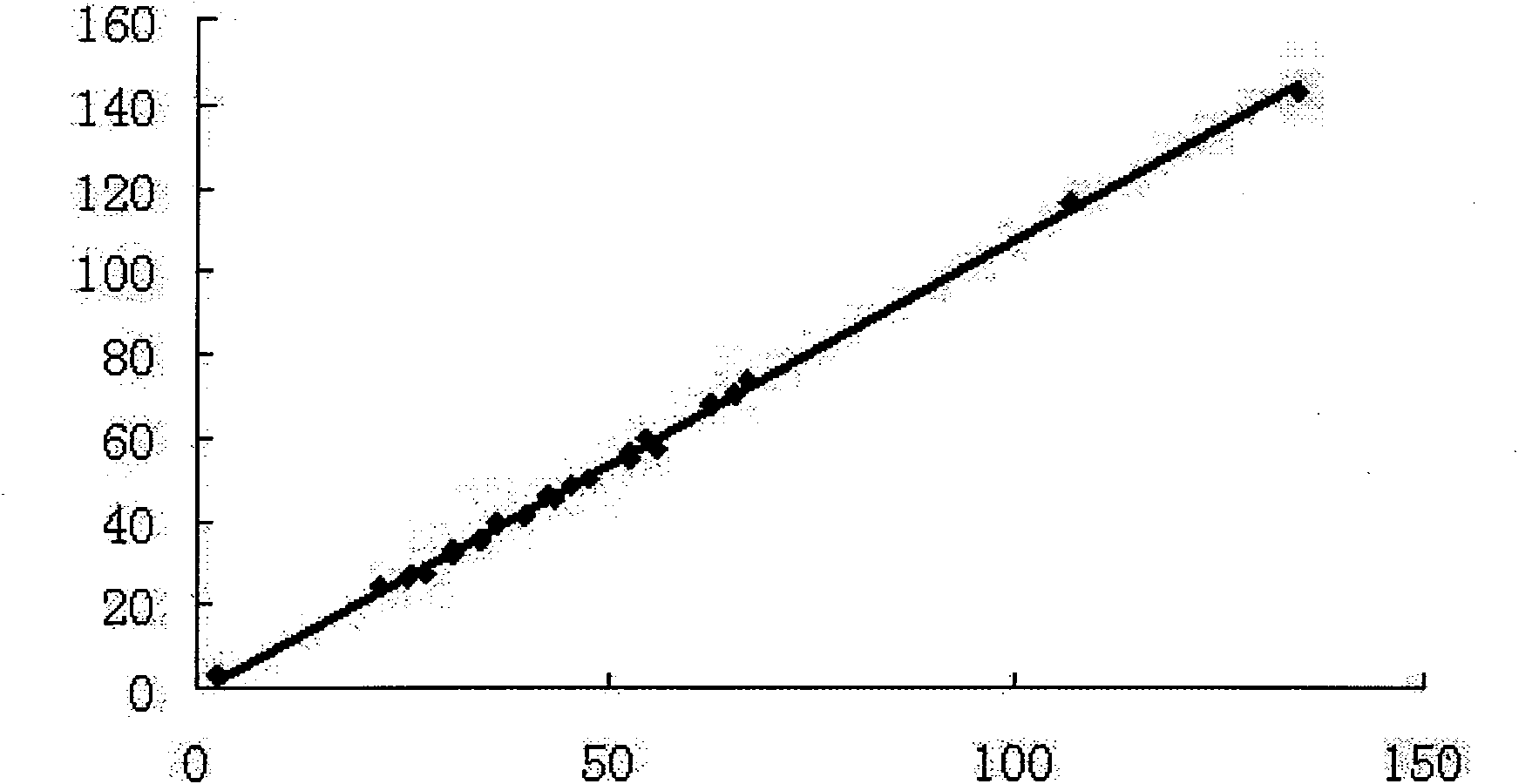
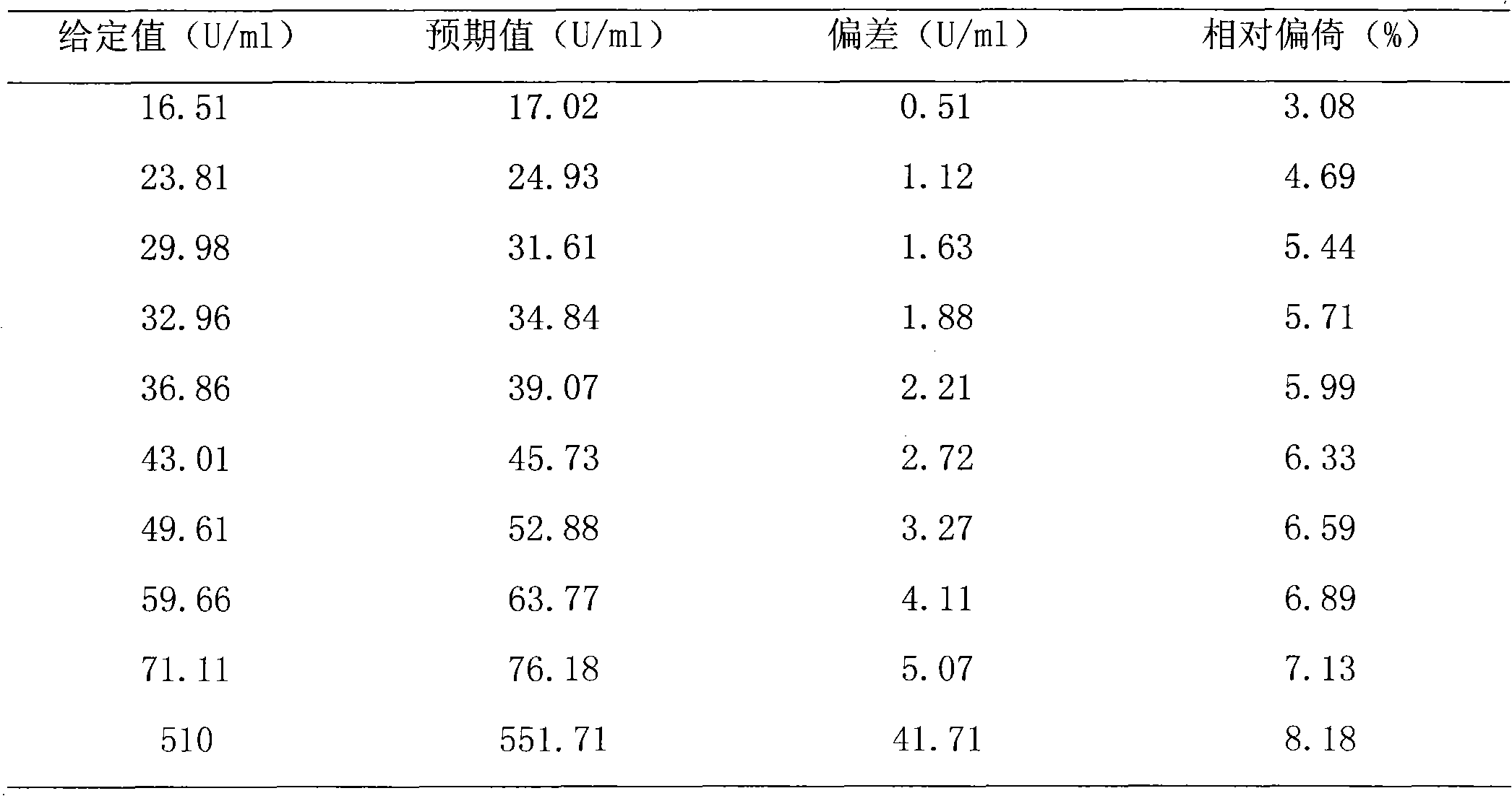
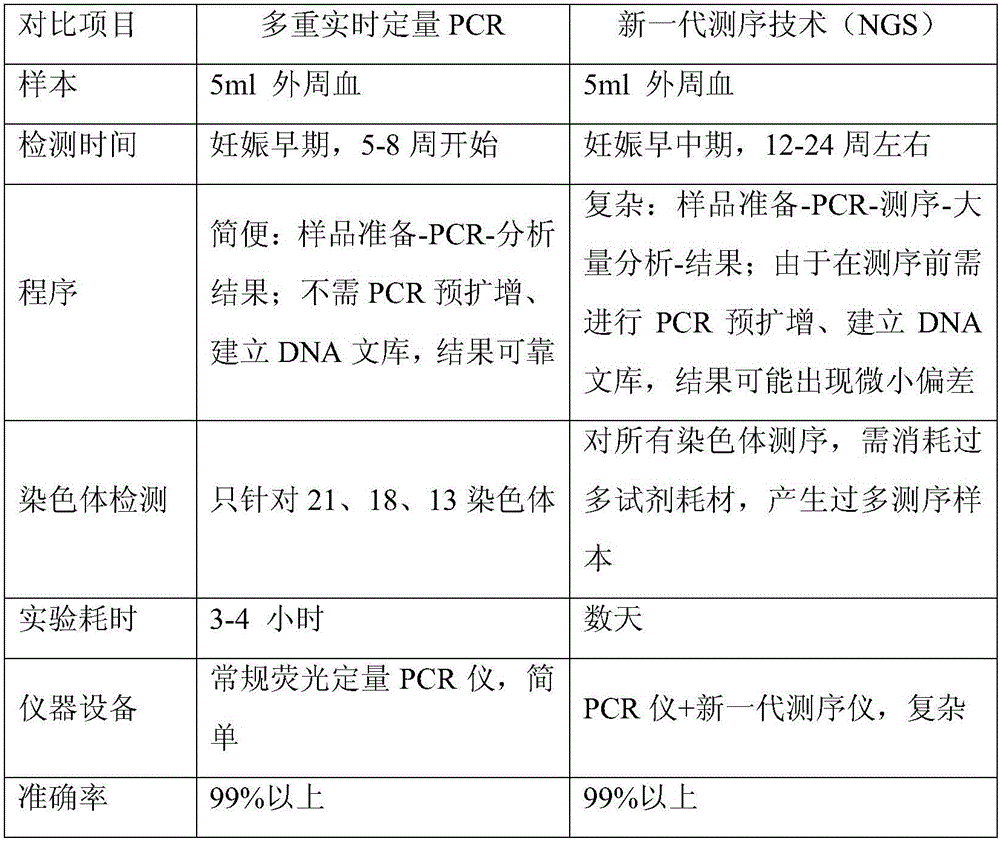
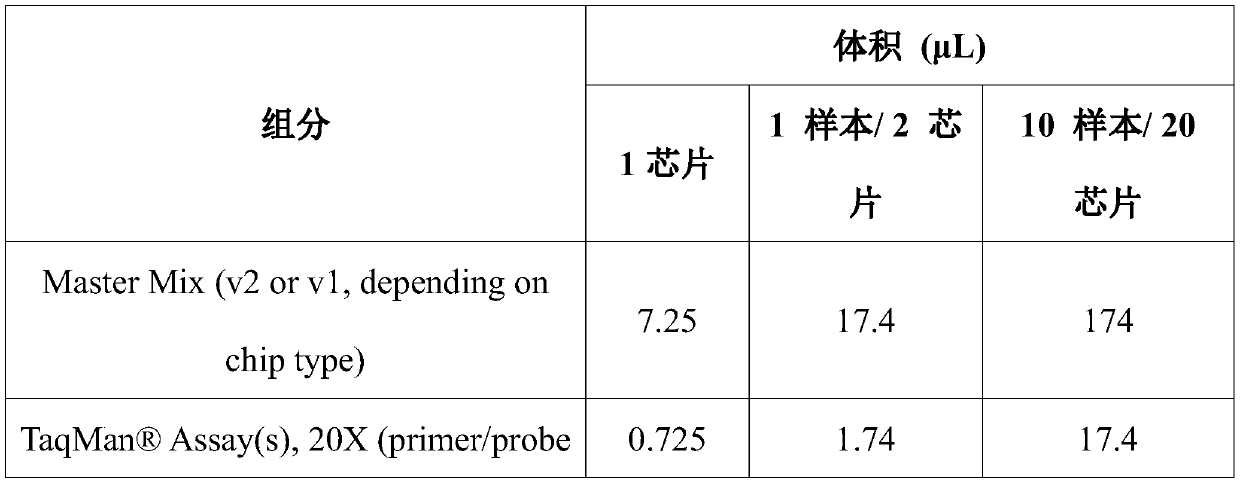
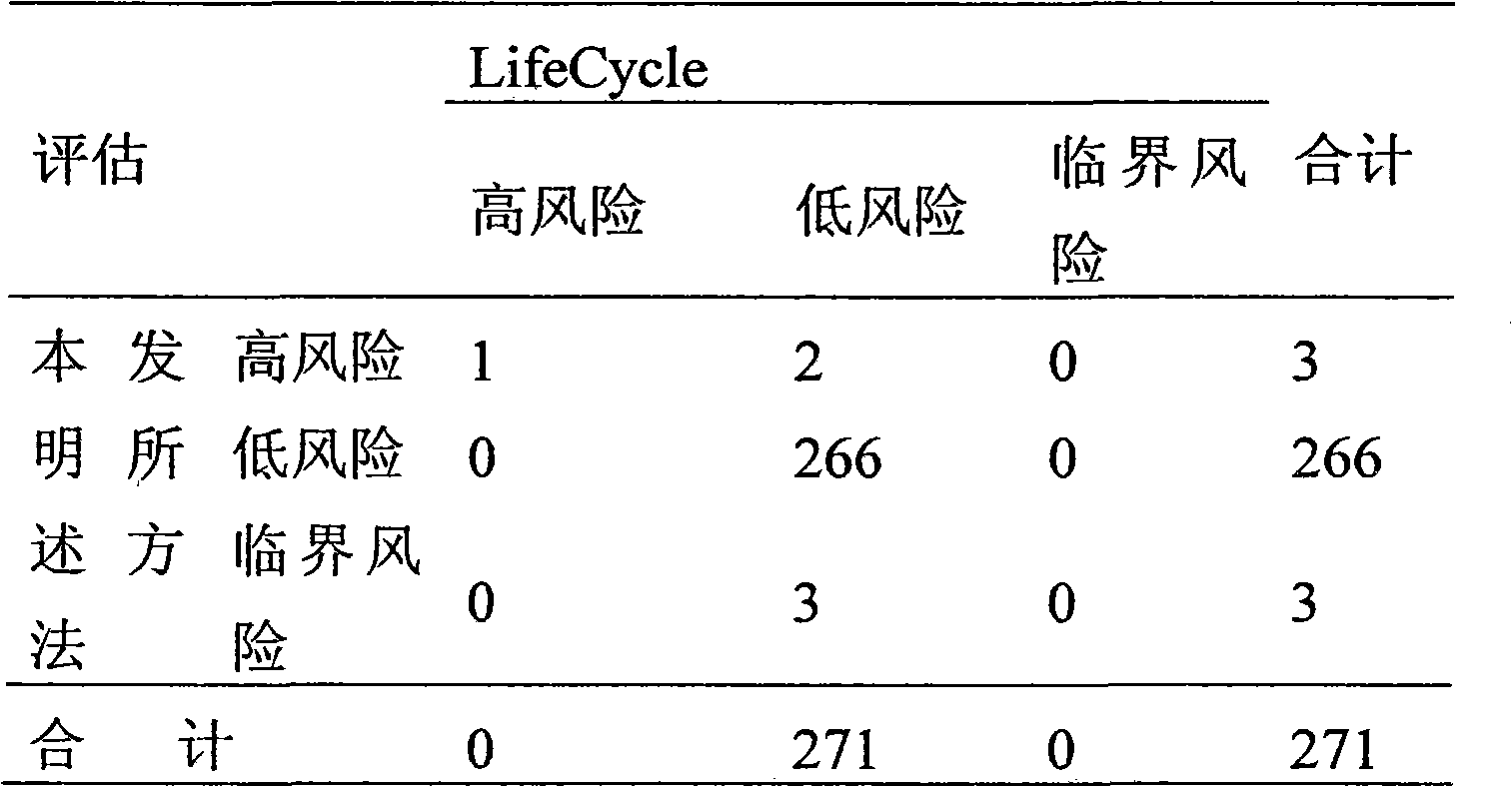
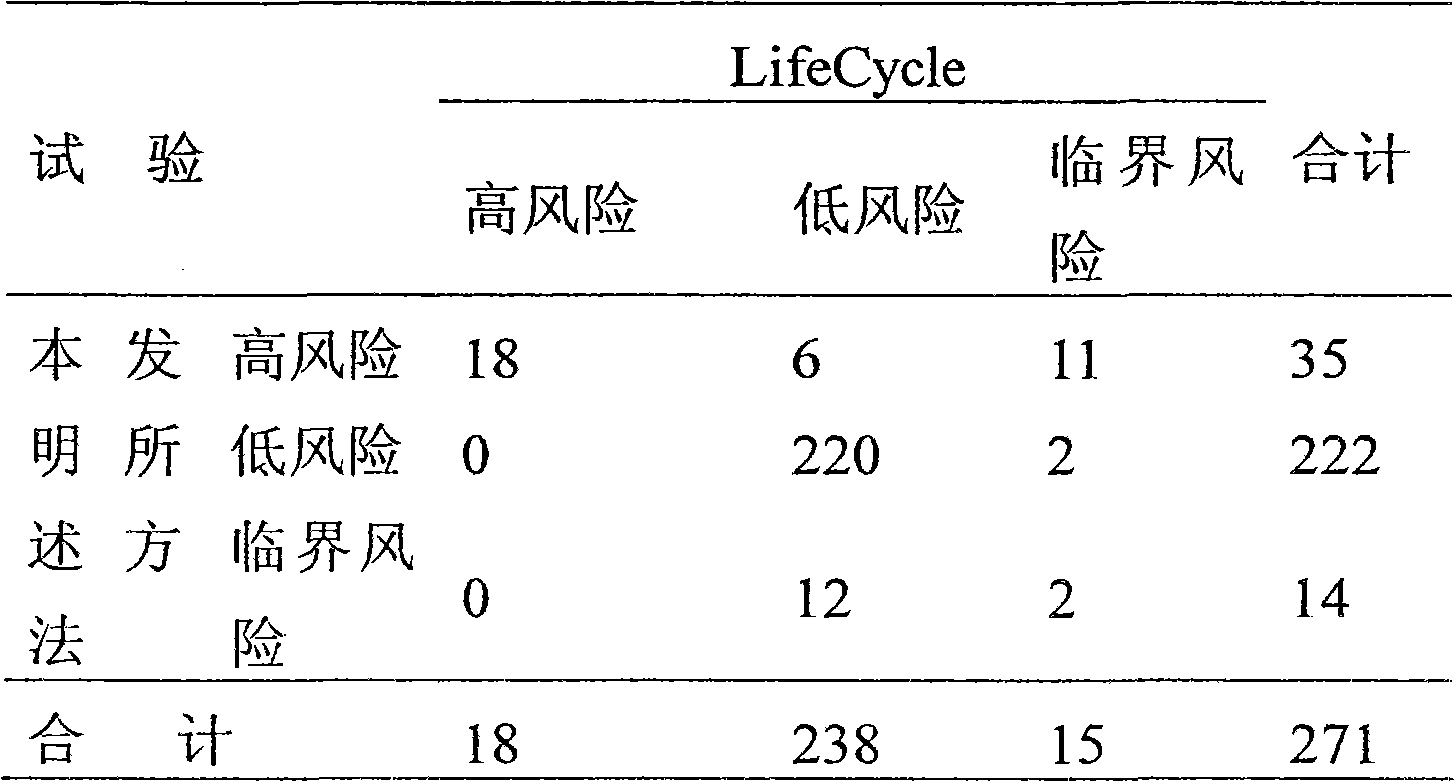
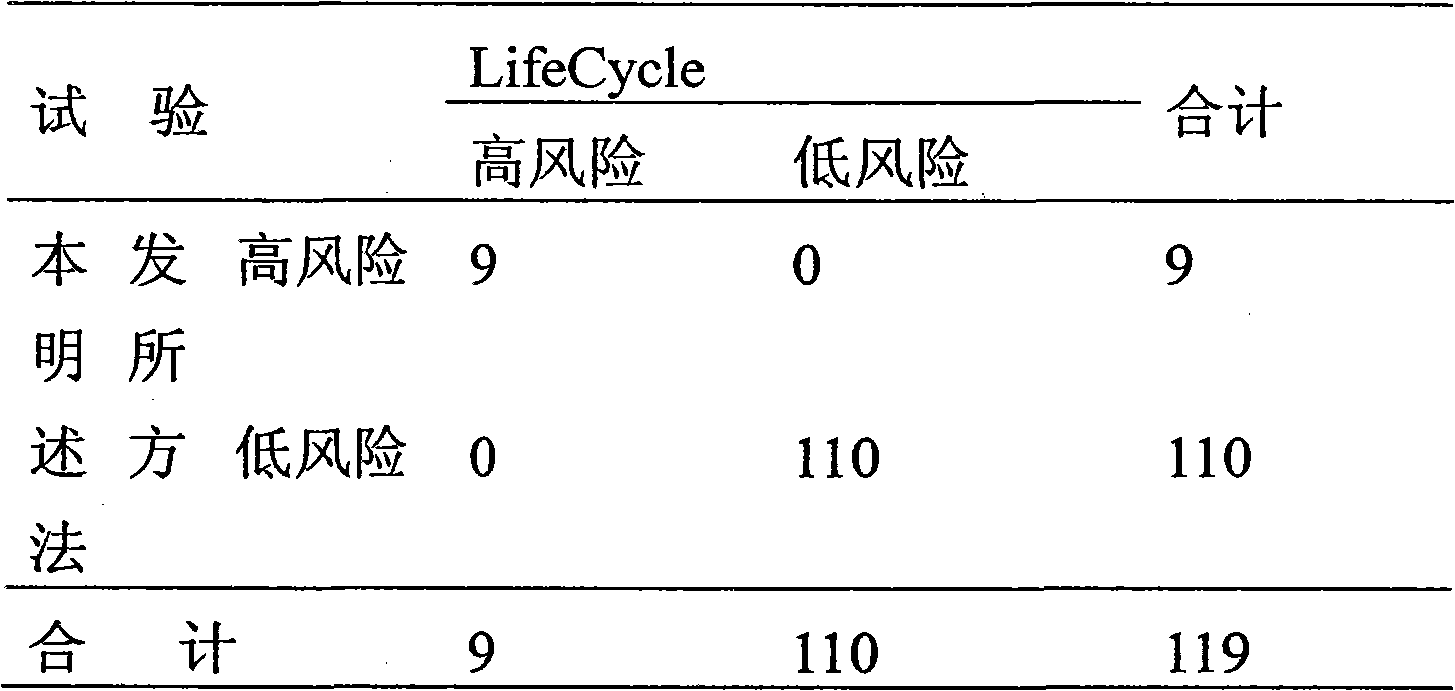
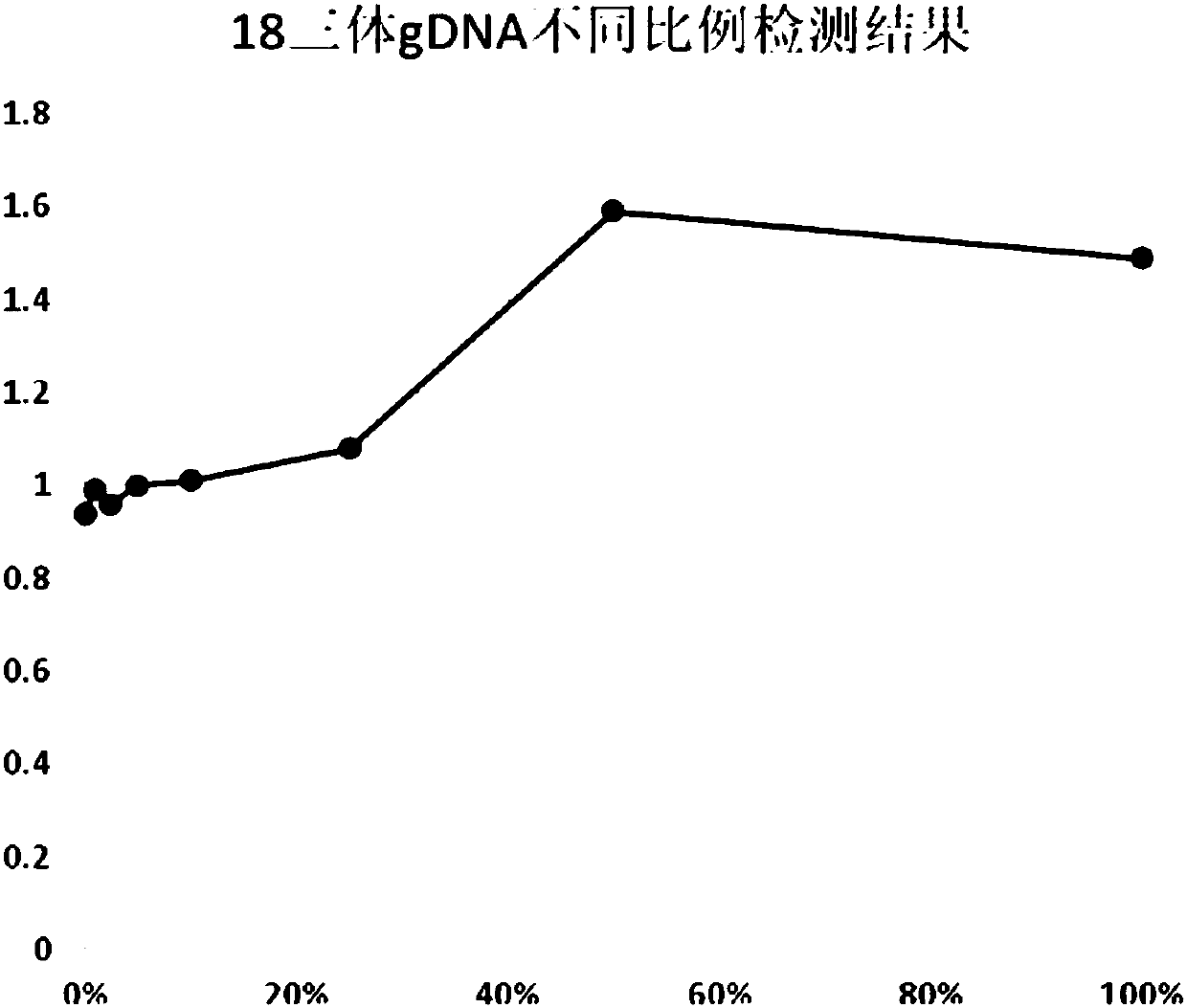
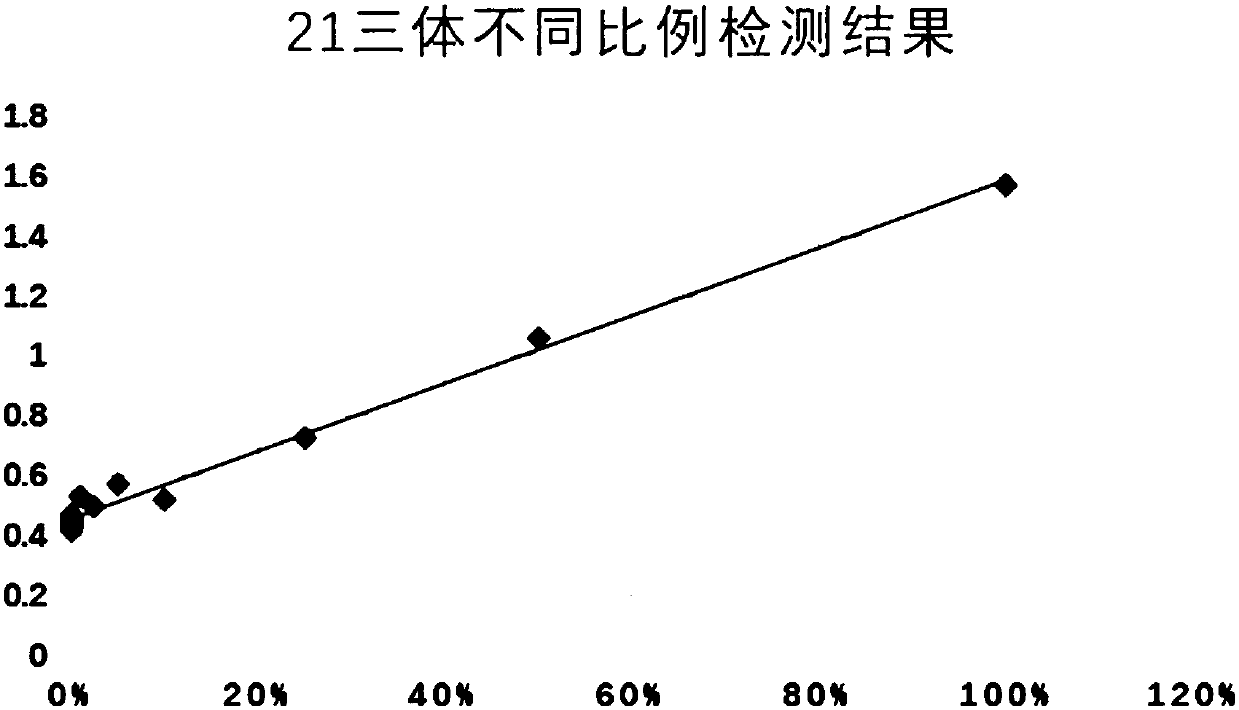
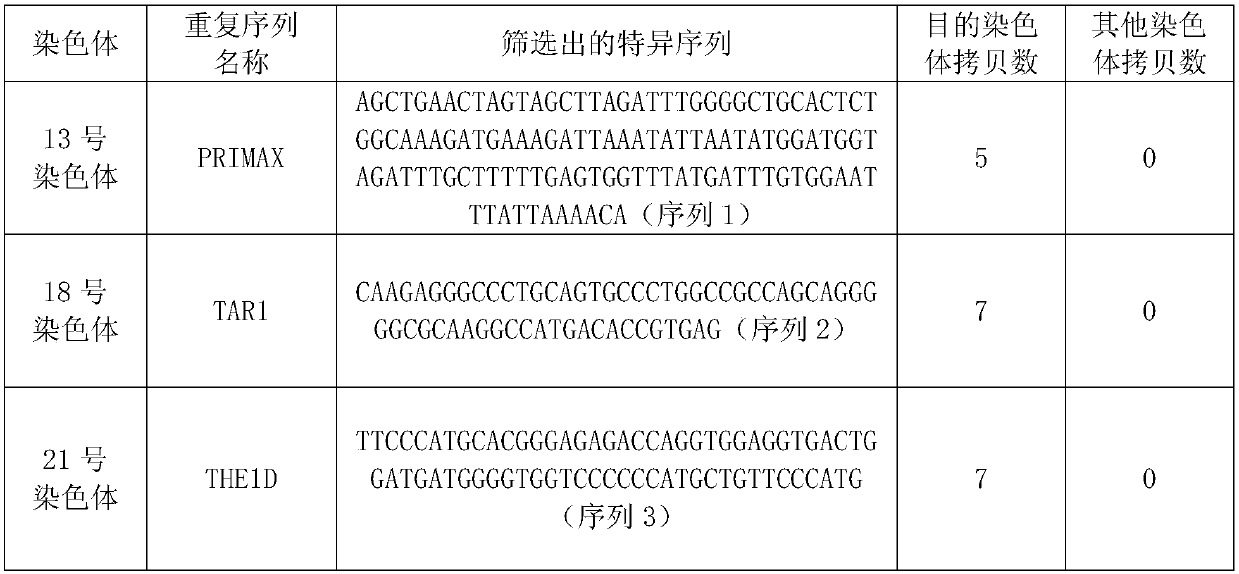
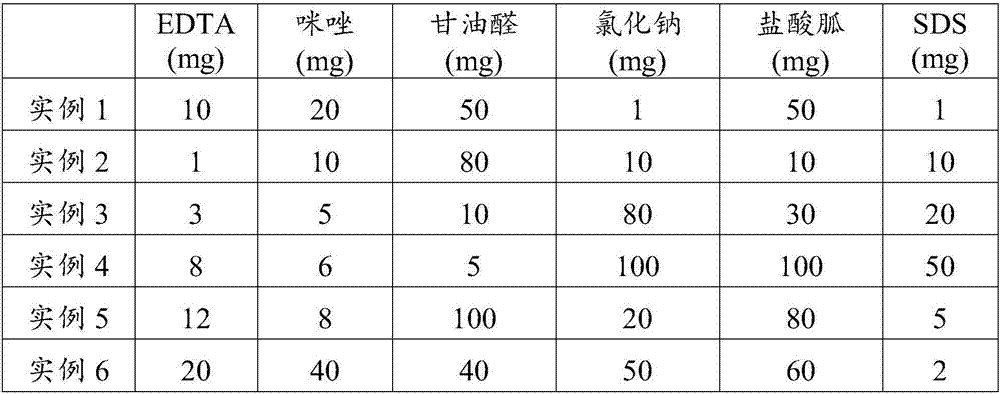
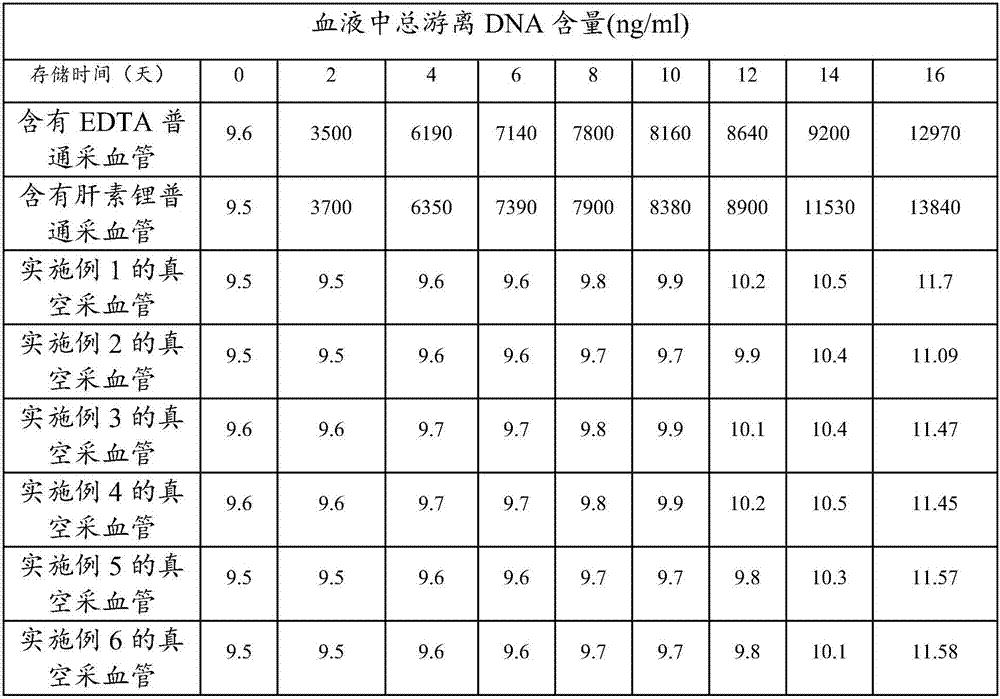
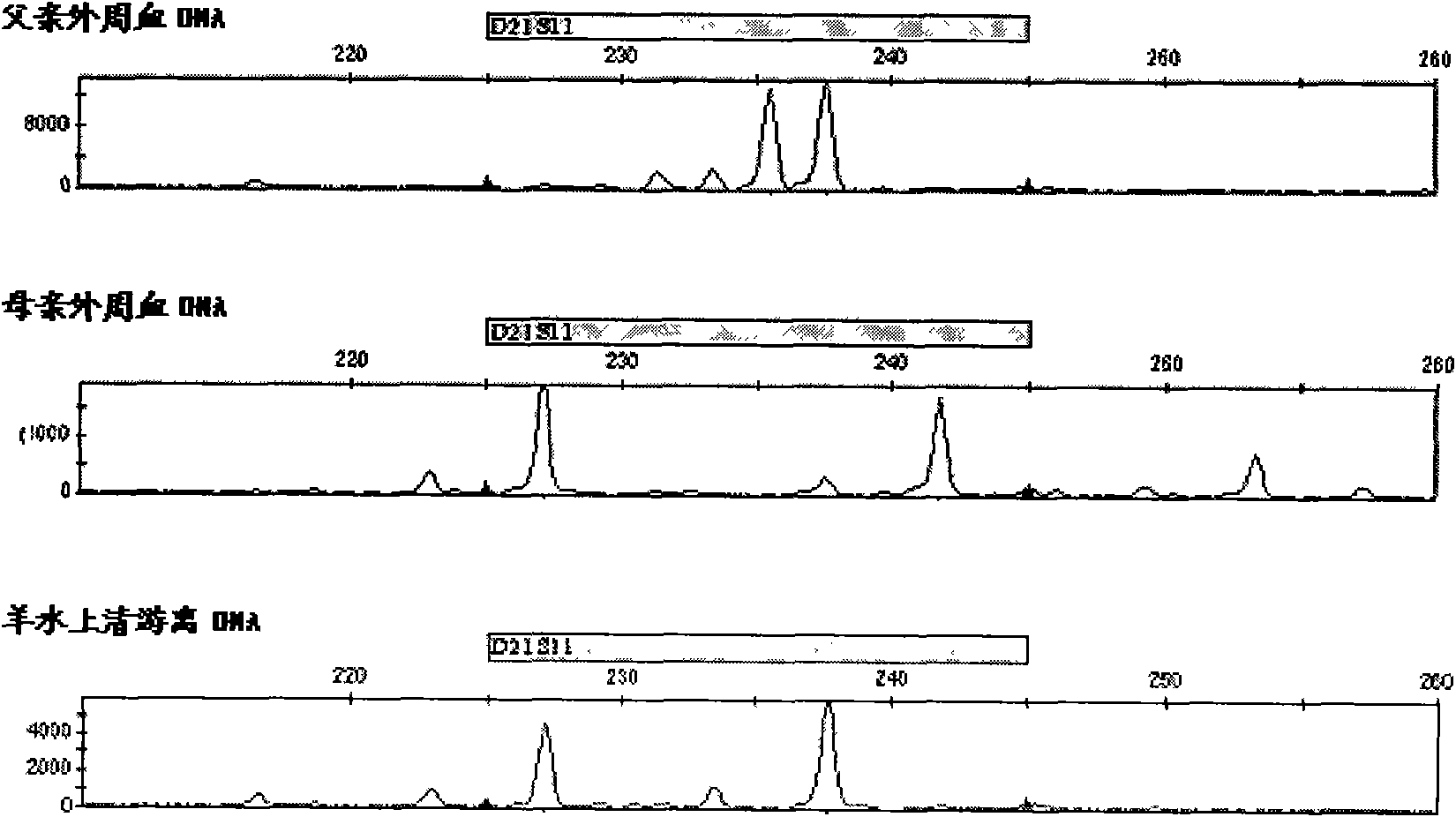
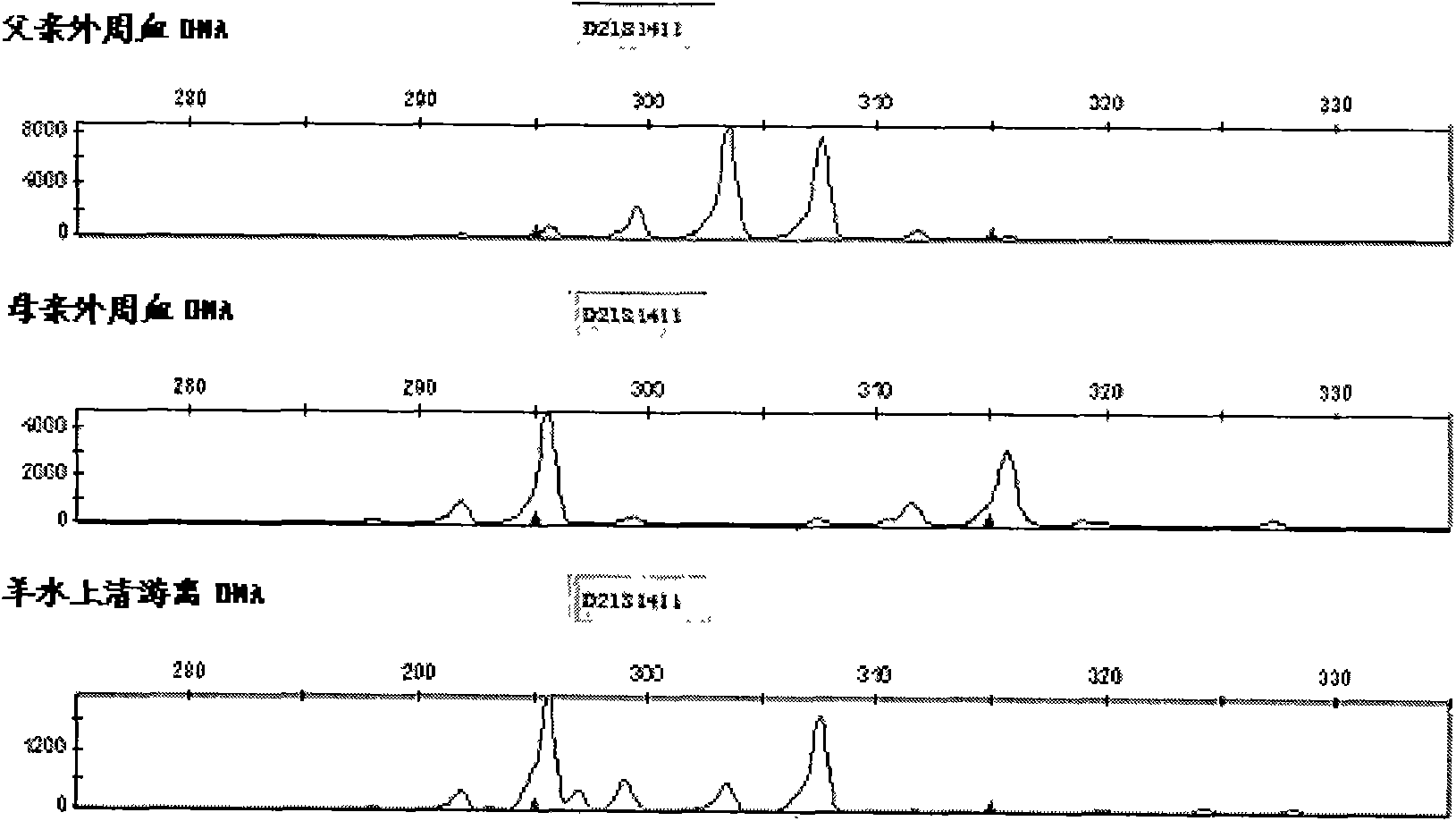
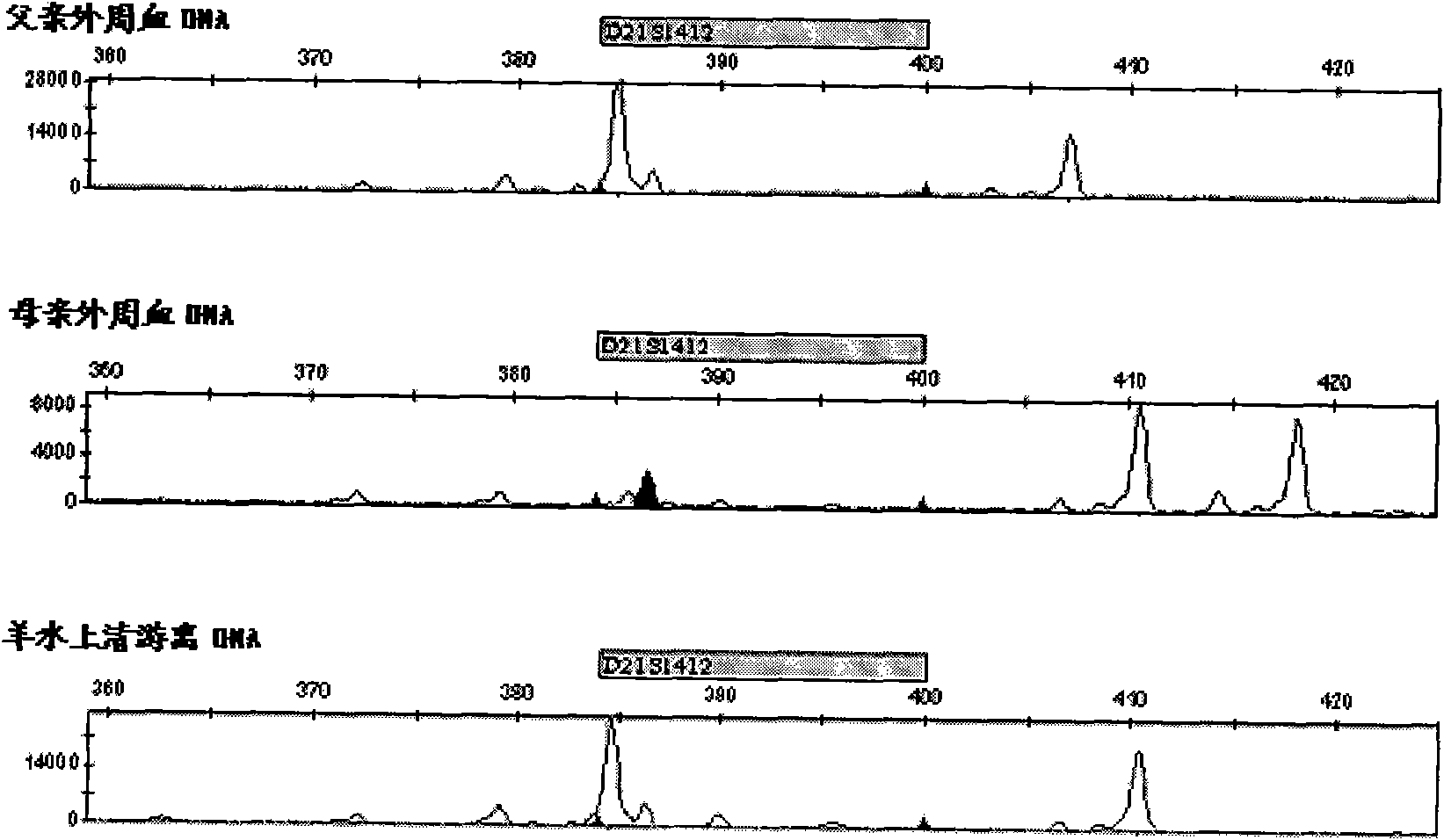
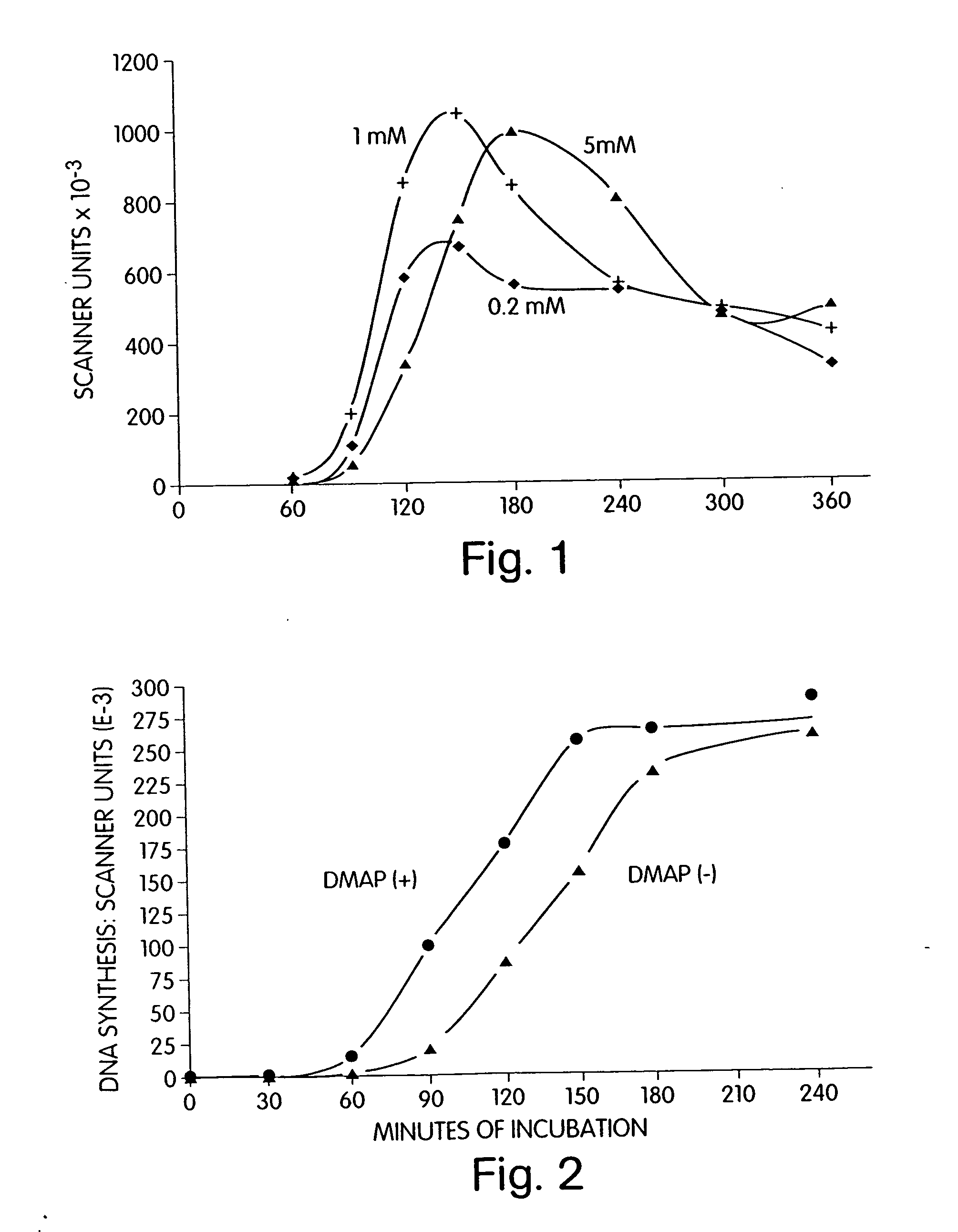
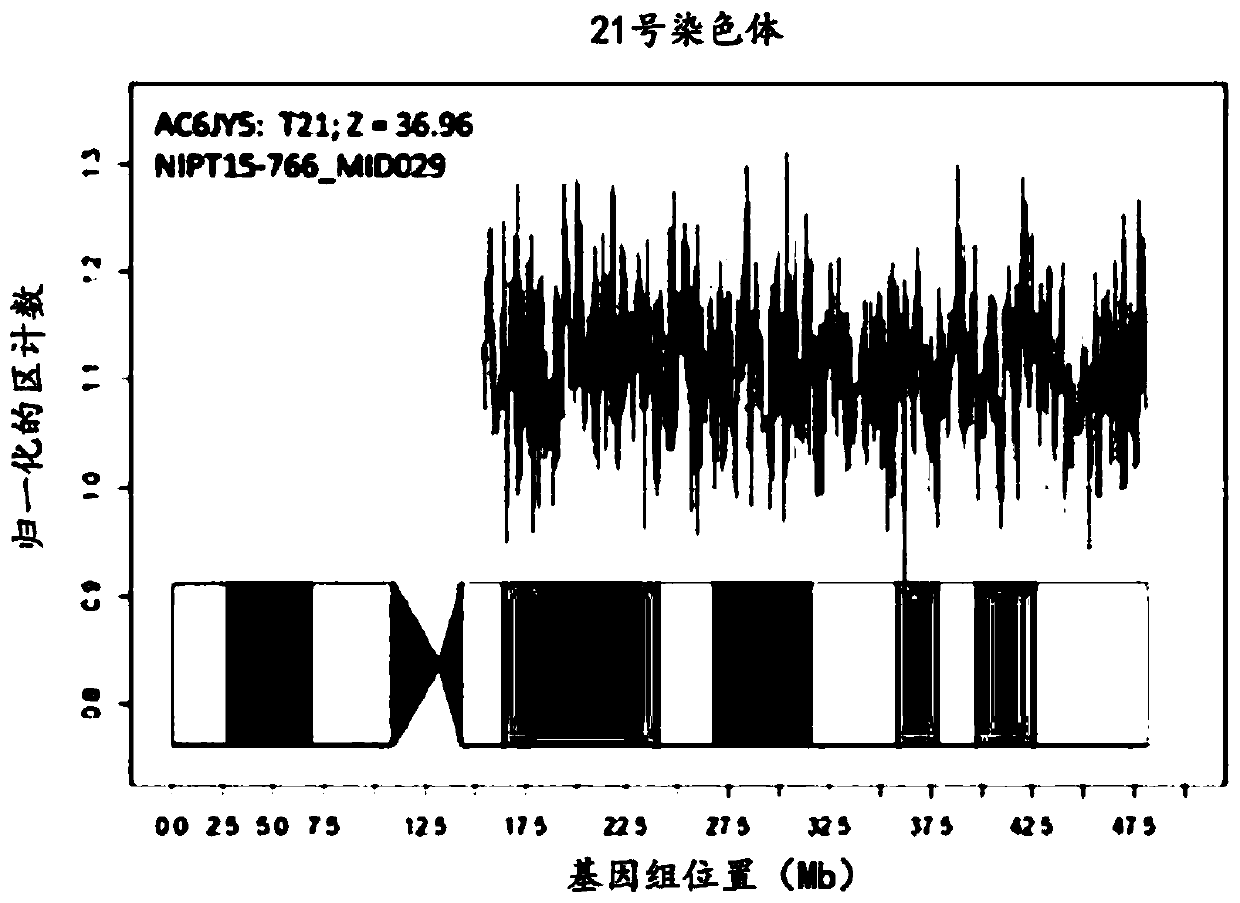
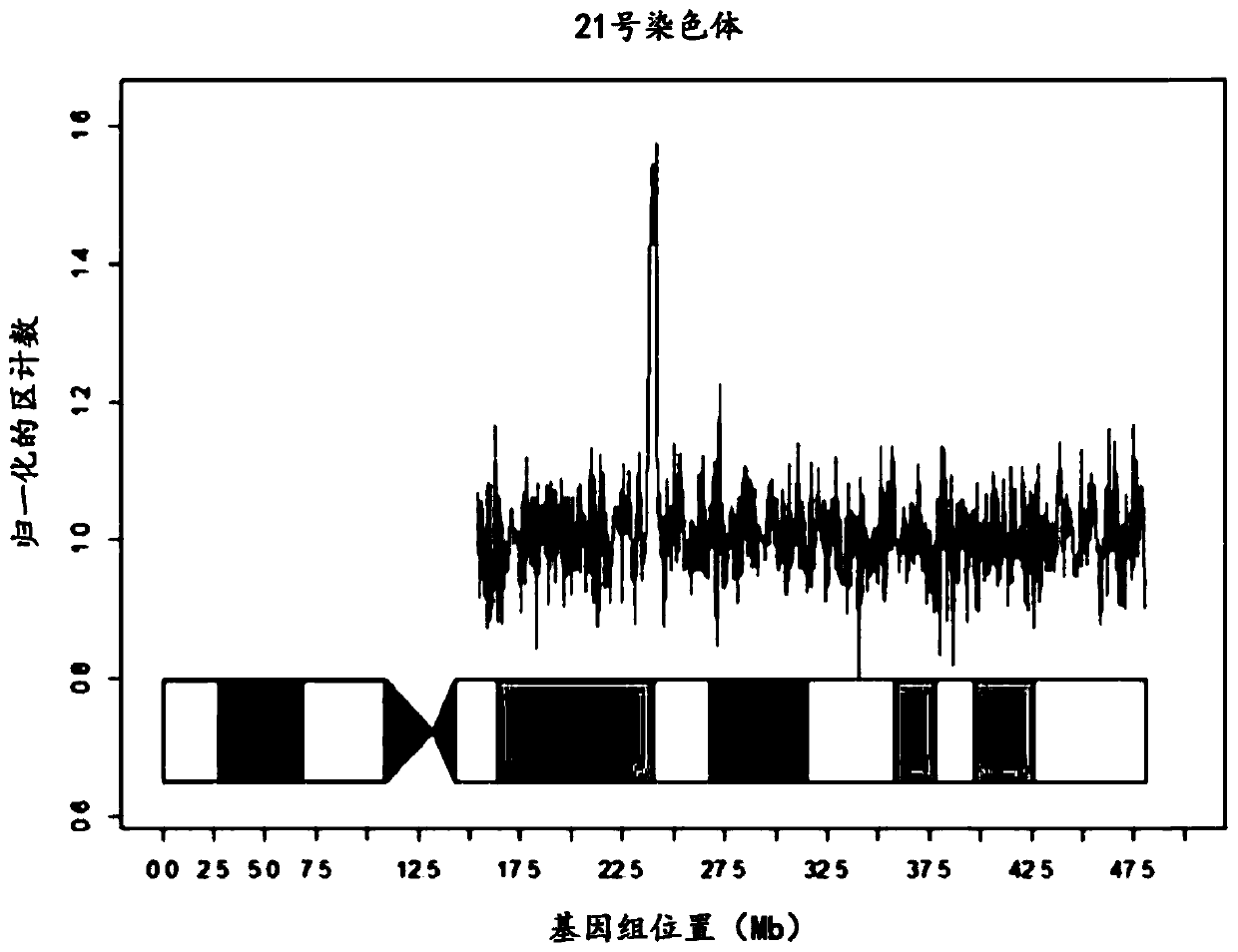
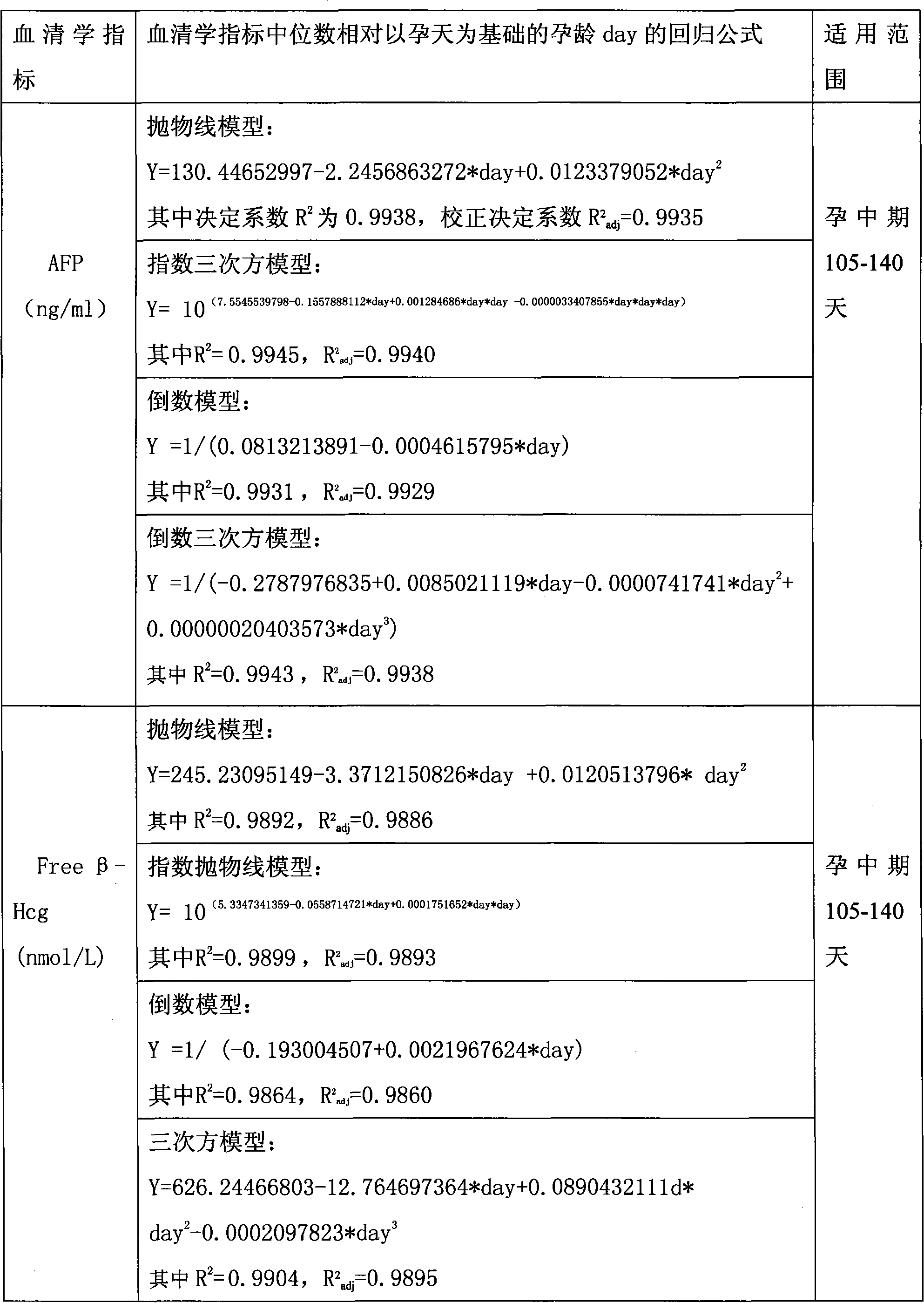
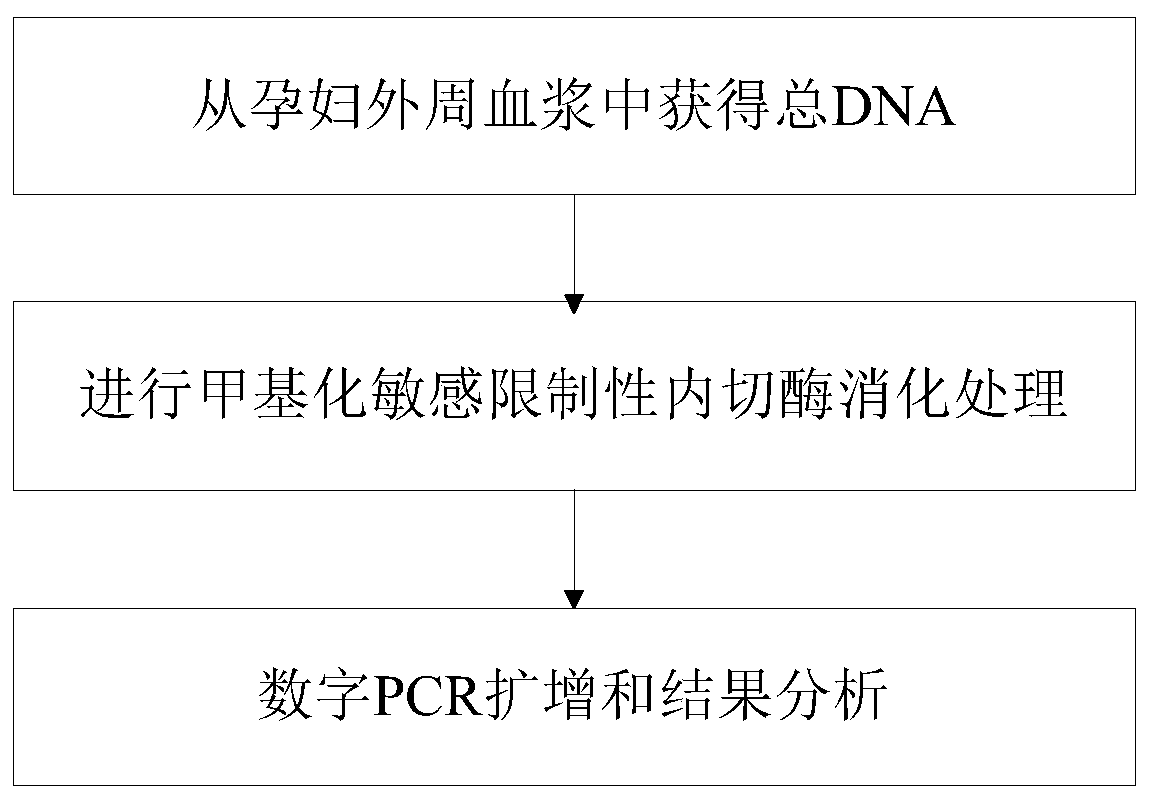
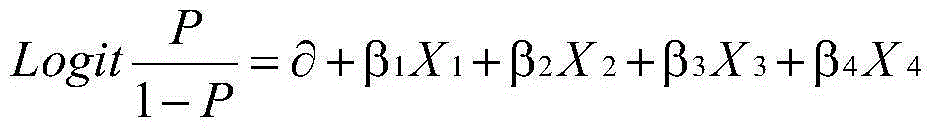Patents
Literature
89 results about "Prenatal screening" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
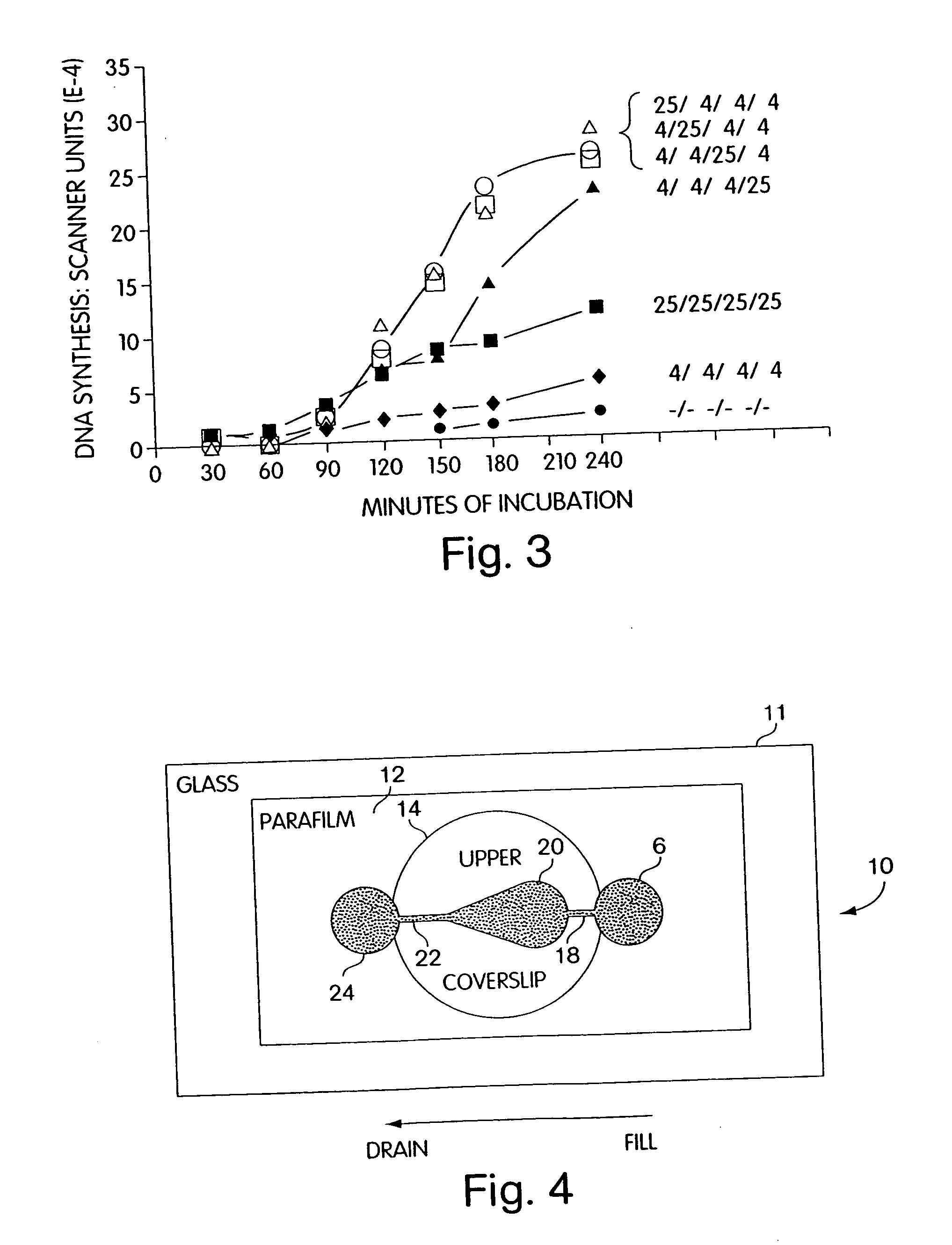
Prenatal screening tests generally are used to assess the likelihood that a baby will be affected by certain conditions. When screening tests indicate that a fetus is at increased risk, prenatal diagnostic tests, which often are invasive, may be performed to confirm the presence of a disorder.
Methods of prenatal screening for trisomy 21
InactiveUS20080188748A1Raise the possibilityUltrasonic/sonic/infrasonic diagnosticsPerson identificationNormal fetusTrisomy
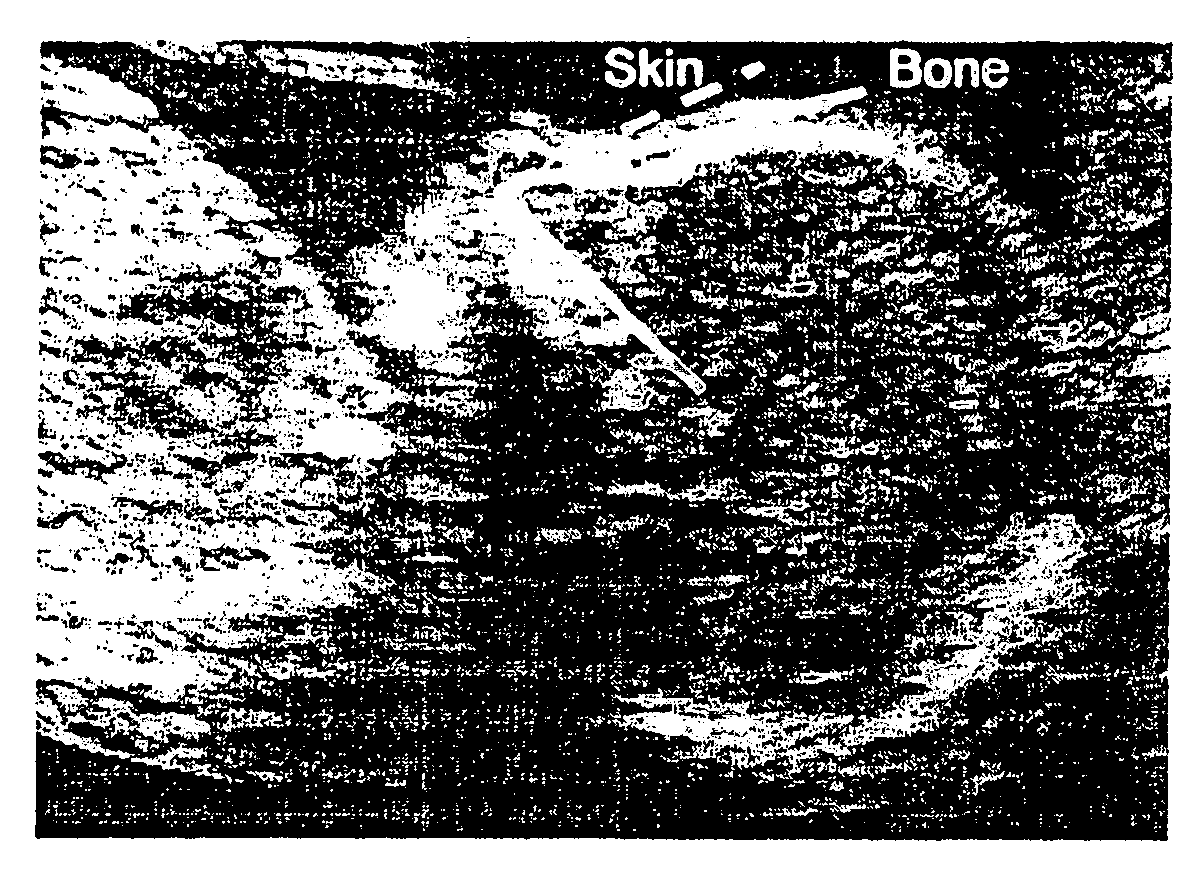
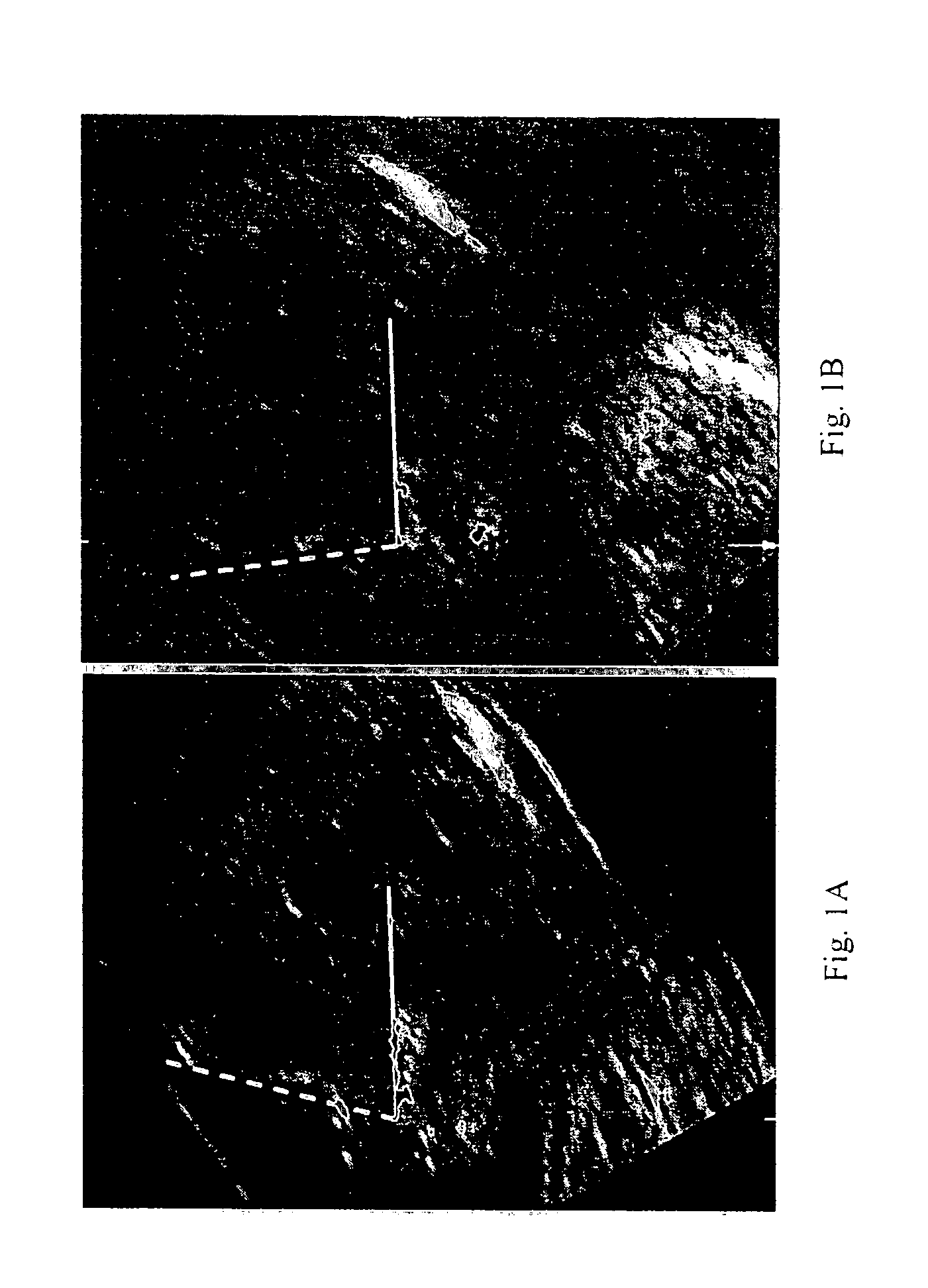
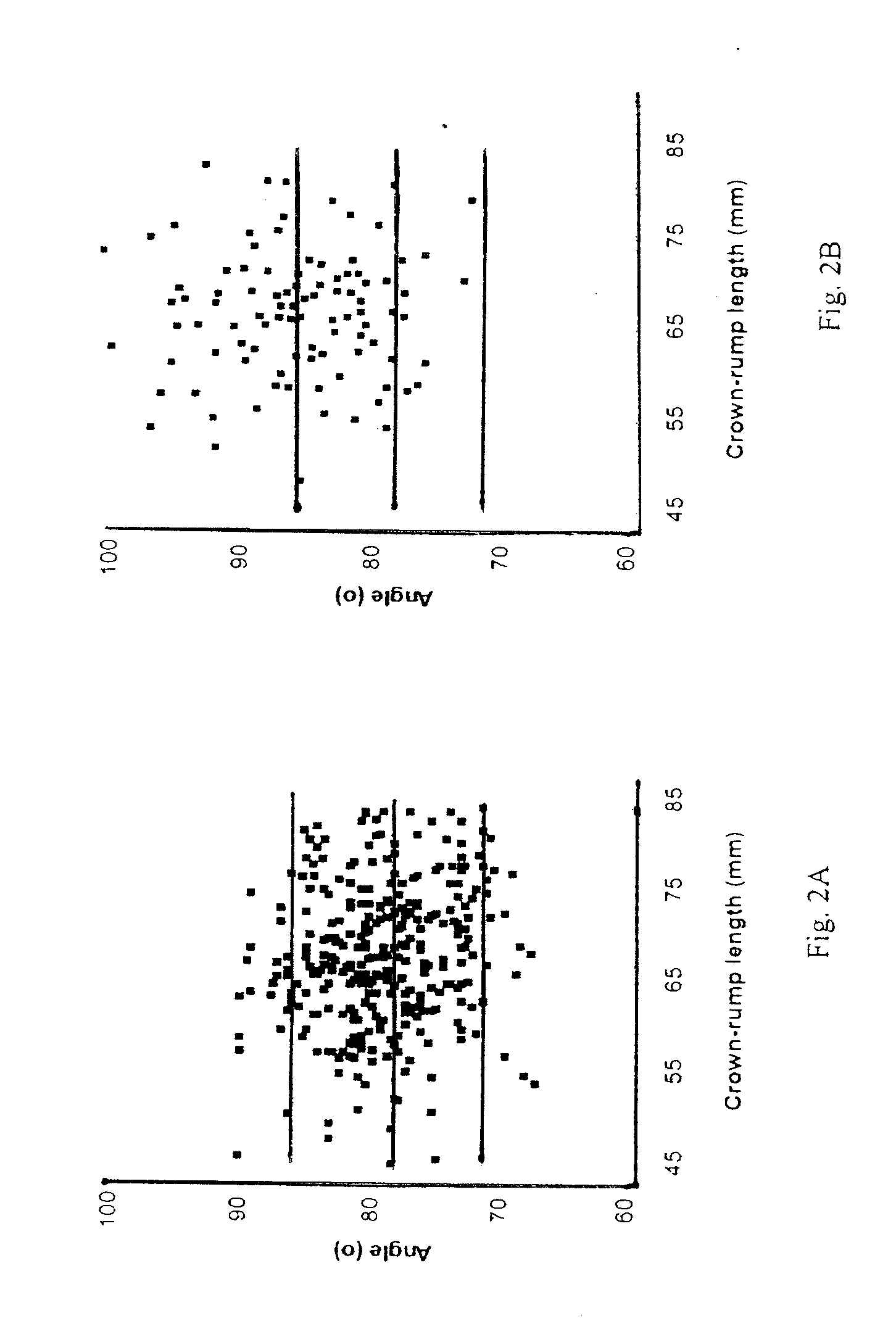
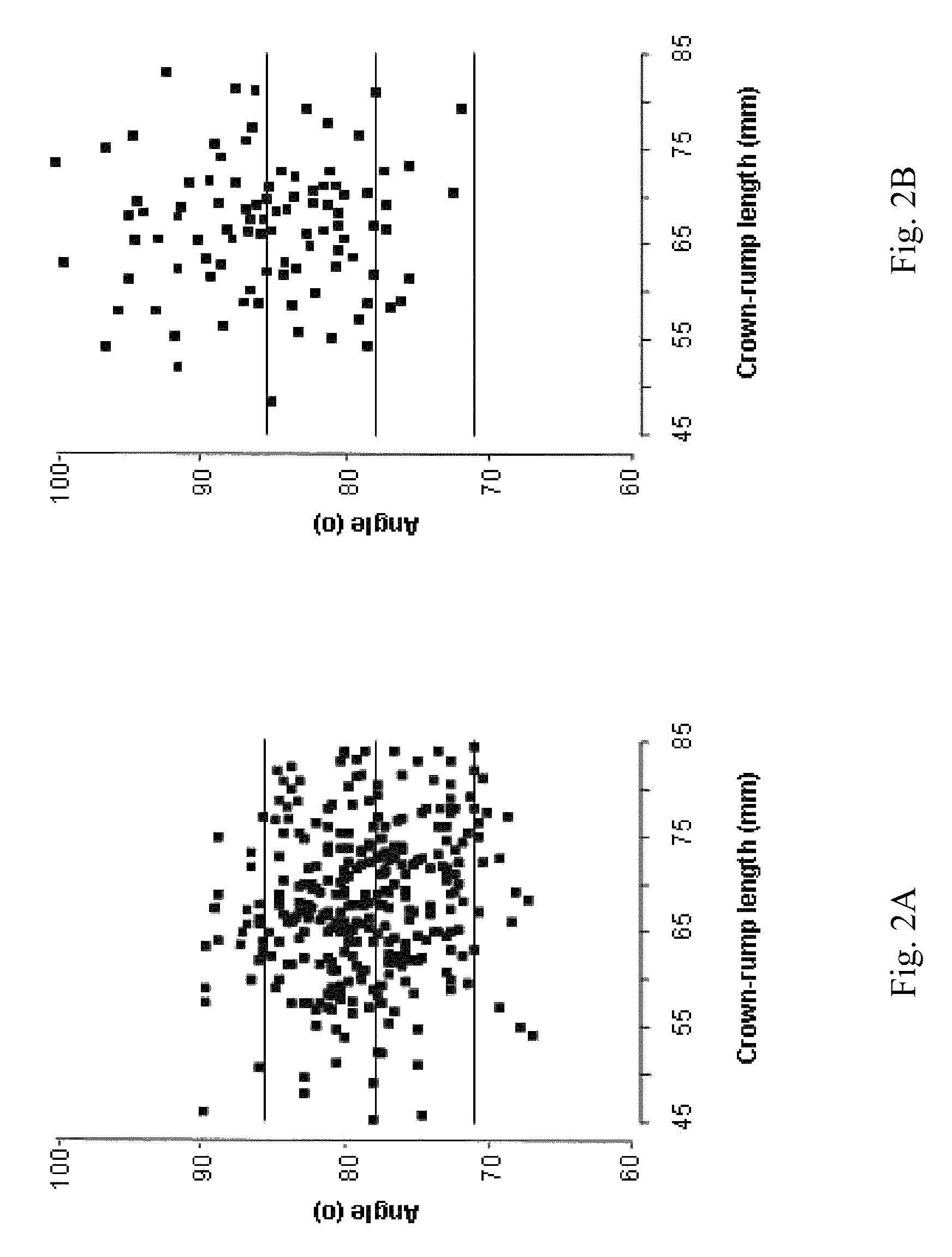
Methods for prenatal screening for trisomy 21 employ examination of the fronto-maxillary facial (FMF) angle of a fetus. In one embodiment, the methods comprise obtaining a two or three dimensional image of a fetal face, measuring the FMFbone angle on the image, and comparing the measured FMFbone angle with an FMFbone angle characteristic of chromosomally normal fetuses, wherein a measured FMFbone angle greater than the FMFbone angle characteristic of chromosomally normal fetuses provides an indication of an increased likelihood of the occurrence of trisomy 21 in the fetus. In another embodiment, the methods comprise obtaining a two or three dimensional image of a fetal face, measuring the FMFskin angle on the image, and comparing the measured