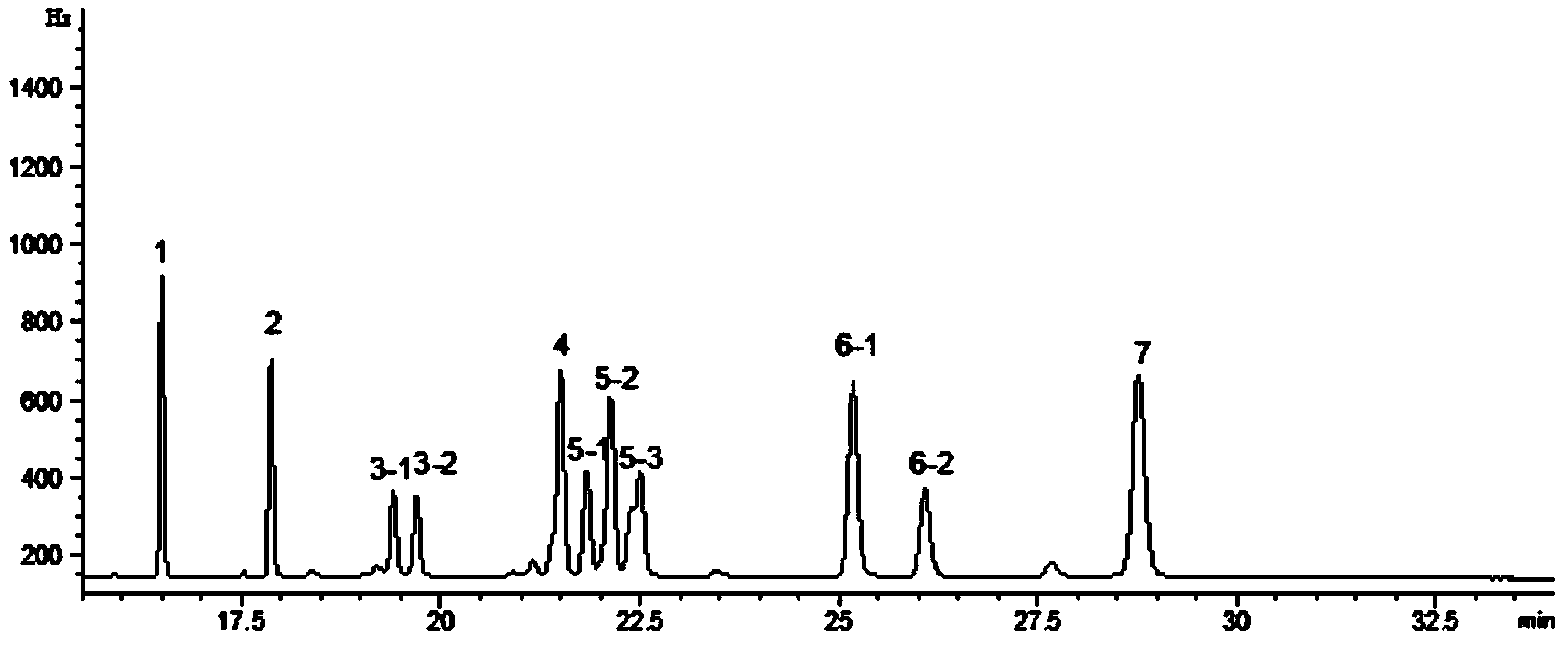
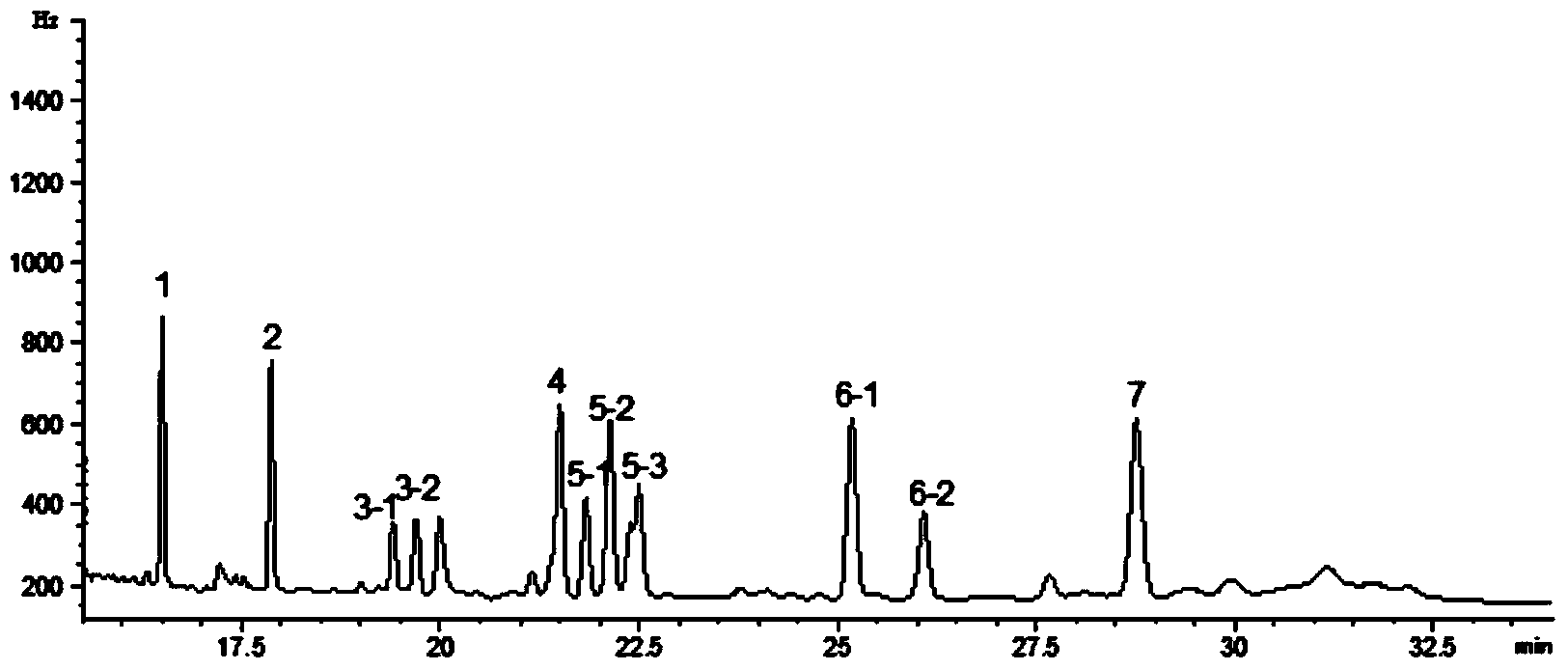
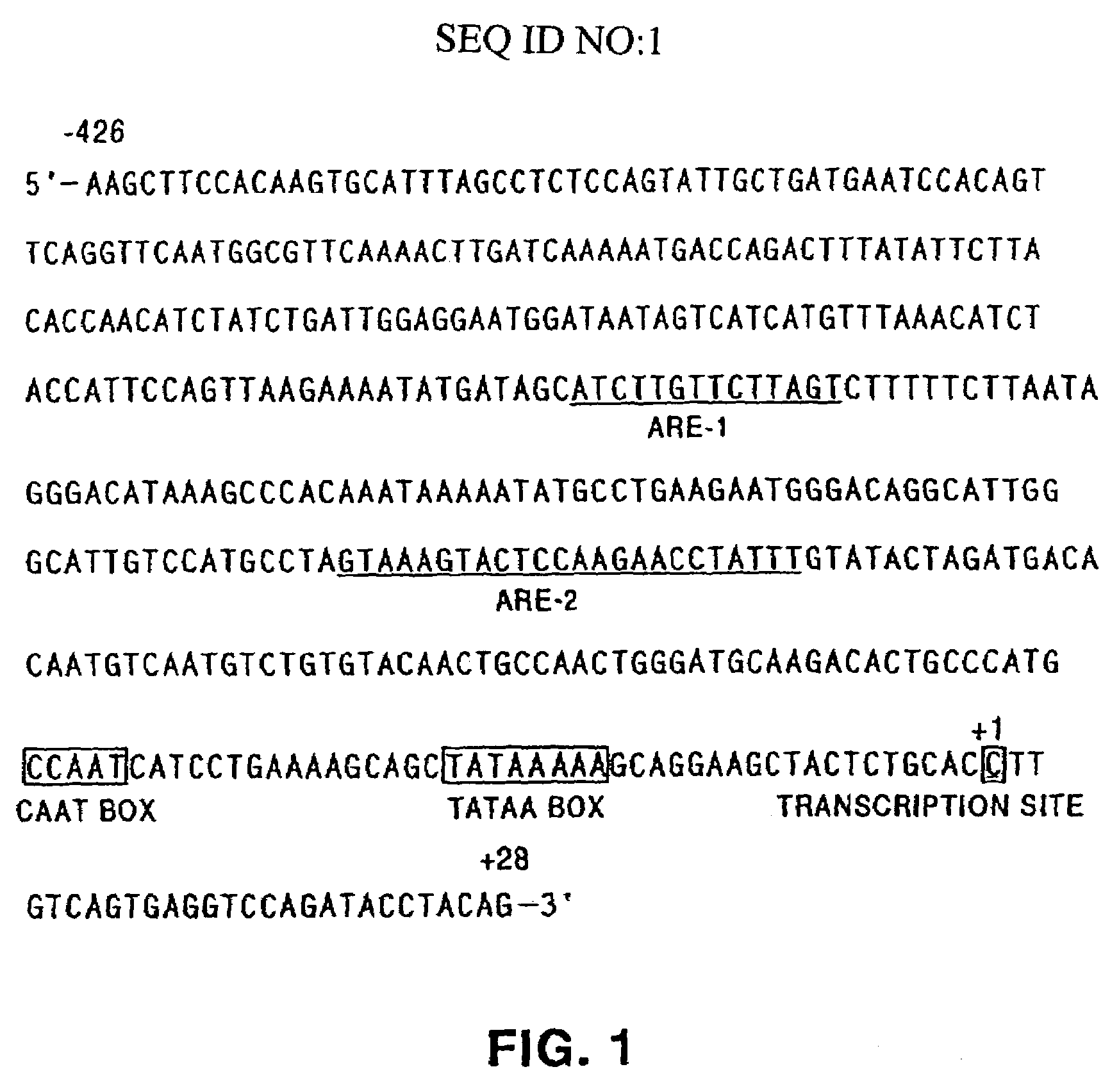
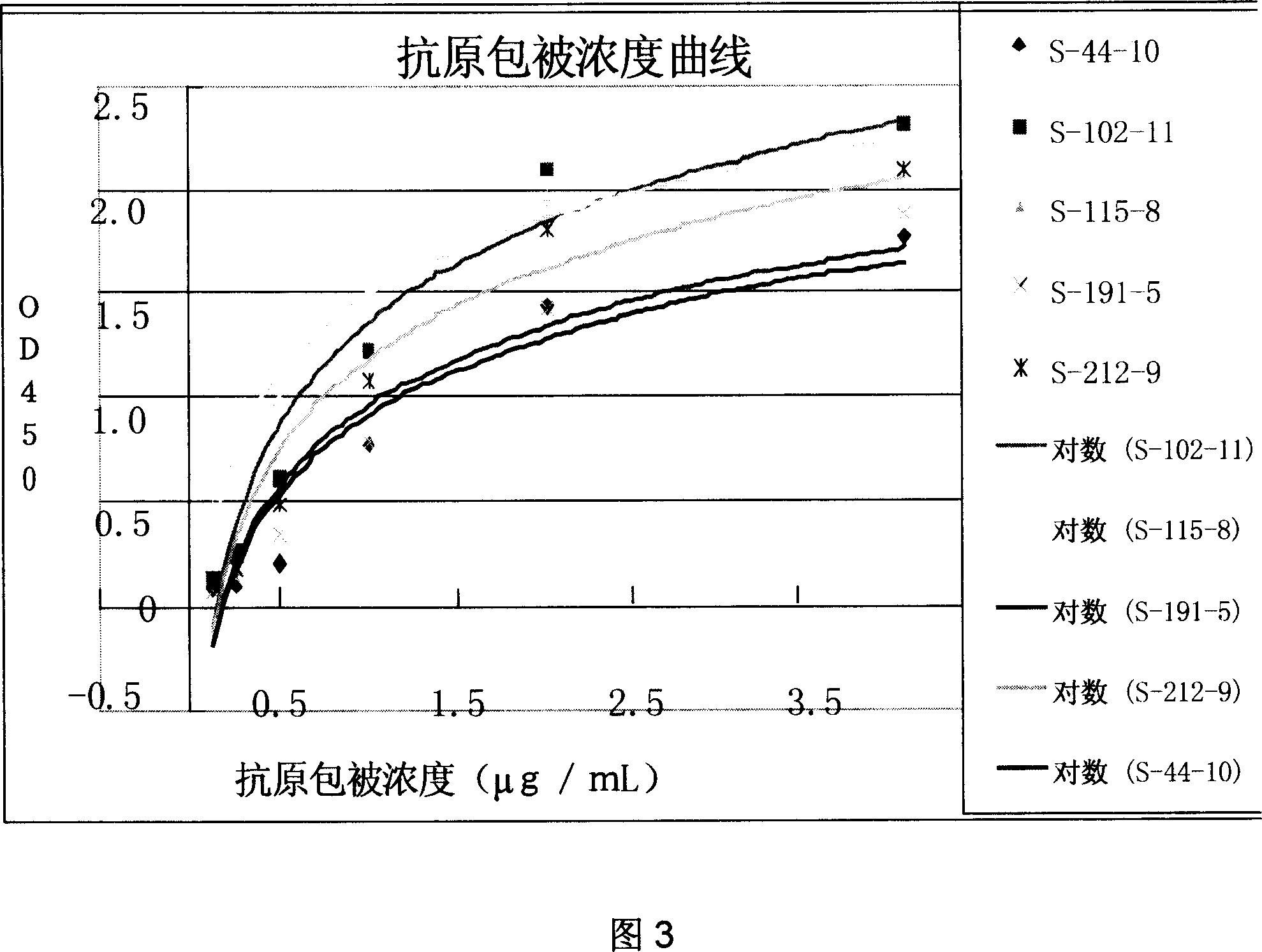
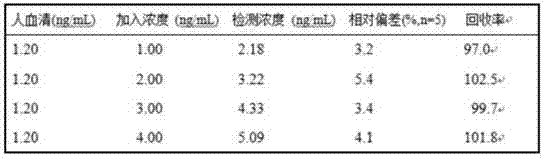
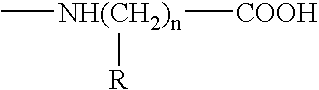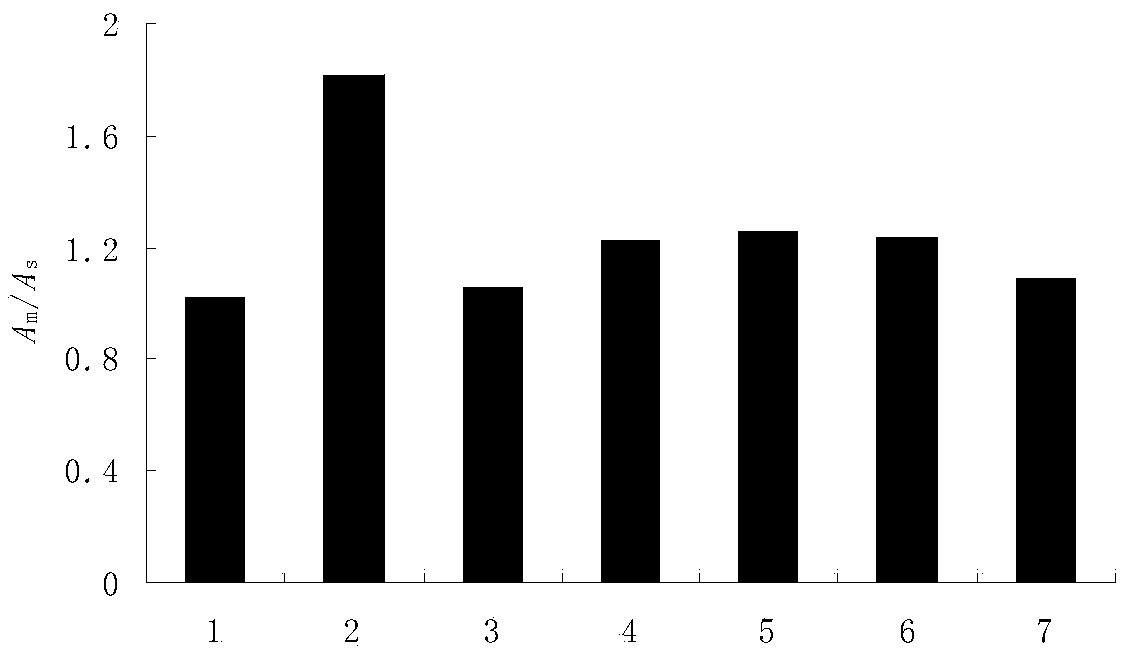Patents
Literature
221 results about "Prostate-specific antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Prostate-specific antigen (PSA), also known as gamma-seminoprotein or kallikrein-3 (KLK3), is a glycoprotein enzyme encoded in humans by the KLK3 gene. PSA is a member of the kallikrein-related peptidase family and is secreted by the epithelial cells of the prostate gland.
Reagents and methods for inducing an immune response to prostate specific antigen
InactiveUS6881405B2Sensitive to proteolytic degradationIncrease productionSnake antigen ingredientsAntibody ingredientsProstate-specific antigenAntibody
Owner:ONCOQUEST INC
Medicament for treating prostate diseases
InactiveUS7582294B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsNatural antibodyHeifer calf
A medicament based on antibodies contains an activated form of ultra-low doses of monoclonal, polyclonal, or natural antibodies to the prostate-specific antigen, the activated form being prepared by multiple consecutive dilutions and exposure to external factors, preferably according to homeopathic technology. In order to obtain the antibodies, the prostate-specific antigen isolated from the prostatic tissues of cattle or prepared synthetically is employed; a mixture of various, mostly centesimal, homeopathic dilutions is used. The method of treating diseases of the urogenital sphere consists in using activated forms of ultra-low doses of antibodies to prostate-specific antigen prepared by multiple consecutive dilutions and exposure to external factors.
Owner:EPSHTEIN OLEG I
Polyamine analog conjugates and quinone conjugates as therapies for cancers and prostate diseases
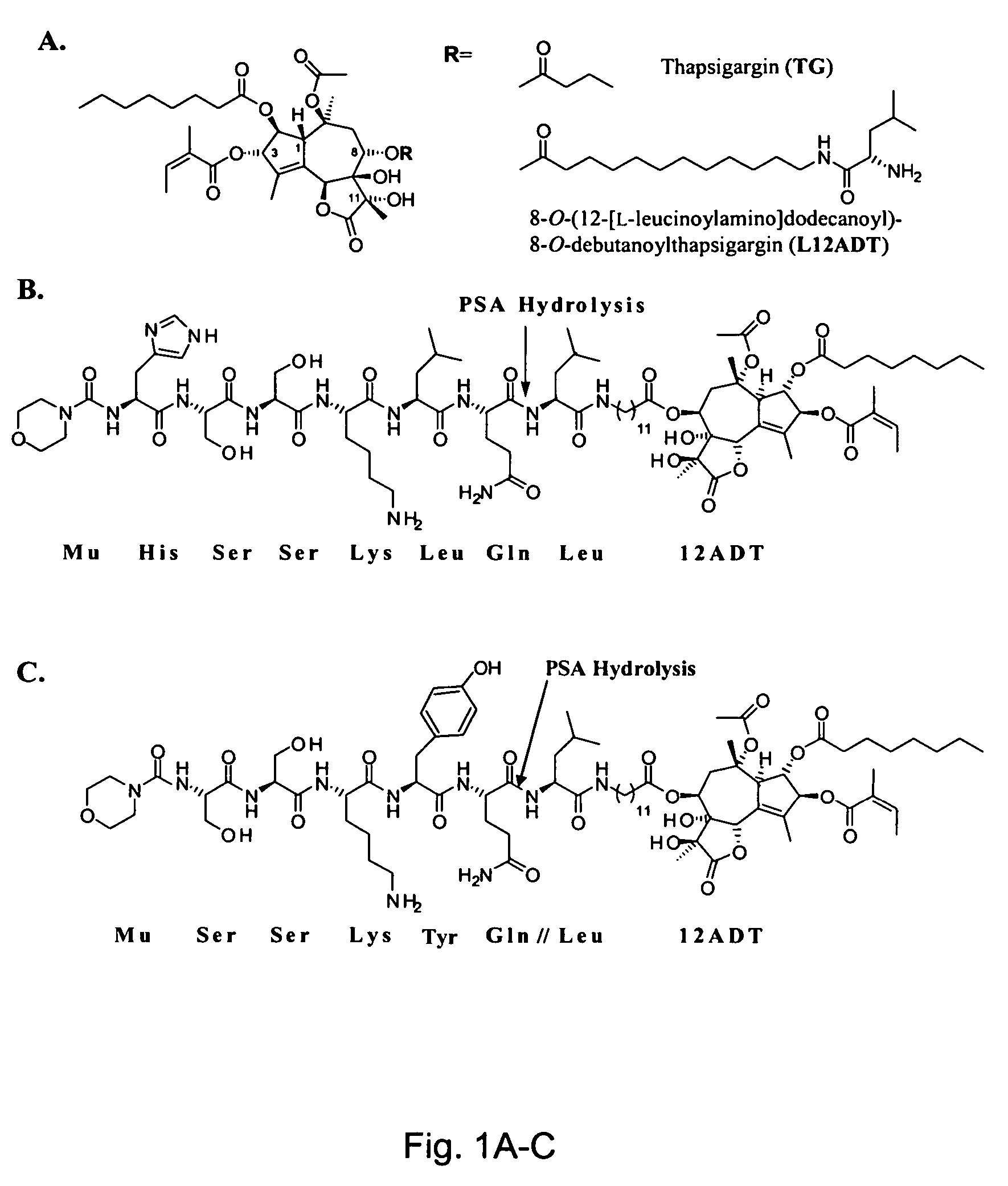
Peptide conjugates in which cytocidal and cytostatic agents, such as polyamine analogs or naphthoquinones, are conjugated to a polypeptide recognized and cleaved by enzymes such as prostate-specific antigen (PSA) and cathepsin B are provided, as well as compositions comprising these conjugates. Methods of using these conjugates in the treatment of prostate diseases are also provided.
Owner:CELLGATE
High throughput multi-antigen microfluidic fluorescence immunoassays
InactiveUS20060263818A1Reduce total integrated backgroundIncreased signal noiseBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenPoint of care
The development of a high-throughput multi-antigen microfluidic fluorescence immunoassay system is illustrated in a 100-chamber PDMS (polydimethylsiloxane) chip which performs up to 5 tests for each of 10 samples. Specificity of detection is demonstrated and calibration curves produced for C-Reactive Protein (CRP), Prostate Specific Antigen (PSA), ferritin, and Vascular Endothelial Growth Factor (VEGF). The measurements show sensitivity at and below levels that are significant in current clinical laboratory practice (with SIN>8 at as low as 10 pM antigen concentration). The chip uses 100 nL per sample for all four tests and provides an improved instrument for use in scientific research and “point-of-care” testing in medicine.
