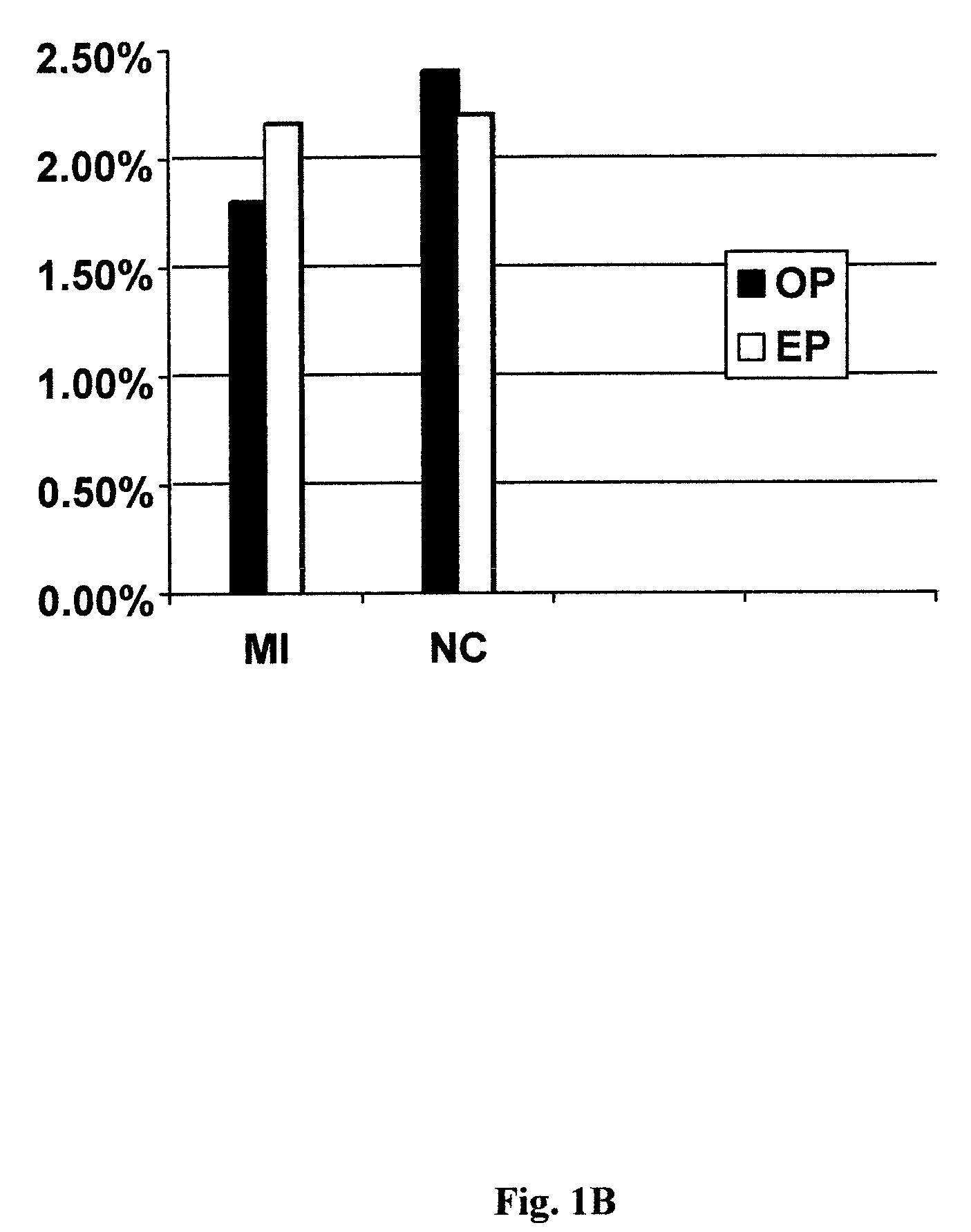
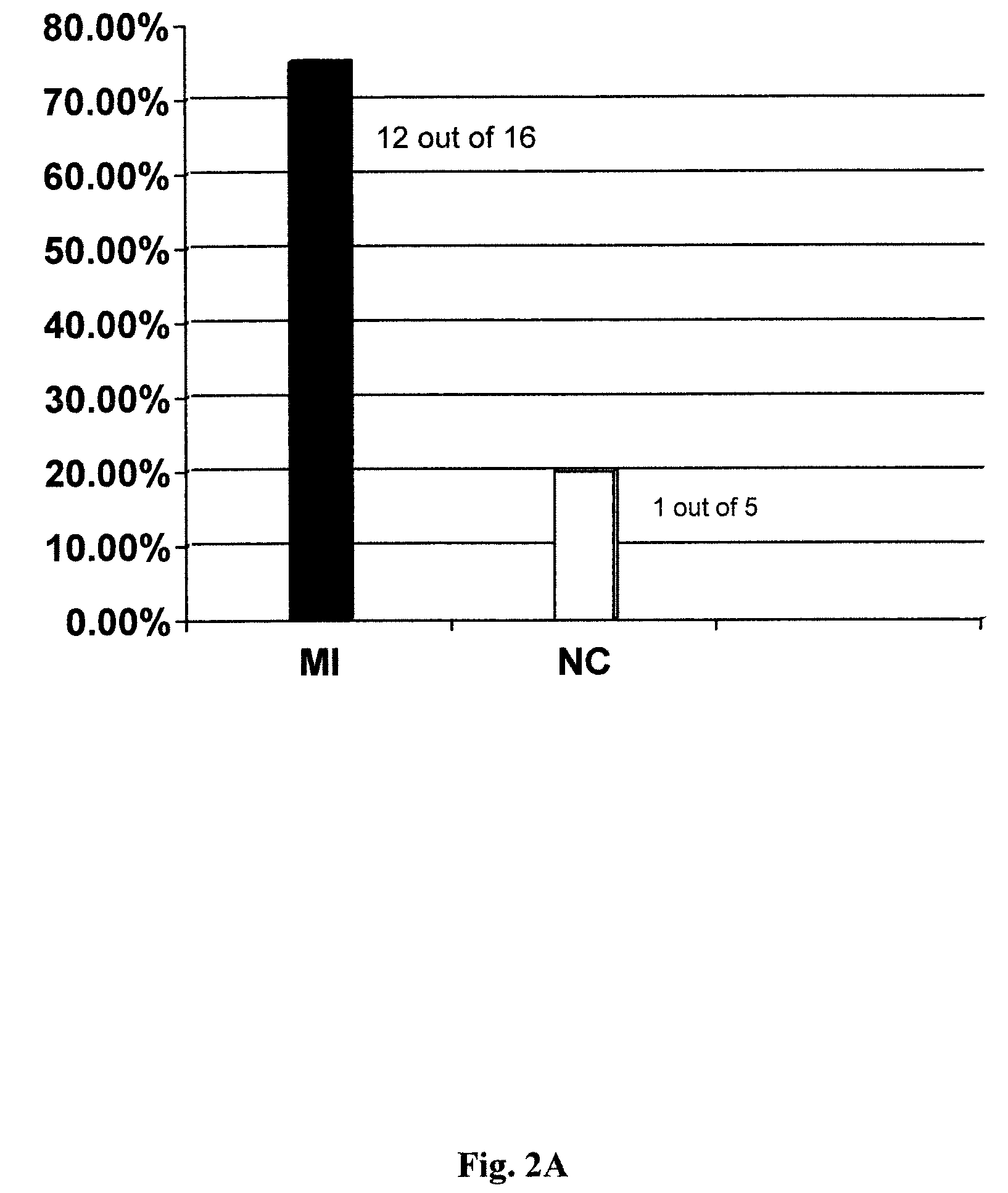
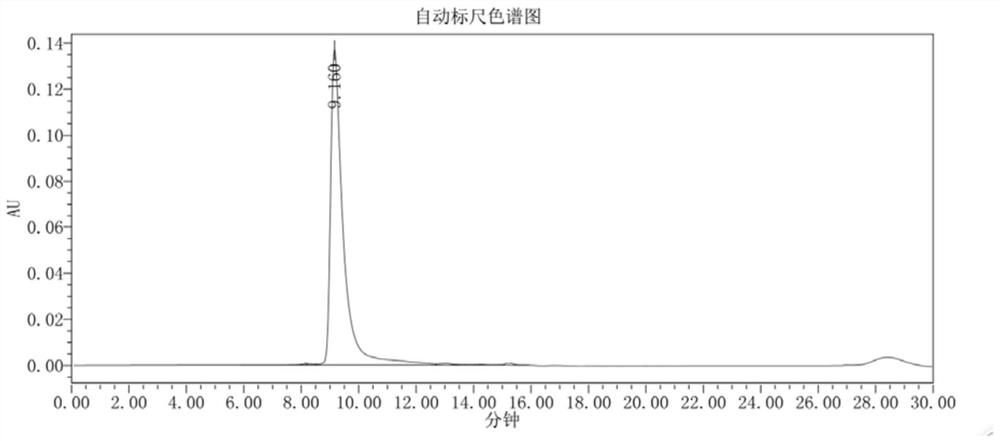
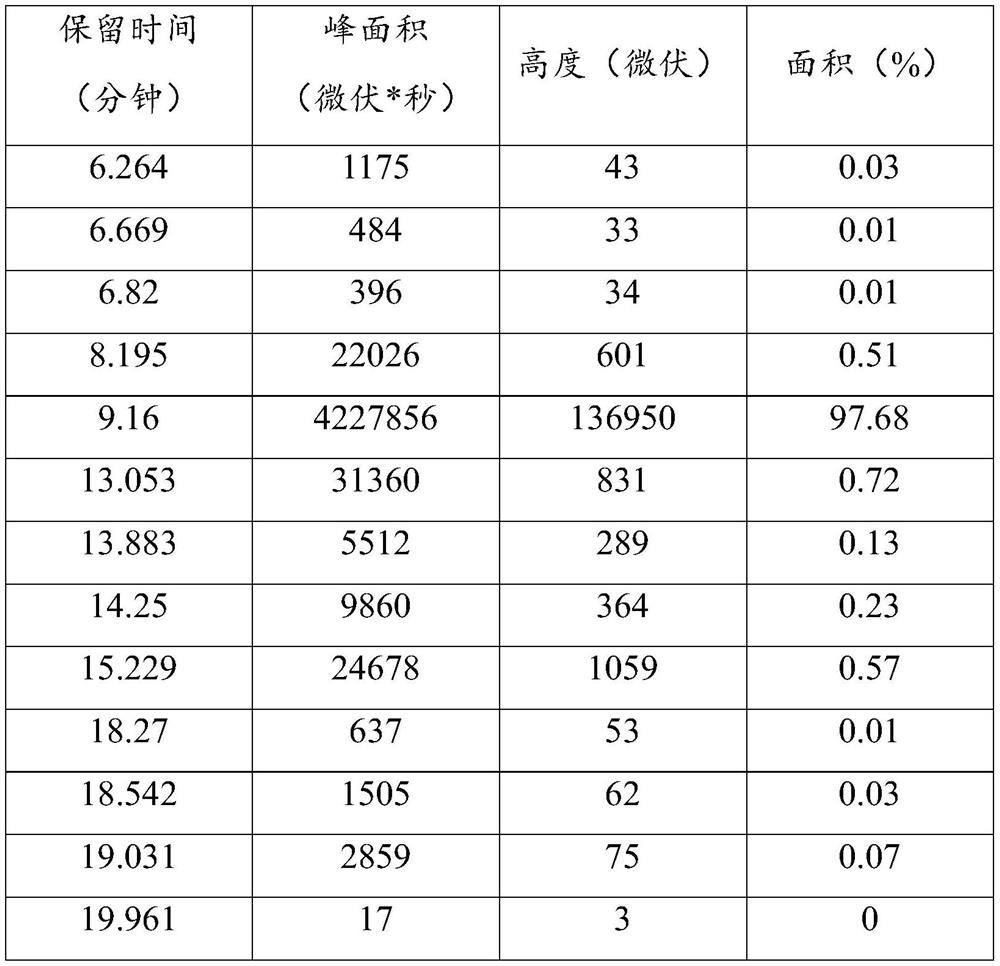
Patents
Literature
32 results about "Factor XIII" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Factor XIII or fibrin stabilizing factor is an enzyme (EC 2.3.2.13) of the blood coagulation system that crosslinks fibrin. Deficiency of this factor (FXIIID) affects clot stability.
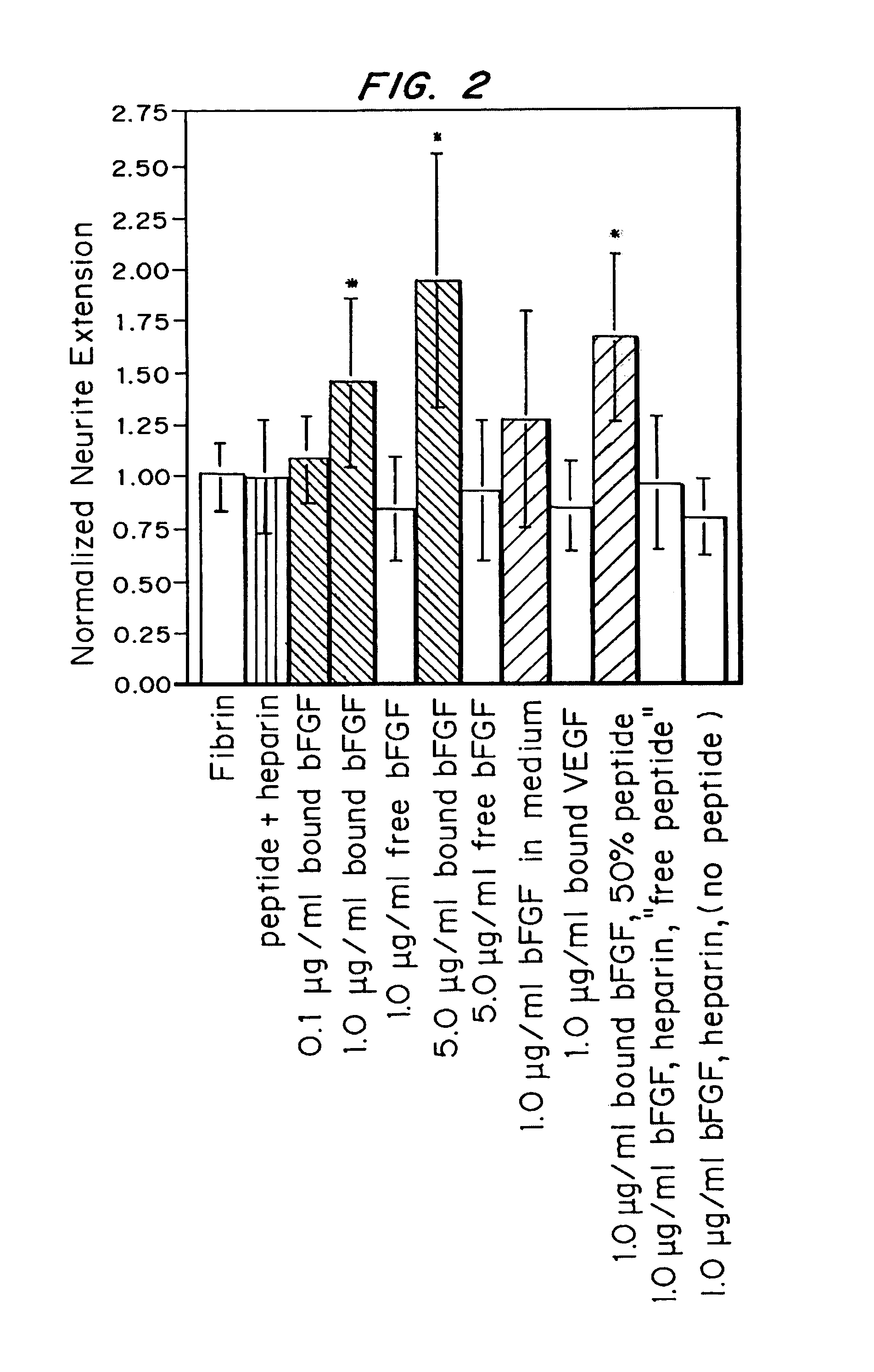
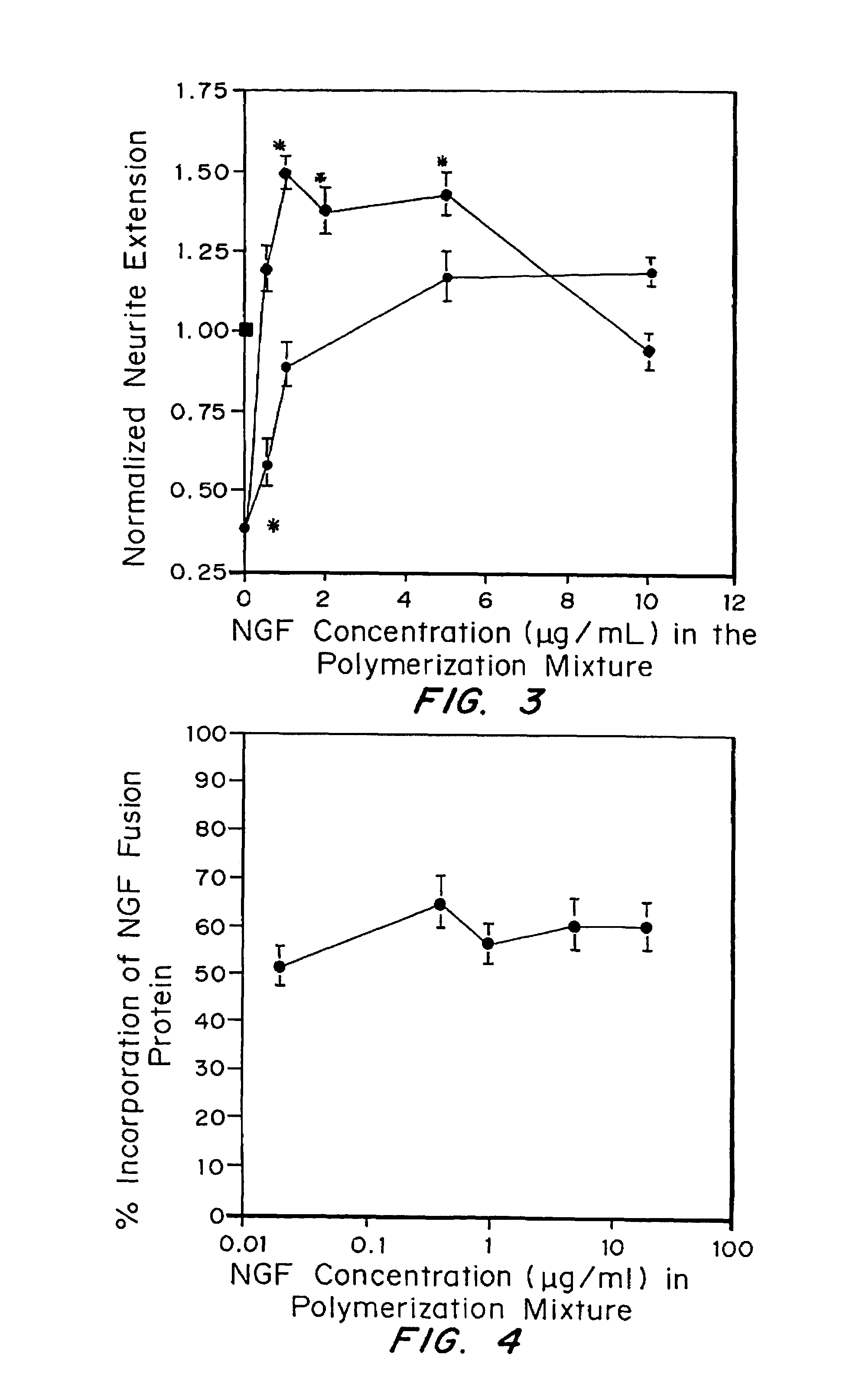
Growth factor modified protein matrices for tissue engineering
InactiveUS7247609B2Reduce drug doseGreat percentagePeptide/protein ingredientsAntibody mimetics/scaffoldsTissue repairDrug release
Proteins are incorporated into protein or polysaccharide matrices for use in tissue repair, regeneration and / or remodeling and / or drug delivery. The proteins can be incorporated so that they are released by degradation of the matrix, by enzymatic action and / or diffusion. As demonstrated by the examples, one method is to bind heparin to the matrix by either covalent or non-covalent methods, to form a heparin-matrix. The heparin then non-covalently binds heparin-binding growth factors to the protein matrix. Alternatively, a fusion protein can be constructed which contains a crosslinking region such as a factor XIIIa substrate and the native protein sequence. Incorporation of degradable linkages between the matrix and the bioactive factors can be particularly useful when long-term drug delivery is desired, for example in the case of nerve regeneration, where it is desirable to vary the rate of drug release spatially as a function of regeneration, e.g. rapidly near the living tissue interface and more slowly farther into the injury zone. Additional benefits include the lower total drug dose within the delivery system, and spatial regulation of release which permits a greater percentage of the drug to be released at the time of greatest cellular activity.
Owner:ETH ZZURICH +1
Method for the detection of risk factors associated with myocardial infarction
A method for determining whether an individual is at an increased risk for myocardial infarction, comprising screening for the presence of Factor II and Factor XIII alleles associated with myocardial infarction. Also provided are kits and primers that specifically hybridize adjacent to the allele-specific regions of the Factor II and Factor XIII genes.
