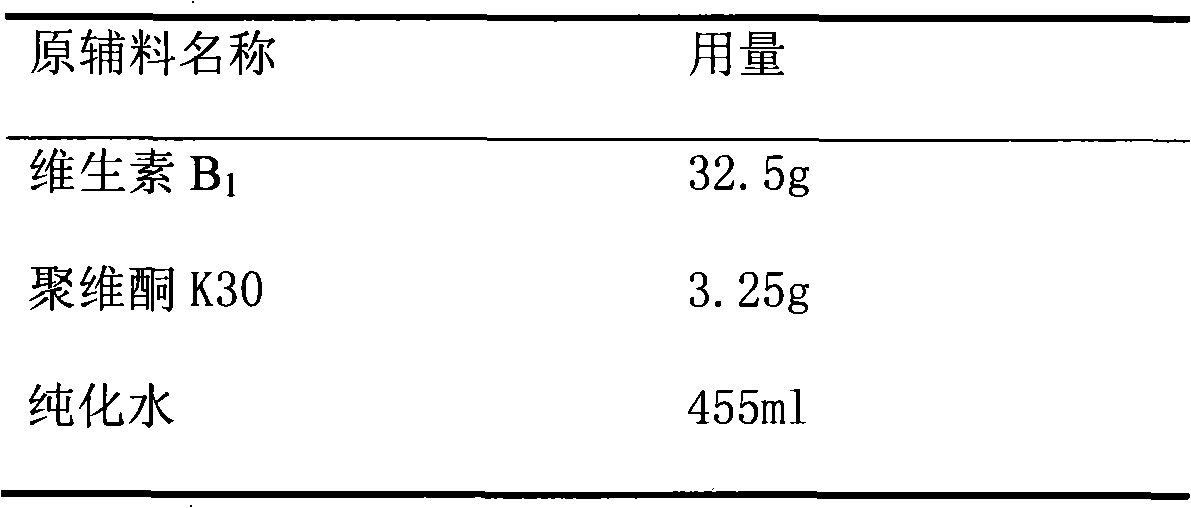
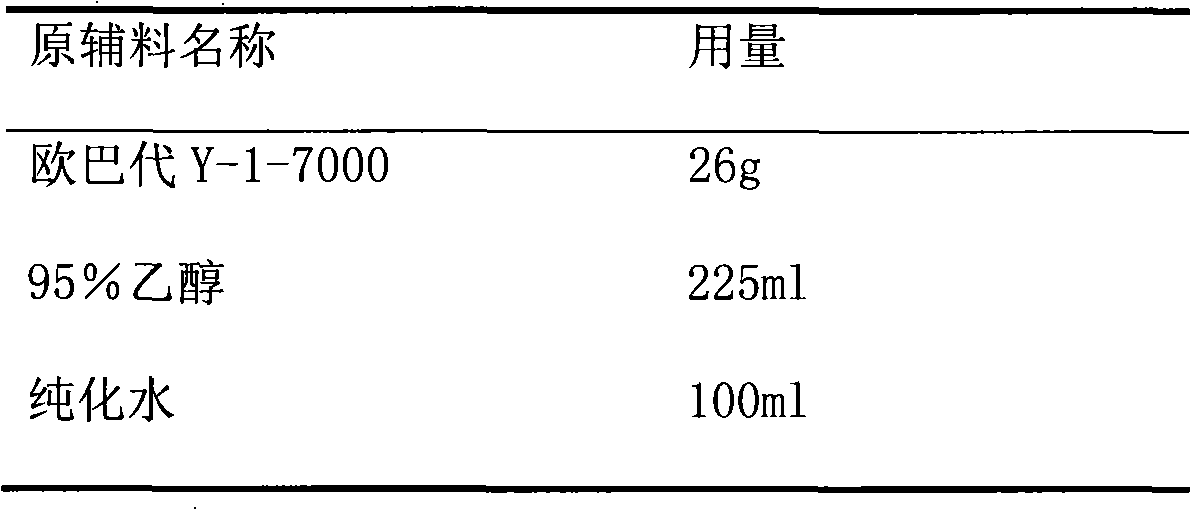
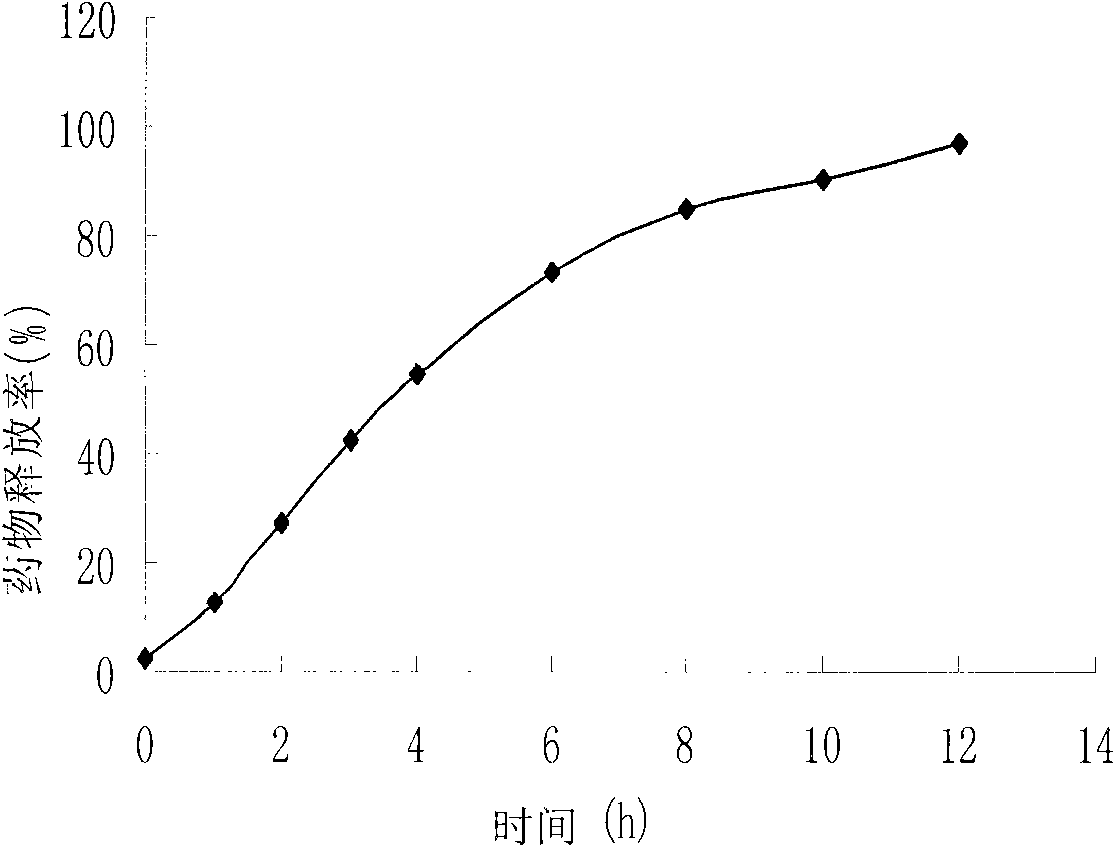
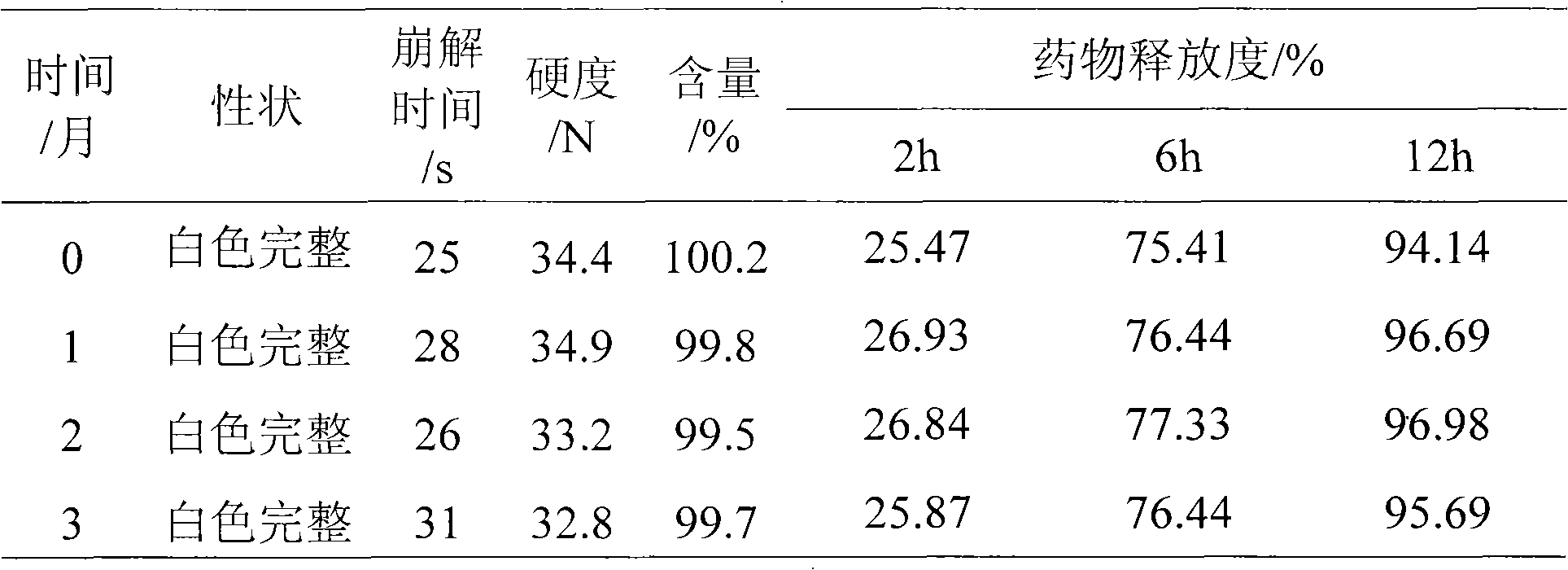
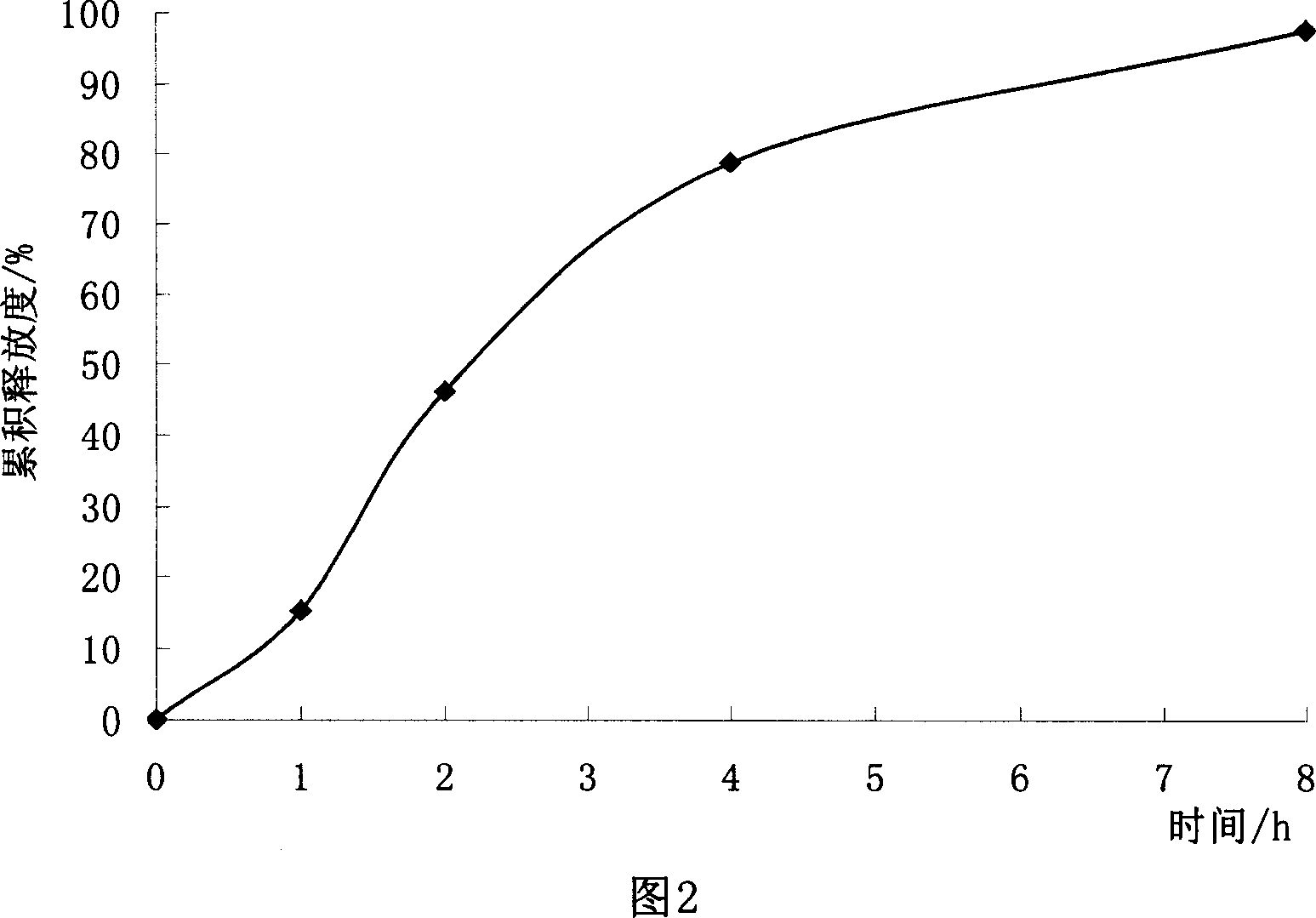
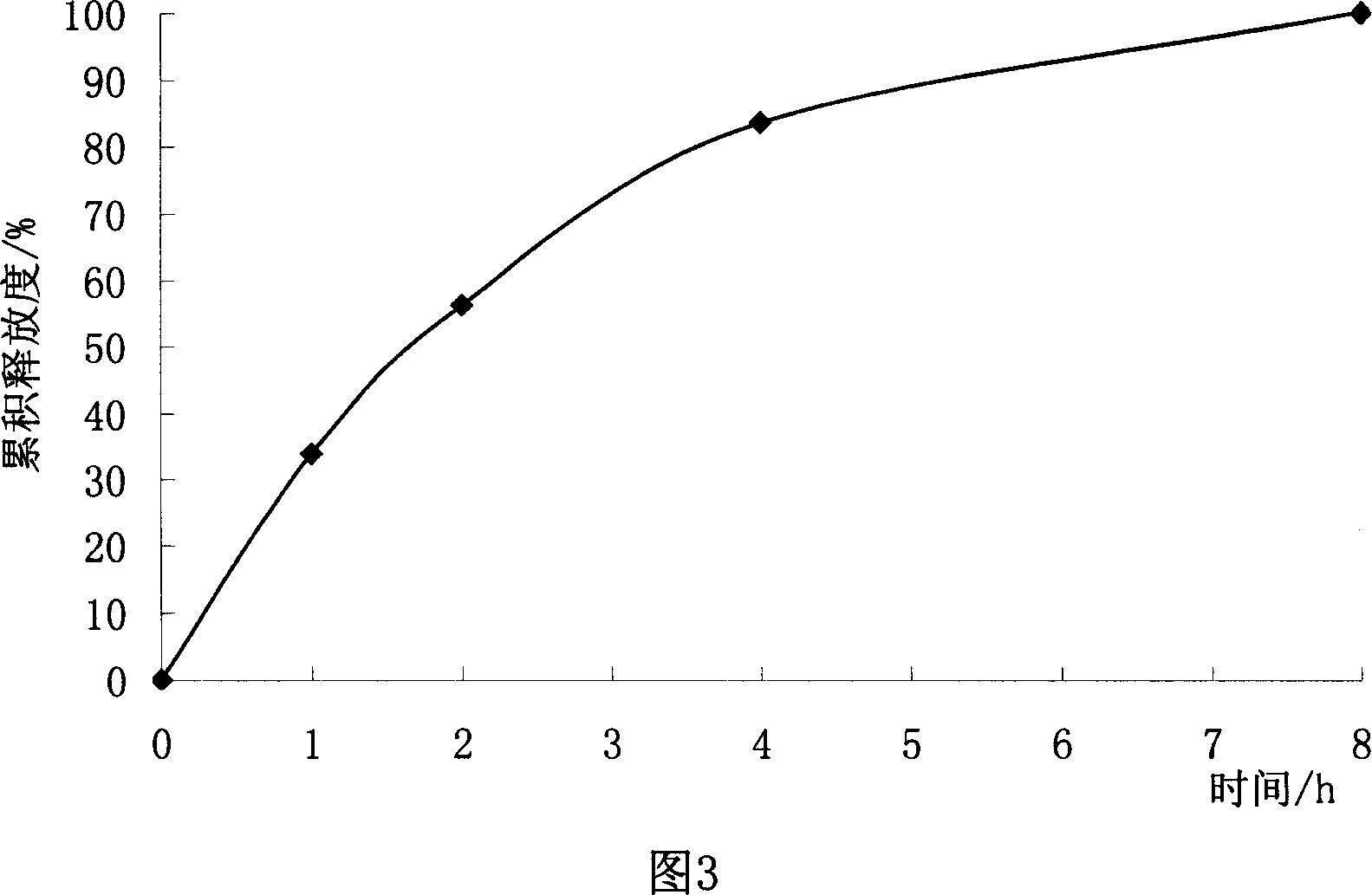
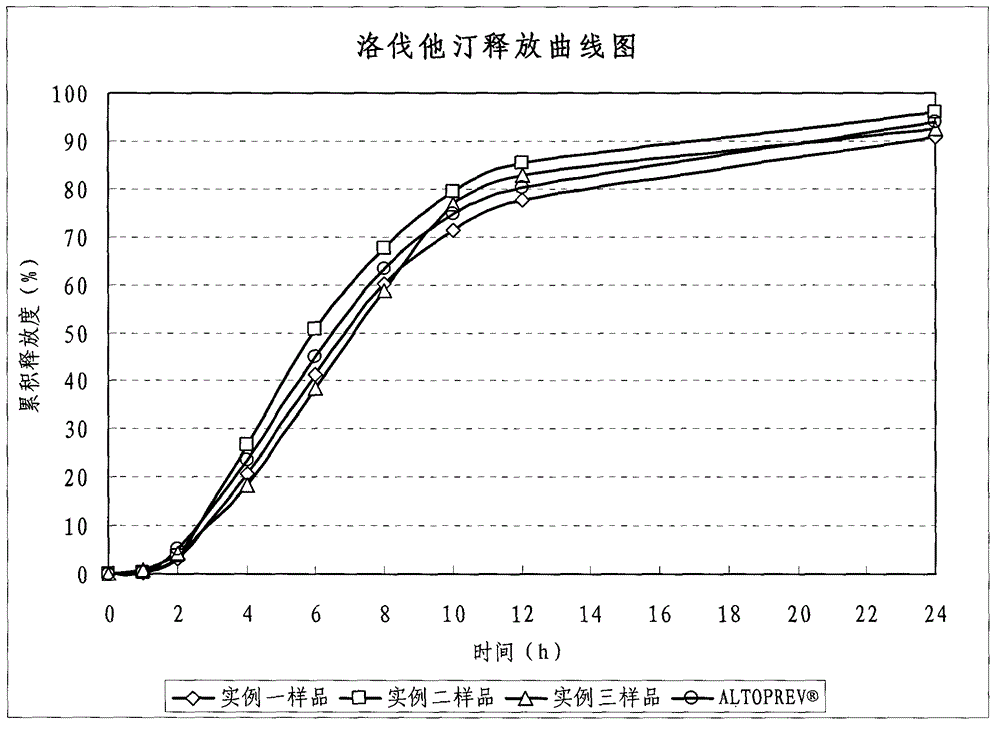
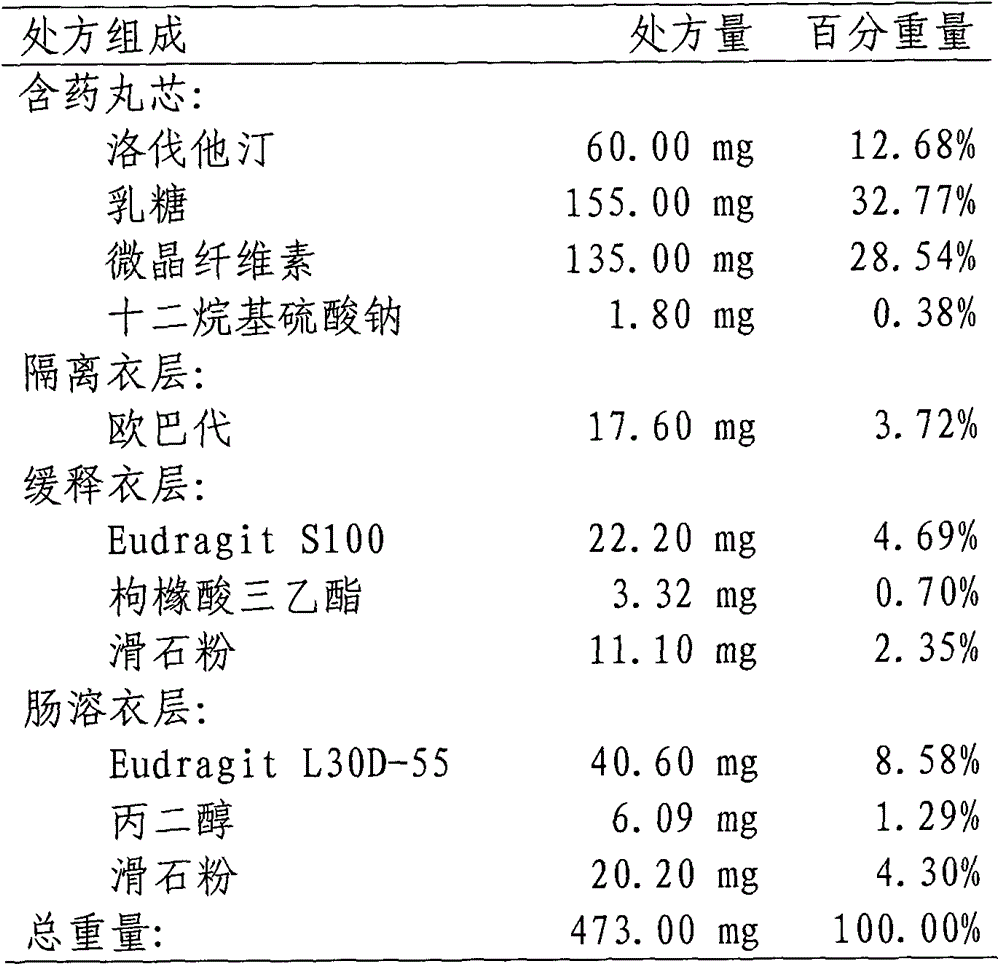
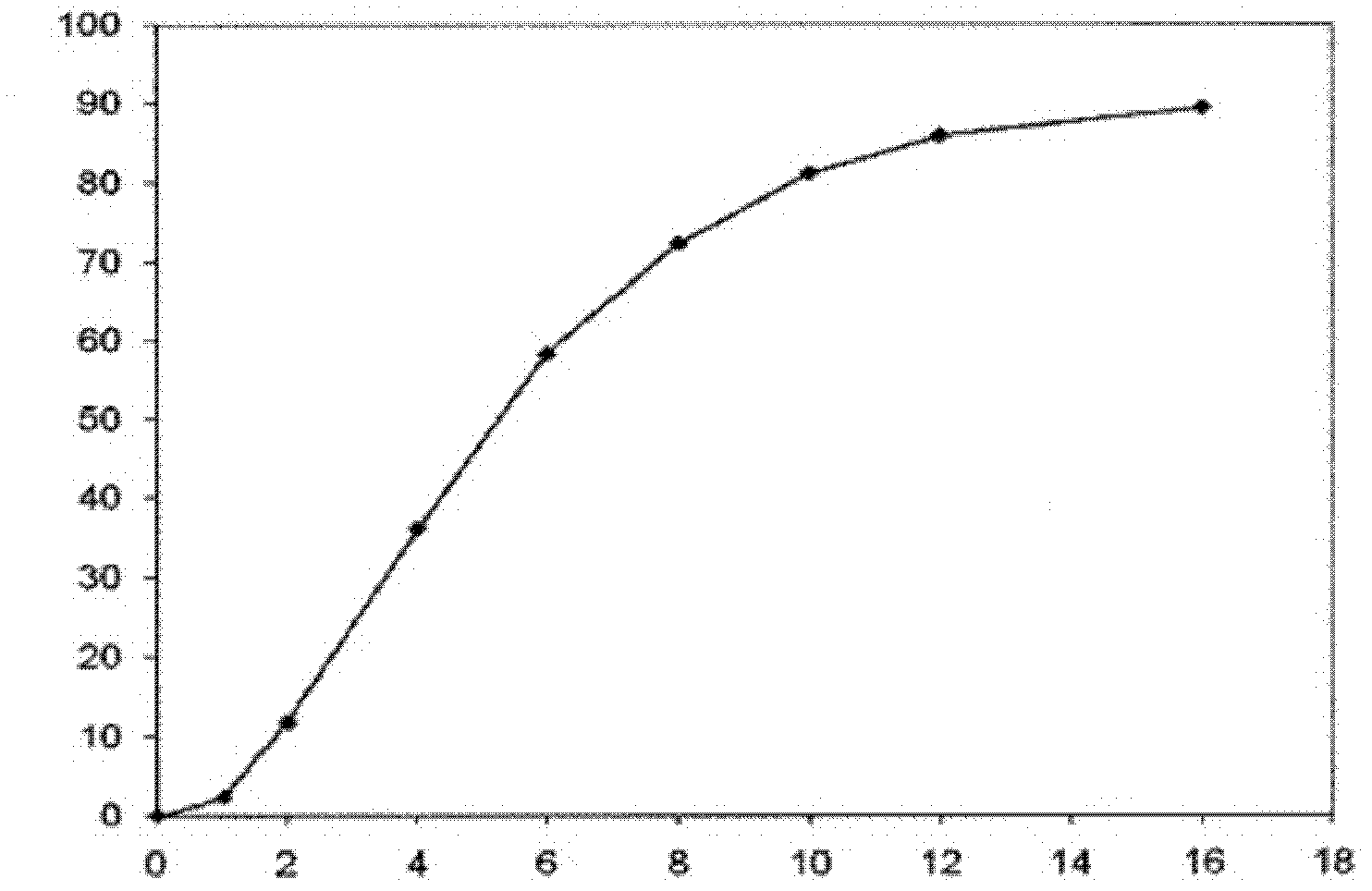
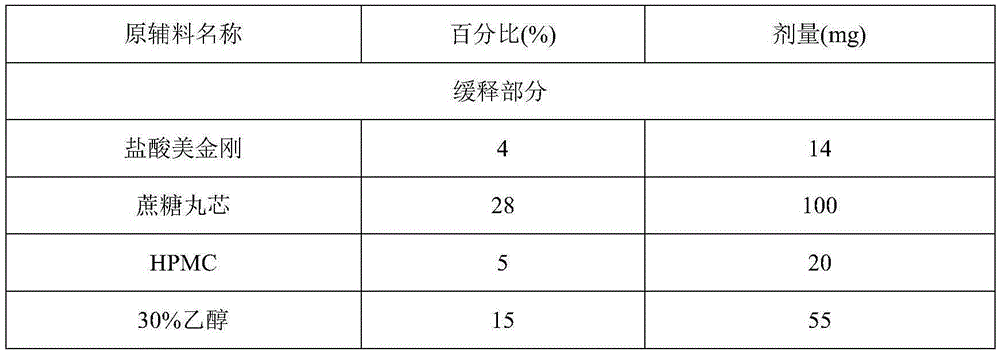
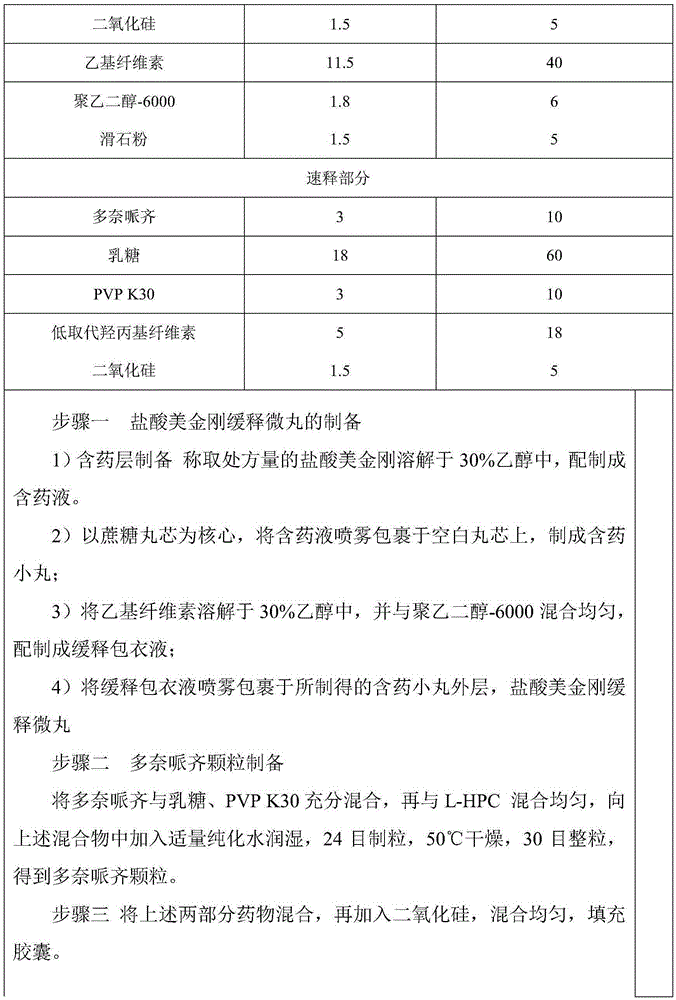
