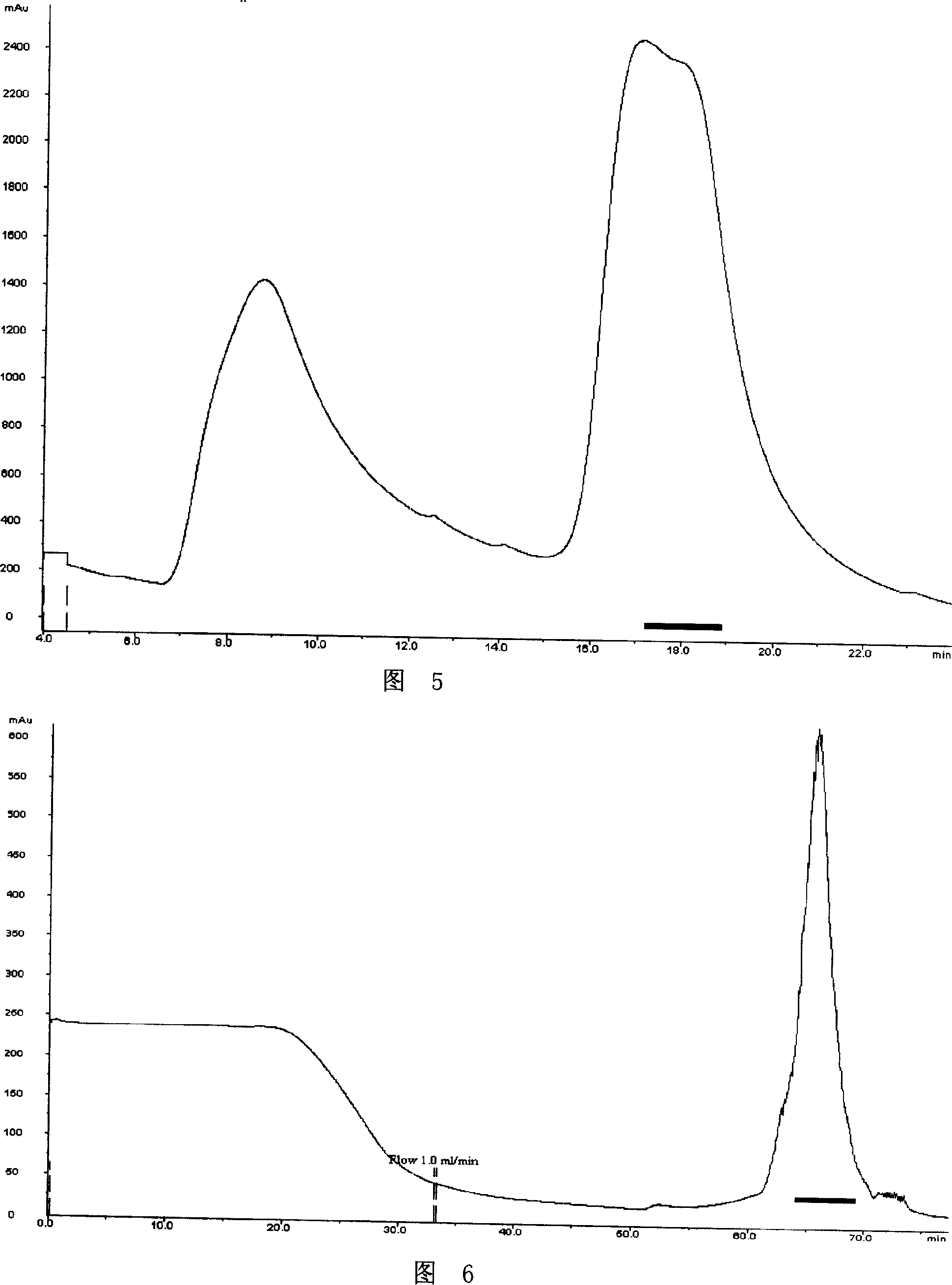
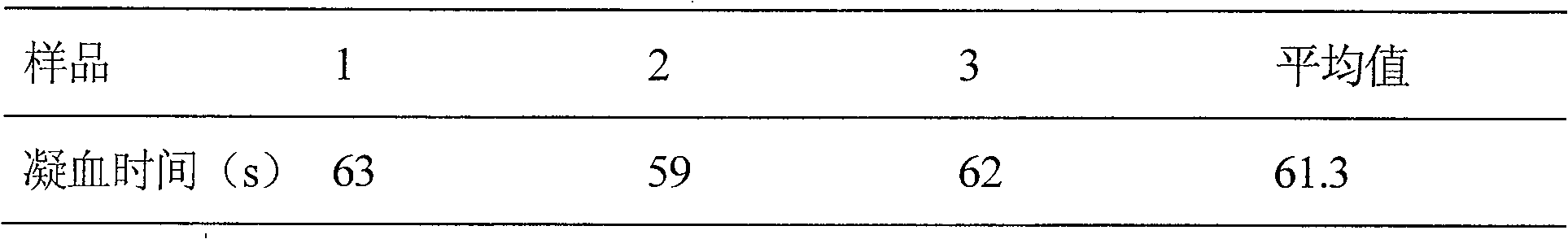Patents
Literature
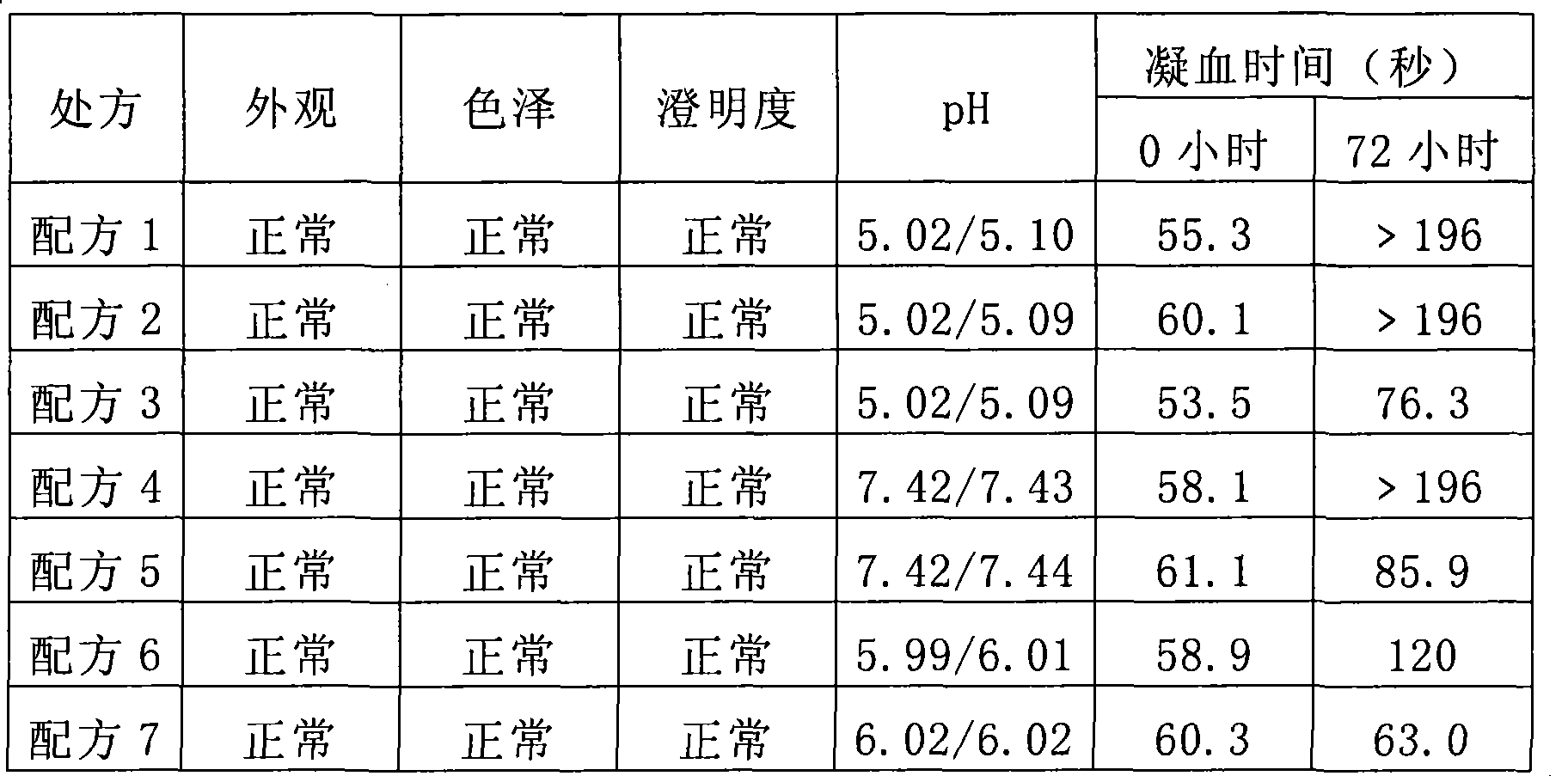
34 results about "Batroxobin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Batroxobin, also known as reptilase, is a snake venom enzyme with Venombin A activity produced by Bothrops atrox and Bothrops moojeni, venomous species of pit viper found east of the Andes in South America. It is a hemotoxin which acts as a serine protease similarly to thrombin, and has been the subject of many medical studies as a replacement of thrombin. Different enzymes, isolated from different species of Bothrops, have been called batroxobin, but unless stated otherwise, this article covers the batroxobin produced by B. moojeni, as this is the most studied variety.
Platelet aggregation function detection kit and detection method
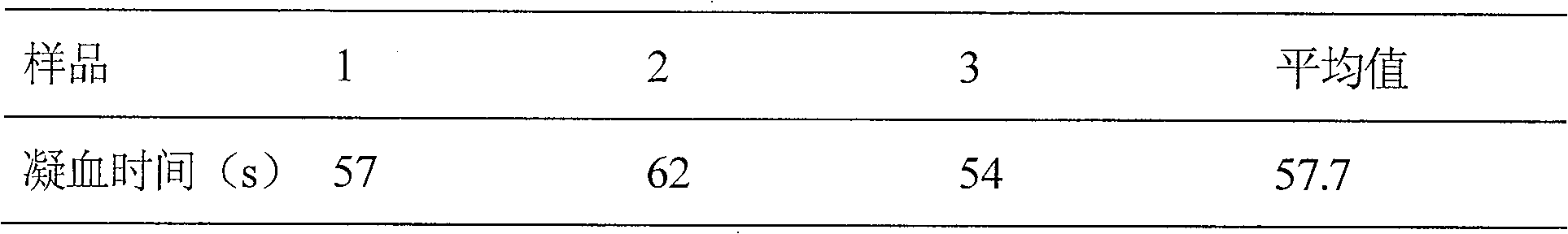
InactiveCN102980993AStrong specificityImprove replicate assay variabilityBiological testingTesting medicinal preparationsRepeat testingBatroxobin
The present invention relates to a platelet aggregation function detection kit, and a method using the kit to detect platelet aggregation function. The detection kit mainly includes the following detection reagents: a sodium citrate whole blood activator, a fibrin activator and a platelet activator. The fibrin activator comprises: recombinant batroxobin and activated clotting factor. By using the kit of the invention to detect, blood sample processing requirements are low, repeat testing variability is small, accuracy and stability are high, use and storage are convenient, detection cost is low, and promotion is easy.
Owner:北京乐普诊断科技股份有限公司
Novel sacculus dilating catheter
The present invention provides a new type balloon dilation catheter which includes ballon and medication material coated on stent. Said medication material comes from one or two and more than two mixtures of heparin sodium, fiber degrading enzyme, serine proteinase, batroxobin, aspirin, genistein, hirudin and its recombined product, colchicine, sirolimus, biolimus, zotarolimus, tracrolimus, pimecrolimus, simvastatin, atorvastatin, pravastatin, ciclosporin, Anti-CD34, dexamethasone, bleomycin, plicamycin, daunomycin, mitomycin C, actinomycin D, taxol, celastrol, methopterin, 5-fluorouracil, cytarabine and 6-purinethol. The balloon is made of macromolecule nylon material, and the stimulation to blood vessel is far lower than the stent with metal structure.
