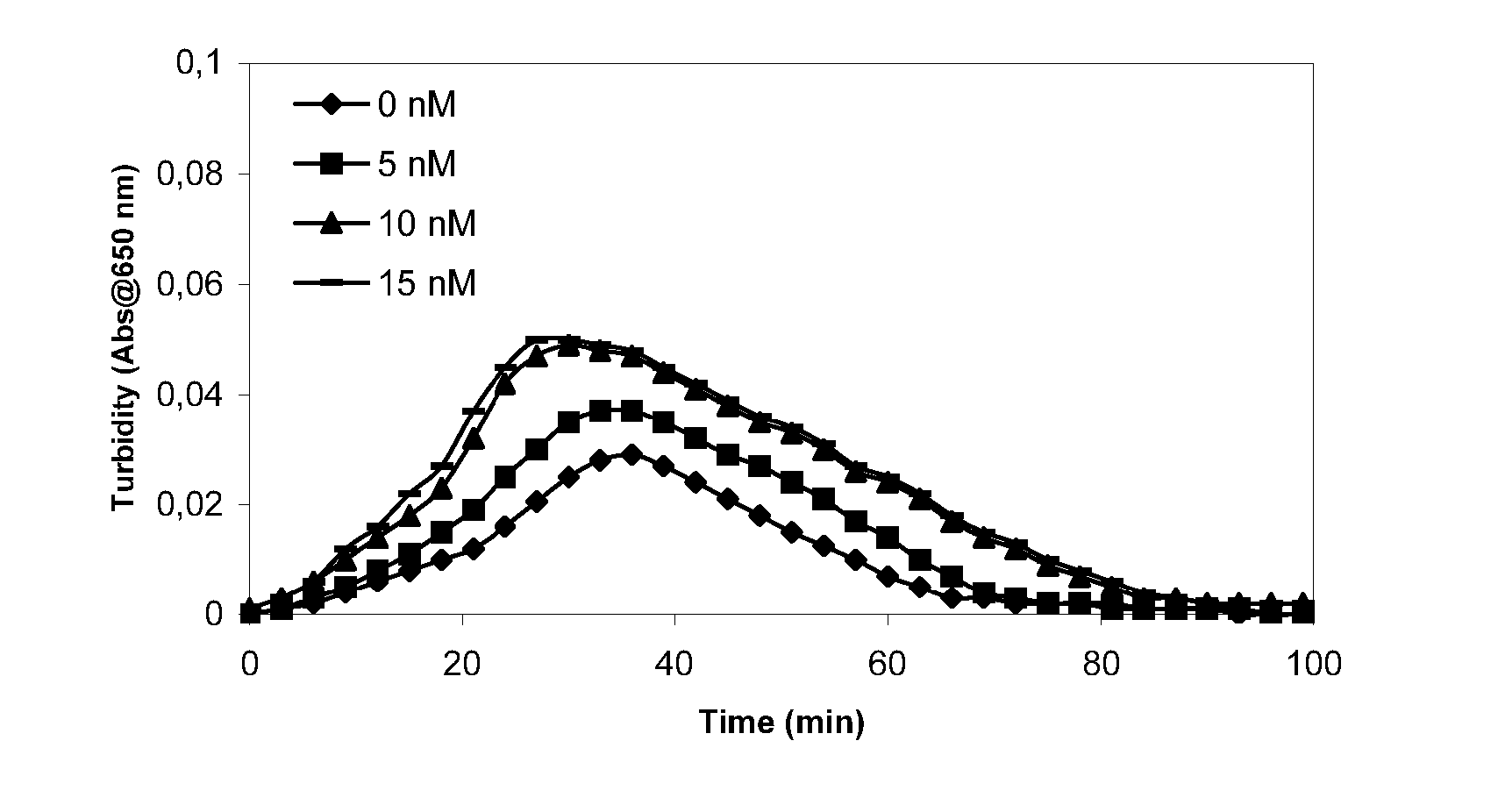
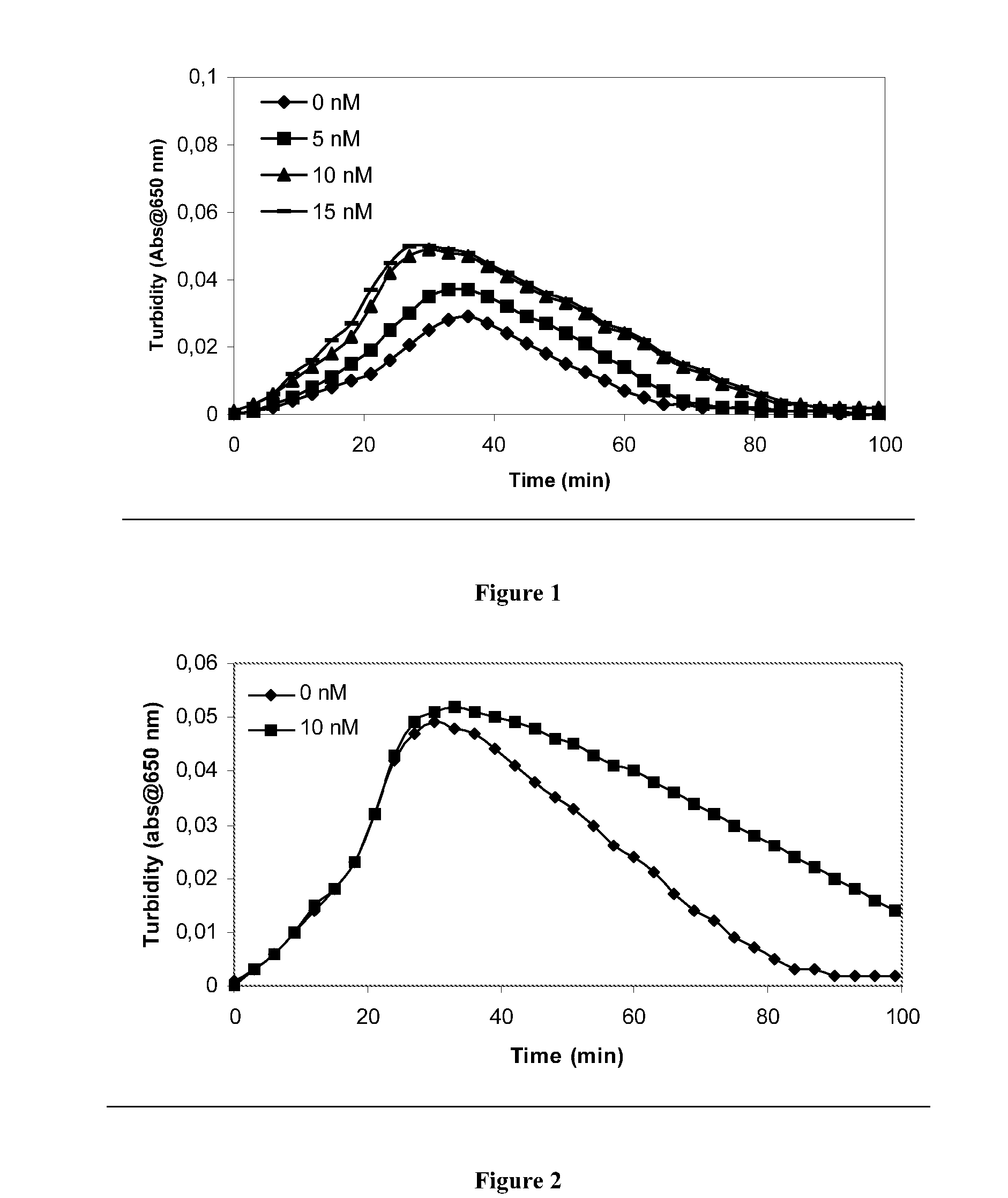
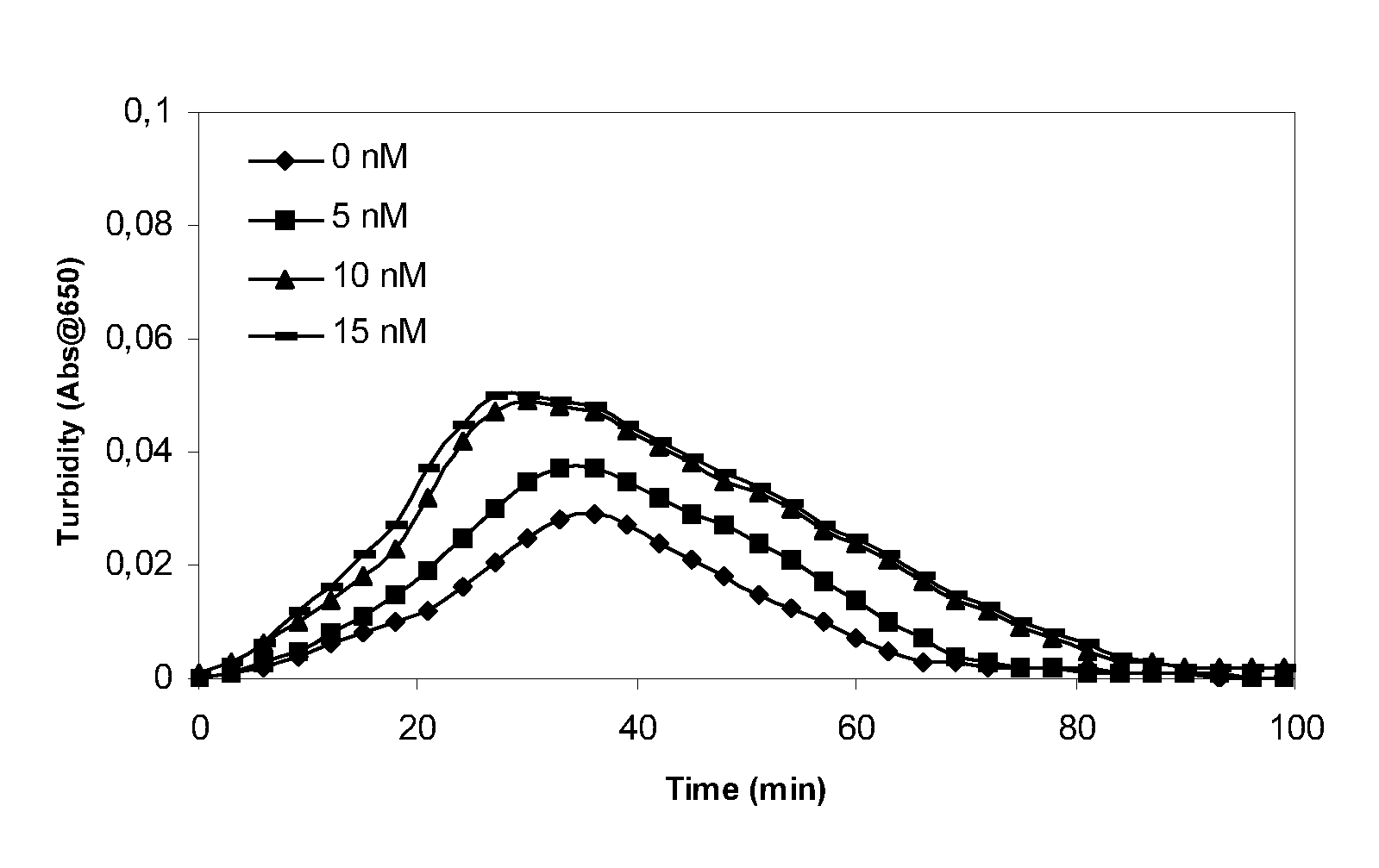
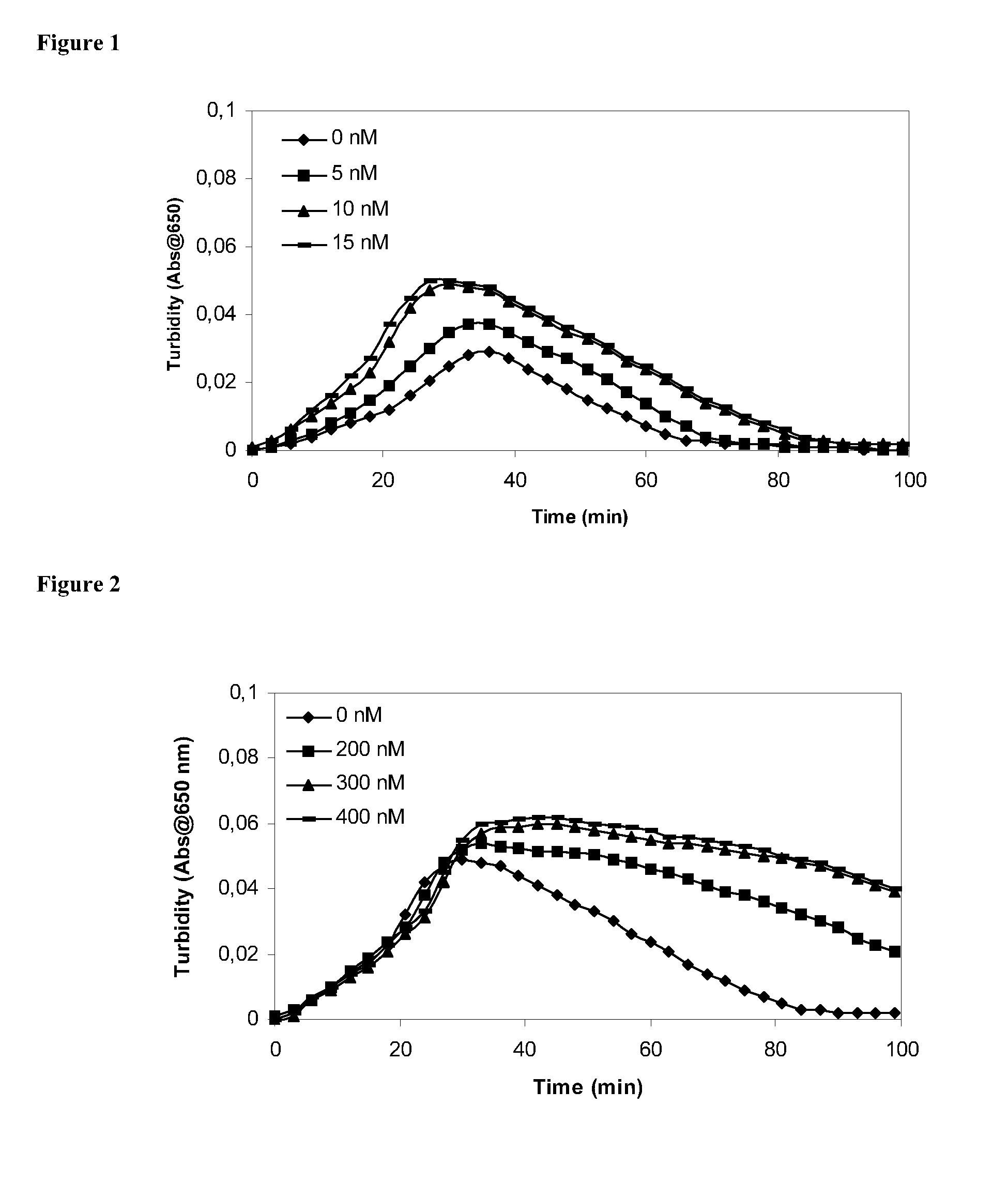
Patents
Literature
40 results about "Bleeding episodes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
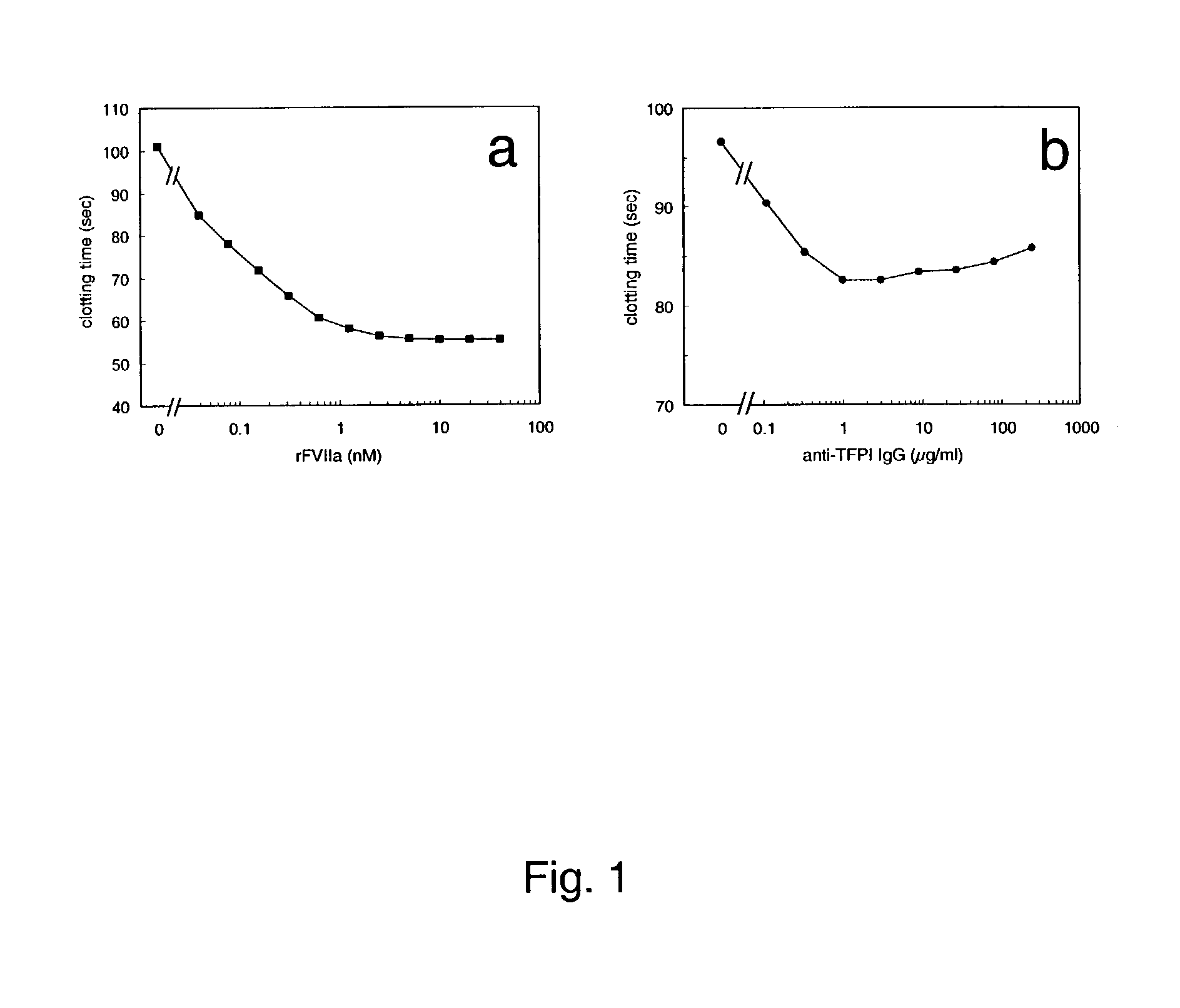
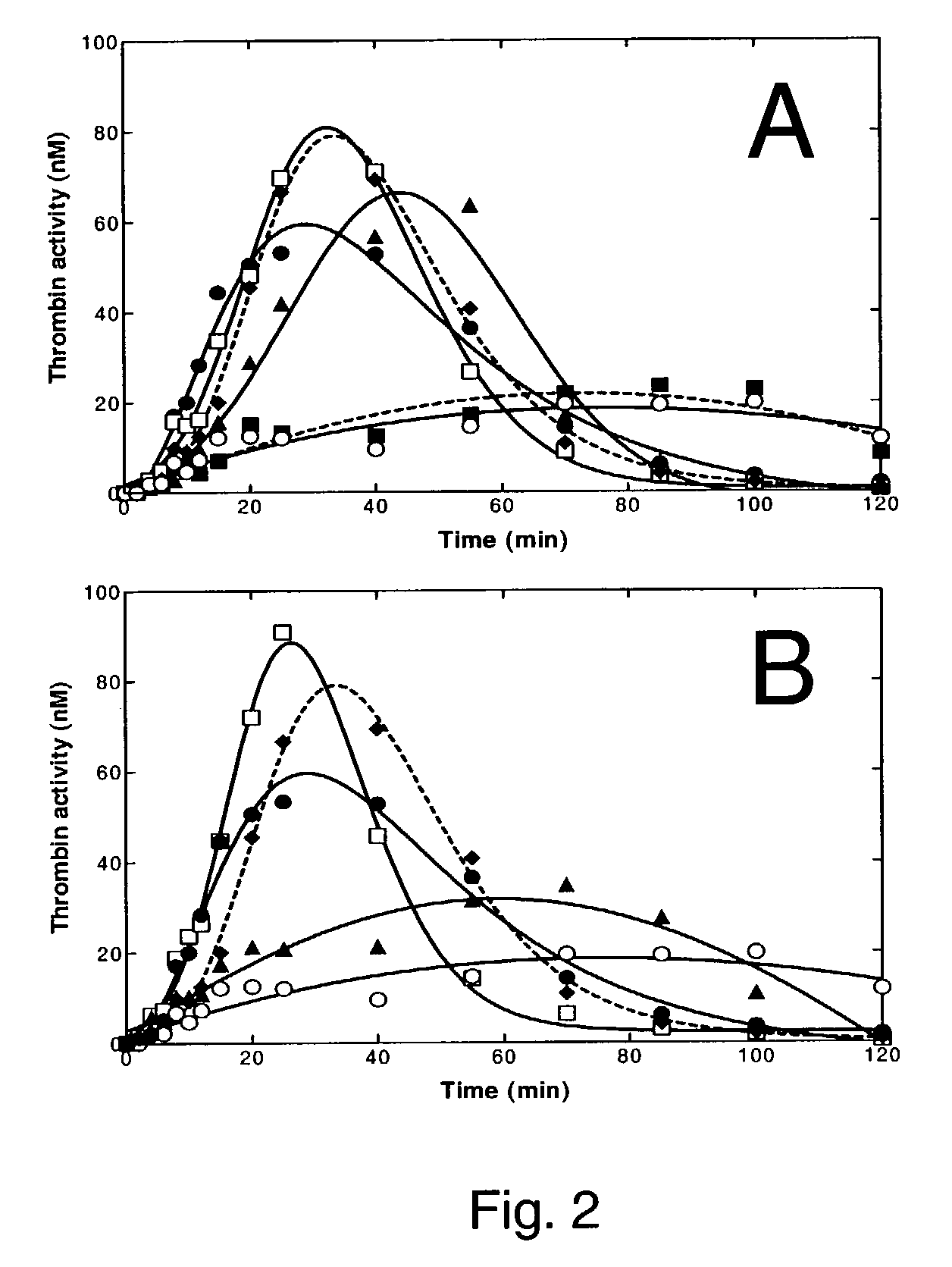
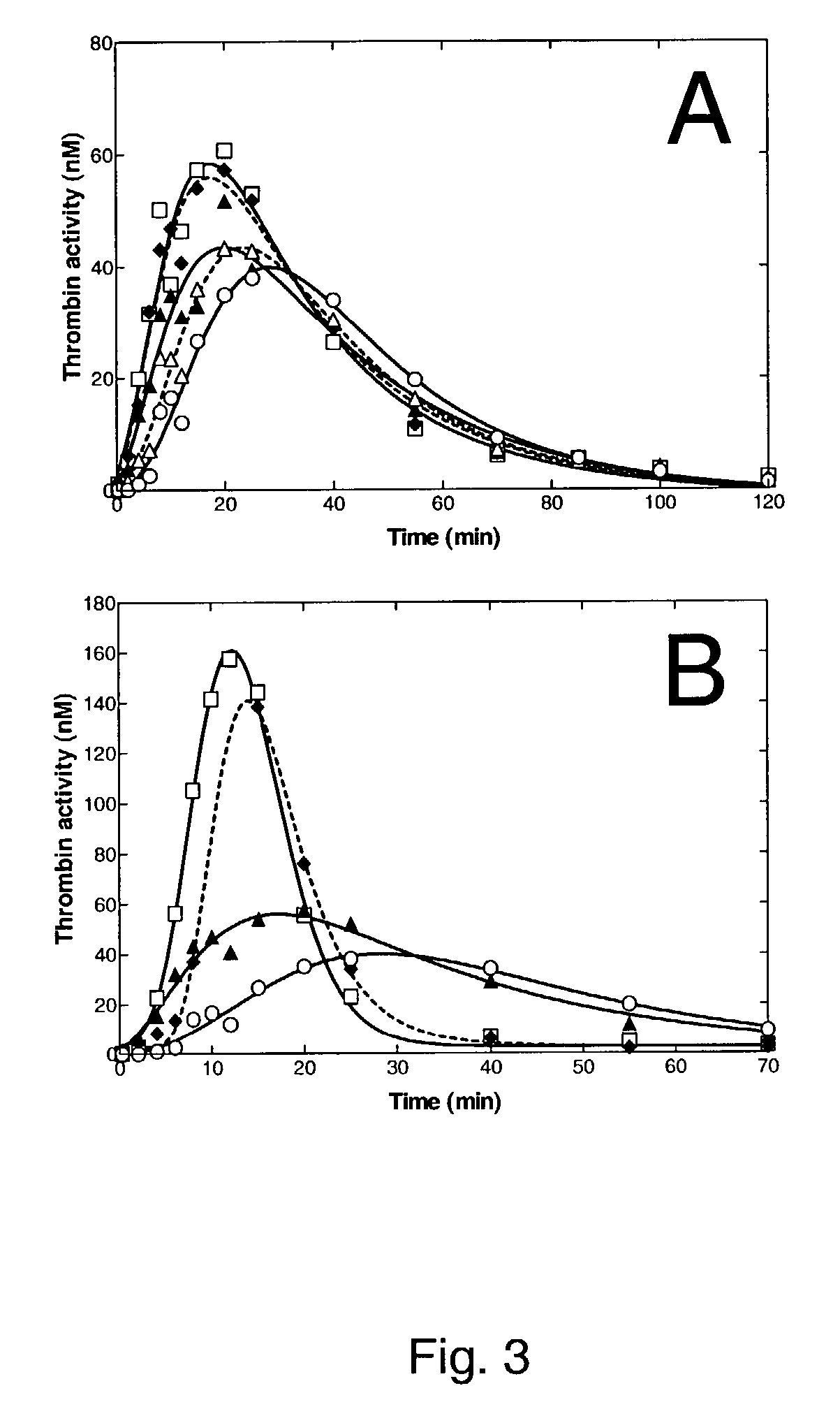
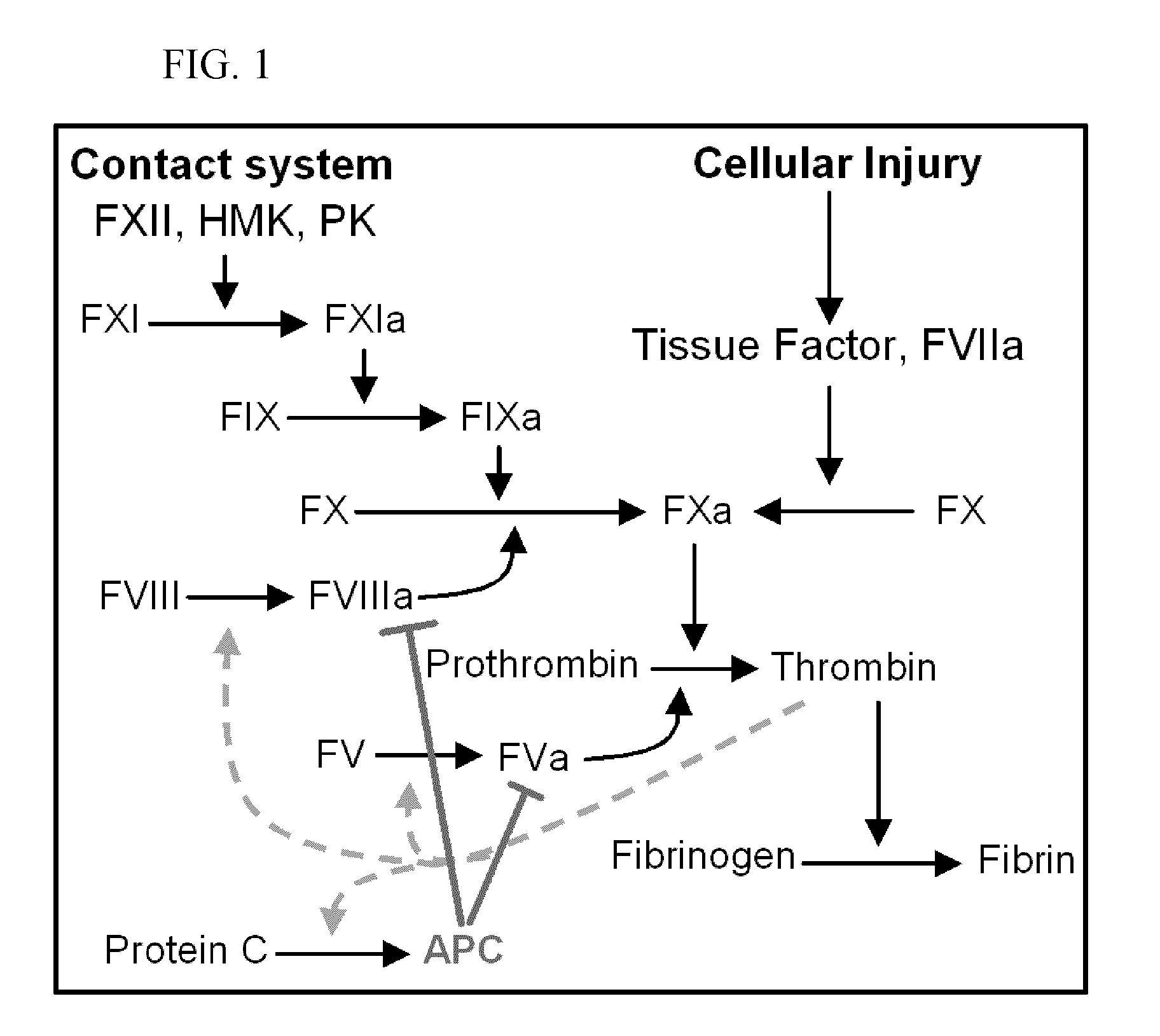
Pharmaceutical composition comprising factor VIIa and anti-TFPI