Owner:GENESIS GROUP
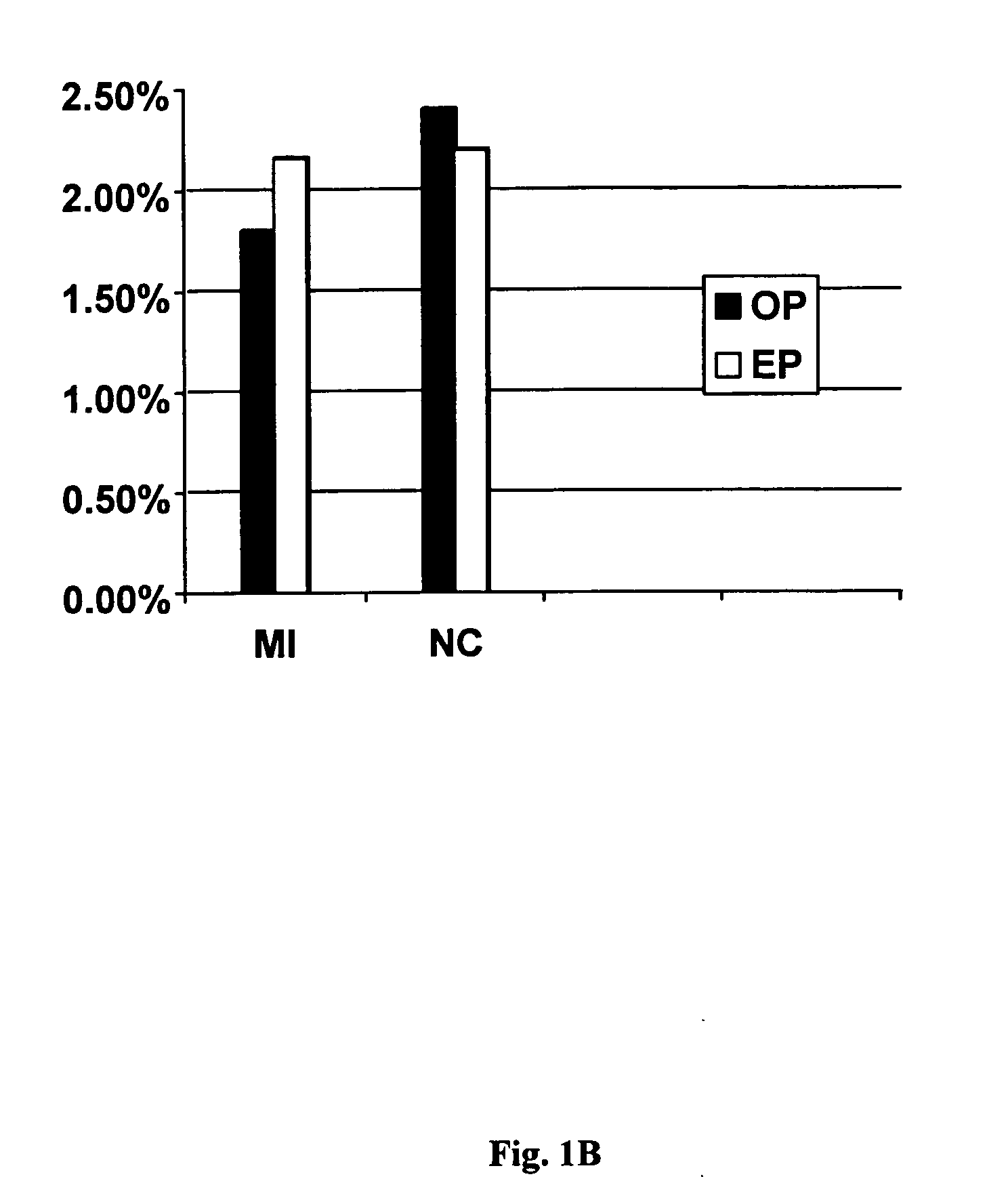
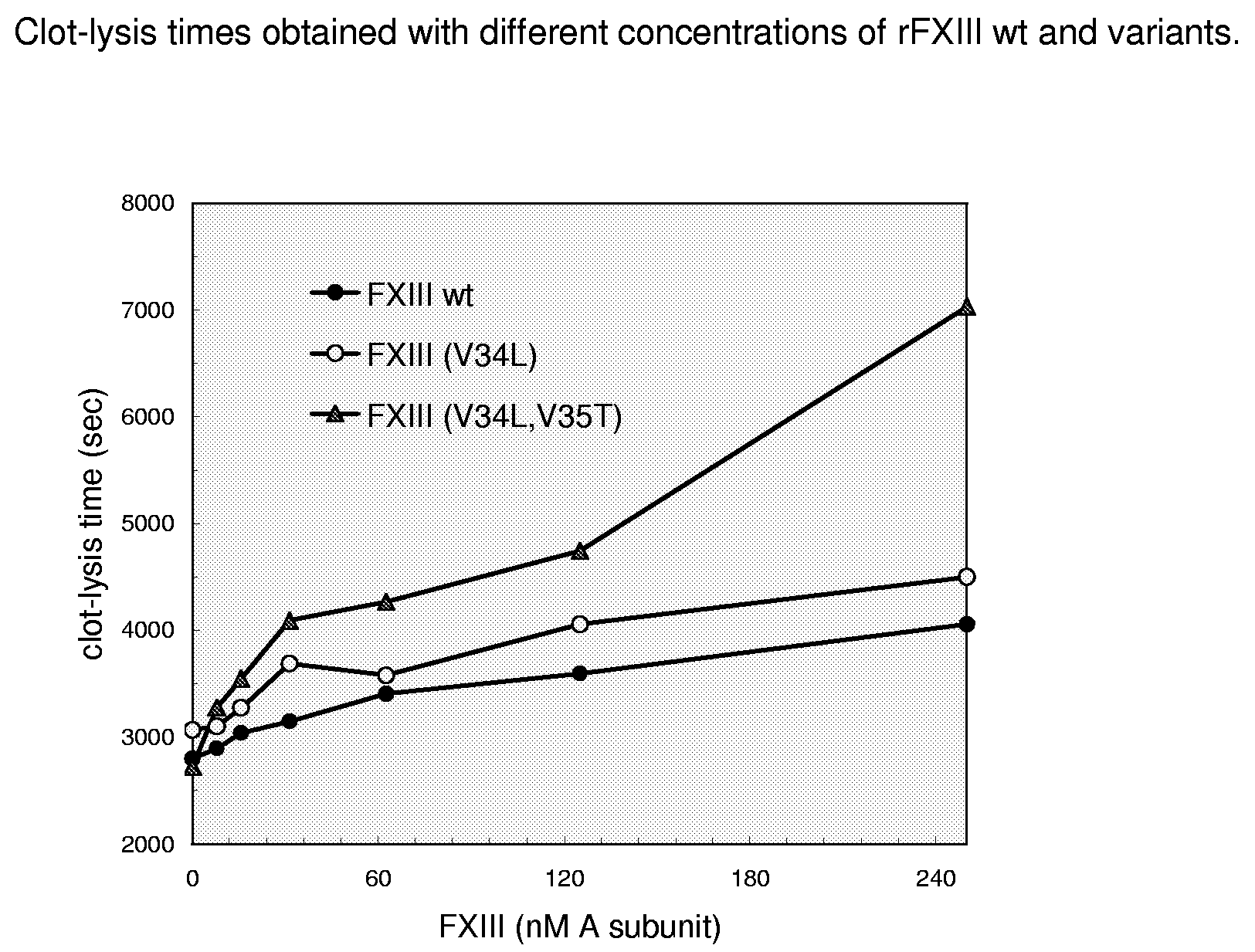
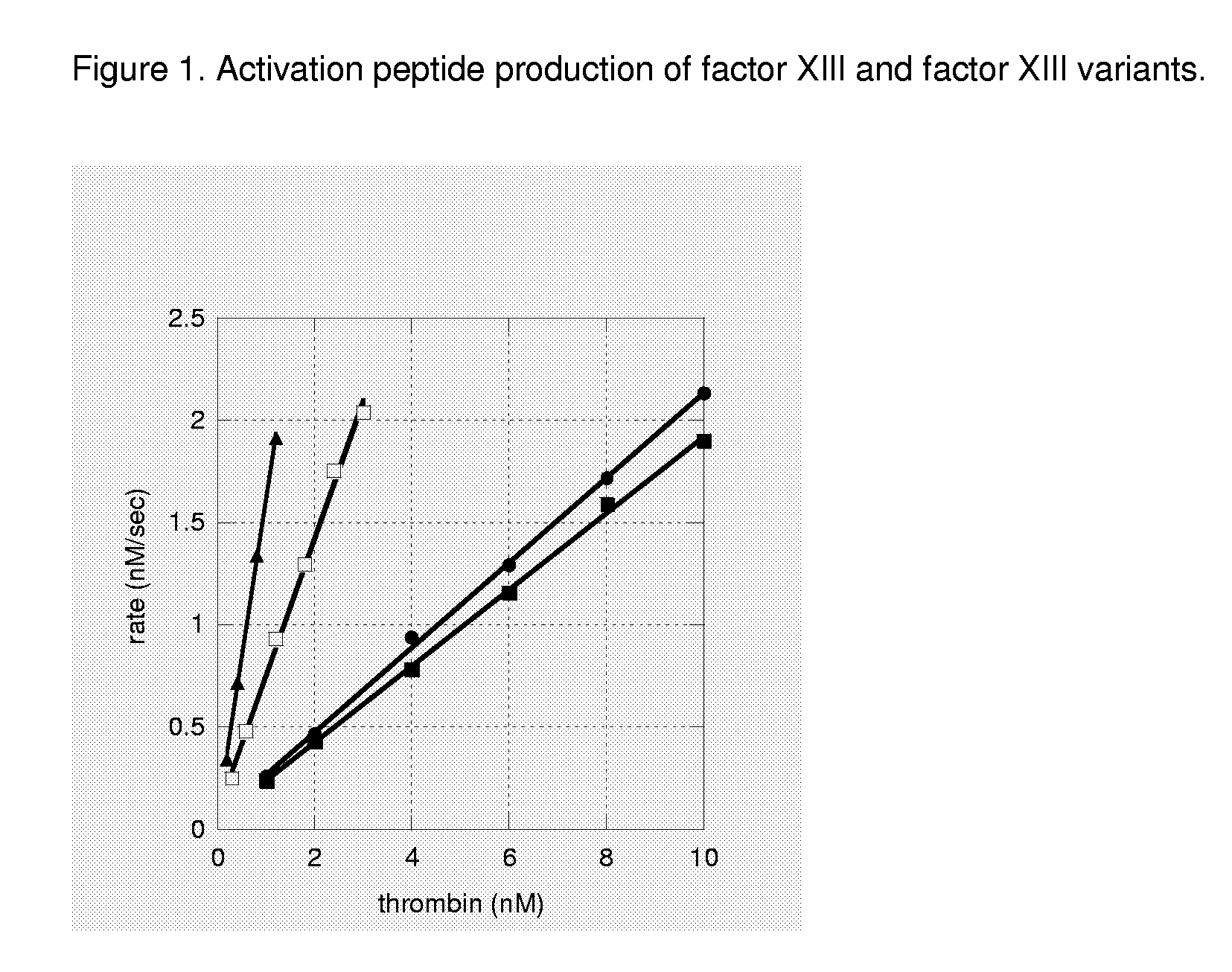
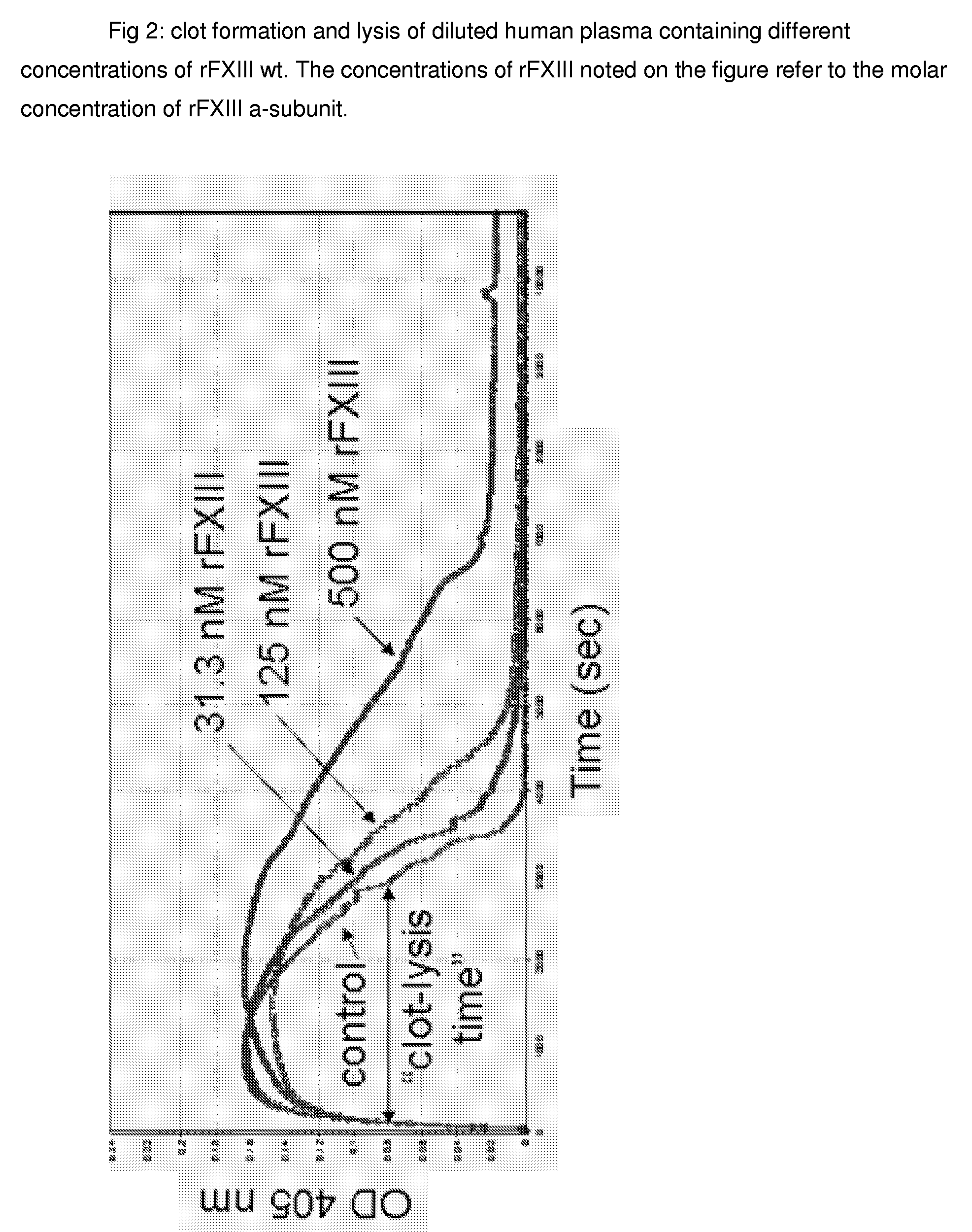
FXIII Variants with Improved Properties
The present invention concerns variant factor XIII, wherein the rate of activation of said variant by thrombin is faster than for wild type FXIII. Methods for enhancing fibrin clot formation, pharmaceutical compositions and the use for the manufacture of medicaments wherein the variant factor XIII is applied are disclosed.
Owner:NOVO NORDISK HEALTH CARE AG
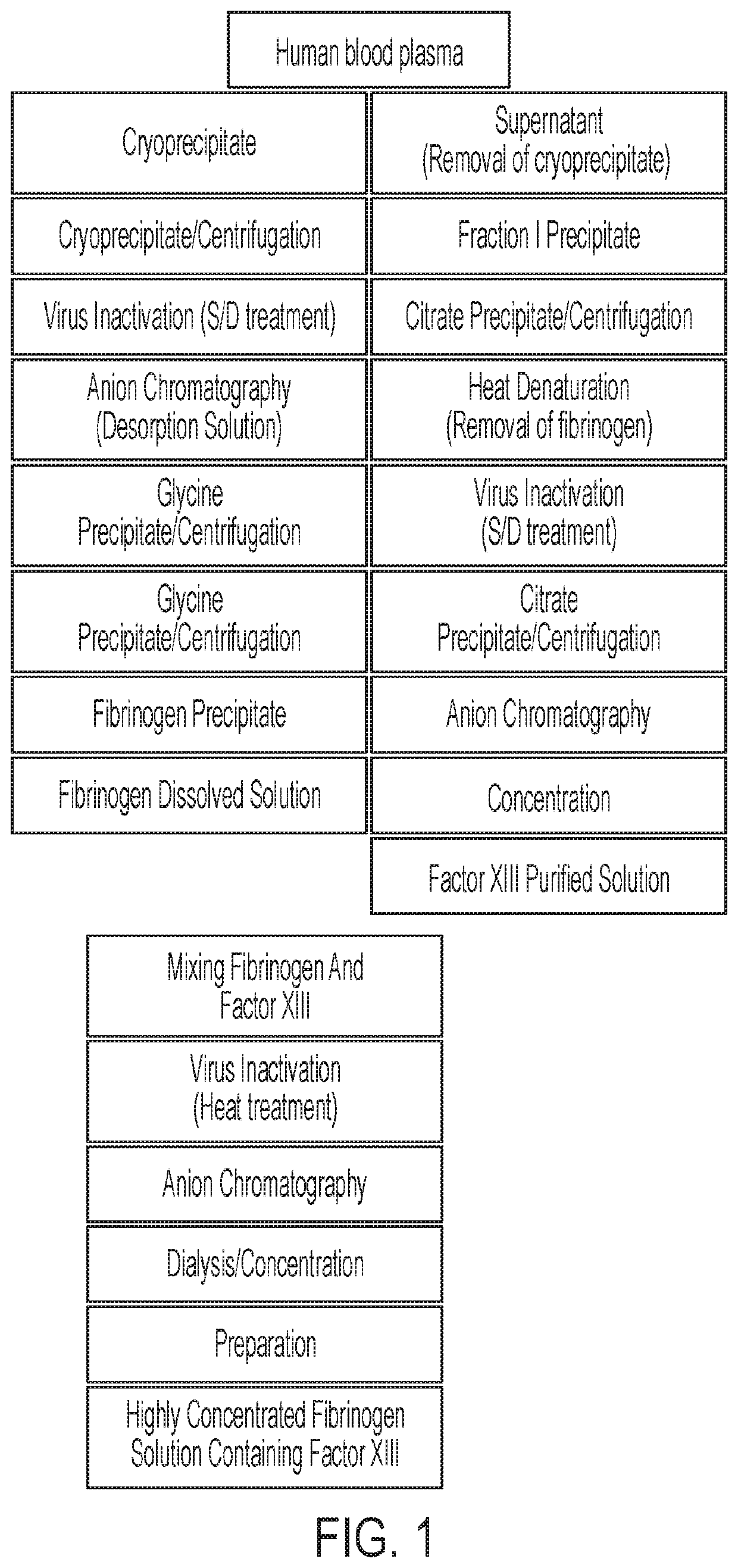
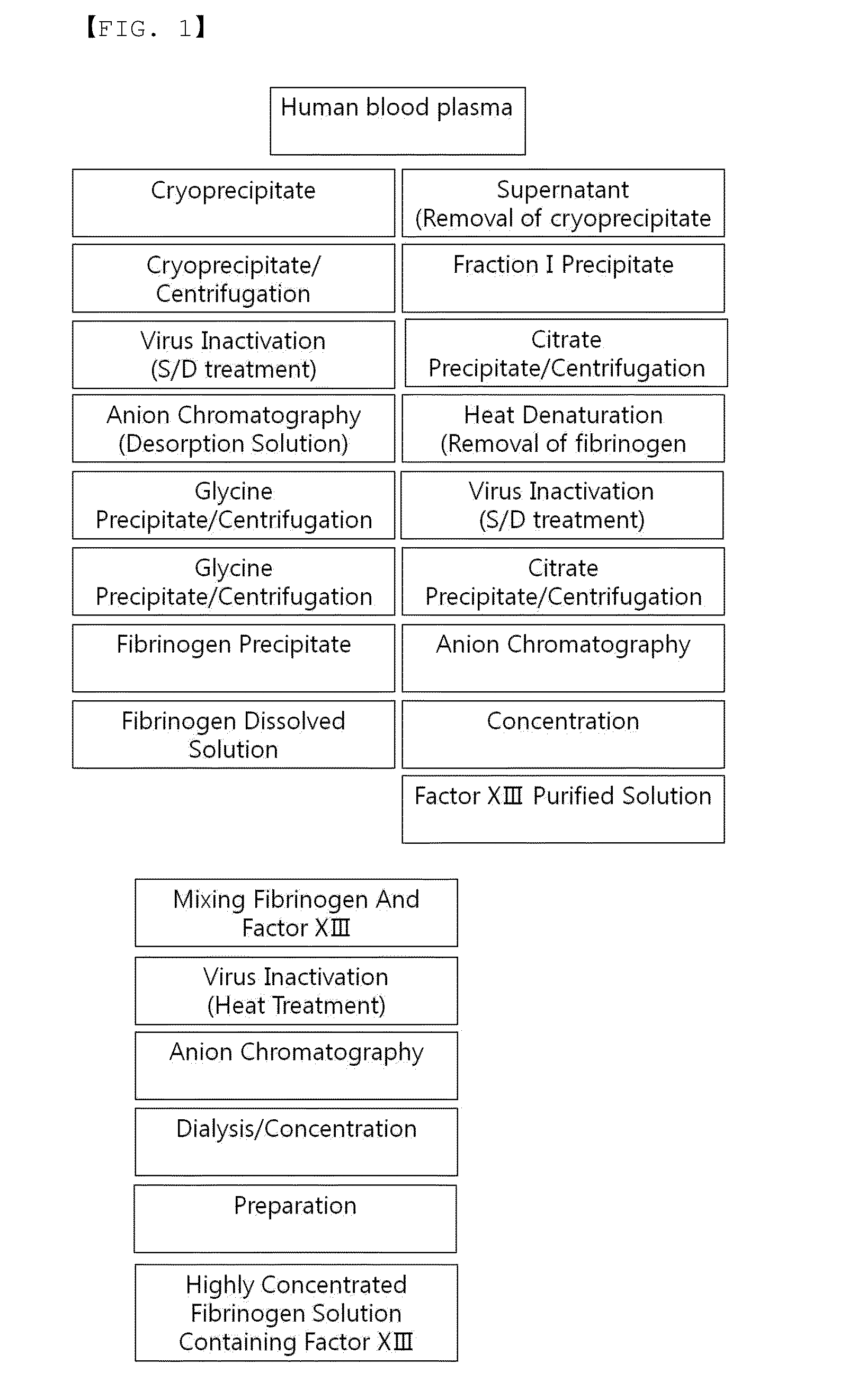
Process for separating proteins fibrinogen, factor XIII and biological glue from a solubilized plasma fraction and for preparing lyophilised concentrates of said proteins
ActiveUS20080207878A1Simple and rapid and low cost processEasy to optimizeFibrinogenPeptide/protein ingredientsFreeze-dryingBlood plasma
Process for separating proteins fibrinogen, Factor XIII and biological glue from a solubilized plasma fraction and for preparing lyophilised concentrates of said proteinsSummaryThe invention is related to a process for separating proteins fibrinogen, Factor XIII and biological glue from a solubilized plasma fraction and for preparing freeze-dried concentrates of said proteins comprising the steps of:chromatographic purification comprising the steps of loading an anion exchanger of weak base type with the said solubilized fraction, previously equilibrated with a buffer of a predetermined ionic strength of an alkaline pH, which allows to retain the biological glue, elution of the biological glue by increasing the ionic strength of the said buffer, andseparation of FXIII from fibrinogen by addition to at least one part of the biological glue eluate of at least one chemical agent precipitating the FXIII, and recovery of the resulting purified fibrinogen containing supernatant solution, anddiafiltration of the fibrinogen, biological glue and resolubilized FXIII solutions, followed by a freeze-drying of said solutions. It is also related to freeze-dried concentrates of the said proteins capable to be obtained by carrying out the process.