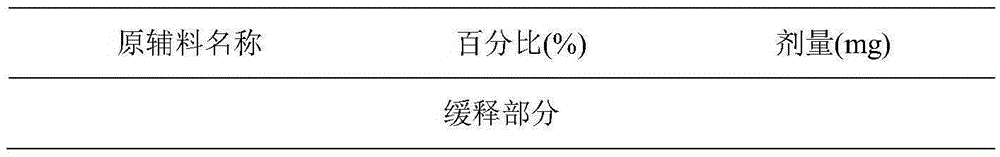
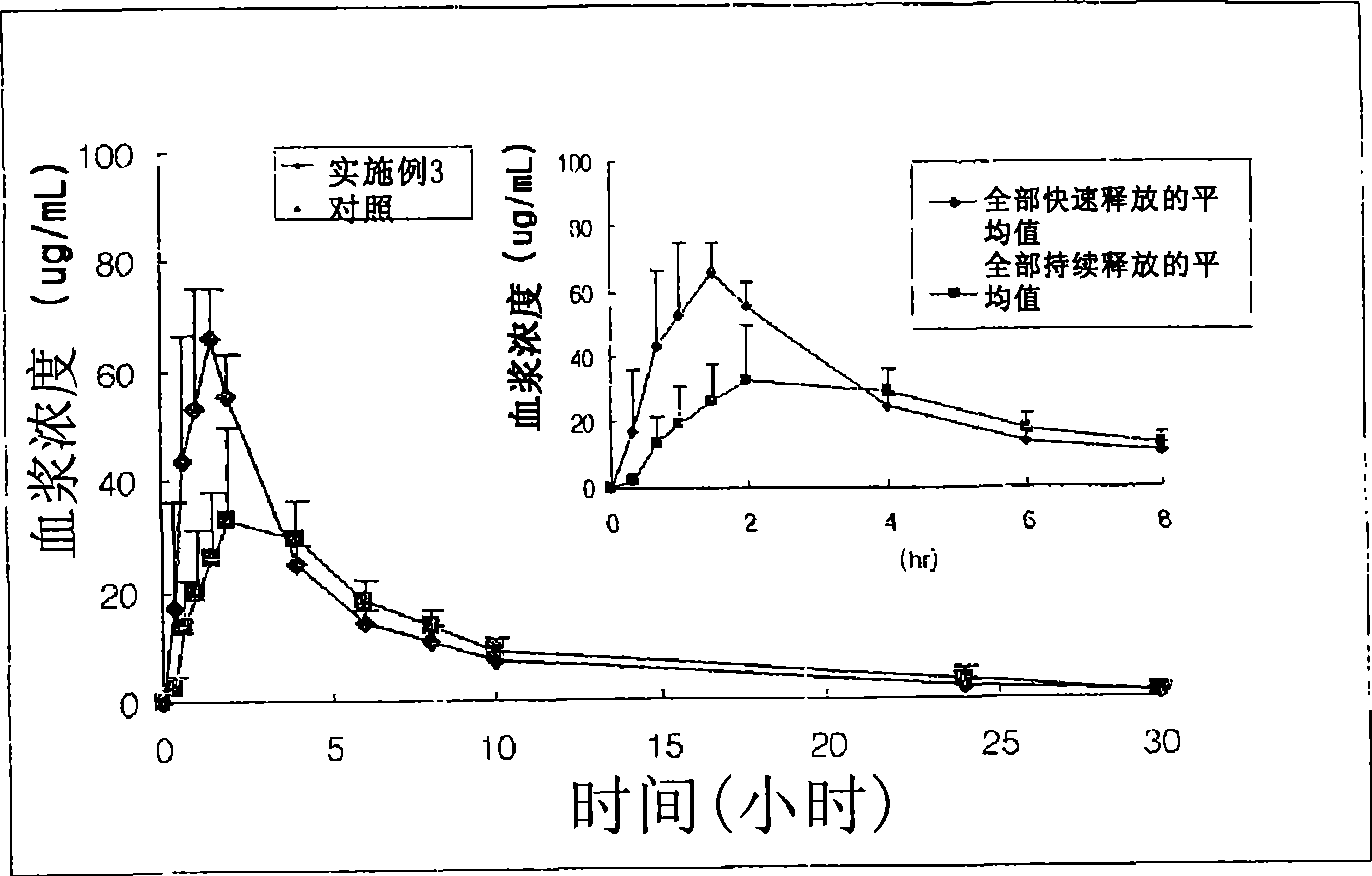
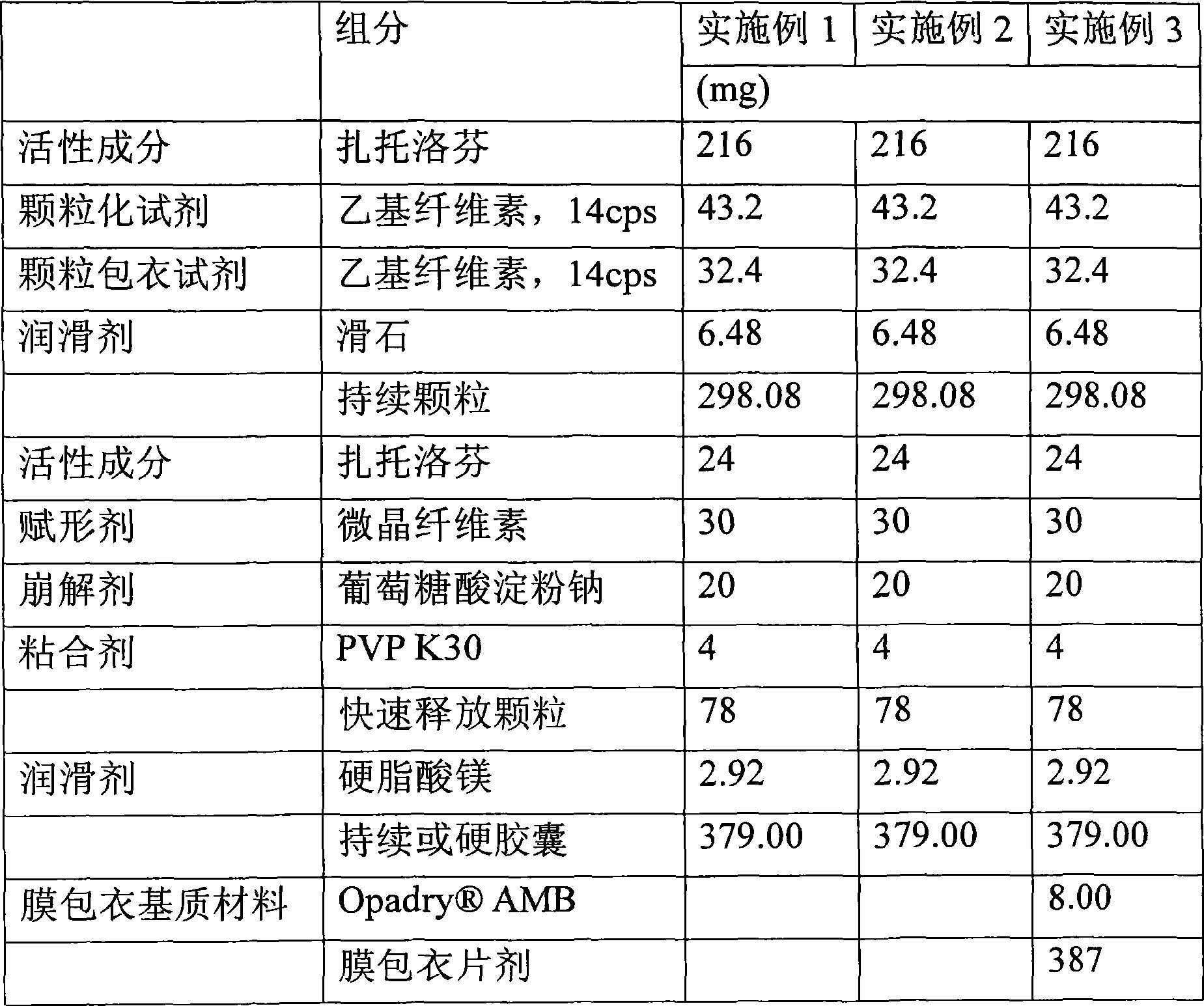
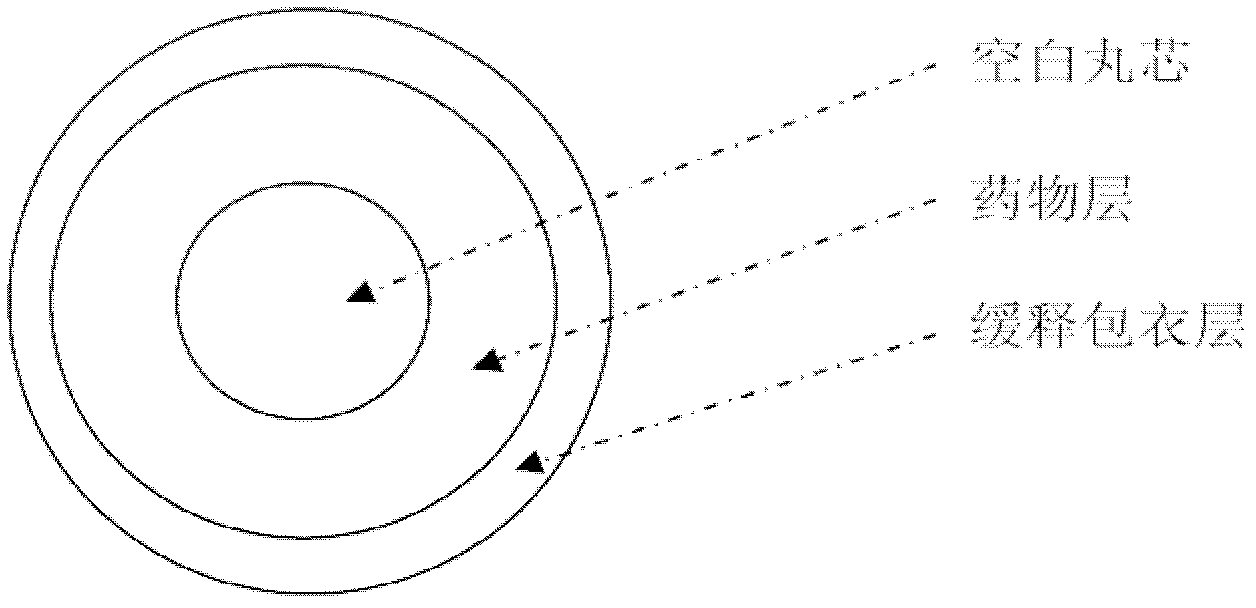
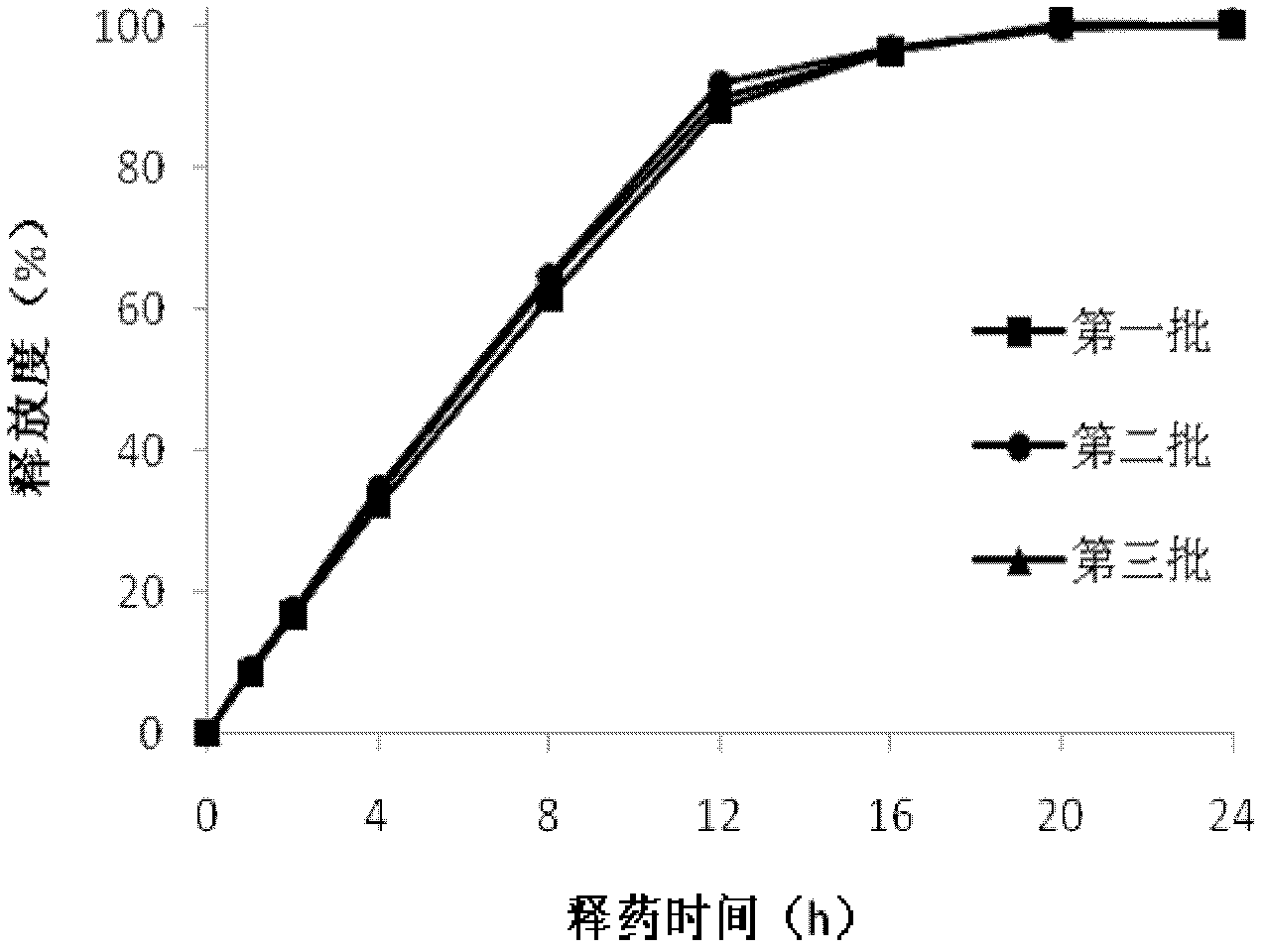
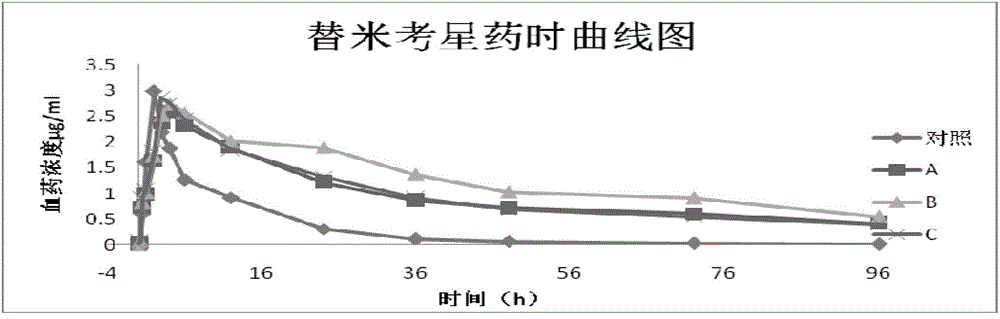
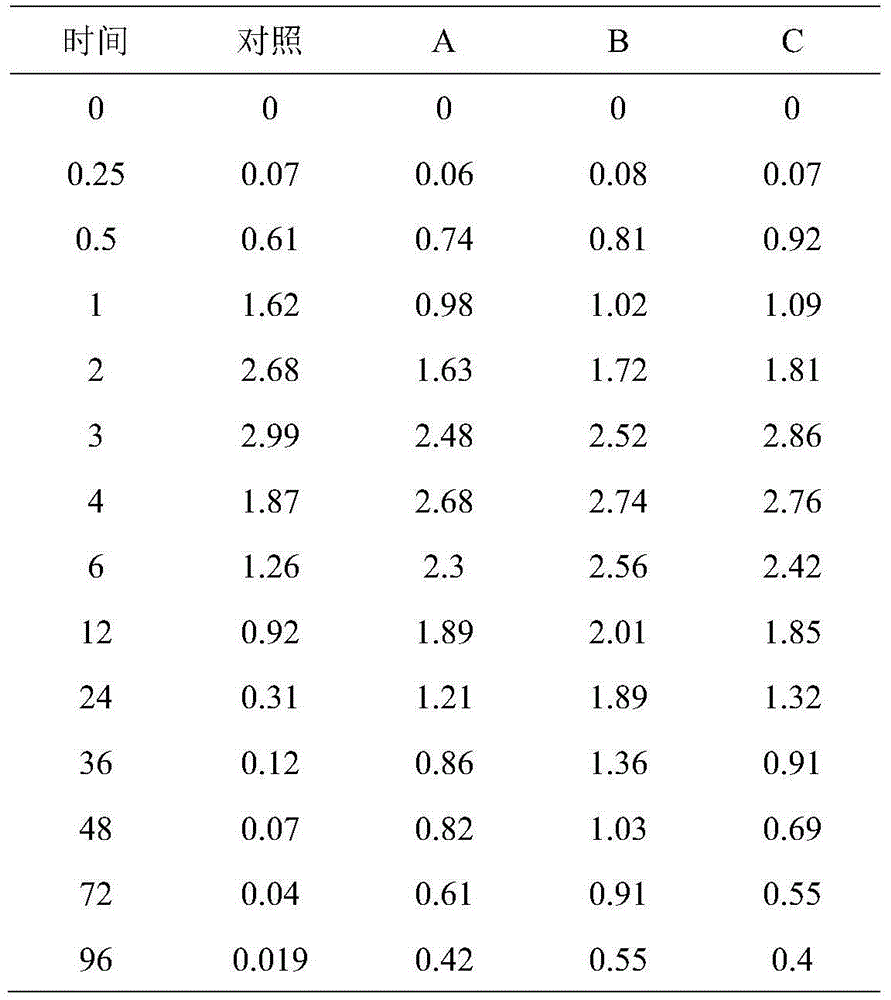
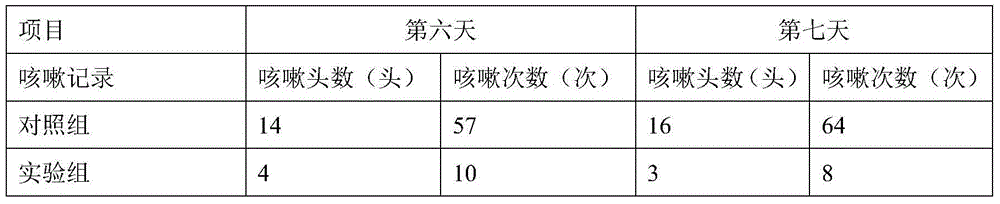
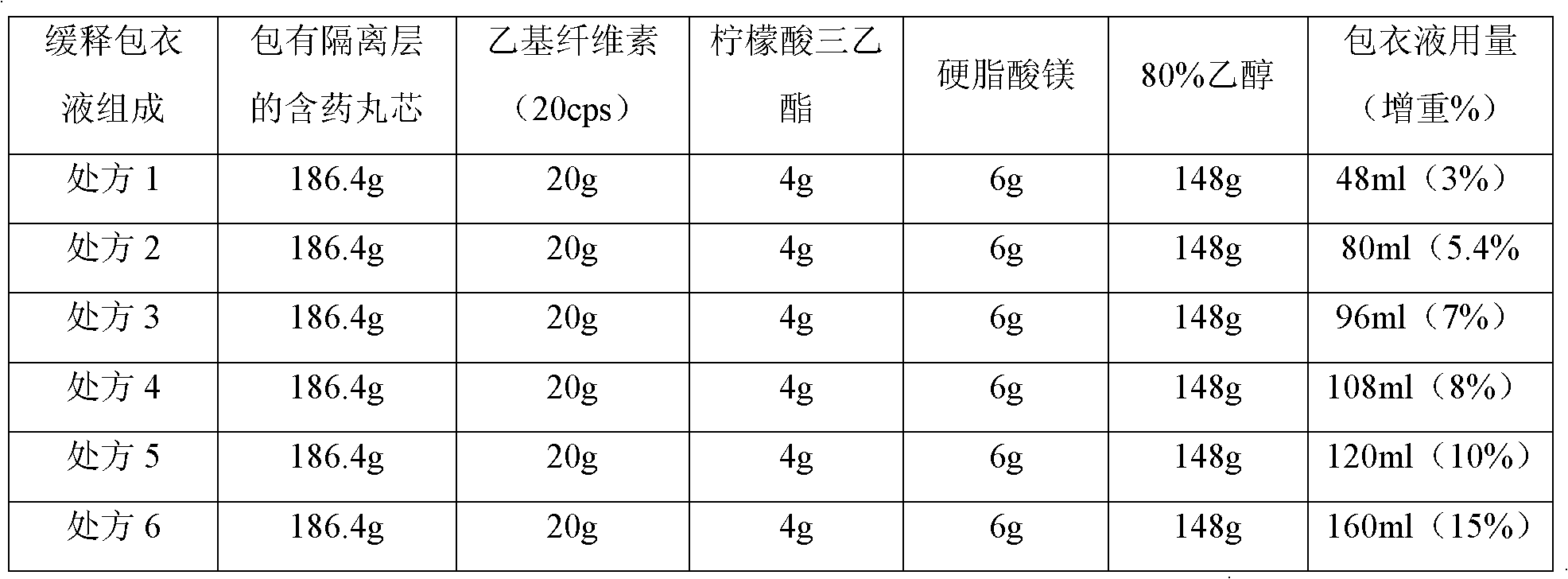
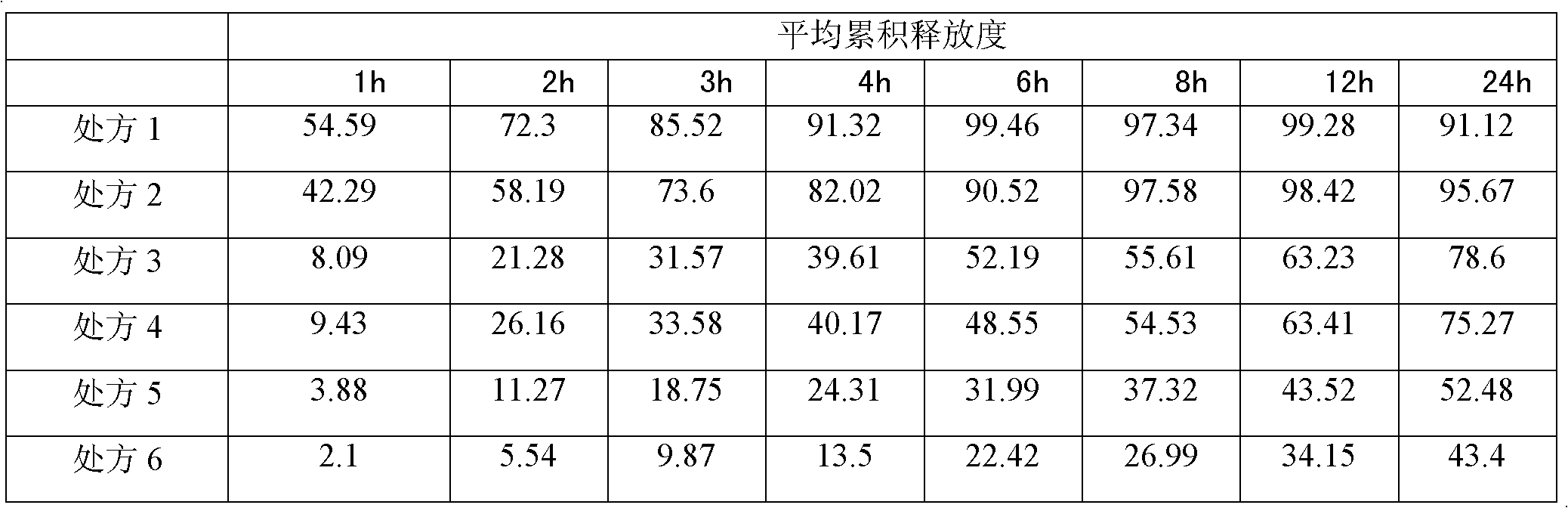
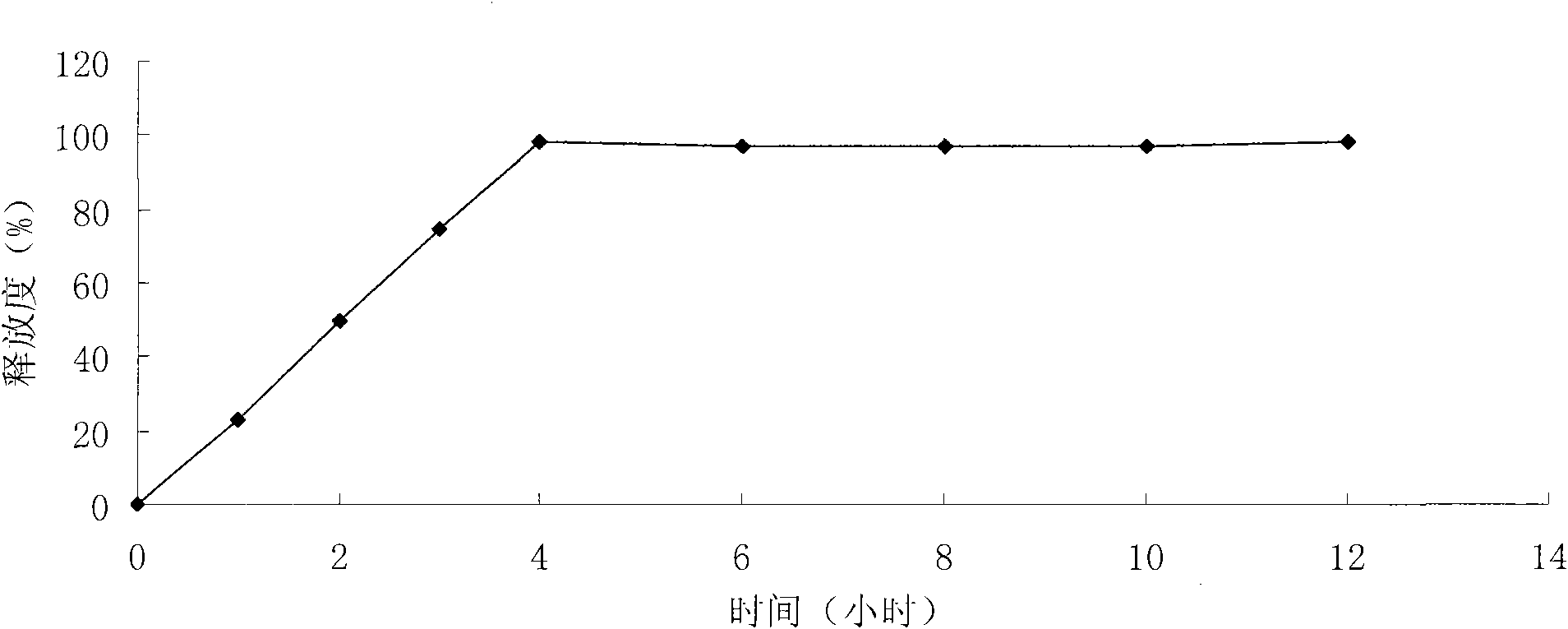
