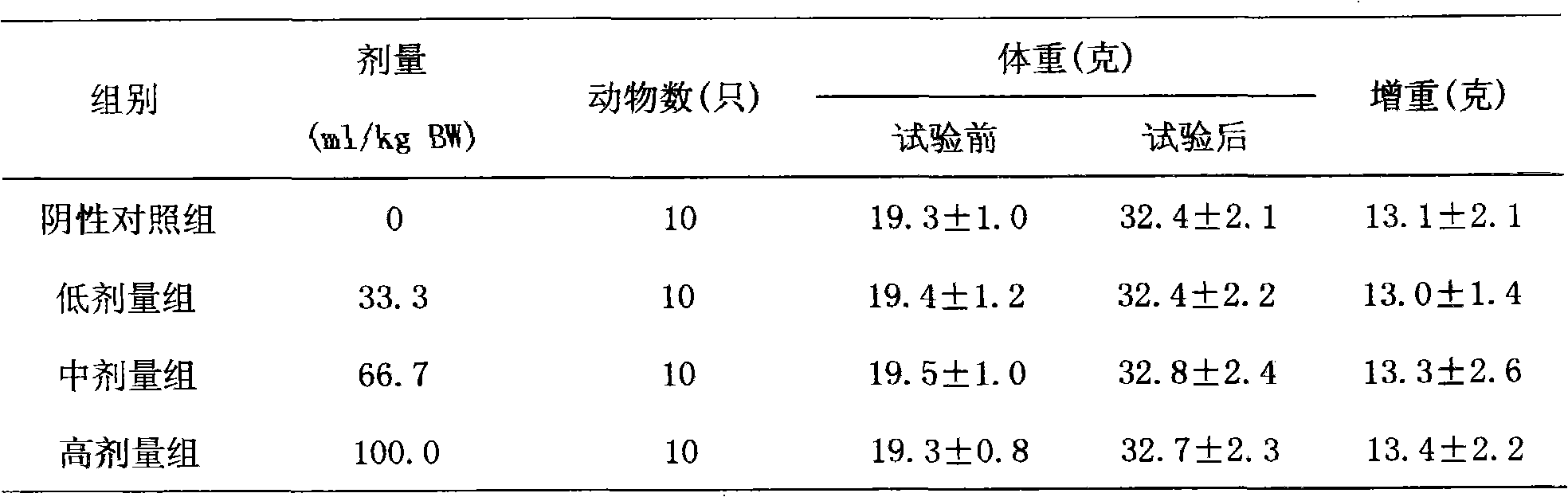
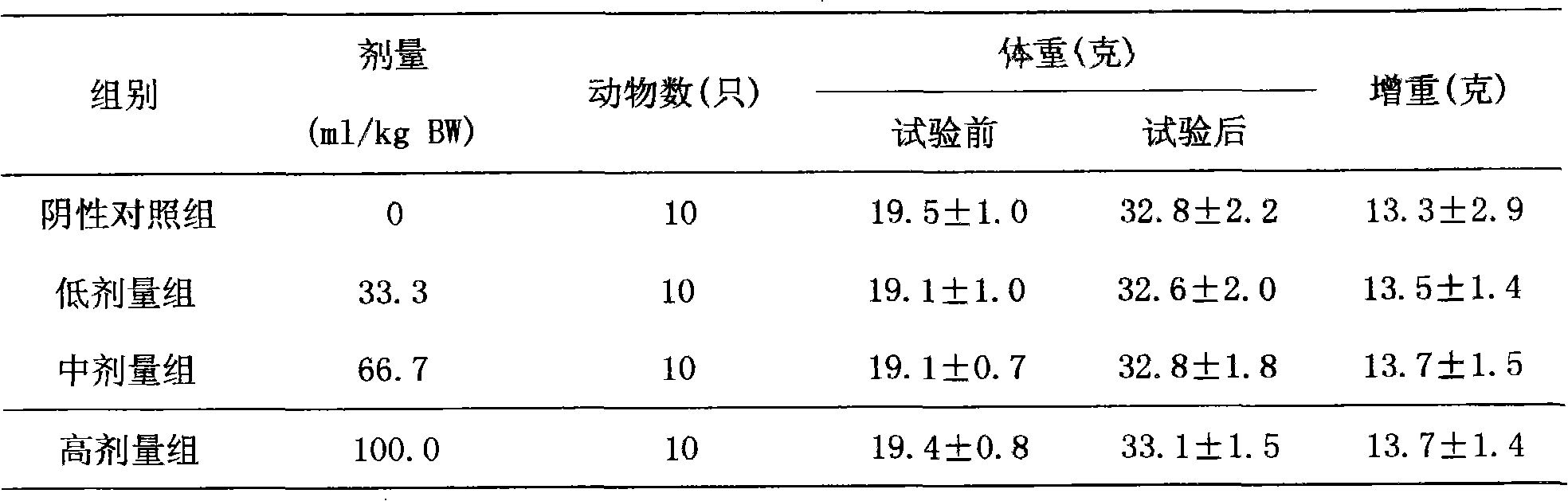
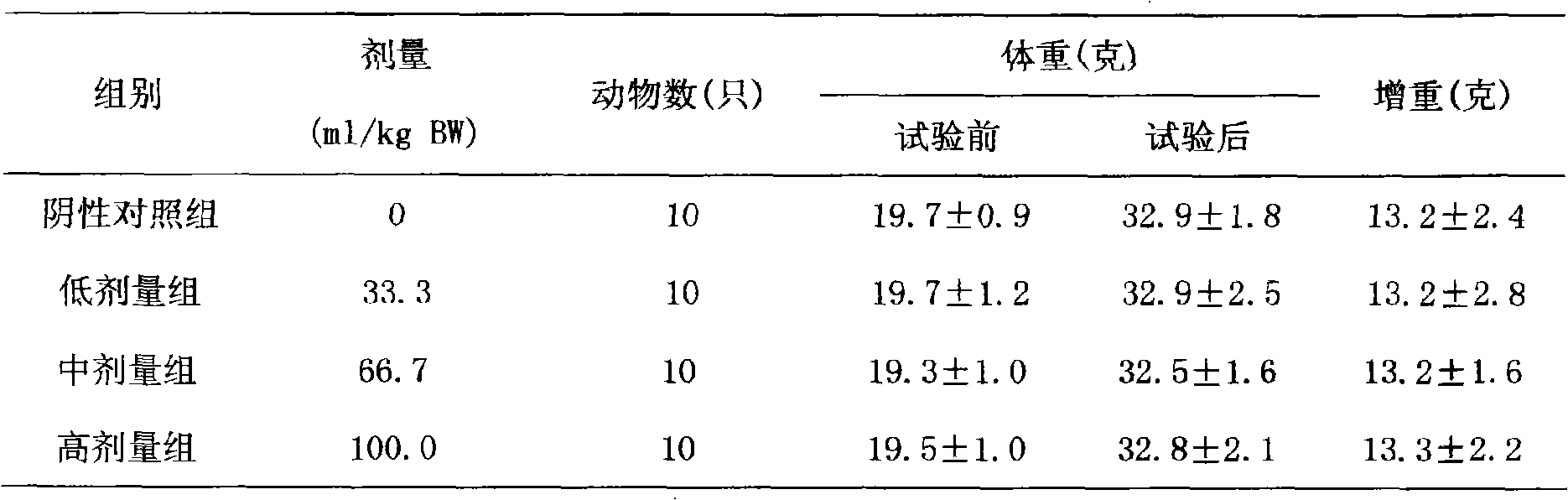
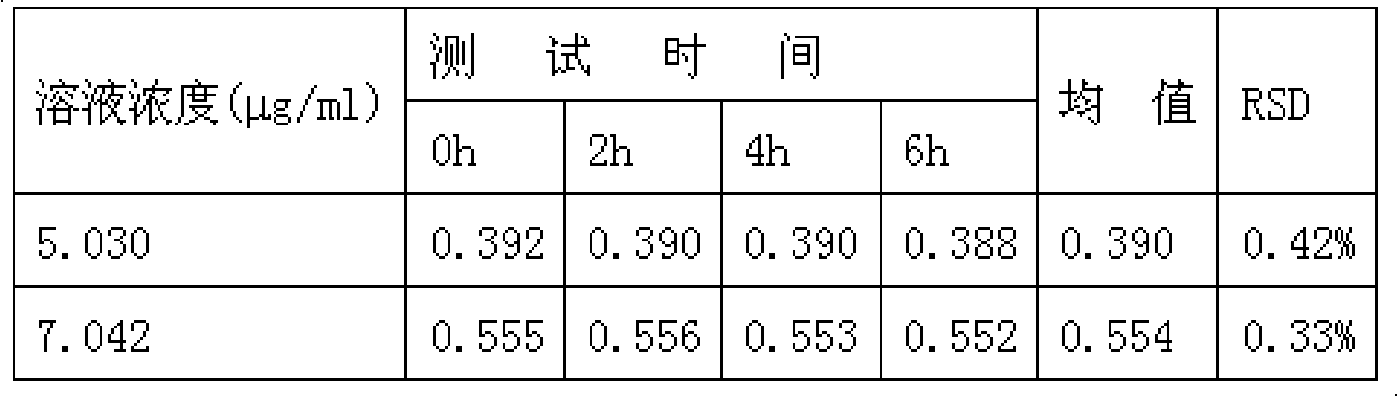
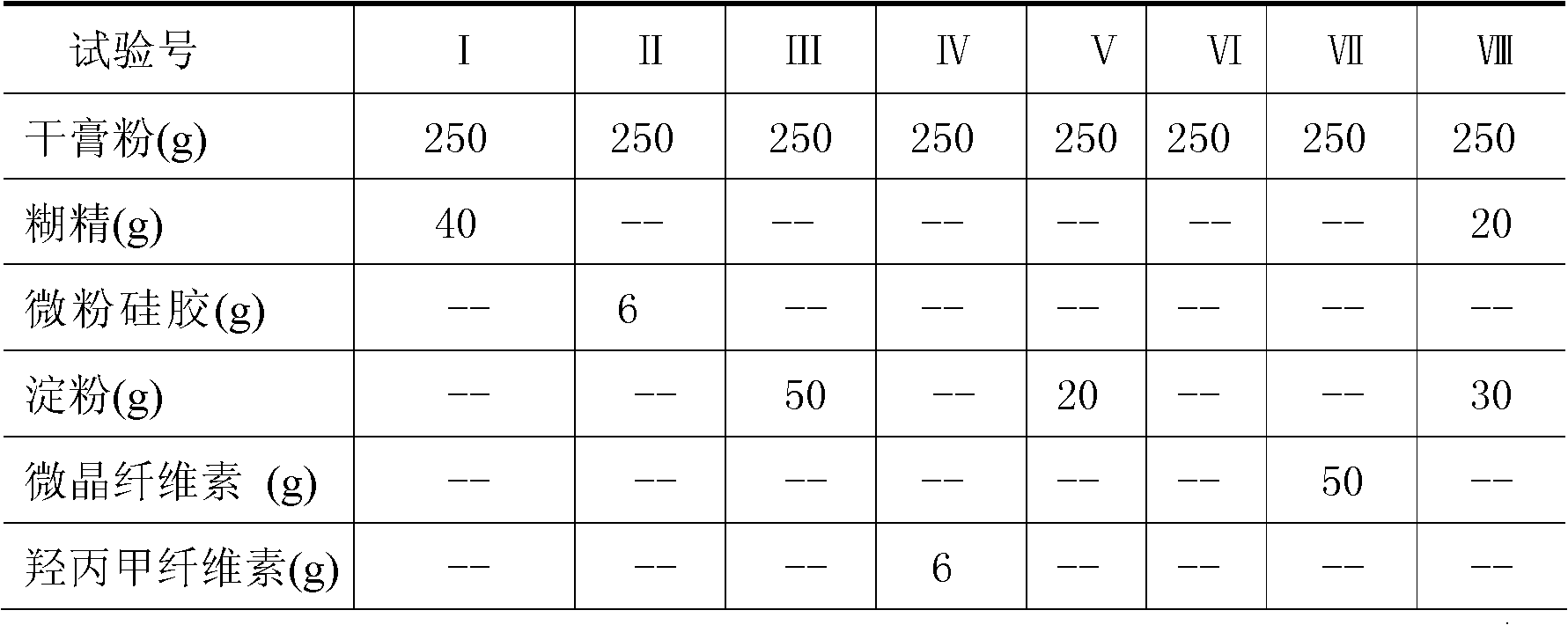
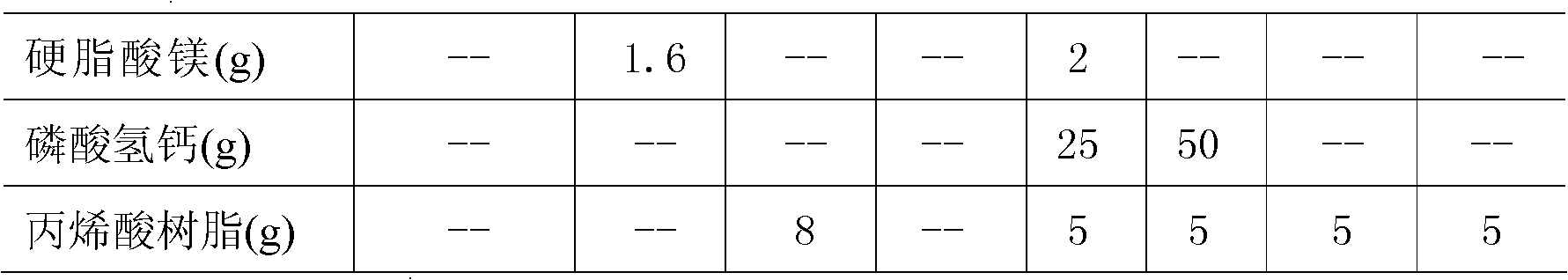
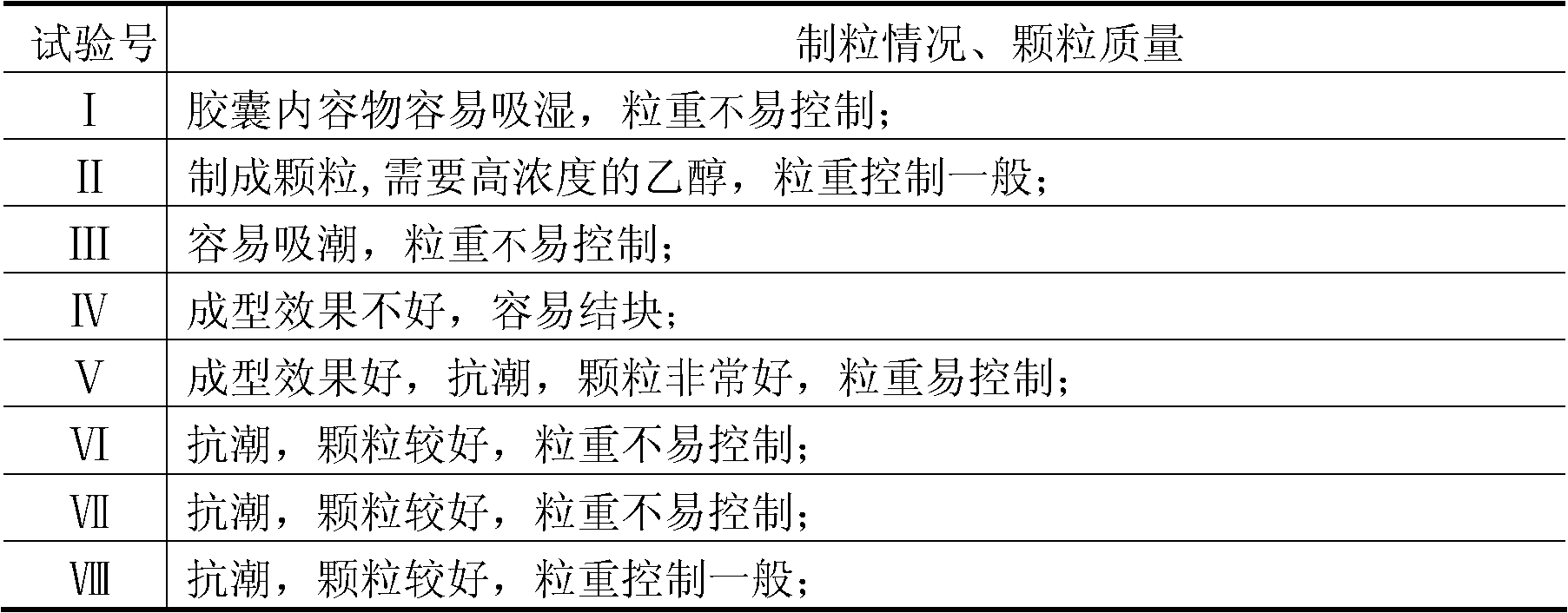
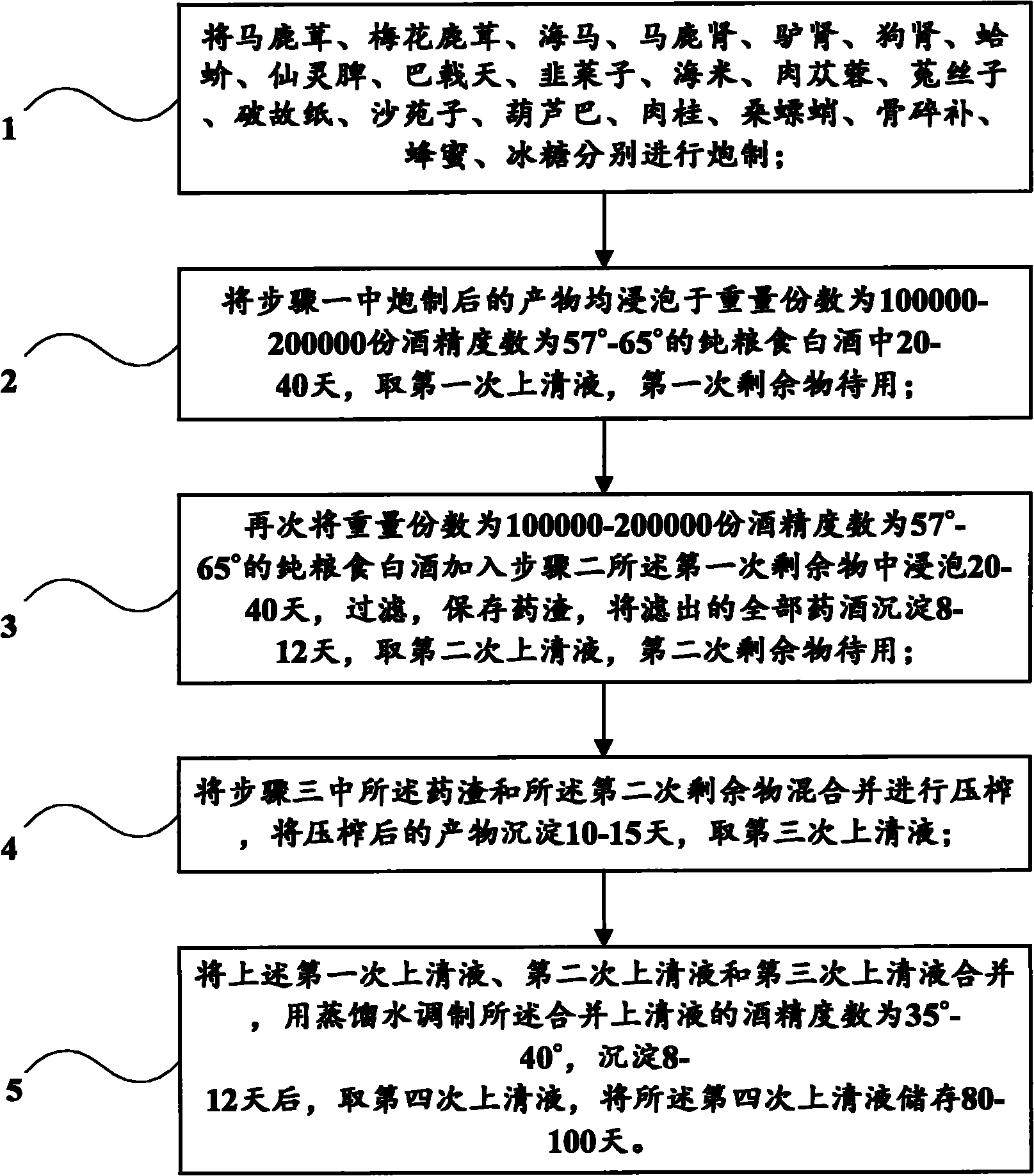
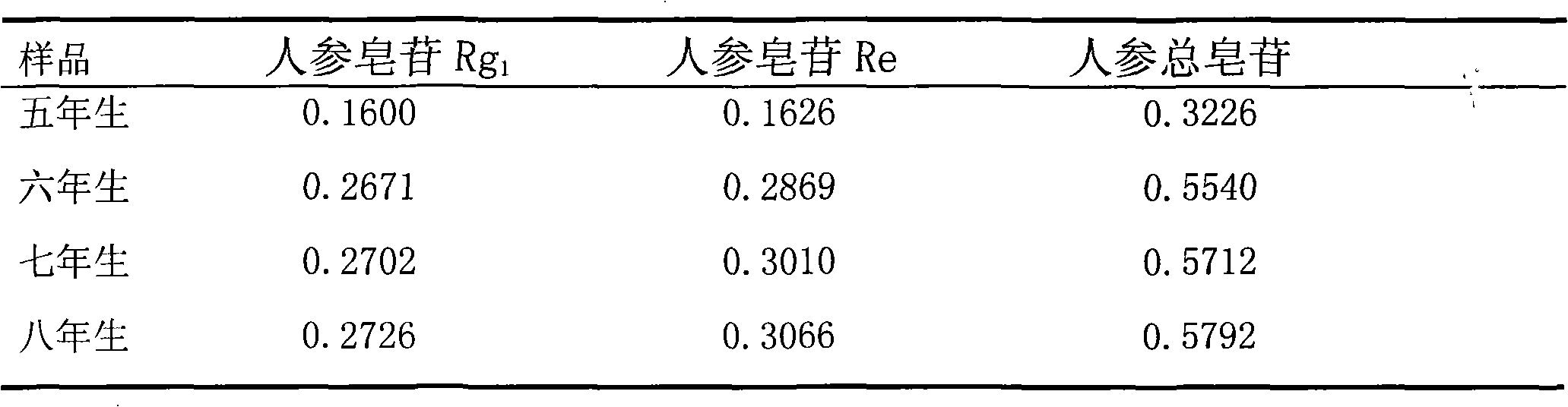
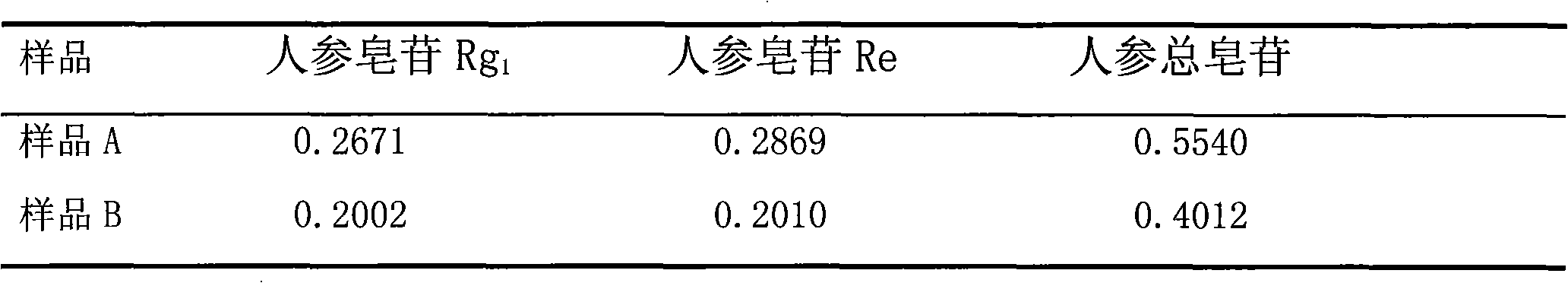
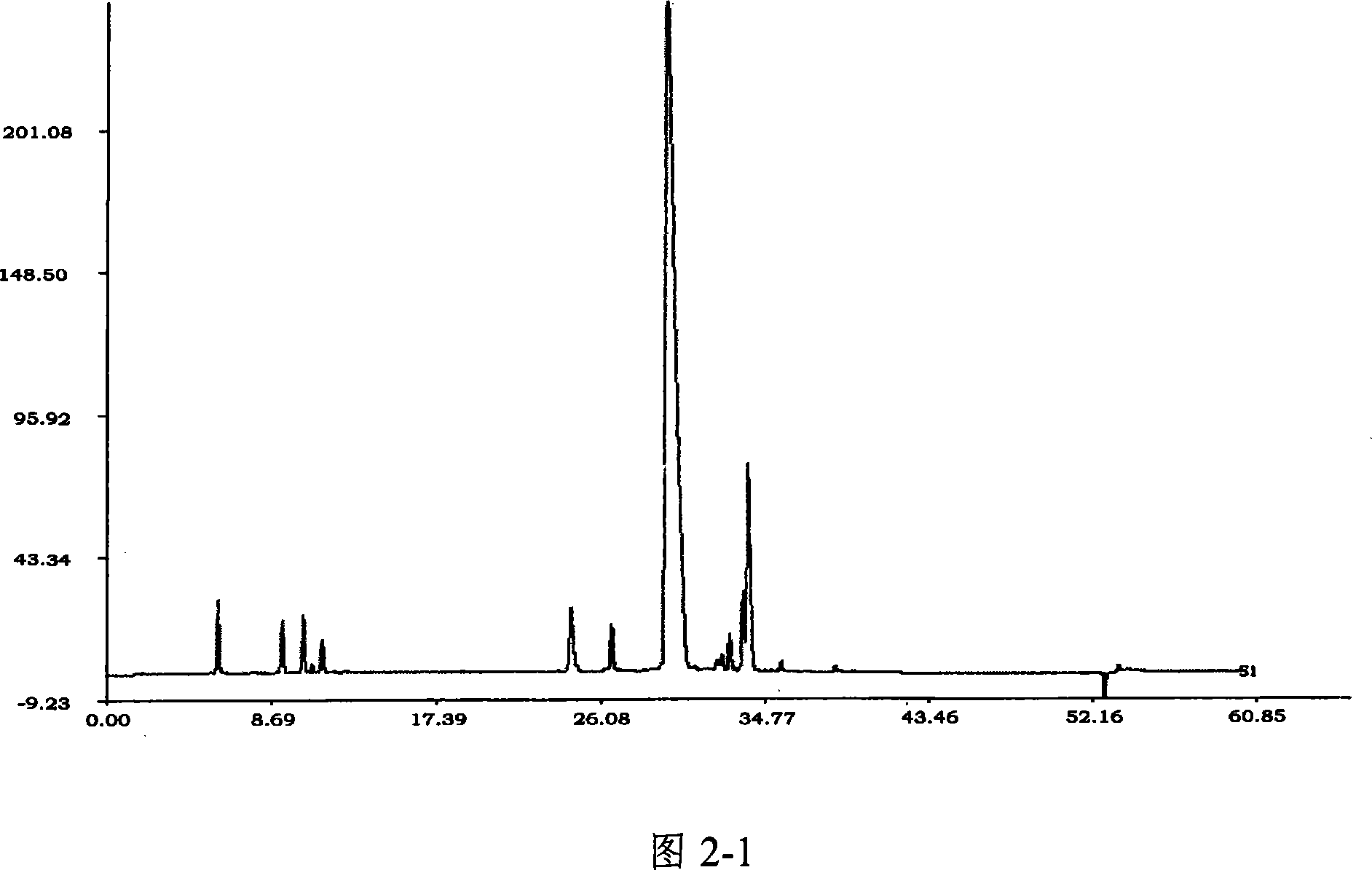
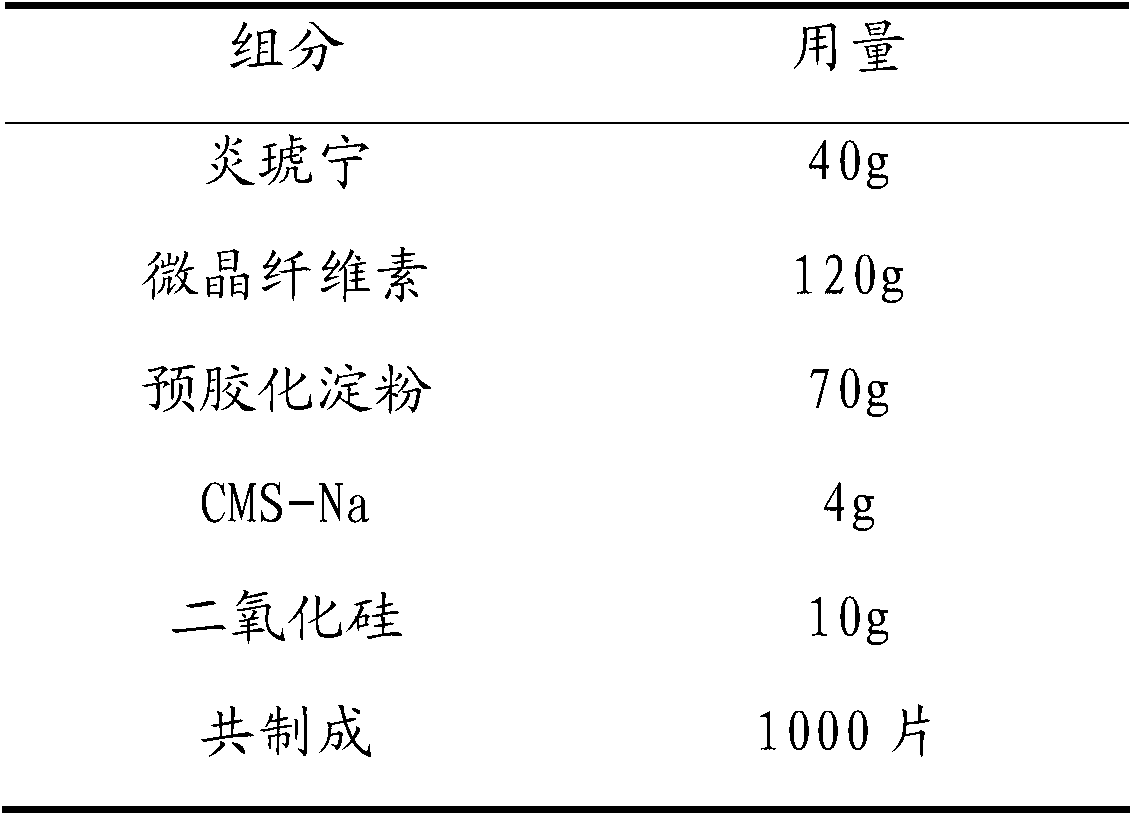
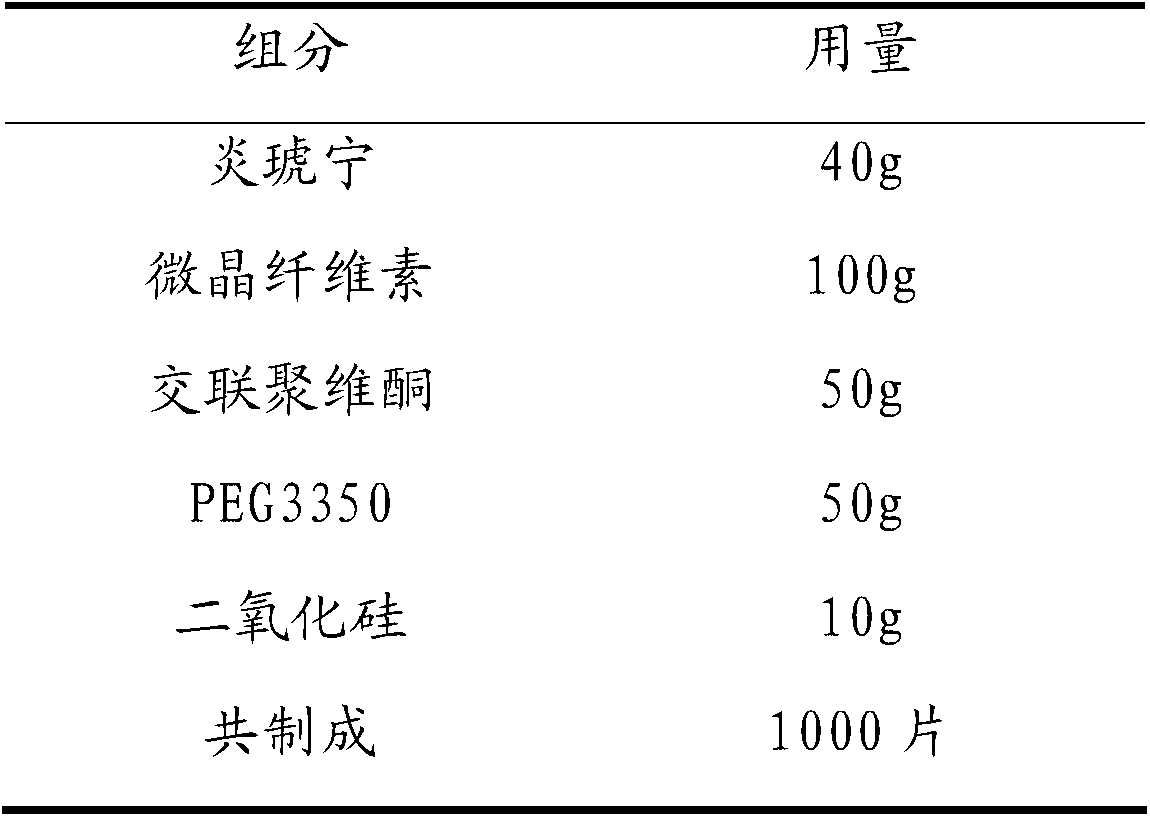
Patents
Literature
1151results about How to "Guaranteed curative effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Health food for reinforcing immune function of human body and preparation technique thereof
ActiveCN101292742ACheap sourceStable sourceImmunological disordersFood preparationPharmacyCordycepin
The invention aims at providing a health-care food enhancing immune function of human body and provides a preparation technique thereof. The health-care food, according to weight proportion, is produced by raw materials comprising 10-50 portions of gen-seng, 10-50 portions of glossy ganoderma and 10-50 portions of zymotic cordycepin powder; the raw materials comprising the gen-seng, the glossy ganoderma and the zymotic cordycepin powder are positioned in a hop-pocket; the processed raw materials are carried out heating and decoction for 1-3 times (each time is for 1-2 hours) after being added with water for soaking for one hour; extraction solution is mixed, filtered and concentrated to defined amount; after being cooled, concentrated solution is carried out high-speed centrifugal impurity removal; the obtained concentrated solution is produced into required preparation independently or by adding with accessories acceptable in medical science with the conventional processes of pharmacy.