FMFskin angle with an FMFskin angle characteristic of chromosomally normal fetuses, wherein a measured FMFskin angle greater than the FMFskin angle characteristic of chromosomally normal fetuses provides an indication of an increased likelihood of the occurrence of trisomy 21 in the fetus. In additional embodiments, both FMFbone angle and FMFskin angle are measured and compared with values characteristic of chromosomally normal fetuses.
Owner:SONEK JIRI D +1
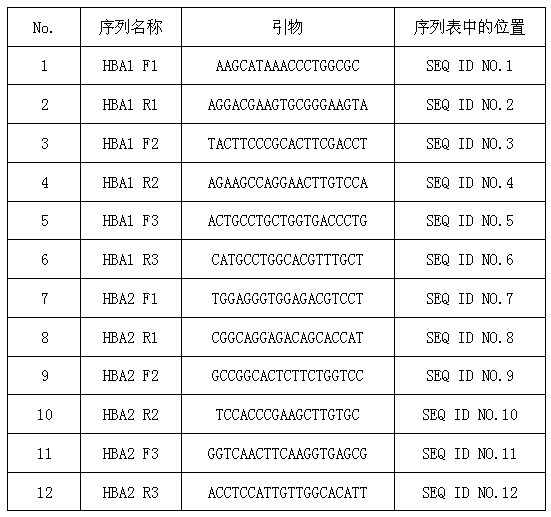
Primer system for detecting gene SNPs (single nucleotide polymorphisms) related to hereditary hearing loss and application of primer system
InactiveCN103276065AHigh detection sensitivityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationNucleotideQuantitative epidemiology
The invention discloses a primer system which is used for simultaneously detecting 10 gene SNPs (single nucleotide polymorphisms) related to hereditary hearing loss. Based on a product prepared by using the primer system, the 10 gene SNPs related to the related to hereditary hearing loss can be simultaneously detected. The product can be used for detecting the gene types of 10 gene SNPs of a testee, and the detection results can be used as a reference to clinical diagnosis and also can be used in the fields, such as epidemiological investigation, prenatal screening and neonatal screening. According to the primer system, 10 gene SNPs on different genes can be simultaneously detected in one reaction system; and therefore compared with technologies, such as sequencing, real-time fluorogenic quantitative PCR (polymerase chain reaction), the primer system is lower in cost and simple and convenient to operate, and the accuracy and sensitivity are improved.
Owner:向华
Application of MDPCR (multiple digital PCR (polymerase chain reaction)) technology to chromosome aneuploidy screening
InactiveCN104846103AShort half-lifeIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseAgricultural science
The invention provides application of an MDPCR (multiple digital PCR (polymerase chain reaction)) technology to chromosome aneuploidy screening. Concretely, the invention discloses a method for performing NIPS (non-invas ive prenatal screening) including Down's syndrome, Edward's syndrome, Patau syndrome and other chromosome abnormality diseases on pregnant women by the MDPCR technology. The method has the advantages that early, safe, non-invas, accurate and fast effects are achieved, the method is suitable for large-scale detection and clinic application, and the like; intrauterine infection, abortion and death in the pregnancy middle and later periods of the pregnant women can be avoided; the goals of bearing and rearing better children are achieved.