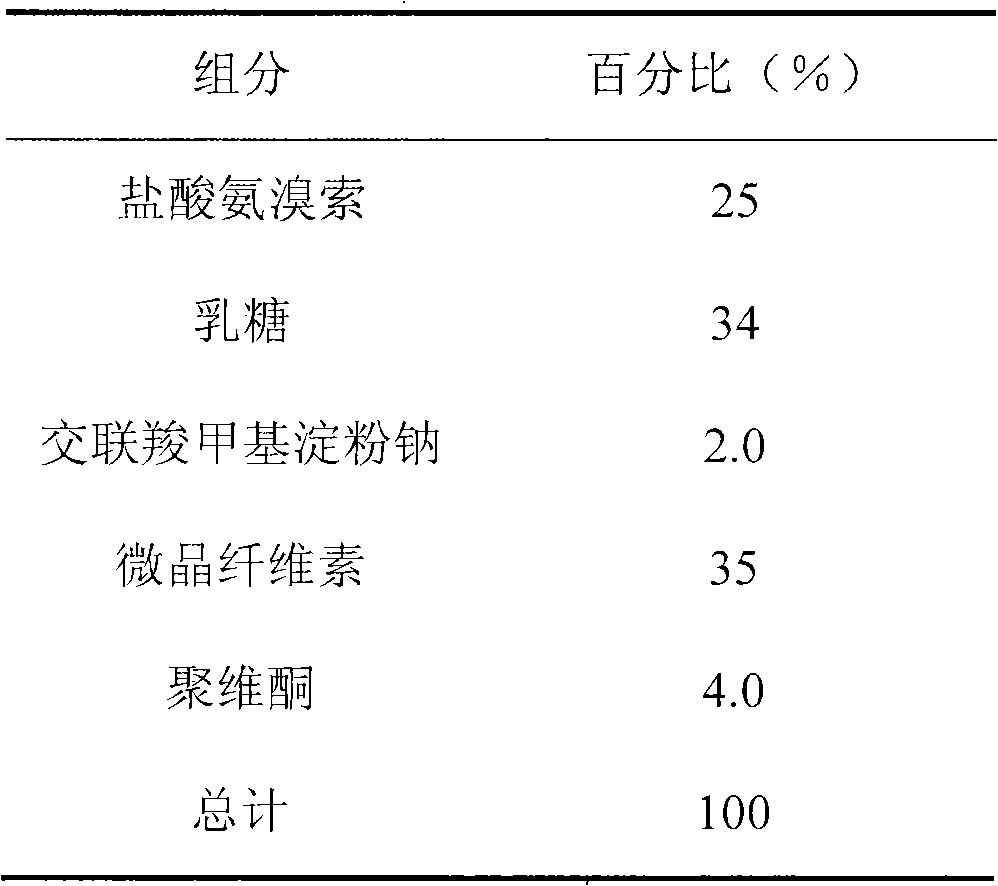
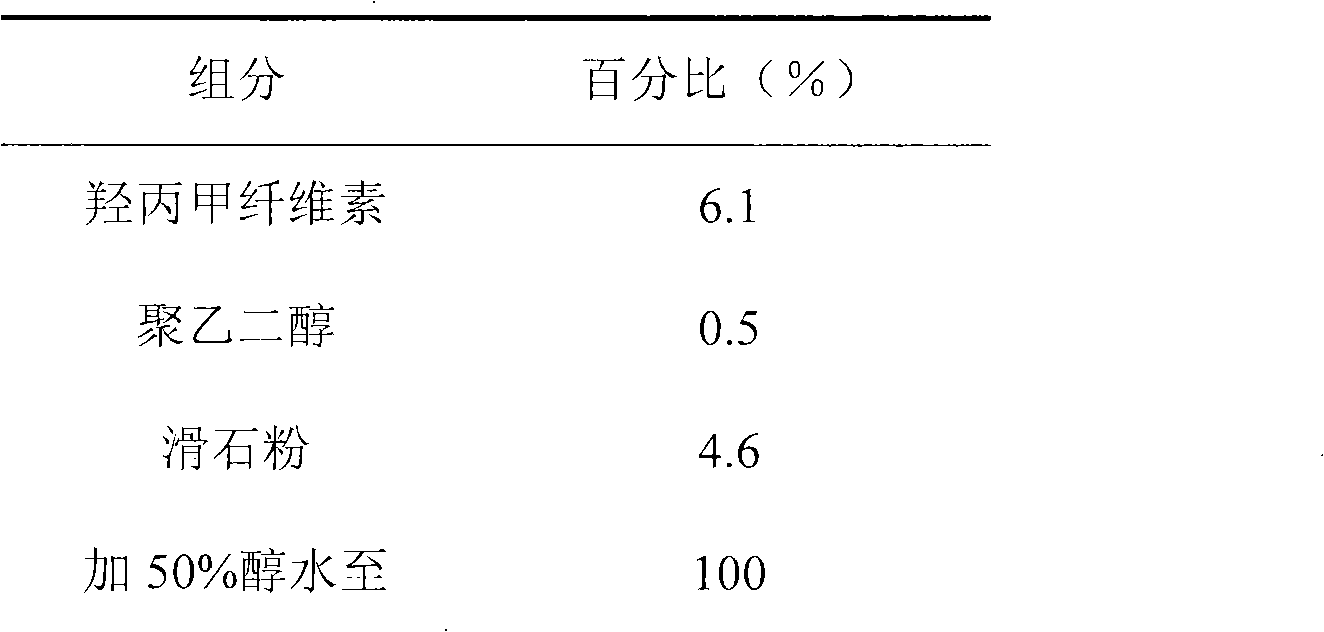
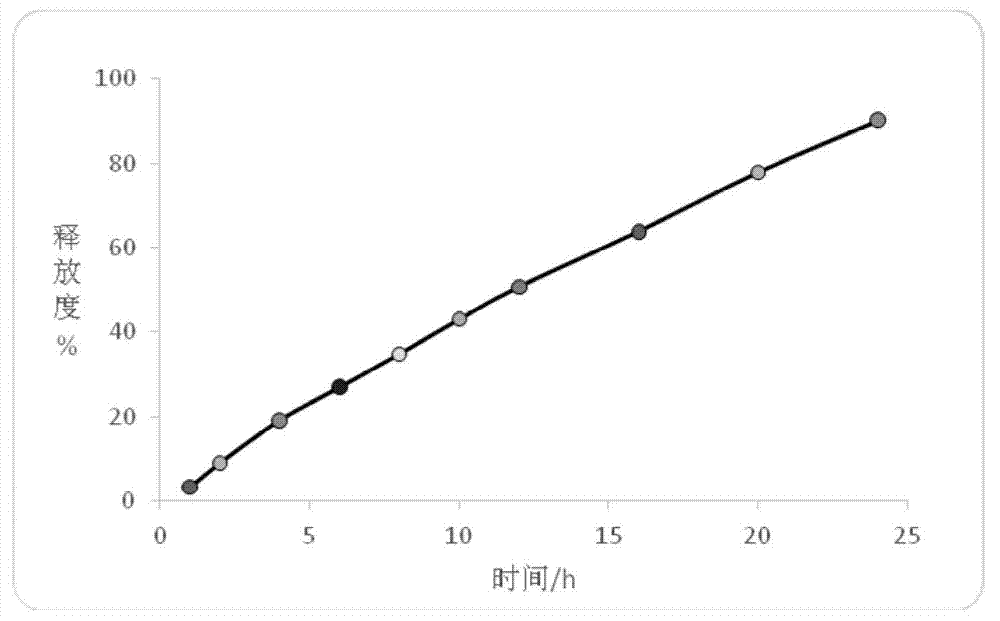
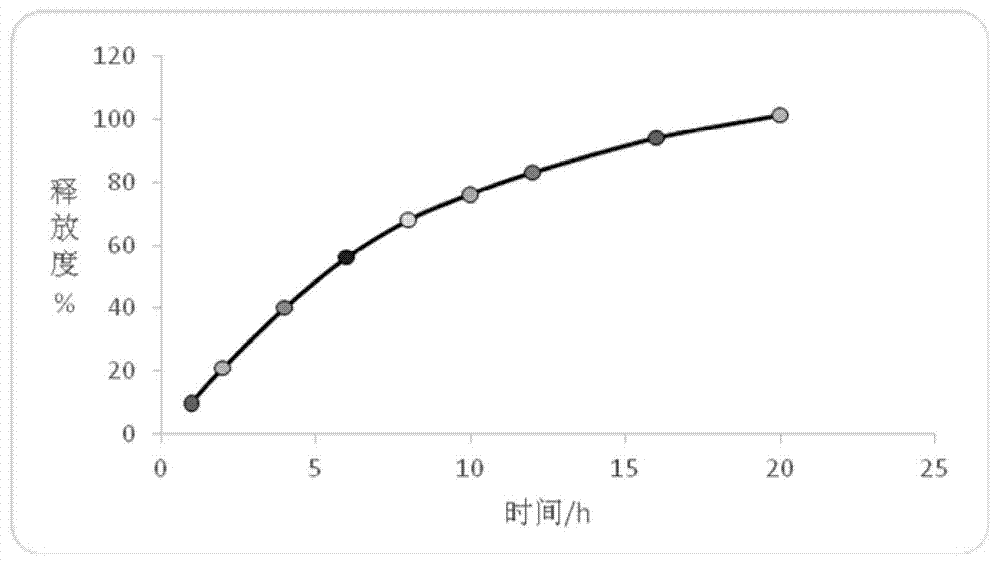
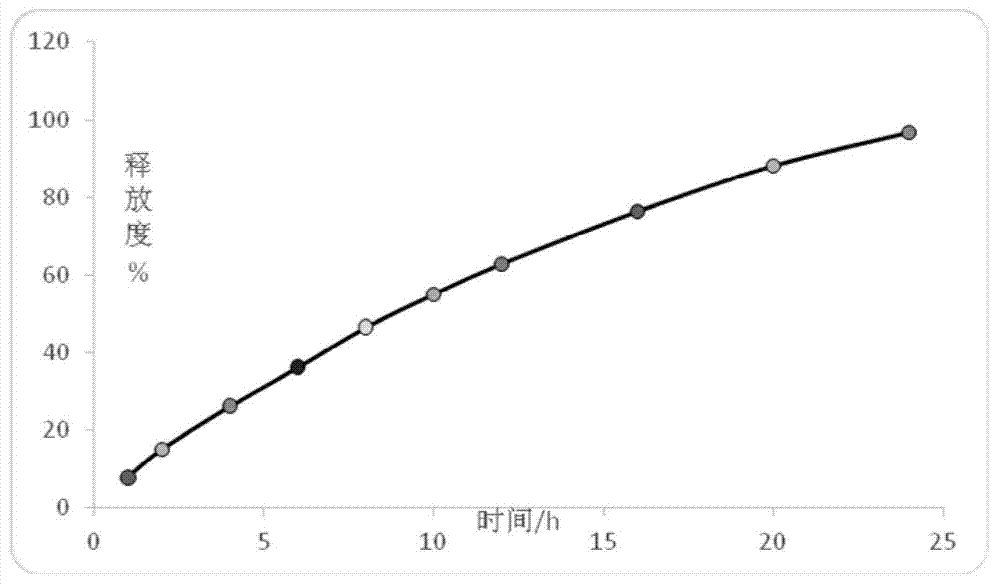
Patents
Literature
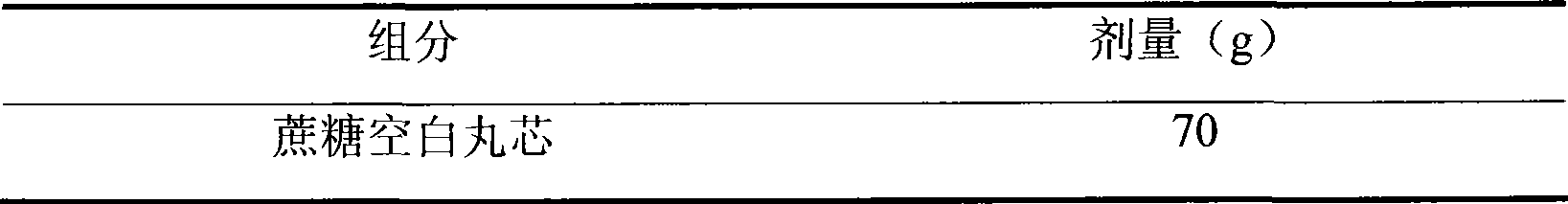
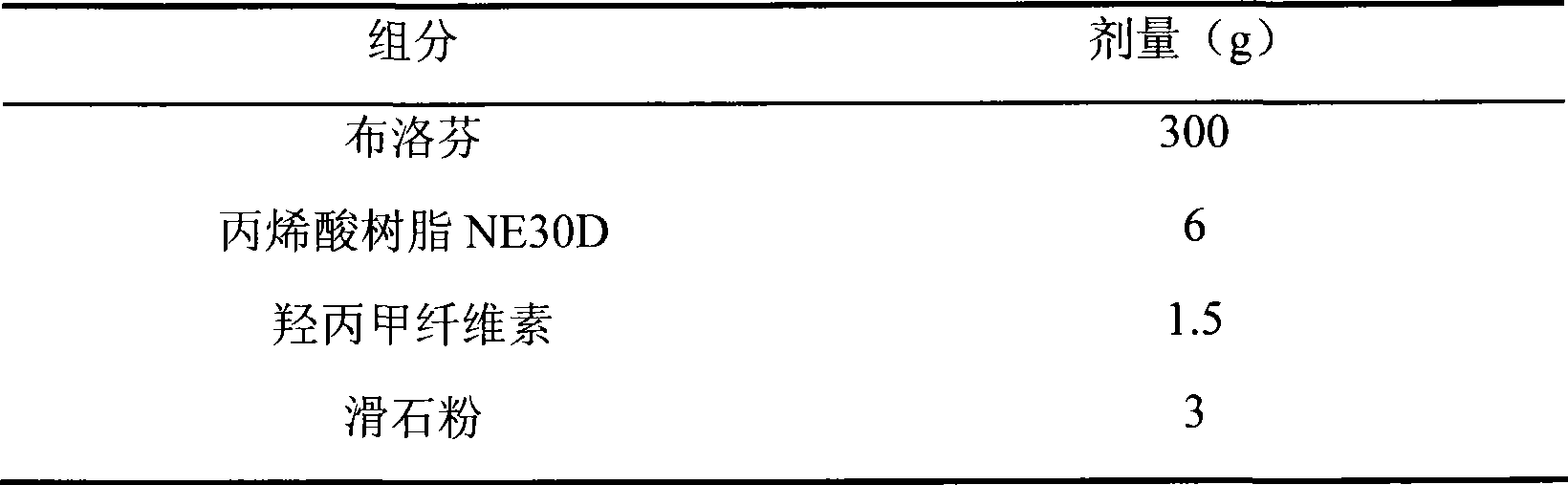
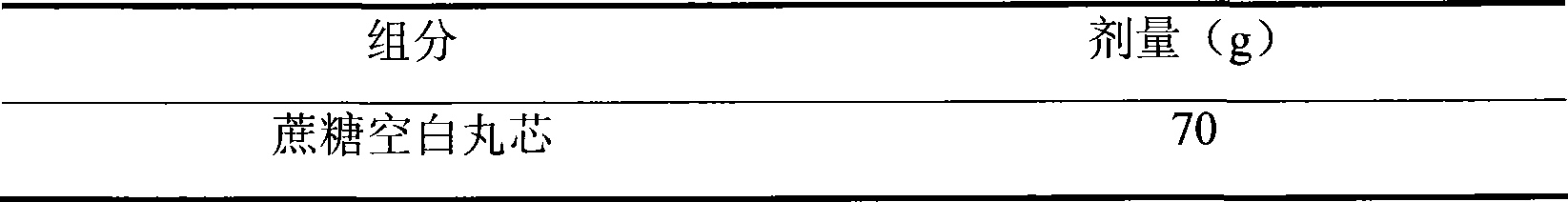
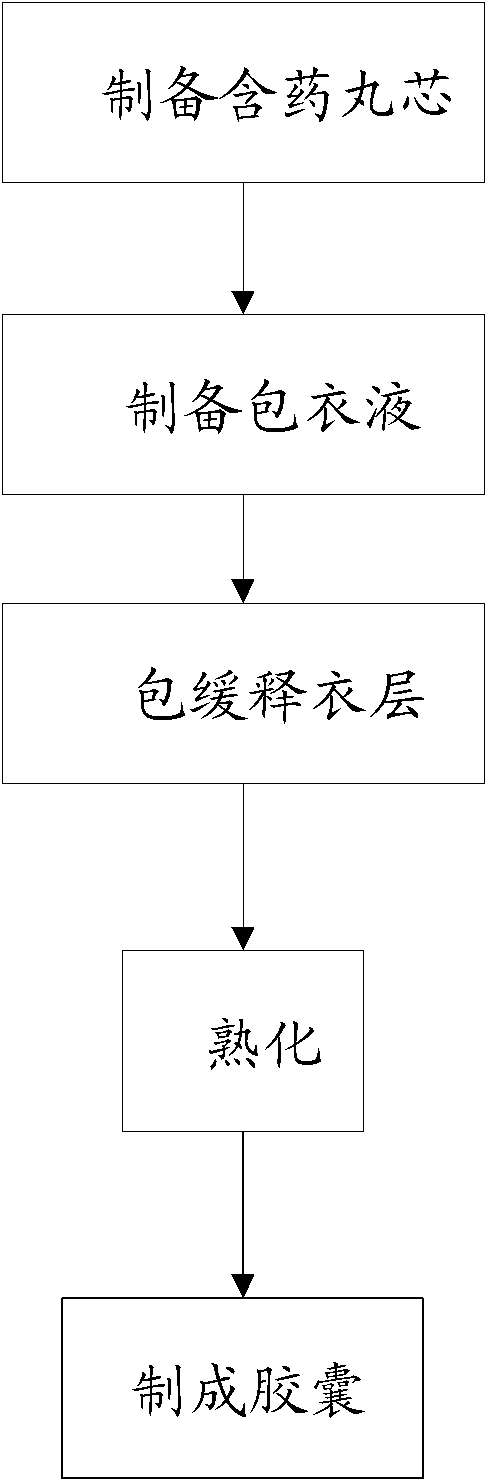
472 results about "Sustained release pellets" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
