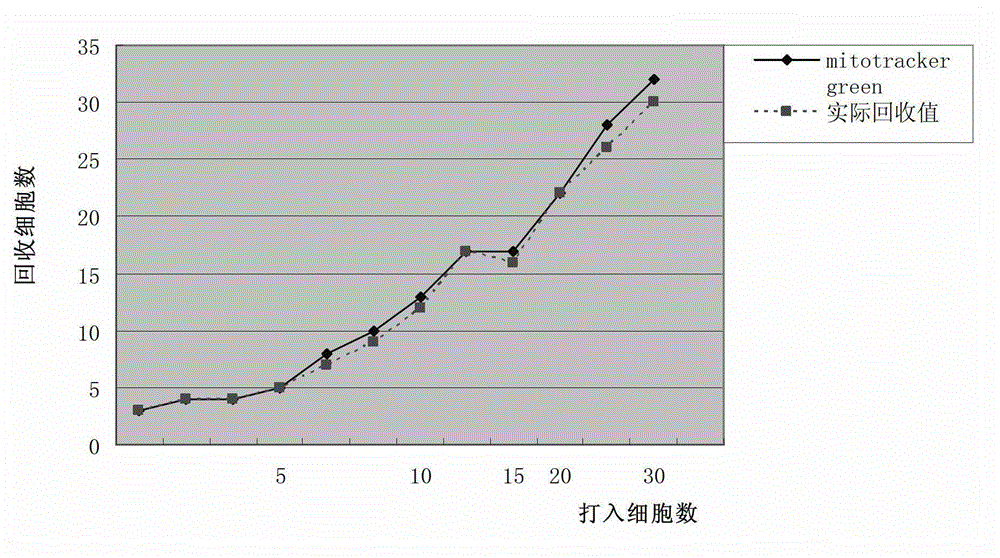
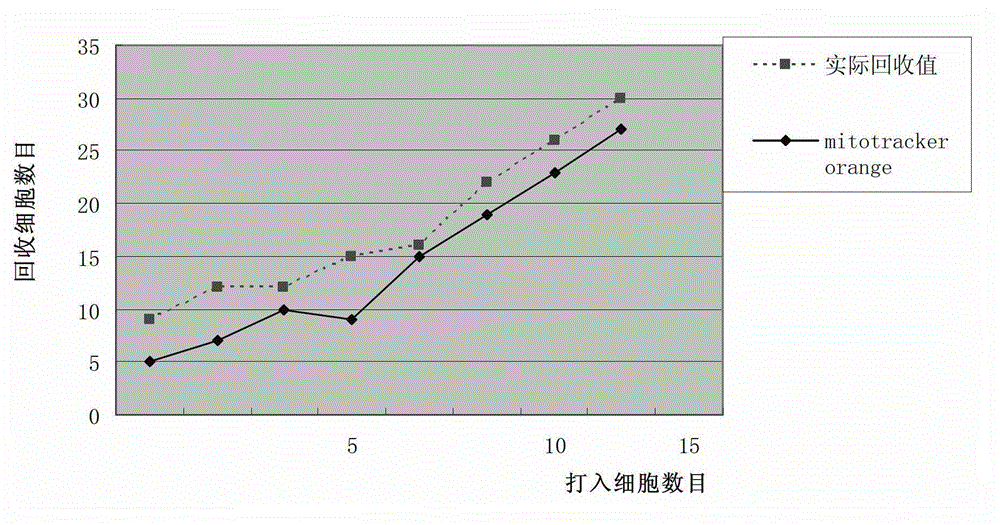
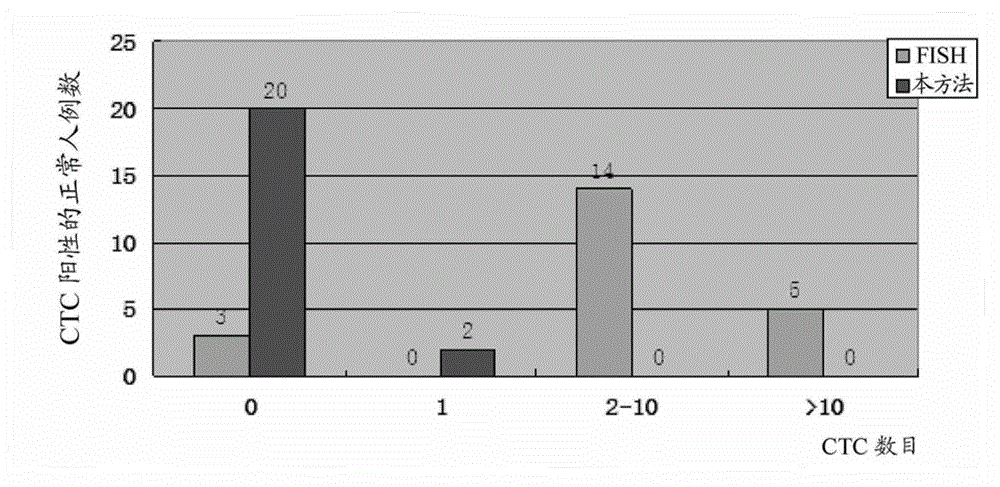
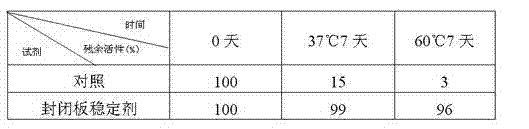
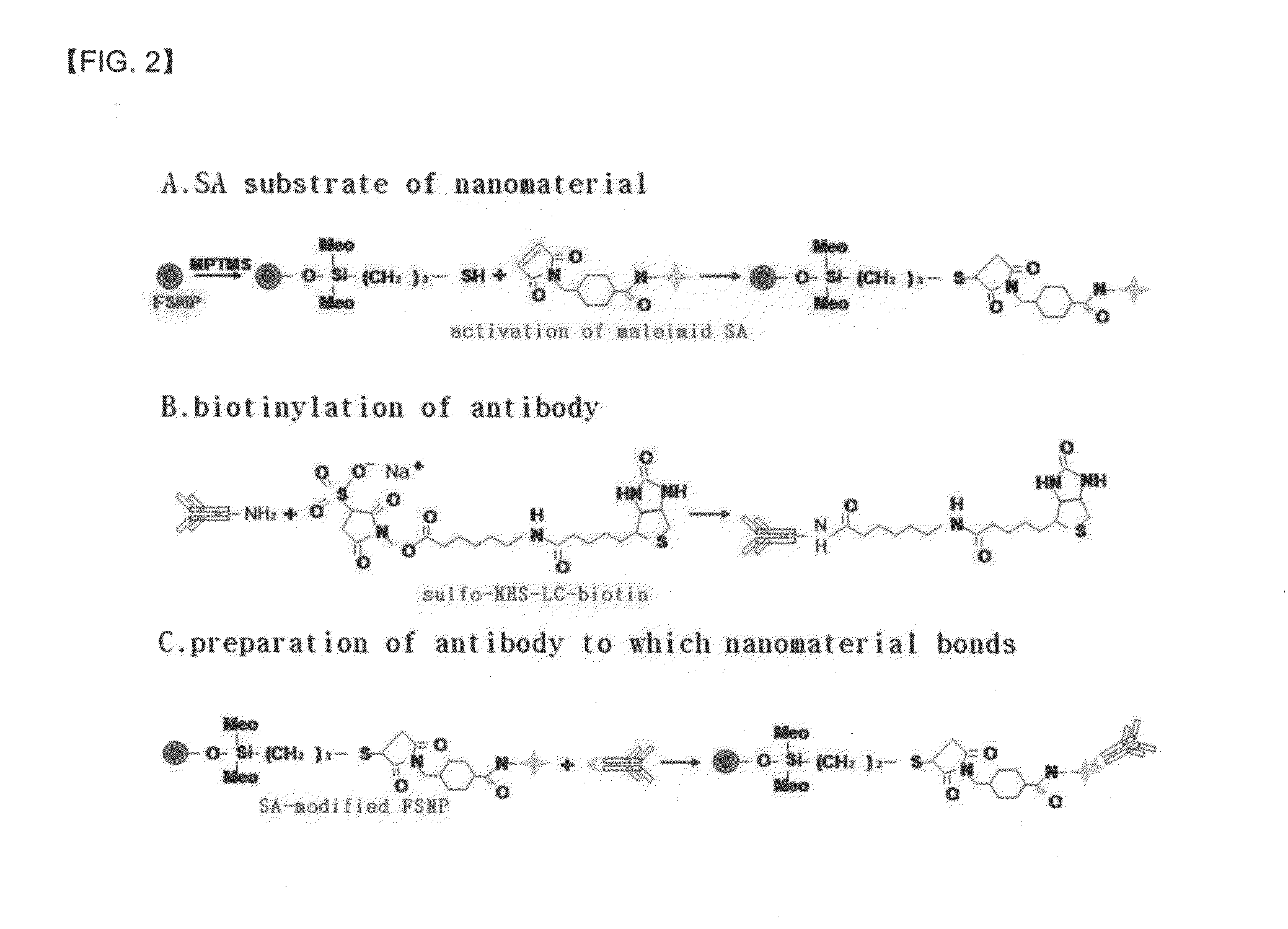Patents
Literature
1277 results about "Immunofluorescence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunofluorescence is a technique used for light microscopy with a fluorescence microscope and is used primarily on microbiological samples. This technique uses the specificity of antibodies to their antigen to target fluorescent dyes to specific biomolecule targets within a cell, and therefore allows visualization of the distribution of the target molecule through the sample. The specific region an antibody recognizes on an antigen is called an epitope. There have been efforts in epitope mapping since many antibodies can bind the same epitope and levels of binding between antibodies that recognize the same epitope can vary. Additionally, the binding of the fluorophore to the antibody itself cannot interfere with the immunological specificity of the antibody or the binding capacity of its antigen. Immunofluorescence is a widely used example of immunostaining (using antibodies to stain proteins) and is a specific example of immunohistochemistry (the use of the antibody-antigen relationship in tissues). This technique primarily makes use of fluorophores to visualise the location of the antibodies.
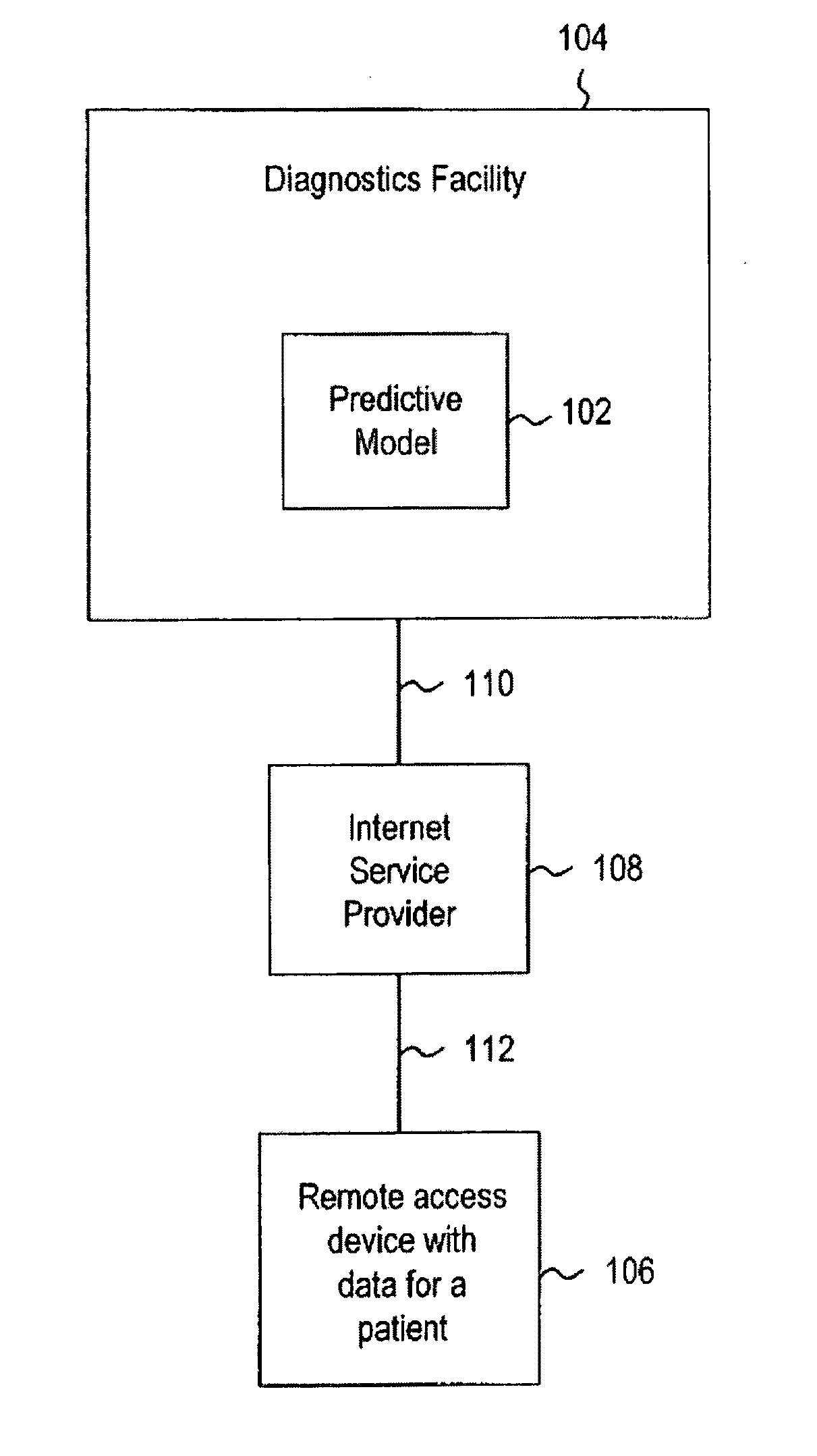
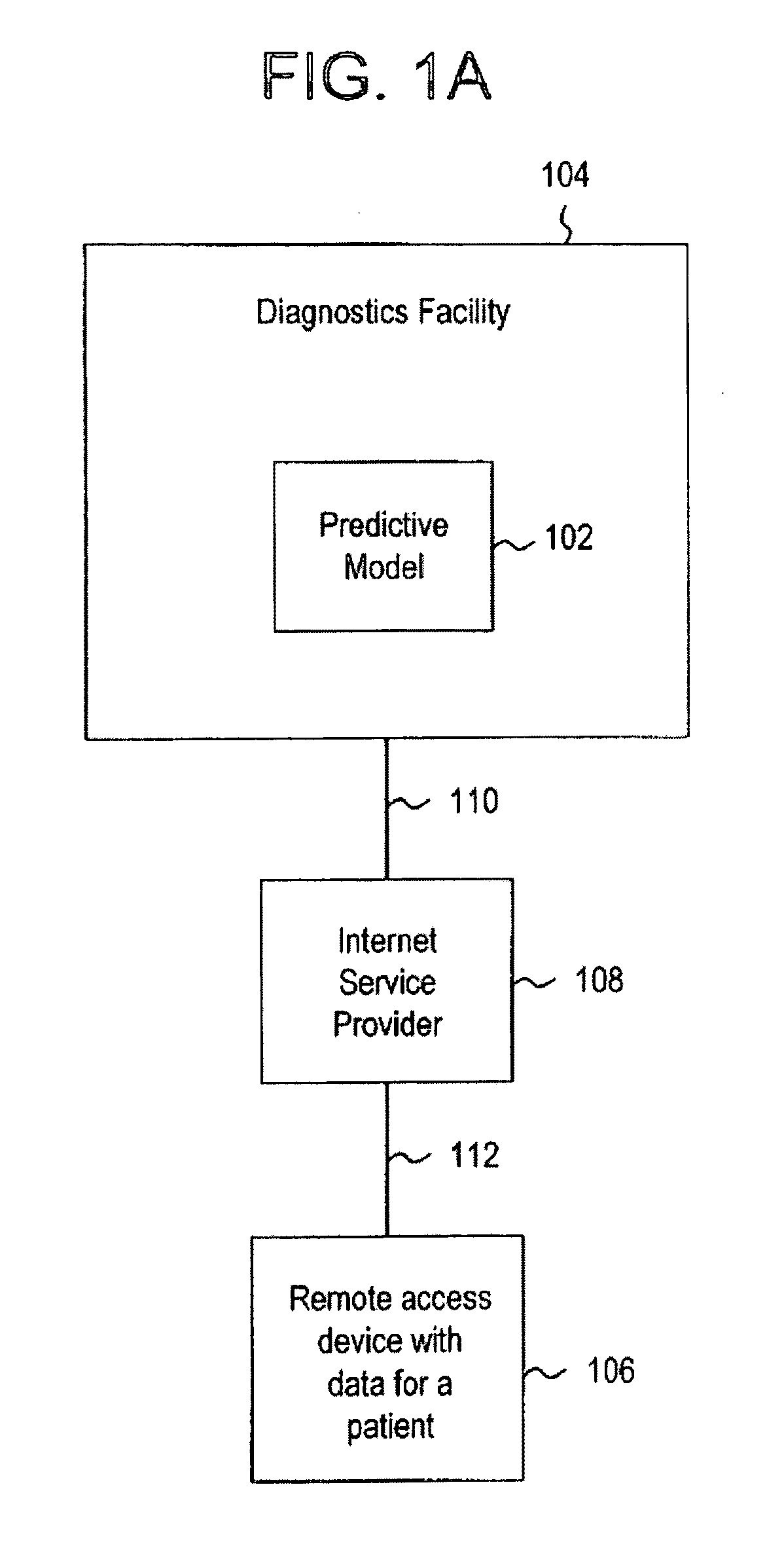
Systems and methods for treating, diagnosing and predicting the occurrence of a medical condition
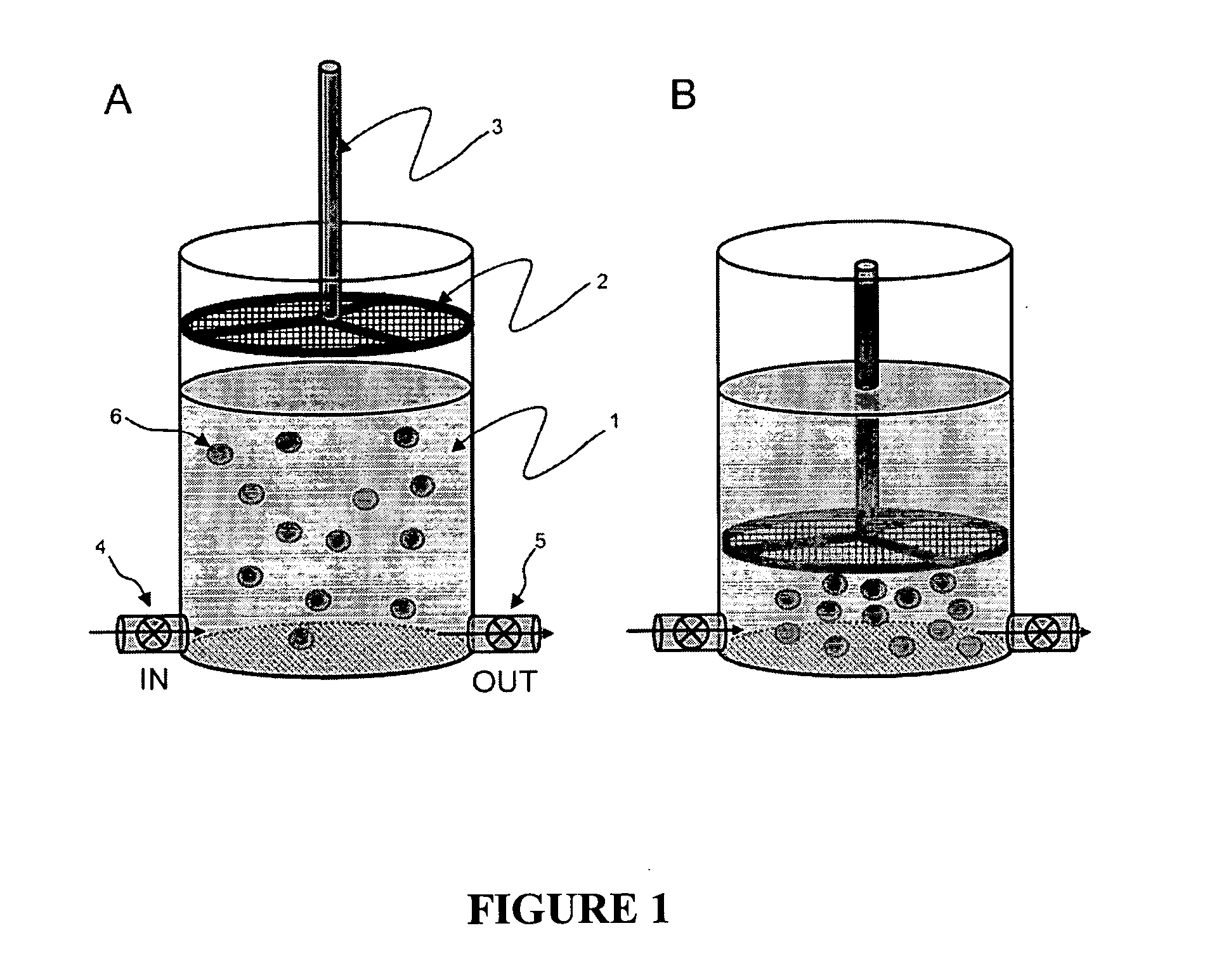
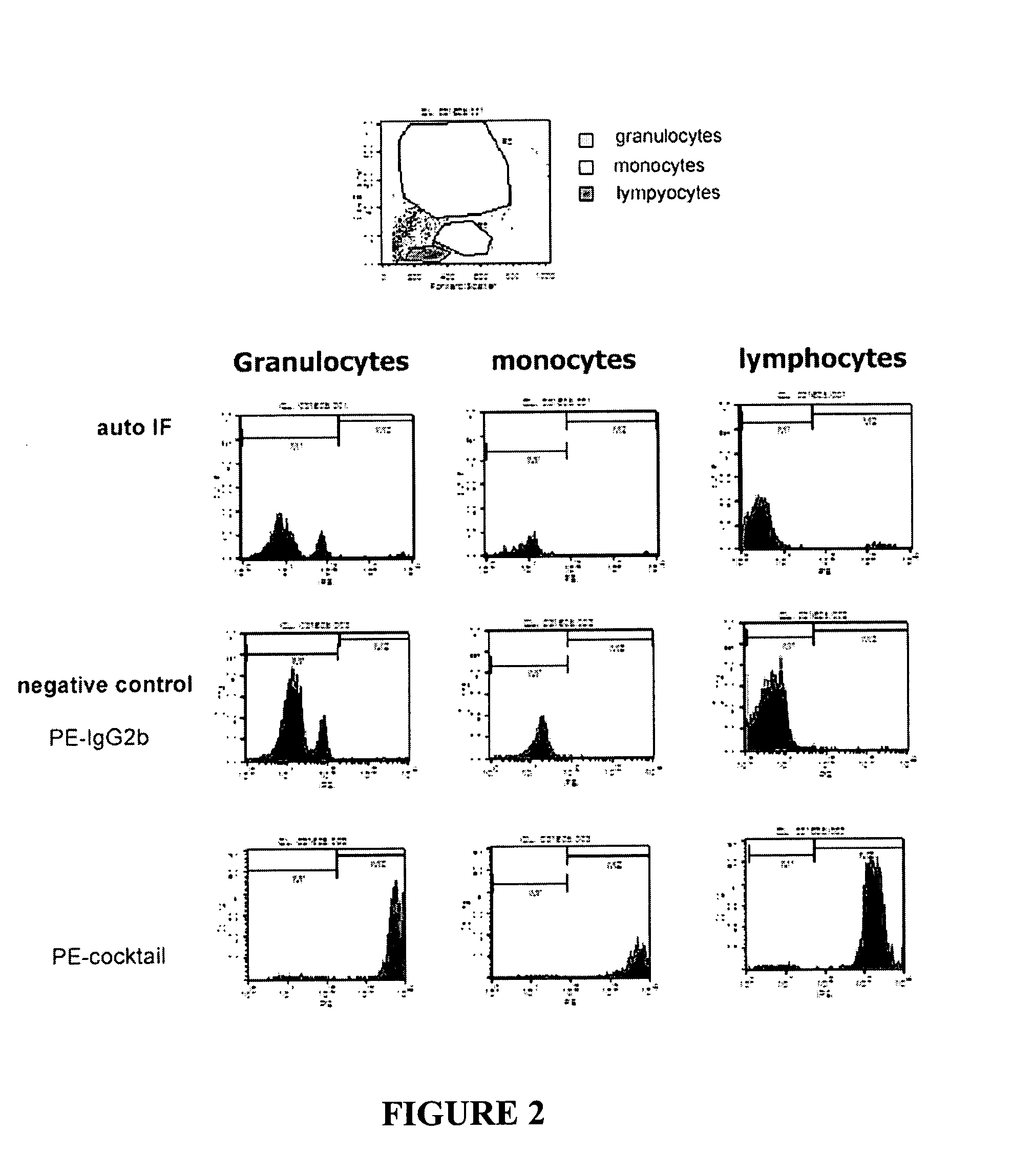
ActiveUS20100184093A1Reliable and accurate image segmentationMedical simulationBioreactor/fermenter combinationsImmunofluorescenceClinical information
Clinical information, molecular information and / or computer-generated morphometric information is used in a predictive model for predicting the occurrence of a medical condition. In an embodiment, a model predicts whether a patient is likely to have a favorable pathological stage of prostate cancer, where the model is based on features including one or more (e.g., all) of preoperative PSA, Gleason Score, a measurement of expression of androgen receptor (AR) in epithelial and stromal nuclei and / or a measurement of expression of Ki67-positive epithelial nuclei, a morphometric measurement of a ratio of area of epithelial nuclei outside gland units to area of epithelial nuclei within gland units, and a morphometric measurement of area of epithelial nuclei distributed away from gland units. In some embodiments, quantitative measurements of protein expression in cell lines are utilized to objectively assess assay (e.g., multiplex immunofluorescence (IF)) performance and / or to normalize features for use within a predictive model.