Owner:NANJING JENOMED BIOTECH CO LTD
Primer system for detecting gene SNP related to genetic deafness, and use thereof
ActiveCN103352073AHigh detection sensitivityImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationAccuracy improvementFluorescence
The present invention discloses a primer system for concurrently detecting 10 gene polymorphism sites related to genetic deafness. Based on the product prepared by the primer system, 10 gene polymorphism sites related to genetic deafness can be concurrently detected. Use of the product, genotypes of 10 gene polymorphism sites of a subject are detected, and the detection results can be used for assisted clinical diagnosis, and can further be used for epidemiological investigation, and can also be used for prenatal screening, neonate screening and other fields. With the present invention, 10 gene polymorphism sites on different genes can be concurrently detected in a reaction system, such that advantages of low cost, convenient operation, accuracy improvement and sensitivity improvement are provided compared with sequencing, real-time fluorescence quantitative PCR and other technologies.
Owner:BIOYONG TECH
AFP (Alpha-Fetoprotein) testing kit (time-resolved fluoroimmunoassay) for prenatal screening and preparation method thereof
ActiveCN101819206AImprove accuracyHigh sensitivityBiological testingBlood specimenPrenatal screening
The invention relates to an alpha-fetoprotein testing kit (time-resolved fluoroimmunoassay) applicable for mid-pregnancy prenatal screening and a preparation method thereof. The kit comprises the following main components of an experimental buffer solution, a concentrated washing solution, an enhancement solution, a reaction plate, a standard product and a quality control product of filter paper dried blood spots and a europium marker. The method for preparing the kit according to the invention comprises the following steps of: 1. preparing the experimental buffer solution, the concentrated washing solution and the enhancement solution; 