Owner:上海赢生医疗科技有限公司
Purified high-specific-activity recombinant batroxobin
ActiveCN101275126AImprove matching accuracyHigh specific activityPeptide/protein ingredientsHydrolasesDisulfide bondingHigh specific activity
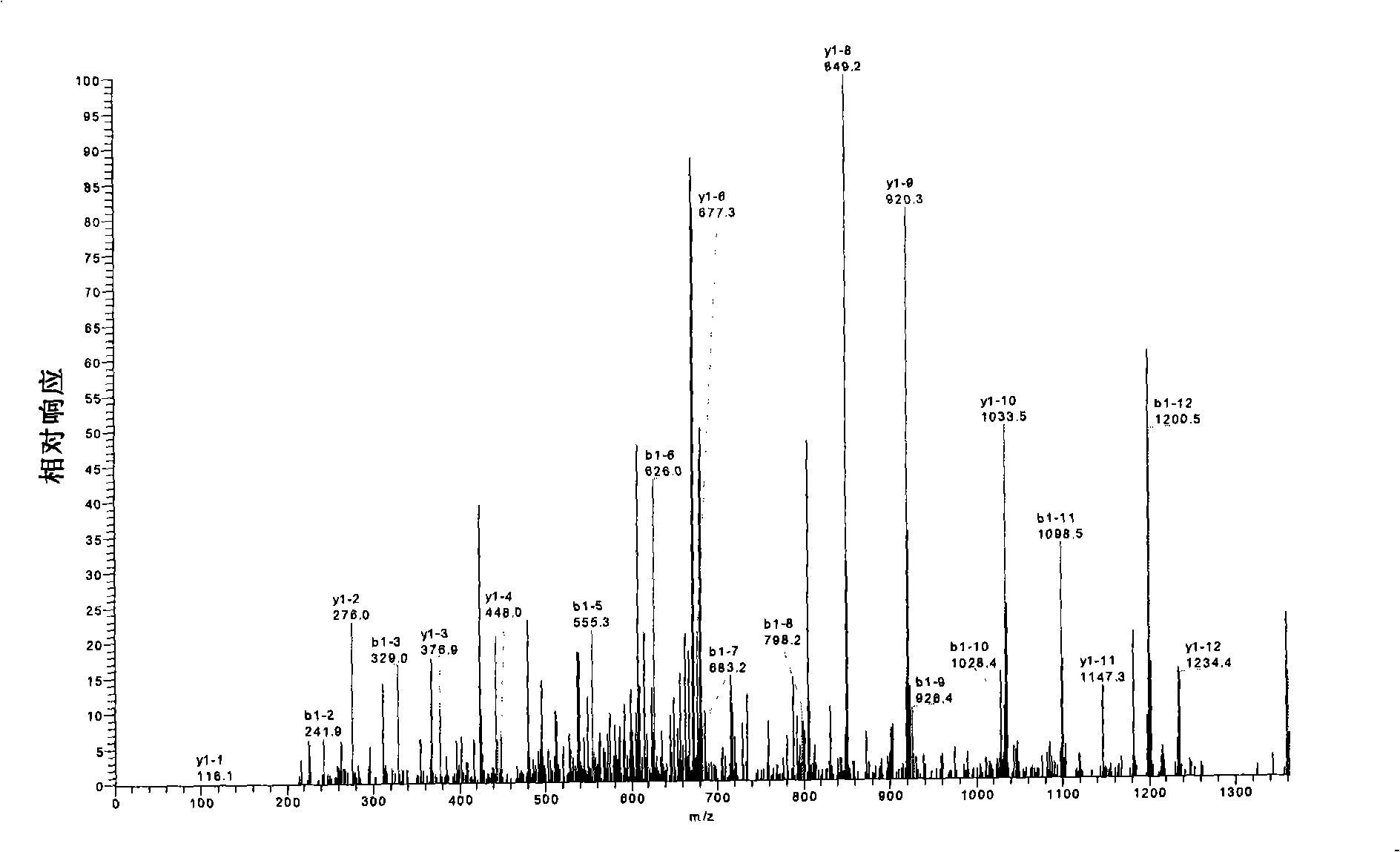
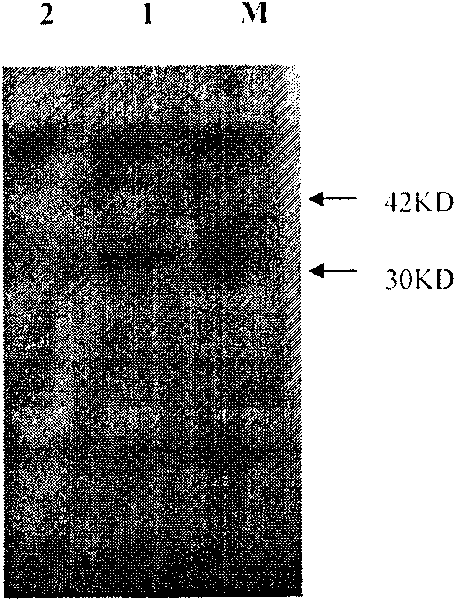
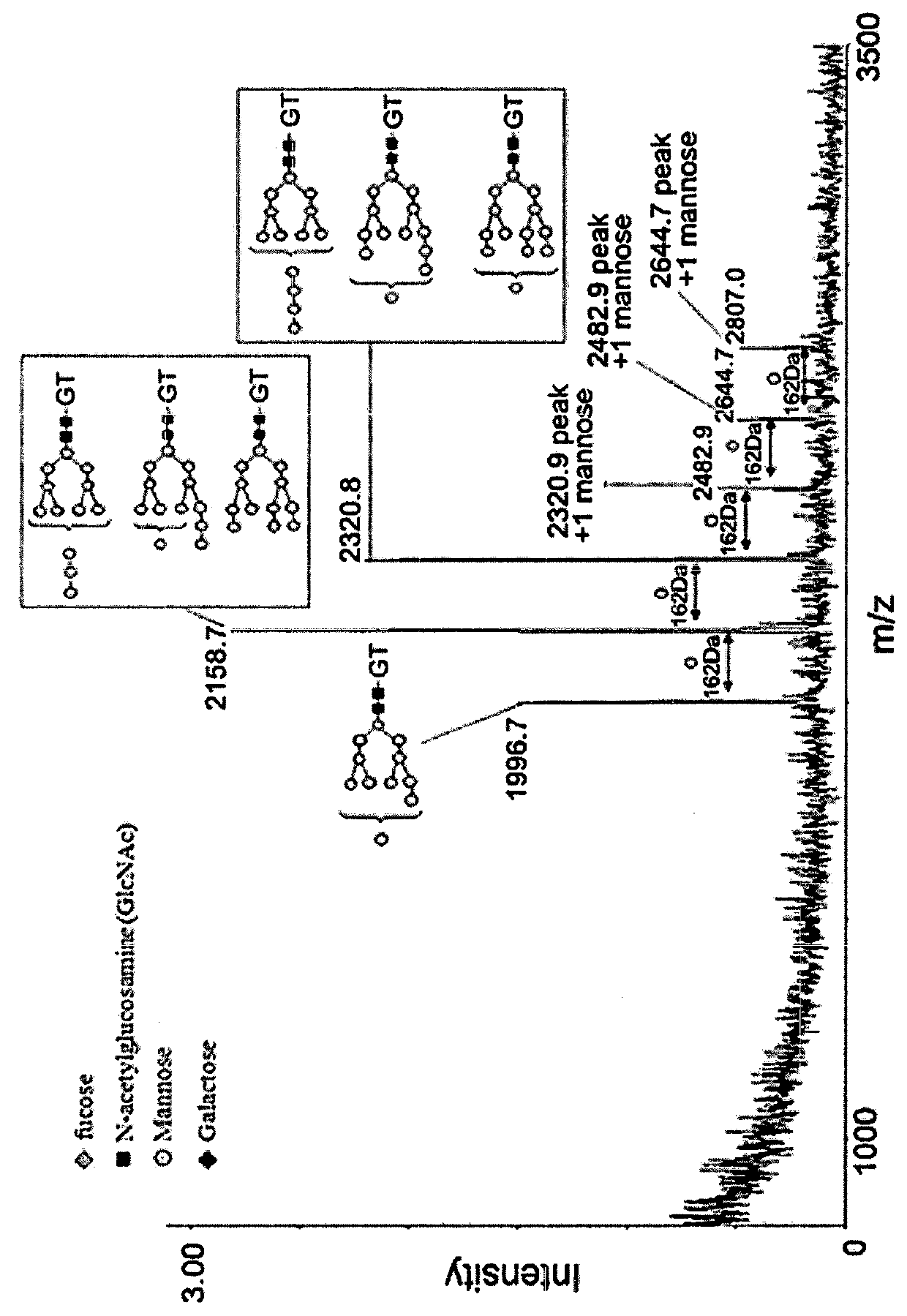
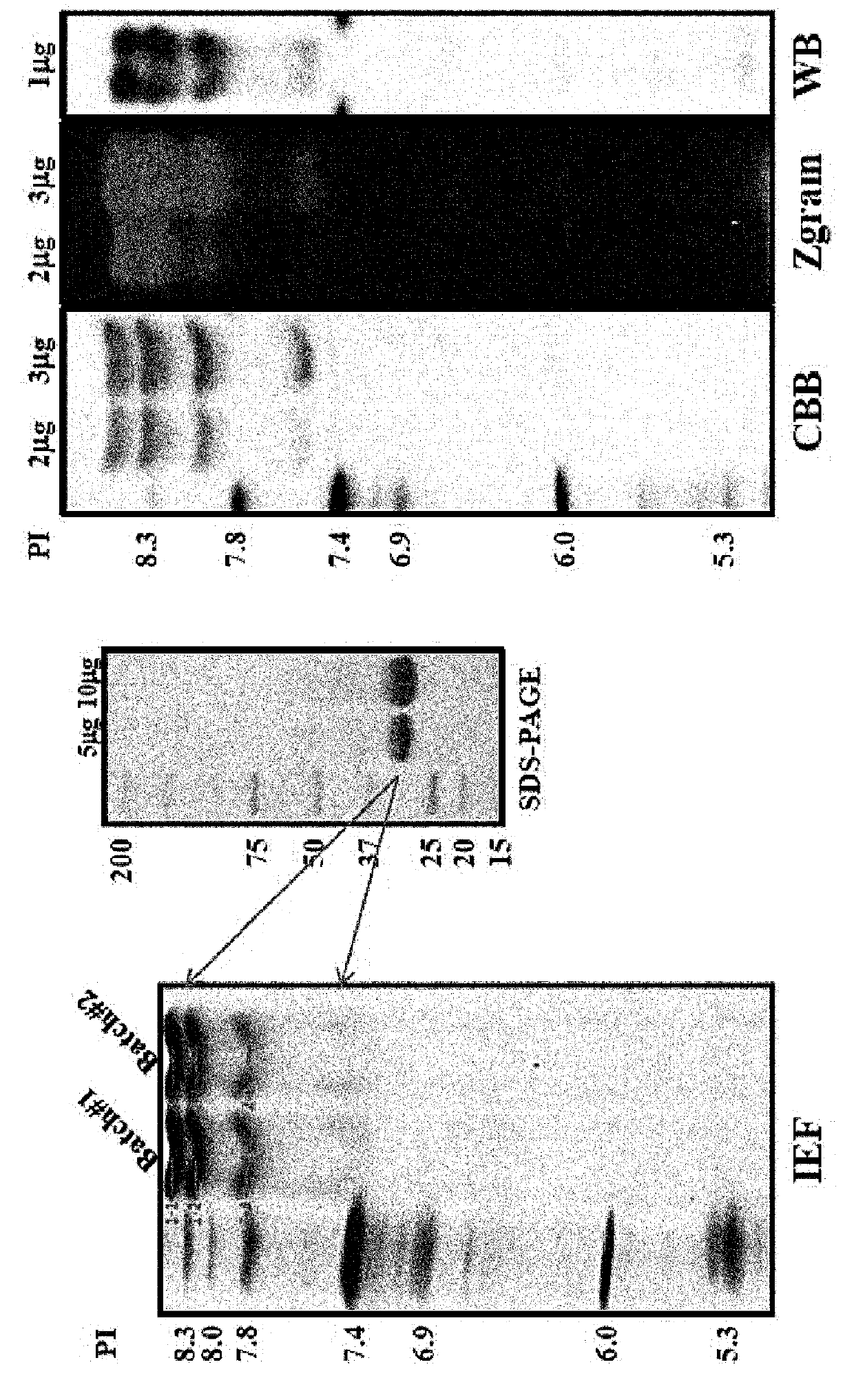
The present invention provides a recombined-batroxobin, having following characteristics: (a) the molecular weight is 29-32Kda; (b) at least 90% batroxobin in the recombined-batroxobin has correct 6 pairs of disulfide bonding; Cys7-Cys139, Cys26-Cys42, Cys74-Cys230, Cys118-Cys184, Cys150-Cys163 and Cys174-Cys199; (c) the 146 position and the 225 position in the SEQ ID NO:1 are suffered from glycosylation; (d) the specific activity of the batroxobin is more than 1500KU / mg.
Owner:SHANGHAI TENRY PHARMCEUTICAL CO LTD
Process for preparing an autologous platelet gel and membrane thereof
A process is disclosed for preparing an autologous platelet gel and membranes thereof comprising mixing a platelet concentrate with a calcium salt and batroxobin. This process encompassing the use of batroxobin as the gel activator allows to overcome the prior art processes drawbacks connected with the use for the same purpose of human of bovine thrombin. A kit is also described for carrying out this process.
Owner:SACCHI MARIA CRISTINA
Fibrinogen plate assay for detection of thrombin, thrombin-like enzyme and thrombin inhibitor
InactiveCN1763219AOt affected by turbidityUndisturbedMicrobiological testing/measurementBiological testingTurbidityBiology
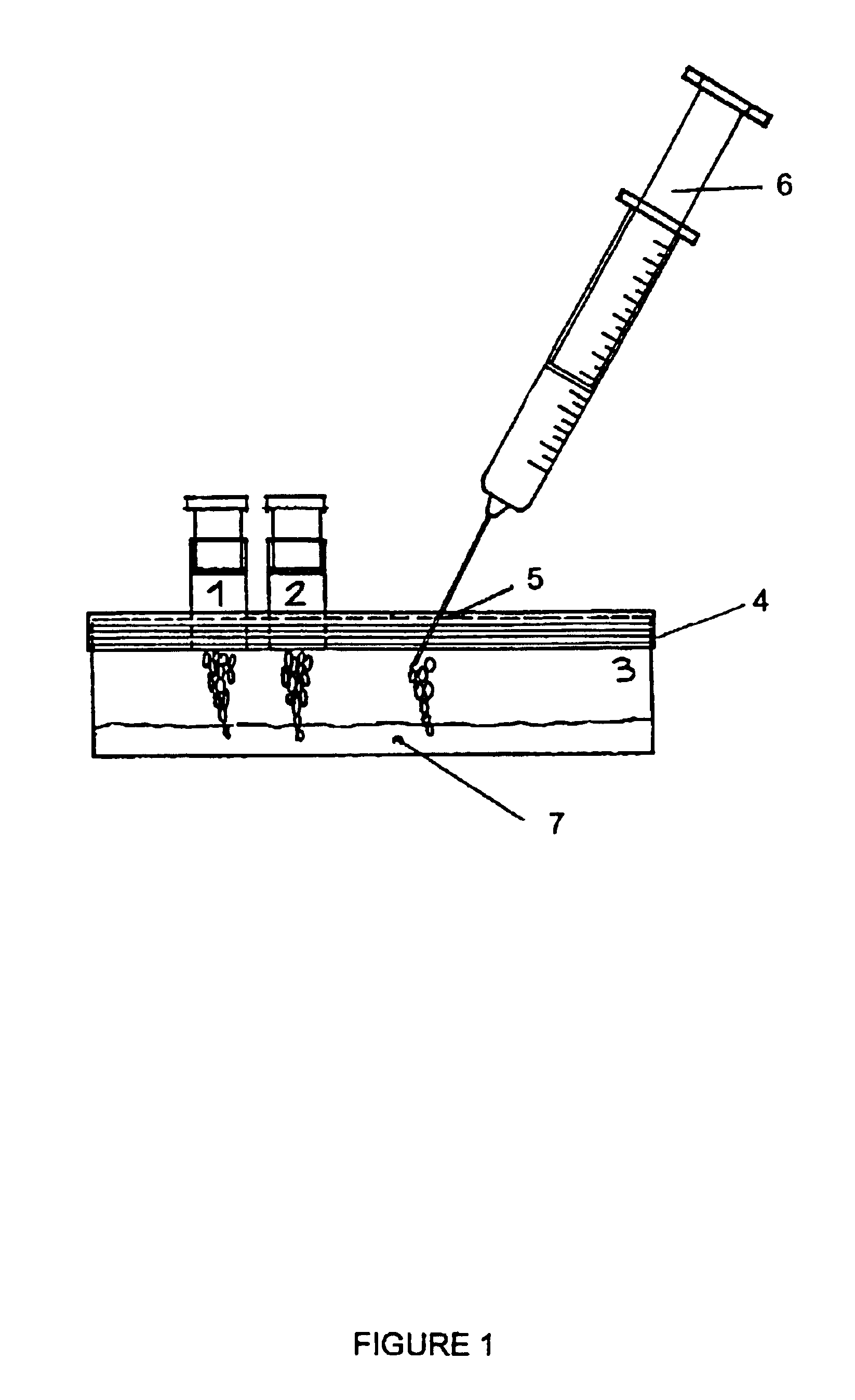
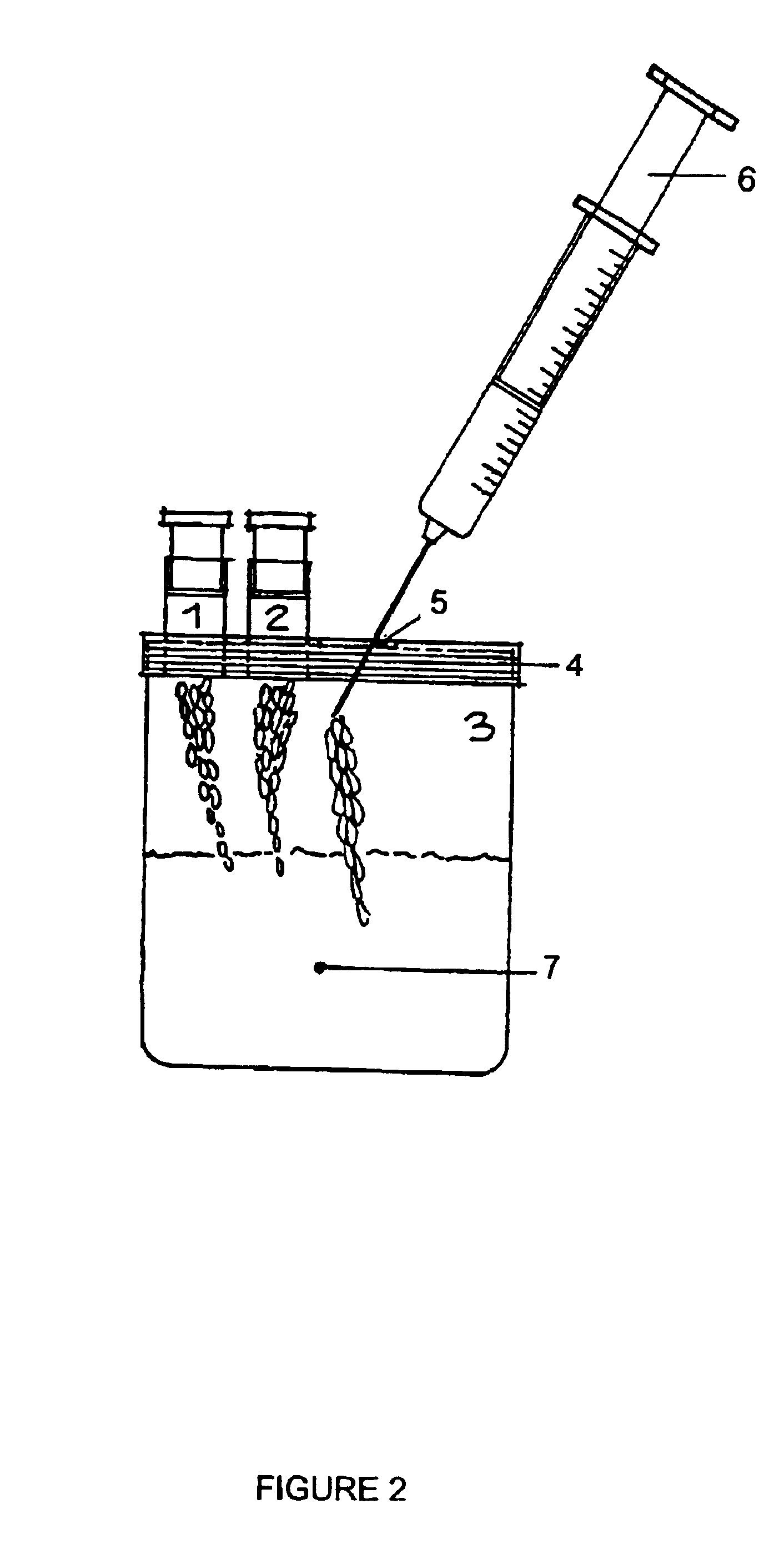
The fibrinogen plate method for detecting thrombase, batroxobin and thrombase inhibitor includes preparing fibrinogen plate with agarose and fibrinogen and quantitative detection via the diffusion reaction of standard sample and detected sample on the plate. The detection method of the present invention has simple operation, high sensitivity, being intuitive and stable, no interference of the turbidity and color of the detected sample and low cost. The detection method may be realized simply in lab with thermotank.