Owner:AUREON LAB INC +1
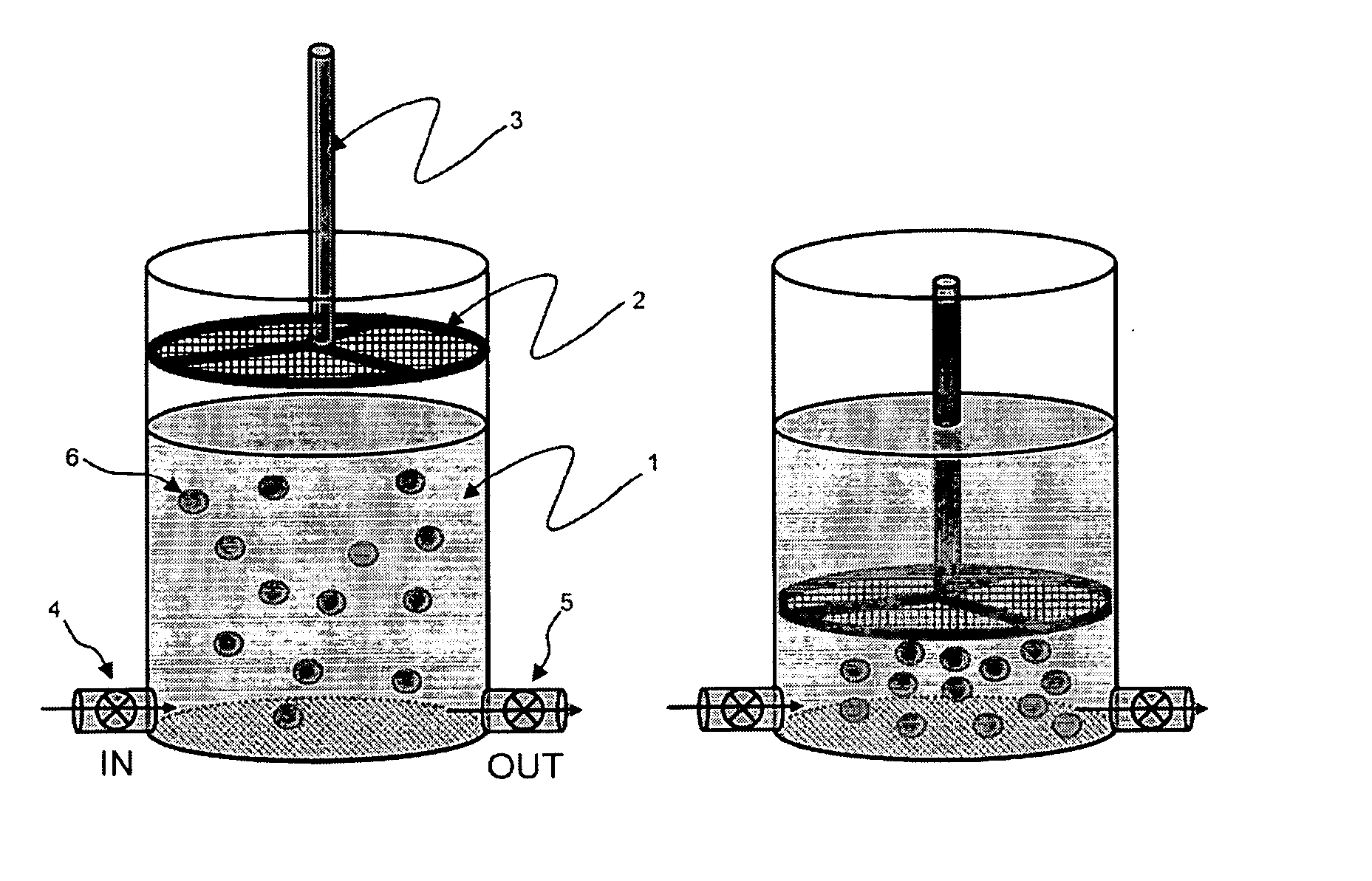
Methods and compositions for detecting rare cells from a biological sample
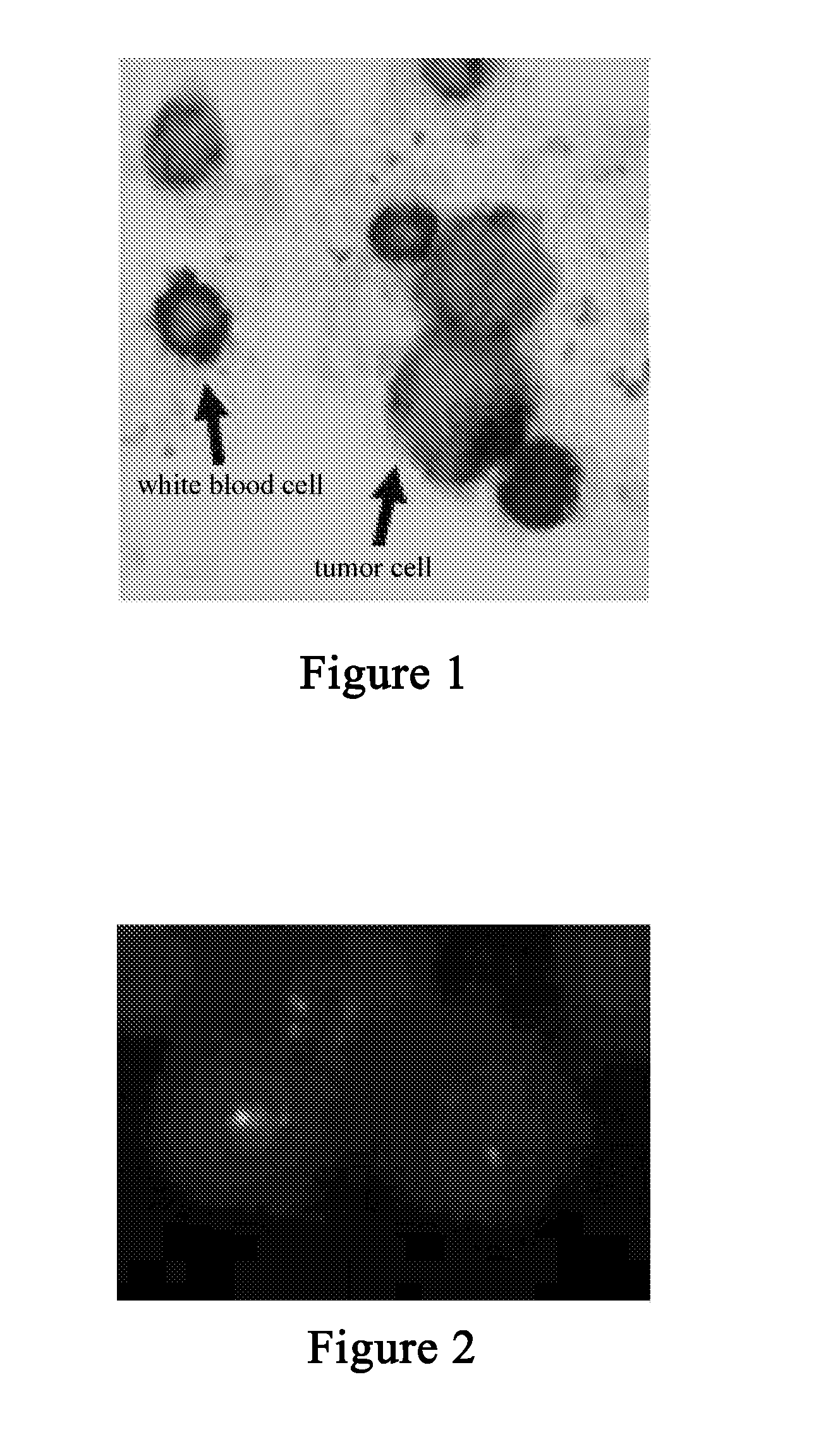
InactiveUS20080057505A1Strong specificityEasy to identifyMicrobiological testing/measurementBiomass after-treatmentHematopoietic cellWhite blood cell
The present invention provides methods and compositions for isolating and detecting rare cells from a biological sample containing other types of cells. In particular, the present invention includes a debulking step that uses a microfabricated filters for filtering fluid samples and the enriched rare cells can be used in a downstream process such as identifies, characterizes or even grown in culture or used in other ways. The invention also include a method of determining the aggressiveness of the tumor or of the number or proportion of cancer cells in the enriched sample by detecting the presence or amount of telomerase activity or telomerase nucleic acid or telomerase expression after enrichment of rare cells. This invention further provides an efficient and rapid method to specifically remove red blood cells as well as white blood cells from a biological sample containing at least one of each of red blood cells and white blood cells, resulting in the enrichment of rare target cells including circulating tumor cells (CTC), stromal cells, mesenchymal cells, endothelial cells, fetal cells, stem cells, non-hematopoietic cells etc from a blood sample. The method is based upon combination of immuno-microparticles (antibody coated microparticles) and density-based separation. The final enriched target cells can be subjected to a variety of analysis and manipulations, such as flowcytometry, PCR, immunofluorescence, immunocytochemistry, image analysis, enzymatic assays, gene expression profiling analysis, efficacy tests of therapeutics, culturing of enriched rare cells, and therapeutic use of enriched rare cells. In addition, depleted plasma protein and white blood cells can be optionally recovered, and subjected to other analysis such as inflammation studies, gene expression profiling, etc.
