Patents
Literature
189 results about "Factor VII" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Factor VII (EC 3.4.21.21, blood-coagulation factor VIIa, activated blood coagulation factor VII, formerly known as proconvertin) is one of the proteins that causes blood to clot in the coagulation cascade. It is an enzyme of the serine protease class. A recombinant form of human factor VIIa (eptacog alfa [activated], NovoSeven) has U.S. Food and Drug Administration approval for uncontrolled bleeding in hemophilia patients. It is sometimes used unlicensed in severe uncontrollable bleeding, although there have been safety concerns. A biosimilar form of recombinant activated factor VII (AryoSeven) is also available, but does not play any considerable role in the market.
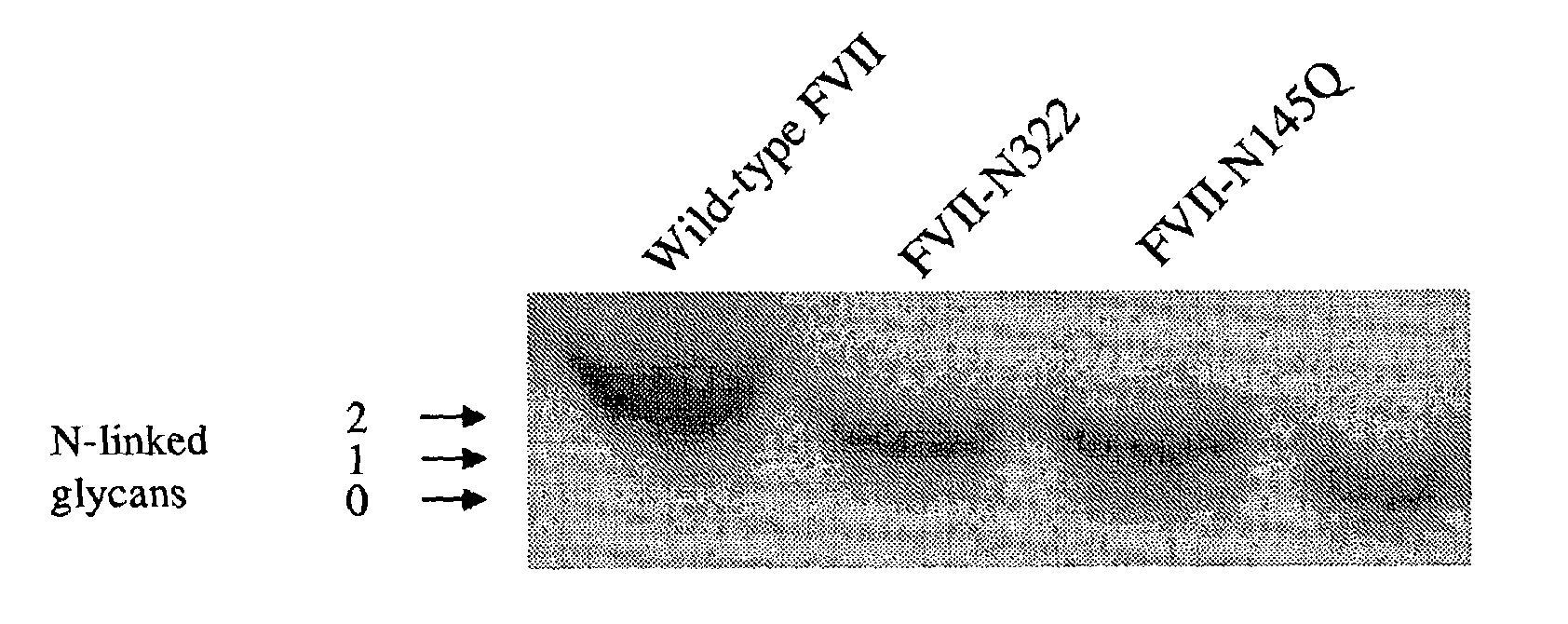
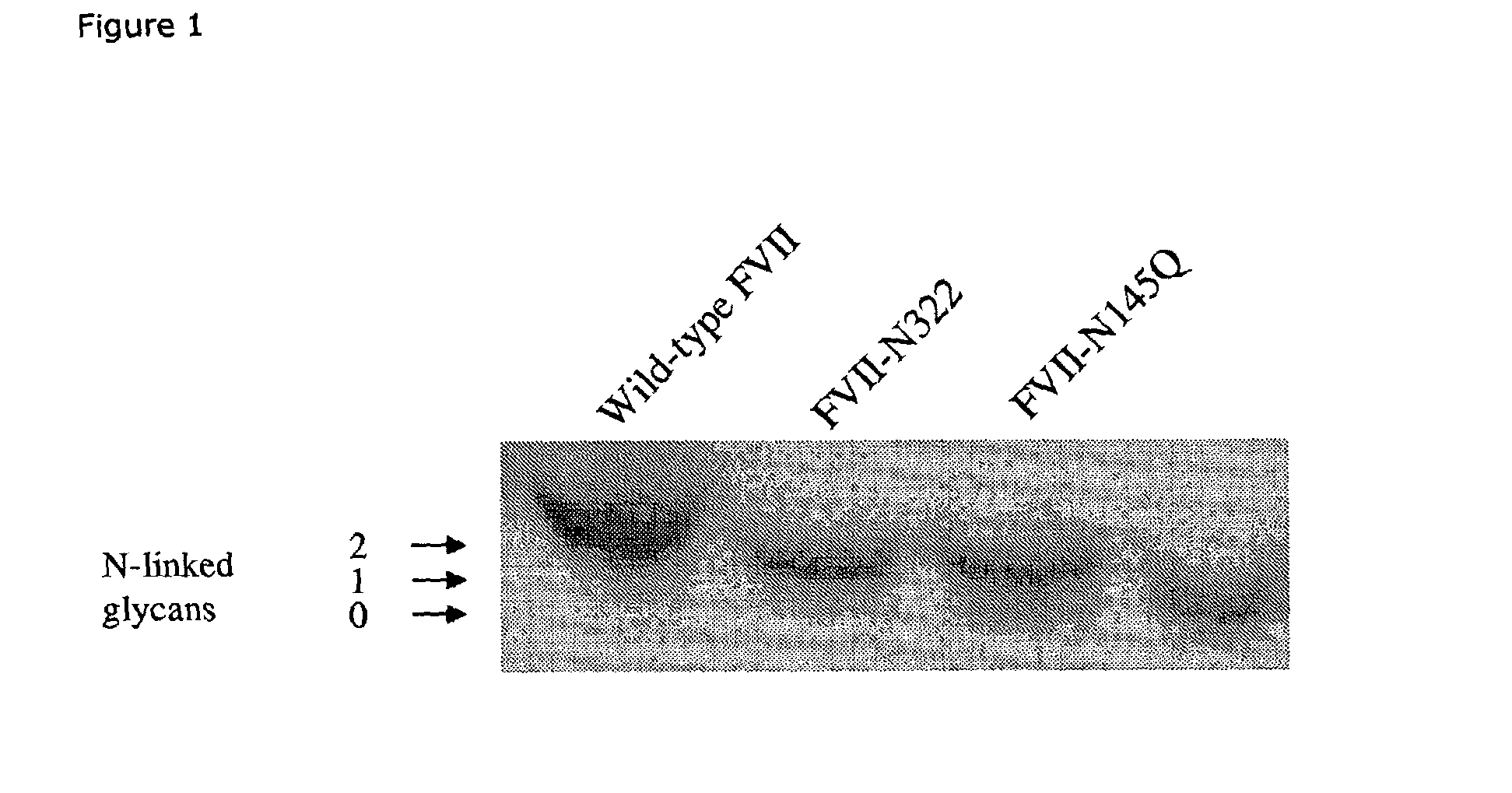
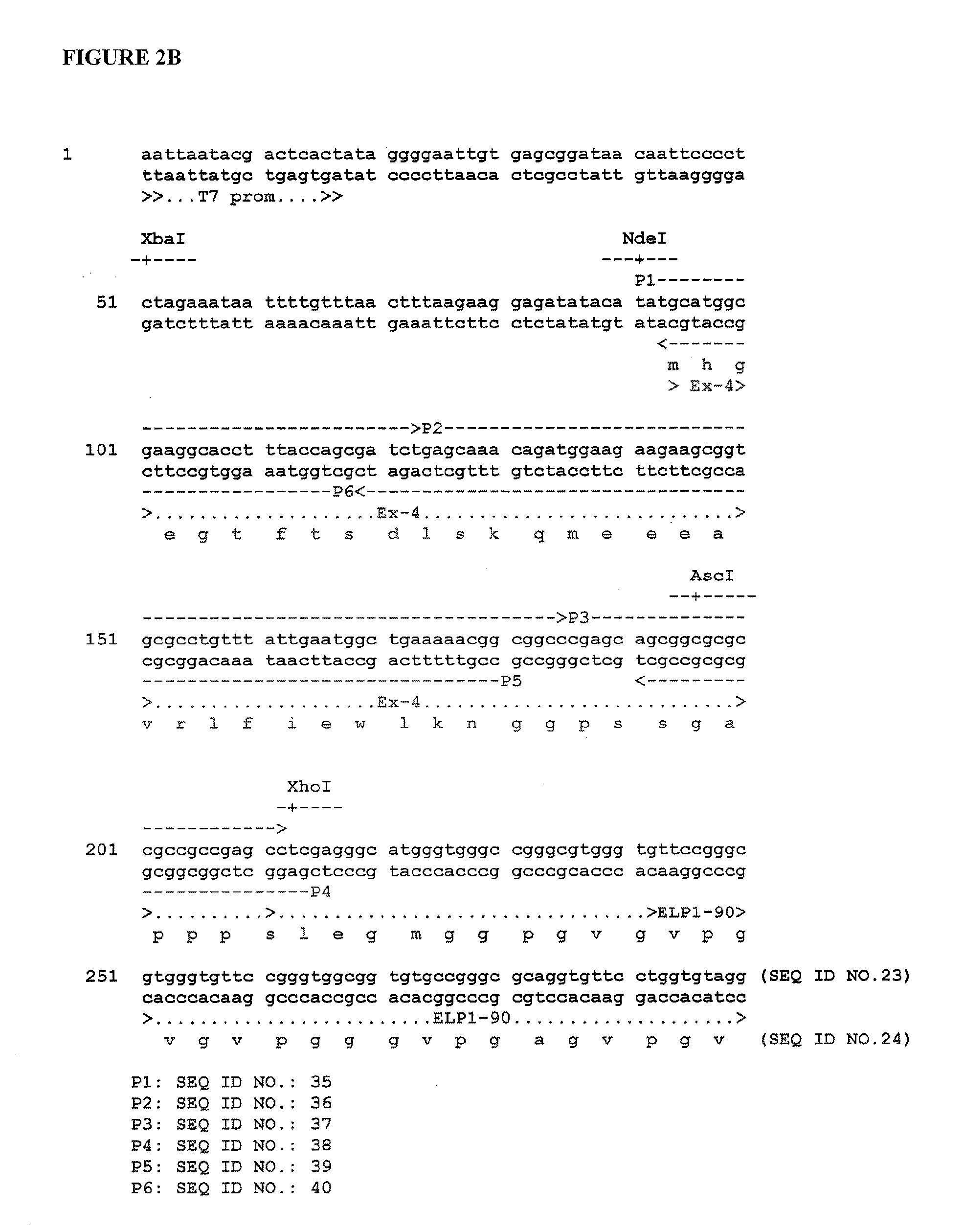
Therapeutic agents comprising elastin-like peptides
ActiveUS20100022455A1Enhanced advantageImprove stabilityBacteriaPeptide/protein ingredientsSolubilityTherapeutic protein
The present invention provides therapeutic agents and compositions comprising elastin-like peptides (ELPs) and therapeutic proteins. In some embodiments, the therapeutic protein is a GLP-1 receptor agonist, insulin, or Factor VII / VIIa, including functional analogs. The present invention further provides encoding polynucleotides, as well as methods of making and using the therapeutic agents. The therapeutic agents have improvements in relation to their use as therapeutics, including, inter alia, one or more of half-life, clearance and / or persistance in the body, solubility, and bioavailability.
Owner:DUKE UNIV
Human Coagulation Factor VII Polypeptides
InactiveUS20090055942A1Prolong half-life in vivoPeptide/protein ingredientsFermentationFactor VIIClotting factor
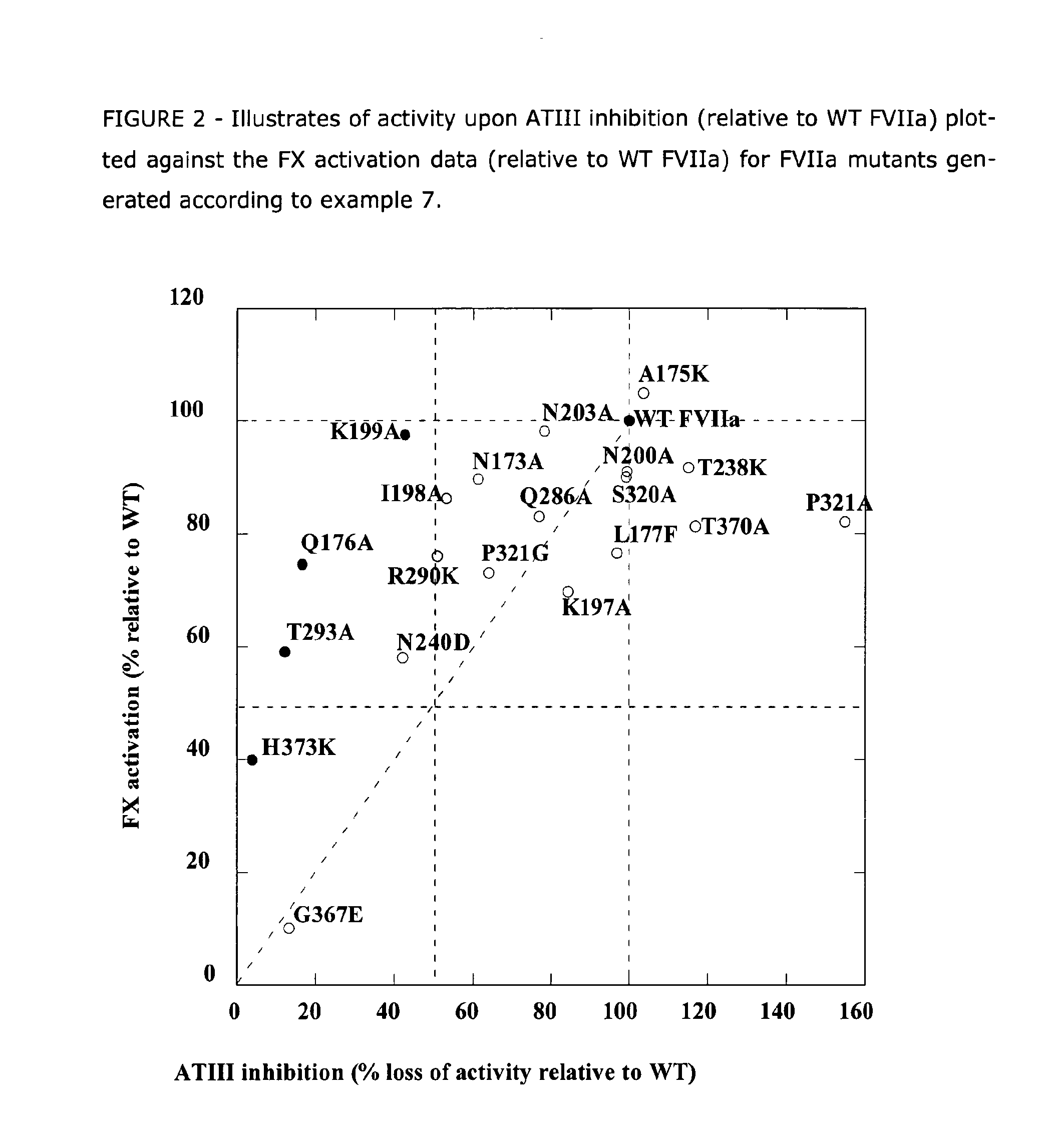
The present invention relates to novel human coagulation Factor Vila variants having substitutions of one or more amino acids at a position selected from the group consisting of position 172, 173, 175, 176, 177, 196, 197, 198, 199, 200, 203, 235, 237, 238, 239, 240, 286, 287, 288, 289, 290, 291, 292, 293, 294, 295, 297, 299, 319, 320, 321, 327, 341, 363, 364, 365, 366, 367, 370, 373 corresponding to amino acid positions of SEQ ID NO:1 and wherein said Factor VII polypeptide exhibits increased resistance to inactivation by an endogenous inhibitor of said FVII polypeptide relative to wild-type human FVIIa.