Owner:JIANGZHONG PHARMA
Metformin hydrochloride enteric-coated tablets quality control method
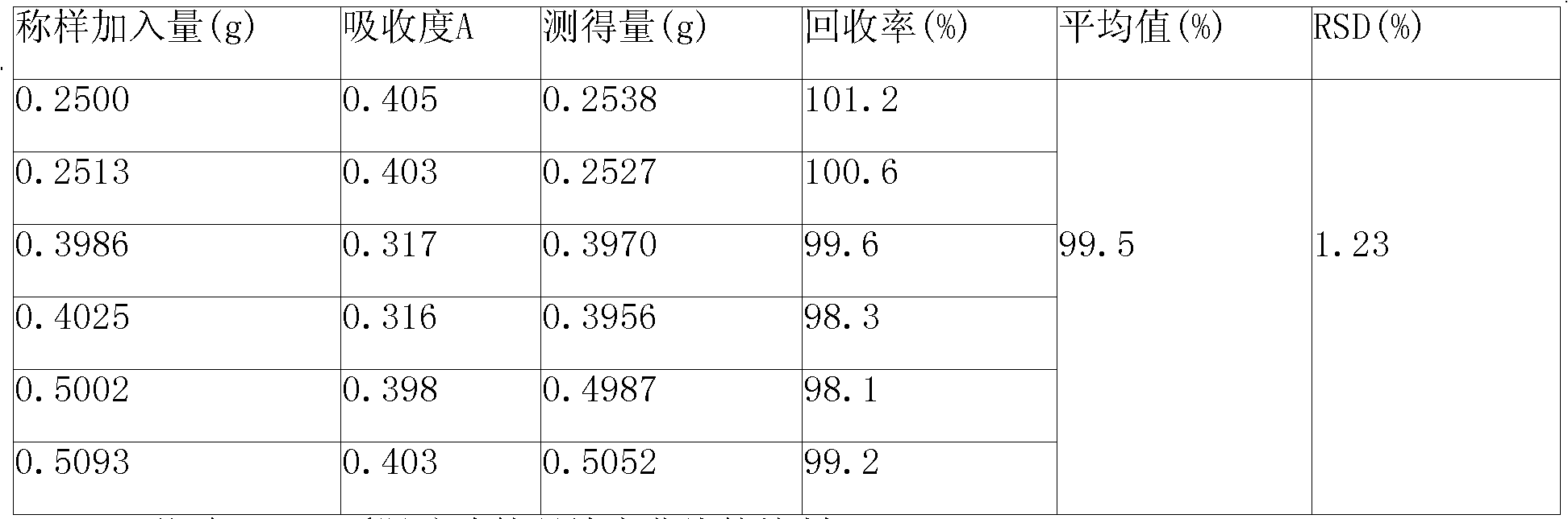
ActiveCN101339178AFacilitated releaseGuaranteed to dissolveComponent separationColor/spectral properties measurementsPhosphateMetformin Hydrochloride
The invention discloses a quality control method of metformin hydrochloride enteric coated tablet, comprising the aspects of character, identification, examination and content measurement; wherein, release examination comprises the release quantity examination of acid in hydrochloric acid solution of 0.1 mol / l and the release quantity examination in phosphate buffer with the pH value of 6.8; the examination of relevant substances comprises the following steps: dicyandiamide is taken as reference, sulfonic group cation exchange bonded silica is taken as filler, ammonium dihydrogen phosphate solution of 1.7 percent with the pH value of 3 is mobile phase and the high performance liquid chromatography is used for examining the relevant substances. The invention controls the release quantity of the metformin hydrochloride enteric coated tablet in gastric juice strictly, reduces the adverse reaction of patients effectively, improves the release quantity of the metformin hydrochloride enteric coated tablet in the buffer solution (simulated intestinal juice) and ensures the dissolution of the enteric coated tablet in the intestinal juice effectively; the invention also adds the examination of dicyandiamide impurity under the examination item and enhances the safety of the medicine.