Owner:AVIVA BIOSCI
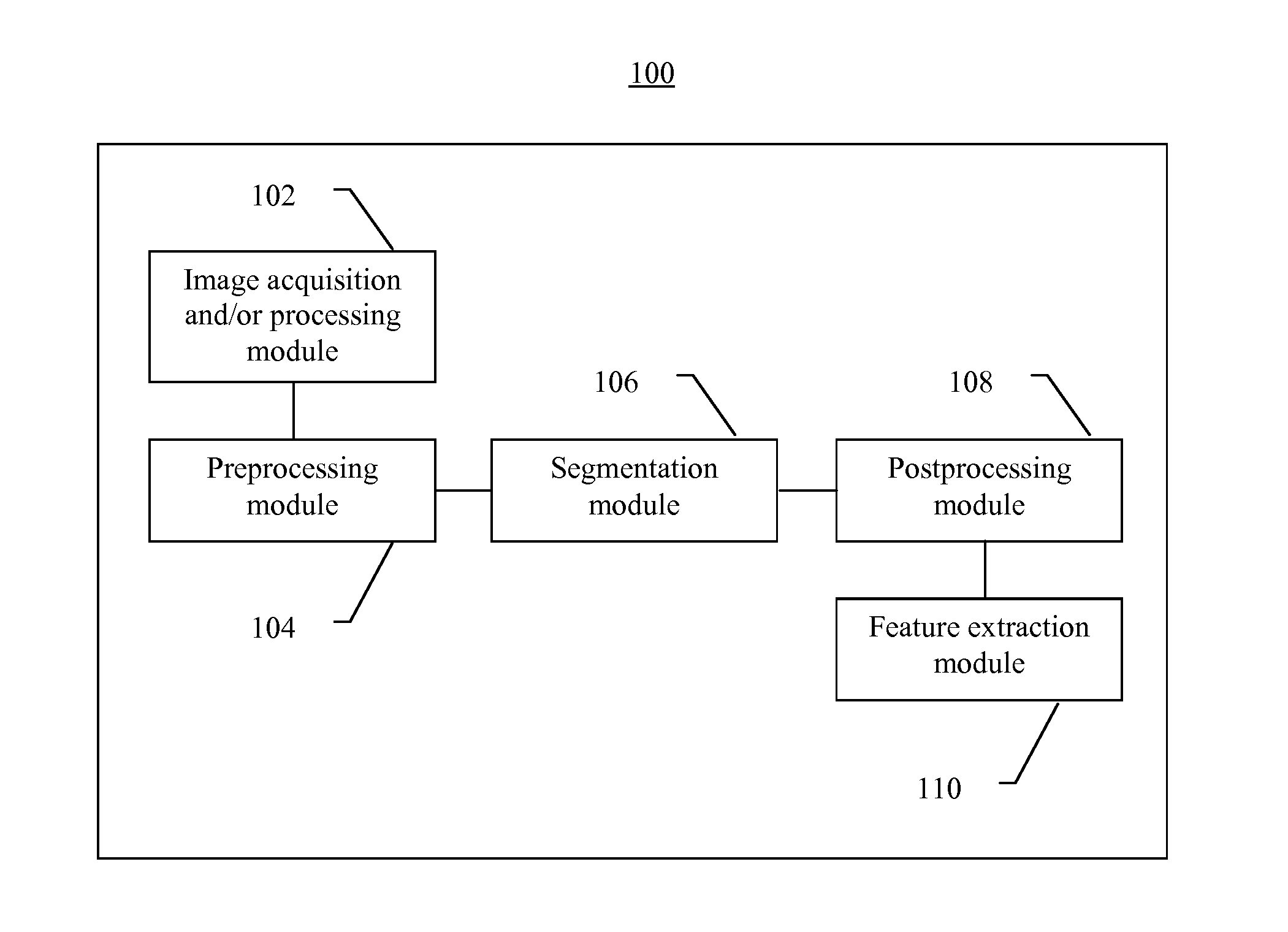
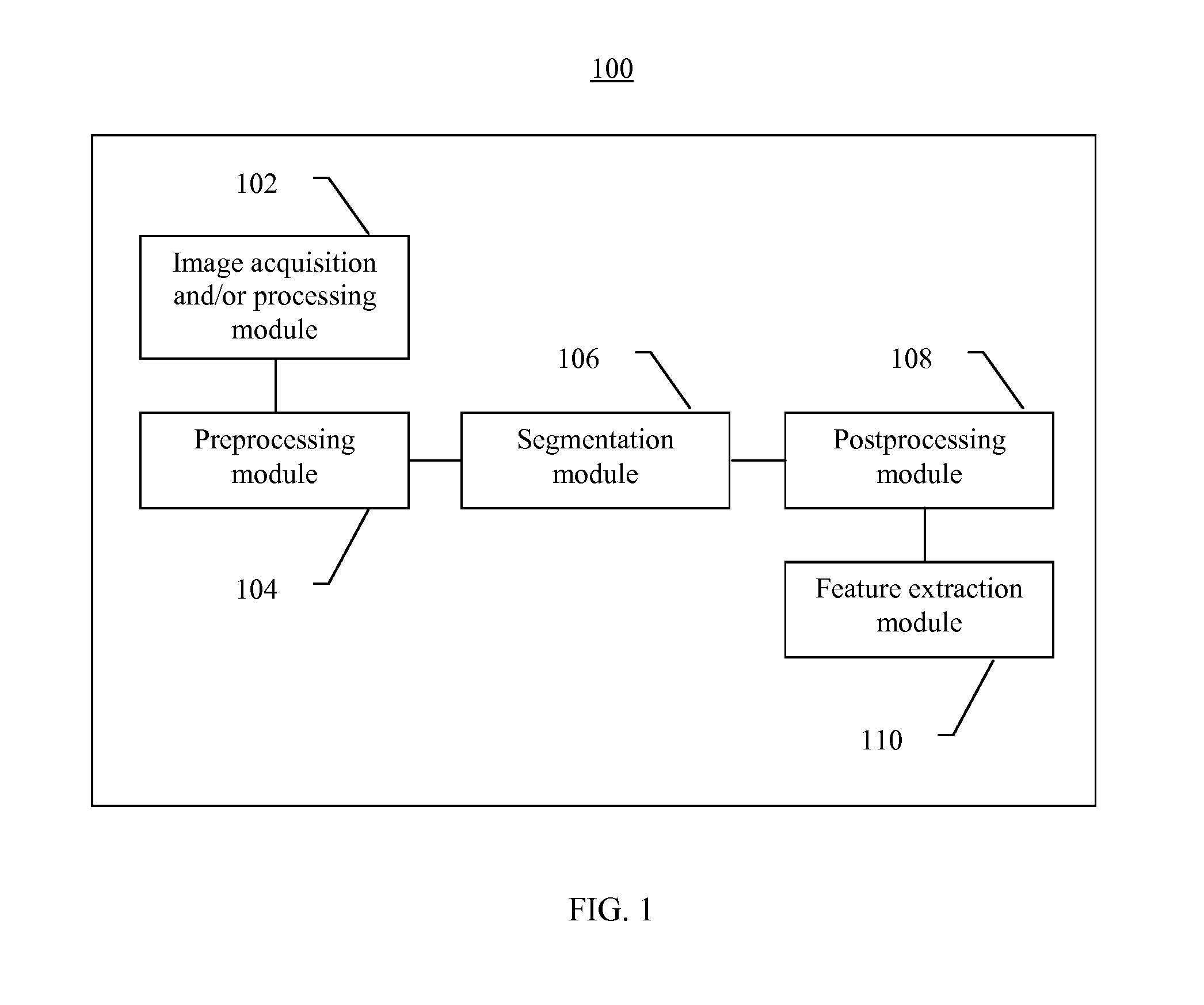
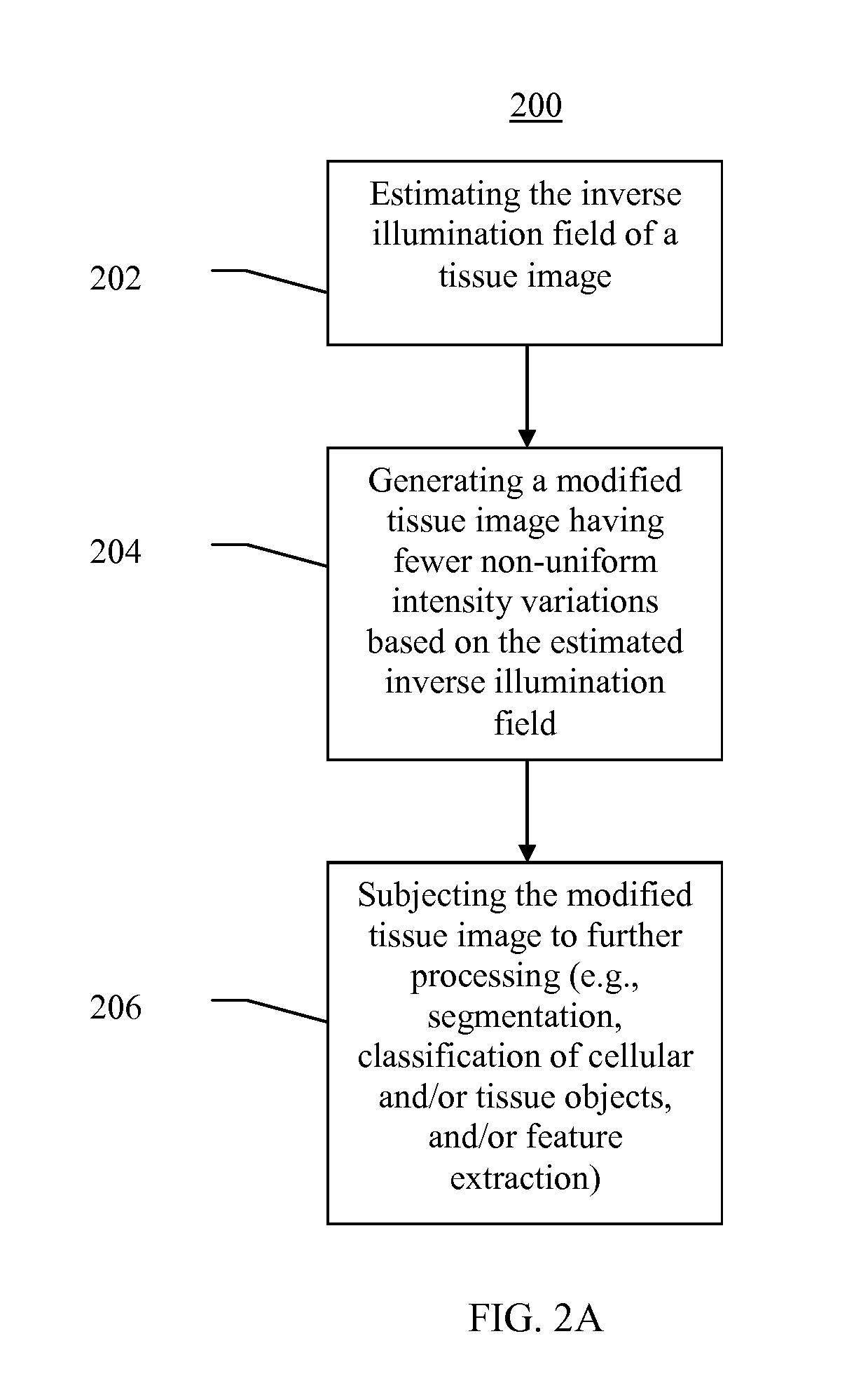
Systems and methods for segmentation and processing of tissue images and feature extraction from same for treating, diagnosing, or predicting medical conditions
ActiveUS20130230230A1Reduce non-uniform variationEliminate needImage enhancementImage analysisDiseaseFeature extraction
Apparatus, methods, and computer-readable media are provided for segmentation, processing (e.g., preprocessing and / or postprocessing), and / or feature extraction from tissue images such as, for example, images of nuclei and / or cytoplasm. Tissue images processed by various embodiments described herein may be generated by Hematoxylin and Eosin (H&E) staining, immunofluorescence (IF) detection, immunohistochemistry (IHC), similar and / or related staining processes, and / or other processes. Predictive features described herein may be provided for use in, for example, one or more predictive models for treating, diagnosing, and / or predicting the occurrence (e.g., recurrence) of one or more medical conditions such as, for example, cancer or other types of disease.
Owner:AUREON LAB INC +1
Cell enriching, separating and extracting method and instrument and single cell analysis method
ActiveCN102690786AEnrichment reachedEfficient collectionMicrobiological testing/measurementBiomass after-treatmentImmunofluorescence stainingMagnetic bead
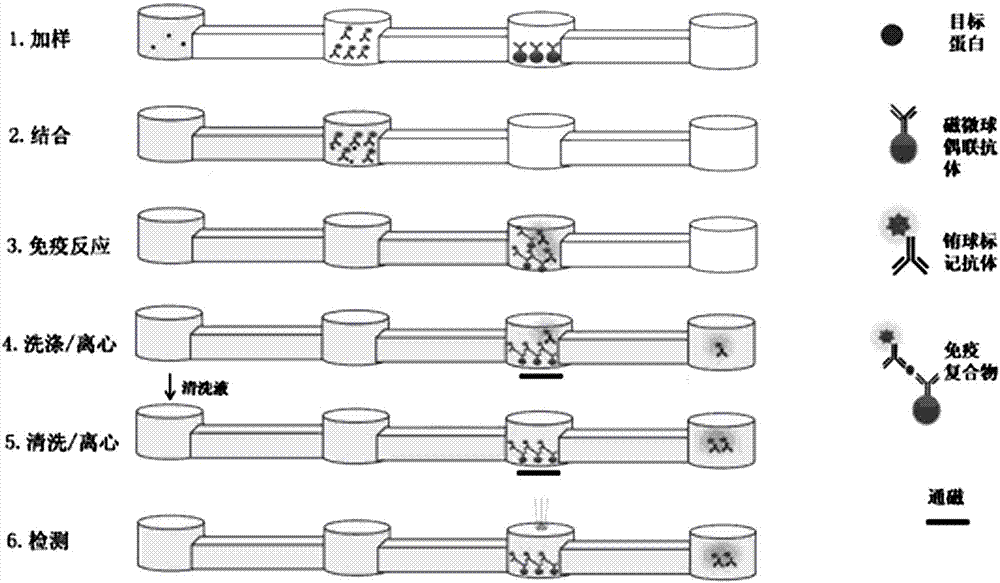
The invention discloses a method and an automatic instrument device for enriching and extracting target cells and separating single cells by using a positive magnetic bead method and performing immunity and molecular biology identification and analysis on single cells. By the method and the instrument device, the on / off of a capture magnet and a release magnet is controlled by various methods to complete target cell searching, capturing, cleaning and releasing operation once or for multiple times, so that the target cell detection sensitivity and stability are improved. When the capture magnet searches and captures the target cells, the search line is circular, square, comb-shaped, S-shaped or U-shaped. In addition, the captured substances are filtered, most free micro magnetic beads are removed, the purity of the product is further improved and the product can be used as a good experimental material. By combining a special filter and the adsorption of the capture magnet, more than 95 percent of free micro magnetic beads can be effectively removed. Meanwhile, the types of the single cells are identified and biological characteristics of the single cells are analyzed by an immunofluorescence staining method and a method in molecular biology, and effective biological indexes are provided for clinical diagnosis and treatment of cancers.
Owner:GD TECH INC
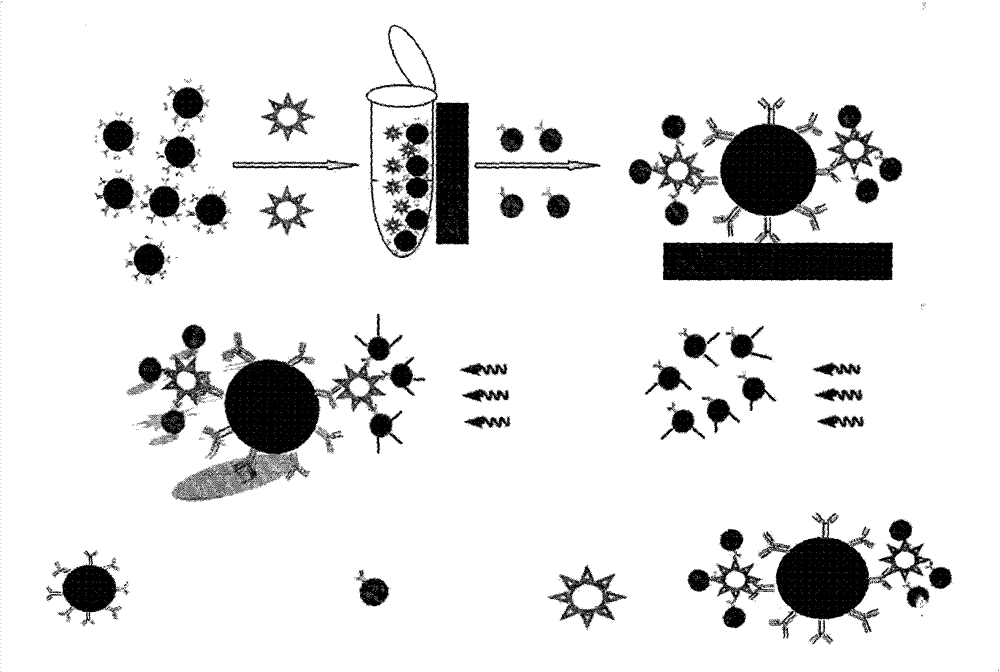
Magnetic fluorescent kit for rapidly detecting microbes as well as preparation method and use method thereof
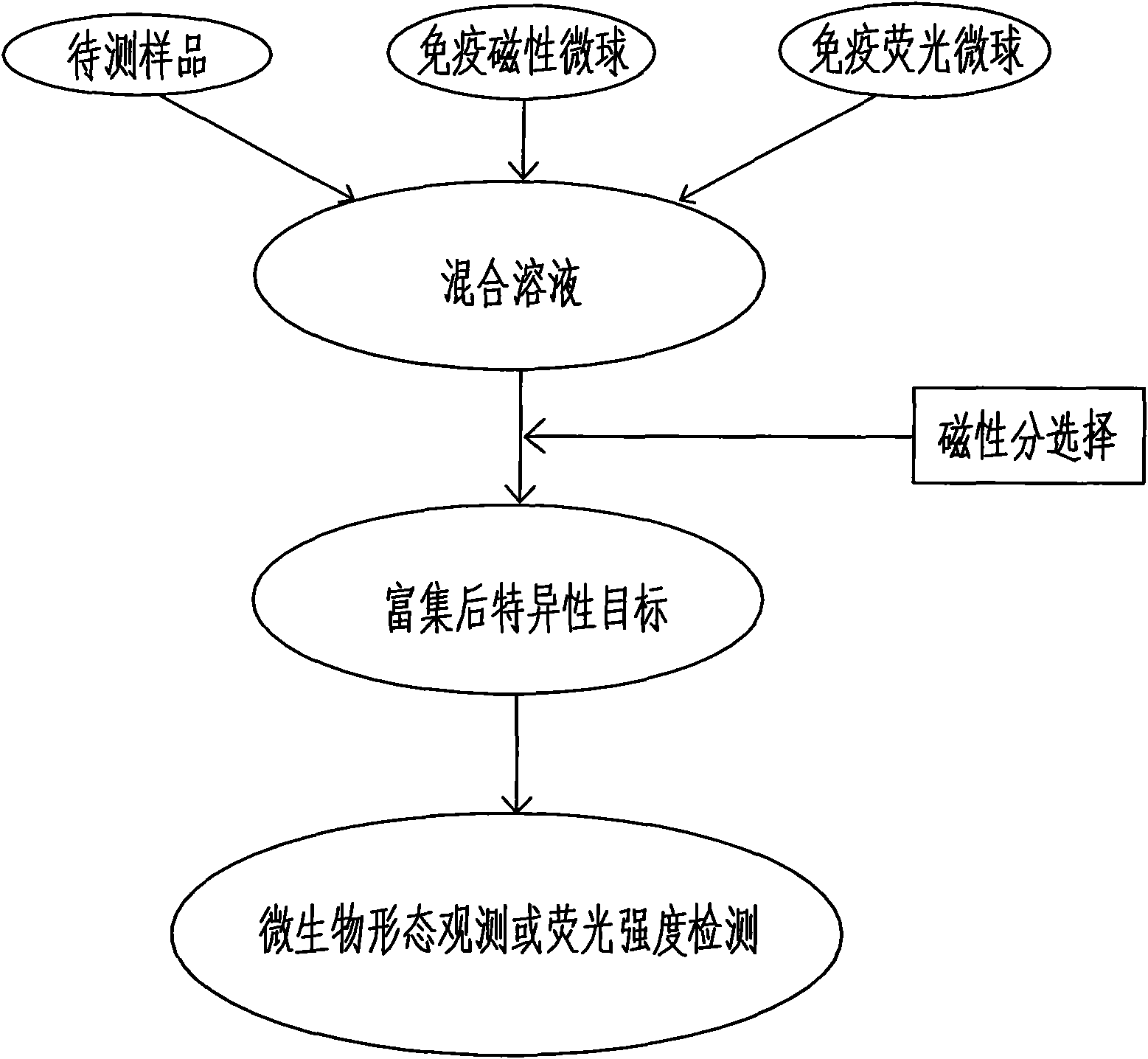
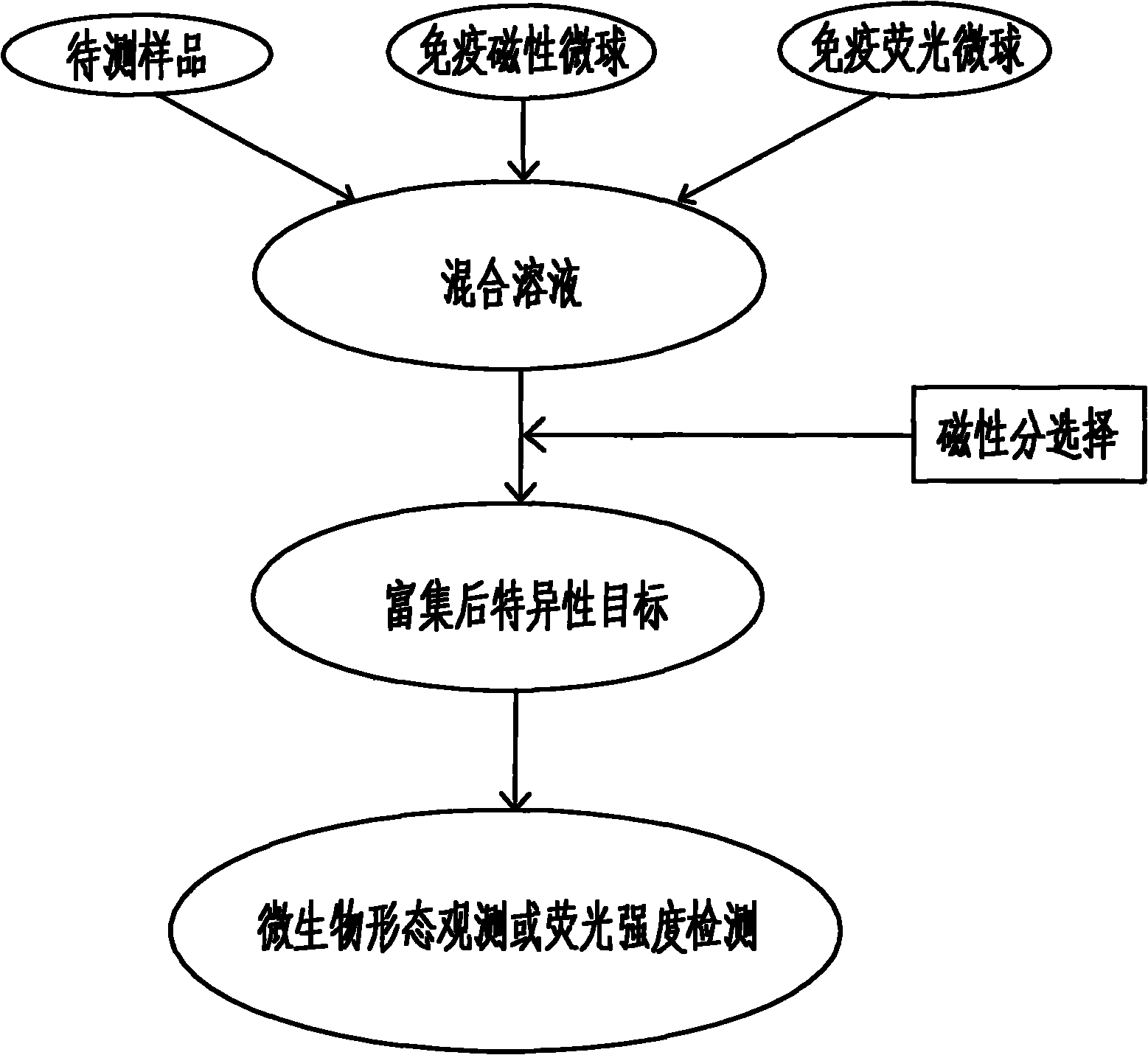
InactiveCN102253193AEfficient separationRapid Quantitative AnalysisBiological testingFluorescence/phosphorescenceImmunofluorescenceMicrosphere
The invention discloses a magnetic fluorescent kit for rapidly detecting microbes as well as a preparation method and a use method thereof. The kit comprises two components: 1) immunomagnetic microspheres specifically bound with the microbes to be detected; and 2) immunofluorescent microspheres specifically bound with the microbes to be detected. The preparation method comprises the following steps: (1) preparation of the immunomagnetic microspheres; and (2) preparation of the immunofluorescent microspheres. The use method comprises the following steps: (1) adding the sample to be detected, lyophilized powder of the immunomagnetic microspheres and the immunofluorescent microspheres to a buffer solution; (2) ensuring the surfaces of the identified microbes to have antigenic determinants simultaneously bound with the immunomagnetic microspheres and the immunofluorescent microspheres; (3) enriching the microbes bound with the immunomagnetic microspheres through magnetic separation of the immunomagnetic microspheres; and (4) qualitatively and quantitatively judging the microbes by measuring the fluorescence intensity of the immunofluorescent microspheres bound with the separated and enriched microbes. The kit, the preparation method and the use method have the advantages of rapidness, quantitative property and wide scope of application.