Owner:GUANGXI MEDICAL UNIVERSITY
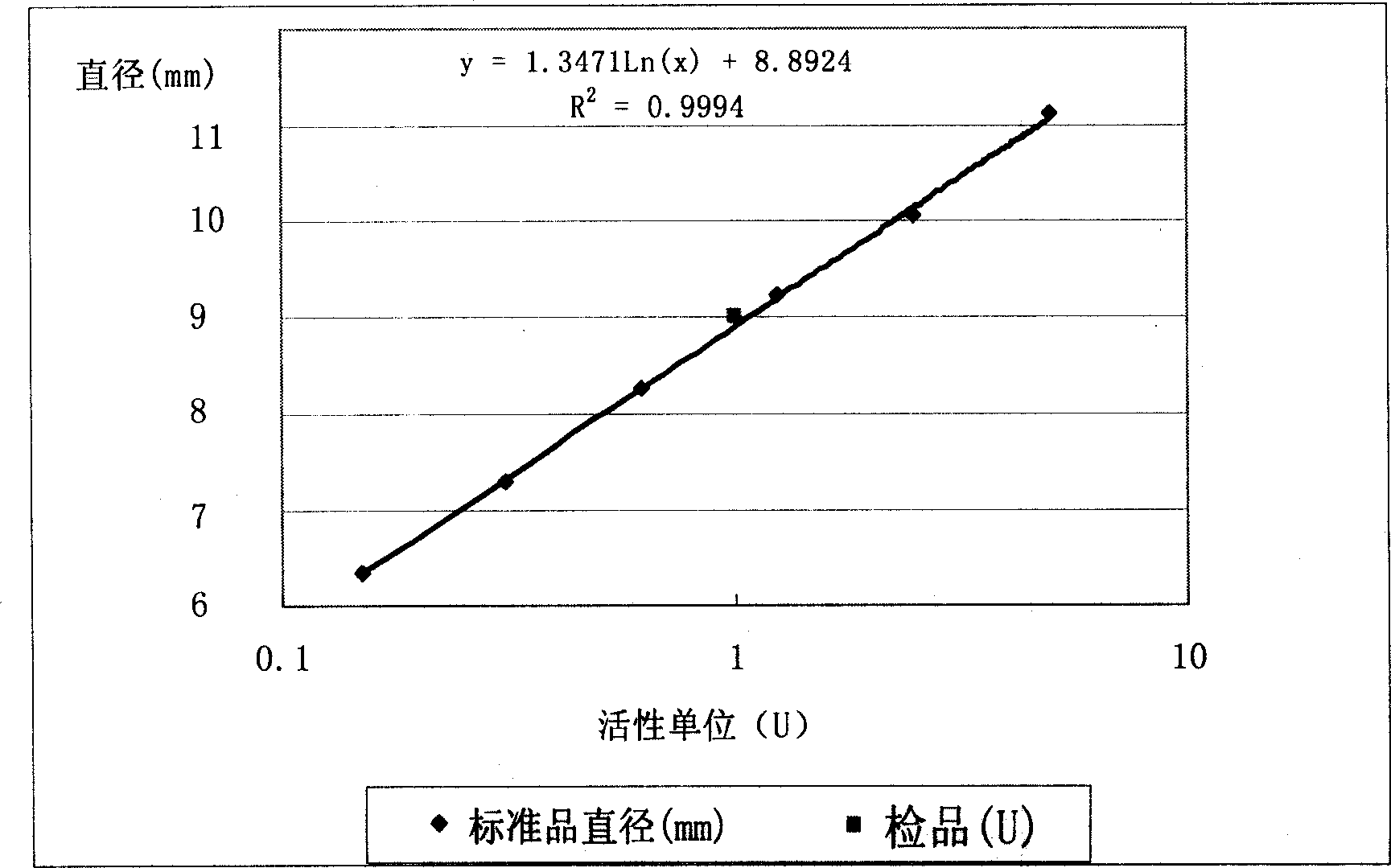
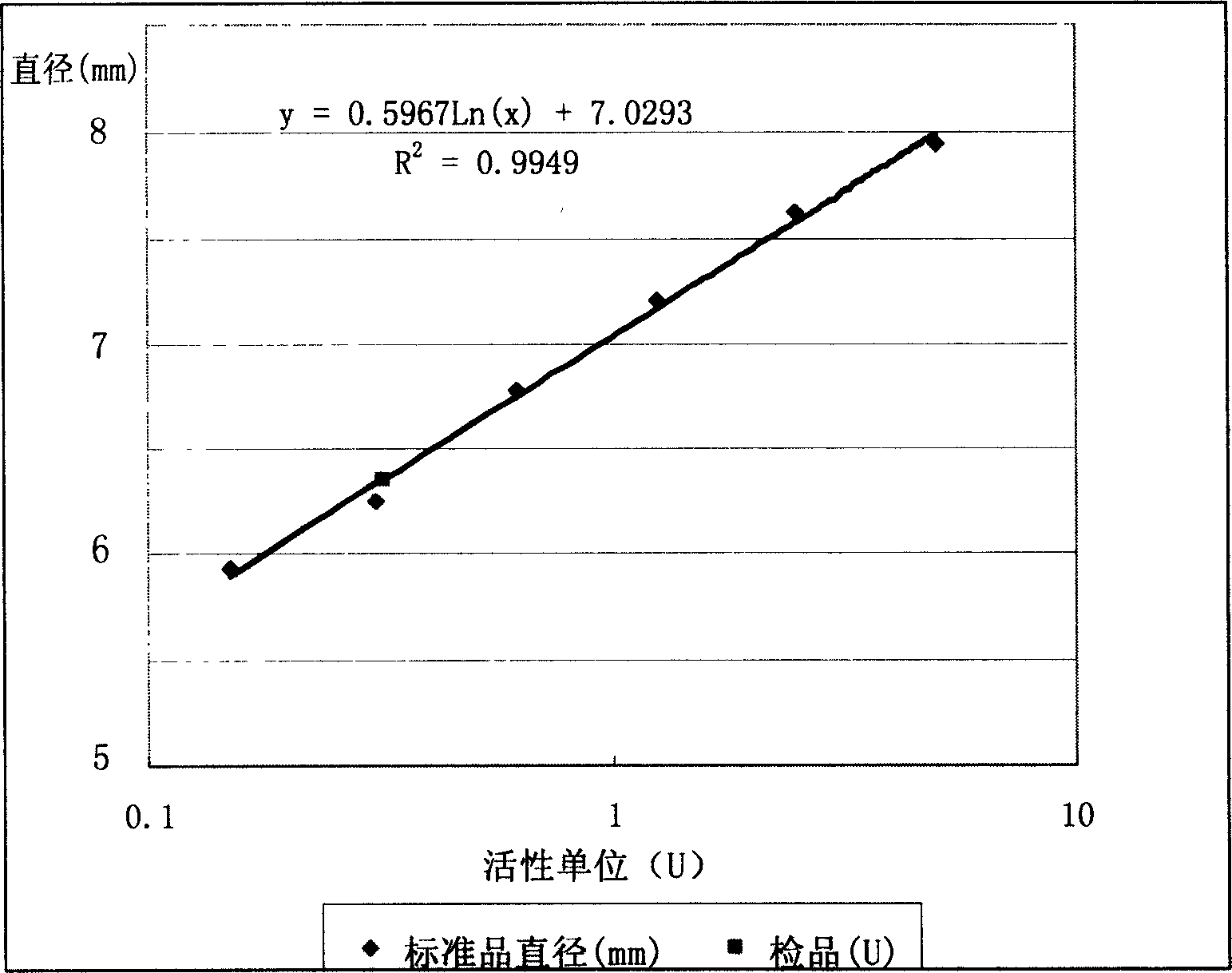
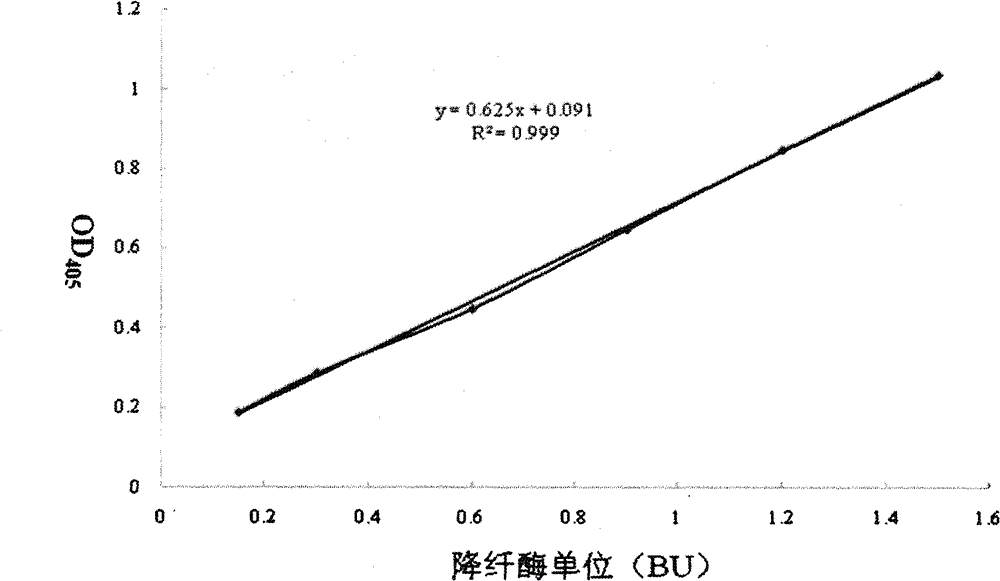
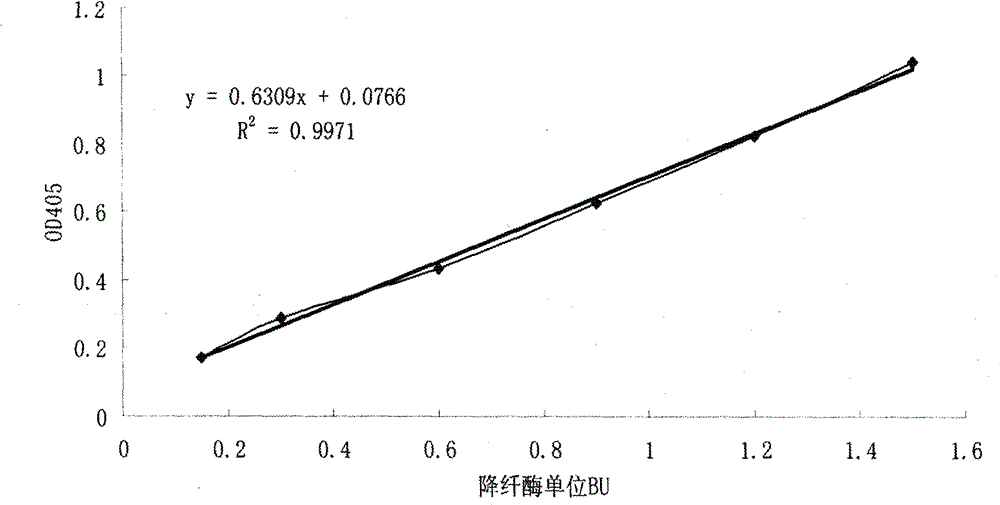
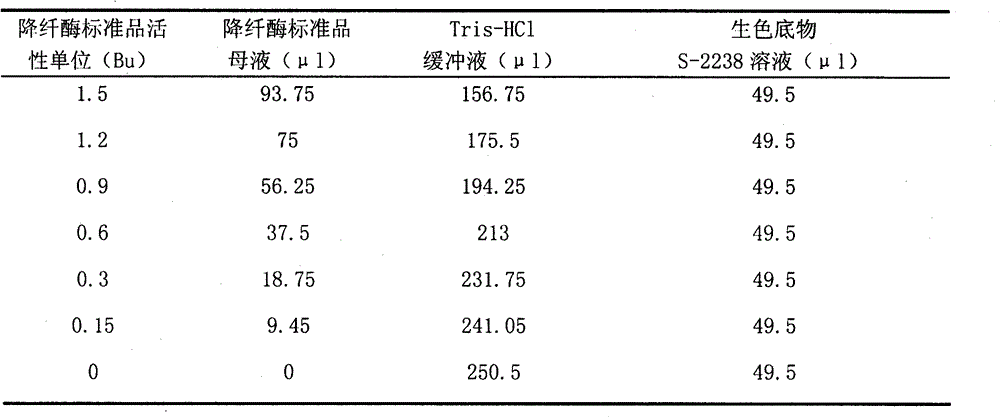
Method for detecting activity of batroxobin
InactiveCN102798632AMaterial analysis by observing effect on chemical indicatorChromogenic substrate assayReaction curve
The invention discloses a method for quickly and specifically detecting the activity of batroxobin. A chromogenic substrate assay is adopted, a microplate reader is used, a linear reaction curve is obtained in a certain reaction time and a certain-activity standard substance range, a linear relation between a light absorption value of a product and the amount of the batroxobin is established, and the corresponding enzymatic activity of a sample is calculated. In addition, the recovery rate and accuracy of the standard curve are carefully researched. The method is high in accuracy, simple in steps and convenient to impellent in a laboratory, and is suitable for quality control of batroxobin raw materials and hemocoagulase preparations.
