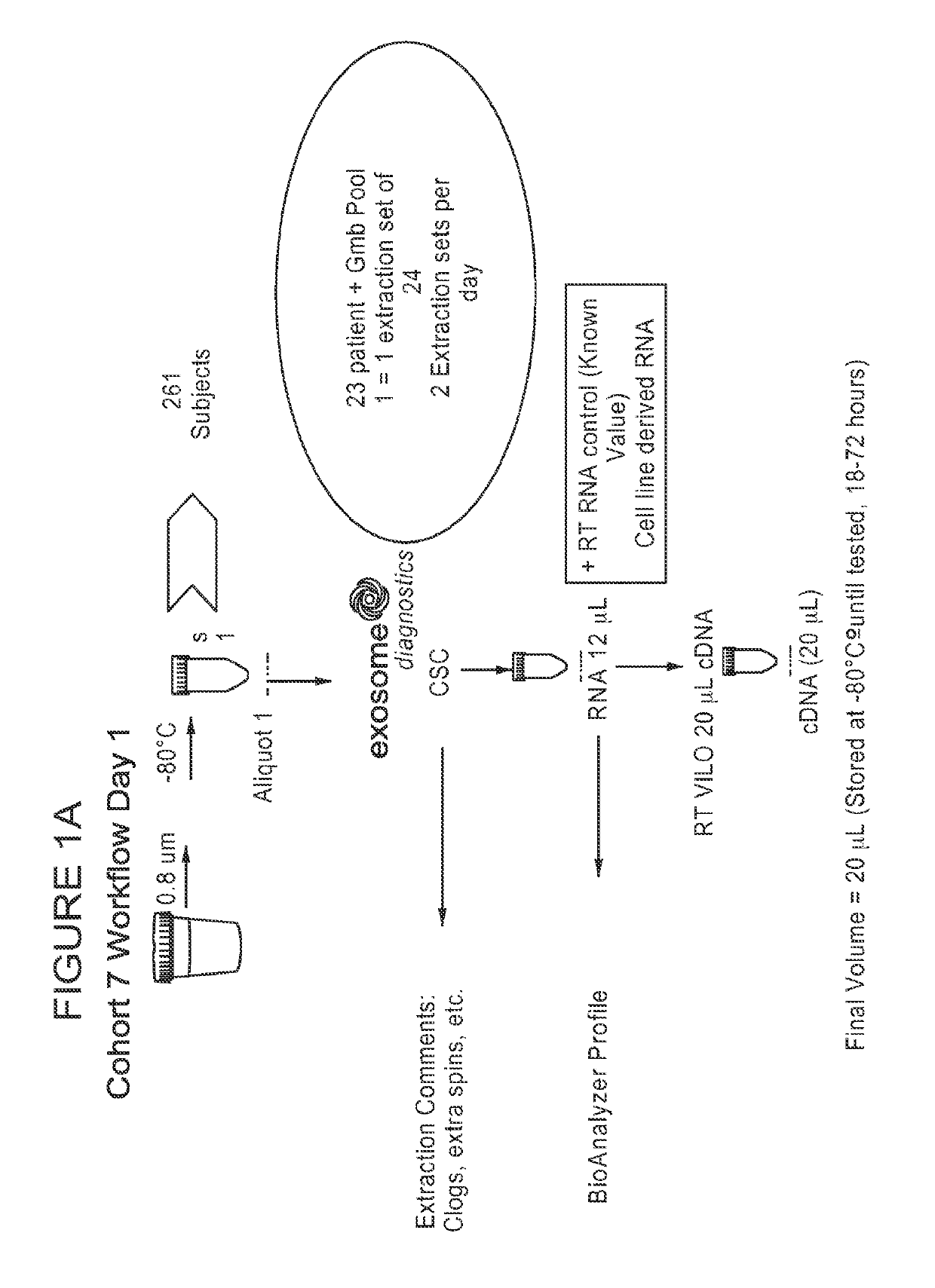
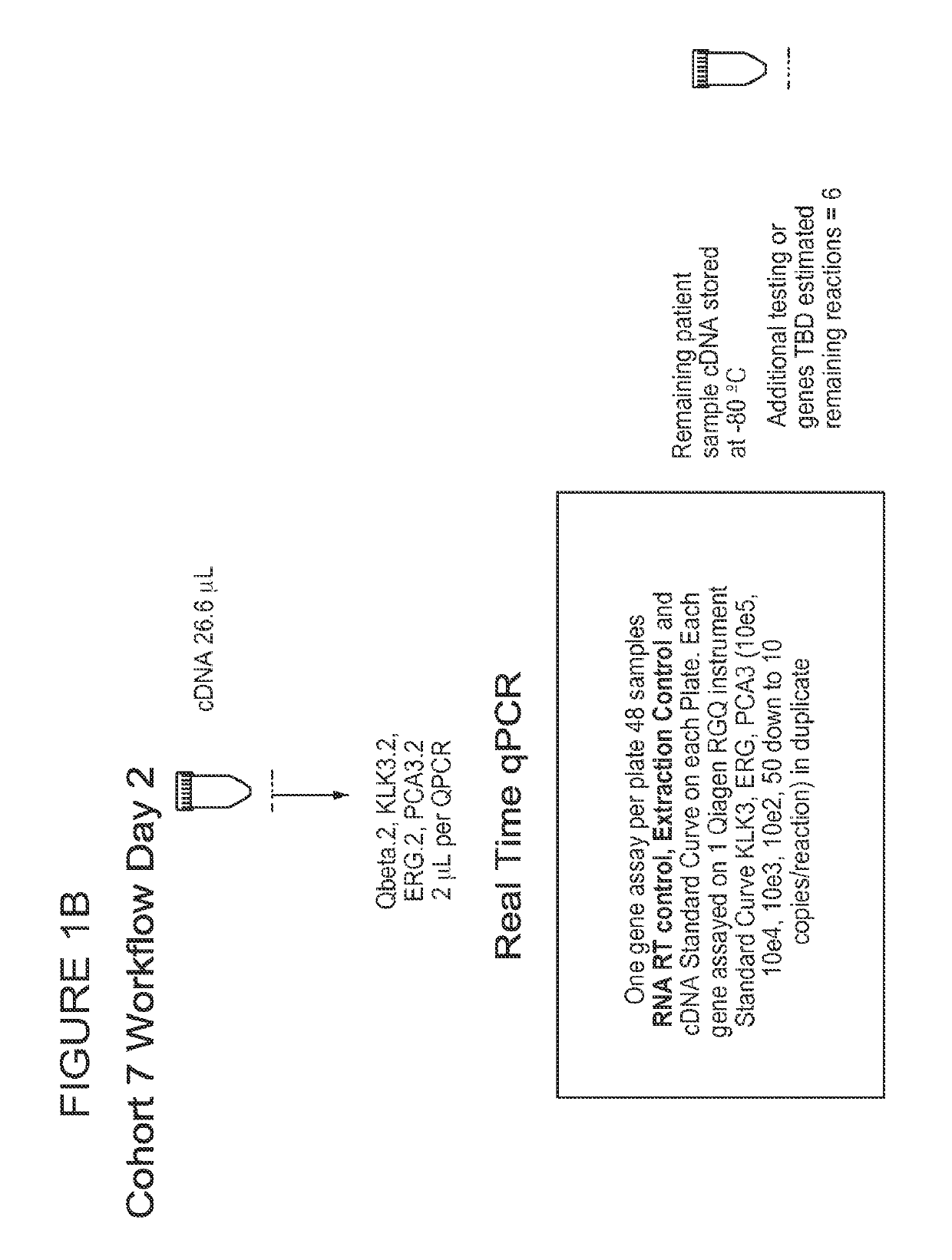
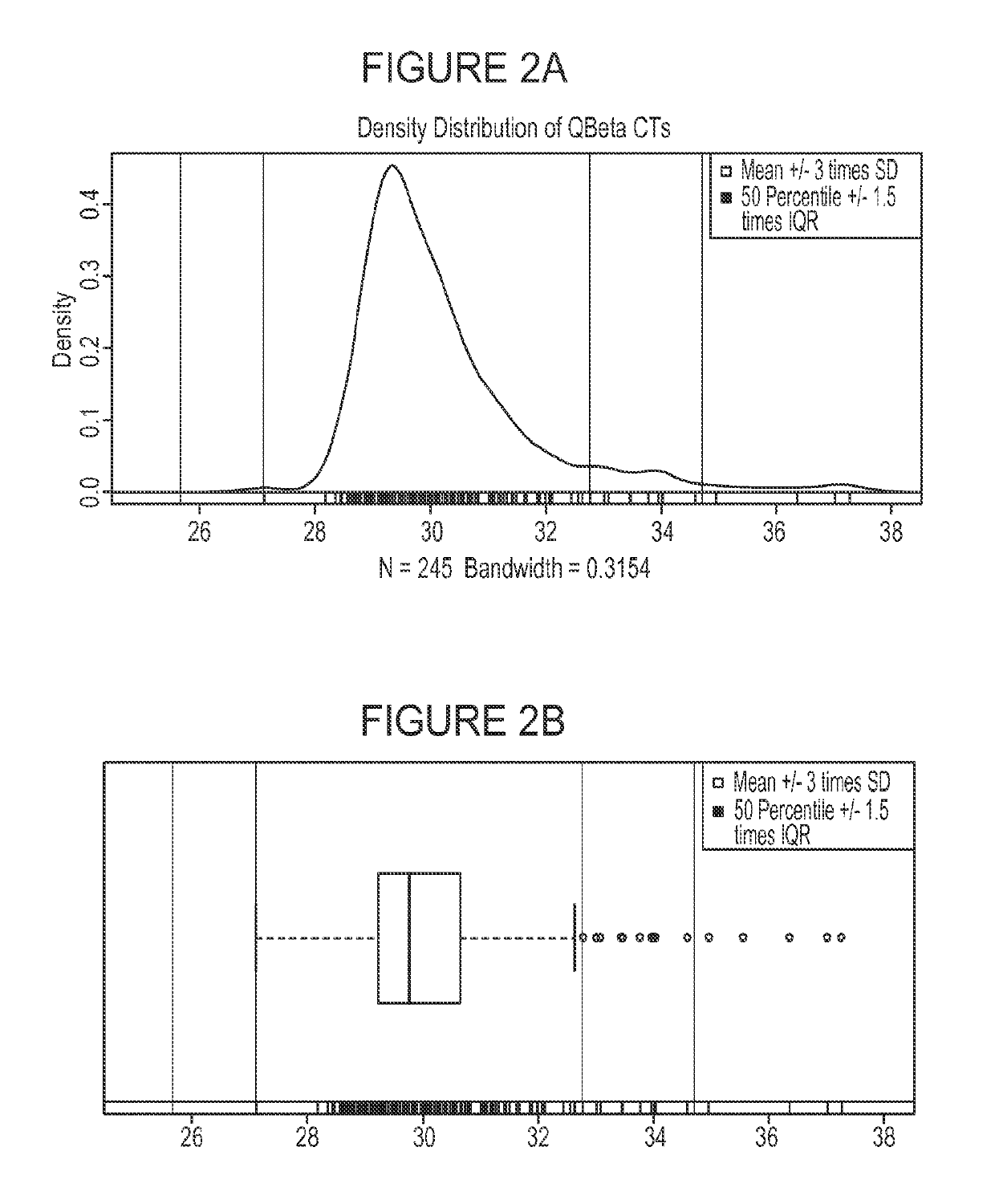
Patents
Literature
31 results about "Gleason scores" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
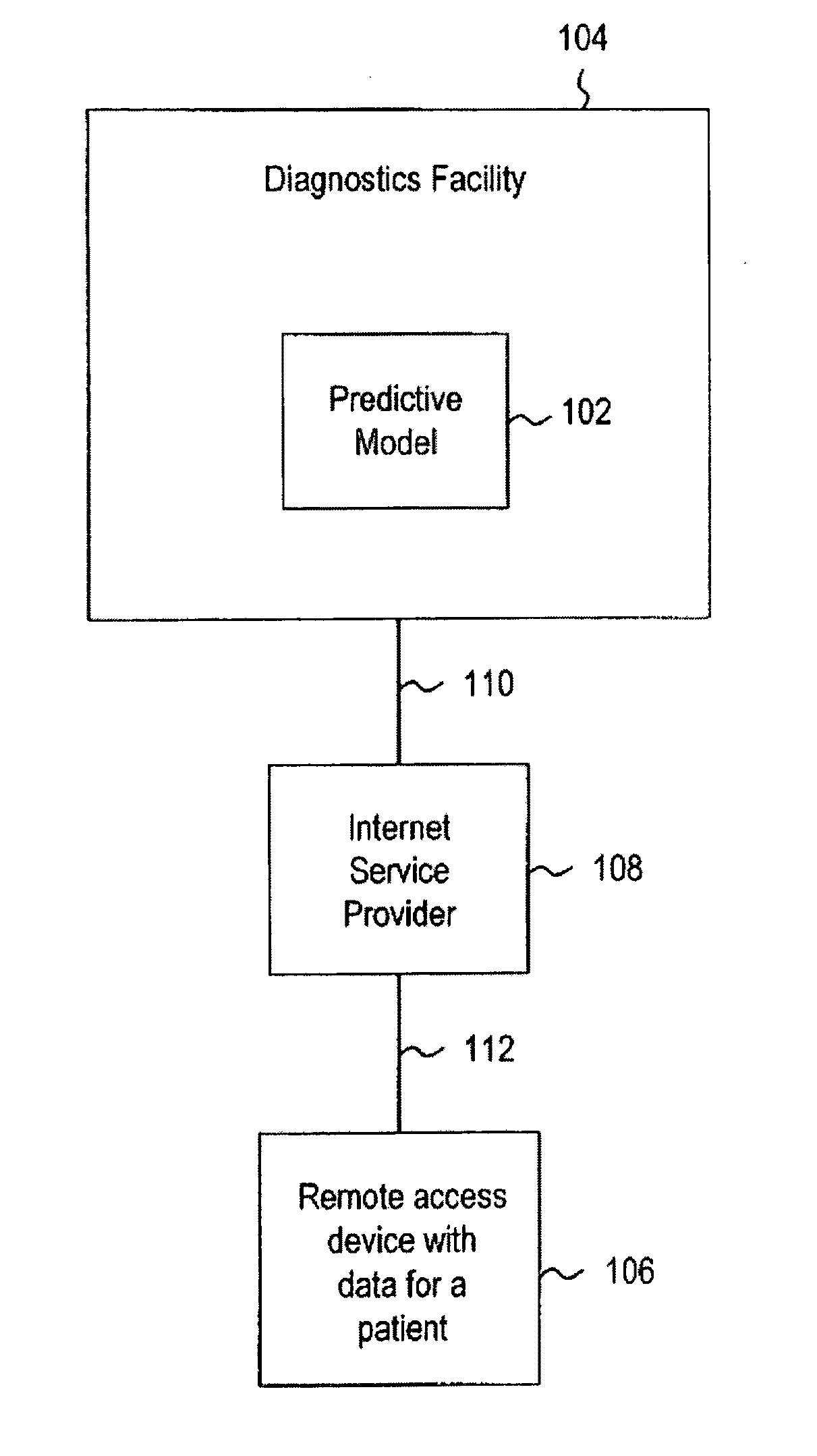
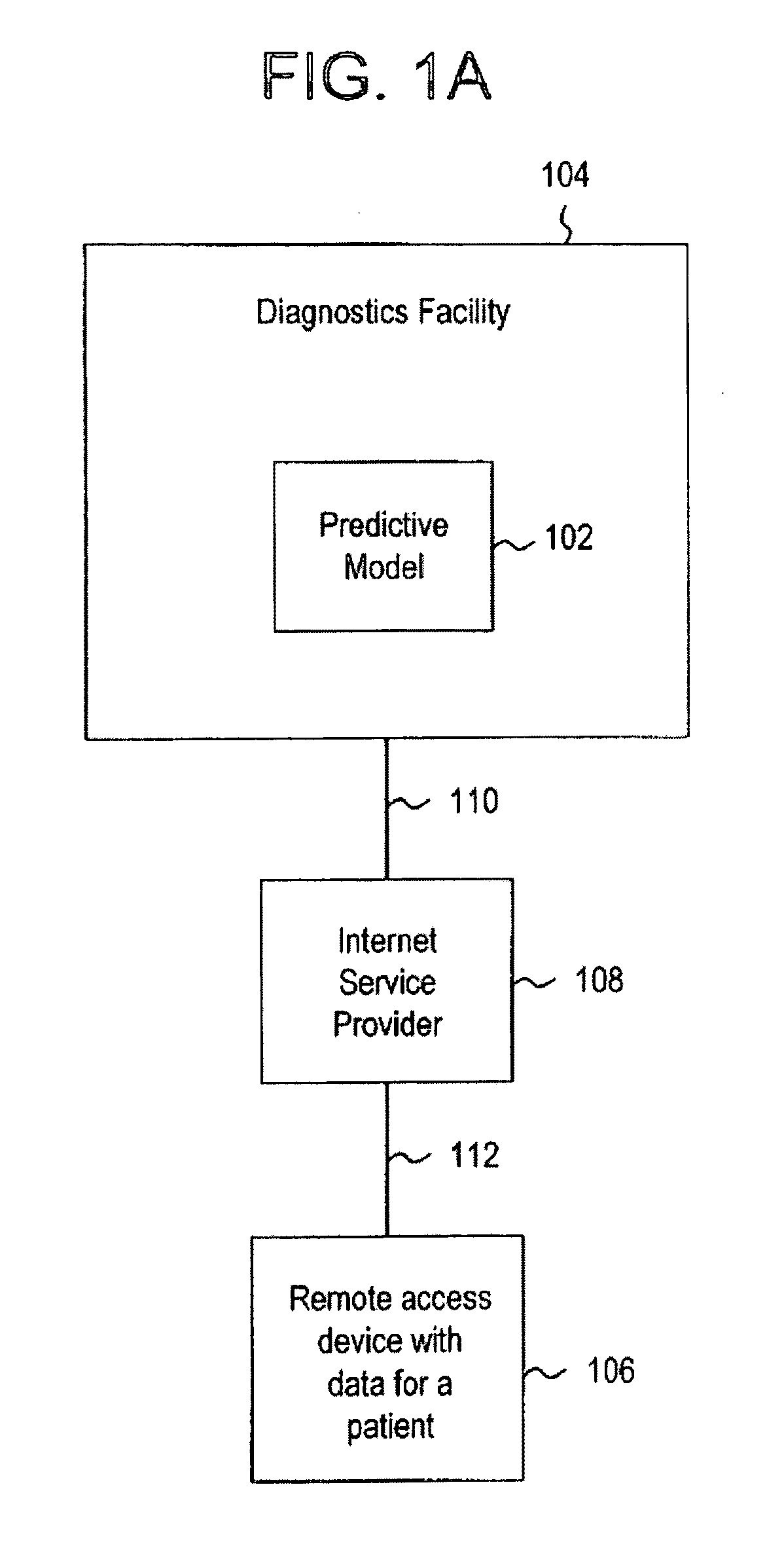
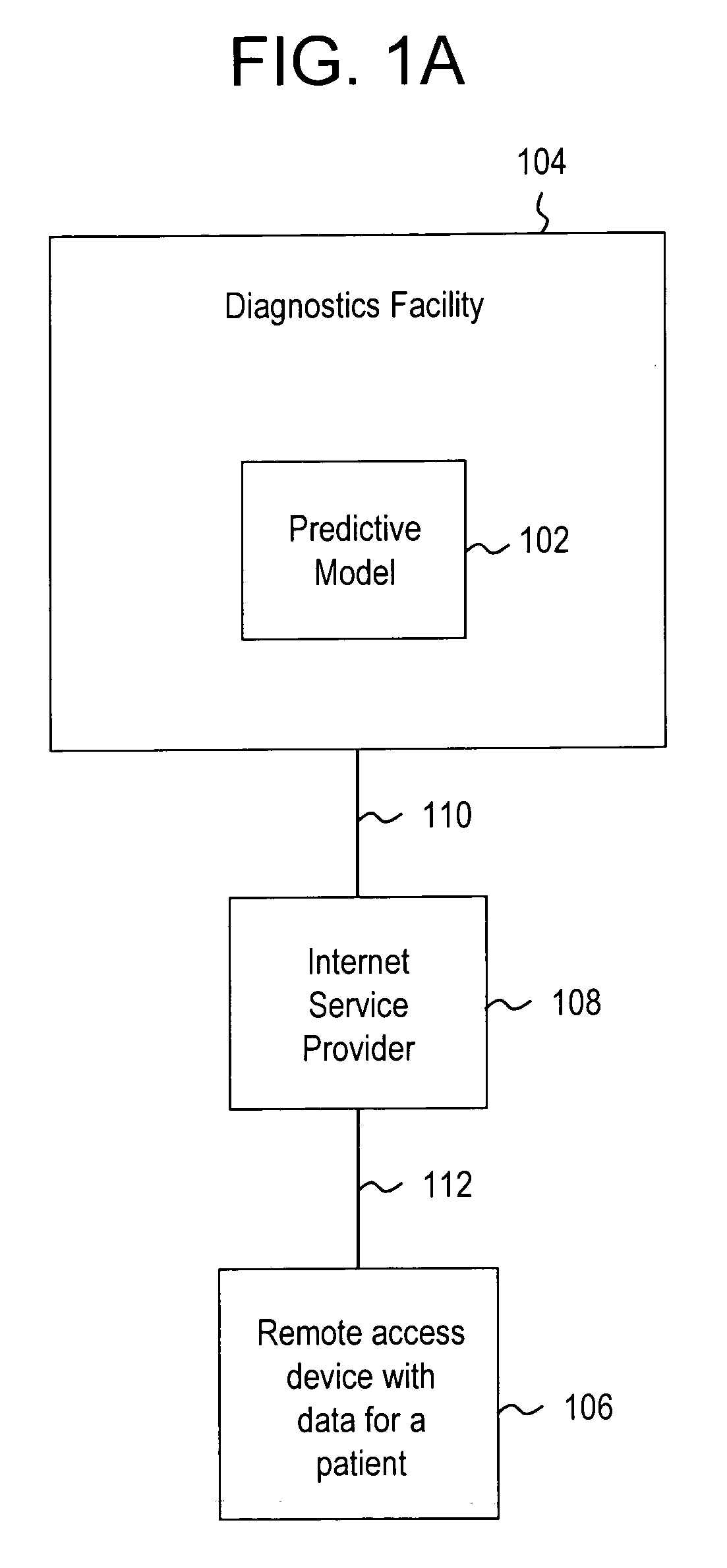
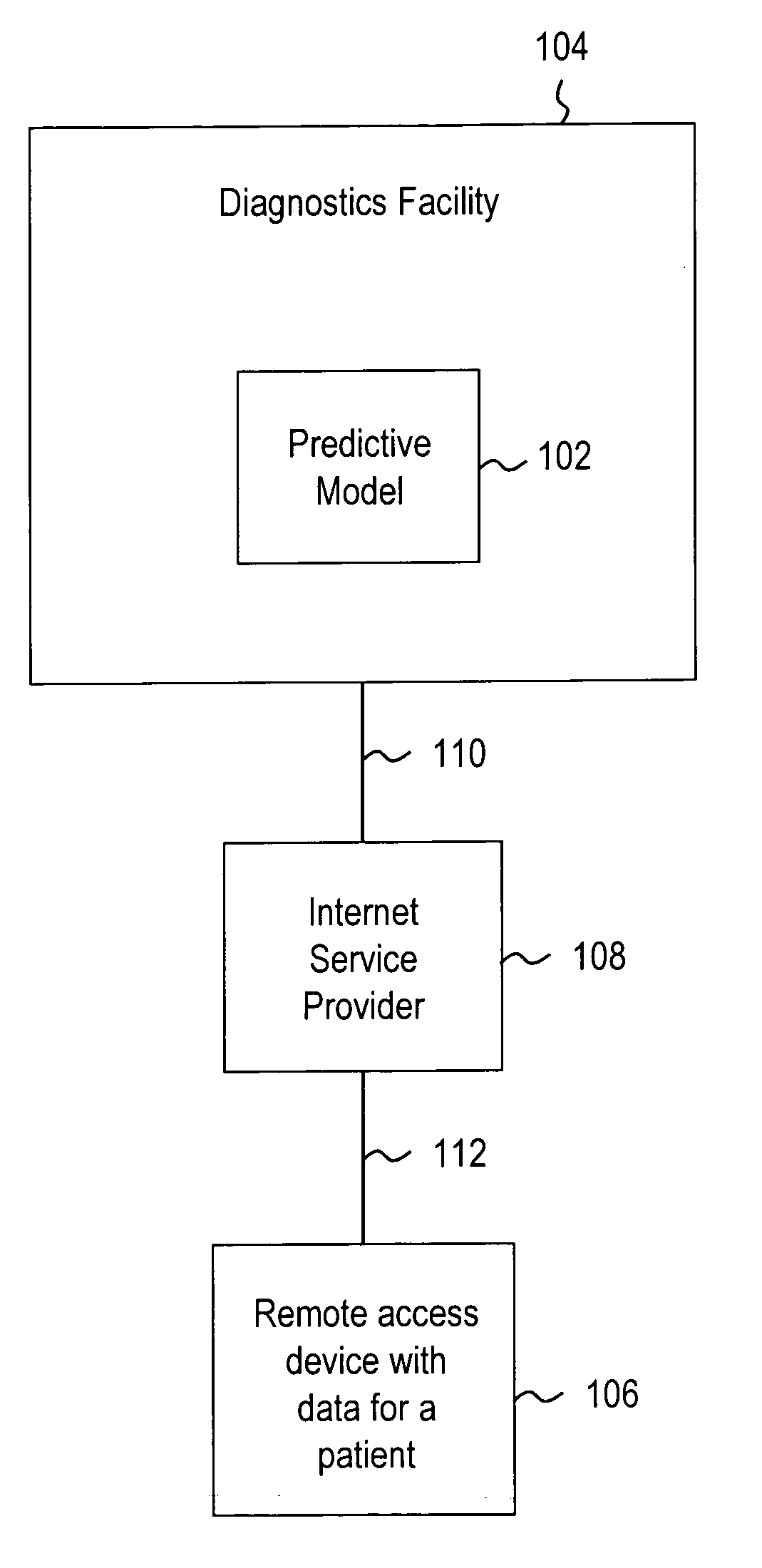
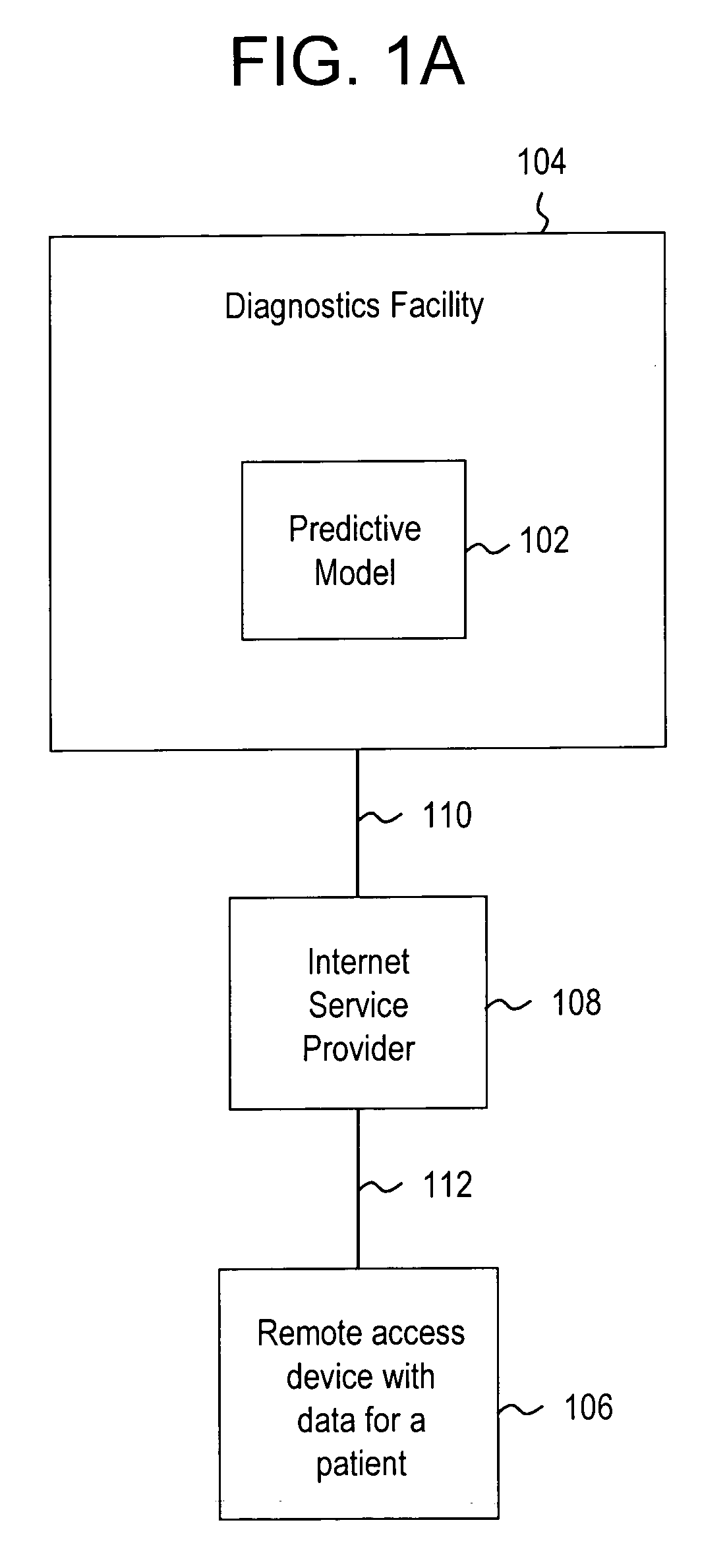
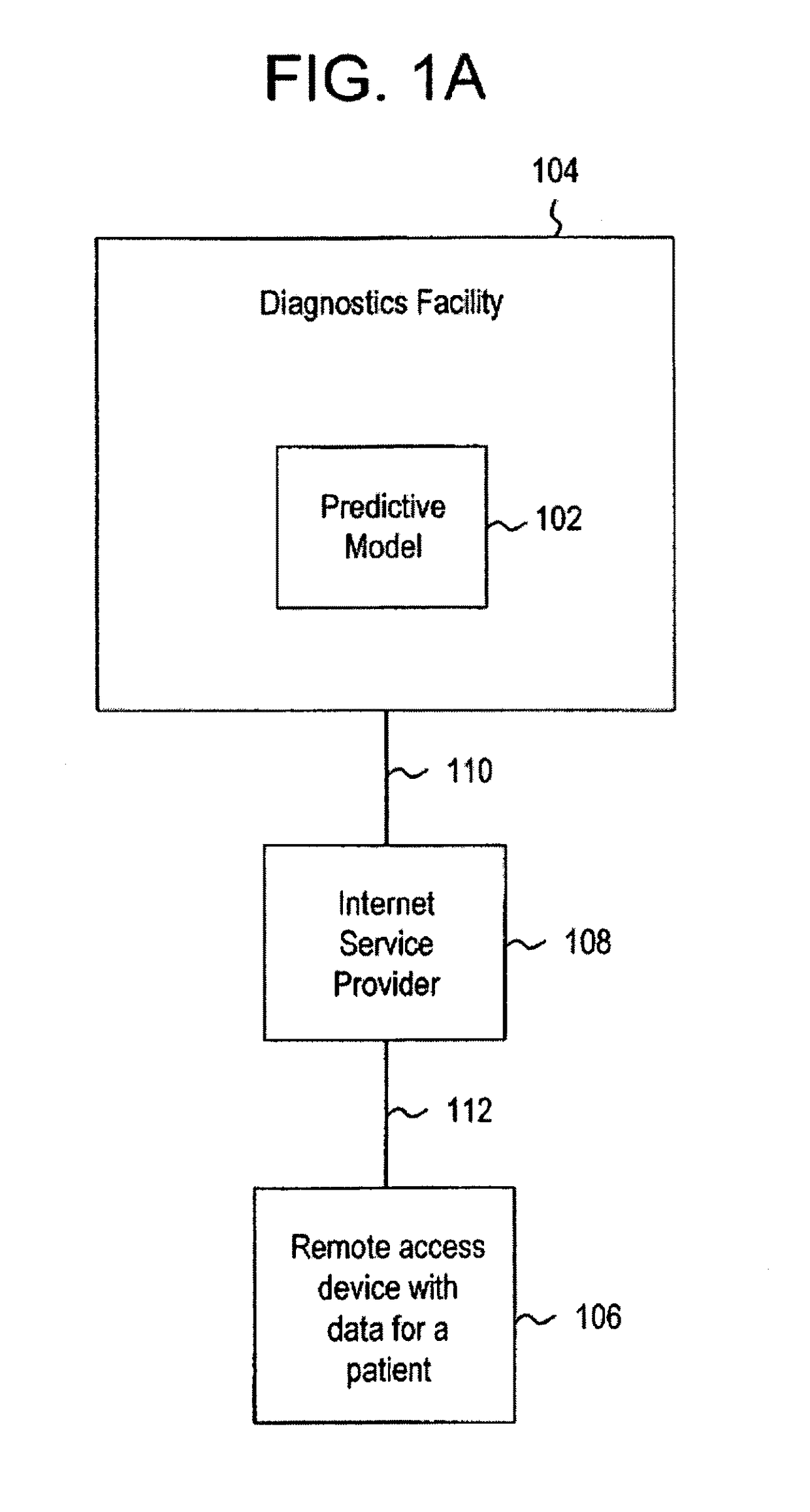
Systems and methods for treating, diagnosing and predicting the occurrence of a medical condition
ActiveUS20100184093A1Reliable and accurate image segmentationMedical simulationBioreactor/fermenter combinationsImmunofluorescenceClinical information
Clinical information, molecular information and / or computer-generated morphometric information is used in a predictive model for predicting the occurrence of a medical condition. In an embodiment, a model predicts whether a patient is likely to have a favorable pathological stage of prostate cancer, where the model is based on features including one or more (e.g., all) of preoperative PSA, Gleason Score, a measurement of expression of androgen receptor (AR) in epithelial and stromal nuclei and / or a measurement of expression of Ki67-positive epithelial nuclei, a morphometric measurement of a ratio of area of epithelial nuclei outside gland units to area of epithelial nuclei within gland units, and a morphometric measurement of area of epithelial nuclei distributed away from gland units. In some embodiments, quantitative measurements of protein expression in cell lines are utilized to objectively assess assay (e.g., multiplex immunofluorescence (IF)) performance and / or to normalize features for use within a predictive model.
