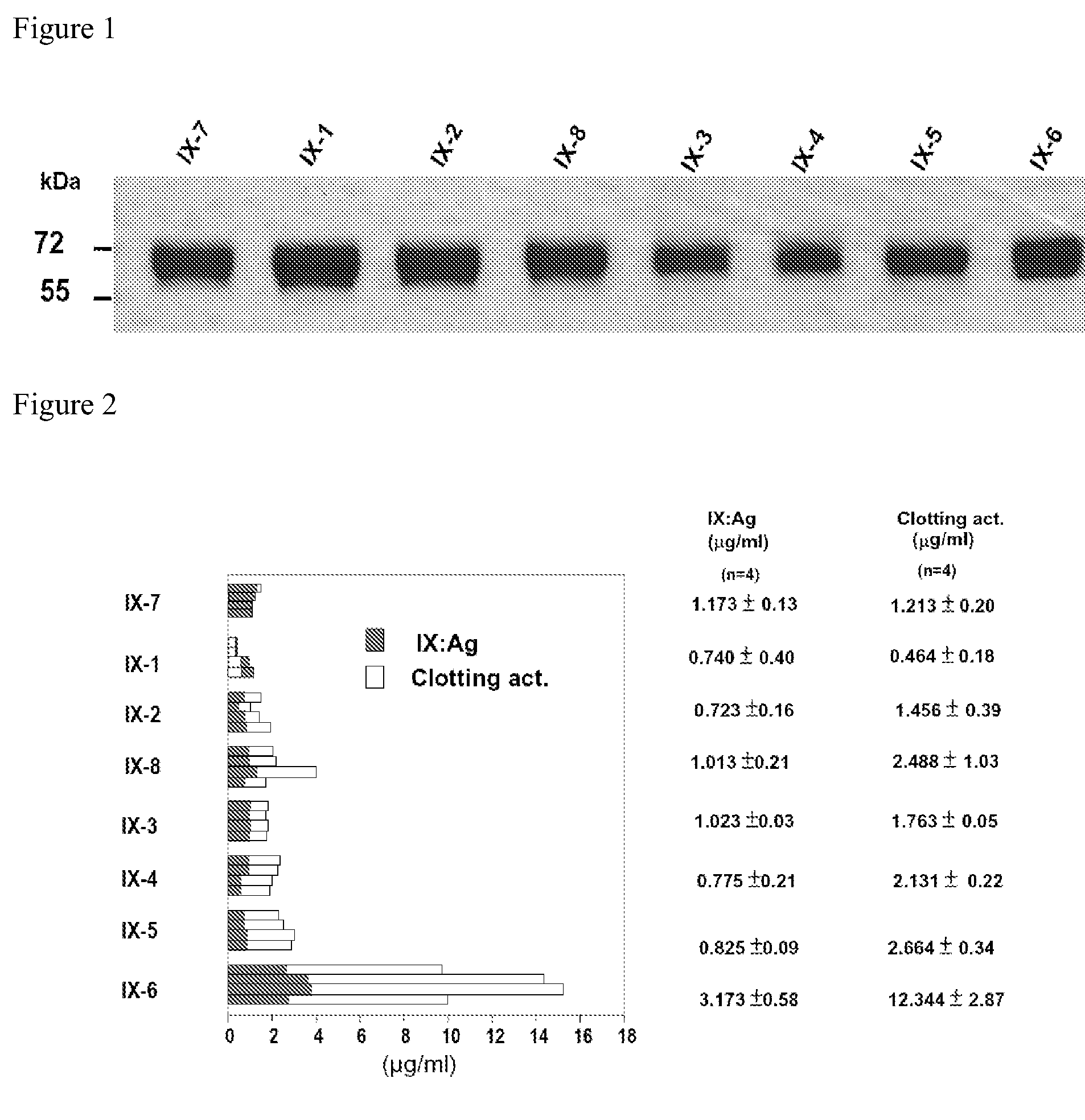
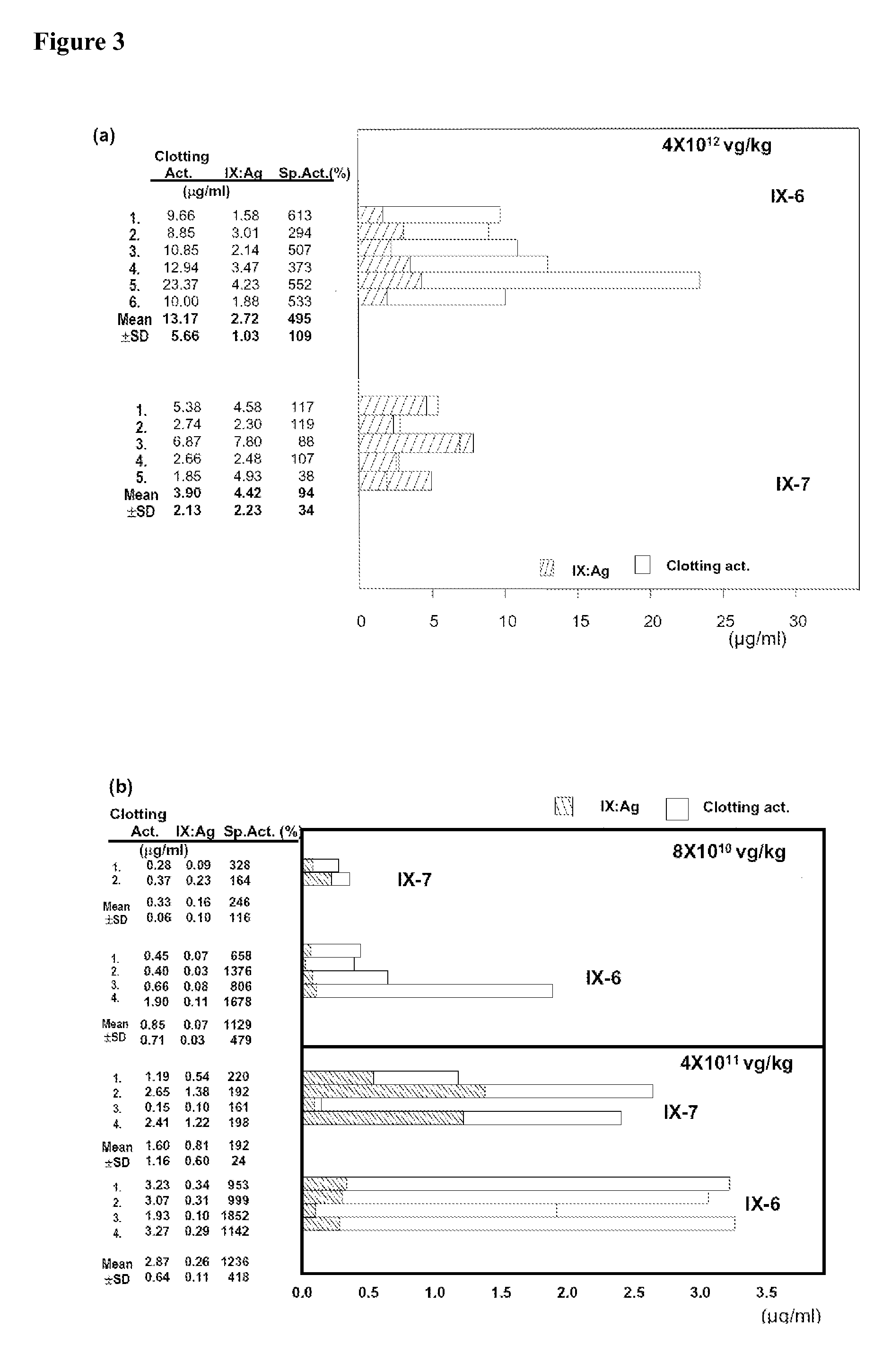
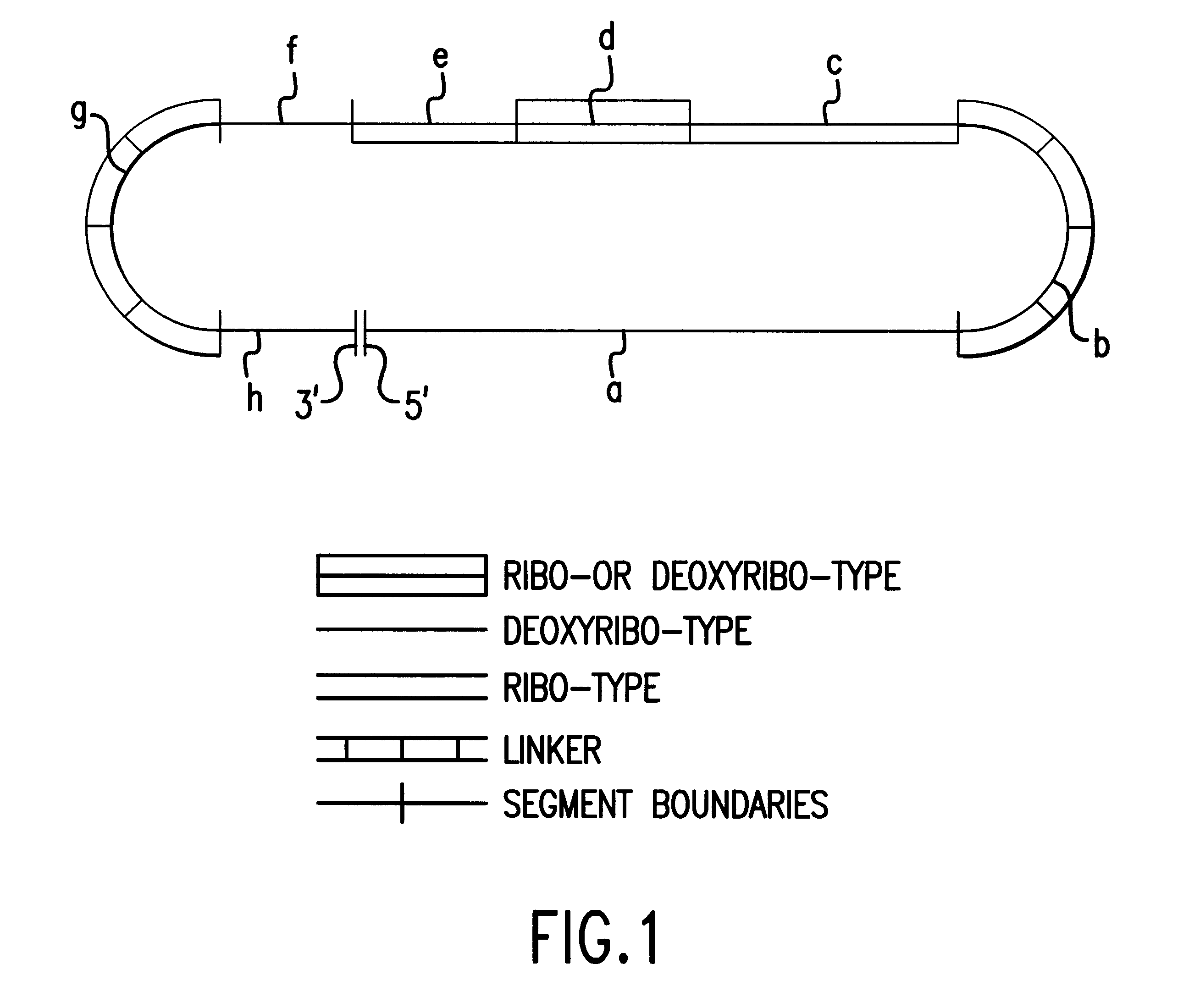
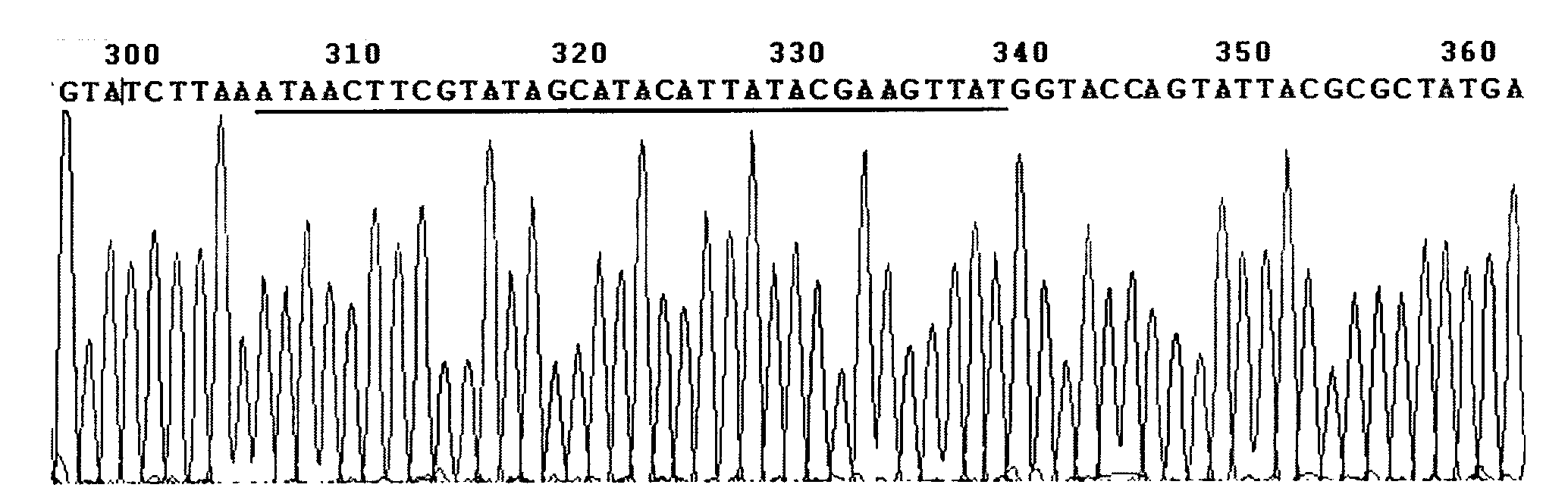
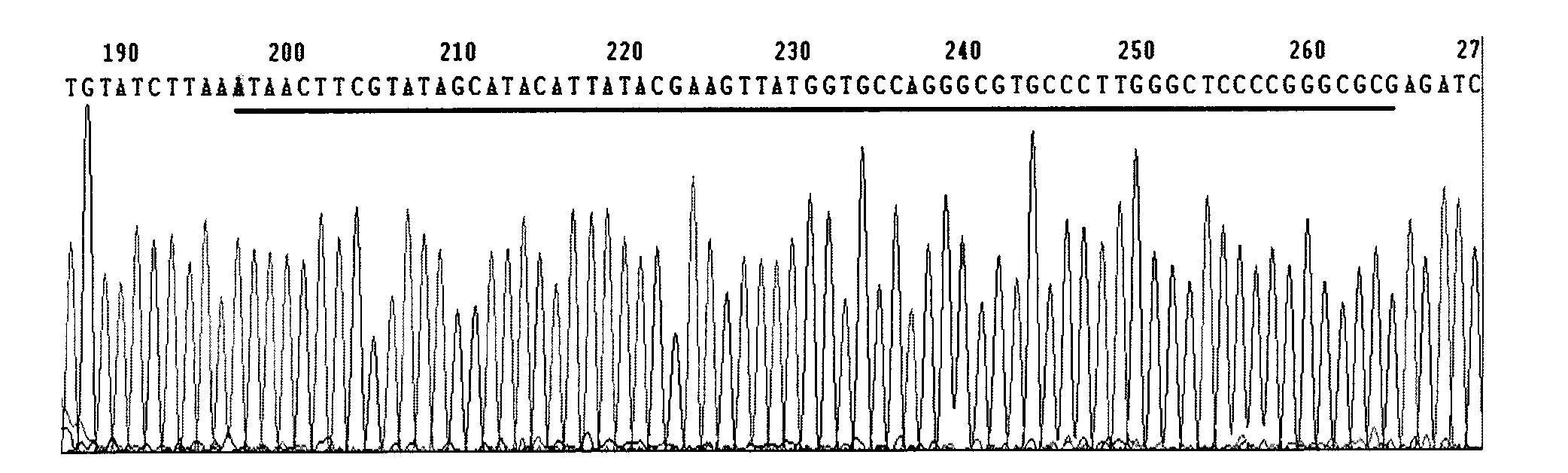
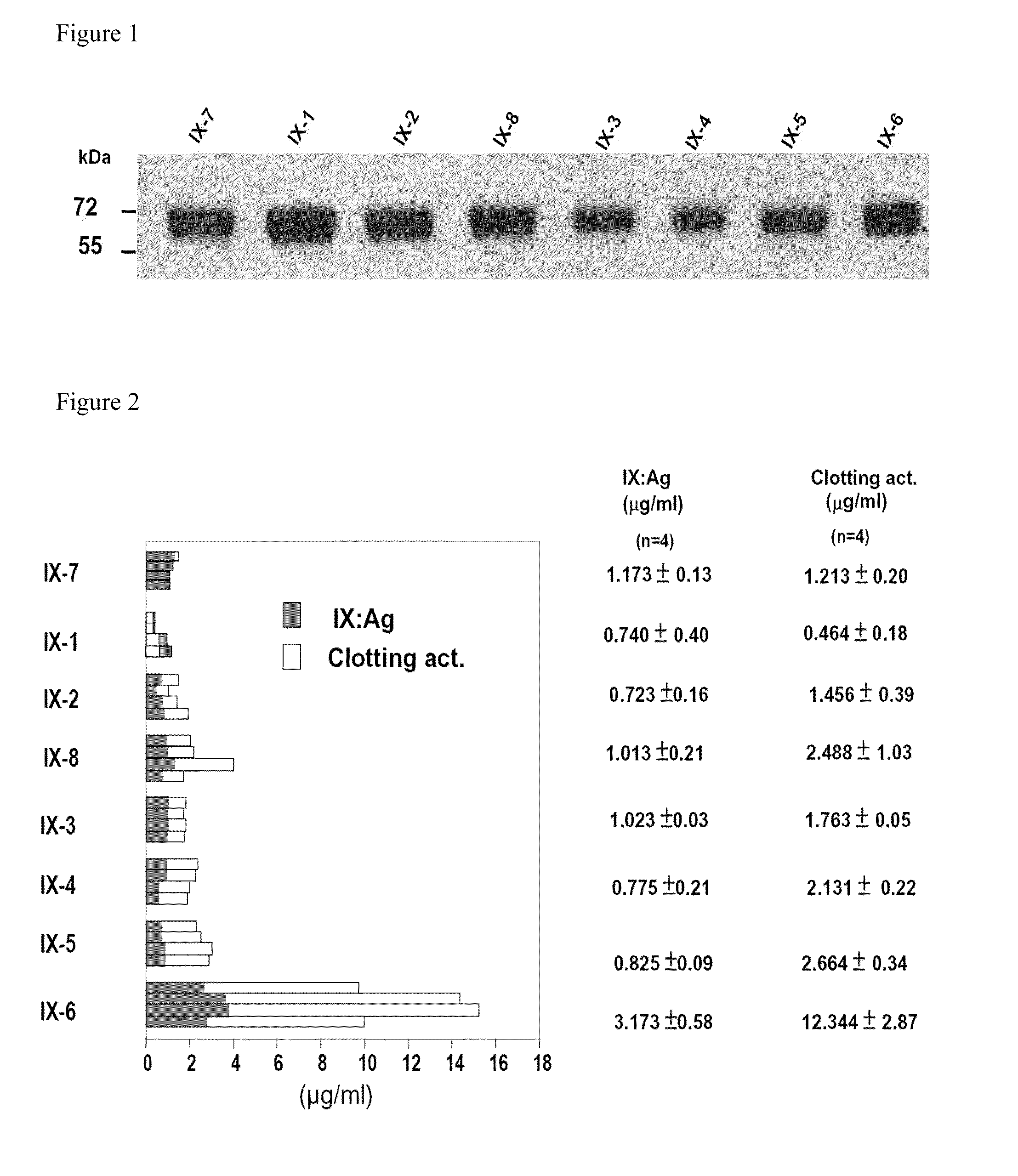
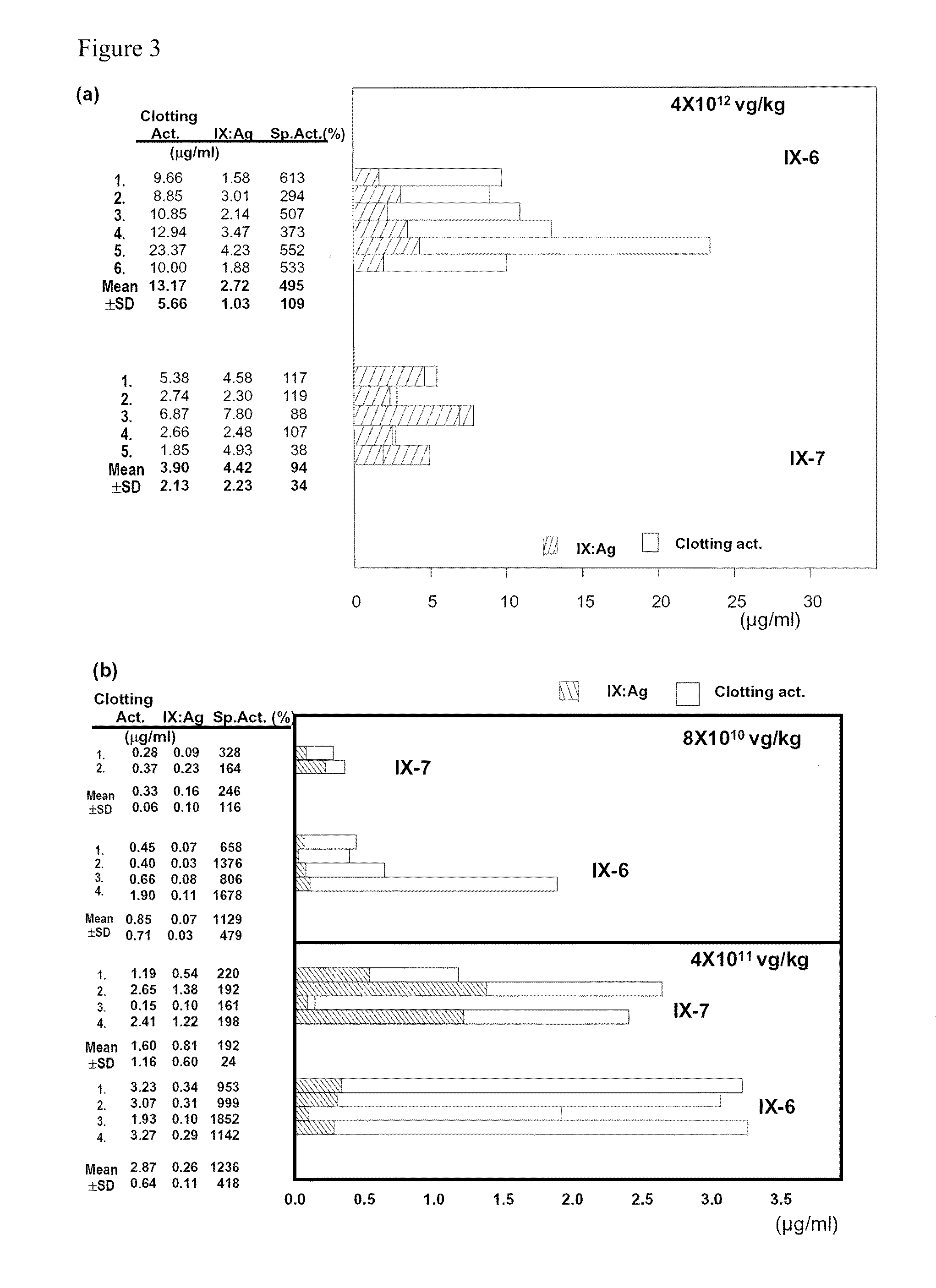
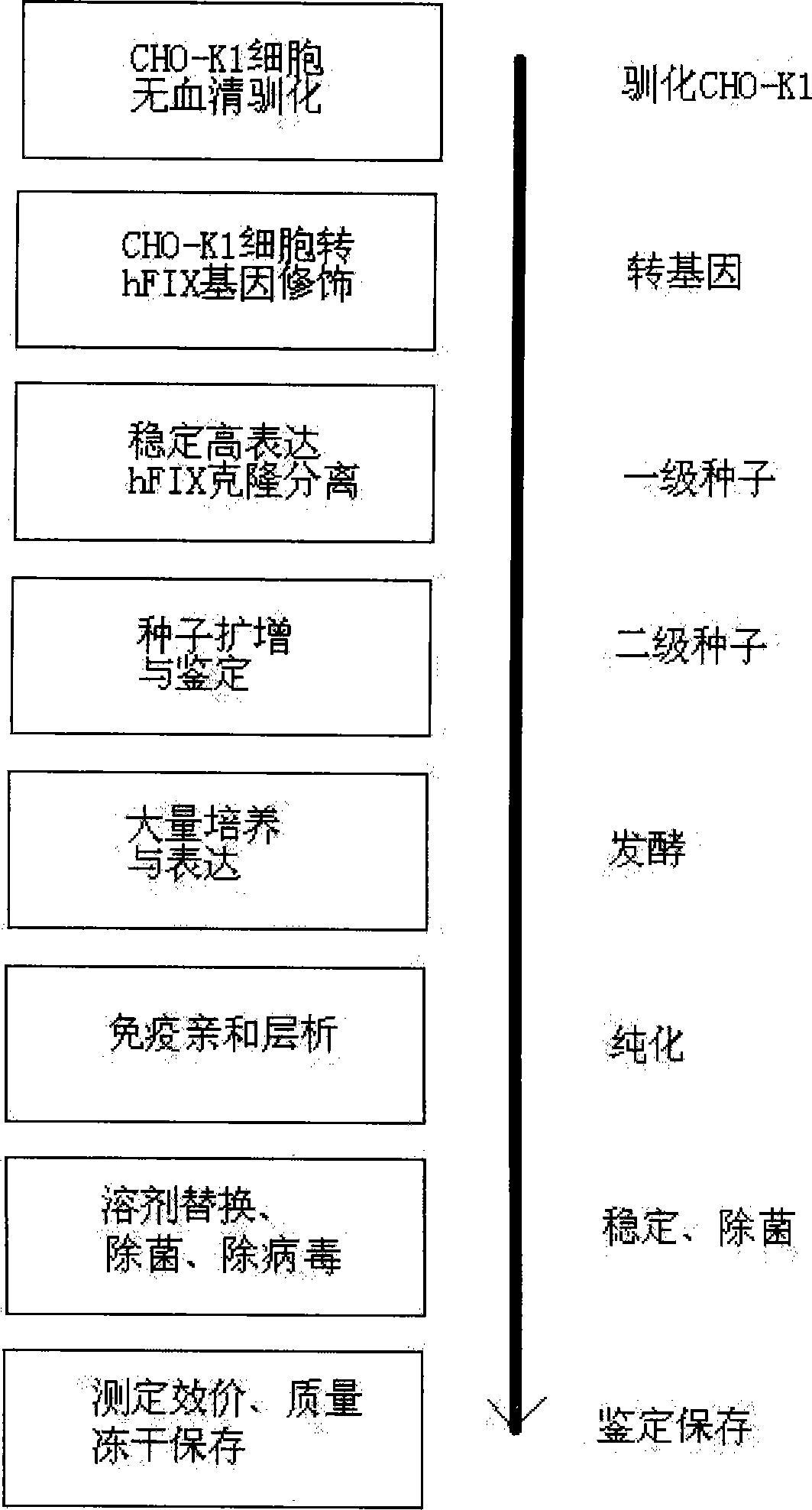
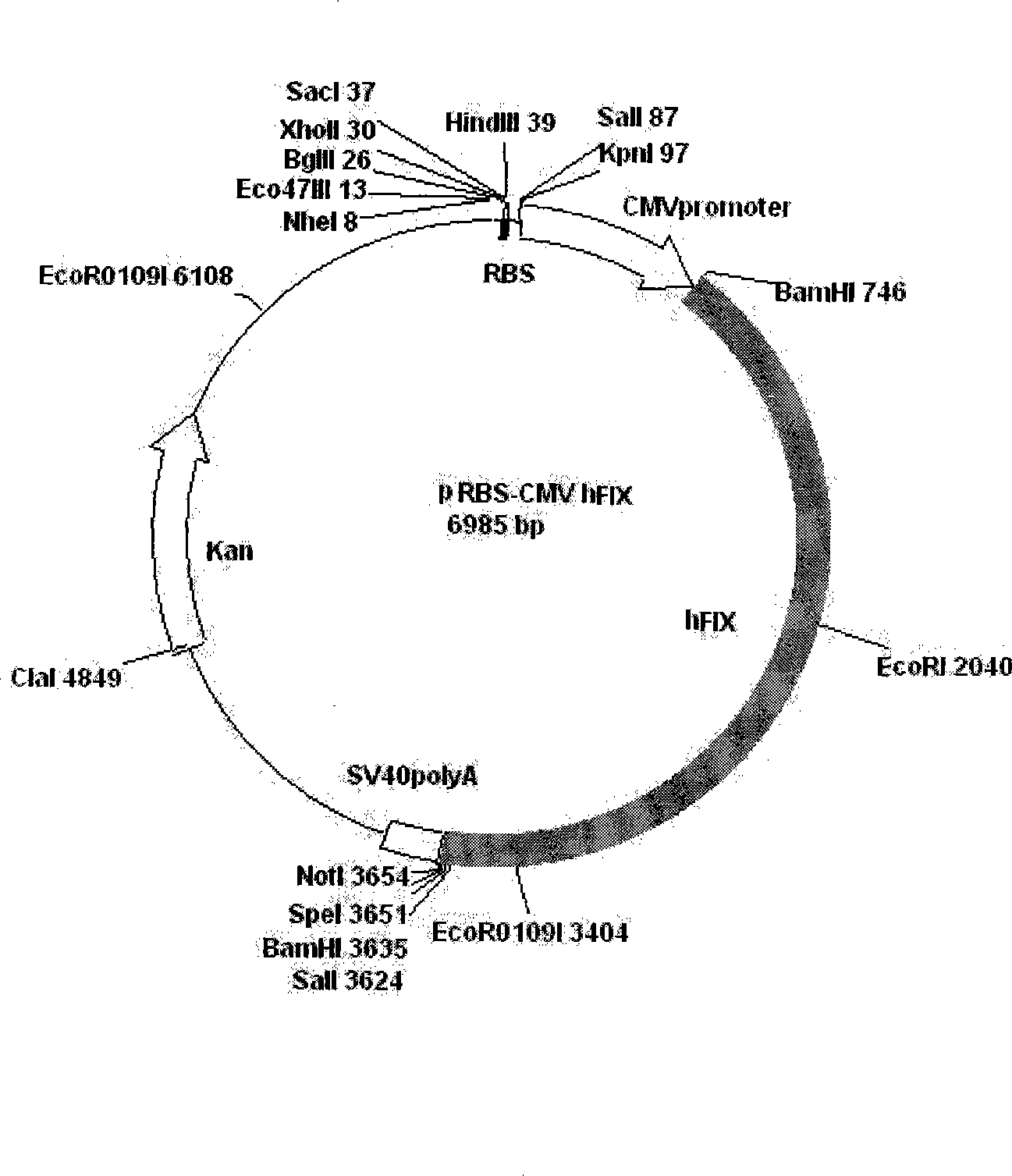
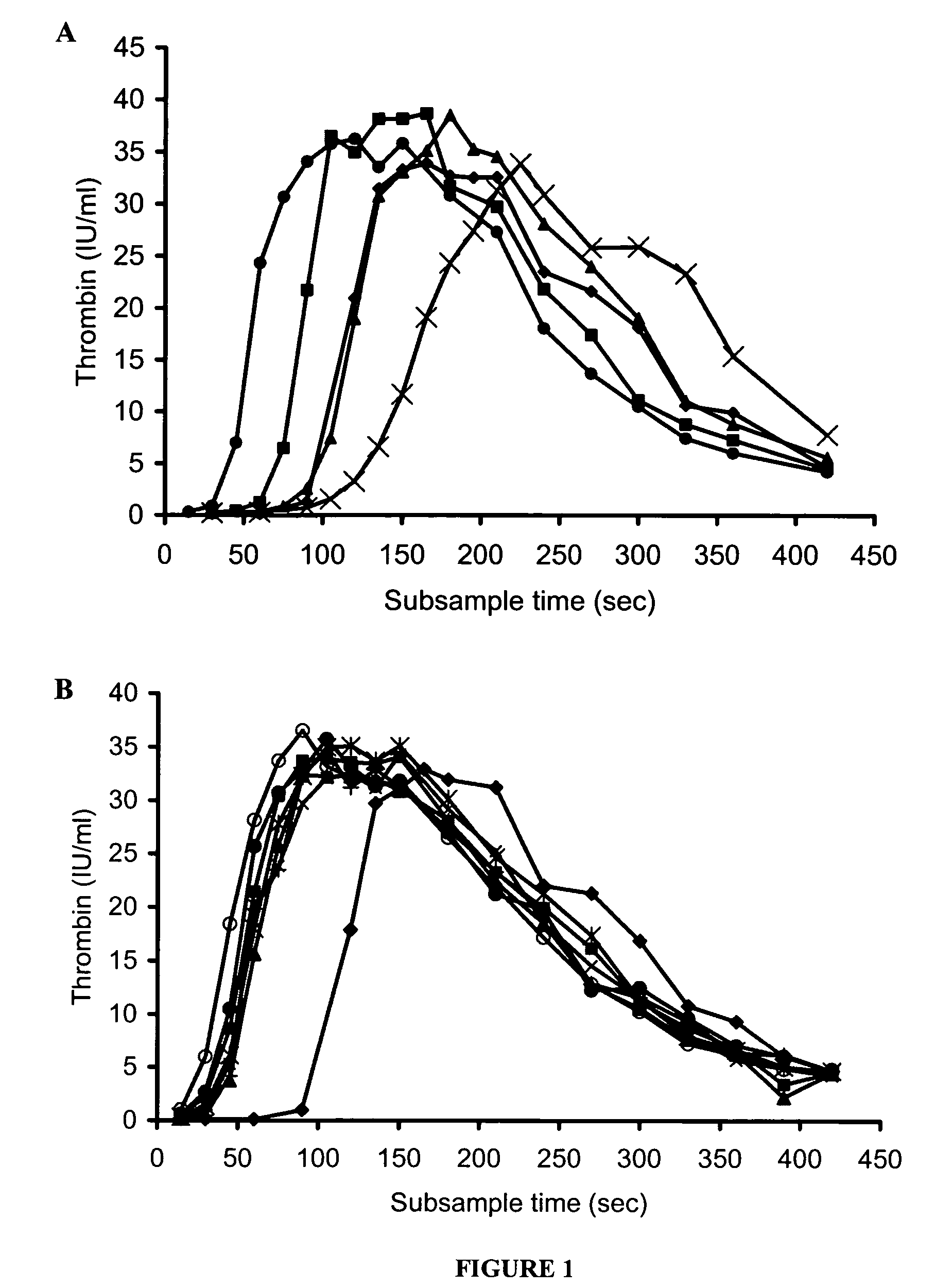
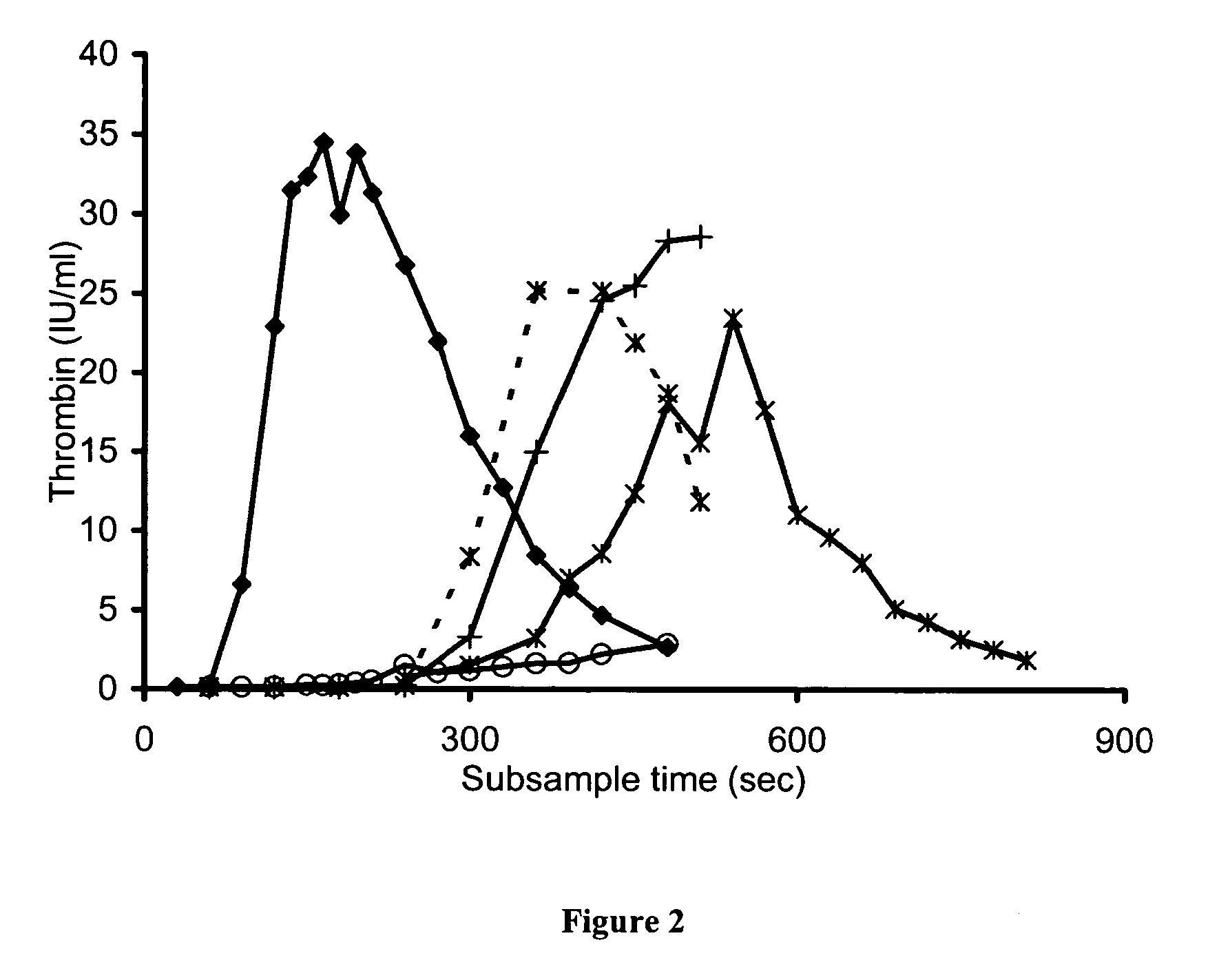
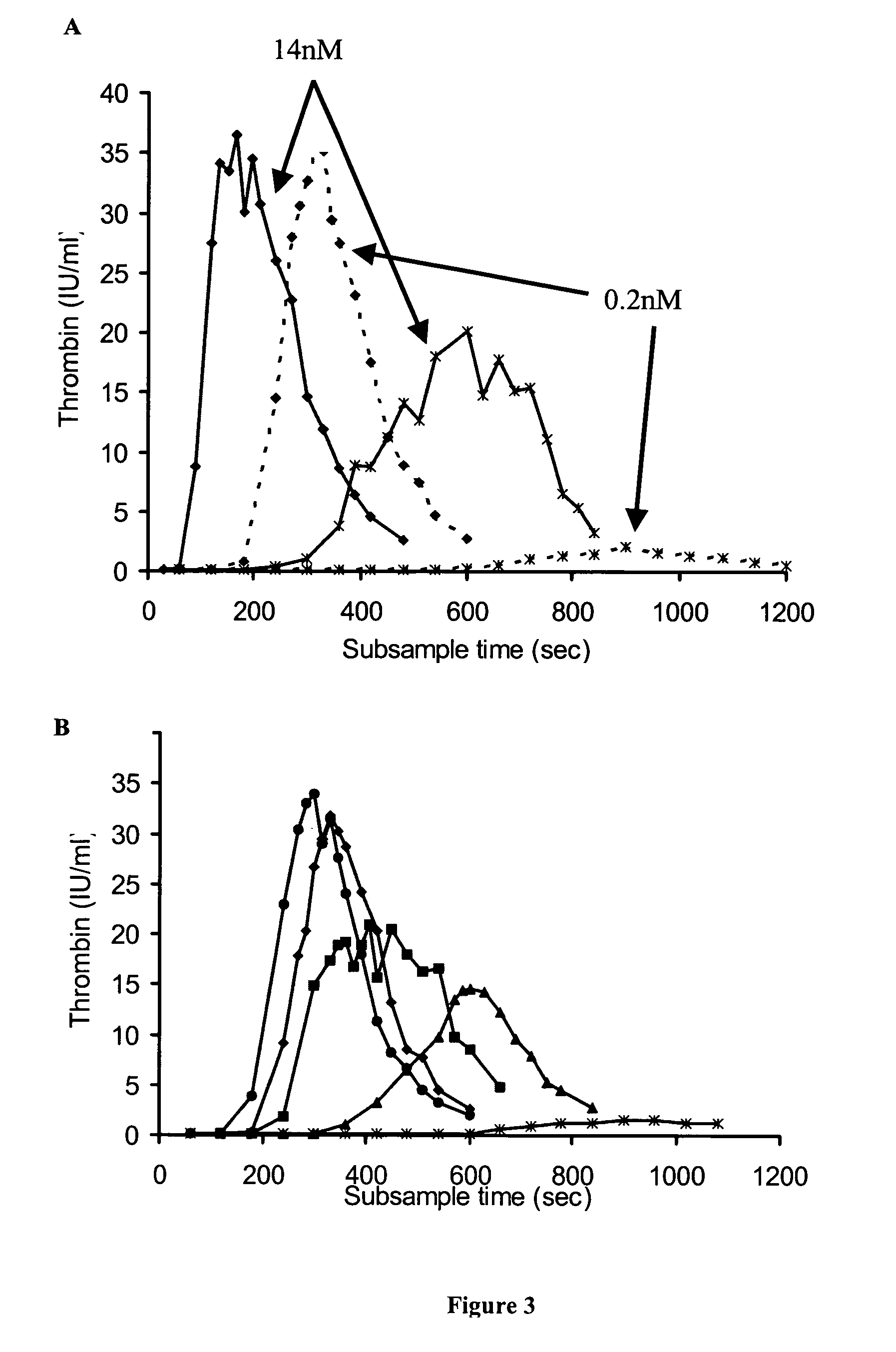
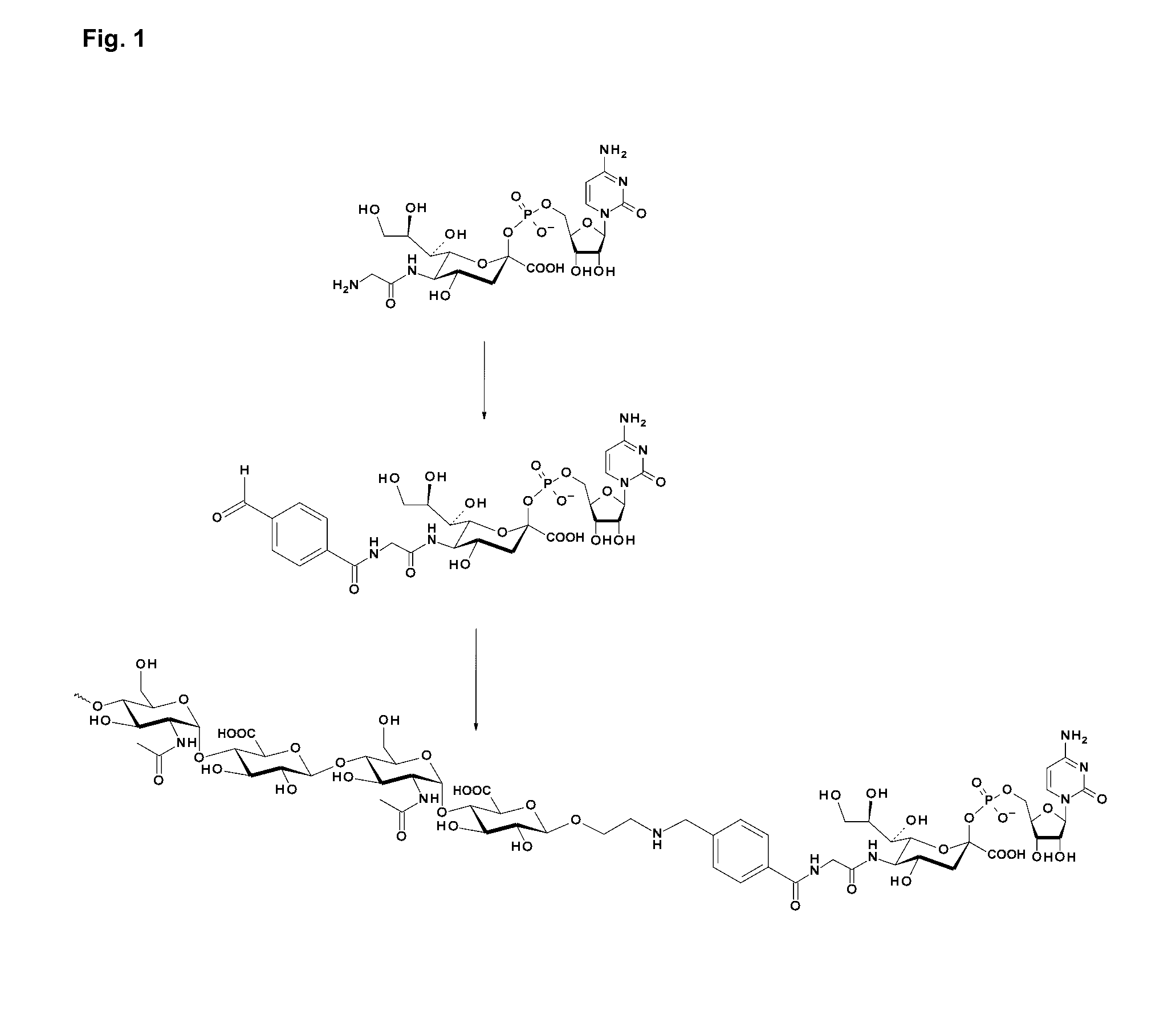
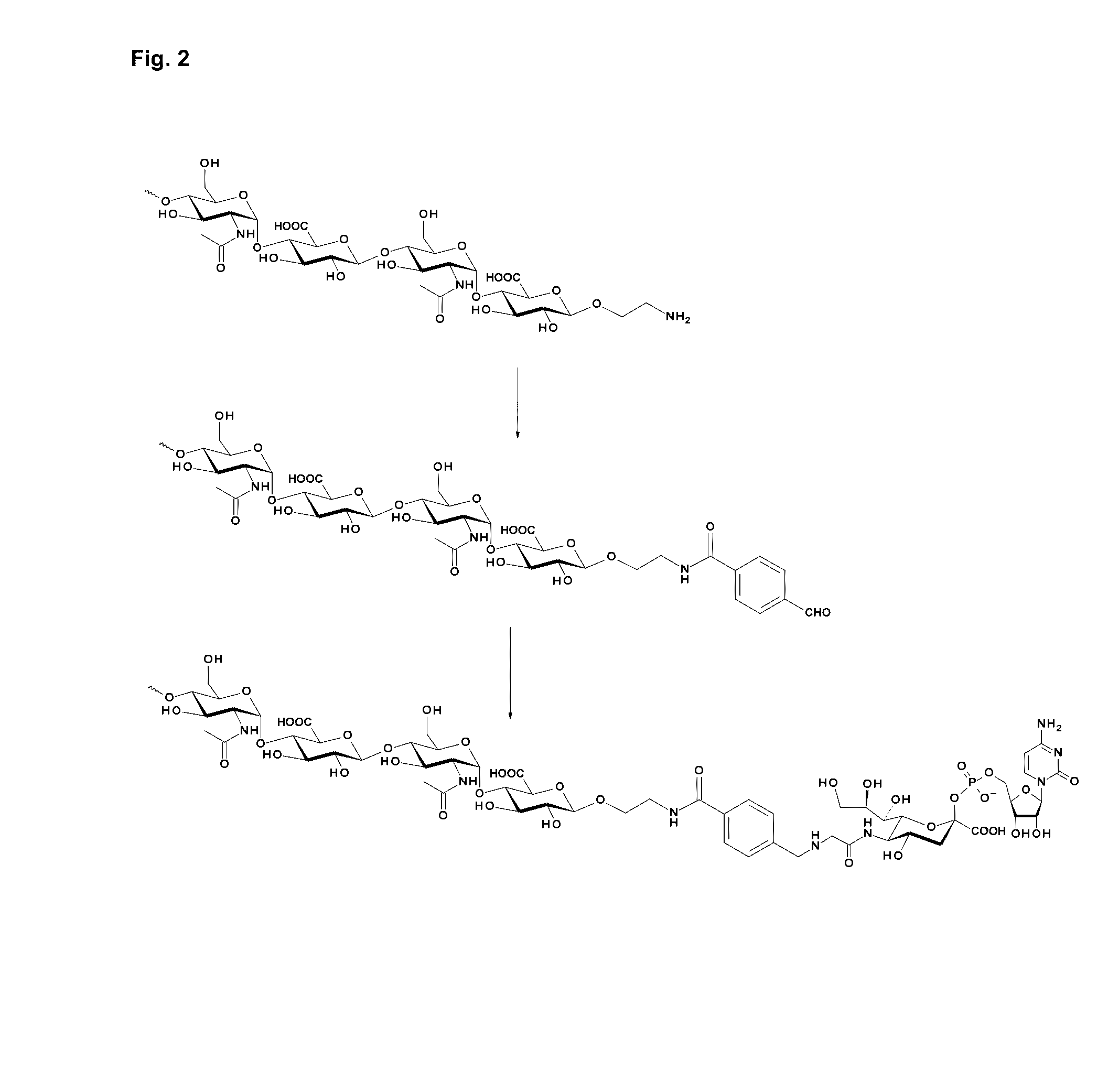
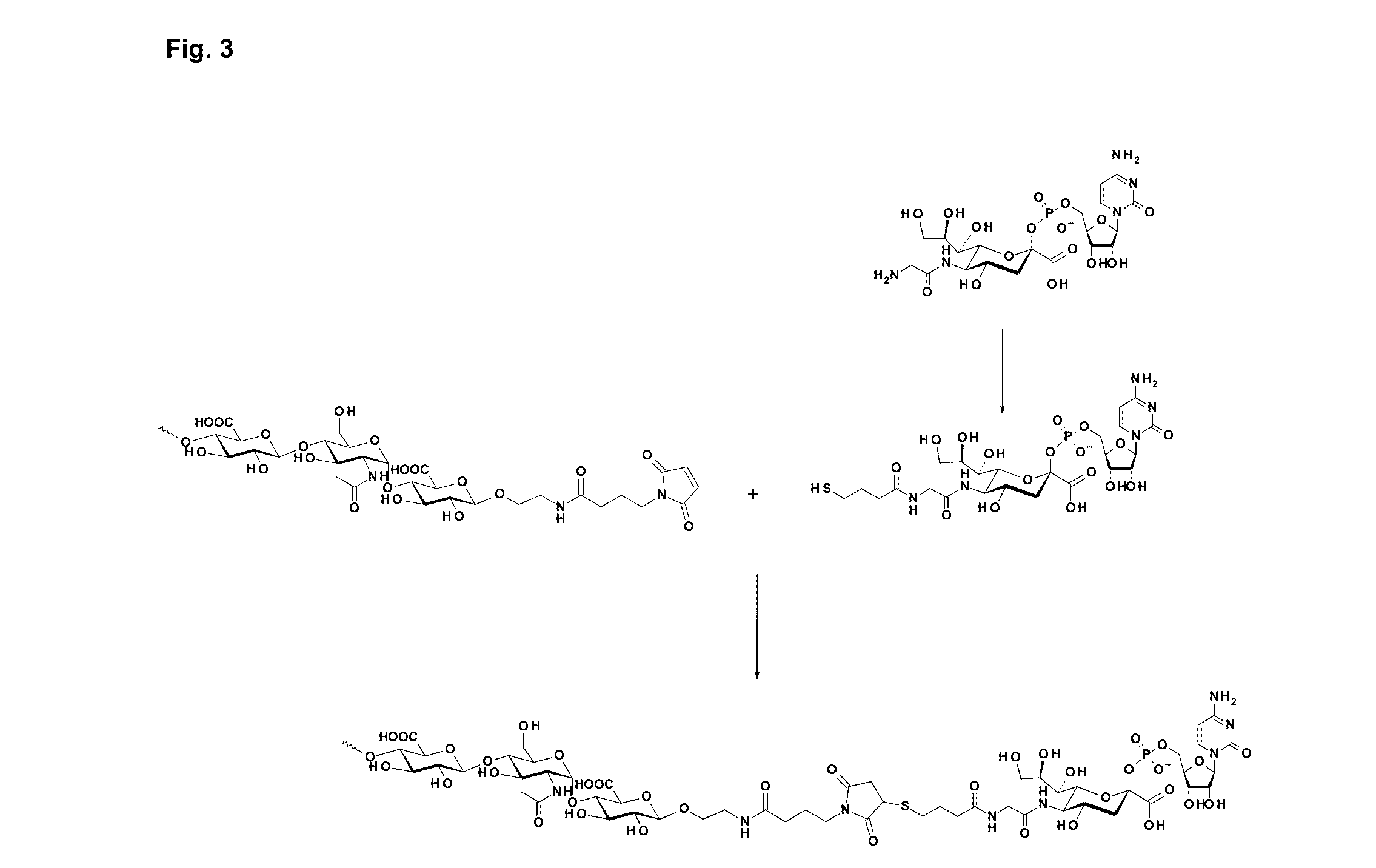
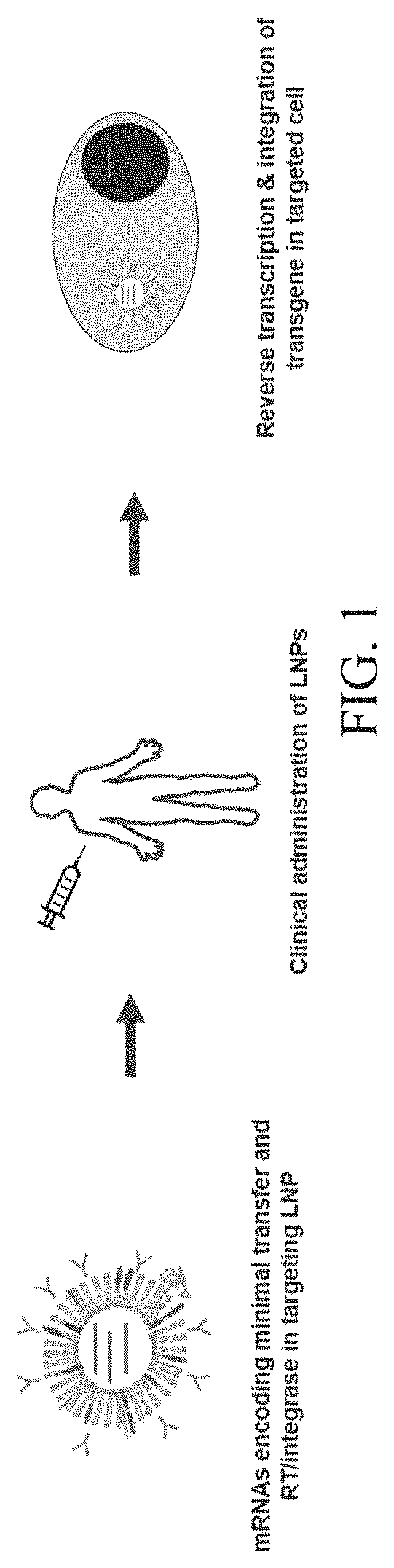
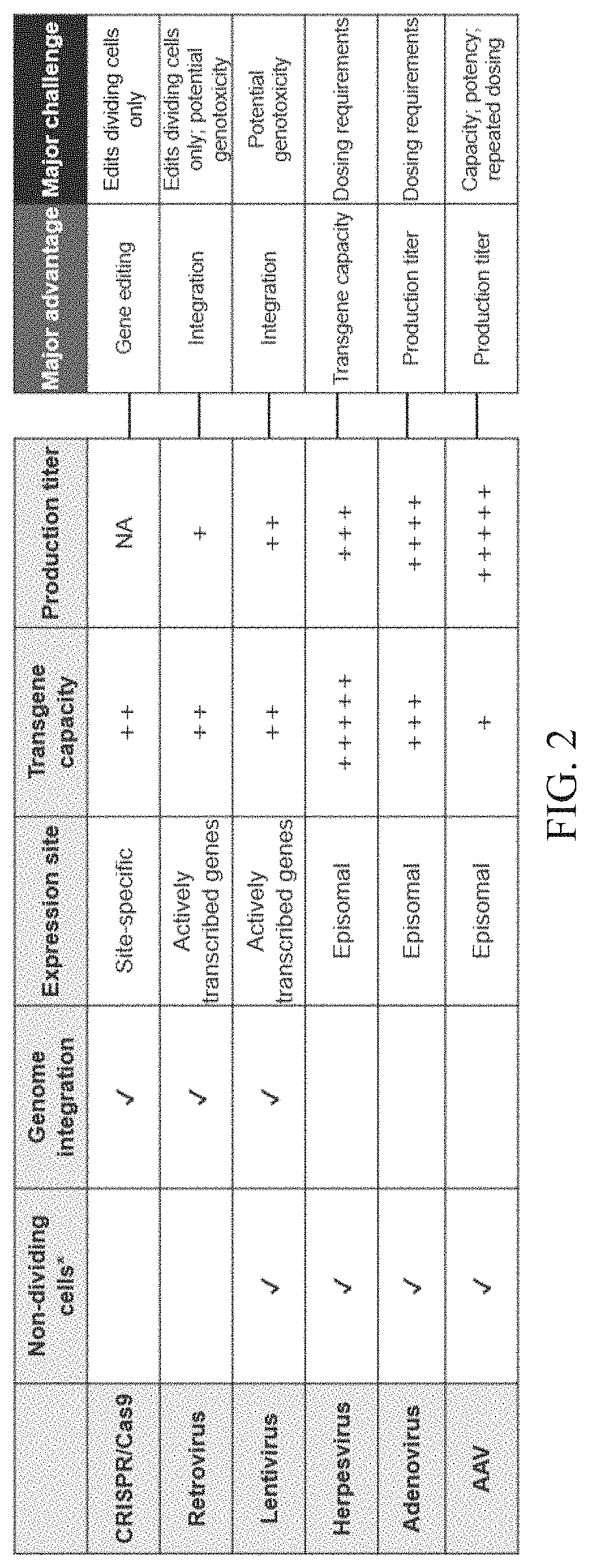
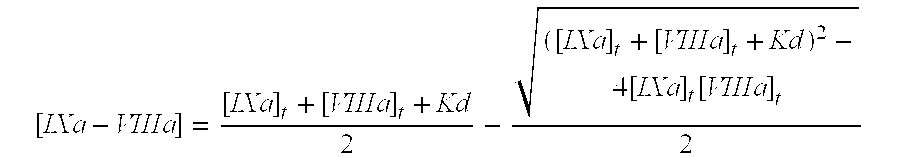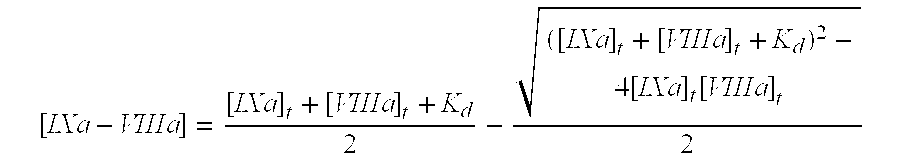Patents
Literature
35 results about "Haemophilia B" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Haemophilia B is a blood clotting disorder causing easy bruising and bleeding due to an inherited mutation of the gene for factor IX, and resulting in a deficiency of factor IX. It is less common than factor VIII deficiency (haemophilia A).
Recombinant human factor ix and use thereof
ActiveUS20080167219A1Easy to adaptPeptide/protein ingredientsGenetic material ingredientsWild typeFactor ii