2. coating the reaction plate; 3. preparing the standard product and the quality control product; 4. preparing the europium marker; 5. separately loading; 6. sticking labels; and 7. assembling into a finished product. The invention has the characteristics that the accuracy and the sensitivity of a detected result are high, the stability is good, and a detecting method is economical, convenient and safe and has no traumatic property, has high automation degree, and the like. A filter paper dried blood spot technology is applied to prenatal screening work, which is beneficial to blood specimen collection, storage and transportation and ensures the experimental reliability.
Owner:GUANGZHOU FENGHUA BIOENG
Kit for multiplex real-time quantitative PCR (polymerase chain reaction) detection of chromosome aneuploid and application of kit
ActiveCN106191233AShort half-lifeIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPrenatal screeningS syndrome
The invention discloses a kit for multiplex real-time quantitative PCR (polymerase chain reaction) detection of chromosome aneuploid and application of the kit. The kit comprises n1 primer pairs used for amplifying multiple genes and non-genetic locus of a target chromosome and related probes and n2 primer pairs used for amplifying multiple genes and non-genetic locus of a reference chromosome and related probes, wherein the target chromosome is a chromosome subject to chromosome quantitative variation diseases, the reference chromosome is one or multiple chromosomes, and n1 and n2 are greater than or equal to 1 respectively. The kit can be used for noninvasive prenatal screening of Down's syndrome, Edward's syndrome, PaDow's syndrome and other chromosome abnormality diseases of pregnant women. The kit has the advantages of early stage, safety, noninvasiveness, accuracy, quickness and suitability for large-scale detection and clinical application, intrauterine infection, abortion and death of fetuses at middle and late stages of pregnancy of women can be avoided, and the objective of bearing and rearing better children is achieved.
Owner:SHANGHAI JENOMED BIOTECH CO LTD
Mutations in the C7orf11 (TTDN1) gene causative of non-photosensitive trichothiodystrophy
InactiveUS20060172322A1Alleviate and treat and prevent diseaseFungiBacteriaDiseaseAntiendomysial antibodies