Owner:NOVO NORDISK AS
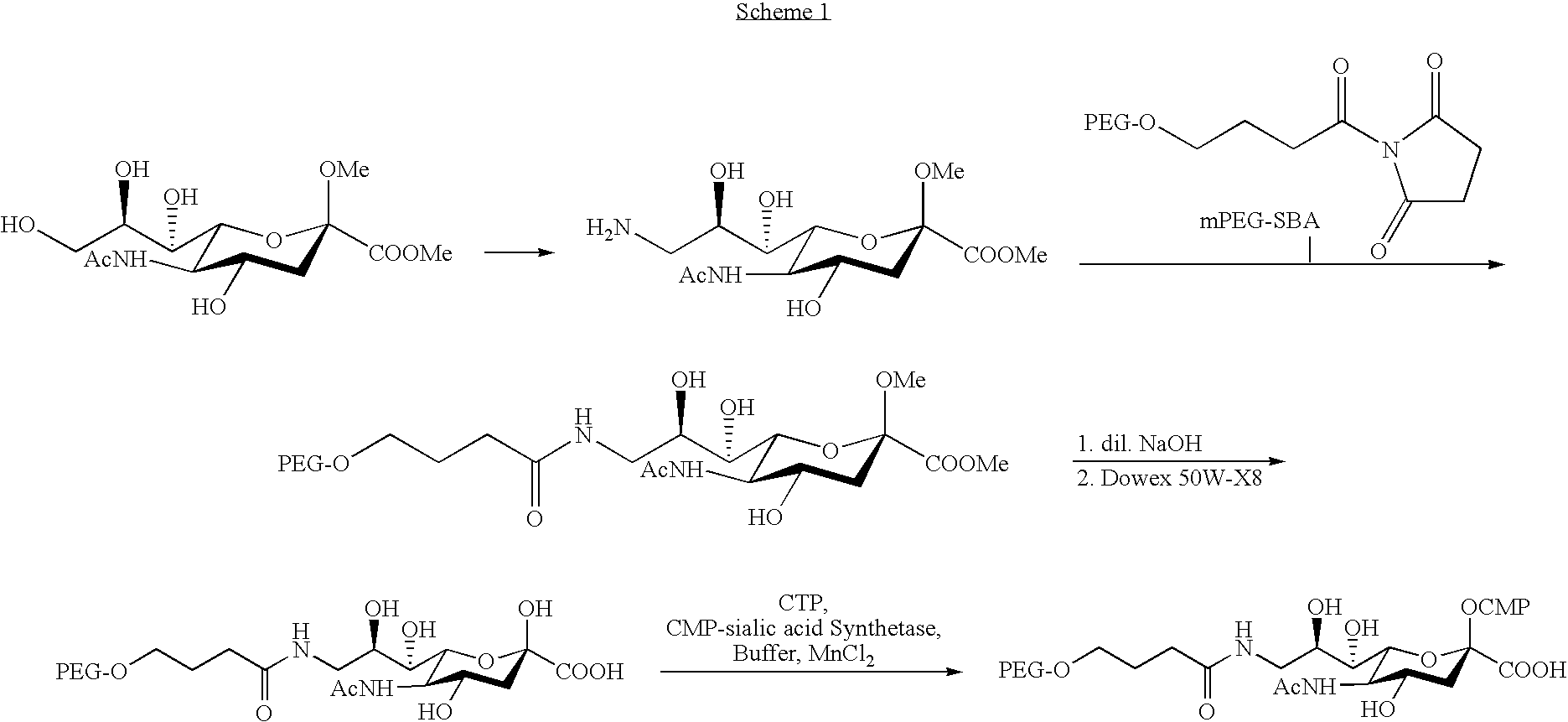
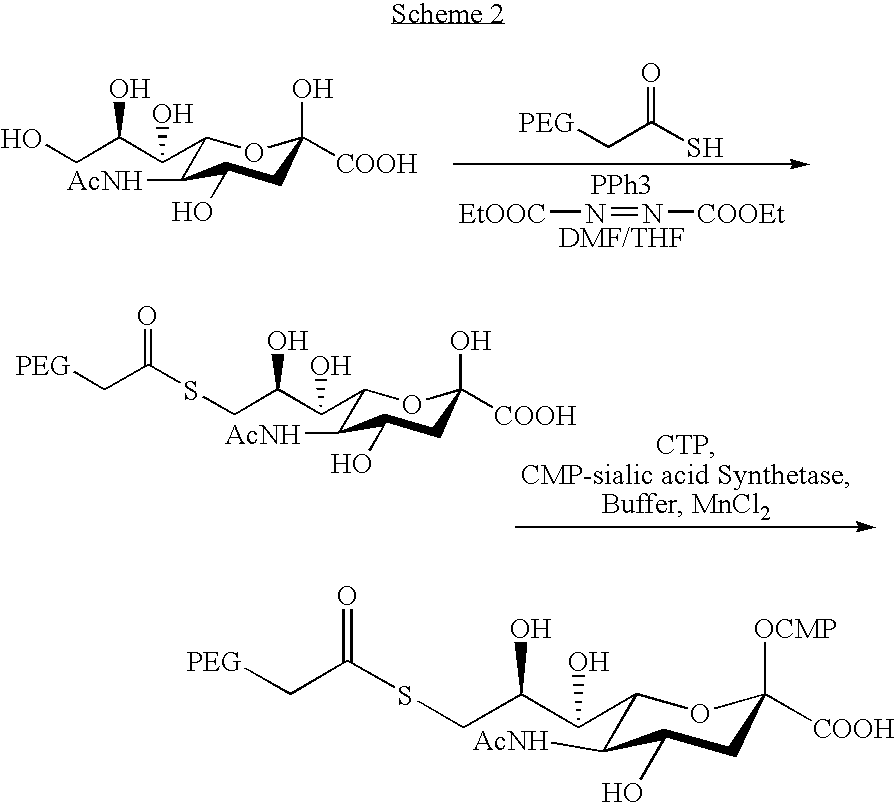
Pegylated factor VII glycoforms
InactiveUS20050113565A1Improve functional propertiesFactor VIIPeptide/protein ingredientsFactor VIIOligosaccharide
The invention concerns a preparation comprising a plurality of Factor VII polypeptides or Factor VII-related polypeptides, wherein the polypeptides comprise asparagine-linked and / or serine-linked oligosaccharide chains, and wherein at least one oligosaccharide group is covalently attached to at least one polymeric group.
Owner:KLAUSEN NIELS +3
Compositions and methods for inhibiting expression of factor vii gene
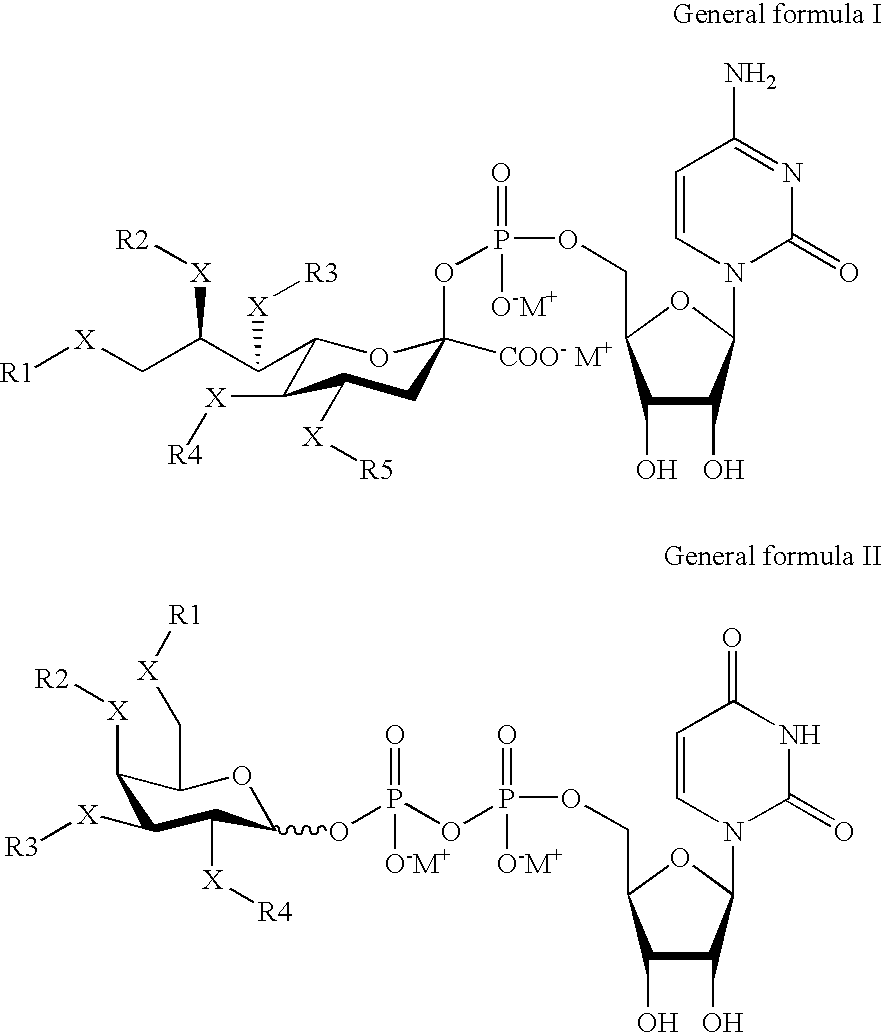
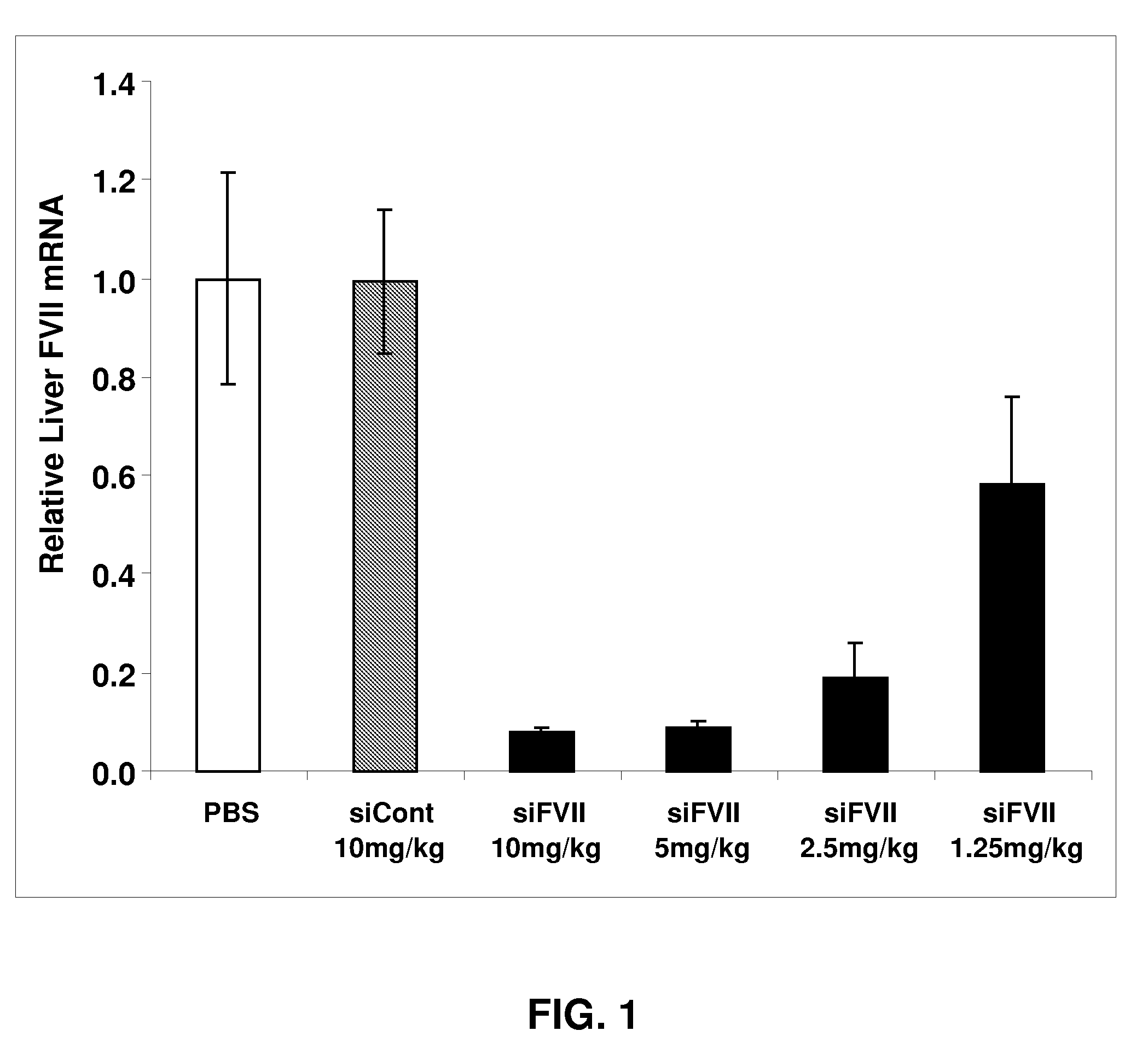
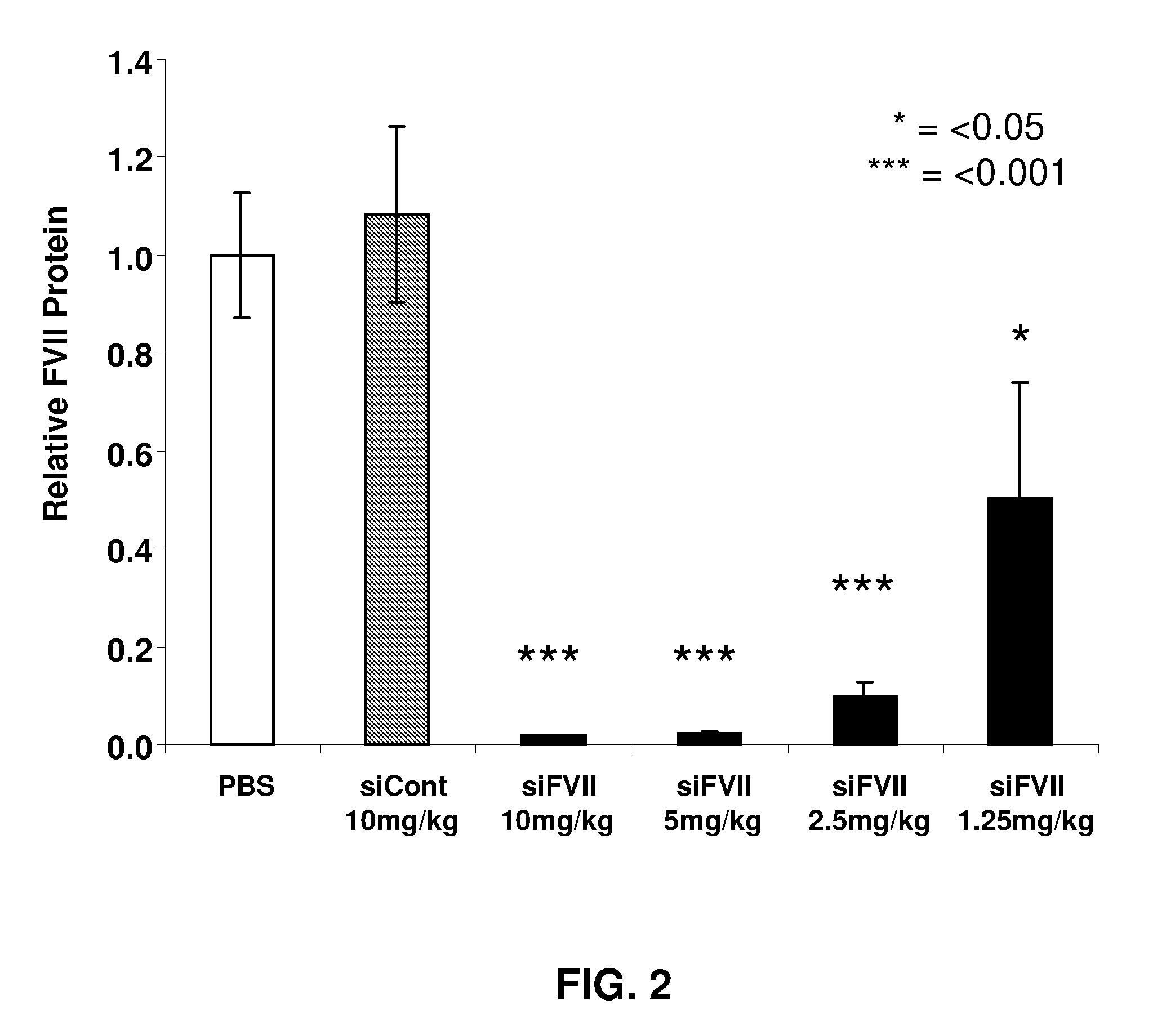
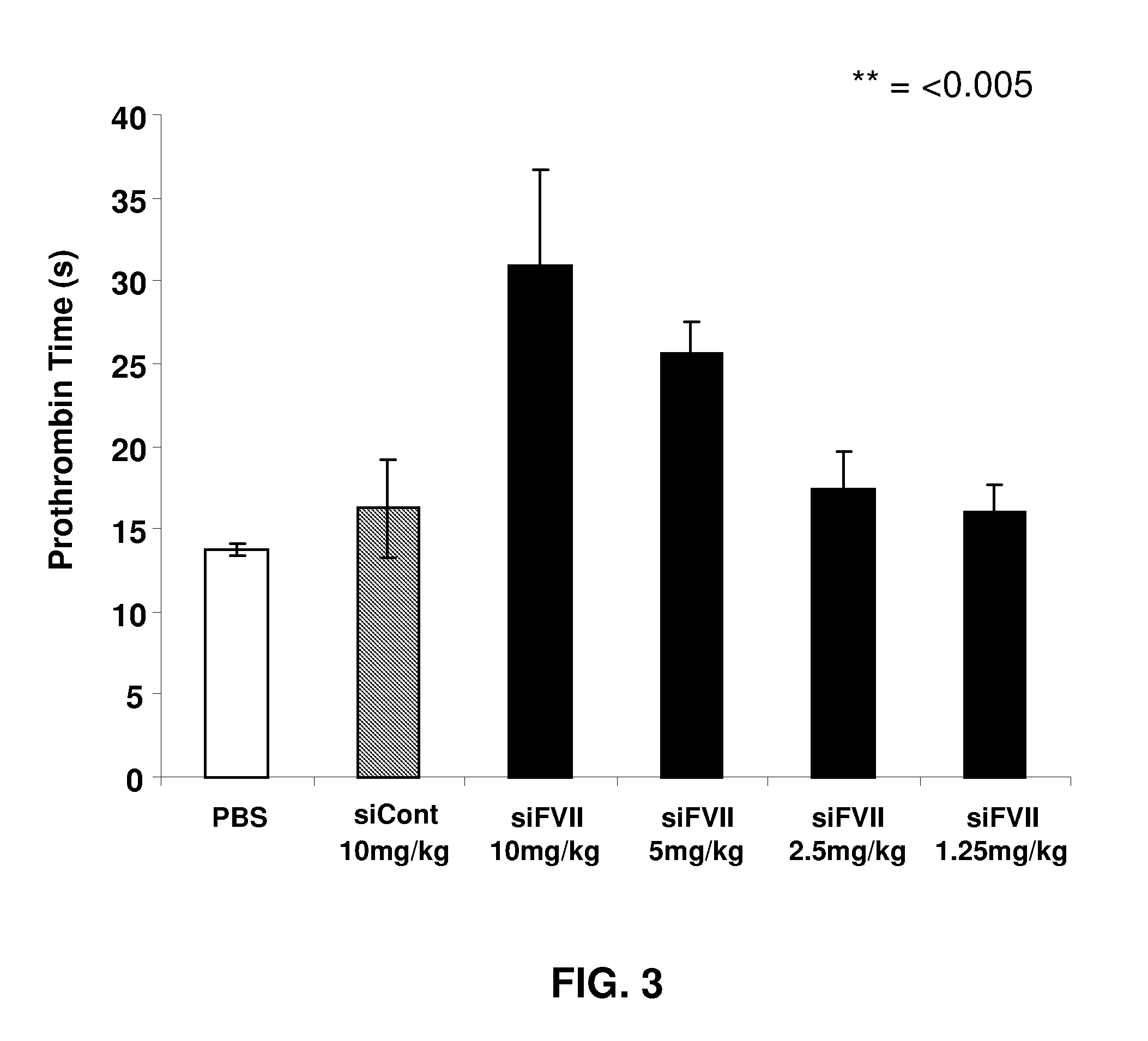
ActiveUS20090264511A1Decrease in levelLower protein levelOrganic active ingredientsAntipyreticDouble strandFactor VII
The invention relates to a double-stranded ribonucleic acid (dsRNA) for inhibiting the expression of the Factor VII gene.