The present invention aims at converting factor IX into a molecule with enhanced activity which provides an alternative for replacement therapy and gene therapy for hemophilia B. Using recombinant techniques, factor IX with replacement at positions 86, 277, and 338 exhibits better clotting activity than recombinant wild type factor IX.
Owner:LIN SHU WHA
Hepatocellular chimeraplasty
InactiveUS6524613B1Decrease in levelReduce riskOrganic active ingredientsBiocideGenetic ChangeFhit gene
The present invention concerns compositions and methods for the introduction of specific genetic changes in endogenous genes of the cells of an animal. The genetic changes are effected by oligonucleotides or oligonucleotide derivatives and analogs, which are generally less than about 100 nucleotides in length. The invention provides for macromolecular carriers, optionally incorporating ligands for clathrin coated pit receptors. In one embodiment the ligand is a lactose or galactose and the genetic changes are made in hepatocytes. By means of the invention up to 40% of the copies of a target gene have been changed in vitro. Repair of mutant genes having a Crigler-Najjar like phenotype and Hemophilia B phenotype were observed.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV +1
Liquid, aqueous, pharmaceutical compositions of factor VII polypeptides
InactiveUS20060063714A1Good storage stabilityPeptide/protein ingredientsInorganic non-active ingredientsFactor VIIaClotting factor deficiency
The invention relates to a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (e.g. human Factor VIIa) and a buffering agent; wherein the molar ratio of non-complexed calcium ions (Ca2+) to the Factor VII polypeptide is lower than 0.5. The composition may further comprise a stabilizing agent (e.g. copper or magnesium ions, benzamidine, or guanidine), a non-ionic surfactant, a tonicity modifying agent, an antioxidant and a preservative. The composition is useful for treating a Factor VII-responsive syndrome, such as bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor XI deficiency, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy.
Owner:NOVO NORDISK AS
Methods for treating blood coagulation disorders
InactiveUS7615537B2Optimally promote proper disulfide bondingPrecise BondingBiocideVirusesFactor VIIaA-DNA
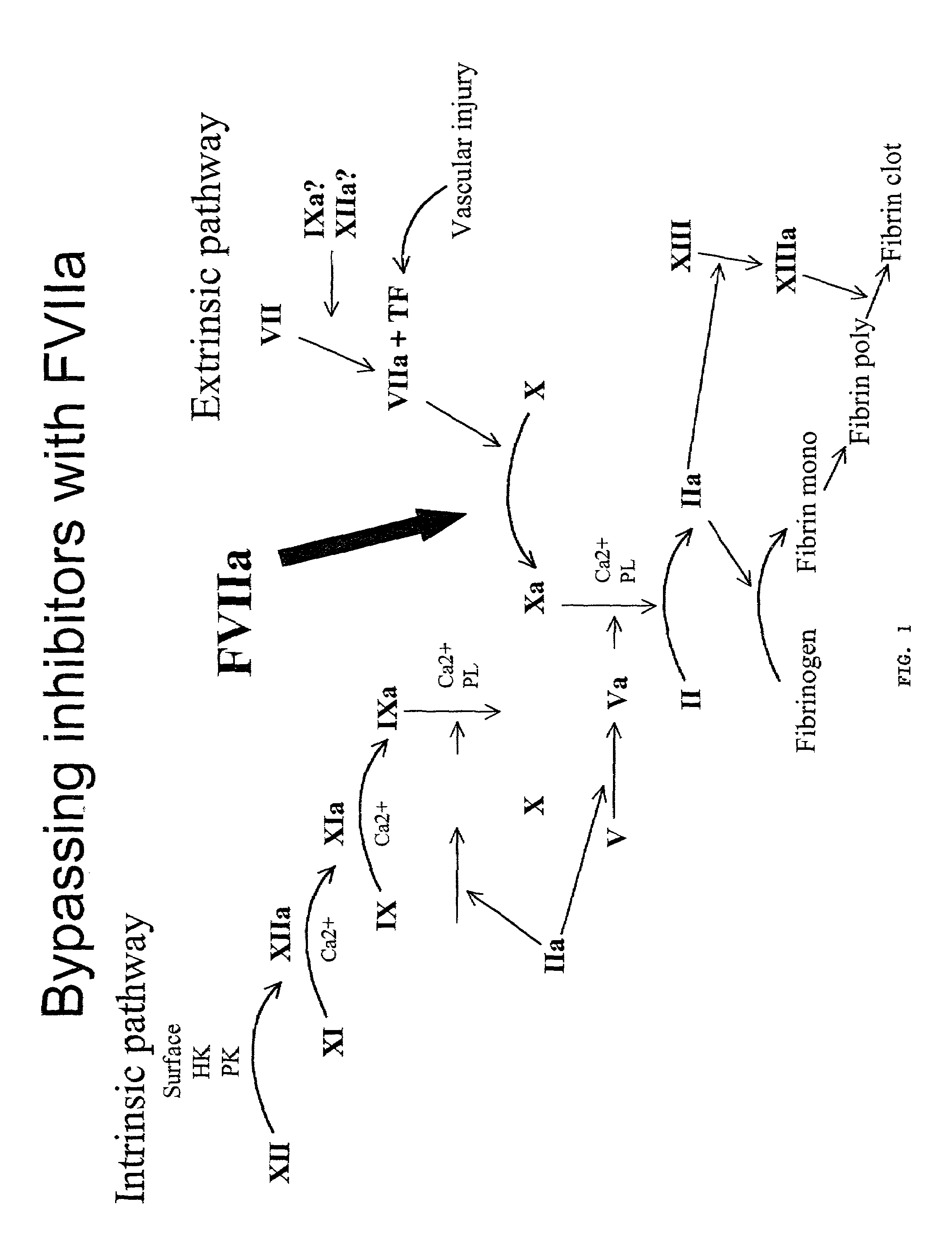
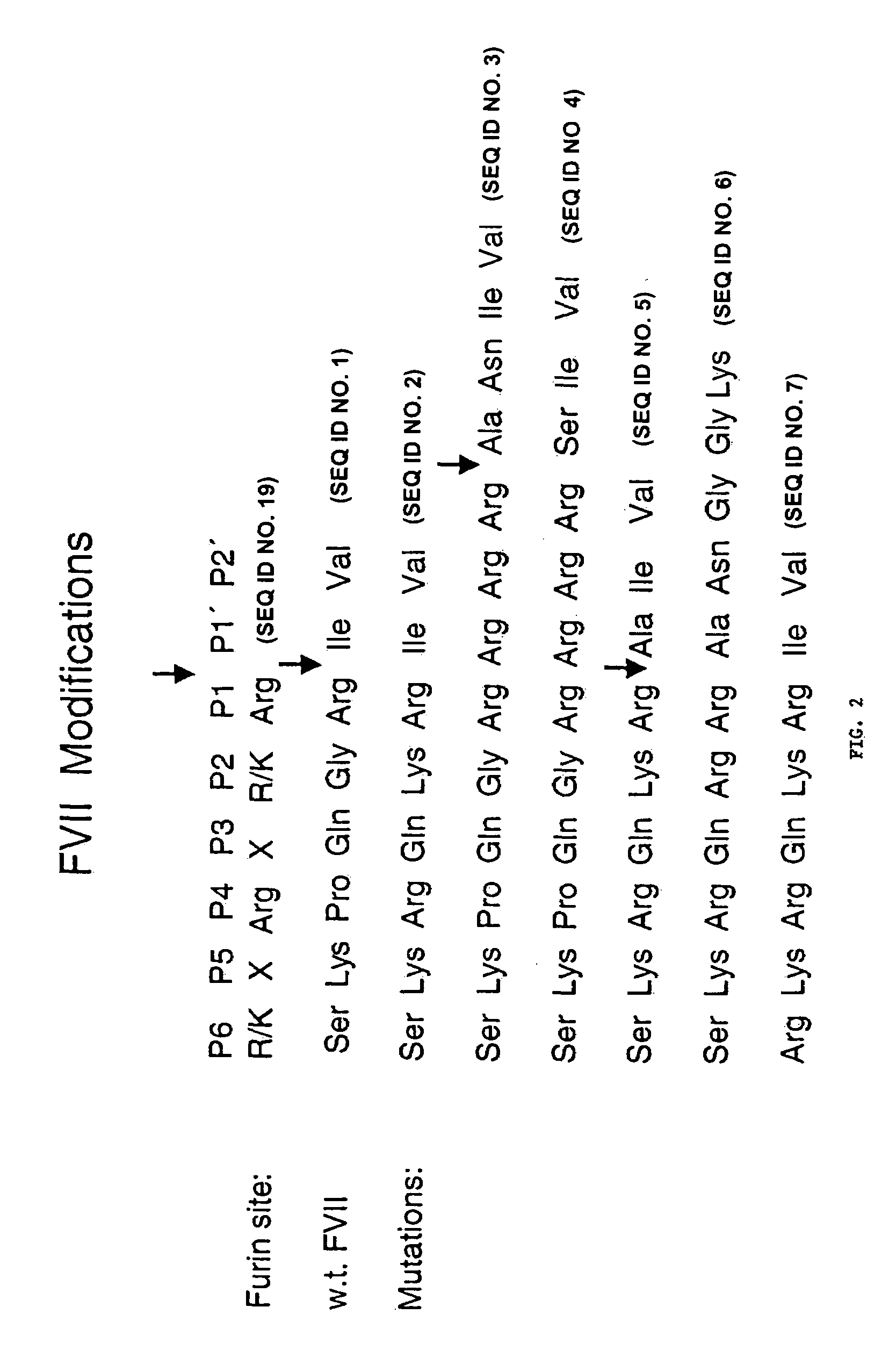
The present invention relates to a method of treating an individual having a blood coagulation defect (e.g., hemophilia A, hemophilia B), comprising administering to the individual an effective amount of a DNA vector encoding modified Factor VII (FVII), wherein the modified Factor VII leads to generation of Factor VIIa in vivo. In a particular embodiment, the invention pertains to a method of treating an individual having a blood coagulation defect comprising administering to the individual an effective amount of a nucleic acid encoding a modified FVII wherein the modified FVII comprises a signal which codes for precursor cleavage by furin at the activation cleavage site of the modified FVII. The invention also relates to a method of treating an individual having a blood coagulation disorder comprising administering to the individual an effective amount of a nucleic acid encoding the light chain of human FVII and a nucleic acid encoding the heavy chain of human FVII operably linked to a leader sequence. Compositions, expression vectors and host cells comprising nucleic acid which encodes a modified Factor VII, wherein the modified Factor VII leads to generation of Factor VIIa in vivo is also encompassed by the present invention.