Owner:贵州天安药业股份有限公司
Antibacterial antivirus Chinese medicinal composition for animal
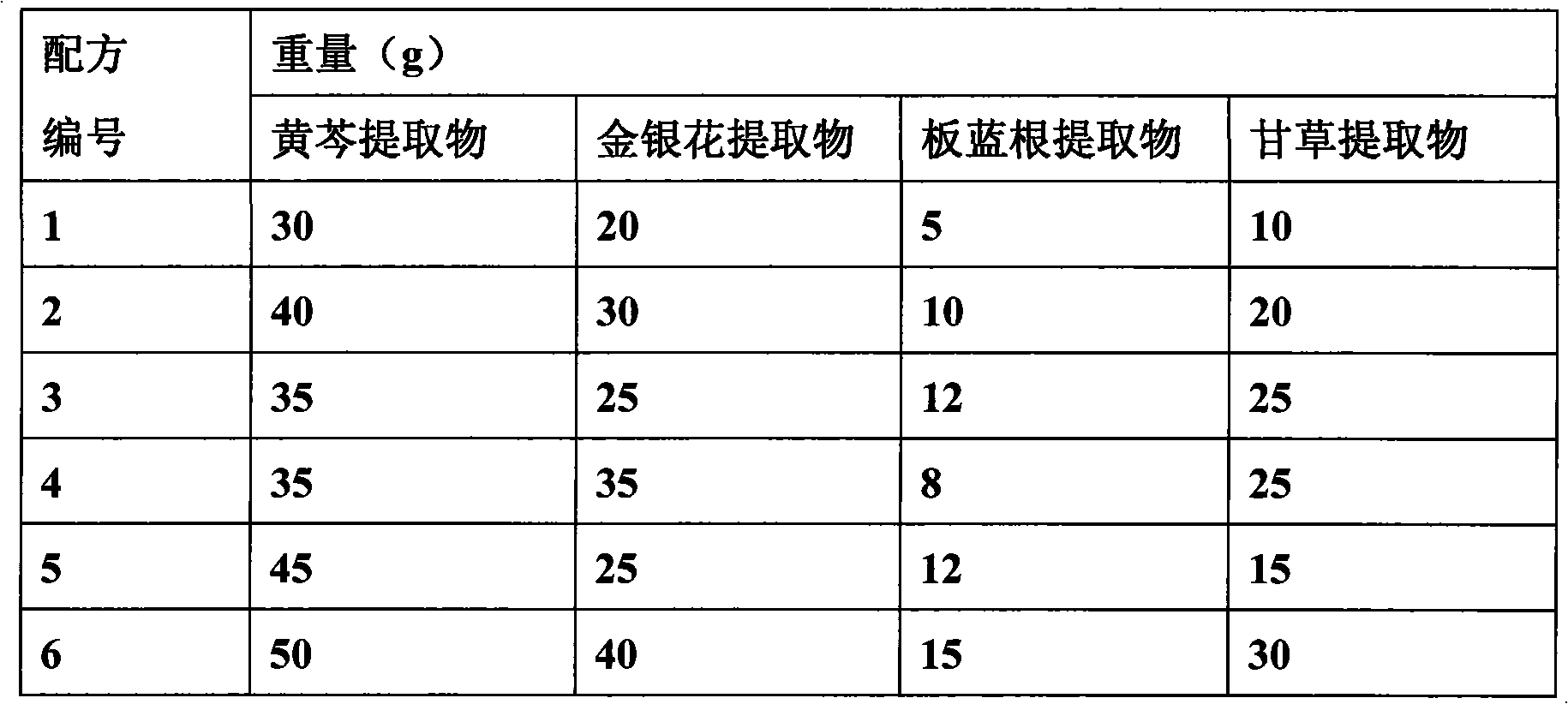
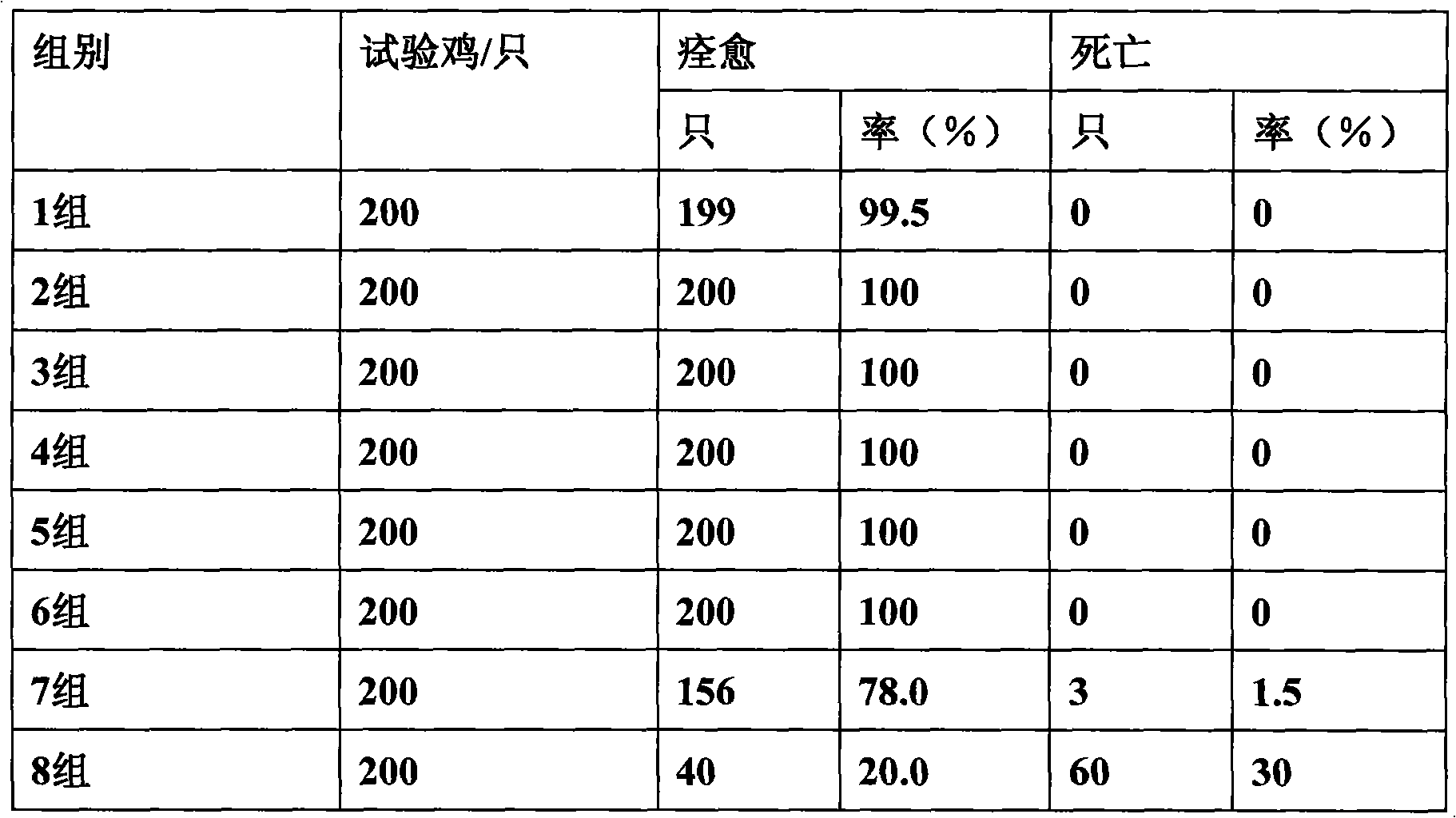
ActiveCN101584747AReasonable compositionStrong broad-spectrum antibacterialAntibacterial agentsFood processingPoultry diseaseBacteroides
The invention discloses an antibacterial antivirus Chinese medicinal composition for the animal, comprising the following main medicament ingredients: scutellaria extract, honeysuckle extract, isatis root extract and glycyrrhiza extract. the pharmaceutical composition of the invention can be made into premix, injection, oral liquid or granula, also can be made into feedstuff premix adding into the feedstuff. the chinese medicinal composition of the invention is a pure Chinese medicinal compound preparation which has the antibacterial, anti-inflammation, antivirus and immunity-reinforced functionsand wide application range, can effectively prevent and treat various livestock and poultry diseases caused by the virus and the bacteria, such as piggyyellow-white dysentery, swine plague, infectious pleuropneumonia, asthma, canker, circovirus, swine influenza, white diarrhea, newcastle disease, bursa of Fabricius, egg drop syndrome, duck serositis and the like, and has fast effect and remarkable curative effect.
Owner:BEIJING DABEINONG ANIMAL HEALTH TECH
Preparation method of Yunkang capsule
ActiveCN102512621AGuaranteed curative effectKeep it workingCapsule deliverySexual disorderEUCOMMIA ULMOIDES BARKDioscorea polystachya
The invention which relates to a preparation method of a Yunkang capsule belongs to the technical field of medicines. The Yunkang capsule of the invention is prepared from the following raw traditional Chinese medicines: 80-120 parts of donkey-hide gelatin, 160-200 parts of Chinese yam, 90-130 parts of Himalayan Teasel Root, 120-160 parts of Radix Astragali, 90-130 parts of Chinese angelica, 120-160 parts of Rhizoma Cibotii, 90-130 parts of Cuscuta chinensis Lam., 50-90 parts of Chinese Taxillus Twig, 90-130 parts of Eucommia ulmoides, 90-130 parts of Malaytea Scurfpea Fruit, 90-130 parts of Radix Codonopsis, 120-160 parts of Poria cocos, 90-130 parts of Rhizoma Atractylodis Macrocephalae, 120-160 parts of rehmannia, 90-110 parts of Cornus officinalis, 120-160 parts of wolfberry fruit, 50-90 parts of Dark Plum Fruit, 90-130 parts of white peony root, 50-90 parts of Fructus Amomi, 50-90 parts of Fructus Alpiniae Oxyphyllae, 90-130 parts of Radix Boehmeriae, 5-20 parts of Argy Wormwood Leaf and 50-90 parts of Radix Scutellariae.
Owner:JIANGXI JIMINKEXIN PHARMA +1
Health-care medicated wine and preparation method thereof
InactiveCN101940702AImprove immunityRelieve drynessAnthropod material medical ingredientsDigestive systemSide effectKidney
The invention relates to a health-care medicated wine and a preparation method thereof. The health-care medicated wine comprises the following components: cervus elaphus linnaeus, spotted deer antler slice, sea horse, kidneys of horse and deer, kidney of donkey, kidney of dog, gecko, herba epimedii, morinda officinalis, Chinese chive seed, dried shrimps, cistanche, the seed of Chinese dodder, babchi seed, flastem milkvetch seed, fenugreek, cinnamon, ootheca mantidis, rhizoma drynariae, honey, crystal sugar and white spirit. In the invention, by adopting the traditional compatibility principle, the health-care medicated wine has the functions of tonifying Qi and strengthening kidney, replenishing essence and nourishing medulla, regulating the balance of Yin and Yang, enhancing sex function, improving immunity and the like, and has the advantages of exquisite material selection, strict preparation process and no bad and side effects.
Owner:内蒙古永业生物技术有限责任公司
Personalized three-dimensional (3D) printing-based brain seed implantation guidance system
PendingCN107126619APrecision therapyPrecisely calculate the doseRadiation therapyPersonalizationGuidance system
The invention relates to a 3D printing-based personalized cranial particle implantation guide system, including a head-fixed vacuum pad, particle implantation planning software, a personalized skull perforation template, a personalized particle implantation puncture template, template making and Printing software, 3D printer. The skull perforation template of the present invention is used in conjunction with the particle implantation puncture template, so that the intersection point of each particle implantation needle body is at the perforation of the skull, and the puncture needle passes through the skull smoothly, and the particle implantation needle column of the template can accurately guide the puncture needle. The puncture direction avoids blood vessels and important central nerves, accurately punctures to the lesion, reduces the difficulty of the operation, shortens the operation time, and reduces the risk of the operation.
Owner:于江平
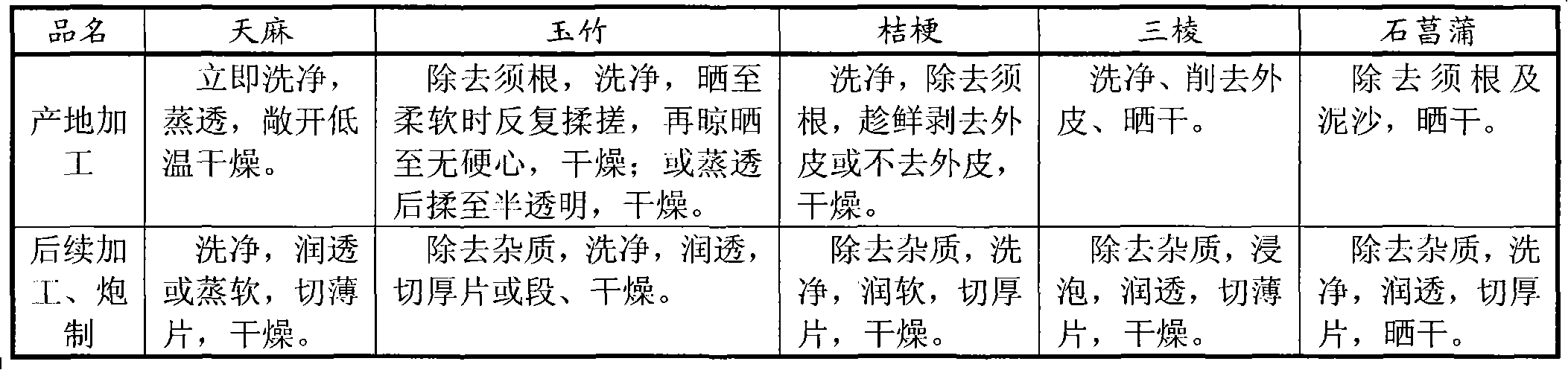
Fresh processing technology of traditional Chinese plant medicine at producing site
InactiveCN102000119AQuality improvementGuaranteed curative effectPlant ingredientsSocial benefitsBiology
The invention relates to the filed of traditional Chinese plant medicine processing technologies at the producing site, in particular to a fresh processing technology of a traditional Chinese plant medicine at the producing site. The technology comprises the following steps of: harvesting, cleaning, cutting, inactivating and drying the medicinal material, or harvesting, cleaning, cutting and drying the medicinal material. The multiple and repeated procedures of cleaning and drying the medicinal material at the producing site and cleaning, softening, cutting and drying the medicinal material at a medicine piece plant of the prior art are directly changed into the process of cleaning, cutting and drying at the producing site. Thus, the invention achieves the purposes of simplifying the processing, subsequent processing and preparing procedures of the traditional Chinese medicinal material at the producing site, saving energy consumption, reducing comprehensive production cost, ensuring medicinal material quality and increasing peasant incomes and has obvious economical and social benefits.
Owner:HANGZHOU HAISHAN PHARMA EQUIP
Health-care food capsule and preparation method thereof
ActiveCN102511800AReasonable processing methodAnti-menopausal syndromeFood shapingFood preparationBiotechnologyGlycerol
The invention provides a health-care food capsule, which consists of a content and a gelatine skin, wherein the content is a mixture which is prepared from raw materials according to the following part by weight: 12-22 of maca powder, 5-15 of wolfberry extract, 5-15 of Chinese angelica extract, 2-8 of acanthopanax extract, 2-8 of epimedium extract, 1-5 of ginseng extract, 15-25 of corn oil and 35-50 of tea seed oil. The gelatine skin is prepared from edible gelatine, glycerin and purified water according to the weight ratio of 1 / 1 / 0.5. The content is obtained through crushing, burdening, mixing, grinding and degassing the raw materials. The gelatine skin takes the edible gelatine, the glycerin and the purified water as gelatine skin raw materials, the gelatine liquor is obtained after mixing, gelating and heating the gelatine skin raw materials,, then the content and the gelatine liquor in the capsule are produced through the pelleting of an automated machine, and a product has a reasonable and practicable processing method. The health-care food capsule has the effects of alleviating climacteric syndrome, increasing the sexual capacity and relieving physical fatigue, is easy to beabsorbed by the human body, has no toxic and side effects, and can be orally taken for a long time.
Owner:厦门鹰君药业有限公司
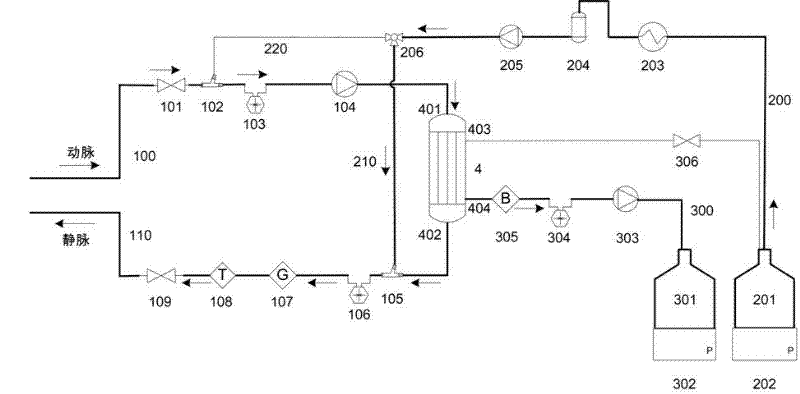
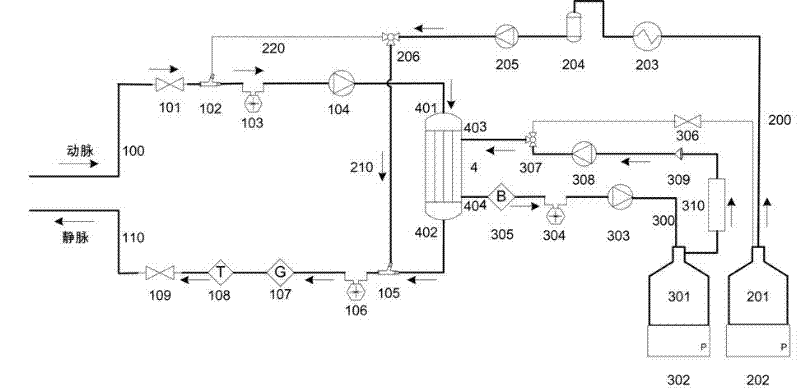
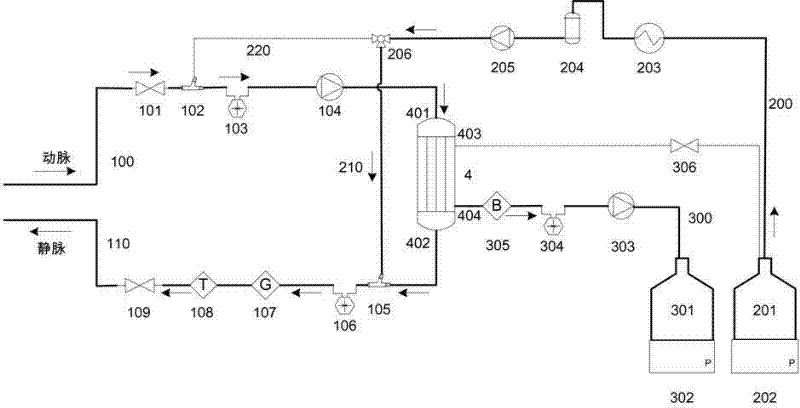
Portable blood purifying system
InactiveCN102389594AEfficient removal capacityImprove purification efficiencyDialysis systemsExtracorporeal circulationLiquid waste
The invention relates to a portable blood purifying system, which comprises a high throughput dialyzer with an arterial duct, a venous duct, a washing liquid tube and a transfusion tube; the arterial duct is in turn connected in series with an arterial valve, an arterial pressure sensor and a blood pump; the venous duct is in turn connected in series with a venous valve, a venous temperature sensor, a venous bubble detector and a venous pressure sensor, so that an external circulation unit is formed; the washing liquid tube is connected in series with a pre-charging valve and is communicated with a washing liquid container; the transfusion tube is in turn connected in series with a temperature controller, a transfusion tube bubble collector and a transfusion pump, and is communicated with a waste liquid container, so that a waste liquid processing unit is formed; and the system also comprises a transfusion pump, a temperature control device, a transfusion tube, a gating valve and a first transfusion branch and a second transfusion tube branch, so that a transfusion unit is formed. The system is simplified in structure and convenient to carry; and the system is suitable for patients to do short-time dialysis for several times or night dialysis treatment at home as household treatment equipment, so that toxin tolerance of dialysis patients during dialysis period is relieved while medical cost is reduced.