Owner:ALNYLAM PHARMA INC
Therapeutic agents comprising a GLP-1 receptor agonist and elastin-like peptide
ActiveUS8178495B2Improve stabilityImprove solubilityBacteriaPeptide/protein ingredientsSolubilityTherapeutic protein
The present invention provides therapeutic agents and compositions comprising elastin-like peptides (ELPs) and therapeutic proteins. In some embodiments, the therapeutic protein is a GLP-1 receptor agonist, insulin, or Factor VII / VIIa, including functional analogs. The present invention further provides encoding polynucleotides, as well as methods of making and using the therapeutic agents. The therapeutic agents have improvements in relation to their use as therapeutics, including, inter alia, one or more of half-life, clearance and / or persistance in the body, solubility, and bioavailability.
Owner:DUKE UNIV
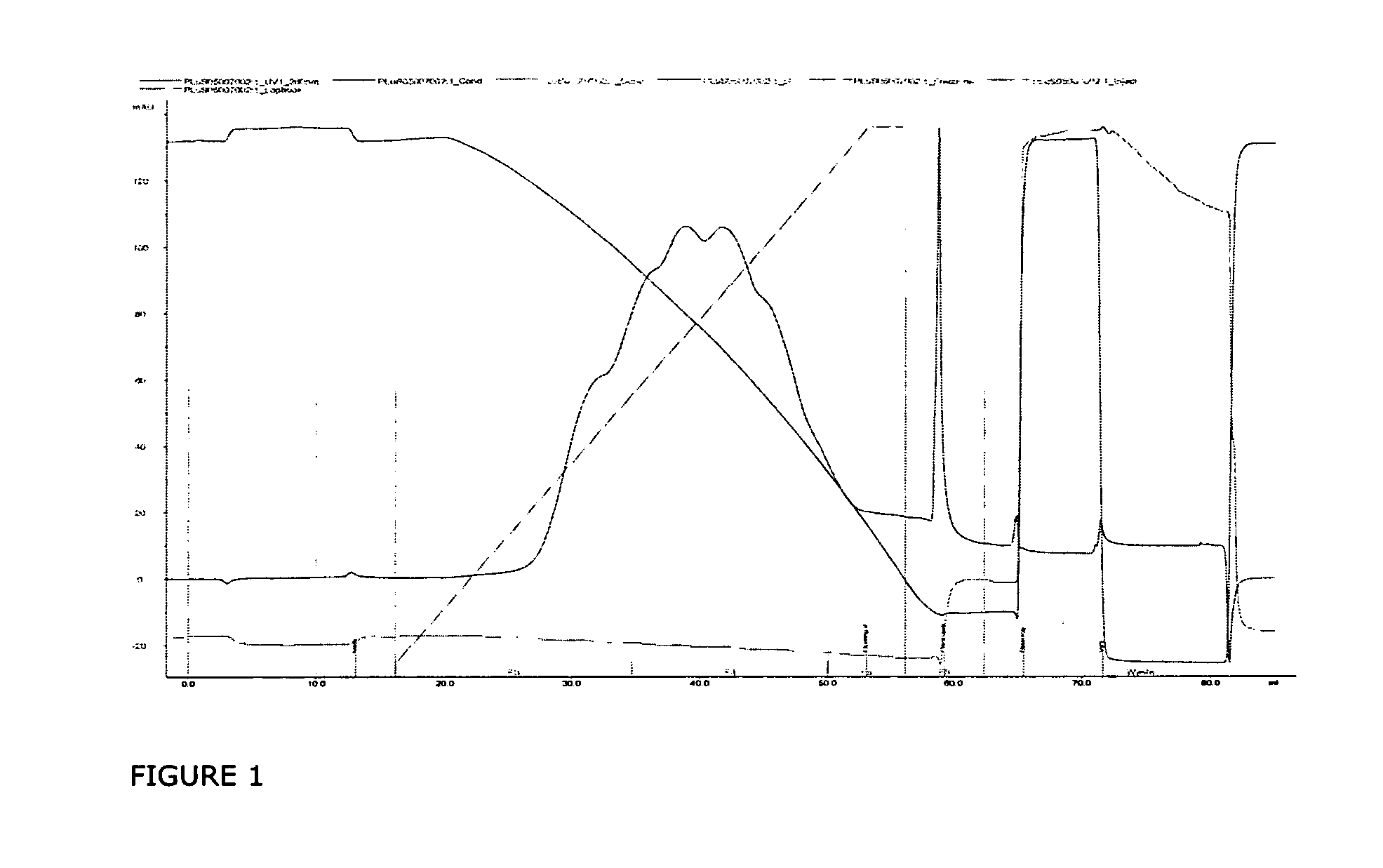
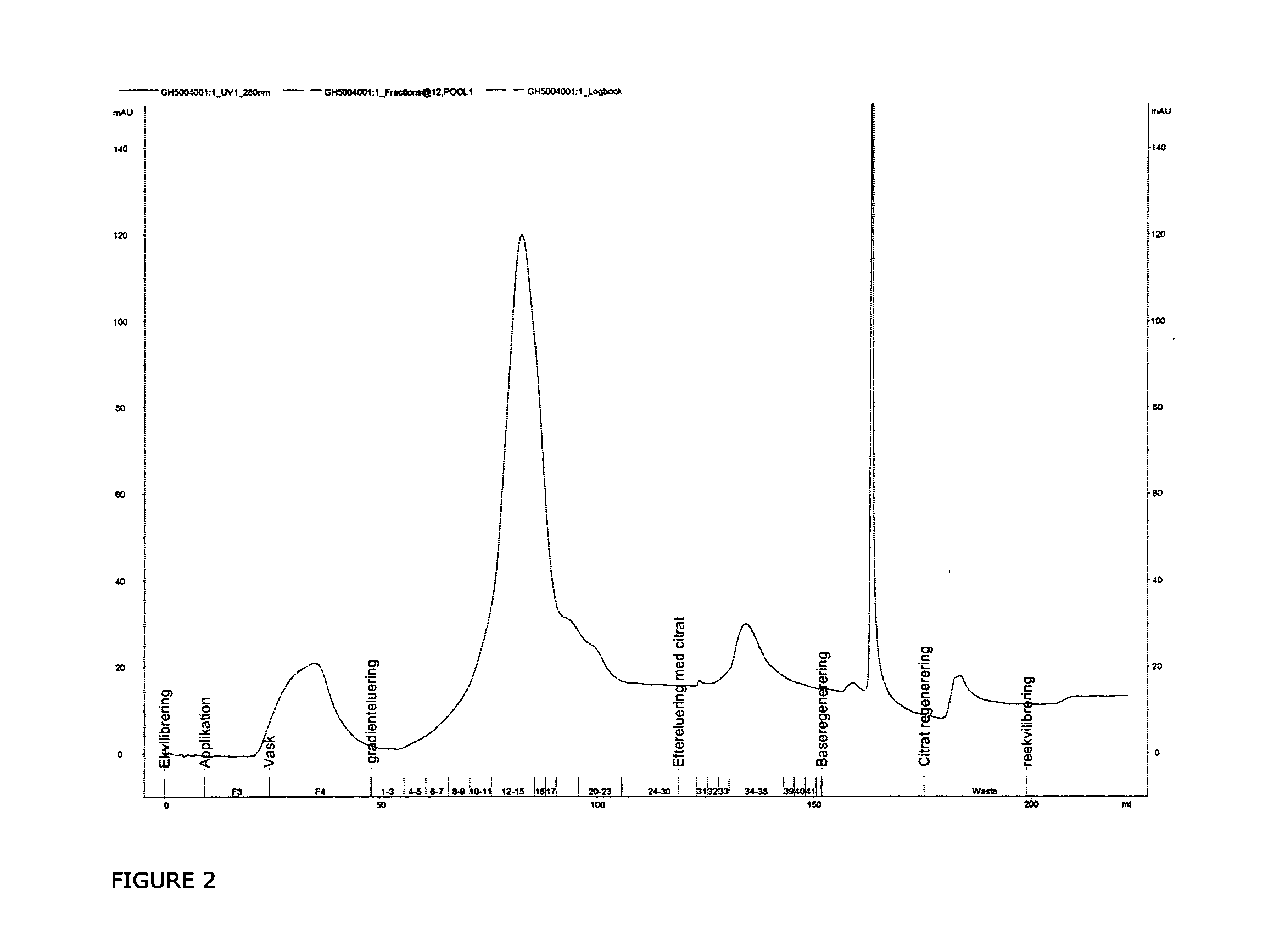
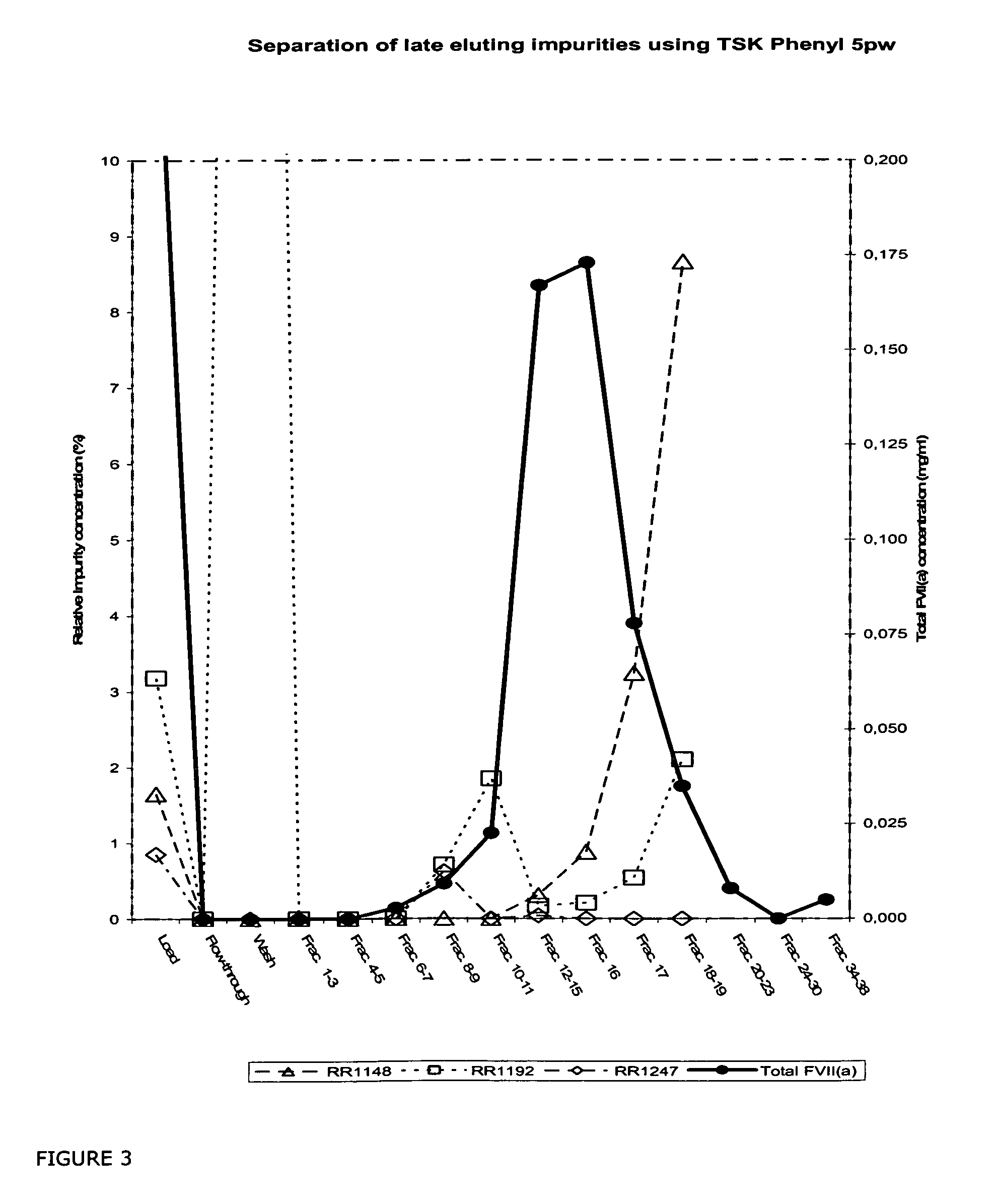
Hydrophobic interaction chromatography purification of factor VII polypeptides
InactiveUS20070037966A1Reduce the presence of impuritiesReduce contentMammal material medical ingredientsPeptide preparation methodsFactor iiRelated impurities
The invention described herein provides new methods of preparing purified Factor VII polypeptide drug substances in large quantities (industrial scale levels) that are associated with reduced content of product-related impurities (e.g., late eluting peaks) and / or that exhibit a relatively uniform glycosylation pattern.