Owner:EMERGING THERAPEUTICS SHANGHAI CO LTD
Immunofluorescence chromatography test paper for CRP (C-reaction protein)/SAA (Serum amyloid A protein) quantitative combined detection and preparation method of immunofluorescence chromatography test paper
InactiveCN105092861AAccurate detectionSolve the problem of CRP/SAA contentDisease diagnosisBiological testingImmunofluorescenceVenous blood
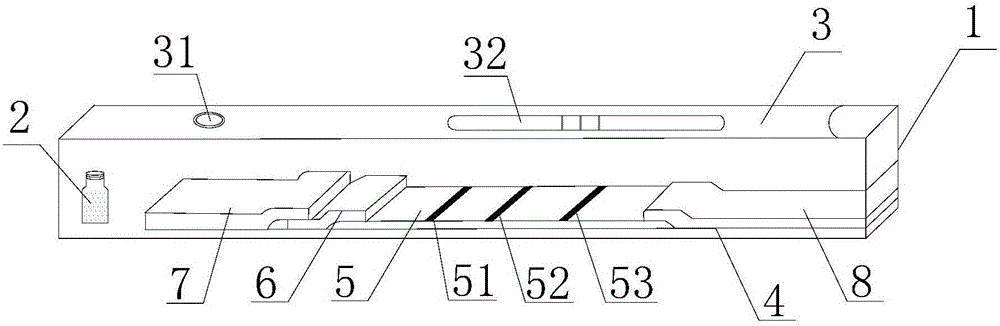
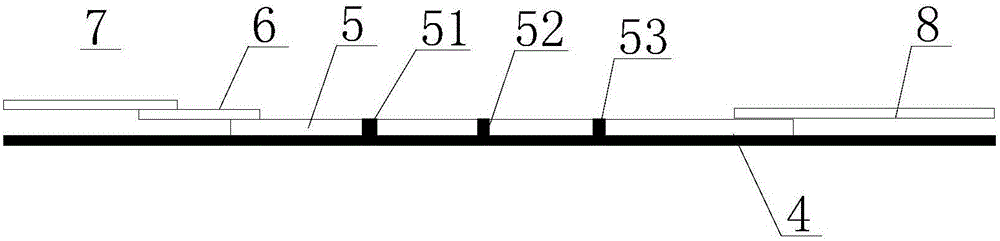
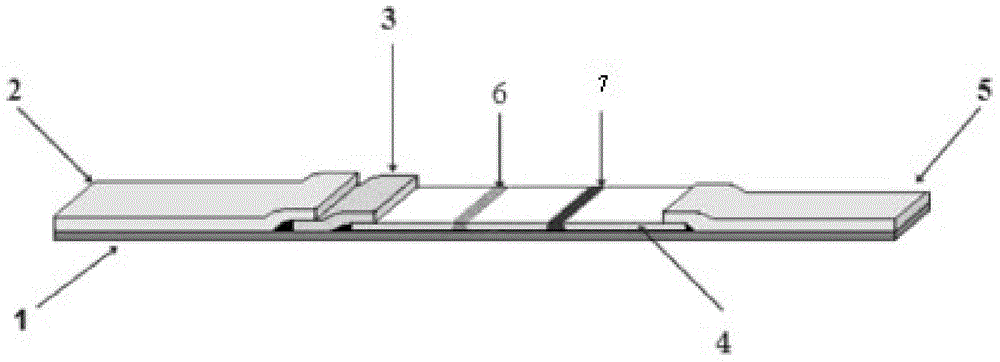
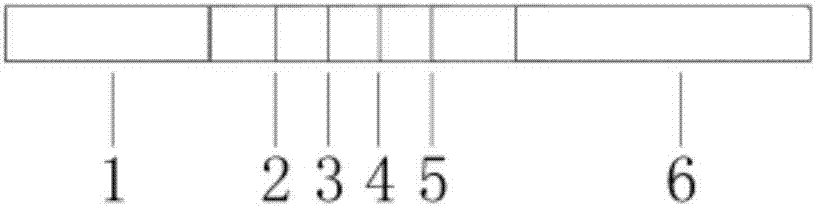
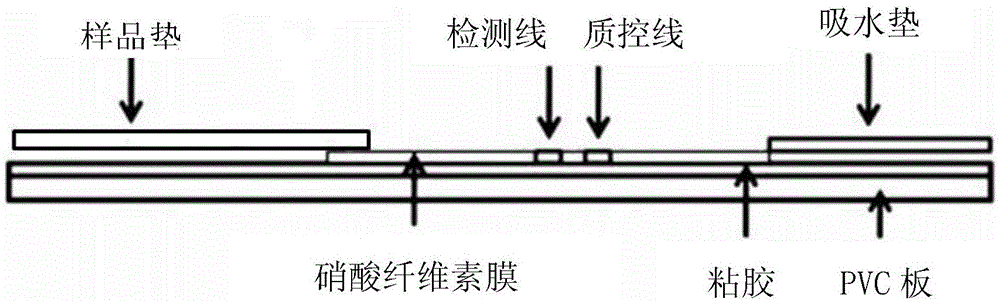
The invention discloses immunofluorescence chromatography test paper for CRP (C-reaction protein) / SAA (Serum amyloid A protein) quantitative combined detection, aiming at providing a test strip capable of realizing quantitative combined detection on the content of CRP / SAA in human peripheral blood and quantitative combined detection on the content of CRP / SAA in venous blood. The immunofluorescence chromatography test paper is technically characterized by comprising a box with a cover, wherein a detection test strip and a blood diluent bottle are arranged in the box, the detection test strip comprises a bottom lining, the bottom lining is provided with a nitrocellulose membrane, one end of the nitrocellulose membrane is connected with a fluorescent microsphere marked antibody fixation pad, the fluorescent microsphere marked antibody fixation pad is connected with a sample pad, the other end of the nitrocellulose membrane is connected with an absorption pad, the fluorescent microsphere marked antibody fixation pad is coated by a CRP monoclonal antibody and an SAA monoclonal antibody, a CRP detection line coated by a CRP monoclonal antibody, an SAA detection line coated by an SAA monoclonal antibody, and a quality control line coated by a goat-anti-mouse IgG polyclonal antibody are arranged on the nitrocellulose membrane in parallel. The immunofluorescence chromatography test paper belongs to the technical field of biological medicines.
Owner:GUANGZHOU WEIMI BIOLOGICAL SCI & TECH
Multiple-antigen synchronous detection method of quantum dot mark fluorescent immune
InactiveCN101441212ASolving the Problem of Simultaneous Fluorescence ImmunoassaysLow costBiological testingFluorescence/phosphorescenceAntigenFluorescence
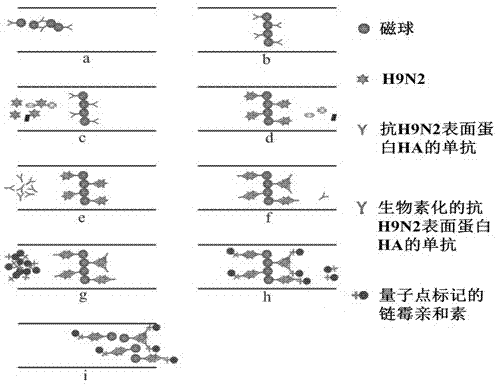
The invention relates to a quantum-dot-labeled immunofluorescence multi-antigen simultaneous detection method, belonging to the detection technical field. The method of the invention comprises the steps that the objects, i.e., the antibodies of the antigen to be detected are respectively connected to the quantum dot and the nano-particle like magnetic nano-particle by using the fluorescence characteristics of the quantum dot, like multiple-wavelength excitation, high-strength fluorescence emission, narrow emission peak, symmetrical peak shape, and stable luminescence; the antigen to be detected and an excessive amount of quantum-dot-labeled antibody are added; by immune reaction, the objects , i.e., the antibodies of the antigen to be detected, are jointed with the antibodies fixed on the quantum dot and the nano-particle like magnetic nano-particle to result in the joint of the quantum dot and the nano-particle like magnetic nano-particle, so as to form the composition of the antigen and the antibody, i.e., complex; the complex and the unreacted quantum-dot-labeled substance, i.e., free substance are separated, the intensity of fluorescence signal of the free substance is detected, and quantitative detection is carried out for the antigen to be detected. The invention has high sensitivity and wide detection range, and can simultaneously detect multiple antigens with simple operation and at a low cost.
Owner:SHANGHAI JIAO TONG UNIV
Micro-fluidic chip immunofluorescence rapid detection kit as well as preparation and detection methods thereof
PendingCN107044972AReduce dosageEasy to integrateFluorescence/phosphorescenceCoatingsMicrofluidic chipImmunofluorescence
The invention discloses a micro-fluidic chip immunofluerescence rapid detection kit as well as preparation and detection methods thereof. The kit comprises a micro-fluidic chip; the micro-fluidic chip comprises one or more detection units; the detection units are arranged on a radius axis of the micro-fluidic chip and are arranged in a divergence manner by taking the micro-fluidic chip as a center point; and a sample feeding groove, a fluorescence probe groove, a reaction detection groove, a waste liquid groove and microchannels which are mutually connected are sequentially arranged on the detection units from the center point of the chip to the edge. By using the kit, the dosage of a reagent is small, the integration degree is high, and a reaction systems is a homogeneous phase; and all the reagents are solidified in the chip in advance, and antigen-antibody reaction is carried out in a relatively closed space, so that crossed contamination and environmental pollution do not occur, and the kit has the advantages of high efficiency, high flux, high sensitivity, rapidness, sensitiveness, stability and the like.
Owner:沈阳微流控生物科技有限公司
Use of dimly fluorescing nucleic acid dyes in the identification of nucleated cells
InactiveUS6329158B1Easy to identifyMicrobiological testing/measurementChemiluminescene/bioluminescenceFluorescenceImmunofluorescence
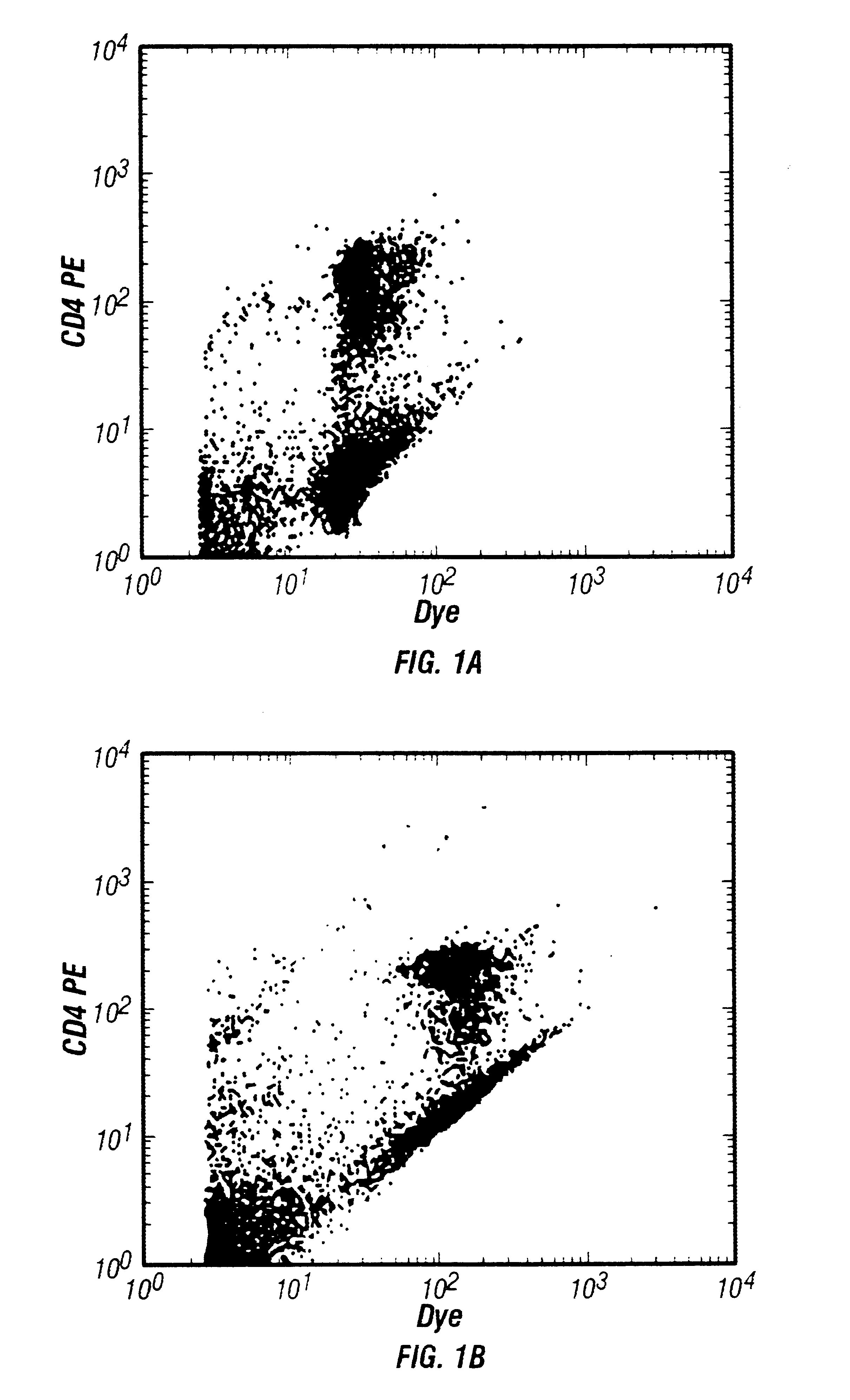
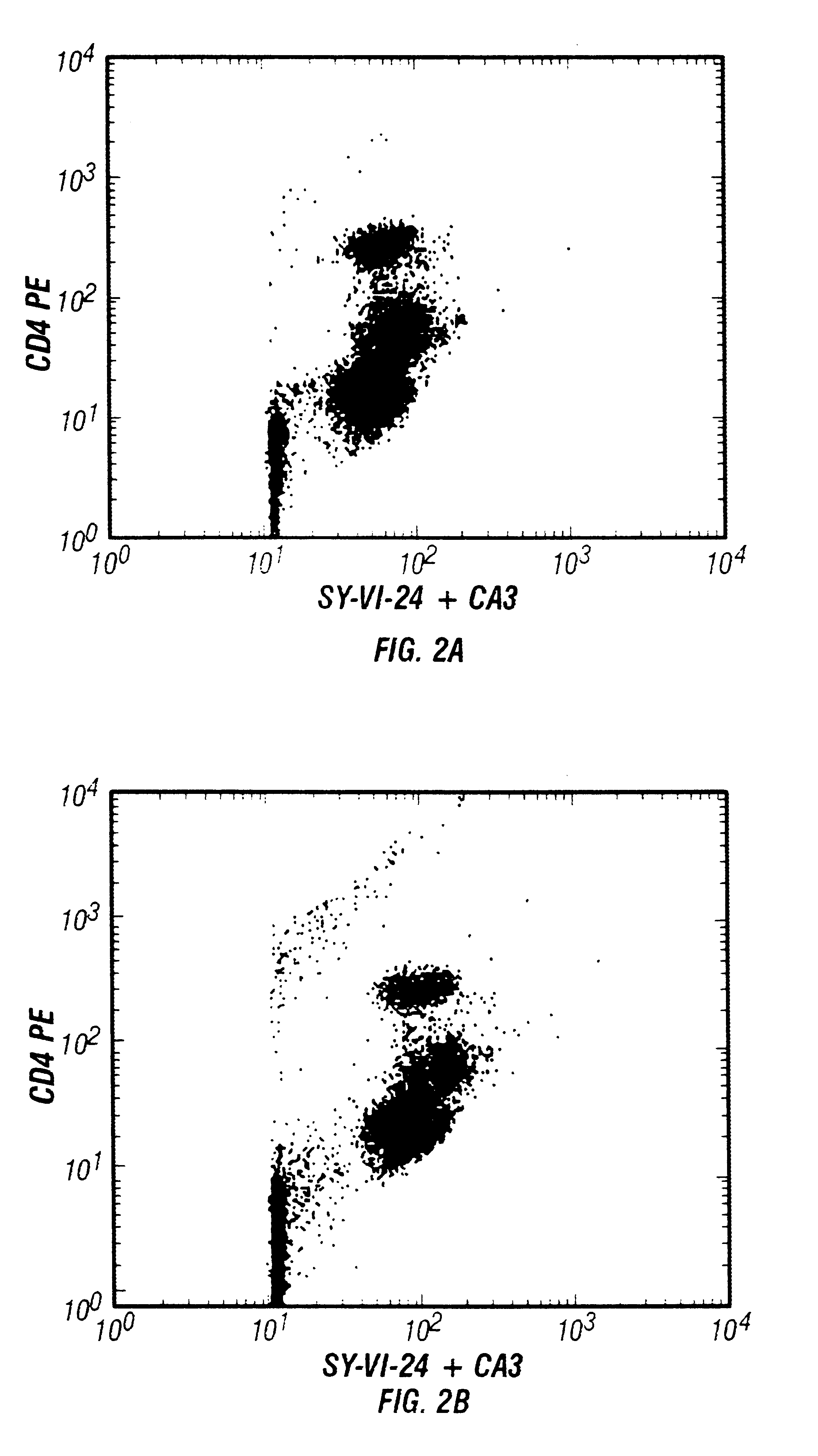
This invention presents improved methodology for identification of nucleated cells in flow cytometric analysis when immunofluorescent dyes are also used. Briefly, in the method nucleic acids are stained with a fluorescent dye which can then be used to identify the nucleated cells by measurement of fluorescence on a flow cytometer. The improvement presented by this invention is the use of a saturating (or near saturating) amount of a nucleic acid dye, or mixture of dyes, which gives low fluorescence at excitation conditions, so as not to greatly interfere with the signals of the immunofluorescent dyes.