Owner:GENZYME CORP
Liquid, aqueous pharmaceutical composition of Factor VII polypeptides
InactiveUS20060166882A1Improve stabilityHeavy metal active ingredientsBiocideClotting factor deficiencyOxidation state
Owner:NOVO NORDISK HEALTH CARE AG
Administration of plant expressed oral tolerance agents
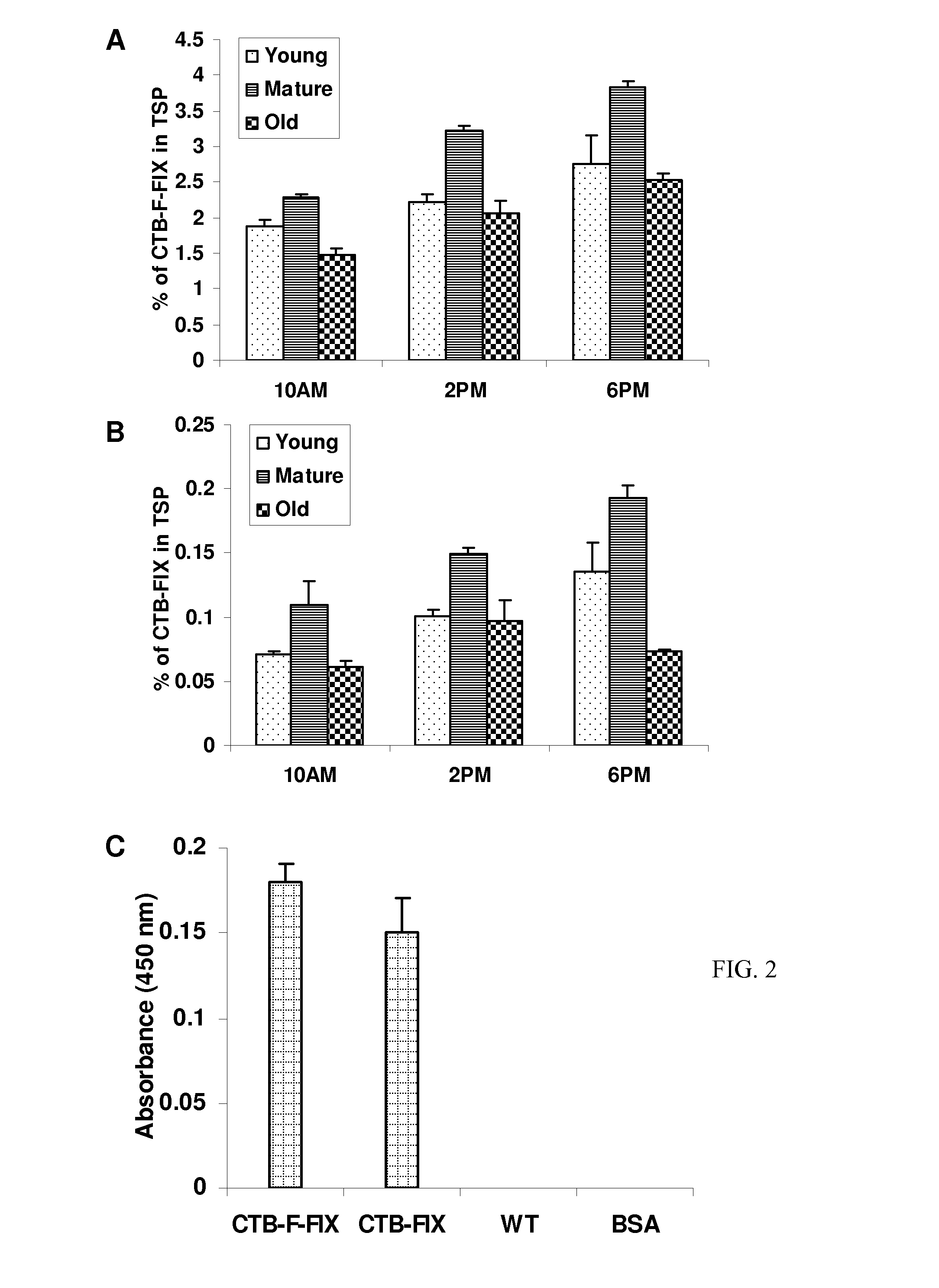
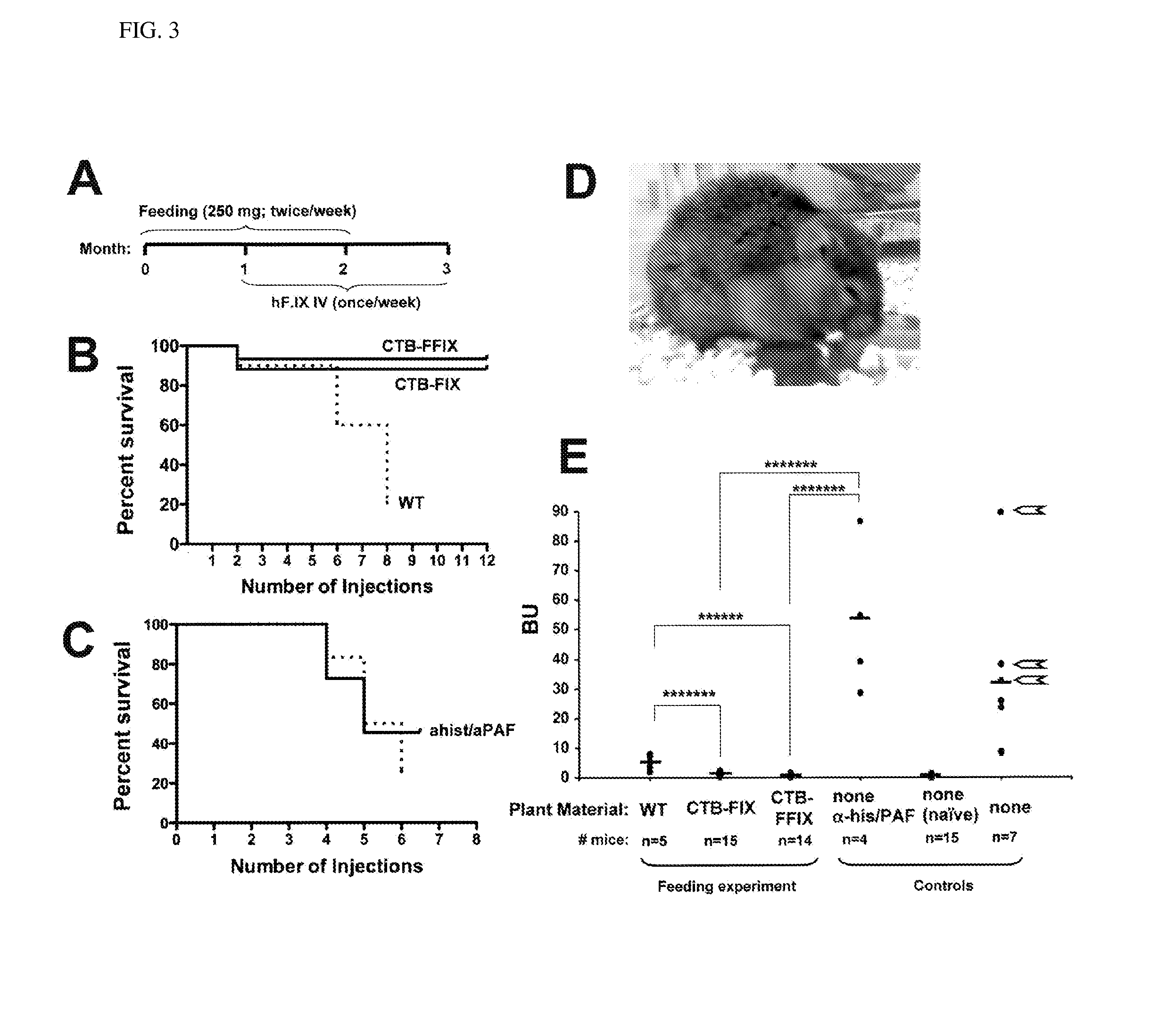
Protein replacement therapy for patients with hemophilia or other inherited protein deficiencies is often complicated by pathogenic antibody responses, including antibodies that neutralize the therapeutic protein or that predispose to potentially life-threatening anaphylactic reactions by formation of IgE. Using murine hemophilia B as a model, we have developed a prophylactic protocol against such responses that is non-invasive and does not include immune suppression or genetic manipulation of the patient's cells. Oral delivery of coagulation factor IX (F. IX) expressed in chloroplasts, bioencapsulated in plant cells, effectively blocked formation of inhibitory antibodies in protein replacement therapy. Inhibitor titers were mostly undetectable and up to 100-fold lower in treated mice when compared to controls. Moreover, this treatment eliminated fatal anaphylactic reactions that occurred after 4 to 6 exposures to intravenous F. IX protein. While only 20-25% of control animals survived after 6-8 F. IX doses, 90-95% of tolerized mice survived 12 injections without signs of allergy or anaphylaxis. This high-responder strain of hemophilia B mice represents the first hemophilic animal model to study anaphylactic reactions. The plant material was effective over a range of oral antigen doses (equivalent to 5-80 μg recombinant F.IX / kg), and controlled inhibitor formation and anaphylaxis long-term, up to 7 months. Oral antigen administration caused a deviant immune response that suppressed formation of IgE and inhibitory antibodies. This cost-effective and efficient approach to oral delivery of protein antigens to the gut should be applicable to several genetic diseases that are prone to pathogenic antibody responses during treatment.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Liquid, Aqueous Pharmaceutical Composition of Factor VII Polypeptides
InactiveUS20100166730A1Improve stabilityHeavy metal active ingredientsPeptide/protein ingredientsClotting factor deficiencyOxidation state
The present invention is directed to liquid, aqueous pharmaceutical compositions containing Factor VII polypeptides, and methods for preparing and using such compositions, as well as vials containing such compositions, and the use of such compositions in the treatment of a Factor VII-responsive syndrome, e.g., bleeding disorders, including those caused by clotting Factor deficiencies (e.g. haemophilia A, haemophilia B, coagulation Factor VII deficiency); by thrombocytopenia or von Willebrand's disease, or by clotting Factor inhibitors, and intra cerebral haemorrhage, or excessive bleeding from any cause. The preparations may also be administered to patients in association with surgery or other trauma or to patients receiving anticoagulant therapy. More particularly, the invention relates to liquid compositions stabilised against chemical and / or physical degradation. The main embodiment is represented by a liquid, aqueous pharmaceutical composition comprising a Factor VII polypeptide (i); a buffering agent (ii) suitable for keeping pH in the range of from about 4.0 to about 9.0; at least one metal-containing agent (iii), wherein said metal is selected from the group consisting of first transition series metals of oxidation state +II, except zinc, such as chromium, manganese, iron, cobalt, nickel, and copper; and a non-ionic surfactant (iv).
Owner:NOVO NORDISK HEALTH CARE AG
Factor IX Variants with Clotting Activity in Absence of Their Cofactor and/or With Increased F.IX Clotting Activity and Their Use for Treating Bleeding Disorders
The present invention relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has increased F.IX clotting activity compared to wildtype. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
Composition
InactiveUS20040235737A1Use of compositionReduce the amount requiredFactor VIIPeptide/protein ingredientsMedicineAntibody
The present invention relates to the use of FIXa and FVIII in the preparation of a composition for the treatment of haemophilia A or haemophilia B in a subject which does not present with anti-FVIII antibodies. The present invention further relates to a composition comprising FIXa and a composition comprising FVIII for simultaneous, simultaneous separate or sequential use in the treatment of haemophilia A or haemophilia B in a subject which does not present with anti-FVIII antibodies.