Owner:NOVO NORDISK AS
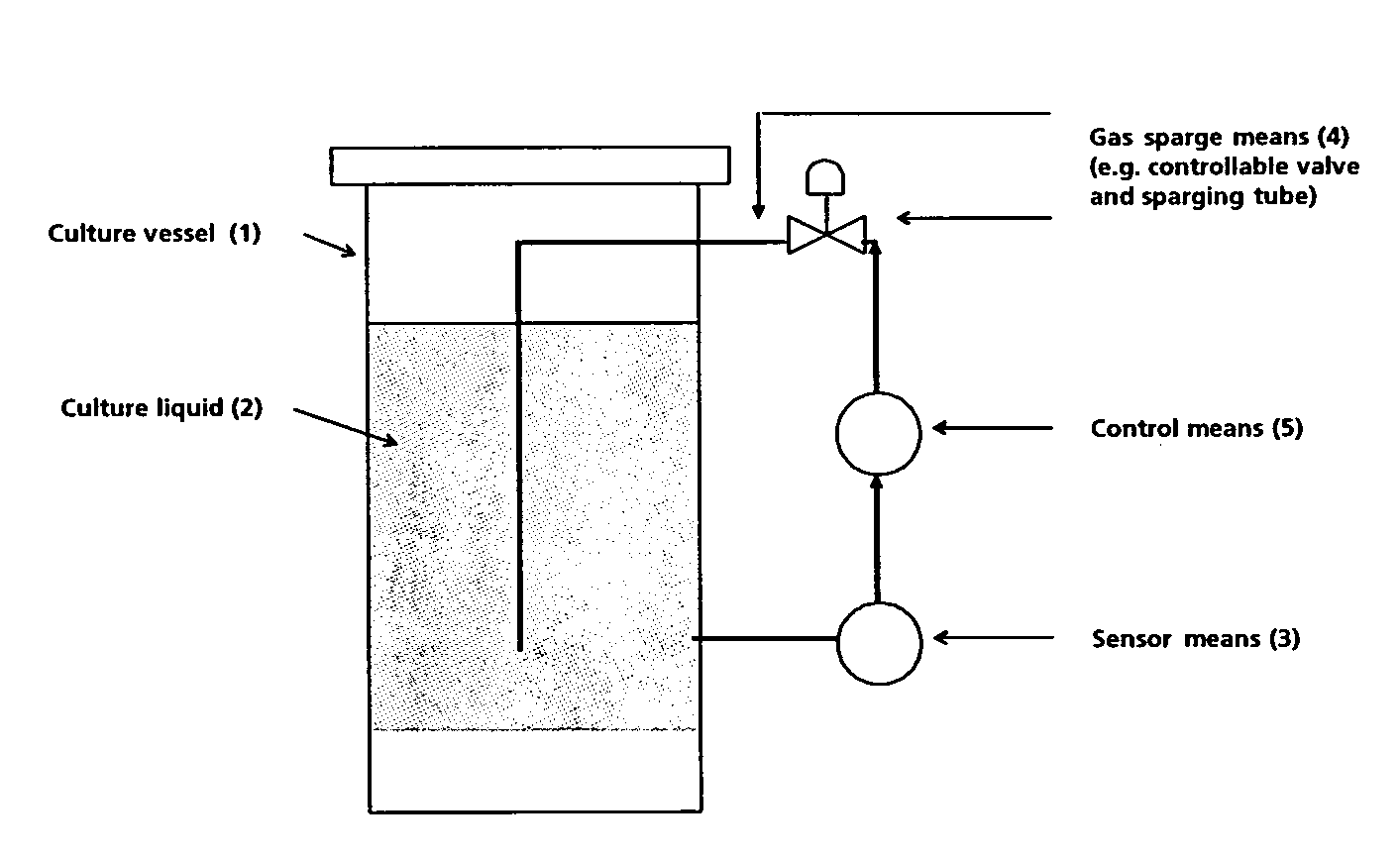
Method for large-scale production of polypeptide in eukaryote cells and a culture vessel suitable therefor
InactiveUS7521210B2Bioreactor/fermenter combinationsBiological substance pretreatmentsFactor VIIaPh control
The invention provides a method for large-scale production of a polypeptide, such as a Factor VII or Factor VIIa polypeptide, in eukaryote cells, such as mammalian cells, contained in a culture liquid, said method comprising: monitoring the concentration of dissolved CO2 in the culture liquid, and constantly or intermittently sparging atmospheric air through the culture liquid, wherein the sparging rate of the air is controlled in relation to the monitored concentration of dissolved CO2 in the culture liquid. The method reduces or eliminates the use of bases while providing an excellent pH control. The invention also provides a culture vessel suitable for the methods.
Owner:NOVO NORDISK HEALTH CARE AG
Glycosylation-Disrupted Factor VII Variants
InactiveUS20080058255A1Improve biological activityRapid clearancePeptide/protein ingredientsSurgical drugsNucleotideFactor VII
The present invention relates to human coagulation Factor VII polypeptides, as well as polynucleotide constructs encoding such polypeptides, vectors and host cells comprising and expressing the polynucleotide, pharmaceutical compositions comprising Factor VII polypeptides, uses and methods of treatment; and any additional inventive features related thereto.
Owner:NOVO NORDISK AS
Therapeutic agents comprising elastic peptides
ActiveUS20110123487A1Improve bioavailabilityStability biological actionFungiBacteriaSolubilityTherapeutic protein
The present invention provides therapeutic agents and compositions comprising elastic peptides and therapeutic proteins. Such peptides exhibit a flexible, extended conformation. In some embodiments, the therapeutic protein is a GLP-1 receptor agonist (e.g., GLP-1, exendin), insulin, or Factor VII / VIIa, including functional analogs. The present invention further provides encoding polynucleotides, as well as methods of making and using the therapeutic agents. The therapeutic agents have improvements in relation to their use as therapeutics, including, inter alia, one or more of half-life, clearance and / or persistence in the body, solubility, and bioavailability.
Owner:DUKE UNIV
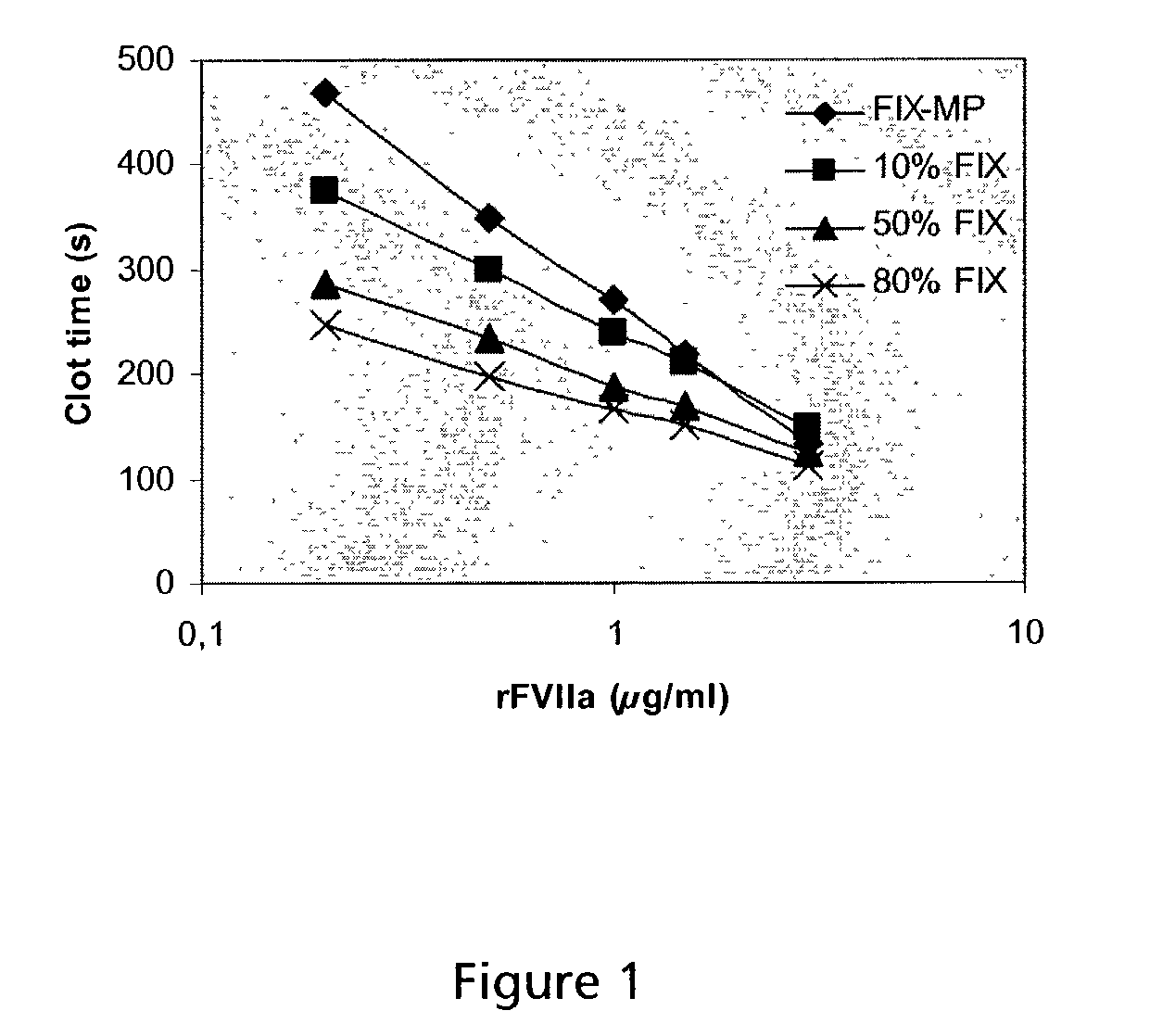
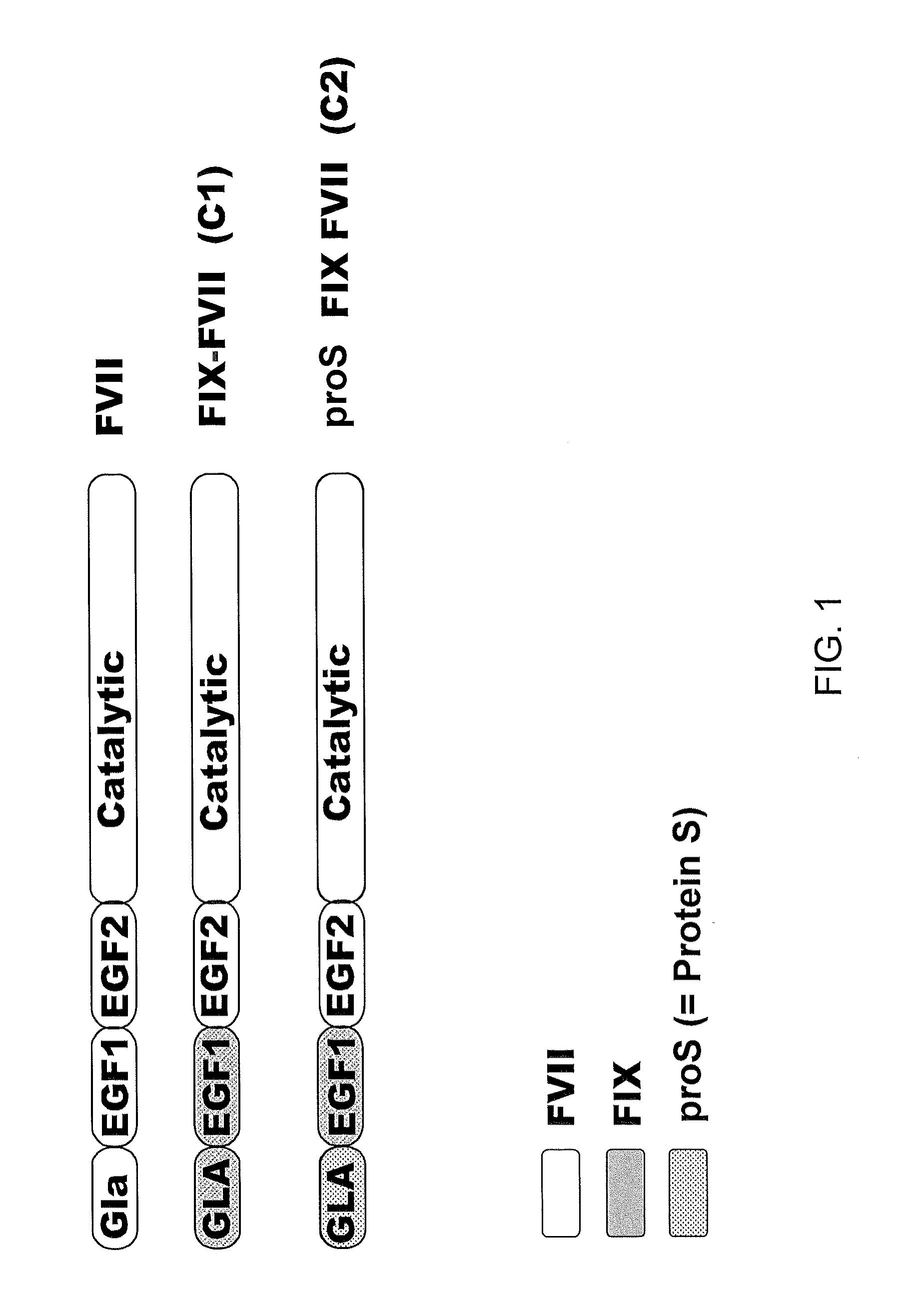
Combined use of factor VII polypeptides and factor IX polypeptides
InactiveUS20030203845A1Effective treatmentPeptide/protein ingredientsPharmaceutical drugBleeding episodes
The invention concerns a pharmaceutical preparation comprising a factor VII or factor VII-related polypeptide and a factor IX or factor IX-related polypeptide. The invention also concerns use of a factor VII or factor VII-related polypeptide and a factor IX or factor IX-related polypeptide for manufacture of a medicament for pharmaceutical use as well as methods for prevention or treatment of bleeding episodes in subjects.
Owner:NOVO NORDISK AS
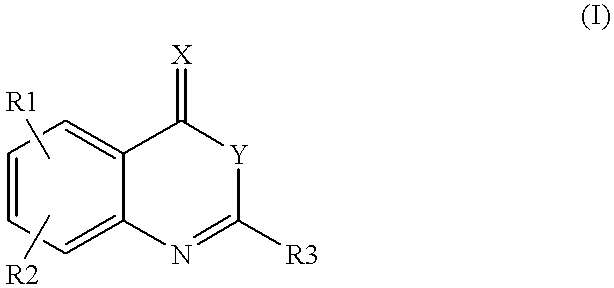
Heterocyclic compounds regulating clotting
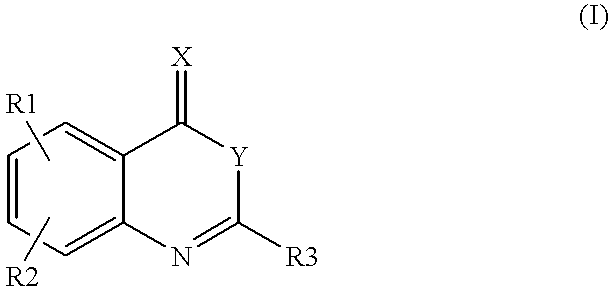
InactiveUS6180625B1Inhibition formationPreventing initiationOrganic active ingredientsOrganic chemistryFactor VIIaFactor VII
Compounds of formula (I)as factor VII-tissue factor inhibitors as well as novel benzoxazin derivatives are disclosed, wherein R1, R2, R3, X and Y are as defined in the specification. These compounds, and pharmaceutically acceptable salts thereof, have been shown to be inhibitors of factor VIIa-tissue factor activity and have anticoagulant properties. These compounds are useful for treating deficiencies of blood clotting factors or the effects of inhibitors to blood clotting factors. Methods for inhibiting clotting activity are also disclosed.