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
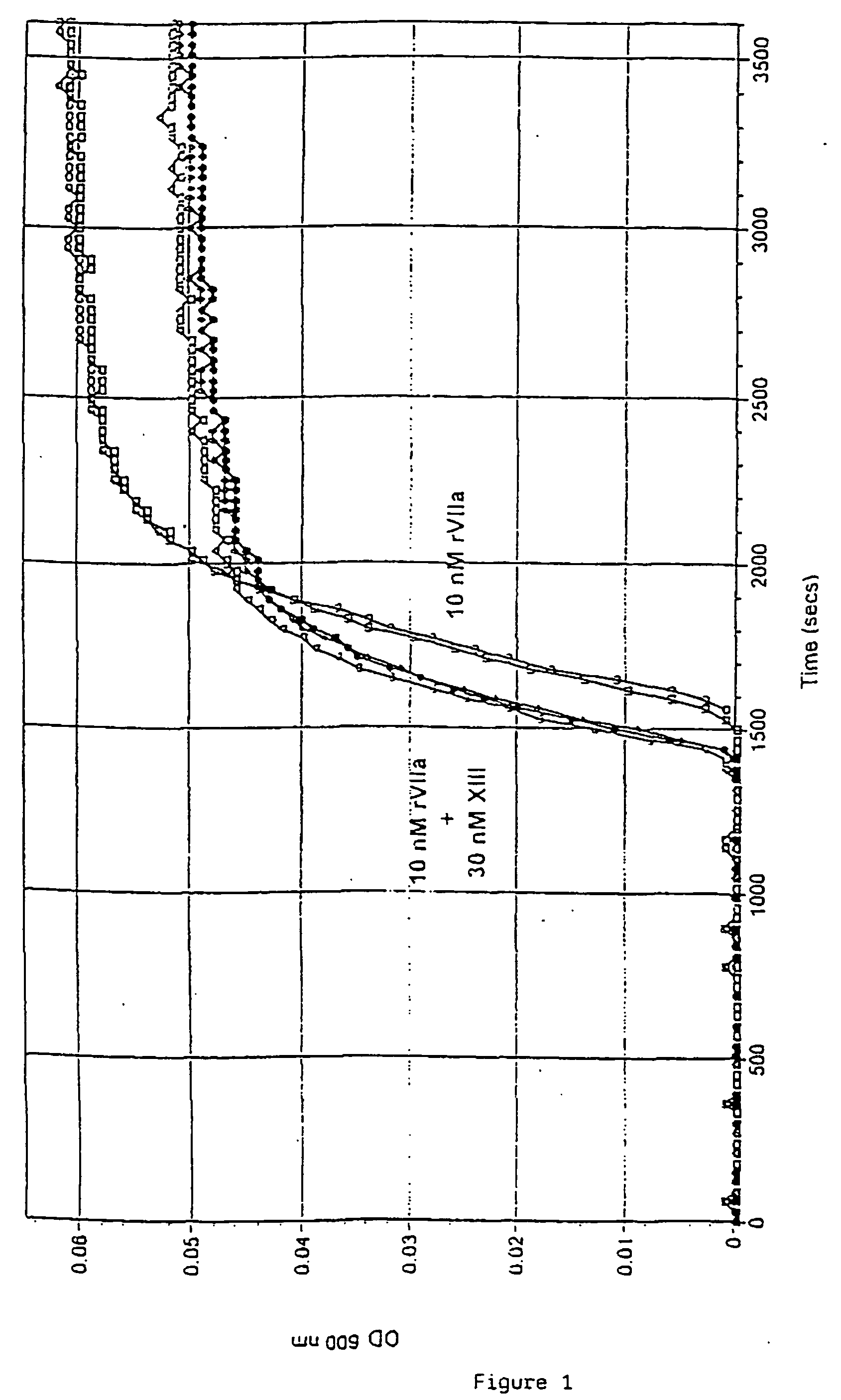
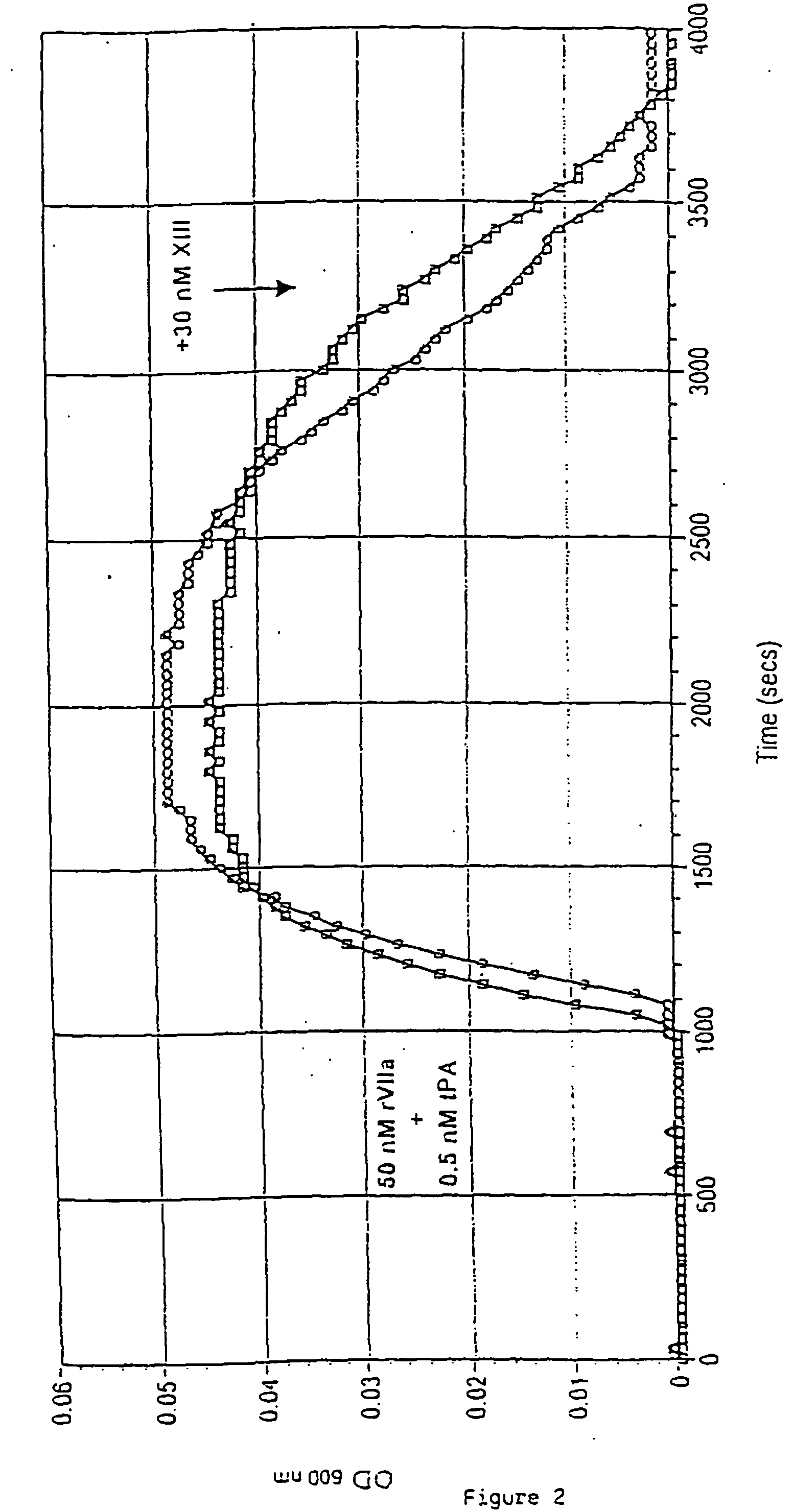
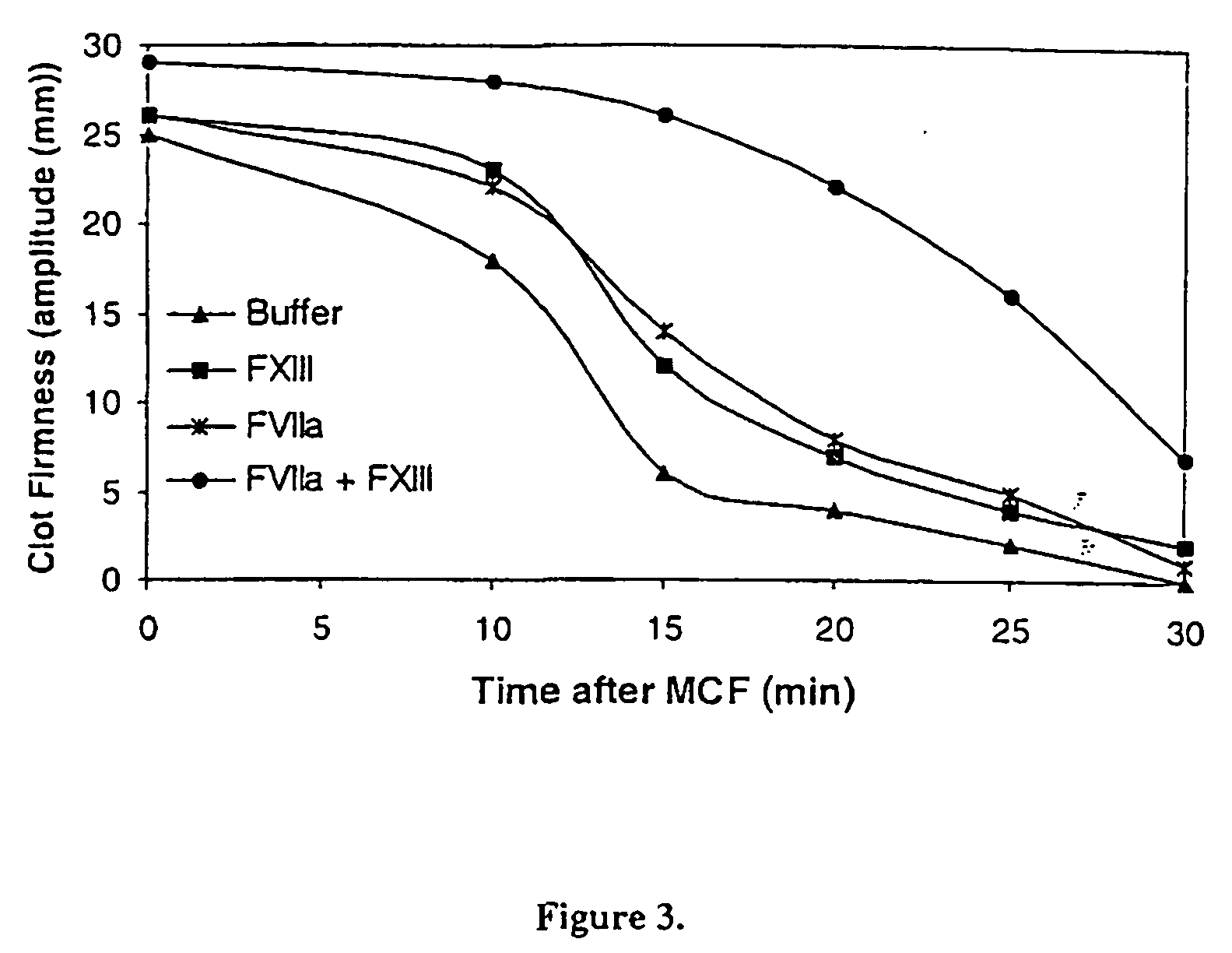
Pharmaceutical composition comprising a factor VIIa and a factor XIII
InactiveUS20060199766A1Shorten clotting timeProlonging clot lysis timeFactor VIIPeptide/protein ingredientsFactor VIIaBleeding episodes
The present invention relates to the use of a factor VIIa and a factor XIII in the treatment or pro-phylaxis of bleeding episodes.
Owner:NOVO NORDISK AS
Growth factor modified protein matrices for tissue engineering
InactiveUS7601685B2Slow drug releaseGreat percentagePeptide/protein ingredientsPeptide sourcesTissue repairDelivery system
Proteins are incorporated into protein or polysaccharide matrices for use in tissue repair, regeneration and / or remodeling and / or drug delivery. The proteins can be incorporated so that they are released by degradation of the matrix, by enzymatic action and / or diffusion. As demonstrated by the examples, one method is to bind heparin to the matrix by either covalent or non-covalent methods, to form a heparin-matrix. The heparin then non-covalently binds heparin-binding growth factors to the protein matrix. Alternatively, a fusion protein can be constructed which contains a crosslinking region such as a factor XIIIa substrate and the native protein sequence. Incorporation of degradable linkages between the matrix and the bioactive factors can be particularly useful when long-term drug delivery is desired, for example in the case of nerve regeneration, where it is desirable to vary the rate of drug release spatially as a function of regeneration, e.g. rapidly near the living tissue interface and more slowly farther into the injury zone. Additional benefits include the lower total drug dose within the delivery system, and spatial regulation of release which permits a greater percentage of the drug to be released at the time of greatest cellular activity.
Owner:UNIV ZURICH +1
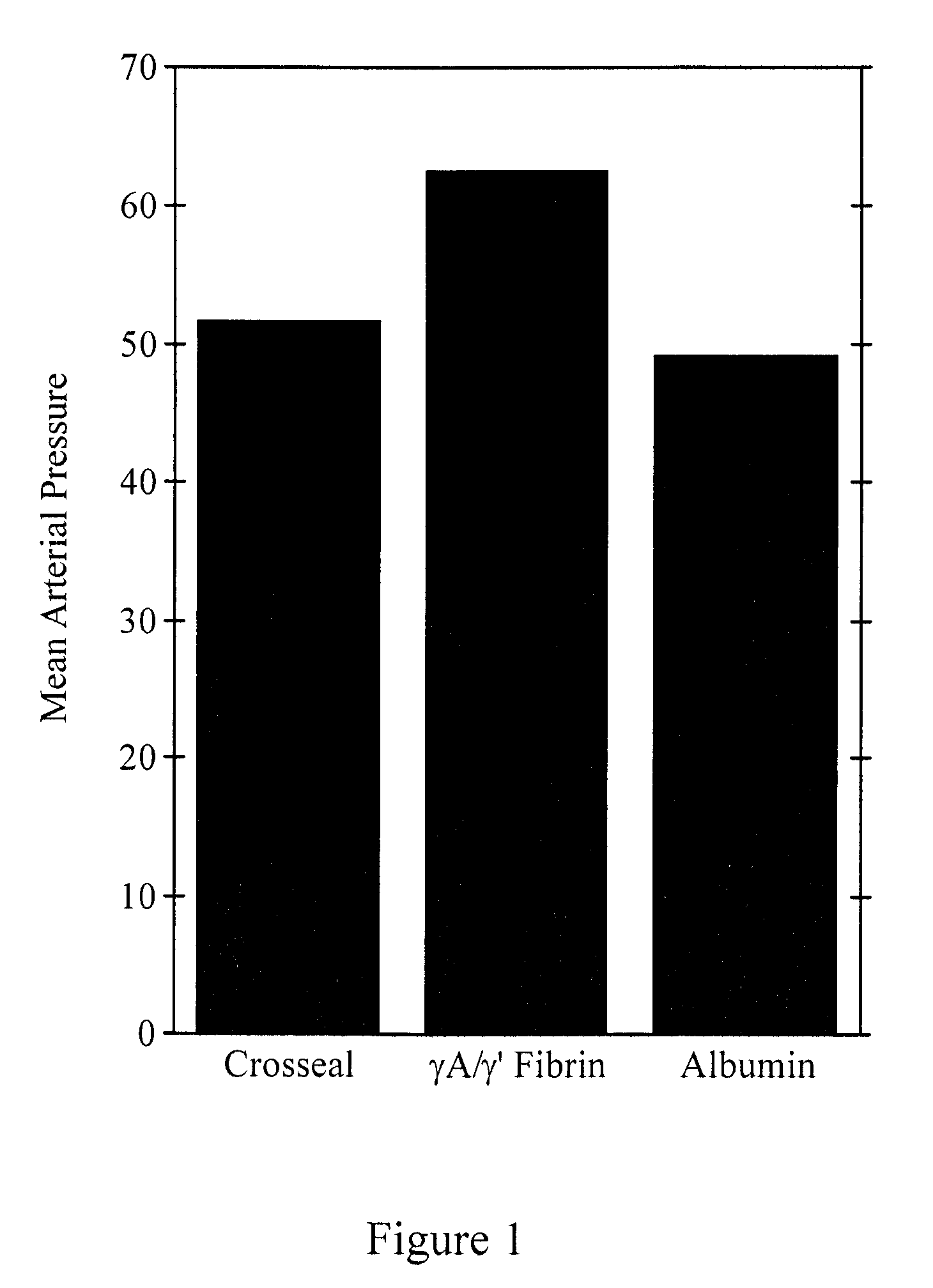
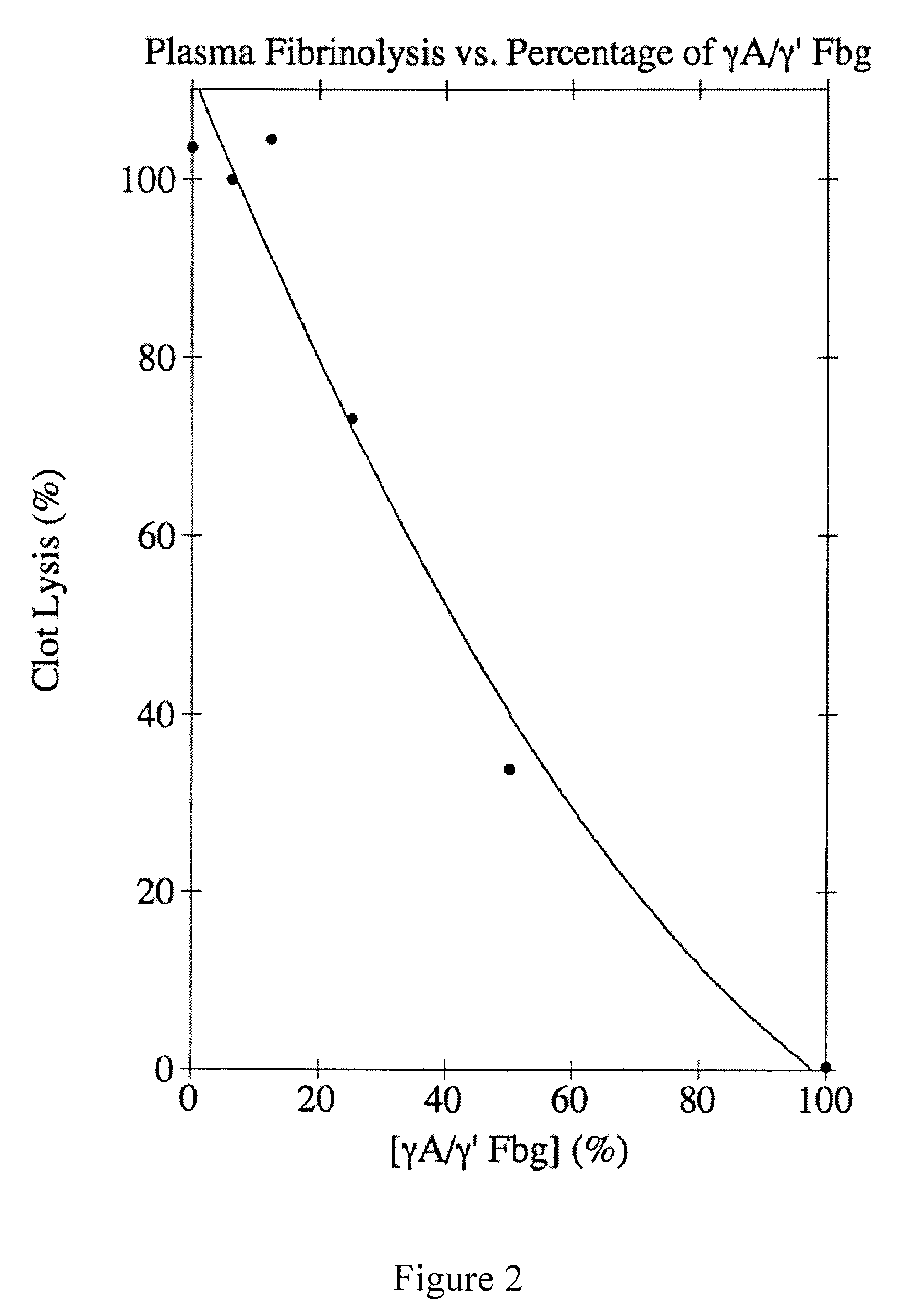
Degradation-resistant fibrinogen sealants
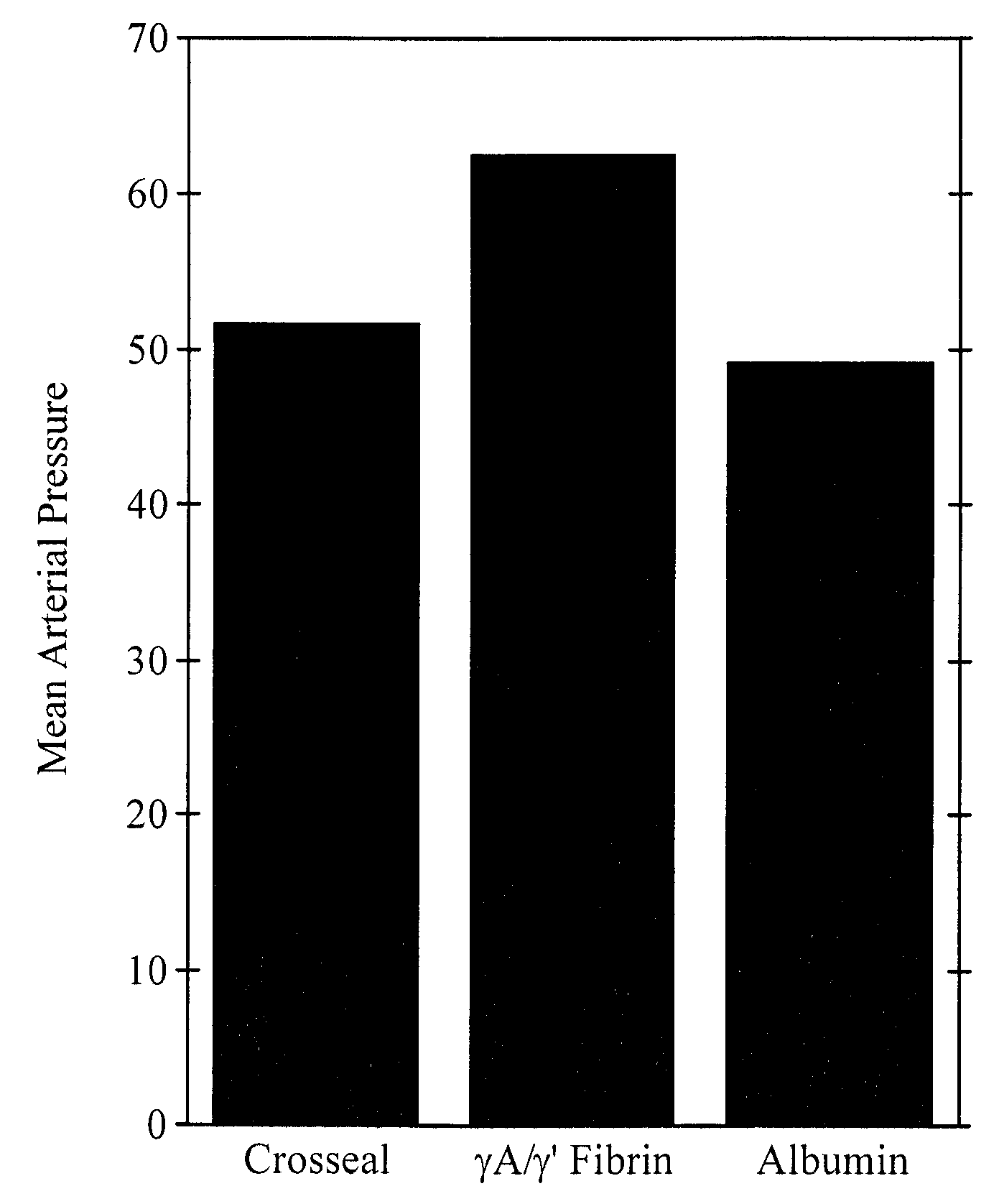
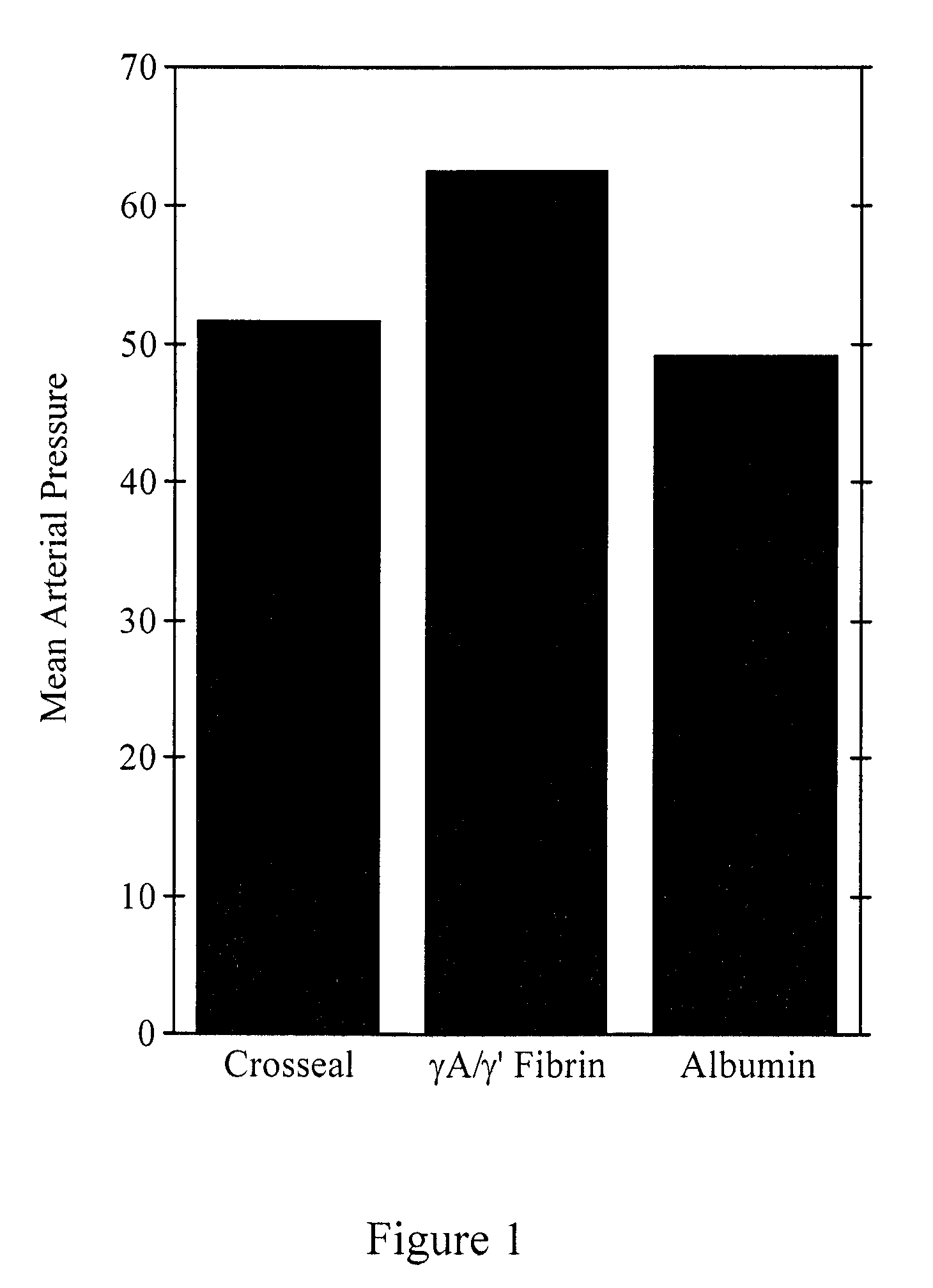
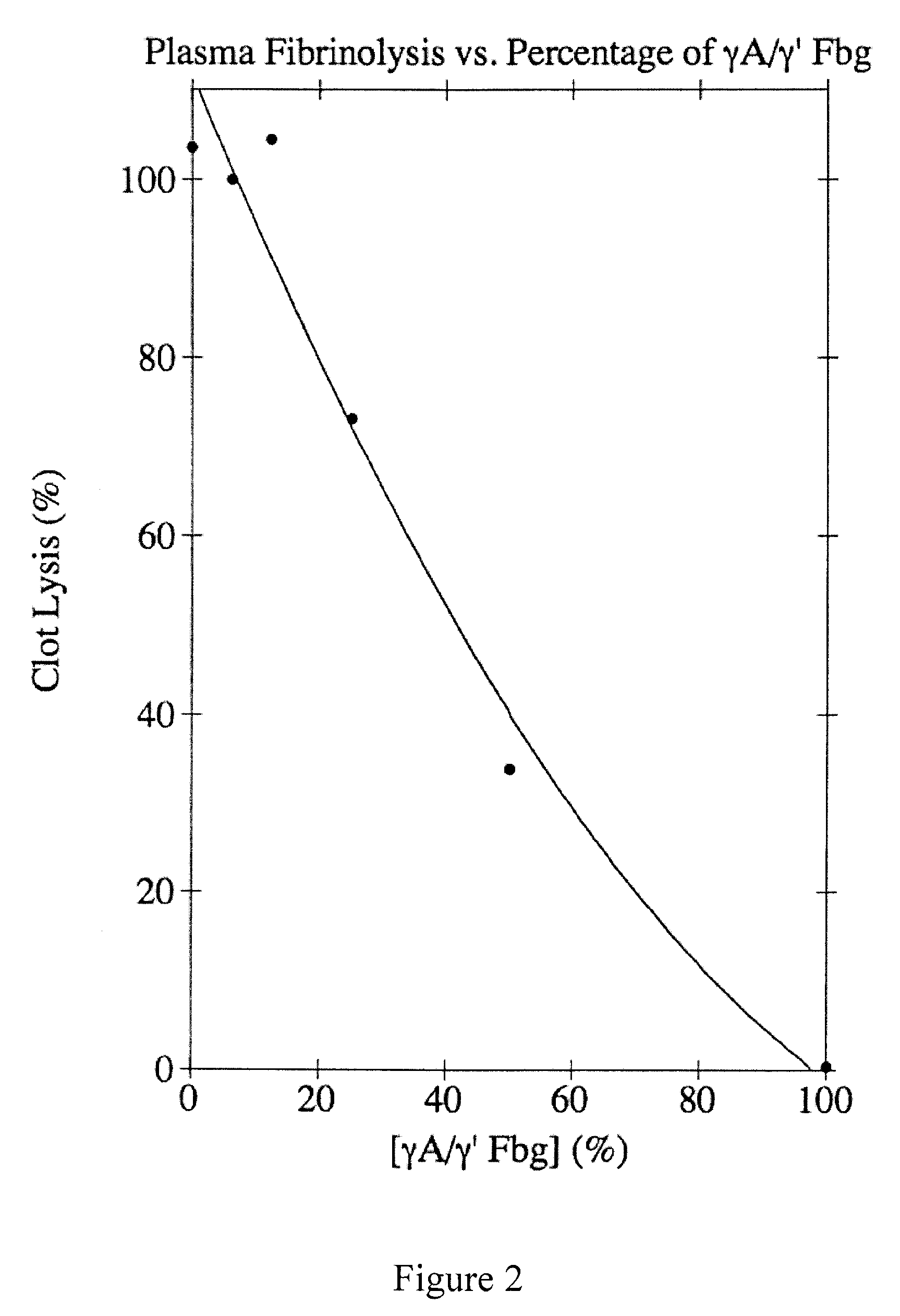
Provided are degradation-resistant fibrinogen sealants comprising a first composition comprising one or more of fibrinogen γA / γ′ heterodimers and / or fibrinogen γ′ / γ′ homodimers and a second composition comprising thrombin and, optionally, degradation-resistant fibrinogen sealants disclosed herein may further comprise Factor XIII and calcium. Degradation-resistant fibrinogen sealants are suitable for the treatment of trauma, particularly vascular trauma.
