Beta-human chorionic gonadotrophin test kit (time-resolved fluoroimmunoassay) for prenatal screening and preparation method thereof
A chorionic gonadotropin and prenatal screening technology, applied in biological testing, material inspection products, etc., can solve the problems of insufficient understanding of prenatal screening, lagging economic technology, unbalanced development, etc., and achieve reliability and rigor. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of the prenatal screening β-human chorionic gonadotropin assay kit (time-resolved fluorescent immunoassay) of the present invention
[0036] Components of the β-human chorionic gonadotropin assay kit (time-resolved fluorescent immunoassay) for prenatal screening include:
[0037] Experiment buffer: made with 10.0ml / L~100.0ml / L calf serum and buffer;
[0038] Concentrated lotion: made with 1.0ml / L~10.0ml / L Tween-20 and buffer;
[0039] Enhancement solution: made with 1-5mg / L β-naphthoyltrifluoroacetone, 15-30mg / L tri-n-octylphosphine oxide and buffer.
[0040] Monoclonal antibody-coated plate: β-human chorionic gonadotropin monoclonal antibody-coated plate Dilute β-human chorionic gonadotropin monoclonal antibody to 1-10 μg / ml with buffer solution containing 1-10 ppm preservative The coating solution is prepared by coating a microwell blank plate, sealing it with a blocking solution containing 1.0g / L-10.0g / L protective agent, and then drying it. ...
Embodiment 2
[0054] Embodiment 2 The using method of kit of the present invention
[0055] The specific operations of the β-human chorionic gonadotropin assay kit (time-resolved fluorescent immunoassay) for the prenatal screening prepared in the above embodiment 1 are as follows:
[0056] Equilibrate the number of microwell reaction strips required by the reagent to room temperature; mix 40ml of the above-mentioned concentrated washing solution and 960ml of purified water to form a working washing solution; Marker working solution.
[0057] Punch the standard, quality control or sample into the solid-phase antibody-coated reaction plate with a hole puncher with a diameter of 3.0 mm, and add 100-200 μl of the above-mentioned β-HCG experimental buffer to each well; the reaction plate is at room temperature, Slowly shake and incubate for 1-4 hours; wash the plate 6 times with the above-mentioned working washing solution, and pat dry; add 100-200 μl of the above-mentioned β-HCG marker working...