Owner:AUREON LAB INC +1
Systems and methods for treating, diagnosing and predicting the occurence of a medical condition
InactiveUS20070099219A1Medical simulationMicrobiological testing/measurementAbnormal tissue growthBiopsy
Methods and systems are provided that use clinical information, molecular information and computer-generated morphometric information in a predictive model for predicting the occurrence (e.g., recurrence) of a medical condition, for example, cancer. In an embodiment, a model that predicts prostate cancer recurrence is provided, where the model is based on features including seminal vesicle involvement, surgical margin involvement, lymph node status, androgen receptor (AR) staining index of tumor, a morphometric measurement of epithelial nuclei, and at least one morphometric measurement of stroma. In another embodiment, a model that predicts clinical failure post prostatectomy is provided, wherein the model is based on features including biopsy Gleason score, lymph node involvement, prostatectomy Gleason score, a morphometric measurement of epithelial cytoplasm, a morphometric measurement of epithelial nuclei, a morphometric measurement of stroma, and intensity of androgen receptor (AR) in racemase (AMACR)-positive epithelial cells.
Owner:AUREON LAB INC +2
Systems and methods for treating diagnosing and predicting the occurrence of a medical condition
InactiveUS20100088264A1Medical automated diagnosisKnowledge representationGleason gradeComputer generation
Methods and systems are provided that use clinical information, molecular information and computer-generated morphometric information in a predictive model for predicting the occurrence (e.g., recurrence) of a medical condition, for example, cancer. In an embodiment, a model that predicts prostate cancer recurrence is provided, where the model is based on features including one or more (e.g., all) of biopsy Gleason score, seminal vesicle invasion, extracapsular extension, preoperative PSA, dominant prostatectomy Gleason grade, the relative area of AR+ epithelial nuclei, a morphometric measurement of epithelial nuclei, and a morphometric measurement of epithelial cytoplasm. In another embodiment, a model that predicts clinical failure post-prostatectomy is provided, wherein the model is based on features including one or more (e.g., all) of dominant prostatectomy Gleason grade, lymph node invasion status, one or more morphometric measurements of lumen, a morphometric measurement of cytoplasm, and average intensity of AR in AR+ / AMACR− epithelial nuclei.
Owner:AUREON LAB INC +1
Systems and methods for treating, diagnosing and predicting the occurrence of a medical condition
InactiveCN101689220AMedical automated diagnosisSpecial data processing applicationsGleason gradeBiopsy
Methods and systems are provided that use clinical information, molecular information and computer-generated morphometric information in a predictive model for predicting the occurrence (e.g., recurrence) of a medical condition, for example, cancer. In an embodiment, a model that predicts prostate cancer recurrence is provided, where the model is based on features including one or more (e.g., all)of biopsy Gleason score, seminal vesicle invasion, extracapsular extension, preoperative PSA, dominant prostatectomy Gleason grade, the relative area of AR+ epithelial nuclei, a morphometric measurement of epithelial nuclei, and a morphometric measurement of epithelial cytoplasm. In another embodiment, a model that predicts clinical failure post-prostatectomy is provided, wherein the model is based on features including one or more (e.g., all) of dominant prostatectomy Gleason grade, lymph node invasion status, one or more morphometric measurements of lumen, a morphometric measurement of cytoplasm, and average intensity of AR in AR+ / AMACR- epithelial nuclei.
Owner:AUREON LAB INC
Systems and methods for treating, diagnosing and predicting the occurrence of a medical condition