Owner:SCHERER AXEL +3
Peptides for targeting the prostate specific membrane antigen
InactiveUS20070254316A1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenProstate specific membrane
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Tumor activated prodrugs
InactiveUS7635682B2Improve featuresImprove anti-tumor efficacyPeptide/protein ingredientsImmunoglobulinsMedicineProstate-specific antigen
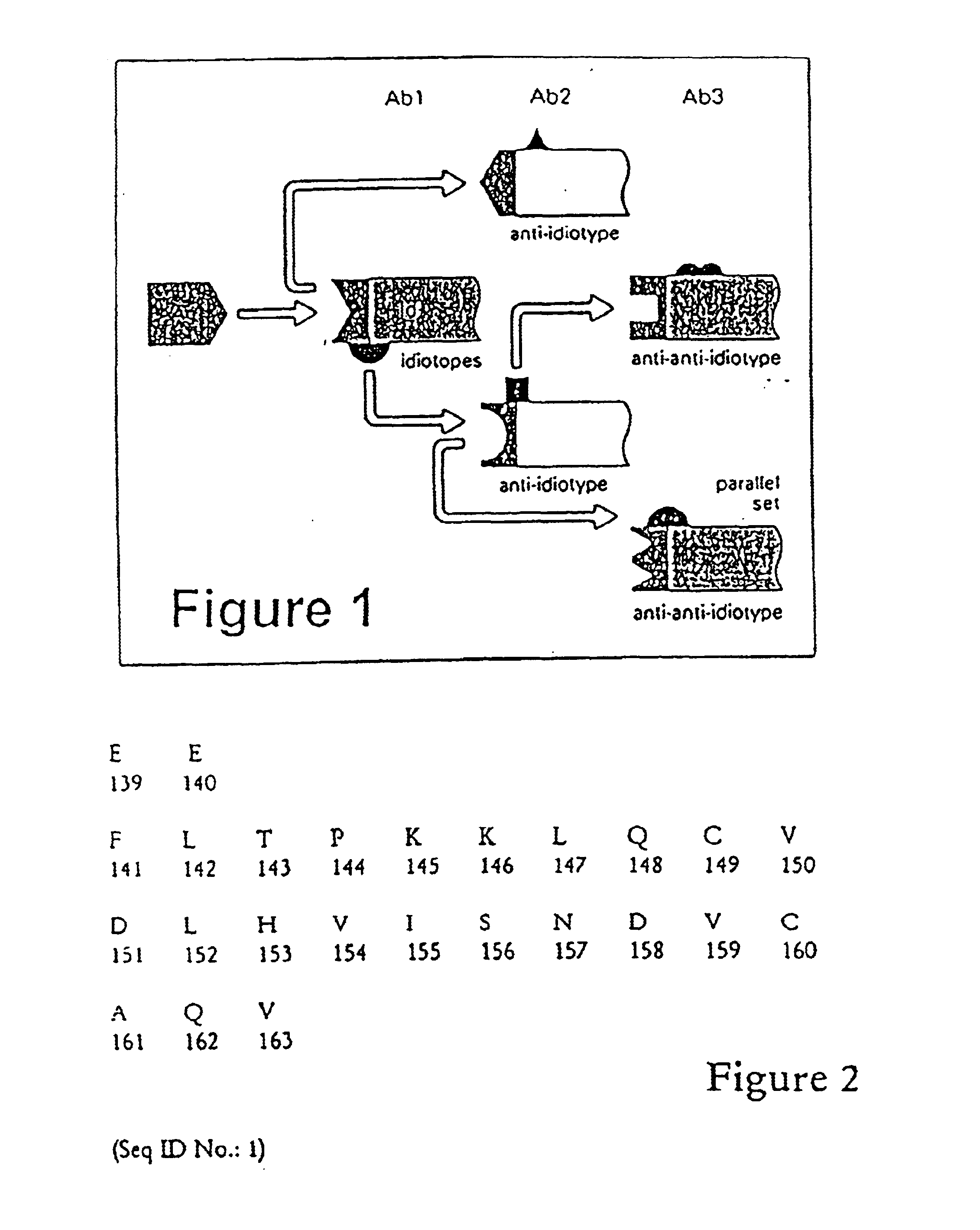
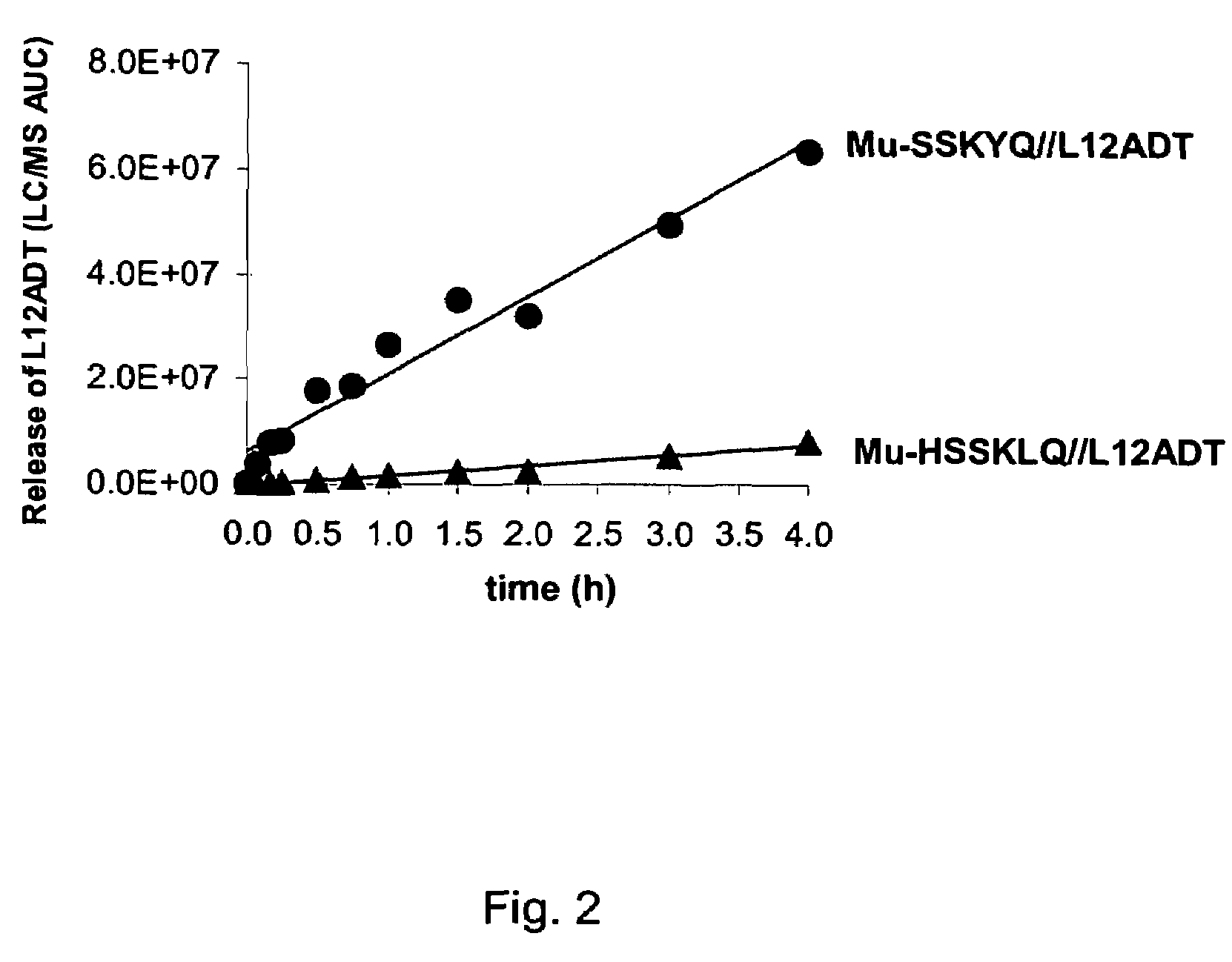
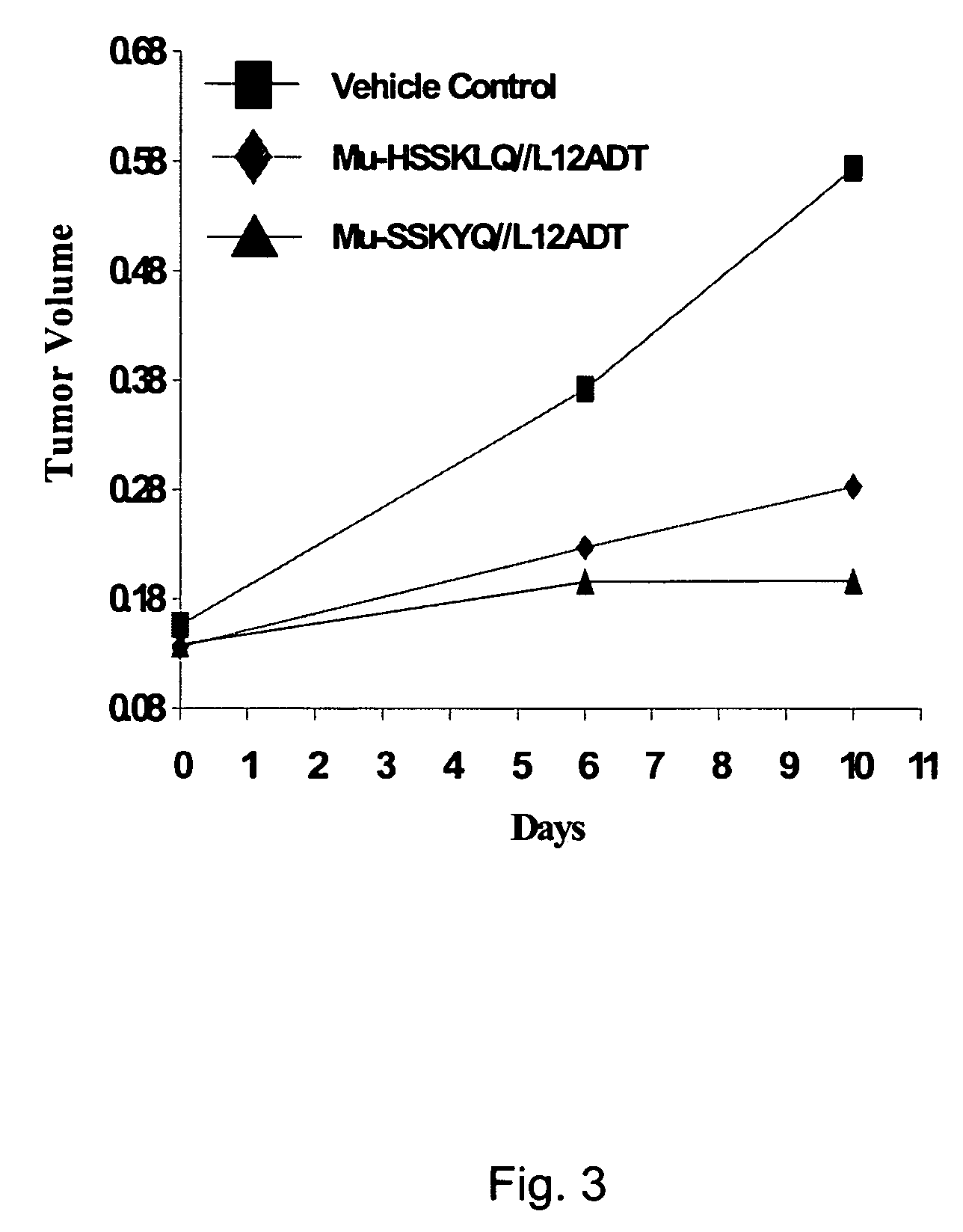
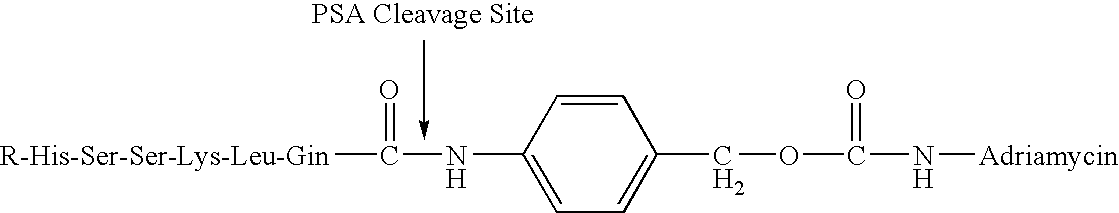
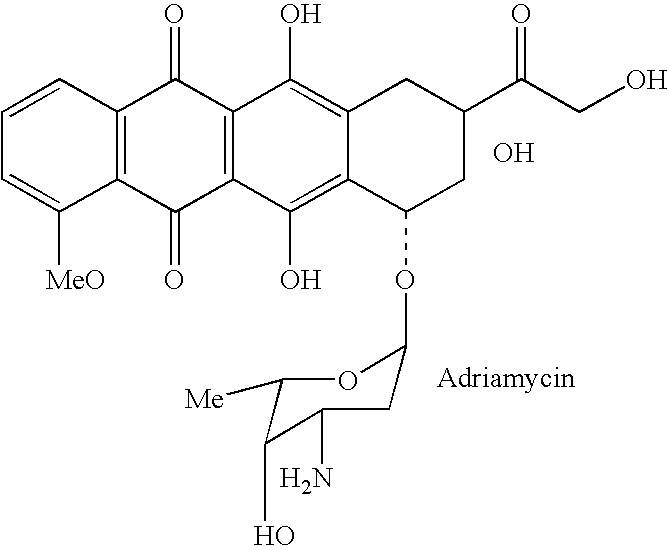
The instant invention provides compositions comprising a prodrug, the prodrug comprising a therapeutically active drug; and a peptide selected from the group consisting of the sequences: Ser-Ser-Lys-Tyr-Gln (SEQ ID NO:1);Gly-Lys-Ser-Gln-Tyr-Gln (SEQ ID NO:2); and Gly-Ser-Ala-Lys-Tyr-Gln (SEQ ID NO:3) wherein the peptide is linked to the therapeutically active drug to inhibit the therapeutic activity of the drug, and wherein the therapeutically active drug is cleaved from the peptide upon proteolysis by an enzyme having a proteolytic activity of prostate specific antigen (PSA). The invention further provides methods of making and using the claimed compositions.
Owner:INSPYR THERAPEUTICS INC
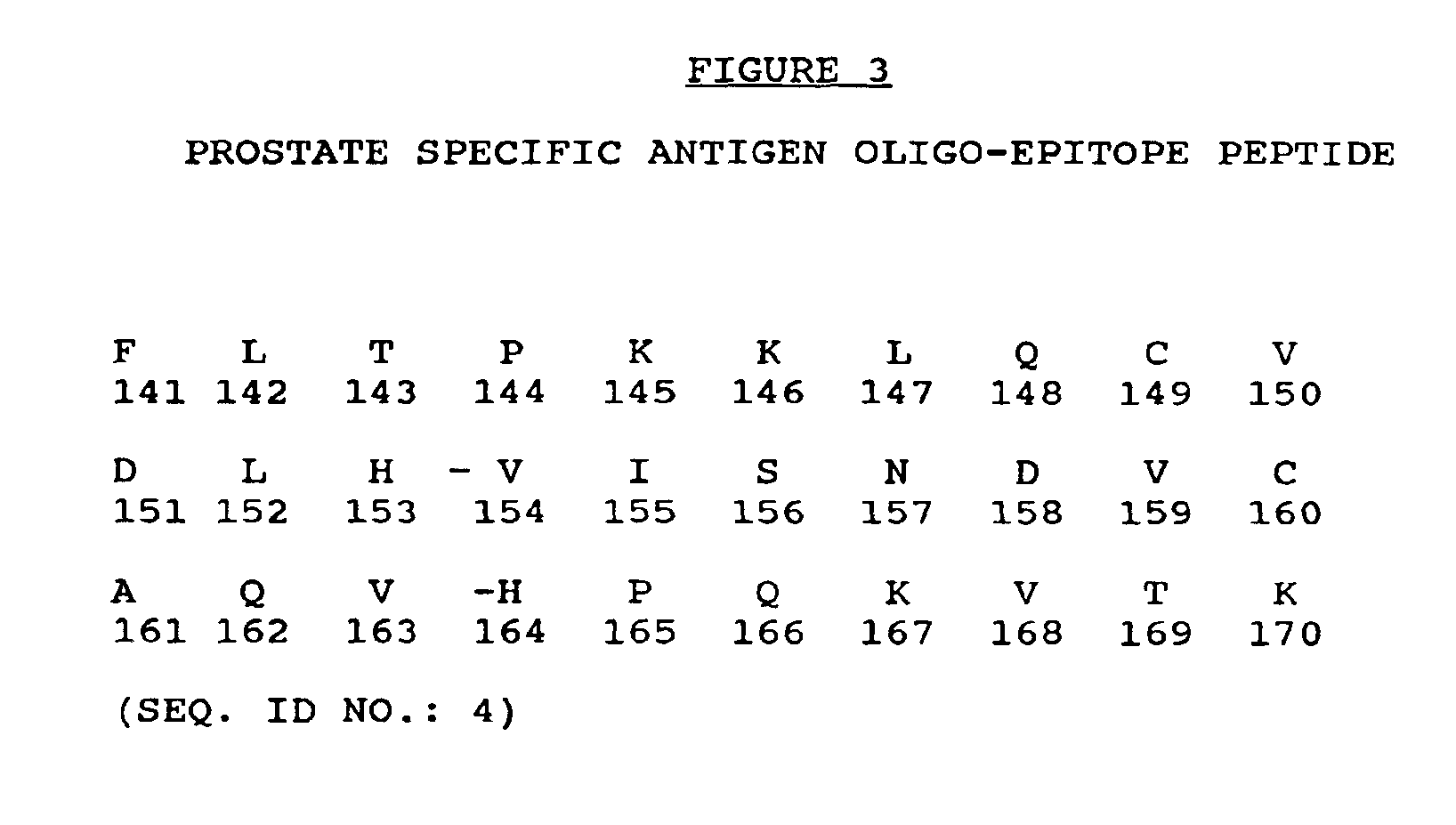
Prostate specific antigen oligo-epitope peptide
The invention is a prostate specific antigen oligo-epitope peptide which comprises more than one PSA epitope peptide, which conforms to one or more human HLA class I motifs. The prostate specific antigen oligo-epitope peptide in combination with various HLA-class I molecules or interactions with various T-cell receptors elicits PSA specific cellular immune responses. The prostate specific antigen oligo-epitope peptide is useful as an immunogen in the prevention or treatment of prostatic cancer, in the inhibition of prostatic cancer cells and in the establishment and characterization of PSA-specific cytotoxic T-cell lines.
Owner:HEALTH & HUMAN SERVICES DEPT OF THE GOVERNMENT AS REPRESENTED BY THE SEC
Methods of prognosis of prostate cancer
The present invention applies classical survival analysis to genome-wide gene expression profiles of prostate cancers and preoperative prostate-specific antigen levels from prostate cancer patient, to identify prognostic markers of disease relapse that provide additional predictive value relative to prostate-specific antigen concentration. The present invention provides a method of determining prognosis of prostate cancer and predicting prostate cancer outcome of a patient. The method comprises the steps of first establishing the threshold value of at least one prognostic gene of prostate cancer. Then, the amount of the prognostic gene from a prostate tissue of a prostate cancer patient is determined. The amount of the prognostic gene present in that patient is compared with the established threshold value of the prognostic gene, whereby the prognostic outcome of the patient is determined.