Owner:NOVO NORDISK AS
Factor VII or VIIa - like molecules
InactiveUS20060019336A1Prolong half-life in vivoReduce sensitivityAntibacterial agentsFungiFactor VIIaFactor VII
Conjugates of Factor VII (FVII) and Factor VIIa (FVIIA) are provided, as are methods for preparing them. Methods for producing novel polypeptides contributing to the production of such conjugates are provided. Methods of treatment by administering a FVII or FVIIa conjugate are provided.
Owner:BAYER HEALTHCARE LLC
Pegylated Factor VII Glycoforms
InactiveUS20080039373A1Improve functional propertiesFactor VIIPeptide/protein ingredientsFactor VIIOligosaccharide
The invention concerns a preparation comprising a plurality of Factor VII polypeptides or Factor VII-related polypeptides, wherein the polypeptides comprise asparagine-linked and / or serine-linked oligosaccharide chains, and wherein at least one oligosaccharide group is covalently attached to at least one polymeric group.
Owner:NOVO NORDISK AS
Pharmaceutical composition comprising factor VII polypeptides and factor V polypeptides
InactiveUS7125846B2Improved and reliable and widely applicableGood coagulationPeptide/protein ingredientsMammal material medical ingredientsFactor iiBleeding episodes
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide, and factor V or a factor V-related polypeptide, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Factor VII polypeptide having factor VII:C activity
InactiveUS6919311B2Improve stabilityExtended half-lifeFactor VIIHydrolysed protein ingredientsLow-density lipoproteinFactor ii