Owner:黄昱
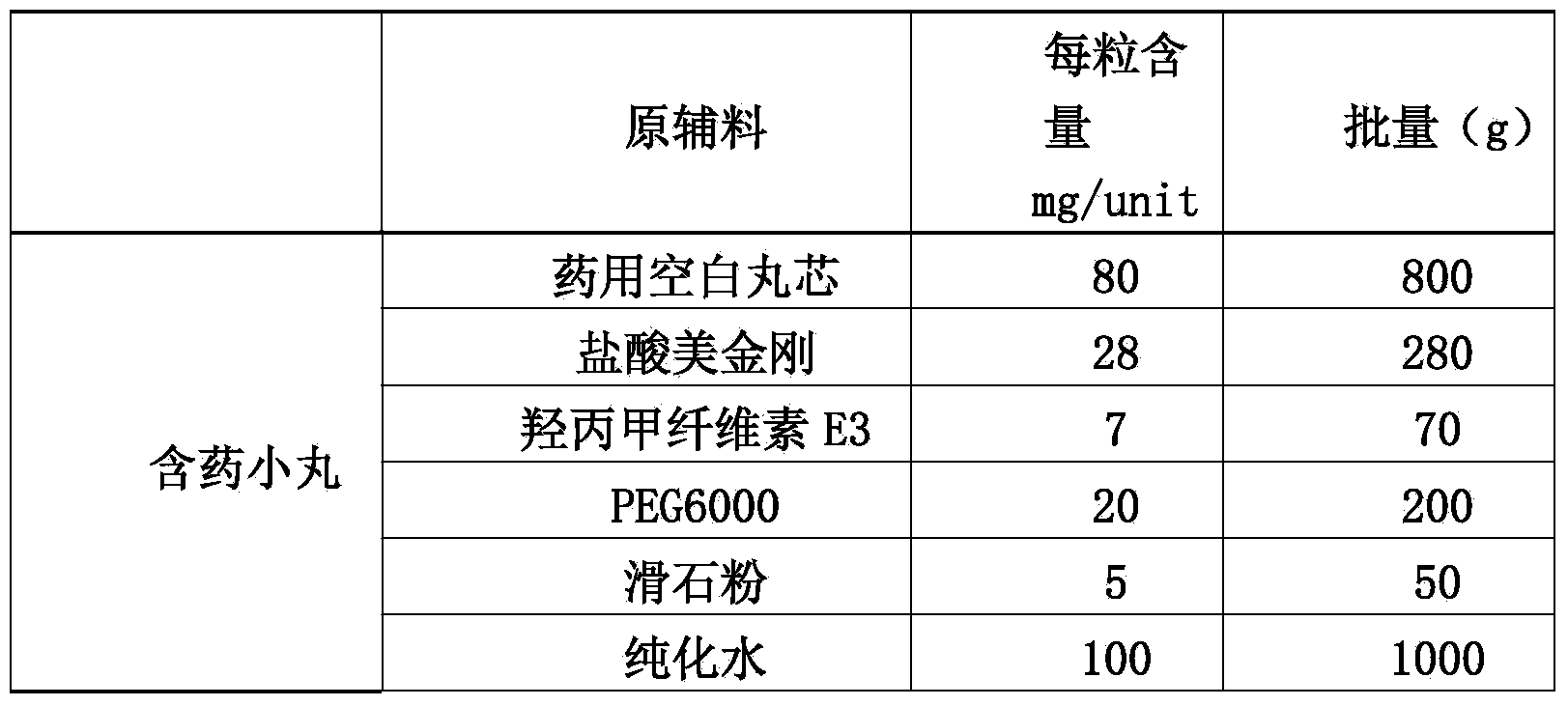
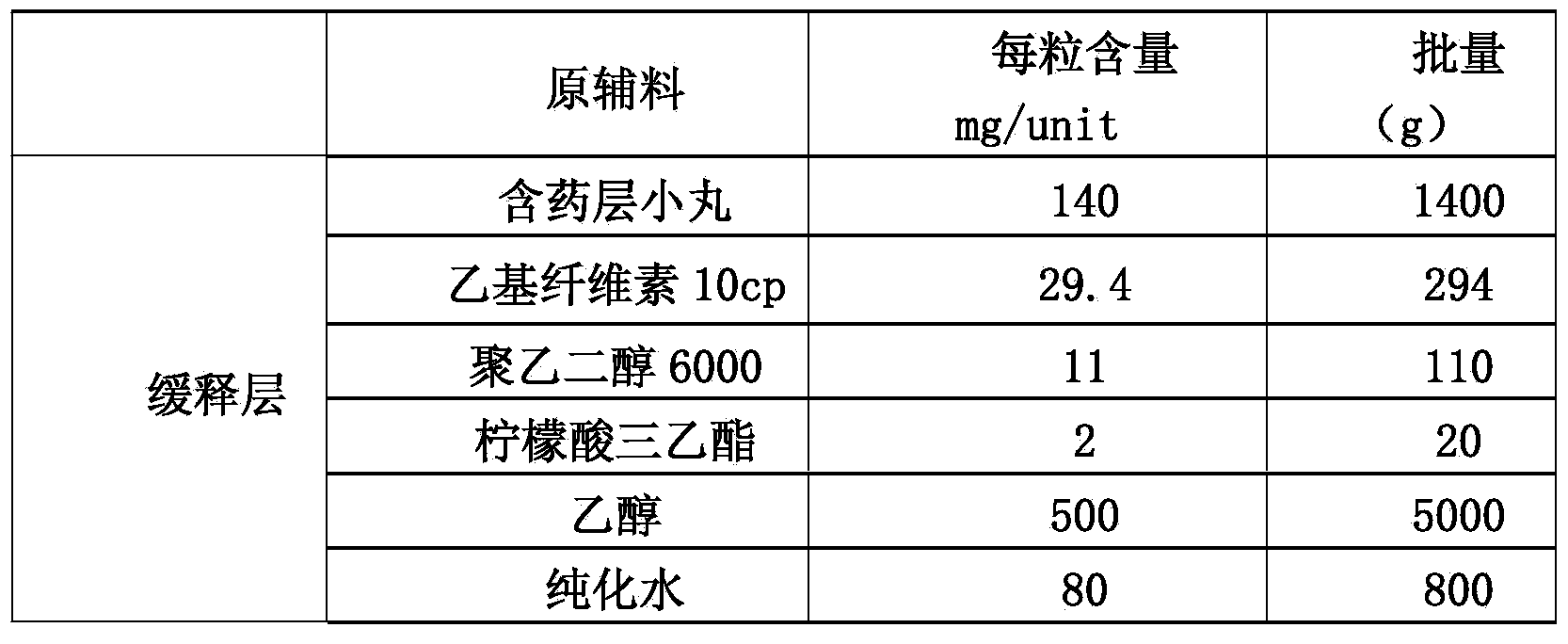
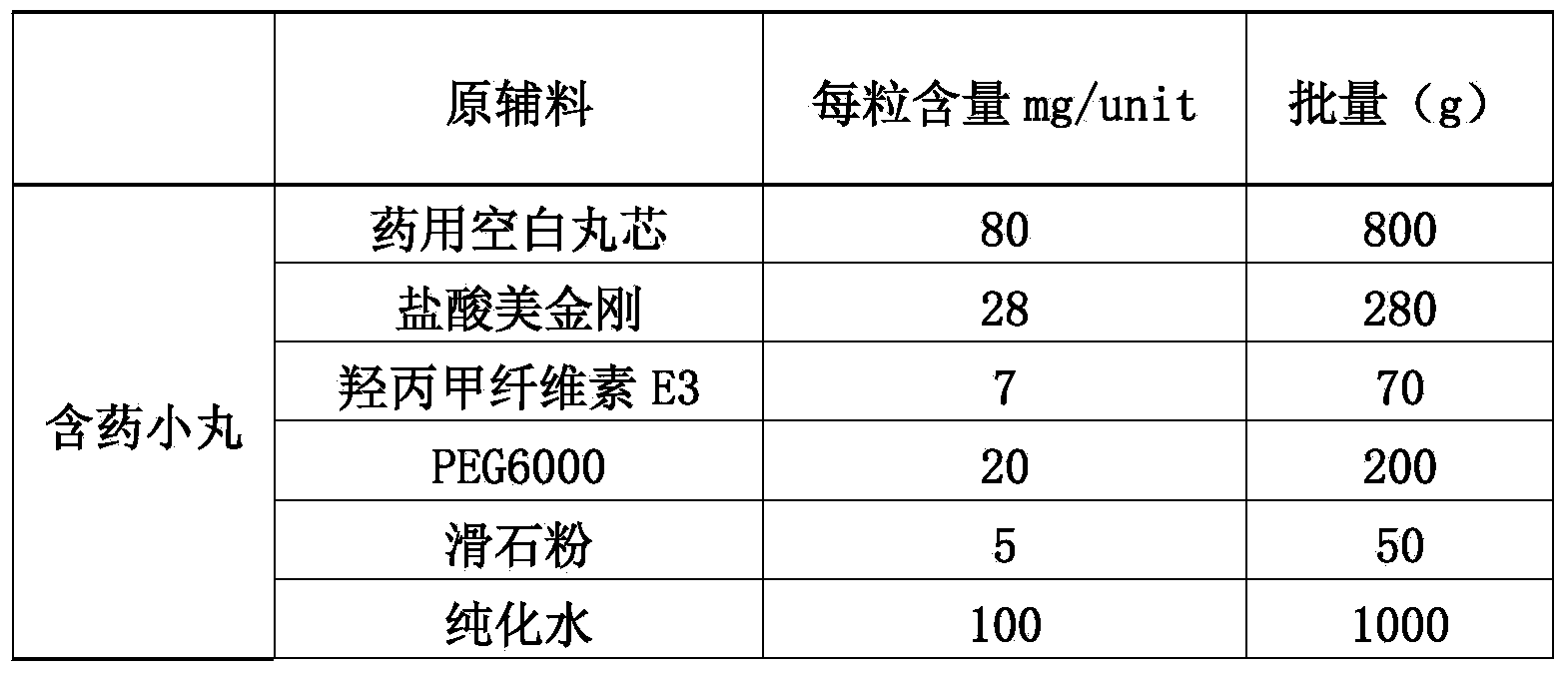
Memantine hydrochloride slow-release pill and preparation method thereof
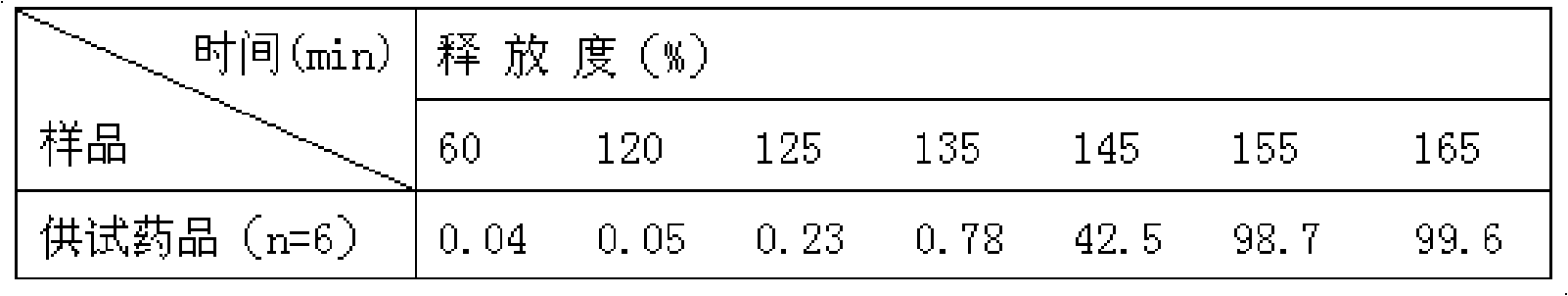
ActiveCN104013592ASolve the problem of easy releaseReduce the risk of sudden releaseNervous disorderPharmaceutical product form changeMemantine HydrochlorideCurative effect
The invention discloses a memantine hydrochloride slow-release pill and a preparation method thereof. Packing inside a capsule is a memantine hydrochloride slow-release pill; the memantine hydrochloride slow-release pill sequentially comprises a hollow pill core, a main medicine layer containing memantine hydrochloride, an isolated coating layer and a slow-release coating layer from inside to outside. The memantine hydrochloride slow-release pill is good in stability and free of an initial burst release phenomenon, the condition that the medicine is lastingly, evenly and slowly released is ensured, and the curative effect of the product is improved.