Owner:GARVAN INST OF MEDICAL RES
Drug complex for treatment of metastatic prostate cancer
InactiveUS20050233948A1Improve efficacyLow toxicityTripeptide ingredientsSaccharide peptide ingredientsProstate cancer cellAntigen
A drug complex for delivery of a drug or other agent to a target cell, comprising a targeting carrier molecule which is selectively distributed to a specific cell type or tissue containing the specific cell type; a linker which is acted upon by a molecule which is present at an effective concentration in the environs of the specific cell type; and a drug or an agent to be delivered to the specific cell type. In particular, a drug complex for delivering a cytotoxic drug to prostate cancer cells, comprising a targeting carrier molecule which is selectively delivered to prostate tissue, bone or both; a peptide which is a substrate for prostate specific antigen; and a cytotoxic drug which is toxic to androgen independent prostate cancer cells.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC +2
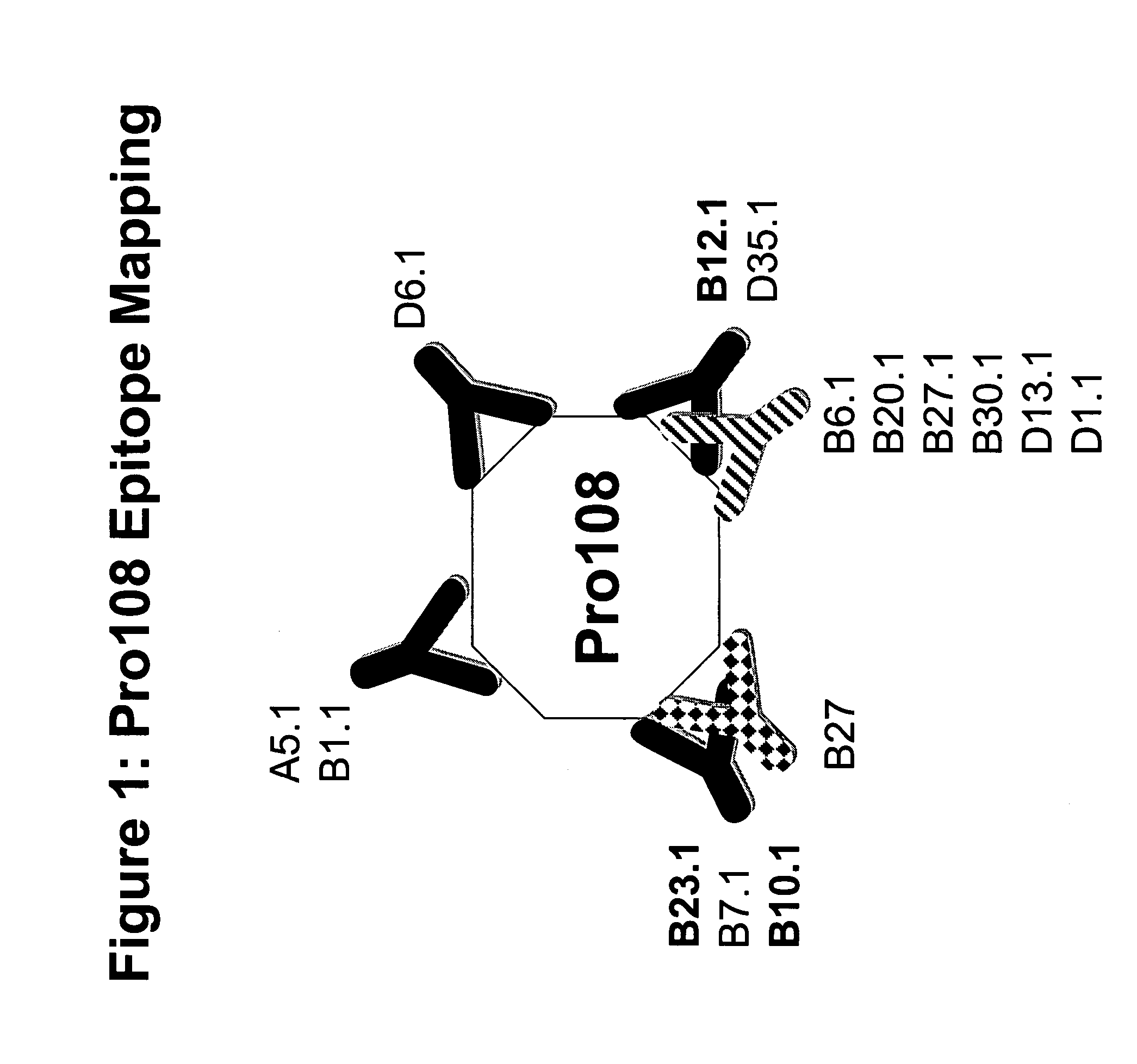
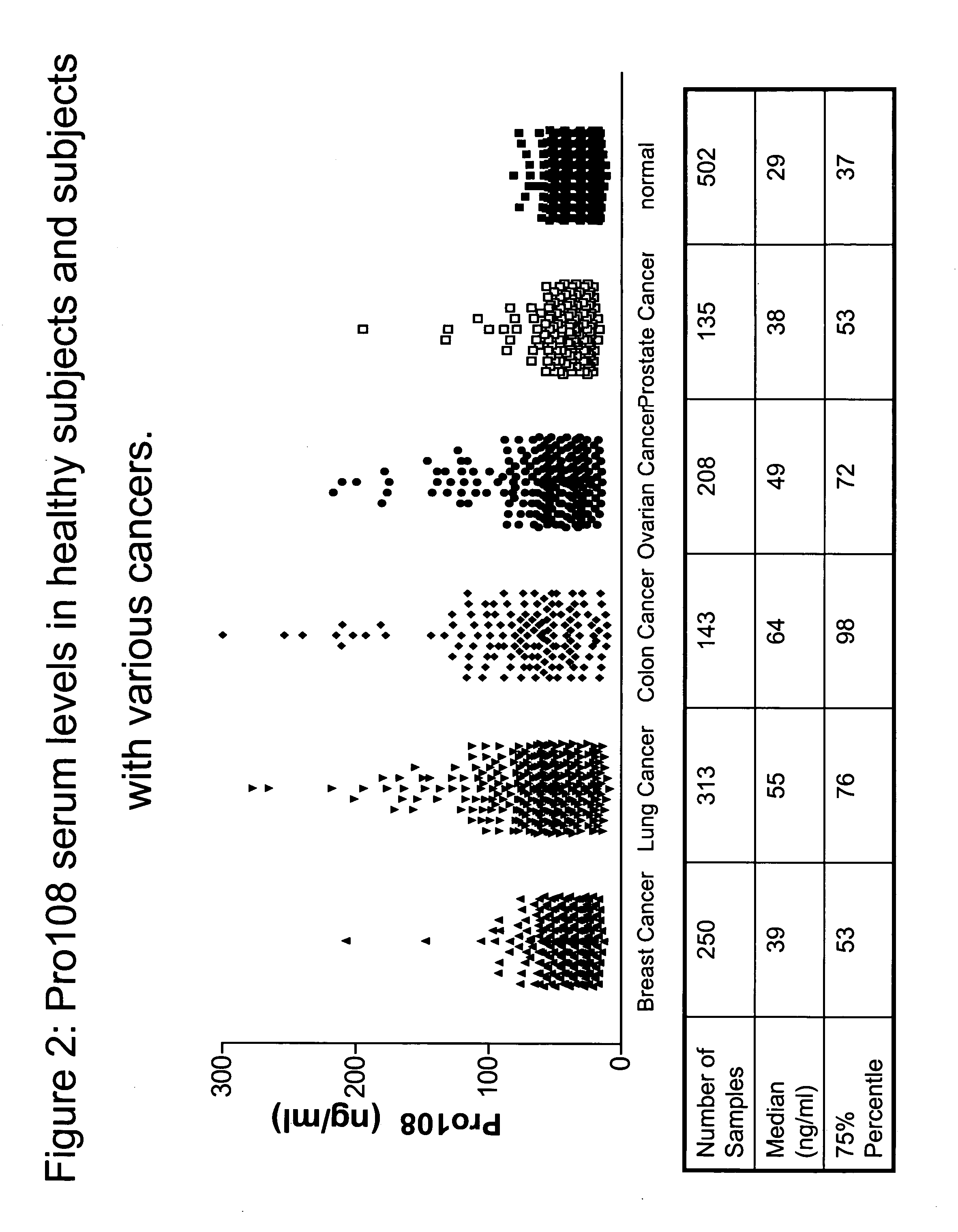
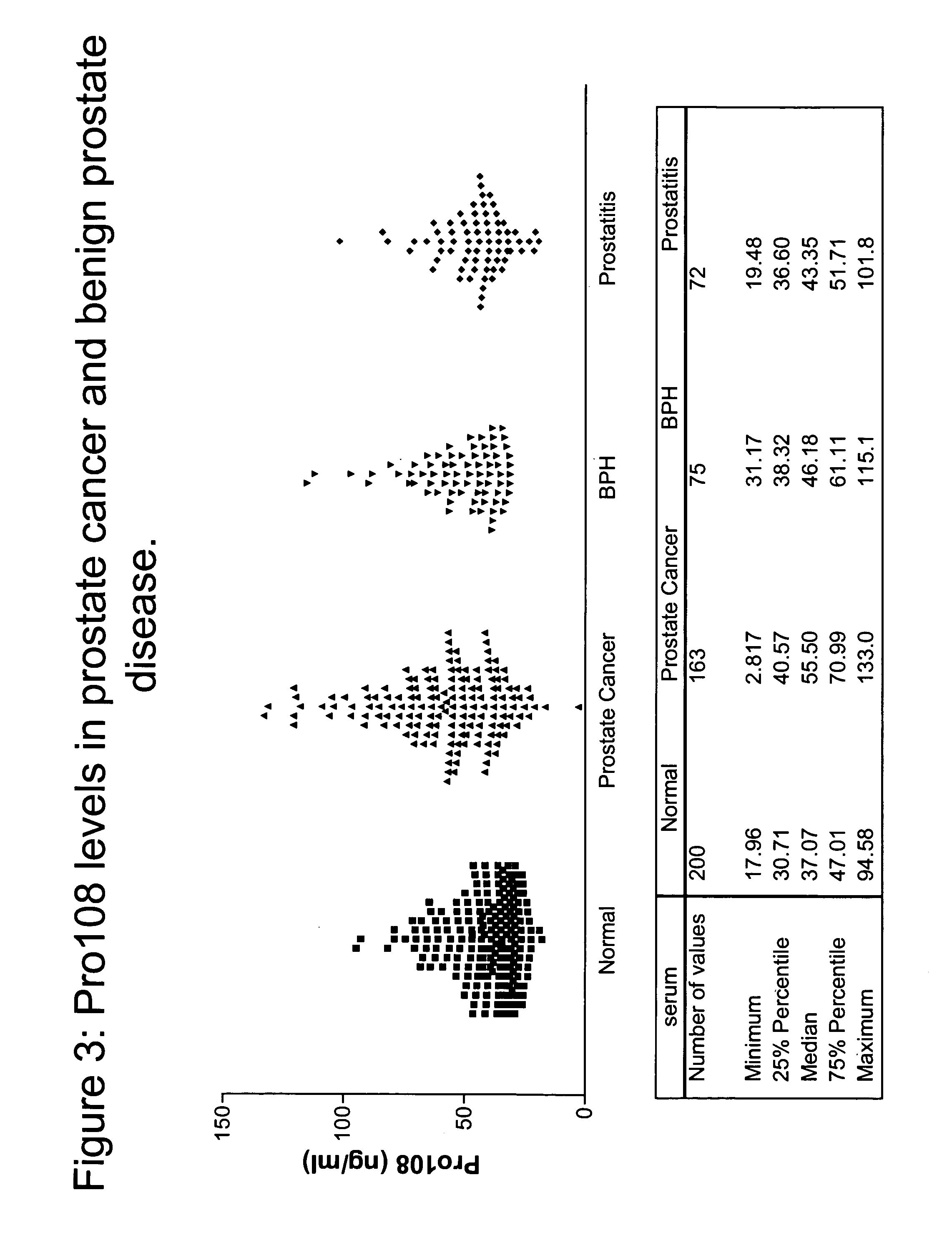
Pro108 antibody compositions and methods of use and use of Pro108 to assess cancer risk
InactiveUS7294704B2Reduce riskHigh profileAnimal cellsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellMammal
Owner:DIAZYME LAB INC
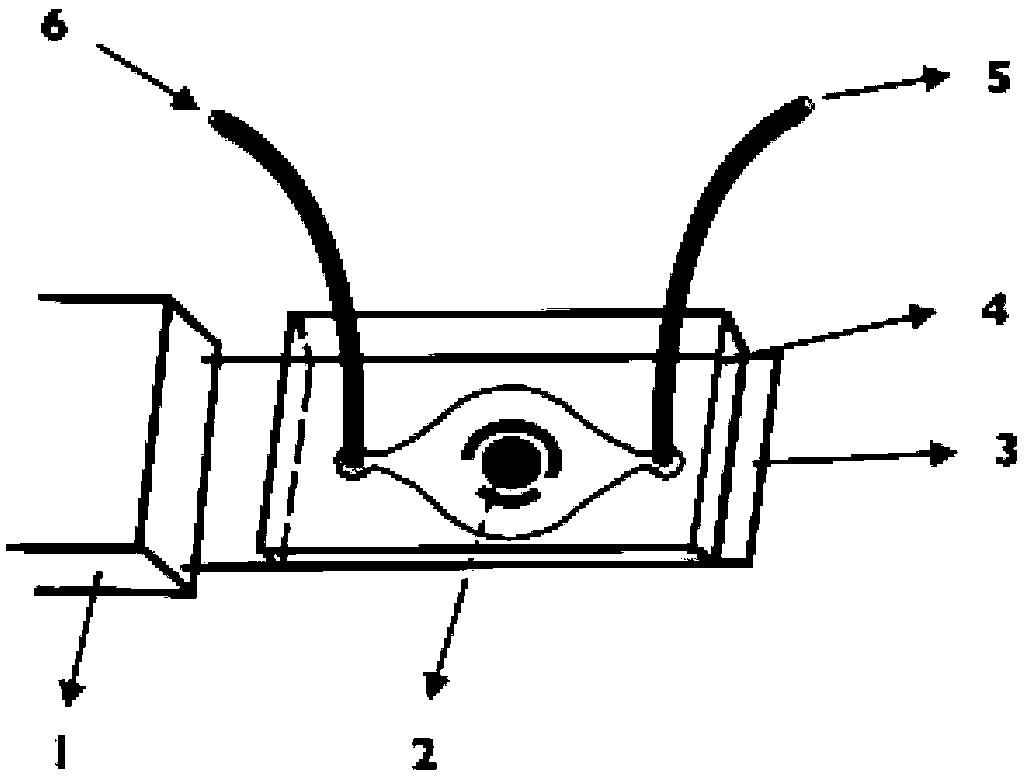
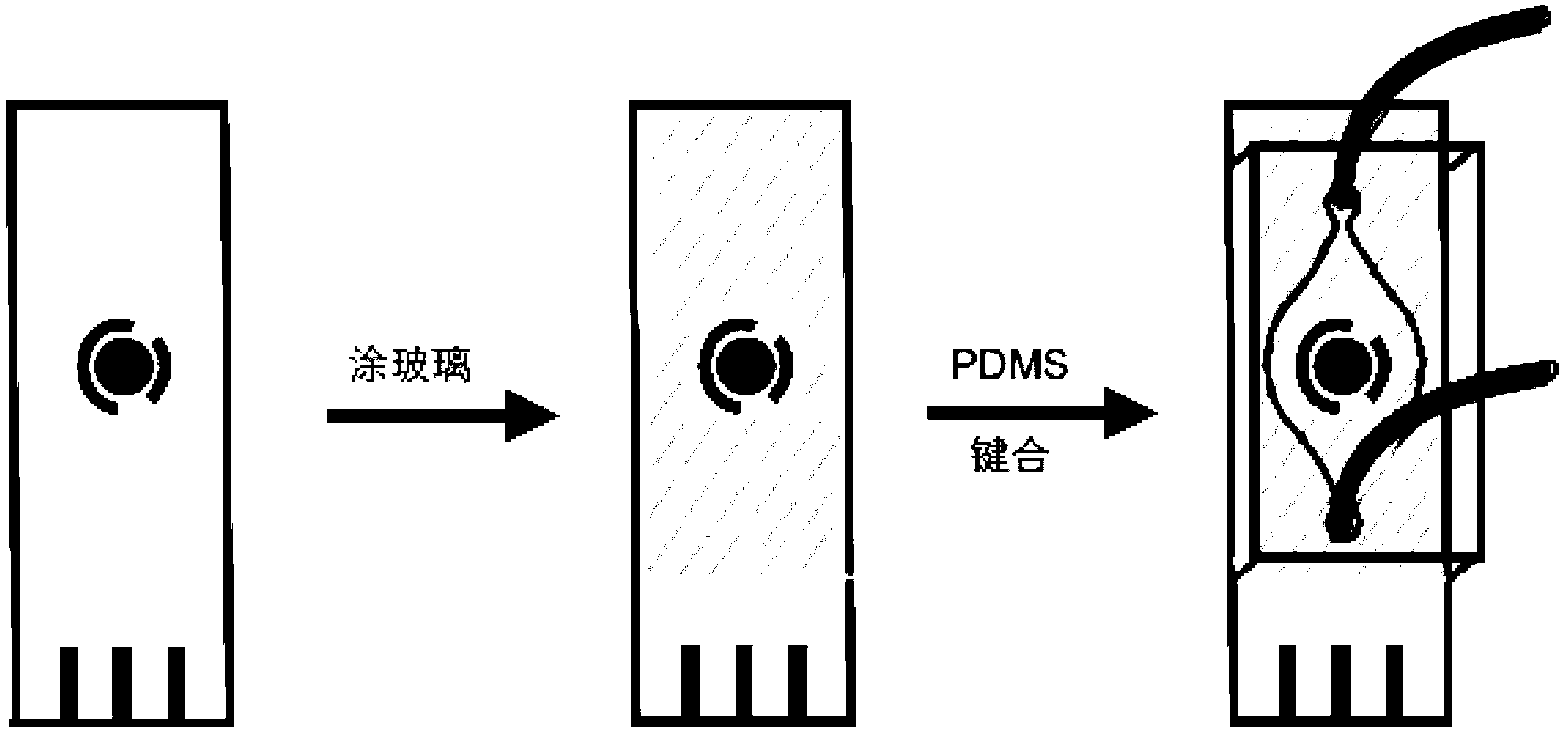
Preparation method and application of electrochemical micro-fluidic sensing chip
ActiveCN103182334AEasy to manufactureReduce weightMaterial analysis by electric/magnetic meansLaboratory glasswaresElectrochemical detectorStandardization
The invention provides a preparation method and the application of an electrochemical micro-fluidic sensing chip. The preparation method comprises the following steps: directly coating an improved glass solution on a commercial standard printed electrode; and performing vacuum plasma treatment on a PDMS (Polydimethylsiloxane) chip with pre-designed pipelines and the printed electrode coated with the glass solution together, and directly bonding the PDMS chip on the commercial standard printed electrode to form a novel electrochemical microfluidic sensing platform. A sensor provided by the invention can perform ultrasensitive detection on various sample analytes in a biological fluid sample, taking the detection of a prostate cancer marker PSA (Prostate-specific Antigen) in human serum as an example, a coulomb amperometry is used for detection, and a result shows that the detection sensitivity can reach 0.84 pg / mL which is improved by two magnitudes than the standardized clinical testing requirement of 0.1 ng / mL, so that the sensor has superhigh detection sensitivity and accuracy, which are higher than those of other electrochemical detection devices, is convenient in operation, and can integrates sample processing, separation and the like on one micro electrochemical microfluidic sensing chip.
Owner:SHANGHAI JIAO TONG UNIV
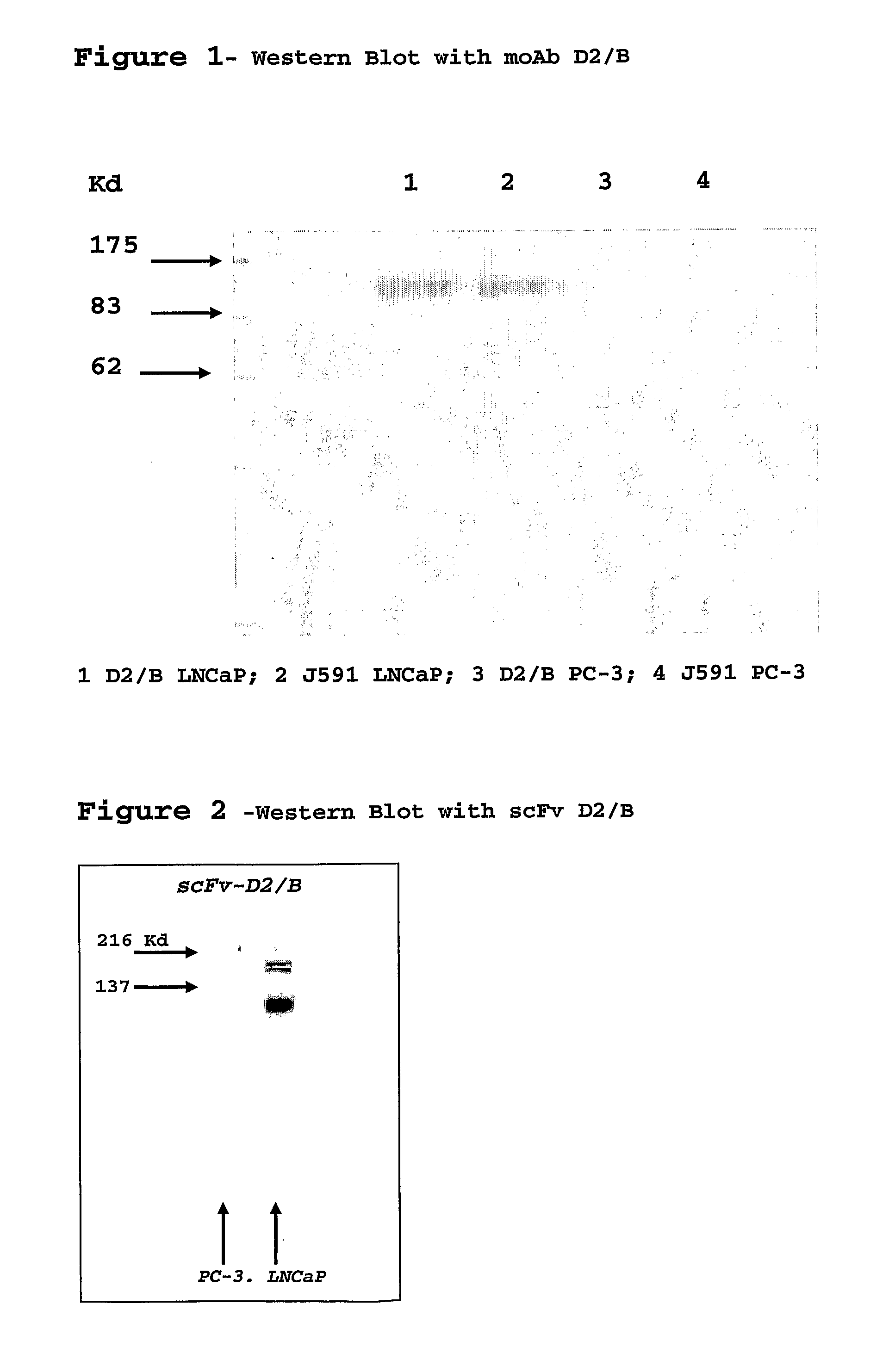
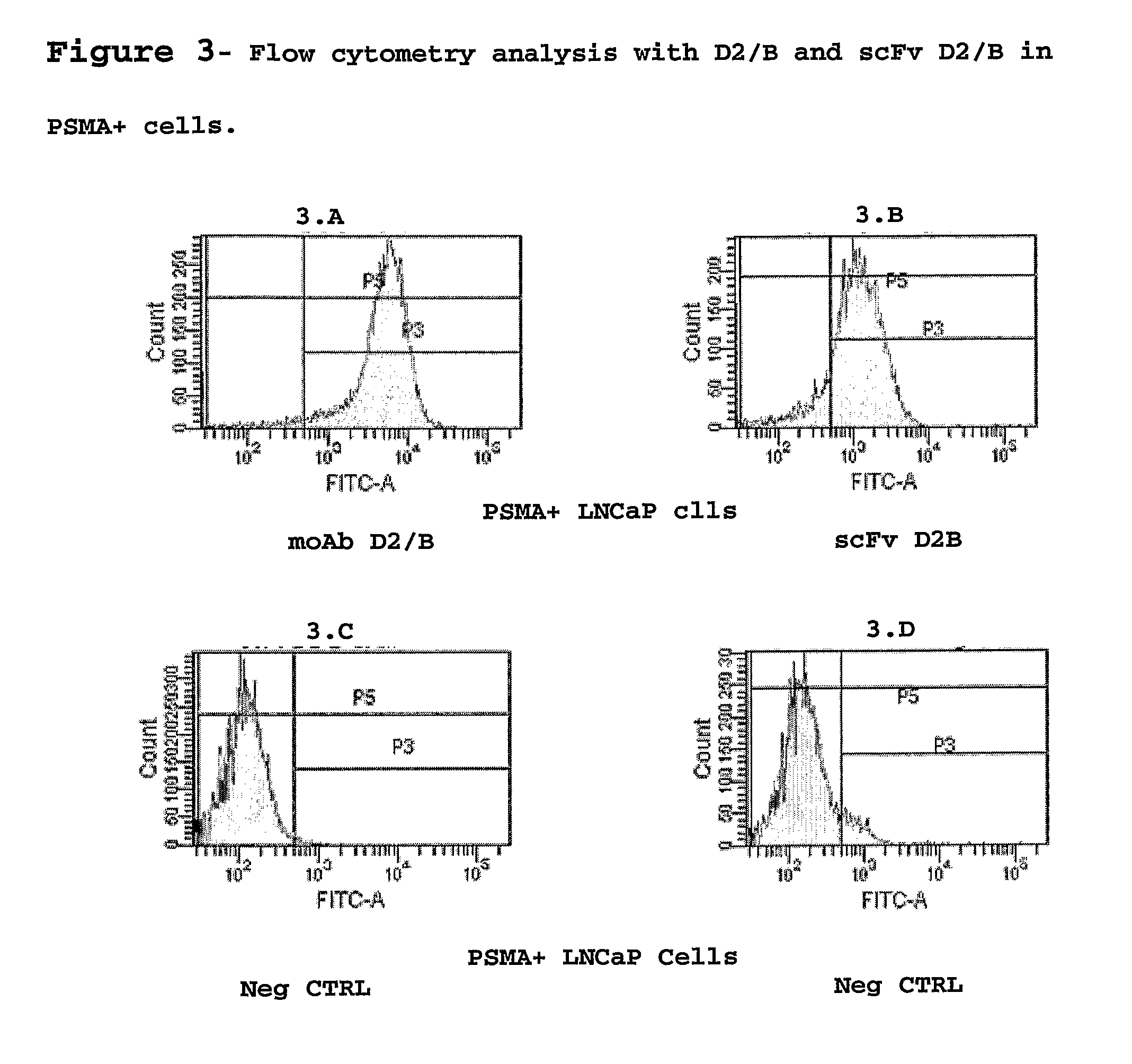
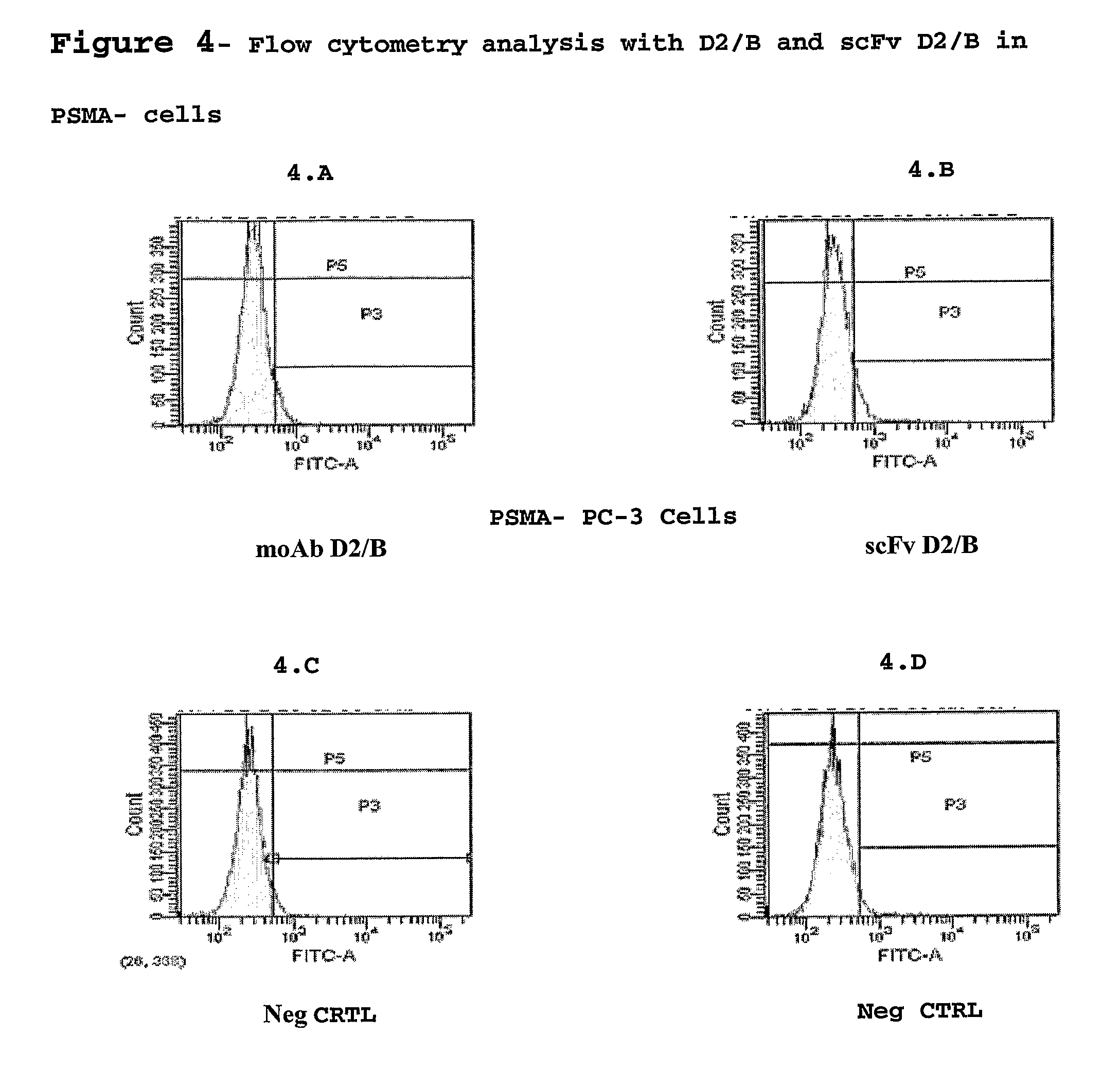
Monoclonal antibody specific for the extracellular domain of prostate specific membrane antigen
InactiveUS7476513B2VirusesIn-vivo radioactive preparationsImmunoenzymatic assayProstate specific membrane
The present invention relates to monoclonal antibodies that bind to the extracellular domain of prostate-specific membrane antigen (PSMA), hybridoma cell lines producing the antibodies, and methods of using such antibodies for diagnosis and treatment of cancer. In particular, thirty-five monoclonal antibodies reactive with PSMA expressed on the cell surface are exemplified. Additionally, the present invention relates to a novel protein variant (PSM′) of PSMA detected by a number of the antibodies of the invention. The hydrolase activity of PSMA and PSM′ allows the use of an immunoenzymatic assay for their detection.