The present invention relates to the use of factor VIIa and TFPI inhibitor in the treatment or prophylaxis of bleeding episodes or coagulative treatment.
Owner:NOVO NORDISK HEALTHCARC A G
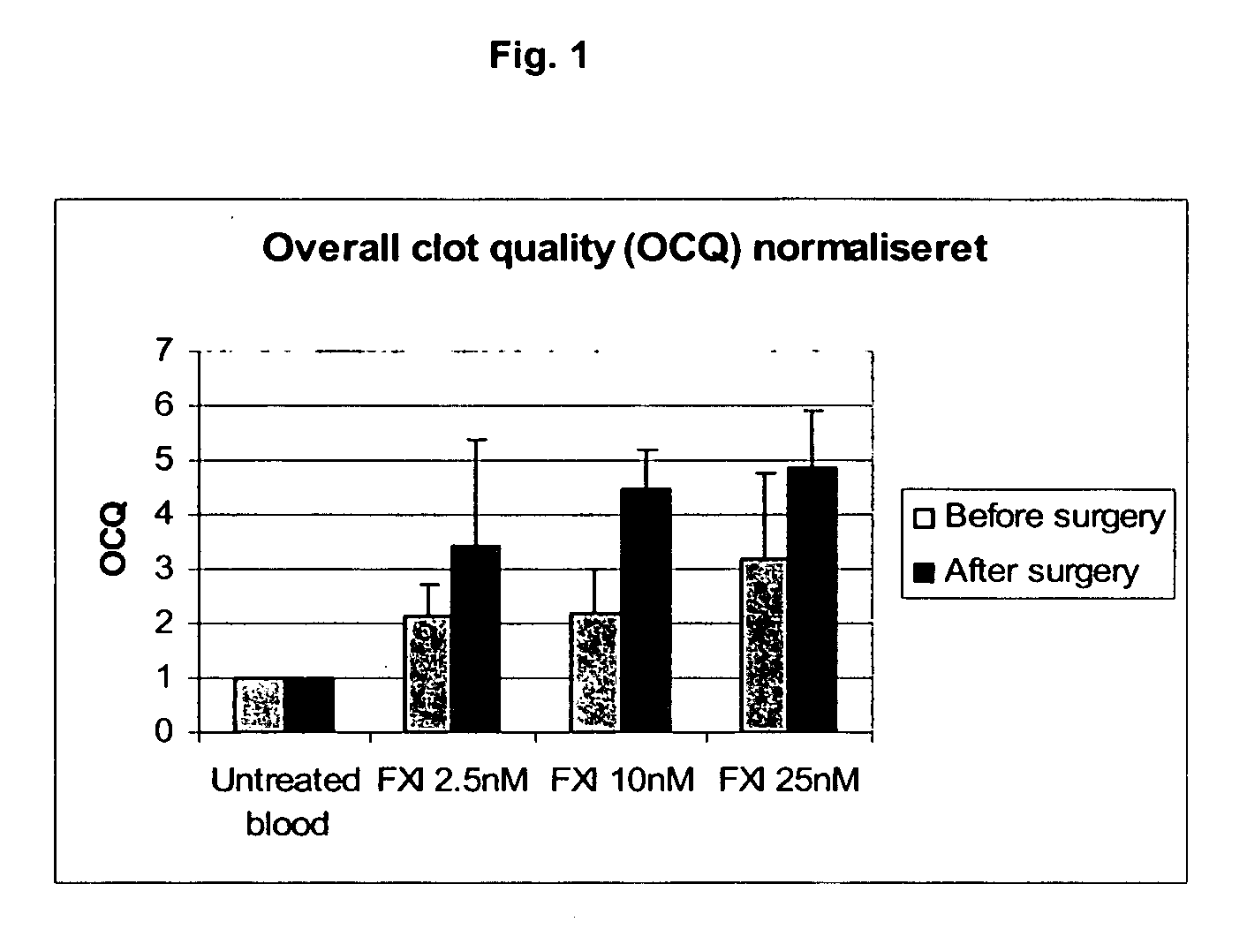
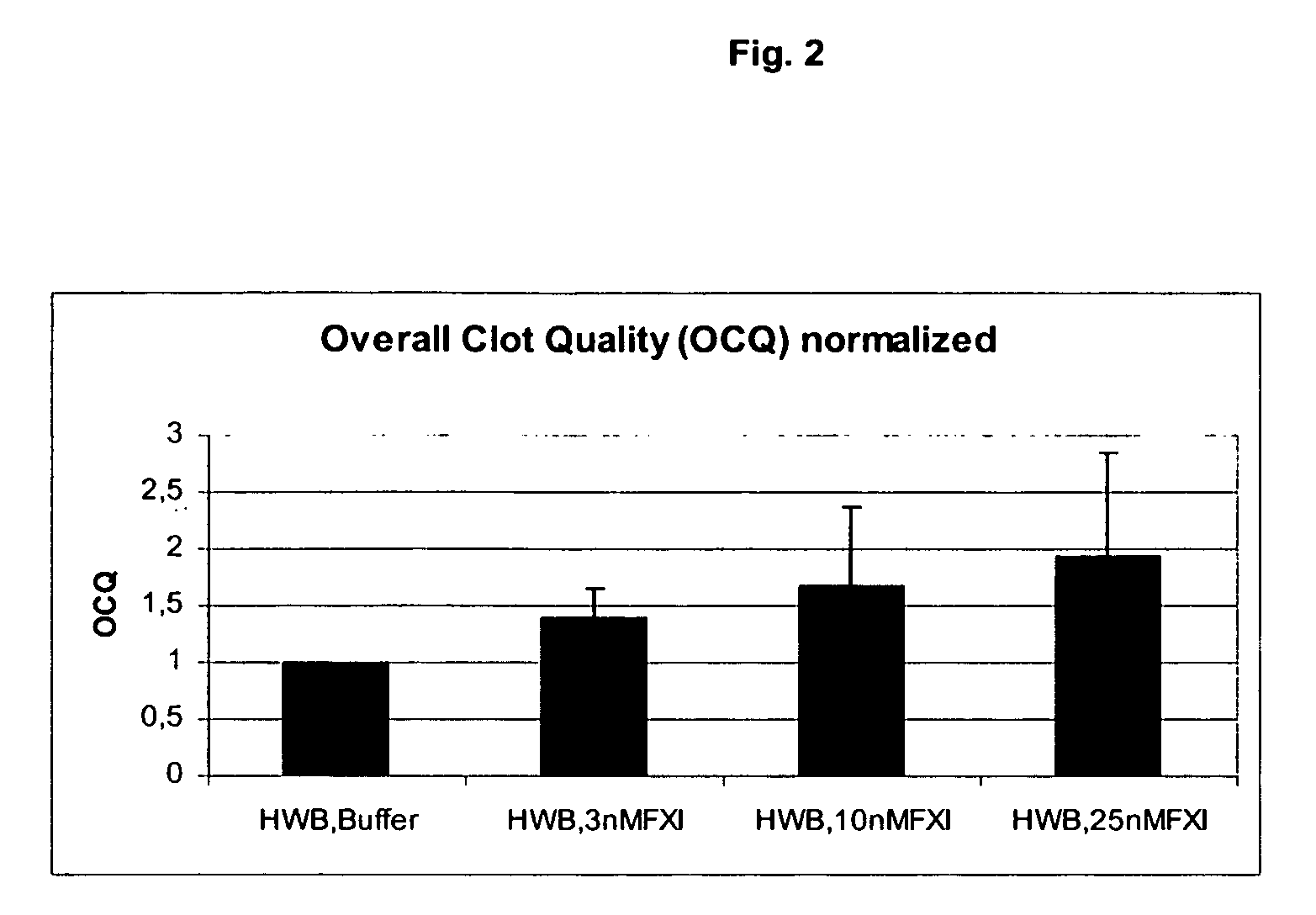
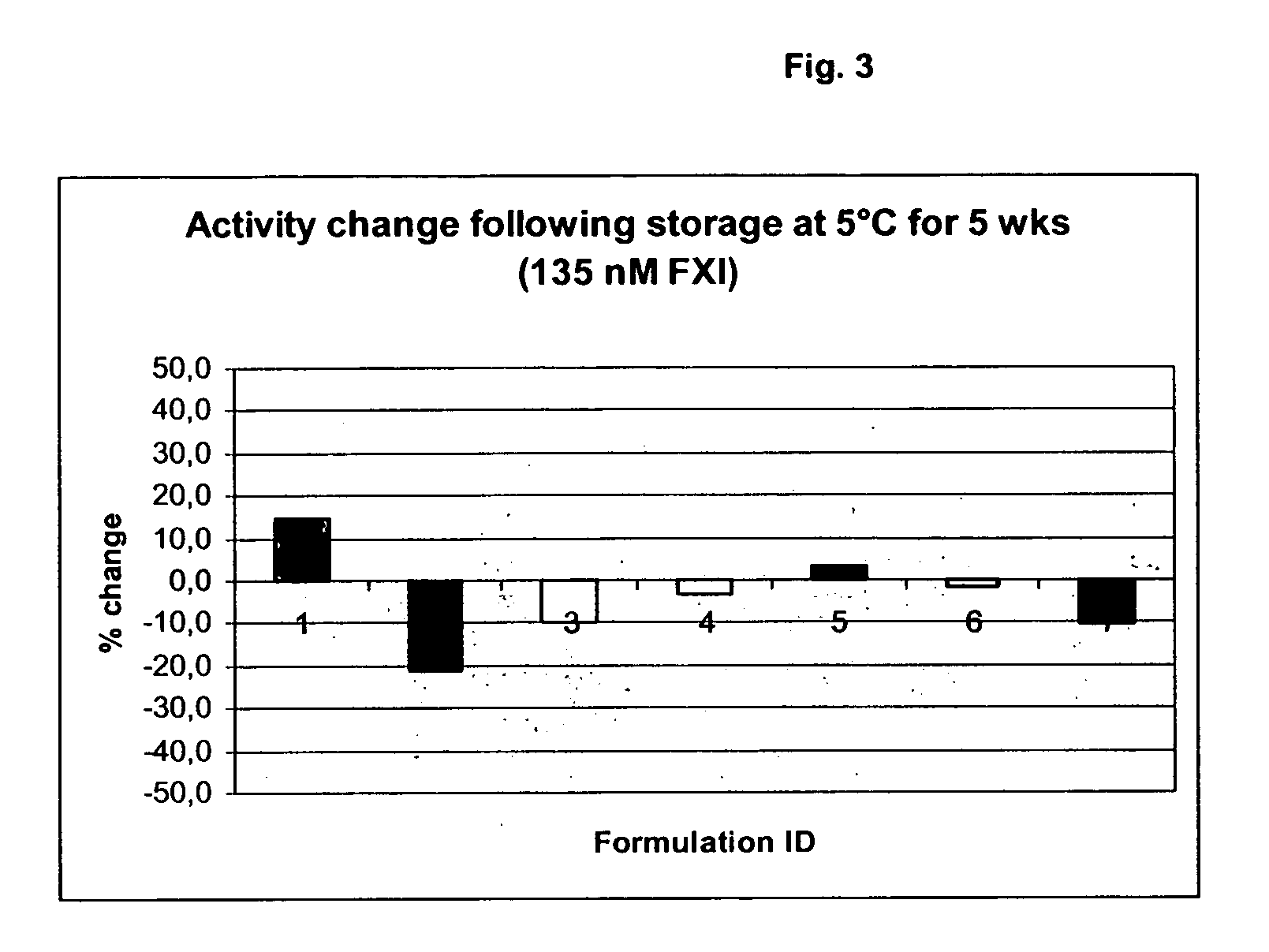
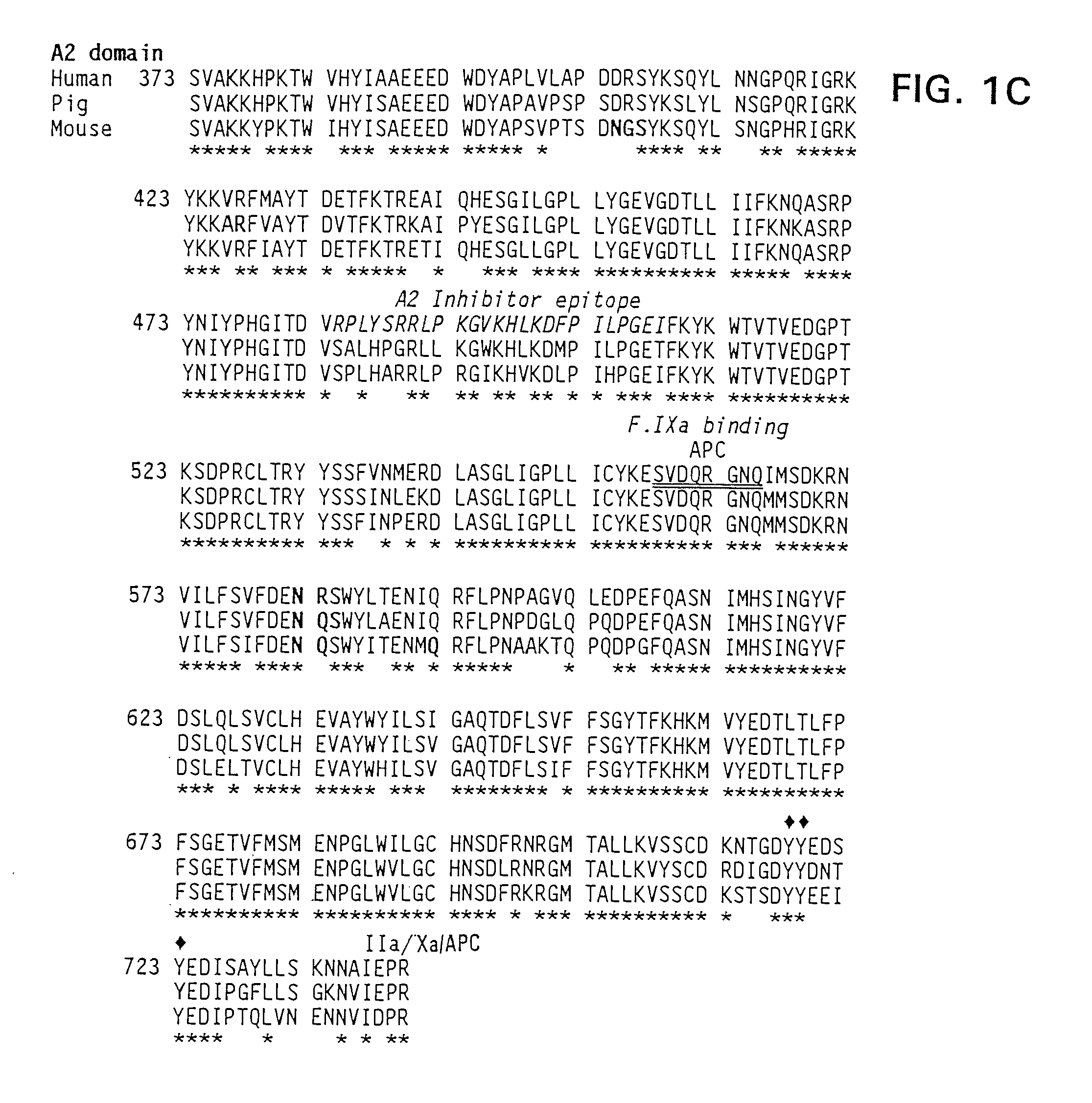
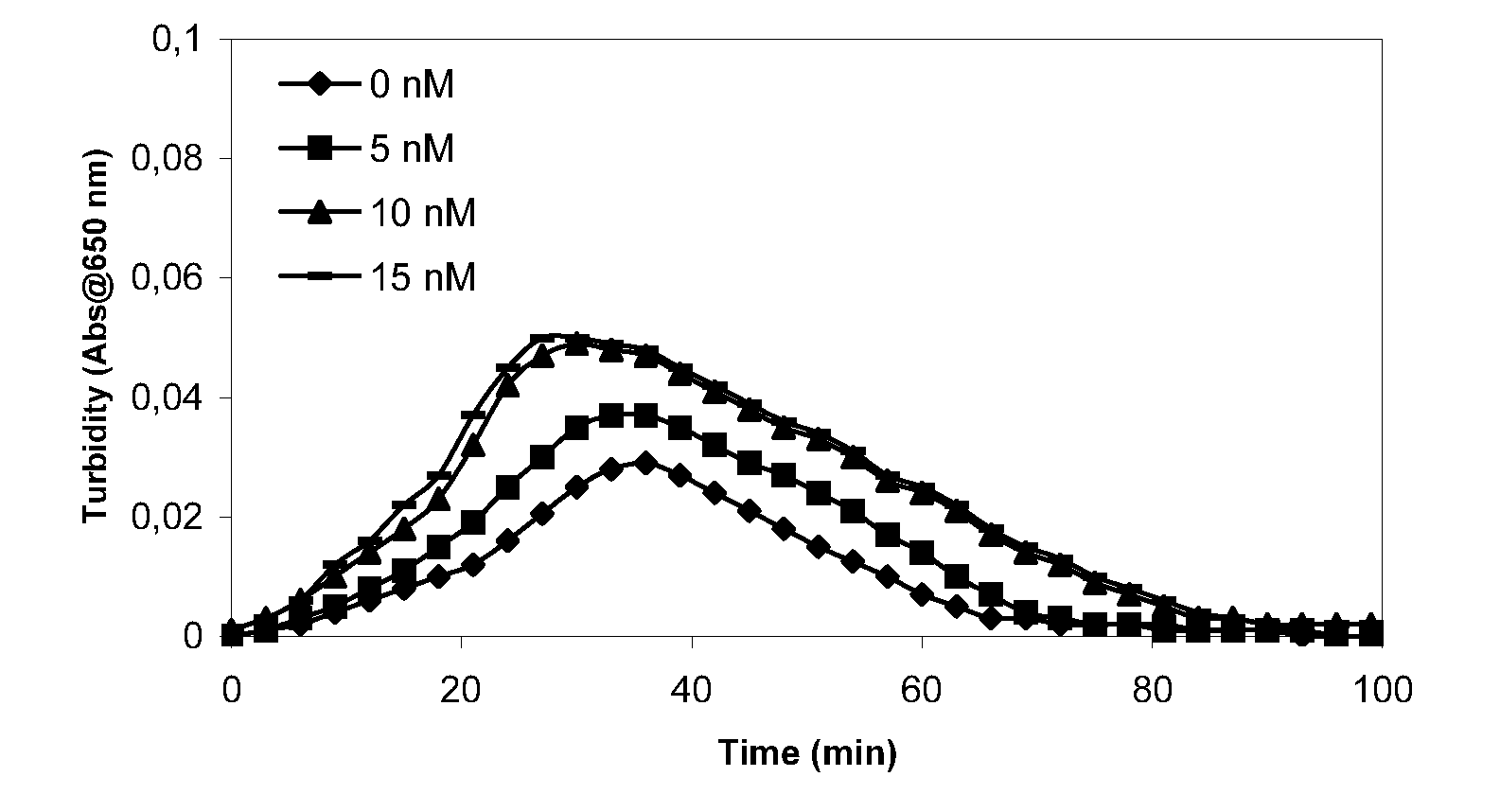
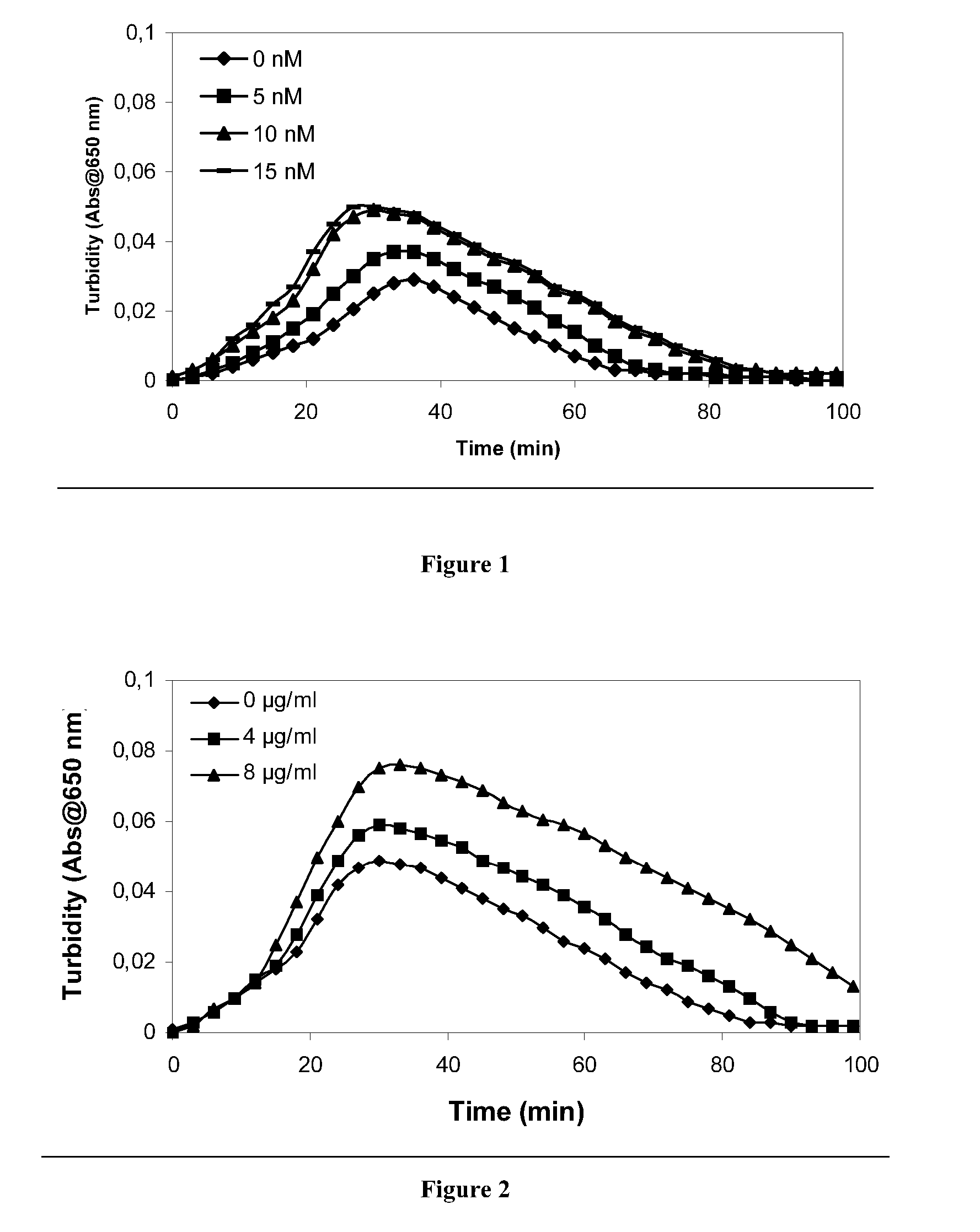
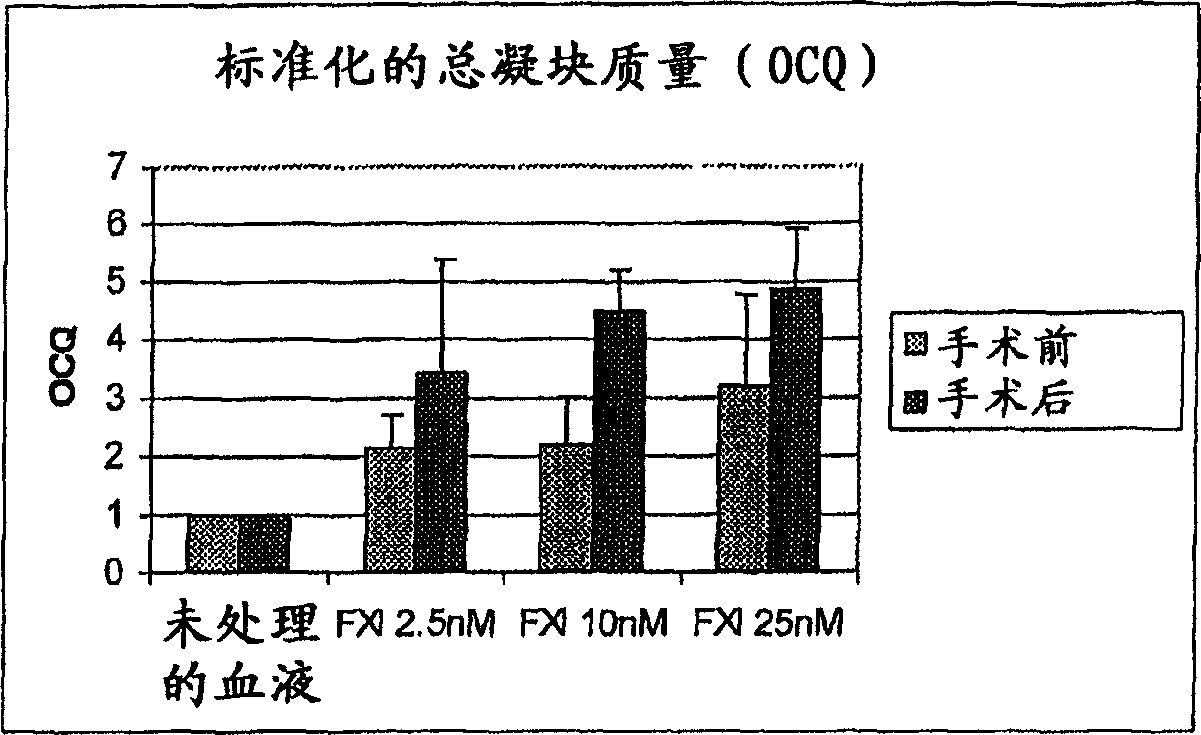
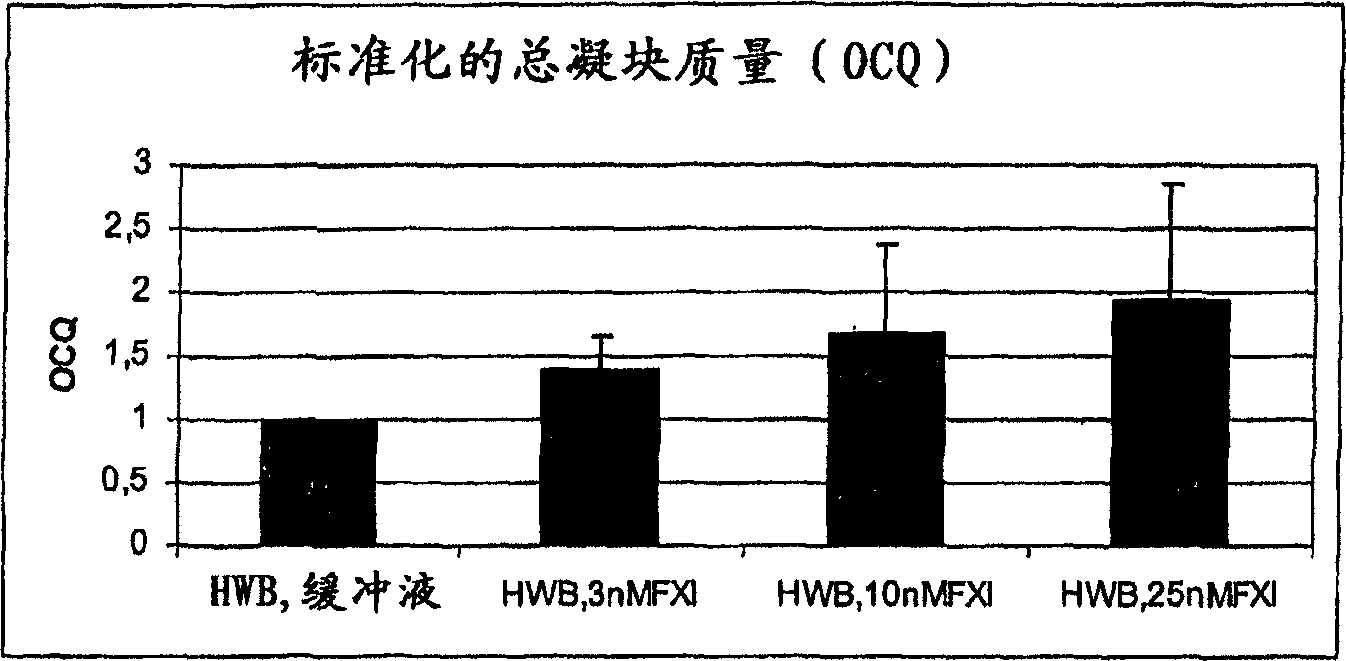
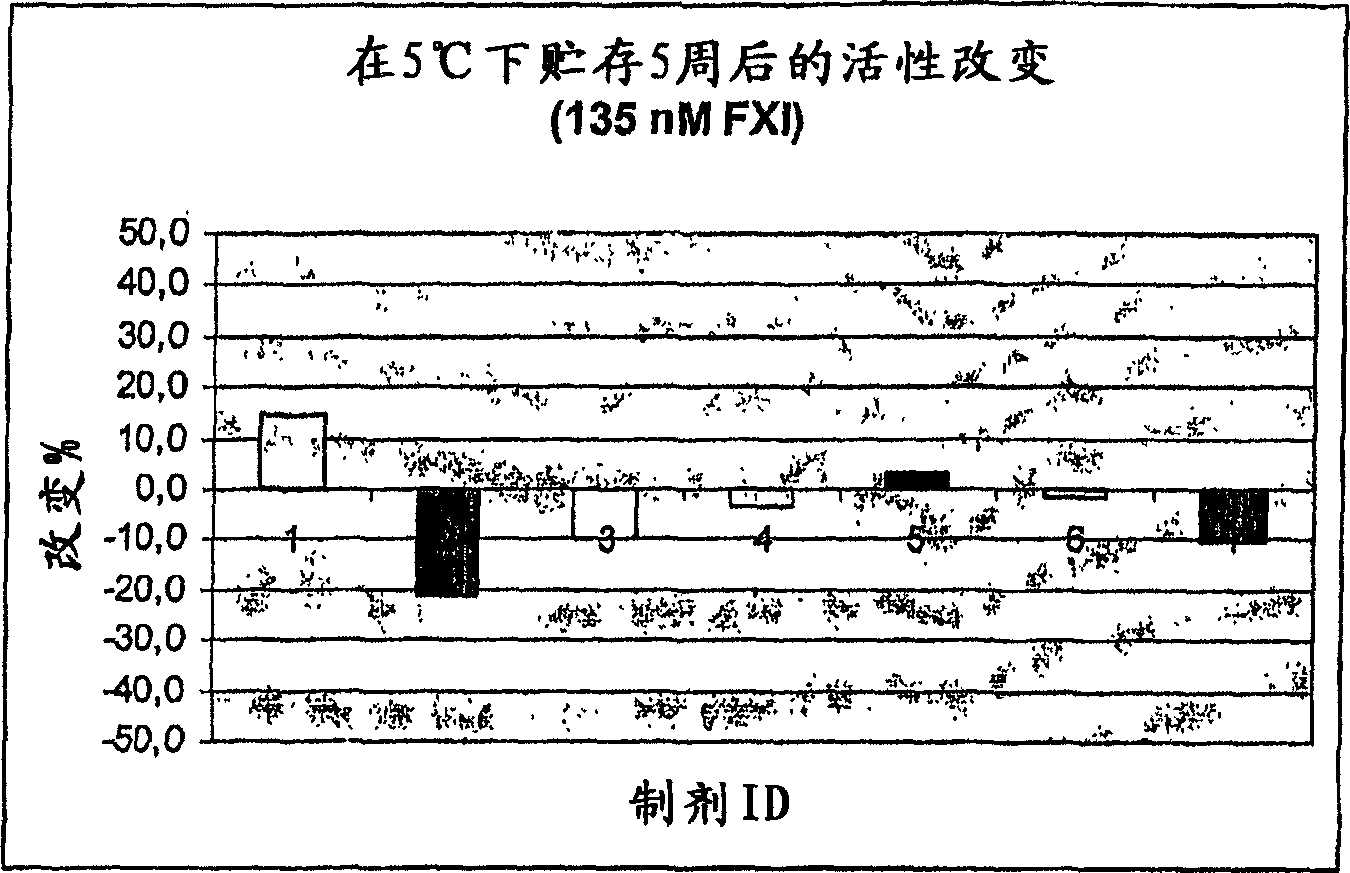
Therapeutic use of factor XI
InactiveUS20050181978A1Shorten clotting timeGood hemostasisPeptide/protein ingredientsBlood disorderMedicineFactor XI
The present invention provides methods and compositions for treating bleeding episodes. The methods are carried out by administering to a patient in need thereof a preparation comprising a factor XI polypeptide, in an amount effective for such treatment. The methods of the invention result in one or more of: reduced clotting time; enhancement of hemostasis; increase in clot lysis time; increase in clot strength; and / or increase in overall clot quality (OCQ) in said patient.