Owner:BECTON DICKINSON & CO
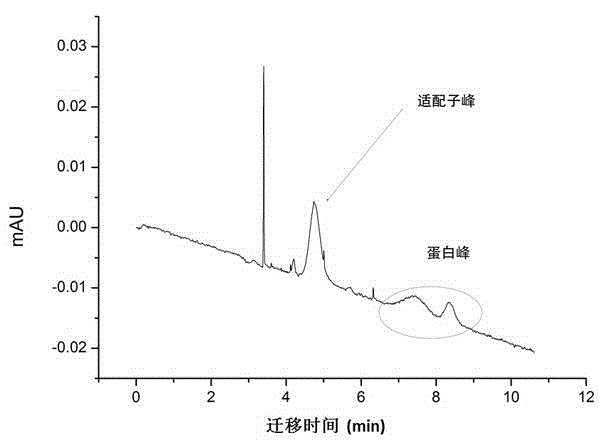
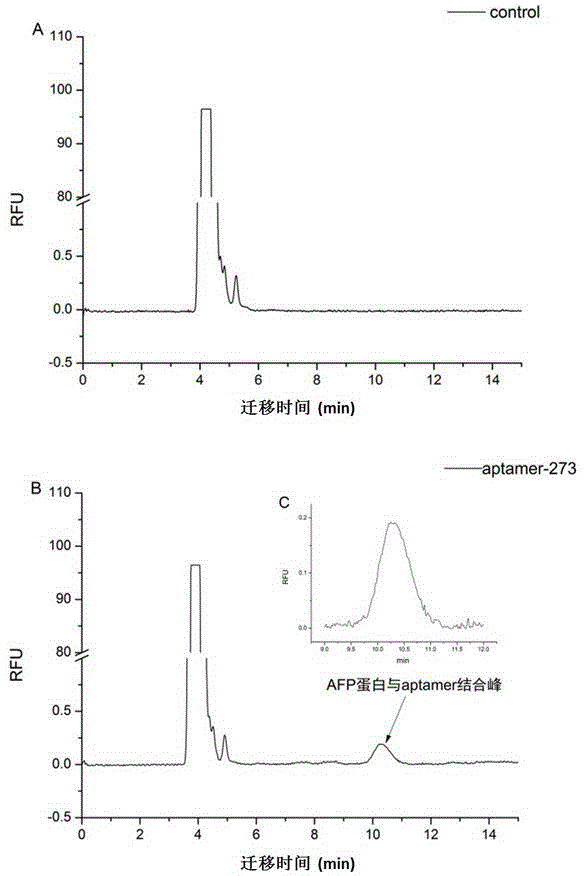
Method for screening aptamer specifically bound with alpha-fetoprotein
InactiveCN104131105AStrong interactionIncrease workloadMicrobiological testing/measurementDNA preparationAptamerFetuin b
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV +1
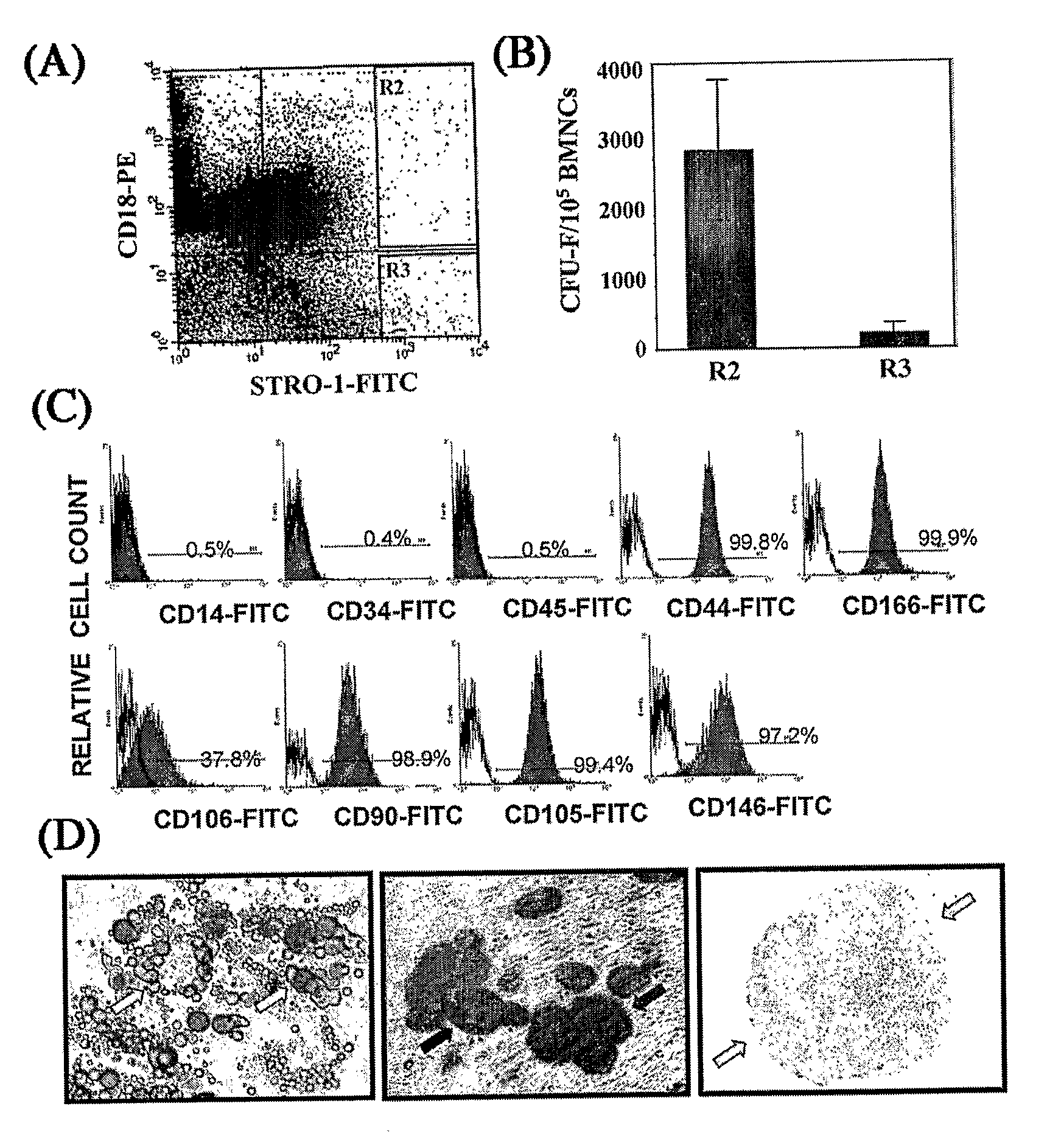
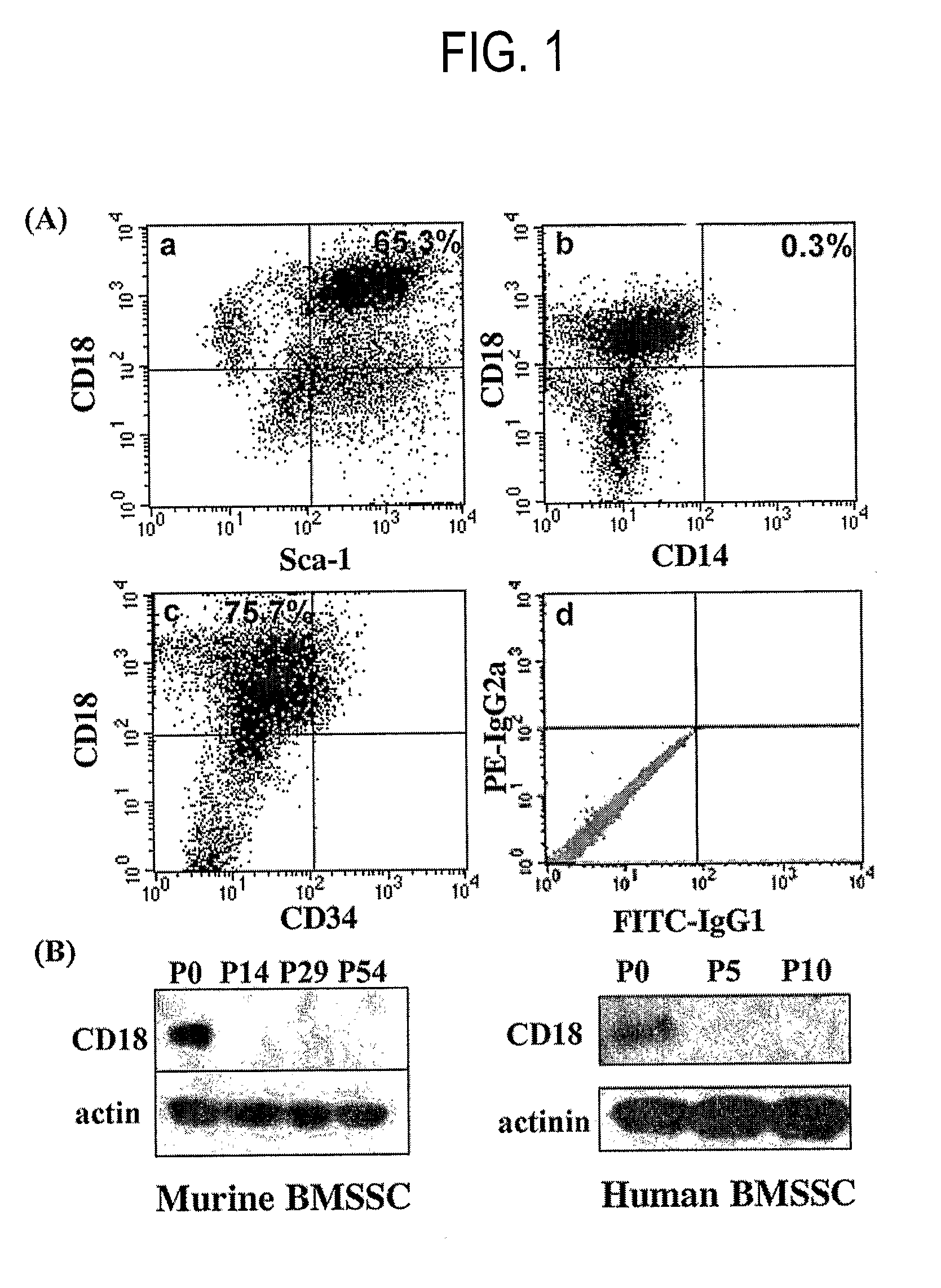
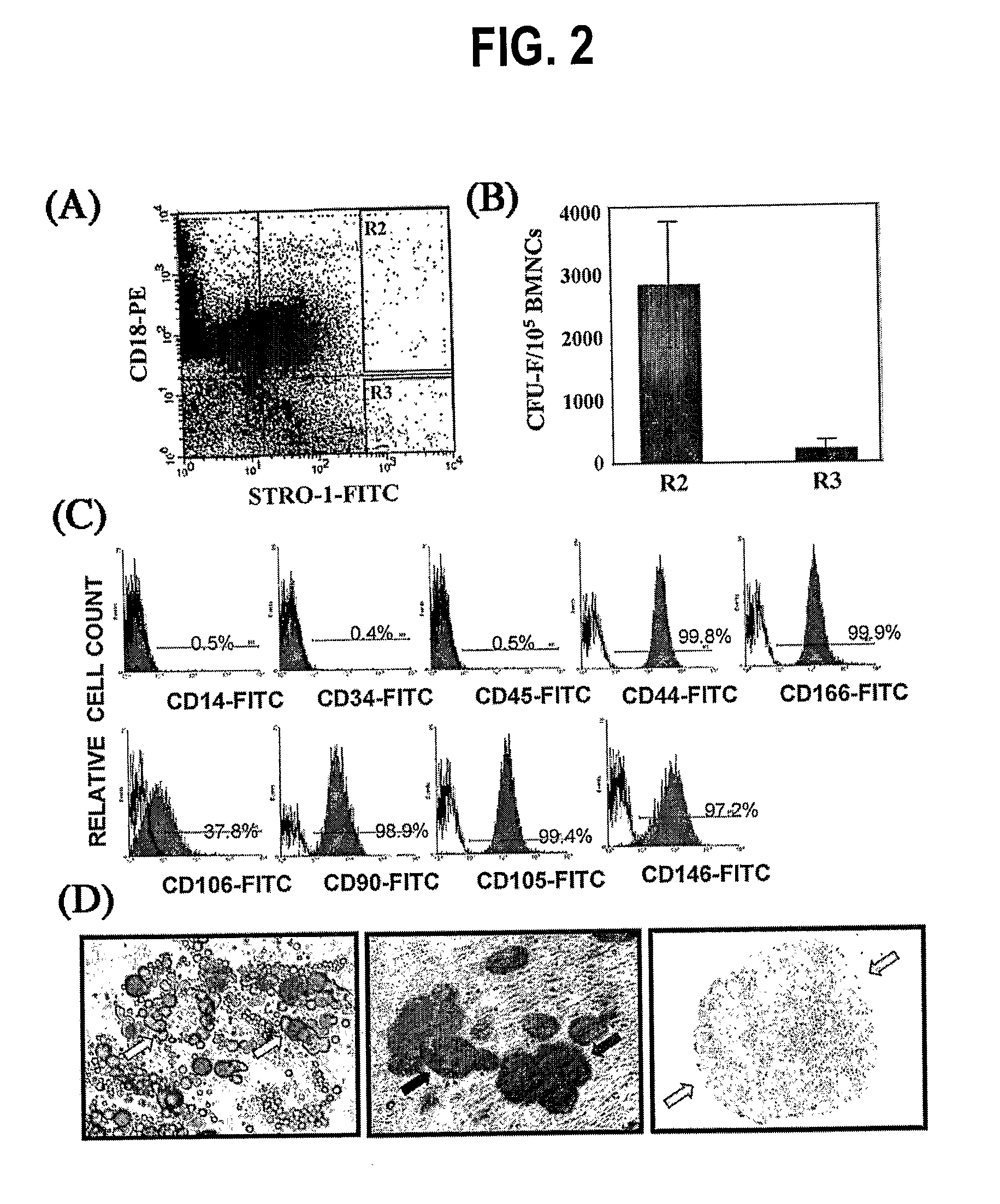
Integrin CD18 is a novel stromal stem cell marker and functions to promote osteogenesis
InactiveUS7732126B2Bioreactor/fermenter combinationsBiological substance pretreatmentsImmunoperoxidase StainingCancer research
The present invention is directed to a new bone marrow stromal stem cell (BMSSC) marker, CD18, for use in selecting a population of cells enriched in BMSSCs, from bone marrow cells, adipose cells, or peripheral blood. The invention is further directed to methods for selecting a population of cells enriched in BMSSCs based on the selective expression of CD18 on their surface, using techniques known in the art such as fluorescent assisted cell sorting, an immunomagnetic method, flow microfluorimetry, immunofluorescence, immunoperoxidase staining, radioimmunoassay and immunoaffinity chromatography. The invention is further directed to the BMSSCs isolated based on CD18 expression, and their use to treat various diseases. In one aspect, the HMSSCs are transformed with a vector having a normal gene for CD18, and the transformed BMSSCs are administered to treat bone degenerative diseases and diseases of bone involving abnormal expression of CD18 expression of CD18.
Owner:UNITED STATES OF AMERICA +1
A method for immunofluorescence staining of suspension cells
InactiveCN102288471AGood dyeing resultPreparing sample for investigationImmunofluorescenceFluorescent staining
The invention relates to an immunofluorescence staining method for suspension cells. The immunofluorescence staining method for the suspension cells is characterized by comprising the following steps of: collecting cells into a centrifuge tube; performing 800-gram centrifugation to collect the cells; respectively adding paraformaldehyde to fix, Triton-X100 to pass through and 1 percent bovine serum albumin (BSA) to close; adding a primary antibody and a secondary antibody sequentially to incubate; washing by an 800-gram centrifugation method after the operation of each step; performing diamino phenyl indole (DAPI) staining; and dripping on a glass slide and closing the glass slide to observe. The immunofluorescence staining method for the suspension cells has the advantages that: the immunofluorescence staining process is performed in the centrifuge tube, so the problems that the suspension cells grow on the glass slide difficultly and drop off easily in the test process are solved; the staining result is good; and the method is suitable for the suspension cells and cells which are adhered to the wall difficultly.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Method for detecting non-humoral rare karyotes, and kit thereof
ActiveCN104007257AHigh detection sensitivityImprove featuresBiological material analysisBiological testingBiological bodySurface marker
The invention discloses a new method for detecting non-humoral rare karyotes. Non-humoral rare karyotes in a biological body fluid are obtained through many means, and the combined use of the antibody of a humoral cell surface marker and the specific probe of the non-humoral rare karyotes is carried out to discriminate the enriched rare karyotes. Compared with commercial discriminating methods existing in the present market, the method disclosed in the invention well combines a fluorescent in situ hybridization (FISH) method for processing the non-humoral rare karyotes with the immunofluorescence dyeing for processing humoral rare karyotes, realizes the systemic associative processing of a same specimen sheet, and can realize the intelligible observation of two processing results.
Owner:CYTTEL BIOSCI BEIJING
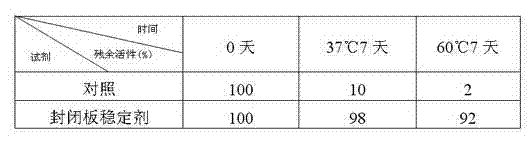
Sealing and stabilizing agent for microporous board
The invention discloses a sealing and stabilizing agent for a microporous board. In terms of 100ml water, 0.1-3g of protein, 3-20g of saccharide, 0.01-1g of organic polymer, 0.01-0.05ml of nonionic surfactant, 0.01-0.1g of preservative and 10-100mmol of buffer solution are contained. The sealing and stabilizing agent disclosed by the invention is researched and designed on account of the characteristic that antigen or antibodies coated on a stationary phase are easy to inactivate. The sealing and stabilizing agent includes not only sealing components but also organic components and inorganic components, wherein the sealing components can be used for effectively sealing excessive loci on a sealing board, and the organic components and the inorganic component can be used for effectively stabilizing the antigen or the antibodies, so that the sealing purpose and the stabilizing purpose can be organically combined together through one-step operation. The sealing and stabilizing agent disclosed by the invention can be applied to immunological detection methods such as enzymelinked immunosorbent assay, chemiluminesent immunoassay, time resolved fluoroimmunoassay and the like, wherein the microporous board serves as the stationary phase; and the sealing and stabilizing agent can be used for effectively stabilizing the activity of the antigen or the antibodies coated on a stabilizing board.