Owner:ER SQUIBB & SONS INC
Methods for diagnosis and prognosis of cancer
InactiveUS20060105343A1Increases specificity and sensitivityHigh expressionSugar derivativesMicrobiological testing/measurementAntigenAbnormal tissue growth
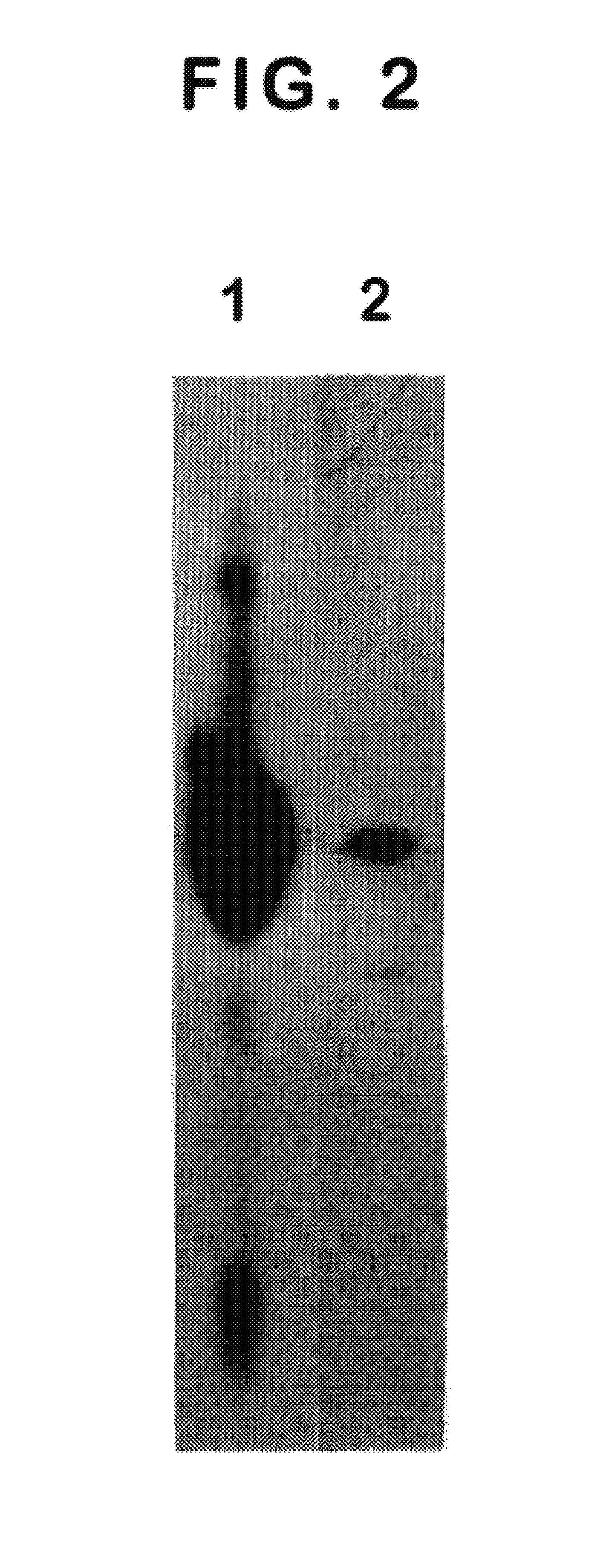
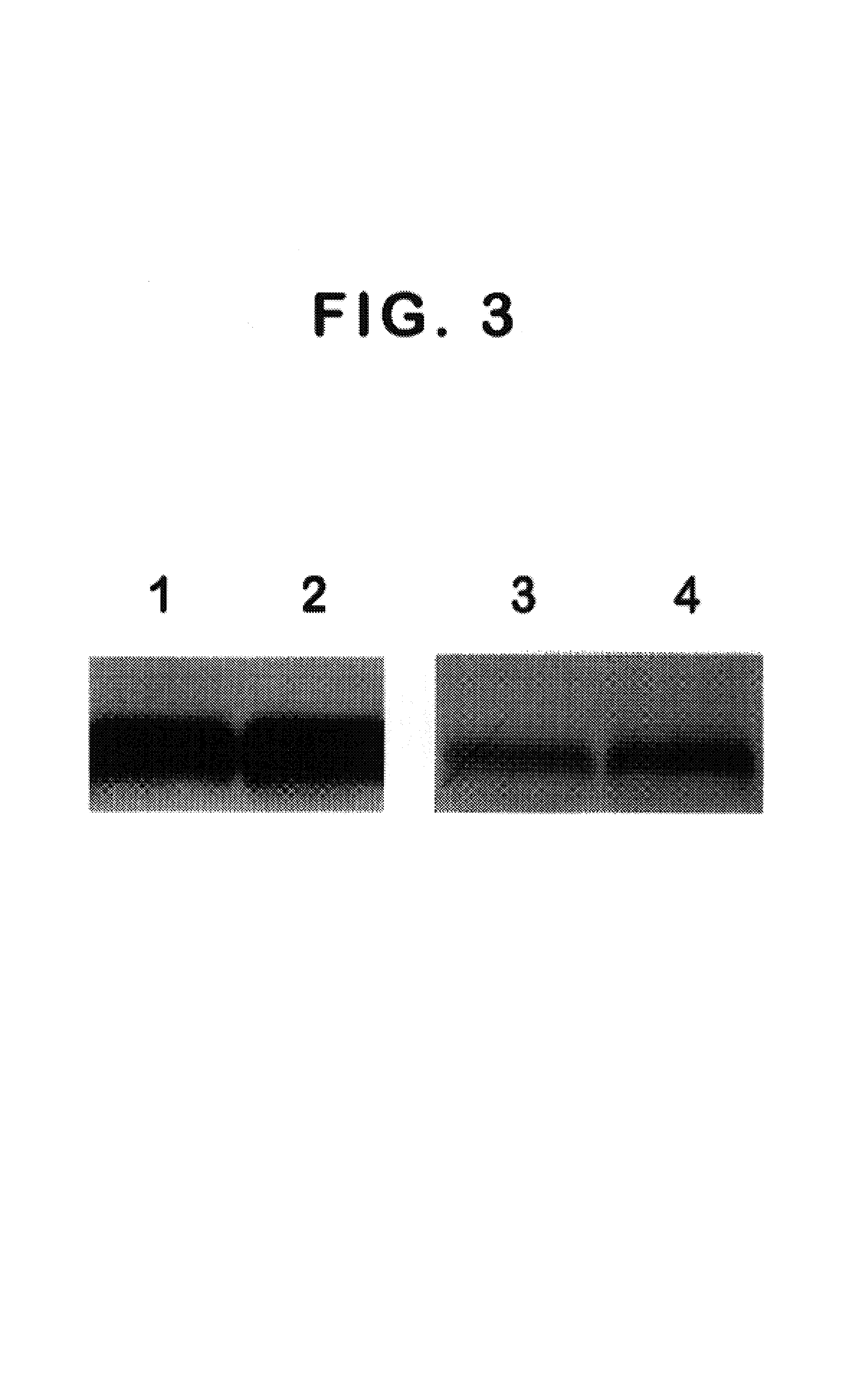
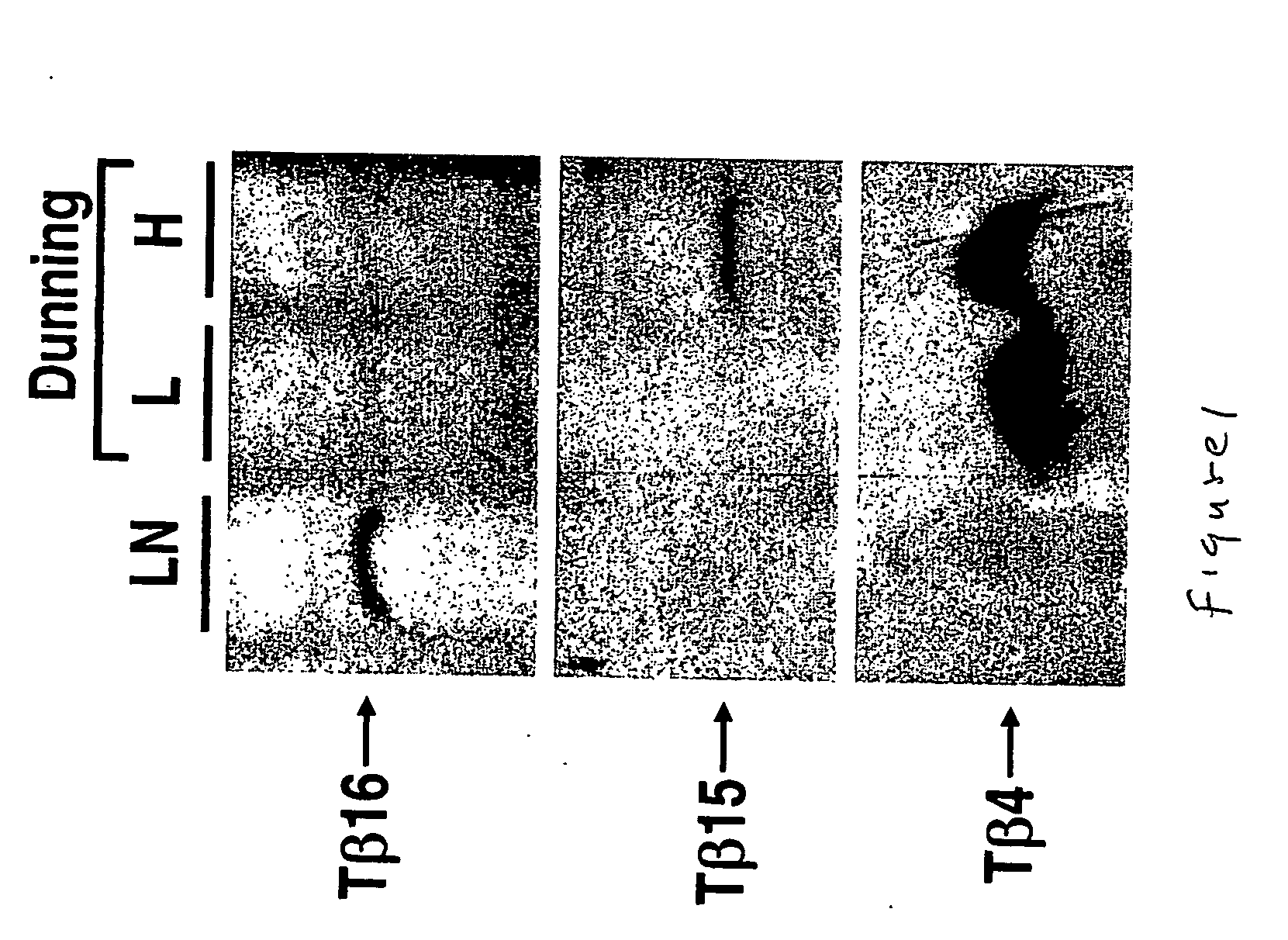
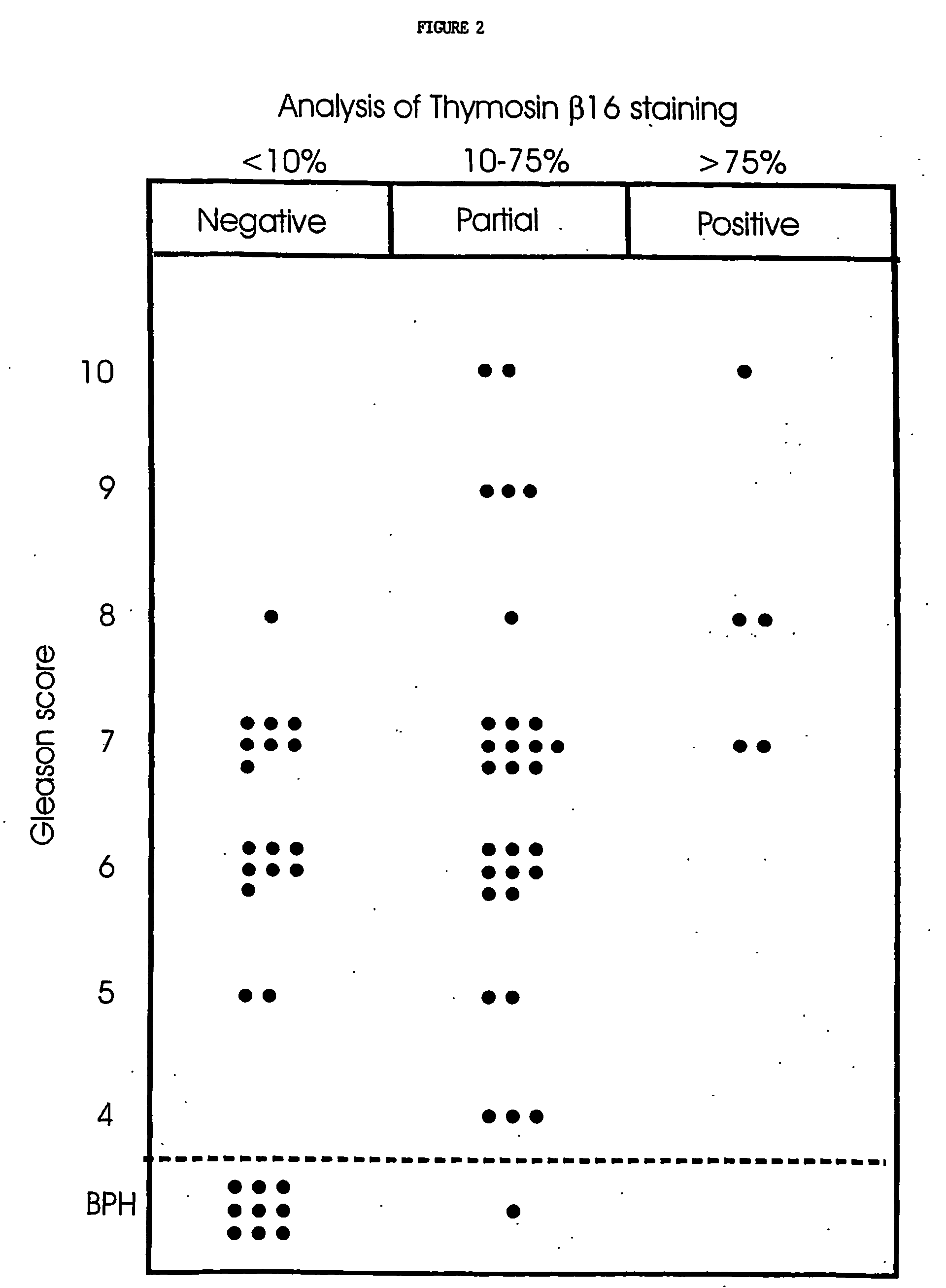
We have discovered a protein in humans, herein referred to as thymosin R16 (SEQ ID NO: 1), that is expressed in human prostate cancer tumors but not in specimens of benign prostate hyperplasma (BPH) tissues. In contrast, prostate specific antigen (PSA), the gold standard of prostate cancer diagnosis, is highly expressed in BPH tissues. Increased expression of thymosin (316 has a high correlation to disease state in a number of cancers including prostate cancer and cancers of epithelial origin. Accordingly, method of diagnosing and prognosing cancer in a patient by measuring the level of thymosin (316 in a biological test sample obtained from the patient are provided.