Owner:ZHAOKE PHARMA HEFEI
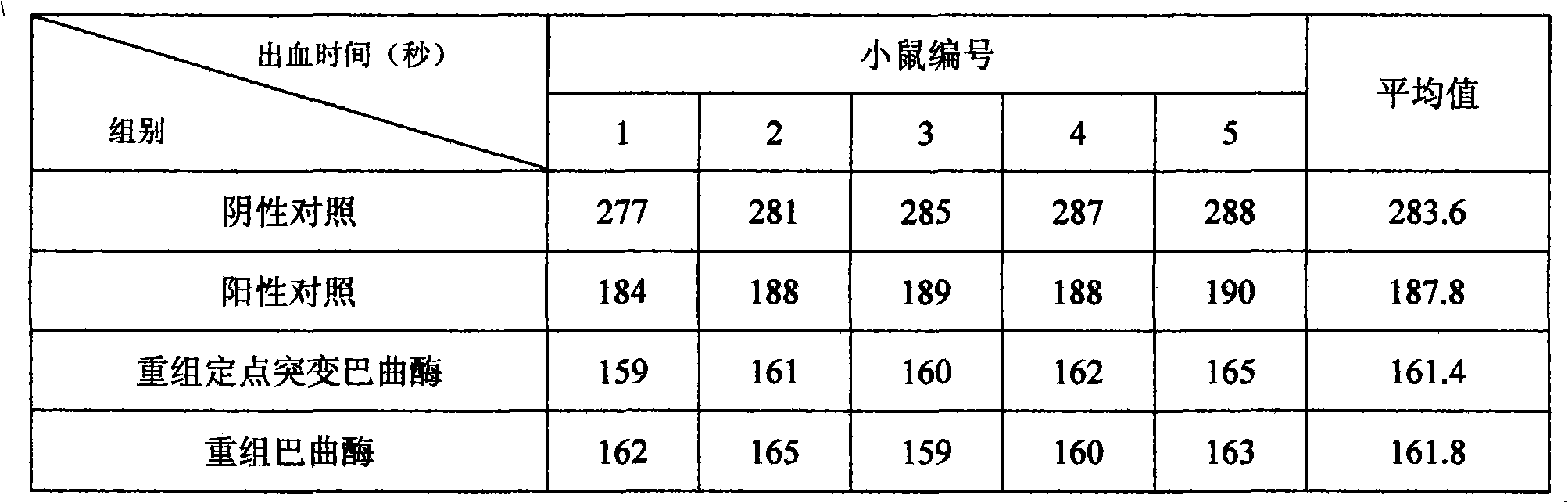
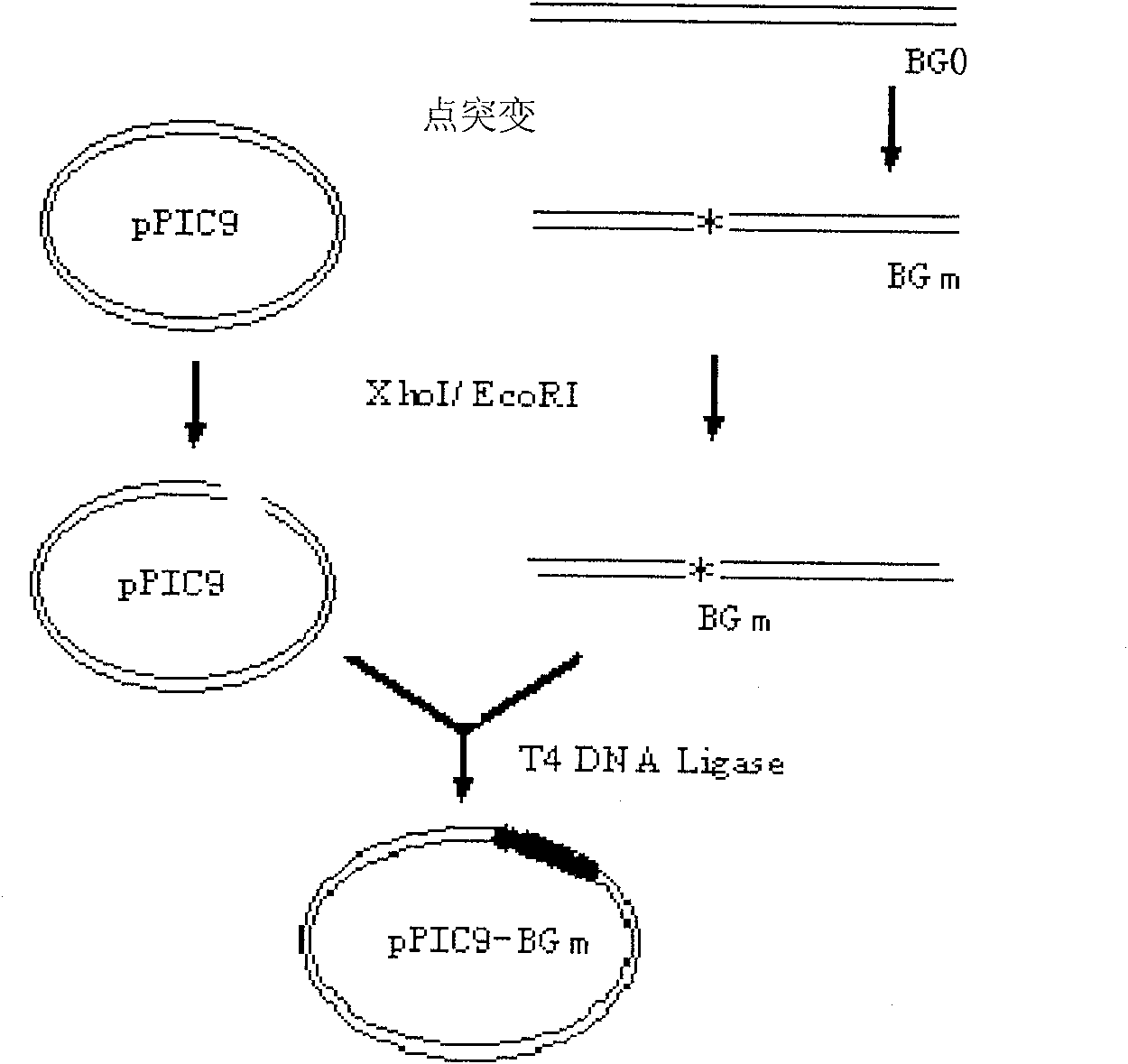
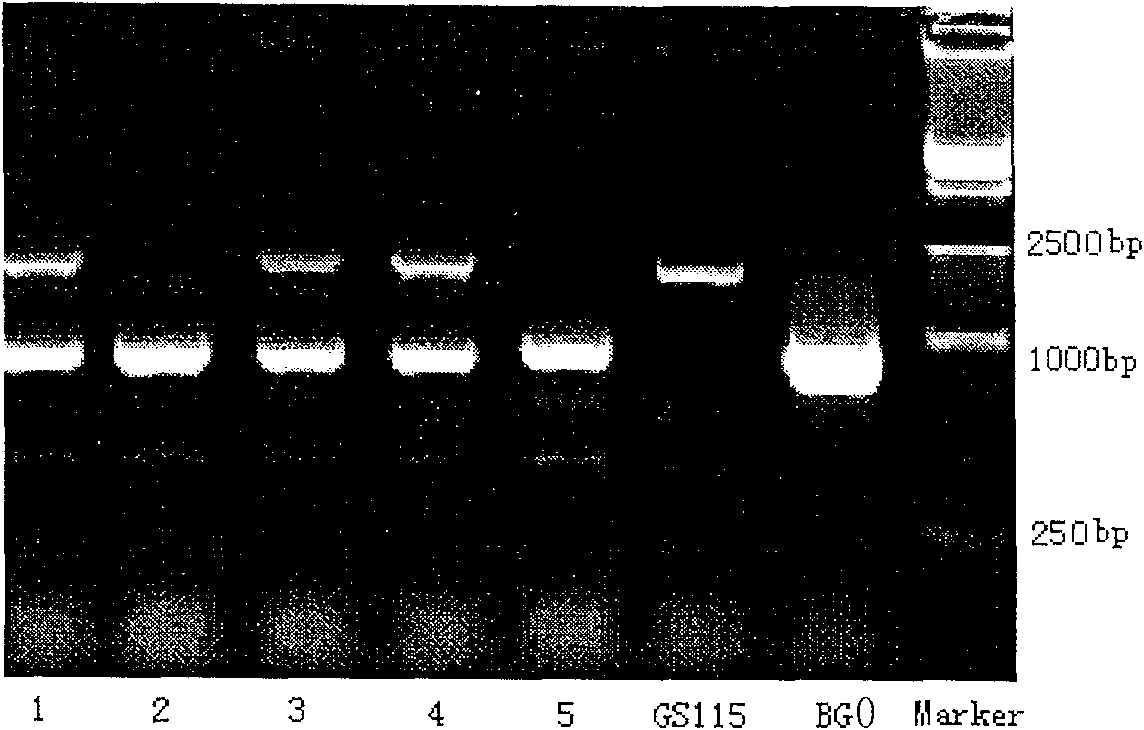
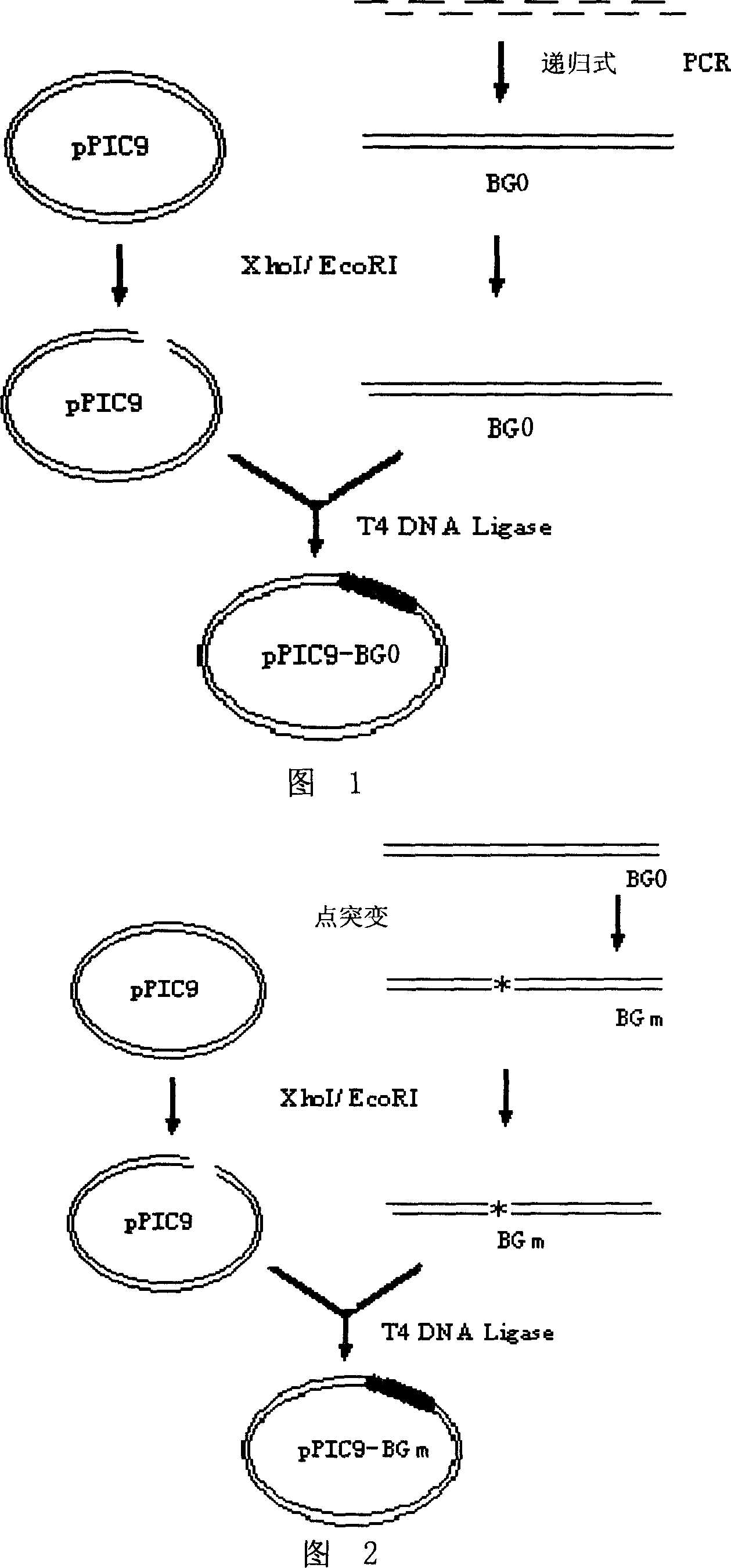
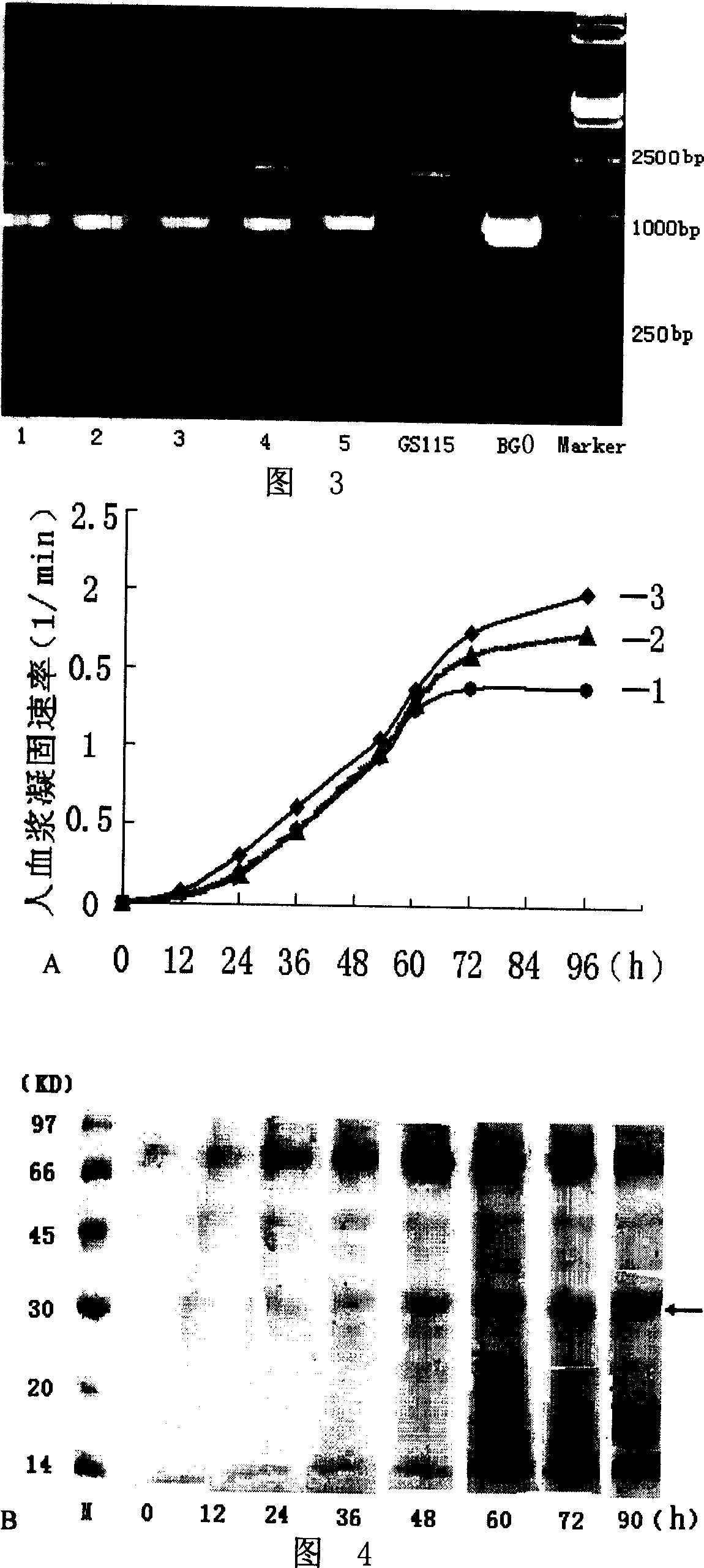
Site-directed mutagenesis genetic engineering batroxobin and uses thereof
The invention relates to a site-directed mutagenesis genetic engineering batroxobin is capable of clotting animal plasma containing an anti-coagulant agent. The site-directed mutagenesis genetic engineering batroxobin is characterized in that: compared with the amino acid sequence of a natural batroxobin, the amino acid sequence of the site-directed mutagenesis genetic engineering batroxobin has a mutation from Arg to Lys at the 45th digit. The site-directed mutagenesis genetic engineering batroxobin having higher bioactivity can be produced in a large scale through a secreted expression mode.