Owner:ROJKJAER RASMUS +4
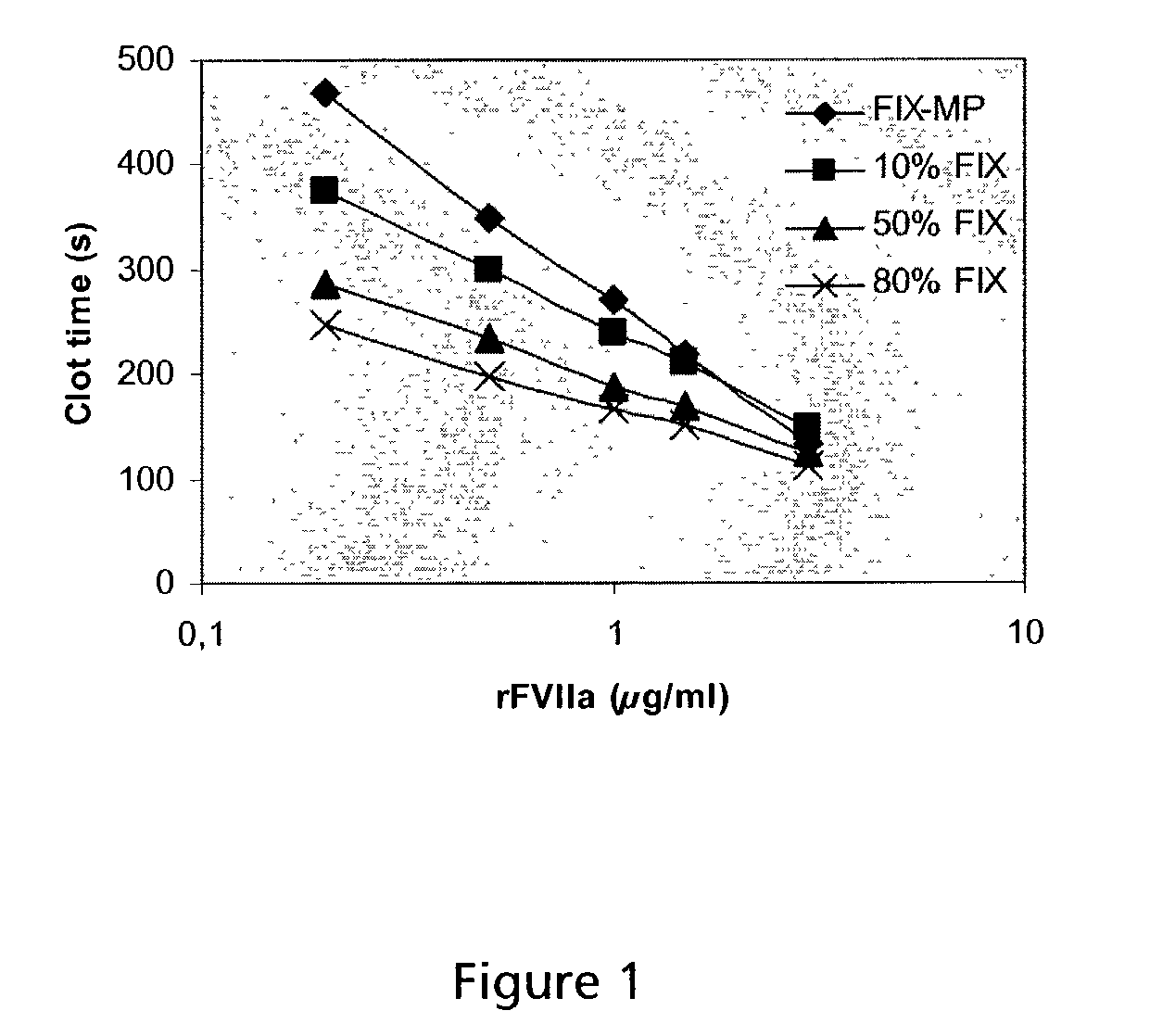
Combined use of factor VII polypeptides and factor IX polypeptides
InactiveUS20030203845A1Effective treatmentPeptide/protein ingredientsPharmaceutical drugBleeding episodes
The invention concerns a pharmaceutical preparation comprising a factor VII or factor VII-related polypeptide and a factor IX or factor IX-related polypeptide. The invention also concerns use of a factor VII or factor VII-related polypeptide and a factor IX or factor IX-related polypeptide for manufacture of a medicament for pharmaceutical use as well as methods for prevention or treatment of bleeding episodes in subjects.
Owner:NOVO NORDISK AS
Pharmaceutical composition comprising factor VII polypeptides and factor V polypeptides
InactiveUS7125846B2Improved and reliable and widely applicableGood coagulationPeptide/protein ingredientsMammal material medical ingredientsFactor iiBleeding episodes
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide, and factor V or a factor V-related polypeptide, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Pharmaceutical composition comprising factor VII polypeptides and alpha2-antiplasmin polypeptides
InactiveUS7078479B2Improved and reliable and widely applicableGood coagulationPeptide/protein ingredientsMammal material medical ingredientsPharmaceutical drugBleeding episodes
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide and alpha2-antiplasmin or an alpha2-antiplasmin-related polypeptide, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
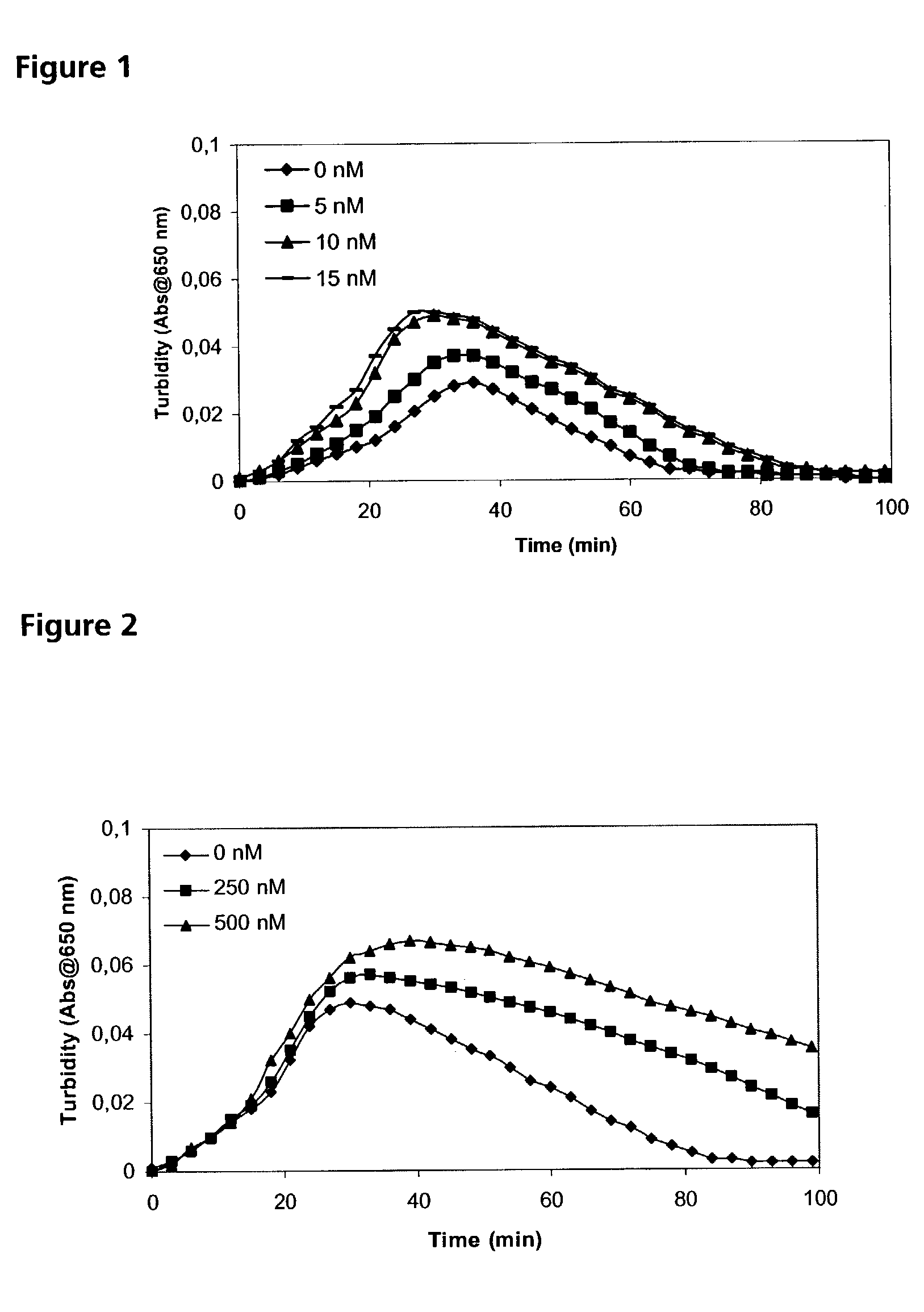
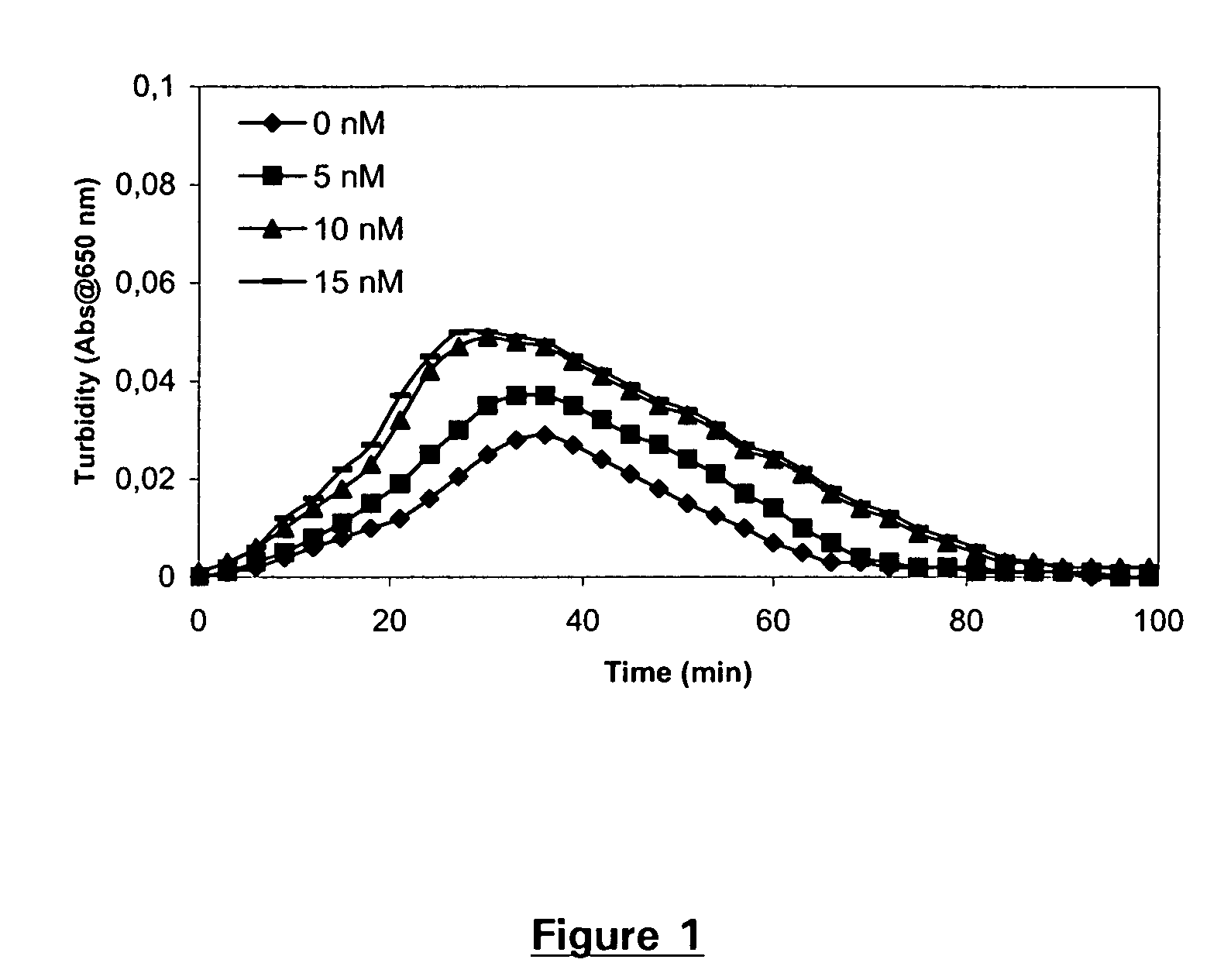
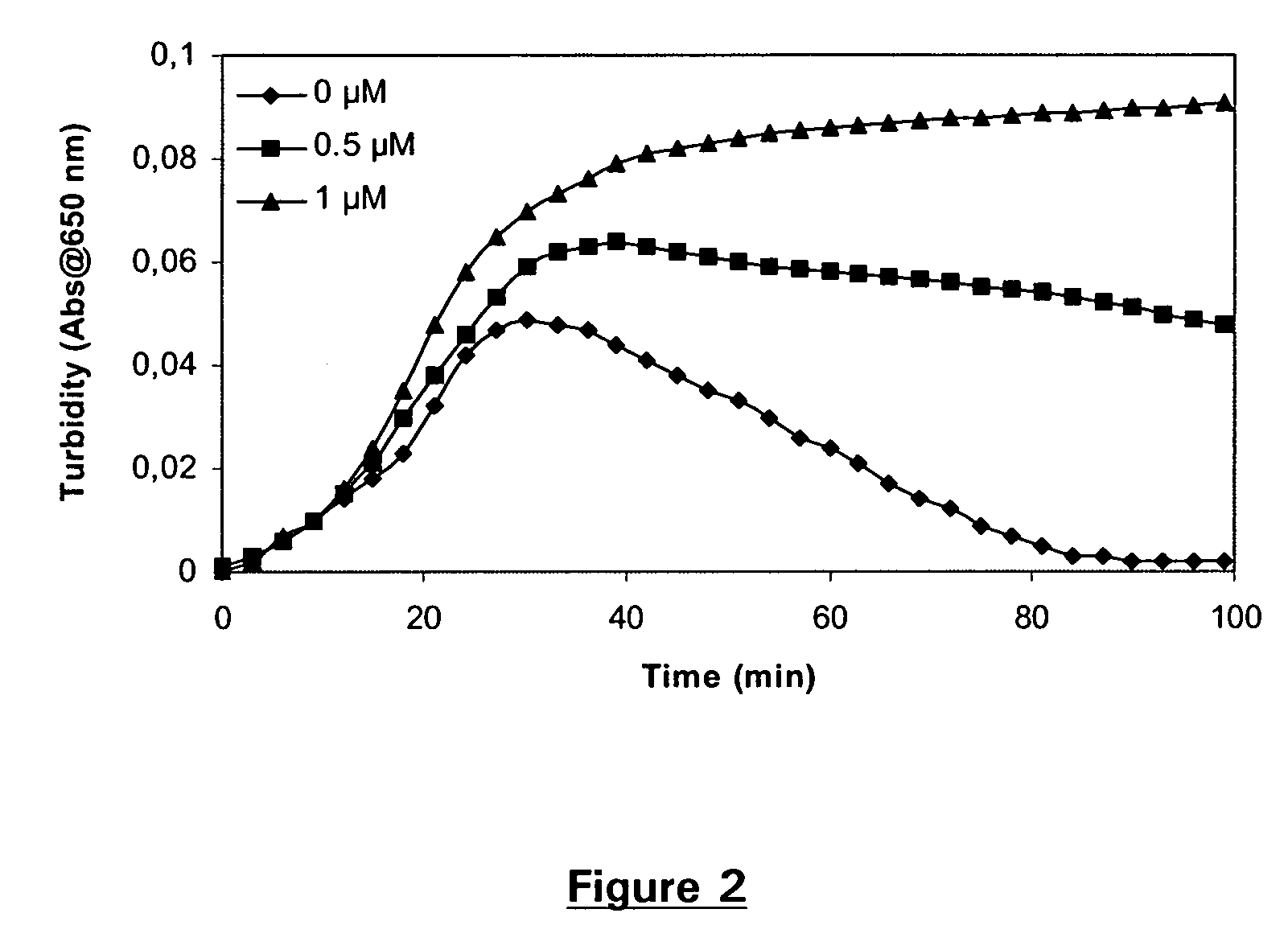
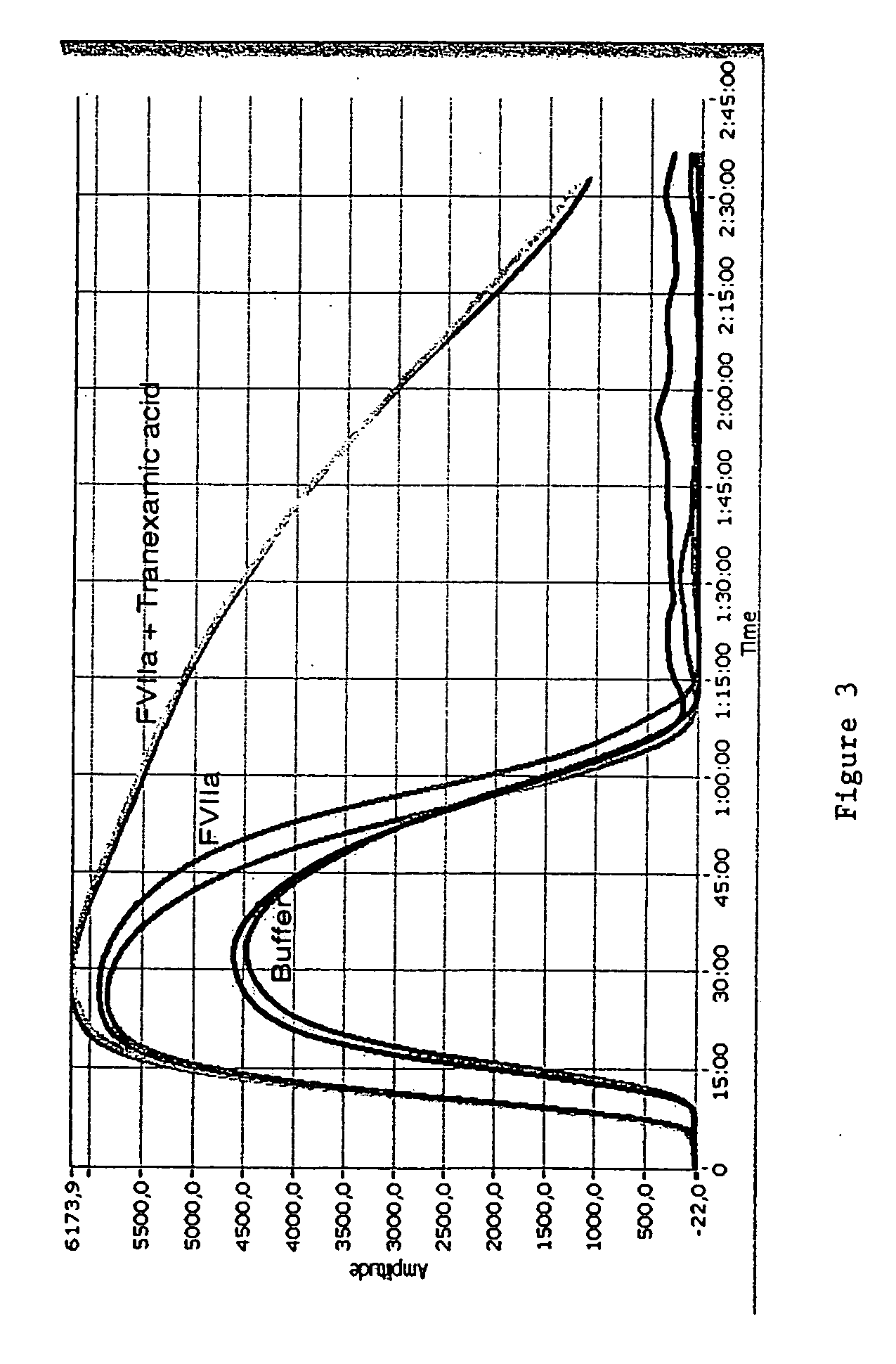
Pharmaceutical composition comprising factor VII polypeptides and tranexamic acid
The present invention relates to compositions comprising factor VII or a factor VII-related polypeptide and tranexamic acid, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Single-dose administration of factor VIIa
InactiveUS7419949B2Peptide/protein ingredientsPharmaceutical delivery mechanismFactor VIIaBleeding episodes
The present invention provides methods for preventing and / or treating bleeding episodes by administering a single dose of Factor VIIa or a Factor VIIa equivalent. Preferably, the single dose comprises between about 150 and about 500 μg / kg Factor VIIa or Factor VIIa equivalent.
Owner:NOVO NORDISK AS
Modified factor VIII
Modified porcine factor VIII is disclosed in which most of the B domain has been removed through genetic engineering. This modified factor VIII is particularly useful for treatment of hemophiliacs, especially those undergoing bleeding episodes.