Owner:ZHEJIANG JINGXIN PHARMA
Traditional Chinese medicine preparation for treating peptic ulcer and preparation method thereof
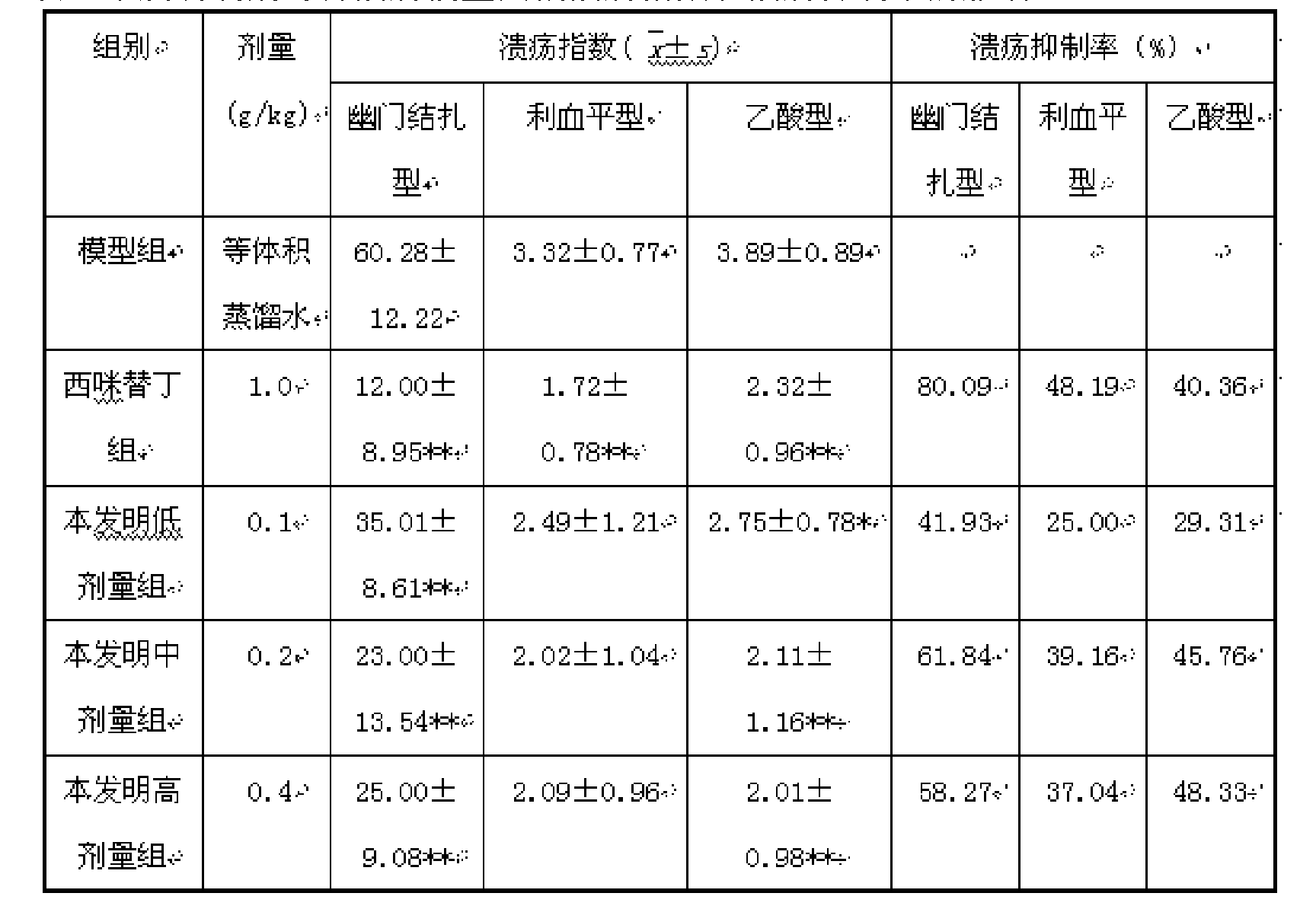
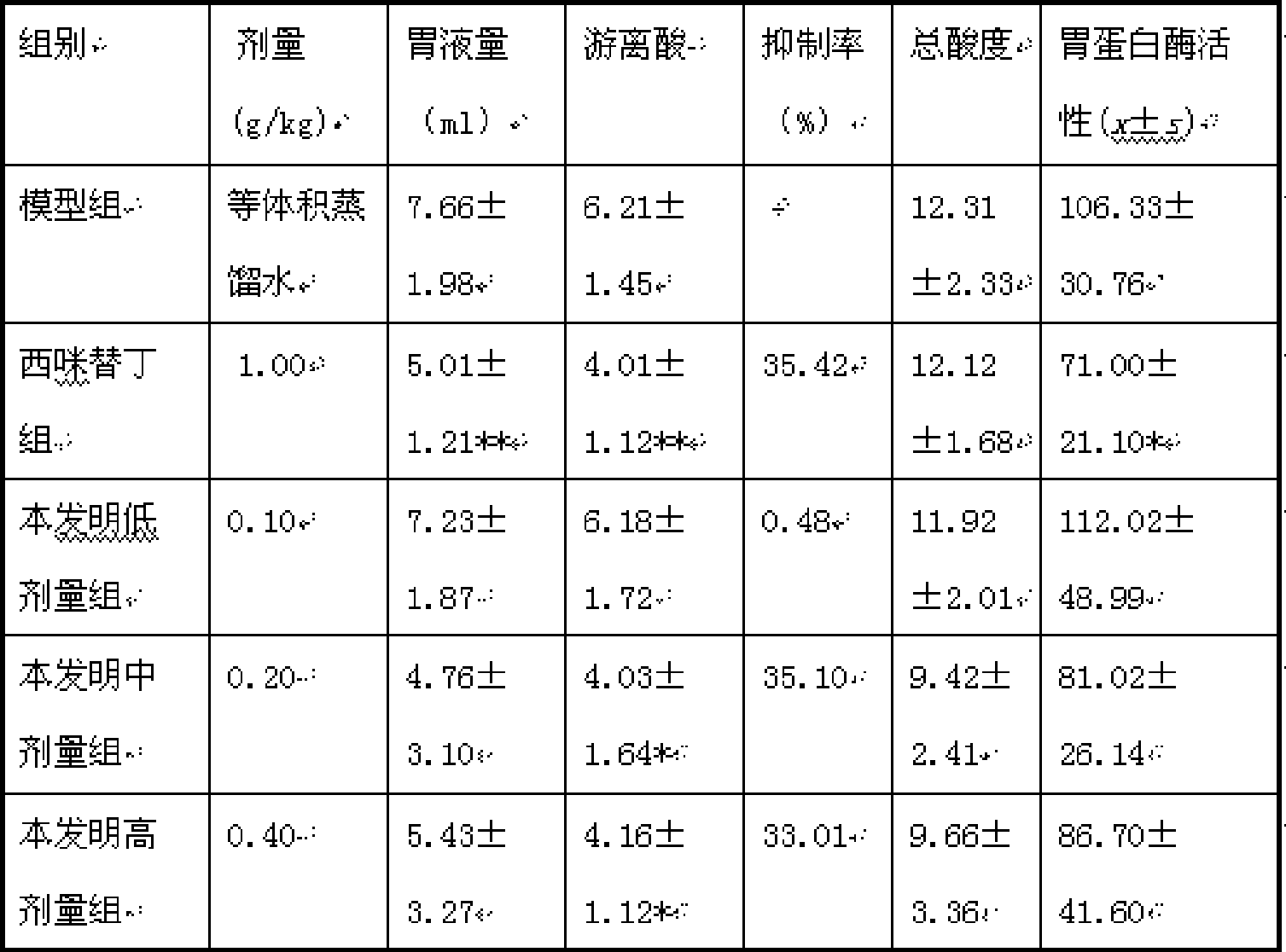
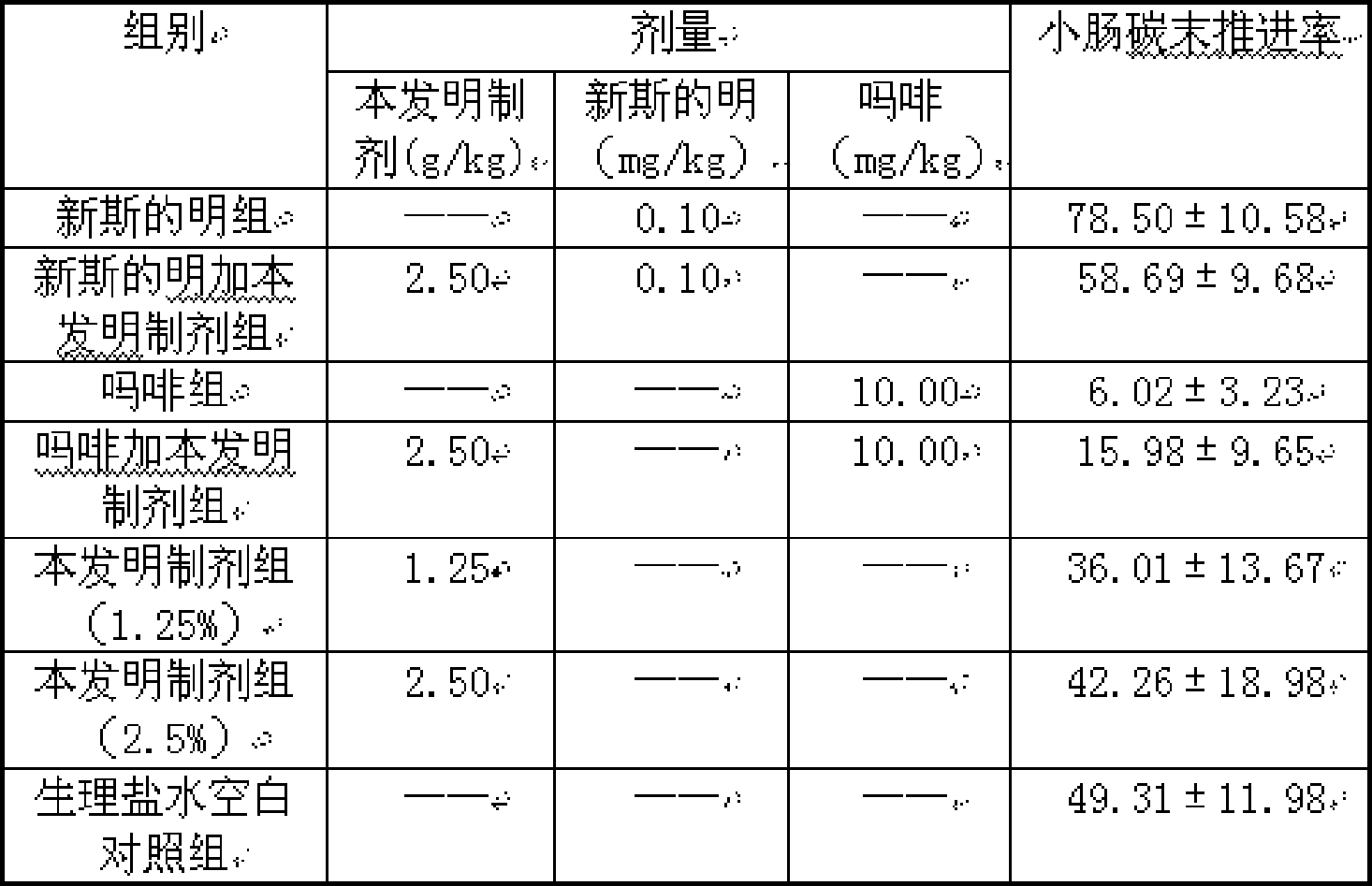
InactiveCN101428086AGuaranteed curative effectTake a small doseDigestive systemMammal material medical ingredientsDiseaseTherapeutic effect
The invention discloses a traditional Chinese medicine preparation for curing peptic ulcer, and a preparation method thereof. The traditional Chinese medicine preparation is prepared by taking 1 to 10 parts of white peony root, 1 to 20 parts of white atractylodes rhizome, 1 to 20 parts of atractylodes rhizome and 1 to 10 parts of evodia fruit as the basic bulk drugs according to the parts by weight, and also can be combined with 1 to 10 parts of chickens gizzard-membrane, 1 to 20 parts of rhizoma corydalis, 1 to 20 parts of Chinese cinnamon and 15 to 20 parts of auckandia root. Compared with the prior art, the traditional Chinese medicine preparation has the effects of strengthening the spleen and stomach and promoting qi-circulation and relieving pain, and has good treatment effect for the gastric and intestinal diseases with main symptoms of gastric ulcer, doudenal ulcer, gastritis, abdominal pain and abdominal distention; and the traditional Chinese medicine preparation can reduce the dose for the patients to the utmost extent on the premise of ensuring the therapeutic effect of the drug.
Owner:ZUNYI MEDICAL UNIVERSITY
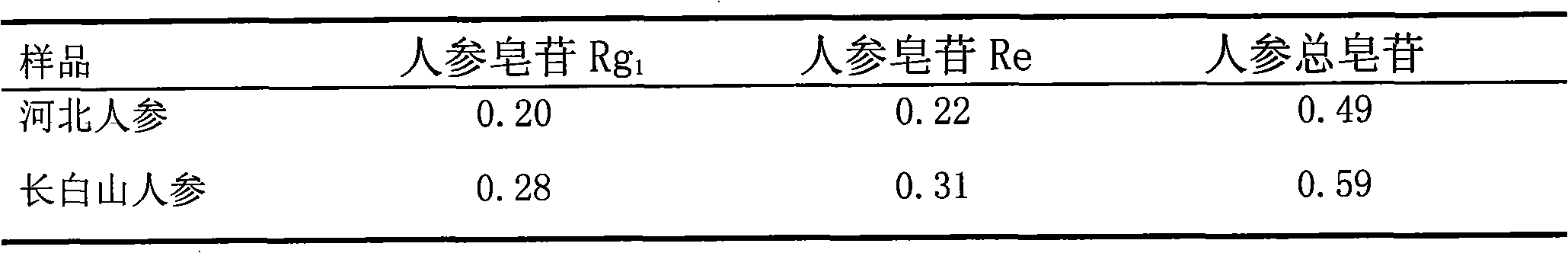
Quality control method for ginseng in Yixinshu Chinese medicinal preparation
InactiveCN101596275AQuality improvementGuaranteed curative effectComponent separationCardiovascular disorderGinsengBody fluid
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Method for preparing a Shuanhuanglian injection and the component detecting method
ActiveCN101040915AAvoid destructionHigh yieldComponent separationRespiratory disorderBiotechnologyShuang-huang-lian
The invention provides a method for preparing an injection, comprising that extracting respectively the scutellaria root, honeysuckle flower and capsule of weeping forsythia, then preparing the injection. The invention also provides a component check method of the injection, for improving the quality control level, assure the treatment effect, and support the product detection.
Owner:HARBIN ZHENBAO PHARMA
Wall-breaking and superfine grinding process and device for better ground traditional Chinese medicine decoction piece
ActiveCN105344428AImprove qualityStable and controllable qualityGrain treatmentsLiquid-crystal displayMedicine
The invention belongs to the field of medicinal material grinding and smashing equipment and method, and particularly relates to a wall-breaking and superfine vibration grinder for a better ground traditional Chinese medicine decoction piece, a traditional Chinese medicine decoction piece preparation method adopting the grinder, and the better ground traditional Chinese medicine decoction piece. The grinder comprises a motor (1), a coupler (2), a rotating spindle (3), a bearing pedestal (4), a drum holder (5), a drum (6), a supporting spring (7), a base, an excitation vibration absorber, a controller, a timer, a liquid crystal display operating panel, a central processing unit and a silencing case. By means of the specific rotating speed and the specific motion trace of the motor of the grinder, the grinder achieves the advantages of energy conservation, high efficiency and smaller sizes of smashed grains.
Owner:SHAANXI NIANQINGBAO PHARMA CO LTD
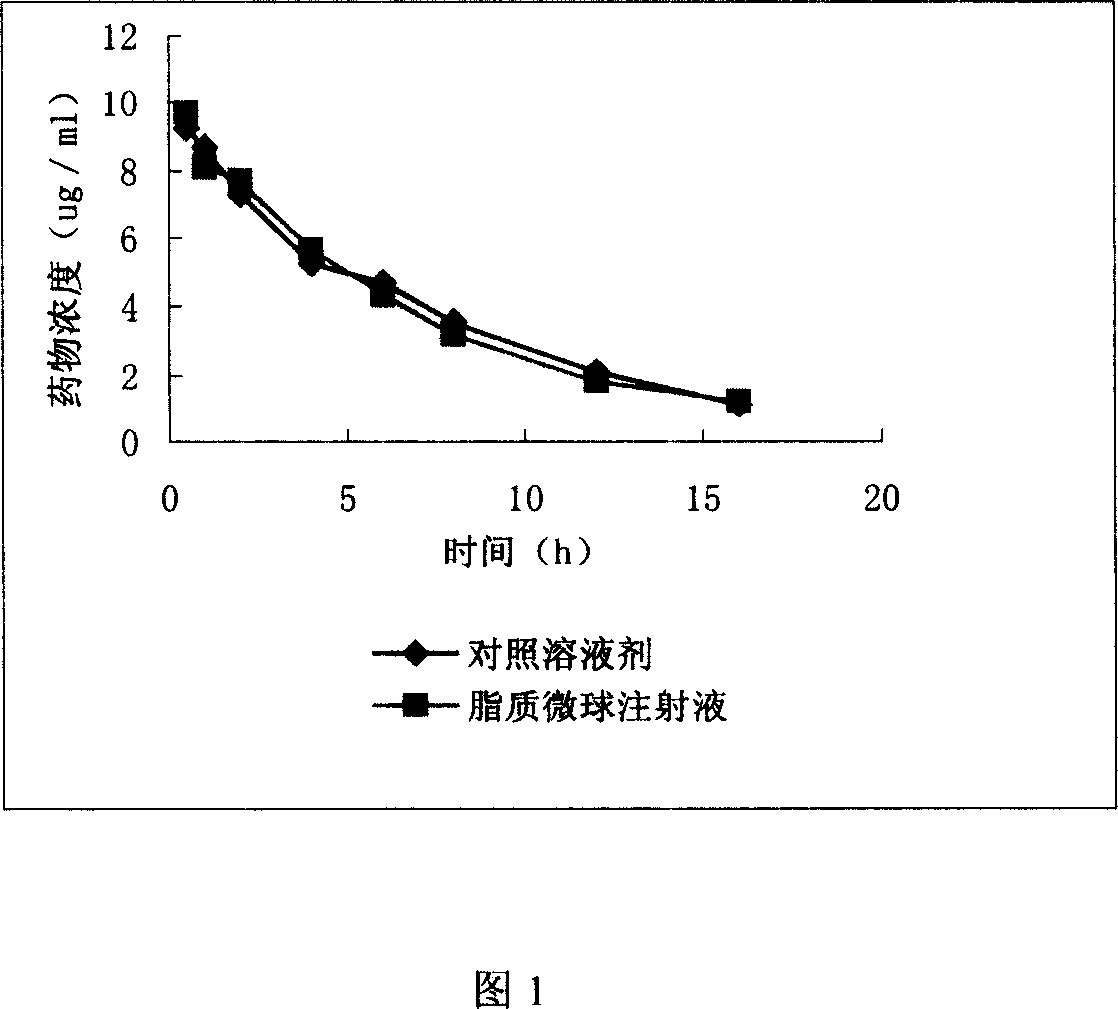
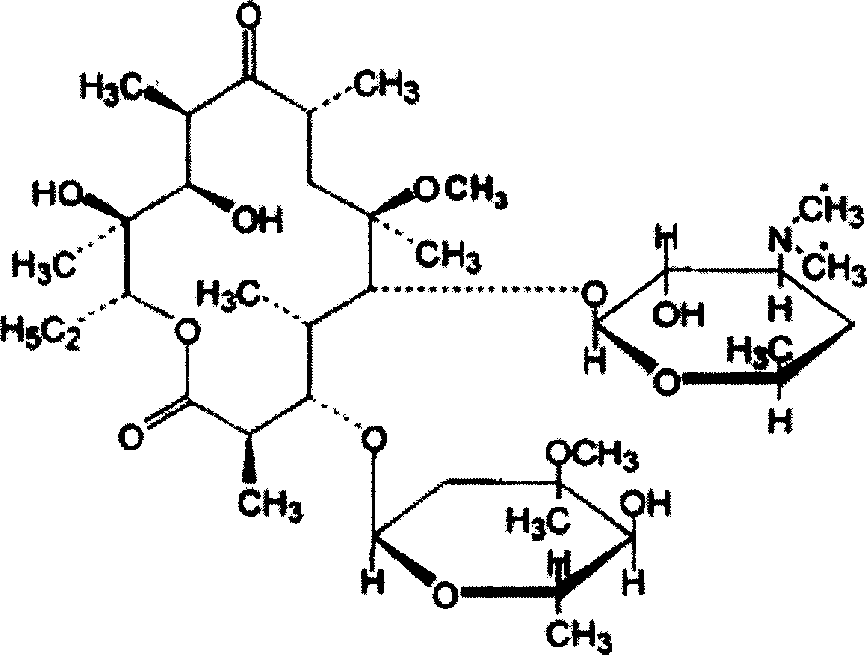
Clarithromycin liposome microsphere injection and its prepn. method
InactiveCN1947720AHigh drug loadingGuaranteed curative effectAntibacterial agentsOrganic active ingredientsMicrosphereCurative effect
A lipide microball type injection of clarithromycin with high stability and low toxin is prepared from the oil for injection, clarithromycin, surfactant, glycerin, oil-phase solubilizer, metallic chelating agent, and the water for injection. Its preparing process is also disclosed.
Owner:刘玉辉
Flower red pills and their preparation
InactiveCN1552371AThe production process is perfect and reasonableRaise quality standardsAntipyreticAnalgesicsCurative effectUtilization rate
A Chinese medicine 'Huahong pill' for treating blood stasis, leukorrhagia and hypogastralgia is an improvement to existing relative medicine in the form of capsule. Its preparing process is also disclosed. Its advantages are high curative effect, high release speed and high biologic utilization rate.
Owner:毛友昌
Compounded medicine for wholely preventing and treating cardio-cerebral blood vessel diseases and use thereof
InactiveCN101024082ASmall doseLittle side effectsPharmaceutical delivery mechanismOil/fats/waxes non-active ingredientsDiseaseSide effect
The present invention relates to a compound medicine for preventing and curing angiocardiopathy and cerebrovascular disease, belonging to the field of chemical medicine. It is characterized by that said compound medicine includes sexual hormones medicine, lipid-reducing medicine, hypotenser, hypoglycemic medicine and anticoagulant medicine. Said compound medicine can be made into various dosage forms. Besides, said compound medicine also can be used for curing the diseases of climacteric syndrome, osteoporosis, endometrial carcinoma, diabetes and metabolic syndrome, etc.
Owner:张士东
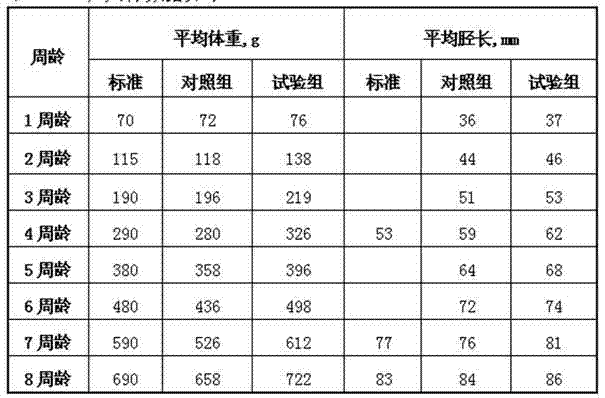
Complete feed for laying hens at brood time and preparation method thereof
The invention belongs to the technical field of feed for laying hens and particularly relates to complete feed for laying hens at brood time and a preparation method thereof. The complete feed comprises, by weight, 37-44% of corn, 18-22% of extruded corn, 0.4-0.6% of soybean oil, 18-20% of soybean meal, 9-11% of extruded soybeans, 1.5-2.5% of fish meal, 3.5-4.5% of fermented soybean meal, 1.1-1.2% of mountain flour, 1.4-1.7% of calcium hydrophosphate, 0.14-0.16% of methionine, 0.09-0.13% of choline chloride, 0.1-0.2% of baking soda, 0.2-0.3% of salt, 0.13-0.16% of microecologics, 0.01-0.03% of liquid phytase, 0.03-0.05% of liquid xylanase, 0.1-0.2% of acidifier, 0.1-0.2% of glycine betaine, 0.004-0.006% of antioxidant, 0.015-0.035% of 4% enramycin, 0.03-0.04% of vitamin complex and 0.08-0.09% of composite microelement. The complete feed can meet nutrition requirements of the laying hens at the brood time, improves immunity of the laying hens at the brood time, has remarkable effects on increasing weight and shin length of the laying hens at the brood time, enables the laying hens to reach the standard in advance, and provides a good foundation for achieving high yield at an egg producing period.