The invention is the demonstration that mutations of the C7orf11 nucleic acid sequence leads to the development of non-photosensitive trichothiodystrophy. The invention comprises methods of screening for and detection of non-photosensitive TTD carriers, prenatal screening and diagnosis, diagnosis of non-photosensitive TTD, drug and gene therapy, manufacture of the protein, and antibodies as well as development of animal models of disease for the testing of pharmaceutical agents.
Owner:HOSPITAL FOR SICK CHILDREN
Method, kit and analysis system for synchronous prenatal screening of chromosomes and monogenic diseases
ActiveCN111951890AMicrobiological testing/measurementBiostatisticsPrenatal screeningChromosome microdeletion
The invention provides a detection method, a kit and a system for noninvasive prenatal screening of fetal chromosome copy number variation, fetal chromosome microdeletion / microduplication and / or dominant single gene mutation. The invention further provides a design method of a targeted capture probe. The detection method is used for non-invasive prenatal screening of fetuses. Compared with existing non-invasive prenatal screening detection methods, the application range of clinical gene detection can be expanded, and the detection accuracy is improved.
Owner:BEIJING BIOBIGGEN TECH CO LTD +1
Alpha-thalassemia screening kit and application thereof in prenatal screening
ActiveCN103421903AReasonable primer designAchieving Prenatal ScreeningMicrobiological testing/measurementDiseaseObstetrics
The invention discloses an alpha-thalassemia screening kit and application thereof in prenatal screening and belongs to the technical field of prenatal screening of alpha-thalassemia. The alpha-thalassemia screening kit comprises a negative control sample, a positive control sample, PCRmasterMIX, and a PCR primer. Application of the alpha-thalassemia screening kit includes: amplifying fetal DNA in maternal peripheral blood by the designed primer so as to perform prenatal screening on alpha-thalassemia. The maternal peripheral blood is collected for prenatal screening of alpha-thalassemia, with no need for puncturing the amnion cavity and inserting pile tissue and with no injury to fetus, and the alpha-thalassemia screening kit is safe and reliable; accuracy is up to 99.99%; the technical blank of noninvasive prenatal screening of alpha-thalassemia is filled, and fewer children with diseases are born.