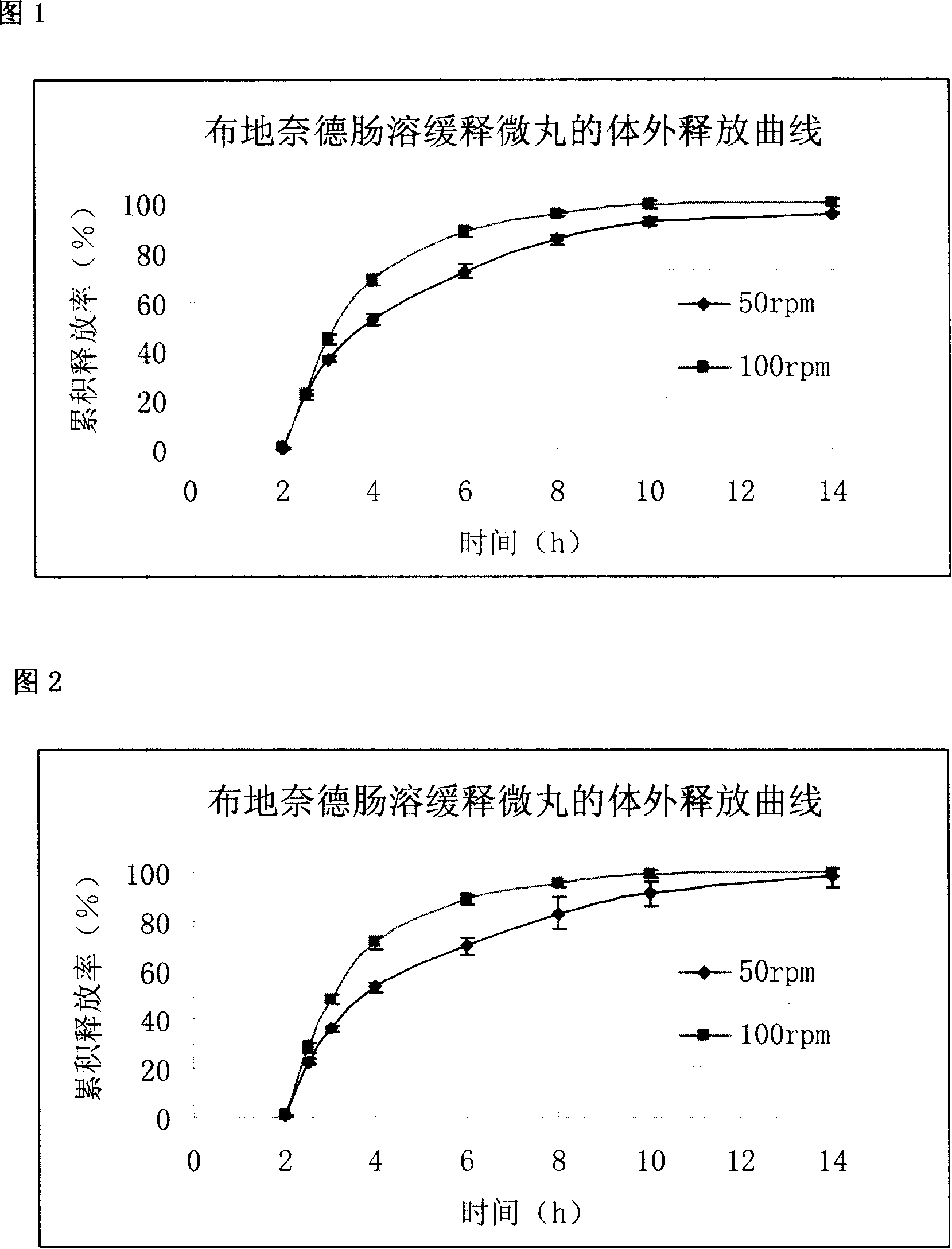
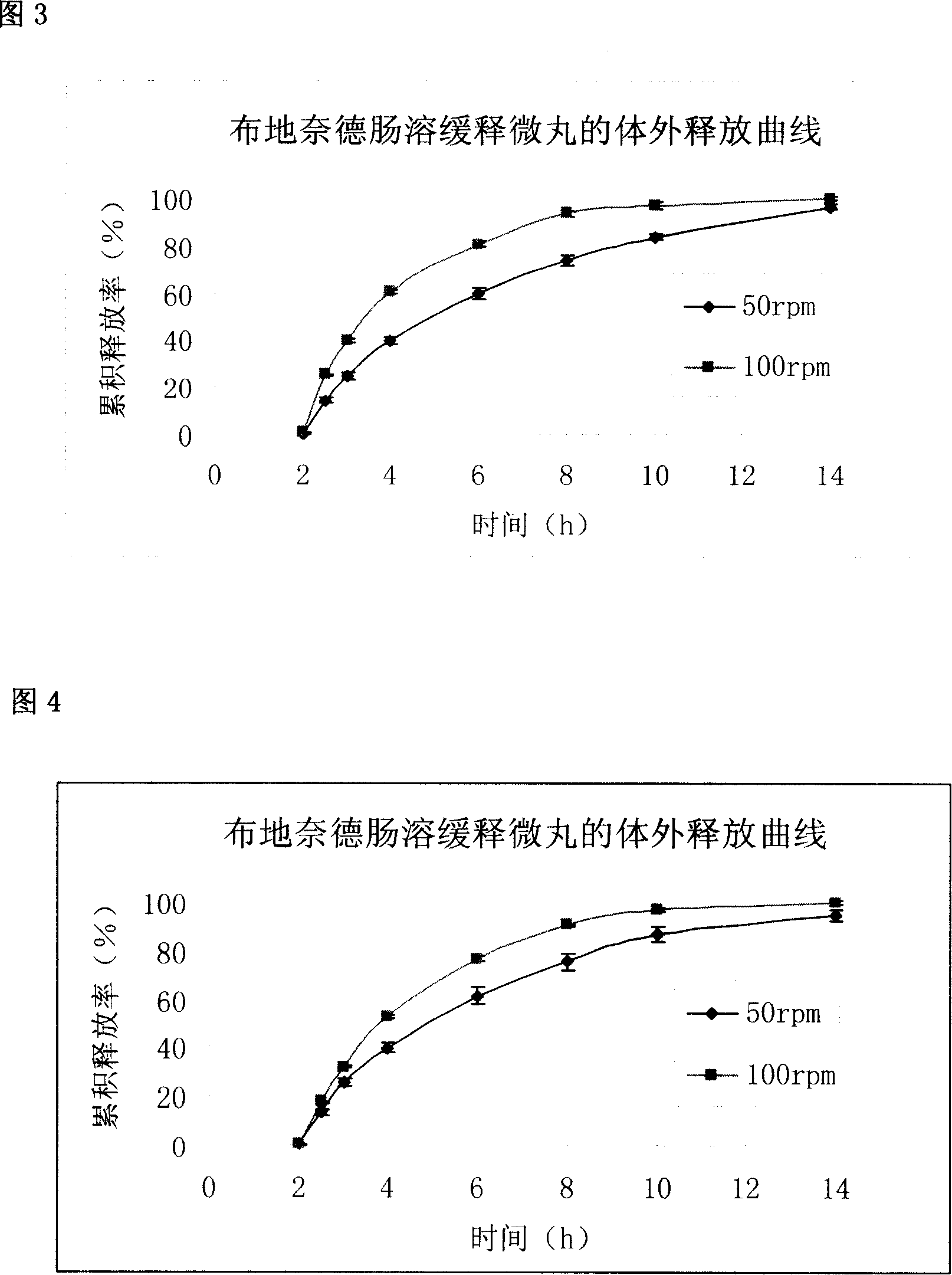
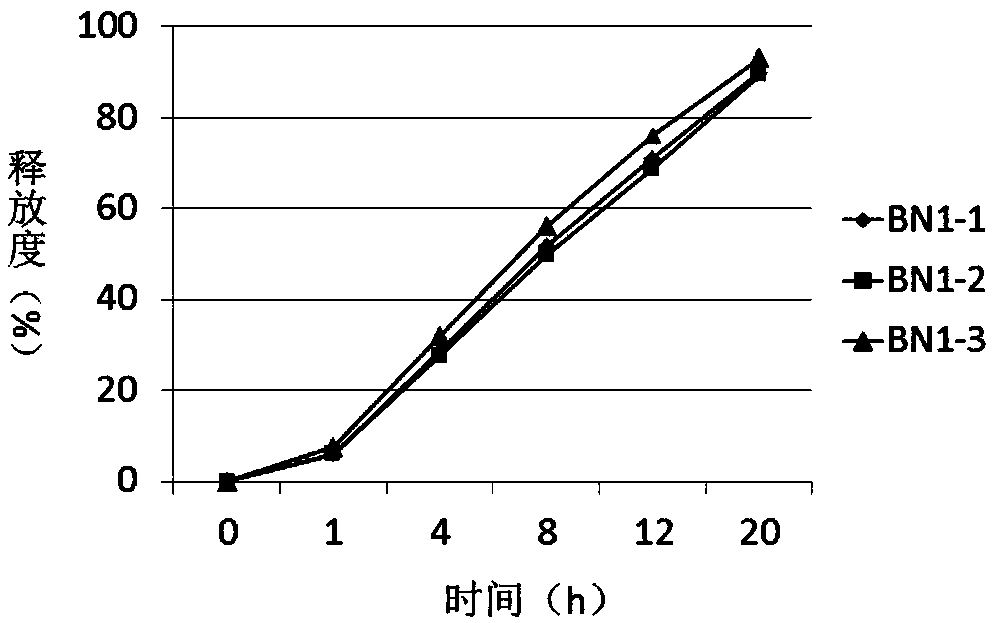
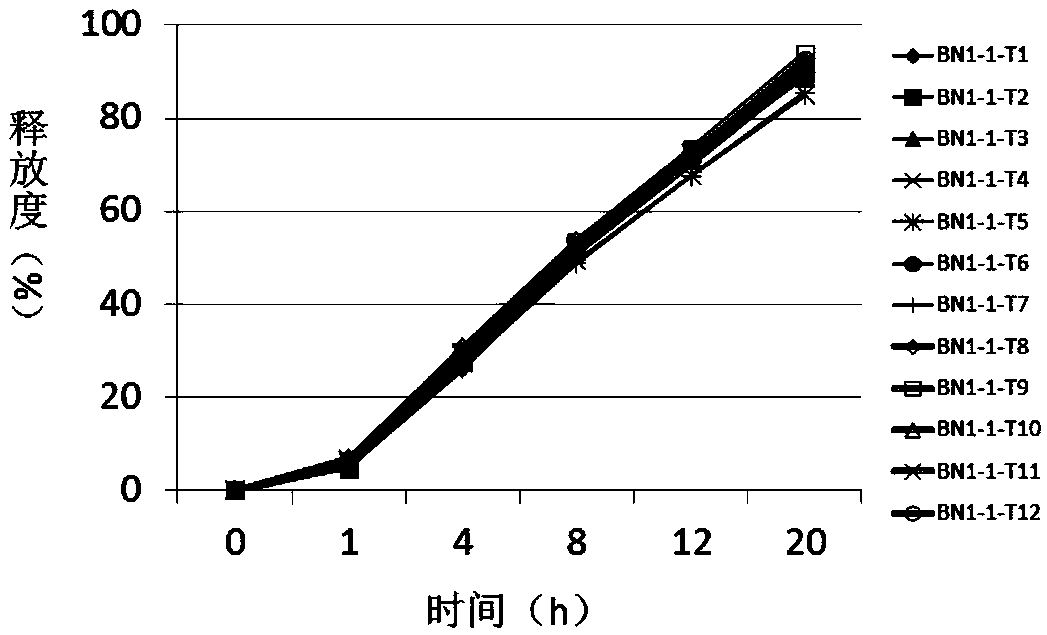
Sustained release pellets of Budesonide could be prepared by extrusion and spheronization which released the drug in intestinal pH for an intestine to treat inflammatory bowel disease.
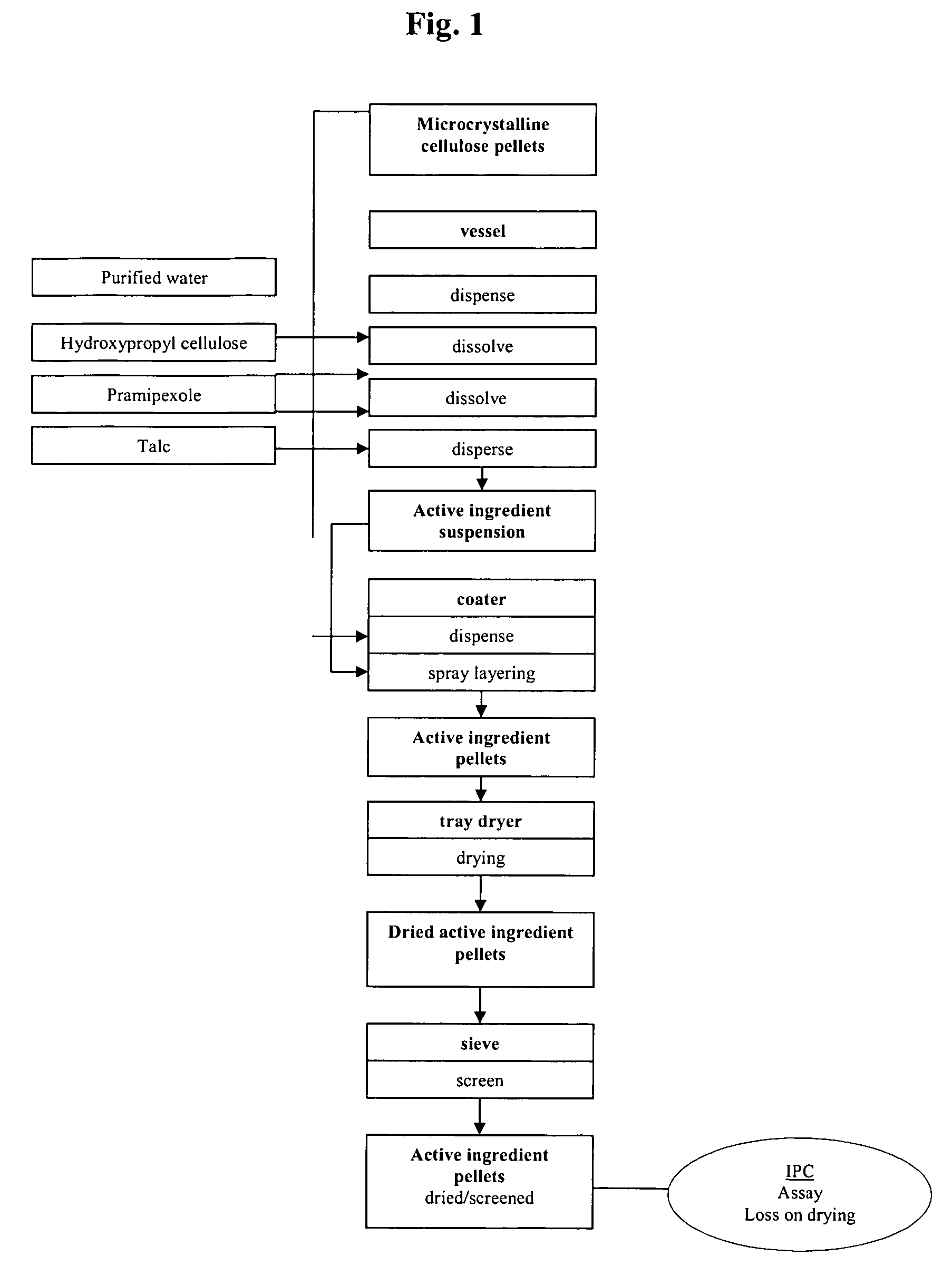
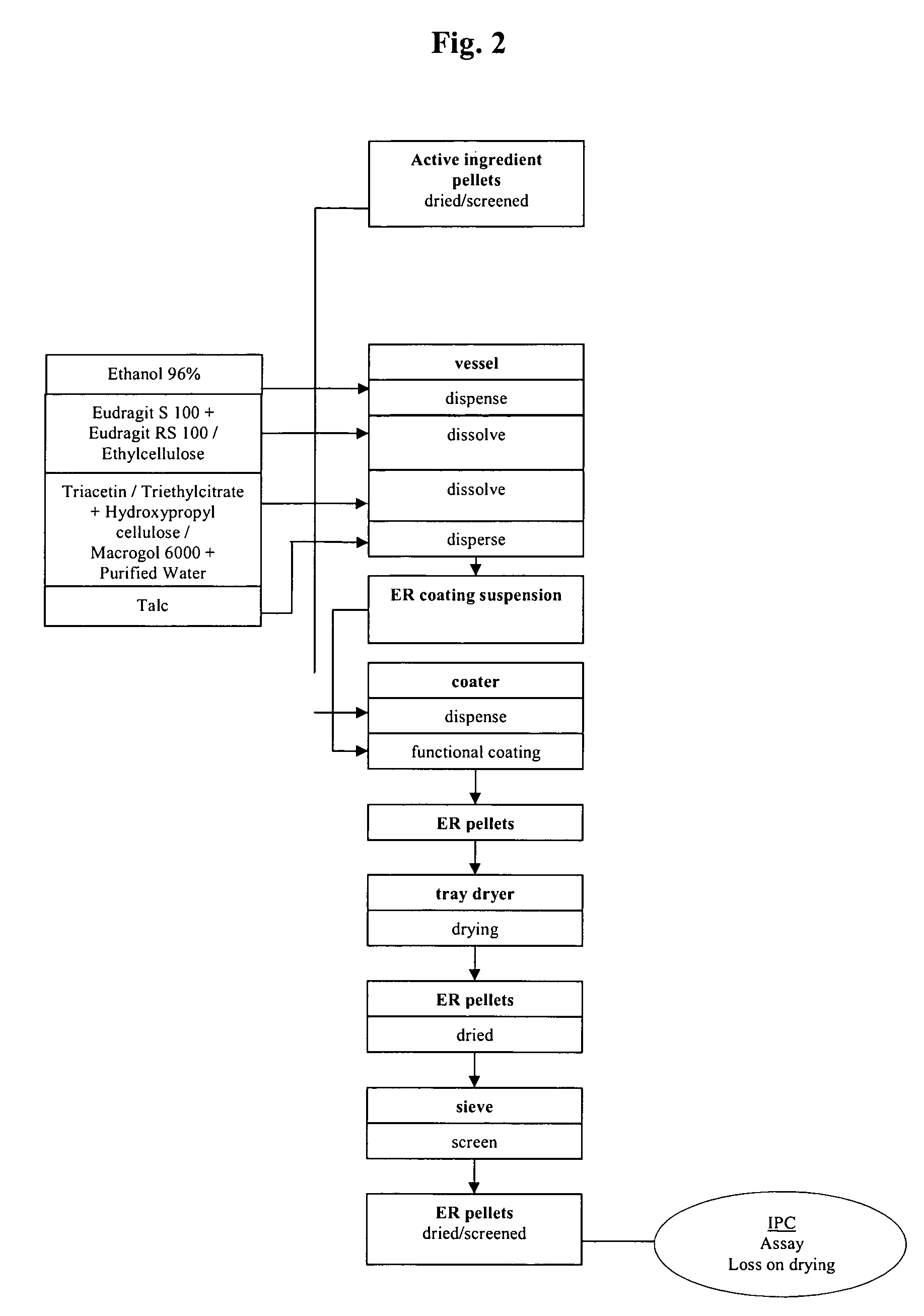