Owner:河南商都生物技术股份有限公司
Oral preparation containing andrographolide and preparation method thereof
InactiveCN103070843AImprove toleranceAvoid direct accessOrganic active ingredientsAntiviralsEnteric-coated granulesSide effect
The invention relates to an oral preparation containing andrographolide and a preparation method thereof, belonging to the field of medicines. The oral preparation containing andrographolide is in dosage forms of enteric-coated granules, enteric-coated tablets, enteric-coated capsules and enteric-coated dispersible tablets which are prepared by mixing andrographolide and pharmaceutically acceptable auxiliary materials. The prepared oral preparation can reduce incidence of adverse reactions and side effects thereof and improve the medication safety while ensuring the therapeutic effect, and uses the andrographolide more widely.
Owner:司鹏 +1
Process for extracting, separating and refining amygdalin
InactiveCN1365979ATake advantage ofGuaranteed curative effectSugar derivativesSugar derivatives preparationAlcoholEther
A process for extracting, separating and refining amygdalin includes such steps as squeezing bitter apricot kernels to remove oil, then extracting in alcohol three times, pressure reduction filtering, merging the filtrate, pressure reduction recovery of alcohol, laying aside to educe out white cluster crystal of amygdalin, filtering to obtain the coarse product, dissolving in alcohol, filtering, cold storage, educing out amygdalin crystal, pressure reduction filtering, and washing crystal with ether or alcohol to obtain the pure product. Its adgantages are high purity (more than 99%), and high extraction rate.
Owner:药都制药集团股份有限公司
Quality control method of total glycosides single preparation of white paeony roots
ActiveCN102138985AQuality assuranceGuaranteed curative effectComponent separationPlant ingredientsClinical efficacyCurative effect
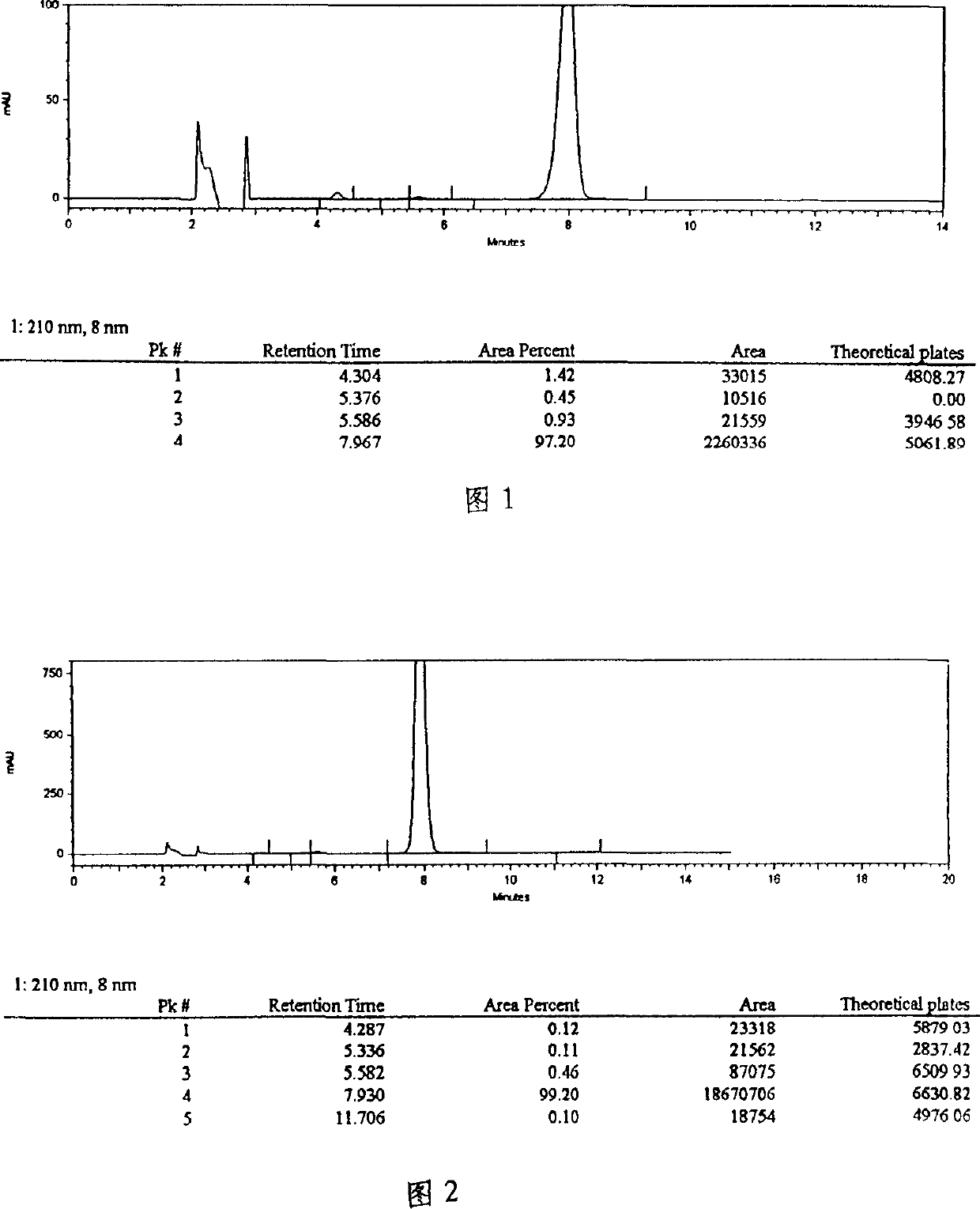
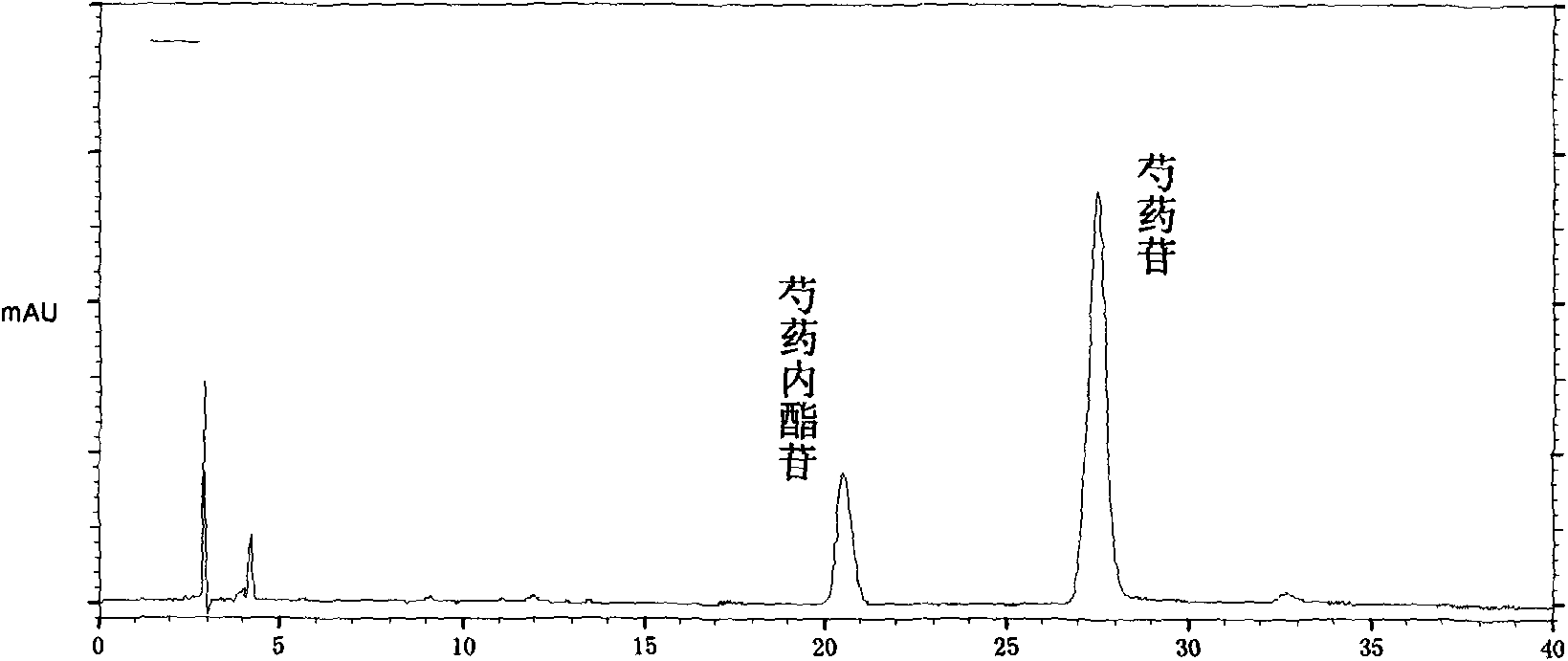
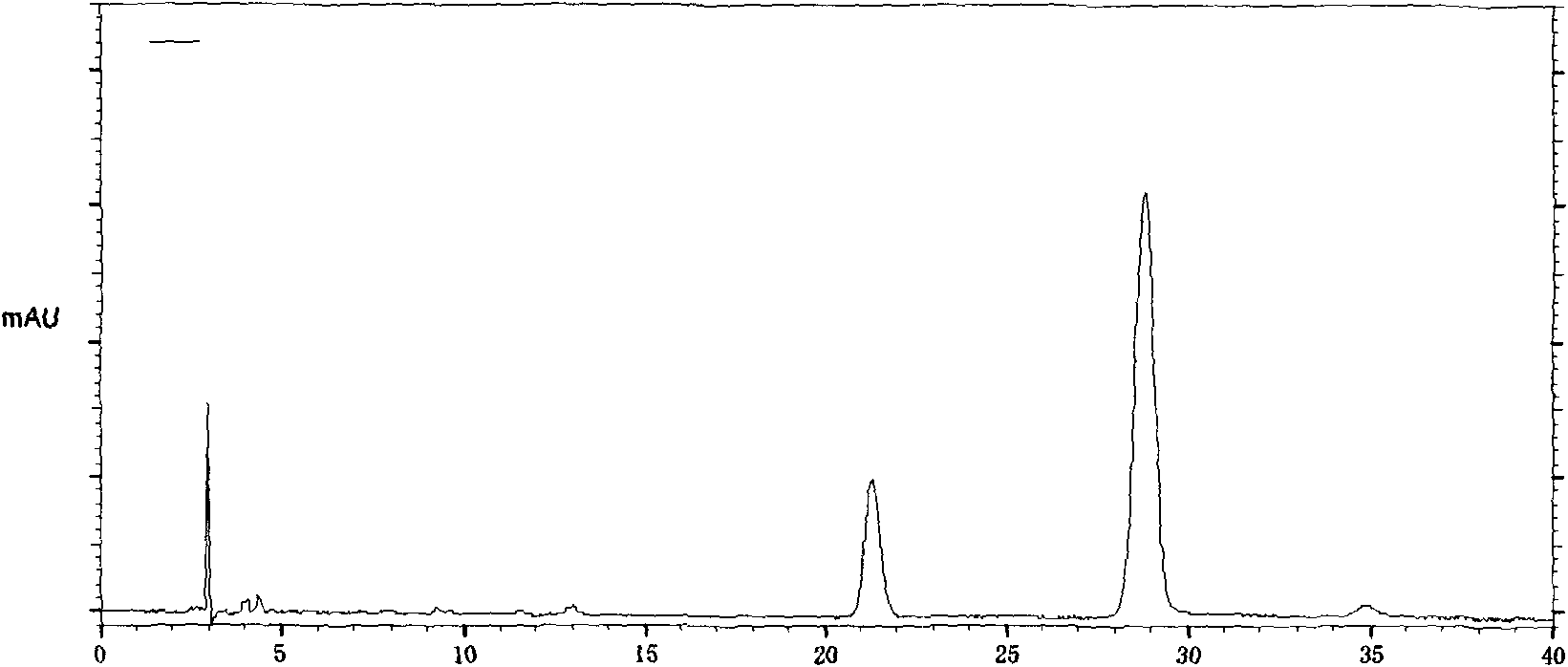
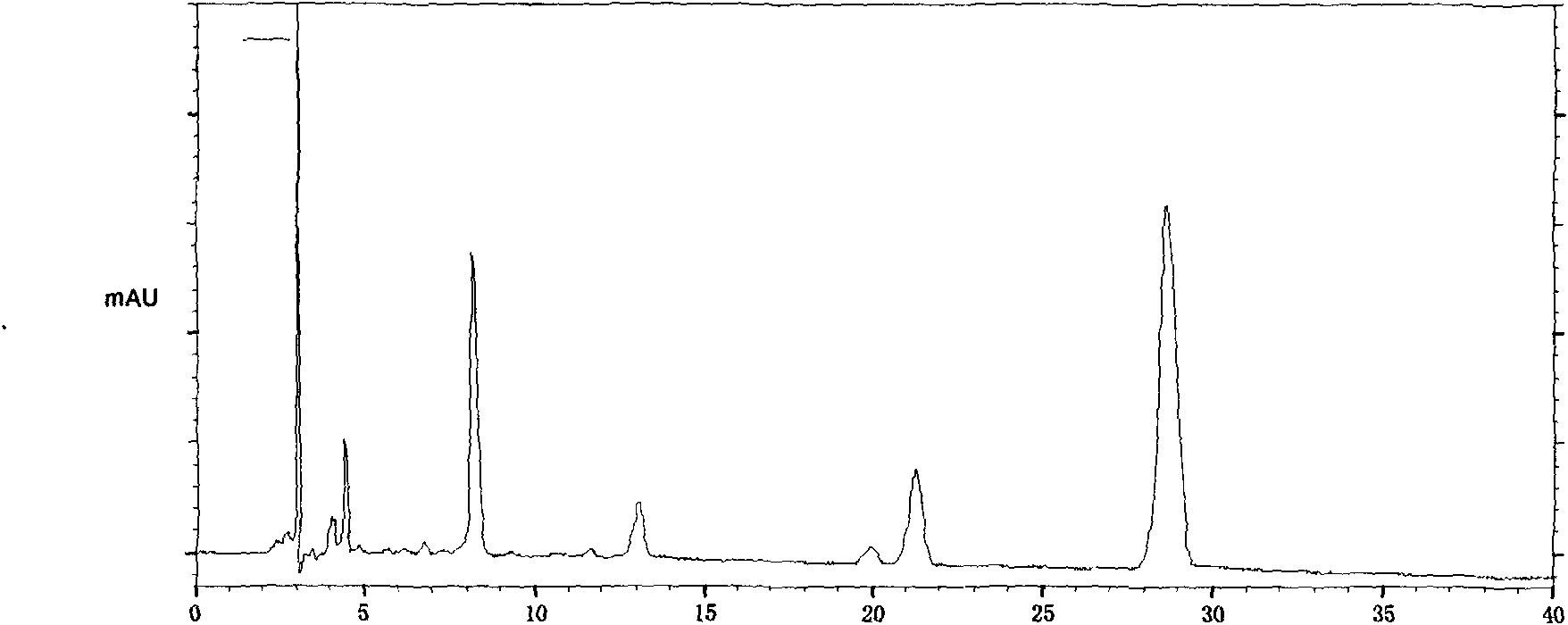
The invention provides a quality control method of a preparation of white paeony roots. The method comprises the following steps of: establishing a synchronous content measuring method of white paeony root herbs, white paeony root total glycosides and paeoniflorin and albiflorin in the white paeony root preparation by adopting the same liquid-phase chromatography condition; and accurately measuring the contents of the paeoniflorin and the albiflorin. The method is simple to preprocess a sample, keeps complete characteristic components and provides a stable sample solution and has higher accuracy, favorable reproduction and a certain specificity; characteristic peaks in the obtained fingerprint map have favorable separation effect, the fingerprint maps of the white paeony root herbs, the white paeony root total glycosides and the preparation have favorable relativity; the standard fingerprint map of the white paeony root herbs is established at a new angle by utilizing the relativity research of the fingerprint maps, can be used for identifying the qualities of the white paeony root herbs and improving the controllability of the production process of the white paeony root preparation and is favorable to ensuring the quality stability and the clinical curative effect of the white paeony root preparation.
Owner:NINGBO LIWAH PHARM CO LTD
Tibetan medicine for major functions of acute-chronic sprain-contusion, rheumatism, rheumatoid diseases, and its preparing method
The present invention relates to a Chinese Tibet medicine for mainly curing the diseases of acute and chronic sprain and contusion, rheumatism and rheumatoid disease and its preparation method. It is characterized by that said preparation method includes the following steps: utilizing the Chinese Tibet medicinal materials of lamiophlomis rotate, Jidou, turmeric, zanthoxylum husk, water buffalo horn and myricaria germanica as raw material, making them undergo the processes of decocting, vacuum freeze-drying, pulverizing and making them into any pharmaceutically-acceptable dosage form, including oral preparation or preparation for external application, in particular, it can be made into the adhesive plaster, ointment preparation, spray, gel preparation, film preparation and resin plaster, etc.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
Reduced type glutathione lozenge
InactiveCN1408427ARich in capillariesFast blood flowNervous disorderDigestive systemEnteropeptidaseAdhesive
The reduced glutathione lozenge consists of flutathione as main componnet, stuffing, disintegrating agent, adhesive, absorption promoter, corrective and lubricant. The present invention produces reduced glutathione lozenge for absorption via oral cavity mucous membrane and entering blood circulation via jugular vein and superior vena cava, and this can aovid the decomposition of glutathione by intestinal peptidase and ensure high biological utilization and high curative effect.