Owner:邯郸市康业生物科技有限公司
Prenatal Screening
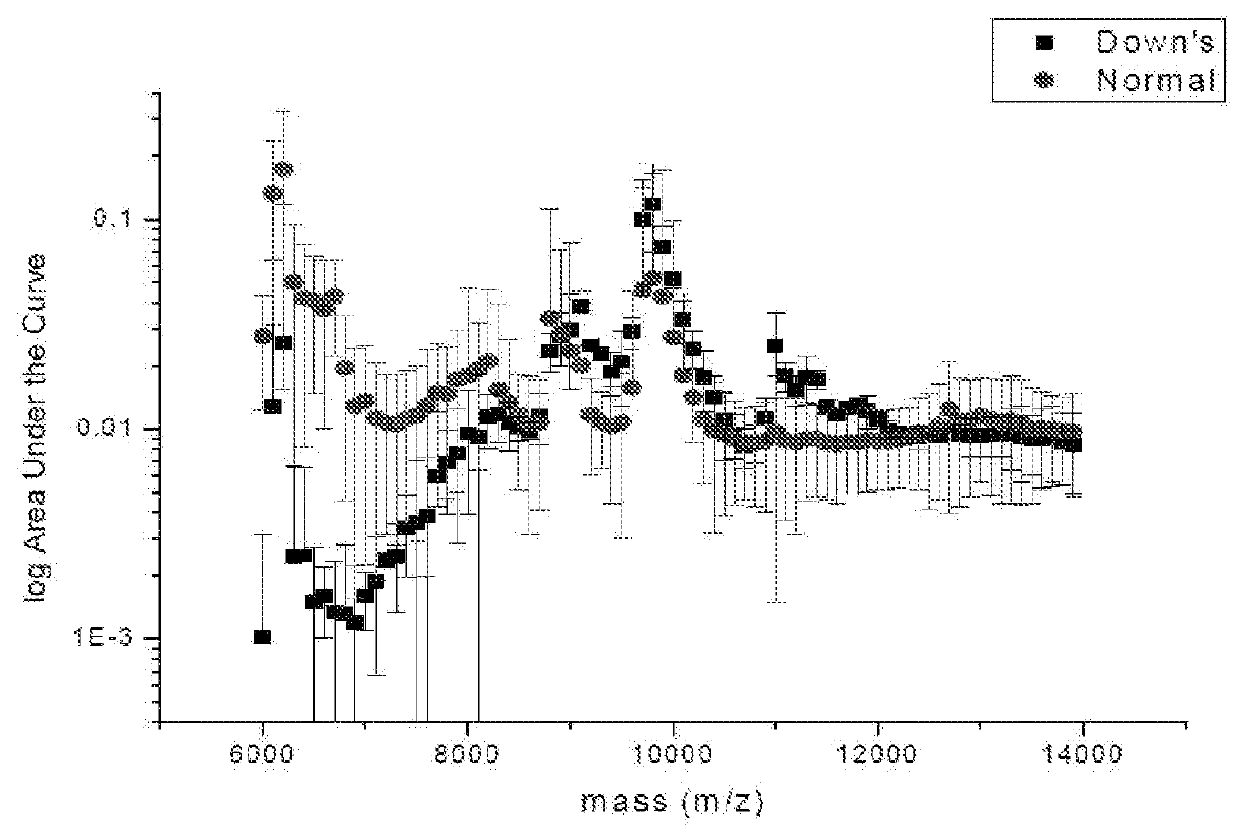
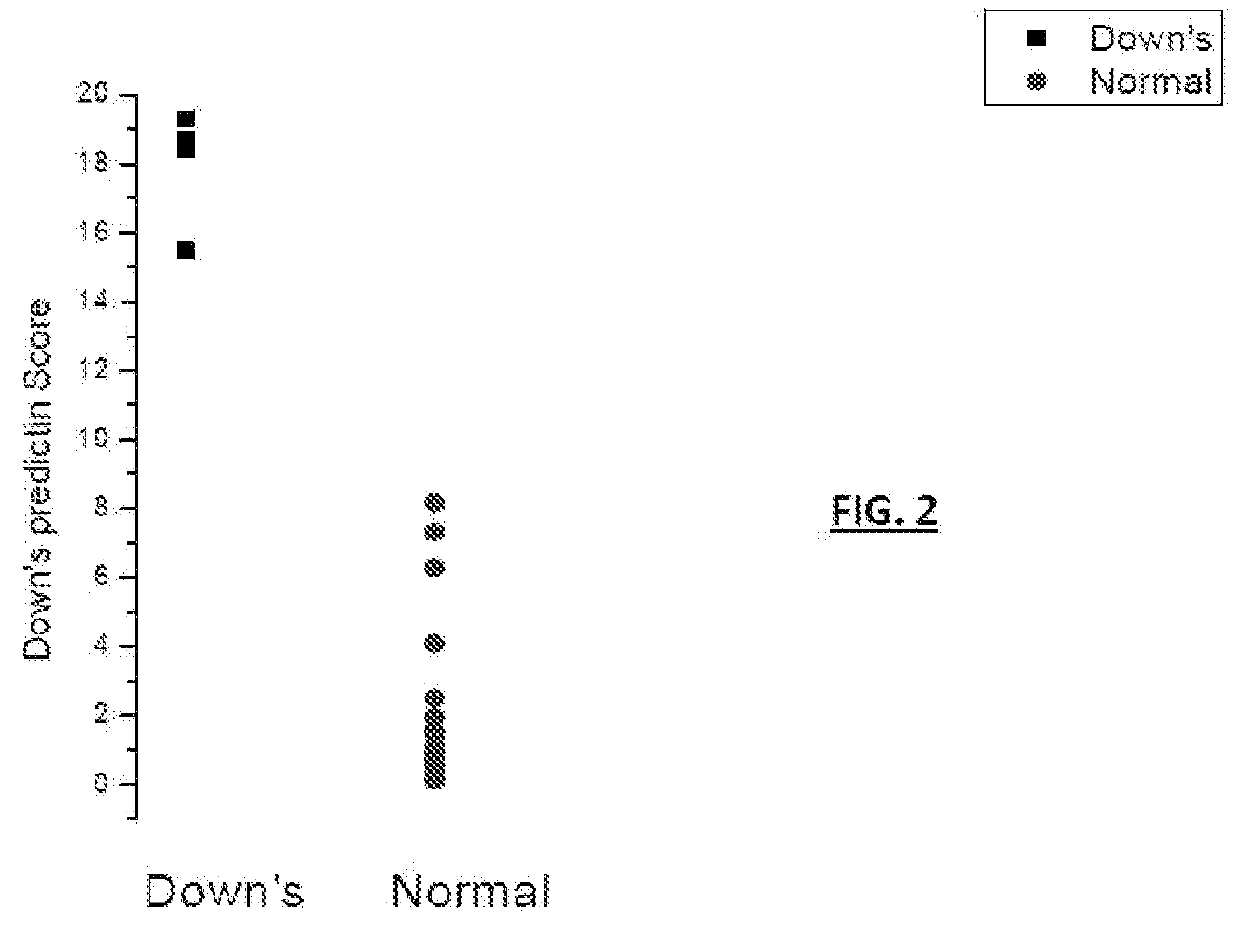
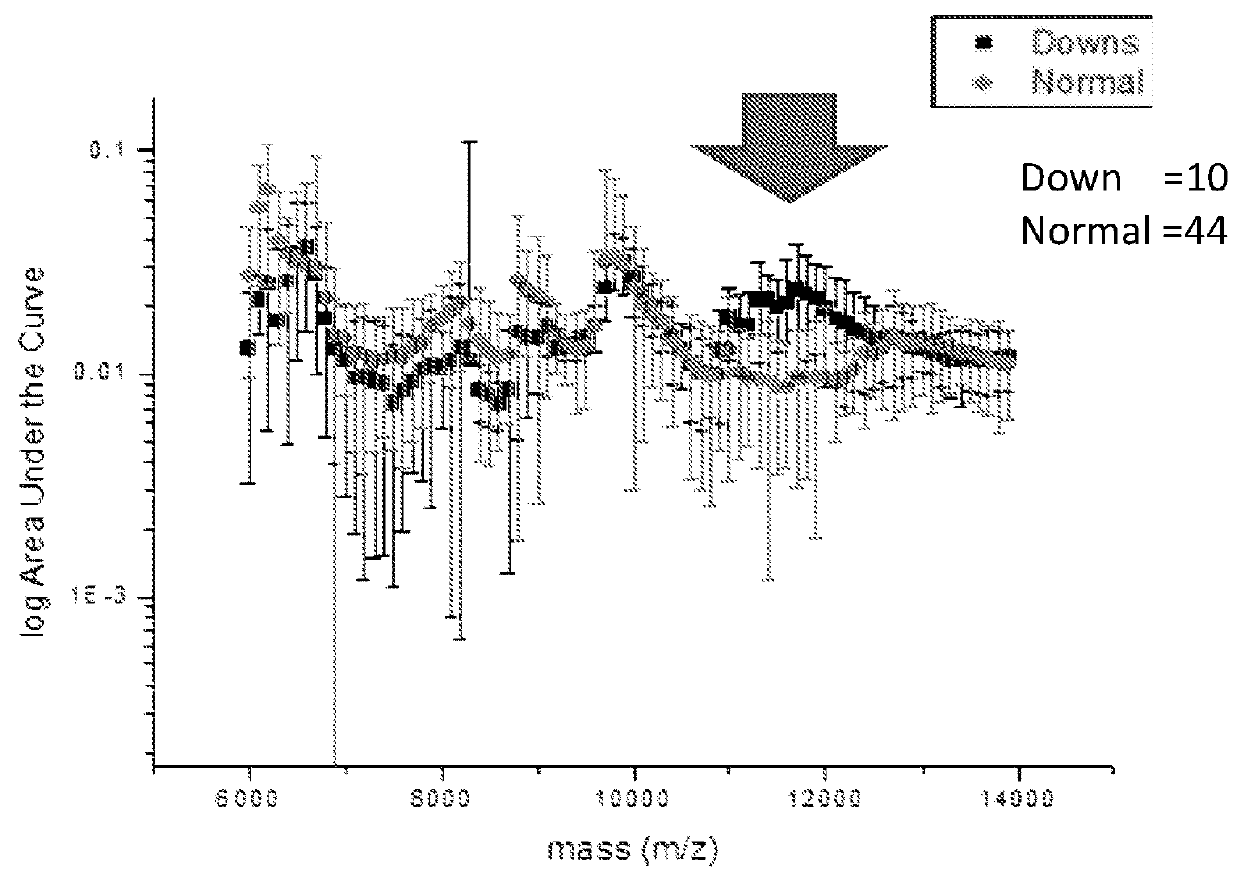
ActiveUS20160054293A1Easy to calculateSmall sizeTime-of-flight spectrometersParticle spectrometer methodsDiseaseFetal abnormality
The present invention relates to a method for screening maternal urine samples for changes in the pattern of mass spectral fingerprinting which have been found to be characteristic of fetal aneuploidies such as Down's Syndrome and have application for the screening of other fetal abnormalities and disorders of pregnancy including gestational trophoblastic diseases.
Owner:MAP IP HOLDING LTD
Down's syndrome risk assessment method for prenatal screening in pregnant metaphase
InactiveCN105069277AEligible for prenatal screeningImprove screening effectSpecial data processing applicationsDoubling testObstetrics
The present invention discloses a Down's syndrome risk assessment method for prenatal screening in pregnant metaphase, which includes the following steps: 1) collecting serological index testing results of pregnant women who meet the requirement of age, gestation age and weight; 2) determining gestation ages; 3) determining a median of a serological index of normal pregnant women of each of pregnancy days to obtain a regression formula of the median of the serological index of the normal pregnant women of each of pregnancy days relative to pregnancy days; 4) determining a multiple of the median of the tested pregnant women; 5) adjusting the multiple of the median of the tested pregnant women by the regression formula; 6) establishing a coefficient of a risk assessment formula of Down's syndrome by Logistic regression; 7) establishing the risk assessment formula of a triple test scheme and a double test scheme of Down's syndrome. The method based on pregnancy days and applied to prenatal screening aims at Chinese pregnant women, and has high positive rate and low false positive.
Owner:NINGBO UNIV
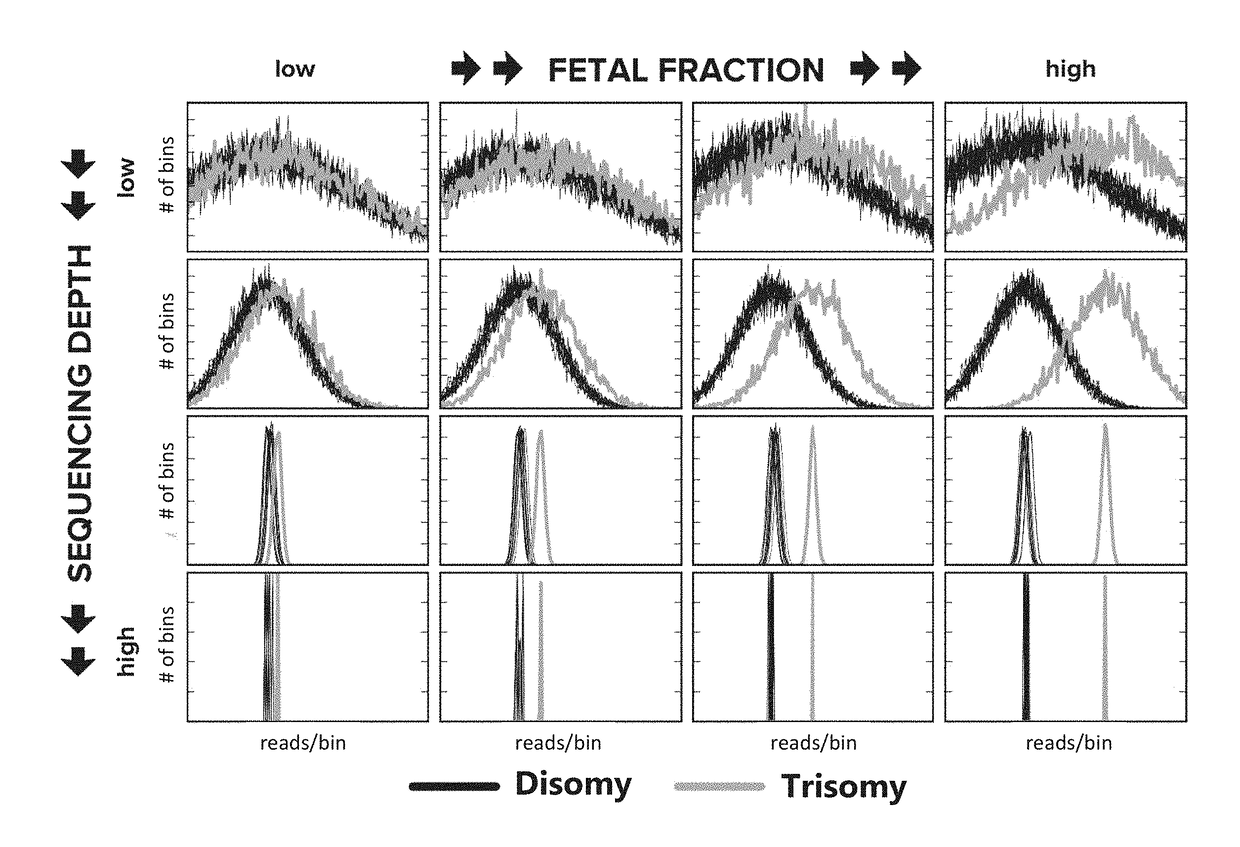
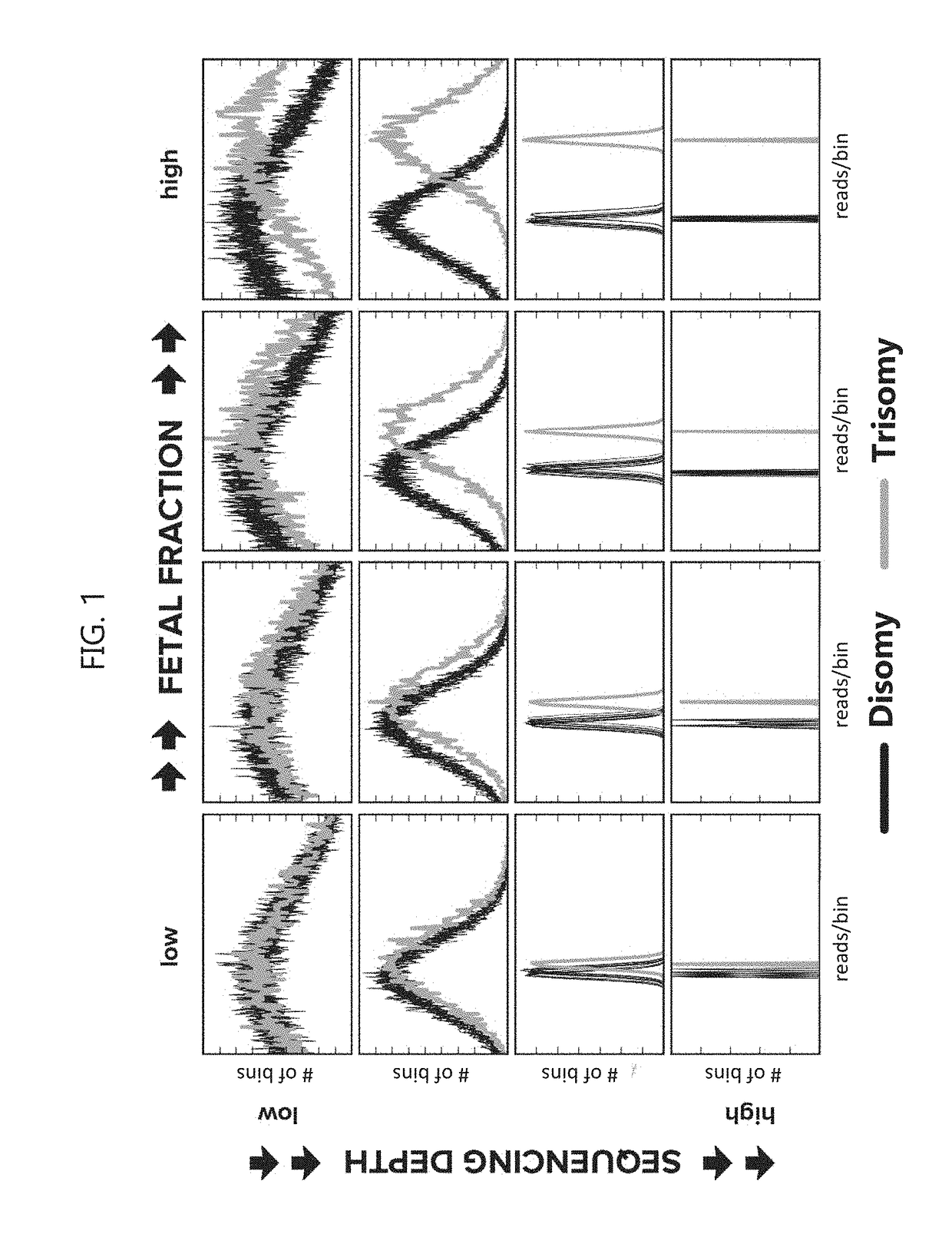
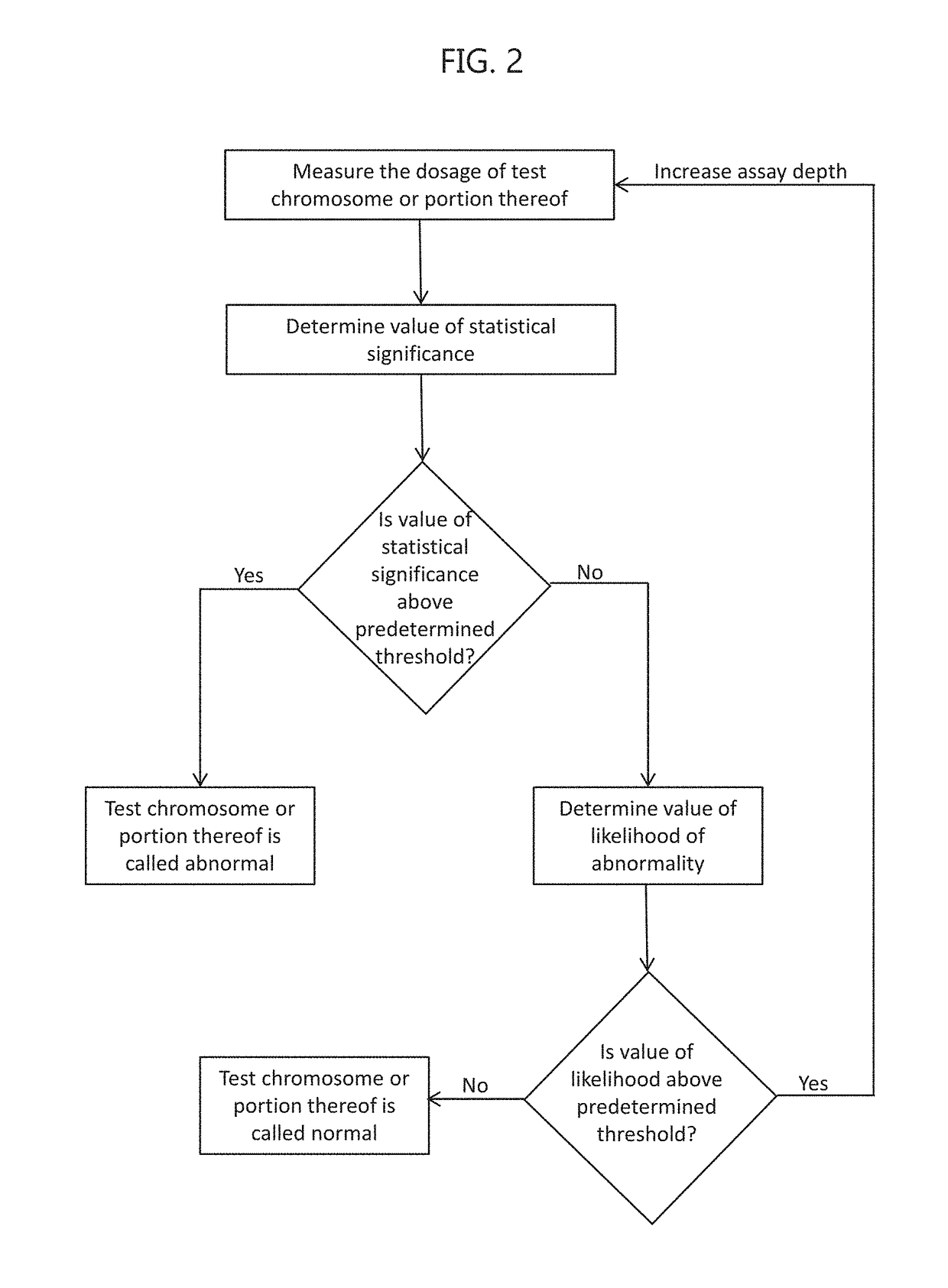
Noninvasive prenatal screening using dynamic iterative depth optimization
Fetal maternal samples taken from pregnant women include both maternal cell-free DNA and fetal cell-free DNA. Described herein are methods for determining a chromosomal abnormality of a test chromosome or a portion thereof in a fetus by analyzing a test maternal sample of a woman carrying said fetus, wherein the test maternal sample comprises fetal cell-free DNA and maternal cell-free DNA. The chromosomal abnormality can be, for example, aneuploidy or the presence of a microdeletion. In some embodiments, the chromosomal abnormality is determined by measuring a dosage of the test chromosome or portion thereof in the test maternal sample, measuring a fetal fraction of cell-free DNA in the test maternal sample, and determining an initial value of likelihood that the test chromosome or the portion thereof in the fetal cell-free DNA is abnormal based on the measured dosage, an expected dosage of the test chromosome or portion thereof, and the measured fetal fraction.