Factor VIII polypeptides having FVIII:C activity that contain modifications in the A3 and / or C1 and / or C2 domains of the sequence of the light chain of Factor VIII, characterized by the binding affinity to low density lipoprotein receptor protein, and methods for producing the same.
Owner:STICHTING SANQUIN BLOEDVOORZIENING
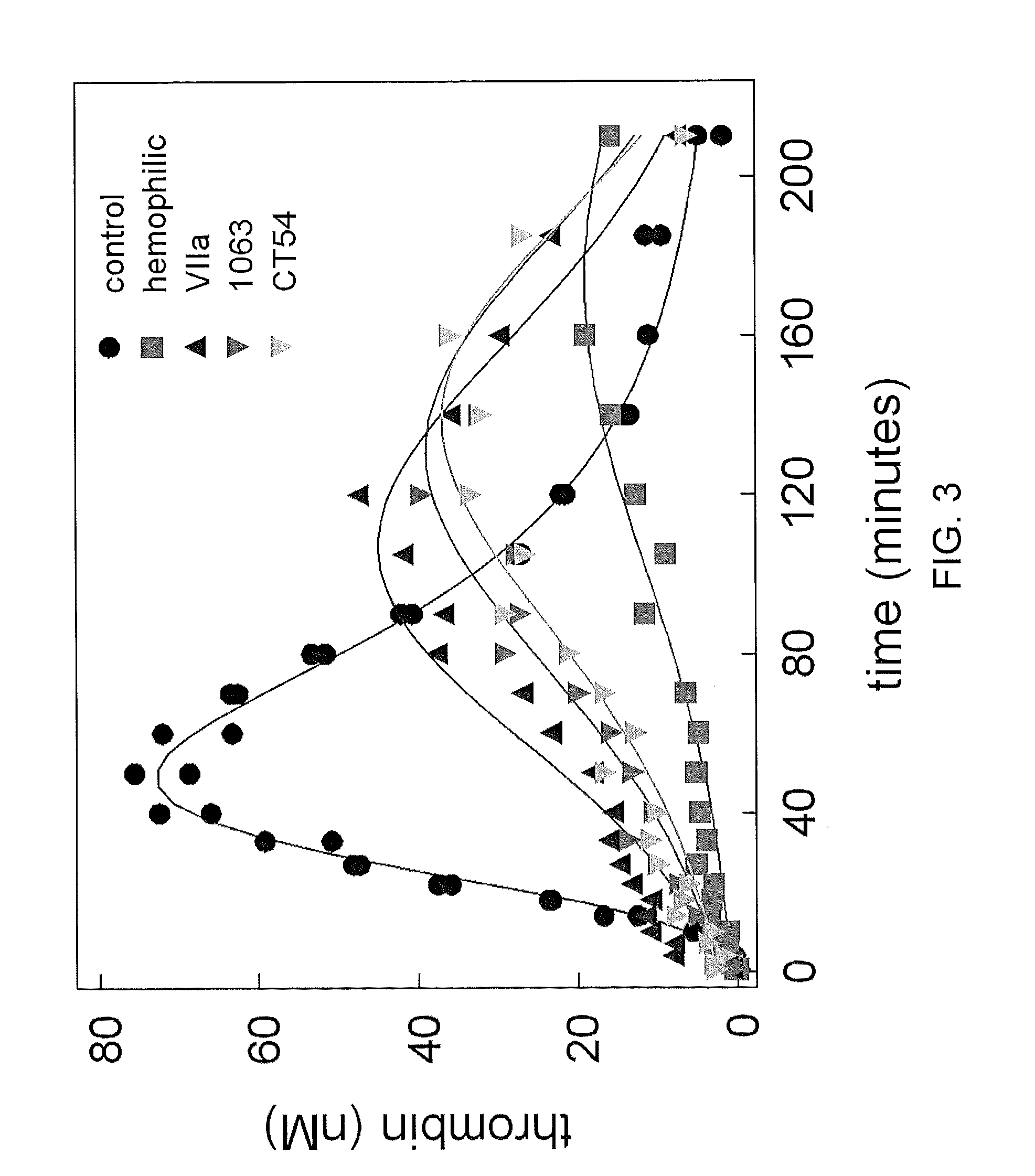
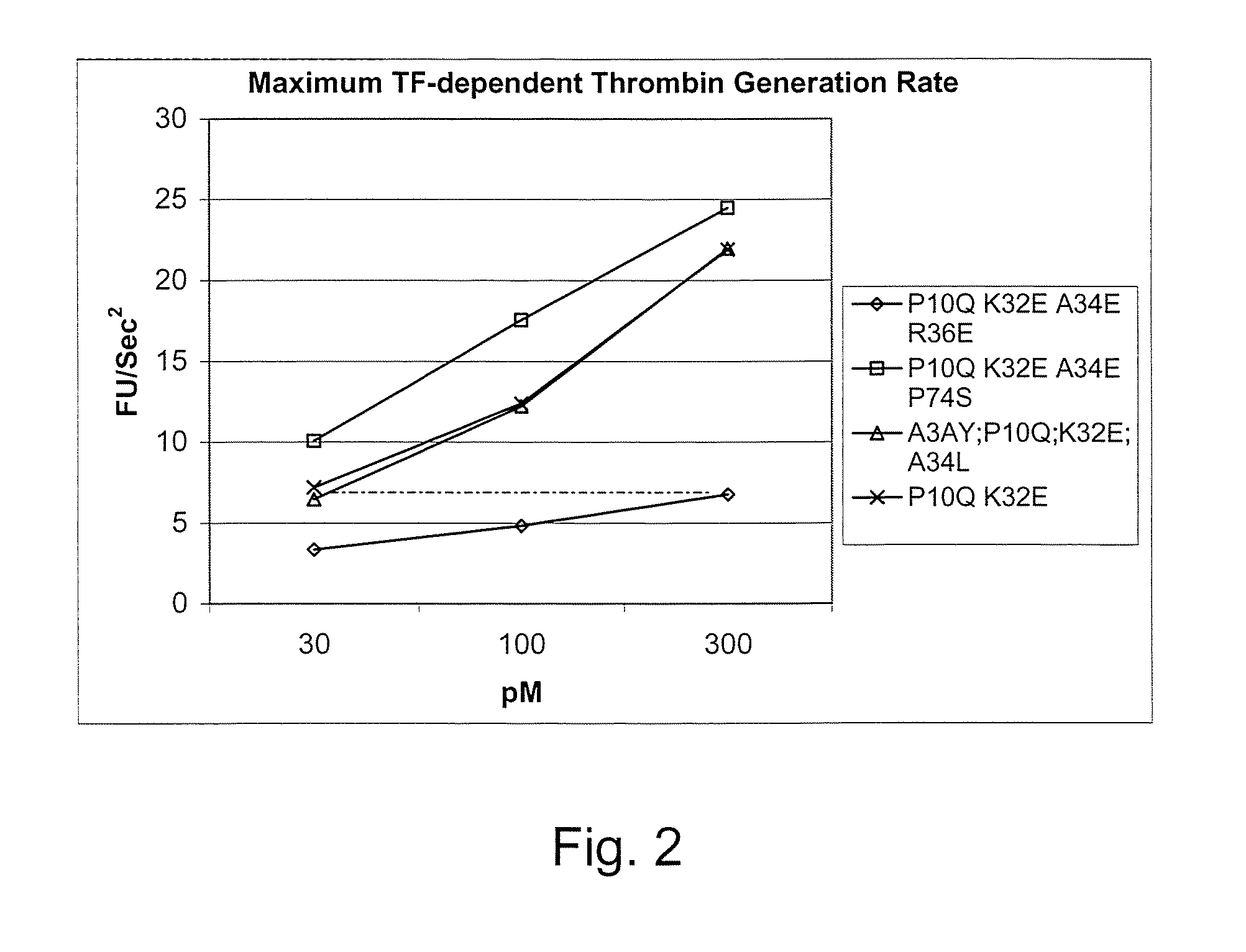
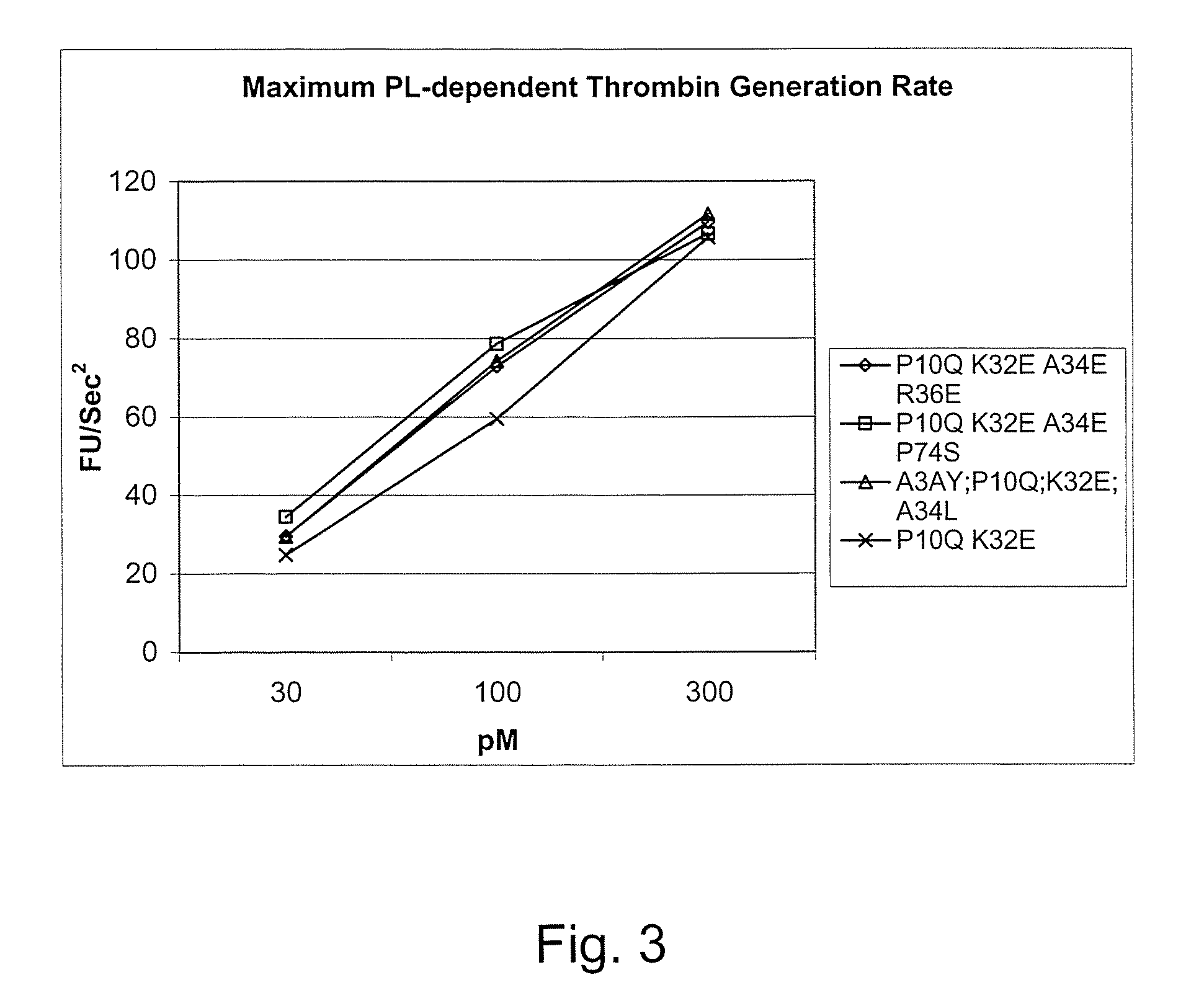
Fvii or fviia gla domain variants
InactiveUS20060240526A1Reduce doseGood curative effectSaccharide peptide ingredientsMammal material medical ingredientsFactor VIIaAmino acid substitution
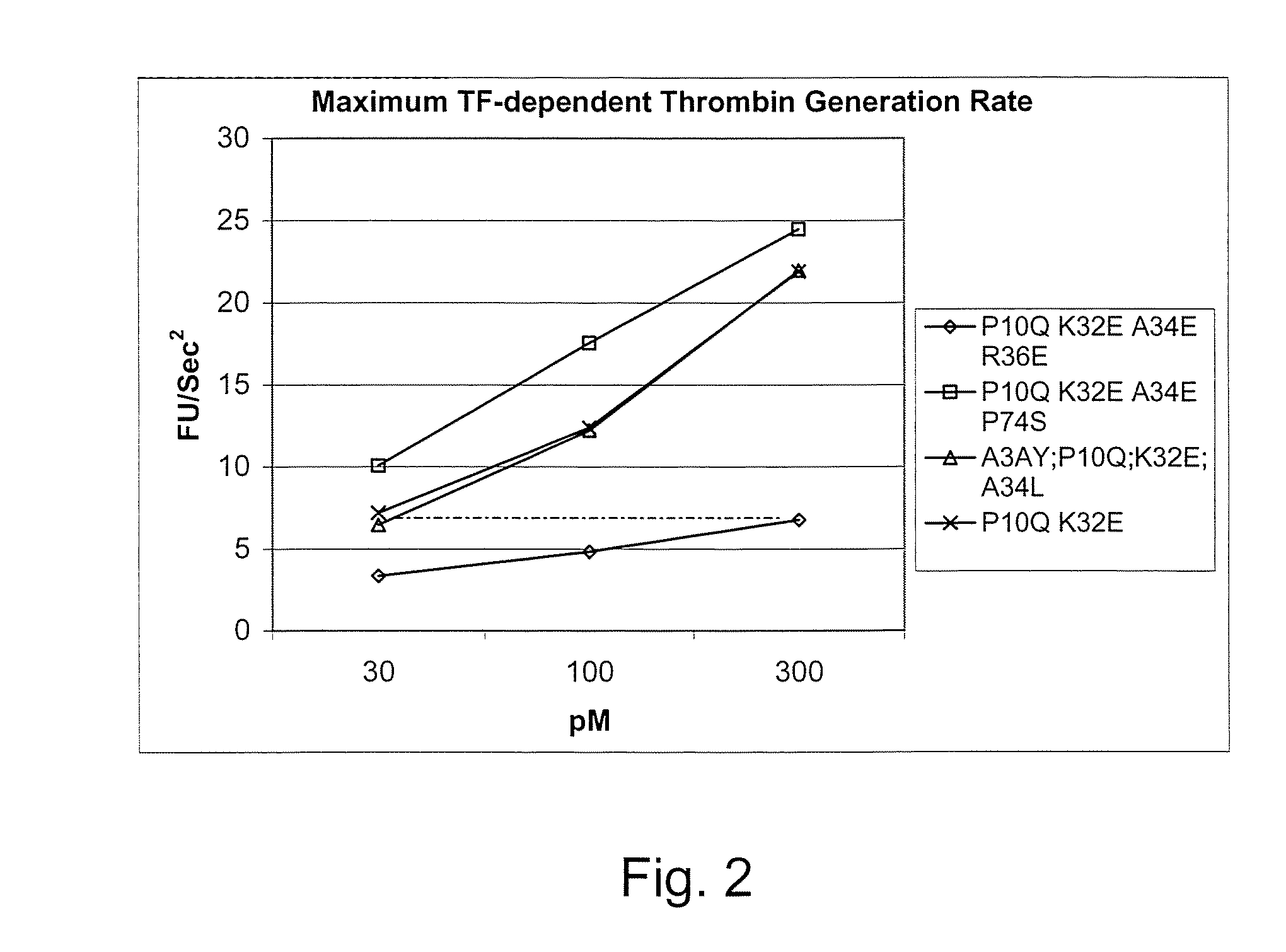
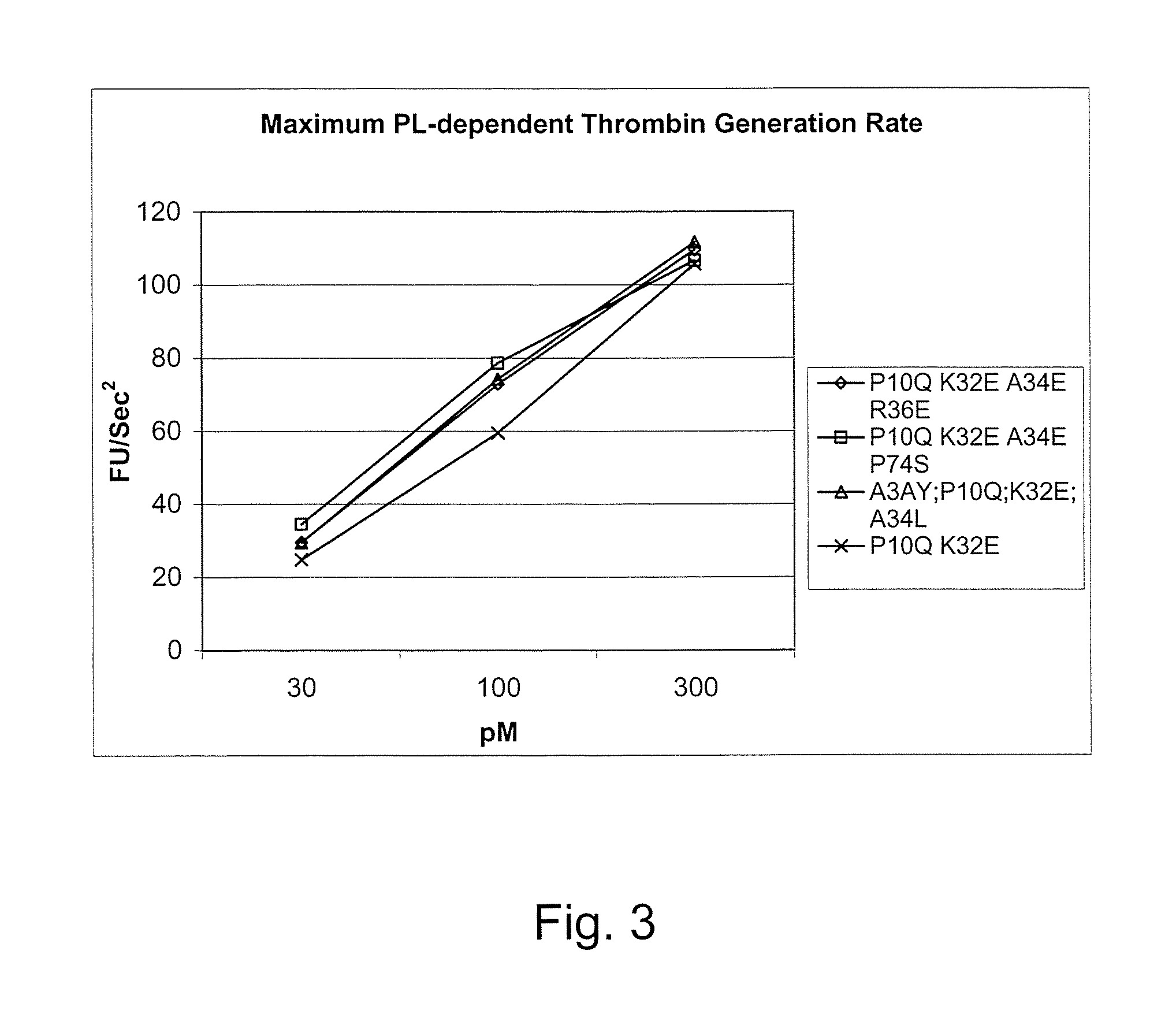
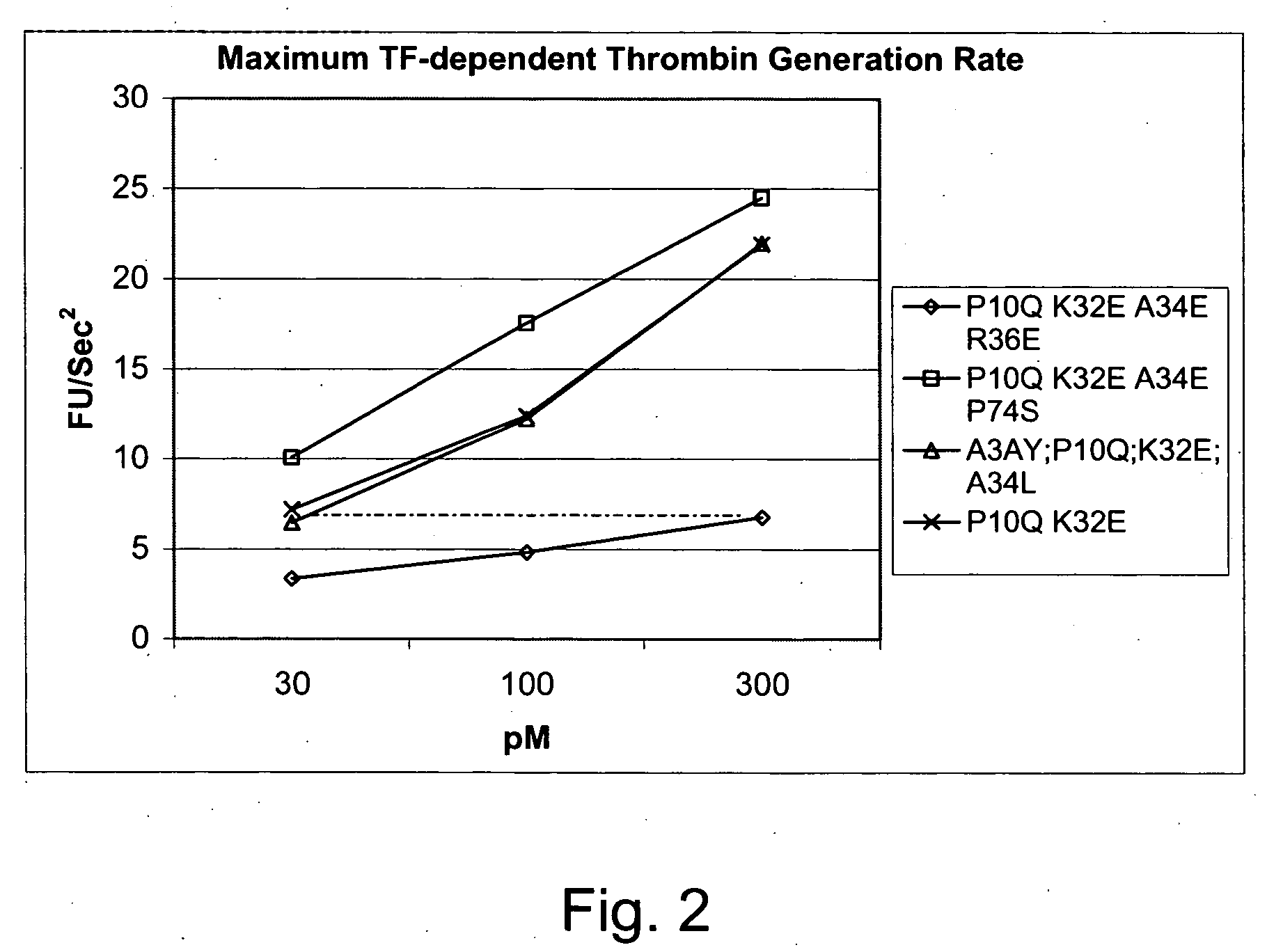
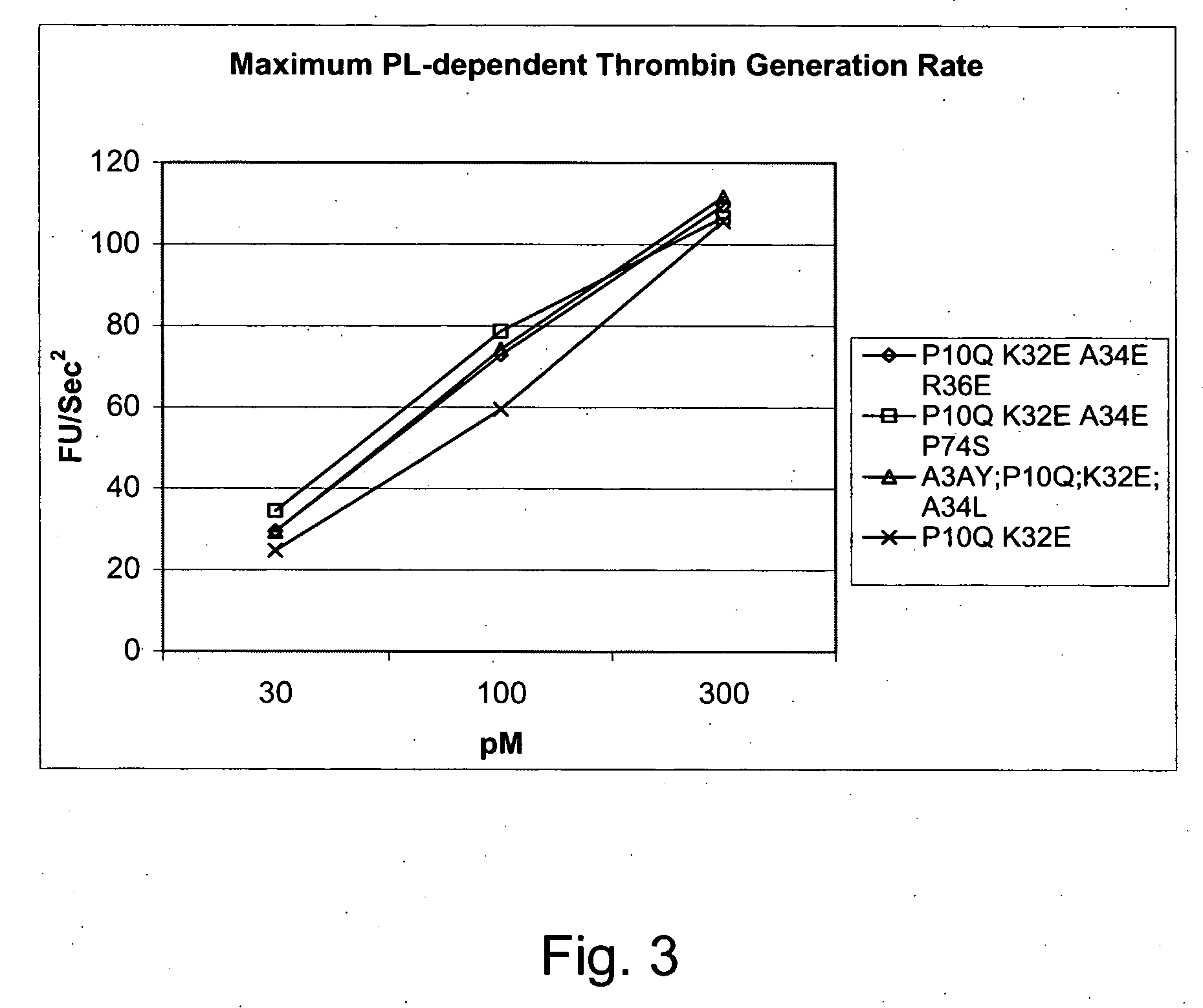
Gla domain variants of human Factor VII or human Factor VIIa, comprising 1-15 amino acid modifications relative to human Factor VII or human Factor VIIa, wherein a hydrophobic amino acid residue has been introduced by substitution in position 34; or having an amino acid substitution in position 36; and use of the variants for the treatment of intracerebral haemorrhage (ICH) or trauma.
Owner:BAYER HEALTHCARE LLC
Liquid composition of modified factor VII polypeptides
InactiveUS20070049523A1Chemically and physically stablePeptide/protein ingredientsPharmaceutical delivery mechanismMagnesium saltFactor VII
The invention provides a liquid, aqueous composition, comprising (i) a modified factor VII polypeptide; (ii) an agent suitable for keeping pH in the range of from about 4.0 to about 8.0; (iii) an antioxidant; and (iv) an agent selected from the list of: a calcium salt, a magnesium salt, or a mixture thereof.