Owner:EMORY UNIVERSITY
Pharmaceutical composition comprising factor VII polypeptides and protein C inhibitors
InactiveUS20060013812A1Improved and reliable and widely applicableGood coagulationFactor VIIPeptide/protein ingredientsMedicineProtein C inhibitor
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide and a protein C inhibitor, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Pharmaceutical composition comprising factor VII polypeptides and tissue plasminogen inhibitors
InactiveUS20050266006A1Effective treatmentPeptide/protein ingredientsAntibody ingredientsTPA InhibitorsBleeding episodes
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide and a tPA inhibitor, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
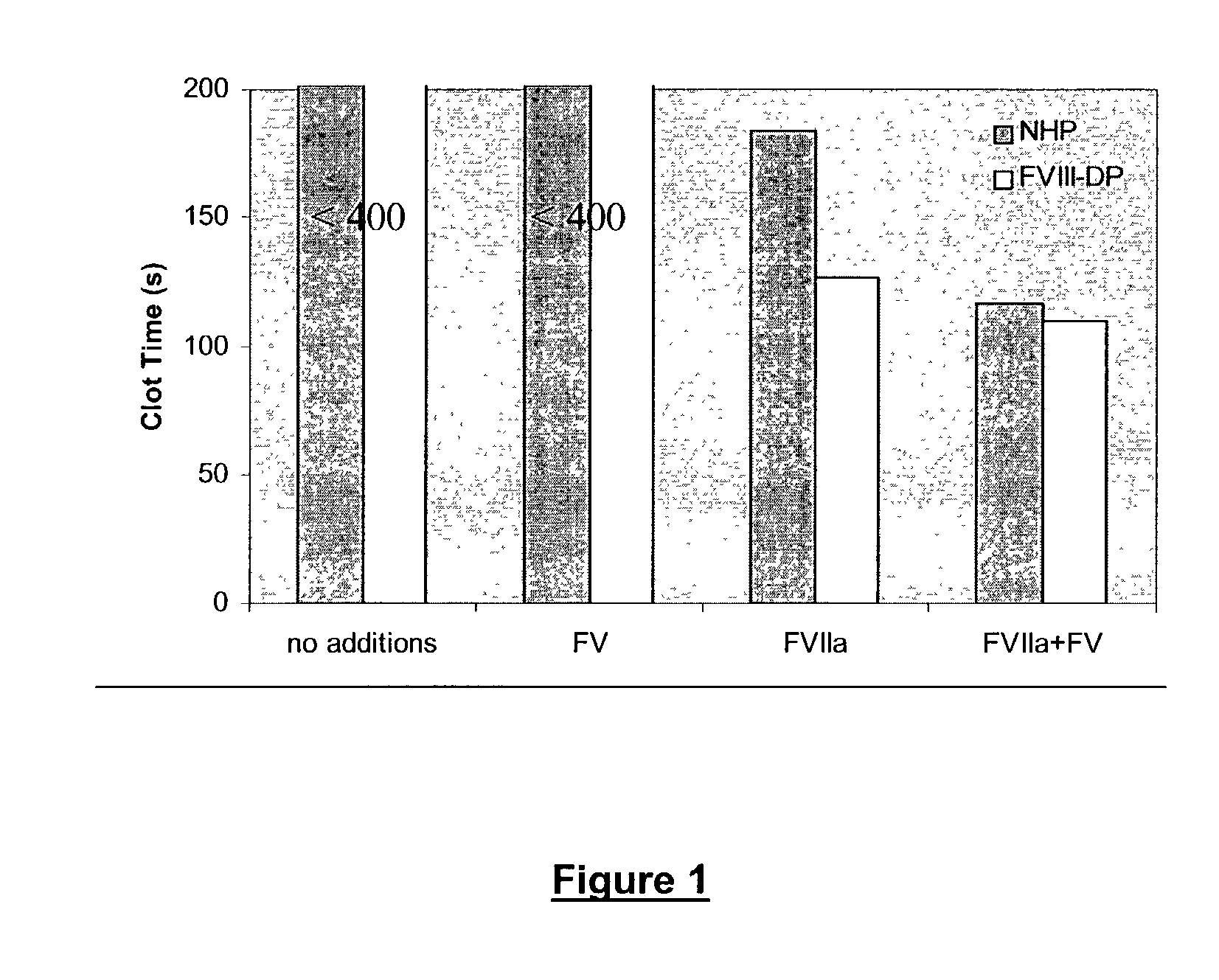
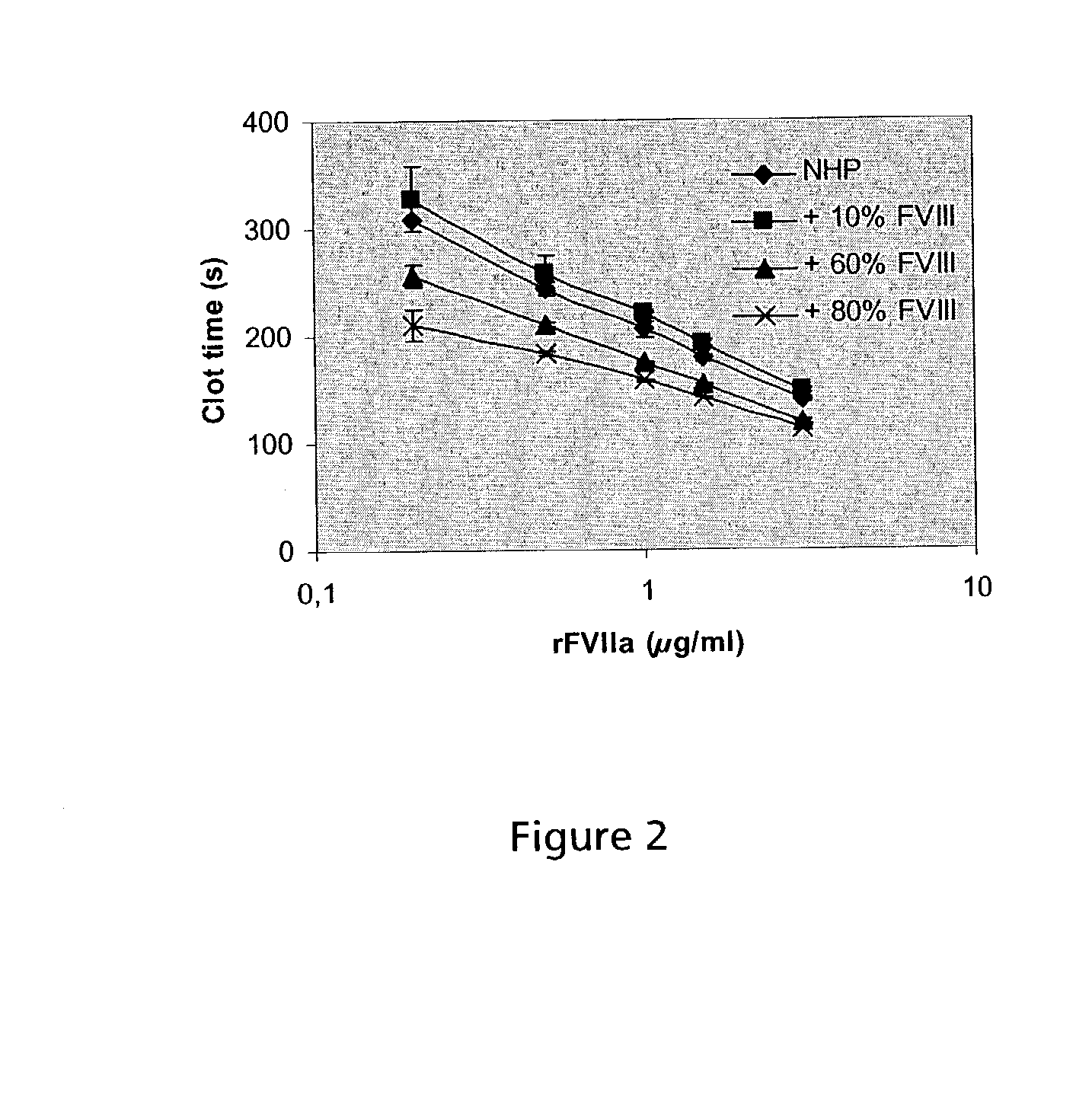
Combined use of VII polypeptides and factor VIII polypeptides
InactiveUS20030199444A1Effective treatmentGood effectFactor VIIPeptide/protein ingredientsCombined useFactor ii
The invention concerns a pharmaceutical preparation comprising a factor VII or factor VII-related polypeptide and a factor VIII or factor VIII-related polypeptide. The invention also concerns use of a factor VII or factor VII-related polypeptide and a factor VIII or factor VIII-related polypeptide for manufacture of a medicament for pharmaceutical use as well as methods for prevention or treatment of bleeding episodes in subjects.
Owner:NOVO NORDISK AS
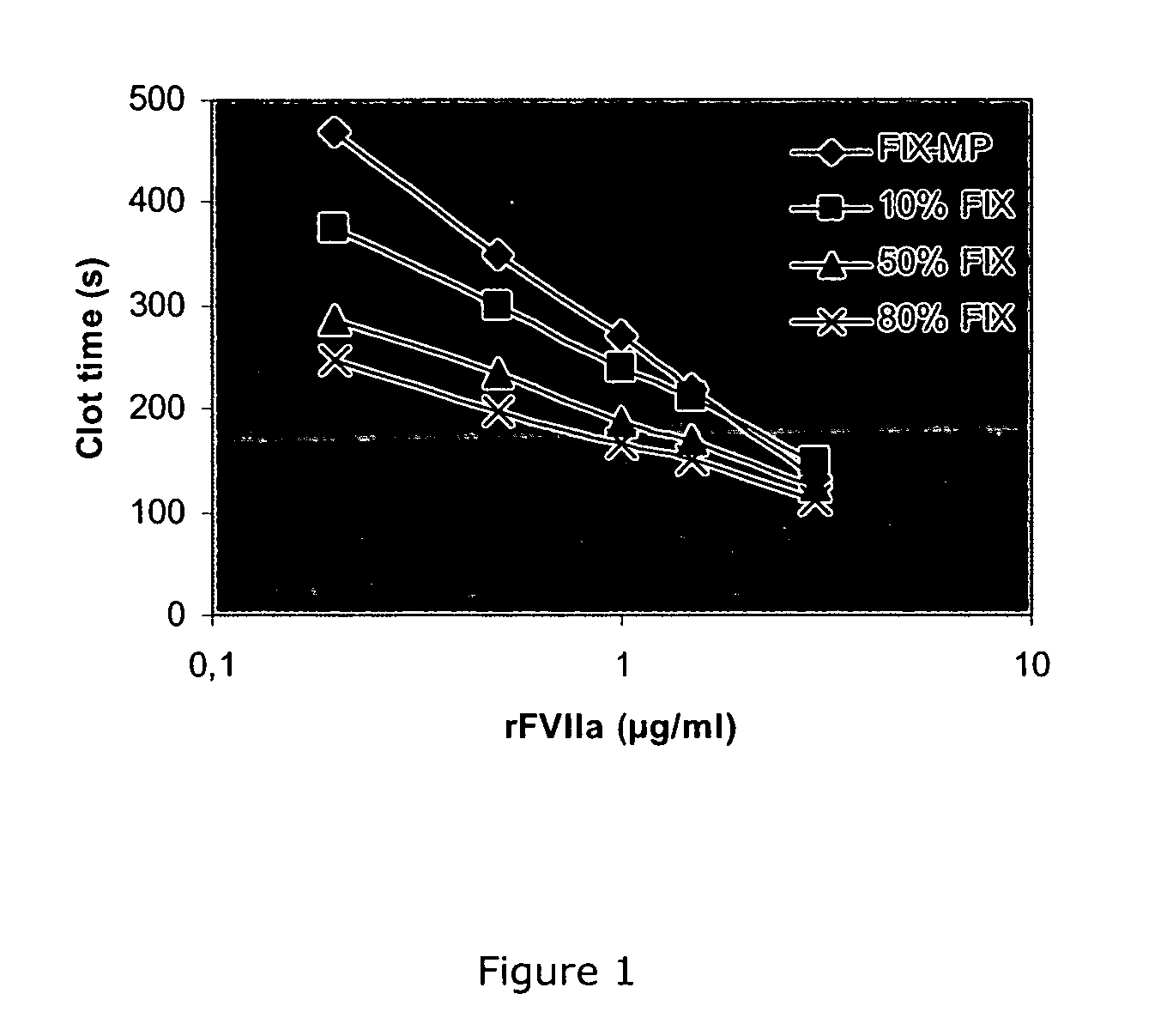
Combined use of factor VII polypeptides and factor IX polypeptides
The invention concerns a pharmaceutical preparation comprising a factor VII or factor VII-related polypeptide and a factor IX or factor IX-related polypeptide. The invention also concerns use of a factor VII or factor VII-related polypeptide and a factor IX or factor IX-related polypeptide for manufacture of a medicament for pharmaceutical use as well as methods for prevention or treatment of bleeding episodes in subjects.