Methods and systems are provided that use clinical information, molecular information and computer-generated morphometric information in a predictive model for predicting the occurrence (e.g., recurrence) of a medical condition, for example, cancer. In an embodiment, a model that predicts prostate cancer recurrence is provided, where the model is based on features including seminal vesicle involvement, surgical margin involvement, lymph node status, androgen receptor (AR) staining index of tumor, a morphometric measurement of epithelial nuclei, and at least one morphometric measurement of stroma. In another embodiment, a model that predicts clinical failure post prostatectomy is provided, wherein the model is based on features including biopsy Gleason score, lymph node involvement, prostatectomy Gleason score, a morphometric measurement of epithelial cytoplasm, a morphometric measurement of epithelial nuclei, a morphometric measurement of stroma, and intensity of androgen receptor (AR) in racemase (AMACR)-positive epithelial cells.
Owner:AUREON LAB INC +2
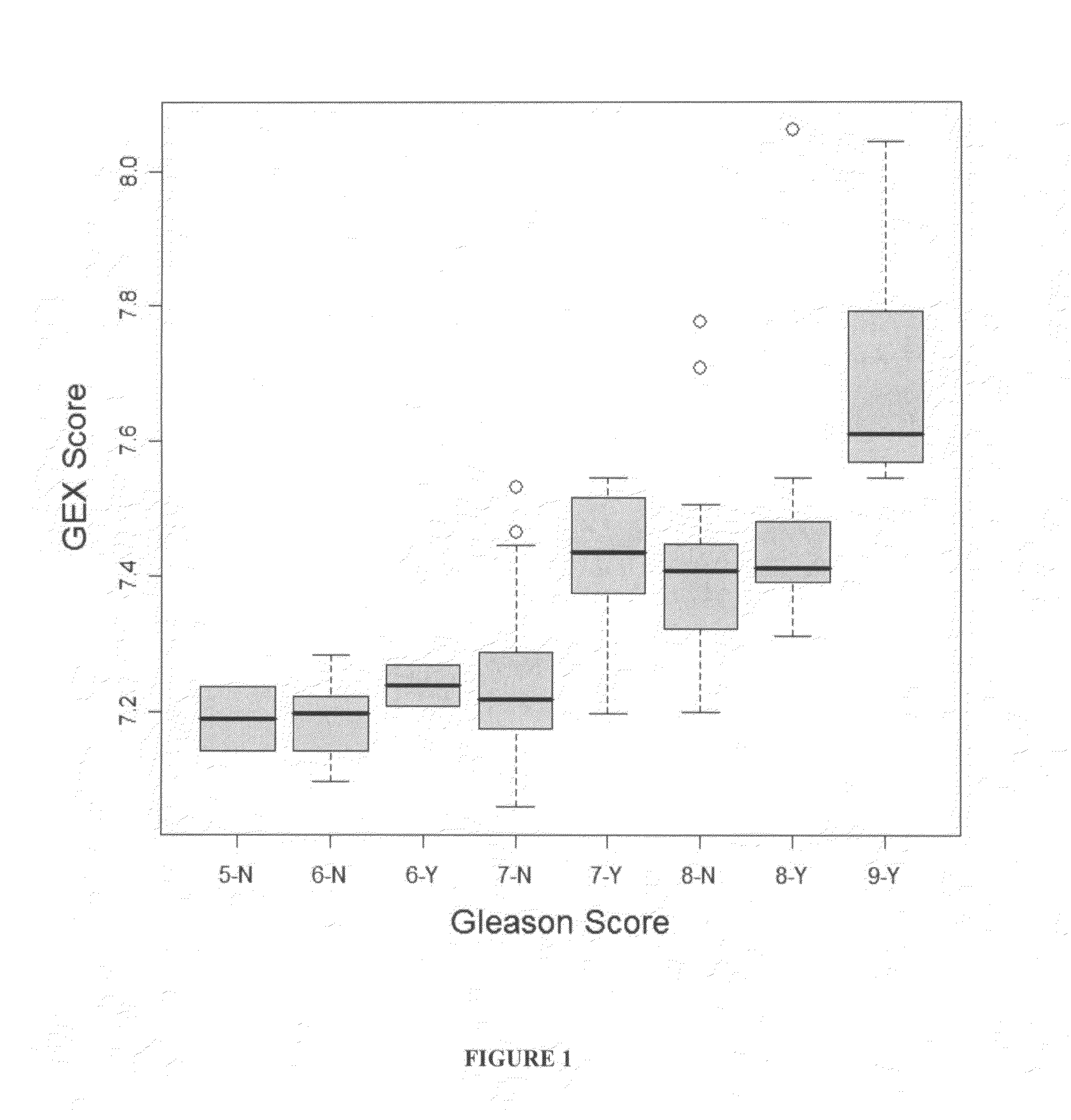
Gene expression profiles to predict relapse of prostate cancer
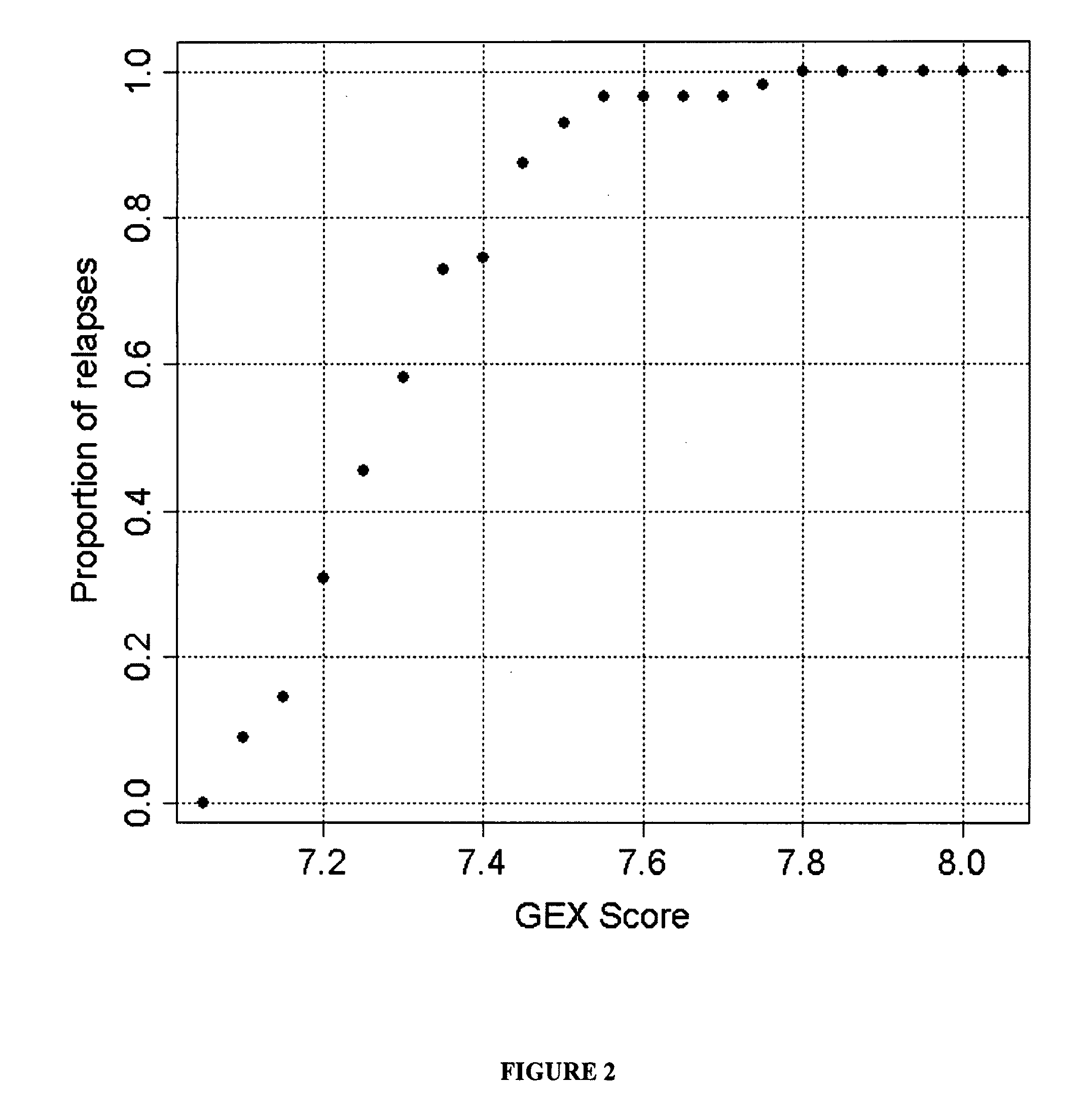
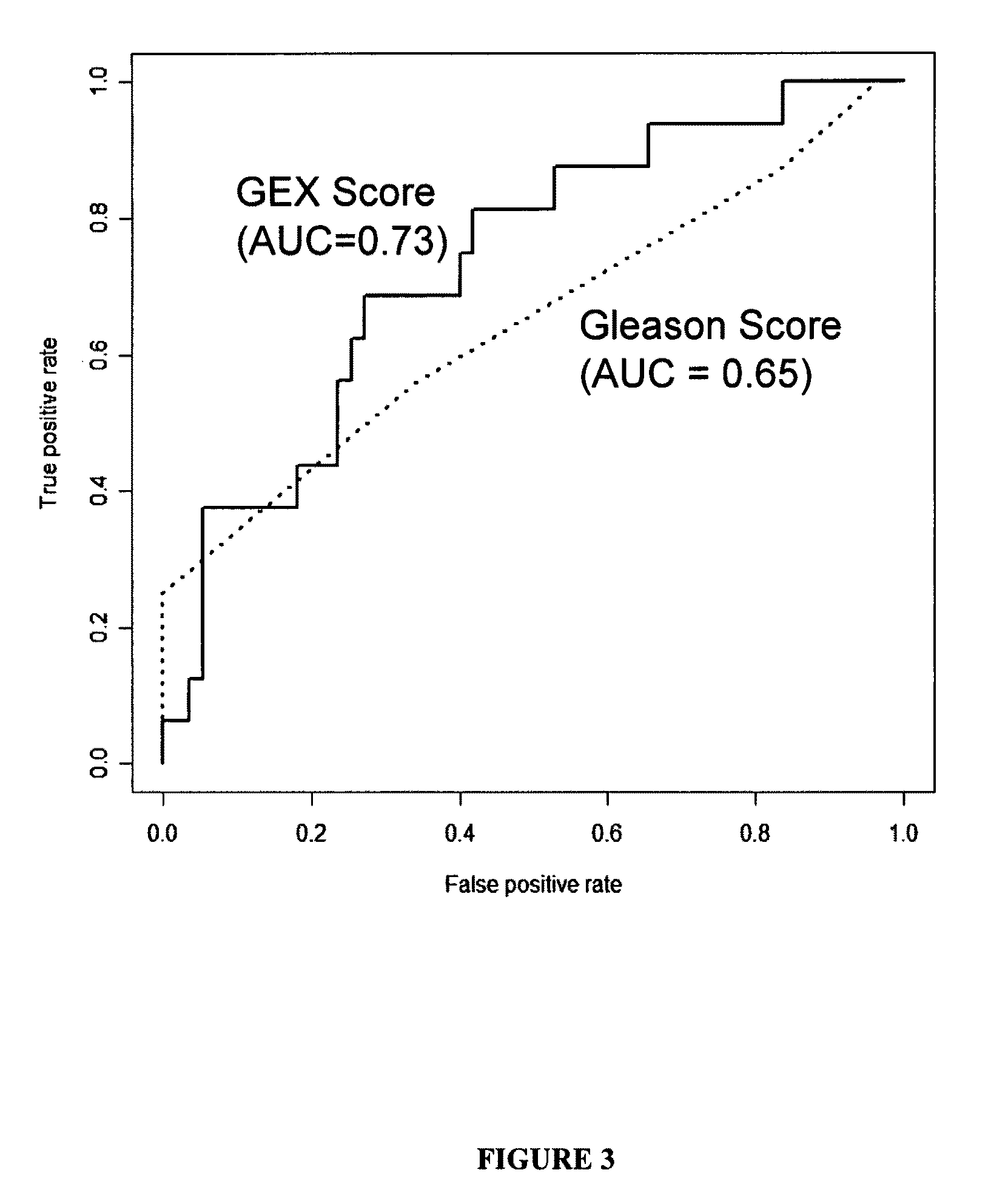
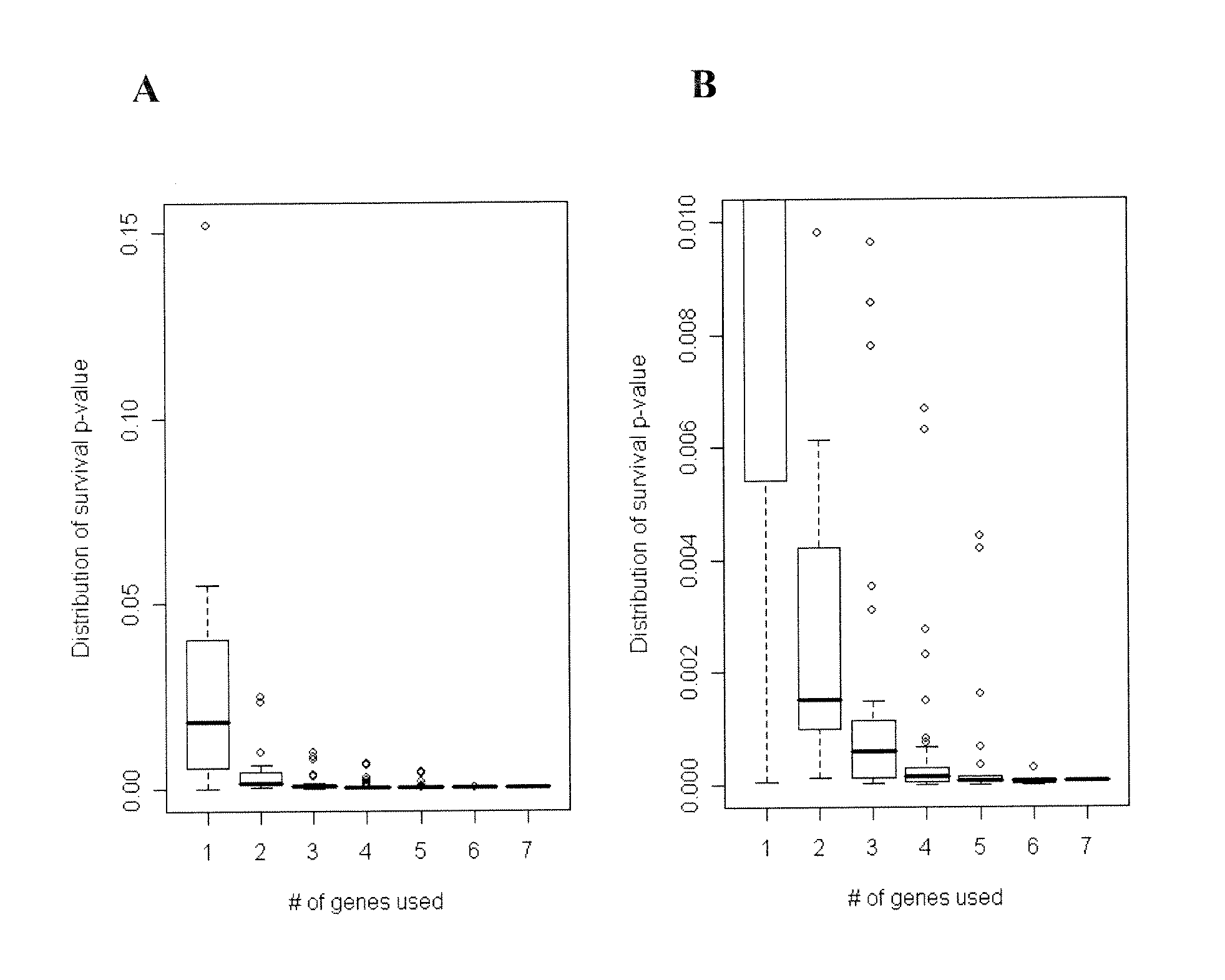
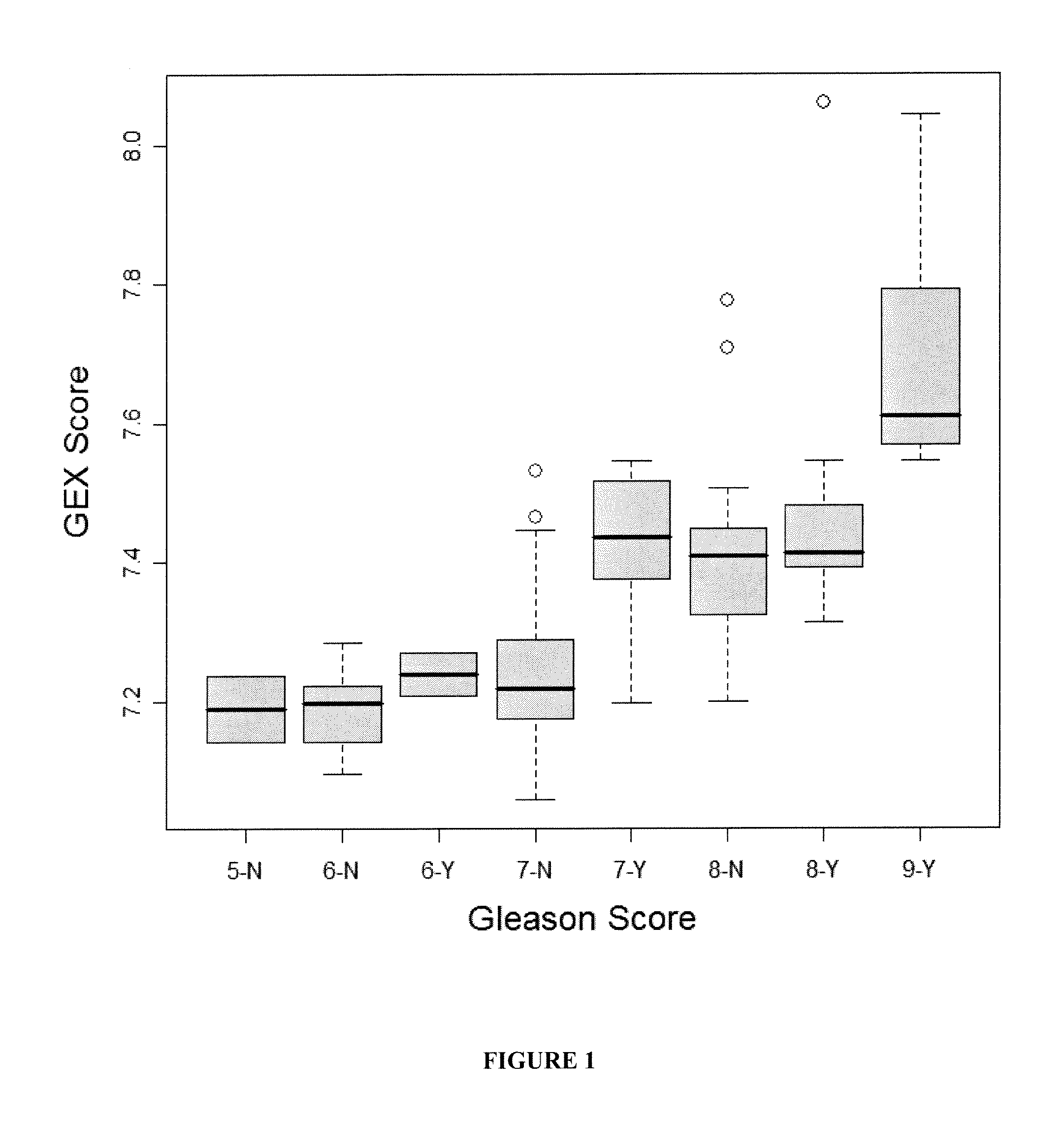
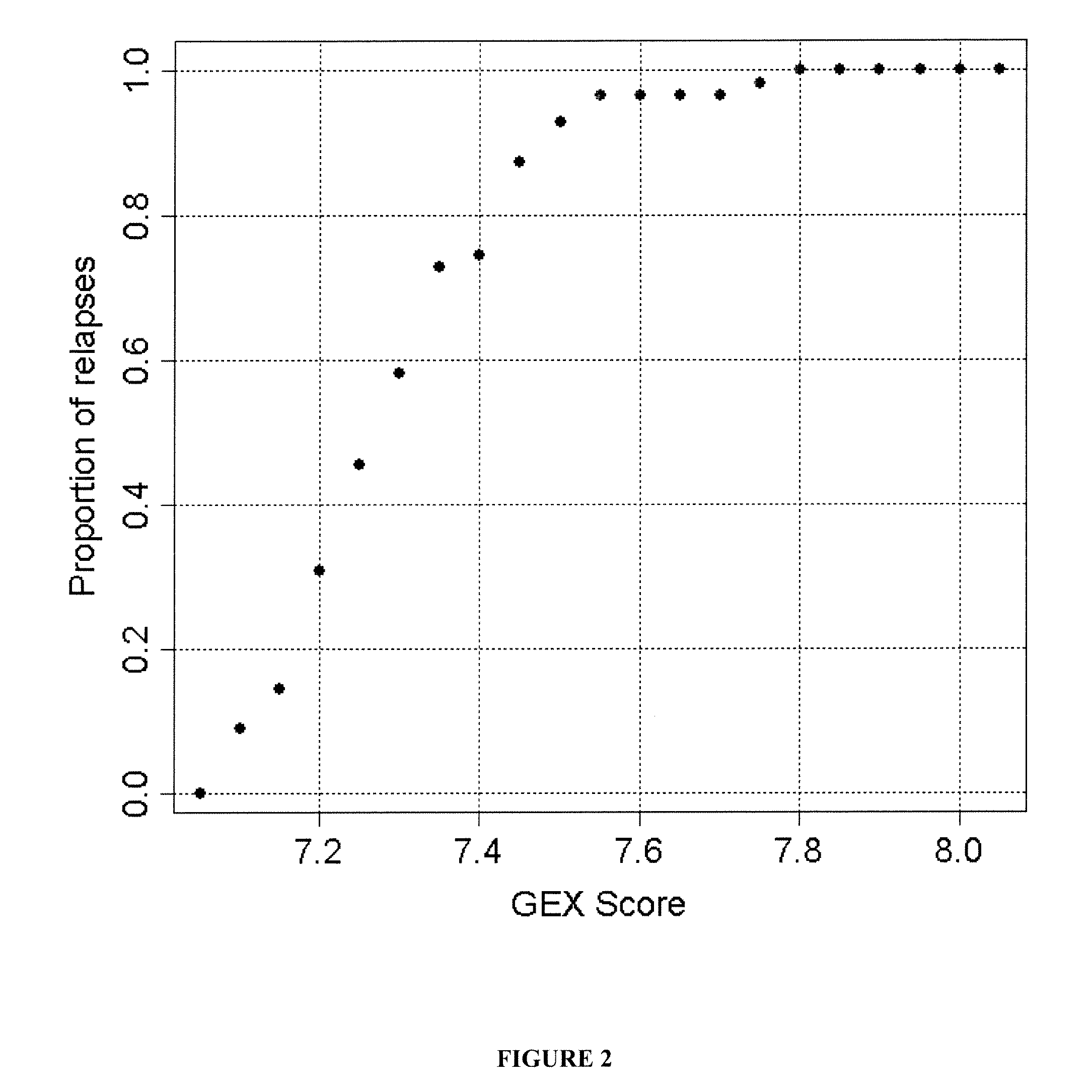
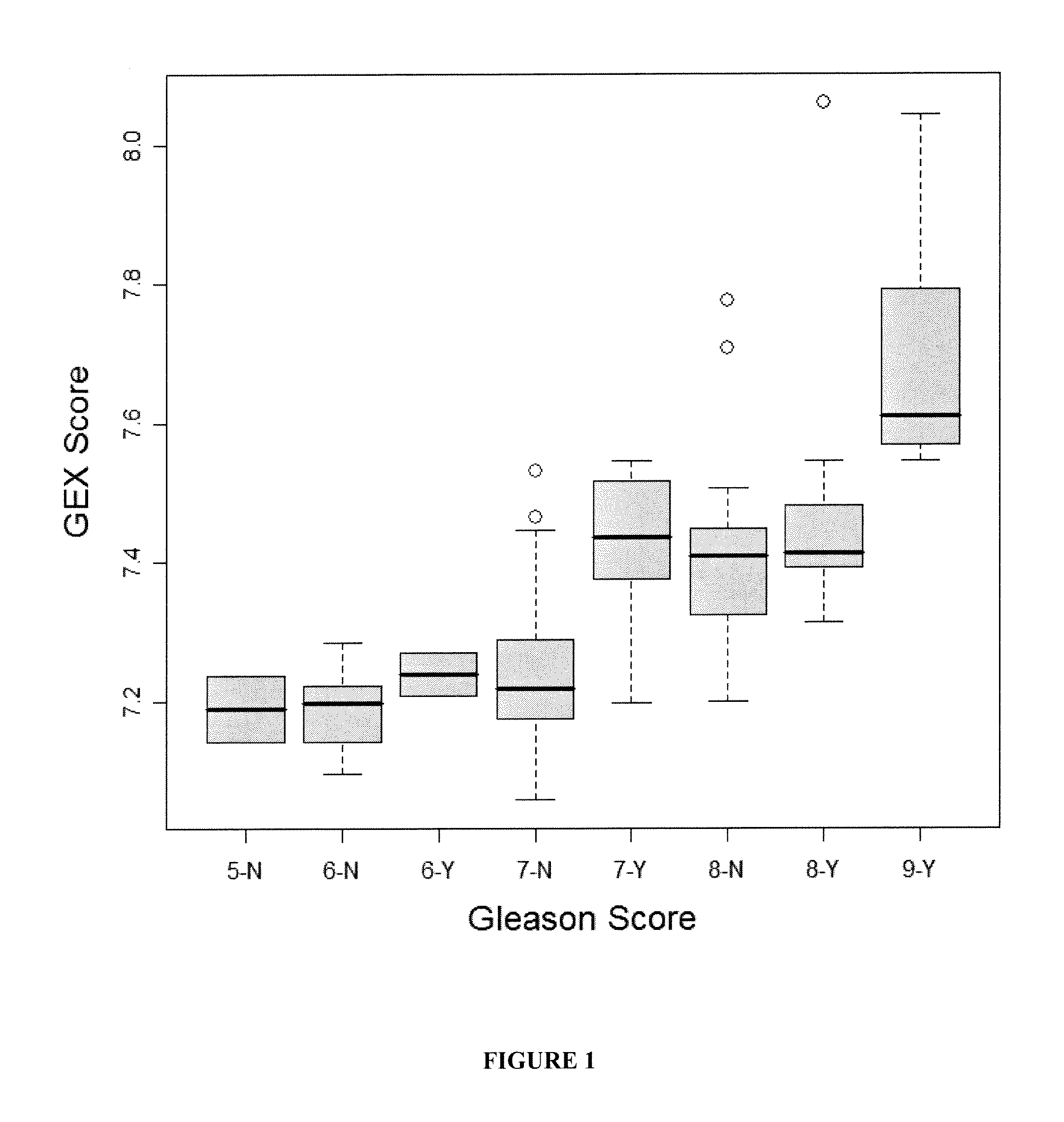
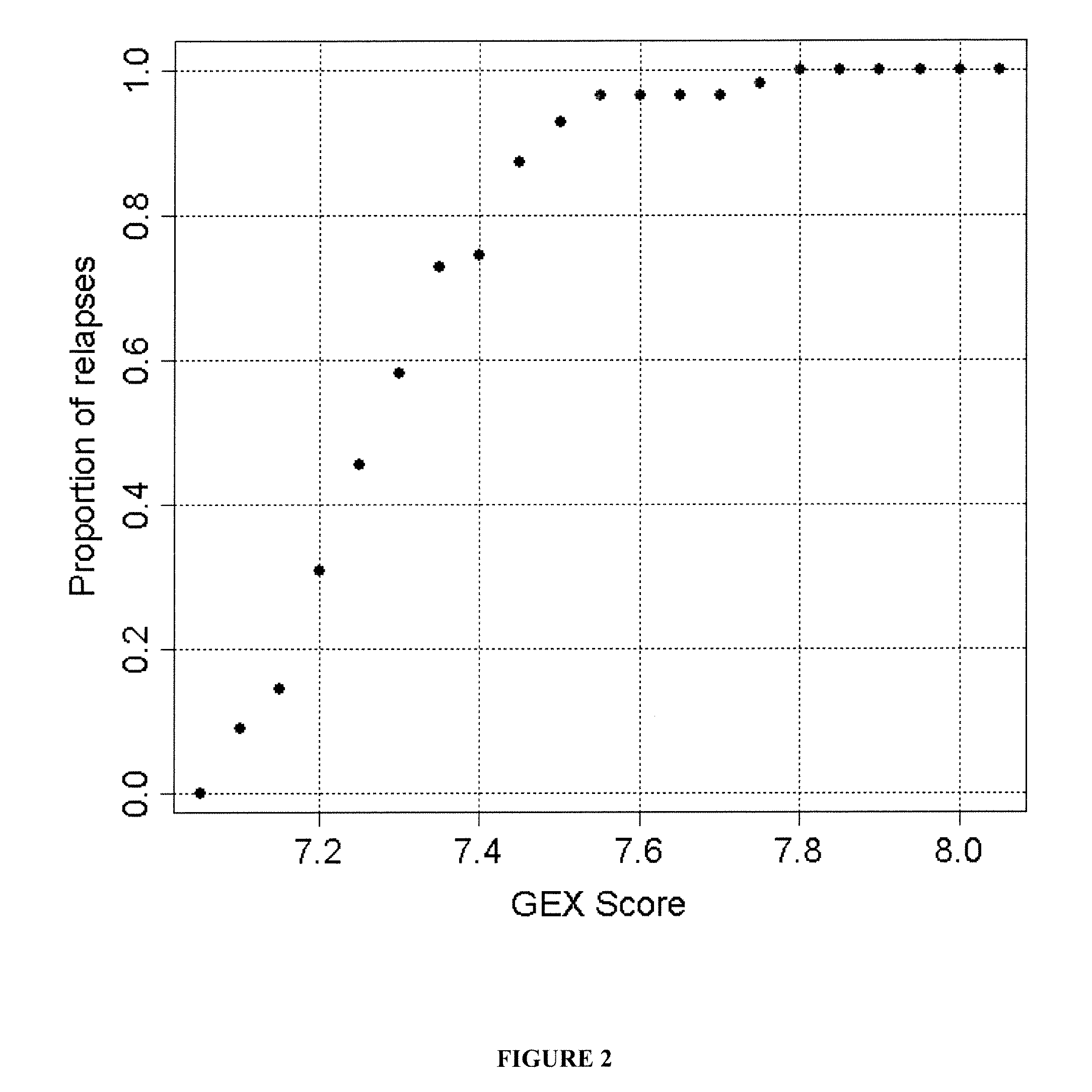
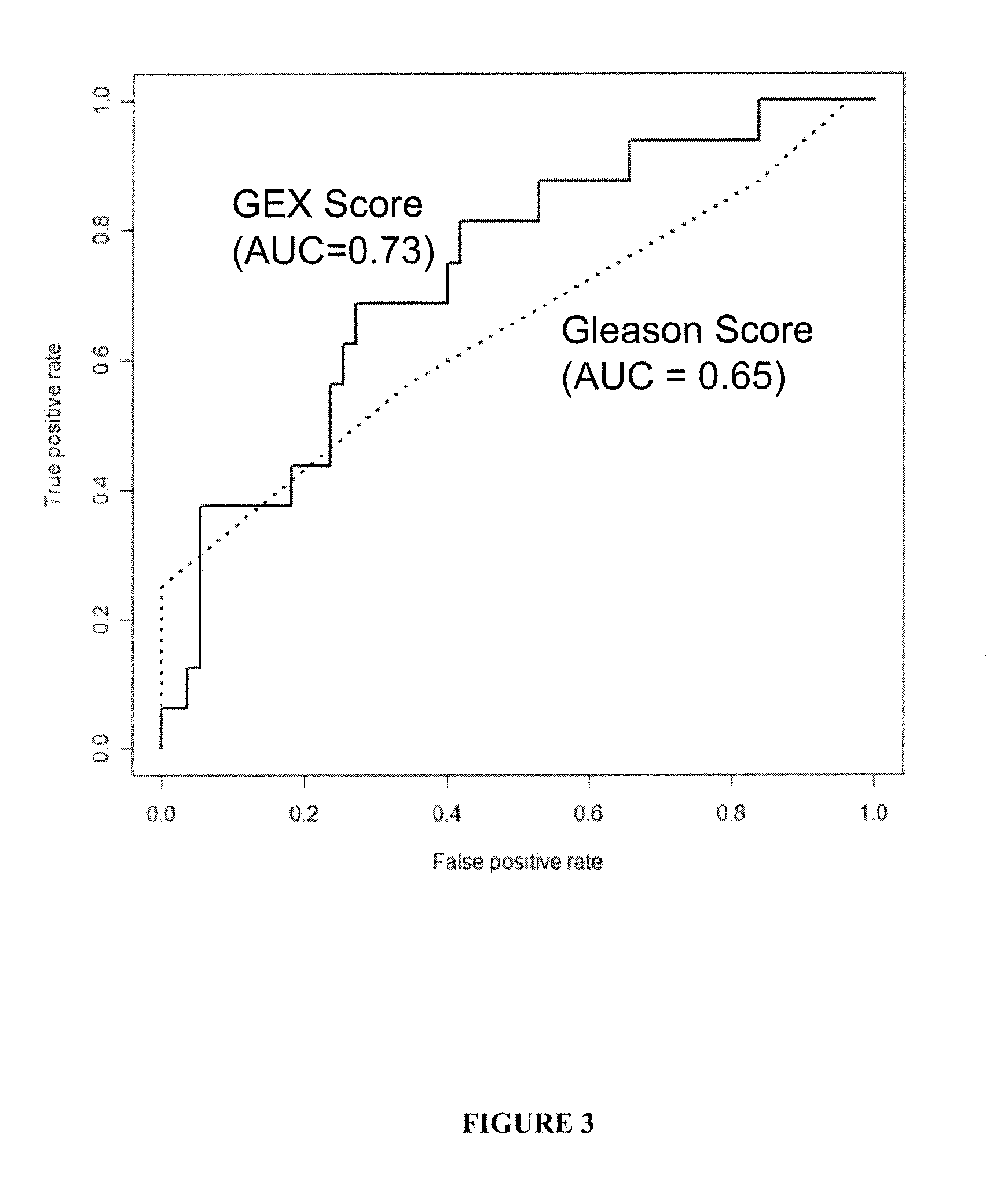
The present invention provides a method for preparing a reference model for cancer relapse prediction that provides higher resolution grading than Gleason score alone. The method encompasses obtaining from different individuals a plurality of prostate carcinoma tissue samples of known clinical outcome representing different Gleason scores; selecting a set of signature genes having an expression pattern that correlates positively or negatively in a statistically significant manner with the Gleason scores; independently deriving a prediction score that correlates gene expression of each individual signature gene with Gleason score for each signature gene in said plurality of prostate carcinoma tissue samples; deriving a prostate cancer gene expression (GEX) score that correlates gene expression of said set of signature genes with the Gleason score based on the combination of independently derived prediction scores in the plurality of prostate cancer tissue samples; and correlating said GEX score with the clinical outcome for each prostate carcinoma tissue sample. A set of signature genes is provided that encompasses all or a sub-combination of GI_2094528, KIP2, NRG1, NBL1, Prostein, CCNE2, CDC6, FBP1, HOXC6, MKI67, MYBL2, PTTG1, RAMP, UBE2C, Wnt5A, MEMD, AZGP1, CCK, MLCK, PPAP2B, and PROK1. Also provided a methods for predicting the probability of relapse of cancer in an individual and methods for deriving a prostate cancer gene expression (GEX) score for a prostate carcinoma tissue sample obtained from an individual.
Owner:ILLUMINA INC
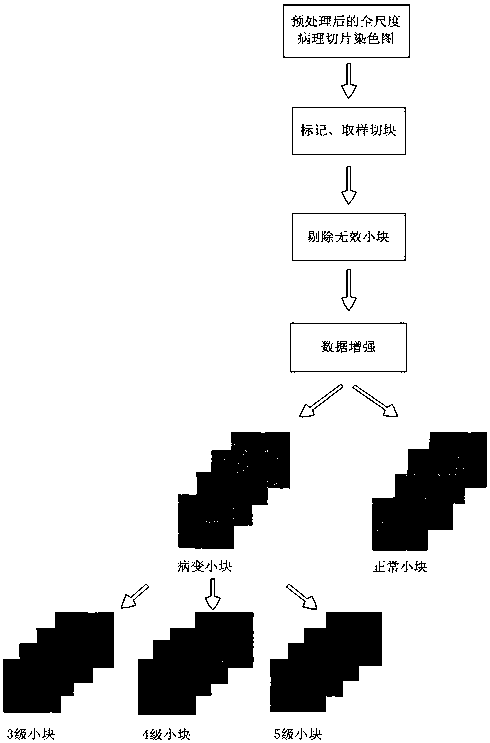
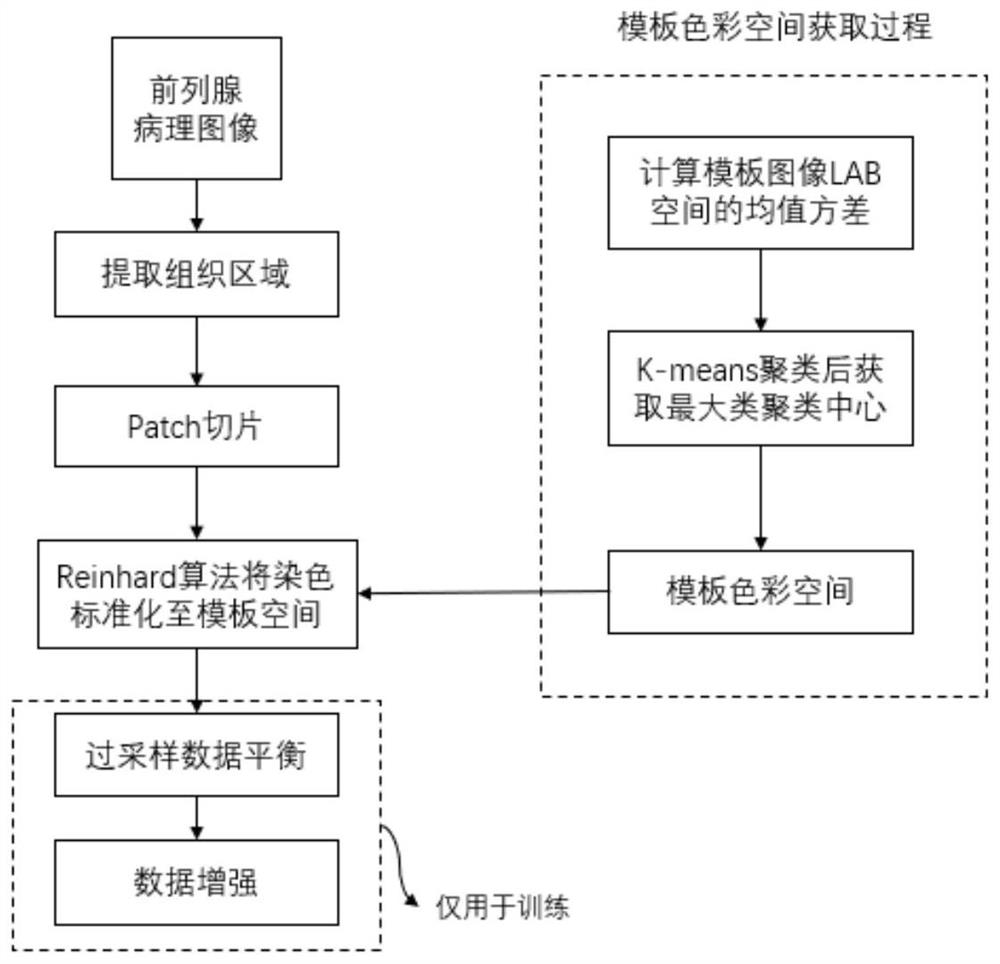
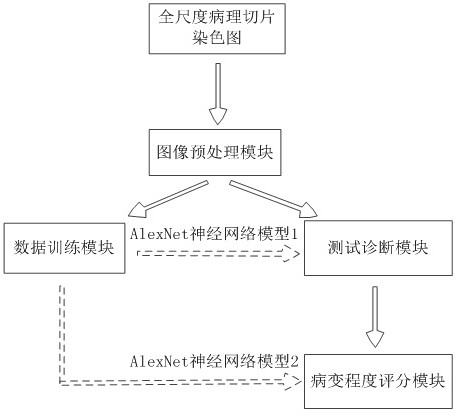
Rapid diagnosis and scoring method for full-scale pathological section based on deep learning
ActiveCN108305249ASolve the problem of size limitationImplement diagnosticsImage enhancementImage analysisNetwork modelScore method
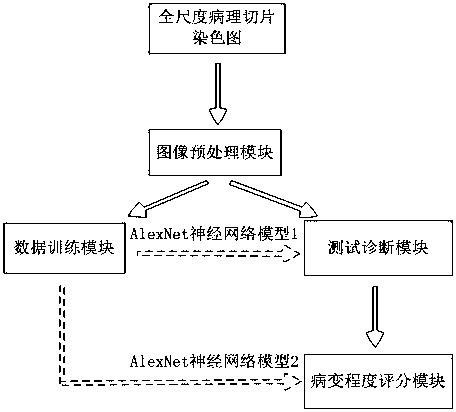
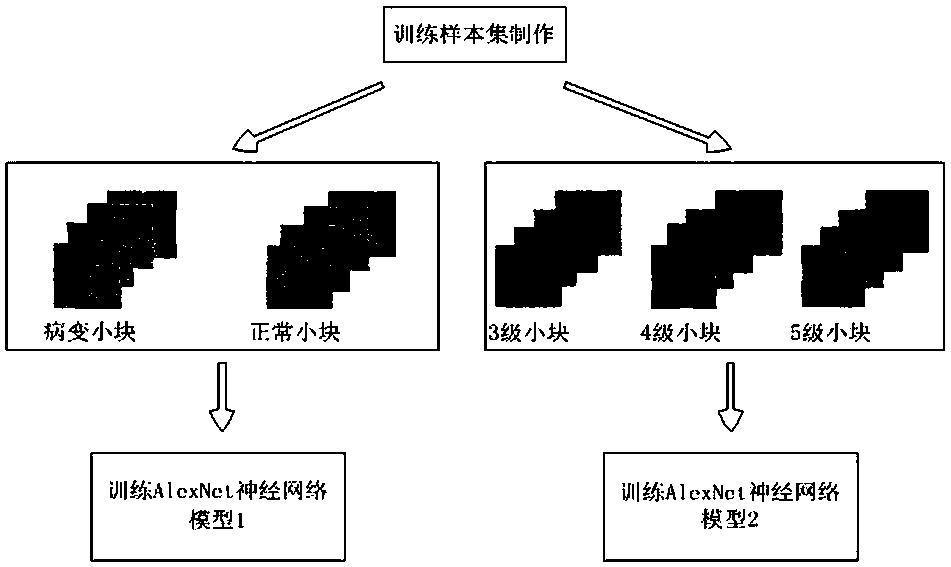
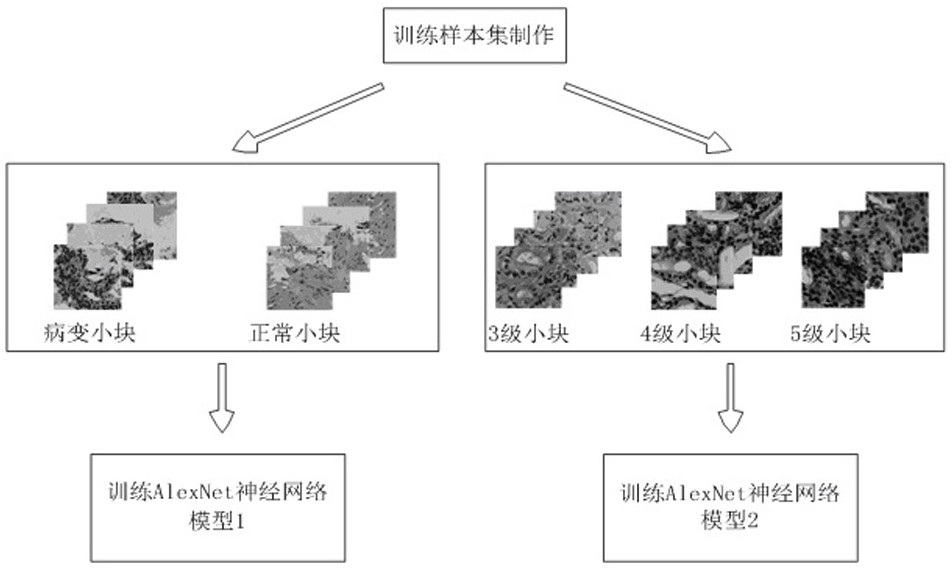
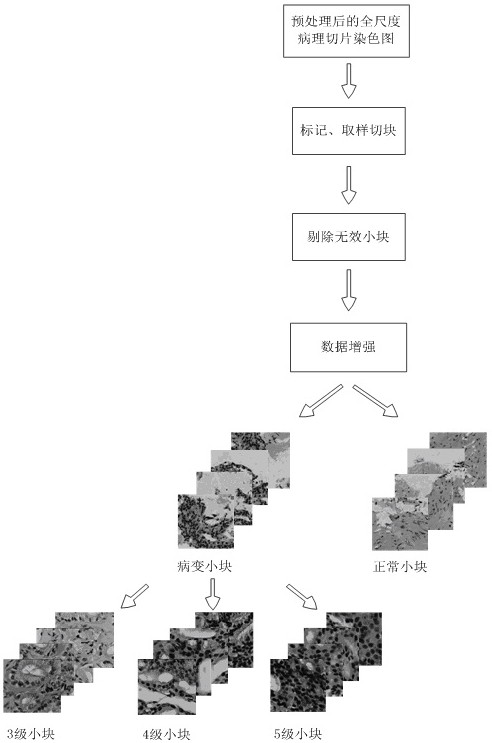
The invention relates to a rapid diagnosis and scoring method for a full-scale pathological section based on deep learning. Preprocessing is carried out on a full-scale pathological section staining map; the node number of a full-connection layer and an output layer of a traditional AlexNet neural network is changed to meet the needs of practical problems, a marked training sample set is selectedto train two AlexNet neural network models for diagnosis and scoring, and high-dimensional feature information of a lesion area is extracted; with the two improved AlexNet neural network models aftertraining, the full-scale pathological section staining map is diagnosed and scored; and according to a diagnosis predicted probability, a probability heat map is drawn and the lesion area is identified visually, statistics of proportions of small sampling block numbers with different lesion degrees is carried out, and the lesion degree of a tissue is scored. Therefore, the diagnosis and Gleason scoring of the full-scale pprostate tissue pathological section are realized automatically; and the accuracy rate and the calculation rate exceed the average level of the artificial diagnosis substantially.