Owner:NAT INST FOR BIOLOGICAL STANDARDS & CONTROL
Adenovirus vector for gene therapy on hemophilia B and application thereof
The invention relates to an adenovirus vector for gene therapy on hemophilia B and application thereof, and the adenovirus vector can simultaneously express hFIX and red fluorescent protein; both sides of a tandem gene cassette of the hFIX and the red fluorescent protein are respectively provided with a loxp site of a Cre integrase action site in the same direction, and an action site attB of site-specific integrase PhiC31 is arranged in the gene cassette. The invention obtains a recombinant adenovirus vector which can be cyclized by DNA marked by the red fluorescent protein, and lays the foundation for the recombinant adenovirus vector to be further integrated into a main genome through attb sequences to exert the lasting function for treating hemophilia B. The adenovirus vector or adenovirus particles packaged outside the adenovirus vector is applied in the treatment of hemophilia B.
Owner:INST OF HEMATOLOGY & BLOOD HOSPITAL CHINESE ACAD OF MEDICAL SCI
Recombinant human factor ix and use thereof
ActiveUS20100081712A1Easy to adaptOrganic active ingredientsPeptide/protein ingredientsFactor iiWild type
The present invention aims at converting factor IX into a molecule with enhanced activity which provides an alternative for replacement therapy and gene therapy for hemophilia B. Using recombinant techniques, factor IX with replacement at positions 86, 277, and 338 exhibits better clotting activity than recombinant wild type factor IX.
Owner:LIN SHU WHA
Non-blood serum preparation of recombinant human blood coagulation factor IX
ActiveCN101481692AReduce pollutionHigh production yieldPeptide/protein ingredientsBiological testingDiseaseFactor ii
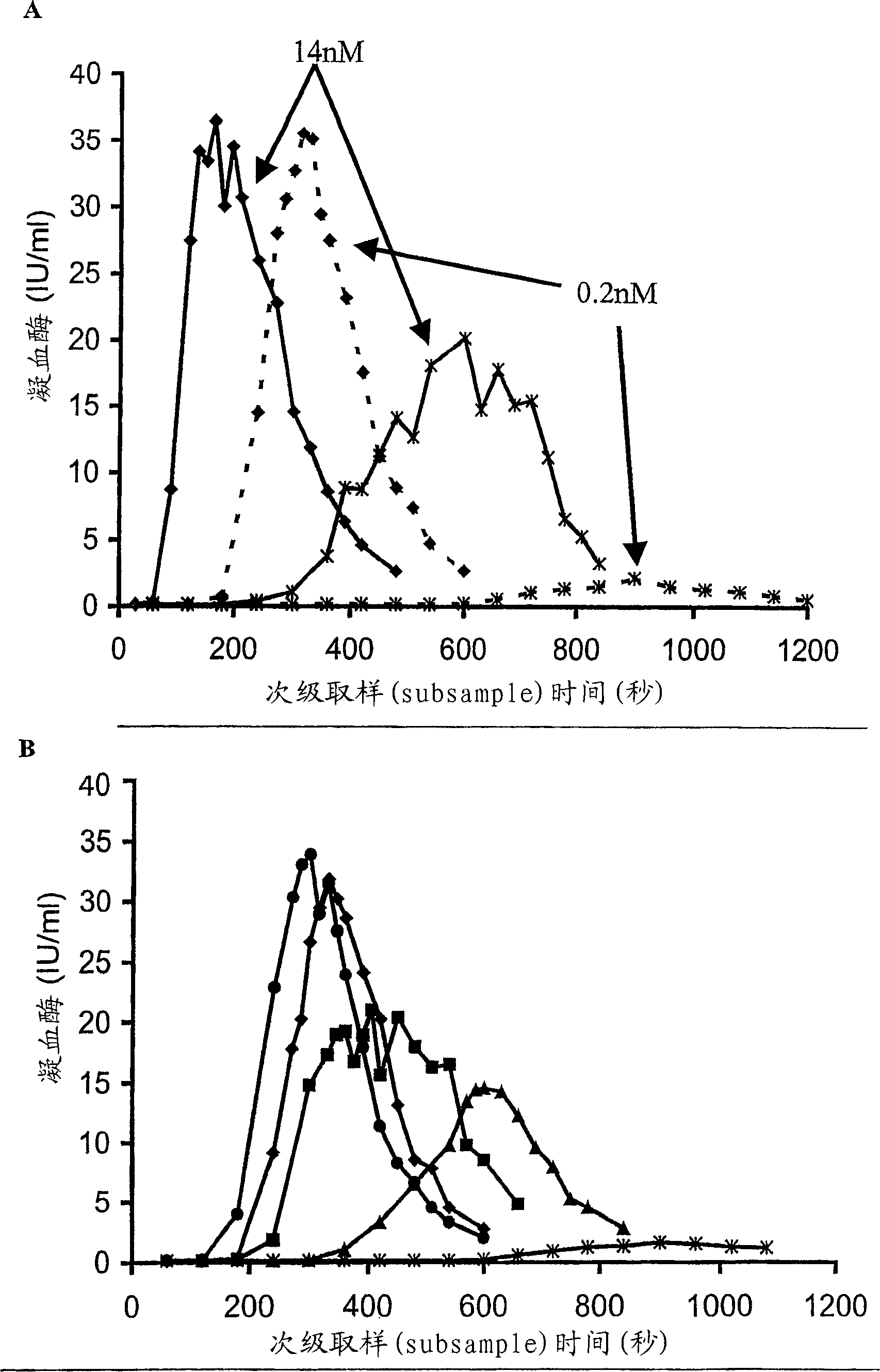
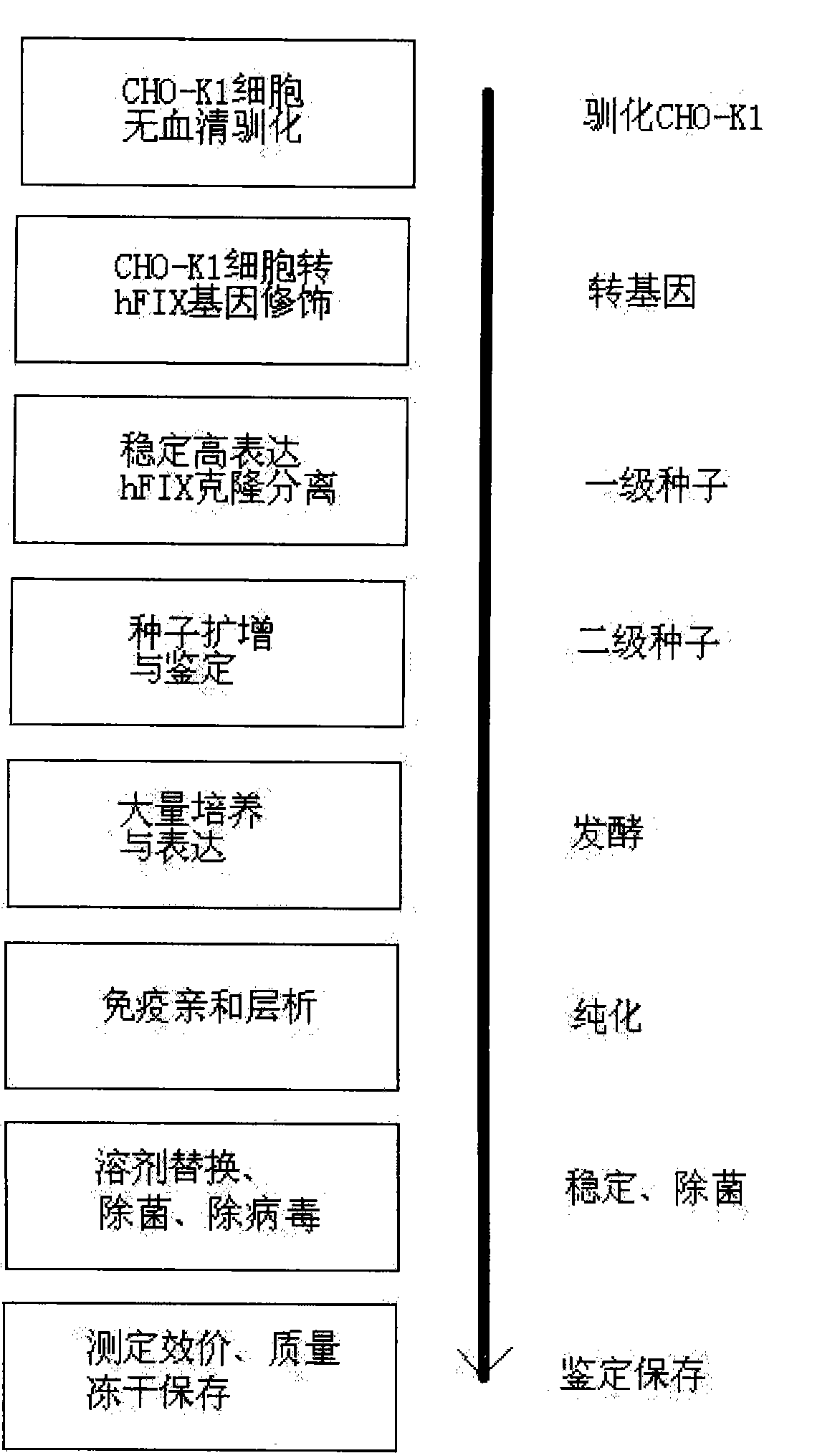
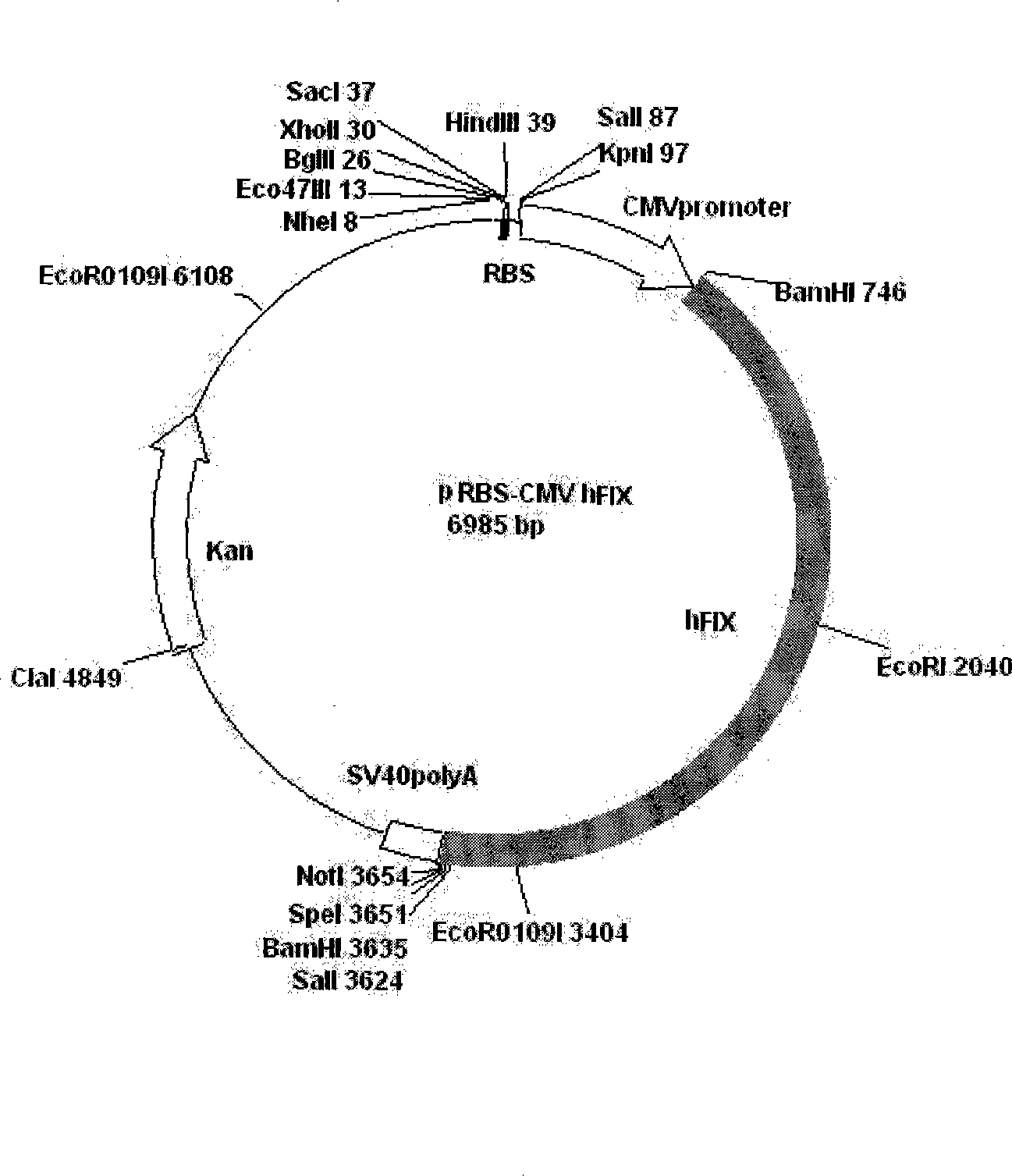
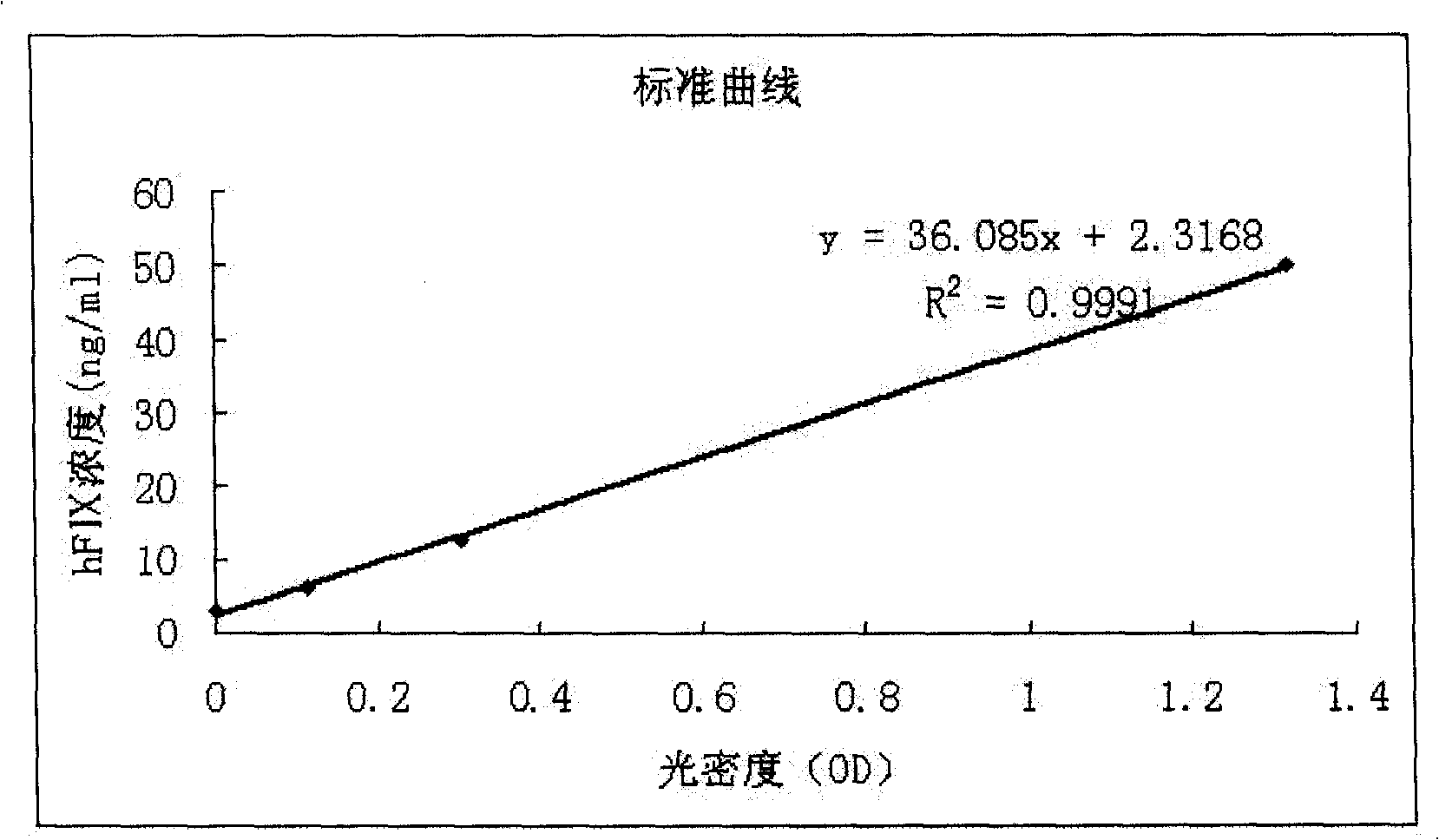
The invention relates to the preparation of recombinant human coagulation factor IX and an application thereof. The recombinant hFIX protein and hFIX of human blood source have the same molecular composition and biological activity and can be eased by being used for hemophilia B mouse model. The recombinant hFIX protein can be used as a standard in the tests for clinically detecting hFIX and the like, and can also cure diseases requiring supplementing hFIX, such as hemophilia B, etc.
Owner:浙江耶大生物医药有限公司
Pharmaceutical composition of hemocoagulase and application of pharmaceutical composition
PendingCN108785665APromote aggregationAggregate recoveryPeptide/protein ingredientsPharmaceutical delivery mechanismFIBRINOGEN/THROMBINHaemophilia B
The invention discloses a pharmaceutical composition of hemocoagulase. The pharmaceutical composition is characterized in that the pharmaceutical composition is prepared from 0.00005 to 0.0005 percentof a hemocoagulase composition and the balance of a pharmaceutically acceptable carrier. The composition is applied to preparation of a medicine for treating Haemophilia B (coagulation factor IX deficiency). Active components in the hemocoagulase comprise hemocoagulase, i.e., a thrombin-like enzyme (batroxobin), and a phospholipid dependent coagulation factor X activating agent (FXA). A bleedingstopping process of the hemocoagulase does not depend on a coagulation factor IX and is mainly used for fibrin and an activated coagulation factor X, and has the effect of treating bleeding of the haemophilia B. After the hemocoagulase is used for treating, platelet aggregation can be remarkably enhanced, and the formation of blood clots or fibrin clots in a patient with the haemophilia B is recovered.
Owner:ZHAOKE PHARMA HEFEI
Coagulation factor IX conjugates
The present invention relates to Factor IX polypeptides conjugated to heparosan (HEP) polymers, methods for the manufacture thereof and uses of such conjugates. The resultant conjugates may be used - for example - in the treatment or prevention of bleeding disorders such as haemophilia B.
Owner:NOVO NORDISK HEALTH CARE AG
Factor IX gene therapy
ActiveUS10413598B2Improving potency and transduction efficiencyImprove securitySugar derivativesPeptide/protein ingredientsNucleotideIntein
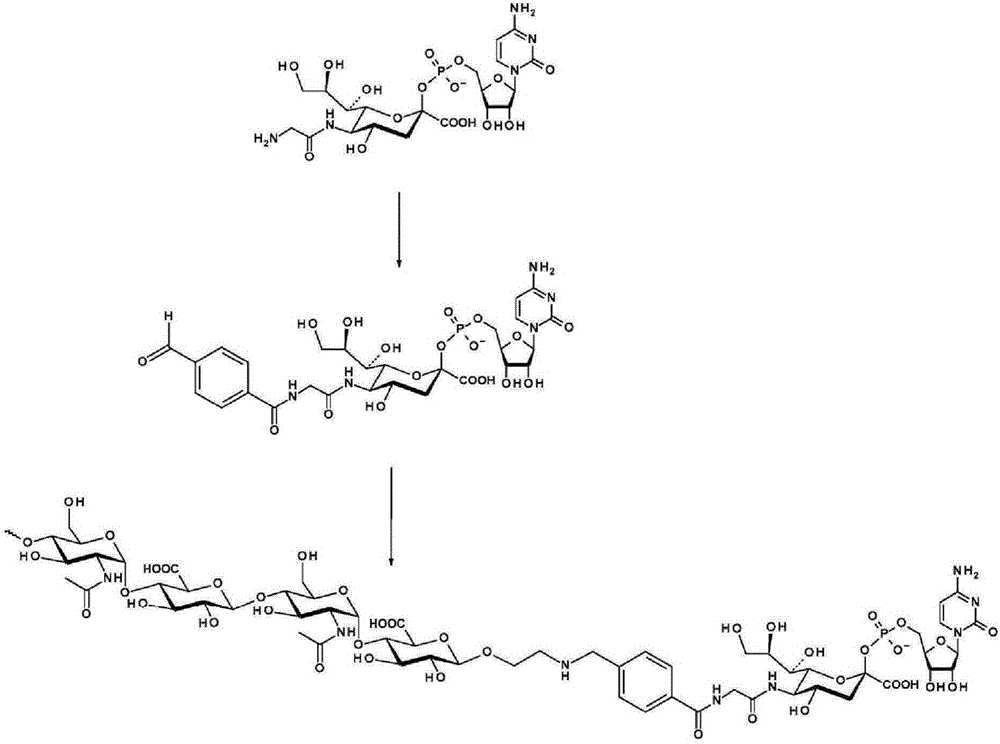
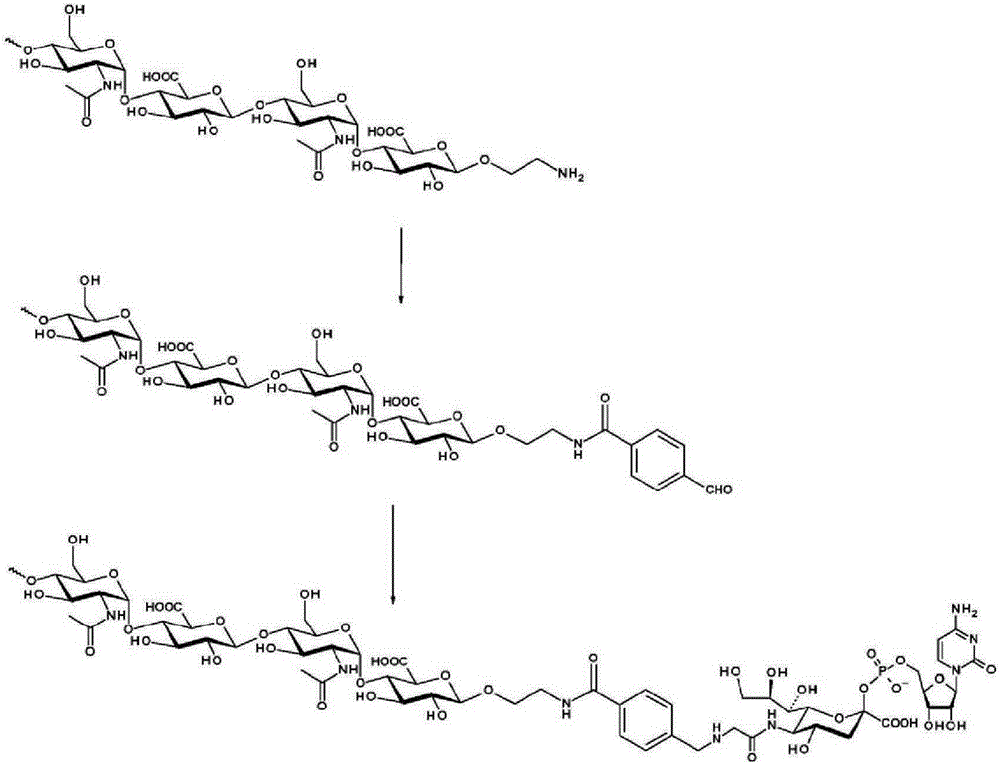
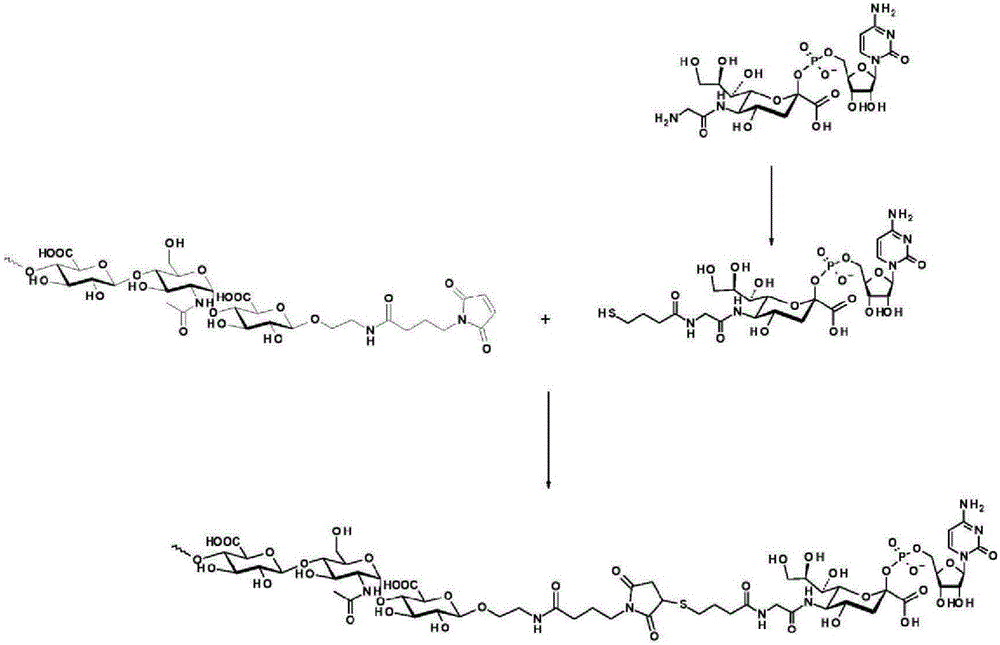
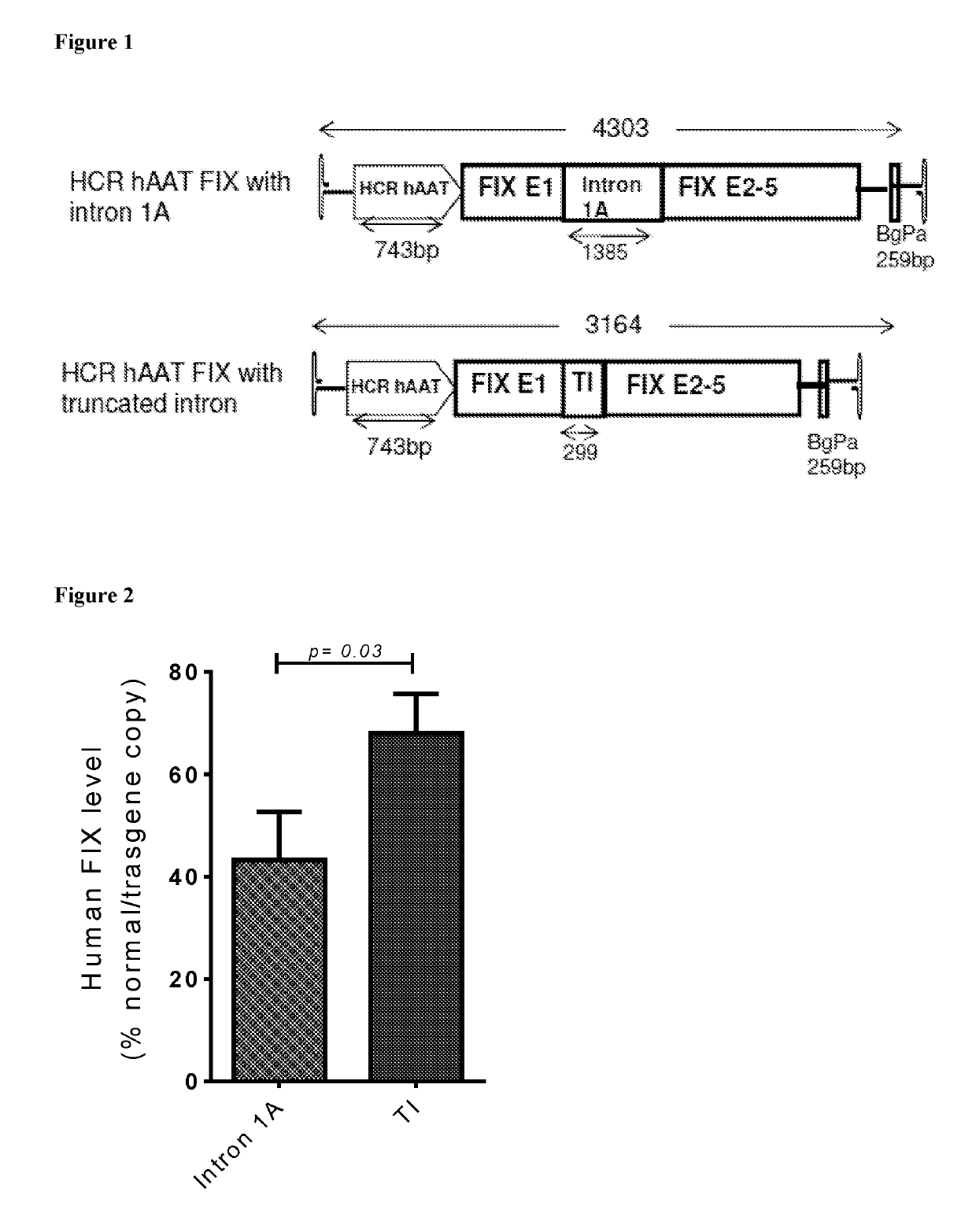
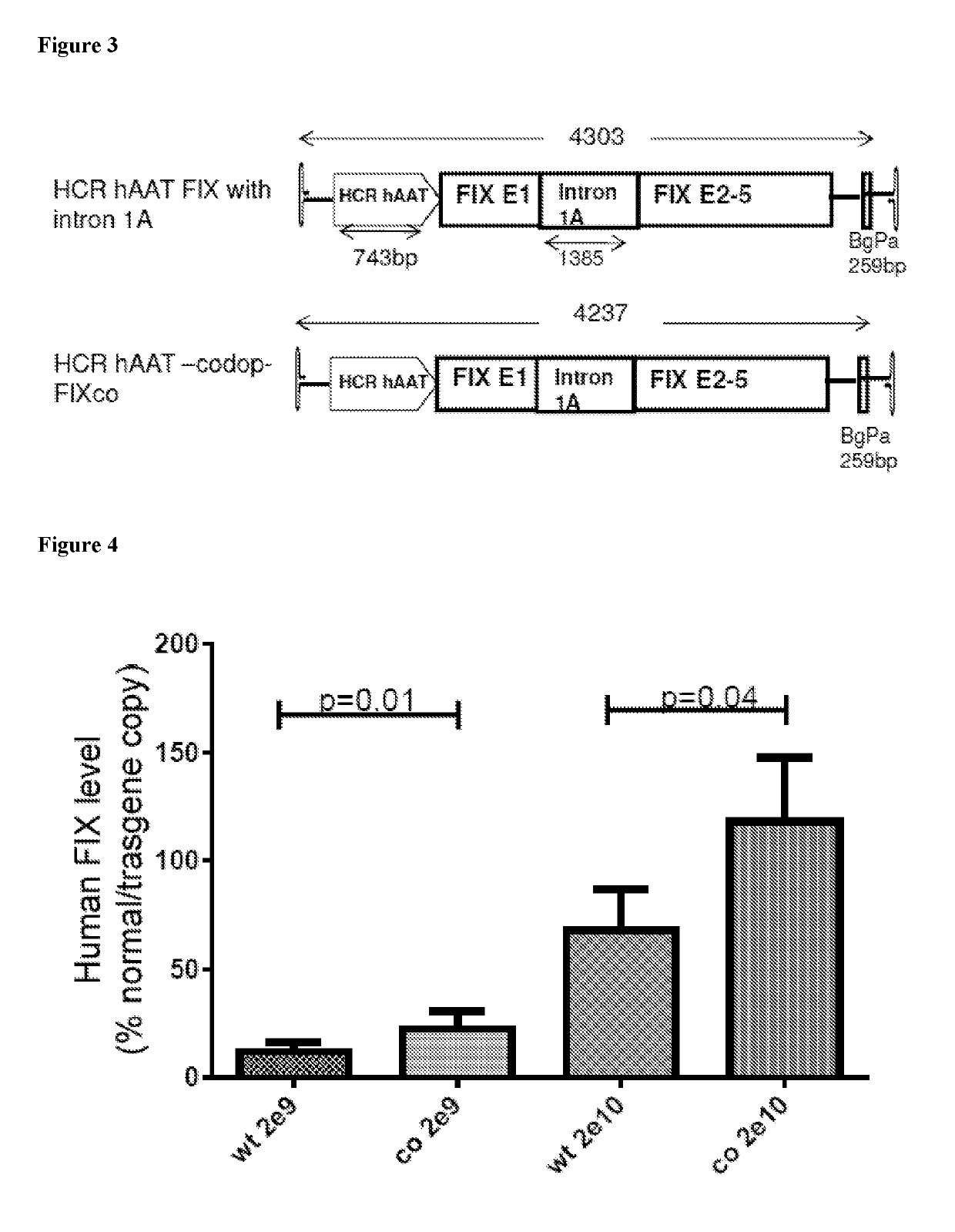