Owner:GRAND LIFE SCI (LIAONING) CO LTD
Thrombin-like recombinant baxtroxobin expressed by pichia sp.and production method thereof
A recombinant thrombin-like enzyme batroxobin expressed by yeast, a production method thereof, and use of the batroxobin as a hemostatic agent or antithrombotic agent is disclosed. In order to express recombinant thrombin-like enzyme, an expression vector is prepared by inserting cDNA encoding the enzyme, the transformed cell is prepared by introducing the expression vector into the cell, the transformed cell then is incubated and the recombinant thrombin-like enzyme is obtained from the cell. The recombinant thrombin-like enzyme may be usefully used as a hemostatic agent because the enzyme effectively decrease bleeding time and blood coagulation time and does not affect various blood coagulation factors. The recombinant thrombin-like enzyme is also useful for a hemostatic agent or antithrombotic agent comprising the recombinant thrombin-like enzyme as an active component
Owner:BIOBUD
Hemostasis composition containing batroxobin and preparation method thereof
InactiveCN101797378AReduce the chance of infectionAvoid bleeding symptomsPeptide/protein ingredientsAluminium/calcium/magnesium active ingredientsThrombin activityBatroxobin
The invention provides a novel hemostasis composition which comprises fibrinogen or hemaleucin, and batroxobin, wherein the ratio of the batroxobin to the fibrinogen or hemaleucin is 0.01:100IU:1mg. Because a clot formed by the batroxobin can not cause contraction, and the batroxobin can not cause crossimmunity reaction, the hemostasis composition can overcome multiple defects existing in hemostasis products containing thrombin or prothrombin.
Owner:BEIJING SAISHENG PHARMA
Batroxobin and its preparing process and specific coding gene
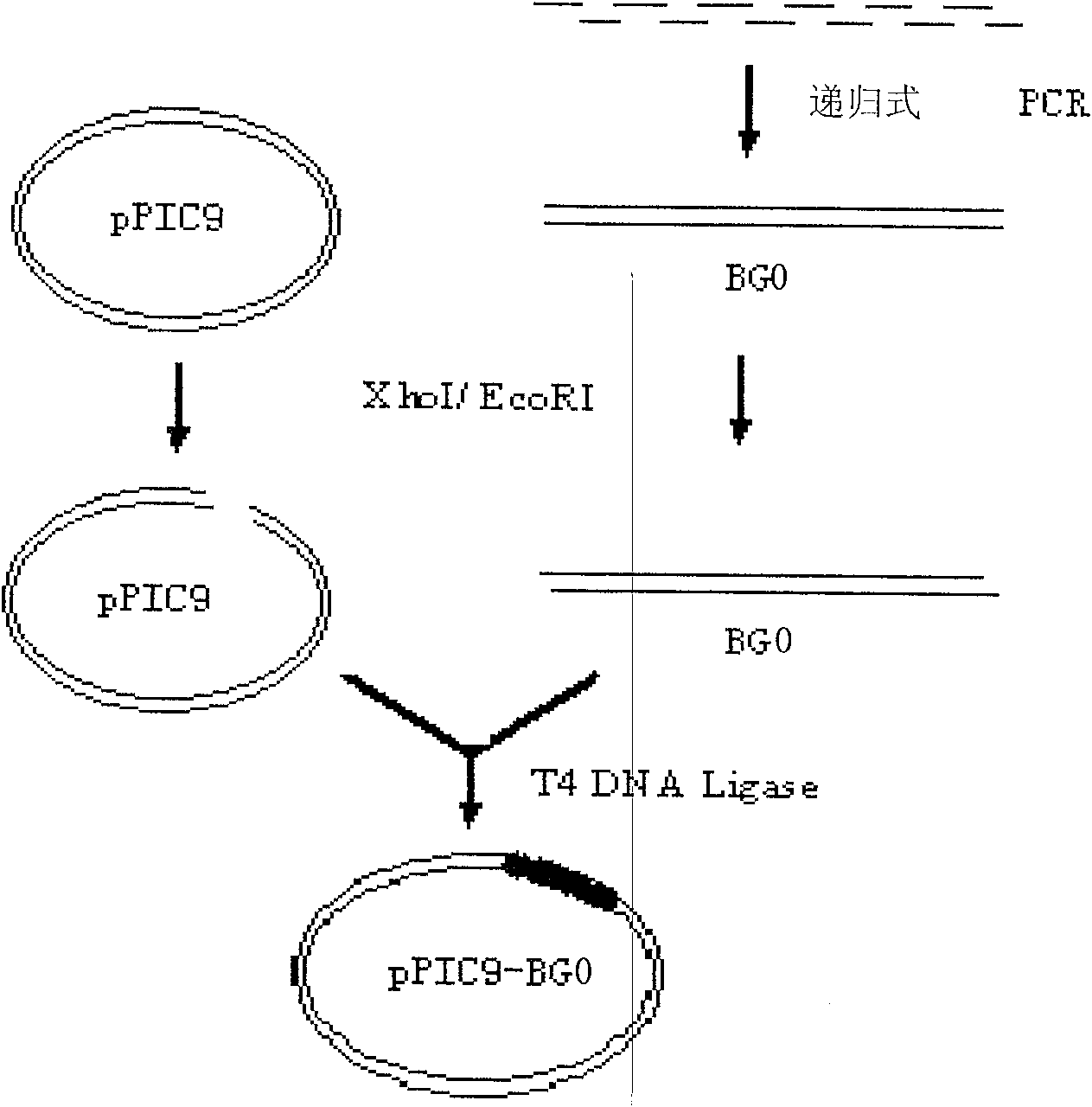
The invention discloses batroxobin, its preparation method and special coding gene. The batroxobin coding gene is one of the following nucleotide sequences: 1) sequence 2 in the sequence listing; 2) sequence 4 in the sequence listing; 3) sequence 6 in the sequence listing; 4) sequence 8 in the sequence listing . The method for preparing batroxobin comprises introducing the coding gene described in any one of claims 1-3 into yeast or mammalian cell line for expression through a eukaryotic expression vector to obtain batroxobin protein. The method of the present invention has successfully expressed the batroxobin protein with high biological activity, and the yeast yield after purification reaches 10 μg / ml or 14 batroxobin units (14BU / ml) per milliliter of fermentation broth, and the specific activity is 1400BU / mg . This yield exceeds other published and reported expression levels and is suitable for large-scale production.
Owner:JOINN LAB (SUZHOU) INC
Batroxobin and its preparing process and specific coding gene
The present invention discloses batroxobin and its preparation process and specific coding gene. The batroxobin coding gene possesses one of the following nucleotide sequence: 1. the sequence 2 in the sequence list; 2. the sequence 2 in the sequence list with 1-3 mutant nucleotides; and 3. the nucleotide sequence obtained through connecting IL-2 signal peptide gene sequence to the 5' end of the said sequence. During preparation process of batroxobin, any one coding gene of the nucleotide sequence 1-3 is introduced through eukaryon expression vector into yeast or mammal cell line for expression to obtain batroxobin protein. The process of the present invention expresses batroxobin protein in high bioactivity successfully, and obtains purified yeast yield of 10 mcg / ml or 14 BU / ml and in specific activity of 1400 BU / mg. The present invention has high yield and is suitable for industrial production.
Owner:JOINN LAB (SUZHOU) INC
Compound stypticum
ActiveCN101138632AReduce dosageGuaranteed curative effectPeptide/protein ingredientsBlood disorderBone Marrow Stromal CellWhite blood cell
A compound medicine for arresting bleeding is characterized in that the compound medicine is a mixture of a batroxobin and an interleukin-11; the clinical dosage of the batroxobin is 0.01-10u and that of IL-11 is 0.06-50mg; the batroxobin is the batroxobin from snake venom of Brazilian spearhead adder, white-eyebrow adder or acutus adder, or the batroxobin from recombinant Brazilian spearhead adder, white-eyebrow adder or acutus adder obtained by genetic engineering; the interleukin-11 is produced by human bone-marrow stromal cells (fibroblasts) and interstitial cells, or the recombinant IL-11 by genetic engineering. The medicine for arresting bleeding can not only ensure the curative effect but also decrease the amount of protease so as to reduce the rate of adverse immune response.
Owner:GRAND LIFE SCI (LIAONING) CO LTD
Preparation comprising batroxobin for inhibiting local invasion of malignant tumors
A preparation for inhibiting local invasion of malignant tumors is provided which comprises batroxobin and therefore can inhibit local invasion of malignant tumors. A preparation for encapsulating malignant tumor tissues is also provided which comprises batroxobin and therefore can cause or promote formation of capsule-like tissue around malignant tumor tissues.
Owner:TOBISHI PHARMA CO LTD
Purified recombinant batroxobin with high specific activity
ActiveUS20100047229A1High activityEasy to controlPeptide/protein ingredientsHydrolasesHigh specific activityBatroxobin
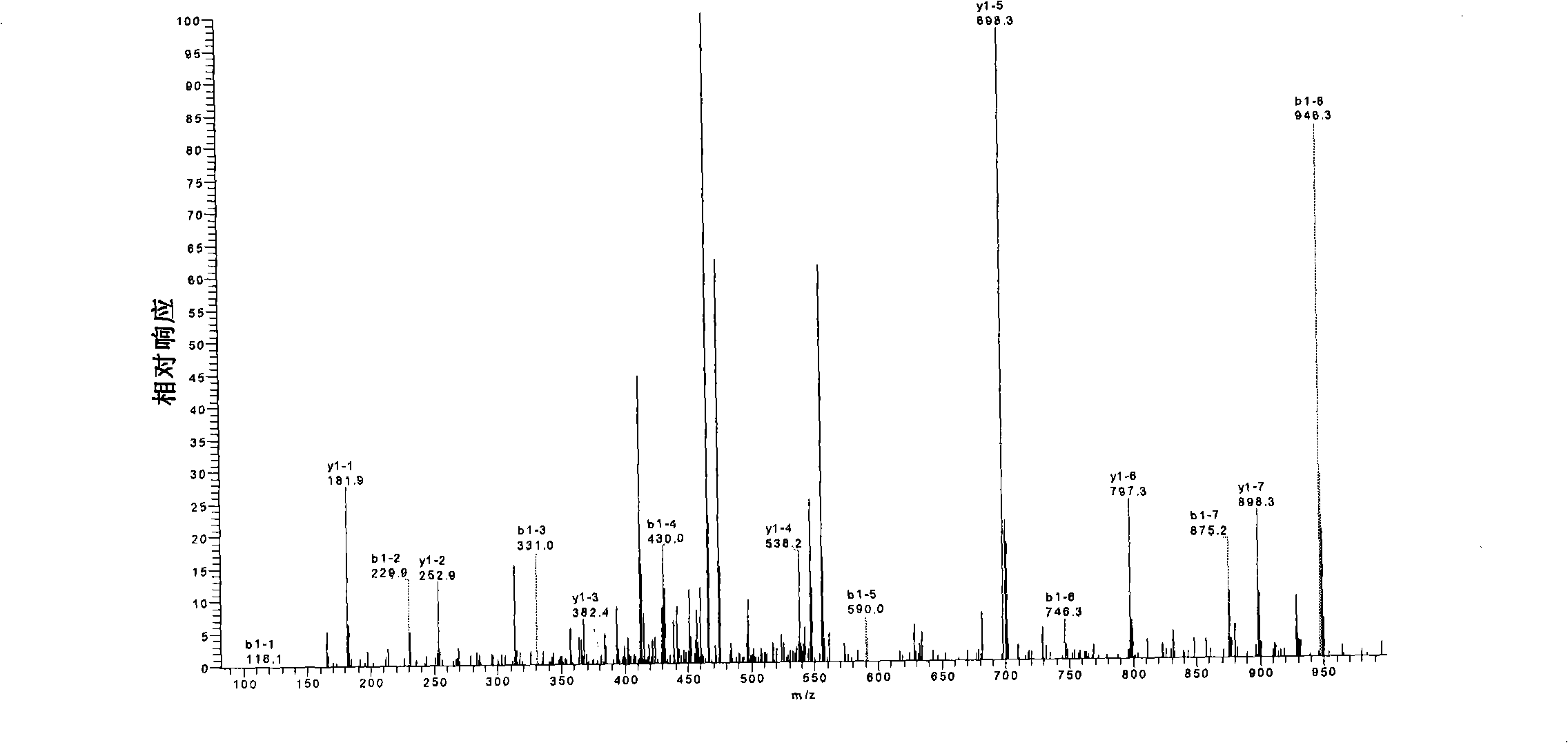
A purified recombinant batroxobin with high specific activity, which has the following properties: (a) the batroxobin has a molecular weight of 29-32 kDa; (b) at least 90% of the batroxobin have 6 pairs of disulfide bonds which correctly match at Cys7-Cys139, Cys26-Cys42, Cys74-Cys230, Cys118-Cys184, Cys150-Cys163 and Cysl174-Cys199; (c) positions 146 and 225 in SEQ ID NO:1 are modified as N-glycosylation; and (d) the specific activity of the batroxobin is equal to or greater than 1500 KU / mg protein.
Owner:SHANGHAI TENRY PHARMCEUTICAL CO LTD
Purified recombinant batroxobin with high specific activity
ActiveUS7939067B2High activityEasy to controlHydrolasesPeptide/protein ingredientsHigh specific activityBatroxobin
A purified recombinant batroxobin with high specific activity, which has the following properties: (a) the batroxobin has a molecular weight of 29-32 kDa; (b) at least 90% of the batroxobin have 6 pairs of disulfide bonds which correctly match at Cys7-Cys139, Cys26-Cys42, Cys74-Cys230, Cys118-Cys184, Cys150-Cys163 and Cysl174-Cys199; (c) positions 146 and 225 in SEQ ID NO:1 are modified as N-glycosylation; and (d) the specific activity of the batroxobin is equal to or greater than 1500 KU / mg protein.
Owner:SHANGHAI TENRY PHARMCEUTICAL CO LTD
Synthesis of batroxobin gene and preparation method of expression product thereof
InactiveCN101705240AExcellent non-polar environmentEnhance electrostatic interactionHydrolasesMicroorganism based processesPurification methodsNucleotide
The invention relates to a batroxobin protein which is derived from the venom of a brothrops atrox and prepared by adopting a recombinant DNA technology. The coding gene of the batroxobin protein has one of the following nucleotide sequences of: (1) sequence 3 in a sequence table; (2) sequence 4 in the sequence table, wherein a coding batroxobin amino acid sequence is relative to a natural batroxobin amino acid sequence, and the 41 bit of His and the 178 bit of Ser are mutated to be Glu. By utilizing the fermentation and purification method optimized by the invention, the high bioactive batroxobin protein is successfully expressed in a secretion expression way. The batroxobin with recombinant expression can be used as a main ingredient for hemostatics.
Owner:YANGTZE RIVER PHARMA GRP BEIJING HAIYAN PHARMA
Oriented mutant gene engineering barr kinase and its use
A directed mutation gene engineering batroxobin and its use are disclosed. The process is carried out by deleting 10 amino acids at C-end of batroxobin amino-acid sequence and mutating No.133 Tyr to Glu. It has better biological activity.