Owner:FUJIAN NORMAL UNIV
System for evaluating a pathological stage of prostate cancer
ActiveUS9779213B2Reliable and accurate image segmentationBioreactor/fermenter combinationsBiological substance pretreatmentsComputer generationStandardization
Clinical information, molecular information and / or computer-generated morphometric information is used in a predictive model for predicting the occurrence of a medical condition. In an embodiment, a model predicts whether a patient is likely to have a favorable pathological stage of prostate cancer, where the model is based on features including one or more (e.g., all) of preoperative PSA, Gleason Score, a measurement of expression of androgen receptor (AR) in epithelial and stromal nuclei and / or a measurement of expression of Ki67-positive epithelial nuclei, a morphometric measurement of a ratio of area of epithelial nuclei outside gland units to area of epithelial nuclei within gland units, and a morphometric measurement of area of epithelial nuclei distributed away from gland units. In some embodiments, quantitative measurements of protein expression in cell lines are utilized to objectively assess assay (e.g., multiplex immunofluorescence (IF)) performance and / or to normalize features for use within a predictive model.
Owner:AUREON LAB INC +1
Expression Profiles to Predict Relapse of Prostate Cancer
InactiveUS20110153534A1Microbiological testing/measurementDigital computer detailsReference modelImage resolution
The present invention provides a method for preparing a reference model for cancer relapse prediction that provides higher resolution grading than Gleason score alone. The method encompasses obtaining from different individuals a plurality of prostate carcinoma tissue samples of known clinical outcome representing different Gleason scores; selecting a set of signature genes having an expression pattern that correlates positively or negatively in a statistically significant manner with the Gleason scores; independently deriving a prediction score that correlates gene expression of each individual signature gene with Gleason score for each signature gene in said plurality of prostate carcinoma tissue samples; deriving a prostate cancer gene expression (GEX) score that correlates gene expression of said set of signature genes with the Gleason score based on the combination of independently derived prediction scores in the plurality of prostate cancer tissue samples; and correlating said GEX score with the clinical outcome for each prostate carcinoma tissue sample. A set of signature genes is provided that encompasses all or a sub-combination of GI_2094528, KIP2, NRG1, NBL1, Prostein, CCNE2, CDC6, FBP1, HOXC6, MKI67, MYBL2, PTTG1, RAMP, UBE2C, Wnt5A, MEMD, AZGP1, CCK, MLCK, PPAP2B, and PROK1. Also provided a methods for predicting the probability of relapse of cancer in an individual and methods for deriving a prostate cancer gene expression (GEX) score for a prostate carcinoma tissue sample obtained from an individual.
Owner:ILLUMINA INC
Expression profiles to predict relapse of prostate cancer
InactiveUS8110363B2Microbiological testing/measurementDigital computer detailsProstate cancerOncology
The present invention provides a method for preparing a reference model for cancer relapse prediction that provides higher resolution grading than Gleason score alone. The method encompasses obtaining from different individuals a plurality of prostate carcinoma tissue samples of known clinical outcome representing different Gleason scores; selecting a set of signature genes having an expression pattern that correlates positively or negatively in a statistically significant manner with the Gleason scores; independently deriving a prediction score that correlates gene expression of each individual signature gene with Gleason score for each signature gene in said plurality of prostate carcinoma tissue samples; deriving a prostate cancer gene expression (GEX) score that correlates gene expression of said set of signature genes with the Gleason score based on the combination of independently derived prediction scores in the plurality of prostate cancer tissue samples; and correlating said GEX score with the clinical outcome for each prostate carcinoma tissue sample. A set of signature genes is provided that encompasses all or a sub-combination of GI_2094528, KIP2, NRG1, NBL1, Prostein, CCNE2, CDC6, FBP1, HOXC6, MKI67, MYBL2, PTTG1, RAMP, UBE2C, Wnt5A, MEMD, AZGP1, CCK, MLCK, PPAP2B, and PROK1. Also provided a methods for predicting the probability of relapse of cancer in an individual and methods for deriving a prostate cancer gene expression (GEX) score for a prostate carcinoma tissue sample obtained from an individual.
Owner:ILLUMINA INC
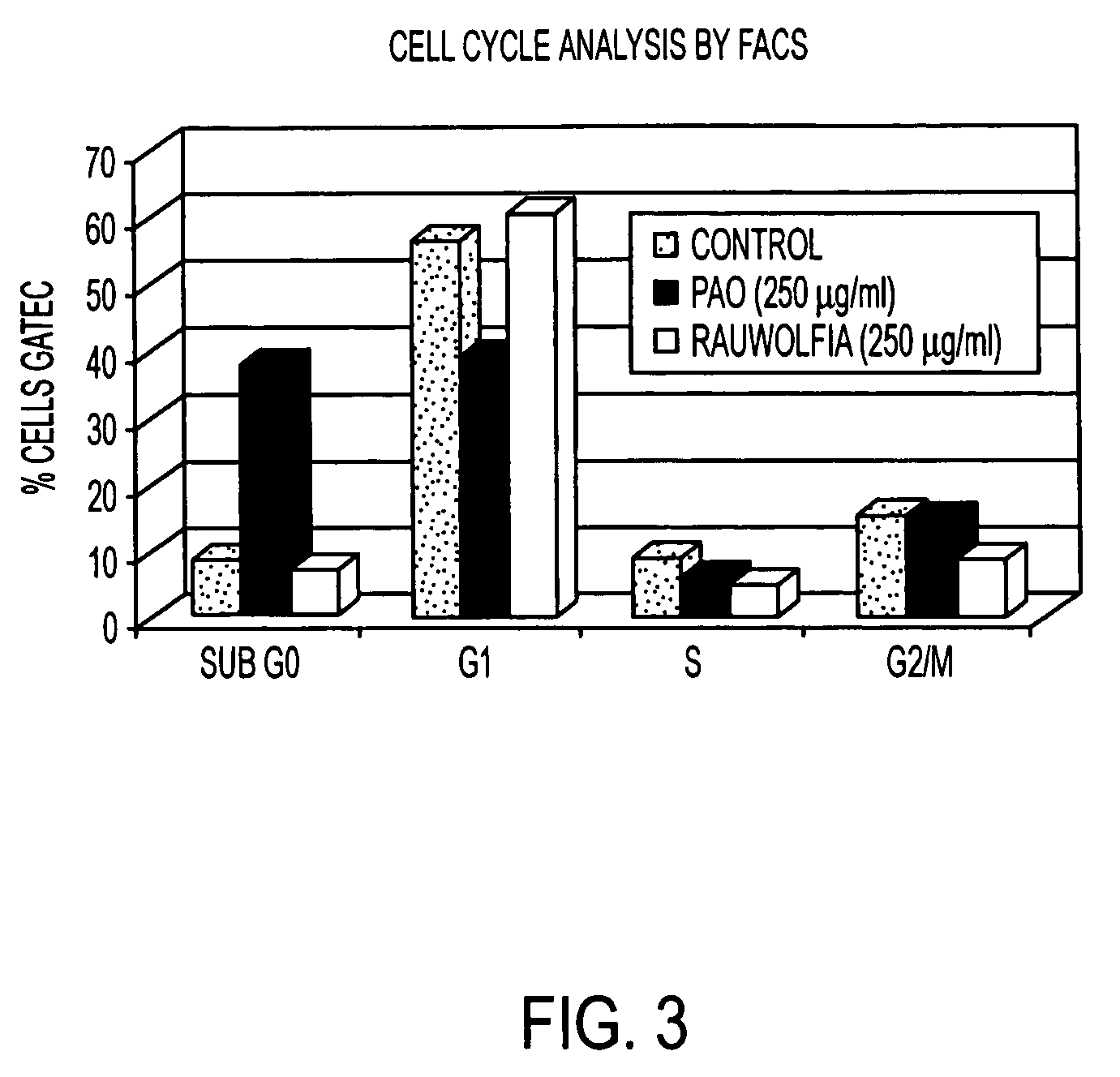
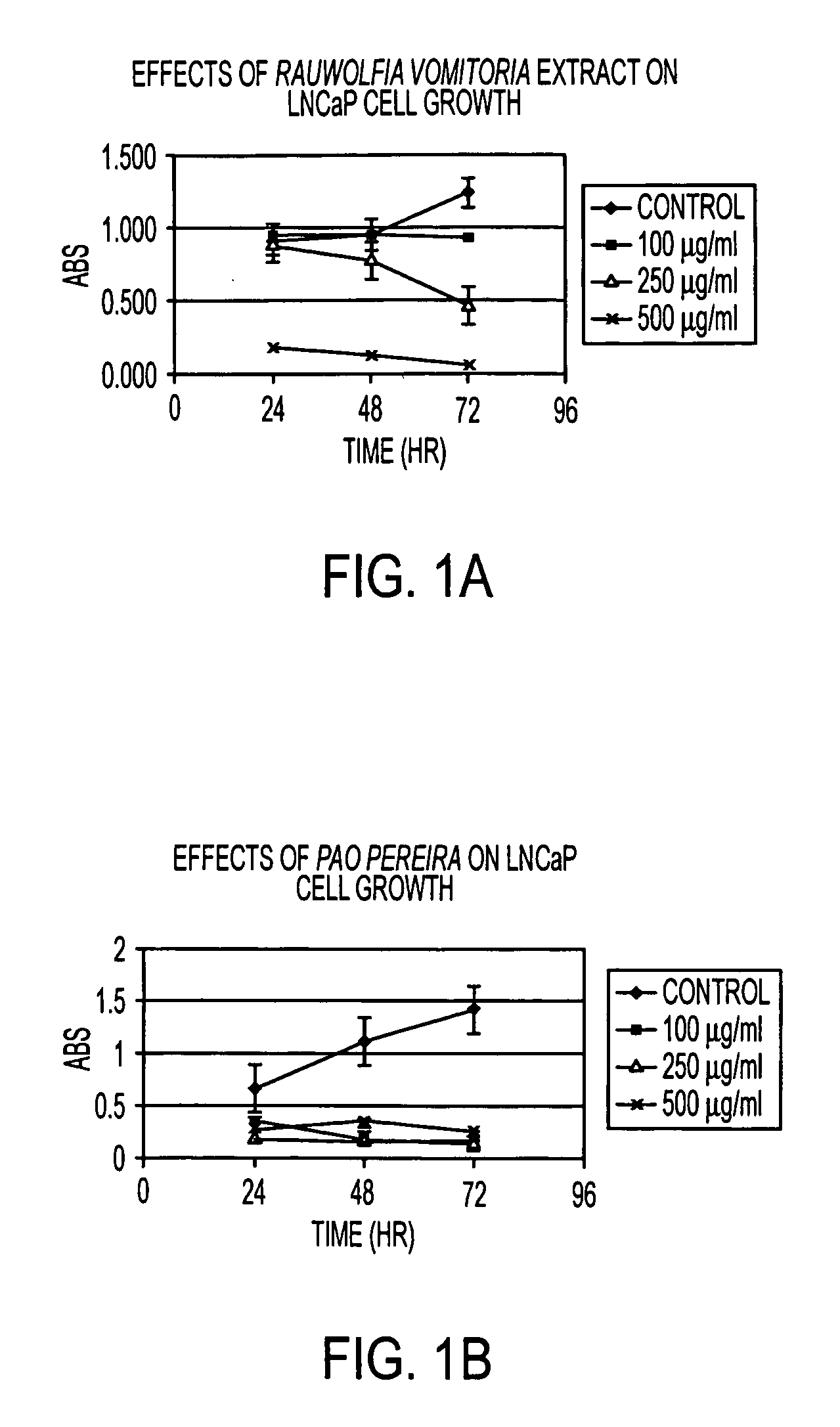
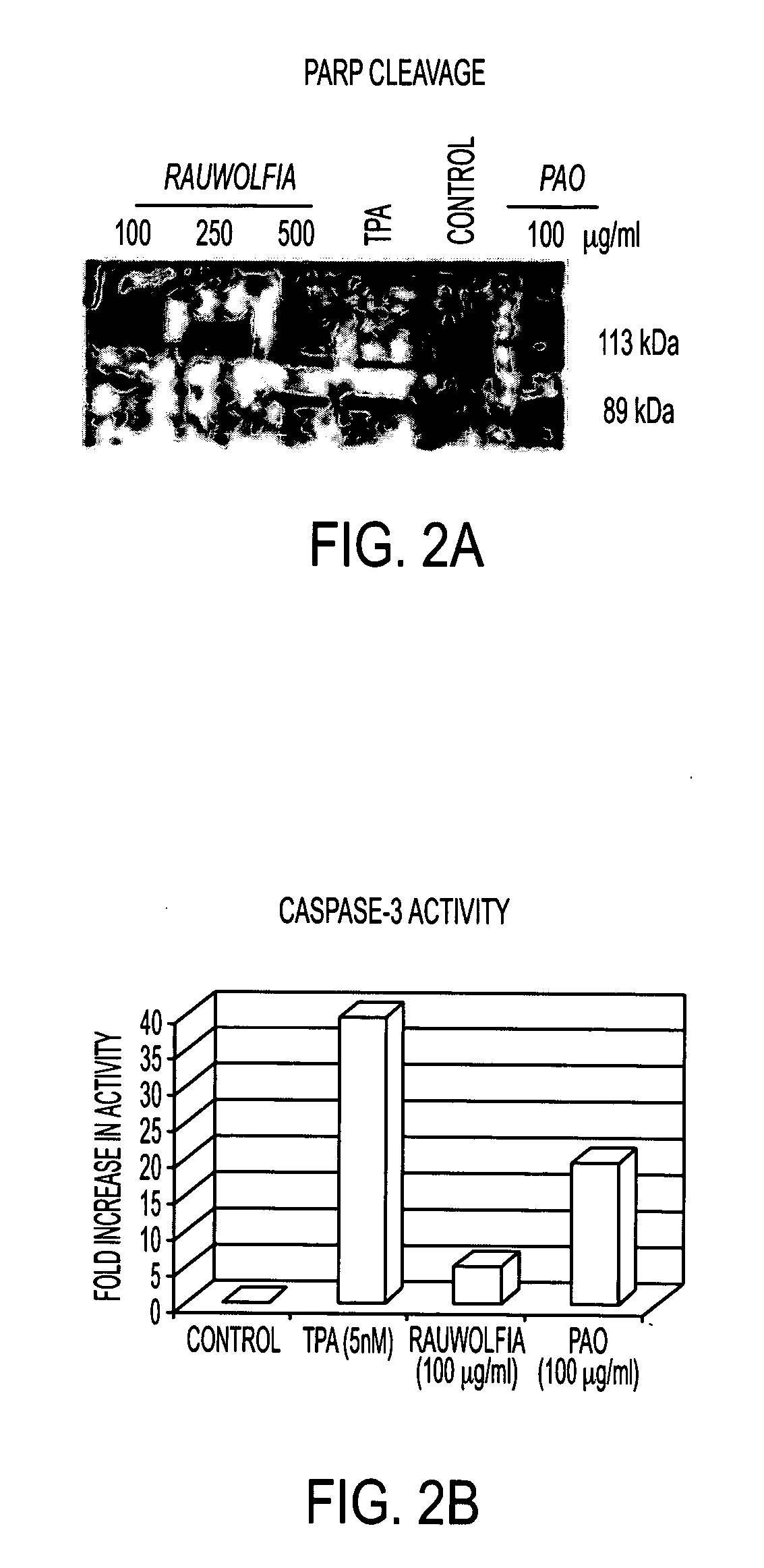
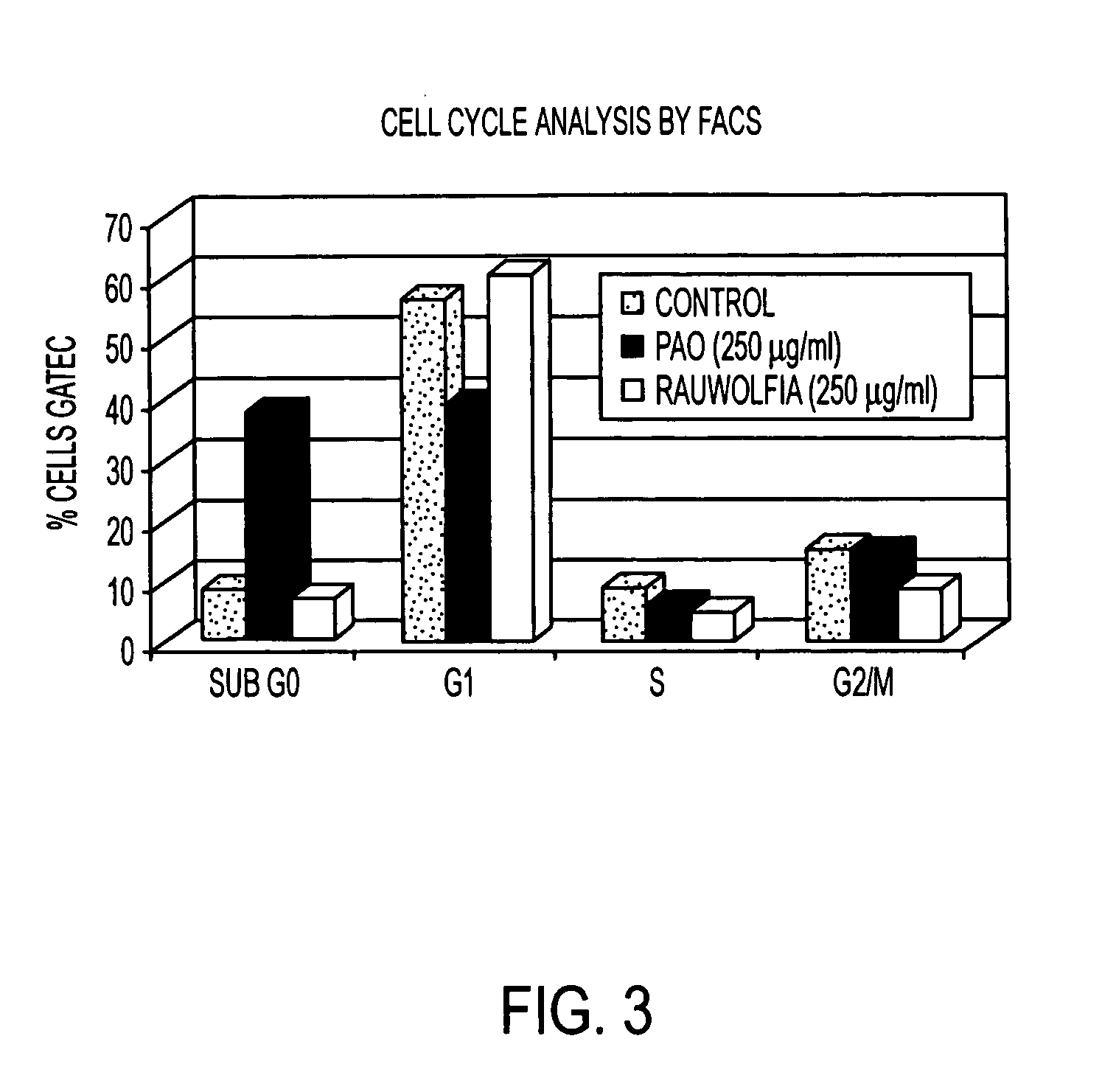
Flavopereirine and alstonine combinations in the treatment and prevention of prostate cancer
InactiveUS7341749B2Alleviating and avoiding onsetInhibit progressBiocideUnknown materialsDiseaseDouble-time
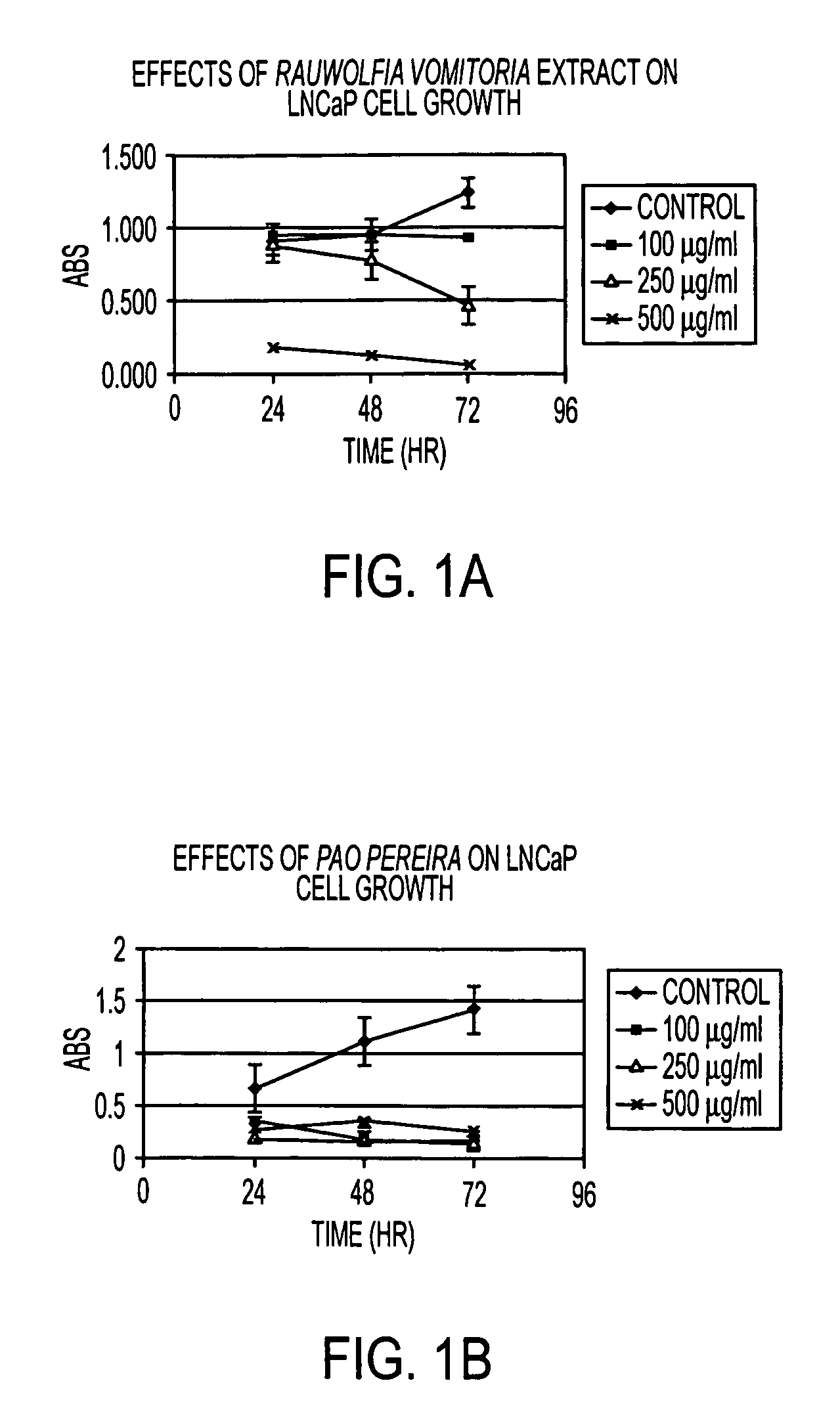
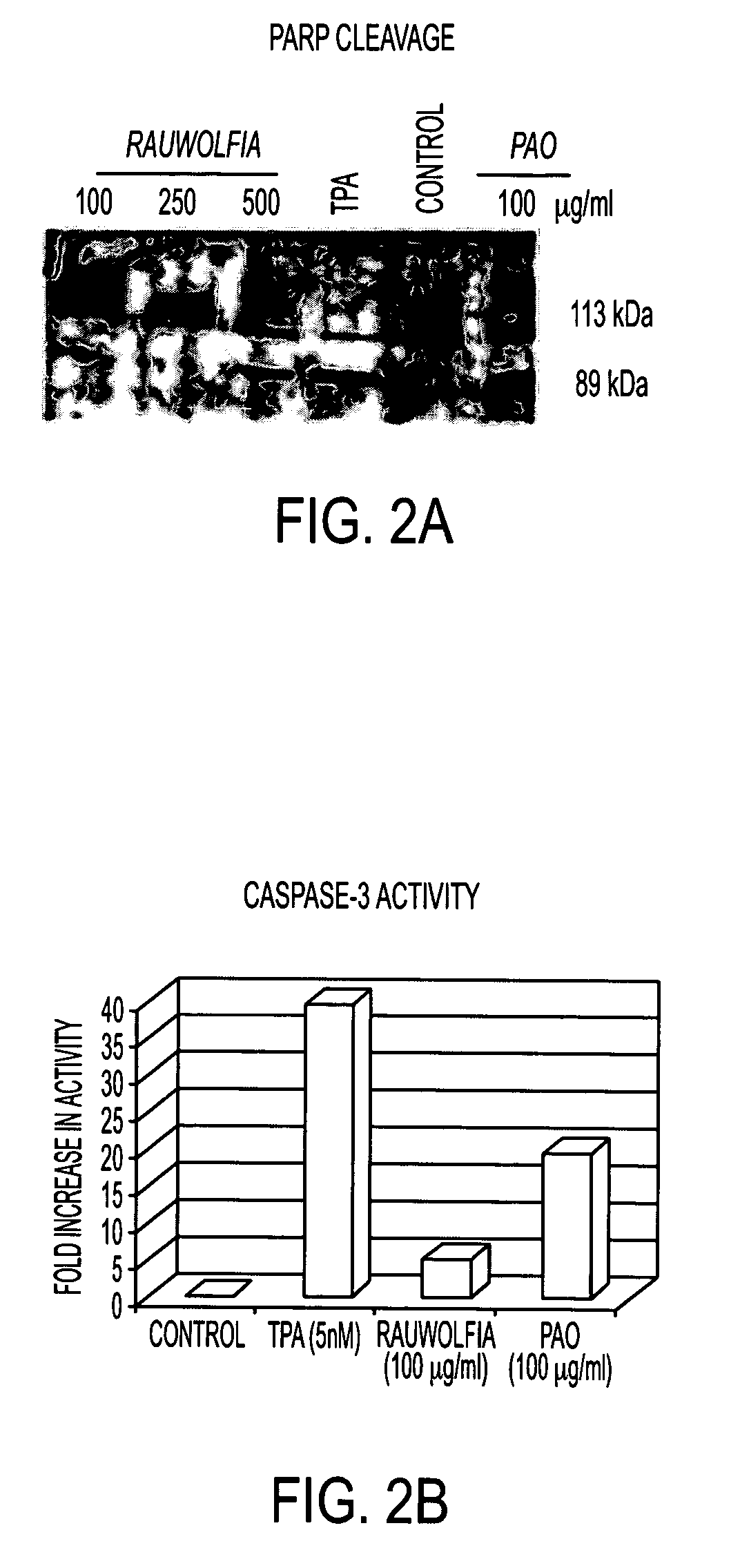
A method and composition for preventing prostate cancer and / or reducing PSA levels and / or alleviating the symptoms of BPH (Benign Prostatic Hyperplasia) or PIN (prostatic intraepithelial neoplasia) by administration of an effective amount of a mixture of flavopereirine and alstonine. A method and composition for treating low-grade prostate cancer and preventing the onset of metastatic disease and / or reducing the doubling time of PSA levels in men with positive biopsies showing relatively low Gleason scores and morphologies characteristic of non-invasive, slow-progressing prostate cancer. The flavopereirine and alstonine can be in the form of natural extracts derived from Pao Pereira and Rauwolfia Vomitoria, respectively. Alternatively, these two active compounds can be administered in purified form. The composition can be in included in a kit along with instructions for use in a treatment regimen.