Owner:CHILDRENS MEDICAL CENT CORP
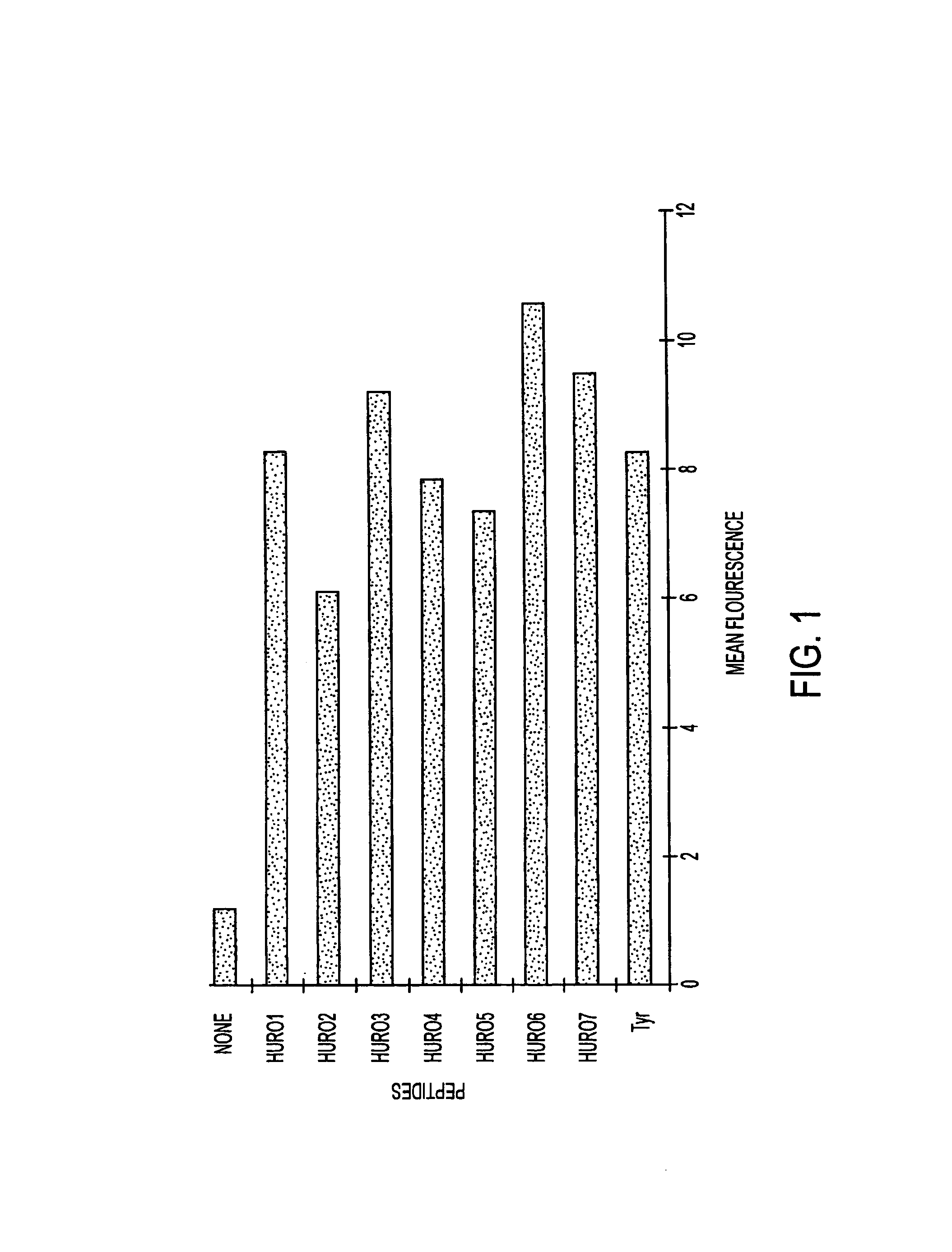
HLA binding peptides and their uses
InactiveUS7252829B1Effective combinationTumor rejection antigen precursorsSsRNA viruses positive-senseImmunodeficiency virusBinding peptide
The present invention provides the means and methods for selecting immunogenic peptides and the immunogenic peptide compositions capable of specifically binding glycoproteins encoded by HLA alleles and inducing T cell activation in T cells restricted by the allele. The peptides are useful to elicit an immune response against a desired antigen. The immunogenic peptide compositions of the present invention comprise immunogenic peptides having an HLA binding motif, where the peptide is from a target antigen. Target antigens of the present invention include prostate specific antigen (PSA), hepatitis B core and surface antigens (HBVc, HBVs) hepatitis C antigens, Epstein-Barr virus antigens, melanoma antigens (e.g., MAGE-1), human immunodeficiency virus (HIV) antigens, human papilloma virus (HPV) antigens, Lassa virus, mycobacterium tuberculosis (MT), p53, CEA, trypanosome surface antigen (TSA) and Her2 / neu. An example of an immunogenic peptide of the present invention corresponds to a peptide less than about 15 amino acids in length that comprises an HLA-A2.1 binding motif, where the peptide comprises the p53 sequence SMPPPGTRV.
Owner:OSE PHARMA INT
Highly sensitive system and methods for analysis of prostate specific antigen (PSA)
InactiveUS20090087860A1Increase the number ofDisease diagnosisImmunoglobulinsAntigenProstate-specific antigen
The invention described herein provides methods, compositions, kits, and systems for the sensitive detection of prostate specific antigen. Such methods, compositions, kits, and systems are useful in diagnosis, prognosis, and determination of methods of treatment in conditions that involve release of prostate specific antigen.
Owner:SINGULEX
Enhanced diagnostic potential of prostate-specific antigen expressing cells
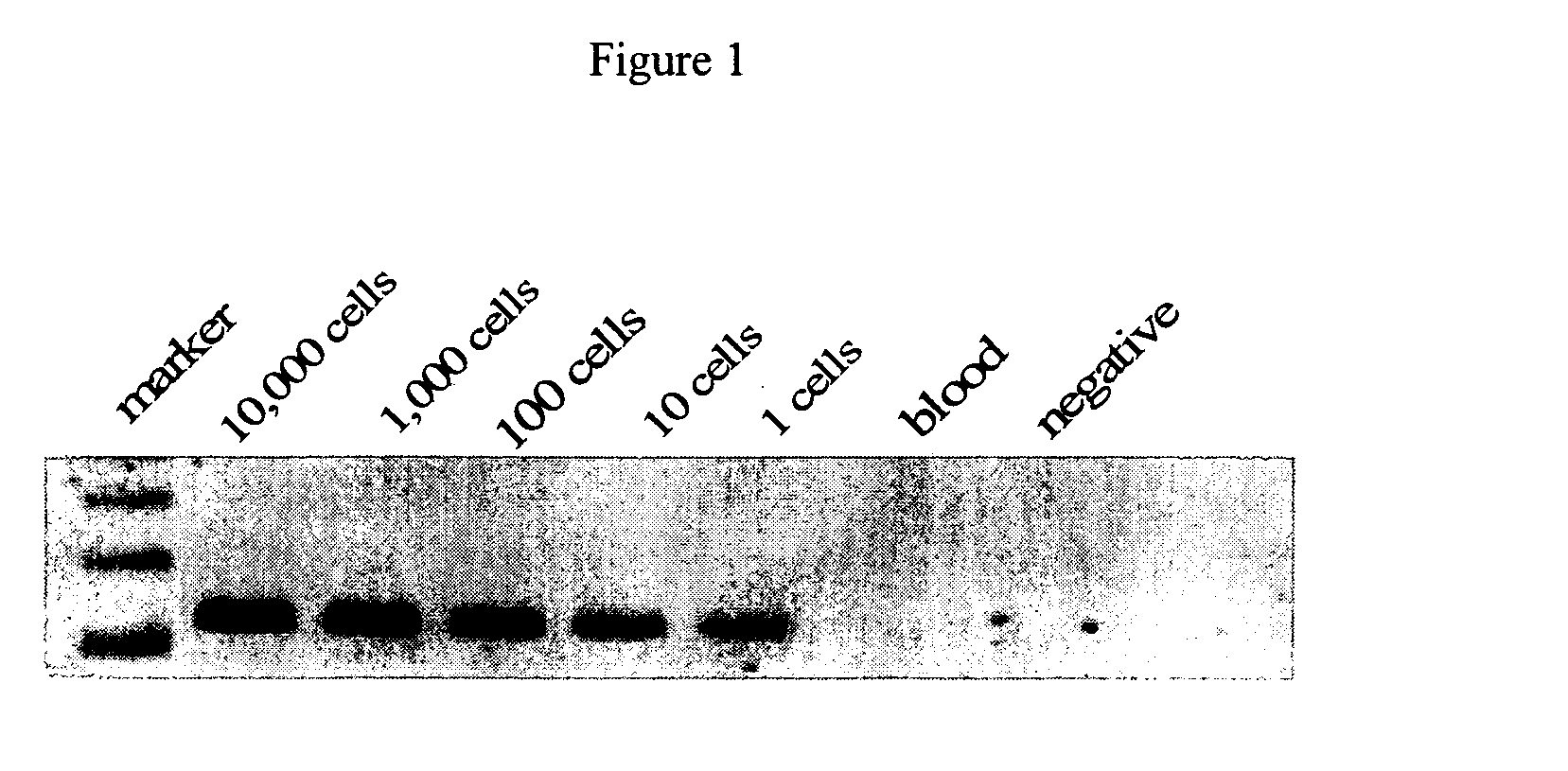
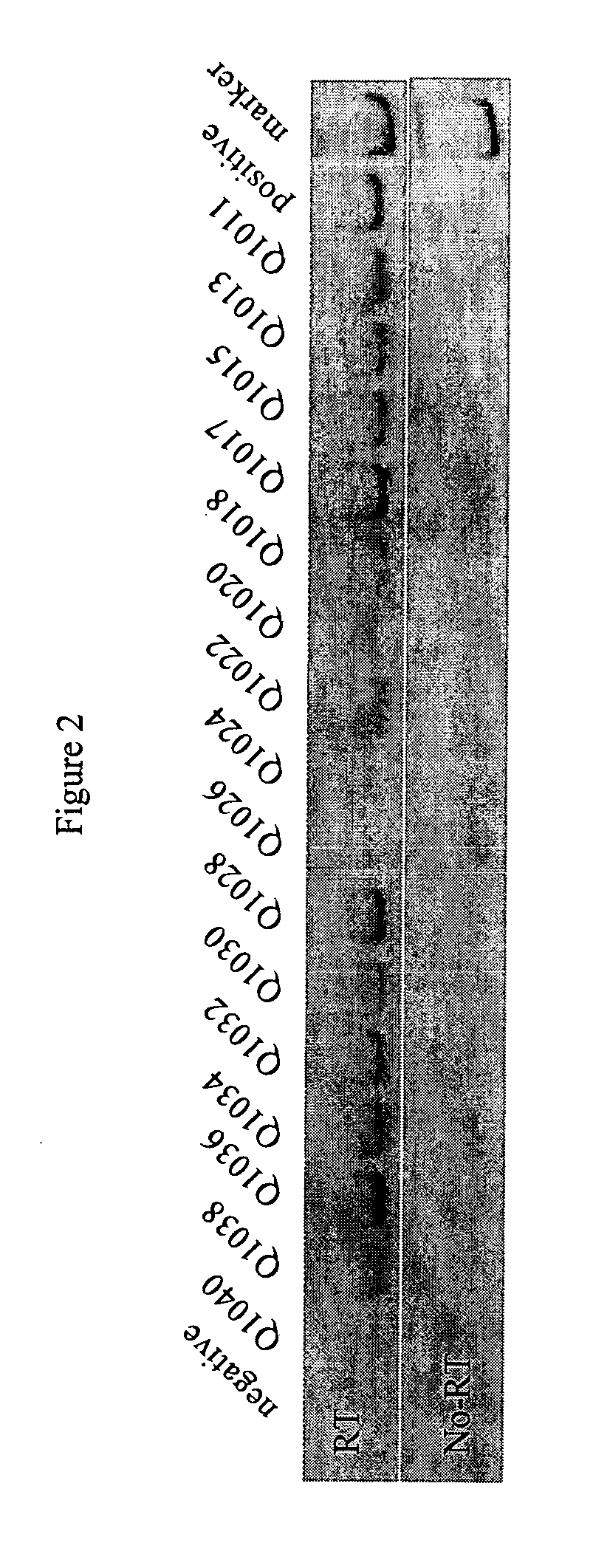
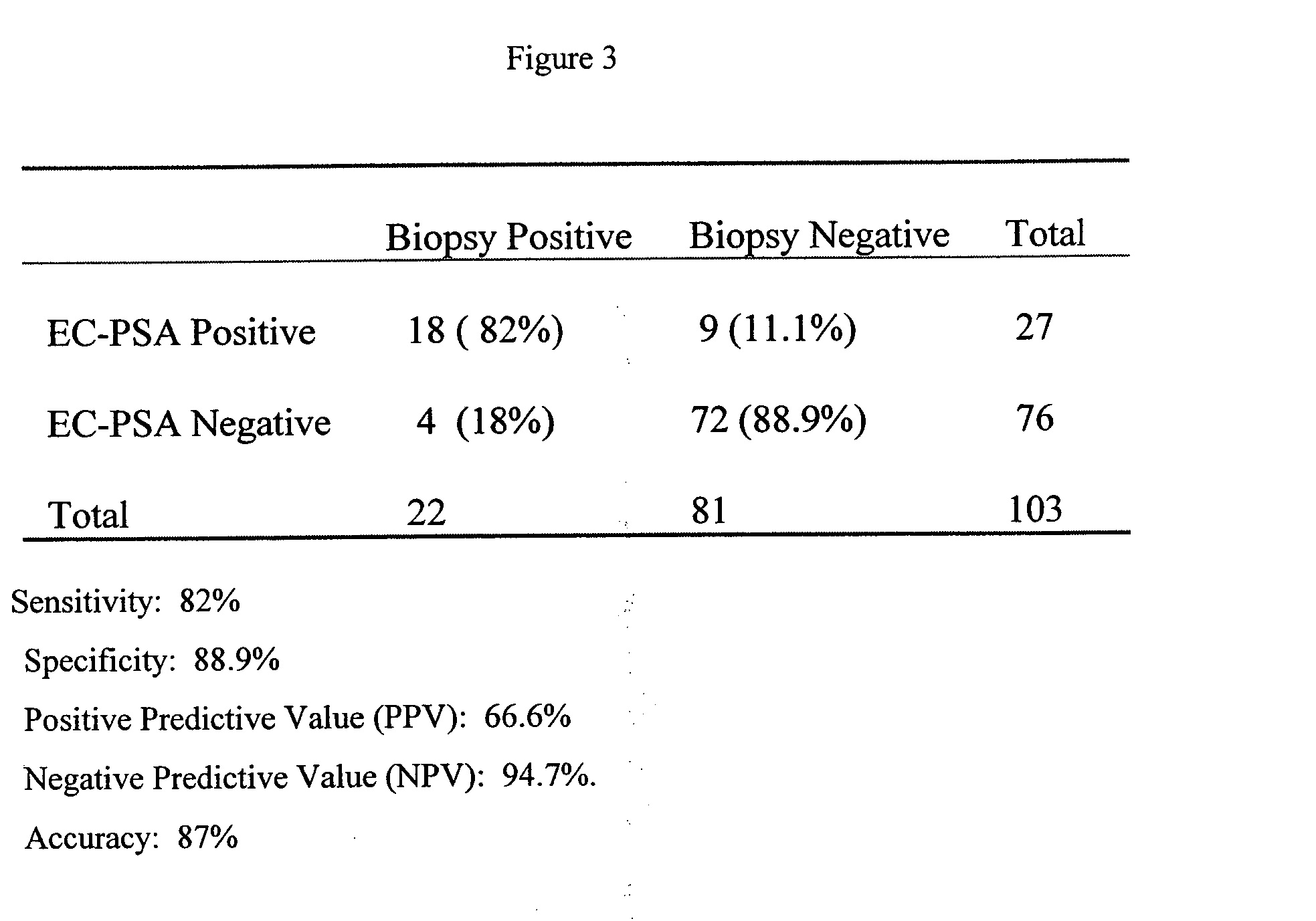
The present invention is directed to sensitive and specific methods and kits for the detection of prostate cancer in a patient. The invention uses RT-PCR to detect the expression or change in expression of PSA in epithelial cells enriched from whole blood. The methods and kits on the invention represent a a significant improvement over previous methods of CaP diagnosis. According to one embodiment, prostate epithelial cells are isolated from blood and the RNA subjected to RT-PCR. The resulting cDNA is subjected to traditional PCR amplification with primers able to distinguish between the genomic copy of the gene and the cDNA copy resulting from CaP gene expression. This method provides an assay which is more sensitive, specific and reproducible as compared to conventional methods. Results disclosed herein demonstrate a correlation between the presence of PSA-expressing prostate epithelial cells with cancer recurrence which provides a diagnostic tool for the early detection of prostate disease.
Owner:GAO CHUN L +2
Insulin-like growth factor system and cancer
InactiveUS6448086B1Microbiological testing/measurementDisease diagnosisInsulin-like growth factorAntigen
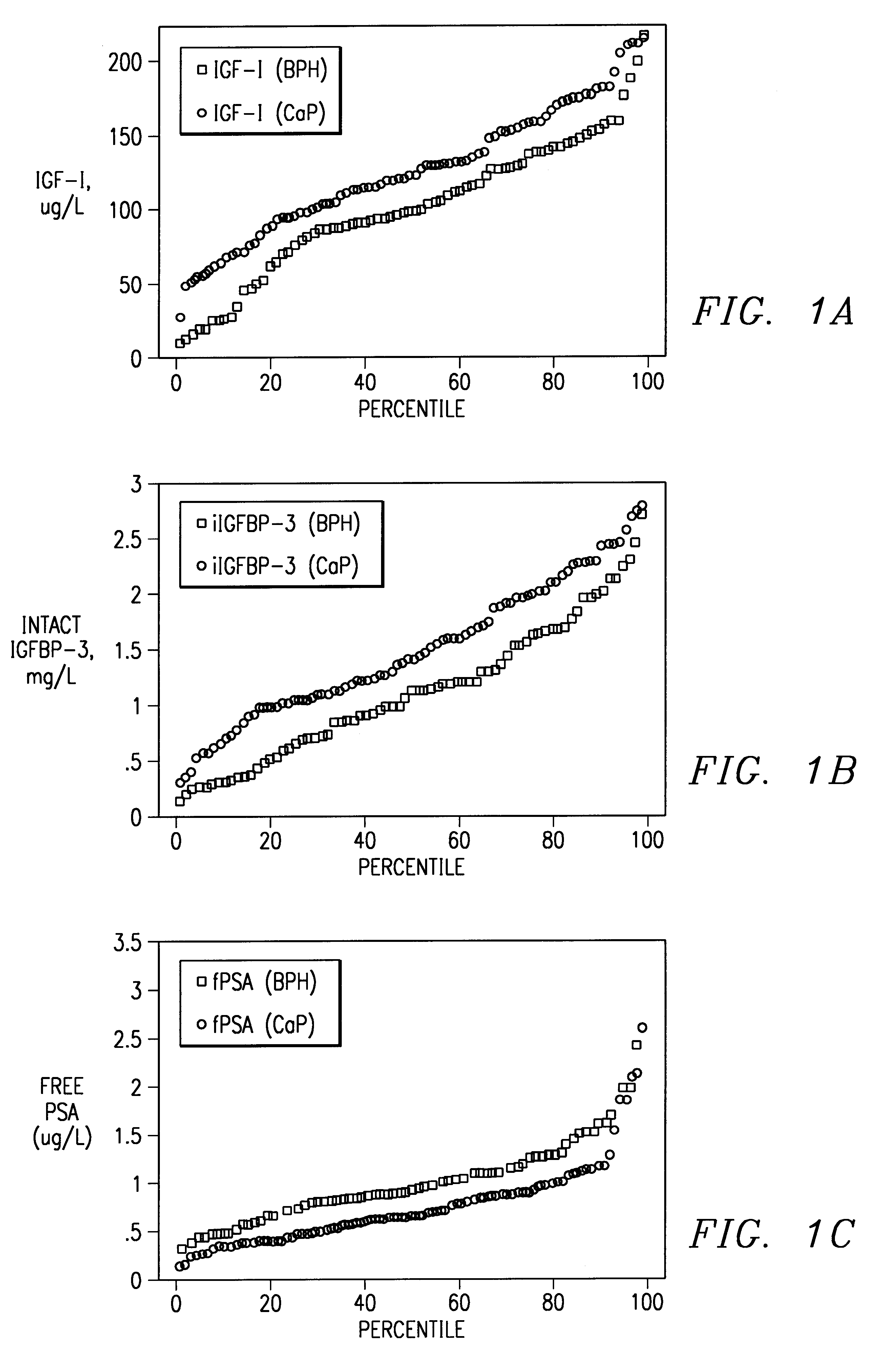
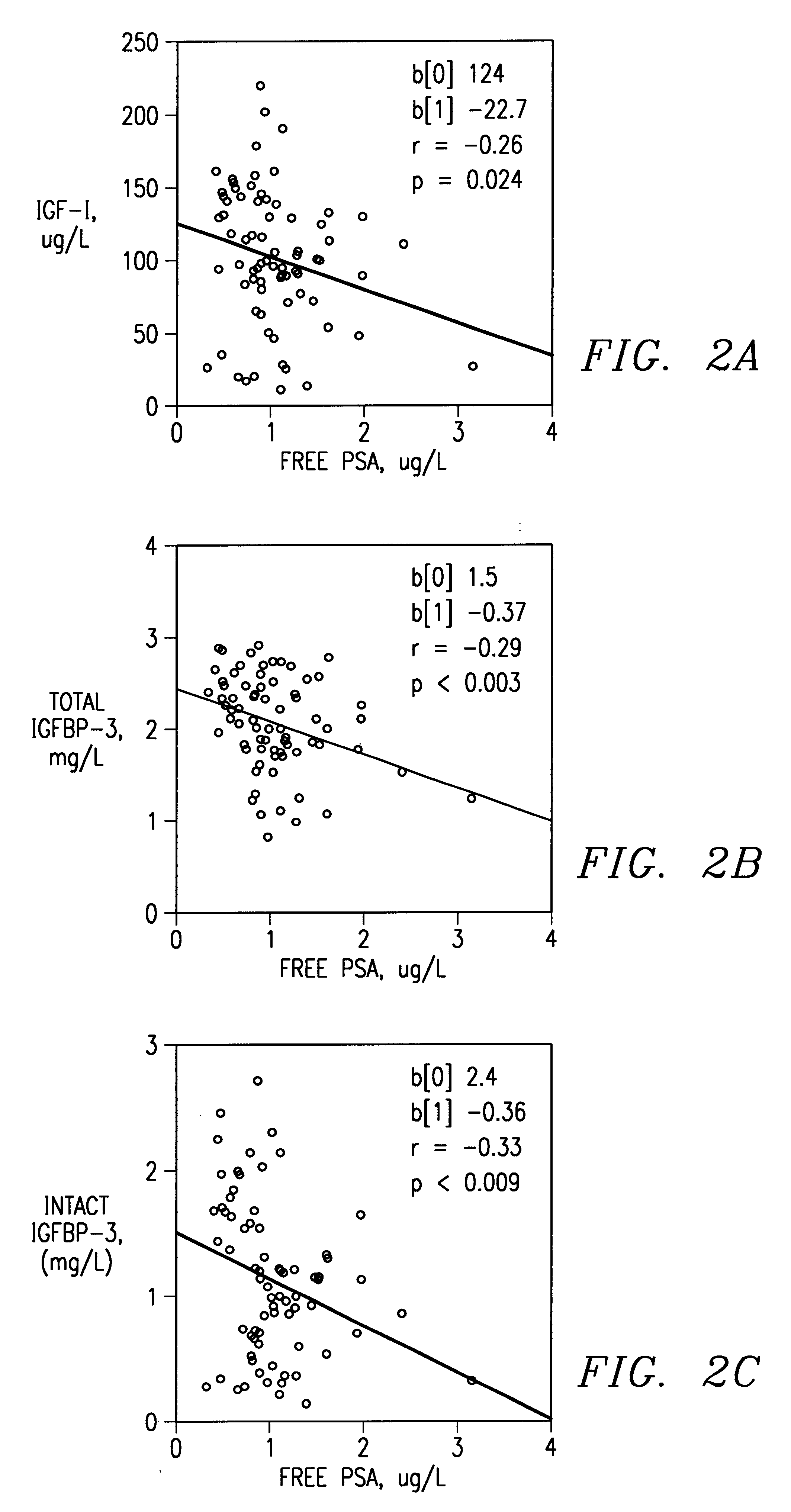
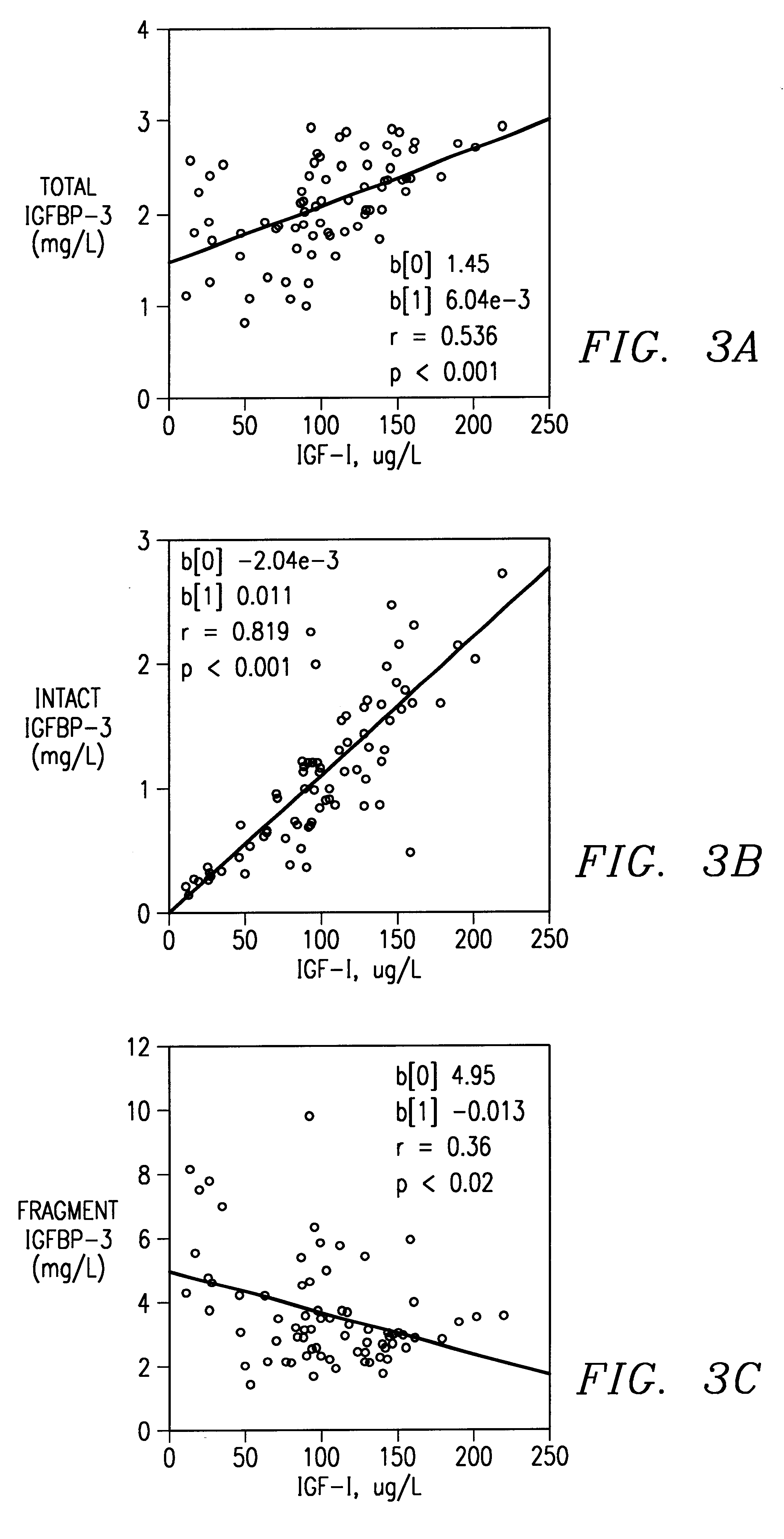
Method of monitoring or diagnosing disease conditions, including disease of the prostate, that involve measuring a combination of tumor markers and at least one component of the IGF axis. The invention is exemplified with prostate cancer and benign prostatic hyperplasia, the tumor marker prostate specific antigen, and the insulin-like growth factors and their binding proteins.