Owner:GUANGZHOU YOUDI BIOTECH CO LTD
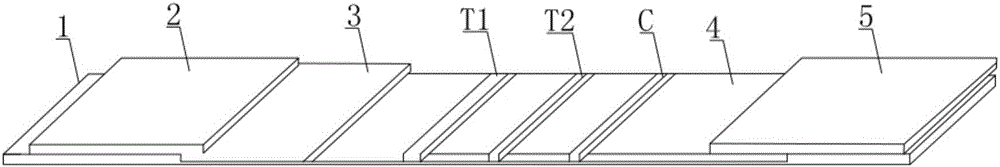
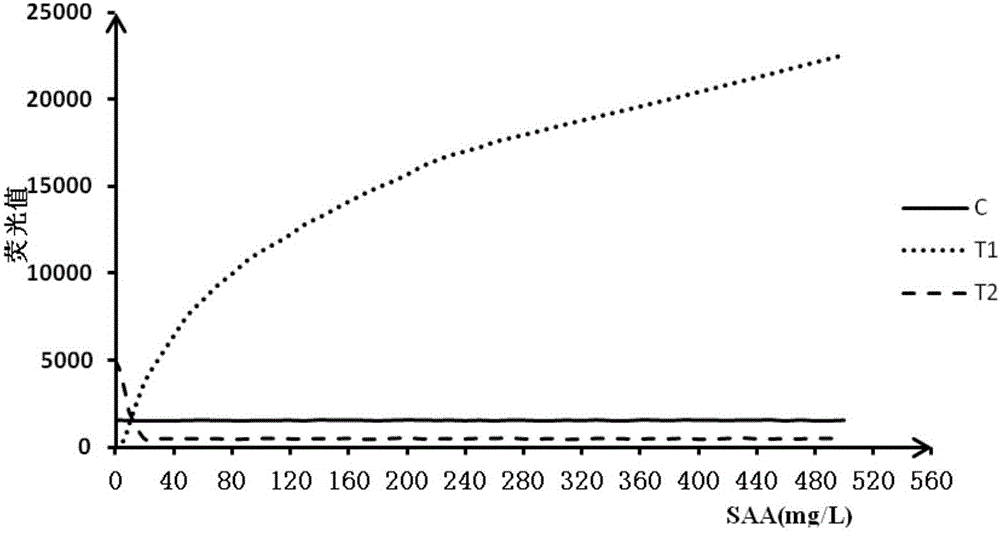
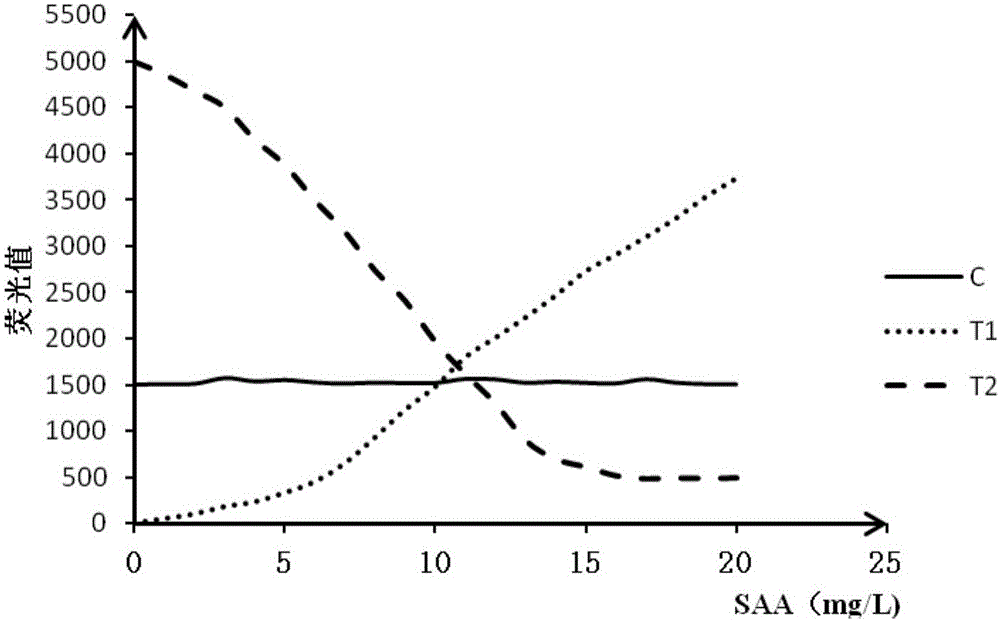
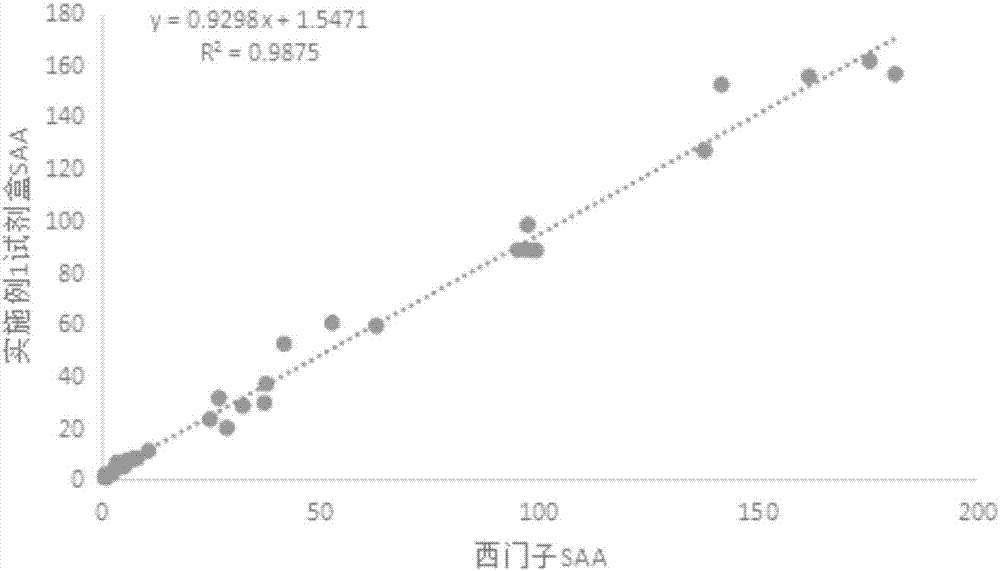
Double detection line SAA (Serum amyloid A protein) immunofluorescence chromatography quantitative detection reagent and preparation method thereof
InactiveCN105785038AExpand the scope of detectionHigh sensitivityDisease diagnosisBiological testingAntigenSAA protein
The invention discloses a double detection line SAA (Serum amyloid A protein) immunofluorescence chromatography quantitative detection reagent and a preparation method thereof. The double detection line SAA immunofluorescence chromatography quantitative detection reagent can simultaneously promote sensitivity and detection scope and can be applied to clinical detection for SAA level in patients of acute inflammation and chronic inflammation. According to the technical key points, the reagent comprises a base plate, a combining cushion, a coating film and an absorbing cushion, wherein a sample cushion is connected with the base plate; anti-SAA monoclonal antibody 1 and chick IgY are sprayed on the combining cushion and are marked with a same fluorescent microsphere; a detection line T1, a detection line T2 and a quality control C line are arranged on the coating film; anti-SAA monoclonal antibody 2 is coated with the detection line T1; antigen SAA protein is coated with the detection line T2; goat-anti-chick IgY is coated with the quality control C line. The reagent belongs to the technical field of in-vitro diagnostic reagents.
Owner:GUANGZHOU WEIMI BIOLOGICAL SCI & TECH
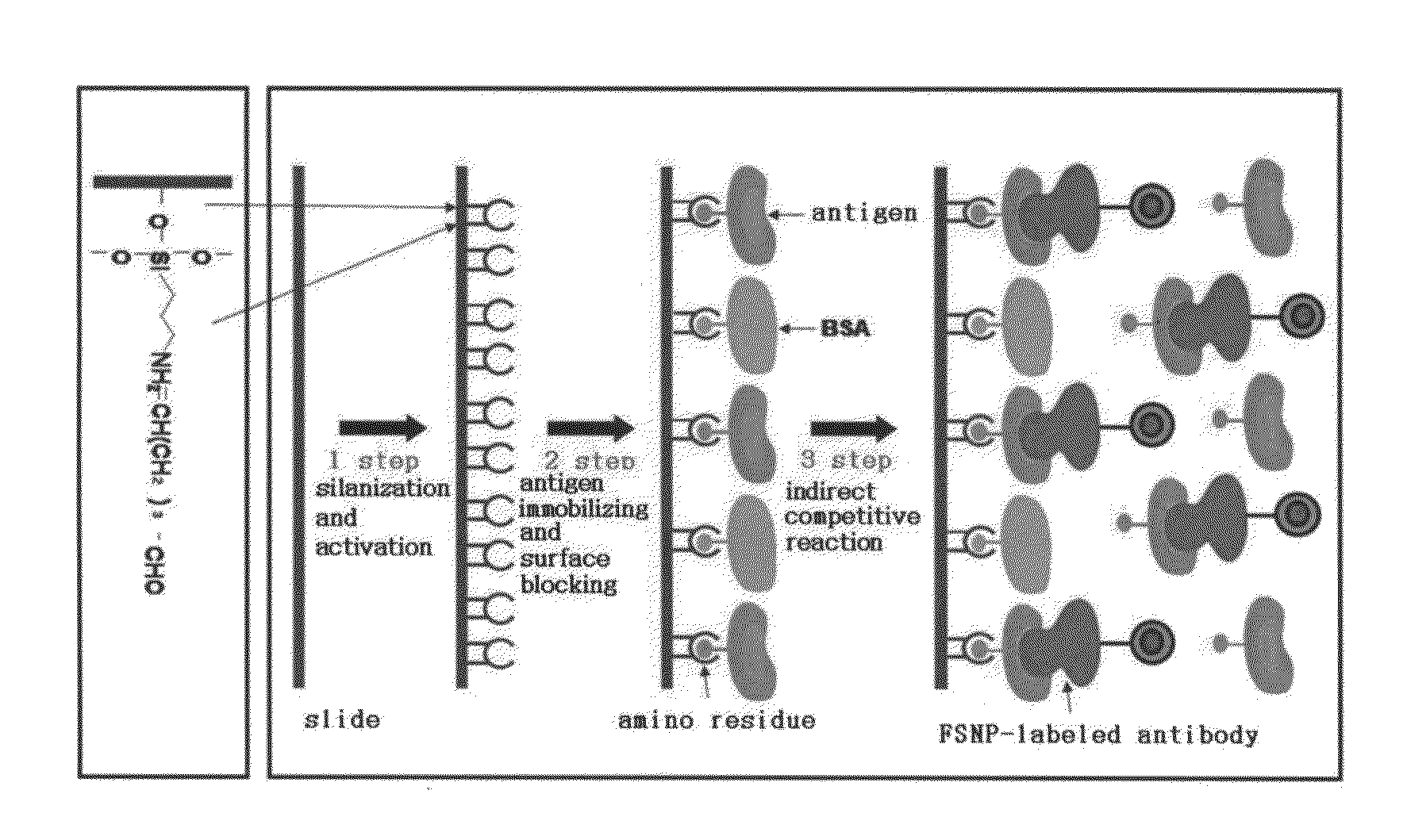
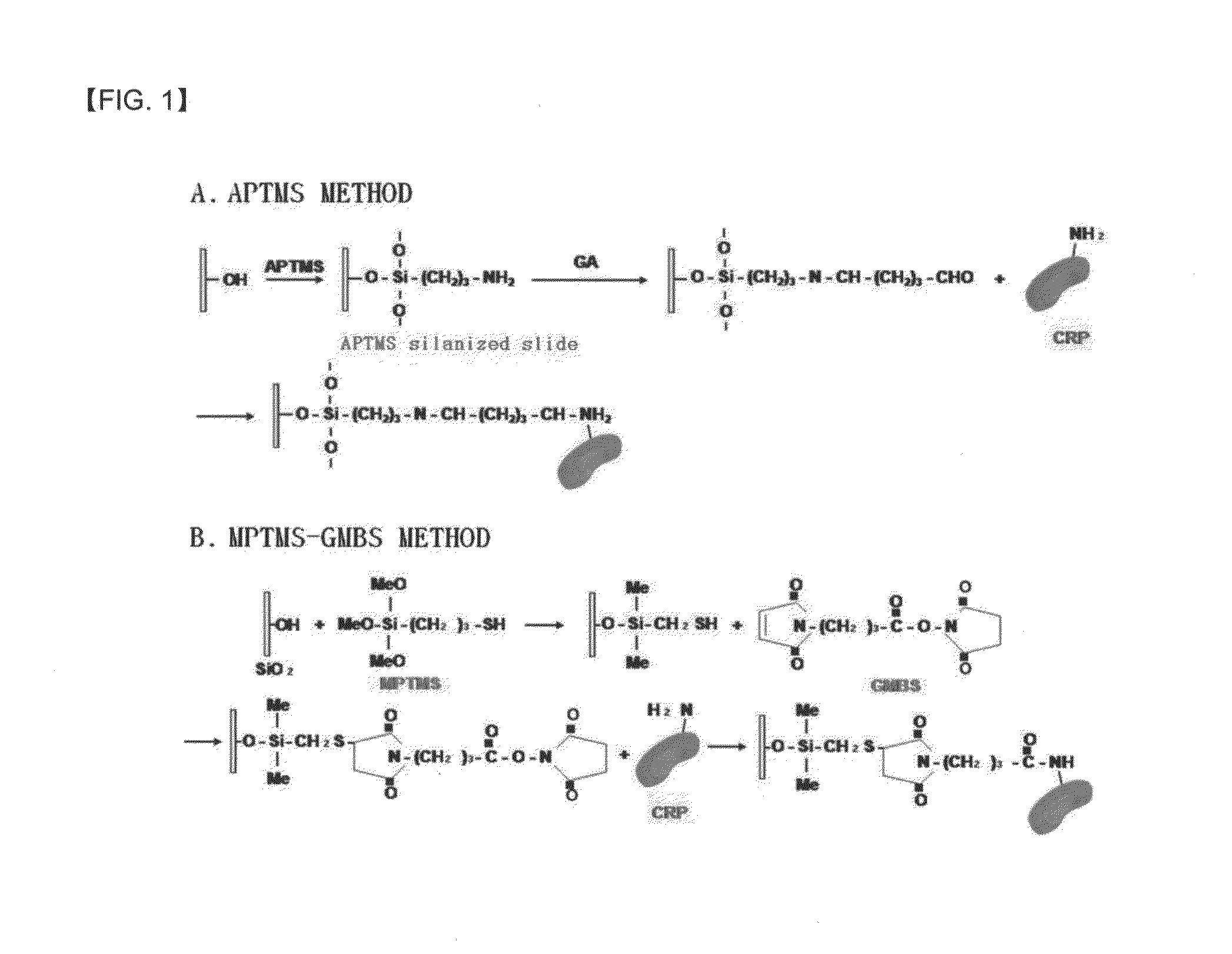
Preparation method of antigen-immobilized immuno- fluorescence slide and immuno-fluoroscence slide prepared thereby
InactiveUS20130029428A1Easily and highly-sensitively measuringBiological material analysisMammal material medical ingredientsAntigenBiotin-streptavidin complex
A method of preparing an antigen-immobilized immuno-fluorescence slide, the method comprising: immobilizing a C-reactive protein on a slide to prepare a protein chip; mixing an antibody that specifically binds to a target protein, with streptavidin to label the antibody with a fluorescent nanoparticle; immuno-reacting the antibody by competitive mixing, assaying with a fluorescence camera, wherein the immobilizing of the C-reactive protein on the slide comprises: modifying the slide with 3-aminopropyltrimethoxysilane to prepare a modified slide; hydrating the slide modified with 3-aminopropyltrimethoxysilane; activating the modified slide by using a glutaraldehyde solution; dissolving a C-reactive protein at a concentration of 0.01-0.5 mg / ml in a 30-70 mM phosphate buffer solution (pH 6.5-7.8) to prepare an antigen solution for immobilization; placing a petri dish comprising the slide on a spotting guide and spotting 1-100 μl of the antigen solution on spotting points; and performing a reaction on the slide prepared as described above for 1-6 hours to immobilize the antigen, and an immune-fluorescence slide prepared by using the method.
Owner:KOREA FOOD RES INST
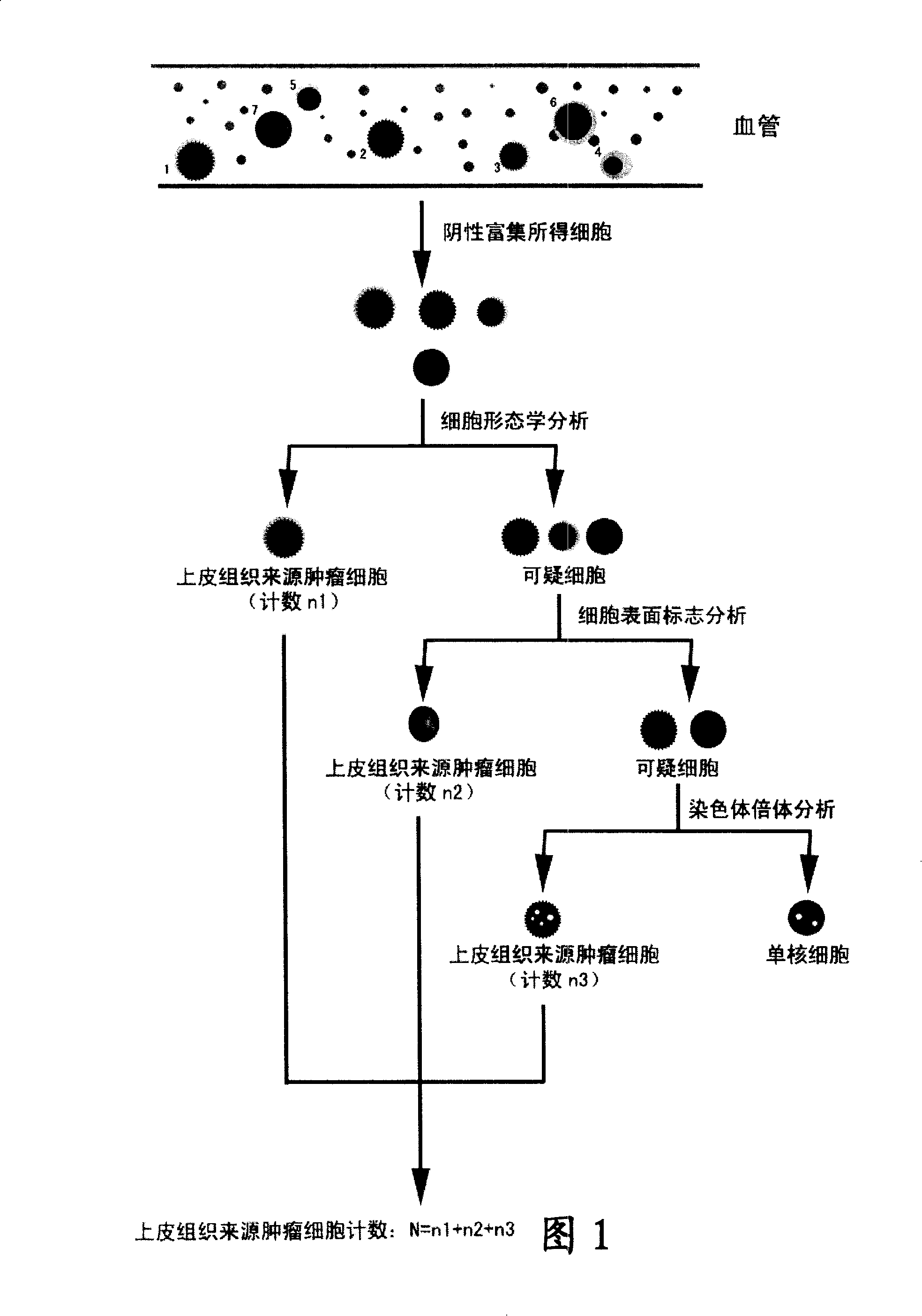
Cytochemical staining method being compatible with immunofluorescence analysis and uses thereof
ActiveCN101226118AMicrobiological testing/measurementPreparing sample for investigationImmunofluorescenceCytochemical staining
The invention relates to a cell chemical dyeing method compatible with immunofluorescence analysis and relative applications. The invention further relates to a method for checking epithelial tissue source tumor cell in human peripheral blood sample, which uses the cell chemical dyeing method compatiable with immunofluorescence analysis. The inventive check method can simply, quickly and accurately check the tumor cells of epithelial tissue source in blood circulation from the karyocyte separated from small human periphery blood sample.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI +1
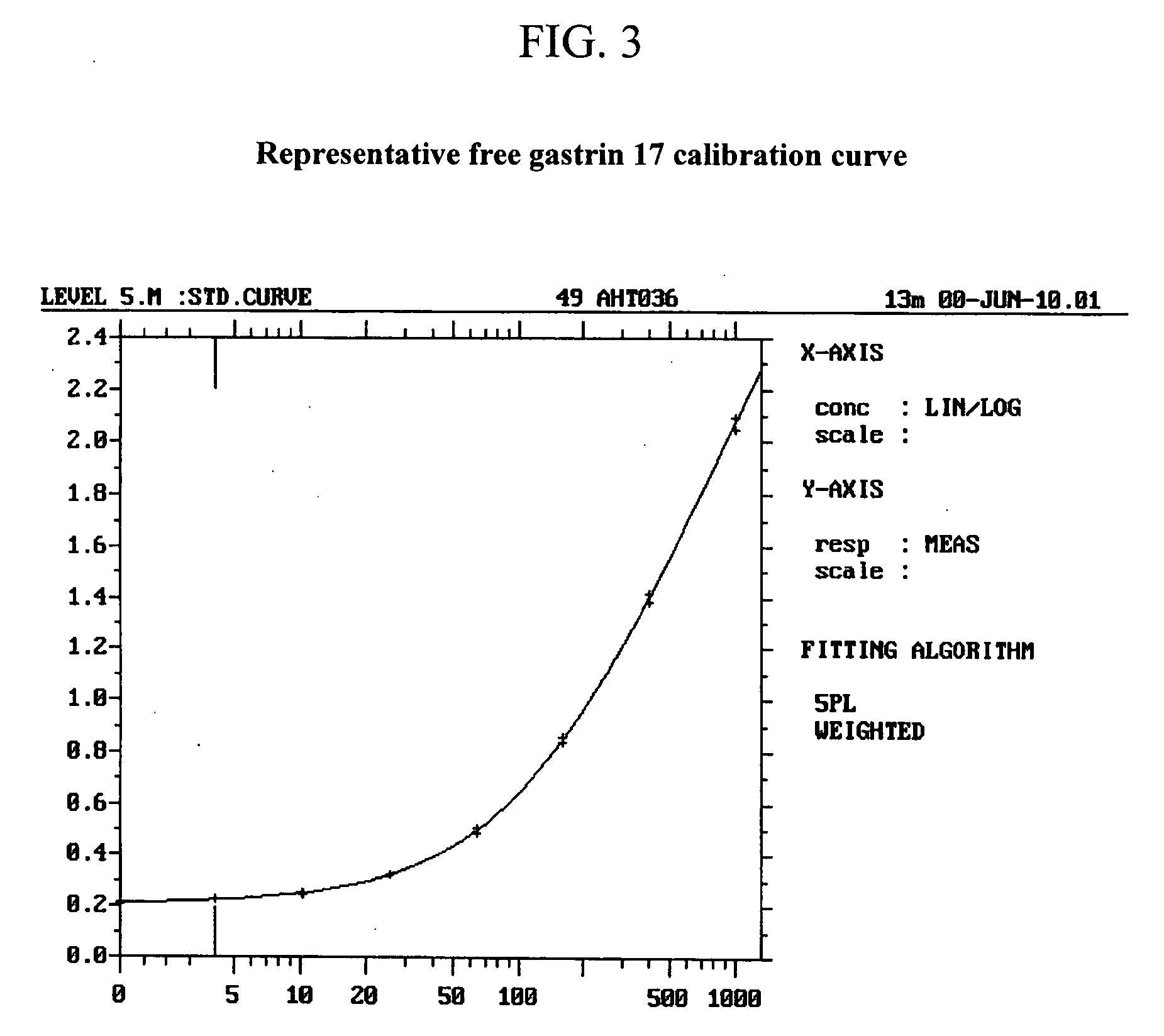
Monoclonal antibodies to gastrin hormone
InactiveUS20060020119A1Microbiological testing/measurementDigestive systemImmunofluorometric AssaysGlycine extended gastrin
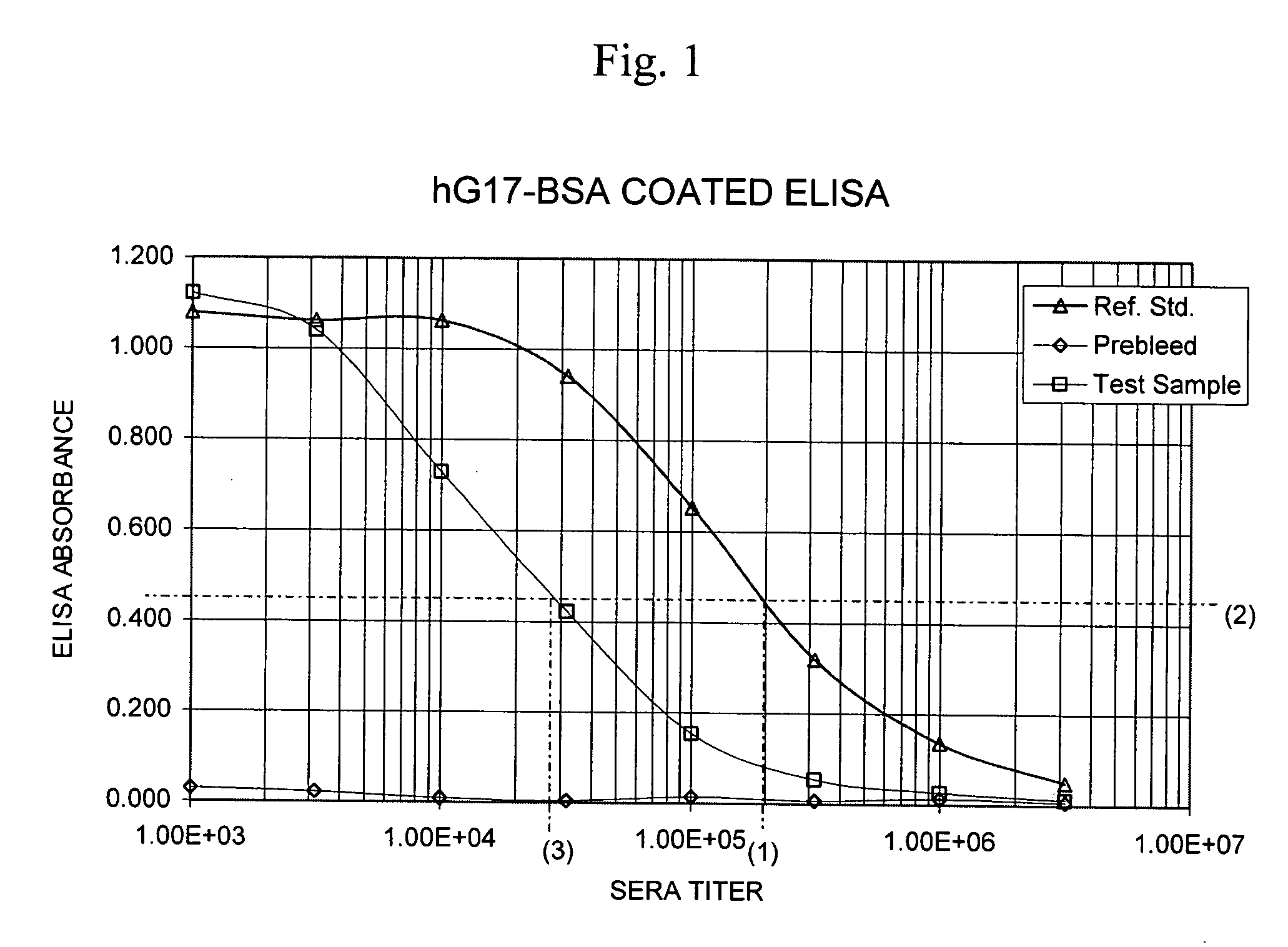
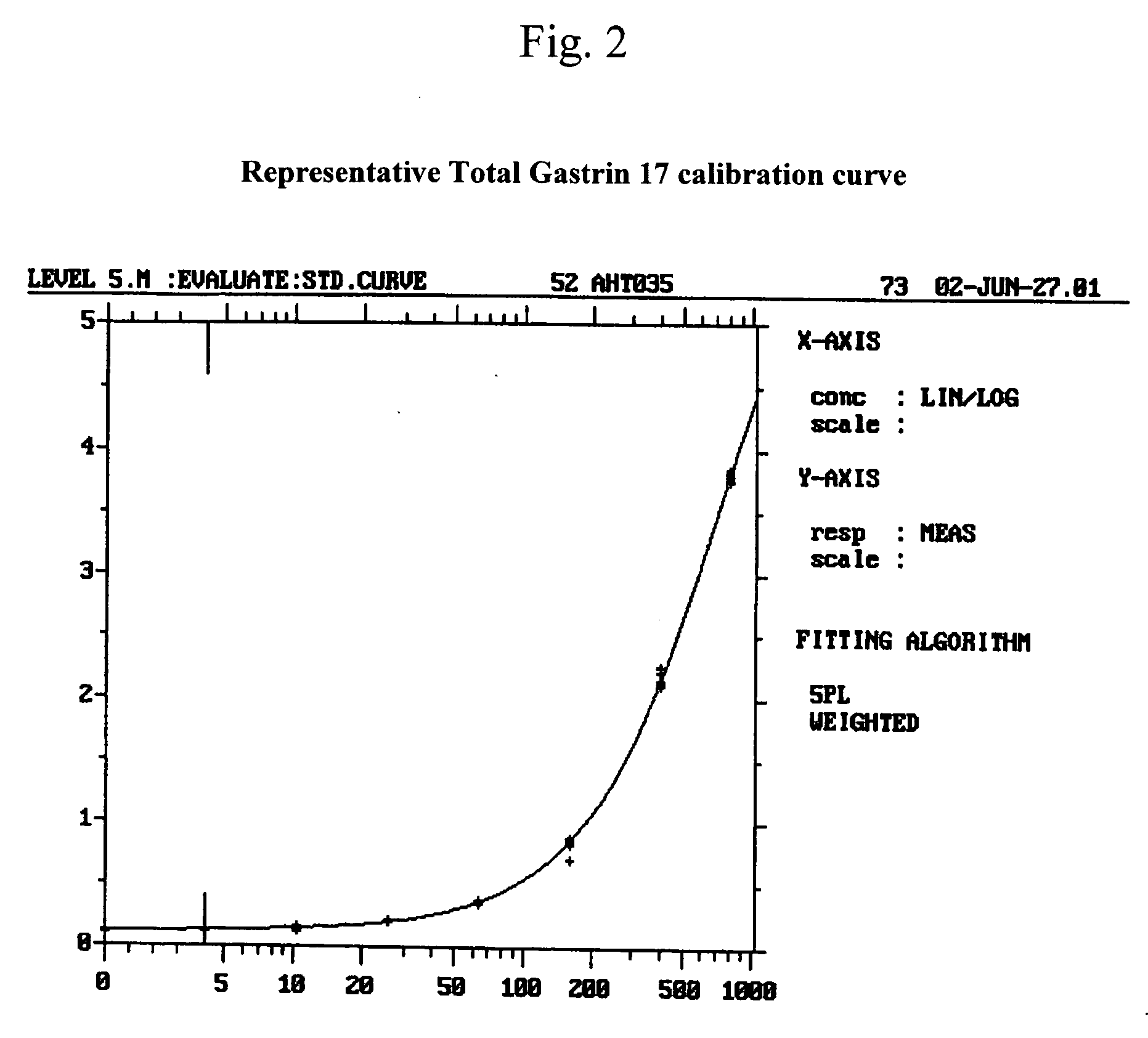
The present invention provides monoclonal antibodies (MAbs) selective for the N-termini and C-termini of the gastrin hormone forms, gastrin-17 (G17), glycine-extended gastrin-17 (G17-Gly), gastrin-34 (G34) and glycine-extended gastrin-34 (G34-Gly); and the hybridomas that produce these MAbs. Also provided are panels of MAbs useful for the detection and quantitation of gastrin-17 (G17), glycine-extended gastrin-17 (G17-Gly), gastrin-34 (G34) and glycine-extended gastrin-34 (G34-Gly). These assays are useful for monitoring a gastrin-mediated disease or condition, or for monitoring the progress of a course of therapy. The invention further provides solid phase assays including immunohistochemical (IHC) and immunofluorescence (IF) assays suitable for detection and visualization of gastrin species in solid samples, such as biopsy samples or tissue slices. Pharmaceutical compositions of the MAbs of the invention are also provided, along with methods of diagnosis, prevention and treatment of gastrin-mediated diseases or conditions. Methods of evaluating a gastrin hormone-blocking treatment are described. The course of a gastrin-mediated disease or condition may be monitored in a patient by means of assay methods provided.
Owner:CANCER ADVANCES INC
Immunofluorescence detection test strip and preparation method thereof for rapid quantitative detection of porcine epidemic diarrhea viruses
ActiveCN104804082AEasy to operateRealize quantitative detectionImmunoglobulins against virusesFluorescence/phosphorescenceAntigenEpidemic diarrhea
The invention discloses an immunofluorescence detection test strip and a preparation method thereof for rapid quantitative detection of porcine epidemic diarrhea viruses. The test strip comprises a sample cushion, a combination cushion, a chromatography film and a water-absorbing cushion, wherein the combination cushion is provided with a fluorescent microsphere labelled anti-PEDV (Porcine Epidemic Diarrhea Viruses) single-domain antibody; the single-domain antibody has high specificity and high sensibility against the antigen PEDV. The test strip is used for detecting PEVD viruses in breeding pig manure or PEDV pollution in feeding stuff plasma proteins on the basis of the newfound immunology principle of the specific single-domain antibody and antigen, can be used for on-site rapid detection or laboratory detection by simply taking 5-10 min; when the test strip is used in combination with a fluorescence quantitative detector, quantitative detection can be realized; the test strip is simple and convenient to operate, operators don't need professional training, a special laboratory is not needed, the limitation of the conventional detection method is overcome, and the test strip has good market prospects.
Owner:GUANGDONG HAID ANIMAL HUSBANDRY & VETERINARY RES INST
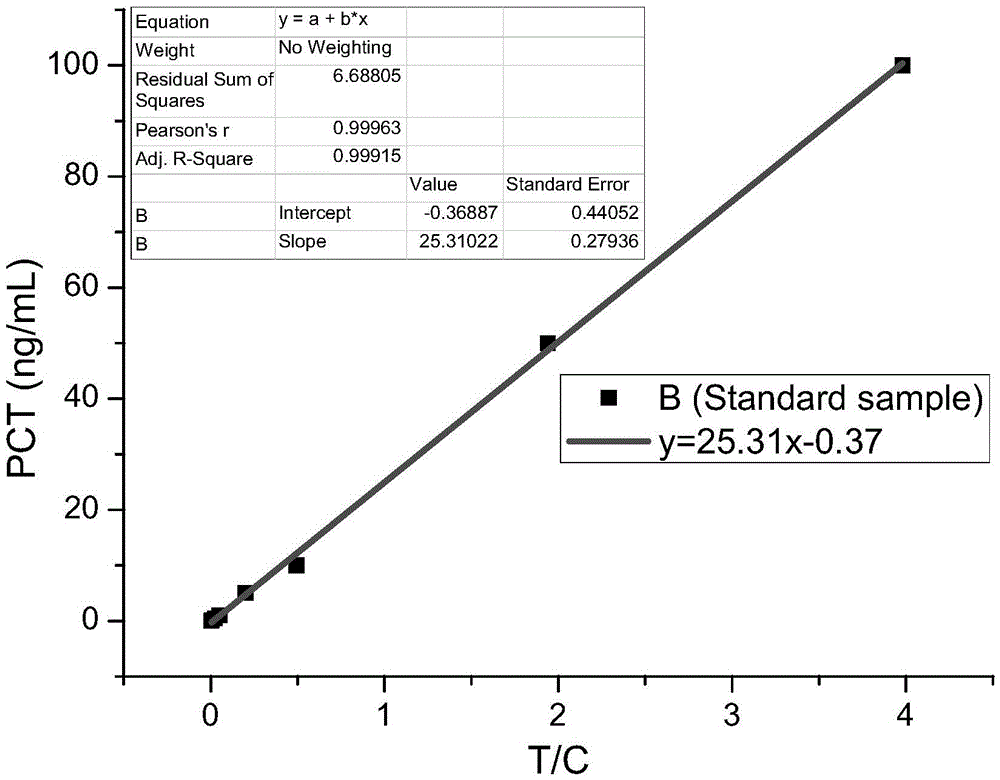
