Memantine hydrochloride slow-release pill and preparation method thereof
A technology of memantine hydrochloride and sustained-release pills, which is applied in sugar-coated pills, pharmaceutical formulas, and devices that make drugs into special physical or ingestible forms, and can solve problems such as being easily affected by humid environments Burst release, improved stability, guaranteed curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: In order to study the impact of the isolation layer on the dissolution of the sustained-release capsule under high humidity conditions, two kinds of pellets were prepared, namely, drug-containing pellet A without isolation layer (or isolation coating layer) and drug-containing pellet B with isolation layer , and then poured into the capsules to form corresponding slow-release capsules A and slow-release capsules B, and put them into a closed container with a relative humidity of 92.5% to investigate the dissolution results in 5 and 10 days.
[0042] One, the preparation of sustained-release capsule A
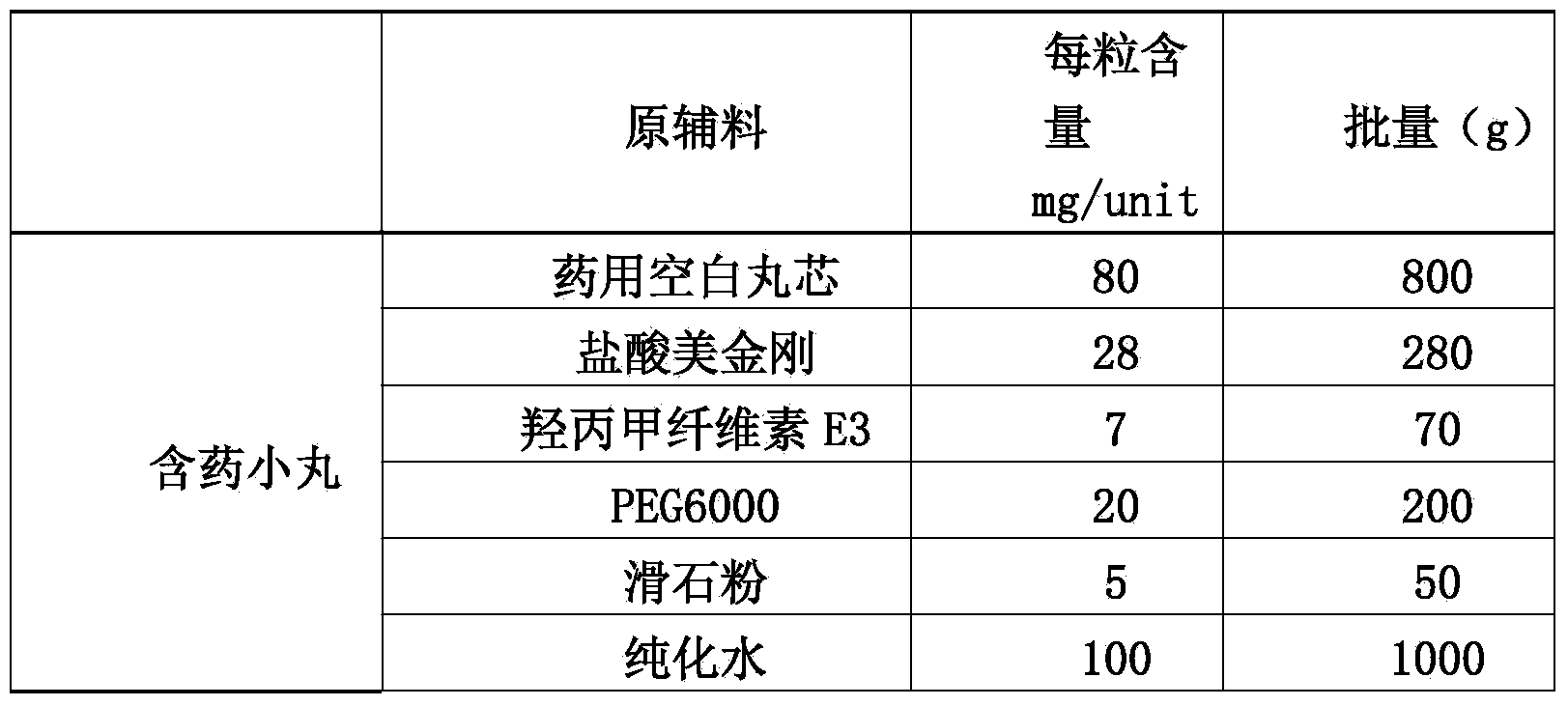
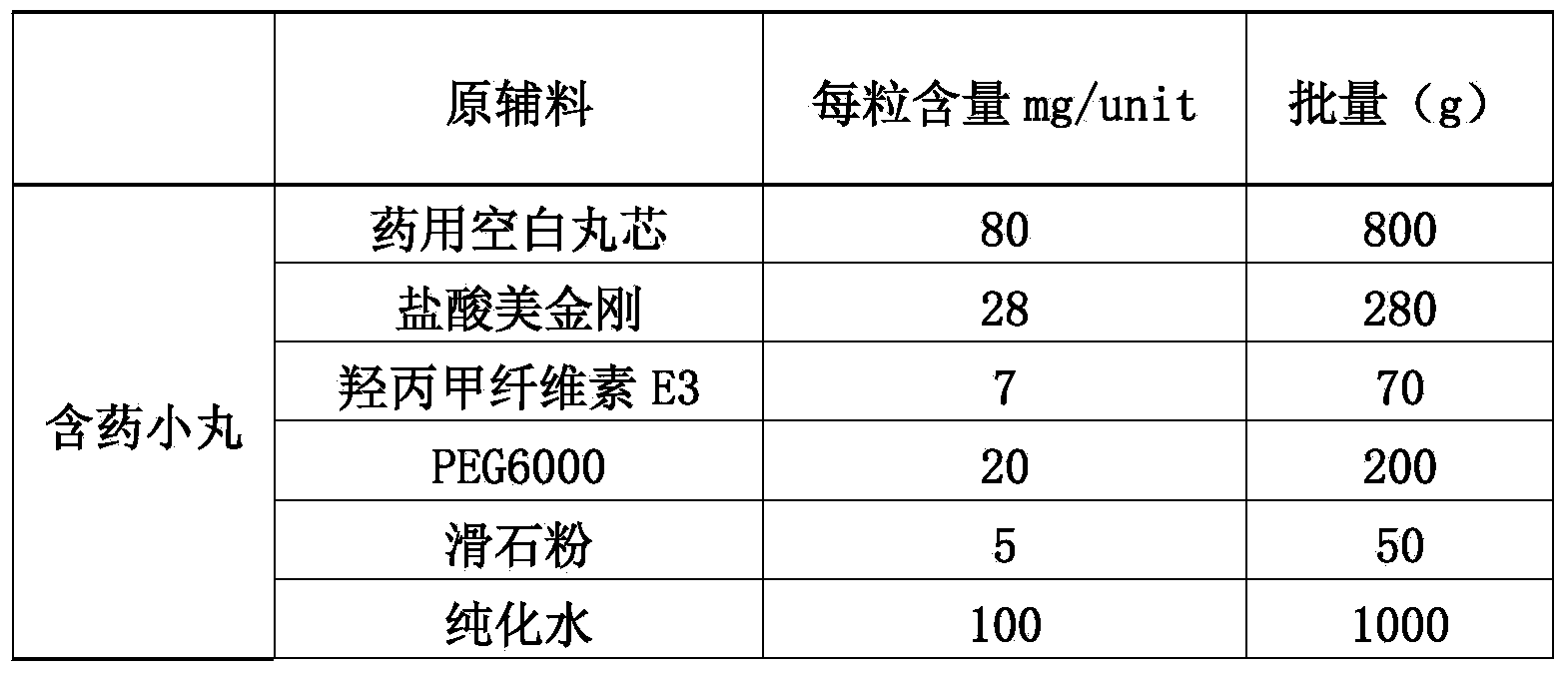
[0043] (1) Preparation of drug-containing pellets of memantine hydrochloride:
[0044]
[0045] 1.1 Weigh hypromellose E3 into a container filled with purified water at 80°C to 90°C, stir to disperse, then add cold purified water, and stir while adding, to obtain I;
[0046] 1.2 Put polyethylene glycol 6000 and memantine hydrochloride in a container, add...