Owner:MOLECULAR INT RES +1
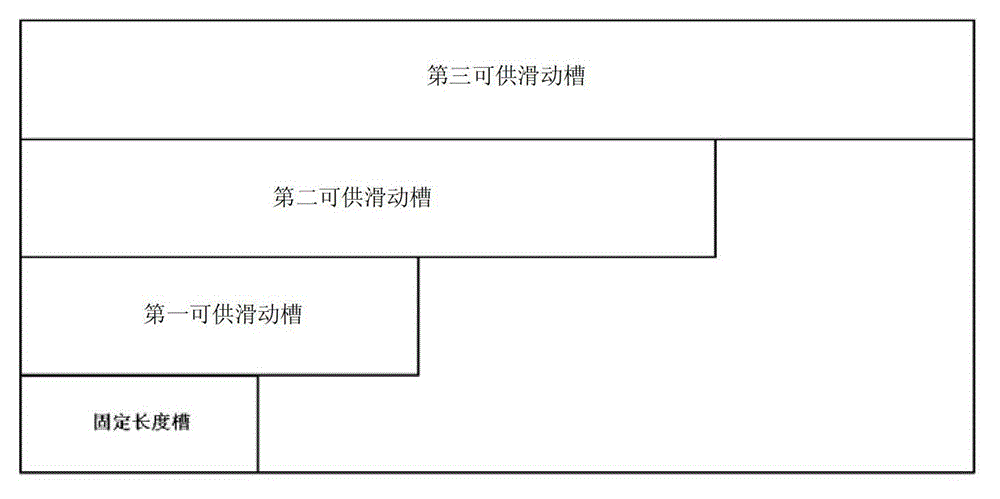
Device for predicting bone metastasis risk of newly-diagnosed prostate cancer
InactiveCN102968558AAvoid overuseClinically convenientSpecial data processing applicationsNewly diagnosedMedicine
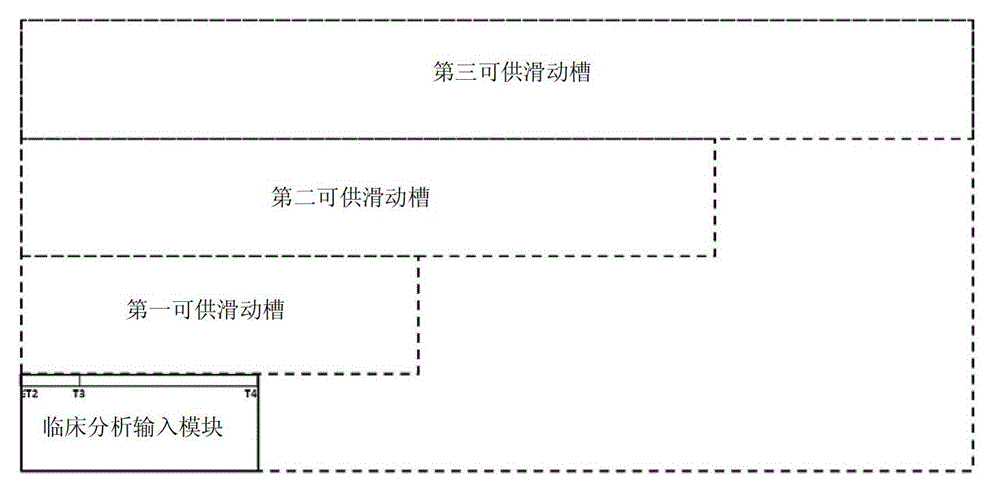
The invention discloses a device for predicting the bone metastasis risk of newly-diagnosed prostate cancer. According to the device, the risk of bone metastasis is predicted, bone scanning is carried out on patients with high bone metastasis risk and the overuse of the bone scanning is avoided under the condition of not increasing missed diagnosis. The technical scheme provided by the invention is that the device comprises a storage module, four input modules, a processing module and an output module, wherein four input slots are formed in the storage module and are a slot with fixed length, a first slot for sliding, a second slot for sliding and a third slot for sliding respectively; the four input modules are a clinical stage input module placed in the slot with the fixed length, a Gleason scoring input module placed in the first slot for sliding, a PSA (Prostate-Specific Antigen) value input module placed in the second slot for sliding and an age input module placed in the third slot for sliding; the processing module is used for adjusting the positions of the four input modules in the input slots of the storage module to obtain an output locus; and the output module is provided with a staff gauge for predicting a risk value of the bone metastasis, and a corresponding locus of the output locus on the staff gauge is a predicted risk value.
Owner:叶定伟
Characterizing prostate cancer
InactiveCN101220392AMicrobiological testing/measurementBiological testingRecurrent CancerProstate sample
The invention relates to identify prostate cancer. The invention provides a method for predicting the recurrence or aggressiveness of prostate cancer, comprising a) determining the Gleason score of a prostate sample, and b) determining the methylation status of a Marker in a biological sample for those patients having a Gleason score of 7 or greater; wherein methylation that exceeds a pre-determined value is indicative of an aggressive or recurrent cancer and methylation that does not exceed such pre-determined value is indicative of indolent cancer. The invention also provides a kit used for predicting the course or aggressiveness of the prostate cancer, which comprising nucleic acid amplification test reagent and a synopsis which instructs the patients having the Gleason score of 7 or greater in the use of the reagent.
Owner:VERIDEX LCC
Prognostic marker for prostate cancer and methods and kits for using same
InactiveUS20050026196A1Accurate prognosisMicrobiological testing/measurementBiological testingTumor SampleOncology
A method for predicting whether a patient suffering from a prostate cancer is at risk of experiencing a cancer progression or recurrence which comprises the steps of : obtaining a Gleason score of said prostate tumor sample; assessing the proportion of NF-kB localized in the nuclei of said tumor sample with regard to all NF-kB present in said tumor sample.
Owner:CENT HOSPITALER UNIV DE MONTREAL +1
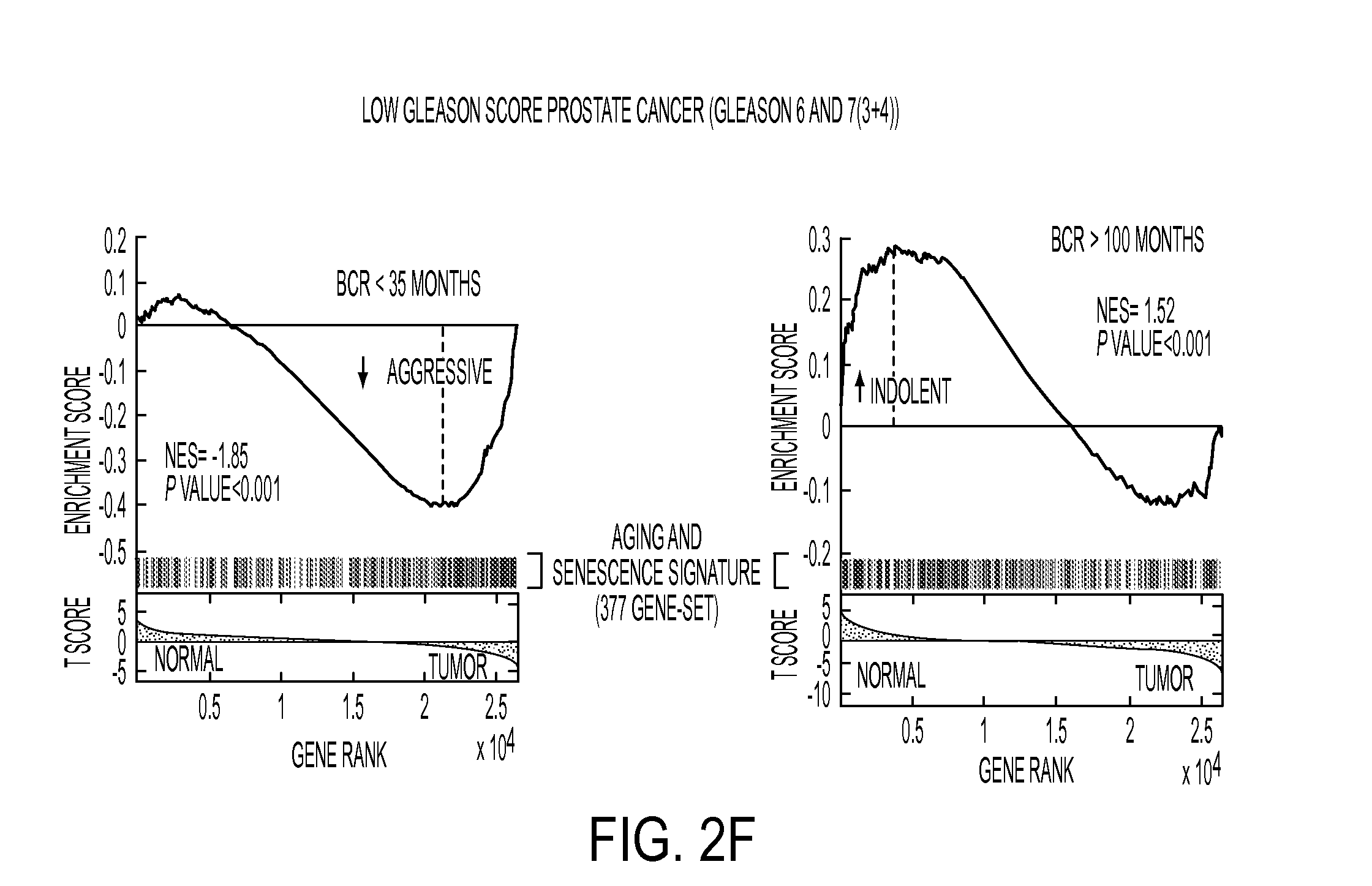
Diagnostic Markers of Indolent Prostate Cancer
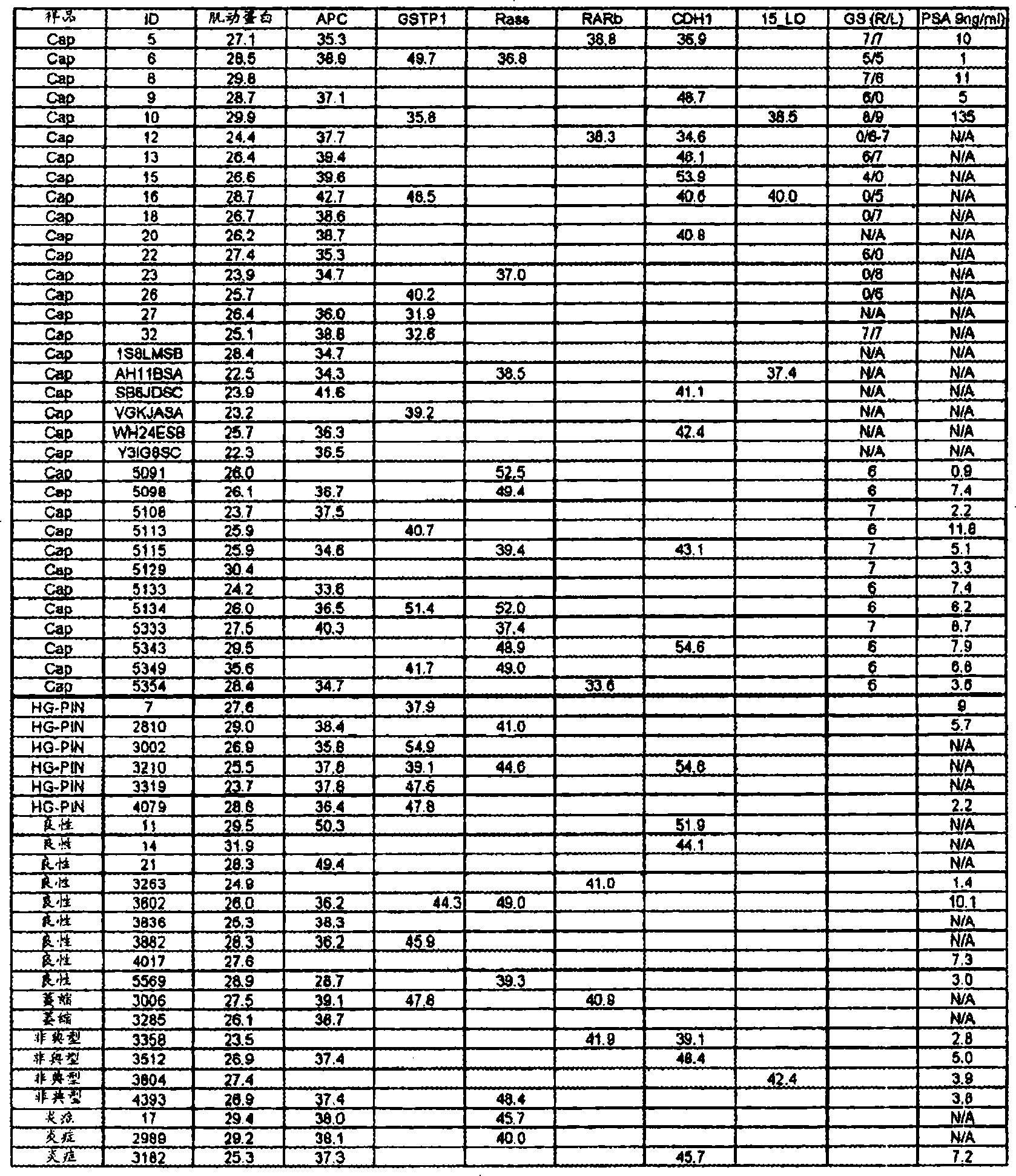
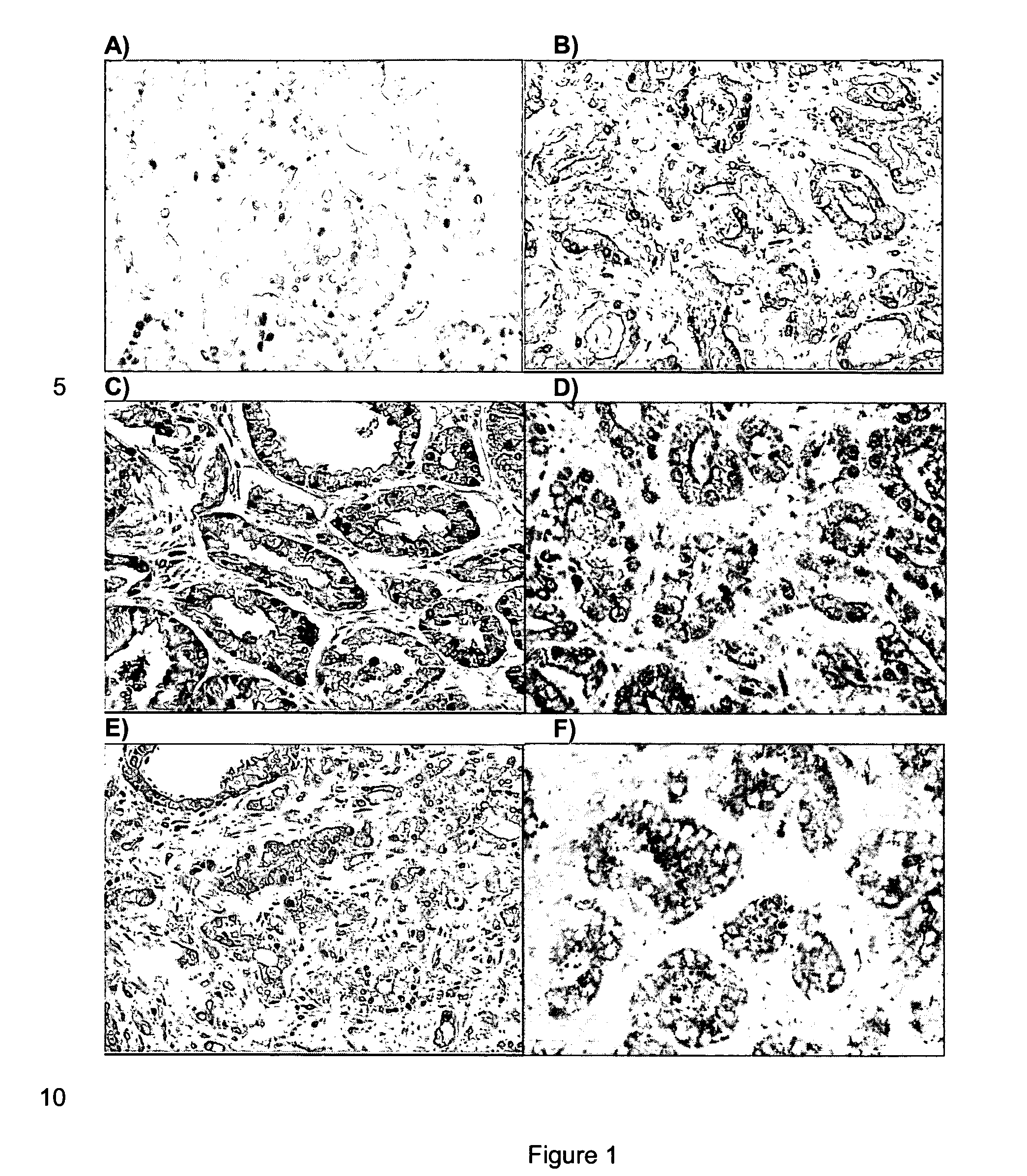
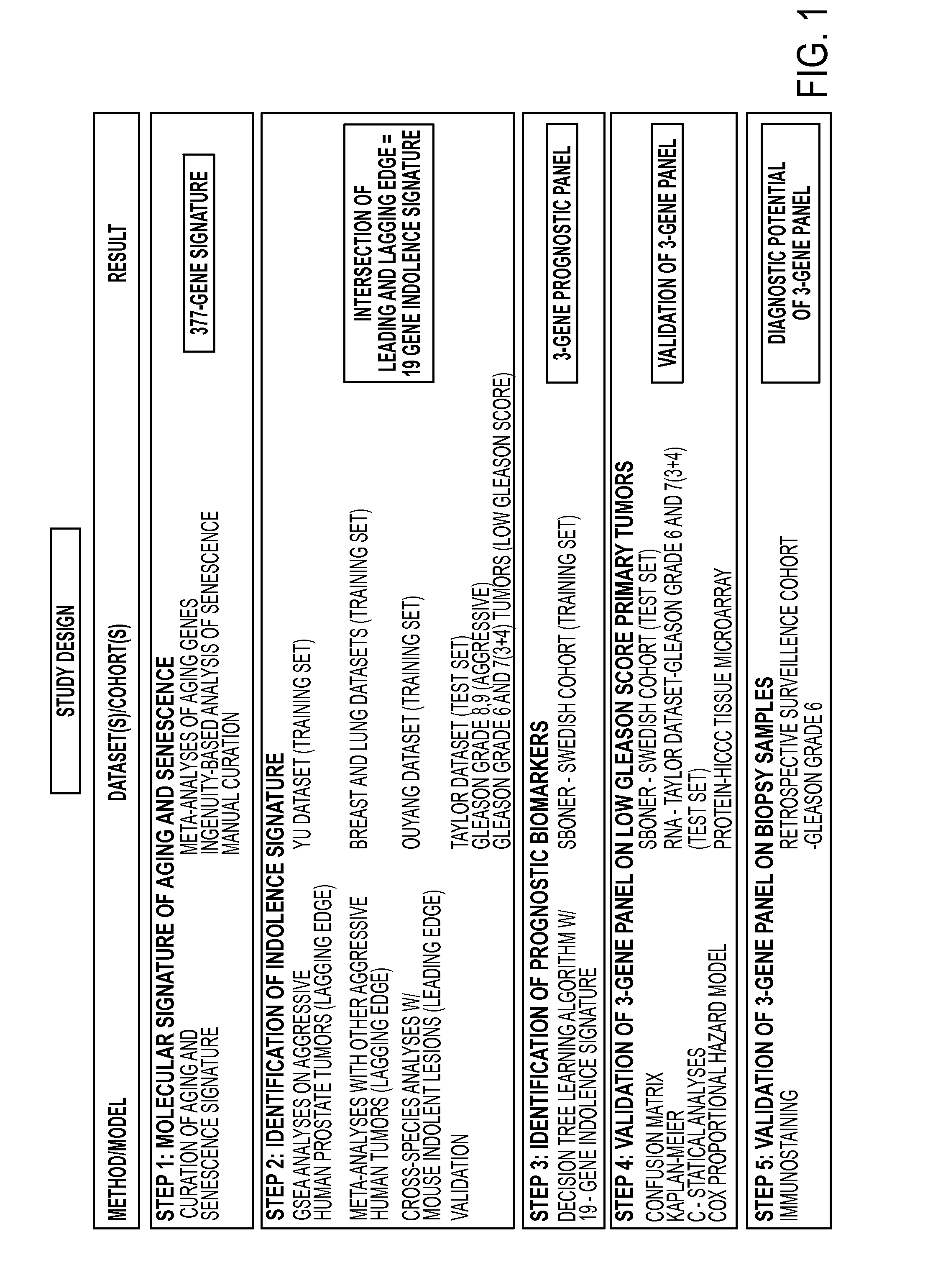
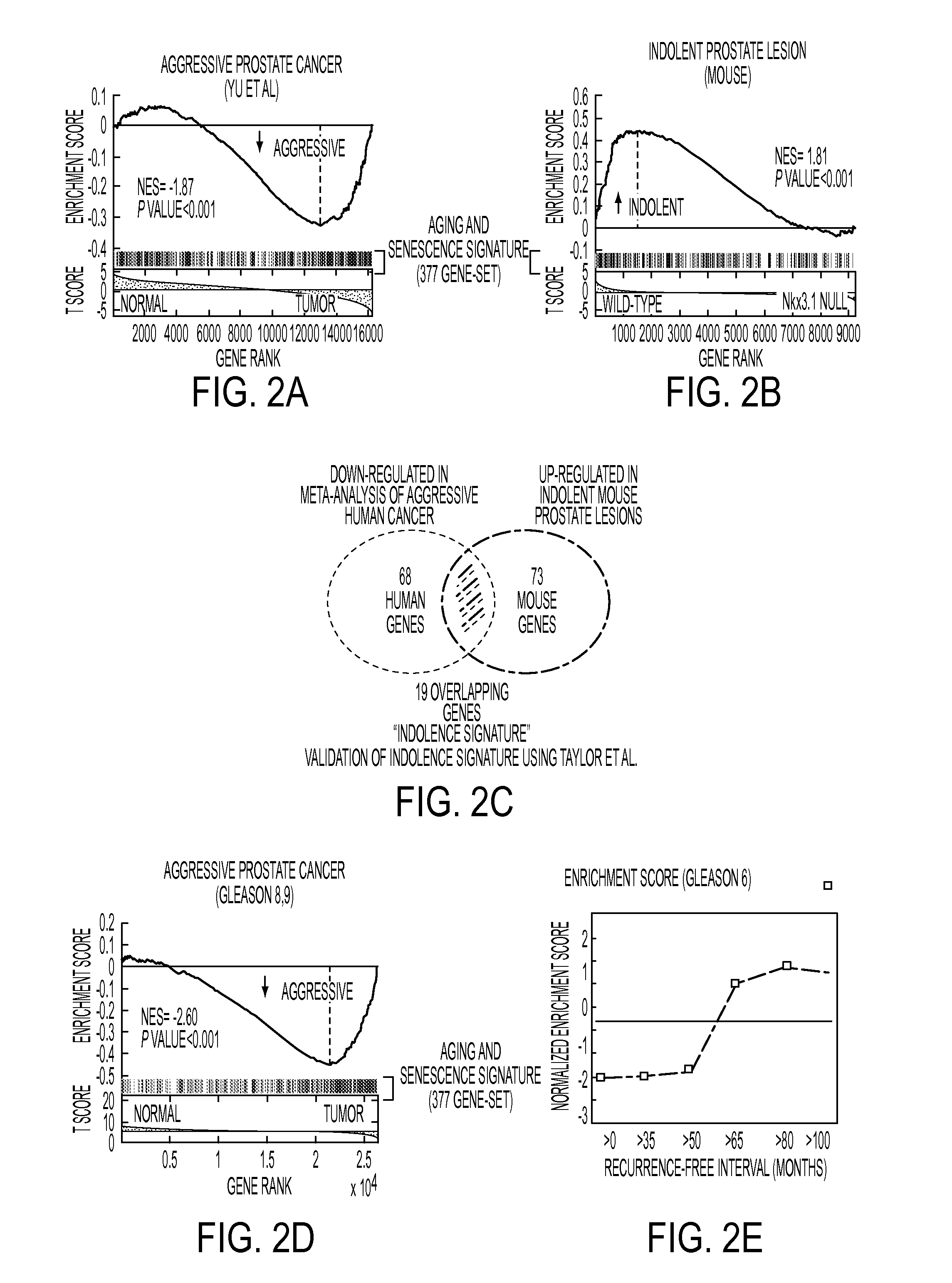
A 3-gene prognostic panel has been identified that together accurately predicted the outcome of low Gleason score prostate tumors as either truly indolent or at a high risk of becoming aggressive. The 3-gene prognostic panel was validated on independent cohorts confirmed its independent prognostic value, as well as its ability to improve prognosis with currently used clinical nomograms. Expression of the 3-gene prognostic panel was determined by quantifying mRNA or protein encoded by the panel (collectively referred to as “prognostic biomarkers”). The prognostic biomarkers were discovered to be up-regulated in indolent tumors and down-regulated in aggressive forms of prostate cancer.