Owner:GRAND LIFE SCI (LIAONING) CO LTD
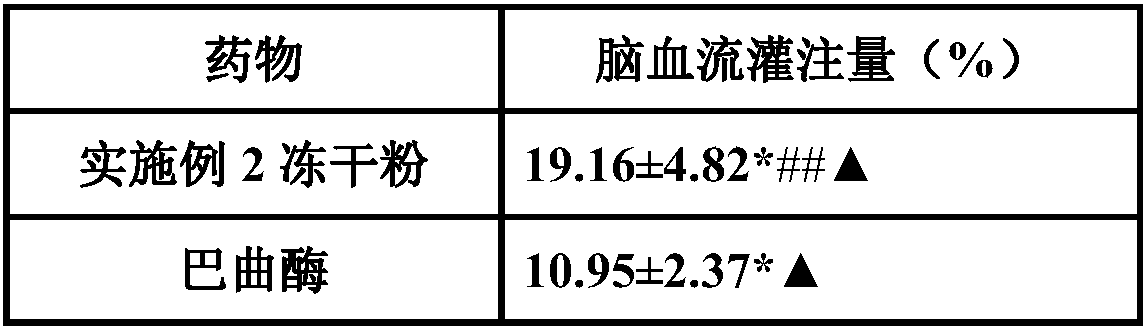
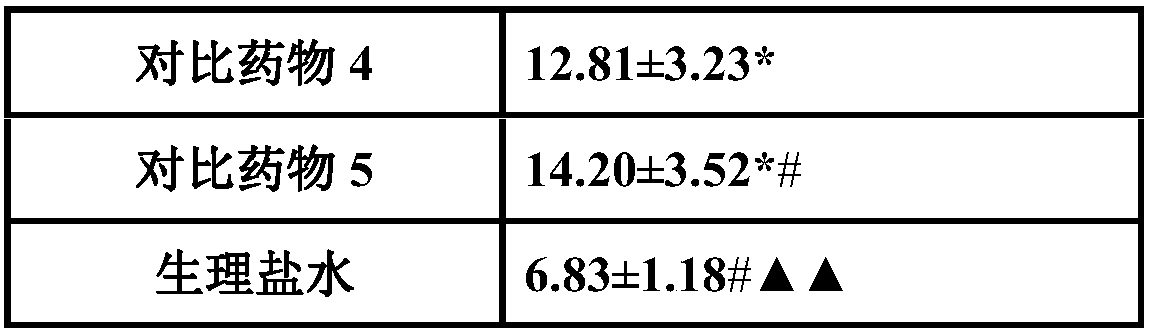
Medicinal composition for treating acute cerebral ischemic stroke
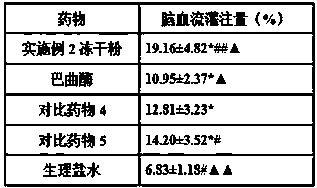
InactiveCN107648598AEnhance the antithrombotic effect in vivoReduce bleeding riskOrganic active ingredientsPowder deliveryGinkgolideTherapeutic effect
The invention belongs to the field of medicines, and particularly relates to a medicinal composition for treating acute cerebral ischemic stroke. The medicinal composition is prepared from pharmaceutically acceptable medicinal auxiliary materials, batroxobin, citicoline, ginkgolide and protoporphyrin sodium. The dosage form of the medicinal composition is preferably lyophilized powder for injection. The protoporphyrin sodium in the medicinal composition has a significant effect of enhancing the batroxobin functions of preventing thrombosis and improving cerebral blood perfusion, so that improvement on the cerebral ischemic stroke treating effect of the batroxobin is benefited.
Owner:张惊宇
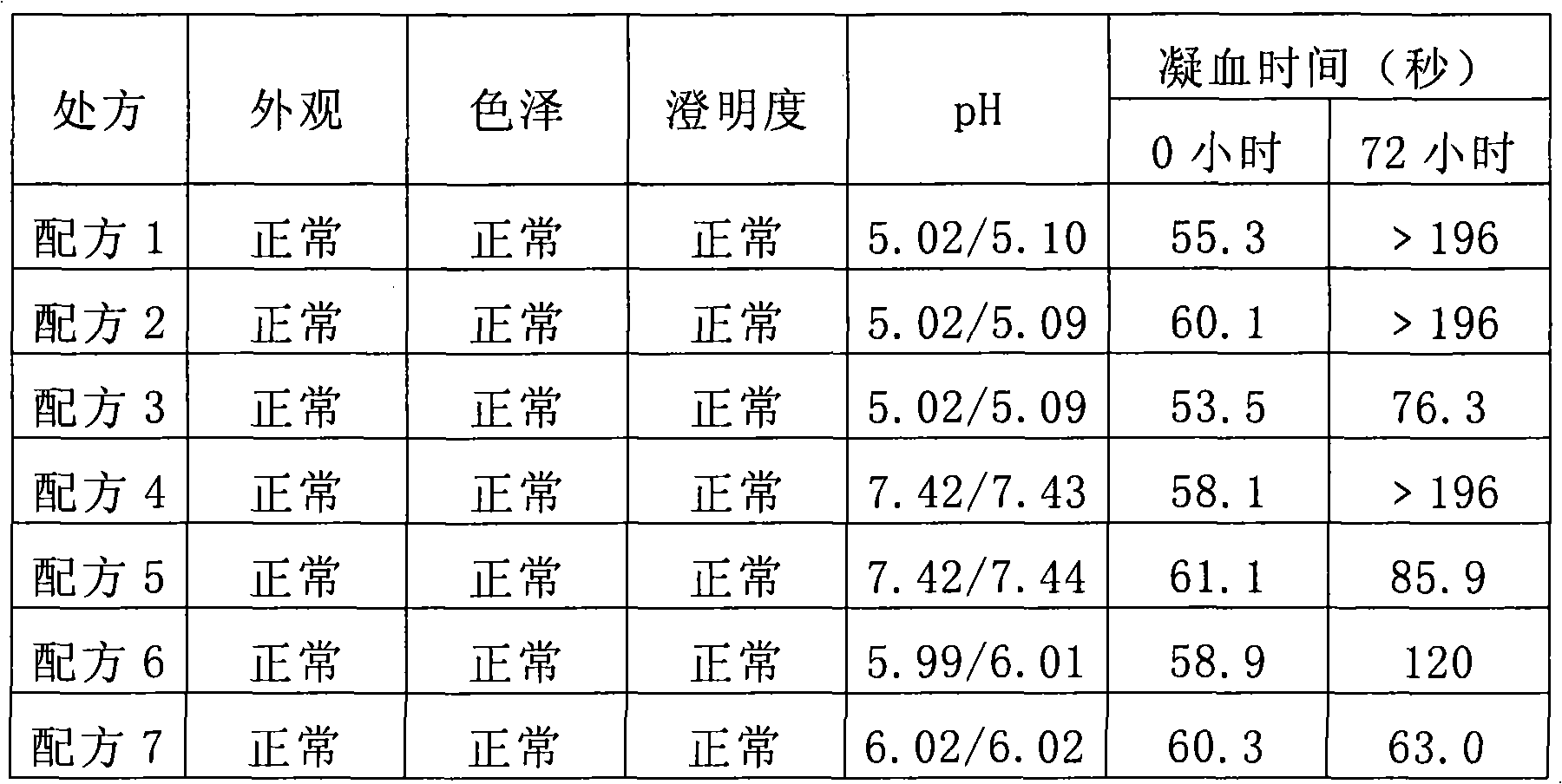
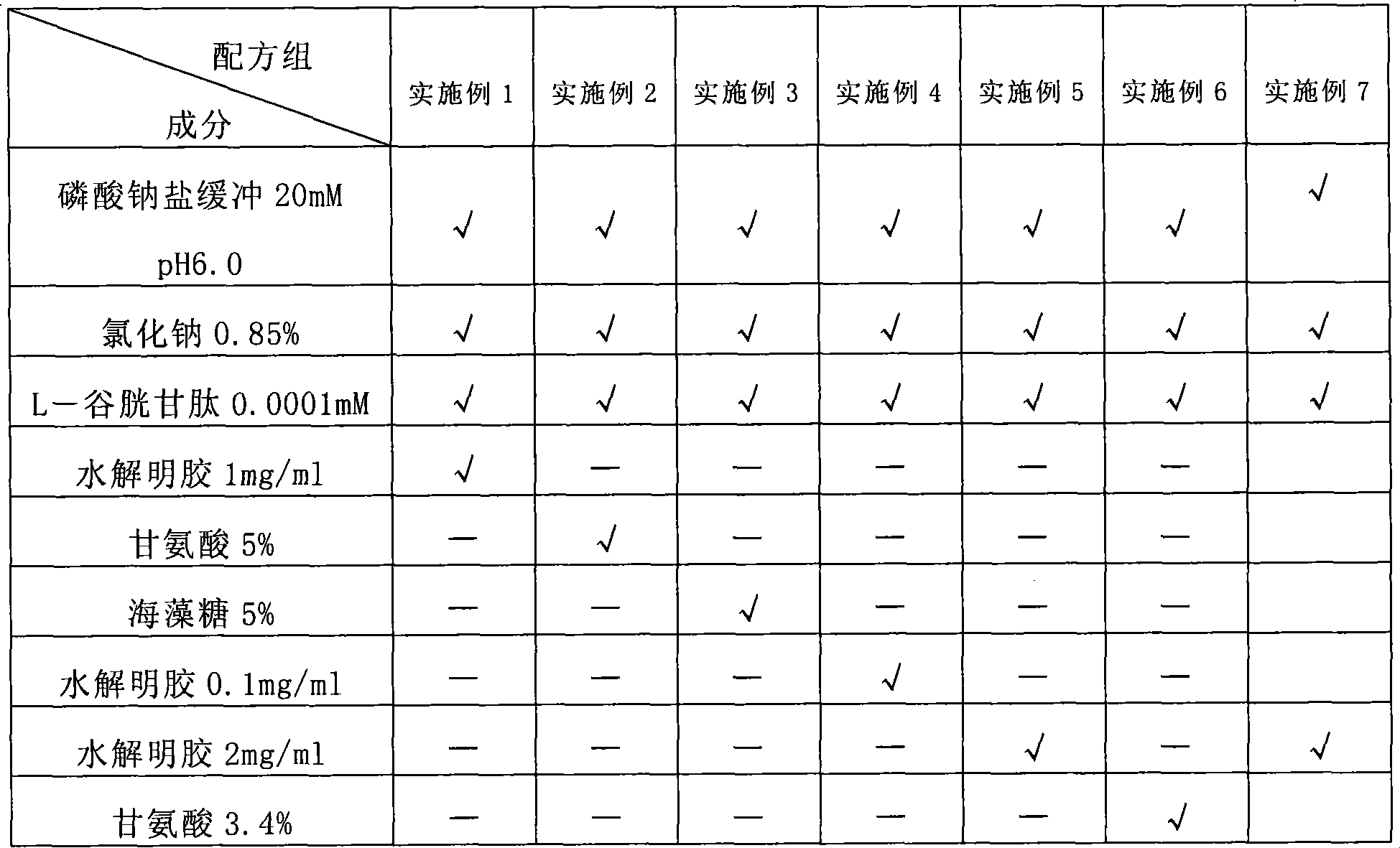
Stable Batroxobin medicament composition
ActiveCN101274096AMaintain biological activityReduce manufacturing costPowder deliveryPeptide/protein ingredientsLiquid stateGelatin
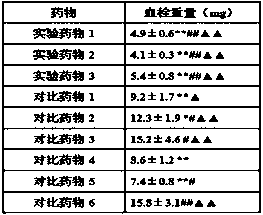
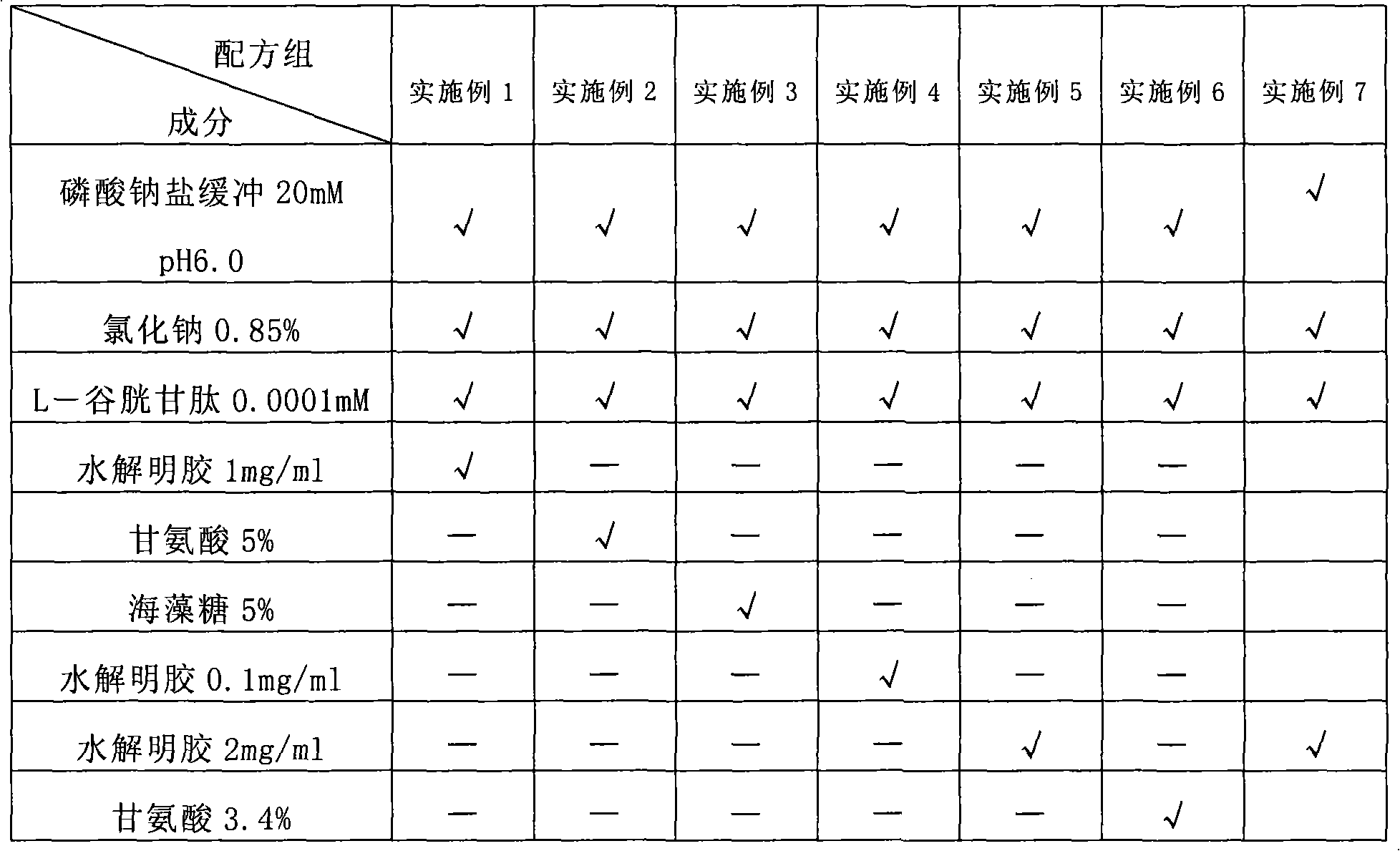
The invention discloses a composition containing batroxobin, which comprises hydrolyzed gelatin of 0.1 to 5 parts by weight. The composition disclosed by the invention still has high stability and biological activity even if being preserved in a liquid state.