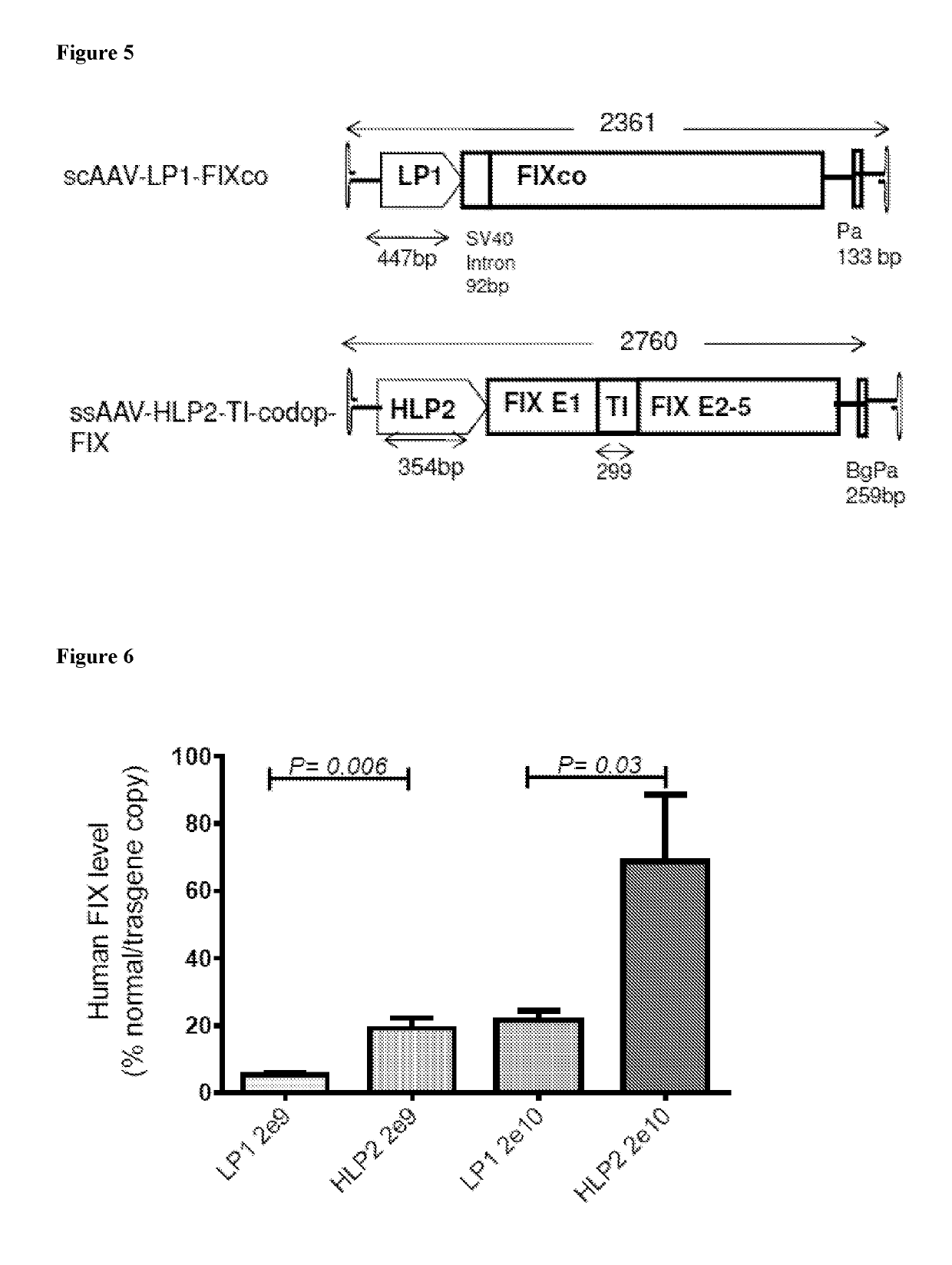
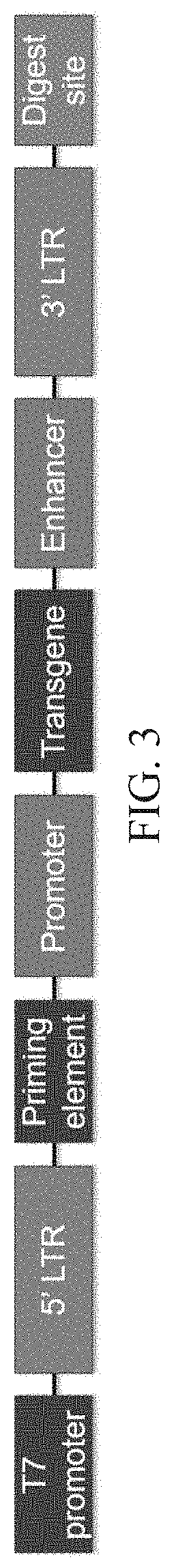
The invention relates to a new, more potent, coagulation factor IX (FIX) expression cassette for gene therapy of haemophilia B (HB). Disclosed is a vector for expressing factor IX protein, the vector comprising a promoter, a nucleotide sequence encoding for a functional factor IX protein and an intron sequence, wherein the intron sequence is positioned between exon 1 and exon 2 of the nucleotide sequence encoding for a functional factor IX protein, and wherein the intron sequence has at least 80% identity to the sequence of SEQ ID NO. 1 as disclosed herein.
Owner:UCL BUSINESS PLC
Gene therapy agent for Haemophilia B and its preparation method
The invention relates to site-specific integrating expression vectors for therapy Hemophilia B and to methods of preparing them. A vector of the invention contains a human Factor IX gene in a vector constructed using as a chromosome targeting sequence a polynucleotide without any important physiological function-related gene homologous to DNA on the short arms of human group D and human group G chromosomes. The vector of the invention provides high stability of Factor IX expression, high expression efficiency, no immunogenicity and safety in use.
Owner:XIA JIAHUI
Modified factor ix polypeptides and uses thereof
The invention relates to modified Factor IX polypeptides such as Factor IX polypeptides with one or more amino acid substitutions. The invention also relates to methods of making modified Factor IX polypeptides, and methods of using modified Factor IX polypeptides, for example, to treat patients afflicted with hemophilia B.
Owner:BAYER HEALTHCARE LLC
Novel gene therapy agent for haemophilia b and its preparation method
The invention deals with the gene drugs for therapy Hemophilia B and its method of preparation. It contains human source gene vector-FIX recombinant constructed using DNA sequence as leading sequence of therapy gene without important physiological function-related gene on the short arms of human group D,G chromosomes or to which DNA sequence is homologous. This invention also provides the method of preparing gene drug. The therapy gene of gene drug for hemophilia B has a high stability of expression, safety, high expression efficiency and no immunogenecity.
Owner:XIA JIAHUI
Modified factor IX polypeptides and uses thereof
The present invention relates to modified Factor IX polypeptides such as Factor IX polypeptides with one or more introduced glycosylation sites. The modified Factor IX polypeptides may exhibit increased in vitro or in vivo stability such as a longer plasma half-life. The invention also relates to methods of making modified Factor IX polypeptides, and methods of using modified Factor IX polypeptides, for example, to treat patients afflicted with hemophilia B.
Owner:BAYER HEALTHCARE LLC +1
Method for treating Hemophilia B
Use of factor XIII for treating hemophilia B. A patient having hemophilia B is treated by administering factor XIII, generally in conjunction with factor IX.
Owner:ZYMOGENETICS INC
Compositions comprising coagulation factors IXA and VIII for the treatment of haemophilia A or B
Owner:NAT INST FOR BIOLOGICAL STANDARDS & CONTROL
Coagulation Factor IX Conjugates
InactiveUS20170035890A1Extended half-lifeProlong half-life in vivoPeptide/protein ingredientsEnzyme stabilisationPolymerHaemophilia B
Owner:NOVO NORDISK HEALTH CARE AG
Nucleic acid therapeutics for genetic disorders
PendingUS20220096525A1Improve stabilityOrganic active ingredientsPeptide/protein ingredientsThalassemiaTransgene
Provided herein, are compositions based on retroviruses (e.g., lentiviruses) comprising one or more nucleic acid molecules encoding retroviral Pol polyprotein components and a nucleic acid molecule comprising one or more transgene sequences flanked by long terminal repeat sequences, for delivery of the one or more transgenes to a target cell ex vivo or in vivo. The compositions are useful for delivering to a target cell (e.g., hematopoietic stem cells (HSCs), liver cells, ocular cells, muscle cells, epithelial cells, T cells, etc.) and / or stably expressing any transgene (e.g., beta-globin, Factor VIII, RP GTPase regulator (RPGR), dystrophin, cystic fibrosis transmembrane conductance regulator (CFTR), a chimeric antigen receptor, etc.) with a biological effect to treat and / or ameliorate the symptoms associated with any disorder related to gene expression (e.g., sickle cell disease, beta-thalassemia, haemophilia B, retinitis pigmentosa, Duchenne muscular dystrophy, cystic fibrosis, cancer, etc.).