Embodiment 3
[0058] The analytical performance evaluation index of embodiment 3 kit of the present invention
[0059] The performance evaluation indexes of the β-human chorionic gonadotropin assay kit (time-resolved fluorescent immunoassay) of the prenatal screening prepared in the above embodiment 1 are as follows:
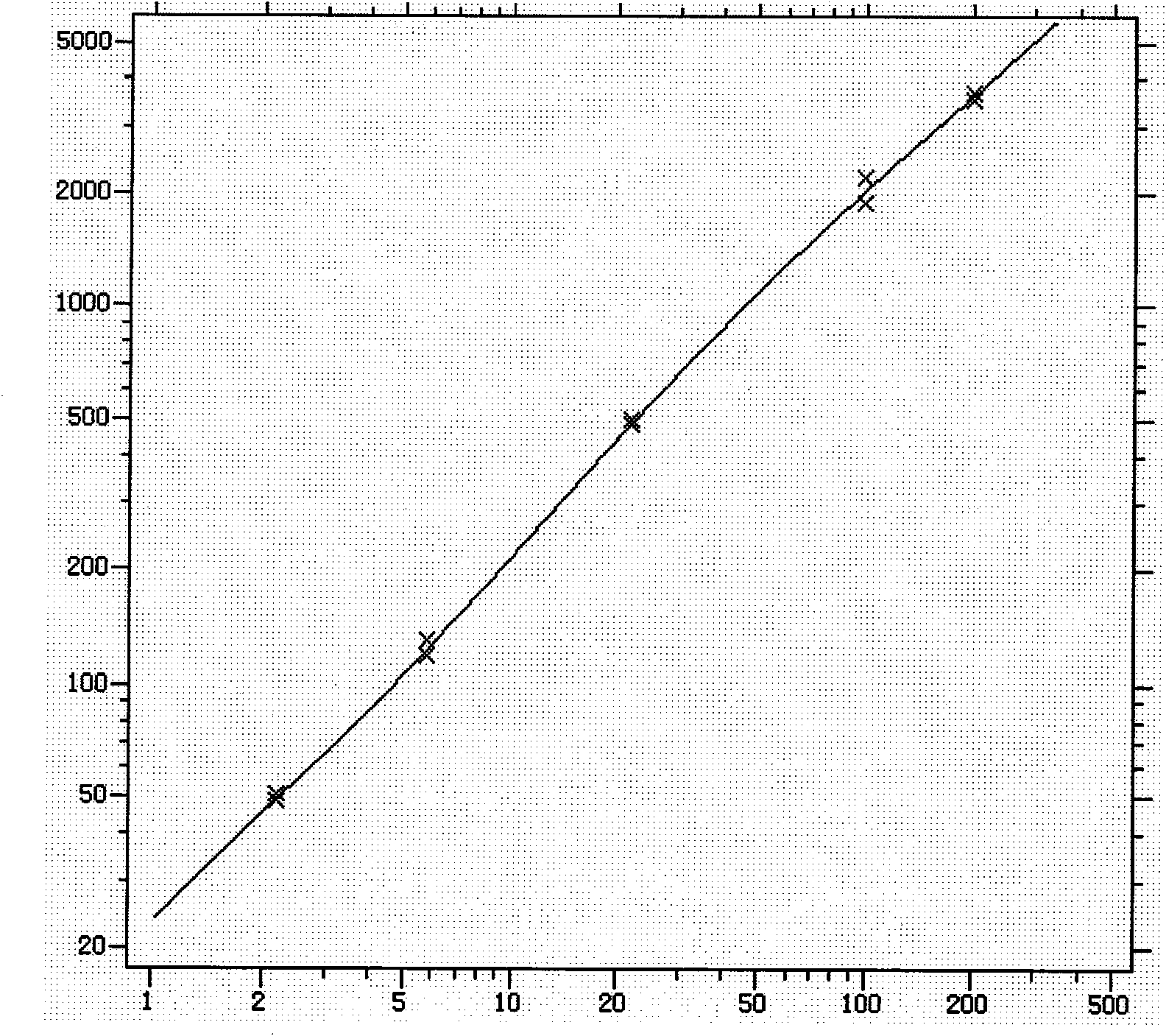
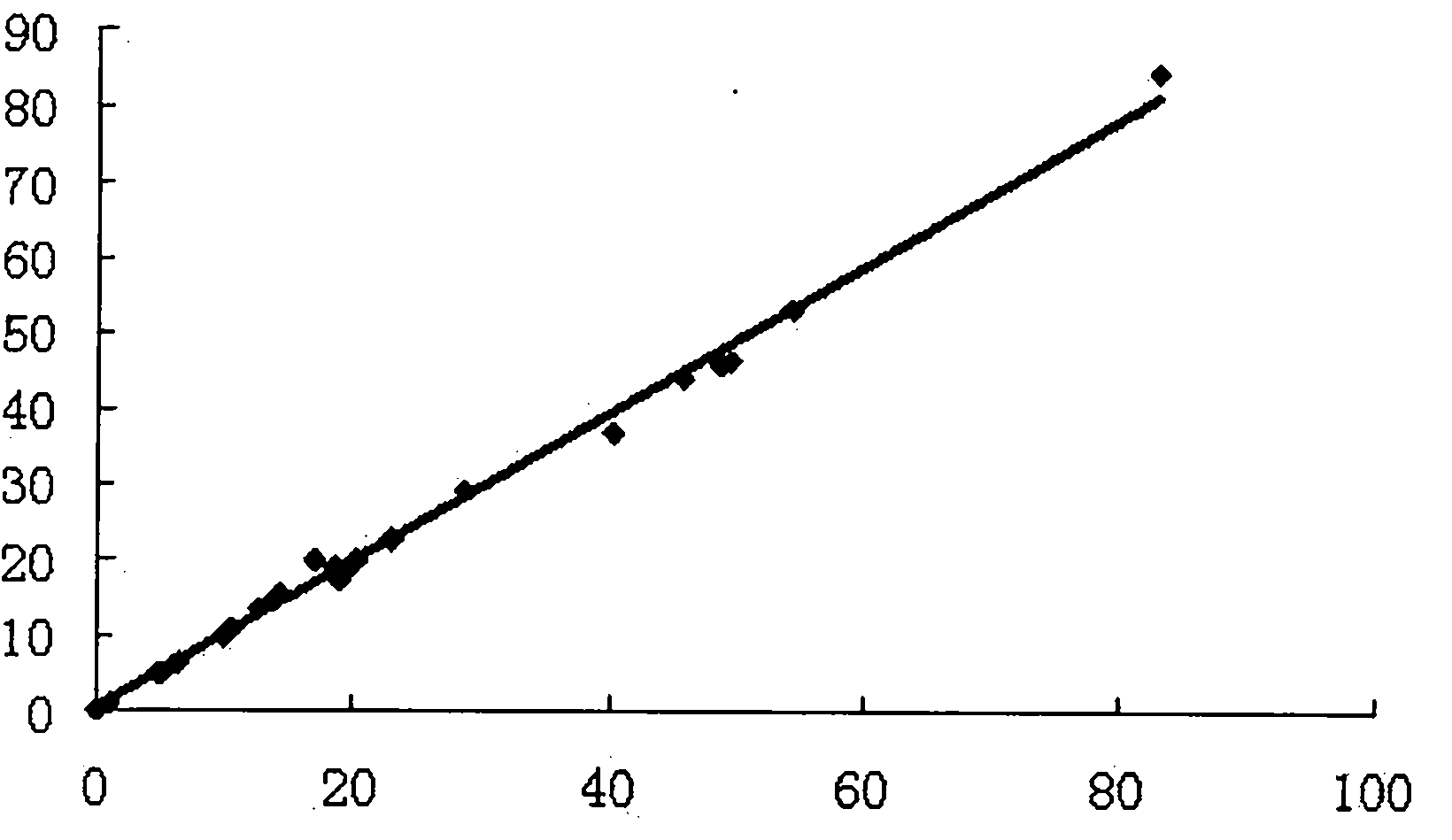
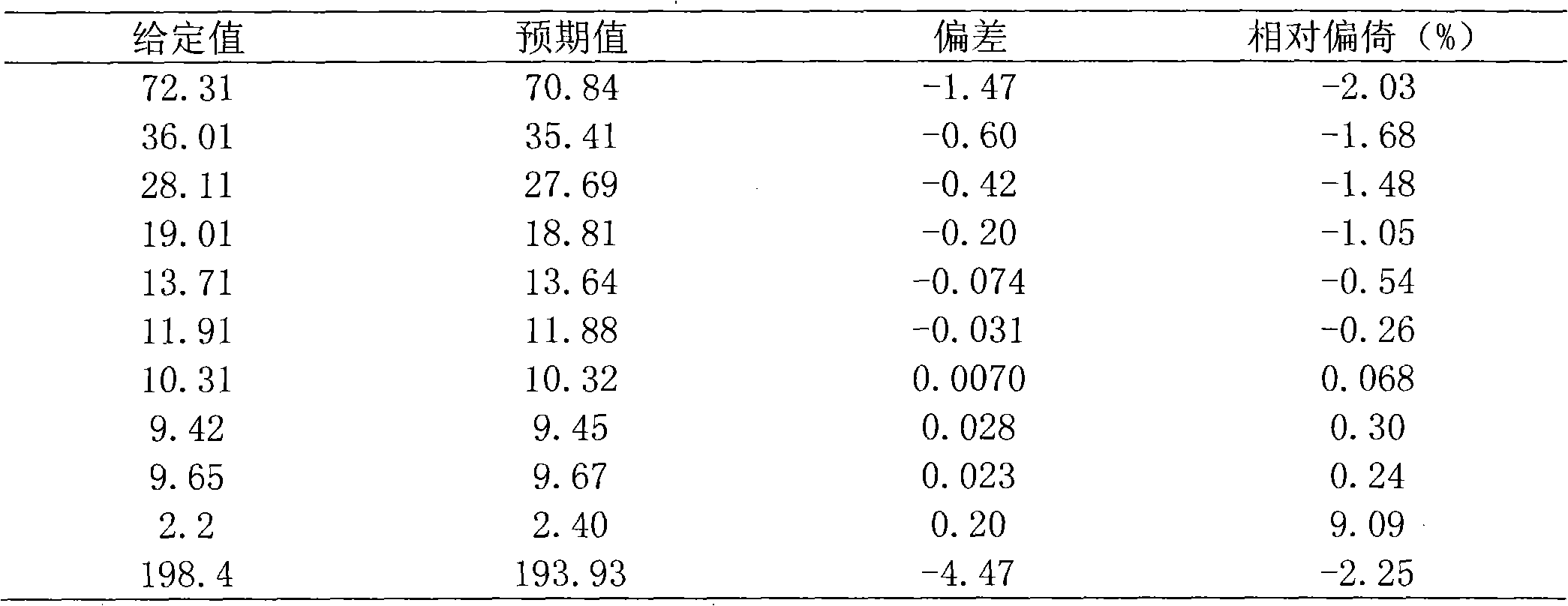
[0060] 1. Dose response curve and linear range
[0061] Using the LOG-LOGB axis transformation mode and the SPLINE fitting algorithm, the logarithm of the content of the standard is the abscissa, and the logarithm of the fluorescence value of the corresponding reaction well is the ordinate, and the standard curve is drawn.
[0062] Within the measurement range of the β-HCG kit, the linear correlation coefficient of the dose-response curve should not be lower than 0.9900.
[0063] 2. Sensitivity
[0064] Detect 20 times with zero standard, calculate its concentration mean (x) and standard deviation (s), and take x±2s as the detection limit, then the sensitivity of the β-HCG de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com