Owner:OREGON HEALTH & SCI UNIV
Degradation-resistant fibrinogen sealants
Provided are degradation-resistant fibrinogen sealants comprising a first composition comprising one or more of fibrinogen γA / γ′ heterodimers and / or fibrinogen γ′ / γ′ homodimers and a second composition comprising thrombin and, optionally, degradation-resistant fibrinogen sealants disclosed herein may further comprise Factor XIII and calcium. Degradation-resistant fibrinogen sealants are suitable for the treatment of trauma, particularly vascular trauma.
Owner:OREGON HEALTH & SCI UNIV
Method for treating inflammatory bowel disease
InactiveUS20070122384A1Salicyclic acid active ingredientsPeptide/protein ingredientsUlcerative colitisEnteropathy
A method for treating inflammatory bowel disease by administering interferon beta in conjunction with factor XIII to a mammal afflicted with the disease. The IBD may either be ulcerative colitis or Crohn's disease. The factor XIII used may be recombinant or nonrecombinant. The interferon beta used can be naturally occurring interferon beta, interferon beta-1a or interferon beta-1b.
Owner:ZYMOGENETICS INC
Process for separating proteins fibrinogen, factor XIII and biological glue from a solubilized plasma fraction and for preparing lyophilised concentrates of said proteins
ActiveUS8598319B2Easy to optimizeIncrease profitabilityFibrinogenPeptide/protein ingredientsFiberFreeze-drying
Owner:LABE FR DU FRACTIONNEMENT & DES BIOTECH SA
Growth factor modified protein matrices for tissue engineering
InactiveUS20070179093A1Great percentageFibrinogenPeptide/protein ingredientsTissue repairDrug release
Proteins are incorporated into protein or polysaccharide matrices for use in tissue repair, regeneration and / or remodeling and / or drug delivery. The proteins can be incorporated so that they are released by degradation of the matrix, by enzymatic action and / or diffusion. As demonstrated by the examples, one method is to bind heparin to the matrix by either covalent or non-covalent methods, to form a heparin-matrix. The heparin then non-covalently binds heparin-binding growth factors to the protein matrix. Alternatively, a fusion protein can be constructed which contains a crosslinking region such as a factor XIIIa substrate and the native protein sequence. Incorporation of degradable linkages between the matrix and the bioactive factors can be particularly useful when long-term drug delivery is desired, for example in the case of nerve regeneration, where it is desirable to vary the rate of drug release spatially as a function of regeneration, e.g. rapidly near the living tissue interface and more slowly farther into the injury zone. Additional benefits include the lower total drug dose within the delivery system, and spatial regulation of release which permits a greater percentage of the drug to be released at the time of greatest cellular activity.
Owner:ETH ZZURICH +1
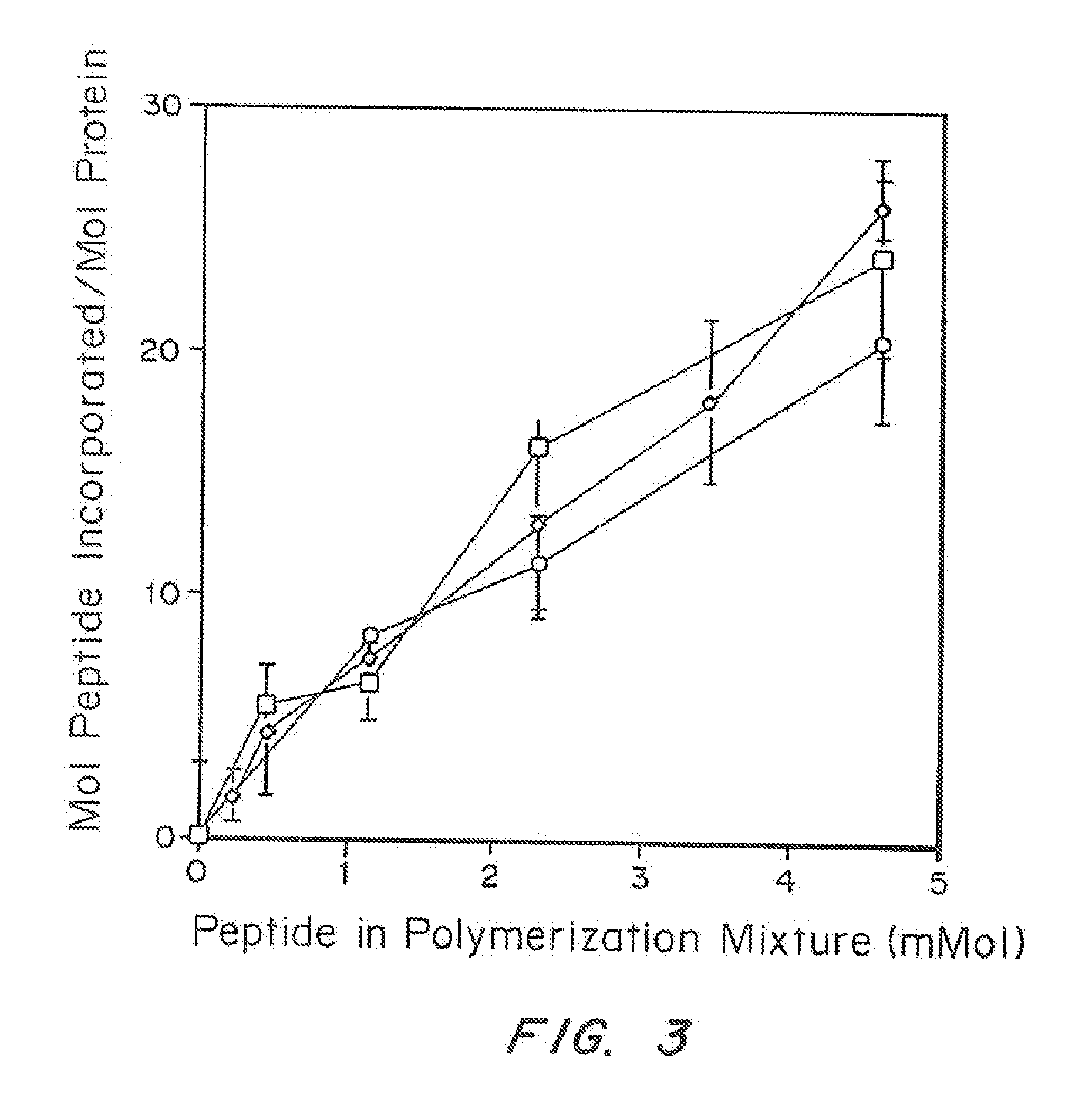
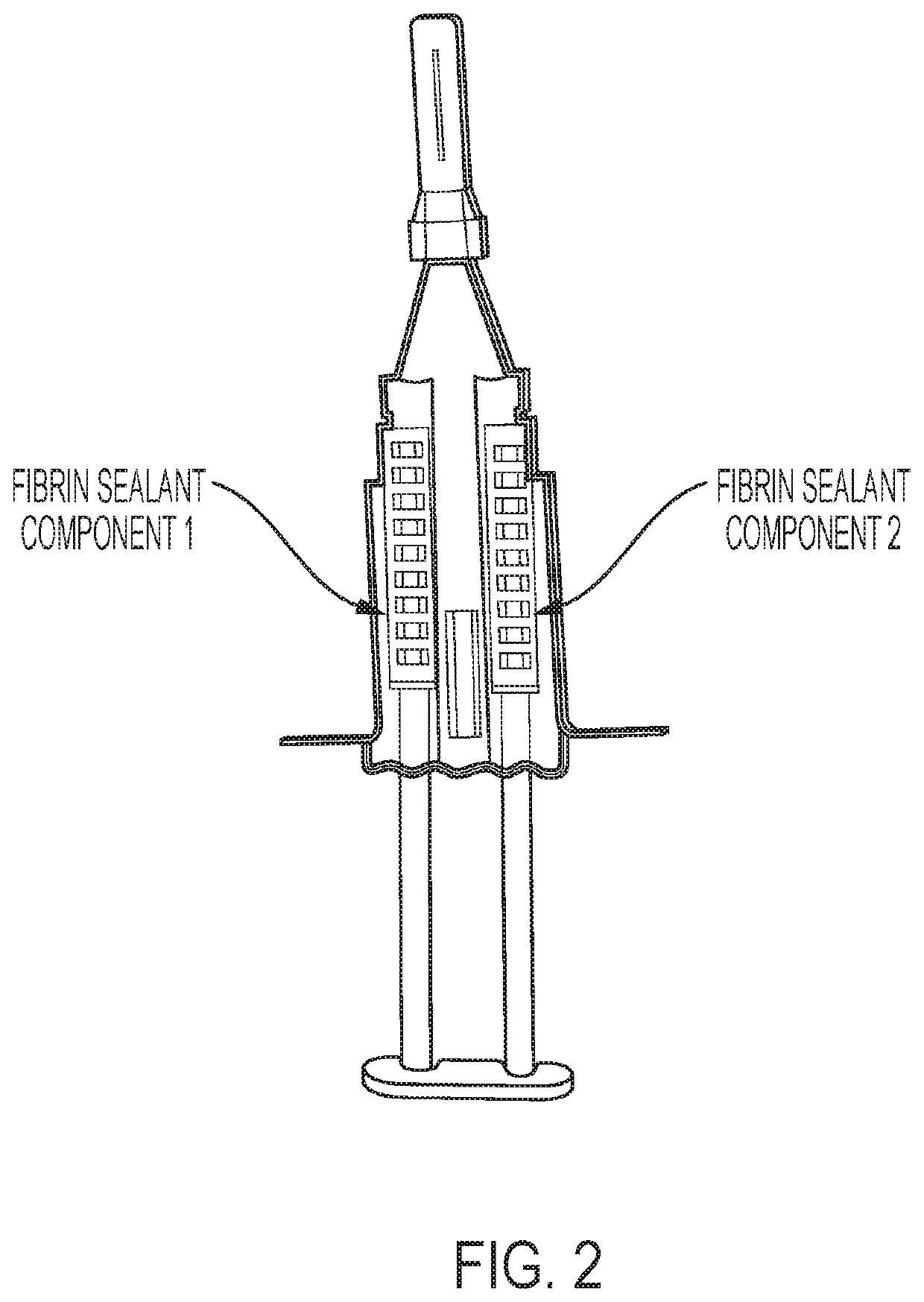
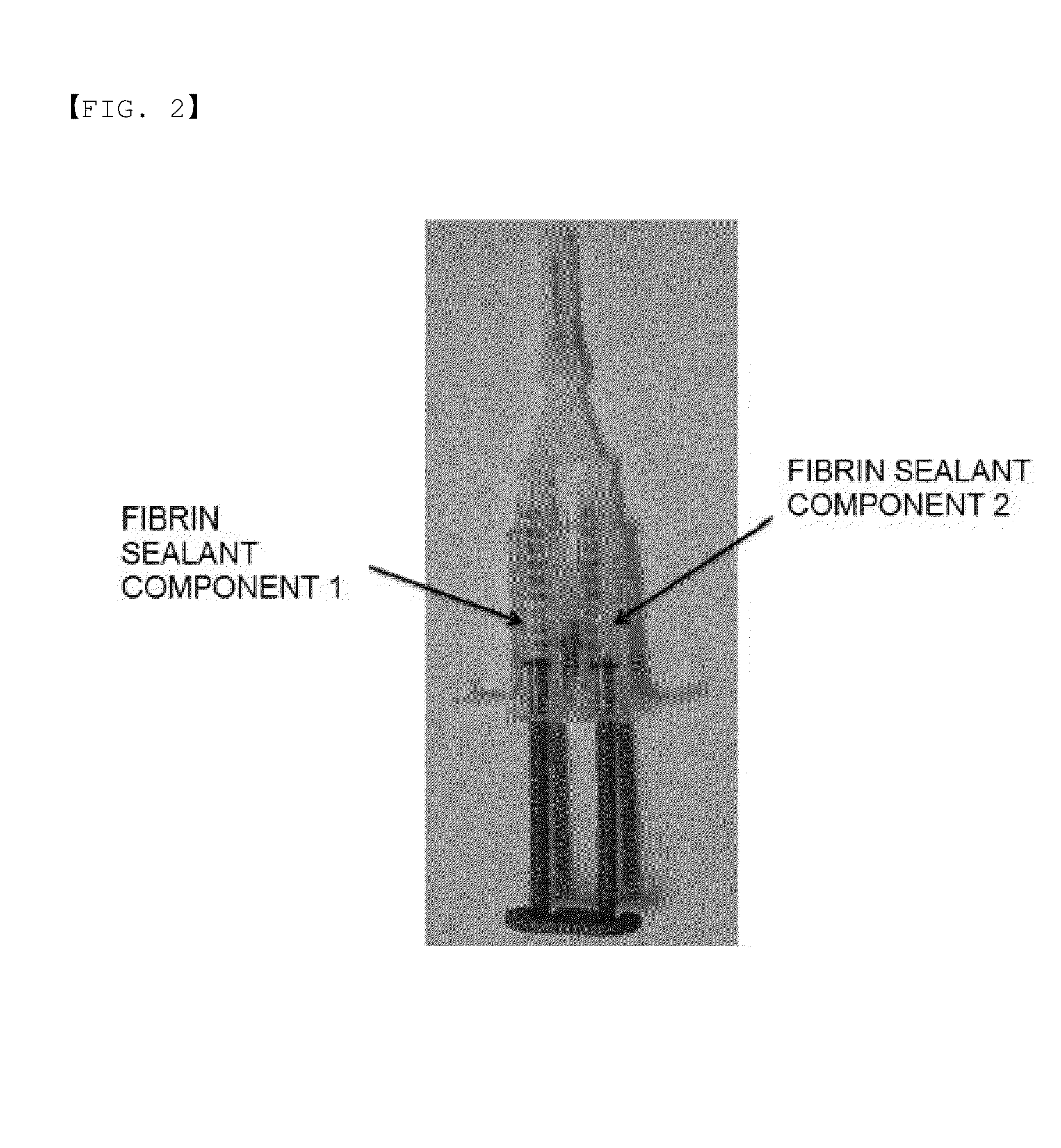
Method for preparing highly concentrated fibrinogen solution and method for preparing fibrin sealant by using thereof
ActiveUS10493133B2Economically and efficiently and rapidly obtainedConvenient for clinical operationFibrinogenSurgical adhesivesFiberFiltration
A method for preparing a highly concentrated fibrinogen solution includes adding amino acid or amino acid derivatives, and / or salts to a lowly concentrated fibrinogen solution, followed by a ultra-filtration concentration. Factor XIII can be added either before or after the ultra-filtration to give a fibrin sealant component 1 containing the highly concentrated fibrinogen solution. The fibrin sealant component 1 could be preserved for a long time at room temperature and be used without a reconstitution. Fibrin sealant component 2 is a solution containing thrombin and calcium. A fibrin sealant product may be provided in a vial type in which the fibrin sealant components 1 and 2 are each filled in separate vials or in a re-filled syringe type wherein the fibrin sealant components 1 and 2 are each filled in separate syringes connected with each other to be instantly used.