Owner:BECKMAN COULTER INC
Agglutination accelerator for immunological measurement
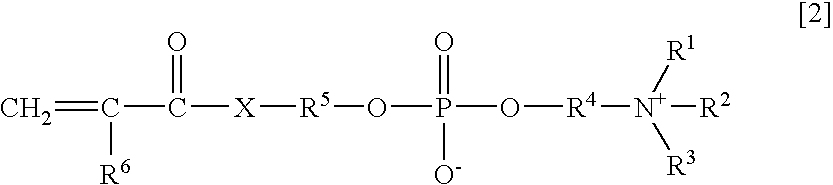
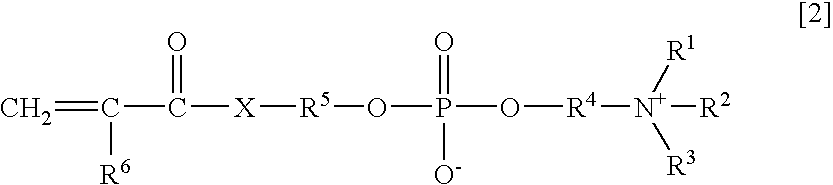
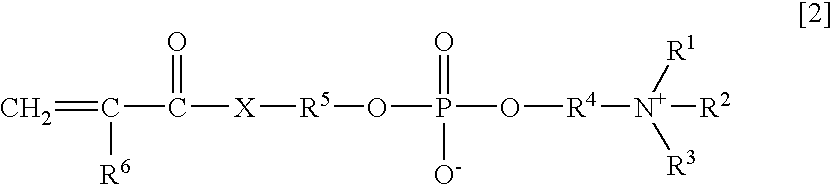
An object of the present invention is to provide an immunoassay of PSA using an agglutination accelerator, which has an agglutination accelerating effect equal to or stronger than the known agglutination accelerator; hardly generates non-specific turbidity; and hardly generates salting out even in a solution with a high salt concentration. The present invention relates to an immunoassay of a prostate-specific antigen comprising performing an antigen-antibody reaction in the presence of a polymer having a monomer unit derived from a monomer represented by the following general formula [2]: (wherein R<1>-R<3 >are each independently a hydrogen atom or an alkyl group optionally having a hydroxyl group; R<4 >is an alkylene group; R<5 >is an alkylene group optionally having a substituent and optionally having an oxygen atom in a chain; R<6 >is a hydrogen atom or a methyl group, and X is an oxygen atom or a -NH- group), and a kit of reagent for an immunoassay comprising a reagent containing an agglutination accelerator for the immunoassay.
Owner:FUJIFILM WAKO PURE CHEM CORP
Isolated monoclonal antibody or fragment thereof binding prostate specific membrane antigen, conjugates and uses thereof
ActiveUS8703918B2Ultrasonic/sonic/infrasonic diagnosticsAntibody mimetics/scaffoldsProstate specific membraneAntibody fragments
An isolated monoclonal antibody or fragment thereof binding prostate specific membrane antigen (PSMA) preferably in its native form on the surface of tumour cells. A conjugate of the antibody with an active ingredient and modified forms of the antigen-binding antibody fragment are also provided. The complete antibody and the antigen-recognising fragment thereof are used alone or conjugated for the treatment and the diagnosis of tumours or tissues associated to the tumour overexpressing the PSMA antigen, preferably prostatic neoplastic diseases.
Owner:MARCO COLOMBATTI +2
Nanotechnology-based trace protein detection method
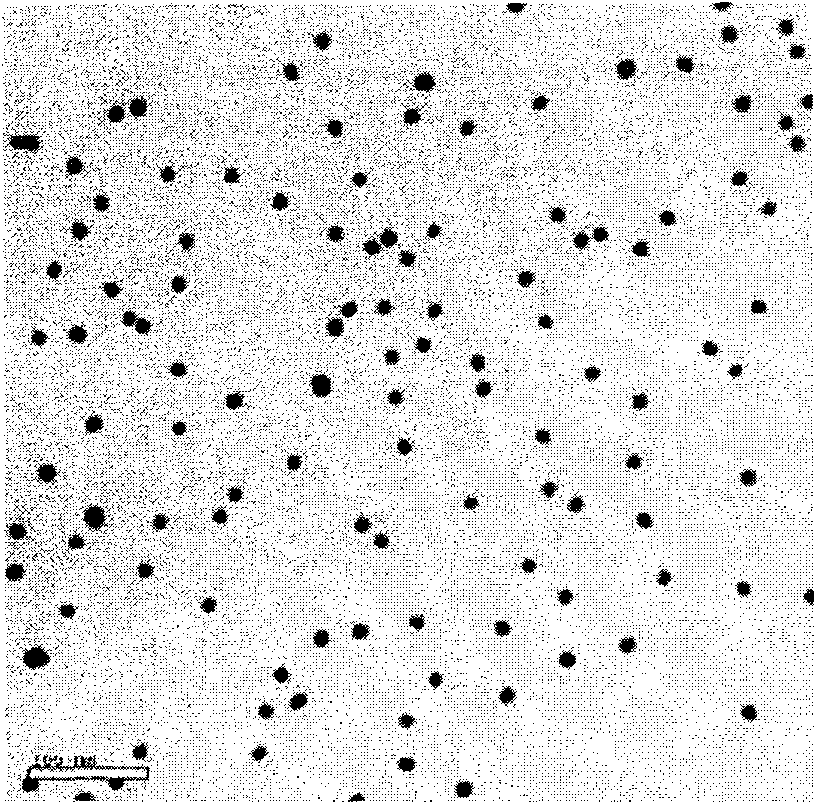
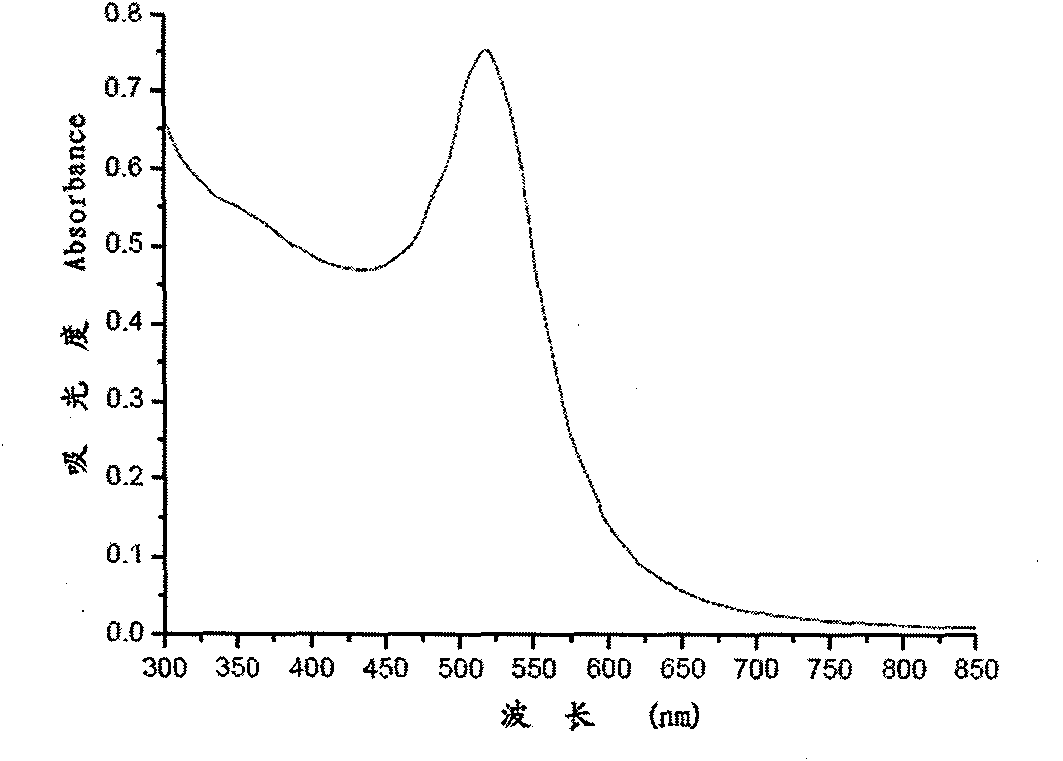
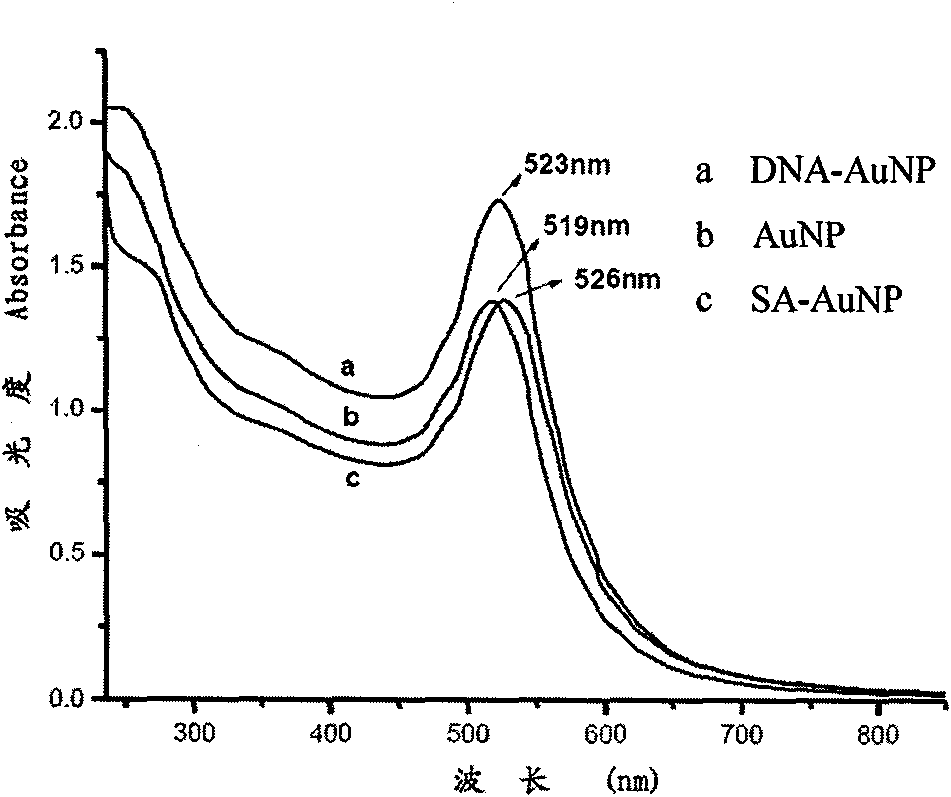
InactiveCN101943703AWide linear rangeLow detection limitColor/spectral properties measurementsBiological testingAntigenBiotin-streptavidin complex
The invention relates to a nanotechnology-based trace protein detection method, which combines enzyme-linked immunosorbent assay technology, tyramine signal amplification technology and the aggregation phenomenon of gold nanoparticles modified by different biological molecules, so an experimental method used for detecting trace proteins such as prostate specific antigen (PSA) and the like is established. The method comprises the following steps of: fixing an antibody aiming at the protein to be detected (such as the PSA) on the surface of a substrate; incubating another antibody with horseradish peroxidase (HRP) activity of the protein to be detected (such as the PSA) after capturing the protein to be detected in a sample, wherein the HRP catalyzes biotin-tyramide to generate biotin deposition under certain conditions; further amplifying a signal by using the aggregation phenomenon of the gold nanoparticles modified by biotin-labeled DNA and the gold nanoparticles modified by streptavidin; performing silver staining; and performing data analysis on an experimental result by using software. The method has the advantages of extremely low detection limit, wider detection range, capacity of detecting the antigen in a rabbit serum with complex compositions, and important application prospect.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
HLA binding peptides and their uses
InactiveUS20090012004A1Effective combinationSsRNA viruses positive-senseTumor rejection antigen precursorsImmunodeficiency virusBinding peptide
The present invention provides the means and methods for selecting immunogenic peptides and the immunogenic peptide compositions capable of specifically binding glycoproteins encoded by HLA alleles and inducing T cell activation in T cells restricted by the allele. The peptides are useful to elicit an immune response against a desired antigen. The immunogenic peptide compositions of the present invention comprise immunogenic peptides having an HLA binding motif, where the peptide is from a target antigen. Target antigens of the present invention include prostate specific antigen (PSA), hepatitis B core and surface antigens (HBVc, HBVs) hepatitis C antigens, Epstein-Barr virus antigens, melanoma antigens (e.g., MAGE-1), human immunodeficiency virus (HIV) antigens, human papilloma virus (HPV) antigens, Lassa virus, mycobacterium tuberculosis (MT), p53, CEA, trypanosome surface antigen (TSA) and Her2 / neu.