Owner:NOVO NORDISK AS
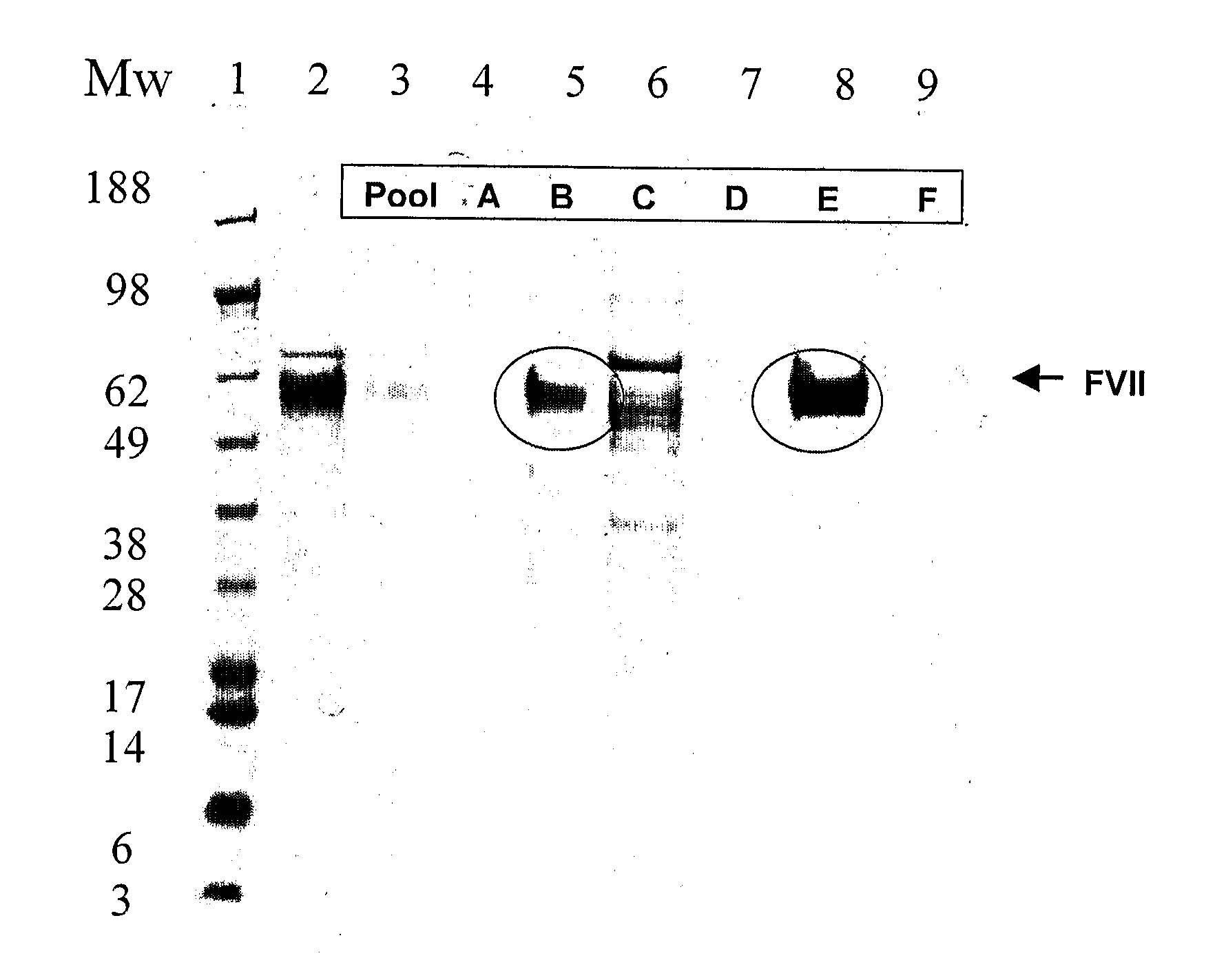
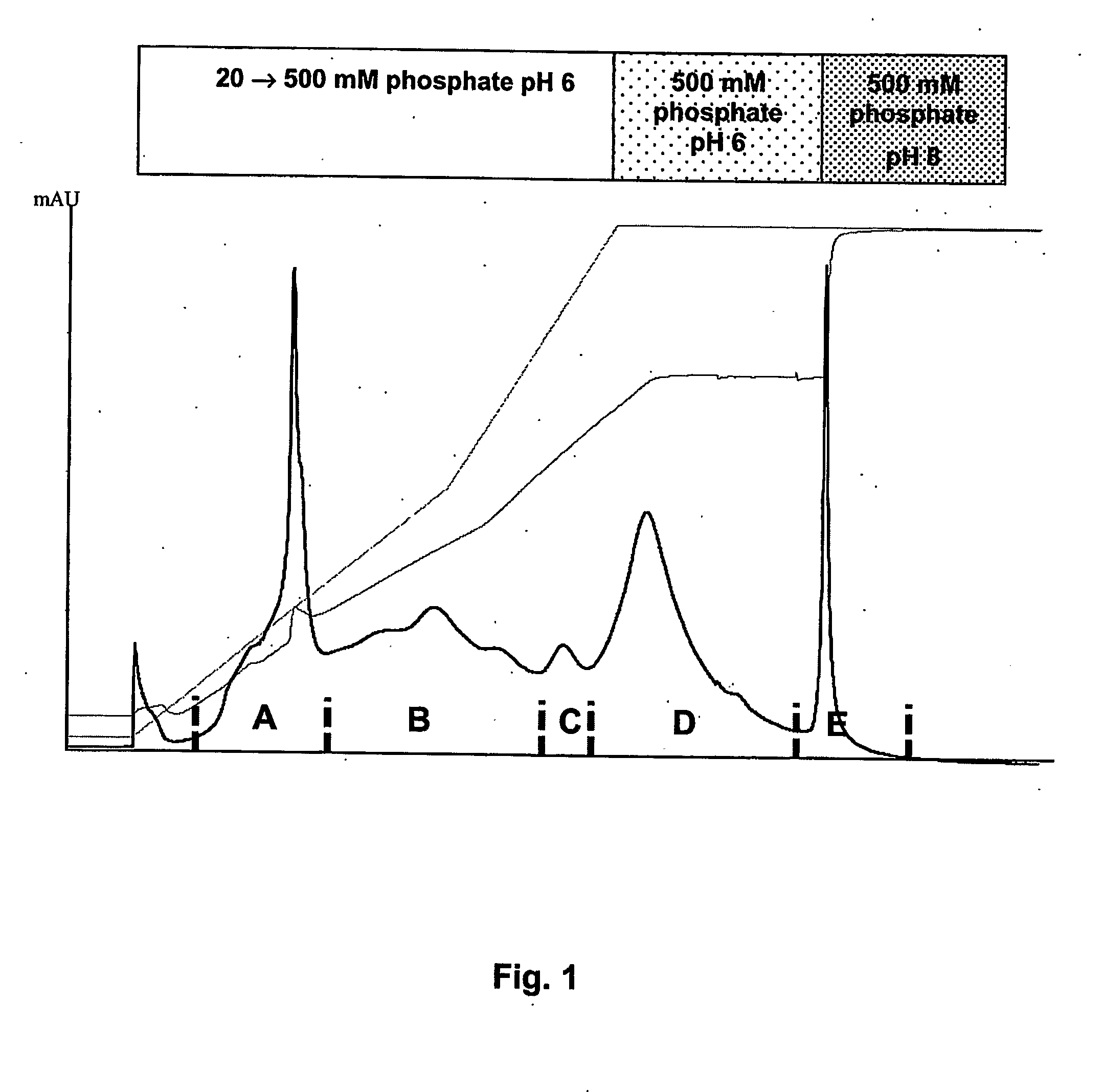
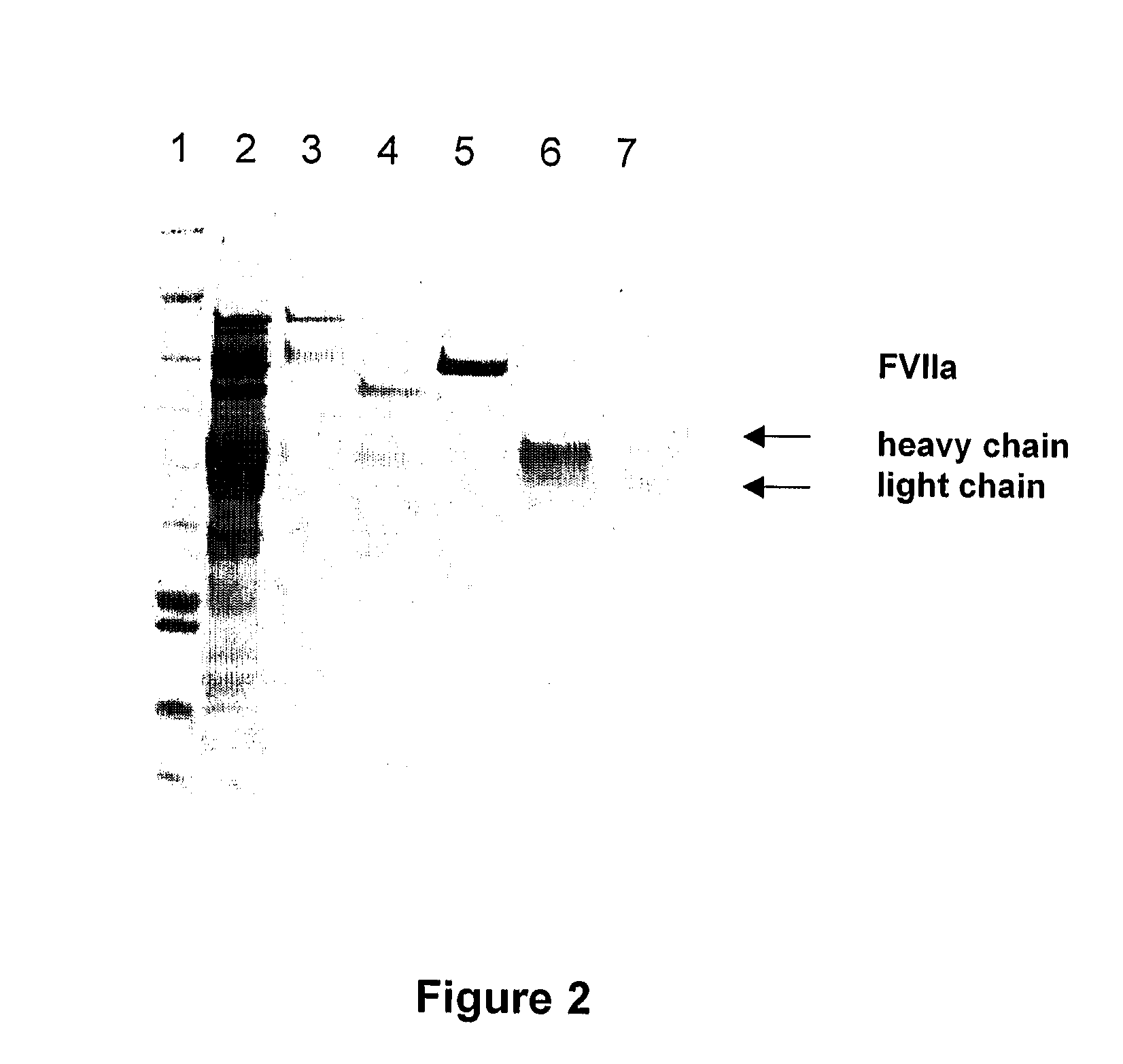
Method for purification of factor vii
A method for purifying recombinant Factor VII (rFVII) or recombinant activated Factor VII (rFVIIa), comprising subjecting the rFVII or rFVIIa to liquid chromatography on a hydroxyapatite (HAP) column.
Owner:BAYER HEALTHCARE LLC
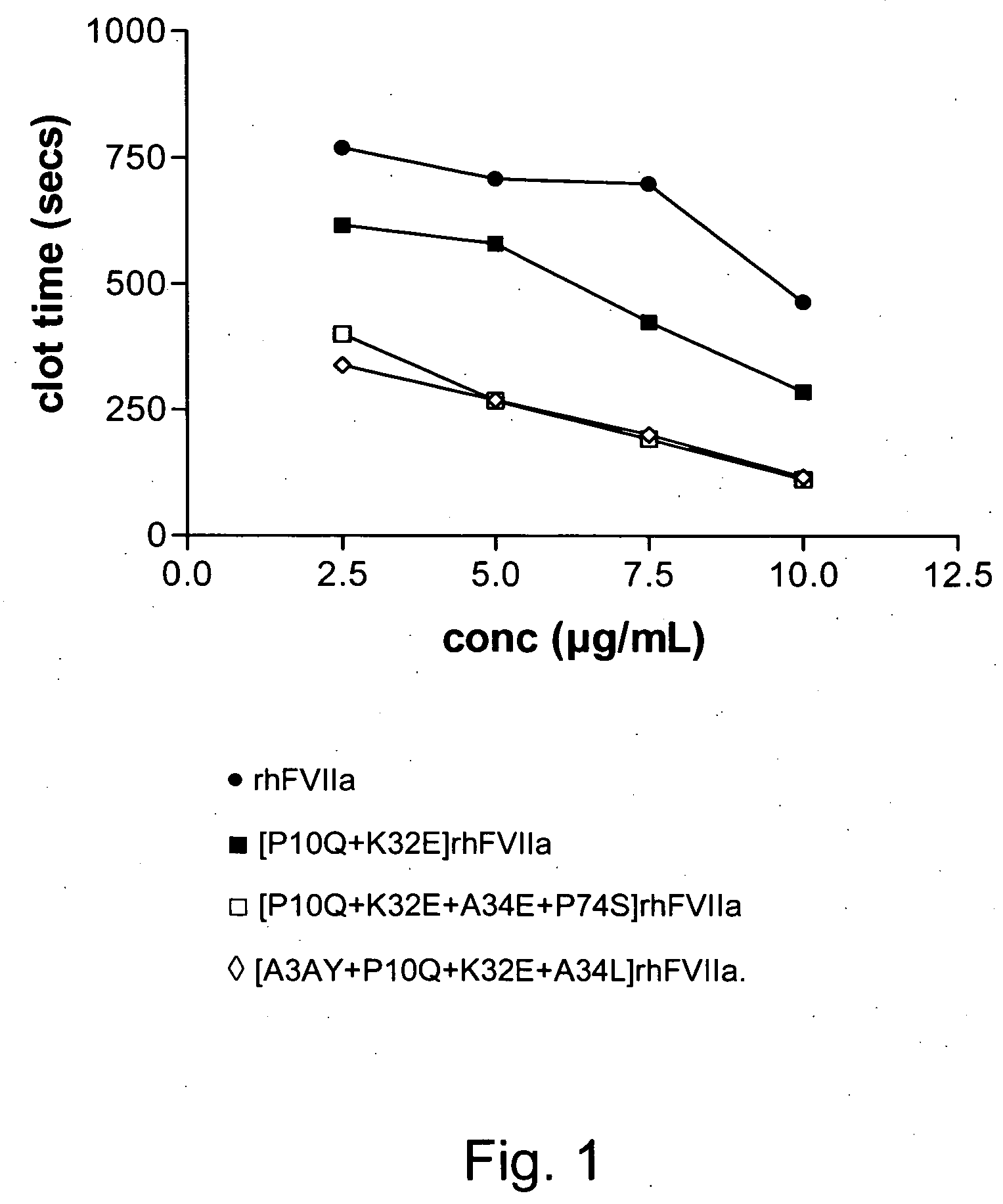
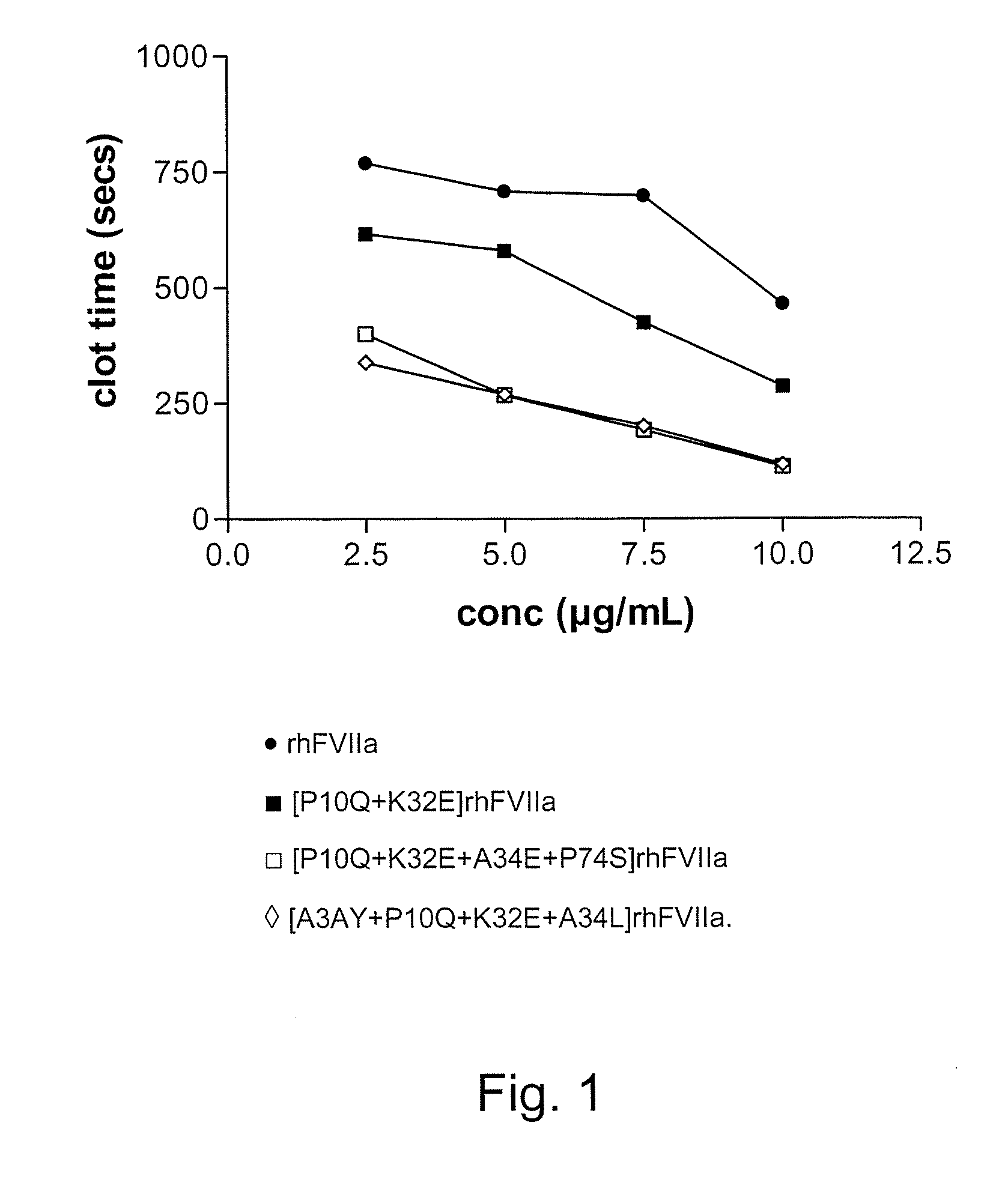
Factor vii or viia polypeptide variants
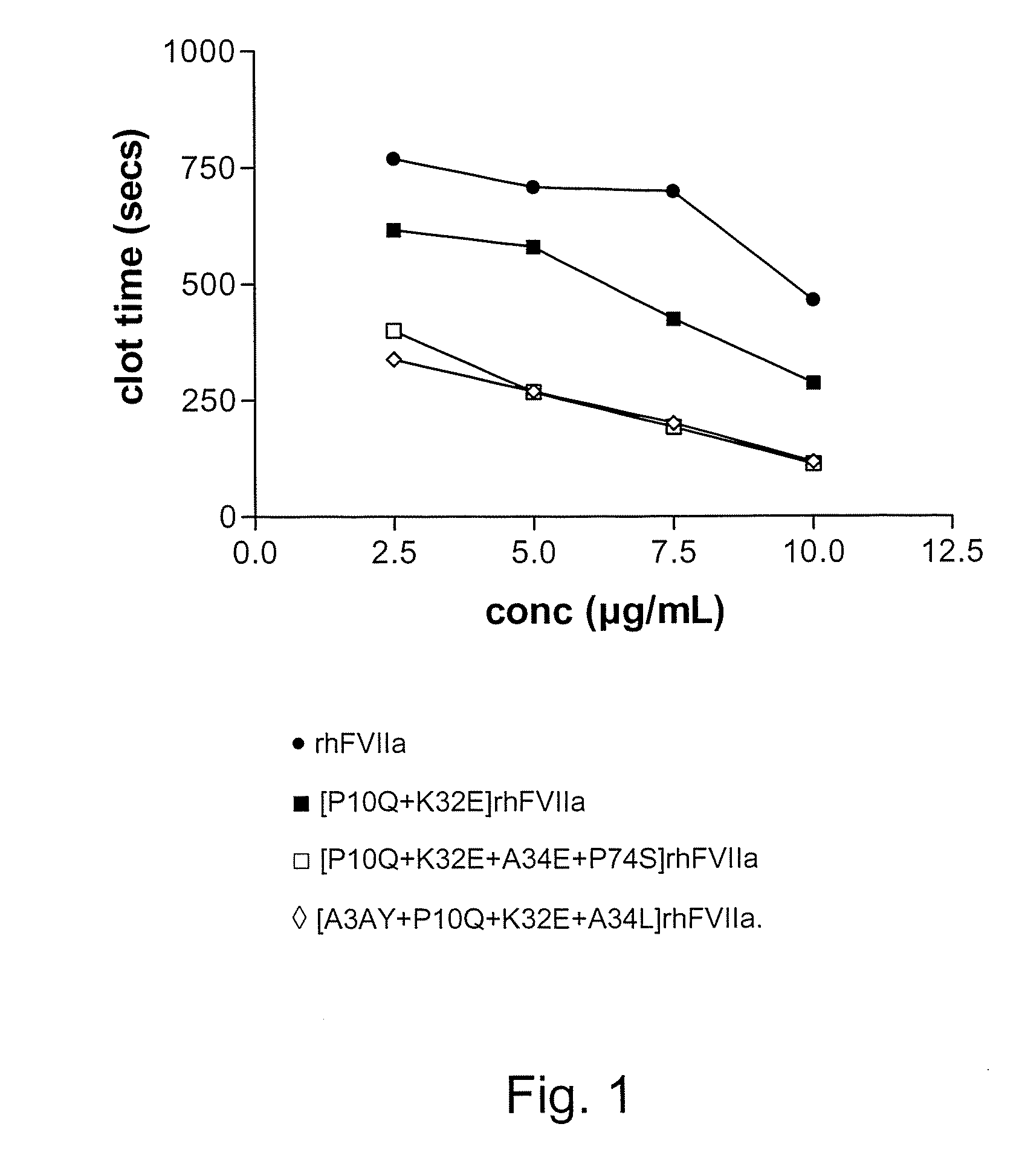
The present invention relates to novel polypeptide variants of factor VII (FVII) or factor VIIa (FVIIa) polypeptides, where said variants comprise an amino acid substitution in position 10 and 32 and where said variants further comprise a sugar moiety covalently attached to an introduced in vivo N-glycosylation site located outside the Gla domain. Such polypeptide variants are useful in therapy, in particular for the treatment of a variety of coagulation-related disorders, such as trauma.