Immunofluorescence chromatography kit for quantitative detection of SAA (serum amyloid A), CRP (C-reactive protein) and PCT (procalcitonin) and preparation method of immunofluorescence chromatography kit
The invention discloses an immunofluorescence chromatography kit for quantitatively detecting SAA, CRP, and PCT and a preparation method thereof, which includes a fluorescent immunochromatography test strip and a fluorescent substance. Nitrocellulose membrane, one end of the nitrocellulose membrane is connected with a sample pad, and the other end of the nitrocellulose membrane is connected with an absorption pad; the nitrocellulose membrane is provided with detection lines for detecting the contents of SAA, CRP, and PCT in parallel. ; The fluorescent substance includes a fluorescent substance coupled to a rabbit IgG polyclonal antibody, a fluorescent substance coupled to a paired SAA monoclonal antibody, a fluorescent substance coupled to a paired CRP monoclonal antibody, and a paired PCT monoclonal antibody. of fluorescent substances. This kit can simultaneously detect the contents of SAA, CRP, and PCT conveniently, accurately, and with high sensitivity.
Owner:深圳市惠安生物科技有限公司
Kit for time resolution fluorescent quantitative detection on PCT
InactiveCN105548567AAvoid Fluorescent EffectsEasy to detectBiological material analysisBiological testingFreeze-dryingRare earth
The invention discloses a kit for time resolution fluorescent quantitative detection on PCT. The kit comprises a fluorescent microsphere antibody complex and an immune fluorescent test paper card, wherein the fluorescent microsphere antibody complex is prepared by marking rare earth fluorescent microsphere with a PCT monoclonal antibody to form a compound and adding the compound on a pipette tip, and is used as a detection antibody after freeze-drying; the immune fluorescent test paper card comprises a detection test paper card; the detection test paper card consists of a sample pad, a nitrocellulose membrane and a water absorbing pad which are adhered to a lining plate in sequence in a lap joint manner; the position of a detection line on the nitrocellulose membrane is wrapped by another PCT monoclonal antibody; the position of a quality control line is wrapped by goat anti-mouse polyclonal antibody. By adopting the kit, the fluorescence influence caused by a sample self can be avoided, the rare earth fluorescent microsphere is adopted as a marking carrier, good stability can be achieved, the microsphere can be connected with the antibody through a covalent bond, and a stable marking product can be generated. The kit is rapid, simple and convenient in detecting the sample and high in sensitivity, and full quantitative detection can be achieved.
Owner:武汉菲恩生物科技有限公司
Monoclonal Antibodies to Progastrin
The present invention provides progastrin-binding molecules specific for progastrin that do not bind gastrin-17(G17), gastrin-34(G34), glycine-extended gastrin-17(G17-Gly), or glycine-extended gastrin-34(G34-Gly). Further, the invention provides monoclonal antibodies (MAbs) selective for sequences at the N-terminus and the C-terminus of the gastrin precursor molecule, progastrin and the hybridomas that produce these MAbs. Also provided are panels of MAbs useful for the detection and quantitation of progastrin and gastrin hormone species in immuno-detection and quantitation assays. These assays are useful for diagnosing and monitoring a gastrin-promoted disease or condition, or for monitoring the progress of a course of therapy. The invention further provides solid phase assays including immunohistochemical (IHC) and immunofluorescence (IF) assays suitable for detection and visualization of gastrin species in solid samples, such as biopsy samples or tissue slices. The progastrin-binding molecules are useful therapeutically for passive immunization against progastrin in progastrin-promoted diseases or conditions. Also provided are surrogate reference standard (SRS) molecules that are peptide chains of from about 10 to about 35 amino acids, wherein the SRS molecule comprises at least two epitopes found in a protein of interest of greater than about 50 amino acids. Such SRS molecules are useful as standards in place of authentic proteins of interest.
Owner:CANCER ADVANCES INC
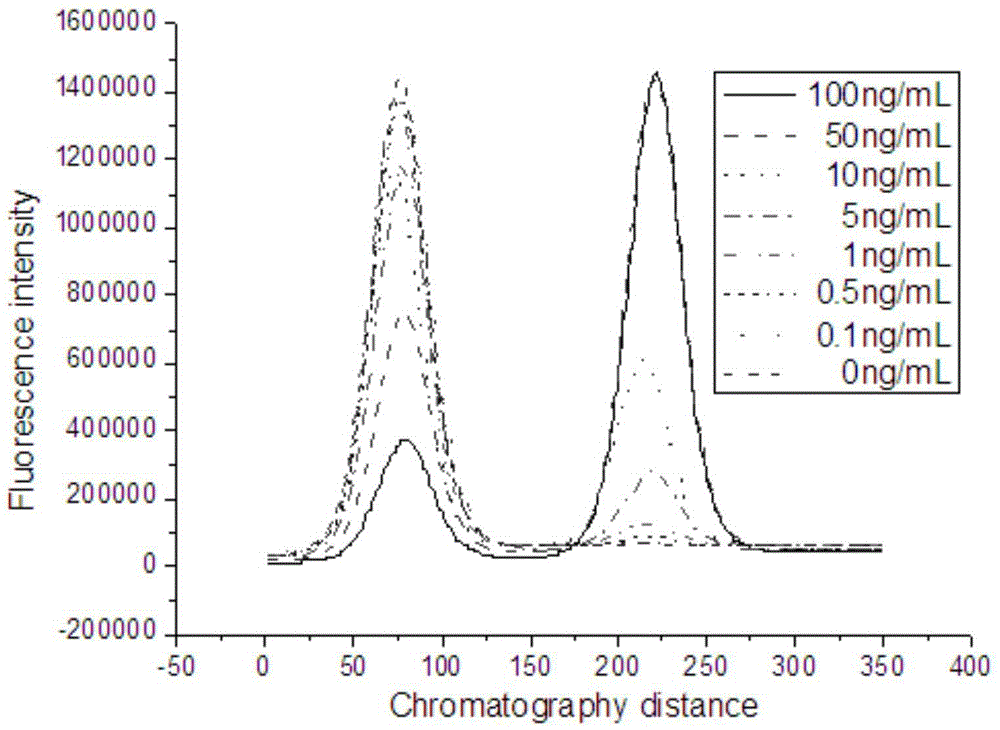
Method for detecting biomacromolecule based on magnetic separation-quantum dot immunofluorescence sensing and reagent preparation method
InactiveCN103543260AAvoid background fluorescence interferenceIncrease fluorescence signal valueCoatingsMaterial analysisImmunofluorescenceTest sample
The invention provides a method for detecting a biomacromolecule based on magnetic separation-quantum dot immunofluorescence sensing and a reagent preparation method. The method is characterized by comprising the following steps: with superparamagnetic nano beads MBs as carriers, coupling the carriers with an antibody Ab1 of a biomacromolecule to be detected to prepare a capturing antibody MB-Ab1; coupling a quantum dot with an antibody Ab2 of the biomacromolecule to be detected to prepare a quantum dot fluorescence probe QDs-Ab2; enriching the biomacromolecule Ag to be detected in a test sample by using the MB-Ab1 to prepare a serial compound MB-Ab1-Ag; reacting the MB-Ab1-Ag with the QDs-Ab2 to generate an MB-Ab1-Ag-Ab2-QDs and carrying out magnetic separation to separate the MB-Ab1-Ag-Ab2-QDs from a liquid-phase QDs-Ab2 immunofluorescence probe; and measuring QDs-Ab2 fluorescence intensity X in a liquid phase and indirectly measuring C by utilizing an inverse relation represented between the concentration C of the biomacromolecule to be detected and the X. The method is simple and convenient to operate, good in stability and high in detection sensitivity.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Integrated Method for Enriching and Detecting Rare Cells from Biological Body Fluid Sample
InactiveUS20110195413A1Good cell shapeHigh recovery rateBioreactor/fermenter combinationsBiological substance pretreatmentsStainingSorbent
The present invention relates to an integrated method for enriching and detecting rare cells in biological body fluid sample. The enriching method comprises: (a) removing plasma protein by centrifugation; (b) optionally adding a red cell lysis solution to carry out red cell lysis so as to remove the red blood cells; (c) adding immunomicrospheres or immunoadsorbent to incubate; and (d) carrying out density centrifugation based on a special cell separation medium for separating the circulating rare cells, residual red blood cells after removing red blood cells and the white blood cells combined on the immunomicrospheres. The method for detecting the enriched rare cells according to the present invention comprises combining immunohistochemistry based staining with immunofluorescence, or bicolor, tricolor or multicolor staining based on immunohistochemistry, and observing and identifying by fluorescence or ordinary optical microscope or a scanner based on microscope principle. The novel and unique method for enriching and staining has been proved to have low cost and can rapidly, effectively and high specifically enrich and quantitatively detect the rare cells in blood.