Owner:GREEN CROSS HLDG CORP
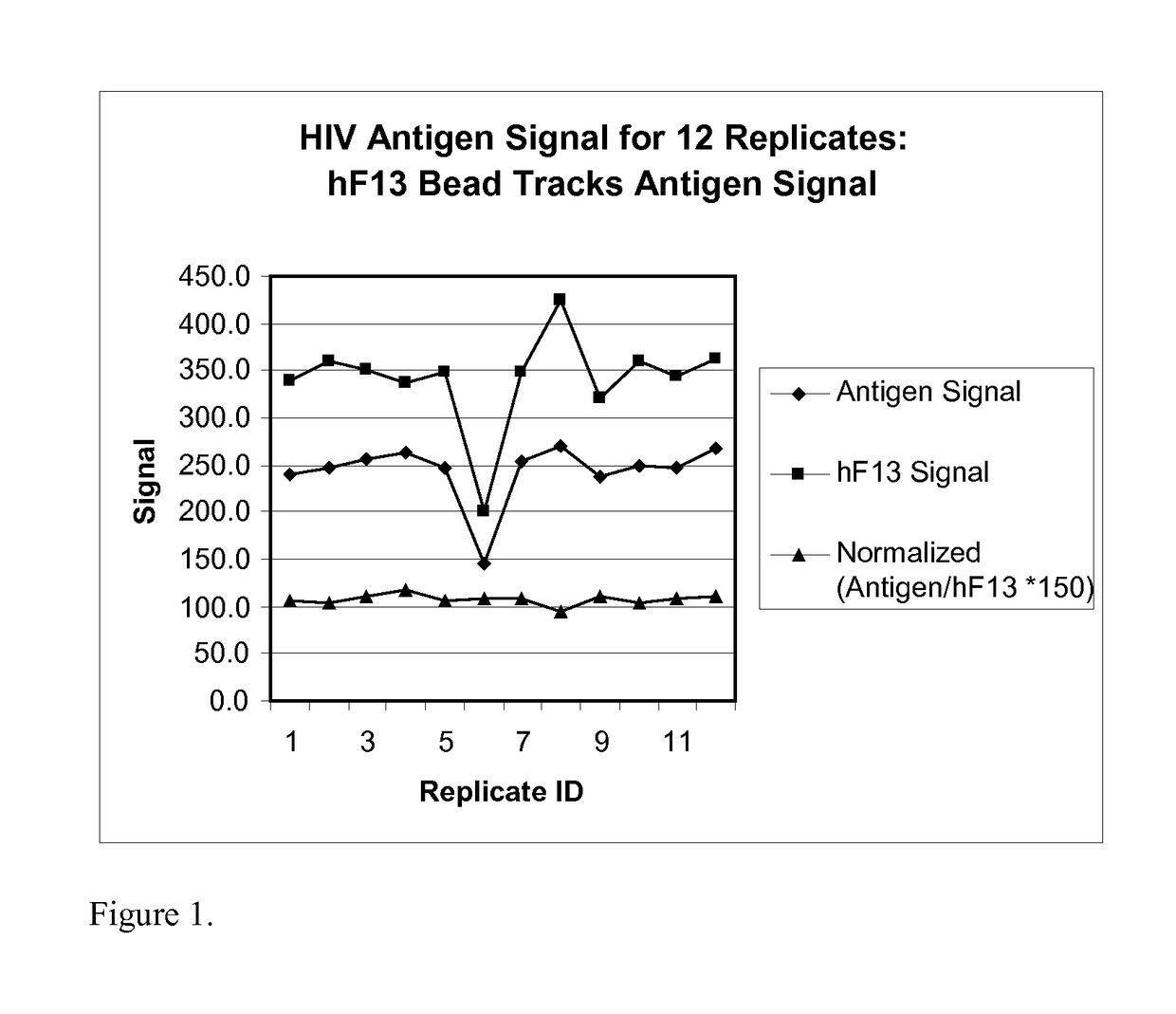
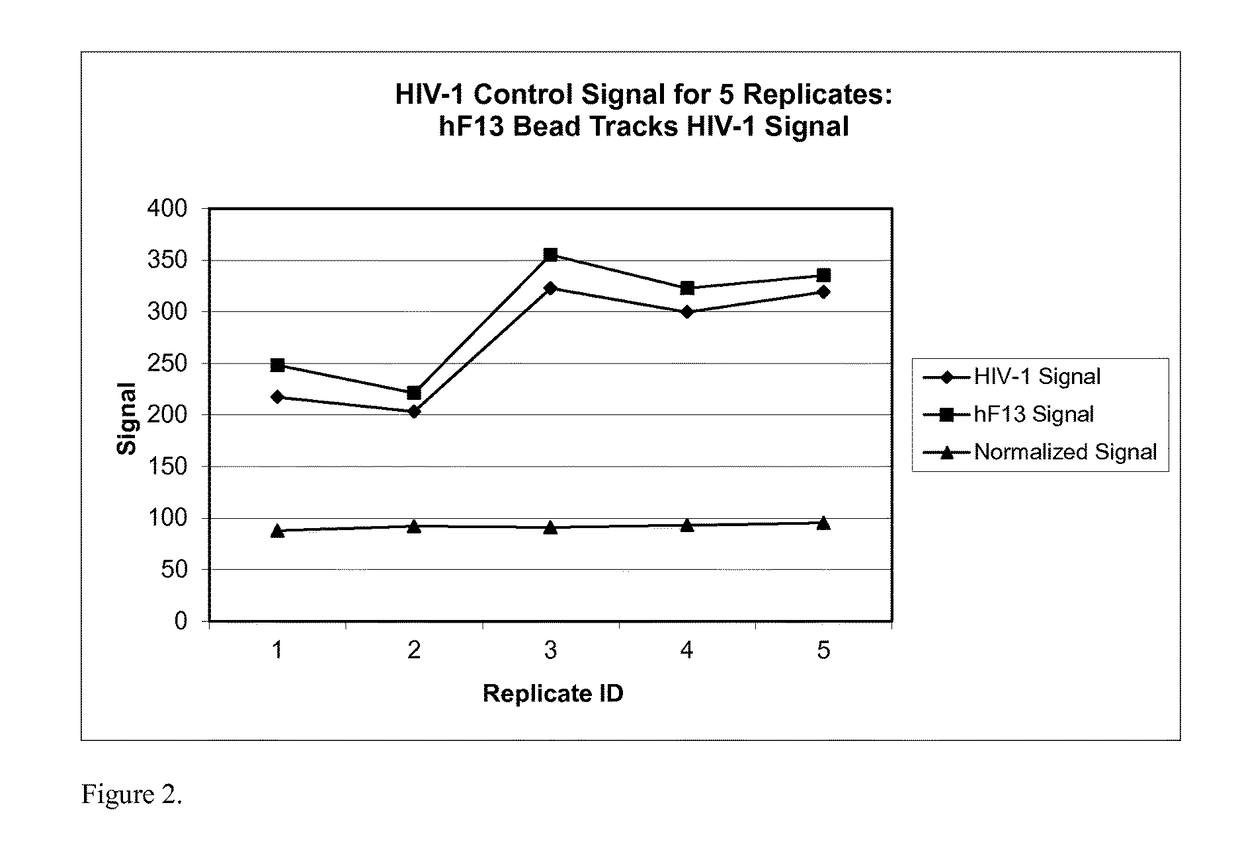
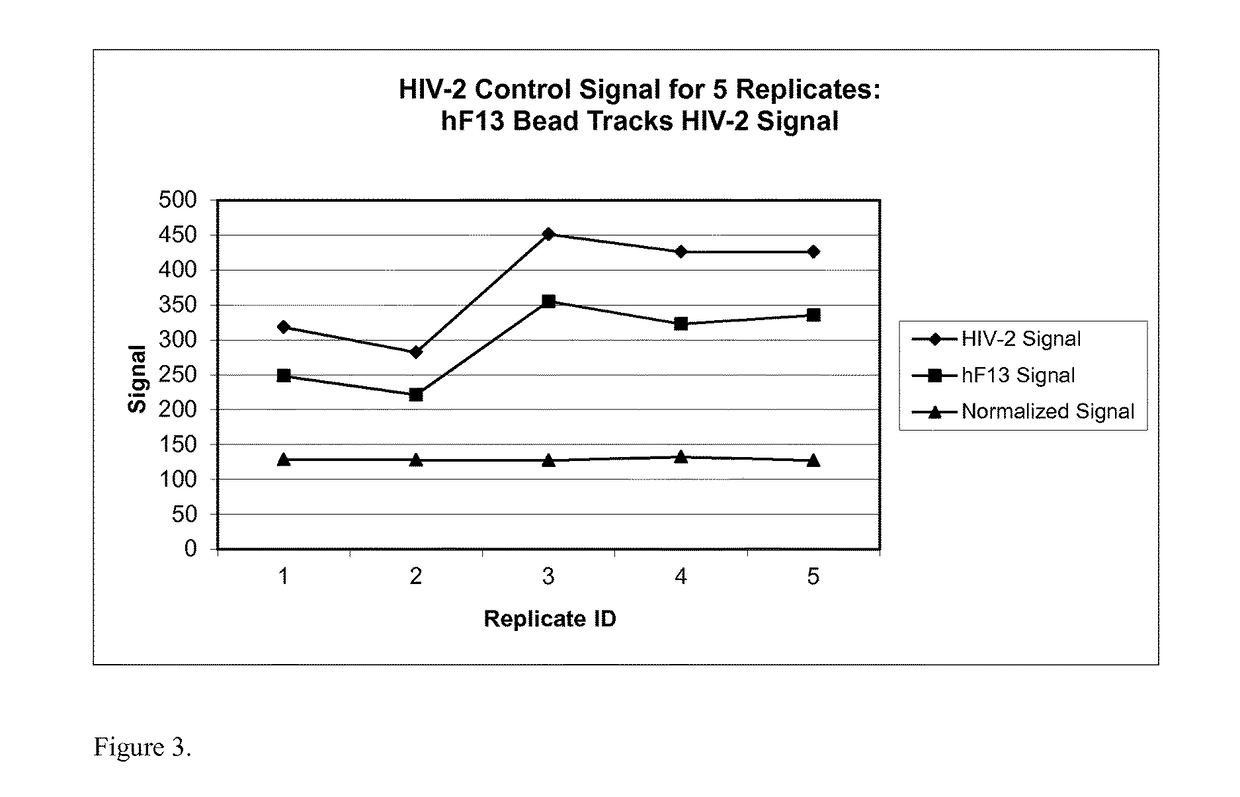
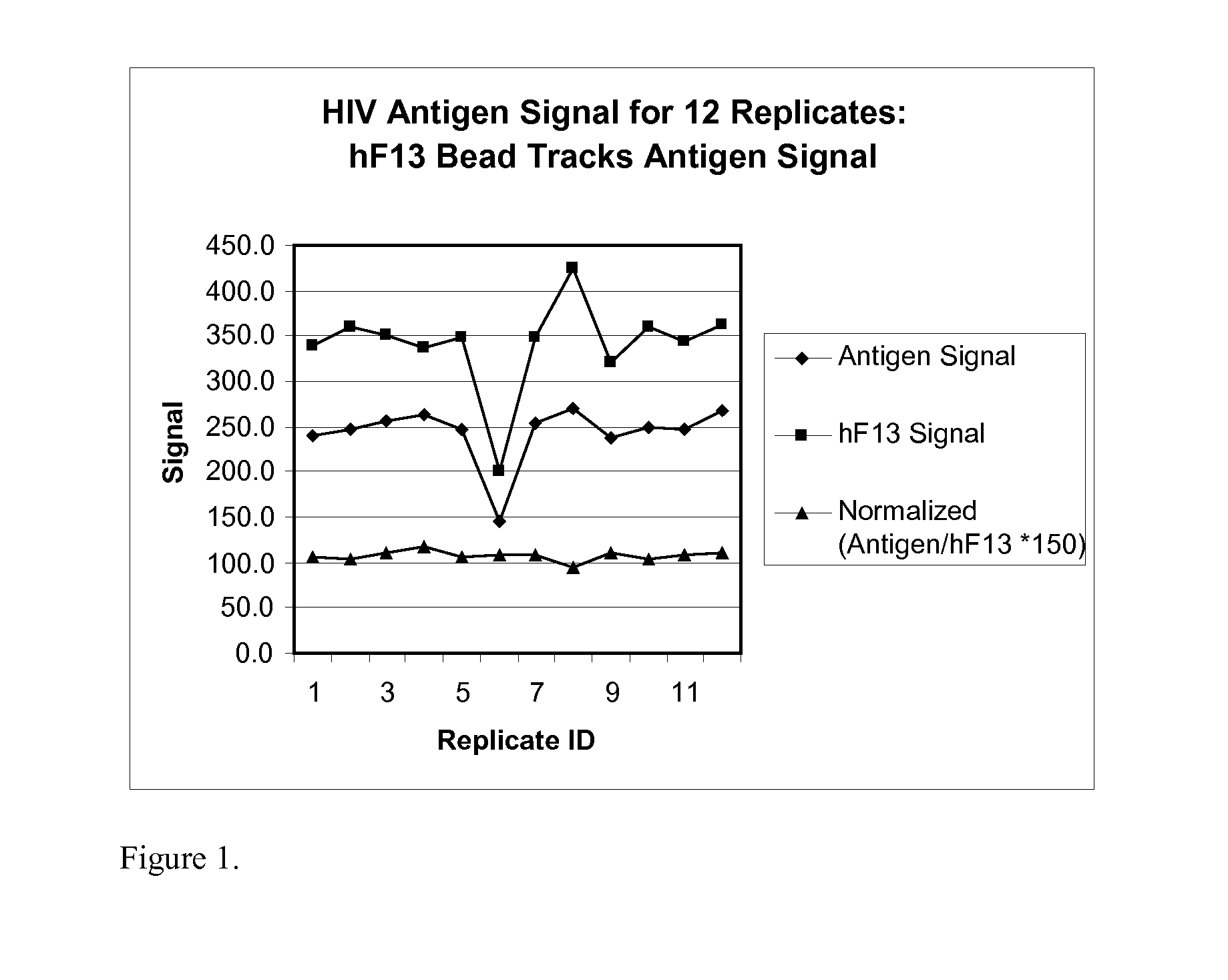
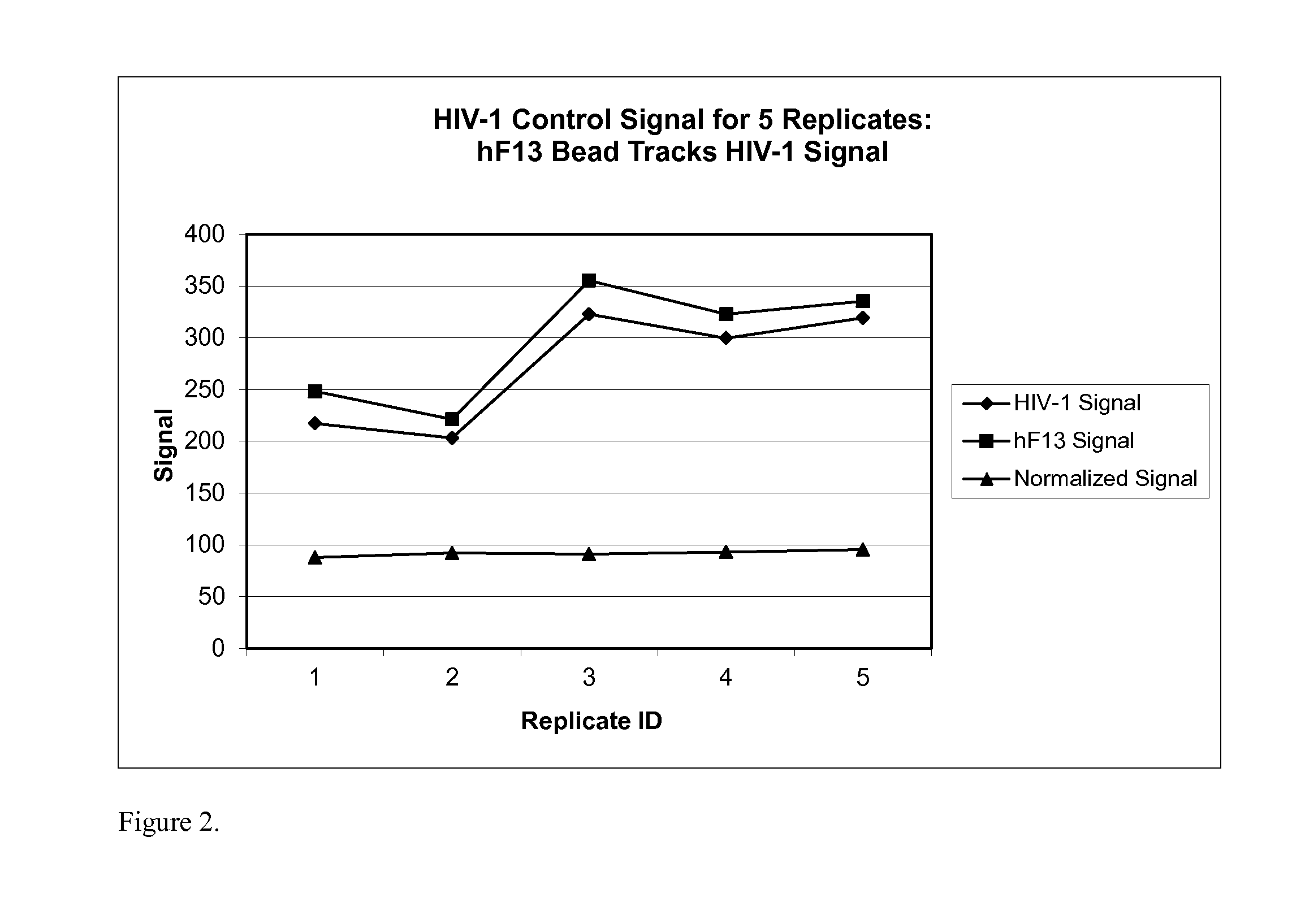
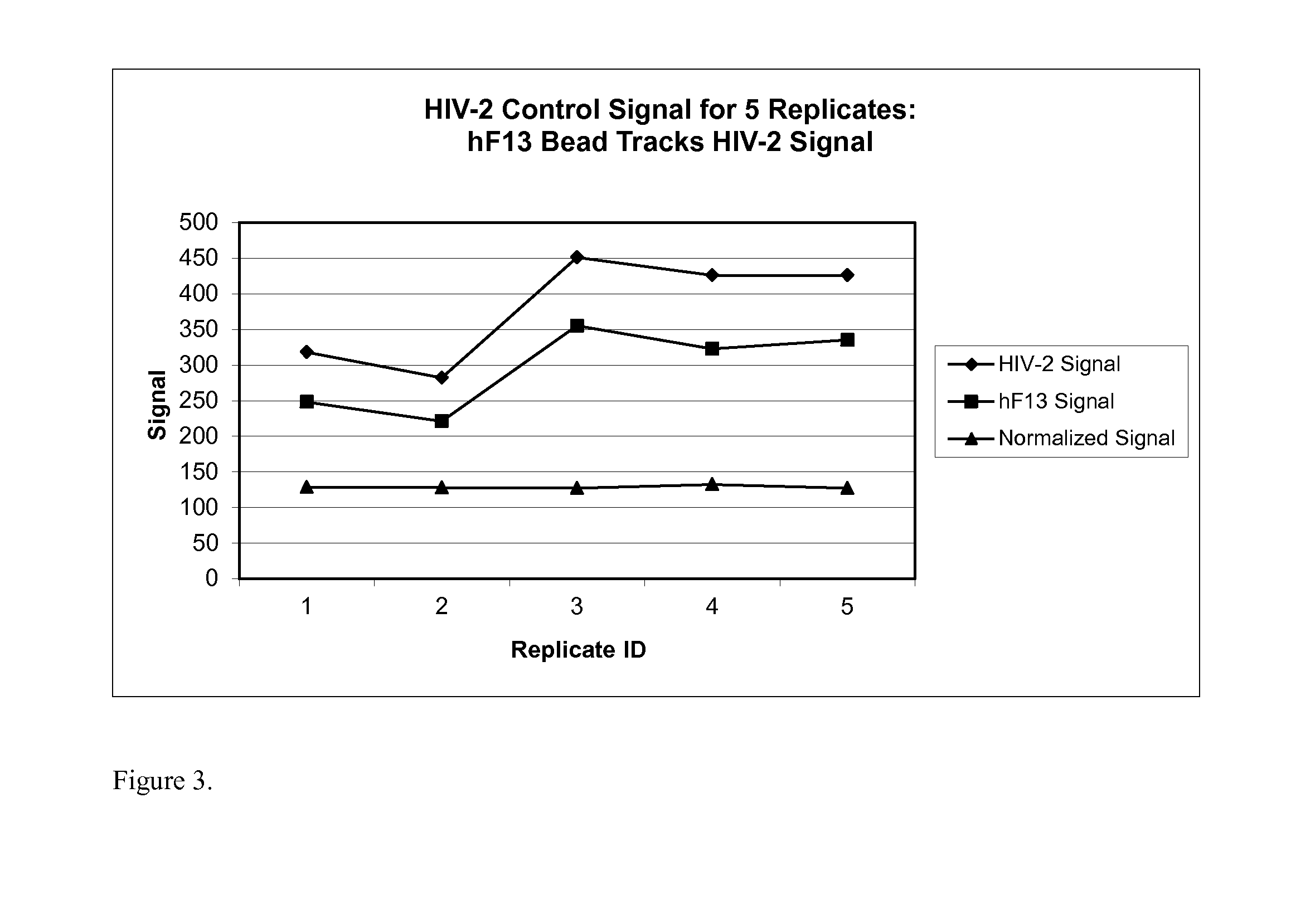
Human factor XIII as a normalization control for immunoassays
ActiveUS9658220B2Improve analytical performancePeptide librariesLibrary screeningAssayBioinformatics
The present disclosure provides compositions and methods that are useful for normalizing the amount of signal detected in an assay, such as an immunoassay. The compositions and methods are useful for improving the accuracy of immunoassays, such as immunoassays that detect whether a subject is infected with a retrovirus such as HIV.
Owner:BIO RAD LAB INC
Method for preparing highly concentrated fibrinogen solution and method for preparing fibrin sealant by using thereof
ActiveUS20140328822A1Economically and efficiently and rapidly obtainedConvenient for clinical operationFibrinogenSurgical adhesivesFiberFiltration
A method for preparing a highly concentrated fibrinogen solution includes adding amino acid or amino acid derivatives, and / or salts to a lowly concentrated fibrinogen solution, followed by a ultra-filtration concentration. Factor XIII can be added either before or after the ultra-filtration to give a fibrin sealant component 1 containing the highly concentrated fibrinogen solution. The fibrin sealant component 1 could be preserved for a long time at room temperature and be used without a reconstitution. Fibrin sealant component 2 is a solution containing thrombin and calcium. A fibrin sealant product may be provided in a vial type in which the fibrin sealant components 1 and 2 are each filled in separate vials or in a re-filled syringe type wherein the fibrin sealant components 1 and 2 are each filled in separate syringes connected with each other to be instantly used.
Owner:GREEN CROSS HLDG
Human factor xiii as a normalization control for immunoassays
ActiveUS20130345081A1Improve analytical performancePeptide librariesLibrary screeningFactor XIIIStandardization
The present disclosure provides compositions and methods that are useful for normalizing the amount of signal detected in an assay, such as an immunoassay. The compositions and methods are useful for improving the accuracy of immunoassays, such as immunoassays that detect whether a subject is infected with a retrovirus such as HIV.
Owner:BIO RAD LAB INC
Method for treating von willebrand's disease
Use of factor XIII for treating von Willebrand's disease. A patient having von Willebrand's disease is treated by administering factor XIII generally in conjunction with factor VIII concentrate, 1-desamino-8-D-arginine vasopressin (DDAVP) or desmopressin.
Owner:ZYMOGENETICS INC
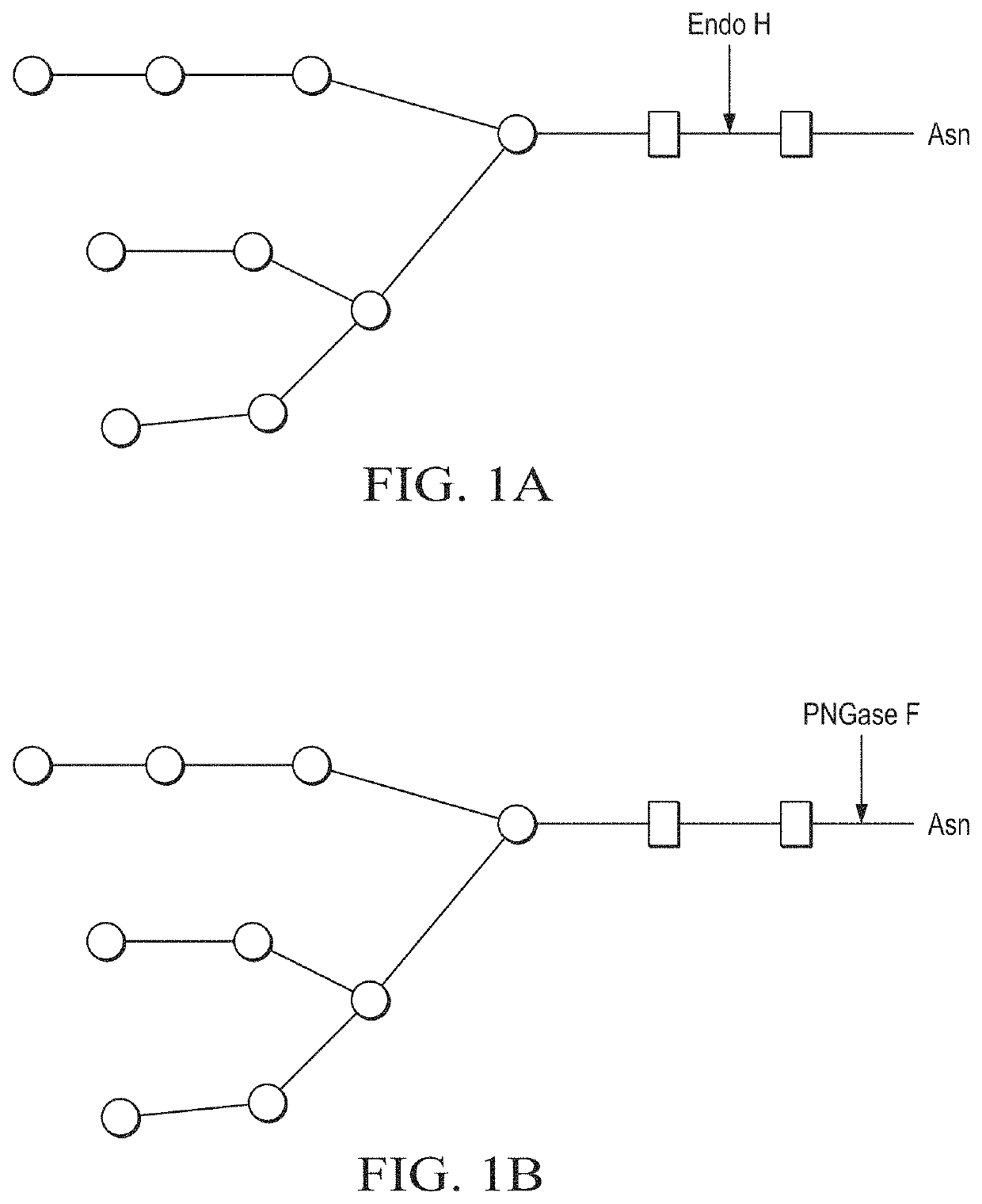
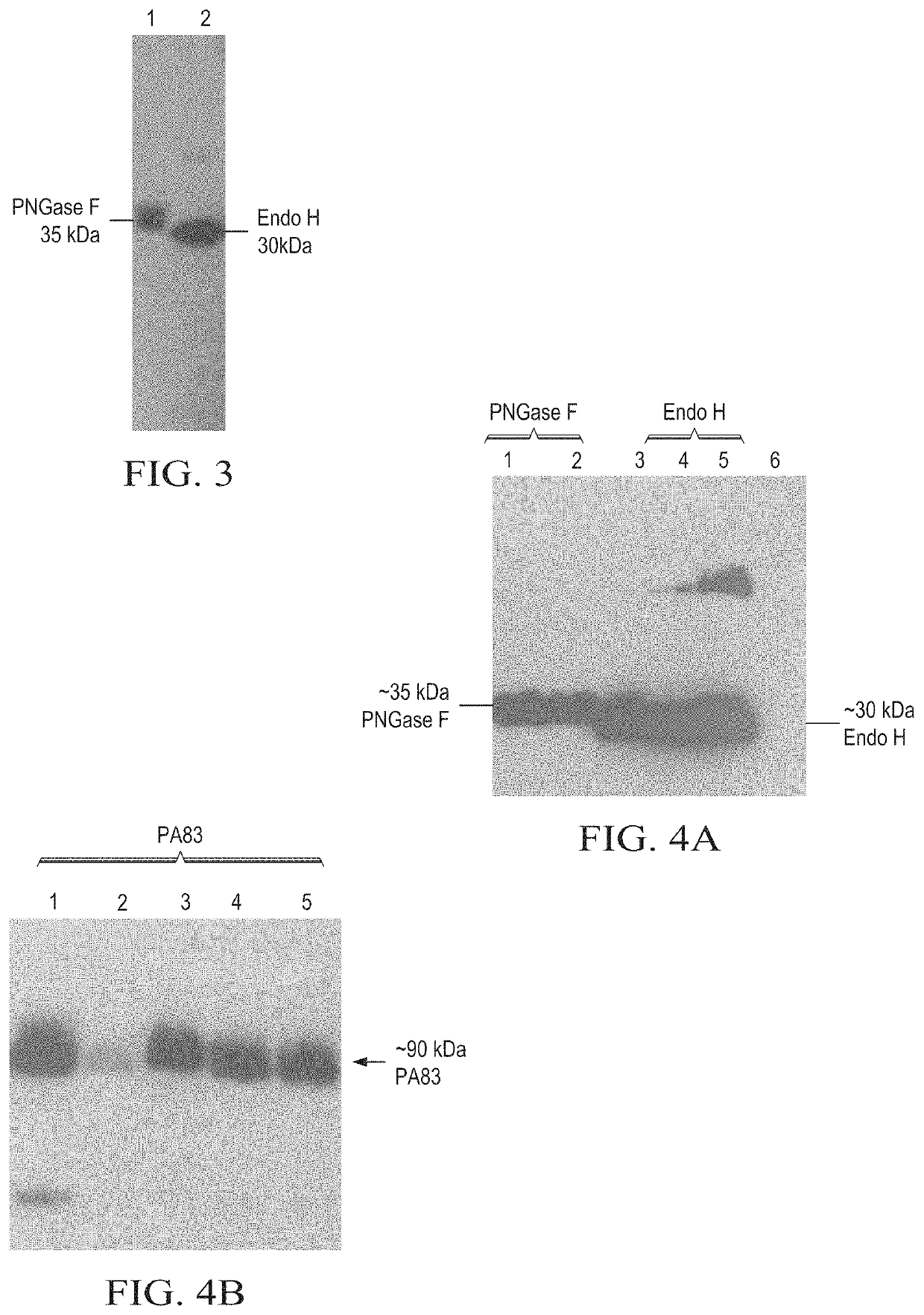
Production of in vivo N-deglycosylated recombinant proteins by co-expression with endo H
ActiveUS11041163B2Improve the level ofReduced functionalityAntibacterial agentsHydrolasesMalariaImmunogenicity
Owner:MAMMEDOV TARLAN
Method for the detection of risk factors associated with myocardial infarction
A method for determining whether an individual is at an increased risk for myocardial infarction, comprising screening for the presence of Factor II and Factor XIII alleles associated with myocardial infarction. Also provided are kits and primers that specifically hybridize adjacent to the allele-specific regions of the Factor II and Factor XIII genes.