Owner:GREENLIGHT BIOSCIENCES INC
Gene therapy for treating hemophilia b
ActiveUS20190076550A1Safe deliveryImprove responseSugar derivativesNon-active genetic ingredientsPeripheral veinsGene
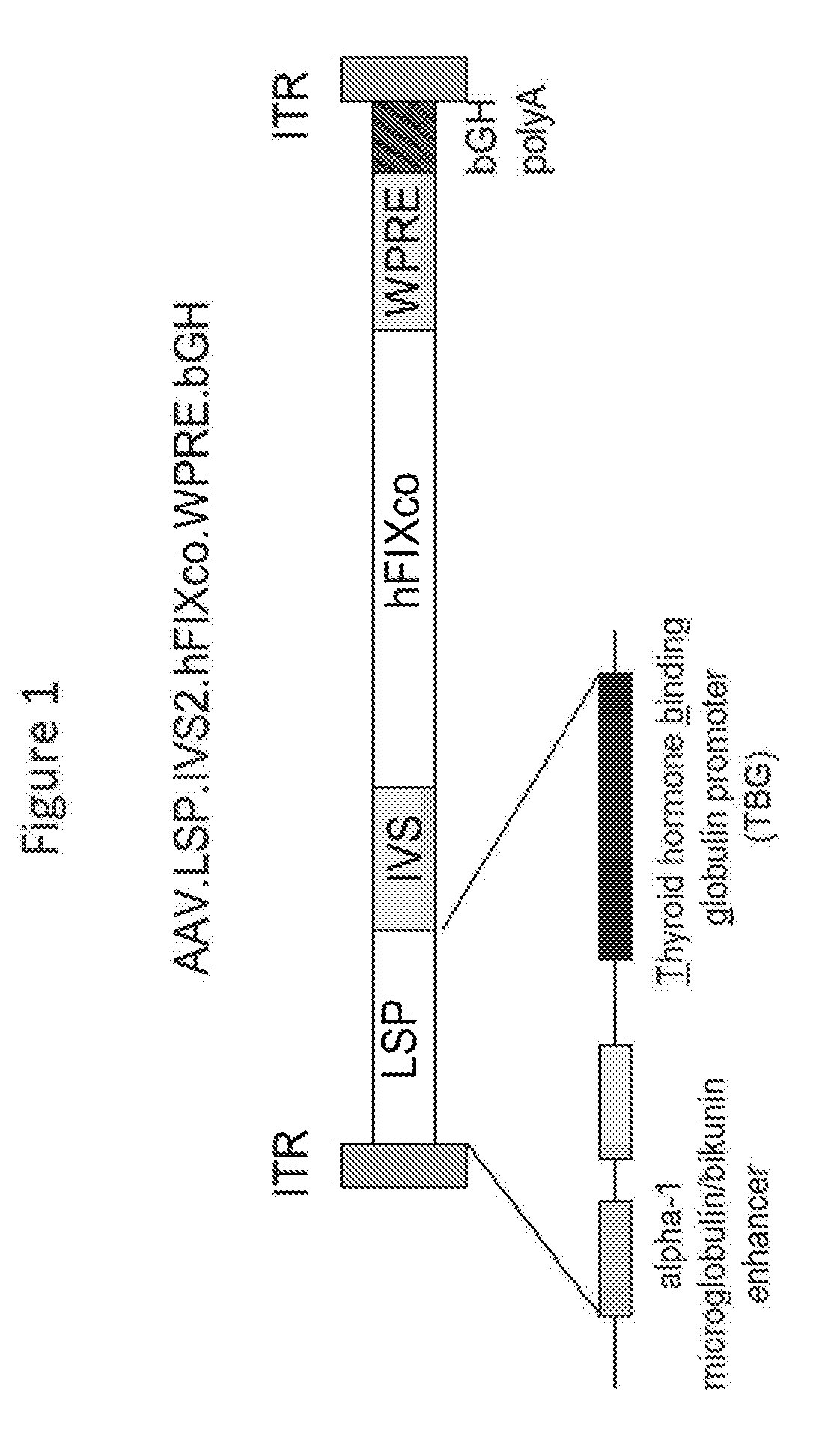
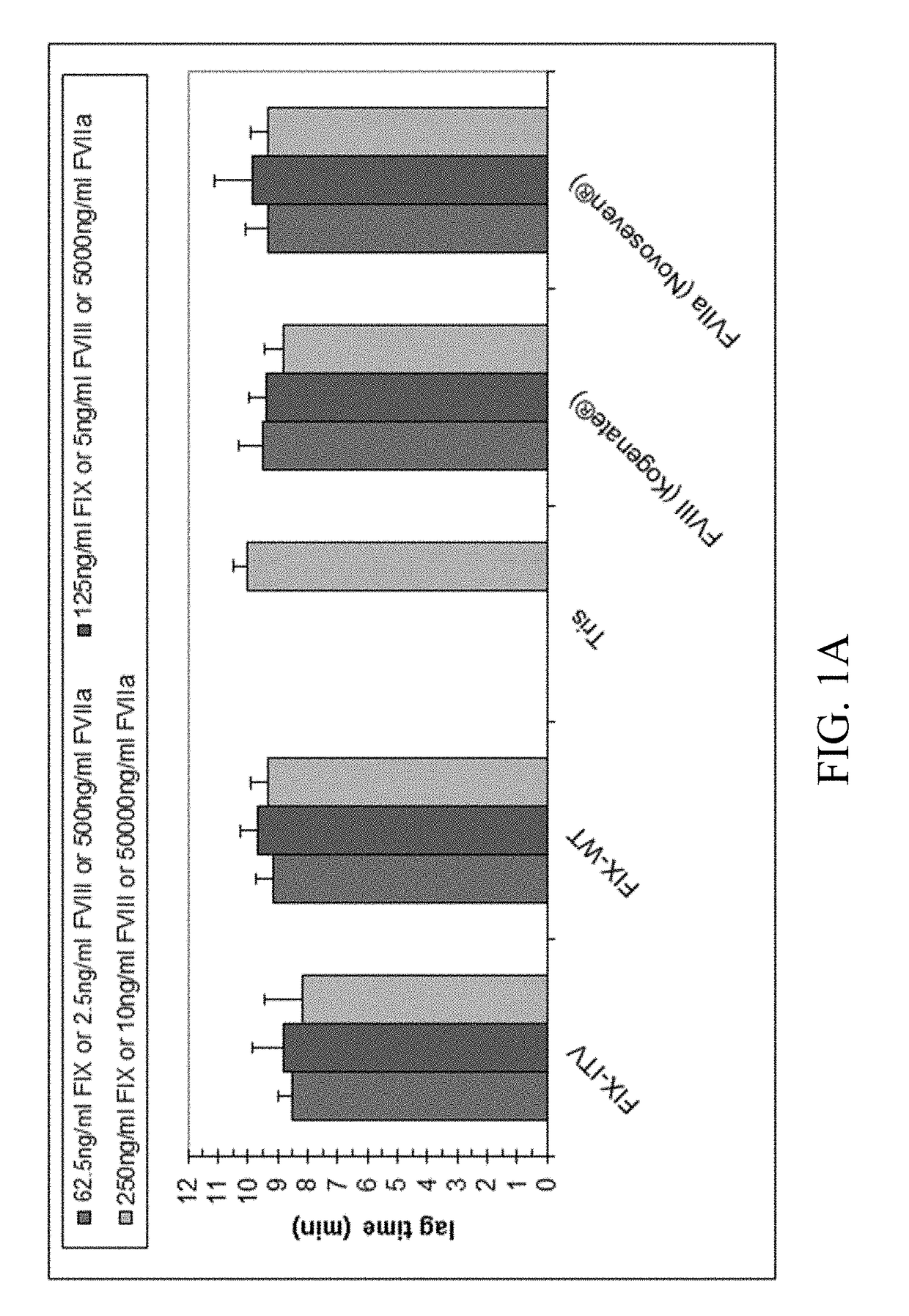
Compositions and regimens useful in treating hemophilia B are provided. The method involves administering to the human subject via a peripheral vein by infusion of a suspension of replication deficient recombinant adeno-associated virus (rAAV).
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Factor ix variants with clotting activity in absence of their cofactor and/or with increased f.ix clotting activity and their use for treating bleeding disorders
ActiveUS20180251744A1Correct hemophilic phenotypeSpecific activityCompound screeningApoptosis detectionActivated factor IXWild type
The present invention relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has clotting activity in absence of its cofactor. The present invention furthermore relates to variants of factor IX (F.IX) or activated factor IX (F.IXa), wherein the variant is characterized in that it has increased F.IX clotting activity compared to wildtype. The present invention furthermore relates to the use of these variants for the treatment and / or prophylaxis of bleeding disorders, in particular hemophilia A and / or hemophilia B or hemophilia caused or complicated by inhibitory antibodies to F.VIII. The present invention also relates to further variants of factor IX (F.IX) which have desired properties and can, thus be tailored for respective specific therapeutic applications.
Owner:DRK BLUTSPENDEDIENST BADEN WURTTEMBERG HESSEN GGMBH
Methods for treating blood coagulation disorders
InactiveUS20110034539A1Precise BondingPolypeptide with localisation/targeting motifOrganic active ingredientsFactor VIIaA-DNA
The present invention relates to a method of treating an individual having a blood coagulation defect (e.g., hemophilia A, hemophilia B), comprising administering to the individual an effective amount of a DNA vector encoding modified Factor VII (FVII), wherein the modified Factor VII leads to generation of Factor VIIa in vivo. In a particular embodiment, the invention pertains to a method of treating an individual having a blood coagulation defect comprising administering to the individual an effective amount of a nucleic acid encoding a modified FVII wherein the modified FVII comprises a signal which codes for precursor cleavage by furin at the activation cleavage site of the modified FVII. The invention also relates to a method of treating an individual having a blood coagulation disorder comprising administering to the individual an effective amount of a nucleic acid encoding the light chain of human FVII and a nucleic acid encoding the heavy chain of human FVII operably linked to a leader sequence. Compositions, expression vectors and host cells comprising nucleic acid which encodes a modified Factor VII, wherein the modified Factor VII leads to generation of Factor VIIa in vivo is also encompassed by the present invention.
Owner:WADSWORTH SAMUEL +1
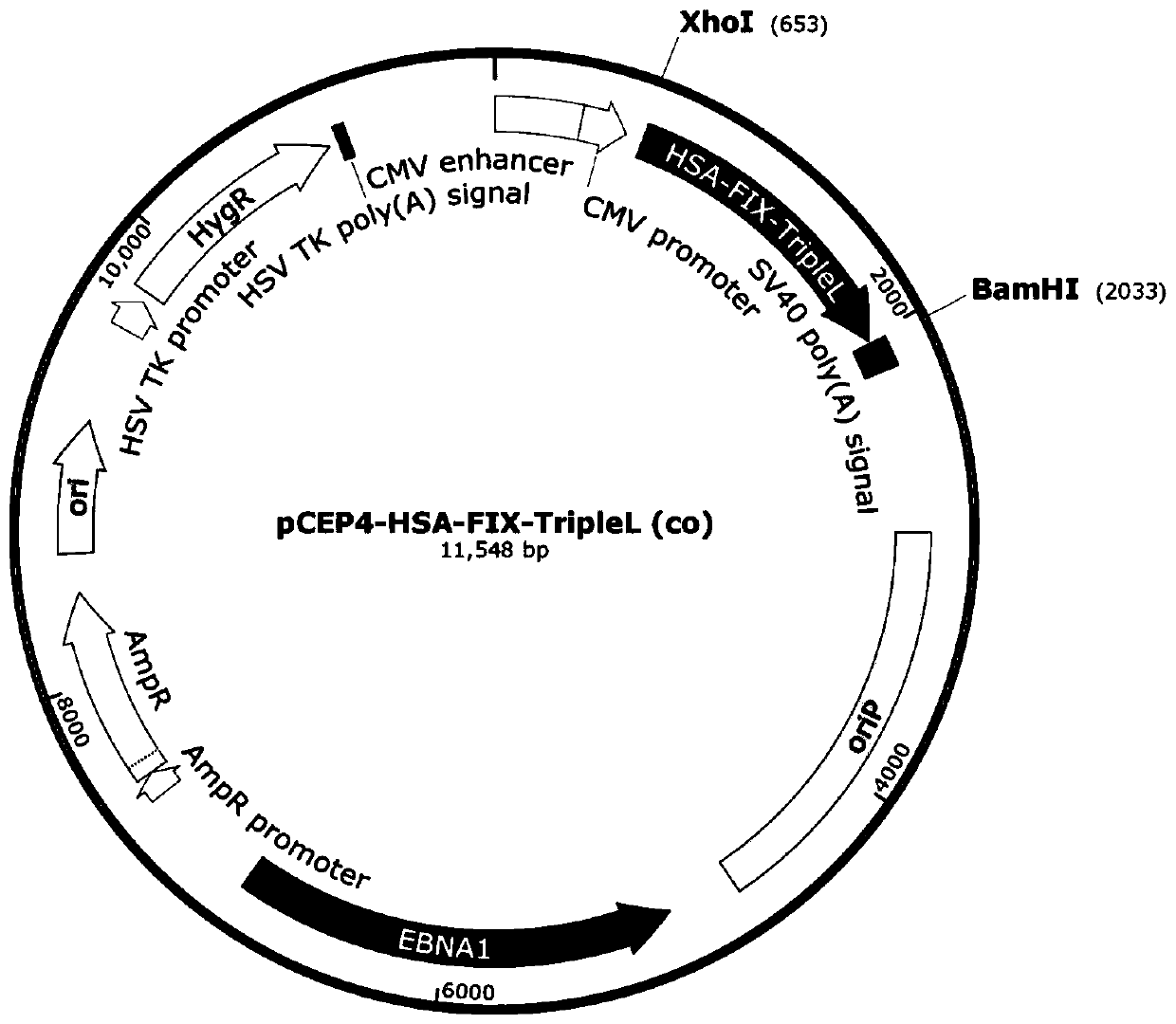
Vector for expressing recombinant blood coagulation factor FIX and construction method and applications thereof
ActiveCN110423776AContinuous expressionStable expressionPeptide/protein ingredientsGenetically modified cellsPlasmid VectorHost genome
The invention belongs to the field of gene engineering, and particularly relates to a vector for expressing a recombinant blood coagulation factor FIX and a construction method and applications thereof. Aiming at the problems of insecurity of viruses and packaging complexity of adeno-associated viruses, the invention provides a safe cell which is not integrated into a host genome, can be copied incells and is used for hemophilia gene therapy and a construction method and applications of the cell. The plasmid vector comprises an EBV gene for encoding EBV nucleoprotein EBNA-1, an oriP replication starting point of EBV, a blood coagulation factor hFIX encoding sequence and the like. The recombinant vector disclosed by the invention is a gene therapy product which introduces the blood coagulation factor into cells to treat hemophilia for the first time, and lays a foundation for the vector to further safely treat hemophilia B.
Owner:广州辉园苑医药科技有限公司
Method of treating haemophilia by inducing tolerance to blood factors
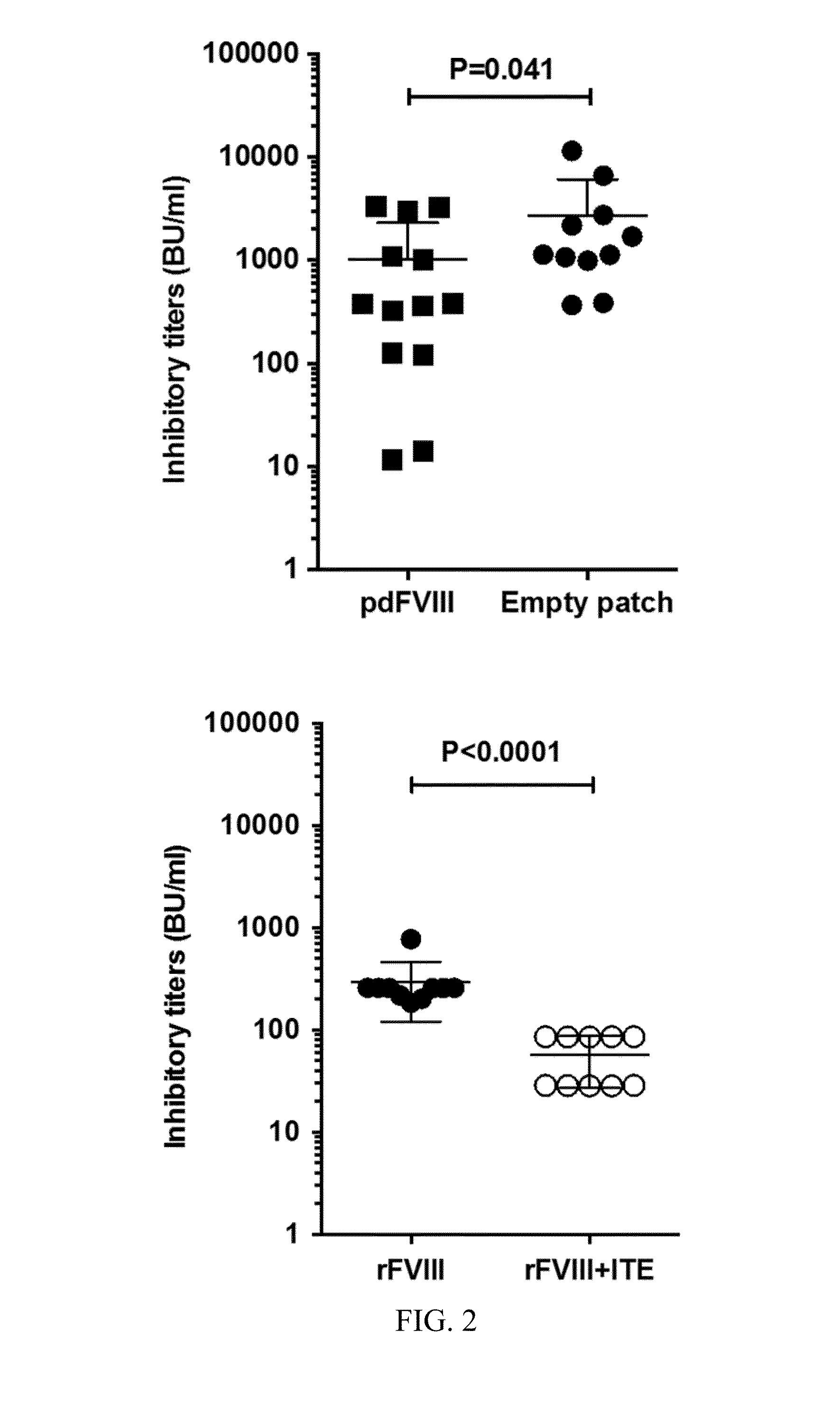
InactiveUS20160220644A1Relieve symptomsGood curative effectPeptide/protein ingredientsImmunological disordersTolerabilityImmune tolerance
The present invention relates to the treatment of haemophilia. More specifically, the invention relates to a new method of inducing an immune tolerance against at least one blood factor used to treat haemophilia through the epicutaneous route by application of a skin patch device comprising a blood factor in order to continue haemophilia treatment. The present invention also relates to the skin patch device containing Factor VIII.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Compositions comprising factor ó° and factor IXa for treating haemophilia
InactiveCN1826136AEconomical treatmentLow immunogenicityPeptide/protein ingredientsBlood disorderMedicineFactor ii
The present invention relates to the use of FIXa and FVIII in the preparation of a composition for the treatment of haemophilia A or haemophilia B in a subject which does not present with anti-FVIII antibodies. The present invention further relates to a composition comprising FIXa and a composition comprising FVIII for simultaneous, simultaneous separate or sequential use in the treatment of haemophilia A or haemophilia B in a subject which does not present with anti-FVIII antibodies.
Owner:NAT INST FOR BIOLOGICAL STANDARDS & CONTROL
Non-blood serum preparation of recombinant human blood coagulation factor IX
ActiveCN101481692BReduce pollutionProduct yield is highPeptide/protein ingredientsBiological testingMolecular compositionCures diseases
The invention relates to the preparation of recombinant human coagulation factor IX and an application thereof. The recombinant hFIX protein and hFIX of human blood source have the same molecular composition and biological activity and can be eased by being used for hemophilia B mouse model. The recombinant hFIX protein can be used as a standard in the tests for clinically detecting hFIX and the like, and can also cure diseases requiring supplementing hFIX, such as hemophilia B, etc.
Owner:浙江耶大生物医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com