Owner:SHANGHAI TENRY PHARMCEUTICAL CO LTD
Method for fermenting traditional Chinese medicine with batroxobin yeast
InactiveCN105535949AReduce blood viscosityInhibition formationPeptide/protein ingredientsAerosol deliveryYeastMedicinal herbs
The invention belongs to the field of traditional Chinese medicine, and aims to provide a method for fermenting traditional Chinese medicine with batroxobin yeast. The method can effectively improve a medicinal material use rate and enhance the medicinal effect. For realizing the purposes, an adopted technical solution comprises: placing prepared medicinal materials for external application into a container, adding water for cooking for 3-5h, then naturally cooling to 28-36 DEG C, adding batroxobin and yeast, and preserving temperature and fermenting for 1-3h, wherein a ratio of the batroxobin to the medicinal materials is 500-1000BU to 100g, a ratio of the yeast to the medicinal materials is 2-3g to 100g. The batroxobin can effectively dissolve fibers in the traditional Chinese herbal medicines, cause effective components wrapped in the fibers in the medicinal materials to be released, improve the effective medicinal material use rate and enhance the medicinal effect. The batroxobin has the effects of reducing blood viscosity, dissolving fibrinogen, inhibiting thrombogenesis and dissolving thrombus, and can promote blood of a human body to flow and improve the absorbing ability of the human body to the medicine.
Owner:重庆维礼骑士医院管理中心(有限合伙)
A kind of pharmaceutical composition for treating acute ischemic stroke
InactiveCN107648598BEnhance the antithrombotic effect in vivoReduce bleeding riskOrganic active ingredientsPowder deliveryThrombusGinkgolide
The invention belongs to the field of medicines, and particularly relates to a medicinal composition for treating acute cerebral ischemic stroke. The medicinal composition is prepared from pharmaceutically acceptable medicinal auxiliary materials, batroxobin, citicoline, ginkgolide and protoporphyrin sodium. The dosage form of the medicinal composition is preferably lyophilized powder for injection. The protoporphyrin sodium in the medicinal composition has a significant effect of enhancing the batroxobin functions of preventing thrombosis and improving cerebral blood perfusion, so that improvement on the cerebral ischemic stroke treating effect of the batroxobin is benefited.
Owner:张惊宇
Stable Batroxobin medicament composition
ActiveCN101274096BMaintain biological activityReduce manufacturing costPowder deliveryPeptide/protein ingredientsLiquid stateGelatin
The invention discloses a composition containing batroxobin, which comprises hydrolyzed gelatin of 0.1 to 5 parts by weight. The composition disclosed by the invention still has high stability and biological activity even if being preserved in a liquid state.
Owner:SHANGHAI TENRY PHARMCEUTICAL CO LTD
Method for extracting single component batroxobin from Bothrops atrox poison
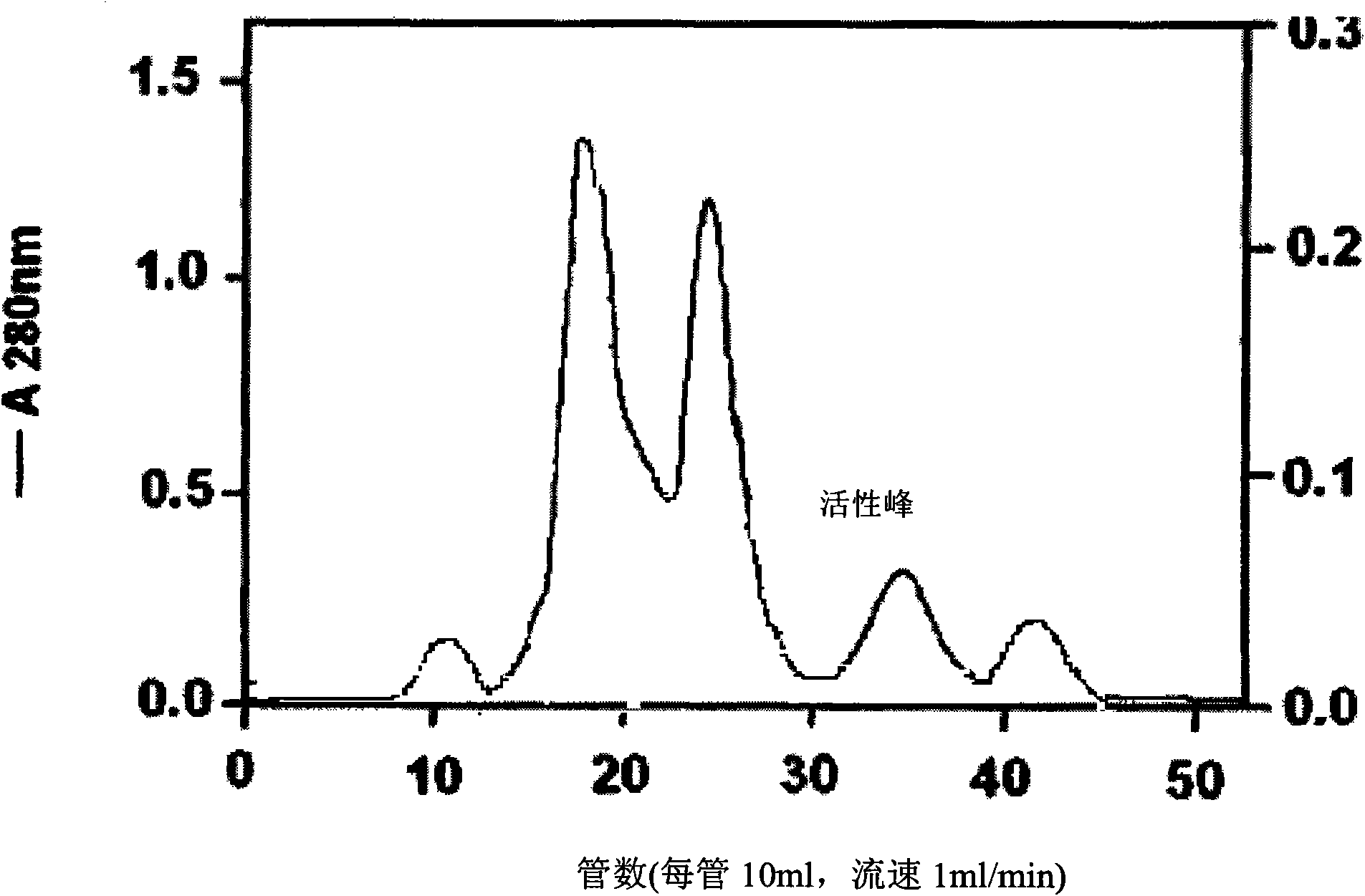
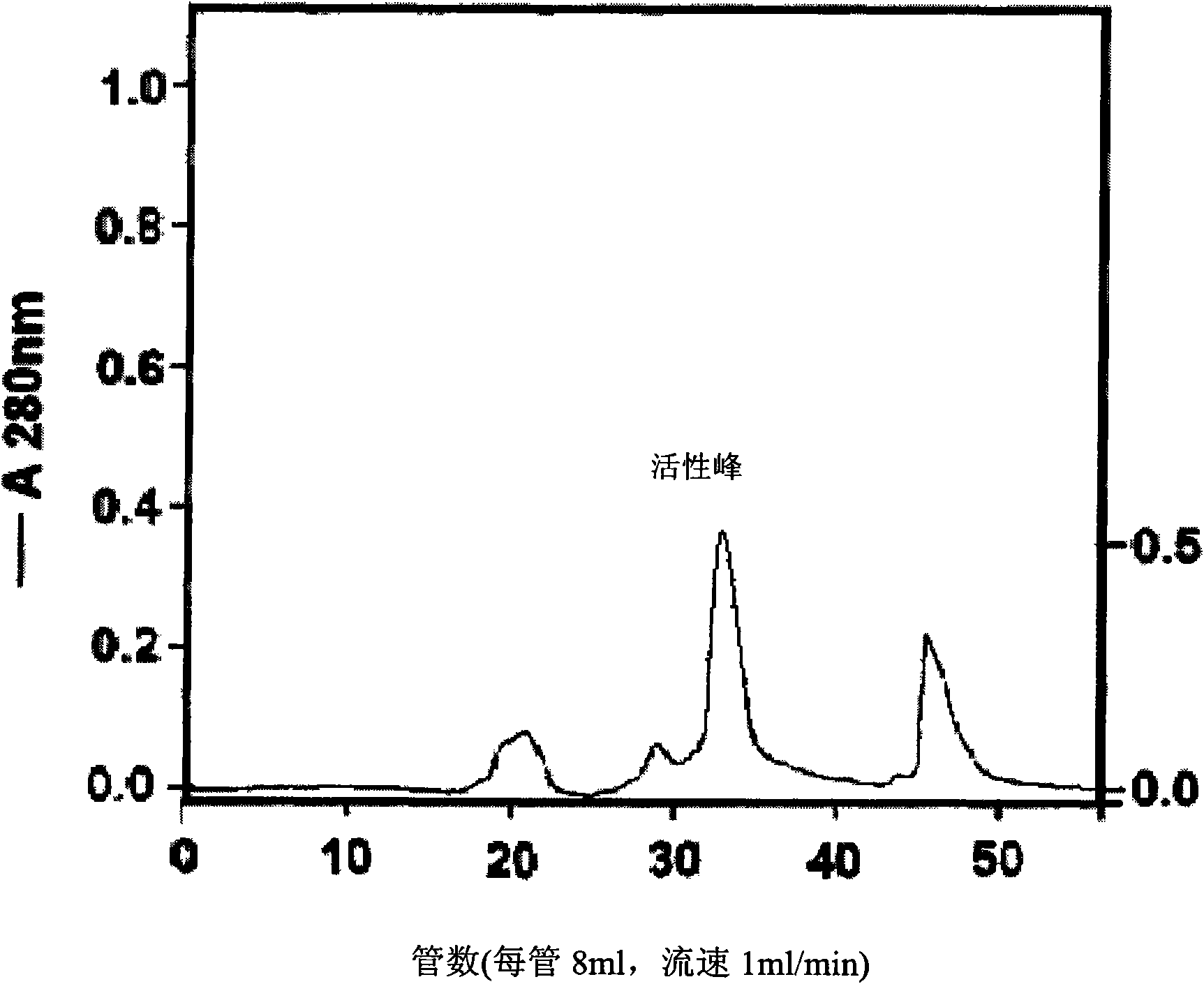
The invention discloses a method for extracting batroxobin with fibrinolytic and thrombolytic effect or hemostatic effect from snake venom, which belongs to the technical field of medicine. The steps are as follows: the snake venom is dissolved by soaking and centrifuged; the supernatant is subjected to affinity column chromatography; the eluate is directly flowed through Sephadex G25 desalting chromatography; the eluate containing the active ingredient is embedded and concentrated; The concentrated sample is subjected to molecular sieve chromatography to obtain batroxobin with a single component. The method has the characteristics of being suitable for large-scale production, simple and convenient operation, short production cycle, high product purity and the like.
Owner:QILU PHARMA CO LTD
Mixed carbon source induced pichia pastoris expression recombinant batroxobin and purification method thereof
PendingCN113862246AHigh recovery rateClosely connectedFungiHydrolasesPichia pastorisContinuous fermentation
The batroxobin is a hemostatic which is widely used at present. The invention relates to production of recombinant batroxobin by continuous fermentation induction of a mixed carbon source of methanol and glucose. And the recombinant batroxobin in a fermentation supernatant is purified by adopting an ionic membrane filtration and high-recovery-rate chromatography method.