Owner:福安药业集团庆余堂制药有限公司
Shenqi hypoglycemic preparation HPLC standard finger print and construction method thereof
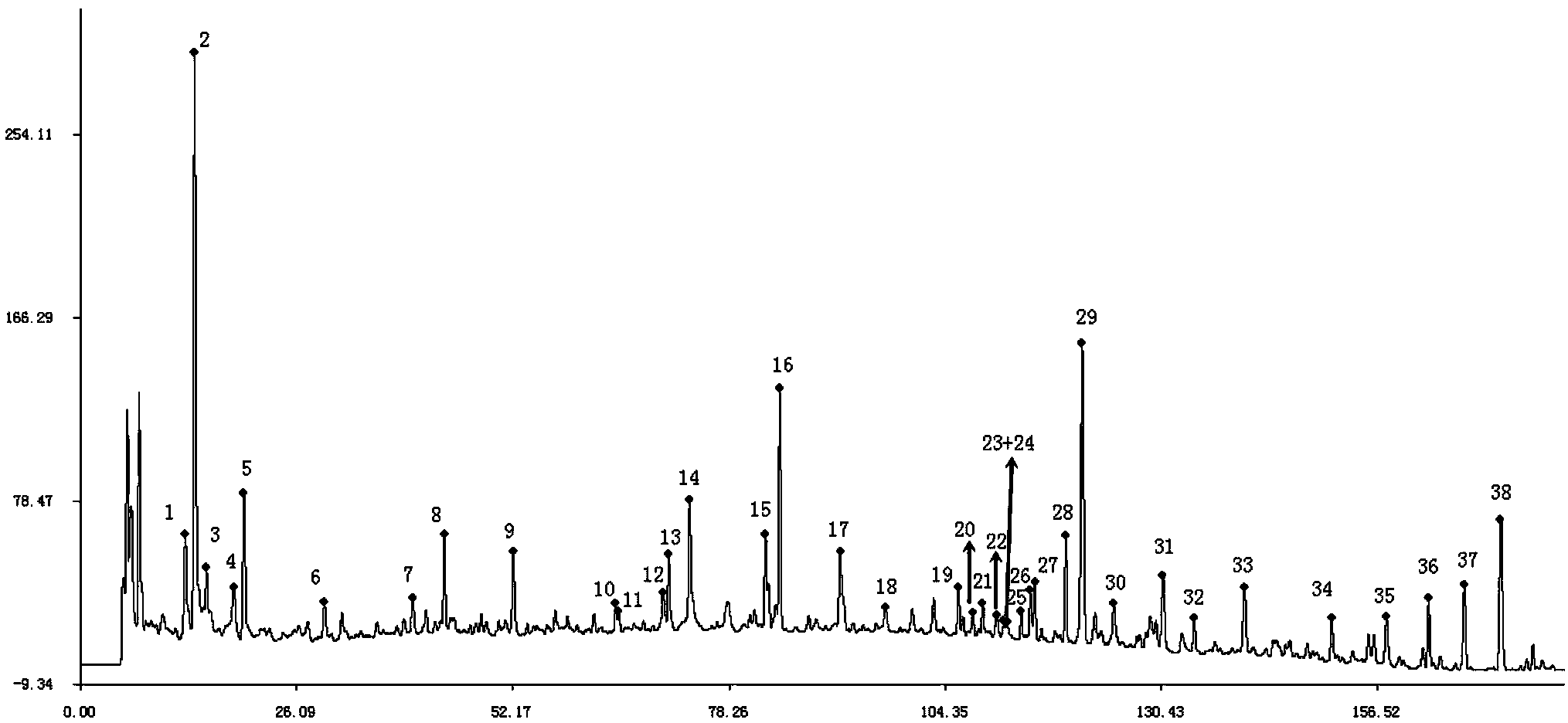
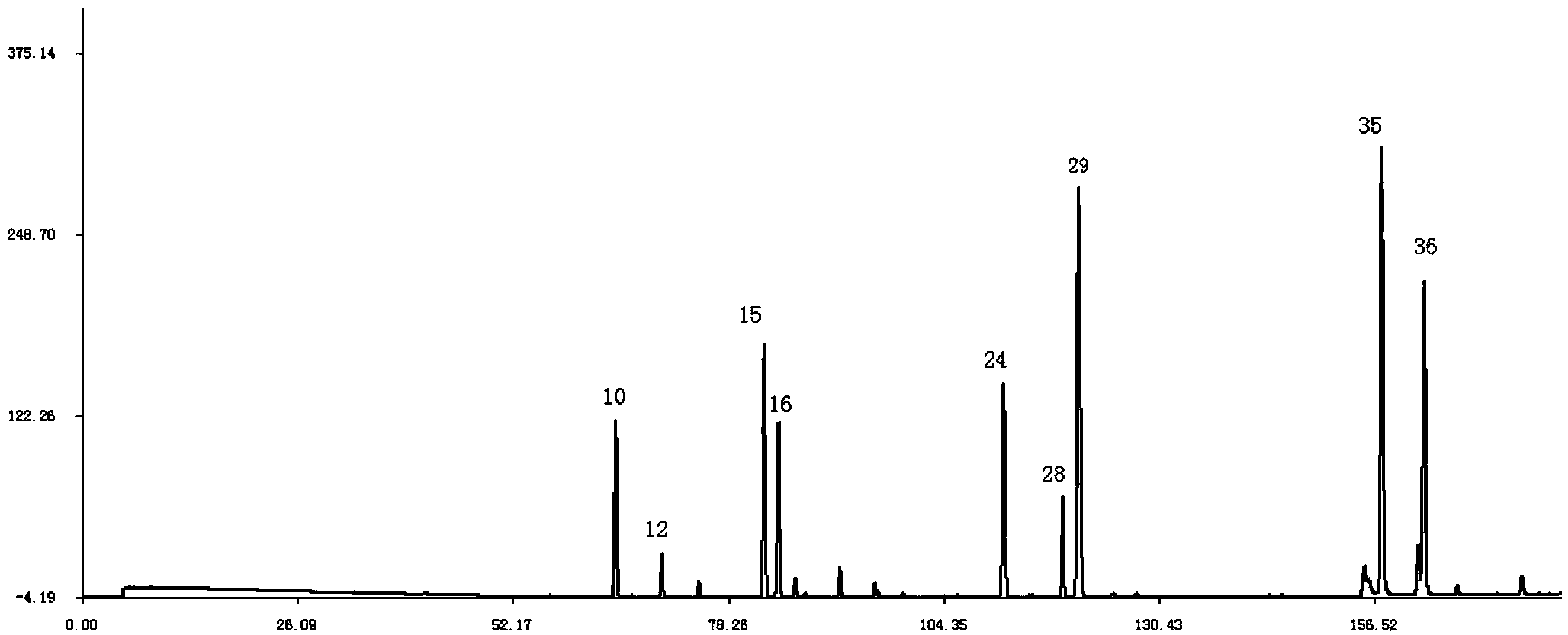
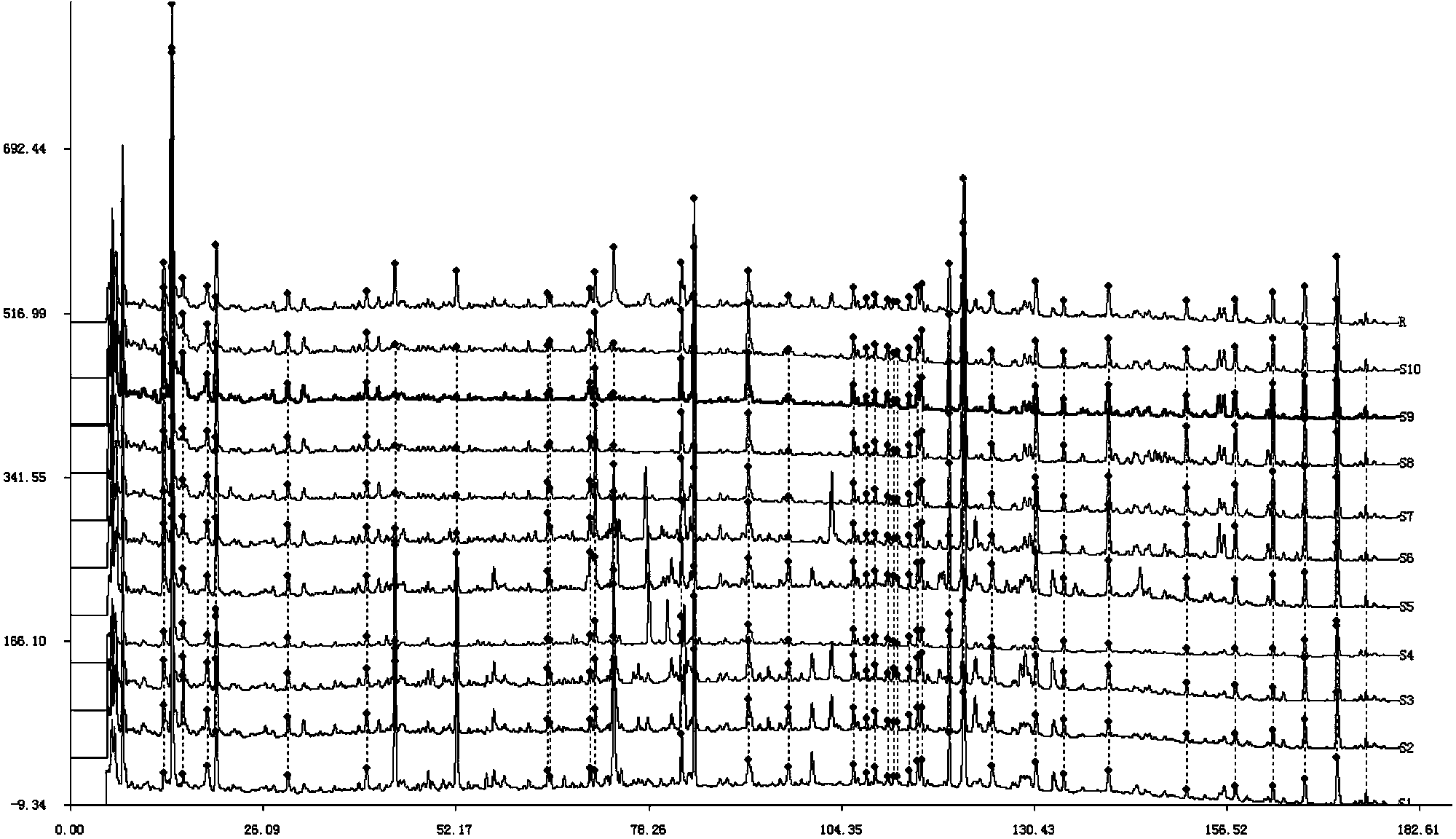
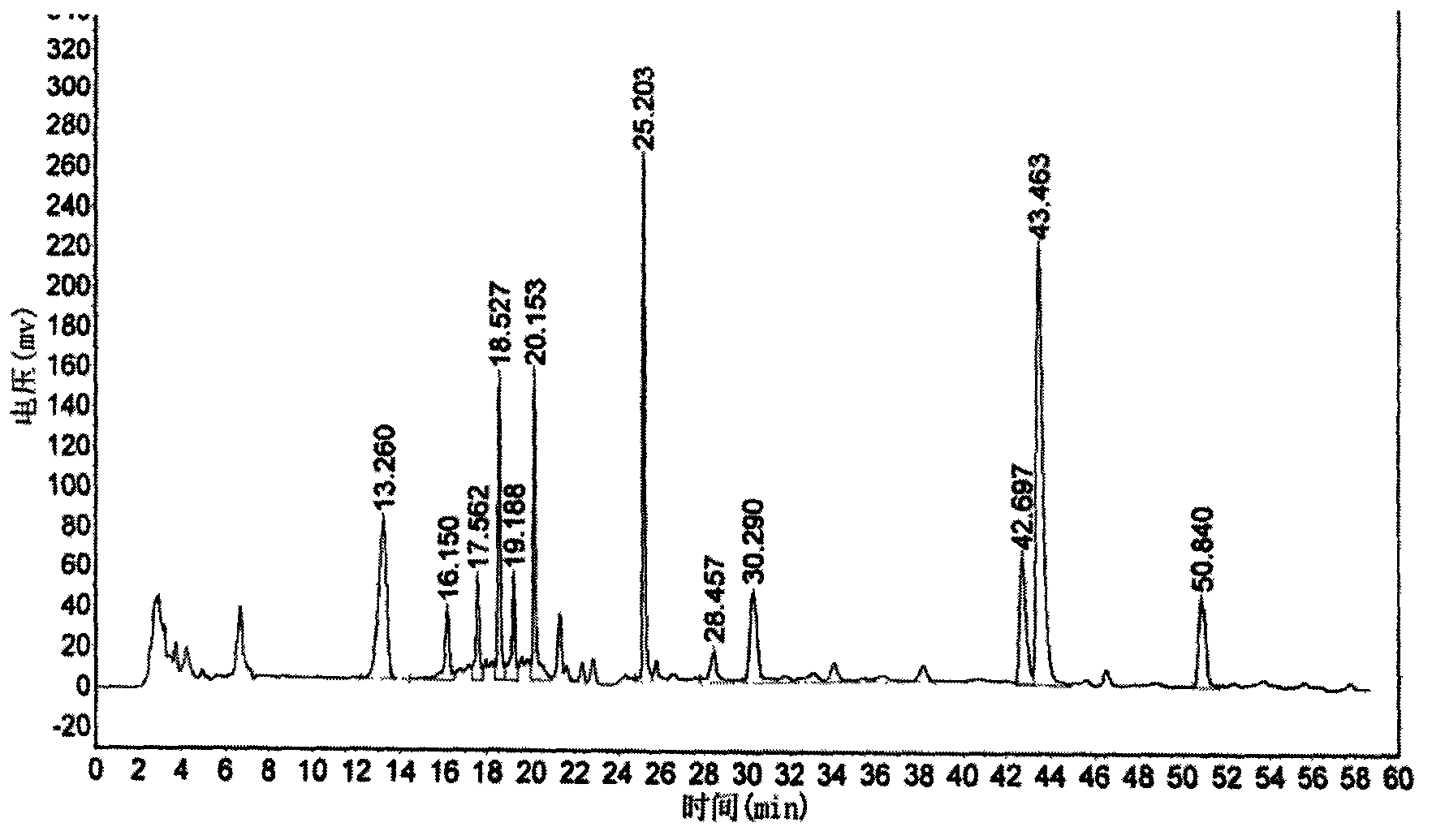
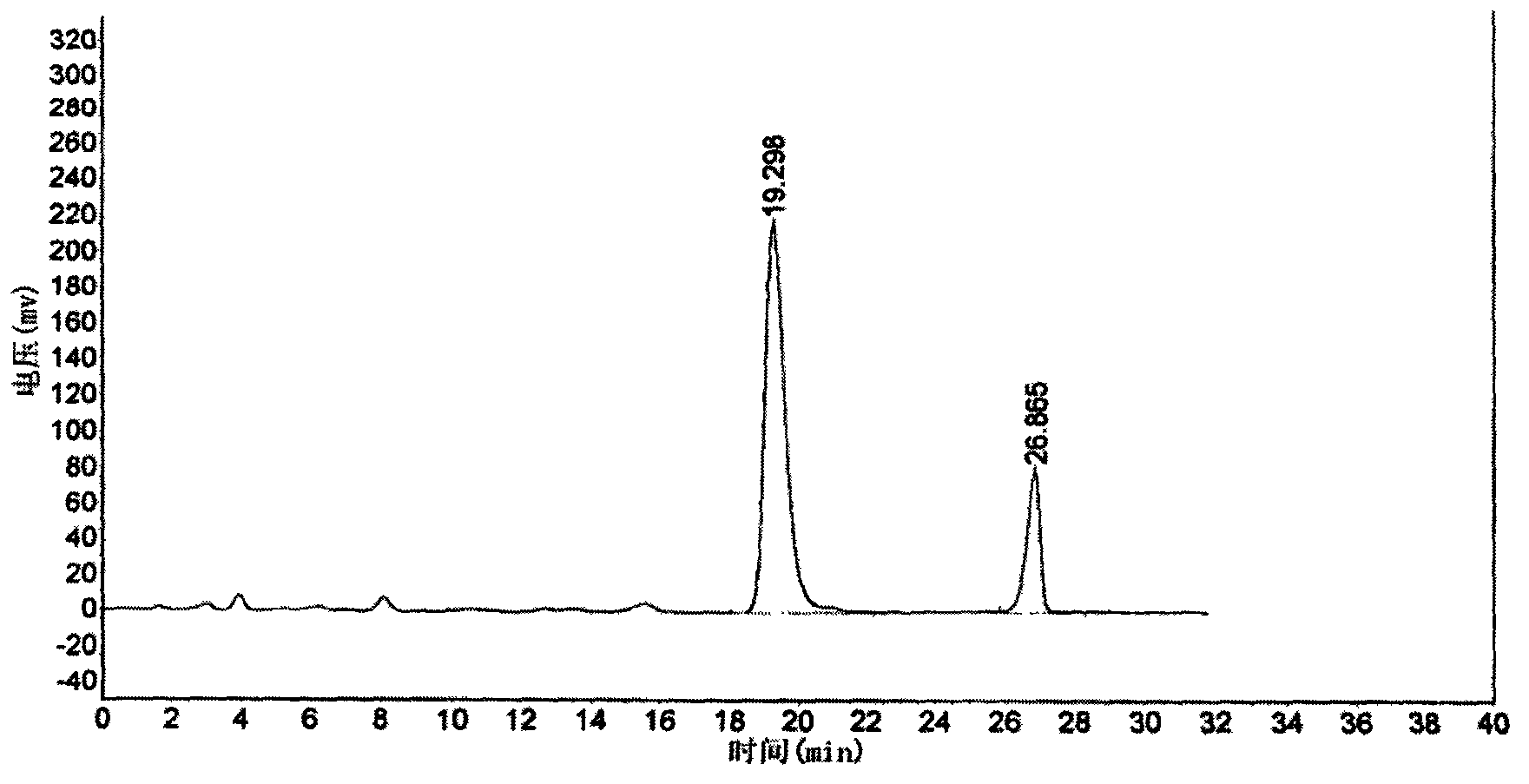
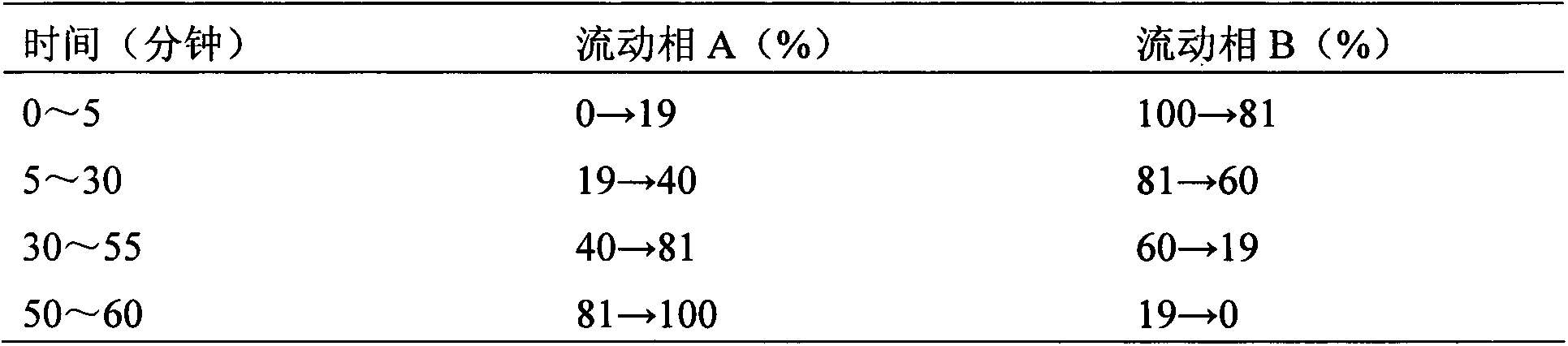
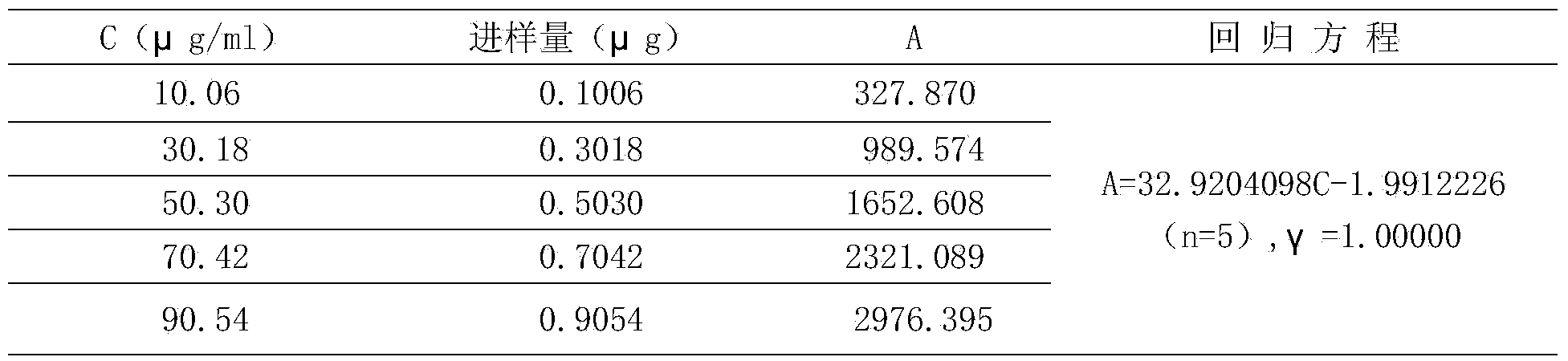
The present invention discloses a Shenqi hypoglycemic preparation HPLC standard finger print and a construction method thereof. The construction method comprises the following steps: preparing test sample solutions and a reference solution, measuring the test sample solutions and the reference solution by using the HPLC method and the linear gradient elution process, so as to obtain the Shenqi hypoglycemic preparation finger prints, wherein the chromatographic condition is that a mobile phase A is acetonitrile, a mobile phase B is 0.1% (v / v) phosphoric acid solution, the detection wavelength is 203nm, the column temperature is 25-35 DEG C and the flow rate is 0.8-1.2mL / min. The common characteristic peaks of at least ten finger prints are used as the characteristic peaks of the standard finger print; the standard finger print comprises 38 characteristic peaks, and the numbers of the characteristic peaks of saponin of ginseng stem and leaf, astragalus, Chinese magnoliaving, rehmannia root and raspberry are 13, 4, 7, 3 and 4 respectively. The Shenqi hypoglycemic preparation HPLC standard finger print and the construction method thereof can comprehensively and accurately evaluate the whole quality of the Shenqi hypoglycemic preparation, and facilitate to ensure the quality and the clinical curative effect of the Shenqi hypoglycemic preparation.