Owner:NOVO NORDISK HEALTH CARE AG
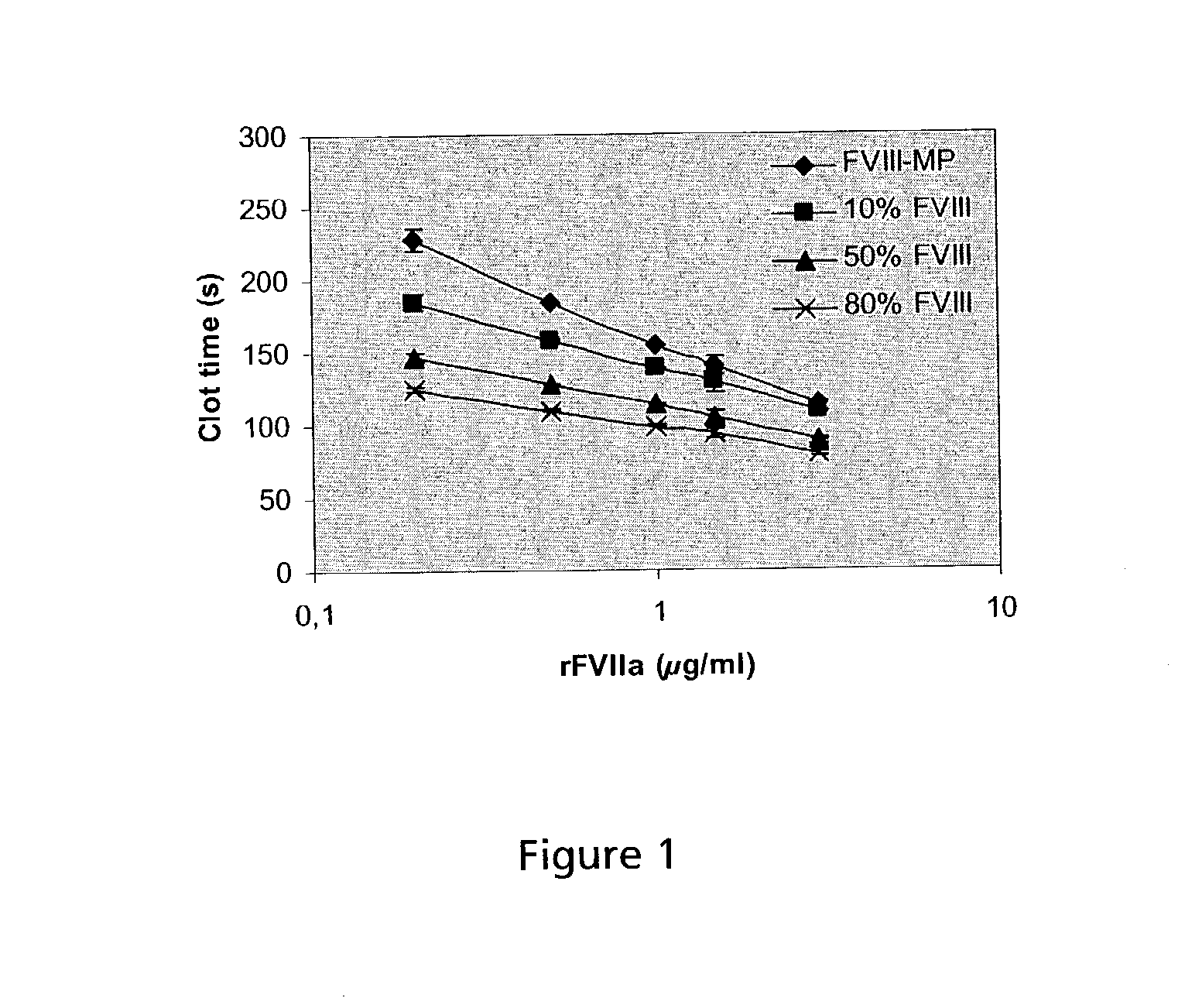
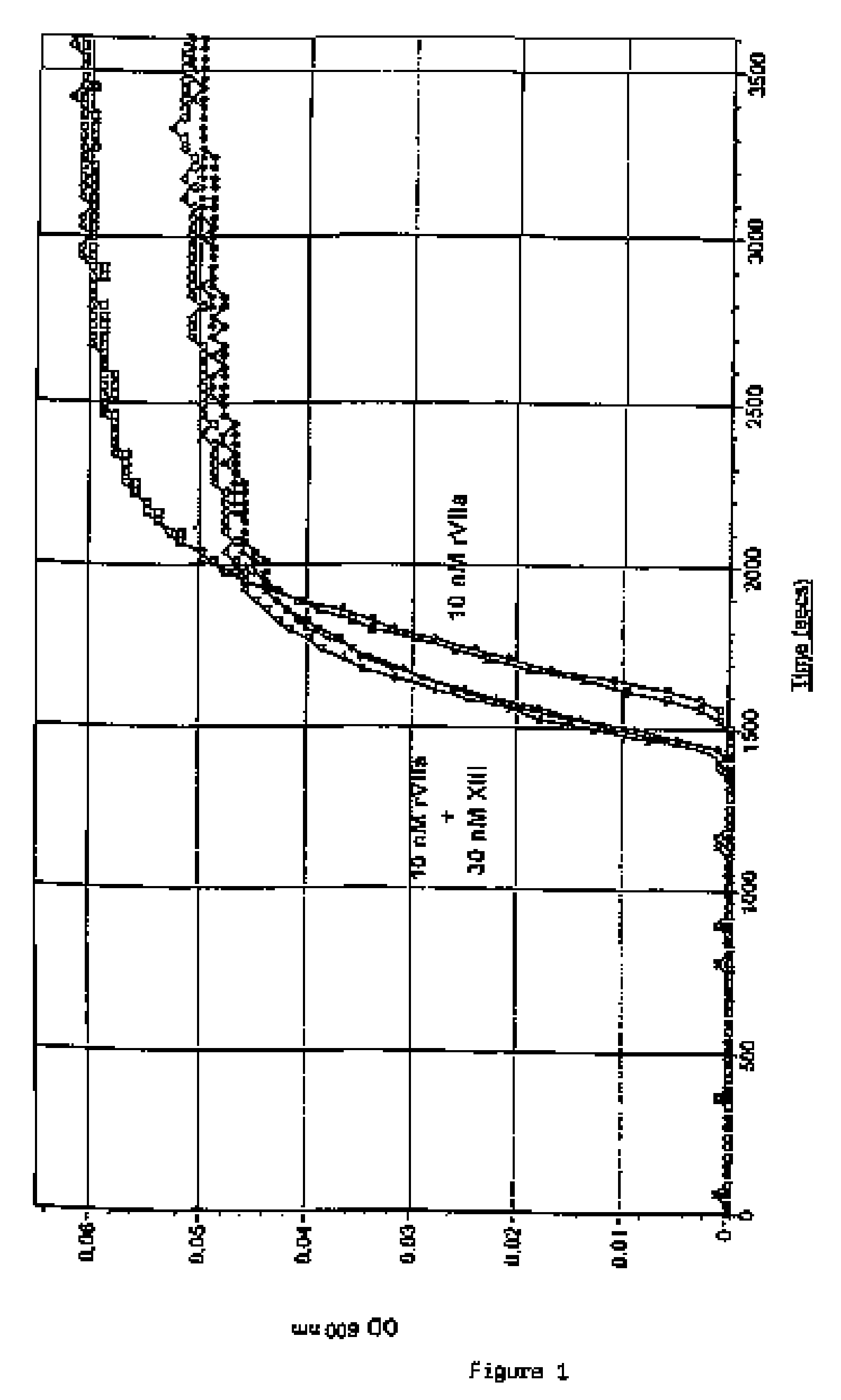
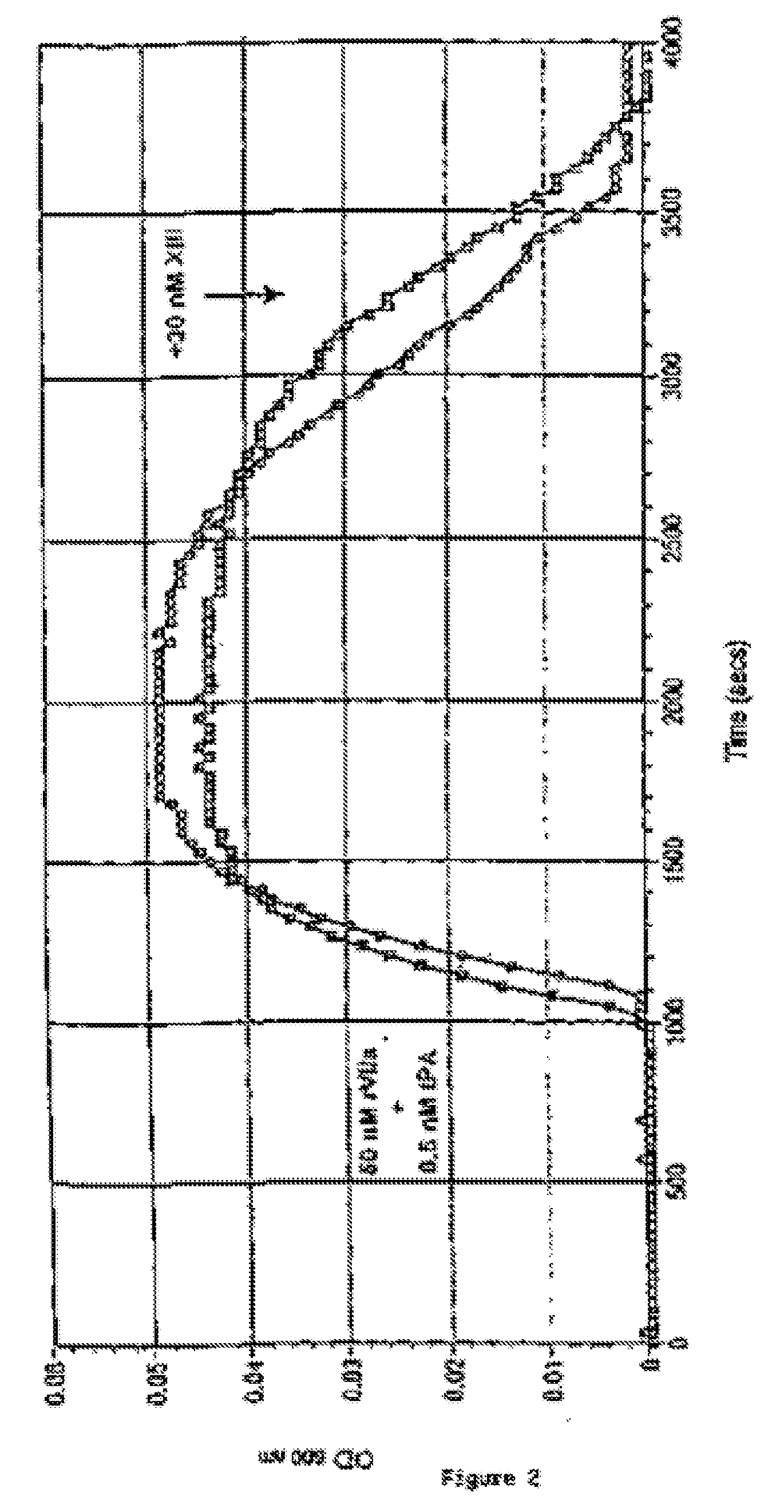
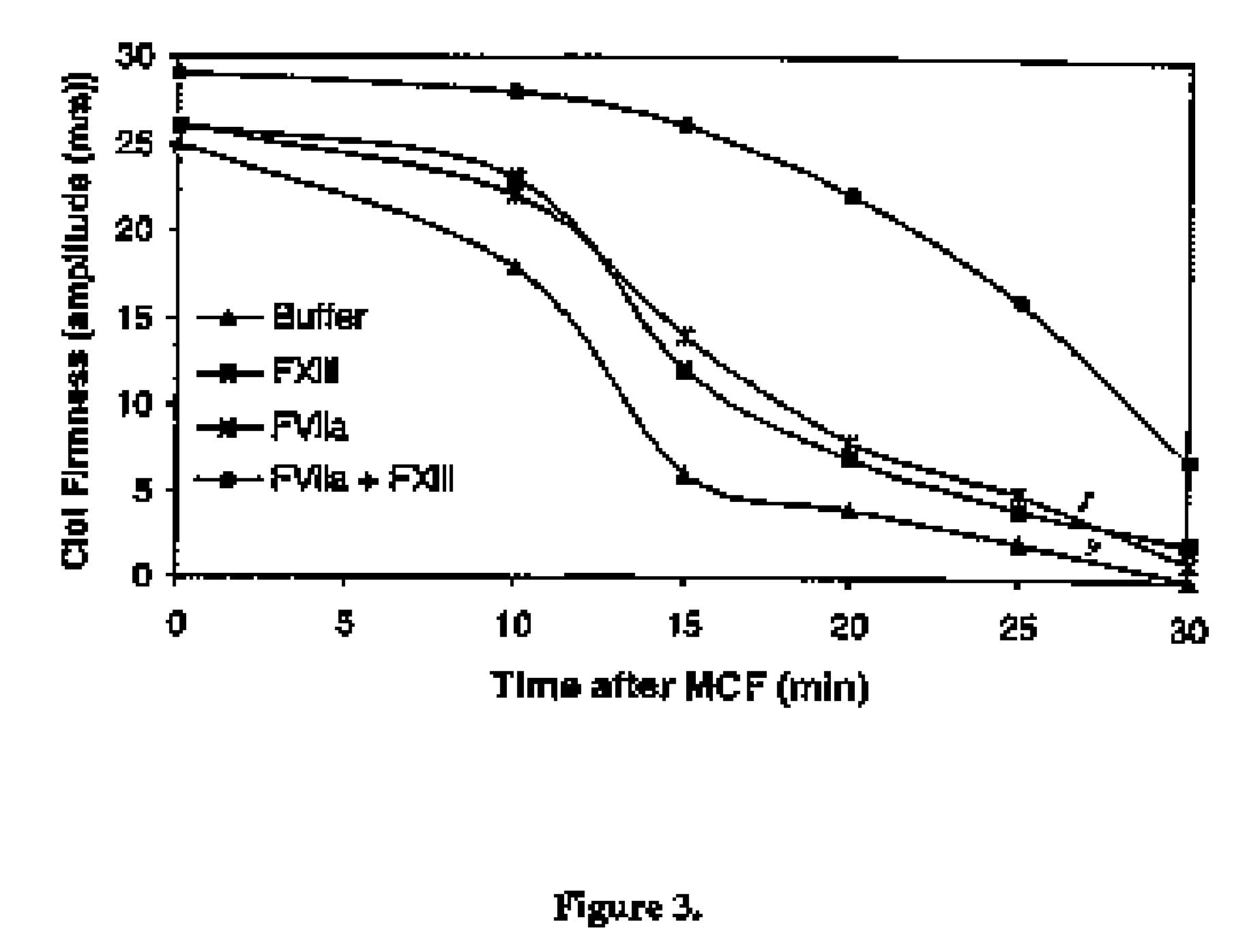
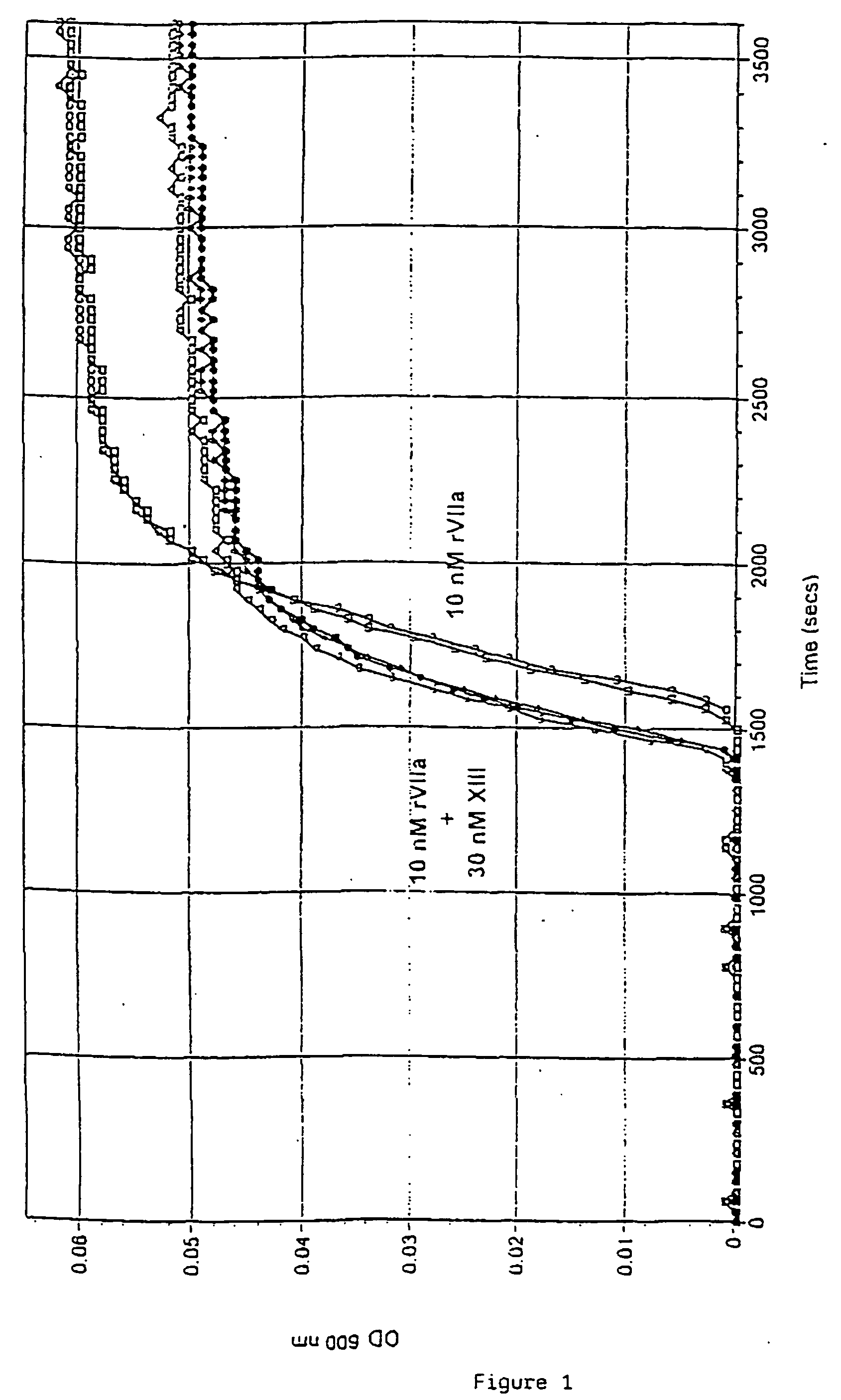
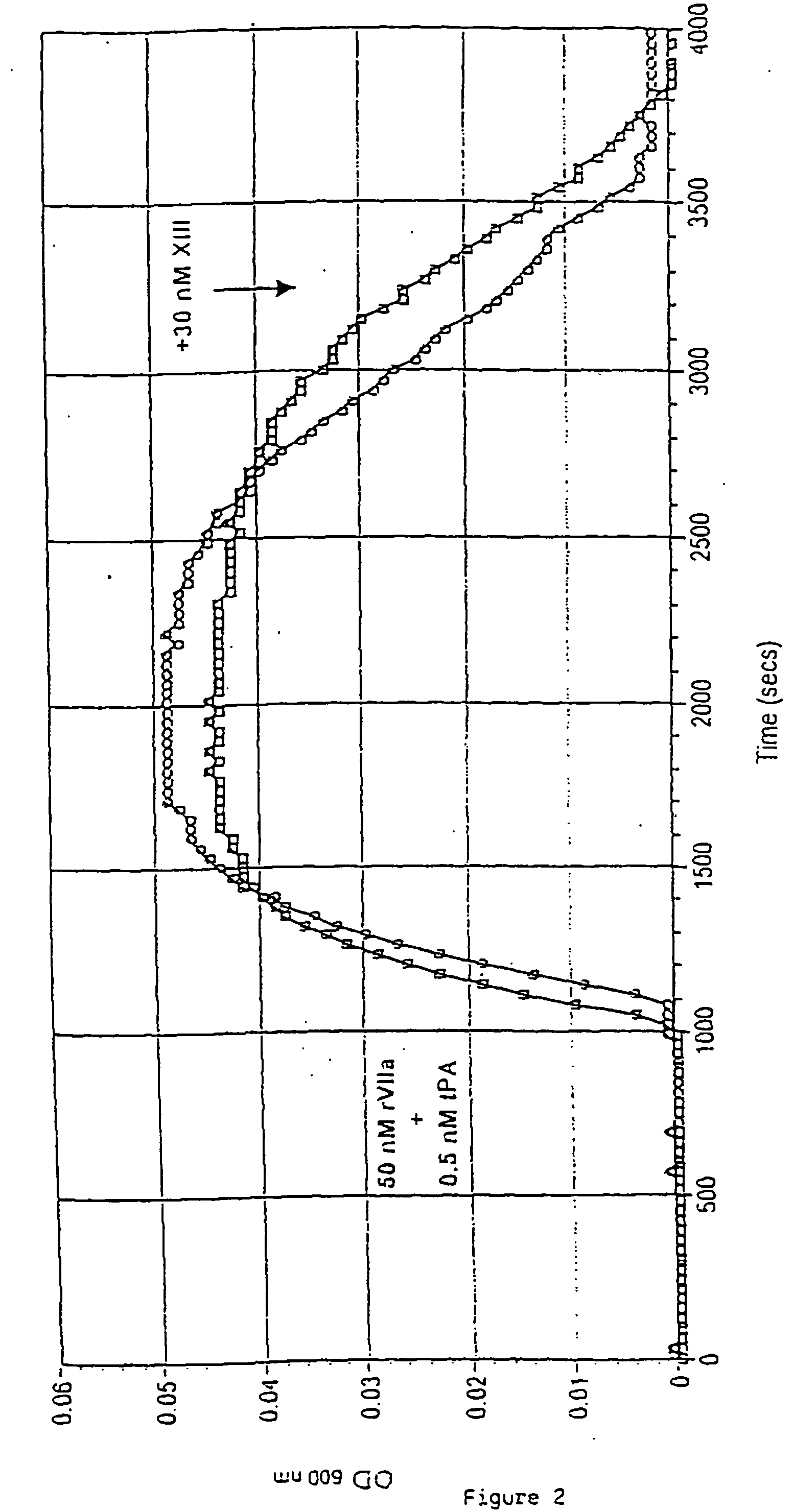
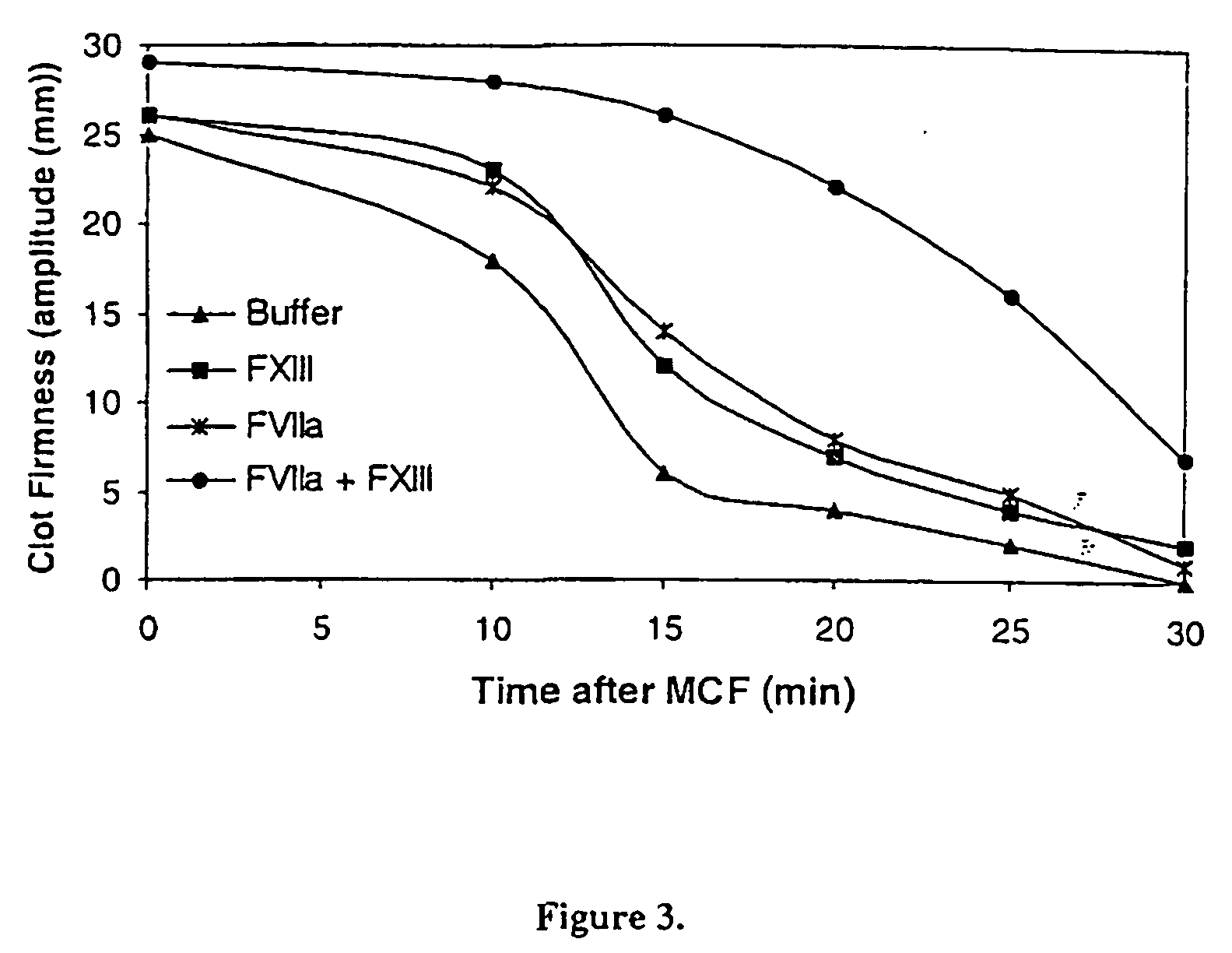
PHARMACEUTICAL COMPOSITION COMPRISING A FACTOR VIIa AND A FACTOR XIII
InactiveUS20070280920A1Effective treatmentHigh levelPeptide/protein ingredientsSurgical drugsFactor VIIaPharmaceutical drug
The present invention relates to the use of a factor VIIa and a factor XIII in the treatment or prophylaxis of bleeding episodes.
Owner:NOVO NORDISK HEALTH CARE AG
Modified Factor VIII
InactiveUS20070135342A1Superior coagulant activityFactor VIIHydrolasesCombinatorial chemistryBleeding episodes
Modified porcine factor VIII is disclosed in which most of the B domain has been removed through genetic engineering. This modified factor VIII is particularly useful for treatment of hemophiliacs, especially those undergoing bleeding episodes.
Owner:EMORY UNIVERSITY
Protein c for use in maintaining hemostasis
InactiveUS20100184672A1Effective hemostasisGood hemostasisPeptide/protein ingredientsGenetic material ingredientsPlatelet disorderInjury cause
It is disclosed herein that protein C functions as a hemostatic agent. Thus, provided is a method of preventing, treating or ameliorating abnormal bleeding in a subject, comprising administering to the subject a protein C polypeptide or polynucleotide. Abnormal bleeding can result from a bleeding disorder, such as hemophilia or a platelet disorder, or from a bleeding episode, such as from a traumatic injury.