Embodiment 2
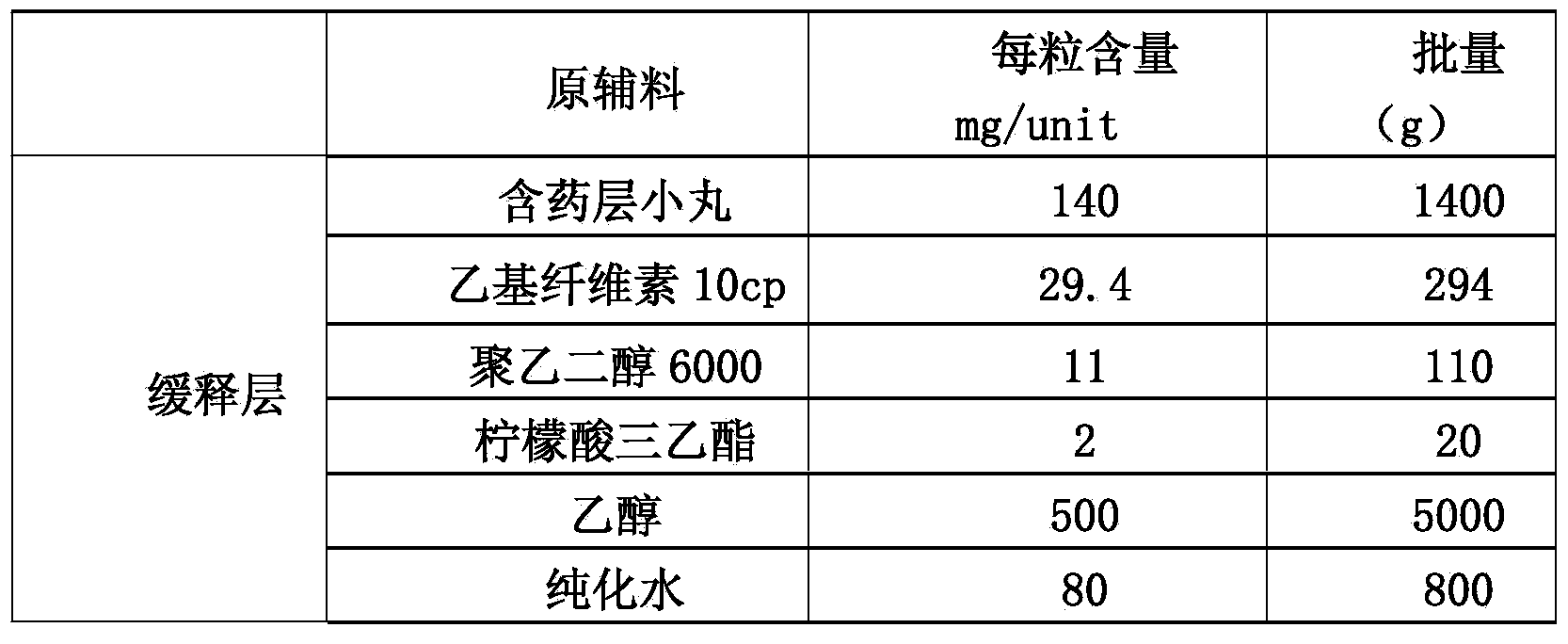
[0083] Embodiment 2: the preparation method of memantine hydrochloride sustained-release capsules:
[0084] (1): Preparation of main drug layer pellets containing memantine hydrochloride (i.e. drug-containing pellets):
[0085]
[0086] 1.1 Weigh hydroxypropyl cellulose and add it to a container filled with purified water at 80°C to 90°C, stir to disperse, then add cold purified water, and stir while adding to obtain I.
[0087] 1.2 Put polyethylene glycol and memantine hydrochloride in a container, add purified water to dissolve, and obtain II.
[0088] 1.3 Then add (I) into (II), stir evenly, then add talcum powder while stirring, stir to disperse the talc powder evenly, pass through an 80-mesh sieve, keep stirring gently with the agitator, and obtain the drug-containing coating solution for later use.
[0089] 1.4 Put the blank pellet core into the material cylinder of the fluidized bed by manual feeding, start heating, and when the temperature of the material is kept a...
Embodiment 3
[0104] Embodiment 3: the preparation method of memantine hydrochloride sustained-release capsules
[0105] (1) Preparation of main drug layer pellets:
[0106]
[0107] 1.1 Weigh hydroxypropyl cellulose and add it to a container filled with purified water at 80°C to 90°C, stir to disperse, then add cold purified water, and stir while adding to obtain I.
[0108] 1.2 Put polyethylene glycol and memantine hydrochloride in a container, add purified water to dissolve, and obtain II.
[0109] 1.3 Then add (I) into (II), stir evenly, then add talcum powder while stirring, and stir to disperse the talcum powder evenly. Pass through an 80-mesh sieve and keep stirring gently with the agitator to obtain a drug-containing coating solution for later use.
[0110] 1.4 Put the blank pellet core into the material cylinder of the fluidized bed by manual feeding, start heating, and when the temperature of the material is kept above 35°C, turn on the atomization pressure (1.6BAR), adjust t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com