Owner:MYRIAD WOMENS HEALTH INC
Fetal chromosome library construction kit and method and application thereof
ActiveCN105624798ANo lossNo pollutionMicrobiological testing/measurementLibrary creationGeneticsPrenatal screening
The invention relates to the field of noninvasive prenatal screening detection, in particular to a fetal chromosome library construction kit and method and application thereof.The kit includes a reagent for DNA extraction and a reagent for DNA library construction.Compared with other free DNA library construction kits in the markets at home and abroad, the fetal chromosome library construction kit can directly extract free DNA from the plasma of a pregnant woman to perform sequencing library construction, can completely and systematically achieve sample free DNA extraction and library construction, can be used for detecting whether fetal chromosomes are aneuploid or not through high-throughput noninvasive screening, is convenient to operate, simple, rapid, efficient and accurate in detection result and high in sensitivity and has an important value.
Owner:北京爱普益医学检验中心有限公司
Quality control product, preparation method thereof, kit and method for detecting trisomy 21 and 18 syndromes of fetuses
PendingCN110106247AGuaranteed detection sensitivityShort detection cycleMicrobiological testing/measurementTrisomyQuality control
The invention relates to a quality control product, a preparation method thereof, a kit and a method for detecting trisomy 21 and 18 syndromes of fetuses. The digital PCR technology with single-molecule detection sensitivity and absolute quantitative capability is used, and a new simple and effective chromosome aneuploid non-invasive prenatal detection method is developed. By using peripheral blood of a pregnant woman, non-invasive prenatal detection can be completed on one sample within about 5 hours. By adopting the non-invasive prenatal detection technology based on the digital PCR, accurate, rapid and simple prenatal screening can be realized. The accuracy of the detection kit reaches 99%, the cost is only 1 / 5 that of the NIPT technology, and the detection time is 5 hours.
Owner:苏州行知康众生物科技有限公司
Beta-human chorionic gonadotrophin test kit (time-resolved fluoroimmunoassay) for prenatal screening and preparation method thereof
The invention discloses a beta-human chorionic gonadotrophin test kit (time-resolved fluoroimmunoassay) applicable for early-pregnancy and mid-pregnancy prenatal screening and a preparation method thereof. The kit comprises the following main components of an experimental buffer solution, a concentrated washing solution, an enhancement solution, a reaction plate, a standard product and a quality control product of filter paper dried blood spots and a europium marker. The method for preparing the kit according to the invention comprises the following steps of: 1. preparing the experimental buffer solution, the concentrated washing solution and the enhancement solution; 2. coating the reaction plate; 3. preparing the standard product and the quality control product; 4. preparing the europium marker; 5. separately loading; 6. sticking labels; and 7. assembling into a finished product. The invention has the characteristics that the accuracy and the sensitivity of a detected result are high, the stability is good, and a detecting method is economical, convenient and safe and has no traumatic property and the like. A filter paper dried blood spot technology is applied to prenatal screening work, which is beneficial to blood specimen collection, storage and transportation and ensures the experimental reliability.
Owner:广州市丰华生物股份有限公司
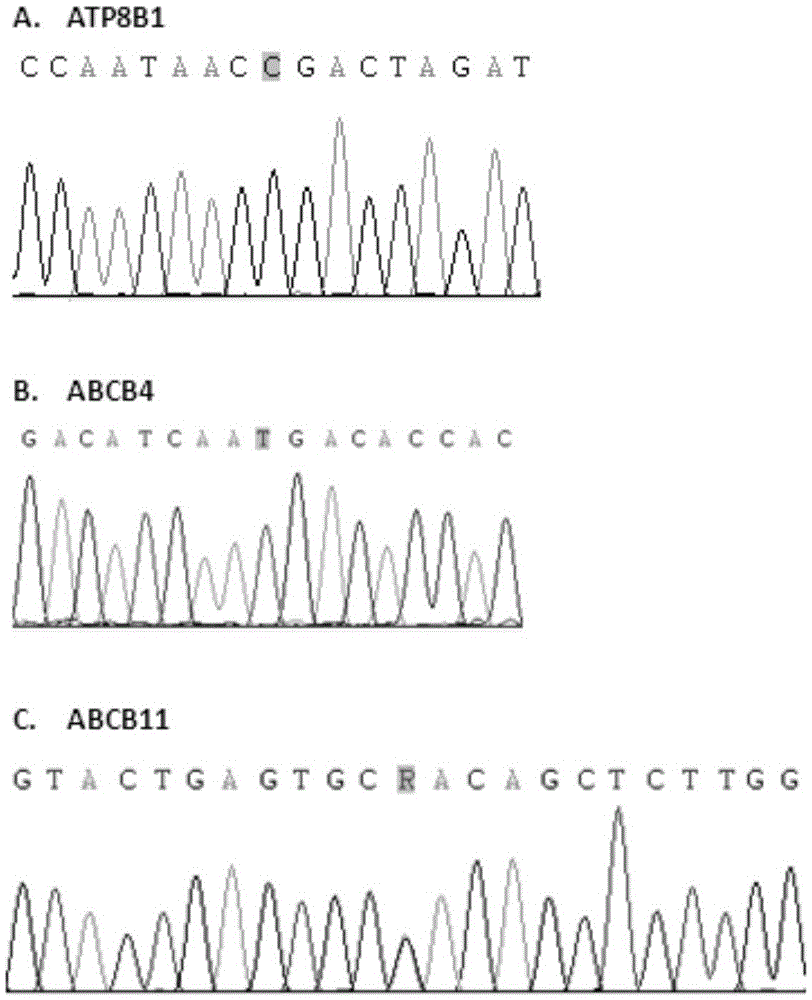
Kit for detecting gene mutation of progressive familial intrahepatic choleatasia (PFIC) and detection method of kit
ActiveCN105463083AReduce morbidityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationPrenatal screeningDNA
The invention relates to a kit for detecting gene mutation of progressive familial intrahepatic choleatasia (PFIC) and a detection method of the kit. The kit comprises a PCR amplification reaction agent and a PCR primer for amplifying ATP8B1 gene / ABCB4 gene / ABCB11 gene in sample DNA. The detection method comprises the steps of sample collection, PCR amplification and sequencing. The kit and the detection method can be used for detecting the mutation sites of ATP8B1 gene / ABCB4 gene / ABCB11 gene, and are good in specificity and high in sensitivity; after family members are screened, the risk of disease is clear; prenatal screening and intervention are carried out, so that the incidence of PFIC of the descendants is reduced; a method of the kit is simple; and the kit and the detection method have good application prospects.
Owner:SHANGHAI SIMPLEGENE CLINICAL LAB CO LTD
Probe set and kit used for detecting related genes of congenital cataract
InactiveCN106282369AIncrease coverageImprove efficiencyMicrobiological testing/measurementDNA/RNA fragmentationCongenital cataractsPrenatal screening
The invention discloses a probe set and a kit used for detecting the related genes of a congenital cataract. The probe set comprises a plurality of probe sequences which respectively aim at the exon non-repeated areas of 225 related genes of the congenital cataract. The invention also provides the kit which contains the probe set and is used for regional capture and next generation sequencing. According to the probe set and the kit, the found 225 related genes of the congenital cataract can be comprehensively detected in one time, the coverage rate and the efficiency of molecular diagnosis can be improved, specificity is good, and sensitivity is high, and the probe set and the kit have an important meaning for the prenatal screening, the early diagnosis and the accurate prevention and control of the congenital cataract.
Owner:ZHONGSHAN OPHTHALMIC CENT SUN YAT SEN UNIV
Method for prenatal screening chromosome abnormality and application thereof
InactiveCN101603083AImprove the detection rateReduce false positive rateMicrobiological testing/measurementSerum markersObstetrics
The invention discloses a method for prenatal screening chromosome abnormality and an application thereof. The invention comprises the following steps: totally calculating various measurement indicators of normal pregnant women in China to obtain the median of serum marker of single pregnancy people in each gestational week, the median of serum marker of double pregnancy people in each gestational week and the median of translucent thickness of fetal nape of normal pregnant women in each gestational week; detecting the serum marker, fetal number, translucent thickness of fetal nape of the detected pregnant women to determine the gestational weeks of the pregnant women; correcting according to the weight of the detected pregnant women and finally calculating the multiples of the median value (MoM value) of serum marker; then comparing correspondingly the multiples of the median of serum marker of the detected women with the median of serum marker of normal pregnant women according to the gestational weeks of the pregnant women and fetal number, calculating the likelihood ratio of the serum marker of the detected pregnant women to obtain the risk rates of 21 trisome, 18 trisome or 13 trisome and the risk rate of NTD risk.
Owner:GUANGDONG WOMEN & CHILDREN HOSPITAL +1
Kit for non-invasive prenatal screening for trisomy syndrome and application thereof
ActiveCN109694907ALow costShort detection cycleMicrobiological testing/measurementDNA/RNA fragmentationTrisomy E SyndromeTrisomy 13 Syndrome
The invention discloses a kit for non-invasive prenatal screening for trisomy syndrome and an application thereof. The kit of the present invention includes a primer pair and a probe for prenatal screening of trisomy 13 syndrome, a primer pair for prenatal screening of trisomy 18 syndrome, and a primer pair and a probe for prenatal screening of trisomy 21 syndrome. The invention also discloses a non-invasive prenatal screening method for trisomy syndrome. Compared with a second-generation sequencing method, the screening method has the advantages of low cost, short detection period, high detection sensitivity, convenience and absolute quantification.
Owner:SHENZHEN HUADA GENE INST
Blood cfDNA (cell free DNA) preservative, vacuum blood collection tube and preparation method of vacuum blood collection tube
InactiveCN107574225AProtects cell-free DNAEffectively fixedMicrobiological testing/measurementDiagnostic recording/measuringBlood Collection TubeCell free
The invention discloses a blood cfDNA (cell free DNA) preservative and a vacuum blood collection tube constituted by the blood cfDNA preservative, wherein the preservative consists of the following components in parts by weight: 1-200 parts of EDTA, 1-500 parts of imidazole, 1-200 parts of glyceraldehyde, 1-1000 parts of sodium chloride, 1-500 parts of guanidine hydrochloride, 1-1000 parts of SDSand 5000-7000 parts of purified water. The blood cfDNA vacuum blood collection tube is the vacuum blood collection tube containing the preservative. With the application of the blood cfDNA preservative provided by the invention, RNase enzyme activity can be effectively inhibited and free DNA in blood can be protected. The blood cfDNA vacuum blood collection tube is capable of inhibiting the RNaseenzyme activity and protecting the free DNA in the blood, and the vacuum blood collection tube is also capable of effectively immobilizing leukocyte and preventing the leukocyte from getting cracked,so that the circumstance that the free DNA is contaminated by the cracked leukocyte is prevented; the preservation duration of a blood sample at room temperature is effectively prolonged, and the vacuum blood collection tube is quite conducive to the long-distance transportation of a plasma sample; therefore, an effective preservation method is provided for collection, storage and transportation of tumor early screening samples or fetus prenatal screening and diagnosis samples.
Owner:北京恩吉思生物科技有限公司
21-trisomy syndrome prenatal screening kit
InactiveCN101560565AEasy to getEasy to cultivate successMicrobiological testing/measurementDNA/RNA fragmentationAgricultural scienceTrisomy
The invention discloses a 21-trisomy syndrome prenatal screening kit. The kit provided by the invention comprises a primer pair A, a primer pair B, a primer pair C and a primer pair D; the primer A consists of nucleotides represented by a sequence 1 in a sequence table and nucleotides represented by a sequence 2 in the sequence table; the primer pair B consists of nucleotides represented by a sequence 3 in the sequence table and nucleotides represented by a sequence 4 in the sequence table; the primer pair C consists of nucleotides represented by a sequence 5 in the sequence table and nucleotides represented by a sequence 6 in the sequence table; and the primer pair D consists of nucleotides represented by a sequence 7 in the sequence table and the nucleotides represented by a sequence 8 in the sequence table. The kit of the invention applied to 21-trisomy syndrome prenatal screening has the advantages of innovating specimen material, making prenatal screening earlier, reducing specimen acquisition amount, reducing diagnosis time, and judging extra chromosome resources. The kit has far-reaching significance for finding the causes of chromosomal disorder and in-time prevention and control of the chromosomal disorder.
Owner:PEKING UNIV THIRD HOSPITAL
Prenatal screening
InactiveUS20060021076A9New breed animal cellsMicrobiological testing/measurementMicroscope slideDividing cell
The present invention concerns products and methods particularly useful for activating and analyzing non-dividing cell nuclei. The featured products include activating egg extracts, cytostatic factor (CSF) extracts, kits containing these extracts, and a microchamber microscope slide useful in analyzing nucleus activation.
Owner:BRANDEIS UNIV
Methods of prenatal screening for trisomy 21
InactiveUS7922660B2Raise the possibilityUltrasonic/sonic/infrasonic diagnosticsPerson identificationTrisomyPrenatal screening
Owner:SONEK JIRI D +1
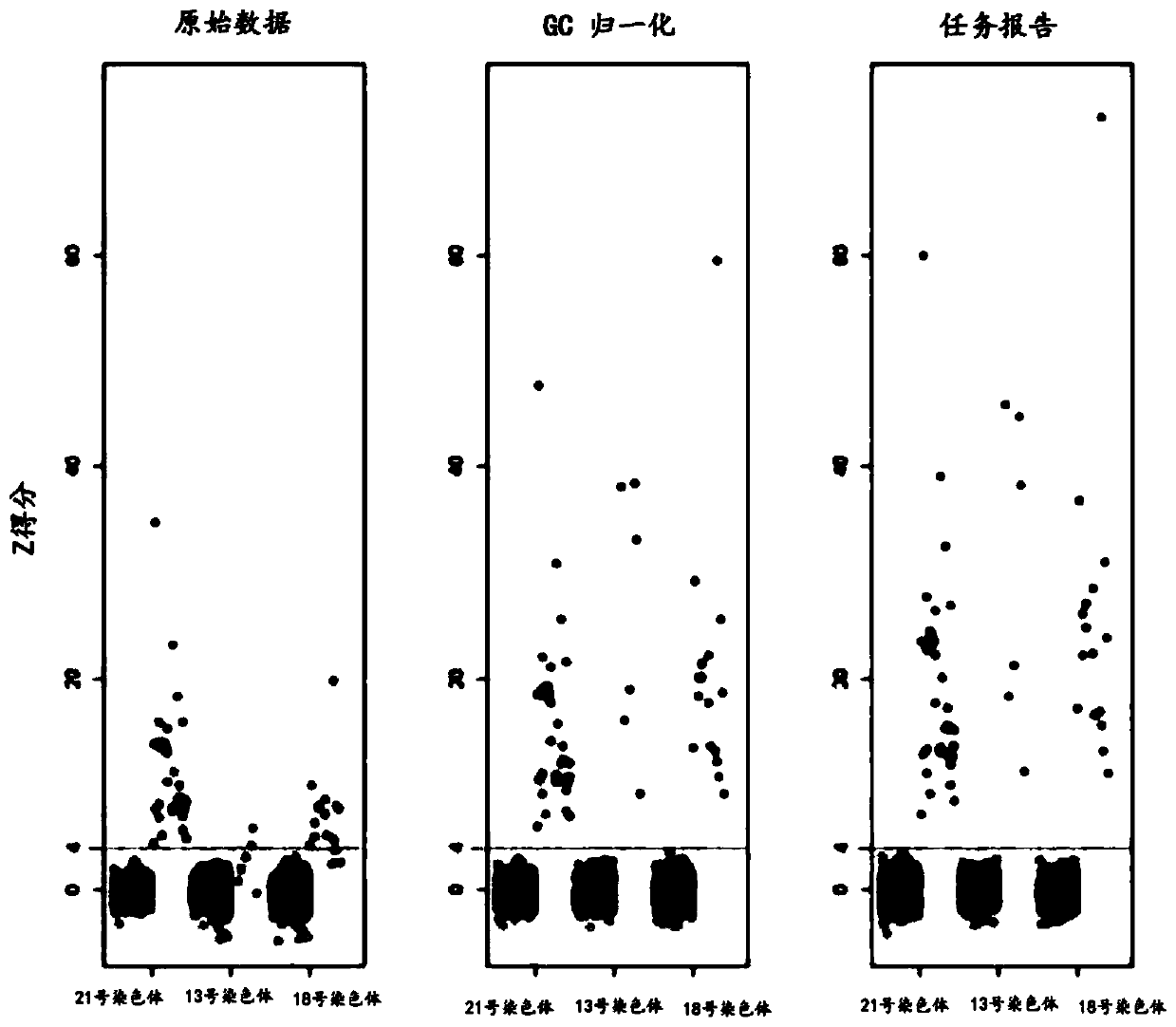
Method for non-invasive prenatal screening for aneuploidy
The present disclosure provides methods for non-invasive prenatal screening (NIPS) of fetal aneuploidies. The present methods are based on analyzing cell-free fetal DNA (cff DNA) found in a pregnant woman's circulation through the next generation sequencing (NGS) technology. Particularly, the present methods analyze the relative abundance of different fetal genomic fragments present in the maternal sample, where the fragments can be aligned to particular chromosomal locations of the fetal genome. The relative abundance information is indicative as to whether a particular chromosome is overrepresented or underrepresented in a fetal genome as compared to normal individuals, and thus can be used to detect fetal aneuploidy. Additionally, methods for increasing the positive predictive values (PPV) of NIPS by excluding false-positive detections are also provided.