Owner:OSE PHARMA INT
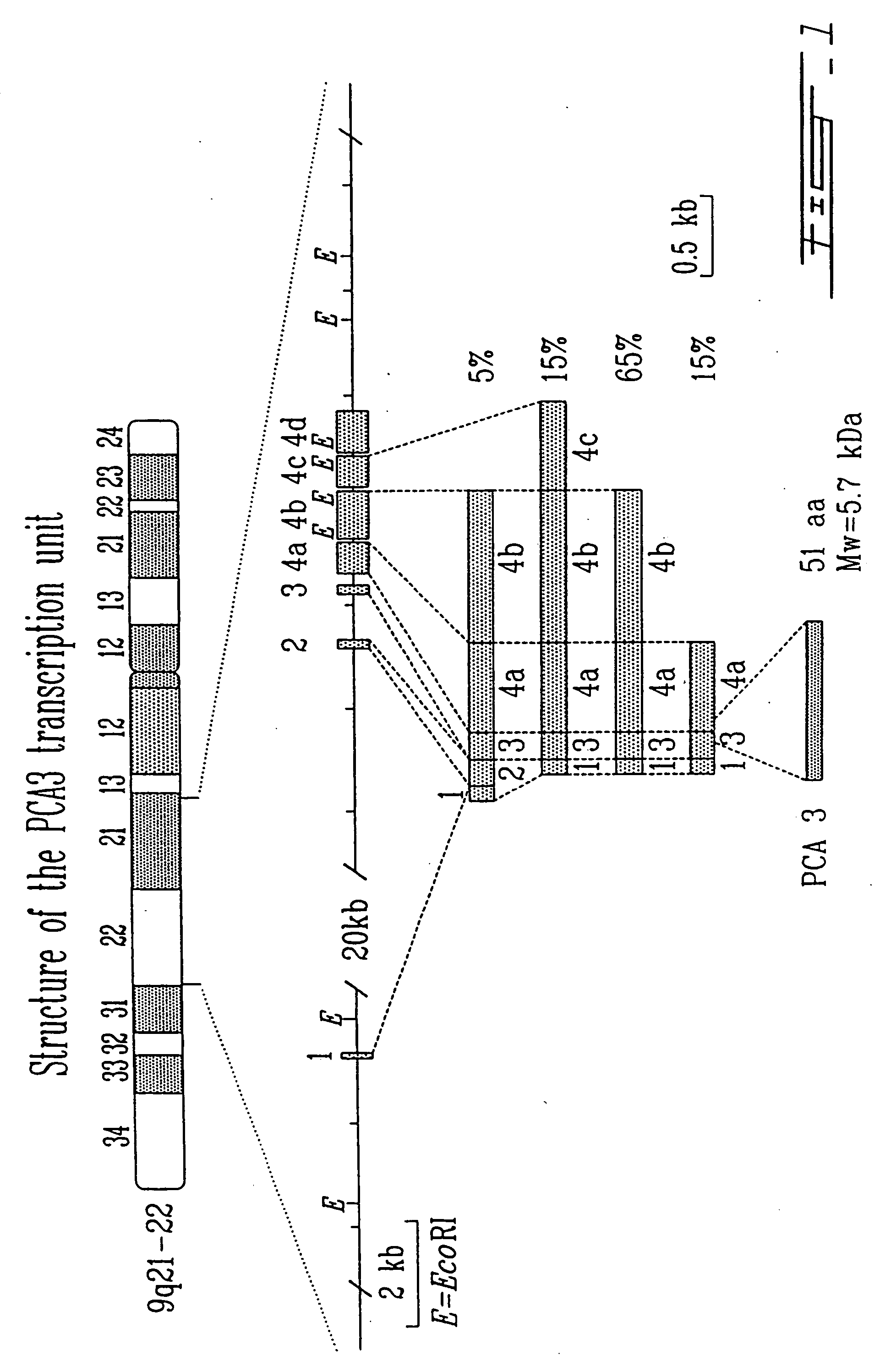
PCA3, PCA3 genes, and methods of use
The present invention relates, in general, to a prostate-specific antigen, PCA3. In particular, the present invention relates to nucleic acid molecules coding for the PCA3 protein; purified PCA3 proteins and polypeptides; recombinant nucleic acid molecules; cells containing the recombinant nucleic acid molecules; antibodies having binding affinity specifically to PCA3 proteins and polypeptides; hybridomas containing the antibodies; nucleic acid probes for the detection of nucleic acids encoding PCA3 proteins; a method of detecting nucleic acids encoding PCA3 proteins or polypeptides in a sample; kits containing nucleic acid probes or antibodies; bioassays using the nucleic acid sequence, protein or antibodies of this invention to diagnose, assess or prognose a mammal afflicted with prostate cancer; therapeutic uses; and methods of preventing prostate cancer in an animal.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Sequence Variants Associated with Prostate Specific Antigen Levels
InactiveUS20120150032A1Ultrasonic/sonic/infrasonic diagnosticsMicrobiological testing/measurementAntigenProstate-specific antigen
Owner:DECODE GENETICS EHF
Methods and kits for the diagnosis of acute coronary syndrome
InactiveUS20070003981A1Quick checkAccurate diagnosisMicrobiological testing/measurementDisease diagnosisComplement 3Factor VII
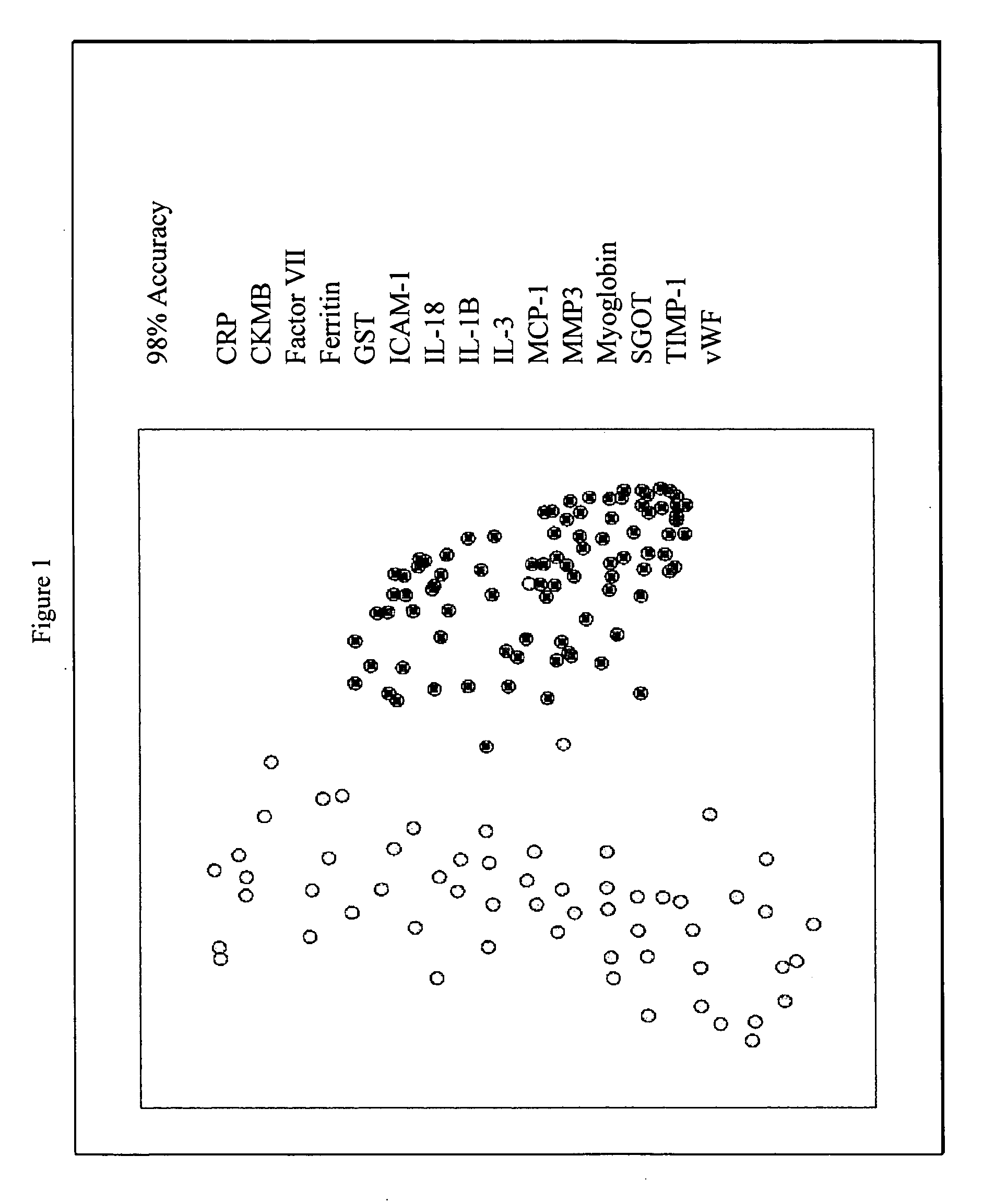
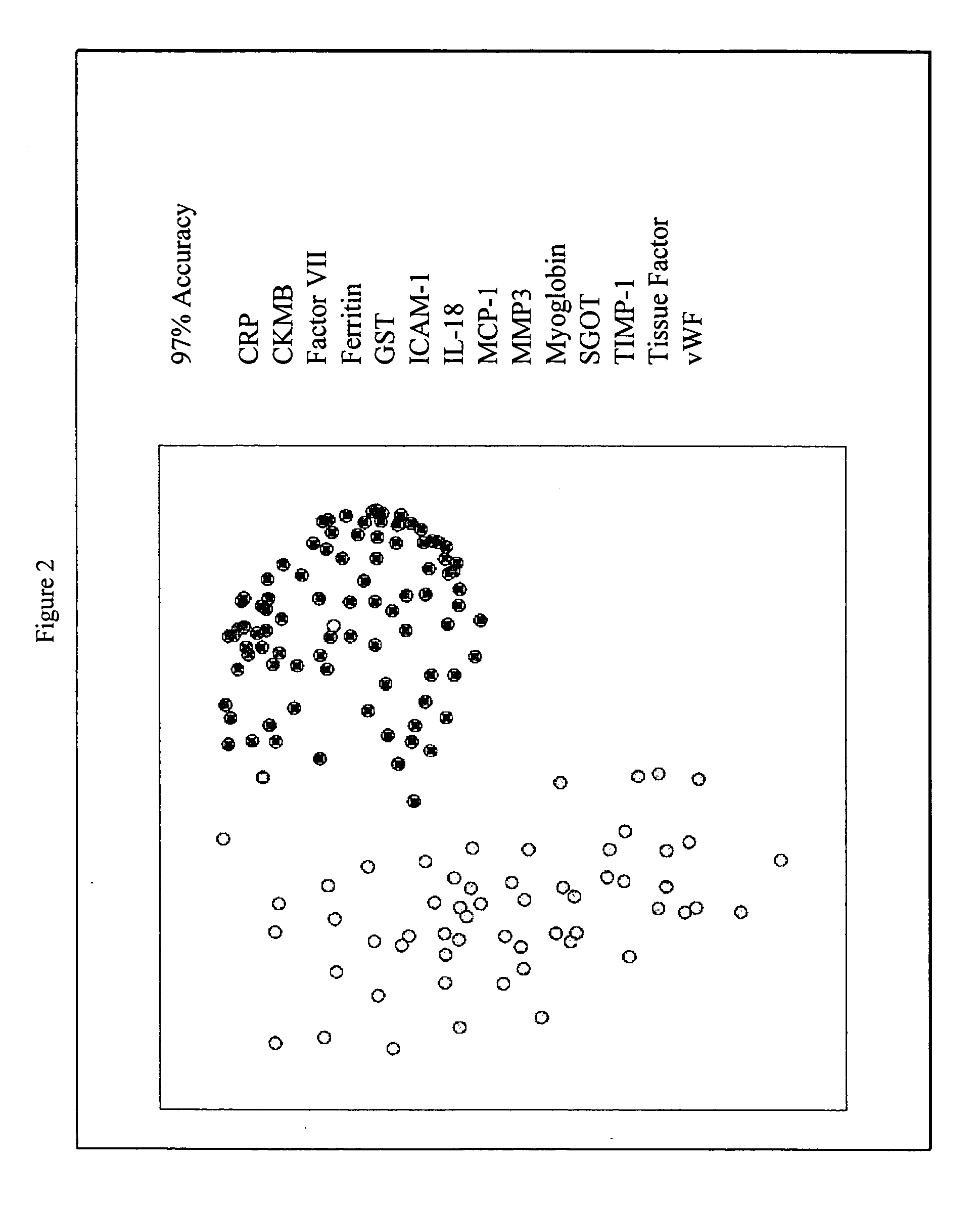
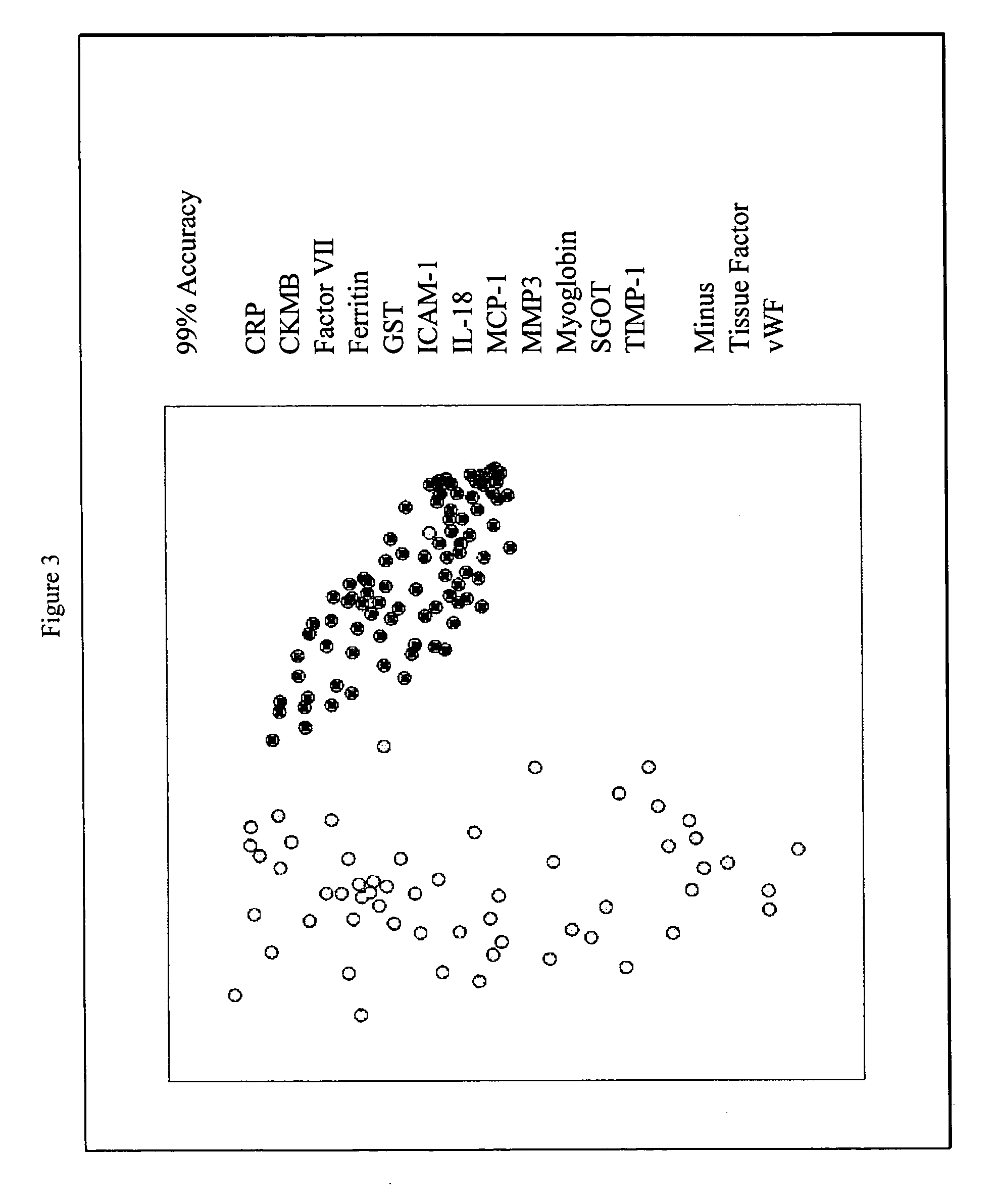
Provided are methods for the detection and diagnosis of acute coronary syndrome or ACS. The methods are based on the discovery that abnormal levels of selected analytes in sample fluid, typically blood samples, of patients who are at risk are supportive of a diagnosis of ACS. At least two new biomarkers for ACS are thus disclosed, MMP-3 and SGOT. Altogether the concentrations of twelve analytes provide a sensitive and selective picture of the patient's condition, namely, whether the patient is suffering a heart attack. Other important biomarkers for ACS are described, including but not limited to IL-18, Factor VII, ICAM-1, Creatine Kinase-MB, MCP-1, Myoglobin, C Reactive Protein, von Willebrand Factor, TIMP-1, Ferritin, Glutathione S-Transferase, Prostate Specific Antigen (free), IL-3, Tissue Factor, alpha-Fetoprotein, Prostatic Acid Phosphatase, Stem Cell Factor, MIP-1-beta, Carcinoembryonic Antigen, IL-13, TNF-alpha, IgE, Fatty Acid Binding Protein, ENA-78, IL-1-beta, Brain-Derived Nerotrophic Factor, Apolipoprotein A1, Serum Amyloid P, Growth Hormone, Beta-2 microglobulin, Lipoprotein (a), MMP-9, Thyroid Stimulating hormone, alpha-2 Macroglobulin, Complement 3, IL-7, Leptin, and IL-6. Kits containing reagents to assist in the analysis of fluid samples are also described.
Owner:RULES BASED MEDICINE
Tumor associated antigen peptides and use of same as anti-tumor vaccines
The present invention relates to tumor associated antigen (TAA) peptides and to use of same, of polynucleotides encoding same and of cells presenting same as anti-tumor vaccines. More particularly, the present invention relates to tumor associated antigen peptides derived from Uroplakin Ia, Ib, II and III, Prostate specific antigen (PSA), Prostate acid phosphatase (PAP) and Prostate specific membrane antigen (PSMA), BA-46 (Lactadherin), Mucin (MUC-1), and Teratocarcinoma-derived growth factor (CRIPTO-1) and the use of same as anti-tumor vaccines to prevent or cure bladder, prostate, breast or other cancers, carcinomas in particular. Most particularly, the present invention relates to tumor associated antigen peptides which are presentable to the immune system by HLA-A2 molecules.
Owner:YEDA RES & DEV CO LTD
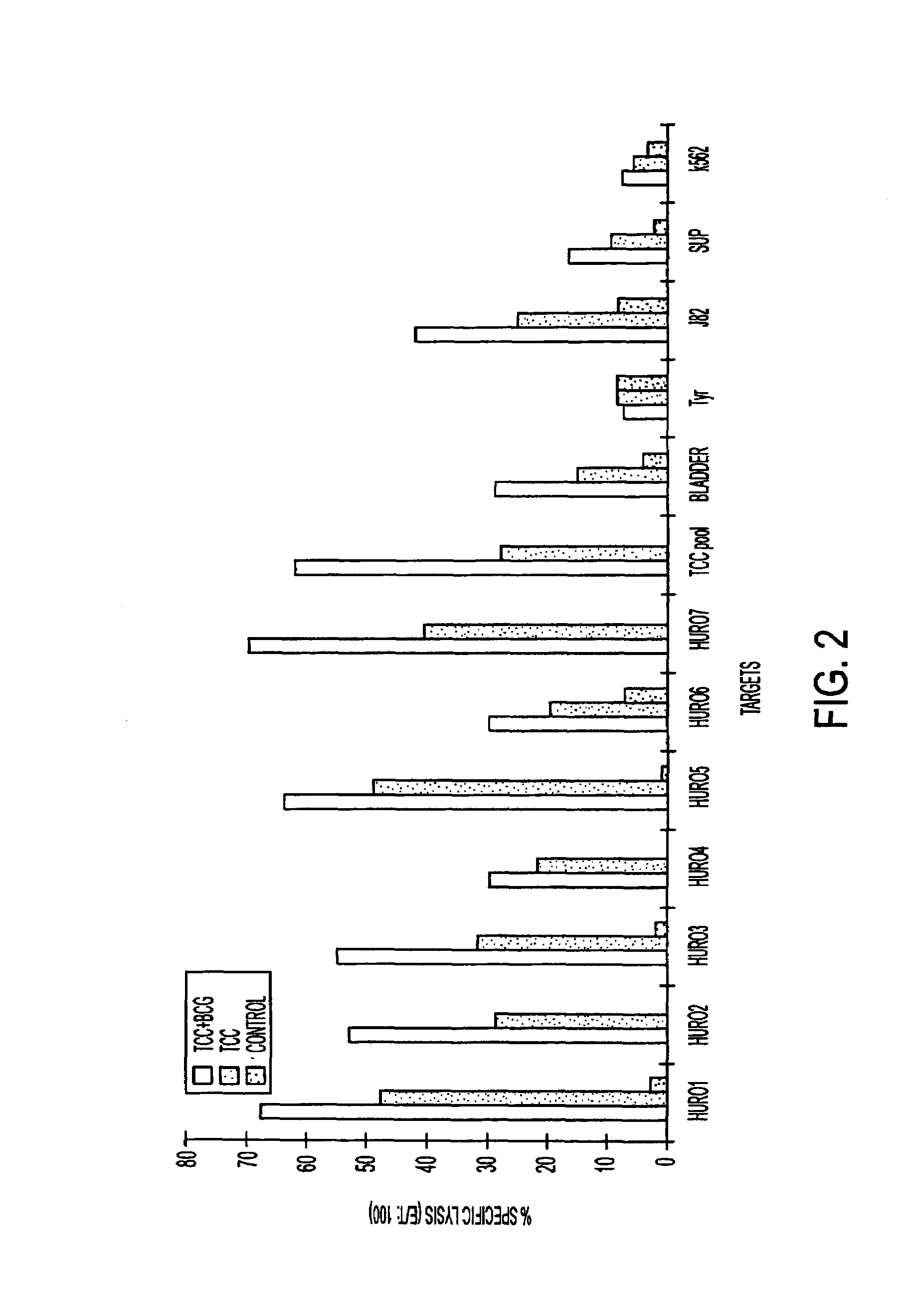
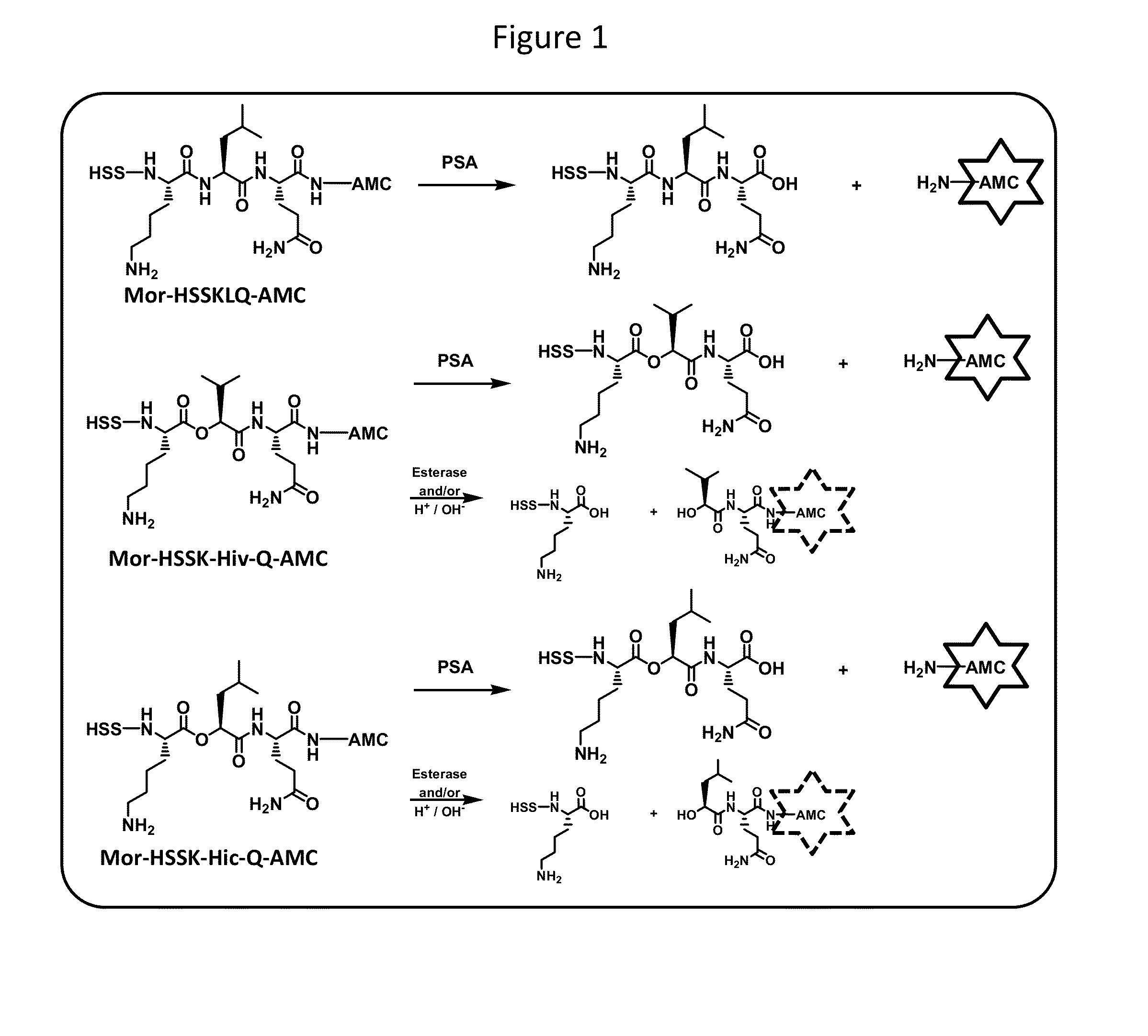
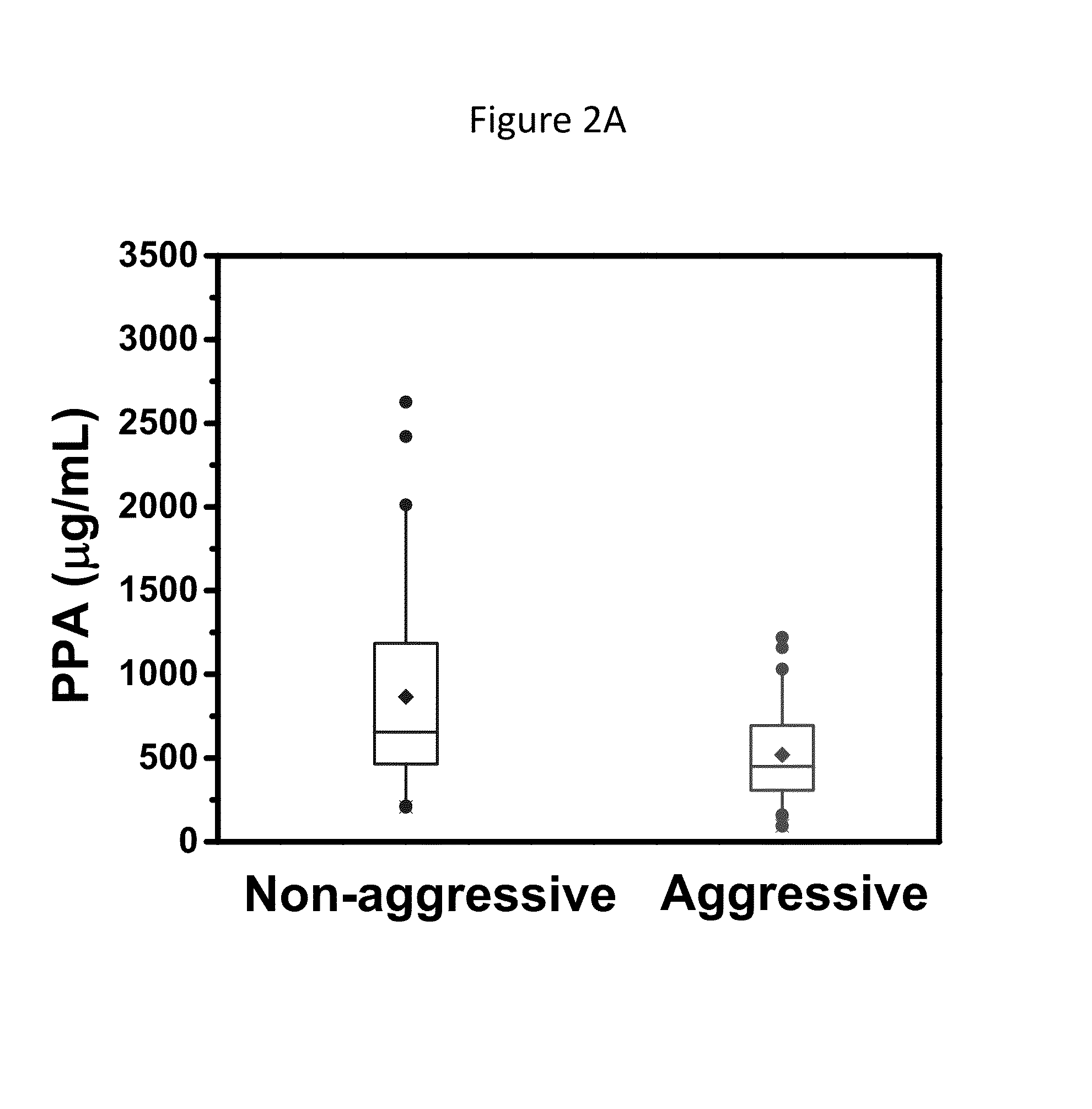
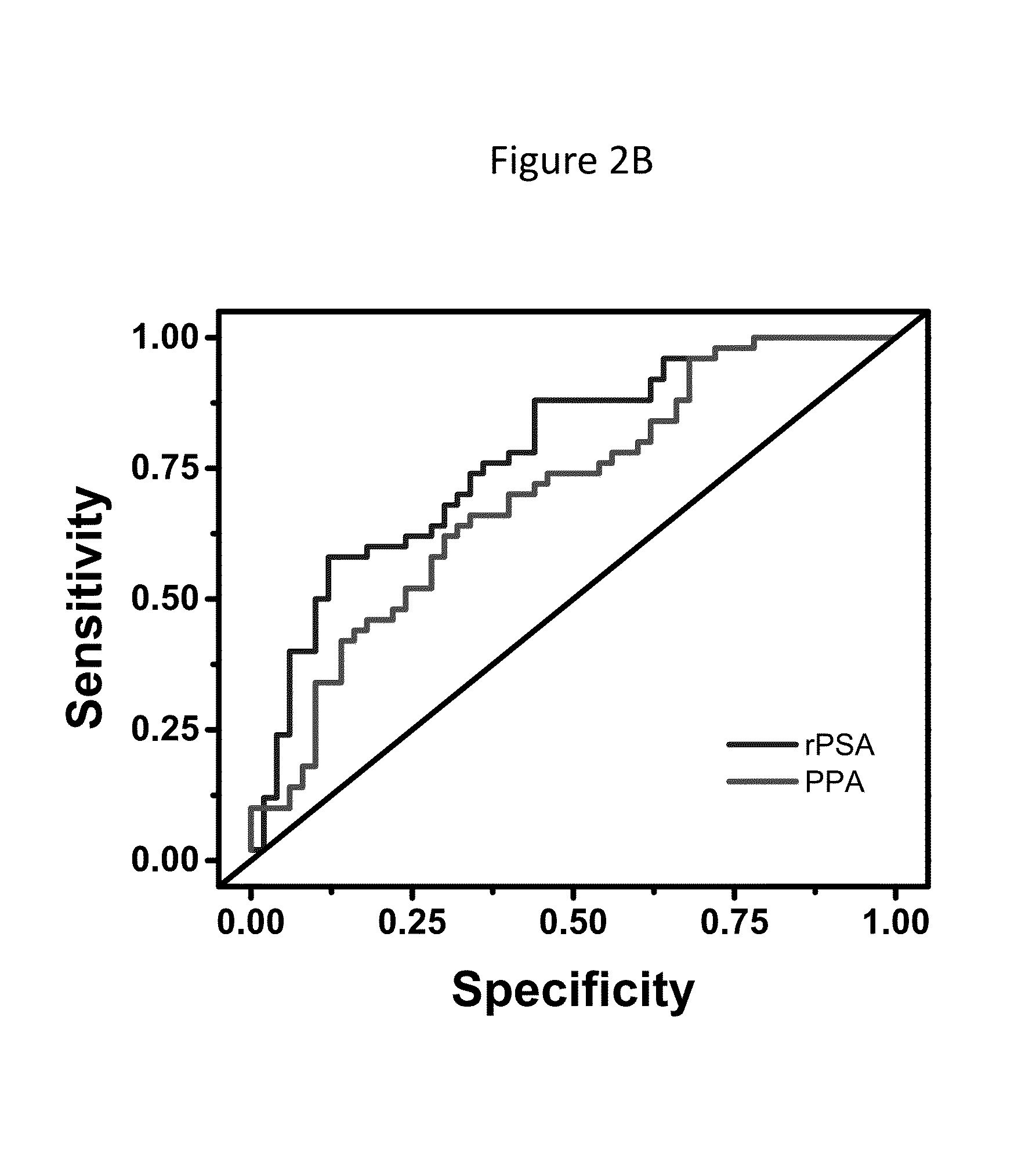
Psa enzymatic activity: a new biomarker for assessing prostate cancer aggressiveness
InactiveUS20140134658A1Improved Positive Predictive ValueUndetectable cross-reactivityMicrobiological testing/measurementDisease diagnosisProstate-specific antigenOncology
The disclosure provides for methods to determine prognosis, aggressiveness, and progression of prostate cancer with the ability to differentiate between aggressive and non-aggressive prostate cancer. The method utilizes the differential enzymatic activity in patient samples, for example of enzymatically active prostate specific antigen (PSA) activity, in order to determine the aggressiveness and prognosis of prostate cancer, and monitor the progression of prostate cancer therapy. The invention also encompasses assay platforms (e.g., optical or electrochemical) that specifically detect PSA-triggered peptide cleavage events.