Owner:NEWLAB CLINICAL RES
Compositions useful as fibrin sealants
InactiveCN1260720AInhibition of polymerizationReduce transmissionSurgical adhesivesPeptide/protein ingredientsMedicineFactor X
Novel fibrin monomer compositions which are solutions including additional coharvested components, such as prothrombin and Factor XIII, are useful in fibrin sealant applications. Preferably, the compositions are autologous to the patient receiving the sealant and these compositions may also include coharvested plasminogen, Factor X, antithrombin III and / or fibronectin.
Owner:BRISTOL MYERS SQUIBB CO
Method for treating Hemophilia B
Use of factor XIII for treating hemophilia B. A patient having hemophilia B is treated by administering factor XIII, generally in conjunction with factor IX.
Owner:ZYMOGENETICS INC
Separation and purification method of blood coagulation factor xiii and its application
ActiveCN110314414BHigh purityImprove extraction efficiencySolid sorbent liquid separationPeptide preparation methodsAntigenAntiendomysial antibodies
The invention provides an immunoaffinity chromatography column filler, an immunoaffinity chromatography column, a method for separating and purifying blood coagulation factor XIII and applications thereof, and relates to the technical field of preparation of functional blood coagulation factors. The immunoaffinity chromatography column filler is mainly obtained by coupling an anticoagulation factor XIII monoclonal antibody with an affinity chromatography medium. According to the principle of high-specificity selection of antigen and antibody, during the purification process of coagulation factor XIII on the immunoaffinity chromatography column containing this filler, the monoclonal antibody against coagulation factor XIII can specifically adsorb coagulation factor XIII, thus effectively The purity and extraction efficiency of the blood coagulation factor XIII are improved, and the purity of the prepared blood coagulation factor XIII is as high as 95%, which can be widely used in the preparation of viscoelastic coagulation analysis and detection reagents.
Owner:宝锐生物科技泰州有限公司
Method for treating coumarin-induced hemorrhage
Use of factor XIII for treating coumarin-induced hemorrhage or bleeding. The coumarin may be warfarin or dicoumarol. A patient having coumarin-induced bleeding is treated with factor XIII alone or in conjunction with vitamin K.
Owner:ZYMOGENETICS INC
Apparatus, Systems And Methods For Integrative Photo-Optical/Mechanical Test For Noncontact Measurement Of Polymerization
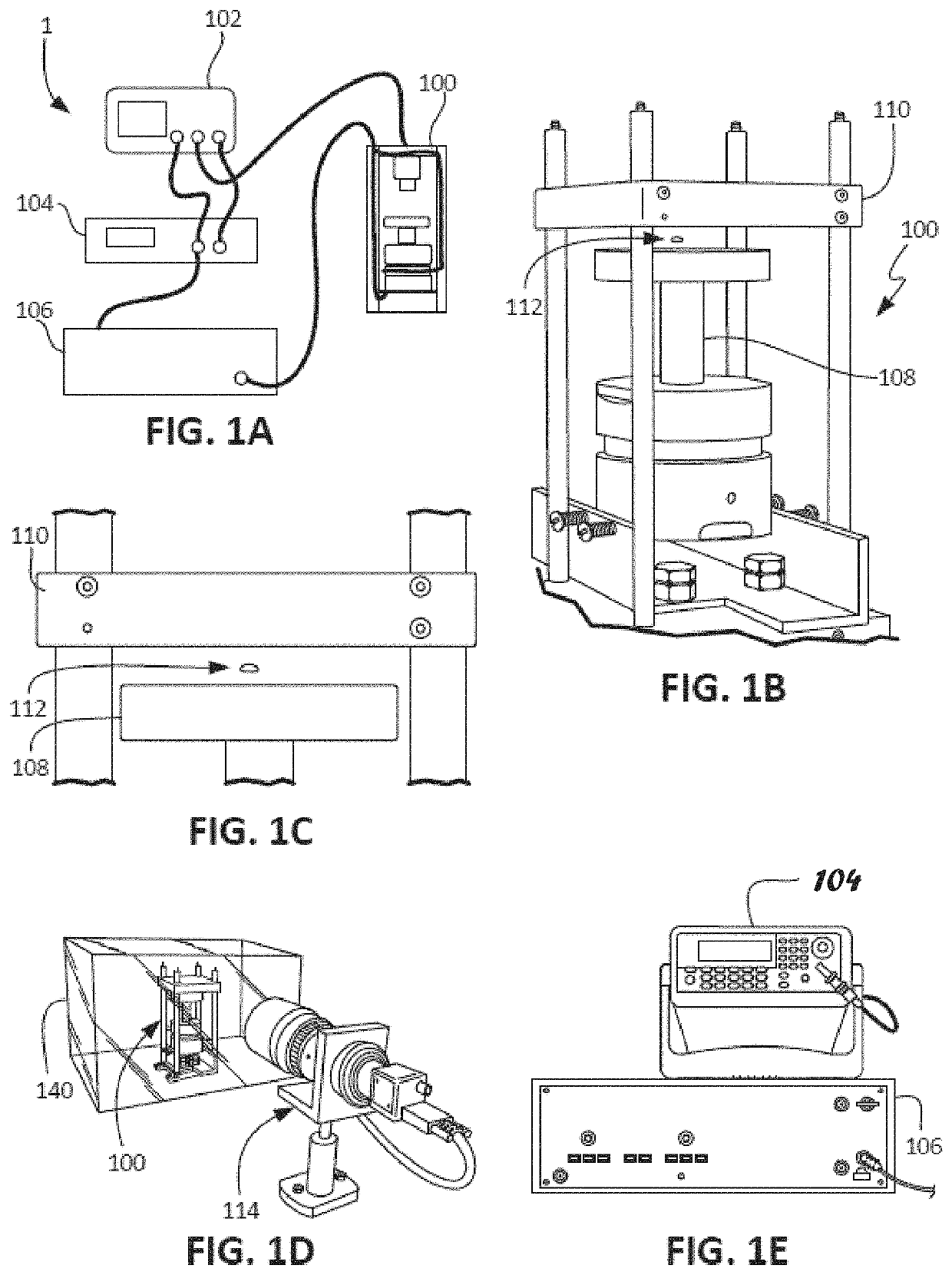
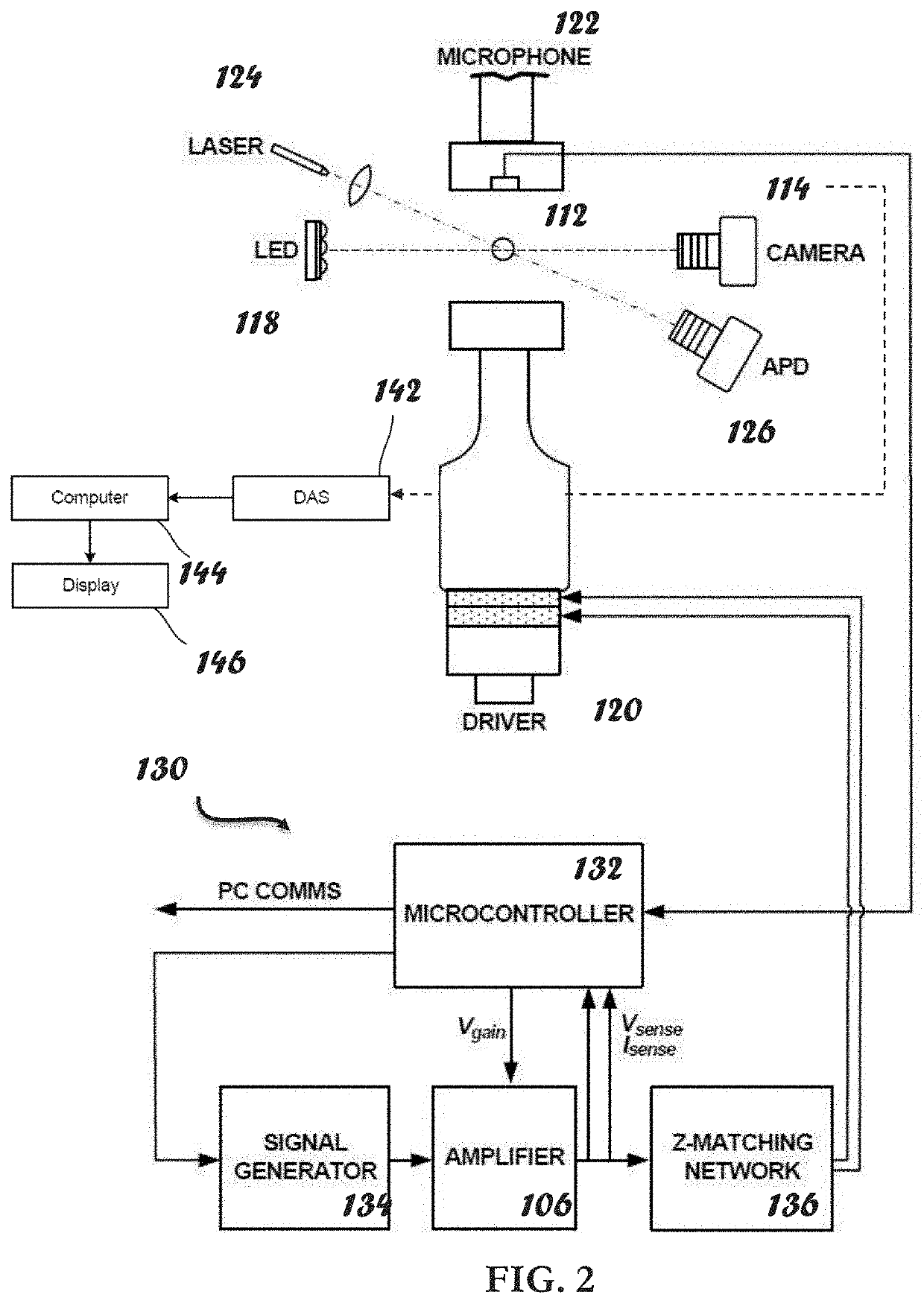
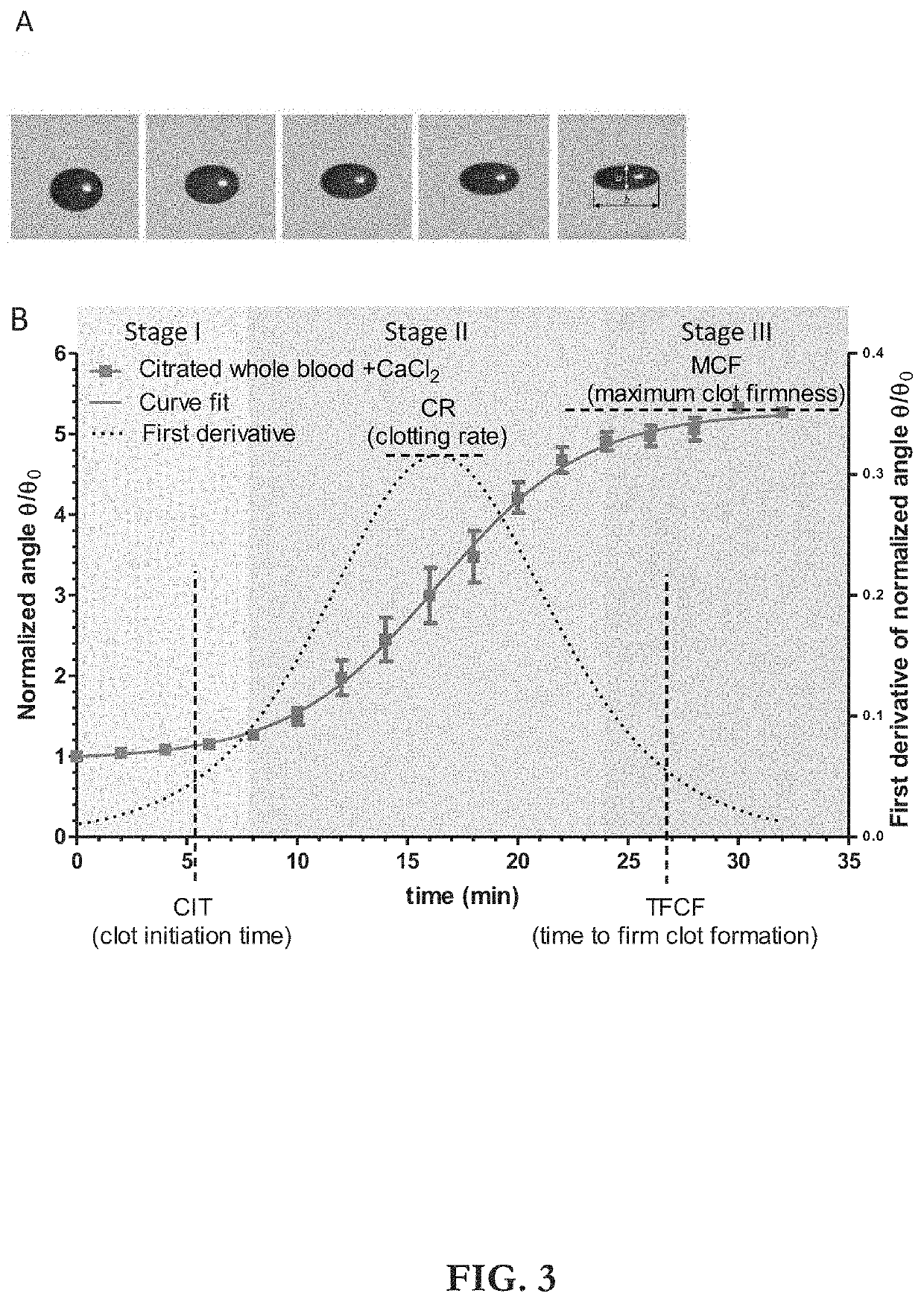
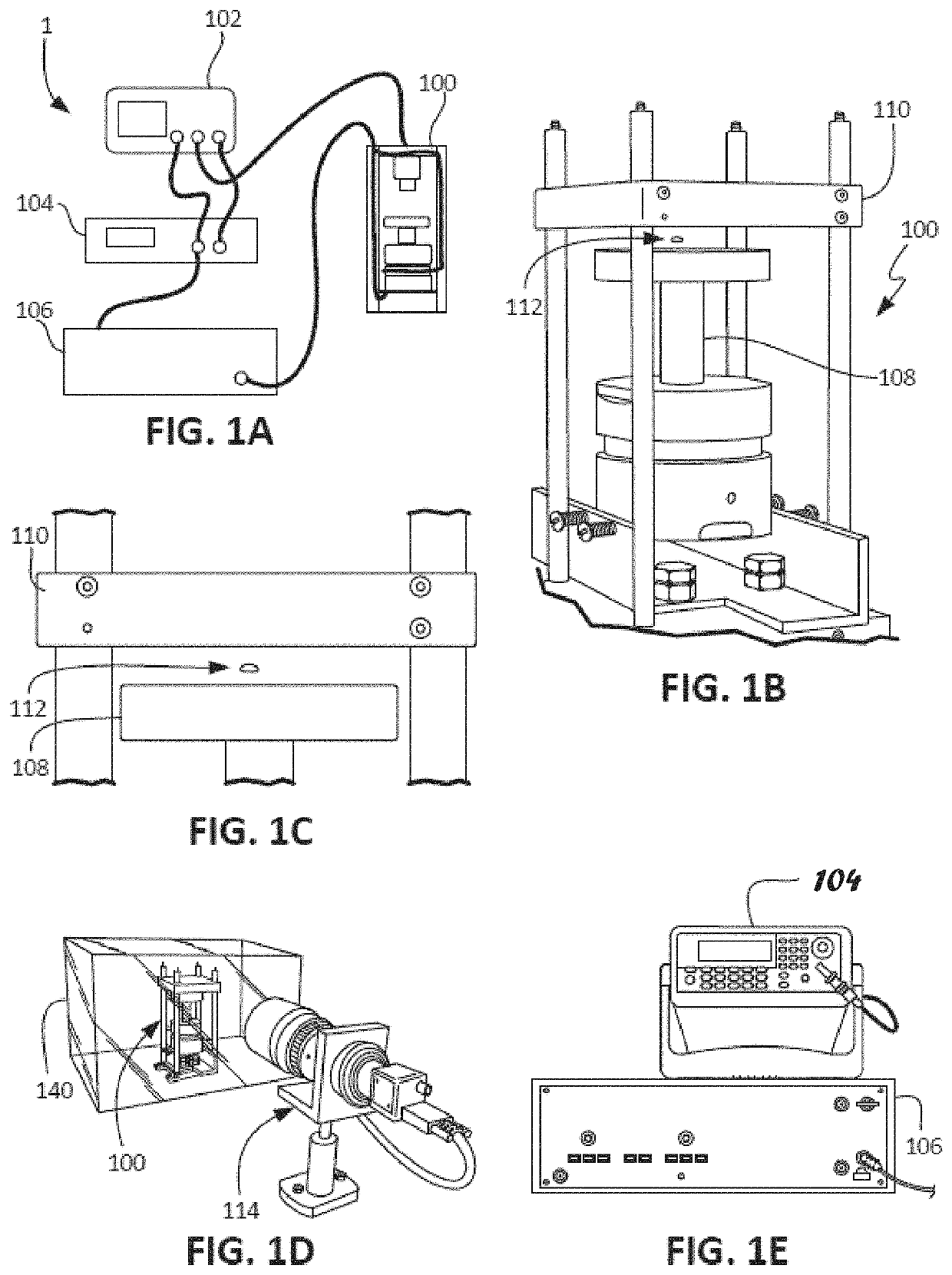
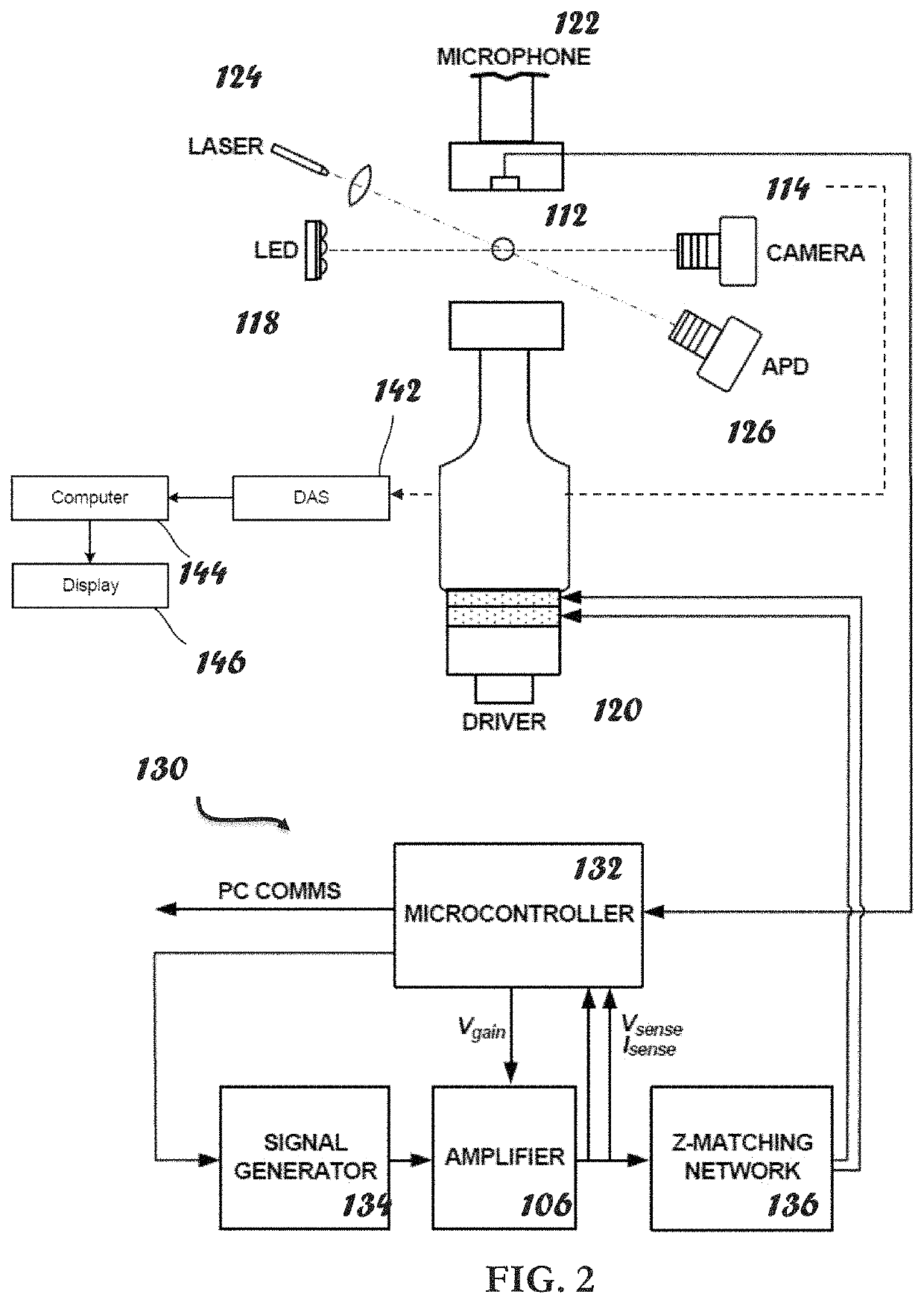
ActiveUS20210109083A1Material analysis by observing effect on chemical indicatorBiological testingOptical testAssay
Owner:THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND
Method for treating inflammatory bowel disease
A method for treating inflammatory bowel disease by administering interferon beta in conjunction with factor XIII to a mammal afflicted with the disease. The IBD may either be ulcerative colitis or Crohn's disease. The factor XIII used may be recombinant or nonrecombinant. The interferon beta used can be naturally occurring interferon beta, interferon beta-1a or interferon beta-1b.
Owner:ZYMOGENETICS INC
Method for inhibiting the formation of seromas using factor XIII
Use of factor XIII for inhibiting the formation of seromas by administering factor XIII. The factor XIII can be administered locally at the site of a wound or surgery or administered systemically. If the factor XIII is administered locally, it can be activated or non-activated and may be administered in conjunction with activated thrombin.