Owner:广东万年青制药股份有限公司
Medicinal preparation for treating nerve-root cervical spondylopathy, and preparation method and quality detection method thereof
InactiveCN104887771AMeet needsVarious dosage formsNervous disorderComponent separationClinical efficacyCervical spondylopathy
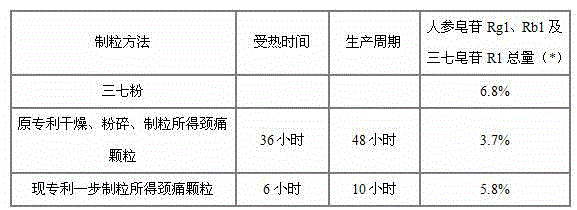
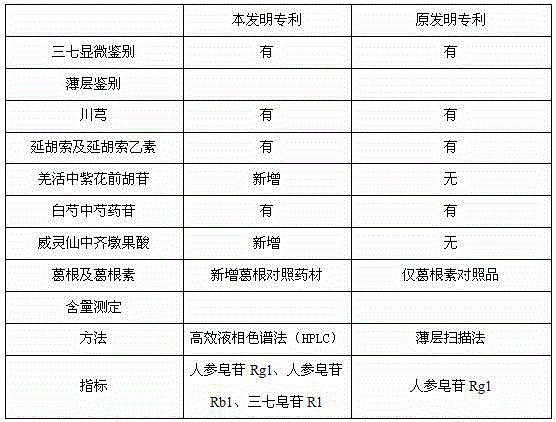
The invention relates to a medicinal preparation for treating nerve-root cervical spondylopathy, and a preparation method and a quality detection method thereof. The invention is an extension of an original invention. The medicinal preparation comprises a granule, a tablet and a capsule, the preparation method is an optimized and screened production technology based on the original invention, a modern new device, a new process and a new technique are adopted to realize industrial production; and quality standard researches are completed and improved on the basis of original standards, HPLC is adopted to simultaneously determine the content of ginsenoside Rg1, ginsenoside Rb1 and notoginsenoside R1 in a finished product, and thin layer discrimination of all medicines is carried out to comprehensively control the quality, so the clinic curative effects are guaranteed.
Owner:SHANDONG MINGREN FURUIDA PHARMA
Lyophilized viper antivenin and preparation method thereof
ActiveCN101816789AHigh potencyGuaranteed curative effectAntinoxious agentsMammal material medical ingredientsMass ratioBlood plasma
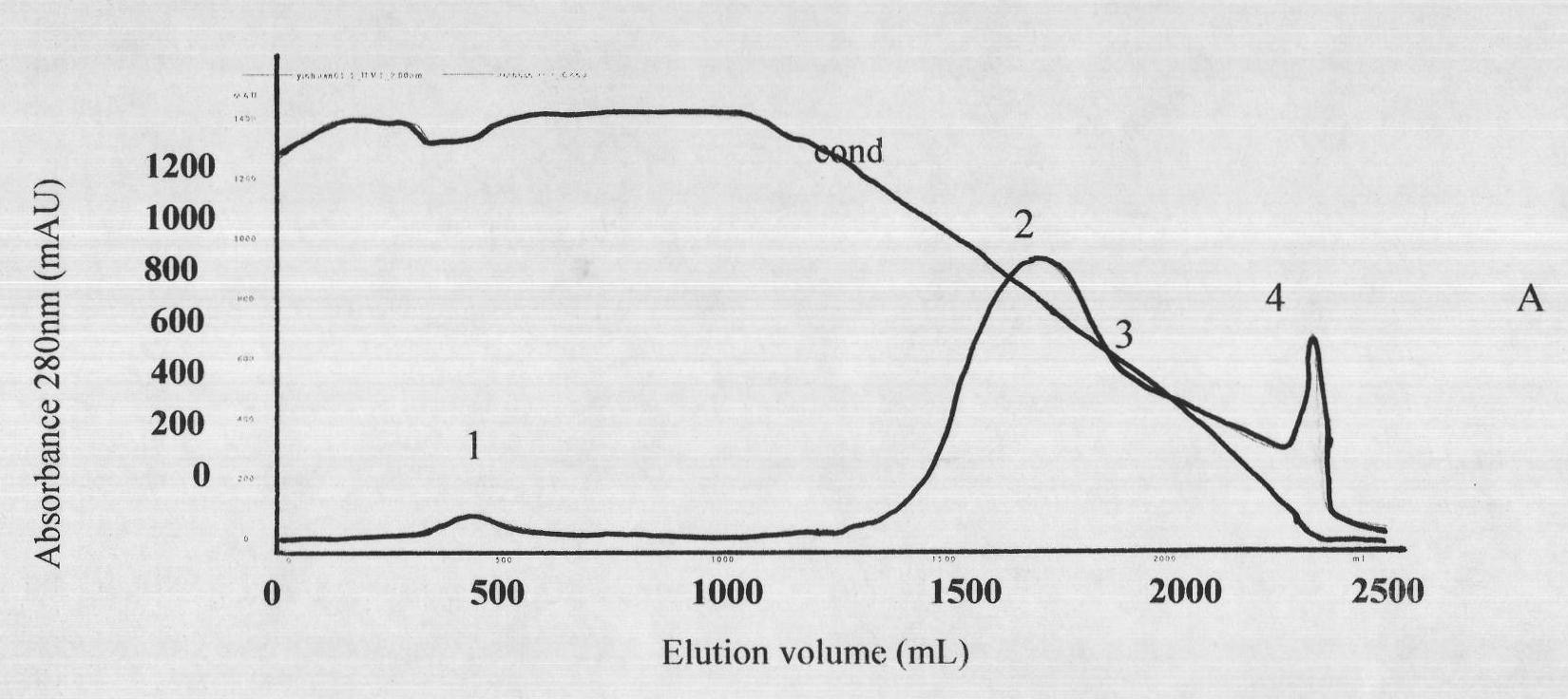
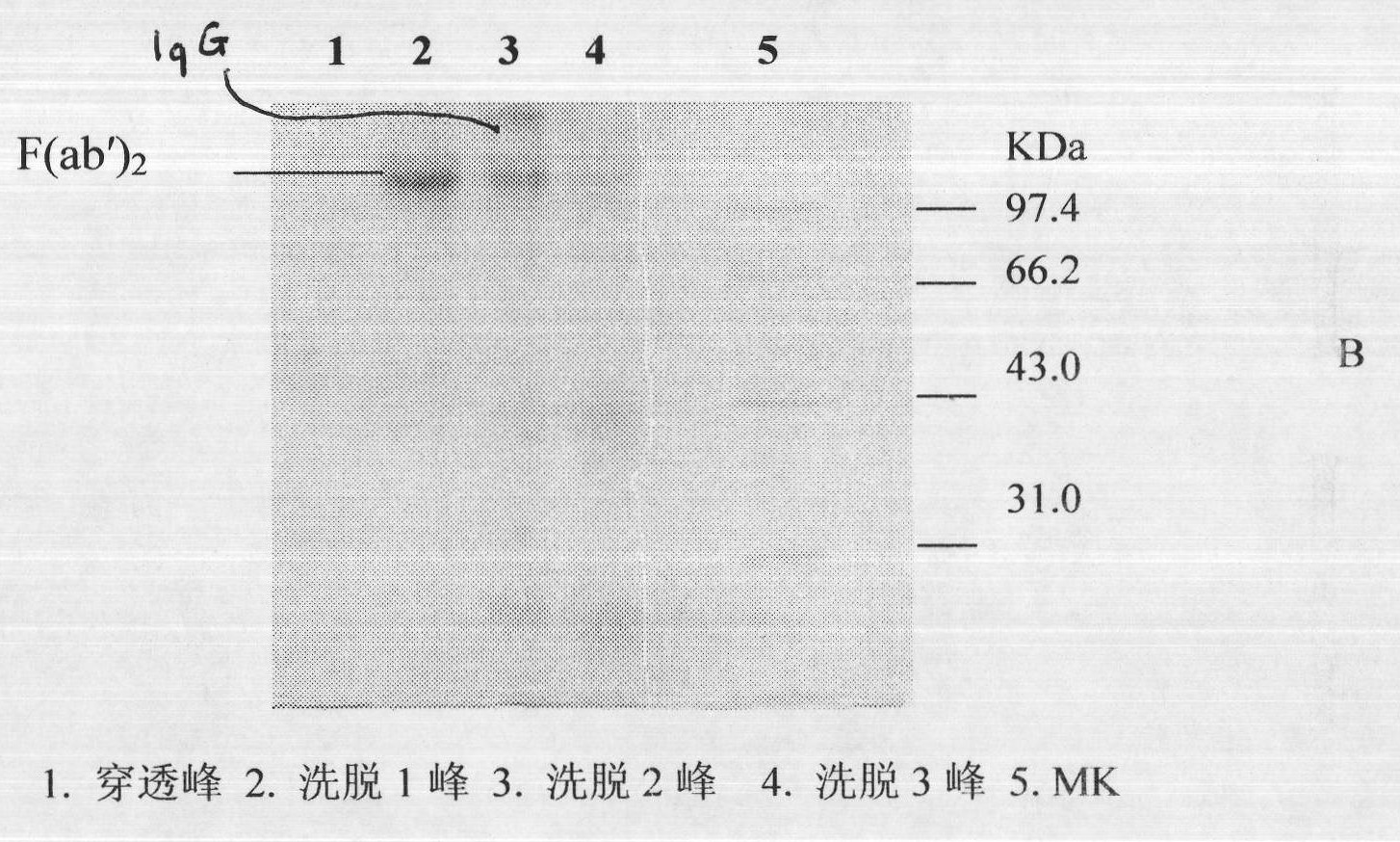
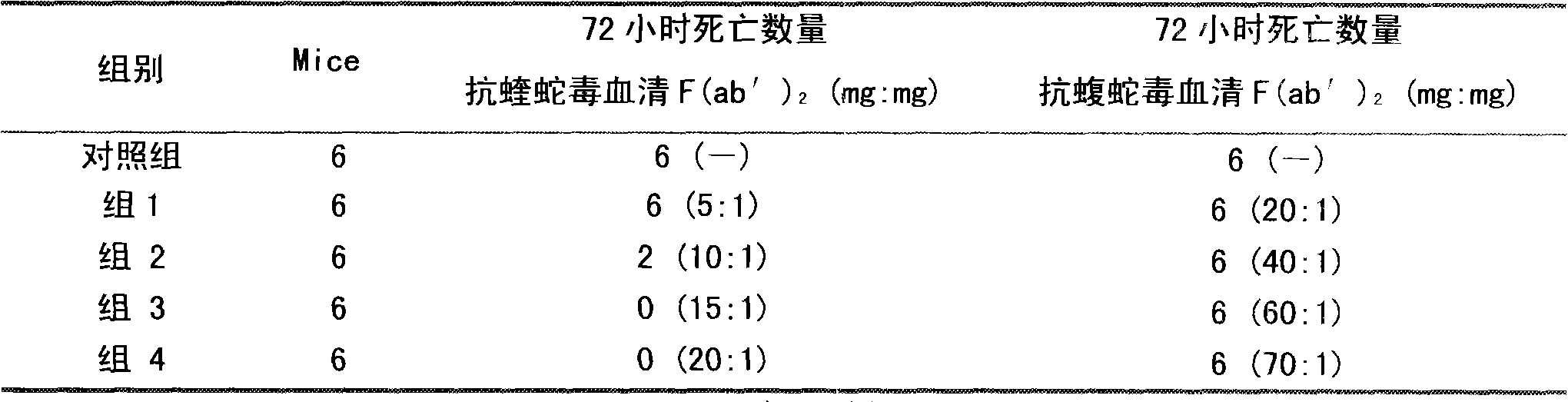
The invention discloses a lyophilized viper antivenin and a preparation method thereof, belonging to biochemical products, more particularly relating to an antivenin and a preparation technology thereof. The mass ratio of the antivenin and viper venom is 15:1, which can specifically neutralize the viper venom injected to mouse; as determined by immunodiffusion, the immunoprecipitation line appears in case that the ratio of viper antivenin F(ab')2 in lyophilized form to viper venom is 8:1; and other detected items conform to the quality standard of antivenin in Chinese Pharmacopoeia 2010. The detection of Phenyl-Sepharose (low-sub) FF column chromatography result shows that: activity is centralized at eluting peak 1, micromolecule impurity proteins are centralized at penetration peal, eluting peak 2 and eluting peak 3. According to the technology of the invention, immune blood plasma is resulted from viper venom immune horse, IgG is prepared by salting out the immune blood plasma, and F(ab')2 active fragment is obtained after the IgG is subject to enzymolysis and purification by a hydrophobic column. The lyophilized viper antivenin has strong specificity, high potency and more than 85% of the purity of antivenin F(ab')2 in lyophilized form.
Owner:浙江健博生物科技股份有限公司
Six-flavor hematinic capsule, its quality control method and application thereof
ActiveCN103381217ARealize authenticationAccurate measurementSenses disorderComponent separationBlurred visionLithospermum
The invention discloses a six-flavor hematinic capsule, its quality control method and an application thereof. The six-flavor hematinic capsule is prepared by adding auxiliary materials into raw materials consisting of Chinese angelica, Ligusticum wallichii, Radix Astragali, prepared rehmannia root, lithospermum and white peony root. According to the quality control method of the six-flavor hematinic preparation, whether the six-flavor hematinic capsule contains white peony root, Chinese angelica, Radix Astragali, prepared rehmannia root, lithospermum, Ligusticum wallichii and components is identified by thin layer chromatography; by high-performance liquid chromatography, in vitro dissolution behavior of the six-flavor hematinic capsule is determined, and effective component groups are identified by fingerprint; verbascoside and calycosin glucoside are used as reference substances to simultaneously determine contents in the six-flavor hematinic capsule; and the content of a volatile component ligustilide in the six-flavor hematinic capsule is determined by a gas chromatographic method. The method provided by the invention is simple to operate, is accurate and advanced, has good linear relation, reappearance, precision, stability and recovery rate, can be adopted to effectively control product quality and guarantee curative effect of the product. The invention also provides an application in the preparation of a medicine for treating eye damage and blurred vision.
Owner:GUANGDONG GUOYUAN GUOYAO PHARMA CO LTD
Method for detecting Jingfang granules
ActiveCN103954724AMonitor qualityGuaranteed curative effectComponent separationDrugLigusticum chuanxiong
The invention relates to a method for detecting Jingfang granules. The method comprises the steps of character identifying, checking and measuring content, wherein the step of identifying comprises identification of schizonepeta, radix angelicae pubescentis, ligusticum wallichii, fructus aurantii and bupleurum longiradiatum; the step of measuring content refers to a step of measuring content of ephedrine hydrochloride. Aiming at the problem that the condition that fewer raw materials are added or corresponding raw materials are not added by illegal manufacturer or a counterfeit drug bupleurum longiradiatum is mixed cannot be monitored because active ingredients of the granules cannot be correspondingly detected by a set of perfect detection method at present, a scientific, reasonable, practical and feasible component identification and content measurement method is formulated, the clinical effects of the Jingfang granules are guaranteed, quality indexes of several medicinal materials are clear, and application of various medicinal materials in the Jingfang granules is ensured, so that the curative effect of the drug is guaranteed. Moreover, the counterfeit drug bupleurum longiradiatum in radix bupleuri can be effectively detected, and a phenomenon that health of patients is endangered by two toxic ingredients, including bupleurotoxin and acetylbupleurotoxin, contained in bupleurum longiradiatum is avoided.
Owner:九寨沟天然药业股份有限公司
Compound dendrobium candidum blood-nourishing and liver-softening capsule and preparation method thereof
InactiveCN101703695AKeep active ingredientsSave solventDigestive systemCapsule deliverySalvia miltiorrhizaLycium barbarum fruit
The invention provides a dendrobium candidum blood-nourishing and liver-softening capsule and a preparation method thereof, wherein the dendrobium candidum blood-nourishing and liver-softening capsule takes seven types of traditional Chinese medicines of dendrobium candidum, radix rehmanniae, Chinese angelica, white paeony root, salvia miltiorrhiza bunge, barbary wolfberry fruit and adenophora stricta as raw materials. The main technique comprises the following steps: firstly crushing the radix rehmanniae and the white paeony root into powder for later use, combining and mixing evenly the extracts of the Chinese angelica, the dendrobium candidum, the barbary wolfberry fruit and the like, reducing the pressure under low temperature, drying and filling into a capsule. The invention can nourish the blood and soften the liver, and has the advantages of stable manufacture technique, simple operation, short production cycle and the like.
Owner:浙江森宇实业有限公司
Detection method of common goldenrop particles
InactiveCN103954723AQuality reflectionEasy to useComponent separationBupleurum longiradiatumClinical efficacy
The invention relates to a detection method of common goldenrop particles. The detection method comprises inspection items of identifying characters and measuring content. The identification item comprises identification of scutellaria baicalensis, identification of liquorice, identification of radix bupleuri and identification of bupleurum longiradiatum; the item of measuring content is used for measuring the content of baicalin. In order to solve the problem of incapability of monitoring insufficiency or absence of corresponding raw materials of illegal manufacturers and monitoring the interfusion of bupleurum longiradiatum caused by the lack of one set of complete detection method for detecting active ingredients of common goldenrop particles at present, the method disclosed by the invention can be used for identifying components and measuring content scientifically, reasonably and feasibly; the method is capable of guaranteeing the clinical efficacy of common goldenrop particles, making clear quality indexes of several medicine materials and guaranteeing the use of all medicine materials in the common goldenrop particles, thereby guaranteeing the curative effect of the common goldenrop particles; in addition, bupleurum longiradiatum serving as a counterfeit drug of radix bupleuri can also be effectively detected, thereby avoiding the damage to the health of patients caused by two toxic ingredients comprising radix bupleuri toxin and acetyl radix bupleuri toxin in bupleurum longiradiatum.
Owner:SICHUAN FENGCHUN PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com