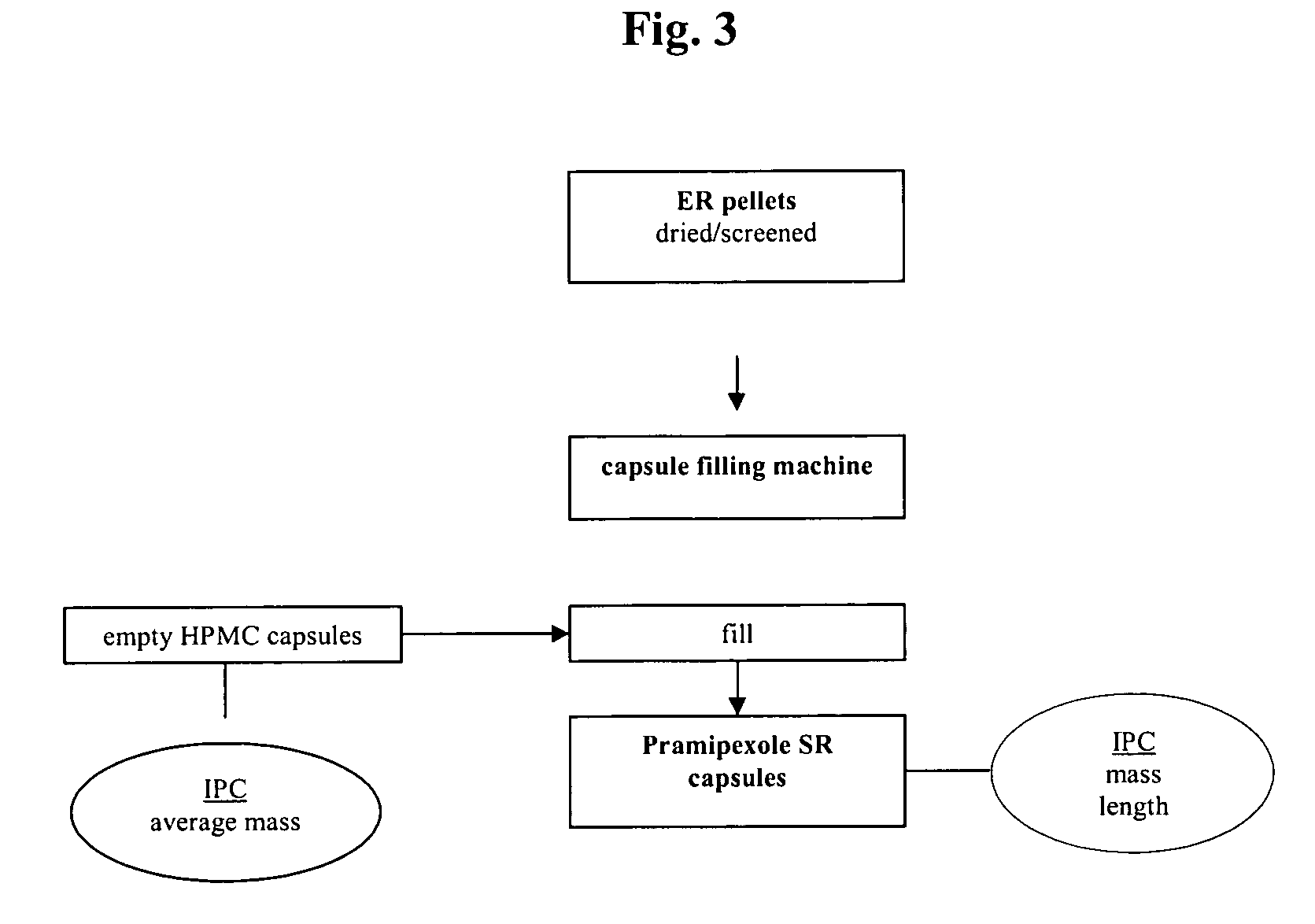
Extended release pellet formulation containing pramipexole or a pharmaceutically acceptable salt thereof
InactiveUS20060051419A1Improve complianceImprove convenienceOrganic active ingredientsNervous disorderPharmaceutical preservativesPramipexole
An extended release pellet comprising an active ingredient selected from pramipexole and the pharmaceutically acceptable salts thereof, and at least one release-modifying excipient.