Owner:ZYMOGENETICS INC
Apparatus, systems and methods for integrative photo-optical/mechanical test for noncontact measurement of polymerization
ActiveUS10823723B2Material analysis by observing effect on chemical indicatorColor/spectral properties measurementsOptical testAssay
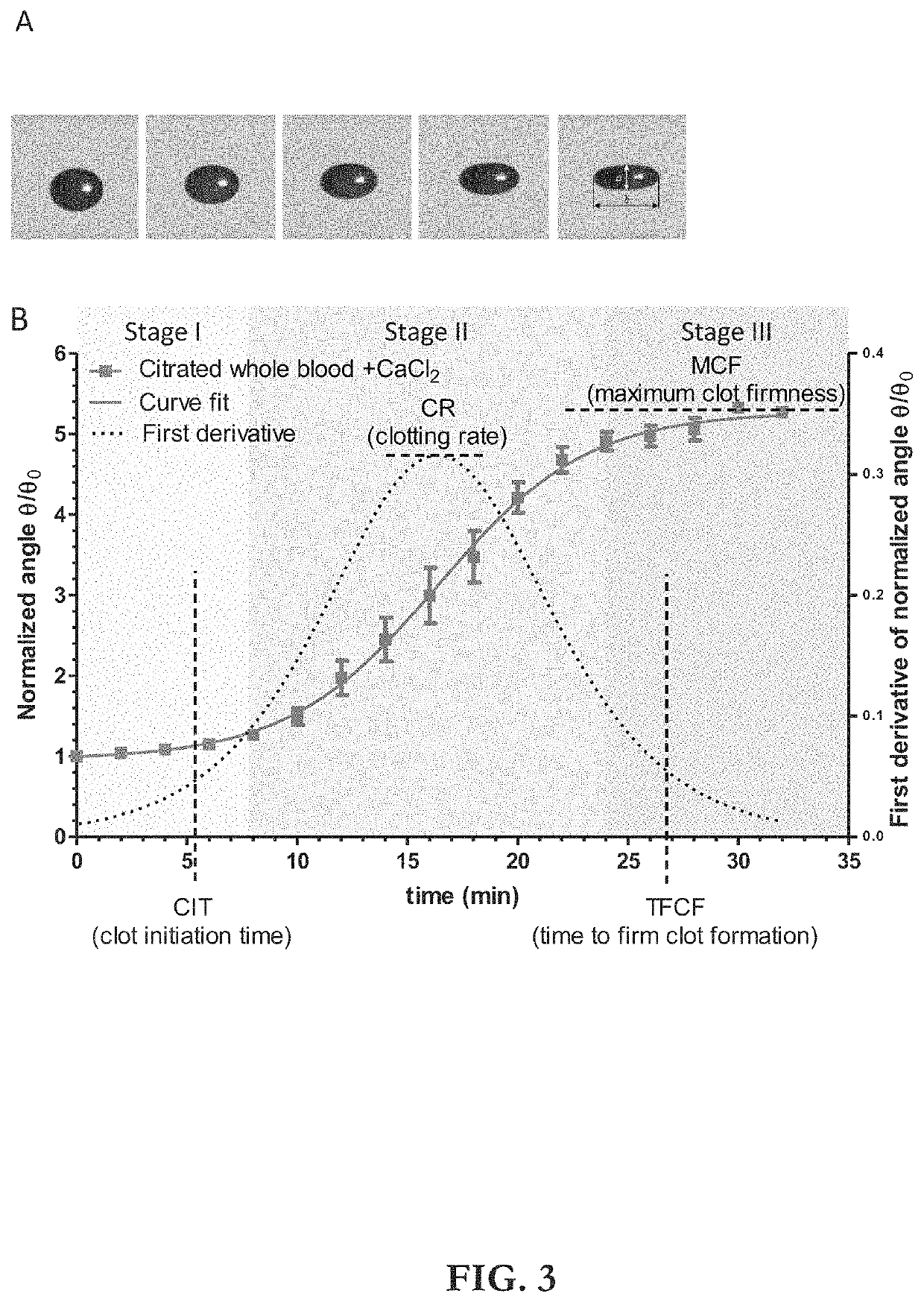
The disclosed apparatus, systems and methods relate to ATPA technology that provides a method for the real-time assessment of the polymerization of a sample, e.g., whole blood or blood plasma coagulation, by a non-contact acoustic tweezing device. The acoustic tweezing technology integrates photo-optical tests used in plasma coagulation assays with mechanical (viscoelastic) tests used in whole blood analysis. Its key disruptive features are the increased reliability and accuracy due to non-contact measurement, low sample volume requirement, relatively short procedure time (less than 10 minutes), and the ability to assess the level of Factor XIII function from measurements of the fibrin network formation time.
Owner:THE ADMINISTRATORS OF THE TULANE EDUCATIONAL FUND
FXIII detection for verifying serum sample and sample size and for detecting dilution
InactiveUS20050106640A1Microbiological testing/measurementBiological material analysisSerum samplesSources of error
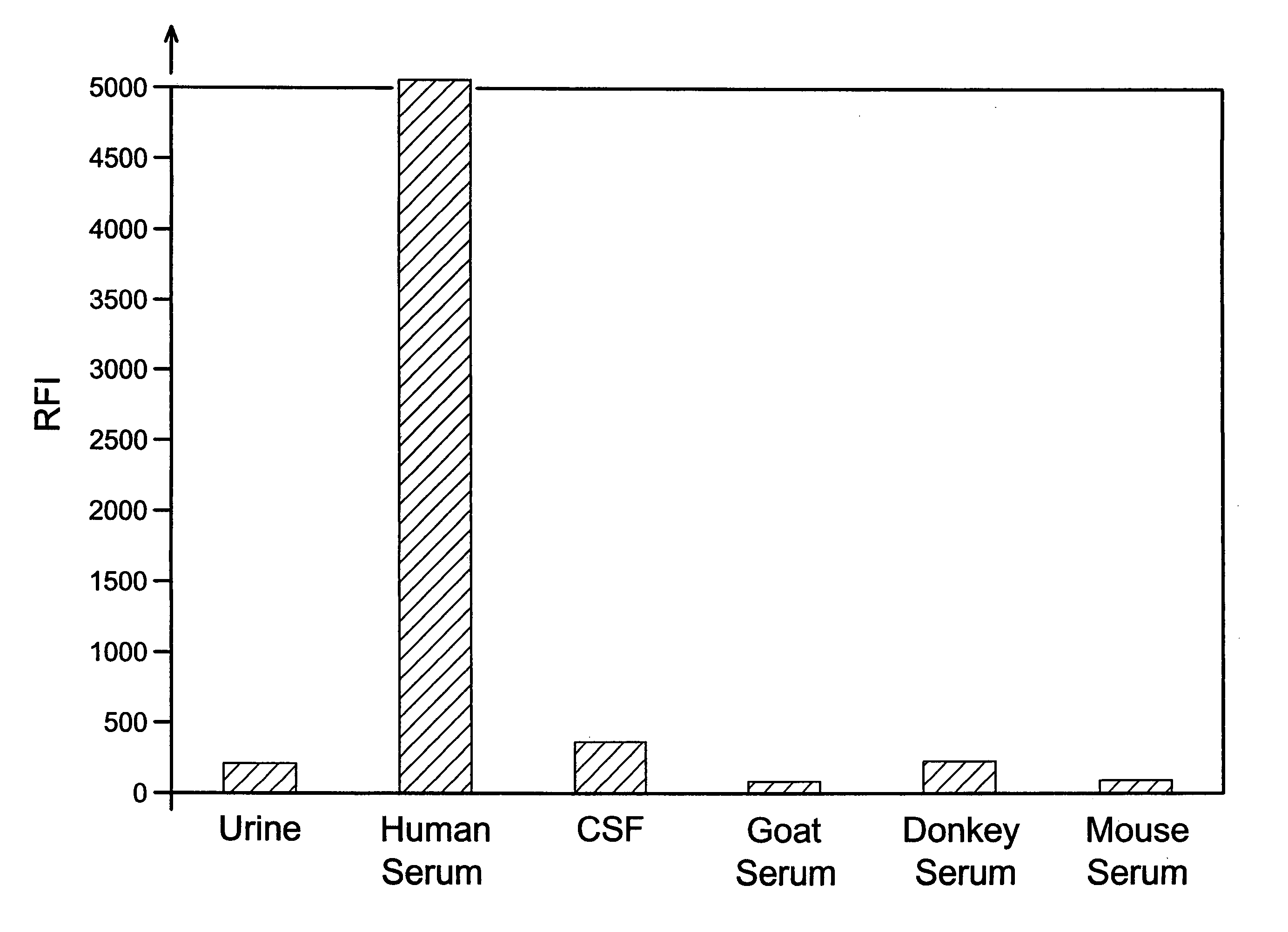
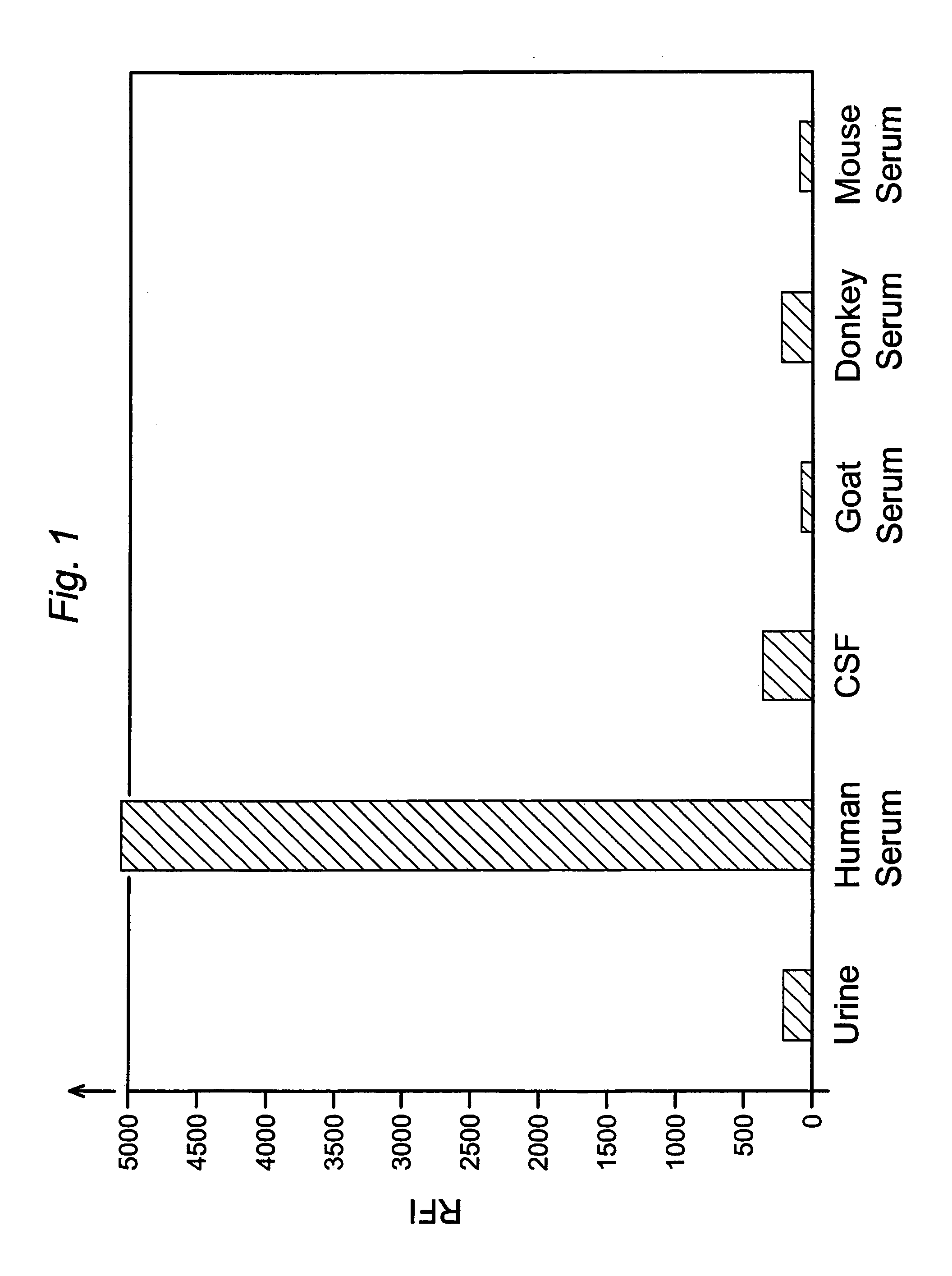
Analyses of serum samples for the presence and amount of either of the two subunits of human Factor XIII protein are used as a means of eliminating a significant source of error that arises in the testing of serum and plasma. For serum samples, a negative result of an analysis for the presence of subunit a is a means of verifying that a sample is indeed serum, while a negative or positive result for subunit a serves to distinguish serum (negative) from plasma (positive). A positive result for the presence of subunit b is a means of verifying that the sample is either serum or plasma and not any other biological fluid. A quantitative analysis of subunit b is a means of verifying that the sample is of the intended volume rather than having been reduced in volume due to improper sampling. A quantitative analysis of subunit b is also a means of verifying the dilution of a sample of either serum or plasma.
Owner:BIO RAD LAB INC
Method of quality control testing a Factor XIII containing sample
InactiveUS9080200B2Accurate and sensitive determinationAccurate measurementComponent separationMicrobiological testing/measurementQuality controlIon exchange