Owner:BEIJING GREATSUN BIO PHARM TECH CO LTD
Effects of batroxobin in promoting survival of ischemic super-long random flap
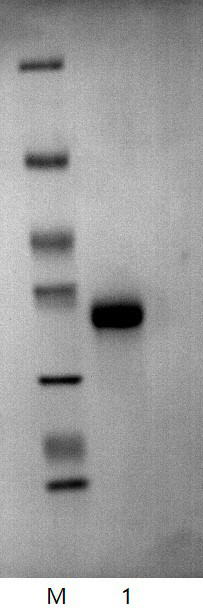
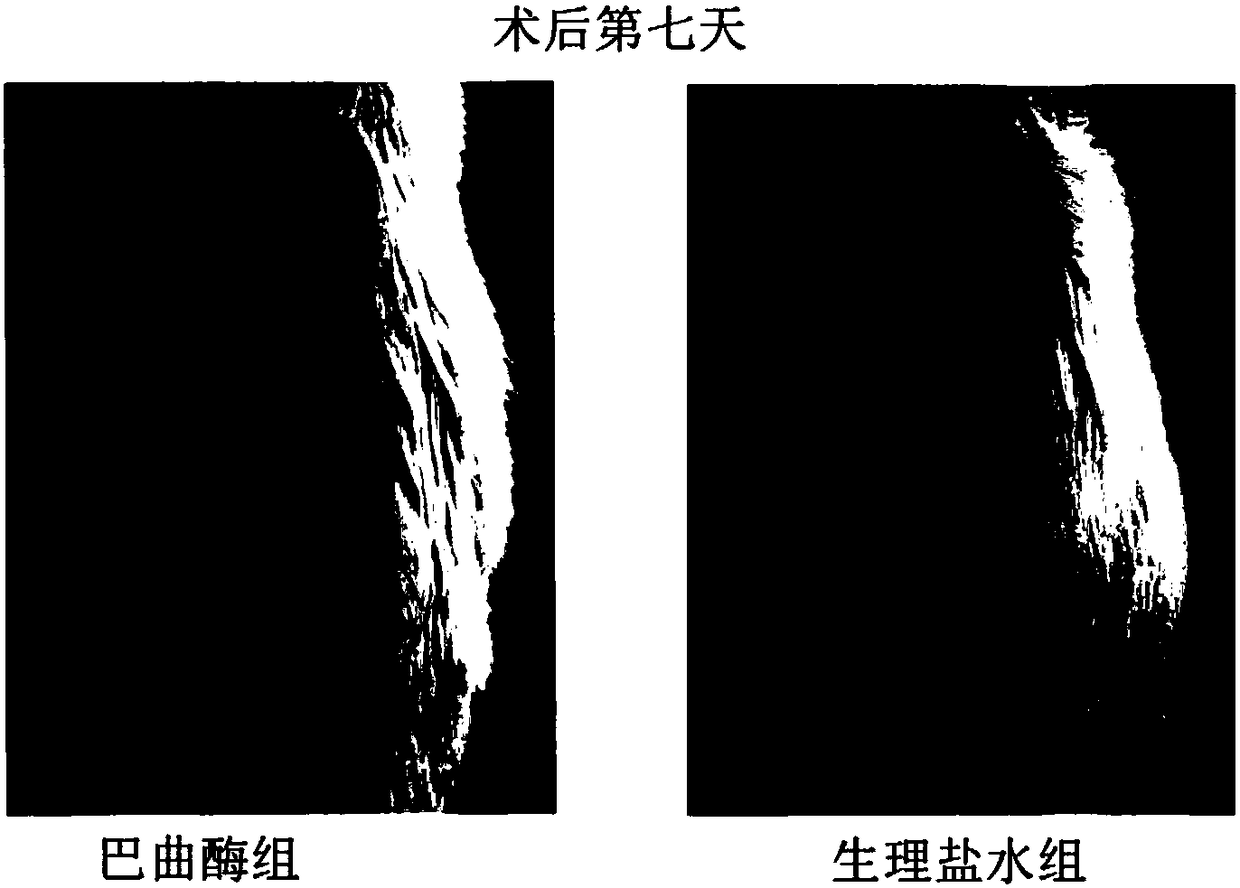
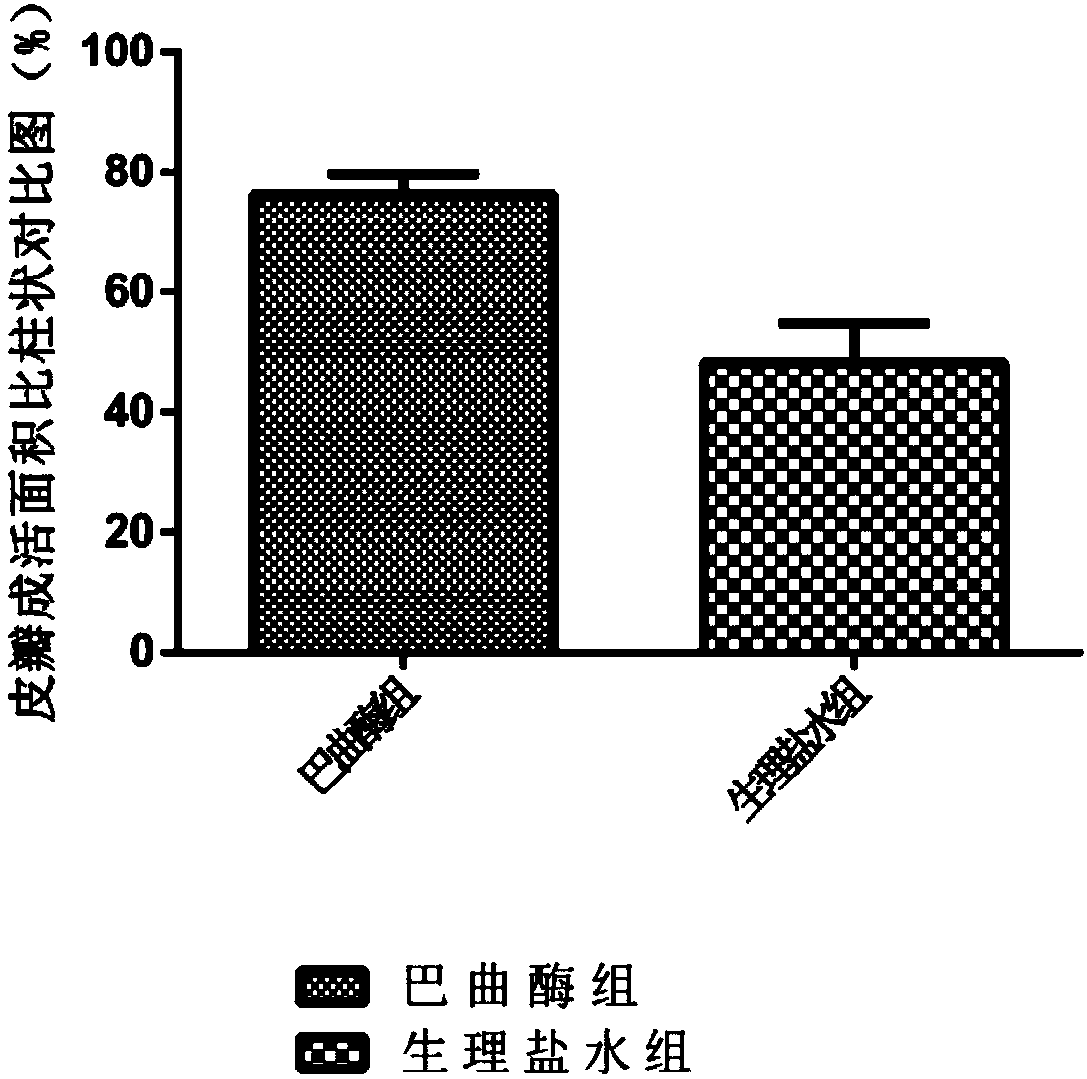
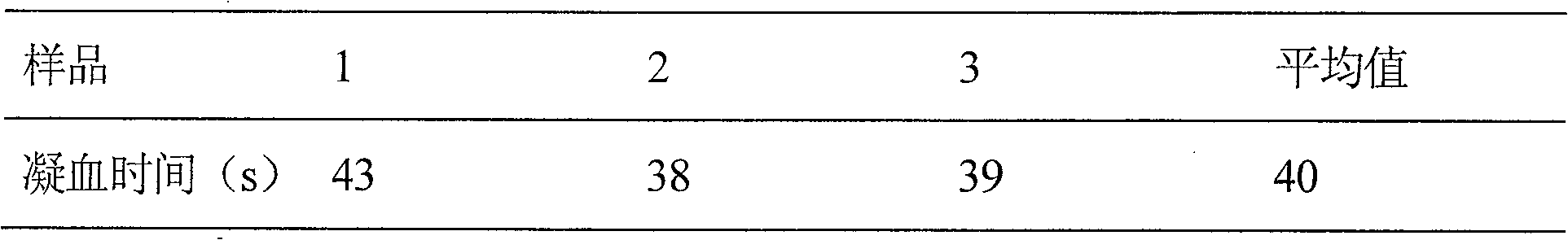
InactiveCN108159405AIncrease living areaConducive to survivalPeptide/protein ingredientsCardiovascular disorderRegimenMedicine
The invention relates to effects of batroxobin in promoting the survival of ischemic super-long random flap. A tail vein injection mode is used in the process of promoting the survival of the ischemicsuper-long random flap; a use method is that tail vein injection of 5BU / kg pure batroxobin is performed every day, with 5 to 7 days as one treatment course. A conclusion can be drawn through a largenumber of animal experiments; the survival area ratio of the flap can be greatly improved by using the batroxobin, so that the survival of the ischemic super-long random flap can be well promoted.
Owner:THE SECOND HOSPITAL AFFILIATED TO WENZHOU MEDICAL COLLEGE
Compound stypticum
ActiveCN100574802CReduce dosageGuaranteed curative effectPeptide/protein ingredientsBlood disorderBone Marrow Stromal CellWhite blood cell
A compound medicine for arresting bleeding is characterized in that the compound medicine is a mixture of a batroxobin and an interleukin-11; the clinical dosage of the batroxobin is 0.01-10u and that of IL-11 is 0.06-50mg; the batroxobin is the batroxobin from snake venom of Brazilian spearhead adder, white-eyebrow adder or acutus adder, or the batroxobin from recombinant Brazilian spearhead adder, white-eyebrow adder or acutus adder obtained by genetic engineering; the interleukin-11 is produced by human bone-marrow stromal cells (fibroblasts) and interstitial cells, or the recombinant IL-11 by genetic engineering. The medicine for arresting bleeding can not only ensure the curative effect but also decrease the amount of protease so as to reduce the rate of adverse immune response.
Owner:GRAND LIFE SCI (LIAONING) CO LTD
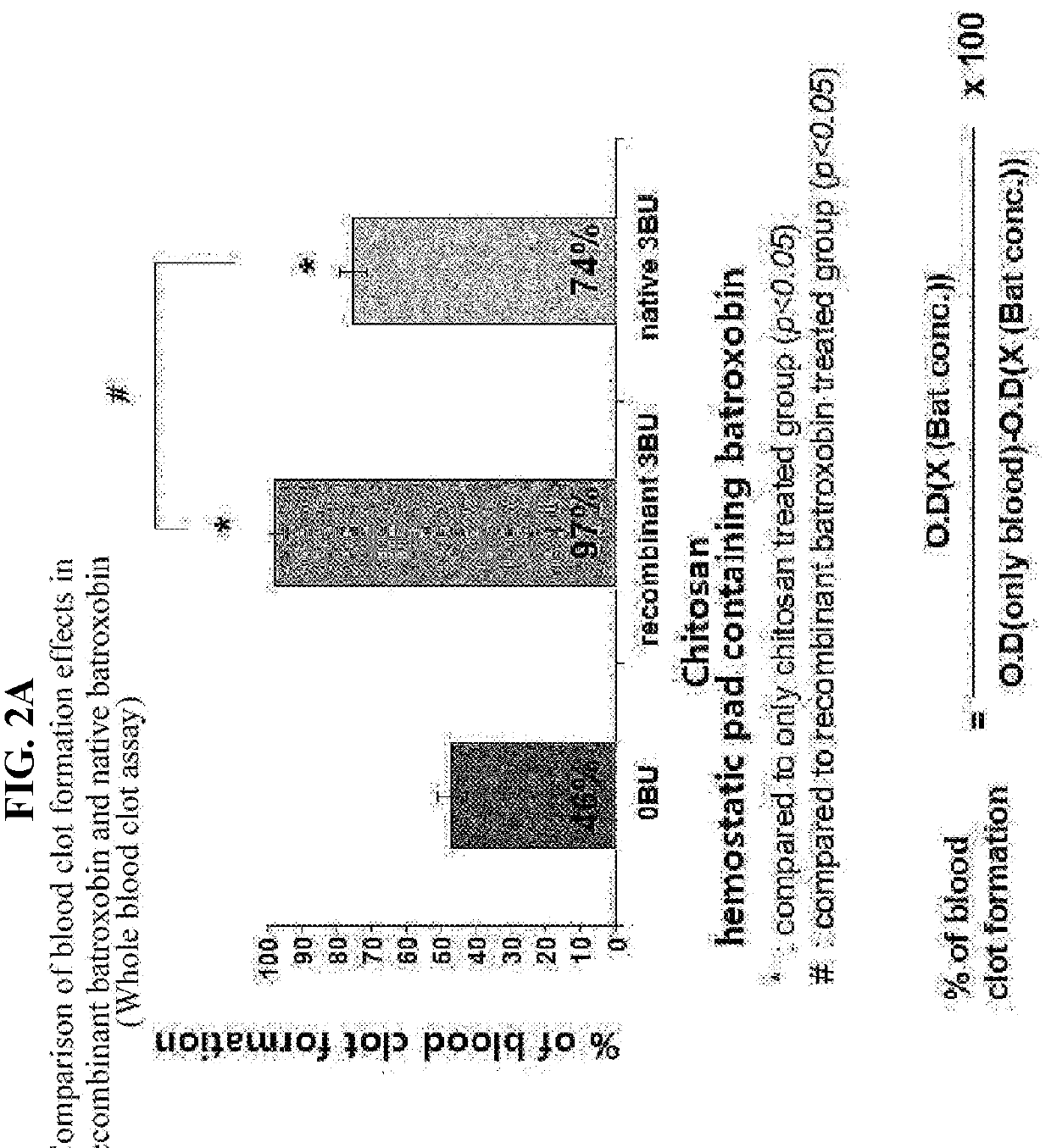
Recombinant batroxobin mixed composition and a hemostatic powder or hemostatic pad comprising same
ActiveUS20180265857A1Good hemostasisPeptide/protein ingredientsMacromolecular non-active ingredientsHEMOSTATIC POWDERMedicine
The present invention relates to a novel recombinant batroxobin mixture, a hemostatic composition comprising the same, and a method for preparing the same. According to the present invention, the hemostatic composition of the present invention has an excellent hemostatic effect by suppressing rebleeding, and maintains activity thereof even when prepared in a solid form, and thus, the composition of the present invention can be easily used by being applied as a topical hemostatic agent.
Owner:NC BIT INC
Preparation comprising batroxobin for inhibiting local invasion of malignant tumors
InactiveUS7691371B2Promote formationPeptide/protein ingredientsHydrolasesCell invasionAbnormal tissue growth
A preparation for inhibiting local invasion of malignant tumors is provided which comprises batroxobin and therefore can inhibit local invasion of malignant tumors. A preparation for encapsulating malignant tumor tissues is also provided which comprises batroxobin and therefore can cause or promote formation of capsule-like tissue around malignant tumor tissues.
Owner:TOBISHI PHARMA CO LTD
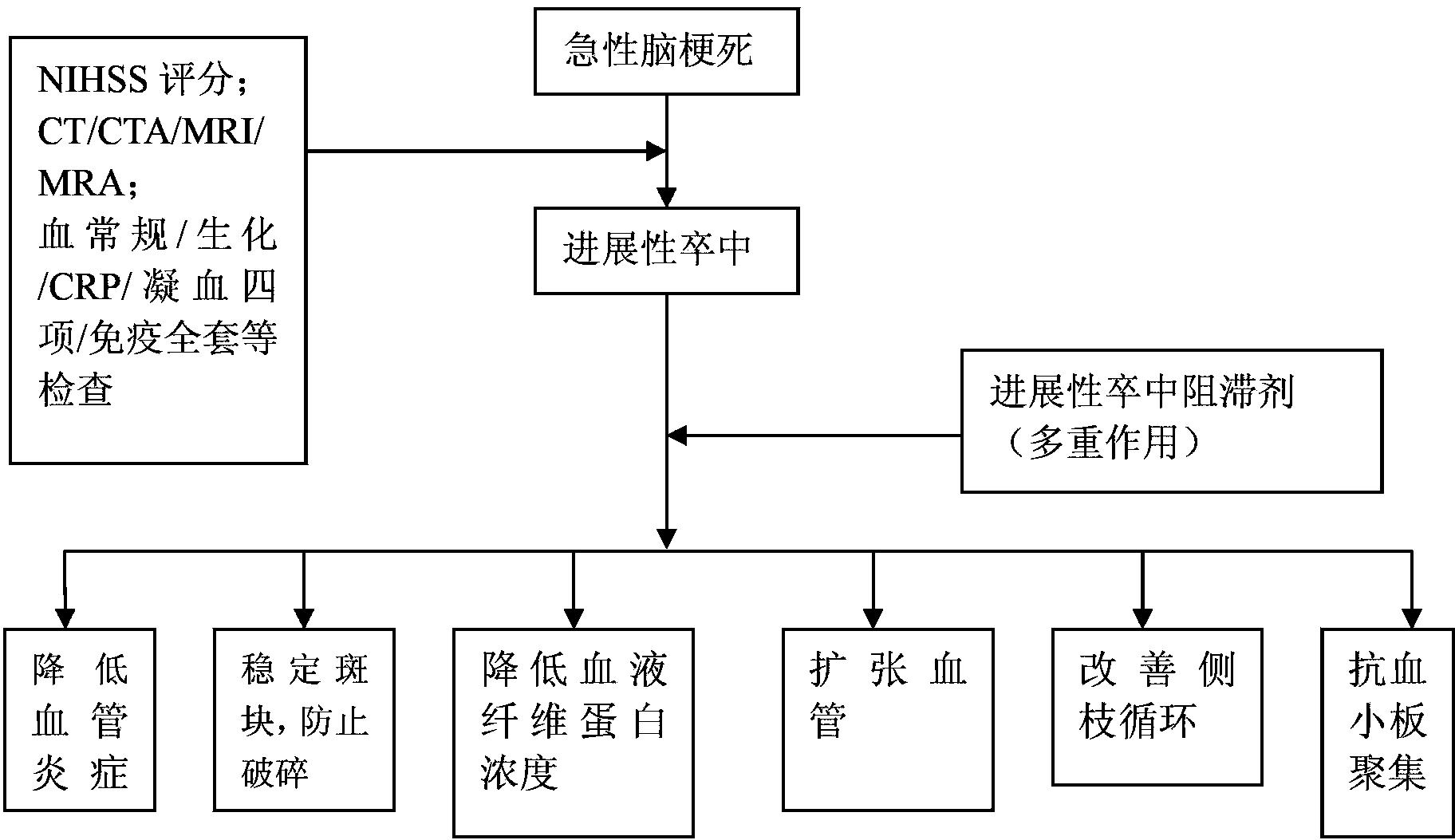
Progressive stroke retardant
InactiveCN103357000AReduce protein concentrationAvoid breakingPeptide/protein ingredientsHeterocyclic compound active ingredientsRisk strokeBatroxobin
The invention discloses a progressive stroke retardant which is characterized in that the dosage per day comprises the following components of: 100-300mg of bayaspirin, 75-300mg of clopidogrel, 10-20mg of Lipitor, 5-20BU of batroxobin and 15-45 Danshen dropping pills. According to the provided progressive stroke retardant, multiple targets act on multiple progressive parts of stroke, and the progressive stroke retardant has obvious effects of stabilizing, preventing and breaking inflammatory response and pathogenic plaques of offending vessels, reducing the blood fibrin concentration, dilating blood vessels, improving collateral circulation and resisting platelet aggregation and has obvious effects on progressive diseases, disease outcome and prognosis of progressive stroke patients.
Owner:童绥君
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com