Owner:NIH DEITR
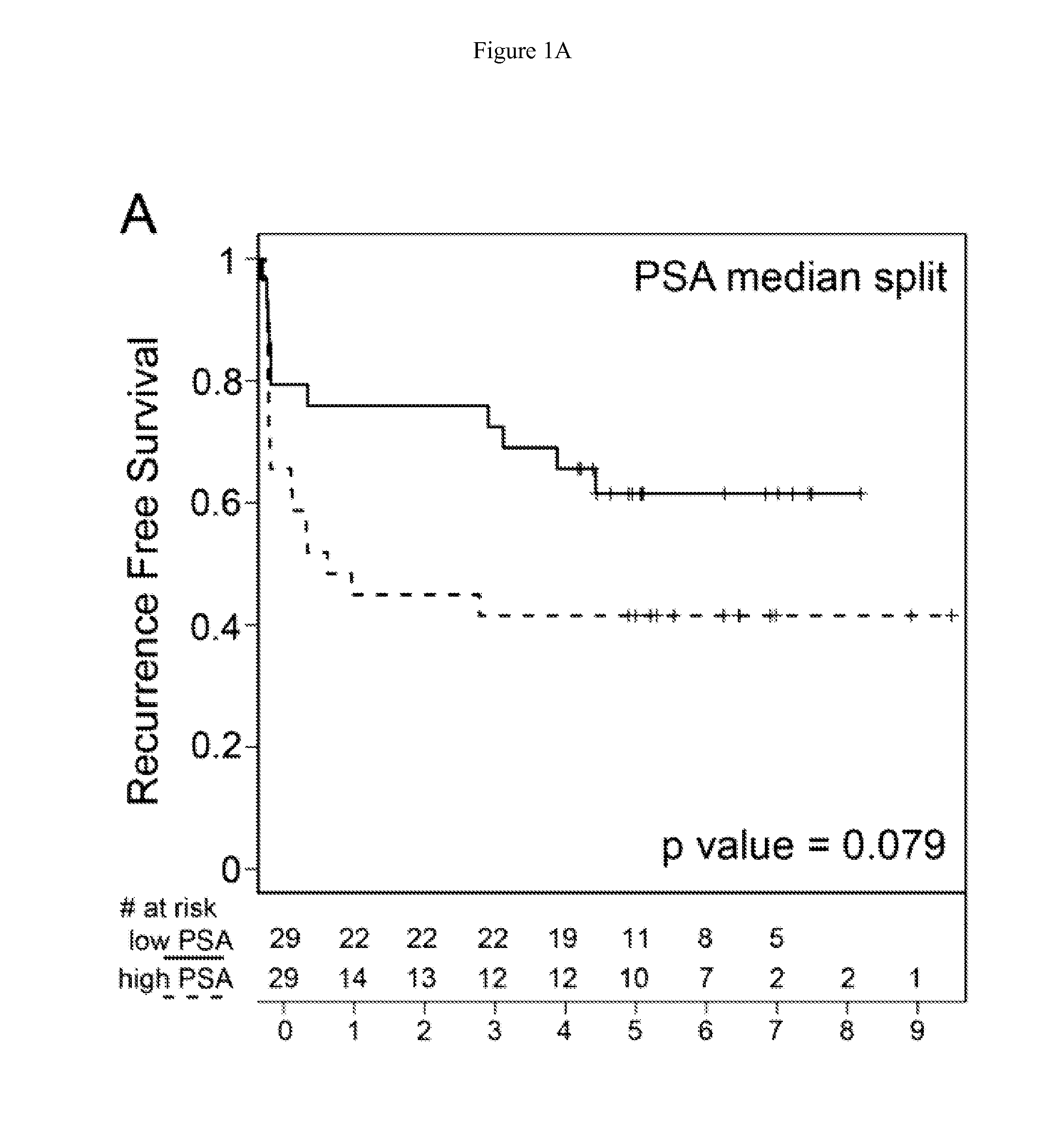
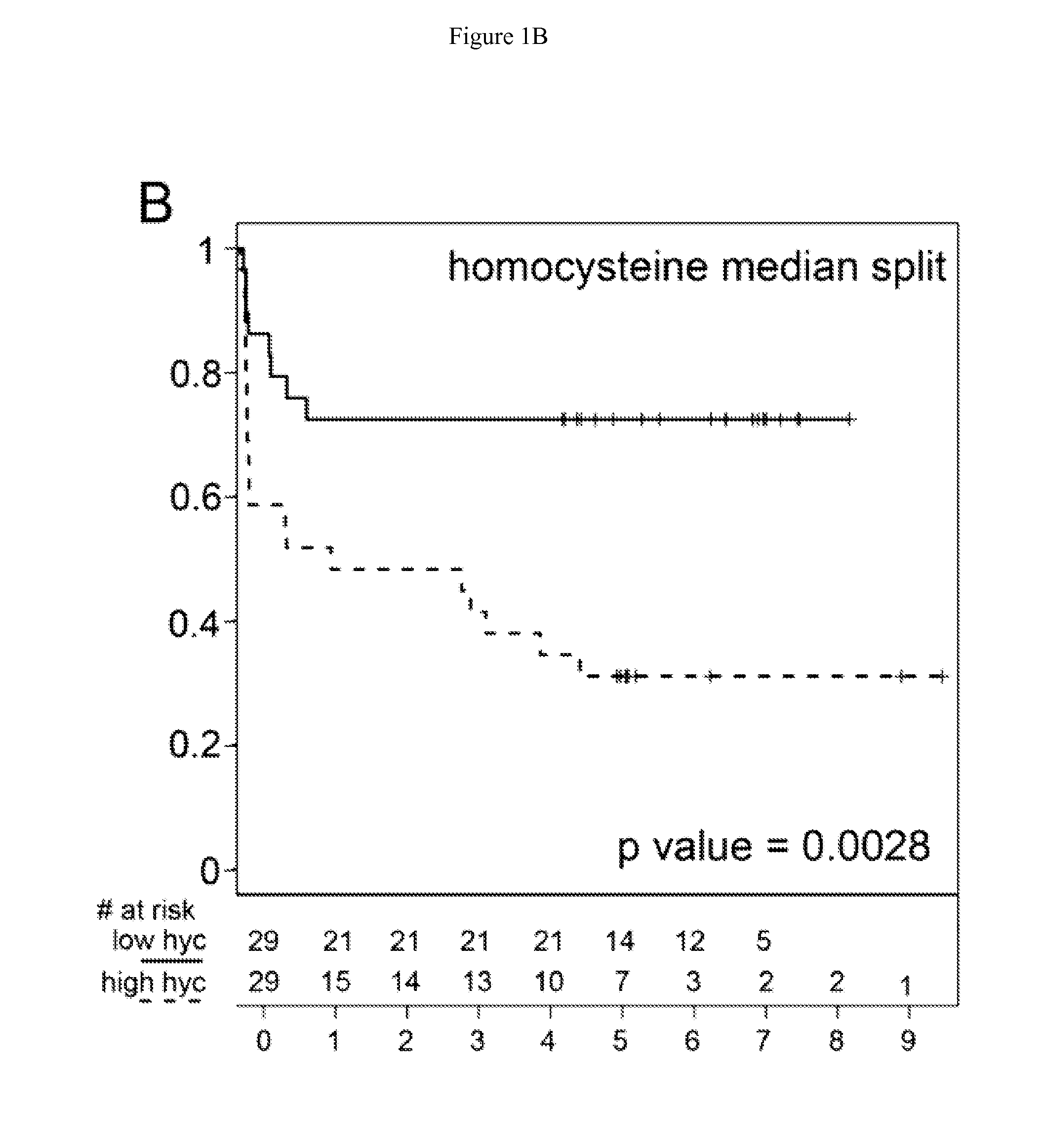
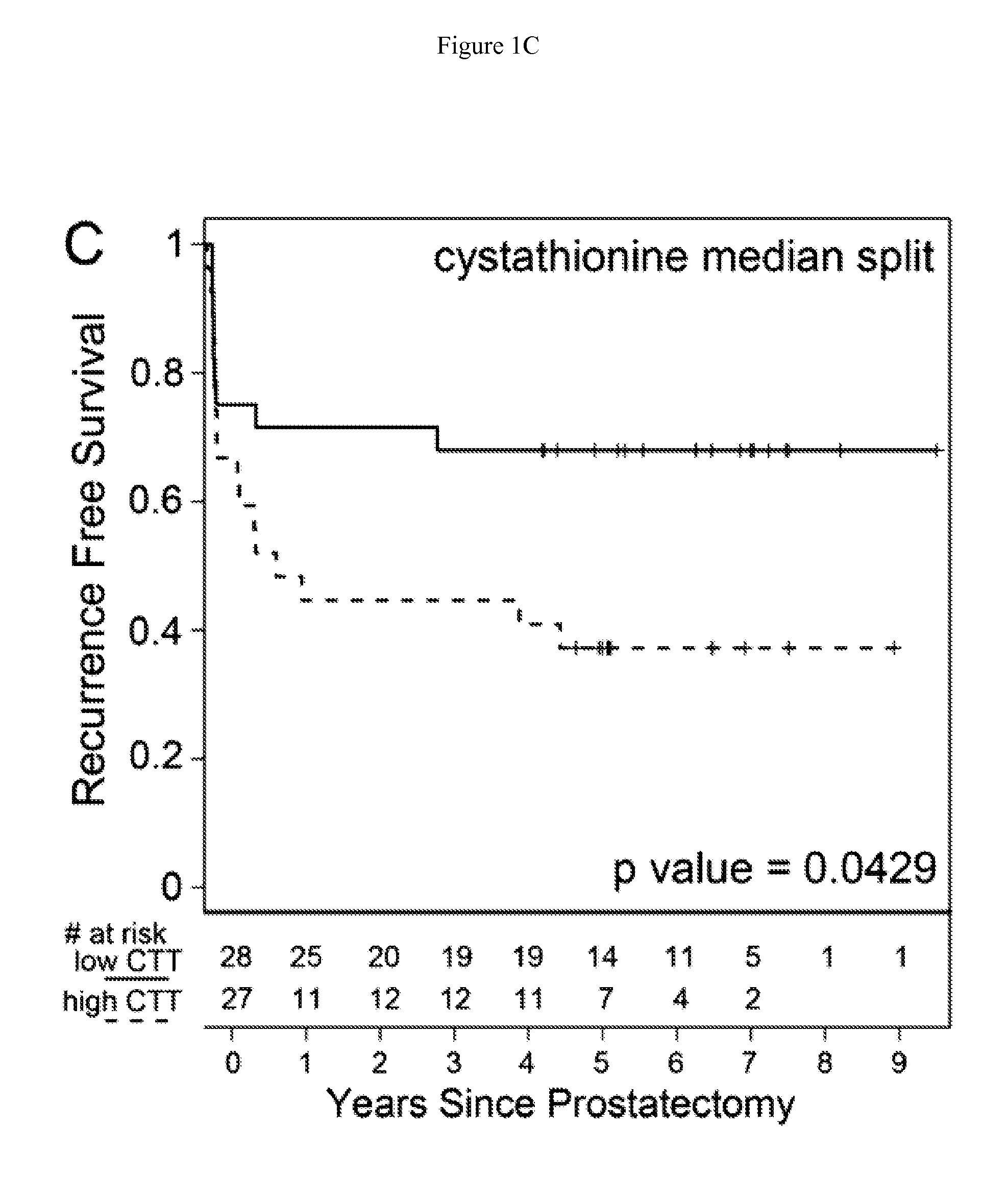
Methionine metabolites predict aggressive cancer progression
InactiveUS20150204882A1Increase probabilityImmobilised enzymesBioreactor/fermenter combinationsMetaboliteProper treatment
Owner:CEDARS SINAI MEDICAL CENT
Methionine metabolites predict aggressive cancer progression
InactiveUS20140045193A1Increase probabilityMicrobiological testing/measurementDisease diagnosisMetaboliteMethionine biosynthesis
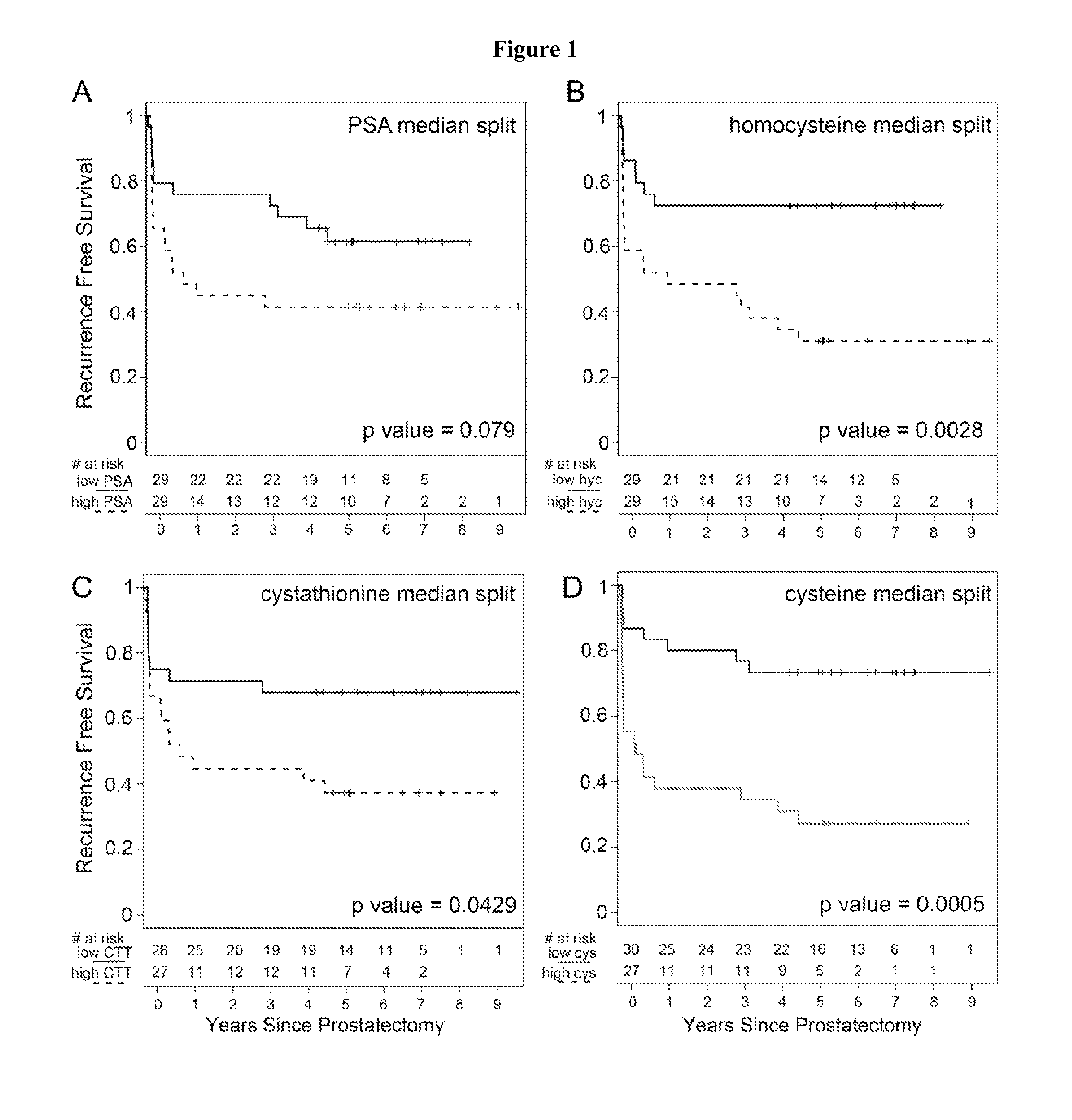
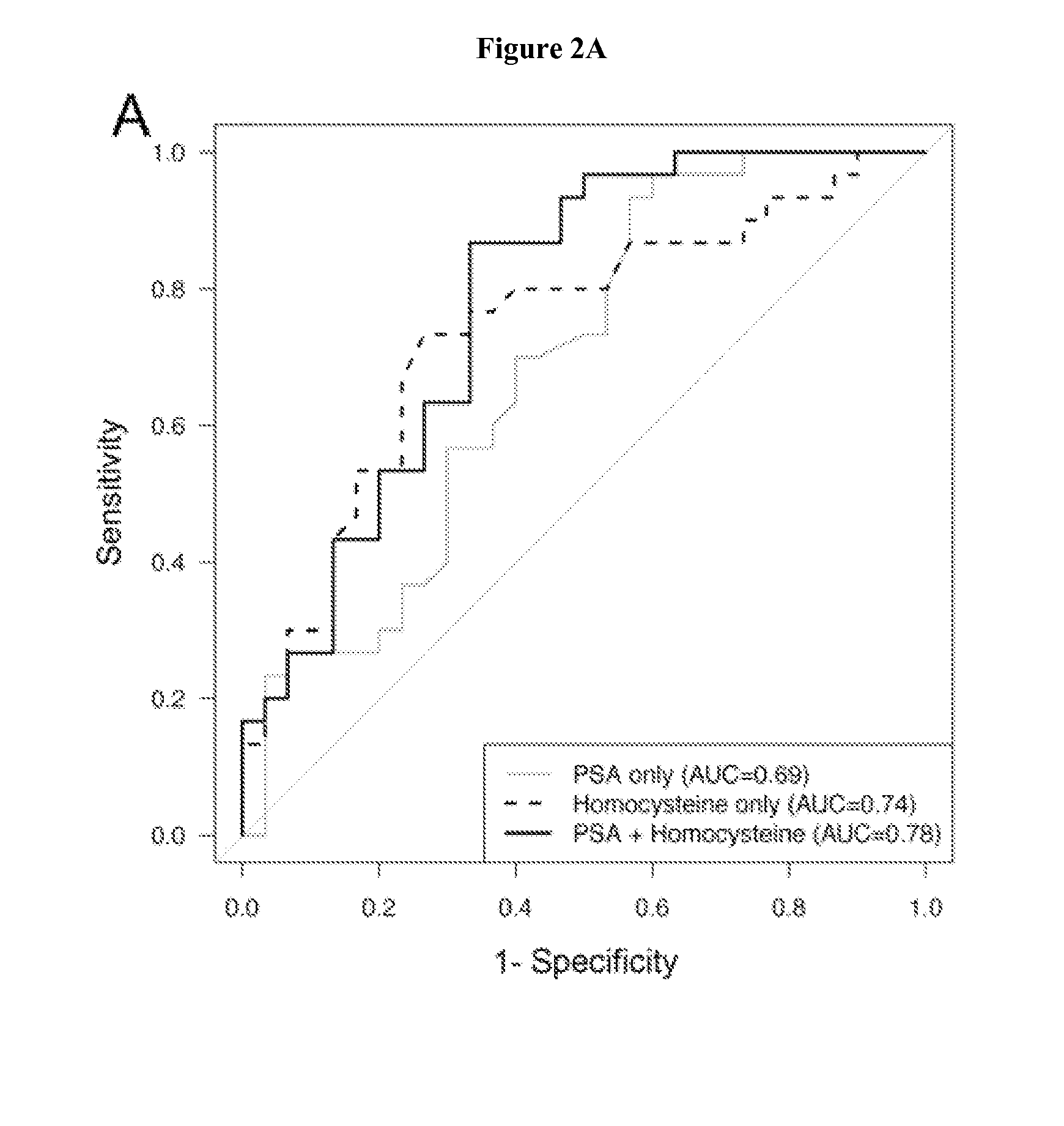
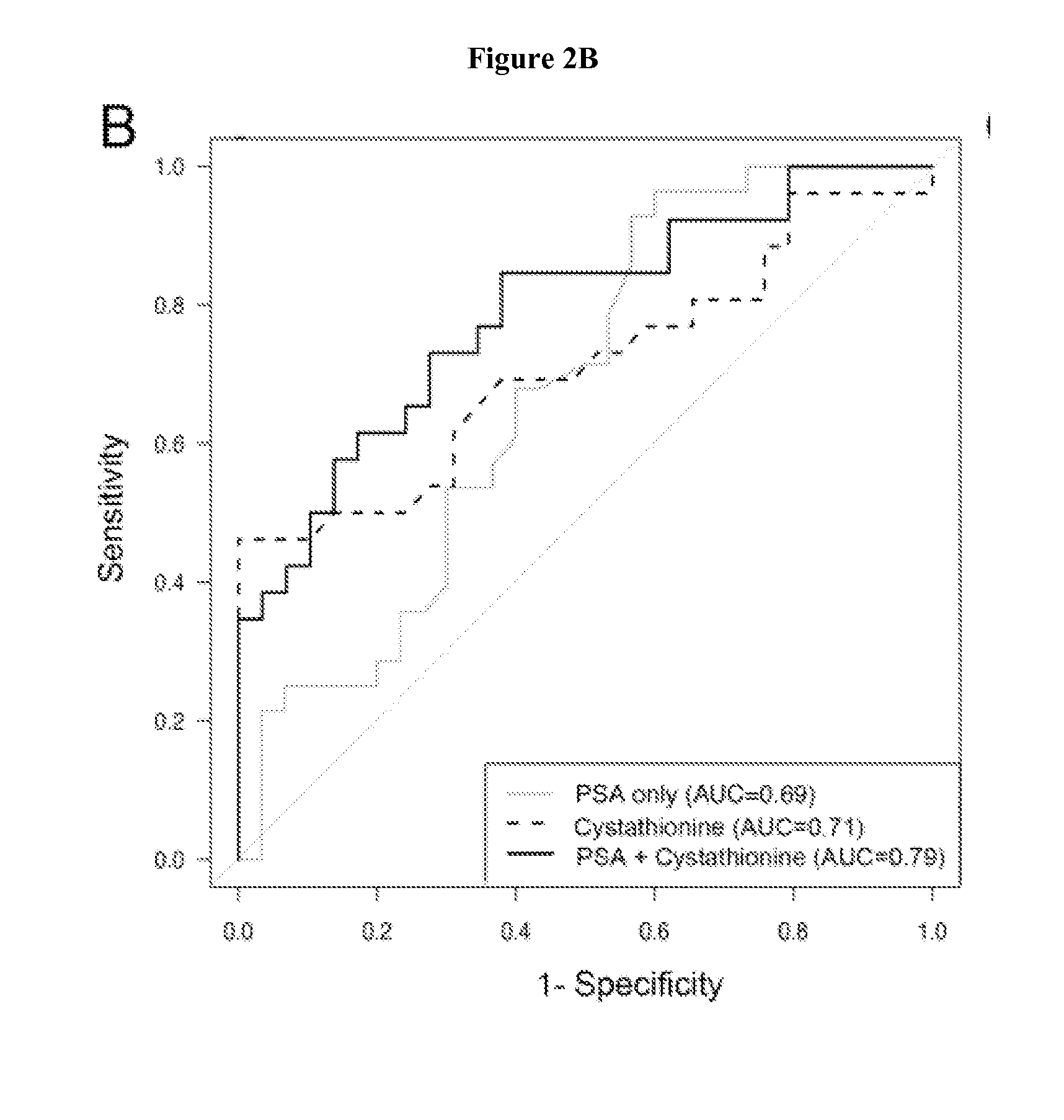
The invention relates to the use of enzymes and nanorods to detect cysteine level in a patient sample and relates to the use of the detected cysteine level to predict cancer recurrence in the patient. The invention is directed to systems and methods of detecting cysteine level in a sample from a subject, wherein the systems or methods can further comprise measuring at least one additional parameter, such as PSA level, Gleason score and clinical stage. The invention is directed to systems and methods of predicting the probability of a recurrence of a cancer in a subject, wherein the systems or methods can further comprise measuring at least one additional parameter, such as PSA level, Gleason score and clinical stage. This invention is directed to systems and methods of predicting the probability of a recurrence of a cancer in a subject and treating the subject, wherein the systems or methods can further comprise measuring at least one additional parameter, such as PSA level, Gleason score and clinical stage.
Owner:CEDARS SINAI MEDICAL CENT
Method and system for determining the risk of occurrence of prostate cancer
ActiveUS9858389B2Reliable and accurate image segmentationMedical simulationMedical data miningMultiplexProstate cancer
Clinical information, molecular information and / or computer-generated morphometric information is used in a predictive model for predicting the occurrence of a medical condition. In an embodiment, a model predicts risk of prostate cancer progression in a patient, where the model is based on features including one or more (e.g., all) of preoperative PSA, dominant Gleason Grade, Gleason Score, at least one of a measurement of expression of AR in epithelial and stromal nuclei and a measurement of expression of Ki67-positive epithelial nuclei, a morphometric measurement of average edge length in the minimum spanning tree (MST) of epithelial nuclei, and a morphometric measurement of area of non-lumen associated epithelial cells relative to total tumor area. In some embodiments, the morphometric information is based on image analysis of tissue subject to multiplex immunofluorescence and may include characteristic(s) of a minimum spanning tree (MST) and / or a fractal dimension observed in the images.