Owner:CYTTEL BIOSCI BEIJING
Method for preparing live vaccines of hog cholera and product thereof
InactiveCN101879311ASmall batch-to-batch quality varianceStable production processInactivation/attenuationAntiviralsVaccine ProductionFreeze-drying
The invention discloses a method for preparing live vaccines of hog cholera and a product thereof. The preparation method comprises the following steps of: (1) culturing porcine passage cell lines; (2) inoculating the porcine passage cell lines with live vaccine production seed viruses of the hog cholera to obtain attenuated vaccine strains of the hog cholera; (3) performing virus multiplication on the attenuated vaccine strains of the hog cholera; (4) measuring the virus titer of multiplication virus suspension by adopting an immunofluorescence method; and (5) adding a freeze-drying protective agent and antibiotics into the virus suspension which is detected to be qualified for vaccine matching and freeze-drying. The preparation method has the advantages of producing the live vaccines of the hog cholera by using the cell lines so as to achieve small quality differences among batches and the characteristics of simple and stable process, easy operation, high yield, low cost, the feasibility and extendibility of industrial production and the like, and measuring the virus titer of the multiplication virus suspension by adopting the immunofluorescence method so as to achieve sensitive, fast, specific and accurate detection, high repeatability and reliable results. The live vaccines of the hog cholera prepared by the method can completely protect pigs from the attacks of violent hog cholera viruses.
Owner:武华
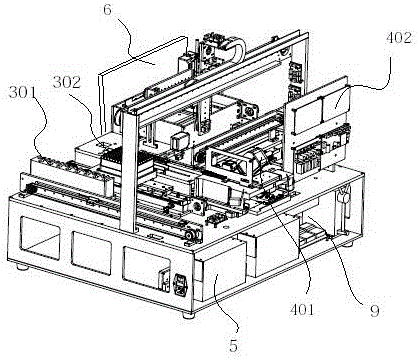
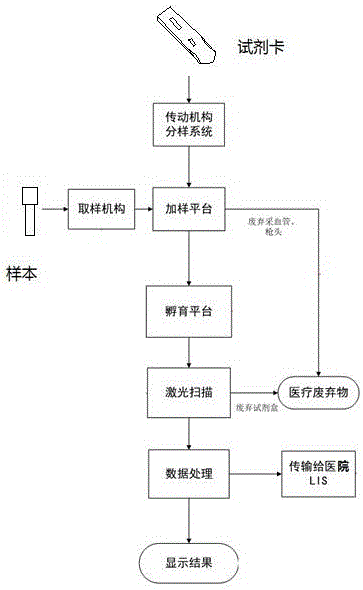
Full-automatic immunofluorescence detection device
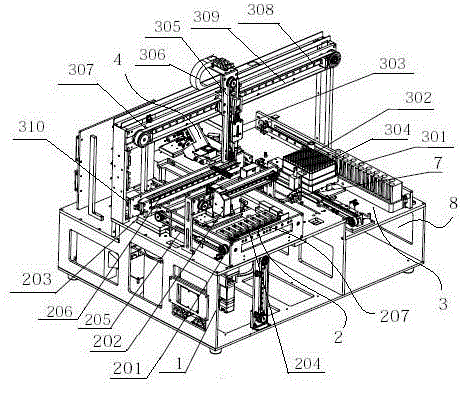
ActiveCN105067806AImprove detection efficiencyImprove sampling accuracyMaterial analysisTemperature controlImmunofluorescence
The invention discloses a full-automatic immunofluorescence detection device, which comprises a main frame. The main frame is provided with a test operation area, a sample adding area and a detection area. The detection area consists of a reagent card tray transmission mechanism, a scanning mechanism and a temperature controlled incubation mechanism. The sample adding area consists of a sample loading mechanism, a Tip head mechanism and a sample adding mechanism. The detection area consists of an optical detection mechanism and detection software. The scanning mechanism and the temperature controlled incubation mechanism are connected to the detection software, the scanning mechanism is disposed at the upper end of the reagent card tray transmission mechanism, the rear end of the reagent card tray transmission mechanism is equipped with a sample adding platform, reagent cards are sent into the temperature controlled incubation mechanism through a push-card-into-incubation mechanism and an incubation card push mechanism, and the temperature controlled incubation mechanism is provided with a laser scanning mechanism. By adopting the full-automatic immunofluorescence detection device provided by the invention, the detection process is automatically completed mechanically, the efficiency is improved, tedious manual operation is saved, automatic detection is realized, and the detection time is saved. Therefore, the full-automatic immunofluorescence detection device is convenient for application to emergency rooms, laboratory departments and other public places, community hospitals and other occasions, has high cost performance and strong market competitiveness, and fills the domestic blank.
Owner:RELIA BIOTECH JIANGSU
Method for detecting double-chain DNA resistant antibody using gold magnetic particle as carrier
ActiveCN101165491AImprove coating efficiencyImprove adsorption capacityMicrobiological testing/measurementMaterial analysisAnti-dsDNA antibodiesMicroparticle
The method comprises: 1) the GoldMag nano-particles are mixed with the double strand DNA; under certain condition, the double strand DNA is fixed on the surface of GoldMag nano-particles, or the GoldMag nano-particles are used to extract the DNA from the human blood, saliva and semen and to fix the extracted DNA on the surface of GoldMag nano-particles; 2) using indirect ELISA method, immumofluorescence method or chemiluminescent method to detect the anti double strand DNA antibody existed in the sampler under test.
Owner:XIAN GOLDMAG NANOBIOTECH
Polypeptide specifically combined with HepG2 cell surface
The invention discloses a polypeptide specifically combined with a HepG2 cell surface. According to the invention, four polypeptide segments are selected by utilizing a phage display random dodecapeptide library, the amino acid sequences of the four polypeptide segments are respectively LLADTTHHRPWT, LLADTPHHRPWT, FGWVTPHHELRS and SLSDLTHMGPWP. According to the invention, a polypeptide sequence combined with a liver cancer HepG2 cell is selected by utilizing a phage polypeptide display technology, and ELISA (enzyme-linked immunosorbent assay) identifies the affinity of phage clone and the liver caner cell, thus eight phage clones are obtained; four polypeptide sequences are obtained by sequencing, wherein the common amino acid sequence (basic sequence) is ***D(V)TT(P)HH*P(L)W(R)*; homology analysis indicates that the basic sequence of the polypeptide is possibly amino acid determinant on a ligand protein combined with a tumor cell surface receptor; cell immunofluorescence further identities that the target result of the positive clone of the phage prompts that the positive clone of the phage can be specifically combined with the HepG2 cell; and the selected specific polypeptide of the liver cancer HepG2 cell provides an experiment basis for early diagnosis of liver cancer, targeting delivery of an antitumor medicine and research and development of a targeting short peptide medicine.
Owner:SHAANXI NORMAL UNIV
Pathogen detection method based on micro-fluidic chip
InactiveCN102854304AReduce dosageImprove maneuverabilityBiological testingImmune profilingEngineering
The invention relates to a pathogen detection method based on a micro-fluidic chip. The method adopts a micro-fluidic chip integrated with a micro-magnetic field as a reaction container, adopts magnetic balls as solid phase carriers, and adopts quantum dots modified by streptavidin (SA-QDs) as fluorescent markers; under the action of the micro-magnetic field, magnetic balls are captured at specific parts in a chip channel, and thus a micro-reaction zone is formed; pathogens are captured through a sandwich immunization reaction; with the interaction between biotin and streptavidin, the immunofluorescence quantitative analysis of the pathogens is realized. The detection method combines the micro-fluidic chip, magnetic immunoassay, and fluorescence detection, has the advantages of rapidness, high efficiency, and few sample using amount for the micro-fluidic chip, high specificity and strong manoeuvrability for magnetic immunoassay, and excellent optical properties for quantum dots, and is a multi-objective real-time pathogen detection method.
Owner:WUHAN UNIV
Improved method for dyeing immunofluorescence cell
InactiveCN101329230AReduce usageReduce stainsPreparing sample for investigationImmunofluorescenceStaining
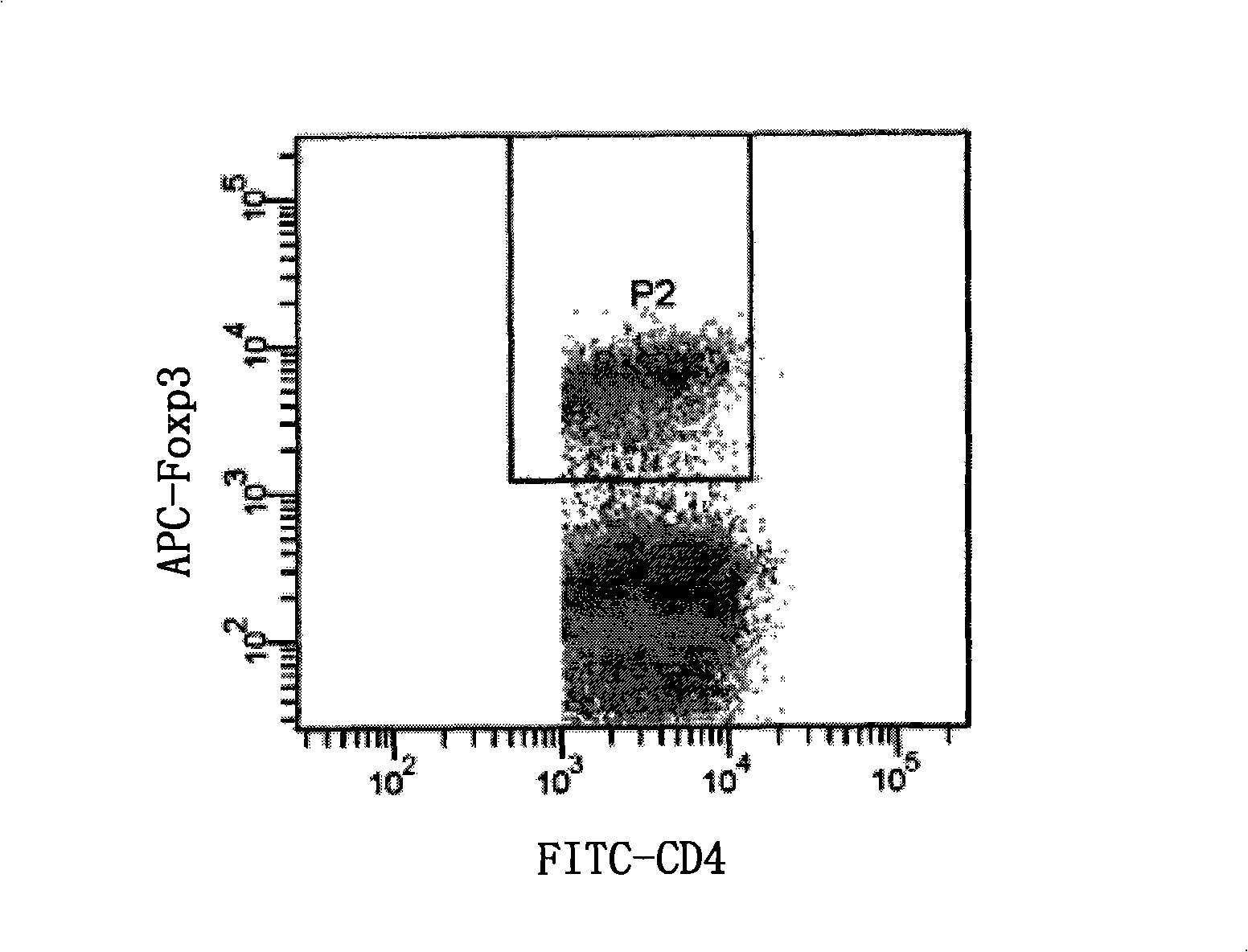
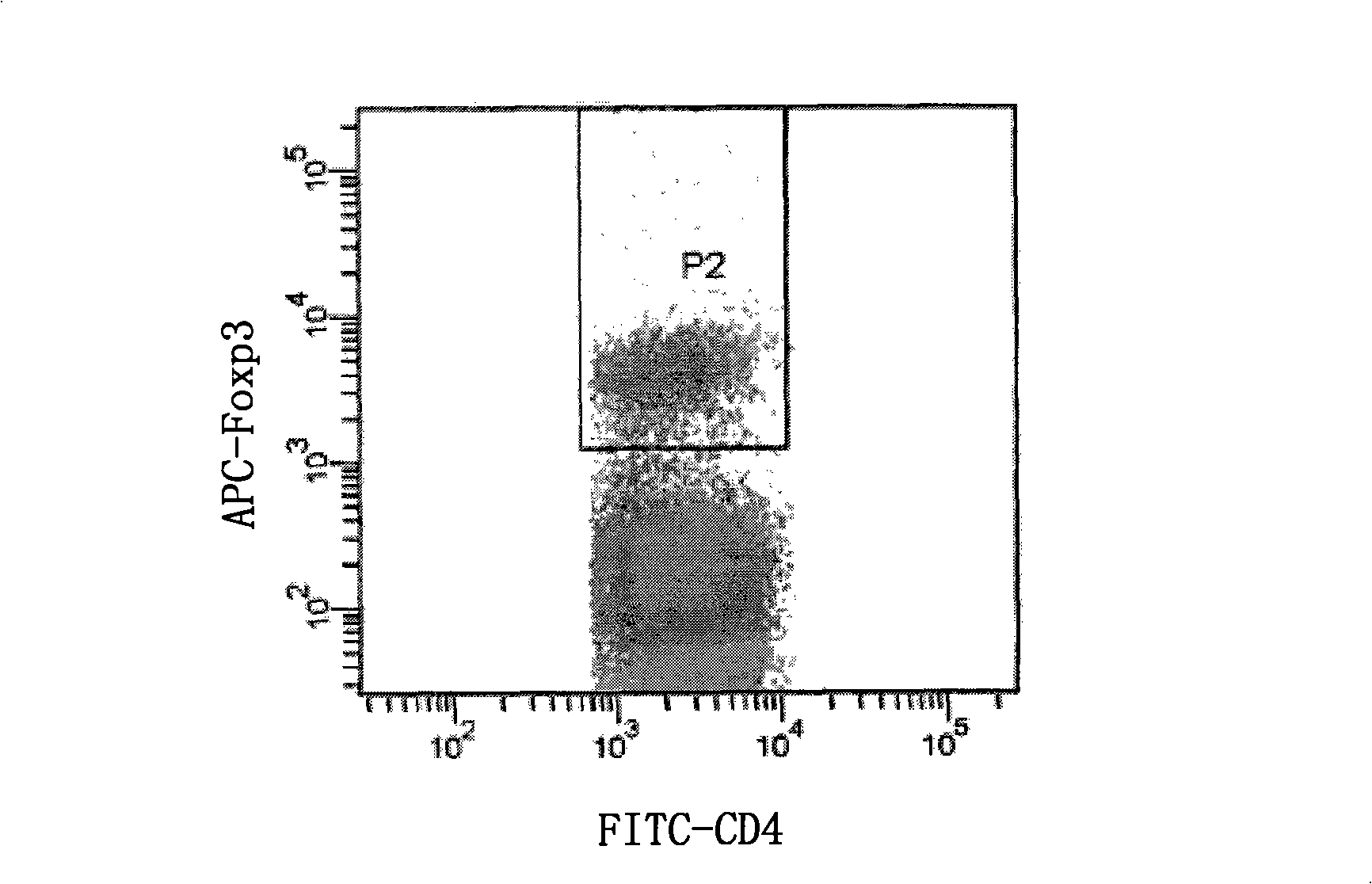
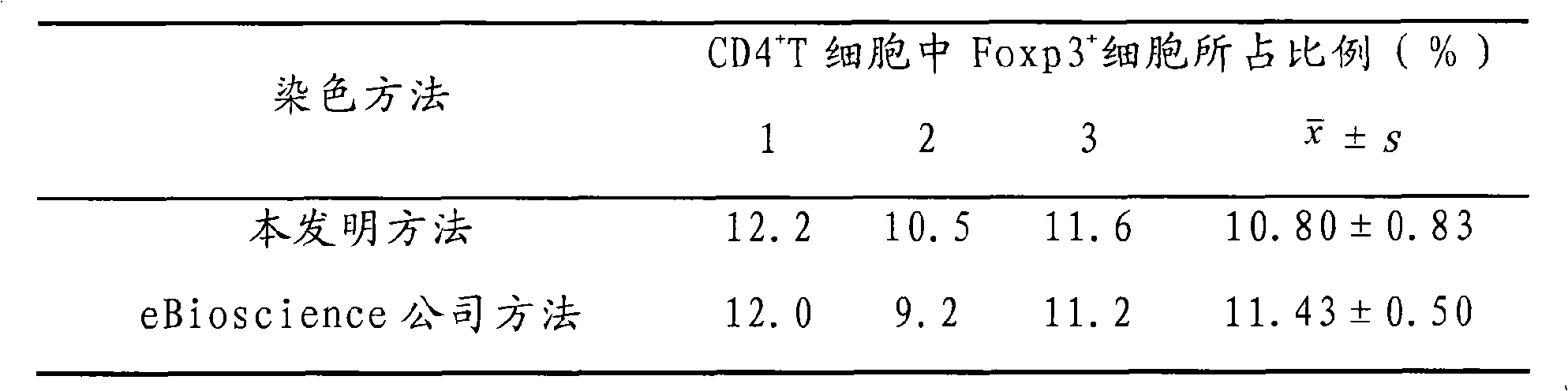
The invention discloses an improved immune fluorescyte staining method, comprising 5 steps: fixing / permeating, permeating, surface staining, staining in nucleus, washing and weight dropping; the method of the invention is an improvement to the Foxp3 immune fluorescyte staining method of eBioscience Company, changes a two-step multiple staining method into a one-step multiple staining method, and carries out the staining on the surface of the cell and in the nucleus simultaneously, thus having the advantages of simple and convenient operation, quick staining, saving reagent, and reducing the cost, etc. The research shows that the method of the invention has no remarkable difference with the method of that of the eBioscience Company, can be used for the staining on the surface of the cell and in the nucleus simultaneously and has extremely good application prospect and promotional value.
Owner:ARMY MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com