Owner:BOEHRINGER INGELHEIM INT GMBH
Floating type pellets in stomach and preparation method thereof
ActiveCN101288659ASustained releaseLow densityCapsule deliveryMacromolecular non-active ingredientsIntestinal absorptionSustained release pellets
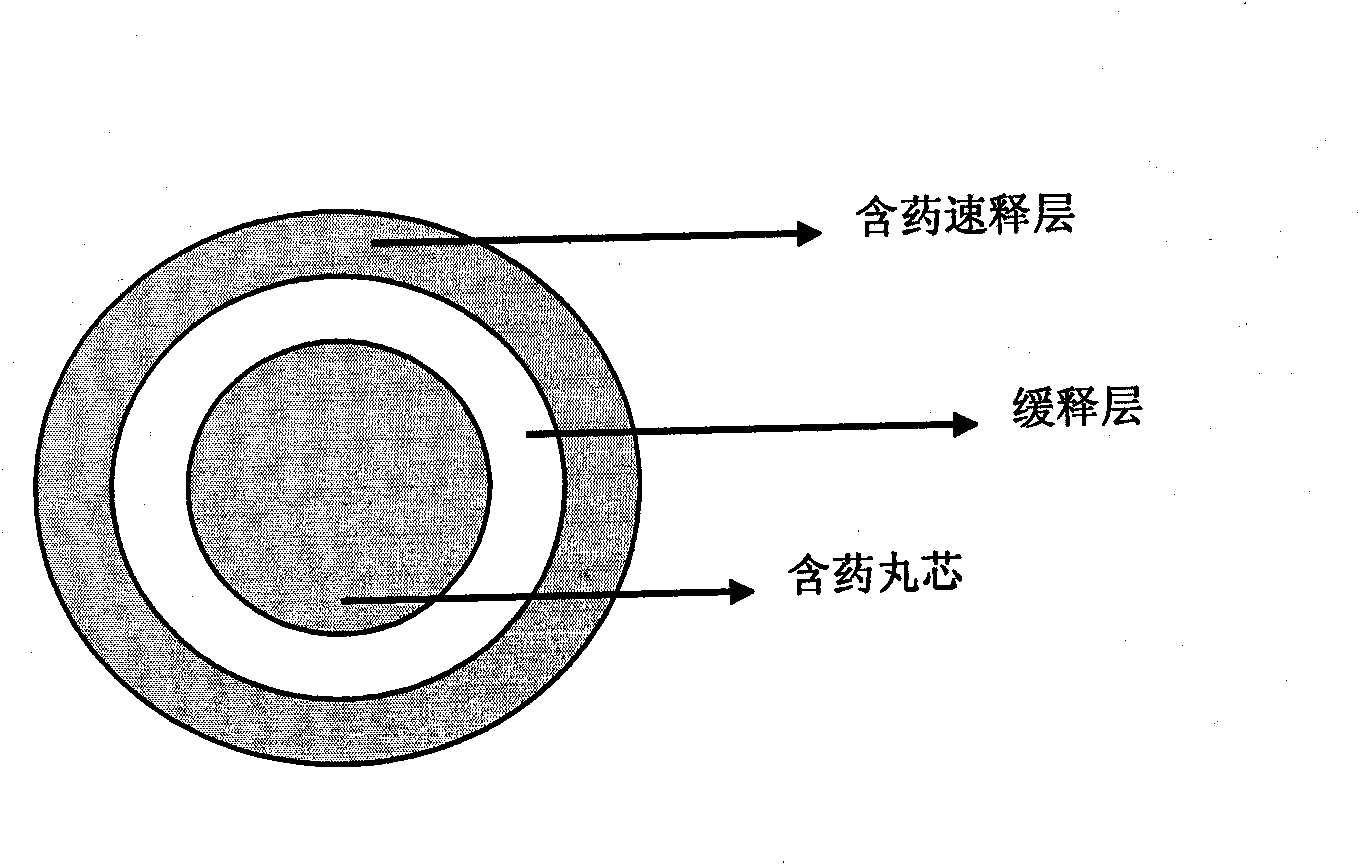
The invention relates to the technical field of medicine, in particular to a stomach floating type pellet and a preparation method thereof. The stomach floating type pellet comprises a pellet core, a drug layer and a sustained-release layer; the density of the pellet is smaller than 1g / cm<3>, so that the drug can float in the stomach, sustainably release the drug ingredients; stomach floating type capsules can be prepared by loading the pellets into capsules. The preparation type is suitable for drugs mainly absorbed in the stomach, in particular to the drugs that are absorbed better in the stomach than in the enteric canal. When the drugs are prepared into stomach floating type pellets or stomach floating type capsules, compared with ordinary preparation, the times of administration is reduced and the relatively stable blood concentration of the drugs is kept in vivo, thus ensuring the sustained release of the drugs, improving the bioavailability and reducing the dosage. At the same time, as the stomach floating type pellet is a granose system, the drug release behavior is the collective behavior of each pellet, so that coating failure of a plurality of pellets can not lead to failure of the whole dosage, thus having higher safety. The sustained-release pellet is prepared by adopting general technique and is easy for realizing industrial production.
Owner:特康药业集团有限公司
Water-insoluble medicine sustained-release pellet, sustained-release orally disintegrating tablet thereof and preparation method thereof
InactiveCN101862297ASmall particle sizeHigh strengthOrganic active ingredientsAntipyreticSustained release pelletsSide effect
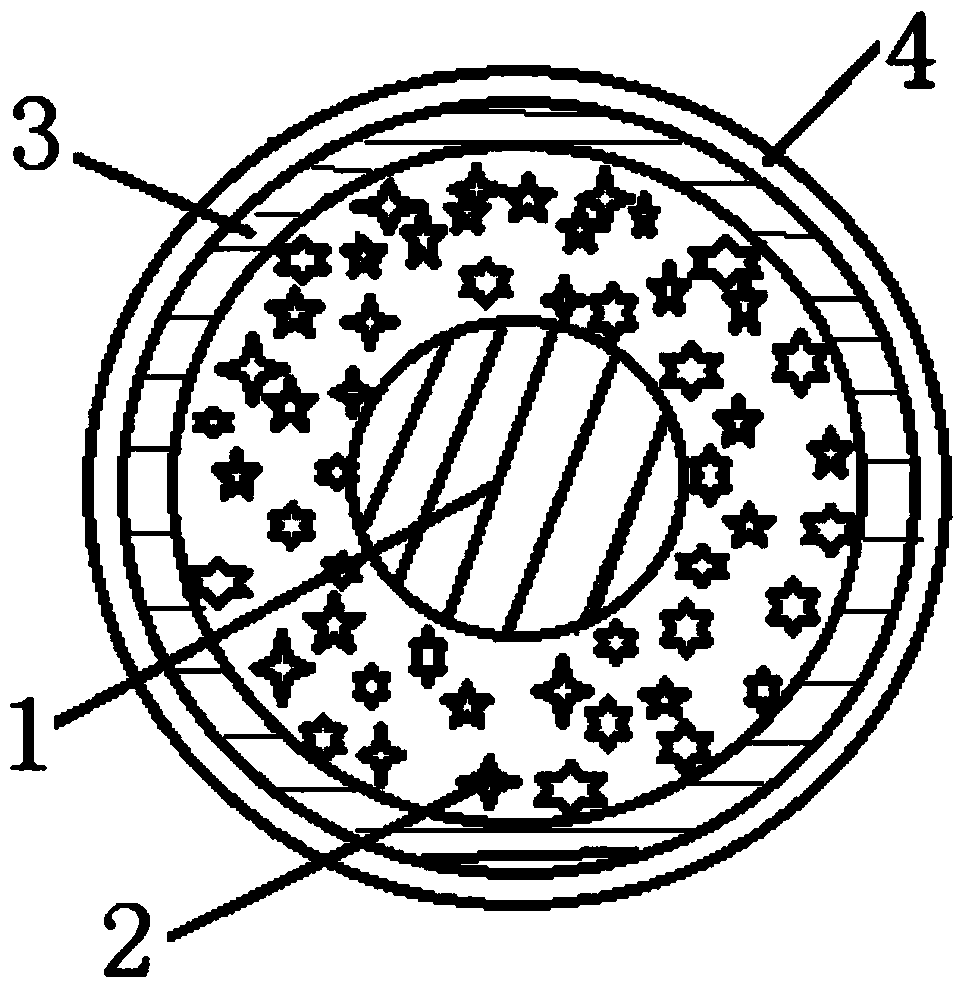
The invention discloses a water-insoluble medicine sustained-release pellet, a sustained-release orally disintegrating tablet thereof and a preparation method thereof. The water-insoluble medicine sustained-release pellet comprises a hollow pellet core, a medicine layer, an insulation layer and a sustained-release layer, wherein the sustained-release layer comprises the following ingredients: 54 to 88 percent of sustained-release materials, 2 to 30 percent of antitackiness agents and 1 to 30 percent of pore-foaming agents, wherein the percentage is the mass percentage in the sustained-release layer, wherein the sustained-release materials are one kind or several kinds of materials selected from ethyl acrylate and methyl methacrylate copolymers, polyvinyl acetate and ethyl cellulose. Through regulating the coating combinations and filling auxiliary materials to be pressed into orally disintegrating tablets, the medicine can be slowly released for more than 8 to 13 hours, so the stable blood medicine concentration can be maintained, the side effect is reduced, the medicine taking times can be reduced, and the medicine taking is convenient. The invention conforms to zero-grade release, has the advantages of high final accumulated medicine release amount, high efficacy, strong selectivity, good mouth feeling, simple production steps and high efficiency, and can be applied to large-scale production.
Owner:SHANGHAI INST OF PHARMA IND +1
Slow/controlled release pellet composition containing ginkgo leaf extracts and preparation method thereof
InactiveCN101375869ASmall toxicityStable blood concentrationGranular deliveryGinkgophyta medical ingredientsSustained release pelletsHard Capsule
The invention belongs to the field sustained / controlled-release preparations, in particular to an oral sustained / controlled-release pellet combination containing ginkgo biloba extract and a preparation method. The oral sustained / controlled-release pellet combination is composed of (A) a core containing a pill; (B) an insulating coating layer; (C) a sustained-release coating layer; (D) and an enteric-coated coating layer. The invention is the traditional Chinese medicine multi-component sustained-release pellet combination which is taken once by 24 hours and the multi-unit sustained-release pellet combined preparation with the different drug release systems, the core containing the pill is prepared by adopting the extrusion pill rolling method, a novel sustained-release multi-layer coating technology and a fluidized bed are utilized for coating the sustained-release pellet, the rapid-release part and the sustained-release part of the coated pellet are mixedly filled into a hard capsule or pressed into a pellet tablet. The sustained-release pellet has stable coating process and good reproducibility, thereby being applicable to the industrial mass production; and the drug quality of the preparation is stable through the long-term storage. The in vitro release test shows that the multiple components of the traditional Chinese medicine can achieve the sustained-release role, the sustained-release preparation can significantly increase the transmembrane absorption and the stability of various effective active ingredients by oral drug administration, the curve of plasma drug concentration in vivo is smooth, and the design purpose of 24-hour sustained-release is achieved.
Owner:CHINA PHARM UNIV
Sustained releasing preparation of vilamin C and its preparing method
InactiveCN1582922AGood sustained release effectOrganic active ingredientsMetabolism disorderVitamin CPlasticizer
A slow-releasing VC in the form of micropills is composed of pill and coated layer. Said pill is prepared from core, VC, antioxidizing agent, synergistic, adhesive and other auxiliary. Said coated layer is prepared from slow-releasing material, plasticizer, pore forming agent and defoaming agent.
Owner:范敏华
Sustained-release micro-pellet of trimetazidine and preparation process thereof
InactiveCN1994280AImprove liquidityUniform absorption rate in the bodyOrganic active ingredientsPharmaceutical non-active ingredientsTrimetazidine DihydrochlorideSustained release pellets
The invention relates to a slow-release micro drop whose active component is Humeitashen or other salt, wherein it is formed by element and film layer that controlling the drug release, whose weight ratio is 20:1-5:1; the Humeitashen content of element is 10-60%. The invention mainly uses protrusion method to prepare drop, and uses fluidize bed to pack.
Owner:SHANDONG INST OF PHARMA IND
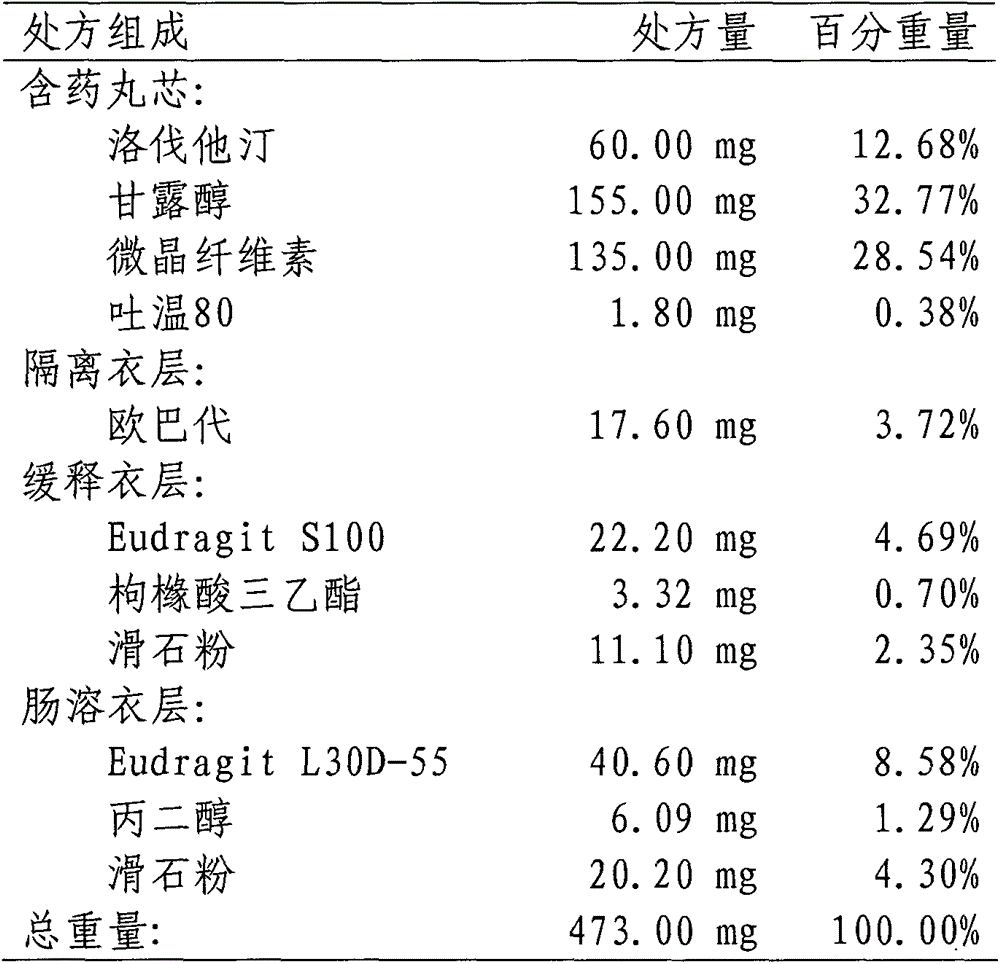
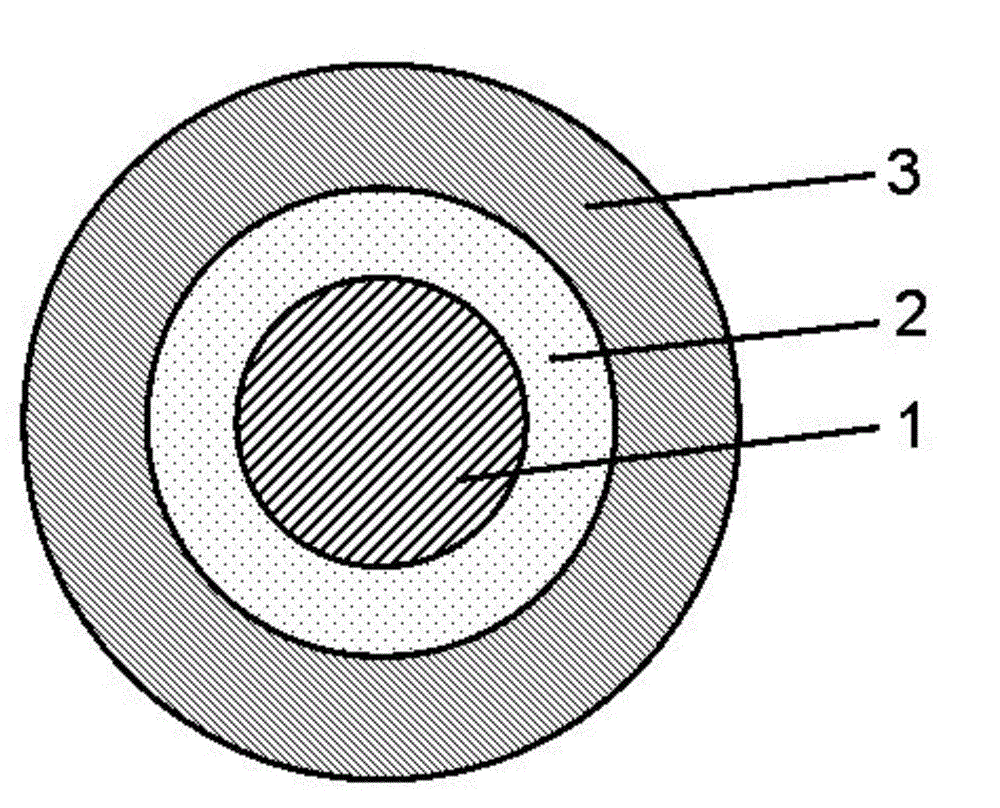
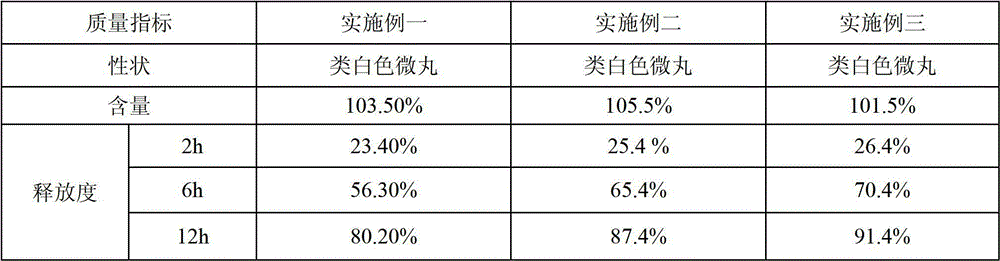
Lovastatin enteric coated sustained-release pellet capsule and preparation method thereof
InactiveCN103142552AUniform absorption rateSmall differences in individual bioavailabilityMetabolism disorderGranular deliverySustained release pelletsSide effect
The invention discloses a lovastatin-containing enteric coated sustained-release pellet capsule and a preparation method thereof. The lovastatin-containing enteric coated sustained-release pellet capsule comprises two parts, namely, an enteric coated sustained-release pellet and a hollow capsule, wherein the enteric coated sustained-release pellet comprises 55-86% of medicine-containing pellet core, 2-5% of isolation coating layer, 2-15% of a sustained-release coating layer and 10-25% of enteric-coated coating layer by weight. The prepared lovastatin enteric coated sustained-release pellet capsule is uniform in granule size and stable in medicine release; the medicine is not released in gastric acid but is slowly and constantly released in intestinal tracts and livers, has the characteristic of targeted medicine release, is small in irritation to gastrointestinal tracts, and can reduce the toxic and side effects of the medicine and reduce the number of the medicine administrations, so that the compliance of patients is improved; and meanwhile as the enteric coated sustained-release pellet preparation is composed of hundreds of pellets of uniform granule sizes, the sudden release of the whole preparation is not caused by the breakage of individual pellets, so that the medicine is safer than a sustained-release tablet, smaller in irritation to gastrointestinal tracts and more stable in blood concentration, and the clinical effectiveness and security are effectively improved.