Owner:QUEST DIAGNOSTICS INVESTMENTS INC
Spray for disinfection of prenatal screening diagnosis center
The invention relates to a spray for the disinfection of a prenatal screening diagnosis center. The spray is prepared from the following components and pharmaceutically acceptable carriers: smilax, fructus broussonetiae, rhizoma anemones raddeanae, lantana camara, rhizoma zingiberis, bunge cherry seed, oroxylum indicum, white hyacinth bean, flos daturae, folium viticis negundo, campsis grandiflora, rhizoma osmundae, herba sidae rhombifoliae, fructus xanthii, folium eriobotryae, fructus tribuli, radix gentianae macrophyllae, justicia procumbens, manchurian lilac bark, hemsleya macrosperma, murraya jasminorage, rhizoma atractylodis macrocephalae, tangerine seed, herba lespedezae cuneatae, fructus galangae, rhizoma dioscoreae hypoglaucae, cortex ailanthi, the root of Chinese pulsatilla, chingma abutilon seed, thlaspi arvense, entada phaseoloides, common bombax flower, centipeda minima, celosia cristata, pubescent holly root, discolor cinquefoil herb, mung bean, nux prinsepiae, cacumen platycladi, peucedanum decursivum maxim, flatstem milkvetch seed, folium apocyni veneti, radix saposhnikoviae, Japanese ardisia herb, radix berberidis, radix polygonati officinalis and saffron. The spray for the disinfection of the prenatal screening diagnosis center provided by the invention has an obvious effect and is low in cost and very suitable for clinical large-scale application.
Owner:周洪亮
Method for correcting multiple of median of serum marker in second-trimester prenatal screening
InactiveCN101936981AEligible for prenatal screeningReduce false positive rateBiological testingSerum markersObstetrics
The invention discloses a method for correcting multiple of median of a serum marker in second-trimester prenatal screening. The method is characterized by comprising the following steps of: (1) acquiring related data, such as a serum marker detection result, weight and the like of a pregnant woman with age, gestational age and weight meeting the requirement; (2) determining the gestational age (day) based on gestational day; (3) determining the median of the serum marker of a normal pregnant woman group on each gestational day to obtain a regression formula of the median of the serum marker of the normal pregnant woman group relative to the day on each gestational day; (4) determining the multiple of the median of a detected pregnant woman; and (5) correcting the multiple of the median of the detected pregnant woman by using a weight regression formula. The method is applied to the gestational day-based prenatal screening established special for a Chinese pregnant woman group, and has the advantages of high detection ratio and low false positive.
Owner:NINGBO UNIV +2
Method for detecting absolute copy number of fetal free DNA in maternal plasma on basis of digital PCR and kit for detecting absolute copy number of fetal free DNA in maternal plasma on basis of digital PCR
PendingCN111154841AThe experimental system is stableThe experimental method is simple and reliableMicrobiological testing/measurementReference genesAntepartum diagnosis
Owner:江苏圣极基因科技有限公司
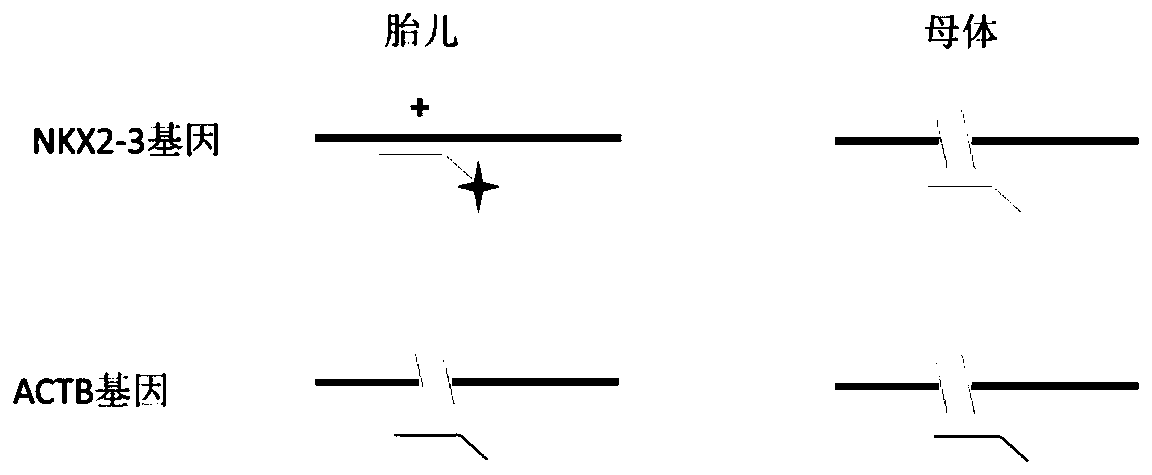
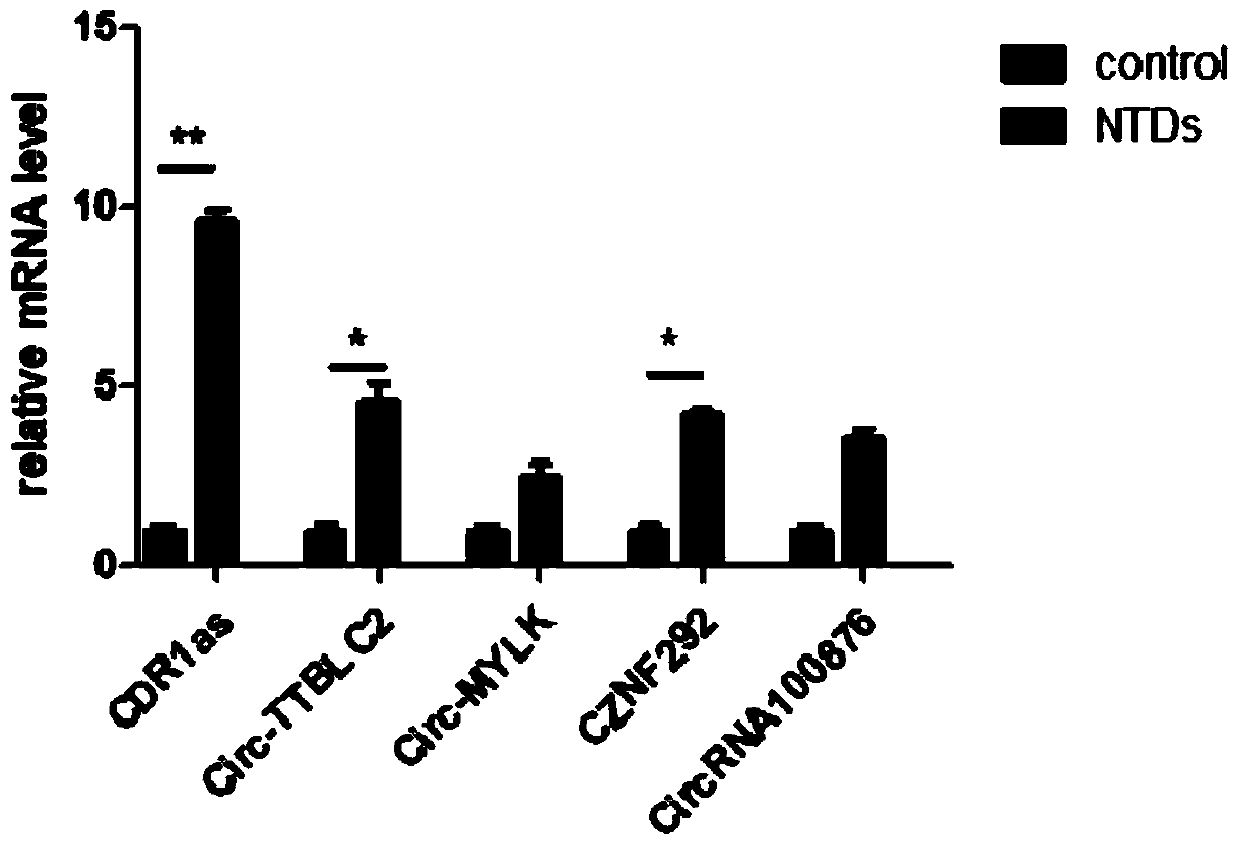
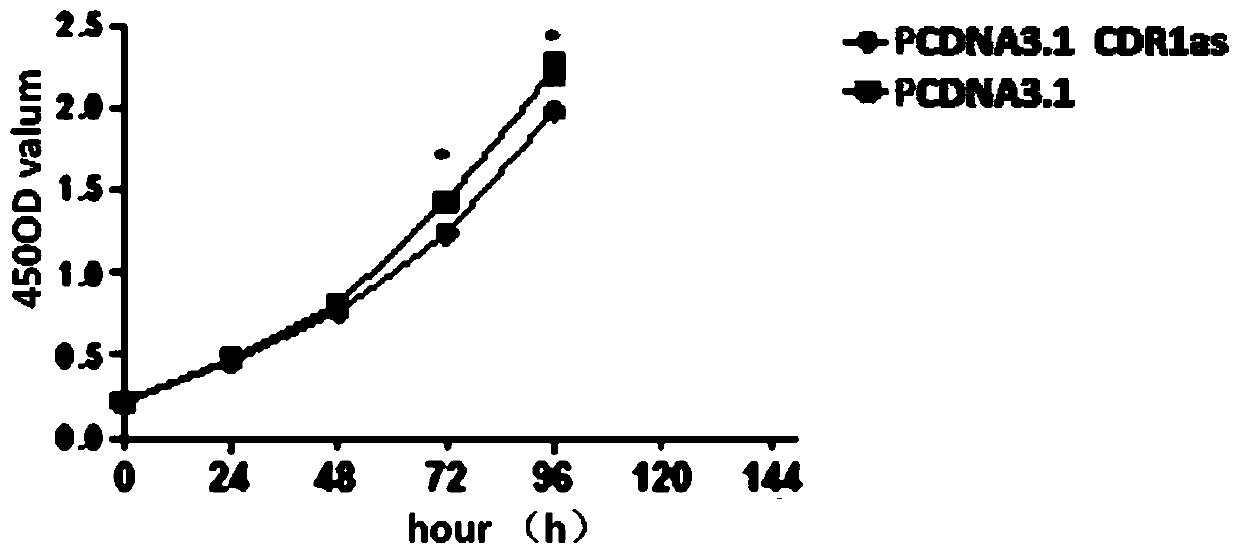
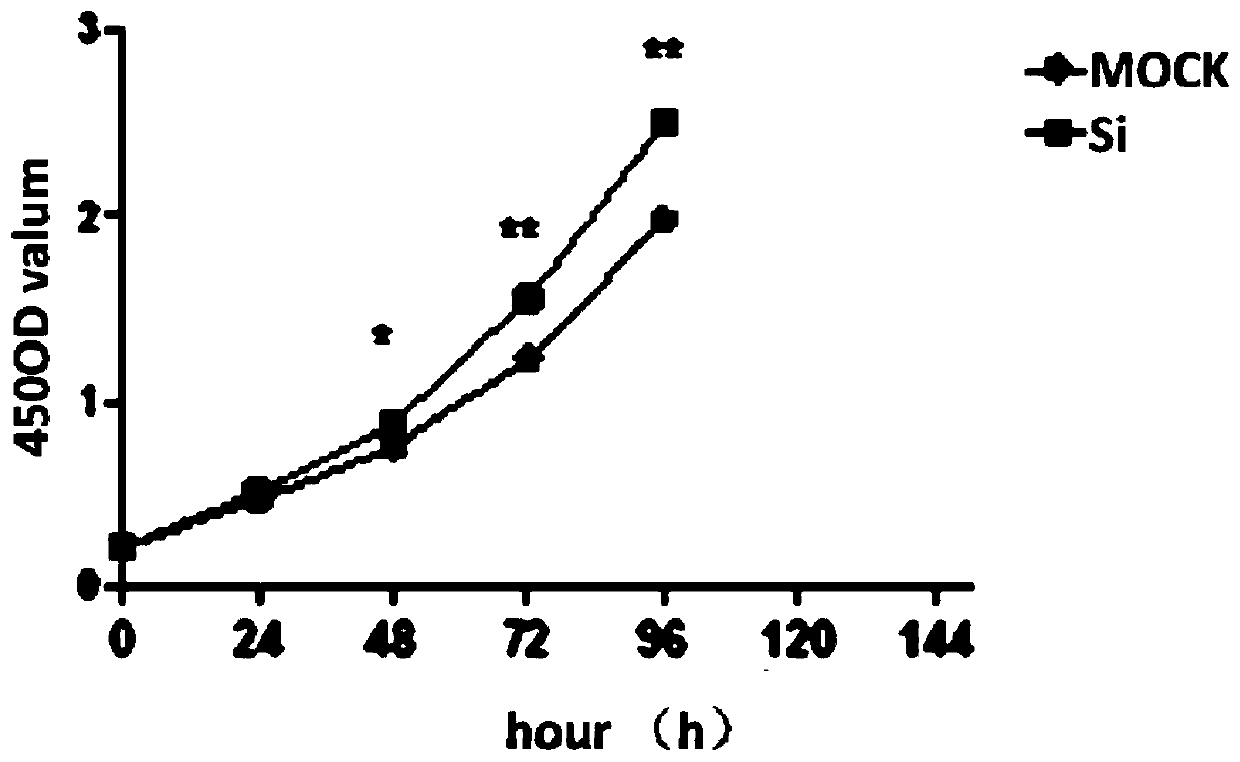
Application of CDR1as to prenatal screening of neural tube defects, and product and method for performing prenatal screening of neural tube defects
ActiveCN110016504AEasy to detectReduce professional requirementsMicrobiological testing/measurementAgainst vector-borne diseasesCongenital malformationsRoutine screening
The invention provides an application of CDR1as to prenatal screening of neural tube defects, and a product and method for performing prenatal screening of neural tube defects, and relates to the technical field of clinical diagnosis. The invention provides an application of circRNA-CDR1as as a marker for prenatal screening of neural tube defects. Through research, the inventor finds out that the,circRNA-CDR1as is notably highly expressed in the tissue of NTDs child patients, the circRNA-CDR1as can be separately or can be united with other markers to be used for conventional screening of congenital malformation at the early pregnancy, and has great significance to families and society. According to the product and method for performing prenatal screening of neural tube defects, through detecting the expression number of the circRNA-CDR1as, NTDs is diagnosed, the detection cost is low, the detection is accurate and quick, and the NTDs can be diagnosed at the early pregnancy.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
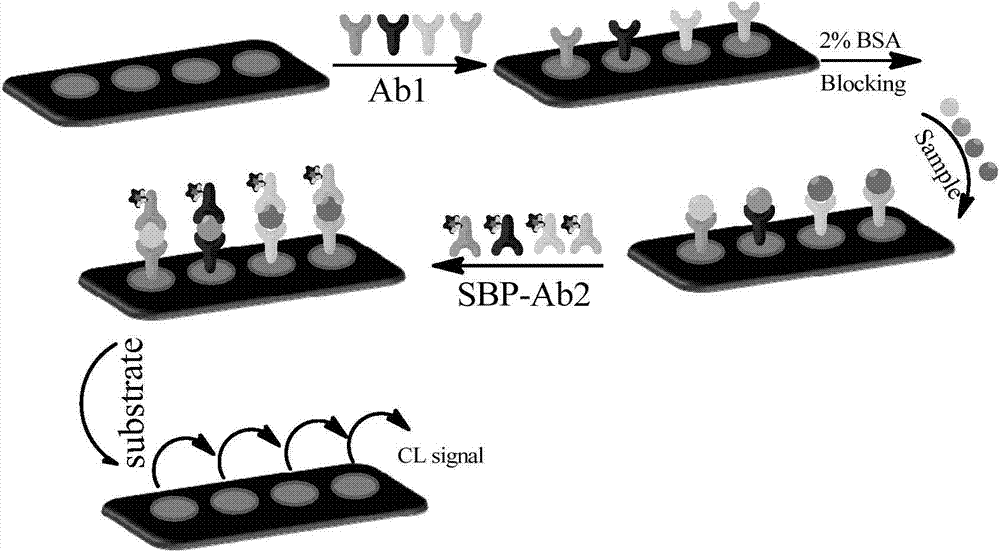
Soybean peroxidase immune biochip and application of thereof to detection of serum marks during down syndrome prenatal screening
InactiveCN103901217AHigh luminous intensityWide range of actionChemiluminescene/bioluminescenceDisease diagnosisPeroxidasePrenatal screening
The invention discloses a soybean peroxidase immune biochip and application thereof to detection of serum marks during down syndrome prenatal screening. The application comprises the following steps: 1,preparing a soybean protein enzyme labelled antibody; 2, modifying a slide, namely performing hydroxylation, amino silanization and formylation on the surface of the slide; and 3, constructing a sandwich immune model on the surface of the biochip, namely covering the surface of the slide with the antibody, closing a non-specific active site, adding serum to be detected, performing incubation and washing, adding the enzyme labelled antibody, then performing incubation and washing again, adding a luminous substrate, and acquiring a luminous signal through CCD (charge coupled device) imaging. Four indexes, namely AFP (alpha fetoprotein), HCG (human chorionic gonadotropin), uE3 and PAPP (pregnancy associated plasma protein)-A, are selected as down syndrome serum marks. The SBP (soybean peroxidase) is wide in substrate working range, high in heat resistance, high in acid-alkaline stability and wide in pH adaptation range. An enhancer is added into a Luminol-H2O system, so that a chemical luminous signal can be amplified by nearly 100 times, and the detection sensitivity is improved.
Owner:靖江市人民医院 +1
Phenylketonuria (PKU) screening kit and application thereof to prenatal screening
InactiveCN103276077AShorten screening timeScreening is quick and easyMicrobiological testing/measurementGeneticsPrenatal screening
The invention discloses a phenylketonuria (PKU) screening kit and application thereof to prenatal screening, and belongs to the technical field of PKU screening. The application method comprises the following steps of: (1) extracting free fetal DNA (Deoxyribonucleic Acid) from peripheral blood of pregnant women; (2) taking the fetal DNA as a template to carry out polymerase chain reaction (PCR) amplification; (3) detecting a PCR product through agarose gel electrophoresis and purifying the PCR product through enzyme reaction; (4) carrying out sequencing reaction on the purified PCR product; (5) purifying a PCR sequencing reaction product; (6) sequencing the purified PCR sequencing reaction product; and (7) comparing the sequencing result with positive and negative control samples. The kit has the beneficial effects that the kit can achieve automatic sequencing, thus shortening the screening time and enabling PKU screening to be simpler, more convenient and faster; the technical gap of prenatal PKU screening is filled; the birth rate of sick children is reduced; and the kit causes no trauma to the fetuses and is safe and reliable.
Owner:邯郸市康业生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com