Owner:OREGON HEALTH & SCI UNIV +1
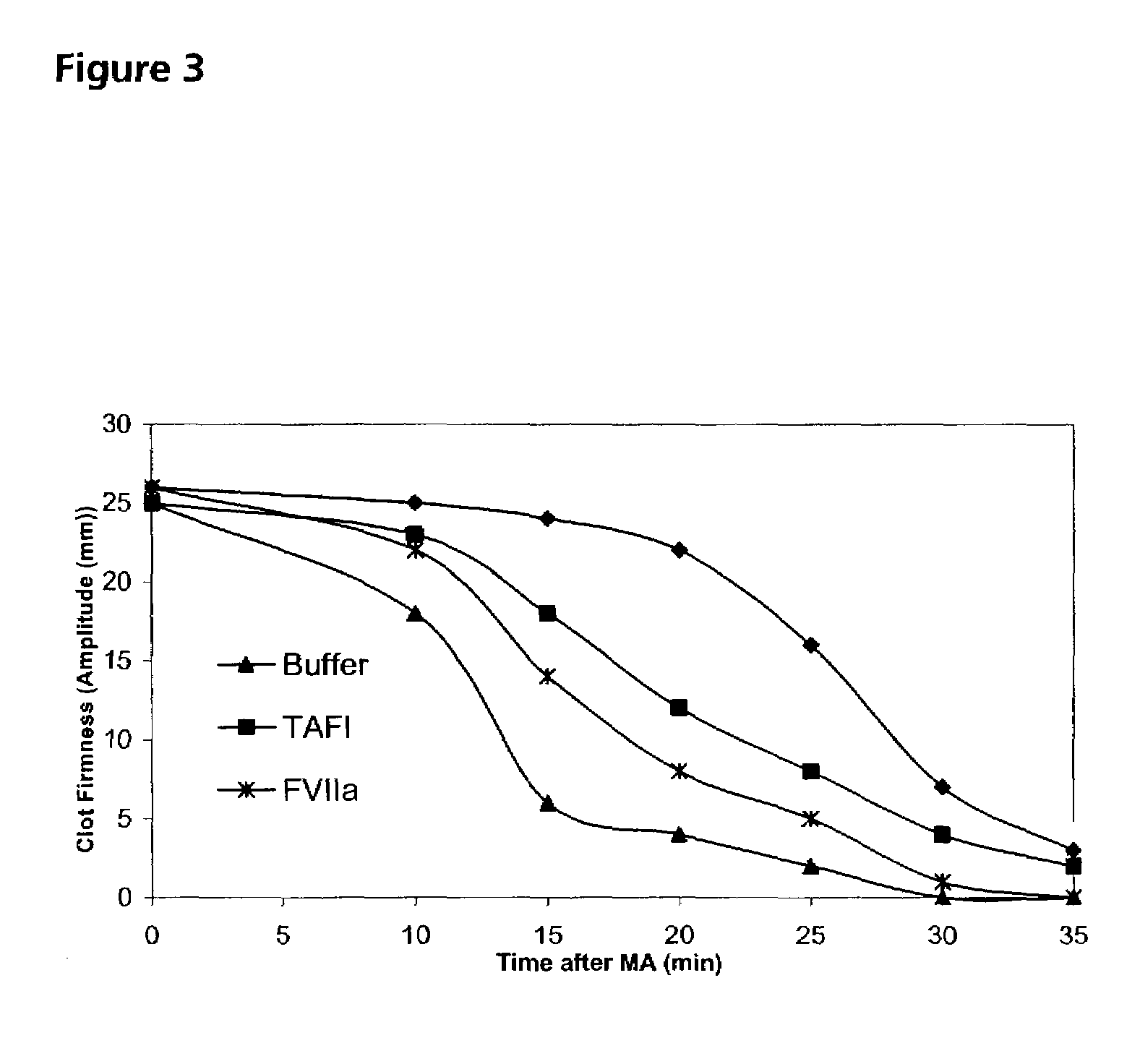
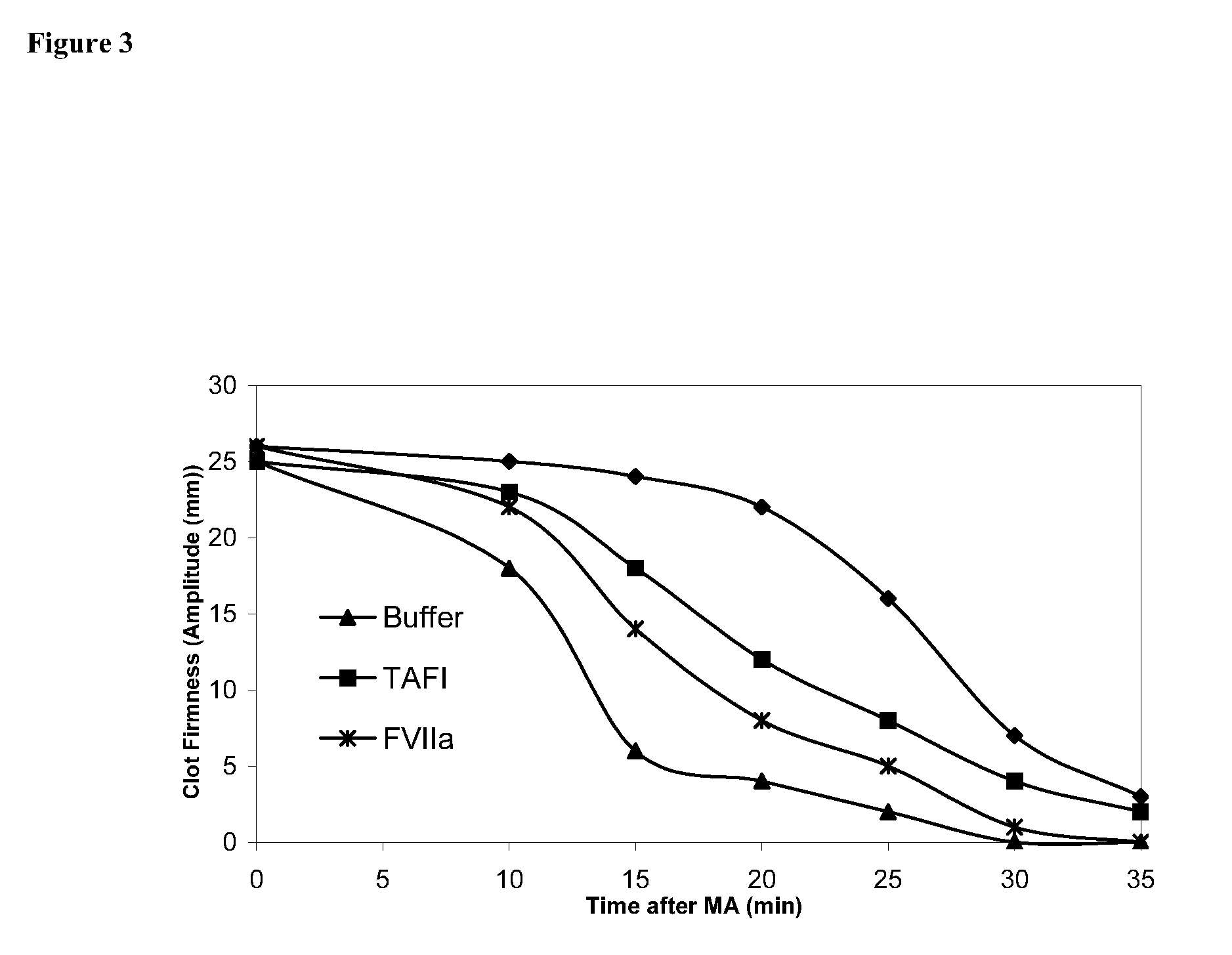
Pharmaceutical composition comprising factor VII polypeptides and TAFI polypeptides
InactiveUS7291587B2Effective treatmentPeptide/protein ingredientsDepsipeptidesPharmaceutical drugBleeding episodes
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide, and TAFI or a TAFI-related polypeptide, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
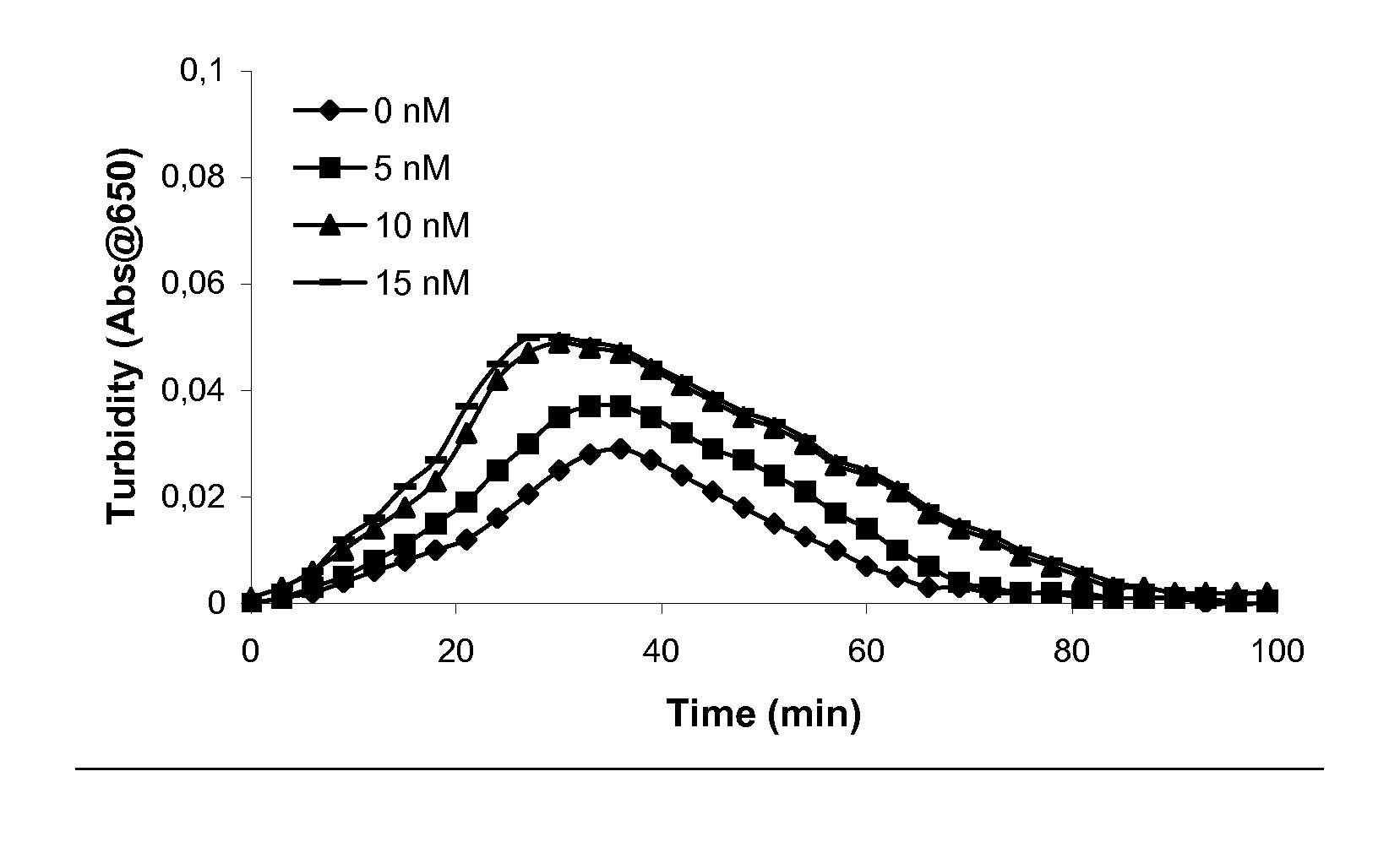
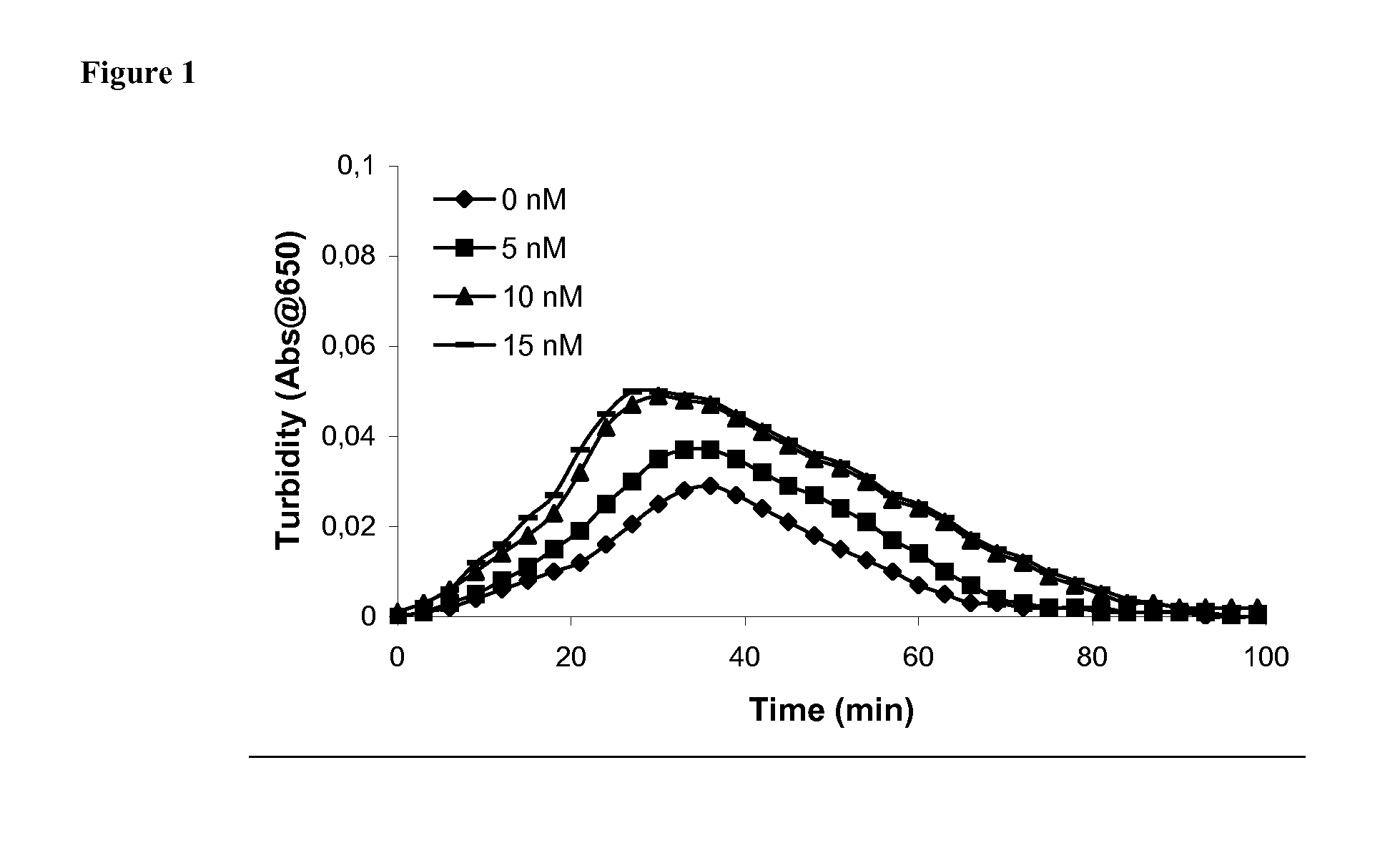
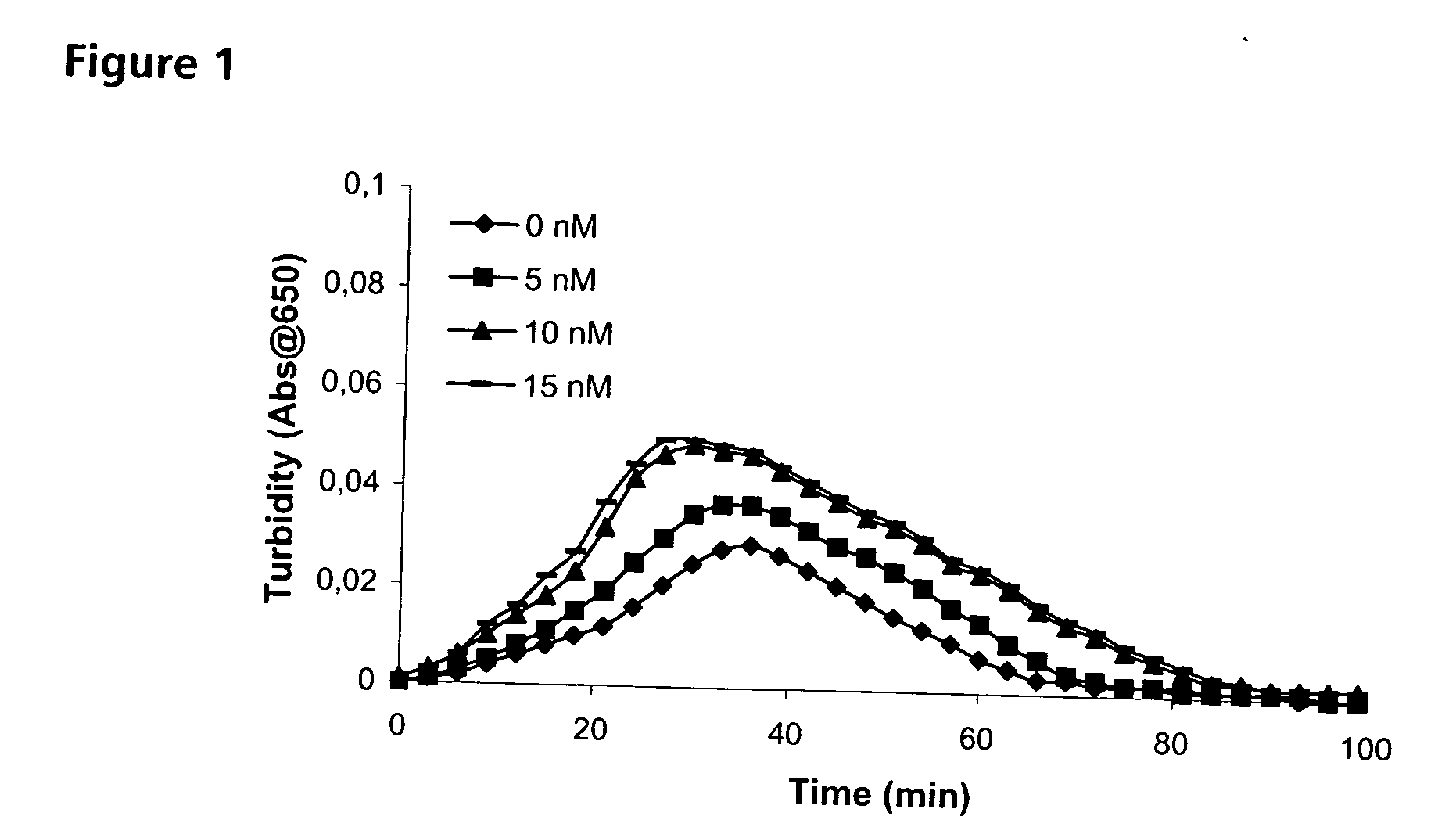
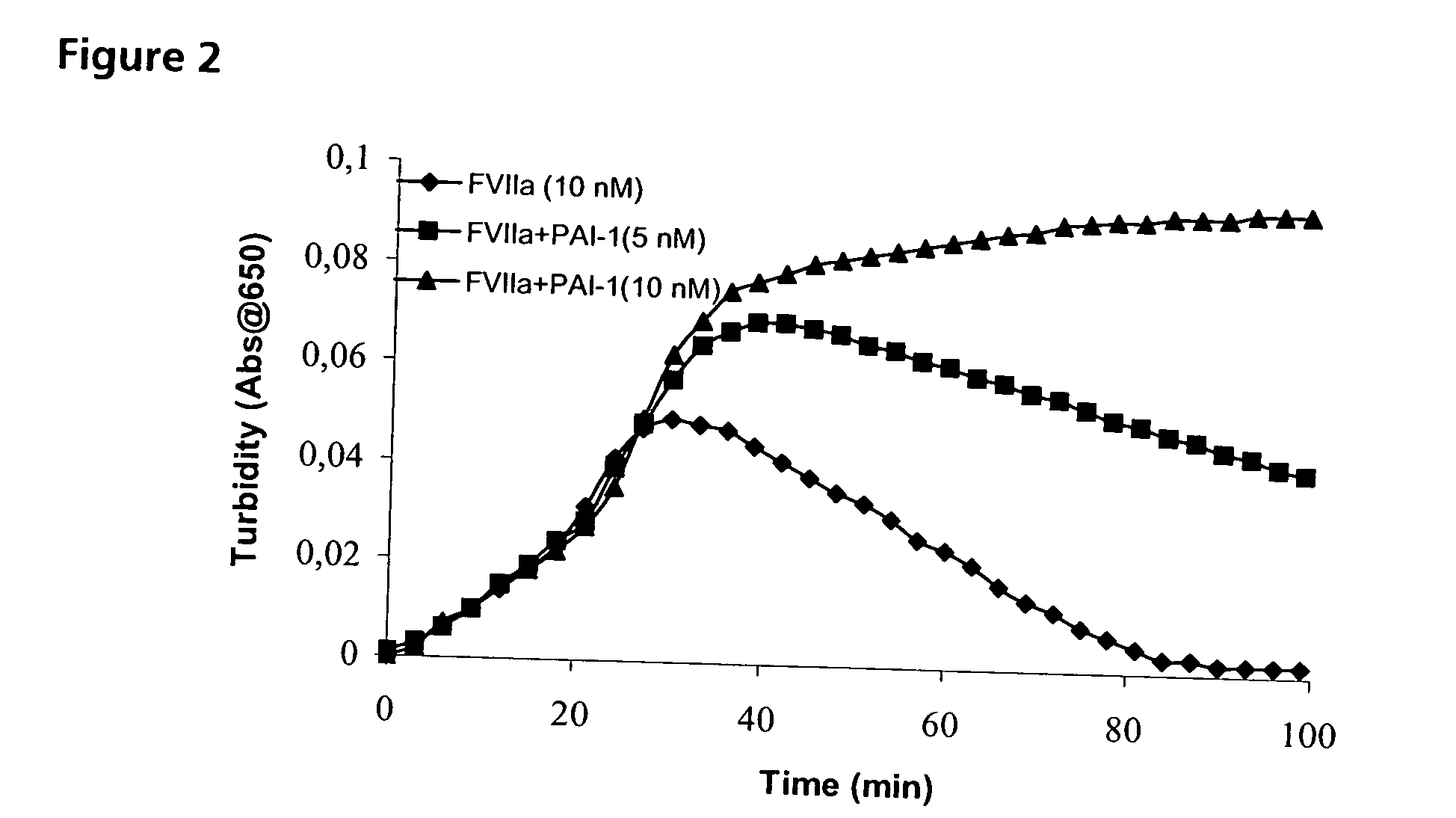
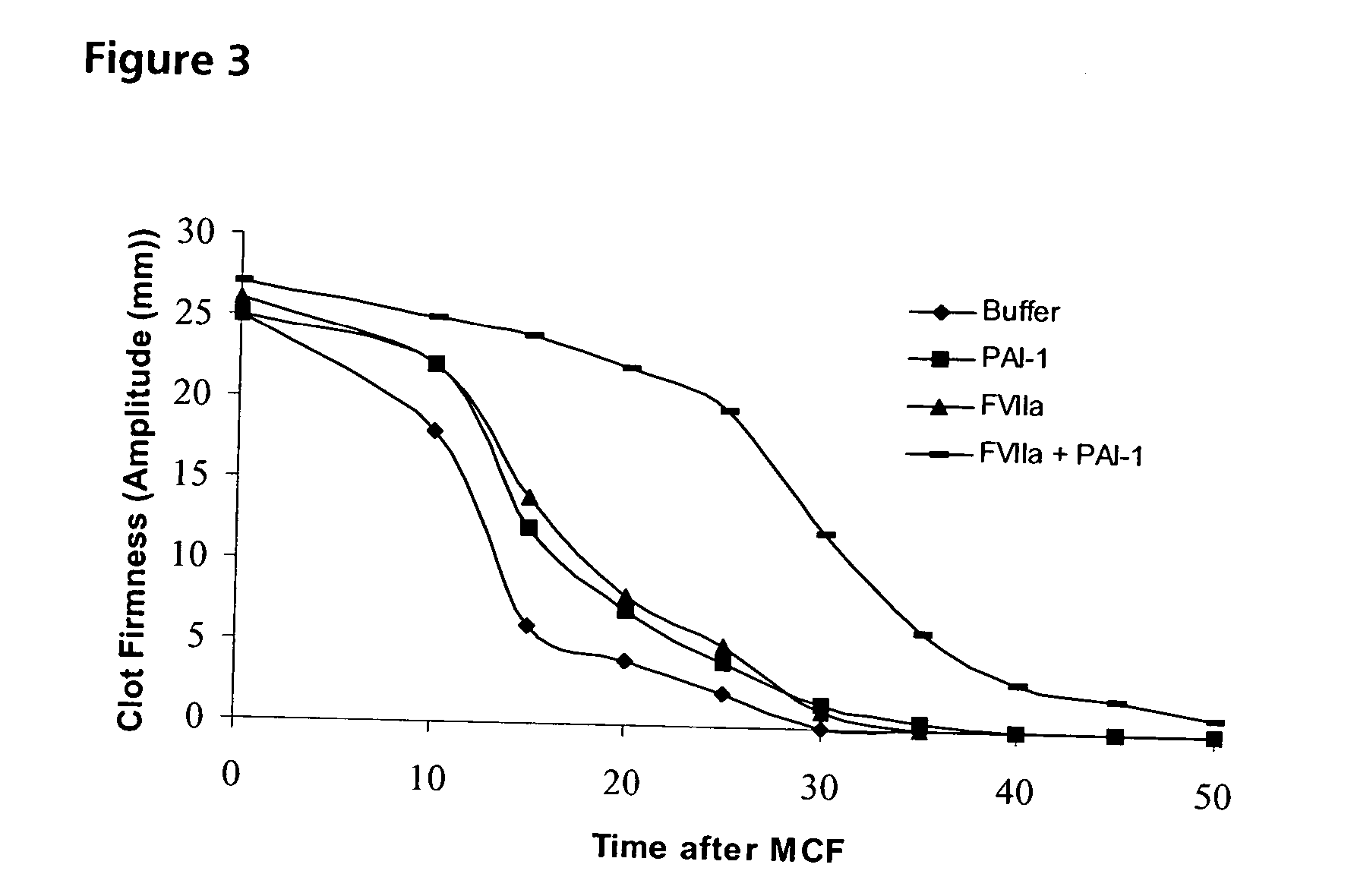
Pharmaceutical Composition Comprising Factor VII Polypeptides and PAI-1 Polypeptide
InactiveUS20070219135A1Effective treatmentPeptide/protein ingredientsDepsipeptidesMedicineBleeding episodes
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide, and PAI-1 or a PAI-1-related polypeptide, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK HEALTH CARE AG
Single-Dose Administration of Factor VIIa
InactiveUS20080261886A1Peptide/protein ingredientsPharmaceutical delivery mechanismFactor VIIaMedicine
The present invention provides methods for preventing and / or treating bleeding episodes by administering a single dose of a Factor VIIa equivalent. Preferably, the single dose comprises between about 150 and about 500 ug / kg Factor VIIa equivalent.