Owner:AUREON LAB INC +1
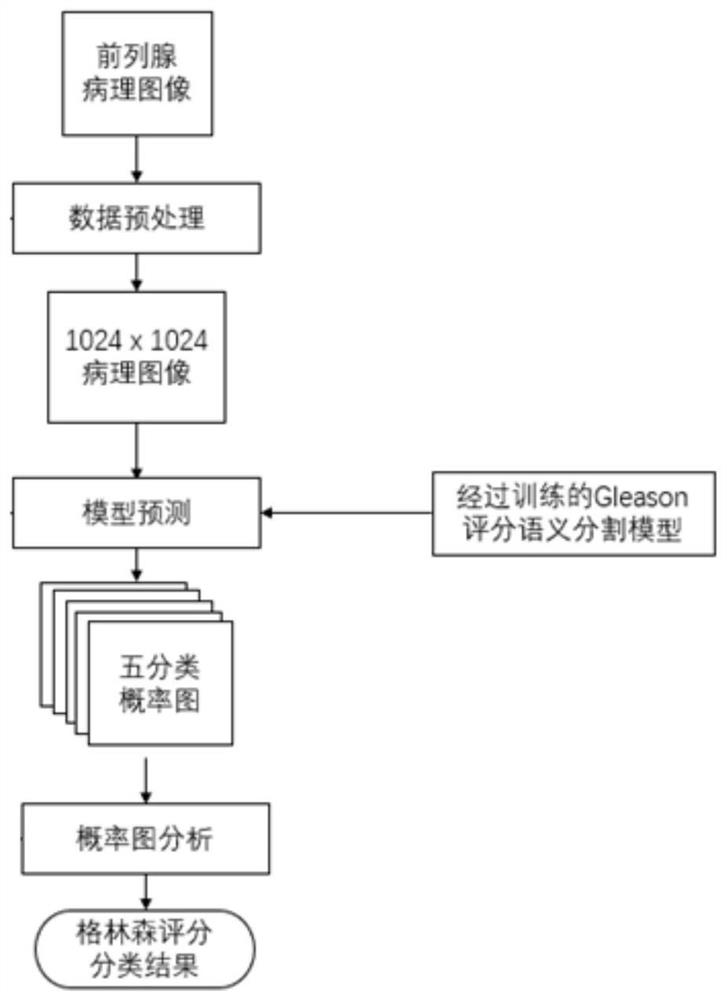
Rapid Gleason scoring system for prostate digital pathological image
The invention provides a rapid Gleason scoring system for a prostate digital pathological image, and the system is characterized in that the system comprises a data preprocessing unit which is used for data preprocessing of the prostate digital pathological image; a pre-training weight loaded semantic segmentation network used for outputting a five-channel probability graph according to the patchslices output by the data preprocessing unit; and a Gleason scoring unit used for carrying out post-processing on the five-channel probability graph and then carrying out calculation by utilizing an ISUP grading rule to obtain a Gleason score of the prostate digital pathological image. According to the method, the deep neural network technology is combined with the characteristics of the prostatedigital pathological image, the Gleason score is rapidly and automatically analyzed and predicted, subjective judgment errors are reduced, and more accurate Gleason score reference is provided.
Owner:WONDERS INFORMATION +1
Flavopereirine and alstonine combinations in the treatment and prevention of prostate cancer
InactiveUS20050266107A1Contributes to effectiveness of preventiveAlleviating and avoiding onsetBiocideUnknown materialsDiseaseDouble-time
A method and composition for preventing prostate cancer and / or reducing PSA levels and / or alleviating the symptoms of BPH (Benign Prostatic Hyperplasia) or PIN (prostatic intraepithelial neoplasia) by administration of an effective amount of a mixture of flavopereirine and alstonine. A method and composition for treating low-grade prostate cancer and preventing the onset of metastatic disease and / or reducing the doubling time of PSA levels in men with positive biopsies showing relatively low Gleason scores and morphologies characteristic of non-invasive, slow-progressing prostate cancer. The flavopereirine and alstonine can be in the form of natural extracts derived from Pao Pereira and Rauwolfia Vomitoria, respectively. Alternatively, these two active compounds can be administered in purified form. The composition can be in included in a kit along with instructions for use in a treatment regimen.
Owner:MOLECULAR INT RES +1
Methods of treating a subject with a high gleason score prostate cancer
Owner:EXOSOME DIAGNOSTICS
A device for predicting the risk of bone metastases in newly diagnosed prostate cancer
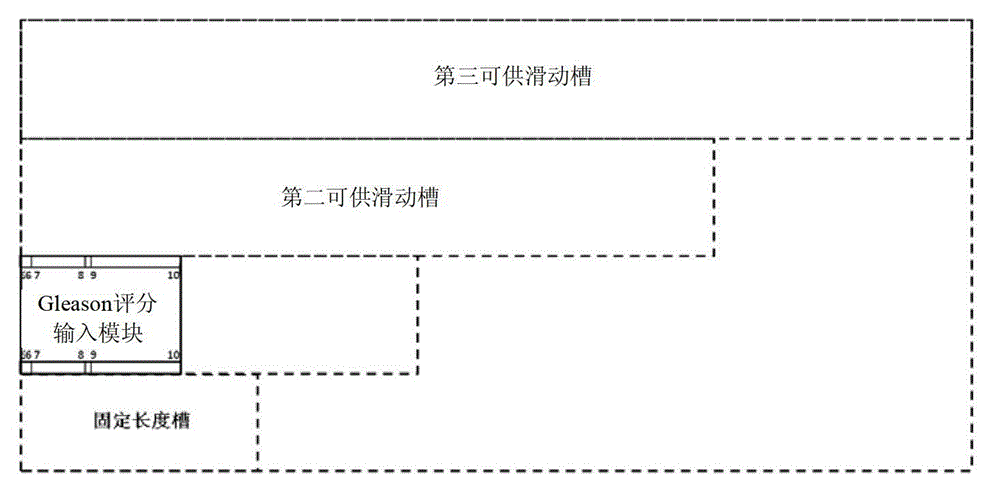
InactiveCN102968558BAvoid overuseClinically convenientSpecial data processing applicationsNewly diagnosedMedicine
The invention discloses a device for predicting the bone metastasis risk of newly-diagnosed prostate cancer. According to the device, the risk of bone metastasis is predicted, bone scanning is carried out on patients with high bone metastasis risk and the overuse of the bone scanning is avoided under the condition of not increasing missed diagnosis. The technical scheme provided by the invention is that the device comprises a storage module, four input modules, a processing module and an output module, wherein four input slots are formed in the storage module and are a slot with fixed length, a first slot for sliding, a second slot for sliding and a third slot for sliding respectively; the four input modules are a clinical stage input module placed in the slot with the fixed length, a Gleason scoring input module placed in the first slot for sliding, a PSA (Prostate-Specific Antigen) value input module placed in the second slot for sliding and an age input module placed in the third slot for sliding; the processing module is used for adjusting the positions of the four input modules in the input slots of the storage module to obtain an output locus; and the output module is provided with a staff gauge for predicting a risk value of the bone metastasis, and a corresponding locus of the output locus on the staff gauge is a predicted risk value.
Owner:叶定伟
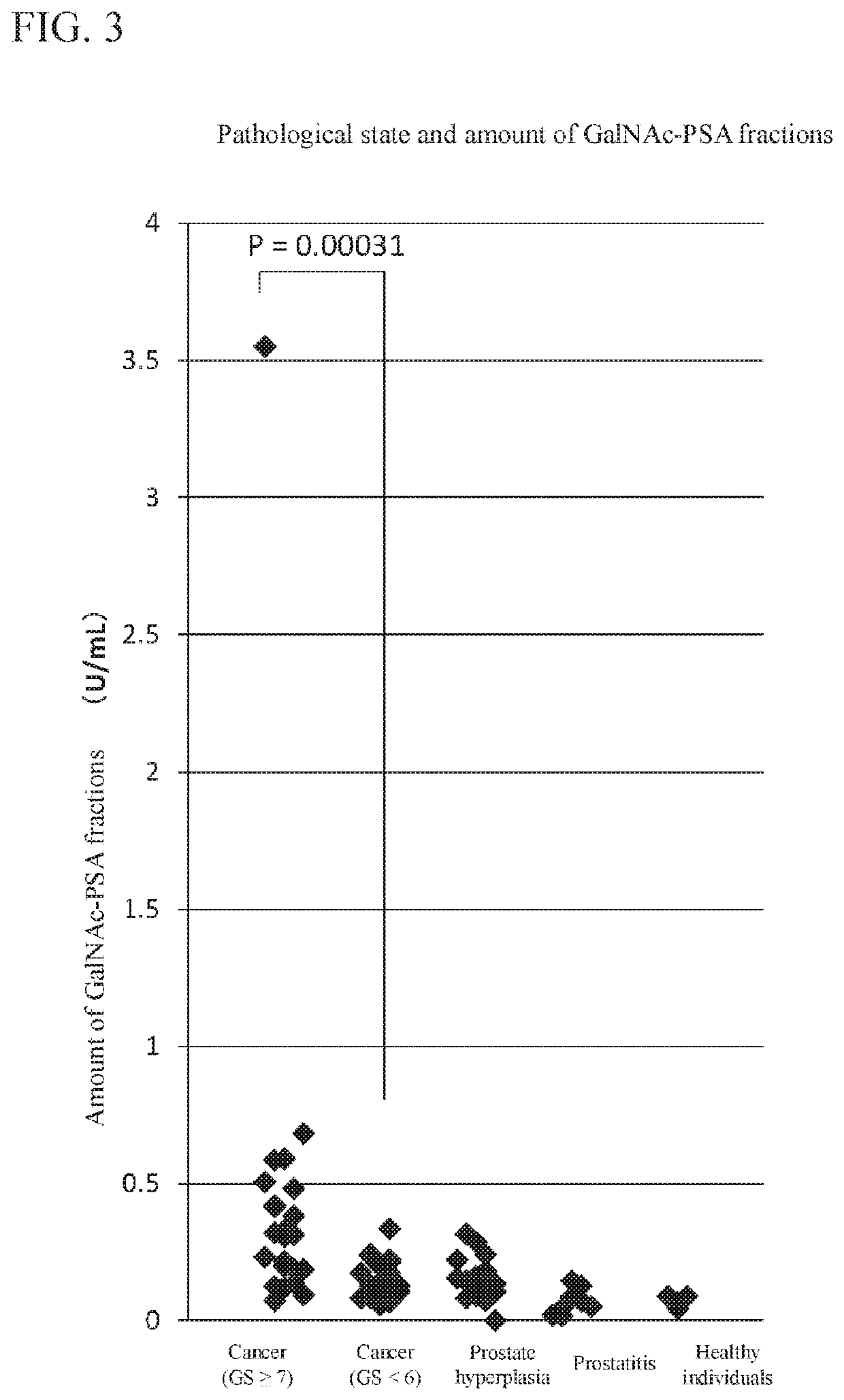
Method for estimating pathological tissue diagnosis result (gleason score) of prostate cancer
ActiveUS20180348223A1Heavy burdenReduce intrusionBiological testingDetection of post translational modificationsAntigenWisteria floribunda
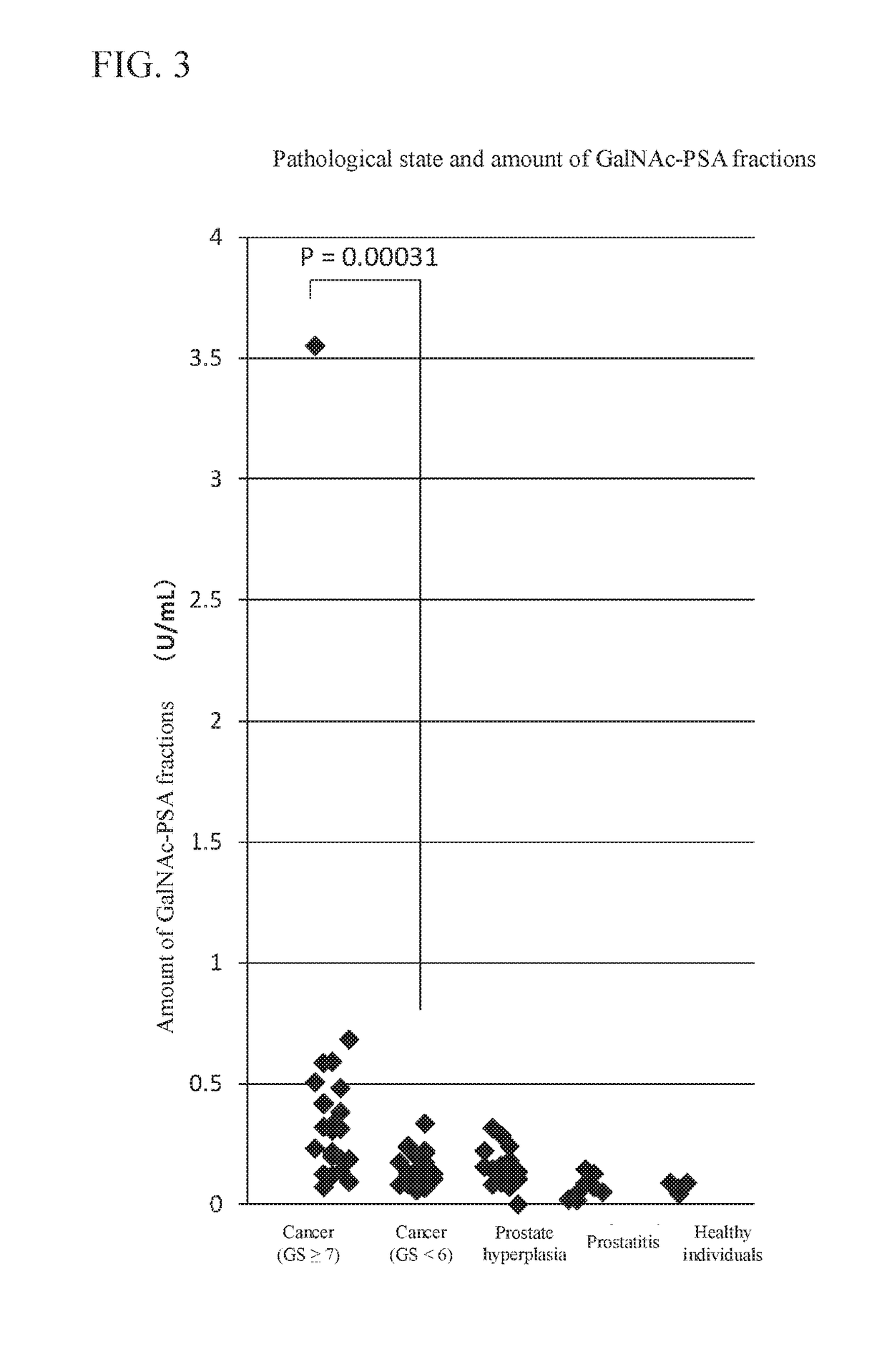
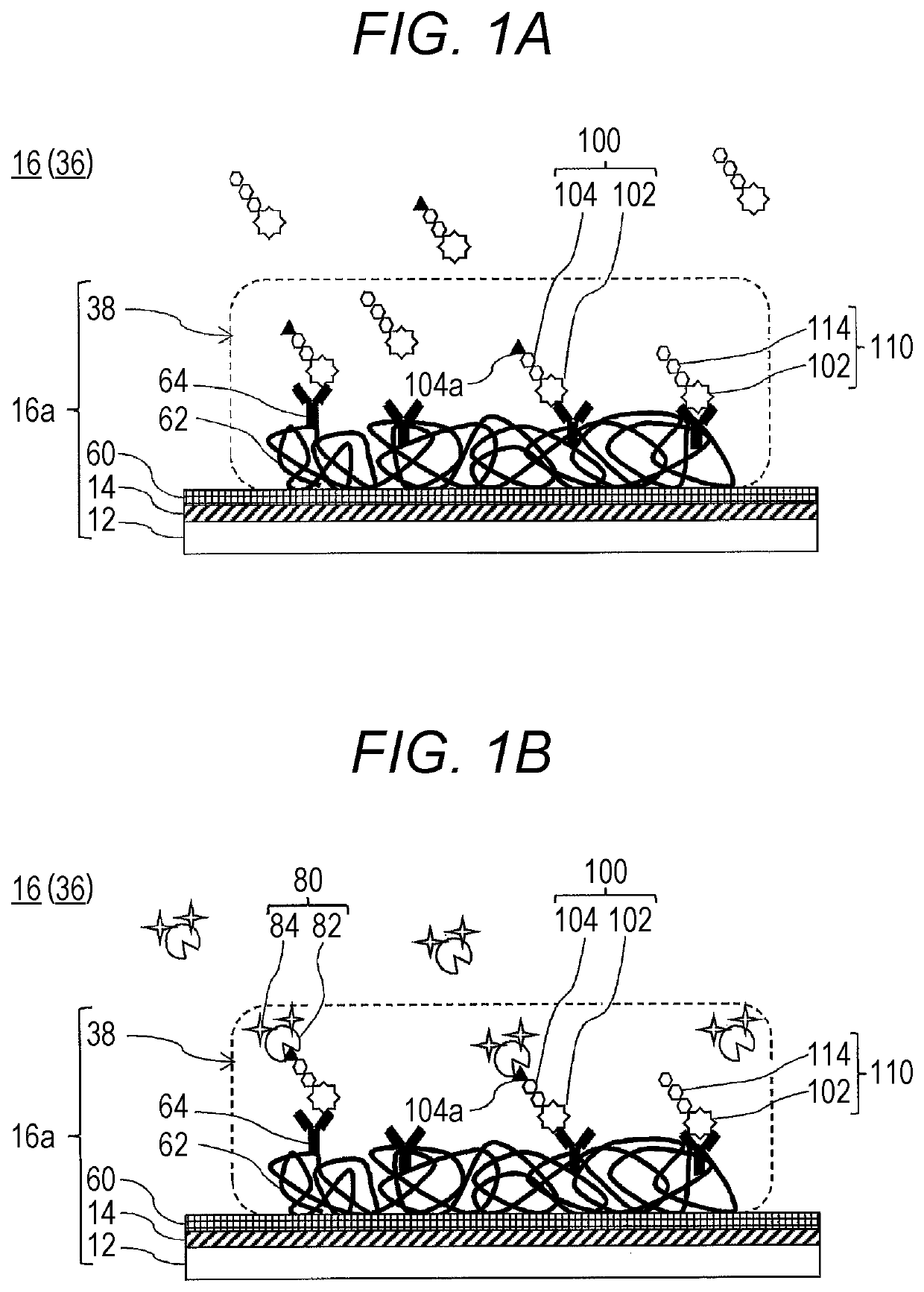
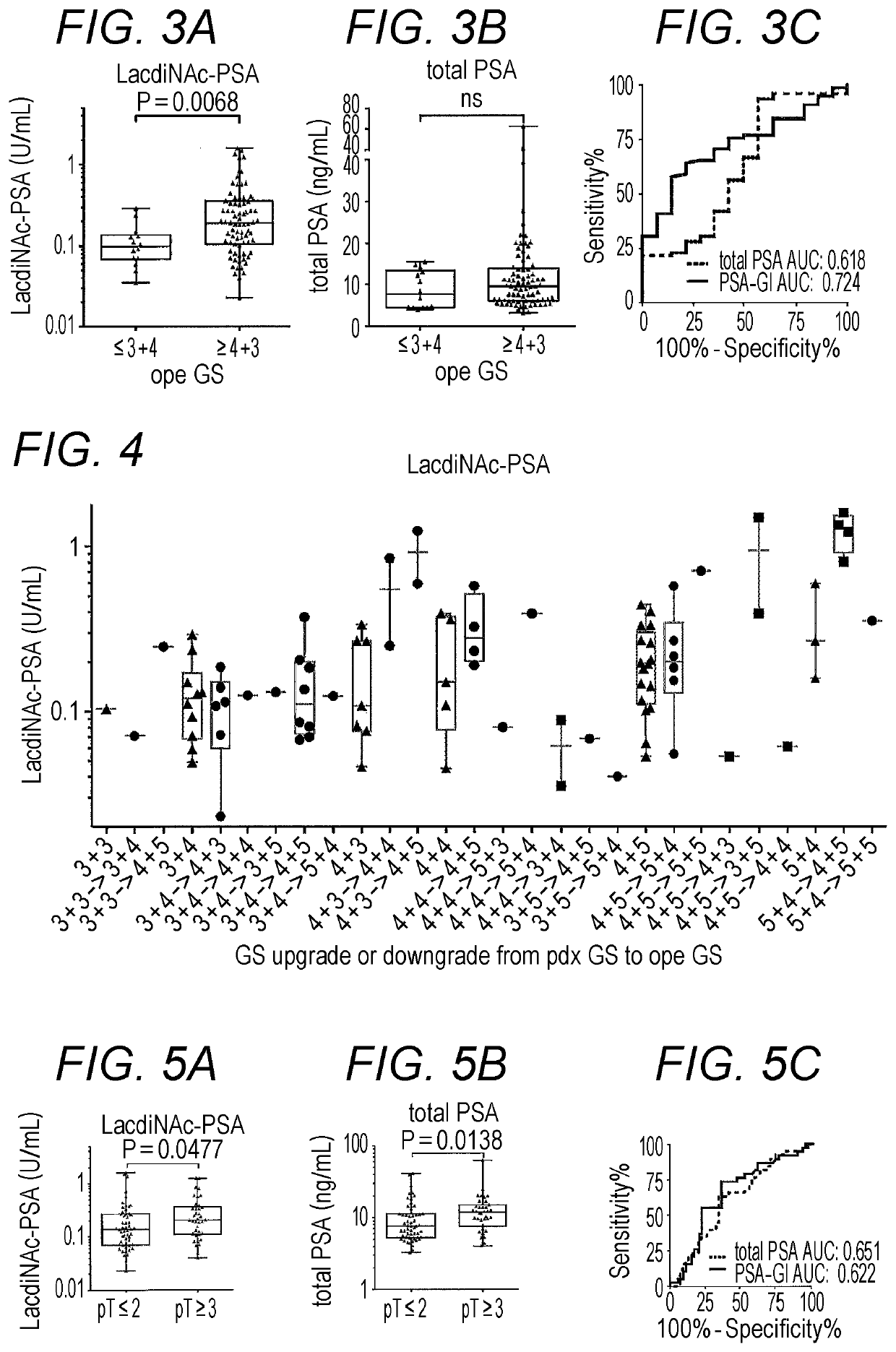
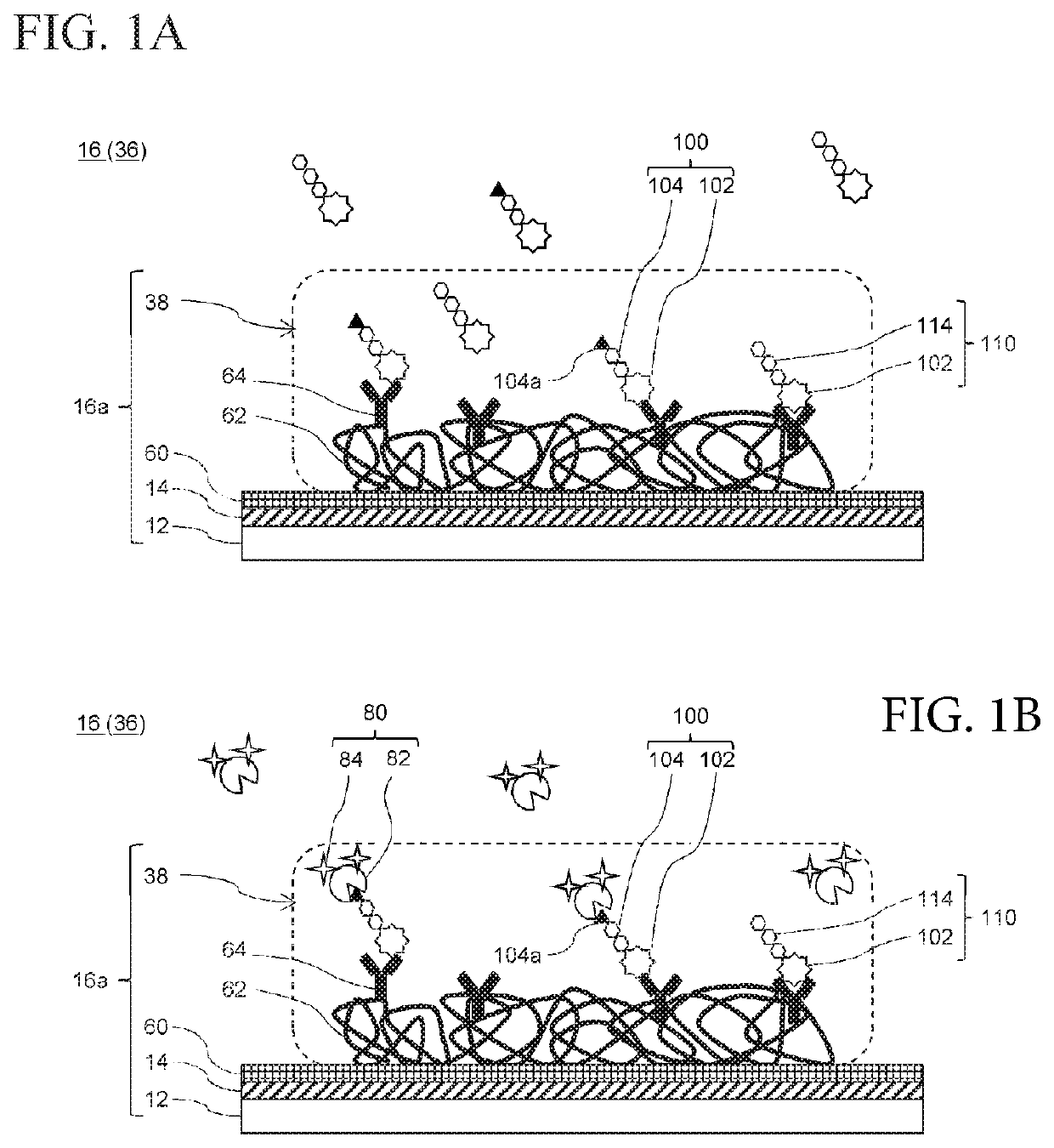
Provided is a method of obtaining an index value used for pathological tissue diagnosis of prostate cancer, which method has low invasiveness and can be performed at a low cost. The method is a method of estimating a Gleason score that represents the malignancy of prostate cancer, which method includes: measuring the content of a prostate-specific antigen having an N-acetylgalactosamine residue at a non-reducing terminal of a sugar chain in a sample; and estimating that the Gleason score is 7 or higher when the thus measured value is larger than a threshold value, or estimating that the Gleason score is 6 or lower when the measured value is smaller than a threshold value. The prostate-specific antigen is preferably quantified by a method including the step of binding a molecule having an affinity for β-N-acetylgalactosamine residue, such as Wisteria floribunda lectin, soybean agglutinin, Vicia Villosa lectin or an anti-β-N-acetylgalactosamine antibody, to the prostate-specific antigen.
Owner:OTSUKA PHARM CO LTD
Metabolic profiling in tissue and serum is indicative of tumor differentiation in prostate cancer
InactiveUS20150310169A1Enhancing risk predictionHealth-index calculationMicrobiological testing/measurementMetabolic profileGleason Score 6
The invention provides methods and products to detect the presence of unidentified high grade prostate tumors in a subject with a Gleason score 7 prostate tumor. The method comprises obtaining a biological sample from a subject in need thereof, measuring a profile of metabolites in the biological sample, wherein the metabolites are differentially expressed in Gleason score 6 versus Gleason score 8 prostate tumors, and classifying the profile of the metabolites to assign a grade to the sample based on the profile of the metabolites.