Owner:OHMX CORP +1
Quantitative detection method for pyrethriods pesticides in fresh tea
ActiveCN103913528ABroad specificityIncrease flexibilityComponent separationCapillary columnAccuracy and precision
The invention discloses a quantitative detection method for pyrethriods pesticides in fresh tea. The quantitative detection method is characterized by comprising the following steps: ultrasonically extracting a fresh tea sample by an acetonitrile solution containing 1% of acetic acid in percentage by volume, then purifying by using an appropriate amount of mixed filler of PSA (Prostate Specific Antigen), C18 and GCB, carrying out nitrogen blowing to concentrate liquid supernatant to be nearly dry, dissolving with normal hexane and making up to volume, increasing programmed temperature and separating by using an HP-5 quartz capillary column, carrying out GC / muECD, and finally quantifying by using a matrix external standard method. Through confirmation by test, the method is wide in linear range; the technical indexes such as detection sensitivity, accuracy and precision meet the requirements of analysis of residues; the pretreatment operation is simple, convenient and quick; through the method, a reliable manner is provided for analysis and research for detecting the residues of the pyrethriods pesticides in the fresh tea.
Owner:江苏太湖地区农业科学研究所
Adenovirus vectors specific for cells expressing androgen receptor and methods of use thereof
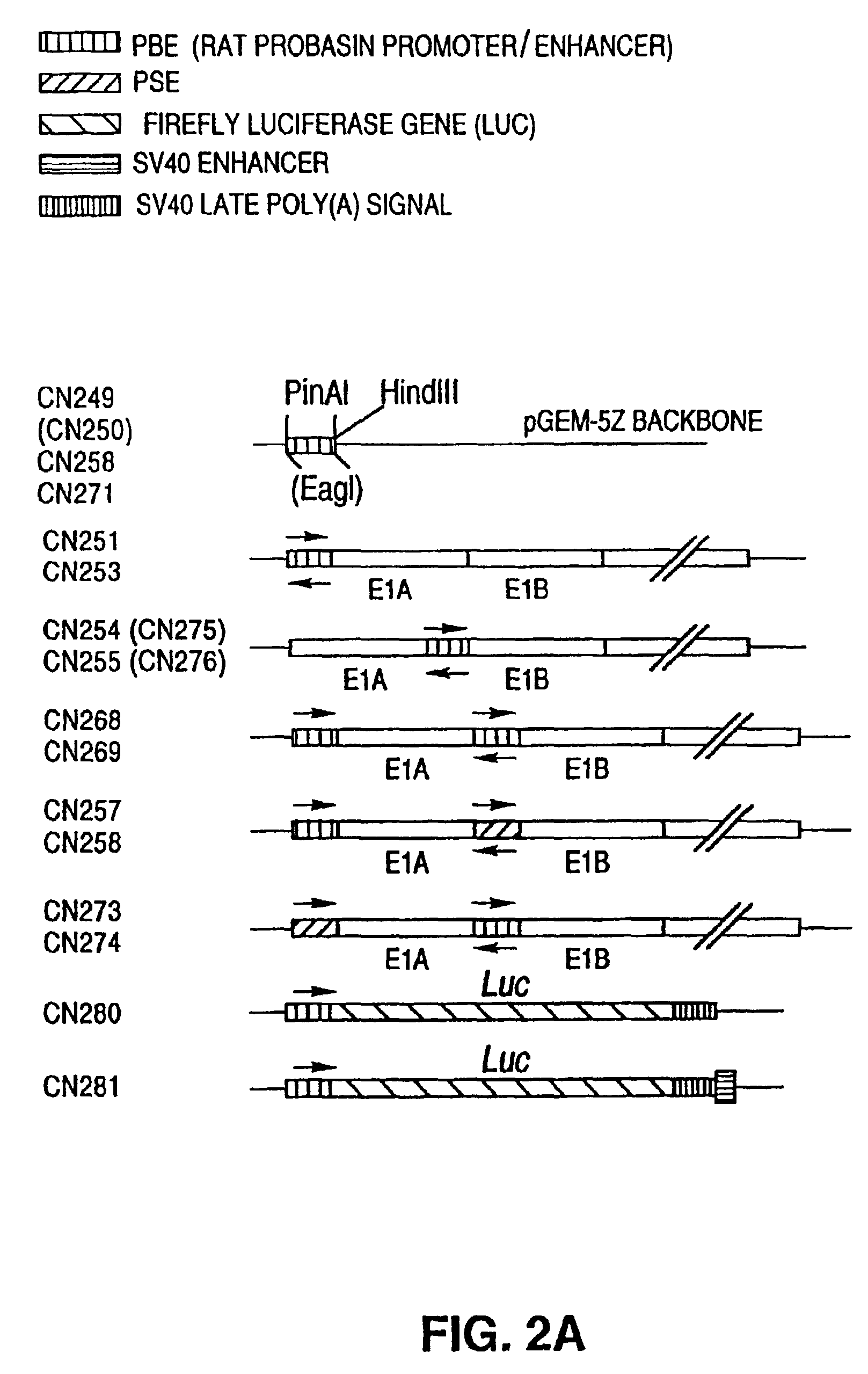
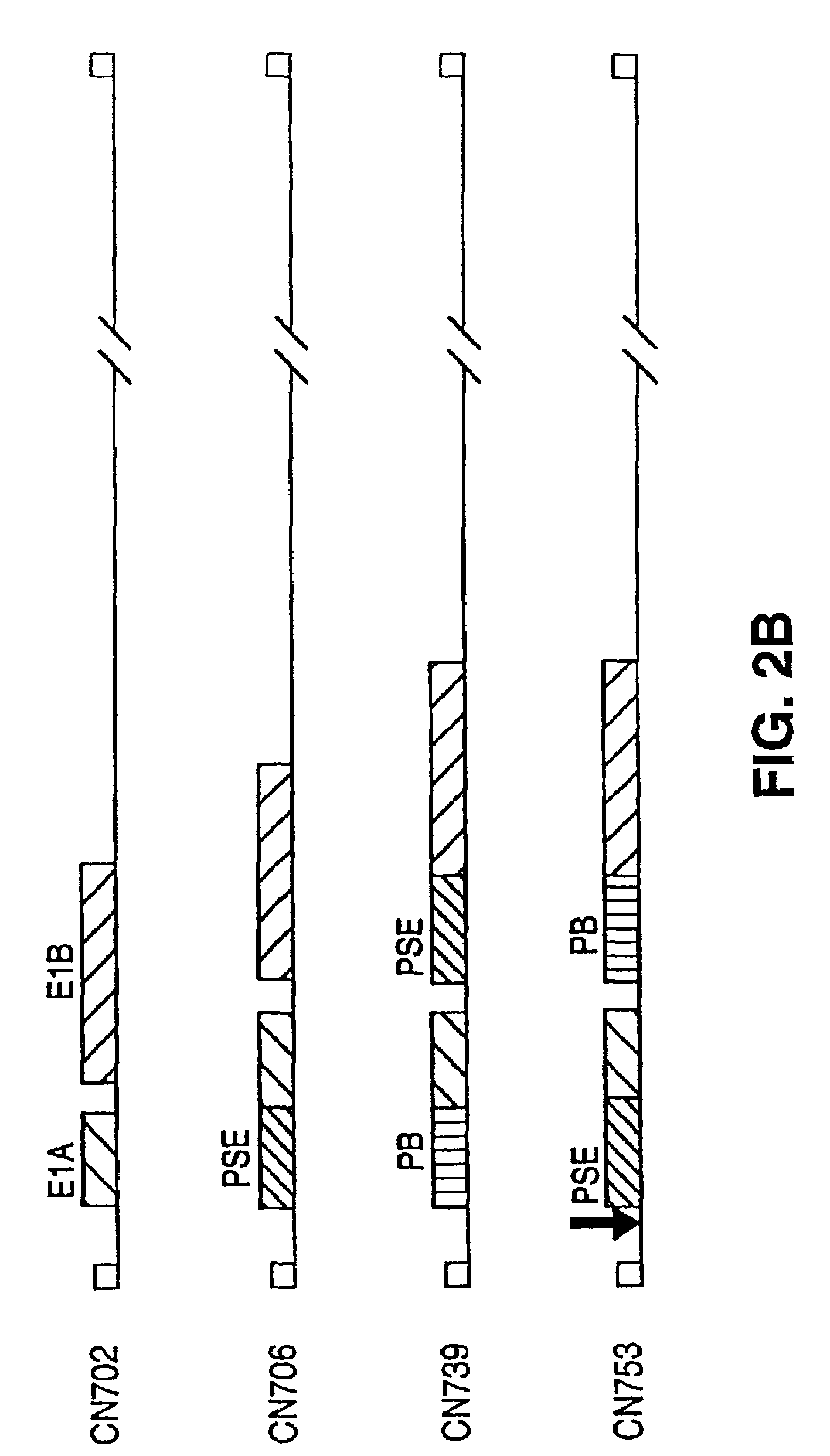
Replication-competent adenovirus vectors specific for cells which allow a probasin transcriptional response element (PB-TRE) to function, such as cells which express the androgen receptor (AR), and methods of use of such viruses are provided. These viruses comprise an adenoviral gene under control of a transcriptional regulatory portion of a PB-TRE, which is in turn dependent upon AR expression. The gene can be, for example, a gene required for viral replication or the adenovirus death protein gene (ADP). The viruses can also comprise at least one additional adenoviral gene under control of at least one additional prostate-specific transcriptional response element, such as that controlling prostate-specific antigen expression (PSA-TRE). Thus, virus replication can be restricted to target cells exhibiting prostate-specific gene expression, particularly prostate carcinoma cells.
Owner:CELLS GENESYS INC
Anti-prostate-specific-antigen PSA monoclone antibody and its use
InactiveCN101070345AWide and sensitive detection rangeGood antibody propertiesImmunoglobulins against animals/humansBiological testingProstate-specific antigenBiological macromolecule
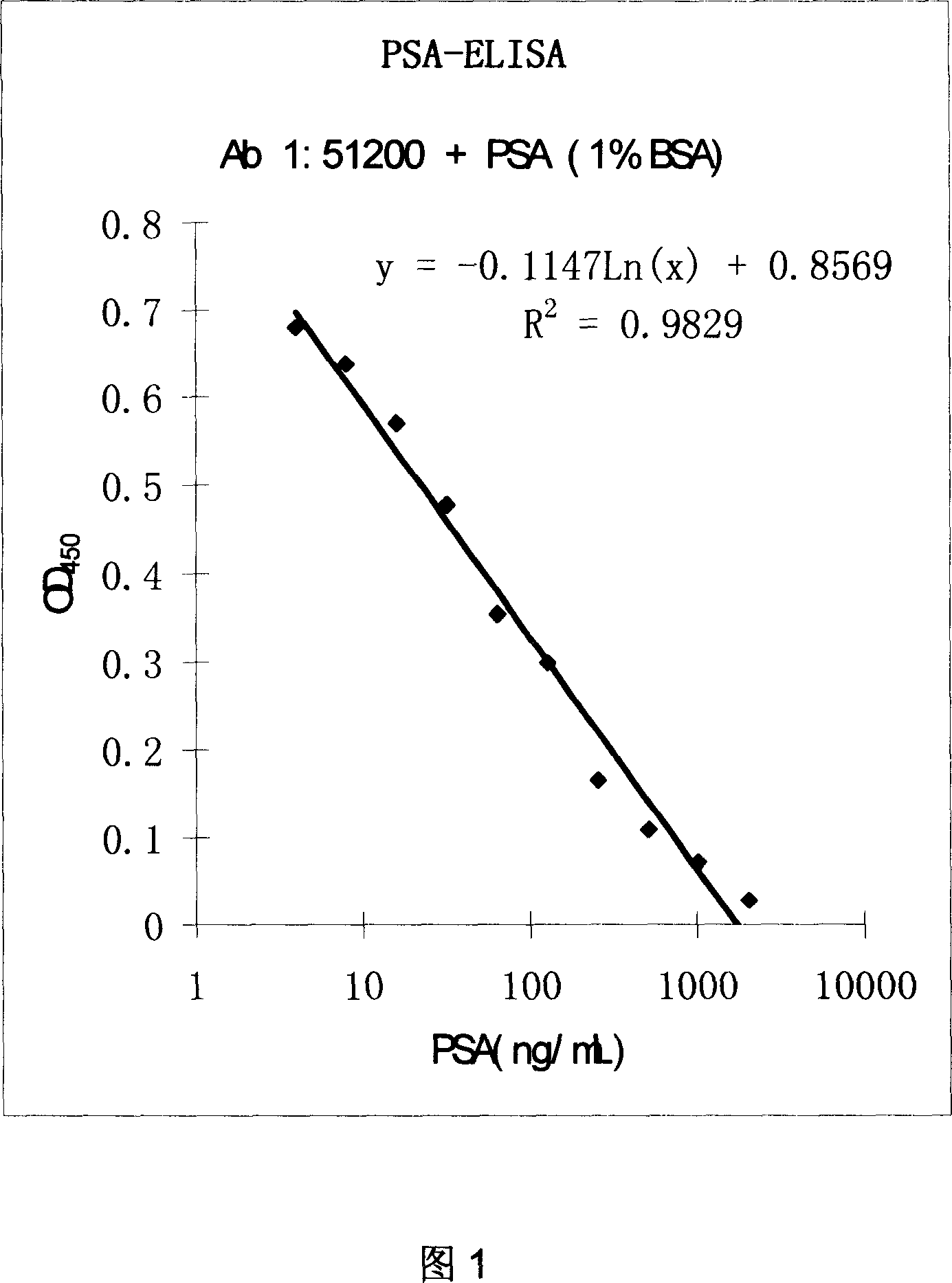
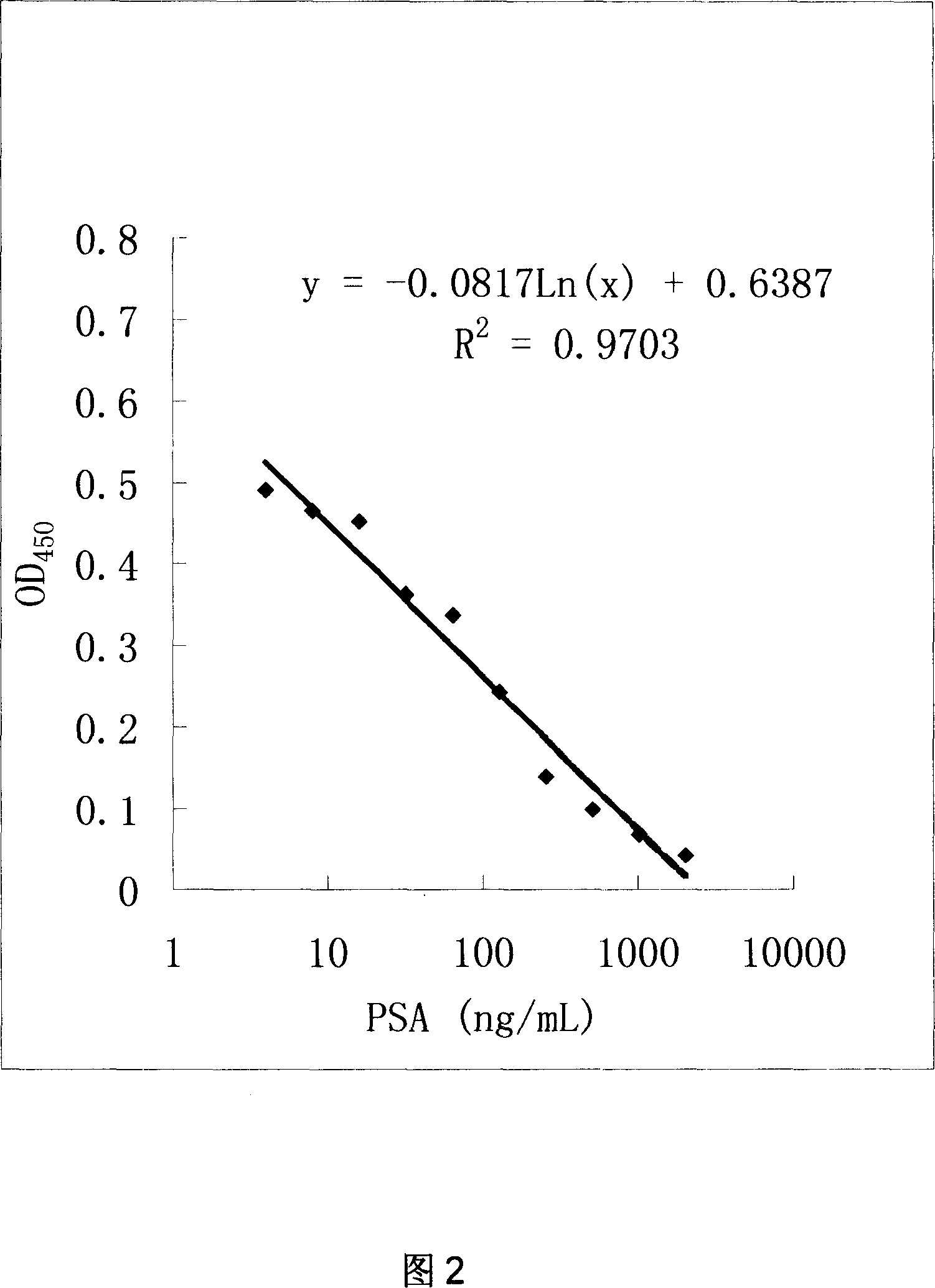
This invention relates to three kinds of monoclonal antibody of prostate specific antigen(PSA), and corresponding hybridoma cell strain. The preservation number respectively are: CCTCC-C200620, CCTCC-C200621 and CCTCC-C200622. The detection range of this monoclonal antibody can reach 0 to 2048ng / mL, possess feature of sensitivity, idiosyncratic and low cost. The invention has important application prospects at cytobiology, biomacromolecule detection and medicine clinical diagnosis etc.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
Preparation method and application of prostate tumor marker immunosensor
InactiveCN103675279AHigh sensitivityEnhanced electrochemical signalMaterial analysisAntigenPhosphate
The invention relates to a preparation method of a prostate tumor marker immunosensor. The preparation method comprises the following steps: (1) dissolving silver-hybridized NH2-MCM-48 into a phosphate buffered solution, uniformly dispersing in an ultrasonic mode, further adding a prostate specific antigen second antibody, oscillating at the temperature of 4 DEG C, centrifuging to remove the supernate to obtain a second antibody marker Ag@NH2-MCM-48 / Ab2; (2) firstly, modifying the surface of a glassy carbon electrode as a working electrode by using an amino graphene-ferrocenecarboxaldehyde composite material solution, subsequently modifying by using glutaraldehyde, further modifying by using a prostate specific antigen-anti-solution, dropping a bovine serum albumin solution, continuously modifying prostate specific antigen, and finally modifying Ag@NH2-MCM-48 / Ab1 so as to obtain the prostate tumor marker immunosensor. The immunosensor prepared by using the preparation method is applied to measurement on the prostate specific antigen, and is high in sensitivity and good in specificity.
Owner:SHANDONG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com