Owner:广州科的信医药技术有限公司
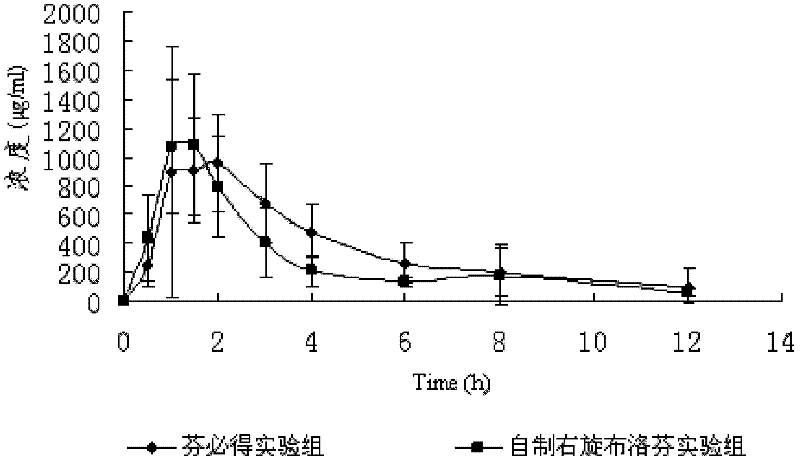
Dexibuprofen sustained-release pellet and preparation method thereof
ActiveCN102228441ASolve adhesionAvoid stickingOrganic active ingredientsAntipyreticSustained release pelletsBioavailability
The invention discloses a dexibuprofen sustained-release pellet and a preparation method thereof. The dexibuprofen sustained-release pellet comprises a celphere, a drug layer and an external coating. In the terms of the total weight of the pellet, the celphere accounts for 15 % to 30 %. The drug layer consists of dexibuprofen, a binding agent and talcum powder, wherein the dexibuprofen accounts for 70-85 %, the binding agent accounts for 1-10 %, and the talcum powder accounts for 0.1-3 %. The external coating consists of a coating material and the talcum powder, the coating material accounts for 0.5-3 % and the talcum powder accounts for 0.1-3 %. The invention further provides the preparation method of the dexibuprofen sustained-release pellet. In the invention, the dexibuprofen sustained-release pellet has the advantages of good stability, high bioavailability, good mobility and wide application prospect and is beneficial to subpackage of preparations or is further pressed in a form of a tablet.
Owner:湖北舒邦药业有限公司
Vitamin C sustained-release pellets and method for preparing same
ActiveCN102908319AHigh drug loadingLarge particle sizeOrganic active ingredientsMetabolism disorderSustained release pelletsVitamin C
A vitamin C sustained-release pellet applied to the vitamin C sustained-release preparation field and a method for preparing the same are disclosed. The vitamin C sustained-release pellet is composed of a vitamin C sustained-release pill and a sustained-release coating, wherein the vitamin C sustained-release pill is composed of a mother nucleus and a lamination layer, or composed of a vitamin C and vitamin C pill accessory, or composed of vitamin C; the sustained-release coating is composed of a sustained-release coating material and a sustained-release coating accessory, or composed of the sustained-release coating material; the vitamin C sustained-release pill accessory is one or two selected from a filler and a binder; the sustained-release coating accessory is one or two selected from a plasticizer and an antisticking agent; the weight percentage content of the vitamin C in the mother nucleus is the same as that in the lamination layer; and the filler is one or several selected from microcrystalline cellulose, powdered sugar, starch, dextrin and lactose. The vitamin C sustained-release pellet disclosed by the invention is simple in prescription, free of metal-chelator or antioxidant, great in unit volume drug loading capacity, good in stability and capable of keeping sustained release for a long time; and the preparation method of the vitamin C sustained-release pellet is short in operation time and low in cost.
Owner:SHENYANG NO 1 PHARMA FACTORY DONGBEI PHARMA GRP
Sustained-release pellet containing ibuprofen
InactiveCN101467989AOrganic active ingredientsAntipyreticSustained release pelletsControlled release
The invention relates to a sustained-release pellet containing ibuprofen having an empty core and a medicament-contained coating layer. The inventive pellet can control release of medicament in a better way and stay good stability within a comparatively long time.
Owner:北京德众万全医药科技有限公司
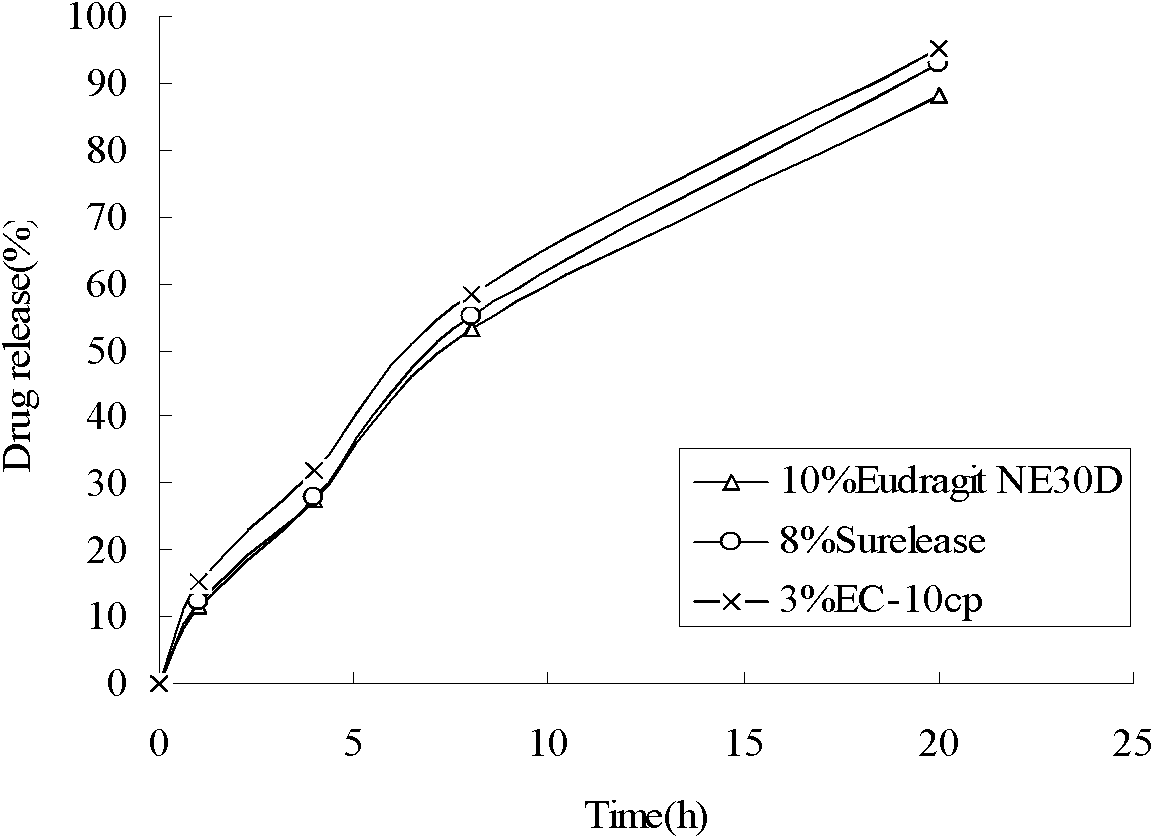
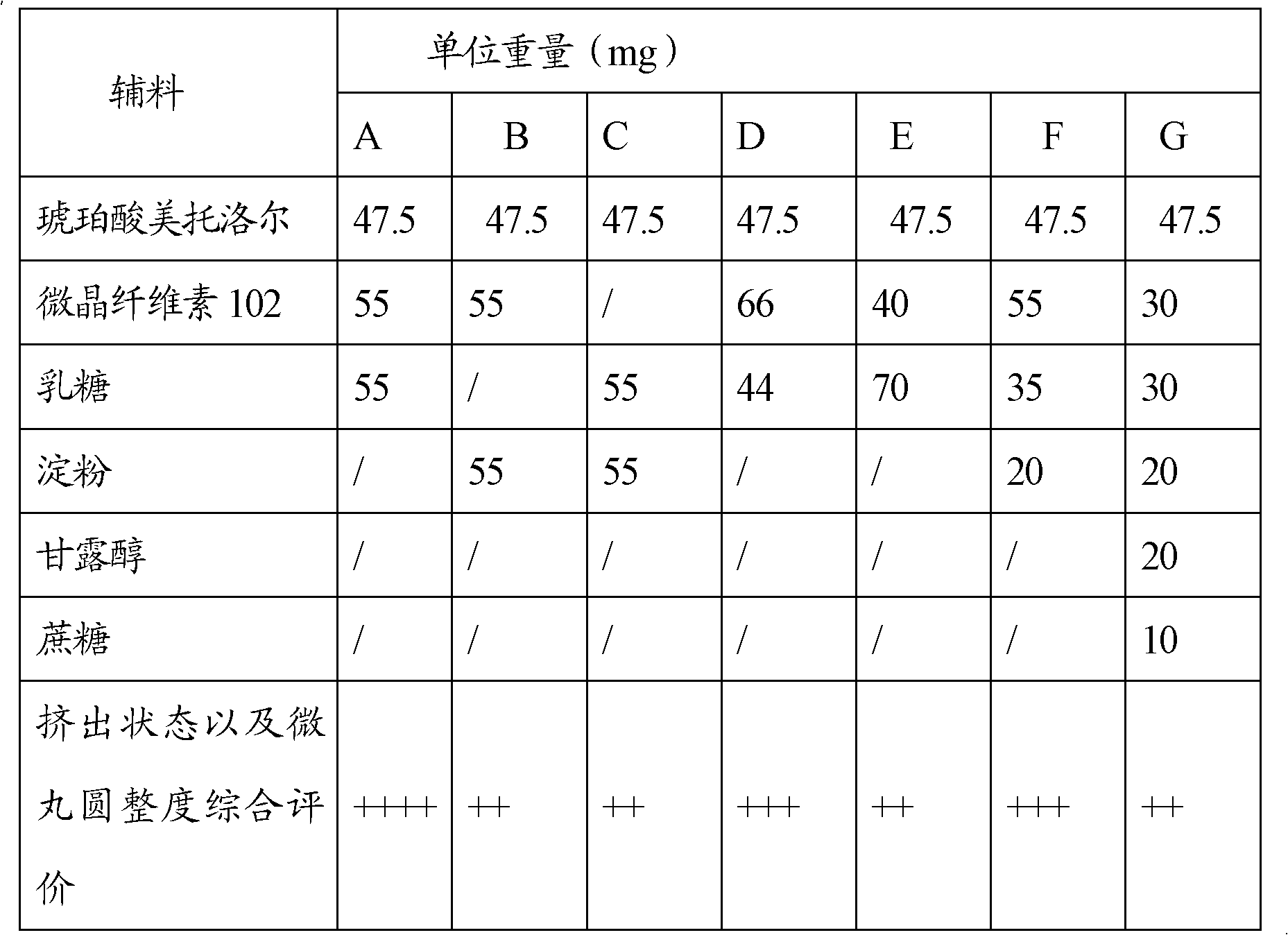
Sustained-release capsules of metoprolol succinate and preparation method thereof
ActiveCN102274205AStable drug releaseSimple processOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsMedicine
The invention discloses a metoprolol succinate sustained-release capsule, consisting of a capsule shell and contents; the contents comprise a metoprolol succinate sustained-release pellet; the metoprolol succinate sustained-release pellet comprises a metoprolol succinate contained pill core and a sustained-release coating layer coated outside the metoprolol succinate contained pill core; the metoprolol succinate contained pill core is prepared by using metoprolol succinate as the active ingredient and mixing with an auxiliary material, wherein the weight ratio of the metoprolol succinate to the auxiliary material is 1:1.5 to 1:3. The metoprolol succinate sustained-release medicine disclosed by the invention is stable and the expected effects of all release behaviors in acid, alkaline and water can be achieved. The invention further discloses a method for preparing the metoprolol succinate sustained-release capsule; compared with the prior art, the process is simpler and has good operability and reproducibility.
Owner:佛山市隆信医药科技有限公司
Memantine hydrochloride sustained-release capsule and preparation method thereof
ActiveCN103181914ASustained releaseSmooth releaseNervous disorderPharmaceutical non-active ingredientsSustained release pelletsMemantine Hydrochloride
The invention provides a preparation method of a memantine hydrochloride sustained-release preparation. According to the present invention, sustained-release pellets are obtained by sustained-release coating of drug-containing pellets containing memantine hydrochloride. The drug-containing pellets are obtained by extrusion spheronization or solution medicine-feeding or suspension medicine-feeding, and are nearly circular in shape. The shape and granularity-controllable drug-containing pellets are subjected to sustained-release coating and the thickness of the film formed by coating can also be controlled. The invention realizes the controllability of the coating film and the spherical pellets, and reproducibility of stability of releasing the memantine hydrochloride is controlled under the circumstance of guaranteeing nonoccurrence of crystal form of memantine hydrochloride. The preparation of the invention can provide sustained release in the form of a single dose within 24 hours. The drug penetrates and diffuses to the release medium through the film pores. Because the size is small, the medicine taking is less susceptible to foods and the efficacy is improved. The production method of the present invention is simple, is suitable for industrial production, and has a great application value.
Owner:SHANGHAI FOSUN PHARMA DEV CO LTD
Memantine hydrochloride sustained release-donepezil quick release compound capsule
InactiveCN105326837AQuality is easy to controlHigh yieldNervous disorderPharmaceutical delivery mechanismSustained release pelletsMemantine Hydrochloride
Owner:BEIJING VENTUREPHARM BIOTECH
Budesonide intestines sustained release dextromethorphan pellets and method of manufacturing the same
InactiveCN101108171ARegulated release rateGood film formingOrganic active ingredientsAntipyreticSustained release pelletsMedicine
The invention belongs to the technical field of medicinal preparation and relates to an enteric preparation and sustained or controlled release preparation, in particular to an enteric controlled release micro-granule with budesonide and its preparation method. The invention, which comprises a quick release celphere, a controlled release layer and an enteric layer, takes budesonide as active ingredient of the drug to coordinate with medicinal carrier supplementary materials. After the release level testing, it is proved that the enteric controlled release micro-granule, which adopts different enteric materials, controlled release material and different coating conditions, does not release in stomach but began to release slowly after entering the small intestine with even release level. Therefore, the enteric controlled release micro-granule in the invention can effectively prevent the release in stomach, ensure the slow release in small intestine and the release of drug in the affected section of small incestine, ileocecus and colon.
Owner:FUDAN UNIV
Pantoprazole and its sodium salt enteric sustained-release pellet preparation
InactiveCN101428005AEliminate local irritationLarge distribution areaOrganic active ingredientsDigestive systemSustained release pelletsIsolation layer
The invention discloses pantoprazole and the sodium salt enteric sustained-release micropill preparation thereof. The preparation comprises pantoprazole-containing micropills, an isolation layer, a sustained-release layer, another isolation layer and an enteric layer from inside to outside in sequence; the weight of the first isolation layer is 0.5% to 40% of that of the pantoprazole-containing micropills, the weight of the sustained-release layer is 5% to 100% of that of the pantoprazole-containing micropills, the weight of the second isolation layer is 0.5% to 40% of that of the pantoprazole-containing micropills, and the weight of the enteric layer is 20% to 200% of that of the pantoprazole-containing micropills. The pantoprazole enteric sustained-release micropills can stably release the drug.
Owner:ZHEJIANG UNIV
Metroprolol succinate sustained release tablet and preparation method thereof
InactiveCN107595795AModerate fluidityStable release rateOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSustained Release Tablet
The invention belongs to the technical field of drug preparations, and relates to a Metoprolol succinate sustained release tablet and a preparation method thereof. The sustained release tablet includes a tablet film coating material and a tablet core coated with the coating, the tablet core comprises a pellet and accessories, the pellet comprises, from the inside to the outside, a blank pill core,a drug-loading coating layer, a sustained release coating layer and a protective coating layer, the drug-loading coating layer comprises Metoprolol succinate and a drug-loading pellet coating adhesive, the sustained release coating layer comprises a sustained release pellet coating layer coating material, a pore forming agent, a plasticizer and an anti adhesion agent, the protective coating layerincludes a protection pellet coating adhesive, the accessories include a filler, a disintegrating agent and a lubricating agent. The sustained layer of the release tablet is externally wrapped with the protective layer, and the problems of slow release ability reduction and unqualified content uniformity caused by excess fluidity and the like due to the damage of the sustained release layer can be solved.