Owner:NOVO NORDISK HEALTH CARE AG
Pharmaceutical composition comprising a factor VIIa and a factor XIII
InactiveUS20060199766A1Shorten clotting timeProlonging clot lysis timeFactor VIIPeptide/protein ingredientsFactor VIIaBleeding episodes
The present invention relates to the use of a factor VIIa and a factor XIII in the treatment or pro-phylaxis of bleeding episodes.
Owner:NOVO NORDISK AS
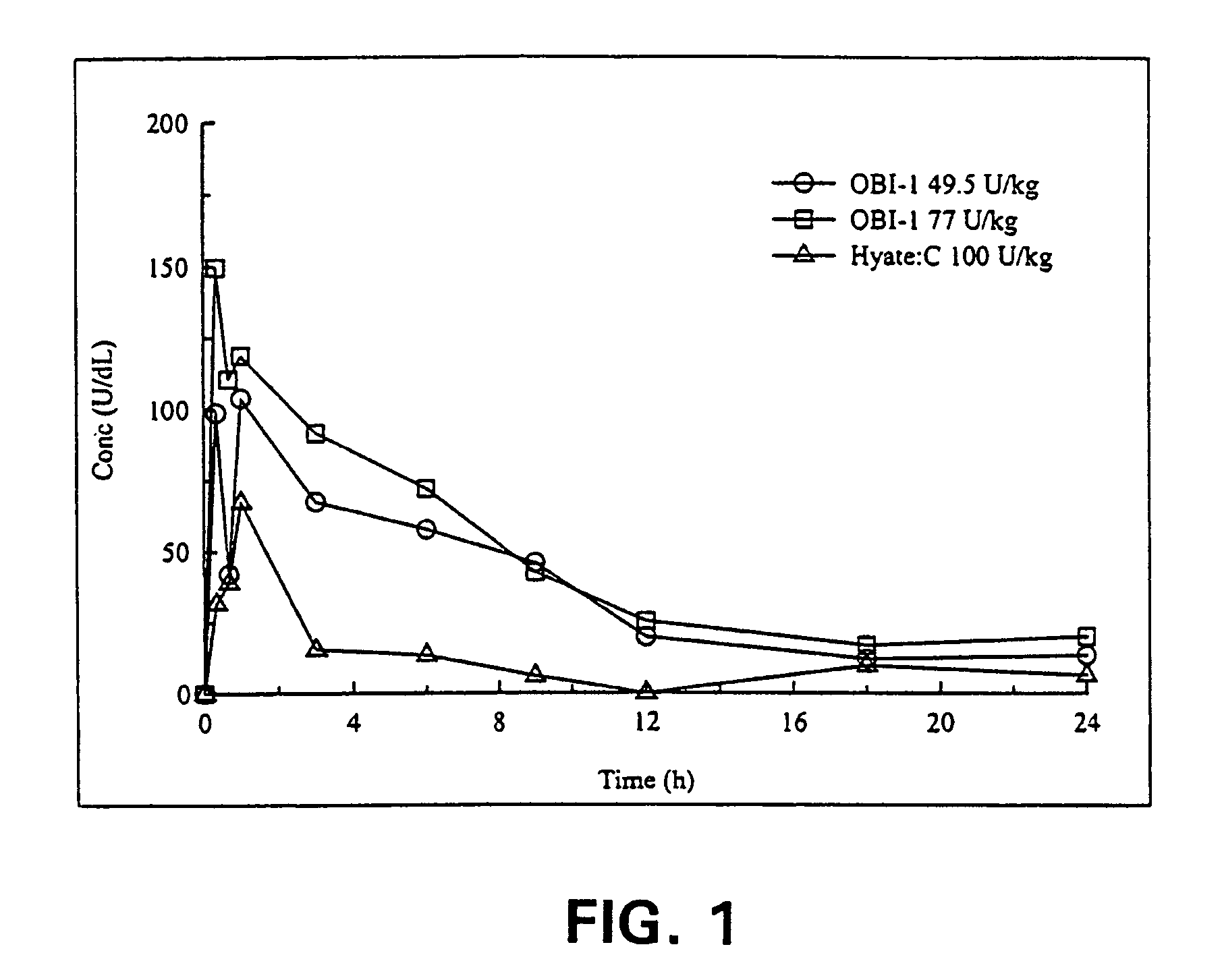
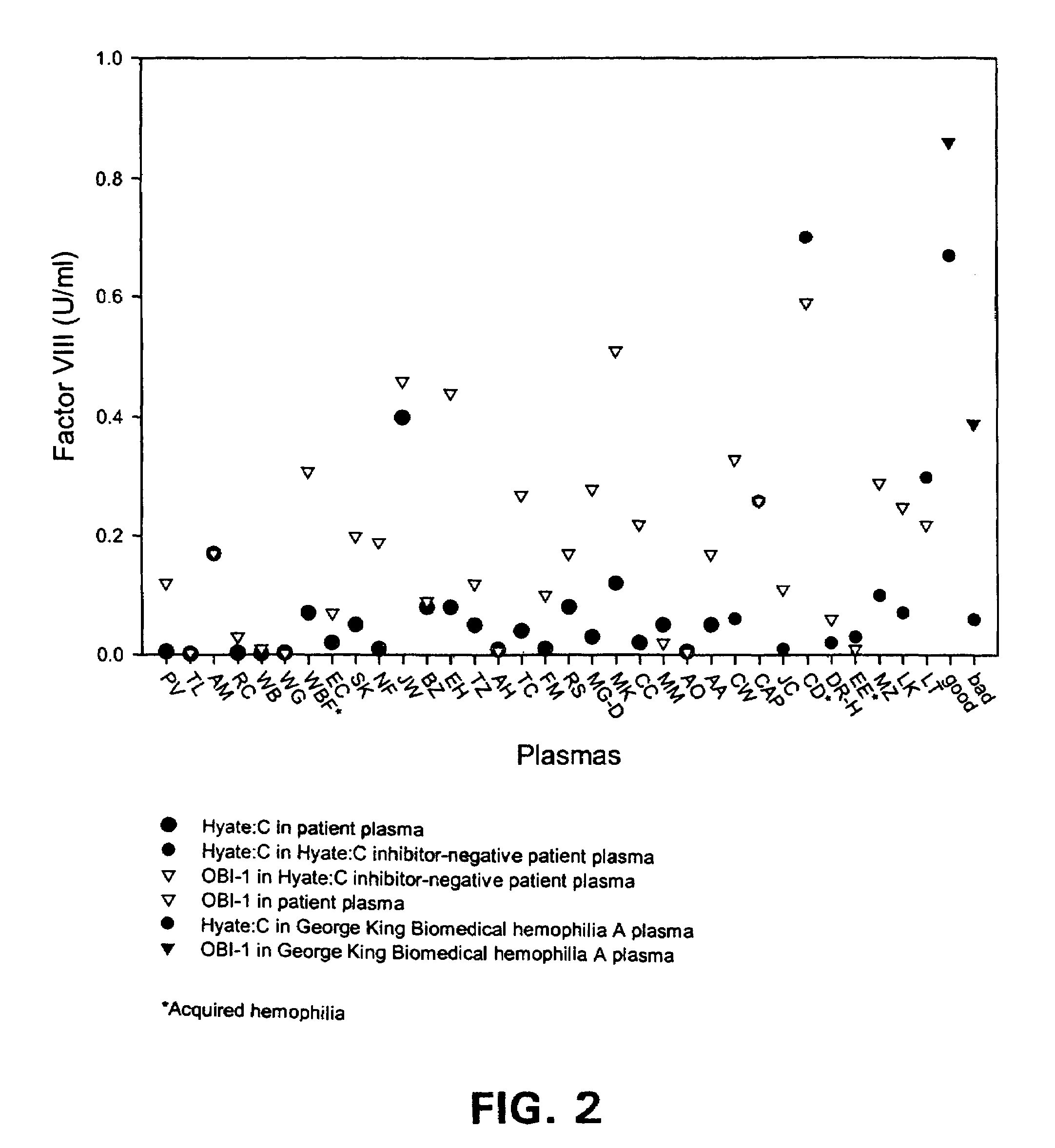
Method of administering porcine B-domainless fVIII
ActiveUS7576181B2Improve bioavailabilityQuick controlFactor VIIPeptide/protein ingredientsFactor VIII deficiencyMedicine
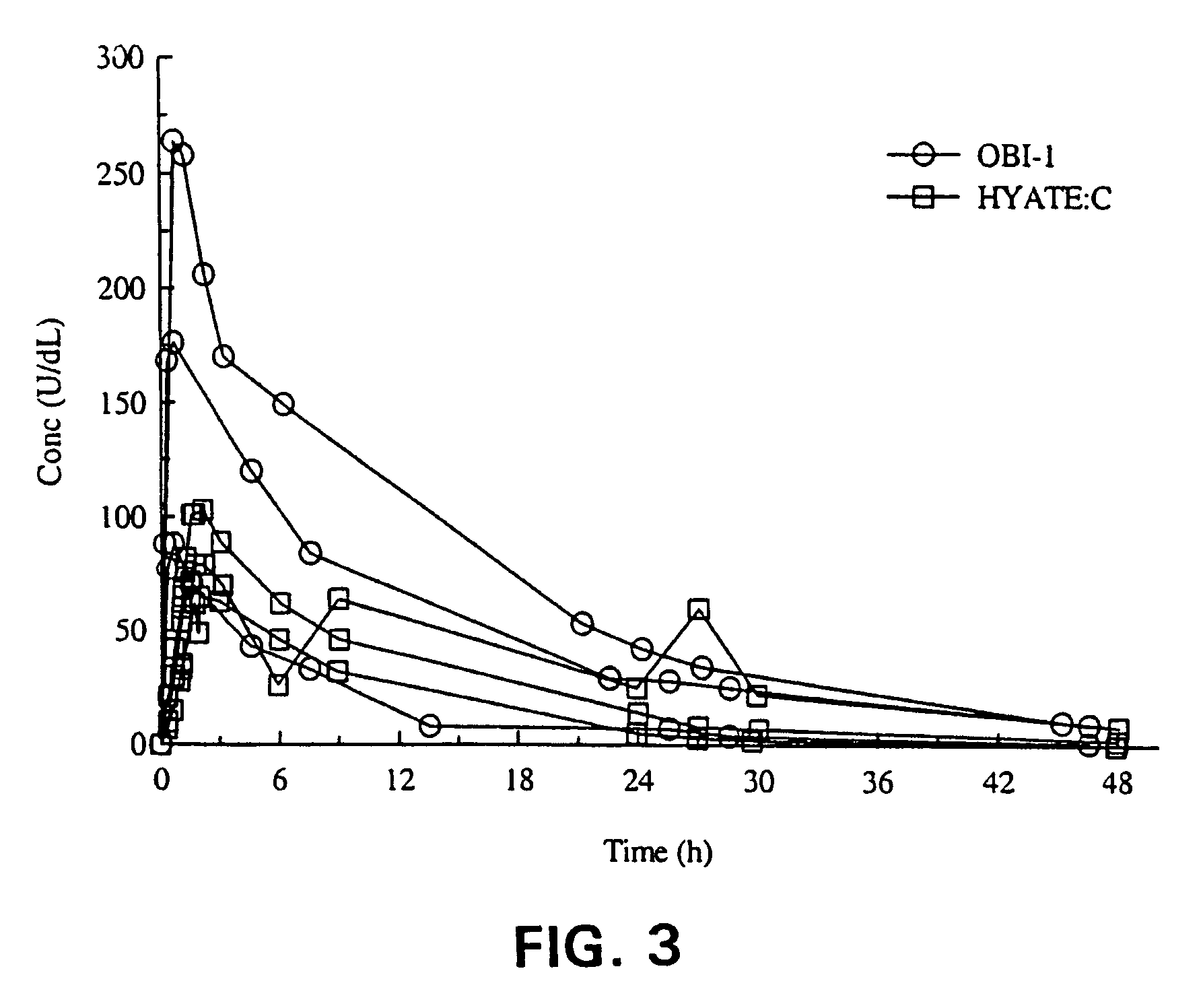
The present invention provides a method of administering porcine B-domainless factor VIII (OBI-1) to a patient having factor VIII deficiency to provide more rapid and effective protection against bleeding episodes, compared to formerly available methods, or to provide more effective protection to such patients during non-bleeding periods. This invention is based on the discovery that the recombinant B-domainless porcine fVIII, termed OBI-1, has greater bioavailability compared to the natural porcine fVIII partially purified from porcine plasma, termed HYATE:C. Therefore, the inventive method employs lower unit doses of OBI-1, including, alternatively, omission of antibody-neutralizing dosage, or has longer intervals between the administration, compared to HYATE:C, to provide equivalent protection in patients having fVIII deficiency. The invention further provides pharmaceutical compositions and kits containing OBI-1 in combination with a pharmaceutically acceptable carrier, that are useful for treating patients in need of fVIII more effectively.
Owner:EMORY UNIVERSITY +1
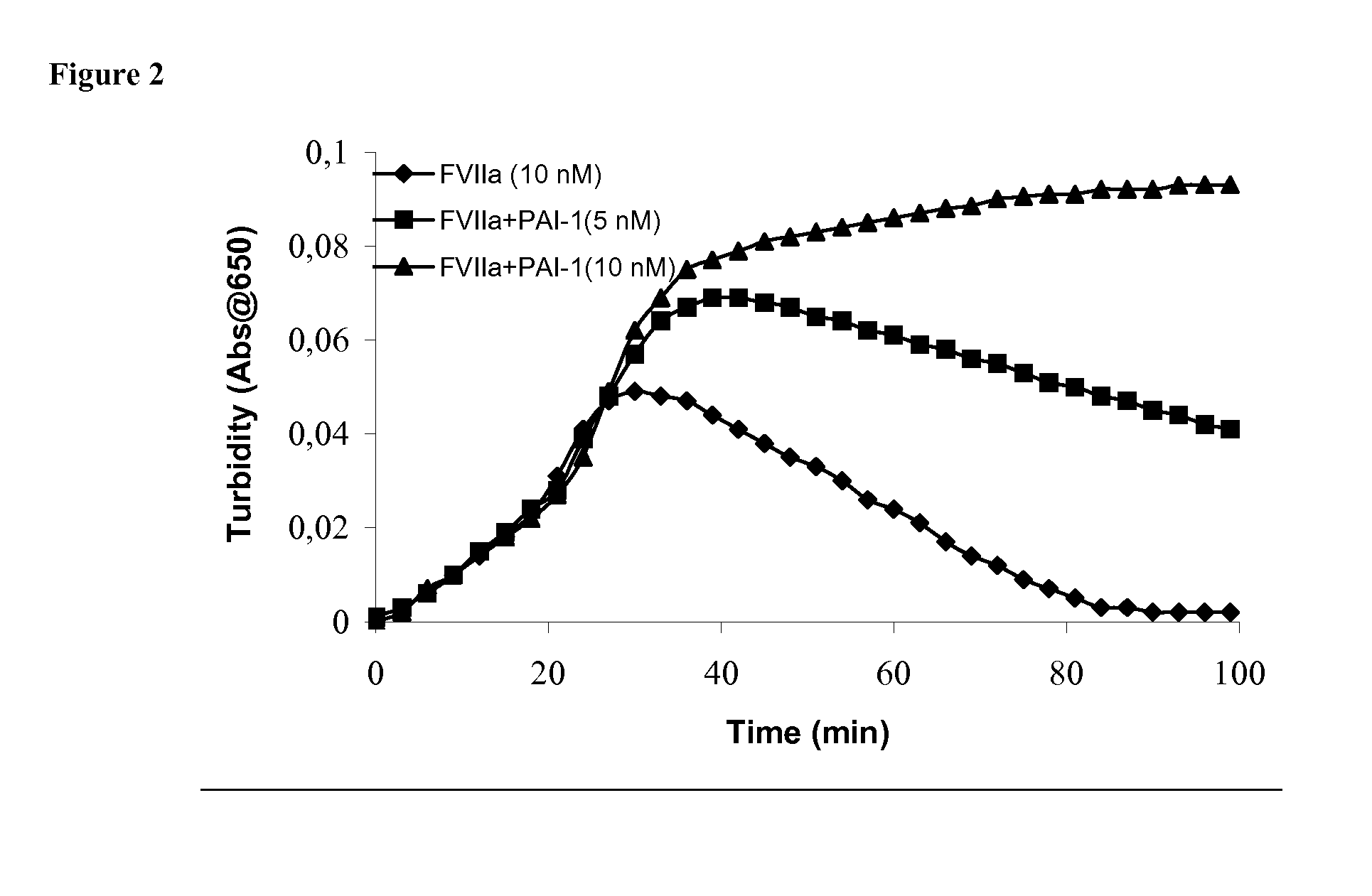
Pharmaceutical composition comprising factor VII polypeptide and PAI-1 polypeptide
InactiveUS20060030531A1Improved and reliable and widely applicableGood coagulationPeptide/protein ingredientsDepsipeptidesMedicineBleeding episodes
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide, and PAI-1 or a PAI-1-related polypeptide, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Pharmaceutical Composition Comprising Factor VII Polypeptides and Protein S Inhibitors
InactiveUS20080057059A1Improved and reliable and widely applicableGood coagulationFactor VIIPeptide/protein ingredientsMedicineProtein S
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide and a protein S inhibitor, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Use of Factor VIIa Analogues with Increased Activity
InactiveUS20090191180A1High activityEffective treatmentPeptide/protein ingredientsAntiinfectivesFactor VIIaPlatelet
The invention relates to methods for treatment of bleeding episodes in a subject with thrombocytopenia.
Owner:NOVO NORDISK AS
Pharmaceutical Composition Comprising Factor VII Polypeptides and Protein C Inhibitors
InactiveUS20080102064A1Effective treatmentPeptide/protein ingredientsSurgical drugsMedicineProtein C inhibitor
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide and a protein C inhibitor, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK HEALTH CARE AG
Therapeutic use of factor XI
The present invention provides methods and compositions for treating bleeding episodes. The methods are carried out by administering to a patient in need thereof a preparation comprising a factor XI polypeptide, in an amount effective for such treatment. The methods of the invention result in one or more of: reduced clotting time; enhancement of hemostasis; increase in clot lysis time; increase in clot strength; and / or increase in overall clot quality (OCQ) in said patient.
Owner:NOVO NORDISK AS
Endometrial sample collector
A non-invasive endometrial sample collector has an outer body of absorbent material configured for insertion into a vaginal cavity of a patient such that a distal end of the body is positioned proximate a uterine cervix of the patient. The collector has an internal collection assembly disposed within the outer body of absorbent material. The internal collection assembly includes a funnel having an opening at the distal end of the outer body configured to face the uterine cervix when the outer body is positioned in the vaginal cavity, and a reservoir in fluid communication with the funnel. During a menstruation cycle of the patient when endometrial tissue cells are shed within menstrual fluid that passes through the uterine cervix, or during any type of normal or abnormal bleeding episode, at least a portion of said fluid is directed to the reservoir via the funnel under the force of gravity.
Owner:INNOVA TECH
Pharmaceutical Composition Comprising Factor VII Polypeptides And Thrombomodulin Polypeptides
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide and thrombomodulin or a thrombomodulin-related polypeptide, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK AS
Pharmaceutical Composition Comprising Factor VII Polypeptides and TAFI Polypeptides
InactiveUS20080069810A1Effective treatmentFactor VIIPeptide/protein ingredientsMedicineBleeding episodes
The present invention relates to a composition comprising factor VII or a factor VII-related polypeptide, and TAFI or a TAFI-related polypeptide, and the use thereof for treating bleeding episodes.
Owner:NOVO NORDISK HEALTH CARE AG
Single-dose administration of Factor VIIa
InactiveUS9029324B2Peptide/protein ingredientsPharmaceutical delivery mechanismFactor VIIaBleeding episodes
The present invention provides methods for preventing and / or treating bleeding episodes by administering a single dose of a Factor VIIa equivalent. Preferably, the single dose comprises between about 150 and about 500 ug / kg Factor VIIa equivalent.
Owner:NOVO NORDISK HEALTH CARE AG
Pharmaceutical composition comprising a factor VII polypeptide and epsilon-aminocaproic acid
InactiveUS20060293241A1Effective treatmentBiocideOrganic active ingredientsEpsilon-Aminocaproic AcidBleeding episodes
The present invention relates to compositions or kits comprising factor VII or a factor VII-related polypeptide and epsilon-aminocaproic acid, and the use thereof for the treatment of bleeding episodes or enhancement of hemostasis.
Owner:NOVO NORDISK AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com