Owner:DANA FARBER CANCER INST INC +1
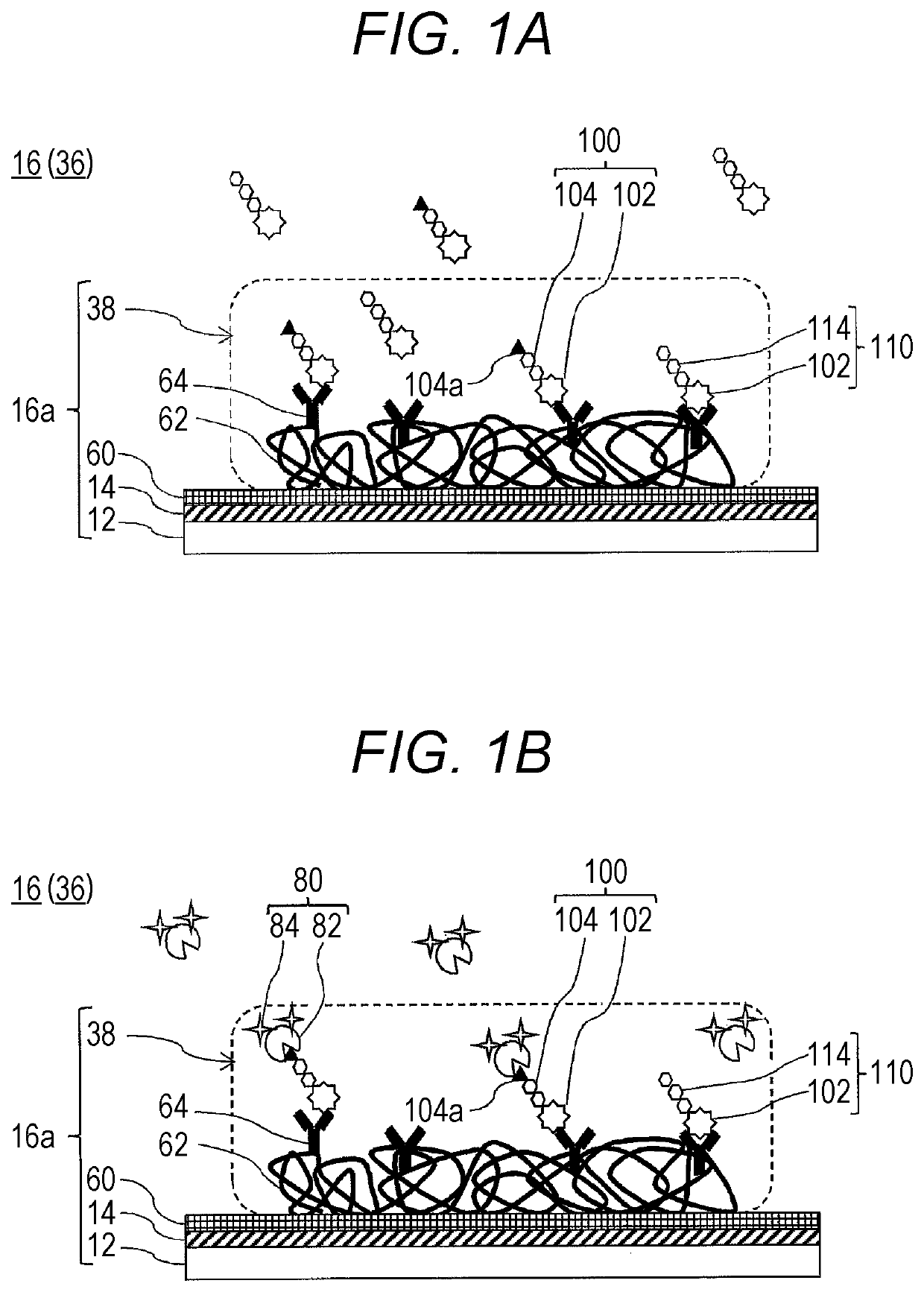
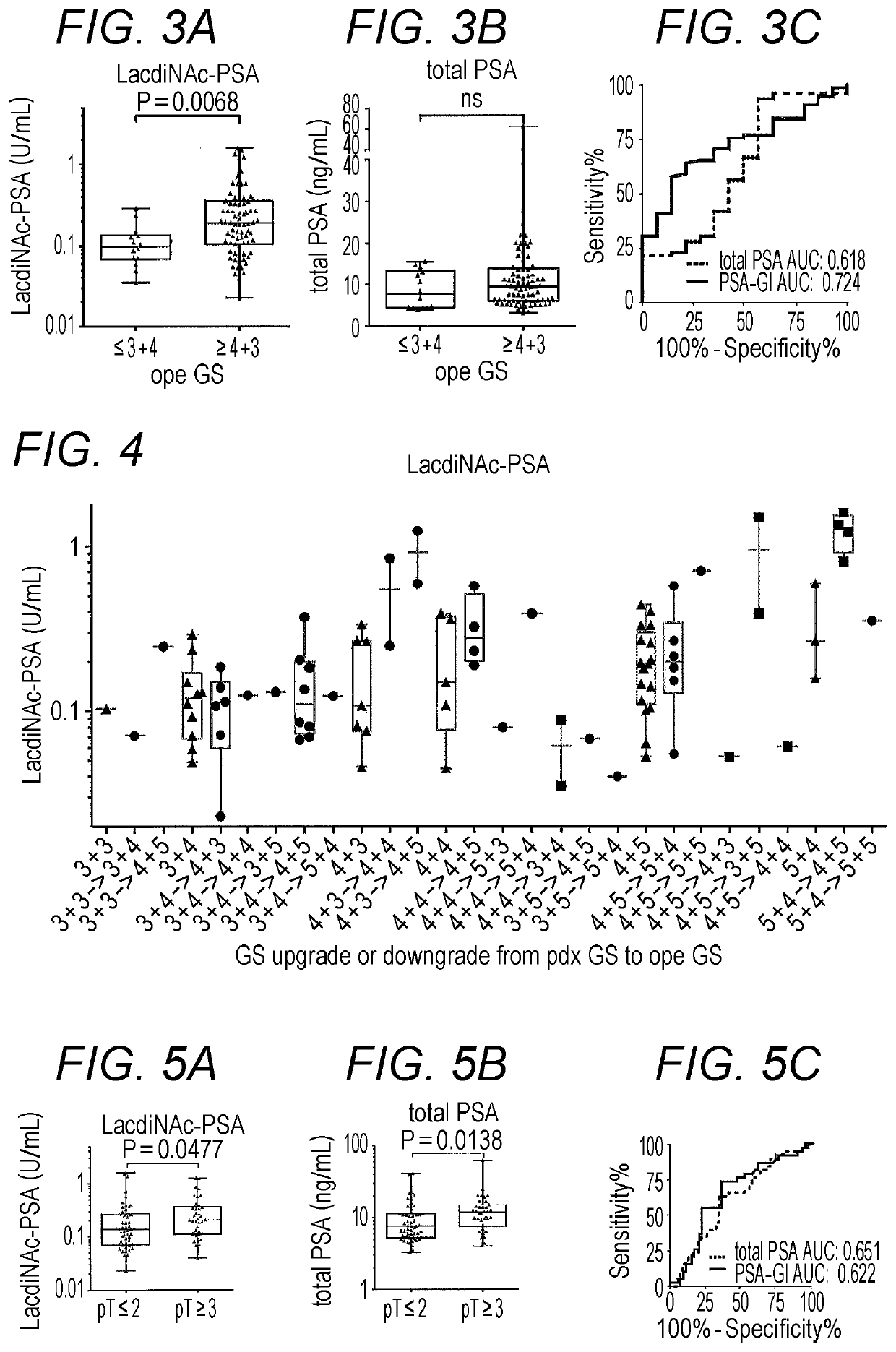
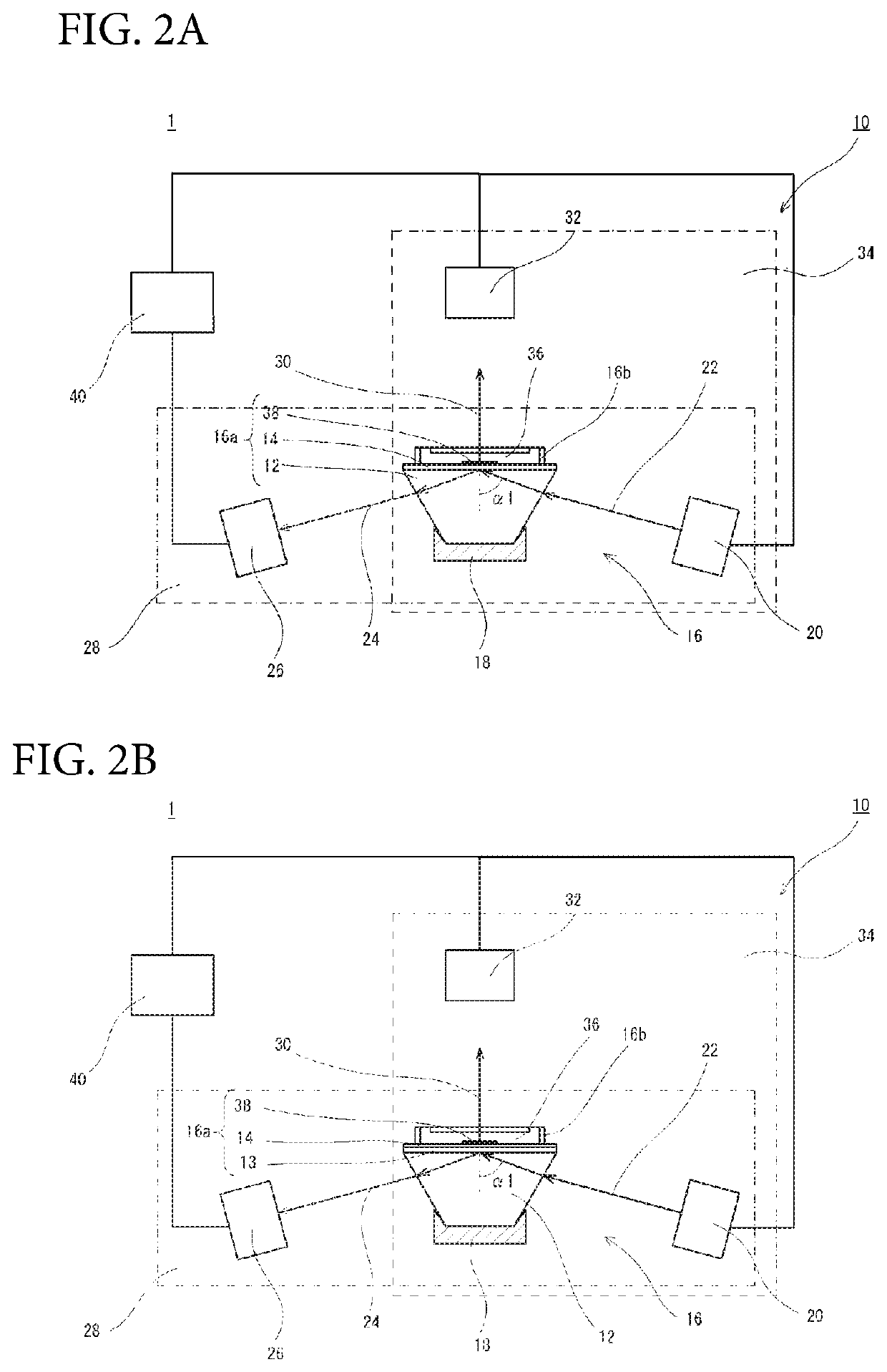
Method For Estimating Gleason Score Of Prostate Cancer, Method For Estimating Pathological Stage, And Method For Acquiring Supplementary Information, All On The Basis Of Specific PSA Content In Specimen
ActiveUS20190383818A1Reduce intrusionSuppressing medical costDisease diagnosisFluorescence/phosphorescenceTotal removalProstate-specific antigen
The present invention provides methods that are for acquiring various types of supplementary information used for diagnosis or treatment of prostate cancer, and that can be implemented in a less-invasive manner at a low cost. Provided are, by measuring the content of prostate specific antigen (PSA) having a β-N-acetylgalactosamine residue at a non-reducing terminal of a sugar chain in a specimen, and comparing the measured value with a threshold value, (1) a method for estimating whether a Gleason score (primary pattern and secondary pattern) is not less than or less than a prescribed value, (2) a method for estimating whether the pathological stage (pT) is not less than or less than a prescribed value, and (3) a method for acquiring information for assessment indicating diagnosis or treatment should be actively conducted because a GS at gross total removal is expected to be higher than a GS at biopsy.
Owner:HIROSAKI UNIVERSITY +1
Rapid diagnosis and scoring method for full-scale pathological slices based on deep learning
ActiveCN108305249BSolve the problem of size limitationImplement diagnosticsImage enhancementImage analysisStainingHeat map
Owner:FUJIAN NORMAL UNIV
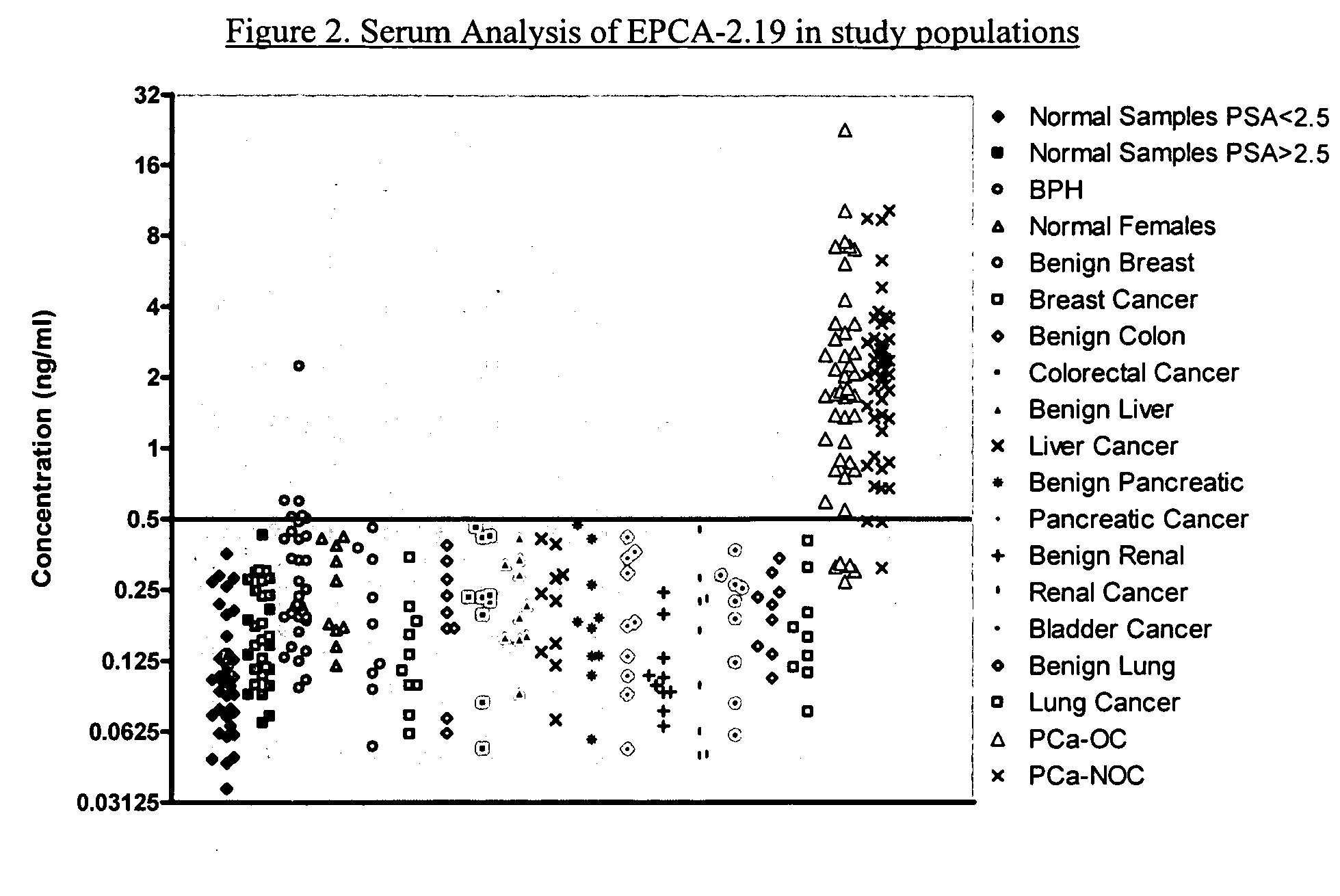
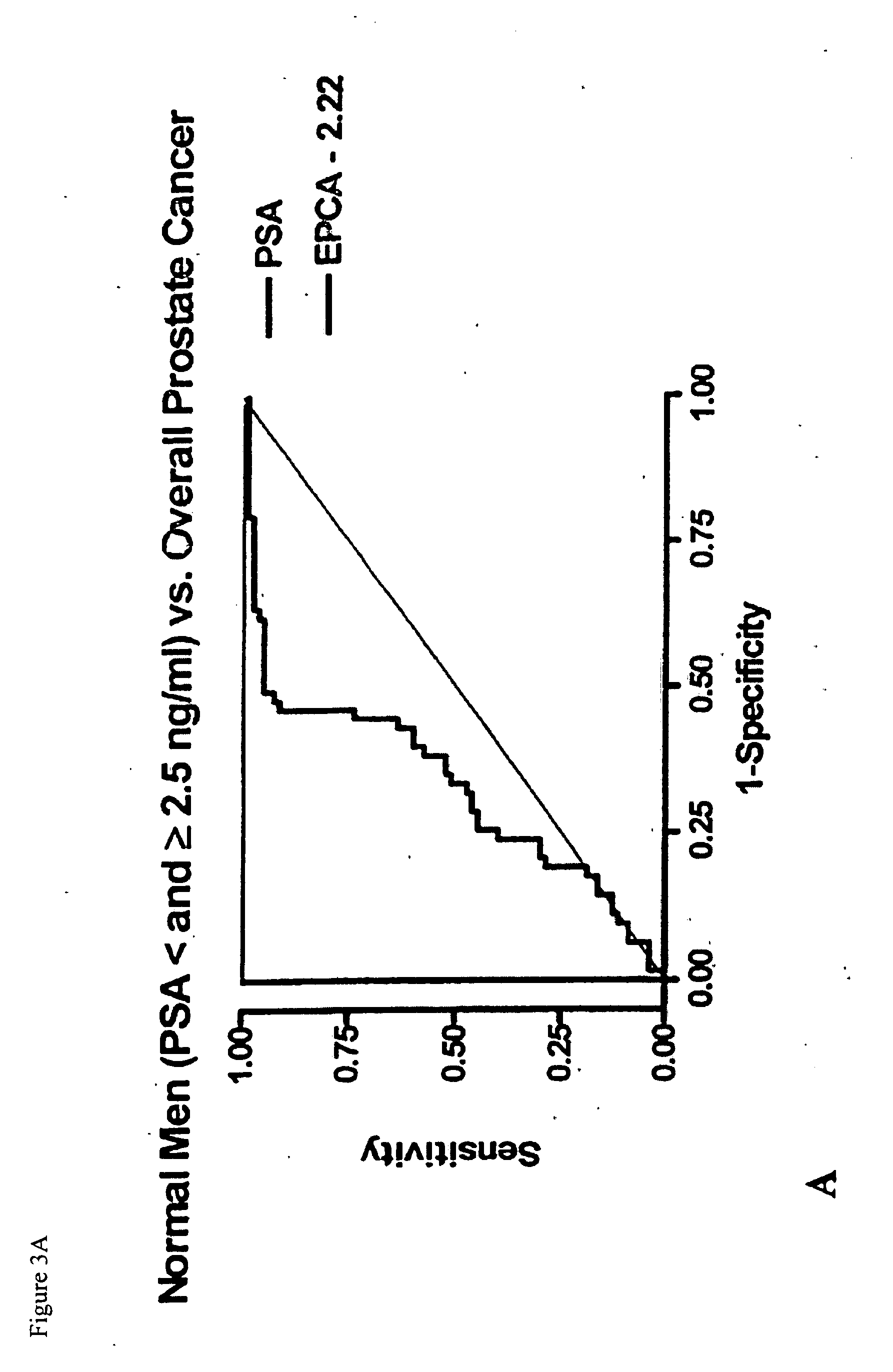
Early prostate cancer antigen-2: a novel serum specific marker for prostate cancer detection
Early Prostate Cancer Antigen-2 (EPCA-2) is a prostate cancer biomarker, newly identified via proteomics-based examination of alterations in nuclear structure. EPCA-2 shows a higher overall specificity than PSA. Thus, an immunoassay based on antibodies against an epitope of EPCA-2 more accurately differentiates between confined and non-confined disease than PSA or Gleason score.
Owner:UNIVERSITY OF PITTSBURGH
Method for estimating Gleason score of prostate cancer, method for estimating pathological stage, and method for acquiring supplementary information, all on the basis of specific PSA content in specimen
ActiveUS11137403B2Heavy burdenReduce intrusionDisease diagnosisFluorescence/phosphorescenceAntigenPsa antigen
The present invention provides methods that are for acquiring various types of supplementary information used for diagnosis or treatment of prostate cancer, and that can be implemented in a less-invasive manner at a low cost. Provided are, by measuring the content of prostate specific antigen (PSA) having a β-N-acetylgalactosamine residue at a non-reducing terminal of a sugar chain in a specimen, and comparing the measured value with a threshold value, (1) a method for estimating whether a Gleason score (primary pattern and secondary pattern) is not less than or less than a prescribed value, (2) a method for estimating whether the pathological stage (pT) is not less than or less than a prescribed value, and (3) a method for acquiring information for assessment indicating diagnosis or treatment should be actively conducted because a GS at gross total removal is expected to be higher than a GS at biopsy.
Owner:HIROSAKI UNIVERSITY +1
Method for estimating pathological tissue diagnosis result (Gleason score) of prostate cancer
ActiveUS11105807B2Heavy burdenReduce intrusionBiological testingDetection of post translational modificationsAntigenPsa antigen
Owner:OTSUKA PHARM CO LTD
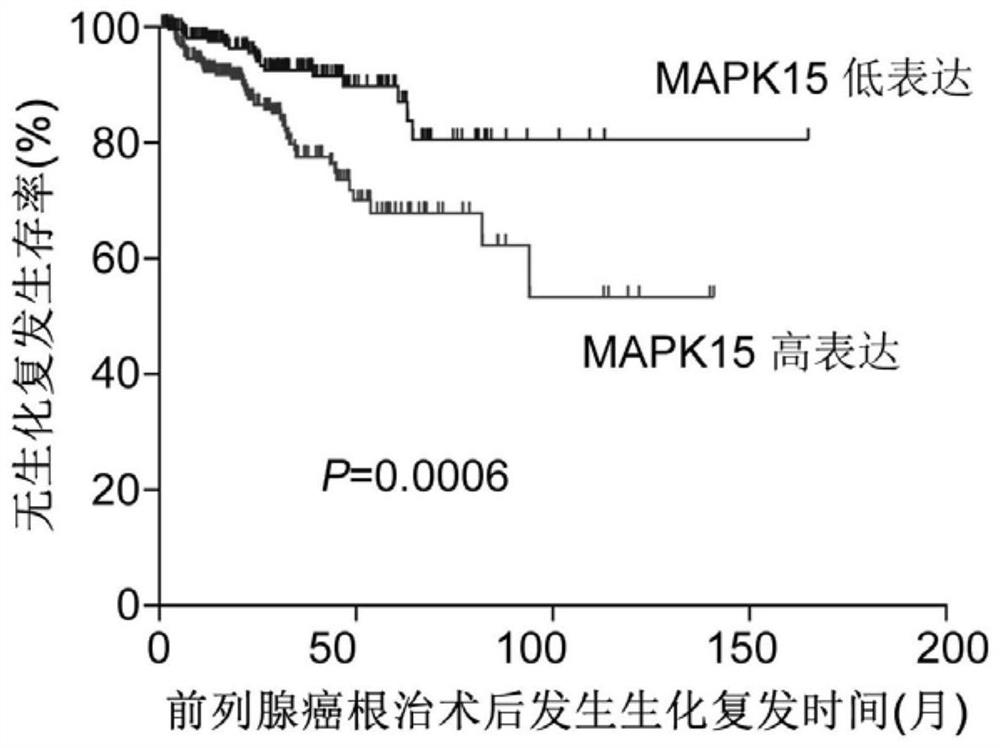
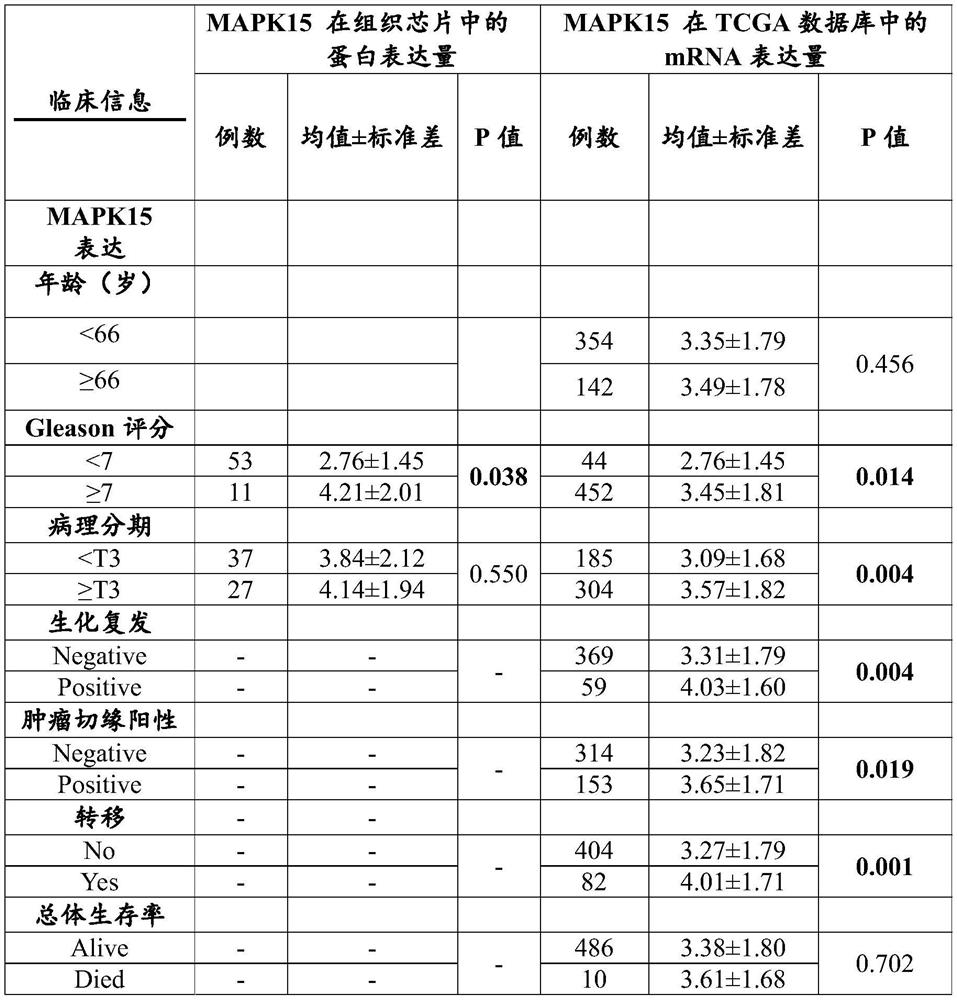
Application of MAPK15 protein in prediction of malignancy degree or prognosis degree of prostate cancer
The invention relates to the technical field of prostate cancer detection, in particular to application of MAPK15 protein in prediction of malignancy degree or prognosis degree of prostate cancer. The invention discloses application of a molecule for detecting MAPK15 protein in preparation of a kit or a detection reagent for predicting the malignancy degree or prognosis degree of prostatic cancer. The invention further discloses a kit for predicting the malignancy degree or prognosis degree of the prostate cancer. The kit comprises an endogenous peroxidase blocking agent, animal non-immune serum, a primary antibody, a biotin-labeled secondary antibody, streptomyces avitin protein-peroxidase, a DAB color developing agent and a phosphate buffer salt solution. The MAPK15 is used as a marker for detecting PSA biochemical recurrence, tumor metastasis and Gleason score (GS) after the prostate cancer operation, and a feasible detection method is provided for predicting prostate cancer progression.
Owner:广州金研生物医药研究院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com