Owner:北京华素制药股份有限公司
Minocycline hydrochloride sustained-release capsule and preparation method thereof
ActiveCN103054832AImprove stabilitySimple manufacturing processAntibacterial agentsTetracycline active ingredientsSustained release pelletsAdhesive
The invention relates to a minocycline hydrochloride sustained-release capsule and a preparation method thereof. A substance in the capsule is a sustained-release pellet which is prepared from the following raw and accessory material by weight: minocycline hydrochloride, a filler, an adhesive, an isolating layer film forming material, a plasticizer A, an opacifying agent, a sustained-release material, a plasticizer B, an antiadherent and a pore forming agent. The preparation method comprises the following steps: preparing a drug-loaded pellet core according to a formula; preparing an isolating layer pellet from the pellet core; preparing the sustained-release pellet from the isolating layer pellet; and filling the sustained-release pellet into a capsule so as to obtain the minocycline hydrochloride sustained-release capsule. According to the invention, the opacifying agent is added into an isolating layer, so stability of the photosensitive drug minocycline is improved; a sustained-release layer is prepared by using an optimized prescription, so usage amount of a coating material is substantially reduced; and the method provided by the invention has good reproducibility and is applicable to industrial production.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
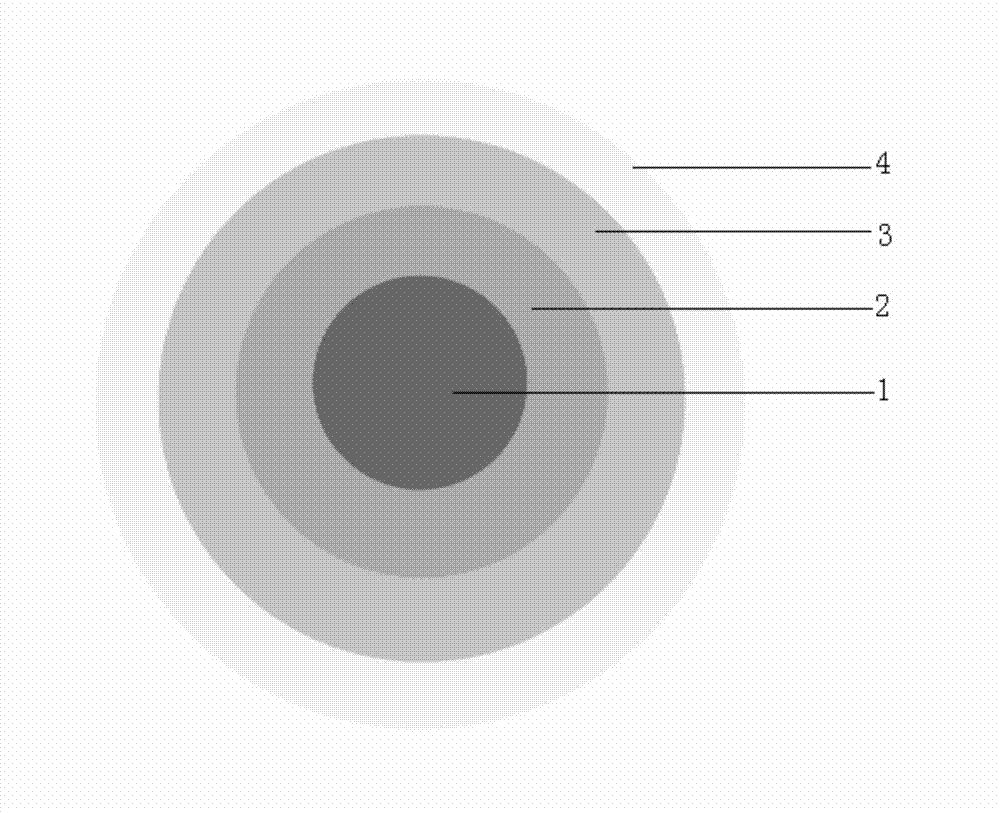
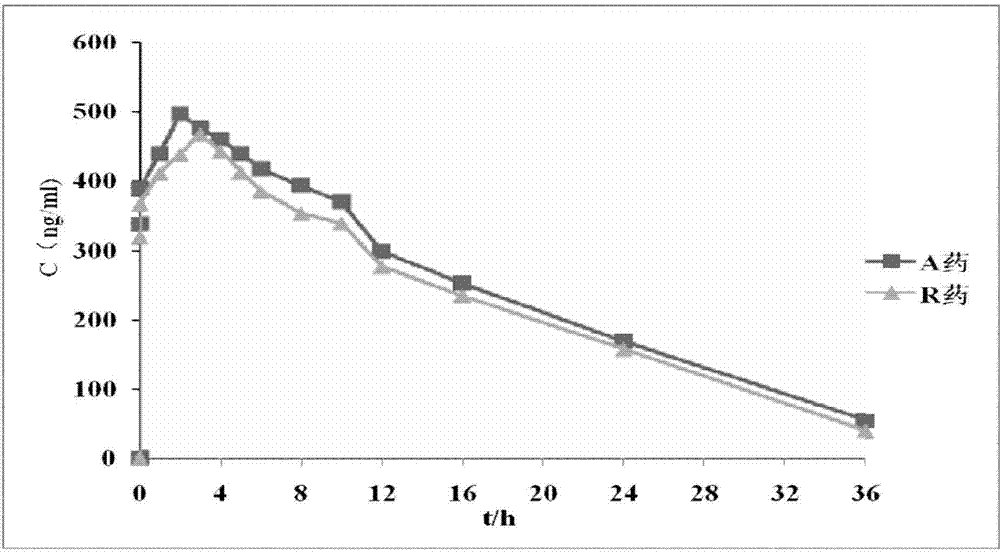
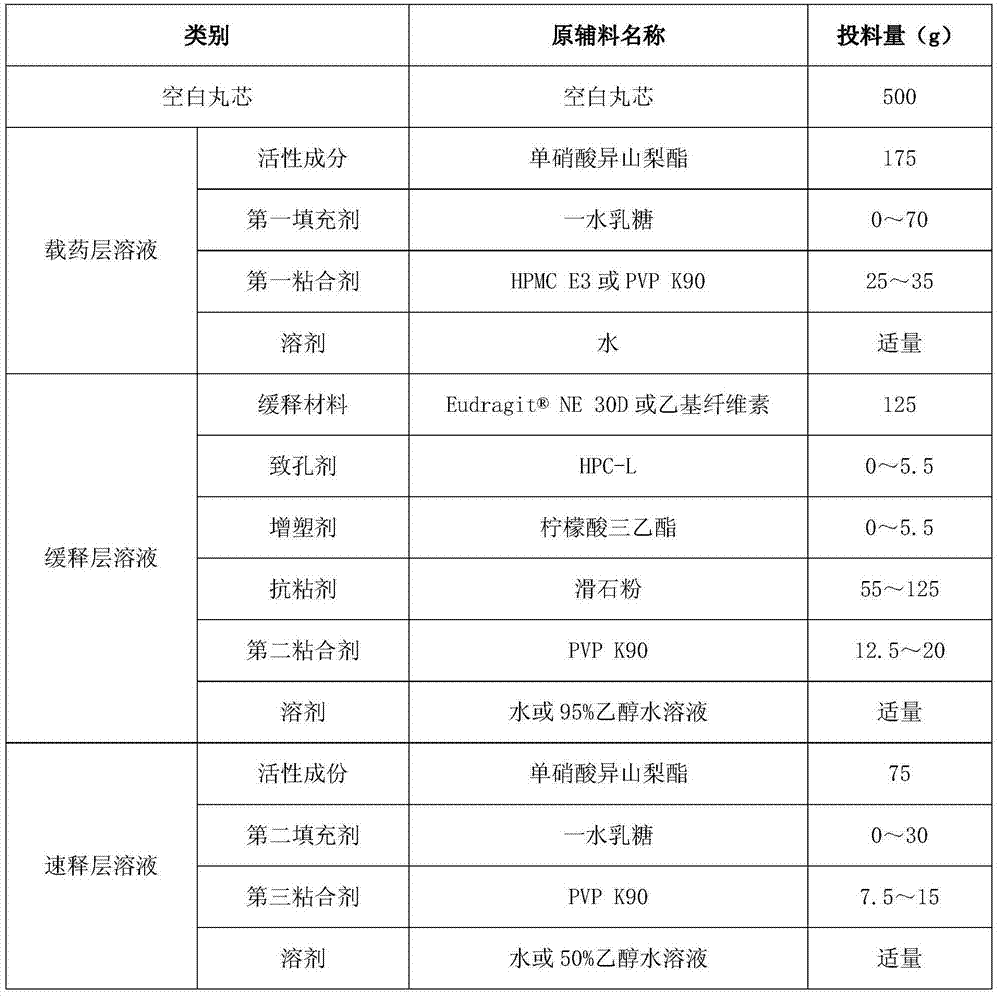
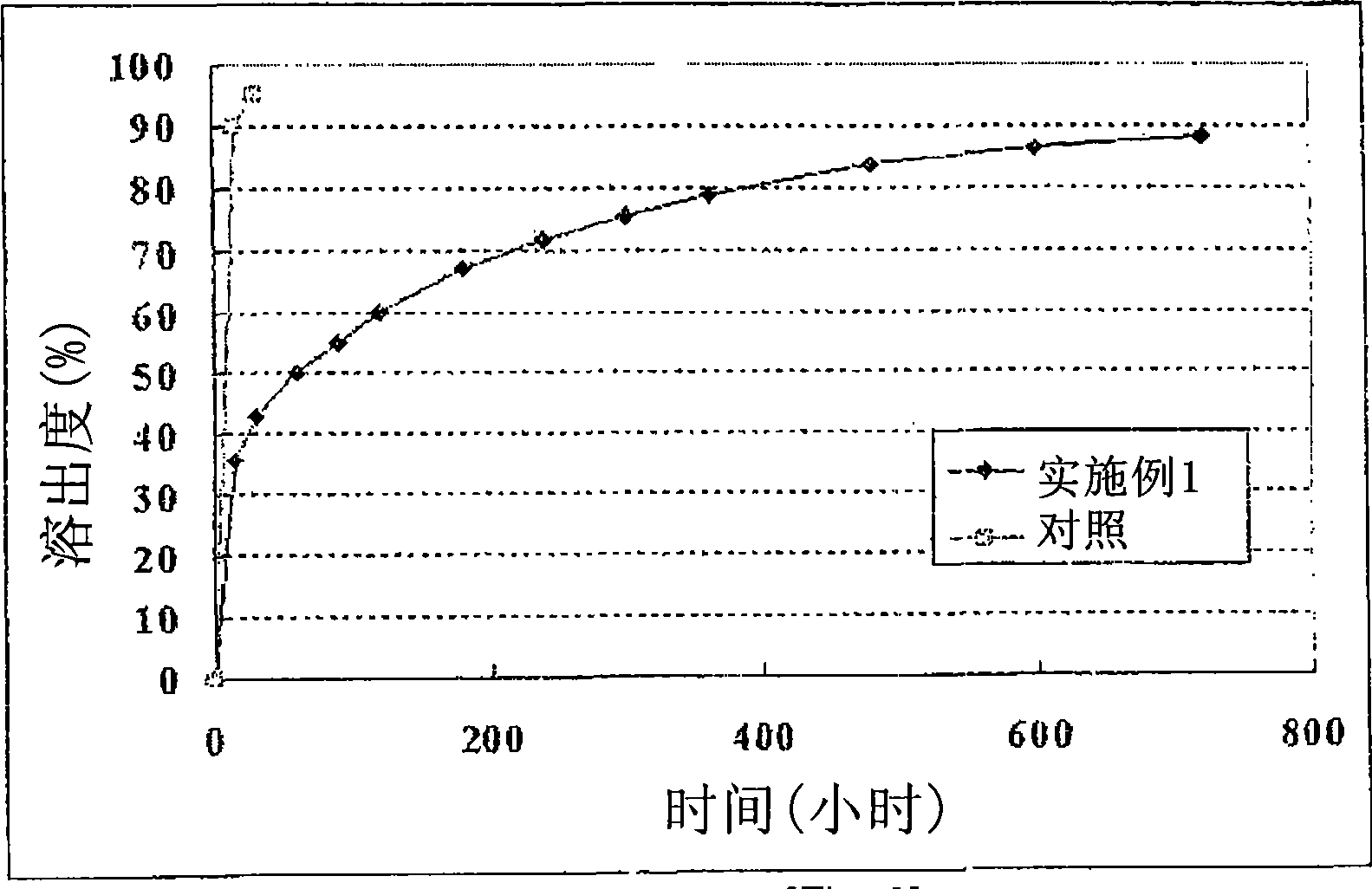
Isosorbide mononitrate sustained-release pallets, preparation prepared from same and preparation method for isosorbide mononitrate sustained-release pallets
ActiveCN103610650AStable in natureQuality improvementPharmaceutical non-active ingredientsGranular deliverySustained release pelletsAdhesive
The invention relates to the field of medicaments, and in particular relates to isosorbide mononitrate sustained-release pallets, a preparation prepared from the same and a preparation method for the isosorbide mononitrate sustained-release pallets. Each sustained-release pallet consists of a blank pallet core, a medicament-carrying layer, a sustained-release layer and a quick-release layer, wherein the medicament-carrying layer consists of isosorbide mononitrate, a first filling agent and a first adhesive; the sustained-release layer consists of a sustained-release material, a pore-forming agent, an anti-sticking agent, a plasticizer and a second adhesive; the quick-release layer consists of isosorbide mononitrate, a second filling agent and a third adhesive. The isosorbide mononitrate sustained-release pallets and the preparation prepared from the same are stable in property, reliable in quality and high in bioavailability, remarkable changes in the in-vitro release of a medicament caused by the crystallization of isosorbide mononitrate can be avoided, and the stability of the isosorbide mononitrate sustained-release pallets and the preparation prepared from the same is improved.
Owner:珠海润都制药股份有限公司
Multiple unit type sustained release oral formulation and process for the preparation thereof
Disclosed is a multiple unit type sustained release oral formulation comprising sustained release pellets formed from granules containing an active ingredient and a water-insoluble polymer, the granules being coated with a sustained release base material; and rapid release granules containing the active ingredient, and a method for preparing the same.
Owner:CJ CHEILJEDANG CORP
Topiramate sustained-release drug composition, method for preparing same and application of Topiramate sustained-release drug composition
ActiveCN102579367AOrganic active ingredientsNervous disorderSustained release pelletsSustained release drug
The invention belongs to the field of pharmaceutical chemicals, and relates to a Topiramate sustained-release drug composition, a method for preparing the same and an application of the Topiramate sustained-release drug composition. Specifically, the drug layer of the Topiramate sustained-release drug composition does not contain a bonding agent. Concretely, the Topiramate sustained-release drug composition is a sustained-release pellet. The Topiramate sustained-release drug composition has the advantages of good sustained-release effect, strong controllability, high stability, good repeatability, simple prescription, easiness in operation and preparation and the like.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Allopurinol sustained-release pellet and preparation method and preparation thereof
InactiveCN103417506AIncrease contentSimple recipeOrganic active ingredientsSkeletal disorderSustained release pelletsMedicine
The invention relates to the technical field of medicine, in particular to an allopurinol sustained-release pellet, and a preparation method and a preparation thereof. The allopurinol sustained-release pellet comprises the following components in parts by weight: 30-95 parts of allopurinol and 1-20 parts of anionic polymer, and further comprises a diluent or a pellet core. According to the allopurinol sustained-release pellet, the anionic polymer serves as both an adhesive and a sustained release material, and no additional adhesive is needed, so that the types and consumption of auxiliary materials are reduced, and the relative content of allopurinol in the pellet is improved. In addition, as the anionic polymer is applied in the pellet skeleton, the step of coating the sustained-release material is omitted, and accordingly, the preparation steps are reduced. Therefore, the allopurinol sustained-release pellet simplified not only the formula but also the preparation technology.
Owner:HYBIO PHARMA
Nicotine-containing sustained-release pellet and heatless smokeless cigarette containing pellet
ActiveCN104824850AUniform and stable releaseSolve non-volatileTobacco smoke filtersTobacco devicesSustained release pelletsWater baths
The invention provides a nicotine-containing sustained-release pellet. The nicotine-containing sustained-release pellet preparation method comprises steps: 1, by metering with weight parts, 60 to 90 parts of polyethylene glycol with molecular weight being 2000 to 6000 or 60 to 90 parts of a mixture with a different molecular weight is taken, heating and melting in a water bath at a temperature of 60 to 70 DEG C are carried out, 10 to 40 parts of nicotine by weight are then added, stirring and uniform mixing are carried out, and a mixed solution is formed; 2, an automatic pill dropping machine is used for dropping the above mixed solution in liquid paraffin, condensation and curing processing are carried out, and the nicotine-containing sustained-release pellet is acquired, wherein the liquid paraffin has a temperature between -4 DEG C to 0 DEG C so as to enable the dropped liquid drop to be quickly condensed and formed. The invention also provides a heatless smokeless cigarette containing the nicotine-containing sustained-release pellet. The problem that nicotine is not likely to volatilize or instable to volatilize when the nicotine is directly added to essence and flavor can be solved, no heat is generated during use, and no smoke, no tar or no carbon monoxide is generated.
Owner:HUBEI CHINA TOBACCO IND +1
Process for preparing tilmicosin pellets
ActiveCN104586774AEasy to useIncrease blood concentrationAntibacterial agentsOrganic active ingredientsSustained release pelletsFluidized bed
The invention provides a process for preparing tilmicosin pellets. The process comprises the following steps: a, uniformly mixing tilmicosin with a diluting agent and a disintegrating agent, adding ethanol, wetting, screening, rounding the particles in a rounding machine, and drying to obtain matrix type sustained-release pellets I; b, dissolving adhesive and carbomer in a 80% ethanol solution, adding tilmicosin into the ethanol solution to constantly stir to form a uniform suspension, adding the pellets I which are prepared in the step a and serve as pill cores into a fluidized bed turntable, spraying the suspension on the surfaces of the pill cores, drying, and collecting the dried pellets II; c, dissolving a coating agent in 80% ethanol, coating the pellets II, and drying to obtain the tilmicosin pellets. The tilmicosin pellets are stable and convenient to use, the bitter taste can be covered, and the pellets can be pressed into pellet tables for pets. The process is suitable for industrial production and popularization application.
Owner:CHENGDU QIANKUN VETERINARY PHARMA
Clonidine hydrochloride sustained release micropill preparation
ActiveCN102138906AExtended half-lifeExtension of timeOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsPharmaceutical formulation
The invention relates to the field of medicinal preparations, in particular to a clonidine hydrochloride sustained release preparation and a preparation method thereof. The clonidine hydrochloride sustained release preparation is characterized in that: a sustained release coating membrane is divided into two layers, wherein a sustained release coating membrane of an inner layer contains a pore-forming agent, and a sustained release coating membrane on an outer layer does not contain the pore-forming agent; and a weight ratio of a sustained release coating on the inner layer to a sustained release coating of the outer layer is (1:1)-(2:5), and the weight of sustained release coatings on inner and outer layers are increased by 7 to 8 percent. In the sustained release preparation, two sustained release layers are coated, so that medicaments are released slowly, safely and effectively.
Owner:HEFEI COSOURCE PHARMA CO LTD
Ambroxol hydrochloride sustained-release pellet and preparation method
ActiveCN101862305AOrganic active ingredientsPharmaceutical non-active ingredientsSustained release pelletsPulmonary circulation diseases
The invention relates to the composition of an ambroxol HCl-containing sustained-release pellet and a preparation method. The sustained-release pellet consists of two parts which are a quick-release pellet core and a sustained-release coating film respectively, can achieve the effect of long-term release approaching to zero-order release, is mainly used for treating acute and chronic respiratory diseases, and is a medicament of first choice for dispelling phlegm.
Owner:AVENTIS PHARMA HAINAN
Novel enteric sustained-release pellet for feed and preparation method thereof
ActiveCN102308916AReduce weight ratioReduce diarrhea rateAnimal feeding stuffAccessory food factorsSustained release pelletsCellulose acetate
The invention relates to a novel enteric sustained-release zinc oxide-oligosaccharide composite pellet for feed and a preparation method thereof. The novel enteric sustained-release zinc oxide-oligosaccharide composite pellet for feed comprises: by weight, 38 to 75% of zinc oxide powder, 15 to 35% of oligosaccharide, 2 to 14% of starch, 1.5 to 5.5% of cellulose acetate-phthalate, 0.05 to 0.1% of sodium alginate, 4.5 to 9.5% of talcum powder, and 0.15 to 0.5% of diethyl phthalate. The novel enteric sustained-release zinc oxide-oligosaccharide composite pellet for feed has the advantages of good effects of dissolution in the enteric canal and good treatment effects.
Owner:珠海天凯生物科技有限公司
Long-acting sustained-release pellet and preparation method thereof
InactiveCN103565751AEvenly wrappedReduced release rateOrganic active ingredientsGranular deliverySustained release pelletsBlood concentration
The invention discloses a long-acting sustained-release pellet and a preparation method thereof. The long-acting trimetazidine hydrochloride sustained-release pellet comprises a drug-containing core and a sustained-release coating layer from inside to outside sequentially, wherein an optional isolation coating layer is arranged between the drug-containing core and the sustained-release coating layer; the isolation coating layer accounts for 0-15 percent of the mass of the drug-containing core, and the sustained-release coating layer accounts for 5-30 percent of the mass of the drug-containing core; a gastrointestinal adhesive is contained in the sustained-release coating layer. According to the trimetazidine hydrochloride sustained-release pellet, the prescription is meticulously designed, the drug-containing core is uniformly coated by utilizing the sustained-release coating material, so that slow and uniform drug release of the drug-containing core can be maintained, the active component releasing speed can be reduced, the time for reaching the peak can be postponed, the pellet can be constantly and stably released in 24 hours, the blood concentration stability can be maintained, and the phenomenon of insufficient blood concentration in morning ischemia can be avoided. The administration frequency can be reduced to once a day, and the compliance of patients can be improved.
Owner:AC PHARMA CO LTD
Trimetazidine hydrochloride sustained-release capsule and preparation method thereof
InactiveCN104473905AOrganic active ingredientsSenses disorderControlled releaseSustained release pellets
The invention discloses a trimetazidine hydrochloride sustained-release capsule and a preparation method thereof, belonging to the technical field of medicines. The sustained-release capsule is prepared by filling sustained-release pellets containing trimetazidine in capsules, wherein the sustained-release pellets are a framework control preparation. The trimetazidine hydrochloride sustained-release capsule prepared by the preparation method is simple in process and low in cost. As the capsule is in a multiunit control release system of drug, the user does not need to worry about abrupt release of drug. Compared with preparations such as sustained release tablets, the trimetazidine hydrochloride sustained-release capsule is safe and reliable in medication and high in medication compliance.
Owner:万全万特制药(厦门)有限公司
Zolpidem tartrate controlled-release pellet and preparation method thereof
InactiveCN101884619AInhibitory concentrationAvoid timeOrganic active ingredientsNervous disorderSustained release pelletsControl release
The invention provides an officinal composite containing zolpidem tartrate, and the weight of the active ingredients of the officinal composite is between 1mg and 20mg. The invention is characterized in that the officinal composite is a controlled-release pellet which mainly consists of a pill core and a controlled-release layer with a controlled-release function or consists of a faster-working quick-release outer layer part, a controlled-release layer maintaining slow release and a pill core. The preparation prepared by the invention can prolong drug release time by 6h and play roles in reducing blood concentration fluctuation and prolonging drug action time.
Owner:BEIJING HONGWAN PHARMA TECH
Floating and sustained-release pellet, pharmaceutical composition containing pellet and preparation method of pharmaceutical composition
ActiveCN104288106ALow densityExtended floating timeOrganic active ingredientsNervous disorderSustained release pelletsMedicine
The invention discloses a Pregabalin gastric floating and sustained-release pellet. The pellet comprises a medicine-containing pellet core, a floating supporting layer and a sustained-release coating layer sequentially from inside to outside, wherein the external surface of the medicine-containing pellet core is wrapped by the floating supporting layer, and the external surface of the floating supporting layer is wrapped by the sustained-release coating layer; the medicine-containing pellet core contains a retarding agent, and the floating support layer contains a waxy material and a hydrophilic material. According to the Pregabalin gastric floating and sustained-release pellet disclosed by the invention, the risk that a medicine is suddenly released and is completely emptied can be lowered, and the bioavailability of the medicine and the adaptability of patient taking can be improved. Meanwhile, the invention further provides a pharmaceutical composition containing the gastric floating and sustained-release pellet and a preparation method of the pharmaceutical composition.
Owner:AC PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com