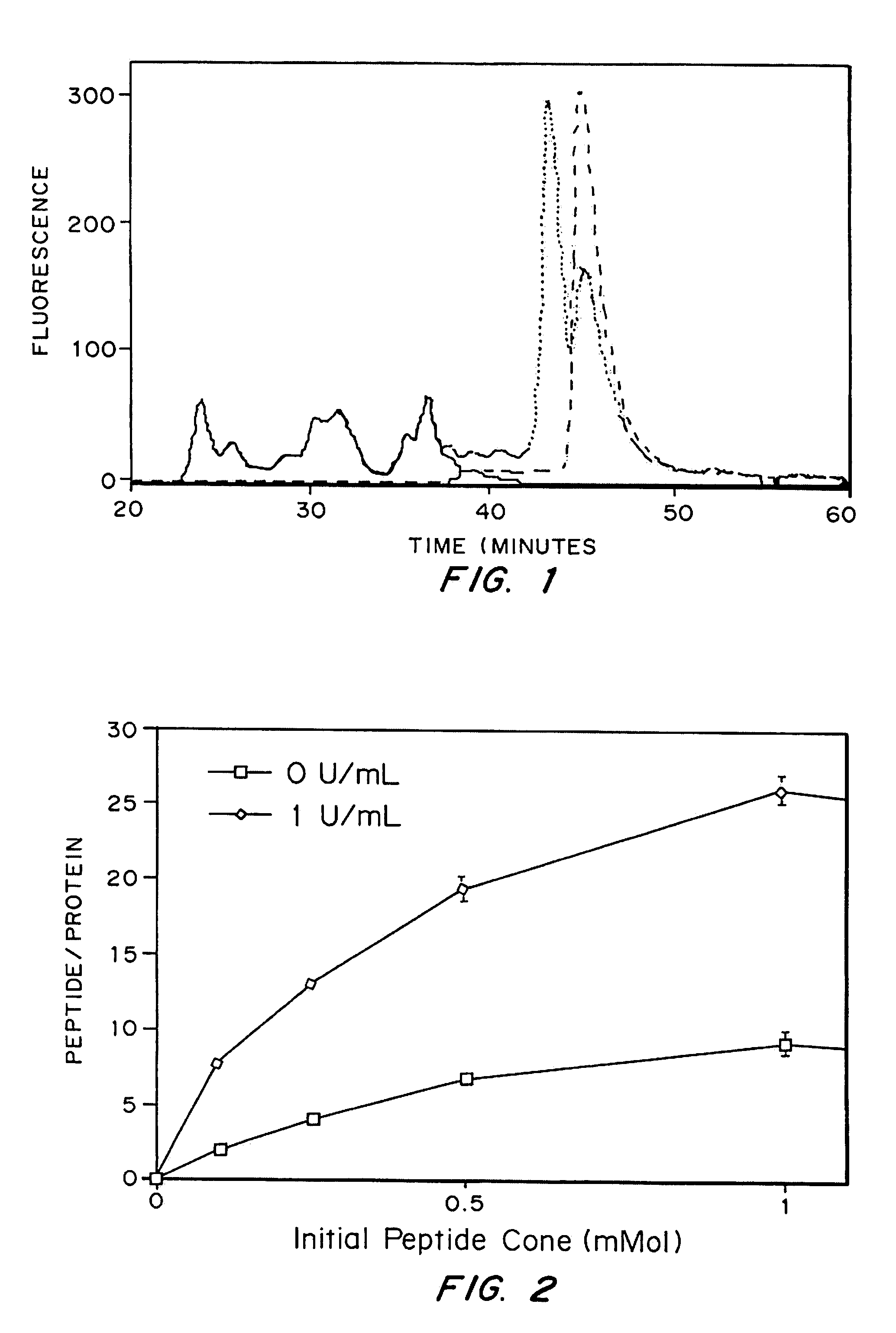
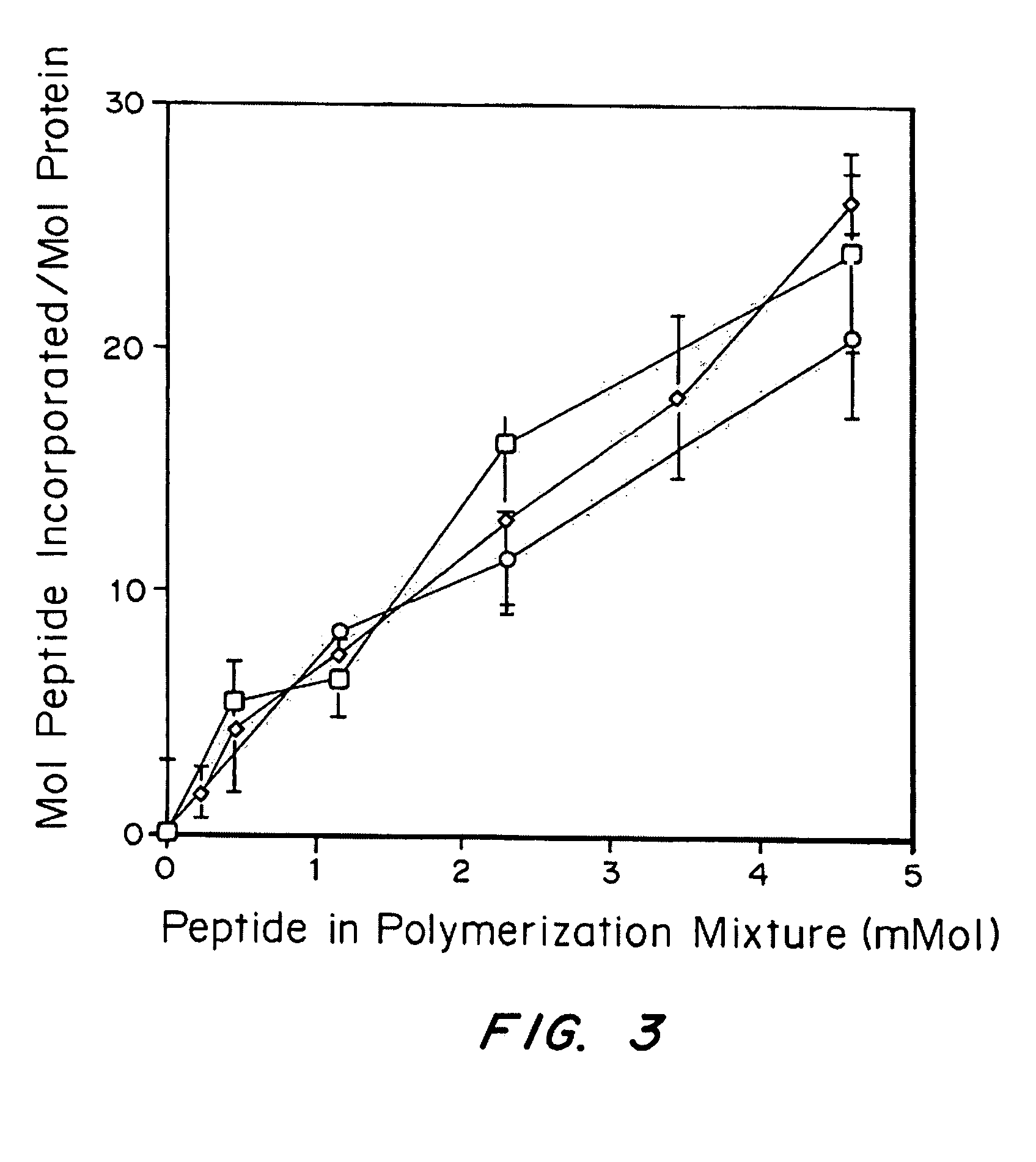
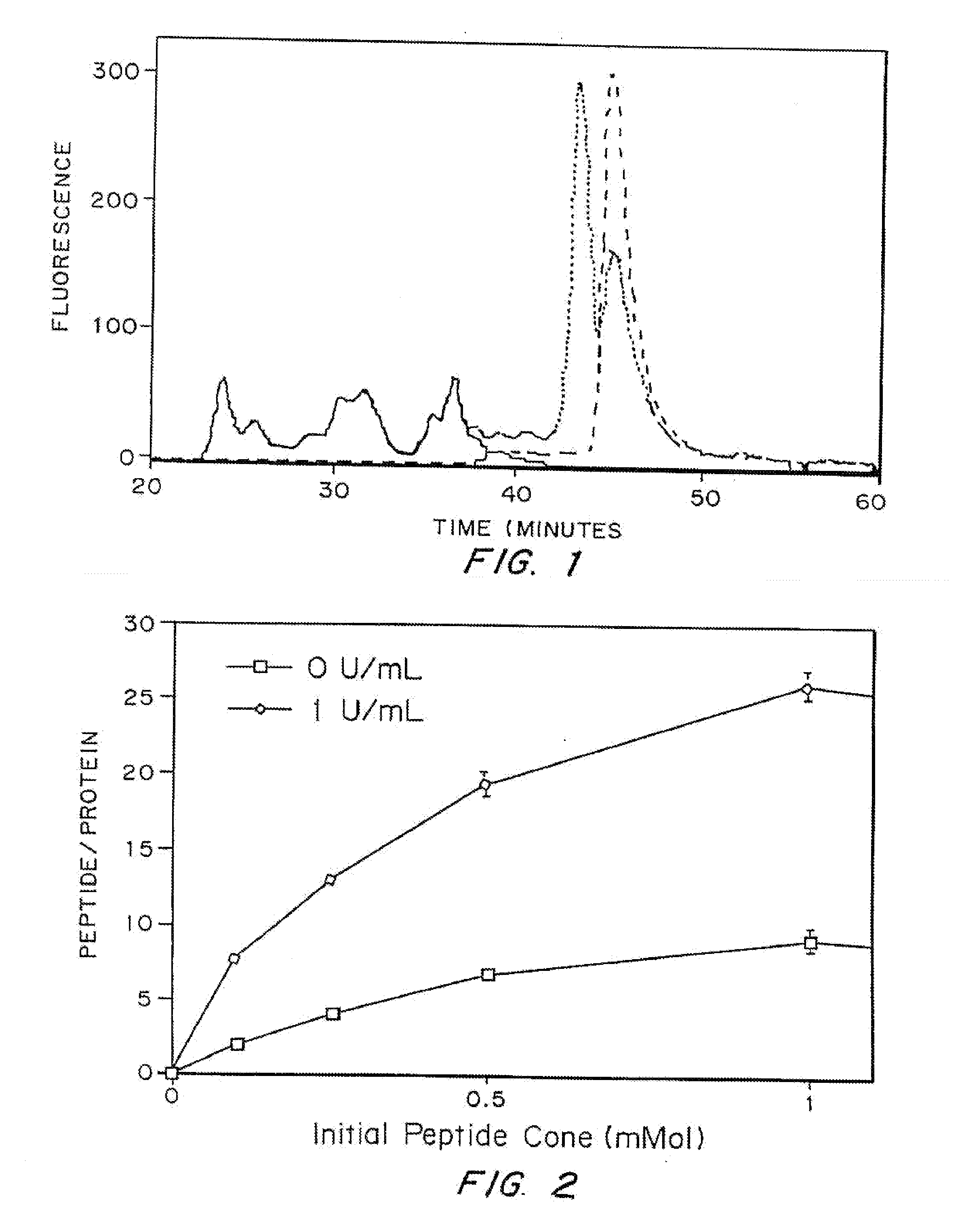
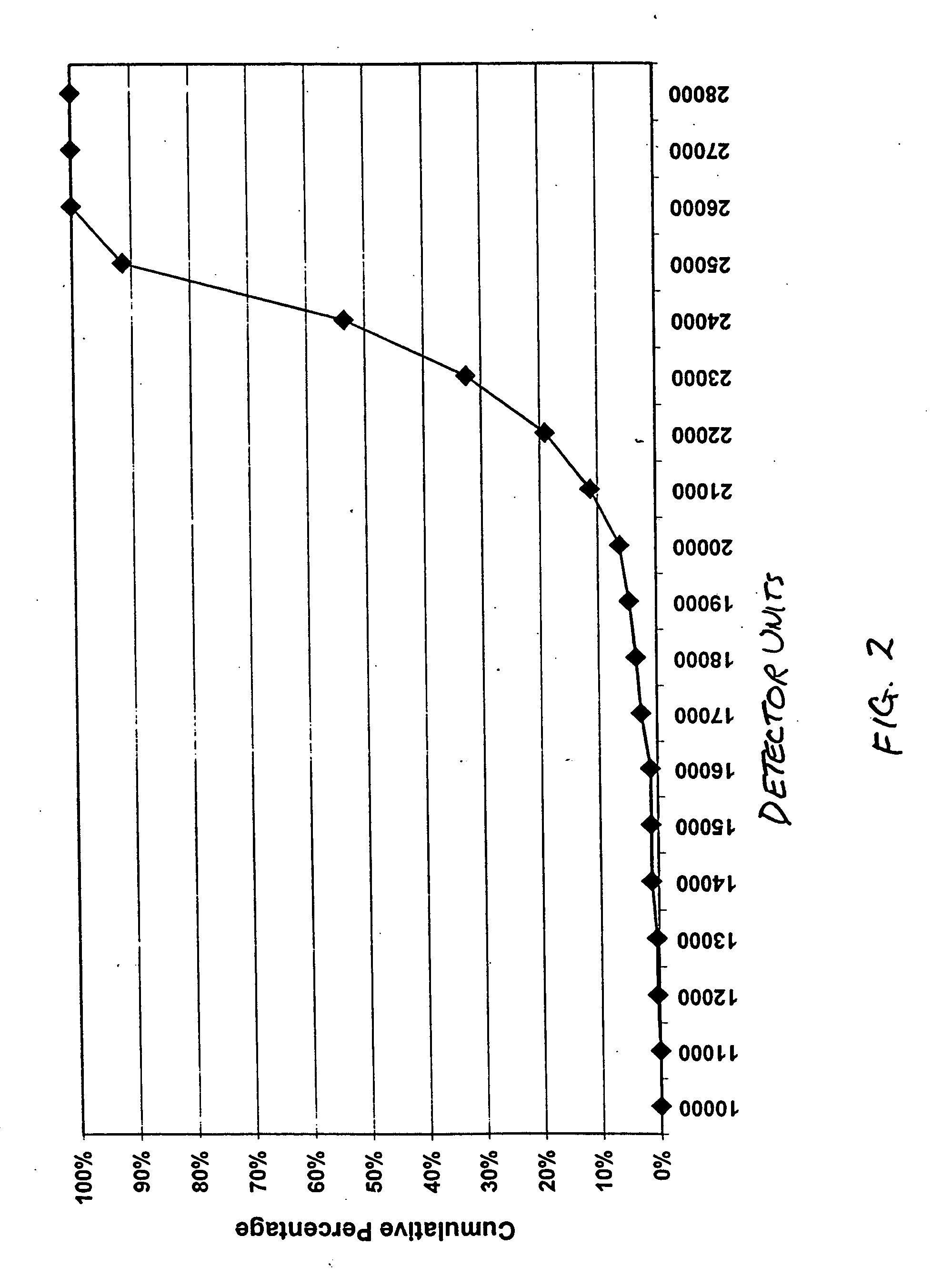
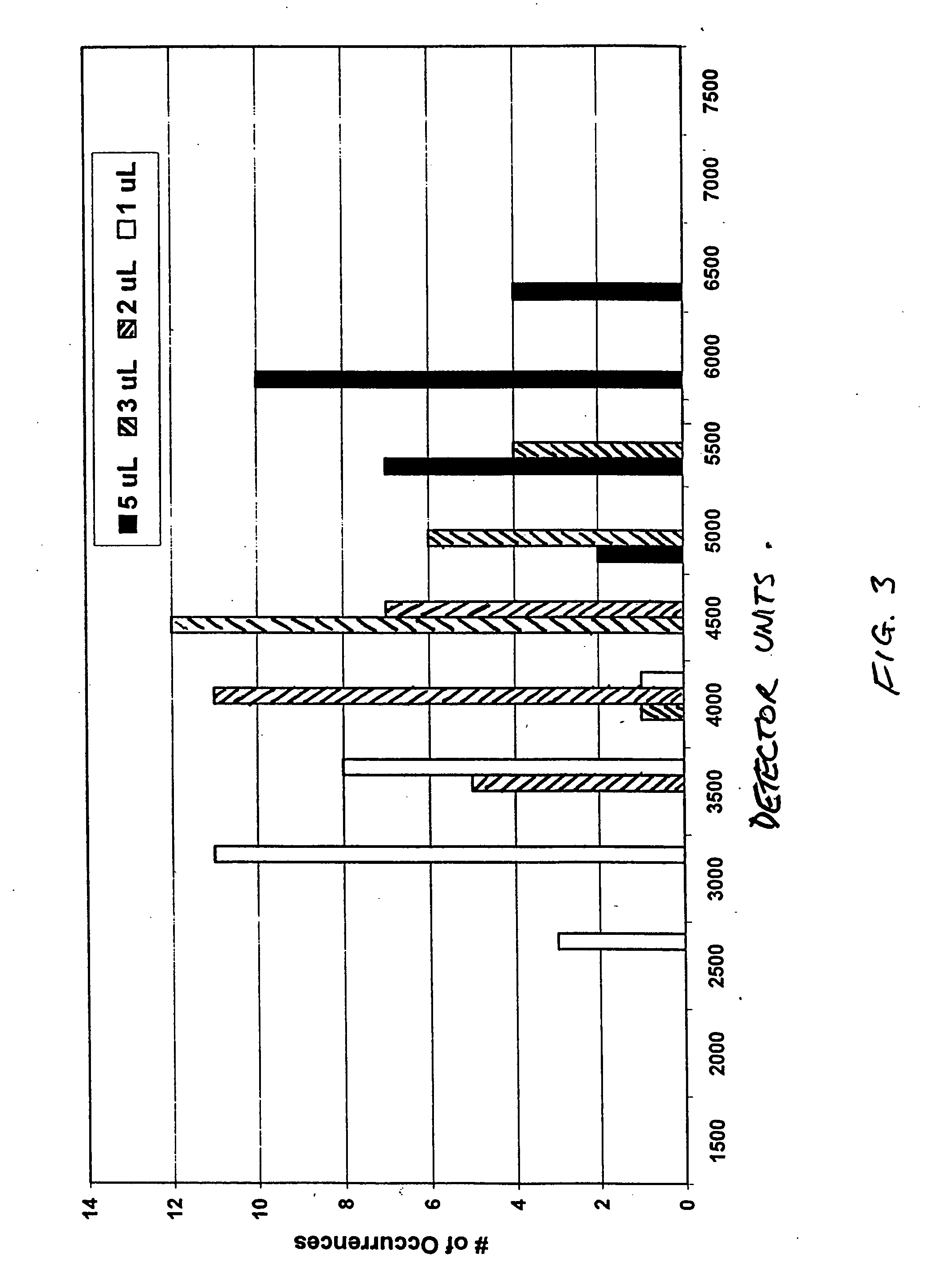
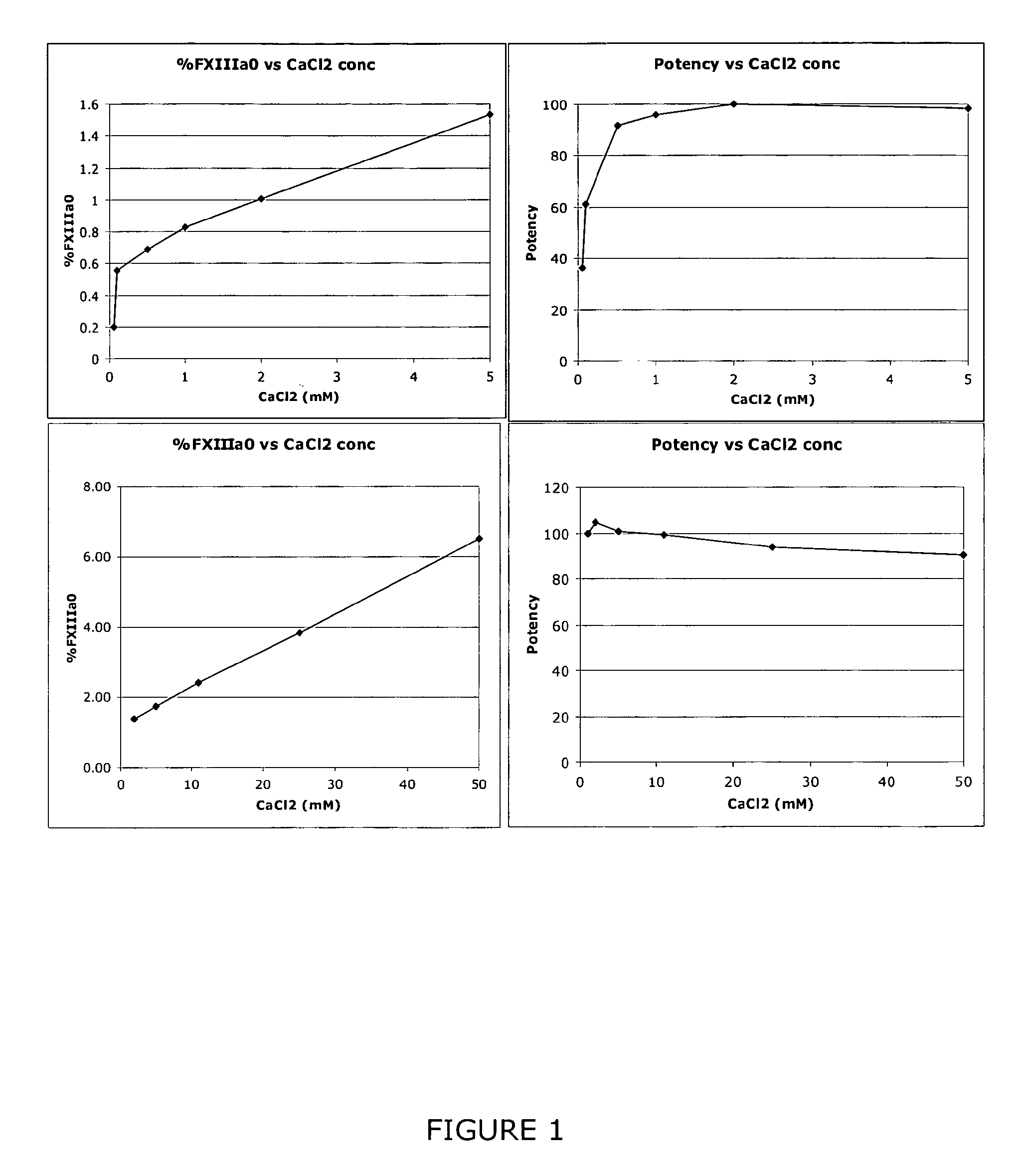
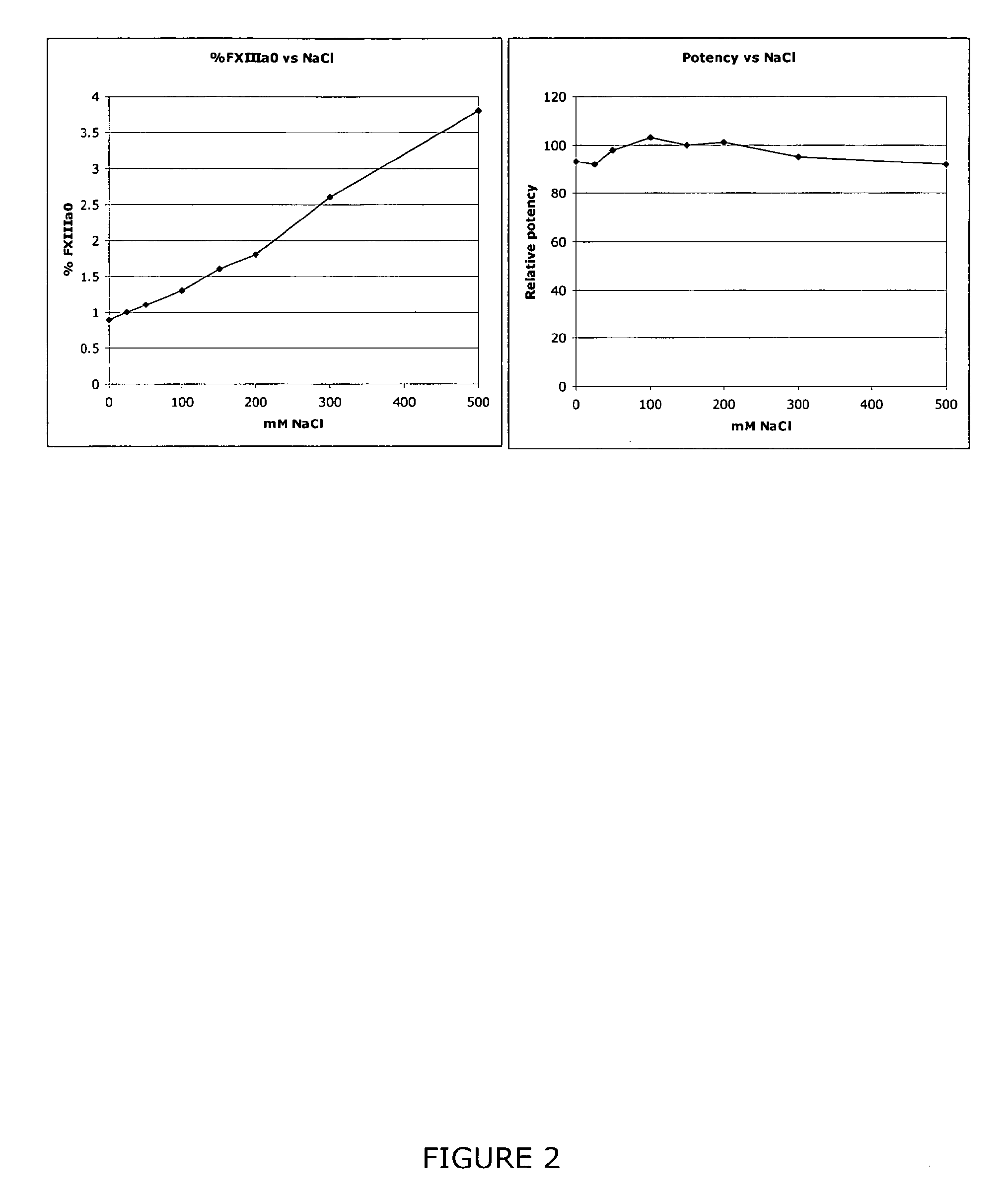
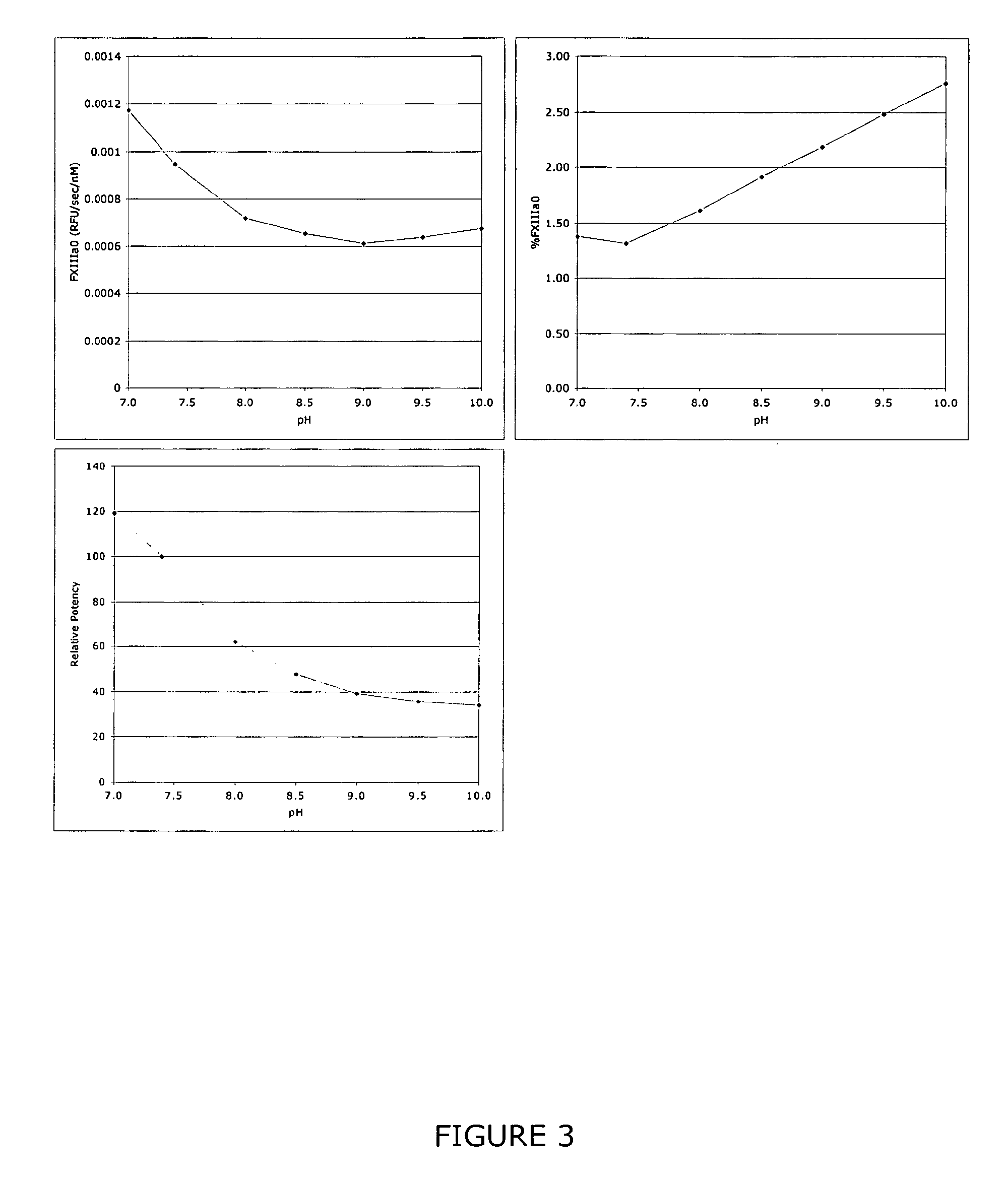
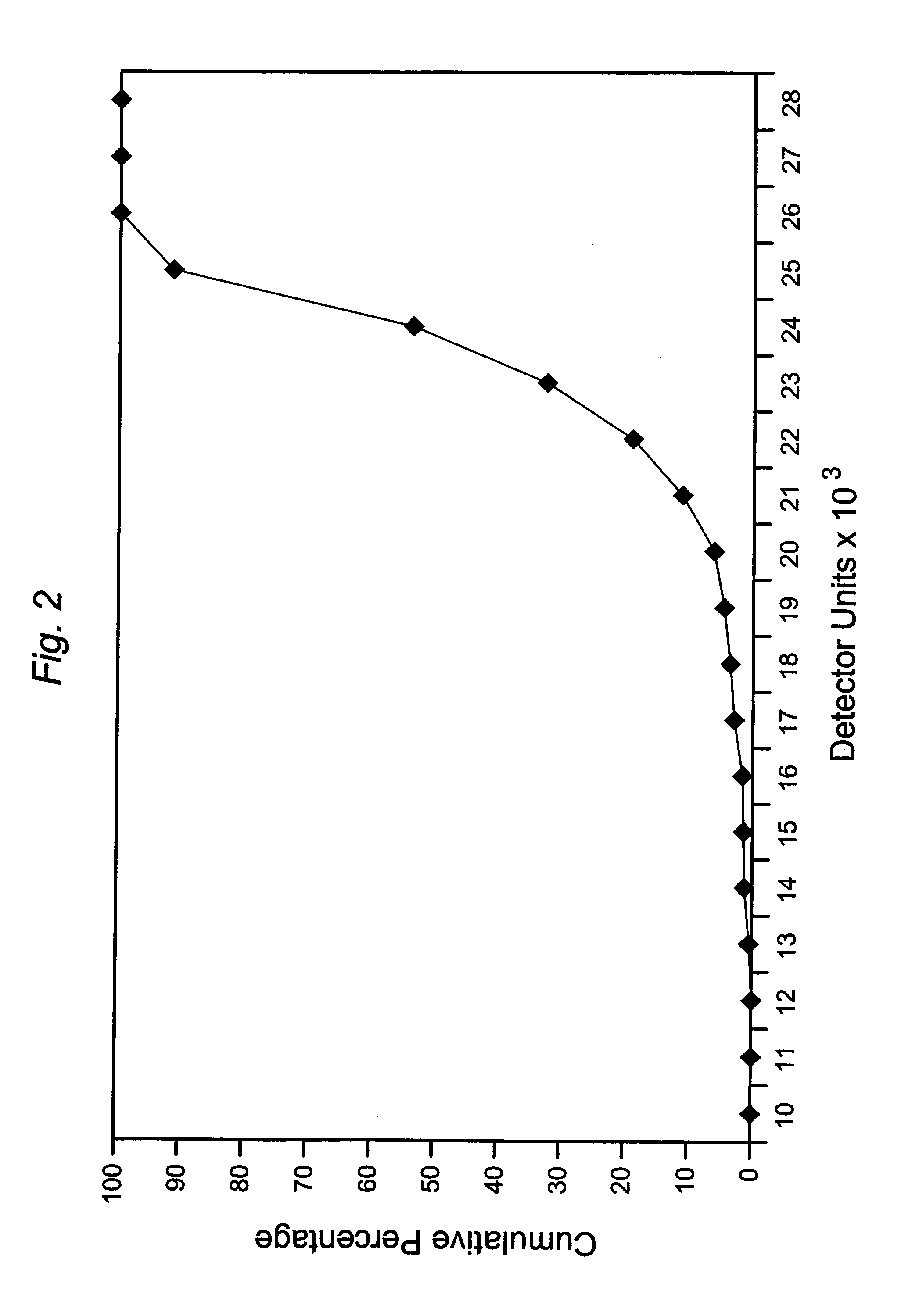
The invention relates to a method of quality control testing a Factor XIII (FXIII) containing sample which comprises the step of detecting the presence of and / or measuring the concentration of pre-activated FXIII (FXIIIao) in said sample and to a quality control kit for determining the quality of a Factor XIII (FXIII) containing sample. Preferably, an anion-exchange chromatographic column is used, as well as the fluorescent substrate Abz-NE (Cad-Dnp) EQVS PLTLLK-OH.
Owner:NOVO NORDISK HEALTH CARE AG
Method for inhibiting the formation of seromas using factor XIII
Use of factor XIII for inhibiting the formation of seromas by administering factor XIII. The factor XIII can be administered locally at the site of a wound or surgery or administered systemically. If the factor XIII is administered locally, it can be activated or non-activated and may be administered in conjunction with activated thrombin.
Owner:ZYMOGENETICS INC
FXIII detection for verifying serum sample and sample size and for detecting dilution
InactiveUS7138245B2Microbiological testing/measurementBiological material analysisSerum igeSerum samples
Analyses of serum samples for the presence and amount of either of the two subunits of human Factor XIII protein are used as a means of eliminating a significant source of error that arises in the testing of serum and plasma. For serum samples, a negative result of an analysis for the presence of subunit a is a means of verifying that a sample is indeed serum, while a negative or positive result for subunit a serves to distinguish serum (negative) from plasma (positive). A positive result for the presence of subunit b is a means of verifying that the sample is either serum or plasma and not any other biological fluid. A quantitative analysis of subunit b is a means of verifying that the sample is of the intended volume rather than having been reduced in volume due to improper sampling. A quantitative analysis of subunit b is also a means of verifying the dilution of a sample of either serum or plasma.
Owner:BIO RAD LAB INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com