Owner:BAYER HEALTHCARE LLC
Pharmaceutical composition comprising factor VII polypeptides and alpha2-antiplasmin polypeptides
InactiveUS7078479B2Improved and reliable and widely applicableGood coagulationPeptide/protein ingredientsMammal material medical ingredientsPharmaceutical drugBleeding episodes
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide and alpha2-antiplasmin or an alpha2-antiplasmin-related polypeptide, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Chimeric factor vii molecules
ActiveUS20100330059A1Reduce riskLow affinityPeptide/protein ingredientsSemiconductor/solid-state device detailsFactor VIIImmunology
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Factor VII or VIIa - Like Molecules
InactiveUS20060228782A1Improved propertyReduce sensitivityAntibacterial agentsFungiFactor VIIaFactor VII
Conjugates of Factor VII (FVII) and Factor VIIa (FVIIA) are provided, as are methods for preparing them. Methods for producing novel polypeptides contributing to the production of such conjugates are provided. Methods of treatment by administering a FVII or FVIIa conjugate are provided.
Owner:BAYER HEALTHCARE LLC
FVII or FVIIa Gla domain variants
InactiveUS20050164932A1Reduce doseGood curative effectNervous disorderSaccharide peptide ingredientsFactor VIIaAmino acid substitution
Gla domain variants of human Factor VII or human Factor VIIa, comprising 1-15 amino acid modifications relative to human Factor VII or human Factor VIIa, wherein a hydrophobic amino acid residue has been introduced by substitution in position 34; or having an amino acid substitution in position 36; and use of the variants for the treatment of intracerebral haemorrhage (ICH) or trauma.
Owner:BAYER HEALTHCARE LLC
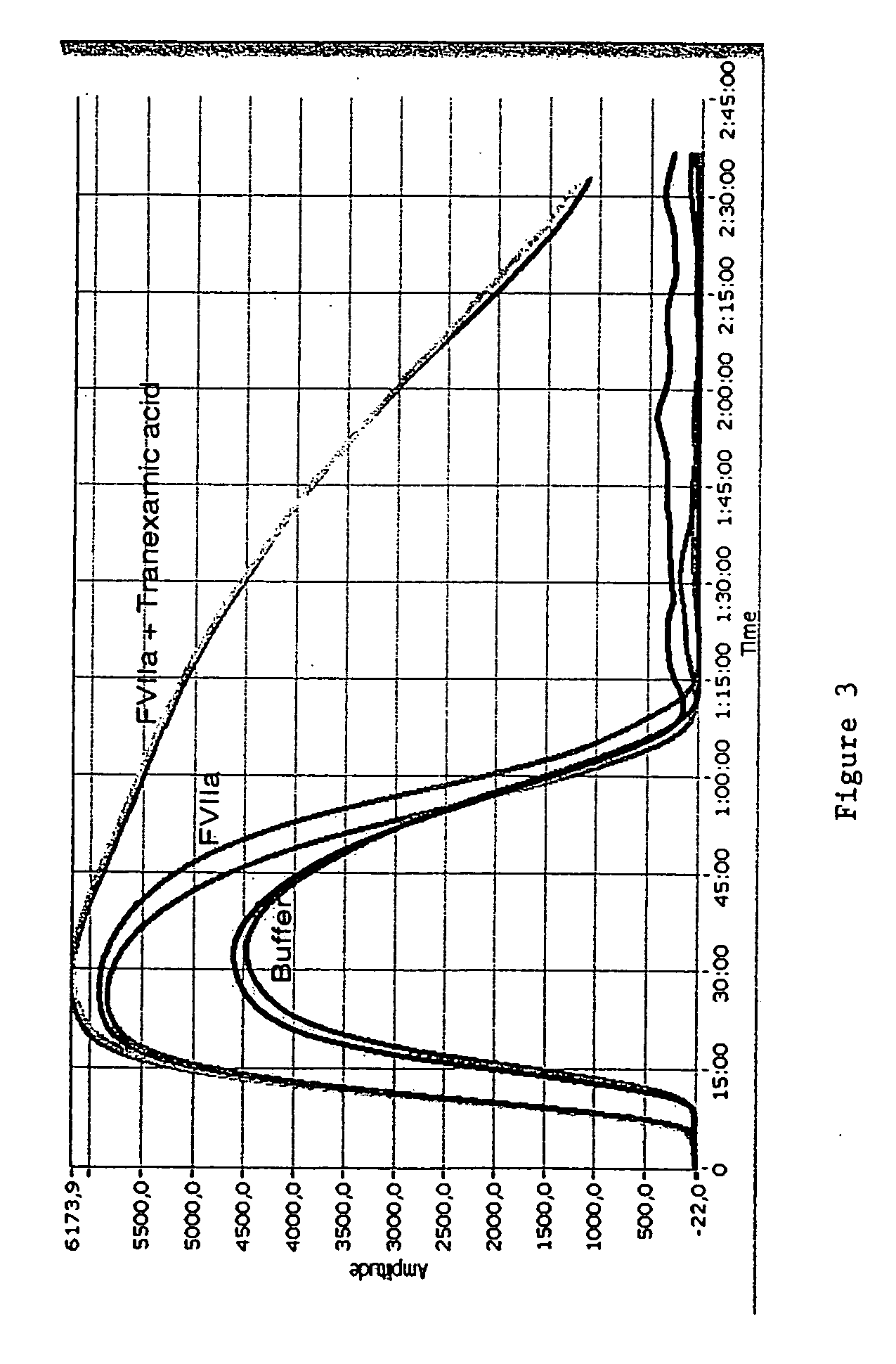
Pharmaceutical composition comprising factor VII polypeptides and tranexamic acid
The present invention relates to compositions comprising factor VII or a factor VII-related polypeptide and tranexamic acid, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Fvii or fviia gla domain variants
InactiveUS20060241041A1Reduce doseGood curative effectFibrinogenPeptide/protein ingredientsFactor VIIaAmino acid substitution
Gla domain variants of human Factor VII or human Factor VIIa, comprising 1-15 amino acid modifications relative to human Factor VII or human Factor VIIa, wherein a hydrophobic amino acid residue has been introduced by substitution in position 34; or having an amino acid substitution in position 36; and use of the variants for the treatment of intracerebral haemorrhage (ICH) or trauma.
Owner:BAYER HEALTHCARE LLC
Pharmaceutical composition comprising factor VII polypeptides and protein C inhibitors
InactiveUS20060013812A1Improved and reliable and widely applicableGood coagulationFactor VIIPeptide/protein ingredientsMedicineProtein C inhibitor
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide and a protein C inhibitor, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Factor VII polypeptides for preventing formation of inhibitors in subjects with haemophilia
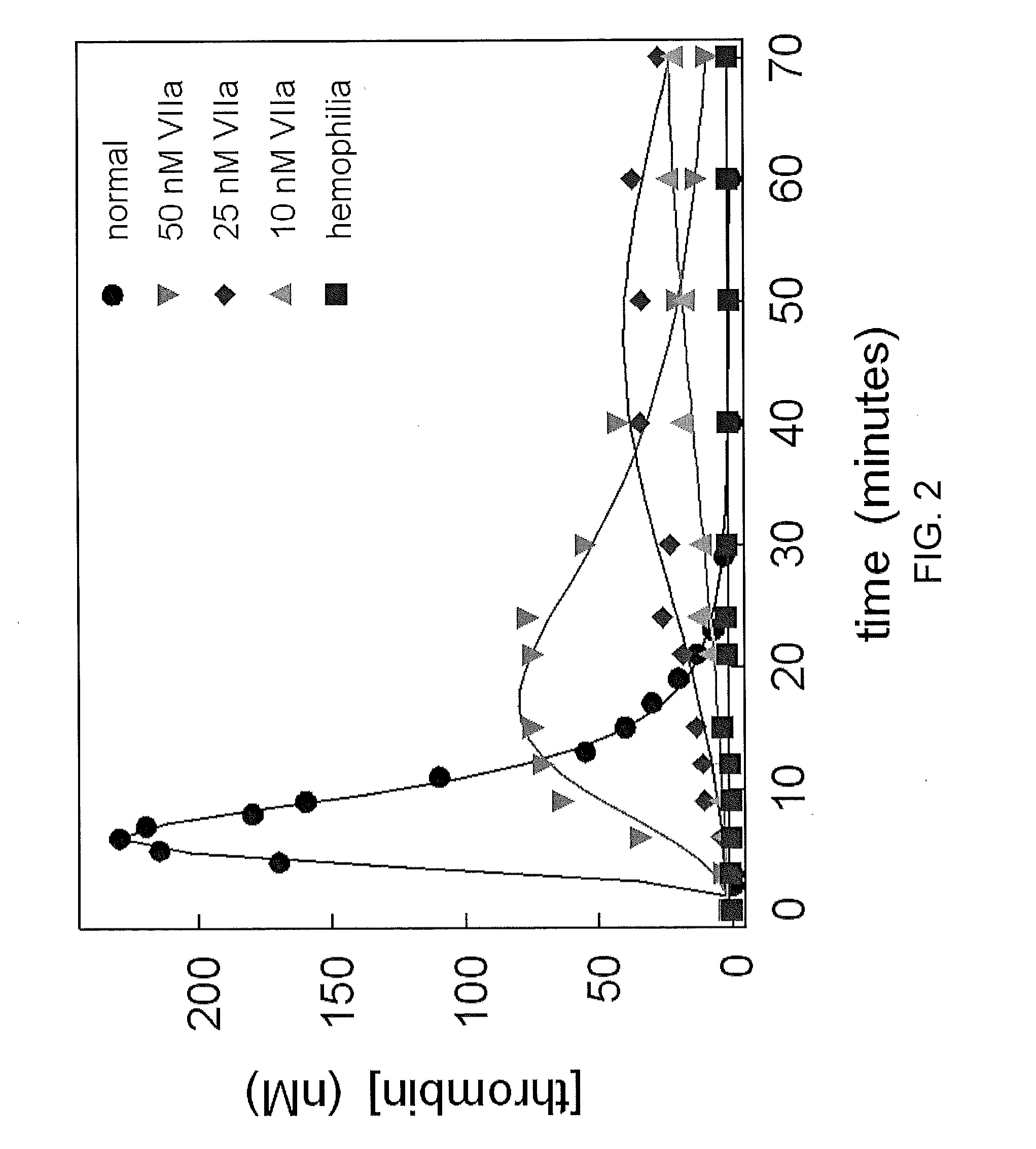
InactiveUS20050032690A1Inhibition formationImprove stabilityOrganic active ingredientsFactor VIIFactor VIIaBlood coagulation factor VIII
The invention provides a method for preventing formation of inhibitors to blood coagulation factor VIII or factor IX in a subject having haemophilia, the method comprising administering (via intravenous, subcutaneous, intradermal, or intramuscular routes) to a previously untreated subject an effective dosage of factor VIIa or a factor VII-related polypeptide.
Owner:NOVO NORDISK AS
Factor VII or VIIa Polypeptide Variants
InactiveUS20060270002A1Improve bioavailabilityIncrease the areaFungiBacteriaFactor VIIaAmino acid substitution
The present invention relates to novel polypeptide variants of factor VII (FVII) or factor VIIa (FVIIa) polypeptides, where said variants comprise an amino acid substitution in position 10 and 32 and where said variants further comprise a sugar moiety covalently attached to an introduced in vivo N-glycosylation site located outside of the Gla domain. Such polypeptide variants are useful in therapy, in particular for the treatment of a variety of coagulation-related disorders, such as trauma.
Owner:BAYER HEALTHCARE LLC
Factor VII or VIIa Polypeptide Variants
InactiveUS20060270001A1Improve bioavailabilityIncrease the areaFungiBacteriaFactor VIIaAmino acid substitution
The present invention relates to novel polypeptide variants of factor VII (FVII) or factor VIIa (FVIIa) polypeptides, where said variants comprise an amino acid substitution in position 10 and 32 and where said variants further comprise a sugar moiety covalently attached to an introduced in vivo N-glycosylation site located outside of the Gla domain. Such polypeptide variants are useful in therapy, in particular for the treatment of a variety of coagulation-related disorders, such as trauma.
Owner:BAYER HEALTHCARE LLC
Factor VII or VIIa Polypeptide Variants
InactiveUS20060270000A1Improve bioavailabilityIncrease the areaFungiNervous disorderFactor VIIaDisease
The present invention relates to novel polypeptide variants of factor VII (FVII) or factor VIIa (FVIIa) polypeptides, where said variants comprise an amino acid substitution in position 10 and 32 and where said variants further comprise a sugar moiety covalently attached to an introduced in vivo N-glycosylation site located outside of the Gla domain. Such polypeptide variants are useful in therapy, in particular for the treatment of a variety of coagulation-related disorders, such as trauma.
Owner:BAYER HEALTHCARE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com